Abstract
Background
Patients with Sickle Cell Anemia (SCA) have an increased prevalence of nephropathy and mortality from chronic kidney disease (CKD).
Methods
We evaluated the association of hyperuricemia and nocturnal hypertension with lower estimated glomerular filtration rate (eGFR) by cystatin-C among patients 10-21 years old with HbSS or SB0 thalassemia during a non-acute clinic visit. eGFR was obtained in 83 participants, uric acid in 81, and 24 hour ambulatory blood pressure monitoring (ABPM) was performed in 44 participants. Vital signs, CBC, CMP, LDH, medications, and urine microalbumin/creatinine were measured. Hyperuricemia was defined as a uric acid level ≥5.5 mg/dL. Nocturnal hypertension was defined as >25 % of nocturnal readings >95th percentile according to norms established by the American Heart Association Statement on ABPM in children and adolescents.
Results
The mean eGFR was statistically significantly lower among patients with hyperuricemia than with normal uric acid levels (143 vs 161 mL/min/1.73m2, respectively). Fourteen of 44 (32 %) participants had systolic nocturnal hypertension and 12 of 44 (27 %) had diastolic nocturnal hypertension. The mean eGFR was statistically significantly lower among participants with nocturnal systolic and diastolic hypertension than normal nocturnal blood pressure. In a regression model, nocturnal hypertension and hyperuricemia were associated with a lower eGFR.
Conclusion
Two risk factors for CKD, nocturnal hypertension and hyperuricemia, were associated with lower eGFR in older children and adolescent patients with SCA, and warrant long-term studies of their association with progression to CKD in this population.
Keywords: Sickle Cell Disease, Nephropathy, Hypertension, Hyperuricemia, Kidney, Children
Introduction
Patients with Sickle Cell Anemia (SCA) can develop renal injury at an early age and progress to death from renal failure [1-3]. While approaches to estimate glomerular filtration rate (eGFR) have changed over time, data suggests that infants with SCA develop a hyperfiltration phase which plateaus during childhood [4-6]. During adolescence, eGFR begins to decline in some patients and around 10 % of adolescent patients with SCA develop a GFR <90 ml/min/1.73m2 [5, 7, 8]. Progression to chronic kidney disease (CKD) and renal failure in adults with SCA is devastating; 10 to 20 % of all deaths in SCA patients are associated with kidney disease and 30 % of SCA deaths from irreversible organ failure are complicated by renal failure [9-11]. Adult patients with HbSS develop renal failure at an earlier age (median 23.1 yrs) than adult patients with HbSC (median 49.9), the mean survival of SCA patients after developing end-stage renal disease (ESRD) is less than 3 years, and dialysis and renal transplant outcomes in patients with SCA are poor [2, 3, 12].
While it is clear that many young adult patients with SCA develop a decline in their eGFR and progress to CKD, current studies fail to identify consistent risk factors and interventions that modify disease progression [13, 14]. Nocturnal hypertension and hyperuricemia are well-established, independent risk factors for cardiovascular disease and progression to CKD in children without SCA [15-19]. Ambulatory blood pressure monitoring (ABPM), which identifies nocturnal hypertension, is superior to casual in-clinic blood pressure monitoring for identifying children at high risk for end-organ damage [20]. Two prior studies have identified a high prevalence of nocturnal hypertension in SCA, yet no data are available to define the association between nocturnal hypertension and eGFR in SCA [21, 22]. Hyperuricemia has been associated with hypertension and cardiovascular events in other diseases but, despite the high prevalence of hyperuricemia in SCA, the association of hyperuricemia and sickle cell nephropathy has not been explored [18, 19]. Hyperuricemia has been associated with leg ulcers and pulmonary hypertension in SCA [23-25].
With the knowledge that adolescent SCA patients begin their decline in eGFR and the independent strong associations of nocturnal hypertension and hyperuricemia with progression to CKD in other diseases, we investigated in adolescent SCA patients our hypothesis that nocturnal hypertension and hyperuricemia are associated with lower eGFR [16-18, 20]. In addition, we evaluated other clinical and laboratory variables that prior studies suggest may be associated with a lower eGFR [5, 8, 13, 14, 26-28].
Methods
The Institutional Review Board at the University of Alabama at Birmingham (UAB) approved this study of the evaluation of risk factors for the progression to sickle cell nephropathy and written consent and assent was obtained from every parent and child enrolled. In exploring the concept for a randomized controlled trial to correct abnormal nocturnal blood pressure in adolescent patients with SCA, this study was performed to determine the association between nocturnal hypertension, hyperuricemia, as well as other laboratory results, and clinical information on eGFR in patients ≥10 years. All patients with HbSS or SB0 thalassemia (age >5 yrs) who attend the Pediatric Sickle Cell Clinics at UAB/Children’s of Alabama were eligible to enroll in this cohort study but 24 hour ABPM is deferred until patients are ≥10 years. Participants were recruited during their routinely scheduled well-visits from either the hospital based Pediatric SCA clinic in Birmingham, AL or at a UAB Pediatric SCA Satellite clinic.
All participants were expected to complete institutional standard of care annual testing that includes: blood pressure obtained by a DINAMAP device in a sitting position, a complete blood count (CBC) with reticulocyte count, Metabolic Profile, Urine studies (microalbumin/creatinine), LDH. Participants in the cohort had uric acid performed as a study procedure. All blood and urine were obtained and processed at the clinical laboratory at the University of Alabama/ Children’s of Alabama or at the Satellite clinic laboratory during their first non-acute SCA clinic visit after consent/assent were signed. In-clinic systolic and diastolic hypertension was defined by blood pressure >95th percentile for age, height, and sex [29]. Glomerular filtration rate was estimated by serum cystatin C (eGFR) using BN ProSpec System (log GFR=1.962+ [1.123 × log (1/cystatin C)]. Microalbuminuria was defined as >30 mg/g Cr using a Siemens DCA Vantage Analyzer. Hyperuricemia was defined as a uric acid ≥5.5 mg/dL [30]. Uric acid, urine microalbumin and cystatin C were not performed at satellite SCA clinics. Of the 120 participants enrolled in the SCA Nephropathy cohort, 94 participants were ≥10 years old. eGFR was obtained in 83 participants, uric acid in 81, and 24 hour ABPM was performed in 44 of these participants ≥10 years old. In addition, chart review was performed to identify patient age, gender, vital signs, current SCA modifying therapy (no therapy, chronic transfusion, or hydroxyurea).
Adolescent participants (≥10 years of age) were eligible to complete 24 hour ABPM regardless of their current blood pressure in clinic. Participants who underwent ABPM had their monitor placed by the research team at the end of their non-acute SCA clinic visit. All ABPM procedures conformed to the 2014 American Heart Association guidelines for cuff placement, monitoring, and quality assurance in children and adolescents [20]. Participants completed the monitoring and a self-report diary that included start and end times of activities and sleeping. As no established norms on ABPM exist specifically for patients with SCA, we used the defined norms for 95th percentile blood pressure for awake, sleeping and 24 hour blood pressure norms based on age and gender percentile from the general population [20]. Nocturnal hypertension, as identified by blood pressure load, was defined as >25 % of nocturnal BP readings >95th percentile according to norms established by the American Heart Association Statement on ABPM in children and adolescents [20]. Percentage nocturnal dipping was defined as (mean awake minus mean sleeping BP) divided by mean awake BP. Attenuated nocturnal dipping was defined by <10 % to ≥0 % dip in mean BP while sleeping. Reverse dipping was defined by <0 % dip while sleeping (blood pressure increased while sleeping). Among the 94 participants, 47 had ABPM placed and 44 completed ABPM per AHA guidelines for quality assurance (3 participants removed the BP cuff at night). Patients received a 25 dollar payment upon completion of the ABPM.
Descriptive statistics were expressed as mean and standard deviation (SD). Variables were compared between patients with and without nocturnal hypertension, with and without hyperuricemia, and current SCA modifying therapy using t-test for normally distributed continuous variables, Wilcoxon for non-normally distributed continuous variables, and chi-square for discrete variables. Analysis of variance (ANOVA) was used to compare differences in variables across categories of normal, attenuated, and reverse dip of systolic blood pressure (SBP), as well as categorical variables of SCA modifying therapy. If ANOVA was statistically significant, Tukey’s honest significant difference test was performed to further evaluate differences. Box plots were generated to compare the distribution of eGFR by hyperuricemia, SCA modifying therapy, and nocturnal systolic hypertension. P-values <0.05 were considered statistically significant. The variables selected for regression modelling were based on biological plausibility for their association with eGFR. Stepwise forward linear regression was used to determine variables associated with eGFR using a p-value <0.1 was required to enter the model. All analyses were conducted using JMP10 (Cary, NC) and SAS9.4 (Cary, NC).
Results
The 94 participants evaluated in this study were between the ages of 10.0 and 21 years (mean 15.2 years, SD 3.0). Eighty six participants had HbSS and 8 had HbSB0 thalassemia. Fourteen participants (15 %) were not receiving a SCA modifying therapy, 37 (39 %) were on hydroxyurea, and 43 (46 %) were on transfusion therapy (24 on simple transfusion and 19 on exchange transfusions) (Supplemental Table I). The most prevalent reasons for treatment among the 80 participants receiving an SCA modifying therapy included 48 participants for central nervous system (CNS) disease, 21 for pain, and 8 for recurrent acute chest crisis (ACS). The mean eGFR was 158.2 mL/min/1.73m2 ± 30.2 for the cohort. The maximum eGFR was 256 mL/min/1.73m2 and the minimum eGFR was 82 mL/min/1.73m2.
Ambulatory Blood Pressure Monitoring
Among the 44 participants ≥10 years who completed 24 hour ABPM, 32 % had systolic nocturnal hypertension and 27 % had diastolic nocturnal hypertension (Table I). The only variables that were statistically different between the 44 participants (≥10 years) who completed ABPM and the 50 additional participants in the cohort (10-21 years) who did not complete ABPM were hemoglobin (8.6±1.4 vs 9.5±1.5 g/dL) and ANC (5618±2315 vs 4100±2512 × 106/L) (Supplemental Table II).
Table I.
Baseline characteristics of all patients and those who completed 24 hour Ambulatory Blood Pressure Monitoring
| Normal Nocturnal SBP n=30 |
Systolic Nocturnal Hypertension n=14 |
p-value | Normal Nocturnal SBP Dip (Dip >10%) n=13 |
Attenuated Nocturnal SBP Dip (Dip 0-10 %) n=21 |
Reverse Nocturnal SBP Dip (Dip <0 %) n=10 |
p-value all pairs | |
|---|---|---|---|---|---|---|---|
| Age (years) | 15.1 (2.8) | 14.5 (3.1) | 0.56 | 15.0 (2.8) | 14.6 (2.9) | 15.3 (3.3) | 0.8 |
| BMI | 20.0 (4.7) | 23.2 (5.5) | 0.05 | 20.8 (4.9) | 19.7 (5.1) | 24 (4.6) | 0.09 |
| WBC × 109/L | 11.6(3.4) | 11.8 (2.8) | 0.80 | 10.8 (3.1) | 12.5 (3.5) | 11.1 (2.4) | 0.3 |
| ANC × 109/L | 5.4 (2.4) | 6.1(2.1) | 0.32 | 4.9 (2.3) | 6.1 (2.3) | 5.6 (2.2) | 0.3 |
| Hemoglobin g/L | 82 (1.3) | 95 (1.3) | 0.004* | 85 (1.3) | 83 (1.3) | 95 (1.4) | 0.09 |
| Platelet × 109/L | 436 (174) | 356 (94) | 0.12 | 501 (207) | 384 (114) | 350 (116) | 0.03* |
| LDH (U/L) | 1440 (776) | 942(284) | 0.03* | 1321 (607) | 1415 (828) | 939 (320) | 0.2 |
| Uric Acid (mg/dL) | 5.1 (1.6) | 4.4 (1.5) | 0.27 | 4.5 (0.7) | 5.2 (1.9) | 4.6 (1.6) | 0.4 |
| eGFR mL/min/1.73m2 | 168 (30) | 140 (31) | 0.006* | 177 (33) | 160 (24) | 134 (31) | 0.003*# |
Albumin/Creatinine reported as median. All other values are reported as mean and (standard deviation)
p-value < 0.05 reported as significantly different.
eGFR: Reverse Nocturnal Dip is significantly different from Normal SBP Dip (p=0.002) and Attenuated SBP Nocturnal Dip using Tukey HSD.(p=0.05)
SPB= systolic blood pressure, BMI= body mass index, WBC= white blood cells, ANC= absolute neutrophil count, LDH= lactate dehydrogenase, Alb/Cr= Albumin creatinine, eGFR= estimated GFR by Cystatin C
Participants with systolic nocturnal hypertension had a lower eGFR (140±31 mL/min/1.73m2) than participants without systolic nocturnal hypertension (168±30 mL/min/1.73m2) (Table I). Higher hemoglobin and lower LDH were also associated with systolic nocturnal hypertension. In-clinic hypertension did not correlate with nocturnal hypertension; only three of the 14 (21 %) participants with systolic nocturnal hypertension and four of the 12 participants with diastolic nocturnal hypertension were also identified as having an abnormal in-clinic blood pressure. Furthermore, only two participants in our cohort had ambulatory daytime hypertension.
Attenuated nocturnal dipping and reverse nocturnal dipping were present in 31/44 participants (70 %) for SBP. Participants with reverse nocturnal SBP dipping had a lower eGFR (134±31 mL/min/1.73m2) than participants with attenuated nocturnal dipping (160±24 mL/min/1.73m2) and normal nocturnal dipping (177±33 mL/min/1.73m2). Lower platelet count (p=0.03) was also associated with abnormal systolic nocturnal dipping (Table I).
Hyperuricemia
The mean uric acid for 81 participants in this study was 4.9 (SD 1.5) mg/dL. Twenty four participants (30 %) had hyperuricemia. Participants with hyperuricemia had a statistically significantly lower eGFR (143±23 mL/min/1.73m2) than participants with a normal uric acid (161±31 mL/min/1.73m2, p=0.02) (Figure 1a). Among participants with normal nocturnal SBP, those with hyperuricemia (≥5.5 mg/dL) had a lower eGFR than participants with normal uric acid (143±29 vs 176±30 mL/min/1.73m2, p=0.02) (Figure 1c). No statistical difference was noted in eGFR among participants based on sickle cell disease modifying therapy overall or when stratified by nocturnal hypertension (Figures 1b, 1d).
Figure 1.
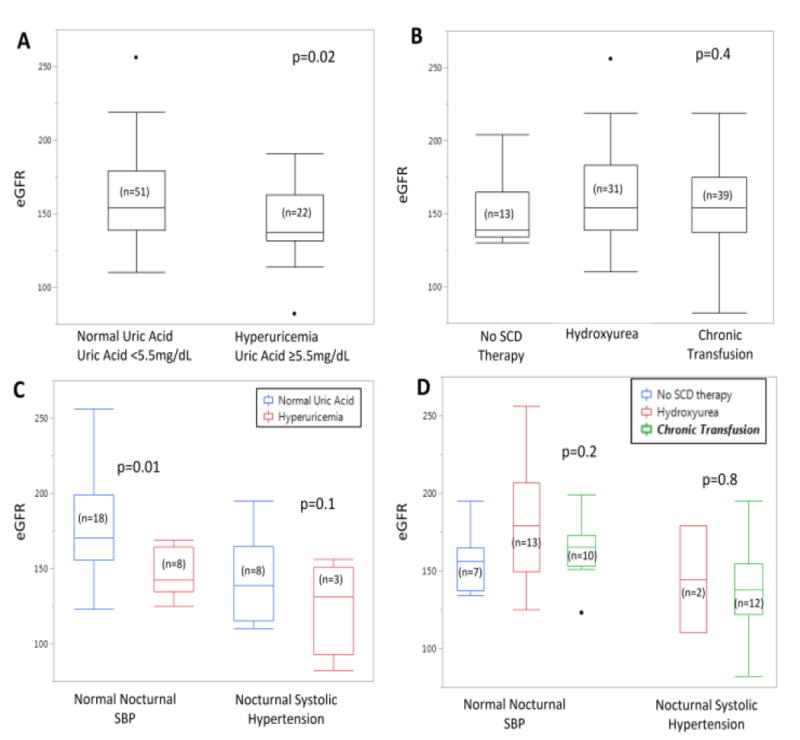
a: p-value comparison of eGFR by Uric Acid between groups
b: p-value ANOVA of eGFR across all 3 therapy group
c: p-value comparison of eGFR by hyperuricemia within nocturnal blood pressure group
d: p-value comparison of eGFR by therapy group within nocturnal blood pressure group
HTN= hypertension BP= Blood Pressure SCD= Sickle Cell Disease HU= Hydroxyurea
Microalbuminuria
We identified no statistical relationship between ABPM and microalbuminuria. Three of 14 (21 %) participants with systolic nocturnal hypertension had microalbuminuria, as well as 8 of 29 (28 %) patients without nocturnal systolic hypertension (p=0.7). Two of 10 (20 %) of patients with reverse systolic dipping also had microalbuminuria, three of 18 (14 %) patients with attenuated systolic dipping had microalbuminuria, and six of 12 patients with normal nocturnal dipping had microalbuminuria (p=0.08). For all participants ≥10 years, we also identified no statistical difference in prevalence of microalbuminuria by hyperuricemia; 6 of 22 (27 %) patients with hyperuricemia also had microalbuminuria and 12 of 55 (22 %) with normal uric acid had microalbuminuria (p=0.6).
Sickle Cell Disease Modifying Therapy
Participants on hydroxyurea had lower white blood cell count than participants on no disease modifying therapy or chronic transfusion (Supplemental Table I). Participants on chronic transfusion had higher hemoglobin and lower LDH than participants on hydroxyurea or no disease modifying therapy (Supplemental Table I). Uric acid levels were statistically lower among participants on chronic transfusion (4.4 mg/dL) as compared to hydroxyurea (5.3 mg/dL) or no therapy (5.6 mg/dL) (p=0.01) (Supplemental Table I). Clinic blood pressure was not significantly different based on SCA modifying therapy. However using ABPM, patients on chronic transfusion had higher nocturnal SBP and DBP, and lower SBP and DBP dip than patients on hydroxyurea or no therapy.
Regression modeling
We conducted linear regression modelling to evaluate risk factors for lower eGFR. Among all statistically significant laboratory and 24 ABPM variables analyzed in stepwise regression, only dichotomous variables of abnormal nocturnal blood pressure load (p=0.001) and hyperuricemia (p=0.006) were associated with lower eGFR (Table II). Elevation in white blood cell count was entered into the final model but did not reach statistical significance (p=0.09).
Table II.
Linear Regression for the outcome of estimated glomerular filtration rate (eGFR)
| Full Model R2 adjusted=0.28 |
Forward Stepwise Selection P=0.1 to enter model R2 adjusted=0.38 |
|||||
|---|---|---|---|---|---|---|
| Variables | Beta Coefficient Estimate | Standard Error | p-value | Beta Coefficient Estimate | Standard Error | p-value |
| Age | -1.52 | 2.15 | 0.5 | |||
| BMI | -1.34 | 1.33 | 0.3 | |||
| WBC | -1.66 | 2.63 | 0.6 | -2.34 | 1.36 | 0.09 |
| ANC | -0.001 | 0.003 | 0.6 | |||
| Hemoglobin | 2.09 | 5.21 | 0.7 | |||
| Platelet | -0.01 | 0.05 | 0.7 | |||
| LDH | -0.006 | 0.009 | 0.5 | |||
| Uric Acid | 9.97 | 6.26 | 0.15 | 13.48 | 4.76 | 0.008 |
| Nocturnal Systolic Hypertension | 21.1 | 8.06 | 0.02 | 17.62 | 4.77 | 0.001 |
| Nocturnal Diastolic Hypertension | -5.92 | 8.39 | 0.5 | |||
| Microalbuminuria | -6.2 | 7.30 | 0.5 | |||
| SCA Modifying Therapy (vs. Transfusion) | ||||||
| No SCA therapy | -18.20 | 10.66 | 0.11 | |||
| Hydroxyurea | 18.4 | 10.30 | 0.11 | |||
BMI= Body Mass Index, WBC= White Blood Cell Count, ANC= Absolute Neutrophil Count, LDH=Lactate Dehydrogenase, SBP= Systolic Blood Pressure, DBP= Diastolic Blood Pressure, SCA= Sickle Cell Anemia
Discussion
Patients with SCA are at high risk from morbidity and mortality associated with sickle cell nephropathy. Early identification of patients at risk for progressive disease and developing interventions to prevent progression is vital to enhance the longevity and quality of life of patients with sickle cell disease. As nocturnal hypertension and hyperuricemia are well-established risk factors for the development of CKD in non-SCA populations, this cohort was designed to analyze these risk factors in adolescent SCA patients, as this age group is the first to experience declining eGFR [5]. At cohort entry, SCA patients identified with nocturnal hypertension and hyperuricemia have a markedly lower eGFR. Of interest to patients and clinicians, both of these risk factors (hypertension and hyperuricemia) have FDA approved interventions that can be tested in an SCA clinical trial.
Identifying patients with hypertension based on clinic BP suggests risk for sickle cell nephropathy in adults [13, 31]. Clinic hypertension has also been identified as a risk factor in both overt and silent strokes [32-34]. While in-clinic BP is convenient and may portend clinical complications, the gold standard strategy for identifying risk for end-organ damage in other disease has been to perform 24 hour ABPM [20]. Our cohort confirms prior pediatric studies that demonstrate a lack of correlation between hypertension defined by in-clinic BP monitoring and 24 hour ABPM [21, 35]. In addition, our cohort mirrors the results of two prior studies which suggested that SCA patients have a higher prevalence of nocturnal hypertension and lack of nocturnal dipping in pediatric SCA [21, 22]. The prevalence of non-dipping in this SCA study (70 % abnormal SBP dipping and 50 % abnormal DBP dipping) is higher than obese pediatric patients (42 % and 17 % abnormal SBP and DBP dipping) or patients with diabetes (20-40 % abnormal dipping) [36-38]. Finally, our study suggests that nocturnal hypertension and abnormal nocturnal dipping have strong associations with a lower eGFR (33 mL/min/1.73m2 lower for patients with nocturnal hypertension) and may be, as well-established in several other disease states, a strong predictor of adverse renal and cardiovascular outcomes.
A second important finding in this data is the association of and potential causal role for hyperuricemia on progression to SCA nephropathy and hypertension. Hyperuricemia in SCA patients has been associated with leg ulcers and pulmonary hypertension [24, 25]. In non-SCA pediatric and adult populations, hyperuricemia has been associated with a decrease in eGFR and progression to CKD [17, 39-41]. Our data independently links hyperuricemia to eGFR and predicts that a uric acid level ≥5.5 mg/dL lowers eGFR by 28 mL/min/1.73m2. Animal models suggest a casual role of hyperuricemia on progression to CKD. Hyperuricemia in animals leads to an initial phase of hypertension which is reversible due to increased renal release and reduction in plasma nitric oxide; the second, irreversible phase is associated with vascular smooth muscle proliferation and inflammation, which leads to arteriosclerosis causing chronic hypoperfusion, tubulointerstitial inflammation and fibrosis [18]. As SCA patients have known abnormalities of their endothelial system, an increase in uric acid that exacerbates endothelial dysfunction could also contribute to progression to SCA nephropathy. In addition to its potential direct effect on CKD, uric acid has become a well-established risk factor for hypertension in children without SCA, and uric acid reduction can ameliorate elevations in blood pressure [18, 42]. If additional studies confirm that hyperuricemia contributes to hypertension and progression to CKD in SCA, early introduction of medications that reduce uric acid should be evaluated prospectively in future pediatric SCA clinical trials.
We did not demonstrate a protective role for hydroxyurea or blood transfusion in this cross-sectional study, but will continue to evaluate these participants prospectively. One potential concerning finding is that the group receiving monthly blood transfusion had a significantly higher percentage of patients with nocturnal hypertension and nocturnal dipping. As a convenience sample was used for the current study, participants had ABPM performed after their clinic visit which included either simple transfusion or red cell exchange transfusion. We could postulate that transfusion volume accounted for this hypertension. However, only one out of 21 patients (5 %) were identified with ambulatory daytime hypertension after their transfusion as compared to 12 patients (57 %) with nocturnal hypertension after transfusion and an additional 16 transfusion patients (76 %) having an increase in blood pressure while sleeping. An alternative concern for the transfusion patients would be the hypothesis that patients on chronic transfusion have suffered a more severe clinical course that includes kidney damage. Similar to patients on chronic transfusion for stroke who, despite strict HbS control, continue to develop CNS vasculopathy and new strokes, these patients may represent a subset of patients who may require novel therapies or SCA transplant to prevent continued organ damage [43, 44].
Some pediatric SCA studies have identified an association between in-clinic BP and microalbuminuria, but many other studies failed to replicate this association [5, 13, 14, 28]. We did not demonstrate an association of nocturnal hypertension or hyperuricemia with microalbuminuria. In a large non-SCA adult ABPM registry of almost 100,000 patients, nocturnal hypertension was associated with albuminuria [45]. As only 20 % of our pediatric cohort were identified with microalbuminuria at the time of their study visit, but as many as 70 % of adults develop microalbuminuria, we may still identify an association between nocturnal hypertension and progression to proteinuria or microalbuminuria over time [46]. In large studies of hyperuricemia, abnormal uric acid level has a greater impact on development of CKD, defined by eGFR, than albuminuria [17, 47].
This study has some limitations worth noting. First, not all participants completed 24 hour ABPM and the ABPM has not yet been repeated to confirm the reproducibility of nocturnal hypertension and nocturnal dipping. While the reproducibility of nocturnal hypertension is high in pediatric populations, nocturnal blood pressure dipping has lower reproducibility [48]. Second, our data identifies a lower eGFR in patients with abnormal nocturnal hypertension and abnormal uric acid, but those eGFR values may be within the normal range. While we are hypothesizing that similar to the strong evidence in non-SCA diseases that links nocturnal hypertension and abnormal uric acid to CKD, this has not yet been proven in SCA. Future studies are needed to determine if these risk factors for lower eGFR are also associated with continued progression to CKD. Third, we used cystatin C to estimate GFR, rather than nuclear medicine techniques. While we acknowledge that nuclear medicine techniques may be the current gold standard for directly measuring GFR (inulin and iothalamate), cystatin C does correlate with 99mTc-DTPA in SCD, although with a negative bias, and represents usual standard of care monitoring in our SCA clinic setting [5]. Additionally, the use of annual cystatin C in our five year cohort will provide an internally consistent estimate of GFR.
Among participants with SCA screened for 24 hour ABPM without regard to clinic hypertension, we identified a large percentage of children with nocturnal hypertension and abnormal nocturnal dipping. Our data has identified that nocturnal hypertension and abnormal uric acid are associated with a lower eGFR. These two risk factors have proven interventions and could be important to prevent the high morbidity and mortality associated with SCA nephropathy. We will continue to follow this cohort for five years to better understand a potential relationship between CKD and uric acid or nocturnal hypertension. In addition, we are performing a feasibility trial of losartan to reverse nocturnal hypertension in patients (NCT 02373241).
Supplementary Material
Key Points.
Nocturnal hypertension and hyperuricemia are established risk factors for nephropathy in other diseases and may play a role in SCA nephropathy.
Acknowledgments
The authors would like to acknowledge Children’s of Alabama/ Kaul Pediatric Research Institute for funding the initial participants in the cross sectional study and the NIH (K23HL127100-01) and American Society of Hematology Scholar award for funding the ongoing cohort. The authors would like to thank the participants living with sickle cell disease that are volunteering for this study. The authors would like to thank the additional members of the Pediatric Sickle Cell team (Lee Hilliard, MD, Christina Bemrich-Stolz, MD, MSPH, Kristen Osborn CRNP, Susan Dobbins, CRNP, Heather Carlton, CRNP, Michelle Alleman CRNP, Jeanine Dumas RN, MSN, and the SCA clinic nurses) for providing excellent care and assisting with obtaining labs. The authors would like to thank SCA research interns Kavita Tripathi, Hannah Ware, Rakesh Patel, and Prasannalaxmi, Palabindela for assisting in performing 24 hour ABPM.
Footnotes
Compliance with ethical standards
The Institutional Review Board at the University of Alabama at Birmingham (UAB) approved this study of the evaluation of risk factors for the progression to sickle cell nephropathy and written consent and assent was obtained from every parent and child enrolled.
Authorship Statement
JL wrote the first draft or the manuscript. All five authors participated in editing the drafts of this manuscript. PM, GC, and DF assisted JL in the design and analysis of the manuscript and serve as primary mentors for JL’s K23 award and TH serves as an advisory panel member.
Conflict of Interest Statement
Dr. Cutter discloses the following: Data and Safety Monitoring Boards: Apotek, Biogen-Idec, Cleveland Clinic (Vivus), Glaxo Smith Klein Pharmaceuticals, Gilead Pharmaceuticals, Modigenetech/Prolor, Merck/Ono Pharmaceuticals, Merck, Merck/Pfizer, Neuren, Sanofi-Aventis, Teva, , Washington University, NHLBI (Protocol Review Committee), NINDS, NICHD (OPRU oversight committee). Consulting or Advisory Boards: Consortium of MS Centers (grant), D3 (Drug Discovery and Development), Genzyme, Jannsen Pharmaceuticals, Klein-Buendel Incorporated, Medimmune, Novartis, Opexa Therapeutics, Receptos, Roche, EMD Serono, Teva pharmaceuticals, Transparency Life Sciences. Dr. Cutter is employed by the University of Alabama at Birmingham and President of Pythagoras, Inc. a private consulting company located in Birmingham AL.
References
- 1.McClellan AC, Luthi JC, Lynch JR, Soucie JM, Kulkarni R, Guasch A, Huff ED, Gilbertson D, McClellan WM, DeBaun MR. High one year mortality in adults with sickle cell disease and end-stage renal disease. Br J Haematol. 2012;159:360–367. doi: 10.1111/bjh.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nielsen L, Canoui-Poitrine F, Jais JP, Dahmane D, Bartolucci P, Bentaarit B, Gellen-Dautremer J, Remy P, Kofman T, Matignon M, Suberbielle C, Jacquelinet C, Wagner-Ballon O, Sahali D, Lang P, Damy T, Galacteros F, Grimbert P, Habibi A, Audard V. Morbidity and mortality of sickle cell disease patients starting intermittent haemodialysis: a comparative cohort study with non- Sickle dialysis patients. Br J Haematol. 2016;174:148–152. doi: 10.1111/bjh.14040. [DOI] [PubMed] [Google Scholar]
- 3.Powars DR, Elliott-Mills DD, Chan L, Niland J, Hiti AL, Opas LM, Johnson C. Chronic renal failure in sickle cell disease: risk factors, clinical course, and mortality. Ann Intern Med. 1991;115:614–620. doi: 10.7326/0003-4819-115-8-614. [DOI] [PubMed] [Google Scholar]
- 4.Alvarez O, Miller ST, Wang WC, Luo Z, McCarville MB, Schwartz GJ, Thompson B, Howard T, Iyer RV, Rana SR, Rogers ZR, Sarnaik SA, Thornburg CD, Ware RE BABY HUG Investigators. Effect of hydroxyurea treatment on renal function parameters: results from the multi-center placebo-controlled BABY HUG clinical trial for infants with sickle cell anemia. Pediatr Blood Cancer. 2012;59:668–674. doi: 10.1002/pbc.24100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aygun B, Mortier NA, Smeltzer MP, Hankins JS, Ware RE. Glomerular hyperfiltration and albuminuria in children with sickle cell anemia. Pediatr Nephrol. 2011;26:1285–1290. doi: 10.1007/s00467-011-1857-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lebensburger JD, Miller ST, Howard TH, Casella JF, Brown RC, Lu M, Iyer RV, Sarnaik S, Rogers ZR, Wang WC BABY HUG Investigators. Influence of severity of anemia on clinical findings in infants with sickle cell anemia: analyses from the BABY HUG study. Pediatr Blood Cancer. 2012;59:675–678. doi: 10.1002/pbc.24037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bodas P, Huang A, O’Riordan MA, Sedor JR, Dell KM. The prevalence of hypertension and abnormal kidney function in children with sickle cell disease -a cross sectional review. BMC Nephrol. 2013;14:237. doi: 10.1186/1471-2369-14-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McPherson Yee M, Jabbar SF, Osunkwo I, Clement L, Lane PA, Eckman JR, Guasch A. Chronic kidney disease and albuminuria in children with sickle cell disease. Clin J Am Soc Nephrol. 2011;6:2628–2633. doi: 10.2215/CJN.01600211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamideh D, Alvarez O. Sickle cell disease related mortality in the United States (1999-2009) Pediatr Blood Cancer. 2013;60:1482–1486. doi: 10.1002/pbc.24557. [DOI] [PubMed] [Google Scholar]
- 10.Platt OS, Brambilla DJ, Rosse WF, Milner PF, Castro O, Steinberg MH, Klug PP. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994;330:1639–1644. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 11.Powars DR, Chan LS, Hiti A, Ramicone E, Johnson C. Outcome of sickle cell anemia: a 4-decade observational study of 1056 patients. Medicine (Baltimore) 2005;84:363–376. doi: 10.1097/01.md.0000189089.45003.52. [DOI] [PubMed] [Google Scholar]
- 12.Ojo AO, Govaerts TC, Schmouder RL, Leichtman AB, Leavey SF, Wolfe RA, Held PJ, Port FK, Agodoa LY. Renal transplantation in end-stage sickle cell nephropathy. Transplantation. 1999;67:291–295. doi: 10.1097/00007890-199901270-00018. [DOI] [PubMed] [Google Scholar]
- 13.Gosmanova EO, Zaidi S, Wan JY, Adams-Graves PE. Prevalence and progression of chronic kidney disease in adult patients with sickle cell disease. J Investig Med. 2014;62:804–807. doi: 10.1097/01.JIM.0000446836.75352.72. [DOI] [PubMed] [Google Scholar]
- 14.Saraf SL, Zhang X, Kanias T, Lash JP, Molokie RE, Oza B, Lai C, Rowe JH, Gowhari M, Hassan J, Desimone J, Machado RF, Gladwin MT, Little JA, Gordeuk VR. Haemoglobinuria is associated with chronic kidney disease and its progression in patients with sickle cell anaemia. Br J Haematol. 2014;164:729–739. doi: 10.1111/bjh.12690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitsnefes M, Ho PL, McEnery PT. Hypertension and progression of chronic renal insufficiency in children: a report of the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS) J Am Soc Nephrol. 2003;14:2618–2622. doi: 10.1097/01.asn.0000089565.04535.4b. [DOI] [PubMed] [Google Scholar]
- 16.Mitsnefes MM, Kimball TR, Daniels SR. Office and ambulatory blood pressure elevation in children with chronic renal failure. Pediatr Nephrol. 2003;18:145–149. doi: 10.1007/s00467-002-1030-z. [DOI] [PubMed] [Google Scholar]
- 17.Rodenbach KE, Schneider MF, Furth SL, Moxey-Mims MM, Mitsnefes MM, Weaver DJ, Warady BA, Schwartz GJ. Hyperuricemia and Progression of CKD in Children and Adolescents: The Chronic Kidney Disease in Children (CKiD) Cohort Study. Am J Kidney Dis. 2015;66:984–992. doi: 10.1053/j.ajkd.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. The N Engl J Med. 2008;359:1811–1821. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feig DI. Serum uric acid and the risk of hypertension and chronic kidney disease. Curr Opin Rheumatol. 2014;26:176–185. doi: 10.1097/BOR.0000000000000033. [DOI] [PubMed] [Google Scholar]
- 20.Flynn JT, Daniels SR, Hayman LL, Maahs DM, McCrindle BW, Mitsnefes M, Zachariah JP, Urbina EM American Heart Association Atherosclerosis, Hypertension and Obesity in Youth Committee of the Council on Cardiovascular Disease in the Young. Update: ambulatory blood pressure monitoring in children and adolescents: a scientific statement from the American Heart Association. Hypertension. 2014;63:1116–1135. doi: 10.1161/HYP.0000000000000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Becker AM, Goldberg JH, Henson M, Ahn C, Tong L, Baum M, Buchanan GR. Blood pressure abnormalities in children with sickle cell anemia. Pediatr Blood Cancer. 2014;61:518–522. doi: 10.1002/pbc.24843. [DOI] [PubMed] [Google Scholar]
- 22.Shatat IF, Jakson SM, Blue AE, Johnson MA, Orak JK, Kalpatthi R. Masked hypertension is prevalent in children with sickle cell disease: a Midwest Pediatric Nephrology Consortium study. Pediatr Nephrol. 2013;28:115–120. doi: 10.1007/s00467-012-2275-9. [DOI] [PubMed] [Google Scholar]
- 23.Aloni MN, Ngiyulu RM, Gini-Ehungu JL, Nsibu CN, Ekila MB, Lepira FB, Nseka NM. Renal function in children suffering from sickle cell disease: challenge of early detection in highly resource-scarce settings. PLoS One. 2014;9:e96561. doi: 10.1371/journal.pone.0096561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Minniti CP, Taylor JGt, Hildesheim M, O’Neal P, Wilson J, Castro O, Gordeuk VR, Kato GJ. Laboratory and echocardiography markers in sickle cell patients with leg ulcers. Am J Hematol. 2011;86:705–708. doi: 10.1002/ajh.22065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joshi K, Anjum F, Gowda S, Damania D, Graham-Hill S, Gillette P, Zein J, Jamaleddine G, Demetis S, Wadgaonkar R. Uric Acid as a potential biomarker of pulmonary arterial hypertension in patients with sickle cell disease. Indian J Hematol Blood Transfus. 2011;27:96–100. doi: 10.1007/s12288-011-0072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King L, MooSang M, Miller M, Reid M. Prevalence and predictors of microalbuminuria in Jamaican children with sickle cell disease. Arch Dis Child. 2011;96:1135–1139. doi: 10.1136/archdischild-2011-300628. [DOI] [PubMed] [Google Scholar]
- 27.Aban I, Baddam S, Hilliard LM, Howard TH, Feig D, Lebensburger JD. Severe anemia early in life as a risk factor for sickle cell kidney disease. Blood. 2017;129:385–387. doi: 10.1182/blood-2016-09-738104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lebensburger J, Johnson SM, Askenazi DJ, Rozario NL, Howard TH, Hilliard LM. Protective role of hemoglobin and fetal hemoglobin in early kidney disease for children with sickle cell anemia. Am J Hematol. 2011;86:430–432. doi: 10.1002/ajh.21994. [DOI] [PubMed] [Google Scholar]
- 29.Pegelow CH, Colangelo L, Steinberg M, Wright EC, Smith J, Phillips G, Vichinsky E. Natural history of blood pressure in sickle cell disease: risks for stroke and death associated with relative hypertension in sickle cell anemia. Am J Med. 1997;102:171–177. doi: 10.1016/s0002-9343(96)00407-x. [DOI] [PubMed] [Google Scholar]
- 30.Feig DI, Johnson RJ. Hyperuricemia in childhood primary hypertension. Hypertension. 2003;42:247–252. doi: 10.1161/01.HYP.0000085858.66548.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gordeuk VR, Sachdev V, Taylor JG, Gladwin MT, Kato G, Castro OL. Relative systemic hypertension in patients with sickle cell disease is associated with risk of pulmonary hypertension and renal insufficiency. Am J Hematol. 2008;83:15–18. doi: 10.1002/ajh.21016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeBaun MR, Sarnaik SA, Rodeghier MJ, Minniti CP, Howard TH, Iyer RV, Inusa B, Telfer PT, Kirby-Allen M, Quinn CT, Bernaudin F, Airewele G, Woods GM, Panepinto JA, Fuh B, Kwiatkowski JK, King AA, Rhodes MM, Thompson AA, Heiny ME, Redding-Lallinger RC, Kirkham FJ, Sabio H, Gonzalez CE, Saccente SL, Kalinyak KA, Strouse JJ, Fixler JM, Gordon MO, Miller JP, Noetzel MJ, Ichord RN, Casella JF. Associated risk factors for silent cerebral infarcts in sickle cell anemia: low baseline hemoglobin, sex, and relative high systolic blood pressure. Blood. 2012;119:3684–3690. doi: 10.1182/blood-2011-05-349621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohene-Frempong K, Weiner SJ, Sleeper LA, Miller ST, Embury S, Moohr JW, Wethers DL, Pegelow CH, Gill FM. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 1998;91:288–294. [PubMed] [Google Scholar]
- 34.Lebensburger JD, Hilliard LM, McGrath TM, Fineberg NS, Howard TH. Laboratory and clinical correlates for magnetic resonance imaging (MRI) abnormalities in pediatric sickle cell anemia. J Child Neurol. 2011;26:1260–1264. doi: 10.1177/0883073811405054. [DOI] [PubMed] [Google Scholar]
- 35.Dionne JM, Turik MM, Hurley RM. Blood pressure abnormalities in children with chronic kidney disease. Blood Press Monit. 2008;13:205–209. doi: 10.1097/MBP.0b013e3283052fd0. [DOI] [PubMed] [Google Scholar]
- 36.Westerstahl M, Hedvall Kallerman P, Hagman E, Ek AE, Rossner SM, Marcus C. Nocturnal blood pressure non-dipping is prevalent in severely obese, prepubertal and early pubertal children. Acta Paediatr. 2014;103:225–230. doi: 10.1111/apa.12479. [DOI] [PubMed] [Google Scholar]
- 37.Lee SH, Kim JH, Kang MJ, Lee YA, Won Yang S, Shin CH. Implications of nocturnal hypertension in children and adolescents with type 1 diabetes. Diabetes Care. 2011;34:2180–2185. doi: 10.2337/dc11-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Atabek ME, Akyurek N, Eklioglu BS, Alp H. Impaired systolic blood dipping and nocturnal hypertension: an independent predictor of carotid intima-media thickness in type 1 diabetic patients. J Diabetes Complications. 2014;28:51–55. doi: 10.1016/j.jdiacomp.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 39.Goicoechea M, Garcia de Vinuesa S, Verdalles U, Verde E, Macias N, Santos A, Perez de Jose A, Cedeno S, Linares T, Luno J. Allopurinol and progression of CKD and cardiovascular events: long-term follow-up of a randomized clinical trial. Am J Kidney Dis. 2015;65:543–549. doi: 10.1053/j.ajkd.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 40.Zhu P, Liu Y, Han L, Xu G, Ran JM. Serum uric acid is associated with incident chronic kidney disease in middle-aged populations: a meta-analysis of 15 cohort studies. PLoS One. 2014;9:e100801. doi: 10.1371/journal.pone.0100801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodenbach KE, Schneider MF, Furth SL, Moxey-Mims MM, Mitsnefes MM, Weaver DJ, Warady BA, Schwartz GJ. Hyperuricemia and Progression of CKD in Children and Adolescents: The Chronic Kidney Disease in Children (CKiD) Cohort Study. Am J Kidney Dis. 2015;66:984–992. doi: 10.1053/j.ajkd.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA. 2008;300:924–932. doi: 10.1001/jama.300.8.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hulbert ML, McKinstry RC, Lacey JL, Moran CJ, Panepinto JA, Thompson AA, Sarnaik SA, Woods GM, Casella JF, Inusa B, Howard J, Kirkham FJ, Anie KA, Mullin JE, Ichord R, Noetzel M, Yan Y, Rodeghier M, Debaun MR. Silent cerebral infarcts occur despite regular blood transfusion therapy after first strokes in children with sickle cell disease. Blood. 2011;117:772–779. doi: 10.1182/blood-2010-01-261123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Griessenauer CJ, Lebensburger JD, Chua MH, Fisher WS, 3rd, Hilliard L, Bemrich-Stolz CJ, Howard TH, Johnston JM. Encephaloduroarteriosynangiosis and encephalomyoarteriosynangiosis for treatment of moyamoya syndrome in pediatric patients with sickle cell disease. J Neurosurg Pediatr. 2015;16:64–73. doi: 10.3171/2014.12.PEDS14522. [DOI] [PubMed] [Google Scholar]
- 45.de la Sierra A, Gorostidi M, Banegas JR, Segura J, de la Cruz JJ, Ruilope LM. Nocturnal hypertension or nondipping: which is better associated with the cardiovascular risk profile? Am J Hypertens. 2014;27:680–687. doi: 10.1093/ajh/hpt175. [DOI] [PubMed] [Google Scholar]
- 46.Guasch A, Navarrete J, Nass K, Zayas CF. Glomerular involvement in adults with sickle cell hemoglobinopathies: Prevalence and clinical correlates of progressive renal failure. J Am Soc Nephrol. 2006;17:2228–2235. doi: 10.1681/ASN.2002010084. [DOI] [PubMed] [Google Scholar]
- 47.Odden MC, Amadu AR, Smit E, Lo L, Peralta CA. Uric acid levels, kidney function, and cardiovascular mortality in US adults: National Health and Nutrition Examination Survey (NHANES) 1988-1994 and 1999-2002. Am J Kidney Dis. 2014;64:550–557. doi: 10.1053/j.ajkd.2014.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Acosta AA, McNiece KL. Ambulatory blood pressure monitoring: a versatile tool for evaluating and managing hypertension in children. Pediatr Nephrol. 2008;23:1399–1408. doi: 10.1007/s00467-008-0766-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


