Abstract
This study has investigated the metabolic effects of catechin-rich green tea (GT) and its formulation with ascorbic acid (AA) on the Zucker rat model of type 2 diabetes. AA is used to protect the GT catechins during digestion and increase bioavailability. Thirty two Zucker diabetic fatty (ZDF) rats were randomly divided into four groups (n=8 in each group) and treated with water, GT, AA and GT+AA respectively for five weeks. Urinary metabolic profiles were determined using 1H NMR spectroscopy. Fourteen metabolites were identified and their 24-hr excretions were quantified. Changes in the 14 metabolites demonstrated differential treatment effects on the metabolism of ZDF rats. GT and AA were found to be able to independently reduce urinary excretions of most metabolites that were over-excreted in the control diabetic rats, such as oxidative stress marker metabolites and TCA cycle metabolites. GT showed a great potential in controlling metabolic acidosis by suppressing the excretion of lactic acid and acetic acid from diabetic rats and GT+AA showed a remarkably stronger suppression than GT while AA was unable to suppress these two acids. Further investigation is needed to better understand the role of GT and/or formulated GT in altering the metabolic pathways in the diabetic animal model as well as in humans.
Keywords: Metabolomics, Metabolic Profiling, NMR spectroscopy, Diabetes, Green tea, Ascorbic acid
Introduction
Type 2 diabetes, also known as non-insulin-dependent diabetes mellitus (NIDDM), is a disorder characterized by high blood glucose levels in the context of insulin resistance and relative insulin deficiency. In the United States, 8.3 percent of the total population has diabetes, among which 90 percent are type 2, according to the American Diabetes Association [1]. Type 2 diabetes is often managed by increased exercise and altered diet; however, medications are also needed as the disease progresses. The total cost of diabetes in the U.S. was $ 245 billion in 2012 [1]. Much of the cost and morbidity of diabetes is due to long term complications including heart disease, kidney disease, neuropathy and blindness. Cost-effective methods are needed not only for preventing the disease from happening, stopping or slowing its progression, and reducing symptoms, but also for rapid and accurate screening/monitoring disease status. Currently, the early diagnosis of diabetes and monitoring of disease progression and treatment efficacy involve blood tests such as the glucose tolerance test and HbA1c. However, few noninvasive, low cost methods are available to monitor the severity of tissue damage due to persistent hyperglycemia prior to the presentation of overt complications. Development and validation of more efficient and economical methods of evaluating treatment efficacy hold the potential to greatly improve the effectiveness of treatment and/or preventative measures that would reduce the debilitating and costly long term complications of diabetes.
The use of green tea as a beverage and food ingredient dates back to five thousand years ago in ancient China and other Far East countries. Over the last few decades green tea has been the subject of many clinical and non-clinical studies to determine the extent of its long-purported health benefits, with some evidence suggesting regular green tea drinkers have lower risks and incidence of obesity, diabetes, heart disease and certain types of cancer [2–5]. An epidemiological study showed that long-term tea intake was associated with reduced prevalence of type 2 diabetes mellitus among elderly people from Mediterranean islands [6]. Chronically administered oral green tea was shown to have some benefit in Zucker diabetic rats but the response was not as great as intraperitoneal-administered green tea [7]. This may due to the poor stability and/or poor absorption of catechins, which are believed to be the active ingredients in green tea. About 80% of catechins are destroyed in the digestive process when tea is consumed alone. However, it has been shown that formulation with ascorbic acid can preserve the catechins during the digestive process [8].
Metabolomics promises immense potential for better understanding the biological processes associated with a number of diseases such as diabetes, and may have a significant impact on diagnosis, control and therapy [9–14]. NMR spectroscopy has been shown to be highly useful in obtaining nonselective, information-rich global metabolite profiles of biological samples with minimal sample preparation at low cost [11,15,16]. NMR-based metabolic profiles of biofluids contain latent information on the more subtle effects of diet and physiological variation. Ultimately, these profiles may also provide a rapid non-invasive method for diagnosing disease, monitoring disease progression and evaluating the effectiveness of treatment. NMR-based metabolomics has been applied to nutritional studies extensively [17–19]. A recent study showed the capability of NMR-based metabolite profiling for investigating the metabolism and bioavailability of black tea components in healthy human subjects [20]. A second NMR-based study focused on the human metabolic response to chamomile tea ingestion [21]. A clear differentiation between the samples obtained before and after chamomile treatment was achieved, which was found to be associated with the depletion of creatinine and the elevation of hippurate, glycine, and other important metabolites. We have previously applied 1H NMR-based metabolomics to study type 1 diabetes and successfully detected a number of candidate biomarkers and disturbed metabolic pathways that are promising for characterizing type 1 diabetes [13,22].
In this study, we have used a Zucker rat model of type 2 diabetes to investigate the metabolic effects of green tea versus green tea formulated with ascorbic acid. Ascorbic acid has been used to improve catechin bioavailability [8]. The use of this model to study diabetes has clear advantages over clinical time-course studies as well as human therapy trials because of cost and importantly a reduced subject variations or “biological noise” as well as rapid feedback. Metabolic profiling indicated that green tea, ascorbic acid and their combination had sizable effects on metabolic processes and these effects were different. We have also showed that this NMR-based metabolic profiling of urine is a sensitive promising tool for non-invasively screening subjects for age-related diseases such as diabetes, evaluating disease progression and the effectiveness of treatments.
Materials and Methods
Samples
All animal study protocols were approved by the Purdue Animal Care and Use Committee. Thirty two Zucker diabetic fatty (ZDF) rats were obtained at 6 weeks of age from Charles River Laboratory and randomly assigned to one of 4 groups (n=8 for each group): 1) Green tea (GT), 2) Ascorbic acid (AA), 3) GT+AA and 4) Water (Control). The GT powder contains 5% caffeine, 12% EGC ((-) epigallocatechin), 16% EGCG (epigallocatechin gallate), 4% EC((-) epicatechin) and 8% ECG ((-)epicatechin gallate) (wt/wt). Rats were individually housed in metabolic cages in a climate controlled room with 12 hour dark/light cycles. The rats were fed Purina 5008 rat chow (PMI Nutrition International, Framingham, MA) and provided with deionized drinking water. The food contains 23.5% protein, 7.5% fat, 49.4% carbohydrate, and 3.8% fiber. Rats had access to food and water ad libitum. The rats were gavaged daily for 5 weeks with one of the following supplements: A) ultrapure water only, B) AA (17 mg/kg), C) GT (85 mg/kg), D) GT+AA (85 mg/kg+17 mg/kg) for 5 weeks. The daily dose was divided into two equal parts and was administered in the morning and afternoon. All doses were reconstituted in water just prior to the delivery of the dose. This was done to assure that there was no degradation of the sample dose for either the AA or the GT catechins. AA was purchased from Sigma-Aldrich, Corp. (St. Louis, MO) and the GT was a hot water extract (A gift from the Nestle Product Technology Center, Marysville, OH). Urine samples were collected, in refrigerated vials, weekly throughout the study. The total urine volume for each rat excreted over 24 hour was collected in one container, weighed and stored at −80°C until NMR analysis.
1H NMR Spectroscopy
Each thawed urine sample (500 μl) was mixed with phosphate buffer (75 μl, 0.5 M, pH 7.0), D2O (75 μl), 3-(trimethylsilyl)propionic acid-d4 sodium salt (TSP) (5 μl in D2O, 0.11 μmol), and sodium azide (5 μl in D2O, 12.3 nmol). Resulting solutions were centrifuged; 500 μL supernatants were then transferred to 5-mm NMR tubes. All 1H NMR experiments were carried out at 25 °C on a Bruker DRX 500 MHz spectrometer equipped with an HCN 1H inverse detection probe with triple axis magnetic field gradients. 1H NMR spectra were acquired using the standard one-dimensional NOESY pulse sequence with water presaturation during the recycle delay of 3 s and a mixing time of 100 ms. Each dataset was averaged over 32 transients using 32,000k points. The time domain data were Fourier transformed after multiplying by a 0.3 Hz line broadening exponential window function, and the spectra were phase and baseline corrected using Bruker XWINNMR software (version 3.5).
Identification and quantitation of metabolites
A total of 14 metabolites were identified in the 1H NMR spectra based on their characteristic chemical shifts and multiplicities. Assignments of metabolites with overlap or small differences in chemical shifts (such as for fumarate, and allantoin) were confirmed by comparing 1H NMR spectra for urine before and after the addition of small quantities of synthetic standard compounds. Individual metabolite were then quantified from integrated peak areas, taking into account the NMR signal intensity and number of protons for both metabolites and the reference, TSP, and corrected using the volume of urine excreted in 24 h.
Data analysis
Primarily analysis was performed by calculating fold change values that are the ratios of 24-hour excreted metabolite quantities for each rat at each supplementation week to their concentrations at the baseline week (prior to the treatment) and the ratios were presented with a colored heat map (Figure 2).
Figure 2.

Fold changes in metabolite excretion over time under four treatments: water, AA, GT and GT+AA. This was to eliminate systematic variations and emphasize treatments-induced variations. Color intensities reflected the natural logarithmic fold-change as referenced to the first week.
The effects of GT and GT formulation to the metabolism of studied rats were also evaluated by comparing the mean concentration changes (versus baseline) among treatments using the generalized estimating equation modification to linear regression models to account for the longitudinal nature of the data. Statistical analyses were performed using SAS software (version 8.2; SAS Institute Inc., Cary, NC).
Results
Figure 1 shows typical 1H NMR spectra of urine from rats with four different treatments: 1) GT, 2) AA, 3) GT+AA and 4) Water (Control). Fourteen metabolites were identified and quantified: formate, hippurate, fumarate, urea, allantoin, creatinine, glucose, 2-ketoglutarate, dimethylglycine, citrate, succinate, acetate, alanine and lactate. Catechins and their metabolites were not detected by 1H NMR, most likely due to their relatively low levels in urine. Preliminary analysis of the data showed that the AA treatment decreased glucose tolerance only slightly, GT and GT+AA increased glucose tolerance slightly (data not shown). Therefore metabolic changes seen were due to mechanisms other than changes in the degree of hyperglycemia.
Figure 1.
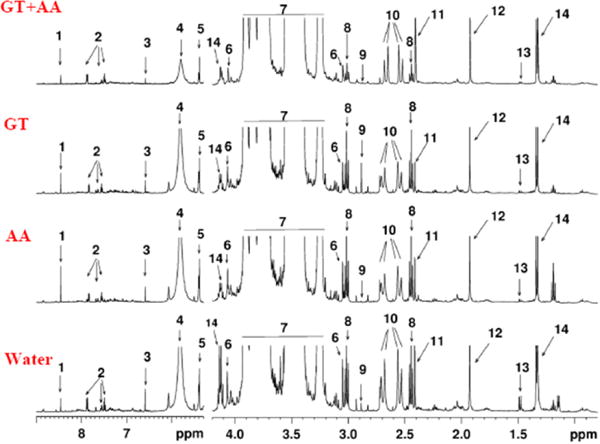
Typical 1H NMR spectra of urine samples from Zucker diabetic rats under four treatments: water, AA, GT, GT+AA; 14 altered metabolites are annotated: 1: formate, 2: hippurate, 3: fumarate, 4: urea, 5: allantoin, 6: creatinine, 7: glucose, 8: 2-ketoglutarate, 9: dimethylglycine, 10: citrate, 11: succinate, 12: acetate, 13: alanine, 14: lactate.
Figure 2 depicted the fold changes in 24-hour excreted quantities of metabolites as compared to baseline levels over time under the four treatments. Red indicates the highest increase and blue indicates the lowest increase. It was seen that daily average excretion of all 14 metabolites increased from their baseline week regardless of treatments. Such an enhanced metabolism was also demonstrated by the temporal response curves of individual metabolites using mean quantities estimated by generalized linear mixed model (Supplementary Figures 1a–c). Remarkably, with the control/water treatment (Figure 2), the daily glucose excretion showed an increase up to 8 fold and the daily excretion of creatinine and other metabolites increased continuously over time up to 32 or 64 folds by the end of study course (from dark blue to orange or red). Other metabolites also increased as substantially as creatinine over time. With GT treatment, many metabolites showed a significantly reduced excretion (as shown in disappearance of red and orange region) as compared to control/water (hippurate p=0.0370, fumarate p=0.0462, urea p=0.0363, allantoin p=0.0428, creatinine p=0.0579, glucose p=0.0214,, citrate p=0.0409) by week 3 and the AA treatment showed a similar metabolic effect to the GT treatment except for lactate and acetate, the excretion of which increased at much higher rates than all other treatments as in shown in orange/red bands. The GT+AA treatment showed remarkably different effects to the metabolism of ZDF rats: 1) Urinary excretions of lactate (p=0.0273) and acetate (p=0.0206) were dramatically reduced from Week 1 compared to control (two blue bands) and these changes were completely opposite to the AA treatment-induced changes; 2) Urinary excretions of hippurate, fumarate, urea, allantoin, creatinine, 2-ketoglutarate, dimethylglycine and citrate peaked by Week 3 (as high as the maximum excretion level in control rats at Week 6) and decreased over time from Week 4.
Discussion
The ZDF rats showed enhanced metabolite urinary excretions without any treatment. This suggested that glucose metabolism was impaired, as is attributable to the development of insulin insensitive diabetes, and the impaired glucose metabolism may then further stimulate a profound disturbance to the whole metabolic system. The elevated urinary excretion of all 14 metabolites may be due to a progressive impairment of renal tubular function of the ZDF rats, therefore a reduced ability in reabsorbing these metabolites. Other factors such as osmotic diuresis due to hyper glycemia could also contribute to the enhanced metabolite excretion. The enhanced excretion of creatinine could be in part attributed from the natural growth of rat muscle cells. The increased alanine and lactate excretions could be associated with increased gluconeogenic and glycolytic activities, respectively. The increased excretion of citrate, 2-ketoglutarate, fumarate, succinate could indicate the increased TCA cycle activity. Allantoin, a product of nonenzymatic urate oxidation, known to increase with type 2 diabetes progression, was an indication of high oxidative stress [23–25]. The increase of hippurate excretion could be due to increased hepatic availability of its precursors, mainly acetyl-CoA, in ZDF rats and reflect a metabolic acidosis. It was shown that metabolic acidosis stimulates hippurate synthesis in the liver and kidney and leads to increases of hippurate in plasma concentration and fractional excretion by the kidney [26].
This study demonstrated substantial metabolic effects of a catechin-rich GT formula, AA as well as the formulation of GT with AA on the ZDF rats. The beneficial effects of GT are usually attributed to the antioxidant activity of its catechin contents. Catechins are the most abundant polyphenols of GT. Catechins have been shown to counteract oxidative stress, help prevent obesity, and alleviate insulin insensitivity in type 2 diabetic patients [27,28]. AA is an enzymatic cofactor in many biosynthesis processes and works as an important antioxidant that can protect against oxidative stress caused by non-enzymatic glycation and metabolic stress in diabetic subjects [29]. Diabetes often induces oxidative stress that causes damage to multiple organs, leading to various complications. In this study, we have found reduced excretion rates of allantoin (an oxidative stress indicator) with GT treatment and AA treatment as compared to control. In addition to allantoin, the over-excretions of other important metabolites from several pathways (e.g. TCA cycle, glucose metabolism, amino acid metabolism) in non-treated ZDF rats (control) were also attenuated by GT or AA treatments. This suggested an effective modulation of GT or AA to the energy metabolism of ZDF rats.
Over-excretions of lactate and acetate in the non-treated control ZDF rats provided evidence of metabolic acidosis [30]. GT showed a moderate effect in controlling metabolic acidosis as shown by lowering the excretion of lactate and acetate (Figure 2). GT+AA showed a stronger effect than GT in controlling metabolic acidosis by an enhanced suppression of urinary lactate and acetate that occurred from the beginning of the treatment. On the contrary, AA treatment tended to exacerbate metabolic acidosis by enhancing the urinary excretion of lactate and acetate from ZDF diabetic rats. AA has been shown to play an important role in modulating lactate metabolism [31,32], and is believed to help muscles release greater quantities of lactic acid, although more concrete scientific proof is still needed. Whether or not the use of AA causes metabolic acidosis is not yet concluded, however, patients with metabolic acidosis are often conserved about the use of AA because its overdose can sometimes worsen acidosis conditions. This rat model study suggested that the combination of GT with AA could provide a better solution for metabolic acidosis in diabetic patients.
The goal of this study was to investigate the metabolic effects of GT versus GT formulated with AA on type 2 diabetes using ZDF rats. This study showed that metabolic profiles of the rats were altered differently when they were treated with GT and AA separately from when treated by GT and AA together. GT+AA seemed to have a synergistic effect in reducing metabolic acidosis in the diabetic rats as shown by continuously suppressing lactate and acetate over the 5-week treatment period. GT+AA, although reduced the excretion of almost all observed metabolites at week 5, enhanced the excretion of many of them for two or three weeks at the middle stage of the treatment (week 2~week 4) (Figure 2). Such a mid-stage enhancement effect was not found in the ZDF rats that were treated with GT and AA separately. It is known that both GT catechins and AA are antioxidants and can increase insulin sensitivity in patients with insulin resistance or type 2 diabetes [2,3,27]. Previous studies on rats have also shown that AA can significantly enhance the intestinal absorption of catechins [8,33]. Therefore, it was anticipated that the combined treatment could improve a metabolic control and the mid-stage perturbation was not expected. At present, the explanations for the perturbation remain unclear. They might be related to factors such as the severity of diabetes in the ZDF rats (e.g. hyperglycemia, hyperlipidemia, insulin resistance, and nephropathy), imbalance in oxidative/antioxidative status, the intricate metabolic adaption of the biological system to large levels of multi-antioxidant inputs, and the possible competition of antioxidant activities in removing oxidative stress. The GT+AA formulation treatment may be improved by modifications of the treatment starting time, antioxidant proportions of the formulation as well as total antioxidant dosage.
Conclusion
The 1H NMR-based metabolomics approach provides a great tool to aid in understanding the treatment of Zucker diabetic rats with GT formulations. This study also provides evidence for the concept of using urine for possible rapid and inexpensive screenings and detailed monitoring of disease status and treatment efficacy among type 2 diabetic population. In addition to further NMR-based metabolite profiling, MS-based methods such as GC-MS and LC-MS can be used in the future to detect more metabolites and provide more detailed information. Further investigation is needed to better understand the role of GT and/or formulated GT in altering the metabolic pathways in the diabetic animal model as well as in humans. It is expected that these studies will provide more evidences that GT formulations ameliorate type 2 diabetes.
Supplementary Material
Acknowledgments
The authors thank Pamela Lachcik for assistance with the animal studies. This project was funded by the Purdue-UAB Botanicals Center for Age Related Diseases NIH-NCCAM Grant P50-AT00477.
Abbreviations
- NMR
Nuclear Magnetic Resonance
- GT
Green Tea
- AA
Ascorbic Acid
- ZDF
Zucker Diabetic Fat
References
- 1.http://www.diabetes.org/diabetes-basics/diabetes-statistics/
- 2.Kao YH, Hiipakka RA, Liao S. Modulation of obesity by a green tea catechin. Am J Clin Nutr. 2000;72:1232–1234. doi: 10.1093/ajcn/72.5.1232. [DOI] [PubMed] [Google Scholar]
- 3.Ramadan G, El-Beih NM, Abd El-Ghffar EA. Modulatory effects of black v. green tea aqueous extract on hyperglycaemia, hyperlipidaemia and liver dysfunction in diabetic and obese rat models. Br J Nutr. 2009;102:1611–1619. doi: 10.1017/S000711450999208X. [DOI] [PubMed] [Google Scholar]
- 4.Hsieh SR, Tsai DC, Chen JY, Tsai SW, Liou YM. Green tea extract protects rats against myocardial infarction associated with left anterior descending coronary artery ligation. Pflugers Arch. 2009;458:631–642. doi: 10.1007/s00424-009-0655-1. [DOI] [PubMed] [Google Scholar]
- 5.Sturgeon JL, Williams M, van Servellen G. Efficacy of green tea in the prevention of cancers. Nurs Health Sci. 2009;11:436–446. doi: 10.1111/j.1442-2018.2009.00476.x. [DOI] [PubMed] [Google Scholar]
- 6.Panagiotakos DB, Lionis C, Zeimbekis A, Gelastopoulou K, Papairakleous N, et al. Long-term tea intake is associated with reduced prevalence of (type 2) diabetes mellitus among elderly people from Mediterranean islands: MEDIS epidemiological study. Yonsei Med J. 2009;50:31–38. doi: 10.3349/ymj.2009.50.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janle EM, Portocarrero C, Zhu Y, Zhou Q. Effect of long-term oral administration of green tea extract on weight gain and glucose tolerance in Zucker diabetic (ZDF) rats. J Herb Pharmacother. 2005;5:55–65. [PubMed] [Google Scholar]
- 8.Peters CM, Green RJ, Janle EM, Ferruzzi MG. Formulation with ascorbic acid and sucrose modulates catechin bioavailability from green tea. Food Res Int. 2010;43:95–102. doi: 10.1016/j.foodres.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maher AD, Lindon JC, Nicholson JK. 1H NMR-based metabonomics for investigating diabetes. Future Med Chem. 2009;1:737–747. doi: 10.4155/fmc.09.54. [DOI] [PubMed] [Google Scholar]
- 10.Bain JR, Stevens RD, Wenner BR, Ilkayeva O, Muoio DM, et al. Metabolomics applied to diabetes research: moving from information to knowledge. Diabetes. 2009;58:2429–2443. doi: 10.2337/db09-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gowda GA, Zhang S, Gu H, Asiago V, Shanaiah N, et al. Metabolomics-based methods for early disease diagnostics. Expert Rev Mol Diagn. 2008;8:617–633. doi: 10.1586/14737159.8.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denkert C, Bucher E, Hilvo M, Salek R, Oresic M, et al. Metabolomics of human breast cancer: new approaches for tumor typing and biomarker discovery. Genome Med. 2012;4:37. doi: 10.1186/gm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang S, Nagana Gowda GA, Asiago V, Shanaiah N, Barbas C, et al. Correlative and quantitative 1H NMR-based metabolomics reveals specific metabolic pathway disturbances in diabetic rats. Anal Biochem. 2008;383:76–84. doi: 10.1016/j.ab.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patti GJ, Yanes O, Siuzdak G. Innovation: Metabolomics: the apogee of the omics trilogy. Nat Rev Mol Cell Biol. 2012;13:263–269. doi: 10.1038/nrm3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang S, Nagana Gowda GA, Ye T, Raftery D. Advances in NMR-based biofluid analysis and metabolite profiling. Analyst. 2010;135:1490–1498. doi: 10.1039/c000091d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye T, Mo H, Shanaiah N, Gowda GA, Zhang S, et al. Chemoselective 15N tag for sensitive and high-resolution nuclear magnetic resonance profiling of the carboxyl-containing metabolome. Anal Chem. 2009;81:4882–4888. doi: 10.1021/ac900539y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gibney MJ, Walsh M, Brennan L, Roche HM, German B, et al. Metabolomics in human nutrition: opportunities and challenges. Am J Clin Nutr. 2005;82:497–503. doi: 10.1093/ajcn.82.3.497. [DOI] [PubMed] [Google Scholar]
- 18.Rezzi S, Ramadan Z, Fay LB, Kochhar S. Nutritional metabonomics: applications and perspectives. J Proteome Res. 2007;6:513–525. doi: 10.1021/pr060522z. [DOI] [PubMed] [Google Scholar]
- 19.Wishart SD. Metabolomics: applications to food science and nutrition research. Trends in Food Science & Technology. 2008;19:482–493. [Google Scholar]
- 20.Daykin CA, Van Duynhoven JP, Groenewegen A, Dachtler M, Van Amelsvoort JM, et al. Nuclear magnetic resonance spectroscopic based studies of the metabolism of black tea polyphenols in humans. J Agric Food Chem. 2005;53:1428–1434. doi: 10.1021/jf048439o. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Tang H, Nicholson JK, Hylands PJ, Sampson J, et al. A metabonomic strategy for the detection of the metabolic effects of chamomile (Matricaria recutita L.) ingestion. J Agric Food Chem. 2005;53:191–196. doi: 10.1021/jf0403282. [DOI] [PubMed] [Google Scholar]
- 22.Lanza IR, Zhang S, Ward LE, Karakelides H, Raftery D, et al. Quantitative metabolomics by H-NMR and LC-MS/MS confirms altered metabolic pathways in diabetes. PLoS One. 2010;5:e10538. doi: 10.1371/journal.pone.0010538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benzie IF, Chung Wy, Tomlinson B. Simultaneous measurement of allantoin and urate in plasma: analytical evaluation and potential clinical application in oxidant:antioxidant balance studies. Clin Chem. 1999;45:901–904. [PubMed] [Google Scholar]
- 24.Gruber J, Tang SY, Jenner AM, Mudway I, Blomberg A, et al. Allantoin in human plasma, serum, and nasal-lining fluids as a biomarker of oxidative stress: avoiding artifacts and establishing real in vivo concentrations. Antioxid Redox Signal. 2009;11:1767–1776. doi: 10.1089/ars.2008.2364. [DOI] [PubMed] [Google Scholar]
- 25.Yardim-Akaydin S, Sepici A, Ozkan Y, Torun M, SimÅŸek B, et al. Oxidation of uric acid in rheumatoid arthritis: is allantoin a marker of oxidative stress? Free Radic Res. 2004;38:623–628. doi: 10.1080/10715760410001694044. [DOI] [PubMed] [Google Scholar]
- 26.Dzúrik R, Spustová V, Krivosíková Z, Gazdíková K. Hippurate participates in the correction of metabolic acidosis. Kidney Int Suppl. 2001;78:S278–281. doi: 10.1046/j.1523-1755.2001.59780278.x. [DOI] [PubMed] [Google Scholar]
- 27.Crespy V, Williamson G. A review of the health effects of green tea catechins in in vivo animal models. J Nutr. 2004;134:3431S–3440S. doi: 10.1093/jn/134.12.3431S. [DOI] [PubMed] [Google Scholar]
- 28.Rizvi SI, Zaid MA, Anis R, Mishra N. Protective role of tea catechins against oxidation-induced damage of type 2 diabetic erythrocytes. Clin Exp Pharmacol Physiol. 2005;32:70–75. doi: 10.1111/j.1440-1681.2005.04160.x. [DOI] [PubMed] [Google Scholar]
- 29.Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes. 1999;48:1–9. doi: 10.2337/diabetes.48.1.1. [DOI] [PubMed] [Google Scholar]
- 30.Omole OO, Nappert G, Naylor JM, Zello GA. Both L- and D-lactate contribute to metabolic acidosis in diarrheic calves. J Nutr. 2001;131:2128–2131. doi: 10.1093/jn/131.8.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castro MA, Angulo C, Brauchi S, Nualart F, Concha II. Ascorbic acid participates in a general mechanism for concerted glucose transport inhibition and lactate transport stimulation. Pflugers Arch. 2008;457:519–528. doi: 10.1007/s00424-008-0526-1. [DOI] [PubMed] [Google Scholar]
- 32.Castro MA, Beltrán FA, Brauchi S, Concha II. A metabolic switch in brain: glucose and lactate metabolism modulation by ascorbic acid. J Neurochem. 2009;110:423–440. doi: 10.1111/j.1471-4159.2009.06151.x. [DOI] [PubMed] [Google Scholar]
- 33.Majchrzak D, Mitter S, Elmadfa I. The effect of ascorbic acid on total antioxidant activity of black and green teas. Food Chem. 2004;88:447–451. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


