ABSTRACT
The distinguishing factors that characterize the host response to infection with virulent Mycobacterium tuberculosis (M.tb) are largely confounding. We present an infection study with 2 genetically closely related M.tb strains that have vastly different pathogenic characteristics. The early host response to infection with these detergent-free cultured strains was analyzed through RNAseq in an attempt to provide information on the subtleties which may ultimately contribute to the virulent phenotype. Murine bone marrow derived macrophages (BMDMs) were infected with either a hyper- (R5527) or hypovirulent (R1507) Beijing M. tuberculosis clinical isolate. RNAseq revealed 69 differentially expressed host genes in BMDMs during comparison of these 2 transcriptomes. Pathway analysis revealed activation of the stress-induced and growth inhibitory Gadd45 signaling pathway in hypervirulent infected BMDMs. Upstream regulators of interferon activation such as and IRF3 and IRF7 were predicted to be upregulated in hypovirulent-infected BMDMs. Additional analysis of the host immune response through ELISA and qPCR included the use of human THP-1 macrophages where a robust proinflammatory response was observed after infection with the hypervirulent strain. RNAseq revealed 2 early-response genes (ier3 and saa3) and 2 host-defense genes (oasl1 and slpi) that were significantly upregulated by the hypervirulent strain. The role of these genes under M.tb infection conditions are largely unknown but here we provide validation of their presence with use of qPCR and Western blot. Further analysis into their biological role during infection with virulent M.tb is required.
KEYWORDS: host-response, infection, mycobacterium tuberculosis, RNAseq, virulence
Introduction
The initial response of the host to Mycobacterium tuberculosis ultimately determines the establishment of an infection.1 Evidence is accumulating for strain and lineage-specific virulence in M.tb2 which further complicates our understanding of the factors which ultimately govern the ability to cause disease in humans.3 The initial infection-related events may provide information on the subtleties which ultimately contribute to the virulent phenotype.
Two well-known studies of the host response to hypo- and hypervirulent M.tb infection were based on the clinical isolates CDC15514 and HN878,5 respectively. It was determined that the hypovirulent CDC1551 was highly immunogenic which contributed to rapid and stable control of lung bacillary loads and subsequent improved survival. In contrast, the hypervirulent HN878 failed to induce a significant Th1 response which translated into increased bacilliary load and early death. This virulence was associated with the presence of a phenolic glycolipid (PGL) that was able to suppress proinflammatory cytokines such as TNF-α, IL-6 and IL-12,6 however it was eventually revealed that although it modulates the immune response, it does not itself confer hypervirulence.7 Further research revealed that the cytokine response initiated by both hypo-and hypervirulent strains is not predictable (reviewed by8). Thus our understanding of the processes involved in the acute and chronic inflammatory response during TB infection is still inadequate and it is therefore necessary to find other components which may be associated with virulence. Much work has been performed in an attempt to define the immune response to infection,4-7 however these studies focus on strains which differ genetically and therefore pin-pointing factors associated with virulence becomes challenging.
In this study, we assessed the early transcription profiles of the interplay between the host and 2 genetically closely related M.tb Beijing genotype strains with vastly different pathogenic characteristics. In doing so we were able to assess a subtle difference in the host response to infection with these strains. We also evaluated in more detail a select number of differentially regulated gene products in the context of their functional role during infection in a mouse in vitro model and repeated this with human THP-1 cells to ensure that the response we were observing was not species specific. We observe that during the early stages of infection, the hypervirulent strain induces a robust pro-inflammatory cytokine response characterized by the secretion of IL-6, IL-12B and IL-1B, as well as the RANTES. Additionally we isolated oasl1, ier3 and saa3 as early response genes that have largely unknown roles during M.tb infection.
Results and discussion
The characterization of virulence in M.tb can be related to various bacterial cell components, however characterizing virulence in the context of the host response to infection is challenging. Studies attempting to elucidate a particular host response to hypo and hypervirulent M.tb infection have used strains that are not closely associated with one another and are often from entirely different lineages. Here we present an infection study using 2 closely related M.tb strains that possess very different pathogenic characteristics with regards to their ability to transmit and cause disease in humans and kill mice.9-11 A previous study determined that mice infected with the hypervirulent Beijing strain did not survive 5 weeks post-infection whereas more than 80% of mice infected with the hypovirulent strain survived 4 months post-infection,11 indicating significant differences in virulence. Both of these strains are members of sublineage 7 and only differ by 2 IS6110 insertions,11 thus when comparing the 2 host responses, the differences we observe are more likely to be related to the virulent phenotype, thus excluding any other host responses which would otherwise be induced due to differences in lineage and other genotypical and phenotypical variances.
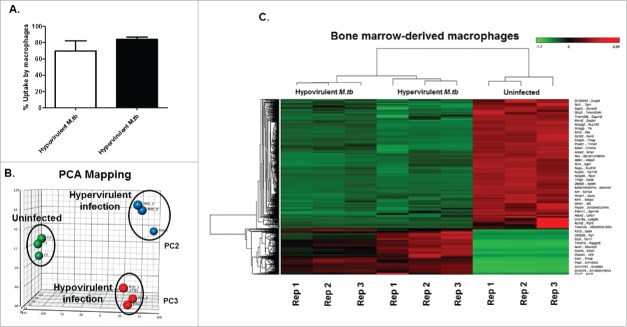
To this end, we assessed the early host response to infection (MOI = 1) with both strains using RNAseq at 12 h post-infection (Fig. 1). No significant difference was observed in the uptake of both strains (Fig. 1A). Principal component analysis (PCA) was conducted on filtered gene expression data with the circles representing individual samples that are visualized according to infection status (uninfected BMDMs, BMDMs infected with either R5527 hypervirulent M.tb or R1507 hypovirulent M.tb). PCA shows that each infection state (infected/uninfected) is distinctly clustered. Additionally, PCA indicates that the RNAseq-derived transcriptome profile of BMDMs infected with the closely related Beijing M.tb strains indicate a differential transcriptional host-response to infection. The adjacent heat map indicating differentially expressed genes was generated using 3 replicate samples of control (uninfected), BMDMs infected with hypervirulent M.tb and BMDMs infected with hypovirulent M.tb (Fig. 1B). When comparing uninfected BMDMs (control) to BMDMs infected with hypervirulent M.tb using the filters described (Materials and Methods), 2241 differentially expressed genes were identified (Table S1). When uninfected BMDMs were compared to BMDMs infected with hypovirulent M.tb, 2488 differentially expressed genes were identified (Table S2). RNAseq comparisons of the 2 infected-state transcriptome profiles recovered 69 differentially expressed genes between the cell infection models (Table S3). The top 20 up- and downregulated genes are presented in Table 1.
Figure 1.
Global transcriptome profile of BMDMs infected with either a hyper-(R5527) or hypovirulent (R1507) M.tb. (A). Percentage uptake of Hypovirulent and hypervirulent M.tb 4 hr post-infection in BMDM (MOI = 1). (B). Principal component analysis of uninfected BMDMs (Green, C1-C3), BMDMs infected with hypervirulent M.tb (Blue, R55-1-R55-3) and hypovirulent M.tb (Red, R50-1-R50-3) which are independently clustered. (C). Heatmap visualization of differentially expressed transcripts as analyzed by RNA-seq. Transcripts with significant fold changes, based on both fold change and FDR adjusted P-value threshold, are shown in the heat map. Gene names are indicated to the right of the heat map and bacterial growth conditions are shown at the top. Red = upregulation, green = downregulation. Dendrogram indicates sample clustering. Differentially expressed genes defined as having an FC > 2 .0 and FDR < 0 .05 in both the common and tagwise dispersion estimate analysis. Analysis was conducted on 3 biological replicates (C1, 2, 3, R55-1, 2, 3 and R150-1, 2, 3).
Table 1.
Top 20 up- and downregulated genes in BMDMs infected with Hypervirulent M.tb vs. BMDMs infected with Hypovirulent M.tb.
| Gene symbol |
Total reads (mean) |
P-value |
FDR step up |
Fold change (Hyper vs. Hypo) |
|---|---|---|---|---|
| Upregulated | ||||
| Hdc | 3.93E + 02 | 5.92E − 05 | 1.65E − 02 | 12.14 |
| Ccl5 | 1.16E + 05 | 1.65E − 04 | 2.57E − 02 | 10.54 |
| U90926 | 9.80E + 02 | 4.16E − 04 | 3.74E − 02 | 9.00 |
| Peg10 | 1.32E + 02 | 2.03E − 04 | 2.89E − 02 | 4.95 |
| Oasl1 | 1.48E + 04 | 6.44E − 04 | 4.63E − 02 | 4.63 |
| Gbp5 | 9.21E + 03 | 7.34E − 04 | 4.83E − 02 | 4.27 |
| Il27 | 2.78E + 02 | 4.38E − 04 | 3.83E − 02 | 4.25 |
| Saa3 | 3.38E + 05 | 1.55E − 04 | 2.48E − 02 | 4.14 |
| Lcn2 | 2.19E + 04 | 6.42E − 05 | 1.67E − 02 | 4.05 |
| Gbp4 | 3.73E + 02 | 2.58E − 04 | 3.25E − 02 | 3.63 |
| Cxcl10 | 3.13E + 04 | 6.96E − 05 | 1.69E − 02 | 3.55 |
| Cfb | 3.50E + 04 | 3.62E − 04 | 3.63E − 02 | 3.50 |
| Arhgef37 | 3.60E + 02 | 5.51E − 04 | 4.26E − 02 | 3.39 |
| Tbx10 | 9.70E + 01 | 7.82E − 04 | 4.98E − 02 | 3.22 |
| Tnfsf10 | 1.62E + 03 | 2.51E − 04 | 3.24E − 02 | 3.15 |
| Plekha4 | 1.65E + 02 | 4.09E − 04 | 3.74E − 02 | 3.14 |
| Pydc3 | 1.15E + 03 | 2.94E − 04 | 3.37E − 02 | 3.12 |
| Arid5a | 1.00E + 03 | 1.37E − 04 | 2.35E − 02 | 3.05 |
| 4930413G21Rik | 1.35E + 02 | 6.03E − 04 | 4.50E − 02 | 2.98 |
| Gbp7 | 1.12E + 04 | 6.53E − 06 | 4.83E − 03 | 2.77 |
| Isg15 | 1.84E + 04 | 4.62E − 04 | 3.91E − 02 | 2.60 |
| Downregulated | ||||
| Smpd3 | 9.50E + 01 | 3.63E − 04 | 3.63E − 02 | −4.65 |
| Ift74 | 2.92E + 02 | 3.34E − 04 | 3.55E − 02 | −3.77 |
| Mpzl1 | 3.29E + 02 | 7.63E − 04 | 4.91E − 02 | −3.75 |
| Rapgef5 | 6.38E + 03 | 5.52E − 04 | 4.26E − 02 | −3.70 |
| Gemin6 | 2.36E + 02 | 3.89E − 04 | 3.70E − 02 | −3.55 |
| Itga8 | 1.08E + 03 | 5.22E − 05 | 1.58E − 02 | −3.48 |
| Slc13a3 | 3.69E + 04 | 5.79E − 04 | 4.38E − 02 | −3.27 |
| Cd36 | 6.45E + 04 | 4.01E − 05 | 1.38E − 02 | −3.04 |
| Rpl22l1 | 1.64E + 03 | 8.31E − 05 | 1.80E − 02 | −2.86 |
| Arhgap26 | 1.05E + 03 | 1.48E − 04 | 2.46E − 02 | −2.78 |
| Fkbp3 | 1.32E + 03 | 3.04E − 04 | 3.40E − 02 | −2.77 |
| Tfrc | 9.16E + 03 | 9.94E − 06 | 5.89E − 03 | −2.62 |
| Zfp932 | 4.26E + 02 | 3.86E − 05 | 1.37E − 02 | −2.46 |
| Il10 | 4.67E + 02 | 4.79E − 05 | 1.51E − 02 | −2.43 |
| Lpl | 1.80E + 05 | 1.00E − 04 | 2.01E − 02 | −2.35 |
| Ispd | 1.05E + 02 | 4.91E − 04 | 4.00E − 02 | −2.32 |
| Zfp874b | 6.39E + 02 | 7.43E − 04 | 4.83E − 02 | −2.29 |
| Mir17hg | 3.70E + 02 | 4.41E − 04 | 3.83E − 02 | −2.26 |
| Myo1b | 2.01E + 03 | 5.36E − 04 | 4.23E − 02 | −2.19 |
| Zfp715 | 3.75E + 03 | 2.17E − 04 | 2.97E − 02 | −2.18 |
| Dkc1 | 2.43E + 03 | 3.42E − 04 | 3.55E − 02 | −2.12 |
The canonical pathways were then compared in BMDMs infected with both strains. Canonical pathway analysis (Table 2) reveals activation of similar pathways during infection with both hypo and hypervirulent strains, apart from growth arrest and DNA damage-inducible 45 (Gadd45) signaling which is induced by infection with the hypervirulent M.tb strain. This family of genes, including Gadd45α, Gadd45β, and Gadd45γ, are stress sensors that modulate responses of mammalian cells to physiological stresses.12,13 It is thus growth inhibitory under conditions of stress and suggests that infection with the hypervirulent M.tb strain is perceived as threatening to the host cell, a response which is not observed by infection with the hypovirulent strain. Interestingly, when we assessed various upstream transcriptional regulators of the enriched pathways and their predicted activation (Table 3), both interferon regulatory factor (IRF) 3 and 7 are predicted to be activated after infection with the hypovirulent M.tb strain. Literature suggests that these regulatory factors induce the expression of type-1 interferons14 which control infection progression.
Table 2.
Top Canonical pathways in BMDMs during infection with either hypo- or hypervirulent M.tb.
| Hypervirulent infected BMDMs | ||
|---|---|---|
| Pathway | p-value | Overlap |
| Role of BRCA1 in DNA Damage Response | 4.79E − 14 | 44.9% 35/78 |
| Hereditary Breast Cancer Signaling | 6.50E − 14 | 36.5 % 46/126 |
| Mismatch Repair in Eukaryotes | 6.91E − 14 | 93.8 % 15/16 |
| Cell Cycle Control of Chromosomal Replication | 2.18E − 13 | 73.1% 19/26 |
| GADD45 Signaling | 2.93E − 10 | 73.7 % 14/19 |
| Hypovirulent infected BMDMs | ||
| Pathway |
p-value |
Overlap |
| Cell Cycle Control of Chromosomal Replication | 1.36E − 12 | 73.1 % 19/26 |
| Role of BRCA1 in DNA Damage Response | 1.59E − 10 | 41.0 % 32/78 |
| Hereditary Breast Cancer Signaling | 7.45E − 09 | 31.7 % 40/126 |
| Mismatch Repair in Eukaryotes | 1.34E − 08 | 75.0 % 12/16 |
| Cell Cycle:G2M DNA Damage Checkpoint Regulation | 4.75E − 07 | 40.8% 20/49 |
Table 3.
Upstream regulators and their predicted activation in BMDMs during infection with either hypo- or hypervirulent M.tb.
| HYPOVIRULENT M.tb infection | ||
|---|---|---|
| Upstream regulator | p value of overlap | Predicted activation |
| PTGR4 | 1.85E − 49 | Inhibited |
| CSF2 | 2.37E − 48 | Activated |
| TICAM1 | 2.48E − 32 | Activated |
| IRF3 | 7.70E − 31 | Activated |
| IRF7 | 1.02E − 28 | Activated |
| |
HYPERVIRULENT M.tb infection |
|
| PTGR4 | 7.41E − 52 | Inhibited |
| CSF2 | 1.30E − 45 | Activated |
| TICAM1 | 2.31E − 38 | Activated |
| IRF3 | 2.87E − 38 | Activated |
| TLR4 | 8.98E − 37 | Activated |
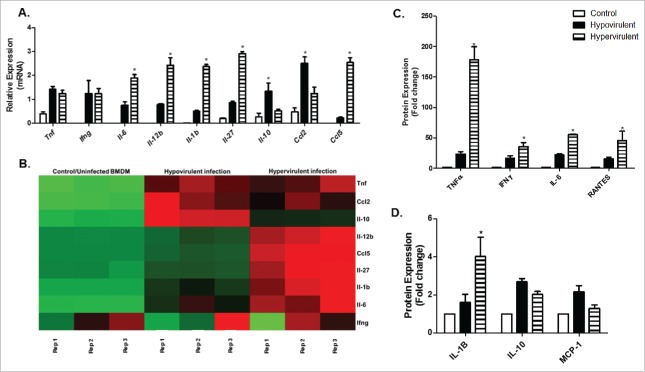
To probe further into the host response induced by these 2 closely related strains, we assessed the cytokine and chemokine response profile to infection (Fig. 2). We analyzed a number of differentially expressed cytokine and chemokine genes and the corresponding secreted proteins in both mouse (BMDM) and human (THP-1) macrophages (Fig. 2 and Fig. 3). Fold-change comparisons of all differentially expressed genes analyzed through RNAseq and qPCR are represented in Table 4. In both human and mouse macrophages, infection with the hypervirulent strain induces the transcription of genes encoding the pro-inflammatory cytokines Il-6, Il-12b and Il-1b, as well as the chemokine Ccl5 (RANTES). Although there are no differences observed in the transcribed mRNA levels of Ifnγ and tnfα, a significant difference is observed in the secreted protein detected by ELISA. This typical interferon-related immune response is not only typical of infection with the Beijing strains,3 but this signature appears to be relevant to active pulmonary tuberculosis patients.15,16 It is suggested that the production of interferons is related to the presence of the ESX-1 secretion system which functions to promote bacterial replication during infection.17 This robust inflammatory response indicates that the hypervirulent strain is highly pro-inflammatory during the early stages of infection.
Figure 2.
qPCR based validation and corresponding secreted proteins of differentially expressed cytokines and chemokines in BMDM after M.tb infection. A. Relative mRNA expression (fold change) of various cytokines and chemokines induced by BMDMs following infection with hypo- and hypervirulent M.tb as analyzed through qPCR (n = 3). B. Corresponding heatmap as generated by RNAseq under the same infection conditions (Red-Upregulation, Green-downregulation). C and D. Corresponding secreted cytokines and chemokines in BMDMs under the same infection conditions as measured by ELISA (n = 6). The means and standard error of a minimum of 3 independent experiments are shown, * indicates significance p < 0.05. Legend corresponds to all 3 graphs (A, C and D).
Figure 3.
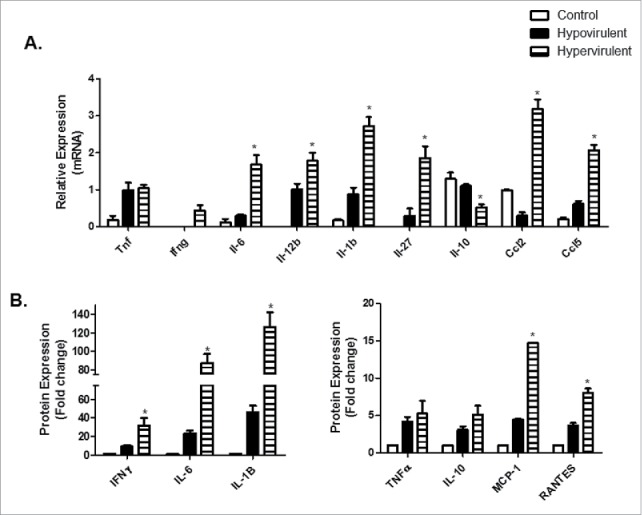
qPCR based validation and corresponding secreted proteins of differentially expressed cytokines and chemokines in THP-1 macrophages after infection with hypo- and hypervirulent M.tb. A. Relative expression (fold change) of various cytokines and chemokines induced by THP-1 macrophages following infection with hypo- and hypervirulent M.tb as analyzed through qPCR (n = 3). B. Corresponding secreted cytokines and chemokines in THP-1s under the same infection conditions measure by ELISA (n = 6). The means and standard error of a minimum of 3 independent experiments are shown, *indicates significance p < 0.05. Legend corresponds to all graphs (A and B).
Table 4.
Selected differentially expressed genes as analyzed by RNAseq and qPCR.
| Fold change* | ||
|---|---|---|
| Gene | RNAseq | qPCR |
| Tnf | 1.04 | 1.67 |
| Il-6 | 4.89 | 2.06 |
| Il-12b | 3.56 | 7.31 |
| Il-1b | 2.87 | 3.19 |
| Il-27 | 4.25 | 3.56 |
| Il10 | 0.40 | 0.35 |
| Ccl2 | 0.39 | 0.75 |
| Ccl5 | 10.54 | 9.32 |
| Ier3 | 2.22 | 2.05 |
| Oasl1 | 4.63 | 3.94 |
| Slpi | 8.18 | 3.60 |
| Saa3 | 4.14 | 3.50 |
Note.
Hypervirulent infected BMDMs vs. Hypovirulent infected BMDMs
Interestingly, of all the cytokines investigated, we could not detect the IL-12p70 bioactive heterodimer in the cell culture supernatant of both human and mouse macrophages, even though Il-12b gene expression was strongly induced after infection. A similar observation was made in murine macrophages infected with Salmonella dublin where the production of IL-12p70 was not detected, although secretion of the 40 kDa subunit was observed.18 Others have observed that endogenous IL-12 production or exogenous IL-12 administration results in increased resistance against mycobacterial infection.19,20 IL-12p70 induces the release of IFN-γ17 and under normal circumstances initiates T cell activation which will determine the eventual effector phenotype of the effector T cell.21 This is perhaps one mechanism utilized by mycobacteria to evade optimal cell-mediated immune responses is the ability to preferentially minimize IL-12p70 secretion. On the other hand, IL-12p40 is said to be able to function independently and act as a competitive antagonist22 where it is able to competitively bind to the common receptor component IL-12Rβ1,22 inhibit IL-23-mediated functions and antagonize the effects of the IL-12 heterodimer.23 This is therefore an alternative explanation as to the ability of virulent M.tb to evade the host immune response and persist intracellularly.
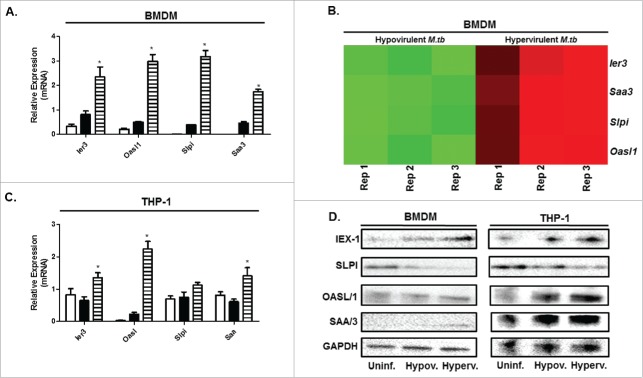
A previous study focusing on in vivo infections with the hypo and hypervirulent M.tb strains used in this study observed that bacillary loads in mice infected with the hypervirulent strain were 10-fold higher than those infected with hypovirulent strain.10 The fact that the hypervirulent strain is able to persist and replicate in vivo suggests that the mycobacteria are able to maintain a favorable cellular milieu, despite the secretion of proinflammatory immune modulators. A possible explanation for this may be the observed transcriptional upregulation of the immediate early response 3 (ier3) gene in both human and mouse macrophages after infection with hypervirulent strain in this study (Fig. 4) Ier3 is upregulated under conditions of cellular stress and inflammation24 and was recently observed to play a role as feedback inhibitor during LPS-stimulation, thereby protecting the macrophage from cell death induced by successive secretion of proinflammatory cytokines.25,26 The observed increase in this transcriptional response may suggest that ier3 transcription is not only related to virulence, but ensures macrophage survival through translational derepression25 which is consequently beneficial to mycobacterium survival. This may be the case during the early response to infection with virulent M.tb as observed in our infection model. To the best of our knowledge, this is the first study documenting a possible role for ier3 under M.tb infection conditions and requires further investigations into its role in possibly conferring intracellular M.tb survival. Another early response gene that was stimulated post-infection was the serum amyloid A (saa) gene. We observed its expression to be upregulated after infection with M.tb in both mouse and human macrophages, particularly after infection with the hypervirulent M.tb strain. In this study we observed an upregulation in transcription of this gene and its protein component in both human and mouse macrophages infected with M.tb and is in agreement with previous studies.27,28 SAA has been detected in a number of pulmonary infections, including tuberculosis and is involved in the induction of TNFα, IL-6, IL-8 and IL-1β secretion after infection.29
Figure 4.
Virulence-specific gene expression confirmed through qPCR and Western blot in BMDM and THP-1 macrophages infected with hypo- and hypervirulent M.tb. A. Relative mRNA expression (fold change) of selected differentially expressed genes induced by BMDMs following infection with hypervirulent M.tb after 12 h of infection as analyzed through qPCR (n = 4). B. Corresponding heatmap as generated by RNAseq under the same infection conditions in BMDMs (Red-Upregulation, Green-downregulation), Rep = Replicate. C. Relative mRNA expression (fold change) of the same virulence-specific genes induced by THP-1s following infection with hypervirulent M.tb after 12 h of infection as analyzed through qPCR (n = 4). D. Western blot of corresponding proteins expressed by BMDMs and THP-1s under the same infection conditions, GAPDH was used as a loading control (n = 4), Uninf. = Uninfected BMDM and THP-1, Hypov.= Hypovirulent infection, Hyperv. = Hypervirulent infection. *p < 0.05 indicates significance, a minimum of 3 biological replicates were performed for each experiment.
In the context of host defense, we focused on 2 genes encoding pattern recognition receptors (PRR) in which a virulence specific response to infection was observed. The first PRR was secretory leukocyte protease inhibitor gene (slpi). It was previously observed to have potent antimycobacterial activity30 and attaches to the surface of the mycobacteria through binding of mannan-capped lipoarabinomannans and phosphatidylinositol mannoside.31 Its expression is related to virulence32 which is in agreement with our findings (Fig. 4), however the accumulation of mRNA in BMDMs and THP-1 cells does not correspond to protein expression levels of SLPI in hypervirulent infected BMDM and THP-1 cells (Fig. 4.D). Absence of the SLPI protein component was also observed after herpes simplex virus infection.33 Previous studies revealed that its expression is inhibited by IFNγ,34 and since we observed increased IFNγ secretion in hypervirulent infected macrophages (Fig. 2 and Fig. 3), this could explain our result. In the context of M.tb survival however, abrogation of the functional protein component possibly contributes to the favorable intracellular milieu.
The second PRR of interest in this study was the oasl1 gene. Its function has been extensively studied in response to viral infection.14,35,36 Recent data deleting oasl1, a negative regulator of IFN-I signaling, suggests that sustained IFN-I signaling may be beneficial to control what will become a persistent viral infection.14,35 Irf7, a predicted upstream regulator of interferon signaling, is inhibited by oasl1,14,35,37 a downstream interferon-stimulated gene. In this study we observed that oasl1 is highly expressed after infection with the hypervirulent M.tb strain in both human and mouse macrophages (Table 1 and Fig. 4) which could account for Irf7 having poor prediction for activation as analyzed by IPA. We indicate a possible role for oasl1 during M.tb infection which certainly warrants further investigations. Interestingly, its biological role during M.tb infection has not yet been characterized, however a recent comparative analysis of the host transcriptome observed an over-representation of Oasl which was dependent not only on the presence of the region of difference 1 (RD1) in M.tb, but on virulence (when compared to BCG).38 Although it was one of the top upregulated genes, the authors did not expand on this finding. This result is in accordance with our own, however here we validate its expression and show that it is independent of species and dependent on the virulent phenotype.
Collectively our results reveal a virulence-specific host response to infection through global transcriptome analysis. Further research elucidating the role of each of these virulence-specific host genes are required under M.tb infection conditions since these are not well documented as yet.
Materials and methods
Cells and culture medium
Murine bone marrow derived precursor cells from the femurs of 6–8 week-old C57Bl/6 female mice were isolated as previously described39 and diluted in RPMI-1640 (containing L-glutamine and Na-bicarbonate; Sigma, USA) supplemented with 10% L-cell conditioned medium (source of CSF-1) and 10% heat-inactivated FBS (Biochrom, Germany) as growth medium and incubated at 37°C, 5% CO2. For infection experiments, cells were seeded into 6-well tissue culture dishes (Nunc, Thermo Scientific, USA) at 5 × 105 cells per well. The murine bone marrow derived precursor cells were allowed 5 d to adhere and differentiate into macrophages, where after the undifferentiated cells were washed away and the medium was refreshed. Medium was refreshed every second day and bacterial infection was carried out on day 7.
Human macrophage-like cells, THP-1(ATCC-88081201), were cultured in RPMI-1640 supplemented with 10% heat-inactivated foetal calf serum (Biochrome, Germany) and incubated at 37°C, 5% CO2. For infection experiments, THP-1 cells were seeded in 6-well plates (Nunc, Germany) at 2 X106 cells per well and differentiated into macrophage-like cells with Phorbol 12-Myristate 13-Acetate (PMA; Sigma Aldrich, USA) at a final concentration of 100 nM for 48 hours.
Bacterial strains and infection conditions
Genetically closely related hyper (R5527) and hypovirulent (R1507) Beijing M. tuberculosis clinical isolates11 were used for infection. These isolates were cultured from TB-positive patient's sputum samples, originally collected for diagnostic purposes. Cultures were genotyped by IS6110 DNA fingerprinting and spoligotyping using established international standardised methods. Mycobacteria were cultured in 7H9 (supplemented with 10% OADC, 0.5% glycerol) without Tween 80.40,41 BMDMs and THP-1s were infected with either the hyper- or hypovirulent M.tb strain at a MOI = 1 using the “syringe settle filtrate” (SSF) method40 and allowed 4 h for uptake. The cells were then washed 3 times with phosphate buffered saline (PBS) to remove any extracellular M.tb, and incubated for an additional 8 hours in complete medium (12 h in total).Uninfected BMDMs and THP-1s served as the control/uninfected samples.
RNA extraction and mRNA enrichment
Total RNA from BMDM and THP-1 cells were extracted using the RNeasy® Plus Mini Kit (Cat. No. 74134, Qiagen, Limburg, Netherlands) according to the manufacturer's instructions immediately following the 12 h infection period. The “gDNA eliminator” column included in this kit was used to remove genomic DNA in all samples. For each experiment, RNA quality and quantity was assessed using the Agilent 2100 Bioanalyser. Only RNA with a RNA integrity Number (RIN) above 9.0 were used for RNAseq and qPCR experiments. mRNA enrichment was achieved using the Dynabeads® mRNA DIRECT™ Kit (Cat. No. 61012, Ambion, Life Technologies, Oslo, Norway) according to the manufacturer's instructions. The enriched mRNA was then frozen immediately at −80°C until RNAseq was performed. Three biological replicates for RNA-seq (each biological replicate run in triplicate) and qPCR were used (each biological replicate run in duplicate).
RNA-seq
A barcoded RNA library was constructed for each of the 3 biological replicates in triplicate using the AB Library Builder™ Whole Transcriptome Core Kit for 5500 Genetic Analysis Systems (Cat. No. 4472690, Applied Biosystems, Life Technologies). The concentrations of the libraries were normalized using qPCR. To prevent any potential bias being introduced during emulsion PCR or sequencing, the 9 libraries were mixed prior to emulsion PCR using 2 E120 modules and the SOLiD® EZ Bead™ System (Cat. No. 4448419, Applied Biosystems, Life Technologies). The libraries were loaded onto 2 flow cells for sequencing after enrichment. A SOLiD™5500xl was used for paired-end sequencing (75/35 bp). The run was continuously monitored for data quality using standard tools in the Instrument Control software.
RNAseq data analysis was performed using LifeScope 2.5 (http://www.lifetechnologies.com/lifescope) and Partek Flow (Partek Inc., St Louis, MO, USA, build 4.0.15). Version GRCm38/mm10 of the mouse reference genome were used to map reads. The total number of reads mapped by LifeScope was extracted from the BAMSTATS output, along with the number of unmapped reads and reads with a mapQV of less than 10. The mapped reads were exported as .bam files into the Partek Flow Software tool. The post-alignment QC module of Partek Flow was used to visualize the average base quality score per position as well as the mapping quality per alignment. The mapped reads were quantified using the RefSeq transcripts-2015-02-02 annotation for quantification using the Partek E/M method (Methods S1) and strict paired-end compatibility was enforced with a requirement for junction reads to match defined annotated introns. All RNA-seq data have been deposited in the NCBI Gene Expression Omnibus (GEO) database with experiment series accession number [GSE78706].
Gene expression level analysis
A stringent gene expression filtering criterion was applied to the sense strand expression data to remove lowly expressed genes, thereby reducing Type I error. Differential gene expression analysis was achieved using Partek Flow and the Gene Specific Analysis (GSA) algorithm (Methods S1). False discovery rates (FDR) were also calculated and only regions with a minimum coverage of at least one were considered. All data was normalized using the FPKM method. Only regions with a FDR < 0.05 and fold change ≥ 2 or ≤ −2 were considered for hierarchical clustering. Both the samples and the genes were clustered.
Pathway analyses
Ingenuity Pathway Analysis (IPA, http://www.ingenuity.com) was used to assess the canonical pathways regulated during hypo- and hyperviruelnt M.tb infection in BMDM cells. Curated pathways from the IPA Knowledge Base that were significantly associated with the dataset were revealed as well as cascade of upstream transcriptional regulators and their predicted activation under the infection conditions described in Materials and Methods.
Quantitative qPCR
For cDNA synthesis, 0.5 µg RNA was converted to cDNA using the Quantitect® Reverse Transcription Kit (Cat. No. 205311, Qiagen, Limburg, Netherlands). To ensure the removal of genomic DNA, “gDNA wipe-out buffer” was added to RNA (included in the kit) prior to the RNA conversion step. qPCR amplification was performed in 96-well plates and run on a LightCycler® 96 system (Roche, Germany). LightCycler® 480 SYBR Green I Master (Cat. No. 04887352001, Roche, Germany) was used for various differentially expressed genes using QuantiTect® primer assays (Qiagen, Limburg, Netherlands - See Supplementary Materials S1) at a reaction volume of 20 µl. The reference genes ubc, b2m and g6pd were chosen according to stable expression levels from RNAseq data and confirmed through qPCR. The amplification procedure entailed 45 cycles of 95°C for 10 s followed by 60°C for 10s and finally 72°C for 10s. Gene expression fold-changes were computed for hypovirulent infected, hypervirulent infected and uninfected macrophages using calibrated normalized relative quantities using the equation N = N0 × 2Cp (LightCycler®96 software, Roche). All qPCRs were done on RNA extracted from 3 additional experiments. All biological replicates were run in triplicate with a positive control (calibrator) and a non-reverse transcription control in accordance with the MIQE Guidelines.
Cytokine and chemokine ELISAs
ELISArray for human (CMEH0707A) and Mouse (CME0708A) cytokines were purchased from Qiagen (Limburg, Netherlands) specific for TNFα, IFNγ, IL-6, RANTES, MCP-1, IL-12, IL-1B, IL-10. After the infection period, cell culture medium was removed and frozen at −80°C until analysis. ELISAs were conducted according to the manufacturer's instructions.
Western blotting
After the infection period, protein was extracted using RIPA buffer (150 mM NaCl, 1.0% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS and 50 mM Tris) containing Complete Protease Inhibitor Cocktail Tablets (Cat. No. 04693116001, Roche Diagnostics, South Africa). Proteins of interest for mouse and human were detected with antibodies (Santa Cruz Biotechnology) specific for OASLl1 (sc-98385), OASL (sc-98313), SLPI (sc-28803), SAA (sc-20651), IEX-1 (sc-33171) and the reference protein GAPDH (sc-32233). Corresponding secondary antibody used was goat anti-rabbit IgG-HRP (sc-2030).
Animal housing and ethics
The mice used for this study were housed 3 per cage in a temperature-controlled room with a 12-h light-dark cycle and had free access to food and water. Approval for this study was granted by the Stellenbosch University Animal Ethics committee on Animal Care and Use and complies with the South African Animal Protection Act (Act no 71, 1962). Animal Ethics No. SU-ACUD14-00041.
Statistical analysis
Statistical significance was performed with GraphPad Prism software. ANOVA was used for comparisons involving 3 or more groups. All values expressed as means ± SEM with a p < 0.05 considered as significant. “n” values signify the number of biological replicates performed for each experiment.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This project was funded by the Department of Science and Technology/National Research Foundation (DST/NRF) and the South African Medical Research Council (SAMRC). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
References
- [1].Ehlers MRW, Daffé M. Interactions between Mycobacterium tuberculosis and host cells: are mycobacterial sugars the key? Trends Microbiol 1998; 6:328-335; http://dx.doi.org/ 10.1016/S0966-842X(98)01301-8 [DOI] [PubMed] [Google Scholar]
- [2].Reiling N, Homolka S, Walter K, Brandenburg J, Niwinski L, Ernst M, Herzmann C, Lange C, Diel R, Ehlers S, et al.. Clade-specific virulence patterns of Mycobacterium tuberculosis complex strains in human primary macrophages and aerogenically infected mice. MBio 2013; 4:e00250-00213; PMID:23900170; http://dx.doi.org/ 10.1128/mBio.00250-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wu K, Dong D, Fang H, Levillain F, Jin W, Mei J, Gicquel B, Du Y, Wang K, Gao Q, et al.. An interferon-related signature in the transcriptional core response of human macrophages to Mycobacterium tuberculosis infection. PLoS One 2012; 7:e38367; http://dx.doi.org/ 10.1371/journal.pone.0038367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Manca C, Tsenova L, Barry CE 3rd, Bergtold A, Freeman S, Haslett PA, Musser JM, Freedman VH, Kaplan G. Mycobacterium tuberculosis CDC1551 induces a more vigorous host response in vivo and in vitro, but is not more virulent than other clinical isolates. J Immunol 1999; 162:6740-6; PMID:10352293 [PubMed] [Google Scholar]
- [5].Manca C, Tsenova L, Bergtold A, Freeman S, Tovey M, Musser JM, Barry CE 3rd, Freedman VH, Kaplan G. Virulence of a Mycobacterium tuberculosis clinical isolate in mice is determined by failure to induce Th1 type immunity and is associated with induction of IFN-α /β. Proc Natl Acad Sci U S A 2001; 98:5752-7; PMID:11320211; http://dx.doi.org/ 10.1073/pnas.091096998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Reed MB, Domenech P, Manca C, Su H, Barczak AK, Kreiswirth BN, Kaplan G, Barry CE 3rd. A glycolipid of hypervirulent tuberculosis strains that inhibits the innate immune response. Nature 2004; 430:84-7 [DOI] [PubMed] [Google Scholar]
- [7].Sinsimer D, Huet G, Manca C, Tsenova L, Koo MS, Kurepina N, Kana B, Mathema B, Marras SA, Kreiswirth BN, et al.. The phenolic glycolipid of Mycobacterium tuberculosis differentially modulates the early host cytokine response but does not in itself confer hypervirulence. Infect Immun 2008; 76:3027-36; PMID:18443098; http://dx.doi.org/ 10.1128/IAI.01663-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hernández-Pando R, Marquina-Castillo B, Barrios-Payán J, Mata-Espinosa D. Use of mouse models to study the variability in virulence associated with specific genotypic lineages of Mycobacterium tuberculosis. Infect Genet Evol 2012; 12:725-31; http://dx.doi.org/ 10.1016/j.meegid.2012.02.013 [DOI] [PubMed] [Google Scholar]
- [9].Meissner-Roloff RJ, Koekemoer G, Warren RM. A metabolomics investigation of a hyper-and hypo-virulent phenotype of Beijing lineage M. tuberculosis. Metabolomics 2012; 8:1194-203; http://dx.doi.org/ 10.1007/s11306-012-0424-6 [DOI] [Google Scholar]
- [10].de Souza GA, Fortuin S, Aguilar D, Pando RH, McEvoy CR, van Helden PD, Koehler CJ, Thiede B, Warren RM, et al.. Using a label-free proteomics method to identify differentially abundant proteins in closely related hypo-and hypervirulent clinical Mycobacterium tuberculosis Beijing isolates. Mol Cell Proteomics 2010; 9:2414-23; PMID:20190197; http://dx.doi.org/ 10.1074/mcp.M900422-MCP200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Aguilar D, Hanekom M, Mata D, Gey van Pittius NC, van Helden PD, Warren RM, Hernandez-Pando R. Mycobacterium tuberculosis strains with the Beijing genotype demonstrate variability in virulence associated with transmission. Tuberculosis 2010; 90:319-25; PMID:20832364; http://dx.doi.org/ 10.1016/j.tube.2010.08.004 [DOI] [PubMed] [Google Scholar]
- [12].Liebermann DA, Hoffman B. Gadd45 in stress signaling. J Mol Signal 2008; 3:15; PMID:18789159; http://dx.doi.org/ 10.1186/1750-2187-3-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Salvador JM, Brown-Clay JD, Fornace AJ Jr in Gadd45 Stress Sensor Genes 1-19 (Springer, 2013) [DOI] [PubMed] [Google Scholar]
- [14].Lee MS, Kim B, Oh GT, Kim YJ. OASL1 inhibits translation of the type I interferon-regulating transcription factor IRF7. Nat Immunol 2013; 14:346-55; PMID:23416614; http://dx.doi.org/ 10.1038/ni.2535 [DOI] [PubMed] [Google Scholar]
- [15].Berry MP, Graham CM, McNab FW, Xu Z, Bloch SA, Oni T, Wilkinson KA, Banchereau R, Skinner J, Wilkinson RJ, et al.. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature 2010; 466:973-7; http://dx.doi.org/ 10.1038/nature09247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ordway D, Henao-Tamayo M, Harton M, Palanisamy G, Troudt J, Shanley C, Basaraba RJ, Orme IM. The hypervirulent Mycobacterium tuberculosis strain HN878 induces a potent TH1 response followed by rapid down-regulation. J Immunol 2007; 179:522-31; PMID:17579073; http://dx.doi.org/ 10.4049/jimmunol.179.1.522 [DOI] [PubMed] [Google Scholar]
- [17].Stanley SA, Johndrow JE, Manzanillo P, Cox JS. The Type I IFN response to infection with Mycobacterium tuberculosis requires ESX-1-mediated secretion and contributes to pathogenesis. J Immunol 2007; 178:3143-52; PMID:17312162; http://dx.doi.org/ 10.4049/jimmunol.178.5.3143 [DOI] [PubMed] [Google Scholar]
- [18].Bost KL, Clements JD. Intracellular Salmonella dublin induces substantial secretion of the 40-kgdalton subunit of interleukin-12 (IL-12) but minimal secretion of IL-12 as a 70-kgdalton protein in murine macrophages. Infect Immun 1997; 65:3186-92; PMID:9234773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cooper A, Roberts AD, Rhoades ER, Callahan JE, Getzy DM, Orme IM. The role of interleukin-12 in acquired immunity to Mycobacterium tuberculosis infection. Immunology 1995; 84:423; PMID:7751026 [PMC free article] [PubMed] [Google Scholar]
- [20].Flynn J, Goldstein MM, Triebold KJ, Sypek J, Wolf S, Bloom BR. IL-12 increases resistance of BALB/c mice to Mycobacterium tuberculosis infection. J Immunol 1995; 155:2515-24; PMID:7650381 [PubMed] [Google Scholar]
- [21].Khader SA, Pearl JE, Sakamoto K, Gilmartin L, Bell GK, Jelley-Gibbs DM, Ghilardi N, deSauvage F, Cooper AM. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-γ responses if IL-12p70 is available. J Immunol 2005; 175:788-95; PMID:16002675; http://dx.doi.org/ 10.4049/jimmunol.175.2.788 [DOI] [PubMed] [Google Scholar]
- [22].Mattner F, Fischer S, Guckes S, Jin S, Kaulen H, Schmitt E, Rüde E, Germann T. The interleukin‐12 subunit p40 specifically inhibits effects of the interleukin‐12 heterodimer. Euro J Immunol 1993; 23:2202-8; PMID:8103745; http://dx.doi.org/ 10.1002/eji.1830230923 [DOI] [PubMed] [Google Scholar]
- [23].Shimozato O, Ugai S, Chiyo M, Takenobu H, Nagakawa H, Wada A, Kawamura K, Yamamoto H, Tagawa M. The secreted form of the p40 subunit of interleukin (IL)‐12 inhibits IL‐23 functions and abrogates IL‐23‐mediated antitumour effects. Immunology 2006; 117:22-8; PMID:16423037; http://dx.doi.org/ 10.1111/j.1365-2567.2005.02257.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Arlt A, Schäfer H. Role of the immediate early response 3 (IER3) gene in cellular stress response, inflammation and tumorigenesis. Eur J Cell Biol 2011; 90:545-52; PMID:21112119; http://dx.doi.org/ 10.1016/j.ejcb.2010.10.002 [DOI] [PubMed] [Google Scholar]
- [25].Schott J, Reitter S, Philipp J, Haneke K, Schäfer H, Stoecklin G. Translational Regulation of Specific mRNAs Controls Feedback Inhibition and Survival during Macrophage Activation. PLoS Genet 2014; 10:e1004368; http://dx.doi.org/ 10.1371/journal.pgen.1004368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Vinuesa CG, Preiss T. Inflammation: Gone with Translation. PLoS Genet 2014; 10:e1004442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Koo MS, Manca C, Yang G, O'Brien P, Sung N, Tsenova L, Subbian S, Fallows D, Muller G, Ehrt S, et al.. Phosphodiesterase 4 inhibition reduces innate immunity and improves isoniazid clearance of Mycobacterium tuberculosis in the lungs of infected mice. PLoS One 2011; 6:e17091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Essone PN, Chegou NN, Loxton AG, Stanley K, Kriel M, van der Spuy G, Franken KL, Ottenhoff TH, Walzl G. Host cytokine responses induced after overnight stimulation with novel M. tuberculosis infection phase-dependent antigens show promise as diagnostic candidates for TB disease. PLoS One 2014; 9:e102584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Song C, Hsu K, Yamen E, Yan W, Fock J, Witting PK, Geczy CL, Freedman SB. Serum amyloid A induction of cytokines in monocytes/macrophages and lymphocytes. Atherosclerosis 2009; 207:374-83; PMID:19535079; http://dx.doi.org/ 10.1016/j.atherosclerosis.2009.05.007 [DOI] [PubMed] [Google Scholar]
- [30].Nishimura J, Saiga H, Sato S, Okuyama M, Kayama H, Kuwata H, Matsumoto S, Nishida T, Sawa Y, Akira S, et al.. Potent antimycobacterial activity of mouse secretory leukocyte protease inhibitor. J Immunol 2008; 180:4032-9; PMID:18322212; http://dx.doi.org/ 10.4049/jimmunol.180.6.4032 [DOI] [PubMed] [Google Scholar]
- [31].Gomez SA, Argüelles CL, Guerrieri D, Tateosian NL, Amiano NO, Slimovich R, Maffia PC, Abbate E, Musella RM, Garcia VE, et al.. Secretory leukocyte protease inhibitor: a secreted pattern recognition receptor for mycobacteria. Am J Respir Crit Care Med 2009; 179:247-53; PMID:19011154; http://dx.doi.org/ 10.1164/rccm.200804-615OC [DOI] [PubMed] [Google Scholar]
- [32].Beisiegel M, Mollenkopf HJ, Hahnke K, Koch M, Dietrich I, Reece ST, Kaufmann SH. Combination of host susceptibility and Mycobacterium tuberculosis virulence define gene expression profile in the host. Euro J Immunol 2009; 39:3369-84; http://dx.doi.org/ 10.1002/eji.200939615 [DOI] [PubMed] [Google Scholar]
- [33].Fakioglu E, Wilson SS, Mesquita PM, Hazrati E, Cheshenko N, Blaho JA, Herold BC. Herpes simplex virus downregulates secretory leukocyte protease inhibitor: a novel immune evasion mechanism. J Virol 2008; 82:9337-44; PMID:18667508; http://dx.doi.org/ 10.1128/JVI.00603-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Jin FY, Nathan C, Radzioch D, Ding A. Secretory leukocyte protease inhibitor: A macrophage product induced by and antagonistic to bacterial lipopolysaccharide. Cell 1997; 88:417-26; http://dx.doi.org/ 10.1016/S0092-8674(00)81880-2 [DOI] [PubMed] [Google Scholar]
- [35].Lee MS, Park CH, Jeong YH, Kim YJ, Ha SJ. Negative regulation of type I IFN expression by OASL1 permits chronic viral infection and CD8 (+) T-cell exhaustion. PLoS Pathog 2013; 9:e1003478; http://dx.doi.org/ 10.1371/journal.ppat.1003478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zhu J, Ghosh A, Sarkar SN. OASL—a new player in controlling antiviral innate immunity. Curr Opin Virol 2015; 12:15-9; PMID:25676874; http://dx.doi.org/ 10.1016/j.coviro.2015.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Papatriantafyllou M. Cytokines: IRF7 lost in translation. Nat Rev Immunol 2013; 13:221; PMID:23449338; http://dx.doi.org/ 10.1038/nri3425 [DOI] [PubMed] [Google Scholar]
- [38].Etna MP, Giacomini E, Pardini M, Severa M, Bottai D, Cruciani M, Rizzo F, Calogero R, Brosch R, Coccia EM. Impact of Mycobacterium tuberculosis RD1-locus on human primary dendritic cell immune functions. Sci Rep 2015; 5:17078; PMID:26602835; http://dx.doi.org/ 10.1038/srep17078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].de Chastellier C, Lang T, Thilo L. Phagocytic processing of the macrophage endoparasite, Mycobacterium avium, in comparison to phagosomes which contain Bacillus subtilis or latex beads. Eur J Cell Biol 1995; 68:167-82; PMID:8575463 [PubMed] [Google Scholar]
- [40].Leisching G, Pietersen RD, Mpongoshe V, van Heerden C, van Helden P, Wiid I, Baker B. The Host Response to a Clinical MDR Mycobacterial Strain Cultured in a Detergent-Free Environment: A Global Transcriptomics Approach. PLoS One 2016; 11:e0153079; http://dx.doi.org/ 10.1371/journal.pone.0153079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Leisching G, Pietersen RD, Wiid I, Baker B. Virulence, biochemistry, morphology and host-interacting properties of detergent-free cultured mycobacteria: An update. Tuberculosis 2016; 100:53-60; PMID:27553410; http://dx.doi.org/ 10.1016/j.tube.2016.07.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.