Abstract
Mathematical modelling has become an established tool for studying the dynamics of biological systems. Current applications range from building models that reproduce quantitative data to identifying systems with predefined qualitative features, such as switching behaviour, bistability or oscillations. Mathematically, the latter question amounts to identifying parameter values associated with a given qualitative feature. We introduce a procedure to partition the parameter space of a parameterized system of ordinary differential equations into regions for which the system has a unique or multiple equilibria. The procedure is based on the computation of the Brouwer degree, and it creates a multivariate polynomial with parameter depending coefficients. The signs of the coefficients determine parameter regions with and without multistationarity. A particular strength of the procedure is the avoidance of numerical analysis and parameter sampling. The procedure consists of a number of steps. Each of these steps might be addressed algorithmically using various computer programs and available software, or manually. We demonstrate our procedure on several models of gene transcription and cell signalling, and show that in many cases we obtain a complete partitioning of the parameter space with respect to multistationarity.
Author summary
Mathematical modelling has become an important tool in biology. As modelling requires separating the essential from the ordinary, there is never just one model but a collection thereof. To understand biology through modelling it is therefore crucial to be able to tell which of these models are capable of reproducing an observed behaviour and which are not. For example, to understand cellular decision making, models allowing multiple equilibria are studied and one asks which models allow for this behaviour. Here we describe a procedure that links the existence of a unique and of multiple equilibria to the sign of a single expression. We demonstrate the usefulness of the procedure by applying it to models of gene transcription and cellular signalling.
Introduction
Mathematical models in the form of parameterized systems of ordinary differential equations (ODEs) are valuable tools in biology. Often, qualitative properties of the ODEs are associated with macroscopic biological properties and biological functions [1–4]. It is therefore important that we are able to analyse mathematical models with respect to their qualitative features and to understand when these properties arise in models. With the growing adaptation of differential equations in biology, an automated screening of ODE models for parameter dependent properties and discrimination of parameter regions with different properties would be a very useful tool for biology, and perhaps even more for synthetic biology [5]. Even though it is currently not conceivable how and if this task can be efficiently formalized, we view the procedure presented here as a first step in this direction.
Multistationarity, that is, the capacity of the system to rest in different positive equilibria depending on the initial state of the system, is an important qualitative property. Biologically, multistationarity is linked to cellular decision making and ‘memory’-related on/off responses to graded input [2–4]. It has been suggested that different stable equilibria of a cell represent different cell types [6, 7]. Whole-cell modelling provides an opportunity to understand the number and type of the stable equilibria of the cell and could potentially give insight into the different cell types that a particular cell can differentiate into and transition between. Currently, this is an important open question in biology [8]. Moreover, the existence of multiple equilibria is often a design objective in synthetic biology [9, 10]. Various mathematical methods, developed in the context of reaction network theory, can be applied to decide whether multistationarity exists for some parameter values or not at all, or to pinpoint specific values for which it does occur [11–20]. Some of these methods are freely available as software tools [21, 22].
It is a hard mathematical problem to delimit parameter regions for which multistationarity occurs. Often it is solved by numerical investigations and parameter sampling, guided by biological intuition or by case-by-case mathematical approaches. A general approach, in part numerical, is based on a certain bifurcation condition [18, 19, 23, 24]. Alternatively, for polynomial ODEs, a decomposition of the parameter space into regions with different numbers of equilibria could be achieved by Cylindrical Algebraic Decomposition (a version of quantifier elimination) [25]. This method, however, scales very poorly and is thus only of limited help in biology, where models tend to be large in terms of the number of variables and parameters.
Here we present two new theoretical results pertaining to multistationarity (Theorem 1 and Corollary 2). The results are in the context of reaction network theory and generalize ideas in [26, 27]. We consider a parameterized ODE system defined by a reaction network and compute a single polynomial in the species concentrations with coefficients depending on the parameters of the system. The theoretical results relate the capacity for multiple equilibria or a single equilibrium to the signs of the polynomial as a function of the parameters and the variables (concentrations).
The theoretical results apply to dissipative reaction networks (networks for which all trajectories eventually remain in a compact set) without boundary equilibria in stoichiometric compatibility classes with non-empty interior. These conditions are met in many reaction network models of molecular systems. We show by example that the results allow us to identify regions of the parameter space for which multiple equilibria exist and regions for which only one equilibrium exists. Subsequently this leads to the formulation of a general procedure for detecting regions of mono- and multistationarity. The procedure verifies the conditions of the theoretical results and further, calculates the before-mentioned polynomial. A key ingredient is the existence of a positive parameterization of the set of positive equilibria. Such a parameterization is known to exist for many classes of reaction networks, for example, systems with toric steady states [14] and post-translational modification systems [28, 29].
The conditions of the procedure might be verified manually or algorithmically according to computational criteria. The algorithmic criteria are, however, only sufficient for the conditions to hold. For example, a basic condition is that of dissipativity. To our knowledge there is not a sufficient and necessary computational criterion for dissipativity, but several sufficient ones. If these fail, then the reaction network might still be dissipative, which might be verified by other means. By collecting the algorithmic criteria, the procedure can be formulated as a fully automated procedure (an algorithm) that partitions the parameter space without any manual intervention. The algorithm might however terminate indecisively if some of the criteria are not met.
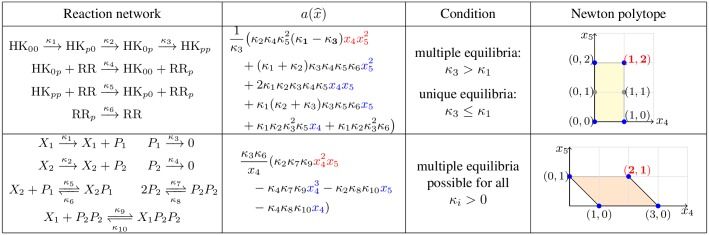
Table 1 shows two examples of reaction network motifs that occur frequently in intracellular signalling: a two-site protein modification by a kinase–phosphatase pair and a one-site modification of two proteins by the same kinase–phosphatase pair. These reaction networks are in the domain of the automated procedure and conditions for mono- and multistationarity can be found without any manual intervention. The conditions discriminating between a unique and multiple equilibria highlight a delicate relationship between the catalytic and Michaelis-Menten constants of the kinase and the phosphatase with the modified protein as a substrate (the kc- and kM-values). If the condition for multiple equilibria is met, then multiple equilibria occur provided the total concentrations of kinase, phosphatase and substrate are in suitable ranges (values thereof can be computed as part of the procedure).
Table 1. Conditions for unique and multiple equilibria in post-translational modification of proteins.
| Motif | Condition |
|---|---|
|
|
Multiple: b(κ) < 0, Unique: b(κ) ≥ 0 |
|
|
b1(κ) = kc1kc4 − kc2kc3 b2(κ) = kc1kc4(kM2 + kM3) − kc2kc3(kM1 + kM4) Multiple: b1(κ) < 0, Unique: b1(κ) ≥ 0 and b2(κ) ≥ 0 |
The symbols kci and kMi denote respectively the catalytic and the Michaelis-Menten constants of the i-th modification step (i = 1: phosphorylation of A, i = 2: dephosphorylation of Ap, i = 3: phosphorylation of B or Ap, i = 4: dephosphorylation of Bp or App). All parameter values satisfying the conditions in the second column yield multiple (unique) equilibria for some (all) values of the conserved quantities. For the second motif, we cannot decide on the number of equilibria for b1(κ) ≥ 0 and b2(κ) < 0. See §6.1 and §6.2 in the S1 File for details.
The paper has three main sections: a theoretical section, a section about the procedure and an application section. We close the paper with two brief sections discussing computational limitations, related work and future directions. In the theoretical section we first introduce notation and mathematical background material. We then give the theorem and the corollary that links the number of equilibria to the sign of the determinant of the Jacobian of a certain function, which is derived from the ODE system associated with a reaction network. In the second section we state the procedure, derive the algorithm and comment on the feasibility and verifiability of the conditions. Finally, in the application section we apply the procedure to several examples. The S1 File has six sections. All proofs are relegated to §1–4 together with background material. In §5 we elaborate further on how the conditions of the procedure/algorithm can be verified. In §6 we provide details of the algorithmic analysis of the examples in Table 1. Also we include a further monostationary example for illustration of the algorithm.
Results
Theory
In this part of the manuscript we present the theoretical results. We start by introducing the basic formalism of reaction networks. Theorem 1, Corollary 1 and 2 below apply to dissipative networks without boundary equilibria and concern the (non)existence of multiple equilibria in some stoichiometric compatibility class. Corollary 2 assumes the existence of a positive parameterization of the set of positive equilibria. Before stating the results these five concepts are formally defined.
Reaction networks
A reaction network, or simply a network, consists of a set of species {X1, …, Xn} and a set of reactions of the form:
| (1) |
where αij, βij are non-negative integers. The left hand side is called the reactant, while the right hand side is called the product. We let be the stoichiometric matrix of the network, defined as Nij = βij − αij, that is, the (i, j)-th entry encodes the net production of species Xi in reaction Rj. We refer to the ‘running example’ in Fig 1 for an illustration of the definitions.
Fig 1. Running example.
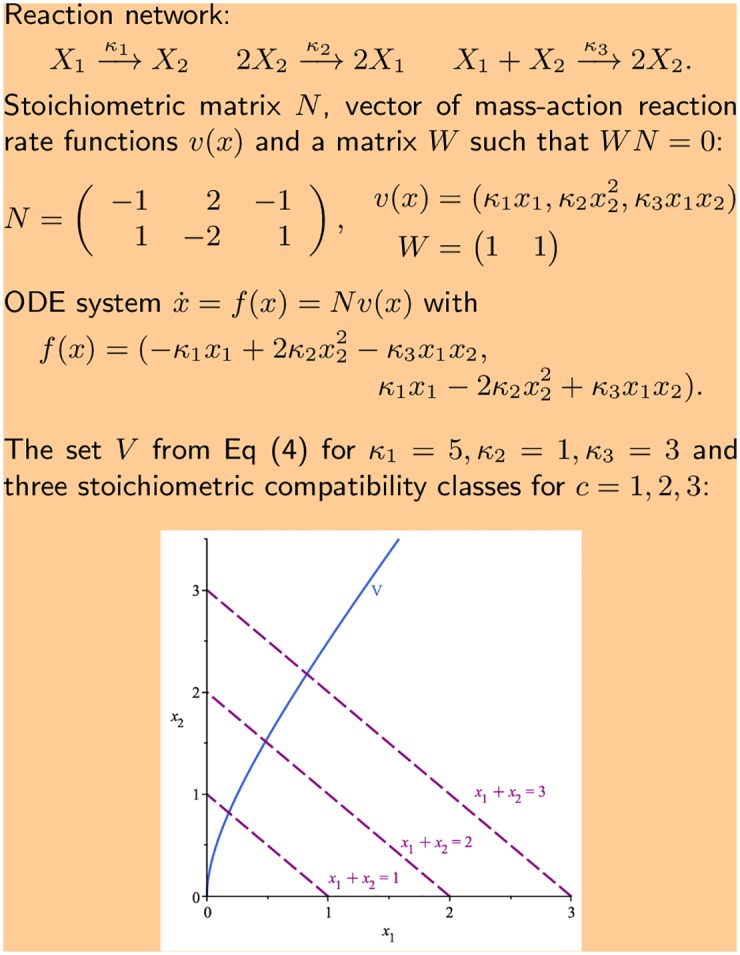
Example network with two species, X1 and X2, and three reactions with mass-action kinetics.
The concentrations of the species X1, …, Xn are denoted by lower-case letters x1, …, xn and we let x = (x1, …, xn). We denote by (), the positive (non-negative) orthant in . The evolution of the concentrations with respect to time is modeled as an ODE system derived from a set of reaction rate functions. A reaction rate function for reaction Rj is a -function that models the (non-negative) speed of the reaction. We further assume that
| (2) |
that is, the reaction only takes place in the presence of all reactant species. We refer to the set of reaction rate functions as the kinetics.
A particular important example of a kinetics is that of mass-action kinetics. In this case the reaction rate functions are given by
where κj is a positive number called the reaction rate constant and we assume 00 = 1. Other important examples are Michaelis-Menten kinetics and Hill kinetics. All three types of kinetics fulfil the assumption in Eq (2).
For a choice of reaction rate functions v = (v1, …, vℓ), the ODE system modelling the species concentrations over time with initial condition x(0) = x0, is
| (3) |
Under assumption Eq (2), the orthants and are forward-invariant under f in Eq (3) [30, Theorem 5.6], [31, Section 16]. Forward-invariance implies that the solutions to the ODE system stays in (resp. ) for all positive times if the initial condition is in (resp. ).
The trajectories of the ODEs in Eq (3) are confined to the so-called stoichiometric compatibility classes, which are defined as follows. Let s = rank(N) be the rank of the network and d = n − s be the corank. Further, let be any matrix of full rank d such that WN = 0, see Fig 1 for an example. This matrix is zero-dimensional if N has full rank n. For each , there is an associated stoichiometric compatibility class defined as
This set is empty if . The positive stoichiometric compatibility class is defined as the relative interior of , that is, the intersection of with the positive orthant:
The sets and are convex. Since by construction Wx is conserved over time and determined by the initial condition, then and are also forward-invariant.
An equation of the form ω ⋅ x = c′ for some ω ∈ im(N)⊥ and is called a conservation relation. In particular, Wx = c forms a system of d conservation relations.
For the running example in Fig 1, the rank of the network is s = 1 and the corank is d = 1. The matrix W in the figure leads to the conservation relation x1 + x2 = c. Here the stoichiometric compatibility class has non-empty interior, that is, , if and only if c > 0.
In the following, to ease the notation, we implicitly assume a reaction network comes with a kinetics (a set of reaction rate functions) and the associated ODE system.
Dissipative and conservative reaction networks
A reaction network is dissipative if, for all stoichiometric compatibility classes , there exists a compact set where the trajectories of eventually enter (see §3.2 in the S1 File). A reaction network is conservative if there exists a conservation relation with only positive coefficients, or, equivalently, if for all species Xi there is a conservation relation such that the coefficient of xi is positive and all other coefficients are non-negative. This is equivalent to the stoichiometric compatibility classes being compact sets [32]. Hence, in particular, a conservative reaction network is dissipative because we can choose the attracting compact set to be the stoichiometric compatibility class itself. Because of the conservation relation x1 + x2 = c, the reaction network of the running example is conservative.
Equilibria
Given the ODE in Eq (3), the set of non-negative equilibria is the set of points for which f(x) vanishes:
| (4) |
We are interested in the positive equilibria in each stoichiometric compatibility class, that is, in the set . Generically, this set consists of isolated points obtained as the simultaneous positive solutions to the equations
| (5) |
Fig 1 shows a representation of the set V together with examples of stoichiometric compatibility classes for the running example. The figure suggests that the set V intersects each stoichiometric compatibility class in exactly one point.
We introduce some definitions: a network admits multiple equilibria (or is multistationary) if there exists such that contains at least two points, that is, the system in Eq (5) has at least two positive solutions. Equilibria belonging to but not to for some c are boundary equilibria. A boundary equilibrium has at least one coordinate equal to zero.
The function φc(x)
Some of the n equations in the system f(x) = 0 might be redundant. Indeed, every vector ω ∈ im(N)⊥ fulfils ω ⋅ f(x) = 0, and hence gives a linear relation among the entries of f(x). As a consequence, there are (at least) as many independent linear relations as rows of W, that is, d, and there are at most s = n − d linearly independent equations in the system f(x) = 0. Thus d of the equations are redundant. By removing these from f(x) = 0, the system in Eq (5) becomes a system of n equations in n variables.
In order to systematically choose d equations to remove, we proceed as follows. We choose the matrix of conservation relations to be row reduced and let i1, …, id be the indices of the first non-zero coordinate of each row. Then the scalar product of the j-th row of W with f(x) can be used to express fij(x) as a linear combination of the entries of f(x) with indices different from i1, …, id. It follows that the equations fi1(x) = 0, …, fid(x) = 0 can be removed.
For , we define the -function by
| (6) |
For the running example in Fig 1 the matrix W is already row reduced with i1 = 1. Hence φc is obtained by replacing f1(x) with x1 + x2 − c:
As the function φc(x) is obtained by replacing redundant equations in f(x) = 0 with equations defining , we have
Consequently, a network admits multiple equilibria if the equation φc(x) = 0 has at least two positive solutions for some .
A theorem for unique and multiple equilibria
Let be the Jacobian matrix of φc(x), that is, the matrix with (i, j)-th entry equal to the partial derivative of φc,i(x) with respect to xj. The matrix M(x) does not depend on c, see Eq (6).
We say that an equilibrium is non-degenerate if the Jacobian of φc at x*, M(x*), is non-singular, that is, if det(M(x*)) ≠ 0 [13].
Theorem 1 (Unique and multiple equilibria). Assume the reaction rate functions fulfil Eq (2), let s = rank(N) and let be a stoichiometric compatibility class such that , where . Further, assume that
(i) The network is dissipative.
(ii) There are no boundary equilibria in .
Then the following holds.
(A’) Uniqueness of equilibria. If
then there is exactly one positive equilibrium in . Further, this equilibrium is non-degenerate.
(B’) Multiple equilibria. If
then there are at least two positive equilibria in , at least one of which is non-degenerate. If all positive equilibria in are non-degenerate, then there are at least three and always an odd number.
The proof of Theorem 1 is based on relating det(M(x)) to the Brouwer degree of φc at 0 (see §1-§4 in the S1 File). Note that the only situation that is not covered by Theorem 1 is when sign(det(M(x))) takes the value 0 for some x, but never the value (−1)s+1. The determinant of M(x) is the same as the core determinant in [33, Lemma 3.7]. See also [13, Remark 9.27].
To check whether the sign conditions in part (A’) or (B’) hold requires information about the equilibria in . As such, these conditions are difficult to check. If sign(det(M(x))) is constant for all x in a set containing the positive equilibria, then the condition in (A’) is always fulfilled. In particular, this is the case for injective networks, where sign(det(M(x))) = (−1)s for all [13] (see also [34–39] for related work on injective networks). The latter might be verified or falsified without any knowledge about the equilibria of the system (see the comments to Step 5 and Step 7 in the section “Procedure for finding parameter regions for mono- and multistationarity”).
Corollary 1 (Unique equilibria). Assume that the assumptions of Theorem 1 hold and that sign(det(M(x))) = (−1)s for all . Then there is exactly one positive equilibrium in each stoichiometric compatibility class. Further, this equilibrium is non-degenerate.
The conclusions of Theorem 1 refer specifically to non-degenerate equilibria. Non-degenerate equilibria are always isolated from each other within a given stoichiometric compatibility class, as det(M(x)) ≠ 0 ensures M(x) is locally invertible. In some situations we might be able to “lift” non-degenerate equilibria of a reaction network to another reaction network that in some sense is larger, thereby proving lower bounds on the number of non-degenerate equilibria of the larger reaction network. This is for example the case if the smaller network is embedded in the larger [40, 41], if the smaller network is without inflows/outflows while the larger has all inflows/outflows [42], or if the smaller is obtained by elimination of intermediate species [43]. Conditions for the existence of degenerate equilibria, where det(M(x)) is expected to change sign, are also known [23, 44].
Positive parameterizations and a corollary
Verifying condition (A’) or (B’) is considerably easier if there exists a positive parameterization of the set of all positive equilibria. In this subsection we define such a parameterization and restate Theorem 1 as Corollary 2 in this situation. In the following sections this corollary will become the foundation for the procedure to partition the parameter space into regions with different equilibrium properties.
By a positive parameterization of the set of positive equilibria we mean a surjective function
| (7) |
for some m < n, such that is the vector of free variables. In other words, a positive parameterization implies that x1, …, xn are expressed at equilibrium as functions of :
such that x1, …, xn are positive provided is positive. Thus
| (8) |
Typically, the number of free variables equals the corank of the network, that is m = d = n − s.
We say that a parameterization is algebraic if the components are polynomials or rational functions (quotients of polynomials) and can be given such that the denominator is positive for all . See Fig 2 (Step 6) for an application to the running example. Note that the parameterizations considered here do not make use of the conservation relations.
Fig 2. Step 1-3 check the assumptions of Corollary 1 and Corollary 2.
In step 4 the function φc(x) is constructed and the determinant of M(x) is found. Step 5 is the sign analysis of the polynomial det(M(x)) for . Step 6 establishes a positive parameterization and finds the polynomial . Step 7 is similar to step 5, but for .
A positive equilibrium , , belongs to the stoichiometric compatibility class where
| (9) |
Combining Eqs (8) and (9), it follows that the positive solutions to Eq (5) for a given c are in one-to-one correspondence with the positive solutions to Eq (9), that is,
In order to restate Theorem 1 using the parameterization Φ, we consider the determinant of M(x) evaluated at ,
| (10) |
Corollary 2 (Positive parameterization). Assume the reaction rate functions fulfil Eq (2) and let s = rank(N). Further, assume that
(i) The network is dissipative.
(ii) There are no boundary equilibria in , for all such that .
(iii) The set of positive equilibria admits a positive parameterization as in Eq (7).
Then the following holds.
(A) Uniqueness of equilibria. If
then there is exactly one positive equilibrium in each with . Further, this equilibrium is non-degenerate.
(B) Multiple equilibria. If
then there are at least two positive equilibria in the stoichiometric compatibility class where . Further, at least one of the equilibria is non-degenerate. If all positive equilibria in are non-degenerate, then there are at least three equilibria and always an odd number.
Note that, contrary to Theorem 1, the stoichiometric compatibility class is not fixed in the corollary.
In the next section we formulate a procedure based on Corollary 1 and Corollary 2 to find regions of mono- and multistationarity. Before that we end this section with an application to the running example. The analysis is divided into seven steps which prelude the steps of the procedure.
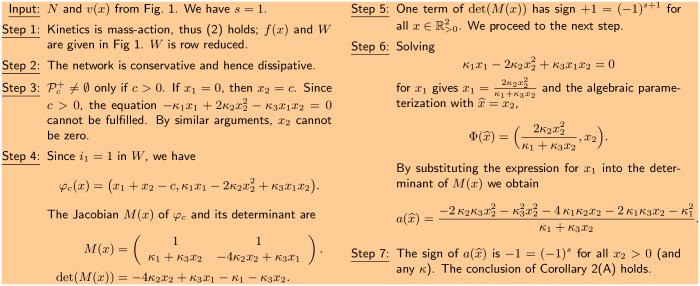
Application of Corollary 1 and Corollary 2 to the running example
We start with the setup given in Fig 1 and first check whether the sign condition of Corollary 1 is fulfilled, in which case there is a single equilibrium in all stoichiometric compatibility classes. The steps of the analysis are illustrated in Fig 2.
The assumptions of the corollary are easily verified in this case. As we are assuming mass-action kinetics, Eq (2) is fulfilled (Fig 2, Step 1). Further the network is conservative, hence dissipative and (i) is fulfilled (Fig 2, Step 2). It is easily seen that there are no boundary equilibria in any stoichiometric compatibility class with non-empty interior (Fig 2, Step 3). Hence (ii) is fulfilled. We then construct φc(x) and calculate the determinant of M(x). It is a polynomial (in fact, a linear function) in x1, x2 with coefficients containing both positive and negative terms (Fig 2, Step 4). By choosing with x1 large enough, the determinant of M(x) is positive (Fig 2, Step 5). Therefore Corollary 1 cannot be applied as s = 1. We note that this conclusion is independent of the specific choice of the parameter vector κ = (κ1, κ2, κ3), so in fact it holds for all parameter values.
Corollary 2 has the same assumptions as Corollary 1. We find a positive parameterization by solving the equilibrium equation for x1. That is, we treat it as an equation in x1, while x2 is treated as a parameter. The function a(x2) obtained by substituting x by Φ(x2) in the determinant of M(x) is given in Fig 2, Step 6. It is clear from the expression of a(x2) that it takes the sign −1 for all x2 > 0. Also this conclusion does not depend on the specific value of κ. By application of Corollary 2(A) with s = 1, we conclude that there exists a unique positive non-degenerate equilibrium in each stoichiometric compatibility class with c > 0, for all values of the reaction rate constants (Fig 2, Step 7). The possibility of multiple equilibria is therefore excluded. In this particular example the existence of a positive parameterization is essential to draw the conclusion.
To illustrate how Corollary 2 can be used to find parameter regions for multistationarity, we consider the polynomial corresponding to the hybrid histidine kinase example worked out in detail below, where n = 6 and s = 4:
Only one of the coefficients of the polynomial in x4, x5 can be negative. If κ3 ≤ κ1, then for all positive . Corollary 2(A) implies that there is a unique positive non-degenerate equilibrium in each stoichiometric compatibility class with non-empty positive part.
Oppositely, we show that for κ3 > κ1, Corollary 2(B) applies. For that, let x4 = T and x5 = T. Then becomes a polynomial in T with negative leading coefficient of degree 3. For T large enough, is negative, and we conclude that there exists such that . Corollary 2(B) implies that there exists a stoichiometric compatibility class that contains at least two positive equilibria. In summary, the region of the parameter space for which multistationarity exists is completely characterized by the inequality κ3 > κ1.
Step 5 and 7 are sign analyses of det(M(x)) and , respectively. These are crucial steps and essential for determining parameter regions with mono- and multistationarity. In general, the sign of a polynomial might be studied by studying the signs of the coefficients of the monomials in the polynomial. If all coefficients have the same sign, then the polynomial is either positive or negative for all , respectively, , depending on the sign, and Corollary 1, respectively, Corollary 2(A) applies. If this is not the case, then Corollary 2(B) might be applicable if we can show that the polynomial has the sign (−1)s+1 for some . In the two examples discussed here, the signs of det(M(x)) and are straightforward to analyse. However, this is not always the case, see the section “Checking the steps of the procedure”.
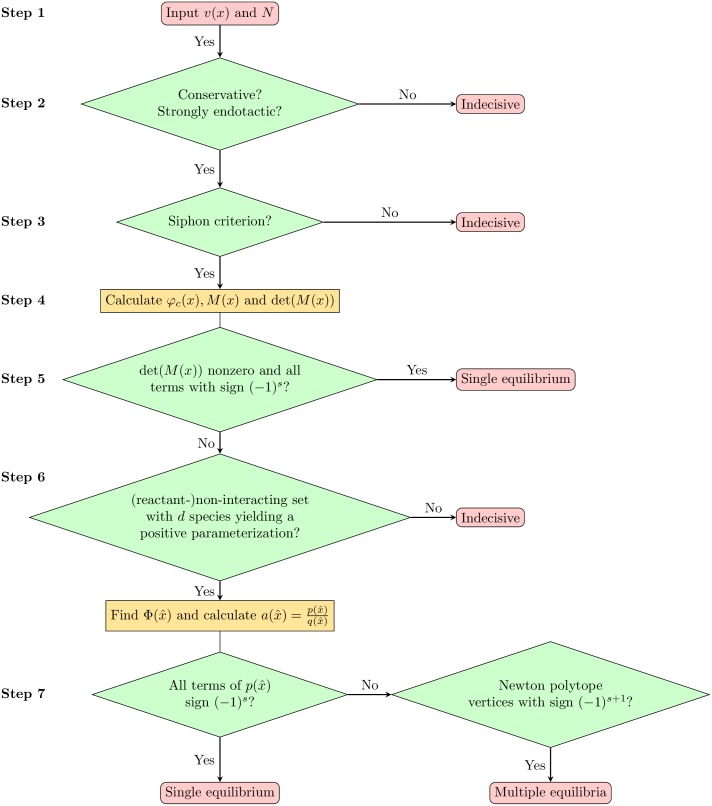
Procedure for finding parameter regions for multistationarity
In the previous subsection we applied Corollary 1 and Corollary 2 to the running example by going through a number of steps corresponding to the conditions of the statements and the calculation of the determinant. In this section we outline the steps formally. Afterwards we discuss the steps and how they can be verified either manually or algorithmically, that is, without user intervention. Finally we devise an algorithm to conclude uniqueness of equilibria or to find regions in the parameter space where multistationarity occurs. We conclude this section with some extra examples that follow the steps of the procedure.
We assume the reaction rate functions v(x) depend on some parameters κ. The reaction rate functions are further assumed to be polynomials (as for mass-action kinetics) or quotients of polynomials (as for Michaelis-Menten and Hill kinetics with integer exponents).
The input to the procedure is v(x) and N (the stoichiometric matrix) and the output is parameter regions for which the network admits multistationarity or uniqueness of equilibria.
Procedure (Identification of parameter regions for multistationarity)
Input: N and v(x) depending on κ.
1. Find f(x), a row reduced matrix W of size d × n such that WN = 0, and check that v(x) vanishes in the absence of one of the reactant species, that is, check that it satisfies Eq (2).
2. Check that the network is dissipative.
3. Check for boundary equilibria in for and .
4. Construct φc(x), M(x) and compute det(M(x)).
5. Analyze the sign of det(M(x)). Find conditions on the parameters κ such that sign(det(M(x))) = (−1)s for all , in which case Corollary 1 holds.
If Corollary 1 does not hold for all κ, continue to the next step.
6. Obtain an algebraic parameterization of the set of positive equilibria for all κ, as in Eq (7), such that the coefficients of the numerator and the denominator of each possibly depend on κ. Compute . By hypothesis, can be written as the quotient of two polynomials in with coefficients depending on κ, whose denominator takes positive values.
7. Analyze the sign of the numerator of .
7a. Identify coefficients with sign (−1)s+1 and coefficients that can have different signs depending on the parameters.
7b. Use the terms corresponding to identified coefficients to construct parameter inequalities such that, whenever these inequalities hold, one has either for all or for at least one , in which case either Corollary 2(A) or (B) holds.
There is no guarantee that all steps of the procedure can be carried out successfully, let alone automatically. While step 1 and 4 usually are straightforward (only computational issues might arise for large networks), step 2, 3, 5, 6 and 7 might in particular require case specific approaches. However, there exist computationally feasible sufficient criteria that guarantee the conditions in each step can be checked efficiently.
Checking the steps of the procedure
Step 2: establishing dissipativity. If the network is not dissipative, then at least one concentration grows to infinity over time. This is typically not the case for realistic networks, but it needs to be ruled out in order to apply the procedure.
We start by checking whether the network is conservative. This implies solving the linear system ωt N = 0 with the constraint ω > 0. Alternatively, conservation relations are often easily established by inspection of the reactions. For example, in many signalling networks, the total concentration of enzyme (free and bounded) and of substrate (phosphoforms) are conserved.
If the network is not conservative, then we check whether it is strongly endotactic [45, 46]. Strongly endotactic reaction networks are in particular permanent, that is, dissipative and the compact set can be chosen such that it does not intersect the boundary of , see [45–47] for details.
If the network is neither conservative nor strongly endotactic, then we can use the following proposition to decide on dissipativity (see the §3.2 in the S1 File).
Proposition 1 (Dissipative network). Let ||⋅|| be a norm in . Assume that for each c with , there exists a vector and a number R > 0 such that ωc ⋅ f(x) < 0 for all with ||x|| > R. Then the network is dissipative.
Thus, we look for vectors ωc with all coordinates positive and such that ωc ⋅ f(x) < 0 for large x. To avoid restricting the parameter values, this computation should be done symbolically.
Step 3: absence of boundary equilibria. For systems of moderate size it is often possible to establish nonexistence of boundary equilibria by arguments similar to those employed in the analysis of the running example: for each i, assume xi = 0, and show that it leads to a contradiction.
A systematic procedure to check for the existence of boundary equilibria relies on computing the so-called minimal siphons of the network [48]. A siphon is a set of species Z ⊆ {X1, …, Xn} fulfilling the following closure property: if Xi ∈ Z and Xi is produced in reaction Rj (that is, βij > 0), then there exists Xk ∈ Z such that Xk is consumed in the same reaction (that is, αkj > 0). A minimal siphon is a siphon that does not properly contain any other siphon.
Proposition 2 (Siphons) ([49, 50]) If for every minimal siphon Z there exists a subset {Xi1, …, Xik} ⊆ Z, and a conservation relation λ1xi1 + … + λkxik = c for some positive λ1, …, λk, then the network has no boundary equilibria in any stoichiometric compatibility class with .
The hypothesis of the proposition can be summarised by saying that each minimal siphon contains the support of a positive conservation relation.
For example, the running example has only one minimal siphon, namely {X1, X2}. The conservation relation x1 + x2 = c fulfils the requirement of Proposition 2, and hence the network has no boundary equilibria in any with c > 0.
More information about using siphons to preclude boundary equilibria is given in the section “Computational issues” below and in §5.1 of the S1 File.
Step 5: determining the sign of det(M(x)). If the kinetics is mass-action, then det(M(x)) is a polynomial in x. In general, if the reaction rate functions are rational functions in x, then so is det(M(x)). In the latter case, if the jth reaction rate function fulfils vj(x) = pj(x)/qj(x) with pj(x) ≥ 0 and qj(x) > 0 for all , then det(M(x)) = p(x)/q(x), where . It follows from the definition of M(x) and by differentiation of vj(x), j = 1, …, ℓ.
We determine conditions on the parameters such that all coefficients of p(x) have sign (−1)s. Then the sign of det(M(x)) is also (−1)s for all and Corollary 1 holds.
Step 6: finding an algebraic positive parameterization. Computer algebra systems like Maple or Mathematica can be used to find a parameterization. One strategy is to solve the equations fi(x) = 0, i ∉ {i1, …, id}, for some subset of (at most) s variables, treating the remaining (at least) d variables as coefficients of the system. If a parameterization found in this way exists but is not positive, another set of variables should be tried out. This can be systematically addressed by trying out all possible subsets of variables. It requires computation and analysis of at most parameterizations. Alternatively, one can compute the circuits of degree one of the matroid associated with the equilibrium equations [51].
In some cases, the network structure implies that a positive parameterization of the set of equilibria exists. A set, say {Xk+1, …, Xn} with n − k elements for some k, is non-interacting if two species never appear on the same side of a reaction and they have coefficient at most one in all reactions. In this case the equilibrium equations fk+1(x) = ⋯ = fn(x) = 0 form a linear system in the variables {xk+1, …, xn}. Provided that the determinant of the coefficient matrix of the linear system is not identically zero, this system can be solved and we obtain a positive parameterization of the non-interacting variables xk+1, …, xn at equilibrium in terms of the remaining variables x1, …, xk [52, 53]. A necessary condition for the determinant of the coefficient matrix not being identically zero is that there is no conservation relation of the form xi1 + ⋯ + xil with i1, …, il ∈ {k + 1, …, n}. If a non-interacting set with k = d exists, that is, with s = n − d elements, then this guarantees the existence of the desired parameterization. In the running example there is not a non-interacting set because both species have coefficient 2 in the reaction 2X1 ⟶ 2X2.
The non-interacting condition can be relaxed in some cases by requiring that none of the species in {Xk+1, …, Xn} appear together in a reactant (these sets are called reactant-non-interacting [54]). Proceeding as above, provided that the determinant of the coefficient matrix is not identically zero, xk+1, …, xn can be expressed at equilibrium in terms of x1, …, xk. Conditions that ensure this is a positive parameterization are given in [54]. In the running example, species X1 is a reactant-non-interacting set and we can obtain a positive parameterization of x1 in terms of x2, see Figs 1 and 2.
If the network admits so-called toric steady states, then a positive parameterization also exists [14].
Step 7: the sign of and the Newton polytope. This is perhaps the hardest step of all. We write with positive for all and would like to determine the sign of . We first look for conditions that ensure uniqueness of positive equilibria by imposing that all coefficients of as a polynomial in have sign (−1)s.
We next identify the monomials of , where the sign of the coefficient, say β, is (−1)s+1 for some parameter values. For each of these monomials we check whether the monomial can “dominate” the sign of . That is to say, if sign(β) = (−1)s+1, then we determine whether there is an such that also has the sign (−1)s+1. If it is the case, then the condition sign(β) = (−1)s+1 is a sufficient condition for multiple equilibria according to Corollary 2(B).
Given a coefficient of a monomial with sign (−1)s+1, it might not be straightforward to decide if the polynomial has the same sign for some value of . (For example, the polynomial x2 − 2xy + y2 = (x − y)2 has one monomial with negative sign, but the polynomial itself can never be negative.) When the number of variables is small, one can attempt to decide the sign as we did in the examples above. Otherwise, our strategy is to determine whether the monomial of interest corresponds to a vertex of the Newton polytope. If that is the case, then the monomial can dominate the sign of (see §5.2 in the S1 File). The Newton polytope of is defined as the convex hull of the exponent vectors corresponding to the monomials of . If α is a vertex of the Newton polytope, then there exists such that the sign of agrees with the sign of the coefficient of the monomial (see §5.2 in the S1 File).
An algorithm. In the previous subsection we have outlined computational criteria that might be used to verify the conditions of the steps in the procedure. These computational criteria are only sufficient, that is, even if they fail the procedure might still work on the given network. For example, a sufficient computational criterion for the absence of boundary equilibria is based on Proposition 2. However, it might happen that Proposition 2 cannot be applied, but that the network nonetheless has no boundary equilibria in stoichiometric compatibility classes with non-empty interior.
We have collected sufficient computational criteria that guarantee the conditions of the procedure are fulfilled. In this way the procedure is formulated as an algorithm with decision diagram shown in Fig 3. If one step of the algorithm fails, then we say that the algorithm ends indecisively. In that case we might check whether the step can be verified by other means.
Fig 3. Decision diagram of the algorithm.
At each step either the condition is fulfilled or the algorithm terminates indecisively. If that is the case, the corresponding condition might still be verified manually and the algorithm resumed from the next following step.
For simplicity, we have restricted to mass-action kinetics. Under this assumption, det(M(x)) is a polynomial in x and the parameters κ, and is a rational function in and κ because the parameterization is assumed to be algebraic.
Applications to selected examples
To illustrate several aspects of the algorithm we provide a detailed step-by-step analysis of a collection of examples.
Two-component system
We have chosen this example to illustrate the situation where an algebraic parameterization is not required, as already det(M(x)) is of constant sign. The algorithm therefore stops successfully at Step 5 (and consequently skips Step 6 and 7).
We consider a simple version of a two-component system consisting of a histidine kinase HK that autophosphorylates and transfers the phosphate group to a response regulator RR, which undergoes autodephosphorylation. The reactions of the network are
We let X1 = HK, X2 = HKp, X3 = RR and X4 = RRp. The stoichiometric matrix N and a row reduced matrix W such that WN = 0 are
The matrix W gives rise to the conservation relations x1 + x2 = c1 and x3 + x4 = c2. With mass-action kinetics, the vector of reaction rates is v(x) = (κ1x1, κ2x2x3, κ3x4), and the function f(x) = Nv(x) is
We apply the algorithm to this network.
Step 1. Mass-action kinetics fulfills assumption in Eq (2) on the vanishing of reaction rate functions. The function f(x) and W are given above. The matrix W is row reduced.
Step 2. The network is conservative since (1, 1, 1, 1) ∈ im(N)⊥. Therefore the network is dissipative.
Step 3. The minimal siphons of the network are {X1, X2} and {X3, X4}. These two sets are the supports of the conservation relations. By Proposition 2, there are no boundary equilibria in any as long as .
Step 4. With our choice of W, we have i1 = 1, i2 = 3. Hence φc is obtained by replacing the components f1(x), f3(x) of f(x) by the expressions derived from the two conservation relations:
The Jacobian M(x) of φc and its determinant are
Step 5. All terms of det(M(x)) have sign +1 = (−1)s, since s = 2, and thus the conclusion of Corollary 1 holds. The network admits exactly one non-degenerate equilibrium point in every stoichiometric compatibility class with non-empty positive part.
Hybrid histidine kinase
This example has been analysed in [55]. Taken with mass-action kinetics the network is known to be multistationary for specific choices of reaction rate constants. We have chosen this example to illustrate how the algorithm can be used to sharpen known results: not only does it establish multistationarity for some parameter values, it provides precise conditions for when it occurs and allows a complete partition of the parameter space into regions with and without multistationarity. It also illustrates the use of an algebraic parameterization, which can be obtained by identifying sets of reactant-non-interacting species, and the use of the Newton polytope in Step 7.
This reaction network is an extension of the two-component system discussed above and it is given in the first row of Fig 4. Specifically, the histidine kinase is assumed to be hybrid, that is, it has two ordered phosphorylation sites [55]. Whenever the second phosphorylation site is occupied, the phosphate group can be transferred to a response protein.
Fig 4. Two examples describing a hybrid histidine kinase (row 1) and a gene transcription network (row 2).
Column 1: the reaction network; Column 2: the function where monomials with coefficients of constant sign (−1)s are in blue, and those that can have sign (−1)s+1 are in red; Column 3: parameter conditions for multistationarity; Column 4: Newton polytope where each point corresponds to the exponent vector of a monomial of the numerator of (e.g. (1, 2) is the exponent vector of the monomial ), blue points are the vertices of the Newton polytope and red numbers indicate the exponents of the red monomials in column 2.
Using the notation X1 = HK00, X2 = HKp0, X3 = HK0p, X4 = HKpp, X5 = RR and X6 = RRp, the stoichiometric matrix N and a row reduced matrix W such that WN = 0 are
The matrix W gives rise to the conservation relations x1 + x2 + x3 + x4 = c1 and x5 + x6 = c2. We assume mass-action kinetics
and the function is
We apply the algorithm to this network.
Step 1. Mass-action kinetics fulfills assumption in Eq (2) on the vanishing of reaction rate functions. The function f(x) and W are given above. The matrix W is row reduced.
Step 2. Since (1, 1, 1, 1, 1, 1) ∈ im(N)⊥ the network is conservative and hence dissipative.
Step 3. The network has two minimal siphons {X1, X2, X3, X4} and {X5, X6}, which are respectively the supports of the two conservation relations. We apply Proposition 2 to conclude that there are no boundary equilibria in any as long as .
Step 4. Since i1 = 1, i2 = 5, the function φc is obtained by replacing the components f1(x), f5(x) of f(x) by the expressions derived from the two conservation relations:
The Jacobian M(x) of φc(x) and its determinant are
Step 5. The sign of the first coefficient of det(M(x)) depends on the parameters. If κ1 ≥ κ3, then the sign is positive and det(M(x)) has sign +1 = (−1)4 (s = 4) as the remaining terms are positive. According to Corollary 1, there is a single non-degenerate equilibrium in each stoichiometric compatibility class with non-empty positive part. If κ1 < κ3, then Corollary 1 cannot be applied. We proceed to the next step to investigate the parameter space further.
Step 6. The set {X1, X2, X3, X6} is reactant-non-interacting and consists of s = 4 elements. We solve the equilibrium equations f1 = f2 = f3 = f6 = 0 for x1, x2, x3, x6. This gives the following algebraic parameterization of the set of equilibria in terms of :
The function , which is det(M(x)) evaluated at Φ(x4, x5), is the polynomial given in the first row of Fig 4.
Step 7. We assume κ1 < κ3, as the case κ1 ≥ κ3 is analysed in Step 5. Only one coefficient of has sign −1 = (−1)s+1 = (−1)5. The monomial associated with this term is . As the point (1, 2) (the degrees of the monomial) is a vertex of the Newton polytope (see Fig 4), then there exists such that the sign of is −1. Corollary 2(B) implies that there exists c = (c1, c2) such that contains at least two positive equilibria.
Multistationarity is thus completely characterized by the inequality κ3 > κ1. This condition states that the reaction rate constant for phosphorylation of the first site of the hybrid kinase is larger if the second site is phosphorylated than if it is not.
Gene transcription network
We consider the gene transcription network given in row 2 of Fig 4. This example has been studied in [56]. The particularities of this example are that the network is dissipative but not conservative, and that it displays multistationarity for all parameters κ. Further, this network illustrates the situation where the algorithm stops inconclusively at some step, but can be resumed after successful manual verification.
The network represents a gene transcription motif with two proteins P1, P2, produced by their respective genes X1, X2, and such that P2 dimerises [56]. Further, the proteins cross regulate each other as depicted in Fig 4. Using the notation X1 = X1, X2 = X2, X3 = P1, X4 = P2, X5 = X2P1, X6 = P2P2, and X7 = X1P2P2 the stoichiometric matrix N and a row reduced matrix W such that WN = 0 are
From W we find the conservation relations x1 + x7 = c1 and x2 + x5 = c2. Here s = 5. We consider mass-action kinetics such that
and f(x) = Nv(x) is the function
We apply the algorithm to this network:
Step 1. Mass-action kinetics fulfills assumption in Eq (2) on the vanishing of reaction rate functions. The function f(x) and W are given above. The matrix W is row reduced.
Step 2. The network is neither conservative nor strongly endotactic. Thus the algorithm terminates inconclusive. We take a manual approach: we pick and observe
Note that x1, x2 are bounded (due to the conservation relations) while x3, x4 can be arbitrarily large. Then, for x3, x4 large enough, ωc ⋅ f(x) < 0 and the network is dissipative by Proposition 1 (as has been shown in [56] by other means).
Step 3. This network has two minimal siphons: {X1, X7} and {X2, X5}, which are the supports of the two conservation relations. Therefore, by Proposition 2, there are no boundary steady states in stoichiometric compatibility class with non-empty positive part.
In section §5.1 in the S1 File we illustrate how to apply a simplification technique, based on the removal of so-called intermediates and catalysts, to check whether Proposition 2 holds for this network.
Step 4. Using that i1 = 1, i2 = 2 for our choice of W, the function φc(x) is:
The matrix M(x) and its determinant are:
Step 5. One coefficient of det(M(x)) has sign (−1)s+1 = 1 for all values of κ. Thus we proceed to the next step.
Step 6. There is not a set of non-interacting species nor reactant-non-interacting with s = 5 elements. Thus the algorithm terminates inconclusively.
We take a manual approach and solve the equilibrium equations f3 = f4 = f5 = f6 = f7 = 0 for x1, x2, x3, x6, x7. This gives the following algebraic parameterization of the set of equilibria in terms of :
Evaluating det(M(x)) at Φ(x4, x5) we obtain the polynomial
Step 7. The coefficient of the monomial of the numerator of a(x4, x5) has sign (−1)s+1 = (−1)6 = 1. Since the monomial is a vertex of the associated Newton polytope (see Fig 4), there exists such that the sign of a(x4, x5) is 1. We conclude from Corollary 2(B) that for all κi > 0 there exists c such that contains at least two positive equilibria.
Special classes of networks
There are several classes of networks for which some of the steps of the procedure are automatically fulfilled. We review some of them here.
Post-Translational Modification (PTM) networks consist of enzymes (Ei), substrates (Si) and intermediate species (Yi) [28]. Allowed reactions are of the form
All intermediates are assumed to be the reactant, respectively, the product of some reaction. These networks are conservative (hence dissipative) and boundary equilibria are precluded provided the underlying substrate network obtained by ignoring enzymes and intermediates is strongly connected [50], see also §5.1 in the S1 File. When equipped with mass-action kinetics, these networks have a non-interacting set with d elements consisting of all enzymes and some of the substrates, namely one per (minimal) conservation relation involving the substrates [28]. Thus, a positive parameterization can always be found under the conditions stated above in Step 6. The class of PTM networks is contained in the class of cascades of PTM networks. Also this class admits a positive parameterization in terms of the concentrations of the enzymes and some of the substrate forms [29].
Cascades of PTM networks might further be generalized to so-called MESSI networks [57]. These networks are all conservative. Easy-to-check conditions for the absence of boundary equilibria and to decide whether the network admits toric steady states (and hence a positive parameterization) are given in [57].
A class of networks that cannot have boundary equilibria in any stoichiometric compatibility class with non-empty interior is given in [58].
The two examples in Table 1 are both PTM networks. Hence they are conservative and positive parameterizations exist. The underlying substrate network is strongly connected (they pass the criterion based on minimal siphons). For both networks the conditions shown in Table 1 are obtained by the algorithm. See §6.1 and §6.2 in the S1 File. For illustration purposes, we apply the algorithm in §6.3 of the S1 File to an additional network and show that it is monostationary.
Computational issues
The computational complexity of some of the steps in the procedure are demanding. Some conditions can be checked using linear algebra and do not depend on parameter values, others depend on parameter values and require symbolic manipulations. In some situations, the calculation can be done for even large networks at the cost of time, while in other situations symbolic software (like Mathematica and Maple) have inherent limits to what it can process. We offer here a few remarks about computational strategies and time complexity.
Dissipativity. There are efficient algorithms to check whether the network is conservative and strongly endotactic, using linear algebra or mixed-integer linear programming [21, 47]. We are not aware of a systematic way to check if Proposition 1 is fulfilled or not.
Finding the minimal siphons of a network requires in general exponential time and there might be exponentially many of these [59]. Different algorithms developed in Petri Net theory can be applied to find the minimal siphons; see for example [48, 49, 59] and references therein. The complexity of this computation can often be substantially reduced by removing so-called intermediates and catalysts from the network [50] (see §5.1 in the S1 File for details).
Finding all non-interacting and reactant-non-interacting sets requires in general exponential time. One strategy is the following. We first remove all species Si for which αij > 1 or βij > 1 for some reaction Rj (the latter constraint is omitted if we are looking for reactant-non-interacting sets only). Then we build non-interacting (reactant-non-interacting) sets by adding new species recursively until no more species can be added without having an interacting pair of species in the set.
Calculation of the symbolic determinant of the matrix M(x), and hence also of , often fails in our experience for networks with more than 20 variables on common laptops [60]. However, this clearly depends on the sparsity of the matrix M(x), that is, on the number and order of the reactions. Strategies to reduce the complexity of the computation by expanding the determinant along the non-symbolic rows (conservation relations) were inspected in [60]. Specialized software like Singular [61] and/or better hardware could probably push what is possible to something closer to 50 variables. At this size, however, what might best be called ‘cognitive limitations’ come into play: symbolic software typically has problems with collecting and simplifying terms ‘the right way’ if there are many variables and/or parameters. And if terms are not collected appropriately it might be difficult, if not impossible, to decide on the sign of the polynomial coefficients. Our approach is therefore best suited to systems of moderate size (say 20-30 variables). Furthermore, it is our experience that large non-linear models tend to be multistationary because of the many non-linear dependencies that typically are present [60].
Positive parameterizations: The worst case scenario involves checking different sets of variables, each with at most d variables.
Finding the vertices of the Newton polytope can be done with existing symbolic software, for example Polymake [62] or Maple, as we demonstrate in the S1 File.
We stress that it is always beneficial to guide the procedure/algorithm whenever possible in the sense that, if something is known for the network, there is no reason to go through many possibilities.
Discussion
The main result of this paper, the procedure to identify parameter regions for unique and multiple equilibria, combines Brouwer degree theory and algebraic geometry. In particular, under the assumptions of Corollary 2, we show that there exist stoichiometric compatibility classes with at least two equilibria if, and only if, a certain multivariate polynomial can attain a specific sign.
Discriminating regions of the parameter space where multistationarity occurs is a hard mathematical problem, theoretically addressable by computationally expensive means [25]. Our approach beautifully overcomes these difficulties by building on a simple idea, the computation of the Brouwer degree of a function related to a dissipative network. Additionally, not only closed-form expressions in the parameters are obtained, but, as illustrated in examples, these expressions are often interpretable in biochemical terms, providing an explanation of why multistationarity occurs.
The procedure applies theoretically to any choice of algebraic reaction rate functions. However, in practice, the procedure works well with mass-action kinetics. For example, we have considered the two-site phosphorylation cycle depicted in the second row of Table 1, but now modelled with Michaelis-Menten kinetics instead of mass-action kinetics. This network is known to be multistationary [63], and the conditions to apply Corollary 1 and Corollary 2 are valid. However, a positive algebraic parameterization does not exist, and hence our approach cannot be used to find parameter conditions for multistationarity.
However, Corollary 1 might be used with rational reaction rate functions for monostationary networks. This is the case for example for the one-site phosphorylation cycle with Michaelis-Menten kinetics [63]. This network has two species and rank one. The sign of det(M(x)) is −1 for all parameter values and all . By Corollary 1, the network admits exactly one positive equilibrium in every stoichiometric compatibility class with for all parameter values.
If a reaction network does not have any conservation relation, then the set of equilibria consists typically of a finite number of points. In this case an algebraic parameterization is an algebraic expression of the equilibria in terms of the parameters of the system. Since m = 0, then consists of a single point and it follows directly that there is a unique equilibrium. Such an expression rarely exists. Therefore the procedure applies mainly to reaction networks with conservation relations. In particular, this rules out reaction networks where each species is produced and degraded.
Several natural questions remain outside the reach of our procedure. Firstly one would like to determine the particular stoichiometric compatibility classes for which there are multiple equilibria. As stated in Corollary 2, if , then defines a stoichiometric compatibility class with multiple equilibria. However, this only establishes c indirectly through . In some situations, it might be possible to find a positive parametrization that uses some of the conservation relations (ideally, all but one) and the stoichiometric compatibility classes with multiple/single equilibria would be determined up to a single parameter.
Secondly, one could ask for parameter regions that differentiate between the precise number of equilibria (that is, 0, 1, 2, …). This question should be seen in conjunction with the previous question: in typical examples, when there are two equilibria in a particular stoichiometric compatibility class, then there exists another class for which there are three. Hence the number of equilibria cannot be separated from the stoichiometric compatibility classes.
A third question concerns the stability of the equilibria, which cannot be obtained from our procedure. It is, however, known that if the sign of the Jacobian evaluated at an equilibrium is (−1)s+1, then it is unstable [34]. This is in particular the case for an equilibrium fulfilling the condition in Corollary 2(B).
We have shown that for some reaction networks our procedure can be formulated as an algorithm. We consider therefore our research a step in the direction of providing ‘black box tools’ to analyse complex dynamical systems. Such tools would easily find their use in systems and synthetic biology, where it is commonplace to consider (many) competing models. A particular problem is to exclude models that cannot explain observed qualitative features, such as multistationarity.
Methods
We used Maple for the symbolic computations, such as finding det(M(x)), the positive parameterizations, and the vertices of the Newton polytope.
Supporting information
In this document we first prove the claims of the main text. Next, we provide details on how to check the steps of the procedure. Finally, we give details of the examples in Table 1 and include an extra example which is a PTM network.
(PDF)
Acknowledgments
Alicia Dickenstein, Timo de Wolff and Bernd Sturmfels are thanked for discussions and the idea to use the vertices of the Newton polytope to study the sign of polynomials. Meritxell Sáez, Amirhossein Sadeghi Manesh, Anne Shiu and Angélica Torres are thanked for their comments on preliminary versions of the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
EF and CW received funding from the Danish Research Council for Independent Research (http://ufm.dk/en/research-and-innovation/councils-and-commissions/the-danish-council-for-independent-research?set_language=en&cl=en). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Gould P, Ugarte N, Domijan M, Costa M, Foreman J, MacGregor D, et al. Network balance via CRY signalling controls the Arabidopsis circadian clock over ambient temperatures. Mol Sys Biol. 2013;9:650 10.1038/msb.2013.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Laurent M, Kellershohn N. Multistability: a major means of differentiation and evolution in biological systems. Trends Biochem Sciences. 1999;24(11):418–422. 10.1016/S0968-0004(99)01473-5 [DOI] [PubMed] [Google Scholar]
- 3. Ozbudak EM, Thattai M, Lim HN, Shraiman BI, Van Oudenaarden A. Multistability in the lactose utilization network of Escherichia coli. Nature. 2004;427(6976):737–740. 10.1038/nature02298 [DOI] [PubMed] [Google Scholar]
- 4. Xiong W, Ferrell JE Jr. A positive-feedback-based bistable ‘memory module’ that governs a cell fate decision. Nature. 2003;426(6965):460–465. 10.1038/nature02089 [DOI] [PubMed] [Google Scholar]
- 5. Marchisio MA, Stelling J. Computational design tools for synthetic biology. Current Opinion Biotech. 2009;20(4):479–485. 10.1016/j.copbio.2009.08.007 [DOI] [PubMed] [Google Scholar]
- 6. Thomas R, Kaufman M. Multistationarity, the basis of cell differentiation and memory. I. Structural conditions of multistationarity and other nontrivial behavior. Chaos. 2001;11(1):170–179. 10.1063/1.1350439 [DOI] [PubMed] [Google Scholar]
- 7. Ferrell JE Jr. Bistability, bifurcations, and Waddington’s epigenetic landscape. Current Biology. 2012;22:R458–R466. 10.1016/j.cub.2012.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mak HC. Clouds, continuums, and cells. Cell Systems. 2017;4(3):251–. 10.1016/j.cels.2017.03.008 [DOI] [PubMed] [Google Scholar]
- 9. Gardner TS, Cantor CR, Collins JJ. Construction of a genetic toggle switch in Escherichia coli. Nature. 2000;403(6767):339–342. 10.1038/35002131 [DOI] [PubMed] [Google Scholar]
- 10. Palani S, Sarkar CA. Synthetic conversion of a graded receptor signal into a tunable, reversible switch. Mol Sys Biol. 2011;7:480 10.1038/msb.2011.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Feinberg M. The existence and uniqueness of steady states for a class of chemical reaction networks. Arch Rational Mech Anal. 1995;132(4):311–370. 10.1007/BF00375614 [DOI] [Google Scholar]
- 12. Feliu E. Injectivity, multiple zeros, and multistationarity in reaction networks. Proc Roy Soc A. 2014;471 (2173). 10.1098/rspa.2014.0530 [DOI] [Google Scholar]
- 13. Wiuf C, Feliu E. Power-law kinetics and determinant criteria for the preclusion of multistationarity in networks of interacting species. SIAM J Appl Dyn Syst. 2013;12:1685–1721. 10.1137/120873388 [DOI] [Google Scholar]
- 14. Pérez Millán M, Dickenstein A, Shiu A, Conradi C. Chemical reaction systems with toric steady states. Bull Math Biol. 2012;74:1027–1065. 10.1007/s11538-011-9685-x [DOI] [PubMed] [Google Scholar]
- 15. Conradi C, Flockerzi D. Multistationarity in mass action networks with applications to ERK activation. J Math Biol. 2012;65(1):107–156. 10.1007/s00285-011-0453-1 [DOI] [PubMed] [Google Scholar]
- 16. Conradi C, Flockerzi D, Raisch J, Stelling J. Subnetwork analysis reveals dynamic features of complex (bio)chemical networks. Proc Nat Acad Sci. 2007;104(49):19175–80. 10.1073/pnas.0705731104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mincheva M, Roussel MR. Graph-theoretic methods for the analysis of chemical and biochemical networks. I. Multistability and oscillations in ordinary differential equation models. J Math Biol. 2007;55(1):61–86. 10.1007/s00285-007-0098-2 [DOI] [PubMed] [Google Scholar]
- 18. Otero-Muras I, Yordanov P, Stelling J. A method for inverse bifurcation of biochemical switches: inferring parameters from dose response curves. BMC Sys Biol. 2014;8:114 10.1186/s12918-014-0114-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Otero-Muras I, Banga JR, Alonso AA. Exploring multiplicity conditions in enzymatic reaction networks. Biotech Prog. 2009;25(3):619–631. 10.1002/btpr.112 [DOI] [PubMed] [Google Scholar]
- 20. Craciun G, Helton JW, Williams RJ. Homotopy methods for counting reaction network equilibria. Mathematical biosciences. 2008;216(2):140–149. 10.1016/j.mbs.2008.09.001 [DOI] [PubMed] [Google Scholar]
- 21. Donnell P, Banaji M, Marginean A, Pantea C. CoNtRol: an open source framework for the analysis of chemical reaction networks. Bioinformatics. 2014;30(11):1633–1634. 10.1093/bioinformatics/btu063 [DOI] [PubMed] [Google Scholar]
- 22.Ellison P, Feinberg M, Ji H, Knight D. Chemical Reaction Network Toolbox, Version 2.2; 2012. Available online at http://www.crnt.osu.edu/CRNTWin
- 23. Otero-Muras I, Banga JR, Alonso AA. Characterizing multistationarity regimes in biochemical reaction networks. PLoS ONE. 2012;7(7):e39194 10.1371/journal.pone.0039194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Otero-Muras I SJ Yordanov P. Chemical Reaction Network Theory elucidates sources of multistability in interferon signaling. PLoS Comput Biol. 2017;13:e1005454 10.1371/journal.pcbi.1005454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chaves M, Sengupta A, Sontag ED. Geometry and topology of parameter space: investigating measures of robustness in regulatory networks. J Math Biol. 2008;59(3):315–358. 10.1007/s00285-008-0230-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Conradi C, Mincheva M. Catalytic constants enable the emergence of bistability in dual phosphorylation. J R S Interface. 2014;11(95). 10.1098/rsif.2014.0158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Conradi C, Mincheva M. Graph-theoretic analysis of multistationarity using degree theory. Math Comput Simulation. 2017;133:76–90. 10.1016/j.matcom.2015.08.010 [DOI] [Google Scholar]
- 28. Thomson M, Gunawardena J. The rational parameterization theorem for multisite post-translational modification systems. J Theor Biol. 2009;261:626–636. 10.1016/j.jtbi.2009.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Feliu E, Wiuf C. Variable elimination in post-translational modification reaction networks with mass-action kinetics. J Math Biol. 2013;66(1):281–310. 10.1007/s00285-012-0510-4 [DOI] [PubMed] [Google Scholar]
- 30.Smirnov GV. Introduction to the theory of differential inclusions. vol. 41 of Graduate Studies in Mathematics. American Mathematical Society, Providence, RI; 2002.
- 31.Amann H. Ordinary Differential Equations: An Introduction to Nonlinear Analysis. De Gruyter studies in mathematics. de Gruyter; 1990. Available from: https://books.google.dk/books?id=DTZ5bVAsyvEC
- 32. Ben-Israel A. Notes on linear inequalities, I: The intersection of the nonnegative orthant with complementary orthogonal subspaces. J Math Anal Appl. 1964;9:303–314. 10.1016/0022-247X(64)90045-9 [DOI] [Google Scholar]
- 33. Helton JW, Klep I, Gomez R. Determinant expansions of signed matrices and of certain Jacobians. SIAM J Matrix Anal A. 2009;31(2):732–754. 10.1137/080718838 [DOI] [Google Scholar]
- 34. Feliu E, Wiuf C. Preclusion of switch behavior in networks with mass-action kinetics. Appl Math Comput. 2012;219:449–1467. 10.1016/j.amc.2012.07.048G [DOI] [Google Scholar]
- 35. Craciun G, Feinberg M. Multiple equilibria in complex chemical reaction networks: I. The injectivity property. SIAM Appl Math. 2005;65(5):1526–1546. 10.1137/S0036139904440278 [DOI] [Google Scholar]
- 36. Mincheva M, Craciun G. Multigraph conditions for multistability, oscillations and pattern formation in biochemical reaction networks. Proceedings of the IEEE. 2008;96(8):1281–1291. 10.1109/JPROC.2008.925474 [DOI] [Google Scholar]
- 37. Müller S, Feliu E, Regensburger G, Conradi C, Shiu A, Dickenstein A. Sign conditions for injectivity of generalized polynomial maps with applications to chemical reaction networks and real algebraic geometry. Found Comput Math. 2016;16:69–97. 10.1007/s10208-014-9239-3 [DOI] [Google Scholar]
- 38. Banaji M, Craciun G. Graph-theoretic criteria for injectivity and unique equilibria in general chemical reaction systems. Adv Appl Math. 2010;44(2):168–184. 10.1016/j.aam.2009.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pantea C, Koeppl H, Craciun G. Global injectivity and multiple equilibria in uni- and bi-molecular reaction networks. Discrete Cont Dyn B. 2012;17:2153–2170. 10.3934/dcdsb.2012.17.2153 [DOI] [Google Scholar]
- 40. Joshi B, Shiu A. Atoms of multistationarity in chemical reaction networks. J Math Chem. 2013;51(1):153–178. 10.1007/s10910-012-0072-0 [DOI] [Google Scholar]
- 41. Joshi B, Shiu A. A survey of methods for deciding whether a reaction network is multistationary. Mathematical Modelling of Natural Phenomena. 2015;10:47–67. 10.1051/mmnp/201510504 [DOI] [Google Scholar]
- 42. Craciun G, Feinberg M. Multiple equilibria in complex chemical reaction networks: extensions to entrapped species models. Syst Biol (Stevenage). 2006;153:179–186. 10.1049/ip-syb:20050093 [DOI] [PubMed] [Google Scholar]
- 43. Feliu E, Wiuf C. Simplifying biochemical models with intermediate species. J R S Interface. 2013;10:20130484 10.1098/rsif.2013.0484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Conradi C, Flockerzi D. Switching in mass action networks based on linear inequalities. SIAM J Appl Dyn Syst. 2012;11(1):110–134. 10.1137/10081722X [DOI] [Google Scholar]
- 45. Craciun G, Nazarov F, Pantea C. Persistence and permanence of mass-action and power-law dynamical systems. SIAM J Appl Math. 2013;73(1):305–329. 10.1137/100812355 [DOI] [Google Scholar]
- 46. Gopalkrishnan M, Miller E, Shiu A. A projection argument for differential inclusions, with application to mass-action kinetics. SIGMA. 2013;9:025 10.3842/SIGMA.2013.025 [DOI] [Google Scholar]
- 47. Johnston MD, Pantea C, Donnell P. A computational approach to persistence, permanence, and endotacticity of biochemical reaction systems. J Math Biol. 2016;72:467–498. 10.1007/s00285-015-0892-1 [DOI] [PubMed] [Google Scholar]
- 48. Angeli D, De Leenheer P, Sontag E. A Petri net approach to the study of persistence in chemical reaction networks. Math Biosci. 2007;210(2):598–618. 10.1016/j.mbs.2007.07.003 [DOI] [PubMed] [Google Scholar]
- 49. Shiu A, Sturmfels B. Siphons in chemical reaction networks. Bull Math Biol. 2010;72(6):1448–1463. 10.1007/s11538-010-9502-y [DOI] [PubMed] [Google Scholar]
- 50. Marcondes de Freitas M, Feliu E, Wiuf C. Intermediates, catalysts, persistence, and boundary steady states. J Math Biol. 2017;74:887–932. 10.1007/s00285-016-1046-9 [DOI] [PubMed] [Google Scholar]
- 51. Gross E, Harrington HA, Rosen Z, Sturmfels B. Algebraic systems biology: A case study for the Wnt pathway. Bull Math Biol. 2015;78(1):21–51. 10.1007/s11538-015-0125-1 [DOI] [PubMed] [Google Scholar]
- 52. Feliu E, Wiuf C. Variable elimination in chemical reaction networks with mass-action kinetics. SIAM J Appl Math. 2012;72:959–981. 10.1137/110847305 [DOI] [PubMed] [Google Scholar]
- 53. Sáez M, Wiuf C, Feliu E. Graphical reduction of reaction networks by linear elimination of species. J Math Biol. 2017;74:195–237. 10.1007/s00285-016-1028-y [DOI] [PubMed] [Google Scholar]
- 54.Sáez M, Feliu E, Wiuf C. Graphical criteria for positive solutions to linear systems, arXiv:1709.01700 (2017)
- 55. Kothamachu VB, Feliu E, Cardelli L, Soyer OS. Unlimited multistability and Boolean logic in microbial signalling. J R S Interface. 2015;12(108). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Siegal-Gaskins D, Franco E, Zhou T, Murray RM. An analytical approach to bistable biological circuit discrimination using real algebraic geometry. J R S Interface. 2015;12(108). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pérez Millán M, Dickenstein A. The structure of MESSI biological systems. arXivorg. 2016;1612.08763.
- 58. Gnacadja G. Reachability, persistence, and constructive chemical reaction networks (part III): a mathematical formalism for binary enzymatic networks and application to persistence. Journal of Mathematical Chemistry. 2011;49(10):2158–2176. 10.1007/s10910-011-9896-2 [DOI] [Google Scholar]
- 59. Nabli F, Martinez T, Fages F, Soliman S. On enumerating minimal siphons in Petri nets using CLP and SAT solvers: theoretical and practical complexity. Constraints. 2016;21(2):251–276. 10.1007/s10601-015-9190-1 [DOI] [Google Scholar]
- 60. Feliu E, Wiuf C. A computational method to preclude multistationarity in networks of interacting species. Bioinformatics. 2013;29:2327–2334. 10.1093/bioinformatics/btt400 [DOI] [PubMed] [Google Scholar]
- 61.Decker W, Greuel GM, Pfister G, Schönemann H. Singular 4-1-0 — A computer algebra system for polynomial computations; 2016. http://www.singular.uni-kl.de
- 62. Gawrilow E, Joswig M. polymake: a framework for analyzing convex polytopes In: Kalai G, Ziegler GM, editors. Polytopes — Combinatorics and Computation. Birkhäuser; 2000. p. 43–74. [Google Scholar]
- 63. Hell J, Rendall AD. A proof of bistability for the dual futile cycle. Nonlinear Analysis-Real World Applications. 2015;24:175–189. 10.1016/j.nonrwa.2015.02.004 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In this document we first prove the claims of the main text. Next, we provide details on how to check the steps of the procedure. Finally, we give details of the examples in Table 1 and include an extra example which is a PTM network.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.