Abstract
The hippocampus is well known as a central site for memory processing—critical for storing and later retrieving the experiences events of daily life so they can be used to shape future behavior. Much of what we know about the physiology underlying hippocampal function comes from spatial navigation studies in rodents, which have allowed great strides in understanding how the hippocampus represents experience at the cellular level. However, it remains a challenge to reconcile our knowledge of spatial encoding in the hippocampus with its demonstrated role in memory-dependent tasks in both humans and other animals. Moreover, our understanding of how networks of neurons coordinate their activity within and across hippocampal subregions to enable the encoding, consolidation, and retrieval of memories is incomplete. In this chapter, we explore how information may be represented at the cellular level and processed via coordinated patterns of activity throughout the subregions of the hippocampal network.
Keywords: hippocampus, learning, memory, oscillations, LFP, network activity, spatial coding, place cells, theta, gamma, sharp-wave ripples
1 Introduction
Decades of study have established the hippocampus as a critical center for memory processing in the brain. The hippocampus, along with several associated brain regions, processes the events of daily life and facilitates the long-term storage of these experiences. The link between the medial temporal lobes and memory was indicated first by Scoville and Milner in 1957 as a result of their evaluation of patient now known as H.M. At the age of 27, H.M. underwent bilateral medial temporal lobectomy in a medical effort to alleviate his intractable epilepsy. While the surgical procedure reduced his seizures, H.M. was also rendered unable to form new episodic memories (Scoville and Milner 1957). Subsequent studies of additional patients with more restricted medial temporal lobe lesions, as well as studies in nonhuman primates, identified the hippocampus and parahippocampal gyrus as the most critical regions for memory function (for review, see Squire and Wixted 2011).
While the hippocampus was becoming established as a memory formation center in primates, a seminal series of studies in rodents revealed that hippocampal neurons were remarkably well tuned to spatial location, suggesting a critical role in encoding space. The first evidence for this view of hippocampal function emerged in 1971, when O’Keefe and Dostrovsky reported that a subset of hippocampal neurons fired when rats occupied a specific location in an environment (O’Keefe and Dostrovsky 1971). These neurons became known as place cells, and were shown to be ubiquitous throughout the hippocampus (Muller et al. 1987; Jung and McNaughton 1993; O’Keefe 1976). Moreover, lesion studies of the rat hippocampus revealed a specific deficit in navigation-based memory tasks, further corroborating a role for the hippocampus in spatial processing (Mishkin 1978; Olton and Papas 1979; Morris et al. 1982). Based on these findings, O’Keefe and Nadel proposed that hippocampal neural activity constituted a cognitive map of space, in which individual place cells function to map out the animal’s location in reference to its spatial environment (O’Keefe and Nadel 1978).
Since this proposal, many studies have demonstrated that hippocampal neural activity can represent far more than simply spatial location, including aspects of contextual information, object recognition, and time (Eichenbaum et al. 1987; Young et al. 1994; Pastalkova et al. 2008; Hok et al. 2007; Moita et al. 2003; Manns and Eichenbaum 2009). For example, beyond providing a framework for linking locations together to form spatial trajectories, the hippocampus can associate multiple objects with a context (Komorowski et al. 2009), and further link a series of events in a temporally specific order to represent a complex experience (Allen et al. 2016). These observations have led to a proposed expansion of the original spatial cognitive map theory, describing the hippocampal network as a more general relational processing system which enables the rapid association of spatial, temporal, and conceptual aspects of experience (Eichenbaum and Cohen 2001; Eichenbaum et al. 2012). This perspective serves to unify the general memory function of the hippocampus from human and primate studies with the extensive demonstration of a spatial processing function in rodent research.
While these conceptual advances have been important, a complete understanding of the role of the hippocampus will require knowledge of how hippocampal neurons cooperate at a network level to encode, store, and retrieve as memories the complex relationships and experiences that characterize daily life. The original discovery of place cells marked a critical step toward this understanding, as it pointed to a neural mechanism for encoding discrete experiences in the hippocampus. Since then, spatial encoding has been used as a model for the formation of representations that could underlie memory (Eichenbaum and Cohen 2014; Schiller et al. 2015). In this chapter, we will therefore focus on spatial learning and memory as a means to understand mnemonic processing more broadly. In particular, we will explore how coordinated patterns of network activity both within and across the subregions of the hippocampus contribute to spatial memory processing.
2 Anatomical Organization of the Hippocampal Network
To fully understand how hippocampal network activity contributes to learning and memory, it is important to have a sense for the underlying anatomy that supports this activity. Others have written excellent and detailed reviews (see van Strien et al. 2009; Witter and Amaral 2004), so our goal here is to highlight the fundamental connections in the hippocampal network that facilitate information processing.
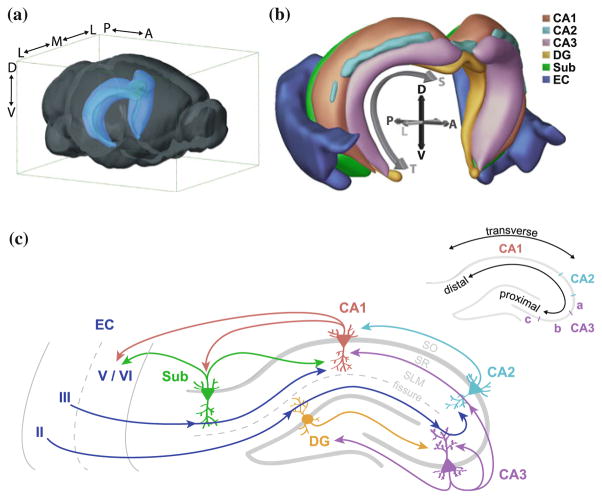
The rodent hippocampal formation is a cashew-shaped structure (Fig. 1a) which includes the dentate gyrus (DG), the subiculum, and the hippocampus proper: CA1, CA2 and CA3 (as defined in Witter 1986; Witter et al. 2000). The key axes often used to describe the hippocampus are dorsoventral (often used synonymously with septotemporal, which describes the long axis from the septal, dorsomedial pole of the hippocampus to its temporal pole; Fig. 1b), transverse, and proximodistal (in which proximity is measured relative to DG, Fig. 1c). These axes can delineate anatomical as well as functional gradients, especially along the dorsoventral axis, as we will discuss later. Each hippocampal subregion is organized into layers, formed by the alignment of the principal neurons (Amaral and Witter 1989; Ishizuka et al. 1995). In the hippocampus proper, principal pyramidal neurons are oriented with their basal dendrites in stratum oriens (SO), pyramidal cell bodies in stratum pyramidale (SP), and the apical dendrites in stratum radiatum (SR) and stratum lacunosum moleculare (SLM; Fig. 1c). Various types of interneurons with distinct morphological and functional properties are interspersed throughout each layer (Klausberger and Somogyi 2008). In the DG, the principal granule cell layer is bordered by a molecular layer separating it from the hippocampal fissure. The two “blades” of the granule cell layer surround the hilus, or polymorphic layer, which is composed of interneurons and hilar mossy cells (Freund and Buzsaki 1996; van Strien et al. 2009). The diverse collection of neuronal populations in each hippocampal subregion strongly influences the activity patterns expressed across the hippocampal network.
Fig. 1.
Anatomical organization of the hippocampal network. a Relative location of the hippocampi within the mouse brain. Blue structures highlight the hippocampus proper (CA1, CA2, CA3, and DG) in each hemisphere. The geometry of the hippocampus is very similar in the rat brain. D dorsal, V ventral, A anterior, P posterior, M medial, L lateral. b Three-dimensional organization of the hippocampal formation and entorhinal cortex. The hippocampal subregions in each hemisphere are nested such that the DG resides most medially, and the EC wraps around the ventroposterior extent of the hippocampal formation, next to the subiculum (Sub). The curved arrow delineates the septotemporal axis (S septal, T temporal). Note that in this representation, the transverse axis lies perpendicular to the septotemporal axis, and thus is similar but not exactly analogous to the coronal plane. a and b are adapted from Brain Explorer 2, © 2015 Allen Institute for Brain Science. Allen Mouse Brain Atlas [Internet]. Available from: http://mouse.brain-map.org. c The hippocampal circuit. Major projections into and within the hippocampal circuit are depicted here, following as closely as possibly the true trajectory of axons through the hippocampal layers (SO stratum oriens, SR stratum radiatum, SLM stratum lacunosum moleculare). For example, EC projections target distal apical dendrites of CA1, CA2, and CA3 neurons in SLM, while CA3 targets the proximal apical dendrites of CA2 and CA1 neurons in SR. Minor projections, as well as interneurons and mossy cells, have been omitted for clarity; however, note that these cells are the targets of the depicted CA3 backprojection to the DG hilus. Arrows represent synapses, but are not weighted by strength. Dotted grey lines represent a subset of layer boundaries, including the hippocampal fissure and the boundary between EC layers II/III and V/VI. The depiction of the EC immediately next to the subiculum is a simplification; note that this exact geometry is only preserved in the horizontal plane of the ventral hippocampus (see panel b). Inset: the transverse and proximodistal axes of the hippocampus. Also shown are approximate subdivisions of CA3a, b, and c
The hippocampal circuit has canonically been described as a trisynaptic pathway, which involves the perforant path inputs of the entorhinal cortex (EC) to the DG, the mossy fiber projection from the DG to CA3, and the Schaffer collateral projection from CA3 to CA1 (Ramón y Cajal 1893; Lorente de Nó 1934, 1933). However, many local, recurrent, and extrahippocampal connections add complexity to the flow of information through the hippocampus, as we will summarize below.
2.1 Hippocampal Inputs
Inputs to the hippocampal formation originate from both cortical and subcortical structures. The hippocampus receives its primary cortical innervation from the entorhinal cortex (Steward and Scoville 1976), via a projection called the perforant pathway (Fig. 1c). EC layer II projects to the apical dendrites of DG granule cells as well as CA3 and CA2 pyramidal cells. While there is some evidence of additional EC input directly onto granule cell bodies (Deller et al. 1996), most EC inputs target the DG molecular layer and SLM of CA2/CA3, with the medial EC (MEC) and the lateral EC (LEC) targeting the more proximal and distal apical dendrites of pyramidal cells, respectively (Witter et al. 1989). The stratification of inputs here may be important for dendritic summation and contribute in specific ways to local LFP (McNaughton and Barnes 1977; Bragin et al. 1995b). In contrast, EC layer III projects to CA1 and the subiculum. In these regions, the subdivision of the MEC and LEC occurs along the proximodistal axis, with the LEC targeting distal CA1 and proximal subiculum, and the MEC targeting proximal CA1 and distal subiculum (van Strien et al. 2009). Direct inputs from sensory and associational cortices primarily target the subiculum, although CA1 has been recently described to receive input directly from the anterior cingulate cortex (Rajasethupathy et al. 2015).
Subcortical inputs to the EC and the hippocampus arise from a variety of structures. The medial septum and diagonal band of Broca (MSDB) send long-range GABAergic and cholinergic afferents to the DG as well as to CA1, CA2 and CA3 (Petsche and Stumpf 1962; Frotscher and Leranth 1985; Freund and Antal 1988; Amaral and Kurz 1985). Additional modulatory inputs come from regions such as the locus coeruleus, the raphe nucleus, and others (Beckstead 1978; Loughlin et al. 1986). CA1 also communicates bidirectionally with the amygdala (Pitkanen et al. 2000; Pikkarainen et al. 1999), which has been long been implicated in emotional forms of learning and conditioned fear memory (Gallagher and Chiba 1996; Paz and Pare 2013; Duvarci and Pare 2014; Janak and Tye 2015). Specifically, the inputs from amygdala to ventral hippocampus have been causally linked to anxiety-like behaviors (Felix-Ortiz and Tye 2014; Felix-Ortiz et al. 2013). CA1 also receives direct input from the nucleus reuniens of the thalamus (Herkenham 1978; Dolleman-Van der Weel and Witter 1996; Vertes et al. 2007) which may influence goal-directed behavior (Ito et al. 2015). For a complete review of hippocampal inputs, see (Witter et al. 1989).
2.2 DG to CA3
Dentate granule cells receive their primary input from the EC and then project to CA3 pyramidal neurons as well as to the other neuronal populations located in the dentate hilus. The DG projection to CA3 is known as the mossy fiber pathway, because of the extensive arborization of granule cell axons and the high density of elaborate postsynaptic spines known as thorny excrescences, which give a “mossy” appearance (Gonzales et al. 2001). In addition to its complex spine structure, CA3 is characterized by heavily recurrent connectivity, meaning that CA3 cells often project onto other CA3 cells (Ishizuka et al. 1990). While recurrence also exists in other hippocampal subregions, it is substantially more prominent in CA3. Specifically, CA3c (Fig. 1c) projects recurrently to the same septotemporal levels of CA3c, while CA3b and CA3a project more extensively within CA3, both across the transverse axis and throughout the septotemporal axis. CA3 is therefore hypothesized to help coordinate activity across the septotemporal extent of the hippocampus (Ishizuka et al. 1990; Li et al. 1994). Furthermore, CA3 projects back to the DG hilus, most strongly from dorsal CA3c and ventral CA3. This back-projection primarily targets excitatory mossy cells and inhibitory interneurons in the hilus, and is therefore hypothesized to indirectly provide both excitation and inhibition of granule cells (Scharfman 2007).
2.3 CA3 to CA1
By far the most intensively studied hippocampal projections are the Schaffer collateral projections from CA3 pyramidal cells to CA1, both ipsilaterally and contralaterally through the hippocampal commissure. The Schaffer collaterals synapse primarily onto the apical dendrites of CA1 pyramidal cells in SR (Fig. 1c), and are stratified by origin: CA3c projects to superficial SR, CA3b to deep SR, and CA3a to SO. Distal CA3 projects to proximal CA1, and proximal CA3 projects to distal CA1 (Laurberg 1979; Ishizuka et al. 1990). Single CA1 pyramidal cells and interneurons receive convergent inputs from both EC layer III and CA3 (Kajiwara et al. 2008; Megias et al. 2001). Although modulated by neural state, the CA3 drive of CA1 is generally thought to be stronger than that of the EC (Spruston 2008).
2.4 CA2
CA2 has received relatively little attention until recently, leaving the functional role of its connections in the hippocampal circuit unclear. CA2 receives input from EC layer II and CA3, as well as strong innervation from the supramammillary nucleus of the hypothalamus (Ishizuka et al. 1990; Chevaleyre and Siegelbaum 2010; Hitti and Siegelbaum 2014; Zhao et al. 2007). Furthermore, neurons in CA2 are extensively recurrently connected and send a strong projection from CA2 to CA1, synapsing primarily in SO and to a lesser degree in SR, and a backprojection from CA2 to CA3 (Hitti and Siegelbaum 2014; Tamamaki et al. 1988; Ishizuka et al. 1990; Cui et al. 2013). Other connections have been more controversial. Notably, individual studies have reported a CA2 to EC layer II projection (Rowland et al. 2013) and a DG to CA2 projection (Kohara et al. 2014), while others do not observe such projections (e.g., Cui et al. 2013).
2.5 CA1
CA1 sends its strongest outputs to the subiculum and to the deep layers of the EC (layers V and VI). The projection to the subiculum is segregated such that proximal CA1 projects most strongly to distal subiculum while distal CA1 projects to proximal subiculum (Amaral et al. 1991). In addition, both CA1 and CA3 project directly to the MSDB (Toth et al. 1993; Toth and Freund 1992; Gulyas et al. 2003; Meibach and Siegel 1977), while other direct projections predominantly from ventral CA1 disperse hippocampal output widely across the brain (Cenquizca and Swanson 2007). Within CA1, local connectivity may have an influence on network patterns and on information processing before signals are sent outward. Specifically, CA1 pyramidal cells synapse laterally onto CA1 interneurons (Takacs et al. 2012; Amaral et al. 1991), which in turn can even project back to CA3 SR and SO as well as to the DG hilus (Sik et al. 1994, 1995). Furthermore, CA1 axons projecting forward to the subiculum extend collaterals that loop back into CA1 SO (Amaral et al. 1991), providing a small amount of recurrent connectivity within CA1.
2.6 Subiculum
The inputs and outputs of the subiculum differ substantially along the dorsal–ventral axis as well as the proximal–distal axis. Dorsal subiculum mostly innervates neocortical regions and receives most inputs from CA1 as well as perirhinal cortex, prefrontal cortex, visual cortex, and MSDB. The ventral subiculum receives the majority of its non-CA1 input from subcortical structures, including hypothalamic nuclei, MSDB, and the amygdala, and returns projections to these regions as well as to the nucleus reuniens of the thalamus and the nucleus accumbens (Witter 2006; Ishizuka 2001; Witter and Amaral 2004). Recently, the early demonstration of a subicular backprojection to CA1 (Kohler 1985; Finch et al. 1983) was confirmed elegantly using Cre-dependent rabies tracing (Sun et al. 2014). Interestingly, both glutamatergic and GABAergic subicular pyramidal neurons innervate all layers of CA1, and the subicular neurons that backproject are the same neurons that receive direct input from CA1. These same cells also receive input from entorhinal cortex, visual cortex, and the MSDB, and both CA1 pyramidal cells and interneurons are targets of this backprojection (Sun et al. 2014). This newly elaborated circuit may provide an important substrate for feedback and fine tuning of hippocampal processing.
3 Electrophysiological Signatures of the Hippocampus
To understand how neural activity in the hippocampal circuit enables the encoding, consolidation, and retrieval of memories, rodent studies of the hippocampus often use place cells as a model for how information can be represented on the single cell and cell ensemble levels. For each subregion of the hippocampal network, we will describe how space is represented at the level of individual neurons, and then how these spatial representations are structured within rhythmic network activity. We will focus primarily on two network patterns that have been implicated in the encoding and retrieval of mnemonic information: theta oscillations and sharp-wave ripples. By understanding how network patterns organize the firing of place cells, we may begin to understand how neural networks may organize information into memories useful for guiding subsequent behavior.
3.1 Place Cells
The most striking feature of hippocampal neurons is their spatial specificity. Their stable, location-based receptive fields, called place fields, are now known to be characteristic of the majority of excitatory hippocampal neurons in all subregions (O’Keefe and Dostrovsky 1971; Jung and McNaughton 1993; Muller and Kubie 1987; Thompson and Best 1990). It is important to note that the characterization of place cells is generally done during locomotion, including much of the information we will discuss in the following sections. However, a wealth of evidence suggests that neural activity maintains its place representations outside of locomotion, across behavioral states (e.g., de Lavilleon et al. 2015; Pavlides and Winson 1989; Kay et al. 2016). Place fields develop over the first few minutes of exploration in a new environment and become more refined with experience (Hill 1978; Wilson and McNaughton 1994; Frank et al. 2004). Although exact definitions vary, a cell’s place field is generally defined as the region in which its firing exceeds 1 Hz or a certain proportion of the cell’s peak firing rate in the environment, such that the place cell fires maximally when the animal is centered in its place field and sparsely or not at all in distant regions of the environment (O’Keefe 1976; Muller et al. 1987). Some place cells have multiple fields, especially in large environments (Fenton et al. 2008; Park et al. 2011). In different environments, different subsets of neurons will become active; this shift in ensemble place activity is called global remapping (Muller et al. 1987; Markus et al. 1995; Lever et al. 2002). A local alteration in an environment (e.g., elimination or addition of a visual cue) might induce rate remapping, in which the active place cell ensemble remains the same, but the firing rates of the ensemble change (Leutgeb et al. 2005b; Anderson and Jeffery 2003; Allen et al. 2012). Rate remapping is thus hypothesized to contribute to the representation of new information within a pre-established spatial framework, while global remapping reflects the creation of an independent spatial representation. Overall, these encoding mechanisms show how neural ensemble activity relates to a representation of the animal’s experience, providing a means to investigate how experience is processed within the hippocampal circuit.
3.2 Theta Oscillations
During movement, place cells fire at specific times relative to a network rhythm known as theta. Theta is a low frequency oscillation (~8 Hz, or more broadly 5–12 Hz) which dominates the local field potential (LFP) during locomotion (Fig. 2) and during periods of active engagement in the environment, such as rearing, exploring objects, and preparation for movement (Green and Arduini 1954; Vanderwolf 1969; Grastyan et al. 1959; Foster et al. 1989). An extensive body of literature has thus described theta as the critical marker of an active, location-encoding behavioral state in rodents. Moreover, as theta is known to coordinate place cell firing in this state, it has long been thought to be an important contributor to hippocampal processing (for review, see Buzsaki 2002).
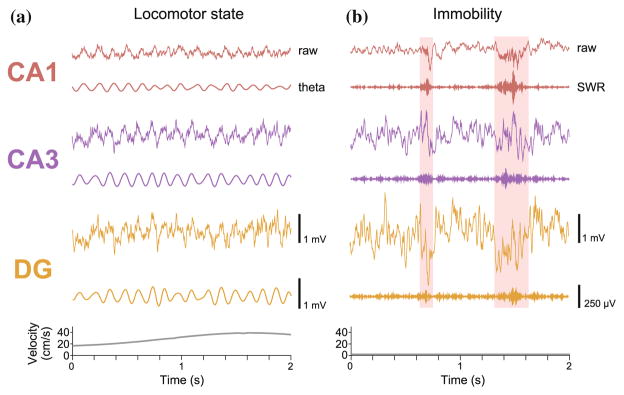
Fig. 2.
LFP signatures of the hippocampal network. To illustrate the distinct features of hippocampal LFP, we show raw and filtered LFP detected simultaneously in a single rat from tetrodes located in the principal layer of each major subregion of the hippocampus. During the locomotor state (left), persistent theta oscillation dominates the raw LFP signal in all three subregions. As seen in both the raw and theta filtered (5–11 Hz) traces, theta amplitude is smallest in CA1, larger in CA3, and largest in the DG. Graph of the rat’s velocity (bottom) shows that during this period, the animal is constantly in motion. In contrast, traces on the right show LFP data acquired during awake immobility. Instead of the highly regular rhythmic activity during locomotion, the LFP signal is far more varied, but also increases in amplitude from CA1 to the DG. SWRs detected in CA1 are highlighted in pink, and are easily distinguishable as periods of increased power in the ripple filtered trace (150–250 Hz). The high frequency component of the SWRs are most dominant in CA1, while the sharp wave component is more visible in CA3 and the DG. Note that a substantial amount of time during immobility does not contain SWRs, during which the rat presumably still maintains spatial representations but perhaps through alternate coding mechanisms. During this period, the velocity plot (bottom) shows that the rat is motionless
It is worth noting that while theta has been observed in mammals other than rodents (Winson 1972; Arnolds et al. 1980), theta is substantially less prominent in bats, cats, monkeys, and humans during analogous periods of locomotion and decision-making (Watrous et al. 2013; Jutras et al. 2013; Ulanovsky and Moss 2007; Kemp and Kaada 1975). For this reason, the specific significance of theta defined as a 5–12 Hz rhythm is not clear. It is possible that an alternative low frequency signal, or irregular but time-locked activity, may perform similar roles in other species (Watrous et al. 2013; Yartsev et al. 2011; Ulanovsky and Moss 2007). To encompass the general behavioral state marked by movement and active sensory engagement across species, we will refer to such periods of activity as the locomotor state. However, as most studies of hippocampal activity to date were conducted in rodents, we will discuss theta in the locomotor state as one potential mechanism for binding spatial and mnemonic representations.
Hippocampal theta is dependent on activity in the MSDB. Lesions of the MSDB abolish theta activity throughout the entire neocortex (Petsche and Stumpf 1962; Mitchell et al. 1982; Stewart and Fox 1990), and theta suppression from MSDB inactivation has been directly linked to spatial working memory impairment (Mitchell et al. 1982; Givens and Olton 1990; Winson and Abzug 1978; Mizumori et al. 1989). However, other regions such as the supramammillary nuclei may also contribute to the pacing of theta (Kocsis and Vertes 1997; Pan and McNaughton 2002). In addition, lesions of the EC drastically reduce theta power in the hippocampus, suggesting that entorhinal input may be critical for supporting a strong hippocampal theta signal (Buzsaki et al. 1983). Interestingly, while MSDB inactivation abolishes theta entirely, place coding in the hippocampus is somewhat abnormal but not absent (Brandon et al. 2014; Mizumori et al. 1989). The functional role of theta in establishing hippocampal representations thus remains somewhat unclear.
In the rodent hippocampus, theta phase and amplitude are variable across layers (Buzsaki et al. 1986; Bullock et al. 1990) and change along the dorsoventral axis within the same layer (Patel et al. 2012). Theta phase is most consistent just above SP, in SO or corpus callosum, where phase changes within the layer are minimal along the dorsoventral axis (Lubenov and Siapas 2009). The phases of theta represent varying levels of excitation and inhibition. At the trough, for example, inhibition from local inhibitory interneurons is thought to be least, permitting strong firing in local pyramidal cells (Csicsvari et al. 1999b). During the locomotor state, the phase of theta at which a neuron fires is also governed by the animal’s proximity to the center of the place field, a phenomenon known as phase precession (O’Keefe and Recce 1993; Skaggs et al. 1996). As an animal enters a place field, that neuron will begin to fire in the later phases of theta, toward the peak of the oscillation. As the animal runs through the neuron’s place field, each spike of the neuron tends to align to a progressively earlier phase of the theta cycle, firing near the trough of theta in the place field center and on the descending phases as the rat moves past the place field center (O’Keefe and Recce 1993; Skaggs et al. 1996). This results in each cycle of theta containing a range of place cell activity ordered by location. During most theta cycles, the place cells that fire are those with fields spanning from just ahead to just behind the current position of the animal, such that this sequence of locations is compressed into one theta cycle (Itskov et al. 2008). This has been suggested as a strategy for compressing experience from the timescale of behavior down to the millisecond timescale of spike-timing-dependent plasticity (Skaggs et al. 1996), potentially enabling the rapid encoding of experience in novel environments (Cheng and Frank 2008) and the efficient transmission of spatial information to downstream brain regions (Skaggs et al. 1996; Olypher et al. 2003; Ego-Stengel and Wilson 2007). However, some theta cycles contain a range of place cells representing locations more distant from the animal, and these have been proposed to play a role in planning future trajectories (Gupta et al. 2012; Wikenheiser and Redish 2015). In general, place cell activity coupled to theta is thought to provide an ongoing representation of location and potentially represent immediate upcoming plans during active behavior.
3.3 Sharp-Wave Ripples (SWRs)
In contrast to the overt rhythmicity of theta during the locomotor state, the hippocampal LFP signal is far more irregular during times of awake immobility (Fig. 2) and slow wave sleep. These periods are punctuated by hippocampal sharp-wave ripples (SWRs) (for review, see Buzsaki 2015). SWRs are perhaps the most synchronous events in the healthy brain, with an estimated 50,000–100,000 neurons discharging over ~50–150 ms in the hippocampus and EC (Chrobak and Buzsaki 1996; Csicsvari et al. 1999a). SWRs have been seen in mammals ranging from mice to humans (Buzsaki et al. 2003; Skaggs et al. 2007; Ulanovsky and Moss 2007; Axmacher et al. 2008), suggesting that their function likely to be conserved. In the rodent, SWRs are characterized by a high frequency ripple oscillation (150–250 Hz) predominantly in the CA1 subregion (Buzsaki et al. 1992) as well as a sharp wave: a simultaneous large negative deflection of the LFP signal detectable throughout most of the hippocampus (Buzsaki 1986). During SWRs, ensembles of place cells become sequentially active in a time-compressed manner, often recapitulating prior experience at high speed (Pavlides and Winson 1989; Lee and Wilson 2002; Skaggs and McNaughton 1996; Buzsaki 1989; Wilson and McNaughton 1994). These sequential reactivations are known as replay events, and are hypothesized to be a key mechanism of hippocampal memory. Complementary to the rapid encoding enabled by compressed place cell sequences within theta cycles, replay during SWRs has been linked to memory consolidation and retrieval. Multiple studies have demonstrated that disrupting SWRs, either during awake immobility or sleep, is sufficient to impair performance on memory-dependent tasks (Girardeau et al. 2009; Ego-Stengel and Wilson 2010; Jadhav et al. 2012; Nokia et al. 2012). Furthermore, SWRs may contribute directly to forming new associations, potentially linking outcomes, such as reward, with the route that leads to them and thus guiding future behavior (Singer and Frank 2009; Foster and Wilson 2006; Lansink et al. 2008, 2009). Distinct patterns of activity concurrent with SWRs have been detected in numerous distant brain areas, suggesting that these events coordinate memory processes across the entire brain (Logothetis et al. 2012).
3.4 Network Activity Outside of Theta and SWRs
While theta and SWRs have been extensively studied as network patterns that facilitate spatial encoding, consolidation and retrieval, relatively little is known about how information in encoded outside of these patterns. During immobility and periods of slow movement, such as when an animal is consuming reward or simply sitting quietly, SWRs only comprise a small fraction (<10 %; Suzuki and Smith 1987; Buzsaki 1989, 2015; Kay et al. 2016) of the ongoing network activity. Recently, a subpopulation of principal neurons in CA2 has been shown to encode location in the absence of locomotion, firing specifically outside of SWRs. Along with neurons in CA1 and CA3, these neurons fire during a transient ~200 ms network pattern with opposite polarity to that of sharp waves. These findings indicate a distinct hippocampal subnetwork dedicated to coding the animal’s current location during immobility and even sleep (Kay et al. 2016). In particular, spatial coding during sleep occurred during periods marked by small amplitude LFP activity distinct from the more commonly studied slow wave sleep and REM sleep states (Vanderwolf 1969; Jarosiewicz et al. 2002; Louie and Wilson 2001; Montgomery et al. 2008; Grosmark et al. 2012). Overall, these observations illustrate that that our understanding of the breadth of brain states relevant to hippocampal processing remains incomplete.
3.5 Gamma Oscillations
During both the locomotor state and quiescent brain states, an additional rhythm can be seen in the 20–110 Hz range, known as gamma. In the cortex, gamma oscillations have been proposed to play a role in binding neural ensembles, contributing to information transfer and spike-timing dependent plasticity (Gerstner et al. 1996; Markram et al. 1997; Fell and Axmacher 2011), and it is likely that they play similar roles in the hippocampus. However, unlike cortical gamma, hippocampal gamma has been subdivided into several frequency bands, each of which associates with specific states and subregions and is driven by distinct inputs and mechanisms (Buzsaki and Wang 2012; Belluscio et al. 2012). Slow (or low) gamma (20–50 Hz) is thought to be driven predominantly by CA3 (Bragin et al. 1995a; Colgin et al. 2009), while fast gamma (50–90 Hz) is thought to be driven by the MEC (Colgin et al. 2009). An even higher gamma band (90–150 Hz), sometimes called the epsilon band, has also been suggested (Csicsvari et al. 1999a; Sullivan et al. 2011; Freeman 2007). During the locomotor state, all three gamma bands can be observed nested within theta cycles, generally with a single gamma band predominating in each cycle. Thus individual theta cycles tend to exhibit either slow or fast gamma in an interleaved fashion, likely dependent on the cognitive demands experienced by the animal. The frequency of gamma coupled to theta may influence the function and content of theta sequences (Colgin et al. 2009; Zheng et al. 2016). During SWRs, slow gamma in particular is transiently increased in power and coherence throughout the hippocampal circuit, and has been proposed as a clocking mechanism to coordinate accurate replay (Carr et al. 2012; Pfeiffer and Foster 2015).
In the remainder of this chapter, we will focus on the unique contributions of each hippocampal subregion to the patterns of cellular and network activity associated with spatial mnemonic processing in the locomotor state and during SWRs.
4 Entorhinal Cortex
The EC is the main conduit of information to the hippocampus, sending projections to every subregion. The EC receives and integrates sensory information from the primary sensory cortices and head direction information from the thalamic nuclei via the pre- and para-subiculum. In turn, the EC receives direct feedback from the hippocampus. In rodents, the EC is comprised of medial and lateral subregions with distinct functional roles and anatomical connectivity, which are likely preserved in primates although the anatomical delineation between regions is less clear (Witter 1993; Kerr et al. 2007). The MEC conveys mostly spatial information, while encoding in the LEC tends to correspond more to objects, object–context associations, cues, and odors (Deshmukh and Knierim 2013).
4.1 Cell Characteristics of the EC
Within the MEC, distinct subpopulations of cells have been shown to represent major features of any environment. Grid cells are the most common, comprising 30 % of MEC cells, and are found in layers II and III (Zhang et al. 2013). The firing field of a grid cell forms a triangular lattice spanning the entire environment (Hafting et al. 2005). Grid cells are organized into modules that share similar grid scale and orientation; those located in dorsal MEC have smaller grid spacing than those in ventral MEC (Stensola et al. 2012). The other two major MEC populations are border cells, which exhibit firing fields specific to the edge of an environment (Savelli et al. 2008; Solstad et al. 2008; Lever et al. 2009), and head direction cells, which show preference for the animal facing a certain direction independent of location (Sargolini et al. 2006; Taube 2007). These populations can overlap, as some neurons, especially in layers III and V of the MEC, show both grid and head direction tuning (Sargolini et al. 2006). While grid, head direction, and border cells have been the most extensively characterized populations, they comprise only 50 % of the neurons in the MEC, and it is unclear what the function of the remaining neural population may be (Zhang et al. 2013; Sasaki et al. 2015). Recently discovered speed cells may comprise part of this population (Kropff et al. 2015), and may be important for the constant updating of an animal’s location on the grid cell map, a process known as path integration (for review, see McNaughton et al. 2006). Together, MEC cells likely provide the animal with a spatial map of the environment and a continuous representation of self-location and transmit that information to the hippocampus.
In contrast to the thoroughly studied MEC, the role of the LEC is much less clear. Current evidence suggests that the LEC may be responsible for encoding the objects, odors, and local cues that occur within an environment rather than mapping space on a global scale (Neunuebel et al. 2013). While objects and local cues can also influence the development of place fields in the MEC, LEC neurons generally lack the spatial tuning frequently seen in MEC neurons (Deshmukh and Knierim 2011; Yoganarasimha et al. 2011). Furthermore, lesions of the LEC have been shown to impair the association of objects with environmental contexts, despite sparing normal object recognition and context recognition (Wilson et al. 2013). This finding, along with others, suggests that the LEC plays a role in linking items and cues with the environment in which they were experienced (Neunuebel et al. 2013). Together, the MEC and LEC are thought to provide the “where” and “what” of an experience to the hippocampus, where it can then be compared to previous experience, integrated with existing frameworks, and stored. In particular, the EC may establish location-based representations of stimuli that could be the basis for the spatial encoding seen in hippocampal cells.
4.2 EC Network Activity
During the locomotor state, theta is prominent in the MEC and strongly entrains neuronal spiking (Deshmukh et al. 2010). Grid cells show theta phase precession (Hafting et al. 2008), and the integrity of grid cell firing is dependent on theta oscillations (Koenig et al. 2011). Surprisingly, although the MEC was initially suspected to be the primary driver of place cell activity, lesions of the MEC do not abolish hippocampal place fields (Van Cauter et al. 2008; Hales et al. 2014). However, MEC lesions do disrupt hippocampal theta phase precession and reduce the spatial specificity and stability of place fields (Van Cauter et al. 2008; Hales et al. 2014; Schlesiger et al. 2015), concomitantly impairing spatial navigation-dependent behavior (Hales et al. 2014). This evidence suggests that the MEC provides critical spatial and temporal cues to refine hippocampal representations of location. Conversely, grid cell integrity is heavily dependent on hippocampal feedback, as inactivation of the hippocampus abolishes grid cell periodicity (Hafting et al. 2008; Bonnevie et al. 2013). In parallel to the reduced spatial encoding observed in the LEC compared to the MEC, the power of the theta rhythm is lower in the LEC than in the MEC or hippocampus, and entrainment of LEC neurons by theta is less prominent. This suggests that theta may be particularly important for coordinating spatial processing across the MEC and hippocampus, and less so for nonspatial object information in the LEC (Deshmukh et al. 2010).
Cells in the layers of the EC that project to the hippocampus are thought to be relatively inactive during SWRs themselves (Chrobak and Buzsaki 1994). However, several studies indicate that the EC, together with the rest of the neocortex, experiences periods of higher firing (up states) and periods of relative inactivity (down states) governed by the neocortical slow oscillation which may influence SWR activity in the hippocampus (Steriade et al. 1993; Sirota et al. 2003; Battaglia et al. 2004a; Isomura et al. 2006). SWRs are more likely to occur during up states (Battaglia et al. 2004a; Sullivan et al. 2011), suggesting that the overall cortical state may influence the ability of the hippocampus to generate SWRs, and this modulation may be conveyed by EC inputs.
5 Dentate Gyrus
Despite its prominent place in the hippocampal circuit, the DG has been one of the least studied of the hippocampal subregions with respect to patterns of network activity. However, it has garnered attention due to several unique characteristics. Most notably, the DG is one of the only regions in the brain which supports persistent neurogenesis throughout life. The regular addition of new neurons to DG circuitry has major implications for network activity in the region (Ge et al. 2008; Schmidt-Hieber et al. 2004) and for behavior dependent on the DG (Dupret et al. 2008; Garthe et al. 2009; Jessberger et al. 2009; Shors et al. 2001; Wojtowicz et al. 2008). A second key feature of the DG is the highly sparse firing of its principal cells, which have very low spontaneous firing rates (Amaral et al. 1990; Jung and McNaughton 1993) and of which only a very small fraction are active in any given environment (Guzowski et al. 1999). It has been proposed that the sparse firing of distributed ensembles and the addition of newborn neurons into those ensembles make the DG uniquely suited to perform pattern separation, a process by which similar experiences are disambiguated and encoded by orthogonal representations (Marr 1971; Clelland et al. 2009).
5.1 Cell Characteristics of the DG
The principal cell type in the DG is the granule cell (GC). These small, tightly packed cells make up the cell layer ‘blades’ of the DG. Neurogenesis in the sub-granular zone lining the border between the GC layer and hilus consistently adds newborn GCs to the GC layer. These newborn cells migrate and integrate into existing GC layer circuitry over a 4–8 week period, during which they show increased excitability compared to mature GCs (Esposito et al. 2005; Ge et al. 2008; Schmidt-Hieber et al. 2004; Gu et al. 2012; Li et al. 2012; Marin-Burgin et al. 2012; Danielson et al. 2016). GCs tend to fire very sparsely (generally less than 0.5 Hz in awake recordings), and have narrow, asymmetric waveforms (Jung and McNaughton 1993). Recordings from putative GCs suggest that they have spatially and directionally specific place fields that are highly stable, although smaller than those found in CA3 and CA1 (Jung and McNaughton 1993; Leutgeb et al. 2007). Moreover, these putative GCs may have more discontiguous sub-place fields than CA3 and CA1 pyramidal cells, although due to dense cellular packing of the DG, this conclusion is confounded by the challenge of identifying the cell type being recorded. It is possible that the cells with multiple sub-place fields may be the hyperexcitable newborn GCs or mossy cells, another excitatory neuronal population in the DG (Danielson et al. 2016; Neunuebel and Knierim 2012).
Small, subtle changes in an environment are sufficient to prompt global remapping of DG ensembles and thus change DG input to CA3, in contrast to CA3 ensembles which adjust slightly but do not remap (Danielson et al. 2016; Leutgeb et al. 2007; Neunuebel and Knierim 2014). This falls in line with the idea of pattern separation, showing that the DG can amplify differences between similar experiences. However, it remains challenging to differentiate the contribution of young and mature GCs to ensemble representations. New GCs seem to be important for pattern separation, as blocking neurogenesis impairs the ability to perform pattern separation, while stimulating neurogenesis enhances it (Nakashiba et al. 2012; Tronel et al. 2012; Creer et al. 2010; Sahay et al. 2011; Clelland et al. 2009). This finding is somewhat contradicted, however, by the hyperactive nature of young GCs, which would seem to undermine the activation of orthogonal ensembles capable of distinguishing similar experiences (Johnston et al. 2016; Danielson et al. 2016). However, young GCs have also been shown to more effectively recruit feedback inhibition than mature GCs (Temprana et al. 2015), which may offset their hyperactivity (McAvoy et al. 2015).
The sparse firing of granule cells is enforced by high levels of GABAergic inhibition from local inhibitory interneurons. Various interneuron subtypes provide both feedback and feedforward inhibition onto GCs by targeting GC bodies or dendrites, respectively (Savanthrapadian et al. 2014). In addition to interneurons, the hilus also contains excitatory mossy cells (Henze and Buzsaki 2007; Scharfman and Myers 2012). As mossy cells are relatively rare (1:100 ratio of mossy cells to granule cells; (Henze and Buzsaki 2007), and because it is unclear how best to distinguish them from GCs, they have not been well characterized electrophysio-logically (Neunuebel and Knierim 2012). Mossy cells receive inputs either directly from the EC or indirectly through GCs, and synapse onto hilar interneurons and remote GCs (Buckmaster et al. 1996; Larimer and Strowbridge 2008). This may allow them to integrate activity across the septotemporal axis of the DG by transferring excitation between GCs, or by suppressing the activity of distant GC populations via feedforward inhibition (Larimer and Strowbridge 2008; Henze and Buzsaki 2007). Together, the interactions between sparsely firing mature GCs, excitable newborn GCs, mossy cells, and interneurons may underlie the DG’s ability to integrate entorhinal inputs into distinct representations of mnemonic experience.
5.2 DG Network Activity During the Locomotor State
Large, clear theta oscillations can be observed in the DG during the locomotor state (Bragin et al. 1995b) (Fig. 2). Theta entrains the spiking of both GCs and interneurons (Skaggs et al. 1996), although newborn GCs may be more weakly modulated (Rangel et al. 2013). Spatially modulated GCs also exhibit theta phase precession (Skaggs et al. 1996). Theta phase, as well as the coherence of DG theta with the rest of the hippocampus, varies by layer, which may be due to the stratification of inputs from different EC layers (Montgomery et al. 2009). Theta power and coherence measures also fluctuate based on the activity being performed, although the significance of these observations remains unclear.
During the locomotor state, gamma oscillations nested within the theta rhythm are larger in the hilus than anywhere else in the hippocampus (Bragin et al. 1995a; Buzsaki 2002; Montgomery and Buzsaki 2007). Like theta, the power and coherence of DG gamma with the rest of the circuit fluctuates with the cognitive demands of activity performed (Montgomery and Buzsaki 2007), but the exact role of DG gamma is not known. A study using current source density analysis (CSD; see Box) showed the largest gamma current sink in the middle third of the DG molecular layer, where axons from the MEC terminate. This current sink disappeared upon lesion of the EC, further suggesting that DG gamma activity is primarily driven by the EC during locomotor state (Bragin et al. 1995a). This study did not differentiate slow gamma from fast gamma; however, as the EC is thought to promote fast rather than slow gamma in the hippocampus (Colgin et al. 2009), the CSD finding may predominantly reflect fast gamma in the DG during the locomotor state.
BOX. Measuring neural activity in an awake, behaving animal.
Recording neural activity during behavior allows us to understand how information is processed during an experience and stored as memories. We will discuss two primary types of data collected during this process: single unit activity and local field potential (LFP).
Single unit activity refers to the action potentials, or spikes, fired by individual neurons. Although spikes can now be recorded in vivo using whole cell patch clamp techniques (Tao et al. 2015) or calcium imaging (Ziv et al. 2013), the predominant method for recording unit activity in vivo is extracellular recording. An action potential alters the ionic charge in the extracellular space, as positive sodium ions flow into the cell and away from the recording electrode. The cell’s depolarization is therefore reflected as a sharp negative deflection on the extracellular electrode, the inverse of an intracellular recording. The amplitude of this deflection, or waveform, is primarily a function of the electrode’s proximity to the cell, as an electrode closer to the cell will measure a larger voltage change. However, because the cell layers of the hippocampus are so densely populated, it can be challenging to distinguish the activity of a single neuron from the surrounding neurons. To address this, hippocampal electrophysiologists typically use recording probes with several closely spaced electrode sites, such as tetrodes, which consist of four insulated electrode wires twisted together (for review, see Buzsaki 2004). Each wire picks up a cell’s spike at a slightly different amplitude due to the different proximity of each wire to the cell. This allows the spikes of the cell to be “clustered” by comparing the recorded amplitudes between pairs of electrode wires (Gray et al. 1995; Jog et al. 2002), thus isolating the spike cluster from those of neighboring cells in amplitude space. In contrast, single site electrodes can be sufficient to isolate cells in less densely packed brain regions such as the cortex.
Once spikes have been clustered to link them to a particular neuron, parameters such as the neuron’s firing rate, inter-spike interval, and spike waveform can be analyzed to better understand its activity. In the hippocampus, pyramidal cells and fast-spiking interneurons can be putatively identified by their different waveform shapes and firing rates (Fox and Ranck 1981). Not all neuronal populations have clearly differentiable waveforms, however, so it is difficult to definitively identify cell types using extracellular recording alone. Further analysis often describes how the timing of spikes is modulated by behavioral events or by local network activity, as reflected by local field potentials.
Local field potentials (LFP) are defined as the extracellular voltage fluctuations at lower frequencies relative to spiking, which reflect neural network oscillations (hippocampal spiking is typically filtered between ~600–6000 Hz, LFP between ~1–400 Hz) (for review, see Buzsaki et al. 2012). The LFP signal is dominated by synaptic and dendritic activity near the recording electrode for two main reasons. First, high frequency action potentials are largely removed by the low-pass filter. Second, and more importantly, dendritic post-synaptic currents occur at slower timescales than action potentials, increasing the chance of events coinciding in time. The ionic flux of many coincident small synaptic events accumulates, resulting in relatively large fluctuations in the LFP. In laminar structures in which the dendrites and cell bodies of principal neurons are segregated, such as the hippocampus, synaptic input often aligns spatially and temporally, resulting in characteristic layer-specific LFP activity. The amplitude of the LFP signal is influenced by the scale, anatomical organization, and synchrony of inputs to a particular layer (Kajikawa and Schroeder 2011), as well as the proximity of the electrode to the site of maximal current flow, which can be measured using current source density analysis (CSD; Mitzdorf 1985). CSD utilizes the change in LFP signal across closely spaced recording sites to help identify the location of inward or outward current flow. A CSD sink is a negative deflection that represents predominantly positive ions moving into a cell (i.e., an input generating local action potentials), and a source is a positive deflection that is typically interpreted as reflecting the compensatory exit of those positive ions from another part of the cell.
There are many methods for analyzing LFP signals to gain an understanding of how network-level activity is organized within and across brain regions. To isolate particular rhythms, LFPs are often decomposed into their time and frequency components. Measuring the relative intensity of different frequency components can be done using spectral analysis, and the interaction between different frequencies of oscillation can be described by cross-frequency coupling parameters (Tort et al. 2010). LFP can also be compared across multiple brain areas using a measure called coherence, which describes the coordinated modulation of the phase or amplitude of the LFP signals, and may reflect common driving inputs or information flow between the regions (Fries 2005). Finally, as mentioned above, the phase preference of single unit spiking can be determined to understand how LFP signals modulate the firing of local neuronal ensembles. Together, action potentials from individual cells (single unit activity) combined with coordinated network signals (LFP) enable detailed description of neural activity within and across brain regions.
5.3 DG Network Activity During SWRs
Since SWRs are thought to originate in CA3 and proceed to CA1, as described later in this chapter, most studies have focused on the SWR-related activity that occurs in those subregions. However, several pieces of evidence suggest that the DG also participates in SWR-associated activity. First, granule cell activity has been observed during SWRs (Penttonen et al. 1997) including reactivation during sleep (Shen et al. 1998), potentially driven by the CA3 backprojection (Scharfman 2007, 1994). Second, state-dependent activity in the DG may affect SWR generation. During slow wave sleep, DG activity can be categorized into “up” and “down” states which correlate with those seen in neocortex (Isomura et al. 2006; Sullivan et al. 2011). As mentioned above, SWRs are more likely to occur during up states than down states (Battaglia et al. 2004a; Isomura et al. 2006; Sullivan et al. 2011). This suggests state-dependent modulation of SWR generation, however, it is unclear whether the DG contributes to this modulation directly, or whether both the DG and CA3 are influenced by EC up/down states in parallel. Finally, a recent study have shown that slow gamma activity in the DG increases during SWRs (Gillespie et al. 2016). This is similar to an SWR-associated transient slow gamma increase observed in CA3 (Carr et al. 2012), which may serve as a critical timing mechanism to organize replay activity during SWRs (Pfeiffer and Foster 2015) as we will further discuss below. In the DG, the power of slow gamma transiently increases during SWRs, as does coherence in this frequency band between DG-CA3 and DG-CA1. These results suggest that slow gamma activity engages all subregions of the hippocampus proper during SWRs, potentially coordinating information flow through the circuit. Disruption of DG circuitry, caused by the loss of hilar interneuron populations, results in impaired SWR-associated slow gamma activity throughout the hippocampal circuit, further indicating that the DG may be actively engaged during SWRs (Gillespie et al. 2016).
Interestingly, there is another pattern of activity, called dentate spikes (DSs), that also occurs in the DG during awake immobility and slow wave sleep (Bragin et al. 1995b; Penttonen et al. 1997). DSs are brief, large-amplitude LFP deflections seen in the hilus and granule cell layer. Two types have been described, one (DS1) which has a broad waveform, shows a phase reversal in the outer molecular layer, and contains some fast gamma activity, and another (DS2) which shows a single narrow LFP peak with a phase reversal in the inner molecular layer (Bragin et al. 1995b). Lesions of the EC eliminate both DS types, and CSD analysis of these events as well as the location of their phase reversals suggests LEC and MEC drive of DS1 and DS2, respectively (Bragin et al. 1995b). Although DSs and SWRs appear during the same behavioral state, they do not coincide. Instead, DSs seem to have the opposite effect on the hippocampus from SWRs; rather than inducing ensemble activity downstream, DSs seem to transiently suppress CA3 and CA1 activity (Bragin et al. 1995b; Penttonen et al. 1997; Buzsaki et al. 2003). Although behavioral correlates of DSs are not well understood, this observation suggests that they may enable a transient blockade of hippocampal output via CA1.
6 CA3 and CA1
CA3 and CA1 are by far the most well-studied subregions of the hippocampus. As activity patterns are highly coordinated across CA3 and CA1, we will discuss their network activity in parallel, while highlighting the distinctions that embody each region’s unique role in hippocampal processing.
6.1 Functional Roles of CA3
CA3 has been functionally implicated in rapid task acquisition (Lee and Kesner 2003; Nakazawa et al. 2003, 2008; Kesner 2007; Lee and Kesner 2004; Cravens et al. 2006), as well as task recall (Nakazawa et al. 2002; Kesner 2007; Lee et al. 2005; Schlesiger et al. 2013), the latter of which is likely facilitated by the retrieval of previously learned patterns. For example, lesions of CA3 impair the ability of animals to use partial cues to trigger memory-based performance of a task acquired in the presence of full cues (Gold and Kesner 2005). This ability to recall a whole memory based on a partial cue is known as pattern completion, and may be a critical neural process for comparing current events to past memories and generalizing across similar experiences (for review, see Leutgeb and Leutgeb 2007; Knierim and Neunuebel 2016; Rolls 2007). Pattern completion may be supported by the trait which most often distinguishes CA3 in the current literature: its relatively high level of recurrent connectivity compared to other hippocampal subregions (Ishizuka et al. 1990; Li et al. 1994; Witter 2007). This recurrence has been presented as anatomical evidence that CA3 acts as an autoassociative network (Marr 1971; Guzowski et al. 2004; Treves and Rolls 1991; Rolls 2007; Papp et al. 2007; Gilbert and Brushfield 2009; McClelland and Goddard 1996; Treves and Rolls 1992, 1994). Autoassociation implies that the activation of a subset of neurons within an ensemble can drive sustained activation of the entire ensemble by propagating excitation through reciprocal connections between cells (Lisman 2003). Such an autoassociative network might also exhibit attractor dynamics (Marr 1971; Mcnaughton and Morris 1987; Kali and Dayan 2000; Leutgeb et al. 2005c; Rolls 2007; Lengyel et al. 2005). In CA3, an ensemble of neurons representing a stored memory could act as the attractor basin; when an input is similar enough to the stored memory, the activity in the network settles on that ensemble. When an external input is sufficiently distinct from the stored pattern, it would outweigh the ongoing activity to transition the pattern of activity to a new group of cells, and thus form a distinct memory (Renno-Costa et al. 2014; Leutgeb and Leutgeb 2007; Colgin et al. 2010). While there is not yet definitive evidence that CA3 functions as a true attractor network, the importance of its recurrent collaterals to pattern completion have been supported by recent findings. Results showing that distal CA3 (where the level of recurrence is highest) shows stronger autoassociation than proximal CA3 (where recurrence is lowest) suggest that the contribution of CA3 subregions to pattern completion depends on the local recurrent connectivity (Lee et al. 2015). The autoassociative nature of CA3 is further supported by the stability of CA3 population representations in response to small changes in environmental cues (Neunuebel and Knierim 2014), indicating that small changes in sensory inputs are insufficient to substantially alter the representation.
6.2 Functional Roles of CA1
The CA1 network represents the final stage of hippocampal processing before information is sent to the subiculum and to the rest of the brain. CA1 continually integrates input received from CA3 and the EC during ongoing experience (Bittner et al. 2015; Spruston 2008; Milstein et al. 2015; Piskorowski and Chevaleyre 2012; Kali and Freund 2005) and permits incremental spatial learning and retrieval even in the absence of CA3 input (Nakashiba et al. 2008). One possible function of CA1 is to compare past experiences stored in and retrieved by CA3 with new information from ongoing experiences transmitted by the EC. In this scenario, CA1 would then create a new representation when there is no past experience that directly corresponds to current input (Lee et al. 2004a; Hasselmo and Schnell 1994; Lisman 1999; Vinogradova 2001; Duncan et al. 2012; Meeter et al. 2005; Meeter et al. 2004). CA1 may therefore compile memories by layering newly learned spatial information onto past and current representations of the global environment.
The possibility that CA1 somehow compares stored and new information is consistent with observations that CA1 responds to novelty (Lisman and Otmakhova 2001; Li et al. 2003; Kumaran and Maguire 2007), specifically by signaling the presence of a novel experience and potentially enhancing the incorporation of novel information into an existing framework (Larkin et al. 2014). In particular, CA1 place cells change their firing rates in response to novel or changing objects (Lenck-Santini et al. 2005; Deshmukh and Knierim 2013; Fyhn et al. 2002; Larkin et al. 2014) and novel spatial environments (Karlsson and Frank 2008; Nitz and McNaughton 2004; VanElzakker et al. 2008). CA1 network patterns, including SWRs and gamma, are also modulated by novelty both during the novel experience and during sleep afterward (Cheng and Frank 2008; Karlsson and Frank 2009; Eschenko et al. 2008; O’Neill et al. 2008; Singer and Frank 2009; Dupret et al. 2010; Kemere et al. 2013; Ramadan et al. 2009). Importantly, novelty-induced increases in firing rate and SWR reactivation appear to be specific to CA1, and not CA3 (Karlsson and Frank 2008), suggesting that the recognition of novelty is a function that emerges uniquely in CA1 or in conjunction with the EC, rather than through input to CA1 from CA3 (Larkin et al. 2014).
6.3 Cell Characteristics of CA3 and CA1
In support of their complementary functional roles, CA3 and CA1 exhibit small but important differences in how their principal cells represent space and other variables. Place cells in the two regions have similar spatial coverage and firing rates (Olton et al. 1978; O’Keefe and Dostrovsky 1971; Best and Ranck 1982) but CA3 is thought to be more strictly responsive to spatial location than CA1 (Barnes et al. 1990; Lee et al. 2004b; Leutgeb et al. 2005a; Knierim et al. 2006; Vazdarjanova and Guzowski 2004; Leutgeb et al. 2004, 2005b). Once CA3 places fields are established in a given environment, firing rates and spatial coverage remain stable over time (Mankin et al. 2012, 2015). CA3 ensembles also show higher sensitivity to absolute location than CA1 ensembles, as distinct populations of CA3 cells can represent distinct spatial locations, even if the local environments in those locations are visually identical (Leutgeb et al. 2004). In contrast, the fields of CA1 cells show prolonged susceptibility to modulation by sensory cues and changes in the environment (e.g., Leutgeb et al. 2004; Vazdarjanova and Guzowski 2004), and thus are more likely to globally remap their firing fields within the same environment than CA3 cells. In addition to encoding spatial information, CA1 place cells can integrate nonspatial information into their firing patterns, usually via rate remapping, to odors, objects, goals, and conditioned stimuli (Eichenbaum et al. 1987; Kobayashi et al. 1997; Kennedy and Shapiro 2009; Hok et al. 2007; Moita et al. 2003; Manns and Eichenbaum 2009; Komorowski et al. 2009; McKenzie et al. 2014; Dupret et al. 2010). The sensitivity of CA1 ensembles is further reflected in novel environments, in which CA1 firing rates start high and then decline along with the proportion of active CA1 cells as an environment becomes familiar. This tunes the population representation to a subset of CA1 neurons (Karlsson and Frank 2008).
During early exposure to an environment, place cells in both CA3 and CA1 may fire in any direction of movement through their place field (Muller et al. 1987). However, over the course of experience on a stereotyped path, such as a linear track, cells tend to develop a directional preference (McNaughton et al. 1983; Frank et al. 2004; Battaglia et al. 2004b). This directional bias is informative, as the ordered firing of unidirectional place cells enables the decoding of not only the spatial trajectory of an animal, but also the animal’s direction of movement. In addition, cells in both CA3 and CA1 are capable of developing path equivalence through experience, in which cells fire similarly in geometrically or behaviorally similar areas of a spatial maze. This path equivalence reflects an ability of CA3/CA1 neurons to generalize across related locations and episodes, rather than an inability to distinguish locations (Singer et al. 2010).
Interestingly, despite the flow of information in the canonical trisynaptic loop, the DG is not required for the spatial specificity of place fields in CA3 (McNaughton et al. 1989). Likewise, neither inactivation of CA3 nor the EC is sufficient to abolish place fields in CA1, yet both result in more diffuse, less spatially tuned place fields in CA1 (Mizumori et al. 1989; Brun et al. 2002, 2008a; Van Cauter et al. 2008; Nakashiba et al. 2008). Together, these results suggest that although CA1 place fields can be derived from either EC or CA3 input, both projections are required for robust spatial specificity. These findings also support a role for CA3 in providing a stable spatial framework onto which other types of information can be layered via the more malleable encoding seen in CA1.
6.4 CA3 and CA1 Network Activity During the Locomotor State
During locomotion, CA3 and CA1 cell activity is tightly coupled to the theta rhythm, with both regions exhibiting temporally compressed place cell sequences via theta phase precession that emerge rapidly during novel experience (Feng et al. 2015). As mentioned previously, theta phase precession is thought to be a mechanism by which online spatial encoding is compressed onto a time scale conducive to neural plasticity (Skaggs et al. 1996). Theta phase precession has likewise been proposed to promote synaptic plasticity between CA3 and CA1 cells, such that synapses are strengthened between cells with overlapping place fields via the repeated coincident firing of those cells during experience (Mehta et al. 2000, 2002; O’Neill et al. 2008; Isaac et al. 2009). Moreover, the theta phase at which a place cell fires indicates how far into the firing field the animal is, providing a temporal code for location. This temporal code, consisting of the precise timing of spikes relative to theta, exists independently from the rate code of the local network. The rate code is defined as the collective firing rates of the local ensemble, which represent both location as well as other nonspatial features due to rate remapping (Mehta et al. 2002; Jensen and Lisman 2000; Huxter et al. 2003). The addition of a temporal code to this framework thus could support precise spatial coding despite firing rates that may be highly variable (Mehta et al. 2002; Ahmed and Mehta 2009; Hopfield 1995).
The phases of theta have functional implications in terms of inputs to the circuit, possibly segregating encoding and retrieval (Hasselmo 2005; Hasselmo et al. 2002; Mizuseki et al. 2009). At the trough of theta, Schaffer collateral synapses are highly susceptible to long-term potentiation (Hyman et al. 2003; Kwag and Paulsen 2009) and receive maximal excitation from the EC, potentially facilitating the encoding of new information (Brankack et al. 1993; Kamondi et al. 1998; Mizuseki et al. 2009; Colgin et al. 2009). At the peak of theta, EC input to CA1 decreases and gives way to maximal CA3 input, but CA3 synapses onto CA1 neurons are more likely to be depressed than potentiated (Hyman et al. 2003; Kwag and Paulsen 2009). This may allow for retrieval without corrupting or restoring the retrieved information (Hasselmo 2005; Hasselmo et al. 2002; Mizuseki et al. 2009). Importantly, the theta referred to in these studies was recorded from the hippocampal fissure, which is ~180° out of phase with the theta recorded in the CA1 pyramidal cell layer (Hasselmo 2005). Recent behavioral evidence supports this hypothesis of input segregation, as inhibition of CA1 at the peak of theta enhanced spatial working memory performance when delivered during the encoding phase of the task, while inhibition during the trough improved performance during the retrieval phase of the task (Siegle and Wilson 2014). This balance of encoding and retrieval within single theta cycles could be due to the dendritic integration of CA3 and EC inputs on CA1 neurons, regulated by waxing and waning inhibition at theta frequencies (Milstein et al. 2015).
Another critical function of theta in CA3 and CA1 may be the exploration of future trajectories and goals. Early in learning, an animal will often pause at decision points on a maze and visually survey possible routes before choosing a trajectory (termed vicarious trial and error, or VTE) (Muenzinger 1938; Tolman 1938). During periods of VTE behavior, the neural representation of location sweeps ahead of the animal as distant place cells activate (Johnson and Redish 2007). These sweeps of activity within a theta cycle, called theta sequences, are distinct from the majority of theta content because they activate representations outside the animal’s current position. Similarly, nonlocal theta sequences can be predictive, reflecting the upcoming behavior of the animal. When deciding between possible reward locations, place cells representing the chosen goal location become active during theta cycles ahead of their place fields, despite often substantial distances from the animal’s current location (Wikenheiser and Redish 2015).
During ongoing locomotion, place cells may also fire at different rates in the same location depending on the animal’s future destination (prospective coding) or past locations (retrospective coding) (Frank et al. 2000; Wood et al. 2000; Ferbinteanu and Shapiro 2003; Ainge et al. 2007; Ito et al. 2015). These types of coding suggest that place field activity can encode not only absolute location, but also the animal’s position relative to an ongoing trajectory (Frank et al. 2000) and thus reflects both upcoming and past experience. Interestingly, the prospective coding phenomenon has also been observed in cells which are modulated by time rather than location (for review see Eichenbaum 2014). These “time cells” were first observed to fire at temporally specific intervals while an animal ran on a treadmill in a singular location, forming sequences which predicted the animal’s upcoming trajectory (Pastalkova et al. 2008). Time cells exhibit theta phase precession and can also have place properties, suggesting that time and place encoding coexist within theta sequences (MacDonald et al. 2011; Kraus et al. 2013). Time cell coupling to the theta rhythm has also been described in the context of odor memory in head-fixed animals, indicating that temporal encoding also exists in the absence of movement (MacDonald et al. 2013). Together the current evidence points to a role for theta in exploring future possibilities and temporally organizing spatial experience.
As mentioned earlier, gamma band activity can be found nested within theta oscillations during the locomotor state (Belluscio et al. 2012; Colgin et al. 2009; Csicsvari et al. 2003; Bragin et al. 1995a). The frequency of both slow and fast gamma bands increases with increasing speed of locomotion, as do theta frequencies, indicating locomotor-driven coupling (Ahmed and Mehta 2012). In both CA1 and CA3, slow gamma shows a subtle increase in frequency at higher velocities, while the frequency of fast gamma is strongly modulated by speed (Ahmed and Mehta 2012; Zheng et al. 2015). In addition to modulating oscillation frequency, movement speed differentially alters the power of slow and fast gamma in rats. With increasing speed, slow gamma power decreases while fast gamma power increases, suggesting continuous modulation of the circuit as the behavioral state of the animal changes (Kemere et al. 2013). However, both slow and fast gamma power were positively modulated by speed in mice, suggesting that gamma power modulation varies across species (Chen et al. 2011). The shift to higher frequencies and power of fast gamma is mirrored by an increase in MEC firing rates at faster speeds (Zheng et al. 2015), suggesting predominant engagement of CA1 by the EC at high speeds and by CA3 at low speeds (Kemere et al. 2013; Zheng et al. 2015).
The coupling of theta with slow and fast gamma has been proposed to underlie dynamic switching between difference sources of information in the hippocampus, modulated by behavioral and cognitive demands. Fast gamma, driven by the MEC, is thought to convey information about current location and state, and coincides with spiking activity enriched for place cells with place fields near the animal’s current position. In contrast, slow gamma, driven by CA3 (Scheffer-Teixeira et al. 2012; Belluscio et al. 2012; Colgin et al. 2009), may be more likely to coincide with place field spiking that represents trajectories extending beyond the current location (Zheng et al. 2016). The theta cycles containing slow gamma are likely to correspond with the nonlocal representations during VTE or trajectory planning, as mentioned above (Johnson and Redish 2007; Wikenheiser and Redish 2015). As further evidence for a role in active information processing, the phase coupling between theta and both slow and fast gamma is increased during awake locomotion as compared to REM sleep (Montgomery et al. 2008). Together, the evidence suggests that dynamic coupling between network oscillations may reflect changing cognitive demand on the hippocampal circuit during active learning and navigation (Montgomery and Buzsaki 2007; Axmacher et al. 2010; Bott et al. 2015; Igarashi et al. 2014; Colgin 2015; Tort et al. 2009).
6.5 CA3 and CA1 Network Activity During SWRs
CA1 and CA3 are the main contributors to the network activity involved in SWRs and play distinct but highly intertwined roles in supporting these events. SWRs are an intrinsic hippocampal pattern, frequently occurring in hippocampal slice preparations in which major hippocampal afferents, such as those from the EC, are generally disrupted (e.g., Maier et al. 2003). Since the discovery of replay during SWRs, hundreds of studies have investigated their origins and functional contributions to memory processes like consolidation and retrieval.
During SWRs, ensembles of neurons are reactivated in a precise, time-compressed sequence that recapitulates experience. Place cell reactivation was originally demonstrated during sleep after the animal had traversed the reactivated cell’s place field (Pavlides and Winson 1989). Ensemble reactivation during sleep was then demonstrated by the finding that pairs of cells with overlapping place fields in a previously explored environment reactivated together more frequently than pairs of cells with distant place fields (Wilson and McNaughton 1994). With improvements in analysis techniques, it became possible to observe the reactivation of more specific neuronal sequences during sleep that recapitulated awake experience (Skaggs and McNaughton 1996; Kudrimoti et al. 1999; Nadasdy et al. 1999; Lee and Wilson 2002). These replay events were shown to occur specifically during SWRs (Kudrimoti et al. 1999; Lee and Wilson 2002). Replay was also suggested to occur during periods of awake immobility (Pavlides and Winson 1989; Kudrimoti et al. 1999) and then confirmed during pauses in awake behavior (Foster and Wilson 2006; Jackson et al. 2006; O’Neill et al. 2006; Diba and Buzsaki 2007). Some studies also reported that SWRs can even occur during movement, particularly in more novel environments (O’Neill et al. 2006; Cheng and Frank 2008). The time-compressed representation of prior experience during SWRs made these network events compelling candidates for neural mechanisms of memory processes.
In support of this theory, several studies have provided causal evidence for the essential role of SWRs in learning and memory. During rest immediately following training sessions on a spatial memory task, disruption of SWRs impaired subsequent task performance and delayed task acquisition (Girardeau et al. 2009; Ego-Stengel and Wilson 2010). These results suggest a critical role for SWRs during sleep in memory consolidation. Disruption of SWRs during awake immobility also had a detrimental effect on spatial task acquisition, impairing the component of the task that most relied on linking experiences across time and making choices based on immediate past experience (Jadhav et al. 2012). This result implicates SWRs in retrieval during ongoing decision-making. Conversely, successful performance of working memory tasks can be correlated with enhanced content or increased incidence of SWRs (Dupret et al. 2010; Eschenko et al. 2006, 2008; Molle et al. 2009; Ramadan et al. 2009) or even predicted by increased place cell reactivation during SWRs (Singer et al. 2013). In addition, experiences such as the exploration of novel environments or receipt of reward increase SWR incidence both in the awake state and during sleep afterwards (Kudrimoti et al. 1999; Karlsson and Frank 2009; Eschenko et al. 2008; Cheng and Frank 2008; Singer and Frank 2009; Wu and Foster 2014), suggesting that particularly salient experiences increase SWR activity. The increase in awake SWRs may facilitate the association of novel spatial trajectories with their outcomes, while the increase in sleep SWRs may support the consolidation of those experiences through persistent reactivation and communication with the neocortex (Carr et al. 2011; O’Neill et al. 2010). Interestingly, SWRs during wakefulness tend to be more accurate in replicating past experience, while SWRs during sleep show lower fidelity (Karlsson and Frank 2009). This may relate to their proposed functional differences: awake SWRs may be critical for the rapid, accurate retrieval of stored experiences to evaluate a current decision, while sleep SWRs may rely on less accurate replay to more flexibly integrate new experiences with existing memory frameworks (Roumis and Frank 2015).
The content of SWR replay is directly dependent on prior experience (Silva et al. 2015), but can also be influenced by many factors with implications for replay function. During sleep SWRs, replay content can be biased by current odor or sound cues, despite the animal not attending to the stimulus (Bendor and Wilson 2012). During awake SWRs, replay often begins at the animal’s current location (Csicsvari et al. 2007; Karlsson and Frank 2009; Davidson et al. 2009). The length of a SWR event has been shown to correlate with the length of the trajectory replayed, and multiple SWRs can chain together with replay spanning across the chain (Davidson et al. 2009). Replay events can be either forward, exactly as the event was initially experience, or reverse, “rewinding” through the steps of a trajectory. The distinction between these two options, especially on mazes in which the animal can traverse each section in both directions, relies on the directionality of the cells involved (Diba and Buzsaki 2007; Foster and Wilson 2006). Because place field activity on a linear track becomes more unidirectional with experience (McNaughton et al. 1983; Frank et al. 2004; Battaglia et al. 2004b), unique ensembles distinguish trajectories in the two directions, differentiating forward and reverse replay (Diba and Buzsaki 2007; Foster and Wilson 2006; Gupta et al. 2010; Csicsvari et al. 2007). Forward replay is more common before an animal embarks on a trajectory, suggesting a potential role for planning or evaluating choices. In contrast, reverse replay is observed more after trajectories are completed, which may be important for associating the location of a reward with the steps taken to reach it (Diba and Buzsaki 2007). While functional and correlative studies have pointed to a role for SWRs in planning and reward associations (Pfeiffer and Foster 2013; Singer et al. 2013; Singer and Frank 2009), none have been able to address directional specificity, so the potential implications of forward and reverse replay remain unclear. In addition to the replay of previous and upcoming trajectories, replay of remote environments and distant locations has also been observed during the awake state (Karlsson and Frank 2009; Davidson et al. 2009). The diversity of SWR content likely enables the flexible consolidation and possibly retrieval functions of hippocampal replay.
Several lines of evidence indicate that SWRs are generated in CA3. During SWRs, synchronous neuronal discharge is seen first in the CA3a and b subregions, and later in CA3c and CA1, suggesting a flow of activity (Csicsvari et al. 2000). More than 10 % of the CA3 population must burst synchronously in order to significantly increase firing rates in CA1 (Csicsvari et al. 2000). This burst in CA3 activity results in the sharp wave of the SWR, a large deflection lasting ~200 ms (Buzsaki 1986) in the LFP signal thought to be caused by the massive depolarization of CA1 pyramidal cells from the influx of CA3 excitation. The sharp wave lasts the duration of the SWR (~100 ms or more; Buzsaki et al. 1992). Once discharged by CA3, the spiking of CA1 pyramidal cells then drives the firing of basket and chandelier interneurons in CA1 (Csicsvari et al. 1999b). The fast feedback between excitation and inhibition in the local network results in the characteristic high frequency ripple oscillation detected in CA1, with principal cells firing at the trough of the oscillation and interneurons firing at the peaks (Buzsaki et al. 1992; Ylinen et al. 1995; Csicsvari et al. 1999b; Cutsuridis and Taxidis 2013). Pharmacological blockade of GABAergic signaling eliminates SWRs, demonstrating a dependence on local inhibition to pace and sustain the high frequency oscillation (Stark et al. 2014). Recently, the sharp wave depolarization of CA1 dendrites was shown to be critical for the long term potentiation of CA3-to-CA1 synapses between cell pairs involved in SWR replay events (Sadowski et al. 2016), although the role of local inhibition in this plasticity remains unknown.
Strikingly, CA1 can generate and maintain brief, high frequency events even when CA3 inputs are removed (Nakashiba et al. 2009). However, these SWR-like events oscillate at a lower frequency than normal SWRs and do not show the ordered reactivation of ensembles indicative of replay (Nakashiba et al. 2009; Stark et al. 2014). This further suggests that CA3 provides important excitatory drive to CA1 that activates the pyramidal cell ensembles underlying meaningful replay events.
Surprisingly, although ensembles active during SWRs often include neurons from the CA3 and CA1 regions of both hemispheres, ripple oscillations themselves are not always coherent across subregions or hemispheres (Csicsvari et al. 1999a; Sullivan et al. 2011; Ylinen et al. 1995). This suggests that a separate network oscillation is necessary for coordinating place cell spiking activity across regions. Recently, a transient increase in slow gamma (20–50 Hz) power was observed in CA1 and CA3 during SWRs which may be an important organizational signal. Specifically, slow gamma coherence between CA3 and CA1 increases during awake SWRs and is positively correlated with higher fidelity of replay, suggesting that slow gamma may coordinate replay activity between the two regions (Carr et al. 2012). As mentioned previously, slow gamma is thought to originate in CA3 (Colgin et al. 2009) and shows highest power in the CA1 SR, where input from CA3 reaches CA1. Evidence of slow gamma in the DG also coherent with CA3 and CA1 during SWRs (Gillespie et al. 2016), suggests that slow gamma activity might coordinate SWR activity throughout the entire hippocampus. With an established role in binding ensembles at a time scale optimal for plasticity (Kopell et al. 2000; Axmacher et al. 2006; Bibbig et al. 2001; Wespatat et al. 2004; Isaac et al. 2009), slow gamma may provide the temporal organization critical for replay during SWRs (Carr et al. 2012; Colgin 2012). Furthermore, recent evidence shows that spatial trajectories during replay events do not proceed at a constant rate, but instead alternate between virtual movement and stillness in a manner time-locked to the slow gamma rhythm (Pfeiffer and Foster 2015). Together, these findings suggest that slow gamma activity in CA1 and CA3 plays an important organizational role during SWRs.
7 CA2
Relative to CA3 and CA1, CA2 has received relatively little attention in the hippocampal literature. CA2 was first characterized as a distinct hippocampal subregion due to the absence of both thorny excrescences on dendrites and afferent mossy fibers from the DG, thus distinguishing it from CA3, while its enlarged somata distinguished it from CA1 (Lorente de Nó 1934; Ishizuka et al. 1995). More recently, CA2 has been distinguished by a variety of molecular markers (San Antonio et al. 2014; Lee et al. 2010; Vellano et al. 2011; Lein et al. 2004, 2005; Zhao et al. 2001), distinct reciprocal connections with the supramammillary nucleus of the hypothalamus (Cui et al. 2013), and the expression of receptors for the neuromodulators vasopressin and adenosine (Young et al. 2006; Ochiishi et al. 1999). Partially because of these markers, there have been some recent advances in our understanding of the physiology and function of CA2. However, molecularly defined CA2 neurons are intermingled with overlapping cells from proximal CA1 and CA3a (Lein et al. 2005; Hitti and Siegelbaum 2014; Dudek et al. 2016), making it a challenge to study CA2 neurons specifically.
Electrophysiological properties of CA2 remained almost completely unexplored until recently, but it is now clear that CA2 is more than simply a relay center between CA3 and CA1 (Sekino et al. 1997; Bartesaghi and Gessi 2004; Jones and McHugh 2011; Mercer et al. 2007). Plasticity at the CA3-CA2 synapse is surprisingly hard to induce, and CA2 cells do not show changes in synaptic strength with conventional protocols of long term potentiation and depression used in CA3 and CA1 (Simons et al. 2009; Zhao et al. 2007). This is partially because CA2 cells have more negative resting membrane potentials than CA3/CA1 neurons, thus requiring greater input current to fire spikes necessary for activity-dependent synaptic plasticity (Zhao et al. 2007). In addition, CA2 interneurons provide feedback and feedforward inhibition to CA3 and CA1, which may be important for circuit function (Mercer et al. 2007, 2012; Valero et al. 2015). CA2 pyramidal neurons are also unique in their dendritic integration. Unlike CA1 neurons, CA2 neurons are more strongly excited by EC layer II synapses onto their distal dendrites than by CA3 synapses onto their proximal dendrites (Chevaleyre and Siegelbaum 2010). CA2 could thus act as a strong relay from EC to CA1, which may account for the persistence of spatial encoding in CA1 in the absence of CA3 input (Chevaleyre and Siegelbaum 2010; Nakashiba et al. 2008). This idea is supported by slice physiology demonstrating strong excitation of CA1 pyramidal cells at synapses from CA2 (Chevaleyre and Siegelbaum 2010; Kohara et al. 2014).
One of the most intriguing functional roles for CA2 is its contribution to social recognition memory (Hitti and Siegelbaum 2014; Stevenson and Caldwell 2014). This was originally proposed based on strong vasopressinergic afferents to CA2 from the paraventricular nucleus of the hypothalamus (Cui et al. 2013) and the potentiation of CA2 responses to vasopressin (Pagani et al. 2015), given vasopressin’s known role in social behavior (Wersinger et al. 2002, 2004; DeVito et al. 2009). This role was specifically tested in mice with inhibited CA2 synaptic output. These mice were significantly impaired in their ability to recognize and distinguish familiar companion mice from novel mice. However, spatial working memory and general sociability was left intact (Hitti and Siegelbaum 2014). The relevance of social experience to CA2 is further supported by recent findings showing that social experience prompts CA2 to globally remap in the absence of any environmental change (Alexander et al. 2016). These studies are the first to link CA2 neural activity with behavioral consequences.
7.1 Cell Characteristics of CA2
CA2 place cells were previously thought to be functionally indistinguishable from CA1 place cells (Martig and Mizumori 2011). Recent studies, however, have suggested unique encoding properties of CA2 cells. Compared to CA1 and CA3 place cells, cells recorded in CA2 tend to have higher mean firing rates, larger spatial coverage of the environment and more spatial firing fields per cell (Lu et al. 2015; Mankin et al. 2015). In addition, they exhibit lower preference for spatial context, such that putative CA2 cells distinguish different environments much more weakly than do CA1 and CA3 cells. Notably, a recent study reports that the CA2 population representation of similar environments decorrelates over extended periods of time, indicating that CA2 cells may facilitate the encoding of similar memory episodes occurring at different time points (Mankin et al. 2015).
Recent evidence suggests that the CA2 neurons described by Mankin and colleagues may comprise a subpopulation of the neurons found at the CA2 anatomical locus. In addition to these neurons, there also exists a subpopulation of cells with spatially specific fields that are predominantly active during periods of low velocity and immobility. These neurons located in CA2 encode the animal’s location in the absence of movement, and continue to encode for location during periods of sleep characterized by desynchronized activity (Kay et al. 2016).
7.2 CA2 Network Activity
Very little is known about network activity in CA2. The frequency of theta detected in CA2 is similar to CA1, and CA2 neurons exhibit comparable levels of theta modulation to CA1 neurons (Kay et al. 2016; Mankin et al. 2015), with slightly slower theta phase precession in cells with enlarged place fields (Mankin et al. 2015). During SWRs, recent findings have highlighted a potentially unique activity pattern in CA2. In a study of a small population of cells, Valero et al. (2015) demonstrated that CA2 neurons hyperpolarize during SWRs in vivo while CA1 and CA3 neurons are depolarized and excited. In line with this evidence, it has been hypothesized that CA2 would be largely suppressed during strong activation of CA3 as is thought to occur during SWRs (Jones and McHugh 2011). Notably, the subpopulation of neurons located in CA2 that were uniquely active during immobility were found to be either unmodulated or negatively modulated by SWRs, raising the possibility that lack of participation in SWRs is a defining property of CA2 neurons (Kay et al. 2016).
8 Subiculum
The subiculum is a major site of hippocampal output and is responsible for distributing information received from CA1 pyramidal cells to the neocortex and subcortical structures, as its projections are generally much more numerous than those directly from CA1 (Meibach and Siegel 1977; Rosene and Van Hoesen 1977; Swanson and Cowan 1977; O’Mara 2006). Lesions of the subiculum result in comparable impairment of learning and memory performance to lesions of the hippocampus proper (Morris et al. 1990). Lesions of both hippocampus and subiculum cause a more severe impairment than either alone, suggesting that the subiculum adds a layer of functionality rather than simply relaying hippocampal output (Morris et al. 1990; Potvin et al. 2006, 2007). The dorsal subiculum is thought to be particularly important for spatial memory, and evidence exists to suggest that subicular neurons integrate many layers of information from CA1 and project rich representations to downstream areas. In addition, the ventral subiculum, with substantial inputs from and outputs to subcortical areas, plays a key role in the inhibition of the hypothalamic–-pituitary–adrenal axis, thus serving as the interface between hippocampal mnemonic activity and the limbic stress response (O’Mara 2006).
8.1 Cell Characteristics of the Subiculum
Historically, subicular principal cells have often been categorized into two populations: bursting cells and regular firing cells (Witter 2006). It has been suggested that bursting cells are more likely to target more spatial areas, like the MEC, while regular spiking cells may send more projections to the amygdala and LEC (Kim and Spruston 2012). While some evidence suggests that subicular neurons lie on a spectrum of “burstiness” regardless of their location in the subiculum (Kim et al. 2012), other findings indicate that burstiness, as well as firing rates and spatial tuning, depends on the cell’s position within the subiculum relative to CA1 (Staff et al. 2000; Sharp and Green 1994; Sharp 2006; Jarsky et al. 2008). Indeed, firing patterns of subicular neurons differ substantially along the proximal-distal axis (Sharp 1996), with pyramidal cells in the proximal subiculum (nearest CA1) exhibiting lower firing rates and relatively smaller firing fields, similar to CA1 place cells, than neurons in the distal subiculum (Kim et al. 2012). The dispersed but rate-modulated firing fields of distal subicular neurons allow them to encode more spatial information than CA1 place cells, and may enable the efficient transfer of this information to the neocortex (Kim et al. 2012). Further in support of this idea, many CA1 neurons are thought to converge onto single subicular neurons, potentially explaining their complex rather than simply location-modulated firing fields. This may allow the subiculum to integrate dispersed CA1 information into a more compressed, rich representation for broad dispersal throughout the brain (O’Mara 2005; Deadwyler and Hampson 2004).
8.2 Subiculum Network Activity
Although generally understudied, several findings inform our understanding of network activity in the subiculum during various behavioral states. During the locomotor state, subicular neurons show robust phase precession similar to CA1 (Kim et al. 2012). During awake immobility and rest, SWRs can be detected in subiculum at the same time as in CA1 (Chrobak and Buzsaki 1994, 1996; Bohm et al. 2015). SWRs differentially modulate populations of subicular neurons; bursting cells tend to be activated during SWRs, while regular-firing cells are suppressed by SWRs (Bohm et al. 2015; Eller et al. 2015). The isolated subiculum has been shown to generate both slow and fast gamma oscillations in vitro (Jackson et al. 2011), suggesting that the subiculum may participate in gamma activity during the locomotor state and SWRs. Beyond this, little is understood of how the subiculum engages in and contributes to network activity in order to facilitate widespread distribution of hippocampal output.
9 Hippocampal Function Along the Dorsoventral Axis
The vast majority of our knowledge about hippocampal activity comes from studies of the dorsal hippocampus (dHPC). This is primarily because it is easily accessible for electrophysiological recording, whereas recording from a deep brain structure such as ventral hippocampus (vHPC) is more technically challenging. Thus far, the hypothesized functional distinction between dHPC and vHPC (which assigns them roles in spatial in spatial and emotional processing, respectively) comes mostly from anatomical connectivity, lesion studies, differences in the spatial specificity of each region’s place fields, and differential genetic expression (Moser and Moser 1998; Dong et al. 2009; Fanselow and Dong 2010; Strange et al. 2014). In particular, genetic markers are expressed in a gradient along the dorsoventral axis and highly differentiates ventral pyramidal neurons from dorsal pyramidal neurons (Cembrowski et al. 2016). Dorsal HPC receives more visual cortical inputs via the EC, while vHPC receives more olfactory and gustatory inputs. In terms of outputs, dHPC projects directly to the deep layers of the EC and mostly indirectly, via the subiculum, to other cortical areas such as the retrosplenial cortex and prefrontal cortices. In contrast, vHPC projects not only to the deep layers of the EC, but also heavily and directly to the medial prefrontal cortex, orbitofrontal cortex, olfactory and auditory cortices, amygdala (Cenquizca and Swanson 2007), nucleus accumbens (Groenewegen et al. 1987; Brog et al. 1993), and hypothalamus (Cenquizca and Swanson 2006). These distinct anatomical outputs suggest that vHPC dominates the hippocampal innervation of areas associated with processing emotional information, such as anxiety and reward. Lesion studies further implicate the dHPC in spatial learning to a greater extent than vHPC (Moser et al. 1993, 1995; Pothuizen et al. 2004; Ferbinteanu and McDonald 2001; Bannerman et al. 1999; Richmond et al. 1999), which is implicated in anxiety (Kjelstrup et al. 2002; Bannerman et al. 2004; Henke 1990; Weeden et al. 2015; Wang et al. 2013; Zhang et al. 2001). However, the results of these studies may depend on the animal’s training protocol, such that the vHPC may be sufficient for spatial learning over longer time periods while dHPC may be required for rapid acquisition of spatial tasks (de Hoz et al. 2003; Loureiro et al. 2012; Bast 2007; Bast et al. 2009). Ventral HPC may also be important for developing representations of environmental context over time (Komorowski et al. 2013) and transferring spatial knowledge across contexts to facilitate learning in new environments based on prior experience (de Hoz and Martin 2014).
9.1 Cell Characteristics Across the Dorsoventral Axis
The landmark electrophysiological finding that distinguished dHPC and vHPC is that place fields increase gradually in size along the dorsoventral axis, spatially restricted in dHPC and reaching vast spatial coverage at the ventral pole (Poucet et al. 1994; Jung et al. 1994; Kjelstrup et al. 2008; Royer et al. 2010; Komorowski et al. 2013; Ciocchi et al. 2015). This is consistent with an increase in the spacing of grid cell fields along the dorsoventral axis of entorhinal cortex (Stensola et al. 2012; Brun et al. 2008b). From these findings it was hypothesized that vHPC could not accurately encode for spatial information using cells with such little spatial specificity. Recently, however, a study of ventral CA1 in mice suggested that the distributed representations of space in vHPC may actually be ideal for transmitting spatial information to downstream regions (Keinath et al. 2014). While single cell spatial selectivity is lower in ventral CA1 due to large place field sizes, the population of ventral CA1 cells encodes an animal’s location just as accurately as the dorsal CA1 population. This is possible via population coding: with some overlap in cells’ place fields, each spatial location will be represented by a specific subset of cells firing at specific rates (Keinath et al. 2014; Kim et al. 2012; Osborne et al. 2008; Olypher et al. 2003). In addition to spatial information, non-spatial correlates seem to increase in strength in vHPC, in contrast to dHPC where representation of variables other than place is limited. For example, some ventral CA3 cells are active in motivationally related, but spatially distinct, areas of an environment such as reward sites (Royer et al. 2010; Ciocchi et al. 2015). vHPC cells also tend to represent contextual similarity between locations (Royer et al. 2010) in a manner that develops with experience (Komorowski et al. 2013). In addition, place cells in vHPC have been shown to track changes in olfactory stimuli (Keinath et al. 2014; Petrulis et al. 2005) and may represent locations associated with elevated anxiety (Royer et al. 2010; Ciocchi et al. 2015). While the functional implications of these representations remain largely unexplored, optogenetic manipulation studies point to a role in anxiety behaviors for the ventral HPC (Kheirbek et al. 2013) and its projections to the medial prefrontal cortex (Padilla-Coreano et al. 2016) and a role for vHPC projections to the nucleus accumbens in promoting reward associations (Britt et al. 2012).
9.2 Network Activity Across the Dorsoventral Axis
Although few studies have recorded simultaneous network activity in dHPC and vHPC, several differences have been observed which may have functional relevance. Notably, the theta rhythm is weaker in power in vHPC and less modulated by run speed than in dHPC (Patel et al. 2012; Royer et al. 2010; Schmidt et al. 2013). However, vHPC place cells still exhibit theta phase precession even though they are large, with smaller incremental phase changes such that phase precession persists across the extent of the place field (Kjelstrup et al. 2008). vHPC theta is also shifted 180° relative to dHPC theta, suggesting that theta may be a wave that propagates along the dorsoventral axis (Patel et al. 2012; Lubenov and Siapas 2009). How this “traveling wave” might coordinate activity across the axis in the context of learning and memory remains an open question.
SWRs in vHPC are largely similar in their fundamental characteristics to SWRs in dHPC, with the exception that SWRs in vHPC are typically smaller in amplitude and slightly lower in oscillation frequency (Patel et al. 2013). This is likely due to the more diffuse pyramidal cell layers and lower burstiness of vHPC cells (Royer et al. 2010; Fanselow and Dong 2010). Ventral HPC SWRs tend to occur independently of dHPC SWRs in sleep, although they can also propagate along the entire dorsoventral axis in either direction. The degree of propagation, or synchrony, between dHPC and vHPC may depend on the amplitude of the SWR (Patel et al. 2013), indicating that larger SWRs engage a greater portion of the hippocampus and therefore may reflect increased cognitive demand during behavior. Interestingly, cells of ventral CA1 with tri-directional projections to the medial prefrontal cortex, amygdala, and nucleus accumbens are activated during SWRs in sleep to a greater degree than cells that project to only one or two of those regions (Ciocchi et al. 2015). This suggests that SWRs in vHPC are indeed important for integrating information and synchronizing transfer to distant hippocampal targets. The characteristics and function of vHPC SWRs in the awake state have yet to be explored.
10 Hippocampal Output
Neural activity time-locked to both hippocampal theta and SWRs has been observed in the downstream targets of the hippocampus, suggesting that these network patterns facilitate the integration of spatial information with other modalities. Theta has been posited as a coordinator of brain regions particularly during periods of attention and working memory. Theta power is positively correlated not only with velocity (Montgomery et al. 2009) but also with working memory demands (Belchior et al. 2014; Richard et al. 2013; Schmidt et al. 2013; Tesche and Karhu 2000). Moreover, theta coherence increases between hippocampus and its projection targets (such as mPFC) during coding phases of working memory tasks (Benchenane et al. 2010; Harris and Gordon 2015; Backus et al. 2016). In addition, phase precession relative to hippocampal theta has been observed in the prefrontal cortex (Jones and Wilson 2005; Siapas et al. 2005) as well as in spatially modulated, reward-predictive cells of the nucleus accumbens (van der Meer and Redish 2011; Malhotra et al. 2012). These results point to theta as a mechanism for broadcasting spatial information to downstream regions and coordinating spatial and non-spatial representations across distant brain areas.
There is also substantial evidence that SWRs engage not only the hippocampus but also its projection targets. SWRs can be detected in the deep layers of the EC following the occurrence of CA1 SWRs (Chrobak and Buzsaki 1994, 1996). Strikingly, SWRs also modulate cell ensembles in distant brain regions, including prefrontal cortex (Siapas and Wilson 1998; Wierzynski et al. 2009; Jadhav et al. 2016), visual cortex (Ji and Wilson 2007), nucleus accumbens (Lansink et al. 2008, 2009), and the ventral tegmental area (Gomperts et al. 2015). Activity time-locked to SWRs recorded elsewhere in the brain suggests that the information contained in SWRs is being communicated or integrated with other memory-related modalities (Chrobak and Buzsaki 1996; Siapas and Wilson 1998; Wierzynski et al. 2009; Sirota et al. 2003). SWR-triggered whole brain MRI in monkeys shows widespread activation of cortical areas and coincident suppression of subcortical areas (Logothetis et al. 2012), and the modulation of cohesive functional networks that have been associated with memory processes (Kaplan et al. 2016). These findings point to a pivotal role for SWRs in coordinating memory processes across the entire brain.
11 Conclusion
Since the identification of the hippocampus as a critical neural center for spatial mnemonic processing, our understanding of memory has expanded in stride with developments in neural recording techniques and subsequent discoveries of hippocampal network activity. In particular, by identifying the spatial aspect of memory as a key to decoding hippocampal activity, and beginning to tease apart the unique contributions of each hippocampal subregion to spatial representations, we can begin to understand the patterns of coordinated neural activity that underlie memory function. In this chapter, we have highlighted two key patterns of activity, theta and sharp-wave ripples, which are largely distinct during exploratory activity and quiescence and specialized within each subregion, but that both coordinate the activation and reactivation of neuronal ensembles with high temporal precision. Although our understanding of these patterns focuses largely on spatial memory, we are gradually appreciating the ability of the hippocampus to build relational maps between diverse types of information. Dense, large scale single unit and LFP recordings, optical recording methods, optogenetics, and many other developing techniques will continue to expand our ability to characterize and manipulate hippocampal network activity in order to further our grasp on hippocampal mnemonic processing.
Acknowledgments
We wish to thank members of the Frank lab for their careful reviews and constructive comments on this book chapter. In particular, we thank Kenny Kay for the hippocampal recording data displayed in Fig. 2.
Contributor Information
Marielena Sosa, Kavli Institute for Fundamental Neuroscience and Department of Physiology, University of California, San Francisco, USA.
Anna K. Gillespie, Kavli Institute for Fundamental Neuroscience and Department of Physiology, University of California, San Francisco, USA
Loren M. Frank, Kavli Institute for Fundamental Neuroscience and Department of Physiology, University of California, San Francisco, USA, Howard Hughes Medical Institute, Maryland, USA
References
- Ahmed OJ, Mehta MR. The hippocampal rate code: anatomy, physiology and theory. Trends Neurosci. 2009;32(6):329–338. doi: 10.1016/j.tins.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed OJ, Mehta MR. Running speed alters the frequency of hippocampal gamma oscillations. J Neurosci. 2012;32(21):7373–7383. doi: 10.1523/JNEUROSCI.5110-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainge JA, Tamosiunaite M, Woergoetter F, Dudchenko PA. Hippocampal CA1 place cells encode intended destination on a maze with multiple choice points. J Neurosci. 2007;27(36):9769–9779. doi: 10.1523/JNEUROSCI.2011-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GM, Farris S, Pirone JR, Zheng C, Colgin LL, Dudek SM. Social and novel contexts modify hippocampal CA2 representations of space. Nat Commun. 2016;7:10300. doi: 10.1038/ncomms10300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen K, Rawlins JN, Bannerman DM, Csicsvari J. Hippocampal place cells can encode multiple trial-dependent features through rate remapping. J Neurosci. 2012;32(42):14752–14766. doi: 10.1523/JNEUROSCI.6175-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen TA, Salz DM, McKenzie S, Fortin NJ. Nonspatial sequence coding in CA1 neurons. J Neurosci. 2016;36(5):1547–1563. doi: 10.1523/JNEUROSCI.2874-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, Dolorfo C, Alvarez-Royo P. Organization of CA1 projections to the subiculum: a PHA-L analysis in the rat. Hippocampus. 1991;1(4):415–435. doi: 10.1002/hipo.450010410. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Ishizuka N, Claiborne B. Neurons, numbers and the hippocampal network. Prog Brain Res. 1990;83:1–11. doi: 10.1016/s0079-6123(08)61237-6. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Kurz J. An analysis of the origins of the cholinergic and noncholinergic septal projections to the hippocampal formation of the rat. J Comp Neurol. 1985;240(1):37–59. doi: 10.1002/cne.902400104. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31(3):571–591. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- Anderson MI, Jeffery KJ. Heterogeneous modulation of place cell firing by changes in context. J Neurosci. 2003;23(26):8827–8835. doi: 10.1523/JNEUROSCI.23-26-08827.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnolds DE, Lopes da Silva FH, Aitink JW, Kamp A, Boeijinga P. The spectral properties of hippocampal EEG related to behaviour in man. Electroencephalogr Clin Neurophysiol. 1980;50(3–4):324–328. doi: 10.1016/0013-4694(80)90160-1. [DOI] [PubMed] [Google Scholar]
- Axmacher N, Elger CE, Fell J. Ripples in the medial temporal lobe are relevant for human memory consolidation. Brain. 2008;131(Pt 7):1806–1817. doi: 10.1093/brain/awn103. [DOI] [PubMed] [Google Scholar]
- Axmacher N, Henseler MM, Jensen O, Weinreich I, Elger CE, Fell J. Cross-frequency coupling supports multi-item working memory in the human hippocampus. Proc Natl Acad Sci USA. 2010;107(7):3228–3233. doi: 10.1073/pnas.0911531107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axmacher N, Mormann F, Fernandez G, Elger CE, Fell J. Memory formation by neuronal synchronization. Brain Res Rev. 2006;52(1):170–182. doi: 10.1016/j.brainresrev.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Backus AR, Schoffelen JM, Szebenyi S, Hanslmayr S, Doeller CF. Hippocampal-prefrontal theta oscillations support memory integration. Curr Biol. 2016;26(4):450–457. doi: 10.1016/j.cub.2015.12.048. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, Zhang WN, Pothuizen HH, Feldon J. Regional dissociations within the hippocampus–memory and anxiety. Neurosci Biobehav Rev. 2004;28(3):273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Yee BK, Good MA, Heupel MJ, Iversen SD, Rawlins JN. Double dissociation of function within the hippocampus: a comparison of dorsal, ventral, and complete hippocampal cytotoxic lesions. Behav Neurosci. 1999;113(6):1170–1188. doi: 10.1037//0735-7044.113.6.1170. [DOI] [PubMed] [Google Scholar]
- Barnes CA, McNaughton BL, Mizumori SJ, Leonard BW, Lin LH. Comparison of spatial and temporal characteristics of neuronal activity in sequential stages of hippocampal processing. Prog Brain Res. 1990;83:287–300. doi: 10.1016/s0079-6123(08)61257-1. [DOI] [PubMed] [Google Scholar]
- Bartesaghi R, Gessi T. Parallel activation of field CA2 and dentate gyrus by synaptically elicited perforant path volleys. Hippocampus. 2004;14(8):948–963. doi: 10.1002/hipo.20011. [DOI] [PubMed] [Google Scholar]
- Bast T. Toward an integrative perspective on hippocampal function: from the rapid encoding of experience to adaptive behavior. Rev Neurosci. 2007;18(3–4):253–281. doi: 10.1515/revneuro.2007.18.3-4.253. [DOI] [PubMed] [Google Scholar]
- Bast T, Wilson IA, Witter MP, Morris RG. From rapid place learning to behavioral performance: a key role for the intermediate hippocampus. PLoS Biol. 2009;7(4):e1000089. doi: 10.1371/journal.pbio.1000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia FP, Sutherland GR, McNaughton BL. Hippocampal sharp wave bursts coincide with neocortical “up-state” transitions. Learn Mem. 2004a;11(6):697–704. doi: 10.1101/lm.73504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia FP, Sutherland GR, McNaughton BL. Local sensory cues and place cell directionality: additional evidence of prospective coding in the hippocampus. J Neurosci. 2004b;24(19):4541–4550. doi: 10.1523/JNEUROSCI.4896-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckstead RM. Afferent connections of the entorhinal area in the rat as demonstrated by retrograde cell-labeling with horseradish peroxidase. Brain Res. 1978;152(2):249–264. doi: 10.1016/0006-8993(78)90254-8. [DOI] [PubMed] [Google Scholar]
- Belchior H, Lopes-Dos-Santos V, Tort AB, Ribeiro S. Increase in hippocampal theta oscillations during spatial decision making. Hippocampus. 2014;24(6):693–702. doi: 10.1002/hipo.22260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluscio MA, Mizuseki K, Schmidt R, Kempter R, Buzsaki G. Cross-frequency phase-phase coupling between theta and gamma oscillations in the hippocampus. J Neurosci. 2012;32(2):423–435. doi: 10.1523/JNEUROSCI.4122-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benchenane K, Peyrache A, Khamassi M, Tierney PL, Gioanni Y, Battaglia FP, Wiener SI. Coherent theta oscillations and reorganization of spike timing in the hippocampal- prefrontal network upon learning. Neuron. 2010;66(6):921–936. doi: 10.1016/j.neuron.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Bendor D, Wilson MA. Biasing the content of hippocampal replay during sleep. Nat Neurosci. 2012;15(10):1439–1444. doi: 10.1038/nn.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best PJ, Ranck JB., Jr Reliability of the relationship between hippocampal unit activity and sensory-behavioral events in the rat. Exp Neurol. 1982;75(3):652–664. doi: 10.1016/0014-4886(82)90032-2. [DOI] [PubMed] [Google Scholar]
- Bibbig A, Faulkner HJ, Whittington MA, Traub RD. Self-organized synaptic plasticity contributes to the shaping of gamma and beta oscillations in vitro. J Neurosci. 2001;21(22):9053–9067. doi: 10.1523/JNEUROSCI.21-22-09053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner KC, Grienberger C, Vaidya SP, Milstein AD, Macklin JJ, Suh J, Tonegawa S, Magee JC. Conjunctive input processing drives feature selectivity in hippocampal CA1 neurons. Nat Neurosci. 2015;18(8):1133–1142. doi: 10.1038/nn.4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohm C, Peng Y, Maier N, Winterer J, Poulet JF, Geiger JR, Schmitz D. Functional diversity of subicular principal cells during hippocampal ripples. J Neurosci. 2015;35(40):13608–13618. doi: 10.1523/JNEUROSCI.5034-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnevie T, Dunn B, Fyhn M, Hafting T, Derdikman D, Kubie JL, Roudi Y, Moser EI, Moser MB. Grid cells require excitatory drive from the hippocampus. Nat Neurosci. 2013;16(3):309–317. doi: 10.1038/nn.3311. [DOI] [PubMed] [Google Scholar]
- Bott JB, Muller MA, Jackson J, Aubert J, Cassel JC, Mathis C, Goutagny R. Spatial reference memory is associated with modulation of theta-gamma coupling in the dentate gyrus. Cereb Cortex. 2015 doi: 10.1093/cercor/bhv177. [DOI] [PubMed] [Google Scholar]
- Bragin A, Jando G, Nadasdy Z, Hetke J, Wise K, Buzsaki G. Gamma (40–100 Hz) oscillation in the hippocampus of the behaving rat. J Neurosci. 1995a;15(1 Pt 1):47–60. doi: 10.1523/JNEUROSCI.15-01-00047.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin A, Jando G, Nadasdy Z, van Landeghem M, Buzsaki G. Dentate EEG spikes and associated interneuronal population bursts in the hippocampal hilar region of the rat. J Neurophysiol. 1995b;73(4):1691–1705. doi: 10.1152/jn.1995.73.4.1691. [DOI] [PubMed] [Google Scholar]
- Brandon MP, Koenig J, Leutgeb JK, Leutgeb S. New and distinct hippocampal place codes are generated in a new environment during septal inactivation. Neuron. 2014;82(4):789–796. doi: 10.1016/j.neuron.2014.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brankack J, Stewart M, Fox SE. Current source density analysis of the hippocampal theta rhythm: associated sustained potentials and candidate synaptic generators. Brain Res. 1993;615(2):310–327. doi: 10.1016/0006-8993(93)90043-m. [DOI] [PubMed] [Google Scholar]
- Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA, Bonci A. Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron. 2012;76(4):790–803. doi: 10.1016/j.neuron.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brog JS, Salyapongse A, Deutch AY, Zahm DS. The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol. 1993;338(2):255–278. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- Brun VH, Leutgeb S, Wu HQ, Schwarcz R, Witter MP, Moser EI, Moser MB. Impaired spatial representation in CA1 after lesion of direct input from entorhinal cortex. Neuron. 2008a;57(2):290–302. doi: 10.1016/j.neuron.2007.11.034. [DOI] [PubMed] [Google Scholar]
- Brun VH, Otnass MK, Molden S, Steffenach HA, Witter MP, Moser MB, Moser EI. Place cells and place recognition maintained by direct entorhinal-hippocampal circuitry. Science. 2002;296(5576):2243–2246. doi: 10.1126/science.1071089. [DOI] [PubMed] [Google Scholar]
- Brun VH, Solstad T, Kjelstrup KB, Fyhn M, Witter MP, Moser EI, Moser MB. Progressive increase in grid scale from dorsal to ventral medial entorhinal cortex. Hippocampus. 2008b;18(12):1200–1212. doi: 10.1002/hipo.20504. [DOI] [PubMed] [Google Scholar]
- Buckmaster PS, Wenzel HJ, Kunkel DD, Schwartzkroin PA. Axon arbors and synaptic connections of hippocampal mossy cells in the rat in vivo. J Comp Neurol. 1996;366(2):271–292. doi: 10.1002/(sici)1096-9861(19960304)366:2<270::aid-cne7>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Bullock TH, Buzsaki G, McClune MC. Coherence of compound field potentials reveals discontinuities in the CA1-subiculum of the hippocampus in freely-moving rats. Neuroscience. 1990;38(3):609–619. doi: 10.1016/0306-4522(90)90055-9. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Hippocampal sharp waves: their origin and significance. Brain Res. 1986;398(2):242–252. doi: 10.1016/0006-8993(86)91483-6. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Two-stage model of memory trace formation: a role for “noisy” brain states. Neuroscience. 1989;31(3):551–570. doi: 10.1016/0306-4522(89)90423-5. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33(3):325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Large-scale recording of neuronal ensembles. Nat Neurosci. 2004;7(5):446–451. doi: 10.1038/nn1233. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Hippocampal sharp wave-ripple: a cognitive biomarker for episodic memory and planning. Hippocampus. 2015 doi: 10.1002/hipo.22488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Anastassiou CA, Koch C. The origin of extracellular fields and currents–EEG, ECoG, LFP and spikes. Nat Rev Neurosci. 2012;13(6):407–420. doi: 10.1038/nrn3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Buhl DL, Harris KD, Csicsvari J, Czeh B, Morozov A. Hippocampal network patterns of activity in the mouse. Neuroscience. 2003;116(1):201–211. doi: 10.1016/s0306-4522(02)00669-3. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Czopf J, Kondakor I, Kellenyi L. Laminar distribution of hippocampal rhythmic slow activity (RSA) in the behaving rat: current-source density analysis, effects of urethane and atropine. Brain Res. 1986;365(1):125–137. doi: 10.1016/0006-8993(86)90729-8. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Horvath Z, Urioste R, Hetke J, Wise K. High-frequency network oscillation in the hippocampus. Science. 1992;256(5059):1025–1027. doi: 10.1126/science.1589772. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Leung LW, Vanderwolf CH. Cellular bases of hippocampal EEG in the behaving rat. Brain Res. 1983;287(2):139–171. doi: 10.1016/0165-0173(83)90037-1. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Wang XJ. Mechanisms of gamma oscillations. Annu Rev Neurosci. 2012;35:203–225. doi: 10.1146/annurev-neuro-062111-150444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr MF, Jadhav SP, Frank LM. Hippocampal replay in the awake state: a potential substrate for memory consolidation and retrieval. Nat Neurosci. 2011;14(2):147–153. doi: 10.1038/nn.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr MF, Karlsson MP, Frank LM. Transient slow gamma synchrony underlies hippocampal memory replay. Neuron. 2012;75(4):700–713. doi: 10.1016/j.neuron.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cembrowski MS, Bachman JL, Wang L, Sugino K, Shields BC, Spruston N. Spatial gene-expression gradients underlie prominent heterogeneity of CA1 pyramidal neurons. Neuron. 2016 doi: 10.1016/j.neuron.2015.12.013. [DOI] [PubMed] [Google Scholar]
- Cenquizca LA, Swanson LW. Analysis of direct hippocampal cortical field CA1 axonal projections to diencephalon in the rat. J Comp Neurol. 2006;497(1):101–114. doi: 10.1002/cne.20985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenquizca LA, Swanson LW. Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex. Brain Res Rev. 2007;56(1):1–26. doi: 10.1016/j.brainresrev.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Resnik E, McFarland JM, Sakmann B, Mehta MR. Speed controls the amplitude and timing of the hippocampal gamma rhythm. PLoS ONE. 2011;6(6):e21408. doi: 10.1371/journal.pone.0021408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S, Frank LM. New experiences enhance coordinated neural activity in the hippocampus. Neuron. 2008;57(2):303–313. doi: 10.1016/j.neuron.2007.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre V, Siegelbaum SA. Strong CA2 pyramidal neuron synapses define a powerful disynaptic cortico-hippocampal loop. Neuron. 2010;66(4):560–572. doi: 10.1016/j.neuron.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrobak JJ, Buzsaki G. Selective activation of deep layer (V–VI) retrohippocampal cortical neurons during hippocampal sharp waves in the behaving rat. J Neurosci. 1994;14(10):6160–6170. doi: 10.1523/JNEUROSCI.14-10-06160.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrobak JJ, Buzsaki G. High-frequency oscillations in the output networks of the hippocampal-entorhinal axis of the freely behaving rat. J Neurosci. 1996;16(9):3056–3066. doi: 10.1523/JNEUROSCI.16-09-03056.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocchi S, Passecker J, Malagon-Vina H, Mikus N, Klausberger T. Brain computation. Selective information routing by ventral hippocampal CA1 projection neurons. Science. 2015;348(6234):560–563. doi: 10.1126/science.aaa3245. [DOI] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325(5937):210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin LL. Slow gamma takes the reins in replay. Neuron. 2012;75(4):549–550. doi: 10.1016/j.neuron.2012.08.006. [DOI] [PubMed] [Google Scholar]
- Colgin LL. Theta-gamma coupling in the entorhinal-hippocampal system. Curr Opin Neurobiol. 2015;31:45–50. doi: 10.1016/j.conb.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin LL, Denninger T, Fyhn M, Hafting T, Bonnevie T, Jensen O, Moser MB, Moser EI. Frequency of gamma oscillations routes flow of information in the hippocampus. Nature. 2009;462(7271):353–357. doi: 10.1038/nature08573. [DOI] [PubMed] [Google Scholar]
- Colgin LL, Leutgeb S, Jezek K, Leutgeb JK, Moser EI, McNaughton BL, Moser MB. Attractor-map versus autoassociation based attractor dynamics in the hippocampal network. J Neurophysiol. 2010;104(1):35–50. doi: 10.1152/jn.00202.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravens CJ, Vargas-Pinto N, Christian KM, Nakazawa K. CA3 NMDA receptors are crucial for rapid and automatic representation of context memory. Eur J Neurosci. 2006;24(6):1771–1780. doi: 10.1111/j.1460-9568.2006.05044.x. [DOI] [PubMed] [Google Scholar]
- Creer DJ, Romberg C, Saksida LM, van Praag H, Bussey TJ. Running enhances spatial pattern separation in mice. Proc Natl Acad Sci USA. 2010;107(5):2367–2372. doi: 10.1073/pnas.0911725107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csicsvari J, Hirase H, Czurko A, Mamiya A, Buzsaki G. Fast network oscillations in the hippocampal CA1 region of the behaving rat. J Neurosci. 1999a;19(16):RC20. doi: 10.1523/JNEUROSCI.19-16-j0001.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csicsvari J, Hirase H, Czurko A, Mamiya A, Buzsaki G. Oscillatory coupling of hippocampal pyramidal cells and interneurons in the behaving rat. J Neurosci. 1999b;19(1):274–287. doi: 10.1523/JNEUROSCI.19-01-00274.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csicsvari J, Hirase H, Mamiya A, Buzsaki G. Ensemble patterns of hippocampal CA3-CA1 neurons during sharp wave-associated population events. Neuron. 2000;28(2):585–594. doi: 10.1016/s0896-6273(00)00135-5. [DOI] [PubMed] [Google Scholar]
- Csicsvari J, Jamieson B, Wise KD, Buzsaki G. Mechanisms of gamma oscillations in the hippocampus of the behaving rat. Neuron. 2003;37(2):311–322. doi: 10.1016/s0896-6273(02)01169-8. [DOI] [PubMed] [Google Scholar]
- Csicsvari J, O’Neill J, Allen K, Senior T. Place-selective firing contributes to the reverse-order reactivation of CA1 pyramidal cells during sharp waves in open-field exploration. Eur J Neurosci. 2007;26(3):704–716. doi: 10.1111/j.1460-9568.2007.05684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Z, Gerfen CR, Young WS., 3rd Hypothalamic and other connections with dorsal CA2 area of the mouse hippocampus. J Comp Neurol. 2013;521(8):1844–1866. doi: 10.1002/cne.23263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutsuridis V, Taxidis J. Deciphering the role of CA1 inhibitory circuits in sharp wave-ripple complexes. Front Syst Neurosci. 2013;7:13. doi: 10.3389/fnsys.2013.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielson NB, Kaifosh P, Zaremba JD, Lovett-Barron M, Tsai J, Denny CA, Balough EM, Goldberg AR, Drew LJ, Hen R, Losonczy A, Kheirbek MA. Distinct contribution of adult-born hippocampal granule cells to context encoding. Neuron. 2016 doi: 10.1016/j.neuron.2016.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TJ, Kloosterman F, Wilson MA. Hippocampal replay of extended experience. Neuron. 2009;63(4):497–507. doi: 10.1016/j.neuron.2009.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hoz L, Knox J, Morris RG. Longitudinal axis of the hippocampus: both septal and temporal poles of the hippocampus support water maze spatial learning depending on the training protocol. Hippocampus. 2003;13(5):587–603. doi: 10.1002/hipo.10079. [DOI] [PubMed] [Google Scholar]
- de Hoz L, Martin SJ. Double dissociation between the contributions of the septal and temporal hippocampus to spatial learning: the role of prior experience. Hippocampus. 2014;24(8):990–1005. doi: 10.1002/hipo.22285. [DOI] [PubMed] [Google Scholar]
- de Lavilleon G, Lacroix MM, Rondi-Reig L, Benchenane K. Explicit memory creation during sleep demonstrates a causal role of place cells in navigation. Nat Neurosci. 2015;18(4):493–495. doi: 10.1038/nn.3970. [DOI] [PubMed] [Google Scholar]
- Deadwyler SA, Hampson RE. Differential but complementary mnemonic functions of the hippocampus and subiculum. Neuron. 2004;42(3):465–476. doi: 10.1016/s0896-6273(04)00195-3. [DOI] [PubMed] [Google Scholar]
- Deller T, Martinez A, Nitsch R, Frotscher M. A novel entorhinal projection to the rat dentate gyrus: direct innervation of proximal dendrites and cell bodies of granule cells and GABAergic neurons. J Neurosci. 1996;16(10):3322–3333. doi: 10.1523/JNEUROSCI.16-10-03322.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh SS, Knierim JJ. Representation of non-spatial and spatial information in the lateral entorhinal cortex. Front Behav Neurosci. 2011;5:69. doi: 10.3389/fnbeh.2011.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh SS, Knierim JJ. Influence of local objects on hippocampal representations: landmark vectors and memory. Hippocampus. 2013;23(4):253–267. doi: 10.1002/hipo.22101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh SS, Yoganarasimha D, Voicu H, Knierim JJ. Theta modulation in the medial and the lateral entorhinal cortices. J Neurophysiol. 2010;104(2):994–1006. doi: 10.1152/jn.01141.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVito LM, Konigsberg R, Lykken C, Sauvage M, Young WS, 3rd, Eichenbaum H. Vasopressin 1b receptor knock-out impairs memory for temporal order. J Neurosci. 2009;29(9):2676–2683. doi: 10.1523/JNEUROSCI.5488-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diba K, Buzsaki G. Forward and reverse hippocampal place-cell sequences during ripples. Nat Neurosci. 2007;10(10):1241–1242. doi: 10.1038/nn1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolleman-Van der Weel MJ, Witter MP. Projections from the nucleus reuniens thalami to the entorhinal cortex, hippocampal field CA1, and the subiculum in the rat arise from different populations of neurons. J Comp Neurol. 1996;364(4):637–650. doi: 10.1002/(SICI)1096-9861(19960122)364:4<637::AID-CNE3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW, Chen L, Fanselow MS, Toga AW. Genomic-anatomic evidence for distinct functional domains in hippocampal field CA1. Proc Natl Acad Sci USA. 2009;106(28):11794–11799. doi: 10.1073/pnas.0812608106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek SM, Alexander GM, Farris S. Rediscovering area CA2: unique properties and functions. Nat Rev Neurosci. 2016;17(2):89–102. doi: 10.1038/nrn.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan K, Ketz N, Inati SJ, Davachi L. Evidence for area CA1 as a match/mismatch detector: a high-resolution fMRI study of the human hippocampus. Hippocampus. 2012;22(3):389–398. doi: 10.1002/hipo.20933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupret D, O’Neill J, Pleydell-Bouverie B, Csicsvari J. The reorganization and reactivation of hippocampal maps predict spatial memory performance. Nat Neurosci. 2010;13(8):995–1002. doi: 10.1038/nn.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupret D, Revest JM, Koehl M, Ichas F, De Giorgi F, Costet P, Abrous DN, Piazza PV. Spatial relational memory requires hippocampal adult neurogenesis. PLoS ONE. 2008;3(4):e1959. doi: 10.1371/journal.pone.0001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvarci S, Pare D. Amygdala microcircuits controlling learned fear. Neuron. 2014;82(5):966–980. doi: 10.1016/j.neuron.2014.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ego-Stengel V, Wilson MA. Spatial selectivity and theta phase precession in CA1 interneurons. Hippocampus. 2007;17(2):161–174. doi: 10.1002/hipo.20253. [DOI] [PubMed] [Google Scholar]
- Ego-Stengel V, Wilson MA. Disruption of ripple-associated hippocampal activity during rest impairs spatial learning in the rat. Hippocampus. 2010;20(1):1–10. doi: 10.1002/hipo.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H. Time cells in the hippocampus: a new dimension for mapping memories. Nat Rev Neurosci. 2014;15(11):732–744. doi: 10.1038/nrn3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. From conditioning to conscious recollection. Oxford University Press; New York: 2001. [Google Scholar]
- Eichenbaum H, Cohen NJ. Can we reconcile the declarative memory and spatial navigation views on hippocampal function? Neuron. 2014;83(4):764–770. doi: 10.1016/j.neuron.2014.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Kuperstein M, Fagan A, Nagode J. Cue-sampling and goal-approach correlates of hippocampal unit activity in rats performing an odor-discrimination task. J Neurosci. 1987;7(3):716–732. doi: 10.1523/JNEUROSCI.07-03-00716.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Sauvage M, Fortin N, Komorowski R, Lipton P. Towards a functional organization of episodic memory in the medial temporal lobe. Neurosci Biobehav Rev. 2012;36(7):1597–1608. doi: 10.1016/j.neubiorev.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eller J, Zarnadze S, Bauerle P, Dugladze T, Gloveli T. Cell type-specific separation of subicular principal neurons during network activities. PLoS ONE. 2015;10(4):e0123636. doi: 10.1371/journal.pone.0123636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschenko O, Molle M, Born J, Sara SJ. Elevated sleep spindle density after learning or after retrieval in rats. J Neurosci. 2006;26(50):12914–12920. doi: 10.1523/JNEUROSCI.3175-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschenko O, Ramadan W, Molle M, Born J, Sara SJ. Sustained increase in hippocampal sharp-wave ripple activity during slow-wave sleep after learning. Learn Mem. 2008;15(4):222–228. doi: 10.1101/lm.726008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito MS, Piatti VC, Laplagne DA, Morgenstern NA, Ferrari CC, Pitossi FJ, Schinder AF. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J Neurosci. 2005;25(44):10074–10086. doi: 10.1523/JNEUROSCI.3114-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65(1):7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix-Ortiz AC, Beyeler A, Seo C, Leppla CA, Wildes CP, Tye KM. BLA to vHPC inputs modulate anxiety-related behaviors. Neuron. 2013;79(4):658–664. doi: 10.1016/j.neuron.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix-Ortiz AC, Tye KM. Amygdala inputs to the ventral hippocampus bidirectionally modulate social behavior. J Neurosci. 2014;34(2):586–595. doi: 10.1523/JNEUROSCI.4257-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell J, Axmacher N. The role of phase synchronization in memory processes. Nat Rev Neurosci. 2011;12(2):105–118. doi: 10.1038/nrn2979. [DOI] [PubMed] [Google Scholar]
- Feng T, Silva D, Foster DJ. Dissociation between the experience-dependent development of hippocampal theta sequences and single-trial phase precession. J Neurosci. 2015;35(12):4890–4902. doi: 10.1523/JNEUROSCI.2614-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton AA, Kao HY, Neymotin SA, Olypher A, Vayntrub Y, Lytton WW, Ludvig N. Unmasking the CA1 ensemble place code by exposures to small and large environments: more place cells and multiple, irregularly arranged, and expanded place fields in the larger space. J Neurosci. 2008;28(44):11250–11262. doi: 10.1523/JNEUROSCI.2862-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferbinteanu J, McDonald RJ. Dorsal/ventral hippocampus, fornix, and conditioned place preference. Hippocampus. 2001;11(2):187–200. doi: 10.1002/hipo.1036. [DOI] [PubMed] [Google Scholar]
- Ferbinteanu J, Shapiro ML. Prospective and retrospective memory coding in the hippocampus. Neuron. 2003;40(6):1227–1239. doi: 10.1016/s0896-6273(03)00752-9. [DOI] [PubMed] [Google Scholar]
- Finch DM, Nowlin NL, Babb TL. Demonstration of axonal projections of neurons in the rat hippocampus and subiculum by intracellular injection of HRP. Brain Res. 1983;271(2):201–216. doi: 10.1016/0006-8993(83)90283-4. [DOI] [PubMed] [Google Scholar]
- Foster DJ, Wilson MA. Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature. 2006;440(7084):680–683. doi: 10.1038/nature04587. [DOI] [PubMed] [Google Scholar]
- Foster TC, Castro CA, McNaughton BL. Spatial selectivity of rat hippocampal neurons: dependence on preparedness for movement. Science. 1989;244(4912):1580–1582. doi: 10.1126/science.2740902. [DOI] [PubMed] [Google Scholar]
- Fox SE, Ranck JB., Jr Electrophysiological characteristics of hippocampal complex-spike cells and theta cells. Exp Brain Res. 1981;41(3–4):399–410. doi: 10.1007/BF00238898. [DOI] [PubMed] [Google Scholar]
- Frank LM, Brown EN, Wilson M. Trajectory encoding in the hippocampus and entorhinal cortex. Neuron. 2000;27(1):169–178. doi: 10.1016/s0896-6273(00)00018-0. [DOI] [PubMed] [Google Scholar]
- Frank LM, Stanley GB, Brown EN. Hippocampal plasticity across multiple days of exposure to novel environments. J Neurosci. 2004;24(35):7681–7689. doi: 10.1523/JNEUROSCI.1958-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman WJ. Definitions of state variables and state space for brain-computer interface: Part 1. Multiple hierarchical levels of brain function. Cogn Neurodyn. 2007;1(1):3–14. doi: 10.1007/s11571-006-9001-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Antal M. GABA-containing neurons in the septum control inhibitory interneurons in the hippocampus. Nature. 1988;336(6195):170–173. doi: 10.1038/336170a0. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6(4):347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci. 2005;9(10):474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Frotscher M, Leranth C. Cholinergic innervation of the rat hippocampus as revealed by choline acetyltransferase immunocytochemistry: a combined light and electron microscopic study. J Comp Neurol. 1985;239(2):237–246. doi: 10.1002/cne.902390210. [DOI] [PubMed] [Google Scholar]
- Fyhn M, Molden S, Hollup S, Moser MB, Moser E. Hippocampal neurons responding to first-time dislocation of a target object. Neuron. 2002;35(3):555–566. doi: 10.1016/s0896-6273(02)00784-5. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Chiba AA. The amygdala and emotion. Curr Opin Neurobiol. 1996;6(2):221–227. doi: 10.1016/s0959-4388(96)80076-6. [DOI] [PubMed] [Google Scholar]
- Garthe A, Behr J, Kempermann G. Adult-generated hippocampal neurons allow the flexible use of spatially precise learning strategies. PLoS ONE. 2009;4(5):e5464. doi: 10.1371/journal.pone.0005464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Sailor KA, Ming GL, Song H. Synaptic integration and plasticity of new neurons in the adult hippocampus. J Physiol. 2008;586(16):3759–3765. doi: 10.1113/jphysiol.2008.155655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstner W, Kempter R, van Hemmen JL, Wagner H. A neuronal learning rule for sub-millisecond temporal coding. Nature. 1996;383(6595):76–81. doi: 10.1038/383076a0. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Brushfield AM. The role of the CA3 hippocampal subregion in spatial memory: a process oriented behavioral assessment. Prog Neuropsychopharmacol Biol Psych. 2009;33(5):774–781. doi: 10.1016/j.pnpbp.2009.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie AK, Jones EA, Lin Y-H, Karlsson MP, Kay K, Yoon SY, Tong LM, Nova P, Carr JS, Frank LM, Huang Y. Apolipoprotein E4 causes age-dependent disruption of slow gamma oscillations during hippocampal sharp-wave ripples. Neuron. 2016;90(4):740–51. doi: 10.1016/j.neuron.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardeau G, Benchenane K, Wiener SI, Buzsaki G, Zugaro MB. Selective suppression of hippocampal ripples impairs spatial memory. Nat Neurosci. 2009;12(10):1222–1223. doi: 10.1038/nn.2384. [DOI] [PubMed] [Google Scholar]
- Givens BS, Olton DS. Cholinergic and GABAergic modulation of medial septal area: effect on working memory. Behav Neurosci. 1990;104(6):849–855. doi: 10.1037//0735-7044.104.6.849. [DOI] [PubMed] [Google Scholar]
- Gold AE, Kesner RP. The role of the CA3 subregion of the dorsal hippocampus in spatial pattern completion in the rat. Hippocampus. 2005;15(6):808–814. doi: 10.1002/hipo.20103. [DOI] [PubMed] [Google Scholar]
- Gomperts SN, Kloosterman F, Wilson MA. VTA neurons coordinate with the hippocampal reactivation of spatial experience. eLife. 2015:4. doi: 10.7554/eLife.05360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales RB, DeLeon Galvan CJ, Rangel YM, Claiborne BJ. Distribution of thorny excrescences on CA3 pyramidal neurons in the rat hippocampus. J Comp Neurol. 2001;430(3):357–368. doi: 10.1002/1096-9861(20010212)430:3<357::aid-cne1036>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Grastyan E, Lissak K, Madarasz I, Donhoffer H. Hippocampal electrical activity during the development of conditioned reflexes. Electroencephalogr Clin Neurophysiol. 1959;11(3):409–430. doi: 10.1016/0013-4694(59)90040-9. [DOI] [PubMed] [Google Scholar]
- Gray CM, Maldonado PE, Wilson M, McNaughton B. Tetrodes markedly improve the reliability and yield of multiple single- unit isolation from multi-unit recordings in cat striate cortex. J Neurosci Methods. 1995;63(1–2):43–54. doi: 10.1016/0165-0270(95)00085-2. [DOI] [PubMed] [Google Scholar]
- Green JD, Arduini AA. Hippocampal electrical activity in arousal. J Neurophysiol. 1954;17(6):533–557. doi: 10.1152/jn.1954.17.6.533. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Vermeulen-Van der Zee E, te Kortschot A, Witter MP. Organization of the projections from the subiculum to the ventral striatum in the rat. A study using anterograde transport of Phaseolus vulgaris leucoagglutinin. Neuroscience. 1987;23(1):103–120. doi: 10.1016/0306-4522(87)90275-2. [DOI] [PubMed] [Google Scholar]
- Grosmark AD, Mizuseki K, Pastalkova E, Diba K, Buzsaki G. REM sleep reorganizes hippocampal excitability. Neuron. 2012;75(6):1001–1007. doi: 10.1016/j.neuron.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Arruda-Carvalho M, Wang J, Janoschka SR, Josselyn SA, Frankland PW, Ge S. Optical controlling reveals time-dependent roles for adult-born dentate granule cells. Nat Neurosci. 2012;15(12):1700–1706. doi: 10.1038/nn.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyas AI, Hajos N, Katona I, Freund TF. Interneurons are the local targets of hippocampal inhibitory cells which project to the medial septum. Eur J Neurosci. 2003;17(9):1861–1872. doi: 10.1046/j.1460-9568.2003.02630.x. [DOI] [PubMed] [Google Scholar]
- Gupta AS, van der Meer MA, Touretzky DS, Redish AD. Hippocampal replay is not a simple function of experience. Neuron. 2010;65(5):695–705. doi: 10.1016/j.neuron.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta AS, van der Meer MA, Touretzky DS, Redish AD. Segmentation of spatial experience by hippocampal theta sequences. Nat Neurosci. 2012;15(7):1032–1039. doi: 10.1038/nn.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, Knierim JJ, Moser EI. Ensemble dynamics of hippocampal regions CA3 and CA1. Neuron. 2004;44(4):581–584. doi: 10.1016/j.neuron.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci. 1999;2(12):1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- Hafting T, Fyhn M, Bonnevie T, Moser MB, Moser EI. Hippocampus-independent phase precession in entorhinal grid cells. Nature. 2008;453(7199):1248–1252. doi: 10.1038/nature06957. [DOI] [PubMed] [Google Scholar]
- Hafting T, Fyhn M, Molden S, Moser MB, Moser EI. Microstructure of a spatial map in the entorhinal cortex. Nature. 2005;436(7052):801–806. doi: 10.1038/nature03721. [DOI] [PubMed] [Google Scholar]
- Hales JB, Schlesiger MI, Leutgeb JK, Squire LR, Leutgeb S, Clark RE. Medial entorhinal cortex lesions only partially disrupt hippocampal place cells and hippocampus-dependent place memory. Cell Rep. 2014;9(3):893–901. doi: 10.1016/j.celrep.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AZ, Gordon JA. Long-range neural synchrony in behavior. Annu Rev Neurosci. 2015;38:171–194. doi: 10.1146/annurev-neuro-071714-034111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME. What is the function of hippocampal theta rhythm?-Linking behavioral data to phasic properties of field potential and unit recording data. Hippocampus. 2005;15(7):936–949. doi: 10.1002/hipo.20116. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Bodelon C, Wyble BP. A proposed function for hippocampal theta rhythm: separate phases of encoding and retrieval enhance reversal of prior learning. Neural Comput. 2002;14(4):793–817. doi: 10.1162/089976602317318965. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Schnell E. Laminar selectivity of the cholinergic suppression of synaptic transmission in rat hippocampal region CA1: computational modeling and brain slice physiology. J Neurosci. 1994;14(6):3898–3914. doi: 10.1523/JNEUROSCI.14-06-03898.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke PG. Hippocampal pathway to the amygdala and stress ulcer development. Brain Res Bull. 1990;25(5):691–695. doi: 10.1016/0361-9230(90)90044-z. [DOI] [PubMed] [Google Scholar]
- Henze DA, Buzsaki G. Hilar mossy cells: functional identification and activity in vivo. Prog Brain Res. 2007;163:199–810. doi: 10.1016/S0079-6123(07)63012-X. [DOI] [PubMed] [Google Scholar]
- Herkenham M. The connections of the nucleus reuniens thalami: evidence for a direct thalamo-hippocampal pathway in the rat. J Comp Neurol. 1978;177(4):589–610. doi: 10.1002/cne.901770405. [DOI] [PubMed] [Google Scholar]
- Hill AJ. First occurrence of hippocampal spatial firing in a new environment. Exp Neurol. 1978;62(2):282–297. doi: 10.1016/0014-4886(78)90058-4. [DOI] [PubMed] [Google Scholar]
- Hitti FL, Siegelbaum SA. The hippocampal CA2 region is essential for social memory. Nature. 2014;508(7494):88–92. doi: 10.1038/nature13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hok V, Lenck-Santini PP, Roux S, Save E, Muller RU, Poucet B. Goal-related activity in hippocampal place cells. J Neurosci. 2007;27(3):472–482. doi: 10.1523/JNEUROSCI.2864-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfield JJ. Pattern recognition computation using action potential timing for stimulus representation. Nature. 1995;376(6535):33–36. doi: 10.1038/376033a0. [DOI] [PubMed] [Google Scholar]
- Huxter J, Burgess N, O’Keefe J. Independent rate and temporal coding in hippocampal pyramidal cells. Nature. 2003;425(6960):828–832. doi: 10.1038/nature02058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman JM, Wyble BP, Goyal V, Rossi CA, Hasselmo ME. Stimulation in hippocampal region CA1 in behaving rats yields long-term potentiation when delivered to the peak of theta and long-term depression when delivered to the trough. J Neurosci. 2003;23(37):11725–11731. doi: 10.1523/JNEUROSCI.23-37-11725.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi KM, Lu L, Colgin LL, Moser MB, Moser EI. Coordination of entorhinal-hippocampal ensemble activity during associative learning. Nature. 2014;510(7503):143–147. doi: 10.1038/nature13162. [DOI] [PubMed] [Google Scholar]
- Isaac JT, Buchanan KA, Muller RU, Mellor JR. Hippocampal place cell firing patterns can induce long-term synaptic plasticity in vitro. J Neurosci. 2009;29(21):6840–6850. doi: 10.1523/JNEUROSCI.0731-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka N. Laminar organization of the pyramidal cell layer of the subiculum in the rat. J Comp Neurol. 2001;435(1):89–110. doi: 10.1002/cne.1195. [DOI] [PubMed] [Google Scholar]
- Ishizuka N, Cowan WM, Amaral DG. A quantitative analysis of the dendritic organization of pyramidal cells in the rat hippocampus. J Comp Neurol. 1995;362(1):17–45. doi: 10.1002/cne.903620103. [DOI] [PubMed] [Google Scholar]
- Ishizuka N, Weber J, Amaral DG. Organization of intrahippocampal projections originating from CA3 pyramidal cells in the rat. J Comp Neurol. 1990;295(4):580–623. doi: 10.1002/cne.902950407. [DOI] [PubMed] [Google Scholar]
- Isomura Y, Sirota A, Ozen S, Montgomery S, Mizuseki K, Henze DA, Buzsaki G. Integration and segregation of activity in entorhinal-hippocampal subregions by neocortical slow oscillations. Neuron. 2006;52(5):871–882. doi: 10.1016/j.neuron.2006.10.023. [DOI] [PubMed] [Google Scholar]
- Ito HT, Zhang SJ, Witter MP, Moser EI, Moser MB. A prefrontal-thalamo-hippocampal circuit for goal-directed spatial navigation. Nature. 2015;522(7554):50–55. doi: 10.1038/nature14396. [DOI] [PubMed] [Google Scholar]
- Itskov V, Pastalkova E, Mizuseki K, Buzsaki G, Harris KD. Theta-mediated dynamics of spatial information in hippocampus. J Neurosci. 2008;28(23):5959–5964. doi: 10.1523/JNEUROSCI.5262-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson J, Goutagny R, Williams S. Fast and slow gamma rhythms are intrinsically and independently generated in the subiculum. J Neurosci. 2011;31(34):12104–12117. doi: 10.1523/JNEUROSCI.1370-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JC, Johnson A, Redish AD. Hippocampal sharp waves and reactivation during awake states depend on repeated sequential experience. J Neurosci. 2006;26(48):12415–12426. doi: 10.1523/JNEUROSCI.4118-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav SP, Kemere C, German PW, Frank LM. Awake hippocampal sharp-wave ripples support spatial memory. Science. 2012;336(6087):1454–1458. doi: 10.1126/science.1217230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav SP, Rothschild G, Roumis DK, Frank LM. Coordinated excitation and inhibition of prefrontal ensembles during awake hippocampal sharp-wave ripple events. Neuron. 2016;90(1):113–127. doi: 10.1016/j.neuron.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janak PH, Tye KM. From circuits to behaviour in the amygdala. Nature. 2015;517(7534):284–292. doi: 10.1038/nature14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarosiewicz B, McNaughton BL, Skaggs WE. Hippocampal population activity during the small-amplitude irregular activity state in the rat. J Neurosci. 2002;22(4):1373–1384. doi: 10.1523/JNEUROSCI.22-04-01373.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarsky T, Mady R, Kennedy B, Spruston N. Distribution of bursting neurons in the CA1 region and the subiculum of the rat hippocampus. J Comp Neurol. 2008;506(4):535–547. doi: 10.1002/cne.21564. [DOI] [PubMed] [Google Scholar]
- Jensen O, Lisman JE. Position reconstruction from an ensemble of hippocampal place cells: contribution of theta phase coding. J Neurophysiol. 2000;83(5):2602–2609. doi: 10.1152/jn.2000.83.5.2602. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Clark RE, Broadbent NJ, Clemenson GD, Jr, Consiglio A, Lie DC, Squire LR, Gage FH. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem. 2009;16(2):147–154. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci. 2007;10(1):100–107. doi: 10.1038/nn1825. [DOI] [PubMed] [Google Scholar]
- Jog MS, Connolly CI, Kubota Y, Iyengar DR, Garrido L, Harlan R, Graybiel AM. Tetrode technology: advances in implantable hardware, neuroimaging, and data analysis techniques. J Neurosci Methods. 2002;117(2):141–152. doi: 10.1016/s0165-0270(02)00092-4. [DOI] [PubMed] [Google Scholar]
- Johnson A, Redish AD. Neural ensembles in CA3 transiently encode paths forward of the animal at a decision point. J Neurosci. 2007;27(45):12176–12189. doi: 10.1523/JNEUROSCI.3761-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston ST, Shtrahman M, Parylak S, Goncalves JT, Gage FH. Paradox of pattern separation and adult neurogenesis: A dual role for new neurons balancing memory resolution and robustness. Neurobiol Learn Mem. 2016;129:60–68. doi: 10.1016/j.nlm.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MW, McHugh TJ. Updating hippocampal representations: CA2 joins the circuit. Trends Neurosci. 2011;34(10):526–535. doi: 10.1016/j.tins.2011.07.007. (S0166-2236(11)00123-8) [DOI] [PubMed] [Google Scholar]
- Jones MW, Wilson MA. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biol. 2005;3(12):e402. doi: 10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung MW, McNaughton BL. Spatial selectivity of unit activity in the hippocampal granular layer. Hippocampus. 1993;3(2):165–182. doi: 10.1002/hipo.450030209. [DOI] [PubMed] [Google Scholar]
- Jung MW, Wiener SI, McNaughton BL. Comparison of spatial firing characteristics of units in dorsal and ventral hippocampus of the rat. J Neurosci. 1994;14(12):7347–7356. doi: 10.1523/JNEUROSCI.14-12-07347.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutras MJ, Fries P, Buffalo EA. Oscillatory activity in the monkey hippocampus during visual exploration and memory formation. Proc Natl Acad Sci USA. 2013;110(32):13144–13149. doi: 10.1073/pnas.1302351110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajikawa Y, Schroeder CE. How local is the local field potential? Neuron. 2011;72(5):847–858. doi: 10.1016/j.neuron.2011.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiwara R, Wouterlood FG, Sah A, Boekel AJ, Baks-te Bulte LT, Witter MP. Convergence of entorhinal and CA3 inputs onto pyramidal neurons and interneurons in hippocampal area CA1–an anatomical study in the rat. Hippocampus. 2008;18(3):266–280. doi: 10.1002/hipo.20385. [DOI] [PubMed] [Google Scholar]
- Kali S, Dayan P. The involvement of recurrent connections in area CA3 in establishing the properties of place fields: a model. J Neurosci. 2000;20(19):7463–7477. doi: 10.1523/JNEUROSCI.20-19-07463.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kali S, Freund TF. Distinct properties of two major excitatory inputs to hippocampal pyramidal cells: a computational study. Eur J Neurosci. 2005;22(8):2027–2048. doi: 10.1111/j.1460-9568.2005.04406.x. [DOI] [PubMed] [Google Scholar]
- Kamondi A, Acsady L, Wang XJ, Buzsaki G. Theta oscillations in somata and dendrites of hippocampal pyramidal cells in vivo: activity-dependent phase-precession of action potentials. Hippocampus. 1998;8(3):244–261. doi: 10.1002/(SICI)1098-1063(1998)8:3<244::AID-HIPO7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Kaplan R, Adhikari MH, Hindriks R, Mantini D, Murayama Y, Logothetis NK, Deco G. Hippocampal sharp-wave ripples influence selective activation of the default mode network. Curr Biol. 2016;26(5):686–691. doi: 10.1016/j.cub.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson MP, Frank LM. Network dynamics underlying the formation of sparse, informative representations in the hippocampus. J Neurosci. 2008;28(52):14271–14281. doi: 10.1523/JNEUROSCI.4261-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson MP, Frank LM. Awake replay of remote experiences in the hippocampus. Nat Neurosci. 2009;12(7):913–918. doi: 10.1038/nn.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay K, Sosa M, Chung JE, Karlsson MP, Larkin MC, Frank LM. A hippocampal network for spatial coding during immobility and sleep. Nature. 2016;531(7593):185–190. doi: 10.1038/nature17144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keinath AT, Wang ME, Wann EG, Yuan RK, Dudman JT, Muzzio IA. Precise spatial coding is preserved along the longitudinal hippocampal axis. Hippocampus. 2014;24(12):1533–1548. doi: 10.1002/hipo.22333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemere C, Carr MF, Karlsson MP, Frank LM. Rapid and continuous modulation of hippocampal network state during exploration of new places. PLoS ONE. 2013;8(9):e73114. doi: 10.1371/journal.pone.0073114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp IR, Kaada BR. The relation of hippocampal theta activity to arousal, attentive behaviour and somato-motor movements in unrestrained cats. Brain Res. 1975;95(2–3):323–342. doi: 10.1016/0006-8993(75)90110-9. [DOI] [PubMed] [Google Scholar]
- Kennedy PJ, Shapiro ML. Motivational states activate distinct hippocampal representations to guide goal-directed behaviors. Proc Natl Acad Sci USA. 2009;106(26):10805–10810. doi: 10.1073/pnas.0903259106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr KM, Agster KL, Furtak SC, Burwell RD. Functional neuroanatomy of the parahippocampal region: the lateral and medial entorhinal areas. Hippocampus. 2007;17(9):697–708. doi: 10.1002/hipo.20315. [DOI] [PubMed] [Google Scholar]
- Kesner RP. Behavioral functions of the CA3 subregion of the hippocampus. Learn Mem. 2007;14(11):771–781. doi: 10.1101/lm.688207. [DOI] [PubMed] [Google Scholar]
- Kheirbek MA, Drew LJ, Burghardt NS, Costantini DO, Tannenholz L, Ahmari SE, Zeng H, Fenton AA, Hen R. Differential control of learning and anxiety along the dorsoventral axis of the dentate gyrus. Neuron. 2013;77(5):955–968. doi: 10.1016/j.neuron.2012.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SM, Ganguli S, Frank LM. Spatial information outflow from the hippocampal circuit: distributed spatial coding and phase precession in the subiculum. J Neurosci. 2012;32(34):11539–11558. doi: 10.1523/JNEUROSCI.5942-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Spruston N. Target-specific output patterns are predicted by the distribution of regular-spiking and bursting pyramidal neurons in the subiculum. Hippocampus. 2012;22(4):693–706. doi: 10.1002/hipo.20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelstrup KB, Solstad T, Brun VH, Hafting T, Leutgeb S, Witter MP, Moser EI, Moser MB. Finite scale of spatial representation in the hippocampus. Science. 2008;321(5885):140–143. doi: 10.1126/science.1157086. [DOI] [PubMed] [Google Scholar]
- Kjelstrup KG, Tuvnes FA, Steffenach HA, Murison R, Moser EI, Moser MB. Reduced fear expression after lesions of the ventral hippocampus. Proc Natl Acad Sci USA. 2002;99(16):10825–10830. doi: 10.1073/pnas.152112399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321(5885):53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knierim JJ, Lee I, Hargreaves EL. Hippocampal place cells: parallel input streams, subregional processing, and implications for episodic memory. Hippocampus. 2006;16(9):755–764. doi: 10.1002/hipo.20203. [DOI] [PubMed] [Google Scholar]
- Knierim JJ, Neunuebel JP. Tracking the flow of hippocampal computation: Pattern separation, pattern completion, and attractor dynamics. Neurobiol Learn Mem. 2016;129:38–49. doi: 10.1016/j.nlm.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Nishijo H, Fukuda M, Bures J, Ono T. Task-dependent representations in rat hippocampal place neurons. J Neurophysiol. 1997;78(2):597–613. doi: 10.1152/jn.1997.78.2.597. [DOI] [PubMed] [Google Scholar]
- Kocsis B, Vertes RP. Phase relations of rhythmic neuronal firing in the supramammillary nucleus and mammillary body to the hippocampal theta activity in urethane anesthetized rats. Hippocampus. 1997;7(2):204–214. doi: 10.1002/(SICI)1098-1063(1997)7:2<204::AID-HIPO7>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Koenig J, Linder AN, Leutgeb JK, Leutgeb S. The spatial periodicity of grid cells is not sustained during reduced theta oscillations. Science. 2011;332(6029):592–595. doi: 10.1126/science.1201685. (332/6029/592) [DOI] [PubMed] [Google Scholar]
- Kohara K, Pignatelli M, Rivest AJ, Jung HY, Kitamura T, Suh J, Frank D, Kajikawa K, Mise N, Obata Y, Wickersham IR, Tonegawa S. Cell type-specific genetic and optogenetic tools reveal hippocampal CA2 circuits. Nat Neurosci. 2014;17(2):269–279. doi: 10.1038/nn.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler C. Intrinsic projections of the retrohippocampal region in the rat brain. I. The subicular complex. J Comp Neurol. 1985;236(4):504–522. doi: 10.1002/cne.902360407. [DOI] [PubMed] [Google Scholar]
- Komorowski RW, Garcia CG, Wilson A, Hattori S, Howard MW, Eichenbaum H. Ventral hippocampal neurons are shaped by experience to represent behaviorally relevant contexts. J Neurosci. 2013;33(18):8079–8087. doi: 10.1523/JNEUROSCI.5458-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komorowski RW, Manns JR, Eichenbaum H. Robust conjunctive item-place coding by hippocampal neurons parallels learning what happens where. J Neurosci. 2009;29(31):9918–9929. doi: 10.1523/JNEUROSCI.1378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopell N, Ermentrout GB, Whittington MA, Traub RD. Gamma rhythms and beta rhythms have different synchronization properties. Proc Natl Acad Sci USA. 2000;97(4):1867–1872. doi: 10.1073/pnas.97.4.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus BJ, Robinson RJ, 2nd, White JA, Eichenbaum H, Hasselmo ME. Hippocampal “time cells”: time versus path integration. Neuron. 2013;78(6):1090–1101. doi: 10.1016/j.neuron.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropff E, Carmichael JE, Moser MB, Moser EI. Speed cells in the medial entorhinal cortex. Nature. 2015;523(7561):419–424. doi: 10.1038/nature14622. [DOI] [PubMed] [Google Scholar]
- Kudrimoti HS, Barnes CA, McNaughton BL. Reactivation of hippocampal cell assemblies: effects of behavioral state, experience, and EEG dynamics. J Neurosci. 1999;19(10):4090–4101. doi: 10.1523/JNEUROSCI.19-10-04090.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran D, Maguire EA. Which computational mechanisms operate in the hippocampus during novelty detection? Hippocampus. 2007;17(9):735–748. doi: 10.1002/hipo.20326. [DOI] [PubMed] [Google Scholar]
- Kwag J, Paulsen O. Bidirectional control of spike timing by GABA(A) receptor-mediated inhibition during theta oscillation in CA1 pyramidal neurons. NeuroReport. 2009;20(13):1209–1213. doi: 10.1097/WNR.0b013e32832f5cc7. [DOI] [PubMed] [Google Scholar]
- Lansink CS, Goltstein PM, Lankelma JV, Joosten RN, McNaughton BL, Pennartz CM. Preferential reactivation of motivationally relevant information in the ventral striatum. J Neurosci. 2008;28(25):6372–6382. doi: 10.1523/JNEUROSCI.1054-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansink CS, Goltstein PM, Lankelma JV, McNaughton BL, Pennartz CM. Hippocampus leads ventral striatum in replay of place-reward information. PLoS Biol. 2009;7(8):e1000173. doi: 10.1371/journal.pbio.1000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larimer P, Strowbridge BW. Nonrandom local circuits in the dentate gyrus. J Neurosci. 2008;28(47):12212–12223. doi: 10.1523/JNEUROSCI.3612-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin MC, Lykken C, Tye LD, Wickelgren JG, Frank LM. Hippocampal output area CA1 broadcasts a generalized novelty signal during an object-place recognition task. Hippocampus. 2014;24(7):773–783. doi: 10.1002/hipo.22268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurberg S. Commissural and intrinsic connections of the rat hippocampus. J Comp Neurol. 1979;184(4):685–708. doi: 10.1002/cne.901840405. [DOI] [PubMed] [Google Scholar]
- Lee AK, Wilson MA. Memory of sequential experience in the hippocampus during slow wave sleep. Neuron. 2002;36(6):1183–1194. doi: 10.1016/s0896-6273(02)01096-6. [DOI] [PubMed] [Google Scholar]
- Lee H, Wang C, Deshmukh SS, Knierim JJ. Neural population evidence of functional heterogeneity along the CA3 transverse axis: pattern completion versus pattern separation. Neuron. 2015;87(5):1093–1105. doi: 10.1016/j.neuron.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Jerman TS, Kesner RP. Disruption of delayed memory for a sequence of spatial locations following CA1- or CA3-lesions of the dorsal hippocampus. Neurobiol Learn Mem. 2005;84(2):138–147. doi: 10.1016/j.nlm.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Lee I, Kesner RP. Differential roles of dorsal hippocampal subregions in spatial working memory with short versus intermediate delay. Behav Neurosci. 2003;117(5):1044–1053. doi: 10.1037/0735-7044.117.5.1044. [DOI] [PubMed] [Google Scholar]
- Lee I, Kesner RP. Differential contributions of dorsal hippocampal subregions to memory acquisition and retrieval in contextual fear-conditioning. Hippocampus. 2004;14(3):301–310. doi: 10.1002/hipo.10177. [DOI] [PubMed] [Google Scholar]
- Lee I, Rao G, Knierim JJ. A double dissociation between hippocampal subfields: differential time course of CA3 and CA1 place cells for processing changed environments. Neuron. 2004a;42(5):803–815. doi: 10.1016/j.neuron.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Lee I, Yoganarasimha D, Rao G, Knierim JJ. Comparison of population coherence of place cells in hippocampal subfields CA1 and CA3. Nature. 2004b;430(6998):456–459. doi: 10.1038/nature02739. [DOI] [PubMed] [Google Scholar]
- Lee SE, Simons SB, Heldt SA, Zhao M, Schroeder JP, Vellano CP, Cowan DP, Ramineni S, Yates CK, Feng Y, Smith Y, Sweatt JD, Weinshenker D, Ressler KJ, Dudek SM, Hepler JR. RGS14 is a natural suppressor of both synaptic plasticity in CA2 neurons and hippocampal-based learning and memory. Proc Natl Acad Sci USA. 2010;107(39):16994–16998. doi: 10.1073/pnas.1005362107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein ES, Callaway EM, Albright TD, Gage FH. Redefining the boundaries of the hippocampal CA2 subfield in the mouse using gene expression and 3-dimensional reconstruction. J Comp Neurol. 2005;485(1):1–10. doi: 10.1002/cne.20426. [DOI] [PubMed] [Google Scholar]
- Lein ES, Zhao X, Gage FH. Defining a molecular atlas of the hippocampus using DNA microarrays and high-throughput in situ hybridization. J Neurosci. 2004;24(15):3879–3889. doi: 10.1523/JNEUROSCI.4710-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenck-Santini PP, Rivard B, Muller RU, Poucet B. Study of CA1 place cell activity and exploratory behavior following spatial and nonspatial changes in the environment. Hippocampus. 2005;15(3):356–369. doi: 10.1002/hipo.20060. [DOI] [PubMed] [Google Scholar]
- Lengyel M, Kwag J, Paulsen O, Dayan P. Matching storage and recall: hippocampal spike timing-dependent plasticity and phase response curves. Nat Neurosci. 2005;8(12):1677–1683. doi: 10.1038/nn1561. [DOI] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315(5814):961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Treves A, Meyer R, Barnes CA, McNaughton BL, Moser MB, Moser EI. Progressive transformation of hippocampal neuronal representations in “morphed” environments. Neuron. 2005a;48(2):345–358. doi: 10.1016/j.neuron.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK. Pattern separation, pattern completion, and new neuronal codes within a continuous CA3 map. Learn Mem. 2007;14(11):745–757. doi: 10.1101/lm.703907. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Barnes CA, Moser EI, McNaughton BL, Moser MB. Independent codes for spatial and episodic memory in hippocampal neuronal ensembles. Science. 2005b;309(5734):619–623. doi: 10.1126/science.1114037. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Moser MB, Moser EI. Place cells, spatial maps and the population code for memory. Curr Opin Neurobiol. 2005c;15(6):738–746. doi: 10.1016/j.conb.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Treves A, Moser MB, Moser EI. Distinct ensemble codes in hippocampal areas CA3 and CA1. Science. 2004;305(5688):1295–1298. doi: 10.1126/science.1100265. [DOI] [PubMed] [Google Scholar]
- Lever C, Burton S, Jeewajee A, O’Keefe J, Burgess N. Boundary vector cells in the subiculum of the hippocampal formation. J Neurosci. 2009;29(31):9771–9777. doi: 10.1523/JNEUROSCI.1319-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever C, Wills T, Cacucci F, Burgess N, O’Keefe J. Long-term plasticity in hippocampal place-cell representation of environmental geometry. Nature. 2002;416(6876):90–94. doi: 10.1038/416090a. [DOI] [PubMed] [Google Scholar]
- Li S, Cullen WK, Anwyl R, Rowan MJ. Dopamine-dependent facilitation of LTP induction in hippocampal CA1 by exposure to spatial novelty. Nat Neurosci. 2003;6(5):526–531. doi: 10.1038/nn1049. [DOI] [PubMed] [Google Scholar]
- Li XG, Somogyi P, Ylinen A, Buzsaki G. The hippocampal CA3 network: an in vivo intracellular labeling study. J Comp Neurol. 1994;339(2):181–208. doi: 10.1002/cne.903390204. [DOI] [PubMed] [Google Scholar]
- Li Y, Aimone JB, Xu X, Callaway EM, Gage FH. Development of GABAergic inputs controls the contribution of maturing neurons to the adult hippocampal network. Proc Natl Acad Sci USA. 2012;109(11):4290–4295. doi: 10.1073/pnas.1120754109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J. Long-term potentiation: outstanding questions and attempted synthesis. Philos Trans R Soc Lond B Biol Sci. 2003;358(1432):829–842. doi: 10.1098/rstb.2002.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE. Relating hippocampal circuitry to function: recall of memory sequences by reciprocal dentate-CA3 interactions. Neuron. 1999;22(2):233–242. doi: 10.1016/s0896-6273(00)81085-5. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46(5):703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Otmakhova NA. Storage, recall, and novelty detection of sequences by the hippocampus: elaborating on the SOCRATIC model to account for normal and aberrant effects of dopamine. Hippocampus. 2001;11(5):551–568. doi: 10.1002/hipo.1071. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Eschenko O, Murayama Y, Augath M, Steudel T, Evrard HC, Besserve M, Oeltermann A. Hippocampal-cortical interaction during periods of subcortical silence. Nature. 2012;491(7425):547–553. doi: 10.1038/nature11618. [DOI] [PubMed] [Google Scholar]
- Lorente de Nó R. Studies on the structure of the cerebral cortex. I. The area entorhinalis. J Psychol Neurol. 1933;45:381–438. [Google Scholar]
- Lorente de Nó R. Studies on the structure of the cerebral cortex. II. Continuation of the study of the ammonic system. J Psychol Neurol. 1934;46:113–177. [Google Scholar]
- Loughlin SE, Foote SL, Bloom FE. Efferent projections of nucleus locus coeruleus: topographic organization of cells of origin demonstrated by three-dimensional reconstruction. Neuroscience. 1986;18(2):291–306. doi: 10.1016/0306-4522(86)90155-7. [DOI] [PubMed] [Google Scholar]
- Louie K, Wilson MA. Temporally structured replay of awake hippocampal ensemble activity during rapid eye movement sleep. Neuron. 2001;29(1):145–156. doi: 10.1016/s0896-6273(01)00186-6. [DOI] [PubMed] [Google Scholar]
- Loureiro M, Lecourtier L, Engeln M, Lopez J, Cosquer B, Geiger K, Kelche C, Cassel JC, Pereira de Vasconcelos A. The ventral hippocampus is necessary for expressing a spatial memory. Brain Struct Funct. 2012;217(1):93–106. doi: 10.1007/s00429-011-0332-y. [DOI] [PubMed] [Google Scholar]
- Lu L, Igarashi KM, Witter MP, Moser EI, Moser MB. Topography of place maps along the CA3-to-CA2 axis of the hippocampus. Neuron. 2015;87(5):1078–1092. doi: 10.1016/j.neuron.2015.07.007. [DOI] [PubMed] [Google Scholar]
- Lubenov EV, Siapas AG. Hippocampal theta oscillations are travelling waves. Nature. 2009;459(7246):534–539. doi: 10.1038/nature08010. [DOI] [PubMed] [Google Scholar]
- MacDonald CJ, Carrow S, Place R, Eichenbaum H. Distinct hippocampal time cell sequences represent odor memories in immobilized rats. J Neurosci. 2013;33(36):14607–14616. doi: 10.1523/JNEUROSCI.1537-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald CJ, Lepage KQ, Eden UT, Eichenbaum H. Hippocampal “time cells” bridge the gap in memory for discontiguous events. Neuron. 2011;71(4):737–749. doi: 10.1016/j.neuron.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier N, Nimmrich V, Draguhn A. Cellular and network mechanisms underlying spontaneous sharp wave-ripple complexes in mouse hippocampal slices. J Physiol. 2003;550(Pt 3):873–887. doi: 10.1113/jphysiol.2003.044602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra S, Cross RW, van der Meer MA. Theta phase precession beyond the hippocampus. Rev Neurosci. 2012;23(1):39–65. doi: 10.1515/revneuro-2011-0064. [DOI] [PubMed] [Google Scholar]
- Mankin EA, Diehl GW, Sparks FT, Leutgeb S, Leutgeb JK. Hippocampal CA2 activity patterns change over time to a larger extent than between spatial contexts. Neuron. 2015;85(1):190–201. doi: 10.1016/j.neuron.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankin EA, Sparks FT, Slayyeh B, Sutherland RJ, Leutgeb S, Leutgeb JK. Neuronal code for extended time in the hippocampus. Proc Natl Acad Sci USA. 2012;109(47):19462–19467. doi: 10.1073/pnas.1214107109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns JR, Eichenbaum H. A cognitive map for object memory in the hippocampus. Learn Mem. 2009;16(10):616–624. doi: 10.1101/lm.1484509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Burgin A, Mongiat LA, Pardi MB, Schinder AF. Unique processing during a period of high excitation/inhibition balance in adult-born neurons. Science. 2012;335(6073):1238–1242. doi: 10.1126/science.1214956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Lubke J, Frotscher M, Sakmann B. Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science. 1997;275(5297):213–215. doi: 10.1126/science.275.5297.213. [DOI] [PubMed] [Google Scholar]
- Markus EJ, Qin YL, Leonard B, Skaggs WE, Mcnaughton BL, Barnes CA. Interactions between location and task affect the spatial and directional firing of hippocampal-neurons. J Neurosci. 1995;15(11):7079–7094. doi: 10.1523/JNEUROSCI.15-11-07079.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr D. Simple memory: a theory for archicortex. Philos Trans R Soc Lond B Biol Sci. 1971;262(841):23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- Martig AK, Mizumori SJ. Ventral tegmental area disruption selectively affects CA1/CA2 but not CA3 place fields during a differential reward working memory task. Hippocampus. 2011;21(2):172–184. doi: 10.1002/hipo.20734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAvoy K, Besnard A, Sahay A. Adult hippocampal neurogenesis and pattern separation in DG: a role for feedback inhibition in modulating sparseness to govern population-based coding. Front Syst Neurosci. 2015;9:120. doi: 10.3389/fnsys.2015.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland JL, Goddard NH. Considerations arising from a complementary learning systems perspective on hippocampus and neocortex. Hippocampus. 1996;6(6):654–665. doi: 10.1002/(SICI)1098-1063(1996)6:6<654::AID-HIPO8>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- McKenzie S, Frank AJ, Kinsky NR, Porter B, Riviere PD, Eichenbaum H. Hippocampal representation of related and opposing memories develop within distinct, hierarchically organized neural schemas. Neuron. 2014;83(1):202–215. doi: 10.1016/j.neuron.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton BL, Barnes CA. Physiological identification and analysis of dentate granule cell responses to stimulation of the medial and lateral perforant pathways in the rat. J Comp Neurol. 1977;175(4):439–454. doi: 10.1002/cne.901750404. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Barnes CA, Meltzer J, Sutherland RJ. Hippocampal granule cells are necessary for normal spatial learning but not for spatially-selective pyramidal cell discharge. Exp Brain Res. 1989;76(3):485–496. doi: 10.1007/BF00248904. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Barnes CA, O’Keefe J. The contributions of position, direction, and velocity to single unit activity in the hippocampus of freely-moving rats. Exp Brain Res. 1983;52(1):41–49. doi: 10.1007/BF00237147. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Battaglia FP, Jensen O, Moser EI, Moser MB. Path integration and the neural basis of the ‘cognitive map’. Nat Rev Neurosci. 2006;7(8):663–678. doi: 10.1038/nrn1932. [DOI] [PubMed] [Google Scholar]
- Mcnaughton BL, Morris RG. Hippocampal Synaptic Enhancement and Information-Storage within a Distributed Memory System. Trends Neurosci. 1987;10(10):408–415. [Google Scholar]
- Meeter M, Murre JM, Talamini LM. Mode shifting between storage and recall based on novelty detection in oscillating hippocampal circuits. Hippocampus. 2004;14(6):722–741. doi: 10.1002/hipo.10214. [DOI] [PubMed] [Google Scholar]
- Megias M, Emri Z, Freund TF, Gulyas AI. Total number and distribution of inhibitory and excitatory synapses on hippocampal CA1 pyramidal cells. Neuroscience. 2001;102(3):527–540. doi: 10.1016/s0306-4522(00)00496-6. [DOI] [PubMed] [Google Scholar]
- Mehta MR, Lee AK, Wilson MA. Role of experience and oscillations in transforming a rate code into a temporal code. Nature. 2002;417(6890):741–746. doi: 10.1038/nature00807. [DOI] [PubMed] [Google Scholar]
- Mehta MR, Quirk MC, Wilson MA. Experience-dependent asymmetric shape of hippocampal receptive fields. Neuron. 2000;25(3):707–715. doi: 10.1016/s0896-6273(00)81072-7. [DOI] [PubMed] [Google Scholar]
- Meibach RC, Siegel A. Efferent connections of the hippocampal formation in the rat. Brain Res. 1977;124(2):197–224. doi: 10.1016/0006-8993(77)90880-0. [DOI] [PubMed] [Google Scholar]
- Mercer A, Eastlake K, Trigg HL, Thomson AM. Local circuitry involving parvalbumin-positive basket cells in the CA2 region of the hippocampus. Hippocampus. 2012;22(1):43–56. doi: 10.1002/hipo.20841. [DOI] [PubMed] [Google Scholar]
- Mercer A, Trigg HL, Thomson AM. Characterization of neurons in the CA2 subfield of the adult rat hippocampus. J Neurosci. 2007;27(27):7329–7338. doi: 10.1523/JNEUROSCI.1829-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstein AD, Bloss EB, Apostolides PF, Vaidya SP, Dilly GA, Zemelman BV, Magee JC. Inhibitory gating of input comparison in the CA1 microcircuit. Neuron. 2015;87(6):1274–1289. doi: 10.1016/j.neuron.2015.08.025. [DOI] [PubMed] [Google Scholar]
- Mishkin M. Memory in monkeys severely impaired by combined but not by separate removal of amygdala and hippocampus. Nature. 1978;273(5660):297–298. doi: 10.1038/273297a0. [DOI] [PubMed] [Google Scholar]
- Mitchell SJ, Rawlins JN, Steward O, Olton DS. Medial septal area lesions disrupt theta rhythm and cholinergic staining in medial entorhinal cortex and produce impaired radial arm maze behavior in rats. J Neurosci. 1982;2(3):292–302. doi: 10.1523/JNEUROSCI.02-03-00292.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitzdorf U. Current source-density method and application in cat cerebral cortex: investigation of evoked potentials and EEG phenomena. Physiol Rev. 1985;65(1):37–100. doi: 10.1152/physrev.1985.65.1.37. [DOI] [PubMed] [Google Scholar]
- Mizumori SJ, McNaughton BL, Barnes CA, Fox KB. Preserved spatial coding in hippocampal CA1 pyramidal cells during reversible suppression of CA3c output: evidence for pattern completion in hippocampus. J Neurosci. 1989;9(11):3915–3928. doi: 10.1523/JNEUROSCI.09-11-03915.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuseki K, Sirota A, Pastalkova E, Buzsaki G. Theta oscillations provide temporal windows for local circuit computation in the entorhinal-hippocampal loop. Neuron. 2009;64(2):267–280. doi: 10.1016/j.neuron.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moita MA, Rosis S, Zhou Y, LeDoux JE, Blair HT. Hippocampal place cells acquire location-specific responses to the conditioned stimulus during auditory fear conditioning. Neuron. 2003;37(3):485–497. doi: 10.1016/s0896-6273(03)00033-3. [DOI] [PubMed] [Google Scholar]
- Molle M, Eschenko O, Gais S, Sara SJ, Born J. The influence of learning on sleep slow oscillations and associated spindles and ripples in humans and rats. Eur J Neurosci. 2009;29(5):1071–1081. doi: 10.1111/j.1460-9568.2009.06654.x. [DOI] [PubMed] [Google Scholar]
- Montgomery SM, Betancur MI, Buzsaki G. Behavior-dependent coordination of multiple theta dipoles in the hippocampus. J Neurosci. 2009;29(5):1381–1394. doi: 10.1523/JNEUROSCI.4339-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SM, Buzsaki G. Gamma oscillations dynamically couple hippocampal CA3 and CA1 regions during memory task performance. Proc Natl Acad Sci USA. 2007;104(36):14495–14500. doi: 10.1073/pnas.0701826104. 0701826104 [pii]/pnas.0701826104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SM, Sirota A, Buzsaki G. Theta and gamma coordination of hippocampal networks during waking and rapid eye movement sleep. J Neurosci. 2008;28(26):6731–6741. doi: 10.1523/JNEUROSCI.1227-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297(5868):681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Morris RG, Schenk F, Tweedie F, Jarrard LE. Ibotenate lesions of hippocampus and/or subiculum: dissociating components of allocentric spatial learning. Eur J Neurosci. 1990;2(12):1016–1028. doi: 10.1111/j.1460-9568.1990.tb00014.x. [DOI] [PubMed] [Google Scholar]
- Moser E, Moser MB, Andersen P. Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. J Neurosci. 1993;13(9):3916–3925. doi: 10.1523/JNEUROSCI.13-09-03916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser MB, Moser EI. Functional differentiation in the hippocampus. Hippocampus. 1998;8(6):608–619. doi: 10.1002/(SICI)1098-1063(1998)8:6<608::AID-HIPO3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Moser MB, Moser EI, Forrest E, Andersen P, Morris RG. Spatial learning with a minislab in the dorsal hippocampus. Proc Natl Acad Sci USA. 1995;92(21):9697–9701. doi: 10.1073/pnas.92.21.9697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muenzinger KF. Vicarious trial and error at a point of choice. I. A general survey of its relation to learning efficiency. J Genet Psychol. 1938;12:75–86. [Google Scholar]
- Muller RU, Kubie JL. The effects of changes in the environment on the spatial firing of hippocampal complex-spike cells. J Neurosci. 1987;7(7):1951–1968. doi: 10.1523/JNEUROSCI.07-07-01951.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller RU, Kubie JL, Ranck JB., Jr Spatial firing patterns of hippocampal complex-spike cells in a fixed environment. J Neurosci. 1987;7(7):1935–1950. doi: 10.1523/JNEUROSCI.07-07-01935.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadasdy Z, Hirase H, Czurko A, Csicsvari J, Buzsaki G. Replay and time compression of recurring spike sequences in the hippocampus. J Neurosci. 1999;19(21):9497–9507. doi: 10.1523/JNEUROSCI.19-21-09497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashiba T, Buhl DL, McHugh TJ, Tonegawa S. Hippocampal CA3 output is crucial for ripple-associated reactivation and consolidation of memory. Neuron. 2009;62(6):781–787. doi: 10.1016/j.neuron.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashiba T, Cushman JD, Pelkey KA, Renaudineau S, Buhl DL, McHugh TJ, Rodriguez Barrera V, Chittajallu R, Iwamoto KS, McBain CJ, Fanselow MS, Tonegawa S. Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell. 2012;149(1):188–201. doi: 10.1016/j.cell.2012.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashiba T, Young JZ, McHugh TJ, Buhl DL, Tonegawa S. Transgenic inhibition of synaptic transmission reveals role of CA3 output in hippocampal learning. Science. 2008;319(5867):1260–1264. doi: 10.1126/science.1151120. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Quirk MC, Chitwood RA, Watanabe M, Yeckel MF, Sun LD, Kato A, Carr CA, Johnston D, Wilson MA, Tonegawa S. Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science. 2002;297(5579):211–218. doi: 10.1126/science.1071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K, Sun LD, Quirk MC, Rondi-Reig L, Wilson MA, Tonegawa S. Hippocampal CA3 NMDA receptors are crucial for memory acquisition of one-time experience. Neuron. 2003;38(2):305–315. doi: 10.1016/s0896-6273(03)00165-x. [DOI] [PubMed] [Google Scholar]
- Neunuebel JP, Knierim JJ. Spatial firing correlates of physiologically distinct cell types of the rat dentate gyrus. J Neurosci. 2012;32(11):3848–3858. doi: 10.1523/JNEUROSCI.6038-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neunuebel JP, Knierim JJ. CA3 retrieves coherent representations from degraded input: direct evidence for CA3 pattern completion and dentate gyrus pattern separation. Neuron. 2014;81(2):416–427. doi: 10.1016/j.neuron.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neunuebel JP, Yoganarasimha D, Rao G, Knierim JJ. Conflicts between local and global spatial frameworks dissociate neural representations of the lateral and medial entorhinal cortex. J Neurosci. 2013;33(22):9246–9258. doi: 10.1523/JNEUROSCI.0946-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitz D, McNaughton B. Differential modulation of CA1 and dentate gyrus interneurons during exploration of novel environments. J Neurophysiol. 2004;91(2):863–872. doi: 10.1152/jn.00614.2003. [DOI] [PubMed] [Google Scholar]
- Nokia MS, Mikkonen JE, Penttonen M, Wikgren J. Disrupting neural activity related to awake-state sharp wave-ripple complexes prevents hippocampal learning. Front Behav Neurosci. 2012;6:84. doi: 10.3389/fnbeh.2012.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe J. Place units in the hippocampus of the freely moving rat. Exp Neurol. 1976;51(1):78–109. doi: 10.1016/0014-4886(76)90055-8. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34(1):171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L. The hippocampus as a cognitive map. Oxford University Press; London: 1978. [Google Scholar]
- O’Keefe J, Recce ML. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus. 1993;3(3):317–330. doi: 10.1002/hipo.450030307. [DOI] [PubMed] [Google Scholar]
- O’Mara S. The subiculum: what it does, what it might do, and what neuroanatomy has yet to tell us. J Anat. 2005;207(3):271–282. doi: 10.1111/j.1469-7580.2005.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Mara S. Controlling hippocampal output: the central role of subiculum in hippocampal information processing. Behav Brain Res. 2006;174(2):304–312. doi: 10.1016/j.bbr.2006.08.018. [DOI] [PubMed] [Google Scholar]
- O’Neill J, Pleydell-Bouverie B, Dupret D, Csicsvari J. Play it again: reactivation of waking experience and memory. Trends Neurosci. 2010;33(5):220–229. doi: 10.1016/j.tins.2010.01.006. [DOI] [PubMed] [Google Scholar]
- O’Neill J, Senior T, Csicsvari J. Place-selective firing of CA1 pyramidal cells during sharp wave/ripple network patterns in exploratory behavior. Neuron. 2006;49(1):143–155. doi: 10.1016/j.neuron.2005.10.037. [DOI] [PubMed] [Google Scholar]
- O’Neill J, Senior TJ, Allen K, Huxter JR, Csicsvari J. Reactivation of experience-dependent cell assembly patterns in the hippocampus. Nat Neurosci. 2008;11(2):209–215. doi: 10.1038/nn2037. [DOI] [PubMed] [Google Scholar]
- Ochiishi T, Saitoh Y, Yukawa A, Saji M, Ren Y, Shirao T, Miyamoto H, Nakata H, Sekino Y. High level of adenosine A1 receptor-like immunoreactivity in the CA2/CA3a region of the adult rat hippocampus. Neuroscience. 1999;93(3):955–967. doi: 10.1016/s0306-4522(99)00179-7. [DOI] [PubMed] [Google Scholar]
- Olton DS, Branch M, Best PJ. Spatial correlates of hippocampal unit activity. Exp Neurol. 1978;58(3):387–409. doi: 10.1016/0014-4886(78)90096-1. [DOI] [PubMed] [Google Scholar]
- Olton DS, Papas BC. Spatial memory and hippocampal function. Neuropsychologia. 1979;17(6):669–682. doi: 10.1016/0028-3932(79)90042-3. [DOI] [PubMed] [Google Scholar]
- Olypher AV, Lansky P, Muller RU, Fenton AA. Quantifying location-specific information in the discharge of rat hippocampal place cells. J Neurosci Methods. 2003;127(2):123–135. doi: 10.1016/s0165-0270(03)00123-7. [DOI] [PubMed] [Google Scholar]
- Osborne LC, Palmer SE, Lisberger SG, Bialek W. The neural basis for combinatorial coding in a cortical population response. J Neurosci. 2008;28(50):13522–13531. doi: 10.1523/JNEUROSCI.4390-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla-Coreano N, Bolkan SS, Pierce GM, Blackman DR, Hardin WD, Garcia-Garcia AL, Spellman TJ, Gordon JA. Direct ventral hippocampal-prefrontal input is required for anxiety-related neural activity and behavior. Neuron. 2016;89(4):857–866. doi: 10.1016/j.neuron.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani JH, Zhao M, Cui Z, Avram SK, Caruana DA, Dudek SM, Young WS. Role of the vasopressin 1b receptor in rodent aggressive behavior and synaptic plasticity in hippocampal area CA2. Mol Psychiatry. 2015;20(4):490–499. doi: 10.1038/mp.2014.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan WX, McNaughton N. The role of the medial supramammillary nucleus in the control of hippocampal theta activity and behaviour in rats. Eur J Neurosci. 2002;16(9):1797–1809. doi: 10.1046/j.1460-9568.2002.02267.x. [DOI] [PubMed] [Google Scholar]
- Papp G, Witter MP, Treves A. The CA3 network as a memory store for spatial representations. Learn Mem. 2007;14(11):732–744. doi: 10.1101/lm.687407. [DOI] [PubMed] [Google Scholar]
- Park E, Dvorak D, Fenton AA. Ensemble place codes in hippocampus: CA1, CA3, and dentate gyrus place cells have multiple place fields in large environments. PLoS ONE. 2011;6(7):e22349. doi: 10.1371/journal.pone.0022349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastalkova E, Itskov V, Amarasingham A, Buzsaki G. Internally generated cell assembly sequences in the rat hippocampus. Science. 2008;321(5894):1322–1327. doi: 10.1126/science.1159775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel J, Fujisawa S, Berenyi A, Royer S, Buzsaki G. Traveling theta waves along the entire septotemporal axis of the hippocampus. Neuron. 2012;75(3):410–417. doi: 10.1016/j.neuron.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel J, Schomburg EW, Berenyi A, Fujisawa S, Buzsaki G. Local generation and propagation of ripples along the septotemporal axis of the hippocampus. J Neurosci. 2013;33(43):17029–17041. doi: 10.1523/JNEUROSCI.2036-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlides C, Winson J. Influences of hippocampal place cell firing in the awake state on the activity of these cells during subsequent sleep episodes. J Neurosci. 1989;9(8):2907–2918. doi: 10.1523/JNEUROSCI.09-08-02907.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz R, Pare D. Physiological basis for emotional modulation of memory circuits by the amygdala. Curr Opin Neurobiol. 2013;23(3):381–386. doi: 10.1016/j.conb.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penttonen M, Kamondi A, Sik A, Acsady L, Buzsaki G. Feed-forward and feed-back activation of the dentate gyrus in vivo during dentate spikes and sharp wave bursts. Hippocampus. 1997;7(4):437–450. doi: 10.1002/(SICI)1098-1063(1997)7:4<437::AID-HIPO9>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Petrulis A, Alvarez P, Eichenbaum H. Neural correlates of social odor recognition and the representation of individual distinctive social odors within entorhinal cortex and ventral subiculum. Neuroscience. 2005;130(1):259–274. doi: 10.1016/j.neuroscience.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Petsche H, Stumpf C. The origin of theta-rhytm in the rabbit hippocampus. Wien Klin Wochenschr. 1962;74:696–700. [PubMed] [Google Scholar]
- Pfeiffer BE, Foster DJ. Hippocampal place-cell sequences depict future paths to remembered goals. Nature. 2013;497(7447):74–79. doi: 10.1038/nature12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer BE, Foster DJ. Place cells. Autoassociative dynamics in the generation of sequences of hippocampal place cells. Science. 2015;349(6244):180–183. doi: 10.1126/science.aaa9633. [DOI] [PubMed] [Google Scholar]
- Pikkarainen M, Ronkko S, Savander V, Insausti R, Pitkanen A. Projections from the lateral, basal, and accessory basal nuclei of the amygdala to the hippocampal formation in rat. J Comp Neurol. 1999;403(2):229–260. [PubMed] [Google Scholar]
- Piskorowski RA, Chevaleyre V. Synaptic integration by different dendritic compartments of hippocampal CA1 and CA2 pyramidal neurons. Cell Mol Life Sci. 2012;69(1):75–88. doi: 10.1007/s00018-011-0769-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkanen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat—A review. Ann Ny Acad Sci. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- Pothuizen HH, Zhang WN, Jongen-Relo AL, Feldon J, Yee BK. Dissociation of function between the dorsal and the ventral hippocampus in spatial learning abilities of the rat: a within-subject, within-task comparison of reference and working spatial memory. Eur J Neurosci. 2004;19(3):705–712. doi: 10.1111/j.0953-816x.2004.03170.x. [DOI] [PubMed] [Google Scholar]
- Potvin O, Allen K, Thibaudeau G, Dore FY, Goulet S. Performance on spatial working memory tasks after dorsal or ventral hippocampal lesions and adjacent damage to the subiculum. Behav Neurosci. 2006;120(2):413–422. doi: 10.1037/0735-7044.120.2.413. [DOI] [PubMed] [Google Scholar]
- Potvin O, Dore FY, Goulet S. Contributions of the dorsal hippocampus and the dorsal subiculum to processing of idiothetic information and spatial memory. Neurobiol Learn Mem. 2007;87(4):669–678. doi: 10.1016/j.nlm.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Poucet B, Thinus-Blanc C, Muller RU. Place cells in the ventral hippocampus of rats. NeuroReport. 1994;5(16):2045–2048. doi: 10.1097/00001756-199410270-00014. [DOI] [PubMed] [Google Scholar]
- Rajasethupathy P, Sankaran S, Marshel JH, Kim CK, Ferenczi E, Lee SY, Berndt A, Ramakrishnan C, Jaffe A, Lo M, Liston C, Deisseroth K. Projections from neocortex mediate top-down control of memory retrieval. Nature. 2015;526(7575):653–659. doi: 10.1038/nature15389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadan W, Eschenko O, Sara SJ. Hippocampal sharp wave/ripples during sleep for consolidation of associative memory. PLoS ONE. 2009;4(8):e6697. doi: 10.1371/journal.pone.0006697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajal SR. Estructura del asta de Ammon y fascia dentata. Ann Soc Esp Hist Nat. 1893:22. [Google Scholar]
- Rangel LM, Quinn LK, Chiba AA, Gage FH, Aimone JB. A hypothesis for temporal coding of young and mature granule cells. Front Neurosci. 2013;7:75. doi: 10.3389/fnins.2013.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renno-Costa C, Lisman JE, Verschure PF. A signature of attractor dynamics in the CA3 region of the hippocampus. PLoS Comput Biol. 2014;10(5):e1003641. doi: 10.1371/journal.pcbi.1003641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard GR, Titiz A, Tyler A, Holmes GL, Scott RC, Lenck-Santini PP. Speed modulation of hippocampal theta frequency correlates with spatial memory performance. Hippocampus. 2013;23(12):1269–1279. doi: 10.1002/hipo.22164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond MA, Yee BK, Pouzet B, Veenman L, Rawlins JN, Feldon J, Bannerman DM. Dissociating context and space within the hippocampus: effects of complete, dorsal, and ventral excitotoxic hippocampal lesions on conditioned freezing and spatial learning. Behav Neurosci. 1999;113(6):1189–1203. doi: 10.1037/0735-7044.113.6.1189. [DOI] [PubMed] [Google Scholar]
- Rolls ET. An attractor network in the hippocampus: theory and neurophysiology. Learn Mem. 2007;14(11):714–731. doi: 10.1101/lm.631207. [DOI] [PubMed] [Google Scholar]
- Rosene DL, Van Hoesen GW. Hippocampal efferents reach widespread areas of cerebral cortex and amygdala in the rhesus monkey. Science. 1977;198(4314):315–317. doi: 10.1126/science.410102. [DOI] [PubMed] [Google Scholar]
- Roumis DK, Frank LM. Hippocampal sharp-wave ripples in waking and sleeping states. Curr Opin Neurobiol. 2015;35:6–12. doi: 10.1016/j.conb.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland DC, Weible AP, Wickersham IR, Wu H, Mayford M, Witter MP, Kentros CG. Transgenically targeted rabies virus demonstrates a major monosynaptic projection from hippocampal area CA2 to medial entorhinal layer II neurons. J Neurosci. 2013;33(37):14889–14898. doi: 10.1523/JNEUROSCI.1046-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer S, Sirota A, Patel J, Buzsaki G. Distinct representations and theta dynamics in dorsal and ventral hippocampus. J Neurosci. 2010;30(5):1777–1787. doi: 10.1523/JNEUROSCI.4681-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski JH, Jones MW, Mellor JR. Sharp-wave ripples orchestrate the induction of synaptic plasticity during reactivation of place cell firing patterns in the hippocampus. Cell Rep. 2016;14(8):1916–1929. doi: 10.1016/j.celrep.2016.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O’Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472(7344):466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Antonio A, Liban K, Ikrar T, Tsyganovskiy E, Xu X. Distinct physiological and developmental properties of hippocampal CA2 subfield revealed by using anti-Purkinje cell protein 4 (PCP4) immunostaining. J Comp Neurol. 2014;522(6):1333–1354. doi: 10.1002/cne.23486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargolini F, Fyhn M, Hafting T, McNaughton BL, Witter MP, Moser MB, Moser EI. Conjunctive representation of position, direction, and velocity in entorhinal cortex. Science. 2006;312(5774):758–762. doi: 10.1126/science.1125572. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Leutgeb S, Leutgeb JK. Spatial and memory circuits in the medial entorhinal cortex. Curr Opin Neurobiol. 2015;32:16–23. doi: 10.1016/j.conb.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savanthrapadian S, Meyer T, Elgueta C, Booker SA, Vida I, Bartos M. Synaptic properties of SOM- and CCK-expressing cells in dentate gyrus interneuron networks. J Neurosci. 2014;34(24):8197–8209. doi: 10.1523/JNEUROSCI.5433-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savelli F, Yoganarasimha D, Knierim JJ. Influence of boundary removal on the spatial representations of the medial entorhinal cortex. Hippocampus. 2008;18(12):1270–1282. doi: 10.1002/hipo.20511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE. Evidence from simultaneous intracellular recordings in rat hippocampal slices that area CA3 pyramidal cells innervate dentate hilar mossy cells. J Neurophysiol. 1994;72(5):2167–2180. doi: 10.1152/jn.1994.72.5.2167. [DOI] [PubMed] [Google Scholar]
- Scharfman HE. The CA3 “backprojection” to the dentate gyrus. Prog Brain Res. 2007;163:627–637. doi: 10.1016/S0079-6123(07)63034-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Myers CE. Hilar mossy cells of the dentate gyrus: a historical perspective. Front Neural Circuits. 2012;6:106. doi: 10.3389/fncir.2012.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer-Teixeira R, Belchior H, Caixeta FV, Souza BC, Ribeiro S, Tort AB. Theta phase modulates multiple layer-specific oscillations in the CA1 region. Cereb Cortex. 2012;22(10):2404–2414. doi: 10.1093/cercor/bhr319. [DOI] [PubMed] [Google Scholar]
- Schiller D, Eichenbaum H, Buffalo EA, Davachi L, Foster DJ, Leutgeb S, Ranganath C. Memory and space: towards an understanding of the cognitive map. J Neurosci. 2015;35(41):13904–13911. doi: 10.1523/JNEUROSCI.2618-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesiger MI, Cannova CC, Boublil BL, Hales JB, Mankin EA, Brandon MP, Leutgeb JK, Leibold C, Leutgeb S. The medial entorhinal cortex is necessary for temporal organization of hippocampal neuronal activity. Nat Neurosci. 2015;18(8):1123–1132. doi: 10.1038/nn.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesiger MI, Cressey JC, Boublil B, Koenig J, Melvin NR, Leutgeb JK, Leutgeb S. Hippocampal activation during the recall of remote spatial memories in radial maze tasks. Neurobiol Learn Mem. 2013;106:324–333. doi: 10.1016/j.nlm.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Schmidt B, Hinman JR, Jacobson TK, Szkudlarek E, Argraves M, Escabi MA, Markus EJ. Dissociation between dorsal and ventral hippocampal theta oscillations during decision-making. J Neurosci. 2013;33(14):6212–6224. doi: 10.1523/JNEUROSCI.2915-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429(6988):184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20(1):11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekino Y, Obata K, Tanifuji M, Mizuno M, Murayama J. Delayed signal propagation via CA2 in rat hippocampal slices revealed by optical recording. J Neurophysiol. 1997;78(3):1662–1668. doi: 10.1152/jn.1997.78.3.1662. [DOI] [PubMed] [Google Scholar]
- Sharp PE. Multiple spatial/behavioral correlates for cells in the rat postsubiculum: multiple regression analysis and comparison to other hippocampal areas. Cereb Cortex. 1996;6(2):238–259. doi: 10.1093/cercor/6.2.238. [DOI] [PubMed] [Google Scholar]
- Sharp PE. Subicular place cells generate the same “map” for different environments: Comparison with hippocampal cells. Behav Brain Res. 2006;174(2):206–214. doi: 10.1016/j.bbr.2006.05.034. [DOI] [PubMed] [Google Scholar]
- Sharp PE, Green C. Spatial correlates of firing patterns of single cells in the subiculum of the freely moving rat. J Neurosci. 1994;14(4):2339–2356. doi: 10.1523/JNEUROSCI.14-04-02339.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Kudrimoti HS, McNaughton BL, Barnes CA. Reactivation of neuronal ensembles in hippocampal dentate gyrus during sleep after spatial experience. J Sleep Res. 1998;7(Suppl 1):6–16. doi: 10.1046/j.1365-2869.7.s1.2.x. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410(6826):372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Siapas AG, Lubenov EV, Wilson MA. Prefrontal phase locking to hippocampal theta oscillations. Neuron. 2005;46(1):141–151. doi: 10.1016/j.neuron.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Siapas AG, Wilson MA. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron. 1998;21(5):1123–1128. doi: 10.1016/s0896-6273(00)80629-7. [DOI] [PubMed] [Google Scholar]
- Siegle JH, Wilson MA. Enhancement of encoding and retrieval functions through theta phase-specific manipulation of hippocampus. eLife. 2014;3:e03061. doi: 10.7554/eLife.03061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sik A, Penttonen M, Ylinen A, Buzsaki G. Hippocampal CA1 interneurons: an in vivo intracellular labeling study. J Neurosci. 1995;15(10):6651–6665. doi: 10.1523/JNEUROSCI.15-10-06651.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sik A, Ylinen A, Penttonen M, Buzsaki G. Inhibitory CA1-CA3-hilar region feedback in the hippocampus. Science. 1994;265(5179):1722–1724. doi: 10.1126/science.8085161. [DOI] [PubMed] [Google Scholar]
- Silva D, Feng T, Foster DJ. Trajectory events across hippocampal place cells require previous experience. Nat Neurosci. 2015;18(12):1772–1779. doi: 10.1038/nn.4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons SB, Escobedo Y, Yasuda R, Dudek SM. Regional differences in hippocampal calcium handling provide a cellular mechanism for limiting plasticity. Proc Natl Acad Sci USA. 2009;106(33):14080–14084. doi: 10.1073/pnas.0904775106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer AC, Carr MF, Karlsson MP, Frank LM. Hippocampal SWR activity predicts correct decisions during the initial learning of an alternation task. Neuron. 2013;77(6):1163–1173. doi: 10.1016/j.neuron.2013.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer AC, Frank LM. Rewarded outcomes enhance reactivation of experience in the hippocampus. Neuron. 2009;64(6):910–921. doi: 10.1016/j.neuron.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer AC, Karlsson MP, Nathe AR, Carr MF, Frank LM. Experience-dependent development of coordinated hippocampal spatial activity representing the similarity of related locations. J Neurosci. 2010;30(35):11586–11604. doi: 10.1523/JNEUROSCI.0926-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirota A, Csicsvari J, Buhl D, Buzsaki G. Communication between neocortex and hippocampus during sleep in rodents. Proc Natl Acad Sci USA. 2003;100(4):2065–2069. doi: 10.1073/pnas.0437938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL. Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience. Science. 1996;271(5257):1870–1873. doi: 10.1126/science.271.5257.1870. [DOI] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL, Permenter M, Archibeque M, Vogt J, Amaral DG, Barnes CA. EEG sharp waves and sparse ensemble unit activity in the macaque hippocampus. J Neurophysiol. 2007;98(2):898–910. doi: 10.1152/jn.00401.2007. [DOI] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL, Wilson MA, Barnes CA. Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus. 1996;6(2):149–172. doi: 10.1002/(SICI)1098-1063(1996)6:2<149::AID-HIPO6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Solstad T, Boccara CN, Kropff E, Moser MB, Moser EI. Representation of geometric borders in the entorhinal cortex. Science. 2008;322(5909):1865–1868. doi: 10.1126/science.1166466. [DOI] [PubMed] [Google Scholar]
- Spruston N. Pyramidal neurons: dendritic structure and synaptic integration. Nat Rev Neurosci. 2008;9(3):206–221. doi: 10.1038/nrn2286. [DOI] [PubMed] [Google Scholar]
- Squire LR, Wixted JT. The cognitive neuroscience of human memory since H.M. Annu Rev Neurosci. 2011;34:259–288. doi: 10.1146/annurev-neuro-061010-113720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staff NP, Jung HY, Thiagarajan T, Yao M, Spruston N. Resting and active properties of pyramidal neurons in subiculum and CA1 of rat hippocampus. J Neurophysiol. 2000;84(5):2398–2408. doi: 10.1152/jn.2000.84.5.2398. [DOI] [PubMed] [Google Scholar]
- Stark E, Roux L, Eichler R, Senzai Y, Royer S, Buzsaki G. Pyramidal cell-interneuron interactions underlie hippocampal ripple oscillations. Neuron. 2014;83(2):467–480. doi: 10.1016/j.neuron.2014.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stensola H, Stensola T, Solstad T, Froland K, Moser MB, Moser EI. The entorhinal grid map is discretized. Nature. 2012;492(7427):72–78. doi: 10.1038/nature11649. [DOI] [PubMed] [Google Scholar]
- Steriade M, Nunez A, Amzica F. A novel slow (< 1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J Neurosci. 1993;13(8):3252–3265. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson EL, Caldwell HK. Lesions to the CA2 region of the hippocampus impair social memory in mice. Eur J Neurosci. 2014;40(9):3294–3301. doi: 10.1111/ejn.12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Scoville SA. Cells of origin of entorhinal cortical afferents to the hippocampus and fascia dentata of the rat. J Comp Neurol. 1976;169(3):347–370. doi: 10.1002/cne.901690306. [DOI] [PubMed] [Google Scholar]
- Stewart M, Fox SE. Do septal neurons pace the hippocampal theta rhythm? Trends Neurosci. 1990;13(5):163–168. doi: 10.1016/0166-2236(90)90040-h. [DOI] [PubMed] [Google Scholar]
- Strange BA, Witter MP, Lein ES, Moser EI. Functional organization of the hippocampal longitudinal axis. Nat Rev Neurosci. 2014;15(10):655–669. doi: 10.1038/nrn3785. [DOI] [PubMed] [Google Scholar]
- Sullivan D, Csicsvari J, Mizuseki K, Montgomery S, Diba K, Buzsaki G. Relationships between hippocampal sharp waves, ripples, and fast gamma oscillation: influence of dentate and entorhinal cortical activity. J Neurosci. 2011;31(23):8605–861. doi: 10.1523/JNEUROSCI.0294-11.2011. 31/23/8605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Nguyen AQ, Nguyen JP, Le L, Saur D, Choi J, Callaway EM, Xu X. Cell-type-specific circuit connectivity of hippocampal CA1 revealed through cre-dependent rabies tracing. Cell Rep. 2014;7(1):269–280. doi: 10.1016/j.celrep.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki SS, Smith GK. Spontaneous EEG spikes in the normal hippocampus. I. Behavioral correlates, laminar profiles and bilateral synchrony. Electroencephalogr Clin Neurophysiol. 1987;67(4):348–359. doi: 10.1016/0013-4694(87)90123-4. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Cowan WM. An autoradiographic study of the organization of the efferent connections of the hippocampal formation in the rat. J Comp Neurol. 1977;172(1):49–84. doi: 10.1002/cne.901720104. [DOI] [PubMed] [Google Scholar]
- Takacs VT, Klausberger T, Somogyi P, Freund TF, Gulyas AI. Extrinsic and local glutamatergic inputs of the rat hippocampal CA1 area differentially innervate pyramidal cells and interneurons. Hippocampus. 2012;22(6):1379–1391. doi: 10.1002/hipo.20974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamamaki N, Abe K, Nojyo Y. Three-dimensional analysis of the whole axonal arbors originating from single CA2 pyramidal neurons in the rat hippocampus with the aid of a computer graphic technique. Brain Res. 1988;452(1–2):255–272. doi: 10.1016/0006-8993(88)90030-3. [DOI] [PubMed] [Google Scholar]
- Tao C, Zhang G, Xiong Y, Zhou Y. Functional dissection of synaptic circuits: in vivo patch-clamp recording in neuroscience. Front Neural Circuits. 2015;9:23. doi: 10.3389/fncir.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JS. The head direction signal: origins and sensory-motor integration. Annu Rev Neurosci. 2007;30:181–207. doi: 10.1146/annurev.neuro.29.051605.112854. [DOI] [PubMed] [Google Scholar]
- Temprana SG, Mongiat LA, Yang SM, Trinchero MF, Alvarez DD, Kropff E, Giacomini D, Beltramone N, Lanuza GM, Schinder AF. Delayed coupling to feedback inhibition during a critical period for the integration of adult-born granule cells. Neuron. 2015;85(1):116–130. doi: 10.1016/j.neuron.2014.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesche CD, Karhu J. Theta oscillations index human hippocampal activation during a working memory task. Proc Natl Acad Sci USA. 2000;97(2):919–924. doi: 10.1073/pnas.97.2.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson LT, Best PJ. Long-term stability of the place-field activity of single units recorded from the dorsal hippocampus of freely behaving rats. Brain Res. 1990;509(2):299–308. doi: 10.1016/0006-8993(90)90555-p. [DOI] [PubMed] [Google Scholar]
- Tolman EC. The determiners of behavior at a choice point. Psychol Rev. 1938;45:1–41. [Google Scholar]
- Tort AB, Komorowski R, Eichenbaum H, Kopell N. Measuring phase-amplitude coupling between neuronal oscillations of different frequencies. J Neurophysiol. 2010;104(2):1195–1210. doi: 10.1152/jn.00106.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tort AB, Komorowski RW, Manns JR, Kopell NJ, Eichenbaum H. Theta-gamma coupling increases during the learning of item-context associations. Proc Natl Acad Sci USA. 2009;106(49):20942–20947. doi: 10.1073/pnas.0911331106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth K, Borhegyi Z, Freund TF. Postsynaptic targets of GABAergic hippocampal neurons in the medial septum-diagonal band of broca complex. J Neurosci. 1993;13(9):3712–3724. doi: 10.1523/JNEUROSCI.13-09-03712.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth K, Freund TF. Calbindin D28 k-containing nonpyramidal cells in the rat hippocampus: their immunoreactivity for GABA and projection to the medial septum. Neuroscience. 1992;49(4):793–805. doi: 10.1016/0306-4522(92)90357-8. [DOI] [PubMed] [Google Scholar]
- Treves A, Rolls E. What determines the capacity of autoassociative memories in the brain? Comp Neural Sys. 1991;2(4):371–397. [Google Scholar]
- Treves A, Rolls ET. Computational constraints suggest the need for two distinct input systems to the hippocampal CA3 network. Hippocampus. 1992;2(2):189–199. doi: 10.1002/hipo.450020209. [DOI] [PubMed] [Google Scholar]
- Treves A, Rolls ET. Computational analysis of the role of the hippocampus in memory. Hippocampus. 1994;4(3):374–391. doi: 10.1002/hipo.450040319. [DOI] [PubMed] [Google Scholar]
- Tronel S, Belnoue L, Grosjean N, Revest JM, Piazza PV, Koehl M, Abrous DN. Adult-born neurons are necessary for extended contextual discrimination. Hippocampus. 2012;22(2):292–298. doi: 10.1002/hipo.20895. [DOI] [PubMed] [Google Scholar]
- Ulanovsky N, Moss CF. Hippocampal cellular and network activity in freely moving echolocating bats. Nat Neurosci. 2007;10(2):224–233. doi: 10.1038/nn1829. [DOI] [PubMed] [Google Scholar]
- Valero M, Cid E, Averkin RG, Aguilar J, Sanchez-Aguilera A, Viney TJ, Gomez-Dominguez D, Bellistri E, de la Prida LM. Determinants of different deep and superficial CA1 pyramidal cell dynamics during sharp-wave ripples. Nat Neurosci. 2015;18(9):1281–1290. doi: 10.1038/nn.4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cauter T, Poucet B, Save E. Unstable CA1 place cell representation in rats with entorhinal cortex lesions. Eur J Neurosci. 2008;27(8):1933–1946. doi: 10.1111/j.1460-9568.2008.06158.x. [DOI] [PubMed] [Google Scholar]
- van der Meer MA, Redish AD. Theta phase precession in rat ventral striatum links place and reward information. J Neurosci. 2011;31(8):2843–2854. doi: 10.1523/JNEUROSCI.4869-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Strien NM, Cappaert NL, Witter MP. The anatomy of memory: an interactive overview of the parahippocampal-hippocampal network. Nat Rev Neurosci. 2009;10(4):272–282. doi: 10.1038/nrn2614. [DOI] [PubMed] [Google Scholar]
- Vanderwolf CH. Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr Clin Neurophysiol. 1969;26(4):407–418. doi: 10.1016/0013-4694(69)90092-3. [DOI] [PubMed] [Google Scholar]
- VanElzakker M, Fevurly RD, Breindel T, Spencer RL. Environmental novelty is associated with a selective increase in Fos expression in the output elements of the hippocampal formation and the perirhinal cortex. Learn Mem. 2008;15(12):899–908. doi: 10.1101/lm.1196508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazdarjanova A, Guzowski JF. Differences in hippocampal neuronal population responses to modifications of an environmental context: evidence for distinct, yet complementary, functions of CA3 and CA1 ensembles. J Neurosci. 2004;24(29):6489–6496. doi: 10.1523/JNEUROSCI.0350-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellano CP, Lee SE, Dudek SM, Hepler JR. RGS14 at the interface of hippocampal signaling and synaptic plasticity. Trends Pharmacol Sci. 2011;32(11):666–674. doi: 10.1016/j.tips.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, Szigeti-Buck K, Leranth C. Nucleus reuniens of the midline thalamus: link between the medial prefrontal cortex and the hippocampus. Brain Res Bull. 2007;71(6):601–609. doi: 10.1016/j.brainresbull.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradova OS. Hippocampus as comparator: role of the two input and two output systems of the hippocampus in selection and registration of information. Hippocampus. 2001;11(5):578–598. doi: 10.1002/hipo.1073. [DOI] [PubMed] [Google Scholar]
- Wang ME, Fraize NP, Yin L, Yuan RK, Petsagourakis D, Wann EG, Muzzio IA. Differential roles of the dorsal and ventral hippocampus in predator odor contextual fear conditioning. Hippocampus. 2013;23(6):451–466. doi: 10.1002/hipo.22105. [DOI] [PubMed] [Google Scholar]
- Watrous AJ, Lee DJ, Izadi A, Gurkoff GG, Shahlaie K, Ekstrom AD. A comparative study of human and rat hippocampal low-frequency oscillations during spatial navigation. Hippocampus. 2013;23(8):656–661. doi: 10.1002/hipo.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeden CS, Roberts JM, Kamm AM, Kesner RP. The role of the ventral dentate gyrus in anxiety-based behaviors. Neurobiol Learn Mem. 2015;118:143–149. doi: 10.1016/j.nlm.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Ginns EI, O’Carroll AM, Lolait SJ, Young WS., 3rd Vasopressin V1b receptor knockout reduces aggressive behavior in male mice. Mol Psychiatry. 2002;7(9):975–984. doi: 10.1038/sj.mp.4001195. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Kelliher KR, Zufall F, Lolait SJ, O’Carroll AM, Young WS., 3rd Social motivation is reduced in vasopressin 1b receptor null mice despite normal performance in an olfactory discrimination task. Horm Behav. 2004;46(5):638–645. doi: 10.1016/j.yhbeh.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Wespatat V, Tennigkeit F, Singer W. Phase sensitivity of synaptic modifications in oscillating cells of rat visual cortex. J Neurosci. 2004;24(41):9067–9075. doi: 10.1523/JNEUROSCI.2221-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierzynski CM, Lubenov EV, Gu M, Siapas AG. State-dependent spike-timing relationships between hippocampal and prefrontal circuits during sleep. Neuron. 2009;61(4):587–596. doi: 10.1016/j.neuron.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikenheiser AM, Redish AD. Hippocampal theta sequences reflect current goals. Nat Neurosci. 2015;18(2):289–294. doi: 10.1038/nn.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DI, Langston RF, Schlesiger MI, Wagner M, Watanabe S, Ainge JA. Lateral entorhinal cortex is critical for novel object-context recognition. Hippocampus. 2013;23(5):352–366. doi: 10.1002/hipo.22095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265(5172):676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- Winson J. Interspecies differences in the occurrence of theta. Behav Biol. 1972;7(4):479–487. doi: 10.1016/s0091-6773(72)80210-4. [DOI] [PubMed] [Google Scholar]
- Winson J, Abzug C. Neuronal transmission through hippocampal pathways dependent on behavior. J Neurophys. 1978;41(3):716–732. doi: 10.1152/jn.1978.41.3.716. [DOI] [PubMed] [Google Scholar]
- Witter MP. A survey of the anatomy of the hippocampal formation, with emphasis on the septotemporal organization of its intrinsic and extrinsic connections. Adv Exp Med Biol. 1986;203:67–82. doi: 10.1007/978-1-4684-7971-3_5. [DOI] [PubMed] [Google Scholar]
- Witter MP. Organization of the entorhinal-hippocampal system: a review of current anatomical data. Hippocampus. 1993;3(Spec):33–44. [PubMed] [Google Scholar]
- Witter MP. Connections of the subiculum of the rat: topography in relation to columnar and laminar organization. Behav Brain Res. 2006;174(2):251–264. doi: 10.1016/j.bbr.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Witter MP. Intrinsic and extrinsic wiring of CA3: indications for connectional heterogeneity. Learn Mem. 2007;14(11):705–713. doi: 10.1101/lm.725207. [DOI] [PubMed] [Google Scholar]
- Witter MP, Amaral DG. Hippocampal formation. In: Paxinos G, editor. The rat nervous system. Elsevier Academic Press; San Diego: 2004. pp. 635–704. [Google Scholar]
- Witter MP, Groenewegen HJ, Lopes da Silva FH, Lohman AH. Functional organization of the extrinsic and intrinsic circuitry of the parahippocampal region. Prog Neurobiol. 1989;33(3):161–253. doi: 10.1016/0301-0082(89)90009-9. [DOI] [PubMed] [Google Scholar]
- Witter MP, Wouterlood FG, Naber PA, Van Haeften T. Anatomical organization of the parahippocampal-hippocampal network. Ann N Y Acad Sci. 2000;911:1–24. doi: 10.1111/j.1749-6632.2000.tb06716.x. [DOI] [PubMed] [Google Scholar]
- Wojtowicz JM, Askew ML, Winocur G. The effects of running and of inhibiting adult neurogenesis on learning and memory in rats. Eur J Neurosci. 2008;27(6):1494–1502. doi: 10.1111/j.1460-9568.2008.06128.x. [DOI] [PubMed] [Google Scholar]
- Wood ER, Dudchenko PA, Robitsek RJ, Eichenbaum H. Hippocampal neurons encode information about different types of memory episodes occurring in the same location. Neuron. 2000;27(3):623–633. doi: 10.1016/s0896-6273(00)00071-4. [DOI] [PubMed] [Google Scholar]
- Wu X, Foster DJ. Hippocampal replay captures the unique topological structure of a novel environment. J Neurosci. 2014;34(19):6459–6469. doi: 10.1523/JNEUROSCI.3414-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yartsev MM, Witter MP, Ulanovsky N. Grid cells without theta oscillations in the entorhinal cortex of bats. Nature. 2011;479(7371):103–107. doi: 10.1038/nature10583. [DOI] [PubMed] [Google Scholar]
- Ylinen A, Bragin A, Nadasdy Z, Jando G, Szabo I, Sik A, Buzsaki G. Sharp wave-associated high-frequency oscillation (200 Hz) in the intact hippocampus: network and intracellular mechanisms. J Neurosci. 1995;15(1 Pt 1):30–46. doi: 10.1523/JNEUROSCI.15-01-00030.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoganarasimha D, Rao G, Knierim JJ. Lateral entorhinal neurons are not spatially selective in cue-rich environments. Hippocampus. 2011;21(12):1363–1374. doi: 10.1002/hipo.20839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young BJ, Fox GD, Eichenbaum H. Correlates of hippocampal complex-spike cell activity in rats performing a nonspatial radial maze task. J Neurosci. 1994;14(11 Pt 1):6553–6563. doi: 10.1523/JNEUROSCI.14-11-06553.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young WS, Li J, Wersinger SR, Palkovits M. The vasopressin 1b receptor is prominent in the hippocampal area CA2 where it is unaffected by restraint stress or adrenalectomy. Neuroscience. 2006;143(4):1031–1039. doi: 10.1016/j.neuroscience.2006.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SJ, Ye J, Miao C, Tsao A, Cerniauskas I, Ledergerber D, Moser MB, Moser EI. Optogenetic dissection of entorhinal-hippocampal functional connectivity. Science. 2013;340(6128):1232627. doi: 10.1126/science.1232627. [DOI] [PubMed] [Google Scholar]
- Zhang WN, Bast T, Feldon J. The ventral hippocampus and fear conditioning in rats: different anterograde amnesias of fear after infusion of N-methyl-D-aspartate or its noncompetitive antagonist MK-801 into the ventral hippocampus. Behav Brain Res. 2001;126(1–2):159–174. doi: 10.1016/s0166-4328(01)00256-x. [DOI] [PubMed] [Google Scholar]
- Zhao M, Choi YS, Obrietan K, Dudek SM. Synaptic plasticity (and the lack thereof) in hippocampal CA2 neurons. J Neurosci. 2007;27(44):12025–12032. doi: 10.1523/JNEUROSCI.4094-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Lein ES, He A, Smith SC, Aston C, Gage FH. Transcriptional profiling reveals strict boundaries between hippocampal subregions. J Comp Neurol. 2001;441(3):187–196. doi: 10.1002/cne.1406. [DOI] [PubMed] [Google Scholar]
- Zheng C, Bieri KW, Hsiao YT, Colgin LL. Spatial sequence coding differs during slow and fast gamma rhythms in the hippocampus. Neuron. 2016;89(2):398–408. doi: 10.1016/j.neuron.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C, Bieri KW, Trettel SG, Colgin LL. The relationship between gamma frequency and running speed differs for slow and fast gamma rhythms in freely behaving rats. Hippocampus. 2015;25(8):924–938. doi: 10.1002/hipo.22415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv Y, Burns LD, Cocker ED, Hamel EO, Ghosh KK, Kitch LJ, El Gamal A, Schnitzer MJ. Long-term dynamics of CA1 hippocampal place codes. Nat Neurosci. 2013;16(3):264–266. doi: 10.1038/nn.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]