Abstract
The insulin receptor (IR) gene undergoes differential splicing that generates two IR isoforms, IR-A and IR-B. The physiological roles of IR isoforms are incompletely understood and appear to be determined by their different binding affinities for insulin-like growth factors (IGFs), particularly for IGF-2. Predominant roles of IR-A in prenatal growth and development and of IR-B in metabolic regulation are well established. However, emerging evidence indicates that the differential expression of IR isoforms may also help explain the diversification of insulin and IGF signaling and actions in various organs and tissues by involving not only different ligand-binding affinities but also different membrane partitioning and trafficking and possibly different abilities to interact with a variety of molecular partners. Of note, dysregulation of the IR-A/IR-B ratio is associated with insulin resistance, aging, and increased proliferative activity of normal and neoplastic tissues and appears to sustain detrimental effects. This review discusses novel information that has generated remarkable progress in our understanding of the physiology of IR isoforms and their role in disease. We also focus on novel IR ligands and modulators that should now be considered as an important strategy for better and safer treatment of diabetes and cancer and possibly other IR-related diseases.
We discuss recent work on the physiology of IR isoforms and their role in disease including new findings on IR ligands and modulators that are relevant to the treatment of diabetes and other disorders.
Essential Points
The insulin receptor (IR) exists in two isoforms, IR-A and IR-B, expressed in different relative abundance in the various organs and tissues
The two IR isoforms have similar binding affinity for insulin but different affinity for insulin-like growth factor (IGF)-2 and proinsulin, which are bound by IR-A but not IR-B
Activation of IR-A by IGF-2 and proinsulin is thought to sustain prenatal growth whereas this ability is less understood in adult life
Tonic IR-A activation by IGF-2 may affect IR-A membrane partitioning and trafficking and its crosstalk with a variety of other membrane molecules
High IR-A expression, which is advantageous in prenatal life, appears to be associated with detrimental effects, such as dysregulated cell proliferation and insulin resistance in adult life
Selective modulation of the two IR isoforms should now be considered as an important strategy for precision medicine
In a previous review, we summarized the available data suggesting that insulin receptor (IR) gene splicing is an evolutionarily conserved mechanism in mammals, responsible for the specificity of insulin and insulin-like growth factor (IGF) signaling. Indeed, accumulating evidence has led to the new concept that the physiological roles of IR isoforms are regulated by their different binding affinities for IGFs, particularly for IGF-2, rather than by their slightly different binding affinities for insulin (1). According to this view, predominant IR-A expression may be important for prenatal growth and development, whereas IR-B expression has a more important role in metabolic insulin action in adults. The differential expression of IR isoforms and their association with the type I IGF-1 receptor (IGF-1R) to form hybrid receptors (HRs) could help explain the diversification of insulin and IGF signaling and actions in various organs and tissues (2).
The IR isoforms, therefore, are relevant components of the network modulating the pleiotropic effects of the insulin/IGF system, which exerts distinct effects on cell growth, differentiation, apoptosis, and metabolism. The specificity of the different biological responses in this system relies on the multiplicity of the involved ligands and receptors, with a fine modulation of the amplitude of each signal depending on specific ligand–receptor interactions (3). Moreover, we underline the concept that dysregulation of the low-specificity IR-A isoform, biologically prevalent in less-differentiated tissues, may promote detrimental effects such as cancer progression when overexpressed in well-differentiated cells.
In the last few years, this view has been consistently confirmed, and new information has led to remarkable progress in our understanding of the physiology of IR isoforms and their role in disease. Crystal structure studies have better clarified the ligand–receptor interaction, with the identification of the primary binding site for insulin (4, 5). These studies may provide a basis for a better understanding of the binding of IR-A to specific ligands such as IGF-2 and proinsulin (6), which have recently been added to the list of naturally occurring IR-A ligands. Additional data have become available regarding the specific roles of IR isoforms in different physiological and pathological conditions, namely, insulin resistance, diabetes, and cancer. Some studies have examined the possibility that insulin analogs used for diabetes therapy may have biased binding affinity for one of the IR isoforms. This possibility is now routinely evaluated in the study of new insulin analogs. At the same time, new approaches have been sought to generate insulin analogs that may have lower affinity for the more mitogenic IR-A. Moreover, several independent studies have confirmed and extended the role of IR-A in cancer, metastatic spread, and cell stemness.
New insights are also available regarding insulin and related ligands in IR signal diversification, which may occur only partially through the formation of IR/IGF-1R HRs. In fact, IR isoform interactions and functional crosstalk with other tyrosine kinase receptors and other membrane molecules are an emerging area (7). These mechanisms may greatly impact our understanding of the regulation of insulin signaling and may provide an opportunity to selectively favor metabolic effects while inhibiting unwanted IR effects. The use of novel allosteric ligands, both antibodies and small molecules, has suggested that it is possible to modulate postreceptor intracellular signaling. Whether some of these molecules might be useful in the treatment of insulin resistance, neurodegenerative diseases, or cancer is still unclear.
In this review, we discuss more recent evidence indicating that our knowledge of the complex and fundamental role of the IR in the physiological processes of development, differentiation, metabolism, and aging, as well as in the disease states of diabetes, cancer, and neurodegeneration, will benefit greatly from a better understanding of the regulation, ligand specificity, crosstalk, and signaling of the two IR isoforms. We also aim to put the available information in a comprehensive context and highlight findings that might be clinically relevant as well as areas that would benefit from more research.
Ligand Binding to the IR and Insulin/IGF-1 Receptor Hybrids
Structural studies have recently elucidated how insulin binds to its receptor, thus providing further insight into the different binding characteristics of IR isoforms and IR/IGF-1R hybrids.
Insulin binding to the IR
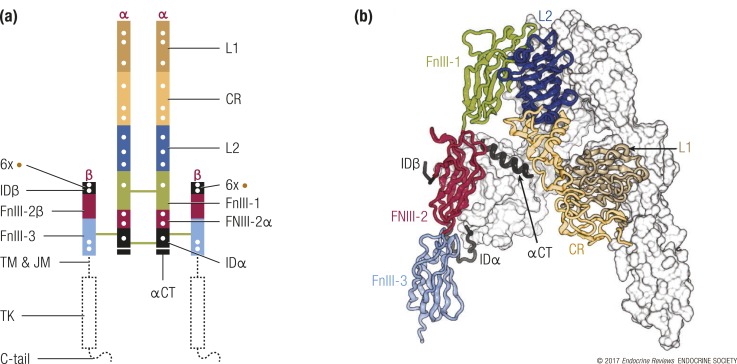
The unliganded three-dimensional structure of the human IR extracellular region (or “ectodomain”) was initially described in 2006 (8, 9), with subsequent improvements in the resolution of the α-chain C-terminal segment and correction of the register of amino acid residues with secondary structural elements within two of the domains (3, 4). Human IR is a disulfide-linked (αβ)2 homodimer, with each monomer consisting of seven extracellular domains. Starting from the N terminus, these domains are the first leucine-rich repeat domain (L1), the cysteine-rich region (CR), the second leucine-rich repeat domain, and the first, second, and third fibronectin type III domains (FnIII-1, FnIII-2, and FnIII-3), with the seventh domain, a relatively disordered insert domain (ID), being located within the canonical CC′ loop of FnIII-2 and containing the α/β furin cleavage site [Fig. 1(a)]. These domains assemble into a twofold symmetric Λ-shape, each “leg” of which comprises the L1–CR–second leucine-rich repeat domain module of one receptor monomer juxtaposed against the FnIII-1–FnIII-2–FnIII-3 module of the alternate receptor monomer (8). A peptide segment (termed αCT) from the C-terminal region of the ID α-chain component (IDα) forms an α-helix on the surface of the central β-sheet (L1-β2) of the L1 domain (10) [Fig. 1(b)]. The αCT segment differs in length in the A and B isoforms of IR, depending on whether it includes (IR-B isoform) or excludes (IR-A isoform) the 12-residue gene product of exon 11. Intermonomer disulfide bonds occur between the FnIII-1 domains and between the IDα segments, whereas within each monomer, a disulfide bond links IDα to the FnIII-3 domain (in turn within the β-chain) [Fig. 1(a)]. Seventeen N-linked glycans are attached to asparagine residues at various positions within each monomer (11, 12), and six O-linked glycans are attached to serine or threonine residues within the N-terminal region (IDβ) of each receptor β-chain (13) [Fig. 1(a)].
Figure 1.
The structure of the IR (αβ)2 homodimer. (a) Location of domains within the IR αβ polypeptide. Interchain disulfide bonds are indicated by solid green lines, N-linked glycosylation sites by white dots, and O-linked glycosylation sites are indicated by brown dots. The N termini of the chains are labeled in red (α or β), and the interchain disulfide bonds are shown as green lines. C-tail, C-terminal tail of the IR β chain; JM, juxtamembrane segment; L2, second leucine-rich repeat domain; TM, transmembrane segment. (b) Λ-shaped assembly of the IR ectodomain. One monomer is depicted as a ribbon, with the domains colored and labeled as in (a); the second is depicted as a white molecular surface. The depiction is based on PDB entry 4ZXB (11).
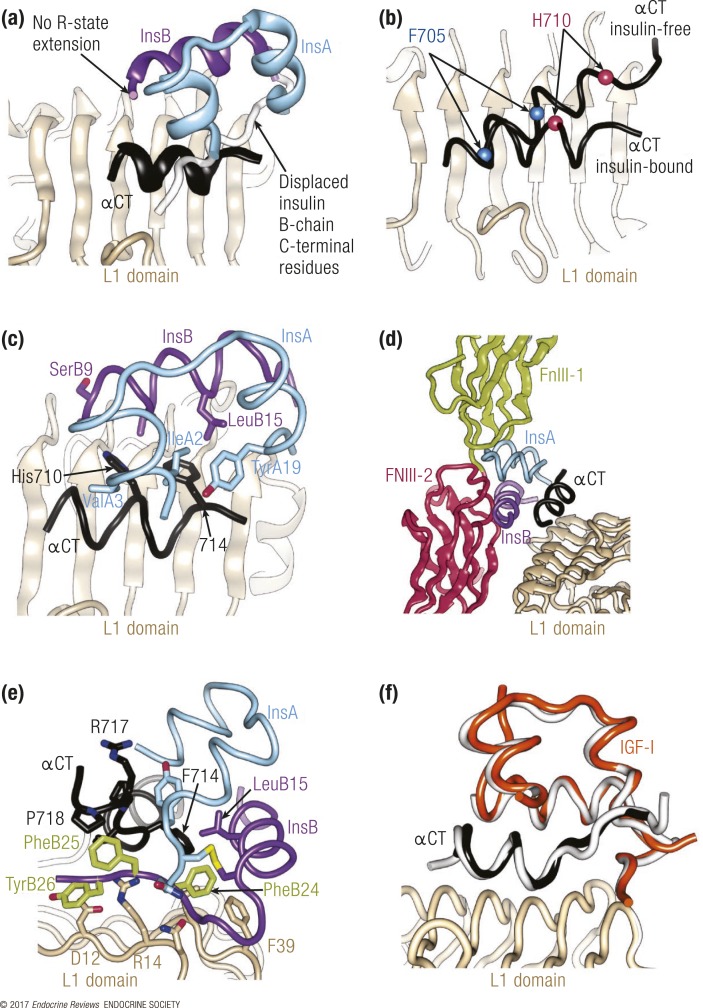
Although the three-dimensional structure of insulin has been known since 1969 (4), the specificity of ligand/receptor engagement has long remained elusive. In 2013, a major advance was made with the determination of the structure of insulin and a high-affinity insulin analog in separate complex with elements of the IR forming the primary hormone-binding site (14). The study used two receptor constructs. The first was the so-called insulin “microreceptor” (μIR), a two-domain L1-CR construct (IR residues 1 to 310) combined with an exogenous αCT peptide spanning residues 704 to 719 of the IR-A receptor isoform in one instance and spanning residues 697 to 719 of the same isoform in another. The second receptor construct was a homodimeric, four-domain L1–CR–second leucine-rich repeat domain–FnIII-1 construct (IR residues 1 to 593), the C terminus of which was covalently attached to the IR-A αCT peptide (residues 704 to 719), known as IR593.αCT. Crystallization of the μIR plus insulin constructs was improved by the attachment of an Fab module from the monoclonal antibody 83-7 (15) to the CR domain of the μIR, whereas crystallization of IR593.αCT plus insulin was optimized by the attachment of an Fab module from monoclonal antibody 83-14 (15) to the FnIII-1 domain of the receptor fragment. Together, these structures, although limited in resolution to 3.9 Å at best, revealed the following. (1) Insulin undergoes a conformational change upon engaging the primary binding site of the receptor, as the B-chain C-terminal segment of insulin folds away from the hormone core [Fig. 2(a)]. Such folding out had long been predicted to be an integral part of hormone binding (16, 17). (2) The so-called R state of insulin, in which the B-chain N-terminal residues form a helical extension of the canonical B-chain α-helix (18, 19), was not observed [Fig. 2(a)], despite the earlier suggestion that such a transition was an integral part of receptor binding [reviewed in Weiss (19)]. (3) The αCT segment undergoes a substantial rearrangement on the L1-β2 surface, including the formation of an additional turn at the C-terminal end of the αCT helix observed in the apo-IR ectodomain structure, removal of turns at the N-terminal end of the same helix, and rotation of the αCT helix axis by ∼34° with respect to its orientation in the apo-IR ectodomain structure [Fig. 2(b)]. A subsequently determined structure of the insulin-free μIR displayed the αCT helix in a similar orientation on the L1-β2 surface to that seen in the structure of the apo-IR ectodomain (5), indicating that this altered arrangement of αCT is indeed a consequence of insulin binding and not a consequence of using domain-deleted constructs. (4) The folding out of the B-chain C-terminal segment of insulin exposes the hydrophobic core of the hormone, which is now engaged by αCT residues His710 and Phe714 [Fig. 2(c)]. (5) Superposition of the hormone/μIR complex over the intact ectodomain results in significant overlap between the hormone and the adjacent fibronectin domain module, implying that accommodation of the bound hormone requires domain movement within the receptor ectodomain [Fig. 2(d)]. It was suggested that insulin may then cross-link to a site at the junction of the first and second fibronectin domains, a view supported by alanine scanning of residues within FnIII-1 and FnIII-2 (20).
Figure 2.
Structural biology of the interaction of insulin with its primary binding site on the receptor. (a) Overview of the insulin plus μIR complex, demonstrating the displacement of the insulin B-chain C-terminal segment (purple ribbon) away from the hormone core. (b) Rearrangement of αCT on the L1-β2 surface upon insulin binding. The C-terminal IR residues His710 and Phe714 are highlighted to show the change in the length and position of αCT upon hormone binding. (c) Engagement of the insulin core by IR residues Phe714 and His 710, highlighting insulin residues that are within 4 Å of these two receptor residues. (d) Steric overlap between bound insulin and the FnIII-1 and FnIII-2 domains upon superposition of the insulin plus μIR complex onto the structure of the apo-IR ectodomain. (e) Detail of the location of the insulin aromatic triplet PheB24-PheB25-TyrB26 within the insulin plus μIR complex. (f) Structure of IGF in complex with the IR L1-CR domain and the IGF-1R αCT segment (colored orange, light brown, and black, respectively), that is, a “hybrid” microreceptor complex, overlaid onto that of the insulin plus μIR complex (white). Unless otherwise indicated, the IR domains are colored as in Fig. 1, the insulin A chain is colored light blue, and the insulin B chain is colored purple.
A further refinement of these structures provided details of the way in which the folded-out B chain of insulin engages the receptor (21), as this aspect was unresolved within the earlier (2013) suite of structures. Of particular interest here is the location of the highly conserved aromatic triplet PheB24-PheB25-TyrB26 of insulin (22) within the hormone receptor complex. In particular, the side chain of PheB24 undergoes rotameric rearrangement as it engages the receptor and is buried within a hydrophobic cavity formed by insulin, the αCT segment, and the L1 domain [Fig. 2(e)]. The side chain of PheB25 is more exposed and is directed away from the L1 surface to engage residues Pro718 and Val715 of IR αCT, whereas the side chain of PheB26 is folded onto the L1 surface where it stacks against the side chain of IR Asp12 [Fig. 2(e)].
Ligand binding to IR/IGF-1R hybrids
The hybrid IR/IGF-1R contains two distinct primary ligand-binding sites: the first comprises the L1 domain of the IR and the αCT segment of the IGF-1R, and the second consists of the L1 domain of the IGF-1R and the αCT segment of the IR. The three-dimensional structure of the first site, as an IGF-1 complex with an IR L1-CR construct and exogenous IGF-1R αCT peptide (IGF-1R residues 691 to 706), has recently been obtained (5). This structure [Fig. 2(f)] is very similar to that of insulin bound to the receptor constructs described previously. In particular, it maintains the arrangement of IR αCT as well as the folding out of the IGF-1 equivalent of the B-chain C-terminal region of insulin. Within the structure, the IGF-1 C domain remains disordered as in the receptor-free form [Fig. 1(d)], but the authors suggest that its topology is such that the αCT segment “threads” through the loop that it forms with respect to the three-helix bundle that is the growth factor core. The physiological relevance of this structure is unclear, as the primary binding site that it reflects (IR L1 plus IGF-1R αCT) has ∼10-fold lower affinity for IGF-1 than does the alternate primary binding site (IGF-1R L1 plus IR αCT) within the HR (23).
Note that all of the previously mentioned IR structures are based on IR-A, that is, they have the shorter αCT segment. In the hybrid microreceptor structure (5), the IGF-1R αCT segment used is equivalent in length to that of IR-A. In all of the ligand-complexed structures, the C-terminal region of αCT is relatively poorly resolved. Therefore, it remains an open question as to whether, within a structure of insulin bound to an IR-B–based receptor construct, the 12-residue exon 11-derived segment of the (longer) αCT polypeptide would be ordered and modulate hormone binding. In the case of IGFs binding to IR-B or an IGF-1R/IR-B HR, the requirement to “thread” the longer αCT segment through the loop formed by the C domain and the growth factor core may underlie the lower affinity of IGFs for these receptor forms (24). We also note the ∼100-fold higher affinity of IGF-2 for IR-A compared with that of IGF-1 (24). One possible reason for this higher affinity is that the C domain of IGF-2 is four residues smaller than that of IGF-1, and consequently it may be more easily accommodated in the volume between the receptor αCT segment and receptor CR domain upon binding to the primary site.
Ligand binding affinity of IR isoforms
The activation of the two IR isoforms may lead to different biological outcome due to differences in ligand affinity, interactions with other molecular partners, internalization rates, and phosphorylation patterns of the IR and its substrates. It is now established that the two IR isoforms have slightly different affinity for native insulin but differ substantially in their affinity for the two IGFs. IR-A has a high affinity for IGF-2 and a low affinity for IGF-1, whereas IR-B has a low affinity for IGF-2 and a very low affinity for IGF-1 (25) (Table 1). As IGF-2 has a similar affinity for both IR-A and the IGF-1R (Table 1), IGF-2 can elicit different actions through either IR-A or the IGF-1R or both, depending on the relative level of the predominantly expressed receptor type and on the relative bioavailability of various ligands.
“Subtle differences in intracellular substrate activation upon either insulin or IGF binding may result in different eventual biological outputs.”
Table 1.
EC50 Values of Insulin, Proinsulin, IGF-2, IGF-2 Precursors, and IGF-1 for IR-A, IR-B, and IGF-1R
| IR-A | IR-B | IGF-1R | References | |
|---|---|---|---|---|
| Insulin | 0.91 ± 0.3 | 1.0 ± 0.4 | nd | Frasca et al. (32) |
| nd | nd | >30 | Pandini et al. (2) | |
| 0.40 ± 0.10 | 0.49 ± 0.05 | >1000 | Sciacca et al. (33) | |
| nd | nd | 383 ± 27 | Versteyhe et al. (34) | |
| 1.57 ± 0.33 | nd | nd | Rajapaksha et al. (35) | |
| 2.7 ± 0.6 | 2.6 ± 0.7 | nd | Pierre-Eugene et al. (36) | |
| Proinsulin | 4.5 ± 0.6 | 31.0 ± 6.3 | >100 | Sacco et al. (37) |
| IGF-2 | 3.3 ± 0.4 | 36.0 ± 3.8 | nd | Frasca et al. (32) |
| nd | nd | 0.6 | Pandini et al. (2) | |
| nd | nd | 13.1 ± 0.7 | Versteyhe et al. (34) | |
| 15.2 ± 0.2 | nd | nd | Rajapaksha et al. (35) | |
| 4 ± 0.4 | nd | 3.4 ± 0.2 | Ziegler et al. (38) | |
| pro-IGF-2 | nd (<IGF-2) | nd | nd | Marks et al. (39) |
| big-IGF-2 | nd (≈IGF-2) | nd | nd | |
| IGF-1 | >30 | >30 | 0.2 ± 0.3 | Pandini et al. (2) |
| nd | nd | 1.49 ± 0.14 | Versteyhe et al. (34) | |
| 34 ± 13 | 50 ± 13 | nd | Pierre-Eugene et al. (36) |
Abbreviations: EC50, ligand concentration (nM) required to achieve 50% of maximal receptor activation; nd, not determined.
Recently, proinsulin has been reported as an additional IR-A ligand. Proinsulin binds to IR-A with a similar affinity as IGF-2 (ligand concentration required to achieve 50% of maximal receptor activation, 4.5 ± 0.6 nM), whereas it has a low affinity for IR-B (Table 1) (6). Its lower affinity for IR-B compared with that for IR-A may align with it being energetically more favorable to thread the C-terminal region of shorter αCT segment of IR-A through the loop formed by the C domain of proinsulin and the remainder of proinsulin than it is to thread that of IR-B upon proinsulin engaging the receptor’s primary ligand binding site. Similar to IGF-2, proinsulin effectively stimulates cell proliferation and migration. However, proinsulin differs from IGF-2 in its low binding affinity for the IGF-1R and IR/IGF-1R hybrids and can be considered a selective IR-A ligand (6) (Table 1). The mechanisms by which IGF-2 binds and activates IR-A and the IGF-1R have also recently been further characterized. Alvino et al. (26) have mapped two distinct receptor sites by site-directed mutagenesis, and, specifically, IGFs have two separate binding surfaces that interact with these two receptor binding sites. Insulin second binding surface, which includes residue HisB10, plays an important role in IR activation and mediates mitogenic signals. Similarly, it has been found that the equivalent binding surface of IGF-2 (in particular, residue Glu12) is important for IR-A binding and activation. The substitution of the positively charged insulin residue HisB10 with a negatively charged amino acid (as in IGF-2) plays a role in IR-A binding affinity and the increased mitogenic effect. Conversely, the introduction of a positive charge at Glu12 of IGF-2 (equivalent to Glu9 of IGF-1) results in a lower affinity for both the IGF-1R and IR-A. Similarly, a positive charge at Glu9 of IGF-1 also results in a lower affinity for the IGF-1R (27). Moreover, insulin residue HisB10 (which is responsible for interaction with Zn2+ in the hexameric, storage form of the hormone) participates in metabolic signaling through IR (26).
Additional studies have identified the C domain of IGFs as the main determinant of binding specificity to the IGF-1R, IR-A, and IR-B. IGF-1 and IGF-2 display a high degree of homology. They have a single chain divided into four domains: B, C, A, and D (from N to C terminus). The A and B domains are similar to insulin, and the C domain sequence is comparable to that of proinsulin, whereas the D domain is specific for IGFs. The C domain of both IGF-1 and IGF-2 is responsible for IR and IGF-1R binding specificity and activation. Moreover, both flanks of the IGF-2 C domain are shown to have an important role in the ability of IGF-2 to bind the IR but not the IGF-1R (28). The mature form of IGF-2 derives from proteolitic cleavage of the E domain at the C terminus of pro–IGF-2. Proteolytic cleavage at alternative sites in the same domain sequence produces intermediate forms termed “big” IGF-2. Pro–IGF-2 and big IGF-2 account for 10% to 20% of total IGF-2 (29).
Ligand binding affinity of IR/IGF-1R hybrids
As the IR exists in two isoforms (IR-A and IR-B), two possible HR combinations may arise: IR-A/IGF-1R (HR-A) and IR-B/IGF-1R (HR-B). Hybrid formation clearly alters the specificity of binding sites, as each hybrid ligand binding site comprises parts from both the constituent IR monomer and the constituent IGF-1R monomer (2, 30, 31).
Ligand activation of HRs induces the phosphorylation of both IR and IGF-1R moieties (2, 31); therefore, it is a matter of discussion whether HR activation may elicit unique intracellular signaling, which may differ from the signaling elicited by IR and/or IGF-1R homodimers. In fact, although the two homodimeric receptors share the same intracellular signaling cascades for the most part, it is widely accepted that subtle differences in intracellular substrate activation upon either insulin or IGF binding may result in different eventual biological outputs (40). The absolute and relative abundances of IR-A, IR-B, and IGF-1R in a given tissue and HR formation, as well as the availability of IGF-1, IGF-2, and insulin, are critical therefore in determining the final signaling network that is activated and the consequent biological output. In this context, it is important to understand whether HR formation is simply regulated by the relative abundance of the IR and IGF-1R (41) or additionally modulated by ligands or other factors, and what the HR affinity is for the different ligands.
So far, attempts to answer these questions have faced significant problems due to the lack of reliable assays and/or of specific antibodies recognizing only HRs or HR activation. HRs were first described (15) by evaluating the proportion of total 125I-labeled IGF-1 binding (a measure of both the IGF-1R and HRs) immunoprecipitated with an anti-IR antibody (antibody 83-7) that recognizes HRs but not the IGF-1R. Subsequently, a more sensitive and specific enzyme-linked immunosorbent assay (ELISA) was developed (2, 41) by first capturing HRs with an IR α-subunit antibody (83-7 antibody) and visualizing them with a biotinylated IGF-1R α-subunit antibody (17-69 antibody). Recently, nuclear reciprocal immunoprecipitation and colocalization experiments by immunofluorescence have been used to demonstrate the presence of HRs in nuclear cell extracts (42). Similarly, a variety of methodologies have been used to measure the binding affinity of HRs to IGF-1, IGF-2, and insulin. These include competition-inhibition assays of 125I–IGF-1 binding after polyethylene glycol precipitation (43) or HR immunocapturing (2, 30) and scintillation proximity binding assays (44). HR autophosphorylation in response to different ligands has been measured by immunoprecipitation (2, 30) and ELISA (2, 41) as well as by bioluminescence resonance energy transfer–based assays (30, 36). Recently, a new ELISA has been described in which HRs are immunocaptured by an IGF-1R antibody (24-31 antibody), and HR activation is then measured by a phosphorylated antibody specific for IR phosphorylation at Y1334 (45).
Although all of these studies support the notion that IGF-1 has the highest affinity (of the three ligands) for HRs, it is unclear whether the affinity of IGF-2 for HRs is similar to that of IGF-1 or sixfold to sevenfold lower, as it is for homodimeric IGF-1R (Table 2). Moreover, studies disagree as to whether IGF-1, IGF-2, and insulin have higher affinities for HR-A than for HR-B. Although Pandini et al. (2) reported a higher affinity for all three ligands for HR-A than for HR-B, these findings have not been confirmed by others (30, 44). However, a recent study has confirmed a higher affinity of IGF-1 for HR-A than for HR-B (45), whereas the insulin affinity was low for both HR-A and HR-B. The IGF-2 affinity to HRs was not assessed (Table 2).
Table 2.
EC50 Values of Insulin, IGF-1, and IGF-2 for IR-A/IGF-1R and IR-B/IGF-1R Hybrids
|
IR-A/IGF-1R |
IR-B/IGF-1R |
Method | References | ||||
|---|---|---|---|---|---|---|---|
| Insulin | IGF-1 | IGF-2 | Insulin | IGF-1 | IGF-2 | ||
| 3.7 ± 0.9 | 0.3 ± 0.2 | 0.6 ± 0.1 | >100 | 2.5 ± 0.5 | 15 ± 0.9 | PACA | Pandini et al. (2) |
| 2.6 ± 1.3 | 0.01 ± 0.01 | nd | 2.8 ± 1.4 | 0.01 ± 0.01 | nd | PACA | Slaaby et al. (44) |
| 1.1 ± 0.2 | 0.02 ± 0.006 | 0.18 ± 0.04 | 1.1 ± 0.3 | 0.02 ± 0.07 | 0.19 ± 0.04 | SPA | |
| 4.6 ± 1.9 | 0.01 ± 0.001 | nd | 5.1 ± 2.3 | 0.01 ± 0.01 | nd | PEG | |
| 70 ± 12 | 0.5 ± 0.2 | 0.7 ± 0.1 | 76 ± 12 | 0.3 ± 0.1 | 0.3 ± 0.1 | PACA | Benyoucef et al. (30) |
| 60 ± 10 | 4.0 ± 0.5 | nd | 40 ± 5 | 4.0 ± 1.0 | nd | BRET | |
| 130 ± 41 | 3.0 ± 0.66 | nd | 70 ± 35 | 2.8 ± 0.66 | nd | BRET | Pierre-Eugene et al. (36) |
| 342 ± 121 | 6 ± 3 | nd | 325 ± 88 | 12 ± 2 | nd | ELISA | Slaaby et al. (45) |
Abbreviations: BRET, bioluminescence resonance energy transfer assay; EC504 ligand concentration (nM) required to achieve 50% of maximal receptor activation; nd, not determined; PACA, plate antibody capture assay; PEG, polyethylene glycol precipitation binding assay; SPA, scintillation proximity binding assay.
IR Isoform Regulation
Although IR isoform expression is tightly regulated, the mechanisms underlying this regulation are still poorly understood. However, several recent studies have yielded significant advances in the field.
Regulation of IR expression
Gene transcription
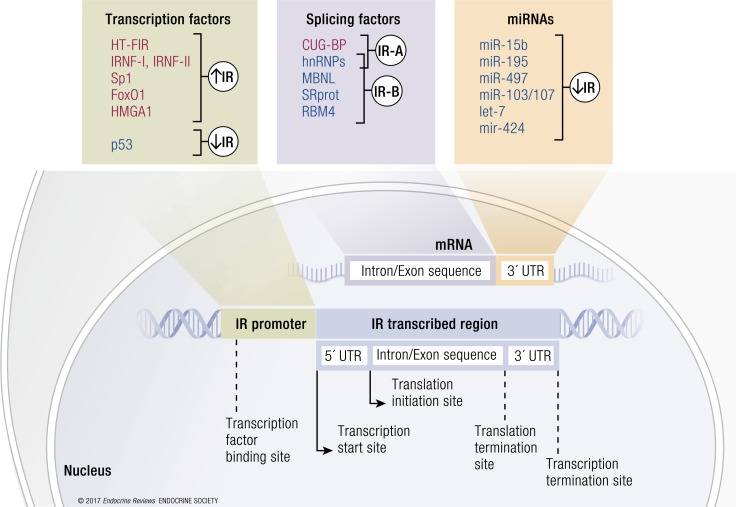
IR expression can be modulated at the promoter level in a developmental- and tissue-specific manner. As previously reviewed (1), the IR promoter activity can be positively regulated in insulin-sensitive tissues by a number of factors, including hepatocyte-specific transcription factor of the IR gene (46), IR nuclear factor I and II (47, 48), and the transcription factors high-mobility group protein A1 (HMGA1) and specificity protein 1 (49), whereas it is negatively modulated by p53 (50) (Fig. 3). It has been recently shown that IR promoter occupancy by HMGA1 is dependent on the histone chaperone nucleophosmin (NPM1), which exerts a chaperoning function on HMGA1-mediated gene expression (51). In turn, insulin signaling may induce HMGA1 phosphorylation and inhibition of its transcriptional activity (52). In Drosophila, IR transcription is also regulated by multiple cis-regulatory elements, which are enhancers located within introns of the IR gene. They are indirectly activated or repressed by FOXO1, allowing a temporal and spatial control of IR gene expression. It has been proposed that FOXO1 initially induces IR expression via the well-known direct binding to the IR gene promoter and by activating enhancers; subsequently, FOXO1 downregulates the IR gene by repressing enhancers. Besides this temporal model, FOXO1 may differentially induce IR gene activators and repressors depending on the spatial binding to each of them, thereby driving a tissue-specific control of IR gene expression. Both mechanisms allow balanced IR levels to be reached (53). Whether this type of regulation occurs also in humans is unknown.
Figure 3.
Schematic representation of regulators of IR isoform expression. The figure summarizes the principal IR regulators acting at the promoter (transcription factors) and mRNA level (splicing factors and miRNAs). Transcription factors act by promoting (in red) or blocking (in blue) IR gene transcription. Several splicing factors and miRNAs are involved in the posttranscriptional regulation of IR expression. Once the IR mRNA is formed, splicing factors remove introns and let exons bind together. They regulate the differential splicing of exon 11, thereby generating IR-A (ex11−) or IR-B (ex11+). Several miRNAs can bind to the 3′-UTR of the IR mRNA, favoring its degradation.
Translation regulation by internal ribosome entry sites
Beyond transcriptional regulation at the promoter level, messenger RNA (mRNA) translation represents a key regulatory mechanism for the expression of many proteins. Translation is most commonly initiated by cap-dependent scanning; alternatively, in several genes, the 5′-untranslated region (UTR) of the mRNA is able to acquire highly complex structures [(internal ribosome entry sites (IRESs)] allowing ribosome recruitment and initiation of translation. This process normally requires polypyrimidine tract-binding protein and can be activated under stress conditions when general translation is reduced (54). It has recently been reported that the 5′-UTR of human IR mRNA contains an IRES element. IR-IRES activity is increased by cell density (55), but it is stimulated by insulin only in nonconfluent cells. It is hypothesized that IR-IRES activity may play a role in tissues, such as brain, where mRNA translation by cap-dependent scanning is less effective.
IR-regulating microRNAs
Noncoding RNAs such as microRNAs (miRNAs) also play a key role in posttranscriptional regulation of gene expression (56). miRNAs typically bind to an mRNA and negatively regulate its stability or translation. Most of these are expressed in a tissue-specific manner, suggesting a particular function, whereas inappropriate regulation of miRNA expression is implicated in the pathogenesis of many diseases, including proliferative, degenerative, and metabolic diseases (57). Recent evidence has shown that the posttranscriptional regulation of the IR is also controlled by miRNAs, with a tissue-specific effect on insulin sensitivity and insulin signaling (58). The expression of certain miRNAs targeting the mRNAs of insulin signaling molecules is aberrantly modulated in obesity and contributes to the pathogenesis of insulin resistance. Obesity induced by a high-fat diet causes insulin resistance with a concomitant upregulation in the liver of several specific miRNAs, including miRNA (miR)-15b (59), miR-195 (60), miR-497 (61), and miR-103/107 (62) (Fig. 3). The overexpression of miR-15b suppresses IR expression by targeting its 3′-UTR, directly impairing insulin signaling and inducing hepatic insulin resistance (60). Saturated fatty acid and a high-fat diet significantly induce miR-195 expression in hepatocytes. In vitro experiments in HepG2 cells have shown that miR-195 suppresses the expression of the IR directly through posttranscriptional suppression (60). miR-497 is upregulated in the livers of rats with high-fat diet–induced metabolic syndrome and contributes to insulin resistance by IR downregulation (61). miR-103/107 destabilizes the IR at the membrane by targeting caveolin-1, a critical regulator of the IR. The expression of miR-103 and miR-107 is upregulated in obese mice in both the liver and adipose tissue, and the gain of miR-103/107 function in either the liver or fat tissue affects glucose homeostasis. In contrast, silencing of miR-103/107 leads to improved glucose homeostasis and insulin sensitivity (62). IR expression is also suppressed by let-7 miRNA family members. These act as tumor suppressors by suppressing oncogenes and cell cycle regulators (63–65) and are downregulated by different proteins, including the RNA-binding proteins Lin28a and Lin28b (66, 67). Overexpression of Lin28b increased IR protein expression in skeletal muscle in vivo, whereas IR levels were reduced by either let-7f transfection or Lin28b knockdown (KD) in human HEK293T cells. This RNA processing pathway regulates insulin sensitivity and glucose metabolism and is tightly coordinated, as let-7 and Lin28a/b individually have modest effects but simultaneously regulate multiple components of IR signaling (68). Another miRNA involved in IR regulation is the mouse miR-322 (and its human ortholog miR-424), expressed in a broad range of tissues. miR-424 downregulates IR and has antiproliferative and prodifferentiation effects (69) (Fig. 3). Further studies are needed to clarify whether these miRNAs may differentially regulate the relative abundance of IR isoforms.
Regulation of IR protein levels
It has been recently reported that the E3 ubiquitin ligase MARCH1 regulates the unstimulated IR pool at the cellular surface by directing IR ubiquitination in several cell types, including hepatocytes and white adipocytes. In turn, MARCH1 is itself insulin regulated through the transcription factor FOXO1. Therefore, the crosstalk between MARCH1 and the IR has different consequences under insulin-sensitive and insulin-resistant conditions. Indeed, in the insulin-sensitive state, IR activation inhibits FOXO1 and, as a consequence, MARCH1 transcription, leading to increased IR content and insulin signaling (70). In contrast, in an insulin-resistant state, insulin fails to inhibit FOXO1, resulting in enhancement of MARCH1 expression, decreased IR levels at the cell membrane, and impaired insulin signaling. Accordingly, MARCH1 is upregulated in the white adipose tissue of obese insulin-resistant patients. However, how the MARCH1 crosstalk with the IR contributes to cellular insulin action has not yet been investigated in vivo. Additionally, further experiments are required to establish whether the role of MARCH1 in IR ubiquitination is conserved in other cellular contexts, such as cancer cells, and whether it may affect both IR isoforms.
Membrane IR protein expression is also regulated by ligand binding through mechanisms involving internalization and degradation of hormone-occupied receptors (71). Interestingly, chronic exposure to hyperinsulinemia associated with obesity and insulin resistance may promote IR downregulation also via induction of endoplasmic reticulum stress and activation of the autophagy pathway (72). More details regarding the mechanisms underlying downregulation of the two IR isoforms, upon acute and prolonged ligand stimulation, are described later in the section titled “Differential signaling and trafficking of IR isoforms.”
IR isoform generation
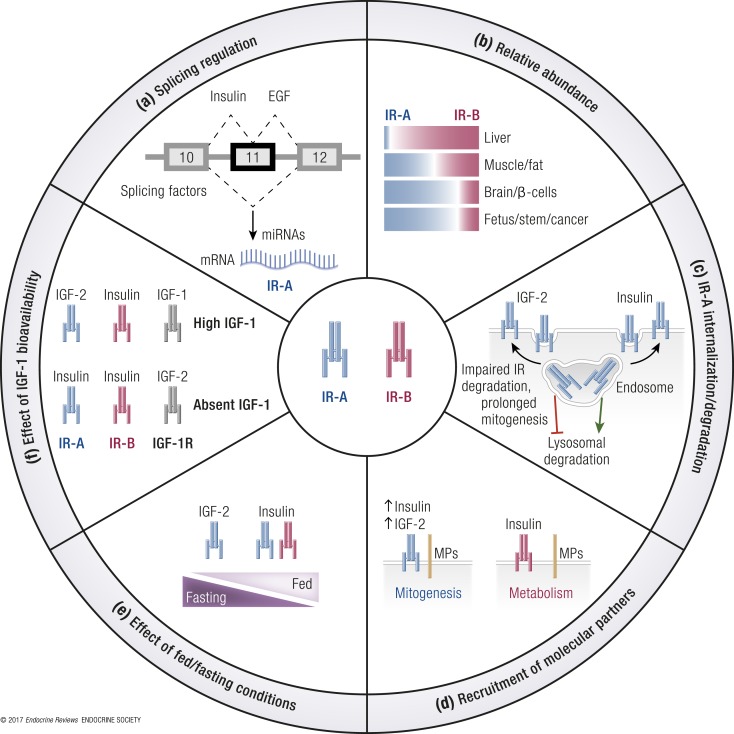
After transcription, premRNA undergoes processing in which exons are joined and introns are removed by a complex of ribonucleoproteins called the “spliceosome.” Splicing regulatory RNA binding proteins bind to the premRNA and promote or suppress spliceosome formation on the alternative splicing sites. The recognition by splicing factors of exons and introns during premRNA splicing relies on regulatory elements located within exons and flanking introns. The mechanism of IR alternative splicing and the identification of regulatory sequences and factors that control the IR-B/IR-A ratio are of critical importance for the full understanding of IR isoform actions.
Alternative splicing regulation of the IR gene
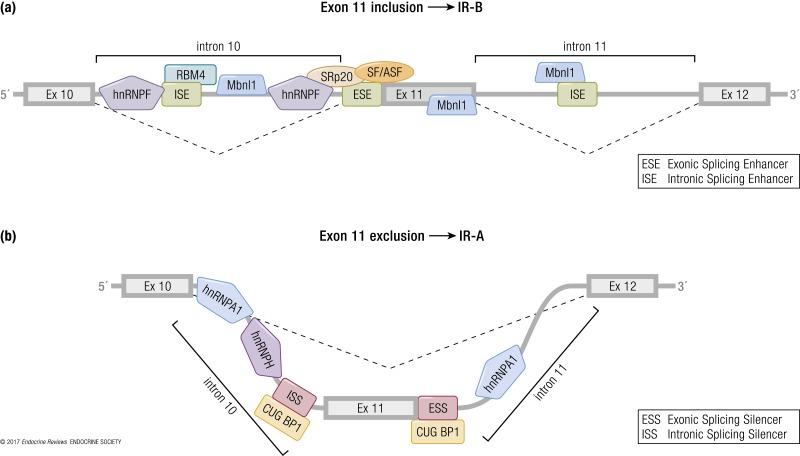
Both intron 10 and the alternatively spliced exon 11 contain regulatory sequences that affect IR splicing both positively and negatively (73, 74) (Fig. 3). Critical sequences regulating the splicing process have been mapped upstream of the breakpoint sequence of intron 10 and in exons 10, 11, and 12. Splicing factors that control exon 11 inclusion/exclusion are expressed in a tissue- and developmental stage–specific manner. Indeed, the resulting IR-A/IR-B ratio reflects the balance between splicing factors including CUG-binding protein (CUGBP) and Elav-like family members, heterogeneous nuclear ribonucleoprotein family proteins (hnRNPs), muscleblind-like protein (MBNL), serine-arginine–rich (SR) proteins, and RBM4 (Figs. 3 and 4).
Figure 4.
Schematic model for alternative IR splicing regulation by splicing factors. IR sequences encoding exons 10, 11, and 12 and introns 10 and 11 are shown. (a) Some splicing factors regulate exon 11 inclusion, thereby modulating a preferential expression of isoform B of the IR. hnRNP F binds to both ends (5′ and 3′) of intron 10. Mbnl1 recognizes an intronic splicing enhancer (ISE) element within intron 11 and binds to two other regions localized in intron 10 and in exon 11. SRp20 and SF2/ASF bind the exonic splicing enhancer (ESE) element within exon 11. RBM4 binds GC-rich sequences in intron 10 and acts synergistically with other IR splicing regulators. (b) Factors regulating IR-A formation. CUGBP1 binds to two silencer sequences, one located at the 3′ end of intron 10 (ISS) and the other one in exon 11 (ESS). hnRNP A1 binds similarly to the 5′ splice site of both intron 10 and intron 11. hnRNP H favors exon 11 skipping by binding a region within intron 10.
CUG-binding protein and Elav-like family members are proteins that regulate mRNA alternative splicing, editing, and translation. CUGBP1, a splicing factor belonging to this group, was the first discovered regulator of IR exon 11 splicing. This factor binds two silencer sequences, one located upstream of exon 11 at the 3′ end of intron 10, and one in the middle of the same exon, promoting exon 11 exclusion and thus favoring IR-A expression (75).
The hnRNPs are another group of proteins involved in IR premRNA splicing and mRNA export, stability, and translation (76). Talukdar et al. (77) identified two hnRNPs able to modulate IR splicing. Specifically, they reported that hnRNPF and hnRNPA1 bind intronic and exonic splicing regulatory elements that are GA-rich, antagonistically regulating the alternative splicing of exon 11. Indeed, hnRNPF binds to both ends of intron 10, resulting in inclusion of exon 11 (promoting IR-B expression), whereas hnRNPA1 binds similarly to intron 10 but also to the 5′ splice site of intron 11, resulting in exclusion of exon 11 (promoting IR-A expression) (77).
Within intron 11, there is an intronic enhancer element highly conserved across species (75). Mbnl1, a splicing factor belonging to the MBNL proteins, recognizes this region and promotes exon 11 inclusion, thus increasing IR-B expression (75, 78). Moreover, Mbnl1 antagonizes CUGBP1 in several alternative splicing events, including that of IR exon 11 (79). Additionally, Mbnl1 interacts with other splicing regulators involved in IR mRNA regulation; for example, it inhibits hnRNPH action (79).
A family of SR proteins plays a crucial role in the alternative splicing of mRNA. They bind to exonic and intronic sites and interact with small nuclear ribonucleoproteins to facilitate their association with splicing sites (80). Specifically, the splicing factors SRp20 and SF2/ASF, belonging to the SR family of proteins, bind to the enhancer sequence at the 5′ end of exon 11 and promote IR-B isoform formation. Sen et al. (75) demonstrated that SRp20 and SF2/ASF antagonize CUGBP1 activity in regulating IR alternative splicing and that the balance of these splicing factors is fundamental to determining the ratio of IR isoform expression.
RBM4 regulates mRNA alternative splicing and translation (81). It promotes exon 11 inclusion and favors IR-B expression by binding to GC-rich motifs. Lin et al. observed that Rbm4 knockout (KO) mouse embryonic fibroblast (MEFs) and adult muscles had higher levels of the exon 11–skipped IR-A isoform (82). RBM4 is involved in the alternative splicing regulation of transcripts involved in muscle cell differentiation, as previously observed in vitro (83).
Insulin modulation of IR gene splicing
Splicing factors are modulated by growth factors, and insulin is a known regulator of the activity and expression of several splicing factors (84, 85). However, the available data on the possible role of insulin in the regulation of IR splicing are controversial (1). Recently, it has been reported that weight loss induced by either bariatric surgery or a very low–calorie diet is associated with an increased relative abundance of IR-B in both subcutaneous and visceral adipose tissue. A multivariate analysis indicated that the reduced fasting insulin level consequent to weight loss was the major determinant of this IR-B increase. The effect of weight loss on IR splicing could be mediated by changes in the expression of various splicing factors. In particular, low insulin levels were strongly associated with reduced hnRNPA1, which is known to inhibit exon 11 inclusion. Weight loss also regulated other splicing factors, such as SF3A1 and SFRS7 (86). Besic et al. (87) studied IR isoform expression in liver specimens from 46 patients with or without type 2 diabetes mellitus (T2DM) undergoing bariatric surgery and found that the IR-A/IR-B ratio was increased in diabetic patients compared with nondiabetics, suggesting a role for hyperinsulinemia in favoring IR exon skipping. As expected, the IR-A/IR-B ratio normalized in 16 of 46 patients 17 ± 5.6 months after bariatric surgery (87). These data confirm previous results obtained in spontaneously obese and diabetic rhesus monkeys indicating that hyperinsulinemia is associated with IR-A expression (1, 88). On this basis, it could be hypothesized that high levels of IGF-2 in cancer may regulate a positive feedback loop through IR-A activation, thus increasing IR-A abundance. In a similar way, epidermal growth factor (EGF) has been shown to increase IR-A in cancer cells (65) (see the paragraph titled “Mechanisms of the increased IR-A/IR-B ratio in cancer”). In contrast, in both human and mouse pancreatic β cells, insulin was reported to induce exon 11 inclusion through the activation of the Ras–mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) signaling pathway and consequent upregulation of the splicing factors serine/arginine-rich splicing factor (SRSF)1 and MBNL1 (89). In this model, glucose metabolism, which stimulates insulin secretion, and constitutively active glucokinase increased exon 11 inclusion as well. However, low glucose favored exon 11 inclusion, whereas high glucose and lipids induced MBNL1 and increased IR exon 11 skipping. The authors of the study hypothesized that in β cells, unlike in other tissues, insulin may favor survival through the IR-B and not the IGF-2/IR-A circuitry (89). It is unclear how these findings fit with previous data indicating that insulin gene transcription by pancreatic cells requires IR-A signaling (90).
Differential IR isoform regulation at the protein level
The relative protein abundance of IR isoforms may also result from differences in their maturation process. The IR is synthesized as a proreceptor and undergoes proteolytic cleavage to become active. This modification occurs in the Golgi compartment, and it is modulated by the convertase furin. The two IR isoforms are both cleaved by furin, but cleavage is essential only for IR-A maturation (91). Indeed, when furin-dependent maturation is inefficient, IR precursors move to the cell surface, where a different convertase, PACE4, selectively supports IR-B maturation. Accordingly, furin inhibition may reduce IR-A maturation and its downstream signaling and cell mitogenic activity (91). On the basis of these data, it could be hypothesized that high expression levels of PACE4 in the liver (92) may contribute to the predominant expression of IR-B in this organ.
Ligand-Dependent Signaling of IR Isoforms
IR-A downstream signaling after IGF-2, IGF-1. and proinsulin binding
Recent evidence indicates that the IR-A isoform may elicit partially different intracellular signaling and biological effects upon binding of different ligands. In cells lacking the IGF-1R and expressing solely the IR-A, IGF-2 induced lower IR-A phosphorylation than did insulin but activated p70S6 kinase (p70S6K) at higher levels than did insulin. Insulin and IGF-2 induced similar peak levels of ERK1/2 and Akt activation. However, ERK1/2 activation was more prolonged after IGF-2 stimulation compared with insulin, whereas Akt activation was more prolonged after insulin than after IGF-2 stimulation (37). These findings are in close agreement with previous data showing that IGF-2 and insulin elicit partially different gene expression patterns through IR-A (93) and may help explain the more potent mitogenic effect but weaker metabolic effect of IGF-2 in respect to insulin (32, 94). In the same cell model, IGF-1 also induced significant ERK and Akt activation despite low-affinity binding and minimal IR-A autophosphorylation. Indeed, both IGF-1 and IGF-2 activated IR-A, inducing higher p70S6K/Akt and ERK1/2/Akt activation ratios than did insulin (37).
“The fine-tuned regulation of proinsulin expression plays an important role during embryonic development and…the excess proinsulin observed in diabetic mothers may be teratogenic.”
IGF-2 precursors (pro–IGF-2 and big IGF-2) are also present in human plasma. Pro–IGF-2 represents ∼13% of the total IGF-2, and big IGF-2 represents ∼16%. Both forms are increased in the plasma of patients with nonislet cell tumor hypoglycemia. Mature IGF-2 and big IGF-2 were equipotent in activating autophosphorylation of both IR isoforms, whereas pro–IGF-2 was less potent (39). Moreover, both mature and big IGF-2 elicited greater Akt activation in cells expressing IR-A than in cells expressing IR-B, in line with the higher affinity of IGF-2 for IR-A (32). Therefore, IR-A is activated by both mature and big IGF-2 rather than by pro–IGF-2.
Proinsulin was approximately equipotent to IGF-2 and insulin in activating cell proliferation and migration in cells expressing only IR-A. The intracellular signaling elicited by proinsulin was similar to that observed with IGF-2, with an increased p70S6K/Akt activation ratio compared with insulin. In fact, proinsulin induced a slower negative feedback mechanism on IR activation, as IR and IR substrate (IRS)-1 degradation required a longer period of stimulation (24 h) compared with insulin (8 h), thus explaining its more potent mitogenic and migratory effects (6). However, the significance of the proinsulin interaction with IR-A in adults is still unclear.
IR isoform ligand bioavailability and pathophysiology implications
Insulin and proinsulin are mainly secreted after a meal and circulate largely in their free forms. Fasting circulating insulin levels are low, ranging from ∼55 to 75 pM (8 to 11 µIU/mL). During digestion, to avoid IR downregulation in target cells, insulin is released from the pancreas within a 3- to 6-minute period of time, generating a blood concentration that varies from >800 pM to <100 pM.
In adults, fasting circulating levels of proinsulin are <10 pM, but they reach 10 to 50 pM or higher in insulin-resistant patients with T2DM (95, 96). Both insulin and proinsulin concentrations are several fold higher in the portal vein than in the peripheral bloodstream and are efficiently removed by the liver. As the liver almost exclusively expresses IR-B, it is exposed to the metabolic effects of insulin and protected from the mitogenic effect of proinsulin and IGFs. Of note, the hepatic removal of proinsulin is ∼10- to 15-fold less than that of insulin, accounting for its prolonged half-life in vivo and its relatively high plasma concentration in the fasting state. Proinsulin may have an important biological role in prenatal/early neonatal life (97). Recently, predominant expression of the translationally inactive intron 1–containing proinsulin mRNA isoform together with low expression of the translationally active proinsulin transcript (Pro1B) has been found in the developing hearts of chick embryos expressing IR-A, where forced expression of Pro1B led to heart malformation. These results suggest that the fine-tuned regulation of proinsulin expression plays an important role during embryonic development and that the excess proinsulin observed in diabetic mothers may be teratogenic (98).
However, the role of proinsulin in adults is still unclear, as circulating proinsulin levels are a fraction of the insulin levels, and proinsulin affinity for the IR-A is approximately fivefold lower than insulin affinity (6). However, proinsulin levels increase with age (99). Moreover, in obesity-related T2DM, islet β-cell dysfunction is characterized by accelerated proinsulin synthesis and dysregulated proinsulin processing, insulin granule formation, and insulin secretion that may cause a high proinsulin/insulin ratio (96). An excess of nutrients and other stressors, such as cytokines, may also increase the proinsulin/insulin ratio (100). Interestingly, fasting proinsulin levels are associated with all-cause and cardiovascular mortality (101) and are predictive of stroke in elderly men (102). In line with these studies, exogenous proinsulin administration was associated with an increased incidence of cardiovascular events (103).
Whereas insulin and proinsulin plasma concentrations vary depending on fasting or feeding conditions, IGF levels in the blood are more stable. More than 90% of IGFs circulate in complexes with members of a family of six different IGF-binding proteins (IGFBPs), which regulate both the half-life and the biological activities of IGFs (104, 105). Most circulating IGF-1 is produced by the liver under the control of growth hormone (GH), which also regulates IGFBP-3 production (the most abundant IGFBP). Circulating levels of IGF-1 can reach 20 nM, and free IGF-1 accounts for ∼1500 pM of that total. IGF-2 is widely expressed and not very responsive to GH, and it is more abundant than IGF-1 in serum, reaching levels of 80 nM. Notably, the free IGF-2 concentration is ∼400 pM, higher than the fasting insulin concentration (106). The physiological role of IGF-2 in adults is still poorly defined and is probably linked to the regulation of trophic, survival, and differentiation signals in skeletal muscle, adipose tissue, bone, and ovary (107). Insulin is involved in the regulation of IGF function both directly, by upregulating hepatic GH receptors and increasing IGF-1 production (108), and indirectly, by increasing the amount of bioavailable IGFs through the inhibition of IGFBP-1, the gene of which is a FOXO1 target (109). Specifically, insulin-activated AKT translocates into the nucleus where it phosphorylates FOXO1, thus inducing its nuclear exclusion and preventing it to bind the insulin response element located in the IGFBP-1 promoter (110). Given the tissue distribution of IR isoforms and the serum concentrations of insulin and IGFs, it can be hypothesized that during fasting, when insulin concentrations are low, IR-B is activated at a low level by insulin, whereas IR-A is mostly activated by IGF-2. In contrast, both IR isoforms are predominantly activated by insulin during a postprandial state, with the possible exception of tissues with low insulin availability and substantial IGF-2 production, as is the case for some areas of the brain (111) (Table 3).
Table 3.
Tissue-Specific Distribution of IR Isoforms and Their Putative Activation by Either Insulin or IGF-2 During Fasting and Fed Conditions
| Tissue/Organ | IR Isoform |
Fasting |
Fed |
||
|---|---|---|---|---|---|
| Insulin | IGF-2 | Insulin | IGF-2 | ||
| Liver | IR-B | + | − | +++ | − |
| Muscle/fat | IR-B | + | − | +++ | − |
| IR-A | ± | +++ | +++ | + | |
| Brain | IR-A | ± | +++ | +++ | + |
Symbols indicate the putative relevance of each ligand on the specific IR isoform.
Differential signaling and trafficking of IR isoforms
Ligand-mediated endocytosis of membrane receptor tyrosine kinases (RTKs) has emerged in recent years as a critical mechanism in the regulation of receptor action and contributes to fine-tuning the intensity and duration of receptor-initiated signaling (112, 113). Ligand-induced polyubiquitination of RTKs targets them for degradation through the lysosomal pathway, thus promoting receptor downregulation (114). However, recent studies have demonstrated that the EGF receptor (EGFR), platelet-derived growth factor receptor, and IGF-1R are not polyubiquitinated but rather monoubiquitinated at multiple sites (multiubiquitination), and this modification ensures receptor sorting and degradation (115–119).
In spite of the important role played by the IR in modulating several physiological and pathological processes, the mechanisms regulating IR ubiquitination, endocytosis, and sorting are still poorly characterized (118, 120). The vast majority of the original experiments on IR internalization were performed before the identification of the IR-A isoform (121) and mostly in adipocytes, which preferentially express the IR-B isoform (120, 121), and demonstrated that IR internalization occurs through both clathrin-dependent and clathrin-independent pathways (120). Therefore, whether the IR-A and IR-B isoforms differ in their endocytosis from the cell surface and the role of their ligands in modulating this process are relevant issues. Based on the observation that IGF-2 is as mitogenic as insulin through the IR-A isoform despite a threefold to fivefold lower affinity than insulin for the receptor and a reduced capacity to induce IR-A phosphorylation (32, 94), studies were performed to evaluate the hypothesis that insulin and IGF-2 might differentially regulate IR-A trafficking, thereby differentially affecting downstream responses (122). Using R−/IR-A cells, which lack the IGF-1R (123) and express solely the human IR-A isoform (124), it has been shown that insulin promotes significant IR-A internalization through clathrin-dependent and clathrin-independent pathways, a process minimally affected by IGF-2 (122). Significantly, the differential internalization was not due to a defect in IR-A ubiquitination, which was comparable after insulin or IGF-2 stimulation (122). Instead, prolonged stimulation of R−/IR-A cells with insulin, but not with IGF-2, preferentially targeted the receptor for degradation (122) through clathrin-dependent endocytosis. Low-affinity insulin analogs behaved similarly to IGF-2 (122). Additionally, upon insulin and IGF-2 stimulation, clathrin-dependent endocytosis was critical for IR-A–dependent activation of Akt, whereas clathrin-independent endocytosis preferentially regulated IR-A–dependent activation of ERKs (122).
These results provided the first characterization of IR-A endocytosis and demonstrated the critical role that this process plays in regulating IR-A activation and downstream biological effects mediated by insulin and IGF-2. Accordingly, a recent study (35) has shown that the ability of various ligands to differentially promote IR-A phosphorylation and internalization rates has a major impact in regulating mitogenic and metabolic responses (35).
Giudice et al. (125) have recently performed similar experiments comparing insulin and IGF-2 actions on IR-B internalization and signaling and demonstrated that IGF-2 promotes faster IR-B internalization than does insulin, which regulates IR-B mitogenic action through endosomes (125). In contrast, upon insulin stimulation, IR-B mostly remains at the cell membrane, thus facilitating an interaction with effector molecules involved in the regulation of IR-B–dependent metabolic responses (126). Hence, IR-B retention at the cell membrane by the expression of a traceable chimeric IR mutant fully retained its ability to activate the Akt pathway (127).
The level of IR phosphorylation seems to play a more important role than ubiquitination in regulating IR-A internalization (35, 120, 122) and may also affect the ability of IR-A to recruit proteins important for receptor internalization, such as Grb10/14 or SH2B1/B2 (128, 129), which bind the IR upon insulin stimulation (128, 130–132) and regulate insulin-dependent IR stability (128, 133). Early studies have shown that IR-A, which has a higher affinity for insulin but has a less potent kinase (134, 135), may internalize and recycle faster than IR-B (136). However, more studies are warranted to clarify better the endocytosis and trafficking of the two IR isoforms after insulin stimulation.
Another layer of complexity in the regulation of IR isoform trafficking and signaling is the fact that the IR, similar to the IGF-1R, can bind proteins of the extracellular matrix, and these interactions have a profound role in regulating ligand binding and ligand-induced receptor activation and signaling (137, 138). In this regard, it has been recently demonstrated that the proteoglycan decorin binds IGF-2 and insulin with a high affinity and proinsulin and IR-A with a threefold lower affinity (25). Although decorin did not affect ligand-induced IR-A phosphorylation and did not modulate IGF-2– and proinsulin-induced IR-A internalization (120), it significantly reduced insulin-mediated IR-A internalization (120), indicating that decorin binds IR-A at the cell surface and may modulate its levels. Importantly, decorin reduced IGF-2–induced Akt activation, enhanced IR-A downregulation after sustained IGF-2 stimulation, and significantly diminished IGF-2–induced cell proliferation (120). In contrast, decorin did not affect insulin- or proinsulin-induced signaling and biological responses downstream of IR-A (120). These results demonstrated that decorin differentially regulates IR-A ligands and they provide a plausible mechanism whereby decorin loss may contribute to tumor formation in cancer systems addicted to an IGF-2/IR-A autocrine loop.
The spindle checkpoint protein human homolog of yeast MAD-2 is also involved in IR endocytosis. The human homolog of yeast MAD-2 constitutively binds the IR through the MAD-2 interacting motif found in the C-terminal cytoplasmic tail of the IR (139). Indeed, MAD-2 binding facilitates the recruitment of the clathrin adaptor AP2 to the IR and constitutive clathrin-mediated IR endocytosis with IR signaling inhibition. Hence, the MAD-2 inhibitory protein p31comet counteracts the IR–MAD-2 interaction and enables signal transduction through the IR. Indeed, liver-specific ablation of p31comet in mice causes insulin resistance, hyperinsulinemia, glucose intolerance and hyperglycemia, and diminished the localization of the IR at the plasma membrane in hepatocytes, affecting nutrient metabolism (139). According to these data, it is possible to speculate that premature IR-B internalization in the liver, prior to insulin binding, may potentially contribute to metabolic disorders such as T2DM. Whether this system may also affect IR-A isoform trafficking in other contexts such as cancer cells is unknown.
Role of IR Isoforms in IR Signaling Diversification and Partitioning
The IR may undergo various types of signal diversification, including binding different ligands, the expression of different isoforms, the formation of HRs with the IGF-1R, and crosstalk with other membrane molecules. Moreover, the IR may exhibit functional activity after nuclear localization. The relevance of IR isoforms in these settings is variably documented and may need further research.
Signaling through HRs
The formation of HR-A and HR-B is certainly a major source of IR signaling diversification (140, 141). Because HRs are often more abundant than IR and IGF-1R homoreceptors, defining their specific signaling output is pivotal to further understanding insulin and IGF signal diversification in many aspects of physiology and disease.
As insulin has a much lower affinity for HRs than for the IR holoreceptor, HR formation in tissues with physiological IR levels may favor insulin resistance. In line with this concept, Chisalita et al. (142) found that in cultured human aortic smooth muscle cells, the presence of HRs impairs IR-mediated metabolic signaling and causes insulin resistance. The authors hypothesized that because of the low number of homodimeric IRs, physiological concentrations of insulin generate a weak downstream signal, whereas both IGF-1 and IGF-2 elicit a strong and unique downstream signal by activating both the IGF-1R and HRs. In accordance with this proposed model, in vascular smooth muscle cells in the basal state, most IRs are sequestered into insulin-insensitive HRs. The formation of HRs therefore contributes to reducing the insulin-mediated downstream events such as phosphatidylinositol 3-kinase (PI3K)/Akt activation and glucose uptake or inhibition of proinflammatory pathways (i.e., NF-κB). In accordance with this interpretation, IGF-1R disruption was followed by more IR holoreceptors, improved insulin sensitivity, reduced NF-κB activation and expression of the inflammatory gene MCP-1 in response to tumor necrosis factor (TNF)-α (143). Similar results were obtained in genetically modified mice with global or endothelium-specific IGF-1R deletion. In these in vivo models, IGF-1R deletion resulted in enhanced insulin-mediated vasorelaxation, endothelial nitric oxide synthase activation, and generation of the antioxidant/anti-inflammatory nitric oxide (144). Complementary in vitro studies conducted in human umbilical vein endothelial cells (144), human cardiac microvascular endothelial cells (145), platelets (146), and preadipocytes (147) corroborated these in vivo data. Overall, these findings strongly suggest that the IGF-1R, by participating in HR formation, may act as a negative regulator of insulin signaling and contribute to insulin resistance, at least in cells of the vascular system and in preadipocytes. However, there is also the possibility that in skeletal muscles, IGFs may partially compensate for insulin resistance by acting through HRs (see also the paragraph titled “Glucose and lipid metabolism”).
HRs are mostly thought to arise randomly and stoichiometrically from existing IRs and IGF-1Rs (141). According to this hypothesis, which is supported by experimental data obtained in placenta, human cancer cell lines, and transfected cells (2, 141), the amount of HRs can be calculated as follows: HRs = 2√IGF-1R√IR. This calculation assumes that the levels of mature receptors expressed at the cell membrane reflect the relative pools in the subcellular compartments where the assembly occurs and does not take into account possible differences in receptor turnover and/or possible posttranslational controls on receptor assembly and processing.
However, there is some scattered evidence that HR assembly may also be modulated. For instance, HCT116 human colon cells were found to express lower HR levels than predicted by the IR and IGF-1R abundance (148). Moreover, Gómez-Hernández et al. (149) have provided evidence that in vascular smooth muscle cells, IGF-2 binding favors an interaction between IR-A and the IGF-1R, enhancing HR-A formation, which would induce cell proliferation in response to IGFs and contribute to the early atherosclerotic process as well as to the development of vascular insulin resistance. Furthermore, it has been suggested that in trophoblast cells, TNF-α may inhibit HR assembly and reduce IGF-1–dependent HR phosphorylation (150). However, the mechanisms by which HR assembly may be modulated have not been investigated.
In contrast with nontransformed cells, malignant cells often express very high levels of HRs, especially HR-A (2). A few studies have provided evidence that in such conditions, insulin may induce biological actions through HRs. For example, in multiple myeloma cell lines and primary cultures, insulin, similar to IGF-1, was able to phosphorylate the IGF-1R and activate Akt and MAPK but not JAK/STAT3 or NF-κB. In these cells, HRs measured by an immunoprecipitation assay in nondenaturing conditions were activated to transduce insulin-dependent signaling and to confer potent prosurvival and proliferative activities at physiological (100 pg/mL) concentrations of insulin. At the same time, HR formation increased the amount of high-affinity receptors for IGF-1 and IGF-2, which also contributed to the transduction of HR-mediated effects (151). Interestingly, several myeloma cell lines express only the IR-A isoform and not the IR-B isoform (152), confirming previous evidence that HR-A might also respond to insulin.
Similarly, insulin, similar to IGF-1 and IGF-2, was able to activate ERK1/2 and proliferation in estrogen-independent HEC-1 endometrial carcinoma cells, which express a high level of HRs (153). These studies have raised concerns that insulin analogs used in the treatment of diabetes could have increased affinity for HRs compared with native insulin. Using a bioluminescence resonance energy transfer assay, a recent study found that only the long-acting insulin analog glargine, but not its active metabolites M1 and M2, binds with a high affinity to both HR-A and HR-B. Interestingly, the short-acting analog Lispro also binds to both HR subtypes with an approximately threefold higher affinity than native insulin, whereas another short-acting insulin analog, Aspart, binds HR-B, but not HR-A, with a twofold higher affinity than native insulin (36).
Finally, HRs were recently detected in the nucleus of human corneal epithelial cells (42). Because the IR, but not the IGF-1R, possesses a putative nuclear localization sequence, it was hypothesized that the IR moiety of HRs guides the IGF-1R to the nucleus through the importin system. Alternatively, HRs may traffic into the nucleus through SUMO modification of either the IGF-1R or IR, as previously described for the IGF-1R (154). The nuclear function of HRs is currently unknown.
“The biological significance of the interaction between the GPER and IR isoforms is still not fully clarified.”
In summary, HR assembly appears to be regulated not only by the relative abundance of IR and IGF-1R hemidimers but also by soluble factors. Clearly, HRs have high-affinity binding sites for IGF-1 and IGF-2. Accordingly, in nontransformed cells of the vascular system with relatively low IR expression, the relevant IR incorporation into HRs may contribute to the impairment of the metabolic and anti-inflammatory effects of insulin and expose cells to the mitogenic and proinflammatory effects of IGF-1 and IGF-2. In contrast, high HR-A expression in certain malignant cells likely transmits not only IGF-1 and IGF-2 signals but also insulin signals. More studies, and possibly new technical advances, are needed to better understand assembly regulation and the ligand affinity and signaling output of HR-A and HR-B in physiology and disease.
Modulation of IR signaling: IR crosstalk with molecular partners
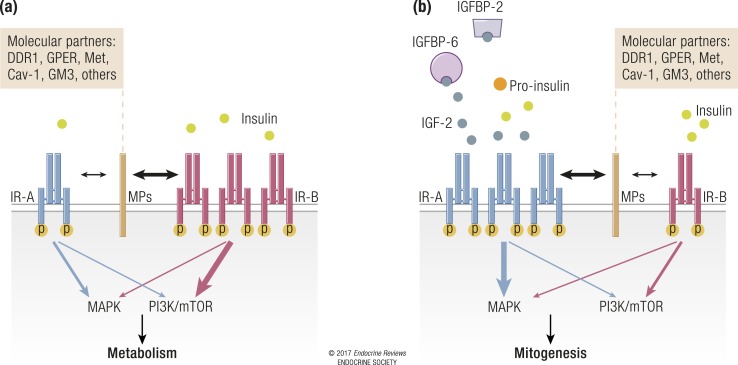
There is increasing evidence that IR-mediated signaling may undergo significant diversification, not only by forming hybrids with the IGF-1R but also by interacting with various other membrane molecules, thus generating molecular networks that might play a key role in physiological actions, such as glucose uptake and metabolism. Dysregulation of these molecular networks may therefore be associated with various disorders such as insulin resistance and cancer.
Transmembrane hormone receptors
There is evidence showing that the IR crosstalk with at least two receptor partners, the hepatocyte growth factor (HGF) receptor Met and the noncanonical estrogen receptor (ER) G protein–coupled ER (GPER), is implicated in the regulation of glucose metabolism in response to insulin.
In primary cultures of human hepatocytes as well as in hepatocarcinoma cells, exposure to either insulin or HGF induced the formation of an IR/Met hybrid complex, which occurred concomitantly with bidirectional activation of both Met and the IR, recruitment of IRS proteins, mostly IRS-2, and stimulation of the PI3K and MAPK signaling pathways (155). In vivo studies demonstrated that after the intraperitoneal injection of insulin in mice, a functional IR-Met crosstalk was crucial for liver glucose homeostasis. Indeed, loss of Met function in mice induced hyperglycemia under fasting conditions and significantly impaired glucose clearance. Conversely, in gain-of-function ob/ob mice, HGF suppressed hepatic glucose production and output and restored insulin sensitivity. This cooperation between IR and Met occurred preferentially in the liver, which expresses high amounts of both receptors and IRS-1/IRS-2, but not in adipose tissue and skeletal muscle, which express lower Met levels (155). These studies did not investigate whether both IR isoforms were equally able to form HRs with Met. Although liver predominantly expresses the IR-B isoform, the preferential formation of Met/IR hybrids in the liver was attributed to the high abundance of both receptors and not to a specific property of IR-B. As both Met and IR are often overexpressed in several malignancies (156), which may also express the corresponding ligands in an autocrine/paracrine manner, it is possible that the described functional crosstalk between the IR and Met might also play a role in cancer and control biological responses such as cell motility, growth, and morphogenesis, rather than just glucose and lipid metabolism. In cancer cells, IRs could possibly participate in the extensive network of Met interactors, which includes, among others, RET, EGFR, integrins, and certain G protein–coupled receptors (157). In view of these data, it is possible that the high circulating HGF levels observed in obesity (158–162) may represent a compensatory mechanism, which would counteract the increased insulin resistance via IR-Met crosstalk. Therefore, it could be hypothesized that the increased HGF levels in obese patients may contribute to cancer progression in tumors overexpressing the IR and Met. Further studies are necessary to fully clarify the implications of the IR-Met crosstalk in cancer.
The GPER is a noncanonical, seven-transmembrane domain receptor involved in rapid estrogen signaling (163). Recent studies have identified an important role of the GPER in glucose metabolism (164). In particular, GPER genetic KO mice develop obesity, insulin resistance and glucose intolerance, and impaired insulin secretion, among other defects. In accordance with these studies, we found that in cancer cells and in fibroblasts from breast cancer patients, insulin upregulates GPER expression and functions through the activation of the protein kinase C δ/MAPK/c-Fos/AP1 transduction pathway. Both IR-A and IR-B isoforms induced GPER upregulation, which in turn enhanced the proliferative and migratory response to insulin and boosted glucose uptake (165). Notably, in cancer-associated fibroblasts, GPER mRNA expression was positively correlated with patient serum insulin levels, highlighting the potential role of the GPER in cancer progression in individuals with hyperinsulinemia. Other studies have shown a similar crosstalk between the GPER and IGF-1R and that these two receptors may directly interact (166–169). The biological significance of the interaction between the GPER and IR isoforms is still not fully clarified and warrants future studies.
Matrix receptors
We have recently used proteomics approaches in cells solely expressing the IR-A isoform to identify several putative molecular partners uniquely recruited to phosphotyrosine protein complexes after cell stimulation with IGF-2 (170). Among these molecules, the nonintegrin collagen receptor discoidin domain receptor 1 (DDR1) was further characterized as a new IR-A molecular partner. In fact, in human breast cancer cells DDR1 associates with the IR after cell stimulation with both insulin and IGF-2 and increases IR expression through multiple mechanisms. As a consequence, DDR1 expression increases IR phosphorylation, downstream signaling, and biological responses, including cell invasion and colony formation, after cell exposure to both insulin and IGF-2 (171). A similar functional crosstalk was observed between DDR1 and the IGF-1R (172). In turn, insulin and IGFs, via the PI3K/Akt/miR-199a-5p pathway, upregulate DDR1, providing a positive feedback loop of insulin/IGF effects (173). These data suggest that in malignancies with an activated IR-A/IGF-2 loop, IR-A could constitutively interact with DDR1, triggering biased signaling.
Caveolins and IR partitioning
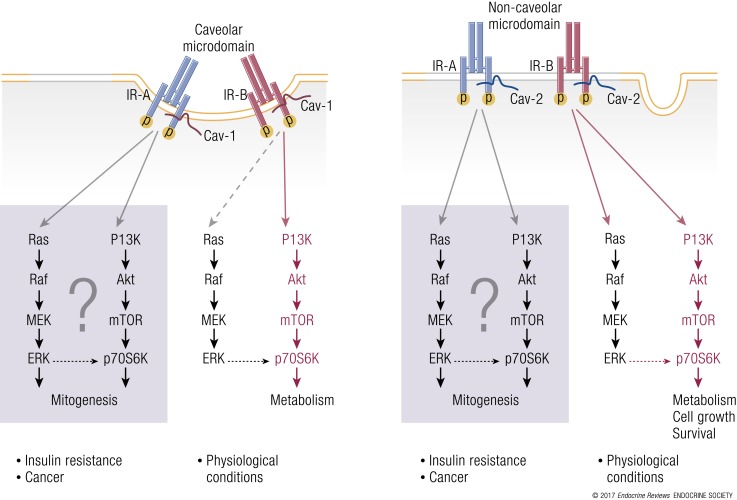
In view of previous observations that IR-A and IR-B may reside in membrane subdomains that are differentially sensitive to cholesterol depletion (90), studies focused on IR interactions with molecules expressed in specific membrane subdomains may help explain the different biological roles of the two IR isoforms. The IR is often found in caveolae, which are caveolin 1 (cav-1)–rich cell membrane invaginations representing a subdomain of lipid rafts that require cholesterol (174). In humans, cav-1–mediated IR signaling is likely required for glucose metabolism. In fact, cav-1 mutations induce generalized lipodystrophy, insulin resistance, and hypertriglyceridemia (175), and cav-1 silencing in skeletal muscle is associated with insulin resistance in vitro and in vivo (176). Moreover, adipocyte differentiation is associated with a strong induction of cav-1, along with a coordinated increase in IR, protein kinase B–Akt, and GLUT-4 expression (177). Likewise, age-dependent insulin resistance in JYD mice is associated with the progressive loss of cav-1 in skeletal muscle (176), as is insulin resistance in mice fed a high-fat diet (178). In agreement with these findings, cav-1 targeting by miR-103 and miR-107, which are increased in obesity, results in reduced IR stability and expression in caveolae-enriched plasma membrane microdomains and insulin resistance (62). Whether cav-1 differentially regulates IR isoforms remains to be determined.
Recent studies have reported a different mode of IR signaling that occurs in a particular subset of membrane microdomains and requires caveolin 2 (cav-2) (179, 180). According to these results, insulin binding induces cav-2 association with the activated IR at the NPEY motif in the IR juxtamembrane region. The fatty acylation status of cav-2 is crucial for membrane localization and interaction with the IR. Cav-2 becomes phosphorylated on Tyr19 and Tyr27 and promotes IRS-1 recruitment to the IR and increased PI3K-Akt and ERK activation, thus playing a key role in glucose uptake, cell survival, and the proliferation of 3TL1 preadipocytes and adipocytes (180). These results were unaffected by cholesterol or ganglioside depletion but were modulated by actin depolymerization. Moreover, Tyr27–cav-2 caused prolonged IR activation by interfering with the IR–SOCS-3 interaction (181). Most of these observations were made in IR-B–transfected cells or in 3TL1 preadipocytes and adipocytes. Therefore, it is important to assess whether cav-2 is equally important for IR-A signaling. As cav-2 is widely expressed and upregulated in various tumor cells (182), it is plausible that cav-2–dependent IR-A signaling may contribute to prolonged IR tyrosine kinase activation and cancer cell mitogenesis and invasion (Fig. 5). Notably, insulin induces the nuclear targeting of ERK and cav-2 in a cav-2–dependent fashion. In contrast, IGF-1 could only induce the nuclear translocation of ERK but not of cav-2 (183).
Figure 5.
IR signal diversification and partitioning by caveolins. (a) IR signaling in caveolae: under physiological conditions, insulin promotes phasic IR-B interaction with cav-1 at caveolar necks and consequent activation of metabolic effects, such as glucose transport and glycogen synthesis. In cells overexpressing IR-A, such as cancer cells, IR-B association with cav-1 may be compromised and switched in favor of the IR-A/cav-1 interaction, which may be biased toward mitogenic stimuli. (b) IR signaling in noncaveolar microdomains: in cav-2– and IR-A–enriched cells, such as certain cancer cells, the cav-2/IR-A interaction may elicit prolonged IR-A phosphorylation with enhanced mTOR/Stat-3 activation and preferential activation of mitogenic and prosurvival stimuli.
Membrane gangliosides
Another molecule that might modulate the differential partitioning of IR isoforms between caveolae and other noncaveolar microdomains is the ganglioside GM3, an important component of membrane microdomain lipid rafts. GM3-enriched microdomains have a different lipid composition than do caveolin-enriched microdomains and are not affected by cholesterol depletion. GM3 physically binds to the IR, and this interaction functionally competes with the IR–cav-1 association (184). Therefore, a TNF-α–mediated GM3 increase causes dissociation of IR–cav-1 complexes and increases IR mobility from caveolar microdomains into GM3-rich microdomains, reducing insulin-mediated signaling (184–187). Conversely, GM3 KO or depletion increases insulin signaling (188). GM3 synthesis and/or metabolism undergoes significant dysregulation in inflammatory and metabolic disorders and cancer (189, 190). Further studies are needed to clarify how GM3 may affect IR isoform partitioning and function in physiologic and pathologic conditions.
Membrane proteoglycans
An additional cell-surface molecule and circulating factor, glypican-4 (Gpc4), has been shown to bind the IR and modulate its signaling and action. Gpc4 belongs to a six-member family of glycosylphosphatidylinositol-anchored heparan sulfate proteoglycans, which function as coreceptors for a variety of growth factors and other transmembrane proteins. Gpc4 interacts with the unoccupied IR under basal conditions and dissociates upon insulin stimulation and receptor activation (191). This Gpc4-IR interaction enhances IR functions, whereas depletion of Gpc4 reduces IR phosphorylation and downstream signaling. Additionally, Gpc4 can be released from adipose tissue and acts as an insulin-sensitizing “adipokine,” which might counteract insulin resistance in obesity. The positive modulatory effect of Gpc4 on the IR may have important consequences in diabetes and cancer. Indeed, these data may encourage the discovery of new antidiabetic drugs. Further studies are required to assess whether Gpc4 may play a role in cancers overexpressing the IR-A isoform.
With the exception of Gpc4, a common feature of the IR crosstalk with these interactors is that they all are ligand-dependent or at least ligand-enhanced. Therefore, the consequences of these crosstalks may be different based on the tissue distribution of IR isoforms and the availability of IR ligands and molecular partners. For instance, in insulin target tissues predominantly expressing IR-B and in insulin-sensitive individuals, IR-B crosstalk with molecular partners is expected to be phasic and dependent on the postprandial insulin surge. In contrast, in insulin-resistant states, IR-B binding to cav-1 may be compromised and the IR-A/IR-B ratio modified in favor of IR-A (192). Hyperinsulinemia may therefore activate IR-A and its steady crosstalk with other available molecular partners with predominantly nonmetabolic actions (Fig. 6). A similar scenario may occur in cancer cells, where both IR-A and IGF-2 are often overexpressed. In this context, IGF-2 may induce continuous IR-A crosstalk with the aforementioned interactors and favor protumoral actions (Fig. 6).
Figure 6.
Schematic representation of the proposed IR signal diversification by ligand-induced crosstalk with molecular partners. (a) In cells and tissues with predominant IR-B expression, IR-B activation by insulin is normally phasic (postprandial) and favors “metabolic” downstream signaling, which may be affected by the IR-B crosstalk with tissue-specific molecular partners (MPs) such as Met, GPER, Cav1/2, DDR1, and GM3. These molecules, in turn, may autonomously activate additional signaling. (b) In cells predominantly expressing IR-A and producing IGF-2, such as fetal or cancer cells, IR-A activation by IGF-2 elicits a steady IR-A interaction with molecular partners (MPs), favoring “nonmetabolic” effects, including mitogenesis and cell migration. In insulin-resistant patients, hyperinsulinemia, and perhaps proinsulin, may elicit similar effects. The line thickness indicates the strength of signaling pathway activation.
Nuclear IR
Several lines of evidence have indicated that many receptor tyrosine kinases, including the IR and IGF-1R, not only function at the cell membrane but also translocate into the nucleus (193, 194), where they may regulate a wide array of biological responses at additional levels (such as genomic control) (193, 194). The initial evidence of IR nuclear translocation was provided in the late 1970s by Goldfine and colleagues, who reported insulin binding to purified nuclei from rat liver (195, 196) and human cultured lymphocytes (197). Similar results were recapitulated by Horvat (198) and Bergeron et al. (199), who demonstrated insulin and growth hormone binding to Golgi fractions purified from liver tissue. Subsequent work identified the nuclear envelope as the major binding site for insulin (200) and supported the notion that intracellular binding sites are immunologically distinct from binding sites at the cell membrane (197).
The mechanisms regulating IR translocation into the nucleus and its nuclear action are still very poorly characterized, and therefore it is a rapidly developing area of study in the IGF-1 field.
In the late 1980s, Podlecki et al. (201) demonstrated that a fraction (∼5%) of the internalized insulin/IR complexes translocated into hepatocyte nuclei in a time- and temperature-dependent manner (201), suggesting that IR association with specific nuclear binding sites may influence the transcription of insulin-dependent genes.
Subsequent studies confirmed that the nuclear translocation of the IR could be detected in vivo. Gletsu et al. (202) showed that obese mice had higher IR levels in the nuclei of hepatocyte cells following insulin stimulation with an oral glucose meal compared with lean mice. Importantly, phosphorylation of nuclear IR increased within 15 minutes of the glucose meal (202), and the phosphorylation levels of a 30-kDa DNA-binding protein were significantly decreased (202). Additonally, enhanced IR translocation to the nuclei was associated with increased expression of malic enzyme, suggesting that nuclear IR may regulate the phosphorylation of insulin response element transcription factors (202). Significantly, Nelson et al. (203) demonstrated that the IR and additional components of the IR signaling pathway, including most members of the MAPK cascade, were corecruited to two prototypic insulin-inducible genes, early growth response 1 and glucokinase, which regulate mitogenic and metabolic responses, respectively.
More recent data have suggested that cellular compartmentalization of the IR may play an important role in determining downstream biological responses. Amaya et al. (204) have in fact demonstrated that insulin-mediated increases in calcium concentration and cell proliferation depended on clathrin- and caveolin-dependent translocation of the IR to the nucleus, as well as on the generation of inositol 1,4,5,-trisphosphate in the nucleus, whereas the metabolic effects of insulin were independent of either of these events (204). Moreover, liver regeneration after partial hepatectomy also relied on the formation of inositol 1,4,5,-trisphosphate in the nucleus, but not in the cytosol. In contrast, hepatic glucose metabolism was not affected by inositol 1,4,5,-trisphosphate levels in the nucleus (204). These results therefore suggested that nuclear IR signaling might have broad clinical implications.
Despite these data indicating a very important role for nuclear IR signaling, whether IR-A and IR-B differ in their ability to translocate into the nucleus has not been established nor has the role that different IR ligands may play in regulating IR isoform nuclear signaling. However, studies performed in 32D hematopoietic cells transfected with either IR-A or IR-B showed that IR-A promoted nuclear translocation of IRS-1 upon insulin and IGF-2 stimulation, whereas IR-B modestly affected IRS-1 nuclear translocation and only upon insulin stimulation (205). This difference was even more pronounced in MEFs, in which IR-B activation by insulin or IGF-2 was unable to induce IRS-1 nuclear localization, which instead was strongly stimulated upon IR-A stimulation by its cognate ligands (206).
Recently, data from Wu et al. (42) have demonstrated that IGF-1 stimulation of corneal epithelial cells promoted nuclear localization of IGF-1R/IR hybrids but not translocation of IGF-1R/IGF-1R hybrids (42), adding a new layer of complexity to the modulation of IGF-1R/IR nuclear signaling.
Collectively, these results support the hypothesis that different IR isoforms and IGF-1R/IR hybrids may differ in their capacity to activate nuclear signaling, which may have an important effect in differentially modulating downstream biological responses in diabetes, metabolism, and cancer.
“The relative abundance of IR isoforms may… play a significant role in regulating development and differentiation.”
Unliganded IR actions
The IR has ontogenetically specialized to play a major role in controlling cell metabolism depending on nutrient availability but has maintained its role in regulating growth and apoptosis. Under optimal environmental conditions, nutrient (glucose) availability stimulates ligand abundance (insulin), which activates the IR and postreceptor signaling cascades, leading to anabolic cell metabolism and antiapoptotic effects favoring cell and tissue growth and differentiation. In contrast, in an environment of suboptimal growth conditions, the restriction of nutrient-derived calories reduces insulin availability and antiapoptotic effects.
Recently, an additional role of the IR (and IGF-1R) in regulating apoptosis in the unliganded state has been described. Engineered cells lacking both the IR and the IGF-1R genes [double KO (DKO)] become resistant to apoptosis induced by TNF-α, etoposide, or serum deprivation (207). This resistance is caused by reduced production of the proapoptotic protein Bax, whereas many antiapoptotic factors are increased in the DKO cells. The mechanism involved in these abnormal changes in the levels of apoptotic effector proteins is unclear, but it is probably posttranscriptional (207). The normal apoptotic response is restored in DKO cells when either the IR or IGF-1R or both are re-expressed in the absence of ligands. Interestingly, mice with liver-specific IR KO (LIRKO mice) show age-dependent liver dysfunction with scattered focal liver dysplasia, which however do not progress to hepatocarcinoma. These structural changes are possibly related to altered liver metabolism and increased oxidative stress (208). Alternatively, it is also possible to hypothesize that decreased apoptosis in cells lacking IR and IGF-1R (3) may also have a role in the genesis of nodular liver hyperplasia in LIRKO mice.
These observations indicate that unliganded IR and IGF-1R membrane proteins have a permissive role in apoptosis, an effect reversed upon ligand binding to the receptors and activation of postreceptor signaling (Fig. 7).
Figure 7.
Three-state model for the IR and IGF-1R control of cell apoptosis. The schematic representation of the complex regulation of cell apoptosis by the IR and IGF-1R in the presence or absence of ligands is shown. Unliganded IR-B might have a more rapid effect on apoptosis.
This permissive role of the IR and IGF-1R in apoptosis was independent from receptor tyrosine kinase activity and postreceptor signaling. In fact, transfecting DKO cells with a kinase-deficient receptor (IR or IGF-1R) protein was as efficient as a wild-type receptor. Moreover, inhibiting both “conventional” postreceptor signaling pathways (PI3K and MAPK) alone or in combination did not affect permissive receptor effects on apoptosis. Currently, the mechanisms underlying the proapoptotic effect of unliganded IR (and IGF-1R) have not been fully clarified, nor has the role therein of IR isoforms.
In a different model where IR, but not IGF-1R, was knocked out in immortalized neonatal hepatocytes and either IR isoform A or isoform B was re-expressed, slightly different results were obtained (209). In response to serum starvation, cells lacking the IR showed enhanced apoptosis associated with an increase in reactive oxygen species (ROS), nuclear translocation of FoxO1, changes in Bcl-xL and Bim, and caspase-3 activation. The expression of either IR-A or IR-B induced a stronger apoptotic process while preventing ROS generation. In these cells, both IR isoforms interacted with Fas/Fas-associated protein with death domain and induced caspase-3 activation. However, in cells expressing IR-A, caspase-3 activation involved cytochrome c release by mitochondria, whereas in cells expressing IR-B, caspase-3 was directly activated by caspase-8, resulting in a more rapid apoptotic process. The mechanisms accounting for the different effects of the two IR isoforms on apoptosis remain unclear. However, the authors hypothesized that cells expressing the IR-A isoform may activate the mitochondrial pathway of apoptosis due to the greater ability of IR-A to bind GLUT-1 and GLUT-2 and facilitate glucose uptake (210). Notably, coexpression of the two IR isoforms in the absence of insulin also resulted in protection from apoptosis (209). Further research is needed to establish the in vivo relevance of these data in both physiologic and pathologic conditions. The relative abundance of IR isoforms may, in fact, play a significant role in regulating development and differentiation, balancing the proapoptotic condition of the unliganded state with the antiapoptotic condition when receptors are ligand activated. The authors suggested that the IR-A/IR-B ratio may regulate apoptosis in hepatocytes during development. If these data are confirmed in other systems, it is possible that cancer cells with a high IR-A/IR-B ratio may undergo apoptosis in response to therapies aimed at blocking IR-A ligands (see also the paragraph titled “The IR-A pathway as a cancer target”).
IR Isoforms and IR/IGF-1R Hybrids in Pathophysiology
Development, differentiation, and aging
The different roles of IR isoforms in mammalian development are underscored by the differences in IR isoform expression patterns between fetal and adult tissues. IR-B is more abundant in several differentiated cells, including epithelial intestinal cells, mammary gland cells, endothelial cells, liver cells, white and brown adipose tissue cells, kidney cells, and thyroid cells, compared with proliferating and precursor cells. In contrast, IR-A is the predominant isoform in all the cell precursors of the previously-mentioned cell types (211). Because IR-A, but not IR-B, binds IGF-2 and proinsulin with a high affinity, this evidence suggests that IR-A may regulate tissue development in response to these growth factors. The recent observation that IR-A binds GLUT-1 (210) may suggest a role for this isoform in basal glucose uptake in fetal tissues as well as in some adult tissue such as the central nervous system (CNS). This hypothesis is supported by studies showing that IGF-2, but not insulin, stimulates glycogen synthesis via IR-A in fetal mouse livers (212).
The role of HRs in fetal development is more elusive. There are no studies specifically addressing their expression and function in fetal tissues. As HR expression depends on the availability of both IR and IGF-1R moieties, they are present in both fetal and adult tissues. Functional studies on HRs performed in rabbit and human tissues, including human placenta, suggest that they enhance the actions of IGFs and confer IR signaling capabilities on them via the activation of IR β-subunit moieties of HRs (141). In vitro evidence (213) indicates that IR-A downregulation is coincident with enhanced IGF-1R phosphorylation due to a decreased formation of IR/IGF-1R hybrids.
The role of IR isoforms, the IGF-1R, and HRs in differentiation has been investigated in several model systems. For instance, preadipocytes express approximately equal levels of the IR and IGF-1R, whereas the IR/IGF-1R ratio increases during their differentiation into mature adipocytes, which express ∼10-fold more IR than IGF-1R. Studies in preadipocytes from brown adipose tissue, where the expression of either IR or IGF-1R was silenced, revealed that the IR and IGF-1R have different roles in regulating the activation of various signaling molecules associated with adipocyte differentiation (147, 214) (Table 4). Significantly, in these cells, one receptor cannot compensate for the lack of the other. However, adipocyte-specific IR and IGF-1R DKO (FIGIRKO) mice are resistant to age-associated and diet-induced obesity and show an almost complete absence of brown fat, further demonstrating that both insulin and IGF-1 signaling are required for the development of white and brown fat (224). The low expression of the IGF-1R, and therefore of HRs, in mature adipocytes might be explained by the fact that these fully differentiated cells no longer require signals through the IGF-1R but only metabolic signals via IR-B. Accordingly, rat preadipocytes predominantly express IR-A, whereas progression through adipogenesis results in an increased IR-B/IR-A ratio and IR-B being significantly higher than IR-A in mature white adipose tissue (215). In agreement with this expression pattern, stimulation of glucose transport in mature adipocytes is considerably more sensitive to insulin than to IGF-1, which can be explained by the fact that mature adipocytes have the IR but not a functional IGF-1R. In agreement with these data, in adult mice, tamoxifen-inducible adipocyte-specific deletion of IR expression caused a rapid and significant reduction in body adiposity, hyperinsulinemia, severe insulin resistance, and diabetes, whereas adipocyte-specific deletion of IGF-1R produced only a marginal effect on adiposity reduction and no effect on glucose homeostasis (225). In preadipocytes, where the IR and IGF-1R are almost equally expressed, both DNA synthesis and glucose transport are sensitive to IGF-1 and to insulin stimulation (147, 214). In this respect, glucose uptake stimulated by IGF-1 in preadipocytes might be mediated by HRs via the IR β-subunit. These data demonstrating the significance of an IR-A to IR-B shift during adipogenesis are in agreement with recent studies showing that multiple alternative splicing events are involved in the development of white or brown adipocytes (226).
Table 4.
Molecular Alterations of IR Isoforms and Related Components in Various Tissues and Organs During Development and Differentiation
| Tissue/Organ | Alterations | References | ||||
|---|---|---|---|---|---|---|
| Fat | h-Preadipocytes | ⇧ IR-A | ⇔ IR/IGF-IR | Back et al. (147, 214) | ||
| h-Mature adipocytes | ⇧ IR-B | ⇧ IR/IGF-IR | ⇩ HRs | Back et al. (147, 214) | ||
| r-Epididymal adipocytes during aging | ⇧ IR-A | ⇩ IR-B | ⇩ IRS-1 | ⇩ IRS-3 | Serrano et al. (215) | |
| Bone | h-Osteoblast precursors | ⇧ IR-A | ⇩ IR-B | Avnet et al. (216) | ||
| h-Mature osteoblasts | ⇧ IR-B | ⇧ IR-B/IR-A | Avnet et al. (216) | |||
| Breast | m-MEC precursors | ⇧ IR-A | ⇩ IR-B | Berlato et al. (217) | ||
| m-Mature MECs | ⇧ IR-B | ⇩ IGF-IR | Berlato et al. (217) | |||
| m-MEC line (HC11) after differentiation | ⇧ IR-B | Berlato et al. (217) | ||||
| m-MECs in postnatal development | ⇧ IR/IGF-IR | ⇩ HRs | Rowzee et al. (218) | |||
| Intestine | m-IECs precursors | ⇧ IR-A | ⇩ IR-B | Andres et al. (219) | ||
| m-Mature IECs | ⇧ IR-B | ⇩ IR-A | ⇧ MBNL2 | ⇩ CUGBP1 | Andres et al. (219) | |
| Endometrium | h-ECs during proliferative phase | ⇧ IR-A | ⇩ IR-B | Flannery et al. (220) | ||
| h-ECs during secretory phase | ⇧ IR-B | ⇩ IR-A | ⇧ IGF-IR | Flannery et al. (220) | ||
| Brain | m-NCs precursors | ⇧ IR-A/IR-B | ⇧ IR-A/IGF-IR | Ziegler et al. (221) | ||
| m-Lineage restricted NCs | ⇩ IR-A | ⇧ IGF-IR | Ziegler et al. (221) | |||
| Endothelium | h-HUVECs during normal pregnancy | ⇧ IR-A | Westermeier et al. (222) | |||
| Thyroid | h-TCs precursors | ⇧ IR-A | ⇩ IR-B | ⇧ IR-A/IR-B | ⇧ IR/IGF-IR | Malaguarnera et al. (223) |
| h-TCs after differentiation | ⇧ IR-B | ⇩ IR-A | ⇩ IR-A/IR-B | ⇩ IR/IGF-IR | Malaguarnera et al. (223) |
Abbreviations: EC, endometrial cell; h, human; IEC, intestinal epithelial cell; m, murine; MEC, mammary epithelial cell; NC, neural cell; r, rat; TC, thyroid cell.
A similar shift from IR-A to IR-B has been observed during osteogenesis, highlighting the crucial role of insulin in osteoblast function (Table 4). Osteoblast precursors mainly express the IR-A isoform, whereas mature human osteoblasts predominantly express IR-B, with the IR-B/IR-A ratio progressively increasing along the osteogenic differentiation of mesenchymal precursors (216).
Similar findings were reported in mammary glands. Mammary gland terminal differentiation is known to require insulin and its regulatory role in milk secretion during lactation. In mice, selective IR silencing in the mammary gland induces a dramatic decrease in alveolar development and differentiation that cannot be compensated for by IGF-1R stimulation, thus confirming the key role of insulin in inhibiting cell proliferation in the mammary gland of the midpregnant mouse (227). Analysis of the expression of the IGF-1R and IR isoforms has shown a substantial increase in IR-B levels (threefold to fourfold) associated with a decrease in IGF-1R expression (sixfold) during terminal differentiation in the developing mammary gland (217) (Table 4). These data were confirmed by the observation that during in vitro differentiation of the HC11 murine mammary epithelial cell line, IR-B undergoes a 10-fold upregulation. Notably, insulin was shown to induce milk constituents and related biosynthetic enzymes solely through IR-B, whereas stimulation of the IGF-1R only stimulated cell growth but not lactogenesis, further supporting the nonredundant role of IR-B in the terminal differentiated mammary epithelium (217).
Other laboratories have confirmed that IR-B expression reaches a maximum during terminal alveolar cell differentiation and is required for lactation (218). Interestingly, both IR-A and IR-B expression exceeded IGF-1R levels at all stages of murine mammary epithelial cells during postnatal development. Unlike IR-B, which plays a key role during differentiation, the IGF-1R is more important than the IR in mediating IGF-2–dependent mammary epithelial cell growth in virgin glands. Interestingly, the total number of HRs did not vary during pregnancy (∼50% of IGF-1R subunits), despite that IGF-1R homodimers decreased with terminal differentiation (218).
Similarly, in highly proliferative intestinal epithelial stem cells and progenitors, IR-A expression was predominant, whereas IR-B levels increased in postmitotic, differentiated intestinal cells (Table 4). These studies support a model in which IR-A expression is enriched in intestinal epithelial stem cells and progenitors of the intestinal crypt, whereas IR-B expression is enhanced in postmitotic, differentiated lineages of the intestinal villus. Significantly, the IR-A to IR-B switch was accompanied by increased expression of the exon 11 splicing enhancer MBNL2 and decreased expression of CUGBP1, the exon 11 splicing silencer (219).
Similarly, IR-A expression was elevated in endometrial cells during the proliferative phase, indicating that the IR-A isoform plays a key role in the initial estrogen-independent endometrial proliferation. In contrast, IR-B increased during the secretory phase of endometrial cells, which is when they provide metabolic support for embryo development (Table 4). These findings highlighted the differential roles of IR isoforms in endometrial physiology and embryo development (220).
Intriguingly, in the CNS, IR-A activation by IGF-2 has been shown to support the expansion of neural stem cell proliferation and self-renewal independently of the IGF-1R or the IGF-2R/6MP, suggesting a unique role for IR-A in brain development (38).
Recent findings have also indicated that the IR drives hematopoietic stem cell differentiation into lymphoid lineages during early lymphopoiesis, which is essential for maintaining a balanced generation of myeloid and lymphoid populations. However, the relative contribution of the two IR isoforms has not been determined (228).
The potential role of insulin in determining cell differentiation and fate has been confirmed in induced pluripotent stem cells from individuals with genetic insulin resistance caused by IR mutations (220). However, the different roles of the two IR isoforms in determining cell fate in pluripotent stem cell populations need to be clarified. In human placental mesenchymal stem cells, both the IR-A/IR-B ratio and IGF-2 expression were found to be increased by low oxygen tension (230), and cell proliferation was regulated by IGF-1R in response to IGF-1 and IR-A in response to IGF-2. However, pluripotency markers were upregulated by IR-A whereas they were reduced by IGF-1R stimulation.
In contrast, no differences were observed in the IGF-1R/IR ratio in freshly isolated (differentiated) compared with cultured (undifferentiated) endothelial cells [human umbilical vein endothelial cellc (HUVECs)]. Changes in receptor expression levels and in the IGF-1R/IR ratio during differentiation may therefore be different in different cell types. In accordance with the receptor expression data, in HUVECs, IGF-1 elicited significant effects on glucose accumulation and thymidine incorporation, suggesting that the insulin signal might be below a threshold required to exert biological effects (222). However, during normal pregnancy, HUVECs predominantly express the IR-A isoform, which mediates the protective effects of insulin for endothelial cell functions (222) (Table 4).
Increasing evidence suggests that IGF-1/insulin signaling plays a pivotal role in the regulation of longevity in invertebrates. In Caenorhabditis elegans and Drosophila melanogaster, the downregulation of IGF-1/insulin signaling significantly extends survival (231).
In humans, it has been shown that GH/IGF-1 secretion and insulin sensitivity decline with aging and that insulin resistance is associated with increased morbidity and mortality (232). The role of the IGF-1 system in relationip to longevity is still controversial. Some reports found an increased plasma IGF-1/IGFBP-3 molar ratio in healthy centenarians compared with aging subjects, suggesting that the higher IGF-1 bioavailability contributes to increased lifespan (233). Alternatively, heterozygous mutations in the IGF-1R gene, associated with high serum IGF-1 levels and reduced activity of the IGF-1R, have been reported in Ashkenazi Jewish centenarians compared with a control population (234).
Moreover, although a positive association between circulating IGF-1 levels and cancer mortality has been found in many studies (235), low total IGF-1 levels have been associated with an increased risk for cardiovascular diseases and diabetes (236). These conflicting results probably reflect the complexity of the IGF system and do not take into account the role of IR isoforms and IR/IGF-1R hybrids. Studies performed in rats indicate that the relative abundance of IR isoforms may also vary during aging. One study reported a decrease in the expression of the IR-B isoform in the epididymal adipose tissue in aging rats as well as a decrease in both the mRNA and protein levels of total IR, IRS-1, and IRS-3 (215). Notably, epididymal adipose tissue is the first among white fat tissues to develop signs of age-related inflammation and insulin resistance (237). These data are in agreement with findings that obese Zucker rats display age-related increases in the proportion of IR-A in the liver, associated with increased endocytosis and degradation of total IR protein (238). Interestingly, it has been reported that approximately a third of splicing factors show age-related expression changes in human fibroblasts and endothelial cells. At least some of these changes are causally associated with changes in ataxia telangiectasia mutated (ATM), a key regulator of the DNA damage response signaling pathway, which increases alongside age-related increases in DNA damage. SR proteins and hnRNPA1, factors involved in IR isoform regulation, are among the ATM-regulated splicing factors (239). Thus, it is reasonable to speculate that IR-B downregulation may contribute to insulin resistance in aging. Further studies are needed to assess whether age-related insulin resistance is associated with increased HRs expression in humans.
Taken together, these data suggest that (1) IR-A is important in fetal development, as it mediates some effects of IGF-2 and proinsulin in embryos; (2) IR-A may increase with aging, thereby contributing to insulin resistance; (3) IR-B increases during cell differentiation, thereby sensitizing tissues to the metabolic effects of insulin; (4) the increase in HRs reduces insulin and proinsulin action and might contribute to the unbalanced insulin/IGF tuning in insulin-resistant subjects; and (5) whereas the IR-B confers signaling specificity on the IGF system, the IR-A isoform and HRs are responsible for signaling redundancy and promiscuity, which are useful in fetal development but detrimental in adults, as they might contribute to insulin resistance and reduced lifespan.
Insulin secretion and the liver β-cell axis
The pivotal stimulatory role of the IR metabolic pathway in β-cell viability and proliferation has been widely demonstrated in several biological models, including KO mice and cultured cells. The contribution of IR isoforms in this context has only recently been unraveled in the complex interplay between β-cells, insulin secretion, and liver metabolism.
The first evidence of this complex network was obtained in inducible LIRKO mice (240). As expected, the loss of hepatic IR expression in these animals was accompanied by progressive hepatic and, in the long term, generalized insulin resistance. More importantly, the mice displayed compensatory hyperinsulinemia and increased β-cell mass, which was related to the degree of hepatic IR loss. Liver IGF-1 and, as a consequence, serum IGF-1 levels were also increased in inducible LIRKO mice. Interestingly, the hyperplastic β-cells of these animals showed IR-A upregulation (241). Proliferation studies performed in these cells showed that the IR-A–expressing cells were more sensitive than those expressing IR-B to the mitogenic effect of IGF-1, acting through the IR-A/IRS-1/2/PI3K pathway. Depletion of the IGF-1R transcript by small interfering RNA did not change the selective effect of IGF-1 on IR-A–expressing cells, indicating that IGF-1–mediated responses occur through a direct interaction with IR-A and not through IR/IGF-1R hybrids. These data suggest that in this model of liver insulin resistance, increased IGF-1 and insulin levels contribute to β-cell hyperplasia through the IR-A (240). However, other liver-derived factors, including the protease inhibitor SerpinB1, may play also a role in β-cell hyperplasia in LIRKO mice (242).
Glucose and lipid metabolism
Early data obtained in HepG2 human hepatoma cells indicated that IR-B was more potent than IR-A in terms of receptor and substrate phosphorylation, suggesting that IR-B rather than IR-A is the receptor with the predominant metabolic commitment (243). Few studies, however, have addressed the specific role of IR isoforms in glucose and lipid metabolism. Data from LIRKO mouse studies have been particularly informative, because they demonstrated that in the mouse liver, ∼95% of the IR is expressed as IR-B (244). Interestingly, the predominant expression of IR-B in the liver is conserved across species (32, 244, 245). LIRKO mice show severe glucose intolerance, hyperinsulinemia with the inability of insulin to suppress hepatic glucose production, and unchanged levels of glucagon. Additionally, these mice have decreased clearance of apolipoprotein B lipoproteins as well as reduction in both serum triglyceride and free fatty acids. These abnormalities in lipids suggest a decreased effect of insulin in liver to promote triglyceride synthesis coupled with an increased action of insulin in adipose tissue to suppress lipolysis (208). These data confirm the key role of hepatic IR-B in glucose and lipid metabolism (208, 246). Similar data were recently obtained using a model of acute, LIRKO in adult mice (247).
In apparent discordance with these findings, it has been recently reported that in fetal mice with inducible IR KO in the liver, adenovirus-mediated IR-A expression was more efficient than IR-B expression in restoring liver glucose uptake, glycogen synthesis, and storage (212). In this model, liver expression of IR-A, but not IR-B, was able to compensate for diabetes in the long term by improving glucose homeostasis and avoiding β-cell hyperplasia due to insulin resistance and subsequent β-cell failure (248). These results could be explained at least in part by the observation that the IR-A isoform has a stronger effect than IR-B in favoring basal glucose uptake by specifically binding the glucose transporter GLUT1/2 and inducing GSK3α/β phosphorylation (248). In view of the predominant expression of IR-B in the adult liver, it seems unlikely that these findings may have physiological relevance in adult animals.
The different roles of IR isoforms in glucose and lipid metabolism have been additionally dissected by the use of isoform-specific insulin analogs. An insulin analog with a preferential affinity for the IR-B isoform exerted a stronger effect on glycogen accumulation in rat hepatocytes and lipogenesis in adipocytes compared with muscle glycogen synthesis (244). In contrast, a different analog with a higher affinity for IR-A was more potent in favoring muscle glycogen synthesis rather than glycogen accumulation in hepatocytes and lipogenesis in adipocytes. Overall, the preferential stimulation of IR-B elicited a stronger metabolic effect not only in hepatocytes and adipocytes that predominantly express IR-B but also in rat skeletal muscle, which predominantly expresses IR-A (244). These data showing a higher metabolic activity of IR-B in skeletal muscle appear to be in accordance with human studies indicating that the IR-A/IR-B ratio is increased in the skeletal muscles of patients with T2DM and/or insulin resistance (249) and that restoration of glucose control by insulin administration or bariatric surgery (87) in T2DM patients favors preferential IR-B expression (see also the paragraph titled “Insulin resistance, gestational diabetes, and diabetes”).
Data from mice with skeletal muscle-specific IR KO (MIRKO) mice are, however, more difficult to interpret in relationship to IR isoform–specific effects, as both isoforms are expressed in mouse skeletal muscle, and the IR-A isoform is prevalent in the soleus muscle (244). MIRKO mice exhibited hypertriglyceridemia and mild obesity but not hyperglycemia (250), despite markedly decreased insulin-stimulated glucose transport and glycogen synthesis in muscle. However, MIRKO mice exhibited increased insulin-stimulated glucose transport in fat that contributed to the development of metabolic syndrome (251).
With respect to the role of the IGF-1R and HRs in glucose homeostasis, our understanding appears incomplete. Interestingly, in IR KO mouse myotubes, IGF-1 increased glucose uptake and glycogen synthesis, indicating that the IGF-1R elicits metabolic signaling in muscle cells (252). Recent studies show that mice with either IR or IGF-1R deletion undergo little or no changes in glucose homeostasis or muscle mass, indicating that each of these two receptors may vicariate the other receptor (253). Surprisingly, mice with combined loss of IR and IGF-1R in muscle displayed dramatically decreased muscle mass but normal glucose and insulin tolerance, owing to enhanced basal glucose uptake into muscle secondary to increased expression and translocation of glucose transporters. When mice with combined loss of IR and IGF-1R in muscle were crossed to mice carrying a dominant-negative IGF-1R, the resultant mice still developed glucose intolerance and dyslipidemia, even in the absence of functional IR or IGF-1R, suggesting that other receptor-interacting proteins in muscle can have a role in glucose homeostasis (253).
In humans, a role for HRs in glucose homeostasis is suggested by data obtained in T2DM patients. In the skeletal muscles of patients with T2DM, the expression of HRs is higher than in control subjects and positively associated with insulin resistance and hyperinsulinemia. As a consequence, skeletal muscle cells from T2DM patients exhibited a lower binding affinity for insulin but a higher affinity for IGF-1 than did cells from control subjects, and these changes were correlated with the percentage of HRs (254).
The involvement of HRs in glucose and lipid metabolism has been confirmed by studies in transgenic animals. Overexpression of a kinase-deficient IR in mouse muscle led to glucose intolerance with increased circulating insulin and triglyceride levels. Likewise, male mice overexpressing a dominant-negative, kinase-dead IGF-1R in muscle [muscle IGF-IR-lysine-arginine (MKR) mice] developed severe glucose intolerance, insulin resistance, and diabetes. Further studies revealed that expression of the kinase-dead IGF-1R (MKR allele) impairs both insulin and IGF-1 signaling in muscle due to the formation of HRs containing the defective allele. These data suggest that the normal glycemia in MIRKO mice might be due to the IGF-1R compensating for the loss of IR signaling in muscle (255). In this respect, it is reasonable to speculate that HR expression in the muscles of insulin-resistant patients might be a compensatory event that restores sensitivity to insulin/IGF signaling in muscle.
Brain activity
The IR is expressed in various regions of fetal and adult brains. The highest expression of the IR in the brain is found in the olfactory bulb, hypothalamus, cerebral cortex, cerebellum, and hippocampus. IR downstream effectors, such as IRS proteins and PI3K isoforms, show discrete expression patterns, mostly overlapping with expression of the IR (256). In neurons, the IR exhibits a lower molecular mass because of exclusive expression of IR-A and reduced glycosylation with sialic acid. In fact, IR ligand binding induces IR interaction with neuraminidase 1, which causes IR desialylation on the β-chain; IR desialylation, in turn, favors IR activation (257, 258). Unlike the IR in peripheral tissues, the brain IR seems not to display negative cooperativity (259, 260).
Insulin enters the CNS through the blood–brain barrier (BBB) by receptor-mediated transport. Studies in rodents have indicated that <1% of peripherally administered insulin reaches the CNS (261), but this percentage may strongly vary among different species (261). The transport of insulin across the BBB is saturable and may be reduced in several conditions, including fasting, obesity, aging, and dexamethasone treatment, whereas it is increased in some models of diabetes mellitus (261). A number of actions have been attributed to insulin in the brain. Recent studies have tried to address the mechanism by which insulin controls cerebral and systemic metabolic homeostasis and found that a key step is insulin-induced glucose uptake across the BBB via hypothalamic astrocytes, which regulate CNS glucose sensing. Indeed, astrocytic IR ablation reduced glucose-induced activation of hypothalamic pro-opiomelanocortin neurons and impaired physiological responses to changes in glucose availability, demonstrating that astrocytic IRs are required for proper glucose and insulin entry into the brain (262). Interestingly, unlike neurons, astrocytes appear to predominantly express the IR-B isoform (263). Moreover, through the IR-B, insulin stimulates cell proliferation, glucose uptake, and glycogen synthesis in astrocytes (264). In contrast, IR-A expression in neurons mediates a variety of nonmetabolic effects. Direct insulin administration into the brain has an anorexigenic effect (265) by both inhibiting the orexigenic neuropeptide Y and stimulating the expression of the anorexigenic peptide α-melanocyte stimulating hormone. Selective inhibition of PI3K prevents insulin-induced anorexia, suggesting that PI3K is an important mediator of this action (266). Another effect of insulin in the brain is the regulation of reproduction, as suggested by the relationship between obesity, hyperinsulinemia, and infertility. Indeed, neuron-selective IR KO mice display reduced fertility due to hypothalamic impairment of gonadotropin-releasing hormone secretion (267). Insulin and IGF-1 stimulate gonadotropin-releasing hormone production in hypothalamic cells both in vitro and in vivo (268). Moreover, insulin activates neurons of the mesolimbic dopaminergic pathway, which is implicated in the motivating, rewarding, and reinforcing properties of natural stimuli such as food (269). In accordance with these results, several studies have indicated a role for insulin in learning and memory. In humans, the systemic infusion of insulin under euglycemic conditions is associated with a significant improvement in verbal and attention capabilities (270), and intranasal insulin administration improves working memory (271) while not affecting peripheral glucose levels, indicating that insulin may control brain functions independently of glycemic changes. In accordance with these studies, insulin-resistant T2DM patients generally exhibit worse learning performance than age-matched control subjects (265).
The IGF-1R and IGF-1 are also coexpressed in various brain regions, suggesting the presence of a paracrine or autocrine loop (256). The IGF-1R is highly expressed in the developing cerebellum, midbrain, olfactory bulb, and in the ventral floorplate of the hindbrain (256). The level of IGF-1R expression decreases to adult levels in the postnatal period and remains high in the choroid plexus, meninges, and vascular sheaths (272). IGF-1 is expressed in the rodent embryo, peaking in the second postnatal week, but continues to be expressed in the adult brain, particularly in neuronal cells (256). Similar to insulin, IGF-1 exerts remarkable neurotrophic effects that involve glutamatergic synapses within the hippocampal circuitries, thereby affecting learning and memory, as well as protection against cellular injury, neurogenesis, angiogenesis, and even amyloid clearance (273). However, in contrast with IR-A, IGF-1R reduces glucose uptake in mouse astrocytes by interacting with GLUT1 and retaining it inside the cell (274).
IGF-2 is expressed mostly in mesenchymal tissues, mainly the meninges and choroid plexus, which is also the main source of IGF-2 in the cerebrospinal fluid (275). IGF-2 also plays a role in memory and learning processes by binding to the IGF-1R and/or IR-A, whereas forced downregulation of IGF-2 in the dorsal hippocampus as well as inhibition of IGF-2 by blocking antibodies blunted long-term memory consolidation (276). In the model of Tg2576 mice, which are affected by a double mutation of amyloid precursor protein (K670N/M671L) and show an early accumulation of β-amyloid, local IGF-2 induced neuronal protection by binding to IR-A and stimulating sustained Akt phosphorylation (277). These data confirm that the IR-A and IGF-2 have a neuroprotective function under conditions of neuronal damage and neurodegeneration.
“The possible role of HRs in the brain… remains elusive.”
Genetically engineered IGF-2 analogs were used to establish which receptor mediates the effect of IGF-2 on stemness of neural precursors in the mouse subventricular zone. Indeed, the V43M analog, which binds with high affinity to M6P/IGF-2R but with very low affinity to IR-A and IGF-1R, failed to promote growth of neural stem cell precursors in vitro. In contrast, F19A, an IGF-2 analog that binds both the IR-A and IGF-1R, but not the M6P/IGF-2R, was able to stimulate the growth of neural stem cells. This action was unaffected by the blockade of IGF-1R with a specific antibody, showing that IR-A, but not IGF-1R, was involved in the promotion of neural stem cell stemness in response to IGF-2 (38).
Studies in transgenic mice indicate different roles of the IR and IGF-IR in brain growth and development. IGF-1R KO results in reduced brain size (278), whereas IR KO does not influence brain development or size (279), suggesting that IR action occurs postnatally. Accordingly, overexpression of IGF-1 results in increased brain size (280), whereas overexpression of IGF-2 induces neonatal body overgrowth (281). This specificity of action may partially depend on the preferential recruitment of IRS-2 by the IGF-1R and of IRS-1 by the IR (282). Accordingly, studies performed in human brain tissue indicated that insulin stimulates IRS-1, but not IRS-2, whereas IGF-1 activates IRS-2, but not IRS-1 (283). These studies suggest that the IGF-1R/IRS-2 pathway is more important in neuronal and brain development, whereas the IR/IRS-1 pathway preferentially impacts neuronal survival and remodeling.
IR and IGF-IR may also differentially modulate IGFBPs. In glial cells, insulin stimulates the expression of IGFBP-2, whereas IGF-1 increases the expression of both IGFBP-2 and IGFBP-3. Both IGFBPs tend to reduce the effects of IGFs on neuronal cells.
The coexpression of both IRs and IGF-IRs in brain cells is expected to induce the assembly of HRs, which, however, have not been studied in the brain. In particular, it would be important to have data concerning HR expression in different cells of brain as well as concerning the relative proportion of HR-A and HR-B. The possible role of HRs in the brain, therefore, remains elusive. It is reasonable to suppose that HRs may confer unique properties by allowing IGF-1 to activate IR β-subunit moieties.
In conclusion, the available evidence indicates that whereas astrocytes express mainly IR-B, neurons express IR-A. The astrocytic IR-B is required for proper glucose and insulin entry into the brain. Insulin and IR-A in neurons are important in food intake, reproduction, and cognition, whereas insulin resistance with reduced insulin transport through the BBB may contribute to neurodegeneration. Brain IGFs are also important in both CNS development and cognition. IGF-2 produced in the CNS may compensate for deficient insulin and IGF-1 signaling by binding IR-A, the IGF-1R, and HRs. As shown in Tg2576 mice, IGF-2, via IR activation, may prevent/attenuate neuronal damage in response to amyloid accumulation.
IR Isoforms and IR/IGF-1R Hybrids in Disease
Several studies have confirmed the relevance of IR-A and HRs in cancer development, progression, and resistance to anticancer therapies. The IR-A/IR-B ratio may also play important roles in insulin resistance, diabetes, and neurodegeneration.
Cancer
IR isoforms and their biological roles in cancer
Several studies in the last few years have confirmed and extended the concept that both IR isoforms are broadly overexpressed in human tumors and that the IR-A/IR-B ratio is generally in favor of the IR-A isoform. Studies have also confirmed that IR isoforms act in strict connection with the IGF-1R. Moreover, in certain cancer histotypes, IR isoforms may play a more important role than the IGF-1R itself. In terms of potential target therapies, these findings have confirmed early evidence that IR isoforms should be taken into account when considering anti-IGF therapies (32, 284).
Mechanisms of increased IR-A expression in cancer
Increased IR-A expression in cancer is modulated at multiple levels of the complex regulatory network involved in IR expression and the generation of IR isoforms (see the paragraph titled “differential IR isoform regulation at the protein level”).
Alteration of transcription factors and/or miRNAs targeting the IR.
Upregulation of the two main positive regulators of IR transcription, specificity protein 1 (285, 286) and HMGA1 (287), as well as functional inactivation of its negative regulator, p53 (288–290), are very common alterations in cancer and are likely to affect IR overexpression [see also Belfiore et al. (1)].
As mentioned previously (see the paragraph titled “IR isoform regulation”), several specific miRNAs, including miR-15b, miR-195, miR-497, miR-103/107, let-7, and miR-424, have been reported to regulate IR expression in pathological conditions such as obesity and insulin resistance. These miRNAs can be dysregulated in cancer and, in principle, could contribute to both IR upregulation and an increased IR-A/IR-B ratio. These miRNAs are generally regarded as tumor suppressors. For instance, miR-15b/16-2 KO mice developed B-cell malignancy with characteristics of human chronic lymphocytic leukemia. Notably, miR-15b/16-2 modulates not only the IR but also the IGF-1R and cyclin D genes (291). The high frequency of the loss of heterozygosity at chromosomal region 17p13.1 (the genomic locus for the miRNA cluster miR-195 and miR-497) may be responsible for the deficient expression of these two miRNAs in cancer (292). Other mechanisms accounting for low miR-497 expression in various tumors (293, 294) include DNA copy number reduction (295) and hypermethylation of CpG islands upstream of the miR-497 locus (296). MiR-195 was identified as a tumor suppressor in non–small cell lung cancer (NSCLC) cells. Indeed, the miR-195 expression level was dramatically decreased in both NSCLC tissues and cell lines, and forced expression of miR-195 suppressed NSCLC cell proliferation and a metastatic phenotype. The IGF-1R was identified as a direct target of miR-195 in NSCLC cells (297). Let-7 miRNA family members act as tumor suppressors, reducing oncogene translation, and cell cycle regulators (63, 64, 298, 299) and are downregulated by the RNA-binding protein Lin28, which is overexpressed in several cancer histotypes and is associated with cancer progression, epithelial–mesenchymal transition (EMT), and cancer stem cells (300). MiR-103/107 is downregulated in gastric cancer cells and modulates multidrug resistance in human gastric carcinoma by downregulating cav-1 (301). MiR-424, also involved in IR regulation, has antiproliferative and prodifferentiation effects (69) and is viewed as a tumor suppressor in various cancers (302, 303).
Finally, dysregulation of miRNAs and splicing factors may be interconnected. For instance, changes in the expression profile of host genes and intronic miRNAs recently observed in cancer cell lines (304) might be caused by aberrant splicing, which is often associated with malignant transformation (305). Additionally, hnRNP proteins are regulated by Myc (306). Conversely, miRNAs may also affect splicing factors. For instance, miRNA-1 inhibits SRSF9 (SRp30c) in bladder cancer (307).
Taken together, these studies strongly suggest that the downregulation of several miRNAs may be involved in determining high IR levels and the increased IR-A/IR-B ratio observed in cancer. However, the evidence is indirect, and more specific studies are required.
Dysregulation of IR degradation.
Dysregulation of IR degradation could, in principle, contribute to IR upregulation in cancer. In light of the effects of the E3 ubiquitin ligase MARCH1 (70) in determining IR plasma membrane pools (see the paragraph titled “Differential IR isoform regulation at the protein level”), it is possible that in cancer cells, the presence of an activated IR-A/IGF-2 loop reduces FOXO1 transcriptional activity and MARCH1 levels, thus increasing membrane IR expression levels and the potentiation of IR-mediated mitogenic effects. Importantly, insulin and IGF-2 considerably differ in their ability to regulate IR-A stability, as prolonged IGF-2 stimulation has a significantly reduced capacity to induce IR-A and IRS-1 degradation compared with insulin (120, 122), thereby evoking sustained and robust mitogenic stimuli.
Mechanisms of the increased IR-A/IR-B ratio in cancer
Alternative splicing is a fundamental mechanism that plays an important role in regulating gene expression in development and the cell response to external and internal stimuli. Notably, alterations in splicing programs are crucially linked to cancer progression. It has been recently demonstrated using next-generation sequencing technologies that various malignancies may develop somatic mutations in several components of the spliceosome complex (308). Although these mutations were originally discovered in hematological malignancies and myelodysplastic syndromes, they were later confirmed in solid tumors. For instance, the gene encoding for the SF3B1 splicing factor is frequently mutated in breast (309) and pancreatic cancer, whereas the splicing factor U2AF1 is altered in lung cancer (310). No study has so far attempted to correlate mutations in splicing factors to changes in the relative abundance of IR isoforms, and no specific mutations in splicing factors correlating with an increased IR-A/IR-B ratio have been identified.
However, in the last few years, our knowledge of the mechanisms associated with increased IR-A expression in cancer has significantly improved. In this context, it was reported that in a panel of 85 human hepatocellular carcinoma (HCC) specimens, the IR-A/IR-B ratio was generally higher than in the adjacent nontumor liver tissue. In these cells, autocrine/paracrine activation of the EGFR and the downstream ERK cascade was associated with upregulation of the expression of the splicing factors CUGBP1, hnRNPH, hnRNPA1, hnRNPA2B1, and SF2/ASF. In contrast, the splicing factor SRp20/SRSF3 was undetectable. These changes in splicing factor expression were strongly associated with a high IR-A/IR-B ratio (311). Whereas CUGBP1, hnRNPH, and hnRNPA1 favored IR exon 11 skipping and IR-A formation in studies on myotonic dystrophy type 1 (DM1; see the paragraph titled “Myotonic dystrophy”), a novel factor, hnRNPA2B1, has been identified that might also cooperate in regulating IR exon 11 skipping. Interestingly, SRp20/SRSF3, which antagonizes CUGBP1-mediated repression of exon 11 inclusion, was undetectable in HCCs. The relevance of the increased level of SF2/ASF remains unclear, although it was demonstrated that this factor might antagonize exon 11 exclusion by CUGBP1 or hnRNPA1 (77, 312), whereas there is also evidence that it antagonizes the function of SRp20/SRSF3 (313). In accordance with these studies, Sen et al. (314) demonstrated that SRp20/SRSF3 expression is either decreased or the protein is mislocalized in human HCCs. Moreover, hepatocyte-specific deletion of SRp20/SRSF3 was associated with impaired hepatocyte maturation and metabolism in early adults and the development of progressive liver steatosis and fibrosis with aging. Loss of SRp20/SRSF3 was associated with increased IGF-2 and IR-A, which promoted proliferation, Wnt/β-catenin signaling, and induction of c-Myc along with aberrant splicing and induction of EMT genes. An analysis of public domain databases indicated that SRp20/SRSF3 might be lost or mutated in a subset of cancers with various histotypes, suggesting that this mechanism may generally contribute to the high IR-A/IR-B ratio in cancer (314). Moreover, hnRNPA2/B1 is overexpressed in human HCC tissues but not in normal liver tissues. Nuclear hnRNPA2/B1 tends to acquire a predominantly cytoplasmic localization with cancer dedifferentiation. However, the relevance of these findings in relation to IR gene splicing was not investigated (315).
“The level of IR-A activation in cancer cells is associated with active downstream signaling pathways and poor patient prognosis.”
Interestingly, insulin itself may induce proteasome-dependent degradation of SRp20/SRSF3, which in turn may lead to increased IGF-2 and IR-A levels (316). The results are also in line with the findings that an increased IR-A/IR-B ratio positively correlates with hyperinsulinemia and hnRNPA1, whereas weight loss and low insulin levels during fasting or after bariatric surgery are associated with a reduced IR-A/IR-B ratio (86, 87).
IR isoform expression in various cancer histotypes
As exemplified by the analysis of IR isoforms and of IGF-1R expression using RNA sequencing data from The Cancer Genome Atlas including 6943 samples representative of 21 tumor types, IR-A expression was found in virtually all tumor types and was particularly high in clear cell renal cell carcinoma. IR-B was also expressed in many tumor samples, with the highest levels observed in clear cell renal cell carcinoma and HCC. High levels of IGF-1R expression were observed in breast, ovarian, prostate, head and neck, and squamous lung cancer and melanoma (317). Herein, we briefly review the most recent studies dealing with the quantitative analysis of IR isoforms and their relevance in specific malignancies (Table 5).
Table 5.
Alterations of IR Isoforms, IGF-IR, and Downstream Pathways in Various Cancer Histotypes
| Histotype | Model | Alterations | References | |||
|---|---|---|---|---|---|---|
| Breast cancer | h-BC specimens | ⇧ IR-A | ⇧ IR-A/IR-B | ⇩ IR-B | Aljada et al. (318); Harrington et al. (319); Law et al. (321) | |
| h-BC specimens [HR-ER+] | ⇩ IGF-IR | ⇧ pIR, pS6K | Harrington et al. (319) | |||
| Prostate cancer | h-PC specimens | ⇧ IR-A | ⇧ IR-A/IR-B | Cox et al. (323); Perks et al. (327); Heni et al. (324) | ||
| h-PC specimens from T2DM patients | ⇩ IGF-IR | ⇔ IR | ⇔ IRS-1/IRS-2 | Heni et al. (324) | ||
| Endometrial cancer | m-EAC | ⇧ IR-A | ⇧ IR-A/IR-B | ⇧ IR-B | ⇧ IGF-IR | Flannery et al. (220) |
| h-EC specimens, xenografts, | ⇧ IR-A/IR-B | ⇧ PI3K | ⇩ MAPK | Wang et al. (328) | ||
| h-EC cell lines | ||||||
| Lung cancer | h-NSCLC specimens | ⇧ IR-A/IR-B | ⇩ IR-B | ⇔⇧ IGF-IR | Jiang et al. (329) | |
| h-NSCLC specimens | ⇧ IR-A/IR-B | ⇔⇧ IGF-IR/IR | Forest et al. (317) | |||
| Colon cancer | h-RA specimens | ⇔⇧ IR-A | ⇩ IR-B | ⇩ IGF-IR | Santoro et al. (330) | |
| h-CRC specimens | ⇧ IR/IGF-IR | ⇧ IR-A/IR-B | Forest et al. (317) | |||
| Stem/progenitor intestinal cells | ⇧ IR-A/IR-B | ⇩ IR-B | Andres et al. (219) | |||
| m-Precancerous CA, | ⇩ IR-B | Andres et al. (219) | ||||
| h-PDCC cell lines | ||||||
| Liver cancer | h-HCC and r-HCC specimens | ⇧ IR | ⇧ IR-A | ⇧ IR-A/ IR-B | ⇩ IR-B | Chettouh et al. (311); Spector et al. (331) |
| h-HCC cell lines | ⇧ IR-A/IR-B | ⇧ ERK | ⇧ CUGBP1; | ⇩ Srp20/SRSF3 | Chettouh et al. (311) | |
| hnRNPH; hnRNPA1; | ||||||
| hnRNPA2B1; | ||||||
| SF2/ASF | ||||||
| m-HCC specimens | ⇩ Srp20/SRSF3 | ⇧ IR-A | ⇧ IGF-2 | ⇧ Wnt/β-catenin; | Sen et al. (314) | |
| c-myc; | ||||||
| EMTgenes; | ||||||
| h-HCC specimens | ⇩ Srp20/SRSF3 | Sen et al. (314) | ||||
| r-HCC specimens | ⇧ IR | ⇧ IGF-IR | ⇧ IRS-1;IRS-2 | ⇧ ERK | Aleem et al. (332) | |
| Viral-related h-HCC specimens and serum | ⇧ IGF-IR | ⇧ IGF-1 | ⇩ IGF-2 | ⇧ ERK | Kasprzak et al. (333) | |
| ⇩ IGFBP-3 | ⇩ IGF-2R/M6PR | |||||
| Multiple myeloma | h-MM cell lines | ⇧ IR-A | Shushanov et al. (334) |
Abbreviations: BC, breast cancer; BC [HR-ER+], hormone refractory estrogen receptor–positive breast cancer; CA, colon adenoma; EAC, endometrial adenocarcinoma; EC, endometrial carcinoma; h, human; m, murine; MM, multiple myeloma; PC, prostate cancer; PDCC, poor differentiated colorectal cancer; r, rat; RA, rectal adenoma.
Breast cancer.
A number of studies using either quantitative reverse transcription polymerase chain reaction or immunostaining have measured IR-A and IR-B isoforms in commercially available breast cancer disease complementary DNA (cDNA) and cancer survey cDNA arrays as well as in human breast cancer specimens. These studies have confirmed that the IR-A isoform is generally present at high levels, whereas the IR-B isoform is expressed at lower levels or downregulated (88, 318, 319). Indeed, the IR-A/IR-B ratio appears to be significantly increased in all breast cancer stages, and the most elevated levels of IR-A were observed in clinical stage IV (64). The highest IR-A levels were detectable in ER+ tumors compared with low IR-A levels in ER− breast tumors, whereas IR-B expression was similar in ER+ and ER− tumors. Interestingly, hormone-resistant ER+ tumors also showed elevated IR expression and a high IR-A/IR-B ratio, whereas IGF-1R expression was significantly lower (319). Moreover, a high IR-A/IR-B ratio was particularly associated with the luminal B subtype of ER+/progesterone receptor+/HER2− breast cancers compared with the luminal A subtype. Notably, the luminal B subtype is associated with a cell proliferation signature and markers of tamoxifen resistance and is clinically characterized by a higher grade, larger tumor size, positive lymph node involvement, increased lymphovascular invasion, and poorer relapse-free survival (320). In accordance with these studies, phosphorylated IR in breast cancer cells is associated with poor patient survival. Indeed, in a large cohort of patients with invasive breast cancer, positive immunostaining of phosphorylated IR/IGF-1R was significantly associated with poor survival. Although the phosphorylation-specific antibody used cannot discriminate between activated IR and IGF-1R, phosphorylated IR/IGF-1R and its downstream signaling partner phospho-S6 correlated with IR levels but not with IGF-1R levels, indicating that IR phosphorylation might play a more important role than IGF-1R phosphorylation in activating downstream signaling and affecting patient prognosis (321). Finally, in a study involving 760 patients with breast cancer, insulin resistance was significantly correlated with obesity, postmenopausal status, and the highly proliferative luminal B/HER2–negative subtype in patients with breast cancer (322).
Taken together, these findings support the concept that elevated levels of IR-A and a high IR-A/IR-B ratio as well as hyperinsulinemia correlate with more aggressive and hormone-resistant breast cancers. Additionally, the level of IR-A activation in cancer cells is associated with active downstream signaling pathways and poor patient prognosis (Table 5).
Prostate cancer.
In a study that evaluated both IR and IGF-1R expression levels by immunostaining in 132 primary prostate cancer samples, both receptors were detected, but the IR was significantly more expressed in cancer than in benign prostate tissue (323). Indeed, a subsequent study found that the IR-A/IR-B ratio, as assessed by quantitative reverse transcription polymerase chain reaction, was significantly higher in prostate cancer tissues and in tumors adjacent to benign prostate tissues compared with benign prostate tissue. In the small subgroup of patients with T2DM, the IGF-1R levels were significantly lower whereas the IR and IRS levels did not change (324).
In prostate cancer cell lines, both insulin and IGF-1 increased cell proliferation and glucose consumption, whereas the same growth factors induced differentiation in noncancerous prostate cells. Similarly, overexpression of the IR-A isoform and IGF-1R increased cancer cell proliferation while inducing differentiation of nontransformed prostate cells. Notably, IR-B expression did not affect cell proliferation, although it favored differentiation in noncancerous prostate cells (325). In vivo studies from the same group indicated that in addition to promoting tumor growth, IGF-1R and IR-A overexpression also enhanced angiogenesis, as suggested by the higher vessel density in a chicken allantoic membrane assay, and induced resistance to chemotherapeutic agents (326). However, in this model, IR-B overexpression stimulated blood vessel formation as well, although to a lesser extent than the IGF-1R and IR-A. A recent study confirmed that IR-A was the predominant isoform in both prostate cancer specimens and cell lines. The relative IR-A abundance was increased by exposure to either insulin or IGF-2 (327) (Table 5).
Endometrial cancer.
Two recent studies evaluated IR isoform expression in endometrial cancer, and both found that IR-A is overexpressed in endometrial cancer and predominantly elicits mitogenic effects (220, 328). One study demonstrated that IR-A was expressed in 78 of 103 (75.7%) endometrial cancer specimens but only in 21 of 60 normal endometrial tissue control specimens (35%). In cancer specimens, the IR-A/total IR ratio was significantly higher than in normal specimens, especially in T2DM patients (328). An endometrial cancer cell line stably transfected with IR-A grew faster than the parental cells both in vitro and in tumor xenografts in immunocompromised mice. In these cells, the PI3K/Akt signaling pathway was preferentially activated, whereas the MAPK pathway was inhibited. This study did not specifically address the role of IR-B.
The second study, carried out in mice, examined the role of both IR isoforms both in endometrial cancer and during the normal endometrial cycle (220). In mouse adenocarcinoma, the expression of IR-A, IR-B, and the IGF-1R was fivefold to sixfold higher than in the normal endometrium. However, IR-A levels increased dramatically during the early proliferative phase, reaching a level ∼20-fold higher than IR-B, indicating that IR-A is the predominant isoform responsible for the initial estrogen-independent endometrial proliferation as well as subsequent cancer. In the early secretory phase, IR-A levels decreased, whereas IR-B and IGF-1R expression increased, possibly indicating that the IR-B isoform might be implicated in the elevated glucose uptake and glycogen synthesis of the normal secretory phase, which supports embryo development. These data suggest, therefore, that the IR-A and IR-B isoforms may have distinct roles in endometrial physiology and cancer (Table 5).
Lung cancer.
The expression of IR-A and IR-B transcripts was analyzed in a large series of 614 NSCLC (355 lung adenocarcinomas and 259 squamous cell carcinomas) and 92 control normal lung specimens included in The Cancer Genome Atlas. In both NSCLC histotypes, the IR-A/IR-B ratio was significantly increased compared with normal lung specimens, and the IR-B levels were decreased on average. Moreover, 11% of the NSCLC specimens expressed only the IR-A isoform (329). The authors also examined two independent panels of NSCLC samples that confirmed the elevated IR-A/IR-B ratio observed in the The Cancer Genome Atlas data. The IGF-1R seemed to have a minor function in NSCLC, as it was overexpressed (more than twofold relative to normal samples) in only 12 of 144 (8%) NSCLC samples.
When evaluating the transcriptional expression of IR isoforms and the IGF-1R in a series of clinical lung cancer samples, it was found that in lung adenocarcinoma, the median IR levels were similar to those of the IGF-1R (350 vs 392 copies/ng of cDNA), whereas in squamous cell carcinoma, the IR levels were lower than those of IGF-1R (362 vs 680 copies/ng of cDNA). The median IR-A/IR-B ratio was 1.9 in lung adenocarcinoma and 2.4 in squamous cell carcinoma (317). When the effects of IR KD in NSCLC cell lines were studied in three different cell lines, all variably expressing IR isoforms, IR KD induced apoptosis and increased the expression of proapoptotic cytokines such as IL-20 and TNF-α, indicating a novel role for the IR in regulating NSCLC cell survival (335) (Table 5).
Colon cancer.
Epidemiologic evidence has linked elevated plasma insulin levels with reduced apoptosis in normal rectal mucosa and an increased adenoma risk (336, 337). In a study carried out in 100 patients with rectal adenoma and 98 normal controls, among patients with high plasma insulin, those with adenomas had a higher mean IR-A/IR-B ratio in their normal rectal mucosa compared with controls, which seemed to result from decreased IR-B and sustained IR-A levels. In contrast, IGF-1R expression was lower in adenomas than in controls (330). In colorectal carcinoma (CRC) samples, the IR was expressed at higher levels than the IGF-1R (413 vs 328 copies/ng of cDNA), and the median IR-A/IR-B ratio was 3.8. In CRC samples, the expression of both IR isoforms was clearly higher than that of the EGFR, a clinically validated target (317). In close agreement with previous findings obtained in the thyroid, stem/progenitor intestinal cells showed a predominance of IR-A expression that switched to IR-B in differentiated enterocytes. Furthermore, IR-B expression was reduced in mouse precancerous adenomas vs normal colon tissue and was dramatically reduced in aggressive, poorly differentiated human colorectal cancer cell lines compared with differentiated colorectal cancer cells, confirming that IR-B may limit proliferation and is associated with differentiation (219) (Table 5).
Liver cancer.
HCC is the most common primary malignancy of the liver, and it is strongly associated with insulin resistance and hyperinsulinemia (332). Several years ago, it was reported that IR expression was significantly higher in human HCC specimens than in normal liver specimens (331). The relevance of the IR in human HCC has been recently confirmed by a study reporting that the IR was significantly overexpressed in 40% of 85 HCC tumors compared with the adjacent nontumor tissue. This IR overexpression was essentially due to increased IR-A levels, whereas IR-B expression was decreased in these tumors compared with control tissues (311). Similar findings were observed in chemically induced rat HCC, but not in regenerating livers after partial hepatectomy. To clarify the IR isoform shift, the authors demonstrated that in human HCC cell lines, but not in normal hepatocytes, activation of the ERK pathway by autocrine or paracrine EGF increased the IR-A/IR-B ratio, with a concomitant deregulation of splicing factors such as CUGBP1, hnRNPH, hnRNPA1, hnRNPA2B1, and SF2/ASF, which are thought to regulate IR exon 11 skipping (311).
In a rat model of HCC induced by N-nitrosomorpholine, IR, IGF-1R, IRS-1, IRS-2, and ERK levels increased in the initial phases of hepatocarcinogenesis, resulting in biochemical changes such as glycogen accumulation and increased proliferation. However, IR and IGF-1R expression decreased in later stages of tumor progression (332). Although the IGF axis is undoubtedly involved in hepatitis C virus–associated carcinogenesis, as indicated by the deregulation of many its components, including the IGF-1R, IGF-1, and IGF-2, the role of IR isoforms has not been clearly defined in this model (333) (Table 5).
Multiple myeloma.
Insulin has been reported as a potent growth and survival factor for primary multiple myeloma cells and human multiple myeloma cell lines. Interestingly, insulin induced cell proliferation starting at a concentration as low as 0.1 ng/mL, 30-fold below physiological concentrations, and required both the IR and the IGF-1R, indicating that its actions occur through IR/IGF-1R hybrids (151). These hybrids likely contain only or predominantly the IR-A isoform, as this is the only isoform expressed by a panel of multiple myeloma cell lines (334) (see also the paragraph titled “Ligand binding to IR/IGF-1R hybrids”). These data might support the view that circulating insulin may elicit unwanted biological actions in malignant cells that overexpress IR-A (Table 5).
The role of IR isoforms and their ligands in carcinogenesis and cancer promotion
A wealth of epidemiological evidence has now established that obesity, metabolic syndrome, and T2DM, all conditions characterized by insulin resistance, are associated with an increased cancer risk and cancer-specific mortality (338, 339). As chronic hyperinsulinemia is a hallmark of these pathological conditions, hyperinsulinemia itself is regarded as playing a crucial role in this context (340). However, epidemiological studies have not precisely established the relative contribution of hyperinsulinemia in cancer patients with concomitant metabolic disorders, as these disorders are associated with alterations of several biological parameters that might also play a role in favoring cancer. Many excellent reviews have addressed specific mechanisms with a possible role in carcinogenesis and cancer progression in patients with obesity, T2DM, and insulin resistance (278, 281, 282). Indeed, in diabetic patients, hyperglycemia is associated with glucose toxicity and AGE product formation and could also impact nutrient availability. Adiposity, especially central adiposity, is associated with an unbalanced adipokine profile, increased secretion of inflammatory cytokines, immunological alterations, and increased androgen to estrogen conversion. Insulin resistance may also cause increased IGF and estrogen bioavailability and alterations in fatty acid metabolism (278, 281, 282).
Herein, we review experimental data concerning the specific roles of insulin and IGF-2 in carcinogenesis and cancer promotion via the IR.
The role of insulin
Insulin is not usually regarded as a carcinogenetic agent. However, some experimental evidence suggests that hyperinsulinemia may also play a role in carcinogenesis through the induction of excess ROS and consequent genetic damage. Low-level bursts of ROS production are part of the physiological response to insulin and other growth factors. However, ROS may also cause genetic damage, including impaired base pairing, base loss, or DNA single-strand breaks or possibly double-strand breaks, which are difficult to repair and very toxic.
Insulin at a concentration of 5 nM caused a significant increase in DNA damage in cultured pig and rat kidney cells (342) and in human colon adenocarcinoma cells and human peripheral lymphocytes (343). This genomic damage was related to Akt activation and was not affected by the glucose concentration in the medium. For human colon adenocarcinoma cells, the lowest toxic insulin concentrations ranged from 0.5 to 1 nM after chronic exposure (343). Notably, in humans, physiological circulating insulin concentrations range from 0.04 nM after overnight fasting to ∼0.2 nM after a meal, but in insulin-resistant patients, insulin levels often increase by fivefold or more. To rule out a concomitant effect of glucose, lean ZDF rats were subjected to the hyperinsulinemic-euglycemic clamp technique, which caused chronic hyperinsulinemia (an average insulin concentration of 1.67 nM) that was also associated with high ROS production and genomic stress and p53 accumulation in the kidneys (342). The sources of ROS were identified as the mitochondria and the reduced NAD phosphate isoforms Nox1 and Nox4 in the colon and kidney, respectively (344).
Conversely, the potential tumor-promoting effect of insulin has long been recognized, especially when acting through IR-A. A number of recent studies have identified some of the molecular mechanisms of insulin action. In C. elegans and in human breast cancer cells, exposure to insulin (100 nM and 2 mM) decreased PTEN protein levels, a result recapitulated by transfection of a constitutively active myristoylated IR β-subunit. As PTEN is an important tumor suppressor, these data identify a possible tumor-promoting effect of hyperinsulinemia. In turn, wild-type PTEN could dephosphorylate the IR, indicating a mutual inhibitory circuitry (345).
Insulin has also been implicated in the stimulation of the Hippo signaling pathway, which regulates cell proliferation via the transcriptional coactivator Yorkie/YAP that binds transcription factors regulating the cell cycle and regulators of apoptotic gene expression. In both Drosophila and HCC cells, insulin stimulation was positively correlated with the Yorkie/YAP pathway, which in turn led to the activation of the insulin/TOR pathway (346).
Activation of IGFs is known to stimulate EMT and cell migration through multiple mechanisms (211). Moreover, in MEFs and mammary epithelial cells, insulin may contribute to EMT by promoting the translocation of TGF-β receptors from intracellular compartments to the plasma membrane through Akt-mediated phosphorylation of AS160, an RabGAP. Cells exposed to insulin might, therefore, show increased sensitivity to autocrine TGF-β, which is a key regulator of EMT (347). Interestingly, insulin stimulates human microvascular endothelial cell migration and tube formation independently of VEGF/VEGFR signaling through the IR/PI3K/Akt/sterol regulatory element–binding protein 1 pathway, leading to the activation of Rac1 (348). These findings are highly consistent with data indicating that overexpression of both IR-A and IR-B is angiogenic in prostate cancer xenografts (326).
In vivo studies.
Several studies have reported that a hyperinsulinemic tumor microenvironment may favor cancer growth through IR activation in cancer cells. Early studies found that benign and malignant hepatocellular neoplasms can be induced by local insulin production caused by long-term (15 to 22 months) low-number intraportal pancreatic islet transplants in autoimmune diabetic rats. Preneoplastic foci developed in the liver acini located downstream of the transplanted islets in rats that were also mildly hyperglycemic, indicating that hyperglycemia could also contribute. However, the preneoplastic foci did not regress in animals that became normoglycemic or hypoglycemic due to excessive insulin production by hyperplastic transplants or in animals with late rejection of islet grafts (349). More direct evidence was provided by experiments with mouse xenografts of MCF-7 human breast cancer cells. When the animals were injected subcutaneously with insulin around the tumor site, the malignant cells were stimulated to grow and migrate faster, effects mainly associated with insulin-dependent activation of the ERK pathway (350). Using the MKR transgenic mouse model, LeRoith’s group has made significant contributions in establishing the role of the IR and its ligands in breast cancer initiation and progression. MKR mice harbor a dominant-negative, kinase-deficient human IGF-1R, which is expressed exclusively in skeletal muscle and inactivates endogenous IR and IGF-1R. Lean female MKR mice are insulin resistant and hyperinsulinemic, but not overtly diabetic, making them a good model to dissociate hyperinsulinemia from cytokine production and metabolic derangements associated with obesity and overt diabetes. Notably, female MKR mice exhibited accelerated mammary gland development and the occurrence of hyperplastic precancerous lesions characterized by increased phosphorylation of the IR, the IGF-1R, and Akt. These tumor-promoting effects could be reversed by pharmacological blockade of IR/IGF-1R signaling by the tyrosine kinase inhibitor BMS-536924 (351).
To better establish whether hyperinsulinemia increased tumor growth specifically through the IR and not through the IGF-1R or HRs, orthotopic mammary tumors were induced in control and hyperinsulinemic MKR mice, which were then treated with the insulin analog AspB10, recombinant human IGF-1, or vehicle. Tumors from mice with endogenous hyperinsulinemia grew faster and showed high IR phosphorylation but not IGF-1R phosphorylation compared with control mice. Chronic AspB10 administration also increased tumor growth and IR (but not IGF-1R) phosphorylation in tumors. IGF-1 led to activation of both the IGF-1R and IR and of HRs (352).
To explore the therapeutic potential of IR blockade, MKR female mice were inoculated with mammary carcinoma Mvt-1 cells, and mice bearing tumors were treated with either the selective IR antagonist S961 or with the dual IR/IGF-1R inhibitor picropodophyllin. Inhibition of the IR alone resulted in severe insulin resistance and enhanced tumor growth secondary to IGF-1R stimulation by high insulin levels. In contrast, dual inhibition of the IR and IGF-1R with picropodophyllin reduced the tumor growth rate with only mild metabolic consequences (353). In further studies, female MKR or control (wild-type) mice were inoculated with mammary carcinoma Mvt-1 cells with or without IR or IGF-1R KD. IR KD cells, but not IGF-1R KD cells, generated significantly smaller tumors in both wild-type and MKR mice compared with control cells. CD24 expression was reduced in the IR KD cells when compared with control cells, and CD24 re-expression could partially restore the tumorigenic capacity of IR KD cells (354). These studies, however, have not specifically addressed the relevance of IR isoforms in insulin-dependent cancer progression.
MKR mice were crossed to immunodeficient Rag1−/− mice to generate Rag/MKR mice, which retained insulin resistance and high circulating insulin levels. When injected with human metastatic MDA435/LCC6 breast cancer cells that express 80% of total IR as IR-A (355), female Rag/MKR mice developed xenograft tumors exhibiting EMT changes, growth, and metastatic spread. These features were reduced by IR silencing, further highlighting the role of the IR in cancer progression and metastasis (356).
The role of IGF-2
Dysregulation of IGF-2 expression and/or its bioavailability is a common event in human cancer (357–359). Although loss of imprinting (LOI) has been extensively studied as the driving mechanism of IGF-2–dependent tumorigenesis, additional mechanisms have recently been described. One of these mechanisms relies on the overexpression of intronic miR-483-5p, an miRNA embedded within the IGF-2 gene itself. In fact, miR-483-5p likely enhances IGF-2 transcription at the fetal promoter through a mechanism involving the RNA helicase DHX9, thereby sustaining a positive feedback loop of IGF-2 expression (360). Moreover, several recent studies have emphasized the role of IR-A as an essential IGF-2 receptor in tumor initiation and progression. Using a transgenic mouse model of pancreatic neuroendocrine tumorigenesis, it has been shown that IGF-2 expression is focally activated concomitantly with the initiation of β-cell hyperproliferation, representing a key survival factor for malignant development. The IR protein was also markedly increased during this multistep tumor progression and was functionally involved in this process, as KD of the IR gene impaired tumor progression and induced apoptosis. Notably, IR gene recombination was efficient in normal or hyperplastic tissues but inefficient in advanced tumors, suggesting its relevance to tumor progression (361).
In a model of lethal prostate cancer, IGF-2 upregulation induced by GATA2 was a key mediator of cancer development and progression by stimulating both the IR and IGF-1R. In fact, the combined inhibition of both receptors restored the chemotherapy response and improved survival in preclinical models (362).
In addition to mature IGF-2, several tumors also express high levels of O-glycosylated, longer isoforms of IGF-2, termed “pro” and “big” IGF-2 (363). These isoforms may bind and activate IR-A and the IGF-1R and perhaps also the IR-B isoform. Notably, these IGF-2 isoforms partially escape mechanisms limiting IGF-2 biological activity, as they form binary complexes with several IGFBPs but have a reduced ability to form more stable ternary complexes with IGFBP-3 and the acid-labile subunit. Moreover, big–IGF-2 isoforms have a lower affinity for M6P/IGF-2R, which regulates IGF-2 degradation. Therefore, the glycosylated, longer forms of IGF-2 exhibit increased bioavailability and may chronically activate the IR with tumorigenic effects (364).
Myotubularin-related protein 7 (MTMR7), a member of the myotubularin lipid phosphatase family, may provide an additional link between insulin, IGF-2, and cancer. MTMR7 is present in the endosomal-lysosomal compartments as well as in soluble form and has been identified as a negative modulator of insulin signaling by inhibiting the production of the lipid second messenger PI3P Indeed, MTMR7 protein expression is downregulated in vitro in colon cancer cells and in tissues of colon cancer patients under conditions of active insulin/IGF-2 signaling (365).
IGF-2 production may be stimulated by antitumor treatments, such as the anti–IGF-1R monoclonal antibody cixutumumab and histone deacetylase inhibitors, and may enhance drug resistance through IR-A binding. In both cases, IGF-2 production is stimulated by STAT3 activation. Increased IGF-2 production may have antiapoptotic effects in cancer cells and stimulate angiogenesis through macrophage and fibroblast recruitment and consequent CXCL8 production and vascular endothelial cell proliferation and migration (366, 367).
The IGF-2/IR-A loop and cell stemness in cancer.
Several lines of evidence have indicated a close relationship between insulin/IGF signaling and cell stemness (211). Indeed, IGFs are involved in the activation of key features of cell stemness, such as EMT, pluripotency, and self-renewal. First, it is now well established that IGF stimulation of immortalized and cancer cells upregulates several EMT-related transcriptional regulators. In particular, the MEK/ERK pathway is involved in the upregulation of Twist and ZEB1 (368). Moreover, GSK3β inhibition by insulin regulates the proteasomal degradation of Slug and Snail (369). EMT may in turn trigger autocrine IGF-1 production, which activates a positive feedback loop between IGF-1R activation and SLUG expression (370, 371). Insulin/IGF signaling also regulates several transcription factors involved in pluripotency, such as p53, Oct-4, and Nanog, and induces phosphorylation and inactivation of p53, which negatively regulates Oct-4 and Nanog (372). Moreover, insulin/IGF signaling, through the PI3K/GSK3β pathway, mediates the formation of a β-catenin/Oct-4/SOX2 complex, which activates the Nanog promoter (373). Additionally, insulin- and IGF-1–dependent activation of the PI3K/Akt/mTOR pathway regulates hypoxia factors 1 and 2, which upregulate Twist and Snail, as well as pluripotency factors, such as Oct-4, SOX2, and Nanog (374). Insulin/IGF signaling is also implicated in significant crosstalk with microenvironment signals of the stem cell niche, such as the Wnt/β-catenin, Notch, and Sonic hedgehog pathways, which play a key role in regulating stem cell self-renewal and differentiation into specialized lineages (211). In agreement with these findings, insulin signaling, via PI3K and FOXO, controls the competence of the Drosophila female germline stem cell niche to respond to Notch ligands and promotes niche stability (375).
“Contrary to the common belief, several lines of evidence now suggest that IGF-2 is the principal ligand of IR-A.”
Most of these studies have examined the IGF-1/IGF-1R axis and neglected the specific role of IR isoforms and IR/IGF-1R hybrids. However, several lines of evidence indicate that the IGF-2/IR-A axis might play a more prevalent role than the IGF-1/IGF-1R axis in some cellular contexts. For instance, progenitor/stem cells from human thyroid cancer specimens cultured as thyrospheres exhibited a higher IR/IGF-1R ratio compared with normal thyrospheres or differentiated thyrocytes (223). Moreover, cancer thyrospheres had a higher relative IR-A abundance (65% to 86%) compared with normal thyrospheres (50% to 65%), differentiated sphere–derived thyrocytes (45%), or normal thyroid primary cultures (40%). IGF-2 was also produced at high levels by both normal and cancer thyrospheres, whereas it was markedly decreased when normal spheres differentiated. In contrast, IGF-1R expression was similar in normal thyrospheres and thyroid primary cultures and was generally lower in cancer thyrospheres. Similarly, IGF-1 expression was much higher in normal than in malignant thyrospheres. Collectively, these data suggest that the IGF-2/IR-A loop might play a predominant role in thyroid cancer stem cells, whereas the IGF-1/IGF-1R loop is more important in normal thyroid cells (223). Interestingly, whereas IGF-1, IGF-2, and insulin significantly stimulated an increase in cancer thyrosphere volume, only IGF-2 affected cancer thyrosphere self-renewal, likely through activation of the IR-A isoform.
Notably, several recent studies have indicated a key role of IGF-2 in the biology of cancer stem cells. IGF-2 is paternally imprinted, and LOI may occur in a variety of human cancers (376). Interestingly, some studies indicate that IGF-2 LOI may occur in CSCs, even in CSCs derived from cells with normal IGF-2 imprinting. These CSCs may present aberrant IGF-2 expression, enhanced colony formation, and greater resistance to chemotherapy and radiotherapy in vitro (377). It is not clear how the normally suppressed maternal IGF-2 allele is reactivated in tumors with IGF-2 LOI. The polycomb protein SUZ12, involved in histone methylation, is critical for the maintenance of normal IGF-2 imprinting. SUZ12 is downregulated in IGF-2 LOI tumors. Loss of SUZ12 is associated with the dysregulation of IGF-2 imprinting in tumors, suggesting that this pathway may be dysfunctional in human tumors and perhaps in cancer stem cells, in which IGF-2 is biallelically expressed (378).
Notably, in invasive mucinous lung adenocarcinoma cells, in which the CD74–Neuregulin 1 fusion gene is a driver, Neuregulin 1 promotes stem-like properties by inducing autocrine IGF-2 expression through the PI3K/Akt/NF-κB signaling pathway (379).
Moreover, the embryonic stem–specific transcription factor ZFP57, which is involved in the anchorage-independent growth of human fibrosarcoma cells and is overexpressed in cancer specimens, acts by regulating the expression of IGF-2 (380). In turn, IGF-2, by phosphorylating and activating STAT3, may induce the pluripotency transcription factor NANOG, activating the transcription of Slug, EMT, and a self-renewal and metastatic phenotype (371). Similar data were confirmed in human hepatocarcinoma cells (373).
Most studies demonstrating a strict correlation between autocrine IGF-2 expression and the emergence/maintenance of stem-like cancer cell features have generally not determined whether the main receptor in delivering the effects of IGF-2 is IR-A or the IGF-1R. Therefore, more studies are needed to precisely define the roles of the IR isoforms in malignant stem cells of various origins. Importantly, however, note that although both the IGF-1R and IR-A have roughly the same affinity for IGF-2, the IGF-1R binds with a higher affinity to IGF-1, which is often abundant in the tumor microenvironment and may saturate IGF-1R binding sites. In contrast, IR-A has a low affinity for IGF-1 but a high affinity for IGF-2, which is very often secreted in an autocrine manner by stem-like cancer cells. Contrary to the common belief, several lines of evidence now suggest that IGF-2 is the principal ligand of IR-A, at least in several models of CSCs.
Insulin resistance, gestational diabetes, and diabetes
Insulin resistance, a condition in which higher than normal concentrations of insulin are required to maintain normoglycemia, is often associated with a number of diseases including obesity, gestational diabetes mellitus (GDM), and T2DM (1). Data on the roles of IR isoforms in insulin-resistant states, including T2DM, are scarce and sometimes contradictory. Because in humans, IR-B is the predominant isoform in the typical target tissues of insulin (i.e., liver, adipose tissue, and muscle), it has been hypothesized that insulin resistance in all or some insulin target tissues is associated with an increased IR-A/IR-B ratio. Early studies, however, did not find significant alterations of the IR-A/IR-B ratio in several forms of insulin resistance (381). However, this view has been challenged by more recent findings. Obesity is the most common condition associated with insulin resistance. It has been recently shown that the improvement of insulin resistance induced by weight loss obtained by a very low–calorie diet or bariatric surgery results in an increase in the IR-B isoform in adipose tissue (86). Changes in the IR-A/IR-B ratio in favor of the more metabolically active IR-B are often related to the reduction of circulating insulin levels and should contribute to improved insulin sensitivity (Table 6).
Table 6.
IR Isoform Relative Abundance in Various Conditions With Insulin Resistance and Hyperinsulinemia
| Disorder | Model | IR-A/IR-B Ratio | References |
|---|---|---|---|
| Obesity | Adipocytes | ⇧ | |
| Kaminska et al. (86) | |||
| Adipocytes after weight loss | ⇩ | ||
| T2DM | Liver | ⇧ | |
| Besic et al. (87) | |||
| Liver after weight loss only when diabetes remission is present | ⇩ | ||
| Gestational diabetes | HUVECs | ⇧ | Westermeier et al. (222) |
| Myotonic dystrophy | Skeletal muscle | ⇧ | Santoro et al. (382) |
Liver insulin resistance plays a central role in the pathogenesis of T2DM, because defective liver gluconeogenesis is the major contributor to the fasting hyperglycemia seen in T2DM. Several studies have reported that liver insulin resistance improves quickly after weight loss. Recently, the expression of IR isoforms has been evaluated in livers from obese patients who underwent bariatric surgery. The authors included individuals with normal glucose tolerance and T2DM patients. The mean IR-A/IR-B ratio in the T2DM group was significantly higher than in the normal glucose tolerance group (87). Approximately 1 year later, after significant weight loss, some individuals had a second liver biopsy, showing that the IR-A/IR-B ratio was reduced only in those that had diabetes in remission (though similar weight loss was observed in patients with persistent diabetes). The improved IR-A/IR-B ratio after T2DM remission was apparently due to decreased levels of IR-A, rather than increased levels of IR-B (87), and was correlated with the resolution of hyperinsulinemia. The possible correlation between the IR-A/IR-B ratio and liver fat content was not studied. These data fit well with those showing an increase of IR-B in the adipose tissue of obese patients after bariatric surgery (86) (Table 6).
Moreover, in immortalized pancreatic β-cells expressing the IR-A isoform, but not in cells expressing IR-B, glucose uptake was increased due to an association between GLUT-1 and GLUT-2 with IR-A (241). These findings are in line with the hypothesis that the IR-A/GLUT complex constitutes a sensor unit allosterically regulated by glucose (383). Glycosylation of this IR-A/GLUT complex may therefore have implications in the reduced insulin secretion and the insulin resistance of diabetes (383).
The IR-A/IR-B ratio has been studied in relationship to the placental vascular alterations observed in GDM. GDM is defined as glucose intolerance with onset during the second or third trimester of pregnancy. In Western countries, the prevalence of GDM has increased to up to >10% of pregnancies, in accordance with the increase in obesity. In most women with GDM, the pathogenesis involves pancreatic β-cell dysfunction in a background of chronic insulin resistance already apparent before pregnancy (384). If not adequately treated, GDM leads to adverse maternal and neonatal outcomes (385). Additionally, pregnant women with GDM have a higher risk of developing postpartum T2DM (386). GDM leads to placental vascular alterations in both the macrovascular and microvascular endothelium (387). Insulin and adenosine cause relaxation of umbilical vein rings, but this effect is reduced in GDM compared with normal pregnancies. In primary cultured HUVECs from normal subjects, both IR-A and IR-B are present, with an IR-A/IR-B ratio in favor of IR-A; this IR-A/IR-B ratio is increased in HUVECs from GDM patients (222) (Table 6). Moreover, HUVECs from GDM patients exhibit increased ERK phosphorylation (222), reduced adenosine transport with an increased extracellular concentration of adenosine, and a mitogenic-like phenotype (388). Insulin exposure corrects these abnormalities, reverting these cells into a metabolic-like phenotype (389).
Whether altered IR isoform expression has a pathophysiological role in human placental tissue in GDM pregnancies and how it affects normal endothelial function during pregnancy remains to be elucidated. It has been hypothesized that insulin resistance in GDM might be causally associated with increased IR-A expression, as previously demonstrated in other diseases [i.e., DM1 and myotonic dystrophy type 2 (DM2)].
Taken together, these data strongly suggest that the IR-A/IR-B ratio is altered in insulin-resistant conditions, including obesity, GDM, and T2DM, and that these alterations contribute to insulin resistance as well as to dysregulated insulin secretion by pancreatic β-cells.
Myotonic dystrophy
DM1 and DM2 are two of the most common muscular dystrophies in adulthood, characterized by myotonia, progressive myopathy, and multiorgan involvement. Patients with DM1 and DM2 present with severe insulin resistance, hyperinsulinemia, glucose intolerance, and a high risk of developing T2DM. DMs may have a complex and not fully understood pathogenesis, but a major mechanism involves marked expansion of an unstable CTG triplet repeat in the 3′-untranslated region of the dystrophia myotonica protein kinase gene. These transcribed CUG repeats sequester certain splicing factors such as MBNL1, which are required for the inclusion of IR exon 11 in the IR-B transcript (Table 6) as well as for the correct splicing of numerous genes (390). As a consequence, DM patients have an increased IR-A/IR-B ratio in skeletal muscles, which has been linked to insulin resistance (14). DMs therefore represent a useful model to better understand the mechanisms of IR isoform generation as well as a model for studying the consequences of predominant expression of IR-A and to correct the abnormal IR splicing. In young nondiabetic DM1 patients, insulin resistance may not be severe but is associated with elevated fasting plasma proinsulin concentrations and a high proinsulin/insulin ratio that is amplified in response to an oral glucose tolerance test (391). Interestingly, these patients showed impairment of the negative feedback of insulin on β-cell secretion. Whether this loss of β-cell sensitivity was due to the altered IR-A/IR-B ratio was not clarified (391).
One intriguing strategy to cure DM patients would be the attempt to restore MBNL1 activity with small molecules to inhibit MBNL1 sequestration. At least some of these molecules seem to have negligible toxicity in mammals (392). Recently, resveratrol, a polyphenolic flavonoid present in grape skins and seeds, red wine, blueberries, and peanuts, has been evaluated, and it enhanced IR exon 11 inclusion and increased IR-B mRNA expression in cultured fibroblasts from DM1 patients (19).
Neurodegeneration and Alzheimer’s disease
Alzheimer’s disease (AD) is the most common cause of dementia worldwide, and its pathogenesis is multifactorial. AD prevalence varies with age: it is ∼11% in people aged 65 or older, and ∼50% in people aged 85 or older. Insulin and IGF-1 play an important role in the CNS, regulating key processes such as energy homeostasis, neuronal survival, longevity, learning, and memory (see the paragraph titled “Brain activity”). Additionally, the IR and IGF-1R are selectively distributed in the brain, with a higher density in the olfactory bulb, hypothalamus, and in two of the main brain areas affected by AD pathology (i.e., the hippocampus and cerebral cortex). A state of insulin resistance has been reported in the brains of AD patients. Indeed, AD patients have reduced brain IR sensitivity and reduced levels of the IR and IGF-1R (20, 21). This peculiar characteristic of brain insulin resistance in AD has been termed “type 3 diabetes” to highlight this mechanism of neurodegeneration.
IR Activation and Inhibition in Therapy: New Advances and Perspectives
Advances have been made in the development of new insulin analogs or allosteric IR activators for the therapy of diabetes and/or neurodegeneration as well as new IR inhibitors for cancer therapy. Additionally, the importance of IR isoform-specific targeting is becoming more appreciated.
New IR ligands and modulators
Insulin binding to the activated IR has pleiotropic effects, triggering metabolic, mitogenic, and antiapoptotic responses (344). Natural or synthetic ligands can change the receptor conformation and activity by interacting with either the natural insulin binding site (orthosteric binding site) or with a site different from the canonical insulin binding site (allosteric binding site).
Insulin analogs
Insulin analogs are orthosteric variants of native insulin developed to efficiently mimic physiologic insulin secretion and achieve improved glycemic control (393, 394). However, due to the structural differences with native insulin, insulin analogs may interact with IR isoforms and the IGF-1R with different binding affinities and dissociation rates, which may affect the activation of downstream signaling cascades. Theoretically, they may elicit imbalanced mitogenic effects compared with native insulin (393, 395). Thus, this aspect has become a matter of concern, because insulin analogs are used in large diabetic populations. For this reason, all insulin analogs have been tested for their mitogenic potential risk in vitro and in vivo before being used in clinical settings.
According to their pharmacokinetics and to the general principle of protein folding and assembly, insulin analogs can be classified as short acting (AspB10, lispro, aspart, and glulisine) and long acting (glargine, detemir, and degludec). Short-acting analogs rapidly disassemble in the subcutaneous injection site, and this facilitates capillary absorption; conversely, long-acting insulin analogs adopt different strategies to provide a slow and continuous insulin delivery in the circulation. Next-generation insulin analogs have been designed to maintain effective glucose regulation but minimal mitogenic potency compared with native insulin. Recently, a novel long-acting insulin analog consisting of the insulin lispro linked to a polyethylene glycol moiety (LY2605541) as well as engineered glucose-responsive insulin analogs (called “smart insulins”) have been designed. Although many of these new formulations are still not yet fully characterized, they will likely enable patients to achieve better metabolic control associated with a safer profile in terms of mitogenic risk.
The first and most studied insulin analog was AspB10, which showed an increased affinity for both the IR and IGF-1R and enhanced cell proliferation and promoted mammary tumors in rats (396–398). Owing to its increased mitogenic action, AspB10 was discontinued in early clinical development. However, the pioneering studies on the molecular safety of this insulin have been of great importance to better understanding the mechanisms implicated in metabolic and mitogenic signaling for the next generation of insulin analogs. Over the years, several cell models have been developed to investigate the potential carcinogenic risk of insulin analogs in vitro. The available data are incomplete and sometimes contradictory, due to different cell types, experimental conditions, and insulin dosages used (399). A high binding affinity for the IGF-1R and prolonged occupancy time on the IR-A isoform have been proposed as possible mechanisms underlying the enhanced mitogenic potential of insulin analogs.
In regard to IR isoforms, it has been demonstrated that all three short-acting insulin analogs have binding affinity and dissociation rates for IR isoforms similar to those of native insulin (33, 400) and induce molecular and biological responses similar to those of human insulin. Although both short- and long-acting analogs phosphorylate IR isoforms at levels comparable to human insulin (33, 400), aspart and lispro but not glulisine induce a more rapid ERK activation through IR-A, whereas all three analogs stimulate a more prolonged Akt activation compared with insulin. Furthermore, these analogs exhibited a mitogenic potential similar to that of insulin, with the exception of glulisine, which was more potent than native insulin, likely due to stimulation of the IGF-1R.
In contrast, the long-acting analogs glargine and detemir behaved differently, as both of them had a reduced binding affinity for the IR-A isoform but a longer dissociation rate in comparison with native insulin (33, 400–402). Only detemir has a lower affinity for the IR-B isoform (33, 401, 403). To date, very few data are available on the insulin degludec. To the best of our knowledge, the relative affinity and dissociation rates of degludec for IR isoforms as well as for the IGF-1R and HRs have not been defined (404, 405). Owing to these molecular properties, long-acting analogs differ from short-acting analogs and native insulin in modulating the downstream biological responses of the receptors. In particular, both glargine and detemir may exert more potent mitogenic stimuli than native insulin by inducing a preferential activation of the ERK pathway and an increased ERK/Akt activation ratio in vitro (33, 401, 402, 406–408). The increased mitogenic effect of glargine can also be attributed to its higher affinity for the IGF-1R compared with insulin (401, 402). These data have been confirmed in MCF-7 cells overexpressing the IGF-1R (MCF-7/IGF-1R cells) (409), where glargine induced a stronger mitogenic response compared with insulin, whereas aspart and lispro had comparable mitogenic potencies similar to native insulin. Notably, glargine is rapidly converted into M1 and M2 metabolites after in vivo administration. Detemir, the M1/M2 glargine metabolites, and glulisine showed reduced mitogenic action compared with insulin (409, 410). Furthermore, glargine stimulated proliferation more potently in MCF-7/IGF-1R cells than in MCF-7 cells overexpressing the IR-A isoform (MCF-7/IR-A cells). These data suggest that at least in this cell model, glargine-induced mitogenic responses are preferentially elicited through the IGF-1R rather than the IR-A isoform (409, 410). Accordingly, insulin analogs might evoke stronger mitogenic responses than human insulin in cells characterized by a high IGF-1R/IR ratio (407, 411–413), although not in all studies (148, 402, 414). However, other studies have instead suggested that the proliferative effects of long-acting analogs may occur preferentially via IRs (especially IR-A), likely due to the decreased dissociation rate from the IR and consequent prolonged IR activation (33). Alternatively, the increased mitogenic properties of an insulin analog might reflect the binding preference for IR-A vs IR-B. For instance, Sciacca et al. (33) reported that detemir displayed a 13-fold higher affinity for IR-A than for IR-B. In support of this notion, human insulin exerts increased mitogenic responses by preferentially binding to the IR-A isoform, especially in cancer cells, which preferentially express this isoform (415). In contrast to these data, Hansen et al. (411) reported similar binding and activation of both IR isoforms for detemir, glargine, and AspB10 (411, 413).
Another possibility to consider is that analogs could mediate tumor growth through HRs, especially HR-A. However, Hansen et al. (411) did not find significant differences among analogs in their relative affinities for the two IR isoforms either in homodimers or as HRs, which showed similar phosphorylation or activation of downstream effectors. However, they demonstrated that the glargine binding affinity for both HR-A and HR-B was threefold higher than that of human insulin and similar to that of AspB10. In contrast, detemir bound both IR/IGF-1R hybrids with a fourfold decreased affinity compared with human insulin. No analog showed an increased mitogenic effect in cells predominantly expressing the IR, whereas glargine exerted an increased mitogenic effect in cells predominantly expressing the IGF-1R (411).
In light of these in vitro results, it is reasonable to assume that binding affinities and the dissociation rates for each of the two IR isoforms, the IGF-1R and HRs, as well as ligand-induced receptor internalization and trafficking all contribute to regulating the different and tissue-specific biological effects of particular insulin analogs.
“It appears that the therapeutic risk of insulin analogs in terms of cancer development and progression is modest at best compared with native insulin.”
Notably, commercially available analogs, unlike AspB10, do not show an increased transforming capacity compared with insulin (33). Additionally, the gene expression profiles after glargine injection in the mammary glands of mice were similar to those induced by insulin (410).
However, the scenario in vivo could be different from what was observed in vitro. For instance, insulin glargine is metabolized to M1 and M2, which induce a mitogenic/metabolic ratio similar to that of native insulin (400), whereas detemir and degludec, which circulate as the albumin-bound form, reach relatively high concentrations with no clear data regarding their free concentrations in vivo (416).
So far, clinical evidence cannot exclude or confirm a relationship between analog use and cancer risk. Four retrospective clinical trials have recently addressed the question of whether use of insulin glargine in diabetic patients is associated with an increased cancer risk. Three of these studies, published simultaneously in 2009 (417–419), speculated a possible association between glargine and cancer risk. One study did not find any relationship with glargine but found an increased cancer risk for all insulin preparations (420). More recently, other studies have not found any correlation between the use of glargine in diabetic patients and cancer risk, suggesting a safety profile of this analog similar to native insulin (421, 422). In 2012, the ORIGIN trial reported no differences between glargine treatment and the standard of care in cancer incidence and cancer-related deaths (423). A systematic review of studies evaluating the effect of long-acting insulin analogs on the risk of cancer (breast, colon, and prostate) found no association between glargine and detemir and cancer in 13 out of 15 studies. Four out of 13 studies reported an increased risk of breast cancer with the use of insulin glargine. However, these clinical data are quite controversial due to many biases and confounders in all studies analyzed (424). Based on the in vitro, in vivo, and epidemiological observations currently available, it appears that the therapeutic risk of insulin analogs in terms of cancer development and progression is modest at best compared with native insulin. However, uncertainty still remains, and future studies should be conducted to address this issue conclusively.
IR modulators
As previously discussed, the issue of selective IR activation is of special interest, due to concerns regarding the possible mitogenic effect of insulin. One possible approach is to design IR modulators that would induce conformational changes of the receptor different from the one induced by the natural ligand to stimulate selective responses in terms of the time, intensity, and quality of downstream signals. For this reason, a number of new IR ligands able to separate the metabolic from the mitogenic IR action have been studied in the last decade.
Autoantibodies to the IR were first identified in patients with rare diseases and severe insulin resistance. In most cases, by binding to the receptor, these antibodies competitively blocked insulin binding and caused severe insulin resistance and diabetes (425). However, in some instances, these orthosteric IR antibodies mimicked the insulin effect and caused hypoglycemia (426). Based on these observations, novel anti-IR monoclonal antibodies were designed, and these antibodies interacted with allosteric sites of the IR, thereby inducing distinct structural states of the receptor and, consequently, different signals. For example, monoclonal antibodies isolated from a human antibody phage display library (427) bind to the IR with a high affinity in different allosteric sites and therefore display different properties. These antibodies include an agonist monoclonal antibody with full glucoregulatory and cholesterol lowering activity but no mitogenic effect in cancer cells (it activates the Akt pathway but only minimally activates the MAPK mitogenic pathway) (428, 429), and a nonagonist antibody that increases the insulin binding affinity of the IR for insulin 20-fold by decreasing the dissociation rate and stabilizing the ligand-bound conformation of the receptor (430).
Additionally, an antagonist antibody has been obtained that reduces insulin binding by 3-fold and, more importantly, reduces insulin postreceptor signaling by 20- to 100-fold (431).
The potential clinical relevance of these allosteric IR modulators is evident, because they demonstrate the possibility of inducing specific modifications of the IR conformation, differentially stimulating the pleiotropic biological signals of IR activation. Moreover, these antibodies have the advantage of being IR specific (no effect on the IGF-1R), with a long half-life and, being human proteins, low immunogenicity (432). The effect of these antibodies on IR isoform activation and signaling has not been studied, but it is unlikely to be relevant, because they bind to allosteric sites of the receptor molecule: their selective activation of IR signaling, therefore, should be independent from the structure/function relationship typical of the IR isoform binding site.
In addition to antibodies, small synthetic peptides have also been obtained for IR modulation. The recent advancements in understanding the structural biology of the interaction between insulin and its primary binding site of the receptor (see the previous paragraph) and the identification of a set of synthetic peptides exhibiting agonist activity in both lipogenesis and glucose uptake (433) can provide novel insights that help to clarify the structure/function relationship of the IR molecule and its pleiotropic effects (434). The first single-chain peptide was synthesized in 2008 (433), bound to the orthosteric site of the IR, and selectively activated the metabolic function (glycogen synthesis) of the IR-A isoform similarly to insulin. In contrast to insulin, however, the Shc and ERK pathways were not activated, and cell proliferation was only slightly stimulated. Small IR-binding compounds have been recently isolated from Gymnema sylvestre, a common herb in South Asia with known antidiabetic properties. These small compounds have a high binding affinity for the IR, interacting with amino acids in the active binding site (435).
In addition to small peptides and antibodies, a novel approach to the selective activation of the IR has been obtained with aptamers. These are single-stranded nucleotides artificially isolated by a new in vitro selection process (SELEX) (436). Aptamers can interact with a high affinity and specificity with specific regions of a protein. The IR agonist aptamer IR-488 causes the preferential phosphorylation of Y1150 of the IR catalytic domain and differentially regulates IR signaling: the Akt (metabolic) pathway but not the MAPK (mitogenic) pathway is stimulated with little effect on cancer cell proliferation. The possible IR isoform-specific action of these aptamers is unknown at present.
In 2011, a mutation at position B25 alone or in combination with a mutation at position B27 in the insulin molecule was identified as the common denominator conferring IR isoform selectivity. By amino acid scanning mutagenesis, two IR isoform-selective insulin analogs were engineered, INS-A and INS-B, with twofold to fourfold differences in relative IR isoform binding affinity compared with human insulin (244, 437). These two insulin analogs were also found to exert isoform-selective effects both in vitro and in vivo. In particular, INS-B showed higher potency in stimulating lipogenesis in rat adipocytes and glycogen accumulation in rat hepatocytes, in accordance with the tissue expression profile of IR-B, which is the predominant isoform in liver and fat tissue. The analog INS-A exerted opposite effects, exerting a stronger effect on muscle glycogen synthesis (244). Covalent intrachain crosslinking within its B22 to B30 segment has been also used to modulate the binding affinity of insulin analogs toward IR isoforms. Using this approach, Vicová et al. (438) have synthesized an insulin analog containing a B26 to B29 triazole bridge that was highly active in binding to both IR isoforms, with a significant preference for IR-B. Moreover, it has been observed that insulin analogs modified at the TyrB26 site may provide structural insight into the structural origins of differential insulin signaling through IR-A and IR-B. For instance, it was found that an AsnB26 analog may preferentially signal through the IR-B (439). Insulin/IGF hybrids have been also used as molecular probes to evaluate the ability of specific amino acid sequences added at the C terminus of the insulin B chain in modulating the differential binding affinity of insulin analogs to the two IR isoforms (440).
These studies are potentially of high clinical importance, because diabetic patients treated with exogenous insulin by subcutaneous injection incur a nonphysiological insulin distribution, as the liver should normally be exposed to significantly higher insulin concentrations than peripheral organs. Owing to the tissue-specific expression of the IR isoforms in humans, where IR-B is highly expressed in the liver whereas most peripheral tissues also express variable IR-A levels, the IR-B–selective insulin analog could be primarily effective in the liver and induce liver-preferential effects. Consequently, this analog could better mimic the natural physiology of insulin distribution, improving blood glucose control and the lipid profile and reducing fat mass and the incidence of hypoglycemia (244, 437). In summary, the design of analogs with specific selectivity for the two IR splicing variants could provide an opportunity to apply tissue-selective insulin therapy on the basis of tissue-specific IR isoform expression profiles (244). Importantly, in this regard, note that an IR-A–selective insulin analog could potentially exert a deleterious effect in cancer cells that overexpress IR-A by inducing a pronounced mitogenic response and favoring cancer progression.
In conclusion, many ways to selectively regulate the IR have been discovered. The conformational changes induced by both orthosteric and allosteric ligands can differentially activate the postreceptor signaling pathways and insulin-dependent gene expression. This may result in a series of advantages that are relevant in terms of clinical applications (Table 7).
Table 7.
New IR Ligands and modulators
| Desiderable characteristics of a selective IR modulator in diabetic patients |
| Full glucoregulatory activity |
| Minimal (or reduced) mitogenic activity |
| No effect on hypoglycemia |
| No effect on weight gain |
| Long-acting |
| Different types of IR modulators |
| Agonists, stimulating selective biological responses |
| Agonists, active with different time courses |
| Agonists, IR isoform specific |
| Agonist, tissue specific |
| Coactivators, increasing the ligand effect (fully or partially) |
| Antagonists, blocking IR activation (fully or partially) |
Despite these recent advances in establishing IR modulators, the enormous potential for fine-tuning the IR signaling processes in target cells is far from being reached. Many issues require further investigation, such as the structure/function interactions at the receptor level, optimization of the complex network of intracellular signaling, the tissue specificity of selective IR activation/inhibition, and the long-term consequences of unbalancing the multitarget IR signaling in the whole organism (441, 442).
Inhibitors of the IR-A pathway
The IR-A pathway as a cancer target
Several studies have now established the importance of the insulin/IGF-2/IR-A pathway as a viable target in malignancies addicted to this signaling. Indeed, in agreement with previous studies demonstrating that the IGF-1R and IR form HRs with an important role in cancer (443), it is now widely accepted that the IGF-1R and IR are critically interconnected. In fact, it has been shown that targeting the IGF-1R alone in IGF-2–producing cancer cells results in increased IR activity through the formation of phospho-IR homodimers (444). Similarly, genetic disruption of the IGF-1R gene in normal MEFs leads to IR pathway hypersensitivity due to increased IR homodimers and IR oligomerization (445). In contrast, IR ablation leads to increased IGF-1R homodimers and enhances signaling through the IGF-1R (213). As previously reported (446), measuring IR and IGF-1R expression levels and the IR/IGF-1R ratio can predict the prevalent receptor action and therapeutic response. Accordingly, tamoxifen-resistant MCF-7 cells predominantly express the IR and are sensitive to IR inhibition, whereas the parental cells express both the IR and IGF-1R and are sensitive to the combined inhibition (447). Notably, IR-A overexpression in tumor cells confers complete resistance to the anti–IGF-1R antibody cixutumumab, whereas IR-B expression confers partial resistance. Total IR expression acts as a biomarker predictive of resistance to anti–IGF-1R antibodies alone or in combination with chemotherapy (317).
Three main strategies have been pursued to target the INS/IGF-2/IR-A pathway. The first approach relies on cotargeting the IR and IGF-1R with dual small molecule TK inhibitors, whereas the second approach directly targets IGF-2 as the common ligand for IR-A and IGF-1R using specific antibodies or specific ligand traps. Lastly, there is the option of specifically targeting the IR-A isoform without affecting the IR-B isoform.
Dual IGF-1R/IR small-molecule TK inhibitors
Evidence in the literature supports the notion that an ATP-competitive dual IGF-1R/IR RTK inhibitor may be more effective than the combination of anti–IGF-1R and anti-IR antibodies (448). Moreover, treatment with the dual IGF-1R/IR inhibitor AZD9362 enhanced the antitumor efficacy of the Akt inhibitor AZD5363 in MCF-7/LTED cells and MCF-7 xenografts in ovariectomized mice, suggesting that this combination therapy would be an effective treatment against hormone-independent ER+ breast cancer (449).
Linsitinib (OSI-906) and BMS-754807 are well-characterized small-molecule dual inhibitors of the IGF-1R and IR. Preclinical data have shown a remarkable efficacy of linsitinib both in cultured cancer cells and in vivo human tumor xenografts. Tumor cell lines with autocrine IGF-2 production (450) and expressing high levels of phosphorylated IGF-1R and IR are most likely to respond to such an inhibitor (450). Several phase I to III studies are currently underway with linsitinib, which has shown acceptable tolerability in patients with a variety of cancer histotypes (https://clinicaltrials.gov/ct2/results?term=linsitinib&pg=1). However, recently published results of a multicenter phase III study using linsitinib in 90 patients with locally advanced or metastatic adrenocortical carcinoma did not show any effect of this drug in increasing either progression-free survival or overall survival (451). Similar results were obtained in preclinical studies with BMS-754807 alone or in combination with other anticancer agents, such as gefitinib, gemcitabine, an ATM-related kinase inhibitor, and cisplatin (452–456). However, although some clinical studies are in progress (https://clinicaltrials.gov/ct2/results?term=BMS-754807+&Search=Search), no evidence of efficacy in a clinical setting has been demonstrated so far.
Similarly, the KW-2450 dual TK inhibitor had significant antitumor activity in preclinical studies. A phase I clinical trial in 13 patients with advanced solid tumors showed an acceptable degree of toxicity, which includes cases with grade 3 hyperglycemia, and was associated with modest antitumor activity (457). Recently, using the dual inhibitor BI 885578, it was observed that the IR-B signaling in muscle recovered better than the IGF-1R/IR-A signaling in GEO colon cancer cells, indicating the possibility of a therapeutical window (458).
Specific targeting of IR-A or total IR.
The identification of mutations in splicing factors in hematological malignancies and solid tumors (309, 310) has reinforced the important role of gene splicing in the establishment of the malignant phenotype. Moreover, various drugs have been developed to normalize the action of mutated splicing factors or to target cells bearing mutated splicing factors for degradation (308, 459). However, no splicing factor mutation has been isolated as a driving factor modulating the high IR-A/IR-B ratio frequently observed in cancer. As a consequence, whether some of the drugs available to offset splicing factor mutations may normalize the IR-A/IR-B ratio in favor of the IR-B isoform in cancer is currently unknown. The differential action on IR isoform protein maturation might provide new target strategies. Data showing that furin cleavage is essential for IR-A maturation whereas IR-B maturation may be supported by the convertase PACE4 (see the paragraph titled “Differential IR isoform regulation at the protein level”) (91) are in line with previous observations reporting that furin inhibition attenuates critical properties of tumor cells (460, 461). This approach might therefore open interesting avenues in cancer prevention and therapy, as furin can be inhibited by a number of polyphenols (catechins, gallic acid, and quercetin) (462).
Nucleic acid–based aptamers are emerging as therapeutic antagonists of disease-associated proteins such as receptor tyrosine kinases. Recently, a nuclease-resistant RNA aptamer that specifically recognizes the IR and inhibits IR-dependent signaling has been described. These data suggest that it could be possible to select high-affinity aptamers that specifically bind the IR-A isoform (463).
As mentioned previously, several specific miRNAs, including miR-15b, miR-195, miR-497, miR-103/107, let-7, and miR-424, regulate IR expression in physiopathological conditions such as obesity and insulin resistance. These miRNAs are dysregulated in cancer and, in principle, could contribute to IR upregulation and an increased IR-A/IR-B ratio.
IGF-2–blocking strategies
In osteosarcoma cells, IGF-2 is expressed at high levels after chemotherapy treatment. Indeed, exposure of osteosarcoma cells to IGF-2 or insulin induced a dormant state associated with resistance to various chemotherapeutic drugs both in vitro and in mice xenografts (464). IGF-2 levels were identified as predictive of tumor sensitivity to anti-EGFR therapy and a determinant of the response to combined IGF-2/EGFR targeting (465).
Sequestration of IGF-2 has been achieved by blocking antibodies that can either specifically bind IGF-2 only or both IGF-1 and IGF-2 or with IGF-2 ligand traps. These approaches do not impair the metabolic effects of insulin but do not antagonize the possible protumorigenic effects of high circulating insulin levels.
MEDI-573 is a fully humanized IgG2 monoclonal antibody that neutralizes both IGF-1 and IGF-2, thus inhibiting IGF-1R and IR-A activation by IGF-2. This antibody has shown potent antitumor efficacy in IGF-driven tumors (466). In mouse models, MEDI-573 efficiently neutralized IGF-2 and significantly inhibited the tumor growth of CRC cells with overexpressed IGF-2 as the major driver but did not affect the growth of cells with physiological levels of IGF-2 (467). Similar results were obtained in sarcoma cells expressing IGF-1 and IGF-2 (467). Phase I dose-escalation studies carried out in patients with advanced solid tumors refractory to standard therapy have shown that MEDI-573 is generally well tolerated. No complete or partial responses were observed, but approximately a third of patients showed stable disease (228, 468).
The high-affinity domain 11 of M6P/IGF2R can be exploited as a soluble ligand antagonist or trap to deplete IGF-2 isoforms from serum and inhibit IGF-2–dependent signaling in vivo. The affinity of domain 11 for IGF-2 may be increased several fold by mutagenesis (469). Recently, novel mutations in the domain 11 binding site have been described, and they increase the binding affinity for IGF-2 by ∼100-fold, suggesting that this mutated domain 11 might function as a more efficient IGF-2 trap (470). A different IGF trap with a high affinity for IGF-1 and IGF-2 and a low affinity for insulin has been engineered by combining the soluble form of the IGF-1R and the Fc portion of IgG1. This IGF trap has shown significant antitumor activity in preclinical models (471). In gastro-entero-pancreatic neuroendocrine tumors that preferentially express IR-A (472), autocrine IGF-2 production leads to IR-A activation. In these tumors, IGF-2 production can be reduced by somatostatin inhibitors and dopamine agonists (473) that are able, therefore, to inhibit the activation of the IR-A/IGF-2 loop.
Conclusions and Perspectives
The complex role of the two IR isoforms in physiology and disease is only partially understood (Fig. 8). During development, cells predominantly express IR-A, most likely to maximize their response to IGFs and to proinsulin. The observation that the two IR isoforms may have opposite effects on cell differentiation warrants further research. In agreement with the data indicating different roles of IR isoforms in development and differentiation, it has been recently reported that two different IRs in insects determine specific effects on wing phenotype and cause alternative development outcomes (474). IR-A also likely induces cell growth in response to IGF-1, as shown in pancreatic β-cells. In adulthood, the predominant expression of IR-A in the brain and of IR-B in the liver is conserved across species, whereas the relative abundance of the two isoforms is more variable in skeletal muscle and adipose tissue. The predominant IR-B expression in the liver is likely protective against the mitogenic effects of high concentrations of insulin, proinsulin, and IGFs, whereas IR-A expression in the brain is possibly more related to the unique effects of insulin and IGF-2 in this organ. The predominant IR-B expression in the liver is likely protective against the mitogenic effects of high concentrations of insulin, proinsulin, and IGFs, whereas IR-A expression in the brain is possibly more related to the unique effects of insulin and IGF-2 in this organ. Alternatively, the variable abundance of IR-A in muscle and adipose cells suggests that the IR-A isoform may mediate the metabolic and nonmetabolic effects of insulin and IGFs in these tissues. Finally, a full understanding of the physiological significance of IR isoforms clearly requires a practical assay to measure the protein expression of the two isoforms as well as a more complete knowledge of the regulation of all of the factors involved in IR gene expression and mRNA splicing and the protein processing of the two IR isoforms. Indeed, so far no antibodies are able to distinguish the two IR isoforms and, therefore, data describing their biology are based on mRNA expression and on cells transfected with or naturally expressing only one isoform.
Figure 8.
IR isoforms affect insulin/IGF signaling diversification. (a) Splicing regulation: IR isoform relative abundance is regulated by numerous splicing factors and miRNAs. Growth factors such as insulin and EGF favor IR-A generation. (b) Relative abundance: The relative abundance of IR isoforms is also regulated by tissue-specific factors. IR-A is predominant in fetal, stem, and cancer cells. (c) IR-A Internalization/degradation: Unlike insulin, IGF-2 binding to IR-A does not target the receptor to lysosomal degradation, allowing for prolonged mitogenic effects. (d) Recruitment of molecular partners: Ligand binding recruits various molecular partners (MPs) to the activated receptor. It can be hypothesized that IR-A tonic activation by IGF-2 or hyperinsulinemia may induce prolonged MP interaction and biased signaling. (e) Effect of fed/fasting conditions: According to ligand binding affinities, it can be predicted that during fasting (low circulating insulin) IGF-2 is the predominant IR-A ligand, whereas in fed conditions (high circulating insulin) both IR isoforms are engaged by insulin. (f) Effect of IGF-1 bioavailability: IR-A is predicted to be the major IGF-2 receptor when IGF-1 is present and saturates the IGF-1R. In contrast, IR-A would preferably bind insulin when IGF-1 bioavailability is low or absent.
One important concept that has recently emerged is that IR isoforms are responsible for the complexity of IR signaling diversification, which involves not only different ligand binding affinities but also different membrane partitioning and trafficking as well as the different abilities of the IR isoforms to interact with a variety of molecular partners and to modify their downstream signaling accordingly. Unliganded IR isoforms may also show functional differences in terms of apoptosis regulation and in facilitating glucose transport.
The dysregulation of this complex network has important roles in several diseases, namely, cancer and diabetes. Therefore, recent advances in the characterization of novel IR ligands and modulators with either agonistic or antagonistic activity should now be considered as an important strategy for better and safer treatment of diabetes and cancer and possibly other IR-related diseases. Our present understanding of these processes is still incomplete, but the possibility of modulating IR activity according to the specific disease context or patient will be a step forward in precision therapy for very common diseases.
Acknowledgments
Financial Support: This work was supported in part by Italian Association for Cancer Research Grants IG 19242 and PON01_01078 to A.B., from Ministero della Salute Grant 67/GR-2010–2319511 to R.M., and from National Institutes of Health Grant R01 CA164462 to A.M. M.C.L. acknowledges support from the Australian National Health and Medical Research Council (NHMRC) Project Grant APP1099595 and support to his Institute from the Victorian State Government Operational Infrastructure Support and the Australian NHMRC Independent Research Institutes Infrastructure Support Scheme.
Acknowledgments
Disclosure Summary: Part of M.C.L.’s research is funded by Sanofi (Germany). M.C.L. is an inventor on a number of patents related to the material presented. The remaining authors have nothing to disclose.
Footnotes
- AD
- Alzheimer’s disease
- ATM
- ataxia telangiectasia mutated
- BBB
- blood–brain barrier
- cav-1
- caveolin 1
- cav-2
- caveolin 2
- cDNA
- complementary DNA
- CNS
- central nervous system
- CR
- cysteine-rich region
- CRC
- colorectal carcinoma
- CUGBP
- CUG binding protein
- αCT
- C-terminal segment of the receptor α-chain
- DDR1
- discoidin domain receptor 1
- DM1
- myotonic dystrophy type 1
- DM2
- myotonic dystrophy type 2
- EGF
- epidermal growth factor
- EGFR
- epidermal growth factor receptor
- ELISA
- enzyme-linked immunosorbent assay
- EMT
- epithelial–mesenchymal transition
- ER
- estrogen receptor
- ERK
- extracellular signal-regulated kinase
- FnIII-1
- FnIII-2, and FnIII-3, first, second, and third fibronectin type III domains
- GDM
- gestational diabetes mellitus
- GH
- growth hormone
- Gpc4
- glypican-4
- GPER
- G protein-coupled estrogen receptor
- HCC
- hepatocellular carcinoma
- HGF
- hepatocyte growth factor
- HMGA1
- high-mobility group protein A1
- hnRNP
- heterogeneous nuclear ribonucleoprotein family protein
- HR
- hybrid receptor
- HUVEC
- human umbilical vein endothelial cell
- ID
- insert domain
- IGF
- insulin-like growth factor
- IGFBP
- insulin-like growth factor–binding protein
- IGF-1R
- type I insulin-like growth factor-1 receptor
- IR
- insulin receptor
- IRES
- internal ribosome entry site
- IRS
- insulin receptor substrate
- KD
- knockdown
- KO
- knockout
- L1
- first leucine-rich repeat domain
- LIRKO
- liver-specific insulin receptor knockout
- LOI
- loss of imprinting
- MAPK
- mitogen-activated protein kinase
- MBNL
- muscleblind-like protein
- MEF
- mouse embryonic fibroblast
- MIRKO
- muscle-specific insulin receptor knockout
- miR/miRNA
- microRNA
- MKR
- muscle IGF-IR-lysine-arginine
- mRNA
- messenger RNA
- NF-κB
- nuclear factor κB
- NSCLC
- non-small cell lung cancer
- p70S6K
- p70S6 kinase
- PI3K
- phosphatidylinositol 3-kinase
- ROS
- reactive oxygen species
- RTK
- receptor tyrosine kinase
- SR
- serine-arginine–rich
- SRSF
- serine/arginine-rich splicing factor
- T2DM
- type 2 diabetes mellitus
- TNF
- tumor necrosis factor
- TK
- tyrosine kinase
- UTR
- untranslated region
- μIR
- insulin “microreceptor”.
References
- 1.Belfiore A, Frasca F, Pandini G, Sciacca L, Vigneri R. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr Rev. 2009;30(6):586–623. [DOI] [PubMed] [Google Scholar]
- 2.Pandini G, Frasca F, Mineo R, Sciacca L, Vigneri R, Belfiore A. Insulin/insulin-like growth factor I hybrid receptors have different biological characteristics depending on the insulin receptor isoform involved. J Biol Chem. 2002;277(42):39684–39695. [DOI] [PubMed] [Google Scholar]
- 3.Boucher J, Tseng YH, Kahn CR. Insulin and Growth Insulin-like. Factor-1 Receptors Act as Ligand-specific Amplitude Modulators of a Common Pathway Regulating Gene Transcription. J Biol Chem. 2010;285(22):17235–17245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Menting JG, Whittaker J, Margetts MB, Whittaker LJ, Kong GK-W, Smith BJ, Watson CJ, Žáková L, Kletvíková E, Jiráček J, Chan SJ, Steiner DF, Dodson GG, Brzozowski AM, Weiss MA, Ward CW, Lawrence MC. How insulin engages its primary binding site on the insulin receptor. Nature. 2013;493(7431):241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menting JG, Lawrence CF, Kong GK-W, Margetts MB, Ward CW, Lawrence MC. Structural Congruency of Ligand Binding to the Insulin and Insulin/Type 1 Insulin-like Growth Factor Hybrid Receptors. Structure. 2015;23(7):1271–1282. [DOI] [PubMed] [Google Scholar]
- 6.Malaguarnera R, Sacco A, Voci C, Pandini G, Vigneri R, Belfiore A. Proinsulin Binds with High Affinity the Insulin Receptor Isoform A and Predominantly Activates the Mitogenic Pathway. Endocrinology. 2012;153(5):2152–2163. [DOI] [PubMed] [Google Scholar]
- 7.Morcavallo A, Gaspari M, Pandini G, Palummo A, Cuda G, Larsen MR, Vigneri R, Belfiore A. Research resource: new and diverse substrates for the insulin receptor isoform a revealed by quantitative proteomics after stimulation with igf-ii or insulin. Mol Endocrinol. 2011;25(8):1456–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKern NM, Lawrence MC, Streltsov VA, Lou MZ, Adams TE, Lovrecz GO, Elleman TC, Richards KM, Bentley JD, Pilling PA, Hoyne PA, Cartledge KA, Pham TM, Lewis JL, Sankovich SE, Stoichevska V, Da Silva E, Robinson CP, Frenkel MJ, Sparrow LG, Fernley RT, Epa VC, Ward CW. Structure of the insulin receptor ectodomain reveals a folded-over conformation. Nature. 2006;443(7108):218–221. [DOI] [PubMed] [Google Scholar]
- 9.Lou M, Garrett TP, McKern NM, Hoyne PA, Epa VC, Bentley JD, Lovrecz GO, Cosgrove LJ, Frenkel MJ, Ward CW The first three domains of the insulin receptor differ structurally from the insulin-like growth factor 1 receptor in the regions governing ligand specificity. Proc Natl Acad Sci USA. 2006;103(33):12429–12434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith BJ, Huang K, Kong G, Chan SJ, Nakagawa S, Menting JG, Hu S-Q, Whittaker J, Steiner DF, Katsoyannis PG, Ward CW, Weiss MA, Lawrence MC. Structural resolution of a tandem hormone-binding element in the insulin receptor and its implications for design of peptide agonists. Proc Natl Acad Sci USA. 2010;107(15):6771–6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Croll TI, Smith BJ, Margetts MB, Whittaker J, Weiss MA, Ward CW, Lawrence MC. Higher-Resolution Structure of the Human Insulin Receptor Ectodomain: Multi-Modal Inclusion of the Insert Domain. Structure. 2016;24(3):469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sparrow LG, Lawrence MC, Gorman JJ, Strike PM, Robinson CP, McKern NM, Ward CW. N-linked glycans of the human insulin receptor and their distribution over the crystal structure. Proteins. 2008;71(1):426–439. [DOI] [PubMed] [Google Scholar]
- 13.Sparrow LG, Gorman JJ, Strike PM, Robinson CP, McKern NM, Epa VC, Ward CW. The location and characterisation of the O-linked glycans of the human insulin receptor. Proteins. 2007;66(2):261–265. [DOI] [PubMed] [Google Scholar]
- 14.Adams MJ, Blundell TL, Dodson EJ, Dodson GG, Vijayan M, Baker EN, Harding MM, Hodgkin DC, Rimmer B, Sheat S. Structure of Rhombohedral 2 Zinc Insulin Crystals. Nature. 1969;224(5218):491–495. [Google Scholar]
- 15.Soos MA, Siddle K, Baron MD, Heward JM, Luzio JP, Bellatin J, Lennox ES. Monoclonal antibodies reacting with multiple epitopes on the human insulin receptor. Biochem J. 1986;235(1):199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hua QX, Shoelson SE, Kochoyan M, Weiss MA. Receptor binding redefined by a structural switch in a mutant human insulin. Nature. 1991;354(6350):238–241. [DOI] [PubMed] [Google Scholar]
- 17.Bentley G, Dodson E, Dodson G, Hodgkin D, Mercola D. Structure of insulin in 4-zinc insulin. Nature. 1976;261(5556):166–168. [DOI] [PubMed] [Google Scholar]
- 18.Derewenda U, Derewenda Z, Dodson EJ, Dodson GG, Reynolds CD, Smith GD, Sparks C, Swenson D. Phenol stabilizes more helix in a new symmetrical zinc insulin hexamer. Nature. 1989;338(6216):594–596. [DOI] [PubMed] [Google Scholar]
- 19.Weiss MA. Chapter 2 The Structure and Function of Insulin. Vitam Horm. 2009;80:33–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whittaker L, Hao C, Fu W, Whittaker J. High-affinity insulin binding: insulin interacts with two receptor ligand binding sites. Biochemistry. 2008;47(48):12900–12909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Menting JG, Yang Y, Chan SJ, Phillips NB, Smith BJ, Whittaker J, Wickramasinghe NP, Whittaker LJ, Pandyarajan V, Wan Z, Yadav SP, Carroll JM, Strokes N, Roberts CT, Ismail-Beigi F, Milewski W, Steiner DF, Chauhan VS, Ward CW, Weiss MA, Lawrence MC. Protective hinge in insulin opens to enable its receptor engagement. Proc Natl Acad Sci USA. 2014;111(33):E3395–E3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blundell TL, Cutfield JF, Cutfield SM, Dodson EJ, Dodson GG, Hodgkin DC, Mercola DA, Vijayan M Atomic positions in rhombohedral 2-zinc insulin crystals. Nature. 1971;231(5304):506–511. [DOI] [PubMed] [Google Scholar]
- 23.Kristensen C, Wiberg FC, Andersen AS. Specificity of insulin and insulin-like growth factor I receptors investigated using chimeric mini-receptors. Role of C-terminal of receptor alpha subunit. J Biol Chem. 1999;274(52):37351–37356. [DOI] [PubMed] [Google Scholar]
- 24.Denley A, Cosgrove LJ, Booker GW, Wallace JC, Forbes BE. Molecular interactions of the IGF system. Cytokine Growth Factor Rev. 2005;16(4–5):421–439. [DOI] [PubMed] [Google Scholar]
- 25.Malaguarnera R, Morcavallo A, Belfiore A. The Insulin and IGF-I Pathway in Endocrine Glands Carcinogenesis. J Oncol. 2012;2012:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alvino CL, Ong SC, McNeil KA, Delaine C, Booker GW, Wallace JC, Forbes BE. Understanding the mechanism of insulin and insulin-like growth factor (IGF) receptor activation by IGF-II. Wilson E, ed. PLoS One 2011;6(11):e27488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magee BA, Shooter GK, Wallace JC, Francis GL. Insulin-like growth factor I and its binding proteins: a study of the binding interface using B-domain analogues. Biochemistry. 1999;38(48):15863–15870. [DOI] [PubMed] [Google Scholar]
- 28.Henderson ST, Brierley GV, Surinya KH, Priebe IK, Catcheside DEA, Wallace JC, Forbes BE, Cosgrove LJ. Delineation of the IGF-II C domain elements involved in binding and activation of the IR-A, IR-B and IGF-IR. Growth Horm IGF Res. 2015;25(1):20–27. [DOI] [PubMed] [Google Scholar]
- 29.Daughaday WH, Trivedi B. Measurement of derivatives of proinsulin-like growth factor-II in serum by a radioimmunoassay directed against the E-domain in normal subjects and patients with nonislet cell tumor hypoglycemia. J Clin Endocrinol Metab. 1992;75(1):110–115. [DOI] [PubMed] [Google Scholar]
- 30.Benyoucef S, Surinya KH, Hadaschik D, Siddle K. Characterization of insulin/IGF hybrid receptors: contributions of the insulin receptor L2 and Fn1 domains and the alternatively spliced exon 11 sequence to ligand binding and receptor activation. Biochem J. 2007;403(3):603–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soos MA, Whittaker J, Lammers R, Ullrich A, Siddle K. Receptors for insulin and insulin-like growth factor-I can form hybrid dimers. Characterisation of hybrid receptors in transfected cells. Biochem J. 1990;270(2):383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frasca F, Pandini G, Scalia P, Sciacca L, Mineo R, Costantino A, Goldfine ID, Belfiore A, Vigneri R. Insulin receptor isoform A, a newly recognized, high-affinity insulin-like growth factor II receptor in fetal and cancer cells. Mol Cell Biol. 1999;19(5):3278–3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sciacca L, Cassarino MF, Genua M, Pandini G, Le Moli R, Squatrito S, Vigneri R. Insulin analogues differently activate insulin receptor isoforms and post-receptor signalling. Diabetologia. 2010;53(8):1743–1753. [DOI] [PubMed] [Google Scholar]
- 34.Versteyhe S, Klaproth B, Borup R, Palsgaard J, Jensen M, Gray SG, De Meyts P. IGF-I, IGF- II, and Stimulate Insulin. Different Gene Expression Responses through Binding to the IGF-I Receptor. Front Endocrinol (Lausanne). 2013;4:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rajapaksha H, Forbes BE. Ligand-Binding Affinity at the Insulin Receptor Isoform-A and Subsequent IR-A Tyrosine Phosphorylation Kinetics are Important Determinants of Mitogenic Biological Outcomes. Front Endocrinol (Lausanne). 2015;6:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pierre-Eugene C, Pagesy P, Nguyen TT, Neuillé M, Tschank G, Tennagels N, Hampe C, Issad T. Effect of Insulin Analogues on Insulin/IGF1 Hybrid Receptors: Increased Activation by Glargine but Not by Its Metabolites M1 and M2. Federici M, ed. PLoS One. 2012;7(7):e41992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sacco A, Morcavallo A, Pandini G, Vigneri R, Belfiore A. Differential signaling activation by insulin and insulin-like growth factors I and II upon binding to insulin receptor isoform A. Endocrinology. 2009;150(8):3594–3602. [DOI] [PubMed] [Google Scholar]
- 38.Ziegler AN, Chidambaram S, Forbes BE, Wood TL, Levison SW. Insulin-like growth factor-II (IGF-II) and IGF-II analogs with enhanced insulin receptor-a binding affinity promote neural stem cell expansion. J Biol Chem. 2014;289(8):4626–4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marks AG, Carroll JM, Purnell JQ, Roberts CT. Plasma Distribution and Activities Signaling. of IGF-II Precursors. Endocrinology. 2011;152(3):922–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu BA, Engelmann BW, Jablonowski K, Higginbotham K, Stergachis AB, Nash PD. SRC Homology 2 Domain Binding Sites in Insulin, IGF-1 and FGF receptor mediated signaling networks reveal an extensive potential interactome. Cell Commun Signal. 2012;10(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pandini G, Vigneri R, Costantino A, Frasca F, Ippolito A, Fujita-Yamaguchi Y, Siddle K, Goldfine ID, Belfiore A. Insulin and insulin-like growth factor-I (IGF-I) receptor overexpression in breast cancers leads to insulin/IGF-I hybrid receptor overexpression: evidence for a second mechanism of IGF-I signaling. Clin Cancer Res. 1999;5(7):1935–1944. [PubMed] [Google Scholar]
- 42.Wu Y-C, Zhu M, Robertson DM. Novel Nuclear Localization and Function Potential. of Insulin-Like Growth Factor-1 Receptor/Insulin Receptor Hybrid in Corneal Epithelial Cells. Fleiszig S, ed. PLoS One. 2012;7(8):e42483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soos MA, Field CE, Siddle K. Purified hybrid insulin/insulin-like growth factor-I receptors bind insulin-like growth factor-I, but not insulin, with high affinity. Biochem J. 1993;290(Pt 2):419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Slaaby R, Schaffer L, Lautrup-Larsen I, Andersen AS, Shaw AC, Mathiasen IS, Brandt J. Hybrid receptors formed by insulin receptor (IR) and insulin-like growth factor I receptor (IGF-IR) have low insulin and high IGF-1 affinity irrespective of the IR splice variant. J Biol Chem. 2006;281(36):25869–25874. [DOI] [PubMed] [Google Scholar]
- 45.Slaaby R. Specific insulin/IGF1 hybrid receptor activation assay reveals IGF1 as a more potent ligand than insulin. Sci Rep. 2015;5:7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshizato K, Shirotani T, Furukawa N, Taguchi T, Motoshima H, Toyonaga T, Hirashima Y, Kawashima J, Ebina Y, Shichiri M, Araki E. Identification of a cis-acting element and a novel trans-acting factor of the human insulin receptor gene in HepG2 and rat liver cells. Biochem Biophys Res Commun. 2001;280(2):428–434. [DOI] [PubMed] [Google Scholar]
- 47.Lee JK, Tam JW, Tsai MJ, Tsai SY. Identification of cis- and trans-acting factors regulating the expression of the human insulin receptor gene. J Biol Chem. 1992;267(7):4638–4645. [PubMed] [Google Scholar]
- 48.Barthel A, Schmoll D, Unterman TG. FoxO proteins in insulin action and metabolism. Trends Endocrinol Metab. 2005;16(4):183–189. [DOI] [PubMed] [Google Scholar]
- 49.Foti D, Iuliano R, Chiefari E, Brunetti A. A nucleoprotein complex containing Sp1, C/EBP beta, and HMGI-Y controls human insulin receptor gene transcription. Mol Cell Biol. 2003;23(8):2720–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Webster NJ, Resnik JL, Reichart DB, Strauss B, Haas M, Seely BL. Repression of the insulin receptor promoter by the tumor suppressor gene product p53: a possible mechanism for receptor overexpression in breast cancer. Cancer Res. 1996;56(12):2781–2788. [PubMed] [Google Scholar]
- 51.Arnoldo L, Sgarra R, Chiefari E, Iiritano S, Arcidiacono B, Pegoraro S, Pellarin I, Brunetti A, Manfioletti G. A novel mechanism of post-translational modulation of HMGA functions by the histone chaperone nucleophosmin. Sci Rep. 2015;5:8552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chiefari E, Nevolo MT, Arcidiacono B, Maurizio E, Nocera A, Iiritano S, Sgarra R, Possidente K, Palmieri C, Paonessa F, Brunetti G, Manfioletti G, Foti D, Brunetti A HMGA1 is a novel downstream nuclear target of the insulin receptor signaling pathway. Sci Rep. 2012;2:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wei Y, Gokhale RH, Sonnenschein A, Montgomery KM, Ingersoll A, Arnosti DN. Complex cis -regulatory landscape of the insulin receptor gene underlies the broad expression of a central signaling regulator. Development. 2016;143(19):3591–3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.López-Lastra M, Rivas A, Barría MI. Protein synthesis in eukaryotes: the growing biological relevance of cap-independent translation initiation. Biol Res. 2005;38(2–3):121–146. [DOI] [PubMed] [Google Scholar]
- 55.Spriggs KA, Cobbold LC, Ridley SH, Coldwell M, Bottley A, Bushell M, Willis AE, Siddle K. The human insulin receptor mRNA contains a functional internal ribosome entry segment. Nucleic Acids Res. 2009;37(17):5881–5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pillai RS, Bhattacharyya SN, Filipowicz W. Repression of protein synthesis by miRNAs: how many mechanisms? Trends Cell Biol. 2007;17(3):118–126. [DOI] [PubMed] [Google Scholar]
- 57.Bartel DP. MicroRNAs: Target Recognition and Regulatory Functions. Cell. 2009;136(2):215–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dumortier O, Hinault C, Van Obberghen E MicroRNAs and Crosstalk Metabolism. in Energy Homeostasis. Cell Metab. 2013;18(3):312–324. [DOI] [PubMed] [Google Scholar]
- 59.Yang W-M, Jeong H-J, Park S-W, Lee W. Obesity-induced miR-15b is linked causally to the development of insulin resistance through the repression of the insulin receptor in hepatocytes. Mol Nutr Food Res. 2015;59(11):2303–2314. [DOI] [PubMed] [Google Scholar]
- 60.Yang W-M, Jeong H-J, Park S-Y, Lee W. Saturated fatty acid-induced miR-195 impairs insulin signaling and glycogen metabolism in HepG2 cells. FEBS Lett. 2014;588(21):3939–3946. [DOI] [PubMed] [Google Scholar]
- 61.Wang X, Wang M, Li H, Lan X, Liu L, Li J, Li Y, Li J, Yi J, Du X, Yan J, Han Y, Zhang F, Liu M, Lu S, Li D. Upregulation of miR-497 induces hepatic insulin resistance in E3 rats with HFD-MetS by targeting insulin receptor. Mol Cell Endocrinol. 2015;416:57–69. [DOI] [PubMed] [Google Scholar]
- 62.Trajkovski M, Hausser J, Soutschek J, Bhat B, Akin A, Zavolan M, Heim MH, Stoffel M. MicroRNAs 103 and 107 regulate insulin sensitivity. Nature. 2011;474(7353):649–653. [DOI] [PubMed] [Google Scholar]
- 63.Mayr C, Hemann MT, Bartel DP Disrupting the Pairing Between let-7 and Hmga2 Enhances Oncogenic Transformation. Science (80-). 2007;315(5818):1576–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kumar MS, Erkeland SJ, Pester RE, Chen CY, Ebert MS, Sharp PA, Jacks T. Suppression of non-small cell lung tumor development by the let-7 microRNA family. Proc Natl Acad Sci USA. 2008;105(10):3903–3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun X, Liu J, Xu C, Tang S-C, Ren H. The insights of Let-7 miRNAs in oncogenesis and stem cell potency. J Cell Mol Med. 2016;20(9):1779–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Newman MA, Thomson JM, Hammond SM. Lin-28 interaction with the Let-7 precursor loop mediates regulated microRNA processing. RNA. 2008;14(8):1539–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee H, Han S, Kwon CS, Lee D. Biogenesis and regulation of the let-7 miRNAs and their functional implications. Protein Cell. 2016;7(2):100–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhu H, Shyh-Chang N, Segrè AV, Shinoda G, Shah SP, Einhorn WS, Takeuchi A, Engreitz JM, Hagan JP, Kharas MG, Urbach A, Thornton JE, Triboulet R, Gregory RI, Altshuler D, Daley GQ, Altshuler D, Daley GQ. The Lin28/let-7 Axis Regulates Glucose Metabolism. Cell. 2011;147(1):81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marchand A, Atassi F, Mougenot N, Clergue M, Codoni V, Berthuin J, Proust C, Trégouët D-A, Hulot J-S, Lompré A-M. miR-322 regulates insulin signaling pathway and protects against metabolic syndrome-induced cardiac dysfunction in mice. Biochim Biophys Acta - Mol Basis Dis. 2016;1862(4):611–621. [DOI] [PubMed] [Google Scholar]
- 70.Nagarajan A, Petersen MC, Nasiri AR, Butrico G, Fung A, Ruan H-B, Kursawe R, Caprio S, Thibodeau J, Bourgeois-Daigneault M-C, Sun L, Gao G, Bhanot S, Jurczak MJ, Green MR, Shulman GI, Wajapeyee N. MARCH1 regulates insulin sensitivity by controlling cell surface insulin receptor levels. Nat Commun. 2016;7:12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gavin JR, Roth J, Neville DM, de Meyts P, Buell DN. Insulin-dependent regulation of insulin receptor concentrations: a direct demonstration in cell culture. Proc Natl Acad Sci USA. 1974;71(1):84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou L, Zhang J, Fang Q, Liu M, Liu X, Jia W, Dong LQ, Liu F. Autophagy-mediated insulin receptor down-regulation contributes to endoplasmic reticulum stress-induced insulin resistance. Mol Pharmacol. 2009;76(3):596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Charlet BN, Savkur RS, Singh G, Philips AV, Grice EA, Cooper TA. Loss of the muscle-specific chloride channel in type 1 myotonic dystrophy due to misregulated alternative splicing. Mol Cell. 2002;10(1):45–53. [DOI] [PubMed] [Google Scholar]
- 74.Kosaki A, Nelson J, Webster NJ. Identification of intron and exon sequences involved in alternative splicing of insulin receptor pre-mRNA. J Biol Chem. 1998;273(17):10331–10337. [DOI] [PubMed] [Google Scholar]
- 75.Sen S, Talukdar I, Liu Y, Tam J, Reddy S, Webster NJG. Muscleblind-like 1 (Mbnl1) promotes insulin receptor exon 11 inclusion via binding to a downstream evolutionarily conserved intronic enhancer. J Biol Chem. 2010;285(33):25426–25437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Geuens T, Bouhy D, Timmerman V. The hnRNP family: insights into their role in health and disease. Hum Genet. 2016;135(8):851–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Talukdar I, Sen S, Urbano R, Thompson J, Yates JR, Webster NJG. hnRNP A1 and hnRNP F modulate the alternative splicing of exon 11 of the insulin receptor gene. PLoS One. 2011;6(11):e27869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Echeverria GV, Cooper TA. Muscleblind-like 1 activates insulin receptor exon 11 inclusion by enhancing U2AF65 binding and splicing of the upstream intron. Nucleic Acids Res. 2014;42(3):1893–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Paul S, Dansithong W, Kim D, Rossi J, Webster NJ, Comai L, Reddy S. Interaction of muscleblind, CUG-BP1 and hnRNP H proteins in DM1-associated aberrant IR splicing. EMBO J. 2006;25(18):4271–4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Boukis LA, Liu N, Furuyama S, Bruzik JP. Ser/Arg-rich protein-mediated communication between U1 and U2 small nuclear ribonucleoprotein particles. J Biol Chem. 2004;279(28):29647–29653. [DOI] [PubMed] [Google Scholar]
- 81.Lu C-C, Chen T-H, Wu J-R, Chen H-H, Yu H-Y, Tarn W- Y. Phylogenetic and Molecular. Characterization of the Splicing Factor RBM4. Gallouzi IE, ed. PLoS One. 2013;8(3):e59092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lin J-C, Yan Y-T, Hsieh W-K, Peng P-J, Su C-H, Tarn W-Y. RBM4 promotes pancreas cell differentiation and insulin expression. Mol Cell Biol. 2013;33(2):319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lin J-C, Tarn W-Y. Multiple roles of RBM4 in muscle cell differentiation. Front Biosci (Schol Ed). 2012;4:181–189. [DOI] [PubMed] [Google Scholar]
- 84.Jiang K, Patel NA, Watson JE, Apostolatos H, Kleiman E, Hanson O, Hagiwara M, Cooper DR. Akt2 regulation of Cdc2-like kinases (Clk/Sty), serine/arginine-rich (SR) protein phosphorylation, and insulin-induced alternative splicing of PKCbetaII messenger ribonucleic acid. Endocrinology. 2009;150(5):2087–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pihlajamäki J, Lerin C, Itkonen P, Boes T, Floss T, Schroeder J, Dearie F, Crunkhorn S, Burak F, Jimenez-Chillaron JC, Kuulasmaa T, Miettinen P, Park PJ, Nasser I, Zhao Z, Zhang Z, Xu Y, Wurst W, Ren H, Morris AJ, Stamm S, Goldfine AB, Laakso M, Patti ME. Expression of the splicing factor gene SFRS10 is reduced in human obesity and contributes to enhanced lipogenesis. Cell Metab. 2011;14(2):208–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kaminska D, Hämäläinen M, Cederberg H, Käkelä P, Venesmaa S, Miettinen P, Ilves I, Herzig K-H, Kolehmainen M, Karhunen L, Kuusisto J, Gylling H, Laakso M, Pihlajamäki J. Adipose tissue INSR splicing in humans associates with fasting insulin level and is regulated by weight loss. Diabetologia. 2014;57(2):347–351. [DOI] [PubMed] [Google Scholar]
- 87.Besic V, Shi H, Stubbs RS, Hayes MT. Aberrant liver insulin receptor isoform a expression normalises with remission of type 2 diabetes after gastric bypass surgery. Sanchez-Margalet V, ed. PLoS One. 2015;10(3):e0119270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huang Z, Bodkin NL, Ortmeyer HK, Zenilman ME, Webster NJ, Hansen BC, Shuldiner AR Altered insulin receptor messenger ribonucleic acid splicing in liver is associated with deterioration of glucose tolerance in the spontaneously obese and diabetic rhesus monkey: analysis of controversy between monkey and human studies. J Clin Endocrinol Metab. 1996;81(4):1552–1556. [DOI] [PubMed] [Google Scholar]
- 89.Malakar P, Chartarifsky L, Hija A, Leibowitz G, Glaser B, Dor Y, Karni R. Insulin receptor alternative splicing is regulated by insulin signaling and modulates beta cell survival. Sci Rep. 2016;6:31222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Leibiger B, Leibiger IB, Moede T, Kemper S, Kulkarni RN, Kahn CR, de Vargas LM, Berggren PO. Selective insulin signaling through A and B insulin receptors regulates transcription of insulin and glucokinase genes in pancreatic beta cells. Mol Cell. 2001;7(3):559–570. [DOI] [PubMed] [Google Scholar]
- 91.Kara I, Poggi M, Bonardo B, Govers R, Landrier J-F, Tian S, Leibiger I, Day R, Creemers JWM, Peiretti F. The paired basic amino acid-cleaving enzyme 4 (PACE4) is involved in the maturation of insulin receptor isoform B: an opportunity to reduce the specific insulin receptor-dependent effects of insulin-like growth factor 2 (IGF2). J Biol Chem. 2015;290(5):2812–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Roebroek AJM, Taylor NA, Louagie E, Pauli I, Smeijers L, Snellinx A, Lauwers A, Van de Ven WJM, Hartmann D, Creemers JWM. Limited Redundancy of the Proprotein Convertase Furin in Mouse Liver. J Biol Chem. 2004;279(51):53442–53450. [DOI] [PubMed] [Google Scholar]
- 93.Pandini G, Conte E, Medico E, Sciacca L, Vigneri R, Belfiore A. IGF-II binding to insulin receptor isoform A induces a partially different gene expression profile from insulin binding. Ann N Y Acad Sci. 2004;1028:450–456. [DOI] [PubMed] [Google Scholar]
- 94.Morrione A, Valentinis B, Xu SQ, Yumet G, Louvi A, Efstratiadis A, Baserga R. Insulin-like growth factor II stimulates cell proliferation through the insulin receptor. Proc Natl Acad Sci USA. 1997;94(8):3777–3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pfützner A, Kunt T, Hohberg C, Mondok A, Pahler S, Konrad T, Lübben G, Forst T. Fasting intact proinsulin is a highly specific predictor of insulin resistance in type 2 diabetes. Diabetes Care. 2004;27(3):682–687. [DOI] [PubMed] [Google Scholar]
- 96.Festa A, Williams K, Hanley AJ, Haffner SM. Beta-cell dysfunction in subjects with impaired glucose tolerance and early type 2 diabetes: comparison of surrogate markers with first-phase insulin secretion from an intravenous glucose tolerance test. Diabetes. 2008;57(6):1638–1644. [DOI] [PubMed] [Google Scholar]
- 97.Shields BM, Knight B, Hopper H, Hill A, Powell RJ, Hattersley AT, Clark PM. Measurement of cord insulin and insulin-related peptides suggests that girls are more insulin resistant than boys at birth. Diabetes Care. 2007;30(10):2661–2666. [DOI] [PubMed] [Google Scholar]
- 98.Martínez-Campos E, Hernández-SanMiguel E, López-Sánchez C, De Pablo F, Hernández-Sánchez C. Alternative splicing variants of proinsulin mRNA and the effects of excess proinsulin on cardiac morphogenesis. FEBS Lett. 2013;587(14):2272–2277. [DOI] [PubMed] [Google Scholar]
- 99.Bryhni B, Arnesen E, Jenssen TG. Associations of age with serum insulin, proinsulin and the proinsulin-to-insulin ratio: a cross-sectional study. BMC Endocr Disord. 2010;10:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nolan CJ, Delghingaro-Augusto V. Reversibility of Defects in Proinsulin Processing and Islet β-Cell Failure in Obesity-Related Type 2 Diabetes. Diabetes. 2016;65(2):352–354. [DOI] [PubMed] [Google Scholar]
- 101.Alssema M, Dekker JM, Nijpels G, Stehouwer CDA, Bouter LM, Heine RJ Hoorn Study. Proinsulin concentration is an independent predictor of all-cause and cardiovascular mortality: an 11-year follow-up of the Hoorn Study. Diabetes Care. 2005;28(4):860–865. [DOI] [PubMed] [Google Scholar]
- 102.Wiberg B, Sundstrom J, Zethelius B, Lind L. Insulin sensitivity measured by the euglycaemic insulin clamp and proinsulin levels as predictors of stroke in elderly men. Diabetologia. 2009;52(1):90–96. [DOI] [PubMed] [Google Scholar]
- 103.Galloway JA, Hooper SA, Spradlin CT, Howey DC, Frank BH, Bowsher RR, Anderson JH Biosynthetic human proinsulin. Review of chemistry, in vitro and in vivo receptor binding, animal and human pharmacology studies, and clinical trial experience. Diabetes Care. 1992;15(5):666–692. [DOI] [PubMed] [Google Scholar]
- 104.Rajaram S, Baylink DJ, Mohan S. Insulin-like growth factor-binding proteins in serum and other biological fluids: regulation and functions. Endocr Rev. 1997;18(6):801–831. [DOI] [PubMed] [Google Scholar]
- 105.Forbes BE, McCarthy P, Norton RS. Insulin-Like Growth Factor Binding Proteins: A Structural Perspective. Front Endocrinol (Lausanne). 2012;3:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Brugts MP, Ranke MB, Hofland LJ, van der Wansem K, Weber K, Frystyk J, Lamberts SWJ, Janssen JAMJL Normal Values of Circulating Insulin-Like Growth Factor-I Bioactivity in the Healthy Population: Comparison with Five Widely Used IGF-I Immunoassays. J Clin Endocrinol Metab. 2008;93(7):2539–2545. [DOI] [PubMed] [Google Scholar]
- 107.Holly JMP, Perks CM. Insulin-like growth factor physiology: what we have learned from human studies. Endocrinol Metab Clin North Am. 2012;41(2):249–263 (v.). [DOI] [PubMed] [Google Scholar]
- 108.Leung K-C, Doyle N, Ballesteros M, Waters MJ, Ho KKY. Insulin Regulation of Human Hepatic Growth Hormone Receptors: Divergent Effects on Biosynthesis and Surface Translocation 1. J Clin Endocrinol Metab. 2000;85(12):4712–4720. [DOI] [PubMed] [Google Scholar]
- 109.Frystyk J, Vestbo E, Skjaerbaek C, Mogensen CE, Orskov H. Free insulin-like growth factors in human obesity. Metabolism. 1995;44(10, Suppl 4):37–44. [DOI] [PubMed] [Google Scholar]
- 110.Guo S, Rena G, Cichy S, He X, Cohen P, Unterman T. Phosphorylation of serine 256 by protein kinase B disrupts transactivation by FKHR and mediates effects of insulin on insulin-like growth factor-binding protein-1 promoter activity through a conserved insulin response sequence. J Biol Chem. 1999;274(24):17184–17192. [DOI] [PubMed] [Google Scholar]
- 111.Suh H-S, Zhao M-L, Derico L, Choi N, Lee SC. Insulin-like growth factor 1 and 2 (IGF1, IGF2) expression in human microglia: differential regulation by inflammatory mediators. J Neuroinflammation. 2013;10(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sigismund S, Confalonieri S, Ciliberto A, Polo S, Scita G, Di Fiore PP. Endocytosis and Signaling Cell. Logistics Shape the Eukaryotic Cell Plan. Physiol Rev. 2012;92(1):273–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Irannejad R, Tsvetanova NG, Lobingier BT, von Zastrow M. Effects of endocytosis on receptor-mediated signaling. Curr Opin Cell Biol. 2015;35:137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Di Fiore PP, De Camilli P. Endocytosis and signaling. an inseparable partnership. Cell. 2001;106(1):1–4. [DOI] [PubMed] [Google Scholar]
- 115.Haglund K, Sigismund S, Polo S, Szymkiewicz I, Di Fiore PP, Dikic I. Multiple monoubiquitination of RTKs is sufficient for their endocytosis and degradation. Nat Cell Biol. 2003;5(5):461–466. [DOI] [PubMed] [Google Scholar]
- 116.Haglund K, Di Fiore PP, Dikic I. Distinct monoubiquitin signals in receptor endocytosis. Trends Biochem Sci. 2003;28(11):598–603. [DOI] [PubMed] [Google Scholar]
- 117.Mosesson Y, Shtiegman K, Katz M, Zwang Y, Vereb G, Szollosi J, Yarden Y. Endocytosis of Receptor Tyrosine Kinases Is Driven by Monoubiquitylation, Not Polyubiquitylation. J Biol Chem. 2003;278(24):21323–21326. [DOI] [PubMed] [Google Scholar]
- 118.Vecchione A, Marchese A, Henry P, Rotin D, Morrione A. The Grb10/Nedd4 complex regulates ligand-induced ubiquitination and stability of the insulin-like growth factor I receptor. Mol Cell Biol. 2003;23(9):3363–3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Monami G, Emiliozzi V, Morrione A. Grb10/Nedd4-mediated multiubiquitination of the insulin-like growth factor receptor regulates receptor internalization. J Cell Physiol. 2008;216(2):426–437. [DOI] [PubMed] [Google Scholar]
- 120.Morcavallo A, Stefanello M, Iozzo RV, Belfiore A, Morrione A. Ligand-Mediated Endocytosis and Trafficking of the Insulin-Like Growth Factor Receptor I and Insulin Receptor Modulate Receptor Function. Front Endocrinol (Lausanne). 2014;5:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.McClain DA. Mechanism and role of insulin receptor endocytosis. Am J Med Sci. 1992;304(3):192–201. [DOI] [PubMed] [Google Scholar]
- 122.Morcavallo A, Genua M, Palummo A, Kletvikova E, Jiracek J, Brzozowski AM, Iozzo RV, Belfiore A, Morrione A. Insulin and insulin-like growth factor II differentially regulate endocytic sorting and stability of the insulin receptor isoform A. J Biol Chem. 2012. doi:M111.252478 [pii]10.1074/jbc.M111.252478 [DOI] [PMC free article] [PubMed]
- 123.Sell C, Dumenil G, Deveaud C, Miura M, Coppola D, DeAngelis T, Rubin R, Efstratiadis A, Baserga R. Effect of a null mutation of the insulin-like growth factor I receptor gene on growth and transformation of mouse embryo fibroblasts. Mol Cell Biol. 1994;14(6):3604–3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Miura M, Surmacz E, Burgaud JL, Baserga R. Different effects on mitogenesis and transformation of a mutation at tyrosine 1251 of the insulin-like growth factor I receptor. J Biol Chem. 1995;270(38):22639–22644. [DOI] [PubMed] [Google Scholar]
- 125.Giudice J, Barcos LS, Guaimas FF, Penas-Steinhardt A, Giordano L, Jares-Erijman EA, Coluccio Leskow F. Insulin and insulin like growth factor II endocytosis and signaling via insulin receptor B. Cell Commun Signal. 2013;11(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Giudice J, Leskow FC, Arndt-Jovin DJ, Jovin TM, Jares-Erijman EA. Differential endocytosis and signaling dynamics of insulin receptor variants IR-A and IR-B. J Cell Sci. 2011;124(Pt 5):801–811. [DOI] [PubMed] [Google Scholar]
- 127.Giudice J, Jares-Erijman EA, Leskow F. Insulin receptor membrane retention by a traceable chimeric mutant. Cell Commun Signal. 2013;11(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Desbuquois B, Carré N, Burnol A-F. Regulation of insulin and type 1 insulin-like growth factor signaling and action by the Grb10/14 and SH2B1/B2 adaptor proteins. FEBS J. 2013;280(3):794–816. [DOI] [PubMed] [Google Scholar]
- 129.Morrione A. Grb10 adapter protein as regulator of insulin-like growth factor receptor signaling. J Cell Physiol. 2003;197(3):307–311. [DOI] [PubMed] [Google Scholar]
- 130.Liu F, Roth RA. Grb-IR: a SH2-domain-containing protein that binds to the insulin receptor and inhibits its function. Proc Natl Acad Sci USA. 1995;92(22):10287–10291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hansen H, Svensson U, Zhu J, Laviola L, Giorgino F, Wolf G, Smith RJ, Riedel H. Interaction between the Grb10 SH2 domain and the insulin receptor carboxyl terminus. J Biol Chem. 1996;271(15):8882–8886. [DOI] [PubMed] [Google Scholar]
- 132.Laviola L, Giorgino F, Chow JC, Baquero JA, Hansen H, Ooi J, Zhu J, Riedel H, Smith RJ. The adapter protein Grb10 associates preferentially with the insulin receptor as compared with the IGF-I receptor in mouse fibroblasts. J Clin Invest. 1997;99(5):830–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ramos FJ, Langlais PR, Hu D, Dong LQ, Liu F. Grb10 mediates insulin-stimulated degradation of the insulin receptor: a mechanism of negative regulation. Am J Physiol Endocrinol Metab. 2006;290(6):E1262–E1266. [DOI] [PubMed] [Google Scholar]
- 134.Yamaguchi Y, Flier JS, Yokota A, Benecke H, Backer JM, Moller DE. Functional properties of two naturally occurring isoforms of the human insulin receptor in Chinese hamster ovary cells. Endocrinology. 1991;129(4):2058–2066. [DOI] [PubMed] [Google Scholar]
- 135.Yamaguchi Y, Flier JS, Benecke H, Ransil BJ, Moller DE. Ligand-binding properties of the two isoforms of the human insulin receptor. Endocrinology. 1993;132(3):1132–1138. [DOI] [PubMed] [Google Scholar]
- 136.Vogt B, Carrascosa JM, Ermel B, Ullrich A, Haring HU. The two isotypes of the human insulin receptor (HIR-A and HIR-B) follow different internalization kinetics. Biochem Biophys Res Commun. 1991;177(3):1013–1018. [DOI] [PubMed] [Google Scholar]
- 137.Iozzo RV, Buraschi S, Genua M, Xu S-Q, Solomides CC, Peiper SC, Gomella LG, Owens RC, Morrione A. Decorin Antagonizes IGF Receptor I (IGF-IR) Function by Interfering with IGF-IR Activity and Attenuating Downstream Signaling. J Biol Chem. 2011;286(40):34712–34721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Morrione A, Neill T, Iozzo RV. Dichotomy of decorin activity on the insulin-like growth factor-I system. FEBS J. 2013;280(10):2138–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Choi E, Zhang X, Xing C, Yu H. Mitotic Checkpoint Regulators Control Insulin Signaling and Metabolic Homeostasis. Cell. 2016;166(3):567–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Soos MA, Siddle K. Immunological relationships between receptors for insulin and insulin-like growth factor I. Evidence for structural heterogeneity of insulin-like growth factor I receptors involving hybrids with insulin receptors. Biochem J. 1989;263(2):553–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bailyes EM, Nave BT, Soos MA, Orr SR, Hayward AC, Siddle K. Insulin receptor/IGF-I receptor hybrids are widely distributed in mammalian tissues: quantification of individual receptor species by selective immunoprecipitation and immunoblotting. Biochem J. 1997;327(Pt 1):209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chisalita SI, Johansson GS, Liefvendahl E, Back K, Arnqvist HJ. Human aortic smooth muscle cells are insulin resistant at the receptor level but sensitive to IGF1 and IGF2. J Mol Endocrinol. 2009;43(6):231–239. [DOI] [PubMed] [Google Scholar]
- 143.Engberding N, San Martin A, Martin-Garrido A, Koga M, Pounkova L, Lyons E, Lassegue B, Griendling KK. Insulin-Like Growth Factor-1 Receptor Expression Masks the Antiinflammatory and Glucose Uptake Capacity of Insulin in Vascular Smooth Muscle Cells. Arterioscler Thromb Vasc Biol. 2009;29(3):408–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Abbas A, Imrie H, Viswambharan H, Sukumar P, Rajwani A, Cubbon RM, Gage M, Smith J, Galloway S, Yuldeshava N, Kahn M, Xuan S, Grant PJ, Channon KM, Beech DJ, Wheatcroft SB, Kearney MT. The insulin-like growth factor-1 receptor is a negative regulator of nitric oxide bioavailability and insulin sensitivity in the endothelium. Diabetes. 2011;60(8):2169–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bäck K, Islam R, Johansson GS, Chisalita SI, Arnqvist HJ. Insulin and IGF1 receptors in human cardiac microvascular endothelial cells: metabolic, mitogenic and anti-inflammatory effects. J Endocrinol. 2012;215(1):89–96. [DOI] [PubMed] [Google Scholar]
- 146.Hunter RW, Hers I. Insulin/IGF-1 hybrid receptor expression on human platelets: consequences for the effect of insulin on platelet function. J Thromb Haemost. 2009;7(12):2123–2130. [DOI] [PubMed] [Google Scholar]
- 147.Bäck K, Brännmark C, Strålfors P, Arnqvist HJ. Differential effects of IGF-I, IGF-II and insulin in human preadipocytes and adipocytes–role of insulin and IGF-I receptors. Mol Cell Endocrinol. 2011;339(1–2):130–135. [DOI] [PubMed] [Google Scholar]
- 148.Lundby A, Bolvig P, Hegelund AC, Hansen BF, Worm J, Lützen A, Billestrup N, Bonnesen C, Oleksiewicz MB. Surface-expressed insulin receptors as well as IGF-I receptors both contribute to the mitogenic effects of human insulin and its analogues. J Appl Toxicol. 2015;35(7):842–850. [DOI] [PubMed] [Google Scholar]
- 149.Gómez-Hernández A, Escribano Ó Perdomo L, Otero YF, García-Gómez G, Fernández S, Beneit N, Benito M. Implication of insulin receptor A isoform and IRA/IGF-IR hybrid receptors in the aortic vascular smooth muscle cell proliferation: role of TNF-α and IGF-II. Endocrinology. 2013;154(7):2352–2364. [DOI] [PubMed] [Google Scholar]
- 150.Hashimoto R, Sakai K, Matsumoto H, Iwashita M. Tumor necrosis factor-alpha (TNF-alpha) inhibits insulin-like growth factor-I (IGF-I) activities in human trophoblast cell cultures through IGF-I/insulin hybrid receptors. Endocr J. 2010;57(3):193–200. [DOI] [PubMed] [Google Scholar]
- 151.Sprynski AC, Hose D, Kassambara A, Vincent L, Jourdan M, Rossi JF, Goldschmidt H, Klein B. Insulin is a potent myeloma cell growth factor through insulin/IGF-1 hybrid receptor activation. Leukemia. 2010;24(11):1940–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shushanov SS, Kravtsova TA. Cytotoxic effect of doxorubicin on human multiple myeloma cells in vitro. Bull Exp Biol Med. 2013;155(2):228–232. [DOI] [PubMed] [Google Scholar]
- 153.Zhang G, Li X, Zhang L, Zhao L, Jiang J, Wang J, Wei L. The expression and role of hybrid insulin/insulin-like growth factor receptor type 1 in endometrial carcinoma cells. Cancer Genet Cytogenet. 2010;200(2):140–148. [DOI] [PubMed] [Google Scholar]
- 154.Deng H, Lin Y, Badin M, Vasilcanu D, Strömberg T, Jernberg-Wiklund H, Sehat B, Larsson O. Over-accumulation of nuclear IGF-1 receptor in tumor cells requires elevated expression of the receptor and the SUMO-conjugating enzyme Ubc9. Biochem Biophys Res Commun. 2011;404(2):667–671. [DOI] [PubMed] [Google Scholar]
- 155.Fafalios A, Ma J, Tan X, Stoops J, Luo J, Defrances MC, Zarnegar R. A hepatocyte growth factor receptor (Met)-insulin receptor hybrid governs hepatic glucose metabolism. Nat Med. 2011;17(12):1577–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Di Renzo MF, Olivero M, Ferro S, Prat M, Bongarzone I, Pilotti S, Belfiore A, Costantino A, Vigneri R, Pierotti MA. Overexpression of the c-MET/HGF receptor gene in human thyroid carcinomas. Oncogene. 1992;7(12):2549–2553. [PubMed] [Google Scholar]
- 157.Lai AZ, Abella JV, Park M. Crosstalk in Met receptor oncogenesis. Trends Cell Biol. 2009;19(10):542–551. [DOI] [PubMed] [Google Scholar]
- 158.Rajpathak SN, Wassertheil-Smoller S, Crandall J, Liu S, Ho GYF. Hepatocyte Growth Factor and Clinical Diabetes in Postmenopausal Women. Diabetes Care. 2010;33(9):2013–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Rehman J, Considine RV, Bovenkerk JE, Li J, Slavens CA, Jones RM, March KL. Obesity is associated with increased levels of circulating hepatocyte growth factor. J Am Coll Cardiol. 2003;41(8):1408–1413. [DOI] [PubMed] [Google Scholar]
- 160.Hiratsuka A, Adachi H, Fujiura Y, Yamagishi S-I, Hirai Y, Enomoto M, Satoh A, Hino A, Furuki K, Imaizumi T. Strong Association between Serum Hepatocyte Growth Factor and Metabolic Syndrome. J Clin Endocrinol Metab. 2005;90(5):2927–2931. [DOI] [PubMed] [Google Scholar]
- 161.Araújo TG, Oliveira AG, Carvalho BM, Guadagnini D, Protzek AOP, Carvalheira JBC, Boschero AC, Saad MJA. Hepatocyte Growth Factor Plays a Key Role in Insulin Resistance-Associated Compensatory Mechanisms. Endocrinology. 2012;153(12):5760–5769. [DOI] [PubMed] [Google Scholar]
- 162.Tsukagawa E, Adachi H, Hirai Y, Enomoto M, Fukami A, Ogata K, Kasahara A, Yokoi K, Imaizumi T. Independent association of elevated serum hepatocyte growth factor levels with development of insulin resistance in a 10-year prospective study. Clin Endocrinol (Oxf). 2013;79(1):43–48. [DOI] [PubMed] [Google Scholar]
- 163.Lappano R, Maggiolini M. G protein-coupled receptors: novel targets for drug discovery in cancer. Nat Rev Drug Discov. 2011;10(1):47–60. [DOI] [PubMed] [Google Scholar]
- 164.Barton M, Prossnitz ER. Emerging roles of GPER in diabetes and atherosclerosis. Trends Endocrinol Metab. 2015;26(4):185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.De Marco P, Romeo E, Vivacqua A, Malaguarnera R, Abonante S, Romeo F, Pezzi V, Belfiore A, Maggiolini M. GPER1 is regulated by insulin in cancer cells and cancer-associated fibroblasts. Endocr Relat Cancer. 2014;21(5):739–753. [DOI] [PubMed] [Google Scholar]
- 166.De Marco P, Bartella V, Vivacqua A, Lappano R, Santolla MF, Morcavallo A, Pezzi V, Belfiore A, Maggiolini M. Insulin-like growth factor-I regulates GPER expression and function in cancer cells. Oncogene. 2013;32(6):678–688. [DOI] [PubMed] [Google Scholar]
- 167.De Marco P, Cirillo F, Vivacqua A, Malaguarnera R, Belfiore A, Maggiolini M. Novel Aspects Concerning the Functional Cross-Talk between the Insulin/IGF-I System and Estrogen Signaling in Cancer Cells. Front Endocrinol (Lausanne). 2015;6:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Pisano A, Santolla MF, De Francesco EM, De Marco P, Rigiracciolo DC, Perri MG, Vivacqua A, Abonante S, Cappello AR, Dolce V, Belfiore A, Maggiolini M, Lappano R. GPER, IGF-IR, and EGFR transduction signaling are involved in stimulatory effects of zinc in breast cancer cells and cancer-associated fibroblasts. Mol Carcinog. 2017;56(2):580–593. doi: 10.1002/mc.22518. [DOI] [PubMed] [Google Scholar]
- 169.Avino S, De Marco P, Cirillo F, Santolla MF, De Francesco EM, Perri MG, Rigiracciolo D, Dolce V, Belfiore A, Maggiolini M, Lappano R, Vivacqua A. Stimulatory actions of IGF-I are mediated by IGF-IR cross-talk with GPER and DDR1 in mesothelioma and lung cancer cells. Oncotarget. 2016;7(33):52710–52728. doi: 10.18632/oncotarget.10348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Morcavallo A, Buraschi S, Xu S-Q, Belfiore A, Schaefer L, Iozzo RV, Morrione A. Decorin differentially modulates the activity of insulin receptor isoform A ligands. Matrix Biol. 2014;35:82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Vella V, Malaguarnera R, Nicolosi M L, Palladino C, Spoleti C, Michele M, Vigneri P, Purrello M, Ragusa M, Morrione A BA. Discoidin domain receptor 1 modulates insulin receptor signaling and biological responses in breast cancer cells. Oncotarget. 2017;Advance pu(May 19):1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Malaguarnera R, Nicolosi ML, Sacco A, Morcavallo A, Vella V, Voci C, Spatuzza M, Xu S-Q, Iozzo RV, Vigneri R, Morrione A, Belfiore A. Novel cross talk between IGF-IR and DDR1 regulates IGF-IR trafficking, signaling and biological responses. Oncotarget. 2015;6(18):16084–16105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Matà R, Palladino C, Nicolosi ML, Lo Presti AR, Malaguarnera R, Ragusa M, Sciortino D, Morrione A, Maggiolini M, Vella V, Belfiore A. IGF-I induces upregulation of DDR1 collagen receptor in breast cancer cells by suppressing MIR-199a-5p through the PI3K/AKT pathway. Oncotarget. 2016;7(7):7683–7700. doi: 10.18632/oncotarget.6524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ikonen E, Heino S, Lusa S. Caveolins and membrane cholesterol. Biochem Soc Trans. 2004;32(Pt 1):121–123. [DOI] [PubMed] [Google Scholar]
- 175.Cao L, Yu Y, Darko I, Currier D, Mayeenuddin LH, Wan X, Khanna C, Helman LJ. Addiction to elevated insulin-like growth factor I receptor and initial modulation of the AKT pathway define the responsiveness of rhabdomyosarcoma to the targeting antibody. Cancer Res. 2008;68(19):8039–8048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Oh YS, Khil L-Y, Cho KA, Ryu SJ, Ha MK, Cheon GJ, Lee TS, Yoon J-W, Jun H-S, Park SC. A potential role for skeletal muscle caveolin-1 as an insulin sensitivity modulator in ageing-dependent non-obese type 2 diabetes: studies in a new mouse model. Diabetologia. 2008;51(6):1025–1034. [DOI] [PubMed] [Google Scholar]
- 177.Palacios-Ortega S, Varela-Guruceaga M, Milagro FI, Martínez JA, de Miguel C. Expression of Caveolin 1 is enhanced by DNA demethylation during adipocyte differentiation. status of insulin signaling. Sanchez-Margalet V, ed. PLoS One. 2014;9(4):e95100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gómez-Ruiz A, de Miguel C, Campión J, Martínez JA, Milagro FI. Time-dependent regulation of muscle caveolin activation and insulin signalling in response to high-fat diet. FEBS Lett. 2009;583(19):3259–3264. [DOI] [PubMed] [Google Scholar]
- 179.Kwon H, Lee J, Jeong K, Jang D, Pak Y. A novel actin cytoskeleton-dependent noncaveolar microdomain composed of homo-oligomeric caveolin-2 for activation of insulin signaling. Biochim Biophys Acta. 2013;1833(10):2176–2189. [DOI] [PubMed] [Google Scholar]
- 180.Kwon H, Lee J, Jeong K, Jang D, Pak Y. Fatty acylated caveolin-2 is a substrate of insulin receptor tyrosine kinase for insulin receptor substrate-1-directed signaling activation. Biochim Biophys Acta - Mol. Cell Res. 2015;1853(5):1022–1034. [DOI] [PubMed] [Google Scholar]
- 181.Kwon H, Pak Y. Prolonged tyrosine kinase activation of insulin receptor by pY27-caveolin-2. Biochem Biophys Res Commun. 2010;391(1):49–55. [DOI] [PubMed] [Google Scholar]
- 182.Lee S, Kwon H, Jeong K, Pak Y. Regulation of cancer cell proliferation by caveolin-2 down-regulation and re-expression. Int J Oncol. 2011;38(5):1395–1402. [DOI] [PubMed] [Google Scholar]
- 183.Kwon H, Jeong K, Hwang EM, Park J-Y, Pak Y. A novel domain of caveolin-2 that controls nuclear targeting: regulation of insulin-specific ERK activation and nuclear translocation by caveolin-2. J Cell Mol Med. 2011;15(4):888–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kabayama K, Sato T, Saito K, Loberto N, Prinetti A, Sonnino S, Kinjo M, Igarashi Y, Inokuchi J-i. Dissociation of the insulin receptor and caveolin-1 complex by ganglioside GM3 in the state of insulin resistance. Proc Natl Acad Sci USA. 2007;104(34):13678–13683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sekimoto J, Kabayama K, Gohara K, Inokuchi J. Dissociation of the insulin receptor from caveolae during TNFα-induced insulin resistance and its recovery by D-PDMP. FEBS Lett. 2012;586(2):191–195. [DOI] [PubMed] [Google Scholar]
- 186.Inokuchi J. Physiopathological function of hematoside (GM3 ganglioside). Proc Jpn Acad, Ser B, Phys Biol Sci. 2011;87(4):179–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lipina C, Hundal HS. Ganglioside GM3 as a gatekeeper of obesity-associated insulin resistance: Evidence and mechanisms. FEBS Lett. 2015;589(21):3221–3227. [DOI] [PubMed] [Google Scholar]
- 188.Wang X-Q, Lee S, Wilson H, Seeger M, Iordanov H, Gatla N, Whittington A, Bach D, Lu J, Paller AS. Ganglioside GM3 depletion reverses impaired wound healing in diabetic mice by activating IGF-1 and insulin receptors. J Invest Dermatol. 2014;134(5):1446–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Inokuchi J. GM3 and diabetes. Glycoconj J. 2014;31(3):193–197. [DOI] [PubMed] [Google Scholar]
- 190.Hakomori S-I, Handa K. GM3 and cancer. Glycoconj J. 2015;32(1–2):1–8. [DOI] [PubMed] [Google Scholar]
- 191.Ussar S, Bezy O, Blüher M, Kahn CR. Glypican-4 enhances insulin signaling via interaction with the insulin receptor and serves as a novel adipokine. Diabetes. 2012;61(9):2289–2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Huang Z, Bodkin NL, Ortmeyer HK, Hansen BC, Shuldiner AR Hyperinsulinemia is associated with altered insulin receptor mRNA splicing in muscle of the spontaneously obese diabetic rhesus monkey. J Clin Invest. 1994;94(3):1289–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Schlessinger J, Lemmon MA. Nuclear signaling by receptor tyrosine kinases: the first robin of spring. Cell. 2006;127(1):45–48. [DOI] [PubMed] [Google Scholar]
- 194.Sarfstein R, Werner H. Minireview: nuclear insulin and insulin-like growth factor-1 receptors: a novel paradigm in signal transduction. Endocrinology. 2013;154(5):1672–1679. [DOI] [PubMed] [Google Scholar]
- 195.Goldfine ID, Smith GJ. Binding of insulin to isolated nuclei. Proc Natl Acad Sci USA. 1976;73(5):1427–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Purrello F, Vigneri R, Clawson GA, Goldfine ID Insulin stimulation of nucleoside triphosphatase activity in isolated nuclear envelopes. Science. 1982;216(4549):1005–1007. [DOI] [PubMed] [Google Scholar]
- 197.Goldfine ID, Smith GJ, Wong KY, Jones AL Cellular uptake and nuclear binding of insulin in human cultured lymphocytes: evidence for potential intracellular sites of insulin action. Proc Natl Acad Sci USA. 1977;74(4):1368–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Horvat A. Insulin binding sites on rat liver nuclear membranes: biochemical and immunofluorescent studies. J Cell Physiol. 1978;97(1):37–47. [DOI] [PubMed] [Google Scholar]
- 199.Bergeron JJ, Posner BI, Josefsberg Z, Sikstrom R. Intracellular polypeptide hormone receptors. The demonstration of specific binding sites for insulin and human growth hormone in Golgi fractions isolated from the liver of female rats. J Biol Chem. 1978;253(11):4058–4066. [PubMed] [Google Scholar]
- 200.Vigneri R, Goldfine ID, Wong KY, Smith GJ, Pezzino V The nuclear envelope. The major site of insulin binding in rat liver nuclei. J Biol Chem. 1978;253(7):2098–2103. [PubMed] [Google Scholar]
- 201.Podlecki DA, Smith RM, Kao M, Tsai P, Huecksteadt T, Brandenburg D, Lasher RS, Jarett L, Olefsky JM. Nuclear translocation of the insulin receptor. A possible mediator of insulin’s long term effects. J Biol Chem. 1987;262(7):3362–3368. [PubMed] [Google Scholar]
- 202.Gletsu NA, Field CJ, Clandinin MT. Obese mice have higher insulin receptor levels in the hepatocyte cell nucleus following insulin stimulation in vivo with an oral glucose meal. Biochim Biophys Acta. 1999;1454(3):251–260. [DOI] [PubMed] [Google Scholar]
- 203.Nelson JD, LeBoeuf RC, Bomsztyk K. Direct recruitment of insulin receptor and ERK signaling cascade to insulin-inducible gene loci. Diabetes. 2011;60(1):127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Amaya MJ, Oliveira AG, Guimarães ES, Casteluber MCF, Carvalho SM, Andrade LM, Pinto MCX, Mennone A, Oliveira CA, Resende RR, Menezes GB, Nathanson MH, Leite MF. The insulin receptor translocates to the nucleus to regulate cell proliferation in liver. Hepatology. 2014;59(1):274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Sciacca L, Prisco M, Wu A, Belfiore A, Vigneri R, Baserga R. Signaling differences from the A and B isoforms of the insulin receptor (IR) in 32D cells in the presence or absence of IR substrate-1. Endocrinology. 2003;144(6):2650–2658. [DOI] [PubMed] [Google Scholar]
- 206.Wu A, Sciacca L, Baserga R. Nuclear translocation of insulin receptor substrate-1 by the insulin receptor in mouse embryo fibroblasts. J Cell Physiol. 2003;195(3):453–460. [DOI] [PubMed] [Google Scholar]
- 207.Boucher J, Macotela Y, Bezy O, Mori MA, Kriauciunas K, Kahn CR. A kinase-independent role for unoccupied insulin and IGF-1 receptors in the control of apoptosis. Sci Signal. 2010;3(151):ra87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson MA, Kahn CR. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell. 2000;6(1):87–97. [PubMed] [Google Scholar]
- 209.Nevado C, Benito M, Valverde AM. Role of insulin receptor and balance in insulin receptor isoforms A and B in regulation of apoptosis in simian virus 40-immortalized neonatal hepatocytes. Mol Biol Cell. 2008;19(3):1185–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Nevado C, Valverde AM, Benito M. Role of insulin receptor in the regulation of glucose uptake in neonatal hepatocytes. Endocrinology. 2006. Available at: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16644916. [DOI] [PubMed]
- 211.Malaguarnera R, Belfiore A. The emerging role of insulin and insulin-like growth factor signaling in cancer stem cells. Front Endocrinol (Lausanne). 2014;5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Diaz-Castroverde S, Baos S, Luque M, Di Scala M, González-Aseguinolaza G, Gómez-Hernández A, Beneit N, Escribano O, Benito M. Prevalent role of the insulin receptor isoform A in the regulation of hepatic glycogen metabolism in hepatocytes and in mice. Diabetologia. 2016;59(12):2702–2710. [DOI] [PubMed] [Google Scholar]
- 213.Brierley GV, Macaulay SL, Forbes BE, Wallace JC, Cosgrove LJ, Macaulay VM. Silencing of the insulin receptor isoform A favors formation of type 1 insulin-like growth factor receptor (IGF-IR) homodimers and enhances ligand-induced IGF-IR activation and viability of human colon carcinoma cells. Endocrinology. 2010;151(4):1418–1427. [DOI] [PubMed] [Google Scholar]
- 214.Bäck K, Arnqvist HJ. Changes in insulin and IGF-I receptor expression during differentiation of human preadipocytes. Growth Horm IGF Res. 2009;19(2):101–111. [DOI] [PubMed] [Google Scholar]
- 215.Serrano R, Villar M, Martinez C, Carrascosa JM, Gallardo N, Andres A. Differential gene expression of insulin receptor isoforms A and B and insulin receptor substrates 1, 2 and 3 in rat tissues: modulation by aging and differentiation in rat adipose tissue. J Mol Endocrinol. 2005;34(1):153–161. [DOI] [PubMed] [Google Scholar]
- 216.Avnet S, Perut F, Salerno M, Sciacca L, Baldini N. Insulin receptor isoforms are differently expressed during human osteoblastogenesis. Differentiation. 2012;83(5):242–248. [DOI] [PubMed] [Google Scholar]
- 217.Berlato C, Doppler W. Selective response to insulin versus insulin-like growth factor-I and -II and up-regulation of insulin receptor splice variant B in the differentiated mouse mammary epithelium. Endocrinology. 2009;150(6):2924–2933. [DOI] [PubMed] [Google Scholar]
- 218.Rowzee AM, Ludwig DL, Wood TL. Insulin-like growth factor type 1 receptor and insulin receptor isoform expression and signaling in mammary epithelial cells. Endocrinology. 2009;150(8):3611–3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Andres SF, Simmons JG, Mah AT, Santoro MA, Van Landeghem L, Lund PK. Insulin receptor isoform switching in intestinal stem cells, progenitors, differentiated lineages and tumors: evidence that IR-B limits proliferation. J Cell Sci. 2013;126(Pt 24):5645–5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Flannery CA, Saleh FL, Choe GH, Selen DJ, Kodaman PH, Kliman HJ, Wood TL, Taylor HS. Differential Expression of IR-A, IR-B and IGF-1R in Endometrial Physiology and Distinct Signature in Adenocarcinoma. J Clin Endocrinol Metab. 2016;101(7):2883–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Ziegler AN, Schneider JS, Qin M, Tyler WA, Pintar JE, Fraidenraich D, Wood TL, Levison SW. IGF-II promotes stemness of neural restricted precursors. Stem Cells. 2012;30(6):1265–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Westermeier F, Salomón C, González M, Puebla C, Guzmán-Gutiérrez E, Cifuentes F, Leiva A, Casanello P, Sobrevia L. Insulin restores gestational diabetes mellitus-reduced adenosine transport involving differential expression of insulin receptor isoforms in human umbilical vein endothelium. Diabetes. 2011;60(6):1677–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Malaguarnera R, Frasca F, Garozzo A, Giani F, Pandini G, Vella V, Vigneri R, Belfiore A. Insulin receptor isoforms and insulin-like growth factor receptor in human follicular cell precursors from papillary thyroid cancer and normal thyroid. J Clin Endocrinol Metab. 2011;96(3):766–774. [DOI] [PubMed] [Google Scholar]
- 224.Boucher J, Mori MA, Lee KY, Smyth G, Liew CW, Macotela Y, Rourk M, Bluher M, Russell SJ, Kahn CR. Impaired thermogenesis and adipose tissue development in mice with fat-specific disruption of insulin and IGF-1 signalling. Nat Commun. 2012;3:902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Sakaguchi M, Fujisaka S, Cai W, Winnay JN, Konishi M, O’Neill BT, Li M, García-Martín R, Takahashi H, Hu J, Kulkarni RN, Kahn CR. Adipocyte Dynamics and Metabolic Reversible. Syndrome in Mice with an Inducible Adipocyte-Specific Deletion of the Insulin Receptor. Cell Metab. 2017;25(2):448–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Lin J-C. Impacts of Alternative Splicing Events on the Differentiation of Adipocytes. Int J Mol Sci. 2015;16(9):22169–22189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Neville MC, Webb P, Ramanathan P, Mannino MP, Pecorini C, Monks J, Anderson SM, MacLean P. The insulin receptor plays an important role in secretory differentiation in the mammary gland. Am J Physiol Endocrinol Metab. 2013;305(9):E1103–E1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Xia P, Wang S, Du Y, Huang G, Satoh T, Akira S, Fan Z. Insulin-InsR signaling drives multipotent progenitor differentiation toward lymphoid lineages. J Exp Med. 2015;212(13):2305–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Iovino S, Burkart AM, Kriauciunas K, Warren L, Hughes KJ, Molla M, Lee Y-K, Patti M-E, Kahn CR. Genetic insulin resistance is a potent regulator of gene expression and proliferation in human iPS cells. Diabetes. 2014;63(12):4130–4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Youssef A, Han VKM. Low Oxygen Tension Modulates the Insulin-Like Growth Factor-1 or -2 Signaling via Both Insulin-Like Growth Factor-1 Receptor and Insulin Receptor to Maintain Stem Cell Identity in Placental Mesenchymal Stem Cells. Endocrinology. 2016;157(3):1163–1174. [DOI] [PubMed] [Google Scholar]
- 231.Taguchi A, White MF. Insulin-like signaling, nutrient homeostasis, and life span. Annu Rev Physiol. 2008;70:191–212. [DOI] [PubMed] [Google Scholar]
- 232.Ausk KJ, Boyko EJ, Ioannou GN. Insulin Resistance Predicts Mortality in Nondiabetic Individuals in the U.S. Diabetes Care. 2010;33(6):1179–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Vitale G, Barbieri M, Kamenetskaya M, Paolisso G. GH/IGF-I/insulin system in centenarians. Mech Ageing Dev. 2017;165(Pt B):107–114. doi: 10.1016/j.mad.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 234.Suh Y, Atzmon G, Cho MO, Hwang D, Liu B, Leahy DJ, Barzilai N, Cohen P. Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proc Natl Acad Sci USA. 2008;105(9):3438–3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8(12):915–928. [DOI] [PubMed] [Google Scholar]
- 236.Aguirre GA, De Ita JR, de la Garza RG, Castilla-Cortazar I. Insulin-like growth factor-1 deficiency and metabolic syndrome. J Transl Med. 2016;14:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Sierra Rojas JX, García-San Frutos M, Horrillo D, Lauzurica N, Oliveros E, Carrascosa JM, Fernández-Agulló T, Ros M. Differential Development of Inflammation and Insulin Resistance in Different Adipose Tissue Depots Along Aging in Wistar Rats: Effects of Caloric Restriction. J Gerontol A Biol Sci Med Sci. 2016;71(3):310–322. [DOI] [PubMed] [Google Scholar]
- 238.Amessou M, Tahiri K, Chauvet G, Desbuquois B. Age-related changes in insulin receptor mRNA and protein expression in genetically obese Zucker rats. Diabetes Metab. 2010;36(2):120–128. [DOI] [PubMed] [Google Scholar]
- 239.Holly AC, Melzer D, Pilling LC, Fellows AC, Tanaka T, Ferrucci L, Harries LW. Changes in splicing factor expression are associated with advancing age in man. Mech Ageing Dev. 2013;134(9):356–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Escribano O, Guillén C, Nevado C, Gómez-Hernández A, Kahn CR, Benito M. Beta-Cell hyperplasia induced by hepatic insulin resistance: role of a liver-pancreas endocrine axis through insulin receptor A isoform. Diabetes. 2009;58(4):820–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Escribano O, Gómez-Hernández A, Díaz-Castroverde S, Nevado C, García G, Otero YF, Perdomo L, Beneit N, Benito M. Insulin receptor isoform A confers a higher proliferative capability to pancreatic beta cells enabling glucose availability and IGF-I signaling. Mol Cell Endocrinol. 2015;409:82–91. [DOI] [PubMed] [Google Scholar]
- 242.El Ouaamari A, Dirice E, Gedeon N, Hu J, Zhou J-Y, Shirakawa J, Hou L, Goodman J, Karampelias C, Qiang G, Boucher J, Martinez R, Gritsenko MA, De Jesus DF, Kahraman S, Bhatt S, Smith RD, Beer H-D, Jungtrakoon P, Gong Y, Goldfine AB, Liew CW, Doria A, Andersson O, Qian W-J, Remold-O’Donnell E, Kulkarni RN. SerpinB1 Promotes Pancreatic β Cell Proliferation. Cell Metab. 2016;23(1):194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Kosaki A, Pillay TS, Xu L, Webster NJ. The B isoform of the insulin receptor signals more efficiently than the A isoform in HepG2 cells. J Biol Chem. 1995;270(35):20816–20823. [DOI] [PubMed] [Google Scholar]
- 244.Vienberg SG, Bouman SD, Sørensen H, Stidsen CE, Kjeldsen T, Glendorf T, Sørensen AR, Olsen GS, Andersen B, Nishimura E. Receptor-isoform-selective insulin analogues give tissue-preferential effects. Biochem J. 2011;440(3):301–308. [DOI] [PubMed] [Google Scholar]
- 245.Seino S, Seino M, Nishi S, Bell GI. Structure of the human insulin receptor gene and characterization of its promoter. Proc Natl Acad Sci USA. 1989;86(1):114–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Biddinger SB, Hernandez-Ono A, Rask-Madsen C, Haas JT, Alemán JO, Suzuki R, Scapa EF, Agarwal C, Carey MC, Stephanopoulos G, Cohen DE, King GL, Ginsberg HN, Kahn CR. Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell Metab. 2008;7(2):125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Titchenell PM, Chu Q, Monks BR, Birnbaum MJ Hepatic insulin signalling is dispensable for suppression of glucose output by insulin in vivo. Nat Commun. 2015;6:7078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Diaz-Castroverde S, Gómez-Hernández A, Fernández S, García-Gómez G, Di Scala M, González-Aseguinolaza G, Fernández-Millán E, González-Rodríguez Á García-Bravo M, Chambon P, Álvarez C, Perdomo L, Beneit N, Escribano O, Benito M. Insulin receptor isoform A ameliorates long-term glucose intolerance in diabetic mice. Dis Model Mech. 2016;9(11):1271–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Mosthaf L, Grako K, Dull TJ, Coussens L, Ullrich A, McClain DA. Functionally distinct insulin receptors generated by tissue-specific alternative splicing. EMBO J. 1990;9(8):2409–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Brüning JC, Michael MD, Winnay JN, Hayashi T, Hörsch D, Accili D, Goodyear LJ, Kahn CR. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol Cell. 1998;2(5):559–569. [DOI] [PubMed] [Google Scholar]
- 251.Kim JK, Michael MD, Previs SF, Peroni OD, Mauvais-Jarvis F, Neschen S, Kahn BB, Kahn CR, Shulman GI. Redistribution of substrates to adipose tissue promotes obesity in mice with selective insulin resistance in muscle. J Clin Invest. 2000;105(12):1791–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Baudry A, Lamothe B, Bucchini D, Jami J, Montarras D, Pinset C, Joshi RL. IGF-1 receptor as an alternative receptor for metabolic signaling in insulin receptor-deficient muscle cells. FEBS Lett. 2001;488(3):174–178. [DOI] [PubMed] [Google Scholar]
- 253.O’Neill BT, Lauritzen HPMM, Hirshman MF, Smyth G, Goodyear LJ, Kahn CR. Differential Role of Insulin/IGF-1 Receptor Signaling in Muscle Growth and Glucose Homeostasis. Cell Reports. 2015;11(8):1220–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Federici M, Porzio O, Lauro D, Borboni P, Giovannone B, Zucaro L, Hribal ML, Sesti G. Increased Abundance of Insulin/Insulin-Like Growth Factor-I Hybrid Receptors in Skeletal Muscle of Obese Subjects Is Correlated with In Vivo Insulin Sensitivity 1. J Clin Endocrinol Metab. 1998;83(8):2911–2915. [DOI] [PubMed] [Google Scholar]
- 255.Yakar S, Kim H, Zhao H, Toyoshima Y, Pennisi P, Gavrilova O, Leroith D. The growth hormone-insulin like growth factor axis revisited: lessons from IGF-1 and IGF-1 receptor gene targeting. Pediatr Nephrol. 2005;20(3):251–254. [DOI] [PubMed] [Google Scholar]
- 256.Lee WH, Javedan S, Bondy CA. Coordinate expression of insulin-like growth factor system components by neurons and neuroglia during retinal and cerebellar development. J Neurosci. 1992;12(12):4737–4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Dridi L, Seyrantepe V, Fougerat A, Pan X, Bonneil E, Thibault P, Moreau A, Mitchell GA, Heveker N, Cairo CW, Issad T, Hinek A, Pshezhetsky AV. Positive Regulation of Insulin Signaling by Neuraminidase 1. Diabetes. 2013;62(7):2338–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Alghamdi F, Guo M, Abdulkhalek S, Crawford N, Amith SR, Szewczuk MR. A novel insulin receptor-signaling platform and its link to insulin resistance and type 2 diabetes. Cell Signal. 2014;26(6):1355–1368. [DOI] [PubMed] [Google Scholar]
- 259.Joost HG. Structural and functional heterogeneity of insulin receptors. Cell Signal. 1995;7(2):85–91. [DOI] [PubMed] [Google Scholar]
- 260.De Meyts P. Insulin/receptor binding: The last piece of the puzzle? BioEssays. 2015;37(4):389–397. [DOI] [PubMed] [Google Scholar]
- 261.Banks WA, Owen JB, Erickson MA. Insulin in the brain: There and back again. Pharmacol Ther. 2012;136(1):82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.García-Cáceres C, Quarta C, Varela L, Gao Y, Gruber T, Legutko B, Jastroch M, Johansson P, Ninkovic J, Yi C-X, Le Thuc O, Szigeti-Buck K, Cai W, Meyer CW, Pfluger PT, Fernandez AM, Luquet S, Woods SC, Torres-Alemán I, Kahn CR, Götz M, Horvath TL, Tschöp MH. Astrocytic Insulin Signaling Couples Brain Glucose Uptake with Nutrient Availability. Cell. 2016;166(4):867–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Garwood CJ, Ratcliffe LE, Morgan SV, Simpson JE, Owens H, Vazquez-Villaseñor I, Heath PR, Romero IA, Ince PG, Wharton SB. Insulin and IGF1 signalling pathways in human astrocytes in vitro and in vivo; characterisation, subcellular localisation and modulation of the receptors. Mol Brain. 2015;8(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Heni M, Hennige AM, Peter A, Siegel-Axel D, Ordelheide A-M, Krebs N, Machicao F, Fritsche A, Häring H-U, Staiger H. Insulin Promotes Glycogen Storage and Cell Proliferation in Primary Human Astrocytes. Maedler K, ed. PLoS One. 2011;6(6):e21594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Gerozissis K. Brain insulin and feeding: a bi-directional communication. Eur J Pharmacol. 2004;490(1–3):59–70. [DOI] [PubMed] [Google Scholar]
- 266.Areias MFC, Prada PO. Mechanisms of insulin resistance in the amygdala: influences on food intake. Behav Brain Res. 2015;282:209–217. [DOI] [PubMed] [Google Scholar]
- 267.Brüning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Müller-Wieland D, Kahn CR. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289(5487):2122–2125. [DOI] [PubMed] [Google Scholar]
- 268.Sliwowska JH, Fergani C, Gawałek M, Skowronska B, Fichna P, Lehman MN. Insulin: Its role in the central control of reproduction. Physiol Behav. 2014;133:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Kullmann S, Heni M, Hallschmid M, Fritsche A, Preissl H, Häring H-U. Brain Insulin Resistance at the Crossroads of Metabolic and Cognitive Disorders in Humans. Physiol Rev. 2016;96(4):1169–1209. [DOI] [PubMed] [Google Scholar]
- 270.Strachan MWJ. Insulin and cognitive function in humans: experimental data and therapeutic considerations. Biochem Soc Trans. 2005;33(5):1037. [DOI] [PubMed] [Google Scholar]
- 271.Shemesh E, Rudich A, Harman-Boehm I, Cukierman-Yaffe T. Effect of Intranasal Insulin on Cognitive Function: A Systematic Review. J Clin Endocrinol Metab. 2012;97(2):366–376. [DOI] [PubMed] [Google Scholar]
- 272.Russo VC, Gluckman PD, Feldman EL, Werther GA. The Insulin-Like Growth Factor System and Its Pleiotropic Functions in Brain. Endocr Rev. 2005;26(7):916–943. [DOI] [PubMed] [Google Scholar]
- 273.Trejo J, Piriz J, Llorens-Martin MV, Fernandez AM, Bolós M, LeRoith D, Nuñez A, Torres-Aleman I. Central actions of liver-derived insulin-like growth factor I underlying its pro-cognitive effects. Mol Psychiatry. 2007;12(12):1118–1128. [DOI] [PubMed] [Google Scholar]
- 274.Hernandez-Garzón E, Fernandez AM, Perez-Alvarez A, Genis L, Bascuñana P, Fernandez de la Rosa R, Delgado M, Angel Pozo M, Moreno E, McCormick PJ, Santi A, Trueba-Saiz A, Garcia-Caceres C, Tschöp MH, Araque A, Martin ED, Torres Aleman I. The insulin-like growth factor I receptor regulates glucose transport by astrocytes. Glia. 2016;64(11):1962–1971. [DOI] [PubMed] [Google Scholar]
- 275.Ye X, Kohtz A, Pollonini G, Riccio A, Alberini CM. Insulin Like Growth Factor 2 Expression in the Rat Brain Both in Basal Condition and following Learning Predominantly Derives from the Maternal Allele. Feil R, ed. PLoS One. 2015;10(10):e0141078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Alberini CM, Chen DY. Memory enhancement: consolidation, reconsolidation and insulin-like growth factor 2. Trends Neurosci. 2012;35(5):274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Pascual-Lucas M, Viana da Silva S, Di Scala M, Garcia-Barroso C, Gonzalez-Aseguinolaza G, Mulle C, Alberini CM, Cuadrado-Tejedor M, Garcia-Osta A. Insulin-like growth factor 2 reverses memory and synaptic deficits in APP transgenic mice. EMBO Mol Med. 2014;6(10):1246–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75(1):73–82. [PubMed] [Google Scholar]
- 279.Tamemoto H, Kadowaki T, Tobe K, Yagi T, Sakura H, Hayakawa T, Terauchi Y, Ueki K, Kaburagi Y, Satoh S, et al. Insulin resistance and growth retardation in mice lacking insulin receptor substrate-1. Nature. 1994;372(6502):182–186. [DOI] [PubMed] [Google Scholar]
- 280.Popken GJ, Hodge RD, Ye P, Zhang J, Ng W, O’Kusky JR, D’Ercole AJ. In vivo effects of insulin-like growth factor-I (IGF-I) on prenatal and early postnatal development of the central nervous system. Eur J Neurosci. 2004;19(8):2056–2068. [DOI] [PubMed] [Google Scholar]
- 281.Sun FL, Dean WL, Kelsey G, Allen ND, Reik W. Transactivation of Igf2 in a mouse model of Beckwith-Wiedemann syndrome. Nature. 1997;389(6653):809–815. [DOI] [PubMed] [Google Scholar]
- 282.Freude S, Leeser U, Müller M, Hettich MM, Udelhoven M, Schilbach K, Tobe K, Kadowaki T, Köhler C, Schröder H, Krone W, Brüning JC, Schubert M. IRS-2 branch of IGF-1 receptor signaling is essential for appropriate timing of myelination. J Neurochem. 2008;107(4):907–917. [DOI] [PubMed] [Google Scholar]
- 283.Yarchoan M, Toledo JB, Lee EB, Arvanitakis Z, Kazi H, Han L-Y, Louneva N, Lee VM-Y, Kim SF, Trojanowski JQ, Arnold SE. Abnormal serine phosphorylation of insulin receptor substrate 1 is associated with tau pathology in Alzheimer’s disease and tauopathies. Acta Neuropathol. 2014;128(5):679–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Scotlandi K, Belfiore A. Targeting the Insulin-Like Growth Factor (IGF) System Is Not as Simple as Just Targeting the Type 1 IGF Receptor. Am Soc Clin Oncol Educ Book. 2012:599–604. [DOI] [PubMed]
- 285.Jiang W, Jin Z, Zhou F, Cui J, Wang L, Wang L. High co-expression of Sp1 and HER-2 is correlated with poor prognosis of gastric cancer patients. Surg Oncol. 2015;24(3):220–225. [DOI] [PubMed] [Google Scholar]
- 286.Wang J, Kang M, Qin Y-T, Wei Z-X, Xiao J-J, Wang R-S. Sp1 is over-expressed in nasopharyngeal cancer and is a poor prognostic indicator for patients receiving radiotherapy. Int J Clin Exp Pathol. 2015;8(6):6936–6943. [PMC free article] [PubMed] [Google Scholar]
- 287.Sumter TF, Xian L, Huso T, Koo M, Chang Y-T, Almasri TN, Chia L, Inglis C, Reid D, Resar LMS. The High Mobility Group A1 (HMGA1) Transcriptome in Cancer and Development. Curr Mol Med. 2016;16(4):353–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Oren M. p53: the ultimate tumor suppressor gene? FASEB J. 1992;6(13):3169–3176. [DOI] [PubMed] [Google Scholar]
- 289.Mantovani F, Walerych D, Del Sal G. Targeting mutant p53 in cancer: a long road to precision therapy. FEBS J. 2017;284(6):837–850. doi: 10.1111/febs.13948. [DOI] [PubMed] [Google Scholar]
- 290.Charni M, Aloni-Grinstein R, Molchadsky A, Rotter V. p53 on the crossroad between regeneration and cancer. Cell Death Differ. 2017;24(1):8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Lovat F, Fassan M, Gasparini P, Rizzotto L, Cascione L, Pizzi M, Vicentini C, Balatti V, Palmieri D, Costinean S, Croce CM. miR-15b/16-2 deletion promotes B-cell malignancies. Proc Natl Acad Sci USA. 2015;112(37):11636–11641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Flavin RJ, Smyth PC, Laios A, O’Toole SA, Barrett C, Finn SP, Russell S, Ring M, Denning KM, Li J, Aherne ST, Sammarae DA, Aziz NA, Alhadi A, Sheppard BL, Lao K, Sheils OM, O’Leary JJ. Potentially important microRNA cluster on chromosome 17p13.1 in primary peritoneal carcinoma. Mod Pathol. 2009;22(2):197–205. [DOI] [PubMed] [Google Scholar]
- 293.Furuta M, Kozaki K, Tanimoto K, Tanaka S, Arii S, Shimamura T, Niida A, Miyano S, Inazawa J. The tumor-suppressive miR-497-195 cluster targets multiple cell-cycle regulators in hepatocellular carcinoma. Deb S, ed. PLoS One. 2013;8(3):e60155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Creevey L, Ryan J, Harvey H, Bray IM, Meehan M, Khan AR, Stallings RL MicroRNA-497 increases apoptosis in MYCN amplified neuroblastoma cells by targeting the key cell cycle regulator WEE1. Mol Cancer. 2013;12(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Guo ST, Jiang CC, Wang GP, Li YP, Wang CY, Guo XY, Yang RH, Feng Y, Wang FH, Tseng H-Y, Thorne RF, Jin L, Zhang XD. MicroRNA-497 targets insulin-like growth factor 1 receptor and has a tumour suppressive role in human colorectal cancer. Oncogene. 2013;32(15):1910–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Li D, Zhao Y, Liu C, Chen X, Qi Y, Jiang Y, Zou C, Zhang X, Liu S, Wang X, Zhao D, Sun Q, Zeng Z, Dress A, Lin MC, Kung H-F, Rui H, Liu L-Z, Mao F, Jiang B-H, Lai L. Analysis of MiR-195 and MiR-497 Expression, Regulation and Role in Breast Cancer. Clin Cancer Res. 2011;17(7):1722–1730. [DOI] [PubMed] [Google Scholar]
- 297.Wang X, Wang Y, Lan H, Li J. MiR-195 inhibits the growth and metastasis of NSCLC cells by targeting IGF1R. Tumour Biol. 2014;35(9):8765–8770. [DOI] [PubMed] [Google Scholar]
- 298.Johnson SM, Grosshans H, Shingara J, Byrom M, Jarvis R, Cheng A, Labourier E, Reinert KL, Brown D, Slack FJ. RAS Is Regulated by the let-7 MicroRNA Family. Cell. 2005;120(5):635–647. [DOI] [PubMed] [Google Scholar]
- 299.Lee YS, Dutta A. The tumor suppressor microRNA let-7 represses the HMGA2 oncogene. Genes Dev. 2007;21(9):1025–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Wang H, Zhao Q, Deng K, Guo X, Xia J. Lin28: an emerging important oncogene connecting several aspects of cancer. Tumour Biol. 2016;37(3):2841–2848. [DOI] [PubMed] [Google Scholar]
- 301.Zhang Y, Qu X, Li C, Fan Y, Che X, Wang X, Cai Y, Hu X, Liu Y. miR-103/107 modulates multidrug resistance in human gastric carcinoma by downregulating Cav-1. Tumour Biol. 2015;36(4):2277–2285. [DOI] [PubMed] [Google Scholar]
- 302.Yao H, Liu X, Chen S, Xia W, Chen X. Decreased expression of serum miR-424 correlates with poor prognosis of patients with hepatocellular carcinoma. Int J Clin Exp Pathol. 2015;8(11):14830–14835. [PMC free article] [PubMed] [Google Scholar]
- 303.Li Q, Qiu X-M, Li Q-H, Wang X-Y, Li L, Xu M, Dong M, Xiao Y-B. MicroRNA-424 may function as a tumor suppressor in endometrial carcinoma cells by targeting E2F7. Oncol Rep. 2015;33(5):2354–2360. [DOI] [PubMed] [Google Scholar]
- 304.Sikand K, Slane SD, Shukla GC Intrinsic expression of host genes and intronic miRNAs in prostate carcinoma cells. Cancer Cell Int. 2009;9(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Venables JP. Unbalanced alternative splicing and its significance in cancer. BioEssays. 2006;28(4):378–386. [DOI] [PubMed] [Google Scholar]
- 306.David CJ, Chen M, Assanah M, Canoll P, Manley JL. HnRNP proteins controlled by c-Myc deregulate pyruvate kinase mRNA splicing in cancer. Nature. 2010;463(7279):364–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Yoshino H, Enokida H, Chiyomaru T, Tatarano S, Hidaka H, Yamasaki T, Gotannda T, Tachiwada T, Nohata N, Yamane T, Seki N, Nakagawa M. Tumor suppressive microRNA-1 mediated novel apoptosis pathways through direct inhibition of splicing factor serine/arginine-rich 9 (SRSF9/SRp30c) in bladder cancer. Biochem Biophys Res Commun. 2012;417(1):588–593. [DOI] [PubMed] [Google Scholar]
- 308.Scott LM, Rebel VI. Acquired mutations that affect pre-mRNA splicing in hematologic malignancies and solid tumors. J Natl Cancer Inst. 2013;105(20):1540–1549. [DOI] [PubMed] [Google Scholar]
- 309.Banerji S, Cibulskis K, Rangel-Escareno C, Brown KK, Carter SL, Frederick AM, Lawrence MS, Sivachenko AY, Sougnez C, Zou L, Cortes ML, Fernandez-Lopez JC, Peng S, Ardlie KG, Auclair D, Bautista-Piña V, Duke F, Francis J, Jung J, Maffuz-Aziz A, Onofrio RC, Parkin M, Pho NH, Quintanar-Jurado V, Ramos AH, Rebollar-Vega R, Rodriguez-Cuevas S, Romero-Cordoba SL, Schumacher SE, Stransky N, Thompson KM, Uribe-Figueroa L, Baselga J, Beroukhim R, Polyak K, Sgroi DC, Richardson AL, Jimenez-Sanchez G, Lander ES, Gabriel SB, Garraway LA, Golub TR, Melendez-Zajgla J, Toker A, Getz G, Hidalgo-Miranda A, Meyerson M. Sequence analysis of mutations and translocations across breast cancer subtypes. Nature. 2012;486(7403):405–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Imielinski M, Berger AH, Hammerman PS, Hernandez B, Pugh TJ, Hodis E, Cho J, Suh J, Capelletti M, Sivachenko A, Sougnez C, Auclair D, Lawrence MS, Stojanov P, Cibulskis K, Choi K, de Waal L, Sharifnia T, Brooks A, Greulich H, Banerji S, Zander T, Seidel D, Leenders F, Ansén S, Ludwig C, Engel-Riedel W, Stoelben E, Wolf J, Goparju C, Thompson K, Winckler W, Kwiatkowski D, Johnson BE, Jänne PA, Miller VA, Pao W, Travis WD, Pass HI, Gabriel SB, Lander ES, Thomas RK, Garraway LA, Getz G, Meyerson M. Mapping the hallmarks of lung adenocarcinoma with massively parallel sequencing. Cell. 2012;150(6):1107–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Chettouh H, Fartoux L, Aoudjehane L, Wendum D, Clapéron A, Chrétien Y, Rey C, Scatton O, Soubrane O, Conti F, Praz F, Housset C, Rosmorduc O, Desbois-Mouthon C. Mitogenic insulin receptor-A is overexpressed in human hepatocellular carcinoma due to EGFR-mediated dysregulation of RNA splicing factors. Cancer Res. 2013;73(13):3974–3986. [DOI] [PubMed] [Google Scholar]
- 312.Sen S, Talukdar I, Webster NJG. SRp20 and CUG-BP1 Modulate Insulin Receptor Exon 11 Alternative Splicing. Mol Cell Biol. 2009;29(3):871–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Jumaa H, Nielsen PJ. The splicing factor SRp20 modifies splicing of its own mRNA and ASF/SF2 antagonizes this regulation. EMBO J. 1997;16(16):5077–5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Sen S, Langiewicz M, Jumaa H, Webster NJG. Deletion of serine/arginine-rich splicing factor 3 in hepatocytes predisposes to hepatocellular carcinoma in mice. Hepatology. 2015;61(1):171–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Cui H, Wu F, Sun Y, Fan G, Wang Q. Up-regulation and subcellular localization of hnRNP A2/B1 in the development of hepatocellular carcinoma. BMC Cancer. 2010;10(1):356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Saeki K, Yasugi E, Okuma E, Breit SN, Nakamura M, Toda T, Kaburagi Y, Yuo A. Proteomic analysis on insulin signaling in human hematopoietic cells: identification of CLIC1 and SRp20 as novel downstream effectors of insulin. Am J Physiol Endocrinol Metab. 2005;289(3):E419–E428. [DOI] [PubMed] [Google Scholar]
- 317.Forest A, Amatulli M, Ludwig DL, Damoci CB, Wang Y, Burns CA, Donoho GP, Zanella N, Fiebig HH, Prewett MC, Surguladze D, DeLigio JT, Houghton PJ, Smith MA, Novosiadly R. Intrinsic Resistance to Cixutumumab Is Conferred by Distinct Isoforms of the Insulin Receptor. Mol Cancer Res. 2015;13(12):1615–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Aljada A, Saleh AM, Al-Aqeel SM, Shamsa HB, Al-Bawab A, Al Dubayee M, Ahmed AA. Quantification of insulin receptor mRNA splice variants as a diagnostic tumor marker in breast cancer. Cancer Biomark. 2015;15(5):653–661. [DOI] [PubMed] [Google Scholar]
- 319.Harrington SC, Weroha SJ, Reynolds C, Suman VJ, Lingle WL, Haluska P. Quantifying insulin receptor isoform expression in FFPE breast tumors. Growth Horm IGF Res. 2012;22(3–4):108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Huang J, Morehouse C, Streicher K, Higgs BW, Gao J, Czapiga M, Boutrin A, Zhu W, Brohawn P, Chang Y, Viner J, LaVallee T, Richman L, Jallal B, Yao Y. Altered Expression of Insulin Receptor Isoforms in Breast Cancer. Batra SK, ed. PLoS One. 2011;6(10):e26177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Law JH, Habibi G, Hu K, Masoudi H, Wang MYC, Stratford AL, Park E, Gee JMW, Finlay P, Jones HE, Nicholson RI, Carboni J, Gottardis M, Pollak M, Dunn SE. Phosphorylated insulin-like growth factor-i/insulin receptor is present in all breast cancer subtypes and is related to poor survival. Cancer Res. 2008;68(24):10238–10246. [DOI] [PubMed] [Google Scholar]
- 322.Nam S, Park S, Park HS, Kim S, Kim JY, Kim S. Il. Association Between Insulin Resistance and Luminal B Subtype Breast Cancer in Postmenopausal Women. Medicine (Baltimore). 2016;95(9):e2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Cox ME, Gleave ME, Zakikhani M, Bell RH, Piura E, Vickers E, Cunningham M, Larsson O, Fazli L, Pollak M. Insulin receptor expression by human prostate cancers. Prostate. 2009;69(1):33–40. [DOI] [PubMed] [Google Scholar]
- 324.Heni M, Hennenlotter J, Scharpf M, Lutz SZ, Schwentner C, Todenhöfer T, Schilling D, Kühs U, Gerber V, Machicao F, Staiger H, Häring H-U, Stenzl A. Insulin receptor isoforms A and B as well as insulin receptor substrates-1 and -2 are differentially expressed in prostate cancer. Sesti G, ed. PLoS One. 2012;7(12):e50953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Heidegger I, Ofer P, Doppler W, Rotter V, Klocker H, Massoner P. Diverse functions of IGF/insulin signaling in malignant and noncancerous prostate cells: proliferation in cancer cells and differentiation in noncancerous cells. Endocrinology. 2012;153(10):4633–4643. [DOI] [PubMed] [Google Scholar]
- 326.Heidegger I, Kern J, Ofer P, Klocker H, Massoner P. Oncogenic functions of IGF1R and INSR in prostate cancer include enhanced tumor growth, cell migration and angiogenesis. Oncotarget. 2014;5(9):2723–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Perks CM, Zielinska HA, Wang J, Jarrett C, Frankow A, Ladomery MR, Bahl A, Rhodes A, Oxley J, Holly JMP. Insulin Receptor Isoform Variations in Prostate Cancer Cells. Front Endocrinol (Lausanne). 2016;7:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Wang C-F, Zhang G, Zhao L-J, Qi W-J, Li X-P, Wang J-L, Wei L-H. Overexpression of the insulin receptor isoform A promotes endometrial carcinoma cell growth. Lobaccaro J-MA, ed. PLoS One. 2013;8(8):e69001. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 329.Jiang L, Zhu W, Streicher K, Morehouse C, Brohawn P, Ge X, Dong Z, Yin X, Zhu G, Gu Y, Ranade K, Higgs BW, Yao Y, Huang J. Increased IR-A/IR-B ratio in non-small cell lung cancers associates with lower epithelial-mesenchymal transition signature and longer survival in squamous cell lung carcinoma. BMC Cancer. 2014;14(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Santoro MA, Andres SF, Galanko JA, Sandler RS, Keku TO, Lund PK. Reduced insulin-like growth factor I receptor and altered insulin receptor isoform mRNAs in normal mucosa predict colorectal adenoma risk. Cancer Epidemiol Biomarkers Prev. 2014;23(10):2093–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Spector SA, Olson ET, Gumbs AA, Friess H, Büchler MW, Seymour NE. Human insulin receptor and insulin signaling proteins in hepatic disease. J Surg Res. 1999;83(1):32–35. [DOI] [PubMed] [Google Scholar]
- 332.Aleem E, Nehrbass D, Klimek F, Mayer D, Bannasch P. Upregulation of the insulin receptor and type I insulin-like growth factor receptor are early events in hepatocarcinogenesis. Toxicol Pathol. 2011;39(3):524–543. [DOI] [PubMed] [Google Scholar]
- 333.Kasprzak A, Adamek A. The insulin-like growth factor (IGF) signaling axis and hepatitis C virus-associated carcinogenesis. [Review] Int J Oncol. 2012;41(6):1919–1931. [DOI] [PubMed] [Google Scholar]
- 334.Shushanov SS, Kravtsova TA, Chernykh YB, Cheikina NN. Insulin Effects on Survival of Human Multiple Myeloma Cells. Bull Exp Biol Med. 2015;159(2):262–265. [DOI] [PubMed] [Google Scholar]
- 335.Frisch CM, Zimmermann K, Zilleßen P, Pfeifer A, Racké K, Mayer P. Non-small cell lung cancer cell survival crucially depends on functional insulin receptors. Endocr Relat Cancer. 2015;22(4):609–621. [DOI] [PubMed] [Google Scholar]
- 336.Keku TO, Lund PK, Galanko J, Simmons JG, Woosley JT, Sandler RS Insulin resistance, apoptosis, and colorectal adenoma risk. Cancer Epidemiol Biomarkers Prev. 2005;14(9):2076–2081. [DOI] [PubMed] [Google Scholar]
- 337.Vidal AC, Lund PK, Hoyo C, Galanko J, Burcal L, Holston R, Massa B, Omofoye O, Sandler RS, Keku TO Elevated C-peptide and insulin predict increased risk of colorectal adenomas in normal mucosa. BMC Cancer. 2012;12(1):389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocr Relat Cancer. 2009;16(4):1103–1123. [DOI] [PubMed] [Google Scholar]
- 339.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–1638. [DOI] [PubMed] [Google Scholar]
- 340.Pisani P. Hyper-insulinaemia and cancer, meta-analyses of epidemiological studies. Arch Physiol Biochem. 2008;114(1):63–70. [DOI] [PubMed] [Google Scholar]
- 341.Becker S, Dossus L, Kaaks R. Obesity related hyperinsulinaemia and hyperglycaemia and cancer development. Arch Physiol Biochem. 2009;115(2):86–96. [DOI] [PubMed] [Google Scholar]
- 342.Othman EM, Kreissl MC, Kaiser FR, Arias-Loza P-A, Stopper H. Insulin-mediated oxidative stress and DNA damage in LLC-PK1 pig kidney cell line, female rat primary kidney cells, and male ZDF rat kidneys in vivo. Endocrinology. 2013;154(4):1434–1443. [DOI] [PubMed] [Google Scholar]
- 343.Othman EM, Leyh A, Stopper H. Insulin mediated DNA damage in mammalian colon cells and human lymphocytes in vitro. Mutat Res. 2013;745–746:34–39. [DOI] [PubMed] [Google Scholar]
- 344.Othman EM, Hintzsche H, Stopper H. Signaling steps in the induction of genomic damage by insulin in colon and kidney cells. Free Radic Biol Med. 2014;68:247–257. [DOI] [PubMed] [Google Scholar]
- 345.Liu J, Visser-Grieve S, Boudreau J, Yeung B, Lo S, Chamberlain G, Yu F, Sun T, Papanicolaou T, Lam A, Yang X, Chin-Sang I. Insulin activates the insulin receptor to downregulate the PTEN tumour suppressor. Oncogene. 2014;33(29):3878–3885. doi: 10.1038/onc.2013.347. [DOI] [PubMed] [Google Scholar]
- 346.Straßburger K, Tiebe M, Pinna F, Breuhahn K, Teleman AA. Insulin/IGF signaling drives cell proliferation in part via Yorkie/YAP. Dev Biol. 2012;367(2):187–196. [DOI] [PubMed] [Google Scholar]
- 347.Budi EH, Muthusamy B-P, Derynck R. The insulin response integrates increased TGF-β signaling through Akt-induced enhancement of cell surface delivery of TGF-β receptors. Sci Signal. 2015;8(396):ra96. [DOI] [PMC free article] [PubMed]
- 348.Liu Y, Petreaca M, Martins-Green M. Cell and molecular mechanisms of insulin-induced angiogenesis. J Cell Mol Med. 2009;13(11–12):4492–4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Dombrowski F, Mathieu C, Evert M. Hepatocellular neoplasms induced by low-number pancreatic islet transplants in autoimmune diabetic BB/Pfd rats. Cancer Res. 2006;66(3):1833–1843. [DOI] [PubMed] [Google Scholar]
- 350.Pan F, Hong L-Q. Insulin promotes proliferation and migration of breast cancer cells through the extracellular regulated kinase pathway. Asian Pac J Cancer Prev. 2014;15(15):6349–6352. [DOI] [PubMed] [Google Scholar]
- 351.Novosyadlyy R, Lann DE, Vijayakumar A, Rowzee A, Lazzarino DA, Fierz Y, Carboni JM, Gottardis MM, Pennisi PA, Molinolo AA, Kurshan N, Mejia W, Santopietro S, Yakar S, Wood TL, LeRoith D. Insulin-mediated acceleration of breast cancer development and progression in a nonobese model of type 2 diabetes. Cancer Res. 2010;70(2):741–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Gallagher EJ, Alikhani N, Tobin-Hess A, Blank J, Buffin NJ, Zelenko Z, Tennagels N, Werner U, LeRoith D. Insulin receptor phosphorylation by endogenous insulin or the insulin analog AspB10 promotes mammary tumor growth independent of the IGF-I receptor. Diabetes. 2013;62(10):3553–3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Rostoker R, Bitton-Worms K, Caspi A, Shen-Orr Z, LeRoith D. Investigating new therapeutic strategies targeting hyperinsulinemia’s mitogenic effects in a female mouse breast cancer model. Endocrinology. 2013;154(5):1701–1710. [DOI] [PubMed] [Google Scholar]
- 354.Rostoker R, Abelson S, Bitton-Worms K, Genkin I, Ben-Shmuel S, Dakwar M, Orr ZS, Caspi A, Tzukerman M, LeRoith D. Highly specific role of the insulin receptor in breast cancer progression. Endocr Relat Cancer. 2015;22(2):145–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Zhang H, Pelzer AM, Kiang DT, Yee D. Down-regulation of type I insulin-like growth factor receptor increases sensitivity of breast cancer cells to insulin. Cancer Res. 2007;67(1):391–397. [DOI] [PubMed] [Google Scholar]
- 356.Zelenko Z, Gallagher EJ, Antoniou IM, Sachdev D, Nayak A, Yee D, LeRoith D. EMT reversal in human cancer cells after IR knockdown in hyperinsulinemic mice. Endocr Relat Cancer. 2016;23(9):747–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Samani AA, Yakar S, LeRoith D, Brodt P. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev. 2007;28(1):20–47. [DOI] [PubMed] [Google Scholar]
- 358.Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4(7):505–518. [DOI] [PubMed] [Google Scholar]
- 359.Khandwala HM, McCutcheon IE, Flyvbjerg A, Friend KE. The effects of insulin-like growth factors on tumorigenesis and neoplastic growth. Endocr Rev. 2000;21(3):215–244. [DOI] [PubMed] [Google Scholar]
- 360.Liu M, Roth A, Yu M, Morris R, Bersani F, Rivera MN, Lu J, Shioda T, Vasudevan S, Ramaswamy S, Maheswaran S, Diederichs S, Haber DA. The IGF2 intronic miR-483 selectively enhances transcription from IGF2 fetal promoters and enhances tumorigenesis. Genes Dev. 2013;27(23):2543–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Ulanet DB, Ludwig DL, Kahn CR, Hanahan D. Insulin receptor functionally enhances multistage tumor progression and conveys intrinsic resistance to IGF-1R targeted therapy. Proc Natl Acad Sci USA. 2010;107(24):10791–10798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Vidal SJ, Rodriguez-Bravo V, Quinn SA, Rodriguez-Barrueco R, Lujambio A, Williams E, Sun X, de la Iglesia-Vicente J, Lee A, Readhead B, Chen X, Galsky M, Esteve B, Petrylak DP, Dudley JT, Rabadan R, Silva JM, Hoshida Y, Lowe SW, Cordon-Cardo C, Domingo-Domenech J. A targetable GATA2-IGF2 axis confers aggressiveness in lethal prostate cancer. Cancer Cell. 2015;27(2):223–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Dynkevich Y, Rother KI, Whitford I, Qureshi S, Galiveeti S, Szulc AL, Danoff A, Breen TL, Kaviani N, Shanik MH, Leroith D, Vigneri R, Koch CA, Roth J. Tumors, IGF-2, and hypoglycemia: insights from the clinic, the laboratory, and the historical archive. Endocr Rev. 2013;34(6):798–826. [DOI] [PubMed] [Google Scholar]
- 364.Greenall SA, Bentley JD, Pearce LA, Scoble JA, Sparrow LG, Bartone NA, Xiao X, Baxter RC, Cosgrove LJ, Adams TE. Biochemical characterization of individual human glycosylated pro-insulin-like growth factor (IGF)-II and big-IGF-II isoforms associated with cancer. J Biol Chem. 2013;288(1):59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Weidner P, Söhn M, Gutting T, Friedrich T, Gaiser T, Magdeburg J, Kienle P, Ruh H, Hopf C, Behrens H-M, Röcken C, Hanoch T, Seger R, Ebert MPA, Burgermeister E. Myotubularin-related protein 7 inhibits insulin signaling in colorectal cancer. Oncotarget. 2016;7(31):50490–50506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Lee J-S, Kang J-H, Boo H-J, Hwang S-J, Hong S, Lee S-C, Park Y-J, Chung T-M, Youn H, Lee SM, Kim BJ, Chung J-K, Chung Y, William WN, Shin YK, Lee H-J, Oh S-H, Lee H-Y. STAT3-mediated IGF-2 secretion in the tumour microenvironment elicits innate resistance to anti-IGF-1R antibody. Nat Commun. 2015;6:8499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Lee S-C, Min H-Y, Jung HJ, Park KH, Hyun SY, Cho J, Woo JK, Kwon SJ, Lee H-J, Johnson FM, Lee H-Y. Essential role of insulin-like growth factor 2 in resistance to histone deacetylase inhibitors. Oncogene. 2016;35(42):5515–5526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Graham TR, Zhau HE, Odero-Marah VA, Osunkoya AO, Kimbro KS, Tighiouart M, Liu T, Simons JW, O’Regan RM. Insulin-like growth factor-I-dependent up-regulation of ZEB1 drives epithelial-to-mesenchymal transition in human prostate cancer cells. Cancer Res. 2008;68(7):2479–2488. [DOI] [PubMed] [Google Scholar]
- 369.Bachelder RE, Yoon S-O, Franci C, de Herreros AG, Mercurio AM. Glycogen synthase kinase-3 is an endogenous inhibitor of Snail transcription: implications for the epithelial-mesenchymal transition. J Cell Biol. 2005;168(1):29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Sivakumar R, Koga H, Selvendiran K, Maeyama M, Ueno T, Sata M. Autocrine loop for IGF-I receptor signaling in SLUG-mediated epithelial-mesenchymal transition. Int J Oncol. 2009;34(2):329–338. [PubMed] [Google Scholar]
- 371.Yao C, Su L, Shan J, Zhu C, Liu L, Liu C, Xu Y, Yang Z, Bian X, Shao J, Li J, Lai M, Shen J, Qian C. IGF/STAT3/NANOG/Slug Signaling Axis Simultaneously Controls Epithelial-Mesenchymal Transition and Stemness Maintenance in Colorectal Cancer. Stem Cells. 2016;34(4):820–831. [DOI] [PubMed] [Google Scholar]
- 372.Golubovskaya VM. FAK and Nanog cross talk with p53 in cancer stem cells. Anticancer Agents Med Chem. 2013;13(4):576–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Shan J, Shen J, Liu L, Xia F, Xu C, Duan G, Xu Y, Ma Q, Yang Z, Zhang Q, Ma L, Liu J, Xu S, Yan X, Bie P, Cui Y, Bian X, Qian C. Nanog regulates self-renewal of cancer stem cells through the insulin-like growth factor pathway in human hepatocellular carcinoma. Hepatology. 2012;56(3):1004–1014. [DOI] [PubMed] [Google Scholar]
- 374.Mimeault M, Batra SK. Altered gene products involved in the malignant reprogramming of cancer stem/progenitor cells and multitargeted therapies. Mol Aspects Med. 2014;39:3–32. doi: 10.1016/j.mam.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Hsu H-J, Drummond-Barbosa D. Insulin signals control the competence of the Drosophila female germline stem cell niche to respond to Notch ligands. Dev Biol. 2011;350(2):290–300. [DOI] [PubMed] [Google Scholar]
- 376.Leick MB, Shoff CJ, Wang EC, Congress JL, Gallicano GI. Loss of imprinting of IGF2 and the epigenetic progenitor model of cancer. Am J Stem Cells. 2012;1(1):59–74. [PMC free article] [PubMed] [Google Scholar]
- 377.Zhao X, Liu X, Wang G, Wen X, Zhang X, Hoffman AR, Li W, Hu J-F, Cui J. Loss of insulin-like growth factor II imprinting is a hallmark associated with enhanced chemo/radiotherapy resistance in cancer stem cells. Oncotarget. 2016;7(32):51349–51364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Wang H, Ge S, Qian G, Li W, Cui J, Wang G, Hoffman AR, Hu J-F. Restoration of IGF2 imprinting by polycomb repressive complex 2 docking factor SUZ12 in colon cancer cells. Exp Cell Res. 2015;338(2):214–221. [DOI] [PubMed] [Google Scholar]
- 379.Murayama T, Nakaoku T, Enari M, Nishimura T, Tominaga K, Nakata A, Tojo A, Sugano S, Kohno T, Gotoh N. Oncogenic Fusion Gene CD74-NRG1 Confers Cancer Stem Cell-like Properties in Lung Cancer through a IGF2 Autocrine/Paracrine Circuit. Cancer Res. 2016;76(4):974–983. [DOI] [PubMed] [Google Scholar]
- 380.Tada Y, Yamaguchi Y, Kinjo T, Song X, Akagi T, Takamura H, Ohta T, Yokota T, Koide H. The stem cell transcription factor ZFP57 induces IGF2 expression to promote anchorage-independent growth in cancer cells. Oncogene. 2015;34(6):752–760. [DOI] [PubMed] [Google Scholar]
- 381.Moller DE, Yokota A, Caro JF, Flier JS. Tissue-specific expression of two alternatively spliced insulin receptor mRNAs in man. Mol Endocrinol. 1989;3(8):1263–1269. [DOI] [PubMed] [Google Scholar]
- 382.Santoro M, Masciullo M, Bonvissuto D, Bianchi MLE, Michetti F, Silvestri G. Alternative splicing of human insulin receptor gene (INSR) in type I and type II skeletal muscle fibers of patients with myotonic dystrophy type 1 and type 2. Mol Cell Biochem. 2013;380(1–2):259–265. [DOI] [PubMed] [Google Scholar]
- 383.Root-Bernstein R, Vonck J. Glucose binds to the insulin receptor affecting the mutual affinity of insulin and its receptor. Cell Mol Life Sci. 2009;66(16):2721–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Desoye G, Hauguel-de Mouzon S. The human placenta in gestational diabetes mellitus. The insulin and cytokine network. Diabetes Care.2007;30 Suppl 2:S120–126. [DOI] [PubMed]
- 385.Metzger BE. Long-term outcomes in mothers diagnosed with gestational diabetes mellitus and their offspring. Clin Obstet Gynecol. 2007;50(4):972–979. [DOI] [PubMed] [Google Scholar]
- 386.Bellamy L, Casas J-P, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet (London, England). 2009;373(9677):1773–1779. [DOI] [PubMed] [Google Scholar]
- 387.Sobrevia L, Abarzúa F, Nien JK, Salomón C, Westermeier F, Puebla C, Cifuentes F, Guzmán-Gutiérrez E, Leiva A, Casanello P. Review: Differential placental macrovascular and microvascular endothelial dysfunction in gestational diabetes. Placenta. 2011;32(Suppl 2):S159–S164. [DOI] [PubMed] [Google Scholar]
- 388.Vásquez G, Sanhueza F, Vásquez R, González M, San Martín R, Casanello P, Sobrevia L. Role of adenosine transport in gestational diabetes-induced L-arginine transport and nitric oxide synthesis in human umbilical vein endothelium. J Physiol. 2004;560(Pt 1):111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Westermeier F, Salomón C, Farías M, Arroyo P, Fuenzalida B, Sáez T, Salsoso R, Sanhueza C, Guzmán-Gutiérrez E, Pardo F, Leiva A, Sobrevia L. Insulin requires normal expression and signaling of insulin receptor A to reverse gestational diabetes-reduced adenosine transport in human umbilical vein endothelium. FASEB J. 2015;29(1):37–49. [DOI] [PubMed] [Google Scholar]
- 390.Udd B, Krahe R. The myotonic dystrophies: molecular, clinical, and therapeutic challenges. Lancet Neurol. 2012;11(10):891–905. [DOI] [PubMed] [Google Scholar]
- 391.Perseghin G, Caumo A, Arcelloni C, Benedini S, Lanzi R, Pagliato E, Sereni LP, Testolin G, Battezzati A, Comi G, Comola M, Luzi L. Contribution of abnormal insulin secretion and insulin resistance to the pathogenesis of type 2 diabetes in myotonic dystrophy. Diabetes Care. 2003;26(7):2112–2118. [DOI] [PubMed] [Google Scholar]
- 392.Wong C-H, Nguyen L, Peh J, Luu LM, Sanchez JS, Richardson SL, Tuccinardi T, Tsoi H, Chan WY, Chan HYE, Baranger AM, Hergenrother PJ, Zimmerman SC. Targeting toxic RNAs that cause myotonic dystrophy type 1 (DM1) with a bisamidinium inhibitor. J Am Chem Soc. 2014;136(17):6355–6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Vajo Z, Fawcett J, Duckworth WC. Recombinant DNA technology in the treatment of diabetes: insulin analogs. Endocr Rev. 2001;22(5):706–717. [DOI] [PubMed] [Google Scholar]
- 394.Hirsch IB, Vega CP. Optimal initiation of insulin in type 2 diabetes. MedGenMed. 2005;7(4):49. [PubMed] [Google Scholar]
- 395.Jensen M, De Meyts P. Molecular mechanisms of differential intracellular signaling from the insulin receptor. Vitam Horm. 2009;80:51–75. [DOI] [PubMed] [Google Scholar]
- 396.Ish-Shalom D, Christoffersen CT, Vorwerk P, Sacerdoti-Sierra N, Shymko RM, Naor D, De Meyts P. Mitogenic properties of insulin and insulin analogues mediated by the insulin receptor. Diabetologia. 1997;40(Suppl 2):S25–S31. [DOI] [PubMed] [Google Scholar]
- 397.De Meyts P, Shymko RM. Timing-dependent modulation of insulin mitogenic versus metabolic signalling. Novartis Found Symp. 2000;227:46–60. [DOI] [PubMed] [Google Scholar]
- 398.Drejer K. The bioactivity of insulin analogues from in vitro receptor binding to in vivo glucose uptake. Diabetes Metab Rev. 1992;8(3):259–285. [DOI] [PubMed] [Google Scholar]
- 399.Smith U, Gale EAM. Does diabetes therapy influence the risk of cancer? Diabetologia. 2009;52(9):1699–1708. [DOI] [PubMed] [Google Scholar]
- 400.Sommerfeld MR, Muller G, Tschank G, Seipke G, Habermann P, Kurrle R, Tennagels N. In vitro metabolic and mitogenic signaling of insulin glargine and its metabolites. PLoS One. 2010;5(3):e9540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Kurtzhals P, Schaffer L, Sorensen A, Kristensen C, Jonassen I, Schmid C, Trub T. Correlations of receptor binding and metabolic and mitogenic potencies of insulin analogs designed for clinical use. Diabetes. 2000;49(6):999–1005. [DOI] [PubMed] [Google Scholar]
- 402.Mayer D, Shukla A, Enzmann H. Proliferative effects of insulin analogues on mammary epithelial cells. Arch Physiol Biochem. 2008;114(1):38–44. [DOI] [PubMed] [Google Scholar]
- 403.Markussen J, Havelund S, Kurtzhals P, Andersen AS, Halstrøm J, Hasselager E, Larsen UD, Ribel U, Schäffer L, Vad K, Jonassen I. Soluble, fatty acid acylated insulins bind to albumin and show protracted action in pigs. Diabetologia. 1996;39(3):281–288. [DOI] [PubMed] [Google Scholar]
- 404.Tennagels N, Werner U. The metabolic and mitogenic properties of basal insulin analogues. Arch Physiol Biochem. 2013;119(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Janssen JAMJL, Varewijck AJ. Insulin Analogs and Cancer: A Note of Caution. Front Endocrinol (Lausanne). 2014;5:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Staiger K, Hennige AM, Staiger H, Haring HU, Kellerer M. Comparison of the mitogenic potency of regular human insulin and its analogue glargine in normal and transformed human breast epithelial cells. Horm Metab Res. 2007;39(1):65–67. [DOI] [PubMed] [Google Scholar]
- 407.Shukla A, Grisouard J, Ehemann V, Hermani A, Enzmann H, Mayer D. Analysis of signaling pathways related to cell proliferation stimulated by insulin analogs in human mammary epithelial cell lines. Endocr Relat Cancer. 2009;16(2):429–441. [DOI] [PubMed] [Google Scholar]
- 408.Weinstein D, Simon M, Yehezkel E, Laron Z, Werner H. Insulin analogues display IGF-I-like mitogenic and anti-apoptotic activities in cultured cancer cells. Diabetes Metab Res Rev. 2009;25(1):41–49. [DOI] [PubMed] [Google Scholar]
- 409.ter Braak B, Siezen CLE, Kannegieter N, Koedoot E, van de Water B, van der Laan JW. Classifying the adverse mitogenic mode of action of insulin analogues using a novel mechanism-based genetically engineered human breast cancer cell panel. Arch Toxicol. 2014;88(4):953–966. [DOI] [PubMed] [Google Scholar]
- 410.ter Braak B, Wink S, Koedoot E, Pont C, Siezen C, van der Laan JW, van de Water B. Alternative signaling network activation through different insulin receptor family members caused by pro-mitogenic antidiabetic insulin analogues in human mammary epithelial cells. Breast Cancer Res. 2015;17(1):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Hansen BF, Glendorf T, Hegelund AC, Lundby A, Lützen A, Slaaby R, Stidsen CE. Molecular Characterisation of Long-Acting Insulin Analogues in Comparison with Human Insulin, IGF-1 and Insulin X10. Atkin SL, ed. PLoS One. 2012;7(5):e34274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Eckardt K, May C, Koenen M, Eckel J. IGF-1 receptor signalling determines the mitogenic potency of insulin analogues in human smooth muscle cells and fibroblasts. Diabetologia. 2007;50(12):2534–2543. [DOI] [PubMed] [Google Scholar]
- 413.Baricevic I, Jones DR, Roberts DL, Lutzen A, Lundby A, Worm J, Hansen BF, Renehan AG. A framework for the in vitro evaluation of cancer-relevant molecular characteristics and mitogenic potency of insulin analogues. Carcinogenesis. 2015;36(9):1040–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Sciacca L, Cassarino MF, Genua M, Vigneri P, Giovanna Pennisi M, Malandrino P, Squatrito S, Pezzino V, Vigneri R. Biological Effects of Insulin and Its Analogs on Cancer Cells With Different Insulin Family Receptor Expression. J Cell Physiol. 2014;229(11):1817–1821. [DOI] [PubMed] [Google Scholar]
- 415.Bonnesen C, Nelander G-M, Hansen BF, Jensen P, Krabbe JS, Jensen MB, Hegelund AC, Svendsen JE, Oleksiewicz MB. Synchronization in G0/G1 enhances the mitogenic response of cells overexpressing the human insulin receptor A isoform to insulin. Cell Biol Toxicol. 2010;26(4):293–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Monnier L, Colette C, Owens D. Basal insulin analogs: from pathophysiology to therapy. What we see, know, and try to comprehend? Diabetes Metab. 2013;39(6):468–476. [DOI] [PubMed] [Google Scholar]
- 417.Colhoun HM. Use of insulin glargine and cancer incidence in Scotland: a study from the Scottish Diabetes Research Network Epidemiology Group. Diabetologia. 2009;52(9):1755–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Hemkens LG, Grouven U, Bender R, Gunster C, Gutschmidt S, Selke GW, Sawicki PT. Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: a cohort study. Diabetologia. 2009;52(9):1732–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Jonasson JM, Ljung R, Talback M, Haglund B, Gudbjornsdottir S, Steineck G. Insulin glargine use and short-term incidence of malignancies-a population-based follow-up study in Sweden. Diabetologia. 2009;52(9):1745–1754. [DOI] [PubMed] [Google Scholar]
- 420.Currie CJ, Poole CD, Gale EA The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52(9):1766–1777. [DOI] [PubMed] [Google Scholar]
- 421.Morden NE, Liu SK, Smith J, Mackenzie TA, Skinner J, Korc M. Further Exploration of the Relationship Between Insulin Glargine and Incident Cancer: A retrospective cohort study of older Medicare patients. Diabetes Care. 2011;34(9):1965–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Ruiter R, Visser LE, van Herk-Sukel MP, Coebergh JW, Haak HR, Geelhoed-Duijvestijn PH, Straus SM, Herings RM, Stricker BH. Risk of cancer in patients on insulin glargine and other insulin analogues in comparison with those on human insulin: results from a large population-based follow-up study. Diabetologia. 2012;55(1):51–62. doi: 10.1007/s00125-011-2312-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Trial Investigators ORIGIN. Gerstein HC, Bosch J, Dagenais GR, Díaz R, Jung H, Maggioni AP, Pogue J, Probstfield J, Ramachandran A, Riddle MC, Rydén LE, Yusuf S. Basal Insulin and Cardiovascular and Other Outcomes in Dysglycemia. N Engl J Med. 2012;367(4):319–328. [DOI] [PubMed] [Google Scholar]
- 424.Wu JW, Filion KB, Azoulay L, Doll MK, Suissa S. Effect of Long-Acting Insulin Analogs on the Risk of Cancer: A Systematic Review of Observational Studies. Diabetes Care. 2016;39(3):486–494. [DOI] [PubMed] [Google Scholar]
- 425.Taylor SI, Accili D, Cama A, Imano E, Kadowaki H, Kadowaki T. Unusual forms of insulin resistance. Annu Rev Med. 1991;42(1):373–379. [DOI] [PubMed] [Google Scholar]
- 426.Taylor SI, Barbetti F, Accili D, Roth J, Gorden P. Syndromes of autoimmunity and hypoglycemia. Autoantibodies directed against insulin and its receptor. Endocrinol Metab Clin North Am. 1989;18(1):123–143. [PubMed] [Google Scholar]
- 427.Issafras H, Bedinger DH, Corbin JA, Goldfine ID, Bhaskar V, White ML, Rubin P, Scannon PJ. Selective allosteric antibodies to the insulin receptor for the treatment of hyperglycemic and hypoglycemic disorders. J Diabetes Sci Technol. 2014;8(4):865–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Bhaskar V, Goldfine ID, Bedinger DH, Lau A, Kuan HF, Gross LM, Handa M, Maddux BA, Watson SR, Zhu S, Narasimha AJ, Levy R, Webster L, Wijesuriya SD, Liu N, Wu X, Chemla-Vogel D, Tran C, Lee SR, Wong S, Wilcock D, White ML, Corbin JA. A fully human, allosteric monoclonal antibody that activates the insulin receptor and improves glycemic control. Diabetes. 2012;61(5):1263–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Bedinger DH, Goldfine ID, Corbin JA, Roell MK, Adams SH. Differential pathway coupling of the activated insulin receptor drives signaling selectivity by XMetA, an allosteric partial agonist antibody. J Pharmacol Exp Ther. 2015;353(1):35–43. [DOI] [PubMed] [Google Scholar]
- 430.Corbin JA, Bhaskar V, Goldfine ID, Bedinger DH, Lau A, Michelson K, Gross LM, Maddux BA, Kuan HF, Tran C, Lao L, Handa M, Watson SR, Narasimha AJ, Zhu S, Levy R, Webster L, Wijesuriya SD, Liu N, Wu X, Chemla-Vogel D, Lee SR, Wong S, Wilcock D, White ML. Improved glucose metabolism in vitro and in vivo by an allosteric monoclonal antibody that increases insulin receptor binding affinity. Maedler K, ed. PLoS One. 2014;9(2):e88684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Corbin JA, Bhaskar V, Goldfine ID, Issafras H, Bedinger DH, Lau A, Michelson K, Gross LM, Maddux BA, Kuan HF, Tran C, Lao L, Handa M, Watson SR, Narasimha AJ, Zhu S, Levy R, Webster L, Wijesuriya SD, Liu N, Wu X, Chemla-Vogel D, Lee SR, Wong S, Wilcock D, Rubin P, White ML. Inhibition of insulin receptor function by a human, allosteric monoclonal antibody: a potential new approach for the treatment of hyperinsulinemic hypoglycemia. MAbs. 2014;6(1):262–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Bezwada P, Zhao J, Der K, Shimizu B, Cao L, Ahene A, Rubin P, Johnson K A Novel Allosteric Insulin Receptor-Activating Antibody Reduces Hyperglycemia without Hypoglycemia in Diabetic Cynomolgus Monkeys. J Pharmacol Exp Ther. 2016;356(2):466–473. [DOI] [PubMed] [Google Scholar]
- 433.Jensen M, Palsgaard J, Borup R, de Meyts P, Schäffer L. Activation of the insulin receptor (IR) by insulin and a synthetic peptide has different effects on gene expression in IR-transfected L6 myoblasts. Biochem J. 2008;412(3):435–445. [DOI] [PubMed] [Google Scholar]
- 434.Lawrence CF, Margetts MB, Menting JG, Smith NA, Smith BJ, Ward CW, Lawrence MC. Insulin Mimetic Peptide Disrupts the Primary Binding Site of the Insulin Receptor. J Biol Chem. 2016;291(30):15473–15481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Hossain MU, Khan MA, Rakib-Uz-Zaman SM, Ali MT, Islam MS, Keya CA, Salimullah M. Treating Diabetes Mellitus: Pharmacophore Based Designing of Potential Drugs from Gymnema sylvestre against Insulin Receptor Protein. Biomed Res Int. 2016;2016:3187647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Yunn N-O, Koh A, Han S, Lim JH, Park S, Lee J, Kim E, Jang SK, Berggren P-O, Ryu SH. Agonistic aptamer to the insulin receptor leads to biased signaling and functional selectivity through allosteric modulation. Nucleic Acids Res. 2015;43(16):7688–7701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Glendorf T, Stidsen CE, Norrman M, Nishimura E, Sørensen AR, Kjeldsen T. Engineering of Insulin Receptor Isoform-Selective Insulin Analogues. Shirihai OS, ed. PLoS One. 2011;6(5):e20288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Viková J, Collinsová M, Kletvíková E, Buděšínský M, Kaplan V, Žáková L, Veverka V, Hexnerová R, Tarazona Aviñó RJ, Straková J, Selicharová I, Vaněk V, Wright DW, Watson CJ, Turkenburg JP, Brzozowski AM, Jiráček J. Rational steering of insulin binding specificity by intra-chain chemical crosslinking. Sci Rep. 2016;6(1):19431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Žáková L, Kletvíková E, Lepšík M, Collinsová M, Watson CJ, Turkenburg JP, Jiráček J, Brzozowski AM. Human insulin analogues modified at the B26 site reveal a hormone conformation that is undetected in the receptor complex. Acta Crystallogr D Biol Crystallogr. 2014;70(10):2765–2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Křížková K, Chrudinová M, Povalová A, Selicharová I, Collinsová M, Vaněk V, Brzozowski AM, Jiráček J, Žáková L. Insulin–Insulin-like Growth Factors Hybrids as Molecular Probes of Hormone:Receptor Binding Specificity. Biochemistry. 2016;55(21):2903–2913. [DOI] [PubMed] [Google Scholar]
- 441.Vigneri R, Squatrito S, Frittitta L. Selective insulin receptor modulators (SIRM): a new class of antidiabetes drugs? Diabetes. 2012;61(5):984–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Bedinger DH, Adams SH. Metabolic, anabolic, and mitogenic insulin responses: A tissue-specific perspective for insulin receptor activators. Mol Cell Endocrinol. 2015;415:143–156. [DOI] [PubMed] [Google Scholar]
- 443.Frasca F, Pandini G, Vigneri R, Goldfine ID. Insulin and hybrid insulin/IGF receptors are major regulators of breast cancer cells. Breast Dis. 2003;17:73–89. [DOI] [PubMed] [Google Scholar]
- 444.Buck E, Gokhale PC, Koujak S, Brown E, Eyzaguirre A, Tao N, Rosenfeld-Franklin M, Lerner L, Chiu MI, Wild R, Epstein D, Pachter JA, Miglarese MR. Compensatory insulin receptor (IR) activation on inhibition of insulin-like growth factor-1 receptor (IGF-1R): rationale for cotargeting IGF-1R and IR in cancer. Mol Cancer Ther. 2010;9(10):2652–2664. [DOI] [PubMed] [Google Scholar]
- 445.Dinchuk JE, Cao C, Huang F, Reeves KA, Wang J, Myers F, Cantor GH, Zhou X, Attar RM, Gottardis M, Carboni JM. Insulin receptor (IR) pathway hyperactivity in IGF-IR null cells and suppression of downstream growth signaling using the dual IGF-IR/IR inhibitor, BMS-754807. Endocrinology. 2010;151(9):4123–4132. [DOI] [PubMed] [Google Scholar]
- 446.Belfiore A. The role of insulin receptor isoforms and hybrid insulin/IGF-I receptors in human cancer. Curr Pharm Des. 2007;13(7):671–686. [DOI] [PubMed] [Google Scholar]
- 447.Chan JY, LaPara K, Yee D. Disruption of insulin receptor function inhibits proliferation in endocrine-resistant breast cancer cells. Oncogene. 2016;35(32):4235–4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Vincent EE, Elder DJE, Curwen J, Kilgour E, Hers I, Tavaré JM. Targeting non-small cell lung cancer cells by dual inhibition of the insulin receptor and the insulin-like growth factor-1 receptor. Agoulnik IU, ed. PLoS One. 2013;8(6):e66963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Fox EM, Kuba MG, Miller TW, Davies BR, Arteaga CL. Autocrine IGF-I/insulin receptor axis compensates for inhibition of AKT in ER-positive breast cancer cells with resistance to estrogen deprivation. Breast Cancer Res. 2013;15(4):R55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Zhao H, Desai V, Wang J, Epstein DM, Miglarese M, Buck E. Epithelial-mesenchymal transition predicts sensitivity to the dual IGF-1R/IR inhibitor OSI-906 in hepatocellular carcinoma cell lines. Mol Cancer Ther. 2012;11(2):503–513. [DOI] [PubMed] [Google Scholar]
- 451.Fassnacht M, Berruti A, Baudin E, Demeure MJ, Gilbert J, Haak H, Kroiss M, Quinn DI, Hesseltine E, Ronchi CL, Terzolo M, Choueiri TK, Poondru S, Fleege T, Rorig R, Chen J, Stephens AW, Worden F, Hammer GD. Linsitinib (OSI-906) versus placebo for patients with locally advanced or metastatic adrenocortical carcinoma: a double-blind, randomised, phase 3 study. Lancet Oncol. 2015;16(4):426–435. [DOI] [PubMed] [Google Scholar]
- 452.Hou X, Huang F, Macedo LF, Harrington SC, Reeves KA, Greer A, Finckenstein FG, Brodie A, Gottardis MM, Carboni JM, Haluska P. Dual IGF-1R/InsR inhibitor BMS-754807 synergizes with hormonal agents in treatment of estrogen-dependent breast cancer. Cancer Res. 2011;71(24):7597–7607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Lee SJ, Kim EJ, Lee HJ, Kim SY, Oh SJ, Ryu JS, Moon DH, Ahn J-H, Kim S-W. A pilot study for the early assessment of the effects of BMS-754807 plus gefitinib in an H292 tumor model by [(18)F]fluorothymidine-positron emission tomography. Invest New Drugs. 2013;31(3):506–515. [DOI] [PubMed] [Google Scholar]
- 454.Awasthi N, Zhang C, Ruan W, Schwarz MA, Schwarz RE. BMS-754807, a small-molecule inhibitor of insulin-like growth factor-1 receptor/insulin receptor, enhances gemcitabine response in pancreatic cancer. Mol Cancer Ther. 2012;11(12):2644–2653. [DOI] [PubMed] [Google Scholar]
- 455.O’Flanagan CH, O’Shea S, Lyons A, Fogarty FM, McCabe N, Kennedy RD, O’Connor R. IGF-1R inhibition sensitizes breast cancer cells to ATM-Related Kinase (ATR) inhibitor and cisplatin. Oncotarget. 2016;7(35):56826–56841. doi: 10.18632/oncotarget.10862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Huang F, Hurlburt W, Greer A, Reeves KA, Hillerman S, Chang H, Fargnoli J, Graf Finckenstein F, Gottardis MM, Carboni JM. Differential mechanisms of acquired resistance to insulin-like growth factor-i receptor antibody therapy or to a small-molecule inhibitor, BMS-754807, in a human rhabdomyosarcoma model. Cancer Res. 2010;70(18):7221–7231. [DOI] [PubMed] [Google Scholar]
- 457.Schwartz GK, Dickson MA, LoRusso PM, Sausville EA, Maekawa Y, Watanabe Y, Kashima N, Nakashima D, Akinaga S. Preclinical and first-in-human phase I studies of KW-2450, an oral tyrosine kinase inhibitor with insulin-like growth factor receptor-1/insulin receptor selectivity. Cancer Sci. 2016;107(4):499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Sanderson MP, Apgar J, Garin-Chesa P, Hofmann MH, Kessler D, Quant J, Savchenko A, Schaaf O, Treu M, Tye H, Zahn SK, Zoephel A, Haaksma E, Adolf GR, Kraut N. BI. 885578, a Novel IGF1R/INSR Tyrosine Kinase Inhibitor with Pharmacokinetic Properties That Dissociate Antitumor Efficacy and Perturbation of Glucose Homeostasis. Mol Cancer Ther. 2015;14(12):2762–2772. [DOI] [PubMed] [Google Scholar]
- 459.Bonnal S, Vigevani L, Valcárcel J. The spliceosome as a target of novel antitumour drugs. Nat Rev Drug Discov. 2012;11(11):847–859. [DOI] [PubMed] [Google Scholar]
- 460.Coppola JM, Bhojani MS, Ross BD, Rehemtulla A. A small-molecule furin inhibitor inhibits cancer cell motility and invasiveness. Neoplasia. 2008;10(4):363–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Basak A, Chen A, Scamuffa N, Mohottalage D, Basak S, Khatib A-M. Blockade of furin activity and furin-induced tumor cells malignant phenotypes by the chemically synthesized human furin prodomain. Curr Med Chem. 2010;17(21):2214–2221. [DOI] [PubMed] [Google Scholar]
- 462.Zhu J, Van de Ven WJM, Verbiest T, Koeckelberghs G, Chen C, Cui Y, Vermorken AJM. Polyphenols can inhibit furin in vitro as a result of the reactivity of their auto-oxidation products to proteins. Curr Med Chem. 2013;20(6):840–850. [PubMed] [Google Scholar]
- 463.Iaboni M, Fontanella R, Rienzo A, Capuozzo M, Nuzzo S, Santamaria G, Catuogno S, Condorelli G, de Franciscis V, Esposito CL. Targeting Insulin Receptor with a Novel Internalizing Aptamer. Mol Ther Nucleic Acids. 2016;5(9):e365. [DOI] [PMC free article] [PubMed]
- 464.Shimizu T, Sugihara E, Yamaguchi-Iwai S, Tamaki S, Koyama Y, Kamel W, Ueki A, Ishikawa T, Chiyoda T, Osuka S, Onishi N, Ikeda H, Kamei J, Matsuo K, Fukuchi Y, Nagai T, Toguchida J, Toyama Y, Muto A, Saya H. IGF2 preserves osteosarcoma cell survival by creating an autophagic state of dormancy that protects cells against chemotherapeutic stress. Cancer Res. 2014;74(22):6531–6541. [DOI] [PubMed] [Google Scholar]
- 465.Zanella ER, Galimi F, Sassi F, Migliardi G, Cottino F, Leto SM, Lupo B, Erriquez J, Isella C, Comoglio PM, Medico E, Tejpar S, Budinská E, Trusolino L, Bertotti A. IGF2 is an actionable target that identifies a distinct subpopulation of colorectal cancer patients with marginal response to anti-EGFR therapies. Sci Transl Med. 2015;7(272):272ra12. [DOI] [PubMed] [Google Scholar]
- 466.Gao J, Chesebrough JW, Cartlidge SA, Ricketts S-A, Incognito L, Veldman-Jones M, Blakey DC, Tabrizi M, Jallal B, Trail PA, Coats S, Bosslet K, Chang YS. Dual IGF-I/II-neutralizing antibody MEDI-573 potently inhibits IGF signaling and tumor growth. Cancer Res. 2011;71(3):1029–1040. [DOI] [PubMed] [Google Scholar]
- 467.Zhong H, Fazenbaker C, Chen C, Breen S, Huang J, Yao X, Ren P, Yao Y, Herbst R, Hollingsworth RE. Overproduction of IGF-2 drives a subset of colorectal cancer cells, which specifically respond to an anti-IGF therapeutic antibody and combination therapies. Oncogene. 2017;36(6):797–806. doi: 10.1038/onc.2016.248. [DOI] [PubMed] [Google Scholar]
- 468.Haluska P, Menefee M, Plimack ER, Rosenberg J, Northfelt D, LaVallee T, Shi L, Yu X-Q, Burke P, Huang J, Viner J, McDevitt J, LoRusso P, LoRusso P. Phase I Dose-Escalation Study of MEDI-573, a Bispecific, Antiligand Monoclonal Antibody against IGFI and IGFII, in Patients with Advanced Solid Tumors. Clin Cancer Res. 2014;20(18):4747–4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Prince SN, Foulstone EJ, Zaccheo OJ, Williams C, Hassan AB. Functional evaluation of novel soluble insulin-like growth factor (IGF)-II-specific ligand traps based on modified domain 11 of the human IGF2 receptor. Mol Cancer Ther. 2007;6(2):607–617. [DOI] [PubMed] [Google Scholar]
- 470.Frago S, Nicholls RD, Strickland M, Hughes J, Williams C, Garner L, Surakhy M, Maclean R, Rezgui D, Prince SN, Zaccheo OJ, Ebner D, Sanegre S, Yu S, Buffa FM, Crump MP, Hassan AB Functional evolution of IGF2:IGF2R domain 11 binding generates novel structural interactions and a specific IGF2 antagonist. Proc Natl Acad Sci USA. 2016;113(20):E2766–E2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Wang N, Rayes RF, Elahi SM, Lu Y, Hancock MA, Massie B, Rowe GE, Aomari H, Hossain S, Durocher Y, Pinard M, Tabariès S, Siegel PM, Brodt P. The IGF-Trap: Novel Inhibitor of Carcinoma Growth and Metastasis. Mol Cancer Ther. 2015;14(4):982–993. [DOI] [PubMed] [Google Scholar]
- 472.van Adrichem RC1. Hofland LJ, Feelders RA, De Martino MC, van Koetsveld PM, van Eijck CH, de Krijger RR, Sprij-Mooij DM, Janssen JA de HW. Chromogranin A, Ki-67 index and IGF-related genes in patients with neuroendocrine tumors. Endocr Connect. 2013;2(4):172–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.van Adrichem RCS, de Herder WW, Kamp K, Brugts MP, de Krijger RR, Sprij-Mooij DM, Lamberts SWJ, van Koetsveld PM, Janssen JAMJL, Hofland LJ. Effects of Somatostatin Analogs and Dopamine Agonists on Insulin-Like Growth Factor 2-Induced Insulin Receptor Isoform A Activation by Gastroenteropancreatic Neuroendocrine Tumor Cells. Neuroendocrinology. 2016;103(6):815–825. [DOI] [PubMed] [Google Scholar]