Abstract
RET receptor tyrosine kinase (RTK) acts as an ontogenetic driver in several human malignancies, including papillary and medullary thyroid carcinoma, lung adenocarcinoma, colorectal carcinoma, Spitzoid neoplasms, salivary gland carcinoma and chronic myeloproliferative disorders, secondary to as diverse genetic lesions as point-mutations, small insertions/deletions, and gene fusions. In other neoplasms, including breast and pancreatic adenocarcinoma, RET over-expression is up-regulated. Thus, small molecule compounds with RET tyrosine kinase inhibitory activity (TKIs) are being investigated for the targeted treatment of these malignancies. Multi-targeted TKIs with the RET inhibitory enzymatic activity of IC50 in the nanomolar range have entered clinical practice, registered for the treatment of medullary thyroid cancer (vandetanib, cabozantinib), radioiodine refractory non medullary thyroid cancer (lenvatinib, sorafenib) or cancers of other sites (sunitinib, ponatinib, regorafenib). This review summarizes mechanisms of RET oncogenic activity and properties of new TKIs that, at the preclinical stage, have demonstrated promising anti-RET activity.
Keywords: thyroid cancer, tyrosine kinase, targeted therapy, MEN, RET, enzyme
RET structure-function
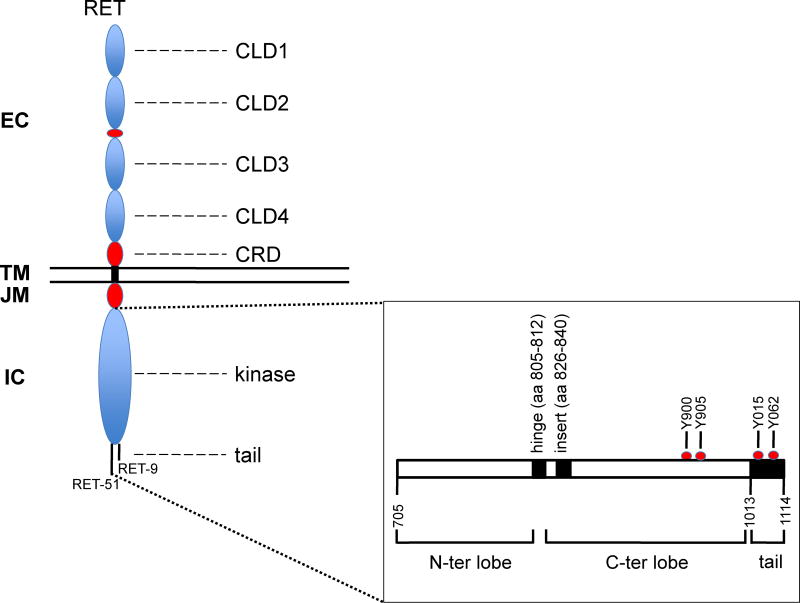
RET (REarranged during Transfection) gene, named after its original identification through a NIH3T3 transfection assay, maps on the long arm of chromosome 10 (10q11.21) and codes for a membrane receptor with tyrosine kinase activity (RTK) (Takahashi et al. 1985). As shown in Figure 1, RET protein features a glycosylated extracellular (EC), a single pass transmembrane (TM), a juxtamembrane (JM), and an intracellular tyrosine kinase (TK) domains. These are followed by two different carboxyterminal tails which are identical in the first 50 aminoacids and then for alternative splicing differe in the last 9 (isoform "short" RET-9) or 51 (isoform "long" RET-51) residues (Ibanez, 2013). Similar to other RTKs such as VEGFR and KIT, the RET TK is splitted in two, by a 14 residues-long kinase insert (Figure 1).
Figure 1.
RET protein structure. Extracellular (EC), transmembrane (TM) and intracellular (IC) portions are depicted with their principal protein domains: the 4 cadherin-like (CLD1–4), the calcium binding and the cysteine-rich (CRD) domains in the EC; the juxtamembrane (JM) and the N-terminal and C-terminal lobes of the RET kinase in the IC. Not represented is the kinase insert splitting the C-terminal lobe. The alternative splicing generating the two alternative RET C-tails (RET-9 and RET-51) thus forming two RET protein isoforms of 1072 and 1114 amino acids long, respectively, are represented. Structural details of the RET kinase (RET-51 isoform) are shown in the bottom right box. Position of activation loop tyrosines (Y900 and Y905) and of C-terminal tyrosine Y1062, the pivotal RET signaling residue is reported.
The extracellular domain of RET contains 4 cadherin (Ca++-dependent adhesion molecules) repeats (CLD1–4), one Ca++ binding site, and one cysteine-rich domain (CRD) adjacent to the plasma-membrane (Figure 1). RET functions as the receptor for soluble covelent dimeric growth factors of the glial cell line-derived neurotrophic growth factor (GDNF) family (GFL); GFL growth factors include GDNF, Neurturin, Persephin and Artemin (Ibanez, 2013). They bind to ancillary GPI (glycosylphosphatidylinositol)-linked co-receptors named GFRα 4, thus forming a bi-partite ligand complex composed by the growth factor and the co-receptor. In turn, the GFL-GFRα complex forms a ternary complex when binds to the RET extracellular domain; this interaction allows re-orientation of the two RET cysteine-rich domains, dimerization and kinase activation (Goodman et al. 2014).
Similar to other kinases, the RET kinase domain is separated in a N-ter and a C-ter lobe by a hinge (aa 805–812); the C-terminal lobe is larger and contains the kinase insert (aa 826–840) (Knowles et al. 2006) (Figure 1). Regulation of RET enzymatic activity depends on a cis-inhibitory mechanism whereby a closed auto-inhibited RET kinase conformation is stabilized by interactions between the N-terminal lobe (in particular, the glycine-rich loop and the αC helix) with the activation loop of the C-terminal lobe (Plaza-Menacho et al. 2014a). Formation of the ternary GFL-GFRα-RET complex may release this auto-inhibited conformation, unleashing RET kinase activity. Phosphorylation of RET at tyrosines Y687 (in the juxtamembrane domain), Y900, Y905 and Y981 (in the kinase domain), and Y1015, Y1029, and Y1062 (in the COOH-tail) is involved in RET signal transduction (Arighi et al. 2005; Santoro et al. 2013; Mulligan, 2014; Plaza-Menacho et al. 2014). These RET auto-phosphorylation events occur in a temporarily ordered manner, with the phosphorylation of Y1062 and Y687 occurring first, and that of Y900, Y905, Y1015, and Y1029 occurring at a later time point (Plaza-Menacho et al. 2014a; Plaza-Menacho et al. 2014b). Therefore, as a variance from other kinases, auto-phosphorylation of activation loop tyrosines (Y900, Y905) (Figure 1), which occurs later than autophosphorylation of Y1062 and Y687, is not essential for the activation of the RET kinase (Knowles at al. 2006; Plaza-Menacho et al. 2014a). Recently, RET has been demonstrated to function as a dual kinase: e.g. be able to phosphorylate not only tyrosine but also serine residues (Bagheri-Yarmand et al. 2015; Plaza-Menacho et al. 2016). Accordingly, the auto-phosphorylation of serine 909 in the RET activation loop also plays a stimulatory role on RET kinase (Plaza-Menacho et al. 2016).
As far as RET intracellular signal transduction, a crucial role has been ascribed to Y1062 (Figure 1), which is able to recruit several intracellular adaptors featuring PTB (phosphotyrosine binding domains), including SHC, FRS2, IRS1/2 and others. This allows RET to activate the two classical RTK signal transduction pathways, e.g. the RAS-MAPK and the PI3K-AKT-mTOR cascades. Accordingly, genetic lesions in components of these cascades (RAS family members, BRAF, PIK3CA) represent major drivers for malignancies, such as thyroid and lung cancer, in alternative to RET activating mutations (Fagin et al. 2016; Chen et al. 2014).
RET is essential for normal development of several tissues, mainly derived from neural crest. In humans, germline loss of function RET with mutations, that impairs its activity, cause abnormal development of the enteric nervous system and congenital megacolon (Hirschsprung's disease, [HSCR]) (Ibanez, 2013). HSCR-associated extracellular RET mutations are localized in CLDs 1–3 and cause RET protein misfolding and retention in ER (endoplamic reticulum), while intracellular HSCR-associated RET mutations knock-down RET kinase and signaling ability (Carlomagno et al. 1996; Pelet et al. 1998; Geneste et al. 1999).
RET in human cancer
Germline gain of function RET mutations cause autosomal dominant inheritance of Multiple Endocrine Neoplasia type 2 (MEN2A and MEN2B) syndromes. These syndromes predispose to medullary thyroid carcinoma (MEN2A and MEN2B), pheochromocytoma (MEN2A and MEN2B), parathyroid hyperplasia (MEN2A only), and intestinal ganglioneuromatosis, corneal nerve thickening and marfanoid abitus (MEN2B only). Cutaneous lichen amyloidosis (CLA) and Hirschsprung's disease are rare phenotypes that can be found in MEN2A (Wells et al. 2013; Wells et al. 2015). In MEN2 syndromes, RET is hit by missense mutations (more rarely small insertions or deletions). The missense mutations typically target extracellular cysteines in the RET CRD domain in MEN2A and Methionine 918 (M918T) in the RET P+1 loop (immediately downstrean the activation loop) in MEN2B. Several additional mutations have been described and correlated with the clinical phenotype (Wells et al. 2015). These mutations activate RET tyrosine kinase and signaling in a ligand-independent manner (Santoro et al. 1995). Cysteine mutations cause disulfide-bonds mediated RET dimerization. Instead, M918T alters mechanism of RET auto-inhibition, by increasing affinity for ATP, accelerating RET autophosphorylation, and enhancing in trans the presentation of RET as a substrate to the other RET monomer (Plaza-Menacho et al. 2014b). Missense mutations similar to those found in MEN2 and sporadic MTC patients (mainly M918T) are found in more than 50% of sporadic MTC cases (Wells et al. 2013). Recently, a RET gene fusion, MYH13-RET, has been described as an alternative mechanism of RET activation in sporadic MTC (Grubbs et al. 2015).
Besides MEN2-associated neoplasm and sporadic MTC, multiple additional cancer types harbour oncogenic RET gene lesions (Kumar-Sinha et al. 2015, Yoshihara et al. 2015). RET gene fusions were initially identified in papillary thyroid carcinoma (PTC), where chromosomal rearrangements, most typically paracentric inversions of the long arm of chromosome 10, cause the fusion of the RET intracellular domain (from exon 12 in the 3'-ter portion of the gene) to the transcriptional promoter and 5'-terminal region of various heterologous gene partners. This leads to aberrant expression and ligand-independent RET kinase activation (Santoro et al. 2013; Mulligan, 2014). RET fusions are found in approximately 7% of sporadic PTC (Fagin et al. 2016), and, more commonly, in radiation-associated PTC (about 60%) (Ricarte-Filho et al. 2013) and in pediatric, adolescent and young adult PTC (27%) (Vanden Borre et al. 2017). Similar fusions have been identified in other cancers, including lung adenocarcinoma (ADC) (1–2%, Chen et al. 2014), colorectal carcinoma (0.2%, Le Rolle et al. 2015), Spitzoid neoplasms (3%, Wiesner et al. 2014), salivary gland carcinoma (1.9% adenocarcinoma and 4.9% ductal carcinoma, Wang et al. 2016) and in single cases of chronic myelomonocitic leukemia (CMML) (Ballerini et al. 2012), primary myelofibrosis (Bossi et al. 2014), gastrointestinal neuroendocrine tumor (Hartmaier et al. 2017), and breast invasive carcinoma (Stransky et al. 2014). In particular, RET fusions involve most commonly CCDC6 and the NCOA4 genes in PTC and KIF5B gene in lung ADC (Santoro et al. 2013; Kohno et al. 2013).
A recent analysis of 4,871 cancer patients has revealed the presence of structural RET gene alterations, including point mutations (38.6%), fusions (30.7%) and amplifications (25%) in multiple cancer types (Kato et al. 2016). Of note, some of these alterations were identified in cancers not previously known to be associated to RET, such as, for example, RET C634R (in breast carcinoma), RET M918T (in paraganglioma and atypical lung carcinoid), RET V804M (in colorectal adenocarcinoma, meningioma, gastrointestinal stromal tumor and hepatocellular carcinoma), and KIF5B-RET (in ovarian epithelial carcinoma) (Kato et al. 2016).
In other human cancers, high levels of RET expression, in the absence of structural alterations, has been reported. As an example, RET is up-regulated in breast carcinoma (Plaza-Menacho et al. 2010, Griseri et al. 2016). In a recent study, RET immunoreactivity was found in HER2+ and basal carcinomas (80%) and in luminal carcinomas (47%) (Nguyen et al. 2015). Moreover, RET was found highly expressed in pancreatic adenocarcinoma and able to trigger their perineural invasion (Amit et al. 2017).
Small molecule tyrosine kinase inhibitors (TKIs)
Following the paradigmatic example of imatinib, as an inhibitor of BCR-ABL kinase, in the treatment of chronic myelogenous leukemia, a large number of TKIs (tyrosine kinase inhibitors) directed against oncogenic tyrosine kinases have entered preclinical and clinical development (Zhang et al. 2009). These drugs are small molecule organic compounds that bind completely or partially to its nucleotide binding pocket in the kinase domain, thus obstructing enzymatic activity.
Depending on the spatial orientation of the activation loop, kinases can adopt an active (so-called "DFG-in", based on the position of the aspartate-phenylalanine-glycine [DFG] motif at the N-terminal of the activation loop) or inactive conformation (so-called "DGF-out" because the DFG is flipped-out). Accordingly, TKIs are subdivided in two major classes depending whether they bind DFG-in (type I) or to the DFG-out (type II) kinase conformational state. Type I TKIs block the active kinase by competing with ATP, while type II inhibitors, by contacting both the ATP binding pocket and an adjacent allosteric site available only in the DFG out conformation, stabilize the kinase in its inactive conformation (Zhao et al. 2014). Less extensively explored, thus far, are alternative binding modes (type III and IV), featured by compounds that bind non-competitively distal to the ATP binding site (Zhao et al. 2014).
Examples of FDA-approved type-I TKIs are Gefitinib, Sunitinib and Vandetanib. Type-II TKIs include the FDA-approved Imatinib, Sorafenib, Cabozantinib and Ponatinib, as well as several additional TKIs under clinical development as Regorafenib and Apatinib. Type II inhibitors might generally be more specific than type I since the allosteric site they contact is less conserved among kinases than the ATP-binding site (Zhao et al. 2014); however, in a pharmacological point of view, type I and II inhibitors may complement each other in terms of different conformation of the kinase they select as well as in terms of different mutations that can confer resistance (Zhao et al. 2014).
Clinically approved RET TKIs
Several multi-kinase inhibitors that have anti-RET activity have been approved for the treatment of thyroid or non thyroid cancers (Bible et al. 2016; Kato et al. 2016; Viola et al. 2016; Bikas et al. 2016; https://pubchem.ncbi.nlm.nih.gov/). These include vandetanib (approved for medullary thyroid carcinoma) (Herbst et al. 2007; Carlomagno et al. 2002; Wells et al. 2010; Vozniak et al. 2012), cabozantinib (approved for medullary thyroid carcinoma and renal cell carcinoma) (Yakes et al. 2011; Bentzien et al. 2013; Elisei et al. 2013; Weitzman et al. 2015; Tannir et al. 2017), lenvatinib (approved for differentiated thyroid carcinoma and renal cell carcinoma) (Matsui et al. 2008; Okamoto et al. 2013; Schlumberger et al. 2015; Cabanillas et al. 2016), ponatinib (approved for chronic myeloid leukemia and Philadelphia chromosome positive acute lymphoblastic leukemia) (O'Hare et al. 2009; Cortes et al. 2012; De Falco et al. 2013; Mologni et al. 2013; Hoy et al. 2014), sunitinib (approved for renal cell carcinoma and imatinib-resistant gastrointestinal stromal tumor) (Mendel et al. 2003; Mologni et al. 2013), regorafenib (approved for colorectal cancer and gastrointestinal stromal tumor) (Ettrich et al. 2014;), and sorafenib (approved for differentiated thyroid carcinoma, renal cell carcinoma and hepatocellular carcinoma) (Wilhelm et al. 2004; Carlomagno et al. 2006; Plaza-Menacho et al. 2007; Thomas et al. 2014; de Castroneves et al. 2016).
Principal features of these drugs are summarized in Table 1. For further information, the reader is referred to the quoted literature. Among them, vandetanib and cabozantinib are those specifically registered for medullary thyroid carcinoma, the human cancer type with a higher proportion of RET oncogenic mutations prevalence (see above) (Wells et al. 2010; Elisei et al. 2013; Sherman et al. 2009; Cabanillas et al. 2014). Clinical responses to these drugs have been also reported for individual RET mutant patients with other malignancies. Partial responses were noted in RET fusion-positive ADC patients treated with cabozantinib or vandetanib (Drilon et al. 2013; Gautschi et al. 2013; Mukhopadhyay et al. 2014; Platt et al. 2015; Falchook et al. 2016; Rosell et al. 2016; Lee et al. 2016; Yoh et al. 2017). In two studies, 28% and 53% of patients with RET fusion-positive ADC featured objective responses to cabozantinib or vandetanib, respectively (Drilon et al. 2013; Yoh et al. 2017). Clinical benefit was also reported in two patients with RET fusion-positive salivary gland carcinoma treated with cabozantinib (Wang et al. 2016) and one patient with RET fusion-positive CMML treated with sorafenib (Ballerini et al. 2012).
Table 1.
Clinically approved TKIs with anti-RET inhibitory activity
| Compound | Other names | 1IUPAC name | 2Targets (IC50 nM) | References |
|---|---|---|---|---|
|
| ||||
| Vandetanib | Zactima; ZD6474 | N-(4-bromo-2-fluorophenyl)-6-methoxy-7-[(1-methylpiperidin-4-yl)methoxy]quinazolin-4-amine | VEGFR2 (40) | Herbst et al. 2007 |
| VEGFR3 (110) | ||||
| RET (130) | ||||
| EGFR (500) | ||||
|
| ||||
| Cabozantinib | BMS-907351; XL-184 | 1-N-[4-(6,7-dimethoxyquinolin-4-yl)oxyphenyl]-1-N'-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide | VEGFR2 (0.035) | Yakes et al. 2011 |
| MET (1.3) | ||||
| KIT (4.6) | ||||
| RET (5.2) | ||||
| AXL (7) | ||||
| FLT3 (11.3) | ||||
| TIE2 (14.3) | ||||
| RON (124) | ||||
|
| ||||
| Lenvatinib | Lenvima; E7080; | 4-[3-chloro-4-(cyclopropylcarbamoylamino)phenoxy]-7-methoxyquinoline-6-carboxamide | RET (1.5) | Okamoto et al. 2013 |
| VEGFR2 (4) | Matsui et al. 2008 | |||
| VEGFR3 (5.2) | Matsui et al. 2008 | |||
| VEGFR1 (22) | Matsui et al. 2008 | |||
| PDGFRβ (39) | Matsui et al. 2008 | |||
| FGFR1 (46) | Matsui et al. 2008 | |||
| PDGFRα (51) | Matsui et al. 2008 | |||
| KIT (100) | Matsui et al. 2008 | |||
|
| ||||
| Ponatinib | Iclusig; AP24534 | 3-(2-imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N-[4-[(4-methylpiperazin-1-yl)methyl]-3-(trifluoromethyl)phenyl]benzamide | LYN (0.24) | O'Hare et al. 2009 |
| ABL (0.37) | O'Hare et al. 2009 | |||
| PDGFRα (1.1) | O'Hare et al. 2009 | |||
| VEGFR2 (1.5) | O'Hare et al. 2009 | |||
| FGFR1 (2.2) | O'Hare et al. 2009 | |||
| SRC (5.4) | O'Hare et al. 2009 | |||
| RET (7) | Mologni et al. 2013 | |||
| KIT (12.5) | O'Hare et al. 2009 | |||
|
| ||||
| Sunitinib | Sutent; SU-11248 | N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fluoro-2-oxo-1H-indol-3-ylidene)methyl]-2,4-dimethyl-1H-pyrrole-3-carboxamide | PDGFRβ (8)* | Mendel et al. 2003 |
| VEGFR2 (9)* | Mendel et al. 2003 | |||
| KIT (1-10)** | Abrams et al. 2003 | |||
| RET (30) | Mologni et al. 2013 | |||
| FLT3 (250) ** | O’Farrell et al. 2003 | |||
| FGFR1 (830)* | Mendel et al. 2003 | |||
|
| ||||
| Regorafenib | Stivarga; BAY 73-4506 | 4-[4-[[4-chloro-3-(trifluoromethyl)phenyl]carbamoylamino]-3-fluorophenoxy]-N-methylpyridine-2-carboxamide | RET (1.5) | Wilhelm et al. 2011 |
| RAF1 (2.5) | ||||
| mVEGFR2 (4.2) | ||||
| KIT (7) | ||||
| VEGFR1 (13) | ||||
| PDGFRβ (22) | ||||
| BRAF (28) | ||||
| mVEGFR3 (46) | ||||
| FGFR1 (202) | ||||
| TIE2 (311) | ||||
|
| ||||
| Sorafenib | Nexavar; BAY 43-9006 | 4-[4-[[4-chloro-3-(trifluoromethyl)phenyl]carbamoylamino]phenoxy]-N-methylpyridine-2-carboxamide | RET (5.9) | Plaza-Menacho et al. 2007 |
| RAF1 (6) | Wilhelm et al. 2004 | |||
| mVEGFR2 (15) | Wilhelm et al. 2004 | |||
| mVEGFR3 (20) | Wilhelm et al. 2004 | |||
| BRAF (22) | Wilhelm et al. 2004 | |||
| mPDGFRb (57) | Wilhelm et al. 2004 | |||
| FLT3 (58) | Wilhelm et al. 2004 | |||
| KIT (68) | Wilhelm et al. 2004 | |||
| VEGFR2 (90) | Wilhelm et al. 2004 | |||
Targets are ranked in descending order based on the efficacy of their inhibition: RET is highlighted. Data from in vitro kinase assays, in some cases from separate experiments (see References), are reported (standard deviations are omitted). Only targets with IC50 smaller than 1,000 nM are reported; based on the different experimental conditions (e.g. type of kinase assay, incubation time, concentration of ATP) direct comparisons between different compounds should be done cautiously.
Biochemical Ki values rather than IC50 are reported.
IC50 in cell-based phosphorylation assays
Though with different relative potencies, all these drugs, besides RET are also able to inhibit VEGFR2, and in several cases other RTKs of the split kinase domain family (PDGFR, VEGFR1 and VEGFR3), thus anticipating an effect on tumor stroma and vasculature besides that on neoplastic cells (Table 1). Though this dual (tumor and stromal cells) activity may be clinically beneficial, it complicates understanding of the specific mechanisms through which these drugs function in thyroid cancer patients (Sherman et al. 2016). SAR (structure-activity relationship) development of the anilinoquinazoline scaffold of vandetanib lead to the synthesis of investigational compounds with improved RET/VEGFR2 selectivity (Newton et al. 2016) that might be exploited to uncouple anti-RET and anti-VEGFR2 activities and discriminate their specific contribution to therapeutic cancer control.
Single amino acid changes at a small number of positions within the RET kinase domain provide resistance mutations to several TKIs inhibitors (Meng et al. 2016). These residues are V804, Y806 and G810 and cluster in a short [V804]-E-[Y806]-A-K-Y-[G810] (residues 804–810) segment of the RET kinase domain close or within the hinge region (residues 805–812) (Carlomagno et al. 2004; Carlomagno et al. 2009; Plaza-Menacho et al. 2007; Mologni et al. 2013; Huang et al. 2016). V804 in RET occupies the position that in ABL (T315) is commonly hit by on-target mutations driving acquired resistance to imatinib (Shah et al. 2002). Such position controls access to the drug binding pocket and for this reason it has been named "gate-keeper" residue (Knowles et al. 2006). In particular, V804M and V804L mutations mediate resistance to both vandetanib and cabozantinib (Carlomagno et al. 2004; Mologni et al 2013). Noteworthy, V804L mutation was identified in Ba/F3 cells expressing KIF5B-RET fusion selected for resistance to cabozantinib (Huang et al. 2016). V804M mutant is refractory also to motesanib (Mologni et al. 2013). Y806C was shown to mediate resistance to vandetanib (Carlomagno et al. 2009). Finally, G810A mutation was identified in Ba/F3 cells expressing KIF5B-RET fusion selected for resistance to vandetanib (Huang et al. 2016).
Both V804 (V804L and V804M) and Y806 (Y806C) RET mutations are pathogenetic lesions identified in medullary thyroid cancer patients regardless treatment with TKIs. Therefore, they may be responsible to primary resistance to the treatment (Wells et al. 2015). At the same time, though not proved yet, if selected during treatment these mutations may also cause a secondary (acquired) resistance. Albeit with a slightly reduced activity with respect to the unmutated kinase, sorafenib (Carlomagno et al. 2006; Plaza-Menacho et al. 2007; Mologni et al. 2013), ponatinib (De Falco et al. 2013; Mologni et al. 2013) and sunitinib (Mologni et al. 2013) keep inhibitory activity against RET V804M mutant. Similarly, sorafenib maintained efficacy at nanomolar doses also for V804L RET (Carlomagno et al. 2006). In contrast, in cell based phosphorylation assays, inhibition of V804L and Y806C RET mutants required higher doses of ponatinib, indicating that these mutants are more refractory than V804M one to TKI inhibition (De Falco et al. 2013). Accordingly, also in cell based proliferation assays, V804L mutation caused an increased IC50 dose for vandetanib, cabozantinib and lenvatinib; the least fold increase (6.2 fold) was seen for ponatinib (Huang et al. 2016). Investigational compounds, ALWII-41-27, XMD15-44 and HG-6-63-01, featuring structural similarity with ponatinib (same tail and hinge fragments and different head fragments) were similalrly active against RET V804M and V804L mutants (Moccia et al. 2015). Finally, in cell based proliferation assays, in the frame of the KIF5B-RET fusion, the G801A mutation, able to mediate resistance to vandetanib, was not affecting RET sensitivity to cabozantinib and was increasing RET sensitivity to inhibition by ponatinib and lenvatinib (Huang et al. 2016).
Novel investigational TKIs with activity against RET
Besides those exploited in patients, additional anti-RET compounds, belonging to different chemical classes, have been identified (Strock et al. 2003; Cuccuru et al. 2004; Akeno-Stuart et al. 2007; Brandt et al. 2010; Konings et al. 2010; Samadi et al. 2012; Frett et al. 2014; Sun et al. 2014; Schwartz et al. 2015; Dunna et al. 2015; Zhang et al. 2016; Han et al. 2016; Duong-Ly et al. 2016; Mologni et al. 2017). In the following section, we will focus on those compounds that have demonstrated, in protein-based or cell-based assays, RET inhibitory activity in the low nanomolar range and for which biochemical selectivity towards other kinases has been systematically explored by enzymatic assays.
Based on the features of the clinically exploited RET TKIs described in the previous section, novel compounds with anti-RET activity have entered preclinical or clinical development with the main aim of identifying more potent (to increase efficacy reducing at the same time off-targets effects) and more specific (to reduce on-targets effects, such as for instance hypertension secondary to VEGFR2 inhibition) anti-RET drugs. On a mechanistic point-of-view, more specific compounds would also help achieving a better qualification of the mechanism of action. Another important issue is to identify compounds that have activity against RET mutants at the gate-keeper and adjacent residues (to overcome primary and acquired resistance) and that, although specific, can still maintain a multitarget inhibitory profile that can anticipate synergistic anti-tumor effects or prevent resistance development in RET-driven neoplasia. This section discusses such properties for the selection of new investigational drugs with RET activity. As the clinically-registered ones (Table 1), most of them feature the classical dual RET/VEGFR2 inhibitory activity, but some exert improved RET selectivity and/or additional target spectrum (Tables 2 and 3).
Table 2.
Investigational anti-RET TKIs
| Compound | Other names | 1IUPAC name | 2Targets (IC50 nM) | References |
|---|---|---|---|---|
|
| ||||
| Apatinib | YN968D1 | N-[4-(1-cyanocyclopentyl)phenyl]-2-(pyridin-4-ylmethylamino)pyridine-3-carboxamide;methanesulfonic acid | VEGFR2 (1) | Tian et al. 2011 |
| RET (13) | ||||
| KIT (429) | ||||
| SRC (530) | ||||
|
| ||||
| Dovitinib | TKI-258 | (3Z)-4-amino-5-fluoro-3-[5-(4-methylpiperazin-1-yl)-1,3-dihydrobenzimidazol-2-ylidene]quinolin-2-one | FLT3 (1) | Lee et al. 2005 |
| KIT (2) | Lee et al. 2005 | |||
| FGFR1 (8) | Lee et al. 2005 | |||
| RET (7) | Lee et al. 2005 | |||
| VEGFR3 (8) | Lee et al. 2005 | |||
| FGFR3 (9) | Lee et al. 2005 | |||
| VEGFR1 (10) | Lee et al. 2005 | |||
| VEGFR2 (13) | Lee et al. 2005 | |||
| PDGFRβ (27) | Lee et al. 2005 | |||
| CSFR (36) | Kang et al. 2015 | |||
|
| ||||
| Motesanib | AMG-706 | N-(3,3-dimethyl-1,2-dihydroindol-6-yl)-2-(pyridin-4-ylmethylamino)pyridine-3-carboxamide | VEGFR1 (2) | Polverino et al. 2006 |
| VEGFR2 (3) | Polverino et al. 2006 | |||
| VEGFR3 (6) | Polverino et al. 2006 | |||
| KIT (8) | Polverino et al. 2006 | |||
| RET (59)* | Coxon et al. 2012 | |||
| PDGFRβ (84) | Polverino et al. 2006 | |||
|
| ||||
| PP121 | — | 1-cyclopentyl-3-(1H-pyrrolo[2,3-b]pyridin-5-yl)pyrazolo[3,4-d]pyrimidin-4-amine | RET (<1) | Apsel et al. 2008 |
| HCK (8) | ||||
| VEGFR (12) | ||||
| mTOR (13) | ||||
| SRC (14) | ||||
| ABL (18) | ||||
| p110α (52) | ||||
| DNAPK (60) | ||||
| p110δ (150) | ||||
| EPHB4 (190) | ||||
| EGFR (260) | ||||
|
| ||||
| AD80 | — | 1-(4-(4-amino-1-isopropyl-1H-pyrazolo[3,4-d]pyrimidin-3-yl)phenyl)-3-(2-fluoro-5-(trifluoromethyl)phenyl)urea | RET (4) | Dar et al. 2012 |
|
| ||||
| CEP-32496 | — | 1-[3-(6,7-dimethoxyquinazolin-4-yl)oxyphenyl]-3-[5-(1,1,1-trifluoro-2-methylpropan-2-yl)-1,2-oxazol-3-yl]urea | VEGFR1 (1) | James et al. 2012 |
| ABL (6) | ||||
| RET (7) | ||||
| LCK (15) | ||||
| EPHA2 (41) | ||||
| VEGFR2 (43) | ||||
| CRAF (146) | ||||
|
| ||||
| Pz-1 | — | N-(5-tert-butyl-1,2-oxazol-3-yl)-2-[4-[5-(1-methylpyrazol-4-yl)benzimidazol-1-yl]phenyl]acetamide | RET (1 <nM) | Frett et al. 2015 |
| VEGFR2 (1 <nM) | ||||
|
| ||||
| Alectinib | CH5424802 | 9-ethyl-6,6-dimethyl-8-(4-morpholin-4-ylpiperidin-1-yl)-11-oxo-5H-benzo[b]carbazole-3-carbonitrile | ALK (1.9) | Kodama et al. 2014 |
| RET (4.8) | ||||
|
| ||||
| Danusertib | PHA-739358 | N-[5-[(2R)-2-methoxy-2-phenylacetyl]-4,6-dihydro-1H-pyrrolo[3,4-c]pyrazol-3-yl]-4-(4-methylpiperazin-1-yl)benzamide | Aurora-A (13) | Carpinelli et al. 2007 |
| ABL (25) | ||||
| RET (31) | ||||
| NTRK1 (31) | ||||
| FGFR1 (47) | ||||
| Aurora-C (61) | ||||
| Aurora-B (79) | ||||
|
| ||||
| RXDX-105 | CEP-32496 | 1-[3-(6,7-dimethoxyquinazolin-4-yl)oxyphenyl]-3-[5-(1,1,1-trifluoro-2-methylpropan-2-yl)-1,2-oxazol-3-yl]urea | VEGFR1 (1)*** | James et al. 2012 |
| KIT (2.4)** | ||||
| ABL (6) | ||||
| RET (7) | ||||
| LCK (15) | ||||
| BRAF (36)** | ||||
| EPHA2 (41) | ||||
| VEGFR2 (43)*** | ||||
| CRAF (146) | ||||
|
| ||||
| compound 6i | — | 3-(5-Cyclopropylisoxazol-3-yl)-1-isopropyl-1H-pyrazolo[3,4-d]-pyrimidin-4-amine | RET (1.4) | Yoon et al. 2015 |
| p70S6K (23) | ||||
| PKA (39) | ||||
| FLT4 (72) | ||||
| MEK2 (82) | ||||
| FGFR (65) | ||||
| VEGFR2 (96) | ||||
| MEK1 (142) | ||||
| HCK (137) | ||||
| RSK1 (311) | ||||
from https://pubchem.ncbi.nlm.nih.gov/ or from the original publication.
Targets are ranked in descending order based on the efficacy of their inhibition. RET is highlighted. Data from in vitro kinase assays, in some cases from separate experiments (see References), are reported (standard deviations are omitted). Only targets with IC50 smaller than 1,000 nM are reported. Based on the different experimental conditions (e.g. type of kinase assay, incubation time, concentration of ATP) direct comparisons between different compounds should be done cautiously.
Limited activity in cell-based phosphorylation assays against RET-C634W (IC501100nM) and RET M918T (IC50 >2500 nM) (Coxon et al. 2012).
Biochemical Kd values for binding affinity are reported rather than IC50.
Largely inactive against VEGFR2 and VEGFR1 in cell based phosphorylation assays (James et al. 2012)
Apatinib is a potent VEGFR2 TKI, approved in the People’s Republic of China for advanced gastric cancer, and in clinical experimentation for gastric, breast, lung and radioiodine-refractory thyroid carcinoma (Tian et al. 2011; Lin et al. 2016; Lin et al. 2017). Apatinib shows additional activity against RET and, to a lower extent, KIT and SRC kinases (Table 2) (Tian et al. 2011). The compound, at microM concentration, reduced phosphorylation of KIF5B-RET in cell based assays and migration/invasion of KIF5B-RET-transfected cells (Lin et al. 2016).
Dovitinib is a potent angiogenesis inhibitor able to target several RTKs expressed in endothelial cells, stromal cells and pericytes with anti-tumor activity in several xenograft models upon daily oral 3–100 mg/kg dosing (Lee et al. 2005) (Table 2). Dovitinib demonstrated also nanomolar activity againts RET; accordingly, it inhibited proliferation of CCDC6-RET fusion-positive LC-2/ad lung carcinoma cells with an IC50 of 200 nM (Kang et al. 2015). SRC activation was associated to resistance to Dovitinib in these cells (Kang et al. 2015). Finally, daily PO dovitinib at 30 mg/kg reduced growth of LC-2/ad xenografts (Kang et al. 2015).
Motesanib is an investigational, DGF-out (type II) oral TKI targeting VEGFRs, PDGFR, KIT, and RET (Polverino et al 2006; Coxon et al. 2012). Though, in enzymatic assays, it inhibited wild type RET at nanomolar doses, in cell based assays it was less efficient at inhibiting phosphorylation of RET mutants (C634W and M918T) (Table 2). Therefore, its effects in thyroid tumor xenografts are probably mainly mediated by the anti-angiogenic activity. In phase 2 clinical study, motesanib showed signs of anti-tumor activity in patients with progressive differentiated thyroid cancer (Sherman et al. 2008) and disease control in patients with progressive or metastatic MTC (Schlumberger et al. 2009).
Through a targeted polypharmacology approach, e.g. engineering compounds with polypharmacological activity against multiple kinases, a pyrazolopyrimidine derivative, PP121, has been optimized featuring nanomolar inhibitory activities not only against RET, VEGFR2 and other TKs but also against serine/threonine (mTOR) and lipid (PI3K) kinases, a combination that may maximize therapeutic index (Table 2) (Apsel et al. 2008). Following a similar conceptual approach and an elegant genetic Drosophila model, compound AD80 with balanced RET, SRC, RAF and S6K inhibitory activities, was identified and shown to effectively reduce growth of RET mutation positive (TT and MZ-CRC-1) medullary thyroid carcinoma cells and at 30 mg/kg oral daily doses it reduced growth of TT cell xenografts (Dar et al. 2012).
A polypharmacology approach was also followed to optimize the relative RET and VEGFR2 kninase inhibitory activities of a novel TKI scaffold generated through a fragment-based chemical screen, thus leading to the identification of Pz-1. This compound is a well-balenced type II RET/VEGFR2 dual TKI, inhibiting both kinases at subnanomolar concentrations (Table 2) (Frett et al. 2015). Importantly, the compound was active also against RET V804M gate-keeper mutant (Frett et al. 2015). Pz-1 also showed significant NTRK1 and NTRK3 kinase inhibitory activities, which may expand the set of cancers potentially targeted by the drug (unpublished results). At as low as 1.0 mg/kg/day oral doses, Pz-1 blunted the formation of tumors induced by RET mutant fibroblasts in the absence of any detectable toxicity at concentrations of up to 100.0 mg/kg and will likely undergo clinical experimentation quite shortly (Frett et al. 2015).
CEP-32496 is a multikinase inhibitor that besides VEGFR family members, inhibits kinase targets as relevant for cancer as ABL, RET, and EPHA2 (Table 2) (James et al. 2012). Significant anti-tumor activity in xenografts was observed at oral doses of 30–100 mg/kg twice daily (James et al. 2012).
Alectinib is a potent ALK kinase inhibitor that was highly active in patients with ALK-rearranged lung adenocarcinomas (Kodama et al. 2014). Alectinib was found to exert also potent activity against wild type RET and RET point mutants associated to MTC (Table 2). It also inhibited at a nanomolar dose RET gate-keeper (V804L and V804M) mutants, albeit with an IC50 6–11 fold higher than wild type RET (Kodama et al. 2014). Importantly, alectinib inhibited proliferation of cell lines expressing RET fusion oncogenes (CCDC6-RET and KIF5B-RET) with an efficacy that was comparable to that of vandetanib and cabozantinib (Kodama et al. 2014). At a 20 and 60 mg/kg daily oral doses, Alectinib strongly inhibited growth of xenografts of the CCDC6-RET positive LC-2/ad lung adenocarcinoma cell line, while it had weak effects in a medullary thyroid carcinoma RET/C634W positive TT cell xenograft model, in which instead compounds like vandetanib, cabozantinib and sorafenib are active (Carlomagno et al. 2002; Carlomagno et al. 2006; Bentzien et al. 2013). As a variance from these drugs (Table 1), alectinib only slightly (at micromolar doses) inhibited VEGFR2 (Kodama et al. 2014). This suggests that the additional activities (against VEGFR2, as an example) may contribute to drug activity in MTC xenograft mouse model (Kodama et al. 2014). Chemical modification of alectinib scaffold, lead to the generation of a series of TKIs with a tetracyclic benzo[b]carbazolone core; one of them, compound 6, also featuring dual ALK and RET activities (Song et al. 2016).
Danusertib is a kinase inhibitor with a peculiar inhibitory profile, able to function at nanomolar doses not only against RET and other tyrosine kinases (ABL and its gate-keeper T315I mutant, FGFR1, NTRK1) but also serine-threonine kinases of the Aurora family (Table 2) (Carpinelli et al. 2007; Modugno et al. 2007; Meulenbeld et al. 2012). Given i.v., the compound exerted significant activity against xenograft models of several tumor types (Carpinelli et al. 2007). The compound had also nanomolar activity against a set of kinases impinging on the MAPK (mitogen activated protein kinases) system, which explained its synergy with the BCR-ABL T315I TKI bosutinib in imatinib-resistant CML (Winter et al. 2012).
RXDX-105 is a potent RAF family inhibitor with modest kinase selectivity binding in a competitive assay to 30% of a 356 kinase panel (Table 2) (James at el. 2012). RXDX-105 was modeled as a DFG-out (e.g. type II) RET inhibitor (Li et al. 2016). Besides wild type RET, the compound also inhibited at low to subnanomolar concentrations CCDC6-RET, NCOA4-RET, PRKAR1ARET, and RET M918T with activity while sparing VEGFR2 and VEGFR1 in cell-based phsophorylation assays (James et al. 2012; Li et al. 2016). However, it displayed reduced activity (about 10 fold) against the gatekeeper mutations RET V804L and RET V804M (Li et al. 2016). At nanomolar doses, RXDX-105 inhibited growth of CCDC6-RET positive lung adenocarcinoma LC-2/ad cells (IC50 = 40 nM) and RET C634W positive medullary thyroid carcinoma TT cells (IC50 = 11 nM). Interestingly, at 30 mg/kg oral BID, the compound exerted significant tumor growth inhibition against two patient-derived (PDX) KIF5B-RET positive lung adenocarcinoma xenografts as well as two colorectal PDX harbouring either the CCDC6-RET or NCOA4-RET gene fusions (Li et al. 2016). Importantly, one RET fusion positive lung adenocarcinoma patient enrolled in a phase Ib trial featured a 78% decrease in tumor burden from baseline when treated with 350 mg daily RXDX-105 (Li et al. 2016).
The pyrazolopyrimidine is a well characterized platform for tyrosine kinase inhibition; PP1, and the closely related PP2, pyrazolopyrimidines are well known SRC family inhibitors with activity also against RET (Hanke et al. 1996; Carlomagno et al. 2002; Carlomagno et al. 2003). Though these compounds have a relatively limited selectivity, medicinal chemistry efforts have succeded in generating promising new derivatives and shown that this scaffold can be used to obtain compounds able to achieve photo-controlled inhibition of RET for experimental purposes (Bliman et al. 2015; Ferreira et al. 2015). Rational design and molecular docking studies lead to the synthesis of a novel related compound (compound 6i) that featured potent inhibitory activity against RET and its V804M and, at a lower extent, V804L mutant; interestingly, compound 6i exhibited an almost 100 fold reduced activity against VEGFR2, though it featured a quite large protein kinase targets (Yoon et al. 2015) (Table 2). Further chemical modifications, mainly directed at enhancing stability, lead to the identification of compound 15l, a potent wild type RET and gatekeeper mutants. Compound 15l featured an exceptional kinase selectivity, at 1 µM concentration, exclusively inhibiting RET among 369 kinases (Yoon et al. 2017).
Acknowledgments
This work has been supported by NIH grant 1R01CA197178-01A1R.
References
- Abrams TJ, Lee LB, Murray LJ, Pryer NK, Cherrington JM. SU11248 inhibits KIT and platelet-derived growth factor receptor beta in preclinical models of human small cell lung cancer. Mol Cancer Ther. 2003 May;2(5):471–8. [PubMed] [Google Scholar]
- Akeno-Stuart N, Croyle M, Knauf JA, Malaguarnera R, Vitagliano D, Santoro M, Stephan C, Grosios K, Wartmann M, Cozens R, Caravatti G, Fabbro D, Lane HA, Fagin JA. The RET kinase inhibitor NVP-AST487 blocks growth and calcitonin gene expression through distinct mechanisms in medullary thyroid cancer cells. Cancer Res. 2007 Jul 15;67(14):6956–64. doi: 10.1158/0008-5472.CAN-06-4605. [DOI] [PubMed] [Google Scholar]
- Amit M, Na'ara S, Leider-Trejo L, Binenbaum Y, Kulish N, Fridman E, Shabtai-Orbach A, Wong RJ, Gil Z. Upregulation of RET induces perineurial invasion of pancreatic adenocarcinoma. Oncogene. 2017 Jan 16; doi: 10.1038/onc.2016.483. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Apsel B, Blair JA, Gonzalez B, Nazif TM, Feldman ME, Aizenstein B, Hoffman R, Williams RL, Shokat KM, Knight ZA. Targeted polypharmacology: discovery of dual inhibitors of tyrosine and phosphoinositide kinases. Nat Chem Biol. 2008 Nov;4(11):691–9. doi: 10.1038/nchembio.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arighi E, Borrello MG, Sariola H. RET tyrosine kinase signaling in development and cancer. Cytokine Growth Factor Rev. 2005 Aug-Oct;16(4–5):441–67. doi: 10.1016/j.cytogfr.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Bagheri-Yarmand R, Sinha KM, Gururaj AE, Ahmed Z, Rizvi YQ, Huang SC, Ladbury JE, Bogler O, Williams MD, Cote GJ, Gagel RF. A novel dual kinase function of the RET proto-oncogene negatively regulates activating transcription factor 4-mediated apoptosis. J Biol Chem. 2015 May 1;290(18):11749–61. doi: 10.1074/jbc.M114.619833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballerini P, Struski S, Cresson C, Prade N, Toujani S, Deswarte C, Dobbelstein S, Petit A, Lapillonne H, Gautier EF, Demur C, Lippert E, Pages P, Mansat-De Mas V, Donadieu J, Huguet F, Dastugue N, Broccardo C, Perot C, Delabesse E. RET fusion genes are associated with chronic myelomonocytic leukemia and enhance monocytic differentiation. Leukemia. 2012 Nov;26(11):2384–9. doi: 10.1038/leu.2012.109. [DOI] [PubMed] [Google Scholar]
- Bentzien F, Zuzow M, Heald N, Gibson A, Shi Y, Goon L, Yu P, Engst S, Zhang W, Huang D, Zhao L, Vysotskaia V, Chu F, Bautista R, Cancilla B, Lamb P, Joly AH, Yakes FM. In vitro and in vivo activity of cabozantinib (XL184), an inhibitor of RET, MET, and VEGFR2, in a model of medullary thyroid cancer. Thyroid. 2013 Dec;23(12):1569–77. doi: 10.1089/thy.2013.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bible KC, Ryder M. Evolving molecularly targeted therapies for advanced-stage thyroid cancers. Nat Rev Clin Oncol. 2016 Jul;13(7):403–16. doi: 10.1038/nrclinonc.2016.19. [DOI] [PubMed] [Google Scholar]
- Bikas A, Vachhani S, Jensen K, Vasko V, Burman KD. Targeted therapies in thyroid cancer: an extensive review of the literature. Expert Rev Clin Pharmacol. 2016 Jul 15;:1–15. doi: 10.1080/17512433.2016.1204230. [DOI] [PubMed] [Google Scholar]
- Bliman D, Nilsson JR, Kettunen P, Andréasson J, Grøtli M. A Caged Ret Kinase Inhibitor and its Effect on Motoneuron Development in Zebrafish Embryos. Sci Rep. 2015 Aug 24;5:13109. doi: 10.1038/srep13109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossi D, Carlomagno F, Pallavicini I, Pruneri G, Trubia M, Raviele PR, Marinelli A, Anaganti S, Cox MC, Viale G, Santoro M, Di Fiore PP, Minucci S. Functional characterization of a novel FGFR1OP-RET rearrangement in hematopoietic malignancies. Mol Oncol. 2014 Mar;8(2):221–31. doi: 10.1016/j.molonc.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt W, Mologni L, Preu L, Lemcke T, Gambacorti-Passerini C, Kunick C. Inhibitors of the RET tyrosine kinase based on a 2-(alkylsulfanyl)-4-(3-thienyl)nicotinonitrile scaffold. Eur J Med Chem. 2010 Jul;45(7):2919–27. doi: 10.1016/j.ejmech.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Cabanillas ME, Habra MA. Lenvatinib: Role in thyroid cancer and other solid tumors. Cancer Treat Rev. 2016 Jan;42:47–55. doi: 10.1016/j.ctrv.2015.11.003. [DOI] [PubMed] [Google Scholar]
- Cabanillas ME, Hu MI, Jimenez C, Grubbs EG, Cote GJ. Treating medullary thyroid cancer in the age of targeted therapy. Int J Endocr Oncol. 2014;1(2):203–216. doi: 10.2217/ije.14.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlomagno F, Anaganti S, Guida T, Salvatore G, Troncone G, Wilhelm SM, Santoro M. BAY 43-9006 inhibition of oncogenic RET mutants. J Natl Cancer Inst. 2006 Mar 1;98(5):326–34. doi: 10.1093/jnci/djj069. [DOI] [PubMed] [Google Scholar]
- Carlomagno F, De Vita G, Berlingieri MT, de Franciscis V, Melillo RM, Colantuoni V, Kraus MH, Di Fiore PP, Fusco A, Santoro M. Molecular heterogeneity of RET loss of function in Hirschsprung's disease. EMBO J. 1996 Jun 3;15(11):2717–25. [PMC free article] [PubMed] [Google Scholar]
- Carlomagno F, Guida T, Anaganti S, Provitera L, Kjaer S, McDonald NQ, Ryan AJ, Santoro M. Identification of tyrosine 806 as a molecular determinant of RET kinase sensitivity to ZD6474. Endocr Relat Cancer. 2009 Mar;16(1):233–41. doi: 10.1677/ERC-08-0213. [DOI] [PubMed] [Google Scholar]
- Carlomagno F, Guida T, Anaganti S, Vecchio G, Fusco A, Ryan AJ, Billaud M, Santoro M. Disease associated mutations at valine 804 in the RET receptor tyrosine kinase confer resistance to selective kinase inhibitors. Oncogene. 2004 Aug 12;23(36):6056–63. doi: 10.1038/sj.onc.1207810. [DOI] [PubMed] [Google Scholar]
- Carlomagno F, Vitagliano D, Guida T, Basolo F, Castellone MD, Melillo RM, Fusco A, Santoro M. Efficient inhibition of RET/papillary thyroid carcinoma oncogenic kinases by 4-amino-5-(4-chloro-phenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP2) J Clin Endocrinol Metab. 2003 Apr;88(4):1897–902. doi: 10.1210/jc.2002-021278. [DOI] [PubMed] [Google Scholar]
- Carlomagno F, Vitagliano D, Guida T, Ciardiello F, Tortora G, Vecchio G, Ryan AJ, Fontanini G, Fusco A, Santoro M. ZD6474, an orally available inhibitor of KDR tyrosine kinase activity, efficiently blocks oncogenic RET kinases. Cancer Res. 2002 Dec 15;62(24):7284–90. [PubMed] [Google Scholar]
- Carlomagno F, Vitagliano D, Guida T, Napolitano M, Vecchio G, Fusco A, Gazit A, Levitzki A, Santoro M. The kinase inhibitor PP1 blocks tumorigenesis induced by RET oncogenes. Cancer Res. 2002 Feb 15;62(4):1077–82. [PubMed] [Google Scholar]
- Chen Z, Fillmore CM, Hammerman PS, Kim CF, Wong KK. Non-small-cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer. 2014 Aug;14(8):535–46. doi: 10.1038/nrc3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes JE, Kantarjian H, Shah NP, Bixby D, Mauro MJ, Flinn I, O'Hare T, Hu S, Narasimhan NI, Rivera VM, Clackson T, Turner CD, Haluska FG, Druker BJ, Deininger MW, Talpaz M. Ponatinib in refractory Philadelphia chromosome-positive leukemias. N Engl J Med. 2012 Nov 29;367(22):2075–88. doi: 10.1056/NEJMoa1205127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coxon A, Bready J, Kaufman S, Estrada J, Osgood T, Canon J, Wang L, Radinsky R, Kendall R, Hughes P, Polverino A. Anti-tumor activity of motesanib in a medullary thyroid cancer model. J Endocrinol Invest. 2012 Feb;35(2):181–90. doi: 10.3275/7609. [DOI] [PubMed] [Google Scholar]
- Cuccuru G, Lanzi C, Cassinelli G, Pratesi G, Tortoreto M, Petrangolini G, Seregni E, Martinetti A, Laccabue D, Zanchi C, Zunino F. Cellular effects and antitumor activity of RET inhibitor RPI-1 on MEN2A-associated medullary thyroid carcinoma. J Natl Cancer Inst. 2004 Jul 7;96(13):1006–14. doi: 10.1093/jnci/djh184. [DOI] [PubMed] [Google Scholar]
- Dar AC, Das TK, Shokat KM, Cagan RL. Chemical genetic discovery of targets and anti-targets for cancer polypharmacology. Nature. 2012 Jun 6;486(7401):80–4. doi: 10.1038/nature11127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Castroneves LA, Negrão MV, de Freitas RM, Papadia C, Lima JV, Jr, Fukushima JT, Simão EF, Kulcsar MA, Tavares MR, Jorge AA, de Castro G, Jr, Hoff PM, Hoff AO. Sorafenib for the Treatment of Progressive Metastatic Medullary Thyroid Cancer: Efficacy and Safety Analysis. Thyroid. 2016 Mar;26(3):414–9. doi: 10.1089/thy.2015.0334. [DOI] [PubMed] [Google Scholar]
- De Falco V, Buonocore P, Muthu M, Torregrossa L, Basolo F, Billaud M, Gozgit JM, Carlomagno F, Santoro M. Ponatinib (AP24534) is a novel potent inhibitor of oncogenic RET mutants associated with thyroid cancer. J Clin Endocrinol Metab. 2013 May;98(5):E811–9. doi: 10.1210/jc.2012-2672. [DOI] [PubMed] [Google Scholar]
- Drilon A, Wang L, Hasanovic A, Suehara Y, Lipson D, Stephens P, Ross J, Miller V, Ginsberg M, Zakowski MF, Kris MG, Ladanyi M, Rizvi N. Response to Cabozantinib in patients with RET fusion-positive lung adenocarcinomas. Cancer Disc. 2013;3:630–5. doi: 10.1158/2159-8290.CD-13-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunna NR, Kandula V, Girdhar A, Pudutha A, Hussain T, Bandaru S, Nayarisseri A. High Affinity Pharmacological Profiling of Dual Inhibitors Targeting RET and VEGFR2 in Inhibition of Kinase and Angiogeneis Events in Medullary Thyroid Carcinoma. Asian Pac J Cancer Prev. 2015;16(16):7089–95. doi: 10.7314/apjcp.2015.16.16.7089. [DOI] [PubMed] [Google Scholar]
- Duong-Ly KC, Devarajan K, Liang S, Horiuchi KY, Wang Y, Ma H, Peterson JR. Kinase Inhibitor Profiling Reveals Unexpected Opportunities to Inhibit Disease-Associated Mutant Kinases. Cell Rep. 2016 Feb 2;14(4):772–81. doi: 10.1016/j.celrep.2015.12.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elisei R, Schlumberger MJ, Müller SP, Schöffski P, Brose MS, Shah MH, Licitra L, Jarzab B, Medvedev V, Kreissl MC, Niederle B, Cohen EE, Wirth LJ, Ali H, Hessel C, Yaron Y, Ball D, Nelkin B, Sherman SI. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol. 2013 Oct 10;31(29):3639–46. doi: 10.1200/JCO.2012.48.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettrich TJ, Seufferlein T. Regorafenib. Recent Results Cancer Res. 2014;201:185–96. doi: 10.1007/978-3-642-54490-3_10. [DOI] [PubMed] [Google Scholar]
- Fagin JA, Wells SA., Jr Biologic and Clinical Perspectives on Thyroid Cancer. N Engl J Med. 2016 Dec 8;375(23):2307. doi: 10.1056/NEJMc1613118. [DOI] [PubMed] [Google Scholar]
- Falchook GS, Ordóñez NG, Bastida CC, Stephens PJ, Miller VA, Gaido L, Jackson T, Karp DD. Effect of the RET Inhibitor Vandetanib in a Patient With RET Fusion-Positive Metastatic Non-Small-Cell Lung Cancer. J Clin Oncol. 2016 May 20;34(15):e141–4. doi: 10.1200/JCO.2013.50.5016. [DOI] [PubMed] [Google Scholar]
- Ferreira R, Nilsson JR, Solano C, Andréasson J, Grøtli M. Design, Synthesis and Inhibitory Activity of Photoswitchable RET Kinase Inhibitors. Sci Rep. 2015 May 6;5:9769. doi: 10.1038/srep09769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frett B, Moccia M, Carlomagno F, Santoro M, Li HY. Identification of two novel RET kinase inhibitors through MCR-based drug discovery: design, synthesis and evaluation. Eur J Med Chem. 2014 Oct 30;86:714–23. doi: 10.1016/j.ejmech.2014.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautschi O, Zander T, Keller FA, Strobel K, Hirschmann A, Aebi S, Diebold J. A patient with lung adenocarcinoma and RET fusion treated with vandetanib. J Thoracic Oncology. 2013;8:e43–4. doi: 10.1097/JTO.0b013e31828a4d07. [DOI] [PubMed] [Google Scholar]
- Geneste O, Bidaud C, De Vita G, Hofstra RM, Tartare-Deckert S, Buys CH, Lenoir GM, Santoro M, Billaud M. Two distinct mutations of the RET receptor causing Hirschsprung's disease impair the binding of signalling effectors to a multifunctional docking site. Hum Mol Genet. 1999 Oct;8(11):1989–99. doi: 10.1093/hmg/8.11.1989. [DOI] [PubMed] [Google Scholar]
- Goodman KM, Kjær S, Beuron F, Knowles PP, Nawrotek A, Burns EM, Purkiss AG, George R, Santoro M, Morris EP, McDonald NQ. RET recognition of GDNF-GFRα1 ligand by a composite binding site promotes membrane-proximal self-association. Cell Rep. 2014 Sep 25;8(6):1894–904. doi: 10.1016/j.celrep.2014.08.040. [DOI] [PubMed] [Google Scholar]
- Griseri P, Garrone O, Lo Sardo A, Monteverde M, Rusmini M, Tonissi F, Merlano M, Bruzzi P, Lo Nigro C, Ceccherini I. Genetic and epigenetic factors affect RET gene expression in breast cancer cell lines and influence survival in patients. Oncotarget. 2016 May 3;7(18):26465–26479. doi: 10.18632/oncotarget.8417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubbs EG, Ng PK, Bui J, Busaidy NL, Chen K, Lee JE, Lu X, Lu H, Meric-Bernstam F, Mills GB, Palmer G, Perrier ND, Scott KL, Shaw KR, Waguespack SG, Williams MD, Yelensky R, Cote GJ. RET fusion as a novel driver of medullary thyroid carcinoma. J Clin Endocrinol Metab. 2015 Mar;100(3):788–93. doi: 10.1210/jc.2014-4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M, Li S, Ai J, Sheng R, Hu Y, Hu Y, Geng M. Discovery of 4-chloro-3-(5-(pyridin-3-yl)-1,2,4-oxadiazole-3-yl)benzamides as novel RET kinase inhibitors. Bioorg Med Chem Lett. 2016 Dec 1;26(23):5679–5684. doi: 10.1016/j.bmcl.2016.10.061. [DOI] [PubMed] [Google Scholar]
- Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, Pollok BA, Connelly PA. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem. 1996 Jan 12;271(2):695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- Hartmaier RJ, Albacker L, Chmielecki J, Bailey M, He J, Goldberg ME, Ramkissoon S, Suh J, Elvin JA, Chiacchia S, Frampton GM, Ross JS, Miller V, Stephens PJ, Lipson D. High-throughput genomic profiling of adult solid tumors reveals novel insights into cancer pathogenesis. Cancer Res. 2017 Feb 24; doi: 10.1158/0008-5472.CAN-16-2479. pii: canres.2479.2016. [DOI] [PubMed] [Google Scholar]
- Herbst RS, Heymach JV, O'Reilly MS, Onn A, Ryan AJ. Vandetanib (ZD6474): an orally available receptor tyrosine kinase inhibitor that selectively targets pathways critical for tumor growth and angiogenesis. Expert Opin Investig Drugs. 2007 Feb;16(2):239–49. doi: 10.1517/13543784.16.2.239. [DOI] [PubMed] [Google Scholar]
- Hoy SM. Ponatinib: a review of its use in adults with chronic myeloid leukaemia or Philadelphia chromosome-positive acute lymphoblastic leukaemia. Drugs. 2014 May;74(7):793–806. doi: 10.1007/s40265-014-0216-6. [DOI] [PubMed] [Google Scholar]
- Huang Q, Schneeberger VE, Luetteke N, Jin C, Afzal R, Budzevich MM, Makanji RJ, Martinez GV, Shen T, Zhao L, Fung KM, Haura EB, Coppola D, Wu J. Preclinical Modeling of KIF5B-RET Fusion Lung Adenocarcinoma. Mol Cancer Ther. 2016 Oct;15(10):2521–2529. doi: 10.1158/1535-7163.MCT-16-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibáñez CF. Structure and physiology of the RET receptor tyrosine kinase. Cold Spring Harb Perspect Biol. 2013 Feb 1;5(2) doi: 10.1101/cshperspect.a009134. pii: a009134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James J, Ruggeri B, Armstrong RC, Rowbottom MW, Jones-Bolin S, Gunawardane RN, Dobrzanski P, Gardner MF, Zhao H, Cramer MD, Hunter K, Nepomuceno RR, Cheng M, Gitnick D, Yazdanian M, Insko DE, Ator MA, Apuy JL, Faraoni R, Dorsey BD, Williams M, Bhagwat SS, Holladay MW. CEP-32496: a novel orally active BRAF(V600E) inhibitor with selective cellular and in vivo antitumor activity. Mol Cancer Ther. 2012 Apr;11(4):930–41. doi: 10.1158/1535-7163.MCT-11-0645. [DOI] [PubMed] [Google Scholar]
- James J, Ruggeri B, Armstrong RC, Rowbottom MW, Jones-Bolin S, Gunawardane RN, Dobrzanski P, Gardner MF, Zhao H, Cramer MD, Hunter K, Nepomuceno RR, Cheng M, Gitnick D, Yazdanian M, Insko DE, Ator MA, Apuy JL, Faraoni R, Dorsey BD, Williams M, Bhagwat SS, Holladay MW. CEP-32496: a novel orally active BRAF(V600E) inhibitor with selective cellular and in vivo antitumor activity. Mol Cancer Ther. 2012 Apr;11(4):930–41. doi: 10.1158/1535-7163.MCT-11-0645. [DOI] [PubMed] [Google Scholar]
- Kang CW, Jang KW, Sohn J, Kim SM, Pyo KH, Kim H, Yun MR, Kang HN, Kim HR, Lim SM, Moon YW, Paik S, Kim DJ, Kim JH, Cho BC. Antitumor Activity and Acquired Resistance Mechanism of Dovitinib (TKI258) in RET-Rearranged Lung Adenocarcinoma. Mol Cancer Ther. 2015 Oct;14(10):2238–48. doi: 10.1158/1535-7163.MCT-15-0350. [DOI] [PubMed] [Google Scholar]
- Kato S, Subbiah V, Marchlik E, Elkin SK, Carter JL, Kurzrock R. RET Aberrations in Diverse Cancers: Next-Generation Sequencing of 4,871 Patients. Clin Cancer Res. 2016 Sep 28; doi: 10.1158/1078-0432.CCR-16-1679. [DOI] [PubMed] [Google Scholar]
- Knowles PP, Murray-Rust J, Kjaer S, Scott RP, Hanrahan S, Santoro M, Ibáñez CF, McDonald NQ. Structure and chemical inhibition of the RET tyrosine kinase domain. J Biol Chem. 2006 Nov 3;281(44):33577–87. doi: 10.1074/jbc.M605604200. [DOI] [PubMed] [Google Scholar]
- Kodama T, Tsukaguchi T, Satoh Y, Yoshida M, Watanabe Y, Kondoh O, Sakamoto H. Alectinib shows potent antitumor activity against RET-rearranged non-small cell lung cancer. Mol Cancer Ther. 2014 Dec;13(12):2910–8. doi: 10.1158/1535-7163.MCT-14-0274. [DOI] [PubMed] [Google Scholar]
- Kohno T, Tsuta K, Tsuchihara K, Nakaoku T, Yoh K, Goto K. RET fusion gene: translation to personalized lung cancer therapy. Cancer Sci. 2013 Nov;104(11):1396–400. doi: 10.1111/cas.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konings IR, de Jonge MJ, Burger H, van der Gaast A, van Beijsterveldt LE, Winkler H, Verweij J, Yuan Z, Hellemans P, Eskens FA. Phase I and pharmacological study of the broad-spectrum tyrosine kinase inhibitor JNJ-26483327 in patients with advanced solid tumours. Br J Cancer. 2010 Sep 28;103(7):987–92. doi: 10.1038/sj.bjc.6605867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar-Sinha C, Kalyana-Sundaram S, Chinnaiyan AM. Landscape of gene fusions in epithelial cancers: seq and ye shall find. Genome Med. 2015 Dec 18;7:129. doi: 10.1186/s13073-015-0252-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Rolle AF, Klempner SJ, Garrett CR, Seery T, Sanford EM, Balasubramanian S, Ross JS, Stephens PJ, Miller VA, Ali SM, Chiu VK. Identification and characterization of RET fusions in advanced colorectal cancer. Oncotarget. 2015 Oct 6;6(30):28929–37. doi: 10.18632/oncotarget.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Lee JK, Ahn MJ, Kim DW, Sun JM, Keam B, Kim TM, Heo DS, Ahn JS, Choi YL, Min HS, Jeon YK, Park K. Vandetanib in pretreated patients with advanced non-small cell lung cancer-harboring RET rearrangement: a phase II clinical trial. Ann Oncol. 2016 Nov 1; doi: 10.1093/annonc/mdw559. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Lee SH, Lopes de Menezes D, Vora J, Harris A, Ye H, Nordahl L, Garrett E, Samara E, Aukerman SL, Gelb AB, Heise C. In vivo target modulation and biological activity of CHIR-258, a multitargeted growth factor receptor kinase inhibitor, in colon cancer models. Clin Cancer Res. 2005 May 15;11(10):3633–41. doi: 10.1158/1078-0432.CCR-04-2129. [DOI] [PubMed] [Google Scholar]
- Li GG, Somwar R, Joseph J, Smith RS, Hayashi T, Martin L, Franovic A, Schairer A, Martin E, Riely GJ, Harris J, Yan S, Wei G, Oliver JW, Patel R, Multani P, Ladanyi M, Drilon A. Antitumor Activity of RXDX-105 in Multiple Cancer Types with RET Rearrangements or Mutations. Clin Cancer Res. 2016 Dec 23; doi: 10.1158/1078-0432.CCR-16-1887. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y, Liu W, Tong J, Liu Y, Xu R, Wang Z, Wang Q, Ouyang X, Yang Y, Ba Y, Liang J, Lin X, Luo D, Zheng R, Wang X, Sun G, Wang L, Zheng L, Guo H, Wu J, Xu N, Yang J, Zhang H, Cheng Y, Wang N, Chen L, Fan Z, Sun P, Yu H. Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Apatinib in Patients With Chemotherapy-Refractory Advanced or Metastatic Adenocarcinoma of the Stomach or Gastroesophageal Junction. J Clin Oncol. 2016 May 1;34(13):1448–54. doi: 10.1200/JCO.2015.63.5995. [DOI] [PubMed] [Google Scholar]
- Lin C, Wang S, Xie W, Zheng R, Gan Y, Chang J. Apatinib inhibits cellular invasion and migration by fusion kinase KIF5B-RET via suppressing RET/Src signaling pathway. Oncotarget. 2016 Sep 13;7(37):59236–59244. doi: 10.18632/oncotarget.10985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Wang C, Gao W, Cui R, Liang J. Overwhelming rapid metabolic and structural response to apatinib in radioiodine refractory differentiated thyroid cancer. Oncotarget. 2017 Feb 2; doi: 10.18632/oncotarget.15036. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui J, Yamamoto Y, Funahashi Y, Tsuruoka A, Watanabe T, Wakabayashi T, Uenaka T, Asada M. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int J Cancer. 2008 Feb 1;122(3):664–71. doi: 10.1002/ijc.23131. [DOI] [PubMed] [Google Scholar]
- Mendel DB, Laird AD, Xin X, Louie SG, Christensen JG, Li G, Schreck RE, Abrams TJ, Ngai TJ, Lee LB, Murray LJ, Carver J, Chan E, Moss KG, Haznedar JO, Sukbuntherng J, Blake RA, Sun L, Tang C, Miller T, Shirazian S, McMahon G, Cherrington JM. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003 Jan;9(1):327–37. [PubMed] [Google Scholar]
- Meng S, Wu H, Wang J, Qiu Q. Systematic Analysis of Tyrosine Kinase Inhibitor Response to RET Gatekeeper Mutations in Thyroid Cancer. Mol Inform. 2016 Oct;35(10):495–505. doi: 10.1002/minf.201600039. [DOI] [PubMed] [Google Scholar]
- Meulenbeld HJ, Mathijssen RH, Verweij J, de Wit R, de Jonge MJ. Danusertib, an aurora kinase inhibitor. Expert Opin Investig Drugs. 2012 Mar;21(3):383–93. doi: 10.1517/13543784.2012.652303. [DOI] [PubMed] [Google Scholar]
- Moccia M, Liu Q, Guida T, Federico G, Brescia A, Zhao Z, Choi HG, Deng X, Tan L, Wang J, Billaud M, Gray NS, Carlomagno F, Santoro M. Identification of Novel Small Molecule Inhibitors of Oncogenic RET Kinase. PLoS One. 2015 Jun 5;10(6):e0128364. doi: 10.1371/journal.pone.0128364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modugno M, Casale E, Soncini C, Rosettani P, Colombo R, Lupi R, Rusconi L, Fancelli D, Carpinelli P, Cameron AD, Isacchi A, Moll J. Crystal structure of the T315I Abl mutant in complex with the aurora kinases inhibitor PHA-739358. Cancer Res. 2007 Sep 1;67(17):7987–90. doi: 10.1158/0008-5472.CAN-07-1825. [DOI] [PubMed] [Google Scholar]
- Mologni L, Gambacorti-Passerini C, Goekjian P, Scapozza L. RET kinase inhibitors: a review of recent patents (2012–2015) Expert Opin Ther Pat. 2017 Jan;27(1):91–99. doi: 10.1080/13543776.2017.1238073. [DOI] [PubMed] [Google Scholar]
- Mologni L, Redaelli S, Morandi A, Plaza-Menacho I, Gambacorti-Passerini C. Ponatinib is a potent inhibitor of wild-type and drug-resistant gatekeeper mutant RET kinase. Mol Cell Endocrinol. 2013 Sep 5;377(1–2):1–6. doi: 10.1016/j.mce.2013.06.025. [DOI] [PubMed] [Google Scholar]
- Morandi A, Martin LA, Gao Q, Pancholi S, Mackay A, Robertson D, Zvelebil M, Dowsett M, Plaza-Menacho I, Isacke CM. GDNF-RET signaling in ER-positive breast cancers is a key determinant of response and resistance to aromatase inhibitors. Cancer Res. 2013 Jun 15;73(12):3783–95. doi: 10.1158/0008-5472.CAN-12-4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay S, Pennell NA, Ali SM, Ross JS, Ma PC, Velcheti V. RET rearranged lung adenocarcinomas with lymphangitic spread, psammoma bodies, and clinical responses to cabozantinib. J Thoracic Oncology. 2014;9:1714–9. doi: 10.1097/JTO.0000000000000323. [DOI] [PubMed] [Google Scholar]
- Mulligan LM. RET revisited: expanding the oncogenic portfolio. Nat Rev Cancer. 2014 Mar;14(3):173–86. doi: 10.1038/nrc3680. [DOI] [PubMed] [Google Scholar]
- Newton R, Bowler KA, Burns EM, Chapman PJ, Fairweather EE, Fritzl SJ, Goldberg KM, Hamilton NM, Holt SV, Hopkins GV, Jones SD, Jordan AM, Lyons AJ, Nikki March H, McDonald NQ, Maguire LA, Mould DP, Purkiss AG, Small HF, Stowell AI, Thomson GJ, Waddell ID, Waszkowycz B, Watson AJ, Ogilvie DJ. The discovery of 2-substituted phenol quinazolines as potent RET kinase inhibitors with improved KDR selectivity. Eur J Med Chem. 2016 Apr 13;112:20–32. doi: 10.1016/j.ejmech.2016.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen M, Miyakawa S, Kato J, Mori T, Arai T, Armanini M, Gelmon K, Yerushalmi R, Leung S, Gao D, Landes G, Haak-Frendscho M, Elias K, Simmons AD. Preclinical Efficacy and Safety Assessment of an Antibody-Drug Conjugate Targeting the c-RET Proto-Oncogene for Breast Carcinoma. Clin Cancer Res. 2015 Dec 15;21(24):5552–62. doi: 10.1158/1078-0432.CCR-15-0468. [DOI] [PubMed] [Google Scholar]
- O'Farrell AM, Abrams TJ, Yuen HA, Ngai TJ, Louie SG, Yee KW, Wong LM, Hong W, Lee LB, Town A, Smolich BD, Manning WC, Murray LJ, Heinrich MC, Cherrington JM. SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo. Blood. 2003 May 1;101(9):3597–605. doi: 10.1182/blood-2002-07-2307. [DOI] [PubMed] [Google Scholar]
- O'Hare T, Shakespeare WC, Zhu X, Eide CA, Rivera VM, Wang F, Adrian LT, Zhou T, Huang WS, Xu Q, Metcalf CA, 3rd, Tyner JW, Loriaux MM, Corbin AS, Wardwell S, Ning Y, Keats JA, Wang Y, Sundaramoorthi R, Thomas M, Zhou D, Snodgrass J, Commodore L, Sawyer TK, Dalgarno DC, Deininger MW, Druker BJ, Clackson T. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell. 2009 Nov 6;16(5):401–12. doi: 10.1016/j.ccr.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelet A, Geneste O, Edery P, Pasini A, Chappuis S, Atti T, Munnich A, Lenoir G, Lyonnet S, Billaud M. Various mechanisms cause RET-mediated signaling defects in Hirschsprung's disease. J Clin Invest. 1998 Mar 15;101(6):1415–23. doi: 10.1172/JCI375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt A, Morten J, Ji Q, Elvin P, Womack C, Su X, Donald E, Gray N, Read J, Bigley G, Blockley L, Cresswell C, Dale A, Davies A, Zhang T, Fan S, Fu H, Gladwin A, Harrod G, Stevens J, Williams V, Ye Q, Zheng L, de Boer R, Herbst RS, Lee JS, Vasselli J. A retrospective analysis of RET translocation, gene copy number gain and expression in NSCLC patients treated with vandetanib in four randomized Phase III studies. BMC Cancer. 2015 Mar 23;15:171. doi: 10.1186/s12885-015-1146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaza-Menacho I, Barnouin K, Barry R, Borg A, Orme M, Chauhan R, Mouilleron S, Martínez-Torres RJ, Meier P, McDonald NQ. RET Functions as a Dual-Specificity Kinase that Requires Allosteric Inputs from Juxtamembrane Elements. Cell Rep. 2016 Dec 20;17(12):3319–3332. doi: 10.1016/j.celrep.2016.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaza-Menacho I, Barnouin K, Goodman K, Martínez-Torres RJ, Borg A, Murray-Rust J, Mouilleron S, Knowles P, McDonald NQ. Oncogenic RET kinase domain mutations perturb the autophosphorylation trajectory by enhancing substrate presentation in trans. Mol Cell. 2014b Mar 6;53(5):738–51. doi: 10.1016/j.molcel.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaza-Menacho I, Mologni L, McDonald NQ. Mechanisms of RET signaling in cancer: current and future implications for targeted therapy. Cell Signal. 2014a Aug;26(8):1743–52. doi: 10.1016/j.cellsig.2014.03.032. [DOI] [PubMed] [Google Scholar]
- Plaza-Menacho I, Mologni L, Sala E, Gambacorti-Passerini C, Magee AI, Links TP, Hofstra RM, Barford D, Isacke CM. Sorafenib functions to potently suppress RET tyrosine kinase activity by direct enzymatic inhibition and promoting RET lysosomal degradation independent of proteasomal targeting. J Biol Chem. 2007 Oct 5;282(40):29230–40. doi: 10.1074/jbc.M703461200. [DOI] [PubMed] [Google Scholar]
- Plaza-Menacho I, Morandi A, Robertson D, Pancholi S, Drury S, Dowsett M, Martin LA, Isacke CM. Targeting the receptor tyrosine kinase RET sensitizes breast cancer cells to tamoxifen treatment and reveals a role for RET in endocrine resistance. Oncogene. 2010 Aug 19;29(33):4648–57. doi: 10.1038/onc.2010.209. [DOI] [PubMed] [Google Scholar]
- Polverino A, Coxon A, Starnes C, Diaz Z, DeMelfi T, Wang L, Bready J, Estrada J, Cattley R, Kaufman S, Chen D, Gan Y, Kumar G, Meyer J, Neervannan S, Alva G, Talvenheimo J, Montestruque S, Tasker A, Patel V, Radinsky R, Kendall R. AMG 706, an oral, multikinase inhibitor that selectively targets vascular endothelial growth factor, platelet-derived growth factor, and kit receptors, potently inhibits angiogenesis and induces regression in tumor xenografts. Cancer Res. 2006 Sep 1;66(17):8715–21. doi: 10.1158/0008-5472.CAN-05-4665. [DOI] [PubMed] [Google Scholar]
- Ricarte-Filho JC, Li S, Garcia-Rendueles ME, Montero-Conde C, Voza F, Knauf JA, Heguy A, Viale A, Bogdanova T, Thomas GA, Mason CE, Fagin JA. Identification of kinase fusion oncogenes in post-Chernobyl radiation-induced thyroid cancers. J Clin Invest. 2013 Nov;123(11):4935–44. doi: 10.1172/JCI69766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosell R, Karachaliou N. RET inhibitors for patients with RET fusion-positive and RET wild-type non-small-cell lung cancer. Lancet Oncol. 2016 Dec;17(12):1623–1625. doi: 10.1016/S1470-2045(16)30557-5. [DOI] [PubMed] [Google Scholar]
- Samadi AK, Bazzill J, Zhang X, Gallagher R, Zhang H, Gollapudi R, Kindscher K, Timmermann B, Cohen MS. Novel withanolides target medullary thyroid cancer through inhibition of both RET phosphorylation and the mammalian target of rapamycin pathway. Surgery. 2012 Dec;152(6):1238–47. doi: 10.1016/j.surg.2012.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro M, Carlomagno F, Romano A, Bottaro DP, Dathan NA, Grieco M, Fusco A, Vecchio G, Matoskova B, Kraus MH, et al. Activation of RET as a dominant transforming gene by germline mutations of MEN2A and MEN2B. Science. 1995 Jan 20;267(5196):381–3. doi: 10.1126/science.7824936. [DOI] [PubMed] [Google Scholar]
- Santoro M, Carlomagno F. Central role of RET in thyroid cancer. Cold Spring Harb Perspect Biol. 2013 Dec 1;5(12):a009233. doi: 10.1101/cshperspect.a009233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, Habra MA, Newbold K, Shah MH, Hoff AO, Gianoukakis AG, Kiyota N, Taylor MH, Kim SB, Krzyzanowska MK, Dutcus CE, de las Heras B, Zhu J, Sherman SI. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015 Feb 12;372(7):621–30. doi: 10.1056/NEJMoa1406470. [DOI] [PubMed] [Google Scholar]
- Schlumberger MJ, Elisei R, Bastholt L, Wirth LJ, Martins RG, Locati LD, Jarzab B, Pacini F, Daumerie C, Droz JP, Eschenberg MJ, Sun YN, Juan T, Stepan DE, Sherman SI. Phase II study of safety and efficacy of motesanib in patients with progressive or symptomatic, advanced or metastatic medullary thyroid cancer. J Clin Oncol. 2009 Aug 10;27(23):3794–801. doi: 10.1200/JCO.2008.18.7815. [DOI] [PubMed] [Google Scholar]
- Schwartz GK, Adkins D, Suk Heist R, Abbott M, Barber SL, Chao RC, Neuteboom STC, Chen I, Christensen J, Bauer TM. A first-in-human phase I/Ib study of receptor tyrosine kinase (RTK) inhibitor, MGCD516, in patients with advanced solid tumors. 2015 ASCO Annual Meeting, J Clin Oncol. 2015;33 (suppl; abstr TPS2621) [Google Scholar]
- Shah NP, Nicoll JM, Nagar B, Gorre ME, Paquette RL, Kuriyan J, Sawyers CL. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell. 2002 Aug;2(2):117–25. doi: 10.1016/s1535-6108(02)00096-x. [DOI] [PubMed] [Google Scholar]
- Sherman SI, Clary DO, Elisei R, Schlumberger MJ, Cohen EE, Schöffski P, Wirth LJ, Mangeshkar M, Aftab DT, Brose MS. Correlative analyses of RET and RAS mutations in a phase 3 trial of cabozantinib in patients with progressive, metastatic medullary thyroid cancer. Cancer. 2016 Dec 15;122(24):3856–3864. doi: 10.1002/cncr.30252. [DOI] [PubMed] [Google Scholar]
- Sherman SI, Wirth LJ, Droz JP, Hofmann M, Bastholt L, Martins RG, Licitra L, Eschenberg MJ, Sun YN, Juan T, Stepan DE, Schlumberger MJ, Motesanib Thyroid Cancer Study Group Motesanib diphosphate in progressive differentiated thyroid cancer. N Engl J Med. 2008 Jul 3;359(1):31–42. doi: 10.1056/NEJMoa075853. [DOI] [PubMed] [Google Scholar]
- Sherman SI. Tyrosine kinase inhibitors and the thyroid. Best Pract Res Clin Endocrinol Metab. 2009 Dec;23(6):713–22. doi: 10.1016/j.beem.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Stransky N, Cerami E, Schalm S, Kim JL, Lengauer C. The landscape of kinase fusions in cancer. Nat Commun. 2014 Sep 10;5:4846. doi: 10.1038/ncomms5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strock CJ, Park JI, Rosen M, Dionne C, Ruggeri B, Jones-Bolin S, Denmeade SR, Ball DW, Nelkin BD. CEP-701 and CEP-751 inhibit constitutively activated RET tyrosine kinase activity and block medullary thyroid carcinoma cell growth. Cancer Res. 2003 Sep 1;63(17):5559–63. [PubMed] [Google Scholar]
- Sun QZ, Xu Y, Liu JJ, Zhang CH, Wang ZR, Zheng RL, Wang WJ, Li LL, Yang SY. Structural modification of an EGFR inhibitor that showed weak off-target activity against RET leading to the discovery of a potent RET inhibitor. Mol Divers. 2014 May;18(2):403–9. doi: 10.1007/s11030-014-9508-8. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Ritz J, Cooper GM. Activation of a novel human transforming gene, ret, by DNA rearrangement. Cell. 1985 Sep;42(2):581–8. doi: 10.1016/0092-8674(85)90115-1. [DOI] [PubMed] [Google Scholar]
- Thomas L, Lai SY, Dong W, Feng L, Dadu R, Regone RM, Cabanillas ME. Sorafenib in metastatic thyroid cancer: a systematic review. Oncologist. 2014 Mar;19(3):251–8. doi: 10.1634/theoncologist.2013-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian S, Quan H, Xie C, Guo H, Lü F, Xu Y, Li J, Lou L. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci. 2011 Jul;102(7):1374–80. doi: 10.1111/j.1349-7006.2011.01939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanden Borre P, Schrock AB, Anderson PM, Morris JC, 3rd, Heilmann AM, Holmes O, Wang K, Johnson A, Waguespack SG, Ou SI, Khan S, Fung KM, Stephens PJ, Erlich RL, Miller VA, Ross JS, Ali SM. Pediatric, Adolescent, and Young Adult Thyroid Carcinoma Harbors Frequent and Diverse Targetable Genomic Alterations, Including Kinase Fusions. Oncologist. 2017 Mar;22(3):255–263. doi: 10.1634/theoncologist.2016-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viola D, Valerio L, Molinaro E, Agate L, Bottici V, Biagini A, Lorusso L, Cappagli V, Pieruzzi L, Giani C, Sabini E, Passannati P, Puleo L, Matrone A, Pontillo-Contillo B, Battaglia V, Mazzeo S, Vitti P, Elisei R. Treatment of advanced thyroid cancer with targeted therapies: ten years of experience. Endocr Relat Cancer. 2016 Apr;23(4):R185–205. doi: 10.1530/ERC-15-0555. [DOI] [PubMed] [Google Scholar]
- Vozniak JM, Jacobs JM. Vandetanib. J Adv Pract Oncol. 2012 Mar;3(2):112–6. [PMC free article] [PubMed] [Google Scholar]
- Wang K, Russell JS, McDermott JD, Elvin JA, Khaira D, Johnson A, Jennings TA, Ali SM, Murray M, Marshall C, Oldham DS, Washburn D, Wong SJ, Chmielecki J, Yelensky R, Lipson D, Miller VA, Stephens PJ, Serracino HS, Ross JS, Bowles DW. Profiling of 149 Salivary Duct Carcinomas, Carcinoma Ex Pleomorphic Adenomas, and Adenocarcinomas, Not Otherwise Specified Reveals Actionable Genomic Alterations. Clin Cancer Res. 2016 Dec 15;22(24):6061–6068. doi: 10.1158/1078-0432.CCR-15-2568. [DOI] [PubMed] [Google Scholar]
- Weitzman SP, Cabanillas ME. The treatment landscape in thyroid cancer: a focus on cabozantinib. Cancer Manag Res. 2015 Aug 19;7:265–78. doi: 10.2147/CMAR.S68373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells SA, Jr, Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, Lee N, Machens A, Moley JF, Pacini F, Raue F, Frank-Raue K, Robinson B, Rosenthal MS, Santoro M, Schlumberger M, Shah M, Waguespack SG, American Thyroid Association Guidelines Task Force on Medullary Thyroid Carcinoma Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015 Jun;25(6):567–610. doi: 10.1089/thy.2014.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells SA, Jr, Gosnell JE, Gagel RF, Moley J, Pfister D, Sosa JA, Skinner M, Krebs A, Vasselli J, Schlumberger M. Vandetanib for the treatment of patients with locally advanced or metastatic hereditary medullary thyroid cancer. J Clin Oncol. 2010 Feb 10;28(5):767–72. doi: 10.1200/JCO.2009.23.6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells SA, Jr, Pacini F, Robinson BG, Santoro M. Multiple endocrine neoplasia type 2 and familial medullary thyroid carcinoma: an update. J Clin Endocrinol Metab. 2013 Aug;98(8):3149–64. doi: 10.1210/jc.2013-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesner T, He J, Yelensky R, Esteve-Puig R, Botton T, Yeh I, Lipson D, Otto G, Brennan K, Murali R, Garrido M, Miller VA, Ross JS, Berger MF, Sparatta A, Palmedo G, Cerroni L, Busam KJ, Kutzner H, Cronin MT, Stephens PJ, Bastian BC. Kinase fusions are frequent in Spitz tumours and spitzoid melanomas. Nat Commun. 2014;5:3116. doi: 10.1038/ncomms4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, Cao Y, Shujath J, Gawlak S, Eveleigh D, Rowley B, Liu L, Adnane L, Lynch M, Auclair D, Taylor I, Gedrich R, Voznesensky A, Riedl B, Post LE, Bollag G, Trail PA. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004 Oct 1;64(19):7099–109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Schütz G, Thierauch KH, Zopf D. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011 Jul 1;129(1):245–55. doi: 10.1002/ijc.25864. [DOI] [PubMed] [Google Scholar]
- Winter GE, Rix U, Carlson SM, Gleixner KV, Grebien F, Gridling M, Müller AC, Breitwieser FP, Bilban M, Colinge J, Valent P, Bennett KL, White FM, Superti-Furga G. Systems-pharmacology dissection of a drug synergy in imatinib-resistant CML. Nat Chem Biol. 2012 Nov;8(11):905–12. doi: 10.1038/nchembio.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin X, Abrams TJ, Hollenbach PW, Rendahl KG, Tang Y, Oei YA, Embry MG, Swinarski DE, Garrett EN, Pryer NK, Trudel S, Jallal B, Mendel DB, Heise CC. CHIR-258 is efficacious in a newly developed fibroblast growth factor receptor 3-expressing orthotopic multiple myeloma model in mice. Clin Cancer Res. 2006 Aug 15;12(16):4908–15. doi: 10.1158/1078-0432.CCR-06-0957. [DOI] [PubMed] [Google Scholar]
- Yakes FM, Chen J, Tan J, Yamaguchi K, Shi Y, Yu P, Qian F, Chu F, Bentzien F, Cancilla B, Orf J, You A, Laird AD, Engst S, Lee L, Lesch J, Chou YC, Joly AH. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther. 2011 Dec;10(12):2298–308. doi: 10.1158/1535-7163.MCT-11-0264. [DOI] [PubMed] [Google Scholar]
- Yoh K, Seto T, Satouchi M, Nishio M, Yamamoto N, Murakami H, Nogami N, Matsumoto S, Kohno T, Tsuta K, Tsuchihara K, Ishii G, Nomura S, Sato A, Ohtsu A, Ohe Y, Goto K. Vandetanib in patients with previously treated RET-rearranged advanced non-small-cell lung cancer (LURET): an open-label, multicentre phase 2 trial. Lancet Respir Med. 2017 Jan;5(1):42–50. doi: 10.1016/S2213-2600(16)30322-8. [DOI] [PubMed] [Google Scholar]
- Yoon H, Kwak Y, Choi S, Cho H, Kim ND, Sim T. A Pyrazolo[3,4-d]pyrimidin-4-amine Derivative Containing an Isoxazole Moiety Is a Selective and Potent Inhibitor of RET Gatekeeper Mutants. J Med Chem. 2016 Jan 14;59(1):358–73. doi: 10.1021/acs.jmedchem.5b01522. [DOI] [PubMed] [Google Scholar]
- Yoon H, Kwak Y, Choi S, Cho H, Kim ND, Sim T. A Pyrazolo[3,4-d]pyrimidin-4-amine Derivative Containing an Isoxazole Moiety Is a Selective and Potent Inhibitor of RET Gatekeeper Mutants. J Med Chem. 2016 Jan 14;59(1):358–73. doi: 10.1021/acs.jmedchem.5b01522. [DOI] [PubMed] [Google Scholar]
- Yoshihara K, Wang Q, Torres-Garcia W, Zheng S, Vegesna R, Kim H, Verhaak RG. The landscape and therapeutic relevance of cancer-associated transcript fusions. Oncogene. 2015 Sep 10;34(37):4845–54. doi: 10.1038/onc.2014.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Lu B, Liu D, Shen R, Yan Y, Yang L, Zhang M, Zhang L, Cao G, Cao H, Fu B, Gong A, Sun Q, Wan H, Zhang L, Tao W, Cao J. EBI-907, a novel BRAF(V600E) inhibitor, has potent oral anti-tumor activity and a broad kinase selectivity profile. Cancer Biol Ther. 2016;17(2):199–207. doi: 10.1080/15384047.2016.1139231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Yang PL, Gray NS. Targeting cancer with small molecule kinase inhibitors. Nat Rev Cancer. 2009 Jan;9(1):28–39. doi: 10.1038/nrc2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Wu H, Wang L, Liu Y, Knapp S, Liu Q, Gray NS. Exploration of type II binding mode: A privileged approach for kinase inhibitor focused drug discovery? ACS Chem Biol. 2014 Jun 20;9(6):1230–41. doi: 10.1021/cb500129t. [DOI] [PMC free article] [PubMed] [Google Scholar]