ABSTRACT
The study was designed to investigate whether serum hepatitis B virus (HBV) RNA is a strong surrogate marker for intrahepatic HBV covalently closed circular DNA (cccDNA) compared with serum HBV DNA, hepatitis B surface antigen (HBsAg), and hepatitis B e antigen (HBeAg) in HBeAg-positive chronic hepatitis B (CHB) patients. Serum HBV RNA, HBV DNA, HBsAg, HBeAg, and intrahepatic cccDNA were quantitatively detected at baseline (n = 82) and 96 weeks (n = 62) after treatment with nucleos(t)ide analogue (NUC) in HBeAg-positive CHB patients. The correlations among serum HBV RNA, HBV DNA, HBsAg, HBeAg, and intrahepatic cccDNA levels were then statistically analyzed. The results showed that pretreatment intrahepatic cccDNA levels correlated better with serum HBV DNA levels (r = 0.36, P < 0.01) than with serum HBV RNA levels (r = 0.25, P = 0.02), whereas no correlations were found between pretreatment intrahepatic cccDNA levels and HBsAg (r = 0.15, P = 0.17) or HBeAg (r = 0.07, P = 0.56) levels. At 96 weeks after NUC treatment, intrahepatic cccDNA levels correlated well with HBsAg levels (r = 0.39, P < 0.01) but not with serum HBV RNA, HBV DNA, and HBeAg levels (all P > 0.05). Besides, the decline in the intrahepatic cccDNA level from baseline to week 96 correlated better with the reduction in the serum HBsAg levels than with the decreases in the levels of the other markers (for the HBsAg decline, r = 0.38, P < 0.01; for the HBV DNA decline, r = 0.35, P = 0.01; for the HBV RNA decline, r = 0.28, P < 0.05; for the HBeAg decline, r = 0.18, P = 0.19). In conclusion, the baseline serum HBV RNA level or its decline after 96 weeks of NUC therapy correlated with the corresponding intrahepatic cccDNA level, while it was less than that seen with serum HBV DNA at baseline and HBsAg (or its decline) at 96 weeks after treatment, respectively.
KEYWORDS: chronic hepatitis B, covalently closed circular DNA, HBV DNA, HBV RNA, hepatitis B surface antigen, nucleos(t)ide analogue
INTRODUCTION
Hepatitis B virus (HBV) infection is a global health problem; an estimated 240 million persons are chronically infected, and about 650,000 people die annually due to chronic hepatitis B (CHB) worldwide (1). Though the currently available antiviral drugs can effectively reduce HBV DNA levels in serum of CHB patients, HBV may not be eliminated due to the persistence of HBV covalently closed circular DNA (cccDNA) in the infected hepatocytes, which represents the key HBV replicative intermediate (2). The fundamental role of intrahepatic cccDNA is as a template for transcription of all viral RNAs, including pregenomic RNA (pgRNA), which further produces the offspring virion DNA (2). Thus, the level of intrahepatic cccDNA reflects the activity of HBV replication. In addition, a previous study also showed that the low level of intrahepatic cccDNA present at the end of treatment could be a strong predictor of sustained response to antiviral therapy among CHB patients (3). However, liver biopsy is needed to detect intrahepatic cccDNA, which limits its use in clinical practice. Therefore, many investigations have been performed to explore noninvasive markers that can effectively indicate the level of HBV cccDNA in the liver (4–7).
The presence of HBV RNA in serum of HBV-infected patients was identified as early as 1996, and it might serve as a new viral marker for CHB patients during nucleos(t)ide analogue (NUC) therapy (8–10). However, the nature and origin of serum HBV RNA were still unclear until a recent study confirmed that serum HBV RNA is pgRNA and that it presents in virus-like particles (11). Moreover, serum HBV RNA has also been reported to be associated with responses to pegylated interferon (peg-IFN) or nucleos(t)ide analogue (NUC) (12, 13). Since serum HBV RNA was transcribed from cccDNA, it might reflect the activity of cccDNA in the liver (11). By using a mouse model, Giersch et al. (14) found that serum HBV RNA levels were correlated with intrahepatic cccDNA levels before treatment (r = 0.89, P < 0.01), while the correlation between them did not exist after 2 to 6 weeks of peg-IFN treatment (r = 0.13, P = 0.73). However, in hepatitis B e antigen (HBeAg)-positive CHB patients, it is still unclear whether serum HBV RNA is a novel surrogate marker for intrahepatic cccDNA before and after NUC treatment.
In the present study, liver biopsies were performed at baseline and 96 weeks after NUC treatment in HBeAg-positive CHB patients, and intrahepatic cccDNA was quantitatively measured. At the same time, serum HBV RNA, HBV DNA, hepatitis B surface antigen (HBsAg), and HBeAg were also quantified before and during the treatment. Furthermore, the correlations between intrahepatic cccDNA and serum HBV RNA levels as well as serum HBV DNA, HBsAg, and HBeAg levels were analyzed to determine whether serum HBV RNA is a strong surrogate marker for intrahepatic cccDNA in HBeAg-positive CHB patients.
RESULTS
Patient characteristics at baseline.
A total of 82 CHB patients (61 male and 21 female) with a median age of 31 years (range, 18 to 56 years) had undergone liver biopsy at baseline. The mean level of intrahepatic cccDNA in 82 patients was 0.67 ± 0.74 log copies/cell. Of the 82 patients, 8.5% (7/82) gave results that were negative (below the lower limit of detection [LoD]) for serum HBV RNA. Serum HBV RNA levels in 82 CHB patients were significantly lower than HBV DNA levels (5.32 ± 1.38 log IU/ml versus 7.48 ± 1.22 log IU/ml, P < 0.01). Liver histopathology assessment data were available for a total of 73 patients at baseline, and 89.0% (65/73) of patients had a necroinflammatory score of ≥2. Liver cirrhosis was present in 17.8% (13/73) of patients, and 79.5% (58/73) of patients had significant fibrosis (fibrosis score, ≥2) before treatment. The characteristics of HBeAg-positive CHB patients at baseline are shown in Table 1.
TABLE 1.
Characteristics of HBeAg-positive CHB patients at baseline and 96 weeks after NUC treatment
| Patient characteristic | Values |
|
|---|---|---|
| Baseline (n = 82) | Week 96 (n = 62) | |
| Median (range) age, yrs | 31 (18–56) | 32 (20–57) |
| No. of males/no. of females | 61/21 | 46/16 |
| HBV RNA level (log IU/ml) | 5.32 ± 1.38 | 2.49 ± 1.00 |
| HBV DNA level (log IU/ml) | 7.48 ± 1.22 | 1.86 ± 0.92 |
| HBsAg level (log IU/ml) | 4.05 ± 0.64 | 3.32 ± 0.90 |
| HBeAg level (log PEIU/ml) | 2.32 ± 1.26 | 0.08 ± 1.13 |
| No. with positive/negative HBeAg status | 82/0 | 44/18 |
| ALT level (U/liter) | 209 ± 175 | 24.9 ± 14.5 |
| AST level (U/liter) | 119 ± 107 | 24.1 ± 11.6 |
| cccDNA level (log copies/cell) | 0.67 ± 0.74 | −0.94 ± 0.60 |
| No. with necroinflammatory score of 1/2/3 | 8/45/20a | 41/18/2b |
| No. with fibrosis score of 0/1/2/3/4 | 2/13/27/18/13a | 3/27/16/7/8b |
| No. infected with genotype B/C/D | 23/57/2 | 19/41/2 |
Necroinflammatory score and fibrosis score data were available from a total of 73 patients at baseline.
Necroinflammatory score and fibrosis score data were available from a total of 61 patients at week 96. The lower limits of detection of serum HBV RNA and serum HBV DNA were 66.67 IU/ml and 20 IU/ml, respectively, and samples below the lower limit were recorded as 66.67 IU/ml and 20 IU/ml, respectively.
Patient characteristics at 96 weeks after NUC treatment.
Patient characteristics at 96 weeks after NUC treatment are shown in Table 1. At week 96, liver biopsy samples were obtained from 62 patients. Intrahepatic cccDNA was detectable in all patients, with a mean level of −0.94 ± 0.60 log copies/cell. The proportions of patients with a hepatic necroinflammatory score of ≥2 and a fibrosis score of ≥2 declined from 89.0% (65/73) and 79.5% (58/73) to 32.8% (20/61) and 50.8% (31/61), respectively (histopathological diagnosis data for 1 patient were lost at week 96). Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were normalized to 24.9 ± 14.5 U/liter and 24.1 ± 11.6 U/liter, respectively. Among 62 CHB patients, 59.7% (37/62) and 51.6% (32/62) had results that were negative for serum HBV RNA and HBV DNA, respectively. The serum HBV RNA level was significantly higher than the HBV DNA level (2.49 ± 1.00 log IU/ml versus 1.86 ± 0.92 log IU/ml, P < 0.01) at week 96. Of the 32 patients with serum HBV DNA-negative results (levels below 20 IU/ml) at week 96, 90.6% (29/32) had results that were negative (below 66.67 IU/ml) for serum HBV RNA; among the 30 patients with serum HBV DNA-positive results at week 96, 73.3% (22/30) had results that were positive for serum HBV RNA. In addition, 22 patients had results that were positive for both serum HBV RNA and HBV DNA, while 29 patients had results that were negative for both serum HBV RNA and HBV DNA. Also, the total rate of concordance of HBV RNA and HBV DNA detection results was 82.3% (51/62) at 96 weeks after treatment, as shown in Table S1 in the supplemental material.
Correlations among serum HBV RNA, HBV DNA, HBsAg, HBeAg, and intrahepatic cccDNA levels at baseline and 96 weeks after NUC treatment.
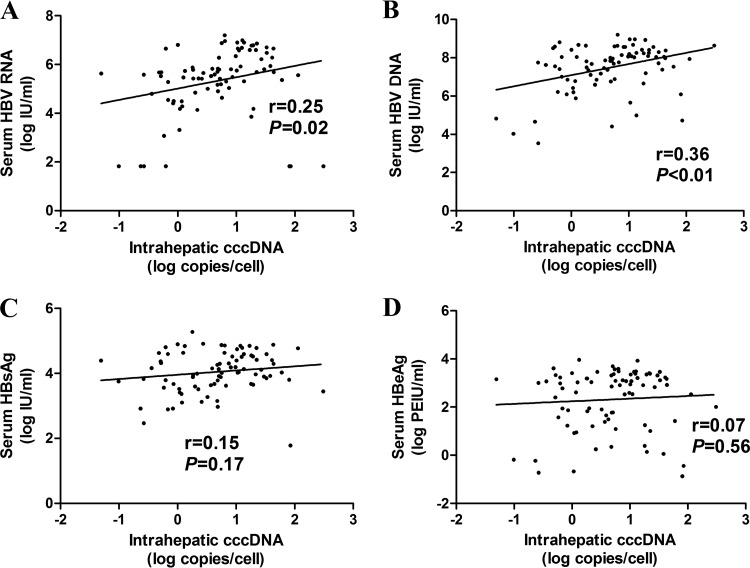
At baseline, the intrahepatic cccDNA level in HBeAg-positive CHB patients correlated better with the serum HBV DNA level (r = 0.36, P < 0.01) than with the serum HBV RNA level (r = 0.25, P = 0.02), whereas no correlations were found between the intrahepatic cccDNA level and the HBsAg (r = 0.15, P = 0.17) and HBeAg (r = 0.07, P = 0.56) levels (Fig. 1). In addition, the serum HBV RNA level correlated with the serum HBsAg level before treatment (r = 0.67, P < 0.01), and the serum HBV RNA level correlated better with the intrahepatic cccDNA level in patients with higher levels of serum HBsAg (r = 0.35, P = 0.03) than in those with lower levels of HBsAg (r = 0.20, P = 0.20) (cutoff value, 4.05 log IU/ml [mean level of HBsAg at baseline]), as shown in Fig. S1 in the supplemental material.
FIG 1.
Correlation between serum HBV RNA (A), HBV DNA (B), HBsAg (C), HBeAg (D), and intrahepatic cccDNA in HBeAg-positive CHB patients at baseline. HBsAg, hepatitis B surface antigen; HBeAg, hepatitis B e antigen; cccDNA, covalently closed circular DNA.
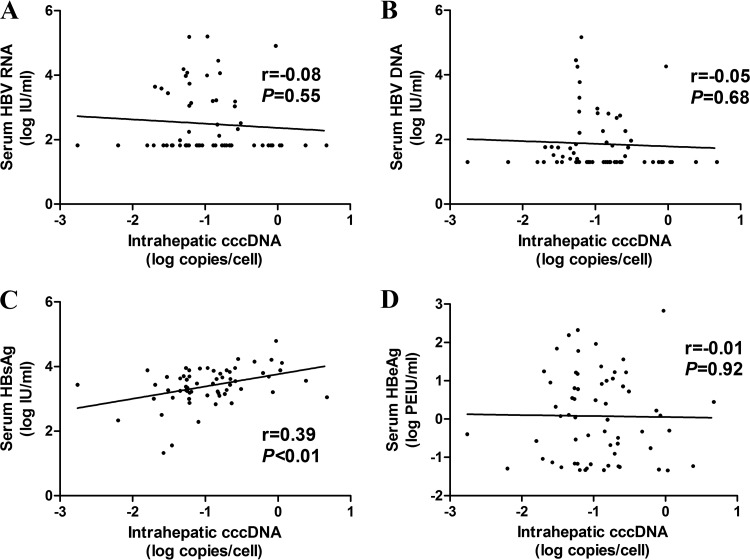
At 96 weeks after NUC treatment, the intrahepatic cccDNA level in CHB patients correlated only with the HBsAg level (r = 0.39, P < 0.01), while no correlations were found between the intrahepatic cccDNA level and serum HBV RNA (r = −0.08, P = 0.55), HBV DNA (r = −0.05, P = 0.68), and HBeAg (r = −0.01, P = 0.92) levels (Fig. 2). In addition, there was no correlation between serum HBV RNA and intrahepatic cccDNA levels in patients with detectable (r = 0.09, P = 0.64) or undetectable (r = 0.01, P = 0.95) serum HBV DNA at 96 weeks after NUC treatment, as shown in Fig. S2.
FIG 2.
Correlation between serum HBV RNA (A), HBV DNA (B), HBsAg (C), HBeAg (D), and intrahepatic cccDNA in CHB patients at 96 weeks after NUC treatment. HBsAg, hepatitis B surface antigen; HBeAg, hepatitis B e antigen; cccDNA, covalently closed circular DNA.
Correlations between the changes in serum HBV RNA, HBV DNA, HBsAg, HBeAg, and intrahepatic cccDNA levels after 96 weeks of NUC treatment.
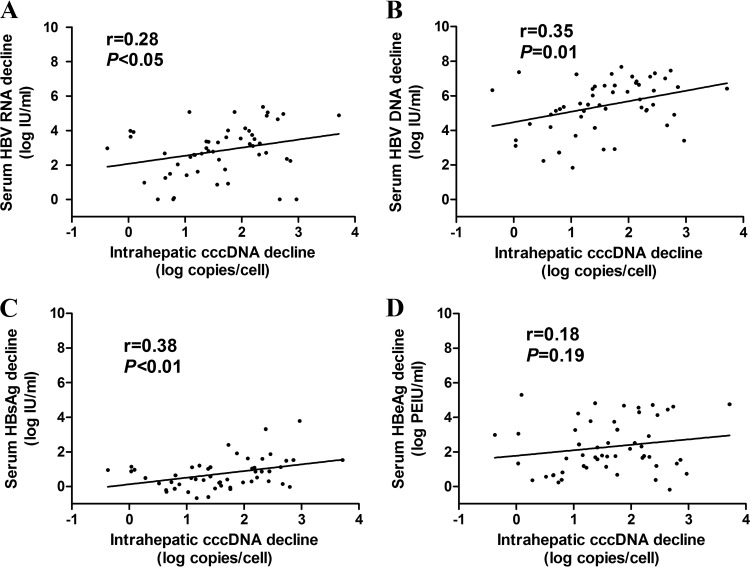
Samples of liver biopsy and paired (baseline and week 96) intrahepatic cccDNA data were obtained from a total of 52 patients both before and after 96 weeks of NUC treatment. Among the 52 patients, serum HBV RNA, HBV DNA, HBsAg, HBeAg, and intrahepatic cccDNA levels decreased by 2.82 ± 1.47 log IU/ml, 5.45 ± 1.49 log IU/ml, 0.74 ± 0.88 log IU/ml, 2.29 ± 1.52 log Paul-Ehrlich-Institute units (PEIU)/ml, and 1.60 ± 0.87 log copies/cell, respectively. The value representing the decline in the intrahepatic cccDNA levels presented the best correlation with the decline in HBsAg levels (r = 0.38, P < 0.01) compared with the declines in the HBV DNA (r = 0.35, P = 0.01) and HBV RNA (r = 0.28, P < 0.05) levels, and no correlation was observed between the declines in the levels of cccDNA and HBeAg (r = 0.18, P = 0.19). The correlations between the changes in serum HBV RNA, HBV DNA, HBsAg, and HBeAg levels and the changes in intrahepatic cccDNA levels from baseline to week 96 in CHB patients are shown in Fig. 3.
FIG 3.
Correlation between the changes in serum HBV RNA (A), HBV DNA (B), HBsAg (C), and HBeAg (D) levels and the changes in levels of intrahepatic cccDNA from baseline to 96 weeks after NUC treatment in CHB patients. HBsAg, hepatitis B surface antigen; HBeAg, hepatitis B e antigen; cccDNA, covalently closed circular DNA.
Dynamic changes in serum HBV RNA, HBV DNA, HBsAg, and HBeAg levels during the 96-week NUC treatment.
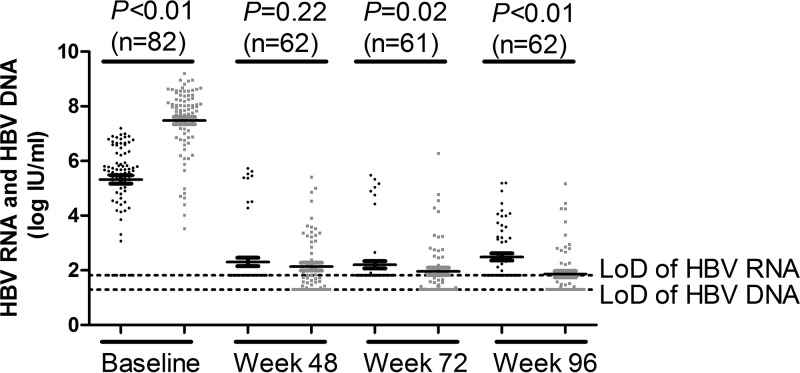
The dynamic changes in serum HBV RNA, HBV DNA, HBsAg, and HBeAg levels during the 96-week NUC treatment are shown in Fig. S3. Overall, serum HBV RNA, HBV DNA, and HBeAg levels showed a sharp decline from baseline to week 48 (5.32 ± 1.38 log IU/ml to 2.31 ± 1.20 log IU/ml for HBV RNA, 7.48 ± 1.22 log IU/ml to 2.14 ± 1.10 log IU/ml for HBV DNA, and 2.32 ± 1.26 log PEIU/ml to 0.39 ± 1.33 log PEIU/ml for HBeAg) and then declined slowly afterwards, whereas serum HBV RNA levels increased slightly at week 96. In contrast, HBsAg levels showed a steady decline during the 96 weeks of NUC treatment (from 4.05 ± 0.64 log IU/ml at baseline to 3.32 ± 0.90 log IU/ml at week 96). Comparing the levels of serum HBV RNA and HBV DNA during the 96-week NUC treatment, HBV RNA levels were significantly lower than HBV DNA levels at baseline (5.32 ± 1.38 log IU/ml versus 7.48 ± 1.22 log IU/ml, P < 0.01), whereas HBV RNA levels were significantly higher than HBV DNA levels at week 72 (2.20 ± 1.03 log IU/ml versus 1.96 ± 1.05 log IU/ml, P = 0.02) and at week 96 (2.49 ± 1.00 log IU/ml versus 1.86 ± 0.92 log IU/ml, P < 0.01), as shown in Fig. 4.
FIG 4.
Comparison of levels of serum HBV RNA with levels of HBV DNA in CHB patients during 96 weeks of NUC treatment. Black dots represent serum HBV RNA; gray squares represent serum HBV DNA. LoD, lower limit of detection.
Comparison of serum HBV RNA, HBV DNA, HBsAg, and HBeAg levels in patients with and without HBeAg loss at 96 weeks after NUC treatment.
The levels of serum HBV RNA, HBV DNA, HBsAg, and HBeAg in patients with and without HBeAg loss at 96 weeks after NUC treatment are shown in Table 2. Serum was serially sampled from a total of 52 patients during the 96-week treatment. After 96 weeks of NUC therapy, 17 of the 52 patients had HBeAg loss, and the remaining 35 patients were still positive for HBeAg. At week 96, serum HBV RNA levels were significantly lower in patients with HBeAg loss than in patients without HBeAg loss (2.03 ± 0.61 log IU/ml versus 2.67 ± 1.10 log IU/ml, P < 0.01), while there was no significant difference in HBV RNA levels between patients with and without HBeAg loss at other time points (all P > 0.05). In contrast, serum HBV DNA, HBsAg, and HBeAg levels were significantly different between patients with and without HBeAg loss from week 48 to week 96 (all P < 0.05).
TABLE 2.
Comparison of serum HBV RNA, HBV DNA, HBsAg, and HBeAg levels in patients with and without HBeAg loss after 96 weeks of NUC treatment
| Patient parameter | Values |
P value | |
|---|---|---|---|
| With HBeAg lossa (n = 17) | Without HBeAg loss (n = 35) | ||
| Baseline | |||
| Age, yrs (median, range) | 27 (21–52) | 31 (19–56) | 0.97 |
| No. of males/no. of females | 14/3 | 27/8 | 0.67 |
| ALT level (U/liter) | 222 ± 164 | 227 ± 178 | 0.92 |
| HBV RNA level (log IU/ml) | 5.20 ± 1.70 | 5.33 ± 1.25 | 0.88 |
| HBV DNA level (log IU/ml) | 7.49 ± 1.51 | 7.26 ± 1.24 | 0.26 |
| HBsAg level (log IU/ml) | 3.89 ± 0.87 | 4.11 ± 0.56 | 0.53 |
| HBeAg level (log PEIU/ml) | 2.33 ± 1.62 | 2.34 ± 1.15 | 0.36 |
| HBV cccDNA level (log copies/cell) | 0.80 ± 0.69 | 0.60 ± 0.79 | 0.34 |
| Week 48 | |||
| HBV RNA level (log IU/ml) | 2.03 ± 0.86 | 2.40 ± 1.30 | 0.26 |
| HBV DNA level (log IU/ml) | 1.83 ± 0.95 | 2.34 ± 1.21 | <0.05 |
| HBsAg level (log IU/ml) | 2.89 ± 1.38 | 3.75 ± 0.49 | <0.01 |
| HBeAg level (log PEIU/ml) | −0.75 ± 0.80 | 0.90 ± 1.25 | <0.01 |
| Week 72 | |||
| HBV RNA level (log IU/ml) | 2.04 ± 0.67 | 2.30 ± 1.18 | 0.73 |
| HBV DNA level (log IU/ml) | 1.78 ± 1.26 | 2.14 ± 1.04 | 0.03 |
| HBsAg level (log IU/ml) | 2.81 ± 1.45 | 3.62 ± 0.56 | <0.01 |
| HBeAg level (log PEIU/ml) | −0.57 ± 1.11 | 0.71 ± 1.11 | <0.01 |
| Week 96 | |||
| HBV RNA level (log IU/ml) | 2.03 ± 0.61 | 2.67 ± 1.10 | <0.01 |
| HBV DNA level (log IU/ml) | 1.55 ± 0.93 | 2.06 ± 0.97 | <0.01 |
| HBsAg level (log IU/ml) | 2.75 ± 1.37 | 3.56 ± 0.51 | <0.01 |
| HBeAg level (log PEIU/ml) | −1.22 ± 0.12 | 0.66 ± 0.94 | <0.01 |
A total of 14 of the 17 patients also achieved HBeAg seroconversion.
In addition, we also performed logistic regression analysis to test whether baseline factors were associated with HBeAg loss after the 96-week NUC treatment. The results showed that no baseline factors were identified, as shown in Table S2. Furthermore, among the 17 patients whose HBeAg levels dropped after 96 weeks of NUC treatment, 14 achieved HBeAg seroconversion. However, similarly to HBeAg loss, logistic regression analysis showed that baseline HBV RNA levels were not associated with HBeAg seroconversion either.
DISCUSSION
In order to clarify whether serum HBV RNA is a good surrogate marker that can effectively reflect the levels of intrahepatic HBV cccDNA present before and after NUC treatment in HBeAg-positive CHB patients, we carried out the current study. To ensure the reproducibility of HBV RNA testing, 3 serum samples were tested 6 times. The coefficients of variation (CV) of HBV RNA measurement for the 3 serum samples were 2.6%, 1.5%, and 3.9%, respectively, which suggested good reproducibility of the assay. The results enabled us to evaluate the clinical significance of HBV RNA levels with confidence. Previous studies reported that serum HBV RNA is transcribed from intrahepatic HBV cccDNA and thus that it may be a potential alternative marker for intrahepatic cccDNA levels (8, 11, 15). Here, we confirmed that serum HBV RNA is a valid marker but that it is nevertheless inferior to serum HBV DNA with respect to reflecting the level of intrahepatic cccDNA before treatment. In addition, using the mean level of HBsAg at baseline as the cutoff value, serum HBV RNA could also reflect the activity of intrahepatic cccDNA in patients with a higher level of HBsAg before treatment (r = 0.35, P = 0.03), while the correlation did not exist in patients with a lower level of HBsAg (r = 0.20, P = 0.20).
Recent studies reported that serum HBV RNA was a useful marker for the safe discontinuation of NUC therapy in CHB patients (11, 16). For instance, Wang et al. (11) reported that viral rebound occurred in all (21/21) patients with positive serum HBV RNA results at the end of NUC treatment, while only 25% (3/12) of patients with negative serum HBV RNA results at the end of NUC treatment showed viral rebound (P < 0.01). Thus, they supposed that serum HBV RNA levels might reflect the presence and transcriptional activity of cccDNA in liver after NUC treatment (11). However, the differences in ALT, AST, and HBsAg levels and HBeAg status between groups of serum HBV RNA-positive and -negative patients were not analyzed. Interestingly, animal experiments revealed that there was no correlation between serum HBV RNA levels and intrahepatic cccDNA levels in HBV-infected mice after peg-IFN treatment (r = 0.13, P = 0.73) (14). In the present study, serum HBV RNA results were negative in 37 (59.7%) of the patients after the 96-week NUC treatment, while intrahepatic cccDNA results were positive in all 62 patients, and there was no correlation between serum HBV RNA levels and intrahepatic cccDNA levels (r = −0.08, P = 0.55), which was in agreement with the results of a recent study conducted in CHB patients who had received NUC treatment (17). However, maybe due to the different detection sensitivities of the two studies with respect to measurement of cccDNA levels, 22.0% (9/41) of patients had cccDNA-negative results after NUC treatment in the study reported by Wang et al. (17). And a chi-square test revealed that detection of serum HBV RNA reflected the status (presence or absence) of intrahepatic cccDNA in the latter study (P < 0.01) (17). On the other hand, we found that patients with HBeAg loss after NUC treatment had a significantly lower serum HBV RNA level at week 96 than patients without HBeAg loss (2.03 ± 0.61 log IU/ml versus 2.67 ± 1.10 log IU/ml, P < 0.01). In addition, serum HBsAg levels were also significantly different at week 96 between HBeAg responders and nonresponders (2.75 ± 1.37 log IU/ml versus 3.56 ± 0.51 log IU/ml, P < 0.01). Compared with serum HBV RNA levels, serum HBsAg levels correlated well with intrahepatic cccDNA levels at 96 weeks after NUC therapy (r = 0.39, P < 0.01). Previous studies also identified a role of serum HBsAg in predicting treatment response during NUC therapy, and a higher HBsAg level at the end of NUC treatment was an independent predictor for posttreatment HBV relapse (18–20), which strongly supported our results indicating that serum HBsAg levels correlated well with intrahepatic cccDNA levels and that the levels might reflect the activity of cccDNA after NUC treatment.
In addition, a recent study revealed that a decline in the levels of serum HBV RNA among patients on treatment was associated with the subsequent response to NUC therapy (12). After the 96-week NUC treatment, we also observed a positive but weak correlation between the changes in serum HBV RNA levels and changes in intrahepatic cccDNA levels (r = 0.28, P < 0.05). Interestingly, compared with serum HBV RNA levels, the changes in serum HBsAg levels again better reflected the changes in intrahepatic cccDNA levels (r = 0.38, P < 0.01). However, the underlying mechanism must still be investigated. Other studies also pointed out that the reduction in HBsAg levels showed a good correlation with the decline in intrahepatic cccDNA levels seen after NUC treatment (5, 21). For example, by detecting intrahepatic cccDNA and serum HBsAg in 26 CHB patients before and after 2 years of lamivudine therapy, Chan et al. (5) found that the reduction in HBsAg levels correlated well with the reduction in cccDNA levels (r = 0.68, P < 0.01). In contrast, a weaker correlation between the reductions in HBsAg and cccDNA levels was found in our study (r = 0.38, P < 0.01). The discrepancies between the two studies might due to the relatively small sample size or the combination of peg-IFN and lamivudine used for treatment in the first 32 weeks in the study by Chan et al. (5).
The antiviral mechanism of NUC drugs is mainly that of acting through inhibiting reverse transcription (RT) of pgRNA into HBV DNA, and the drugs have no direct effect on cccDNA and its transcription (22). Given that serum HBV RNA was shown to be transcribed by intrahepatic cccDNA, NUC drugs may thus have a stronger inhibitory effect on HBV DNA than on HBV RNA. Therefore, in our study, serum HBV DNA levels showed a greater decline than serum HBV RNA levels after 96 weeks of NUC therapy (5.45 ± 1.49 log IU/ml versus 2.82 ± 1.47 log IU/ml, P < 0.01). In addition, serum HBV RNA levels were significantly lower than serum HBV DNA levels at baseline, whereas serum HBV RNA levels were significantly higher than serum HBV DNA levels at week 72 and week 96 after antiviral therapy (at week 72, 2.20 ± 1.03 log IU/ml versus 1.96 ± 1.05 log IU/ml, P = 0.02; at week 96, 2.49 ± 1.00 log IU/ml versus 1.86 ± 0.92 log IU/ml, P < 0.01), as shown in Fig. 4, which was consistent with previous studies (12, 23). On the other hand, it should be noted that the LoD of serum HBV RNA was different from that of serum HBV DNA (66.67 IU/ml for serum HBV RNA; 20 IU/ml for serum HBV DNA). And this may also have contributed to the significantly higher level of serum HBV RNA than of serum HBV DNA at week 96, since more than half of the patients were negative for serum HBV RNA or HBV DNA after the 96-week NUC treatment. Nonetheless, both the positive and negative concordance rates of HBV RNA and HBV DNA reached 82.26% (51/62) after 96 weeks of NUC treatment.
In the present study, we also compared serum HBV RNA levels in patients with and without HBeAg loss during NUC treatment. Our results showed that a significant difference in serum HBV RNA levels between patients with and without HBeAg loss was observed only at week 96 (2.03 ± 0.61 log IU/ml versus 2.67 ± 1.10 log IU/ml, P < 0.01), whereas HBV RNA levels were similar in the two groups at baseline, week 48, and week 72 (all P > 0.05). In addition, van Bömmel et al. (12) also reported that the baseline serum HBV RNA levels were similar between patients with and without HBeAg seroconversion after NUC treatment (P > 0.05). However, at month 3 and month 6 after treatment, patients who had achieved HBeAg seroconversion had significantly lower serum HBV RNA levels than those without HBeAg seroconversion (P < 0.01) (12). Logistic regression analysis in our study showed that pretreatment serum HBV RNA levels were not associated with HBeAg loss after the 96-week NUC therapy. By the use of peg-IFN and adefovir combination therapy, Jansen et al. (13) also found that baseline HBV RNA levels were not associated with HBeAg loss in HBeAg-positive CHB patients. However, in HBeAg-negative CHB patients, the baseline HBV RNA level was an independent predictor for virological response to peg-IFN and adefovir (odds ratio [OR], 0.44; P = 0.02) (13). Moreover, responders to peg-IFN and adefovir showed a more pronounced decline in the HBV RNA load at week 30 than nonresponders (P = 0.01) (13). On the other hand, it should be noted that the declines in the HBV RNA loads seen with peg-IFN based treatment and NUC monotherapy were different and that a stronger decrease in HBV RNA was seen in peg-IFN-based treatment (13, 24). Therefore, the predictive value of serum HBV RNA levels during NUC therapy may need to be further studied.
In conclusion, in the monitoring of antiviral therapies for CHB patients, while avoiding repeated invasive and potentially hazardous biopsies to obtain liver specimens, simple measurements in serum of both HBV DNA and RNA levels are valid, but of these, the HBV DNA level is perhaps more informative. And yet the results of the two assays do not always agree, and the linkages of these assays to biopsy assays for cccDNA do not always correlate. Thus, the issue of the clinical significance of serum HBV RNA can be complex and further studies are needed to reveal its role in the process and pathogenesis of HBV infection.
MATERIALS AND METHODS
Patients.
A cohort of HBeAg-positive CHB patients who received 96-week NUC (lamivudine and adefovir) treatment were investigated in our study (25). Briefly, the inclusion criteria of treatment for CHB patients were as follows: 18 to 65 years old, positive HBsAg for at least 6 months, HBeAg positive and hepatitis B e antibody (anti-HBe) negative, alanine aminotransferase (ALT) level of ≥2× the upper limit of normal, HBV DNA level of ≥105 copies/ml, and no history of antiviral treatment (NUC or IFN) within the previous 6 months. All patients were treated with lamivudine at 100 mg/day orally, and adefovir at 10 mg/day was added for patients with serum HBV DNA levels of >300 copies/ml at week 24 or for whom virological breakthrough occurred during the 96-week treatment. Patients were followed up every 12 weeks from baseline to week 96 during NUC treatment, and serum HBV DNA, HBsAg, HBeAg, and ALT levels were measured. Liver biopsies were performed at baseline and 96 weeks after NUC therapy for cccDNA quantification and histopathology assessment. Patients who had both intrahepatic cccDNA data available and enough serum stored at −80°C for HBV RNA detection were included in our study. Final totals of 82 patients at baseline and 62 patients at week 96 met the criteria and were included in this study. In addition, serum HBV RNA was also quantified at week 48 and week 72 for the 62 patients who had been followed up until week 96 during NUC therapy. The study was approved by the Institutional Review Board of Peking University Health Science Center (approval number IRB00001052-11056) and conducted in accordance with the ethical standards of the Helsinki Declaration. Informed consent was obtained from recruited patients.
Serum HBV DNA, HBsAg, HBeAg, anti-HBe, and ALT measurements.
HBV DNA was quantified by Roche Cobas TaqMan HBV test (Roche Diagnostics, Mannheim, Germany), with a linear range of 20 to 108 IU/ml (1 IU/ml = 5.82 copies/ml). HBV DNA testing results below 20 IU/ml were considered negative. HBsAg, HBeAg, and anti-HBe levels were measured by chemiluminescent microparticle immunoassay (CMIA) using an Architect i2000SR analyzer (Abbott Diagnostics, North Chicago, IL, USA). The HBeAg level was quantified according to the World Health Organization (WHO) HBeAg reference standard (Paul-Ehrlich-Institute, Germany), with a LoD of 0.12 Paul-Ehrlich-Institute units (PEIU)/ml (26). ALT levels were determined according to standard procedures in the clinical laboratories in the hospitals.
Serum HBV RNA quantification.
Serum HBV RNA was quantified using methods reported by previous studies (11, 12), and the procedures were as follows. HBV RNA was extracted by the use of an EasyPure viral RNA kit (TransGen Biotech, Beijing, China) following the manufacturer's instructions. Briefly, total HBV RNA was isolated from 200 μl serum and dissolved in 50 μl RNase-free water. Then, 7.5 μl of the solution was taken from the 50-μl RNA sample and treated with DNase I (Thermo Fisher Scientific, Waltham, MA, USA). The reaction mixture (7.5 μl RNA sample, 1 μl DNase I, 0.5 μl RNase inhibitor, 1 μl 10× DNase buffer; total, 10 μl) was digested at 37°C for 30 min. A 1-μl volume of EDTA was then added to the mixture described above, and the mixture was incubated at 65°C for 10 min to terminate the reaction. Further, the prepared RNA sample (total, 11 μl) was used as a template for reverse transcription (RT) to synthesize the cDNA using a RevertAid First Strand cDNA synthesis kit (Thermo Fisher Scientific, Waltham, MA, USA). The amplification of cDNA was carried out at 42°C for 60 min with a specific primer [5′-ACCACGCTATCGCTACTCAC(dT)17GWAGCTC-3′] as described previously (12). The reaction mixture (total, 20 μl) used for RT consisted of 11 μl RNA sample, 1 μl reverse transcriptase, 1 μl RNase inhibitor, 2 μl deoxynucleoside triphosphate (dNTP) (2.5 mM), 1 μl primer, and 4 μl buffer.
After that, quantitative PCR (qPCR) was applied in an ABI StepOnePlus real-time PCR system (Applied Biosystems, Foster City, CA) by using a hepatitis B viral DNA quantitative fluorescence diagnostic kit (Sansure Biotech, Hunan, China) with a linear range of 5 × 102 IU/ml to 5 × 109 IU/ml (LoD, 100 IU/ml) following the manufacturer's protocol. The LoD of this assay was 100 IU/ml for HBV RNA in the reaction mixture, corresponding to 66.67 IU/ml (100 IU/ml × 20 μl × [50 μl/7.5 μl]/200 μl) HBV RNA in the serum. Accordingly, the testing results reported as “undetermined” or “lower than LoD” were defined as “negative” in this study; otherwise, the results were recorded as “positive.” HBV RNA levels in serum samples below this lower limit were recorded as 66.67 IU/ml in the statistical analyses. The linear quantification range of the assay for serum HBV RNA is 3.33 × 102 IU/ml to 3.33 × 109 IU/ml, where the lower limit of quantification (LLoQ) is 3.33 × 102 IU/ml. Note that there is no WHO RNA standard currently and that HBV RNA quantification was obtained by testing the cDNA in a DNA assay; thus, the unit of HBV RNA in the study was expressed as an equivalent of international units per milliliter.
Intrahepatic cccDNA quantification.
Intrahepatic HBV cccDNA was quantitatively measured according to the method established by our laboratory, and the details of the procedures were described previously (23). Briefly, intrahepatic DNA was extracted using a QIAamp DNA Micro kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Plasmid-safe ATP-dependent DNase (Epicentre Technologies, Madison, WI) selectively hydrolyzes linear double-stranded DNAs and linear and closed circular single-stranded DNAs. The digested DNA was used for intrahepatic HBV cccDNA quantification by an in-house qPCR assay using a LightCycler 480 system (Roche Diagnostics, Mannheim, Germany). The amounts (numbers of copies/cell) of intrahepatic cccDNA were normalized with the β-globin housekeeping gene.
Histopathology assessment of liver tissues.
At baseline and 96 weeks after NUC therapy, liver biopsies were performed and portions of the liver tissues were prepared for histopathology assessment. The necroinflammatory score and fibrosis score were evaluated by experienced pathologists using the METAVIR grading system (27). Briefly, necroinflammatory scores range from 0 to 3, and higher scores indicate more-severe chronic hepatitis; fibrosis scores range from 0 to 4, and a score of 4 indicates cirrhosis.
Statistical analyses.
Continuous variables were expressed as means ± standard deviations (SD) and were compared by the Student t test or Mann-Whitney test. Categorical variables were expressed as counts and percentages and compared by chi-square test. Pearson's correlation analysis was used to analyze the correlations among serum HBV RNA, HBV DNA, HBsAg, HBeAg, and intrahepatic cccDNA levels. All statistical analyses were done by the use of SPSS 19.0 (SPSS, Chicago, IL, USA). All tests were two tailed, and a P value of <0.05 was considered statistically significant.
Supplementary Material
ACKNOWLEDGMENTS
We declare that we have no competing interests.
The study was supported by a Major Science and Technology Special Project of China Twelfth Five-year Plan (no. 2013ZX10002004 and no. 2012ZX10002003-003-013).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.00760-17.
REFERENCES
- 1.World Health Organization. 2015. Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection. http://www.who.int/hepatitis/publications/hepatitis-b-guidelines/en/. [PubMed]
- 2.Nassal M. 2015. HBV cccDNA: viral persistence reservoir and key obstacle for a cure of chronic hepatitis B. Gut 64:1972–1984. doi: 10.1136/gutjnl-2015-309809. [DOI] [PubMed] [Google Scholar]
- 3.Sung JJ, Wong ML, Bowden S, Liew CT, Hui AY, Wong VW, Leung NW, Locarnini S, Chan HL. 2005. Intrahepatic hepatitis B virus covalently closed circular DNA can be a predictor of sustained response to therapy. Gastroenterology 128:1890–1897. doi: 10.1053/j.gastro.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Wong DK, Seto WK, Cheung KS, Chong CK, Huang FY, Fung J, Lai CL, Yuen MF. 28 January 2017. Hepatitis B virus core-related antigen as a surrogate marker for covalently closed circular DNA. Liver Int doi: 10.1111/liv.13346. [DOI] [PubMed] [Google Scholar]
- 5.Chan HL, Wong VW, Tse AM, Tse CH, Chim AM, Chan HY, Wong GL, Sung JJ. 2007. Serum hepatitis B surface antigen quantitation can reflect hepatitis B virus in the liver and predict treatment response. Clin Gastroenterol Hepatol 5:1462–1468. doi: 10.1016/j.cgh.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Guner R, Karahocagil M, Buyukberber M, Kandemir O, Ural O, Usluer G, Inan D, Koksal I, Baykam N, Hizel K, Yamazhan T, Esen S, Tasyaran MA. 2011. Correlation between intrahepatic hepatitis B virus cccDNA levels and other activity markers in patients with HBeAg-negative chronic hepatitis B infection. Eur J Gastroenterol Hepatol 23:1185–1191. doi: 10.1097/MEG.0b013e32834ba13a. [DOI] [PubMed] [Google Scholar]
- 7.Wong DK, Yuen MF, Yuan H, Sum SS, Hui CK, Hall J, Lai CL. 2004. Quantitation of covalently closed circular hepatitis B virus DNA in chronic hepatitis B patients. Hepatology 40:727–737. doi: 10.1002/hep.20353. [DOI] [PubMed] [Google Scholar]
- 8.Rokuhara A, Matsumoto A, Tanaka E, Umemura T, Yoshizawa K, Kimura T, Maki N, Kiyosawa K. 2006. Hepatitis B virus RNA is measurable in serum and can be a new marker for monitoring lamivudine therapy. J Gastroenterol 41:785–790. doi: 10.1007/s00535-006-1856-4. [DOI] [PubMed] [Google Scholar]
- 9.Su Q, Wang SF, Chang TE, Breitkreutz R, Hennig H, Takegoshi K, Edler L, Schroder CH. 2001. Circulating hepatitis B virus nucleic acids in chronic infection: representation of differently polyadenylated viral transcripts during progression to nonreplicative stages. Clin Cancer Res 7:2005–2015. http://clincancerres.aacrjournals.org/content/7/7/2005.long. [PubMed] [Google Scholar]
- 10.Köck J, Theilmann L, Galle P, Schlicht HJ. 1996. Hepatitis B virus nucleic acids associated with human peripheral blood mononuclear cells do not originate from replicating virus. Hepatology 23:405–413. doi: 10.1002/hep.510230303. [DOI] [PubMed] [Google Scholar]
- 11.Wang J, Shen T, Huang X, Kumar GR, Chen X, Zeng Z, Zhang R, Chen R, Li T, Zhang T, Yuan Q, Li PC, Huang Q, Colonno R, Jia J, Hou J, McCrae MA, Gao Z, Ren H, Xia N, Zhuang H, Lu F. 2016. Serum hepatitis B virus RNA is encapsidated pregenome RNA that may be associated with persistence of viral infection and rebound. J Hepatol 65:700–710. doi: 10.1016/j.jhep.2016.05.029. [DOI] [PubMed] [Google Scholar]
- 12.van Bömmel F, Bartens A, Mysickova A, Hofmann J, Krüger DH, Berg T, Edelmann A. 2015. Serum hepatitis B virus RNA levels as an early predictor of hepatitis B envelope antigen seroconversion during treatment with polymerase inhibitors. Hepatology 61:66–76. doi: 10.1002/hep.27381. [DOI] [PubMed] [Google Scholar]
- 13.Jansen L, Kootstra NA, van Dort KA, Takkenberg RB, Reesink HW, Zaaijer HL. 2016. Hepatitis B virus pregenomic RNA is present in virions in plasma and is associated with a response to pegylated interferon alfa-2a and nucleos(t)ide analogues. J Infect Dis 213:224–232. doi: 10.1093/infdis/jiv397. [DOI] [PubMed] [Google Scholar]
- 14.Giersch K, Allweiss L, Volz T, Dandri M, Lutgehetmann M. 2017. Serum HBV pgRNA as a clinical marker for cccDNA activity. J Hepatol 66:460–462. doi: 10.1016/j.jhep.2016.09.028. [DOI] [PubMed] [Google Scholar]
- 15.Hatakeyama T, Noguchi C, Hiraga N, Mori N, Tsuge M, Imamura M, Takahashi S, Kawakami Y, Fujimoto Y, Ochi H, Abe H, Maekawa T, Kawakami H, Yatsuji H, Aisaka Y, Kohno H, Aimitsu S, Chayama K. 2007. Serum HBV RNA is a predictor of early emergence of the YMDD mutant in patients treated with lamivudine. Hepatology 45:1179–1186. doi: 10.1002/hep.21581. [DOI] [PubMed] [Google Scholar]
- 16.Tsuge M, Murakami E, Imamura M, Abe H, Miki D, Hiraga N, Takahashi S, Ochi H, Nelson HC, Ginba H, Matsuyama K, Kawakami H, Chayama K. 2013. Serum HBV RNA and HBeAg are useful markers for the safe discontinuation of nucleotide analogue treatments in chronic hepatitis B patients. J Gastroenterol 48:1188–1204. doi: 10.1007/s00535-012-0737-2. [DOI] [PubMed] [Google Scholar]
- 17.Wang J, Du M, Huang H, Chen R, Niu J, Jiang J, Zhuang H, Lu F. 2017. Reply to: “Serum HBV pgRNA as a clinical marker for cccDNA activity”: consistent loss of serum HBV RNA might predict the “para-functional cure” of chronic hepatitis B. J Hepatol 66:462–463. doi: 10.1016/j.jhep.2016.10.034. [DOI] [PubMed] [Google Scholar]
- 18.Chen CH, Lu SN, Hung CH, Wang JH, Hu TH, Changchien CS, Lee CM. 2014. The role of hepatitis B surface antigen quantification in predicting HBsAg loss and HBV relapse after discontinuation of lamivudine treatment. J Hepatol 61:515–522. doi: 10.1016/j.jhep.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 19.Cai W, Xie Q, An B, Wang H, Zhou X, Zhao G, Guo Q, Gu R, Bao S. 2010. On-treatment serum HBsAg level is predictive of sustained off-treatment virologic response to telbivudine in HBeAg-positive chronic hepatitis B patients. J Clin Virol 48:22–26. doi: 10.1016/j.jcv.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 20.Zoulim F, Carosi G, Greenbloom S, Mazur W, Nguyen T, Jeffers L, Brunetto M, Yu S, Llamoso C. 2015. Quantification of HBsAg in nucleos(t)ide-naive patients treated for chronic hepatitis B with entecavir with or without tenofovir in the BE-LOW study. J Hepatol 62:56–63. doi: 10.1016/j.jhep.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 21.Werle-Lapostolle B, Bowden S, Locarnini S, Wursthorn K, Petersen J, Lau G, Trepo C, Marcellin P, Goodman Z, Delaney WT, Xiong S, Brosgart CL, Chen SS, Gibbs CS, Zoulim F. 2004. Persistence of cccDNA during the natural history of chronic hepatitis B and decline during adefovir dipivoxil therapy. Gastroenterology 126:1750–1758. doi: 10.1053/j.gastro.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 22.Trépo C, Chan HL, Lok A. 2014. Hepatitis B virus infection. Lancet 384:2053–2063. doi: 10.1016/S0140-6736(14)60220-8. [DOI] [PubMed] [Google Scholar]
- 23.Wang M, Qiu N, Lu S, Xiu D, Yu J, Wang XT, Lu F, Li T, Liu X, Zhuang H. 2013. Serum hepatitis B surface antigen is correlated with intrahepatic total HBV DNA and cccDNA in treatment-naive patients with chronic hepatitis B but not in patients with HBV related hepatocellular carcinoma. J Med Virol 85:219–227. doi: 10.1002/jmv.23461. [DOI] [PubMed] [Google Scholar]
- 24.Huang YW, Chayama K, Tsuge M, Takahashi S, Hatakeyama T, Abe H, Hu JT, Liu CJ, Lai MY, Chen DS, Yang SS, Kao JH. 2010. Differential effects of interferon and lamivudine on serum HBV RNA inhibition in patients with chronic hepatitis B. Antivir Ther 15:177–184. doi: 10.3851/IMP1508. [DOI] [PubMed] [Google Scholar]
- 25.Gao Y, Meng Q, Zhang Z, Zhao P, Shang Q, Yuan Q, Li Y, Deng J, Li T, Liu X, Zhuang H. 2016. On-treatment quantitative hepatitis B e antigen predicted response to nucleos(t)ide analogues in chronic hepatitis B. World J Hepatol 8:1511–1520. doi: 10.4254/wjh.v8.i34.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou B, Liu M, Lv G, Zheng H, Wang Y, Sun J, Hou J. 2013. Quantification of hepatitis B surface antigen and e antigen: correlation between Elecsys and Architect assays. J Viral Hepat 20:422–429. doi: 10.1111/jvh.12044. [DOI] [PubMed] [Google Scholar]
- 27.Bedossa P, Poynard T. 1996. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology 24:289–293. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.