Abstract
To assess the true effect of novel therapies for ischaemic stroke, a positive control that can validate the experimental model and design is vital. Hypothermia may be a good candidate for such a positive control, given the convincing body of evidence from animal models of ischaemic stroke. Taking conditions under which substantial efficacy had been seen in a meta-analysis of hypothermia for focal ischaemia in animal models, we undertook three randomised and blinded studies examining the effect of hypothermia induced immediately following the onset of middle cerebral artery occlusion on infarct volume in rats (n = 15, 23, 264). Hypothermia to a depth of 33℃ and maintained for 130 min significantly reduced infarct volume compared to normothermia treatment (by 27–63%) and depended on ischaemic duration (F(3,244) = 21.242, p < 0.05). However, the protective effect varied across experiments with differences in both the size of the infarct observed in normothermic controls and the time to reach target temperature. Our results highlight the need for sample size and power calculations to take into account variations between individual experiments requiring induction of focal ischaemia.
Keywords: Ischaemic stroke, animal models, hypothermia, positive control, ischaemic duration
Introduction
Developing new treatments for brain diseases is difficult. The biological systems we are trying to protect or repair are complex and our knowledge of normal function is incomplete. Nevertheless, there is a growing consensus that our current lack of success necessitates a critical re-evaluation of the methodologies we use. This study examines just one step in this development pathway for stroke: the provision of a positive control in therapeutic intervention studies that can be used across experiments to act as a reference point for testing of therapeutic efficacy.
For thrombotic occlusion models, tissue plasminogen activator, which is effective in both animals1 and man,2,3 can serve this role. However, the majority of stroke studies do not use thrombotic occlusion models or may examine the potential of a therapy in the absence of reperfusion, therefore another positive control is required.
The evidence supporting the concept of cytoprotection is extensive, with convincing evidence that under certain circumstances it is possible to protect brain cells.4,5 In 2006, O’Collins and colleagues identified 1026 agents tested in laboratory models of stroke. Of those agents tested in models of focal ischaemia, 62% improved outcome compared to control.6 However, choosing from these drugs a gold standard for future cytoprotection studies is difficult.
Only hypothermia stands out as having convincing evidence for efficacy in animal models of stroke7–10 and a body of human evidence suggesting it may be cytoprotective in the human brain. Some of the evidence is anecdotal or circumstantial, such as that from reports of individuals who have made remarkable recoveries following cold water drowning.11 Other evidence is much more substantive. Few forms of life saving transplant surgery would be possible without the protective effects of hypothermia during organ harvest and transfer.12,13 Deep hypothermia is routinely used to protect the brain during cardiac and aortic arch surgery, affording 20–30 min of safe surgical time with complete circulatory arrest.14 Mild to moderate hypothermia is routinely used to protect the brain in adults with global ischaemia after cardiac arrest15,16 and in neonates with hypoxic–ischaemic encephalopathy.17,18 There is also clinical evidence of a relationship between body temperature and outcome after human stroke. Lower body temperature upon arrival at hospital after stroke is associated with better outcomes19 and higher temperatures are associated with worse outcomes.19–21 Human clinical trials of hypothermia in stroke are in progress.22,23
In this study, we present our laboratory’s experience with testing hypothermia as a potential positive control. The conditions suggested by meta-analysis of 101 animal studies of focal cerebral ischaemia were used to design the experiments. Specifically, we hypothesised that hypothermia induced to a depth of 33℃ for 130 min initiated immediately after stroke induction would reduce infarct volume by 35%.8 Our purpose here was not to explore the potential clinical utility of hypothermia but to determine the practicality of using hypothermia as a positive control for assessment of future therapeutic candidates. Given changing research staff is a reality of any laboratory, we show here the impact different surgeons can have on detected efficacy. We report that it is indeed practical to use hypothermia as an effective positive control for stroke studies. However, we also found that differences in degree of initial ischaemic insult induced by different surgical teams impacted on the degree of protection afforded. Variability in stroke induction needs to be carefully regulated.
Methods
Experimental design
Experiments 1 and 2 used an identical experimental protocol (Supplementary Figure 1). Hypothermia to 33℃ (or normothermia at 37.4℃) was induced at the onset of transient middle cerebral artery occlusion (MCAo) of 90 min duration. Temperature was controlled for 130 min after occlusion. Twenty-four hours post-MCAo, behavioural deficit was assessed and the brain collected for 2,3,5-triphenyltetrazolium chloride (TTC) staining and infarct analysis. Experiments 1 and 2 differed only in the surgeons inducing focal cerebral ischaemia and assessing brain infarct volume.
Experiment 3 was designed following unexpected results generated by Experiment 2 (Table 1b, Supplementary Figure 2). It aimed to examine the effect of hypothermia on different intensities of ischaemia produced by different occlusion durations, and in doing so explore the reasons for the differences in results obtained between the two surgical teams. In experiment 3, the second surgical team induced transient MCAo of 60, 75, 90 and 120 min duration in cohorts of rats that were then maintained under hypothermic (33℃) or normothermic (37.4℃) conditions for 130 min. Again, behavioural assessments were made at 24 h, immediately prior to tissue collection and TTC staining for infarct delineation.
Table 1.
Summary of outcome measures across all experiments.
| Normothermia | Hypothermia | |||||||
|---|---|---|---|---|---|---|---|---|
| (a) Experiment 1 | ||||||||
| Occlusion duration (min) | 90 | 90 | ||||||
| n | 6 | 6 | ||||||
| Weight at MCAo (g) | 314 ± 18 | 321 ± 13 | ||||||
| Rectal temperature at MCAo (℃) | 37.6 ± 0.2 | 37.5 ± 0.2 | ||||||
| CBF decrement at occlusion (% of baseline) | 69.5 ± 13.0 | 72.7 ± 18.6 | ||||||
| CBF increment at reperfusion (% of pre-occlusion) | 155.6 ± 85.2 | 134.7 ± 57.6 | ||||||
| Behavioural deficit at 24 h (median (Q1–Q3)) | 1.5 (0.8–2.3) | 0.25 (0–1.6) | ||||||
| Infarct volume at 24 h | ||||||||
| Uncorrected (mm3) | 156.3 ± 65.8 | 56.8 ± 50.4* | ||||||
| Corrected for oedema (mm3) | 139.6 ± 56.8 | 52.2 ± 47.8* | ||||||
| Per cent of contralateral hemisphere | 22.7 ± 9.5 | 8.3 ± 6.6% * | ||||||
| (b) Experiment 2 | ||||||||
| Occlusion duration (min) | 90 | 90 | ||||||
| n | 10 | 10 | ||||||
| Weight at MCAo (g) | 329 ± 23 | 350 ± 28 | ||||||
| Rectal temperature at MCAo (℃) | 36.7 ± 1.0 | 36.4 ± 1.1 | ||||||
| CBF decrement at occlusion (% of baseline) | 84.3 ± 11.5 | 82.3 ± 11.7 | ||||||
| CBF increment at reperfusion (% of pre-occlusion) | 107.7 ± 35.0 | 95.3 ± 30.2 | ||||||
| Behavioural deficit at 24 h (median (Q1–Q3)) | 1.8 (0.6–2.0) | 1.5 (1.1–2.0) | ||||||
| Infarct volume at 24 h | ||||||||
| Uncorrected (mm3) | 221.2 ± 62.1 | 183.8 ± 99.7 | ||||||
| Corrected for oedema (mm3) | 195.6 ± 52.5 | 155.8 ± 77.4 | ||||||
| Per cent of contralateral hemisphere | 27.3 ± 6.2 | 23.1 ± 12.4 | ||||||
| (c) Experiment 3 | ||||||||
| Occlusion duration (min) | 60 | 75 | 90 | 120 | 60 | 75 | 90 | 120 |
| n | 31 | 32 | 34 | 31 | 31 | 32 | 31 | 32 |
| Weight at MCAo (g) | 308 ± 19 | 312 ± 19 | 310 ± 16 | 313 ± 13 | 310 ± 20 | 308 ± 23 | 315 ± 15 | 312 ± 22 |
| Rectal temperature at MCAo (℃) | 37.1 ± 0.5 | 37.3 ± 0.6 | 37.0 ± 0.6 | 37.1 ± 0.6 | 37.2 ± 0.7 | 37.0 ± 0.6 | 37.1 ± 0.6 | 37.2 ± 0.9 |
| CBF decrement at occlusion (% of baseline) | 66.6 ± 19.6 | 65.7 ± 17.6 | 68.3 ± 16.4 | 67.3 ± 15.5 | 57.5 ± 30.8 | 63.6 ± 19.1 | 61.8 ± 17.8 | 58.2 ± 23.4 |
| CBF increment at reperfusion (% of pre-occlusion) | 123.3 ± 56.5 | 135.1 ± 44.7 | 141.1 ± 76.3 | 150.6 ± 91.6 | 144.3 ± 68.5 | 157.8 ± 50.1 | 162.7 ± 67.4 | 137.5 ± 63.3 |
| Behavioural deficit at 24 h (median (Q1–Q3)) | 1 (0.3–2) | 1 (0.5–2) | 1.5 (0.6–2) | 1.3 (0.6–1.5) | 0 (0–0.5) | 0.5 (0–1) | 0.5 (0–1.5) | 1 (0.5–2) |
| Infarct volume at 24 h | ||||||||
| Uncorrected (mm3) | 143.3 ± 80.4 | 209.0 ± 81.1 | 247.2 ± 113.7 | 263.8 ± 89.5 | 67.3 ± 64.6* | 92.2 ± 64.6* | 152.7 ± 91.5* | 188.7 ± 88.6* |
| Corrected for oedema (mm3) | 134.2 ± 75.6 | 190.3 ± 38.9 | 218.9 ± 97.4 | 229.8 ± 76.8 | 64.7 ± 66.1* | 85.4 ± 58.1* | 137.5 ± 79.8* | 171.5 ± 79.1 |
| Per cent of contralateral hemisphere | 15.9 ± 8.9 | 23.4 ± 9.2 | 26.5 ± 11.6 | 28.2 ± 9.7 | 8.0 ± 8.2* | 10.4 ± 7.2* | 16.7 ± 9.9* | 20.7 ± 9.5* |
CBF: cerebral blood flow; MCAo: middle cerebral artery occlusion.
All values mean ± standard deviation (except behavioural deficit: median (Q1–Q3)); *p < 0.05.
To reduce selection, performance, detection and attrition biases, care was taken to ensure animals were randomised to experimental group, investigators remained blinded to experimental group throughout surgery and during outcome assessments, and data from all animals used in the experiment are included in the reported outcomes.
Specifically, animals were numbered upon arrival at the animal facility. An investigator independent of the surgical procedure randomised the list of animals to experimental group (using Excel random number generator), with details for each animal being sealed in an envelope opened only once the occluding thread was in place. Given that hypothermia is an active treatment to which the surgeon inducing the lesion can no longer be blinded, behavioural assessments were made by an independent investigator unaware of temperature treatment. Digital images of TTC stained brain slices were recoded to ensure experimental group remained concealed throughout infarct assessment.
Initial sample size calculations (see below for detail) based on prior experiments in spontaneously hypertensive rat (SHR) by the surgeon who performed experiment 1 established that cohorts of six would detect a 35% effect size with a power of 0.8 and alpha = 0.05. The data from experiment 1 were used to provide an updated estimate for experiment 2 of n = 10. Larger baseline infarcts induced by these surgeons required the sample size to be adjusted to n = 30 for experiment 3.
Animals and ethics
All procedures involving animals were approved by the Animal Ethics Committee of Austin Health (Heidelberg, Victoria, Australia) and performed in accordance with institutional and national guidelines (Australian code of practice for the care and use of animals for scientific purposes, 7th edition, 2004) and the Animal Research: Reporting In Vivo Experiments guidelines.24 Male SHRs were purchased from the Animal Resource Centre (Canning Vale, Western Australia) and acclimatised to the Austin Health animal facility conditions (12:12 day:night cycle, ad libitum access to standard rat chow and water) for at least two weeks before induction of ischaemia. Stroke was induced at 15 weeks of age. In total, 302 animals were used for this study.
Anaesthesia
Anaesthesia was initiated with 5% isoflurane mixed with 50% Medical Air:O2 in an enclosed box. Once anaesthetised, animals were transferred to a nose cone (Sound Veterinary Equipment, Rowville, VIC, Australia) and anaesthesia maintained in freely breathing rats with 2% isoflurane in 50% Air:O2 for the rest of the surgical period. Body temperature was maintained at 37℃ via a rectal probe coupled to a heat mat on which the rat lay (manufactured in house). A halogen lamp provided additional warmth if required. Intraperitoneal atropine (Astra Pharmaceutical Pty Ltd) in saline (120 µg) was administered to ease respiration through inhibition of bronchial secretions and salivation throughout surgery. Post-operative analgesia was provided in the form of a single rectal suppository of paracetamol (5 mg of a 20 mg/kg solution) and provision of paracetamol (120 mg/kg body weight) in the drinking water. This dose would be unlikely to exert any major effect on temperature.25 Animals were provided with a 3 ml intraperitoneal injection of 0.9% saline at the end of anaesthesia to help prevent dehydration.
Induction of ischaemia
Cerebral blood flow (CBF) was monitored throughout surgery using Laser Doppler (MoorLab, Devon, UK; in later experiments coupled to iWORX, Dover, NH, USA). A right-angled probe (0.8 mm; MP5b) was positioned over the watershed region between the MCA and ACA territories; 5 mm lateral, 1 mm posterior relative to Bregma.
For all experiments, MCAo was induced using the thread occlusion model, as described by Longa et al.26 with modifications by Spratt et al.27 Briefly, after ligation of branch arteries including the pterygopalatine artery and creation of an external carotid artery stump, a silicone-coated suture (0.35 mm diameter, 2 mm length silicone coating, manufactured in house, Selleys, Padstow NSW; 4-0 nylon, Dynek, Port Adelaide, SA)28 was passed via the right external carotid, and up the internal carotid artery approximately 18 mm from the carotid bifurcation until resistance was felt and a drop in CBF was detected by laser Doppler flowmetry. After the designated occlusion period, the silicone coated suture was gently withdrawn, allowing reperfusion.
Induction of hypothermia
To maximise the likelihood of an effect, body temperature reduction to a target of 33℃ was started within 2 min of occlusion and maintained for 130 min.
Animal body temperature was maintained at 37.4℃ during the surgical dissection until MCAo. Upon insertion of the occluding thread, the experimental group to which the animal was allocated was revealed, at which point induction of hypothermia (to 33℃) was initiated or normothermia (37.4℃) was maintained. Hypothermia was induced via the cooling effect of 70% ethanol sprayed lightly on to the rats’ torso together with fan-induced evaporation. The set point of the temperature control box was decreased to 33℃. Normothermia was maintained using small halogen lamps and the temperature control box coupled to a heat mat (set at 37.4℃). After 130 min of temperature regulation, hypothermic animals were warmed to 37.4℃ and all animals allowed to recover from anaesthesia. Following surgery, animals were housed individually, with self-regulation of temperature.
Assessment of outcome
At the end of each experiment (24 h after induction of ischaemia) neurobehavioural deficit was assessed using the methods described by Petullo et al.29 to score forelimb flexion, torso twisting, lateral push resistance, and general mobility. The rats were then killed by decapitation after isoflurane overdose. The brain was cut into 2 mm coronal sections and rapidly stained with a 1% solution of TTC (Sigma–Aldrich, USA) in normal saline. After 10 min staining per side, sections were fixed in 10% formalin. Digital photographs were taken under fixed lighting conditions and infarct area quantified by researchers blinded to treatment allocation using Image J (NIH). Infarct volumes were derived by calculating the average infarct area between slices and multiplying by the distance between slices. The impact of oedema was accounted for by adjusting the infarct area by the degree of swelling of the ipsilateral hemisphere relative to the contralateral hemisphere. Corrected infarct volume was then expressed as a proportion of the contralateral hemisphere volume.
Statistics and analysis
Statistical analyses were conducted using IBM SPSS Statistics v20 and STATA 13IC. The primary outcome was infarct volume, expressed as a proportion of contralateral hemisphere. Groups were compared using t-tests or ANOVA with Tukey post hoc analysis for between-group comparisons of infarct volume. The effect of hypothermia on behavioural deficit was assessed by generalised odds ratio.30 Sample size and power calculations based on infarct volume data were performed using a web-based calculator (http://www.statisticalsolutions.net/pssZtest_calc.phpl), using an effect size of 35%, power of 0.8 and alpha 0.05. All data are presented as mean ± standard deviation, with the exception of behavioural score, median ± IQR.
Results
Experiment 1: Reduction of infarct volume by hypothermia using the optimised SHR model: A pilot study
Fifteen animals underwent MCAo during this experiment. Three animals died due to subarachnoid haemorrhage during surgery and before assignment to treatment group. These animals were excluded from further analysis. Six animals for each of the hypothermia and normothermia groups received treatment and completed the experiment.
Both normothermic and hypothermic experimental groups were well matched for body weight, temperature at MCAo, degree of CBF decrement at occlusion and degree of reperfusion at thread withdrawal (Table 1a). Hypothermia to a depth of 33℃ was induced rapidly in animals assigned to the treatment group. The average time to reach the target temperature was 14 ± 1.5 min.
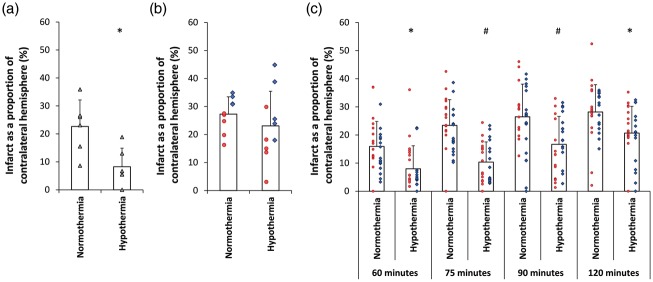
Hypothermia reduced infarct volume by 63.4% (Table 1a, Figure 1(a), (t(10) = 3.039, p = 0.012) (corrected infarct volume expressed as a proportion of the contralateral hemisphere)). For completeness, Table 1 also shows raw infarct volume and oedema corrected volume. In the normothermia group, damage to the striatum and cortex was observed in all animals. In the hypothermia group, ischaemic damage was generally restricted to the striatum, with cortical damage significantly smaller (103.9 ± 53.1 mm3 versus 45.4 ± 35.9 mm3; t(10) = 2.238; p = 0.049).
Figure 1.
Effect of hypothermia treatment on infarct size after tMCAo in the SHR. (a) Experiment 1. Hypothermia reduced ischaemic damage by 63%. (b) Experiment 2. Hypothermia had a reduced protective effect when performed by surgery team 2. (c) Experiment 3. Hypothermia reduced ischaemic damage compared to normothermic controls for 60, 75, 90 and 120 min tMCAo. All data presented as mean ± standard deviation. Individual animals are represented by each data point. Each shape/colour represents a different surgeon. #p < 0.001. *p < 0.05.
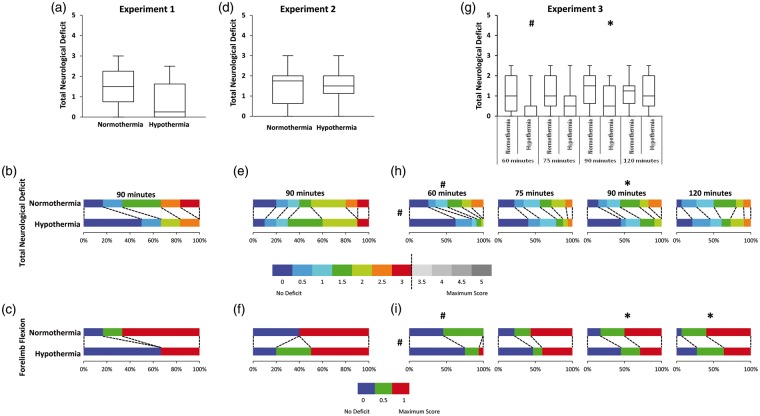
Hypothermia treatment did not influence behavioural deficit when assessed at 24 h (generalised odds ratio = 2.13, 95% CI [0.54, 8.38], p = 0.249) (Figure 2(a) and (b)). Of the test components, forelimb flexion was found to have the greatest influence (Figure 2(c)). Within the normothermic group, 83% of animals sustained forelimb flexion at 24 h, whereas only 33% of hypothermic animals had evidence of forelimb flexion.
Figure 2.
Behavioural deficit assessed using a five-point neuroscore at 24 h after stroke. Box plots of total score at 24 h (a, d, g – Experiments 1, 2, 3). Proportion of animals with each score broken into 0.5 unit increments (b, e, h – Experiments 1, 2, 3). No animal scored greater than 3. Proportion of animals with deficits in forelimb flexion (c, f, i – Experiments 1, 2, 3). #p < 0.001. *p < 0.05.
Experiment 2: Replication study with two different surgeons
Experiment 2 was similar in design to experiment 1, but performed by a different surgical team consisting of two surgeons. These surgeons were well experienced in inducing stroke using the thread occlusion model in rats. Ten animals per cohort were required, based on sample size calculations from experiment 1. A total of 23 animals were used in this experiment. Three animals died due to surgical error before induction of MCAo or randomisation to treatment group. Ten animals were randomised to each treatment group, with five from each surgeon in each group. There were no differences between normothermia and hypothermia groups in terms of weight, CBF change or temperature at MCAo (Table 1b).
Infarct volume in the hypothermic group was 15.4% lower than in the normothermic group, but this was not statistically significant (Table 1b, Figure 1(b), t(17) = 0.970, p = 0.346). Post hoc power analysis revealed reduced power of 0.57. There was no difference between normothermia and hypothermia cohorts in terms of behavioural deficit at 24 h (Figure 2(d) to (f)). Post hoc examination of the experiment suggested two factors that may have contributed to hypothermia having a smaller effect on infarct volume than in experiment 1. The first was the time to target temperature, being 19 ± 3.3 min, significantly slower than the 14 ± 1.5 min for the previous experiment (F(1,3) = 12.12, p = 0.004). The second factor that may have contributed to a smaller effect of hypothermia was the trend towards larger infarcts in the normothermia group compared to the previous experiment (221.2 mm3 versus 156.3 mm3, F(1,13) = 3.76, p = 0.07) (Supplementary Figure 2). This is consistent with the larger drop in CBF at MCAo between the two experiments (t(29) = − 2.521, p = 0.017).
Experiment 3: Effect of hypothermia across different durations of occlusion
To try to explain the difference in results between experiments 1 and 2 we hypothesised that the extent of ischaemic damage (and therefore infarct volume) could influence the protective potential of hypothermia. A third experiment was designed to examine the effect of occlusion duration (so varying infarct size) and hypothermia treatment initiated at MCAo onset on infarct volume. Stroke induction surgery was performed by the same surgeons as experiment 2.
A total of 264 animals were used in this experiment. Ten animals died before the 24 h endpoint, all of which had either 90 or 120 min occlusion duration, with subarachnoid haemorrhage being the most common cause of death. Details of experimental group numbers are presented in Table 1c. Two hundred and fifty-four animals reached their designated endpoint, with 31–34 animals per experimental group (Table 1c). In this experiment of 254 animals which completed the experiment, just nine had no detectible infarction. This equates to an experimental failure rate (no infarct induced) of just 3.5%.
Experimental groups were well matched for weight, temperature immediately prior to MCAo and CBF drop at thread insertion (Table 1c). Animals in the hypothermia group reached 33℃ within 14 ± 4.5 min of MCAo onset. This is not significantly different to that of experiment 1 (p = 0.930).
When the primary outcome of infarct as a proportion of contralateral hemisphere was examined, the protective effect of hypothermia was maintained (Table 1c, Figure 1(c)). Two-way ANOVA found significant effects of occlusion duration (F(3,244) = 21.242, p < 0.001) and temperature (F(1,244) = 65.609, p < 0.001) on infarct volume, with no significant interaction between the two (F(3,244) = 1.143, p = 0.332). Tukey post hoc comparisons indicated a protective effect of hypothermia for 60 min (49.7%, M = 7.99 CI [4.98, 10.99] p = 0.021), 75 min (55.5%, M = 10.37 CI [7.78, 12.95] p < 0.001), 90 min (36.9%, M = 16.69 CI [13.04, 20.34] p = 0.001) and 120 min (26.6%, M = 20.69 CI [17.21, 24.18] p = 0.038) occlusion durations.
Behavioural deficit was significantly improved in hypothermia-treated animals (pooled generalised odds ratio = 1.802, 95% CI [1.36, 2.39], p < 0.001)) (Figure 2(g) and (h)), i.e. a random animal from the hypothermia-treated group has an odds of a better outcome (lower behavioural score) 1.8 times higher than a random animal from the normothermia group. When stratified by occlusion duration, hypothermia-treated animals had significantly greater odds of a better outcome after 60 min (odds ratio = 2.86, 95% CI [1.58, 5.17], p = 0.0002) and 90 min tMCAo (odds ratio = 2.16, 95% CI [1.23, 3.79], p = 0.006) (Figure 2(h)).30 When forelimb flexion was examined, hypothermia treatment resulted in lower deficit (pooled generalised odds ratio = 1.979, 95% CI [1.50, 2.61], p < 0.001) (Figure 2(i)). When stratified by occlusion duration, hypothermia treatment resulted in a greater odds of a better outcome in forelimb flexion for 60 min (odds ratio = 2.82, 95% CI [1.58, 5.01], p = 0.000), 90 min (odds ratio = 1.89, 95% CI [1.09, 3.31], p = 0.021) and 120 min tMCAo (odds ratio = 1.87, 95% CI [1.09, 3.21], p = 0.027).
Discussion
In this series of experiments, we have used the conditions suggested by meta-analysis to test the potential of hypothermia to act as a positive control in experiments designed to assess the ability of new drugs to reduce infarct size after transient MCAo in rats. Our results confirm the practicality of doing so. However, during the course of these experiments we noted critical facets of modelling that need to be controlled to ensure this utility. The first small pilot study showed hypothermia to a depth of 33℃ initiated at the onset of ischaemia for 130 min duration significantly reduced ischaemic damage by 63%. However, a second small study conducted by a different surgical team showed a diminished protective effect (15%). Because control infarct volumes were substantially larger in this second experiment (221 mm3 versus 156 mm3) we hypothesised that this caused the diminished protective effect. Follow up in the third larger study by the second surgical team controlled infarct volume by varying occlusion duration to 60, 75, 90 or 120 min. The resulting infarcts could be significantly protected by 50, 56, 37 and 27%, respectively.
For a positive control to be useful, efficacy must be shown across multiple research teams, both within the same laboratory over time and across different laboratories. Differences in the protective effect of hypothermia between experiments 1 and 2 (performed by different surgical teams) highlight the importance of controlling within laboratory experimental conditions. It also suggests that such practicalities (as multiple surgical teams) will need to be carefully considered for multicentre preclinical testing programmes such as Multi-PART (http://www.dcn.ed.ac.uk/multipart/about.html).
Using variability data from surgical team 2 allowed experiment 3 to be powered appropriately given the shift in pattern of control infarct volume. This experience highlights both the importance of appropriately powering experiments and using data from the individual surgeons for these sample size estimates. If larger animal studies are to be undertaken (such as envisaged in Multi-PART), multiple surgeons will invariably be involved. Being aware and taking into account the variation within and between surgeons is important for ensuring studies are appropriately powered.
What causes these differences between experiments is not clear and requires clarification. Slight variations in performance between individual surgeons may be responsible. For example, using the feeling of resistance when the occluding thread reaches the origin of the MCA as the arbiter of experimental success is subjective, potentially allowing differing degrees of blockage of the MCA.31,32 The use of laser Doppler with objective inclusion and exclusion criteria is designed to overcome this. However, as CBF drop at MCAo does not correlate well with infarct volume, criteria for inclusion or exclusion of animals based on Laser Doppler must be carefully considered.33 Nonetheless, laser Doppler is a useful tool for identifying and excluding inadvertent subarachnoid haemorrhage.34
Differences between batches of animals over time may also contribute to such results. Subtle differences in the branching of the MCA or in blood pressure (in the SHR) may lead to varying infarct size.35,36 However, neither MCA branching nor blood pressure were assessed in these experiments. Another factor that could have contributed to the differing protective effect of hypothermia was a difference in time to reach target temperature. This was significantly slower (19 ± 3.3 min) in the second experiment. In both experiments where hypothermia significantly reduced infarct size, hypothermia to a depth of 33℃ was reached within 15 min. Whilst only being a difference of a few minutes, the actual rate of temperature decline may be important.
Across all experiments presented here, hypothermia protected smaller infarcts to a greater extent than large infarcts. This is consistent with most expectations; patients with larger more evolved lesions are likely to do less well than patients with small initial lesions and large penumbras. The greatest benefits are thus likely to be obtained by those patients with the earliest recanalisation. This is certainly consistent with animal data suggesting hypothermia treatment for stroke is greatest in the context of transient MCAo, suggesting that it is likely to work in concert with reperfusion strategies.8
Given the consistent reduction in infarct volume, we believe hypothermia is a viable option for use as a positive control for future studies of experimental stroke. Whilst we focussed our experimental model here on thread occlusion in the SHR (as infarcts can be consistently induced28), hypothermia has the potential to be applied across different experimental models, and to animals of different species, strains and co-morbidities. Furthermore, given its multiple hypothesised modes of action,37,38 hypothermia has the potential to be protective across different degrees of damage (as demonstrated here) and differing locations. It has a distinct advantage over other candidates for use as a positive control in that it is free and able to be induced and maintained using standard surgical monitoring equipment. Whilst the timing of hypothermia onset used here is not clinically relevant, as a positive control in could be an easy inclusion to most experimental designs where temperature control is already an integral part of most experiments.
Whilst this study demonstrates the potential of hypothermia as a positive control for experimental stroke studies, it is limited here to the thread occlusion model in young, male SHR rats, who experience large striatal and cortical infarcts. Exploration of the benefit of hypothermia as a control in other models, and in animals of different strains, sex, age and co-morbidities is an important next step. Similarly, expanding the repertoire of behavioural tests and the timing of assessment into the weeks and months post-stroke would enhance the link between animal models and clinical stroke and align with the STAIR guidelines.39 In this study, assessment of general disability 24 h after stroke using a standard neuroscore together with assessment of infarct size provided proof of principle that hypothermia could offer an effective positive control. However, 24 h may not be the ideal time to make infarct volume or behavioural assessments because of persistence of anaesthetic and surgical effects. The current STAIR guidelines recommend that therapeutic assessments include studies conducted two weeks or longer after stroke to confirm a sustained benefit.39 Whilst our study was limited to the first day following stroke, others have shown hypothermia to improve neurological deficit and histological damage in the weeks post-stroke.40–44 This supports the idea to use hypothermia as a positive control for both acute and chronic studies. Nevertheless, our systematic review and meta-analysis of therapeutic hypothermia in animal models of ischaemic stroke shows sustained benefit out to two months after stroke and shows that the benefits detected at 24 h and two months are equivalent.8 Expansion of these studies to include tests more closely linking regional neural injury to outcome, for example forelimb motor cortex and paw dexterity assessed for extended periods after stroke, would provide even more powerful confirmation of these effects, and negate any confounding effects of general anaesthesia that may influence the behavioural assessment used here.
Conclusions
Whilst instigating hypothermia treatment at the onset of stroke is rarely possible, it provided us with the best opportunity to investigate the utility of hypothermia as a practical positive control for future experimental animal studies. A gold standard positive control such as hypothermia would help validate the animal model, experimental design and highlight the relative merits of a new treatment. Here we found that robust protection could be achieved and that randomising and blinding these experiments appropriately with a small team was easily achievable. We also found that baseline ischaemic challenge was a critical determinant of success. This is entirely consistent with a biologically plausible mode of action. The observation that baseline ischaemic challenge was strongly influenced by the surgical team employed is not entirely unexpected but highlights the importance of a priori power calculations tailored to experimental purpose and real world variability data.
Supplementary Material
Acknowledgements
The Florey gratefully acknowledges the operational infrastructure support of the Victorian State Government.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by an NHMRC-EU collaborative grant. H.B. van der Worp is supported by a grant from the Dutch Heart Foundation (2010T075).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
Experimental conception and design: DWH, MRM, HBW, JAF, SFC, SSJR, ALJ, SAS
Randomisation and blinding: SFC
Performed the experiments: JAF, ALJ, SAS, SSJR
Tissue preparation: EA, SFC, ALJ, SAS, JAF
Infarct analysis: ALJ, SAS, JAF
Data analysis: SSJR, SFC, LC, DWH
Manuscript preparation: SSJR, DWH
All authors contributed to revising the manuscript.
Supplementary material
Supplementary material for this paper can be found at http://journals.sagepub.com/doi/suppl/10.1177/0271678X16688704.
References
- 1.Sena ES, Briscoe CL, Howells DW, et al. Factors affecting the apparent efficacy and safety of tissue plasminogen activator in thrombotic occlusion models of stroke: systematic review and meta-analysis. J Cereb Blood Flow Metab 2010; 30: 1905–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lees KR, Bluhmki E, von Kummer R, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet 2010; 375: 1695–1703. [DOI] [PubMed] [Google Scholar]
- 3.Emberson J, Lees KR, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet 2014; 384: 1929–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sosa IJ, Reyes O, Inserni J, et al. Isolation and long-term survival of adult human sensory neurons in vitro. Neurosurgery 1998; 42: 681–685; discussion 5–6. [DOI] [PubMed] [Google Scholar]
- 5.Verwer RW, Hermens WT, Dijkhuizen P, et al. Cells in human postmortem brain tissue slices remain alive for several weeks in culture. FASEB J 2002; 16: 54–60. [DOI] [PubMed] [Google Scholar]
- 6.O’Collins VE, Macleod MR, Donnan GA, et al. 1,026 experimental treatments in acute stroke. Ann Neurol 2006; 59: 467–477. [DOI] [PubMed] [Google Scholar]
- 7.Dumitrascu OM, Lamb J, Lyden PD. Still cooling after all these years: meta-analysis of pre-clinical trials of therapeutic hypothermia for acute ischemic stroke. J Cereb Blood Flow Metab 2016; 36: 1157–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Worp HB, Sena ES, Donnan GA, et al. Hypothermia in animal models of acute ischaemic stroke: a systematic review and meta-analysis. Brain 2007; 130: 3063–3074. [DOI] [PubMed] [Google Scholar]
- 9.Sena ES, Jeffreys AL, Cox SF, et al. The benefit of hypothermia in experimental ischemic stroke is not affected by pethidine. Int J Stroke 2013; 8: 180–185. [DOI] [PubMed] [Google Scholar]
- 10.Froehler MT, Ovbiagele B. Therapeutic hypothermia for acute ischemic stroke. Expert Rev Cardiovasc Ther 2010; 8: 593–603. [DOI] [PubMed] [Google Scholar]
- 11.Samuelson H, Nekludov M, Levander M. Neuropsychological outcome following near-drowning in ice water: two adult case studies. J Int Neuropsychol Soc 2008; 14: 660–666. [DOI] [PubMed] [Google Scholar]
- 12.Maathuis MH, Leuvenink HG, Ploeg RJ. Perspectives in organ preservation. Transplantation 2007; 83: 1289–1298. [DOI] [PubMed] [Google Scholar]
- 13.Niemann CU, Feiner J, Swain S, et al. Therapeutic hypothermia in deceased organ donors and kidney-graft function. N Engl J Med 2015; 373: 405–414. [DOI] [PubMed] [Google Scholar]
- 14.Yan TD, Bannon PG, Bavaria J, et al. Consensus on hypothermia in aortic arch surgery. Ann Cardiothorac Surg 2013; 2: 163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med 2002; 346: 557–563. [DOI] [PubMed] [Google Scholar]
- 16.Arrich J, Holzer M, Herkner H, et al. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database Syst Rev 2009; 4: CD004128. [DOI] [PubMed] [Google Scholar]
- 17.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med 2005; 353: 1574–1584. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs S, Hunt R, Tarnow-Mordi W, et al. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev 2007; 17: CD003311. [DOI] [PubMed] [Google Scholar]
- 19.Reith J, Jorgensen HS, Pedersen PM, et al. Body temperature in acute stroke: relation to stroke severity, infarct size, mortality, and outcome. Lancet 1996; 347: 422–425. [DOI] [PubMed] [Google Scholar]
- 20.Castillo J, Davalos A, Marrugat J, et al. Timing for fever-related brain damage in acute ischemic stroke. Stroke 1998; 29: 2455–2460. [DOI] [PubMed] [Google Scholar]
- 21.Hajat C, Hajat S, Sharma P. Effects of poststroke pyrexia on stroke outcome: a meta-analysis of studies in patients. Stroke 2000; 31: 410–414. [DOI] [PubMed] [Google Scholar]
- 22.van der Worp HB, Macleod MR, Bath PM, et al. EuroHYP-1: European multicenter, randomized, phase III clinical trial of therapeutic hypothermia plus best medical treatment vs. best medical treatment alone for acute ischemic stroke. Int J Stroke 2014; 9: 642–645. [DOI] [PubMed] [Google Scholar]
- 23.Lyden PD, Hemmen TM, Grotta J, et al. Endovascular therapeutic hypothermia for acute ischemic stroke: ICTuS 2/3 protocol. Int J Stroke 2014; 9: 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kilkenny C, Browne WJ, Cuthill IC, et al. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 2010; 8: e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ayoub SS, Botting RM, Goorha S, et al. Acetaminophen-induced hypothermia in mice is mediated by a prostaglandin endoperoxide synthase 1 gene-derived protein. Proc Natl Acad Sci USA 2004; 101: 11165–11169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Longa EZ, Weinstein PR, Carlson S, et al. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989; 20: 84–91. [DOI] [PubMed] [Google Scholar]
- 27.Spratt NJ, Fernandez J, Chen M, et al. Modification of the method of thread manufacture improves stroke induction rate and reduces mortality after thread-occlusion of the middle cerebral artery in young or aged rats. J Neurosci Methods 2006; 155: 285–290. [DOI] [PubMed] [Google Scholar]
- 28.Spratt NJ, Fernandez J, Chen M, et al. Modification of the method of thread manufacture improves stroke induction rate and reduces mortality after thread-occlusion of the middle cerebral artery in young or aged rats. J Neurosci Methods 2006; 155: 285–290. [DOI] [PubMed] [Google Scholar]
- 29.Petullo D, Masonic K, Lincoln C, et al. Model development and behavioral assessment of focal cerebral ischemia in rats. Life Sci 1999; 64: 1099–1108. [DOI] [PubMed] [Google Scholar]
- 30.Churilov L, Arnup S, Johns H, et al. An improved method for simple, assumption-free ordinal analysis of the modified Rankin Scale using generalized odds ratios. Int J Stroke 2014; 9: 999–1005. [DOI] [PubMed] [Google Scholar]
- 31.Belayev L, Alonso OF, Busto R, et al. Middle cerebral artery occlusion in the rat by intraluminal suture. Neurological and pathological evaluation of an improved model. Stroke 1996; 27: 1616–22; discussion 23. [DOI] [PubMed] [Google Scholar]
- 32.Ren Y, Hashimoto M, Pulsinelli WA, et al. Hypothermic protection in rat focal ischemia models: strain differences and relevance to “reperfusion injury”. J Cereb Blood Flow Metab 2004; 24: 42–53. [DOI] [PubMed] [Google Scholar]
- 33.Morris GP, Wright AL, Tan RP, et al. A comparative study of variables influencing ischemic injury in the Longa and Koizumi methods of intraluminal filament middle cerebral artery occlusion in mice. PLoS One 2016; 11: e0148503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmid-Elsaesser R, Zausinger S, Hungerhuber E, et al. A critical reevaluation of the intraluminal thread model of focal cerebral ischemia: evidence of inadvertent premature reperfusion and subarachnoid hemorrhage in rats by Laser-Doppler flowmetry. Editorial comment: evidence of inadvertent premature reperfusion and subarachnoid hemorrhage in rats by Laser-Doppler flowmetry. Stroke 1998; 29: 2162–70. [DOI] [PubMed] [Google Scholar]
- 35.Oliff HS, Weber E, Miyazaki B, et al. Infarct volume varies with rat strain and vendor in focal cerebral ischemia induced by transcranial middle cerebral artery occlusion. Brain Res 1995; 699: 329–331. [DOI] [PubMed] [Google Scholar]
- 36.Yao H, Nabika T. Standards and pitfalls of focal ischemia models in spontaneously hypertensive rats: with a systematic review of recent articles. J Transl Med 2012; 10: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao H, Steinberg GK, Sapolsky RM. General versus specific actions of mild-moderate hypothermia in attenuating cerebral ischemic damage. J Cereb Blood Flow Metab 2007; 27: 1879–1894. [DOI] [PubMed] [Google Scholar]
- 38.Yenari MA, Han HS. Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat Rev Neurosci 2012; 13: 267–278. [DOI] [PubMed] [Google Scholar]
- 39.Fisher M, Feuerstein G, Howells DW, et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke 2009; 40: 2244–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee JH, Wei L, Gu X, et al. Improved therapeutic benefits by combining physical cooling with pharmacological hypothermia after severe stroke in rats. Stroke 2016; 47: 1907–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johansen FF, Hasseldam H, Nybro Smith M, et al. Drug-induced hypothermia by 5HT1A agonists provide neuroprotection in experimental stroke: new perspectives for acute patient treatment. J Stroke Cerebrovasc Dis 2014; 23: 2879–2887. [DOI] [PubMed] [Google Scholar]
- 42.Johansen FF, Hasseldam H, Rasmussen RS, et al. Drug-induced hypothermia as beneficial treatment before and after cerebral ischemia. Pathobiology 2014; 81: 42–52. [DOI] [PubMed] [Google Scholar]
- 43.Kollmar R, Blank T, Han JL, et al. Different degrees of hypothermia after experimental stroke: short- and long-term outcome. Stroke 2007; 38: 1585–1589. [DOI] [PubMed] [Google Scholar]
- 44.Maier CM, Sun GH, Kunis D, et al. Delayed induction and long-term effects of mild hypothermia in a focal model of transient cerebral ischemia: neurological outcome and infarct size. J Neurosurg 2001; 94: 90–96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.