Abstract
Cell-free synthetic biology emerges as a powerful and flexible enabling technology that can engineer biological parts and systems for life science applications without using living cells. It provides simpler and faster engineering solutions with an unprecedented freedom of design in an open environment than cell system. This review focuses on recent developments of cell-free synthetic biology on biological engineering fields at molecular and cellular levels, including protein engineering, metabolic engineering, and artificial cell engineering. In cell-free protein engineering, the direct control of reaction conditions in cell-free system allows for easy synthesis of complex proteins, toxic proteins, membrane proteins, and novel proteins with unnatural amino acids. Cell-free systems offer the ability to design metabolic pathways towards the production of desired products. Buildup of artificial cells based on cell-free systems will improve our understanding of life and use them for environmental and biomedical applications.
Keywords: Cell-free synthetic biology, Cell-free protein synthesis, Protein engineering, Metabolic engineering, Artificial cell, Unnatural amino acids
1. Introduction
Advances in DNA sequencing and gene editing technologies have endowed the synthetic biologist with unprecedented power to program cells at will. The ability of synthetic biology to engineer biological functions holds great promises for applications ranging from biomedical to biofuel research. For the most part, synthetic biology is still tied to the living cell. One major advantage of using the living cell is its self-reproduction. However, the daunting complexity of living cells and the barriers of cell membrane make engineering difficult, and therefore make synthetic biology face four insurmountable challenges [1]: hard to standardize, unwieldy complexity, incompatibility and variability. From the standpoint of synthetic biology, it is highly desirable for these problems to be overcome using a standardized set of better engineering solutions.
To address these challenges, an emerging interdisciplinary approach has been adopted: cell-free synthetic biology. Cell-free synthetic biology system activates biological machinery without the use of living cells. It allows direct control of transcription, translation and metabolism in an open environment. Three types of cell-free systems have been well developed. One is extract-based system. The system is composed of crude extract with basic transcription and translation functions, DNA templates, energy regeneration substrates, amino acids, nucleotides, cofactors, and salts. Most commonly used organisms providing the extracts are Escherichia coli [2], Saccharomyces cerevisiae [3], rabbit reticulocyte [4], wheat germ [5], and insect cell [6]. The other one is purified system, such as the PURE system which consists of a toolbox of purified E. coli translational components [7]. The third one is synthetic enzymatic pathway system, which consists of numerous enzymes for implementing complicated bioreactions [8]. The ability of cell-free systems to harness a cell's capabilities unimpeded by cells opens new opportunities for the academic research and industry applications. Briefly, reduced dependence on cells drives the increase in engineering flexibility. As a result, in vitro cell-free systems have many advantages over traditional in vivo cell systems (Table 1) [8], [9], [10], which include controllable transcription, translation and post-translational modification, convenient high-throughput screening format, accelerated design-build-test-learn cycle, high synthesis rate and product yield, easy production of soluble membrane proteins and complex proteins, easy incorporation of unnatural amino acids (uAAs), high tolerance for toxic substrates or products, and good ability to focus on particular metabolism.
Table 1.
Comparison of in vitro cell-free systems and traditional in vivo cell systems.
| Feature | In vitro cell-free system | In vivo cell system |
|---|---|---|
| Manipulation of transcription and translation | Easy to control in an open environment | Hard because of cell membrane as the barrier |
| Post-translational modification | Hard | Easy |
| Self-replication | Hard | Easy |
| DNA template | Plasmids or PCR products | Plasmids or genomes |
| Synthesis of membrane proteins and complex proteins | Easy synthesis by adding surfactants or adjusting the system environment | Hard synthesis due to limited intracellular environment |
| Incorporation of unnatural amino acids into proteins | Easy | Hard |
| Ability to only produce the desired products | Easy achievement by focusing on the target metabolic pathways | Hard achievement due to complicated cellular metabolism |
| Toxic tolerance | High | Low |
| Integration with materials | Easy | Hard |
| Design-build-test-learn cycle | Two days | Two weeks |
| Biomanufacturing | High production rate | Modest production rate |
| High product yield | Modest product yield | |
| Easy purification process without cell lysis | Cell lysis prior to product purification | |
| Cost | Modest to high | Low to modest |
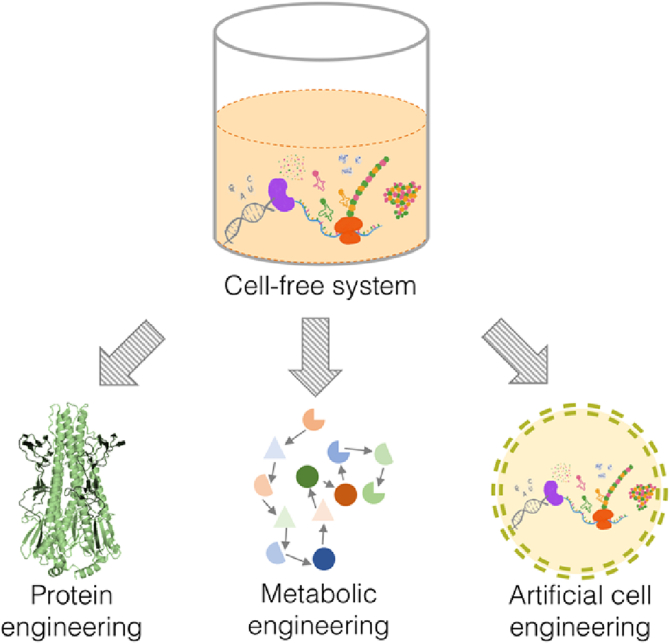
These features make cell-free synthetic biology serve as a versatile platform for engineering biological parts at three different levels of protein, metabolism and cell (Fig. 1). Cell-free synthetic biology can be an enabling technology for innovating medical diagnostics and therapeutics, developing complex metabolic system, making functional biomolecules, and producing sustainable bioenergy and biochemicals.
Fig. 1.
Engineering protein, metabolism and artificial cell in the open cell-free system.
2. Cell-free protein engineering
Protein engineering is an important method to produce valuable proteins for basic and applied research. Currently, engineering proteins still relies on cell-based approaches, but many problems are difficult to deal with. Synthesis of functional proteins in cells usually faces some challenges, including insoluble expression, low protein yield, variability in expression, incorrect folding and low stability. Protein synthesis in the open cell-free system is uncoupled from cell growth and therefore can be directly controlled using greater degrees of manipulation and less complexity [11]. The direct access to the reaction environment of protein synthesis makes engineering proteins much easier. Cell-free expression also allows protein engineering in high-throughput format for discovery of novel biomolecules [12], flexible strategies for post-translational modifications [13] and convenient chemical conjugation [14]. Due to the open nature of cell-free systems, various biological parts or man-made devices can be implemented into cell-free systems to improve the biomanufacturing and expand the applications of proteins [11], [15], [16].
2.1. Synthesis and folding
Proteins are increasingly a key part of modern medical care. A significant proportion of proteins used in biopharmaceutical research and industry are complex proteins, toxic proteins and membrane proteins, which are difficult to produce in vivo. The primary advantage of cell-free systems is the ease of controlling and optimizing the reactions for better protein production.
Expressing complex proteins consisting of hetero subunits in cell-free systems is significantly beneficial as it allows co-translation of multiple mRNA to form bioactive complex proteins [17]. Other efforts to synthesize complex proteins are to encourage correct formation of multiple disulfide bonds. It could be achieved in cell-free systems by pre-treating the cell extracts, using redox buffers, adding disulfide bond forming enzymes or providing the chaperones [9], [18].
Toxic proteins which interfere with cellular metabolic pathways and inhibit cell division are hard to express in high yields in vivo. Restriction endonuclease [19], cytolethal distending toxin [20] and the human microtubule binding protein [21] are typical examples of proteins toxic to cells. Since there is no cell growth, cell-free systems could serve as an excellent platform for the synthesis of those toxic proteins [17].
Constituting a significant fraction (20%–30%) of human genome [22], membrane proteins represent 60% of approved drug targets [23]. Overexpression of them in cells might lead to issues of accumulation as inclusion bodies. The protein concentration in cell-free system is approximately 20-fold less than that in vivo [24]. The dilution appears to be beneficial for protein folding. To assist protein folding, cell-free systems provide an attractive alternative to synthesize membrane proteins in the presence of surfactants or preformed liposomes, which mimics the environment of cellular membranes. It can prevent aggregation and enhance the solubilization of membrane proteins. Many membrane proteins have been successfully expressed in cell-free systems, such as G-protein coupled receptors [25], vaccine antigens [26], [27] and tetracycline pump [28].
2.2. Unnatural amino acids
Conventional protein engineering has been restricted to 20 naturally occurring amino acids. Synthesis of numerous unnatural amino acids (uAAs) provide a wide breadth of possibilities in the design of novel proteins. Up to now, more than 100 different uAAs can be site-specifically incorporated into proteins by biosynthetic methods [29]. Because cell-free synthetic biology system only focuses on the synthesis of the target protein, it can be used as a highly effective synthesis platform for incorporating uAAs into proteins. Based on cell-free systems, two predominant incorporation methods have been developed, including global residue replacement method and stop codon suppression method [30]. In the scheme of global residue replacement, a cell-free reaction is usually conducted in which certain AA is replaced by its analogue. In the stop codon suppression method, orthogonal tRNAs and tRNA synthetases are used to incorporate uAAs at the UAG amber stop codon of mRNAs.
Incorporation of uAAs could provide unlimited novel side-chains for precise posttranslational modifications. In developing antibody-drug conjugates (ADCs), an anticancer drug is coupled to an antibody that specifically targets a certain tumor marker. A stable link between the antibody and anticancer agent is a crucial aspect of an ADC [31]. The first generation of ADCs use linking technologies that conjugate drugs non-selectively to cysteine or lysine residues in the antibody, resulting in a heterogeneous mixture and suboptimal efficacy. The site-specific incorporation of uAA into the antibody by cell-free system generates a site for controlled and stable attachment of the drug [32]. This enables the production of homogeneous ADCs with the antibody precisely linked to the drug and controlled ratios of antibody to drug, allowing the selection of a best-in-class ADC [33]. The uAA incorporation using cell-free system also opens up many other applications, including orientation-controlled immobilization of proteins to scaffolds [30], [34] and functionalized virus-like particles [35].
Proteins play a central role in biological processes and therefore they have been the main targets for discovery of novel enzymes or drugs. However, related studies have run into a bottleneck. One main reason is that conventional cell-based approach is hard to produce active complex proteins, toxic proteins, membrane proteins and unnatural proteins. Increasing the diversity of protein libraries is the first and key step for discovering novel enzymes or drugs. Cell-free system can be a powerful platform for design, synthesis and high-throughput screening of novel proteins.
3. Cell-free metabolic engineering
Now many biotechnology researchers have genetically engineered microbes to synthesize fuels and chemicals. The problem is that when microbes become living chemical factories, they have to spare some resources for cell growth [36]. Moreover, even in a simple bacterium, cellular metabolism is complicated and hard to control. Sometimes changing a few metabolic pathways to improve the chemical production can have negative consequences for the rest of the cell. And the desired products might be poisonous to the microbes. Thus, separating the chemical production from the cell proliferation could be a huge advantage.
Cell-free synthetic biology emerges as an alternative solution for accomplishing a desired biotransformation without concerns of cell growth, complicated metabolism, and side-product formation [8]. Cell-free systems enable flexible modulation and standardization of hybrid enzyme synthesis for cell-free metabolic engineering. Cell-free systems present many biomanufacturing advantages, such as fast synthesis rate, direct reaction control, and tolerance to toxic substrates or products [10]. The open cell-free systems also could greatly decrease the incompatibility problem and make sure high efficiency of every step in the metabolic pathways. Cell-free systems are also industrially scalable [37].
Cell-free metabolic engineering offers unprecedented opportunities to control the overall metabolic pathways for maximal conversion efficiency. In the reaction system, only enzymes directly related to the synthesis of the target product are kept. In the biohydrogen production from sugars, a cell-free synthetic enzyme pathway produces hydrogen at 97% of the maximal theoretical yield [38], [39], which is a 3-fold increase above the theoretical maximum production in microbes [40]. Cell-free synthetic enzymatic pathway systems have been successfully used in enzymatic fuel cells with higher energy-storage density than that of lithium-ion batteries [41]. A key feature of cell-free systems is their tolerance to the presence of higher alcohols. Nontoxic levels of isobutanol have tended to max out at around 2% (v/v) in yeast cells, but use of the cell-free approach pushes that yield to 12% [42], [43]. One more advantage of cell-free metabolic engineering is the potential to implement bioconversion that cannot be catalyzed by living microbes or chemical catalysts, such as the conversion of cellulose to starch [44].
Metabolic engineering approaches have been performed and developed for many years within cells to increase the production of a certain substance. Numerous studies have proved that it is an impossible task to break the nature of cell to reach the theoretical conversion yield. Cell-free systems separate the substance production from the cell growth and offer the ability to design synthetic metabolic pathways towards the production of desired substance. Therefore, cell-free systems provide a significant possibility to produce the substance at the maximal yield for improving the bioindustrial production.
4. Artificial cell engineering
An artificial cell is an engineered capsule in which bioactive materials are encapsulated within a membrane to perform designated functions [45]. Its construction can help to understand the origin of life. Recent research advances suggest that the synthesis of life is now a realistic goal [46]. Its applications range from the environment to the healthcare [47], [48].
Two approaches have been developed for the construction of an artificial cell: top-down and bottom-up. The top-down approach starts from a living organism and makes a cell with the minimum genome possible to live [49]. However, it is expensive, time-consuming and hard to scale up. In contrast, the bottom-up approach starts from scratch and therefore engineering artificial cell becomes much easier. However, it is still challenging. Three basic elements are needed for the construction of a living artificial cell to perform the essential functions of life. These include cell membrane, information-carrying molecule DNA or RNA, and metabolism system [48]. In this context, bottom-up cell-free synthetic biology is becoming a powerful transcription/translation toolbox to engineer artificial cells [50], [51], [52]. A main goal of constructing the artificial cell is its ability to self-organize and self-reproduce [53]. Cell-free synthetic biology could be used to produce artificial cell entities that possess some functions of a living cell. The practical applications include protein synthesis [54], directed protein evolution [55], environmental sensing [47], [56], creation of novel signal pathways [57], and study of biological networks [58].
However, the greatest potential applications of artificial cells lie in the biomedical therapeutics. It is proposed that artificial cells made of cell-free systems could be designed to smartly synthesize and deliver drugs on-site in response to relevant stimuli [10]. This new drug delivery paradigm could promote the innovation of biomedical industry.
5. Conclusions
Cell-free synthetic biology proves a promising tool to overcome inherent limitations of living cells. Its open nature enables flexible biological engineering at both molecular and cellular levels. Because cost remains a top concern in industry, cell-free biosynthesis methodology is well suited for the development of high-value biopharmaceuticals. It is believed that cell-free systems would become more commonly used for basic and applied research in the future.
Despite the promising features of cell-free synthetic biology, challenges still remain. The very first is protein post-translational modification, which is critical in the biology studies and the disease treatment. These modifications include glycosylation, phosphorylation, ubiquitination, nitrosylation, methylation, acetylation, lipidation and proteolysis. The second is how to expand the genetic code to incorporate multiple different uAAs into a single protein. The next is reuse of the cell-free systems. To address these challenges, cell-free synthetic biosystems must be further optimized to flexibly regulate the transcription and translation by gene editing and addition of exogenous substances. A proposed solution for reusing the cell-free system is designing a membrane bioreactor to extend the lifetime of cell-free system by continuously removing the inhibitory molecules. To broaden the applications, cell-free synthetic biology needs to be integrated with other cutting-edge technologies, such as stem cell, 3D printing, microbiome, neuroscience, and artificial intelligence.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Kwok R. Five hard truths for synthetic biology. Nature. 2010;463:288–290. doi: 10.1038/463288a. [DOI] [PubMed] [Google Scholar]
- 2.Shrestha P., Holland T.M., Bundy B.C. Streamlined extract preparation for Escherichia coli-based cell-free protein synthesis by sonication or bead vortex mixing. Biotechniques. 2012;53:163–174. doi: 10.2144/0000113924. [DOI] [PubMed] [Google Scholar]
- 3.Schoborg J.A., Hodgman C.E., Anderson M.J., Jewett M.C. Substrate replenishment and byproduct removal improve yeast cell-free protein synthesis. Biotechnol J. 2014;9:630–640. doi: 10.1002/biot.201300383. [DOI] [PubMed] [Google Scholar]
- 4.Olliver L., Boyd C.D. In vitro translation of mRNA in a rabbit reticulocyte lysate cell-free system. In: Rapley R., editor. The nucleic acid protocols handbook. Humana Press; Totowa, NJ: 2000. pp. 885–890. [Google Scholar]
- 5.Takai K., Sawasaki T., Endo Y. Practical cell-free protein synthesis system using purified wheat embryos. Nat Protoc. 2010;5:227–238. doi: 10.1038/nprot.2009.207. [DOI] [PubMed] [Google Scholar]
- 6.Ezure T., Suzuki T., Ando E. A cell-free protein synthesis system from insect cells. Methods Mol Biol. 2014;1118:285–296. doi: 10.1007/978-1-62703-782-2_20. [DOI] [PubMed] [Google Scholar]
- 7.Shimizu Y., Inoue A., Tomari Y., Suzuki T., Yokogawa T., Nishikawa K. Cell-free translation reconstituted with purified components. Nat Biotechnol. 2001;19:751–755. doi: 10.1038/90802. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y.H. Production of biofuels and biochemicals by in vitro synthetic biosystems: opportunities and challenges. Biotechnol Adv. 2015;33:1467–1483. doi: 10.1016/j.biotechadv.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Carlson E.D., Gan R., Hodgman C.E., Jewett M.C. Cell-free protein synthesis: applications come of age. Biotechnol Adv. 2012;30:1185–1194. doi: 10.1016/j.biotechadv.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swartz J.R. Transforming biochemical engineering with cell-free biology. AIChE J. 2012;58:5–13. [Google Scholar]
- 11.Lee K.H., Kim D.M. Applications of cell-free protein synthesis in synthetic biology: interfacing bio-machinery with synthetic environments. Biotechnol J. 2013;8:1292–1300. doi: 10.1002/biot.201200385. [DOI] [PubMed] [Google Scholar]
- 12.Sawasaki T., Ogasawara T., Morishita R., Endo Y. A cell-free protein synthesis system for high-throughput proteomics. Proc Natl Acad Sci U. S. A. 2002;99:14652–14657. doi: 10.1073/pnas.232580399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tokmakov A.A., Kurotani A., Takagi T., Toyama M., Shirouzu M., Fukami Y. Multiple post-translational modifications affect heterologous protein synthesis. J Biol Chem. 2012;287:27106–27116. doi: 10.1074/jbc.M112.366351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zimmerman E.S., Heibeck T.H., Gill A., Li X., Murray C.J., Madlansacay M.R. Production of site-specific antibody–drug conjugates using optimized non-natural amino acids in a cell-free expression system. Bioconjugate Chem. 2014;25:351–361. doi: 10.1021/bc400490z. [DOI] [PubMed] [Google Scholar]
- 15.Park N., Um S.H., Funabashi H., Xu J., Luo D. A cell-free protein-producing gel. Nat Mater. 2009;8:432–437. doi: 10.1038/nmat2419. [DOI] [PubMed] [Google Scholar]
- 16.Gonen S., DiMaio F., Gonen T., Baker D. Design of ordered two-dimensional arrays mediated by noncovalent protein-protein interfaces. Science. 2015;348:1365–1368. doi: 10.1126/science.aaa9897. [DOI] [PubMed] [Google Scholar]
- 17.Casteleijn M.G., Urtti A., Sarkhel S. Expression without boundaries: cell-free protein synthesis in pharmaceutical research. Int J Pharm. 2013;440:39–47. doi: 10.1016/j.ijpharm.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Goerke A.R., Swartz J.R. Development of cell-free protein synthesis platforms for disulfide bonded proteins. Biotechnol Bioeng. 2008;99:351–367. doi: 10.1002/bit.21567. [DOI] [PubMed] [Google Scholar]
- 19.Goodsell D.S. The molecular perspective: restriction endonucleases. Oncologist. 2002;7:82–83. doi: 10.1634/theoncologist.7-1-82. [DOI] [PubMed] [Google Scholar]
- 20.Ceelen L.M., Decostere A., Ducatelle R., Haesebrouck F. Cytolethal distending toxin generates cell death by inducing a bottleneck in the cell cycle. Microbiol Res. 2006;161:109–120. doi: 10.1016/j.micres.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Betton J.M. Rapid translation system (RTS): a promising alternative for recombinant protein production. Curr Protein Pept Sci. 2003;4:73–80. doi: 10.2174/1389203033380359. [DOI] [PubMed] [Google Scholar]
- 22.Krogh A., Larsson B., von Heijne G., Sonnhammer E.L. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol. 2001;305:567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- 23.Moraes I., Evans G., Sanchez-Weatherby J., Newstead S., Stewart P.D. Membrane protein structure determination - the next generation. Biochim Biophys Acta. 2014;1838:78–87. doi: 10.1016/j.bbamem.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jewett M.C., Swartz J.R. Mimicking the Escherichia coli cytoplasmic environment activates long-lived and efficient cell-free protein synthesis. Biotechnol Bioeng. 2004;86:19–26. doi: 10.1002/bit.20026. [DOI] [PubMed] [Google Scholar]
- 25.Orban E., Proverbio D., Haberstock S., Dotsch V., Bernhard F. Cell-free expression of G-protein-coupled receptors. Methods Mol Biol. 2015;1261:171–195. doi: 10.1007/978-1-4939-2230-7_10. [DOI] [PubMed] [Google Scholar]
- 26.Lu Y., Welsh J.P., Swartz J.R. Production and stabilization of the trimeric influenza hemagglutinin stem domain for potentially broadly protective influenza vaccines. Proc Natl Acad Sci U. S. A. 2014;111:125–130. doi: 10.1073/pnas.1308701110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Welsh J.P., Lu Y., He X.S., Greenberg H.B., Swartz J.R. Cell-free production of trimeric influenza hemagglutinin head domain proteins as vaccine antigens. Biotechnol Bioeng. 2012;109:2962–2969. doi: 10.1002/bit.24581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wuu J.J., Swartz J.R. High yield cell-free production of integral membrane proteins without refolding or detergents. Biochim. Biophys. Acta (BBA) - Biomembr. 2008;1778:1237–1250. doi: 10.1016/j.bbamem.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 29.Gilmore M.A., Steward L.E., Chamberlin A.R. Incorporation of noncoded amino acids by in vitro protein biosynthesis. In: Schmidtchen F.P., Baltzer L., Chamberlin A.R., McDonnell K.A., Famulok M., Gilmore M.A., editors. Implementation and redesign of catalytic function in biopolymers. Springer Berlin Heidelberg; Berlin, Heidelberg: 1999. pp. 77–99. [Google Scholar]
- 30.Lu Y., Welsh J.P., Chan W., Swartz J.R. Escherichia coli-based cell free production of flagellin and ordered flagellin display on virus-like particles. Biotechnol Bioeng. 2013;110:2073–2085. doi: 10.1002/bit.24903. [DOI] [PubMed] [Google Scholar]
- 31.Agarwal P., Bertozzi C.R. Site-specific antibody-drug conjugates: the nexus of bioorthogonal chemistry, protein engineering, and drug development. Bioconjug Chem. 2015;26:176–192. doi: 10.1021/bc5004982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yin G., Garces E.D., Yang J., Zhang J., Tran C., Steiner A.R. Aglycosylated antibodies and antibody fragments produced in a scalable in vitro transcription-translation system. MAbs. 2012;4:217–225. doi: 10.4161/mabs.4.2.19202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ratner M. Celgene wagers on Sutro's cell-free platform to ramp up bispecifics. Nat Biotech. 2014;32 doi: 10.1038/nbt1214-1175. 1175–75. [DOI] [PubMed] [Google Scholar]
- 34.Lu Y., Swartz J.R. Functional properties of flagellin as a stimulator of innate immunity. Sci Rep. 2016;6:18379. doi: 10.1038/srep18379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu Y., Chan W., Ko B.Y., VanLang C.C., Swartz J.R. Assessing sequence plasticity of a virus-like nanoparticle by evolution toward a versatile scaffold for vaccines and drug delivery. Proc Natl Acad Sci U. S. A. 2015;112:12360–12365. doi: 10.1073/pnas.1510533112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu Y., Zhao H., Zhang C., Xing X.-H. Insights into the global regulation of anaerobic metabolism for improved biohydrogen production. Bioresour Technol. 2016;200:35–41. doi: 10.1016/j.biortech.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 37.Zawada J.F., Yin G., Steiner A.R., Yang J., Naresh A., Roy S.M. Microscale to manufacturing scale-up of cell-free cytokine production–a new approach for shortening protein production development timelines. Biotechnol Bioeng. 2011;108:1570–1578. doi: 10.1002/bit.23103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woodward J., Orr M., Cordray K., Greenbaum E. Enzymatic production of biohydrogen. Nature. 2000;405:1014–1015. doi: 10.1038/35016633. [DOI] [PubMed] [Google Scholar]
- 39.Myung S., Rollin J., You C., Sun F., Chandrayan S., Adams M.W.W. In vitro metabolic engineering of hydrogen production at theoretical yield from sucrose. Metab Eng. 2014;24:70–77. doi: 10.1016/j.ymben.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 40.Smith M.T., Wilding K.M., Hunt J.M., Bennett A.M., Bundy B.C. The emerging age of cell-free synthetic biology. FEBS Lett. 2014;588:2755–2761. doi: 10.1016/j.febslet.2014.05.062. [DOI] [PubMed] [Google Scholar]
- 41.Zhu Z., Kin Tam T., Sun F., You C., Percival Zhang Y.H. A high-energy-density sugar biobattery based on a synthetic enzymatic pathway. Nat Commun. 2014;5:3026. doi: 10.1038/ncomms4026. [DOI] [PubMed] [Google Scholar]
- 42.Guterl J.K., Garbe D., Carsten J., Steffler F., Sommer B., Reisse S. Cell-free metabolic engineering: production of chemicals by minimized reaction cascades. ChemSusChem. 2012;5:2165–2172. doi: 10.1002/cssc.201200365. [DOI] [PubMed] [Google Scholar]
- 43.Fischer S. Cell break: how cell-free biology is finally putting the engineering back in bioengineering. IEEE Pulse. 2016;7:13–16. doi: 10.1109/MPUL.2016.2514881. [DOI] [PubMed] [Google Scholar]
- 44.Rollin J.A., Tam T.K., Zhang Y.H.P. New biotechnology paradigm: cell-free biosystems for biomanufacturing. Green Chem. 2013;15:1708–1719. [Google Scholar]
- 45.Chang T.M. Therapeutic applications of polymeric artificial cells. Nat Rev Drug Discov. 2005;4:221–235. doi: 10.1038/nrd1659. [DOI] [PubMed] [Google Scholar]
- 46.Blain J.C., Szostak J.W. Progress toward synthetic cells. Annu Rev Biochem. 2014;83:615–640. doi: 10.1146/annurev-biochem-080411-124036. [DOI] [PubMed] [Google Scholar]
- 47.Osaki T., Takeuchi S. Artificial cell membrane systems for biosensing applications. Anal Chem. 2017;89:216–231. doi: 10.1021/acs.analchem.6b04744. [DOI] [PubMed] [Google Scholar]
- 48.Xu C., Hu S., Chen X. Artificial cells: from basic science to applications. Mater Today. 2016;19:516–532. doi: 10.1016/j.mattod.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hutchison C.A., 3rd, Chuang R.Y., Noskov V.N., Assad-Garcia N., Deerinck T.J., Ellisman M.H. Design and synthesis of a minimal bacterial genome. Science. 2016;351:aad6253. doi: 10.1126/science.aad6253. [DOI] [PubMed] [Google Scholar]
- 50.Shin J., Noireaux V. An E. coli cell-free expression toolbox: application to synthetic gene circuits and artificial cells. ACS Synth Biol. 2012;1:29–41. doi: 10.1021/sb200016s. [DOI] [PubMed] [Google Scholar]
- 51.Kuruma Y., Stano P., Ueda T., Luisi P.L. A synthetic biology approach to the construction of membrane proteins in semi-synthetic minimal cells. Biochim Biophys Acta. 2009;1788:567–574. doi: 10.1016/j.bbamem.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 52.Wu F., Tan C. The engineering of artificial cellular nanosystems using synthetic biology approaches. Wiley Interdisciplin Rev Nanomed Nanobiotechnol. 2014;6:369–383. doi: 10.1002/wnan.1265. [DOI] [PubMed] [Google Scholar]
- 53.Noireaux V., Maeda Y.T., Libchaber A. Development of an artificial cell, from self-organization to computation and self-reproduction. Proc Natl Acad Sci U. S. A. 2011;108:3473–3480. doi: 10.1073/pnas.1017075108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Elani Y., Law R.V., Ces O. Protein synthesis in artificial cells: using compartmentalisation for spatial organisation in vesicle bioreactors. Phys Chem Chem Phys. 2015;17:15534–15537. doi: 10.1039/c4cp05933f. [DOI] [PubMed] [Google Scholar]
- 55.Davidson E.A., Dlugosz P.J., Levy M., Ellington A.D. John Wiley & Sons, Inc; 2001. Directed evolution of proteins in vitro using compartmentalization in emulsions. Current protocols in molecular biology. [DOI] [PubMed] [Google Scholar]
- 56.Zhang R., Ruder W.C. A new environmental biosensor for cell free synthetic biological systems. Biophys. J. 2015;108:481a. [Google Scholar]
- 57.Ho K.K., Murray V.L., Liu A.P. Engineering artificial cells by combining HeLa-based cell-free expression and ultrathin double emulsion template. Methods Cell Biol. 2015;128:303–318. doi: 10.1016/bs.mcb.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Karzbrun E., Tayar A.M., Noireaux V., Bar-Ziv R.H. Programmable on-chip DNA compartments as artificial cells. Science. 2014;345:829–832. doi: 10.1126/science.1255550. [DOI] [PubMed] [Google Scholar]