Abstract
Neuropeptides are a diverse class of intercellular signalling molecules that mediate neuronal regulation of many physiological and behavioural processes. Recent advances in genome/transcriptome sequencing are enabling identification of neuropeptide precursor proteins in species from a growing variety of animal taxa, providing new insights into the evolution of neuropeptide signalling. Here, detailed analysis of transcriptome sequence data from three brittle star species, Ophionotus victoriae, Amphiura filiformis and Ophiopsila aranea, has enabled the first comprehensive identification of neuropeptide precursors in the class Ophiuroidea of the phylum Echinodermata. Representatives of over 30 bilaterian neuropeptide precursor families were identified, some of which occur as paralogues. Furthermore, homologues of endothelin/CCHamide, eclosion hormone, neuropeptide-F/Y and nucleobinin/nesfatin were discovered here in a deuterostome/echinoderm for the first time. The majority of ophiuroid neuropeptide precursors contain a single copy of a neuropeptide, but several precursors comprise multiple copies of identical or non-identical, but structurally related, neuropeptides. Here, we performed an unprecedented investigation of the evolution of neuropeptide copy number over a period of approximately 270 Myr by analysing sequence data from over 50 ophiuroid species, with reference to a robust phylogeny. Our analysis indicates that the composition of neuropeptide ‘cocktails’ is functionally important, but with plasticity over long evolutionary time scales.
Keywords: neuropeptide evolution, brittle star, Ophiuroidea, eclosion hormone, CCHamide, neuropeptide-Y
1. Introduction
The nervous systems of animals use a wide variety of chemicals for neuronal communication. These include amino acids (e.g. glutamate), biogenic amines (e.g. serotonin) and neuropeptides (e.g. vasopressin) among others. Neuropeptides are, by far, the most diverse and they control many physiological/behavioural processes, including feeding, reproduction and locomotion [1–3]. Recent advances in genome/transcriptome sequencing are enabling the identification of neuropeptide precursor proteins in species from a growing variety of animal taxa, providing new insights into the evolution of neuropeptide signalling [4–8]. The echinoderms are notable in this regard because as deuterostomian invertebrates they occupy an ‘intermediate’ phylogenetic position with respect to the vertebrates and intensely studied protostomian invertebrates such as insects (e.g. Drosophila melanogaster) and nematodes (e.g. Caenorhabditis elegans). Accordingly, characterization of neuropeptide signalling systems in echinoderms has recently provided key ‘missing links’ for the determination of neuropeptide relationships and reconstruction of neuropeptide evolution [8–10].
The phylum Echinodermata comprises five extant classes: Echinoidea (sea urchins and sand dollars), Holothuroidea (sea cucumbers), Asteroidea (starfish), Ophiuroidea (brittle stars and basket stars) and Crinoidea (sea lilies and feather stars). Recent molecular phylogenetic studies support the hypothesis that Echinoidea and Holothuroidea are sister groups (Echinozoa), and Asteroidea and Ophiuroidea are also sister groups (Asterozoa), with the Crinoidea basal to the Echinozoa + Asterozoa clade (Eleutherozoa) [11,12]. Echinoderms are marine organisms that have several unique features including pentaradial symmetry as adults, a remarkable ability to autotomize and regenerate body parts, and neurally controlled mutable collagenous tissue [13,14]. Previous transcriptomic analyses have identified neuropeptide precursor complements in Strongylocentrotus purpuratus (purple sea urchin), Apostichopus japonicus (Japanese sea cucumber) and Asterias rubens (common European starfish) [8,15,16]. Furthermore, the identification of neuropeptides in these species has facilitated investigation of the evolution and physiological roles of various neuropeptide signalling systems [8–10,17–21].
The recent progress in transcriptomic/genomic characterization of echinoderm neuropeptide systems has hitherto not been extended to ophiuroids or crinoids. The Ophiuroidea constitutes the largest class among extant echinoderms [22], with a long evolutionary history that extends back to the early Ordovician (around 480 Ma) [23], while the extant radiation dates from the mid-Permian (approx. 270 Ma) [12]. Available molecular data for ophiuroids have increased significantly in recent years with the emergence of numerous transcriptomic studies [20,24–29]. Here, we use transcriptome sequence data from three brittle star species, Ophionotus victoriae, Amphiura filiformis and Ophiopsila aranea, to perform the first comprehensive identification of neuropeptide precursors in ophiuroids. We identify representatives of over 30 neuropeptide families including homologues of endothelin/CCHamide, eclosion hormone (EH), neuropeptide-F/Y (NPF/NPY) and nucleobinin (NUCB)/nesfatin, which are the first to be discovered in a deuterostome/echinoderm.
Transcriptomes have also been employed to investigate the phylogenetic relationships of the ophiuroids, utilizing data from 52 species [12]. In the most comprehensive molecular analysis of ophiuroid phylogeny to date, previous morphology-based classification schemes [30] were rejected in favour of a new phylogeny comprising three primary ophiuroid clades and six orders [12,31,32]. This landmark study and the associated large dataset have provided a unique opportunity to investigate the conservation and diversification of neuropeptide precursor structure over a period of approximately 270 Myr of ophiuroid evolution. Our analysis reveals that the majority of ophiuroid neuropeptide precursors contain a single copy of a neuropeptide, but several precursors comprise multiple copies of identical or non-identical, but structurally related, neuropeptides. Interestingly, the number of neuropeptide copies in the majority of precursors is constant across all the ophiuroid species examined, but examples of clade-specific losses/gains of neuropeptides are also observed. This remarkable conservation in neuropeptide copy number across approximately 270 Myr of ophiuroid evolution indicates that the composition of neuropeptide ‘cocktails’ is functionally important, but with plasticity over long evolutionary time scales.
2. Results and discussion
Here, we have used transcriptome sequence data for the first comprehensive identification of neuropeptide precursors in ophiuroids (figure 1). Representatives of over 30 bilaterian neuropeptide precursor families were identified. Identification of ophiuroid representatives of these neuropeptide precursor types has, in some cases, provided new insights into neuropeptide precursor structure and evolution, as discussed in more detail below. First, however, we will highlight representatives of bilaterian neuropeptide precursor families that have been identified here for the first time in an echinoderm species.
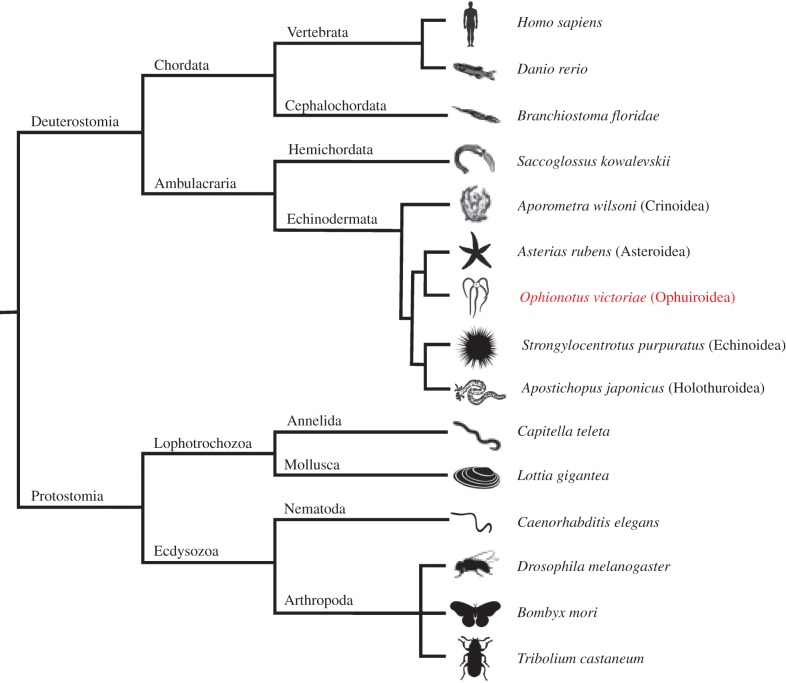
Figure 1.
Bilaterian animal phylogeny. The diagram shows (i) the phylogenetic position of the phylum Echinodermata in the ambulacrarian clade of the deuterostomes and (ii) relationships between the five extant classes of echinoderms, which include the focal class for this study—the Ophiuroidea (e.g. Ophionotus victoriae).
2.1. Discovery of the first echinoderm representatives of bilaterian neuropeptide families
Comprehensive analysis of transcriptome sequence data from three ophiuroid species, O. victoriae, A. filiformis and O. aranea, has enabled the discovery of the first echinoderm representatives of four bilaterian neuropeptide families. Specifically, we have discovered the first deuterostomian homologues of EH (figure 2), the first ambulacrarian homologue of CCHamide/endothelin-type peptides (figure 3a), and the first echinoderm homologues of NPY/NPF (figure 3b) and NUCB/nesfatin (electronic supplementary material, figure S1), as discussed in detail below.
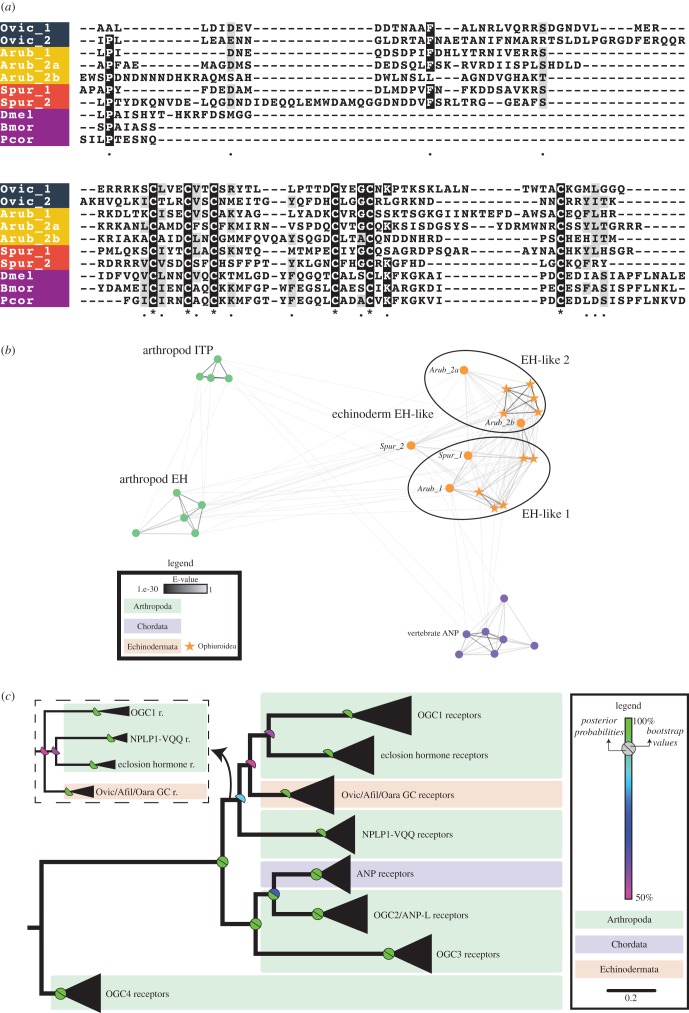
Figure 2.
Eclosion hormone (EH)-type peptides and receptors in echinoderms. (a) Partial multiple sequence alignment of EH-type precursor sequences, excluding the N-terminal signal peptide. (b) Cluster analysis of arthropod EH precursors, echinoderm EH-like precursors, arthropod ion transport peptides (ITPs) and vertebrate atrial natriuretic peptides (ATPs) shows that echinoderm EH-like precursors are more closely related to arthropod EH than ITP. (c) Maximum-likelihood and Bayesian phylogenetic analyses of membrane guanylate cyclase receptors show that EH-like receptors are found in echinoderms, but are absent in vertebrates as seen for the EH-like precursors. OGC1, 2, 3 and 4 are orphan guanylate cyclase receptors found in arthropods [33]. Echinoderm EH-like receptors are clustered with arthropod EH receptors, neuropeptide-like peptide 1-VQQ receptors (NPLP1-VQQ) and OGC1 receptors. The inset shows the alternate topology obtained following Bayesian analysis. Species names: Ophionotus victoriae (Ovic), Asterias rubens (Arub), Strongylocentrotus purpuratus (Spur), Drosophila melanogaster (Dmel), Bombyx mori (Bmor) and Pediculus humanus corporis (Pcor).
Figure 3.
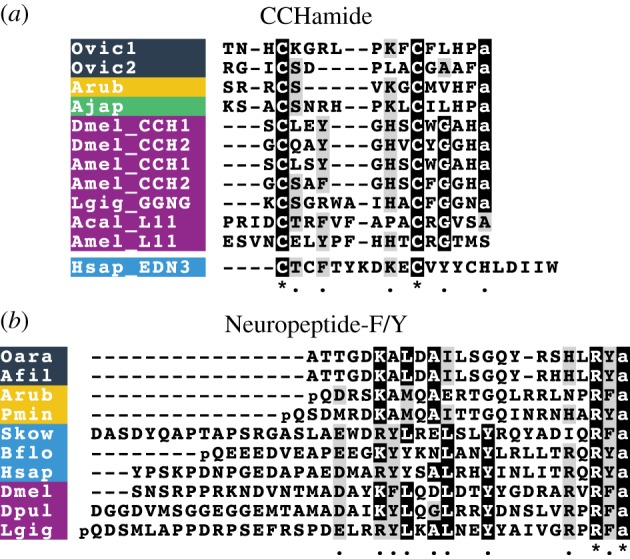
Multiple sequence alignments of (a) CCHamide-type and (b) neuropeptide-F/Y (NPF/NPY) type peptides. Species names: Ophionotus victoriae (Ovic), Asterias rubens (Arub), Apostichopus japonicus (Ajap), Drosophila melanogaster (Dmel), Apis mellifera (Amel), Lottia gigantea (Lgig), Aplysia californica (Acal), Homo sapiens (Hsap), Ophiopsila aranea (Oara), Amphiura filiformis (Afil), Patiria miniata (Pmin), Saccoglossus kowalevskii (Skow), Branchiostoma floridae (Bflo) and Daphnia pulex (Dpul).
2.1.1. Eclosion hormone
EH was first isolated and sequenced in the insects Manduca sexta (tobacco hornworm) and Bombyx mori (silk moth), and was shown to alter the timing of adult emergence [34,35]. EH is one of the main peptide/protein hormones involved in control of ecdysis (i.e. shedding of the cuticle) behaviour in arthropods [36,37]. It binds to and activates a receptor guanylyl cyclase that is expressed in epitracheal Inka cells and causes the secondary release of ecdysis-triggering hormone (ETH) that is also expressed in Inka cells [38,39]. In Drosophila, EH is important for ecdysis, but whether this hormone is essential for ecdysis is not yet clear [40,41]. EH null mutant flies show defects in ecdysis and are unable to reach adulthood, yet some flies in which EH-producing neurons have been genetically ablated (a more extreme manipulation) are able to survive till adulthood. Arthropod EHs have six conserved cysteine residues that form three disulfide bridges [38]. EHs have not been discovered previously outside of arthropods. Interestingly, four EH-like precursors were identified in A. filiformis and O. aranea, and two in O. victoriae (electronic supplementary material, figures S2–S4; GenBank: MF155236; MF155237). The ophiuroid EH-like precursors are orthologous to neuropeptide precursors previously identified in the sea urchin S. purpuratus (Spnp11 and Spnp15, which we now rename as Spur EH1 and Spur EH2, respectively) [16] and the starfish A. rubens (Arnp11, Arnp15 and Arnp15b renamed as Arub EH1, Arub EH2a and Arub EH2b, respectively) [8]. The positions of cysteine residues are conserved across all echinoderm and insect EHs, but aside from this there is little sequence conservation (figure 2a). The echinoderm EH-like precursor sequences were also analysed using a sequence similarity-based clustering approach based on BLASTp e-values using the CLANS software [42]. The analysis shows that echinoderm EH-like precursors (i) cluster in two compact subgroups (echinoderm EH-like precursor 1 and EH-like precursor 2), and (ii) have strong positive BLAST results with arthropod EHs and, to a lesser extent, with arthropod ion transport peptide (ITP) and vertebrate atrial natriuretic peptide (ANP) (figure 2b). ITP precursors also possess six cysteine residues; however, the position of these residues is not conserved with cysteine residues found in echinoderm EH-like precursors (not shown).
To obtain further evidence for the presence of an EH-like signalling system in echinoderms, we performed a phylogenetic analysis of EH-type receptors. Insect EHs mediate their effects by binding to membrane guanylyl cyclase receptors [39]. EH receptors are closely related to vertebrate ANP receptors and various orphan receptors [33]. Specific BLAST searches enabled the identification of transcripts in O. victoriae, A. filiformis and O. aranea that encode proteins similar to arthropod EH receptors. Maximum-likelihood and Bayesian phylogenetic analyses confirmed that these sequences group with the receptor cluster containing EH receptors (figure 2c). The discovery of the first deuterostomian EHs suggests an ancient bilaterian origin of EHs and indicates that these hormones may have other functions in invertebrates aside from their role in ecdysis.
2.1.2. CCHamide
CCHamides are neuropeptides that were discovered relatively recently in the silkworm B. mori [43]. Later, it was found that insects have two CCHamide genes, CCHamide-1 and CCHamide-2, each encoding a single copy of the mature peptide [44]. These peptides are referred to as CCHamides because they contain two cysteine residues and a characteristic histidine-amide C-terminal motif. There are two CCHamide receptors in insects: CCHamide-1 specifically activates one receptor and CCHamide-2 specifically activates the second receptor [44,45]. CCHamide-1 has a physiological role in starvation-induced olfactory modifications [46], whereas CCHamide-2 regulates feeding, growth and developmental timing in flies [45,47]. Recent studies examining the evolution of neuropeptides in the Bilateria have shown that protostomian CCHamides are related to elevenin (another protostomian neuropeptide originally discovered from the mollusc Aplysia californica L11 neuron), lophotrochozoan GGNG peptides, endothelins and gastrin-releasing peptides (GRPs) [6,7,48,49]. The latter two are neuropeptide types that have not been found outside chordates. Furthermore, the degree of sequence/structural conservation varies across these different peptide families. Hence, CCHamides are amidated and have a disulfide bridge, elevenins and endothelins have a disulfide bridge but are non-amidated, and GRPs are amidated but lack the disulfide bridge. Furthermore, CCHamide-1 is located immediately after the signal peptide, whereas there is a dibasic cleavage site separating the signal peptide and CCHamide-2 [44].
Here we have identified two neuropeptide precursors in brittle stars whose sequence and precursor structure resembles those of lophotrochozoan GGNG peptides and insect CCHamide-1 (figure 3a). The CCHamide-like precursor 1 (GenBank: MF155229) identified in O. victoriae is orthologous to an uncharacterized neuropeptide precursor (Arnp25) identified previously in the starfish A. rubens [8], whereas the CCHamide-like precursor 2 (GenBank: MF155230) was only found in brittle stars. Both CCHamide-like precursors in O. victoriae comprise a single copy of a putative cyclic amidated peptide that is flanked by a signal peptide at the N-terminus and a dibasic cleavage site at the C-terminus. Interestingly, both of these peptides lack a penultimate histidine residue, just like the lophotrochozoan GGNG peptides (figure 3a) [48,49].
2.1.3. Neuropeptide-Y/Neuropeptide-F
NPY was first isolated and sequenced from the porcine hypothalamus in 1982 [50,51]. Although the NPY/NPF family of peptides are pleiotropic in nature [52], they are mainly known for their roles in regulation of feeding and stress [3,53,54]. The discovery of NPF in the tapeworm Monieza expansa in 1991 demonstrated for the first time the occurrence of NPY homologues in invertebrates [55]. Here, we have identified the first echinoderm representatives of the NPY/NPF family in brittle stars and starfish (figure 3b; electronic supplementary material, figure S12). The brittle star precursors contain a peptide with a C-terminal RYamide, in common with NPY in vertebrates and an orthologue in the starfish Patiria miniata. By contrast, an orthologue in the starfish A. rubens has a C-terminal RFamide, a feature that it shares with NPY/NPF-type peptides in the hemichordate Saccoglossus kowalevskii and in protostomes. Thus, our findings have revealed that NPY/NPF-type peptides with a C-terminal Yamide motif are not restricted to vertebrates, as has been shown previously in some insects [52]. Echinoderm NPY/NPF-type peptides are located immediately after the signal peptide in the precursor proteins, as is the case in other bilaterian species. Surprisingly, we did not find NPY/NPF-type precursors in the sea urchin S. purpuratus or the sea cucumber A. japonicus. However, we suspect that this may reflect sequence divergence rather than gene loss because a gene encoding an NPY/NPF-type receptor can be found in the S. purpuratus genome [56].
2.1.4. NUCB
Nucleobindins (NUCB1 and NUCB2) are multidomain Ca2+ and DNA-binding proteins. NUCB1 was first discovered in 1992 and thought to play a role in apoptosis and autoimmunity [57]. Interestingly, the NUCB1 precursor has both a signal peptide and a leucine zipper structure, suggesting that it can bind DNA and act as an endocrine factor [58]. NUCB2 is a homologue of NUCB1 and was named based on high sequence similarity between the two precursors [59]. In 2006, an 82 amino acid peptide located in the N-terminal region of NUCB2 was reported. This peptide, Nesfatin-1 (Nucleobindin-2-Encoded Satiety and FAT-Influencing proteiN-1), was discovered as a satiety-inducing factor in the rat hypothalamus [60]. Its role in inhibiting food intake in vertebrates is now well established [59,61]. Moreover, this pleiotropic peptide also modulates other processes, including glucose and lipid metabolism, and cardiovascular and reproductive functions. Recently, nesfatin-1-like peptide derived from NUCB1 was shown to be anorexigenic in goldfish [62]. Surprisingly, the presence of NUCBs in invertebrates other than Drosophila has not been reported until now [63]. Here, we show that NUCB-type precursors are present in echinoderms (electronic supplementary material, figure S1a). Phylogenetic analysis of NUCB precursors reveals that a single copy of the NUCB precursor is found in invertebrate species and gene duplication in the vertebrate lineage gave rise to NUCB1 and NUCB2 (electronic supplementary material, figure S1b). In chordates, the NUCB precursors are predicted to generate three peptides (Nesfatin-1, 2 and 3); however, no biological role has been attributed specifically to nesfatin-2 and nesfatin-3. Interestingly, the prohormone convertase cleavage sites expected to generate Nesfatin-1, 2 and 3 are conserved between echinoderm and chordate NUCBs. Moreover, the O. victoriae precursor (GenBank: MF155235) has an additional predicted cleavage site within the Nesfatin-1-containing region, which is not present in other species (except for D. melanogaster). However, it remains to be determined whether or not this cleavage site in the O. victoriae precursor is functional.
2.2. First comprehensive identification of neuropeptide precursors in ophiuroids
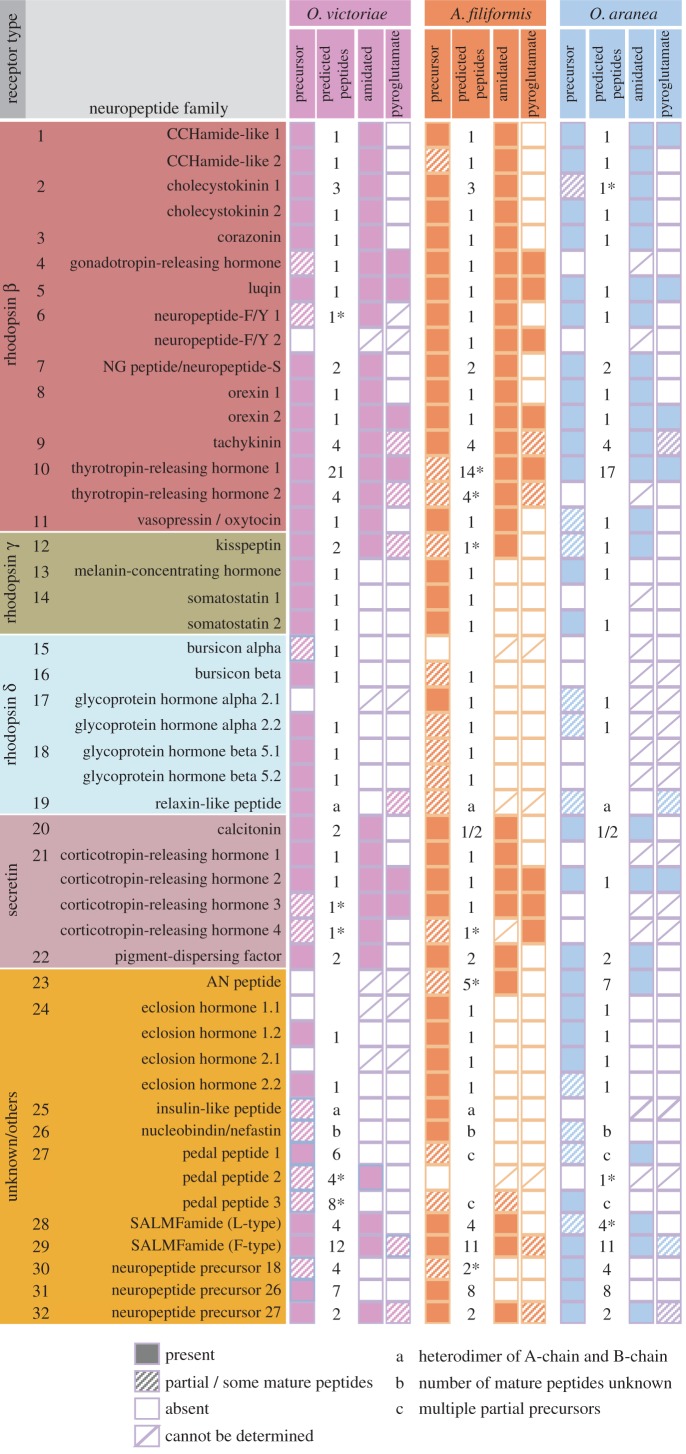
We have identified neuropeptide precursors belonging to 32 families, which represent the first comprehensive analysis of neuropeptide precursors in ophiuroids (figure 4; electronic supplementary material, figures S2–S4). Several of these neuropeptide families have been identified previously in echinoderms and include homologues of AN peptides, bursicon (α and β) (GenBank: MF155260; MF155227), calcitonin (GenBank: MF155228), cholecystokinin (CCK) (GenBank: MF155231; MF155232) [15], corazonin (GenBank: MF155233) [10], corticotropin-releasing hormone (CRH) (GenBank: MF155234, MF155235, MF155261, MF155262), glycoprotein hormones (α2 and β5) (GenBank: MF155238; MF155239; MF155240) [64], gonadotropin-releasing hormone (GnRH) (GenBank: MF155263) [10], insulin-like peptide (GenBank: MF155264) [64], kisspeptin (KP) (GenBank: MF155241) [8], luqin (GenBank: MF155242) [7], melanin-concentrating hormone (MCH) (GenBank: MF155243) [8], NG peptides (neuropeptide-S) (GenBank: MF155244) [9,65], orexin (GenBank: MF155245; MF155246) [6,8], pedal peptides (GenBank: MF155247; MF155266; MF155267) [16], pigment-dispersing factor (PDF) (GenBank: MF155248) [8], relaxin-like peptide (GenBank: MF155249) [66], SALMFamides (L-type and F-type) (GenBank: MF155250; MF155268) [19,20,67], somatostatin (GenBank: MF155252; MF155253) [8], tachykinin (GenBank: MF155254) [8], thyrotropin-releasing hormone (TRH) (GenBank: MF155255; MF155256) [16] and vasopressin/oxytocin (GenBank: MF155257) [64,65] (figures 5–7; electronic supplementary material, figures S5–S10). With the exception of MCH (which may be unique to deuterostomes) [6,8], AN peptides and SALMFamides (which thus far have only been identified in echinoderms), the origins of all of the neuropeptide precursors identified here in ophiuroids pre-date the divergence of protostomes and deuterostomes [6,7]. Of the three species examined here, the neuropeptide precursor complement of O. victoriae was the most complete (figure 4); therefore, this species is used as a representative ophiuroid for sequence alignments, except in a few cases where a neuropeptide precursor was not found in O. victoriae. Below we highlight several interesting and/or unusual features of ophiuroid neuropeptides and neuropeptide precursors.
Figure 4.
Summary of neuropeptide precursors identified in Ophionotus victoriae, Amphiura filiformis and Ophiopsila aranea. Neuropeptide precursors are classified based on the type of G-protein coupled receptor (GPCR) their constituent peptides are predicted to activate (see Mirabeau and Joly [6]). Some peptides bind to receptors other than GPCRs and these are grouped with peptides where the receptor is unknown. Ophiuroids have neuropeptide precursors from up to 32 families. The number of putative mature peptides derived from each precursor has been indicated along with the presence of amidation and pyroglutamation.
Figure 5.
Multiple sequence alignments of mature peptides belonging to selected neuropeptide families. (a) Corazonin alignment; (b) gonadotropin-releasing hormone (GnRH) alignment; (c) orexin alignment; (d) luqin alignment; (e) vasopressin/oxytocin (VP/OT) alignment; (f) Ovnp18 alignment; (g) MCH alignment; (h) NG peptide alignment; (i) pigment-dispersing factor (PDF) alignment (see electronic supplementary material, figure S10 for a multiple sequence alignment of PDF-type precursors). Species names: Ophionotus victoriae (Ovic), Asterias rubens (Arub), Strongylocentrotus purpuratus (Spur), Apostichopus japonicus (Ajap), Saccoglossus kowalevskii (Skow), Branchiostoma floridae (Bflo), Anopheles gambiae (Agam), Daphnia pulex (Dpul), Strigamia maritima (Smar), Lottia gigantea (Lgig) and Homo sapiens (Hsap).
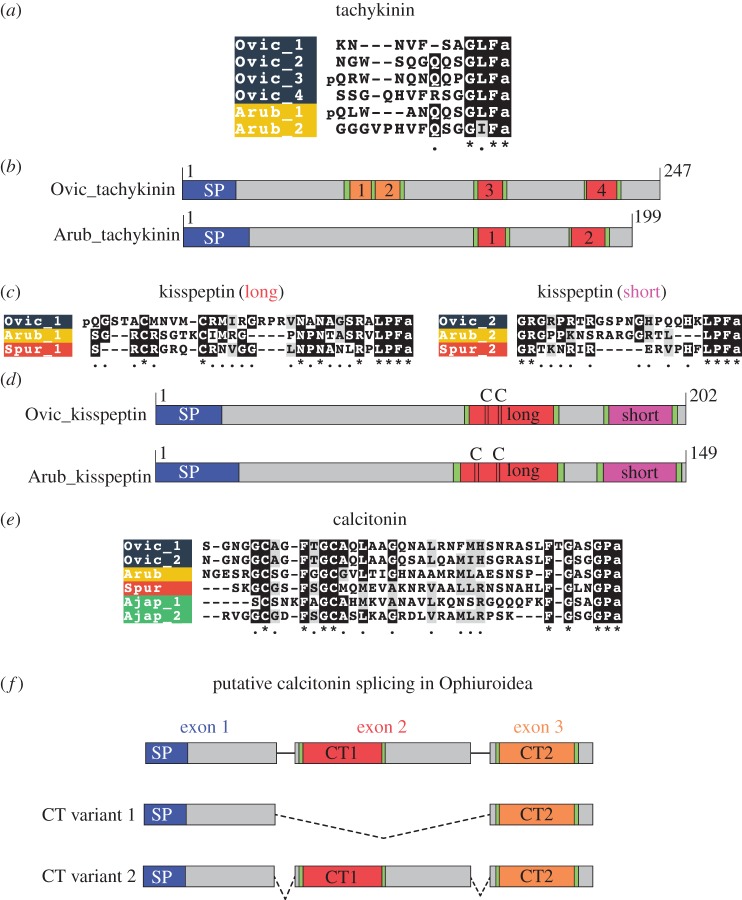
Figure 7.
Comparative analysis of ophiuroid tachykinin, KP and calcitonin-type precursors and neuropeptides. (a) Alignment of tachykinin-type peptides in O. victoriae (Ophiuroidea) and A. rubens (Asteroidea). (b) Schematic diagrams of the O. victoriae and A. rubens tachykinin precursors showing the location of the signal peptide (SP) and predicted neuropeptides (labelled 1–4). (c) Alignments of the long and short forms of kisspeptin (KP)-type neuropeptides in O. victoriae, A. rubens and S. purpuratus (Echinoidea). (d) Schematic diagrams of the O. victoriae and A. rubens KP precursors showing the locations of the SP, short and long orthocopies and cysteine (C) residues. (e) Alignment of calcitonin-type peptides from O. victoriae, A. rubens, S. purpuratus and A. japonicus (Holothuroidea). (f) Predicted alternative splicing of the calcitonin gene in ophiuroids, with the location of the SP and neuropeptides (CT1 and CT2) labelled. Species names: Ophionotus victoriae (Ovic), Asterias rubens (Arub), Strongylocentrotus purpuratus (Spur) and Apostichopus japonicus (Ajap).
2.3. Neuropeptide precursors that occur in multiple forms in Ophionotus victoriae
2.3.1. Thyrotropin-releasing hormone-type precursors
TRH (also known as thyrotropin-releasing factor or thyroliberin) was first isolated and sequenced in the 1960s [68–70]. In mammals, TRH is produced in the hypothalamus, and stimulates the release of thyroid-stimulating hormone (TSH) and prolactin from the anterior pituitary [71,72]. The recent discovery of a TRH receptor in the annelid Platynereis dumerilii indicates that the evolutionary origin of this neuropeptide signalling system predates the divergence of protostomes and deuterostomes [73].
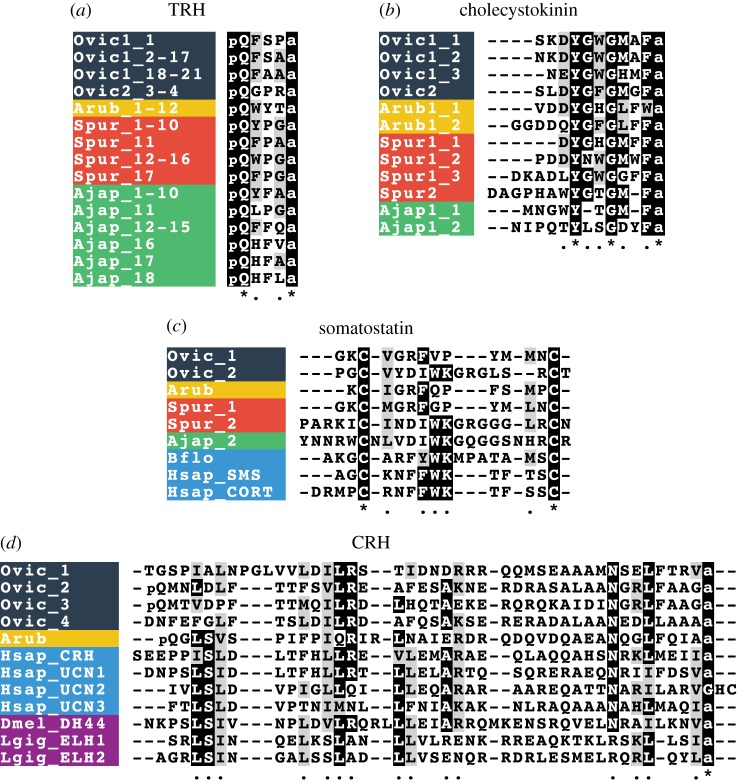
The human TRH precursor contains six copies of the tripeptide pQHPamide [74]. Precursor proteins comprising multiple copies of TRH-like peptides have been identified previously in the sea urchin S. purpuratus, the sea cucumber A. japonicus and the starfish A. rubens [8,15,16], with a single TRH-type precursor found in each of these species. Interestingly, here we identified two TRH-type precursors (OvTRHP1 and OvTRHP2) in O. victoriae (electronic supplementary material, figure S2; figure 6a). OvTRHP1 comprises 21 copies of putative TRH-like tetrapeptides with the motif pQXXXamide (where X is variable). OvTRHP2, on the other hand, comprises two copies of the putative tetrapeptide pQGPRamide and two longer peptides that also have a C-terminal GPRamide motif but lack the N-terminal pyroglutamate.
Figure 6.
Alignments of neuropeptides derived from precursors that exist in multiple forms in ophiuroids. (a) Thyrotropin-releasing hormone (TRH) alignment; (b) Cholecystokinin (CCK) alignment; (c) somatostatin alignment; (d) Corticotropin-releasing hormone (CRH) alignment. Species names: Ophionotus victoriae (Ovic), Asterias rubens (Arub), Strongylocentrotus purpuratus (Spur), Apostichopus japonicus (Ajap), Branchiostoma floridae (Bflo), Homo sapiens (Hsap), Drosophila melanogaster (Dmel) and Lottia gigantea (Lgig).
2.3.2. Cholecystokinin-type precursors
A CCK-type peptide (formerly pancreozymin) was first sequenced in the 1960s [75]. CCK-type peptides play numerous roles in feeding and digestion-related physiology. CCK mediates satiety, and stimulates the release of digestive enzymes and gall bladder contractions [76–78]. CCK-type peptides are involved in mechanisms of learning and memory, and analgesia [79]. A neuropeptide precursor comprising two CCK-like peptides was recently identified in the starfish A. rubens [8]. Here, we have identified two CCK-type precursors in O. victoriae (OvCCKP1 and OvCCKP2) and orthologues of both of these precursors were also identified in the sea urchin S. purpuratus (electronic supplementary material, figure S2) [16]. The CCK-type precursor 1 comprises three CCK-like peptides in both O. victoriae and S. purpuratus, and this precursor is similar to the A. rubens CCK-type precursor, which comprises two CCK-like peptides. By contrast, the CCK-type precursor 2 comprises a single CCK-like peptide in both O. victoriae and S. purpuratus. Interestingly, the sequence of the S. purpuratus CCK-type precursor 2 was reported previously as part of a genome-wide search for neuropeptides [80], but the authors of this study did not identify it as a CCK-type precursor. However, based on the presence of a conserved tyrosine residue and a C-terminal F-amide motif in the predicted neuropeptide derived from this protein, it is evident that it belongs to the family of CCK-type precursors (figure 6b). A search of a preliminary genome assembly of the starfish P. miniata (http://www.echinobase.org) [81] did not reveal a gene encoding a CCK-type precursor 2. Therefore, it appears that this neuropeptide precursor type may have been lost in the Asteroidea; nevertheless, further analysis of a wider range of starfish species will be required to draw definitive conclusions. With a broader evolutionary perspective, CCK-type peptides in deuterostomes are orthologues of sulfakinin (SK)-type neuropeptides found in insects [6,7]. Interestingly, insects have a single SK precursor, which comprises two neuropeptides, SK-1 and SK-2 [82], and this may reflect the ancestral condition in the common ancestor of protostomes and deuterostomes. Thus, the occurrence of two CCK-type peptides on a single precursor in A. rubens and insects may be an ancestral characteristic, and the occurrence of two CCK-type precursors that comprise one and three CCK-type peptides appears to be a derived characteristic.
2.3.3. Somatostatin-type precursors
Somatostatin was first isolated and sequenced from sheep hypothalamus in 1973 [83]. This peptide inhibits the release of pituitary hormones such as growth hormone, prolactin and TSH [84]. Moreover, it also inhibits the release of gastrointestinal (CCK and gastrin among others) and pancreatic (insulin and glucagon) hormones [85–87]. Aside from its effects on release of hormones, somatostatin also has central actions that influence motor activity [85]. Here, we have identified two somatostatin-type precursors (OvSSP-1 and OvSSP-2) in O. victoriae (electronic supplementary material, figure S2; figure 6c). Homologues of both of these precursors are present in the sea urchin S. purpuratus (electronic supplementary material, figure S2; figure 6c), one of which was previously referred to as Spnp16 [16]. By comparison, only a single somatostatin-type precursor has been found in the starfish A. rubens, which is an orthologue of OvSSP-1 [8]. All somatostatin-type precursors comprise a single copy of the bioactive neuropeptide, which is located in the C-terminal region of the precursor [88,89]. Interestingly, the type-1 somatostatins in echinoderms have a phenylalanine residue located in the middle part of the peptide, and this conserved feature is found in human somatostatin. Conversely, type-2 somatostatins in echinoderms lack the phenylalanine residue, but have a neighbouring tryptophan-lysine (WK) motif that is also conserved in human and Branchiostoma floridae somatostatins (figure 6c). The deuterostomian somatostatins are orthologous to the allatostatin-C neuropeptide family in arthropods [7]. This family of peptides comprises three precursor types: allatostatin-C, allatostatin-CC and the recently discovered allatostatin-CCC [89,90]. Both allatostatin-C and allatostatin-CC are non-amidated, like somatostatins; however, allatostatin-CCC has a C-terminal amide. Hence, non-amidated peptides may be representative of the ancestral condition in the common ancestor of protostomes and deuterostomes, with the amidated allatostatin-CCC probably having evolved only within the arthropod lineage [90]. It remains to be determined whether or not the duplication of somatostatin-type precursors in echinoderms and the duplication of allatostatin-C (to give rise to allatostatin-CC) represent independent duplications. Further insights into this issue may be obtained if the receptors for somatostatin-type peptides in echinoderms are deorphanized.
2.3.4. Corticotropin-releasing hormone-type precursors
CRH-type peptides are a family of related neuropeptides that include CRH, urocortins and urotensin-I in chordates, egg-laying hormone (ELH) in lophotrochozoans and diuretic hormone 44 (DH44) in arthropods [6,7]. Arthropods usually have a single DH44 precursor, which comprises a single copy of the mature peptide. In some insects, such as Tribolium castaneum and B. mori, alternative splicing of DH44 transcripts results in multiple mature peptide isoforms of varying lengths [43,91]. The situation in lophotrochozoans is more complex, with several species having multiple precursors and some of these precursors comprising multiple ELH mature peptides [4,92]. A single CRH-type precursor was found previously in the starfish A. rubens, whereas here we have identified four CRH-type precursors in O. victoriae (electronic supplementary material, figure S2; figure 6d). Thus, expanded families of CRH-type peptides and receptors appear to have evolved independently in multiple animal lineages, including chordates and ophiuroid echinoderms [93,94].
2.4. Diversity in neuropeptide precursor structure: new insights from ophiuroids
2.4.1. Tachykinins
The mammalian neuropeptide substance P was the first tachykinin-type peptide to be isolated and sequenced [95–97]. Subsequently, tachykinin-type peptides were discovered in other animals including tunicates [98], insects [99,100], annelids [101] and molluscs [102]. Tachykinin-type peptides regulate various physiological processes, including muscle contractility [103], nociception [104] and stress responses [105], among others [106]. Analysis of genomic/transcriptomic sequence data from the sea urchin S. purpuratus and the sea cucumber A. japonicus did not identify candidate tachykinin-type precursors [6,7,15,16]. However, recently a putative tachykinin-type precursor was discovered in the starfish A. rubens (ArTKP), indicating that this signalling system does occur in some echinoderms [8]. Here, we have identified orthologues of ArTKP in O. victoriae and other ophiuroids (figures 4 and 7a). Collectively, these findings indicate that this signalling system has been retained in the Asterozoa but lost in the Echinozoa. Comparison of the structure of the asterozoan tachykinin-type precursors reveals that the A. rubens precursor (ArTKP) comprises two putative mature peptides, whereas the O. victoriae precursor comprises four mature peptides (figure 7b). It remains to be determined, however, which of these two conditions represent the ancestral state in the common ancestor of the Asterozoa. Further insights into this issue may be obtained if sequence data from a variety of starfish species are analysed.
2.4.2. Kisspeptins
KP (formerly known as metastin) is encoded by the KiSS1 gene in humans. KiSS1 was originally discovered as a gene that may suppress the metastatic potential of malignant melanoma cells [107]. Subsequently, it was found to play a vital role in regulating the onset of puberty. Thus, in vertebrates, KP binds to its receptor GPR54 to stimulate pituitary release of GnRH [108]. The first KP-type precursors to be identified in non-chordates were discovered recently in ambulacrarians—the echinoderms A. rubens and S. purpuratus and the hemichordate S. kowalevskii [8]. Accordingly, here we have identified KP-type precursors in O. victoriae and other ophiuroids. All of the ambulacrarian precursor proteins comprise two KP-type peptides, and the first putative neuropeptide in the echinoderm precursors has two cysteine residues at the N-terminus, which could form an N-terminal disulfide bridge similar that of calcitonin-type peptides (see below). By contrast, the second putative neuropeptide does not contain any cysteine residues and is typically shorter than the first peptide (figure 7c,d). Interestingly, comparison of the sequences of the first (long) and second (short) KP-type peptides in echinoderms reveals that the long and short peptides share less sequence similarity with each other within a species than they do with respective peptides in other species (figure 7c). This indicates that the duplication event that gave rise to the occurrence of the long and short peptides occurred before the divergence of the Asterozoa and Echinozoa. Interestingly, previous studies have revealed that there has been an expansion of KP-type receptors in ambulacraria (S. purpuratus and S. kowalevskii) and in the cephalochordate, B. floridae, with 16 KP receptors present in the latter [6,56]. Further studies are now needed to identify the proteins that act as receptors for the KP-type peptides identified here in ophiuroids and previously in other echinoderms [8].
2.4.3. Calcitonin
Calcitonin was first discovered in 1962 by Copp & Cheney [109]. The sequencing of the porcine calcitonin in 1968 revealed that this polypeptide is composed of 32 amino acids [110]. In vertebrates, calcitonin is produced by the thyroid gland [111] and regulates calcium (Ca2+) levels in the blood, antagonizing the effects of parathyroid hormone [112,113]. The evolutionary antiquity of calcitonin-related peptides was first revealed with the discovery that a diuretic hormone in insects (DH31) is a calcitonin-like peptide [114]. However, DH31 shares modest sequence similarity with vertebrate calcitonins and lacks the N-terminal disulfide bridge that is characteristic of calcitonin-type peptides in vertebrates. More recently, it has been discovered that both DH31-type and vertebrate calcitonin-type neuropeptides occur in some protostomian invertebrates, including the annelid P. dumerilii and the insect Locusta migratoria [4,115]. Hence, it is proposed that an ancestral-type calcitonin precursor gene duplicated in the common ancestor of protostomes to give rise to DH31- and calcitonin-type peptides, but with subsequent loss of calcitonin-type peptides in some protostomes. Consistent with this hypothesis, calcitonin-type precursors, but not DH31-type precursors, have been identified in deuterostomian invertebrates, including echinoderms [8,15,16,116].
An interesting feature of calcitonin/DH31 precursors is the occurrence of multiple splice variants. In vertebrates, alternative splicing of the calcitonin gene results in two transcripts: one transcript encodes calcitonin and the other transcript encodes calcitonin gene-related peptide [117]. Furthermore, a complex interplay of receptors and accessory proteins determines the pharmacological profile of these peptides [118,119]. Alternative splicing of DH31 and calcitonin precursors in insects has also been previously reported [115,120,121]. Interestingly, alternative splicing of insect calcitonin genes also generates variants that give rise to different mature peptides [115]. However, unlike the calcitonin gene, DH31 splice variants all produce an identical mature peptide [120,121].
Our analysis of the ophiuroid transcriptomes also identified two transcript variants for calcitonin (figure 7e,f). Based on our analysis of transcript sequences, ophiuroid calcitonin genes comprise at least three putative coding regions or ‘exons’. It is unclear if these three coding regions represent three or more exons due to the lack of genomic data, but for the sake of simplicity, we refer to them here as ‘exons’. Transcript variant 1 comprises ‘exons’ 1 and 3 but lacks ‘exon’ 2, whereas transcript variant 2 contains all three ‘exons’. Interestingly, ‘exons’ 2 and 3 both encode a calcitonin-type peptide. Hence, transcript variant 1 encodes a precursor that produces one calcitonin-type peptide and transcript variant 2 encodes two non-identical calcitonin-type peptides. These alternatively spliced transcripts were found in several brittle star species (figure 8), and thus this may represent an ancient and conserved feature, although transcript variant 1 was not found in O. victoriae.
Figure 8.
Comparison of neuropeptide copy numbers across the Ophiuroidea for precursors comprising multiple copies of neuropeptides. Neuropeptide precursors were mined from 52 ophiuroid transcriptomes, with the phylogeny adapted from O'Hara et al. [12]. Am_laud: Amphiophiura laudata, Am_spat: Amphiophiura spatulifera, Am_cipu: Amphioplus cipus, Am_cten: Amphioplus ctenacantha, Am_squa: Amphipholis squamata, Am_cons1: Amphiura constricta 1, Am_cons2: Amphiura constricta 2, As_love: Asteronyx loveni, As_bidw: Asteroschema bidwillae, As_tubi: Asteroschema tubiferum, Ba_hero: Bathypectinura heros, Cl_cana: Clarkcoma canaliculata, Gl_sp_no: Glaciacantha sp. nov., Go_pust: Gorgonocephalus pustulatum, Mi_grac: Microphiopholis gracillima, Op_fune: Ophiacantha funebris, Op_abys: Ophiactis abyssicola, Op_resi: Ophiactis resiliens, Op_savi: Ophiactis savignyi, Op_vall: Ophiernus vallincola, Op_pilo: Ophiocentrus pilosus, Op_wend: Ophiocoma wendtii, Op_oedi: Ophiocreas oedipus, Op_tube: Ophiocypris tuberculosis, Op_appr: Ophioderma appressum, Op_bisc: Ophiolepis biscalata, Op_impr: Ophiolepis impressa, Op_brev: Ophioleuce brevispinum, Op_perf: Ophiolimna perfida, Op_prol: Ophiologimus prolifer, Op_obst: Ophiomoeris obstricta, Op_lyma: Ophiomusium lymani, Op_aust: Ophiomyxa australis, Op_vivi: Ophiomyxa sp. cf. vivipara, Op_fasc: Ophionereis fasciata, Op_reti: Ophionereis reticulata, Op_scha: Ophionereis schayeri, Op_cyli: Ophiopeza cylindrica, Op_filo: Ophiophragmus filograneus, Op_wurd: Ophiophragmus wurdemanii, Op_liod: Ophiophrura liodisca, Op_john: Ophiophycis johni, Op_lame: Ophioplax lamellosa, Op_iner: Ophiopleura inermis, Op_plic: Ophioplinthaca plicata, Op_bisp: Ophioplocus bispinosus, Op_macu: Ophiopsammus maculata, Op_angu: Ophiothrix angulata, Op_caes: Ophiothrix caespitosa, Op_exim_1: Ophiotreta eximia 1, Op_exim_2: Ophiotreta eximia 2, Op_sp_no: Ophiura sp. nov.
Previous studies have identified precursors comprising a single calcitonin-type peptide in the starfish A. rubens and the sea urchin S. purpuratus [8,16], and a precursor comprising two calcitonin-type peptides in the sea cucumber A. japonicus [15]. Informed by the identification here of two transcript types in ophiuroids (transcript variants 1 and 2), we have now discovered that two transcript types also occur in A. japonicus transcriptome. Hence, alternative splicing of calcitonin-type precursor genes can be traced back in the echinoderm lineage to the common ancestor of the Asterozoa and Echinozoa, but with subsequent loss of this characteristic in some lineages.
2.4.4. GPA2 and GPB5
The vertebrate glycoprotein hormone family comprises luteinizing hormone follicle-stimulating hormone, chorionic gonadotropin, TSH and the recently discovered thyrostimulin (TS) [122,123]. TS is a heterodimer composed of two subunits, glycoprotein alpha 2 (GPA2) and glycoprotein beta 5 (GPB5). Orthologues of GPA2 and GPB5 have been identified and characterized in the insect D. melanogaster [124] and in other invertebrates, including echinoderms [125]. Insect GPA2 and GPB5 both contain 10 conserved cysteine residues that are important in forming a heterodimeric cysteine-knot structure. Surprisingly, A. japonicus GPA2 contains only seven cysteine residues (having lost residues 7, 8 and 9), whereas O. victoriae GPB5.1, A. rubens GPB5.1 and S. purpuratus GPB5 all contain eight cysteine residues (having lost the final two cysteine residues) (electronic supplementary material, figure S5). It is difficult to predict the structural differences that may arise in the heterodimer due to this variability in the number of cysteine residues. The possibility of GPA2 and/or GPB5 monomers or homodimers exerting their own biological functions has not been ruled out [126]. Additional investigations are needed to investigate if GPA2 and GPB5 are co-localized in echinoderms and if the monomers and dimers (both homo and hetero) exert different effects.
2.5. Uncharacterized neuropeptides
In addition to the neuropeptides discussed above, we have also identified three neuropeptide precursors that could not be classified into any known neuropeptide families. These include O. victoriae neuropeptide precursor (Ovnp) 18 (O. victoriae orthologue of Spnp18 in S. purpuratus) [16], Ovnp26 and Ovnp27, with the latter two identified for the first time in echinoderms. The choice of nomenclature for Ovnp26 and Ovnp27 is based on a previously used numerical nomenclature in S. purpuratus and/or A. rubens, which goes up to Arnp25 in A. rubens.
2.5.1. Ovnp18
Ovnp18 comprises four copies of a predicted mature peptide with the sequence LFWVD and the C-terminal region of the precursor (partial sequence) contains at least four cysteine residues (figure 5f; GenBank MF155258). Interestingly, this precursor type only comprises a single mature peptide in A. rubens, S. purpuratus and A. japonicus, and the C-terminal region contains 9, 8 and 8 cysteine residues, respectively (data not shown) [8,15,16].
2.5.2. Ophionotus victoriae neuropeptide precursor 26
Ovnp26 was identified following an analysis of O. victoriae transcriptome sequence using NpSearch [8]. Orthologues of Ovnp26 (GenBank: MF155259) were identified in other brittle stars but not in other echinoderms (electronic supplementary material, figures S2–S4). Ovnp26 comprises seven copies of peptides with a conserved C-terminal GW motif, whereas orthologues in O. aranea and A. filiformis are predicted to generate eight copies of the mature peptide. Some of the mature peptides have a C-terminal SGW motif, which is similar to the C-terminus of predicted mature peptides derived from O. victoriae pedal peptide precursor 3 (electronic supplementary material, figure S7). However, the lack of sequence similarity in other parts of the peptide suggests that the C-terminal similarity may reflect convergence rather than homology.
2.5.3. Ophionotus victoriae neuropeptide precursor 27
Ovnp27 (GenBank: MF155251) was identified following a Hidden Markov Model (HMM)-based search for SIFamide-type peptides [127,128], albeit with a high E-value. This neuropeptide precursor comprises two putative amidated mature peptides that are located immediately after the signal peptide (electronic supplementary material, figures S2–S4), as seen in SIFamide precursors [129]. The first peptide of the O. victoriae precursor has a C-terminal IFamide motif just like in insect SIFamides (electronic supplementary material, figure S9). However, there is no sequence similarity with SIFamides in the rest of the peptide. This coupled with the fact that SIFamide-type receptors have not been identified in echinoderms [6] suggests that the sequence similarity that peptides derived from Ovnp27-type precursors share with SIFamides may reflect convergence rather than homology.
2.6. Neuropeptide precursors not found in brittle stars
Our analysis of ophiuroid transcriptome sequence data did not reveal orthologues of the Spnp9 precursor from S. purpuratus or the Arnp21, Arnp22, Arnp23 and Arnp24 precursors from A. rubens [8,16]. An Spnp9 orthologue is found in A. japonicus but not in A. rubens [15]; therefore, this neuropeptide precursor type may be restricted to the Echinozoa. Orthologues of Arnp21–24 have not been found in O. victoriae, S. purpuratus or A. japonicus, which suggest that these may be Asteroidea-specific precursors.
Previous studies have shown that receptors for leucokinin, ETH, QRFP, parathyroid hormone, galanin/allatostatins-A and Neuromedin-U/CAPA are present in ambulacraria [6,7,15]. The presence of these receptors suggests that their cognate ligands should also be present in ambulacraria. However, our search approaches failed to identify any proteins in ophiuroids that resemble precursors of these neuropeptides.
2.7. Evolutionary conservation and variation of neuropeptide copy number in the Ophiuroidea
Many neuropeptide precursors comprise several structurally similar but non-identical bioactive peptides (i.e. the precursor protein gives rise to a neuropeptide ‘cocktail’). This feature of neuropeptide precursors occurs throughout metazoans. But how do these ‘cocktails’ of neuropeptides evolve and what is their functional significance? Are the copies of mature peptides functionally redundant or do they have their own specific functions? These are important questions in neuroendocrinology for which answers remain elusive.
Evidence that neuropeptide copy number may be functionally important has been obtained from comparison of the sequences of neuropeptide precursors in 12 Drosophila species, the common ancestor of which dates back approximately 50 Myr [130]. The number of peptide copies in each neuropeptide precursor was found to be identical (except for the FMRFamide precursor) when compared between the 12 species, suggesting that stabilizing selection has acted to conserve neuropeptide ‘cocktails’ in the Drosophila lineage.
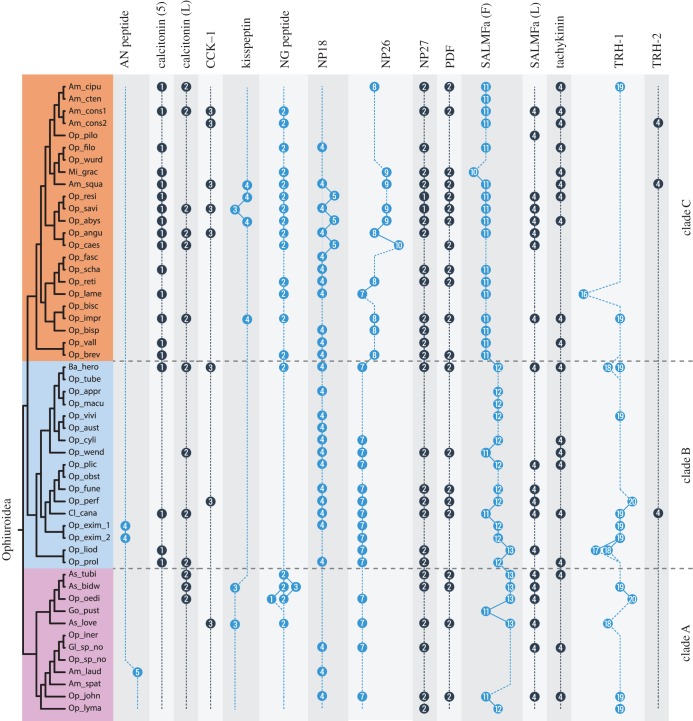
Here, a comparison of O. victoriae, A. filiformis and O. aranea neuropeptide precursors and their putative mature peptides revealed that 14 neuropeptide precursors comprised multiple neuropeptide copies. In certain cases, the number of the mature peptides derived from a particular precursor varied across species, whereas in other cases the numbers remained constant (figure 4). Interestingly, these three species belong to two of the three major clades of brittle stars that evolved approximately 270 Ma [12]. While O. victoriae belongs to the order Ophiurida (in clade A), A. filiformis and O. aranea belong to the order Amphilepidida (in clade C). Hence, this prompted us to examine the evolution of neuropeptides and neuropeptide copy number variation at a higher level of phylogenetic resolution. To do this, we used a unique dataset comprising 52 ophiuroid transcriptomes. These transcriptomes were recently used as part of a phylotranscriptomic approach to reconstruct the phylogeny of ophiuroids, generating a robust phylogenetic tree that comprises three major clades [12]. Hence, this dataset allowed us to explore the evolution of neuropeptide precursors in the context of an established phylogenetic framework spanning over an unprecedented time scale of approximately 270 Myr.
We selected for analysis neuropeptide precursors comprising more than a one putative mature neuropeptide, which include AN peptide, calcitonin, CCK1, KP, np18, np26, np27, NG peptide, PDF, SALMFamide (L-type and F-type), tachykinin and TRH (1 and 2). Pedal peptide precursors (1, 2 and 3) were excluded from the analysis because orthology relationships between these precursors could not be established with confidence across all species (data not shown). We used O. victoriae representatives of these neuropeptide precursor families and the A. filiformis AN peptide precursor to mine 52 ophiuroid transcriptomes using BLAST. Multiple sequence alignments were generated based on the search hits (electronic supplementary material, figure S11) and the number of predicted mature peptides was compared (figure 8). Interestingly, the number of peptides within the majority of precursors remained constant across all the species examined, which share a common ancestor estimated to date from approximately 270 Ma [12].
Some studies that have investigated the physiological significance of neuropeptide ‘cocktails’ indicate that neuropeptides derived from the same precursor protein are functionally redundant. For example, this was found for myomodulin neuropeptides in the mollusc Aplysia californica using the accessory radula closer muscle preparation as a bioassay [131], and for FMRFamide-related neuropeptides in D. melanogaster when analysing effects on nerve-stimulated contraction of larval body wall muscles [132]. However, the authors of the latter study cautiously highlighted the need to ‘search for additional functions or processes in which these peptides may act differentially’. Importantly, studies employing the use of multiple bioassays have obtained data indicating that neuropeptides derived from a single precursor protein are not functionally redundant. For example, when the actions of 14 structurally related neuropeptides derived from a precursor of Mytilus inhibitory peptide-related peptides in Aplysia were tested on three organ preparations (oesophagus, penis retractor and body wall), it was found that the rank order of potency for the peptides differed between preparations [133]. Similarly, when assaying the effects of allatostatin neuropeptides in cockroaches, tissue-specific differences in potency were observed [134]. The conservation of peptide copy number across a time scale of approximately 270 Myr in the Ophiuroidea supports the idea that the occurrence of multiple copies of identical or structurally related neuropeptides is functionally important.
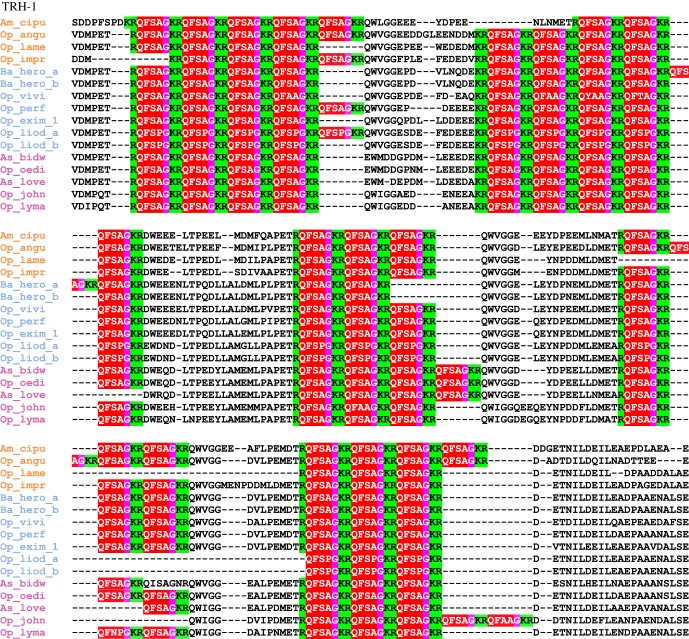
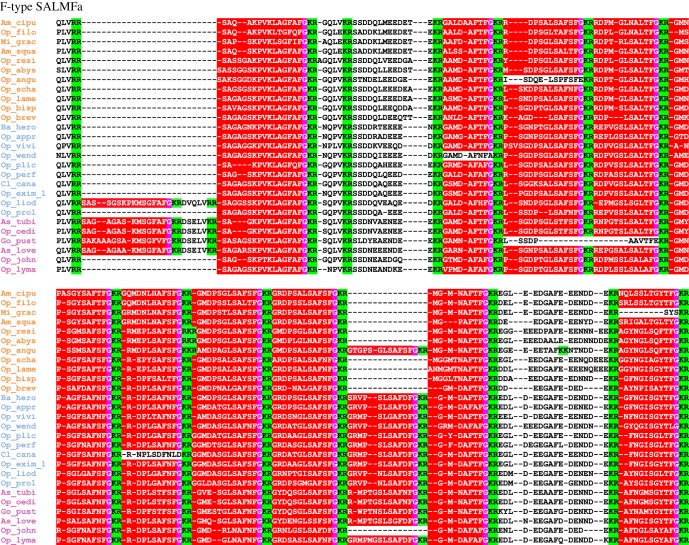
For those neuropeptide precursors that did exhibit variation in neuropeptide copy number, TRH-type precursors exhibited the highest variation, with numbers ranging from 16 to 20 copies (figure 9). F-type SALMFamide precusors also showed variation in copy numbers (figure 10), but loss of peptides was more frequent in F-type SALMFamide precursors than in TRH-type precursors. Furthermore, detailed analysis of sequence alignments for these precursors revealed that loss of neuropeptide copies is usually a consequence of non-synonymous mutations in codons for residues that form dibasic cleavage sites or for glycine residues that are substrates for the C-terminal amidation. This is not surprising because the C-terminal amide in smaller-sized peptides is usually important for receptor binding and activation. What is unclear at the moment is how the peptide copy number increases within a given precursor. Perhaps the increase in peptide copy number occurs as a result of unequal crossing-over during recombination [130].
Figure 9.
A partial multiple sequence alignment of ophiuroid TRH-1 precursors showing clade-specific gain/loss of neuropeptide copies. Mono- and dibasic cleavage sites are highlighted in green, mature peptides in red with the glycine residue for amidation in pink. Species have been grouped and coloured (clade A in purple, clade B in blue and clade C in orange) based on the phylogeny determined by O'Hara et al. [12].
Figure 10.
A partial multiple sequence alignment of ophiuroid F-type SALMFamide precursors showing clade-specific gain/loss of neuropeptide copies. Dibasic cleavage sites are highlighted in green, and mature peptides in red with the glycine residue for amidation in pink. Species have been grouped and coloured (clade A in purple, clade B in blue and clade C in orange) based on the phylogeny determined by O'Hara et al. [12].
The number of peptides within the F-type SALMFamide precursors appears to be clade-specific. Thus, the average/median number of F-type SALMFamides in precursors from clade A is 13, clade B is 12 and clade C is 11, with a few exceptions (figure 8). Similarly, the number of peptides within NP26-type precursors also appears to be clade-specific. Hence, the number of peptides is highly stable at seven peptides within clades A and B, but a high variation in peptide copy number is observed in clade C. When examining peptide copy number within clades, there are a few cases where the number of peptides within a given precursor for certain species appears to be an exception/outlier. For instance, 16 copies of the mature peptide in Ophioplax lamellosa TRH-1 precursor are distinctly different from the 19 copies found in other species within that clade (clade C). Likewise, Ophiactis savignyi only has three copies of KP-type peptides compared with four copies found in other species of that clade (figure 8).
It could be argued that misalignments during transcriptome assembly may have influenced the number of predicted peptides found in a given precursor. However, it is unlikely that misalignments have affected the predicted sequences of neuropeptide precursors comprising multiple copies of peptides that are similar but non-identical, which applies to the majority of the precursor proteins analysed here in ophiuroids. The only exception to this are the TRH-type precursors, where the encoded peptide sequences are short and often identical, even at the nucleotide level (data not shown). Another limitation of using transcriptome data is that the sequences of neuropeptide precursors may be partial or unknown for some species, and where this applies a peptide copy number is not shown in figure 8. An extreme example of this is the AN peptide precursor, where complete precursor sequences were only obtained from the three reference species and three other species. However, for the majority of precursor types, sequence data were obtained from a variety of species from each of the three clades of ophiuroids. For example, complete F-type SALMFamide precursor sequences were found in most of the investigated species (39 species + 3 reference species).
3. Conclusion
Here, we report the first detailed analysis of the neuropeptide precursor complement of ophiuroids and the most comprehensive identification of echinoderm neuropeptide precursors to date. We have identified novel representatives of several bilaterian neuropeptide families in echinoderms for the first time, which include orthologues of endothelin/CCHamide, EH, NPF/NPY and NUCB/nesfatin. Furthermore, analysis of precursor proteins comprising multiple copies of identical or related neuropeptides across approximately 270 Myr of ophiuroid evolution indicates that the precise composition of neuropeptide ‘cocktails’ is functionally important as evident from the conservation of neuropeptide copy number for multiple precursors.
4. Material and methods
4.1. Sequencing and assembly of transcriptomes
Ophiuroid transcriptomes used in this study were sequenced and assembled as reported previously [12,20,24].
4.2. Identification of neuropeptide precursors in ophiuroids
To identify neuropeptide precursors in O. victoriae, A. filiformis and O. aranea, sequences of neuropeptide precursors identified previously in other echinoderms (including the starfish, A. rubens, the sea urchin S. purpuratus and the sea cucumber, A. japonicus) were used as queries for tBLASTn analysis of a transcriptome database, using an e-value of 1000. Sequences identified as potential neuropeptide precursors by BLAST were translated using the ExPASy Translate tool (http://web.expasy.org/translate/) and then analysed for features of neuropeptide precursors. Specifically, sequences were evaluated based on (i) the presence of an N-terminal signal peptide (using Signal P v. 4.1 with the sensitive cut-off of 0.34) and (ii) the presence of monobasic or dibasic cleavage sites flanking the putative bioactive peptide(s).
To identify novel neuropeptide precursors or highly divergent precursors with low sequence similarity to known precursors, we used two additional approaches. In the first approach, we used NpSearch [8], software that identifies putative neuropeptide precursors based on various characteristics (presence of signal peptide and dibasic cleavage sites among others). In the second approach, NpHMMer (http://nphmmer.sbcs.qmul.ac.uk/), an HMM-based software, was used to identify neuropeptides not found using the above approaches.
Neuropeptide precursors identified in O. victoriae (which represented a more comprehensive neuropeptide precursor repertoire compared to A. filiformis and O. aranea) were then submitted as queries for BLAST analysis of sequence data from 52 Ophiuroidea species, using an E-value of 1 × 10−6. BLAST hits were then further analysed using an automated ruby script (available at https://github.com/IsmailM/ophiuroid_neuropeptidome). Each BLAST hit was translated using BioRuby, and the open reading frame (ORF) containing the BLAST high-scoring segment pair (HSP) was extracted. These ORFs were then examined for the presence of a signal peptide using Signal P 4.1 using a sensitive cut-off of 0.34. All sequences were then aligned using MAFFT, with the number of maximum iterations set to 1000 to ensure an optimal alignment. These alignments were then further optimized by manually adjusting the location of the bioactive peptide and cleavage sites. Finally, the alignments were annotated using different colours for the signal peptide (blue), the bioactive peptide(s) (red) and cleavage sites (green).
4.3. Phylogenetic and clustering analyses of sequence data
Phylogenetic analysis of membrane guanylyl cyclase receptors and nucleobindins was performed using maximum-likelihood and Bayesian methods. Prior to these analyses, corresponding multiple alignments were trimmed using BMGE [135] with the following options: BLOSUM30, max – h = 1, −b = 1, as described previously [10,94]. The maximum-likelihood method was implemented in the PhyML program (v. 3.1/3.0 aLRT). The WAG substitution model was selected assuming an estimated proportion of invariant sites (of 0.112) and four gamma-distributed rate categories to account for rate heterogeneity across sites. The gamma shape parameter was estimated directly from the data. Reliability for internal branch was assessed using the bootstrapping method (500 bootstrap replicates). The Bayesian inference method was implemented in the MrBayes program (v. 3.2.3). The number of substitution types was fixed to 6. The Poisson model was used for substitution, while rate variation across sites was fixed to ‘invgamma’. Four Markov chain Monte Carlo (MCMC) chains were run for 100 000 generations, sampling every 100 generations, with the first 500 sampled trees discarded as ‘burn-in’. Finally, a 50% majority rule consensus tree was constructed.
CLANS analysis was performed on echinoderm EH-like, arthropod EH, arthropod ITP and vertebrates ANP precursors based on all-against-all sequence similarity (BLAST searches) using the BLOSUM 45 matrix (https://toolkit.tuebingen.mpg.de/clans/) [42] and the significant HSPs. Neuropeptide precursors were clustered in a three-dimensional graph represented here in two dimensions.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgement
The authors acknowledge Zuraiha Waffa and Giulia Oluwabunmi Olayemi for their assistance with sequence alignments.
Data accessibility
Raw sequence data used to assemble the transcriptomes have been deposited in the NCBI Sequence Read Archive (SRA) under the accession no. SRP107914 (https://www.ncbi.nlm.nih.gov/sra/?term=SRP107914) and in the NCBI BioProject under the accession no. PRJNA311384 (https://www.ncbi.nlm.nih.gov/bioproject/311384).
Authors' contributions
M.Z., T.D.O. and M.R.E. designed the research; I.M. generated HMMs; M.Z., I.M., L.A.Y.G., J.D., N.A. and A.F.H. identified the neuropeptide precursors; M.Z., I.M., L.A.Y.G., J.D. and N.A. analysed the data; M.Z., J.D. and M.R.E. wrote the manuscript with input from other authors. M.Z. and M.R.E. supervised the study.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by Leverhulme Trust grant no. (RPG-2013-351) and a BBSRC grant no. (BB/M001644/1) awarded to M.R.E. L.A.Y.G. is supported by a PhD studentship awarded by CONACYT (studentship no. 418612). I.M. is supported by the Biotechnology and Biological Sciences Research Council (grant no. BB/M009513/1).
References
- 1.Nassel DR, Winther AM. 2010. Drosophila neuropeptides in regulation of physiology and behavior. Prog. Neurobiol. 92, 42–104. (doi:10.1016/j.pneurobio.2010.04.010) [DOI] [PubMed] [Google Scholar]
- 2.Argiolas A, Melis MR. 2013. Neuropeptides and central control of sexual behaviour from the past to the present: a review. Prog. Neurobiol. 108, 80–107. (doi:10.1016/j.pneurobio.2013.06.006) [DOI] [PubMed] [Google Scholar]
- 3.Sohn JW, Elmquist JK, Williams KW. 2013. Neuronal circuits that regulate feeding behavior and metabolism. Trends Neurosci. 36, 504–512. (doi:10.1016/j.tins.2013.05.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conzelmann M, Williams EA, Krug K, Franz-Wachtel M, Macek B, Jekely G. 2013. The neuropeptide complement of the marine annelid Platynereis dumerilii. BMC Genomics 14, 906 (doi:10.1186/1471-2164-14-906) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Veenstra JA. 2016. Neuropeptide evolution: chelicerate neurohormone and neuropeptide genes may reflect one or more whole genome duplications. Gen. Comp. Endocrinol. 229, 41–55. (doi:10.1016/j.ygcen.2015.07.014) [DOI] [PubMed] [Google Scholar]
- 6.Mirabeau O, Joly JS. 2013. Molecular evolution of peptidergic signaling systems in bilaterians. Proc. Natl Acad. Sci. USA 110, E2028–E2037. (doi:10.1073/pnas.1219956110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jekely G. 2013. Global view of the evolution and diversity of metazoan neuropeptide signaling. Proc. Natl Acad. Sci. USA 110, 8702–8707. (doi:10.1073/pnas.1221833110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Semmens DC, Mirabeau O, Moghul I, Pancholi MR, Wurm Y, Elphick MR. 2016. Transcriptomic identification of starfish neuropeptide precursors yields new insights into neuropeptide evolution. Open Biol. 6, 150224 (doi:10.1098/rsob.150224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Semmens DC, Beets I, Rowe ML, Blowes LM, Oliveri P, Elphick MR. 2015. Discovery of sea urchin NGFFFamide receptor unites a bilaterian neuropeptide family. Open Biol. 5, 150030 (doi:10.1098/rsob.150030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian S, Zandawala M, Beets I, Baytemur E, Slade SE, Scrivens JH, Elphick MR. 2016. Urbilaterian origin of paralogous GnRH and corazonin neuropeptide signalling pathways. Sci. Rep. 6, 28788 (doi:10.1038/srep28788) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Telford MJ, Lowe CJ, Cameron CB, Ortega-Martinez O, Aronowicz J, Oliveri P, Copley RR. 2014. Phylogenomic analysis of echinoderm class relationships supports Asterozoa. Proc. R. Soc. B 281, 20140479 (doi:10.1098/rspb.2014.0479) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Hara TD, Hugall AF, Thuy B, Moussalli A. 2014. Phylogenomic resolution of the class Ophiuroidea unlocks a global microfossil record. Curr. Biol. 24, 1874–1879. (doi:10.1016/j.cub.2014.06.060) [DOI] [PubMed] [Google Scholar]
- 13.Hyman LH. 1955. The invertebrates, vol. 4: Echinodermata. New York, NY: MacGraw-Hill. [Google Scholar]
- 14.Wilkie I. 2001. Autotomy as a prelude to regeneration in echinoderms. Microsc. Res. Tech. 55, 369–396. (doi:10.1002/jemt.1185) [DOI] [PubMed] [Google Scholar]
- 15.Rowe ML, Achhala S, Elphick MR. 2014. Neuropeptides and polypeptide hormones in echinoderms: new insights from analysis of the transcriptome of the sea cucumber Apostichopus japonicus. Gen. Comp. Endocrinol. 197, 43–55. (doi:10.1016/j.ygcen.2013.12.002) [DOI] [PubMed] [Google Scholar]
- 16.Rowe ML, Elphick MR. 2012. The neuropeptide transcriptome of a model echinoderm, the sea urchin Strongylocentrotus purpuratus. Gen. Comp. Endocrinol. 179, 331–344. (doi:10.1016/j.ygcen.2012.09.009) [DOI] [PubMed] [Google Scholar]
- 17.Mayorova TD, et al. 2016. Localization of neuropeptide gene expression in larvae of an echinoderm, the starfish Asterias rubens. Front. Neurosci. 10, 553 (doi:10.3389/fnins.2016.00553) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim CH, Kim EJ, Go HJ, Oh HY, Lin M, Elphick MR, Park NG. 2016. Identification of a novel starfish neuropeptide that acts as a muscle relaxant. J. Neurochem. 137, 33–45. (doi:10.1111/jnc.13543) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones CE, Zandawala M, Semmens DC, Anderson S, Hanson GR, Janies DA, Elphick MR. 2016. Identification of a neuropeptide precursor protein that gives rise to a ‘cocktail’ of peptides that bind Cu(II) and generate metal-linked dimers. Biochim. Biophys. Acta 1860, 57–66. (doi:10.1016/j.bbagen.2015.10.008) [DOI] [PubMed] [Google Scholar]
- 20.Elphick MR, Semmens DC, Blowes LM, Levine J, Lowe CJ, Arnone MI, Clark MS. 2015. Reconstructing SALMFamide neuropeptide precursor evolution in the phylum echinodermata: ophiuroid and crinoid sequence data provide new insights. Front. Endocrinol. 6, 2 (doi:10.3389/fendo.2015.00002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin M, Mita M, Egertova M, Zampronio CG, Jones AM, Elphick MR. 2016. Cellular localization of relaxin-like gonad-stimulating peptide expression in Asterias rubens: new insights into neurohormonal control of spawning in starfish. J. Comp. Neurol. 525, 1599–1617. (doi:10.1002/cne.24141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stöhr S, O'Hara TD, Thuy B. 2012. Global diversity of brittle stars (Echinodermata: Ophiuroidea). PLoS ONE 7, e31940 (doi:10.1371/journal.pone.0031940) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shackleton JD. 2005. Skeletal homologies, phylogeny and classification of the earliest asterozoan echinoderms. J. Syst. Palaeontol. 3, 29–114. (doi:10.1017/S1477201905001525) [Google Scholar]
- 24.Delroisse J, Mallefet J, Flammang P. 2016. De novo adult transcriptomes of two European brittle stars: spotlight on opsin-based photoreception. PLoS ONE 11, e0152988 (doi:10.1371/journal.pone.0152988) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delroisse J, Ortega-Martinez O, Dupont S, Mallefet J, Flammang P. 2015. De novo transcriptome of the European brittle star Amphiura filiformis pluteus larvae. Mar. Genomics 23, 109–121. (doi:10.1016/j.margen.2015.05.014) [DOI] [PubMed] [Google Scholar]
- 26.Burns G, Thorndyke MC, Peck LS, Clark MS. 2013. Transcriptome pyrosequencing of the Antarctic brittle star Ophionotus victoriae. Mar. Genomics 9, 9–15. (doi:10.1016/j.margen.2012.05.003) [DOI] [PubMed] [Google Scholar]
- 27.Cannon JT, Kocot KM, Waits DS, Weese DA, Swalla BJ, Santos SR, Halanych KM. 2014. Phylogenomic resolution of the hemichordate and echinoderm clade. Curr. Biol. 24, 2827–2832. (doi:10.1016/j.cub.2014.10.016) [DOI] [PubMed] [Google Scholar]
- 28.Vaughn R, Garnhart N, Garey JR, Thomas WK, Livingston BT. 2012. Sequencing and analysis of the gastrula transcriptome of the brittle star Ophiocoma wendtii. Evodevo 3, 19 (doi:10.1186/2041-9139-3-19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Purushothaman S, Saxena S, Meghah V, Swamy CV, Ortega-Martinez O, Dupont S, Idris M. 2015. Transcriptomic and proteomic analyses of Amphiura filiformis arm tissue-undergoing regeneration. J. Proteomics 112, 113–124. (doi:10.1016/j.jprot.2014.08.011) [DOI] [PubMed] [Google Scholar]
- 30.Smith AB, Paterson GL, Lafay B. 1995. Ophiuroid phylogeny and higher taxonomy: morphological, molecular and palaeontological perspectives. Zool. J. Linn. Soc. 114, 213–243. (doi:10.1111/j.1096-3642.1995.tb00117c.x) [Google Scholar]
- 31.Thuy B, Stohr S. 2016. A new morphological phylogeny of the Ophiuroidea (Echinodermata) accords with molecular evidence and renders microfossils accessible for cladistics. PLoS ONE 11, e0156140 (doi:10.1371/journal.pone.0156140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Hara TD, Hugall AF, Thuy B, Stohr S, Martynov AV. 2017. Restructuring higher taxonomy using broad-scale phylogenomics: the living Ophiuroidea. Mol. Phylogenet. Evol. 107, 415–430. (doi:10.1016/j.ympev.2016.12.006) [DOI] [PubMed] [Google Scholar]
- 33.Vogel KJ, Brown MR, Strand MR. 2013. Phylogenetic investigation of peptide hormone and growth factor receptors in five dipteran genomes. Front. Endocrinol. 4, 193 (doi:10.3389/fendo.2013.00193) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Truman JW, Riddiford LM. 1970. Neuroendocrine control of ecdysis in silkmoths. Science 167, 1624–1626. (doi:10.1126/science.167.3925.1624) [DOI] [PubMed] [Google Scholar]
- 35.Kataoka H, Troetschler RG, Kramer SJ, Cesarin BJ, Schooley DA. 1987. Isolation and primary structure of the eclosion hormone of the tobacco hornworm, Manduca sexta. Biochem. Biophys. Res. Commun. 146, 746–750. (doi:10.1016/0006-291X(87)90592-4) [DOI] [PubMed] [Google Scholar]
- 36.Zhou L, Li S, Wang Z, Li F, Xiang J. 2017. An eclosion hormone-like gene participates in the molting process of Palaemonid shrimp Exopalaemon carinicauda. Dev. Genes Evol. 227, 189–199. (doi:10.1007/s00427-017-0580-9) [DOI] [PubMed] [Google Scholar]
- 37.Ewer J. 2005. Behavioral actions of neuropeptides in invertebrates: insights from Drosophila. Horm. Behav. 48, 418–429. (doi:10.1016/j.yhbeh.2005.05.018) [DOI] [PubMed] [Google Scholar]
- 38.Zitnan D, Kim YJ, Zitnanova I, Roller L, Adams ME. 2007. Complex steroid-peptide-receptor cascade controls insect ecdysis. Gen. Comp. Endocrinol. 153, 88–96. (doi:10.1016/j.ygcen.2007.04.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang JC, Yang RB, Adams ME, Lu KH. 2009. Receptor guanylyl cyclases in Inka cells targeted by eclosion hormone. Proc. Natl Acad. Sci. USA 106, 13 371–13 376. (doi:10.1073/pnas.0812593106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McNabb SL, Baker JD, Agapite J, Steller H, Riddiford LM, Truman JW. 1997. Disruption of a behavioral sequence by targeted death of peptidergic neurons in Drosophila. Neuron 19, 813–823. (doi:10.1016/S0896-6273(00)80963-0) [DOI] [PubMed] [Google Scholar]
- 41.Kruger E, Mena W, Lahr EC, Johnson EC, Ewer J. 2015. Genetic analysis of Eclosion hormone action during Drosophila larval ecdysis. Development 142, 4279–4287. (doi:10.1242/dev.126995) [DOI] [PubMed] [Google Scholar]
- 42.Frickey T, Lupas A. 2004. CLANS: a Java application for visualizing protein families based on pairwise similarity. Bioinformatics 20, 3702–3704. (doi:10.1093/bioinformatics/bth444) [DOI] [PubMed] [Google Scholar]
- 43.Roller L, Yamanaka N, Watanabe K, Daubnerova I, Zitnan D, Kataoka H, Tanaka Y. 2008. The unique evolution of neuropeptide genes in the silkworm Bombyx mori. Insect. Biochem. Mol. Biol. 38, 1147–1157. (doi:10.1016/j.ibmb.2008.04.009) [DOI] [PubMed] [Google Scholar]
- 44.Hansen KK, Hauser F, Williamson M, Weber SB, Grimmelikhuijzen CJ. 2011. The Drosophila genes CG14593 and CG30106 code for G-protein-coupled receptors specifically activated by the neuropeptides CCHamide-1 and CCHamide-2. Biochem. Biophys. Res. Commun. 404, 184–189. (doi:10.1016/j.bbrc.2010.11.089) [DOI] [PubMed] [Google Scholar]
- 45.Ida T, et al. 2012. Isolation of the bioactive peptides CCHamide-1 and CCHamide-2 from Drosophila and their putative role in appetite regulation as ligands for G protein-coupled receptors. Front. Endocrinol. 3, 177 (doi:10.3389/fendo.2012.00177) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Farhan A, Gulati J, Grobetae-Wilde E, Vogel H, Hansson BS, Knaden M. 2013. The CCHamide 1 receptor modulates sensory perception and olfactory behavior in starved Drosophila. Sci. Rep. 3, 2765 (doi:10.1038/srep02765) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ren GR, et al. 2015. CCHamide-2 is an orexigenic brain-gut peptide in Drosophila. PLoS ONE 10, e0133017 (doi:10.1371/journal.pone.0133017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Veenstra JA. 2010. Neurohormones and neuropeptides encoded by the genome of Lottia gigantea, with reference to other mollusks and insects. Gen. Comp. Endocrinol. 167, 86–103. (doi:10.1016/j.ygcen.2010.02.010) [DOI] [PubMed] [Google Scholar]
- 49.Oumi T, Ukena K, Matsushima O, Ikeda T, Fujita T, Minakata H, Nomoto K. 1995. The GGNG peptides: novel myoactive peptides isolated from the gut and the whole body of the earthworms. Biochem. Biophys. Res. Commun. 216, 1072–1078. (doi:10.1006/bbrc.1995.2730) [DOI] [PubMed] [Google Scholar]
- 50.Tatemoto K, Carlquist M, Mutt V. 1982. Neuropeptide Y—a novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature 296, 659–660. (doi:10.1038/296659a0) [DOI] [PubMed] [Google Scholar]
- 51.Tatemoto K. 1982. Neuropeptide Y: complete amino acid sequence of the brain peptide. Proc. Natl Acad. Sci. USA 79, 5485–5489. (doi:10.1073/pnas.79.18.5485) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nassel DR, Wegener C. 2011. A comparative review of short and long neuropeptide F signaling in invertebrates: any similarities to vertebrate neuropeptide Y signaling? Peptides 32, 1335–1355. (doi:10.1016/j.peptides.2011.03.013) [DOI] [PubMed] [Google Scholar]
- 53.Reichmann F, Holzer P. 2016. Neuropeptide Y: a stressful review. Neuropeptides 55, 99–109. (doi:10.1016/j.npep.2015.09.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haas DA, George SR. 1989. Neuropeptide Y-induced effects on hypothalamic corticotropin-releasing factor content and release are dependent on noradrenergic/adrenergic neurotransmission. Brain Res. 498, 333–338. (doi:10.1016/0006-8993(89)91112-8) [DOI] [PubMed] [Google Scholar]
- 55.Maule AG, Shaw C, Halton DW, Thim L, Johnston CF, Fairweather I, Buchanan KD. 1991. Neuropeptide-F—a novel parasitic flatworm regulatory peptide from Moniezia expansa (Cestoda, Cyclophyllidea). Parasitology 102, 309–316. (doi:10.1017/S0031182000062648) [Google Scholar]
- 56.Elphick MR, Mirabeau O. 2014. The evolution and variety of RFamide-type neuropeptides: insights from deuterostomian invertebrates. Front. Endocrinol. 5, 93 (doi:10.3389/fendo.2014.00093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kanai Y, Tanuma S. 1992. Purification of a novel B cell growth and differentiation factor associated with lupus syndrome. Immunol. Lett. 32, 43–48. (doi:10.1016/0165-2478(92)90197-V) [DOI] [PubMed] [Google Scholar]
- 58.Miura K, Titani K, Kurosawa Y, Kanai Y. 1992. Molecular cloning of nucleobindin, a novel DNA-binding protein that contains both a signal peptide and a leucine zipper structure. Biochem. Biophys. Res. Commun. 187, 375–380. (doi:10.1016/S0006-291X(05)81503-7) [DOI] [PubMed] [Google Scholar]
- 59.Gonzalez R, Mohan H, Unniappan S. 2012. Nucleobindins: bioactive precursor proteins encoding putative endocrine factors? Gen. Comp. Endocrinol. 176, 341–346. (doi:10.1016/j.ygcen.2011.11.021) [DOI] [PubMed] [Google Scholar]
- 60.Oh IS, et al. 2006. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature 443, 709–712. (doi:10.1038/nature05162) [DOI] [PubMed] [Google Scholar]
- 61.Dore R, Levata L, Lehnert H, Schulz C. 2017. Nesfatin-1: functions and physiology of a novel regulatory peptide. J. Endocrinol. 232, R45–R65. (doi:10.1530/JOE-16-0361) [DOI] [PubMed] [Google Scholar]
- 62.Sundarrajan L, Blanco AM, Bertucci JI, Ramesh N, Canosa LF, Unniappan S. 2016. Nesfatin-1-like peptide encoded in nucleobindin-1 in goldfish is a novel anorexigen modulated by sex steroids, macronutrients and daily rhythm. Sci. Rep. 6, 28377 (doi:10.1038/srep28377) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Otte S, Barnikol-Watanabe S, Vorbruggen G, Hilschmann N. 1999. NUCB1, the Drosophila melanogaster homolog of the mammalian EF-hand proteins NEFA and nucleobindin. Mech. Dev. 86, 155–158. (doi:10.1016/S0925-4773(99)00103-3) [DOI] [PubMed] [Google Scholar]
- 64.Burke RD, et al. 2006. A genomic view of the sea urchin nervous system. Dev. Biol. 300, 434–460. (doi:10.1016/j.ydbio.2006.08.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Elphick MR, Rowe ML. 2009. NGFFFamide and echinotocin: structurally unrelated myoactive neuropeptides derived from neurophysin-containing precursors in sea urchins. J. Exp. Biol. 212, 1067–1077. (doi:10.1242/jeb.027599) [DOI] [PubMed] [Google Scholar]
- 66.Mita M, Yoshikuni M, Ohno K, Shibata Y, Paul-Prasanth B, Pitchayawasin S, Isobe M, Nagahama Y. 2009. A relaxin-like peptide purified from radial nerves induces oocyte maturation and ovulation in the starfish, Asterina pectinifera. Proc. Natl Acad. Sci. USA 106, 9507–9512. (doi:10.1073/pnas.0900243106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Elphick MR, Reeve JR Jr, Burke RD, Thorndyke MC. 1991. Isolation of the neuropeptide SALMFamide-1 from starfish using a new antiserum. Peptides 12, 455–459. (doi:10.1016/0196-9781(91)90083-2) [DOI] [PubMed] [Google Scholar]
- 68.Schally AV, Bowers CY, Redding TW, Barrett JF. 1966. Isolation of thyrotropin releasing factor (TRF) from porcine hypothalamus. Biochem. Biophys. Res. Commun. 25, 165–169. (doi:10.1016/0006-291X(66)90574-2) [DOI] [PubMed] [Google Scholar]
- 69.Boler J, Enzmann F, Folkers K, Bowers CY, Schally AV. 1969. The identity of chemical and hormonal properties of the thyrotropin releasing hormone and pyroglutamyl-histidyl-proline amide. Biochem. Biophys. Res. Commun. 37, 705–710. (doi:10.1016/0006-291X(69)90868-7) [DOI] [PubMed] [Google Scholar]
- 70.Burgus R, Dunn TF, Desiderio D, Guillemin R. 1969. Molecular structure of the hypothalamic hypophysiotropic TRF factor of ovine origin: mass spectrometry demonstration of the PCA-His-Pro-NH2 sequence. C. R. Acad. Sci. Hebd. Seances Acad. Sci. D 269, 1870–1873. [PubMed] [Google Scholar]
- 71.Guillemin R, Yamazaki E, Gard DA, Jutisz M, Sakiz E. 1963. In vitro secretion of thyrotropin (TSH): stimulation by a hypothalamic peptide (TRF). Endocrinology 73, 564–572. (doi:10.1210/endo-73-5-564) [DOI] [PubMed] [Google Scholar]
- 72.Bowers CR, Redding TW, Schally AV. 1965. Effect of thyrotropin releasing factor (TRF) of ovine, bovine, porcine and human origin on thyrotropin release in vitro and in vivo. Endocrinology 77, 609–616. (doi:10.1210/endo-77-4-609) [DOI] [PubMed] [Google Scholar]
- 73.Bauknecht P, Jekely G. 2015. Large-scale combinatorial deorphanization of Platynereis neuropeptide GPCRs. Cell Rep. 12, 684–693. (doi:10.1016/j.celrep.2015.06.052) [DOI] [PubMed] [Google Scholar]
- 74.Wilber JF, Yamada M, Kim UJ, Feng P, Carnell NE. 1992. The human prepro thyrotropin-releasing hormone (TRH) gene: cloning, characterization, hormonal regulation, and gene localization. Trans. Am. Clin. Climatol. Assoc. 103, 111–119. [PMC free article] [PubMed] [Google Scholar]
- 75.Mutt V, Jorpes JE. 1968. Structure of porcine cholecystokinin-pancreozymin. I. Cleavage with thrombin and with trypsin. Eur. J. Biochem. 6, 156–162. (doi:10.1111/j.1432-1033.1968.tb00433.x) [DOI] [PubMed] [Google Scholar]
- 76.Johnson LP, Magee DF. 1965. Inhibition of gastric motility by a commercial duodenal mucosal extract containing cholecystokinin and pancreozymin. Nature 207, 1401 (doi:10.1038/2071401a0) [DOI] [PubMed] [Google Scholar]
- 77.Cooke AR. 1967. Effect of pancreozymin preparations on gastric secretion. Nature 214, 729 (doi:10.1038/214729a0) [DOI] [PubMed] [Google Scholar]
- 78.Jorpes E, Mutt V. 1966. Cholecystokinin and pancreozymin, one single hormone? Acta Physiol. Scand. 66, 196–202. (doi:10.1111/j.1748-1716.1966.tb03185.x) [DOI] [PubMed] [Google Scholar]
- 79.Beinfeld MC. 2006. CCK/Gastrin A2. In Handbook of biologically active peptides (ed. Kastin AJ.), pp. 715–720. Burlington, MA: Academic Press. [Google Scholar]
- 80.Menschaert G, Vandekerckhove TT, Baggerman G, Landuyt B, Sweedler JV, Schoofs L, Luyten W, Van Criekinge W. 2010. A hybrid, de novo based, genome-wide database search approach applied to the sea urchin neuropeptidome. J. Proteome Res. 9, 990–996. (doi:10.1021/pr900885k) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cameron RA, Samanta M, Yuan A, He D, Davidson E. 2009. SpBase: the sea urchin genome database and web site. Nucleic Acids Res. 37, D750–D754. (doi:10.1093/nar/gkn887) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yu N, Nachman RJ, Smagghe G. 2013. Characterization of sulfakinin and sulfakinin receptor and their roles in food intake in the red flour beetle Tribolium castaneum. Gen. Comp. Endocrinol. 188, 196–203. (doi:10.1016/j.ygcen.2013.03.006) [DOI] [PubMed] [Google Scholar]
- 83.Brazeau P, Vale W, Burgus R, Ling N, Butcher M, Rivier J, Guillemin R. 1973. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science 179, 77 (doi:10.1126/science.179.4068.77) [DOI] [PubMed] [Google Scholar]
- 84.Epelbaum J. 1986. Somatostatin in the central nervous system: physiology and pathological modifications. Prog. Neurobiol. 27, 63–100. (doi:10.1016/0301-0082(86)90012-2) [DOI] [PubMed] [Google Scholar]
- 85.Viollet C, Lepousez G, Loudes C, Videau C, Simon A, Epelbaum J. 2008. Somatostatinergic systems in brain: networks and functions. Mol. Cell. Endocrinol. 286, 75–87. (doi:10.1016/j.mce.2007.09.007) [DOI] [PubMed] [Google Scholar]
- 86.Schlegel W, Raptis S, Harvey RF, Oliver JM, Pfeiffer EF. 1977. Inhibition of cholecystokinin-pancreozymin release by somatostatin. Lancet 2, 166–168. (doi:10.1016/S0140-6736(77)90182-9) [DOI] [PubMed] [Google Scholar]
- 87.Hayes JR, Johnson DG, Koerker D, Williams RH. 1975. Inhibition of gastrin release by somatosatin in vitro. Endocrinology 96, 1374–1376. (doi:10.1210/endo-96-6-1374) [DOI] [PubMed] [Google Scholar]
- 88.Tostivint H, Lihrmann I, Vaudry H. 2008. New insight into the molecular evolution of the somatostatin family. Mol. Cell. Endocrinol. 286, 5–17. (doi:10.1016/j.mce.2008.02.029) [DOI] [PubMed] [Google Scholar]
- 89.Veenstra JA. 2009. Allatostatin C and its paralog allatostatin double C: the arthropod somatostatins. Insect. Biochem. Mol. Biol. 39, 161–170. (doi:10.1016/j.ibmb.2008.10.014) [DOI] [PubMed] [Google Scholar]
- 90.Veenstra JA. 2016. Allatostatins C, double C and triple C, the result of a local gene triplication in an ancestral arthropod. Gen. Comp. Endocrinol. 230–231, 153–157. (doi:10.1016/j.ygcen.2016.04.013) [DOI] [PubMed] [Google Scholar]
- 91.Li B, et al. 2008. Genomics, transcriptomics, and peptidomics of neuropeptides and protein hormones in the red flour beetle Tribolium castaneum. Genome Res. 18, 113–122. (doi:10.1101/gr.6714008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stewart MJ, et al. 2014. Neuropeptides encoded by the genomes of the Akoya pearl oyster Pinctata fucata and Pacific oyster Crassostrea gigas: a bioinformatic and peptidomic survey. BMC Genomics 15, 840 (doi:10.1186/1471-2164-15-840) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cardoso JC, Felix RC, Bergqvist CA, Larhammar D. 2014. New insights into the evolution of vertebrate CRH (corticotropin-releasing hormone) and invertebrate DH44 (diuretic hormone 44) receptors in metazoans. Gen. Comp. Endocrinol. 209, 162–170. (doi:10.1016/j.ygcen.2014.09.004) [DOI] [PubMed] [Google Scholar]
- 94.Lee HR, Zandawala M, Lange AB, Orchard I. 2016. Isolation and characterization of the corticotropin-releasing factor-related diuretic hormone receptor in Rhodnius prolixus. Cell. Signal. 28, 1152–1162. (doi:10.1016/j.cellsig.2016.05.020) [DOI] [PubMed] [Google Scholar]
- 95.Chang MM, Leeman SE. 1970. Isolation of a sialogogic peptide from bovine hypothalamic tissue and its characterization as substance P. J. Biol. Chem. 245, 4784–4790. [PubMed] [Google Scholar]
- 96.Studer RO, Trzeciak A, Lergier W. 1973. Isolierung und Aminosäuresequenz von Substanz P aus Pferdedarm. Helv. Chim. Acta 56, 860–866. (doi:10.1002/hlca.19730560307) [Google Scholar]
- 97.V Euler VE, Gaddum JH. 1931. An unidentified depressor substance in certain tissue extracts. J. Physiol. 72, 74–87. (doi:10.1113/jphysiol.1931.sp002763) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Satake H, et al. 2004. Tachykinin and tachykinin receptor of an ascidian, Ciona intestinalis: evolutionary origin of the vertebrate tachykinin family. J. Biol. Chem. 279, 53 798–53 805. (doi:10.1074/jbc.M408161200) [DOI] [PubMed] [Google Scholar]
- 99.Schoofs L, Holman GM, Hayes TK, Nachman RJ, De Loof A. 1990. Locustatachykinin I and II, two novel insect neuropeptides with homology to peptides of the vertebrate tachykinin family. FEBS Lett. 261, 397–401. (doi:10.1016/0014-5793(90)80601-E) [DOI] [PubMed] [Google Scholar]
- 100.Jiang H, et al. 2013. Natalisin, a tachykinin-like signaling system, regulates sexual activity and fecundity in insects. Proc. Natl Acad. Sci. USA 110, E3526–E3534. (doi:10.1073/pnas.1310676110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kawada T, Satake H, Minakata H, Muneoka Y, Nomoto K. 1999. Characterization of a novel cDNA sequence encoding invertebrate tachykinin-related peptides isolated from the echiuroid worm, Urechis unicinctus. Biochem. Biophys. Res. Commun. 263, 848–852. (doi:10.1006/bbrc.1999.1465) [DOI] [PubMed] [Google Scholar]
- 102.Kanda A, Iwakoshi-Ukena E, Takuwa-Kuroda K, Minakata H. 2003. Isolation and characterization of novel tachykinins from the posterior salivary gland of the common octopus Octopus vulgaris. Peptides 24, 35–43. (doi:10.1016/S0196-9781(02)00274-7) [DOI] [PubMed] [Google Scholar]
- 103.Kovac JR, Chrones T, Preiksaitis HG, Sims SM. 2006. Tachykinin receptor expression and function in human esophageal smooth muscle. J. Pharmacol. Exp. Ther. 318, 513–520. (doi:10.1124/jpet.106.104034) [DOI] [PubMed] [Google Scholar]
- 104.Im SH, Takle K, Jo J, Babcock DT, Ma Z, Xiang Y, Galko MJ. 2015. Tachykinin acts upstream of autocrine Hedgehog signaling during nociceptive sensitization in Drosophila. eLife 4, e10735 (doi:10.7554/eLife.10735) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kahsai L, Kapan N, Dircksen H, Winther AM, Nassel DR. 2010. Metabolic stress responses in Drosophila are modulated by brain neurosecretory cells that produce multiple neuropeptides. PLoS ONE 5, e11480 (doi:10.1371/journal.pone.0011480) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nassel DR. 1999. Tachykinin-related peptides in invertebrates: a review. Peptides 20, 141–158. (doi:10.1016/S0196-9781(98)00142-9) [DOI] [PubMed] [Google Scholar]
- 107.Lee JH, Miele ME, Hicks DJ, Phillips KK, Trent JM, Weissman BE, Welch DR. 1996. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J. Natl Cancer Inst. 88, 1731–1737. (doi:10.1093/jnci/88.23.1731) [DOI] [PubMed] [Google Scholar]
- 108.Messager S, et al. 2005. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc. Natl Acad. Sci. USA 102, 1761–1766. (doi:10.1073/pnas.0409330102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Copp DH, Cheney B. 1962. Calcitonin—a hormone from the parathyroid which lowers the calcium-level of the blood. Nature 193, 381–382. (doi:10.1038/193381a0) [DOI] [PubMed] [Google Scholar]
- 110.Potts JT Jr, Niall HD, Keutmann HT, Brewer HB Jr, Deftos LJ. 1968. The amino acid sequence of porcine thyrocalcitonin. Proc. Natl Acad. Sci. USA 59, 1321–1328. (doi:10.1073/pnas.59.4.1321) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Foster GV, Baghdiantz A, Kumar MA, Slack E, Soliman HA, Macintyre I. 1964. Thyroid origin of calcitonin. Nature 202, 1303–1305. (doi:10.1038/2021303a0) [DOI] [PubMed] [Google Scholar]
- 112.Friedman J, Raisz LG. 1965. Thyrocalcitonin: inhibitor of bone resorption in tissue culture. Science 150, 1465–1467. (doi:10.1126/science.150.3702.1465) [DOI] [PubMed] [Google Scholar]
- 113.Bonga SEW, Pang PK. 1991. Control of calcium regulating hormones in the vertebrates: parathyroid hormone, calcitonin, prolactin, and stanniocalcin. Int. Rev. Cytol. 128, 139–213. (doi:10.1016/S0074-7696(08)60499-4) [DOI] [PubMed] [Google Scholar]
- 114.Furuya K, Milchak RJ, Schegg KM, Zhang J, Tobe SS, Coast GM, Schooley DA. 2000. Cockroach diuretic hormones: characterization of a calcitonin-like peptide in insects. Proc. Natl Acad. Sci. USA 97, 6469–6474. (doi:10.1073/pnas.97.12.6469) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Veenstra JA. 2014. The contribution of the genomes of a termite and a locust to our understanding of insect neuropeptides and neurohormones. Front. Physiol. 5, 454 (doi:10.3389/fphys.2014.00454) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sekiguchi T, et al. 2016. Evidence for conservation of the calcitonin superfamily and activity-regulating mechanisms in the basal chordate Branchiostoma floridae: insights into the molecular and functional evolution in chordates. J. Biol. Chem. 291, 2345–2356. (doi:10.1074/jbc.M115.664003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Amara SG, Jonas V, Rosenfeld MG, Ong ES, Evans RM. 1982. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature 298, 240–244. (doi:10.1038/298240a0) [DOI] [PubMed] [Google Scholar]
- 118.McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, Solari R, Lee MG, Foord SM. 1998. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature 393, 333–339. (doi:10.1038/30666) [DOI] [PubMed] [Google Scholar]
- 119.Evans BN, Rosenblatt MI, Mnayer LO, Oliver KR, Dickerson IM. 2000. CGRP-RCP, a novel protein required for signal transduction at calcitonin gene-related peptide and adrenomedullin receptors. J. Biol. Chem. 275, 31 438–31 443. (doi:10.1074/jbc.M005604200) [DOI] [PubMed] [Google Scholar]
- 120.Zandawala M, Paluzzi JP, Orchard I. 2011. Isolation and characterization of the cDNA encoding DH(31) in the kissing bug, Rhodnius prolixus. Mol. Cell. Endocrinol. 331, 79–88. (doi:10.1016/j.mce.2010.08.012) [DOI] [PubMed] [Google Scholar]
- 121.Zandawala M. 2012. Calcitonin-like diuretic hormones in insects. Insect. Biochem. Mol. Biol. 42, 816–825. (doi:10.1016/j.ibmb.2012.06.006) [DOI] [PubMed] [Google Scholar]
- 122.Nakabayashi K, Matsumi H, Bhalla A, Bae J, Mosselman S, Hsu SY, Hsueh AJ. 2002. Thyrostimulin, a heterodimer of two new human glycoprotein hormone subunits, activates the thyroid-stimulating hormone receptor. J. Clin. Invest. 109, 1445–1452. (doi:10.1172/JCI14340) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Roch GJ, Sherwood NM. 2014. Glycoprotein hormones and their receptors emerged at the origin of metazoans. Genome Biol. Evol. 6, 1466–1479. (doi:10.1093/gbe/evu118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sudo S, Kuwabara Y, Park JI, Hsu SY, Hsueh AJ. 2005. Heterodimeric fly glycoprotein hormone-alpha2 (GPA2) and glycoprotein hormone-beta5 (GPB5) activate fly leucine-rich repeat-containing G protein-coupled receptor-1 (DLGR1) and stimulation of human thyrotropin receptors by chimeric fly GPA2 and human GPB5. Endocrinology 146, 3596–3604. (doi:10.1210/en.2005-0317) [DOI] [PubMed] [Google Scholar]
- 125.Rocco DA, Paluzzi JP. 2016. Functional role of the heterodimeric glycoprotein hormone, GPA2/GPB5, and its receptor, LGR1: an invertebrate perspective. Gen. Comp. Endocrinol. 234, 20–27. (doi:10.1016/j.ygcen.2015.12.011) [DOI] [PubMed] [Google Scholar]
- 126.Cahoreau C, Klett D, Combarnous Y. 2015. Structure function relationships of glycoprotein hormones and their subunits' ancestors. Front. Endocrinol. 6, ARTN 26 (doi:10.3389/fendo.2015.00026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Verleyen P, Huybrechts J, Baggerman G, Van Lommel A, De Loof A, Schoofs L. 2004. SIFamide is a highly conserved neuropeptide: a comparative study in different insect species. Biochem. Biophys. Res. Commun. 320, 334–341. (doi:10.1016/j.bbrc.2004.05.173) [DOI] [PubMed] [Google Scholar]
- 128.Yasuda A, Yasuda-Kamatani Y, Nozaki M, Nakajima T. 2004. Identification of GYRKPPFNGSIFamide (crustacean-SIFamide) in the crayfish Procambarus clarkii by topological mass spectrometry analysis. Gen. Comp. Endocrinol. 135, 391–400. (doi:10.1016/j.ygcen.2003.10.001) [DOI] [PubMed] [Google Scholar]
- 129.Verleyen P, Huybrechts J, Schoofs L. 2009. SIFamide illustrates the rapid evolution in Arthropod neuropeptide research. Gen. Comp. Endocrinol. 162, 27–35. (doi:10.1016/j.ygcen.2008.10.020) [DOI] [PubMed] [Google Scholar]
- 130.Wegener C, Gorbashov A. 2008. Molecular evolution of neuropeptides in the genus Drosophila. Genome Biol. 9, R131 (doi:10.1186/gb-2008-9-8-r131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Brezina V, Bank B, Cropper EC, Rosen S, Vilim FS, Kupfermann I, Weiss KR. 1995. Nine members of the myomodulin family of peptide cotransmitters at the B16-ARC neuromuscular junction of Aplysia. J. Neurophysiol. 74, 54–72. [DOI] [PubMed] [Google Scholar]
- 132.Hewes RS, Snowdeal EC III, Saitoe M, Taghert PH. 1998. Functional redundancy of FMRFamide-related peptides at the Drosophila larval neuromuscular junction. J. Neurosci. 18, 7138–7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fujisawa Y, et al. 1999. The Aplysia Mytilus inhibitory peptide-related peptides: identification, cloning, processing, distribution, and action. J. Neurosci. 19, 9618–9634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lange AB, Bendena WG, Tobe SS. 1995. The effect of the 13 Dip-Allatostatins on myogenic and induced contractions of the cockroach (Diploptera punctata) hindgut. J. Insect. Physiol. 41, 581–588. (doi:10.1016/0022-1910(95)00008-I) [Google Scholar]
- 135.Criscuolo A, Gribaldo S. 2010. BMGE (Block Mapping and Gathering with Entropy): a new software for selection of phylogenetic informative regions from multiple sequence alignments. BMC Evol. Biol. 10, 1 (doi:10.1186/1471-2148-10-210) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw sequence data used to assemble the transcriptomes have been deposited in the NCBI Sequence Read Archive (SRA) under the accession no. SRP107914 (https://www.ncbi.nlm.nih.gov/sra/?term=SRP107914) and in the NCBI BioProject under the accession no. PRJNA311384 (https://www.ncbi.nlm.nih.gov/bioproject/311384).