Abstract
Sex differences between women and men are often overlooked and underappreciated when studying the cardiovascular system. It has been long assumed that men and women are physiologically similar, and this notion has resulted in women being clinically evaluated and treated for cardiovascular pathophysiological complications as men. Currently, there is increased recognition of fundamental sex differences in cardiovascular function, anatomy, cell signaling, and pathophysiology. The National Institutes of Health have enacted guidelines expressly to gain knowledge about ways the sexes differ in both normal function and diseases at the various research levels (molecular, cellular, tissue, and organ system). Greater understanding of these sex differences will be used to steer future directions in the biomedical sciences and translational and clinical research. This review describes sex-based differences in the physiology and pathophysiology of the vasculature, with a special emphasis on sex steroid receptor (estrogen and androgen receptor) signaling and their potential impact on vascular function in health and diseases (e.g., atherosclerosis, hypertension, peripheral artery disease, abdominal aortic aneurysms, cerebral aneurysms, and stroke).
Keywords: estrogen receptors, androgen receptors, cardiovascular system, sex steroid hormones, sex differences
in recent years, greater attention has been placed on the impact of biological sex and hormonal status with regard to predisposition for cardiovascular disease (CVD) and response to therapy. Women generally have a lower risk for developing CVD compared with men of similar age (189), but this protection is lost during menopause (209), suggesting the importance of sex steroid hormone signaling. Although estrogens are viewed as female sex hormones and androgens are viewed as male sex hormones, estrogen and androgen signaling govern a multitude of physiological processes in both women and men. Current biomedical research focuses on the protective role of estrogen in the vasculature, with many fewer studies on androgen signaling. Here, we summarize current scientific literature of both estrogen [estradiol (E2)] signaling and androgen [testosterone and dihydrotestosterone (DHT)] signaling in the pathophysiology of vascular disorders of public health significance, such as hypertension, atherosclerosis, peripheral artery disease (PAD), aneurysms, migraines, and ischemic stroke.
Estrogen Receptors
Estrogen, also referred to as E2, is a classical female sex hormone synthesized primarily in the granulosa cells of ovaries in females and Sertoli cells in males. The physiological effects of E2 are carried out through the activation of various estrogen receptors (ERs), of which there are at least three forms: estrogen receptor-α (ERα), estrogen receptor-β (ERβ) (36, 67), and membrane-bound G protein-coupled estrogen receptor (GPR30/GPER-1) (140, 185).
ERα and ERβ are well-studied nuclear steroid receptors associated with the plasma membrane, cytoplasm, and nucleus in cardiomyocytes, vascular smooth muscle cells (VSMCs), and vascular endothelial cells throughout the mammalian cardiovascular system (177). Both receptor types function as ligand-activated transcription factors and therefore exert long-term genomic effects by modifying gene expression through direct interaction between highly conserved DNA binding domains of nuclear ERs and estrogen response elements (ERE) located near the promoter of target genes (176). Distinct transcriptional activation functions (AF1 and AF2) recruit other regulatory proteins to DNA-bound estrogen receptors and can either operate individually or in unison to further modify gene transcription (176).
GPER-1 is expressed in the endothelium and smooth muscle of the vasculature (97), in intercalated and tubular cells of the kidney, and in high concentrations in the hypothalamic-pituitary-adrenal axis (79). Whether aldosterone is a ligand for GPER-1 remains controversial (79).
Besides genomic signaling, estrogen receptors also elicit cellular responses that occur too rapidly to be attributed to transcriptional regulation. These were originally referred to as “rapid, nongenomic” estrogen signals because they involve ion channels and enzymatic pathways after estrogen receptor activation (175, 178, 250). Estrogen receptors are associated with the cell membrane in various tissues. In some cell types, ERα is found in caveolae (invaginations of the cell membrane) and associates with large protein complexes (Fig. 1) (156). Other signaling factors are also located here and promote signaling cascades after E2 binds to ERα. In addition, rapid responses mediated by estrogen receptors in the cytosol and membrane can still influence gene expression (207). Therefore, either the term “nonnuclear-initiated steroid signaling” or “membrane-initiated steroid signaling” may be more appropriate (102).
Fig. 1.

Rapid estrogen receptor (ER) and androgen receptor (AR) signaling pathways in vascular endothelial cells (ECs) and vascular smooth muscle cells (VSMCs). ERs and ARs interact with caveolin (Cav)-1 and Src. These signaling complexes facilitate rapid downstream signaling by phosphorylation. ECM, extracellular matrix; E2, estradiol.
Androgen Receptors
Testosterone, the androgen synthesized in testicular Leydig cells and ovarian theca cells, is converted to the more potent androgen DHT by the enzyme 5α-reductase. Both androgens are ligands for androgen receptors (ARs), transcription factors that mediate androgen signaling (218). Testosterone is also converted to E2 by the enzyme aromatase, demonstrating its importance as a steroid metabolite in estrogen receptor signaling.
Androgen receptors, like estrogen receptors, belong to the nuclear receptor superfamily (105). Two variants, AR-A and AR-B (281), are found in most tissues, with each showing varying expression levels depending on tissue type. For example, AR-B predominates in most tissue types where both receptor subtypes are present, although not much is known about how they differ in distribution throughout the body (281). Regardless, ARs are expressed in many vascular cell types, such as smooth muscle cells (SMCs), endothelial cells, and blood cells like macrophages and platelets (151).
In the absence of ligands, ARs are localized with heat shock proteins (87), cytoskeletal elements (154), and other chaperones (44) within the cytoplasm. Once bound to testosterone or DHT, androgen receptors undergo conformational changes and act as ligand-inducible transcription factors to regulate genomic actions via interactions with cis-acting androgen response elements (ARE) (124). Various coregulators also play a role in AR transcription, which influence their ligand and DNA binding capabilities (92). “Classical androgen signaling” refers to the genomic effects mediated by activated cytosolic ARs (240). Although androgens mediate male sexual differentiation, androgen signaling is also tied to cell proliferation, differentiation, metabolism, apoptosis, and protein secretion in many different tissues in both women and men (196).
Studies over the past two decades have also demonstrated that androgens can activate nongenomic rapid signaling pathways after binding to membrane-associated or cytosolic ARs (106, 240). This can then trigger the release of intracellular Ca2+ and activate various kinases such as MAPK (106), Akt (19), PKA, and PKC (82). Membrane-associated ARs in aortic endothelial cells specifically interact with Src kinase (c-Src) and the caveolae structural protein caveolin-1 (Fig. 1) (155, 299), possibly a necessary step before the initiation of rapid AR signaling originating from the plasma membrane. Other studies have suggested that membrane-associated AR is a G protein-coupled receptor that interacts with Gnα11 (240), but as this was observed in spermatogenic cells (240), it may not completely mirror cardiovascular cell types. The effects of AR rapid signaling mechanisms appear significant in aspects of vascular physiology and pathophysiology as well.
Hypertension
The development and progression of hypertension are undoubtedly multifactorial and may be explained, at least in part, by lifetime patterns of sex hormone signaling. Both clinical and epidemiological studies have confirmed sex differences in that the onset and progression of hypertension, a disorder that often precedes more serious cardiovascular complications (267), are more prevalent in men than women (233). Although in both sexes increased blood pressure often accompanies aging, postmenopausal women experience more rapid age-related elevations in blood pressure compared with men (209, 294). Estrogen has been thought to contribute to the lower prevalence of hypertension in women compared with age-matched men (204).
Estrogen signaling.
Estrogen is synthesized in the central nervous system (CNS) from cholesterols or converted from aromatizable androgens in presynaptic terminals (57). Additionally, estrogens are able to peripherally diffuse into the CNS (12). In men, testosterone is converted to E2 in the brain via aromatase (235). Estrogen signaling in the brain is especially complex, and estrogen receptor subtype expression is cell type specific (119).
ERα and ERβ are expressed in the CNS in specific nuclei that regulate heart rate and blood pressure (246). They may be involved in regulating the renin-angiotensin system (RAAS), based on findings that renin, angiotensinogen, angiotensin-converting enzymes (ACE1 and ACE2), and angiotensin II receptors [ANG II type 1 and type 2 (AT1 and AT2) receptors] localize and act within these same brain regions (138, 174). Various animal studies have supported the hypothesis that E2 regulates the RAAS within the CNS by showing that E2 infusion effectively reduced both ANG II- and aldosterone-induced hypertension in male and ovariectomized female rodents (286, 289, 291). When estrogen was injected into the solitary tract nucleus (NTS), rostral ventrolateral medulla (RVLM), and parabrachial nucleus of ovariectomized female and male rats, sympathetic nervous system baroreflex control was enhanced with a subsequent decrease in resting blood pressure (230, 231). In contrast, when E2 was injected into the paraventricular nucleus (PVN) in male rats, no effect was seen on heart rate or blood pressure (91). Consequently, specific nuclei in the forebrain and brain stem are sites where estrogen signaling interacts with the central RAAS differently, depending on biological sex and hormonal status.
ERα and ERβ regulate blood pressure differently (208, 289, 304). In female mice, when central ERα was activated, it protected against ANG II-induced hypertension (289). In female and male mice, activating central ERβ preserved resting blood pressure by regulating RVLM voltage-gated Ca2+ currents (239, 275). In female rats, ERβ knockdown in the PVN and RVLM using siRNA-ERβ increased aldosterone-induced hypertension (292), and ERα knockdown in female rats under the same conditions showed no effect on blood pressure (292). These results further support that, in females and males, specific ER subtypes mediate E2’s protective effects on blood pressure within different CNS nuclei.
Overactivation of the RAAS is thought to promote hypertension onset and progression. When inappropriately activated, oxidative stress increases along with vascular constriction and remodeling (56). Recent evidence shows that RAAS constituents, such as ANG II and aldosterone, stimulate the production of ROS in the brain by activating NADPH oxidase (284). This may promote hypertension, since aldosterone-induced ROS in brain regions such as the subfornical organ (SFO) and PVN increases both sympathetic nerve activity and blood pressure (287, 288, 303).
Contrarily, there is evidence that female sex hormones protect against hypertension by directly regulating the RAAS in various organ systems (133).
In support of the antihypertensive effects of estrogen signaling, studies have shown that estrogen activity within the SFO can inhibit intracellular ROS production, thereby decreasing ANG II-induced hypertension (292, 293). Furthermore, intracellular ROS production increases after knockdown of ERα or ERβ expression with siRNA (292). These findings point to a novel estrogenic intracellular signaling mechanism through which estrogen receptor activation interferes with ROS production to mitigate hypertension.
Nitric oxide (NO) plays a significant role in the CNS signaling pathways that control blood pressure (17). Neuronal NO synthase (nNOS), which is responsible for NO production, was found at higher concentrations in the SFO and PVN of female mice compared with male mice (290). When nNOS inhibitors were administered, central ANG II’s hypertensive effects were exacerbated in female but not male mice (290). After ANG II infusion, nNOS levels increased in intact female mice but not in ovariectomized female or male mice (290).
Numerous studies have also confirmed that female sex hormones protect against hypertension via direct signaling in the vasculature. In both humans and murine models, estrogen promotes vasodilation (Fig. 2) by stimulating NO production in the endothelium as a means of regulating blood pressure (191). These rapid cellular responses suggest that estrogen’s vascular actions can occur through nongenomic mechanisms carried out by membrane-associated estrogen receptors and cellular signal transduction (Fig. 3).
Fig. 2.

Estrogens and androgens influence vascular tone. Estrogen enhances the effect of vasorelaxation in female and male vessels. The influence of androgens on vascular tone is more complex as androgen signaling has been demonstrated to promote vasorelaxation in clinical and in vitro studies but elevate blood pressure in vivo. NO, nitric oxide.
Fig. 3.
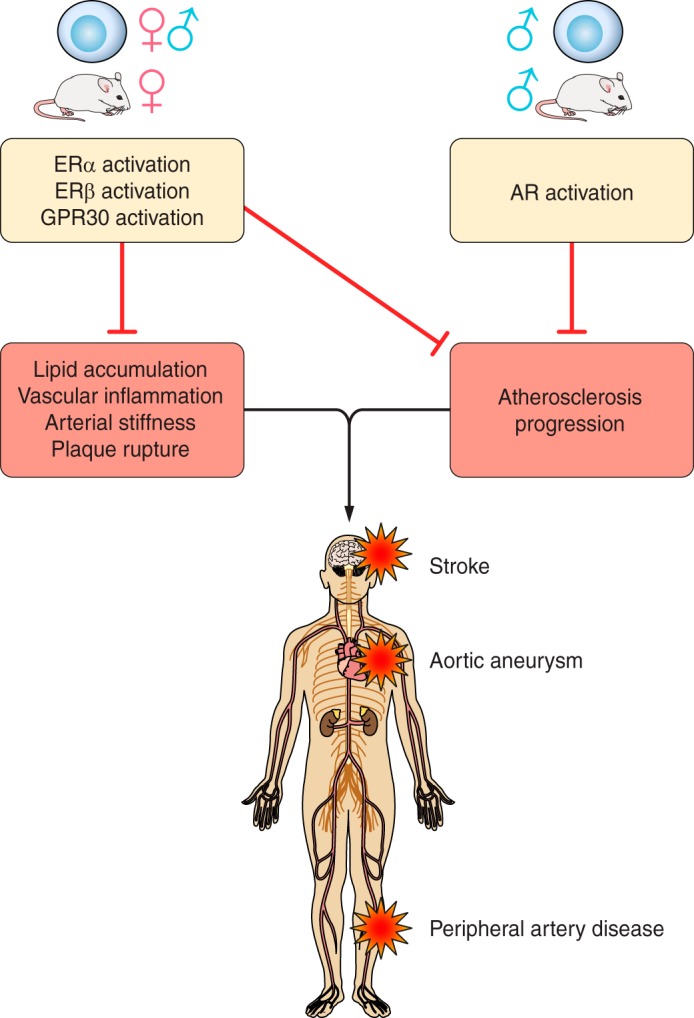
Schematic view of ER and AR signaling in vascular pathophysiology. ERs protect against hypertension and other vascular injury diseases such as atherosclerosis, stroke, cerebral aneurysms, and abdominal aortic aneurysms. AR signaling protects against atherosclerosis and possibly hypertension. However, increased androgen signaling is associated with elevated stroke risk; furthermore, androgen signaling experimentally exacerbates the formation of abdominal aortic aneurysms. GPR30, G protein-coupled estrogen receptor.
When ERα is activated, it promotes vasodilation by rapidly stimulating endothelial NO synthase (eNOS). For example, when eNOS was activated in pulmonary endothelial cells shortly after E2 treatment, kinase inhibitors and ERα antagonist, but not gene transcription inhibitor, significantly interfered with eNOS stimulation (49), suggesting rapid eNOS activation by a nonnuclear ERα isoform. Furthermore, when extranuclear ERα was stimulated, it activated eNOS after binding with an estrogen conjugate excluded from the cell nucleus (46). Taken together, these findings implicate the necessity of activating membrane-bound ERα (Figs. 2 and 3). However, the antihypertensive potency of this mechanism is largely dependent on the type of artery being studied, since both ERα and eNOS localization and expression levels vary between different vascular beds (227).
The kidneys also show similar antihypertensive effects from estrogen signaling, since NO also participates in renal control of extracellular fluid homeostasis (173). Because females produce more NO than males due to naturally higher estrogen levels, free Na+ intake is higher in female versus male animals (205). Yet, clinical studies have demonstrated that the prevalence for salt sensitivity begins to increase once women transition into menopause and estrogen levels decline (280). This is likely because estrogen signaling increases NO bioavailability and decreases the AT1-to-AT2 receptor ratio, thereby preserving renal Na+ handling (108). Clinical studies have supported this hypothesis, where, in premenopausal women, salt loading during estrogen peaks resulted in a reduced filtration fraction and sustained renal vasodilation (206), whereas in postmenopausal women salt loading resulted in an increased filtration fraction (205).
Estrogen signaling may also contribute to blood pressure through renal-specific regulation of ACE2. This enzyme counteracts ANG II’s vasoconstrictor activity via conversion to ANG-(1–7) (118). Although these findings would seem to suggest that naturally higher amounts of estrogen in females contribute to hypertension via downregulation of renal ACE2, normotensive mice showed that basal ACE2 activity was lower in female compared with male mice (150). ERα is the receptor subtype that mediates estrogen regulation of renal ACE1/ACE2. For example, when ovariectomized apolipoprotein (Apo)E knockout (ApoE−/−) mice possessing wild-type ERα (AAee) were chronically treated with estrogen for 3 mo, renal ACE mRNA was reduced by 1.7-fold, whereas the same treatment in ovariectomized ApoE−/− ERα knockout mice (ααee) resulted in a 2.1-fold increase (30). Furthermore, in AAee mice, E2 treatment decreased renal ACE2 mRNA and AT1 receptor mRNA, but this was not observed in ααee mice. In AAee mice, E2 treatment resulted in an 81-fold increase of AT2 receptor mRNA, which was not observed in ααee mice (30).
Unfortunately, the rodent models used in the previously described studies did not model a hypertensive disorder, which is of note in that estrogen signaling in diseased states seems to regulate enzymes differently. Female rat models of renal hypertension demonstrate that estrogen signaling actually upregulates ACE2 activity in the kidneys to oppose ANG II-induced vasoconstriction. In female Sprague-Dawley rats with renal wrap on a phytoestrogen-free, high-Na+ diet (modeling renal hypertension), both ACE2 mRNA levels and protein activity were reduced after ovariectomy. This resulted in renal injury, which was dampened after ANG-(1–7) infusion into ovariectomized rats (123). Moreover, in both intact and ovariectomized female rats given E2, there was decreased inhibition of ACE2 expression and progression of renal injury was significantly attenuated (123). Therefore, estrogen signaling appears to be important in upregulating ACE2 activity as a protective measure against renal hypertension.
GPER-1 is also found in endothelial cells and SMCs (21) and localizes in both arteries and veins of the human vasculature (99). When activated, GPER-1 has been shown to regulate blood pressure and protect against hypertension (Fig. 3). For example, incubation of porcine epicardial coronary artery rings with the selective GPER-1 agonist G1 promoted vasodilation and dampened endothelin-1-mediated vasoconstriction (180). In addition, GPER-1 agonists administered to both female and male rats resulted in endothelium-dependent vasodilation and reduced superoxide levels (31). Studies in knockout GPER-1 animals showed no effect of chronic GPER-1 deficiency on NO bioavailability but did show an increased VSMC response to endothelium-derived vasoconstrictor prostanoids (179). These findings point to a novel, NO-independent role of GPER-1 in regulating blood pressure and control of vascular hypertension (Fig. 3).
The vascular effects of GPER-1 have been studied in various rat models of hypertension, such as the mRen2.Lewis rat (276). When ovariectomized female mRen2.Lewis rats were infused with G1 for 2 wk, they showed reduced blood pressure (146). E2 showed similar effects as G1 in stimulating this GPER-1-mediated effect (145). In contrast, male mRen2.Lewis rats treated with G1 showed no significant changes in blood pressure. When the same female rat model was infused with G15, a GPER-1 antagonist, vasodilation was significantly inhibited, even with E2 administration (145). This points to a possible therapeutic application of agonizing GPER-1 in females to combat prostanoid- and ANG II-induced elevations in blood pressure.
In summary, in females, estrogen signaling has been shown to elicit different antihypertensive mechanisms through specific estrogen receptor subtypes, with ERα being the main mediator of NO production in the vascular endothelium and a regulator of renal ACE2 activity. GPER-1 may be primarily responsible for attenuating increased VSMC contraction to cyclooxygenase (COX)-derived prostanoid vasoconstrictors. A major limitation of many of these animal studies is their exclusion of hypertensive male animals since administering exogenous estrogen is likely not a feasible hypertension treatment in males. For males, future research is needed to examine the effects of selective estrogen receptor modulators.
Androgen signaling.
The effects of androgen signaling and hypertension are more complex and not as well understood as estrogen signaling. Studies using different rat models have suggested that androgens may promote vascular diseases such as hypertension, but clinical findings often demonstrated decreased androgen levels in males with cardiovascular-related diseases, including hypertension (162) (Fig. 2). The conflicting results between animal and clinical studies suggest an optimal physiological threshold of circulating androgens required to circumvent the onset of hypertension (Fig. 2).
Several studies have demonstrated that hypoandrogenism may be linked to hypertension in older men. In a nested case control study on 11,606 men aged 40−79 yr, surveyed from 1993 to 1997 and followed up to 2003, endogenous testosterone concentrations were found to be inversely related to cardiovascular disease mortality (127). Another study on older men found that total testosterone levels were inversely associated with systolic blood pressure and mortality risk over the following 20 yr of life after adjusting for preexisting conditions (137). Moreover, a short-term crossover study found that testosterone replacement therapy decreased diastolic blood pressure in obese men (167).
Chronic androgen administration exacerbated hypertension in rat animal studies. In a study using hypoandrogenic, obese male Zucker rats supplemented with exogenous testosterone for 10 wk improved both body weight and lipid profiles but increased blood pressure (63). These results conflict with human studies that have suggested that normal androgen levels are antihypertensive, which may be due to studies often using young instead of aged animals to model hypertension, a chronic disease that usually manifests in older human populations.
Additionally, inherent challenges exist in comparing physiological levels of circulating androgens between rodent models and humans. Androgen studies on animals are often acute due to short animal viability and high experimental costs, and responses may vary between animal strains. Therefore, results from these studies may not completely mirror clinical and epidemiological observations of androgen imbalance in humans. Even so, studies on rodents and cell culture have provided valuable information on the mechanisms of androgen signaling that raise blood pressure.
Conflicting results from animal studies may also be partially due to the genomic effects of androgen signaling in the kidneys. In one study, blood pressure, plasma renin activity, and ANG mRNA levels were higher in intact male rats compared with matched female rats (48). Orchiectomy of young male rats blunted hypertension development and lowered both plasma renin and renal ANG mRNA levels, whereas testosterone replacement restored these levels back to baseline (48). The results from this study indicate that androgen signaling elevates blood pressure and can promote hypertension by upregulating RAAS constituents in the kidneys. This may occur through the genomic effects of “classical” androgen signaling, since some of these studies observed elevated RAAS constituent mRNA levels in intact males. Another study observed that androgen signaling stimulated Na+ reabsorption in the proximal tubules of the kidneys (129). This may result from upregulation of intrarenal ANG II and subsequent expression of Na+/H+ exchanger 3, the primary transporter for Na+ reabsorption in the proximal tubule (217). These effects would be expected to shift pressure-natriuresis to increase extracellular volume and blood pressure.
Androgens have also been shown to contribute to ANG II-induced hypertension in male animals by increasing renal inflammation and immune cell infiltration. For example, in a study using male and female Rag-1–/– mice, which lack both T cells and B cells, systolic blood pressure was similar in both sexes in response to ANG II (215). However, when male T cells were transferred into male Rag-1–/– mice, significant increases in systolic blood pressure in response to ANG II were seen compared with female mice (215). This study also demonstrated that male mice had more renal lymphocyte infiltration than female mice in both the control and ANG II-treated groups (215). This study highlights an innate difference in the immune response to androgen signaling between males and females and implies that males are more susceptible to renal inflammation and ANG II-induced hypertension.
Very few studies have examined androgen signaling and hypertension in females. However, some studies have suggested that androgens are important in blood pressure regulation just as they are in men. Women with polycystic ovarian syndrome have been shown to exhibit excess androgen levels and are often hypertensive (221). For women with hyperandrogenemia, androgen-induced oxidative stress in the kidneys may account for an increased incidence of hypertension. For instance, previous studies on male rats have demonstrated that androgen signaling upregulates 20-HETE, a vasoconstricting metabolite of arachidonic acid. This increases renal microvascular reactivity, which can then activate NF-κB, increase superoxide production, and deplete bioavailable NO, thereby increasing blood pressure (192, 282). Very few studies have examined this mechanism in female rodents. However, one study demonstrated that chronic DHT administration in female and male rats increased systolic blood pressure and 20-HETE levels in both sexes (244). This suggests that increased blood pressure in women with hyperandrogenemia may be caused by androgen-mediated renal upregulation of vasoconstrictors such as 20-HETE (244). However, the doses of DHT used in this study were significantly higher than serum androgen levels commonly seen in women with hyperandrogenemia. Therefore, more studies that mimic serum androgen levels in women are needed to determine if this is a plausible explanation for increased blood pressure.
While in vivo studies have suggested that androgens contribute to hypertension through renal inflammation, upregulation of RAAS constituents, and modulation of pressure natriuresis, human and in vitro studies have suggested that androgen signaling in endothelial cells and VSMCs of arteries can exert antihypertensive effects (Figs. 2 and 3). For example, testosterone rapidly activates eNOS and increases NO release from vascular endothelial cells through the phosphatidylinositol 3-kinase/Akt signaling pathway (299). These results point to a membrane complex of ARs colocalized with caveolin-1 and c-Src, which mediate this mechanism (299). Endothelium-independent vasodilation has been demonstrated in other studies, where androgen signaling induced VSMC relaxation by inactivating voltage-gated Ca2+ channels (101, 188) and activating K+ channels (37, 236). This collective evidence demonstrates that direct androgen signaling in the vasculature can also elicit antihypertensive effects.
Further research needs to weigh the beneficial actions of androgen signaling in vascular beds against deleterious effects in the kidneys and other tissues to determine if physiological levels of androgens are truly antihypertensive. In females, the effects of hyperandrogenism need to be determined to elucidate the cellular mechanisms that cause hypertension.
Atherosclerosis
Atherosclerosis is characterized by the buildup of fatty plaques in arteries that can potentially rupture and impede blood flow to the heart and other organs. Vascular inflammation, lipid accumulation, intimal fibrosis, arterial stiffness, and plaque rupture are all key factors in the pathogenesis of atherosclerosis, which can eventually lead to more serious cardiovascular insults such as heart attack and stroke (103). The pathogenesis of atherosclerosis involves interactions between endothelial cells and SMCs of the arterial wall and infiltrating cells such as macrophages (144). These infiltrating cells require cellular adhesion molecules, such as VCAM-1, to bind to the endothelium (60). Significant sex disparities exist in the prevalence of atherosclerotic coronary artery disease (CAD). Compared with women, men have a significantly higher risk of developing CAD (47, 263) and dying from it at younger ages (189). Greater levels of adhesion molecules are expressed in the vascular tissue of males compared with females, which may contribute to greater male atherosclerotic predisposition (65, 172). However, the chances of women developing atherosclerosis greatly increase postmenopause (269), which suggests a role for steroid hormone receptor signaling in the sex differences for atherosclerosis.
Estrogen signaling.
The underlying physiological actions of estrogen are important determinants of cardiovascular physiology and development of vascular disease (271). ERα is considered to be the estrogen receptor subtype primarily responsible for mediating estrogen signaling and protecting against vascular injury, fibrosis, and atherosclerosis (114, 160). Elevated expression of ERα in the vasculature of premenopausal women correlates with low atherosclerosis incidence rates (153). However, other estrogen receptor subtypes may mediate protection against atherosclerosis as well. Platelets, which are heavily involved in thrombosis, express ERβ and AR and also respond to sex hormone signaling (90). In addition, the role of GPER-1 in the context of atherosclerosis has also been reported (45).
The vascular calcification process involved in CAD is lipid sensitive (62), and premenopausal female protection from atherosclerosis may be partly due to the beneficial effects of estrogen on lipid metabolism. High circulating levels of estrogens in females correlate with increased high-density lipoprotein (HDL) and decreased low-density lipoprotein (LDL) cholesterol levels, which have an overall beneficial effect on the lipid profile (131). Consistent with these findings, ovariectomy worsened lipid profiles by increasing LDL and reducing HDL cholesterol levels in female rat models of atherosclerosis (131, 203).
Estrogen exerts effects on LDLs and their receptors (LDL-Rs) to improve the lipid profile. Thus, the lack of LDL binding to its receptor results in hypercholesterolemia and subsequently increases the risk for atherosclerosis (93). E2 enhances LDL-R expression and stimulates sterol-27-hydroxylase activity, which inhibits LDL production (55, 258), demonstrating that estrogen signaling regulates both the LDL ligand and its receptor in hindering preliminary atherogenesis. In addition, estrogen’s phenolic structure is effective at preventing the oxidation of LDL cholesterol (252, 273). E2’s ability to regulate LDL is also effective for preventing disruption of the endothelial barrier initiated by vascular accumulation of minimally modified LDL (86).
Estrogens are also able to regulate lipoprotein activity and structure during atherosclerosis (184). For example, estrogen has been shown to inhibit macrophage uptake of acetylated LDL cholesterol, which implies that estrogen signaling may affect scavenger receptor function (253). Clinical studies on postmenopausal estrogen replacement therapy have demonstrated that estrogen upregulates ApoAI mRNA levels in the liver and also stimulates ApoE production (274). Furthermore, estrogen signaling facilitates reverse cholesterol transport by stimulating the production of the ABCA1 transporter (247). On the other hand, estrogen signaling hinders expression of scavenger receptor B1 (SR-B1), which prolongs the duration of circulating HDLs and improves the lipid profile (248). Taken together, this evidence also demonstrates mechanisms by which estrogen signaling can hinder initial plaque formation (Fig. 3).
Inflammation plays a large role in the pathogenesis of atherosclerosis (77), and multiple studies using cell cultures have demonstrated anti-inflammatory effects of estrogen signaling on immune cells. Indeed, many of the infiltrates found in atherosclerotic plaques, such as macrophages, T cells, B cells, and mast cells express sex hormone receptors (90, 269). This denotes a possible influence of estrogen on the complex inflammatory components of CAD pathogenesis. Macrophages are the primary infiltrates found in atherosclerotic plaques (54), and the general consensus is that estrogen exerts anti-inflammatory effects on macrophages.
For example, E2 has been shown to reduce lipoprotein oxidation in healthy macrophages and push them toward the anti-inflammatory M2 phenotype (202) by inhibiting oxidation of LDL (224) and increasing ApoE expression (277). In vitro studies have also demonstrated that estrogens inhibit endothelial activation and adhesion of neutrophils and monocytes to the endothelium. For example, E2 and various estrogen metabolites dampen expression of adhesion molecules (e.g., CD40, CD40L, E-selectin, P-selectin, VCAM-1, and ICAM-1) (Fig. 4) after being exposed to various factors that promote atherogenesis (88, 220, 243, 260). One possible mechanism behind this effect involves estrogen-mediated inhibition of NADPH oxidase activity, which decreases ROS production (272). Additionally, estrogen signaling blunts monocytic expression of factors such as integrins that facilitate binding to VCAM-1 and ICAM-1 (220). Taken together, this evidence indicates that estrogen signaling can protect against atherosclerotic plaque formation by pushing immune cells toward an anti-inflammatory phenotype and preventing their adhesion to the vascular endothelium.
Fig. 4.
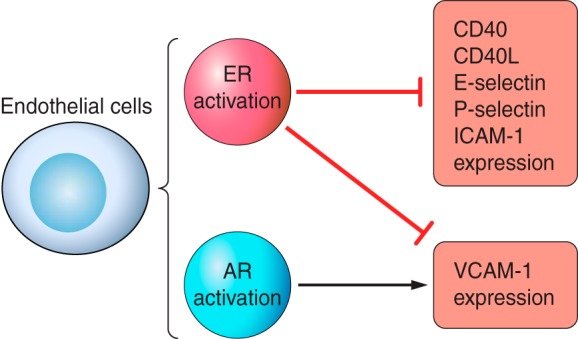
Role of ER and AR signaling on adhesion molecule expression in ECs. ER signaling decreases the expression of adhesion molecules in ECs after exposure to atherogenic-promoting factors. However, VCAM-1 expression is stimulated by male-specific AR in ECs. Thus, male vessels may be more susceptible to inflammatory cell infiltration in atherosclerosis.
ERα was previously thought to mediate most of the atheroprotective effects of estrogen signaling, since early experiments demonstrated that ERα knockout in female ApoE−/− mice was detrimental to atherosclerotic lesion size and increased serum cholesterol compared with controls, even when they were administered exogenous E2 (114). However, studies in later years have demonstrated that estrogens are still able to exert antiatherogenic effects in females with full-length ERα knockout on high-cholesterol diets (270). In male rodents, ERα deletion is actually protective against atherosclerosis in some instances (268). This suggests that either different sex hormone receptors or isoforms of ERα are able to mediate estrogen’s antiatherogenic effects.
Recent studies using mouse models of atherosclerosis have indicated that GPER-1 activation may aid in the prevention of disease by exerting beneficial effects on lipid metabolism. As previously mentioned, dyslipidemia is a strong component of coronary atherosclerosis (20), and unfavorable lipid profiles, such as increases in triglycerides and decreases in HDL cholesterol, have been observed in studies using female GPER1-deficient mice (237). A study using female mice under atherogenic conditions found that genetic and pharmacologic knockout of GPER-1 resulted in a multitude of proatherogenic outcomes, such as increased LDL cholesterol levels, elevated inflammation from macrophage and T cell activation, and a reduction in bioavailable NO production (182). Although both human endothelial cells and VSMCs displayed intracellular expression patterns of GPER-1, this receptor specifically mediated eNOS activity in the vascular endothelium (182). This evidence not only confirms an atheroprotective role of GPER-1 in animals but in human vascular tissue as well.
GPER-1 activation reduces other major components of atherosclerotic pathogenesis such as VSMC proliferation and vascular inflammation. For example, activation of GPER-1 inhibits in vitro proliferation of cultured human VSMCs (98, 142), which is a key step in atherosclerotic plaque formation (20). Additionally, a genetic knockout study (237) on GPER-1 in aged male mice demonstrated that its absence is positively correlated with a proinflammatory state. Next, a study (182) in ovariectomized mice demonstrated that when the GPER-1 agonist G1 was administered, there was significantly reduced atherosclerosis and vascular inflammation. Furthermore, GPER-1’s anti-inflammatory effects have been affirmed through other in vitro studies using endothelial cells and immune cells such as macrophages (45, 223). A molecular genetic study (9) has demonstrated that GPER-1 can be cloned from human lymphoblast cDNA libraries. In another study (223), GPER-1 activation in macrophages resulted in decreased expression of Toll-like receptor-4. The biochemical mechanisms underlying GPER-1 modulation of inflammatory processes are not fully understood yet. However, results from some investigations suggest that the forkhead box P3 protein (FOXOp3) pathway, which is tied to immune cell secretion of IL-10, is involved (33, 34).
Thus far, the body of evidence from preclinical studies on GPER-1 has shown that activation of this receptor improves the lipid profile and dampens the inflammatory component of atherosclerotic pathogenesis. Therefore, GPER-1 has the potential to be a therapeutic target for preventing and treating CAD in men and postmenopausal women (181).
In summary, evidence shows that estrogen signaling can hinder the formation of initial fatty streaks germane to atherosclerosis by improving lipid profiles and dampening inflammation through the ER subtypes. Nevertheless, atherosclerosis is a multifactorial disorder that involves many cell types of both the vascular architecture and circulating blood. Although studies have identified some of the protective effects of ER activation in different cell types, more research is needed to fully understand the signaling pathways that estrogen receptors elicit in the context of atherosclerosis. Furthermore, few in vivo studies have examined the effects of estrogen signaling in male animals, and it is likely that males respond differently to estrogen signaling. Since administering exogenous E2 to men is considered therapeutically impractical, estrogen receptor knockout mouse models and selective estrogen receptor modulators should be used in future research studies to delineate the mechanisms of vascular estrogen signaling in males.
Androgen signaling.
The effects of androgen signaling in the context of atherosclerosis still remain poorly understood. However, there is a general consensus that normal physiological levels of endogenous testosterone are atheroprotective. In fact, low androgen levels in men are associated with accelerated atherosclerosis. For example, clinical studies have shown that markers of atherosclerosis, such as intima-media thickness, are inversely correlated to endogenous androgen levels in men (161, 190, 266). Furthermore, evidence from the Rotterdam study population confirmed an inverse relationship between total bioavailable testosterone and the extent of abdominal aortic calcified deposits in men over 55 yr old (66). Other human studies have demonstrated that androgen deficiency is tied to endothelial dysfunction in men. For instance, clinical data demonstrate that flow-mediated brachial artery dilation in middle-age men was positively associated with circulating testosterone levels (7).
Some clinical studies have linked hyperandrogenemia in women to an increased risk of developing subclinical atherosclerosis. For example, the Coronary Artery Risk Development in Young Adults Women’s Study found that women with polycystic ovarian syndrome that manifested as both anovulation and hyperandrogenism had a 2.70 odds ratio for coronary artery calcification (38). Therefore, clinically, normal physiological levels of endogenous androgens may be protective against atherosclerosis (Fig. 3), since increased risk is associated with hypoandrogenism in men and hyperandrogenism in women.
Biomedical research on atherosclerosis using rabbits and rodents has proven to be difficult since some important characteristics of human atherogenesis are not recapitulated in animal models. Lipid metabolism in rabbits mirrors human physiology more closely than that of rodents, and, as a result, several studies have used rabbit models of atherosclerosis rather than mice to better understand androgen signaling in the context of human atherosclerosis. Nevertheless, research studies with both rabbit and rodent models have confirmed atheroprotective effects of normal androgen levels in males, but the underlying biochemical mechanisms still remain largely unclear.
In studies using male rabbits sustained on a high-cholesterol diet, reduced atherosclerosis in the aortic vessel as a result of exogenous testosterone administration was seen (9, 32). These effects were largely independent of plasma lipid levels (9). Another study using castrated LDL-R-deficient male mice demonstrated that the inhibitory effects of testosterone on fatty streak development were diminished subsequent to treatment with aromatase inhibitors (195). This suggests that atheroprotection from testosterone may be partially attributed to its conversion to E2 by aromatase (195). On the contrary, some studies using castrated male rabbits have demonstrated that DHT, a nonaromatizable androgen, is also able to hinder progression of atherosclerosis by suppressing foam cell formation (216). These results point to a protective mechanism involving AR activation.
As mentioned previously, ARs are expressed in all cell types of the vasculature (132, 151). However, sex differences exist in AR concentrations expressed in vascular tissues. For example, in males, higher AR expression was seen in rat VSMCs (109), human mesenteric artery cells (65), and macrophages (171). Androgens may exert atheroprotective effects in males through multiple mechanisms, such as AR ligand activation (Fig. 3), conversion to E2 via aromatase, or other unknown pathways. Some investigations have directly verified the atheroprotective effects of androgens through both AR-dependent and AR-independent mechanisms.
For example, administration of exogenous testosterone decreased atherosclerotic lesions in both AR knockout and wild-type mice that underwent gonadectomy (29). However, the atherosclerotic lesion sizes in AR knockout mice were still significantly larger than those seen in wild-type mice (29) and there were no traces of aromatase mRNA in the femoral artery or liver of the animals after quantitative PCR analysis (29), which ruled out protective mechanisms brought on by conversion of testosterone to estrogen. This clearly demonstrates that androgens are able to hinder atherosclerosis through mechanisms of both AR-dependent and AR-independent activation.
On the other hand, stimulation with DHT in cultured human umbilical vein endothelial cells from male donors results in increased expression of the VCAM-1 gene and increased levels of the cell surface protein (171). These results are abrogated in cells of female origin as a result of lower AR presence and inhibition of VCAM-1 mRNA and subsequent protein expression by E2 (171). This androgenic stimulation of VCAM-1 in endothelial cells, which is amplified by male-specific AR levels (Fig. 4), may imply that males are more susceptible to immune cell infiltration in the pathogenesis of atherosclerosis.
In summary, normal physiological levels of androgens appear to be atheroprotective in both females and males. However, the cell signaling pathways and mechanisms underlying this androgen-mediated protection are poorly understood. Since current evidence suggests that androgen signaling affects atherosclerosis through multiple AR-dependent and AR-independent pathways, more in vitro studies are needed to better understand the different mechanisms behind androgen-mediated atheroprotection. Furthermore, there is very little research on androgen signaling and vascular disorders in females, creating a unique opportunity for future studies into these areas of vascular research. More research in female rodent models on androgen signaling and atherosclerosis is crucial for developing better therapies.
No animal model perfectly mirrors atherosclerotic development in humans so limitations exist whenever animals are used for translational research. Inducing atherosclerosis in rodents with short lifespans often requires special diets that result in serum cholesterol levels that greatly surpass those found in human disease states. Using these research models inherently limits our ability to translate what often takes decades to develop in humans.
It is important to note that wild-type mice are resistant to developing atherosclerotic lesions. Therefore, it is appropriate and often necessary to use both genetic and dietary modifications for murine models of atherosclerosis. In the animal studies discussed thus far, LDL-R−/− and ApoE−/− mice were used; they are well established and the most commonly used murine models of atherosclerosis. In fact, ApoE−/− mice develop atherosclerosis even under normal dietary conditions. One clear advantage of this mouse model is that it develops atherosclerotic lesions in sites similar to humans such as the aortic root, aortic arch, carotid, and coronary arteries (24, 116). However, the model lacks the frequency of plaque rupture and thrombosis that often accompanies atherosclerosis in humans. One potential strategy to overcome this limitation in future research on sex differences in atherosclerosis would be to cross ApoE−/− mice with mice deficient for SR-B1, as these hybrid mice would likely exhibit rapid coronary artery occlusion, myocardial infarction, and other characteristics that closely resemble advanced human CAD (262).
Observed atherosclerotic plaques in rabbits are highly similar to humans in terms of inflammation (1). Histological analysis of aortic plaques in rabbits reports macrophage accumulation, thin fibrous caps, and lipid-rich cores. These features are also characteristic to humans (1). Still, rabbit models of atherosclerosis are often not inbred or as easily genetically manipulated compared with mice, leading to more variations in response to high-cholesterol diets. Note that larger animals, such as pigs, are known to have cardiovascular anatomy and physiology quite comparable with humans, so this may be a model to consider. Moreover, studies on sex differences in atherosclerosis may benefit from the use of larger mammals.
PAD
PAD is a progressive circulatory disorder characterized by artery and vein blockage not in close proximity to the heart. PAD most often occurs in blood vessels of the legs and feet, and blockage can arise from thrombosis or narrowing of the arteries due to atherosclerosis. Much like CAD, PAD is more prevalent in men than women at younger ages, but the incidence rises in women after menopause (200). In addition, women generally display more severe PAD compared with men at the time of clinical diagnosis and experience greater complications (122). A point-prevalence study of 5,080 subjects in Sweden found that women had a higher prevalence of both asymptomatic PAD (12.6% vs. 9.4%) and PAD with limb ischemia (1.5% vs 0.8%) compared with men at clinical diagnosis (242).
Epidemiological studies have also demonstrated associations between sex hormone levels and PAD. For example, a cross-sectional investigation on 3,034 middle-aged participants from the Framingham Heart Study found that lower total testosterone and sex hormone-binding globulin concentrations in men were associated with prevalent PAD, but none of the analyses on female participants yielded significant results (104). Another investigation on 921 elderly participants from the InCHIANTI study found that low sex hormone-binding globulin levels in men and high testosterone levels in women were significantly associated with PAD, although E2 was not significantly associated with PAD for either sex (159). These results agree with other epidemiological studies that demonstrated an inverse relationship between serum androgen levels and PAD symptoms in older men (295). Collectively, these studies suggest that androgens may protect men but not women from PAD.
Estrogen signaling.
Although many epidemiological and clinical studies have not found significant associations between serum estrogen levels and PAD, evidence from animal studies has shown that estrogen signaling is antithrombotic and can protect against PAD. For example, Abu-Fanne et al. (3) treated female ovariectomized mice with E2, raloxifene, or placebo for 4 wk before inducing thrombosis and vascular occlusion. Their results demonstrated that ovariectomy significantly amplified thrombosis, whereas E2 or raloxifene treatment was antithrombotic in both ovariectomized and intact female mice (3). The antithrombotic effects of both treatments were attributed to suppression of platelet adhesion and upregulation of the COX-2 pathway (3).
Estrogen signaling facilitates neovascularization and recovery from ischemic PAD. From one study investigating the effects of ovariectomy in female BALB/c mice followed by surgically induced hindlimb ischemia (HLI), ovariectomized mice had low plasma levels of E2 coupled with a reduced blood perfusion index and capillary density compared with controls at 21 days postinduction of HLI (169). Furthermore, ovariectomized mice exhibited suppressed eNOS expression as early as 3 days postinduction of HLI (169). This implies that eNOS is an essential downstream target of the estrogen signaling pathway that promotes angiogenesis.
Proangiogenic effects of estrogen have been observed in male animals as well. In an investigation using a male mouse model of HLI, exogenous estradiol administration increased mobilization and incorporation of endothelial progenitor cells (EPCs) into sites of neovascularization in a dose-dependent manner (228). There was improved blood flow recovery and decreased limb necrosis in the E2-treated group compared with the placebo-treated group (228). E2-induced neovascularization was also attributed to eNOS stimulation (120) and subsequent matrix metalloproteinase (MMP)-9 activation in the bone marrow (121).
Androgen signaling.
Studies on animals and cell culture have indicated that androgens may aid in recovery from PAD-induced ischemic stress by promoting angiogenesis. For example, Yoshida et al. (297) demonstrated that AR knockout in both male and female murine models of HLI impaired angiogenesis, which resulted in increased skeletal muscle apoptosis, paired with an increased incidence of autoamputation in both sexes. AR knockout reduced phosphorylation of Akt and eNOS even though there was an increase in VEGF expression in the ischemic limb tissue. Furthermore, siRNA knockdown of AR in cultured vascular endothelial cells significantly decreased VEGF-mediated phosphorylation of Akt and stimulation of eNOS, thereby demonstrating cross-talk between androgen signaling and VEGF signaling pathways (297).
Although androgen signaling was found to be angiogenic in both males and females in the study, prior investigations found only angiogenic potency of androgen signaling in males. For instance, Sieveking et al. (241) demonstrated that in hypoxic conditions, castration in male mouse models of HLI blunted angiogenesis. Furthermore, administration of DHT in vitro augmented male, but not female, endothelial cell migration, proliferation, and tubulogenesis in a dose-dependent manner (241). These proangiogenic effects of androgen signaling in males were also dependent on increased expression of both VEGF and one of its receptors, KDR, which mediates most of the angiogenic actions of VEGF in the endothelium (241). In support of these findings, castration of male mice significantly blunted neovascularization after HLI was induced (241).
In summary, in males, endogenous androgen signaling facilitates neovascularization by activating the Akt signaling pathway and upregulating expression of VEGF and its receptors in endothelial cells (241, 297). Therefore, in males, androgens may be therapeutic in circumventing hypoxic conditions with occlusive PAD. However, there is conflicting evidence on the angiogenic potential of androgens in females. These differences may stem from using varying rodent models, since Sieveking et al. (241) used ovariectomized mice while Yoshida et al. (297) used AR knockout mice for their in vivo experiments. Estrogen signaling, however, has been shown to be angiogenic in both sexes by increasing both EPC mobility and incorporation into neovascularization sites via eNOS stimulation and MMP-9 activity in bone marrow (120, 121, 169, 228). Although PAD’s clinical aspects are well defined, greater emphasis needs to be placed on delineating the mechanisms and impact of AR signaling on PAD in females.
Abdominal Aortic Aneurysms
Aneurysms are characterized by the localized yet permanent dilation and weakening of the arterial wall. Both cerebral and abdominal aortic aneurysms (AAA) share multiple risk factors (e.g., smoking, atherosclerosis, and high blood pressure) (8, 35) but show sexual dimorphisms in their prevalence and age of onset (23, 64).
AAA is a localized ballooning of the abdominal aorta (113). Epidemiological studies of Western populations have confirmed that AAA is much more prevalent in men than women. For example, the Rotterdam Study, a large cross-sectional study on 5283 individuals in The Netherlands, found that AAA prevalence was 4.1% in men compared with 0.7% in women (214). The underlying causes of males being predisposed are still not completely clear because of the disease’s complex pathogenesis. However, key pathological characteristics of AAA development include the degradation of collagen and elastin in the vessel media and adventitia, increased release of chemokines and cytokines, and infiltration of immune cells into the aortic wall (13, 40).
Estrogen signaling.
High levels of circulating female sex hormones have been shown to protect against experimental AAA development by reducing inflammation and matrix protease activity in aortic tissue. Aortas of female rats in an elastase model of AAA displayed less MMP-9, fewer infiltrating macrophages, and overall decreased medial wall destruction compared with matched male rats (6). Furthermore, protection against AAA was abolished after female rat aortas were transplanted into male rats, whereas female rat aortas transplanted into female mouse recipients remained protected from developing AAA (6).
Similar results have been observed in other animal studies. Plasminogen activator inhibitor (PAI)-1 is involved in inhibiting plasmin and subsequent MMP-9 and MMP-2 production, as these MMPs contribute to the breakdown of the aortic wall. Thus, higher levels of PAI-1 in female animals show protection against AAA development (68). Similarly, another study using an elastase model of AAA found that rodents with higher levels of estrogen exhibited lower MMP-9 and MMP-2 levels and decreased immune cell infiltration in AAA tissue compared with rodents with lower estrogen levels due to ovariectomy (283). Significantly slower aneurysm dilatation rates in the rodent group with higher estrogen levels suggested that estrogen blunted AAA progression by reducing immune cell infiltration into the aortic wall while simultaneously dampening vascular tissue production of MMP-9 and MMP-2 (283).
Sex differences in MMP activity may originate in the phosphatidylinositol 3-kinase/Akt signaling pathway (89). For example, male rat aortic SMCs treated with elastase exhibit higher levels of phosphorylated Akt/total Akt than female cells (89). This translates to elevated pro-MMP-9 and active MMP-2 in male rat aortic SMCs. In addition, immunohistochemistry of male and female AAA patient tissue revealed increased p308/total Akt ratios in men compared with women (89). This study highlights the differences in cell signaling pathways that could explain sex differences in MMP activity and male predisposition to developing AAA.
NO is known to promote vasodilation and inhibit VSMC proliferation and leukocyte adhesion (130, 168). Increased NO production from estrogen signaling is also thought to protect against AAA; the incidence in ovariectomized NOS2−/− female mice is 80% compared with wild-type ovariectomized mice at 40% (139).
ERα may mediate the protective effects of estrogen signaling for AAAs (Fig. 3). In a study using the elastase perfusion mouse model, smaller and less frequent aneurysms were observed in female mice. Mouse aortas from female mice showed increased ERα mRNA and protein levels, which correlated with lower MMP activity (136). Also, ERα levels were 80% higher in samples from female patients with AAA compared with men (136). These results indicate that ERα protects against AAA. More research on the downstream signaling mechanisms of estrogen receptor activation in vascular cells are needed to better understand the influence on AAA.
Androgen signaling.
Studies on male animals have shown that higher levels of androgen signaling exacerbate AAA formation (Fig. 3). Androgen signaling upregulates expression of RAAS constituents (80), and many studies use ANG II infusion in mice with hyperlipidemia to model AAA. In studies using ApoE−/− and LDL-R−/− mice infused with ANG II, a significantly greater incidence of AAAs was observed in male mice compared with female mice (163). Similar results were reported by Henriques et al. (107), where ANG II-induced atherosclerosis was similar between male and female mice, although male mice displayed a higher incidence of AAA in addition to greater aneurysm size when compared with matched female mice. Castration of male mice reduced ANG II-induced AAA formation to that of intact female mice but exacerbated atherosclerosis (107). Ovariectomy in female mice did not significantly affect the incidence of ANG II-induced AAA compared with female mice that underwent sham operations.
Thus, while androgens are primarily responsible for the sex differences in ANG II-induced AAA, the data also suggest that androgen signaling contributes to male AAA susceptibility through mechanisms outside of the RAAS. Henriques et al. (107) quantified plasma renin concentrations in the ANG II mouse model and reported that castration did not significantly change plasma renin concentration despite decreased AAA incidence but did result in an observable decrease in ANG II receptor density in the spleen. However, the decreased receptor densities in the spleen were similar in both ANG II-infused sham and castrated mice despite the significant differences in AAA incidence observed between the two groups (107). Still, these results come from analyzing spleen tissue and androgen signaling may have differential effects on RAAS constituents via direct signaling in the vasculature. Further studies are still needed to confirm any effects of androgen signaling on RAAS constituents in this context.
Other in vivo studies have suggested that androgen signaling may predispose males to AAA by upregulating MMP activity. For instance, castration of male ApoE-deficient mice blunted the progression of ANG II-induced AAAs (301). Removal of endogenous androgens halted the expansion of the vascular lumen but had no effect on the external dimensions of aneurysms compared with male mice that underwent sham operations (301). Halting of aortic lumen expansion postcastration might be mediated by upregulation of transforming growth factor-β and PAI-1 gene expression in aortic SMCs (301). A different study found that, in female mice, the increased expression of PAI-1 attenuated developing AAA via reduced serum plasmin levels (68). Downstream effects of reduced plasmin in female mice included reduced MMP activity and differential regulation of VSMC apoptosis (68). Thus, it is possible that increased androgen signaling in males may reduce PAI-1 expression and increase MMP activity associated with degradation and remodeling of the vascular wall.
In female rodent models, administration of exogenous testosterone seems to worsen AAA outcome as well. For example, when female neonatal mice were exposed to testosterone, they were no longer protected from developing AAA in adulthood (via increased AT1a expression in abdominal aortic tissue) (302). Consequently, they suffered long-lasting, predisposing effects of androgen signaling in the context of AAA independent of serum androgens in adulthood (302). Furthermore, testosterone treatment in both female and male rats resulted in similar increased immune cell infiltration and aortic expansion, although male rats experienced more AAA rupture at earlier time points compared with female rats (52).
Although results from animal studies have increased our understanding into the pathogenesis of AAA in humans, the use of current animal models to study a largely multifactorial disease is challenging. In the elastase-induced mouse model, there is delayed medial elastin degradation with aneurysmal development apparent around 14 days postinfusion. Here, the majority of immune infiltrates consists of macrophages. The main limitation of elastase perfusion to model AAA is the involvement of mechanical stress at the aneurysm site during the perfusion process.
In contrast, ANG II infusion in ApoE−/− mice results in large suprarenal aortic aneurysms (61) by inducing inflammation in the aortic wall through AT1 receptor signaling (43), which results in accelerated macrophage recruitment and extracellular matrix degradation via MMPs (164, 234). A main advantage of this model is the RAAS involvement to induce aneurysm formation, since evidence already confirms a role of this physiological system in human aneurysms. However, a limitation to the ANG II-induced murine model of AAA is that aneurysms develop in suprarenal locations while humans usually experience infrarenal AAAs.
Large mammalian animal models, such as pigs, may be one strategy for overcoming some of these problems. In fact, there is an already established porcine model of AAA that combines balloon angioplasty and a solution of elastase/collagenase. These characteristics model those similar to humans: gradual expansion of an AAA by degrading elastic fibers, infiltrating immune cells, and VSMC loss (187). Yet, while using pigs may be more ideal for AAA studies, challenges include high costs, special requirements for housing and surgical equipment, and lower expected sample sizes. Nevertheless, large animals may be the most appropriate research models to use when translating findings to human biology.
Cerebral Aneurysms
Epidemiological studies have indicated that postmenopausal women are at greater risk than premenopausal women for subarachnoid hemorrhage (64). Furthermore, in postmenopausal women, hormone replacement therapy with estrogenic components appears to be protective against cerebral aneurysms (186). Thus, estrogen signaling may also protect women from the development and rupture of cerebral aneurysms (64, 186).
Various studies using rodent models of intracranial aneurysms support this hypothesis. In one study where female rats were subjected to common carotid artery ligation, estrogen deficiency coupled with hypertension significantly increased the risk for intracranial aneurysm (257). Here, hypertensive, ovariectomized rats displayed significant decreases in eNOS mRNA in the cerebral vascular wall (257). This resulted in endothelial dysfunction from disrupted NO bioavailability and increased NADPH oxidase subunit expression in cerebral aneurysm tissue (257). ERβ expression was increased while ERα expression was decreased in ovariectomized rats with intracranial aneurysms (257).
ERβ may mediate estrogen-dependent protection from cerebral aneurysms. For example, in an ovariectomized female mouse model of intracranial aneurysm, treatment of exogenous E2 or a selective ERβ agonist was effective in preventing rupture (256). This indicated that ERβ is the receptor subtype mediating the protective effects of estrogen signaling via increased cerebral vascular NO production (256). Another comparable study yielded similar results when using selective ER agonists and ERβ knockout mice. Here, ovariectomized wild-type mice treated with the ERβ agonist showed a significantly reduced incidence of cerebral aneurysms (255). In contrast, other ovariectomized mouse groups (ERβ knockout, treated with ERα agonist, or given E2) demonstrated no such reductions (255). Moreover, the protective effects of activated ERβ were eliminated after administration of NOS inhibitors (255). This supports that ERβ is the primary mediator of estrogenic protection from subarachnoid hemorrhage through production of bioavailable NO.
Cerebral aneurysms are more prevalent in females than males, and activating ERβ in cerebral arteries appears to be therapeutic in preventing hemorrhage. However, more studies are needed to thoroughly understand the effects of sex steroid hormone signaling on the pathogenesis of cerebral aneurysms and how this knowledge can be applied to manage this vascular disorder in patients. In particular, further research needs to be carried out on the effects of androgen signaling in cerebral aneurysms.
Migraines
Migraines and other headache-related events predominate in females (249, 251). The American Migraine Prevalence and Prevention study, which collected data on over 160,000 study participants in all age and racial groups, found a significantly higher prevalence of migraines in women compared with men (251). Although women and men reported similarities in headache pain and frequency, women reported more migraine-specific symptoms and greater disability (251). Migraines affect both sexes equally until puberty (147), when prevalence in women begins to rise. However, a reversal is usually seen once women complete menopausal transition (84). Therefore, differences in migraine prevalence between women and men after puberty may be related to the physiological effects of estrogen within the CNS.
Clinical studies mainly rely on observations of normal hormonal fluctuations and changes such as menstruation and menopause (278) as opposed to induced hormonal states using contraceptives or hormone replacement therapy (4, 158, 194). A disadvantage may be the varying pharmacological effects between different contraceptives and hormone replacement therapies, which result in inconsistent hormonal states and levels between study participants.
Sudden drops in estrogen have historically been suspected to trigger migraines (157, 245). For instance, from two studies evaluating the effects of estradiol valerate/dienogest on headaches (158, 194), the frequency of headaches and use of headache medicine were reduced in female subjects (158, 194).
Aura, the perceptual distortions that sometimes precede migraine onset (104a), is associated with higher estrogen concentrations (39). A retrospective study on 23 women demonstrated that extended cycle dosing of 15 μg ethinyl estradiol/0.120 mg etonogestrel contraceptive to maintain estrogen levels below that of a normal menstrual cycle significantly reduced aura and migraine frequency (39). Other studies where exogenous estrogenic hormones decreased migraine severity demonstrated that supplementing exogenous sex hormones can dramatically affect the frequency and severity of headaches and migraines in women.
A few genetic studies have considered the relative effects of estrogen receptor signaling on migraines. Rodriguez-Acevedo et al. (225) examined patient genetics by taking serum samples and surveys from 600 individuals (76 experiencing migraine) living on Norfolk Island, a genetically homogenous community with a high prevalence of migraines. In this study, researchers found an association between migraines and 10 markers related to the ESR1 gene that encodes for ERα (225), demonstrating that heritable differences in estrogen receptor signaling contribute to the prevalence of migraines in some populations.
Many migraine studies have used male animals as models for a predominantly female disorder (26, 211). While no animal model is able to accurately mirror pain and related symptoms that can be accurately measured in human studies, measuring vasospasm or neuronal activation in the different rat models does provide important insights into migraine pathophysiology. One study measured meningeal inflammation according to activation of dural mast cells (25), which promotes intracranial nociceptive activation. Mast cell density in female rats was remarkably higher compared with male rats except in the proestrus phase of peaked estrogen levels (25). Furthermore, dural mast cell density was greater in female rats during the estrus and diestrus phases, which is marked by decreased estrogen levels (25). Ovariectomized female rats exhibited decreased mast cell density, but increased mast cell density after exogenous estradiol was administered. This increase was time dependent, with effects diminishing after 72 h (25).
Another study evaluated estrogen’s effect on migraines using a rat model where nitroglycerin (NTG) was used to induce neuronal activation (96). During the proestrus phase of the estrus cycle where estrogen levels peak (75), female rats exhibited greater NTG-induced neuronal activation in several brain regions, such as the PVN and supraoptic nucleus of the hypothalamus, which resulted in greater pain sensitivity (96). Female rats also exhibited greater NTG-induced neuronal activation compared with intact males, implying that they experienced greater migraines (96). Ovariectomy reduced neuronal activation in the female rat brain, but when exogenous estrogen was administered, neuronal activation levels rose again to those of intact female rats (96).
Studies examining migraine in rats present conflicting results. Boes and Levy (25) found that decreases in migraine markers were associated with the proestrus phase, while Greco et al. (96), using NTG-induced neuronal activation, found that migraine in females was exacerbated during the proestrus phase. That vaginal smears were used to determine rat hormonal states instead of directly measuring serum levels of circulating sex hormones may account for the contradiction. This highlights some of the difficulties in translating animal migraine studies to humans. Unfortunately, little to no research has been carried out on the different estrogen receptors. Different contributions of the estrogen receptor subtypes in animals would certainly prove beneficial, as the varying effects of estrogen in the CNS and in regard to migraines are probably mediated by receptor-specific mechanisms.
In summary, even though migraine is a predominantly female disorder, and recent evidence suggests that female sex hormones play a large role in onset, the underlying mechanisms of how estrogen signaling exacerbates the situation remain unclear. Since expression of estrogen receptor subtypes is cell specific in the CNS (119), and estrogen signaling exerts a wide range of physiological effects throughout the body, the roles of sex hormones in migraines are probably multifactorial. Additional clinical and preclinical studies are needed to understand the cell signaling pathways and ER subtypes that contribute to the onset of migraines in women.
Stroke
Clinical and epidemiological evidence suggests that sex differences play a significant role in the physiological response to stroke in the young and elderly. Studies have shown that, compared with women, there is an ~33% higher incidence of stroke in men (14). Being male is a nonmodifiable risk factor for hemorrhagic and ischemic stroke observed not only in adulthood but also in neonatal, perinatal, and childhood populations (15, 27, 94, 95, 219). Sex disparities in ischemic stroke outcomes also exist, with women having better outcomes than men (112, 125, 238). However, the epidemiology of stroke changes for women over the age of 75 yr, when the stroke incidence increases and eventually surpasses that of age-matched men (212, 222). Elderly women also experience more severe strokes and greater disability compared with age-matched men (14, 85, 199, 226). The protective effects for premenopausal women suggest that gonadal sex hormones play significant roles in the physiological outcomes of ischemic injury.
Childhood stroke is rare but does exist. Prepubescent girls are more resistant to ischemic damage than boys (148, 166). Although androgen levels are relatively low in prepubescent children, neonatal and infant boys experience two postnatal testosterone surges (111), which may account for stroke being more common in boys than girls (152). Animal studies have yielded similar results in that, compared with age-matched female mice, neonatal male mice experience greater long-term disability such as brain volume loss and greater behavioral dysfunction after hypoxic-ischemic stroke (110, 135). Therefore, early organizational effects of sex hormones in the CNS have gained recent attention to better understand how females are protected from stroke during prenatal and early postnatal development (53).
In females, brain organization is influenced by the absence of E2, since E2 produced by the ovaries binds to α-fetoprotein and is unable to cross the blood-brain barrier (18). Sensitivity to ischemia may be at least partially molded early in life after the organizational effects of gonadal sex steroids (210). For example, neonatal male rats treated with testosterone propionate have reduced infarct sizes and increased E2 levels poststroke (210). These effects were not observed in neonatal male rats treated with nonaromatizable androgens. This suggests that increased expression of aromatase and serum E2 levels may protect against ischemic damage (210). However, these findings somewhat contradict the previous hypothesis that neonatal testosterone surges predispose males to pediatric stroke. Since conversion of testosterone to E2 seems to be protective in the vasculature and detrimental in the CNS, more research is needed to fully assess how much early developmental effects of sex hormones in the CNS affect sex predisposition and response to pediatric ischemic stroke.
Stroke outcomes vary depending on the sex and age of the animals subjected to ischemic injury. Young female rodents are more resistant to both global (100) and focal (10) ischemic damage, but they become more susceptible to ischemic insult than male rodents after 15 mo of age (69, 141, 149). The loss of circulating estrogen and gonadal senescence is possibly responsible (141).
Various studies show that estradiol dampens inflammatory responses (210, 254, 259). TNF, a proinflammatory cytokine, acts on neuronal receptors (72, 78) as well as glial cells (70) and endothelial cells (22). Even though this cytokine is intended to aid in repair, levels are dramatically elevated in stroke patients (58), and an increasing body of evidence shows that increased TNF expression has neurotoxic effects (134, 261). Therefore, anti-TNF therapies are being investigated as treatment options. Postmenopausal women display higher levels of circulating TNF (126), suggesting that estrogen is able to reduce its expression. Female rats with low estrogen levels exhibit higher TNF levels compared with controls; furthermore, physiological levels are effective at inhibiting TNF secretion (143).
Estrogen is also able to modulate the inflammatory response after stroke by interfering with NF-κB, a transcription factor that mediates inflammatory signaling in neurons and other cell types (76, 170). The full relationship between NF-κB and stroke is still not fully understood since NF-κB has a dual role of both inducing proinflammatory cytokine expression (41, 201) and cell survival signaling (59, 81). However, hypoxic conditions (229) and oxidative stress (71), both characteristic of stroke, have been shown to increase NF-κB expression. In vitro experiments have confirmed that when E2 activates ERβ, it promotes IκB-α, which is responsible for dampening NF-κB signaling (285). Administering estradiol increases the binding of E2-ER complexes at the promoter sites of the vascular inflammatory genes monochemoattractant protein (MCP)-1 and cytokine-induced neutrophil chemoattractant-2β, which interfere with NF-κB signaling (285). Thus increased estrogen signaling here may benefit by dampening subsequent inflammatory cascades (Fig. 3).
Much like estrogens, androgens’ effects may contribute to sex disparities in stroke incidence. Several studies have shown androgens as having both beneficial and detrimental effects to the CNS after ischemic insult (50, 51, 193). Even though estrogen-mediated protection is thought to contribute largely to sex differences in stroke risk, high testosterone levels in young men may also contribute. The South London Stroke register shows that, compared with age-matched women, men between the ages of 14 and 44 yr have a higher incidence of stroke (279). To date, there has been limited clinical research on the relationship between androgens and stroke. However, some evidence has shown that anabolic steroid use increases vascular tone (73). Furthermore, a young man with a history of anabolic steroid abuse showed signs of stroke and other adverse cardiovascular events (298). Even though this evidence is not from large-scale studies, it still points to high androgen levels as a predisposing factor for stroke. Surprisingly, epidemiological data have shown that the female and male incidence of stroke begin to equalize in patients past 54 yr old (222). The gradual decline of testosterone levels in men as they age might be a contributor.
Although high androgen levels may contribute to stroke risk, clinical evidence links low levels of circulating androgens to increased stroke risk in men as well (296). For example, a prospective cohort study of 22,310 prostate cancer patients revealed a higher stroke risk in men who underwent androgen deprivation therapy (16), which may predispose men to ischemic brain injury. The data conflict with other studies that have linked high testosterone levels to increased stroke risk, suggesting a physiological threshold of circulating androgen levels protects against ischemic brain injury.
Rodent models of ischemic stroke have provided some insight into the influence of androgens in CNS injury that cannot be derived from clinical observations. As previously mentioned, male rodents often exhibit greater cerebral ischemic damage compared with female rodents of the same age (165). In studies using middle cerebral artery occlusion (MCAO) to model ischemic stroke, castrated young male rats and mice had decreased infarct areas (50, 265), suggesting that endogenous androgens exacerbated ischemic brain injury. Other studies have also demonstrated that very low doses of androgens complement the reduced infarct sizes observed in castrated male animals, while high doses worsen ischemic injury (265). Under these conditions, there was a dose-dependent response to androgen replacement.
There is ample evidence that AR activation is involved in multiple signaling pathways that mediate cell death or survival. Some experimental studies have demonstrated that AR signaling can be neuroprotective (197, 213). For example, low androgen doses result in activation of the MAPK/ERK pathway to encourage cell survival (197). Further evidence suggests that androgen signaling can increase the antioxidant catalase (5) and cAMP response element-binding protein activity (198) in the CNS. In contrast, androgen signaling can oppose cell survival and therefore be detrimental when faced with ischemic CNS injury (Fig. 3). For example, testosterone is able to induce all proapoptotic p53 genes in oligodendrocytes (42). Studies using cultured hippocampal cells and oligodendrocytes have also demonstrated that exogenous testosterone exacerbates excitotoxicity by increasing glutamate-induced Ca2+ influxes (42, 83, 115). In addition, some evidence has shown that androgen signaling activates target genes in the mouse brain after MCAO that may increase inflammation and disruption of the blood-brain barrier, thereby worsening CNS damage (50).
Conflicting bodies of evidence have demonstrated that androgen signaling modulates pathways that either promote or oppose cell survival in the event of ischemic brain injury. Nevertheless, there seems to be a dose-dependent relationship between androgens and stroke outcome, as observed in both clinical and animal studies. This makes it difficult to define a concrete role for androgens in the event of ischemic stroke.
Recent advances in preclinical stem cell transplantation research have shown promising results for future treatment of ischemic stroke. More specifically, selected studies have demonstrated that intracranial human neural stem cell transplantation improves ischemic stroke outcome through multiple anti-inflammatory and neurotrophic bystander effects (74, 117). Since stem cells can either be biologically male (XY) or female (XX), the sex of stem cells used in these newly developing therapies may also play a role in their expression of sex hormone receptors and response to the host’s hormonal environment.
Unfortunately, few studies have thoroughly investigated sex-specific stem cell effects in the context of neurovascular insults between females and males. However, a few investigations have demonstrated that significant differences exist between female and male stem cell behavior. For example, stromal stem cells found in menstrual blood are biologically female and display a high proliferative rate coupled with an immature phenotype containing similar markers as embryonic cells (11). In comparison, stem cells derived from Sertoli cells of the testis demonstrate anti-inflammatory properties most likely meant to prevent immune cell rejection of germ cells (28, 232). Although many of these studies examined the intrinsic properties of stem cells and their sex for transplantation, how the biological sex of grafted stem cells would ultimately affect behavior if transplanted into different hormonal environments between female and male hosts remains to be further studied. This poses a unique opportunity for future investigations on the interplay between stem cell sex and the host hormonal environment in cell therapies for neurovascular disorders.
Using rodents in experimental stroke studies creates inherent challenges when comparing results across this area of vascular research. It is well established that stroke occurs often in humans with preexisting disorders such as hypertension and diabetes (128, 264), yet experimental studies on ischemic stroke often use young animals without similar comorbidities (50, 265). Therefore, future research on sex differences in ischemic stroke may benefit from the use of aged hypertensive and/or diabetic rodents that are already stroke prone before MCAO or other techniques used to induce brain ischemia.
Conclusions
A large body of research indicates that estrogens and androgens have a multitude of complex effects on vascular physiology that often affect females and males differently. The physiological effects of androgen signaling in the vasculature appear to be even more complex and remain poorly understood. Many clinical studies have reported higher incidences of CVD with age-related androgen decline in men, but animal studies have often demonstrated that androgen signaling contributes to cardiovascular pathogenesis. Moreover, the relationship between androgen signaling and vascular physiology in women is even less understood and has not been as thoroughly investigated. This highlights the growing need for more research focusing on different cell signaling pathways and downstream targets that are regulated through AR and the different estrogen receptor subtypes. This will be a crucial step toward understanding sex differences in the pathogenesis of vascular disorders.
GRANTS
This work was supported by Tulane University Building Interdisciplinary Research Careers in Women's Health Grant 2K12HD043451-11, Louisiana Board of Regents Support Fund-Research and Development RCS Grant LEQSF(2016–2019)-RD-A-22, and startup funds from the Department of Pharmacology, Tulane University School of Medicine.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.C.B. and S.C.K. prepared figures; A.C.B., J.-P.L., and M.H.H. drafted manuscript; A.C.B., K.-J.Y., J.-P.L., and M.H.H. edited and revised manuscript; A.C.B., S.C.K., K.-J.Y., J.-P.L., and M.H.H. approved final version of manuscript.
REFERENCES
- 1.Abela OG, Ahsan CH, Alreefi F, Salehi N, Baig I, Janoudi A, Abela GS. Plaque rupture and thrombosis: the value of the atherosclerotic rabbit model in defining the mechanism. Curr Atheroscler Rep 18: 29, 2016. doi: 10.1007/s11883-016-0587-0. [DOI] [PubMed] [Google Scholar]
- 3.Abu-Fanne R, Brzezinski A, Golomb M, Grad E, Foldes AJ, Shufaro Y, Varon D, Brill A, Lotan C, Danenberg HD. Effects of estradiol and raloxifene on arterial thrombosis in ovariectomized mice. Menopause 15: 98–104, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Aegidius K, Zwart JA, Hagen K, Schei B, Stovner LJ. Oral contraceptives and increased headache prevalence: the Head-HUNT Study. Neurology 66: 349–353, 2006. doi: 10.1212/01.wnl.0000196481.57994.09. [DOI] [PubMed] [Google Scholar]
- 5.Ahlbom E, Prins GS, Ceccatelli S. Testosterone protects cerebellar granule cells from oxidative stress-induced cell death through a receptor mediated mechanism. Brain Res 892: 255–262, 2001. doi: 10.1016/S0006-8993(00)03155-3. [DOI] [PubMed] [Google Scholar]
- 6.Ailawadi G, Eliason JL, Roelofs KJ, Sinha I, Hannawa KK, Kaldjian EP, Lu G, Henke PK, Stanley JC, Weiss SJ, Thompson RW, Upchurch GR Jr. Gender differences in experimental aortic aneurysm formation. Arterioscler Thromb Vasc Biol 24: 2116–2122, 2004. doi: 10.1161/01.ATV.0000143386.26399.84. [DOI] [PubMed] [Google Scholar]
- 7.Akishita M, Hashimoto M, Ohike Y, Ogawa S, Iijima K, Eto M, Ouchi Y. Low testosterone level is an independent determinant of endothelial dysfunction in men. Hypertens Res 30: 1029–1034, 2007. doi: 10.1291/hypres.30.1029. [DOI] [PubMed] [Google Scholar]
- 8.Alcorn HG, Wolfson SK Jr, Sutton-Tyrrell K, Kuller LH, O’Leary D. Risk factors for abdominal aortic aneurysms in older adults enrolled in the Cardiovascular Health Study. Arterioscler Thromb Vasc Biol 16: 963–970, 1996. doi: 10.1161/01.ATV.16.8.963. [DOI] [PubMed] [Google Scholar]
- 9.Alexandersen P, Haarbo J, Byrjalsen I, Lawaetz H, Christiansen C. Natural androgens inhibit male atherosclerosis: a study in castrated, cholesterol-fed rabbits. Circ Res 84: 813–819, 1999. doi: 10.1161/01.RES.84.7.813. [DOI] [PubMed] [Google Scholar]
- 10.Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD, Grady PA. Gender-linked brain injury in experimental stroke. Stroke 29: 159–165, 1998. doi: 10.1161/01.STR.29.1.159. [DOI] [PubMed] [Google Scholar]
- 11.Allickson JG, Sanchez A, Yefimenko N, Borlongan CV, Sanberg PR. Recent studies assessing the proliferative capability of a novel adult stem cell identified in menstrual blood. Open Stem Cell J 3: 4–10, 2011. doi: 10.2174/1876893801103010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aloisi AM. Gonadal hormones and sex differences in pain reactivity. Clin J Pain 19: 168–174, 2003. doi: 10.1097/00002508-200305000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Anidjar S, Dobrin PB, Chejfec G, Michel JB. Experimental study of determinants of aneurysmal expansion of the abdominal aorta. Ann Vasc Surg 8: 127–136, 1994. doi: 10.1007/BF02018860. [DOI] [PubMed] [Google Scholar]
- 14.Appelros P, Stegmayr B, Terént A. Sex differences in stroke epidemiology: a systematic review. Stroke 40: 1082–1090, 2009. doi: 10.1161/STROKEAHA.108.540781. [DOI] [PubMed] [Google Scholar]
- 15.Armstrong-Wells J, Johnston SC, Wu YW, Sidney S, Fullerton HJ. Prevalence and predictors of perinatal hemorrhagic stroke: results from the Kaiser pediatric stroke study. Pediatrics 123: 823–828, 2009. doi: 10.1542/peds.2008-0874. [DOI] [PubMed] [Google Scholar]
- 16.Azoulay L, Yin H, Benayoun S, Renoux C, Boivin JF, Suissa S. Androgen-deprivation therapy and the risk of stroke in patients with prostate cancer. Eur Urol 60: 1244–1250, 2011. doi: 10.1016/j.eururo.2011.08.041. [DOI] [PubMed] [Google Scholar]
- 17.Babich EV, Chertok VM, Kotsuba AE. Nitroxidergic neurons in nuclei of the medulla oblongata in hypertensive and normotensive rats. Bull Exp Biol Med 148: 193–195, 2009. doi: 10.1007/s10517-009-0671-3. [DOI] [PubMed] [Google Scholar]
- 18.Bakker J, De Mees C, Douhard Q, Balthazart J, Gabant P, Szpirer J, Szpirer C. Alpha-fetoprotein protects the developing female mouse brain from masculinization and defeminization by estrogens. Nat Neurosci 9: 220–226, 2006. doi: 10.1038/nn1624. [DOI] [PubMed] [Google Scholar]
- 19.Baron S, Manin M, Beaudoin C, Leotoing L, Communal Y, Veyssiere G, Morel L. Androgen receptor mediates non-genomic activation of phosphatidylinositol 3-OH kinase in androgen-sensitive epithelial cells. J Biol Chem 279: 14579–14586, 2004. doi: 10.1074/jbc.M306143200. [DOI] [PubMed] [Google Scholar]
- 20.Barton M. Cholesterol and atherosclerosis: modulation by oestrogen. Curr Opin Lipidol 24: 214–220, 2013. doi: 10.1097/MOL.0b013e3283613a94. [DOI] [PubMed] [Google Scholar]
- 21.Barton M. Position paper: the membrane estrogen receptor GPER–clues and questions. Steroids 77: 935–942, 2012. doi: 10.1016/j.steroids.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Bebo BF Jr, Linthicum DS. Expression of mRNA for 55-kDa and 75-kDa tumor necrosis factor (TNF) receptors in mouse cerebrovascular endothelium: effects of interleukin-1 beta, interferon-gamma and TNF-alpha on cultured cells. J Neuroimmunol 62: 161–167, 1995. doi: 10.1016/0165-5728(95)00113-5. [DOI] [PubMed] [Google Scholar]
- 23.Bengtsson H, Sonesson B, Bergqvist D. Incidence and prevalence of abdominal aortic aneurysms, estimated by necropsy studies and population screening by ultrasound. Ann N Y Acad Sci 800: 1–24, 1996. doi: 10.1111/j.1749-6632.1996.tb33294.x. [DOI] [PubMed] [Google Scholar]
- 24.Bentzon JF, Falk E. Atherosclerotic lesions in mouse and man: is it the same disease? Curr Opin Lipidol 21: 434–440, 2010. doi: 10.1097/MOL.0b013e32833ded6a. [DOI] [PubMed] [Google Scholar]
- 25.Boes T, Levy D. Influence of sex, estrous cycle, and estrogen on intracranial dural mast cells. Cephalalgia 32: 924–931, 2012. doi: 10.1177/0333102412454947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bolay H, Berman NE, Akcali D. Sex-related differences in animal models of migraine headache. Headache 51: 891–904, 2011. doi: 10.1111/j.1526-4610.2011.01903.x. [DOI] [PubMed] [Google Scholar]
- 27.Bonduel M, Sciuccati G, Hepner M, Pieroni G, Torres AF, Frontroth JP, Tenembaum S. Arterial ischemic stroke and cerebral venous thrombosis in children: a 12-year Argentinean registry. Acta Haematol 115: 180–185, 2006. doi: 10.1159/000090932. [DOI] [PubMed] [Google Scholar]
- 28.Borlongan CV, Cameron DF, Saporta S, Sanberg PR. Intracerebral transplantation of testis-derived sertoli cells promotes functional recovery in female rats with 6-hydroxydopamine-induced hemiparkinsonism. Exp Neurol 148: 388–392, 1997. doi: 10.1006/exnr.1997.6513. [DOI] [PubMed] [Google Scholar]
- 29.Bourghardt J, Wilhelmson AS, Alexanderson C, De Gendt K, Verhoeven G, Krettek A, Ohlsson C, Tivesten A. Androgen receptor-dependent and independent atheroprotection by testosterone in male mice. Endocrinology 151: 5428–5437, 2010. doi: 10.1210/en.2010-0663. [DOI] [PubMed] [Google Scholar]
- 30.Brosnihan KB, Hodgin JB, Smithies O, Maeda N, Gallagher P. Tissue-specific regulation of ACE/ACE2 and AT1/AT2 receptor gene expression by oestrogen in apolipoprotein E/oestrogen receptor-alpha knock-out mice. Exp Physiol 93: 658–664, 2008. doi: 10.1113/expphysiol.2007.041806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Broughton BR, Miller AA, Sobey CG. Endothelium-dependent relaxation by G protein-coupled receptor 30 agonists in rat carotid arteries. Am J Physiol Heart Circ Physiol 298: H1055–H1061, 2010. doi: 10.1152/ajpheart.00878.2009. [DOI] [PubMed] [Google Scholar]
- 32.Bruck B, Brehme U, Gugel N, Hanke S, Finking G, Lutz C, Benda N, Schmahl FW, Haasis R, Hanke H. Gender-specific differences in the effects of testosterone and estrogen on the development of atherosclerosis in rabbits. Arterioscler Thromb Vasc Biol 17: 2192–2199, 1997. doi: 10.1161/01.ATV.17.10.2192. [DOI] [PubMed] [Google Scholar]
- 33.Brunsing RL, Owens KS, Prossnitz ER. The G protein-coupled estrogen receptor (GPER) agonist G-1 expands the regulatory T-cell population under TH17-polarizing conditions. J Immunother 36: 190–196, 2013. doi: 10.1097/CJI.0b013e31828d8e3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brunsing RL, Prossnitz ER. Induction of interleukin-10 in the T helper type 17 effector population by the G protein coupled estrogen receptor (GPER) agonist G-1. Immunology 134: 93–106, 2011. doi: 10.1111/j.1365-2567.2011.03471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burleson AC, Turitto VT. Identification of quantifiable hemodynamic factors in the assessment of cerebral aneurysm behavior. On behalf of the Subcommittee on Biorheology of the Scientific and Standardization Committee of the ISTH. Thromb Haemost 76: 118–123, 1996. [PubMed] [Google Scholar]
- 36.Burns KA, Korach KS. Estrogen receptors and human disease: an update. Arch Toxicol 86: 1491–1504, 2012. doi: 10.1007/s00204-012-0868-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cairrão E, Alvarez E, Santos-Silva AJ, Verde I. Potassium channels are involved in testosterone-induced vasorelaxation of human umbilical artery. Naunyn Schmiedebergs Arch Pharmacol 376: 375–383, 2008. doi: 10.1007/s00210-007-0213-3. [DOI] [PubMed] [Google Scholar]
- 38.Calderon-Margalit R, Siscovick D, Merkin SS, Wang E, Daviglus ML, Schreiner PJ, Sternfeld B, Williams OD, Lewis CE, Azziz R, Schwartz SM, Wellons MF. Prospective association of polycystic ovary syndrome with coronary artery calcification and carotid-intima-media thickness: the Coronary Artery Risk Development in Young Adults Women’s study. Arterioscler Thromb Vasc Biol 34: 2688–2694, 2014. doi: 10.1161/ATVBAHA.114.304136. [DOI] [PubMed] [Google Scholar]
- 39.Calhoun A, Ford S, Pruitt A. The impact of extended-cycle vaginal ring contraception on migraine aura: a retrospective case series. Headache 52: 1246–1253, 2012. doi: 10.1111/j.1526-4610.2012.02211.x. [DOI] [PubMed] [Google Scholar]
- 40.Campa JS, Greenhalgh RM, Powell JT. Elastin degradation in abdominal aortic aneurysms. Atherosclerosis 65: 13–21, 1987. doi: 10.1016/0021-9150(87)90003-7. [DOI] [PubMed] [Google Scholar]
- 41.Carroll JE, Howard EF, Hess DC, Wakade CG, Chen Q, Cheng C. Nuclear factor-kappa B activation during cerebral reperfusion: effect of attenuation with N-acetylcysteine treatment. Brain Res Mol Brain Res 56: 186–191, 1998. doi: 10.1016/S0169-328X(98)00045-X. [DOI] [PubMed] [Google Scholar]
- 42.Caruso A, Di Giorgi Gerevini V, Castiglione M, Marinelli F, Tomassini V, Pozzilli C, Caricasole A, Bruno V, Caciagli F, Moretti A, Nicoletti F, Melchiorri D. Testosterone amplifies excitotoxic damage of cultured oligodendrocytes. J Neurochem 88: 1179–1185, 2004. doi: 10.1046/j.1471-4159.2004.02284.x. [DOI] [PubMed] [Google Scholar]
- 43.Cassis LA, Rateri DL, Lu H, Daugherty A. Bone marrow transplantation reveals that recipient AT1a receptors are required to initiate angiotensin II-induced atherosclerosis and aneurysms. Arterioscler Thromb Vasc Biol 27: 380–386, 2007. doi: 10.1161/01.ATV.0000254680.71485.92. [DOI] [PubMed] [Google Scholar]
- 44.Castoria G, D’Amato L, Ciociola A, Giovannelli P, Giraldi T, Sepe L, Paolella G, Barone MV, Migliaccio A, Auricchio F. Androgen-induced cell migration: role of androgen receptor/filamin A association. PLoS One 6: e17218, 2011. doi: 10.1371/journal.pone.0017218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chakrabarti S, Davidge ST. G-protein coupled receptor 30 (GPR30): a novel regulator of endothelial inflammation. PLoS One 7: e52357, 2012. doi: 10.1371/journal.pone.0052357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chambliss KL, Wu Q, Oltmann S, Konaniah ES, Umetani M, Korach KS, Thomas GD, Mineo C, Yuhanna IS, Kim SH, Madak-Erdogan Z, Maggi A, Dineen SP, Roland CL, Hui DY, Brekken RA, Katzenellenbogen JA, Katzenellenbogen BS, Shaul PW. Non-nuclear estrogen receptor alpha signaling promotes cardiovascular protection but not uterine or breast cancer growth in mice. J Clin Invest 120: 2319–2330, 2010. doi: 10.1172/JCI38291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Channer KS, Jones TH. Cardiovascular effects of testosterone: implications of the “male menopause”? Heart 89: 121–122, 2003. doi: 10.1136/heart.89.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen YF, Naftilan AJ, Oparil S. Androgen-dependent angiotensinogen and renin messenger RNA expression in hypertensive rats. Hypertension 19: 456–463, 1992. doi: 10.1161/01.HYP.19.5.456. [DOI] [PubMed] [Google Scholar]
- 49.Chen Z, Yuhanna IS, Galcheva-Gargova Z, Karas RH, Mendelsohn ME, Shaul PW. Estrogen receptor alpha mediates the nongenomic activation of endothelial nitric oxide synthase by estrogen. J Clin Invest 103: 401–406, 1999. doi: 10.1172/JCI5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheng J, Alkayed NJ, Hurn PD. Deleterious effects of dihydrotestosterone on cerebral ischemic injury. J Cereb Blood Flow Metab 27: 1553–1562, 2007. doi: 10.1038/sj.jcbfm.9600457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheng J, Uchida M, Zhang W, Grafe MR, Herson PS, Hurn PD. Role of salt-induced kinase 1 in androgen neuroprotection against cerebral ischemia. J Cereb Blood Flow Metab 31: 339–350, 2011. doi: 10.1038/jcbfm.2010.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cho BS, Woodrum DT, Roelofs KJ, Stanley JC, Henke PK, Upchurch GR Jr. Differential regulation of aortic growth in male and female rodents is associated with AAA development. J Surg Res 155: 330–338, 2009. doi: 10.1016/j.jss.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cohen-Bendahan CC, van de Beek C, Berenbaum SA. Prenatal sex hormone effects on child and adult sex-typed behavior: methods and findings. Neurosci Biobehav Rev 29: 353–384, 2005. doi: 10.1016/j.neubiorev.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 54.Colin S, Chinetti-Gbaguidi G, Staels B. Macrophage phenotypes in atherosclerosis. Immunol Rev 262: 153–166, 2014. doi: 10.1111/imr.12218. [DOI] [PubMed] [Google Scholar]
- 55.Colvin PL., Jr Estrogen increases low-density lipoprotein receptor-independent catabolism of apolipoprotein B in hyperlipidemic rabbits. Metabolism 45: 889–896, 1996. doi: 10.1016/S0026-0495(96)90165-1. [DOI] [PubMed] [Google Scholar]
- 56.Cooper SA, Whaley-Connell A, Habibi J, Wei Y, Lastra G, Manrique C, Stas S, Sowers JR. Renin-angiotensin-aldosterone system and oxidative stress in cardiovascular insulin resistance. Am J Physiol Heart Circ Physiol 293: H2009–H2023, 2007. doi: 10.1152/ajpheart.00522.2007. [DOI] [PubMed] [Google Scholar]
- 57.Cornil CA, Ball GF, Balthazart J. Functional significance of the rapid regulation of brain estrogen action: where do the estrogens come from? Brain Res 1126: 2–26, 2006. doi: 10.1016/j.brainres.2006.07.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cui G, Wang H, Li R, Zhang L, Li Z, Wang Y, Hui R, Ding H, Wang DW. Polymorphism of tumor necrosis factor alpha (TNF-alpha) gene promoter, circulating TNF-alpha level, and cardiovascular risk factor for ischemic stroke. J Neuroinflammation 9: 235, 2012. doi: 10.1186/1742-2094-9-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Culmsee C, Siewe J, Junker V, Retiounskaia M, Schwarz S, Camandola S, El-Metainy S, Behnke H, Mattson MP, Krieglstein J. Reciprocal inhibition of p53 and nuclear factor-kappaB transcriptional activities determines cell survival or death in neurons. J Neurosci 23: 8586–8595, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cybulsky MI, Iiyama K, Li H, Zhu S, Chen M, Iiyama M, Davis V, Gutierrez-Ramos JC, Connelly PW, Milstone DS. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J Clin Invest 107: 1255–1262, 2001. doi: 10.1172/JCI11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Daugherty A, Manning MW, Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest 105: 1605–1612, 2000. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davies MR, Lund RJ, Mathew S, Hruska KA. Low turnover osteodystrophy and vascular calcification are amenable to skeletal anabolism in an animal model of chronic kidney disease and the metabolic syndrome. J Am Soc Nephrol 16: 917–928, 2005. doi: 10.1681/ASN.2004100835. [DOI] [PubMed] [Google Scholar]
- 63.Davis DD, Ruiz AL, Yanes LL, Iliescu R, Yuan K, Moulana M, Racusen LC, Reckelhoff JF. Testosterone supplementation in male obese Zucker rats reduces body weight and improves insulin sensitivity but increases blood pressure. Hypertension 59: 726–731, 2012. doi: 10.1161/HYPERTENSIONAHA.111.180943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Rooij NK, Linn FH, van der Plas JA, Algra A, Rinkel GJ. Incidence of subarachnoid haemorrhage: a systematic review with emphasis on region, age, gender and time trends. J Neurol Neurosurg Psychiatry 78: 1365–1372, 2007. doi: 10.1136/jnnp.2007.117655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Death AK, McGrath KC, Sader MA, Nakhla S, Jessup W, Handelsman DJ, Celermajer DS. Dihydrotestosterone promotes vascular cell adhesion molecule-1 expression in male human endothelial cells via a nuclear factor-kappaB-dependent pathway. Endocrinology 145: 1889–1897, 2004. doi: 10.1210/en.2003-0789. [DOI] [PubMed] [Google Scholar]
- 66.Demirbag R, Yilmaz R, Ulucay A, Unlu D. The inverse relationship between thoracic aortic intima media thickness and testosterone level. Endocr Res 31: 335–344, 2005. doi: 10.1080/07435800500449494. [DOI] [PubMed] [Google Scholar]
- 67.Deroo BJ, Buensuceso AV. Minireview: Estrogen receptor-beta: mechanistic insights from recent studies. Mol Endocrinol 24: 1703–1714, 2010. doi: 10.1210/me.2009-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.DiMusto PD, Lu G, Ghosh A, Roelofs KJ, Su G, Zhao Y, Lau CL, Sadiq O, McEvoy B, Laser A, Diaz JA, Wakefield TW, Henke PK, Eliason JL, Upchurch GR Jr. Increased PAI-1 in females compared with males is protective for abdominal aortic aneurysm formation in a rodent model. Am J Physiol Heart Circ Physiol 302: H1378–H1386, 2012. doi: 10.1152/ajpheart.00620.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dinapoli VA, Benkovic SA, Li X, Kelly KA, Miller DB, Rosen CL, Huber JD, O’Callaghan JP. Age exaggerates proinflammatory cytokine signaling and truncates signal transducers and activators of transcription 3 signaling following ischemic stroke in the rat. Neuroscience 170: 633–644, 2010. doi: 10.1016/j.neuroscience.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dopp JM, Mackenzie-Graham A, Otero GC, Merrill JE. Differential expression, cytokine modulation, and specific functions of type-1 and type-2 tumor necrosis factor receptors in rat glia. J Neuroimmunol 75: 104–112, 1997. doi: 10.1016/S0165-5728(97)00009-X. [DOI] [PubMed] [Google Scholar]
- 71.Dudek EJ, Shang F, Taylor A. H(2)O(2)-mediated oxidative stress activates NF-kappa B in lens epithelial cells. Free Radic Biol Med 31: 651–658, 2001. doi: 10.1016/S0891-5849(01)00634-7. [DOI] [PubMed] [Google Scholar]
- 72.Dziewulska D, Mossakowski MJ. Cellular expression of tumor necrosis factor a and its receptors in human ischemic stroke. Clin Neuropathol 22: 35–40, 2003. [PubMed] [Google Scholar]
- 73.Ebenbichler CF, Sturm W, Gänzer H, Bodner J, Mangweth B, Ritsch A, Sandhofer A, Lechleitner M, Föger B, Patsch JR. Flow-mediated, endothelium-dependent vasodilatation is impaired in male body builders taking anabolic-androgenic steroids. Atherosclerosis 158: 483–490, 2001. doi: 10.1016/S0021-9150(01)00465-8. [DOI] [PubMed] [Google Scholar]
- 74.Eckert A, Huang L, Gonzalez R, Kim HS, Hamblin MH, Lee JP. Bystander effect fuels human induced pluripotent stem cell-derived neural stem cells to quickly attenuate early stage neurological deficits after stroke. Stem Cells Transl Med 4: 841–851, 2015. doi: 10.5966/sctm.2014-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Emanuele MA, Wezeman F, Emanuele NV. Alcohol’s effects on female reproductive function. Alcohol Res Health 26: 274–281, 2002. [PMC free article] [PubMed] [Google Scholar]
- 76.Emmanouil M, Taoufik E, Tseveleki V, Vamvakas SS, Probert L. A role for neuronal NF-κB in suppressing neuroinflammation and promoting neuroprotection in the CNS. Adv Exp Med Biol 691: 575–581, 2011. doi: 10.1007/978-1-4419-6612-4_60. [DOI] [PubMed] [Google Scholar]
- 77.Fairweather D, Petri MA, Coronado MJ, Cooper LT. Autoimmune heart disease: role of sex hormones and autoantibodies in disease pathogenesis. Expert Rev Clin Immunol 8: 269–284, 2012. doi: 10.1586/eci.12.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Figiel I, Dzwonek K. TNFalpha and TNF receptor 1 expression in the mixed neuronal-glial cultures of hippocampal dentate gyrus exposed to glutamate or trimethyltin. Brain Res 1131: 17–28, 2007. doi: 10.1016/j.brainres.2006.10.095. [DOI] [PubMed] [Google Scholar]
- 79.Filardo EJ, Thomas P. Minireview: G protein-coupled estrogen receptor-1, GPER-1: its mechanism of action and role in female reproductive cancer, renal and vascular physiology. Endocrinology 153: 2953–2962, 2012. doi: 10.1210/en.2012-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fischer M, Baessler A, Schunkert H. Renin angiotensin system and gender differences in the cardiovascular system. Cardiovasc Res 53: 672–677, 2002. doi: 10.1016/S0008-6363(01)00479-5. [DOI] [PubMed] [Google Scholar]
- 81.Foehr ED, Lin X, O’Mahony A, Geleziunas R, Bradshaw RA, Greene WC. NF-kappa B signaling promotes both cell survival and neurite process formation in nerve growth factor-stimulated PC12 cells. J Neurosci 20: 7556–7563, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Foradori CD, Weiser MJ, Handa RJ. Non-genomic actions of androgens. Front Neuroendocrinol 29: 169–181, 2008. doi: 10.1016/j.yfrne.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Foradori CD, Werner SB, Sandau US, Clapp TR, Handa RJ. Activation of the androgen receptor alters the intracellular calcium response to glutamate in primary hippocampal neurons and modulates sarco/endoplasmic reticulum calcium ATPase 2 transcription. Neuroscience 149: 155–164, 2007. doi: 10.1016/j.neuroscience.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 84.Freeman EW, Sammel MD, Lin H, Gracia CR, Kapoor S. Symptoms in the menopausal transition: hormone and behavioral correlates. Obstet Gynecol 111: 127–136, 2008. doi: 10.1097/01.AOG.0000295867.06184.b1. [DOI] [PubMed] [Google Scholar]
- 85.Fukuda M, Kanda T, Kamide N, Akutsu T, Sakai F. Gender differences in long-term functional outcome after first-ever ischemic stroke. Intern Med 48: 967–973, 2009. doi: 10.2169/internalmedicine.48.1757. [DOI] [PubMed] [Google Scholar]
- 86.Gardner G, Banka CL, Roberts KA, Mullick AE, Rutledge JC. Modified LDL-mediated increases in endothelial layer permeability are attenuated with 17 beta-estradiol. Arterioscler Thromb Vasc Biol 19: 854–861, 1999. doi: 10.1161/01.ATV.19.4.854. [DOI] [PubMed] [Google Scholar]
- 87.Georget V, Térouanne B, Nicolas JC, Sultan C. Mechanism of antiandrogen action: key role of hsp90 in conformational change and transcriptional activity of the androgen receptor. Biochemistry 41: 11824–11831, 2002. doi: 10.1021/bi0259150. [DOI] [PubMed] [Google Scholar]
- 88.Geraldes P, Gagnon S, Hadjadj S, Merhi Y, Sirois MG, Cloutier I, Tanguay JF. Estradiol blocks the induction of CD40 and CD40L expression on endothelial cells and prevents neutrophil adhesion: an ERalpha-mediated pathway. Cardiovasc Res 71: 566–573, 2006. doi: 10.1016/j.cardiores.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 89.Ghosh A, Lu G, Su G, McEvoy B, Sadiq O, DiMusto PD, Laser A, Futchko JS, Henke PK, Eliason JL, Upchurch GR Jr. Phosphorylation of AKT and abdominal aortic aneurysm formation. Am J Pathol 184: 148–158, 2014. doi: 10.1016/j.ajpath.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gilliver SC. Sex steroids as inflammatory regulators. J Steroid Biochem Mol Biol 120: 105–115, 2010. doi: 10.1016/j.jsbmb.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 91.Gingerich S, Krukoff TL. Estrogen in the paraventricular nucleus attenuates L-glutamate-induced increases in mean arterial pressure through estrogen receptor beta and NO. Hypertension 48: 1130–1136, 2006. doi: 10.1161/01.HYP.0000248754.67128.ff. [DOI] [PubMed] [Google Scholar]
- 92.Glass CK, Rosenfeld MG. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev 14: 121–141, 2000. [PubMed] [Google Scholar]
- 93.Goldstein JL, Brown MS. The LDL receptor defect in familial hypercholesterolemia. Implications for pathogenesis and therapy. Med Clin North Am 66: 335–362, 1982. doi: 10.1016/S0025-7125(16)31424-9. [DOI] [PubMed] [Google Scholar]
- 94.Golomb MR, Dick PT, MacGregor DL, Curtis R, Sofronas M, deVeber GA. Neonatal arterial ischemic stroke and cerebral sinovenous thrombosis are more commonly diagnosed in boys. J Child Neurol 19: 493–497, 2004. doi: 10.1177/08830738040190070301. [DOI] [PubMed] [Google Scholar]
- 95.Golomb MR, Fullerton HJ, Nowak-Gottl U, Deveber G; International Pediatric Stroke Study Group . Male predominance in childhood ischemic stroke: findings from the international pediatric stroke study. Stroke 40: 52–57, 2009. doi: 10.1161/STROKEAHA.108.521203. [DOI] [PubMed] [Google Scholar]
- 96.Greco R, Tassorelli C, Mangione AS, Smeraldi A, Allena M, Sandrini G, Nappi G, Nappi RE. Effect of sex and estrogens on neuronal activation in an animal model of migraine. Headache 53: 288–296, 2013. doi: 10.1111/j.1526-4610.2012.02249.x. [DOI] [PubMed] [Google Scholar]
- 97.Gros R, Ding Q, Liu B, Chorazyczewski J, Feldman RD. Aldosterone mediates its rapid effects in vascular endothelial cells through GPER activation. Am J Physiol Cell Physiol 304: C532–C540, 2013. doi: 10.1152/ajpcell.00203.2012. [DOI] [PubMed] [Google Scholar]
- 98.Haas E, Bhattacharya I, Brailoiu E, Damjanović M, Brailoiu GC, Gao X, Mueller-Guerre L, Marjon NA, Gut A, Minotti R, Meyer MR, Amann K, Ammann E, Perez-Dominguez A, Genoni M, Clegg DJ, Dun NJ, Resta TC, Prossnitz ER, Barton M. Regulatory role of G protein-coupled estrogen receptor for vascular function and obesity. Circ Res 104: 288–291, 2009. doi: 10.1161/CIRCRESAHA.108.190892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Haas E, Meyer MR, Schurr U, Bhattacharya I, Minotti R, Nguyen HH, Heigl A, Lachat M, Genoni M, Barton M. Differential effects of 17beta-estradiol on function and expression of estrogen receptor alpha, estrogen receptor beta, and GPR30 in arteries and veins of patients with atherosclerosis. Hypertension 49: 1358–1363, 2007. doi: 10.1161/HYPERTENSIONAHA.107.089995. [DOI] [PubMed] [Google Scholar]
- 100.Hall ED, Pazara KE, Linseman KL. Sex differences in postischemic neuronal necrosis in gerbils. J Cereb Blood Flow Metab 11: 292–298, 1991. doi: 10.1038/jcbfm.1991.61. [DOI] [PubMed] [Google Scholar]
- 101.Hall J, Jones RD, Jones TH, Channer KS, Peers C. Selective inhibition of L-type Ca2+ channels in A7r5 cells by physiological levels of testosterone. Endocrinology 147: 2675–2680, 2006. doi: 10.1210/en.2005-1243. [DOI] [PubMed] [Google Scholar]
- 102.Hammes SR, Levin ER. Extranuclear steroid receptors: nature and actions. Endocr Rev 28: 726–741, 2007. doi: 10.1210/er.2007-0022. [DOI] [PubMed] [Google Scholar]
- 103.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol 12: 204–212, 2011. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 104.Haring R, Travison TG, Bhasin S, Vasan RS, Wallaschofski H, Davda MN, Coviello A, Murabito JM. Relation between sex hormone concentrations, peripheral arterial disease, and change in ankle-brachial index: findings from the Framingham Heart Study. J Clin Endocrinol Metab 96: 3724–3732, 2011. doi: 10.1210/jc.2011-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104a.Headache Classification Subcommittee of the International Headache Society The International Classification of Headache Disorders: 2nd edition. Cephalalgia 24, Suppl 1: 9–160, 2004. [DOI] [PubMed] [Google Scholar]
- 105.Heinlein CA, Chang C. Androgen receptor (AR) coregulators: an overview. Endocr Rev 23: 175–200, 2002. doi: 10.1210/edrv.23.2.0460. [DOI] [PubMed] [Google Scholar]
- 106.Heinlein CA, Chang C. The roles of androgen receptors and androgen-binding proteins in nongenomic androgen actions. Mol Endocrinol 16: 2181–2187, 2002. doi: 10.1210/me.2002-0070. [DOI] [PubMed] [Google Scholar]
- 107.Henriques TA, Huang J, D’Souza SS, Daugherty A, Cassis LA. Orchidectomy, but not ovariectomy, regulates angiotensin II-induced vascular diseases in apolipoprotein E-deficient mice. Endocrinology 145: 3866–3872, 2004. doi: 10.1210/en.2003-1615. [DOI] [PubMed] [Google Scholar]
- 108.Hernandez Schulman I, Raij L. Salt sensitivity and hypertension after menopause: role of nitric oxide and angiotensin II. Am J Nephrol 26: 170–180, 2006. doi: 10.1159/000092984. [DOI] [PubMed] [Google Scholar]
- 109.Higashiura K, Mathur RS, Halushka PV. Gender-related differences in androgen regulation of thromboxane A2 receptors in rat aortic smooth-muscle cells. J Cardiovasc Pharmacol 29: 311–315, 1997. doi: 10.1097/00005344-199703000-00002. [DOI] [PubMed] [Google Scholar]
- 110.Hill CA, Fitch RH. Sex differences in mechanisms and outcome of neonatal hypoxia-ischemia in rodent models: implications for sex-specific neuroprotection in clinical neonatal practice. Neurol Res Int 2012: 867531, 2012. doi: 10.1155/2012/867531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hines M. Early androgen influences on human neural and behavioural development. Early Hum Dev 84: 805–807, 2008. doi: 10.1016/j.earlhumdev.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hintz SR, Kendrick DE, Vohr BR, Kenneth Poole W, Higgins RD; NICHD Neonatal Research Network . Gender differences in neurodevelopmental outcomes among extremely preterm, extremely-low-birthweight infants. Acta Paediatr 95: 1239–1248, 2006. doi: 10.1080/08035250600599727. [DOI] [PubMed] [Google Scholar]
- 113.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM Jr, White CJ, White J, White RA, Antman EM, Smith SC Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B; American Association for Vascular Surgery/Society for Vascular Surgery; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Society of Interventional Radiology; ACC/AHA Task Force on Practice Guidelines Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease; American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; Vascular Disease Foundation . ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 113: e463–e654, 2006. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 114.Hodgin JB, Krege JH, Reddick RL, Korach KS, Smithies O, Maeda N. Estrogen receptor alpha is a major mediator of 17beta-estradiol’s atheroprotective effects on lesion size in Apoe−/− mice. J Clin Invest 107: 333–340, 2001. doi: 10.1172/JCI11320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Holmes S, Abbassi B, Su C, Singh M, Cunningham RL. Oxidative stress defines the neuroprotective or neurotoxic properties of androgens in immortalized female rat dopaminergic neuronal cells. Endocrinology 154: 4281–4292, 2013. doi: 10.1210/en.2013-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hu W, Polinsky P, Sadoun E, Rosenfeld ME, Schwartz SM. Atherosclerotic lesions in the common coronary arteries of ApoE knockout mice. Cardiovasc Pathol 14: 120–125, 2005. doi: 10.1016/j.carpath.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 117.Huang L, Wong S, Snyder EY, Hamblin MH, Lee JP. Human neural stem cells rapidly ameliorate symptomatic inflammation in early-stage ischemic-reperfusion cerebral injury. Stem Cell Res Ther 5: 129, 2014. doi: 10.1186/scrt519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ingelfinger JR. Angiotensin-converting enzyme 2: implications for blood pressure and kidney disease. Curr Opin Nephrol Hypertens 18: 79–84, 2009. doi: 10.1097/MNH.0b013e32831b70ad. [DOI] [PubMed] [Google Scholar]
- 119.Ishunina TA, Sluiter AA, Swaab DF, Verwer RW. Transcriptional activity of human brain estrogen receptor-α splice variants: evidence for cell type-specific regulation. Brain Res 1500: 1–9, 2013. doi: 10.1016/j.brainres.2012.12.050. [DOI] [PubMed] [Google Scholar]
- 120.Iwakura A, Luedemann C, Shastry S, Hanley A, Kearney M, Aikawa R, Isner JM, Asahara T, Losordo DW. Estrogen-mediated, endothelial nitric oxide synthase-dependent mobilization of bone marrow-derived endothelial progenitor cells contributes to reendothelialization after arterial injury. Circulation 108: 3115–3121, 2003. doi: 10.1161/01.CIR.0000106906.56972.83. [DOI] [PubMed] [Google Scholar]
- 121.Iwakura A, Shastry S, Luedemann C, Hamada H, Kawamoto A, Kishore R, Zhu Y, Qin G, Silver M, Thorne T, Eaton L, Masuda H, Asahara T, Losordo DW. Estradiol enhances recovery after myocardial infarction by augmenting incorporation of bone marrow-derived endothelial progenitor cells into sites of ischemia-induced neovascularization via endothelial nitric oxide synthase-mediated activation of matrix metalloproteinase-9. Circulation 113: 1605–1614, 2006. doi: 10.1161/CIRCULATIONAHA.105.553925. [DOI] [PubMed] [Google Scholar]
- 122.Jackson EA, Munir K, Schreiber T, Rubin JR, Cuff R, Gallagher KA, Henke PK, Gurm HS, Grossman PM. Impact of sex on morbidity and mortality rates after lower extremity interventions for peripheral arterial disease: observations from the Blue Cross Blue Shield of Michigan Cardiovascular Consortium. J Am Coll Cardiol 63: 2525–2530, 2014. doi: 10.1016/j.jacc.2014.03.036. [DOI] [PubMed] [Google Scholar]
- 123.Ji H, Menini S, Zheng W, Pesce C, Wu X, Sandberg K. Role of angiotensin-converting enzyme 2 and angiotensin(1-7) in 17beta-oestradiol regulation of renal pathology in renal wrap hypertension in rats. Exp Physiol 93: 648–657, 2008. doi: 10.1113/expphysiol.2007.041392. [DOI] [PubMed] [Google Scholar]
- 124.Jin HJ, Kim J, Yu J. Androgen receptor genomic regulation. Transl Androl Urol 2: 157–177, 2013. doi: 10.3978/j.issn.2223-4683.2013.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Johnston MV, Hagberg H. Sex and the pathogenesis of cerebral palsy. Dev Med Child Neurol 49: 74–78, 2007. doi: 10.1017/S0012162207000199.x. [DOI] [PubMed] [Google Scholar]
- 126.Kamada M, Irahara M, Maegawa M, Ohmoto Y, Takeji T, Yasui T, Aono T. Postmenopausal changes in serum cytokine levels and hormone replacement therapy. Am J Obstet Gynecol 184: 309–314, 2001. doi: 10.1067/mob.2001.109940. [DOI] [PubMed] [Google Scholar]
- 127.Khaw KT, Dowsett M, Folkerd E, Bingham S, Wareham N, Luben R, Welch A, Day N. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European prospective investigation into cancer in Norfolk (EPIC-Norfolk) Prospective Population Study. Circulation 116: 2694–2701, 2007. doi: 10.1161/CIRCULATIONAHA.107.719005. [DOI] [PubMed] [Google Scholar]
- 128.Khoury JC, Kleindorfer D, Alwell K, Moomaw CJ, Woo D, Adeoye O, Flaherty ML, Khatri P, Ferioli S, Broderick JP, Kissela BM. Diabetes mellitus: a risk factor for ischemic stroke in a large biracial population. Stroke 44: 1500–1504, 2013. doi: 10.1161/STROKEAHA.113.001318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kienitz T, Quinkler M. Testosterone and blood pressure regulation. Kidney Blood Press Res 31: 71–79, 2008. doi: 10.1159/000119417. [DOI] [PubMed] [Google Scholar]
- 130.Kim KH, Moriarty K, Bender JR. Vascular cell signaling by membrane estrogen receptors. Steroids 73: 864–869, 2008. doi: 10.1016/j.steroids.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Knopp RH, Paramsothy P, Retzlaff BM, Fish B, Walden C, Dowdy A, Tsunehara C, Aikawa K, Cheung MC. Sex differences in lipoprotein metabolism and dietary response: basis in hormonal differences and implications for cardiovascular disease. Curr Cardiol Rep 8: 452–459, 2006. doi: 10.1007/s11886-006-0104-0. [DOI] [PubMed] [Google Scholar]
- 132.Koizumi H, Yu J, Hashimoto R, Ouchi Y, Okabe T. Involvement of androgen receptor in nitric oxide production induced by icariin in human umbilical vein endothelial cells. FEBS Lett 584: 2440–2444, 2010. doi: 10.1016/j.febslet.2010.04.049. [DOI] [PubMed] [Google Scholar]
- 133.Komukai K, Mochizuki S, Yoshimura M. Gender and the renin-angiotensin-aldosterone system. Fundam Clin Pharmacol 24: 687–698, 2010. doi: 10.1111/j.1472-8206.2010.00854.x. [DOI] [PubMed] [Google Scholar]
- 134.Lambertsen KL, Biber K, Finsen B. Inflammatory cytokines in experimental and human stroke. J Cereb Blood Flow Metab 32: 1677–1698, 2012. doi: 10.1038/jcbfm.2012.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lan WC, Priestley M, Mayoral SR, Tian L, Shamloo M, Penn AA. Sex-specific cognitive deficits and regional brain volume loss in mice exposed to chronic, sublethal hypoxia. Pediatr Res 70: 15–20, 2011. doi: 10.1203/PDR.0b013e31821b98a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Laser A, Ghosh A, Roelofs K, Sadiq O, McEvoy B, DiMusto P, Eliason J, Upchurch GR Jr. Increased estrogen receptor alpha expression in experimental aortic aneurysms in females compared with males. J Surg Res 186: 467–474, 2014. doi: 10.1016/j.jss.2013.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab 93: 68–75, 2008. doi: 10.1210/jc.2007-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lavoie JL, Sigmund CD. Minireview: overview of the renin-angiotensin system–an endocrine and paracrine system. Endocrinology 144: 2179–2183, 2003. doi: 10.1210/en.2003-0150. [DOI] [PubMed] [Google Scholar]
- 139.Lee JK, Borhani M, Ennis TL, Upchurch GR Jr, Thompson RW. Experimental abdominal aortic aneurysms in mice lacking expression of inducible nitric oxide synthase. Arterioscler Thromb Vasc Biol 21: 1393–1401, 2001. doi: 10.1161/hq0901.095750. [DOI] [PubMed] [Google Scholar]
- 140.Levin ER. G protein-coupled receptor 30: estrogen receptor or collaborator? Endocrinology 150: 1563–1565, 2009. doi: 10.1210/en.2008-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lewis DK, Thomas KT, Selvamani A, Sohrabji F. Age-related severity of focal ischemia in female rats is associated with impaired astrocyte function. Neurobiol Aging 33: 1123.e1–1123.e16, 2012. doi: 10.1016/j.neurobiolaging.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li F, Yu X, Szynkarski CK, Meng C, Zhou B, Barhoumi R, White RE, Heaps CL, Stallone JN, Han G. Activation of GPER induces differentiation and inhibition of coronary artery smooth muscle cell proliferation. PLoS One 8: e64771, 2013. doi: 10.1371/journal.pone.0064771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Liao SL, Chen WY, Chen CJ. Estrogen attenuates tumor necrosis factor-alpha expression to provide ischemic neuroprotection in female rats. Neurosci Lett 330: 159–162, 2002. doi: 10.1016/S0304-3940(02)00754-1. [DOI] [PubMed] [Google Scholar]
- 144.Libby P. Current concepts of the pathogenesis of the acute coronary syndromes. Circulation 104: 365–372, 2001. doi: 10.1161/01.CIR.104.3.365. [DOI] [PubMed] [Google Scholar]
- 145.Lindsey SH, Carver KA, Prossnitz ER, Chappell MC. Vasodilation in response to the GPR30 agonist G-1 is not different from estradiol in the mRen2.Lewis female rat. J Cardiovasc Pharmacol 57: 598–603, 2011. doi: 10.1097/FJC.0b013e3182135f1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lindsey SH, Cohen JA, Brosnihan KB, Gallagher PE, Chappell MC. Chronic treatment with the G protein-coupled receptor 30 agonist G-1 decreases blood pressure in ovariectomized mRen2.Lewis rats. Endocrinology 150: 3753–3758, 2009. doi: 10.1210/en.2008-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lipton RB, Bigal ME. Migraine: epidemiology, impact, and risk factors for progression. Headache 45, Suppl 1: S3–S13, 2005. doi: 10.1111/j.1526-4610.2005.4501001.x. [DOI] [PubMed] [Google Scholar]
- 148.Liu F, McCullough LD. Interactions between age, sex, and hormones in experimental ischemic stroke. Neurochem Int 61: 1255–1265, 2012. doi: 10.1016/j.neuint.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Liu F, Yuan R, Benashski SE, McCullough LD. Changes in experimental stroke outcome across the life span. J Cereb Blood Flow Metab 29: 792–802, 2009. doi: 10.1038/jcbfm.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Liu J, Ji H, Zheng W, Wu X, Zhu JJ, Arnold AP, Sandberg K. Sex differences in renal angiotensin converting enzyme 2 (ACE2) activity are 17β-oestradiol-dependent and sex chromosome-independent. Biol Sex Differ 1: 6, 2010. doi: 10.1186/2042-6410-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Liu PY, Death AK, Handelsman DJ. Androgens and cardiovascular disease. Endocr Rev 24: 313–340, 2003. doi: 10.1210/er.2003-0005. [DOI] [PubMed] [Google Scholar]
- 152.Lo W, Stephens J, Fernandez S. Pediatric stroke in the United States and the impact of risk factors. J Child Neurol 24: 194–203, 2009. doi: 10.1177/0883073808322665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Losordo DW, Kearney M, Kim EA, Jekanowski J, Isner JM. Variable expression of the estrogen receptor in normal and atherosclerotic coronary arteries of premenopausal women. Circulation 89: 1501–1510, 1994. doi: 10.1161/01.CIR.89.4.1501. [DOI] [PubMed] [Google Scholar]
- 154.Loy CJ, Sim KS, Yong EL. Filamin-A fragment localizes to the nucleus to regulate androgen receptor and coactivator functions. Proc Natl Acad Sci USA 100: 4562–4567, 2003. doi: 10.1073/pnas.0736237100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lu ML, Schneider MC, Zheng Y, Zhang X, Richie JP. Caveolin-1 interacts with androgen receptor. A positive modulator of androgen receptor mediated transactivation. J Biol Chem 276: 13442–13451, 2001. doi: 10.1074/jbc.M006598200. [DOI] [PubMed] [Google Scholar]
- 156.Lu Q, Pallas DC, Surks HK, Baur WE, Mendelsohn ME, Karas RH. Striatin assembles a membrane signaling complex necessary for rapid, nongenomic activation of endothelial NO synthase by estrogen receptor alpha. Proc Natl Acad Sci USA 101: 17126–17131, 2004. doi: 10.1073/pnas.0407492101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.MacGregor EA, Frith A, Ellis J, Aspinall L, Hackshaw A. Incidence of migraine relative to menstrual cycle phases of rising and falling estrogen. Neurology 67: 2154–2158, 2006. doi: 10.1212/01.wnl.0000233888.18228.19. [DOI] [PubMed] [Google Scholar]
- 158.Macìas G, Merki-Feld GS, Parke S, Mellinger U, Serrani M. Effects of a combined oral contraceptive containing oestradiol valerate/dienogest on hormone withdrawal-associated symptoms: results from the multicentre, randomised, double-blind, active-controlled HARMONY II study. J Obstet Gynaecol 33: 591–596, 2013. doi: 10.3109/01443615.2013.800851. [DOI] [PubMed] [Google Scholar]
- 159.Maggio M, Cattabiani C, Lauretani F, Artoni A, Bandinelli S, Schiavi G, Vignali A, Volpi R, Ceresini G, Lippi G, Aloe R, De Vita F, Giallauria F, McDermott MM, Ferrucci L, Ceda GP. The relationship between sex hormones, sex hormone binding globulin and peripheral artery disease in older persons. Atherosclerosis 225: 469–474, 2012. doi: 10.1016/j.atherosclerosis.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mahmoodzadeh S, Leber J, Zhang X, Jaisser F, Messaoudi S, Morano I, Furth PA, Dworatzek E, Regitz-Zagrosek V. Cardiomyocyte-specific estrogen receptor alpha increases angiogenesis, lymphangiogenesis and reduces fibrosis in the female mouse heart post-myocardial infarction. J Cell Sci Ther 5: 153, 2014. doi: 10.4172/2157-7013.1000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mäkinen J, Järvisalo MJ, Pöllänen P, Perheentupa A, Irjala K, Koskenvuo M, Mäkinen J, Huhtaniemi I, Raitakari OT. Increased carotid atherosclerosis in andropausal middle-aged men. J Am Coll Cardiol 45: 1603–1608, 2005. doi: 10.1016/j.jacc.2005.01.052. [DOI] [PubMed] [Google Scholar]
- 162.Malan NT, Smith W, von Känel R, Hamer M, Schutte AE, Malan L. Low serum testosterone and increased diastolic ocular perfusion pressure: a risk for retinal microvasculature. Vasa 44: 435–443, 2015. doi: 10.1024/0301-1526/a000466. [DOI] [PubMed] [Google Scholar]
- 163.Manning MW, Cassi LA, Huang J, Szilvassy SJ, Daugherty A. Abdominal aortic aneurysms: fresh insights from a novel animal model of the disease. Vasc Med 7: 45–54, 2002. doi: 10.1191/1358863x02vm413ra. [DOI] [PubMed] [Google Scholar]
- 164.Manning MW, Cassis LA, Daugherty A. Differential effects of doxycycline, a broad-spectrum matrix metalloproteinase inhibitor, on angiotensin II-induced atherosclerosis and abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol 23: 483–488, 2003. doi: 10.1161/01.ATV.0000058404.92759.32. [DOI] [PubMed] [Google Scholar]
- 165.Manwani B, Liu F, Scranton V, Hammond MD, Sansing LH, McCullough LD. Differential effects of aging and sex on stroke induced inflammation across the lifespan. Exp Neurol 249: 120–131, 2013. doi: 10.1016/j.expneurol.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Manwani B, McCullough LD. Estrogen in ischaemic stroke: the debate continues. Eur J Neurol 19: 1276–1277, 2012. doi: 10.1111/j.1468-1331.2012.03746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mårin P, Holmäng S, Gustafsson C, Jönsson L, Kvist H, Elander A, Eldh J, Sjöström L, Holm G, Björntorp P. Androgen treatment of abdominally obese men. Obes Res 1: 245–251, 1993. doi: 10.1002/j.1550-8528.1993.tb00618.x. [DOI] [PubMed] [Google Scholar]
- 168.Massart F, Marini F, Menegato A, Del Monte F, Nuti M, Butitta F, Ferrari M, Balbarini A, Brandi ML. Allelic genes involved in artery compliance and susceptibility to sporadic abdominal aortic aneurysm. J Steroid Biochem Mol Biol 92: 413–418, 2004. doi: 10.1016/j.jsbmb.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 169.Matsubara K, Harada H, Ando N, Watada S, Obara H, Matsumoto K, Kitagawa Y. Estrogen deficiency attenuates neovascularization in a murine model of hindlimb ischemia. J Surg Res 178: 1022–1028, 2012. doi: 10.1016/j.jss.2012.04.067. [DOI] [PubMed] [Google Scholar]
- 170.Matthews JR, Hay RT. Regulation of the DNA binding activity of NF-kappa B. Int J Biochem Cell Biol 27: 865–879, 1995. doi: 10.1016/1357-2725(95)00071-V. [DOI] [PubMed] [Google Scholar]
- 171.McCrohon JA, Death AK, Nakhla S, Jessup W, Handelsman DJ, Stanley KK, Celermajer DS. Androgen receptor expression is greater in macrophages from male than from female donors. A sex difference with implications for atherogenesis. Circulation 101: 224–226, 2000. doi: 10.1161/01.CIR.101.3.224. [DOI] [PubMed] [Google Scholar]
- 172.McCrohon JA, Jessup W, Handelsman DJ, Celermajer DS. Androgen exposure increases human monocyte adhesion to vascular endothelium and endothelial cell expression of vascular cell adhesion molecule-1. Circulation 99: 2317–2322, 1999. doi: 10.1161/01.CIR.99.17.2317. [DOI] [PubMed] [Google Scholar]
- 173.McGuire BB, Watson RW, Pérez-Barriocanal F, Fitzpatrick JM, Docherty NG. Gender differences in the renin-angiotensin and nitric oxide systems: relevance in the normal and diseased kidney. Kidney Blood Press Res 30: 67–80, 2007. doi: 10.1159/000099150. [DOI] [PubMed] [Google Scholar]
- 174.McKinley MJ, Albiston AL, Allen AM, Mathai ML, May CN, McAllen RM, Oldfield BJ, Mendelsohn FA, Chai SY. The brain renin-angiotensin system: location and physiological roles. Int J Biochem Cell Biol 35: 901–918, 2003. doi: 10.1016/S1357-2725(02)00306-0. [DOI] [PubMed] [Google Scholar]
- 175.Mendelsohn ME. Genomic and nongenomic effects of estrogen in the vasculature. Am J Cardiol 90, 1A: 3F–6F, 2002. doi: 10.1016/S0002-9149(02)02418-9. [DOI] [PubMed] [Google Scholar]
- 176.Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science 308: 1583–1587, 2005. doi: 10.1126/science.1112062. [DOI] [PubMed] [Google Scholar]
- 177.Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med 340: 1801–1811, 1999. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- 178.Mendelsohn ME, Karas RH. Rapid progress for non-nuclear estrogen receptor signaling. J Clin Invest 120: 2277–2279, 2010. doi: 10.1172/JCI43756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Meyer MR, Amann K, Field AS, Hu C, Hathaway HJ, Kanagy NL, Walker MK, Barton M, Prossnitz ER. Deletion of G protein-coupled estrogen receptor increases endothelial vasoconstriction. Hypertension 59: 507–512, 2012. doi: 10.1161/HYPERTENSIONAHA.111.184606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Meyer MR, Baretella O, Prossnitz ER, Barton M. Dilation of epicardial coronary arteries by the G protein-coupled estrogen receptor agonists G-1 and ICI 182,780. Pharmacology 86: 58–64, 2010. doi: 10.1159/000315497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Meyer MR, Barton M. ERalpha, ERbeta, and gpER: novel aspects of oestrogen receptor signalling in atherosclerosis. Cardiovasc Res 83: 605–610, 2009. doi: 10.1093/cvr/cvp187. [DOI] [PubMed] [Google Scholar]
- 182.Meyer MR, Fredette NC, Howard TA, Hu C, Ramesh C, Daniel C, Amann K, Arterburn JB, Barton M, Prossnitz ER. G protein-coupled estrogen receptor protects from atherosclerosis. Sci Rep 4: 7564, 2014. doi: 10.1038/srep07564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Meyer MR, Haas E, Barton M. Need for research on estrogen receptor function: importance for postmenopausal hormone therapy and atherosclerosis. Gend Med 5, Suppl A: S19–S33, 2008. doi: 10.1016/j.genm.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 185.Meyer MR, Prossnitz ER, Barton M. The G protein-coupled estrogen receptor GPER/GPR30 as a regulator of cardiovascular function. Vascul Pharmacol 55: 17–25, 2011. doi: 10.1016/j.vph.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Mhurchu CN, Anderson C, Jamrozik K, Hankey G, Dunbabin D, Longstreth WT, Nelson LM; Australasian Cooperative Research on Subarachnoid Hemorrhage Study (ACROSS) Group . Hormonal factors and risk of aneurysmal subarachnoid hemorrhage: an international population-based, case-control study. Stroke 32: 606–612, 2001. doi: 10.1161/01.STR.32.3.606. [DOI] [PubMed] [Google Scholar]
- 187.Molácek J, Treska V, Kobr J, Certík B, Skalický T, Kuntscher V, Krízková V. Optimization of the model of abdominal aortic aneurysm–experiment in an animal model. J Vasc Res 46: 1–5, 2009. doi: 10.1159/000135659. [DOI] [PubMed] [Google Scholar]
- 188.Montaño LM, Calixto E, Figueroa A, Flores-Soto E, Carbajal V, Perusquía M. Relaxation of androgens on rat thoracic aorta: testosterone concentration dependent agonist/antagonist L-type Ca2+ channel activity, and 5beta-dihydrotestosterone restricted to L-type Ca2+ channel blockade. Endocrinology 149: 2517–2526, 2008. doi: 10.1210/en.2007-1288. [DOI] [PubMed] [Google Scholar]
- 189.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB; Writing Group Members; American Heart Association Statistics Committee; Stroke Statistics Subcommittee . Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation 133: e38–e360, 2016. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 190.Muller M, van den Beld AW, Bots ML, Grobbee DE, Lamberts SW, van der Schouw YT. Endogenous sex hormones and progression of carotid atherosclerosis in elderly men. Circulation 109: 2074–2079, 2004. doi: 10.1161/01.CIR.0000125854.51637.06. [DOI] [PubMed] [Google Scholar]
- 191.Murphy E. Estrogen signaling and cardiovascular disease. Circ Res 109: 687–696, 2011. doi: 10.1161/CIRCRESAHA.110.236687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Nakagawa K, Marji JS, Schwartzman ML, Waterman MR, Capdevila JH. Androgen-mediated induction of the kidney arachidonate hydroxylases is associated with the development of hypertension. Am J Physiol Regul Integr Comp Physiol 284: R1055–R1062, 2003. doi: 10.1152/ajpregu.00459.2002. [DOI] [PubMed] [Google Scholar]
- 193.Nakano T, Hurn PD, Herson PS, Traystman RJ. Testosterone exacerbates neuronal damage following cardiac arrest and cardiopulmonary resuscitation in mouse. Brain Res 1357: 124–130, 2010. doi: 10.1016/j.brainres.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Nappi RE, Terreno E, Sances G, Martini E, Tonani S, Santamaria V, Tassorelli C, Spinillo A. Effect of a contraceptive pill containing estradiol valerate and dienogest (E2V/DNG) in women with menstrually-related migraine (MRM). Contraception 88: 369–375, 2013. doi: 10.1016/j.contraception.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 195.Nathan L, Shi W, Dinh H, Mukherjee TK, Wang X, Lusis AJ, Chaudhuri G. Testosterone inhibits early atherogenesis by conversion to estradiol: critical role of aromatase. Proc Natl Acad Sci USA 98: 3589–3593, 2001. doi: 10.1073/pnas.051003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Nelson PS, Clegg N, Arnold H, Ferguson C, Bonham M, White J, Hood L, Lin B. The program of androgen-responsive genes in neoplastic prostate epithelium. Proc Natl Acad Sci USA 99: 11890–11895, 2002. doi: 10.1073/pnas.182376299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Nguyen TV, Yao M, Pike CJ. Androgens activate mitogen-activated protein kinase signaling: role in neuroprotection. J Neurochem 94: 1639–1651, 2005. doi: 10.1111/j.1471-4159.2005.03318.x. [DOI] [PubMed] [Google Scholar]
- 198.Nguyen TV, Yao M, Pike CJ. Dihydrotestosterone activates CREB signaling in cultured hippocampal neurons. Brain Res 1298: 1–12, 2009. doi: 10.1016/j.brainres.2009.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Niewada M, Kobayashi A, Sandercock PA, Kamiński B, Członkowska A; International Stroke Trial Collaborative Group . Influence of gender on baseline features and clinical outcomes among 17,370 patients with confirmed ischaemic stroke in the international stroke trial. Neuroepidemiology 24: 123–128, 2005. doi: 10.1159/000082999. [DOI] [PubMed] [Google Scholar]
- 200.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG; TASC II Working Group . Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg 45, Suppl S: S5–S67, 2007. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 201.Nurmi A, Lindsberg PJ, Koistinaho M, Zhang W, Juettler E, Karjalainen-Lindsberg ML, Weih F, Frank N, Schwaninger M, Koistinaho J. Nuclear factor-kappaB contributes to infarction after permanent focal ischemia. Stroke 35: 987–991, 2004. doi: 10.1161/01.STR.0000120732.45951.26. [DOI] [PubMed] [Google Scholar]
- 202.Okwan-Duodu D, Umpierrez GE, Brawley OW, Diaz R. Obesity-driven inflammation and cancer risk: role of myeloid derived suppressor cells and alternately activated macrophages. Am J Cancer Res 3: 21–33, 2013. [PMC free article] [PubMed] [Google Scholar]
- 203.Oliver MF, Boyd GS. Effect of bilateral ovariectomy on coronary-artery disease and serum-lipid levels. Lancet 2: 690–694, 1959. doi: 10.1016/S0140-6736(59)92129-4. [DOI] [PubMed] [Google Scholar]
- 204.Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol 286: R233–R249, 2004. doi: 10.1152/ajpregu.00338.2003. [DOI] [PubMed] [Google Scholar]
- 205.Pechère-Bertschi A, Burnier M. Female sex hormones, salt, and blood pressure regulation. Am J Hypertens 17: 994–1001, 2004. doi: 10.1016/j.amjhyper.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 206.Pechère-Bertschi A, Maillard M, Stalder H, Brunner HR, Burnier M. Blood pressure and renal haemodynamic response to salt during the normal menstrual cycle. Clin Sci (Lond) 98: 697–702, 2000. doi: 10.1042/cs0980697. [DOI] [PubMed] [Google Scholar]
- 207.Pedram A, Razandi M, Aitkenhead M, Hughes CC, Levin ER. Integration of the non-genomic and genomic actions of estrogen. Membrane-initiated signaling by steroid to transcription and cell biology. J Biol Chem 277: 50768–50775, 2002. doi: 10.1074/jbc.M210106200. [DOI] [PubMed] [Google Scholar]
- 208.Pelzer T, Jazbutyte V, Hu K, Segerer S, Nahrendorf M, Nordbeck P, Bonz AW, Muck J, Fritzemeier KH, Hegele-Hartung C, Ertl G, Neyses L. The estrogen receptor-alpha agonist 16alpha-LE2 inhibits cardiac hypertrophy and improves hemodynamic function in estrogen-deficient spontaneously hypertensive rats. Cardiovasc Res 67: 604–612, 2005. doi: 10.1016/j.cardiores.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 209.Pérez-López FR, Chedraui P, Gilbert JJ, Pérez-Roncero G. Cardiovascular risk in menopausal women and prevalent related co-morbid conditions: facing the post-Women’s Health Initiative era. Fertil Steril 92: 1171–1186, 2009. doi: 10.1016/j.fertnstert.2009.06.032. [DOI] [PubMed] [Google Scholar]
- 210.Persky RW, Liu F, Xu Y, Weston G, Levy S, Roselli CE, McCullough LD. Neonatal testosterone exposure protects adult male rats from stroke. Neuroendocrinology 97: 271–282, 2013. doi: 10.1159/000343804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Peterlin BL, Gupta S, Ward TN, Macgregor A. Sex matters: evaluating sex and gender in migraine and headache research. Headache 51: 839–842, 2011. doi: 10.1111/j.1526-4610.2011.01900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Petrea RE, Beiser AS, Seshadri S, Kelly-Hayes M, Kase CS, Wolf PA. Gender differences in stroke incidence and poststroke disability in the Framingham heart study. Stroke 40: 1032–1037, 2009. doi: 10.1161/STROKEAHA.108.542894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Pike CJ, Nguyen TV, Ramsden M, Yao M, Murphy MP, Rosario ER. Androgen cell signaling pathways involved in neuroprotective actions. Horm Behav 53: 693–705, 2008. doi: 10.1016/j.yhbeh.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Pleumeekers HJ, Hoes AW, van der Does E, van Urk H, Hofman A, de Jong PT, Grobbee DE. Aneurysms of the abdominal aorta in older adults. The Rotterdam Study. Am J Epidemiol 142: 1291–1299, 1995. doi: 10.1093/oxfordjournals.aje.a117596. [DOI] [PubMed] [Google Scholar]
- 215.Pollow DP, Uhrlaub J, Romero-Aleshire M, Sandberg K, Nikolich-Zugich J, Brooks HL, Hay M. Sex differences in T-lymphocyte tissue infiltration and development of angiotensin II hypertension. Hypertension 64: 384–390, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Qiu Y, Yanase T, Hu H, Tanaka T, Nishi Y, Liu M, Sueishi K, Sawamura T, Nawata H. Dihydrotestosterone suppresses foam cell formation and attenuates atherosclerosis development. Endocrinology 151: 3307–3316, 2010. doi: 10.1210/en.2009-1268. [DOI] [PubMed] [Google Scholar]
- 217.Quigley R. Androgens stimulate proximal tubule transport. Gend Med 5, Suppl A: S114–S120, 2008. doi: 10.1016/j.genm.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 218.Radmayr C, Lunacek A, Schwentner C, Oswald J, Klocker H, Bartsch G. 5-alpha-reductase and the development of the human prostate. Indian J Urol 24: 309–312, 2008. doi: 10.4103/0970-1591.42610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Raju TN, Nelson KB, Ferriero D, Lynch JK; NICHD-NINDS Perinatal Stroke Workshop Participants . Ischemic perinatal stroke: summary of a workshop sponsored by the National Institute of Child Health and Human Development and the National Institute of Neurological Disorders and Stroke. Pediatrics 120: 609–616, 2007. doi: 10.1542/peds.2007-0336. [DOI] [PubMed] [Google Scholar]
- 220.Rauschemberger MB, Sandoval MJ, Massheimer VL. Cellular and molecular actions displayed by estrone on vascular endothelium. Mol Cell Endocrinol 339: 136–143, 2011. doi: 10.1016/j.mce.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 221.Reckelhoff JF. Polycystic ovary syndrome: androgens and hypertension. Hypertension 49: 1220–1221, 2007. doi: 10.1161/HYPERTENSIONAHA.107.088138. [DOI] [PubMed] [Google Scholar]
- 222.Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, Khatiwoda A, Lisabeth L. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol 7: 915–926, 2008. doi: 10.1016/S1474-4422(08)70193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Rettew JA, McCall SH IV, Marriott I. GPR30/GPER-1 mediates rapid decreases in TLR4 expression on murine macrophages. Mol Cell Endocrinol 328: 87–92, 2010. doi: 10.1016/j.mce.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 224.Rifici VA, Khachadurian AK. The inhibition of low-density lipoprotein oxidation by 17-beta estradiol. Metabolism 41: 1110–1114, 1992. doi: 10.1016/0026-0495(92)90295-L. [DOI] [PubMed] [Google Scholar]
- 225.Rodriguez-Acevedo AJ, Maher BH, Lea RA, Benton M, Griffiths LR. Association of oestrogen-receptor gene (ESR1) polymorphisms with migraine in the large Norfolk Island pedigree. Cephalalgia 33: 1139–1147, 2013. doi: 10.1177/0333102413486321. [DOI] [PubMed] [Google Scholar]
- 226.Roquer J, Campello AR, Gomis M. Sex differences in first-ever acute stroke. Stroke 34: 1581–1585, 2003. doi: 10.1161/01.STR.0000078562.82918.F6. [DOI] [PubMed] [Google Scholar]
- 227.Rubanyi GM, Freay AD, Kauser K, Sukovich D, Burton G, Lubahn DB, Couse JF, Curtis SW, Korach KS. Vascular estrogen receptors and endothelium-derived nitric oxide production in the mouse aorta. Gender difference and effect of estrogen receptor gene disruption. J Clin Invest 99: 2429–2437, 1997. doi: 10.1172/JCI119426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ruifrok WP, de Boer RA, Iwakura A, Silver M, Kusano K, Tio RA, Losordo DW. Estradiol-induced, endothelial progenitor cell-mediated neovascularization in male mice with hind-limb ischemia. Vasc Med 14: 29–36, 2009. doi: 10.1177/1358863X08096666. [DOI] [PubMed] [Google Scholar]
- 229.Rupec RA, Baeuerle PA. The genomic response of tumor cells to hypoxia and reoxygenation. Differential activation of transcription factors AP-1 and NF-kappa B. Eur J Biochem 234: 632–640, 1995. doi: 10.1111/j.1432-1033.1995.632_b.x. [DOI] [PubMed] [Google Scholar]
- 230.Saleh MC, Connell BJ, Saleh TM. Autonomic and cardiovascular reflex responses to central estrogen injection in ovariectomized female rats. Brain Res 879: 105–114, 2000. doi: 10.1016/S0006-8993(00)02757-8. [DOI] [PubMed] [Google Scholar]
- 231.Saleh MC, Connell BJ, Saleh TM. Medullary and intrathecal injections of 17beta-estradiol in male rats. Brain Res 867: 200–209, 2000. doi: 10.1016/S0006-8993(00)02313-1. [DOI] [PubMed] [Google Scholar]
- 232.Sanberg PR, Saporta S, Borlongan CV, Othberg AI, Allen RC, Cameron DF. The testis-derived cultured Sertoli cell as a natural Fas-L secreting cell for immunosuppressive cellular therapy. Cell Transplant 6: 191–193, 1997. [DOI] [PubMed] [Google Scholar]
- 233.Sandberg K, Ji H. Sex differences in primary hypertension. Biol Sex Differ 3: 7, 2012. doi: 10.1186/2042-6410-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Saraff K, Babamusta F, Cassis LA, Daugherty A. Aortic dissection precedes formation of aneurysms and atherosclerosis in angiotensin II-infused, apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 23: 1621–1626, 2003. doi: 10.1161/01.ATV.0000085631.76095.64. [DOI] [PubMed] [Google Scholar]
- 235.Schulster M, Bernie AM, Ramasamy R. The role of estradiol in male reproductive function. Asian J Androl 18: 435–440, 2016. doi: 10.4103/1008-682X.173932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Seyrek M, Yildiz O, Ulusoy HB, Yildirim V. Testosterone relaxes isolated human radial artery by potassium channel opening action. J Pharmacol Sci 103: 309–316, 2007. doi: 10.1254/jphs.FP0060883. [DOI] [PubMed] [Google Scholar]
- 237.Sharma G, Hu C, Brigman JL, Zhu G, Hathaway HJ, Prossnitz ER. GPER deficiency in male mice results in insulin resistance, dyslipidemia, and a proinflammatory state. Endocrinology 154: 4136–4145, 2013. doi: 10.1210/en.2013-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Sheiner E, Levy A, Katz M, Hershkovitz R, Leron E, Mazor M. Gender does matter in perinatal medicine. Fetal Diagn Ther 19: 366–369, 2004. doi: 10.1159/000077967. [DOI] [PubMed] [Google Scholar]
- 239.Shih CD. Activation of estrogen receptor beta-dependent nitric oxide signaling mediates the hypotensive effects of estrogen in the rostral ventrolateral medulla of anesthetized rats. J Biomed Sci 16: 60, 2009. doi: 10.1186/1423-0127-16-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Shihan M, Bulldan A, Scheiner-Bobis G. Non-classical testosterone signaling is mediated by a G-protein-coupled receptor interacting with Gnα11. Biochim Biophys Acta 1843: 1172–1181, 2014. doi: 10.1016/j.bbamcr.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 241.Sieveking DP, Lim P, Chow RW, Dunn LL, Bao S, McGrath KC, Heather AK, Handelsman DJ, Celermajer DS, Ng MK. A sex-specific role for androgens in angiogenesis. J Exp Med 207: 345–352, 2010. doi: 10.1084/jem.20091924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Sigvant B, Wiberg-Hedman K, Bergqvist D, Rolandsson O, Andersson B, Persson E, Wahlberg E. A population-based study of peripheral arterial disease prevalence with special focus on critical limb ischemia and sex differences. J Vasc Surg 45: 1185–1191, 2007. doi: 10.1016/j.jvs.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 243.Simoncini T, De Caterina R, Genazzani AR. Selective estrogen receptor modulators: different actions on vascular cell adhesion molecule-1 (VCAM-1) expression in human endothelial cells. J Clin Endocrinol Metab 84: 815–818, 1999. doi: 10.1210/jcem.84.2.5570. [DOI] [PubMed] [Google Scholar]
- 244.Singh H, Schwartzman ML. Renal vascular cytochrome P450-derived eicosanoids in androgen-induced hypertension. Pharmacol Rep 60: 29–37, 2008. [PubMed] [Google Scholar]
- 245.Somerville BW. Estrogen-withdrawal migraine. I. Duration of exposure required and attempted prophylaxis by premenstrual estrogen administration. Neurology 25: 239–244, 1975. doi: 10.1212/WNL.25.3.239. [DOI] [PubMed] [Google Scholar]
- 246.Spary EJ, Maqbool A, Batten TF. Oestrogen receptors in the central nervous system and evidence for their role in the control of cardiovascular function. J Chem Neuroanat 38: 185–196, 2009. doi: 10.1016/j.jchemneu.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 247.Srivastava RA. Estrogen-induced regulation of the ATP-binding cassette transporter A1 (ABCA1) in mice: a possible mechanism of atheroprotection by estrogen. Mol Cell Biochem 240: 67–73, 2002. doi: 10.1023/A:1020604610873. [DOI] [PubMed] [Google Scholar]
- 248.Stangl H, Graf GA, Yu L, Cao G, Wyne K. Effect of estrogen on scavenger receptor BI expression in the rat. J Endocrinol 175: 663–672, 2002. doi: 10.1677/joe.0.1750663. [DOI] [PubMed] [Google Scholar]
- 249.Stewart WF, Lipton RB, Celentano DD, Reed ML. Prevalence of migraine headache in the United States. Relation to age, income, race, and other sociodemographic factors. JAMA 267: 64–69, 1992. doi: 10.1001/jama.1992.03480010072027. [DOI] [PubMed] [Google Scholar]
- 250.Stirone C, Boroujerdi A, Duckles SP, Krause DN. Estrogen receptor activation of phosphoinositide-3 kinase, akt, and nitric oxide signaling in cerebral blood vessels: rapid and long-term effects. Mol Pharmacol 67: 105–113, 2005. doi: 10.1124/mol.104.004465. [DOI] [PubMed] [Google Scholar]
- 251.Stovner L, Hagen K, Jensen R, Katsarava Z, Lipton R, Scher A, Steiner T, Zwart JA. The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia 27: 193–210, 2007. doi: 10.1111/j.1468-2982.2007.01288.x. [DOI] [PubMed] [Google Scholar]
- 252.Sugioka K, Shimosegawa Y, Nakano M. Estrogens as natural antioxidants of membrane phospholipid peroxidation. FEBS Lett 210: 37–39, 1987. doi: 10.1016/0014-5793(87)81293-0. [DOI] [PubMed] [Google Scholar]
- 253.Sulistiyani, St Clair RW. Effect of 17 beta-estradiol on metabolism of acetylated low-density lipoprotein by THP-1 macrophages in culture. Arterioscler Thromb Vasc Biol 17: 1691–1700, 1997. doi: 10.1161/01.ATV.17.9.1691. [DOI] [PubMed] [Google Scholar]
- 254.Suzuki S, Brown CM, Wise PM. Neuroprotective effects of estrogens following ischemic stroke. Front Neuroendocrinol 30: 201–211, 2009. doi: 10.1016/j.yfrne.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Tada Y, Makino H, Furukawa H, Shimada K, Wada K, Liang EI, Murakami S, Kudo M, Kung DK, Hasan DM, Kitazato KT, Nagahiro S, Lawton MT, Hashimoto T. Roles of estrogen in the formation of intracranial aneurysms in ovariectomized female mice. Neurosurgery 75: 690–695, 2014. doi: 10.1227/NEU.0000000000000528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Tada Y, Wada K, Shimada K, Makino H, Liang EI, Murakami S, Kudo M, Shikata F, Pena Silva RA, Kitazato KT, Hasan DM, Kanematsu Y, Nagahiro S, Hashimoto T. Estrogen protects against intracranial aneurysm rupture in ovariectomized mice. Hypertension 63: 1339–1344, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Tamura T, Jamous MA, Kitazato KT, Yagi K, Tada Y, Uno M, Nagahiro S. Endothelial damage due to impaired nitric oxide bioavailability triggers cerebral aneurysm formation in female rats. J Hypertens 27: 1284–1292, 2009. doi: 10.1097/HJH.0b013e328329d1a7. [DOI] [PubMed] [Google Scholar]
- 258.Taylor JM, Borthwick F, Bartholomew C, Graham A. Overexpression of steroidogenic acute regulatory protein increases macrophage cholesterol efflux to apolipoprotein AI. Cardiovasc Res 86: 526–534, 2010. doi: 10.1093/cvr/cvq015. [DOI] [PubMed] [Google Scholar]
- 259.Tenenbaum M, Azab AN, Kaplanski J. Effects of estrogen against LPS-induced inflammation and toxicity in primary rat glial and neuronal cultures. J Endotoxin Res 13: 158–166, 2007. doi: 10.1177/0968051907080428. [DOI] [PubMed] [Google Scholar]
- 260.Thor D, Zhang R, Anderson L, Bose DD, Dubé GP, Rahimian R. Effects of 17 β-estradiol on lipopolysacharride-induced intracellular adhesion molecule-1 mRNA expression and Ca2+ homeostasis alteration in human endothelial cells. Vascul Pharmacol 53: 230–238, 2010. doi: 10.1016/j.vph.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Tobinick E, Kim NM, Reyzin G, Rodriguez-Romanacce H, DePuy V. Selective TNF inhibition for chronic stroke and traumatic brain injury: an observational study involving 629 consecutive patients treated with perispinal etanercept. CNS Drugs 26: 1051–1070, 2012. doi: 10.1007/s40263-012-0013-2. [DOI] [PubMed] [Google Scholar]
- 262.Trigatti BL, Fuller M. HDL signaling and protection against coronary artery atherosclerosis in mice. J Biomed Res 30: 94–100, 2015. doi: 10.7555/JBR.30.20150079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Tunstall-Pedoe H, Kuulasmaa K, Mähönen M, Tolonen H, Ruokokoski E, Amouyel P. Contribution of trends in survival and coronary-event rates to changes in coronary heart disease mortality: 10-year results from 37 WHO MONICA project populations. Monitoring trends and determinants in cardiovascular disease. Lancet 353: 1547–1557, 1999. doi: 10.1016/S0140-6736(99)04021-0. [DOI] [PubMed] [Google Scholar]
- 264.Turin TC, Okamura T, Afzal AR, Rumana N, Watanabe M, Higashiyama A, Nakao Y, Nakai M, Takegami M, Nishimura K, Kokubo Y, Okayama A, Miyamoto Y. Hypertension and lifetime risk of stroke. J Hypertens 34: 116–122, 2016. doi: 10.1097/HJH.0000000000000753. [DOI] [PubMed] [Google Scholar]
- 265.Uchida M, Palmateer JM, Herson PS, DeVries AC, Cheng J, Hurn PD. Dose-dependent effects of androgens on outcome after focal cerebral ischemia in adult male mice. J Cereb Blood Flow Metab 29: 1454–1462, 2009. doi: 10.1038/jcbfm.2009.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.van den Beld AW, Bots ML, Janssen JA, Pols HA, Lamberts SW, Grobbee DE. Endogenous hormones and carotid atherosclerosis in elderly men. Am J Epidemiol 157: 25–31, 2003. doi: 10.1093/aje/kwf160. [DOI] [PubMed] [Google Scholar]
- 267.Vasan RS, Larson MG, Leip EP, Evans JC, O’Donnell CJ, Kannel WB, Levy D. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med 345: 1291–1297, 2001. doi: 10.1056/NEJMoa003417. [DOI] [PubMed] [Google Scholar]
- 268.Villablanca A, Lubahn D, Shelby L, Lloyd K, Barthold S. Susceptibility to early atherosclerosis in male mice is mediated by estrogen receptor alpha. Arterioscler Thromb Vasc Biol 24: 1055–1061, 2004. doi: 10.1161/01.ATV.0000130467.65290.d4. [DOI] [PubMed] [Google Scholar]
- 269.Villablanca AC, Jayachandran M, Banka C. Atherosclerosis and sex hormones: current concepts. Clin Sci (Lond) 119: 493–513, 2010. doi: 10.1042/CS20100248. [DOI] [PubMed] [Google Scholar]
- 270.Villablanca AC, Tenwolde A, Lee M, Huck M, Mumenthaler S, Rutledge JC. 17beta-estradiol prevents early-stage atherosclerosis in estrogen receptor-alpha deficient female mice. J Cardiovasc Transl Res 2: 289–299, 2009. doi: 10.1007/s12265-009-9103-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Vitale C, Mendelsohn ME, Rosano GM. Gender differences in the cardiovascular effect of sex hormones. Nat Rev Cardiol 6: 532–542, 2009. doi: 10.1038/nrcardio.2009.105. [DOI] [PubMed] [Google Scholar]
- 272.Wagner AH, Schroeter MR, Hecker M. 17beta-estradiol inhibition of NADPH oxidase expression in human endothelial cells. FASEB J 15: 2121–2130, 2001. doi: 10.1096/fj.01-0123com. [DOI] [PubMed] [Google Scholar]
- 273.Walsh BA, Mullick AE, Walzem RL, Rutledge JC. 17Beta-estradiol reduces tumor necrosis factor-alpha-mediated LDL accumulation in the artery wall. J Lipid Res 40: 387–396, 1999. [PubMed] [Google Scholar]
- 274.Walsh BW, Li H, Sacks FM. Effects of postmenopausal hormone replacement with oral and transdermal estrogen on high density lipoprotein metabolism. J Lipid Res 35: 2083–2093, 1994. [PubMed] [Google Scholar]
- 275.Wang G, Drake CT, Rozenblit M, Zhou P, Alves SE, Herrick SP, Hayashi S, Warrier S, Iadecola C, Milner TA. Evidence that estrogen directly and indirectly modulates C1 adrenergic bulbospinal neurons in the rostral ventrolateral medulla. Brain Res 1094: 163–178, 2006. doi: 10.1016/j.brainres.2006.03.089. [DOI] [PubMed] [Google Scholar]
- 276.Wang H, Jessup JA, Zhao Z, Da Silva J, Lin M, MacNamara LM, Ahmad S, Chappell MC, Ferrario CM, Groban L. Characterization of the cardiac renin angiotensin system in oophorectomized and estrogen-replete mRen2.Lewis rats. PLoS One 8: e76992, 2013. doi: 10.1371/journal.pone.0076992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Wang JM, Irwin RW, Brinton RD. Activation of estrogen receptor alpha increases and estrogen receptor beta decreases apolipoprotein E expression in hippocampus in vitro and in vivo. Proc Natl Acad Sci USA 103: 16983–16988, 2006. doi: 10.1073/pnas.0608128103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Wang SJ, Fuh JL, Lu SR, Juang KD, Wang PH. Migraine prevalence during menopausal transition. Headache 43: 470–478, 2003. doi: 10.1046/j.1526-4610.2003.03092.x. [DOI] [PubMed] [Google Scholar]
- 279.Wang Y, Rudd AG, Wolfe CD. Age and ethnic disparities in incidence of stroke over time: the South London Stroke Register. Stroke 44: 3298–3304, 2013. doi: 10.1161/STROKEAHA.113.002604. [DOI] [PubMed] [Google Scholar]
- 280.Weinberger MH, Miller JZ, Luft FC, Grim CE, Fineberg NS. Definitions and characteristics of sodium sensitivity and blood pressure resistance. Hypertension 8: II127–II134, 1986. doi: 10.1161/01.HYP.8.6_Pt_2.II127. [DOI] [PubMed] [Google Scholar]
- 281.Wilson CM, McPhaul MJ. A and B forms of the androgen receptor are expressed in a variety of human tissues. Mol Cell Endocrinol 120: 51–57, 1996. doi: 10.1016/0303-7207(96)03819-1. [DOI] [PubMed] [Google Scholar]
- 282.Wu CC, Cheng J, Zhang FF, Gotlinger KH, Kelkar M, Zhang Y, Jat JL, Falck JR, Schwartzman ML. Androgen-dependent hypertension is mediated by 20-hydroxy-5,8,11,14-eicosatetraenoic acid-induced vascular dysfunction: role of inhibitor of kappaB Kinase. Hypertension 57: 788–794, 2011. doi: 10.1161/HYPERTENSIONAHA.110.161570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Wu XF, Zhang J, Paskauskas S, Xin SJ, Duan ZQ. The role of estrogen in the formation of experimental abdominal aortic aneurysm. Am J Surg 197: 49–54, 2009. doi: 10.1016/j.amjsurg.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 284.Xiao X, Zhang C, Ma X, Miao H, Wang J, Liu L, Chen S, Zeng R, Chen Y, Bihl JC. Angiotensin-(1-7) counteracts angiotensin II-induced dysfunction in cerebral endothelial cells via modulating Nox2/ROS and PI3K/NO pathways. Exp Cell Res 336: 58–65, 2015. doi: 10.1016/j.yexcr.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Xing D, Feng W, Miller AP, Weathington NM, Chen YF, Novak L, Blalock JE, Oparil S. Estrogen modulates TNF-alpha-induced inflammatory responses in rat aortic smooth muscle cells through estrogen receptor-beta activation. Am J Physiol Heart Circ Physiol 292: H2607–H2612, 2007. doi: 10.1152/ajpheart.01107.2006. [DOI] [PubMed] [Google Scholar]
- 286.Xue B, Badaue-Passos D Jr, Guo F, Gomez-Sanchez CE, Hay M, Johnson AK. Sex differences and central protective effect of 17beta-estradiol in the development of aldosterone/NaCl-induced hypertension. Am J Physiol Heart Circ Physiol 296: H1577–H1585, 2009. doi: 10.1152/ajpheart.01255.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Xue B, Beltz TG, Johnson RF, Guo F, Hay M, Johnson AK. PVN adenovirus-siRNA injections silencing either NOX2 or NOX4 attenuate aldosterone/NaCl-induced hypertension in mice. Am J Physiol Heart Circ Physiol 302: H733–H741, 2012. doi: 10.1152/ajpheart.00873.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Xue B, Beltz TG, Yu Y, Guo F, Gomez-Sanchez CE, Hay M, Johnson AK. Central interactions of aldosterone and angiotensin II in aldosterone- and angiotensin II-induced hypertension. Am J Physiol Heart Circ Physiol 300: H555–H564, 2011. doi: 10.1152/ajpheart.00847.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Xue B, Pamidimukkala J, Lubahn DB, Hay M. Estrogen receptor-alpha mediates estrogen protection from angiotensin II-induced hypertension in conscious female mice. Am J Physiol Heart Circ Physiol 292: H1770–H1776, 2007. doi: 10.1152/ajpheart.01011.2005. [DOI] [PubMed] [Google Scholar]
- 290.Xue B, Singh M, Guo F, Hay M, Johnson AK. Protective actions of estrogen on angiotensin II-induced hypertension: role of central nitric oxide. Am J Physiol Heart Circ Physiol 297: H1638–H1646, 2009. doi: 10.1152/ajpheart.00502.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Xue B, Zhang Z, Beltz TG, Guo F, Hay M, Johnson AK. Estrogen regulation of the brain renin-angiotensin system in protection against angiotensin II-induced sensitization of hypertension. Am J Physiol Heart Circ Physiol 307: H191–H198, 2014. doi: 10.1152/ajpheart.01012.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Xue B, Zhang Z, Beltz TG, Johnson RF, Guo F, Hay M, Johnson AK. Estrogen receptor-β in the paraventricular nucleus and rostroventrolateral medulla plays an essential protective role in aldosterone/salt-induced hypertension in female rats. Hypertension 61: 1255–1262, 2013. doi: 10.1161/HYPERTENSIONAHA.111.00903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Xue B, Zhao Y, Johnson AK, Hay M. Central estrogen inhibition of angiotensin II-induced hypertension in male mice and the role of reactive oxygen species. Am J Physiol Heart Circ Physiol 295: H1025–H1032, 2008. doi: 10.1152/ajpheart.00021.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Yanes LL, Reckelhoff JF. Postmenopausal hypertension. Am J Hypertens 24: 740–749, 2011. doi: 10.1038/ajh.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Yeap BB, Alfonso H, Chubb SA, Handelsman DJ, Hankey GJ, Golledge J, Flicker L, Norman PE. Lower plasma testosterone or dihydrotestosterone, but not estradiol, is associated with symptoms of intermittent claudication in older men. Clin Endocrinol (Oxf) 79: 725–732, 2013. [DOI] [PubMed] [Google Scholar]
- 296.Yeap BB, Hyde Z, Almeida OP, Norman PE, Chubb SA, Jamrozik K, Flicker L, Hankey GJ. Lower testosterone levels predict incident stroke and transient ischemic attack in older men. J Clin Endocrinol Metab 94: 2353–2359, 2009. doi: 10.1210/jc.2008-2416. [DOI] [PubMed] [Google Scholar]
- 297.Yoshida S, Aihara K, Ikeda Y, Sumitomo-Ueda Y, Uemoto R, Ishikawa K, Ise T, Yagi S, Iwase T, Mouri Y, Sakari M, Matsumoto T, Takeyama K, Akaike M, Matsumoto M, Sata M, Walsh K, Kato S, Matsumoto T. Androgen receptor promotes sex-independent angiogenesis in response to ischemia and is required for activation of vascular endothelial growth factor receptor signaling. Circulation 128: 60–71, 2013. doi: 10.1161/CIRCULATIONAHA.113.001533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Youssef MY, Alqallaf A, Abdella N. Anabolic androgenic steroid-induced cardiomyopathy, stroke and peripheral vascular disease. BMJ Case Rep 2011, jun30 1: bcr1220103650, 2011. doi: 10.1136/bcr.12.2010.3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Yu J, Akishita M, Eto M, Koizumi H, Hashimoto R, Ogawa S, Tanaka K, Ouchi Y, Okabe T. Src kinase-mediates androgen receptor-dependent non-genomic activation of signaling cascade leading to endothelial nitric oxide synthase. Biochem Biophys Res Commun 424: 538–543, 2012. doi: 10.1016/j.bbrc.2012.06.151. [DOI] [PubMed] [Google Scholar]
- 300.Yu J, Akishita M, Eto M, Ogawa S, Son BK, Kato S, Ouchi Y, Okabe T. Androgen receptor-dependent activation of endothelial nitric oxide synthase in vascular endothelial cells: role of phosphatidylinositol 3-kinase/akt pathway. Endocrinology 151: 1822–1828, 2010. doi: 10.1210/en.2009-1048. [DOI] [PubMed] [Google Scholar]
- 301.Zhang X, Thatcher S, Wu C, Daugherty A, Cassis LA. Castration of male mice prevents the progression of established angiotensin II-induced abdominal aortic aneurysms. J Vasc Surg 61: 767–776, 2015. doi: 10.1016/j.jvs.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Zhang X, Thatcher SE, Rateri DL, Bruemmer D, Charnigo R, Daugherty A, Cassis LA. Transient exposure of neonatal female mice to testosterone abrogates the sexual dimorphism of abdominal aortic aneurysms. Circ Res 110: e73–e85, 2012. doi: 10.1161/CIRCRESAHA.111.253880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Zhang ZH, Yu Y, Kang YM, Wei SG, Felder RB. Aldosterone acts centrally to increase brain renin-angiotensin system activity and oxidative stress in normal rats. Am J Physiol Heart Circ Physiol 294: H1067–H1074, 2008. doi: 10.1152/ajpheart.01131.2007. [DOI] [PubMed] [Google Scholar]
- 304.Zhu Y, Bian Z, Lu P, Karas RH, Bao L, Cox D, Hodgin J, Shaul PW, Thoren P, Smithies O, Gustafsson JA, Mendelsohn ME. Abnormal vascular function and hypertension in mice deficient in estrogen receptor beta. Science 295: 505–508, 2002. doi: 10.1126/science.1065250. [DOI] [PubMed] [Google Scholar]


