Abstract
For decades, linden trees (basswoods or lime trees), and particularly silver linden (Tilia tomentosa), have been linked to mass bee deaths. This phenomenon is often attributed to the purported occurrence of the carbohydrate mannose, which is toxic to bees, in Tilia nectar. In this review, however, we conclude that from existing literature there is no experimental evidence for toxicity to bees in linden nectar. Bee deaths on Tilia probably result from starvation, owing to insufficient nectar resources late in the tree's flowering period. We recommend ensuring sufficient alternative food sources in cities during late summer to reduce bee deaths on silver linden. Silver linden metabolites such as floral volatiles, pollen chemistry and nectar secondary compounds remain underexplored, particularly their toxic or behavioural effects on bees. Some evidence for the presence of caffeine in linden nectar may mean that linden trees can chemically deceive foraging bees to make sub-optimal foraging decisions, in some cases leading to their starvation.
Keywords: bumblebee, ecotoxicology, pollinator decline, urban ecology
1. Introduction
Pollinators face increasing pressure from anthropogenic environmental impacts including land use intensification, climate change and pesticides [1]. Concurrently, agricultural and urban environments can support abundant and species-rich pollinator communities if suitable floral resources are available [2–4]. Accurate knowledge about how plant species benefit or harm pollinators is therefore of central importance for creating pollinator-friendly environments. For example, non-native plants interact with native pollinators and the whole ecosystem, with direct or indirect effects that benefit or hinder pollinators and ecosystem services they provide [5]. Non-native plant species can have negative consequences for local non-adapted pollinators where toxins occur in nectar, as shown for the invasive Rhododendron ponticum in the British Isles [6].
Linden or lime trees (Tilia sp., Malvaceae) have at times been regarded as either beneficial food sources or deadly traps for bees. In antiquity, linden trees were regarded as bountiful food plants for honeybees [7]. Linden trees have been planted in Europe to support honeybees since medieval times [8] and are productive nectar sources [3,9]. Conversely, since at least the sixteenth century, other authors have suggested linden can harm bees [10,11]. The potential dual nature of linden is most apparent by reoccurring mass deaths on flowering linden trees with sometimes thousands of dead bees (table 1). Silver linden (Tilia tomentosa Moench) are most often associated with bee deaths and have been asserted in numerous accounts to produce toxic nectar [12,16,21,24–28].
Table 1.
Accounts of bee deaths on linden (Tilia sp.).
| Tilia species | no. dead bees | no. trees | dead bees/tree | city | country | date | % Bombus | % Apis | main species | notes | reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| T. tomentosa | 417 | 13 | 32 | Bonn | Germany | 29 July 1975 | 100 | 0 | B. terrestris | A. mellifera not recorded | Madel [12] |
| T. tomentosa | 1937 | 2 | 968 | Münster | Germany | 14 July 2000 | 83 | 17 | B. terrestris | Illies [13] | |
| T. tomentosa | 1833 | 5 | 367 | Eberswalde | East Germany | 13 August 1987 | 79 | 21 | B. terrestris | Donath [14] | |
| T. tomentosa | 716 | 9 | 80 | Berlin | East Germany | July 1988 | 61 | 39 | B. lucorum | Donath [14] | |
| T. tomentosa | 49 | 25 | 2 | Casel | East Germany | 08 August 1987 | 100 | 0 | B. lucorum | incomplete collection? | Donath [14] |
| T. tomentosa | 1637 | 1 | 1637 | Steinfurt-Borghorst | Germany | July 1990 | 99.5 | 0.5 | B. terrestris | Mühlen et al. [15] | |
| T. tomentosa | 1702 | 1 | 1702 | Steinfurt-Borghorst | Germany | 07.July 1991 | ? | ? | B. terrestris | same tree as above | Mühlen et al. [15] |
| T. tomentosa | 1412 | 1 | 1412 | Steinfurt-Borghorst | Germany | July 1992 | ? | ? | B. terrestris | same tree as above | Mühlen et al. [15] |
| T. tomentosa | 2210 | 1 | 2210 | Münster | Germany | July 1992 | ? | ? | B. terrestris | Mühlen et al. [15] | |
| T. tomentosa | 300 | 1 | 300 | Torun | Poland | 2003 | 83 | 17 | B. terrestris | Pawlikowski [16] | |
| T. tomentosa | 1608 | 1 | 1608 | Dülmen | Germany | July 1993 | 100 | 0 | ? | A. mellifera not recorded | Surholt & Baal [17] |
| T. tomentosa | 1603 | ? | ? | Osnabrück | Germany | 1994 | 100 | 0 | B. terrestris | A. mellifera not recorded | Zucchi [18] |
| T. tomentosa | 141 | 1 | 141 | Gera | East Germany | July 1989 | 89 | 11 | B. terrestris | incomplete collection | Breinl [19] |
| T. tomentosa ‘Petiolaris’ | ‘hundreds’ | 1 | ‘hundreds’ | Innsbruck | Austria | July 1994 | ? | ? | Bombus spp. | Schedl [20] | |
| T. tomentosa ‘Petiolaris’ | 403 | 1 | 403 | Richmond | UK | 05 August 2016 | 99 | 1 | B. terrestris | Kew Gardens | H. Koch 2016, personal observation |
| T. tomentosa ‘Petiolaris’ | ? | 1 | ? | Tortworth | UK | 1908 | ? | ? | ? | dead bees ‘manured ground’ | Elwes & Henry [21] |
| T. tomentosa ‘Petiolaris’ | 660 | 1 | 660 | Gera | East Germany | July 1989 | 67 | 33 | B. terrestris | Botanical Garden | Breinl [19] |
| T. tomentosa ‘Petiolaris’ | 86 | 1 | 86 | Gera | East Germany | July 1989 | 83 | 17 | B. terrestris | incomplete collection | Breinl [19] |
| T. × euchlora | 247 | 30 | 8 | Luckau | East Germany | 02 August 1987 | 71 | 29 | B. hypnorum | Donath [14] | |
| T. × euchlora | 82 | 11 | 7 | Erfurt | East Germany | 24 July 1988 | 93 | 7 | B. lapidarius | Donath [14] | |
| T. × euchlora | 983 | 1 | 983 | Steinfurt-Borghorst | Germany | July 1990 | 97 | 3 | B. terrestris | Mühlen et al. [15] | |
| T. × euchlora | 816 | 70 | 12 | Gera | East Germany | July 1989 | 72 | 28 | B. terrestris | incomplete collection | Breinl [19] |
| T. × euchlora | 336 | 1 | 336 | Gera | East Germany | July 1989 | 89 | 11 | B. terrestris | incomplete collection | Breinl [19] |
| T. × euchlora | 372 | 50 | 7 | Gera | East Germany | July 1989 | 67 | 33 | B. terrestris | incomplete collection | Breinl [19] |
| T. cordata | 10 | 1 | 10 | Münster | Germany | 22 June 2000 | 80 | 20 | B. terrestris | Illies [13] | |
| T. cordata | 534 | 4 | 134 | Steinfurt-Borghorst | Germany | July 1990 | 77 | 23 | ? | Mühlen et al. [15] | |
| T. cordata | 12 | 1 | 12 | Torun | Poland | 2003 | 50 | 50 | B. terrestris | Pawlikowski [16] | |
| T. cordata | 50 000 | 55 | 909 | Wilsonville | USA | 13 June 2013 | 100 | 0 | B. vosnesenskii | killed by Dinotefuran? | Black & Vaughan [22] |
| T. platyphyllos | 40 | 2 | 20 | Münster | Germany | 15 June 2000 | 71 | 29 | B. terrestris | Illies [13] | |
| T. platyphyllos | 78 | 1 | 78 | Berlin | East Germany | 13 June 1988 | 5 | 92 | A. mellifera | Donath [14] | |
| T. platyphyllos | 373 | 4 | 93 | Steinfurt-Borghorst | Germany | July 1990 | 62 | 38 | ? | Mühlen et al. [15] | |
| T. spp. | 608 | 102 | 6 | Linz | Austria | 04 August 1978 | 41 | 59 | A. mellifera | incomplete collection | Pfitzner [23] |
Silver linden (figure 1) originates from southeastern Europe, but is planted widely outside its native range across Europe and North America [8,21]. Linden are among the most common urban trees throughout Europe and North America [29], and so have the greatest potential to affect urban pollinators. Their high drought and pest tolerance qualifies silver linden as excellent urban trees [30,31]. Given the importance of urban habitats and trees for pollinator populations [2,3], it is necessary to review whether linden trees have detrimental effects on bees, and how these may arise.
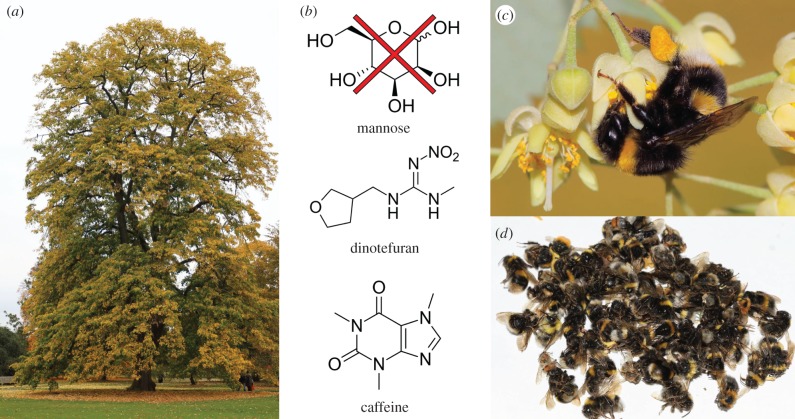
Figure 1.
(a) Silver linden (T. tomentosa ‘Petiolaris’) at the Royal Botanic Gardens, Kew, UK; (b) chemicals implied in bee deaths; (c) buff-tailed bumblebee (B. terrestris) worker foraging on T. tomentosa; (d) dead bees (B. terrestris, B. hypnorum, B. lucorum, Apis mellifera) collected during 1 day (29 July 2016) under flowering T. tomentosa.
Dead bees under flowering linden have been reported from the UK [21], Switzerland [32], Germany [12–15], Norway [33], Poland [16], Austria [20,23] and the USA [28,34] (table 1). The Crimean linden (Tilia × euchlora), a putative hybrid between Tilia cordata and Tilia dasystyla [8], is also associated with bee deaths (table 1). Small-leaved linden (T. cordata), large-leaved linden (Tilia platyphyllos) and their hybrid common linden (Tilia × europaea) are generally not linked to this phenomenon, with the exception of a recent bumblebee kill under T. cordata in Oregon (USA; table 1).
Bumblebees are most affected, accounting for over 75% of dead bees [12,35] (table 1). Short-tongued bumblebee species like Bombus terrestris dominate (table 1 and figure 1). Fewer honeybees (Apis mellifera) die, even though they forage as abundantly on the tree as bumblebees [13,16].
While dead bees under T. tomentosa and other linden trees are still recorded in many countries, uncertainty and confusion prevails over the causes. Here, we categorize and assess the published explanations under five hypotheses, examine their plausibility considering existing research, and identify key research gaps (table 2).
Table 2.
Hypotheses explaining bee deaths on Tilia.
| hypothesis | prediction | supporting evidence | opposing evidence | research need |
|---|---|---|---|---|
| 1. toxic Tilia metabolites | toxic metabolites in Tilia nectar or pollen with lethal or sub-lethal effects on bees | affected bees appear paralysed before dying [12]; suggestion of mannose (toxic to bees) in Tilia nectar based on limited paper-chromatographic investigations [12,36] | no detection of mannose by gas chromatography in T. tomentosa nectar or dead bees; no experimental evidence for toxicity of T. tomentosa nectar [35,37] | detailed chemical analysis of Tilia pollen and nectar metabolites, experimental tests of toxicity |
| 2. insecticides | insecticide (e.g. neonicotid) application to Tilia trees killing bee foragers | prior application of neonicotinoids to Tilia recorded in isolated cases [28,38] | phenomenon existed before use of neonicotinoids [12,14,21], most cases without known previous insecticide application (table 1) | persistence of neonicotinoids in Tilia and exposure of bees from Tilia pollen and nectar when neonicotinoids are applied outside flowering period |
| 3. natural causes: predators/old age | dead bees owing to background mortality from e.g. predators and old age | T. tomentosa flowers during the end of the colony cycle of some bumblebee species; birds and wasps observed preying on bees on flowering Tilia [15] | majority of dead bees are not old, bee deaths also occur without predator attacks [15] | additional quantification of background mortality from predation or old age of bees foraging on Tilia |
| 4. starvation | dead bees owing to insufficient nectar resources during T. tomentosa flowering period causing starvation | most deaths occur at end of Tilia flowering period when nectar production is very limited [13,17,39], foragers on T. tomentosa have depleted body sugar reserves [35], dying bees can recover when fed Tilia nectar [40], scarcity of alternative nectar resources during T. tomentosa flowering suggested [17,35] | bee deaths can occur when alternative food sources are available [18,19] | comparison of bumblebee mortality on T. tomentosa and nearby plants flowering simultaneously; comparison of colony resource intake and mortality in comparable landscapes with and without T. tomentosa |
| 5. chemical deception | chemical deception (e.g. by volatiles, caffeine) causes overvaluation of Tilia as resource and increased foraging persistence once nectar is depleted, leading to starvation | the presence of caffeine in Tilia honey [41], caffeine modulates bee foraging, increasing persistent return to depleted food sources and causing overvaluation of sugar rewards [42–44], the presence of volatile compounds in Tilia flower scent that act as foraging recruitment pheromones in bumblebees [41,45,46] | known Tilia flower volatiles are common in plants not associated with bee deaths [47] | analysis of volatiles from T. tomentosa flowers; exposure of bees to caffeine on Tilia and effects on foraging behaviour; interaction of T. tomentosa volatiles and caffeine in reward association learning |
| 6. interactive effects | bee deaths owing to interaction of factors in hypotheses 1–5 | plausible, but not investigated | not investigated | interactions between factors in hypotheses 1–5 should be studied |
2. Toxic Tilia metabolites
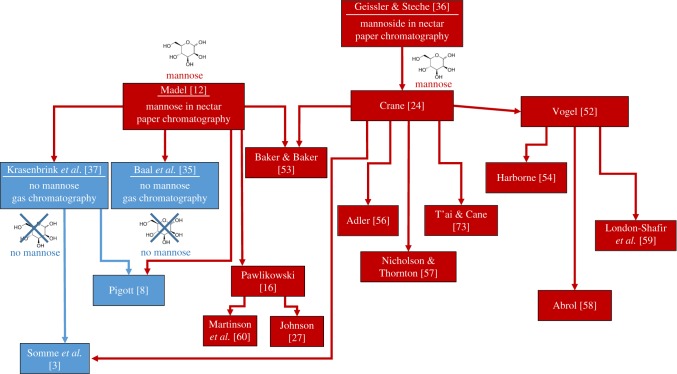
A widely held belief and historic explanation of bee deaths under Tilia is that components in nectar poison bees, first suggested by Elwes & Henry [21]. Geissler & Steche [36] and Madel [12] proposed that the presence of the monosaccharide mannose (figure 1) in T. tomentosa nectar was responsible, after von Frisch [48] and Staudenmayer [49] had discovered toxicity of mannose to honeybees and bumblebees. This toxic effect results from a metabolic disease, in which an intermediate product, mannose 6-phosphate, accumulates and adenosine triphosphate is depleted, resulting in paralysis and death [50]. However, Madel's assertion [12] that this explained T. tomentosa toxicity was supported by scant detail about the detection of mannose beyond stating that he had conducted preliminary paper-chromatographic investigations. Biological evidence was limited to a feeding trial with eight bumblebees caged with seven T. tomentosa flowers without control [12]. All bumblebees tested died within 12 h, leading Madel to conclude T. tomentosa nectar was toxic. However, Baal et al. [35] showed nectar of seven flowers was inadequate for eight caged bumblebees, meeting less than 2% of their energetic demand, and suggested starvation explained Madel's results [35]. Geissler & Steche [36] analysed sugars with paper chromatography and did not detect mannose in linden (T. platyphyllos) nectar. A hydrolysed linden nectar sample revealed a sugar bound as a glycoside that was tentatively identified as mannose based on relative retention time, but was not clearly distinguishable from galactose. Via a colorimetric test, Geissler & Steche [36] also detected a sugar in dead bees collected under linden they concluded to be galactose or mannose. Notably, Geissler & Steche [36] pointed out their identifications were tentative, as they could not isolate sufficient sugar quantities for more refined analytical procedures. Subsequent chemical analyses, described below, discount these earlier proposed identifications. Despite this, Crane [24] later popularized the idea that mannose was responsible, erroneously presenting the riddle of bee deaths on linden as solved (figure 2).
Figure 2.
Citation pattern of scientific papers discussing bee poisoning on T. tomentosa by mannose. Underlined citations are original research studies investigating nectar mannose presence. Red coloured publications suggest mannose as causative agent, blue coloured publications suggest alternative causes.
Baal et al. [35] and Krasenbrink et al. [37] re-examined the nectar sugar chemistry of T. tomentosa and other Tilia species using gas chromatography of derivatized sugars, following standard methods by Sweely et al. [51]. Chromatograms published in Baal et al. [35] and Krasenbrink et al. [37] demonstrate their methods distinguished mannose from other nectar sugars. Glucose, fructose, sucrose and mannose were furthermore enzymatically quantified [35]. These analyses showed unequivocally that mannose was absent in nectar of T. tomentosa (n = 36 trees), T. platyphyllos (n = 20), T. cordata (n = 12) and T × euchlora (n = 14). Only the non-toxic sugars sucrose, glucose and fructose were detected. Since mannose might be produced as a nectar metabolite by bees [36], Baal et al. [35] analysed guts, abdomina and heads/thoraxes of 80 dying bumblebees from flowering T. tomentosa and T × euchlora, but recorded no mannose in the bumblebees. Finally, Baal et al. [35] fed T. tomentosa nectar to 30 caged B. terrestris, and again mannose was absent from guts and haemolymph. Bumblebees fed on T. tomentosa nectar for 5 days showed no adverse effects. Baal et al. [35] thus disproved the hypothesis of mannose poisoning by T. tomentosa. Nevertheless, non-nutritive sugars in Tilia nectar, including sugar moieties in glycosides [36], deserve further study. We suggest carbohydrate chemistry of linden nectar and pollen will become clearer through more accurate and sensitive methods including nuclear magnetic resonance spectroscopy.
Despite the lack of evidence, the received wisdom of mannose poisoning by T. tomentosa nectar continues to prevail as fact in much scientific and technical literature (figure 2), including reviews [24,27,52–58], original research papers [16,59,60], horticultural and botanical guides [25,26], pest control [61] and governmental advisories [38].
The non-sugar chemistry of T. tomentosa nectar and pollen remains largely unstudied. Naef et al. [41] and Frérot et al. [62] described the volatile nectar constituents from the related T. cordata and found secondary compounds including terpenoids, flavonoids and a novel cyclohexa-1,3-diene-1-carboxylic acid and its β-gentiobiosyl ester. The disaccharide gentiobiose occurs in crops of honeybees foraging on T. tomentosa [63], and in linden honey [64]. Gentiobiose is most likely the product of enzymatic cleavage of the β-gentiobiosyl moiety of the above-mentioned glycoside in Tilia nectar [62]. Effects of gentiobiose on bees are unknown, but the feeding trials by Baal et al. [35] (see above) suggest no adverse effects should be expected.
Bumblebees collect pollen on linden [65] (figure 1), but the importance of T. tomentosa pollen remains unknown. Melville [66] observed that only bumblebees and not honeybees collected pollen from T. tomentosa, and speculated a toxic compound in the pollen could explain why the majority of dying bees are bumblebees. However, no published pollen chemistry analysis beyond amino acids and sterols in Somme et al. [3] exists. It remains unknown if foraging bees directly consume Tilia pollen on the tree, or rather carry pollen back externally to the nest as larval food.
We conclude the available evidence shows mannose does not occur in Tilia nectar and therefore cannot explain mass bee deaths on Tilia. There is no convincing experimental evidence for toxicity of T. tomentosa nectar or pollen to bees. However, the exposure of bees foraging on T. tomentosa flowers to toxic compounds other than mannose cannot be completely excluded, given the incomplete knowledge of Tilia pollen and nectar metabolites, and the limited experimental tests of T. tomentosa forage on bumblebee individual or colony health. Plant metabolites in T. tomentosa nectar and pollen therefore need to be analysed further, and their potentially lethal or sub-lethal effects on bees should be tested experimentally.
3. Insecticides
Although T. tomentosa does not poison bees, insecticide application to the tree can. Tilia trees are occasionally treated with insecticides against aphids. Several instances of bumblebee deaths under T. cordata have recently occurred in Oregon, USA. In one outstanding case, over 50 000 bumblebees died under T. cordata trees in Wilsonville, Oregon [28]. Owing to the widespread misconception about the presence of toxic sugars in linden nectar (see above), some sources erroneously suggested naturally occurring nectar toxins caused these bee kills (e.g. [61]). The Oregon Department of Agriculture judged the neonicotinoid dinotefuran (figure 1), that had been applied to the trees prior to the event, as the cause [38]. Neonicotinoids are potent neurotoxins for honeybees and bumblebees [67]. Even when applied outside the flowering period, neonicotinoids can persist in plant tissues and subsequently occur at concentrations detrimental to bees in pollen and nectar [67]. The neonicotinoid use on flowering trees like Tilia spp. should therefore be prohibited.
Bee deaths under linden trees predate the introduction of neonicotinoid insecticides in the 1990s [12,14,21]. Neonicotinoids therefore cannot explain this phenomenon more broadly, but can account for isolated recent cases. The widespread confusion over the erroneously presumed presence of toxic mannose in Tilia nectar (see above) could however misguide policy-makers and pest control professionals (e.g. [38,61]).
4. Death by natural causes: predators and old age
Tilia tomentosa flowers later than other linden species, between mid-July and early August for Europe [13,15,66]. Large trees can accommodate thousands of foraging bees [13]. Many bumblebee species approach the end of their colony cycle at this point in the season. The high bee population on a mass flowering tree like T. tomentosa may see significant numbers of older bumblebees dying of natural causes, giving an impression of toxicity. However, Mühlen et al. [15] classified only 6% of 4116 dead bumblebees collected under T. tomentosa as old, based on characters like loss of pile and wing wear. The vast majority of dead individuals consisted of younger age classes, including young bumblebee queens. These findings led Mühlen et al. [15] to discount old age as a major cause of bee deaths.
Predators including great tits and wasps attack bees on flowering linden trees [15]. Mühlen et al. [15] found 76.1% of 10 984 dead bees from T. tomentosa had damage indicating predator feeding. Mühlen et al. [15] found high variability between trees and seasons for predator damage, with some trees having high death counts but few signs of predation. This suggested predators mostly attacked dying or dead bees, and predation was only a secondary factor.
In conclusion, natural deaths owing to old age or predators account for some of the observed bee deaths, but appear insufficient to fully explain the many thousands of deaths recorded by Mühlen et al. [15] and others.
5. Starvation
The late flowering period of T. tomentosa can coincide with a scarcity of nectar resources in the wider landscape [35]. After the often more abundant linden species T. platyphyllos, T × europaea and T. cordata (generally not linked to bee deaths) have stopped flowering, bees concentrate foraging on the rarer T. tomentosa owing to missing alternative nectar sources. The large honeybee and bumblebee populations at the flowering time of T. tomentosa then face intense competition for remaining nectar [35].
In a detailed temporal study of nectar production, foraging bee species and dead bees covering the flowering period of T. tomentosa, Illies [13] observed an increase of dead bumblebees towards the end of the flowering period. During this time, flowers secrete less nectar, but bumblebees continue visiting [13,39]. This drop in available nectar may lead to large-scale starvation [39]. Similarly, Surholt & Baal [17] monitored foragers of a B. terrestris colony close to a T. tomentosa tree throughout its 11 day flowering period, and found that, coinciding with the cessation of nectar production by the tree at day 8, bumblebee foragers returned from foraging trips without collected nectar. Individually marked workers from the colony were at this point found dead or dying beneath the tree, and the colony died of starvation [17]. Support for the ‘starvation hypothesis’ also comes from the analysis of sugar reserves in bumblebees' bodies [35]. Foragers dying under T. tomentosa had less than a third of the energy reserves left compared with foragers on T. cordata or T. platyphyllos [35]. Surholt et al. [40] reported that paralysed bumblebees under T. tomentosa recover when provided with T. tomentosa nectar. Bumblebees feeding on this nectar recovered fully after 30–40 min [40]. Honeybees may be better able to deal with late summer nectar shortage because of available honey stores in the colony, possibly explaining fewer dead honeybees under T. tomentosa compared to bumblebees [13].
Baal et al. [35], Surholt & Baal [17] and Illies [13] thus presented a compelling case for the bee mass deaths on T. tomentosa resulting from starvation, and considering current evidence this seems the most likely explanation. However, why this happens is still unknown. The best management decision to avert dead bees under Tilia should be to increase late season floral resources in urban environments. This would reduce competition between honeybees and bumblebees. By contrast, felling of T. tomentosa would be counterproductive by further reducing available nectar resources and leading to increased bee losses [13]. Linden including silver linden are valuable nectar sources for bees [3,9].
Doubts remain, however, whether simple starvation owing to insufficient alternative food sources completely explains the phenomenon. Zucchi [18] suggested bee deaths occur under T. tomentosa in areas with alternative flowering forage plants based on observations in a flower-rich park in Osnabrück and observations by Breinl [19] in a botanical garden in Gera (both in Germany). Similarly, we observed 403 dead bumblebees over the flowering period of a single T. tomentosa tree at the Royal Botanic Gardens, Kew (Richmond, UK) in July 2016, when many other nectar providing plants were still flowering in the surrounding garden (table 1 and figure 1).
Bees have been shown to adopt an ideal free distribution across resources [68,69]. This would suggest that if bees are starving on T. tomentosa, they should be starving to an equal extent on other flowering plants simultaneously. Bee deaths on T. tomentosa would thus only be a ‘canary in a coal mine’, highlighting a general lack of nectar resources in a particular area. To test the ‘starvation hypothesis’, bumblebee mortality on T. tomentosa and surrounding flowering plants should be compared, and bumblebee colonies foraging in comparable landscapes with and without T. tomentosa should be monitored for their food intake and starvation. If bumblebee deaths on T. tomentosa are owing to simple starvation, similar levels should be observed on T. tomentosa and other plants, or in colonies foraging in comparable landscapes with or without T. tomentosa. If, however, elevated rates of individual or colony mortality are observed in the presence of T. tomentosa, starvation alone cannot account for the observed phenomenon, and alternative hypotheses outlined in this review need to be considered.
6. Chemical deception
Plants can chemically manipulate pollinator behaviour against the pollinators' best interests, to optimize pollination services at minimal cost. Bee orchids (Ophrys spp.) offer bees no nectar reward, but instead mimic female bee sex pheromones to trick corresponding male bees into visiting and transferring pollen [70]. Other plant species may still offer nectar rewards, but chemically induce pollinators to over-value these rewards and visit with greater frequency than would be optimal for pollinators [42].
Despite the continued interest in the bee deaths on T. tomentosa, the floral chemistry including nectar, pollen and floral volatiles remains understudied. Bumblebees, and to a lesser extent honeybees, are attracted to linden even at the end of the flowering period, when little nectar is produced [39]. The potent scent of T. tomentosa has long been noted [21]. Illies [13] speculated T. tomentosa scent may mimic unknown bumblebee pheromones, causing bees to visit without receiving nectar rewards and thus act as a ‘scent trap’. Returning bumblebee (B. terrestris) foragers emit three pheromones within the colonies that recruit idle workers to start foraging: eucalyptol, farnesol and ocimene [71]. All three compounds occur in flower volatiles or nectar of Tilia species [41,45,46]. Exposure to these volatiles either on the tree or in the colony through returning foragers with Tilia scent could exploit the bumblebees' sensory bias and increase foraging intensity even at times of low nectar production. However, all three volatiles are common among European flowering plants [47]. This suggests that, while the volatiles could have been selected in plants to act as innate stimuli attracting foraging bumblebees, any behavioural effects would not necessarily be unique to Tilia. The specific volatiles emitted by T. tomentosa flowers should be investigated and compared to species of Tilia that are not associated with bee deaths. Their effects on bumblebee foraging behaviour and persistence to return to empty flowers should furthermore be tested experimentally with artificial flowers.
Intriguingly, Naef et al. [41] reported caffeine (figure 1) in T. cordata nectar, and Mathon et al. [72] detected caffeine in Tilia sp. flower tea. Additional studies should verify if, and at what concentrations foraging bees are exposed to caffeine or related alkaloids across different Tilia species. We propose that recent experimental studies investigating the effect of caffeine on bees could help explain the mystery behind bee deaths. Wright et al. [43] demonstrated caffeine enhances odour memory associated with food rewards in honeybees, predicting this induced greater floral fidelity. This was later demonstrated in free-flying honeybees by Couvillon et al. [42], who showed caffeine-laced sugar water increased foraging intensity and recruitment behaviour. Notably, caffeine increased persistence of honeybee foragers to return to previously rewarding but subsequently empty feeders, and increased site specificity, i.e. reducing searching behaviour for other food rewards around the caffeine-laced feeder. Caffeine may allow plants to reduce their nectar investments by misleading bees into making sub-optimal foraging decisions, depleting honey stores despite increased foraging activity [42]. Thomson et al. [44] demonstrated nectar caffeine also affects bumblebee foraging behaviour, with ecologically relevant caffeine levels (10−5 M) leading to increased deposition of a pollen substitute on artificial flowers.
Given sub-optimal honeybee foraging under the influence of caffeine [42], could T. tomentosa similarly manipulate bumblebees to visit after cessation of nectar secretion, until they starve? Certainly, caffeine exposure of bees foraging on Tilia, and its resulting effects should be investigated. Studying T. tomentosa volatiles and their effects on bees, alongside interactive effects with caffeine on scent-reward association learning [43] using artificial flowers (cf. [44]), could help bring two of the more plausible explanations together to understand this extraordinary natural phenomenon.
7. Interactive effects
The interaction of stressors such as pesticides and nutritional deficits is more damaging to pollinators than each stressor in isolation [1]. Similarly, interactions of factors in the preceding five hypotheses could increase bee mortality on T. tomentosa. For example, if compounds in T. tomentosa paralyse bees, they would be more vulnerable to predation. Nutritionally stressed bees may be more susceptible to effects of toxic metabolites in nectar or pollen. Tilia tomentosa metabolites could interact with insecticides causing additive or synergistic toxic effects. Chemical deception of T. tomentosa may be more effective if fewer alternative flowering resources are available in the contiguous landscape. These interactive effects should be considered and tested experimentally.
8. Conclusion
There is no convincing evidence for direct toxicity of T. tomentosa nectar or pollen to bees. Mannose does not occur in T. tomentosa nectar, and the hypothesis of mannose poisoning by foraging bees on this tree has been refuted. In isolated cases, neonicotinoid treatment against aphids can explain some mass bee death events, and insecticide treatment of Tilia trees should be prohibited. In general, starvation of bees owing to insufficient nectar availability is the most likely cause of bee deaths on T. tomentosa. Yet, as the event occurs in the presence of alternative food sources in gardens, starvation alone may not explain the deaths. Starvation rates of individual bees and bee colonies in landscapes with and without T. tomentosa trees associated with bee deaths should be investigated. Ensuring alternative floral resources in late summer during T. tomentosa flowering could be the best way of avoiding associated bee deaths. Tilia tomentosa flower chemistry (including nectar, pollen and volatiles) remains incompletely known, and should be analysed and experimentally tested for bumblebee toxicity. Further research should determine if T. tomentosa can chemically manipulate bee foraging behaviour. A combination of caffeine and Tilia volatiles could lead to sub-optimal foraging in bees, in some cases leading ultimately to starvation.
Data accessibility
This article has no additional data.
Authors' contributions
H.K. drafted the manuscript. H.K. and P.C.S. revised the manuscript, gave their final approval, and are accountable for its content.
Competing interests
We have no competing interests.
Funding
This work was funded by a Peter Sowerby Foundation grant to P.C.S. with the Ann Sowerby Fellowship in Pollinator Health to H.K.
References
- 1.Vanbergen AJ, et al. 2013. Threats to an ecosystem service: pressures on pollinators. Front. Ecol. Environ. 11, 251–259. ( 10.1890/120126) [DOI] [Google Scholar]
- 2.Baldock KC, et al. 2015. Where is the UK's pollinator biodiversity? The importance of urban areas for flower-visiting insects. Proc. R. Soc. B 282, 20142849 ( 10.1098/rspb.2014.2849) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Somme L, Moquet L, Quinet M, Vanderplanck M, Michez D, Lognay G, Jacquemart AL. 2016. Food in a row: urban trees offer valuable floral resources to pollinating insects. Urban Ecosyst. 19, 1149–1161. ( 10.1007/s11252-016-0555-z) [DOI] [Google Scholar]
- 4.Carvell C, et al. 2017. Bumblebee family lineage survival is enhanced in high-quality landscapes. Nature 543, 547–549. ( 10.1038/nature21709) [DOI] [PubMed] [Google Scholar]
- 5.Stout JC, Tiedeken EJ. 2017. Direct interactions between invasive plants and native pollinators: evidence, impacts and approaches. Funct. Ecol. 31, 38–46. ( 10.1111/1365-2435.12751) [DOI] [Google Scholar]
- 6.Tiedeken EJ, Egan PA, Stevenson PC, Wright GA, Brown MJF, Power EF, Farrell I, Matthews SM, Stout JC. 2016. Nectar chemistry modulates the impact of an invasive plant on native pollinators. Funct. Ecol. 30, 885–893. ( 10.1111/1365-2435.12588) [DOI] [Google Scholar]
- 7.Columella LJM. 1954 On agriculture, volume II: books 5-9. Translated by E. S. Forster, Edward H. Heffner. Loeb Classical Library 407. Harvard University Press, Cambridge, MA, USA.
- 8.Pigott D. 2012. Lime-trees and basswoods: a biological monograph of the genus Tilia. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 9.Beutler R, Wahl O. 1936. Über das Honigen der Linde in Deutschland. J. Comp. Physiol. A 23, 301–331. ( 10.1007/BF00338202) [DOI] [Google Scholar]
- 10.Bock H. 1551. Kreüter buch. Strassburg, France: Wendel Rihel. [Google Scholar]
- 11.Munting A. 1696. Naauwkeurige beschryving der aardgewassen. Leiden & Utrecht, Netherlands: Pieter van der Aa & François Halma. [Google Scholar]
- 12.Madel G. 1977. Vergiftungen von Hummeln durch den Nektar der Silberlinde Tilia tomentosa Moench, Bonn. Zool. Beitr. 28, 149–154. [Google Scholar]
- 13.Illies I. 2005. Verhaltensbiologische Untersuchungen zur Trachtnutzung und zum Sammelverhalten von Bienen (Hymenoptera, Apoidea). PhD dissertation, Ruhr-University Bochum, Germany. [Google Scholar]
- 14.Donath H. 1989. Vergiftung von Insekten durch den Blütenbesuch an fremdländischen Lindenarten in der DDR. Entomol. Nachr. Ber. 33, 111–116. [Google Scholar]
- 15.Mühlen W, Riedel V, Baal T, Surholt B. 1994. Insektensterben unter blühenden Linden. Nat. Landsch. 69, 95–100. [Google Scholar]
- 16.Pawlikowski T. 2010. Pollination activity of bees (Apoidea: Apiformes) visiting the flowers of Tilia cordata Mill. and Tilia tomentosa Moench in an urban environment. J. Apic. Sci. 54, 73–79. [Google Scholar]
- 17.Surholt B, Baal T. 1995. Die Bedeutung blühender Silberlinden für Insekten im Hochsommer. Nat. Landsch. 70, 252–258. [Google Scholar]
- 18.Zucchi H. 1996. Ist die Silberlinde rehabilitiert? Zur Diskussion um das Hummelsterben an spätblühenden Linden. Nat. Landsch. 71, 47–50. [Google Scholar]
- 19.Breinl K. 1990. Zur Gefährdung blütenbesuchender Insekten durch Krimlinden und Silberlinden: Untersuchungen in Gera. Veröff. Museen Gera, Naturwiss. R. 17, 74–81. [Google Scholar]
- 20.Schedl W. 2015. Stechimmen II im Botanischen Garten Innsbruck (Tirol, Östereich): Artengarnitur, Blütenbesuch, Phänologie (Insecta: Hymenoptera). Linzer Biol. Beitr. 47, 939–954. [Google Scholar]
- 21.Elwes HJ, Henry A. 1913. The trees of Great Britain & Ireland. Edinburgh, UK: Privately printed. [Google Scholar]
- 22.Black SH, Vaughan M.2013. Pesticide causes largest mass bumble bee death on record. Xerces Soc. Invert. Conserv. Portland, OH, USA. See http://www.xerces.org/2013/06/21/pesticide-causes-largest-mass-bumble-bee-death-on-record .
- 23.Pfitzner G. 1978. Auffallendes Hummel- und Bienensterben in einer Lindenallee! Apollo 53/54, 8–9. [Google Scholar]
- 24.Crane E. 1977. On the scientific front: dead bees under lime trees. Bee World 58, 129–130. ( 10.1080/0005772X.1977.11097662) [DOI] [Google Scholar]
- 25.Hillier J, Coombes A. 2002. The Hillier manual of trees & shrubs. Newton Abbot, UK: David & Charles. [Google Scholar]
- 26.Johnson O, More D. 2006. Collins tree guide. New York, NY: Harper Collins Publishers. [Google Scholar]
- 27.Johnson RM. 2015. Honey bee toxicology. Annu. Rev. Entomol. 60, 415–434. ( 10.1146/annurev-ento-011613-162005) [DOI] [PubMed] [Google Scholar]
- 28.Hopwood J, Code A, Vaughan M, Biddinger D, Shepherd M, Black SH, Mader E, Mazzacano C. 2016. How neonicotinoids can kill bees, 2nd edn Portland, OR: The Xerces Society for Invertebrate Conservation. [Google Scholar]
- 29.Grote R, et al. 2016. Functional traits of urban trees: air pollution mitigation potential. Front. Ecol. Environ. 14, 543–550. ( 10.1002/fee.1426) [DOI] [Google Scholar]
- 30.Raupp MJ, Cumming AB, Raupp EC. 2006. Street tree diversity in eastern North America and its potential for tree loss to exotic borers. Arboric. Urban For. 32, 297–304. [Google Scholar]
- 31.Roloff A, Korn S, Gillner S. 2009. The climate-species-matrix to select tree species for urban habitats considering climate change. Urban For. Urban Greening 8, 295–308. ( 10.1016/j.ufug.2009.08.002) [DOI] [Google Scholar]
- 32.Maurizio A. 1943. Bienenschäden während der Lindentracht. Schweiz. Bienen Ztg. 66, 376–380. [Google Scholar]
- 33.Løken A. 1991. Planter som forgifter humler og bier. Insekt-Nytt 16, 17–19. [Google Scholar]
- 34.Rao S, Poinar G, Henley D. 2017. A scientific note on rare parasitism of the bumble bee pollinator, Bombus impatiens, by a mermithid nematode, Pheromermis sp. (Nematoda: Mermithidae). Apidologie 48, 75 ( 10.1007/s13592-016-0451-9) [DOI] [Google Scholar]
- 35.Baal T, Denker B, Mühlen W, Surholt B. 1994. Die Ursachen des Massensterbens von Hummeln unter spätblühenden Linden. Nat. Landsch. 69, 412–418. [Google Scholar]
- 36.Geissler G, Steche W. 1962. Natürliche Trachten als Ursache für Vergiftungserscheinungen bei Bienen und Hummeln. Z. Bienenforsch. 6, 77–92. [Google Scholar]
- 37.Krasenbrink A, Popp M, Denker B. 1994. Nektarzusammensetzung von Tilia tomentosa (Moench) und anderen Lindenarten/-hybriden. Z. Ökol. Nat.schutz 3, 237–242. [Google Scholar]
- 38.Brown K. 2015. Pesticide advisory: permanent rule prohibiting the use of dinotefuran, imidacloprid, thiamethoxam, and clothianidin on linden trees. Portland, OR: Oregon Department of Agriculture; See https://www.oregon.gov/ODA/shared/Documents/Publications/PesticidesPARC/AdvisoryPermanentNeoNicRule.pdf. [Google Scholar]
- 39.Illies I, Mühlen W. 2007. The foraging behaviour of honeybees and bumblebees on late blooming lime trees (Tilia spec) (Hymenoptera: Apidae). Entomol. Gen. 30, 155–165. ( 10.1127/entom.gen/30/2007/155) [DOI] [Google Scholar]
- 40.Surholt B, Denker B, Baal T, Mühlen W. 1992. Ist Silberlindennektar für Hummeln giftig? Ein Video-Protokoll von Freilandexperimenten. Apidologie 23, 335–337. [Google Scholar]
- 41.Naef R, Jaquier A, Velluz A, Bachofen B. 2004. From the linden flower to linden honey-volatile constituents of linden nectar, the extract of bee-stomach and ripe honey. Chem. Biodivers. 1, 1870–1879. ( 10.1002/cbdv.200490143) [DOI] [PubMed] [Google Scholar]
- 42.Couvillon MJ, Al Toufailia H, Butterfield TM, Schrell F, Ratnieks FLW, Schürch R. 2015. Caffeinated forage tricks honeybees into increasing foraging and recruitment behaviors. Curr. Biol. 25, 2815–2818. ( 10.1016/j.cub.2015.08.052) [DOI] [PubMed] [Google Scholar]
- 43.Wright G, Baker D, Palmer M, Stabler D, Mustard JA, Power EF, Borland AM, Stevenson PC. 2013. Caffeine in floral nectar enhances a pollinator's memory of reward. Science 339, 1202–1204. ( 10.1126/science.1228806) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thomson JD, Draguleasa MA, Tan MG. 2015. Flowers with caffeinated nectar receive more pollination. Arthropod Plant Interact. 9, 1–7. ( 10.1007/s11829-014-9350-z) [DOI] [Google Scholar]
- 45.Farré-Armengol G, Filella I, Llusià J, Peñuelas J. 2015. Pollination mode determines floral scent. Biochem. Syst. Ecol. 61, 44–53. ( 10.1016/j.bse.2015.05.007) [DOI] [Google Scholar]
- 46.Buchbauer G, Remberg B, Jirovetz L, Nikiforov A. 1995. Comparative headspace analysis of living and fresh cut lime tree flowers (Tiliae flores). Flavour Frag. J. 10, 221–224. ( 10.1002/ffj.2730100316) [DOI] [Google Scholar]
- 47.Knudsen JT, Eriksson R, Gershenzon J, Ståhl B. 2006. Diversity and distribution of floral scent. Bot. Rev. 72, 1–120. ( 10.1663/0006-8101(2006)72[1:DADOFS]2.0.CO;2) [DOI] [Google Scholar]
- 48.von Frisch K. 1928. Versuche über den Geschmackssinn der Bienen. Naturwissenschaften 16, 307–315. ( 10.1007/BF01501569) [DOI] [Google Scholar]
- 49.Staudenmayer T. 1939. Die Giftigkeit der Mannose für Bienen und andere Insekten. J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 26, 644–668. ( 10.1007/BF00341096) [DOI] [Google Scholar]
- 50.Sols A, Cadenas E, Alvarado F. 1960. Enzymatic basis of mannose toxicity in honey bees. Science 131, 297–298. ( 10.1126/science.131.3396.297) [DOI] [PubMed] [Google Scholar]
- 51.Sweeley CC, Bentley R, Makita M, Wells WW. 1963. Gas-liquid chromatography of trimethylsilyl derivatives of sugars and related substances. J. Am. Chem. Soc. 85, 2497–2507. ( 10.1021/ja00899a032) [DOI] [Google Scholar]
- 52.Vogel S. 1978. Floral ecology. In Progress in botany/Fortschritte der Botanik (eds Behnke H-D, Lüttge U, Esser K, Kadereit JW, Runge M), pp. 453–481. Berlin, Germany: Springer. [Google Scholar]
- 53.Baker HG, Baker I. 1983. A brief historical review of the chemistry of floral nectar. In The biology of nectaries (eds Bentley B, Elias T), pp. 126–152. New York, NY: Columbia University Press. [Google Scholar]
- 54.Harborne JB. 1993. Introduction to ecological biochemistry. London, UK: Academic Press. [Google Scholar]
- 55.Roulston TH, Cane JH. 2000. Pollen nutritional content and digestibility for animals. In Pollen and pollination (eds Dafni A, Hesse M, Pacini E), pp. 187–209. Vienna, Austria: Springer. [Google Scholar]
- 56.Adler LS. 2000. The ecological significance of toxic nectar. Oikos 91, 409–420. ( 10.1034/j.1600-0706.2000.910301.x) [DOI] [Google Scholar]
- 57.Nicolson SW, Thornburg RW. 2007. Nectar chemistry. In Nectaries and nectar (eds Nicolson SW, Nepi M, Pacini E), pp. 215–264. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 58.Abrol DP. 2012. Biochemical basis of plant-pollination interaction. In Pollination biology (ed. Abrol DP.), pp. 413–458. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 59.London-Shafir I, Shafir S, Eisikowitch D. 2003. Amygdalin in almond nectar and pollen-facts and possible roles. Plant Syst. Evol. 19, 87–95. ( 10.1007/s00606-003-0272-y) [DOI] [Google Scholar]
- 60.Martinson VG, Magoc T, Koch H, Salzberg SL, Moran NA. 2014. Genomic features of a bumble bee symbiont reflect its host environment. Appl. Environ. Microbiol. 80, 3793–3803. ( 10.1128/AEM.00322-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Conlon J. 2014. From where I sit: notes from the AMCA technical advisor. Wing Beats 25, 40–42. [Google Scholar]
- 62.Frérot E, Velluz A, Decorzant E, Naef R. 2006. From linden flower to linden honey. Part 2. Chem. Biodivers. 3, 94–100. ( 10.1002/cbdv.200690012) [DOI] [PubMed] [Google Scholar]
- 63.Von der Ohe W, Pechhacker H, Von der Ohe K, Käferböck K. 1993. Chemismus und Pollenrepräsentanz der Lindentracht. Apidologie 24, 478–479. [Google Scholar]
- 64.Gašić U, Šikoparija B, Tosti T, Trifković J, Milojković-Opsenica D, Natić M, Tešić Ž. 2014. Phytochemical fingerprints of lime honey collected in Serbia. J. AOAC Int. 97, 1259–1267. ( 10.5740/jaoacint.SGEGasic) [DOI] [PubMed] [Google Scholar]
- 65.Corbet SA, Unwin DM, Prŷs-Jones OE. 1979. Humidity, nectar and insect visits to flowers, with special reference to Crataegus, Tilia and Echium. Ecol. Entomol. 4, 9–22. ( 10.1111/j.1365-2311.1979.tb00557.x) [DOI] [Google Scholar]
- 66.Melville R. 1949. The limes as amenity trees and bee pasturage. Kew Bull. 4.2, 147–152. ( 10.2307/4113669) [DOI] [Google Scholar]
- 67.Goulson D. 2013. An overview of the environmental risks posed by neonicotinoid insecticides. J. Appl. Ecol. 50, 977–987. ( 10.1111/1365-2664.12111) [DOI] [Google Scholar]
- 68.Dreisig H. 1995. Ideal free distributions of nectar foraging bumblebees. Oikos 72, 161–172. ( 10.2307/3546218) [DOI] [Google Scholar]
- 69.Goulson D. 1999. Foraging strategies of insects for gathering nectar and pollen, and implications for plant ecology and evolution. Perspect. Plant Ecol. Evol. Syst. 2, 185–209. ( 10.1078/1433-8319-00070) [DOI] [Google Scholar]
- 70.Schiestl FP, Ayasse M, Paulus HF, Löfstedt C, Hansson BS, Ibarra F, Francke W. 1999. Orchid pollination by sexual swindle. Nature 399, 421–422. ( 10.1038/20829) [DOI] [Google Scholar]
- 71.Granero A, Guerra Sanz J, Egea Gonzalez F, Vidal JLM, Dornhaus A, Ghani J, Serrano AR, Chittka L. 2005. Chemical compounds of the foraging recruitment pheromone in bumblebees. Naturwissenschaften 92, 371–374. ( 10.1007/s00114-005-0002-0) [DOI] [PubMed] [Google Scholar]
- 72.Mathon C, Edder P, Christen P, Bieri S. 2014. Unexpected occurrence of caffeine in sleep-inducing herbal teas. Chimia 68, 705–709. ( 10.2533/chimia.2014.705) [DOI] [PubMed] [Google Scholar]
- 73.T'ai HR, Cane JH. 2000. Pollen nutritional content and digestibility for animals. In Pollen and pollination (eds Dafni A, Hesse M, Pacini E), pp. 187–209. Vienna, Austria: Springer. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.