Abstract
Recent advances in cancer immunology, such as the discovery of immune checkpoint inhibitors, have validated immune cells as potential key players for effective cancer treatment. The efficacy of these therapies seems to be codependent on a tumor-reactive T lymphocyte response. For many years, numerous attempts and strategies in developing vaccines to generate tumor-reactive T cells have yielded poor results in the clinic due to suboptimal immunogenicity and the inability to overcome an immunosuppressive tumor microenvironment. In this review, we summarize past and current advances in T cell vaccines and describe our experience in developing optimized methods for antigen/adjuvant selection and vaccine administration in order to induce powerful anti-tumor responses.
1. Introduction
Undoubtedly, vaccines are effective in preventing infections by recruiting various components of the immune system against numerous pathogens. Since the immune system has the ability to recognize transformed malignant cells and limit tumor growth, immunotherapy has now become an effective way to treat cancer. Amongst various components of the immune system T cells and in particular CD8 cytotoxic T lymphocytes (CTLs) are the most effective elements in recognizing alterations occurring in transformed cells. The antigens recognized by T cells correspond to peptides that associate MHC molecules. Such peptides result from processed proteins from the infectious microorganisms or derived from abnormally expressed gene products in malignant cells. Tumor-reactive T cells are frequently present in cancer patients in the form of tumor-infiltrating lymphocytes (TILs), which normally do not control the disease [1]. However, ex vivo TIL expansion and reintroduction into the patients has demonstrated remarkable therapeutic effects in some patients [2]. Unfortunately TIL therapy is technically challenging, expensive and not all cancers contain TILs. Thus, there is a critical need for other means to generate tumor reactive T cells with a simpler and more cost effective strategy such as vaccination.
Checkpoint pathways regulate T cells by blocking their function, presumably to prevent pathological autoimmune responses [3]. Antibodies that inhibit two of these immune checkpoint blockades, CTLA4 and PD-1, have shown notable anti-tumor effects [4,5]. However, the proportion of patients that respond favorably to checkpoint blockade inhibitors (CBIs) is low and is confined to particular types of cancer. Because CBIs require the presence of an existing pool of tumor-reactive T lymphocytes, many believe that patients not responding to CBI lack these T cells. Thus, the expectation is that T cell inducing vaccines should increase the effectiveness and expand the applicability of CBIs.
2. Types of T cell vaccines
Various strategies have been used to develop vaccines to generate tumor-reactive T cells (Table 1). This work developed from early pioneering observations in mice where killed tumor cells vaccines prevented the growth of subsequent challenges with live tumor cells [6,7]. Nevertheless these vaccines were less effective when administered into animals bearing established tumors. Vaccines consisting of tumors expressing immune-stimulating cytokines improved their anti-tumor effects [8], but unfortunately the clinical results were not outstanding [9–12]. Thus, major efforts are devoted to designing more effective vaccines by utilizing defined tumor antigens (TAgs).
Table 1.
Development of vaccines to generate tumor-reactive T cells
| Strategy | General Overview | Reference |
|---|---|---|
| Cell-based vaccines | ||
| Tumor cells/lysate | Killed autologous tumor, cancer cell lines or cell lysates | 7, 38 |
| Genetically modified tumor cells | Tumor cell lines bioengineered to express cytokines (e.g. GM-CSF, IL- 4) | 8–12, 39 |
| Dendritic cells | DCs are isolated or prepared ex vivo and loaded with TAg and then injected to patients | 40 |
| Microorganism-based vaccines | ||
| Recombinant viruses | Attenuated viruses that encode TAg | 41–43 |
| Recombinant bacteria | Attenuated, TAg-expressing bacteria (Listeria, Salmonella) | 44–45 |
| Recombinant yeast | Recombinant yeast particles expressing TAg on their surface enhance and promote presentation of TAg by APCs while avoiding risks associated with live pathogen vaccine models | 46 |
| Subunit vaccines | ||
| Peptides, proteins | Short, or long TAg-derived; synthetic peptides including helper epitopes; carbohydrate- mimetic peptides | 20, 21, 26, 30–33, 35, 47 |
| DNA or RNA | DNA plasmids or RNA encoding TAg or identified T cell epitopes injected to transduce host cells to express TAg | 48, 49 |
| Heat shock proteins | HSP-TAg complexes isolated from patient tumor extracts to target TAg to APCs | 50 |
3. Antigen selection
The identification of proteins that function as TAgs for T cells and their corresponding peptide epitopes facilitated developing more refined T cell vaccines. Practically, TAgs for T cells are grouped into 4 types (Table 2): A) Products of oncogenic viruses; B) Developmental or germ cell products; 3) Tissue-specific differentiation antigens; 4) Products of genetic alterations derived from malignant transformation.
Table 2.
Types of tumor antigens for T cells
| TAg Type | Pros | Cons | Examples |
|---|---|---|---|
| A. Products of oncogenic viruses | High immunogenicity (no tolerance) | The target tumors are limited | EBV, HBV, HPV |
| B. Developmental or germ cell products | Lower risk of recognizing self- antigens in normal tissue (low tolerance) TAgs can be pharmacologically induced in tumor cells |
Possibility of adverse effects to reproductive organs??? | Tumor-testis antigens (MAGE-A3, NY- ESO1) |
| C. Tissue-specific differentiation antigens | High specificity, lower risk of off- target effects | The target tumors are limited, possibility of adverse effects to normal tissues Low immunogenicity (tolerance) |
Melanosomal proteins (gp100, Trp1, Melan- A/Mart-1), prostatic proteins (PSA, PAP) |
| D. Products of genetic alterations derived from malignant transformation | High immunogenicity | The identification of neoepitopes in each individual (i.e. expensive and complicated) | a) Overexpressed gene products (HER2/NEU, CEA b) Mutation-derived epitopes (P53, Ras, EGFRvIII, BCR-ABL, point mutations/neoepitopes) |
Tumor whole exome sequencing has allowed identifying mutations that potentially represent tumor-specific T cell epitopes [13]. Yet, such mutations must occur in protein-coding regions and be contained within peptides binding to MHC molecules. There are numerous algorithms used to predict whether a peptide binds to a specific MHC allele [14,15] and quantitative peptide/MHC-binding assays can be used to demonstrate the formation of peptide/MHC complexes [16]. However, whether a mutated MHC binding peptide is effective in generating tumor-reactive T cells depends on whether the mutated epitopes are generated by the tumor and expressed as surface peptide/MHC complexes. This fundamental requirement is challenging to demonstrate since it requires proof of the mutated peptide presence bound to the tumor MHC molecule, for example by mass spectrometry sequencing of peptides eluted from purified MHC proteins [17]. Alternatively, attesting tumor cell recognition by T cells specific for mutated peptide would demonstrate this prerequisite. Because these approaches are technically challenging, many choose to select mutated peptides solely on the basis on MHC binding predictions to produce peptide cocktail vaccines, not taking into consideration the possibility that peptides not corresponding to true tumor T cell epitopes could generate irrelevant T cell responses that may diminish the effectiveness of the vaccine.
Although there are multiple pros and cons related to the use of TAgs from each group (Table 2), there is still no clear evidence that antigens from one of these types will be more effective than the others. The most significant issue for selecting a TAg is related to potential immune tolerance that could affect the quality of the T cell response (capacity to recognize the tumor cell). Nevertheless, there are examples of successfully eliciting anti-tumor T cell responses to “self-antigens”, when immune tolerance was predicted [18–21].
4. Immunization strategy
Whatever TAg type is selected, diverse vaccination approaches have been explored, such as recombinant proteins, recombinant viruses, DNA vaccines and synthetic peptides. These vaccines are directly administered in vivo with the expectation that professional antigen-presenting cells (pAPCs) such as dendritic cells (DCs) will capture vaccine components and stimulate T cell responses. In other cases the vaccine components are loaded ex vivo onto DCs to produce cell-based vaccines. The goal is that the DCs presenting the peptide/MHC complexes will stimulate T cells via their T cell receptor (TCR), inducing them to proliferate and become effector cells. However, it is evident that effective T cell activation leading to expansion, survival and effector function requires more than simple TCR stimulation (Signal 1). DCs need appropriate activation to provide immune costimulatory signals that promote T cell survival and proliferation after TCR stimulation. Costimulation occurs either in the form of cell surface receptor-ligand interactions between T cells and DCs (Signal 2), or via cytokines (Signal 3). Signal 1 without Signals 2 and 3 fails to generate effective T cell responses and leads to T cell dysfunction [22,23]. During an immune response to an infectious agent, DC activation results from stimulation of pattern recognition receptors (PRRs) such as toll-like receptors (TLRs), and RIG-I-like receptors (RLR) by microbial components [24]. Helper CD4 T lymphocytes (HTLs) further promote DC activation through CD40L/CD40 [25]. Consequently, effective CTL vaccines must contain immune adjuvants that stimulate PRRs, and should stimulate HTLs. Agonistic antibodies to CD40 have been used to enhance the effectiveness of CTL-inducing vaccines that may not trigger HTL responses [20,25,26].
We believe that examining how the immune system responds to an acute infection could facilitate developing effective cancer vaccines that elicit large and lasting CTL responses. During the course of an immune response to infectious pathogen, the activated CTLs will encounter antigen again on infected non-professional APCs cells (e.g., epithelial cells). Under these circumstances, production of type-I interferon (IFN-I) by the infected cells will function as Signal 3 allowing the CTLs to survive and continue to expand until the infection is resolved [27,28]. These basic concepts could be applied for developing cancer vaccines.
5. Optimization of peptide vaccines
Many favor synthetic peptides as the source of TAg for developing vaccines because they are easy to manufacture and characterize in a cost-effective manner for the clinic. Unfortunately, numerous clinical studies using peptide vaccines have resulted in suboptimal therapeutic benefit in most patients [29–31]. We attribute this failure to two major causes: 1) suboptimal immunogenicity, and 2) the presence of an immunosuppressive tumor microenvironment.
Historically vaccines were developed towards eliciting antibodies to prevent infections. Thus, vaccine optimization and evaluation relied in measuring antibody titers, leading to the selection of immune adjuvants and modes of administration, inducing high antibody titers that correlated with protection. Regrettably, cancer vaccine development has followed the same approach to induce CTL responses using the same adjuvants and modes of administration. Proteins and peptides corresponding TAgs are administered with adjuvants such as alum or emulsified in incomplete Freund’s adjuvant and injected subcutaneously to generate CTLs. Moreover, evaluating CTL responses is far more complex than measuring antibody titters, and some approaches lead to erroneous assumptions of the vaccine’s immunogenicity. Ideally, one should quantify numbers of antigen-specific tumor-reactive CTLs before and after vaccination. However, CTL tumor-reactivity assays are difficult to perform since these necessitate a substantial amount of blood and require tumor cells as target cells. Thus, many studies use assays measuring T cell responses (cytokine release) to TAg used in the vaccine (peptide, protein) presented by conventional APCs, or assess the presence of antigen-specific T cells by flow cytometry using peptide/MHC tetramer staining. These methods do not evaluate the ability of the vaccine-induced antigen-reactive T cells to recognize tumor cells. Some conclude that a vaccine is immunogenic solely in the basis of “statistically significant” differences in numbers of antigen-reactive T cells between pre- and post-vaccination samples without taking into account whether these differences are truly substantial to hold any biological relevance. What constitutes a biological relevant CTL response leading to a meaningful therapeutic benefit? Many factors will influence the antitumor effectiveness of the T cell response. In addition to the capacity of the CTLs to recognize the tumor cells (quality), the ability of the T cells to traffic and infiltrate the tumor and to withstand the inhibitory microenvironment will dictate the necessary numbers of CTLs required controlling tumor growth. Nevertheless, one can predict that the efficacy of the vaccine’s antitumor effect will depend on the magnitude and quality of the CTL response.
Based on the work of other [26], we developed a synthetic peptide vaccination strategy (TriVax) that induced rapid and vast CTL responses in mice, where in many instances 50–80% of all CD8 T cells were specific for the peptide immunogen and recognized tumor cells [20,32]. In addition to peptide, TriVax contains a PRR agonist (poly-IC) and a DC-activating antibody (anti-CD40 mAb). Later we described a simpler vaccine (BiVax), using amphiphilic peptides that self-assembled into nanostructures, which were administered with poly-IC without the anti-CD40 mAb [33]. Two sequential immunizations (prime/boost) were required for generating large CTL responses. These vaccines had to be injected systemically (intravenously or intramuscularly), which disseminates the antigen and adjuvant throughout the lymphoid organs, optimizing the recruitment of naive CTLs. Amongst several PRRs tested as adjuvants, poly-IC was unique in promoting vast CTL expansions [20,33]. The effectiveness of poly-IC as an adjuvant relies in its capacity to stimulate TLR3, shown to be important during priming, and its ability to stimulate MDA5, a cytoplasmic RLR responsible for generating IFN-I [34], which was critical for the boost CTL expansions. TriVax and BiVax were effective against established tumors in mice. In a model of human papilloma virus (HPV)-induced tumors, therapeutic immunization using CTL epitope HPV16-E749–57 resulted in eradication of tumors [32]. With the less immunogenic B16-F10 murine melanoma vaccines using several melanosomal derived CTL epitopes (Trp2180–188, Trp1455–463 and gp10025–33) significantly reduced growth of established tumors and tumors were rejected when vaccines were combined with PD-1 blockade [20,33].
Some propose the use of long synthetic peptide vaccines because this would force antigen presentation by DCs [35]. Although this strategy makes sense, in our view the use of potent adjuvants and appropriate routes of administration are more critical than simple peptide length. We believe that physical properties of peptides resulting from their amino acid sequence and composition will determine the immunogenicity of a vaccine. For example the minimal CTL epitope HPV16-E749–57 (RAHYNIVTF) was significantly more immunogenic (10 to 100-fold) with BiVax as compared to 2 long peptides, HPV16-E745–57 and HPV16-E743–77 [33]. The minimal epitope is amphiphilic, since one end is hydrophilic (RAHYN) while the other is hydrophobic (IVTF), and when this peptide is resuspended in an aqueous solvent it self-associates into virus resembling nanoparticles. On the other hand, the elongated peptides HPV16-E745–57 and HPV16-E743–77 are not amphiphilic and are water soluble, which could result in rapid clearance and lower immunogenicity. Following this rationale, the immunogenicity of several non-amphiphilic minimal CTL epitopes was improved by elongating them to form amphiphiles [33].
In addition to helping CTL responses, CD4 HTLs display direct antitumor effector function. In many instances HTLs are effective in killing MHC class II (MHC-II) expressing tumor cells [36], but in other cases, HTLs can eradicate MHC-II negative tumor cells [37]. Our group has been involved in identifying MHC-II binding peptides from numerous human tumor antigens capable of functioning as HTL epitopes [19]. Many of these peptides function as promiscuous epitopes (can be presented by several MHC-II alleles), potentially allowing their use as vaccines in broad patient populations. Following the model used with CTL vaccines, we reported the use of TriVax for eliciting substantive HTL responses in mice [21]. As with CTLs, the induction of HTLs by TriVax required a systemic prime-boost peptide administration and relied on IFN-I. HTL responses by TriVax were more effective with a TLR7 agonist instead of poly-IC and were enhanced by an OX40 agonist antibody. With a peptide derived from Trp1 (Trp1113–127), TriVax was able to overcome immune tolerance and induced HTLs capable of recognizing and killing MHC-II-expressing (interferon-gamma treated) B16 melanoma cells. TriVax immunization with the Trp1 HTL epitope into mice with established B16 tumors resulted in significant reductions in the rate of tumor growth [21].
6. Future challenges for peptide vaccines
Overall, our results demonstrate that peptide vaccines can be an effective way of inducing strong CTL and HTL anti-tumor responses in mice when administered with the appropriate adjuvants and immunization routes. However, it remains to be determined whether TriVax, BiVax or similar vaccination strategies will be effective in patients. There are several necessary prerequisites in order to take TriVax or BiVax into the clinic. Although synthetic peptides are relatively easy and cost effective to produce under good manufacturing practices (GMP), amphiphilic peptides, especially those containing palmitic acid chains can pose a challenge to purify using conventional methods. While currently there are no FDA-approved humanized anti-human CD40 agonistic antibodies, there are several under development, which could be used for TriVax. A GMP grade formulation of poly-IC (Hiltonol™ from Oncovir, Inc.) that is stabilized with poly-lysine and carboxymethylcellulose (poly-ICLC) has been extensively used in the clinic as an experimental drug to induce IFN-I to treat multiple sclerosis and glioblastoma and has been recently used as an immune adjuvant. We have experimental evidence in mice that poly-ICLC is a more potent adjuvant for T cell responses as compared to poly-IC (E. Celis, unpublished observations). Lastly, there is some reticence for administering vaccines intravenously due to safety concerns. Nevertheless, both TriVax and BiVax are effective in mice when injected via an intramuscular route, which also disseminates the peptide, poly-IC and antibody systemically to reach most antigen-specific T cell precursors.
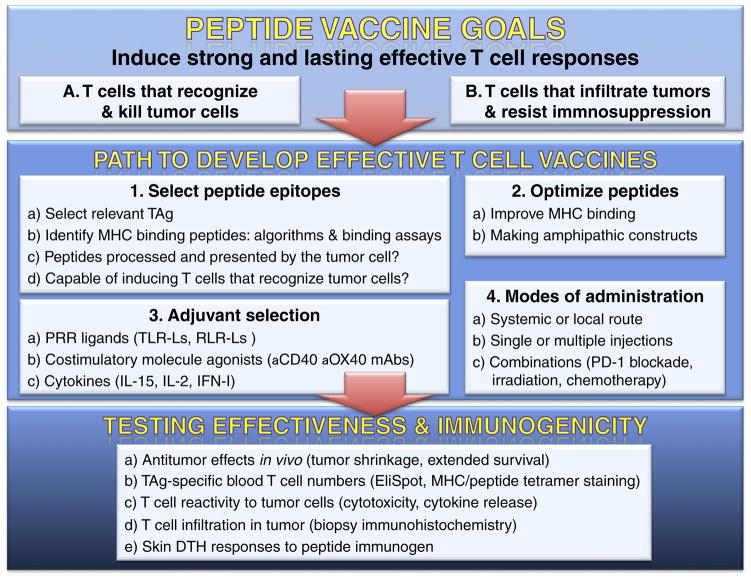
Figure 1. Rational path to designing and testing effective T cell epitope-based vaccines for cancer.
The goals of tumor vaccines are to induce strong and lasting antitumor T cells that can recognize and kill tumor cells (A), and overcome immunosuppression in the tumor microenvironment (B). The 4 steps to develop the vaccines: 1) Epitope selection is critical to develop effective T cell epitope-based vaccines. TAg potential epitopes that bind to an MHC molecule are predicted using computer-based algorithms and can be validated with binding assays. The presence of the peptide epitope on tumor cells can be assessed with mass spec sequencing of MHC eluted peptides. Epitope immunogenicity is established by in vitro T cell stimulation assays or in vivo by vaccinating HLA transgenic mice. 2) To enhance immunogenicity the amino acid sequence of epitope can be modified to increase MHC binding or enhance amphiphilicity. 3) The selection of appropriate adjuvants, costimulatory agonists and cytokines will determine the magnitude and duration of the T cell responses. 4) The mode of vaccine administration (injection route, boosters) and the possibility combining the vaccine with adjunct treatments are factors to consider for achieving effective antitumor responses. The immunogenicity and effectiveness of vaccines should be monitored by measuring realistic antitumor effects in vivo (a), changes in TAg-specific T cells pre- and post-vaccination (b), assessing T cell reactivity to tumor cells (c), infiltration of T cells in tumors (d), and evaluating skin delayed type hypersensitivity (DTH) responses in the vaccinees.
Highlights.
Cancer vaccines have not generated effective anti-tumor responses due to suboptimal immunogenicity.
Peptide vaccines with appropriate adjuvants are the most promising strategy to induce tumor-reactive T cells and treat cancer.
The combination of optimized peptides, strong adjuvants and costimulatory molecule stimulation together with systemic immunizations results in robust T cell responses.
Therapeutic peptide-based vaccines can reduce the tumor growth by generating tumor-specific CD8 CTLs or CD4 HTLs.
Blocking the immunosuppressive tumor microenvironment potentiates the effectiveness of cancer vaccines.
Acknowledgments
This work was supported by grant from the National Cancer Institute of the National Institutes of Health, R01CA157303, and by start-up funds from the Georgia Cancer Center and the Georgia Research Alliance (GRA).
Abbreviations
- APC
antigen-presenting cell
- CBI
checkpoint inhibitor
- CTL
cytotoxic T lymphocyte
- DC
dendritic cell
- GMP
good manufacturing practices
- HTL
helper T lymphocyte
- HPV
human papilloma virus
- IFN-I
type-I interferon
- pAPC
professional antigen presenting cell
- PRR
pathogen recognition receptor
- RIG-I
retinoic acid inducible gene-I
- RLR
RIG-I-like receptor
- TAg
tumor antigen
- TCR
T cell receptor
- TIL
tumor-infiltrating lymphocyte
- TLR
toll-like receptor
Footnotes
Conflicts of interest
Esteban Celis has filed patent applications based on the use of synthetic peptides and poly-IC combinatorial vaccines. The rights of the patent applications have been transferred to the Moffitt Cancer Center (Tampa, FL). Other authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
*of special interest
**of outstanding interest
- *1.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, Rosenberg SA. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–1544. doi: 10.1182/blood-2008-12-195792. This study showed that the majority of tumor-infiltrating T lymphocytes that express PD-1, are functionally impaired. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **2.Andersen R, Donia M, Ellebaek E, Borch TH, Kongsted P, Iversen TZ, Holmich LR, Hendel HW, Met O, Andersen MH, et al. Long-Lasting Complete Responses in Patients with Metastatic Melanoma after Adoptive Cell Therapy with Tumor-Infiltrating Lymphocytes and an Attenuated IL2 Regimen. Clin Cancer Res. 2016;22:3734–3745. doi: 10.1158/1078-0432.CCR-15-1879. This study demonstrated that adoptively transferred T lymphocytes effectively diminished tumor growth in humans. [DOI] [PubMed] [Google Scholar]
- 3.Pico de Coaña Y, Choudhury A, Kiessling R. Checkpoint blockade for cancer therapy: revitalizing a suppressed immune system. Trends Mol Med. 2015;21:482–491. doi: 10.1016/j.molmed.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Sharma P, Retz M, Siefker-Radtke A, Baron A, Necchi A, Bedke J, Plimack ER, Vaena D, Grimm MO, Bracarda S, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017 doi: 10.1016/S1470-2045(17)30065-7. [DOI] [PubMed] [Google Scholar]
- 5.Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, Christensen CL, Mikse OR, Cherniack AD, Beauchamp EM, Pugh TJ, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 2013;3:1355–1363. doi: 10.1158/2159-8290.CD-13-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *6.Klein G. Tumor-specific transplantation antigens: G. H. A. Clowes memorial lecture. Cancer Res. 1968;28:625–635. Classic work and accomplishments by one of the pioneers in tumor immunology vaccination. [PubMed] [Google Scholar]
- **7.Scheffer SR, Nave H, Korangy F, Schlote K, Pabst R, Jaffee EM, Manns MP, Greten TF. Apoptotic, but not necrotic, tumor cell vaccines induce a potent immune response in vivo. Int J Cancer. 2003;103:205–211. doi: 10.1002/ijc.10777. This paper describes how not all modes of tumor cell death are immunogenic. [DOI] [PubMed] [Google Scholar]
- *8.Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan RC. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. Preclinical data that resulted in the development of GVAX vaccines (refs. 9–12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Small E, Demkow T, Gerritson W, Rolland R, Hoskin P, Smith D, Parker C, Chondros D, Ma J, Hege K. ASCO GU. 2009. A phase III trial of GVAX immunotherapy for prostate cancer in combination with docetaxel vs. docetaxel plus prednisone in symptomatic, castration-resistant prostate cancer (CRPC) [Google Scholar]
- 10.Gupta R, Emens LA. GM-CSF-secreting vaccines for solid tumors: moving forward. Discov Med. 2010;10:52–60. [PMC free article] [PubMed] [Google Scholar]
- 11.Higano C, Saad F, Somer B, Curti B, Petrylak D, Drake C, Schnell F, Redfern D, Schrijvers D, Sacks N. ASCO GU. 2009. A phase III trial of GVAX immunotherapy for prostate cancer vs. docetaxel plus prednisone in asymptomatic castration-resistant prostate cancer (CRPC) [Google Scholar]
- 12.Salgia R, Lynch T, Skarin A, Lucca J, Lynch C, Jung K, Hodi FS, Jaklitsch M, Mentzer S, Swanson S, et al. Vaccination with irradiated autologous tumor cells engineered to secrete granulocyte-macrophage colony-stimulating factor augments antitumor immunity in some patients with metastatic non-small-cell lung carcinoma. J Clin Oncol. 2003;21:624–630. doi: 10.1200/JCO.2003.03.091. [DOI] [PubMed] [Google Scholar]
- *13.Castle JC, Kreiter S, Diekmann J, Lower M, van de Roemer N, de Graaf J, Selmi A, Diken M, Boegel S, Paret C, et al. Exploiting the mutanome for tumor vaccination. Cancer Res. 2012;72:1081–1091. doi: 10.1158/0008-5472.CAN-11-3722. This paper identified somatic point mutations in B16 mouse melanoma and showed induction of anti-tumor T cell responses after epitope vaccination. [DOI] [PubMed] [Google Scholar]
- 14.Karosiene E, Lundegaard C, Lund O, Nielsen M. NetMHCcons: a consensus method for the major histocompatibility complex class I predictions. Immunogenetics. 2012;64:177–186. doi: 10.1007/s00251-011-0579-8. [DOI] [PubMed] [Google Scholar]
- 15.Paul S, Sidney J, Sette A, Peters B. TepiTool: A Pipeline for Computational Prediction of T Cell Epitope Candidates. Curr Protoc Immunol. 2016;114:18 19 11–18 19 24. doi: 10.1002/cpim.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buus S, Sette A, Colon SM, Miles C, Grey HM. The relation between major histocompatibility complex (MHC) restriction and the capacity of Ia to bind immunogenic peptides. Science. 1987;235:1353–1358. doi: 10.1126/science.2435001. [DOI] [PubMed] [Google Scholar]
- *17.Yadav M, Jhunjhunwala S, Phung QT, Lupardus P, Tanguay J, Bumbaca S, Franci C, Cheung TK, Fritsche J, Weinschenk T, et al. Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature. 2014;515:572–576. doi: 10.1038/nature14001. very powerful approach to demonstrate the presence of mutated peptides bound to the tumor MHC molecules. [DOI] [PubMed] [Google Scholar]
- *18.Kawashima I, Tsai V, Southwood S, Takesako K, Sette A, Celis E. Identification of HLA-A3-restricted cytotoxic T lymphocyte epitopes from carcinoembryonic antigen and HER-2/neu by primary in vitro immunization with peptide-pulsed dendritic cells. Cancer Res. 1999;59:431–435. Example of the predictive approach to identify and validate T cell epitopes for CTLs. [PubMed] [Google Scholar]
- *19.Kobayashi H, Celis E. Peptide epitope identification for tumor-reactive CD4 T cells. Curr Opin Immunol. 2008;20:221–227. doi: 10.1016/j.coi.2008.04.011. Review describing predictive approach for identifying HTL epitopes and correlates the presence of proximal or imbeded CTL epitopes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **20.Cho HI, Celis E. Optimized peptide vaccines eliciting extensive CD8 T-cell responses with therapeutic antitumor effects. Cancer Res. 2009;69:9012–9019. doi: 10.1158/0008-5472.CAN-09-2019. Description of the TriVax approach to generate vast CTL responses using peptide, poly-IC and anti-CD40 antibodies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *21.Kumai T, Lee S, Cho HI, Sultan H, Kobayashi H, Harabuchi Y, Celis E. Optimization of Peptide Vaccines to Induce Robust Antitumor CD4 T-cell Responses. Cancer Immunol Res. 2016 doi: 10.1158/2326-6066.CIR-16-0194. This study demonstrated that the combination of synthetic peptide, TLR7 ligand, CD40 agonist, and OX40 agonist elicits a huge number of antitumor CD4 T lymphocytes with cytotoxic activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schurich A, Pallett LJ, Lubowiecki M, Singh HD, Gill US, Kennedy PT, Nastouli E, Tanwar S, Rosenberg W, Maini MK. The third signal cytokine IL-12 rescues the anti-viral function of exhausted HBV-specific CD8 T cells. PLoS Pathog. 2013;9:e1003208. doi: 10.1371/journal.ppat.1003208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curtsinger JM, Lins DC, Mescher MF. Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. J Exp Med. 2003;197:1141–1151. doi: 10.1084/jem.20021910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumagai Y, Akira S. Identification and functions of pattern-recognition receptors. J Allergy Clin Immunol. 2010;125:985–992. doi: 10.1016/j.jaci.2010.01.058. [DOI] [PubMed] [Google Scholar]
- *25.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. Classic paper demonstrating the importance of CD40 ligation in the generation of effective CTL responses. [DOI] [PubMed] [Google Scholar]
- *26.Ahonen CL, Doxsee CL, McGurran SM, Riter TR, Wade WF, Barth RJ, Vasilakos JP, Noelle RJ, Kedl RM. Combined TLR and CD40 triggering induces potent CD8+ T cell expansion with variable dependence on type I IFN. J Exp Med. 2004;199:775–784. doi: 10.1084/jem.20031591. First report of a peptide vaccine producing a substantial T cell response by combining the antigen with TLR ligand and anti-CD40 antibodies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *27.Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J Immunol. 2005;174:4465–4469. doi: 10.4049/jimmunol.174.8.4465. [DOI] [PubMed] [Google Scholar]
- 28.Kohlmeier JE, Cookenham T, Roberts AD, Miller SC, Woodland DL. Type I interferons regulate cytolytic activity of memory CD8(+) T cells in the lung airways during respiratory virus challenge. Immunity. 2010;33:96–105. doi: 10.1016/j.immuni.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *29.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. This controversial paper discusses and questions whether cancer vaccines will work against established tumors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Markovic SN, Suman VJ, Ingle JN, Kaur JS, Pitot HC, Loprinzi CL, Rao RD, Creagan ET, Pittelkow MR, Allred JB, et al. Peptide vaccination of patients with metastatic melanoma: improved clinical outcome in patients demonstrating effective immunization. Am J Clin Oncol. 2006;29:352–360. doi: 10.1097/01.coc.0000217877.78473.a4. [DOI] [PubMed] [Google Scholar]
- 31.Legat A, Maby-El Hajjami H, Baumgaertner P, Cagnon L, Abed Maillard S, Geldhof C, Iancu EM, Lebon L, Guillaume P, Dojcinovic D, et al. Vaccination with LAG-3Ig (IMP321) and Peptides Induces Specific CD4 and CD8 T-Cell Responses in Metastatic Melanoma Patients--Report of a Phase I/IIa Clinical Trial. Clin Cancer Res. 2016;22:1330–1340. doi: 10.1158/1078-0432.CCR-15-1212. [DOI] [PubMed] [Google Scholar]
- 32.Barrios K, Celis E. TriVax-HPV: an improved peptide-based therapeutic vaccination strategy against human papillomavirus-induced cancers. Cancer Immunol Immunother. 2012;61:1307–1317. doi: 10.1007/s00262-012-1259-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **33.Cho HI, Barrios K, Lee YR, Linowski AK, Celis E. BiVax: a peptide/poly-IC subunit vaccine that mimics an acute infection elicits vast and effective anti-tumor CD8 T-cell responses. Cancer Immunol Immunother. 2013;62:787–799. doi: 10.1007/s00262-012-1382-6. In this paper we showed that poly-IC is a unique adjuvant for peptide vaccines because it stimulates both TLR3 and MDA5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takeuchi O, Akira S. MDA5/RIG-I and virus recognition. Curr Opin Immunol. 2008;20:17–22. doi: 10.1016/j.coi.2008.01.002. [DOI] [PubMed] [Google Scholar]
- *35.Bijker MS, van den Eeden SJ, Franken KL, Melief CJ, Offringa R, van der Burg SH. CD8+ CTL priming by exact peptide epitopes in incomplete Freund’s adjuvant induces a vanishing CTL response, whereas long peptides induce sustained CTL reactivity. J Immunol. 2007;179:5033–5040. doi: 10.4049/jimmunol.179.8.5033. These authors are proponents of using elongated peptides and avoiding minimal peptide epitopes in vaccines. [DOI] [PubMed] [Google Scholar]
- 36.Kumai T, Matsuda Y, Ohkuri T, Oikawa K, Ishibashi K, Aoki N, Kimura S, Harabuchi Y, Celis E, Kobayashi H. c-Met is a novel tumor associated antigen for T-cell based immunotherapy against NK/T cell lymphoma. Oncoimmunology. 2015;4:e976077. doi: 10.4161/2162402X.2014.976077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *37.Mumberg D, Monach PA, Wanderling S, Philip M, Toledano AY, Schreiber RD, Schreiber H. CD4(+) T cells eliminate MHC class II-negative cancer cells in vivo by indirect effects of IFN-gamma. Proc Natl Acad Sci U S A. 1999;96:8633–8638. doi: 10.1073/pnas.96.15.8633. Interesting and important report showing that HTL recognition of antigen in tumor-infiltrating stromal cells can lead to anti-tumor effects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sosman JA, Sondak VK. Melacine: an allogeneic melanoma tumor cell lysate vaccine. Expert Rev Vaccines. 2003;2:353–368. doi: 10.1586/14760584.2.3.353. [DOI] [PubMed] [Google Scholar]
- 39.Li B, Simmons A, Du T, Lin C, Moskalenko M, Gonzalez-Edick M, VanRoey M, Jooss K. Allogeneic GM-CSF-secreting tumor cell immunotherapies generate potent anti-tumor responses comparable to autologous tumor cell immunotherapies. Clin Immunol. 2009;133:184–197. doi: 10.1016/j.clim.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 40.Wei H, Wang S, Zhang D, Hou S, Qian W, Li B, Guo H, Kou G, He J, Wang H, et al. Targeted delivery of tumor antigens to activated dendritic cells via CD11c molecules induces potent antitumor immunity in mice. Clin Cancer Res. 2009;15:4612–4621. doi: 10.1158/1078-0432.CCR-08-3321. [DOI] [PubMed] [Google Scholar]
- 41.He XP, Su CQ, Wang XH, Pan X, Tu ZX, Gong YF, Gao J, Liao Z, Jin J, Wu HY, et al. E1B-55kD-deleted oncolytic adenovirus armed with canstatin gene yields an enhanced anti-tumor efficacy on pancreatic cancer. Cancer Lett. 2009;285:89–98. doi: 10.1016/j.canlet.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 42.Indraccolo S, Habeler W, Tisato V, Stievano L, Piovan E, Tosello V, Esposito G, Wagner R, Uberla K, Chieco-Bianchi L, et al. Gene transfer in ovarian cancer cells: a comparison between retroviral and lentiviral vectors. Cancer Res. 2002;62:6099–6107. [PubMed] [Google Scholar]
- 43.Pang S, Kang MK, Kung S, Yu D, Lee A, Poon B, Chen IS, Lindemann B, Park NH. Anticancer effect of a lentiviral vector capable of expressing HIV-1 Vpr. Clin Cancer Res. 2001;7:3567–3573. [PubMed] [Google Scholar]
- 44.Ikonomidis G, Paterson Y, Kos FJ, Portnoy DA. Delivery of a viral antigen to the class I processing and presentation pathway by Listeria monocytogenes. J Exp Med. 1994;180:2209–2218. doi: 10.1084/jem.180.6.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Echchannaoui H, Bianchi M, Baud D, Bobst M, Stehle JC, Nardelli-Haefliger D. Intravaginal immunization of mice with recombinant Salmonella enterica serovar Typhimurium expressing human papillomavirus type 16 antigens as a potential route of vaccination against cervical cancer. Infect Immun. 2008;76:1940–1951. doi: 10.1128/IAI.01484-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pan Y, Li X, Kang T, Meng H, Chen Z, Yang L, Wu Y, Wei Y, Gou M. Efficient delivery of antigen to DCs using yeast-derived microparticles. Sci Rep. 2015;5:10687. doi: 10.1038/srep10687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weber JS, Hua FL, Spears L, Marty V, Kuniyoshi C, Celis E. A phase I trial of an HLA-A1 restricted MAGE-3 epitope peptide with incomplete Freund’s adjuvant in patients with resected high-risk melanoma. J Immunother. 1999;22:431–440. doi: 10.1097/00002371-199909000-00007. [DOI] [PubMed] [Google Scholar]
- 48.Trimble CL, Morrow MP, Kraynyak KA, Shen X, Dallas M, Yan J, Edwards L, Parker RL, Denny L, Giffear M, et al. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2b trial. Lancet. 2015;386:2078–2088. doi: 10.1016/S0140-6736(15)00239-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sebastian M, Papachristofilou A, Weiss C, Fruh M, Cathomas R, Hilbe W, Wehler T, Rippin G, Koch SD, Scheel B, et al. Phase Ib study evaluating a self-adjuvanted mRNA cancer vaccine (RNActive(R)) combined with local radiation as consolidation and maintenance treatment for patients with stage IV non-small cell lung cancer. BMC Cancer. 2014;14:748. doi: 10.1186/1471-2407-14-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shevtsov M, Multhoff G. Heat Shock Protein-Peptide and HSP-Based Immunotherapies for the Treatment of Cancer. Front Immunol. 2016;7:171. doi: 10.3389/fimmu.2016.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]