Abstract
Highly ordered interactions between immune and metabolic responses are evolutionarily conserved and paramount for tissue and organismal health. Disruption of these interactions underlies the emergence of many pathologies, particularly chronic non-communicable diseases such as obesity and diabetes. Here, we examine decades of research identifying the complex immunometabolic signaling networks and the cellular and molecular events that occur in the setting of altered nutrient and energy exposures and offer a historical perspective. Furthermore, we describe recent advances such as the discovery that a broad complement of immune cells play a role in immunometabolism and the emerging evidence that nutrients and metabolites modulate inflammatory pathways. Lastly, we discuss how this work may eventually lead to tangible therapeutic advancements to promote health.
Impact of Immunity on Metabolism
Energy management is required for every biological function, and thus metabolism is an essential component of life. In addition, since the emergence of the first unicellular organisms, there has been a need for protection from environmental insults, leading to the evolution of the immune system. Thus, metabolism and immunity have been interwoven since the beginning of life, and, in the broadest manner, one could say that the timeline of immunometabolism is ancient, at least a few billion years old (Figure 1). The contemporary study of this intimate relationship dates to the end of the 19th century, when physicians recognized metabolic pathologies associated with infections. As early as 1884, it was noted that patients with meningitis exhibit a transient diabetic syndrome, and in fact the frequency of diabetes was so high that meningitis diagnoses were sometimes overlooked and patients treated only for hyperglycemia (Fox et al., 1947). Loss of secretion or action of insulin is a critical component of diabetes. Insight into the mechanism at play was missing until experiments carried out in the 1980s, when it was shown in dogs that treatment with lipopolysaccharide (LPS) from gram-negative bacteria caused resistance to insulin by abrogating the ability of insulin to induce glucose uptake in the muscle (Raymond et al., 1981). In the same period it was recognized that acute infection in human patients was associated with decreased binding of insulin to the insulin receptor of isolated blood cells (Drobny et al., 1984).
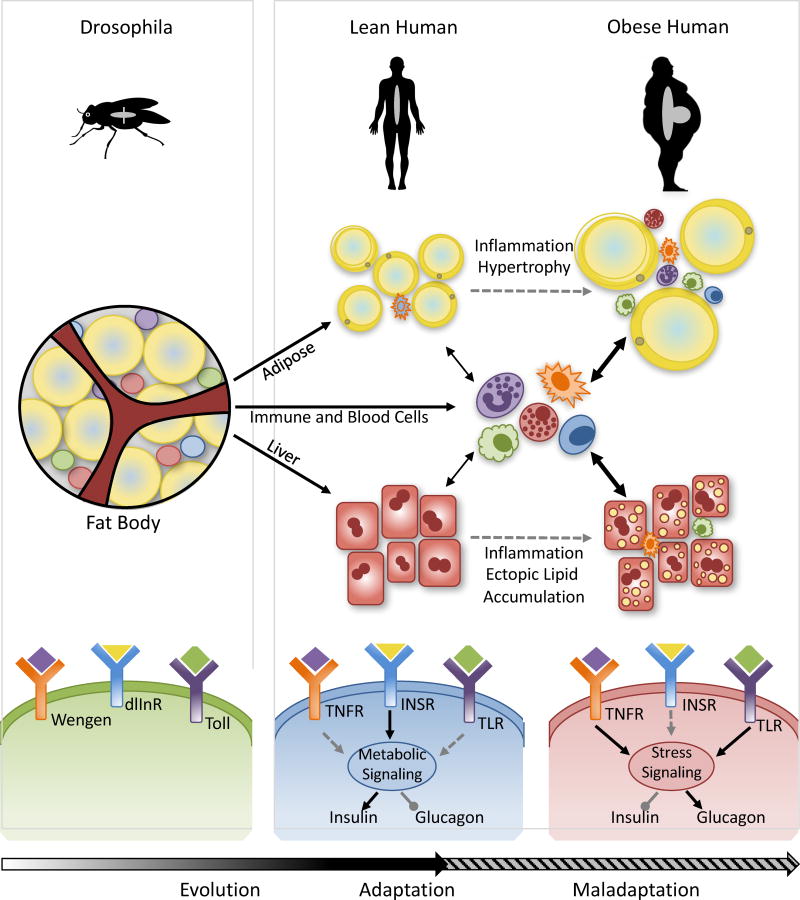
Figure 1. The evolution of immunometabolism.
Over the course of evolution, the Drosophila fat body, where liver, adipose tissue and the principle immune organ is situated in a single structure, has given rise to the distinct metabolic and immune organs observed in modern mammals. However, despite this seeming delegation of tasks, highly regulated interactions and crosstalk that are required to maintain immune and metabolic homeostasis remain as part of this evolutionary history. In the setting of obesity, this gives rise to activation and infiltration of immune cells into metabolic tissues and chronic activation of inflammatory pathways in both stromal and immune components, triggering stress kinase activation which impinges on the signaling of metabolic hormones such as insulin, and leading to impaired glucose and lipid homeostasis. The fundamental principles of this transition from an "immunometabolic" adaptive to maladaptive state could be depicted in a simple framework wherein the pathogen sensing, immune signaling and metabolic responses are signaled through Toll-like, TNF (tumor necrosis factor), and, insulin receptors. Each one of the highly conserved signaling components that construct this principle framework of metaflammation could be enriched and expanded with many more molecules and signaling networks in higher organisms and humans. Wengen (Drosophila TNF receptor), dlInR (Drosophila insulin receptor), Toll (Toll receptor), TNFR (TNF receptor), INSR (Insulin receptor), TLR (Toll like receptor).
Similarly, in the 1960s it began to be understood that obese subjects are simultaneously hyperinsulinemic and display insulin resistance; although glucose is rapidly taken up into muscle in lean subjects following insulin infusion, this effect is blunted in obese patients (Rabinowitz and Zierler, 1962). This state of chronic insulin resistance is a central component of metabolic syndrome, and predisposes individuals to developing type 2 diabetes. Thus, by the early to mid 1900s, two concepts were emerging independently: that obese subjects have insulin resistance and are predisposed to diabetes; and that insulin resistance, glucose intolerance, dyslipidemia and other metabolic problems occur in the setting of infection. The possibility of the existence of a “non-antibody antagonist” of insulin action causing diabetes was also raised during this period, however, there were no indications that such molecule may be an immune mediator (Berson and Yalow, 1958).
It had previously been observed that some diabetic patients treated with aspirin exhibited rapid improvements in glucose homeostasis (Ebstein W, 1876); however, this occurred well before the understanding that aspirin functions as an anti-inflammatory agent and inhibitor of the cyclooxygenase enzymes (Vane, 1971). These observations later inspired studies connecting the anti-diabetic effects of high dose salsalate with inflammatory signaling (discussed below). In addition, careful pathologists examining tissues of animal models of obesity in the 1960s had reported the infiltration of immune cells, such as macrophages and mast cells, into the adipose tissue (Hausberger, 1966; Hellman, 1965), but this mostly escaped the attention of scientists, including myself, until much later. Hence, despite all of these early observations and lines of evidence, the potential for an immunological nature of metabolic disease and its relation to obesity was largely disregarded for decades, and the cellular and molecular mechanisms that lead to abnormal insulin action, production, and glucose metabolism in obesity remained elusive. In the past thirty years, however, research defining the molecular and cellular players that connect immunity to metabolism in this respect has rapidly expanded, and together with the metabolic impact on immune cell function that will be discussed later in this article, yielded an exciting new field of study now dubbed “immunometabolism” (Figure 2).
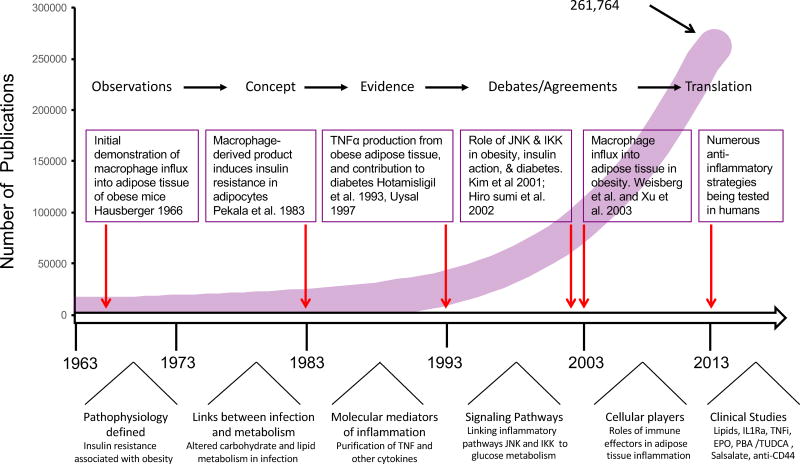
Figure 2. Timeline of immunometabolism.
Summary of the major findings that have given rise to the field of immunometabolism. The line graph reflects the total number of publications (261,764) describing links between immunity and metabolism over the last several decades, found with the search terms “metabolism and inflammation” in PubMed. The major findings that laid the ground work for this field are highlighted. At the bottom of the figure processes that marked each decade are illustrated. The arrows point to the critical discoveries that have initiated and stimulated the expansion of the field of immune-metabolism. Publications in this field have proliferated rapidly in the last decades, as the field has recognized the impact of innate and adaptive immunity in metabolism, identified signaling networks and molecular mediators as well as immunomodulatory effects of lipid species and progressed towards new translational avenues with exciting proof of principle studies. These issues are covered in more detail in the manuscript.
Signaling pathways connecting immunity and glucose metabolism- a brief history
Some of the most important early insights into the molecular connections between immunity, metabolism, and insulin action came from the discovery in the mid-1980s that conditioned medium from macrophages incubated with LPS could induce resistance to insulin-induced glucose uptake and lipoprotein lipase expression in adipocytes (Mahoney et al., 1985; Pekala et al., 1983). These noteworthy observations made by Anthony Cerami and colleagues not only indicated a connection between the immune response and insulin responsiveness, but also were among the first to show an interaction between macrophages and adipocytes, to propose the presence of mediators exchanged between these cells, and to biochemically define the locus of insulin resistance as a post-receptor signaling defect (Mahoney et al., 1985; Pekala et al., 1983). This seminal work represents an important cornerstone in the contemporary timeline of the field of immunometabolism. One of the macrophage-produced factors that may be responsible for this effect was later identified as the inflammatory cytokine tumor necrosis factor (TNF) (Beutler et al., 1985) which had previously been described as a cytotoxic factor (Carswell et al., 1975; Helson et al., 1975) linked to severe metabolic phenotypes in cancer, including dyslipidemia and cachexia (Oliff et al., 1987). During this period, Kenneth Feingold and Carl Grunfeld, as well as Charles Lang and Gregory Bagby, performed critical experiments to illustrate the in vivo metabolic effects of TNF, laying the foundation of our understanding of the metabolic impact of cytokines, particularly on glucose and lipid homeostasis (Feingold et al., 1989; Lang et al., 1992).
The critical observations of the inflammatory origin of obesity and diabetes came from studies in the early 1990s, from which evidence began to emerge that the adipose tissue of obese rodents exhibited inflammatory changes and expressed increased levels of TNF in both the adipocyte and stromal-vascular fractions (Hotamisligil et al., 1993). Soon after, similar observations showed increased TNF expression in the adipose tissue of obese humans, as reported by two simultaneous and independent publications (Hotamisligil et al., 1995; Kern et al., 1995), and subsequently it was shown that TNF expression was also elevated in the muscle tissue of obese humans (Saghizadeh et al., 1996). Many groups also demonstrated that neutralization of TNF in obese rats or mice resulted in increased insulin sensitivity and improved glucose metabolism (Borst and Bagby, 2002; Hotamisligil et al., 1993; Liang et al., 2008) (for a complete list, see www.metaflammation.org), whereas publications from the Grunfeld group, Jerry Olefsky’s group, and Nawfal Istafan and colleagues all showed that administration of TNF caused insulin resistance and impaired glucose metabolism in animal models (Feingold et al., 1989; Ling et al., 1994; Miles et al., 1997). These studies identified differences in glucose fluxes between acute and chronic TNF exposures, as well as the target tissues responsible for impaired insulin action, increased glucose production, and alterations in disposal. Similarly, induction of insulin resistance and modulation of lipid metabolism was also demonstrated when TNF was infused into healthy human subjects (Plomgaard et al., 2005; Plomgaard et al., 2008; Van der Poll et al., 1991).
At this point in the early 1990s, many groups were pursuing the mechanisms underlying impaired insulin resistance and glucose metabolism in the inflammatory setting. Before the discovery that obesity features inflammation in adipose tissue, Jacqueline Stephens and Phillip Pekala had reported the suppression of glucose transporter 4 (GLUT4) in adipocytes by TNF in a series of elegant studies (Stephens and Pekala, 1991). Earlier work had also shown the stimulation of GLUT1 in cells that did not express GLUT4 upon TNF treatment, which explained the acute increase in glucose uptake in certain contexts upon exposure to inflammatory stimuli such as TNF (Cornelius et al., 1990). These studies illustrated a complex pattern of regulation of glucose fluxes by TNF in which immune, metabolic or stromal cells respond differently to inflammatory signals with temporal dependencies, as had been demonstrated in vivo (Spitzer et al., 1989). However, none of these findings were sufficient to explain how inflammatory mediators impacted systemic insulin action and how those relate to impaired glucose metabolism in obesity and diabetes. The next set of critical studies illustrated that TNF can inhibit signaling cascades downstream of the insulin receptor, as first reported by Feinstein et al., in liver cells in 1993 (Feinstein et al., 1993). This was also shown in adipocytes (Hotamisligil et al., 1994) and upon chronic exposure, resembling what was seen in adipose tissue in obesity. Further efforts to understand the mechanisms by which TNF blocked insulin signaling were inspired by the findings from Yannick Le Marchand-Brustel’s lab showing that blocking phosphatase action by okadaic acid treatment led to increased serine phosphorylation of IRS1 and blocked insulin action in cells (Jullien et al., 1993). Serine phosphorylation of insulin receptor substrate 1 (IRS1) was demonstrated upon cytokine stimulation, as first reported by Kanety et al. in liver cells (Kanety et al., 1995), in our studies with adipocytes and other reconstituted cell types (Hotamisligil et al., 1996), and subsequently in vivo in metabolic tissues.
The key evidence linking immunity to metabolism came a year later, in 1997, when two independent studies with independent mouse lines reported that genetic absence of TNF function resulted in reduced insulin resistance and improved glucose tolerance (Uysal et al., 1997; Ventre et al., 1997). These findings were supported by many other independent studies in multiple experimental models over time (for example, see (da Costa et al., 2016) and a complete list at www.metaflammation.org). While less definitive human genetics associations in which TNF polymorphisms have been linked to obesity, diabetes and other aspects of metabolic syndrome, including polycystic ovary syndrome and sleep apnea also started to emerge (Huang et al., 2012a; Sookoian et al., 2005). Many other inflammatory cytokines and mediators such as interleukin 1β (IL-1β) have since been implicated in the pathogenesis of insulin action or secretion (Maedler et al., 2002), leading to thousands of publications. These aspects have been reviewed in depth elsewhere (Donath, 2014; Feve and Bastard, 2009; Hotamisligil, 2017; Tack et al., 2012), and are not detailed here.
Thus, in the early 2000s the field began to understand that in obesity, hundreds of immune mediators are abnormally produced or regulated which contribute to altered metabolic status. Consistent with this complexity, most individual manipulations in mouse models have produced partial results in terms of resolution of disease, or in few occasions, produced differing and even conflicting results. For example, independent investigators have observed conflicting findings regarding the effect of loss of function of a component of inflammatory signaling on systemic metabolism (Hotamisligil, 2017). Similarly, type 1 diabetes research features highly instructive experiences of seemingly contrasting conclusions from which many important lessons can be drawn (Green and Flavell, 1999; Jacob et al., 1990; Koulmanda et al., 2012). This challenge is of course not limited only to immunological pathways and applies to other models and mechanisms that have been examined (for example, (Jornayvaz et al., 2011; Monetti et al., 2007), and may, at least in part, be related to the nature of cytokine actions on glucose metabolism, and to inadvertent flaws in the nature of genetic models and other external modifiers. Interestingly, more consistent results have been observed upon antibody, chemical, or RNAi-mediated suppression of inflammatory mediators than in genetic models ((Hotamisligil, 2017) and www.metaflammation.org). It is now widely recognized that physiological studies in mice are subject to many modifying effects emanating from the diet, genetic background, gut microbiota, circadian considerations, environmental conditions and enrichment, and others (Eisenbarth et al., 2016; Jornayvaz et al., 2011; Thurmond et al., 2015; Woods and Begg, 2015), and we should all practice caution and humility when interpreting or re-interpreting phenotypic results.
Nevertheless, it was clear at the time that there was need to explore the intracellular signaling pathways that regulate the expression of and response to proinflammatory cytokines, and the mechanisms through which these signals converge on common nodes to support metaflammation. This effort led to the discovery of c-Jun N-terminal kinase (JNK) as a very critical signaling pathway that is downstream of many inflammatory signals in obesity and type 2 diabetes (Hirosumi et al., 2002). JNK activity is elevated in adipose tissue, liver and muscle of obese mice, and Jnk1−/− and Jnk1+/−Jnk2−/− mice are protected from diet-induced insulin resistance (Hirosumi et al., 2002; Tuncman et al., 2006). Perhaps most critically, a mutation in the MAPK81P1 locus which leads to constitutive JNK activation is linked to a Mendelian form of diabetes in humans (Waeber et al., 2000). It is reported that TNF activates JNK via the TNF Receptor II-associated protein TRAF2 (Reinhard et al., 1997), and in turn JNK promotes serine phosphorylation of IRS-1 and inhibits insulin signaling (Aguirre et al., 2002). Interestingly, TRAF2 can also interact with the endoplasmic reticulum (ER)-resident protein IRE1, indicating a link between the unfolded protein response and JNK activation (Urano et al., 2000) and demonstrating that in addition to stress signals JNK can potentially serve to integrate a diverse array of pathways converging on metabolic control (Hotamisligil, 2010). Subsequent studies from the laboratories of Roger Davis and Jens Bruning have been highly instructive in identifying the most relevant cell types in which JNK activation affects systemic metabolism (Sabio et al., 2008; Tsaousidou et al., 2014). Highlighting the translational relevance of this work, increased JNK phosphorylation has been demonstrated in the adipose tissue of obese humans (Boden et al., 2008) and peptide or small molecule inhibitors of JNK generate metabolic benefits (Kaneto et al., 2004; Yan et al., 2017). In summary, pathological JNK activation is a benchmark event in immunometabolic signaling in obesity.
Many cytokines such as TNF also activate nuclear factor kappa B (NF-κB) (Lowenthal et al., 1989; Osborn et al., 1989), which is a central modulator of inflammatory responses, regulating the expression of proinflammatory cytokines including TNF itself and other genes that are part of the immune response (reviewed in (Barnes and Karin, 1997). The upstream activator kinase of NF-κB is IKK, which is also reported to phosphorylate IRS-1 at serine residues to block insulin signaling (Gao et al., 2002). It is possible that alternative mechanisms, such as production of molecules that regulate inflammatory resolution, also couple this signaling pathway to metabolic deterioration, and studies on these mechanisms in further detail are warranted. High doses of salicylates block NF-κB signaling (Kopp and Ghosh, 1994) and improve insulin signaling and glucose homeostasis in rodent models of obesity (Yuan et al., 2001). In the mid-2000s, work from Michael Karin’s and Steve Shoelson’s groups provided further evidence for the role of these metaflammatory pathways using genetic models, and demonstrated improved glucose homeostasis in mice heterozygous for IKK (Yuan et al., 2001) as well as in mice with myeloid-specific or liver-specific deletion of IKK (Arkan et al., 2005). Reciprocally, expression of a constitutively active IKK in liver induces expression of inflammatory cytokines and the development of insulin resistance (Cai et al., 2005). There is also evidence that inflammation of the central nervous system contributes to dysregulated metabolism in obesity, as hypothalamic-specific deletion of IKK also improves glucose tolerance and insulin sensitivity in high fat-fed mice (Zhang et al., 2008). Interestingly, adipocyte-specific loss of IKKβ impairs glucose metabolism, leading to the suggestion that adipose tissue inflammation may not be uniformly related to impaired insulin action and glucose intolerance (Park et al., 2016). However, careful examination of this model revealed that complete blockade of IKK-NFκB activity results in an unexpected and massive adipose tissue inflammation, due to the role of this pathway in resolution of inflammation, in part through production of anti-inflammatory cytokine IL-13 (Kwon et al., 2014). Finally, recent studies also demonstrated a critical role for the atypical IκB kinases in insulin sensitivity and glucose metabolism with important translational implications for human disease (Reilly et al., 2013). The balance between inflammatory promotion versus resolution can yield unexpected results due to hormetic effects of cytokine treatments or even in models of individual genetic deletion of inflammatory molecules themselves (Campbell et al., 2001). These studies also underscore the critical importance of balanced inflammatory signaling in adipose tissue and the fact that prodigious manipulation of signaling nodes or mediators may not always yield the predicted, canonical inflammatory, and consequently metabolic, outcomes in the whole body.
Beyond JNK and IKK, multiple other intermediate signaling kinases are also involved in linking inflammatory pathways to insulin action, including PKC, PKR, CAMK, AMPK, mTOR, JAK, PKA, ERK, p38 and other MAP kinases (Copps and White, 2012; Fullerton et al., 2013; Gonzalez-Teran et al., 2016; Hotamisligil, 2006). These kinases can be activated by insulin, in response to lipid mediators, cytokines, or by sympathetic stimulation, and the resultant phosphorylation of the insulin signaling pathway components can be either activating or inhibitory. These pathways are also critical in integrating lipid mediators and abnormal lipid metabolism with insulin action, with or without engaging immune pathways (Petersen et al., 2016). Although all of these signaling pathways are not covered in detail here, it is important to point out that their redundant and overlapping nature highlight the complexity of insulin signaling regulation and its critical importance to organismal homeostasis. One of the most critical integrating mechanisms for immunometabolic integration resides in the ER, and dysfunction of this organelle plays a critical role in metabolic homeostasis as well as disease (Ozcan et al., 2004). Interestingly, a chronic inflammatory environment also impairs the function of the ER (Yang et al., 2015), thus presenting a vicious cycle that prevents resolution of these pathological chronic responses (Cao et al., 2016; Hotamisligil, 2010; Zhang and Kaufman, 2008).
Innate immune cellular mediators of inflammation
Although a role for proinflammatory cytokines in mediating insulin resistance in obesity had been established since the 1990s, and indeed the presence of macrophages in obese adipose tissue was initially reported in the 1960s (Hausberger, 1966; Hellman, 1965), it was not until 2003 that these observations captured the full attention of the research community. At that time, Anthony Ferrante’s and Hong Chen’s groups independently observed a marked upregulation of inflammatory gene expression in the adipose tissue of obese mice, and associated these changes with an influx of macrophages that supported the inflammatory milieu (Weisberg et al., 2003; Xu et al., 2003). Further analysis revealed that adipose tissue macrophages from lean mice have high levels of arginase 1 and IL-10 expression, whereas diet-induced obesity results in increased expression of iNOS and TNF by adipose tissue macrophages (Lumeng et al., 2007a), which contributes to the reduction in adipose tissue insulin sensitivity (Lumeng et al., 2007b). Macrophage accumulation in adipose tissue is the result of recruitment, retention, and proliferation of these cells at this site (Hill et al., 2014). The abundant presence of tissue resident macrophages that are polarized towards repair and maintenance functions in lean adipose tissue suggests a physiological role for these cells in homeostasis, and indicates that they are reprogrammed into an inflammatory phenotype in the setting of metabolic stress and during obesity, leading to functional deterioration.
More recent research has provided detailed insight into the activation and functional impact of adipose tissue macrophages (ATMs). For example, proteomic profiling revealed that although metabolic stress and classical macrophage activation by LPS and IFNγ both induce the expression of inflammatory cytokines such as TNF, this seems to occur via separate pathways (Kratz et al., 2014). In this study, Kratz et al. showed that classical macrophage markers of inflammation such as CD38 and CD274 are not induced in response to metabolic stimuli, which indicates that alternative activation pathways, including fatty acid-driven PPARγ signaling, may underlie metaflammatory phenotypes (Kratz et al., 2014). Interestingly, recent studies also support the concept that chronic ER stress in obesity may lead to inflammatory polarization in adipose tissue macrophages (Shan et al., 2017). It was also demonstrated that GPS2, a component of the co-repressor complex, restrains expression of pro-inflammatory mediators in ATMs. Expression of GPS2 is decreased in the ATMs of obese humans, and accordingly the ATMs are more sensitive to activation. In agreement with this model, GPS2-deficient mice display exaggerated systemic inflammation and worsened glucose and insulin intolerance in response to high fat diet challenge (Fan et al., 2016). Recently, Galectin-3, a lectin produced by macrophages, was also demonstrated to promote adipose tissue inflammation and glucose intolerance. Furthermore, both genetic deletion and pharmacological inhibition of this molecule resulted in resolution of adipose tissue inflammation and marked improvements in insulin sensitivity and glucose tolerance (Li et al., 2016). These studies, along with related findings from many other groups, indicate the potential that specific metaflammatory pathways may exist that will offer novel therapeutic strategies for metabolic disease.
There has also been a growing recognition that in addition to macrophages, other innate immune cell lineages also contribute to the regulation of metabolism. For example, Carey Lumeng’s group recently interrogated the role of adipose tissue dendritic cells (ATDCs), which in previous studies may have been indistinguishable from macrophages because of their expression of F4/80 and CD45, but which can be defined as CD64−, CD11c+ (Cho et al., 2016). This group showed that ATDCs accumulate in adipose tissue of HFD-fed mice and in the subcutaneous adipose tissue of obese humans, and blocking their accumulation improves insulin sensitivity in obese mice (Cho et al., 2016). In further support of this action, Jay Heinekcke’s group has produced evidence that dendritic cells constrain healthy expansion of adipose tissue, and depletion of these cells improves glucose homeostasis in lean mice (Pamir et al., 2015). Jerry Olefsky’s laboratory has also demonstrated an important role for neutrophils in obesity-related insulin resistance, showing that genetic deletion of neutrophil elastase reduces macrophage influx into the adipose tissue of obese mice and results in improved insulin sensitivity (Talukdar et al., 2012).
It is important to note that innate immune cells also play important roles in healthy tissue physiology, and indeed adipose tissue also naturally harbors immune cells which may contribute to tissue homeostasis. Adipose tissue resident immune cells play a critical role in the healthy expansion and remodeling of the tissue during weight gain (Lee et al., 2013; Wernstedt Asterholm et al., 2014), and have recently been shown to modulate brown adipose tissue innervation (Wolf et al., 2017). Immune cells in adipose tissue may also regulate energy expenditure: cold exposure is reported to result in alternative activation of adipose tissue macrophages (Nguyen et al., 2011) and recruitment of eosinophils (Qiu et al., 2014). Relatedly, type 2 innate lymphoid cells (ILC2s) promote the differentiation of beige adipocytes from adipocyte precursors (Brestoff et al., 2015; Lee et al., 2015) to protect against diet-induced insulin resistance (Molofsky et al., 2013). Alternatively activated macrophages have been proposed to promote adaptive thermogenesis directly via their production of catecholamines (Nguyen et al., 2011), although independent studies found that adipose tissue macrophages from cold-exposed mice do not express the key catecholamine synthesis enzyme tyrosine hydroxylase (TH) (Chang et al., 2016; Fischer et al., 2017; Spadaro et al., 2017), and TH deletion in hematopoietic cells neither affected adipose tissue browning nor energy expenditure in response to cold (Fischer et al., 2017). In contrast, polarization towards a more proinflammatory activation directly inhibits adipose tissue browning (Chung et al., 2017) and in turn, browning has been described to limit adipose tissue inflammation (Cohen et al., 2014). However, regardless of whether macrophages directly promote thermogenesis, all of these studies confirm the presence of immune cells resident in adipose tissue in homeostatic and adaptive settings. As further indication that the number of immune cells in the tissue does not always directly correlate with adverse phenotypes, immune cells have been shown to acutely accumulate in adipose tissue during weight loss in response to lipolysis (Kosteli et al., 2010).
Although we focus here on adipose tissue inflammation in obesity as the origin of this concept, similar phenomena occur in other tissues and have been demonstrated to contribute to alterations in systemic glucose homeostasis. For example, type 2 diabetes has been associated with an increase in the number of islet-associated macrophages which impair the function of beta cells (Ehses et al., 2007). Natural Killer T (NKT) cells are depleted in the livers of mice with diet-induced obesity, which may promote hepatic production of inflammatory cytokines and liver inflammation (Li et al., 2005). Obesity is associated with an influx of macrophages to muscle tissue (Hong et al., 2009), and TNF levels in muscle are higher in diabetic subjects compared with insulin-sensitive individuals (Saghizadeh et al., 1996). As mentioned earlier, high fat feeding is associated with the activation of JNK and IKK signaling in the hypothalamus (De Souza et al., 2005). Taken together, these studies demonstrate that obesity is associated with changes in many immune cell types at multiple sites of critical metabolic function, with a cumulative detrimental effect on systemic insulin action as well as glucose and lipid homeostasis. It is also important to recognize that stromal components (adipocytes, beta cells, hepatocytes, etc.) also produce immune mediators often attributed to immune cells, and thus our measurement of metaflammation reflects a cumulative output of both the immune effector cells and the stroma.
Adaptive immune cellular mediators of inflammation
Over the past decade, evidence has accumulated that the adaptive immune system also participates in the inflammatory response to obesity. Beginning in 2008, multiple groups observed the infiltration of T cells- both T-helper and cytotoxic- into the adipose tissue of obese mice and humans (Nishimura et al., 2009; Rausch et al., 2008; Yang et al., 2010). CD4+FOXP3+ T regulatory (Treg) cells were also documented in the adipose tissue of lean mice, and suggested to promote insulin sensitivity (Feuerer et al., 2009; Kolodin et al., 2015; Winer et al., 2009). B cells have been implicated in modulating insulin resistance: B cells accumulate in the adipose tissue of obese mice (Winer et al., 2009), the B cells from obese mice produce a more inflammatory repertoire of cytokines, and obese mice with B cell deficiency have reduced insulin resistance (DeFuria et al., 2013). Importantly, transfer of B cells from obese donor mice results in impaired insulin action and glucose homeostasis in the recipients (Winer et al., 2011). By contrast, tolerance-promoting B regulatory cells have also been identified in adipose tissue, and their numbers are decreased in models of obesity (Nishimura et al., 2013). These cells limit adipose tissue inflammation and their adoptive transfer is metabolically beneficial. Similarly, type 1 NKT cells (Ji et al., 2012) and invariant natural killer T (iNKT) cells (Lynch, 2014), which are both thought to have anti-inflammatory roles, decrease in abundance in adipose tissue in the setting of obesity. These topics are covered in recent excellent reviews (Chawla et al., 2011; Sell et al., 2012; Sonnenberg and Artis, 2015) and will not be covered here in detail (Figure 3).
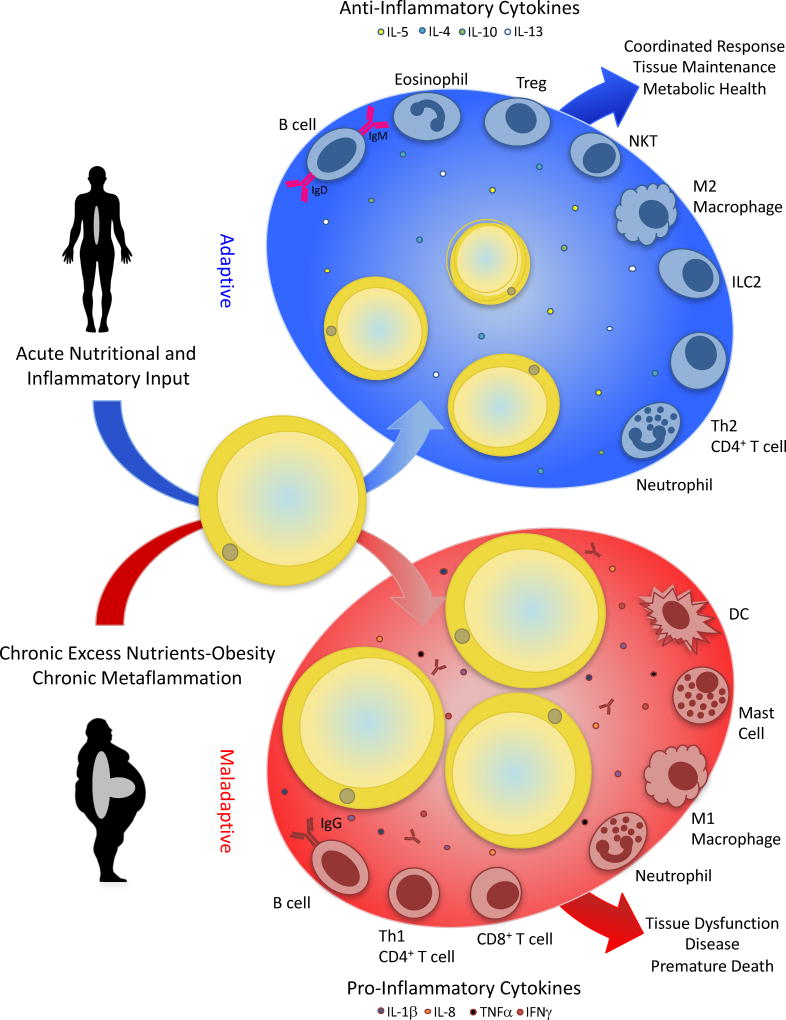
Figure 3. Adipose tissue-immune cell interactions.
Interactions with immune effectors and stromal components are critical for tissue maintenance and health. Immune cells and their interactions with adipocytes, for example, are critical for adipose tissue homeostasis and response to acute inflammatory signals. The expression of multiple anti-inflammatory cytokines, and the presence of alternatively activated macrophages, immunomodulatory Tregs, and other cell types in the adipose tissue in the lean state has been well documented and may have adaptive roles in tissue health and maintenance. In the setting of obesity, chronic overnutrition and metabolic stresses trigger an inflamed state in the adipose tissue, characterized by enhanced immune infiltration, skewing of macrophage polarization towards the inflammatory phenotype, altered B cell antigen production, and expression of pro-inflammatory cytokines. In this case, the maladaptive chronic, non-resolving metabolic inflammation (metaflammation) contributes to tissue dysfunction, disease, and premature death, as best exemplified in obesity and associated metabolic complications. Although adipose tissue is depicted in this scheme as the first discovered and most studied site, similar interactions and transitions are observed in other key metabolic organs, including liver, pancreas, and brain.
The extent to which adaptive immunity contributes to insulin sensitivity in a context dependent manner may require further studies. In a careful time course analysis, Strissel et al. showed that the influx of CD3+ T cells into adipose tissue is a late event, occurring after more than 20 weeks of high fat feeding in mice and well after the onset of insulin resistance (Strissel et al., 2010), and Vishwa Dixit’s group demonstrated that specific depletion of CD3+ T cells from epididymal adipose tissue did not alter insulin sensitivity in adult obese mice (Yang et al., 2010). Similarly, although NKT cells have also been observed to accumulate in the adipose of obese mice, NKT cell deficiency does not protect from diet-induced insulin resistance (Ji et al., 2012). Finally, mice lacking both B and T cells (SCID mice) are not protected against obesity-induced glucose intolerance (Ballak et al., 2013), indicating the complex role of these immune cells in both controlling and promoting inflammatory responses. It is interesting to note that within the adipose tissue environment the T cell receptor repertoire shows significant bias in CD4+ T cells, which supports the possibility that specific antigens presented only in the adipose environment may lead to clonal expansion (Morris et al., 2013). Interestingly, CD8+ T cells have been shown to accumulate in the livers of obese mice and contribute to the development of metabolic dysfunction, but their accumulation and activation is believed to occur in response to interferon signaling, rather the presence of a specific antigen (Ghazarian, 2017). Identification of the tissue-specific differences in inflammatory activation may allow better dissection of functional programming of immune effectors associated with metabolic tissue inflammatory state. Finally, it should be noted that both stromal cells and immune effectors contribute to the inflammatory output within metabolic tissues.
Effects of nutrients and metabolites on inflammation
In the 1960s, work from Eric Newsholme’s laboratory began to reveal the important role of circulating lipids in modulating insulin sensitivity, demonstrating that lipids and fatty acids reduced insulin-induced glucose uptake in isolated heart muscle (Randle et al., 1963; Randle et al., 1964). This phenomenon was later shown in experiments in rats and humans (Jenkins et al., 1988; Johnson et al., 1992). However, the mechanism remained unclear for decades, until the innate immune component toll like receptor 4 (TLR4) was identified as a receptor for saturated and polyunsaturated fatty acids (Lee et al., 2003), suggesting that lipids themselves may act as immunomodulatory molecules. Later, it was shown that TLR4 deficiency selectively protected mice from diets high in saturated fat (Davis et al., 2008), and furthermore TLR signaling may play a role in the central control of metabolism, as deletion of the TLR adaptor molecule MyD88 in the central nervous system protects mice from diet induced insulin resistance (Kleinridders et al., 2009).
A further example of the crosstalk between nutrients and inflammation began to become clear in the last several years based on the study of the inflammasome, which produced compelling evidence linking abnormal inflammasome activation to metabolic deterioration (Henao-Mejia et al., 2014). In 2010, Jürg Tschopp’s laboratory demonstrated that mice deficient in the inflammasome scaffold protein Nlrp3 displayed enhanced insulin sensitivity (Zhou et al., 2010), and other groups demonstrated similar results in mice with genetic deletions of other inflammasome components shortly thereafter (Stienstra et al., 2010; Stienstra et al., 2011; Vandanmagsar et al., 2011). Concurrently, Jenny PY Ting’s group demonstrated that palmitate, a saturated fatty acid that is known to be present at an increased level in the circulation of high fat diet-fed mice, activates the inflammasome and induces IL-1β and IL-18 secretion from macrophages (Wen et al., 2011). This study also illustrated that blocking inflammasome (IL1β) and non-inflammasome (TNF) pathways simultaneously had an additive effect, yielding greater metabolic benefit then single inhibition of either. Emerging evidence suggests that activation of the inflammasome may also be integrated with TLR signaling, as palmitate-induced induction of IL-1β expression can be inhibited by blocking TLRs (Snodgrass et al., 2013). Other molecules such as Protein Kinase R (PKR) which can sense pathogens as well as nutrient signals to alter inflammatory and metabolic responses have also been shown to serve as an important regulator of inflammasome activation (Boriushkin et al., 2016; Hett et al., 2013; Xie et al., 2016).
Notably, other critical mechanisms also clearly contribute to the role of lipid-induced modulation of insulin resistance, possibly through mechanisms independent of cytokine or TLR signaling. For example, Gerald Shulman’s group has demonstrated that lipid infusion leads to the accumulation of fatty acyl-CoA and diacylglycerol (DAG) in skeletal muscle, which activates Protein Kinase C θ (PKCθ), resulting in inhibition of IRS-1 through serine phosphorylation (Griffin et al., 1999; Szendroedi et al., 2014; Yu et al., 2002). Both of these signals are also clearly linked to inflammatory outcomes. However, neither the sufficiency of this pathway to mediate diet-induced insulin resistance, nor the precise identity of the mechanism are fully understood. Animal models investigating the role of PKCθ in the development of insulin resistance have had complex results (Kim et al., 2004b; Serra et al., 2003), and IRS1 serine phosphorylation sites are too numerous to reduce to single sites for definitive experimentation. In recent elegant work, the Shulman group has also characterized the role of additional PKC isoforms and recently used mass spectrometry to demonstrate that the insulin receptor is a target of PKCε in the liver (Petersen et al., 2016). Supporting the metabolic relevance of this biology, mutation of the target threonine on the insulin receptor protects mice from diet-induced insulin resistance (Petersen et al., 2016). Hence, accumulation of these and possibly other detrimental lipid species are of key importance in metabolic deterioration in obesity.
We now also know that some lipids can exert anti-inflammatory effects locally and systemically and regulate metabolism. For instance, the de novo lipogenesis product palmitoleate (C16:1n7) can block lipid-induced inflammatory activation of macrophages, leading to beneficial metabolic outcomes in mouse models of obesity or metabolic disease (Cao et al., 2008; Cimen et al., 2016; Erbay et al., 2009; Talbot et al., 2014). Importantly, palmitoleate administration in humans also generates significant metabolic benefits (Bernstein et al., 2014). Barbara Kahn’s laboratory identified fatty acid hydroxy fatty acids (FAHFAs) also regulated during lipogenesis, and demonstrated that treatment with FAHFAs was sufficient to reduce adipose tissue inflammation and improve glucose homeostasis in obese mice (Yore et al., 2014). In addition, extensive work has highlighted the potent anti-inflammatory actions of omega-3 fatty acids and their metabolites resolvins (Oh et al., 2010; Serhan, 2014). Interestingly, the Olefsky group has demonstrated that omega-3 fatty acids exert their immunomodulatory effects through cell surface receptors, specifically the G-coupled protein receptor GPR120. This research demonstrated omega-3 fatty acids inhibit inflammation and improve glucose homeostasis in mice with diet-induced obesity, in a GPR120-dependent manner (Oh et al., 2010). Expanding on this finding, Olefsky’s group demonstrated that a small molecule agonist of GPR120 improved insulin sensitivity and reduced steatosis in obese mice (Oh et al., 2010). Finally, there is strong evidence that this pathway of nutrient sensing and metaflammatory response is conserved in humans, as a loss of function mutation in GPR120 has been linked to increased risk of obesity (Ichimura et al., 2012). Hence, anti-inflammatory lipids offer great promise for translational opportunities.
Besides circulating lipids, new research demonstrates that other nutrient and metabolite changes can contribute to insulin resistance. Perhaps most notably, comprehensive analysis of serum from lean and obese subjects has revealed that the metabolites that correlated most closely with insulin resistance were derived not from lipids but branched chain amino acids (BCAAs) (Newgard et al., 2009). Furthermore, supplementing high fat diet with BCAAs induced insulin resistance despite reduced food intake and weight gain, and was associated with increased activity of JNK in muscle (Newgard et al., 2009). Interestingly, a recent report showed that exposure to inflammatory cytokines blocked the uptake and metabolism of BCAAs in adipocytes, which could underlie the elevated levels observed in obese patients (Burrill et al., 2015). In addition, exposure to the macromolecules synthesized by pathogenic and commensal species can also have marked immunomodulatory and metabolic consequences. For example, a glycan found on parasitic helminths has been shown to induce anti-inflammatory cytokine production, which reduces adipose tissue inflammation and improves systemic insulin sensitivity and glucose metabolism (Bhargava et al., 2012).
Furthermore, adipocytes produce numerous peptide hormones with the capacity to alter immune responses. A prime example is leptin; leptin-deficient ob/ob mice demonstrate enhanced sensitivity to LPS-induced lethality, and delivery of exogenous leptin extends survival in mice dosed with LPS (Faggioni et al., 1999). In vitro it has been shown that leptin treatment of macrophages enhances LPS-induced expression of TNFα and IL-6 (Loffreda et al., 1998). Similarly, RBP4 is an adipocyte product that binds to vitamin A (retinol) that appears to regulate both inflammation and glucose metabolism (Yang et al., 2005) and elevated levels of RBP4 have been demonstrated in both obese mouse models and humans (Graham et al., 2006; Yang et al., 2005). The adipocyte hormone resistin induces secretion TNFα and IL-12 from macrophages (Silswal et al., 2005), and neutralizing resistin improves insulin sensitivity in obese mice (Steppan et al., 2001). Other adipocyte products function to protect against inflammation; for example adiponectin blocks leptin-induced TNFα expression in macrophages (Zhao et al., 2005). Our group showed that adipocyte-produced aP2 (FABP4) plays an important hormonal role in glucose homeostasis (Cao et al., 2013) and its genetic or antibody-mediated blockade results in improved glucose homeostasis and metabolic outcomes. Earlier studies have demonstrated a significant role for aP2 in modulating macrophage inflammatory responses and cardiometabolic pathologies (Erbay et al., 2009; Furuhashi et al., 2008). While it is not yet known whether the hormonal form of aP2 also exerts immunomodulatory activity, the biology and function of this lipid binding protein offers a compelling mode of immunometabolic integration between metabolic and immune cells which may be targeted for therapeutic purposes.
Metabolism as a determinant of immune cell phenotype
Just as it has been clearly demonstrated that energy and nutrient excess and the resulting metabolic stress can trigger metaflammation and alter immune response to promote disease, it has also long been recognized that other alterations that occur during energy and nutrient deficiency can impair immunity. Similarly, specialized metabolic conditions, such as pregnancy, cold adaptation, migration or hibernation can also present challenges for the immune system. There is much to learn about immunometabolic regulation in future studies on these conditions. In addition to systemic changes in metabolism, the mounting of an immune response and functional programming within a cell is associated with innate metabolic changes (O'Neill and Hardie, 2013). One of the most well recognized of these changes is the activation of anaerobic glycolysis, which is a common feature of inflammatory activation of T cells (Chang et al., 2013; Wang and Green, 2012), dendritic cells (Everts et al., 2014), and macrophages (Tannahill et al., 2013). In macrophages, the glycolytic phenotype offers many advantages in the setting of obesity, including enhanced nitric oxide production and reduced reliance on oxygen, a survival advantage in hypoxic environments such as obese adipose tissue (Ghesquiere et al., 2014). In addition, metabolite profiling of activated macrophages has shown that the accumulation of Kreb’s cycle intermediates is important for the production of inflammatory cytokines (Jha et al., 2015). This arm of immunometabolism is rapidly expanding, and has the potential to lead to many exciting therapeutic intervention strategies.
The overlap between metabolic and immune signaling pathways means that altered metabolic states can have an important impact on immune function. For example, obesity is associated with altered immune responses including reduced T cell responsiveness (Tanaka et al., 1993), and morbidly obese patients have abnormal bactericidal activity of polymorphonuclear (PMN) granulocytes, which improves following weight loss surgery (Palmblad et al., 1980). This is in part related to alterations in circulating nutrient and metabolite signals in the setting of obesity, as macrophage polarization is influenced by nutrient sensing pathways such as AMPK and mTORc1. Macrophages lacking the catalytic AMPK subunit AMPKα1 were shown to have defective M2 polarization (Mounier et al., 2013), and AMPKβ1 deficient macrophages are protected from palmitate induced inflammation (Galic et al., 2011). The nutrient and growth factor sensor mTORc1 regulates multiple metabolic pathways through SREBP (Duvel et al., 2010), and constitutive mTORc1 activation results in defective M2 polarization and enhanced proinflammatory response to LPS (Byles et al., 2013). Relatedly, it was also recently shown that mTOR activity is suppressed by the anti-inflammatory cytokine IL-10, resulting in an increase in mitophagy and suppressed inflammasome activation (Ip et al., 2017). Finally, short chain fatty acids, byproducts of microbial fermentation, can drive the differentiation of T cell subsets, and multiple groups have demonstrated the potential for butyrate and propionate produced by commensal bacteria in the gut to promote Treg cell differentiation (Arpaia et al., 2013; Furusawa et al., 2013; Smith et al., 2013).
Translational strategies and therapeutic implications
The complexity of the immunometabolic networks in chronic disease creates challenges to therapeutic translation, and the small number of studies with classical single molecule immune-targeted therapies for metabolic disease have so far yielded some success in the limited human trials conducted (Donath, 2014). Despite these caveats, the results strongly support the clinical value of targeting immunometabolism, highly promising new areas are in development, and the links to human disease as well as mechanisms for intervention have greatly expanded in the recent years (Hotamisligil, 2017). Furthermore, the connections between these systems are being continuously elaborated: robust genomic studies are highlighting novel associations between variations in inflammatory genes and pathways and metabolic phenotypes (Locke et al., 2015; Shungin et al., 2015), and previously well-characterized developmental and homeostatic signaling pathways such as non-cannonical erythropoietin action are being recognized for their ability to alter inflammation and improve systemic metabolism in model systems as well as in humans (Cerami, 2012; Fuster et al., 2014; Ouchi et al., 2010). The status of human research in this area and emerging therapeutic possibilities are further discussed in recent publications (Donath, 2014; Hotamisligil, 2017). Evidence of the importance of inflammation in metabolic disease may also be deduced from the fact that the current anti-diabetic strategies currently in clinical use, including metformin, thiazolidinediones, DPP4 inhibitors, incretins, and lifestyle interventions, have all been shown to reduce inflammation (Kothari et al., 2016; Lancaster and Febbraio, 2014; Scheen et al., 2015). While our main focus in this discussion has been the negative impact of pro-inflammatory mediators on metabolism, in recent years numerous anti-inflammatory cytokines, such as IL-4, (Chang et al., 2012; Ricardo-Gonzalez et al., 2010) IL-10, (Cintra et al., 2008; Kim et al., 2004a) IL-13, (Darkhal et al., 2015; Stanya et al., 2013) and Hemoxigenase-1 (Huang et al., 2012b; Li et al., 2008), have all been demonstrated to positively regulate metabolism. We are also now recognizing that some endogenously synthesized lipids and metabolites exert potent immunomodulatory effects with exciting and immediate translational possibilities (Cao et al., 2008; Serhan, 2014; Spite et al., 2014; Yore et al., 2014). For example, a recent clinical trial demonstrated that treating obese diabetic patients with an inhibitor of IKKε improved glucose control and, in a subgroup of patients, the treatment resulted in reduced inflammatory gene expression and improved insulin sensitivity (Oral et al., 2017). Another recent study utilized a novel immunomodulatory technique utilizing immunological stem cells to improve beta cell function in type 1 and type 2 diabetic patients (Zhao et al., 2017). These emerging targets, and the development of strategies that incorporate multi-pronged approaches, offer exciting and highly promising avenues for therapeutic opportunities for metabolic diseases. Having said that, it is also clear that inflammatory mediators are not the sole drivers of metabolic disorders, and questions and pathways remain to be explored and exploited for the benefit of patients in a safe and effective manner and in continued support of healthy lifestyle recommendations.
Conclusions
As we have discussed here, careful observations and experiments in animal models and cellular systems have revealed that metabolism and immunity are inextricably interwoven. Research in this field has accelerated over the last two decades (Figure 2), and it has become increasingly clear that overlapping and redundant inflammatory pathways play pleiotropic and important roles in metabolism, and that the metabolic state is a critical determinant of immune function. We have learned that components of both the innate and adaptive immune system modulate metabolism, and we have identified key molecules that drive the cellular and systemic responses to nutrients. Most relevant to the current global obesity epidemic, we are now learning how these networks are re-wired in response to the stress imposed by chronic nutrient excess. This excellent progress should allow us to overcome the challenges of designing effective therapeutic and disease preventative strategies at the immune:metabolic interface to improve human health.
Acknowledgments
I am grateful to Dr. Kathryn Claiborn for editorial assistance and thank all members of the Hotamisligil Lab and Sabri Ülker Center for helpful discussions. Special thanks to Dr. Kacey Prentice for designing and drawing figures. Work in the Hotamisligil lab is supported by grants from the National Institutes of Health (DK052539, HL125753, AI116901), the JDRF (2SRA-2016-147-Q-R), and sponsored research agreements from UCB and Servier. We apologize to our colleagues for omitting many valuable references due to space constraints.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguirre V, Werner ED, Giraud J, Lee YH, Shoelson SE, White MF. Phosphorylation of Ser307 in Insulin Receptor Substrate-1 Blocks Interactions with the Insulin Receptor and Inhibits Insulin Action. J Biol Chem. 2002;277:1531–1537. doi: 10.1074/jbc.M101521200. [DOI] [PubMed] [Google Scholar]
- Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. Ikk-Beta Links Inflammation to Obesity-Induced Insulin Resistance. Nature medicine. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, et al. Metabolites Produced by Commensal Bacteria Promote Peripheral Regulatory T-Cell Generation. Nature. 2013;504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballak DB, Stienstra R, Hijmans A, Joosten LA, Netea MG, Tack CJ. Combined B- and T-Cell Deficiency Does Not Protect against Obesity-Induced Glucose Intolerance and Inflammation. Cytokine. 2013;62:96–103. doi: 10.1016/j.cyto.2013.02.009. [DOI] [PubMed] [Google Scholar]
- Barnes PJ, Karin M. Nuclear Factor-Kappab: A Pivotal Transcription Factor in Chronic Inflammatory Diseases. The New England journal of medicine. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- Bernstein AM, Roizen MF, Martinez L. Purified Palmitoleic Acid for the Reduction of High-Sensitivity C-Reactive Protein and Serum Lipids: A Double-Blinded, Randomized, Placebo Controlled Study. J Clin Lipidol. 2014;8:612–617. doi: 10.1016/j.jacl.2014.08.001. [DOI] [PubMed] [Google Scholar]
- Berson SA, Yalow RS. Insulin Antagonists, Insulin Antibodies and Insulin Resistance. The American journal of medicine. 1958;25:155–159. doi: 10.1016/0002-9343(58)90022-6. [DOI] [PubMed] [Google Scholar]
- Beutler B, Greenwald D, Hulmes JD, Chang M, Pan YC, Mathison J, Ulevitch R, Cerami A. Identity of Tumour Necrosis Factor and the Macrophage-Secreted Factor Cachectin. Nature. 1985;316:552–554. doi: 10.1038/316552a0. [DOI] [PubMed] [Google Scholar]
- Bhargava P, Li C, Stanya KJ, Jacobi D, Dai L, Liu S, Gangl MR, Harn DA, Lee CH. Immunomodulatory Glycan Lnfpiii Alleviates Hepatosteatosis and Insulin Resistance through Direct and Indirect Control of Metabolic Pathways. Nature medicine. 2012;18:1665–1672. doi: 10.1038/nm.2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden G, Duan X, Homko C, Molina EJ, Song W, Perez O, Cheung P, Merali S. Increase in Endoplasmic Reticulum Stress-Related Proteins and Genes in Adipose Tissue of Obese, Insulin-Resistant Individuals. Diabetes. 2008;57:2438–2444. doi: 10.2337/db08-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boriushkin E, Wang JJ, Li J, Bhatta M, Zhang SX. P58(Ipk) Suppresses Nlrp3 Inflammasome Activation and Il-1beta Production Via Inhibition of Pkr in Macrophages. Sci Rep. 2016;6:25013. doi: 10.1038/srep25013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst SE, Bagby GJ. Neutralization of Tumor Necrosis Factor Reverses Age-Induced Impairment of Insulin Responsiveness in Skeletal Muscle of Sprague-Dawley Rats. Metabolism: clinical and experimental. 2002;51:1061–1064. doi: 10.1053/meta.2002.34043. [DOI] [PubMed] [Google Scholar]
- Brestoff JR, Kim BS, Saenz SA, Stine RR, Monticelli LA, Sonnenberg GF, Thome JJ, Farber DL, Lutfy K, Seale P, et al. Group 2 Innate Lymphoid Cells Promote Beiging of White Adipose Tissue and Limit Obesity. Nature. 2015;519:242–246. doi: 10.1038/nature14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrill JS, Long EK, Reilly B, Deng Y, Armitage IM, Scherer PE, Bernlohr DA. Inflammation and Er Stress Regulate Branched-Chain Amino Acid Uptake and Metabolism in Adipocytes. Molecular endocrinology. 2015;29:411–420. doi: 10.1210/me.2014-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byles V, Covarrubias AJ, Ben-Sahra I, Lamming DW, Sabatini DM, Manning BD, Horng T. The Tsc-Mtor Pathway Regulates Macrophage Polarization. Nat Commun. 2013;4:2834. doi: 10.1038/ncomms3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and Systemic Insulin Resistance Resulting from Hepatic Activation of Ikk-Beta and Nf-Kappab. Nature medicine. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JS, Prichard L, Schaper F, Schmitz J, Stephenson-Famy A, Rosenfeld ME, Argast GM, Heinrich PC, Fausto N. Expression of Suppressors of Cytokine Signaling During Liver Regeneration. The Journal of clinical investigation. 2001;107:1285–1292. doi: 10.1172/JCI11867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS. Identification of a Lipokine, a Lipid Hormone Linking Adipose Tissue to Systemic Metabolism. Cell. 2008;134:933–944. doi: 10.1016/j.cell.2008.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Sekiya M, Ertunc ME, Burak MF, Mayers JR, White A, Inouye K, Rickey LM, Ercal BC, Furuhashi M, et al. Adipocyte Lipid Chaperone Ap2 Is a Secreted Adipokine Regulating Hepatic Glucose Production. Cell Metab. 2013;17:768–778. doi: 10.1016/j.cmet.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao SS, Luo KL, Shi L. Endoplasmic Reticulum Stress Interacts with Inflammation in Human Diseases. Journal of cellular physiology. 2016;231:288–294. doi: 10.1002/jcp.25098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B. An Endotoxin-Induced Serum Factor That Causes Necrosis of Tumors. Proceedings of the National Academy of Sciences of the United States of America. 1975;72:3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerami A. Tnf and Epo: Major Players in the Innate Immune Response: Their Discovery. Annals of the rheumatic diseases. 2012;71(Suppl 2):i55–59. doi: 10.1136/annrheumdis-2011-200800. [DOI] [PubMed] [Google Scholar]
- Chang CH, Curtis JD, Maggi LB, Jr, Faubert B, Villarino AV, O'Sullivan D, Huang SC, van der Windt GJ, Blagih J, Qiu J, et al. Posttranscriptional Control of T Cell Effector Function by Aerobic Glycolysis. Cell. 2013;153:1239–1251. doi: 10.1016/j.cell.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HR, Kim HJ, Xu X, Ferrante AW., Jr Macrophage and Adipocyte Igf1 Maintain Adipose Tissue Homeostasis During Metabolic Stresses. Obesity. 2016;24:172–183. doi: 10.1002/oby.21354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YH, Ho KT, Lu SH, Huang CN, Shiau MY. Regulation of Glucose/Lipid Metabolism and Insulin Sensitivity by Interleukin-4. International journal of obesity. 2012;36:993–998. doi: 10.1038/ijo.2011.168. [DOI] [PubMed] [Google Scholar]
- Chawla A, Nguyen KD, Goh YP. Macrophage-Mediated Inflammation in Metabolic Disease. Nat Rev Immunol. 2011;11:738–749. doi: 10.1038/nri3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KW, Zamarron BF, Muir LA, Singer K, Porsche CE, DelProposto JB, Geletka L, Meyer KA, O'Rourke RW, Lumeng CN. Adipose Tissue Dendritic Cells Are Independent Contributors to Obesity-Induced Inflammation and Insulin Resistance. Journal of immunology. 2016 doi: 10.4049/jimmunol.1600820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung KJ, Chatzigeorgiou A, Economopoulou M, Garcia-Martin R, Alexaki VI, Mitroulis I, Nati M, Gebler J, Ziemssen T, Goelz SE, et al. A Self-Sustained Loop of Inflammation-Driven Inhibition of Beige Adipogenesis in Obesity. Nature immunology. 2017 doi: 10.1038/ni.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimen I, Kocaturk B, Koyuncu S, Tufanli O, Onat UI, Yildirim AD, Apaydin O, Demirsoy S, Aykut ZG, Nguyen UT, et al. Prevention of Atherosclerosis by Bioactive Palmitoleate through Suppression of Organelle Stress and Inflammasome Activation. Sci Transl Med. 2016;8:358ra126. doi: 10.1126/scitranslmed.aaf9087. [DOI] [PubMed] [Google Scholar]
- Cintra DE, Pauli JR, Araujo EP, Moraes JC, de Souza CT, Milanski M, Morari J, Gambero A, Saad MJ, Velloso LA. Interleukin-10 Is a Protective Factor against Diet-Induced Insulin Resistance in Liver. Journal of hepatology. 2008;48:628–637. doi: 10.1016/j.jhep.2007.12.017. [DOI] [PubMed] [Google Scholar]
- Cohen P, Levy JD, Zhang Y, Frontini A, Kolodin DP, Svensson KJ, Lo JC, Zeng X, Ye L, Khandekar MJ, et al. Ablation of Prdm16 and Beige Adipose Causes Metabolic Dysfunction and a Subcutaneous to Visceral Fat Switch. Cell. 2014;156:304–316. doi: 10.1016/j.cell.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copps KD, White MF. Regulation of Insulin Sensitivity by Serine/Threonine Phosphorylation of Insulin Receptor Substrate Proteins Irs1 and Irs2. Diabetologia. 2012;55:2565–2582. doi: 10.1007/s00125-012-2644-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius P, Marlowe M, Lee MD, Pekala PH. The Growth Factor-Like Effects of Tumor Necrosis Factor-Alpha. Stimulation of Glucose Transport Activity and Induction of Glucose Transporter and Immediate Early Gene Expression in 3t3-L1 Preadipocytes. J Biol Chem. 1990;265:20506–20516. [PubMed] [Google Scholar]
- da Costa RM, Neves KB, Mestriner FL, Louzada-Junior P, Bruder-Nascimento T, Tostes RC. Tnf-Alpha Induces Vascular Insulin Resistance Via Positive Modulation of Pten and Decreased Akt/Enos/No Signaling in High Fat Diet-Fed Mice. Cardiovasc Diabetol. 2016;15:119. doi: 10.1186/s12933-016-0443-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darkhal P, Gao M, Ma Y, Liu D. Blocking High-Fat Diet-Induced Obesity, Insulin Resistance and Fatty Liver by Overexpression of Il-13 Gene in Mice. International journal of obesity. 2015;39:1292–1299. doi: 10.1038/ijo.2015.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JE, Gabler NK, Walker-Daniels J, Spurlock ME. Tlr-4 Deficiency Selectively Protects against Obesity Induced by Diets High in Saturated Fat. Obesity. 2008;16:1248–1255. doi: 10.1038/oby.2008.210. [DOI] [PubMed] [Google Scholar]
- De Souza CT, Araujo EP, Bordin S, Ashimine R, Zollner RL, Boschero AC, Saad MJ, Velloso LA. Consumption of a Fat-Rich Diet Activates a Proinflammatory Response and Induces Insulin Resistance in the Hypothalamus. Endocrinology. 2005;146:4192–4199. doi: 10.1210/en.2004-1520. [DOI] [PubMed] [Google Scholar]
- DeFuria J, Belkina AC, Jagannathan-Bogdan M, Snyder-Cappione J, Carr JD, Nersesova YR, Markham D, Strissel KJ, Watkins AA, Zhu M, et al. B Cells Promote Inflammation in Obesity and Type 2 Diabetes through Regulation of T-Cell Function and an Inflammatory Cytokine Profile. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:5133–5138. doi: 10.1073/pnas.1215840110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donath MY. Targeting Inflammation in the Treatment of Type 2 Diabetes: Time to Start. Nature reviews Drug discovery. 2014;13:465–476. doi: 10.1038/nrd4275. [DOI] [PubMed] [Google Scholar]
- Drobny EC, Abramson EC, Baumann G. Insulin Receptors in Acute Infection: A Study of Factors Conferring Insulin Resistance. The Journal of clinical endocrinology and metabolism. 1984;58:710–716. doi: 10.1210/jcem-58-4-710. [DOI] [PubMed] [Google Scholar]
- Duvel K, Yecies JL, Menon S, Raman P, Lipovsky AI, Souza AL, Triantafellow E, Ma Q, Gorski R, Cleaver S, et al. Activation of a Metabolic Gene Regulatory Network Downstream of Mtor Complex 1. Mol Cell. 2010;39:171–183. doi: 10.1016/j.molcel.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebstein W MJ. Weitere Mitteillungen Uber Die Behandlung Des Diabetes Mellitus Mot Carbisaure Nebst Bemerkunger Uber Die Anwendung Der Salicylsaure Bie Dieser Krankheit. Berl Klin Wochenschr. 1876;13:53–56. [Google Scholar]
- Ehses JA, Perren A, Eppler E, Ribaux P, Pospisilik JA, Maor-Cahn R, Gueripel X, Ellingsgaard H, Schneider MK, Biollaz G, et al. Increased Number of Islet-Associated Macrophages in Type 2 Diabetes. Diabetes. 2007;56:2356–2370. doi: 10.2337/db06-1650. [DOI] [PubMed] [Google Scholar]
- Eisenbarth SC, Williams A, Colegio OR, Meng H, Strowig T, Rongvaux A, Henao-Mejia J, Thaiss CA, Joly S, Gonzalez DG, et al. Corrigendum: Nlrp10 Is a Nod-Like Receptor Essential to Initiate Adaptive Immunity by Dendritic Cells. Nature. 2016;530:504. doi: 10.1038/nature16074. [DOI] [PubMed] [Google Scholar]
- Erbay E, Babaev VR, Mayers JR, Makowski L, Charles KN, Snitow ME, Fazio S, Wiest MM, Watkins SM, Linton MF, et al. Reducing Endoplasmic Reticulum Stress through a Macrophage Lipid Chaperone Alleviates Atherosclerosis. Nature medicine. 2009;15:1383–1391. doi: 10.1038/nm.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everts B, Amiel E, Huang SC, Smith AM, Chang CH, Lam WY, Redmann V, Freitas TC, Blagih J, van der Windt GJ, et al. Tlr-Driven Early Glycolytic Reprogramming Via the Kinases Tbk1-Ikkvarepsilon Supports the Anabolic Demands of Dendritic Cell Activation. Nature immunology. 2014;15:323–332. doi: 10.1038/ni.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faggioni R, Fantuzzi G, Gabay C, Moser A, Dinarello CA, Feingold KR, Grunfeld C. Leptin Deficiency Enhances Sensitivity to Endotoxin-Induced Lethality. Am J Physiol. 1999;276:R136–142. doi: 10.1152/ajpregu.1999.276.1.R136. [DOI] [PubMed] [Google Scholar]
- Fan R, Toubal A, Goni S, Drareni K, Huang Z, Alzaid F, Ballaire R, Ancel P, Liang N, Damdimopoulos A, et al. Loss of the Co-Repressor Gps2 Sensitizes Macrophage Activation Upon Metabolic Stress Induced by Obesity and Type 2 Diabetes. Nature medicine. 2016;22:780–791. doi: 10.1038/nm.4114. [DOI] [PubMed] [Google Scholar]
- Feingold KR, Soued M, Staprans I, Gavin LA, Donahue ME, Huang BJ, Moser AH, Gulli R, Grunfeld C. Effect of Tumor Necrosis Factor (Tnf) on Lipid Metabolism in the Diabetic Rat. Evidence That Inhibition of Adipose Tissue Lipoprotein Lipase Activity Is Not Required for Tnf-Induced Hyperlipidemia. The Journal of clinical investigation. 1989;83:1116–1121. doi: 10.1172/JCI113991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein R, Kanety H, Papa MZ, Lunenfeld B, Karasik A. Tumor Necrosis Factor-Alpha Suppresses Insulin-Induced Tyrosine Phosphorylation of Insulin Receptor and Its Substrates. J Biol Chem. 1993;268:26055–26058. [PubMed] [Google Scholar]
- Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, et al. Lean, but Not Obese, Fat Is Enriched for a Unique Population of Regulatory T Cells That Affect Metabolic Parameters. Nature medicine. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feve B, Bastard JP. The Role of Interleukins in Insulin Resistance and Type 2 Diabetes Mellitus. Nature reviews Endocrinology. 2009;5:305–311. doi: 10.1038/nrendo.2009.62. [DOI] [PubMed] [Google Scholar]
- Fischer K, Ruiz HH, Jhun K, Finan B, Oberlin DJ, van der Heide V, Kalinovich AV, Petrovic N, Wolf Y, Clemmensen C, et al. Alternatively Activated Macrophages Do Not Synthesize Catecholamines or Contribute to Adipose Tissue Adaptive Thermogenesis. Nature medicine. 2017;23:623–630. doi: 10.1038/nm.4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MJ, Kuzma JF, Washam WT. Transitory Diabetic Syndrome Associated with Meningococcic Meningitis. Archives of internal medicine. 1947;79:614–621. doi: 10.1001/archinte.1947.00220120044003. [DOI] [PubMed] [Google Scholar]
- Fullerton MD, Steinberg GR, Schertzer JD. Immunometabolism of Ampk in Insulin Resistance and Atherosclerosis. Molecular and cellular endocrinology. 2013;366:224–234. doi: 10.1016/j.mce.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Furuhashi M, Fucho R, Gorgun CZ, Tuncman G, Cao H, Hotamisligil GS. Adipocyte/Macrophage Fatty Acid-Binding Proteins Contribute to Metabolic Deterioration through Actions in Both Macrophages and Adipocytes in Mice. The Journal of clinical investigation. 2008;118:2640–2650. doi: 10.1172/JCI34750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, et al. Commensal Microbe-Derived Butyrate Induces the Differentiation of Colonic Regulatory T Cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- Fuster JJ, Zuriaga MA, Thi-Minh Ngo D, Farb MG, Aprahamian T, Yamaguchi TP, Gokce N, Walsh K. Non-Canonical Wnt Signaling Promotes Obesity-Induced Adipose Tissue Inflammation and Metabolic Dysfunction Independent of Adipose Tissue Expansion. Diabetes. 2014 doi: 10.2337/db14-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galic S, Fullerton MD, Schertzer JD, Sikkema S, Marcinko K, Walkley CR, Izon D, Honeyman J, Chen ZP, van Denderen BJ, et al. Hematopoietic Ampk Beta1 Reduces Mouse Adipose Tissue Macrophage Inflammation and Insulin Resistance in Obesity. The Journal of clinical investigation. 2011;121:4903–4915. doi: 10.1172/JCI58577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Hwang D, Bataille F, Lefevre M, York D, Quon MJ, Ye J. Serine Phosphorylation of Insulin Receptor Substrate 1 by Inhibitor Kappa B Kinase Complex. J Biol Chem. 2002;277:48115–48121. doi: 10.1074/jbc.M209459200. [DOI] [PubMed] [Google Scholar]
- Ghazarian MR, X S, Nojr MK, Luck H, Zeng K, Lei H, Tsai S, Schroer SA, Park YJ, Chng MHY, Shen L, D'Angelo JA, Horton P, Chapman WC, Brockmeier D, Woo M, Engleman EG, Adeyi O, Hirano N, Jin T, Gehring AJ, Winer S, Winer DA. Type I Interferon Responses Drive Intrahepatic T Cells to Promote Metabolic Syndrome. Sci Immunol. 2017;2 doi: 10.1126/sciimmunol.aai7616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghesquiere B, Wong BW, Kuchnio A, Carmeliet P. Metabolism of Stromal and Immune Cells in Health and Disease. Nature. 2014;511:167–176. doi: 10.1038/nature13312. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Teran B, Matesanz N, Nikolic I, Verdugo MA, Sreeramkumar V, Hernandez-Cosido L, Mora A, Crainiciuc G, Saiz ML, Bernardo E, et al. P38gamma and P38delta Reprogram Liver Metabolism by Modulating Neutrophil Infiltration. The EMBO journal. 2016;35:536–552. doi: 10.15252/embj.201591857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham TE, Yang Q, Bluher M, Hammarstedt A, Ciaraldi TP, Henry RR, Wason CJ, Oberbach A, Jansson PA, Smith U, et al. Retinol-Binding Protein 4 and Insulin Resistance in Lean, Obese, and Diabetic Subjects. N Engl J Med. 2006;354:2552–2563. doi: 10.1056/NEJMoa054862. [DOI] [PubMed] [Google Scholar]
- Green EA, Flavell RA. Tumor Necrosis Factor-Alpha and the Progression of Diabetes in Non-Obese Diabetic Mice. Immunological reviews. 1999;169:11–22. doi: 10.1111/j.1600-065x.1999.tb01302.x. [DOI] [PubMed] [Google Scholar]
- Griffin ME, Marcucci MJ, Cline GW, Bell K, Barucci N, Lee D, Goodyear LJ, Kraegen EW, White MF, Shulman GI. Free Fatty Acid-Induced Insulin Resistance Is Associated with Activation of Protein Kinase C Theta and Alterations in the Insulin Signaling Cascade. Diabetes. 1999;48:1270–1274. doi: 10.2337/diabetes.48.6.1270. [DOI] [PubMed] [Google Scholar]
- Hausberger FX. Pathological Changes in Adipose Tissue of Obese Mice. The Anatomical record. 1966;154:651–660. doi: 10.1002/ar.1091540311. [DOI] [PubMed] [Google Scholar]
- Hellman B. Studies in Obese-Hyperglycemic Mice. Annals of the New York Academy of Sciences. 1965;131:541–558. doi: 10.1111/j.1749-6632.1965.tb34819.x. [DOI] [PubMed] [Google Scholar]
- Helson L, Green S, Carswell E, Old LJ. Effect of Tumour Necrosis Factor on Cultured Human Melanoma Cells. Nature. 1975;258:731–732. doi: 10.1038/258731a0. [DOI] [PubMed] [Google Scholar]
- Henao-Mejia J, Elinav E, Thaiss CA, Flavell RA. Inflammasomes and Metabolic Disease. Annu Rev Physiol. 2014;76:57–78. doi: 10.1146/annurev-physiol-021113-170324. [DOI] [PubMed] [Google Scholar]
- Hett EC, Slater LH, Mark KG, Kawate T, Monks BG, Stutz A, Latz E, Hung DT. Chemical Genetics Reveals a Kinase-Independent Role for Protein Kinase R in Pyroptosis. Nat Chem Biol. 2013;9:398–405. doi: 10.1038/nchembio.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AA, Reid Bolus W, Hasty AH. A Decade of Progress in Adipose Tissue Macrophage Biology. Immunological reviews. 2014;262:134–152. doi: 10.1111/imr.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A Central Role for Jnk in Obesity and Insulin Resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- Hong EG, Ko HJ, Cho YR, Kim HJ, Ma Z, Yu TY, Friedline RH, Kurt-Jones E, Finberg R, Fischer MA, et al. Interleukin-10 Prevents Diet-Induced Insulin Resistance by Attenuating Macrophage and Cytokine Response in Skeletal Muscle. Diabetes. 2009;58:2525–2535. doi: 10.2337/db08-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and Metabolic Disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS. Endoplasmic Reticulum Stress and the Inflammatory Basis of Metabolic Disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation, Metaflammation and Immunometabolic Disorders. Nature. 2017;542:177–185. doi: 10.1038/nature21363. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased Adipose Tissue Expression of Tumor Necrosis Factor-Alpha in Human Obesity and Insulin Resistance. The Journal of clinical investigation. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS, Murray DL, Choy LN, Spiegelman BM. Tumor Necrosis Factor Alpha Inhibits Signaling from the Insulin Receptor. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:4854–4858. doi: 10.1073/pnas.91.11.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. Irs-1-Mediated Inhibition of Insulin Receptor Tyrosine Kinase Activity in Tnf-Alpha- and Obesity-Induced Insulin Resistance. Science. 1996;271:665–668. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose Expression of Tumor Necrosis Factor-Alpha: Direct Role in Obesity-Linked Insulin Resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- Huang J, Liao N, Huang QP, Xie ZF. Association between Tumor Necrosis Factor-Alpha-308g/a Polymorphism and Obstructive Sleep Apnea: A Meta-Analysis. Genetic testing and molecular biomarkers. 2012a;16:246–251. doi: 10.1089/gtmb.2011.0170. [DOI] [PubMed] [Google Scholar]
- Huang JY, Chiang MT, Yet SF, Chau LY. Myeloid Heme Oxygenase-1 Haploinsufficiency Reduces High Fat Diet-Induced Insulin Resistance by Affecting Adipose Macrophage Infiltration in Mice. PloS one. 2012b;7:e38626. doi: 10.1371/journal.pone.0038626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura A, Hirasawa A, Poulain-Godefroy O, Bonnefond A, Hara T, Yengo L, Kimura I, Leloire A, Liu N, Iida K, et al. Dysfunction of Lipid Sensor Gpr120 Leads to Obesity in Both Mouse and Human. Nature. 2012;483:350–354. doi: 10.1038/nature10798. [DOI] [PubMed] [Google Scholar]
- Ip WKE, Hoshi N, Shouval DS, Snapper S, Medzhitov R. Anti-Inflammatory Effect of Il-10 Mediated by Metabolic Reprogramming of Macrophages. Science. 2017;356:513–519. doi: 10.1126/science.aal3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob CO, Aiso S, Michie SA, McDevitt HO, Acha-Orbea H. Prevention of Diabetes in Nonobese Diabetic Mice by Tumor Necrosis Factor (Tnf): Similarities between Tnf-Alpha and Interleukin 1. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:968–972. doi: 10.1073/pnas.87.3.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins AB, Storlien LH, Chisholm DJ, Kraegen EW. Effects of Nonesterified Fatty Acid Availability on Tissue-Specific Glucose Utilization in Rats in Vivo. The Journal of clinical investigation. 1988;82:293–299. doi: 10.1172/JCI113586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha AK, Huang SC, Sergushichev A, Lampropoulou V, Ivanova Y, Loginicheva E, Chmielewski K, Stewart KM, Ashall J, Everts B, et al. Network Integration of Parallel Metabolic and Transcriptional Data Reveals Metabolic Modules That Regulate Macrophage Polarization. Immunity. 2015;42:419–430. doi: 10.1016/j.immuni.2015.02.005. [DOI] [PubMed] [Google Scholar]
- Ji Y, Sun S, Xu A, Bhargava P, Yang L, Lam KS, Gao B, Lee CH, Kersten S, Qi L. Activation of Natural Killer T Cells Promotes M2 Macrophage Polarization in Adipose Tissue and Improves Systemic Glucose Tolerance Via Interleukin-4 (Il-4)/Stat6 Protein Signaling Axis in Obesity. J Biol Chem. 2012;287:13561–13571. doi: 10.1074/jbc.M112.350066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AB, Argyraki M, Thow JC, Cooper BG, Fulcher G, Taylor R. Effect of Increased Free Fatty Acid Supply on Glucose Metabolism and Skeletal Muscle Glycogen Synthase Activity in Normal Man. Clinical science. 1992;82:219–226. doi: 10.1042/cs0820219. [DOI] [PubMed] [Google Scholar]
- Jornayvaz FR, Birkenfeld AL, Jurczak MJ, Kanda S, Guigni BA, Jiang DC, Zhang D, Lee HY, Samuel VT, Shulman GI. Hepatic Insulin Resistance in Mice with Hepatic Overexpression of Diacylglycerol Acyltransferase 2. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:5748–5752. doi: 10.1073/pnas.1103451108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jullien D, Tanti JF, Heydrick SJ, Gautier N, Gremeaux T, Van Obberghen E, Le Marchand-Brustel Y. Differential Effects of Okadaic Acid on Insulin-Stimulated Glucose and Amino Acid Uptake and Phosphatidylinositol 3-Kinase Activity. J Biol Chem. 1993;268:15246–15251. [PubMed] [Google Scholar]
- Kaneto H, Nakatani Y, Miyatsuka T, Kawamori D, Matsuoka TA, Matsuhisa M, Kajimoto Y, Ichijo H, Yamasaki Y, Hori M. Possible Novel Therapy for Diabetes with Cell-Permeable Jnk-Inhibitory Peptide. Nature medicine. 2004;10:1128–1132. doi: 10.1038/nm1111. [DOI] [PubMed] [Google Scholar]
- Kanety H, Feinstein R, Papa MZ, Hemi R, Karasik A. Tumor Necrosis Factor Alpha-Induced Phosphorylation of Insulin Receptor Substrate-1 (Irs-1). Possible Mechanism for Suppression of Insulin-Stimulated Tyrosine Phosphorylation of Irs-1. J Biol Chem. 1995;270:23780–23784. doi: 10.1074/jbc.270.40.23780. [DOI] [PubMed] [Google Scholar]
- Kern PA, Saghizadeh M, Ong JM, Bosch RJ, Deem R, Simsolo RB. The Expression of Tumor Necrosis Factor in Human Adipose Tissue. Regulation by Obesity, Weight Loss, and Relationship to Lipoprotein Lipase. The Journal of clinical investigation. 1995;95:2111–2119. doi: 10.1172/JCI117899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Higashimori T, Park SY, Choi H, Dong J, Kim YJ, Noh HL, Cho YR, Cline G, Kim YB, et al. Differential Effects of Interleukin-6 and-10 on Skeletal Muscle and Liver Insulin Action in Vivo. Diabetes. 2004a;53:1060–1067. doi: 10.2337/diabetes.53.4.1060. [DOI] [PubMed] [Google Scholar]
- Kim JK, Fillmore JJ, Sunshine MJ, Albrecht B, Higashimori T, Kim DW, Liu ZX, Soos TJ, Cline GW, O'Brien WR, et al. Pkc-Theta Knockout Mice Are Protected from Fat-Induced Insulin Resistance. The Journal of clinical investigation. 2004b;114:823–827. doi: 10.1172/JCI22230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinridders A, Schenten D, Konner AC, Belgardt BF, Mauer J, Okamura T, Wunderlich FT, Medzhitov R, Bruning JC. Myd88 Signaling in the Cns Is Required for Development of Fatty Acid-Induced Leptin Resistance and Diet-Induced Obesity. Cell Metab. 2009;10:249–259. doi: 10.1016/j.cmet.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodin D, van Panhuys N, Li C, Magnuson AM, Cipolletta D, Miller CM, Wagers A, Germain RN, Benoist C, Mathis D. Antigen- and Cytokine-Driven Accumulation of Regulatory T Cells in Visceral Adipose Tissue of Lean Mice. Cell Metab. 2015;21:543–557. doi: 10.1016/j.cmet.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp E, Ghosh S. Inhibition of Nf-Kappa B by Sodium Salicylate and Aspirin. Science. 1994;265:956–959. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- Kosteli A, Sugaru E, Haemmerle G, Martin JF, Lei J, Zechner R, Ferrante AW., Jr Weight Loss and Lipolysis Promote a Dynamic Immune Response in Murine Adipose Tissue. The Journal of clinical investigation. 2010;120:3466–3479. doi: 10.1172/JCI42845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothari V, Galdo JA, Mathews ST. Hypoglycemic Agents and Potential Anti-Inflammatory Activity. J Inflamm Res. 2016;9:27–38. doi: 10.2147/JIR.S86917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koulmanda M, Bhasin M, Awdeh Z, Qipo A, Fan Z, Hanidziar D, Putheti P, Shi H, Csizuadia E, Libermann TA, et al. The Role of Tnf-Alpha in Mice with Type 1- and 2-Diabetes. PloS one. 2012;7:e33254. doi: 10.1371/journal.pone.0033254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratz M, Coats BR, Hisert KB, Hagman D, Mutskov V, Peris E, Schoenfelt KQ, Kuzma JN, Larson I, Billing PS, et al. Metabolic Dysfunction Drives a Mechanistically Distinct Proinflammatory Phenotype in Adipose Tissue Macrophages. Cell Metab. 2014;20:614–625. doi: 10.1016/j.cmet.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon H, Laurent S, Tang Y, Zong H, Vemulapalli P, Pessin JE. Adipocyte-Specific Ikkbeta Signaling Suppresses Adipose Tissue Inflammation through an Il-13-Dependent Paracrine Feedback Pathway. Cell reports. 2014;9:1574–1583. doi: 10.1016/j.celrep.2014.10.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster GI, Febbraio MA. The Immunomodulating Role of Exercise in Metabolic Disease. Trends in immunology. 2014;35:262–269. doi: 10.1016/j.it.2014.02.008. [DOI] [PubMed] [Google Scholar]
- Lang CH, Dobrescu C, Bagby GJ. Tumor Necrosis Factor Impairs Insulin Action on Peripheral Glucose Disposal and Hepatic Glucose Output. Endocrinology. 1992;130:43–52. doi: 10.1210/endo.130.1.1727716. [DOI] [PubMed] [Google Scholar]
- Lee JY, Ye J, Gao Z, Youn HS, Lee WH, Zhao L, Sizemore N, Hwang DH. Reciprocal Modulation of Toll-Like Receptor-4 Signaling Pathways Involving Myd88 and Phosphatidylinositol 3-Kinase/Akt by Saturated and Polyunsaturated Fatty Acids. J Biol Chem. 2003;278:37041–37051. doi: 10.1074/jbc.M305213200. [DOI] [PubMed] [Google Scholar]
- Lee MW, Odegaard JI, Mukundan L, Qiu Y, Molofsky AB, Nussbaum JC, Yun K, Locksley RM, Chawla A. Activated Type 2 Innate Lymphoid Cells Regulate Beige Fat Biogenesis. Cell. 2015;160:74–87. doi: 10.1016/j.cell.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YH, Petkova AP, Granneman JG. Identification of an Adipogenic Niche for Adipose Tissue Remodeling and Restoration. Cell Metab. 2013;18:355–367. doi: 10.1016/j.cmet.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Kim DH, Tsenovoy PL, Peterson SJ, Rezzani R, Rodella LF, Aronow WS, Ikehara S, Abraham NG. Treatment of Obese Diabetic Mice with a Heme Oxygenase Inducer Reduces Visceral and Subcutaneous Adiposity, Increases Adiponectin Levels, and Improves Insulin Sensitivity and Glucose Tolerance. Diabetes. 2008;57:1526–1535. doi: 10.2337/db07-1764. [DOI] [PubMed] [Google Scholar]
- Li P, Liu S, Lu M, Bandyopadhyay G, Oh D, Imamura T, Johnson AM, Sears D, Shen Z, Cui B, et al. Hematopoietic-Derived Galectin-3 Causes Cellular and Systemic Insulin Resistance. Cell. 2016;167:973–984 e912. doi: 10.1016/j.cell.2016.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Soloski MJ, Diehl AM. Dietary Factors Alter Hepatic Innate Immune System in Mice with Nonalcoholic Fatty Liver Disease. Hepatology. 2005;42:880–885. doi: 10.1002/hep.20826. [DOI] [PubMed] [Google Scholar]
- Liang H, Yin B, Zhang H, Zhang S, Zeng Q, Wang J, Jiang X, Yuan L, Wang CY, Li Z. Blockade of Tumor Necrosis Factor (Tnf) Receptor Type 1-Mediated Tnf-Alpha Signaling Protected Wistar Rats from Diet-Induced Obesity and Insulin Resistance. Endocrinology. 2008;149:2943–2951. doi: 10.1210/en.2007-0978. [DOI] [PubMed] [Google Scholar]
- Ling PR, Bistrian BR, Mendez B, Istfan NW. Effects of Systemic Infusions of Endotoxin, Tumor Necrosis Factor, and Interleukin-1 on Glucose Metabolism in the Rat: Relationship to Endogenous Glucose Production and Peripheral Tissue Glucose Uptake. Metabolism: clinical and experimental. 1994;43:279–284. doi: 10.1016/0026-0495(94)90093-0. [DOI] [PubMed] [Google Scholar]
- Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, Powell C, Vedantam S, Buchkovich ML, Yang J, et al. Genetic Studies of Body Mass Index Yield New Insights for Obesity Biology. Nature. 2015;518:197–206. doi: 10.1038/nature14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffreda S, Yang SQ, Lin HZ, Karp CL, Brengman ML, Wang DJ, Klein AS, Bulkley GB, Bao C, Noble PW, et al. Leptin Regulates Proinflammatory Immune Responses. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1998;12:57–65. [PubMed] [Google Scholar]
- Lowenthal JW, Ballard DW, Bohnlein E, Greene WC. Tumor Necrosis Factor Alpha Induces Proteins That Bind Specifically to Kappa B-Like Enhancer Elements and Regulate Interleukin 2 Receptor Alpha-Chain Gene Expression in Primary Human T Lymphocytes. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:2331–2335. doi: 10.1073/pnas.86.7.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng CN, Bodzin JL, Saltiel AR. Obesity Induces a Phenotypic Switch in Adipose Tissue Macrophage Polarization. The Journal of clinical investigation. 2007a;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng CN, Deyoung SM, Saltiel AR. Macrophages Block Insulin Action in Adipocytes by Altering Expression of Signaling and Glucose Transport Proteins. American journal of physiology Endocrinology and metabolism. 2007b;292:E166–174. doi: 10.1152/ajpendo.00284.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch L. Adipose Invariant Natural Killer T Cells. Immunology. 2014;142:337–346. doi: 10.1111/imm.12269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, Kaiser N, Halban PA, Donath MY. Glucose-Induced Beta Cell Production of Il-1beta Contributes to Glucotoxicity in Human Pancreatic Islets. The Journal of clinical investigation. 2002;110:851–860. doi: 10.1172/JCI15318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney JR, Jr, Beutler BA, Le Trang N, Vine W, Ikeda Y, Kawakami M, Cerami A. Lipopolysaccharide-Treated Raw 264.7 Cells Produce a Mediator That Inhibits Lipoprotein Lipase in 3t3-L1 Cells. Journal of immunology. 1985;134:1673–1675. [PubMed] [Google Scholar]
- Miles PD, Romeo OM, Higo K, Cohen A, Rafaat K, Olefsky JM. Tnf-Alpha-Induced Insulin Resistance in Vivo and Its Prevention by Troglitazone. Diabetes. 1997;46:1678–1683. doi: 10.2337/diab.46.11.1678. [DOI] [PubMed] [Google Scholar]
- Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A, Chawla A, Locksley RM. Innate Lymphoid Type 2 Cells Sustain Visceral Adipose Tissue Eosinophils and Alternatively Activated Macrophages. The Journal of experimental medicine. 2013;210:535–549. doi: 10.1084/jem.20121964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monetti M, Levin MC, Watt MJ, Sajan MP, Marmor S, Hubbard BK, Stevens RD, Bain JR, Newgard CB, Farese RV., Sr Dissociation of Hepatic Steatosis and Insulin Resistance in Mice Overexpressing Dgat in the Liver. Cell Metab. 2007;6:69–78. doi: 10.1016/j.cmet.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Morris DL, Cho KW, Delproposto JL, Oatmen KE, Geletka LM, Martinez-Santibanez G, Singer K, Lumeng CN. Adipose Tissue Macrophages Function as Antigen-Presenting Cells and Regulate Adipose Tissue Cd4+ T Cells in Mice. Diabetes. 2013;62:2762–2772. doi: 10.2337/db12-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounier R, Theret M, Arnold L, Cuvellier S, Bultot L, Goransson O, Sanz N, Ferry A, Sakamoto K, Foretz M, et al. Ampkalpha1 Regulates Macrophage Skewing at the Time of Resolution of Inflammation During Skeletal Muscle Regeneration. Cell Metab. 2013;18:251–264. doi: 10.1016/j.cmet.2013.06.017. [DOI] [PubMed] [Google Scholar]
- Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA, et al. A Branched-Chain Amino Acid-Related Metabolic Signature That Differentiates Obese and Lean Humans and Contributes to Insulin Resistance. Cell Metab. 2009;9:311–326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, David T, Mukundan L, Brombacher F, Locksley RM, Chawla A. Alternatively Activated Macrophages Produce Catecholamines to Sustain Adaptive Thermogenesis. Nature. 2011;480:104–108. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, et al. Cd8+ Effector T Cells Contribute to Macrophage Recruitment and Adipose Tissue Inflammation in Obesity. Nature medicine. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- Nishimura S, Manabe I, Takaki S, Nagasaki M, Otsu M, Yamashita H, Sugita J, Yoshimura K, Eto K, Komuro I, et al. Adipose Natural Regulatory B Cells Negatively Control Adipose Tissue Inflammation. Cell Metab. 2013 doi: 10.1016/j.cmet.2013.09.017. [DOI] [PubMed] [Google Scholar]
- O'Neill LA, Hardie DG. Metabolism of Inflammation Limited by Ampk and Pseudo-Starvation. Nature. 2013;493:346–355. doi: 10.1038/nature11862. [DOI] [PubMed] [Google Scholar]
- Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. Gpr120 Is an Omega-3 Fatty Acid Receptor Mediating Potent Anti-Inflammatory and Insulin-Sensitizing Effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliff A, Defeo-Jones D, Boyer M, Martinez D, Kiefer D, Vuocolo G, Wolfe A, Socher SH. Tumors Secreting Human Tnf/Cachectin Induce Cachexia in Mice. Cell. 1987;50:555–563. doi: 10.1016/0092-8674(87)90028-6. [DOI] [PubMed] [Google Scholar]
- Oral EA, Reilly SM, Gomez AV, Meral R, Butz L, Ajluni N, Chenevert TL, Korytnaya E, Neidert AH, Hench R, et al. Inhibition of Ikkvarepsilon and Tbk1 Improves Glucose Control in a Subset of Patients with Type 2 Diabetes. Cell Metab. 2017;26:157–170 e157. doi: 10.1016/j.cmet.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn L, Kunkel S, Nabel GJ. Tumor Necrosis Factor Alpha and Interleukin 1 Stimulate the Human Immunodeficiency Virus Enhancer by Activation of the Nuclear Factor Kappa B. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:2336–2340. doi: 10.1073/pnas.86.7.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchi N, Higuchi A, Ohashi K, Oshima Y, Gokce N, Shibata R, Akasaki Y, Shimono A, Walsh K. Sfrp5 Is an Anti-Inflammatory Adipokine That Modulates Metabolic Dysfunction in Obesity. Science. 2010;329:454–457. doi: 10.1126/science.1188280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic Reticulum Stress Links Obesity, Insulin Action, and Type 2 Diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- Palmblad J, Hallberg D, Engstedt L. Polymorphonuclear (Pmn) Function after Small Intestinal Shunt Operation for Morbid Obesity. Br J Haematol. 1980;44:101–108. doi: 10.1111/j.1365-2141.1980.tb01188.x. [DOI] [PubMed] [Google Scholar]
- Pamir N, Liu NC, Irwin A, Becker L, Peng Y, Ronsein GE, Bornfeldt KE, Duffield JS, Heinecke JW. Granulocyte/Macrophage Colony-Stimulating Factor-Dependent Dendritic Cells Restrain Lean Adipose Tissue Expansion. J Biol Chem. 2015;290:14656–14667. doi: 10.1074/jbc.M115.645820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SH, Liu Z, Sui Y, Helsley RN, Zhu B, Powell DK, Kern PA, Zhou C. Ikkbeta Is Essential for Adipocyte Survival and Adaptive Adipose Remodeling in Obesity. Diabetes. 2016;65:1616–1629. doi: 10.2337/db15-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekala P, Kawakami M, Vine W, Lane MD, Cerami A. Studies of Insulin Resistance in Adipocytes Induced by Macrophage Mediator. The Journal of experimental medicine. 1983;157:1360–1365. doi: 10.1084/jem.157.4.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen MC, Madiraju AK, Gassaway BM, Marcel M, Nasiri AR, Butrico G, Marcucci MJ, Zhang D, Abulizi A, Zhang XM, et al. Insulin Receptor Thr1160 Phosphorylation Mediates Lipid-Induced Hepatic Insulin Resistance. The Journal of clinical investigation. 2016;126:4361–4371. doi: 10.1172/JCI86013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomgaard P, Bouzakri K, Krogh-Madsen R, Mittendorfer B, Zierath JR, Pedersen BK. Tumor Necrosis Factor-Alpha Induces Skeletal Muscle Insulin Resistance in Healthy Human Subjects Via Inhibition of Akt Substrate 160 Phosphorylation. Diabetes. 2005;54:2939–2945. doi: 10.2337/diabetes.54.10.2939. [DOI] [PubMed] [Google Scholar]
- Plomgaard P, Fischer CP, Ibfelt T, Pedersen BK, van Hall G. Tumor Necrosis Factor-Alpha Modulates Human in Vivo Lipolysis. The Journal of clinical endocrinology and metabolism. 2008;93:543–549. doi: 10.1210/jc.2007-1761. [DOI] [PubMed] [Google Scholar]
- Qiu Y, Nguyen KD, Odegaard JI, Cui X, Tian X, Locksley RM, Palmiter RD, Chawla A. Eosinophils and Type 2 Cytokine Signaling in Macrophages Orchestrate Development of Functional Beige Fat. Cell. 2014;157:1292–1308. doi: 10.1016/j.cell.2014.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz D, Zierler KL. Forearm Metabolism in Obesity and Its Response to Intra-Arterial Insulin. Characterization of Insulin Resistance and Evidence for Adaptive Hyperinsulinism. The Journal of clinical investigation. 1962;41:2173–2181. doi: 10.1172/JCI104676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randle PJ, Garland PB, Hales CN, Newsholme EA. The Glucose Fatty-Acid Cycle. Its Role in Insulin Sensitivity and the Metabolic Disturbances of Diabetes Mellitus. Lancet. 1963;1:785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- Randle PJ, Newsholme EA, Garland PB. Regulation of Glucose Uptake by Muscle. 8. Effects of Fatty Acids, Ketone Bodies and Pyruvate, and of Alloxan-Diabetes and Starvation, on the Uptake and Metabolic Fate of Glucose in Rat Heart and Diaphragm Muscles. The Biochemical journal. 1964;93:652–665. doi: 10.1042/bj0930652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch ME, Weisberg S, Vardhana P, Tortoriello DV. Obesity in C57bl/6j Mice Is Characterized by Adipose Tissue Hypoxia and Cytotoxic T-Cell Infiltration. International journal of obesity. 2008;32:451–463. doi: 10.1038/sj.ijo.0803744. [DOI] [PubMed] [Google Scholar]
- Raymond RM, Harkema JM, Emerson TE., Jr In Vivo Skeletal Muscle Insulin Resistance During E Coli Endotoxin Shock in the Dog. Circulatory shock. 1981;8:425–433. [PubMed] [Google Scholar]
- Reilly SM, Chiang SH, Decker SJ, Chang L, Uhm M, Larsen MJ, Rubin JR, Mowers J, White NM, Hochberg I, et al. An Inhibitor of the Protein Kinases Tbk1 and Ikk-Varepsilon Improves Obesity-Related Metabolic Dysfunctions in Mice. Nature medicine. 2013;19:313–321. doi: 10.1038/nm.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard C, Shamoon B, Shyamala V, Williams LT. Tumor Necrosis Factor Alpha-Induced Activation of C-Jun N-Terminal Kinase Is Mediated by Traf2. The EMBO journal. 1997;16:1080–1092. doi: 10.1093/emboj/16.5.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricardo-Gonzalez RR, Red Eagle A, Odegaard JI, Jouihan H, Morel CR, Heredia JE, Mukundan L, Wu D, Locksley RM, Chawla A. Il-4/Stat6 Immune Axis Regulates Peripheral Nutrient Metabolism and Insulin Sensitivity. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:22617–22622. doi: 10.1073/pnas.1009152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabio G, Das M, Mora A, Zhang Z, Jun JY, Ko HJ, Barrett T, Kim JK, Davis RJ. A Stress Signaling Pathway in Adipose Tissue Regulates Hepatic Insulin Resistance. Science. 2008;322:1539–1543. doi: 10.1126/science.1160794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghizadeh M, Ong JM, Garvey WT, Henry RR, Kern PA. The Expression of Tnf Alpha by Human Muscle. Relationship to Insulin Resistance. The Journal of clinical investigation. 1996;97:1111–1116. doi: 10.1172/JCI118504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheen AJ, Esser N, Paquot N. Antidiabetic Agents: Potential Anti-Inflammatory Activity Beyond Glucose Control. Diabetes Metab. 2015;41:183–194. doi: 10.1016/j.diabet.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Sell H, Habich C, Eckel J. Adaptive Immunity in Obesity and Insulin Resistance. Nature reviews Endocrinology. 2012;8:709–716. doi: 10.1038/nrendo.2012.114. [DOI] [PubMed] [Google Scholar]
- Serhan CN. Pro-Resolving Lipid Mediators Are Leads for Resolution Physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra C, Federici M, Buongiorno A, Senni MI, Morelli S, Segratella E, Pascuccio M, Tiveron C, Mattei E, Tatangelo L, et al. Transgenic Mice with Dominant Negative Pkc-Theta in Skeletal Muscle: A New Model of Insulin Resistance and Obesity. Journal of cellular physiology. 2003;196:89–97. doi: 10.1002/jcp.10278. [DOI] [PubMed] [Google Scholar]
- Shan B, Wang X, Wu Y, Xu C, Xia Z, Dai J, Shao M, Zhao F, He S, Yang L, et al. The Metabolic Er Stress Sensor Ire1alpha Suppresses Alternative Activation of Macrophages and Impairs Energy Expenditure in Obesity. Nature immunology. 2017;18:519–529. doi: 10.1038/ni.3709. [DOI] [PubMed] [Google Scholar]
- Shungin D, Winkler TW, Croteau-Chonka DC, Ferreira T, Locke AE, Magi R, Strawbridge RJ, Pers TH, Fischer K, Justice AE, et al. New Genetic Loci Link Adipose and Insulin Biology to Body Fat Distribution. Nature. 2015;518:187–196. doi: 10.1038/nature14132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silswal N, Singh AK, Aruna B, Mukhopadhyay S, Ghosh S, Ehtesham NZ. Human Resistin Stimulates the Pro-Inflammatory Cytokines Tnf-Alpha and Il-12 in Macrophages by Nf-Kappab-Dependent Pathway. Biochem Biophys Res Commun. 2005;334:1092–1101. doi: 10.1016/j.bbrc.2005.06.202. [DOI] [PubMed] [Google Scholar]
- Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, Glickman JN, Garrett WS. The Microbial Metabolites, Short-Chain Fatty Acids, Regulate Colonic Treg Cell Homeostasis. Science. 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass RG, Huang S, Choi IW, Rutledge JC, Hwang DH. Inflammasome-Mediated Secretion of Il-1beta in Human Monocytes through Tlr2 Activation; Modulation by Dietary Fatty Acids. Journal of immunology. 2013;191:4337–4347. doi: 10.4049/jimmunol.1300298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg GF, Artis D. Innate Lymphoid Cells in the Initiation, Regulation and Resolution of Inflammation. Nature medicine. 2015;21:698–708. doi: 10.1038/nm.3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sookoian SC, Gonzalez C, Pirola CJ. Meta-Analysis on the G-308a Tumor Necrosis Factor Alpha Gene Variant and Phenotypes Associated with the Metabolic Syndrome. Obes Res. 2005;13:2122–2131. doi: 10.1038/oby.2005.263. [DOI] [PubMed] [Google Scholar]
- Spadaro O, Camell CD, Bosurgi L, Nguyen KY, Youm YH, Rothlin CV, Dixit VD. Igf1 Shapes Macrophage Activation in Response to Immunometabolic Challenge. Cell reports. 2017;19:225–234. doi: 10.1016/j.celrep.2017.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spite M, Claria J, Serhan CN. Resolvins, Specialized Proresolving Lipid Mediators, and Their Potential Roles in Metabolic Diseases. Cell Metab. 2014;19:21–36. doi: 10.1016/j.cmet.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer JJ, Bagby GJ, Meszaros K, Lang CH. Altered Control of Carbohydrate Metabolism in Endotoxemia. Prog Clin Biol Res. 1989;286:145–165. [PubMed] [Google Scholar]
- Stanya KJ, Jacobi D, Liu S, Bhargava P, Dai L, Gangl MR, Inouye K, Barlow JL, Ji Y, Mizgerd JP, et al. Direct Control of Hepatic Glucose Production by Interleukin-13 in Mice. The Journal of clinical investigation. 2013;123:261–271. doi: 10.1172/JCI64941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens JM, Pekala PH. Transcriptional Repression of the Glut4 and C/Ebp Genes in 3t3-L1 Adipocytes by Tumor Necrosis Factor-Alpha. J Biol Chem. 1991;266:21839–21845. [PubMed] [Google Scholar]
- Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The Hormone Resistin Links Obesity to Diabetes. Nature. 2001;409:307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- Stienstra R, Joosten LA, Koenen T, van Tits B, van Diepen JA, van den Berg SA, Rensen PC, Voshol PJ, Fantuzzi G, Hijmans A, et al. The Inflammasome-Mediated Caspase-1 Activation Controls Adipocyte Differentiation and Insulin Sensitivity. Cell Metab. 2010;12:593–605. doi: 10.1016/j.cmet.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stienstra R, van Diepen JA, Tack CJ, Zaki MH, van de Veerdonk FL, Perera D, Neale GA, Hooiveld GJ, Hijmans A, Vroegrijk I, et al. Inflammasome Is a Central Player in the Induction of Obesity and Insulin Resistance. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:15324–15329. doi: 10.1073/pnas.1100255108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strissel KJ, DeFuria J, Shaul ME, Bennett G, Greenberg AS, Obin MS. T-Cell Recruitment and Th1 Polarization in Adipose Tissue During Diet-Induced Obesity in C57bl/6 Mice. Obesity. 2010;18:1918–1925. doi: 10.1038/oby.2010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szendroedi J, Yoshimura T, Phielix E, Koliaki C, Marcucci M, Zhang D, Jelenik T, Muller J, Herder C, Nowotny P, et al. Role of Diacylglycerol Activation of Pkctheta in Lipid-Induced Muscle Insulin Resistance in Humans. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:9597–9602. doi: 10.1073/pnas.1409229111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack CJ, Stienstra R, Joosten LA, Netea MG. Inflammation Links Excess Fat to Insulin Resistance: The Role of the Interleukin-1 Family. Immunological reviews. 2012;249:239–252. doi: 10.1111/j.1600-065X.2012.01145.x. [DOI] [PubMed] [Google Scholar]
- Talbot NA, Wheeler-Jones CP, Cleasby ME. Palmitoleic Acid Prevents Palmitic Acid-Induced Macrophage Activation and Consequent P38 Mapk-Mediated Skeletal Muscle Insulin Resistance. Molecular and cellular endocrinology. 2014;393:129–142. doi: 10.1016/j.mce.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talukdar S, Oh DY, Bandyopadhyay G, Li D, Xu J, McNelis J, Lu M, Li P, Yan Q, Zhu Y, et al. Neutrophils Mediate Insulin Resistance in Mice Fed a High-Fat Diet through Secreted Elastase. Nature medicine. 2012;18:1407–1412. doi: 10.1038/nm.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Inoue S, Isoda F, Waseda M, Ishihara M, Yamakawa T, Sugiyama A, Takamura Y, Okuda K. Impaired Immunity in Obesity: Suppressed but Reversible Lymphocyte Responsiveness. Int J Obes Relat Metab Disord. 1993;17:631–636. [PubMed] [Google Scholar]
- Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, Frezza C, Bernard NJ, Kelly B, Foley NH, et al. Succinate Is an Inflammatory Signal That Induces Il-1beta through Hif-1alpha. Nature. 2013;496:238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurmond DC, Oh E, Miller RA. Potential Site Effects and Transgene Expression Discrepancies in Mouse Lifespan Studies. Cell Metab. 2015;22:346–347. doi: 10.1016/j.cmet.2015.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsaousidou E, Paeger L, Belgardt BF, Pal M, Wunderlich CM, Bronneke H, Collienne U, Hampel B, Wunderlich FT, Schmidt-Supprian M, et al. Distinct Roles for Jnk and Ikk Activation in Agouti-Related Peptide Neurons in the Development of Obesity and Insulin Resistance. Cell reports. 2014;9:1495–1506. doi: 10.1016/j.celrep.2014.10.045. [DOI] [PubMed] [Google Scholar]
- Tuncman G, Hirosumi J, Solinas G, Chang L, Karin M, Hotamisligil GS. Functional in Vivo Interactions between Jnk1 and Jnk2 Isoforms in Obesity and Insulin Resistance. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:10741–10746. doi: 10.1073/pnas.0603509103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. Coupling of Stress in the Er to Activation of Jnk Protein Kinases by Transmembrane Protein Kinase Ire1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from Obesity-Induced Insulin Resistance in Mice Lacking Tnf-Alpha Function. Nature. 1997;389:610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- Van der Poll T, Romijn JA, Endert E, Borm JJ, Buller HR, Sauerwein HP. Tumor Necrosis Factor Mimics the Metabolic Response to Acute Infection in Healthy Humans. Am J Physiol. 1991;261:E457–465. doi: 10.1152/ajpendo.1991.261.4.E457. [DOI] [PubMed] [Google Scholar]
- Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. The Nlrp3 Inflammasome Instigates Obesity-Induced Inflammation and Insulin Resistance. Nature medicine. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vane JR. Inhibition of Prostaglandin Synthesis as a Mechanism of Action for Aspirin-Like Drugs. Nature: New biology. 1971;231:232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- Ventre J, Doebber T, Wu M, MacNaul K, Stevens K, Pasparakis M, Kollias G, Moller DE. Targeted Disruption of the Tumor Necrosis Factor-Alpha Gene: Metabolic Consequences in Obese and Nonobese Mice. Diabetes. 1997;46:1526–1531. doi: 10.2337/diab.46.9.1526. [DOI] [PubMed] [Google Scholar]
- Waeber G, Delplanque J, Bonny C, Mooser V, Steinmann M, Widmann C, Maillard A, Miklossy J, Dina C, Hani EH, et al. The Gene Mapk8ip1, Encoding Islet-Brain-1, Is a Candidate for Type 2 Diabetes. Nature genetics. 2000;24:291–295. doi: 10.1038/73523. [DOI] [PubMed] [Google Scholar]
- Wang R, Green DR. Metabolic Checkpoints in Activated T Cells. Nature immunology. 2012;13:907–915. doi: 10.1038/ni.2386. [DOI] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity Is Associated with Macrophage Accumulation in Adipose Tissue. The Journal of clinical investigation. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, Brickey WJ, Ting JP. Fatty Acid-Induced Nlrp3-Asc Inflammasome Activation Interferes with Insulin Signaling. Nature immunology. 2011;12:408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernstedt Asterholm I, Tao C, Morley TS, Wang QA, Delgado-Lopez F, Wang ZV, Scherer PE. Adipocyte Inflammation Is Essential for Healthy Adipose Tissue Expansion and Remodeling. Cell Metab. 2014;20:103–118. doi: 10.1016/j.cmet.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer DA, Winer S, Shen L, Wadia PP, Yantha J, Paltser G, Tsui H, Wu P, Davidson MG, Alonso MN, et al. B Cells Promote Insulin Resistance through Modulation of T Cells and Production of Pathogenic Igg Antibodies. Nature medicine. 2011;17:610–617. doi: 10.1038/nm.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F, et al. Normalization of Obesity-Associated Insulin Resistance through Immunotherapy. Nature medicine. 2009;15:921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf Y, Boura-Halfon S, Cortese N, Haimon Z, Sar Shalom H, Kuperman Y, Kalchenko V, Brandis A, David E, Segal-Hayoun Y, et al. Brown-Adipose-Tissue Macrophages Control Tissue Innervation and Homeostatic Energy Expenditure. Nature immunology. 2017 doi: 10.1038/ni.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SC, Begg DP. Food for Thought: Revisiting the Complexity of Food Intake. Cell Metab. 2015;22:348–351. doi: 10.1016/j.cmet.2015.08.017. [DOI] [PubMed] [Google Scholar]
- Xie M, Yu Y, Kang R, Zhu S, Yang L, Zeng L, Sun X, Yang M, Billiar TR, Wang H, et al. Pkm2-Dependent Glycolysis Promotes Nlrp3 and Aim2 Inflammasome Activation. Nat Commun. 2016;7:13280. doi: 10.1038/ncomms13280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, et al. Chronic Inflammation in Fat Plays a Crucial Role in the Development of Obesity-Related Insulin Resistance. The Journal of clinical investigation. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H, Gao Y, Zhang Y. Inhibition of Jnk Suppresses Autophagy and Attenuates Insulin Resistance in a Rat Model of Nonalcoholic Fatty Liver Disease. Mol Med Rep. 2017;15:180–186. doi: 10.3892/mmr.2016.5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Youm YH, Vandanmagsar B, Ravussin A, Gimble JM, Greenway F, Stephens JM, Mynatt RL, Dixit VD. Obesity Increases the Production of Proinflammatory Mediators from Adipose Tissue T Cells and Compromises Tcr Repertoire Diversity: Implications for Systemic Inflammation and Insulin Resistance. Journal of immunology. 2010;185:1836–1845. doi: 10.4049/jimmunol.1000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Calay ES, Fan J, Arduini A, Kunz RC, Gygi SP, Yalcin A, Fu S, Hotamisligil GS. Metabolism. S-Nitrosylation Links Obesity-Associated Inflammation to Endoplasmic Reticulum Dysfunction. Science. 2015;349:500–506. doi: 10.1126/science.aaa0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB. Serum Retinol Binding Protein 4 Contributes to Insulin Resistance in Obesity and Type 2 Diabetes. Nature. 2005;436:356–362. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- Yore MM, Syed I, Moraes-Vieira PM, Zhang T, Herman MA, Homan EA, Patel RT, Lee J, Chen S, Peroni OD, et al. Discovery of a Class of Endogenous Mammalian Lipids with Anti-Diabetic and Anti-Inflammatory Effects. Cell. 2014;159:318–332. doi: 10.1016/j.cell.2014.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, Bergeron R, Kim JK, Cushman SW, Cooney GJ, et al. Mechanism by Which Fatty Acids Inhibit Insulin Activation of Insulin Receptor Substrate-1 (Irs-1)-Associated Phosphatidylinositol 3-Kinase Activity in Muscle. J Biol Chem. 2002;277:50230–50236. doi: 10.1074/jbc.M200958200. [DOI] [PubMed] [Google Scholar]
- Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, Shoelson SE. Reversal of Obesity- and Diet-Induced Insulin Resistance with Salicylates or Targeted Disruption of Ikkbeta. Science. 2001;293:1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- Zhang K, Kaufman RJ. From Endoplasmic-Reticulum Stress to the Inflammatory Response. Nature. 2008;454:455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic Ikkbeta/Nf-Kappab and Er Stress Link Overnutrition to Energy Imbalance and Obesity. Cell. 2008;135:61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T, Hou M, Xia M, Wang Q, Zhu H, Xiao Y, Tang Z, Ma J, Ling W. Globular Adiponectin Decreases Leptin-Induced Tumor Necrosis Factor-Alpha Expression by Murine Macrophages: Involvement of Camp-Pka and Mapk Pathways. Cell Immunol. 2005;238:19–30. doi: 10.1016/j.cellimm.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Jiang Z, Delgado E, Li H, Zhou H, Hu W, Perez-Basterrechea M, Janostakova A, Tan Q, Wang J, et al. Platelet-Derived Mitochondria Display Embryonic Stem Cell Markers and Improve Pancreatic Islet Beta-Cell Function in Humans. Stem Cells Transl Med. 2017;6:1684–1697. doi: 10.1002/sctm.17-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-Interacting Protein Links Oxidative Stress to Inflammasome Activation. Nature immunology. 2010;11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]