Abstract
SymptomCare@Home, an integrated symptom monitoring and management system, was designed as part of randomized clinical trials to help patients with cancer who receive chemotherapy in ambulatory clinics and often experience significant symptoms at home. An iterative design process was informed by chronic disease management theory and features of assessment and clinical decision support systems used in other diseases. Key stakeholders participated in the design process: nurse scientists, clinical experts, bioinformatics experts, and computer programmers. Especially important was input from end-users, patients and nurse practitioners participating in a series of studies testing the system. The system includes both a patient and clinician interface and fully integrates two electronic subsystems: a telephone computer linked Interactive Voice Response system and a web-based Decision Support-Symptom Management System. Key features include: (1) daily symptom monitoring; (2) self-management coaching; (3) alerting; and (4) nurse practitioner follow-up. The nurse practitioner is distinctively positioned to provide assessment, education, support, and pharmacologic and non-pharmacologic interventions to intensify management of poorly controlled symptoms at home. SymptomCare@Home is a model for providing telehealth. The system facilitates using evidence-based guidelines as part of a comprehensive symptom management approach. The design process and system features can be applied to other diseases and conditions.
Keywords: cancer, chemotherapy, symptom management, interactive voice response, decision support system, telehealth, nurse practitioner
Background
Cancer chemotherapy effectively destroys cancer cells yet causes highly distressing side effects. Most patients experience more than one symptom; common symptoms include pain, fatigue, trouble sleeping, nausea and vomiting, and anxiety and depression.1 Poorly controlled symptoms have been shown to adversely affect patient functioning and quality of life 2 and may lead to unplanned visits to the outpatient clinic and emergency department. 3,4 Cancer chemotherapy is primarily delivered in the outpatient setting; thus, patients experience and self-manage treatment-related symptoms at home. Effective symptom monitoring and management at home can be challenging due to the number and varied degree of potential symptoms, the lack of effective communication methods between the patient and healthcare provider, and episodic management practices that are often inconsistent with clinical practice guidelines.
Patients typically receive verbal and written instructions about preventing and managing potential chemotherapy side effects, accompanied by a variety of prescriptions, days before the symptoms occur. Instructions are not typically tailored to a patient’s specific symptom pattern. Nurses may follow up with the patient by phone but more often patients must take an active role in notifying their provider when experiencing distressing symptoms. Often patients do not want to “bother” their providers; they may wait until the symptoms are seriously out of control and present at the emergency department. Better ways are needed to proactively monitor and manage symptoms at home, in between clinic visits.
Telehealth, the use of telecommunication and information technologies to provide health care to patients at home,5 is one approach to improving symptom management that has been deployed in several populations. 6-8 Mobile phone and computer-based systems have been used by outpatients receiving chemotherapy as a symptom monitoring and management method, and as an alert system for the cancer care team 1,9-22 (see Table 1). The evidence indicates that these approaches were feasible and acceptable among patients and providers with inconsistent evidence about efficacy.1,22 Computer-based algorithms have successfully been used by clinicians for managing symptoms experienced by patients with cancer in the ambulatory care area 23 and for management of chemotherapy-related symptoms at home.13 A key feature of many telehealth systems is the ability to generate alerts which can stimulate a clinical response. Clinical decision support systems are a type of telehealth software that integrate patient-generated information and alerts with rules, programs, or guidelines to direct patient care.24 However, no systems to date have included a decision support system for home symptom management directed at patients receiving chemotherapy. Moreover, detailed information about the design and development of existing systems is limited.
Table 1. Characteristics of Electronic Symptom Monitoring and Management Systems for Home Use in Patients receiving Chemotherapy.
| System and Reference | Origin | Platform | Symptom Reporting and Monitoring | Alerts | Self-care messages/ Library or Information Resource | Reports | Reminders | Clinician Interface |
|---|---|---|---|---|---|---|---|---|
| Symptom Tracking and Reporting (STAR)11,21 | Memorial Sloan Kettering Cancer Center NYC, NY, USA | Web portal at home | Adapted CTCAE for self-report; timing not specified | No/No | Longitudinal reports viewed by patient and clinician | Email reminders | Clinician could access and follow-up with patient | |
| WebChoice10 | University Hospital Oslo, Norway | Web Site | Symptoms and Quality of Life; timing not specified | No | Tailored/ Information page with links | No | No –Voluntary log in | Clinician could not access; follow-up occurred after patient emailed nurse |
| Symptom Care and Management System12 | National University Cancer Institute Singapore | Web site could use mobile device | Vital signs and 5 symptoms (if screen positive); daily reporting | No | Standard/Educational module | In real time with 7 days of data | Not reported | Clinician could access and follow-up with patient by video-conference only |
| t+ Medical Ltd 13 | Oxford University, Oxford, UK | Mobile phone | Temperature and 5 symptoms (CTCAE); twice daily reporting | Pager | Tailored/No | Real time, cumulative | Not reported | Clinician access and follow-up with patient |
| Advanced Symptom Management System (ASyMS)9,14 | Cancer Care Research Centre,University of Stirling, Stirling, UK | Mobile phone | Temperature and 6 symptoms; twice daily reporting and if sick | Pager | Tailored/No | Nurse views on web | Not reported | Clinician could access and follow-up with patient |
| Tele-oncology service using short message service (SMS) 15 | National Cancer Centre Singapore | Mobile phone, short message service | Nausea and vomiting; daily reporting | Tailored/No | Not reported | Short message service | Clinician could access and pharmacist follow-up with patient | |
| Handheld Symptom Management System 16 | UK based study, Universities Stirling, Glasgow | Personal Digital Assistant | Adapted C-SAS for report of 5 symptoms; at least once a day reporting | Pager or email | Tailored/No | Real time, Nurse views on web | Not reported | Clinician could access and follow-up with patient |
| Communicating Health Assisted by Technology Project (CHAT) 17 | Ohio State University Comprehensive Cancer Center, Columbus, OH, USA | Personal Digital Assistant | Brief Pain Inventory, Fatigue Symptom Inventory, CESD, SF-36; weekly reporting | PDA alarm tells patient to call oncologist | No/No | Summary graph | Via PDA | No |
| Symptom Monitoring and Reporting System for Lung Cancer (SyMon-L) 18 | Clinics in Cook County area (IL) | telephone-based interactive voice response (IVR) technology | 13-item symptom survey (Functional Assessment of Cancer Therapy [FACT] and LungSymptom Index [FLSI]; weekly reporting | No/No | Weekly automated delivery of paper report | Phone | Clinician could not access; follow-up in response to email alert | |
| Telephone Linked Care for Cancer Symptom Monitoring; Automated Symptom Monitoring 1,19 | Huntsman Cancer Institute, University of Utah College of Nursing, Salt Lake City, UT, USA | Interactive Voice response system | 10 symptoms (1-10 numeric scale) with drill down if symptom present; daily reporting | Email or FAX | No/No | Embedded in alert | Automated | No |
| Health Buddy®20 | James Graham Brown Cancer Center University of Louisville, Louisville, KY, USA | Electrical device that attaches to land-line phone | 3-5 questions per day related to 29 symptoms | No | Tailored/No | No | Green light on device | Clinician could access day after symptom reporting and follow-up with patient |
| Home telehealth Program37 | Veteran's Administration Medical Center Gainesville, FL USA | Electrical device that attaches to land-line phone | 5 symptoms; daily | Computer system | Tailored/No | Not reported | Not reported | Clinician could not access; follow-up in response to alert |
NOTE: CTCAE= Common Terminology Criteria for Adverse Events
Objective
In this paper, we describe the development and design features of SymptomCare@Home (SCH), an integrated symptom monitoring and management system, designed as part of a randomized clinical trial (RCT) to improve the symptom experience of patients with cancer receiving chemotherapy. Results of the RCT are reported elsewhere.25 SymptomCare@Home expands upon our previous work in which we developed and tested an automated telephone-based symptom monitoring system to bridge the communication gap between patients and oncology providers about unrelieved symptoms (see Table 1).1 Although patients and providers reported high satisfaction and ease of use, providers rarely contacted patients who reported moderate to severe intensity symptoms (alerts) so symptoms did not improve. Our research team thus sought to improve the system to help clinicians provide evidence based care.
Designing Symptomcare@Home
The SCH system was designed to support implementation of the Chronic Care Model.26 The model’s goal is an informed patient whose care is led by a knowledgeable, proactive health care team resulting in satisfying interactions, quality care, and improved patient outcomes.27 While this comprehensive model represents an entire health care system approach to chronic disease management, we addressed six factors of the model in designing SCH: (1) community, (2) the health care system, (3) self-management support, (4) delivery system design, (5) decision support, and (6) clinical information systems.
The SCH system includes both a patient and clinician interface and fully integrates two electronic systems: (1) an improved version of the telephonic interactive voice response (IVR) symptom monitoring to now include automated self-management coaching, and (2) a decision support symptom management system (DS-SMS). We originally planned a full integration with the electronic health record (EHR) but that was not feasible for purposes of a clinical trial in multiple cancer centers. Such an integration was time-intensive and cost-prohibitive without preliminary data to support the feasibility and efficacy of the new system. We thus created tools in the DS-SMS to allow the clinician to efficiently obtain information from and document in the EHR.
Enhancing the Interactive Voice Response System: Architecture and Content Build
The process of enhancing the IVR system was informed by experience in system development, testing, and implementation as well as empirical data from two studies: an RCT of the symptom monitoring and alerting system in 250 patients1 and a pilot study of self-management messages in 26 women with breast cancer receiving chemotherapy. Systematic feedback via an exit interview with patients and providers in these studies informed changes to improve the usability and acceptability of the system (see Figure 1).
Figure 1. Iterative Development of SymptomCare@Home including the Interactive Voice Response (IVR) symptom monitoring and alerting system and the Decision Support-Symptom Management System (DS-SMS).
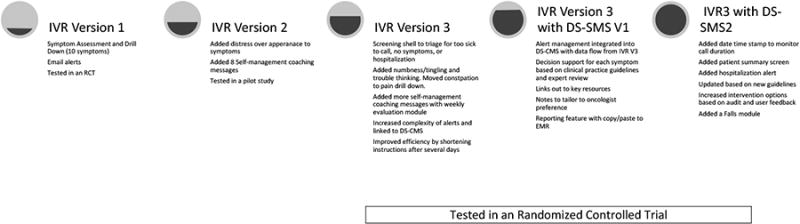
We maintained the basic architecture and logic of screens, drill-down assessments that added more specificity (e.g., number of times vomiting), alerts, and integrated self-management messaging. We addressed patient user concerns of calls being too long when they were very sick by adding a shell filtering structure at the beginning of each call allowing multiple pathways to shorten or opt out of a call. We reviewed updated clinical practice guidelines and engaged clinical experts to validate and expand the content of the IVR system. We augmented and reframed the content to include 12 symptoms and corresponding self-management coaching messages based on current chemotherapy drug toxicities. We added more sophisticated logic to simplify and shorten instructions after the user completed several days of reporting using a longer format with detailed instructions. We increased the sophistication of the algorithms and pathways for generating alerts allowing both simple and complex alerts based on single reports, patterns, or combinations of reports.
Designing the Decision Support-Symptom Management System: Architecture and Content Build
The design of the DS-SMS utilized an iterative and interactive process. We initially collaborated with experts at the Medical Information Systems Unit at Boston University. The team shared examples of patient management systems for childhood asthma and medication adherence in chronic diseases. Our designers then specified the initial structural elements for the SCH system. We simultaneously developed a template of content using a clinical practice guideline for fatigue. This template informed decisions about the structure of the system which was intended to include elements of clinical practice guidelines. Our design team developed mock-up screen shots for review and we made design decisions through a group process that considered workflow, user interface, and data capture.
We then developed content for each screen and each symptom using a standardized approach. Expert oncology nurses built the content through review of published literature and national clinical practice guidelines. Symptom management strategies included both non-pharmacologic and pharmacologic interventions; we thus had to customize the system for consistency with pharmacy formularies within the participating cancer centers. A pharmacist from the Huntsman Cancer Hospital in Salt Lake City, UT reviewed the pharmacologic information for accuracy and consistency with current practice and drug availability. Each participating medical oncologist also reviewed the content. If the physician wished to tailor treatment, and their preference was consistent with the clinical practice guideline, a reminder note was linked to the intervention in the DS-SMS.
Programming
The programming of both systems was completed through a partnership with Datatel Communication Systems (Miami, FL; http://www.datatel-systems.com/). Datatel is a provider of Interactive Voice Response hosted and managed services on the Cloud. The IVR Runs on Datatel’s CryptoIVR Advanced IVR Platform, a propriety platform designed for security and advanced logic handling. The DS-SMS was developed in .NET running on Microsoft IIS 7.5 Web Server; both systems link to a MS SQL Database (.NET and SQL developed by Microsoft, Redmond, WA).
Testing and Refining the System
We systematically tested SCH by inputting plausible and implausible data at each and every data entry point to test the entire system’s logic. In more than 200 test calls, staff reviewed reports and alerts to determine data accuracy and identify any glitches in the flow. The design team collaborated with Datatel to finalize the system and improve the user interface. The improvement process continued into the early implementation phase as Nurse Practitioner (NP) users identified problems and suggested improvements.
Detailed Characteristics of the Integrated System
The design process led to an integrated symptom monitoring and management system (see Figure 2) that was effectively utilized in an RCT with 358 patients who reported daily during their course of chemotherapy. As reported elsewhere, those randomized to SCH had significantly less overall symptom severity , a 40% reduction in moderate symptom days, and a 67% reduction in severe days, compared with usual care (both p < .001)25. We describe here the detailed characteristics of the SCH system which resulted from the design process.
Figure 2. Steps in Implementing the SymptomCare@Home System.
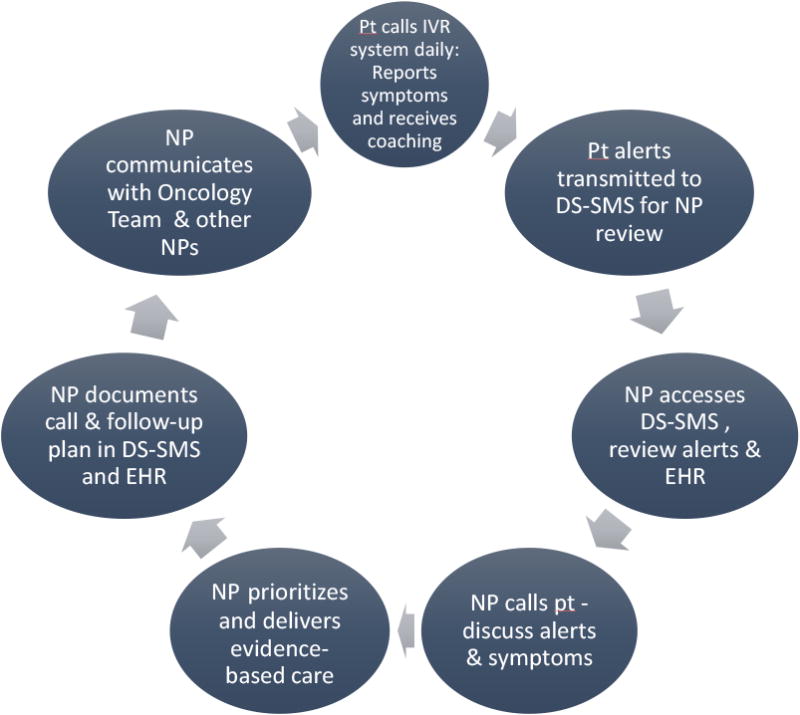
NOTE: Pt = Patient; NP = Nurse Practitioner; EHR=Electronic Health Record; IVR=Interactive Voice Response system; DS-SMS = Decision Support-Symptom Management System
Symptom Monitoring
The IVR symptom monitoring system uses a widely applied technology of telephone-computer interface to monitor daily patient reports of symptom experience, as well as functional and health care utilization information. Each participant called a toll-free number and used a password to log into the system which was stored on a secure and HIPAA-compliant server.
Using extensive branching logic and programming, the SCH system assesses whether a symptom occurred and, based on severity, gathers more detailed clinical information related to distress, associated factors, and current management strategies. The script for nausea and vomiting is displayed in Table 2. The original system monitored fever plus nine common symptoms: pain, fatigue, nausea/vomiting, trouble sleeping, feeling nervous or anxious, feeling blue or down, sore mouth, diarrhea, and constipation. In our redesign, we enhanced the SCH system to also measure distress over appearance, numbness or tingling, and trouble thinking or concentrating, additional symptoms that are prevalent and distressing in patients receiving chemotherapy. Constipation, not usually a side effect of chemotherapy, was moved to a drill down question related to pain.
Table 2. Sample Symptom Care @ Home Symptom Screening and Drill-Down Questions: Nausea and Vomiting Example.
| Nausea and Vomiting Screen |
|---|
| In the past 24 hours have you experienced nausea or vomiting? Press 1 for yes, or 2 for no. |
| If Yes, on a scale of 1 to 10, how would you rate the severity of your nausea during the past 24 hours? (Remember a rating of one means minimal nausea and a rating of 10 means the worst nausea you could have. Please enter the number.) |
| Nausea & Vomiting Drill Down (If severity on screen is 4 or more) |
| Earlier in the call you said that you have experienced nausea or vomiting. How many times have you vomited in the last 24 hours? Please enter the number now. |
| Have you felt dizzy or lightheaded? Press 1 for yes, or 2 for no. |
| Now let's talk about your nausea. If you haven't had any nausea in the past 24 hours, please press 0. If your nausea has occurred only once or twice in the last 24 hours, please press 1. If it comes and goes, press 2. If it has been continuous, press 3. Please enter the number now. |
| Have you taken any prescribed medication for nausea or vomiting in the last 24 hours? Press 1 for yes, or 2 for no. If NO |
|
| In the last 24 hours, how many 8-ounce glasses, which is about a cup of liquid, have you been able to drink and keep down without vomiting? Please enter the number of glasses now. |
A call day existed from midnight to the next midnight and participants were instructed to call the IVR system before noon each day. In the initial study, compliance with daily calling was 65% over an average of 45 call-in days.1 No calls for more than 2 days resulted in a research staff alert to telephone patients to assess for problems. Participants who did not call by noon received an automated reminder call from the IVR system in the afternoon. Calls were personalized to include the patient’s name and a closing message recorded by their provider.
We learned from our original study that sometimes patients didn’t call or complete a call if they were too sick, had no symptoms, or were hospitalized. We thus added a screening question to begin each call by asking participants how they felt in the past 24 hours. Possible answers were: they experienced symptoms (call continued); they were too sick to call; they had no symptoms; or they were hospitalized. If they answered yes to a triage question, the call ended. Using this approach, adherence increased to 90% in this RCT with an average participation time of 77 days. Out of 26,513 calls, 54% were filtered due to No Symptoms, 2.3% were Too Sick to Call, and .02% were Hospitalized.
The IVR system asked the patient if symptoms were present in the past 24 hours and, if present, patients rated severity and distress on a scale of 1 to10, an accepted standard that is commonly used clinically. Questions were stated simply and answered numerically with the touch-tone keypad. If fever was reported, the highest temperature was entered numerically and validated. In addition to symptoms, daily questions assessed functioning: the degree to which symptoms interfered with usual activities, attendance at work (for those employed), and amount of time spent lying down, resting, or sleeping. We subsequently enhanced the system by adding a daily module to monitor occurrence of falls and near falls, including type and context for the fall. Weekly calls assessed resource utilization and interaction with health providers or the health system including emergency department visits or hospitalization.
Self-Management Coaching
Major additions to the IVR system included eight detailed informational self-management coaching messages from the pilot study in women with breast cancer; strategies were based on research evidence and validated by a national panel of medical and master’s prepared nurse oncologists. These included improving fluid intake, hints to improve eating, preventing weight gain, improving concentration and thinking, starting an exercise program, conserving energy (six parts), getting a good night’s sleep (five parts), and improving pain relief. Specific symptom responses triggered integrated, tailored, and sometimes interactive self-management message recordings to instruct the participant on how to better manage their symptoms. Self-management strategies were designed in several different ways. Some messages were programmed to be automatically given when a specific symptom threshold was reached. For example, if a patient reports a fever of 100.5 degrees Fahrenheit or greater, SCH tells the person to report the fever to a physician. Alternatively, optional messages were offered in response to a symptom pattern; the patient could choose to listen or skip the message. For example, if a patient reported moderate levels of fatigue at least several days over the course of a week, she/he was told that research indicates that exercise, such as a walking program, can decrease fatigue in individuals receiving chemotherapy. Patients could choose whether to hear about starting an exercise program; those who listened were guided to set a walking goal for the week. Some self-management strategies were broken into small listening sessions that, once selected, would automatically offer the next session during each successive phone call. For example, energy conservation strategies were made available over a series of 6 days. For some symptoms, patients were asked to choose one of several strategies that might work. For example, if they reported trouble sleeping they could select from five different strategies to improve sleeping. Finally all self-management strategies were available in a “library” menu that patients could elect to listen to at the end of any telephone session. Self-management strategies triggered by complex symptom patterns or presented in multiple components required longer programming time but allowed tailoring to patient symptom and preference. We also added a weekly module to monitor use and helpfulness of self-management recommendations.
Alerting – Connecting Patient Reports to Clinician Management
SymptomCare@Home automatically generates alerts based on pre-established thresholds that vary by symptom. Thresholds were initially established by an expert panel and then revised based on pilot work.19 There are 44 different responses that generate a clinical alert requiring phone follow-up (see Table 3). There are also “information only” alerts and alerts sent only to the research team. The types of clinical alerts include: (1) alerts generated for each occurrence, such as a pain severity rating of 4 or greater on a scale of 1 to 10; (2) alerts requiring a certain pattern of responses such as trouble sleeping at a level of 4 or greater on a scale of 1 to 10 for 3 of the past 7 days; (3) alerts specific to a non-symptom condition such as a fall; (4) general alerts such as “patient feels too sick to call”; and (5) alerts due to implausible responses that require validation or re-entry of data. Each clinical alert was immediately transmitted after the call ended to the DS-SMS.
Table 3. Symptom Care @ Home Symptom Alert Thresholds.
| Symptom | Symptom Severity or Distress on 1 to 10 Scale | Other Alerts |
|---|---|---|
| Nausea and Vomiting | 4 or greater | Vomited 5 or more times |
| Did not take nausea meds – any specified reason | ||
| Fewer than 3 cups of fluid in 24 hours | ||
| Sore Mouth | 4 or greater | Fewer than 3 cups of fluid in 24 hours |
| Diarrhea | 4 or greater | Fewer than 3 cups of fluid in 24 hours |
| More than 6 bouts (new report) | ||
| More than 6 bouts and increasing | ||
| Fever and Chills | 4 or greater (Distress only) | Caller's temp is 100.0° or higher |
| Caller has shaking chills | ||
| Taken medication for fever or pain more than 8 times in 24 hours | ||
| Fatigue | 4 or more on 3 of past 7 days | |
| Trouble Thinking/Concentrating | 4 or more on 3 of past 7 days | |
| Feeling Blue & Down | 7 or greater, or 4 or more on 3 of past 7 days | |
| Feeling Nervous & Anxious | 7 or greater, or 4 or more on 3 of past 7 days | |
| Changes in Appearance | 4 or more on 3 of past 7 days | |
| Pain | 4 or greater | Taken meds, not enough relief |
| Numbness and Tingling | 4 or greater | |
| Trouble Sleeping | 4 or more on 3 of past 7 days | Caller interested in trying sleep medication |
| General Alerts | Too sick to call | |
| Patient wants NP to call today | ||
| Admitted to hospital or seen in an Emergency Department or Urgent Care Clinic or Seen by Oncologist/Nurse (information only) |
Nurse Practitioner Follow-up Using the Decision Support Symptom Management System
The DS-SMS has several functions: (1) provides patient-reported information about unrelieved symptoms and other problems that require attention; (2) allows the NP to manage the workflow and document responses to alert reports; (3) ties an alert-based unrelieved symptom with corresponding follow-up assessment checklists and specific intervention (pharmacologic and non-pharmacologic) based on national symptom guidelines; (4) provides immediate access via links to complete resources such as published guidelines; (5) provides a historical record of the symptom information collected over each cycle of chemotherapy allowing the NP to visualize patient specific symptom trends and patterns via graphs; (6) automatically generates a report of all relevant data (based on checkboxes and text summaries) from each phone encounter.
Symptom assessment and intervention strategies were based on published guidelines from several professional organizations. The primary source (used with permission) was the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology28. These guidelines are based on best available evidence, annually updated by expert panels, focused toward clinical use with decision pathways, outlining specific management and widely adopted within the oncology community. Other sources included evidence-based reviews from the Oncology Nursing Society’s Putting Evidence into Practice Resources program 29; the Symptom Management Guidelines from Cancer Care Ontario30; Clinical Practice Guidelines for the Prevention and Treatment of Mucositis from the Multidisciplinary Association for Supportive Care in Cancer (MASCC)31; and the PDQ resources provided by the US National Cancer Institute32.
The system follows usual work-flow and was organized by symptom (See Supplemental Digital Content 1, Screenshots A-C, SCH_CIN_SD1.PDF). The NPs reviews alerts automatically populated into a Patient Alert Screen in the DS-SMS daily including weekends and holidays (See Screenshot A). Prior to calling each patient, NPs review all pertinent information. The IVR feeds detailed drill down information about the symptoms into the Patient Assessment screen (See Screenshot B); the NPs also have full access to the participants’ EHR, which includes current medications, physician and nurse documentation, orders, labs, histories, and more. Nurse practitioners can also review past symptom alerts and graphs of symptom patterns
After a full review of assessment data and noting important data in a free text field, NPs phone the patient in response to the alert. To start the call, the NP clicks Set Start Phone Call Time Stamp button. The intervention screen contains a list of the symptoms and the guideline-based interventions (brief actions) that the NP uses and checks while talking with the participant (See Screenshot C). If an NP wants detailed information about the intervention, they simply click on the action and receive a drop down of additional text to use as talking points. In addition, a Show Guideline drop-down menu links directly to current published guidelines. The intervention screen also includes a box for free text documentation. The NP completes the call by pressing the Set End Phone Call Time Stamp creating a record of the call length. Next, by closing the alert(s), the NP generates a report of the call indexed by date and retrievable by other NPs and designated staff. The report can also be electronically copied and pasted into the EHR.
Lessons and Improvements
Close communication with end users was critical during the early role out of SCH. We identified and corrected some important issues such as a short time-out that led to the need to re-enter data. During this phase, we added the date/time start stop feature so that we could easily monitor duration of NP phone interaction with patients. Nurse practitioners also requested the addition of a hospitalization alert and a patient summary screen for easy access to information that was in the EHR but frequently needed. These additions improved workflow and efficiency.
An audit and feedback process proved extremely valuable in improving the DS-SMS. For example, we audited the use of pharmacologic interventions in response to pain alerts. We found a low adherence rate, but when we presented the findings to the NPs we learned that they were utilizing pharmacologic approaches that were not captured in the DS-SMS. Often patients already had an appropriate analgesic but were not taking it at all or taking it at less than optimal dose or frequency. We therefore redesigned the pain management intervention screen to capture these types of activities.
There were numerous other lessons learned in implementation that are informative for future system development. Scope of practice for NPs is determined at a state level; therefore, attention to state law will be important in disseminating such a system in multiple states. We also encountered issues with particular types of insurance that did not cover drugs recommended by guidelines. During study implementation, the use of smart phones greatly increased and allowed the NPs to utilize the system wherever they were. Future versions should consider a mobile interface. A further enhancement would include an integrated message board to facilitate communication across the NPs. They utilized phone and email to serve this function but such messaging was not captured in the system data.
We had one unexpected event where the entire system went down due to a power outage. We subsequently developed a back-up system and protocol for handling such an event. Although we anticipated that phone access or the cost of cell phone minutes might be a barrier, we only purchased one cell phone to support participation.
Discussion
Telehealth is becoming an important component of chronic disease management. We have described the process and results of designing an integrated symptom monitoring and management system. Similar to features in other systems (see Table 1), SCH has the capacity to (1) monitor patient symptoms, (2) provide patients with automated, tailored coaching using evidence-based symptom management strategies, and (3) deliver automated alerting to providers about the patient’s symptom status.1,9-20,33 Unique to the SCH system is the integration of a patient assessment and coaching system with a Web-based decision support system to guide symptom management by a NP. The NP is distinctively positioned to provide assessment, education, support, and pharmacologic and non-pharmacologic interventions to better manage symptoms at home. Patient care management by the NP has been shown to improve the health outcomes of patients receiving chemotherapy.25,33
The use of evidence-based guidelines as the foundation for the system guides the NP to deliver quality care. Decision support based on guidelines can facilitate more consistent practice among health care providers. 24,34 Documentation of evidence-based interventions allows for increased communication among cancer care providers about the patient’s symptom severity, distress, and recommended or implemented treatment strategies, thus reducing the potential for fragmented care and improving symptom management.
Implementation of the system requires engagement of both the patient and NP. Because of the dynamic nature of the symptom experience following chemotherapy, the system required daily reporting by the patient. Although this frequency of calling may seem burdensome, the evidence indicated that it was not. Adherence to calling was consistently high. Calls were brief and also contributed to minimizing burden. Future research should examine optimal triggers and frequency for patients to call the system to assure timely detection of new problems given the natural fluctuation of symptom presence and severity throughout a treatment cycle. It would be worthwhile to compare daily calls with asking patients to call the system on days when they experienced moderate or higher symptoms to determine if patients would obtain the same degree of symptom relief. The decision support system provided efficient and organized assessment and management of symptoms congruent with clinical practice guidelines yet allowed clinician judgment and personalized care. As guidelines become more prescriptive, future decision support systems will be able to use advanced programming logic to direct specific interventions for specific types of patients.
The design process was iterative and informed by chronic disease management theory26,27 and examples from IVR and case management systems used in other diseases.6-8,35 Key stakeholders participated in the design process: nurse scientists, clinical experts, bioinformatics experts, and computer programmers. The engagement of end users in using and improving the system is consistent with user-centered design methods.36 This type of user feedback becomes particularly important as systems like SCH are updated and refined. Fortunately, the development and testing of the system within a grant-funded program of research provided resources to design, test and upgrade the system in an iterative way over time.
Advances in technology and digital health indicate many potential improvements in systems such as SCH. Developing integration with the EHR will be essential for translation into the practice setting and linking SCH data with administrative and clinical data. Electronic health record vendors are now developing the capacity to interface with other software programs which will make this challenge less burdensome. There are numerous opportunities to tailor the SCH system. Patients could be provided with optional interfaces such as a Web page, mobile app, or telephone-based IVR, and could choose based on their preferences. Nurse practitioners could also choose a Web or mobile interface. We are currently expanding SCH to include additional symptoms as well as symptom monitoring and coaching that is relevant to other cancer treatments such as surgery, radiation therapy, biotherapy, and immunotherapy. The system has also been adapted to support family caregivers in partnership with hospice care providers. Modules for surveillance during cancer survivorship and palliative care are also needed. Ultimately, the system itself could be designed to tailor the assessment questions and frequency to a specific type of patient and treatment; coaching could be tailored to the patient or family caregiver. Finally, a major challenge in any integrated clinical system is keeping it current with practice and research. It is essential to plan for system updates and upgrades as both clinical content and technology advance. Future designs should consider a user interface that allows content updates without the added expense of programming each time an update is needed.
Conclusion
SymptomCare@Home, designed to support patients receiving chemotherapy, is a model for providing telehealth to patients at home. Patient assessment, self-management coaching, alerting a clinician, and systematic follow-up using evidence-based guidelines are essential features of a comprehensive disease management approach. The design process and system features can be applied to other diseases and conditions.
Supplementary Material
Acknowledgments
Conflicts of Interest and Sources of Funding: This work (KHM, SLB, CE) was supported by research grants from the National Cancer Institute of National Institutes of Health (R01 CA89474 and R01 CA120558). The effort of LHE was supported (in part) by the National Institute of Nursing Research of the National Institutes of Health under Award Number T32NR013456. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contributor Information
Susan L. Beck, University of Utah, College of Nursing and Huntsman Cancer Institute, Salt Lake City, UT USA, @SusanLarsenBeck (Twitter).
Linda H. Eaton, University of Washington of Nursing, Seattle, WA. USA, @lindaeatonRN (Twitter).
Christina Echeverria, University of Utah, College of Nursing, Salt Lake City, UT USA, @ChristinaEch (Twitter).
Kathi H. Mooney, University of Utah, College of Nursing, Huntsman Cancer Institute, Salt Lake City, UT USA.
References
- 1.Mooney KH, Beck SL, Friedman RH, Farzanfar R, Wong B. Automated monitoring of symptoms during ambulatory chemotherapy and oncology providers' use of the information: a randomized controlled clinical trial. Supportive Care in Cancer. 2014;22(9):2343–2350. doi: 10.1007/s00520-014-2216-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patrick DL, Ferketich SL, Frame PS, et al. National Institutes of Health State-of-the-Science Conference Statement: Symptom Management in Cancer: Pain, Depression, and Fatigue, July 15-17, 2002. J Natl Cancer Inst. 2003;95(15):1110–1117. doi: 10.1093/jnci/djg014. [DOI] [PubMed] [Google Scholar]
- 3.McKenzie H, Hayes L, White K, et al. Chemotherapy outpatients' unplanned presentations to hospital: a retrospective study. Support Care Cancer. 2011;19(7):963–969. doi: 10.1007/s00520-010-0913-y. [DOI] [PubMed] [Google Scholar]
- 4.Foltran L, Aprile G, Pisa FE, et al. Risk of unplanned visits for colorectal cancer outpatients receiving chemotherapy: a case-crossover study. Support Care Cancer. 2014;22(9):2527–2533. doi: 10.1007/s00520-014-2234-z. [DOI] [PubMed] [Google Scholar]
- 5.Liss HJ, Glueckauf RL, Ecklund-Johnson EP. Research on telehealth and chronic medical conditions: Critical review, key issues, and future directions. Rehabilitation Psychology. 2002;47(1):8. [Google Scholar]
- 6.Friedman RH, Kazis LE, Jette A, et al. A telecommunications system for monitoring and counseling patients with hypertension. Impact on medication adherence and blood pressure control. Am J Hypertens. 1996;9(4 Pt 1):285–292. doi: 10.1016/0895-7061(95)00353-3. [DOI] [PubMed] [Google Scholar]
- 7.Piette JD, Weinberger M, McPhee SJ. The effect of automated calls with telephone nurse follow-up on patient-centered outcomes of diabetes care: a randomized, controlled trial. Med Care. 2000;38(2):218–230. doi: 10.1097/00005650-200002000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Adams WG, Fuhlbrigge AL, Miller CW, et al. TLC-Asthma: an integrated information system for patient-centered monitoring, case management, and point-of-care decision support. AMIA Annu Symp Proc. 2003:1–5. [PMC free article] [PubMed] [Google Scholar]
- 9.McCann L, Maguire R, Miller M, Kearney N. Patients' perceptions and experiences of using a mobile phone-based advanced symptom management system (ASyMS) to monitor and manage chemotherapy related toxicity. Eur J Cancer Care (Engl) 2009;18(2):156–164. doi: 10.1111/j.1365-2354.2008.00938.x. [DOI] [PubMed] [Google Scholar]
- 10.Ruland CM, Andersen T, Jeneson A, et al. Effects of an internet support system to assist patients with cancer in reducing symptom distress: a randomized controlled trial. Cancer Nurs. 2013;36(1):6–17. doi: 10.1097/NCC.0b013e31824d90d4. [DOI] [PubMed] [Google Scholar]
- 11.Judson TJ, Bennett AV, Rogak LJ, et al. Feasibility of long-term patient self-reporting of toxicities from home via the Internet during routine chemotherapy. J Clin Oncol. 2013;31(20):2580–2585. doi: 10.1200/JCO.2012.47.6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan MF, Ang NK, Cho AA, Chow YL, Taylor B. Online chemotherapy symptom care and patient management system: an evaluative study. Comput Inform Nurs. 2014;32(2):75–83. doi: 10.1097/CIN.0000000000000036. [DOI] [PubMed] [Google Scholar]
- 13.Weaver A, Young A, Rowntree J, et al. Application of mobile phone technology for managing chemotherapy-associated side-effects. Annals of Oncology. 2007;18(11):1887–1892. doi: 10.1093/annonc/mdm354. [DOI] [PubMed] [Google Scholar]
- 14.Kearney N, McCann L, Norrie J, et al. Evaluation of a mobile phone-based, advanced symptom management system (ASyMS) in the management of chemotherapy-related toxicity. Support Care Cancer. 2009;17(4):437–444. doi: 10.1007/s00520-008-0515-0. [DOI] [PubMed] [Google Scholar]
- 15.Yap KY-L, Low HX, Koh KS, Un M, Shih V, Chan A. Feasibility and acceptance of a pharmacist-run tele-oncology service for chemotherapy-induced nausea and vomiting in ambulatory patients with cancer. Telemedicine and e-Health. 2013;19(5):387–395. doi: 10.1089/tmj.2012.0136. [DOI] [PubMed] [Google Scholar]
- 16.McGee MR, Gray P. A handheld chemotherapy symptom management system: results from a preliminary outpatient field trial. Health Informatics Journal. 2005;11(4):243–258. [Google Scholar]
- 17.Post DM, Shapiro CL, Cegala DJ, et al. Improving Symptom Communication Through Personal Digital Assistants: The CHAT (Communicating Health Assisted by Technology) Project. Journal of the National Cancer Institute Monographs. 2013;2013(47):153–161. doi: 10.1093/jncimonographs/lgt027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yount SE, Rothrock N, Bass M, et al. A randomized trial of weekly symptom telemonitoring in advanced lung cancer. Journal of pain and symptom management. 2014;47(6):973–989. doi: 10.1016/j.jpainsymman.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mooney KH, Beck SL, Friedman RH, Farzanfar R. Telephone-Linked Care for Cancer Symptom Monitoring. Cancer practice. 2002;10(3):147–154. doi: 10.1046/j.1523-5394.2002.103006.x. [DOI] [PubMed] [Google Scholar]
- 20.Head BA, Keeney C, Studts JL, Khayat M, Bumpous J, Pfeifer M. Feasibility and acceptance of a telehealth intervention to promote symptom management during treatment for head and neck cancer. The journal of supportive oncology. 2011;9(1):e1. doi: 10.1016/j.suponc.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basch E, Iasonos A, Barz A, et al. Long-term toxicity monitoring via electronic patient-reported outcomes in patients receiving chemotherapy. J Clin Oncol. 2007;25(34):5374–5380. doi: 10.1200/JCO.2007.11.2243. [DOI] [PubMed] [Google Scholar]
- 22.Basch E, Deal AM, Kris MG, et al. Symptom Monitoring With Patient-Reported Outcomes During Routine Cancer Treatment: A Randomized Controlled Trial. J Clin Oncol. 2016;34(6):557–565. doi: 10.1200/JCO.2015.63.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooley ME, Blonquist TM, Catalano PJ, et al. Feasibility of Using Algorithm-Based Clinical Decision Support for Symptom Assessment and Management in Lung Cancer. J Pain Symptom Manage. 2014 doi: 10.1016/j.jpainsymman.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castillo RS, Kelemen A. Considerations for a Successful Clinical Decision Support System. CIN: Computers, Informatics, Nursing. 2013;31(7):319–326. doi: 10.1097/NXN.0b013e3182997a9c. [DOI] [PubMed] [Google Scholar]
- 25.Mooney K, Beck SL, Wong B, Dunson WA, Wujcik D, Whisenant M, Donaldosn G. Automated home monitoring and management of patient-reported symptoms during chemotherapy: Results of the Symptom Care at Home RCT. Cancer Med. 2017 doi: 10.1002/cam4.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness: the chronic care model, Part 2. Jama. 2002;288(15):1909–1914. doi: 10.1001/jama.288.15.1909. [DOI] [PubMed] [Google Scholar]
- 27.Wagner EH, Bennett SM, Austin BT, Greene SM, Schaefer JK, Vonkorff M. Finding common ground: patient-centeredness and evidence-based chronic illness care. J Altern Complement Med. 2005;11(Suppl 1):S7–15. doi: 10.1089/acm.2005.11.s-7. [DOI] [PubMed] [Google Scholar]
- 28.NCCN. NCCN Guidelines for Supportive Care 2005. 2010 https://www.nccn.org/professionals/physician_gls/f_guidelines.asp#supportive.
- 29.ONS. Putting Evidence into Practice Resources 2016. 2014 https://www.ons.org/practice-resources/pep.
- 30.CCO. Symptom Mangement Guides 2016. 2014 https://www.cancercare.on.ca/cms/one.aspx?portalId=1377&pageId=58189.
- 31.MASCC/ISOO. Clinical Practice Guidelines for the Management of Mucositis Secondary to Chemotherapy 2014. 2014 http://www.mascc.org/mucositis-guidelines.
- 32.NCI. Physician Data Query : Cancer Information Summaries: Supportive and Palliative Care 2016. 2014 [Google Scholar]
- 33.Mason H, DeRubeis MB, Foster JC, Taylor JM, Worden FP. Outcomes evaluation of a weekly nurse practitioner-managed symptom management clinic for patients with head and neck cancer treated with chemoradiotherapy. Oncol Nurs Forum. 2013;40(6):581–586. doi: 10.1188/13.ONF.40-06AP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fox J, Patkar V, Chronakis I, Begent R. From practice guidelines to clinical decision support: closing the loop. Journal of the Royal Society of Medicine. 2009;102(11):464–473. doi: 10.1258/jrsm.2009.090010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Young M, Sparrow D, Gottlieb D, Selim A, Friedman R. A telephone-linked computer system for COPD care. Chest. 2001;119(5):1565–1575. doi: 10.1378/chest.119.5.1565. [DOI] [PubMed] [Google Scholar]
- 36.Erwin K, Krishnan JA. Using design methods to provide the care that people want and need. J Comp Eff Res. 2016;5(1):13–15. doi: 10.2217/cer.15.62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


