Abstract
Rationale and objectives
Addiction to psychostimulant methamphetamine (METH) remains a major public health problem in the world. Animal models that use METH self-administration incorporate many features of human drug-taking behavior and are very helpful in elucidating mechanisms underlying METH addiction. These models are also helping to decipher the neurobiological substrates of associated neuropsychiatric complications. This review summarizes our work on the influence of METH self-administration on dopamine systems, transcriptional and immune responses in the brain.
Methods
We used the rat model of METH self-administration with extended access (15 hours/day for 8 consecutive days) to investigate the effects of voluntary METH intake on the markers of dopamine system integrity and changes in gene expression observed in the brain at 2 hours – 1 month after cessation of drug exposure.
Results
Extended access to METH self-administration caused changes in the rat brain that are consistent with clinical findings reported in neuroimaging and post-mortem studies of human METH addicts. In addition, gene expression studies using striatal tissues from METH self-administering rats revealed increased expression of genes involved in CREB signaling pathway and in the activation of neuroinflammatory response in the brain.
Conclusion
These data show an association of METH exposure with activation of neuroplastic and neuroinflammatory cascades in the brain. The neuroplastic changes may be involved in promoting METH addiction. Neuroinflammatory processes in the striatum may underlie cognitive deficits, depression, and parkinsonism reported in METH addicts. Therapeutic approaches that include suppression of neuroinflammation may be beneficial to addicted patients.
Keywords: Self-administration, Striatum, Cortex, Dopamine, Neurotoxicity, Gene expression, Neuroinflammation
Introduction
Methamphetamine (METH) is a highly addictive psychostimulant that is abused throughout the world. METH abuse is a serious public health problem because of its association with adverse neuropsychiatric consequences that include addiction, psychosis, and cognitive impairments in humans (Cadet and Bisagno 2013; Dean et al. 2013; Henry et al. 2010; Huckans et al. 2015; Panenka et al. 2013; Rusyniak 2013; Sadek et al. 2007; van Holst and Schilt 2011). Some of the cognitive abnormalities are thought to be related to potential METH-induced neurodegenerative changes (Cadet et al. 2014a; London et al. 2015; Scott et al. 2007). However, much more work is needed before firm conclusions can be made regarding cognitive deficits in METH-addicted individuals because some studies failed to confirm cognitive deficits in METH users (see Hart et al. 2012 for an extensive discussion). Nevertheless, findings from imaging and postmortem studies describing decreases in the density of striatal dopamine transporters (DAT), reductions in tyrosine hydroxylase (TH) levels, as well as decreases in the concentrations of dopamine (DA) in the brains of chronic METH abusers (Sekine et al. 2003; Volkow et al. 2001b, 2001c; Wilson et al. 1996) are consistent with drug-induced pathologies in the brains of these patients (Cadet et al. 2014a). In fact, clinical abnormalities in some patients might reflect damage to DA neurons and may be responsible for the appearance of parkinsonism in a subset of METH abusers (Callaghan et al. 2010, 2012; Curtin et al. 2015). Differences in cognitive findings may also reflect different levels of individual vulnerability, quantity of METH use, and patterns of drug administration. This conclusion is supported by recent studies showing that patterns of METH injections can lead to different impact on monoaminergic systems in the rodent brain (Cadet et al. 2009a, 2009b; Danaceau et al. 2007; Graham et al. 2008).
In the present paper, we will review some data on the effects of METH self-administration on dopaminergic systems in the brain. We will also describe transcriptional changes induced by METH self-administration that may be involved in the development of METH addiction. In addition, we will discuss how some of these transcriptional changes that impact neuroinflammatory processes may be involved in the biochemical cascades that produce secondary clinical syndromes including parkinsonism in human patients. The review will be based on data published by our laboratory and by other research groups that used brain tissues obtained from rats that self-administered METH under extended-access regimen. We chose to focus attention on the dorsal striatum because it is the area that is the most affected by METH toxic and/or plastic changes in the brain and because it is an integral part of the circuitry that regulates reward and habit forming, core elements of addiction (Belin et al. 2013; Everitt and Robbins 2013; Volkow and Morales 2015; Wise and Koob 2014).
The majority of studies that have reported on the effects of METH on brain monoaminergic systems have used non-contingent (experimenter-administered) drug treatment (Carvalho et al. 2012; Fleckenstein et al. 2007; Halpin et al. 2014; Krasnova and Cadet 2009; Moratalla et al. 2016). Non-contingent preclinical models are popular options to study mechanisms underlying METH effects in the brain because they reliably reproduce the changes in the markers of DA system integrity found in the brains of human METH addicts. Moreover, these models are methodologically easier, more affordable, and can be achieved faster than METH self-administration models. However, several factors limit the relevance of non-contingent models to compulsive and chronic METH use in humans. First, non-contingent METH models do not take into account primary reinforcing effects of METH, do not reproduce drug-taking behaviors, and do not allow studies of an animal’s dynamic changes in drug intake over time. Second, these models are also problematic because METH-induced deficits in the markers of DA systems are usually bigger than those reported in human imaging (Sekine et al. 2003; Volkow et al. 2001b, 2001c) and post-mortem (Wilson et al. 1996) studies. Third, because non-contingent preclinical models do not contain any behavioral parameters, they do not allow investigators to test if METH-induced toxic and/or neuroplastic changes might correlate with vulnerability to relapse to METH self-administration. In contrast, intravenous METH self-administration provides greater face validity with respect to patterns of human METH abuse by allowing to model drug-taking behaviors in humans and to evaluate primary rewarding effects of METH. Thus, several recent studies have examined the long-term effects of METH self-administration on dopaminergic, noradrenergic, serotoninergic, glutamatergic systems (Galinato et al. 2015; Krasnova et al. 2010, 2013; McFadden et al. 2012, 2014, 2015; Schwendt et al. 2009, 2012). METH self-administration has been reported to compromise cognitive functions (Recinto et al. 2012; Reichel et al. 2012; Rogers et al. 2008) and cortical electrophysiology (Parsegian et al. 2011). Withdrawal from METH self-administration is also associated with poor survival of hippocampal progenitor cells (Recinto et al. 2012). However, there is limited information on the role of transcriptional changes caused by METH self-administration in the development and/or maintenance of METH addiction. There is also limited information about the effects of drug withdrawal on various biochemical and molecular parameters in the brain. In what follows, we summarized studies that focused on the effects of METH self-administration on brain DA systems, transcriptional changes, and immune responses in the brain.
METH Intake and Changes in Body Weight
In the experiments reviewed here, we used an extended access to METH self-administration to investigate the effects of voluntary METH intake on dopaminergic systems in the brain (Krasnova et al. 2010, 2013). Rats were allowed to self-administer METH (0.1 mg/kg/infusion, i.v.) during daily 15-hour sessions for 8 consecutive days. Animals in a yoked control group received infusions of saline whenever rats in the test group self-administered METH. The rats were euthanized at 2 hours, 24 hours, 7 days, 14 days and 1 month after cessation of drug exposure. More details of the extended-access self-administration model can be found in two papers on the subject (Krasnova et al. 2010, 2013). Detailed experimental protocols for tissue collection, monoamine and protein extraction, HPLC analysis and Western blot can also be found in our publications on this subject (Cadet et al. 2009a; Krasnova et al. 2007, 2010, 2013).
As reported by Krasnova et al. (2010), the average METH intake in rats during 15-hour daily sessions gradually increased throughout the course of self-administration starting by the third session (Fig. 1A). This increase in comparison to the first and second sessions continued throughout the eighth session. Rats further escalated their drug intake during sixth session when compared with the third session. Rats maintained METH self-administration at this level during seventh and eighth sessions (Fig. 1A). The average daily drug intake reached 16.2±1.1 mg/kg/day, and the total cumulative METH intake over 8 sessions was 89.8±7.4 mg/kg. The escalation of METH intake obtained in our study is consistent with other reports showing increase in drug intake by rats exposed to extended self-administration sessions (Astarita et al. 2015; Galinato et al. 2015; Kitamura et al. 2006; Li et al. 2015; Schwendt et al. 2009). The escalation of METH intake by rats over the course of self-administration is similar to increase of drug consumption over time in human abusers and resembles a transition from controlled (limited) to uncontrolled (binge and run) METH use that is typical of human addicts during the development of METH dependence (Cho and Melega, 2002; Kramer et al., 1967; Simon et al. 2002). The effect of METH self-administration on body weight in rats is shown in Fig. 1B. There were significant decreases in body weights in the group of METH self-administering rats, with the animals losing about 16% of their weights by the eighth self-administration session. The yoked saline group maintained stable weights throughout the experiment. The differences in body weights between the two groups were significant starting at the fourth session. The observed weight loss in the METH self-administration group is consistent with reports of marked anorexia and substantial weight loss in human METH abusers (Albertson et al. 1999; Kramer et al. 1967; Neale et al. 2009).
Fig. 1.
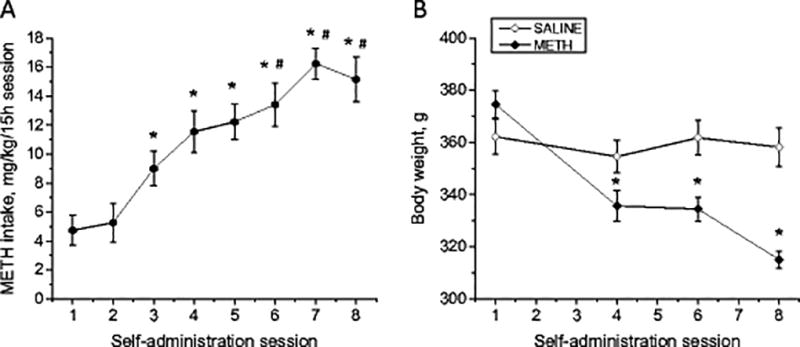
Changes in METH intake and body weight over the course of METH self-administration in rats. (A) Increase of METH intake in rats. Symbols show average METH intake during 8 consecutive self-administration sessions (means ± SEM; n = 11). Rats increased METH intake on sessions 3–8 compared with sessions 1 and 2. Animals further increased their drug intake on session 6 in comparison to session 3 and stayed at that level until cessation of METH self-administration. Data were analyzed by one-way ANOVA for repeated measures, followed by Tukey’s multiple comparison tests: * p<0.05 in comparison with sessions 1 and 2, # p<0.05 in comparison with session 3. (B) Decreases in body weight in animals that self-administered METH (means ± SEM; n = 6–9). Data analyzed by two-way ANOVA for repeated measures, followed by Tukey’s multiple comparison test: * p<0.05 in comparison to saline group. Figure adapted from Krasnova et al. 2010.
METH-Induced Changes in the Markers of Dopamine System Integrity in the Rat Brain
Postmortem analyses of brain tissues obtained from METH addicts revealed significant decreases in DA concentrations in the caudate-putamen (Wilson et al. 1996). To investigate whether METH self-administration causes similar changes in dopaminergic terminals in our rat model, we measured the levels of DA and its metabolites in the striatum. Indeed, METH caused significant decreases in DA levels in the striatum of animals euthanized at 2 hours, 24 hours, at 7, 14 days and at 1 month after cessation of drug self-administration (Fig. 2A). 3,4-dihydroxyindoleacetic acid (DOPAC) levels were also significantly reduced by METH at 2 hours, 24 hours and 7 days, while homovanillic acid (HVA) levels were significantly decreased only at the 7-day time point (Fig. 2A). In addition to post-mortem evidence of decreased DA levels, neuroimaging studies have also found significant reduction in the levels of DAT in the striatum of human METH users (Sekine et al. 2003; Volkow et al. 2001b, 2001c). Post-mortem studies also showed decreases in DAT and TH protein levels in the brains of chronic METH abusers (Wilson et al. 1996). Therefore, we measured the expression of these proteins in the striatum and found that METH caused significant decreases in TH and DAT levels in animals euthanized at 14 days after cessation of drug self-administration (Fig. 2B, C). Because METH addiction is also associated with reductions in D2 DA receptors in the human caudate (Volkow et al. 2001a; Wang et al. 2012), we examined the possibility that similar changes might occur in the dorsal striatum of rats exposed to extended access to METH self-administration. As shown in Figs. 2D, E, we found transient increases in D1 DA receptor protein levels at 2 h that returned to control 24 h after cessation of drug exposure. In contrast, D2 DA receptor protein levels were decreased after 1 month of withdrawal from METH self-administration (Fig. 2D, E), similar to observations in human brains (Volkow et al. 2001a; Wang et al. 2012). Clinical fMRI studies have also reported potential reactive astrogliosis in the brains of METH abusers (Bae et al. 2006; Thompson et al. 2004). Because preclinical studies using non-contingent METH administration detected increased astrogliosis in the brain (Krasnova and Cadet 2009), we also measured the levels of glial fibrillary acidic protein (GFAP), a marker of reactive astrogliosis, in the striatum of animals euthanized at 7 days after cessation of METH self-administration. In agreement with clinical and previous preclinical findings, rats exposed to METH self-administration also showed increased GFAP levels in the striatum (Fig. 2F, G).
Fig. 2.
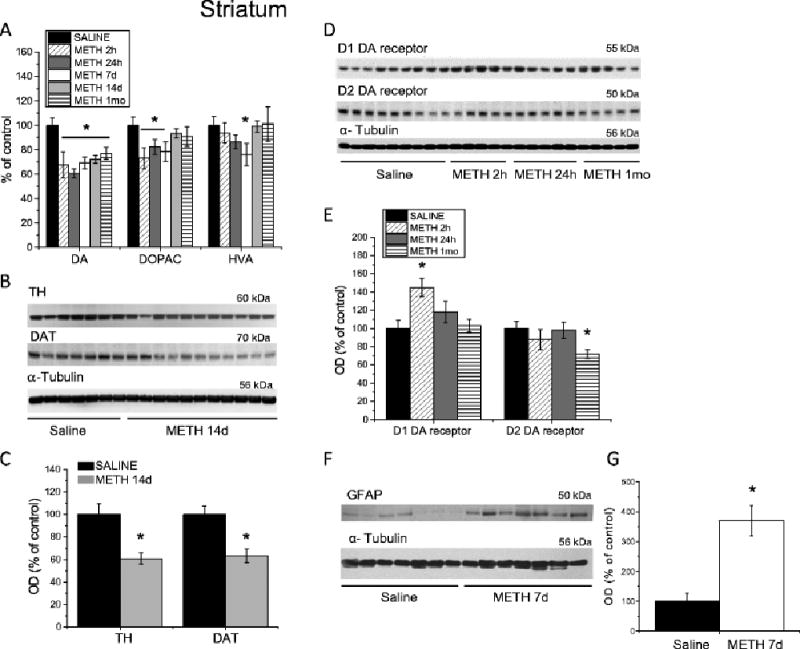
Changes in the markers of DA system integrity and GFAP in the dorsal striatum following METH self-administration. Rats self-administered METH for eight 15-h sessions and were euthanized at 2 h, 24 h, 7 days, 14 days or 1 month withdrawal. (A) METH caused significant decreases in striatal DA levels up to 1 month post-drug. DOPAC concentrations were decreased at 2 h, 24 h, and 7 days, but returned to control levels after 14 days of withdrawal. HVA levels were transiently decreased at 7 days after cessation of METH self-administration, but normalized at 14 days post-drug. (B) Representative immunoblots showing TH and DAT protein levels in the striatum at 14 days after METH withdrawal. (C) Quantitative analyses demonstrated reductions in DAT and TH protein levels in the striatum of METH-treated rats. (D) Representative immunoblots of D1 and D2 DA receptor protein levels. (E) METH self-administration caused increases in D1 DA receptor protein levels at 2 hours post-drug; D2 DA receptor protein levels were decreased after 1 month withdrawal. (F) A representative immunoblot demonstrating GFAP levels in rat striatum 7 days following cessation of METH self-administration. (G) Quantitative analyses of the Western blots show increases in GFAP levels in the METH-treated rats. N = 7–11 per group. Data are expressed as percent (protein optical density, monoamine levels) of mean ± SEM values of saline group. Data were analyzed by ANOVA followed by PLSD. * p < 0.05, significantly different from saline group. Figure adapted from Krasnova et al. 2010, 2013.
Because METH is also known to influence markers of cortical monoaminergic systems (Johnson-Davis et al. 2003; Krasnova et al. 2009; Son et al. 2013), we examined the effects of METH self-administration in the cortex. In contrast to the acute effects of METH in the striatum, METH self-administration caused more gradual decreases in DA levels in the rat frontal cortex that reached significance after 14 days withdrawal (Fig. 3A). DOPAC and HVA concentrations were not affected (Fig. 3A). Next, we measured TH and DAT protein levels in the cortices of animals euthanized 14 days after cessation of METH exposure. Similar to the striatum, METH self-administration caused significant decreases in cortical TH and DAT protein levels (Fig. 3B, C). We also found that METH induced significant increases in GFAP levels in the cortex 7 days after cessation of drug exposure (Fig. 3D and E). The main findings of our study and other reports showing effects of extended access to METH self-administration on the markers of DA system integrity in the brain are summarized in Table 1.
Fig. 3.
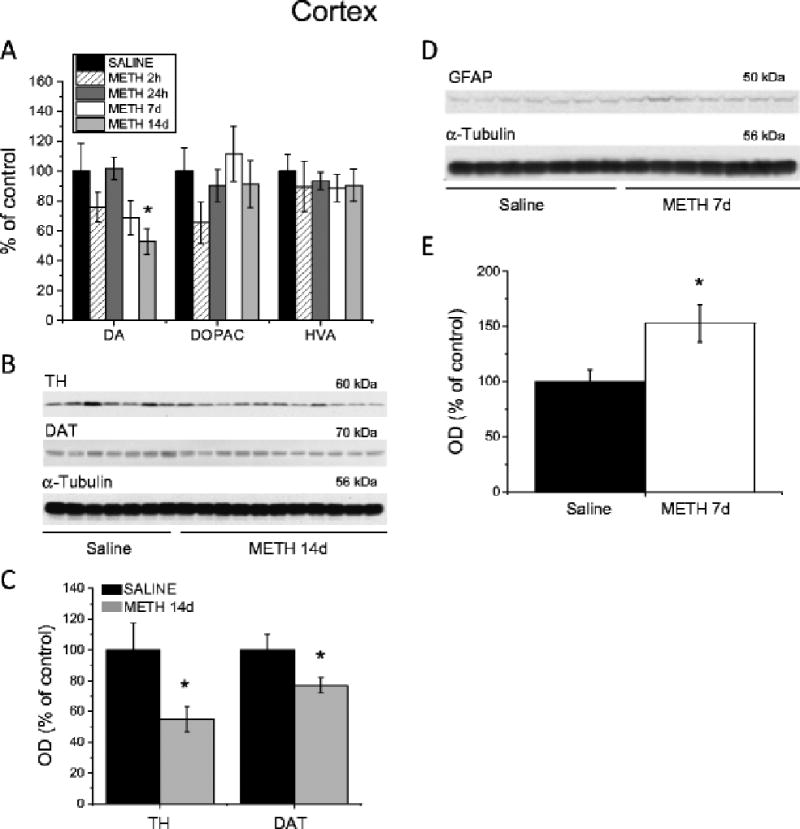
Effects of METH self-administration on the markers of dopamine system integrity and GFAP in the cortex. (A) METH self-administration resulted in long-term reductions in DA concentrations in the cortex. (B) Representative immunoblots of TH and DAT protein expression in the cortex 14 days after cessation of METH exposure. (C) Quantification of METH effects showed decreases in TH and DAT protein levels in the cortex. (D) A representative immunoblot showing GFAP expression in the cortex 7 days post-drug. (E) Quantitative analyses showed increases in GFAP levels in the METH self-administering rats. Data are shown as percent (protein optical density, monoamine levels) of mean ± SEM values of saline group. Data were analyzed by ANOVA followed by PLSD. * p < 0.05, significantly different from saline group. Figure adapted from Krasnova et al. 2010.
Table 1.
Long-term effects of METH self-administration on the markers of DA systems in the brain.
| METH exposure regimen |
METH intake |
Time-points studied |
Brain area | Main findings | Reference |
|---|---|---|---|---|---|
| 15 hours/day, 8 days | up to 14.5 mg/kg/day | 24 hours | Striatum | ↓DA, ↓DOPAC levels | Krasnova et al., 2010 |
| 7 days | Striatum | ↓DA, ↓DOPAC, ↓HVA levels | |||
| ↑GFAP protein levels | |||||
| Cortex | ↑GFAP protein levels | ||||
| 14 days | Striatum | ↓DA levels | |||
| ↓TH, ↓DAT protein levels | |||||
| Cortex | ↓DA levels | ||||
| ↓TH, ↓DAT protein levels | |||||
| 15 hours/day, 8 days | up to 14.0 mg/kg/day | 2 hours | Striatum | ↓DA, ↓DOPAC levels | Krasnova et al., 2013 |
| ↑D1 DAR protein levels | |||||
| 24 hours | Striatum | ↓DA, ↓DOPAC levels | |||
| 1 month | Striatum | ↓DA levels | |||
| ↓D2 DAR protein levels | |||||
| 8 hours/day, 7 days | up to 10 mg/kg/day | 8 days | Striatum | ↓DAT uptake | McFadden et al. 2012 |
| ↓DAT immunoreactivity | |||||
| 30 days | Striatum | ↓DAT uptake | |||
| 1 hour/day, 7–10 days + 6 hours/day, 12–14 day | up to 7 mg/kg/day | 14 days | Striatum | ↓DAT protein levels | Schwendt et al. 2009 |
| Prefrontal cortex | ↓DAT protein levels |
Taken together, the behavioral and biochemical effects obtained in our rat METH self-administration model with extended access to the drug are reminiscent of observations in some human METH addicts. We have reasoned that the development of drug addiction might be related to structural and functional changes in the brain caused by epigenetically induced changes in gene expression. Therefore, we sought to determine if METH self-administering rats show changes in the expression of genes known to be implicated in synaptic plasticity and cognitive functions.
Transcriptional Changes in the Striatum Caused by METH Self-Administration
The transition from occasional use of psychostimulants to psychostimulant dependence may involve a shift of control over drug intake from the ventral to dorsal striatum when the use of drugs becomes habitual and compulsive (Everitt and Robbins 2013). This transition to addictive behaviors appears to depend on transcriptional and neuroplastic changes in the brain (Nestler 2013; Volkow and Morales 2015). Similarly, several studies have reported that METH can significantly influence the expression of many genes in the dorsal striatum after both acute and chronic administration of the drug (Cadet et al. 2009b; Kodama et al. 1998; Krasnova et al. 2011; McCoy et al. 2011; Wang et al. 1995). Although these studies have shown that administration of METH might be associated with transcriptional changes, much remains to be done to improve our knowledge about the molecular mechanisms underlying METH addiction. We and others have proposed that METH-induced transcriptional and neuroplastic changes might underlie altered cellular and synaptic functions and behavioral responses to the drug (Aguilar-Valles et al. 2014; Jayanthi et al. 2014; Krasnova et al. 2013). Together, these changes in gene expression and associated changes in protein levels might cause cognitive deficits found in some METH addicts (Cadet and Bisagno 2013); see Fig. 4 for a hypothetical scheme).
Fig. 4.
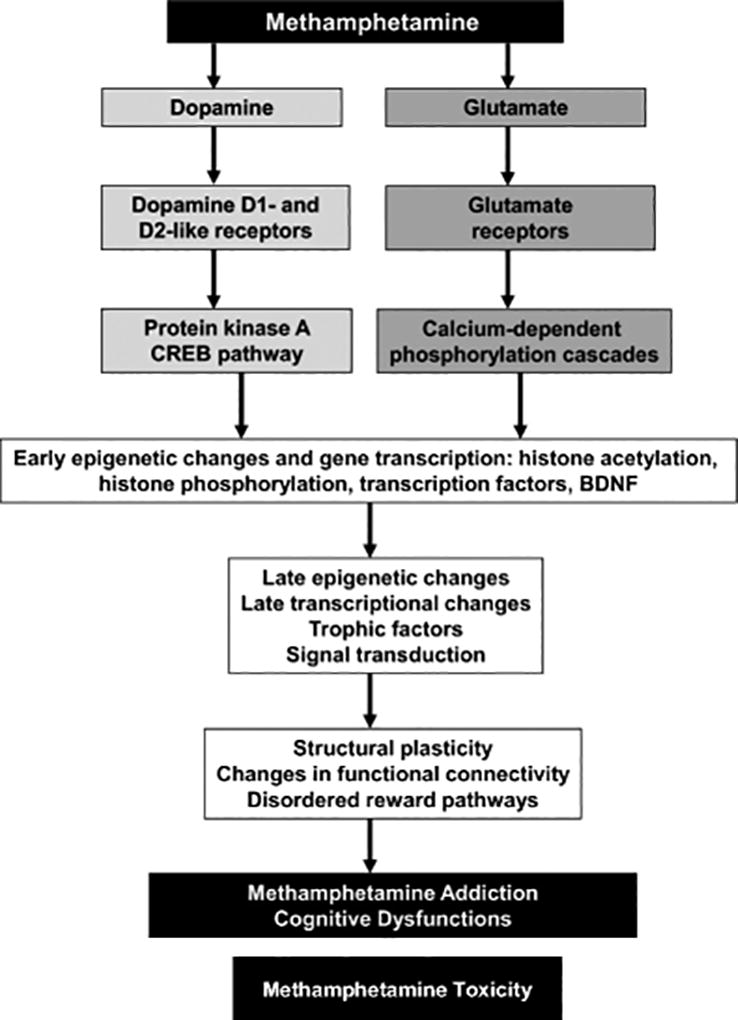
Transcriptional and epigenetic mechanisms are involved in METH addiction and toxicity. Biochemical and behavioral effects of METH include activation of dopaminergic and glutamatergic pathways together with other neurotransmitter systems that might participate in the development of addiction and toxicity. Activation of these neurotransmitter systems is followed by activation or inhibition of transcriptional and epigenetic events that underlie compulsive abuse of the drug. These behaviors might be secondary to subcortical hyperconnection syndrome caused by cortical disinhibition and specific cognitive changes in human METH addicts.
Many previous studies of transcriptional changes caused by with METH exposure have focused on the effects of non-contingent METH treatment on gene expression in various brain regions (Cadet et al. 2009b, 2014b; Jayanthi et al. 2014; Kodama et al. 1998; Krasnova et al. 2011; McCoy et al. 2011; Thomas et al. 2004a; Wang et al. 1995). However, very few studies have been conducted on transcriptional effects of METH self-administration (Bosch et al. 2015; Krasnova et al. 2013; Li et al. 2015). In the experiments reviewed here, we studied global gene expression in striatal tissues using Illumina 22K Rat microarrays. Experimental protocols for tissue collection, RNA extraction, and performance of microarray analyses can be found in our previous publications on this subject (Krasnova et al. 2008, 2013) and will not be described here. As reported by Krasnova et al. (2013), we found that 543 and 266 transcripts were differentially expressed in the striatum 2 and 24 hours after cessation of METH exposure (1.7-fold change versus control, p < 0.05). Using similar criteria, our laboratory was able to replicate microarray expression data from dorsal striatum with RT-PCR (Cadet et al. 2009b; Krasnova et al. 2008). The findings that the drug causes changes in the expression of a large number of transcripts are consistent with various clinical manifestations of METH-addicted patients (Dean et al. 2013; Rusyniak 2013; van Holst and Schilt 2011). These clinical symptoms include deficits in executive and memory functions, depression, and psychosis (Rusyniak 2013; van Holst and Schilt 2011). In addition, clinical studies have shown that METH addicts are at higher risk to develop parkinsonism later in life (Callaghan et al. 2010, 2012; Curtin et al. 2015).
For the microarray data described here, Krasnova et al. (2013) used RT-PCR to validate METH-induced changes in the expression of several transcription factors, neuropeptides, and plasticity-related genes (Table 2, Fig. 5). We analyzed the genes differently expressed in the striatum at 2 and 24 hours after the cessation of METH self-administration for networks and molecular functions using Ingenuity Pathways Analysis (Ingenuity Systems). We found that METH can regulate many biological processes in the dorsal striatum. Specifically, METH caused upregulation of transcripts that play roles in cell-to-cell signaling and interaction (Fig. 6). It is of interest, that several genes, which participate in cell-to-cell signaling and in CREB signaling, including GABA B receptor 2 (GABBR2), G protein-coupled inward rectifying potassium channel subunit 2 (GIRK2) gene, KCNJ6, and hypocretin receptor 1 (HCRTR1) were upregulated by the drug (Fig. 6). Accumulated evidence suggests that acquisition of addictive behaviors involves persistent changes in synaptic strength within reward circuits and changes in DA neuron signaling (Nestler 2013, Volkow and Morales 2015). Therefore, early drug-induced neuroadaptations play critical role in remodeling of the reward circuits in the brain and facilitate the development of addiction. GABBR2 and GIRK2, in particular, may participate in mechanisms underlying drug-induced synaptic plasticity, because activation of GABBR2 and GIRKs mediate DA neuronal excitability (Cruz et al. 2004; Labouebe et al. 2007). Exposure to psychostimulants leads to increased DA neuron excitability (Henry et al. 1989; White and Wang 1984), implicating GABBR and GIRK2 channels in the response to addictive drugs (Luscher and Slesinger 2010). Indeed, chronic AMPH exposure enhances GABBR transmission in the brain during early withdrawal (Giorgetti et al. 2002). Moreover, GIRK channels may also play role in drug taking behavior, because mice lacking GIRK channels self-administer less cocaine (Morgan et al. 2003) and show reduced withdrawal symptoms after chronic exposure to morphine (Cruz et al. 2008). Moreover, GIRK2 mRNA levels are increased in the cortex of human cocaine addicts (Lehrmann et al. 2003).
Table 2.
Partial list of transcripts changed after METH self-administration in the striatum.
| Transcript name | Symbol | Expression ratio METH/Saline
|
|
|---|---|---|---|
| 2h | 24h | ||
| Brain derived neurotrophic factor | Bdnf | 3.28 | 1.33 |
| cAMP responsive element modulator | Crem | 1.72 | 1.02 |
| FBJ murine osteosarcoma viral oncogene homolog | C-fos | 1.87 | 1.29 |
| FBJ osteosarcoma oncogene B | Fosb | 1.72 | 1.27 |
| Follistatin | Fst | 2.20 | 1.24 |
| Jun oncogene | C-jun | 1.84 | 1.06 |
| Nerve growth factor, gamma | Ngfg | −2.31 | 2.22 |
| Neuropeptide Y | Npy | 1.74 | 1.31 |
| Neurotensin | Nts | 3.08 | 1.05 |
| Prodynorphin | Pdyn | 1.79 | 1.26 |
| Syntaxin 1A | Stx1a | 2.30 | −1.02 |
| Synapsin II | Syn2 | 2.12 | −1.11 |
Fig. 5.
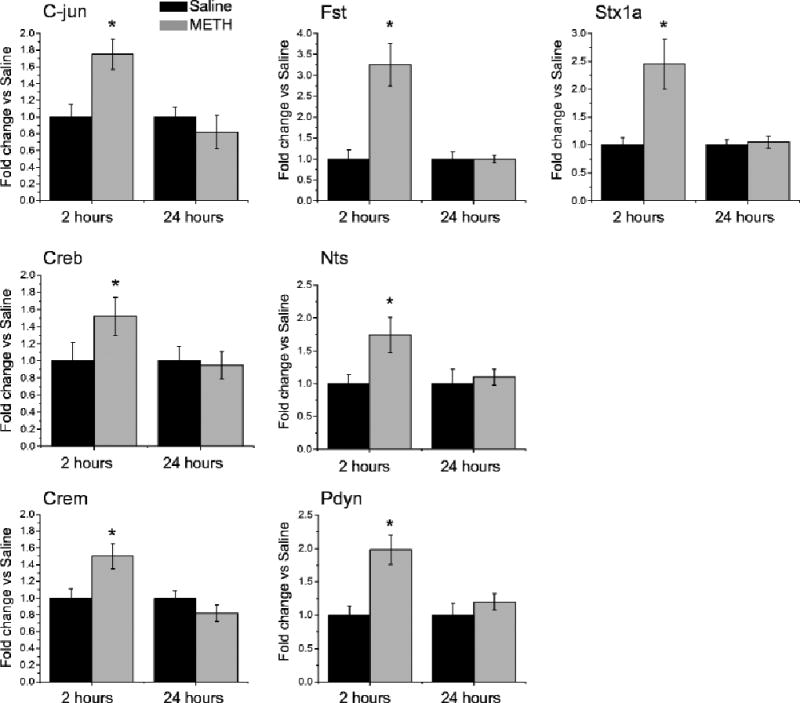
Validation of METH-induced expression of genes identified by microarray experiments using RT-PCR. Values represent means ± SEM in comparison to saline group. Consistent with microarray data, METH self-administration induced increases in the expression of C-jun and Crem transcription factors, Fst, Nts and Pdyn neuropeptides and Stx1A involved in the synaptic functions at 2 h, which were normalized at 24 h after cessation of drug exposure. N = 6 per group. * p < 0.05 in comparison to saline group (Student’s t-test). Figure adapted from Krasnova et al. 2013.
Fig. 6.
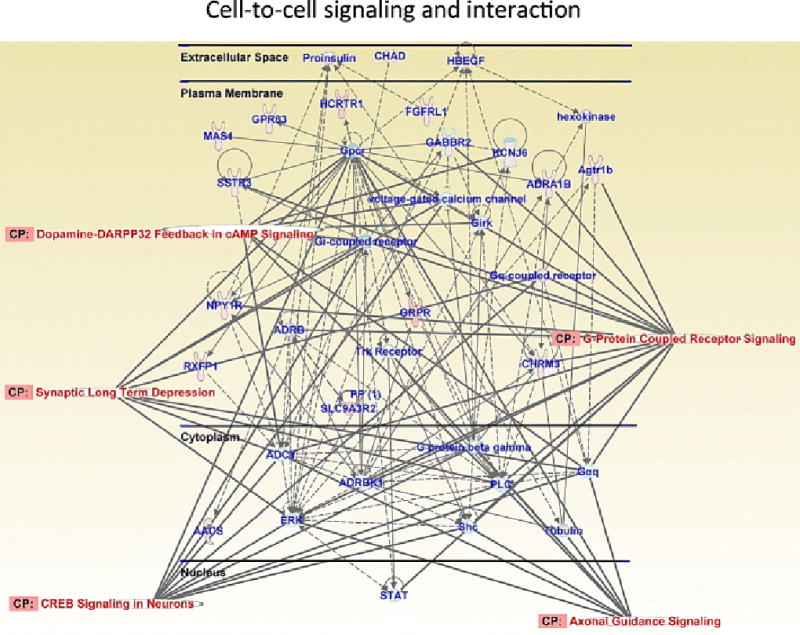
METH self-administration causes differential expression of genes involved in cell-to-cell signaling and interaction at 2 hours after cessation of drug exposure. This network includes genes involved in G-protein coupled receptor signaling, dopamine-DARPP32 signaling, axonal guidance signaling, synaptic long-term depression and CREB signaling. Figure adapted from Cadet et al. 2015; Krasnova et al. 2013.
In addition to DA and GABBR-GIRK signaling, other neurotransmitters may play role in the METH-induced neuroplastic and behavioral effects. Specifically, hypocretin and hypocretin receptor, HCRTR1 that is also upregulated by METH self-administration in the striatum (Fig. 6) may mediate the response to METH. Hypocretin-1 and hypocretin-2 are hypothalamic neuropeptides discovered in the late 1990s (de Lecea et al. 1998; Sakurai et al. 1998). Hypocretin receptors, HCRTR1 and HCRTR2, are located in numerous brain areas implicated in drug reinforcement circuits outside of the hypothalamus (Nambu et al. 1999; Peyron et al. 1998). Hypocretin transmission appears to be a key signaling mechanism involved in relapse to drug seeking after periods of abstinence (Aston-Jones et al. 2009; Borgland et al. 2010; Harris et al. 2005). HCRTR1, in particular, may play an important role in behavioral aspects of addiction because HCRTR1 antagonist has been shown to decrease cocaine self-administration in rats (Hollander et al. 2012; Prince et al. 2015) and to attenuate reinstatement of cocaine-seeking behavior (Bentzley and Aston-Jones 2015; Boutrel et al. 2005; Mahler et al. 2013). Importantly, HCRTR1 knockout mice self-administer less cocaine than wild type animals (Hollander et al. 2012). Therefore, the upregulation of HCRTR1 expression in our METH self-administration model suggests that this receptor may also play role in METH taking behavior and relapse to METH seeking. HCRTRs, in particular, may trigger CREB signaling pathway, because activation of these receptors causes PKC-mediated phosphorylation of ERK and CREB (Guo and Feng 2012) (Fig. 6). ERK is a kinase that plays a key role in the regulating neural and behavioral processes mediated by DA and glutamate pathways (Shiflett and Balleine 2011). Once activated, ERK causes CREB phosphorylation and enhances expression of c-Fos (Thomas and Huganir 2004; Valjent et al. 2005). The involvement of CREB signaling in METH addiction is supported by our finding of METH-induced increased CREB phosphorylation in the rat striatum (Krasnova et al. 2013). CREB is a transcription factor phosphorylated by different kinases including PKA and PKC (Johannessen and Moens 2007). CREB phosphorylation also promotes the recruitment of co-activators, such as CREB-binding protein (CBP)/p300, to the basal transcriptional machinery, a process that is followed by increased expression of CREB target genes such as Arc, c-fos, Egr1, Fosb and BDNF (Barco et al. 2005; Beaumont et al. 2012). In agreement with these observations, we found that METH self-administration caused increases in c-fos and BDNF expression in the striatum after 2 hours of withdrawal from the drug (Table 2, Fig. 5). These data are consistent with the report showing increases in c-Fos protein expression in the dorsal striatum and cortex after 3 weeks of METH self-administration at 2-h daily sessions (Cornish et al. 2012). This model is different from that used in our study in which rats had access to the drug for 15 hours/day, 8 consecutive days (Krasnova et al. 2013). However, in both models, METH likely caused these effects via stimulation of striatal DA receptors, followed by activation of different kinases, phosphorylation of CREB, and consequent CREB-mediated transcription (Cadet et al. 2010; Carlezon et al. 2005). This idea is supported by our findings that METH self-administration was accompanied by increased recruitment of phosphorylated CREB on the promoters of c-fos, fosB, and BDNF (Krasnova et al. 2013). In addition, these data show that c-fos, fosB, and BDNF genes might be co-regulated at both epigenetic and transcriptional levels and may work together to maintain neuroplastic changes that form substrates for METH addiction. The METH-induced increases in BDNF mRNA expression are accompanied by elevated BDNF protein levels at 2 hours post-drug (Krasnova et al. 2013). Our findings of increased BDNF expression are in agreement with data of McFadden et al. (2014) who also reported that METH self-administration caused elevated BDNF expression in the rat hippocampus. It is interesting that increases in C-fos and Bdnf mRNA levels appear to persist in c-Fos-positive striatal neurons and may be associated with drug relapse after long periods of withdrawal from METH self-administration (Li et al. 2015). Thus, METH self-administration might influence the expression of certain genes in various brain regions including the cortex, striatum, and hippocampus (Cornish et al. 2012; Krasnova et al. 2013; Li et al. 2015; McFadden et al. 2014). These results are also consistent with studies that reported increases in BDNF levels in the plasma of chronic METH users (Kim et al. 2005). Moreover, BDNF signaling may play an important role in producing plastic changes that lead to addiction (Russo et al. 2009) via mechanism that involves changes in the expression of synapsin and synaptophysin, which play roles in synaptic functions (Bykhovskaia 2011; Kwon and Chapman 2011). Our findings that METH does increase the expression of syntaxin 1A (Table 2, Fig. 5), synaptophysin and synapsins (Krasnova et al. 2013) provide further evidence that altered synaptic plasticity forms the core of METH addiction. Synapsins are a family of phosphoproteins that are located in presynaptic terminals (Greengard et al. 1993). They promote synaptogenesis and regulate vesicle dynamics and neurotransmitter release (Hilfiker et al. 1999; Kao et al. 2002) that depend on phosphorylation/ dephosphorylation events (Hosaka et al. 1999; Menegon et al. 2006). Thus, our findings of METH-induced changes in the expression of these synaptic proteins might be relevant to the report that repeated METH exposure causes changes in the density of dendritic spines on medium spiny neurons (Jedynak et al. 2007), that depend on activation of the BDNF-tyrosine kinase receptor, type 2 (TrkB) signaling pathway (Rauskolb et al. 2010). The data obtained in the studies of transcriptional changes caused by METH self-administration in the striatum are summarized in Table 3.
Table 3.
Effects of METH self-administration and withdrawal on transcriptional regulation in the rat striatum.
| METH exposure regimen |
METH intake |
Time-points studied |
Data obtained | Reference |
|---|---|---|---|---|
| 2 hours/day for 3 weeks | 4 ± 0.9 mg/kg/day | immediately after cessation of self-administration | ↑c-Fos immunoreactivity | Cornish et al. 2012 |
| ↑FosB/ΔFosB immunoreactivity | ||||
| 15 hours/day, 8 days | up to 14.0 mg/kg/day | 2 hours | ↑C-jun, ↑C-fos, ↑Fosb mRNA | Krasnova et al., 2013 |
| ↑Creb, ↑Crem mRNA | ||||
| ↑Stx1a, ↑Syn2, ↑Syp mRNA | ||||
| ↑Bdnf, ↓Ngfg mRNA | ||||
| ↑GABBR2, ↑KCNJ6, ↑HCRTR1 mRNA | ||||
| ↑ΔFosB, ↑pCREB protein levels | ||||
| 24 hours | ↑Ngfg mRNA, | |||
| ↑NFAT-mediated signaling | ||||
| ↑IL-6 signaling, ↑IL-10 signaling | ||||
| ↑ΔFosB, ↑pCREB protein levels | ||||
| 1 month | ↓cFos, ↓ΔFosB protein levels | |||
| ↓CREB protein, ↓pCREB protein | ||||
| 9 hours/day, 10 days | up to 7 mg/kg/day | 1 month | ↑Fos, ↑Egr1, ↑Arc mRNA | Li et al., 2015 |
| ↑Gria1, ↑Gria3, ↑Grin2a, ↑Grm1 mRNA | ||||
| ↑Bdnf, ↑Trkb mRNA | ||||
| ↑Hdac3, ↑Hdac4, ↑Hdac5, ↑Dnmt3a mRNA | ||||
| ↓Gria2 mRNA in Fos-positive striatal neurons |
METH-Induced Inflammatory Response the Brain
Another network upregulated by METH self-administration in the striatum includes genes that participate in inflammatory response, inflammatory disease and neurological disease (Fig. 7). Inflammatory response in the CNS is primarily regulated by microglia – resident immune cells that protect the brain against injury and damage (Graeber and Streit 2010; Streit et al. 2005). However, overactivation of microglial cells can result in release a variety of cytokines, reactive oxygen and nitrogen species that are known to cause neuronal damage (Beardsley and Hauser 2014). In particular, exposure to psychostimulants causes inflammatory response that may play a role in METH-induced neuronal injury (Beardsley and Hauser 2014). In our study, METH self-administration was associated with activation of nuclear factor of activated T-cells (NFAT)-mediated immune response (Fig.7). NFATs are transcription factors that regulate inflammatory responses in microglia by undergoing nuclear translocation in response to inflammatory stimulus (Nagamoto-Combs and Combs 2010). Previous study from our laboratory has shown that METH treatment caused shuttling of NFATs from cytosol to the nucleus followed by increases in the expression of Fas ligand (Jayanthi et al. 2005). Therefore, METH may also cause neurotoxic effects via activating NFAT/Fas ligand death cascade in the striatum (Jayanthi et al. 2005). Because NFATs are also known to be involved in the regulation of cytokine expression (Rao et al. 1997), they may contribute to METH-induced neuronal damage by mediating increased cytokine expression in microglia. Indeed, in addition to NFAT, METH also caused activation interleukin-6 (IL-6) signaling in the striatum (Fig.7). Another recent study in rats (Astarita et al. 2015) that used extended access to METH self-administration linked the inflammatory changes with increased production of sphingolipid messenger ceramide in the dorsal striatum and peripheral tissues (e.g., skeletal muscle). In this study, inhibitors of ceramide formation prevented METH-induced acceleration of genetic programs that are engaged during chronic inflammation and cellular senesce that contribute to the rapid health decline in METH users.
Fig. 7.
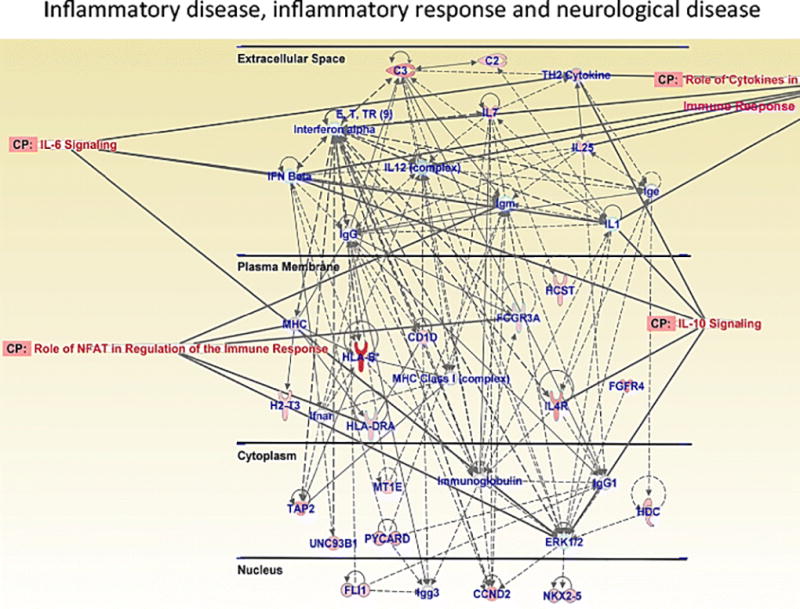
24 hour withdrawal from METH self-administration causes upregulated expression of genes involved in inflammatory response, inflammatory disease and neurological disease. This network includes genes involved in interleukin-6 signaling, interleukin-10 signaling and NFAT-regulated immune response. Figure adapted from Cadet et al. 2015; Krasnova et al. 2013.
Numerous studies have shown that METH exposure results in activation of microglia in the brain areas that show neuronal degeneration, including the dorsal striatum (Asanuma et al. 2004; Escubedo et al. 1998; Thomas and Kuhn 2005b; Thomas et al. 2004b). Microgliosis has been linked to neurodegeneration via pro-inflammatory mechanisms (Ehrlich et al. 1998; Gadient and Otten 1997; McGuire et al. 2001). Evidence has shown that microglia may play role in METH-induced neurotoxicity, including reductions of striatal DA levels and damage to striatal DA terminals (Thomas and Kuhn 2005a). In addition, reserpine and clorgyline that exacerbate METH toxicity also cause further increases in METH-induced microglial activation in the striatum (Thomas et al. 2008). In contrast, attenuation of METH toxicity by NMDA receptor antagonist MK-801 and dextromethorphan is accompanied by reduction in microglial activation (Thomas and Kuhn 2005b). In addition, the anti-inflammatory drugs ketoprofen and indomethacin as well as the antibiotic, minocycline, can also protect against METH-induced microgliosis and neurotoxicity (Asanuma et al. 2004; Zhang et al. 2006). Furthermore, minocycline was protective against the deleterious effects of METH on DAT levels in monkeys (Hashimoto et al. 2007). Microglial cells might potentiate METH-related damage by releasing pro-inflammatory cytokines such as IL-1β (Loftis et al. 2011; Numachi et al. 2007), tumor necrosis factor α (TNF-α) and IL-6 (Goncalves et al. 2008, 2010) (Fig. 7). Consistent with these ideas, METH neurotoxicity and an increase in a marker of microglial activation PK11195 were attenuated in IL-6 knockout mice (Ladenheim et al. 2000).
It is important to note that METH-induced microgliosis has been shown to precede the development of pathological changes in striatal DA neurons (LaVoie et al. 2004), suggesting that microglial activation is involved in the development of METH-induced neurological changes and is not simply a reaction to neurodegeneration. In a human imaging study, a marker for activated microglia, PK1195 binding, was increased in abstinent METH users and correlated inversely with the duration of METH abstinence (Sekine et al. 2008). This microgliosis persisted in the brains of METH addicts even after two years of abstinence (Sekine et al. 2008), suggesting that microgliosis may participate in long-term neurological effects of the drug (Cadet and Bisagno 2014), including parkinsonism (Callaghan et al. 2012) that involves neuroinflammatory processes (Long-Smith et al. 2009). Further evidence for the participation of glial cells in the pathological substrate of METH addiction was provided by a recent imaging study showing that METH-dependent women exhibited severe reductions in glial tricarboxylic acid cycle rate (Sailasuta et al. 2010).
Several studies have also shown that, in addition to microglia, astrocytes can also display hypertrophy and proliferation in the brain after METH treatment (Granado et al. 2011; Raineri et al. 2012; Robson et al. 2014). Moreover, extended access to METH self-administration in our model caused reactive astrogliosis in the dorsal striatum and cortex, as shown by increased GFAP protein levels (Figs. 2F, 2G, 3D, 3E, also see Krasnova et al. 2010). Reactive astrocytes can secrete cytokines and chemokines (Ramesh et al. 2013) that participate in METH-induced neuroinflammatory responses (Borgmann and Ghorpade, 2015). The data showing evidence of METH-induced inflammatory response in the brain are summarized in Table 4.
Table 4.
METH-induced inflammatory response in the brain.
| Species, METH dose |
Time-points studied |
Brain area | Data obtained | Reference |
|---|---|---|---|---|
| BALB/c mice 4 mg/kg × 4 times, every 2 hours | 3 days | Striatum | Microgliosis shown by ↑CD11b immunoreactivity | Asanuma et al. 2004 |
| Sprague-Dawley rats 10 mg/kg × 4 times, every 2 hours | 72 hours | Striatum, hippocampus, cerebellum | Microgliosis shown by ↑peripheral benzodiazepine receptor (PBR) density | Escubedo et al. 1998 |
| C57BL/6J mice, 30 mg/kg, single i.p. injection | 30’ | Striatum | ↑IL-6 mRNA | Goncalves et al. 2008 |
| Hippocampus | ↑TNF-α mRNA | |||
| Frontal cortex | ↑IL-6, TNF-α mRNA | |||
| 1 hour | Striatum, | ↑IL-6 mRNA | ||
| Hippocampus | ↑IL-6 mRNA | |||
| Frontal cortex | ↑IL-6, TNF-α mRNA | |||
| 2 hours | Frontal cortex | ↑IL-6 mRNA | ||
| C57BL/6J mice, 30 mg/kg, single i.p. injection | 1, 24 hours | Hippocampus | Astrogliosis shown by ↑GFAP immunoreactivity, ↑GFAP protein levels Microgliosis shown by ↑CD11b immunoreactivity, ↑TNFR1 protein levels | Goncalves et al. 2010 |
| 7 days | Hippocampus | Microgliosis shown by ↑CD11b immunoreactivity, ↑TNF-α protein levels | ||
| Wild-type mice 5 mg/kg × 3 times every 3 hours | 1 day | Striatum | Astrogliosis shown by ↑GFAP immunoreactivity, Microgliosis shown by ↑Mac-1 and ↑Iba-1 immunoreactivity, ↑GFAP, ↑Iba-1, ↑TNF-α mRNA, ↑IL-15 immunoreactivity | Granado et al. 2011 |
| Sprague-Dawley rats METH exposure for 15 h/day, 8 days METH intake up to 14.5 mg/kg/day | 7 days | Striatum, Cortex | Astrogliosis shown by ↑GFAP protein levels | Krasnova et al. 2010 |
| C57BL/6J mice 10 mg/kg × 4 times, every 2 hours | 3, 14 days | Striatum | Microgliosis shown by ↑PBR density | Ladenheim et al. 2000 |
| Sprague-Dawley rats 15 mg/kg × 4 times, every 2 hours | 1–6 days | Striatum, parietal cortex, piriform cortex | Microgliosis shown by ↑OX42 immunoreactivity | LaVoie et al. 2004 |
| C57BL/6J mice 1 mg/kg, i.p. for 7 days | 72 hours | Striatum | ↑IL-2 mRNA | Loftis et al. 2011 |
| Hippocampus | ↑IL-1β, ↑IL-2, ↑IL-6 mRNA | |||
| Frontal cortex | ↓IL-1β mRNA | |||
| 3 weeks | Hippocampus | ↑IL-1β, ↑L-10 mRNA | ||
| C57BL/6J X 129Sv/J mice, 45 mg/kg, i.p single injection | 1 hour | Hypothalamus | ↑IL-1β mRNA | Numachi et al. 2007 |
| C57BL/6 mice 5 mg/kg × 4 times every 2 hours | 48 hours | Striatum | Astrogliosis shown by ↑GFAP immunoreactivity Microgliosis shown by ↑ILB-4 immunoreactivity | Raineri et al. 2012 |
| 6 days | Striatum | Astrogliosis shown by ↑GFAP immunoreactivity | ||
| Swiss Webster mice 5 mg/kg × 4 times every 2 hours | 12, 24 hours 72 hours | Striatum Striatum | ↑GFAP mRNA Astrogliosis shown by ↑GFAP immunoreactivity | Robson et al. 2014 |
| C57BL/6J mice 40 mg/kg single i.p. injection | 12 hours 24 hours 48–72 hours | Striatum | ↑IL-1α mRNA ↑GFAP mRNA Astrogliosis shown by ↑GFAP protein levels | Thomas et al. 2004a |
| C57BL/6J mice 10 mg/kg × 4 times, every 2 hours | 24–72 hours | Striatum | Microgliosis shown by ↑ILB4 immunoreactivity | Thomas et al. 2004b |
Emerging research has suggested that neuroinflammatory effects may play roles in the rewarding properties of addictive drugs, including METH, and potentially contribute to development of METH addiction (Cadet and Bisagno 2014). In particular, Narita et al. (2006) have shown that injection of astrocyte-conditioned medium, which contained monocyte chemoattractant protein-5 and TNF receptor 1, into the nucleus accumbens or cingulate cortex increased METH-induced conditioned place preference in mice. In contrast, pretreatment with the suppressor of immune responses, propentofylline, attenuated rewarding effects of METH (Narita et al. 2006). In addition to cytokines and chemokines, astrocytes may also secrete several growth factors, including glial cell derived neurotrophic factor (GDNF), that play neuroprotective roles in the brain (Rocha et al. 2012). Specifically, astrocyte-derived GDNF has been shown to provide trophic support for DA neurons and to inhibit microglial activation, thus reducing microglial phagocytic activity and the production of reactive oxygen species in the brain (Rocha et al. 2012). GDNF appears to also modulate the rewarding effects of METH because partial reduction of GDNF expression in GDNF heterozygous knockout mice was shown to potentiate METH self-administration and to increase vulnerability to drug- and cue-induced reinstatement of METH-seeking behavior (Yan et al. 2007). In contrast, overexpression of GDNF in the striatum reduced METH self-administration as well as cue- and drug-primed METH seeking behavior in mice (Yan et al. 2013). Together, these data raise the possibility that astrocytes may contribute to the synaptic plasticity underlying METH-induced rewarding effects and maintenance of METH addiction.
In a different set of studies, ibudilast, a pharmacological agent that reduces glial activation, was shown to decrease METH-induced locomotor activity and sensitization (Snider et al., 2012), to reduce METH self-administration (Snider et al. 2013), and to attenuate prime- and stress-induced reinstatement of METH seeking in rats (Beardsley et al. 2010). These behavioral effects of ibudilast against various components of METH addiction might be mediated via suppression of neuroinflammatory responses because ibudilast can reduce the production of pro-inflammatory cytokines including IL-1β, IL-6 and TNF-α as well as increase the levels of the anti-inflammatory cytokine, IL-10, in activated microglia (Kawanokuchi et al. 2004; Mizuno et al. 2004). Additional preclinical studies showed that the antibiotic, minocycline, blocked METH-induced condition place preference in mice, suggesting that the drug may reduce rewarding METH effects in these animals (Fujita et al. 2012). Moreover, minocycline also attenuated METH self-administration in rats (Snider et al. 2013), an effect that might be mediated, in part, by its anti-inflammatory properties. These studies demonstrate that ibudilast and minocycline might be effective in producing cessation of METH intake and achieving abstinence in human METH users. These drugs might also reduce relapse during clinical treatment for METH dependence. Both drugs have been used safely as medications for other conditions for decades and initial findings of their positive effects against various aspects of METH use disorder are very encouraging.
In addition to playing roles in rewarding effects of METH, changes in immune function may also contribute to the development and persistence of cognitive deficits found in chronic human METH abusers. Indeed, increased plasma levels of pro-inflammatory cytokines IL-1β, IL-2, IL-6, and TNF-α and chemokines MCP-1, MIP-1α, and MIP-1β were significantly associated with greater neurocognitive dysfunction in human METH users (Loftis et al. 2011). The neuropsychiatric impairments found in human METH addicts that include cognitive deficits, depression, and anxiety and last following abstinence are associated with poor treatment outcomes, including increased relapse rates, lower treatment retention rates, and reduced daily functioning (Sadek et al. 2007; Zorick et al. 2010). Together, these results suggest that medications that counteract METH-induced neuroinflammation and microglial activation may help to reduce METH-induced neurological complications, thereby improving treatment outcomes in METH dependence.
Summary
Neurocognitive deficits associated with METH abuse in humans may result from some neuropathological changes observed in the brains of these patients (Cadet et al. 2014a; London et al. 2015). These pathological abnormalities include dysfunctions of DA terminals, astrogliosis and microgliosis in the brains of METH addicts (Sekine et al. 2008; Thompson et al. 2004; Volkow et al. 2001b, 2001c; Wilson et al. 1996; see Cadet et al. 2014a for an extensive review). Neuroimaging studies provide suggestive evidence that these abnormalities and related deficits in cognitive control and decision-making may play role in promoting compulsive drug use and present a critical barrier to success in the treatment of METH addiction (London et al. 2015). It is important to note that this argument does imply that all METH users suffer from cognitive deficits since individual resilient factors might work to protect the brains of some METH addicts from the potential negative effects of this drug. However, the argument supports the idea that these patients deserve thorough neurological evaluations that may help to determine which METH dependent individuals may need cognitive enhancement during therapeutic interventions.
Cytokines and chemokines released by activated microglia appear to also play important roles in METH-induced neuronal injury and neuropsychiatric impairments, which include cognitive deficits, depression and anxiety (Downey and Loftis 2014). These deficits may persist even after prolonged abstinence and may serve as triggers for repeated relapses (Sadek et al. 2007; Zorick et al. 2010). Immunotherapeutic strategies that target specific neuroinflammatory pathways may not only reduce neurotoxic effects of the drug by promoting neuronal repair but may also improve neurocognitive functions in these patients. Therefore, the present review suggests the need for more research to further investigate the role that neuroinflammatory processes might play during the transition from occasional drug taking to pathological habitual drug use. The role that these substances might play in promoting stress- and cue-induced relapse also remains to be investigated. A better understanding of these processes may help us to develop better therapeutic approaches that are beneficial to METH addicted individuals.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute on Drug Abuse, NIH, DHHS
Footnotes
Conflict of Interest The authors declare that they do not have any conflicts of interest (financial or otherwise) related to data presented in this manuscript.
References
- Aguilar-Valles A, Vaissiere T, Griggs EM, Mikaelsson MA, Takacs IF, Young EJ, Rumbaugh G, Miller CA. Methamphetamine-associated memory is regulated by a writer and an eraser of permissive histone methylation. Biol Psychiatry. 2014;76:57–65. doi: 10.1016/j.biopsych.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertson TE, Derlet RW, Van Hoozen BE. Methamphetamine and the expanding complications of amphetamines. West J Med. 1999;170:214–219. [PMC free article] [PubMed] [Google Scholar]
- Asanuma M, Miyazaki I, Higashi Y, Tsuji T, Ogawa N. Specific gene expression and possible involvement of inflammation in methamphetamine-induced neurotoxicity. Ann NY Acad Sci. 2004;1025:69–75. doi: 10.1196/annals.1316.009. [DOI] [PubMed] [Google Scholar]
- Astarita G, Avanesian A, Grimaldi B, Realini N, Justinova Z, Panlilio LV, Basit A, Goldberg SR, Piomelli D. Methamphetamine accelerates cellular senescence through stimulation of de novo ceramide biosynthesis. PLos One. 2015;10:e0116961. doi: 10.1371/journal.pone.0116961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Smith RJ, Moorman DE, Richardson KA. Role of lateral hypothalamic orexin neurons in reward processing and addiction. Neuropharmacology. 2009;56(Suppl 1):112–121. doi: 10.1016/j.neuropharm.2008.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae SC, Lyoo IK, Sung YH, Yoo J, Chung A, Yoon SJ, Kim DJ, Hwang J, Kim SJ, Renshaw PF. Increased white matter hyperintensities in male methamphetamine abusers. Drug Alcohol Depend. 2006;81:83–88. doi: 10.1016/j.drugalcdep.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Barco A, Patterson SL, Alarcon JM, Gromova P, Mata-Roig M, Morozov A, Kandel ER. Gene expression profiling of facilitated L-LTP in VP16-CREB mice reveals that BDNF is critical for the maintenance of LTP and its synaptic capture. Neuron. 2005;48:123–137. doi: 10.1016/j.neuron.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Beardsley PM, Hauser KF. Glial modulators as potential treatments of psychostimulant abuse. Adv Pharmacol. 2014;69:1–69. doi: 10.1016/B978-0-12-420118-7.00001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsley PM, Shelton KL, Hendrick E, Johnson KW. The glial cell modulator and phosphodiesterase inhibitor, AV411 (ibudilast), attenuates prime- and stress-induced methamphetamine relapse. Eur J Pharmacol. 2010;637:102–108. doi: 10.1016/j.ejphar.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont TL, Yao B, Shah A, Kapatos G, Loeb JA. Layer-specific CREB target gene induction in human neocortical epilepsy. J Neurosci. 2012;32:14389–14401. doi: 10.1523/JNEUROSCI.3408-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Belin-Rauscent A, Murray JE, Everitt BJ. Addiction: failure of control over maladaptive incentive habits. Curr Opin Neurobiol. 2013;23:564–572. doi: 10.1016/j.conb.2013.01.025. [DOI] [PubMed] [Google Scholar]
- Bentzley BS, Aston-Jones G. Orexin-1 receptor signaling increases motivation for cocaine-associated cues. Eur J Neurosci. 2015;41:1149–1156. doi: 10.1111/ejn.12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Ungless MA, Bonci A. Convergent actions of orexin/hypocretin and CRF on dopamine neurons: Emerging players in addiction. Brain Res. 2010;1314:139–144. doi: 10.1016/j.brainres.2009.10.068. [DOI] [PubMed] [Google Scholar]
- Borgmann K, Ghorpade A. HIV-1, methamphetamine and astrocytes at neuroinflammatory Crossroads. Front Microbiol. 2015;6:1143. doi: 10.3389/fmicb.2015.01143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch PJ, Benton MC, Macartney-Coxson D, Kivell BM. mRNA and microRNA analysis reveals modulation of biochemical pathways related to addiction in the ventral tegmental area of methamphetamine self-administering rats. BMC Neurosci. 2015;16:43. doi: 10.1186/s12868-015-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, de Lecea L. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci USA. 2005;102:19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bykhovskaia M. Synapsin regulation of vesicle organization and functional pools. Semin Cell Dev Biol. 2011;22:387–392. doi: 10.1016/j.semcdb.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Bisagno V. The primacy of cognition in the manifestations of substance use disorders. Front Neurol. 2013;4:189. doi: 10.3389/fneur.2013.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, Bisagno V. Glial-neuronal ensembles: partners in drug addiction-associated synaptic plasticity. Front Pharmacol. 2014;5:204. doi: 10.3389/fphar.2014.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, Bisagno V, Milroy CM. Neuropathology of substance use disorders. Acta Neuropathol. 2014a;127:91–107. doi: 10.1007/s00401-013-1221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, Brannock C, Ladenheim B, McCoy MT, Krasnova IN, Lehrmann E, Becker KG, Jayanthi S. Enhanced upregulation of CRH mRNA expression in the nucleus accumbens of male rats after a second injection of methamphetamine given thirty days later. PLoS One. 2014b;9:e84665. doi: 10.1371/journal.pone.0084665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, Jayanthi S, McCoy MT, Beauvais G, Cai NS. Dopamine D1 receptors, regulation of gene expression in the brain, and neurodegeneration. CNS Neurol Disord Drug Targets. 2010;9:526–538. doi: 10.2174/187152710793361496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, Krasnova IN, Ladenheim B, Cai NS, McCoy MT, Atianjoh FE. Methamphetamine preconditioning: differential protective effects on monoaminergic systems in the rat brain. Neurotox Res. 2009a;15:252–259. doi: 10.1007/s12640-009-9026-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, McCoy MT, Cai NS, Krasnova IN, Ladenheim B, Beauvais G, Wilson N, Wood W, Becker KG, Hodges AB. Methamphetamine preconditioning alters midbrain transcriptional responses to methamphetamine-induced injury in the rat striatum. PLoS One. 2009b;4:e7812. doi: 10.1371/journal.pone.0007812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan RC, Cunningham JK, Sajeev G, Kish SJ. Incidence of Parkinson's disease among hospital patients with methamphetamine-use disorders. Mov Disord. 2010;25:2333–2339. doi: 10.1002/mds.23263. [DOI] [PubMed] [Google Scholar]
- Callaghan RC, Cunningham JK, Sykes J, Kish SJ. Increased risk of Parkinson's disease in individuals hospitalized with conditions related to the use of methamphetamine or other amphetamine-type drugs. Drug Alcohol Depend. 2012;120:35–40. doi: 10.1016/j.drugalcdep.2011.06.013. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Carvalho M, Carmo H, Costa VM, Capela JP, Pontes H, Remiao F, Carvalho F, Bastos Mde L. Toxicity of amphetamines: an update. Arch Toxicol. 2012;86:1167–1231. doi: 10.1007/s00204-012-0815-5. [DOI] [PubMed] [Google Scholar]
- Cho AK, Melega WP. Patterns of methamphetamine abuse and their consequences. J Addict Dis. 2002;21:21–34. doi: 10.1300/j069v21n01_03. [DOI] [PubMed] [Google Scholar]
- Cornish JL, Hunt GE, Robins L, McGregor IS. Regional c-Fos and FosB/DeltaFosB expression associated with chronic methamphetamine self-administration and methamphetamine-seeking behavior in rats. Neuroscience. 2012;206:100–114. doi: 10.1016/j.neuroscience.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Cruz HG, Berton F, Sollini M, Blanchet C, Pravetoni M, Wickman K, Luscher C. Absence and rescue of morphine withdrawal in GIRK/Kir3 knock-out mice. J Neurosci. 2008;28:4069–4077. doi: 10.1523/JNEUROSCI.0267-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz HG, Ivanova T, Lunn ML, Stoffel M, Slesinger PA, Luscher C. Bi-directional effects of GABA(B) receptor agonists on the mesolimbic dopamine system. Nat Neurosci. 2004;7:153–159. doi: 10.1038/nn1181. [DOI] [PubMed] [Google Scholar]
- Curtin K, Fleckenstein AE, Robison RJ, Crookston MJ, Smith KR, Hanson GR. Methamphetamine/amphetamine abuse and risk of Parkinson's disease in Utah: a population-based assessment. Drug Alcohol Depend. 2015;146:30–38. doi: 10.1016/j.drugalcdep.2014.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danaceau JP, Deering CE, Day JE, Smeal SJ, Johnson-Davis KL, Fleckenstein AE, Wilkins DG. Persistence of tolerance to methamphetamine-induced monoamine deficits. Eur J Pharmacol. 2007;559:46–54. doi: 10.1016/j.ejphar.2006.11.045. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean AC, Groman SM, Morales AM, London ED. An evaluation of the evidence that methamphetamine abuse causes cognitive decline in humans. Neuropsychopharmacology. 2013;38:259–274. doi: 10.1038/npp.2012.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey LA, Loftis JM. Altered energy production, lowered antioxidant potential, and inflammatory processes mediate CNS damage associated with abuse of the psychostimulants MDMA and methamphetamine. Eur J Pharmacol. 2014;727:125–129. doi: 10.1016/j.ejphar.2014.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich LC, Hu S, Sheng WS, Sutton RL, Rockswold GL, Peterson PK, Chao CC. Cytokine regulation of human microglial cell IL-8 production. J Immunol. 1998;160:1944–1948. [PubMed] [Google Scholar]
- Escubedo E, Guitart L, Sureda FX, Jiménez A, Pubill D, Pallàs M, Camins A, Camarasa J. Microgliosis and down-regulation of adenosine transporter induced by methamphetamine in rats. Brain Res. 1998;814:120–126. doi: 10.1016/s0006-8993(98)01065-8. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. From the ventral to the dorsal striatum: devolving views of their roles in drug addiction. Neurosci Biobehav Rev. 2013;37:1946–1954. doi: 10.1016/j.neubiorev.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol. 2007;47:681–698. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Kunitachi S, Iyo M, Hashimoto K. The antibiotic minocycline prevents methamphetamine-induced rewarding effects in mice. Pharmacol Biochem Behav. 2012;101:303–306. doi: 10.1016/j.pbb.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Gadient RA, Otten UH. Interleukin-6 (IL-6)--a molecule with both beneficial and destructive potentials. Progr Neurobiol. 1997;52:379–390. doi: 10.1016/s0301-0082(97)00021-x. [DOI] [PubMed] [Google Scholar]
- Galinato MH, Orio L, Mandyam CD. Methamphetamine differentially affects BDNF and cell death factors in anatomically defined regions of the hippocampus. Neuroscience. 2015;286:97–108. doi: 10.1016/j.neuroscience.2014.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgetti M, Hotsenpiller G, Froestl W, Wolf ME. In vivo modulation of ventral tegmental area dopamine and glutamate efflux by local GABA(B) receptors is altered after repeated amphetamine treatment. Neuroscience. 2002;109:585–59. doi: 10.1016/s0306-4522(01)00510-3. [DOI] [PubMed] [Google Scholar]
- Goncalves J, Baptista S, Martins T, Milhazes N, Borges F, Ribeiro CF, Malva JO, Silva AP. Methamphetamine-induced neuroinflammation and neuronal dysfunction in the mice hippocampus: preventive effect of indomethacin. Eur J Neurosci. 2010;31:315–326. doi: 10.1111/j.1460-9568.2009.07059.x. [DOI] [PubMed] [Google Scholar]
- Goncalves J, Martins T, Ferreira R, Milhazes N, Borges F, Ribeiro CF, Malva JO, Macedo TR, Silva AP. Methamphetamine-induced early increase of IL-6 and TNF-alpha mRNA expression in the mouse brain. Ann NY Acad Sci. 2008;1139:103–111. doi: 10.1196/annals.1432.043. [DOI] [PubMed] [Google Scholar]
- Graeber MB, Streit WJ. Microglia: biology and pathology. Acta Neuropathol. 2010;119:89–105. doi: 10.1007/s00401-009-0622-0. [DOI] [PubMed] [Google Scholar]
- Graham DL, Noailles PA, Cadet JL. Differential neurochemical consequences of an escalating dose-binge regimen followed by single-day multiple-dose methamphetamine challenges. J Neurochem. 2008;105:1873–1885. doi: 10.1111/j.1471-4159.2008.05269.x. [DOI] [PubMed] [Google Scholar]
- Granado N, Lastres-Becker I, Ares-Santos S, Oliva I, Martin E, Cuadrado A, Moratalla R. Nrf2 deficiency potentiates methamphetamine-induced dopaminergic axonal damage and gliosis in the striatum. Glia. 2011;59:1850–1863. doi: 10.1002/glia.21229. [DOI] [PubMed] [Google Scholar]
- Greengard P, Valtorta F, Czernik AJ, Benfenati F. Synaptic vesicle phosphoproteins and regulation of synaptic function. Science. 1993;259:780–785. doi: 10.1126/science.8430330. [DOI] [PubMed] [Google Scholar]
- Guo Y, Feng P. OX2R activation induces PKC-mediated ERK and CREB phosphorylation. Exp Cell Res. 2012;318:2004–2013. doi: 10.1016/j.yexcr.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpin LE, Collins SA, Yamamoto BK. Neurotoxicity of methamphetamine and 3,4-methylenedioxymethamphetamine. Life Sci. 2014;97:37–44. doi: 10.1016/j.lfs.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Hart CL, Marvin CB, Silver R, Smith EE. Is cognitive functioning impaired in methamphetamine users? A critical review. Neuropsychopharmacology. 2012;37:586–608. doi: 10.1038/npp.2011.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Tsukada H, Nishiyama S, Fukumoto D, Kakiuchi T, Iyo M. Protective effects of minocycline on the reduction of dopamine transporters in the striatum after administration of methamphetamine: a positron emission tomography study in conscious monkeys. Biol Psychiatry. 2007;61:577–581. doi: 10.1016/j.biopsych.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Henry BL, Minassian A, Perry W. Effect of methamphetamine dependence on everyday functional ability. Addict Behav. 2010;35:593–598. doi: 10.1016/j.addbeh.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry DJ, Greene MA, White FJ. Electrophysiological effects of cocaine in the mesoaccumbens dopamine system: repeated administration. J Pharmacol Exp Ther. 1989;251:833–839. [PubMed] [Google Scholar]
- Hilfiker S, Pieribone VA, Czernik AJ, Kao HT, Augustine GJ, Greengard P. Synapsins as regulators of neurotransmitter release. Philos Trans R Soc Lond B Biol Sci. 1999;354:269–279. doi: 10.1098/rstb.1999.0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander JA, Pham D, Fowler CD, Kenny PJ. Hypocretin-1 receptors regulate the reinforcing and reward-enhancing effects of cocaine: pharmacological and behavioral genetics evidence. Front Behav Neurosci. 2012;6:47. doi: 10.3389/fnbeh.2012.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosaka M, Hammer RE, Sudhof TC. A phospho-switch controls the dynamic association of synapsins with synaptic vesicles. Neuron. 1999;24:377–387. doi: 10.1016/s0896-6273(00)80851-x. [DOI] [PubMed] [Google Scholar]
- Huckans M, Fuller BE, Chalker AL, Adams M, Loftis JM. Plasma inflammatory factors are associated with anxiety, depression, and cognitive problems in adults with and without methamphetamine dependence: An exploratory protein array study. Front Psychiatry. 2015;6:178. doi: 10.3389/fpsyt.2015.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayanthi S, Deng X, Ladenheim B, McCoy MT, Cluster A, Cai NS, Cadet JL. Calcineurin/NFAT-induced up-regulation of the Fas ligand/Fas death pathway is involved in methamphetamine-induced neuronal apoptosis. Proc Natl Acad Sci USA. 2005;102:868–873. doi: 10.1073/pnas.0404990102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayanthi S, McCoy MT, Chen B, Britt JP, Kourrich S, Yau HJ, Ladenheim B, Krasnova IN, Bonci A, Cadet JL. Methamphetamine downregulates striatal glutamate receptors via diverse epigenetic mechanisms. Biol Psychiatry. 2014;76:47–56. doi: 10.1016/j.biopsych.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedynak JP, Uslaner JM, Esteban JA, Robinson TE. Methamphetamine-induced structural plasticity in the dorsal striatum. Eur J Neurosci. 2007;25:847–853. doi: 10.1111/j.1460-9568.2007.05316.x. [DOI] [PubMed] [Google Scholar]
- Johannessen M, Moens U. Multisite phosphorylation of the cAMP response element-binding protein (CREB) by a diversity of protein kinases. Front Biosci. 2007;12:1814–1832. doi: 10.2741/2190. [DOI] [PubMed] [Google Scholar]
- Johnson-Davis KL, Fleckenstein AE, Wilkins DG. The role of hyperthermia and metabolism as mechanisms of tolerance to methamphetamine neurotoxicity. Eur J Pharmacol. 2003;482:151–154. doi: 10.1016/j.ejphar.2003.09.063. [DOI] [PubMed] [Google Scholar]
- Kao HT, Song HJ, Porton B, Ming GL, Hoh J, Abraham M, Czernik AJ, Pieribone VA, Poo MM, Greengard P. A protein kinase A-dependent molecular switch in synapsins regulates neurite outgrowth. Nat Neurosci. 2002;5:431–437. doi: 10.1038/nn840. [DOI] [PubMed] [Google Scholar]
- Kawanokuchi J, Mizuno T, Kato H, Mitsuma N, Suzumura A. Effects of interferon-beta on microglial functions as inflammatory and antigen presenting cells in the central nervous system. Neuropharmacology. 2004;46:734–742. doi: 10.1016/j.neuropharm.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Kim DJ, Roh S, Kim Y, Yoon SJ, Lee HK, Han CS, Kim YK. High concentrations of plasma brain-derived neurotrophic factor in methamphetamine users. Neurosci Lett. 2005;388:112–115. doi: 10.1016/j.neulet.2005.06.042. [DOI] [PubMed] [Google Scholar]
- Kitamura O, Wee S, Specio SE, Koob GF, Pulvirenti L. Escalation of methamphetamine self-administration in rats: a dose-effect function. Psychopharmacology. 2006;186:48–53. doi: 10.1007/s00213-006-0353-z. [DOI] [PubMed] [Google Scholar]
- Kodama M, Akiyama K, Ujike H, Shimizu Y, Tanaka Y, Kuroda S. A robust increase in expression of arc gene, an effector immediate early gene, in the rat brain after acute and chronic methamphetamine administration. Brain Res. 1998;796:273–283. doi: 10.1016/s0006-8993(98)00349-7. [DOI] [PubMed] [Google Scholar]
- Kramer JC, Fischman VS, Littlefield DC. Amphetamine abuse. Pattern and effects of high doses taken intravenously. JAMA. 1967;201:305–309. doi: 10.1001/jama.201.5.305. [DOI] [PubMed] [Google Scholar]
- Krasnova IN, Betts ES, Dada A, Jefferson A, Ladenheim B, Becker KG, Cadet JL, Hohmann CF. Neonatal dopamine depletion induces changes in morphogenesis and gene expression in the developing cortex. Neurotox Res. 2007;11:107–130. doi: 10.1007/BF03033390. [DOI] [PubMed] [Google Scholar]
- Krasnova IN, Cadet JL. Methamphetamine toxicity and messengers of death. Brain Res Rev. 2009;60:379–407. doi: 10.1016/j.brainresrev.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnova IN, Chiflikyan M, Justinova Z, McCoy MT, Ladenheim B, Jayanthi S, Quintero C, Brannock C, Barnes C, Adair JE, Lehrmann E, Kobeissy FH, Gold MS, Becker KG, Goldberg SR, Cadet JL. CREB phosphorylation regulates striatal transcriptional responses in the self-administration model of methamphetamine addiction in the rat. Neurobiol Dis. 2013;58:132–143. doi: 10.1016/j.nbd.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnova IN, Hodges AB, Ladenheim B, Rhoades R, Phillip CG, Cesena A, Ivanova E, Hohmann CF, Cadet JL. Methamphetamine treatment causes delayed decrease in novelty-induced locomotor activity in mice. Neurosci Res. 2009;65:160–165. doi: 10.1016/j.neures.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnova IN, Justinova Z, Ladenheim B, Jayanthi S, McCoy MT, Barnes C, Warner JE, Goldberg SR, Cadet JL. Methamphetamine self-administration is associated with persistent biochemical alterations in striatal and cortical dopaminergic terminals in the rat. PLoS One. 2010;5:e8790. doi: 10.1371/journal.pone.0008790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnova IN, Ladenheim B, Hodges AB, Volkow ND, Cadet JL. Chronic methamphetamine administration causes differential regulation of transcription factors in the rat midbrain. PLoS One. 2011;6:e19179. doi: 10.1371/journal.pone.0019179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnova IN, Li SM, Wood WH, McCoy MT, Prabhu VV, Becker KG, Katz JL, Cadet JL. Transcriptional responses to reinforcing effects of cocaine in the rat hippocampus and cortex. Genes Brain Behav. 2008;7:193–202. doi: 10.1111/j.1601-183X.2007.00338.x. [DOI] [PubMed] [Google Scholar]
- Kwon SE, Chapman ER. Synaptophysin regulates the kinetics of synaptic vesicle endocytosis in central neurons. Neuron. 2011;70:847–854. doi: 10.1016/j.neuron.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labouebe G, Lomazzi M, Cruz HG, Creton C, Lujan R, Li M, Yanagawa Y, Obata K, Watanabe M, Wickman K, Boyer SB, Slesinger PA, Luscher C. RGS2 modulates coupling between GABAB receptors and GIRK channels in dopamine neurons of the ventral tegmental area. Nat Neurosci. 2007;10:1559–1568. doi: 10.1038/nn2006. [DOI] [PubMed] [Google Scholar]
- Ladenheim B, Krasnova IN, Deng X, Oyler JM, Polettini A, Moran TH, Huestis MA, Cadet JL. Methamphetamine-induced neurotoxicity is attenuated in transgenic mice with a null mutation for interleukin-6. Mol Pharmacol. 2000;58:1247–1256. doi: 10.1124/mol.58.6.1247. [DOI] [PubMed] [Google Scholar]
- LaVoie MJ, Card JP, Hastings TG. Microglial activation precedes dopamine terminal pathology in methamphetamine-induced neurotoxicity. Exp Neurol. 2004;187:47–57. doi: 10.1016/j.expneurol.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Lehrmann E, Oyler J, Vawter MP, Hyde TM, Kolachana B, Kleinman JE, Huestis MA, Becker KG, Freed WJ. Transcriptional profiling in the human prefrontal cortex: evidence for two activational states associated with cocaine abuse. Pharmacogenomics J. 2003;3:27–40. doi: 10.1038/sj.tpj.6500146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Rubio FJ, Zeric T, Bossert JM, Kambhampati S, Cates HM, Kennedy PJ, Liu QR, Cimbro R, Hope BT, Nestler EJ, Shaham Y. Incubation of methamphetamine craving is associated with selective increases in expression of Bdnf and trkb, glutamate receptors, and epigenetic enzymes in cue-activated fos-expressing dorsal striatal neurons. J Neurosci. 2015;35:8232–8244. doi: 10.1523/JNEUROSCI.1022-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftis JM, Choi D, Hoffman W, Huckans MS. Methamphetamine causes persistent immune dysregulation: a cross-species, translational report. Neurotox Res. 2011;20:59–68. doi: 10.1007/s12640-010-9223-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London ED, Kohno M, Morales AM, Ballard ME. Chronic methamphetamine abuse and corticostriatal deficits revealed by neuroimaging. Brain Res. 2015;1628:174–185. doi: 10.1016/j.brainres.2014.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long-Smith CM, Sullivan AM, Nolan YM. The influence of microglia on the pathogenesis of Parkinson's disease. Prog Neurobiol. 2009;89:277–287. doi: 10.1016/j.pneurobio.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Luscher C, Slesinger PA. Emerging roles for G protein-gated inwardly rectifying potassium (GIRK) channels in health and disease. Nat Rev Neurosci. 2010;11:301–315. doi: 10.1038/nrn2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Smith RJ, Aston-Jones G. Interactions between VTA orexin and glutamate in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2013;226:687–698. doi: 10.1007/s00213-012-2681-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy MT, Jayanthi S, Wulu JA, Beauvais G, Ladenheim B, Martin TA, Krasnova IN, Hodges AB, Cadet JL. Chronic methamphetamine exposure suppresses the striatal expression of members of multiple families of immediate early genes (IEGs) in the rat: normalization by an acute methamphetamine injection. Psychopharmacology. 2011;215:353–365. doi: 10.1007/s00213-010-2146-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden LM, Hadlock GC, Allen SC, Vieira-Brock PL, Stout KA, Ellis JD, Hoonakker AJ, Andrenyak DM, Nielsen SM, Wilkins DG, Hanson GR, Fleckenstein AE. Methamphetamine self-administration causes persistent striatal dopaminergic alterations and mitigates the deficits caused by a subsequent methamphetamine exposure. J Pharmacol Exp Ther. 2012;340:295–303. doi: 10.1124/jpet.111.188433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden LM, Vieira-Brock PL, Hanson GR, Fleckenstein AE. Methamphetamine self-administration attenuates hippocampal serotonergic deficits: role of brain-derived neurotrophic factor. Int J Neuropsychopharmacol. 2014;17:1315–1320. doi: 10.1017/S1461145714000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden LM, Vieira-Brock PL, Hanson GR, Fleckenstein AE. Prior methamphetamine self-administration attenuates the dopaminergic deficits caused by a subsequent methamphetamine exposure. Neuropharmacology. 2015;93:146–154. doi: 10.1016/j.neuropharm.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire SO, Ling ZD, Lipton JW, Sortwell CE, Collier TJ, Carvey PM. Tumor necrosis factor alpha is toxic to embryonic mesencephalic dopamine neurons. Exp Neurol. 2001;169:219–230. doi: 10.1006/exnr.2001.7688. [DOI] [PubMed] [Google Scholar]
- Menegon A, Bonanomi D, Albertinazzi C, Lotti F, Ferrari G, Kao HT, Benfenati F, Baldelli P, Valtorta F. Protein kinase A-mediated synapsin I phosphorylation is a central modulator of Ca2+-dependent synaptic activity. J Neurosci. 2006;26:11670–11681. doi: 10.1523/JNEUROSCI.3321-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T, Kurotani T, Komatsu Y, Kawanokuchi J, Kato H, Mitsuma N, Suzumura A. Neuroprotective role of phosphodiesterase inhibitor ibudilast on neuronal cell death induced by activated microglia. Neuropharmacology. 2004;46:404–411. doi: 10.1016/j.neuropharm.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Moratalla R, Khairnar A, Simola N, Granado N, García-Montes JR, Porceddu PF, Tizabi Y, Costa G, Morelli M. Amphetamine-related drugs neurotoxicity in humans and in experimental animals: Main mechanisms. Prog Neurobiol. 2015 doi: 10.1016/j.pneurobio.2015.09.011. [DOI] [PubMed] [Google Scholar]
- Morgan AD, Carroll ME, Loth AK, Stoffel M, Wickman K. Decreased cocaine self-administration in Kir3 potassium channel subunit knockout mice. Neuropsychopharmacology. 2003;28:932–938. doi: 10.1038/sj.npp.1300100. [DOI] [PubMed] [Google Scholar]
- Nagamoto-Combs K, Combs CK. Microglial phenotype is regulated by activity of the transcription factor, NFAT (nuclear factor of activated T cells) J Neurosci. 2010;30:9641–9646. doi: 10.1523/JNEUROSCI.0828-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K. Distribution of orexin neurons in the adult rat brain. Brain Res. 1999;827:243–260. doi: 10.1016/s0006-8993(99)01336-0. [DOI] [PubMed] [Google Scholar]
- Narita M, Miyatake M, Narita M, Shibasaki M, Shindo K, Nakamura A, Kuzumaki N, Nagumo Y, Suzuki T. Direct evidence of astrocytic modulation in the development of rewarding effects induced by drugs of abuse. Neuropsychopharmacology. 2006;31:2476–2488. doi: 10.1038/sj.npp.1301007. [DOI] [PubMed] [Google Scholar]
- Neale A, Abraham S, Russell J. "Ice" use and eating disorders: a report of three cases. Int J Eeat Disord. 2009;42:188–191. doi: 10.1002/eat.20587. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Cellular basis of memory for addiction. Dialogues Clin Neurosci. 2013;15:431–443. doi: 10.31887/DCNS.2013.15.4/enestler. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numachi Y, Ohara A, Yamashita M, Fukushima S, Kobayashi H, Hata H, Watanabe H, Hall FS, Lesch KP, Murphy DL, Uhl GR, Sora I. Methamphetamine-induced hyperthermia and lethal toxicity: role of the dopamine and serotonin transporters. Eur J Pharmacol. 2007;572:120–128. doi: 10.1016/j.ejphar.2007.06.022. [DOI] [PubMed] [Google Scholar]
- Panenka WJ, Procyshyn RM, Lecomte T, MacEwan GW, Flynn SW, Honer WG, Barr AM. Methamphetamine use: a comprehensive review of molecular, preclinical and clinical findings. Drug Alcohol Depend. 2013;129:167–179. doi: 10.1016/j.drugalcdep.2012.11.016. [DOI] [PubMed] [Google Scholar]
- Parsegian A, Glen WB, Jr, Lavin A, See RE. Methamphetamine self-administration produces attentional set-shifting deficits and alters prefrontal cortical neurophysiology in rats. Biol Psychiatry. 2011;69:253–259. doi: 10.1016/j.biopsych.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince CD, Rau AR, Yorgason JT, Espana RA. Hypocretin/Orexin regulation of dopamine signaling and cocaine self-administration is mediated predominantly by hypocretin receptor 1. ACS Chem Neurosci. 2015;6:138–146. doi: 10.1021/cn500246j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineri M, Gonzalez B, Goitia B, Garcia-Rill E, Krasnova IN, Cadet JL, Urbano FJ, Bisagno V. Modafinil abrogates methamphetamine-induced neuroinflammation and apoptotic effects in the mouse striatum. PLoS One. 2012;7:e46599. doi: 10.1371/journal.pone.0046599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh G, MacLean AG, Philipp MT. Cytokines and chemokines at the crossroads of neuroinflammation, neurodegeneration, and neuropathic pain. Mediators Inflamm. 2013;2013:480739. doi: 10.1155/2013/480739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- Rauskolb S, Zagrebelsky M, Dreznjak A, Deogracias R, Matsumoto T, Wiese S, Erne B, Sendtner M, Schaeren-Wiemers N, Korte M, Barde YA. Global deprivation of brain-derived neurotrophic factor in the CNS reveals an area-specific requirement for dendritic growth. J Neurosci. 2010;30:1739–1749. doi: 10.1523/JNEUROSCI.5100-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recinto P, Samant AR, Chavez G, Kim A, Yuan CJ, Soleiman M, Grant Y, Edwards S, Wee S, Koob GF, George O, Mandyam CD. Levels of neural progenitors in the hippocampus predict memory impairment and relapse to drug seeking as a function of excessive methamphetamine self-administration. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37:1275–1287. doi: 10.1038/npp.2011.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Ramsey LA, Schwendt M, McGinty JF, See RE. Methamphetamine-induced changes in the object recognition memory circuit. Neuropharmacology. 2012;62:1119–1126. doi: 10.1016/j.neuropharm.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson MJ, Turner RC, Naser ZJ, McCurdy CR, O'Callaghan JP, Huber JD, Matsumoto RR. SN79, a sigma receptor antagonist, attenuates methamphetamine-induced astrogliosis through a blockade of OSMR/gp130 signaling and STAT3 phosphorylation. Exp Neurol. 2014;254:180–189. doi: 10.1016/j.expneurol.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha SM, Cristovão AC, Campos FL, Fonseca CP, Baltazar G. Astrocyte-derived GDNF is a potent inhibitor of microglial activation. Neurobiol Dis. 2012;47:407–415. doi: 10.1016/j.nbd.2012.04.014. [DOI] [PubMed] [Google Scholar]
- Rogers JL, De Santis S, See RE. Extended methamphetamine self-administration enhances reinstatement of drug seeking and impairs novel object recognition in rats. Psychopharmacology. 2008;199:615–624. doi: 10.1007/s00213-008-1187-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Mazei-Robison MS, Ables JL, Nestler EJ. Neurotrophic factors and structural plasticity in addiction. Neuropharmacology. 2009;56(Suppl 1):73–82. doi: 10.1016/j.neuropharm.2008.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusyniak DE. Neurologic manifestations of chronic methamphetamine abuse. Psychiatr Clin North Am. 2013;36:261–275. doi: 10.1016/j.psc.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadek JR, Vigil O, Grant I, Heaton RK, Group H. The impact of neuropsychological functioning and depressed mood on functional complaints in HIV-1 infection and methamphetamine dependence. J Clin Eexp Neuropsychol. 2007;29:266–276. doi: 10.1080/13803390600659384. [DOI] [PubMed] [Google Scholar]
- Sailasuta N, Abulseoud O, Harris KC, Ross BD. Glial dysfunction in abstinent methamphetamine abusers. J Cereb Blood Flow Metab. 2010;30:950–960. doi: 10.1038/jcbfm.2009.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Schwendt M, Reichel CM, See RE. Extinction-dependent alterations in corticostriatal mGluR2/3 and mGluR7 receptors following chronic methamphetamine self-administration in rats. PLoS One. 2012;7:e34299. doi: 10.1371/journal.pone.0034299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwendt M, Rocha A, See RE, Pacchioni AM, McGinty JF, Kalivas PW. Extended methamphetamine self-administration in rats results in a selective reduction of dopamine transporter levels in the prefrontal cortex and dorsal striatum not accompanied by marked monoaminergic depletion. J Pharmacol Exp Ther. 2009;331:555–562. doi: 10.1124/jpet.109.155770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, Grant I. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev. 2007;17:275–297. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Minabe Y, Ouchi Y, Takei N, Iyo M, Nakamura K, Suzuki K, Tsukada H, Okada H, Yoshikawa E, Futatsubashi M, Mori N. Association of dopamine transporter loss in the orbitofrontal and dorsolateral prefrontal cortices with methamphetamine-related psychiatric symptoms. Am J Psychiatry. 2003;160:1699–1701. doi: 10.1176/appi.ajp.160.9.1699. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Ouchi Y, Sugihara G, Takei N, Yoshikawa E, Nakamura K, Iwata Y, Tsuchiya KJ, Suda S, Suzuki K, Kawai M, Takebayashi K, Yamamoto S, Matsuzaki H, Ueki T, Mori N, Gold MS, Cadet JL. Methamphetamine causes microglial activation in the brains of human abusers. J Neurosci. 2008;28:5756–5761. doi: 10.1523/JNEUROSCI.1179-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiflett MW, Balleine BW. Molecular substrates of action control in cortico-striatal circuits. Prog Neurobiol. 2011;95:1–13. doi: 10.1016/j.pneurobio.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SL, Richardson K, Dacey J, Glynn S, Domier CP, Rawson RA, Ling W. A comparison of patterns of methamphetamine and cocaine use. J Addict Dis. 2002;21:35–44. doi: 10.1300/j069v21n01_04. [DOI] [PubMed] [Google Scholar]
- Snider SE, Hendrick ES, Beardsley PM. Glial cell modulators attenuate methamphetamine self-administration in the rat. Eur J Pharmacol. 2013;701:124–130. doi: 10.1016/j.ejphar.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider SE, Vunck SA, van den Oord EJ, Adkins DE, McClay JL, Beardsley PM. The glial cell modulators, ibudilast and its amino analog, AV1013, attenuate methamphetamine locomotor activity and its sensitization in mice. Eur J Pharmacol. 2012;679:75–80. doi: 10.1016/j.ejphar.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son JH, Kuhn J, Keefe KA. Perseverative behavior in rats with methamphetamine-induced neurotoxicity. Neuropharmacology. 2013;67:95–103. doi: 10.1016/j.neuropharm.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Conde JR, Fendrick SE, Flanary BE, Mariani CL. Role of microglia in the central nervous system's immune response. Neurol Res. 2005;27:685–691. doi: 10.1179/016164105X49463a. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Francescutti-Verbeem DM, Kuhn DM. The newly synthesized pool of dopamine determines the severity of methamphetamine-induced neurotoxicity. J Neurochem. 2008;105:605–616. doi: 10.1111/j.1471-4159.2007.05155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DM, Francescutti-Verbeem DM, Liu X, Kuhn DM. Identification of differentially regulated transcripts in mouse striatum following methamphetamine treatment--an oligonucleotide microarray approach. J Neurochem. 2004a;88:380–393. doi: 10.1046/j.1471-4159.2003.02182.x. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Kuhn DM. Attenuated microglial activation mediates tolerance to the neurotoxic effects of methamphetamine. J Neurochem. 2005a;92:790–797. doi: 10.1111/j.1471-4159.2004.02906.x. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Kuhn DM. MK-801 and dextromethorphan block microglial activation and protect against methamphetamine-induced neurotoxicity. Brain Res. 2005b;1050:190–198. doi: 10.1016/j.brainres.2005.05.049. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Walker PD, Benjamins JA, Geddes TJ, Kuhn DM. Methamphetamine neurotoxicity in dopamine nerve endings of the striatum is associated with microglial activation. J Pharmacol Exp Ther. 2004b;311:1–7. doi: 10.1124/jpet.104.070961. [DOI] [PubMed] [Google Scholar]
- Thomas GM, Huganir RL. MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci. 2004;5:173–183. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, Lee JY, Toga AW, Ling W, London ED. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci. 2004;24:6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Pascoli V, Svenningsson P, Paul S, Enslen H, Corvol JC, Stipanovich A, Caboche J, Lombroso PJ, Nairn AC, Greengard P, Herve D, Girault JA. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci USA. 2005;102:491–496. doi: 10.1073/pnas.0408305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Holst RJ, Schilt T. Drug-related decrease in neuropsychological functions of abstinent drug users. Curr Drug Abuse Rev. 2011;4:42–56. doi: 10.2174/1874473711104010042. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001a;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, Gatley SJ, Miller E, Hitzemann R, Ding YS, Logan J. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci. 2001b;21:9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Logan J, Wong C, Miller EN. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry. 2001c;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Morales M. The Brain on Drugs: From Reward to Addiction. Cell. 2015;162:712–725. doi: 10.1016/j.cell.2015.07.046. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Smith L, Volkow ND, Telang F, Logan J, Tomasi D, Wong CT, Hoffman W, Jayne M, Alia-Klein N, Thanos P, Fowler JS. Decreased dopamine activity predicts relapse in methamphetamine abusers. Mol Psychiatry. 2012;17:918–925. doi: 10.1038/mp.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JQ, Smith AJ, McGinty JF. A single injection of amphetamine or methamphetamine induces dynamic alterations in c-fos, zif/268 and preprodynorphin messenger RNA expression in rat forebrain. Neuroscience. 1995;68:83–95. doi: 10.1016/0306-4522(95)00100-w. [DOI] [PubMed] [Google Scholar]
- White FJ, Wang RY. Electrophysiological evidence for A10 dopamine autoreceptor subsensitivity following chronic D-amphetamine treatment. Brain Research. 1984;309:283–292. doi: 10.1016/0006-8993(84)90594-8. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM, Schmunk GA, Shannak K, Haycock JW, Kish SJ. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med. 1996;2:699–703. doi: 10.1038/nm0696-699. [DOI] [PubMed] [Google Scholar]
- Wise RA, Koob GF. The development and maintenance of drug addiction. Neuropsychopharmacology. 2014;39:254–262. doi: 10.1038/npp.2013.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Miyamoto Y, Nitta A, Muramatsu S, Ozawa K, Yamada K, Nabeshima T. Intrastriatal gene delivery of GDNF persistently attenuates methamphetamine self-administration and relapse in mice. Int J Neuropsychopharmacol. 2013;16:1559–1567. doi: 10.1017/S1461145712001575. [DOI] [PubMed] [Google Scholar]
- Yan Y, Yamada K, Niwa M, Nagai T, Nitta A, Nabeshima T. Enduring vulnerability to reinstatement of methamphetamine-seeking behavior in glial-cell-line-derived neurotrophic factor mutant mice. FASEB J. 2007;21:1994–2004. doi: 10.1096/fj.06-7772com. [DOI] [PubMed] [Google Scholar]
- Zhang L, Kitaichi K, Fujimoto Y, Nakayama H, Shimizu E, Iyo M, Hashimoto K. Protective effects of minocycline on behavioral changes and neurotoxicity in mice after administration of methamphetamine. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1381–1393. doi: 10.1016/j.pnpbp.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Zorick T, Nestor L, Miotto K, Sugar C, Hellemann G, Scanlon G, Rawson R, London ED. Withdrawal symptoms in abstinent methamphetamine-dependent subjects. Addiction. 2010;105:1809–1818. doi: 10.1111/j.1360-0443.2010.03066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


