Abstract
Breast cancer immunotherapy is a potent treatment option, with antibody therapies such as trastuzumab increasing 2-year survival rates by 50%. However, active immunotherapy through vaccination has generally been clinically ineffective. One potential means of improving vaccine therapy is by delivering breast cancer antigens to dendritic cells (DCs) for enhanced antigen presentation. To accomplish this in vivo, we pseudotyped lentiviral vector (LV) vaccines with a modified Sindbis Virus glycoprotein so that they could deliver genes encoding the breast cancer antigen alpha-lactalbumin (Lalba) or erb-b2 receptor tyrosine kinase 2 (ERBB2 or HER2) directly to resident DCs. We hypothesized that utilizing these DC-targeting lentiviral vectors as a breast cancer vaccine could lead to an improved immune response against self-antigens found in breast cancer tumors. Indeed, single injections of the vaccine vectors were able to amplify antigen-specific CD8 T cells 4–6 fold over naïve mice, similar to the best published vaccine regimens. Immunization of these mice completely inhibited tumor growth in a foreign antigen environment (LV-ERBB2 in wildtype mice), and it reduced the rate of tumor growth in a self-antigen environment (LV-Lalba in wildtype or LV-ERBB2 in MMTV-huHER2 transgenic). These results show that a single injection with targeted lentiviral vectors can be an effective immunotherapy for breast cancer. Furthermore, they could be combined with other immunotherapeutic regimens to improve outcomes for patients with breast cancer.
Keywords: Cancer vaccine, lentiviral vector, breast cancer immunotherapy, ERBB2/Her2, alpha-lactalbumin
Graphical abstract
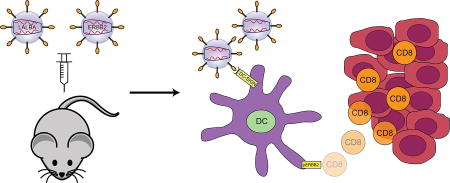
1. Introduction
Breast cancer is the most commonly diagnosed cancer in women, and causes 40,000 deaths per year in the United States [1]. One treatment option for these patients is administration of trastuzumab [2], an antibody that targets the ErbB2 protein (also known as Her2), which is a protein tyrosine kinase of the epidermal growth factor receptor family. Treatment with trastuzumab has reduced death rates by 50% in patients with ErbB2+ breast cancer, and has improved 5-year survival rates to the equivalent of those observed for patients with ErbB2– breast cancer [3]. The success of this passive immunotherapy has inspired multiple approaches towards developing an active immunotherapy treatment, in which patients’ immune systems are stimulated to develop their own immune response to the tumor.
Typically, active immunotherapies have involved vaccination with one or more breast cancer-specific antigens. Antigens can be delivered in many different ways, including peptides, recombinant viral vectors, whole tumor cells or lysates, plasmid DNA, or directly stimulated DCs [4]. To date, these attempts have generally shown limited clinical benefit [5]. One common exception to this pattern is the success of vaccines that utilize DCs to present the target antigen. DCs are the most powerful antigen-presenting cell, and directly including them in any vaccination regimen is likely to create a stronger immune response [6].
Lentiviral vectors (LV) are a versatile tool for gene delivery. Their advantages include efficient transduction of non-dividing cells, low natural anti-vector immunity, and a low potential for genotoxicity due to insertional mutagenesis. More importantly, they can be pseudotyped with many different glycoproteins, which facilitates the direct targeting of these vectors to a specific cell type [7–9]. Previous work in our laboratory has shown that LV pseudotyped with a modified Sindbis glycoprotein can directly target DC-SIGN on the surface of DCs [10]. This targeting was able to elicit a stronger immune response than a non-targeted vector [11]. Here, we sought to utilize LV to deliver breast cancer antigens to DCs in order to stimulate the host’s immune response against a tumor.
A critical element in the development of an effective cancer vaccine is the selection of target antigen. Criteria that define a good target include: (1) presence in the tumor, (2) absence in healthy tissues, (3) immunogenicity, (4) oncogenic potential, (5) evidence for therapeutic efficacy, (6) known epitopes, and (7) existing models for studying vaccine-specific effects [12]. Previous vaccines in breast cancer therapy have targeted Lalba, MUC1, Mammaglobin-A, ErbB2, and NY-BR-1, among others [13–17]. The most advanced have proceeded to Phase II clinical trials [18–20]. In this work, we chose to investigate ErbB2 and Lalba as two potential targets.
As described above, ErbB2 has been a common target of passive immunotherapy. This protein is a membrane-bound receptor tyrosine kinase and is overexpressed in 20 – 30% of human breast cancers [3]. However, it is expressed at low-to-medium levels in only a few healthy tissues. Lalba, on the other hand, is expressed in no healthy tissues except during lactation, while it is expressed at high levels in a subset of human breast cancers, especially triple negative breast cancer [21]. ErbB2’s immunogenicity in mice has been described, and its epitopes are known, while epitopes for Lalba are unknown.
In this study, we have identified Lalba and ERBB2 as two promising candidates for therapeutic vaccination. We engineered each of these two genes into a DC-targeted LV and utilized these vectors for immunization. Mice that were immunized with these LV could generate a T cell immune response to the target antigen. A single immunization was sufficient to prevent or slow the development of tumors in both transplanted and spontaneous mouse models of breast cancer.
2. Materials and Methods
2.1 Plasmids and mice
Lentiviral vectors were created using the FUW LV backbone, which was originally engineered to generate high-titer vectors that can transduce a variety of cell types [22]. LV-Lalba was created by PCR-amplifying the entire mouse Lalba coding sequence (Openbiosystems cDNA, accession #BC069916) and cloning it into FUW using the BamHI and EcoRI restriction sites. LV-ERBB2_ECD was created by PCR-amplifying the extracellular region (amino acids 1–652) of the ERBB2 coding sequence (Genecopoeia #HOC20199, accession #BC156755) and cloning the product into FUW at the HpaI and AscI restriction sites. LV-ERBB2-FL-ki was created by first PCR-amplifying the full-length ERBB2 coding sequence and cloning it into FUW at the HpaI and AscI restriction sites, followed by site-directed mutagenesis of K753A (Quikchange II, Agilent Technologies). LV-GFP was previously published as FUGW [10].
Balb/cJ mice were purchased from Jackson laboratories. MMTV-huHER2 transgenic mice [23] were obtained from Genentech (strain FVB/Tg.MMTV.f.huHER2 #5, South San Francisco, CA). All mice were maintained in the animal facilities at the University of Southern California (USC) under controlled temperature and a 12 h light/dark cycle, with free access to water and standard laboratory chow. All procedures were performed according to the guidelines established in the Guide for the Care and Use of Laboratory Animals [24], and the protocols were approved by the USC IACUC (2011-11623, 2011-11683).
2.2 Production of lentiviral vectors
LV were produced as previously described [25]. Briefly, 293T cells were transiently transfected with a standard calcium phosphate precipitation method. Tissue culture plates (15 cm) were transfected with 37.5 ug of transfer plasmid (LV-Lalba or LV-ERBB2_ECD) along with 18.75 ug of packaging plasmids REV, RRE, and SVGmu. Supernatants were collected at 48 and 72 hours post-transfection, and they were concentrated by ultracentrifugation at 80,000 × g for 90 minutes. Pellets were resuspended in HBSS and titrated on 293T.DC-SIGN cells.
2.3 Construction of 4T1.Lalba and 4T1.ERBB2 cell lines
The 4T1 cell line was purchased from ATCC (#CRL-2539). Antigen positive cell lines (4T1.Lalba or 4T1.ERBB2) were generated by LV transduction of 4T1 cells [26] with either LV-Lalba or LV-ERBB2-FL-ki at a MOI of 10, followed by limiting dilution cloning. Clones were tested for antigen expression by RT-qPCR and/or flow cytometry staining. Primers for qPCR were [forward: TGAGGAAGGTGAAGGTGCTTGGAT, reverse: AGCCATAGGGCATAAGCTGTGTCA] for ERBB2 and [forward: TGGCTATCAAGGCATCTCTTTG, reverse: TCGGGGAACTCACTACTTTTACA] for Lalba. Staining of ErbB2 was measured using an in-house fluorescein-conjugated peptide targeting Her2 (a generous gift of S. Fiacco), incubated at 6 µM for 1 hour at 37 °C.
2.4 Tumor antigen expression analysis
Oncomine™ (Compendia Bioscience™, part of Life Technologies™, Ann Arbor, MI) was used for analysis and visualization. Outlier analysis at the 75th – 95th percentile was performed for 25 independent data sets, and the gene rank for overexpression at the given percentile was determined.
2.5 Immunization and tumor challenge
LV immunization was performed as previously described [27]. Briefly, 1 × 107 TU of concentrated LV stock was injected subcutaneously into the footpad of 8-week-old mice. For intracellular cytokine staining, spleens were harvested at 14 days post-immunization. For tumor challenge, immunizations were administered both prophylactically (14 days prior to tumor challenge) and therapeutically (6 days after challenge).
Tumor challenge was performed by implanting 5 × 105 4T1.ERBB2 or 4T1.Lalba cells orthotopically in mouse mammary glands. Tumor size was measured every 2–3 days with fine-toothed calipers. Tumor volume was calculated as length × width × height / 2. Mice were euthanized when tumors reached 1500 mm3 or became ulcerated.
2.6 Intracellular cytokine staining
Splenocytes (1 × 106) were cultured with an ErbB2 peptide TYLPTNASL [28] (Genscript) at 10 µg/mL, for 6 hours in the following solution: RPMI media, 10% FBS, 10 U/mL penicillin, 100 ug/mL streptomycin, 2 mM L-glutamine, 0.67 uL/mL GolgiPlug™ (BD Biosciences #555029). Cells were washed and stained with antibodies to mouse CD4 and CD8 (Biolegend #130312, #100706). Then they were permeabilized and stained with antibodies to mouse IFNγ (BD Biosciences #554412). Flow cytometry was performed on a MACSQuant Analyzer (Miltenyi Biotec). Analysis of expression was performed using FlowJo software.
2.7 Quantitative RT-PCR
Tumors were excised and dissociated in 0.2% collagenase. RNA was extracted with Trizol (Life technologies) according to the manufacturer’s instructions. Two-step qRT-PCR was performed with the High Capacity cDNA Reverse Transcription kit and the Power SYBR® Green PCR Master Mix (Applied Biosystems). Human ERBB2 expression was normalized to mouse GAPDH expression using the formula, 2−(ERBB2 Ct – GAPDH Ct). Primers for ERBB2 were AACCAAGAGGTGACAGCAGAG and TATTGGCACTGGTAACTGCCC. Primers for GAPDH were GCCTTCCGTGTTCCTACC and CCTCAGTGTAGCCCAAGATG.
2.8 Statistical Analysis
Statistical analysis was performed using GraphPad Prism (GraphPad Software). Two sample comparisons were assessed with the Student’s t test. Survival analyses were performed using the log-rank test. Transplanted tumor growth curves were compared with the two-way ANOVA test.
2.9 IFN-γ Enzyme-linked immunospot assay (ELISpot)
ELISpot was conducted as previously described [27]. Briefly, splenocytes from LV-Lalba- or LV-GFP-immunized mice were plated at 2 × 105 cells/well in 150 µl complete medium + 10 µg/ml cell lysate from 4T1.Lalba cells. After a 24-hour incubation at 37 °C, IFN-γ spots were developed as recommended by the manufacturer (BD Biosciences) and quantified by a Zeiss ELISPOT reader.
Results and Discussion
3.1 Identification and design of lentiviral vectors targeting breast cancer antigens
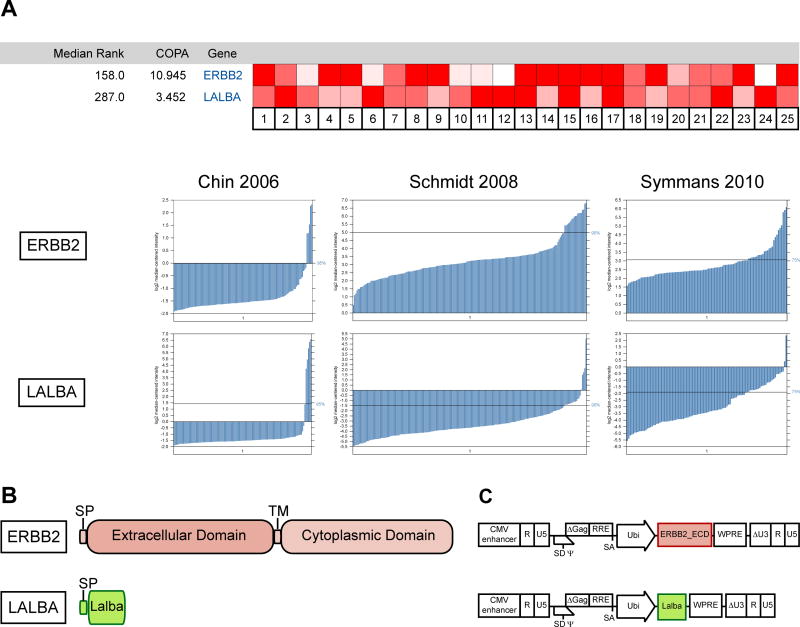
Our first goal in designing breast cancer-specific LV was to select antigens that would be the most promising candidates for a vaccine-mediated immunotherapy. We combined a review of the literature with a meta-analysis using Oncomine software (Life Technologies) to select two antigens most suited to our goals. Both these antigens are highly expressed in subsets of human cancers (Fig. 1A). ErbB2 has been extensively studied, and multiple forms of vaccines have been previously published. Lalba is less well characterized, but one strong advantage for this protein is that it is not expressed in healthy tissues outside of lactation, and thus is an ideal candidate for post-menopausal prophylactic vaccination [14].
Fig. 1.
ErbB2 and Lalba are potential antigens for lentiviral vaccine immunotherapy. (A) Meta-analysis of ERBB2 and LALBA mRNA expression in multiple tumor samples. Cancer Outlier Profile Analysis (COPA) [42] across 25 human breast cancer datasets identified ERBB2 and LALBA as highly overexpressed in a subset of tumor samples, with median gene ranks of 158 and 287, respectively. Brighter red indicates a more significant outlier score for a given dataset of tumor samples. Below, microarray mRNA expression levels from several representative datasets are shown [43–45], demonstrating that ERBB2 or LALBA is overexpressed in a subset of patients with breast cancer. (B) Diagram showing the protein structure of the ERBB2 and LALBA genes. Signal peptides (SP) and transmembrane domains (TM) are indicated. (C) Diagram of the LV constructs used in the experiments (not to scale). The extracellular domain of ErbB2 and the entire coding sequence of mouse Lalba were cloned into a LV construct under the control of the ubiquitin promoter (Ubi). Oncomine™ (Compendia Bioscience™, part of Life Technologies™, Ann Arbor, MI) was used for analysis and visualization.
Each of these two genes was cloned into the FUW LV backbone (Fig. 1C) [22]. The entire mouse Lalba coding sequence was inserted into the LV. In the case of ErbB2, only the extracellular domain of ErbB2 was inserted into the vector, as the dominant epitopes previously identified in mice and humans fall within this region [28,29]. LV particles were produced by transfection into 293T cells according to standard protocols, followed by ultracentrifugation. These particles were pseudotyped with codon-optimized SVGmu [10] to enable their targeting to dendritic cells.
3.2 LV immunization can generate a strong immune response to ErbB2 in a foreign- and self-antigen environment
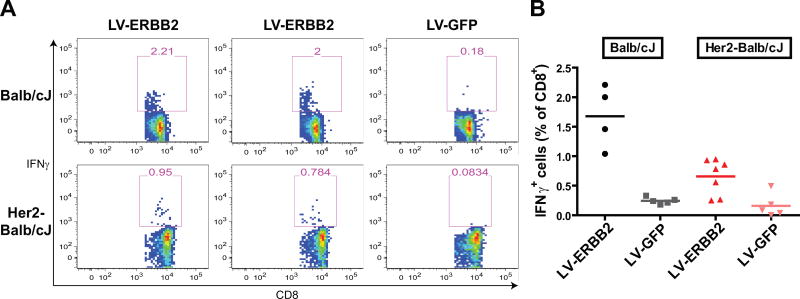
Having produced high-titer LV, we first sought to measure whether immunization with these vectors could generate an antigen-specific immune response. We performed a single immunization of Balb/cJ mice with 1 × 107 TU of LV-ERBB2 and harvested splenocytes two weeks after immunization. By coculturing bulk splenocytes with a peptide of a known ErbB2 H-2 Kd epitope [28], we were able to identify the percentage of CD8+ T cells that were specific for ErbB2 (Fig. 2A and 2B). Mice immunized with LV-ERBB2 generated a marked amount of ErbB2-specific CD8+ T cells as compared with mice immunized with a LV-GFP control LV (1.68% vs. 0.25%, p< 0.001, t test). This compares favorably to previously published DNA, vector and DC vaccines, which have yielded 3–7-fold increases in tumor-specific CD8+ T cells with more complicated immunization regimens [28,30–32].
Fig. 2.
Lentiviral vector vaccines generate a strong immune response to human ErbB2 in both foreign- and self-antigen environments. Intracellular cytokine staining was performed on splenocytes after stimulation with an ErbB2 peptide. The number of IFN-γ-expressing CD8+ cells as a percentage of total CD8+ cells was measured. (A) Representative flow cytometry plots show the percentage of IFN-γ+ cells after gating for CD8+CD4− cells. The top panel shows Balb/cJ mice that were immunized with either the LV-ERBB2 or LV-GFP vaccine. The bottom panel shows MMTV-huHer2-Balb/cJ cross-bred mice immunized with the same conditions. (B) The ICCS data from all mice studied under the 4 conditions described. In both Balb/cJ and huHer2-Balb/cJ cross-bred mice, LV-ERBB2 immunization generated a higher antigen-specific T cell response than LV-GFP (t test, p < 0.001, p < 0.01 respectively).
Such a clear difference was not unexpected, given that the antigen being delivered by the LV is a human antigen, not a mouse antigen. In order to more closely model a “self” tumor antigen setting, we performed the same experiment in mice that were transgenic for human ErbB2. We used hybrid mice from a cross between MMTV-huHER2 transgenic and wild-type BALB/cJ mice. In these offspring, constant expression of human ErbB2 ensures that this antigen will be recognized as a self-antigen, while CD8 T cells are able to recognize the H-2 Kd epitope of our ErbB2 peptide. Again, we found that mice immunized with the LV-ERBB2 vaccine generated more ErbB2-specific CD8 T cells than LV-GFP-immunized mice (Fig. 2, 0.66 vs. 0.16%, p < 0.01, t test). Thus, a single immunization produced a significant T cell immune response in a self-antigen environment.
Without a known epitope for Lalba in mice, it was more difficult to directly assay specific immune responses induced by LV immunization. Using an ELISpot assay with T cells cocultured with cell lysate from Lalba-overexpressing 4T1 cells, we were able to identify a modest increase in the number of IFN-γ+ T cells in mice immunized with LV-Lalba vs. those immunized with the control (Fig. S1, 27.8 vs. 5.3 SFC, p < 0.01, t test).
3.3 LV immunization can inhibit the growth of transplanted tumors
Having determined that a single injection of LV could generate a strong T cell immune response, we hypothesized that such a response would be sufficient to prevent or slow the growth of antigen-positive tumors. We tested this hypothesis using the mouse mammary tumor cell line, 4T1, a well-established model of human breast cancer [33]. We transduced parental 4T1 cells with LV encoding either full-length Lalba or a kinase-deficient version of human ERBB2 [28], followed by clonal isolation via limited dilution. These cell lines demonstrated high levels of antigen expression, as assessed by flow cytometry and RT-PCR (Fig. S2). Notably, the 4T1.ERBB2 cell line demonstrated slower growth kinetics with a higher degree of variation than the 4T1 and 4T1.Lalba cell lines (Fig. S3).
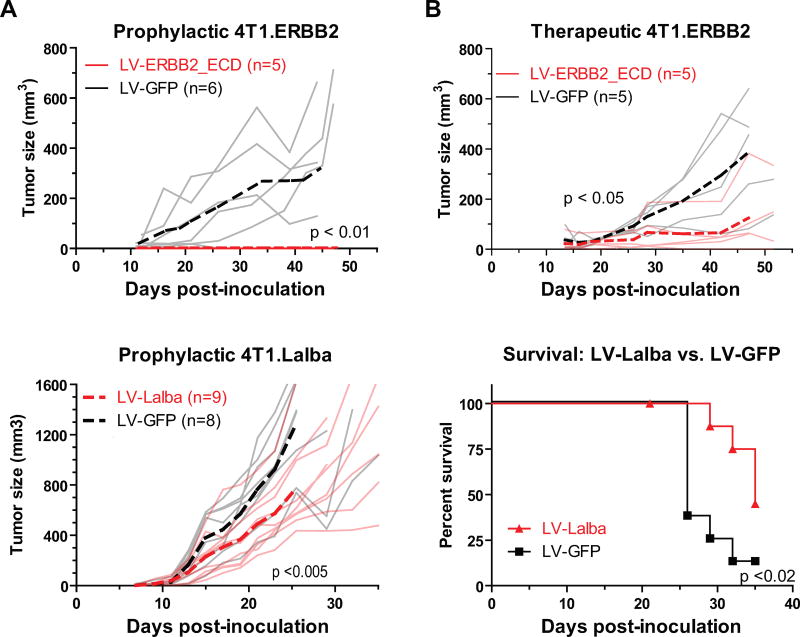
Tumor challenge of immunized subjects was performed in Balb/cJ mice, with vaccines administered either prophylactically or therapeutically. For mice challenged with 4T1.ERBB2, LV-ERBB2 immunization was effective in both a prophylactic and therapeutic setting (Fig. 3A and 3B). None of the mice prophylactically immunized with LV-ERBB2 developed tumors, whereas all control mice developed palpable tumors (p < 0.01, ANOVA). Therapeutic immunization was also effective, as LV-ERBB2 immunization extended tumor doubling time by 1.6-fold (9.3 vs 14.8 days, p < 0.05, ANOVA), and a smaller mean tumor size was detectable by day 42 after inoculation (p < 0.01, t test). We note that several of the control mice displayed reduced tumor growth, including one that controlled tumor growth completely. These mice may be able to control the tumor because these tumors express human ErbB2, a foreign antigen, and as shown in Fig. 2, naïve Balb/cJ mice can develop an immune response to the human antigen.
Fig. 3.
Lentiviral vector vaccines protect Balb/cJ mice against transplanted tumor challenge. Balb/cJ mice were challenged with 4T1.ERBB2 (A and B) or 4T1.Lalba (C and D) tumor cells, and tumor growth was measured over time. LV-ERBB2 immunization was performed prophylactically (A, 14 days before challenge) or therapeutically (B, 6 days after challenge). Tumor size in individual mice is plotted with solid light gray or red lines, while mean tumor size is plotted with dashed lines. A reduction in tumor growth was observed in prophylactic (p < 0.01) and therapeutic settings (p < 0.05, two-way ANOVA). (C and D) LV-Lalba immunization was performed prophylactically (14 days before challenge). Replicates and means are plotted in (C) as above. A survival curve is plotted in (D), using 1000 mm3 as the survival cutoff. A reduction in tumor growth was observed beginning at day 23 (p<0.01, t test), and median survival of immunized mice increased from 26 to 35 days (p<0.02, log-rank test).
For mice challenged with 4T1.Lalba, LV-Lalba immunization was effective when administered prophylactically (Fig. 3C and 3D). With both immunizations, tumors grew in all mice; however, the rate of growth in LV-Lalba mice was reduced, with a smaller mean tumor size detectable at day 23 after inoculation (p < 0.01, t test). These mice survived 35% longer that the control group (35 vs. 26 days, p < 0.02, log-rank test), demonstrating that the antigen-specific immunization was able to delay tumor growth.
3.4 LV immunization can prevent the development of spontaneous ErbB2+ tumors
The above transplantation experiments show that LV immunization can prevent or slow the growth of tumors in a transient model. To better model tumor development in humans, we chose to use MMTV-huHER2 transgenic mice as a model for spontaneous tumor growth. As originally reported by Finkle et al. [23], these mice express high levels of human ErbB2 in mammary glands and other tissues. A majority of females (76%) develop spontaneous mammary gland adenocarcinomas, starting at 23 weeks of age, but these carcinomas can be prevented by passive immunotherapy with anti-ErbB2 antibodies. Here, we sought to test the hypothesis that targeted LV immunization with LV-ERBB2 could prevent tumor development and progression.
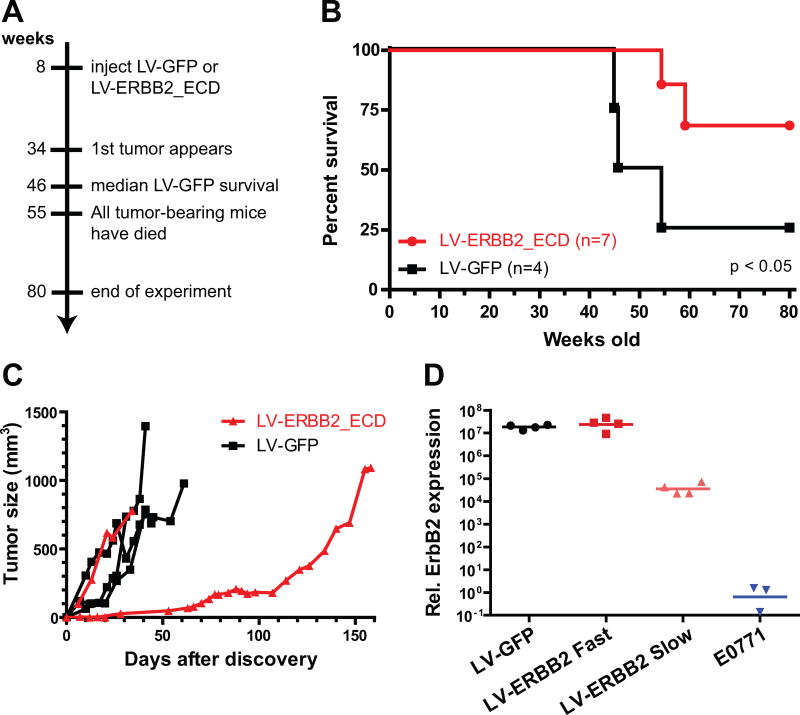
To test this hypothesis, we immunized MMTV-huHER2 transgenic mice with either LV-ERBB2 or LV-GFP prior to tumor onset, at the age of 8 – 10 weeks (Fig. 4A). The mice were observed for 80 weeks, and spontaneous tumor development was observed in 75% of the control group (n=4). Only 29% of the LV-ERBB2 group developed tumors by 55 weeks (n=7), when one tumor-free LV-ERBB2 mouse had to be euthanized due to swelling of the Harderian gland. Vaccination significantly delayed early tumor-induced death (Fig. 4B, p < 0.05, Gehan-Breslow-Wilcoxon test), although the improvement in median survival was less well supported (p < 0.07, log-rank test), as no mice in either group developed tumors after week 52.
Fig. 4.
LV vaccines protect transgenic huHer2 mice against spontaneous tumor growth. (A) Transgenic MMTV-huHer2 mice were immunized with LV-ERBB2 or LV-GFP at 8 weeks of age. Tumor development in these mice was monitored for 80 weeks. (B) Survival is shown as the time to develop 750 mm3 tumors. LV-ERBB2 immunization led to long-term survival of > 80 weeks in the majority of mice, while LV-GFP led to early death in 75% of the mice (Gehan-Breslow-Wilcoxon, p < 0.05). (C) The rate of tumor growth was measured after first palpation, with all 3 observed tumors growing rapidly in the LV-GFP group, while 1 out of 2 tumors in the LV-ERBB2 immunized group developed much more slowly. (D) Human ERBB2 mRNA expression was measured from tumor extracts. Expression levels in the fast growing tumors (LV-GFP and LV-ERBB2 Fast) were higher than those in LV-ERBB2 Slow (t test, p < 0.02), while expression in LV-ERBB2 Slow was higher than background levels (vs. E0771, t test, p < 0.04).
Of the mice that did develop tumors, most exhibited rapid tumor growth. However, one of the LV-ERBB2 tumors grew at a very slow rate (Fig. 4C). This tumor demonstrated reduced ErbB2 expression (Fig. 4D), indicating that this tumor may have escaped from an antigen-specific immune response.
Conclusions
In this study, we have created a lentiviral vector vaccine that can be used to target breast cancer-specific antigens. We demonstrated that these vaccines can produce a T cell immune response after a single injection in mice. Furthermore, these injections are sufficient to prevent or slow the growth of tumors in multiple models of breast cancer. These vaccines represent a possible active immunotherapy approach to the treatment of breast cancer.
One advantage of DC-targeted lentiviral vectors as immunization reagents is that they are able to achieve high levels of protection with only a single injection. In order to elicit strong immune responses, DNA vaccines must generally be injected multiple times over the course of an experiment [32,35–38]. In contrast, all our experiments assayed the immune response to a single intra-footpad injection of the lentiviral vaccine. Previous work in our laboratory has shown that boosting immunizations can elicit even stronger immune responses, with minimal anti-vector immunogenicity [27]. Thus, these LV vaccines have the potential to elicit a much stronger immune response than a DNA vaccine. A strong response is critical when designing an anticancer immunotherapy because tumors often contain immunosuppressive microenvironments [39].
Our spontaneous challenge results adhere closely to those of a DNA and cell-based vaccine that were tested previously in the same strain of mice [34]. According to that work, DNA vaccination led to 65% tumor-free survival at 80 weeks and cell-based vaccination led to approximately 40% tumor-free survival, compared to our results, which yielded 69% tumor-free survival at 80 weeks. The major advantage of the LV approach is that it can achieve equal levels of protection with a single injection, whereas the cell-based immunization required twice-weekly injections for the lifetime of the mice, and the DNA vaccine required 16 injections over 80 weeks. This advantage is counterbalanced by questions surrounding the safety of LV vaccines.
Because lentiviral vectors are derived from HIV-1, a human pathogen, they have rarely been used directly in humans. However, lentiviral vectors have been engineered with numerous safety controls, and in the limited set of trials that have reported results utilizing lentiviral vectors for gene delivery, neither oncogenesis nor development of replication-competent lentivirus has been observed [40]. Recently, results from a Phase I clinical trial (NCT02122861) of an NY-ESO-1 lentiviral vector vaccine showed strong T cell responses and no serious adverse events [41]. This study and others like it will provide the necessary foundation for determining whether these powerful and flexible lentiviral vectors can be used in future cancer vaccines.
Supplementary Material
Highlights.
A dendritic cell-targeted lentivector vaccine delivers breast cancer antigens
Immunization with the ERBB2 antigen generates a strong CD8 T cell response
A single immunization protects against mammary tumors in a self-antigen environment
Acknowledgments
We thank Drs. Steve Fiacco and Richard Roberts (USC) for providing reagents. This work was supported by the National Institutes of Health (R01AI068978, R01CA170820, R01EB017206 and P01CA132681), a translational acceleration grant from the Joint Center for Translational Medicine, and the National Cancer Institute (P30CA014089). P.D.B is supported by a postdoctoral fellowship from the National Cancer Center.
Non-standard abbreviations
- LV
Lentiviral vector
- MOI
Multiplicity of infection
- COPA
Cancer outlier profile analysis
- SFC
Spot-forming cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethical standards
All procedures were performed according to the guidelines established in the Guide for the Care and Use of Laboratory Animals[24], and the protocols were approved by the USC IACUC (2011-11623, 2011-11683). The manuscript does not contain clinical studies or patient data.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA: a cancer journal for clinicians. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. New England Journal of Medicine. 2001;344(11):783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 3.Dawood S, Broglio K, Buzdar AU, Hortobagyi GN, Giordano SH. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J Clin Oncol. 2010;28(1):92–98. doi: 10.1200/JCO.2008.19.9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palena C, Schlom J. Vaccines against human carcinomas: strategies to improve antitumor immune responses. J Biomed Biotechnol. 2010;2010:380697. doi: 10.1155/2010/380697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klebanoff CA, Acquavella N, Yu Z, Restifo NP. Therapeutic cancer vaccines: are we there yet? Immunological Reviews. 2011;239(1):27–44. doi: 10.1111/j.1600-065X.2010.00979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nature reviews. 2012;12(4):265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bryson PD, Wang P. Lentivector Vaccines. In: Lattime EC, Gerson SL, editors. Gene therapy of cancer: translational approaches from preclinical studies to clinical implementation. 3. Academic Press; Amsterdam: 2014. [Google Scholar]
- 8.Breckpot K, Aerts JL, Thielemans K. Lentiviral vectors for cancer immunotherapy: transforming infectious particles into therapeutics. Gene Ther. 2007;14(11):847–862. doi: 10.1038/sj.gt.3302947. [DOI] [PubMed] [Google Scholar]
- 9.Ageichik A, Buchholz CJ, Collins MK. Lentiviral vectors targeted to MHC II are effective in immunization. Human gene therapy. 2010;22(10):1249–1254. doi: 10.1089/hum.2010.184. [DOI] [PubMed] [Google Scholar]
- 10.Yang L, Yang H, Rideout K, Cho T, Joo KI, Ziegler L, Elliot A, Walls A, Yu D, Baltimore D, Wang P. Engineered lentivector targeting of dendritic cells for in vivo immunization. Nature biotechnology. 2008;26(3):326–334. doi: 10.1038/nbt1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang HG, Hu BL, Xiao L, Wang P. Dendritic cell-directed lentivector vaccine induces antigen-specific immune responses against murine melanoma. Cancer Gene Ther. 2011;18(5):370–380. doi: 10.1038/cgt.2011.13. [DOI] [PubMed] [Google Scholar]
- 12.Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, Mellman I, Prindiville SA, Viner JL, Weiner LM, Matrisian LM. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15(17):5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Narayanan K, Jaramillo A, Benshoff ND, Campbell LG, Fleming TP, Dietz JR, Mohanakumar T. Response of established human breast tumors to vaccination with mammaglobin-A cDNA. J Natl Cancer Inst. 2004;96(18):1388–1396. doi: 10.1093/jnci/djh261. [DOI] [PubMed] [Google Scholar]
- 14.Jaini R, Kesaraju P, Johnson JM, Altuntas CZ, Jane-Wit D, Tuohy VK. An autoimmunemediated strategy for prophylactic breast cancer vaccination. Nature medicine. 2010;16(7):799–803. doi: 10.1038/nm.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilewski T, Adluri S, Ragupathi G, Zhang S, Yao TJ, Panageas K, Moynahan M, Houghton A, Norton L, Livingston PO. Vaccination of high-risk breast cancer patients with mucin-1 (MUC1) keyhole limpet hemocyanin conjugate plus QS-21. Clin Cancer Res. 2000;6(5):1693–1701. [PubMed] [Google Scholar]
- 16.Seil I, Frei C, Sultmann H, Knauer SK, Engels K, Jager E, Zatloukal K, Pfreundschuh M, Knuth A, Tseng-Chen Y, Jungbluth AA, Stauber RH, Jager D. The differentiation antigen NY-BR-1 is a potential target for antibody-based therapies in breast cancer. Int J Cancer. 2007;120(12):2635–2642. doi: 10.1002/ijc.22620. [DOI] [PubMed] [Google Scholar]
- 17.Ladjemi MZ, Jacot W, Chardes T, Pelegrin A, Navarro-Teulon I. Anti-HER2 vaccines: new prospects for breast cancer therapy. Cancer Immunol Immunother. 2010;59(9):1295–1312. doi: 10.1007/s00262-010-0869-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arlen PM, Pazdur M, Skarupa L, Rauckhorst M, Gulley JL. A randomized phase II study of docetaxel alone or in combination with PANVAC-V (vaccinia) and PANVAC-F (fowlpox) in patients with metastatic breast cancer (NCI 05-C-0229) Clinical breast cancer. 2006;7(2):176–179. doi: 10.3816/CBC.2006.n.032. [DOI] [PubMed] [Google Scholar]
- 19.Tiriveedhi V, Tucker N, Herndon J, Li L, Sturmoski M, Ellis M, Ma C, Naughton M, Lockhart AC, Gao F, Fleming T, Goedegebuure P, Mohanakumar T, Gillanders WE. Safety and Preliminary Evidence of Biologic Efficacy of a Mammaglobin-A DNA Vaccine in Patients with Stable Metastatic Breast Cancer. Clinical Cancer Research. 2014;20(23):5964–5975. doi: 10.1158/1078-0432.ccr-14-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mittendorf EA, Clifton GT, Holmes JP, Schneble E, van Echo D, Ponniah S, Peoples GE. Final report of the phase I/II clinical trial of the E75 (nelipepimut-S) vaccine with booster inoculations to prevent disease recurrence in high-risk breast cancer patients. Annals of Oncology. 2014;25(9):1735–1742. doi: 10.1093/annonc/mdu211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tuohy VK. Retired self-proteins as vaccine targets for primary immunoprevention of adult-onset cancers. Expert review of vaccines. 2014;13(12):1447–1462. doi: 10.1586/14760584.2014.953063. [DOI] [PubMed] [Google Scholar]
- 22.Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D. Germline transmission and tissuespecific expression of transgenes delivered by lentiviral vectors. Science (New York, NY. 2002;295(5556):868–872. doi: 10.1126/science.1067081. [DOI] [PubMed] [Google Scholar]
- 23.Finkle D, Quan ZR, Asghari V, Kloss J, Ghaboosi N, Mai E, Wong WL, Hollingshead P, Schwall R, Koeppen H, Erickson S. HER2-targeted therapy reduces incidence and progression of midlife mammary tumors in female murine mammary tumor virus huHER2-transgenic mice. Clin Cancer Res. 2004;10(7):2499–2511. doi: 10.1158/1078-0432.ccr-03-0448. [DOI] [PubMed] [Google Scholar]
- 24.National Research Council (U.S.). Committee for the Update of the Guide for the Care and Use of Laboratory Animals., Institute for Laboratory Animal Research (U.S.), National Academies Press (U.S.) Guide for the care and use of laboratory animals 2011 [Google Scholar]
- 25.Liu Y, Tai A, Joo KI, Wang P. Visualization of DC-SIGN-mediated entry pathway of engineered lentiviral vectors in target cells. PLoS One. 2013;8(6):e67400. doi: 10.1371/journal.pone.0067400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bryson PD, Zhang C, Lee CL, Wang P. A tetracycline-regulated cell line produces high-titer lentiviral vectors that specifically target dendritic cells. J Vis Exp. 2013;76 doi: 10.3791/50606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dai B, Xiao L, Bryson PD, Fang J, Wang P. PD-1/PD-L1 blockade can enhance HIV-1 Gag-specific T cell immunity elicited by dendritic cell-directed lentiviral vaccines. Mol Ther. 2012;20(9):1800–1809. doi: 10.1038/mt.2012.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Triulzi C, Vertuani S, Curcio C, Antognoli A, Seibt J, Akusjarvi G, Wei WZ, Cavallo F, Kiessling R. Antibody-dependent natural killer cell-mediated cytotoxicity engendered by a kinase-inactive human HER2 adenovirus-based vaccination mediates resistance to breast tumors. Cancer research. 2010;70(19):7431–7441. doi: 10.1158/0008-5472.CAN-10-0493. [DOI] [PubMed] [Google Scholar]
- 29.Czerniecki BJ, Koski GK, Koldovsky U, Xu S, Cohen PA, Mick R, Nisenbaum H, Pasha T, Xu M, Fox KR, Weinstein S, Orel SG, Vonderheide R, Coukos G, DeMichele A, Araujo L, Spitz FR, Rosen M, Levine BL, June C, Zhang PJ. Targeting HER-2/neu in early breast cancer development using dendritic cells with staged interleukin-12 burst secretion. Cancer research. 2007;67(4):1842–1852. doi: 10.1158/0008-5472.CAN-06-4038. [DOI] [PubMed] [Google Scholar]
- 30.Jacob JB, Quaglino E, Radkevich-Brown O, Jones RF, Piechocki MP, Reyes JD, Weise A, Amici A, Wei WZ. Combining human and rat sequences in her-2 DNA vaccines blunts immune tolerance and drives antitumor immunity. Cancer research. 2010;70(1):119–128. doi: 10.1158/0008-5472.CAN-09-2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mossoba ME, Walia JS, Rasaiah VI, Buxhoeveden N, Head R, Ying C, Foley JE, Bramson JL, Fowler DH, Medin JA. Tumor protection following vaccination with low doses of lentivirally transduced DCs expressing the self-antigen erbB2. Mol Ther. 2008;16(3):607–617. doi: 10.1038/sj.mt.6300390. [DOI] [PubMed] [Google Scholar]
- 32.Whittington PJ, Radkevich-Brown O, Jacob JB, Jones RF, Weise AM, Wei WZ. Her-2 DNA versus cell vaccine: immunogenicity and anti-tumor activity. Cancer Immunol Immunother. 2009;58(5):759–767. doi: 10.1007/s00262-008-0599-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pulaski BA, Ostrand-Rosenberg S. Reduction of Established Spontaneous Mammary Carcinoma Metastases following Immunotherapy with Major Histocompatibility Complex Class II and B7.1 Cell-based Tumor Vaccines. Cancer research. 1998;58(7):1486–1493. [PubMed] [Google Scholar]
- 34.De Giovanni C, Nicoletti G, Quaglino E, Landuzzi L, Palladini A, Ianzano ML, Dall'ora M, Grosso V, Ranieri D, Laranga R, Croci S, Amici A, Penichet ML, Iezzi M, Cavallo F, Nanni P, Lollini PL. Vaccines against human HER2 prevent mammary carcinoma in mice transgenic for human HER2. Breast Cancer Res. 2014;16(1):R10. doi: 10.1186/bcr3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei WZ, Shi WP, Galy A, Lichlyter D, Hernandez S, Groner B, Heilbrun L, Jones RF. Protection against mammary tumor growth by vaccination with full-length, modified human ErbB-2 DNA. Int J Cancer. 1999;81(5):748–754. doi: 10.1002/(sici)1097-0215(19990531)81:5<748::aid-ijc14>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 36.Nakashima H, Terabe M, Berzofsky JA, Husain SR, Puri RK. A novel combination immunotherapy for cancer by IL-13Ralpha2-targeted DNA vaccine and immunotoxin in murine tumor models. J Immunol. 2011;187(10):4935–4946. doi: 10.4049/jimmunol.1102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liao D, Liu Z, Wrasidlo WJ, Luo Y, Nguyen G, Chen T, Xiang R, Reisfeld RA. Targeted therapeutic remodeling of the tumor microenvironment improves an HER-2 DNA vaccine and prevents recurrence in a murine breast cancer model. Cancer research. 2011;71(17):5688–5696. doi: 10.1158/0008-5472.CAN-11-1264. [DOI] [PubMed] [Google Scholar]
- 38.Cho HI, Celis E. Design of immunogenic and effective multi-epitope DNA vaccines for melanoma. Cancer Immunol Immunother. 2012;61(3):343–351. doi: 10.1007/s00262-011-1110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science (New York, NY. 2011;331(6024):1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 40.McGarrity GJ, Hoyah G, Winemiller A, Andre K, Stein D, Blick G, Greenberg RN, Kinder C, Zolopa A, Binder-Scholl G, Tebas P, June CH, Humeau LM, Rebello T. Patient monitoring and follow-up in lentiviral clinical trials. The journal of gene medicine. 2013;15(2):78–82. doi: 10.1002/jgm.2691. [DOI] [PubMed] [Google Scholar]
- 41.Somaiah N, Block MS, Kim JW, Shapiro G, Hwu P, Eder JP, Jones RL, Gnjatic S, Lu H, Hsu FJ. Phase I, first-in-human trial of LV305 in patients with advanced or metastatic cancer expressing NY-ESO-1. ASCO Annual Meeting Proceedings. 2015;15(suppl):3021. [Google Scholar]
- 42.Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun X-W, Varambally S, Cao X, Tchinda J, Kuefer R, Lee C, Montie JE, Shah RB, Pienta KJ, Rubin MA, Chinnaiyan AM. Recurrent Fusion of TMPRSS2 and ETS Transcription Factor Genes in Prostate Cancer. Science (New York, NY. 2005;310(5748):644–648. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 43.Chin K, DeVries S, Fridlyand J, Spellman PT, Roydasgupta R, Kuo WL, Lapuk A, Neve RM, Qian Z, Ryder T, Chen F, Feiler H, Tokuyasu T, Kingsley C, Dairkee S, Meng Z, Chew K, Pinkel D, Jain A, Ljung BM, Esserman L, Albertson DG, Waldman FM, Gray JW. Genomic and transcriptional aberrations linked to breast cancer pathophysiologies. Cancer Cell. 2006;10(6):529–541. doi: 10.1016/j.ccr.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 44.Schmidt M, Bohm D, von Torne C, Steiner E, Puhl A, Pilch H, Lehr HA, Hengstler JG, Kolbl H, Gehrmann M. The humoral immune system has a key prognostic impact in node-negative breast cancer. Cancer research. 2008;68(13):5405–5413. doi: 10.1158/0008-5472.CAN-07-5206. [DOI] [PubMed] [Google Scholar]
- 45.Symmans WF, Hatzis C, Sotiriou C, Andre F, Peintinger F, Regitnig P, Daxenbichler G, Desmedt C, Domont J, Marth C, Delaloge S, Bauernhofer T, Valero V, Booser DJ, Hortobagyi GN, Pusztai L. Genomic index of sensitivity to endocrine therapy for breast cancer. J Clin Oncol. 2010;28(27):4111–4119. doi: 10.1200/JCO.2010.28.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.