Abstract
Pannexins are a three-member family of vertebrate plasma membrane spanning molecules that have homology to the invertebrate gap junction forming proteins, the innexins. However, pannexins do not form gap junctions but operate as plasma membrane channels. The best-characterized member of these proteins, Pannexin1 (Panx1) was suggested to be functionally associated with purinergic P2X and N-methyl-D-aspartate (NMDA) receptor channels. Activation of these receptor channels by their endogenous ligands leads to cross-activation of Panx1 channels. This in turn potentiates P2X and NMDA receptor channel signaling. Two potentiation concepts have been suggested: enhancement of the current responses and/or sustained receptor channel activation by ATP released through Panx1 pore and adenosine generated by ectonucleotidase-dependent dephosphorylation of ATP. Here we summarize the current knowledge and hypotheses about interactions of Panx1 channels with P2X and NMDA receptor channels.
1. Introduction
Gap junctions are clusters of intercellular channels that provide cells with a means of communicating directly with their neighbors. In vertebrates, the well-known connexin (Cx) family accounts for assembly of gap junctions [1]. In invertebrates, innexins (Inxs) were originally characterized as the structural proteins of gap junctions. Both Cxs and Inxs are members of large multigene families; there are over 20 Cxs in mammals [2] and 8 and 25 Inxs in flies and worms, respectively [3]. Intriguingly, Inxs homologues have also been found in vertebrate genomes and named pannexins (Panxs). In contrast to Cxs and Inxs, Panxs are only a three-member family of proteins, which are termed Panx1, Panx2, and Panx3 [4]. The human Panx1 gene possesses an alternatively spliced exon 5, leading to the generation of two isoforms, termed Panx1b and Panx1bv [5, 6]. The unnamed truncated versions of Panx1 transcripts were also found to be present in mouse osteoblast [7]. We reported recently that rat pituitary gland expresses not only the full size isoform of Panx1, but also two novel splice isoforms, termed Panx1c and Panx1d; the presence of alternative splicing sites at exons 2 and 4 accounted for formation of Panx1c and Panx1d variants, respectively [8]. Others reported about the expression of Panx1 and Panx3 isoforms in the male reproductive tract of rats, which are also formed by alternative splicing [9]. A mutation encoding a truncated form of the Panx1 channel, termed Panx11–89, was recently identified in highly metastatic breast cancer cells [10].
Cxs, Inxs, and Panxs share a similar topology and secondary and tertiary structures. Each subunit of these proteins is composed of four transmembrane segments connected by two extracellular loops, one intracellular loop, and intracellularly located amino- and carboxyl-terminal ends [11]. The Cx family proteins assemble into hexameric arrangements, termed connexons or hemichannels, in the endoplasmic reticulum or Golgi apparatus and subsequently get transported to the plasma membrane [1]. Hemichannels from one cell dock with hemichannels from adjacent cells, forming gap junctional intercellular channels at cell-cell contact regions. These channels provide a pathway for the passage of ions, metabolites, small molecules and second messengers from cell to cell, without exposure to the extracellular environment [12]. Undocked Cx hemichannels are also found at the plasma membrane where they operate as large-pore plasma membrane channels [13].
Like Cxs, Panx1 undergoes hexameric oligomerization early in the secretory pathway [14], whereas Panx2 most likely forms octamers [15]. However, Panxs are visualized at cell membranes where there are no opposing cells [16] and function as ion channels [14, 17]. Therefore, the term hemichannels, which supposes an incomplete assembly state of half a channel, is not appropriate for Panxs and hereafter we will use the term “channels” for Panxs, and the terms “gap junction channels” and “hemichannels” for Cx proteins [18]. Furthermore, all three Panxs are glycosylated at their extracellular loops, i.e. at the position of 86 and 71 in the first extracellular loop of mouse Panx2 and Panx3, respectively, and at the position of 254 in the second extracellular loop of mouse Panx1, to different degrees [19]. Finally, glycosylation regulates Panxs intermixing and cellular localization [20]. In addition to plasma membrane localization, Panx1 wild type and splice forms are found intracellularly [8], as well as Panx3, which has a functional role in releasing calcium from the endoplasmic reticulum [21].
Cx junctional channels are gated by voltage and calcium, which are mediated by motifs that lie within or are exposed to the pore lumen [22]. Under resting conditions, Cx hemichannels display a low open probability. These channels are activated by plasma membrane depolarization, a decrease in extracellular calcium concentration, increase in intracellular calcium concentrations, changes in phosphorylation status, mechanical stimulation, ischemia conditions, and upon application of proinflamatory cytokines [21, 22]. Panx1 channels usually keep a closed state at resting conditions while depolarization of the membrane potential by voltage [23] and high extracellular potassium [24], increase in intracellular calcium [25], and mechanical stress [26] activate these channels. Effects of intracellular calcium on Panx1 gating are biphasic, stimulatory at lower concentrations and inhibitory at higher concentrations [27]. Various Cx mutations can interfere with the channel functions, leading to various diseases, including oculo-dento-digital dysplasia [1].
In physiological situations, two ligand-gated receptor channels appear to control gating of Panx1: purinergic P2X receptor (P2XR) channels and N-methyl-D-aspartate (NMDA) receptor (NMDAR) channels [28]. Furthermore, activation of calcium-mobilizing P2Y receptors (P2YRs) by extracellular adenosine 5′-triphosphate (ATP) [25], protease-activated receptors by thrombin [29, 30], and fibroblast growth factor receptors [31, 32] open Panx1 channels, probably reflecting the role of intracellular calcium in their gating. Insulin receptor activation in adipocytes also opens Panx1 channels, which facilitate glucose uptake [33]. Human Panx1 channels are also activated during apoptosis [34] through a mechanism of pro-apoptotic protease caspase 3/11-mediated cleavage of its pore-associated C-terminal autoinhibitory region [35, 36] and in ischemic-like conditions, reflecting activation of NMDAR [37]. The proinflammatory microenvironment [38, 39], including human immunodeficient virus infection [40], may also favor activation of Panx1 channels, and c-Jun kinase N-terminal kinase may play a role in this process [41].
Both Cxs and Panxs have been suggested to have large pores with exclusion limit up to 1 kDa, i.e. the size of pores that are potentially permeable for over 35,000 cellular molecules [12, 18, 42]. Among others, these channels are permeable for ions (calcium, sodium, potassium, chloride), metabolic substrates (glucose, lactose, ATP), and intracellular messengers (inositol 1,4,5-trisphophate and cyclic adenosine monophosphate) [43–48]. Such permeability of Cx hemichannels and Panx channels is consistent with their potential roles in autocrine and paracrine signaling in tissues by releasing molecules that act as agonists for plasma membrane receptors. It has also been suggested that Panx1 operates as an anion-selective channels, which is consistent with permeability of this channel pore to ATP [49]. Others suggested that ATP is too large to pass through Panx1 channel pore and that other pathway accounts for this release as documented in mammalian taste cells devoid of Panx1 [50, 51].
This brief comparison of structure, gating, and permeability of Cx gap channels/hemichannels and Panx channels clearly indicates difficulty in dissociating their functions in single cells, tissues, and organs co-expressing them. This includes current assays for investigation of Cx hemichannels and Panx channels involving cellular dye uptake and release as well as ATP release [18]. Additional complexity in studies with these proteins using dyes comes from finding that some P2XRs can facilitate dye uptake independently of Panx1 in an ATP-dependent manner [52, 53]. Further complication in studies with Panxs is that they are blocked by many of the same compounds that inhibit gap junction channels and hemichannels, including carbenoxolone, mefloquine and flufenamic acid, further suggesting that they may share common gating mechanism [54]. However, there are several non-gap junction related compounds that have been shown to block Panx1, including several transport inhibitors, chloride channel blockers, mitochondrial inhibitors, P2X7R ligands, inflammasome inhibitors and malaria drugs [55]. Furthermore, the development of Panx1 knockout mouse lines provides the cell models that allow for the distinction between Cxs and Panxs [56].
Multiple recent reviews covered in detail Cxs and Panxs structure, trafficking, post-translation modification, and channel gating and functions [4, 19, 21, 57–60]. This issue of the Journal also contains articles discussing in details Panx channels gating (chapter 5), their transcriptional and post-transcriptional regulation (chapter 8), and their roles in cell apoptosis (chapter 14), immune response (chapter 15), and cerebral ischemia (chapter 24). The focus in our review is on the evolving and potentially transformative aspects of Panxs physiology that have drawn considerable attention recently, i.e. interactions of Panx1 with P2XRs and NMDARs. The topics include: crosstalk between Panx1 and purinergic receptors, the role of Panxs in ATP release, Panx1 acting as a mega-pore for P2XRs, interactions between Panx1 and NMDAR, the role of Src family kinases in NMDAR-dependent opening of Panx1, and mechanisms of potentiation of NMDAR signaling by Panx1. For additional information on these topics see review articles [28, 61, 62] and references within.
2. Crosstalk between Panx1 and purinergic receptors
ATP and its degradation products, adenosine 5′-diphosphate (ADP) and adenosine, are important extracellular signaling molecules that participate in numerous biological processes in both physiological and pathophysiological conditions [63]. The potential relevance of ATP as an extracellular ligand for plasma membrane receptors was originally introduced by Geoffrey Burnstock in 1972 [64], but was received with skepticism until the first receptor was cloned in 1993 [65]. It is now well established that ATP activates two families of receptors, P2XRs and P2YRs. P2XRs are receptor channels organized as homotrimers and heterotrimer; seven mammalian P2X subunits, denoted P2X1 through P2X7, and several spliced forms of these subunits have been identified. P2XR activation leads to inward currents accompanied with increase in intracellular calcium concentrations [66]. P2YRs are G protein-coupled receptor family of proteins, in mammals composed of eight members: P2Y1R, P2Y2R, P2Y4R, P2Y6R, P2Y11R, P2Y12R, P2Y13R, and P2Y14R. Phylogenetically, P2YRs form two subgroups; members of the first group (1, 2, 4 and 6) signal through Gq/11 pathways, whereas the second group of receptors (12, 13, and 14) couples to different G proteins, including Gi/o, Gs and G16 [67]. P2XRs and P2YRs were found nearly in all mammalian cells with receptor preference based on the tissue/cell types [63]. The duration and distance of extracellular actions of ATP are limited by ectonucleotidases, four families of ectoenzymes that hydrolyze extracellular nucleotides to the respective nucleosides [68, 69]. In addition to ATP, its metabolite ADP acts as agonists for P2YRs in a receptor-specific manner, whereas ectonucleotidase-derived adenosine monophosphate (AMP) does not act as an agonist. However, AMP degradation product, adenosine, is a natural agonist for four nucleoside-activated G protein-coupled receptors, termed as P1 or adenosine receptors (ARs): A1R, A2AR, A2BR, and A3R [70].
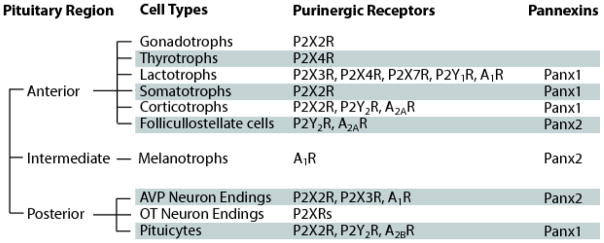
Purinergic receptors and Panx1 are ubiquitously co-expressed in a wide range of tissues, including nervous system, immune tissues, and neuroendocrine cells [5, 25, 71]. Figure 1 summarizes the cellular distribution of purinergic receptors and Panxs in the pituitary gland. The relationship between Panx1 and purinergic receptors in other tissues co-expressing them is also very complex. It has been suggested that both P2XR and P2YR can stimulate Panx1 channels. Among P2XRs, it appears that activated P2X7R stimulates Panx1 [72–76]. It has also been proposed that synergistic participation of P2X7R and Panx1 plays important roles in several physiological mechanisms, including calcium wave propagation [77], inflammation [78], and apoptosis [34]. Calcium mobilizing P2YRs also activate Panx1 [25]. As discussed in details in section 3, activated Panx1 channels provide a pathway for release of ATP in the extracellular space, which accounts for sustained activation of P2XRs and P2YRs. For example, Panx1 was proposed to mediate ATP release from neural progenitor cells and thus regulate their proliferation through purinergic receptors [79]. The possibility that Panx1 channel pore is a mega-pore of P2XRs has also been raised (section 4). Furthermore, ectonucleotidase metabolize ATP, generating ADP, an agonist for some P2YRs, and adenosine, a common agonist for ARs. Thus, there is a functional association between Panx1 and purinergic receptors.
Fig. 1.
Co-expression of purinergic receptors and pannexins in the pituitary gland. Pituitary cells secrete ATP [150, 151] and the role of Panx1 in ATP release in these cells is established [8, 80]. The released ATP activates numerous P2XRs and P2YRs expressed in pituitary cells [152, 153]. Ectonucleotidases are also operative in pituitary cells [150, 151] and provide a pathway for generation of adenosine and subsequent activation of adenosine receptors, which are also expressed in pituitary cells [154]. AVP, arginine-vasopressin; OT, oxytocin.
The association between Panx1 and P2XRs could also be physical. Using coimmunoprecipitation approach, the physical associations between Panx1 and P2XRs (including P2X2R, P2X3R, P2X4R and P2X7R) have been shown in endogenous expression system, such as macrophages, astrocytes and other cell types, and in exogenous expression system, such as HEK293 cells, arguing against overexpression as the mechanism for establishment of physical coupling [8, 24, 72, 80–84].
There was also a progress in clarifying the mechanisms by which activated P2X7R cross-activate Panx1. Two pathways, which differ in the mode of P2XR and Panx1 interactions, are possible: a direct connection between channels or through messengers acting as intermediaries. These pathways are not mutually exclusive. Physical coupling of P2XR and Panx1 provides the possibility that conformational changes associated with P2XR activation co-activate Panx1 channel, which in turn facilitates ATP release in close proximity of P2XR ectodomain and sustain P2XR-dependent signaling. Consistent with this hypothesis, it has been reported that Panx1 facilitates ATP release and is co-translocated with P2X1R and P2X4R to the immune synapse of primary T cells upon TCR/CD28 stimulation for regulation of T-cell activation [85]. Indirect coupling includes the role for intracellular messengers triggered by initial activation of purinergic receptors through Ca2+-dependent and –independent mechanisms. Extracellular Ca2+ was suggested to play a negative role in association of P2X7R and Panx1 [81], and Ca2+ wave propagation was suggested to play a role in association of P2YR with Panx1 by calcium-dependent opening of the channel pore [25]. The proline-rich C-terminal region of P2X7R was suggested to play an important role in calcium-independent activation of Panx1 by Src-tyrosine kinase [74].
3. The role of Panx1 in release of ATP
Extracellular ATP was initially found to be co-secreted at synaptic cleft with other neurotransmitters utilizing the exocytotic secretory pathway [71]. It is now well established that ATP is accumulated and stored in the synaptic vesicles in presynaptic terminals, rapidly (in a millisecond time scale) released, and acts as an excitatory transmitter in several central nervous system (CNS) regions [86]. It has also been shown that nucleotides act not only as neurotransmitters, but also as paracrine factors delivered by diffusion that require several seconds, rather than a few milliseconds, to activate the receptors [87]. Several molecular mechanisms for such ATP release were proposed, including lytic (cell disruption), vesicular, and non-vesicular release [88].
Panx1 was identified as an important contributor for non-vesicular ATP release in extracellular medium, along its concentration gradient [26, 89]. The role of Panx1 in the release of ATP has been shown in numerous cells in normal physiological, stress or pathological conditions [90]. Panx1 channel is ubiquitously expressed in the mammalian CNS, including the retina, olfactory bulb, neocortex, hippocampus, cerebellum, spinal cord, and pituitary gland [23, 80, 91, 92]. The role of Panx1 in ATP release was shown in CNS, including astrocytes and microglia [93–95], cochlea [96], and pituitary cells [80]. Panx1 has been identified as a major mediator of brain damage after ischemia [97], a contributor of seizures [98], and a tumor suppressor [99]. The role of Panx1 in ATP release in traumatic brain injury was also reported [100, 101].
The role of Panx1 in ATP release was observed in many other cell types, including taste buds [102], skeletal and smooth muscle cells [103], urinary bladder [104], kidney [105], airway epithelial cells [106], endothelial cells [29], chondrocytes [107], adipocytes [33], and erythrocytes [108]. Panx1 is also implicated in the release of ATP from immune cells following immune stimulation with lipopolysaccharide [109], T-cell receptor stimulation [85, 110], and osmotic stress [111]. It plays important roles in inflammasome [24] and T cell [110] activation, innate immune responses [112], apoptosis [34], monocyte [41] and lymphocyte [93] migration, leukocyte adhesion and emigration [38], and macrophage recruitment to promote the clearance of dead or dying cells [34]. Interestingly, recent studies indicated that Panx1 and Cx43 are also involved in the immune challenged-induced ATP release in lower vertebrate species Paralichthys olivaceus [113, 114], suggesting a conserved role of these proteins in ATP release remains from lower vertebrates to mammals throughout the evolution.
Even though the role of Panx1 in ATP release has been well recognized, the regulatory mechanism of this process is not fully understood. It has been reported that high levels of extracellular ATP can serve as a negative feedback regulator in controlling Panx1 channel activity [115]. S-nitrosylation, a posttranslational modification on cysteine(s), can also inhibit Panx1-mediated ATP release [116]. In addition, an isoform-dependent mechanism in regulating Panx1 activity could exist. In pituitary cells, two shorter Panx1 isoforms, termed Panx1c and Panx1d, may serve as dominant-negative effectors to attenuate the ATP release of the wild-type Panx1 through formation of a complex that interferes with the function of the wild type channel [8]. The truncated Panx1 channel lacking C-terminal tail, named Panx11–89, had also been found in metastatic breast cancer cells and is capable of augmenting the wild-type Panx1 channel-mediated ATP release [10]. Furthermore, Panx2 is able to form heteromeric channels with Panx1 and thus decrease the Panx1 currents [23]. Finally, a recent study by Boyce et al. revealed that ATP is the most potent stimulator of Panx1 activity and internalization, the latter in a cholesterol-dependent, but clathrin, caveolin and dynamin independent pathway [117].
4. Panx1 as the mega-pore of P2XRs
All P2XR channels pores are permeable for monovalent and divalent inorganic cations [66] and we termed this state as open-1 [118]. Among P2XRs, P2X2R, P2X4R, and P2X7R subtypes are also known to operate in a mode that has features of a mega pore i.e. that these channels can conduct larger organic molecules, like N-methyl-D-glucamine cation and some fluorescent dyes; we termed this mode as open-2 [118]. During continuous ATP application, a transition from open-1 to open-2 state was initially proposed and termed pore dilation [119–121]. However, the open-2 state of P2XR resembles the Panx1 channel pore permeability and has a linear current voltage relationship [122], similar to Panx1 current activated by NMDA [123]. These and several other lines of evidence supported the hypothesis that Panx1 activation accounts for open-2 state. For detailed description of these data see [28, 61, 124].
However, in cells lacking detectable endogenous Panx1, the existence of open-2 state of P2X2R and P2X7R was demonstrated and was associated with the physical movement of the receptor C-terminal [125–127]. P2X4R channel is not permeable to N-methyl-D-glucamine when activated by ATP, but allosterically induced sensitization of this receptor causes transition from open-1 to open-2 state [128]. The dilation of P2X2R has been questioned, but not the ability of this channel to conduct N-methyl-D-glucamine independently of Panx1 expression [129]. It has also been shown that antagonists carbenoxolone and interference RNA targeting Panx1 did not affect P2X7R macroscopic currents and thus questioned the association of Panx1 with P2X7R pore in murine macrophages [130]. Furthermore, activation of P2X7R in microglial cells from Panx1 knockout mice showed biphasic current, indicative of open-2 state [127]. Finally, physical coupling between P2X1R or P2X3R and Panx1 has also been shown [80], but during prolonged stimulations with ATP, these channels desensitize completely without entering into open-2 state [131]. Thus, the open-2 state is an intrinsic property of some members of P2XR channels, independent of their crosstalk with Panx1.
5. Crosstalk between Panx1 with NMDA receptor channels
NMDAR is a glutamate-gated receptor channel with high calcium permeability that plays important roles in different CNS functions. The name indicates specificity of this glutamate receptor subtype to bind the selective synthetic agonist NMDA. In physiological conditions, binding of two ligands, glutamate and glycine or D-serine, combined with membrane depolarization to dislodge and repel Mg2+ and Zn2+ ions from the pore, is required for activation of this non-selective cation channel. NMDAR activation is critical for the development of CNS, generation of rhythms for breathing and locomotion, and synaptic plasticity, a cellular mechanism underlying the processes of learning and memory. Abnormal expression levels and/or altered NMDAR function have been implicated in numerous neurological disorders and pathological conditions, including stroke, hypoxia, ischemia, head trauma, Huntington’s, Parkinson’s, and Alzheimer’s diseases, epilepsy, neuropathic pain, alcoholism, schizophrenia, and mood disorders [132, 133].
The work of Thompson and colleagues first indicated that there could be a functional link between Panx1 and NMDARs. In 2006, they first suggested that Panx1 is activated in isolated hippocampal neurons in response to oxygen and glucose deprivation, generating primary and secondary currents that depolarized the membrane, leading to neuronal death [37]. Later, they showed that NMDAR-induced secondary current and dye efflux could be abrogated by Panx1 knockdown and Panx1 inhibitory peptide [123, 134, 135]. Because Panx1 may facilitate neuronal depolarization, leading to hyperexcitability, it has been suggested that the channel could also participate, exacerbate, or cause a seizure. In vitro the experimental status epilepticus could be generated by stimulation of hippocampal slices with NMDA [123, 136]. Such neuronal activity in hippocampal slices was significantly reduced after blocking the activity of Panx1 with interference RNA, via knockout, or with application of Panx1 antagonists [123]. These observations suggested that NMDAR activation opens Panx1 channels, which in turn potentiates the NMDAR signaling.
A functional link between NMDAR activation and Panx1 was also proposed to play an important role in neuropathic pain in the spinal cord [4, 42] and traumatic brain injury [137]. Pharmacological blockage of Panx1 in spinal cord was found to reduce both the mechanical hyperalgesia and the spinal nociceptive transmission in neuropathic rats [138]. Two other studies also showed that carbenoxolone attenuated mechanical hypersensitivity in models of pathological pain [139, 140]. Because carbenoxolone is a nonselective inhibitor of gap junctions that also blocks Panx1, these reports provide only an indirect link regarding the possible participation of Panx1 in chronic pain. However, others found that the nerve injury increased the number of Panx1-immunoreactive neurons in dorsal root ganglion. Their study also indicated that the increased of Panx1 expression in this ganglion has a pathological correlation with pain hypersensitivity induced by nerve injury [141]. Furthermore, neuronal Panx1 was proposed to closely operate with astroglial Cx43 hemichannels in co-culture of neurons and astrocytes. The authors suggested that during ischemic events, astrocytes exposed to amyloid-β-peptide release ATP and glutamate, which then activate Panx1 in neurons and elicit neuronal cell death; only inhibition of both NMDAR and P2XRs could abolish neuronal mortality [142].
6. NMDAR opens Panx1 channels via Src family kinase
Two lines of evidence support the hypothesis that NMDAR opens Panx1 channel via tyrosine kinases derived from sarcomas, or Src kinases; an amino acid sequence similar to a consensus site for Src has been identified in Panx1 C-terminal and a peptide sequence that binds to C-terminal between the 305–318 amino acids is able to block the opening of Panx1 during anoxic depolarization [134]. In addition, Src kinase phosphorylation and activation of Panx1 requires only ligand binding to NMDAR, that is, it does not depend on NMDAR channel activity, as this effect was also observed in the presence of pharmacological and physiological pore block. Moreover, both in vitro and in vivo disruption of the NMDAR-Src-Panx1 complex was shown to be protective in ischemia [143]. Finally, Src kinases have been proposed to provide a mechanism of NMDAR activation in chronic pain conditions. A peptide consisting of amino acids 40–49 of Src fused to the protein transduction domain of the HIV Tat protein was shown to prevent pain behaviors induced by formalin and reverse pain hypersensitivity produced by injection of complete Freund’s adjuvant or by peripheral nerve injury [144].
7. Mechanisms of Panx1-dependent potentiation of NMDAR signaling
The work by Thomson’s laboratory suggests that the potentiating effect of ionotropic NMDARs occurs through enhancement of currents by activating Panx1 and TRPM channels, leading to anoxic depolarization [62, 145]. Because the opening of Panx1 channels is associated with ATP release, additional physiological consequences of such release are possible. Specifically, ATP released through Panx1 is rapidly degraded by ubiquitously present ectonucleotidases, resulting in extracellular accumulation of adenosine, which could modulate glutamate release by activating its own G protein-coupled receptors. In the striatum, supraphysiological concentration of adenosine activates presynaptic A2A receptors in glutamatergic synapses and facilitates glutamate release, thus potentiating NMDAR signaling [146]. Panx1 activity was also linked to adenosine signaling through A1 receptors in the CA3 subregion of the hippocampus. ATP is released directly from CA3 neurons and dephosphorylated to adenosine, which activates A1Rs to diminish neuronal excitability [147]. The Panx1 knockout mice exhibit increased excitability and prolonged and enhanced long term potentiation responses in the CA1 subregion. Adenosine application and NMDAR-blocking normalized this phenotype, suggesting that absence of Panx1 causes chronic extracellular ATP/adenosine depletion, thus facilitating postsynaptic receptor activation [148]. This suggests that, due to Panx1 loss, animals have low extracellular levels of ATP/adenosine in the hippocampus and consequently impaired A1R signaling, which leads to increased glutamate release. Recently, it was also observed that altered adenosine signaling is the basis of observed differences in sleep-wakefulness cycle and behavior in Panx1 knockout mice [149].
8. Conclusions
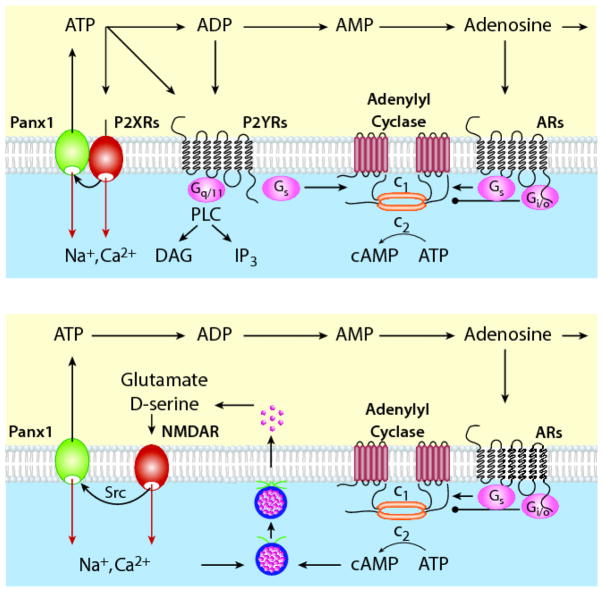
Figure 2 summarizes the bidirectional coupling of Panx1 with P2XRs and NMDARs. The evidence that Panx1 potentiates activity of P2XRs and NMDARs is strong. The NMDAR activation of Panx1 occurs through Src kinase signaling pathway and this signaling pathway may also be involved in the crosstalk between P2X7R and Panx1. Enhancement of the NMADR current appears to be the main mechanism for Panx1-dependent potentiation of receptor signaling. The ability of activated Panx1 to release ATP has been reported by numerous laboratories in different cell types expressing these channels and is consistent with operation of Panx1 as a nonselective or anionic channel. Such release is important not only for sustained activation of P2XRs but also for activation of P2YRs. The release of ATP by Panx1, combined with ectonucleotidase activity, may also account for activation of adenosine receptors, coupled with facilitation of NMDAR signaling events.
Fig. 2.
The crosstalk between Panx1 and ligand-gated receptor channels. Top panel: Interactions between Panx1 and P2XRs. Activated P2XRs cross-activate Panx1 channels probably through the Src family kinases. This leads to potentiation of P2XR signaling. Two pathways have been proposed: enhancement of the current (red arrows) and continued activation of P2XRs and co-activation of P2YRs, and ARs (black arrows). Opening of the Panx1 channel facilitates ATP release, which acts as agonist at P2XRs and P2YRs before ectonucleotidases metabolize it into ADP, AMP, and adenosine. ADP also acts as natural ligand for some P2YRs, whereas adenosine is natural ligand for ARs. P2YRs signals through Gq/11 and Gs signaling pathway, whereas ARs receptors facilitate adenylyl cyclase activity through Gs coupling and inhibit this enzyme through Gi/o coupling. Both autocrine and paracrine mode of actions were reported. This system is operative in many tissues, including the pituitary gland (see Fig. 1). Bottom panel: Interactions between Panx1 and N-methyl-D-aspartate receptor (NMDAR) channels. Activated NMDAR cross-activates Panx1 through the Src family kinases, which in turn potentiate the NMDAR signaling, by enhancement of the current (red arrows) and/or through ATP released from Panx1 and dephosphorylated to adenosine, which binds to adenosine receptors to diminish neuronal excitability (Gi/o coupling) or provides sustained NMDR activity, probably through release of glutamate and D-serine (Gs-coupling). ARs, adenosine receptors; P2XRs, ATP-gated purinergic P2X receptor channels; P2YRs, purinergic G protein-coupled P2Y receptors; PLC, phospholipase C; DAG diacylglycerol; IP3, inositol (1,4,5) trisphosphate; NMDAR, N-methyl-D-aspartate receptor.
Highlights.
The hexameric protein Pannexin1 operates as a plasma membrane channel.
Activated P2X and N-methyl-D-aspartate receptors cross activate Pannexin1 channels.
Turn on Panexin1 potentiates P2X and N-methyl-D-aspartate receptor signaling.
Acknowledgments
This work was supported by the National Natural Science Foundation of China No. 31572645 (S.L.), Serbian Ministry of Education, Science and Technology Project No. III41014 (B.I.), and the Intramural Research Program of the National Institute of Child Health and Human Development Project ZIA HD 000195-22 (S.S).
Abbreviations
- ARs
adenosine receptors
- ATP
adenosine 5′-triphosphate
- ADP
adenosine 5′-diphosphate
- AMP
adenosine monophosphate
- CNS
central nervous system
- Cxs
connexins
- Inxs
innexins
- NMDA
N-Methyl-D-Aspartate
- NMDAR
NMDA receptor
- P2XRs
ATP-gated P2X receptor channels
- P2YRs
G protein-coupled purinergic receptors
- Panxs
pannexins
Footnotes
Disclosure Statement: The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Laird DW. Life cycle of connexins in health and disease. Biochem J. 2006;394:527–543. doi: 10.1042/BJ20051922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Willecke K, Eiberger J, Degen J, Eckardt D, Romualdi A, Guldenagel M, Deutsch U, Sohl G. Structural and functional diversity of connexin genes in the mouse and human genome. Biol Chem. 2002;383:725–737. doi: 10.1515/BC.2002.076. [DOI] [PubMed] [Google Scholar]
- 3.Phelan P. Innexins: members of an evolutionarily conserved family of gap-junction proteins. Biochim Biophys Acta. 2005;1711:225–245. doi: 10.1016/j.bbamem.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Penuela S, Gehi R, Laird DW. The biochemistry and function of pannexin channels. Biochim Biophys Acta. 2013;1828:15–22. doi: 10.1016/j.bbamem.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 5.Baranova A, Ivanov D, Petrash N, Pestova A, Skoblov M, Kelmanson I, Shagin D, Nazarenko S, Geraymovych E, Litvin O, Tiunova A, Born TL, Usman N, Staroverov D, Lukyanov S, Panchin Y. The mammalian pannexin family is homologous to the invertebrate innexin gap junction proteins. Genomics. 2004;83:706–716. doi: 10.1016/j.ygeno.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 6.Ma W, Hui H, Pelegrin P, Surprenant A. Pharmacological characterization of pannexin-1 currents expressed in mammalian cells. J Pharmacol Exp Ther. 2009;328:409–418. doi: 10.1124/jpet.108.146365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Penuela S, Bhalla R, Gong XQ, Cowan KN, Celetti SJ, Cowan BJ, Bai D, Shao Q, Laird DW. Pannexin 1 and pannexin 3 are glycoproteins that exhibit many distinct characteristics from the connexin family of gap junction proteins. J Cell Sci. 2007;120:3772–3783. doi: 10.1242/jcs.009514. [DOI] [PubMed] [Google Scholar]
- 8.Li S, Tomic M, Stojilkovic SS. Characterization of novel Pannexin 1 isoforms from rat pituitary cells and their association with ATP-gated P2X channels. Gen Comp Endocrinol. 2011;174:202–210. doi: 10.1016/j.ygcen.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turmel P, Dufresne J, Hermo L, Smith CE, Penuela S, Laird DW, Cyr DG. Characterization of pannexin1 and pannexin3 and their regulation by androgens in the male reproductive tract of the adult rat. Mol Reprod Dev. 2011;78:124–138. doi: 10.1002/mrd.21280. [DOI] [PubMed] [Google Scholar]
- 10.Furlow PW, Zhang S, Soong TD, Halberg N, Goodarzi H, Mangrum C, Wu YG, Elemento O, Tavazoie SF. Mechanosensitive pannexin-1 channels mediate microvascular metastatic cell survival. Nat Cell Biol. 2015;17:943–952. doi: 10.1038/ncb3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dahl G, Keane RW. Pannexin: from discovery to bedside in 11+/−4 years? Brain Res. 2012;1487:150–159. doi: 10.1016/j.brainres.2012.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris AL. Connexin channel permeability to cytoplasmic molecules. Prog Biophys Mol Biol. 2007;94:120–143. doi: 10.1016/j.pbiomolbio.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodenough DA, Paul DL. Beyond the gap: functions of unpaired connexon channels. Nat Rev Mol Cell Biol. 2003;4:285–294. doi: 10.1038/nrm1072. [DOI] [PubMed] [Google Scholar]
- 14.Boassa D, Ambrosi C, Qiu F, Dahl G, Gaietta G, Sosinsky G. Pannexin1 channels contain a glycosylation site that targets the hexamer to the plasma membrane. J Biol Chem. 2007;282:31733–31743. doi: 10.1074/jbc.M702422200. [DOI] [PubMed] [Google Scholar]
- 15.Ambrosi C, Gassmann O, Pranskevich JN, Boassa D, Smock A, Wang J, Dahl G, Steinem C, Sosinsky GE. Pannexin1 and Pannexin2 channels show quaternary similarities to connexons and different oligomerization numbers from each other. J Biol Chem. 2010;285:24420–24431. doi: 10.1074/jbc.M110.115444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beckmann A, Grissmer A, Krause E, Tschernig T, Meier C. Pannexin-1 channels show distinct morphology and no gap junction characteristics in mammalian cells. Cell Tissue Res. 2016;363:751–763. doi: 10.1007/s00441-015-2281-x. [DOI] [PubMed] [Google Scholar]
- 17.Huang Y, Grinspan JB, Abrams CK, Scherer SS. Pannexin1 is expressed by neurons and glia but does not form functional gap junctions. Glia. 2007;55:46–56. doi: 10.1002/glia.20435. [DOI] [PubMed] [Google Scholar]
- 18.Esseltine JL, Laird DW. Next-Generation Connexin and Pannexin Cell Biology. Trends Cell Biol. 2016;26:944–955. doi: 10.1016/j.tcb.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Penuela S, Lohman AW, Lai W, Gyenis L, Litchfield DW, Isakson BE, Laird DW. Diverse post-translational modifications of the pannexin family of channel-forming proteins. Channels (Austin) 2014;8:124–130. doi: 10.4161/chan.27422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Penuela S, Bhalla R, Nag K, Laird DW. Glycosylation regulates pannexin intermixing and cellular localization. Mol Biol Cell. 2009;20:4313–4323. doi: 10.1091/mbc.E09-01-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Decrock E, De Bock M, Wang N, Bultynck G, Giaume C, Naus CC, Green CR, Leybaert L. Connexin and pannexin signaling pathways, an architectural blueprint for CNS physiology and pathology? Cell Mol Life Sci. 2015;72:2823–2851. doi: 10.1007/s00018-015-1962-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris AL, Contreras JE. Motifs in the permeation pathway of connexin channels mediate voltage and Ca (2+) sensing. Front Physiol. 2014;5:113. doi: 10.3389/fphys.2014.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bruzzone R, Hormuzdi SG, Barbe MT, Herb A, Monyer H. Pannexins a family of gap junction proteins expressed in brain. Proc Natl Acad Sci U S A. 2003;100:13644–13649. doi: 10.1073/pnas.2233464100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silverman WR, de Rivero Vaccari JP, Locovei S, Qiu F, Carlsson SK, Scemes E, Keane RW, Dahl G. The pannexin 1 channel activates the inflammasome in neurons and astrocytes. J Biol Chem. 2009;284:18143–18151. doi: 10.1074/jbc.M109.004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Locovei S, Wang J, Dahl G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 2006;580:239–244. doi: 10.1016/j.febslet.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Bao L, Locovei S, Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 2004;572:65–68. doi: 10.1016/j.febslet.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 27.De Vuyst E, Wang N, Decrock E, De Bock M, Vinken M, Van Moorhem M, Lai C, Culot M, Rogiers V, Cecchelli R, Naus CC, Evans WH, Leybaert L. Ca(2+) regulation of connexin 43 hemichannels in C6 glioma and glial cells. Cell Calcium. 2009;46:176–187. doi: 10.1016/j.ceca.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 28.Bravo D, Maturana CJ, Pelissier T, Hernandez A, Constandil L. Interactions of pannexin 1 with NMDA and P2X7 receptors in central nervous system pathologies: Possible role on chronic pain. Pharmacol Res. 2015;101:86–93. doi: 10.1016/j.phrs.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 29.Godecke S, Roderigo C, Rose CR, Rauch BH, Godecke A, Schrader J. Thrombin-induced ATP release from human umbilical vein endothelial cells. Am J Physiol Cell Physiol. 2012;302:C915–923. doi: 10.1152/ajpcell.00283.2010. [DOI] [PubMed] [Google Scholar]
- 30.Seminario-Vidal L, Kreda S, Jones L, O’Neal W, Trejo J, Boucher RC, Lazarowski ER. Thrombin promotes release of ATP from lung epithelial cells through coordinated activation of rho- and Ca2+-dependent signaling pathways. J Biol Chem. 2009;284:20638–20648. doi: 10.1074/jbc.M109.004762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bennett MV, Garre JM, Orellana JA, Bukauskas FF, Nedergaard M, Saez JC. Connexin and pannexin hemichannels in inflammatory responses of glia and neurons. Brain Res. 2012;1487:3–15. doi: 10.1016/j.brainres.2012.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garre JM, Yang G, Bukauskas FF, Bennett MV. FGF-1 Triggers Pannexin-1 Hemichannel Opening in Spinal Astrocytes of Rodents and Promotes Inflammatory Responses in Acute Spinal Cord Slices. J Neurosci. 2016;36:4785–4801. doi: 10.1523/JNEUROSCI.4195-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adamson SE, Meher AK, Chiu YH, Sandilos JK, Oberholtzer NP, Walker NN, Hargett SR, Seaman SA, Peirce-Cottler SM, Isakson BE, McNamara CA, Keller SR, Harris TE, Bayliss DA, Leitinger N. Pannexin 1 is required for full activation of insulin-stimulated glucose uptake in adipocytes. Mol Metab. 2015;4:610–618. doi: 10.1016/j.molmet.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chekeni FB, Elliott MR, Sandilos JK, Walk SF, Kinchen JM, Lazarowski ER, Armstrong AJ, Penuela S, Laird DW, Salvesen GS, Isakson BE, Bayliss DA, Ravichandran KS. Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature. 2010;467:863–867. doi: 10.1038/nature09413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sandilos JK, Chiu YH, Chekeni FB, Armstrong AJ, Walk SF, Ravichandran KS, Bayliss DA. Pannexin 1, an ATP release channel, is activated by caspase cleavage of its pore-associated C-terminal autoinhibitory region. J Biol Chem. 2012;287:11303–11311. doi: 10.1074/jbc.M111.323378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Engelhardt K, Schmidt M, Tenbusch M, Dermietzel R. Effects on channel properties and induction of cell death induced by c-terminal truncations of pannexin1 depend on domain length. J Membr Biol. 2015;248:285–294. doi: 10.1007/s00232-014-9767-4. [DOI] [PubMed] [Google Scholar]
- 37.Thompson RJ, Zhou N, MacVicar BA. Ischemia opens neuronal gap junction hemichannels. Science. 2006;312:924–927. doi: 10.1126/science.1126241. [DOI] [PubMed] [Google Scholar]
- 38.Lohman AW, Leskov IL, Butcher JT, Johnstone SR, Stokes TA, Begandt D, DeLalio LJ, Best AK, Penuela S, Leitinger N, Ravichandran KS, Stokes KY, Isakson BE. Pannexin 1 channels regulate leukocyte emigration through the venous endothelium during acute inflammation. Nat Commun. 2015;6:7965. doi: 10.1038/ncomms8965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Orellana JA, Montero TD, von Bernhardi R. Astrocytes inhibit nitric oxide-dependent Ca(2+) dynamics in activated microglia: involvement of ATP released via pannexin 1 channels. Glia. 2013;61:2023–2037. doi: 10.1002/glia.22573. [DOI] [PubMed] [Google Scholar]
- 40.Orellana JA, Velasquez S, Williams DW, Saez JC, Berman JW, Eugenin EA. Pannexin1 hemichannels are critical for HIV infection of human primary CD4+ T lymphocytes. J Leukoc Biol. 2013;94:399–407. doi: 10.1189/jlb.0512249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiao F, Waldrop SL, Bronk SF, Gores GJ, Davis LS, Kilic G. Lipoapoptosis induced by saturated free fatty acids stimulates monocyte migration: a novel role for Pannexin1 in liver cells. Purinergic Signal. 2015;11:347–359. doi: 10.1007/s11302-015-9456-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang J, Ma M, Locovei S, Keane RW, Dahl G. Modulation of membrane channel currents by gap junction protein mimetic peptides: size matters. Am J Physiol Cell Physiol. 2007;293:C1112–1119. doi: 10.1152/ajpcell.00097.2007. [DOI] [PubMed] [Google Scholar]
- 43.Cotrina ML, Lin JH, Alves-Rodrigues A, Liu S, Li J, Azmi-Ghadimi H, Kang J, Naus CC, Nedergaard M. Connexins regulate calcium signaling by controlling ATP release. Proc Natl Acad Sci U S A. 1998;95:15735–15740. doi: 10.1073/pnas.95.26.15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jorgensen NR, Geist ST, Civitelli R, Steinberg TH. ATP- and gap junction-dependent intercellular calcium signaling in osteoblastic cells. J Cell Biol. 1997;139:497–506. doi: 10.1083/jcb.139.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goldberg GS, Lampe PD, Nicholson BJ. Selective transfer of endogenous metabolites through gap junctions composed of different connexins. Nat Cell Biol. 1999;1:457–459. doi: 10.1038/15693. [DOI] [PubMed] [Google Scholar]
- 46.Hernandez VH, Bortolozzi M, Pertegato V, Beltramello M, Giarin M, Zaccolo M, Pantano S, Mammano F. Unitary permeability of gap junction channels to second messengers measured by FRET microscopy. Nat Methods. 2007;4:353–358. doi: 10.1038/nmeth1031. [DOI] [PubMed] [Google Scholar]
- 47.Niessen H, Harz H, Bedner P, Kramer K, Willecke K. Selective permeability of different connexin channels to the second messenger inositol 1,4,5-trisphosphate. J Cell Sci. 2000;113(Pt 8):1365–1372. doi: 10.1242/jcs.113.8.1365. [DOI] [PubMed] [Google Scholar]
- 48.Weissman TA, Riquelme PA, Ivic L, Flint AC, Kriegstein AR. Calcium waves propagate through radial glial cells and modulate proliferation in the developing neocortex. Neuron. 2004;43:647–661. doi: 10.1016/j.neuron.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 49.Ma W, Compan V, Zheng W, Martin E, North RA, Verkhratsky A, Surprenant A. Pannexin 1 forms an anion-selective channel. Pflugers Arch. 2012;463:585–592. doi: 10.1007/s00424-012-1077-z. [DOI] [PubMed] [Google Scholar]
- 50.Romanov RA, Bystrova MF, Rogachevskaya OA, Sadovnikov VB, Shestopalov VI, Kolesnikov SS. The ATP permeability of pannexin 1 channels in a heterologous system and in mammalian taste cells is dispensable. J Cell Sci. 2012;125:5514–5523. doi: 10.1242/jcs.111062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Romanov RA, Rogachevskaia OA, Kolesnikova AS, Khokhlov AA, Kolesnikov SS. Permeability of pannexin 1 channels to large anions. Ross Fiziol Zh Im I M Sechenova. 2012;98:1578–1586. [PubMed] [Google Scholar]
- 52.Hansen DB, Ye ZC, Calloe K, Braunstein TH, Hofgaard JP, Ransom BR, Nielsen MS, MacAulay N. Activation, permeability, and inhibition of astrocytic and neuronal large pore (hemi)channels. J Biol Chem. 2014;289:26058–26073. doi: 10.1074/jbc.M114.582155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hansen DB, Braunstein TH, Nielsen MS, MacAulay N. Distinct permeation profiles of the connexin 30 and 43 hemichannels. FEBS Lett. 2014;588:1446–1457. doi: 10.1016/j.febslet.2014.01.036. [DOI] [PubMed] [Google Scholar]
- 54.Bruzzone R, Barbe MT, Jakob NJ, Monyer H. Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes. J Neurochem. 2005;92:1033–1043. doi: 10.1111/j.1471-4159.2004.02947.x. [DOI] [PubMed] [Google Scholar]
- 55.Dahl G, Qiu F, Wang J. The bizarre pharmacology of the ATP release channel pannexin1. Neuropharmacology. 2013;75:583–593. doi: 10.1016/j.neuropharm.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hanstein R, Negoro H, Patel NK, Charollais A, Meda P, Spray DC, Suadicani SO, Scemes E. Promises and pitfalls of a Pannexin1 transgenic mouse line. Front Pharmacol. 2013;4:61. doi: 10.3389/fphar.2013.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kelly JJ, Simek J, Laird DW. Mechanisms linking connexin mutations to human diseases. Cell Tissue Res. 2015;360:701–721. doi: 10.1007/s00441-014-2024-4. [DOI] [PubMed] [Google Scholar]
- 58.Laird DW. The gap junction proteome and its relationship to disease. Trends Cell Biol. 2010;20:92–101. doi: 10.1016/j.tcb.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 59.Barbe MT, Monyer H, Bruzzone R. Cell-cell communication beyond connexins: the pannexin channels. Physiology (Bethesda) 2006;21:103–114. doi: 10.1152/physiol.00048.2005. [DOI] [PubMed] [Google Scholar]
- 60.Dubyak GR. Both sides now: multiple interactions of ATP with pannexin-1 hemichannels. Focus on “A permeant regulating its permeation pore: inhibition of pannexin 1 channels by ATP”. Am J Physiol Cell Physiol. 2009;296:C235–241. doi: 10.1152/ajpcell.00639.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.MacVicar BA, Thompson RJ. Non-junction functions of pannexin-1 channels. Trends Neurosci. 2010;33:93–102. doi: 10.1016/j.tins.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 62.Isakson BE, Thompson RJ. Pannexin-1 as a potentiator of ligand-gated receptor signaling. Channels (Austin) 2014;8:118–123. doi: 10.4161/chan.27978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Burnstock G, Fredholm BB, North RA, Verkhratsky A. The birth and postnatal development of purinergic signalling. Acta Physiol (Oxf) 2010;199:93–147. doi: 10.1111/j.1748-1716.2010.02114.x. [DOI] [PubMed] [Google Scholar]
- 64.Burnstock G. Purinergic nerves. Pharmacol Rev. 1972;24:509–581. [PubMed] [Google Scholar]
- 65.Webb TE, Simon J, Krishek BJ, Bateson AN, Smart TG, King BF, Burnstock G, Barnard EA. Cloning and functional expression of a brain G-protein-coupled ATP receptor. FEBS Lett. 1993;324:219–225. doi: 10.1016/0014-5793(93)81397-i. [DOI] [PubMed] [Google Scholar]
- 66.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 67.Fischer W, Krugel U. P2Y receptors: focus on structural, pharmacological and functional aspects in the brain. Curr Med Chem. 2007;14:2429–2455. doi: 10.2174/092986707782023695. [DOI] [PubMed] [Google Scholar]
- 68.Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim Biophys Acta. 2008;1783:673–694. doi: 10.1016/j.bbamcr.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 69.Baqi Y. Ecto-nucleotidase inhibitors: recent developments in drug discovery. Mini Rev Med Chem. 2015;15:21–33. doi: 10.2174/1389557515666150219115141. [DOI] [PubMed] [Google Scholar]
- 70.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 71.Burnstock G. Purinergic signalling: from discovery to current developments. Exp Physiol. 2014;99:16–34. doi: 10.1113/expphysiol.2013.071951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. Embo J. 2006;25:5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Locovei S, Scemes E, Qiu F, Spray DC, Dahl G. Pannexin1 is part of the pore forming unit of the P2X(7) receptor death complex. FEBS Lett. 2007;581:483–488. doi: 10.1016/j.febslet.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Iglesias R, Locovei S, Roque A, Alberto AP, Dahl G, Spray DC, Scemes E. P2X7 receptor-Pannexin1 complex: pharmacology and signaling. Am J Physiol Cell Physiol. 2008;295:C752–760. doi: 10.1152/ajpcell.00228.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim JE, Kang TC. The P2X7 receptor-pannexin-1 complex decreases muscarinic acetylcholine receptor-mediated seizure susceptibility in mice. J Clin Invest. 2011;121:2037–2047. doi: 10.1172/JCI44818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gulbransen BD, Bashashati M, Hirota SA, Gui X, Roberts JA, MacDonald JA, Muruve DA, McKay DM, Beck PL, Mawe GM, Thompson RJ, Sharkey KA. Activation of neuronal P2X7 receptor-pannexin-1 mediates death of enteric neurons during colitis. Nat Med. 2012;18:600–604. doi: 10.1038/nm.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Scemes E, Suadicani SO, Dahl G, Spray DC. Connexin and pannexin mediated cell-cell communication. Neuron Glia Biol. 2007;3:199–208. doi: 10.1017/S1740925X08000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pelegrin P. Targeting Interleukin-1 Signaling in Chronic Inflammation: Focus on P2X(7) Receptor and Pannexin-1. Drug News Perspect. 2008;21:424–433. doi: 10.1358/dnp.2008.21.8.1265800. [DOI] [PubMed] [Google Scholar]
- 79.Wicki-Stordeur LE, Dzugalo AD, Swansburg RM, Suits JM, Swayne LA. Pannexin 1 regulates postnatal neural stem and progenitor cell proliferation. Neural Dev. 2012;7:11. doi: 10.1186/1749-8104-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li S, Bjelobaba I, Yan Z, Kucka M, Tomic M, Stojilkovic SS. Expression and roles of pannexins in ATP release in the pituitary gland. Endocrinology. 2011;152:2342–2352. doi: 10.1210/en.2010-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Poornima V, Madhupriya M, Kootar S, Sujatha G, Kumar A, Bera AK. P2X7 receptor-pannexin 1 hemichannel association: effect of extracellular calcium on membrane permeabilization. J Mol Neurosci. 2012;46:585–594. doi: 10.1007/s12031-011-9646-8. [DOI] [PubMed] [Google Scholar]
- 82.Hung SC, Choi CH, Said-Sadier N, Johnson L, Atanasova KR, Sellami H, Yilmaz O, Ojcius DM. P2X4 assembles with P2X7 and pannexin-1 in gingival epithelial cells and modulates ATP-induced reactive oxygen species production and inflammasome activation. PLoS One. 2013;8:e70210. doi: 10.1371/journal.pone.0070210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pan HC, Chou YC, Sun SH. P2X7 R-mediated Ca(2+) -independent d-serine release via pannexin-1 of the P2X7 R-pannexin-1 complex in astrocytes. Glia. 2015;63:877–893. doi: 10.1002/glia.22790. [DOI] [PubMed] [Google Scholar]
- 84.Kanjanamekanant K, Luckprom P, Pavasant P. Mechanical stress-induced interleukin-1beta expression through adenosine triphosphate/P2X7 receptor activation in human periodontal ligament cells. J Periodontal Res. 2013;48:169–176. doi: 10.1111/j.1600-0765.2012.01517.x. [DOI] [PubMed] [Google Scholar]
- 85.Woehrle T, Yip L, Elkhal A, Sumi Y, Chen Y, Yao Y, Insel PA, Junger WG. Pannexin-1 hemichannel-mediated ATP release together with P2X1 and P2X4 receptors regulate T-cell activation at the immune synapse. Blood. 2010;116:3475–3484. doi: 10.1182/blood-2010-04-277707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pankratov Y, Lalo U, Verkhratsky A, North RA. Vesicular release of ATP at central synapses. Pflugers Arch. 2006;452:589–597. doi: 10.1007/s00424-006-0061-x. [DOI] [PubMed] [Google Scholar]
- 87.Browne LE, Jiang LH, North RA. New structure enlivens interest in P2X receptors. Trends Pharmacol Sci. 2010;31:229–237. doi: 10.1016/j.tips.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Burnstock G. Purinergic signalling. Br J Pharmacol. 2006;147(Suppl 1):S172–181. doi: 10.1038/sj.bjp.0706429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dahl G. ATP release through pannexon channels. Philos Trans R Soc Lond B Biol Sci. 2015;370 doi: 10.1098/rstb.2014.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Eugenin EA. Role of connexin/pannexin containing channels in infectious diseases. FEBS Lett. 2014;588:1389–1395. doi: 10.1016/j.febslet.2014.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Weickert S, Ray A, Zoidl G, Dermietzel R. Expression of neural connexins and pannexin1 in the hippocampus and inferior olive: a quantitative approach. Brain Res Mol Brain Res. 2005;133:102–109. doi: 10.1016/j.molbrainres.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 92.Ray A, Zoidl G, Weickert S, Wahle P, Dermietzel R. Site-specific and developmental expression of pannexin1 in the mouse nervous system. Eur J Neurosci. 2005;21:3277–3290. doi: 10.1111/j.1460-9568.2005.04139.x. [DOI] [PubMed] [Google Scholar]
- 93.Velasquez S, Eugenin EA. Role of Pannexin-1 hemichannels and purinergic receptors in the pathogenesis of human diseases. Front Physiol. 2014;5:96. doi: 10.3389/fphys.2014.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Iglesias R, Dahl G, Qiu F, Spray DC, Scemes E. Pannexin 1: the molecular substrate of astrocyte “hemichannels”. J Neurosci. 2009;29:7092–7097. doi: 10.1523/JNEUROSCI.6062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Beckel JM, Argall AJ, Lim JC, Xia J, Lu W, Coffey EE, Macarak EJ, Shahidullah M, Delamere NA, Zode GS, Sheffield VC, Shestopalov VI, Laties AM, Mitchell CH. Mechanosensitive release of adenosine 5′-triphosphate through pannexin channels and mechanosensitive upregulation of pannexin channels in optic nerve head astrocytes: a mechanism for purinergic involvement in chronic strain. Glia. 2014;62:1486–1501. doi: 10.1002/glia.22695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen J, Zhu Y, Liang C, Chen J, Zhao HB. Pannexin1 channels dominate ATP release in the cochlea ensuring endocochlear potential and auditory receptor potential generation and hearing. Sci Rep. 2015;5:10762. doi: 10.1038/srep10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cisneros-Mejorado A, Gottlieb M, Cavaliere F, Magnus T, Koch-Nolte F, Scemes E, Perez-Samartin A, Matute C. Blockade of P2X7 receptors or pannexin-1 channels similarly attenuates postischemic damage. J Cereb Blood Flow Metab. 2015;35:843–850. doi: 10.1038/jcbfm.2014.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Santiago MF, Veliskova J, Patel NK, Lutz SE, Caille D, Charollais A, Meda P, Scemes E. Targeting pannexin1 improves seizure outcome. PLoS One. 2011;6:e25178. doi: 10.1371/journal.pone.0025178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lai CP, Bechberger JF, Thompson RJ, MacVicar BA, Bruzzone R, Naus CC. Tumor-suppressive effects of pannexin 1 in C6 glioma cells. Cancer Res. 2007;67:1545–1554. doi: 10.1158/0008-5472.CAN-06-1396. [DOI] [PubMed] [Google Scholar]
- 100.Wang X, Arcuino G, Takano T, Lin J, Peng WG, Wan P, Li P, Xu Q, Liu QS, Goldman SA, Nedergaard M. P2X7 receptor inhibition improves recovery after spinal cord injury. Nat Med. 2004;10:821–827. doi: 10.1038/nm1082. [DOI] [PubMed] [Google Scholar]
- 101.Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 102.Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc Natl Acad Sci U S A. 2007;104:6436–6441. doi: 10.1073/pnas.0611280104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Buvinic S, Almarza G, Bustamante M, Casas M, Lopez J, Riquelme M, Saez JC, Huidobro-Toro JP, Jaimovich E. ATP released by electrical stimuli elicits calcium transients and gene expression in skeletal muscle. J Biol Chem. 2009;284:34490–34505. doi: 10.1074/jbc.M109.057315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Beckel JM, Daugherty SL, Tyagi P, Wolf-Johnston AS, Birder LA, Mitchell CH, de Groat WC. Pannexin 1 channels mediate the release of ATP into the lumen of the rat urinary bladder. J Physiol. 2015;593:1857–1871. doi: 10.1113/jphysiol.2014.283119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hanner F, Lam L, Nguyen MT, Yu A, Peti-Peterdi J. Intrarenal localization of the plasma membrane ATP channel pannexin1. Am J Physiol Renal Physiol. 2012;303:F1454–1459. doi: 10.1152/ajprenal.00206.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ransford GA, Fregien N, Qiu F, Dahl G, Conner GE, Salathe M. Pannexin 1 contributes to ATP release in airway epithelia. Am J Respir Cell Mol Biol. 2009;41:525–534. doi: 10.1165/rcmb.2008-0367OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Iwamoto T, Nakamura T, Doyle A, Ishikawa M, de Vega S, Fukumoto S, Yamada Y. Pannexin 3 regulates intracellular ATP/cAMP levels and promotes chondrocyte differentiation. J Biol Chem. 2010;285:18948–18958. doi: 10.1074/jbc.M110.127027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Locovei S, Bao L, Dahl G. Pannexin 1 in erythrocytes: function without a gap. Proc Natl Acad Sci U S A. 2006;103:7655–7659. doi: 10.1073/pnas.0601037103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yang D, He Y, Munoz-Planillo R, Liu Q, Nunez G. Caspase-11 Requires the Pannexin-1 Channel and the Purinergic P2X7 Pore to Mediate Pyroptosis and Endotoxic Shock. Immunity. 2015;43:923–932. doi: 10.1016/j.immuni.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schenk U, Westendorf AM, Radaelli E, Casati A, Ferro M, Fumagalli M, Verderio C, Buer J, Scanziani E, Grassi F. Purinergic control of T cell activation by ATP released through pannexin-1 hemichannels. Sci Signal. 2008;1:ra6. doi: 10.1126/scisignal.1160583. [DOI] [PubMed] [Google Scholar]
- 111.Woehrle T, Yip L, Manohar M, Sumi Y, Yao Y, Chen Y, Junger WG. Hypertonic stress regulates T cell function via pannexin-1 hemichannels and P2X receptors. J Leukoc Biol. 2010;88:1181–1189. doi: 10.1189/jlb.0410211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Maslieieva V, Thompson RJ. A critical role for pannexin-1 in activation of innate immune cells of the choroid plexus. Channels (Austin) 2014;8:131–141. doi: 10.4161/chan.27653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li S, Li X, Chen X, Geng X, Sun J. ATP release channel Pannexin1 is a novel immune response gene in Japanese flounder Paralichthys olivaceus. Fish Shellfish Immunol. 2014;40:164–173. doi: 10.1016/j.fsi.2014.06.034. [DOI] [PubMed] [Google Scholar]
- 114.Li S, Peng W, Chen X, Geng X, Zhan W, Sun J. Expression and role of gap junction protein connexin43 in immune challenge-induced extracellular ATP release in Japanese flounder (Paralichthys olivaceus) Fish Shellfish Immunol. 2016;55:348–357. doi: 10.1016/j.fsi.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 115.Qiu F, Dahl G. A permeant regulating its permeation pore: inhibition of pannexin 1 channels by ATP. Am J Physiol Cell Physiol. 2009;296:C250–255. doi: 10.1152/ajpcell.00433.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lohman AW, Weaver JL, Billaud M, Sandilos JK, Griffiths R, Straub AC, Penuela S, Leitinger N, Laird DW, Bayliss DA, Isakson BE. S-nitrosylation inhibits pannexin 1 channel function. J Biol Chem. 2012;287:39602–39612. doi: 10.1074/jbc.M112.397976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Boyce AK, Kim MS, Wicki-Stordeur LE, Swayne LA. ATP stimulates pannexin 1 internalization to endosomal compartments. Biochem J. 2015;470:319–330. doi: 10.1042/BJ20141551. [DOI] [PubMed] [Google Scholar]
- 118.Rokic MB, Stojilkovic SS. Two open states of P2X receptor channels. Front Cell Neurosci. 2013;7:215. doi: 10.3389/fncel.2013.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7) Science. 1996;272:735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- 120.Khakh BS, Bao XR, Labarca C, Lester HA. Neuronal P2X transmitter-gated cation channels change their ion selectivity in seconds. Nat Neurosci. 1999;2:322–330. doi: 10.1038/7233. [DOI] [PubMed] [Google Scholar]
- 121.Virginio C, MacKenzie A, Rassendren FA, North RA, Surprenant A. Pore dilation of neuronal P2X receptor channels. Nat Neurosci. 1999;2:315–321. doi: 10.1038/7225. [DOI] [PubMed] [Google Scholar]
- 122.Egan TM, Samways DS, Li Z. Biophysics of P2X receptors. Pflugers Arch. 2006;452:501–512. doi: 10.1007/s00424-006-0078-1. [DOI] [PubMed] [Google Scholar]
- 123.Thompson RJ, Jackson MF, Olah ME, Rungta RL, Hines DJ, Beazely MA, MacDonald JF, MacVicar BA. Activation of pannexin-1 hemichannels augments aberrant bursting in the hippocampus. Science. 2008;322:1555–1559. doi: 10.1126/science.1165209. [DOI] [PubMed] [Google Scholar]
- 124.Pelegrin P, Surprenant A. The P2X(7) receptor-pannexin connection to dye uptake and IL-1beta release. Purinergic Signal. 2009;5:129–137. doi: 10.1007/s11302-009-9141-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yan Z, Li S, Liang Z, Tomic M, Stojilkovic SS. The P2X7 receptor channel pore dilates under physiological ion conditions. J Gen Physiol. 2008;132:563–573. doi: 10.1085/jgp.200810059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chaumont S, Khakh BS. Patch-clamp coordinated spectroscopy shows P2X2 receptor permeability dynamics require cytosolic domain rearrangements but not Panx-1 channels. Proc Natl Acad Sci U S A. 2008;105:12063–12068. doi: 10.1073/pnas.0803008105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rigato C, Swinnen N, Buckinx R, Couillin I, Mangin JM, Rigo JM, Legendre P, Le Corronc H. Microglia proliferation is controlled by P2X7 receptors in a Pannexin-1-independent manner during early embryonic spinal cord invasion. J Neurosci. 2012;32:11559–11573. doi: 10.1523/JNEUROSCI.1042-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zemkova H, Khadra A, Rokic MB, Tvrdonova V, Sherman A, Stojilkovic SS. Allosteric regulation of the P2X4 receptor channel pore dilation. Pflugers Arch. 2015;467:713–726. doi: 10.1007/s00424-014-1546-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li M, Toombes GE, Silberberg SD, Swartz KJ. Physical basis of apparent pore dilation of ATP-activated P2X receptor channels. Nat Neurosci. 2015;18:1577–1583. doi: 10.1038/nn.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Alberto AV, Faria RX, Couto CG, Ferreira LG, Souza CA, Teixeira PC, Froes MM, Alves LA. Is pannexin the pore associated with the P2X7 receptor? Naunyn Schmiedebergs Arch Pharmacol. 2013;386:775–787. doi: 10.1007/s00210-013-0868-x. [DOI] [PubMed] [Google Scholar]
- 131.Coddou C, Yan Z, Obsil T, Huidobro-Toro JP, Stojilkovic SS. Activation and regulation of purinergic P2X receptor channels. Pharmacological Reviews. 2011;63:641–683. doi: 10.1124/pr.110.003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- 133.Kemp JA, McKernan RM. NMDA receptor pathways as drug targets. Nat Neurosci. 2002;5(Suppl):1039–1042. doi: 10.1038/nn936. [DOI] [PubMed] [Google Scholar]
- 134.Weilinger NL, Tang PL, Thompson RJ. Anoxia-induced NMDA receptor activation opens pannexin channels via Src family kinases. J Neurosci. 2012;32:12579–12588. doi: 10.1523/JNEUROSCI.1267-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Weilinger NL, Maslieieva V, Bialecki J, Sridharan SS, Tang PL, Thompson RJ. Ionotropic receptors and ion channels in ischemic neuronal death and dysfunction. Acta Pharmacol Sin. 2013;34:39–48. doi: 10.1038/aps.2012.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chen QX, Perkins KL, Choi DW, Wong RK. Secondary activation of a cation conductance is responsible for NMDA toxicity in acutely isolated hippocampal neurons. J Neurosci. 1997;17:4032–4036. doi: 10.1523/JNEUROSCI.17-11-04032.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Prochnow N. Relevance of gap junctions and large pore channels in traumatic brain injury. Front Physiol. 2014;5:31. doi: 10.3389/fphys.2014.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bravo D, Ibarra P, Retamal J, Pelissier T, Laurido C, Hernandez A, Constandil L. Pannexin 1: a novel participant in neuropathic pain signaling in the rat spinal cord. Pain. 2014;155:2108–2115. doi: 10.1016/j.pain.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 139.Xu Q, Cheong YK, Yang F, Tiwari V, Li J, Liu J, Raja SN, Li W, Guan Y. Intrathecal carbenoxolone inhibits neuropathic pain and spinal wide-dynamic range neuronal activity in rats after an L5 spinal nerve injury. Neurosci Lett. 2014;563:45–50. doi: 10.1016/j.neulet.2014.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang H, Cao Y, Chiang CY, Dostrovsky JO, Sessle BJ. The gap junction blocker carbenoxolone attenuates nociceptive behavior and medullary dorsal horn central sensitization induced by partial infraorbital nerve transection in rats. Pain. 2014;155:429–435. doi: 10.1016/j.pain.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang Y, Laumet G, Chen SR, Hittelman WN, Pan HL. Pannexin-1 Up-regulation in the Dorsal Root Ganglion Contributes to Neuropathic Pain Development. J Biol Chem. 2015;290:14647–14655. doi: 10.1074/jbc.M115.650218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Orellana JA, Froger N, Ezan P, Jiang JX, Bennett MV, Naus CC, Giaume C, Saez JC. ATP and glutamate released via astroglial connexin 43 hemichannels mediate neuronal death through activation of pannexin 1 hemichannels. J Neurochem. 2011;118:826–840. doi: 10.1111/j.1471-4159.2011.07210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Weilinger NL, Lohman AW, Rakai BD, Ma EM, Bialecki J, Maslieieva V, Rilea T, Bandet MV, Ikuta NT, Scott L, Colicos MA, Teskey GC, Winship IR, Thompson RJ. Metabotropic NMDA receptor signaling couples Src family kinases to pannexin-1 during excitotoxicity. Nat Neurosci. 2016;19:432–442. doi: 10.1038/nn.4236. [DOI] [PubMed] [Google Scholar]
- 144.Liu XJ, Gingrich JR, Vargas-Caballero M, Dong YN, Sengar A, Beggs S, Wang SH, Ding HK, Frankland PW, Salter MW. Treatment of inflammatory and neuropathic pain by uncoupling Src from the NMDA receptor complex. Nat Med. 2008;14:1325–1332. doi: 10.1038/nm.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Thompson RJ. Pannexin channels and ischaemia. J Physiol. 2015;593:3463–3470. doi: 10.1113/jphysiol.2014.282426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ciruela F, Casado V, Rodrigues RJ, Lujan R, Burgueno J, Canals M, Borycz J, Rebola N, Goldberg SR, Mallol J, Cortes A, Canela EI, Lopez-Gimenez JF, Milligan G, Lluis C, Cunha RA, Ferre S, Franco R. Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1-A2A receptor heteromers. J Neurosci. 2006;26:2080–2087. doi: 10.1523/JNEUROSCI.3574-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kawamura M, Jr, Ruskin DN, Masino SA. Metabolic autocrine regulation of neurons involves cooperation among pannexin hemichannels, adenosine receptors, and KATP channels. J Neurosci. 2010;30:3886–3895. doi: 10.1523/JNEUROSCI.0055-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Prochnow N, Abdulazim A, Kurtenbach S, Wildforster V, Dvoriantchikova G, Hanske J, Petrasch-Parwez E, Shestopalov VI, Dermietzel R, Manahan-Vaughan D, Zoidl G. Pannexin1 stabilizes synaptic plasticity and is needed for learning. PLoS One. 2012;7:e51767. doi: 10.1371/journal.pone.0051767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kovalzon VM, Moiseenko LS, Ambaryan AV, Kurtenbach S, Shestopalov VI, Panchin YV. Sleep-wakefulness cycle and behavior in pannexin1 knockout mice. Behav Brain Res. 2017;318:24–27. doi: 10.1016/j.bbr.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 150.Tomic M, Jobin RM, Vergara LA, Stojilkovic SS. Expression of purinergic receptor channels and their role in calcium signaling and hormone release in pituitary gonadotrophs. Integration of P2 channels in plasma membrane- and endoplasmic reticulum-derived calcium oscillations. J Biol Chem. 1996;271:21200–21208. doi: 10.1074/jbc.271.35.21200. [DOI] [PubMed] [Google Scholar]
- 151.He ML, Gonzalez-Iglesias AE, Tomic M, Stojilkovic SS. Release and extracellular metabolism of ATP by ecto-nucleotidase eNTPDase 1–3 in hypothalamic and pituitary cells. Purinergic Signal. 2005;1:135–144. doi: 10.1007/s11302-005-6208-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Stojilkovic SS. Purinergic regulation of hypothalamopituitary functions. Trends Endocrinol Metab. 2009;20:460–468. doi: 10.1016/j.tem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bjelobaba I, Janjic MM, Stojilkovic SS. Purinergic signaling pathways in endocrine system. Auton Neurosci. 2015;191:102–116. doi: 10.1016/j.autneu.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rees DA, Scanlon MF, Ham J. Adenosine signalling pathways in the pituitary gland: one ligand, multiple receptors. J Endocrinol. 2003;177:357–364. doi: 10.1677/joe.0.1770357. [DOI] [PubMed] [Google Scholar]