A novel porcine model of injury and repair using radiofrequency ablation has been developed, allowing for reproducible injury to the esophagus to study repair in an animal model with esophageal submucosal glands, a key anatomical feature and missing in rodent models but possibly harboring progenitor cells. There is a strong translational component to this porcine model given the anatomical and physiological similarities between pigs and humans.
Keywords: esophagus, injury, repair, esophageal submucosal gland, proliferation
Abstract
Esophageal injury is a risk factor for diseases such as Barrett’s esophagus (BE) and esophageal adenocarcinoma. To improve understanding of signaling pathways associated with both normal and abnormal repair, animal models are needed. Traditional rodent models of esophageal repair are limited by the absence of esophageal submucosal glands (ESMGs), which are present in the human esophagus. Previously, we identified acinar ductal metaplasia in human ESMGs in association with both esophageal injury and cancer. In addition, the SOX9 transcription factor has been associated with generation of columnar epithelium and the pathogenesis of BE and is present in ESMGs. To test our hypothesis that ESMGs activate after esophageal injury with an increase in proliferation, generation of a ductal phenotype, and expression of SOX9, we developed a porcine model of esophageal injury and repair using radiofrequency ablation (RFA). The porcine esophagus contains ESMGs, and RFA produces a consistent and reproducible mucosal injury in the esophagus. Here we present a temporal assessment of this model of esophageal repair. Porcine esophagus was evaluated at 0, 6, 18, 24, 48, and 72 h and 5 and 7 days following RFA and compared with control uninjured esophagus. Following RFA, ESMGs demonstrated an increase in ductal phenotype, echoing our prior studies in humans. Proliferation increased in both squamous epithelium and ESMGs postinjury with a prominent population of SOX9-positive cells in ESMGs postinjury. This model promises to be useful in future experiments evaluating mechanisms of esophageal repair.
NEW & NOTEWORTHY A novel porcine model of injury and repair using radiofrequency ablation has been developed, allowing for reproducible injury to the esophagus to study repair in an animal model with esophageal submucosal glands, a key anatomical feature and missing in rodent models but possibly harboring progenitor cells. There is a strong translational component to this porcine model given the anatomical and physiological similarities between pigs and humans.
View this article's corresponding video summary at https://youtu.be/oeLgffG2Pk4.
gastresophageal reflux disease (GERD) is prevalent worldwide and is a risk factor for the development of Barrett’s esophagus (BE), a process of abnormal repair after esophageal epithelial injury characterized by generation of intestinal metaplasia with a columnar phenotype (32). BE is also associated with the development of esophageal adenocarcinoma (EAC) (8). Because the 5-yr survival rate for esophageal cancer is <20% (21), prevention may represent the best strategy for improving health. One strategy for preventing long-term esophageal disease could include supporting healthy repair after injury.
Repair mechanisms following esophageal injury remain poorly understood. Multiple investigations in rodent models have established stem/progenitor populations of cells derived from the squamous epithelium of the esophagus (2, 6, 7, 9, 25, 41). Yet these models are limited by the anatomical absence of esophageal submucosal glands (ESMGs). Given the association of esophageal injury with carcinogenesis (32), additional knowledge is needed in esophageal repair processes. The normal human esophagus is lined with a stratified, nonkeratinized squamous epithelium that provides a protective barrier against exposure to potentially caustic substances (31). The barrier is further protected by secretions from ESMGs, including mucins, bicarbonate, epidermal growth factor, and prostaglandins (31). In addition to what is known regarding protective secretions from ESMGs, a few studies have implicated ESMGs or their ducts as a potential source of cells for esophageal repair.
With the use of detailed histological analyses, ESMGs and ducts have been described in close proximity to overlying patches of BE (5). Ducts from ESMGs have also been observed in direct continuity with squamous islands within the submucosa as well as with overlying islands of squamous epithelium within areas of BE (5). The observation that ESMGs and ducts are associated with both squamous epithelium and BE has further support given the shared clonality in one patient between ESMG ducts with BE glands as well as shared clonality of ESMG ducts with squamous epithelium (30). Because of these strong associations between ESMGs and ducts with overlying esophageal mucosa, our group previously evaluated ESMG morphology from different patient populations. Compared with autopsy controls, ESMGs in patients with either esophageal injury (ulcer) or esophageal cancer were more likely to demonstrate a ductal phenotype within the ESMGs, similar to acinar ductal metaplasia reported in other organs such as pancreas (36). In particular, areas of epithelial ulceration in human esophagectomy samples were the most likely to be associated with acinar ductal metaplasia in the underlying ESMG (12). When the esophagus heals normally, ulcers are replaced with squamous epithelium, yet in abnormal repair, BE may result (4, 40). Several theories exist regarding the progenitor origin of BE including transdifferentiation of squamous epithelium and migration of progenitors from the gastresophageal junction and the ESMGs (28). While the cell of origin for BE is still unknown, SOX9 has been implicated as an important transcription factor in the pathogenesis of BE. Wang et al. (40) described positive epithelial expression of SOX9 in patient samples with BE, BE/dysplasia, and EAC and suggested that mesenchymal activation in the epithelium is associated with epithelial activation of SOX9, leading to columnar transcription. In addition, SOX9 has been described in ESMG ducts and myoepithelium (17); however, changes in ESMG SOX9 expression after esophageal injury remain unknown.
While observational studies in humans generated hypotheses about the role of ESMGs in esophageal repair, to understand the prospective temporal course of ESMG contributions to esophageal repair a relevant animal model is needed because the common rodent models, mice and rats, lack these glands (26). In a canine model from the 1980s, after cardioplasty to induce reflux, squamous stripping above the gastroesophageal junction, and administration of pentagastrin to augment acid production, columnar reepithelialization occurred that was contiguous with ducts from ESMGs (14). In addition, to determine proliferation in a canine cardioplasty model, the thymidine analog bromodeoxyuridine was administered and the labeling index in ESMGs increased from 0 to 0.35% in the setting of epithelial acid-related injury (38). As with human models, these canine studies suggest that ESMGs can contribute to esophageal repair with activation after injury. Because pigs are physiologically similar to humans and possess ESMGs, they offer a suitable model for studying esophageal injury and disease (18).
For this pig model, radiofrequency ablation (RFA) was selected to create a reproducible model of esophageal injury and repair. RFA is a technique that was developed in pigs in the 1970s and was refined for use in human esophagus in 2004 (11). RFA is currently used in human patients to treat BE and prevent progression to high-grade dysplasia and EAC (20). Ablation with the Halo system delivers ablation depth of <700 µM, in which the goal is to allow penetration of the epithelium and muscularis mucosa without injuring the submucosa (10). Thus we were able to apply the RFA technique to a new porcine model of esophageal injury and repair, with the goal of delivering a similar depth of ablation by following protocols for the human treatment of BE by RFA. This model allowed us to temporally assess the response of ESMGs to significant esophageal epithelial injury in a manner that is otherwise impossible with human patients or rodent models. Based on our previous observations in humans, we hypothesized that in this porcine model, ESMGs would demonstrate a proliferative response with acinar ductal metaplasia within ESMGs. In addition, based on the association of SOX9 with a columnar phenotype (4, 40), we hypothesized that regenerating ESMGs postinjury would express SOX9 protein. Thus our goals in this initial characterization of the porcine model were to quantify proliferation, determine the extent of the ductular response to injury, and evaluate the status of SOX9 expression within ESMGs.
MATERIALS AND METHODS
Human cases.
Prior approval for use of human esophagectomy cases and autopsy controls were obtained from the Duke University’s Institutional Review Board as described in Garman et al. (12). Archived paraffin-embedded esophageal tissue was obtained with Institutional Review Board approval for histological and immunohistochemical analysis.
Experimental animals and procedures.
Yorkshire crossbred pigs (Sus scrofa) of either sex weighing between 23 and 45 kg were cared for according to North Carolina State University and Duke University Institutional Animal Care and Use Committee-approved protocols (NCSU 13-116-B, Duke A120-14-05) as set forth in the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health. All experiments were performed at the Large Animal Models Core of the Center for Gastrointestinal Biology and Disease (CGIBD) located at North Carolina State University. Pigs were housed in individual pens and maintained on commercial pelleted feed for 3–5 days before anesthesia, endoscopy, and RFA. Feed was withheld overnight preceding the procedure. Pigs were sedated with a combination of xylazine (1.5 mg/kg im) and ketamine (11 mg/kg im) and anesthetized by mask inhalation of 4–5% isoflurane vaporized in 100% O2. Animals were subsequently intubated and maintained on 1–2% isoflurane/O2. A peripheral ear vein was catheterized for administration of lactated Ringers solution at a maintenance rate of 15 ml·kg−1·h−1. Animals were placed on a water-circulated heating pad in dorsal recumbence in preparation for endoscopy. Endoscopy was performed using an Olympus GIF Q140 (Center Valley, PA) endoscope by a board-certified human gastroenterologist experienced with RFA (K. S. Garman).
Radiofrequency ablation.
To create areas of acute esophageal damage, RFA was applied via endoscope to three regions: proximal esophagus, midesophagus, and distal esophagus (gastresophageal junction). The energy dose was derived from previously developed pig protocols (11). A Barrx Flex RFA Energy Generator 1190A-115A was used along with the Halo Barrx 90 RFA Focal Catheter (90–9100; Covidien-Medtronic, Minneapolis, MN), which was attached to the end of the Olympus GIF Q140 endoscope. Energy was delivered at 12 J/cm2 with power density of 40 W/cm2 for a total of two doses to each area and in replicates of two adjacent areas at each of three different segments of the esophagus; this resulted in a combined total of 12 RFA applications per animal. Each treatment area was 260 mm2. RFA application was performed by the same investigator (K. S. Garman) throughout the study.
To determine the pattern of injury and recovery following ablation, a total of three pigs (n = 3) were used for each of seven recovery periods following RFA (0, 6, 18, 24, 48, and 72 h and 5 days). As controls, three sham animals underwent endoscopy with no treatment. For most time points, three animals were used. For the 7-day post-RFA recovery time point, six pigs (n = 6) underwent RFA treatment due to the robust repair response noted at this time point. Between the RFA application and euthanasia, the animals were treated with analgesia (buprenorphine, 0.02–0.05 mg/kg, up to 3 times daily) and were offered a standard laboratory porcine diet. After each defined recovery period, animals were euthanized with an overdosage of pentobarbital (60 mg/kg iv) following initial sedation with xylazine-ketamine as before. Esophageal tissues were collected for histological analyses.
Immunohistochemistry.
Each esophageal ablation site was processed, and portions of the treated ulcer and adjacent border were assessed using immunohistochemistry. Tissue blocks were created that were representative of separate areas of ulcer and normal. In the blocks containing ulcer, a standard area including ulcer border was obtained.
These samples were placed in 10% buffered formalin overnight at room temperature and transferred to refrigerated 70% ethanol (4°C) before being paraffin embedded. Tissues were cut into 5-µm sections and mounted on glass slides. Sections were deparaffinized with xylene and rehydrated stepwise with ethanol and water before peroxidase block (3% peroxide). Sections were then rinsed in TBS and placed in protein block (DAKO X0909) for 30 min. Primary antibodies were applied according to antibody specification in Table 1, which lists all antibodies used along with their source, catalog number, host species, and dilution. DAKO secondary antibody was applied for 1 h at room temperature according to primary antibody species before development with DAKO DAB (K3468). Counterstain was performed with Mayer’s hematoxylin and bluing agent. Slides were dehydrated stepwise in ethanol and water baths before being washed in xylene and mounted with Cytoseal (Thermo 8312–4). Myeloperoxidase (MPO) is an oxidative enzyme contained only within granulocytes, predominantly neutrophils (27). MPO was used to identify inflammation after injury. P63 is a squamous marker that is described to reside in the ducts of ESMGs, is not typically present in BE, and has been well described in the human esophagus (15). Cytokeratin 7 (CK7) is a known marker of BE and has previously been reported in human ESMGs where acinar ductal metaplasia is present (12). CK7 was used to identify ESMG ducts and the ductal phenotype within ESMGs. CK8 is used as a classic marker for BE and is not present in squamous epithelium but has previously been well described in human esophagus (15). MPO, CK7, and CK8 were optimized for this porcine model, and the remainder of antibodies were selected based on a previously established porcine model (19). Phosphohistone H3 (PHH3) was used to mark actively proliferating cells between the G2 and M phases of the cell cycle (19). SOX9 has been described as a driver of columnar differentiation and is found in BE and thus was selected for evaluation in ESMGs in this model (4). SOX9 and CK7 were compared directly between porcine and human esophagus.
Table 1.
Antibodies and their sources, host species and dilutions
| Protein | Source | Catalog No. | Host Species | Dilution 1 |
|---|---|---|---|---|
| CK7 | Dako | M7018 | Mouse monoclonal | 1:1,000 |
| CK8 | Millipore | MAB3414 | Mouse monoclonal | 1:1,000 |
| MPO | Abcam | 9535 | Rabbit polyclonal | 1:25 |
| P63 | Biocare | CM163A | Mouse monoclonal | 1:200 |
| PHH3 | Cell Signaling | 9701 | Rabbit polyclonal | 1:200 |
| SOX9 | Millipore | AB5535 | Rabbit polyclonal | 1:2,000 |
MPO, myeloperoxidase; PHH3, phosphohistone H3; CK, cytokeratin.
Quantification of immunohistochemistry.
To perform quantitative morphometry, Olympus cellSens Dimension 1.13 2009–2015 software was used for automated counting, with defined thresholds in the “Count & Measure” tool. This tool allowed accurate and reproducible quantification of positively labeled individual cells from specific areas of the esophagus. All quantification was performed on images using the UPLFLN 10X objective on an Olympus IX83 and captured with a DP80 camera. Quantitative morphometry by image analysis was performed on ESMGs positive for PHH3 in control and injured tissue with cellSens software. Due to low numbers of normal ESMGs in injured tissue, only activated ESMGs with the ductal phenotype were included for quantitative statistical analysis. Squamous tissue was scored at the border of injury and included neosquamous tissue and the area immediately adjacent to the injury; a total of 3,000 µm of tissue was evaluated immediately adjacent to the ulcerated area for each animal.
Quantification of SOX9, CK8, and P63 was performed by manually counting positive ducts and acini as this was the most accurate way to identify positive features. ESMGs were classified as either containing normal acini or acinar-ductal metaplasia (activated), as they were for PHH3 morphometry.
Statistical analysis was performed with GraphPad Prism 7 for Mac OS X, Version 7.01 (La Jolla, CA) released April 02, 2016. A Kruskal-Wallis test was performed comparing the mean rank to the control group (n = 3) with a corrected Dunn’s test.
RESULTS
Esophageal comparison and antibody reactivity.
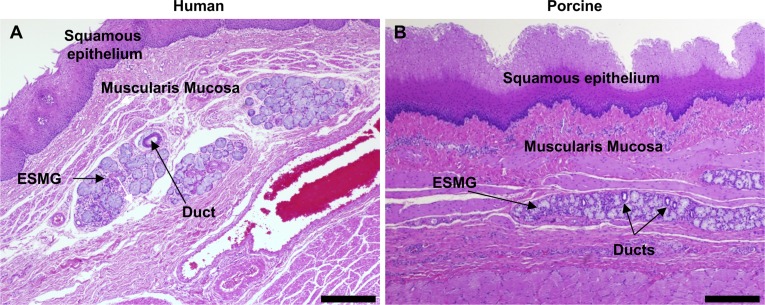
The pig and human esophagus share common esophageal anatomy including similar size and thickness of the esophageal layers from squamous epithelium to the outer muscle layers and adventitia (Fig. 1). While uncommon in humans, pigs have a keratinized layer above the squamous epithelium. Importantly, the esophagus in both pigs and humans contains ESMGs (Fig. 1, A and B). ESMGs are present in the proximal part of the porcine esophagus where they are located in dense longitudinal rows and are not typically found at or just above the gastresophageal junction of the pig. Comparatively, ESMGs are described to be heterogeneously distributed throughout the human esophagus (16) and are often found in the proximity of the gastresophageal junction. In both humans and pigs, ESMGs contain secretory acini and ducts that deliver products from ESMGs to the esophageal lumen.
Fig. 1.
Comparison of esophageal microanatomy in human and porcine tissue. Human esophagus (A) and porcine esophagus (B). Esophageal submucosal glands (ESMGs) are similar in human and porcine esophagus and are located below stratified squamous epithelium and muscularis mucosa. Normal ESMGs contain mucin-producing acini and intercalated ducts. Scale bar = 200 µm.
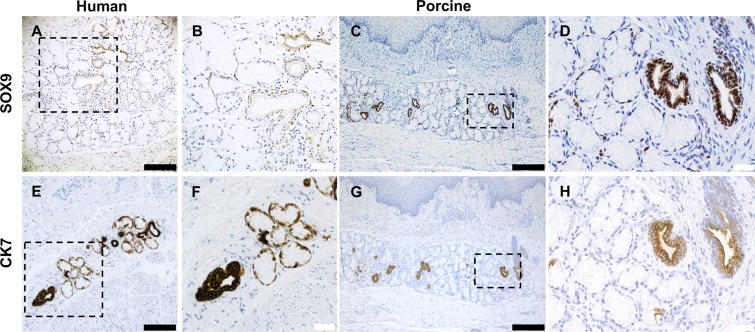
To compare antibody reactivity between both species, we used control human autopsy tissue (n = 30) and control pig tissue (n = 3) to evaluate the staining patterns as shown in representative sections for SOX9 (Fig. 2, A–D) and cytokeratin 7 (CK7) (Fig. 2, E–H). SOX9 is a marker for gastrointestinal progenitor cells and has been shown to identify a putative stem cell population in other gastrointestinal tissues of endodermal origin: liver, exocrine pancreas, and intestine that contribute to self-renewal and repair postinjury (23). SOX9 was present in esophagus as a dark nuclear stain in both the ducts and around the acini (Fig. 2, B and D) in both human and pig. Notably, SOX9 was rare in the basal layers of the squamous epithelium in human and proximal pig uninjured control tissue. CK7 is a cytoplasmic stain that faintly stains the acini in human ESMGs and strongly stains the ducts in both pig and human esophageal tissues. CK7 was first described to have a dark stain in the ducts by Hopwood et al. (22) in 1986. In human tissue, CK7 faintly stained the acini, as expected, and strongly stained the associated duct (Fig. 2, E and F). In pig esophagus, prominent cytoplasmic CK7 identified the ducts present in both ESMGs and epithelium with less robust staining in the ESMG acini (Fig. 2, G and H). Our previous work described an increase in CK7 in human ESMGs with acinar ductal metaplasia (12). Of note, squamous epithelium does not normally stain for CK7 in either pig or human.
Fig. 2.
Comparison of antibody reactivity between human and porcine esophageal tissue. SOX9 and cytokeratin 7 (CK7) antibody staining was compared between human (A, B, E, and F) and pig esophagus (C, D, G, and H). A–D: SOX9 produced a strong nuclear pattern and was identified in ducts and rare cells in the ESMGs in both human and the uninjured pig. E–H: CK7 produced strong cytoplasmic staining in the ducts of both human and pig, with some peripheral staining in the acini in human (E and F); rare CK7 staining was noted in the acini of uninjured pig (G and H). Scale bar = 200 µm (black) and 40 µm (white).
RFA injury and inflammation.
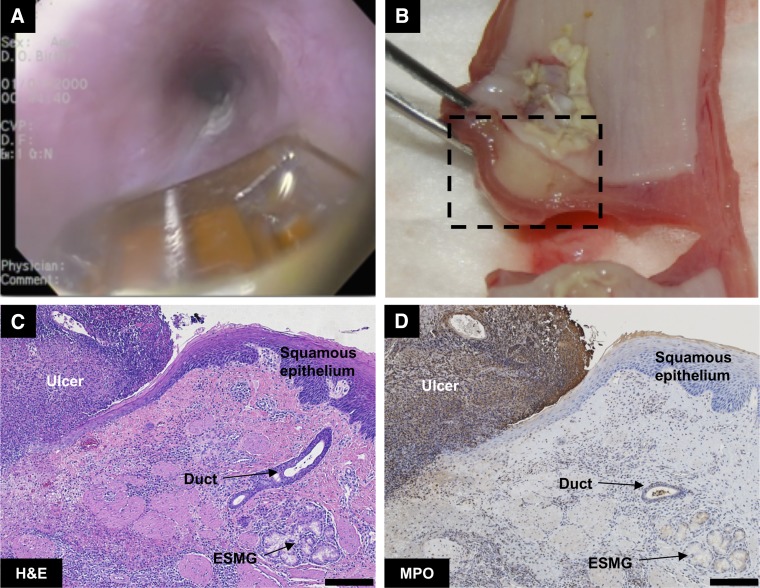
Both gross and histological assessment of the esophagus was performed after RFA. Application of RFA (Fig. 3A) successfully created a consistent and reproducible injury, with an ulcer at the application site and marked subepithelial edema within the esophageal wall 48 h postablation (Fig. 3B). The squamous epithelium was entirely ablated in the direct area of contact with RFA probe with a clear border to the injury (Fig. 3C). The level of injury was consistent with the description of 48-h ablation in pig esophagus at 12 J/cm2 described in Ganz et al. (11), where there was mild edema on gross anatomy. MPO staining was highly visible at the 48-h time point in the ablated tissue in the area of ulceration, indicating infiltration of neutrophils; demarcation was evident between the healthy and ulcerated tissue with clear separation of the ulcerated tissue from the healthy squamous epithelium (Fig. 3D).
Fig. 3.
Assessment of radiofrequency ablation (RFA)-generated wound in porcine esophagus. A: endoscopic view of uninjured porcine esophagus with RFA probe. B: gross appearance in cross section of esophageal injury 48 h postablation with extensive ulceration and mild edema. C: hematoxylin and eosin (H&E) staining 48-h postablation, with a demarcation between ulcerated (left) and healthy tissue (right) where an ESMG and duct are present. D: myeloperoxidase (MPO) staining for neutrophils demonstrated evidence of ulceration on the top left of the image and a clear demarcation at the edge of the injured area. Scale bar = 200 µm.
RFA time course.
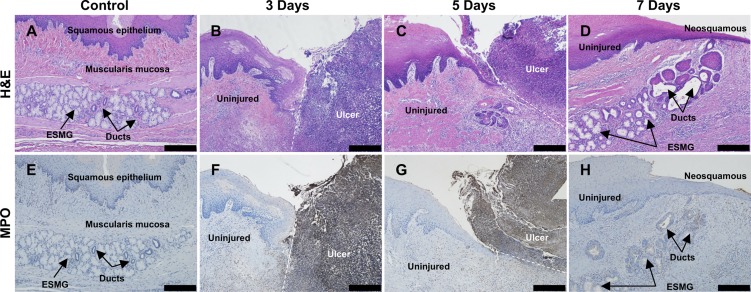
To visualize the anatomical changes, inflammation, and initiation of repair associated with the injury, we evaluated the time course of injury and repair after RFA in the pig model using both histological and gross assessment. Histological evaluation was performed at all of the time points from time 0 h to 7 days in each of the areas of injury. We found high levels of injury (as determined by tissue necrosis, inflammation, and MPO staining) at 72 h, depicted in Fig. 4, B and F, with inflammation noted in the injured tissue (ulcer) adjacent to the normal (uninjured) squamous epithelium. There was no evidence of tissue repair at 72 h; tissue architecture was disrupted, and abundant ulcer exudate was present in the areas of ablation. However, at 5 days, evidence of early repair was noted with regeneration of squamous epithelium over the injured areas (Fig. 4, C and G). More complete repair was present at 7 days (Fig. 4, D and H); damaged tissue had sloughed, and a neosquamous epithelium consisting of a layer of basal cells and 4–5 layers of epithelial cells was present over the injured area. In repairing 7-day tissue (Fig. 4D), the ESMG and its duct were observed to be connected with the neosquamous epithelium, suggesting that the gland may be contributing to the apparent restitution. A stark decrease in MPO staining can also be noted in Fig. 4H, signaling a drop in inflammation as repair mechanisms were activated. In distal esophagus, in areas where ESMGs were not observed, neosquamous epithelium was also observed beginning at 5 days with more complete restitution at 7 days (data not shown).
Fig. 4.
Areas of RFA injured esophageal tissue at 3, 5, and 7 days were compared with control using both H&E and MPO. A: control H&E with normal appearance of an ESMG with overlying squamous epithelium. B: at 3 days, a large ulcer was present (right) with clear demarcation between the uninjured tissue (left) and ablated, ulcerated tissue (right). C: at 5 days, a small area of neosquamous epithelium (left) appeared underneath ulcerated tissue (right) above an ESMG at the junction of injured and uninjured tissue. D: 7 days postablation, neosquamous epithelium appeared along with an ESMG with the ductular phenotype and areas of squamous islands near the neosquamous epithelium. E: uninjured control tissue did not demonstrate MPO-positive cells. F: 3 days after injury, dark MPO staining was present on the right in the ablated area, with little to no staining present in the uninjured tissue (left). G: at 5 days, MPO-stained tissue (right) separated from uninjured, repairing epithelium (left). H: neosquamous epithelium and associated ESMG with little to no positive MPO cells, indicating a reduction in inflammation and resolution of the ulcer. Scale bar = 200 µm.
Proliferative response.
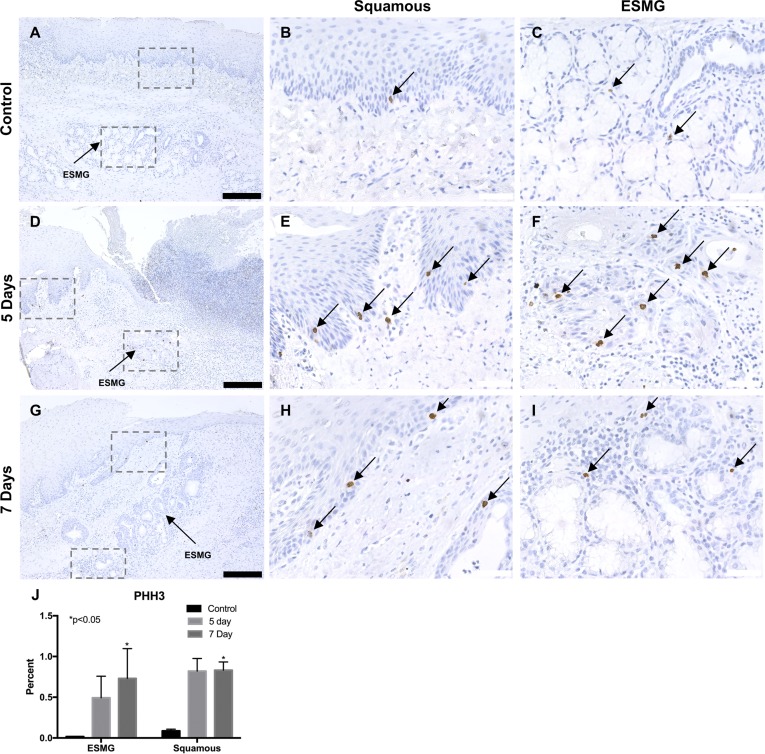
To evaluate cellular proliferation, we used PHH3. We evaluated PHH3-positive cells in the control (n = 3) and injured pigs at the 5-day (n = 3) and 7-day (n = 6) time points in both squamous epithelium and ESMGs as previously described. A total of 34 normal ESMGs were evaluated in control tissue, 4 activated ESMGs at the 5-day time point and 13 activated ESMGs at the 7-day time point. In the uninjured state, control tissue in the esophagus (Fig. 5A) had rare staining in the basal cells of the squamous epithelium (0.09 ± 0.02% positive cells counted; Fig. 5B) and scant PHH3 staining in the ESMGs (0.02 ± 0.003%; Fig. 5C). At 5 days postablation (Fig. 5D), PHH3-positive cells increased to 0.82 ± 0.16% in the squamous epithelium (Fig. 5E) and 0.49 ± 0.27% in the ESMGs (Fig. 5F), indicating increased proliferation in both squamous basal cells and ESMGs compared with control. Seven days postablation (Fig. 5G), PHH3-positive cells increased significantly (P < 0.05) to 0.83 ± 0.10% in the squamous epithelium (Fig. 5H) and 0.73 ± 0.37% in the ESMGs (Fig. 5I). Taken together, these data show an increase in proliferation using the presence of PHH3-positive cells at postablation day 5, and a significant increase (P < 0.05) postablation day 7 in both the squamous epithelium and ESMGs when compared with control, uninjured tissue (Fig. 5J). In areas devoid of ESMGs (distal esophagus), squamous epithelium showed a slightly lower increase in PHH3-positive cells, with an increase 5 days postablation to 0.52 ± 0.22%, and an increase 7 days postablation to 0.61 ± 0.12% compared with uninured control squamous epithelium (0.07 ± 0.00%).
Fig. 5.
Phosphohistone H3 (PHH3), an M-phase marker for proliferation, was used to detect proliferating cells in the porcine esophagus. A–C: in uninjured esophagus, cells were rarely positive for PHH3 in the squamous epithelium (B) and ESMGs (C). D-F: 5 days postablation (n = 3), a marked increase of PHH3-positive cells was noted in both the squamous epithelium (E) and ESMGs (F). G–I: 7 days postablation (n = 6), PHH3-positive cells were present in the squamous epithelium (H) and ESMGs (I). J: after quantification, compared with uninjured control, PHH3 increased significantly in ESMGs 7 days postablation (*P < 0.05) and increased significantly in squamous epithelium 7 days postablation (P < 0.05). Scale bar = 200 µm (black) and 40 µm (white). Percentages reported as means ± SE.
ESMG ductular phenotype.
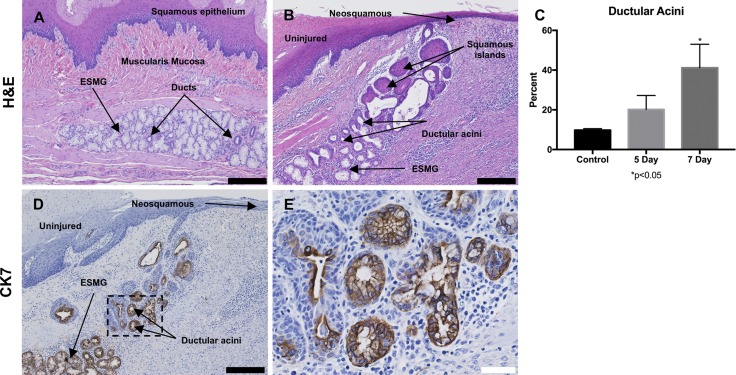
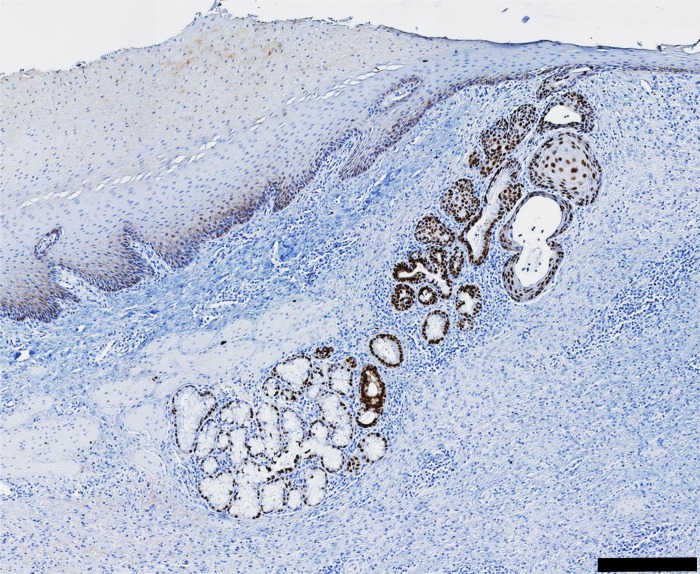
Based on our previous findings in human ESMGs, we were specifically interested in phenotypic changes in the ESMGs and used a scoring system previously developed for human evaluation by our laboratory to determine the proportion of acini that transformed from a normal, mucinous appearance to a ductular phenotype (12). In control tissue, most acini were plump and mucin filled, with a mean 9.76 ± 0.75% ductal acini in uninjured ESMGs (Fig. 6A). After ablation, the ESMGs assumed a ductular appearance with an increased percentage of ductular acini associated with the glands (Fig. 6B). At 5 days postablation, ductular acini increased to 20.18 ± 7.03% compared with control, and at 7 days postablation, ductular acini increased to 41.16 ± 11.85%, which was significantly increased compared with control ESMGs (P < 0.05) (Fig. 6C). To more clearly define these structures, we performed immunohistochemistry with CK7. Dark positive CK7 was noted peripherally in the dilated acini and was noted throughout the ductal acini leading to the squamous epithelium (Fig. 6D). The increase in ductal acini associated with injury echoed the findings in human studies where ESMGs were described to have an increased ductular phenotype and dark positive stain for CK7 associated with high-grade dysplasia and EAC (12). Evaluation of the ductal phenotype observed in ESMGs postinjury revealed that the ductal epithelial cells within ESMGs were cuboidal in appearance, and as the ductal structures approached the overlying regenerating epithelium, the ducts assumed the appearance of squamous islands. These islands were observed in the submucosa and into the squamous epithelium (Fig. 6B). However, areas of regenerating squamous epithelium were not CK7 positive (Fig. 6D).
Fig. 6.
A ductular phenotype was present in injured porcine ESMGs. A: control ESMGs contained mucinous acini with a few small normal ducts to collect acinar secretions. B: 7 days postablation, the ESMGs exhibited an increased ductular phenotype rather than mucinous acini within the ESMGs, with additional squamous islands near the lumen. C: when acinar phenotype was counted (ductal vs. mucinous) and compared with uninjured control, 5 days postablation the ductular phenotype nearly doubled compared with control, and it quadrupled at 7 days to 41.16 ± 11.85% compared with control (*P < 0.05). D: CK7, a ductal marker, stained strongly positive in 7 days postablation ESMGs. E: CK7 in higher magnification image demonstrating acini with mixed phenotype, dilation of the acini, loss of mucin. Multiple layers are present in some ducts, particularly near the overlying repairing squamous epithelium. Scale bar = 200 µm (black) and 40 µm (white). Percentages reported as mean ± SE.
Expression of epithelial markers CK8 and P63 following RFA.
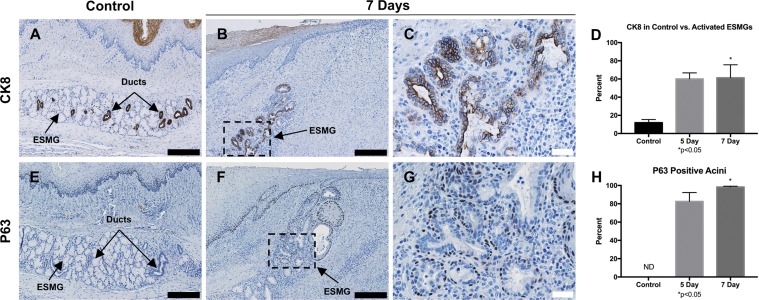
CK8 is a marker for columnar epithelium associated with BE, so we evaluated CK8 expression in control and postablation tissue in the RFA pig model. A total of 41 normal ESMGs were evaluated in control tissue, 4 activated ESMGs at the 5-day time point and 11 activated ESMGs at the 7-day time point. As previously described in human tissue (15), CK8 was not present in the squamous epithelium (Fig. 7A) but densely positive in the ducts and ductular acini within ESMGs. After RFA injury, CK8 staining increased in the ducts and areas of acinar ductal metaplasia (Fig. 7, B and C). In control tissue, 11.99 ± 3.39% of ESMGs were CK8 positive. At 5 days postablation, 60.13 ± 6.53% of activated ESMGs were CK8 positive, and at 7 days postablation, 61.04 ± 14.22% of activated ESMGs were CK8 positive (Fig. 7D).
Fig. 7.
Epithelial markers P63 and CK8 in RFA-injured tissue. A: CK8 expression in control ESMGs had strong expression within the ducts. B and C: after injury, expression of CK8 was strongly present in the ductular acini with a notable expansion. D: in control tissue, 11.99 ± 3.39% of the control ESMGs consisted of CK8-positive ducts. Compared with uninjured controls, CK8 expression increased to 60.13% ± 6.54% in ESMGs 5 days postablation, and to 61.41 ± 14.23% (*P < 0.05) in ESMGs 7 days postablation. E: P63 expression was present on the basal cells in the ducts contained within ESMGs but not detected (ND) in the acini of the uninjured ESMGs. F and G: P63 expression remained active in the ducts associated with ESMGs postablation, and P63-positive cells were found in the acini of injured ESMGs. H: compared with uninjured controls, P63-positive cells were present in 82.64 ± 9.65% of acini in injured ESMGs 5 days postablation, and significantly increased to 98.33 ± 0.88% (P < 0.05) in injured ESMGs 7 days postablation. Scale bar = 200 µm (black) and 40 µm (white). Percentages reported as mean ± SE.
P63 plays a role in the regulation of proliferation and differentiation of squamous epithelium (15). In the human esophagus, P63 is present in the basal and suprabasal layers of the squamous epithelium along with the basal cells of ESMG ducts (15). A similar pattern was found in uninjured porcine esophagus with P63 expression localized to the basal layers of the squamous epithelium and in the basal cells of ducts in ESMGs (Fig. 7E). Notably, in uninjured porcine esophagus, ESMG acini were negative for P63-positive cells. Interestingly, after injury, at both 5 and 7 days postablation, a dramatic increase in P63 expression was noted in ESMG acini with P63-positive cells surrounding acini and areas of acinar ductal metaplasia (Fig. 7, F and G). The ESMG ducts maintained P63-positive staining after injury, although a significant increase was not noted (data not shown). At 5 days postablation, 82.64 ± 9.65% of acini in injured ESMGs contained P63-positive cells. At 7 days postablation, 98.33 ± 0.88% of acini in injured ESMGs contained P63-positive cells (Fig. 7H).
SOX9 expression following RFA.
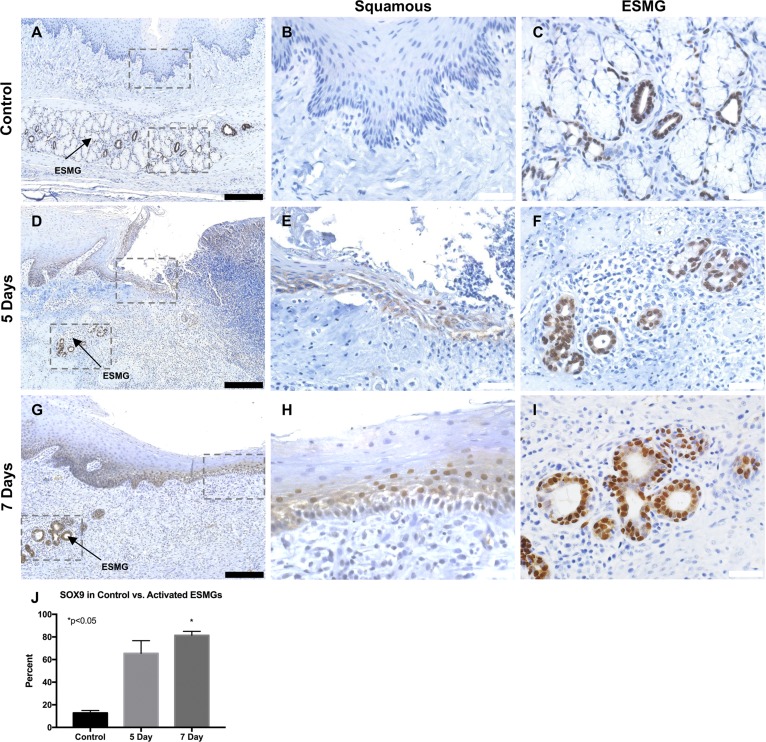
In addition to increased proliferation and a ductular phenotype in ESMGs after injury, we hypothesized that there would be an increase in SOX9-positive cells in the esophagus following epithelial damage. Nuclear SOX9 was evaluated in control (n = 3) and injured pigs at the 5-day (n = 3) and 7-day (n = 6) time point (Fig. 8). The ductular phenotype observed in the ESMGs was densely positive for SOX9 cells, which were defined as having a deep positive nuclear stain.
Fig. 8.
Location of SOX9 expression was assessed in RFA-injured tissue. A–C: In uninjured esophagus, SOX9 was rare in the squamous epithelium (B) but present in the ESMGs and associated ducts (C). D–F: 5 days postablation (n = 3), nuclear positive SOX9 increased in both the squamous epithelium (E) and ESMGs, particularly where the ductal phenotype was noted (F). G–I: 7 days postablation (n = 6), SOX9-positive cells increased in the squamous epithelium (H), and increased in the ESMGs, again where the ductal phenotype was noted (I). J: compared with uninjured controls, in the activated acinar ductal phenotype within ESMGs, SOX9 increased from 12.82 ± 2.11% to 65.42 ± 11.33% 5 days postablation. SOX9-positive ductal acini significantly (*P < 0.05) increased to 81.37 ± 3.62% in ESMGs 7 days postablation, compared with uninjured controls. Scale bar = 200 µm (black) and 40 µm (white). Percentages reported as mean ± SE.
In control tissue, the uninjured esophagus showed rare and sporadic SOX9 staining in both the squamous epithelium (Fig. 8, A and B) and acini and ducts within ESMGs (Fig. 8C). Five and seven days postablation (Fig. 8, D and G), SOX9 increased in the squamous epithelium (Fig. 8, E and H) and ducts and acini within ESMGs (Fig. 8, F and I). Inflammatory cells associated with tissue injury were SOX9 negative. In the regenerating flat squamous epithelium, nuclei of the squamous epithelial cells showed a sideways orientation and were positive for nuclear SOX9 just above the basal-most layer in the squamous epithelium (Fig. 8H). In contrast, the basal-most layer of the squamous epithelium showed plump cuboidal cells that appeared to be negative for SOX9 (Fig. 8H). We quantified SOX9 staining in ESMGs in uninjured controls, 5 days postablation and 7 days postablation tissues (Fig. 8J). Some diffuse cytoplasmic SOX9 stain was seen 5 days postablation; however, we focused on nuclear SOX9 for the descriptions and quantification. A total of 29 normal ESMGs were evaluated in control tissue, 4 activated ESMGs at 5 days and 9 activated ESMGs at 7 days. For quantification, acini within ESMGs were described as mucinous (negative for SOX9), ductal (100% positive for SOX9), or mixed (mucinous with >50% positive SOX9 ductal cells) and scored. In the control tissue, 12.83 ± 2.11% of ESMGs contained SOX9-positive acini or ducts. In contrast, at 5 days postablation, 65.42 ± 11.33% of activated ESMGs contained SOX9-positive acini with the ductal phenotype and this persisted at 7 days postablation to 81.37 ± 3.61% (P < 0.05) when compared with uninjured control tissue.
In summary, control tissue contained nuclear SOX9 staining that was present in ducts and acini of ESMGs, with rare expression in the squamous esophagus. As reepithelialization began 5 days postablation, an increase in SOX9-positive cells was seen and continued to be seen 7 days postablation in both the squamous epithelium and ESMGs, particularly in ductal features that emerged in EMSGs postablation (Fig. 8I). This overall trend of an increase in SOX9-positive cells (Fig. 8J) potentially indicates activation of progenitor populations aiding in repair of the squamous epithelium.
Finally, the nuclear SOX9 staining was readily apparent in the ducts and glands and was also noted in the repairing squamous epithelium, including in the squamous island, particularly in the basal-most layer as well as rare cells in the center of the island (Fig. 9). In addition, there was continuity of cells from the ductular-appearing ESMGs to the neosquamous epithelium.
Fig. 9.
Continuity was observed from ductular ESMG connecting with neosquamous epithelium. Ductular acini were visible and positive for SOX9, 7 days postablation. The ductular acini appeared in continuity with repairing squamous epithelium and SOX9-positive cells were common. Scale bar = 200 µm.
DISCUSSION
We have developed a porcine model of esophageal injury to better understand the role of ESMGs in esophageal repair. The location of stem and progenitor cells in the esophagus has been a matter of notable debate and has important implications for understanding the process of esophageal repair, metaplasia and carcinogenesis. Observational studies in humans (5, 12) and work in rodent animal models (2, 6, 7, 9, 25, 41) have been at the epicenter of esophageal research in stem and progenitor cell biology and metaplasia. It has been established that basal cells of the squamous epithelium proliferate in response to esophageal injury (7, 25). Mouse models have demonstrated that a population of cells at the squamous-columnar junction in the mouse forestomach could serve as progenitors (35, 41). We identified increased proliferation within the basal layer of the squamous epithelium in the porcine model as well. However, due to the absence of ESMGs in mice and rats, previous esophageal research in rodents has not provided any information on the role of ESMGs as an additional potential protected source of esophageal stem and progenitor cells capable of response to injury.
Other animal models that contain ESMGs have also provided important insights into esophageal research. Opossums and canines have both been used in esophageal research (13), and in a canine model of esophageal injury and repair in 1988, Gillen et al. (14) showed that mucosal defects were repaired by the squamous epithelium under normal circumstances and by columnar epithelium in the presence of acid alone or in a combination of acid and bile. Most notably, the authors found that regenerating columnar epithelium contained cells lining the ESMG ducts. This led them to hypothesize that ESMG ducts likely harbor a multipotent stem cell population (14). While canine models include compelling results, due to the difficulty in handling and length of disease progression (1–5 yr), to our knowledge, these models are not in active use in current research (26).
Human studies have demonstrated that cells localized to the basal layer of the squamous epithelium in the esophagus are label retaining, slow cycling, and renew approximately every 11 days (34). Coad et al. (5) found that ESMGs and associated ducts were commonly detected beneath regions of BE and that squamous islands associated with BE were always continuous with an underlying ESMG duct, raising the possibility that in humans, the ESMGs and ducts contain progenitors capable of forming both phenotypes of overlying epithelium. To further support that hypothesis, Leedham et. al. (30) identified clonality between the ESMG ducts and metaplastic BE crypts, providing molecular evidence for the cellular origin of BE within ESMG ducts. Additionally, in a single patient, ESMG ducts shared clonality with both squamous epithelium and BE (30). These findings, in addition to prior animal studies, led us to hypothesize that, in response to injury, ESMGs and associated ducts harbor a population of cells that becomes activated and contributes to repair of injured squamous epithelium.
To test this hypothesis, we developed a porcine model of esophageal injury and repair. Pigs have been established as an excellent model for gastrointestinal research (13, 18, 26, 39) and are a promising model for studying the esophagus due to the presence of ESMGs. RFA is a technology currently being used to treat BE in patients and was originally developed in the pig (11). Similarities in esophageal anatomy between pigs and human (Fig. 1) and similar antibody reactivity (Fig. 2) encouraged us to pursue the pig as a model for esophageal injury and repair. Our results establish that RFA induces a reproducible esophageal injury in pig esophagus (Fig. 3). Limitations to this model include the lack of molecular tools such as transgenic, knockin, and knockout models as are available in mice as well as the availability and cost of husbandry facilities for pigs. This initial characterization of our porcine model has focused on proliferation, evaluation of the ductal phenotype of the ESMGs we had observed in humans, and pattern of SOX9 expression after esophageal injury.
We found that ESMGs and ducts had low levels of proliferation in the uninjured state; the squamous epithelium revealed rare PHH3-positive cells in the basal layer of the esophagus (Fig. 5, A–C). However, postablation, PHH3 increased in the basal layer of the squamous epithelium in the esophagus, consistent with previous studies indicating a progenitor population in the basal cells. These findings are similar to results from a small cohort of human patients who were administered 5-iodo-2′-deoxyuridine while awaiting esophagectomy and in whom a small number of label-retaining cells was identified in the basal layer of the squamous epithelium (34). Unfortunately, that study did not report 5-iodo-2′-deoxyuridine labeling in the ESMGs. When we specifically evaluated proliferation in the ESMGs in the porcine model, PHH3 was very rare in the ESMGs in the uninjured state. However, after injury, PHH3 increased in ESMGs in areas where epithelial injury had occurred. Our findings are consistent with previous surgically induced models of reflux in dogs where columnar epithelium was seen adjacent to ESMGs and uptake of bromodeoxyuridine, while absent in ESMGs at baseline, increased after injury, indicating proliferation (14, 37).
In addition to proliferation, we assessed the presence of the transcription factor SOX9 in our model given the association of SOX9 with both esophageal injury and BE (4, 40). Importantly, SOX9 is not strongly expressed in normal human squamous epithelium but is present in BE and EAC (40). SOX9 has also been reported in ESMG ducts myoepithelial cells around ESMG acini (17). In an uninjured state in the porcine model, SOX9 was rare in the squamous epithelium (Fig. 8B). Previously, the SOX9 transcription factor has been reported in a conditional Shh-transgenic 3D reconstitution model where SOX9 increases in the basal layer of the squamous epithelium (40). Furthermore, ectopic SOX9 expression in mouse esophageal squamous epithelium is associated with an increase in BE markers (4). Similar to the strong SOX9 expression in squamous esophageal cells that was described in the murine reconstitution model, in the porcine model, we found the presence of dark nuclear-positive SOX9 cells appeared 5 days postablation at the leading edge of the neosquamous epithelium (Fig. 8, D and E). Interestingly, at both 5 and 7 days, SOX9 was identified in the cells immediately above the basal-most cell layer, a difference from the mouse 3D reconstitution model from Shh-transgenic mice (40).
To our knowledge this model is the first to describe robust SOX9 expression in ESMGs during regeneration. Specifically, while proliferation was low in ESMGs at baseline, more SOX9-positive cells were found in the ESMGs compared with the basal layers of the squamous epithelium. SOX9 expression in the ESMGs markedly increased following injury, and continuity was observed between ESMGs and areas of squamous repair (Fig. 9). Our findings thus support evolution to a more inclusive hypothesis about esophageal repair: while it has been postulated that a population of esophageal progenitor cells reside in the basal layer of the epithelium (7, 24, 34), it is also possible that ESMG-associated progenitors become activated after injury and play a contributing role in esophageal epithelial repair. As with the mouse studies, the BE markers CK7 and CK8 also increased in ESMGs along with SOX9.
Our previous work in humans demonstrated an association of a ductal phenotype in the ESMGs with both esophageal cancer and esophageal injury. The features of the ductal phenotype observed within the ESMGs after injury in this porcine model closely resemble the acinar ductal metaplasia that has been described in association with both inflammation and progression to cancer in other organs such as stomach, pancreas, and prostate (3, 33). Importantly, acinar ductal metaplasia in other organs is thought to represent a proliferative ductal phenotype that differs from quiescent ducts and is associated with strong SOX9 staining (33), and our findings in porcine ESMGs are consistent with this concept. However, other than in our own observational study in humans, acinar ductal metaplasia has not previously been described in the esophagus. The porcine model we describe here demonstrates the prospective development of a ductal phenotype in ESMGs after injury with strong CK7 expression (Fig. 6) as we described in humans (12) and strong SOX9 expression. The translational potential of this porcine model is supported by the correlative expression of CK8 and P63 compared with the same markers in human esophagus (Fig. 7). Our porcine model will allow future studies into the specific molecular signals that drive esophageal acinar ductal metaplasia.
In humans, profound esophageal injury from acid reflux can lead to patches of ulceration with full-thickness loss of esophageal epithelium, commonly known as erosive esophagitis. In addition, when human patients with BE are treated with RFA, areas of epithelium are ablated and profound esophageal repair is required for reepithelialization. It is known that postablation, acid exposure is associated with persistence of BE (1, 29), suggesting that the environment in which repair occurs may determine cellular phenotype (squamous vs. BE). The porcine model reported here could be useful in testing the molecular implications of known clinical risk factors for BE and EAC such as acid exposure and tobacco exposure, making this a particularly valuable model for studying esophageal repair.
It is clear that ESMGs play an important role in esophageal repair based on human observations, prior work done in canine models (14), and the findings in this porcine model. In particular, we have demonstrated a ductal phenotype in ESMGs after injury and SOX9 expression in ESMGs and neosquamous epithelium after esophageal injury. The presence of ESMGs makes this porcine model uniquely suited for esophageal research given the general physiological and anatomical similarities between pigs and humans (16). With initial characterization of the injury complete, we can move forward to more mechanistic studies in this injury model to enhance the translational potential of this pig model for different esophageal diseases and disorders that involve epithelial injury and repair.
GRANTS
This work was funded by National Institute of Diabetes and Digestive and Kidney Diseases Grant K08-DK-098528 (to K. S. Garman), Center for Gastrointestinal Biology and Disease (CGIBD) Large Animal Models Core Grant P30-DK-034987 (to A. T. Blikslager and L. M. Gonzalez), and CGIBD Pilot Funding Grant P30-DK-034987 (to K. S. Garman).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.K., L.M.G., A.T.B., and K.S.G. conceived and designed research; L.K., L.M.G., T.A.P., G.E.C., A.M.C., A.T.B., and K.S.G. performed experiments; L.K., S.J.M., R.v.F., A.T.B., and K.S.G. analyzed data; L.K., L.M.G., S.J.M., R.v.F., A.T.B., and K.S.G. interpreted results of experiments; L.K., S.J.M., R.v.F., A.T.B., and K.S.G. prepared figures; L.K., A.T.B., and K.S.G. drafted manuscript; L.K., L.M.G., T.A.P., S.J.M., R.v.F., I.H., G.E.C., A.M.C., A.T.B., and K.S.G. edited and revised manuscript; L.K., L.M.G., T.A.P., S.J.M., R.v.F., I.H., G.E.C., A.M.C., A.T.B., and K.S.G. approved final version of manuscript.
REFERENCES
- 1.Akiyama J, Marcus SN, Triadafilopoulos G. Effective intra-esophageal acid control is associated with improved radiofrequency ablation outcomes in Barrett’s esophagus. Dig Dis Sci 57: 2625–2632, 2012. doi: 10.1007/s10620-012-2313-2. [DOI] [PubMed] [Google Scholar]
- 2.Boult J, Roberts K, Brookes MJ, Hughes S, Bury JP, Cross SS, Anderson GJ, Spychal R, Iqbal T, Tselepis C. Overexpression of cellular iron import proteins is associated with malignant progression of esophageal adenocarcinoma. Clin Cancer Res 14: 379–387, 2008. doi: 10.1158/1078-0432.CCR-07-1054. [DOI] [PubMed] [Google Scholar]
- 3.Chen NM, Singh G, Koenig A, Liou GY, Storz P, Zhang JS, Regul L, Nagarajan S, Kühnemuth B, Johnsen SA, Hebrok M, Siveke J, Billadeau DD, Ellenrieder V, Hessmann E. NFATc1 links EGFR signaling to induction of Sox9 transcription and acinar-ductal transdifferentiation in the pancreas. Gastroenterology 148: 1024–1034.e9, 2015. doi: 10.1053/j.gastro.2015.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clemons NJ, Wang DH, Croagh D, Tikoo A, Fennell CM, Murone C, Scott AM, Watkins DN, Phillips WA. Sox9 drives columnar differentiation of esophageal squamous epithelium: a possible role in the pathogenesis of Barrett’s esophagus. Am J Physiol Gastrointest Liver Physiol 303: G1335–G1346, 2012. doi: 10.1152/ajpgi.00291.2012. [DOI] [PubMed] [Google Scholar]
- 5.Coad RA, Woodman AC, Warner PJ, Barr H, Wright NA, Shepherd NA. On the histogenesis of Barrett’s oesophagus and its associated squamous islands: a three-dimensional study of their morphological relationship with native oesophageal gland ducts. J Pathol 206: 388–394, 2005. doi: 10.1002/path.1804. [DOI] [PubMed] [Google Scholar]
- 6.Croagh D, Phillips WA, Redvers R, Thomas RJ, Kaur P. Identification of candidate murine esophageal stem cells using a combination of cell kinetic studies and cell surface markers. Stem Cells 25: 313–318, 2007. doi: 10.1634/stemcells.2006-0421. [DOI] [PubMed] [Google Scholar]
- 7.Doupé DP, Alcolea MP, Roshan A, Zhang G, Klein AM, Simons BD, Jones PH. A single progenitor population switches behavior to maintain and repair esophageal epithelium. Science 337: 1091–1093, 2012. doi: 10.1126/science.1218835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut 63: 871–880, 2014. doi: 10.1136/gutjnl-2012-304269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Epperly MW, Shen H, Jefferson M, Greenberger JS. In vitro differentiation capacity of esophageal progenitor cells with capacity for homing and repopulation of the ionizing irradiation-damaged esophagus. In Vivo 18: 675–685, 2004. [PubMed] [Google Scholar]
- 10.Fleischer DE, Sharma VK. Endoscopic ablation of Barrett’s esophagus using the Halo system. Dig Dis 26: 280–284, 2008. doi: 10.1159/000177009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ganz RA, Utley DS, Stern RA, Jackson J, Batts KP, Termin P. Complete ablation of esophageal epithelium with a balloon-based bipolar electrode: a phased evaluation in the porcine and in the human esophagus. Gastrointest Endosc 60: 1002–1010, 2004. doi: 10.1016/S0016-5107(04)02220-5. [DOI] [PubMed] [Google Scholar]
- 12.Garman KS, Kruger L, Thomas S, Swiderska-Syn M, Moser BK, Diehl AM, McCall SJ. Ductal metaplasia in oesophageal submucosal glands is associated with inflammation and oesophageal adenocarcinoma. Histopathology 67: 771–782, 2015. doi: 10.1111/his.12707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garman KS, Orlando RC, Chen X. Review: Experimental models for Barrett’s esophagus and esophageal adenocarcinoma. Am J Physiol Gastrointest Liver Physiol 302: G1231–G1243, 2012. doi: 10.1152/ajpgi.00509.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gillen P, Keeling P, Byrne PJ, West AB, Hennessy TP. Experimental columnar metaplasia in the canine oesophagus. Br J Surg 75: 113–115, 1988. doi: 10.1002/bjs.1800750208. [DOI] [PubMed] [Google Scholar]
- 15.Glickman JN, Yang A, Shahsafaei A, McKeon F, Odze RD. Expression of p53-related protein p63 in the gastrointestinal tract and in esophageal metaplastic and neoplastic disorders. Hum Pathol 32: 1157–1165, 2001. doi: 10.1053/hupa.2001.28951. [DOI] [PubMed] [Google Scholar]
- 16.Goetsch E. The structure of the mammalian oesophagus. Am J Anat 10: 1–40, 1910. doi: 10.1002/aja.1000100102. [DOI] [Google Scholar]
- 17.Gonzalez G, Huang Q, Mashimo H. Characterization of oncocytes in deep esophageal glands. Dis Esophagus 29: 670–680, 2016. doi: 10.1111/dote.12382. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez LM, Moeser AJ, Blikslager AT. Porcine models of digestive disease: the future of large animal translational research. Transl Res 166: 12–27, 2015. doi: 10.1016/j.trsl.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez LM, Williamson I, Piedrahita JA, Blikslager AT, Magness ST. Cell lineage identification and stem cell culture in a porcine model for the study of intestinal epithelial regeneration. PLoS One 8: e66465, 2013. doi: 10.1371/journal.pone.0066465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haidry R, Lovat L, Sharma P. Radiofrequency ablation for Barrett’s dysplasia: past, present and the future? Curr Gastroenterol Rep 17: 13, 2015. doi: 10.1007/s11894-015-0433-5. [DOI] [PubMed] [Google Scholar]
- 21.Hirst J, Smithers BM, Gotley DC, Thomas J, Barbour A. Defining cure for esophageal cancer: analysis of actual 5-year survivors following esophagectomy. Ann Surg Oncol 18: 1766–1774, 2011. doi: 10.1245/s10434-010-1508-z. [DOI] [PubMed] [Google Scholar]
- 22.Hopwood D, Coghill G, Sanders DS. Human oesophageal submucosal glands. Their detection mucin, enzyme and secretory protein content. Histochemistry 86: 107–112, 1986. doi: 10.1007/BF00492353. [DOI] [PubMed] [Google Scholar]
- 23.Huch M, Clevers H. Sox9 marks adult organ progenitors. Nat Genet 43: 9–10, 2011. doi: 10.1038/ng0111-9. [DOI] [PubMed] [Google Scholar]
- 24.Jankowski JA, Harrison RF, Perry I, Balkwill F, Tselepis C. Barrett’s metaplasia. Lancet 356: 2079–2085, 2000. doi: 10.1016/S0140-6736(00)03411-5. [DOI] [PubMed] [Google Scholar]
- 25.Kalabis J, Oyama K, Okawa T, Nakagawa H, Michaylira CZ, Stairs DB, Figueiredo JL, Mahmood U, Diehl JA, Herlyn M, Rustgi AK. A subpopulation of mouse esophageal basal cells has properties of stem cells with the capacity for self-renewal and lineage specification. J Clin Invest 118: 3860–3869, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kapoor H, Lohani KR, Lee TH, Agrawal DK, Mittal SK. Animal models of Barrett’s esophagus and esophageal adenocarcinoma–past, present, and future. Clin Transl Sci 8: 841–847, 2015. doi: 10.1111/cts.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology 87: 1344–1350, 1984. [PubMed] [Google Scholar]
- 28.Krishnadath KK, Wang KK. Molecular pathogenesis of Barrett esophagus: current evidence. Gastroenterol Clin North Am 44: 233–247, 2015. doi: 10.1016/j.gtc.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 29.Krishnan K, Pandolfino JE, Kahrilas PJ, Keefer L, Boris L, Komanduri S. Increased risk for persistent intestinal metaplasia in patients with Barrett’s esophagus and uncontrolled reflux exposure before radiofrequency ablation. Gastroenterology 143: 576–581, 2012. doi: 10.1053/j.gastro.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leedham SJ, Preston SL, McDonald SA, Elia G, Bhandari P, Poller D, Harrison R, Novelli MR, Jankowski JA, Wright NA. Individual crypt genetic heterogeneity and the origin of metaplastic glandular epithelium in human Barrett’s oesophagus. Gut 57: 1041–1048, 2008. doi: 10.1136/gut.2007.143339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Long JD, Orlando RC. Esophageal submucosal glands: structure and function. Am J Gastroenterol 94: 2818–2824, 1999. doi: 10.1111/j.1572-0241.1999.1422_b.x. [DOI] [PubMed] [Google Scholar]
- 32.McDonald SA, Lavery D, Wright NA, Jansen M. Barrett oesophagus: lessons on its origins from the lesion itself. Nat Rev Gastroenterol Hepatol 12: 50–60, 2015. doi: 10.1038/nrgastro.2014.181. [DOI] [PubMed] [Google Scholar]
- 33.Mills JC, Sansom OJ. Reserve stem cells: Differentiated cells reprogram to fuel repair, metaplasia, and neoplasia in the adult gastrointestinal tract. Sci Signal 8: re8, 2015. doi: 10.1126/scisignal.aaa7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pan Q, Nicholson AM, Barr H, Harrison LA, Wilson GD, Burkert J, Jeffery R, Alison MR, Looijenga L, Lin WR, McDonald SA, Wright NA, Harrison R, Peppelenbosch MP, Jankowski JA. Identification of lineage-uncommitted, long-lived, label-retaining cells in healthy human esophagus and stomach, and in metaplastic esophagus. Gastroenterology 144: 761–770, 2013. doi: 10.1053/j.gastro.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 35.Quante M, Bhagat G, Abrams JA, Marache F, Good P, Lee MD, Lee Y, Friedman R, Asfaha S, Dubeykovskaya Z, Mahmood U, Figueiredo JL, Kitajewski J, Shawber C, Lightdale CJ, Rustgi AK, Wang TC. Bile acid and inflammation activate gastric cardia stem cells in a mouse model of Barrett-like metaplasia. Cancer Cell 21: 36–51, 2012. doi: 10.1016/j.ccr.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strobel O, Rosow DE, Rakhlin EY, Lauwers GY, Trainor AG, Alsina J, Fernández-Del Castillo C, Warshaw AL, Thayer SP. Pancreatic duct glands are distinct ductal compartments that react to chronic injury and mediate Shh-induced metaplasia. Gastroenterology 138: 1166–1177, 2010. doi: 10.1053/j.gastro.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Nieuwenhove Y, Destordeur H, Willems G. Spatial distribution and cell kinetics of the glands in the human esophageal mucosa. Eur J Morphol 39: 163–168, 2001. doi: 10.1076/ejom.39.3.0163. [DOI] [PubMed] [Google Scholar]
- 38.Van Nieuwenhove Y, Willems G. Gastroesophageal reflux triggers proliferative activity of the submucosal glands in the canine esophagus. Dis Esophagus 11: 89–93, 1998. [DOI] [PubMed] [Google Scholar]
- 39.Vulchanova L, Casey MA, Crabb GW, Kennedy WR, Brown DR. Anatomical evidence for enteric neuroimmune interactions in Peyer’s patches. J Neuroimmunol 185: 64–74, 2007. doi: 10.1016/j.jneuroim.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang DH, Clemons NJ, Miyashita T, Dupuy AJ, Zhang W, Szczepny A, Corcoran-Schwartz IM, Wilburn DL, Montgomery EA, Wang JS, Jenkins NA, Copeland NA, Harmon JW, Phillips WA, Watkins DN. Aberrant epithelial-mesenchymal Hedgehog signaling characterizes Barrett’s metaplasia. Gastroenterology 138: 1810–1822, 2010. doi: 10.1053/j.gastro.2010.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang X, Ouyang H, Yamamoto Y, Kumar PA, Wei TS, Dagher R, Vincent M, Lu X, Bellizzi AM, Ho KY, Crum CP, Xian W, McKeon F. Residual embryonic cells as precursors of a Barrett’s-like metaplasia. Cell 145: 1023–1035, 2011. doi: 10.1016/j.cell.2011.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]