Abstract
Photoreceptors are sensory neurons designed to convert light stimuli into neurological responses. This process, called phototransduction, takes place in the outer segments (OS) of rod and cone photoreceptors. OS are specialized sensory cilia, with analogous structures to those present in other nonmotile cilia. Deficient morphogenesis and/or dysfunction of photoreceptor sensory cilia (PSC) caused by mutations in a variety of photoreceptor-specific and common cilia genes can lead to inherited retinal degenerations (IRDs). IRDs can manifest as isolated retinal diseases or syndromic diseases. In this review, we describe the structure and composition of PSC and different forms of ciliopathies with retinal involvement. We review the genetics of the IRDs, which are monogenic disorders but genetically diverse with regard to causality.
Specialized sensory cilia in the photoreceptors of the eye convert light stimuli into neurological responses. Mutations in various photoreceptor-specific and common cilia genes lead to inherited retinal degenerations.
Photoreceptors are sensory neurons designed to convert light stimuli into electrical responses, a process called phototransduction. Phototransduction takes place in the highly specialized compartment of photoreceptors, the outer segment (OS) (Pearring et al. 2013; Molday and Moritz 2015). The OS of the rod and cone photoreceptors differ in structure and protein composition, related to their functional adaptation, in which rods have high sensitivity necessary in dim light and cones are responsible for the high-resolution color vision working in bright light (Lamb and Pugh 2006; Lamb et al. 2007). Research over the past decade on the genetic and molecular components of photoreceptors in vertebrate retinae has led to the clear recognition that photoreceptor OS are specialized sensory cilia (Liu et al. 2007a; Ramamurthy and Cayouette 2009; Khanna 2015). Deficient morphogenesis and/or dysfunction of photoreceptor sensory cilia (PSC) caused by mutations in a variety of photoreceptor-specific and common cilia genes can lead to a group of clinical manifestations, called inherited retinal degenerations (IRDs). In this review, we will discuss the structure and composition of PSC and different forms of ciliopathies with retinal involvement.
SPECIALIZED PHOTORECEPTOR SENSORY CILIA
In vertebrate retina, the visual function depends on the formation of complex sensory cilia of rod and cone photoreceptors. Photoreceptors are highly polarized neurons, composed of four distinct compartments: the OS, the inner segment (IS), the nucleus and a short axon extending to second order neurons (bipolar and horizontal cells) (Fig. 1) (Kennedy and Malicki 2009; Pearring et al. 2013; Molday and Moritz 2015). OS are ciliary organelles with analogous structure to the primary sensory cilia in other cell types (Rosenbaum and Witman 2002; Liu et al. 2007a; Ramamurthy and Cayouette 2009; Khanna 2015). The recognition of PSCs as distinct morphological structures is valuable for the study of photoreceptor cell biology and disease pathogenesis. Studies of genes involved in IRDs and other ciliopathies have identified dozens of novel components of the PSCs. A comprehensive proteomic study of mouse PSCs has identified ∼2000 proteins in this organelle, out of which hundreds are present in other cilia (Liu et al. 2007a). These findings have greatly improved our understanding of how photoreceptor cilia are built and maintained, and how these processes are disrupted in disease.
Figure 1.
Photoreceptor structure. Schematic representation of the rod photoreceptor with sensory cilia components indicated. The drawings to the left of the photoreceptor represent the cross-sectional view of the microtubule structure of the distal axoneme (Ax), proximal Ax, transition zone (TZ), and basal body (BB). RT, rootlet; OS, outer segment; CP, calyceal process; IS, inner segment; Ncl, nucleus; Syn, synapse.
Ciliary Backbone of PSC
The structure of the PSC is analogous to other cilia, where the axoneme arises from the basal body through the transition zone (also called the “connecting cilium”) and extends up to two-thirds of the OS (Fig. 1) (De Robertis 1956; Kaplan et al. 1987). The basal body also nucleates the ciliary rootlet, which extends into the IS, and which is covalently linked to the PSC structure (Yang et al. 2005; Liu et al. 2007a). The basal body contains nine triplet microtubules, two of which extend further to form the axoneme and the third anchors transition fibers linking the basal body to the plasma membrane. The nine doublet microtubules in the transition zone are cross-linked to the surrounding plasma membrane by Y-link structures (Besharse et al. 1985; Horst et al. 1990). These Y-link structures are absent in the rest of the axoneme. The transition zone of rods and cones measures ∼0.3 µm in diameter and 1–1.5 µm in length, which is fairly consistent throughout the species (Besharse et al. 1985). This structure was originally called the “connecting cilium” by De Robertis in 1956, when he was studying some of the first electron micrographs of photoreceptor cells (De Robertis 1956). However, as an analogy with other primary sensory cilia, we refer to this region as a transition zone. Above the transition zone, disc morphogenesis takes place where the surrounding plasma membrane transforms into the disc precursors through membrane evagination (Steinberg et al. 1980; Ding et al. 2015; Pugh 2015). At the distal part of the PSC axoneme, the double microtubules are reduced to singlets (Fig. 1) (Rosenbaum and Witman 2002; Pearring et al. 2013).
Other Structural Components of Outer Segments
PSCs are highly specialized sensory cilia, adapted for light detection by the presence of tightly packed membranous discs containing visual pigments and other phototransduction proteins (Sjostrand 1953; Nickell et al. 2007; Gilliam et al. 2012). PSCs are among the largest of mammalian cilia (Pan et al. 2005) and, like other cilia, they are comprised of a cytoskeleton backbone and a membrane domain, which is distinct from the surrounding plasma membrane (Steinberg et al. 1980; Molday and Molday 1987). In murine rods, the numerous membranous discs in the PSC compartment are stacked at a density of ∼30 discs per micrometer, which is thought to be constant throughout species (Nickell et al. 2007; Gilliam et al. 2012). Such OS organization provides a large surface area for optimized photon capture and rapid signal transduction reactions to occur. Rhodopsin is the most abundant disc membrane protein, organized as rows of dimers with a density of ∼48,000 monomers per µm2 (Fotiadis et al. 2003). With this high density in the disc membranes, rhodopsin plays an important structural role apart from being the main visual pigment in the retina (Wang and Deretic 2014). The rim of the photoreceptor discs contains two tetraspanins: Rds/peripherin-2 (PRPH2) and retinal OS membrane protein 1 (ROM1), which facilitate the folding of the OS discs and are crucial for rim formation and sorting of the OS proteins during the OS biogenesis (Molday et al. 1987; Goldberg and Molday 1996; Arikawa et al. 2011). PRPH2 and its homolog ROM1 both form homodimers and then associate together to form tetrameric complexes, exclusively present at the disc rims (Molday et al. 1987; Goldberg and Molday 1996; Arikawa et al. 2011). Two other membrane proteins prominin 1 (PROM1) and cadherin-related family member 1 (CDHR1) were associated with the open lamellar evaginations in rod and cone discs in Xenopus laevis and mice, respectively (Rattner et al. 2001; Han et al. 2012). PSC are responsible for mediating the sensory transduction of the visual system with a number of proteins involved in this process, including the above-mentioned rhodopsin. Most of these proteins are expressed specifically in PSC and, when mutated, cause nonsyndromic IRDs (Table 1) (Dryja et al. 1990; Farrar et al. 1990; Kajiwara et al. 1991, 1994; Travis et al. 1991; Bascom et al. 1992; Rosenfeld et al. 1992; Dryja et al. 1993; Maw et al. 2000; Yang et al. 2008). Studies of mutant animals have shown that the above-mentioned proteins are essential for OS disc morphogenesis and maintenance (Sanyal et al. 1980; Clarke et al. 2000; Rattner et al. 2001; Dellett et al. 2015).
Table 1.
Genes associated with nonsyndromic retinal degeneration
| Gene | Inheritance pattern | Nonsyndromic form | Syndromic form | Notes and references |
|---|---|---|---|---|
| Genes coding axoneme-associated proteins | ||||
| ARL6 | AR | RP | BBS | New retina-specific exon present (Chiang et al. 2004; Fan et al. 2004; Aldahmesh et al. 2009; Pretorius et al. 2010) |
| BBS1 | AR | RP | BBS | Nishimura et al. 2001; Mykytyn et al. 2002; Estrada-Cuzcano et al. 2012a |
| BBS2 | AR | RP | BBS | Consugar et al. 2014; Shevach et al. 2015 |
| BBS9 | AR | RP | BBS | Nishimura et al. 2005; Abu-Safieh et al. 2012 |
| C2orf71 | AR | RP | - | Putative cilia function, exclusive eye expression (Collin et al. 2010; Nishimura et al. 2010; Kevany et al. 2015) |
| C8orf37 | AR | RP | BBS | Estrada-Cuzcano et al. 2012b; Heon et al. 2016; Khan et al. 2016 |
| CEP164 | AR | LCA | SLS | Chaki et al. 2012 |
| CEP290 | AR | LCA | BBS, JBS, MKS, SLS | den Hollander et al. 2006; Sayer et al. 2006; Valente et al. 2006; Baala et al. 2007a; Helou et al. 2007; Frank et al. 2008; Leitch et al. 2008 |
| CLRN1 | AR | RP | USH | Joensuu et al. 2001; Khan et al. 2011 |
| FAM161A | AR | RP | - | Bandah-Rozenfeld et al. 2010; Langmann et al. 2010 |
| IFT140 | AR | RP, LCA | JATD, MZSDS | Perrault et al. 2012; Schmidts et al. 2013a; Bifari et al. 2015; Xu et al. 2015 |
| IFT172 | AR | RP | BBS, JATD, MZSDS | Halbritter et al. 2013; Bujakowska et al. 2014 |
| IQCB1 | AR | LCA | SLS | Otto et al. 2005; Estrada-Cuzcano et al. 2011; Stone et al. 2011 |
| KIZ | AR | RP | - | El Shamieh et al. 2014 |
| LAC5 | AR | LCA | - | den Hollander et al. 2007 |
| MAK | AR | RP | - | Ozgül et al. 2011; Tucker et al. 2011 |
| NEK2 | AR | RP | Nishiguchi et al. 2013 | |
| OFD1 | XL | RP | OFD, JBS | Ferrante et al. 2001; Coene et al. 2009; Webb et al. 2012 |
| RAB28 | AR | CRD | - | Roosing et al. 2013 |
| RP1 | AR, AD | RP | - | Guillonneau et al. 1999; Pierce et al. 1999 |
| RP1L1 | AR, AD | RP, OMD | - | There are some doubts about this gene being truly associated with IRD, because this gene is highly polymorphic and some mutations were seen in the controls (Bowne et al. 2003; Yamashita et al. 2009; Akahori et al. 2010; Davidson et al. 2013) |
| RP2 | XL | RP | - | Hardcastle et al. 1999; Mears et al. 1999 |
| RPGR | XL | RP, CD, MD | RP with hearing loss and sinorespiratory infections | Extraocular phenotypes may not be related to RPGR, because it mapped to a 43.6-Mb interval with 215 genes including OFD1 (Meindl et al. 1996; Roepman et al. 1996; Ayyagari et al. 2002; Yang et al. 2002; Zito et al. 2003) |
| RPGRIP1 | AR | LCA, CRD | - | Dryja et al. 2001; Hameed et al. 2003 |
| SPATA7 | AR | LCA, RP | - | Wang et al. 2009 |
| TOPORS | AD | RP | - | Chakarova et al. 2007, 2011 |
| TTC8 | AR | RP | BBS | Ansley et al. 2003; Riazuddin et al. 2010 |
| USH2A | AR | RP | USH | Eudy et al. 1998; Rivolta et al. 2000 |
| WDR19 | AR | RP | SLS, CED, JATD | Bredrup et al. 2011; Coussa et al. 2013 |
| Other structural OS proteins | ||||
| CDHR1 | AR | CRD | Henderson et al. 2010; Ostergaard et al. 2010 | |
| EYS | AR | RP | Abd El-Aziz et al. 2008; Collin et al. 2008 | |
| FSCN2 | AD | RP, MD | There are some doubts about this gene being truly associated with IRD, because a frameshift c.208delG is a common polymorphism in the Asian population (Wada et al. 2001, 2003; Zhang et al. 2007; Shin et al. 2010) | |
| PROM1 | AR, AD | RP, MD | Maw et al. 2000; Yang et al. 2008 | |
| PRPH2 | AD, digenic with ROM1 | RP, MD | Kajiwara et al. 1991, 1994; Travis et al. 1991 | |
| ROM1 | AD, digenic with ROM1 | RP | Bascom et al. 1992; Kajiwara et al. 1994 | |
| TULP1 | AR | RP | Banerjee et al. 1998; Hagstrom et al. 1998; Larsson et al. 1998 | |
| Genes involved with the POS sensory function (phototransduction cascade and retinoid cycle in the photoreceptors) | ||||
| ABCA4 | AR | STGD, RP, CRD | Allikmets et al. 1997; Sun and Nathans 1997; Cremers et al. 1998; Martínez-Mir et al. 1998 | |
| CNGA1 | AR | RP | Dryja et al. 1995 | |
| CNGA3 | AR | ACHR | Kohl et al. 1998 | |
| CNGB1 | AR | RP | Bareil et al. 2001 | |
| CNGB3 | AR | ACHM, CD | Kohl et al. 2000 | |
| GNAT1 | AD, AR | CSNB | Dryja et al. 1996 | |
| GNAT2 | AR | ACHM | Aligianis et al. 2002; Kohl et al. 2002 | |
| GRK | AR | CSNB | Yamamoto et al. 1997 | |
| GUCA1A | AD | CD, CRD | Payne et al. 1998; Sokal et al. 1998 | |
| GUCA1B | AD | RP, MD | Sato et al. 2005 | |
| GUCY2D | AR, AD | LCA, CRD | Perrault et al. 1996; Kelsell et al. 1998 | |
| OPN1LW | XL | Deuteranopia, blue cone monochromacy | Nathans et al. 1986; Winderickx et al. 1992; Ayyagari et al. 1999 | |
| OPN1MW | XL | Protanopia, blue cone monochromacy | Nathans et al. 1986; Ayyagari et al. 1999 | |
| OPN1SW | AD | Tritanopia | Nathans et al. 1992; Weitz et al. 1992a,b | |
| PDE6A | AR | RP | Huang et al. 1995 | |
| PDE6B | AR, AD | RP, CSNB | McLaughlin et al. 1993; Gal et al. 1994 | |
| PDE6C | AR | CD, ACHM | Thiadens et al. 2009 | |
| PDE6G | AR | RP | Dvir et al. 2010 | |
| RDH12 | AR, AD | LCA, RP | Janecke et al. 2004; Perrault et al. 2004; Fingert et al. 2008 | |
| RGS9 | AR | Delayed cone adaptation | Nishiguchi et al. 2004 | |
| RGS9BP | AR | Delayed cone adaptation | Nishiguchi et al. 2004 | |
| RHO | AD, AR | RP, CSNB | Dryja et al. 1990; Farrar et al. 1990; Rosenfeld et al. 1992; Dryja et al. 1993 | |
| SAG | AR | RP, CSNB | Fuchs et al. 1995; Nakazawa et al. 1998 | |
ACHM, Achromatopsia; BBS, Bardet–Biedl syndrome; CD, cone dystrophy; CED, cranioectodermal dysplasia, also known as Sensenbrenner syndrome; CRD, cone–rod dystrophy; CSNB, congenital stationary night blindness; JBS, Joubert syndrome; JATD, Jeune asphyxiating thoracic dystrophy; LCA, Leber congenital amaurosis; MKS, Meckel–Gruber syndrome; MZSDS, Mainzer–Saldino syndrome; OFD, oral-facial-digital syndrome; OMD, occult macular dystrophy; RP, retinitis pigmentosa; SLS, Senior–Løken syndrome; STGD, Stargardt disease; USH, Usher syndrome.
PROTEIN TRANSPORT TO PSC
A unique feature of the photoreceptor OS is the high level of its renewal. Each day ∼10% of the OS is shed from the distal tip, which is replaced by new disc formation at the base of the PSC (Young 1967). This necessitates a robust system of protein synthesis in the IS and efficient trafficking of selected proteins to the photoreceptor OS.
Intraflagellar Transport in PSC
The axoneme, initiated at the mother centriole, is built and maintained by extending its distal (+) end (Pedersen and Rosenbaum 2008). Because protein synthesis occurs in the IS, the axoneme building blocks need to be transported to the distal end via intraflagellar transport (IFT) (Rosenbaum and Witman 2002; Pedersen and Rosenbaum 2008; Taschner et al. 2012). The anterograde transport from the base to the tip of the axoneme is mediated by IFT complex B (IFT-B), where kinesin-2 is the motor protein (Rosenbaum and Witman 2002). Kinesin-2 is a heterotrimeric protein composed of Kif3A, Kif3B, and KAP, which is further associated with 14 other IFT proteins that bind cargo molecules (Taschner et al. 2012). Once the axoneme and other PSC components have been delivered to the tip of the cilium, the IFT-B components are recycled back to the base of the cilium by retrograde transport mediated by IFT complex A (IFT-A) (Rosenbaum and Witman 2002). Dynein-2 is the motor protein of IFT-A and it is associated with six other IFT proteins (Taschner et al. 2012). Apart from the IFT complexes, Bardet–Biedl syndrome proteins (BBSome) are also involved in the transport of membrane proteins to the cilium (Taschner et al. 2012; Williams et al. 2014).
Because 10% of the PSC is shed and renewed every day, the necessity for the retrograde transport in this cell type was not clear. However, identification of IRD patients with mutations in genes coding for retrograde transport proteins (e.g., TTC21B) and proteins involved in switching from anterograde to retrograde IFT direction (e.g., IFT172) underlines the importance of transport in both directions for the development and maintenance of PSC (Liu et al. 2010; Davis et al. 2011; Halbritter et al. 2013; Bujakowska et al. 2014).
Transport of Membrane Proteins to PSC
Even though the plasma membrane surrounding the photoreceptor cilium is continuous with the plasma membrane of the cell body, its protein composition is different. In addition, the membranous discs in the rod photoreceptors are distinct from the surrounding plasma membrane (Steinberg et al. 1980; Molday and Molday 1987). This selective protein content in PSC membranes is established by diffusional barriers present at the base of the cilium and within the transition zone (Pearring et al. 2013; Wang and Deretic 2014; Khanna 2015). The molecular composition of the diffusional barrier is not fully understood, although certain proteins like Septin 2 and CEP290 are thought to play an important role (Pearring et al. 2013; Wang and Deretic 2014).
Because rhodopsin is the most abundant protein in the PSC, its photoreceptor OS transport has been studied in detail. After synthesis in the IS endoplasmic reticulum and transport through the Golgi and trans-Golgi network, rhodopsin is sorted into vesicles destined for the OS. This is achieved thanks to the presence of specific sequence signatures (e.g., VXPX and FR motifs), which facilitate interaction with a ciliary targeting molecules Arf4 and ASAP1 (Deretic et al. 2005; Wang and Deretic 2014). Further interaction with FIP3 and small GTPases Rab6, Rab8, and Rab11 directs the vesicle to the base of the OS for fusion with the membrane (Deretic et al. 2005; Pearring et al. 2013; Wang and Deretic 2014). Further, rhodopsin molecules are transported through the transition zone to the site of the disc morphogenesis by two motor proteins, kinesin II and myosin VIIa, as shown in Kif3a and Myo7a knockout mice (Liu et al. 1999; Williams 2002). As mentioned before, kinesin II mediates a microtubule-dependent anterograde IFT (Rosenbaum and Witman 2002). Myosin VIIa is an actin-dependent motor molecule and, in mouse photoreceptors, it locates to the periciliary membrane complex; however, in primates, it locates to the calyceal processes (Sahly et al. 2012). It is, therefore, unclear whether mutations in Myosin VIIa in humans also lead to the aberrant opsin trafficking as shown in mice (Liu et al. 1999). Immunoelectron microscope studies of Rana pipiens frog photoreceptors, revealed that actin is present not only in calyceal processes but also at the sites of disc morphogenesis, suggesting involvement of actin-mediated transport in protein delivery to the forming discs (Chaitin et al. 1984). Little is known about OS targeting of other OS-specific transmembrane proteins, apart from retinol dehydrogenase (RDH8), which also contains the VXPX motif and PRPH2, which has its own OS-targeting sequence (Pearring et al. 2013).
Photoactivated Protein Diffusion
The base of the OS does not contain a selective barrier for the soluble proteins as shown in mice by the light-activated translocation of phototransduction proteins: transducin, arrestin, and recoverin (Sokolov et al. 2002; Calvert et al. 2006). Furthermore, this translocation is thought to be energy-independent, implying that the protein movement occurs by simple diffusion (Nair et al. 2005; Calvert et al. 2010). The diffusion of the proteins is dependent, however, on the steric interactions between the molecules and cell structures, termed steric volume exclusion, which reduces the entry of larger molecular weight proteins to the OS (Najafi and Calvert 2012). Light-mediated translocation of these proteins is thought to play a role in adaptation to different light conditions, in which for instance concentrating transducin in the rod OS in the darkness amplifies the phototransduction signal, and translocation of arrestin to OS in light conditions terminates transducin activation and accelerates photopigment recovery (Pearring et al. 2013). A neuroprotective role for the light-induced protein translocation has also been suggested (Pearring et al. 2013).
RETINAL CILIOPATHIES
Mutations in genes coding for ciliary proteins lead to ciliopathies, rare genetic disorders that may affect one or more organs, including the retina, central nervous system, olfactory epithelium, cardiovascular system, liver, kidney, skeletal system, gonads, and adipose tissue (Goetz and Anderson 2010; Patel and Honoré 2010; Mockel et al. 2011; Waters and Beales 2011). In this review, we will focus on ciliopathies that involve the retina, manifesting most commonly as retinitis pigmentosa (RP) (Hamel 2006; Hartong et al. 2006; Berger et al. 2010) or Leber congenital amaurosis (LCA) (Weleber 2002; Chung and Traboulsi 2009). RP is a condition that primarily affects rod photoreceptors and retinal pigment epithelium. It is the most frequent cause of the IRDs, with a prevalence of ∼1/3500 and accounting for roughly 25% of vision loss in adults (Hamel 2006; Hartong et al. 2006; Berger et al. 2010). It may start in the first or second decade of life, often with nyctalopia and peripheral vision loss as early symptoms, because of the dysfunction of PSCs and photoreceptor cell death in the peripheral retina. In many cases, the disease progresses to include central vision loss as well, because of eventual dysfunction of PSCs and death of photoreceptor cells in the macula (central retina) (Fig. 2) (Hamel 2006; Hartong et al. 2006; Berger et al. 2010). LCA affects rods and cones and leads to vision loss in infancy or early childhood (Weleber 2002; den Hollander et al. 2008; Chung and Traboulsi 2009). LCA is rare, with a population frequency of ∼1/50,000, yet affecting ∼20% of children attending schools for the blind (Weleber 2002; Koenekoop 2004; Berger et al. 2010). Other subtypes of IRD are present in ciliopathy patients and often involve cone photoreceptors and the macula (Michaelides et al. 2006; Estrada-Cuzcano et al. 2012c).
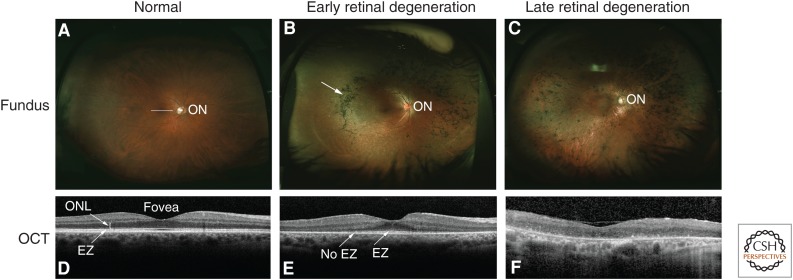
Figure 2.
Clinical features of inherited retinal degeneration (IRD). Fundus photos and optical coherence tomography (OCT) images of normal and diseased retinas are shown. (A) Wide-field fundus photo shows the appearance of a normal retina; the optic nerve (ON) is visible. (B) Fundus image from a patient with early retinal degeneration because of retinitis pigmentosa (RP). The arrow shows the pigment changes, which are characteristic of this disorder. (C) Fundus image from a patient with advanced retinal degeneration because of RP. (D) A cross-sectional image of the center of the normal retina (macula; white line in A) obtained with OCT shows normal retinal layers, including the outer nuclear layer (ONL), where photoreceptor cell nuclei are located. The central indentation is normal, and indicates the fovea. The white band showing the elipsoid zone (EZ, arrow) is generated from the junction of the inner and outer segments of photoreceptor cells, and its presence indicates normal photoreceptor cell and thus PSC structure. (E) The OCT image shows loss of photoreceptor cells peripherally, with the ONL visible only near the fovea. The EZ is evident centrally, but is lost more peripherally, indicating loss of PSCs by the more peripheral photoreceptor cells present. (F) The OCT image shows loss of the ONL and thus all of the photoreceptor cells in the macula of this patient consistent with greatly reduced central vision.
Nonsyndromic Retinal Ciliopathies
As mentioned above, photoreceptor OS can be regarded as specialized cilia designed to detect light and to convert this information into a biochemical signal. Therefore, we consider that all proteins that participate in this sensory function, as well as proteins that build the PSC structure, are in effect cilia proteins. Consequently, we can distinguish two groups of retinal ciliopathies: (1) affecting the structure, and (2) the sensory function of photoreceptor OS.
Mutations in Genes Disrupting POS Structure
There are currently 36 known genes that have been identified to harbor mutations that disrupt PSC structure and can lead to an isolated or syndromic retinal degeneration (Table 1). Thirteen genes that encode axoneme or basal body-associated proteins (C2ORF71, FAM161A, KIZ, LCA5, MAK, NEK2, RAB28, RPGRIP1, RP1, RP1L1, RP2, SPATA7, TOPORS) and seven genes coding for other structural PSC components (CDHR1, EYS, FSCN2, PROM1, PRPH2, ROM1, TULP1) have been exclusively associated with nonsyndromic retinal degeneration (Kajiwara et al. 1991, 1994; Bascom et al. 1992; Banerjee et al. 1998; Hagstrom et al. 1998; Guillonneau et al. 1999; Hardcastle et al. 1999; Mears et al. 1999; Pierce et al. 1999; Maw et al. 2000; Dryja et al. 2001; Wada et al. 2001, 2003; Chakarova et al. 2007; den Hollander et al. 2007; Abd El-Aziz et al. 2008; Collin et al. 2008; Wang et al. 2009; Akahori et al. 2010; Bandah-Rozenfeld et al. 2010; Collin et al. 2010; Henderson et al. 2010; Langmann et al. 2010; Nishimura et al. 2010; Ostergaard et al. 2010; Ozgül et al. 2011; Tucker et al. 2011; Estrada-Cuzcano et al. 2012b; Davidson et al. 2013; Nishiguchi et al. 2013; Roosing et al. 2013; El Shamieh et al. 2014). A query of the human proteome map (Kim et al. 2014) shows that nine of these genes (CDHR1, FSCN2, MAK, PROM1, PRPH2, ROM1, RP1, RP1L1, TULP1) are predominantly expressed in the human retina, which corroborates with the retina-specific phenotype. With the exception of LCA5, which shows no significant expression in any of the assayed tissues, the remaining genes are also significantly expressed in other human tissues, and it remains unclear why mutations in these genes affect specifically the retina.
Two genes stand apart in IRD ciliopathies, USH2A and CLRN1. Mutations in these genes lead to isolated retinal degeneration (Rivolta et al. 2000; Khan et al. 2011) or to deaf–blindness, called Usher syndrome (Eudy et al. 1998; Joensuu et al. 2001). There is only one report of nonsyndromic IRD because of mutations in CLRN1 (Khan et al. 2011); however, mutations in USH2A are the leading cause of the nonsyndromic autosomal recessive IRD, accounting for ∼9% of RP patients (Hartong et al. 2006). These two genes will be further discussed in the section on Usher syndrome.
The remaining 12 genes code for axoneme proteins and are associated with isolated retinopathy or syndromic disease (Table 1). In certain cases, the broad spectrum of phenotypes associated with mutations in a given gene can be explained by the primary mutations in the gene, where mutations in the retina-specific transcripts or hypomorphic alleles may lead to the isolated retinal phenotype (Ansley et al. 2003; den Hollander et al. 2006; Coene et al. 2009; Riazuddin et al. 2010; Webb et al. 2012; Xu et al. 2015). In other cases, the relationship between the primary disease-causing mutation and the phenotype is not clear and epistatic effects of other alleles have been suggested (Badano et al. 2003b, 2006; Estrada-Cuzcano et al. 2012a; Bujakowska et al. 2014). (Both cases will be discussed in detail in the section Broad Phenotypic Spectrum of Ciliopathies and Genetic Modifiers.)
Mutations in Genes Impeding POS Sensory Function
A less obvious subgroup of nonsyndromic retinal ciliopathies are IRDs in which PSC sensory function is affected. This classification is analogous to another ciliopathy, the dominant form of polycystic kidney disease, because of mutations in PKD1 or PKD2, which code for membrane proteins that act as a cilia sensor and a calcium channel, respectively (Ong and Harris 2015). Similarly, genetic mutations that affect phototransduction cascade and retinoid cycle in the photoreceptors are also considered as ciliopathies (Table 1). However, we do not consider as ciliopathies IRDs that are caused by mutations in genes that are expressed in the RPE or code for proteins that function in other compartments of the photoreceptor cell (e.g., splicing factor genes). A comprehensive list of all IRD genes can be found at the Retinal Information Network (RetNet) portal (sph.uth.edu/retnet/home.htm).
Usher Syndrome
Usher syndrome is an autosomal recessive dual impairment of vision and sensorineural hearing with a prevalence of ∼1/25,000 people (Kremer et al. 2006; Millán et al. 2011; Bonnet and El-Amraoui 2012). It is phenotypically and genetically heterogeneous and most of the patients fall into one of the three clinical subtypes of decreasing severity: Usher syndrome type I (USH1), type II (USH2), and type III (USH3).
The most severe form, USH1, is not strictly a ciliopathy because the proteins coded by the six associated genes: MYO7A (Weil et al. 1995), USH1C (Verpy et al. 2000), CDH23 (Bolz et al. 2001), PCDH15 (Ahmed et al. 2001), USH1G (Weil et al. 2003), and CIB2 (Riazuddin et al. 2012), locate to actin-based structures in the periciliary region, called the calyceal processes, and not to cilia themselves (Sahly et al. 2012). The function of these structures is unclear and they might have a structural role supporting the photoreceptor outer segments or be involved in fine tuning of the photoreceptor signaling. Calyceal processes are not present in all of the vertebrates and their absence in rodents may account for the subtle retinal phenotypes in most of the USH1 murine models, in which hearing and vestibular phenotypes are profound (Liu et al. 1999; Di Palma et al. 2001; Libby and Steel 2001; Johnson et al. 2003; Ahmed et al. 2008; Williams 2008; Miyasaka et al. 2013). In the ear, USH1 proteins are involved in the maturation of the stereocilia in the auditory hair cells of the inner-ear cochlea, where they form transient links between the kinocilium and stereocilia during development and the tip links between the mature stereocilia (Kremer et al. 2006; Bonnet and El-Amraoui 2012; Riazuddin et al. 2012; Sahly et al. 2012).
USH2 is caused by mutations in one of three genes: USH2A (Eudy et al. 1998), GPR98 (Weston et al. 2004), and DFNB31 (Mburu et al. 2003). The products of these genes locate to the periciliary membrane complex adjacent to the transition zone of the photoreceptor cilia (Liu et al. 2007b; Sahly et al. 2012). In the cochlea, they form the transient ankle links between the developing sterocilia (reviewed in Bonnet and El-Amraoui 2012).
USH3, the least severe form of Usher, is caused by mutations in CLRN1 or HARS (Joensuu et al. 2001; Puffenberger et al. 2012). In mice, Clarin-1 locates to the base of the photoreceptors cilia and to synaptic ribbons (Zallocchi et al. 2009); however, it does not seem to be essential for the photoreceptor function in rodents, because homozygous Clrn1 knockout mice show no retinal phenotype (Geller et al. 2009). In the cochlea, Clarin-1 locates to the apical and basal aspects of stereocilia depending on the developmental stage (Zallocchi et al. 2009). HARS codes for a histidyl-tRNA synthetase and, currently, the mechanism of the disease is unknown (Puffenberger et al. 2012).
Of the three Usher types, the most frequent is USH2 (56%–57% cases), in which mutations in the USH2A gene are the most common (Millán et al. 2011). USH1 represents 33%–44% of all Usher cases, followed by USH3, with a prevalence of 2% (Yan and Liu 2010; Millán et al. 2011). However, in certain populations (Finland and Ashkenazi Jewish), USH3 reaches 40% of all Usher patients because of founder mutations (Yan and Liu 2010; Millán et al. 2011). Atypical Usher syndromes have also been reported in which the deaf–blindness phenotype is explained by mutations in two different genes: USH2A with PDZD7 and C2orf71 with CEP250 (Table 2) (Ebermann et al. 2010; Khateb et al. 2014). Recently, two groups have reported another form of Usher syndrome because of mutations in CEP78, which manifest as a cone–rod dystrophy accompanied by sensorineural hearing loss involving mainly high frequencies (Nikopoulos et al. 2016; Sharon et al. 2016). Hearing loss can also be part of other syndromes, such as Altrom, as will be discussed later (Mockel et al. 2011).
Table 2.
Genetic modifiers of ciliopathies
| Primary gene | Modifier | Phenotype | Evidence | References |
|---|---|---|---|---|
| PRPH2/RDS p.(Leu185Pro) ROM1 p.(Gly80fs) and p.(Leu114fs) | Digenic inheritance | RP | Three families (11 affected in total) | Kajiwara et al. 1994 |
| BBS2 p.[Tyr24*] and p.[Gln59*] BBS6 p.(Gln147*) | Digenic inheritance | BBS | In one family, the affected sib carried three alleles, whereas the unaffected sib is the only compound heterozygous for the BBS2 mutations; three additional families with a probable triallelic BBS2/BBS6 inheritance | Katsanis et al. 2001 |
| RPE65 p.(Glu102*) | GUCY2D p.(Ile539Val) | More severe RD | One family with two sibs, targeted analysis of: GUCY2D, RPE65, CRX, AIPL1, and RPGRIP1 | Silva et al. 2004 |
| BBS1 p.(Met390Arg) | ARL6 p.(Gly169Ala) | More severe ciliopathy | In one family of two affected sibs, the sister carrying the additional ALR6 allele showed a more severe phenotype | Fan et al. 2004 |
| BBS1 and unknown BBS genotypes | CCDC28B (c.330C>T, p.(=)) (originally noted as C430T) | Increased severity of disease, general mutational load | In three families, variant associated with more severe BBS; variant also enriched in BBS patients (14/226) over controls (4/274) | Badano et al. 2006 |
| Mixed ciliopathy cohort | RPGRIP1L p.(Ala229Thr) | Presence of RD in ciliopathies | Targetted screening of RPGRIP1L; enrichment of 226Thr variant in ciliopathy patients with RD (43/487) over ciliopathy patients without RD (0/115); functional data, showing a decreased biding activity to RPGR | Khanna et al. 2009 |
| BBS mixed cohort | MKS1 p.(Arg123Gln) p.(Asp286Gly) p.(Ile450Thr) p.(Val339Met) (specific to short isoform) | More severe ciliopathy phenotype, seizures in five of six patients | Targetted screening of MKS1 in BBS cohort, heterozygous potentially modifying changes found in six patients from five families (5/155); functionally, variants ranged from mild hypomorphs to null | Leitch et al. 2008 |
| BBS9 and CEP290 | TMEM67 (c.2241G>A) and p.(Ser320Cys) | General mutational load | Potentially pathogenic alleles found in two families: c.2241G>A affects the canonical splice site and is predicted to lead to exon skipping; p.(Ser320Cys) was functionally null | Leitch et al. 2008 |
| PRPH2 | ROM1 p.(Arg229His) and/or ABCA4 p.(Val2050Leu) | Increased macular involvement in RD | One family, eight affected; microarray mutation detection in 16 genes, full sequencing of PRPH2, ROM1, and ABCA4 | Poloschek et al. 2010 |
| USH2A | PDZD7 p.(Arg56fs) | More severe RD | One family with two sibs; targeted analysis of PDZD7 and other Usher genes; digenic inheritance stated but insufficient genetic data to prove it | Ebermann et al. 2010 |
| CEP290 | AHI1 p.(Asn811Lys) and p.(His758Pro) | More severe neurological phenotype | Targeted screening of AHI1 in eight patients; variants detected in two patients | Coppieters et al. 2010 |
| NPHP1 and unknown NPHP genotypes | AHI1 p.(Arg830Trp) | Presence of RD | Targeted screening of AHI1 in 153 NPHP ± RD patients | Louie et al. 2010 |
| Mixed MKS and BBS cohort | C2ORF86 (various changes) | General mutational load | Targeted screening of C2ORF86; enrichment of nonsynonymous coding changes in patients (6/192) versus controls (0/384) | Kim et al. 2010 |
| RPGR | RPGRIP1L p.(Arg744Gln) and IQCB1 p.(Ile393Asn) | Severity of RD | Targeted screening of RPGRIP1, RPGRIP1L, CEP290, and IQCB1 in 98 male patients; the results were marginally significant | Fahim et al. 2011 |
| Mixed ciliopathy cohort | TTC21B (various changes) | General mutational load | Targeted screening of TTC21B; enrichment of pathogenic changes in ciliopathy patients (28/555) over controls (4/305) | Davis et al. 2011 |
| C2orf71 p.(Gln1097*) | CEP250 p.(Arg1155*) | More severe retinal degeneration + hearing loss | Homozygosity mapping in family with seven affected, subsequent WES in two affected; homozygous CEP250 mutation leads to an early-onset severe hearing loss with a mild retinal degeneration and an additional homozygous stop mutation in C2orf71 exacerbated the retinal phenotype in three individuals | Khateb et al. 2014 |
| Mixed BBS cohort | NPHP1 whole gene deletion and p.(Arg5Leu) | General mutational load in ciliopathies | Targeted analysis of NPHP1 in a BBS cohort of 200 families, mutations enriched in the patient population compared with control (incidence of 1.5% (deletion) and 2.5% (missense); functional data for NPHP1-BBS genes interaction | Lindstrand et al. 2014 |
| PRPH2 (c.828+3A>T) | PRPH2 p.[(Glu304;Lys310;Gly338)] haplotype in trans with the causal mutation | More severe retinal phenotype | p.[(Glu304;Lys310;Gly338)] haplotype in trans with the splice site mutation was associated with a more severe phenotype as investigated in 62 patients | Shankar et al. 2016 |
BBS, Bardet–Biedl syndrome; RD, retinal degeneration; RP, retinitis pigmentosa.
Other Syndromic IRDs
Based on the presence of particular symptoms, ciliopathies are subdivided into different subtypes, traditionally named after clinicians who first described them. Here, we will review the major syndromes, which involve the retina. Even though these conditions are considered as distinct clinical entities, it is being increasingly recognized that there is a large phenotypic overlap between these diseases, in which characteristic features of two different syndromes are present in the same patient or distinct ciliopathies co-occur in single families (Lehman et al. 2010; Zaki et al. 2011; Valente et al. 2014). Traditional naming of these conditions is therefore often inaccurate and depends on the clinicians’ training and their specialty. To overcome this bias, we believe that it is crucial to include in the syndrome naming the underlying molecular cause of the disease (e.g., AHI1-associated ciliopathy). However, for the purpose of this review, we describe each syndrome as traditionally called and present the genes associated with them.
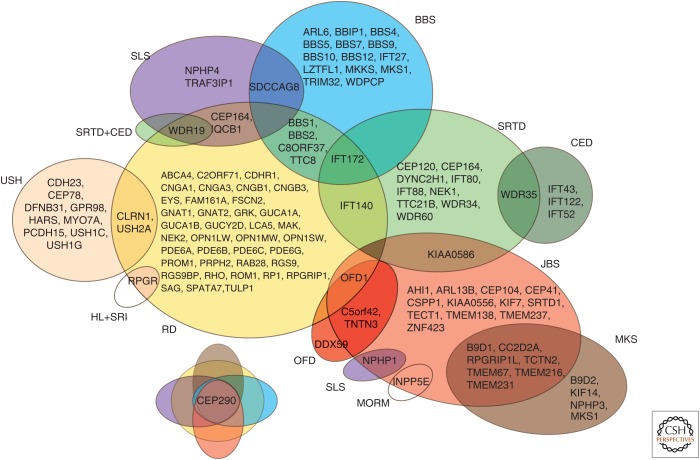
Senior–Løken syndrome (SLS) is an autosomal recessive disease characterized by juvenile nephronophtitis (NPHP) and early-onset retinal degeneration (Løken et al. 1961; Senior et al. 1961). NPHP is a medullary cystic kidney disease leading to the end-stage renal failure later in childhood or in adolescence (Ronquillo et al. 2012). About 10% of NPHP patients also have retinal degeneration (Otto et al. 2005; Mockel et al. 2011). So far, eight genes are associated with SLS (CEP164, CEP290, IQCB1, NPHP1, NPHP4, SDCCAG8, TRAF3IP1, WDR19), although other NPHP-associated genes are mutated in different syndromes involving the retina (Caridi et al. 1998; Otto et al. 2002, 2005, 2010; Sayer et al. 2006; Chaki et al. 2012; Coussa et al. 2013; Bizet et al. 2015). There is a considerable overlap between SLS and other ciliopathies, where almost all SLS genes are associated with other diseases (Fig. 3).
Figure 3.
A Venn diagram showing a phenotypic and genetic overlap between different forms of ciliopathy. BBS, Bardet–Biedl syndrome; CED, cranioectodermal dysplasia (also known as Sensenbrenner syndrome); HL + SRI, hearing loss and sinorespiratory infections; JBS, Joubert syndrome; MKS, Meckel–Gruber syndrome; MORM, mental retardation, truncal obesity, retinal degeneration, and micropenis; OFD, oral-facial-digital syndrome; RD, retinal degeneration—nonsyndromic; SLS, Senior–Løken syndrome; SRTD, short-rib thoracic dysplasia; USH, Usher syndrome.
Joubert syndrome (JBS) is a neurological condition characterized by a distinctive abnormality of the midbrain–hindbrain junction and cerebellar vermis hypoplasia, presenting as the molar tooth sign (MTS) on brain imaging (Maria et al. 1997; Valente et al. 2013). These neurological defects correlate with the clinical presentation of hypotonia, ataxia, abnormal breathing, developmental delay, and abnormal ocular movements. JBS patients may also present with retinal degeneration, renal or hepatic defects, polydactyly, and orofacial dysmorphism (Mockel et al. 2011; Valente et al. 2013). The prevalence of JBS is estimated to be between 1/80,000 and 1/100,000 of live births (Valente et al. 2014). Mutations in 26 genes have been reported to cause JBS (AHI1, ARL13B, B9D1, C5orf42, CC2D2A, CEP104, CEP290, CEP41, CSPP1, INPP5E, KIAA0556, KIAA0586, KIF7, NPHP1, OFD1, RPGRIP1L, SRTD1, TCTN2, TCTN3, TECT1, TMEM67, TMEM138, TMEM216, TMEM231, TMEM237, ZNF423) (Dixon-Salazar et al. 2004; Ferland et al. 2004; Parisi et al. 2004; Sayer et al. 2006; Baala et al. 2007b; Delous et al. 2007; Cantagrel et al. 2008; Gorden et al. 2008; Noor et al. 2008; Bielas et al. 2009; Coene et al. 2009; Edvardson et al. 2010; Dafinger et al. 2011; Garcia-Gonzalo et al. 2011; Huang et al. 2011; Chaki et al. 2012; Lee et al. 2012a,b; Srour et al. 2012a,b; Thomas et al. 2012; Romani et al. 2014; Shaheen et al. 2014; Thomas et al. 2014; Tuz et al. 2014; Sanders et al. 2015). All except one of the above genes cause autosomal-recessive or X-linked disease; ZNF423 has been associated with a dominant JBS form, although this association showed limited genetic evidence (Chaki et al. 2012). ZNF423 is also the only JBS gene, which is not associated with the cilium but with the DNA damage response pathway (Chaki et al. 2012).
Meckel–Gruber syndrome, also known as Meckel syndrome (MKS) is a neonatal lethal autosomal recessive disorder defined by the malformation of the central nervous system (occipital encephalocele), cystic kidneys, and liver fibrosis (Wright et al. 1994; Logan et al. 2011). Other features that may be present are postaxial or preaxial polydactyly, skeletal dysplasia, cleft lip/palate, microphthalmia, optic nerve coloboma, heart defects, genital anomalies, and complete or partial situs inversus (Logan et al. 2011). Mutations in 12 genes have been associated with MKS (B9D1, B9D2, CC2D2A, CEP290, KIF14, MKS1, NPHP3, RPGRIP1L, TCTN2, TMEM67, TMEM216, TMEM231) (Kyttälä et al. 2006; Smith et al. 2006; Baala et al. 2007a; Delous et al. 2007; Bergmann et al. 2008; Tallila et al. 2008; Valente et al. 2010; Dowdle et al. 2011; Hopp et al. 2011; Shaheen et al. 2011, 2013; Filges et al. 2014). There is a considerable genetic overlap between JBS and MKS, in which eight of the genes are shared between the two syndromes and co-occurrence of the two diseases was reported in the same families (Valente et al. 2014). The incidence of MKS varies among populations and it has been estimated as 1/13,250 in the United States, 1/140,000 in the United Kingdom, and 1/9000 in Finland (Logan et al. 2011).
Bardet–Biedl syndrome (BBS) is an autosomal recessive condition defined by rod–cone degeneration, postaxial polydactyly, central obesity, mental retardation, hypogonadism, and renal dysfunction (Beales et al. 1999; Mockel et al. 2011). Other features such as hepatic fibrosis, diabetes mellitus, endocrinological disturbances, heart disease, and short stature may also be present (Beales et al. 1999). Twenty genes have been associated with BBS (ARL6, BBIP1, BBS1, BBS2, BBS4, BBS5, BBS7, BBS9, BBS10, BBS12, CEP290, IFT27, IFT172, LZTFL1, MKKS, MKS1, SDCCAG8, TRIM32, TTC8, WDPCP) (Katsanis et al. 2000; Slavotinek et al. 2000; Mykytyn et al. 2001, 2002; Nishimura et al. 2001, 2005; Ansley et al. 2003; Badano et al. 2003a; Chiang et al. 2004, 2006; Fan et al. 2004; Li et al. 2004; Stoetzel et al. 2006, 2007; Leitch et al. 2008; Kim et al. 2010; Otto et al. 2010; Marion et al. 2012; Aldahmesh et al. 2014; Bujakowska et al. 2014; Scheidecker et al. 2014). Apart from the BBS genes associated with the nonsyndromic IRD (Table 1), a homozygous nonsense mutation (p.S701X) in BBS12 was shown to lead to a late-onset retinal degeneration and postaxial polydactyly but no other BBS-associated clinical features (Pawlik et al. 2010).
There are two phenotypically similar diseases to BBS: Alstrom syndrome (ALMS) and MORM syndrome (mental retardation, truncal obesity, retinal degeneration, and micropenis). ALMS is characterized by cone–rod degeneration, sensorineural hearing loss, childhood obesity, and type 2 diabetes mellitus. ALMS patients often present with cardiomyopathy and other features such as renal, pulmonary, or hepatic disease may also be present. In contrast to BBS, ALMS is not associated with mental retardation, polydactyly, or hypogonadism (Mockel et al. 2011). Mutations in only one gene, ALMS1, have been associated with Alstrom syndrome (Collin et al. 2002). MORM has been described in only one Pakistani family of 14 individuals, in whom a homozygous truncating mutation in INPP5E, was found to cause the disease (Hampshire et al. 2006; Jacoby et al. 2009).
Short-rib thoracic dysplasia (SRTD) with or without polydactyly regroups syndromes formerly known as Mainzer–Saldino (MZSDS), Jeune asphyxiating thoracic dystrophy (JATD), and Ellis–van Creveld (EVC) syndromes. SRTDs are autosomal recessive skeletal ciliopathies, characterized by short ribs, constricted thoracic cage, shortened tubular bones, and a “trident” appearance of the acetabular roof (Huber and Cormier-Daire 2012). The severely constricted thoracic cage leads to respiratory insufficiency, often resulting in death in infancy. Other features that may be present are polydactyly, cleft lip/palate, retinal degeneration, and anomalies of the brain, heart, kidneys, liver, pancreas, intestines, and genitalia (Waters and Beales 2011; Huber and Cormier-Daire 2012). There is a phenotypic and genetic overlap between SRTDs and cranioectodermal dysplasia (CED), also known as Sensenbrenner syndrome. CED is characterized by sagittal craniosynostosis, narrow thorax, short limbs, brachydactyly, protuberant abdomen, and facial and ectodermal anomalies (Huber and Cormier-Daire 2012). Seventeen genes have been associated with these diseases (CEP120, DYNC2H1, EVC, EVC2, IFT52, IFT122, IFT140, IFT172, IFT43, IFT80, KIAA0586, NEK1, TTC21B, WDR19, WDR34, WDR35, WDR60) (Ruiz-Perez et al. 2000; Galdzicka et al. 2002; Beales et al. 2007; Dagoneau et al. 2009; Gilissen et al. 2010; Walczak-Sztulpa et al. 2010; Arts et al. 2011; Bredrup et al. 2011; Davis et al. 2011; Mill et al. 2011; Thiel et al. 2011; Perrault et al. 2012; Halbritter et al. 2013; McInerney-Leo et al. 2013; Schmidts et al. 2013b; Alby et al. 2015; Shaheen et al. 2015; Girisha et al. 2016). Interestingly, some of these genes have also been implicated with a nonsyndromic disease, for example, TTC21B and WDR19 in NPHP (Bredrup et al. 2011; Davis et al. 2011) or IFT172 and IFT140 in RP (Fig. 3) (Bujakowska et al. 2014; Bifari et al. 2015; Xu et al. 2015).
BROAD PHENOTYPIC SPECTRUM OF CILIOPATHIES AND GENETIC MODIFIERS
One of the important aspects of the IRDs is that mutations in the same gene can lead to variable phenotypes (Ferrante et al. 2001; Ansley et al. 2003; Sayer et al. 2006; Perrault et al. 2007; Frank et al. 2008; Leitch et al. 2008; Coene et al. 2009; Riazuddin et al. 2010; Bujakowska et al. 2012; Estrada-Cuzcano et al. 2012a; Webb et al. 2012). In some cases, the severity of disease can be explained by the primary disease-causing mutation. For example, a splice site mutation of a retina-specific exon in TTC8 leads to a nonsyndromic RP (Riazuddin et al. 2010), whereas the gene is most commonly associated with BBS (Ansley et al. 2003). The position of a mutation may also determine the phenotype as in the case of truncating mutations in OFD1. Nonsense mutations downstream from exon 17 lead to an X-linked dominant oral-facial-digital type 1 (OFD1) syndrome, manifesting with malformations of face, oral cavity, and digits in affected females and lethal in males (Ferrante et al. 2001). However, truncating mutations upstream of exon 17 lead to an X-linked recessive JBS (Coene et al. 2009). In addition, hypomorphic alleles can arise by mutations activating cryptic splice sites, which leads to severely reduced levels of wild-type transcripts as in the case of CEP290 (den Hollander et al. 2006) and OFD1 (Webb et al. 2012).
In many cases, however, even a precise genetic diagnosis does not yield a clear genotype–phenotype correlation and the severity of disease can vary greatly even between patients with the same genetic cause of disease. Examples of this include family members that share the same 3bp deletion (c.461_463del) in the PRPH2 gene but show phenotypes varying from RP involving the peripheral retina to macular disease involving only the central retina (Weleber et al. 1993). Similarly, individuals with mutations in the RP1 gene show variable phenotypes, ranging from near normal to profoundly affected by retinal degeneration (Jacobson et al. 2000; Berson et al. 2001). Several genetic modifiers have already been identified in IRD disease (Table 2), in which extreme examples are cases of digenic inheritance of nonsyndromic IRD (Kajiwara et al. 1994) and BBS (Katsanis et al. 2001) or the rescuing effect of the wild-type PRPF31 allele in the dominant PRPF31-associated disease (McGee et al. 1997; Vithana et al. 2003; Rose et al. 2016). Even though more than a dozen of genetic modifiers of IRD disease severity have been reported, our knowledge about these variants is still limited because the studies were conducted on a limited number of patients (sometimes single families) targeting a small number of genes and functional validation was not always performed (Table 2). In addition, no study has yet shown the validity of the previously reported modifiers and therefore they remain to be scrutinized by future research.
CONCLUSIONS
In summary, mutations in many different genes can cause retinal ciliopathies, reflecting the diversity of protein functions required for normal PSC function. As indicated, it is increasingly clear that the phenotypes ascribed to specific genetic forms of disease overlap, and thus a revised system of disease definitions that includes the genetic etiology in the disease name would improve our understanding of these disorders, and their description for patients and clinicians. Further, as we have attempted to illustrate, studies of retinal ciliopathies have provided insights into syndromic disorders, and cilia function in general. Given the ubiquitous presence of cilia on mammalian cells, we anticipate that further study of these disorders and their pathogenesis will continue to inform us about cilia function broadly, and to be informed by the results of cilia in other contexts.
Footnotes
Editors: Wallace Marshall and Renata Basto
Additional Perspectives on Cilia available at www.cshperspectives.org
REFERENCES
- Abd El-Aziz MM, Barragan I, O’Driscoll CA, Goodstadt L, Prigmore E, Borrego S, Mena M, Pieras JI, El-Ashry MF, Safieh LA, et al. 2008. EYS, encoding an ortholog of Drosophila spacemaker, is mutated in autosomal recessive retinitis pigmentosa. Nat Genet 40: 1285–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Safieh L, Al-Anazi S, Al-Abdi L, Hashem M, Alkuraya H, Alamr M, Sirelkhatim MO, Al-Hassnan Z, Alkuraya B, Mohamed JY, et al. 2012. In search of triallelism in Bardet–Biedl syndrome. Eur J Hum Genet 20: 420–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed ZM, Riazuddin S, Bernstein SL, Ahmed Z, Khan S, Griffith AJ, Morell RJ, Friedman TB, Wilcox ER. 2001. Mutations of the protocadherin gene PCDH15 cause Usher syndrome type 1F. Am J Hum Genet 69: 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed ZM, Kjellstrom S, Haywood-Watson RJL, Bush RA, Hampton LL, Battey JF, Riazuddin S, Frolenkov G, Sieving PA, Friedman TB. 2008. Double homozygous waltzer and Ames waltzer mice provide no evidence of retinal degeneration. Mol Vis 14: 2227–2236. [PMC free article] [PubMed] [Google Scholar]
- Akahori M, Tsunoda K, Miyake Y, Fukuda Y, Ishiura H, Tsuji S, Usui T, Hatase T, Nakamura M, Ohde H, et al. 2010. Dominant mutations in RP1L1 are responsible for occult macular dystrophy. Am J Hum Genet 87: 424–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alby C, Piquand K, Huber C, Megarbané A, Ichkou A, Legendre M, Pelluard F, Encha-Ravazi F, Abi-Tayeh G, Bessières B, et al. 2015. Mutations in KIAA0586 cause lethal ciliopathies ranging from a hydrolethalus phenotype to short-rib polydactyly syndrome. Am J Hum Genet 97: 311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldahmesh MA, Safieh LA, Alkuraya H, Al-Rajhi A, Shamseldin H, Hashem M, Alzahrani F, Khan AO, Alqahtani F, Rahbeeni Z, et al. 2009. Molecular characterization of retinitis pigmentosa in Saudi Arabia. Mol Vis 15: 2464–2469. [PMC free article] [PubMed] [Google Scholar]
- Aldahmesh MA, Li Y, Alhashem A, Anazi S, Alkuraya H, Hashem M, Awaji AA, Sogaty S, Alkharashi A, Alzahrani S, et al. 2014. IFT27, encoding a small GTPase component of IFT particles, is mutated in a consanguineous family with Bardet–Biedl syndrome. Hum Mol Genet 23: 3307–3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aligianis IA, Forshew T, Johnson S, Michaelides M, Johnson CA, Trembath RC, Hunt DM, Moore AT, Maher ER. 2002. Mapping of a novel locus for achromatopsia (ACHM4) to 1p and identification of a germline mutation in the alpha subunit of cone transducin (GNAT2). J Med Genet 39: 656–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allikmets R, Singh N, Sun H, Shroyer NF, Hutchinson A, Chidambaram A, Gerrard B, Baird L, Stauffer D, Peiffer A, et al. 1997. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet 15: 236–246. [DOI] [PubMed] [Google Scholar]
- Ansley SJ, Badano JL, Blacque OE, Hill J, Hoskins BE, Leitch CC, Kim JC, Ross AJ, Eichers ER, Teslovich TM, et al. 2003. Basal body dysfunction is a likely cause of pleiotropic Bardet–Biedl syndrome. Nature 425: 628–633. [DOI] [PubMed] [Google Scholar]
- Arikawa K, Molday LL, Molday RS, Williams DS. 2011. Localization of peripherin/RDS in the disk membranes of cone and rod photoreceptors: Relationship to disk membrane morphogenesis and retinal degeneration. J Cell Biol 116: 659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arts HH, Bongers EMHF, Mans DA, van Beersum SEC, Oud MM, Bolat E, Spruijt L, Cornelissen EAM, Schuurs-Hoeijmakers JHM, de Leeuw N, et al. 2011. C14ORF179 encoding IFT43 is mutated in Sensenbrenner syndrome. J Med Genet 48: 390–395. [DOI] [PubMed] [Google Scholar]
- Ayyagari R, Kakuk LE, Coats CL, Bingham EL, Toda Y, Felius J, Sieving P. 1999. Bilateral macular atrophy in blue cone monochromacy (BCM) with loss of the locus control region (LCR) and part of the red pigment gene. Mol Vis 5: 13. [PubMed] [Google Scholar]
- Ayyagari R, Demirci FY, Liu J, Bingham EL, Stringham H, Kakuk LE, Boehnke M, Gorin MB, Richards JE, Sieving PA. 2002. X-linked recessive atrophic macular degeneration from RPGR mutation. Genomics 80: 166–171. [DOI] [PubMed] [Google Scholar]
- Baala L, Audollent S, Martinovic J, Ozilou C, Babron M-C, Sivanandamoorthy S, Saunier S, Salomon R, Gonzales M, Rattenberry E, et al. 2007a. Pleiotropic effects of CEP290 (NPHP6) mutations extend to Meckel syndrome. Am J Hum Genet 81: 170–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baala L, Romano S, Khaddour R, Saunier S, Smith UM, Audollent S, Ozilou C, Faivre L, Laurent N, Foliguet B, et al. 2007b. The Meckel–Gruber syndrome gene, MKS3, is mutated in Joubert syndrome. Am J Hum Genet 80: 186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badano JL, Ansley SJ, Leitch CC, Lewis RA, Lupski JR, Katsanis N. 2003a. Identification of a novel Bardet–Biedl syndrome protein, BBS7, that shares structural features with BBS1 and BBS2. Am J Hum Genet 72: 650–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badano JL, Kim JC, Hoskins BE, Lewis RA, Ansley SJ, Cutler DJ, Castellan C, Beales PL, Leroux MR, Katsanis N. 2003b. Heterozygous mutations in BBS1, BBS2 and BBS6 have a potential epistatic effect on Bardet–Biedl patients with two mutations at a second BBS locus. Hum Mol Genet 12: 1651–1659. [DOI] [PubMed] [Google Scholar]
- Badano JL, Leitch CC, Ansley SJ, May-Simera H, Lawson S, Lewis RA, Beales PL, Dietz HC, Fisher S, Katsanis N. 2006. Dissection of epistasis in oligogenic Bardet–Biedl syndrome. Nature 439: 326–330. [DOI] [PubMed] [Google Scholar]
- Bandah-Rozenfeld D, Mizrahi-Meissonnier L, Farhy C, Obolensky A, Chowers I, Pe’er J, Merin S, Ben-Yosef T, Ashery-Padan R, Banin E, et al. 2010. Homozygosity mapping reveals null mutations in FAM161A as a cause of autosomal-recessive retinitis pigmentosa. Am J Hum Genet 87: 382–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee P, Kleyn PW, Knowles JA, Lewis CA, Ross BM, Parano E, Kovats SG, Lee JJ, Penchaszadeh GK, Ott J, et al. 1998. TULP1 mutation in two extended Dominican kindreds with autosomal recessive retinitis pigmentosa. Nat Genet 18: 177–179. [DOI] [PubMed] [Google Scholar]
- Bareil C, Hamel CP, Delague V, Arnaud B, Demaille J, Claustres M. 2001. Segregation of a mutation in CNGB1 encoding the β-subunit of the rod cGMP-gated channel in a family with autosomal recessive retinitis pigmentosa. Hum Genet 108: 328–334. [DOI] [PubMed] [Google Scholar]
- Bascom RA, Manara S, Collins L, Molday RS, Kalnins VI, McInnes RR. 1992. Cloning of the cDNA for a novel photoreceptor membrane protein (rom-1) identifies a disk rim protein family implicated in human retinopathies. Neuron 8: 1171–1184. [DOI] [PubMed] [Google Scholar]
- Beales PL, Elcioglu N, Woolf AS, Parker D, Flinter FA. 1999. New criteria for improved diagnosis of Bardet–Biedl syndrome: Results of a population survey. J Med Genet 36: 437–446. [PMC free article] [PubMed] [Google Scholar]
- Beales PL, Bland E, Tobin JL, Bacchelli C, Tuysuz B, Hill J, Rix S, Pearson CG, Kai M, Hartley J, et al. 2007. IFT80, which encodes a conserved intraflagellar transport protein, is mutated in Jeune asphyxiating thoracic dystrophy. Nat Genet 39: 727–729. [DOI] [PubMed] [Google Scholar]
- Berger W, Kloeckener-Gruissem B, Neidhardt J. 2010. The molecular basis of human retinal and vitreoretinal diseases. Prog Retin Eye Res 29: 335–375. [DOI] [PubMed] [Google Scholar]
- Bergmann C, Fliegauf M, Brüchle NO, Frank V, Olbrich H, Kirschner J, Schermer B, Schmedding I, Kispert A, Kränzlin B, et al. 2008. Loss of nephrocystin-3 function can cause embryonic lethality, Meckel–Gruber-like syndrome, situs inversus, and renal-hepatic-pancreatic dysplasia. Am J Hum Genet 82: 959–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson EL, Grimsby JL, Adams SM, McGee TL, Sweklo E, Pierce EA, Sandberg MA, Dryja TP. 2001. Clinical features and mutations in patients with dominant retinitis pigmentosa-1 (RP1). Invest Ophthalmol Vis Sci 42: 2217–2224. [PubMed] [Google Scholar]
- Besharse JC, Forestner DM, Defoe DM. 1985. Membrane assembly in retinal photoreceptors. III: Distinct membrane domains of the connecting cilium of developing rods. J Neurosci 5: 1035–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielas SL, Silhavy JL, Brancati F, Kisseleva MV, Al-Gazali L, Sztriha L, Bayoumi RA, Zaki MS, Abdel-Aleem A, Rosti RO, et al. 2009. Mutations in INPP5E, encoding inositol polyphosphate-5-phosphatase E, link phosphatidyl inositol signaling to the ciliopathies. Nat Genet 41: 1032–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bifari IN, Elkhamary SM, Bolz HJ, Khan AO. 2015. The ophthalmic phenotype of IFT140-related ciliopathy ranges from isolated to syndromic congenital retinal dystrophy. Br J Ophthalmol 6: 829–833. [DOI] [PubMed] [Google Scholar]
- Bizet AA, Becker-Heck A, Ryan R, Weber K, Filhol E, Krug P, Halbritter J, Delous M, Lasbennes M-C, Linghu B, et al. 2015. Mutations in TRAF3IP1/IFT54 reveal a new role for IFT proteins in microtubule stabilization. Nat Commun 6: 8666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolz H, von Brederlow B, Ramírez A, Bryda EC, Kutsche K, Nothwang HG, Seeliger M, del C-Salcedó Cabrera M, Vila MC, Molina OP, et al. 2001. Mutation of CDH23, encoding a new member of the cadherin gene family, causes Usher syndrome type 1D. Nat Genet 27: 108–112. [DOI] [PubMed] [Google Scholar]
- Bonnet C, El-Amraoui A. 2012. Usher syndrome (sensorineural deafness and retinitis pigmentosa): Pathogenesis, molecular diagnosis and therapeutic approaches. Curr Opin Neurol 25: 42–49. [DOI] [PubMed] [Google Scholar]
- Bowne SJ, Daiger SP, Malone KA, Heckenlively JR, Kennan A, Humphries P, Hughbanks-Wheaton D, Birch DG, Liu Q, Pierce EA, et al. 2003. Characterization of RP1L1, a highly polymorphic paralog of the retinitis pigmentosa 1 (RP1) gene. Mol Vis 9: 129–137. [PMC free article] [PubMed] [Google Scholar]
- Bredrup C, Saunier S, Oud MM, Fiskerstrand T, Hoischen A, Brackman D, Leh SM, Midtbø M, Filhol E, Bole-Feysot C, et al. 2011. Ciliopathies with skeletal anomalies and renal insufficiency due to mutations in the IFT-A gene WDR19. Am J Hum Genet 89: 634–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujakowska K, Audo I, Mohand-Saïd S, Lancelot ME, Antonio A, Germain A, Leveillard T, Letexier M, Saraiva JP, Lonjou C, et al. 2012. CRB1 mutations in inherited retinal dystrophies. Hum Mutat 33: 306–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujakowska KM, Zhang Q, Siemiatkowska AM, Liu Q, Place E, Falk MJ, Consugar M, Lancelot ME, Antonio A, Lonjou C, et al. 2014. Mutations in IFT172 cause isolated retinal degeneration and Bardet–Biedl syndrome. Hum Mol Genet 24: 230–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert PD, Strissel KJ, Schiesser WE, Pugh EN, Arshavsky VY. 2006. Light-driven translocation of signaling proteins in vertebrate photoreceptors. Trends Cell Biol 16: 560–568. [DOI] [PubMed] [Google Scholar]
- Calvert PD, Schiesser WE, Pugh EN. 2010. Diffusion of a soluble protein, photoactivatable GFP, through a sensory cilium. J Gen Physiol 135: 173–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantagrel V, Silhavy JL, Bielas SL, Swistun D, Marsh SE, Bertrand JY, Audollent S, Attié-Bitach T, Holden KR, Dobyns WB, et al. 2008. Mutations in the cilia gene ARL13B lead to the classical form of Joubert syndrome. Am J Hum Genet 83: 170–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caridi G, Murer L, Bellantuono R, Sorino P, Caringella DA, Gusmano R, Ghiggeri GM. 1998. Renal–retinal syndromes: Association of retinal anomalies and recessive nephronophthisis in patients with homozygous deletion of the NPH1 locus. Am J Kidney Dis 32: 1059–1062. [DOI] [PubMed] [Google Scholar]
- Chaitin MH, Schneider BG, Hall MO, Papermaster DS. 1984. Actin in the photoreceptor connecting cilium: Immunocytochemical localization to the site of outer segment disk formation. J Cell Biol 99: 239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakarova CF, Papaioannou MG, Khanna H, Lopez I, Waseem N, Shah A, Theis T, Friedman J, Maubaret C, Bujakowska K, et al. 2007. Mutations in TOPORS cause autosomal dominant retinitis pigmentosa with perivascular retinal pigment epithelium atrophy. Am J Hum Genet 81: 1098–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakarova CF, Khanna H, Shah AZ, Patil SB, Sedmak T, Murga-Zamalloa CA, Papaioannou MG, Nagel-Wolfrum K, Lopez I, Munro P, et al. 2011. TOPORS, implicated in retinal degeneration, is a cilia-centrosomal protein. Hum Mol Genet 20: 975–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaki M, Airik R, Ghosh AK, Giles RH, Chen R, Slaats GG, Wang H, Hurd TW, Zhou W, Cluckey A, et al. 2012. Exome capture reveals ZNF423 and CEP164 mutations, linking renal ciliopathies to DNA damage response signaling. Cell 150: 533–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang AP, Nishimura D, Searby C, Elbedour K, Carmi R, Ferguson AL, Secrist J, Braun T, Casavant T, Stone EM, et al. 2004. Comparative genomic analysis identifies an ADP-ribosylation factor-like gene as the cause of Bardet–Biedl syndrome (BBS3). Am J Hum Genet 75: 475–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang AP, Beck JS, Yen HJ, Tayeh MK, Scheetz TE, Swiderski RE, Nishimura DY, Braun T, Kim KY, Huang J, et al. 2006. Homozygosity mapping with SNP arrays identifies TRIM32, an E3 ubiquitin ligase, as a Bardet–Biedl syndrome gene (BBS11). Proc Natl Acad Sci 103: 6287–6292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung DC, Traboulsi EI. 2009. Leber congenital amaurosis: Clinical correlations with genotypes, gene therapy trials update, and future directions. J AAPOS 13: 587–592. [DOI] [PubMed] [Google Scholar]
- Clarke G, Goldberg AF, Vidgen D, Collins L, Ploder L, Schwarz L, Molday LL, Rossant J, Szél A, Molday RS, et al. 2000. Rom-1 is required for rod photoreceptor viability and the regulation of disk morphogenesis. Nat Genet 25: 67–73. [DOI] [PubMed] [Google Scholar]
- Coene KLM, Roepman R, Doherty D, Afroze B, Kroes HY, Letteboer SJF, Ngu LH, Budny B, van Wijk E, Gorden NT, et al. 2009. OFD1 is mutated in X-linked Joubert syndrome and interacts with LCA5-encoded lebercilin. Am J Hum Genet 85: 465–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin GB, Marshall JD, Ikeda A, So WV, Russell-Eggitt I, Maffei P, Beck S, Boerkoel CF, Sicolo N, Martin M, et al. 2002. Mutations in ALMS1 cause obesity, type 2 diabetes and neurosensory degeneration in Alström syndrome. Nat Genet 31: 74–78. [DOI] [PubMed] [Google Scholar]
- Collin RWJ, Littink KW, Klevering BJ, van den Born LI, Koenekoop RK, Zonneveld MN, Blokland EAW, Strom TM, Hoyng CB, den Hollander AI, et al. 2008. Identification of a 2 Mb human ortholog of Drosophila eyes shut/spacemaker that is mutated in patients with retinitis pigmentosa. Am J Hum Genet 83: 594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin RWJ, Safieh C, Littink KW, Shalev SA, Garzozi HJ, Rizel L, Abbasi AH, Cremers FPM, den Hollander AI, Klevering BJ, et al. 2010. Mutations in C2ORF71 cause autosomal-recessive retinitis pigmentosa. Am J Hum Genet 86: 783–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consugar MB, Navarro-Gomez D, Place EM, Bujakowska KM, Sousa ME, Fonseca-Kelly ZD, Taub DG, Janessian M, Wang DY, Au ED, et al. 2014. Panel-based genetic diagnostic testing for inherited eye diseases is highly accurate and reproducible, and more sensitive for variant detection, than exome sequencing. Genet Med 17: 253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppieters F, Casteels I, Meire F, De Jaegere S, Hooghe S, van Regemorter N, Van Esch H, Matuleviciene A, Nunes L, Meersschaut V, et al. 2010. Genetic screening of LCA in Belgium: Predominance of CEP290 and identification of potential modifier alleles in AHI1 of CEP290-related phenotypes. Hum Mutat 31: E1709–E1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussa RG, Otto EA, Gee HY, Arthurs P, Ren H, Lopez I, Keser V, Fu Q, Faingold R, Khan A, et al. 2013. WDR19: An ancient, retrograde, intraflagellar ciliary protein is mutated in autosomal recessive retinitis pigmentosa and in Senior–Løken syndrome. Clin Genet 84: 150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremers FP, van de Pol DJ, van Driel M, den Hollander AI, van Haren FJ, Knoers NV, Tijmes N, Bergen AA, Rohrschneider K, Blankenagel A, et al. 1998. Autosomal recessive retinitis pigmentosa and cone–rod dystrophy caused by splice site mutations in the Stargardt’s disease gene ABCR. Hum Mol Genet 7: 355–362. [DOI] [PubMed] [Google Scholar]
- Dafinger C, Liebau MC, Elsayed SM, Hellenbroich Y, Boltshauser E, Korenke GC, Fabretti F, Janecke AR, Ebermann I, Nürnberg G, et al. 2011. Mutations in KIF7 link Joubert syndrome with Sonic Hedgehog signaling and microtubule dynamics. J Clin Invest 121: 2662–2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagoneau N, Goulet M, Geneviève D, Sznajer Y, Martinovic J, Smithson S, Huber C, Baujat G, Flori E, Tecco L, et al. 2009. DYNC2H1 mutations cause asphyxiating thoracic dystrophy and short rib-polydactyly syndrome, type III. Am J Hum Genet 84: 706–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AE, Sergouniotis PI, Mackay DS, Wright GA, Waseem NH, Michaelides M, Holder GE, Robson AG, Moore AT, Plagnol V, et al. 2013. RP1L1 variants are associated with a spectrum of inherited retinal diseases including retinitis pigmentosa and occult macular dystrophy. Hum Mutat 34: 506–514. [DOI] [PubMed] [Google Scholar]
- Davis EE, Zhang Q, Liu Q, Diplas BH, Davey LM, Hartley J, Stoetzel C, Szymanska K, Ramaswami G, Logan CV, et al. 2011. TTC21B contributes both causal and modifying alleles across the ciliopathy spectrum. Nat Genet 43: 189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellett M, Sasai N, Nishide K, Becker S, Papadaki V, Astrid Limb G, Moore AT, Kondo T, Ohnuma SI. 2015. Genetic background and light-dependent progression of photoreceptor cell degeneration in Prominin-1 knockout mice. Investig Ophthalmol Vis Sci 56: 164–176. [DOI] [PubMed] [Google Scholar]
- Delous M, Baala L, Salomon R, Laclef C, Vierkotten J, Tory K, Golzio C, Lacoste T, Besse L, Ozilou C, et al. 2007. The ciliary gene RPGRIP1L is mutated in cerebello-oculo-renal syndrome (Joubert syndrome type B) and Meckel syndrome. Nat Genet 39: 875–881. [DOI] [PubMed] [Google Scholar]
- den Hollander AI, Koenekoop RK, Yzer S, Lopez I, Arends ML, Voesenek KEJ, Zonneveld MN, Strom TM, Meitinger T, Brunner HG, et al. 2006. Mutations in the CEP290 (NPHP6) gene are a frequent cause of Leber congenital amaurosis. Am J Hum Genet 79: 556–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hollander AI, Koenekoop RK, Mohamed MD, Arts HH, Boldt K, Towns KV, Sedmak T, Beer M, Nagel-Wolfrum K, McKibbin M, et al. 2007. Mutations in LCA5, encoding the ciliary protein lebercilin, cause Leber congenital amaurosis. Nat Genet 39: 889–895. [DOI] [PubMed] [Google Scholar]
- den Hollander AI, Roepman R, Koenekoop RK, Cremers FPM. 2008. Leber congenital amaurosis: Genes, proteins and disease mechanisms. Prog Retin Eye Res 27: 391–419. [DOI] [PubMed] [Google Scholar]
- Deretic D, Williams AH, Ransom N, Morel V, Hargrave PA, Arendt A. 2005. Rhodopsin C terminus, the site of mutations causing retinal disease, regulates trafficking by binding to ADP-ribosylation factor 4 (ARF4). Proc Natl Acad Sci 102: 3301–3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis E. 1956. Electron microscope observations on the submicroscopic organization of the retinal rods. J Biophys Biochem Cytol 2: 319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding JD, Salinas RY, Arshavsky VY. 2015. Discs of mammalian rod photoreceptors form through the membrane evagination mechanism. J Cell Biol 211: 495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Palma F, Holme RH, Bryda EC, Belyantseva IA, Pellegrino R, Kachar B, Steel KP, Noben-Trauth K. 2001. Mutations in Cdh23, encoding a new type of cadherin, cause stereocilia disorganization in waltzer, the mouse model for Usher syndrome type 1D. Nat Genet 27: 103–107. [DOI] [PubMed] [Google Scholar]
- Dixon-Salazar T, Silhavy JL, Marsh SE, Louie CM, Scott LC, Gururaj A, Al-Gazali L, Al-Tawari AA, Kayserili H, Sztriha L, et al. 2004. Mutations in the AHI1 gene, encoding jouberin, cause Joubert syndrome with cortical polymicrogyria. Am J Hum Genet 75: 979–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowdle WE, Robinson JF, Kneist A, Sirerol-Piquer MS, Frints SGM, Corbit KC, Zaghloul NA, Zaghloul NA, van Lijnschoten G, Mulders L, et al. 2011. Disruption of a ciliary B9 protein complex causes Meckel syndrome. Am J Hum Genet 89: 94–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryja TP, McGee TL, Reichel E, Hahn LB, Cowley GS, Yandell DW, Sandberg MA, Berson EL. 1990. A point mutation of the rhodopsin gene in one form of retinitis pigmentosa. Nature 343: 364–366. [DOI] [PubMed] [Google Scholar]
- Dryja TP, Berson EL, Rao VR, Oprian DD. 1993. Heterozygous missense mutation in the rhodopsin gene as a cause of congenital stationary night blindness. Nat Genet 4: 280–283. [DOI] [PubMed] [Google Scholar]
- Dryja TP, Finn JT, Peng YW, McGee TL, Berson EL, Yau KW. 1995. Mutations in the gene encoding the α subunit of the rod cGMP-gated channel in autosomal recessive retinitis pigmentosa. Proc Natl Acad Sci 92: 10177–10181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryja TP, Hahn LB, Reboul T, Arnaud B. 1996. Missense mutation in the gene encoding the α subunit of rod transducin in the Nougaret form of congenital stationary night blindness. Nat Genet 13: 358–360. [DOI] [PubMed] [Google Scholar]
- Dryja TP, Adams SM, Grimsby JL, McGee TL, Hong DH, Li T, Andréasson S, Berson EL. 2001. Null RPGRIP1 alleles in patients with Leber congenital amaurosis. Am J Hum Genet 68: 1295–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvir L, Srour G, Abu-Ras R, Miller B, Shalev S, Ben-Yosef T. 2010. Autosomal-recessive early-onset retinitis pigmentosa caused by a mutation in PDE6G, the gene encoding the gamma subunit of rod cGMP phosphodiesterase. Am J Hum Genet 87: 258–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebermann I, Phillips JB, Liebau MC, Koenekoop RK, Schermer B, Lopez I, Schäfer E, Roux AF, Dafinger C, Bernd A, et al. 2010. PDZD7 is a modifier of retinal disease and a contributor to digenic Usher syndrome. J Clin Invest 120: 1812–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvardson S, Shaag A, Zenvirt S, Erlich Y, Hannon GJ, Shanske AL, Gomori JM, Ekstein J, Elpeleg O. 2010. Joubert syndrome 2 (JBTS2) in Ashkenazi Jews is associated with a TMEM216 mutation. Am J Hum Genet 86: 93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Shamieh S, Neuillé M, Terray A, Orhan E, Condroyer C, Démontant V, Michiels C, Antonio A, Boyard F, Lancelot ME, et al. 2014. Whole-exome sequencing identifies KIZ as a ciliary gene associated with autosomal-recessive rod–cone dystrophy. Am J Hum Genet 94: 625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada-Cuzcano A, Koenekoop RK, Coppieters F, Kohl S, Lopez I, Collin RW, De Baere E, Roeleveld D, Marek J, Bernd A, et al. 2011. IQCB1 mutations in patients with Leber congenital amaurosis. Invest Ophthalmol Vis Sci 52: 834–839. [DOI] [PubMed] [Google Scholar]
- Estrada-Cuzcano A, Koenekoop RK, Senechal A, De Baere EBW, de Ravel T, Banfi S, Kohl S, Ayuso C, Sharon D, Hoyng CB, et al. 2012a. BBS1 mutations in a wide spectrum of phenotypes ranging from nonsyndromic retinitis pigmentosa to Bardet–Biedl syndrome. Arch Ophthalmol 130: 1425–1432. [DOI] [PubMed] [Google Scholar]
- Estrada-Cuzcano A, Neveling K, Kohl S, Banin E, Rotenstreich Y, Sharon D, Falik-Zaccai TC, Hipp S, Roepman R, Wissinger B, et al. 2012b. Mutations in C8orf37, encoding a ciliary protein, are associated with autosomal-recessive retinal dystrophies with early macular involvement. Am J Hum Genet 90: 102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada-Cuzcano A, Roepman R, Cremers FPM, den Hollander AI, Mans D. 2012c. Non-syndromic retinal ciliopathies: Translating gene discovery into therapy. Hum Mol Genet 21: R111–R124. [DOI] [PubMed] [Google Scholar]
- Eudy JD, Weston MD, Yao S, Hoover DM, Rehm HL, Ma-Edmonds M, Yan D, Ahmad I, Cheng JJ, Ayuso C, et al. 1998. Mutation of a gene encoding a protein with extracellular matrix motifs in Usher syndrome type IIa. Science 280: 1753–1757. [DOI] [PubMed] [Google Scholar]
- Fahim AT, Bowne SJ, Sullivan LS, Webb KD, Williams JT, Wheaton DK, Birch DG, Daiger SP. 2011. Allelic heterogeneity and genetic modifier loci contribute to clinical variation in males with X-linked retinitis pigmentosa due to RPGR mutations. PLoS ONE 6: e23021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Esmail MA, Ansley SJ, Blacque OE, Boroevich K, Ross AJ, Moore SJ, Badano JL, May-Simera H, Compton DS, et al. 2004. Mutations in a member of the Ras superfamily of small GTP-binding proteins causes Bardet–Biedl syndrome. Nat Genet 36: 989–993. [DOI] [PubMed] [Google Scholar]
- Farrar GJ, McWilliam P, Bradley DG, Kenna P, Lawler M, Sharp EM, Humphries MM, Eiberg H, Conneally PM, Trofatter JA. 1990. Autosomal dominant retinitis pigmentosa: Linkage to rhodopsin and evidence for genetic heterogeneity. Genomics 8: 35–40. [DOI] [PubMed] [Google Scholar]
- Ferland RJ, Eyaid W, Collura RV, Tully LD, Hill RS, Al-Nouri D, Al-Rumayyan A, Topcu M, Gascon G, Bodell A, et al. 2004. Abnormal cerebellar development and axonal decussation due to mutations in AHI1 in Joubert syndrome. Nat Genet 36: 1008–1013. [DOI] [PubMed] [Google Scholar]
- Ferrante MI, Giorgio G, Feather SA, Bulfone A, Wright V, Ghiani M, Selicorni A, Gammaro L, Scolari F, Woolf AS, et al. 2001. Identification of the gene for oral-facial-digital type I syndrome. Am J Hum Genet 68: 569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filges I, Nosova E, Bruder E, Tercanli S, Townsend K, Gibson WT, Röthlisberger B, Heinimann K, Hall JG, Gregory-Evans CY, et al. 2014. Exome sequencing identifies mutations in KIF14 as a novel cause of an autosomal recessive lethal fetal ciliopathy phenotype. Clin Genet 86: 220–228. [DOI] [PubMed] [Google Scholar]
- Fingert JH, Oh K, Chung M, Scheetz TE, Andorf JL, Johnson RM, Sheffield VC, Stone EM. 2008. Association of a novel mutation in the retinol dehydrogenase 12 (RDH12) gene with autosomal dominant retinitis pigmentosa. Arch Ophthalmol 126: 1301–1307. [DOI] [PubMed] [Google Scholar]
- Fotiadis D, Liang Y, Filipek S, Saperstein DA, Engel A, Palczewski K. 2003. Rhodopsin dimers in native disc membranes. Nature 421: 127–128. [DOI] [PubMed] [Google Scholar]
- Frank V, den Hollander AI, Brüchle NO, Zonneveld MN, Nürnberg G, Becker C, Du Bois G, Kendziorra H, Roosing S, Senderek J, et al. 2008. Mutations of the CEP290 gene encoding a centrosomal protein cause Meckel–Gruber syndrome. Hum Mutat 29: 45–52. [DOI] [PubMed] [Google Scholar]
- Fuchs S, Nakazawa M, Maw M, Tamai M, Oguchi Y, Gal A. 1995. A homozygous 1-base pair deletion in the arrestin gene is a frequent cause of Oguchi disease in Japanese. Nat Genet 10: 360–362. [DOI] [PubMed] [Google Scholar]
- Gal A, Orth U, Baehr W, Schwinger E, Rosenberg T. 1994. Heterozygous missense mutation in the rod cGMP phosphodiesterase beta-subunit gene in autosomal dominant stationary night blindness. Nat Genet 7: 64–68. [DOI] [PubMed] [Google Scholar]
- Galdzicka M, Patnala S, Hirshman MG, Cai JF, Nitowsky H, Egeland JA, Ginns EI. 2002. A new gene, EVC2, is mutated in Ellis–van Creveld syndrome. Mol Genet Metab 77: 291–295. [DOI] [PubMed] [Google Scholar]
- Garcia-Gonzalo FR, Corbit KC, Sirerol-Piquer MS, Ramaswami G, Otto EA, Noriega TR, Seol AD, Robinson JF, Bennett CL, Josifova DJ, et al. 2011. A transition zone complex regulates mammalian ciliogenesis and ciliary membrane composition. Nat Genet 43: 776–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller SF, Guerin KI, Visel M, Pham A, Lee ES, Dror AA, Avraham KB, Hayashi T, Ray CA, Reh TA, et al. 2009. CLRN1 is nonessential in the mouse retina but is required for cochlear hair cell development. PLoS Genet 5: 17–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilissen C, Arts HH, Hoischen A, Spruijt L, Mans DA, Arts P, van Lier B, Steehouwer M, van Reeuwijk J, Kant SG, et al. 2010. Exome sequencing identifies WDR35 variants involved in Sensenbrenner syndrome. Am J Hum Genet 87: 418–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliam JC, Chang JT, Sandoval IM, Zhang Y, Li T, Pittler SJ, Chiu W, Wensel TG. 2012. Three-dimensional architecture of the rod sensory cilium and its disruption in retinal neurodegeneration. Cell 151: 1029–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girisha KM, Shukla A, Trujillano D, Bhavani GS, Hebbar M, Kadavigere R, Rolfs A. 2016. A homozygous nonsense variant in IFT52 is associated with a human skeletal ciliopathy. Clin Genet 10.1111/cge.12762. [DOI] [PubMed] [Google Scholar]
- Goetz SC, Anderson KV. 2010. The primary cilium: A signalling centre during vertebrate development. Nat Rev Genet 11: 331–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg AFX, Molday RS. 1996. Subunit composition of the peripherin/RDS–rom-1 disk rim complex from rod photoreceptors: Hydrodynamic evidence for a tetrameric quaternary structure. Biochemistry 35: 6144–6149. [DOI] [PubMed] [Google Scholar]
- Gorden NT, Arts HH, Parisi MA, Coene KLM, Letteboer SJF, van Beersum SEC, Mans DA, Hikida A, Eckert M, Knutzen D, et al. 2008. CC2D2A is mutated in Joubert syndrome and interacts with the ciliopathy-associated basal body protein CEP290. Am J Hum Genet 83: 559–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillonneau X, Piriev NI, Danciger M, Kozak CA, Cideciyan AV, Jacobson SG, Farber DB. 1999. A nonsense mutation in a novel gene is associated with retinitis pigmentosa in a family linked to the RP1 locus. Hum Mol Genet 8: 1541–1546. [DOI] [PubMed] [Google Scholar]
- Hagstrom SA, North MA, Nishina PL, Berson EL, Dryja TP. 1998. Recessive mutations in the gene encoding the tubby-like protein TULP1 in patients with retinitis pigmentosa. Nat Genet 18: 174–176. [DOI] [PubMed] [Google Scholar]
- Halbritter J, Bizet AA, Schmidts M, Porath JD, Braun DA, Gee HY, McInerney-Leo AM, Krug P, Filhol E, Davis EE, et al. 2013. Defects in the IFT-B component IFT172 cause Jeune and Mainzer–Saldino syndromes in humans. Am J Hum Genet 93: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hameed A, Abid A, Aziz A, Ismail M, Mehdi SQ, Khaliq S. 2003. Evidence of RPGRIP1 gene mutations associated with recessive cone-rod dystrophy. J Med Genet 40: 616–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel C. 2006. Retinitis pigmentosa. Orphanet J Rare Dis 1: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire DJ, Ayub M, Springell K, Roberts E, Jafri H, Rashid Y, Bond J, Riley JH, Woods CG. 2006. MORM syndrome (mental retardation, truncal obesity, retinal dystrophy and micropenis), a new autosomal recessive disorder, links to 9q34. Eur J Hum Genet 14: 543–548. [DOI] [PubMed] [Google Scholar]
- Han Z, Anderson DW, Papermaster DS. 2012. Prominin-1 localizes to the open rims of outer segment lamellae in Xenopus laevis rod and cone photoreceptors. Investig Ophthalmol Vis Sci 53: 361–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardcastle AJ, Thiselton DL, Van Maldergem L, Saha BK, Jay M, Plant C, Taylor R, Bird AC, Bhattacharya S. 1999. Mutations in the RP2 gene cause disease in 10% of families with familial X-linked retinitis pigmentosa assessed in this study. Am J Hum Genet 64: 1210–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartong DT, Berson EL, Dryja TP. 2006. Retinitis pigmentosa. Lancet 368: 1795–1809. [DOI] [PubMed] [Google Scholar]
- Helou J, Otto EA, Attanasio M, Allen SJ, Parisi MA, Glass I, Utsch B, Hashmi S, Fazzi E, Omran H, et al. 2007. Mutation analysis of NPHP6/CEP290 in patients with Joubert syndrome and Senior–Løken syndrome. J Med Genet 44: 657–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson RH, Li Z, Abd El Aziz MM, Mackay DS, Eljinini MA, Zeidan M, Moore AT, Bhattacharya SS, Webster AR. 2010. Biallelic mutation of protocadherin-21 (PCDH21) causes retinal degeneration in humans. Mol Vis 16: 46–52. [PMC free article] [PubMed] [Google Scholar]
- Heon E, Kim G, Qin S, Garrison JE, Tavares E, Vincent A, Nuangchamnong N, Scott CA, Slusarski DC, Sheffield VC. 2016. Mutations in C8ORF37 cause Bardet–Biedl syndrome (BBS21). Hum Mol Genet 10.1093/hmg/ddw096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp K, Heyer CM, Hommerding CJ, Henke SA, Sundsbak JL, Patel S, Patel P, Consugar MB, Czarnecki PG, Gliem TJ, et al. 2011. B9D1 is revealed as a novel Meckel syndrome (MKS) gene by targeted exon-enriched next-generation sequencing and deletion analysis. Hum Mol Genet 20: 2524–2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst CJ, Johnson LV, Besharse JC. 1990. Transmembrane assemblage of the photoreceptor connecting cilium and motile cilium transition zone contain a common immunologic epitope. Cell Motil Cytoskeleton 17: 329–344. [DOI] [PubMed] [Google Scholar]
- Huang SHH, Pittler SJJ, Huang X, Oliveira L, Berson ELL, Dryja TPP. 1995. Autosomal recessive retinitis pigmentosa caused by mutations in the α subunit of rod cGMP phosphodiesterase. Nat Genet 11: 468–471. [DOI] [PubMed] [Google Scholar]
- Huang L, Szymanska K, Jensen VL, Janecke AR, Innes AM, Davis EE, Frosk P, Li C, Willer JR, Chodirker BN, et al. 2011. TMEM237 is mutated in individuals with a Joubert syndrome related disorder and expands the role of the TMEM family at the ciliary transition zone. Am J Hum Genet 89: 713–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber C, Cormier-Daire V. 2012. Ciliary disorder of the skeleton. Am J Med Genet Semin Med Genet 160C: 165–174. [DOI] [PubMed] [Google Scholar]
- Jacobson SG, Cideciyan AV, Iannaccone A, Weleber RG, Fishman GA, Maguire AM, Affatigato LM, Bennett J, Pierce EA, Danciger M, et al. 2000. Disease expression of RP1 mutations causing autosomal dominant retinitis pigmentosa. Invest Ophthalmol Vis Sci 41: 1898–1908. [PubMed] [Google Scholar]
- Jacoby M, Cox JJ, Gayral S, Hampshire DJ, Ayub M, Blockmans M, Pernot E, Kisseleva MV, Compère P, Schiffmann SN, et al. 2009. INPP5E mutations cause primary cilium signaling defects, ciliary instability and ciliopathies in human and mouse. Nat Genet 41: 1027–1031. [DOI] [PubMed] [Google Scholar]
- Janecke AR, Thompson DA, Utermann G, Becker C, Hubner CA, Schmid E, McHenry CL, Nair AR, Ruschendorf F, Heckenlively J, et al. 2004. Mutations in RDH12 encoding a photoreceptor cell retinol dehydrogenase cause childhood-onset severe retinal dystrophy. Nat Genet 36: 850–854. [DOI] [PubMed] [Google Scholar]
- Joensuu T, Hämäläinen R, Yuan B, Johnson C, Tegelberg S, Gasparini P, Zelante L, Pirvola U, Pakarinen L, Lehesjoki AE, et al. 2001. Mutations in a novel gene with transmembrane domains underlie Usher syndrome type 3. Am J Hum Genet 69: 673–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KR, Gagnon LH, Webb LS, Peters LL, Hawes NL, Zheng QY, Chang B, Zheng QY. 2003. Mouse models of USH1C and DFNB18: Phenotypic and molecular analyses of two new spontaneous mutations of the Ush1c gene. Hum Mol Genet 12: 3075–3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiwara K, Hahn LB, Mukai S, Travis GH, Berson EL, Dryja TP. 1991. Mutations in the human retinal degeneration slow gene in autosomal dominant retinitis pigmentosa. Nature 354: 480–483. [DOI] [PubMed] [Google Scholar]
- Kajiwara K, Berson EL, Dryja TP. 1994. Digenic retinitis pigmentosa due to mutations at the unlinked peripherin/RDS and ROM1 loci. Science 264: 1604–1608. [DOI] [PubMed] [Google Scholar]
- Kaplan MW, Iwata RT, Sears RC. 1987. Lengths of immunolabeled ciliary microtubules in frog photoreceptor outer segments. Exp Eye Res 44: 623–632. [DOI] [PubMed] [Google Scholar]
- Katsanis N, Beales PL, Woods MO, Lewis RA, Green JS, Parfrey PS, Ansley SJ, Davidson WS, Lupski JR. 2000. Mutations in MKKS cause obesity, retinal dystrophy and renal malformations associated with Bardet–Biedl syndrome. Nat Genet 26: 67–70. [DOI] [PubMed] [Google Scholar]
- Katsanis N, Ansley SJ, Badano JL, Eichers ER, Lewis RA, Hoskins BE, Scambler PJ, Davidson WS, Beales PL, Lupski JR. 2001. Triallelic inheritance in Bardet–Biedl syndrome, a Mendelian recessive disorder. Science 293: 2256–2259. [DOI] [PubMed] [Google Scholar]
- Kelsell RE, Gregory-Evans K, Payne AM, Perrault I, Kaplan J, Yang RB, Garbers DL, Bird AC, Moore AT, Hunt DM. 1998. Mutations in the retinal guanylate cyclase (RETGC-1) gene in dominant cone–rod dystrophy. Hum Mol Genet 7: 1179–1184. [DOI] [PubMed] [Google Scholar]
- Kennedy B, Malicki J. 2009. What drives cell morphogenesis—A look inside the vertebrate photoreceptor. Dev Dyn 238: 2115–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevany BM, Zhang N, Jastrzebska B, Palczewski K. 2015. Animals deficient in C2Orf71, an autosomal recessive retinitis pigmentosa-associated locus, develop severe early-onset retinal degeneration. Hum Mol Genet 24: 2627–2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MI, Kersten FFJ, Azam M, Collin RWJ, Hussain A, Shah STA, Keunen JEE, Kremer H, Cremers FPM, Qamar R, et al. 2011. CLRN1 mutations cause nonsyndromic retinitis pigmentosa. Ophthalmology 118: 1444–1448. [DOI] [PubMed] [Google Scholar]
- Khan AO, Decker E, Bachmann N, Bolz HJ, Bergmann C. 2016. C8orf37 is mutated in Bardet–Biedl syndrome and constitutes a locus allelic to non-syndromic retinal dystrophies. Ophthalmic Genet 8: 1–4. [DOI] [PubMed] [Google Scholar]
- Khanna H. 2015. Photoreceptor sensory cilium: Traversing the ciliary gate. Cells 4: 674–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna H, Davis EE, Murga-Zamalloa CA, Estrada-Cuzcano A, Lopez I, den Hollander AI, Zonneveld MN, Othman MI, Waseem N, Chakarova CF, et al. 2009. A common allele in RPGRIP1L is a modifier of retinal degeneration in ciliopathies. Nat Genet 41: 739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khateb S, Zelinger L, Mizrahi-Meissonnier L, Ayuso C, Koenekoop RK, Laxer U, Gross M, Banin E, Sharon D. 2014. A homozygous nonsense CEP250 mutation combined with a heterozygous nonsense C2orf71 mutation is associated with atypical Usher syndrome. J Med Genet 51: 460–469. [DOI] [PubMed] [Google Scholar]
- Kim SK, Shindo A, Park TJ, Oh EC, Ghosh S, Gray RS, Lewis RA, Johnson CA, Attie-Bittach T, Katsanis N, et al. 2010. Planar cell polarity acts through septins to control collective cell movement and ciliogenesis. Science 329: 1337–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MS, Pinto SM, Getnet D, Nirujogi RS, Manda SS, Chaerkady R, Madugundu AK, Kelkar DS, Isserlin R, Jain S, et al. 2014. A draft map of the human proteome. Nature 509: 575–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenekoop RK. 2004. An overview of leber congenital amaurosis: A model to understand human retinal development. Surv Ophthalmol 49: 379–398. [DOI] [PubMed] [Google Scholar]
- Kohl S, Marx T, Giddings I, Jägle H, Jacobson SG, Apfelstedt-Sylla E, Zrenner E, Sharpe LT, Wissinger B. 1998. Total colourblindness is caused by mutations in the gene encoding the α-subunit of the cone photoreceptor cGMP-gated cation channel. Nat Genet 19: 257–259. [DOI] [PubMed] [Google Scholar]
- Kohl S, Baumann B, Broghammer M, Jägle H, Sieving P, Kellner U, Spegal R, Anastasi M, Zrenner E, Sharpe LT, et al. 2000. Mutations in the CNGB3 gene encoding the β-subunit of the cone photoreceptor cGMP-gated channel are responsible for achromatopsia (ACHM3) linked to chromosome 8q21. Hum Mol Genet 9: 2107–2116. [DOI] [PubMed] [Google Scholar]
- Kohl S, Baumann B, Rosenberg T, Kellner U, Lorenz B, Vadalà M, Jacobson SG, Wissinger B. 2002. Mutations in the cone photoreceptor G-protein α-subunit gene GNAT2 in patients with achromatopsia. Am J Hum Genet 71: 422–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer H, van Wijk E, Märker T, Wolfrum U, Roepman R. 2006. Usher syndrome: Molecular links of pathogenesis, proteins and pathways. Hum Mol Genet 15: R262–R270. [DOI] [PubMed] [Google Scholar]
- Kyttälä M, Tallila J, Salonen R, Kopra O, Kohlschmidt N, Paavola-Sakki P, Peltonen L, Kestilä M. 2006. MKS1, encoding a component of the flagellar apparatus basal body proteome, is mutated in Meckel syndrome. Nat Genet 38: 155–157. [DOI] [PubMed] [Google Scholar]
- Lamb TD, Pugh EN. 2006. Phototransduction, dark adaptation, and rhodopsin regeneration the proctor lecture. Invest Ophthalmol Vis Sci 47: 5137–5152. [DOI] [PubMed] [Google Scholar]
- Lamb TD, Collin SP, Pugh EN. 2007. Evolution of the vertebrate eye: Opsins, photoreceptors, retina and eye cup. Nat Rev Neurosci 8: 960–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmann T, Di Gioia SA, Rau I, Stöhr H, Maksimovic NS, Corbo JC, Renner AB, Zrenner E, Kumaramanickavel G, Karlstetter M, et al. 2010. Nonsense mutations in FAM161A cause RP28-associated recessive retinitis pigmentosa. Am J Hum Genet 87: 376–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson NG, Wang J, Wilhelmsson H, Oldfors A, Rustin P, Lewandoski M, Barsh GS, Clayton DA. 1998. Recessive mutations in TULP1 in RP. Nat Genet 18: 231–236. [DOI] [PubMed] [Google Scholar]
- Lee JE, Silhavy JL, Zaki MS, Schroth J, Bielas SL, Marsh SE, Olvera J, Brancati F, Iannicelli M, Ikegami K, et al. 2012a. CEP41 is mutated in Joubert syndrome and is required for tubulin glutamylation at the cilium. Nat Genet 44: 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Silhavy JL, Lee JE, Al-Gazali L, Thomas S, Davis EE, Bielas SL, Hill KJ, Iannicelli M, Brancati F, et al. 2012b. Evolutionarily assembled cis-regulatory module at a human ciliopathy locus. Science 335: 966–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman AM, Eydoux P, Doherty D, Glass IA, Chitayat D, Chung BYH, Langlois S, Yong SL, Lowry RB, Hildebrandt F, et al. 2010. Co-occurrence of Joubert syndrome and Jeune asphyxiating thoracic dystrophy. Am J Med Genet Part A 152: 1411–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitch CC, Zaghloul NA, Davis EE, Stoetzel C, Diaz-Font A, Rix S, Alfadhel M, Al-Fadhel M, Lewis RA, Eyaid W, et al. 2008. Hypomorphic mutations in syndromic encephalocele genes are associated with Bardet–Biedl syndrome. Nat Genet 40: 443–448. [DOI] [PubMed] [Google Scholar]
- Li JB, Gerdes JM, Haycraft CJ, Fan Y, Teslovich TM, May-Simera H, Li H, Blacque OE, Li L, Leitch CC, et al. 2004. Comparative genomics identifies a flagellar and basal body proteome that includes the BBS5 human disease gene. Cell 117: 541–552. [DOI] [PubMed] [Google Scholar]
- Libby RT, Steel KP. 2001. Electroretinographic anomalies in mice with mutations in Myo7a, the gene involved in human Usher syndrome type 1B. Invest Ophthalmol Vis Sci 42: 770–778. [PubMed] [Google Scholar]
- Lindstrand A, Davis EE, Carvalho CMB, Pehlivan D, Willer JR, Tsai IC, Ramanathan S, Zuppan C, Sabo A, Muzny D, et al. 2014. Recurrent CNVs and SNVs at the NPHP1 locus contribute pathogenic alleles to Bardet–Biedl syndrome. Am J Hum Genet 94: 745–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Udovichenko IP, Brown SD, Steel KP, Williams DS. 1999. Myosin VIIa participates in opsin transport through the photoreceptor cilium. J Neurosci 19: 6267–6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Tan G, Levenkova N, Li T, Pugh EN, Rux JJ, Speicher DW, Pierce EA. 2007a. The proteome of the mouse photoreceptor sensory cilium complex. Mol Cell Proteomics 6: 1299–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Bulgakov OV, Darrow KN, Pawlyk B, Adamian M, Liberman MC, Li T. 2007b. Usherin is required for maintenance of retinal photoreceptors and normal development of cochlear hair cells. Proc Natl Acad Sci 104: 4413–4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Zhang Q, Pierce EA. 2010. Photoreceptor sensory cilia and inherited retinal degeneration. Adv Exp Med Biol 664: 223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan CV, Abdel-Hamed Z, Johnson CA. 2011. Molecular genetics and pathogenic mechanisms for the severe ciliopathies: Insights into neurodevelopment and pathogenesis of neural tube defects. Mol Neurobiol 43: 12–26. [DOI] [PubMed] [Google Scholar]
- Løken AC, Hanssen O, Halvorsen S, Jolster NJ. 1961. Hereditary renal dysplasia and blindness. Acta Paediatr 50: 177–184. [DOI] [PubMed] [Google Scholar]
- Louie CM, Caridi G, Lopes VS, Brancati F, Kispert A, Lancaster MA, Schlossman AM, Otto EA, Leitges M, Gröne HJ, et al. 2010. AHI1 is required for photoreceptor outer segment development and is a modifier for retinal degeneration in nephronophthisis. Nat Genet 42: 175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maria BL, Hoang KB, Tusa RJ, Mancuso AA, Hamed LM, Quisling RG, Hove MT, Fennell EB, Booth-Jones M, Ringdahl DM, et al. 1997. “Joubert syndrome” revisited: Key ocular motor signs with magnetic resonance imaging correlation. J Child Neurol 12: 423–430. [DOI] [PubMed] [Google Scholar]
- Marion V, Stutzmann F, Gérard M, De Melo C, Schaefer E, Claussmann A, Hellé S, Delague V, Souied E, Barrey C, et al. 2012. Exome sequencing identifies mutations in LZTFL1, a BBSome and smoothened trafficking regulator, in a family with Bardet–Biedl syndrome with situs inversus and insertional polydactyly. J Med Genet 49: 317–321. [DOI] [PubMed] [Google Scholar]
- Martínez-Mir A, Paloma E, Allikmets R, Ayuso C, del Rio T, Dean M, Vilageliu L, Gonzàlez-Duarte R, Balcells S. 1998. Retinitis pigmentosa caused by a homozygous mutation in the Stargardt disease gene ABCR. Nat Genet 18: 11–12. [DOI] [PubMed] [Google Scholar]
- Maw MA, Corbeil D, Koch J, Hellwig A, Wilson-Wheeler JC, Bridges RJ, Kumaramanickavel G, John S, Nancarrow D, Röper K, et al. 2000. A frameshift mutation in prominin (mouse)-like 1 causes human retinal degeneration. Hum Mol Genet 9: 27–34. [DOI] [PubMed] [Google Scholar]
- Mburu P, Mustapha M, Varela A, Weil D, El-Amraoui A, Holme RH, Rump A, Hardisty RE, Blanchard S, Coimbra RS, et al. 2003. Defects in whirlin, a PDZ domain molecule involved in stereocilia elongation, cause deafness in the whirler mouse and families with DFNB31. Nat Genet 34: 421–428. [DOI] [PubMed] [Google Scholar]
- McGee TL, Devoto M, Ott J, Berson EL, Dryja TP. 1997. Evidence that the penetrance of mutations at the RP11 locus causing dominant retinitis pigmentosa is influenced by a gene linked to the homologous RP11 allele. Am J Hum Genet 61: 1059–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInerney-Leo AM, Schmidts M, Cortés CR, Leo PJ, Gener B, Courtney AD, Gardiner B, Harris JA, Lu Y, Marshall M, et al. 2013. Short-rib polydactyly and Jeune syndromes are caused by mutations in WDR60. Am J Hum Genet 93: 515–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin MEE, Sandberg MAA, Berson ELL, Dryja TPP. 1993. Recessive mutations in the gene encoding the β-subunit of rod phosphodiesterase in patients with retinitis pigmentosa. Nat Genet 4: 130–134. [DOI] [PubMed] [Google Scholar]
- Mears AJ, Gieser L, Yan D, Chen C, Fahrner S, Hiriyanna S, Fujita R, Jacobson SG, Sieving PA, Swaroop A. 1999. Protein-truncation mutations in the RP2 gene in a North American cohort of families with X-linked retinitis pigmentosa. Am J Hum Genet 64: 897–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meindl A, Dry K, Herrmann K, Manson F, Ciccodicola A, Edgar A, Carvalho MR, Achatz H, Hellebrand H, Lennon A, et al. 1996. A gene (RPGR) with homology to the RCC1 guanine nucleotide exchange factor is mutated in X-linked retinitis pigmentosa (RP3). Nat Genet 13: 35–42. [DOI] [PubMed] [Google Scholar]
- Michaelides M, Hardcastle AJ, Hunt DM, Moore AT. 2006. Progressive cone and cone–rod dystrophies: Phenotypes and underlying molecular genetic basis. Surv Ophthalmol 51: 232–258. [DOI] [PubMed] [Google Scholar]
- Mill P, Lockhart PJ, Fitzpatrick E, Mountford HS, Hall EA, Reijns MAM, Keighren M, Bahlo M, Bromhead CJ, Budd P, et al. 2011. Human and mouse mutations in WDR35 cause short-rib polydactyly syndromes due to abnormal ciliogenesis. Am J Hum Genet 88: 508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millán JM, Aller E, Jaijo T, Blanco-Kelly F, Gimenez-Pardo A, Ayuso C. 2011. An update on the genetics of Usher syndrome. J Ophthalmol 2011: 417217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyasaka Y, Suzuki S, Ohshiba Y, Watanabe K, Sagara Y, Yasuda SP, Matsuoka K, Shitara H, Yonekawa H, Kominami R, et al. 2013. Compound heterozygosity of the functionally null Cdh23v-ngt and hypomorphic Cdh23ahl alleles leads to early-onset progressive hearing loss in mice. Exp Anim 62: 333–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mockel A, Perdomo Y, Stutzmann F, Letsch J, Marion V, Dollfus H. 2011. Retinal dystrophy in Bardet–Biedl syndrome and related syndromic ciliopathies. Prog Retin Eye Res 30: 258–274. [DOI] [PubMed] [Google Scholar]
- Molday RS, Molday LL. 1987. Differences in the protein composition of bovine retinal rod outer segment disk and plasma membranes isolated by a ricin–gold–dextran density perturbation method. J Cell Biol 105: 2589–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molday RS, Moritz OL. 2015. Photoreceptors at a glance. J Cell Sci 128: 4039–4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molday RS, Hicks D, Molday L. 1987. Peripherin: A rim specific membrane protein of rod outer segment discs. Investig Opthalmology Vis Sci 28: 50–61. [PubMed] [Google Scholar]
- Mykytyn K, Braun T, Carmi R, Haider NB, Searby CC, Shastri M, Beck G, Wright AF, Iannaccone A, Elbedour K, et al. 2001. Identification of the gene that, when mutated, causes the human obesity syndrome BBS4. Nat Genet 28: 188–191. [DOI] [PubMed] [Google Scholar]
- Mykytyn K, Nishimura DY, Searby CC, Shastri M, Yen H, Beck JS, Braun T, Streb LM, Cornier AS, Cox GF, et al. 2002. Identification of the gene (BBS1) most commonly involved in Bardet–Biedl syndrome, a complex human obesity syndrome. Nat Genet 31: 435–438. [DOI] [PubMed] [Google Scholar]
- Nair KS, Hanson SM, Mendez A, Gurevich EV, Kennedy MJ, Shestopalov VI, Vishnivetskiy SA, Chen J, Hurley JB, Gurevich VV, et al. 2005. Light-dependent redistribution of arrestin in vertebrate rods is an energy-independent process governed by protein–protein interactions. Neuron 46: 555–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najafi M, Calvert PD. 2012. Transport and localization of signaling proteins in ciliated cells. Vision Res 75: 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa M, Wada Y, Tamai M. 1998. Arrestin gene mutations in autosomal recessive retinitis pigmentosa. Arch Ophthalmol 116: 498–501. [DOI] [PubMed] [Google Scholar]
- Nathans J, Thomas D, Hogness DS. 1986. Molecular genetics of human color vision: The genes encoding blue, green, and red pigments. Science 232: 193–202. [DOI] [PubMed] [Google Scholar]
- Nathans J, Merbs S, Sung CH, Weitz C, Wang Y. 1992. Molecular genetics of human visual pigments. Annu Rev Genet 26: 403–424. [DOI] [PubMed] [Google Scholar]
- Nickell S, Park PSH, Baumeister W, Palczewski K. 2007. Three-dimensional architecture of murine rod outer segments determined by cryoelectron tomography. J Cell Biol 177: 917–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikopoulos K, Farinelli P, Royer-Bertrand B, Bedoni N, Kjellström U, Andreasson S, Tsilimbaris MK, Tsika C, Blazaki S, Rivolta C. 2016. Whole exome sequencing reveals CEP78 as a novel disease gene for cone-rod dystrophy. ARVO Annual Meeting, Abstract 1423 Seattle, WA, May 1–6. [Google Scholar]
- Nishiguchi KM, Sandberg MA, Kooijman AC, Martemyanov KA, Pott JWR, Hagstrom SA, Arshavsky VY, Berson EL, Dryja TP. 2004. Defects in RGS9 or its anchor protein R9AP in patients with slow photoreceptor deactivation. Nature 427: 75–78. [DOI] [PubMed] [Google Scholar]
- Nishiguchi KM, Tearle RG, Liu YP, Oh EC, Miyake N, Benaglio P, Harper S, Koskiniemi-Kuendig H, Venturini G, Sharon D, et al. 2013. Whole genome sequencing in patients with retinitis pigmentosa reveals pathogenic DNA structural changes and NEK2 as a new disease gene. Proc Natl Acad Sci 110: 16139–16144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura DY, Searby CC, Carmi R, Elbedour K, Van Maldergem L, Fulton AB, Lam BL, Powell BR, Swiderski RE, Bugge KE, et al. 2001. Positional cloning of a novel gene on chromosome 16q causing Bardet–Biedl syndrome (BBS2). Hum Mol Genet 10: 865–874. [DOI] [PubMed] [Google Scholar]
- Nishimura DY, Swiderski RE, Searby CC, Berg EM, Ferguson AL, Hennekam R, Merin S, Weleber RG, Biesecker LG, Stone EM, et al. 2005. Comparative genomics and gene expression analysis identifies BBS9, a new Bardet–Biedl syndrome gene. Am J Hum Genet 77: 1021–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura DY, Baye LM, Perveen R, Searby CC, Avila-Fernandez A, Pereiro I, Ayuso C, Valverde D, Bishop PN, Manson FDC, et al. 2010. Discovery and functional analysis of a retinitis pigmentosa gene, C2ORF71. Am J Hum Genet 86: 686–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noor A, Windpassinger C, Patel M, Stachowiak B, Mikhailov A, Azam M, Irfan M, Siddiqui ZK, Naeem F, Paterson AD, et al. 2008. CC2D2A, encoding a coiled-coil and C2 domain protein, causes autosomal-recessive mental retardation with retinitis pigmentosa. Am J Hum Genet 82: 1011–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong ACM, Harris PC. 2015. A polycystin-centric view of cyst formation and disease: The polycystins revisited. Kidney Int 88: 699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostergaard E, Batbayli M, Duno M, Vilhelmsen K, Rosenberg T. 2010. Mutations in PCDH21 cause autosomal recessive cone–rod dystrophy. J Med Genet 47: 665–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto E, Hoefele J, Ruf R, Mueller AM, Hiller KS, Wolf MTF, Schuermann MJ, Becker A, Birkenhäger R, Sudbrak R, et al. 2002. A gene mutated in nephronophthisis and retinitis pigmentosa encodes a novel protein, nephroretinin, conserved in evolution. Am J Hum Genet 71: 1161–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto EA, Loeys B, Khanna H, Hellemans J, Sudbrak R, Fan S, Muerb U, O’Toole JF, Helou J, Attanasio M, et al. 2005. Nephrocystin-5, a ciliary IQ domain protein, is mutated in Senior–Løken syndrome and interacts with RPGR and calmodulin. Nat Genet 37: 282–288. [DOI] [PubMed] [Google Scholar]
- Otto EA, Hurd TW, Airik R, Chaki M, Zhou W, Stoetzel C, Patil SB, Levy S, Ghosh AK, Murga-Zamalloa CA, et al. 2010. Candidate exome capture identifies mutation of SDCCAG8 as the cause of a retinal–renal ciliopathy. Nat Genet 42: 840–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozgül RK, Siemiatkowska AM, Yücel D, Myers CA, Collin RWJ, Zonneveld MN, Beryozkin A, Banin E, Hoyng CB, van den Born LI, et al. 2011. Exome sequencing and cis-regulatory mapping identify mutations in MAK, a gene encoding a regulator of ciliary length, as a cause of retinitis pigmentosa. Am J Hum Genet 89: 253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J, Wang Q, Snell WJ. 2005. Cilium-generated signaling and cilia-related disorders. Lab Invest 85: 452–463. [DOI] [PubMed] [Google Scholar]
- Parisi MA, Bennett CL, Eckert ML, Dobyns WB, Gleeson JG, Shaw DWW, McDonald R, Eddy A, Chance PF, Glass IA. 2004. The NPHP1 gene deletion associated with juvenile nephronophthisis is present in a subset of individuals with Joubert syndrome. Am J Hum Genet 75: 82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A, Honoré E. 2010. Polycystins and renovascular mechanosensory transduction. Nat Rev Nephrol 6: 530–538. [DOI] [PubMed] [Google Scholar]
- Pawlik B, Mir A, Iqbal H, Li Y, Nürnberg G, Becker C, Qamar R, Nürnberg P, Wollnik B. 2010. A novel familial BBS12 mutation associated with a mild phenotype: Implications for clinical and molecular diagnostic strategies. Mol Syndromol 1: 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne AM, Downes SM, Bessant DA, Taylor R, Holder GE, Warren MJ, Bird AC, Bhattacharya SS. 1998. A mutation in guanylate cyclase activator 1A (GUCA1A) in an autosomal dominant cone dystrophy pedigree mapping to a new locus on chromosome 6p21.1. Hum Mol Genet 7: 273–277. [DOI] [PubMed] [Google Scholar]
- Pearring JN, Salinas RY, Baker SA, Arshavsky VY. 2013. Protein sorting, targeting and trafficking in photoreceptor cells. Prog Retin Eye Res 36: 24–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen LB, Rosenbaum JL. 2008. Intraflagellar transport (IFT) role in ciliary assembly, resorption and signalling. Curr Top Dev Biol 85: 23–61. [DOI] [PubMed] [Google Scholar]
- Perrault I, Rozet JM, Calvas P, Gerber S, Camuzat A, Dollfus H, Châtelin S, Souied E, Ghazi I, Leowski C, et al. 1996. Retinal-specific guanylate cyclase gene mutations in Leber’s congenital amaurosis. Nat Genet 14: 461–464. [DOI] [PubMed] [Google Scholar]
- Perrault I, Hanein S, Gerber S, Barbet F, Ducroq D, Dollfus H, Hamel C, Dufier JL, Munnich A, Kaplan J, et al. 2004. Retinal dehydrogenase 12 (RDH12) mutations in leber congenital amaurosis. Am J Hum Genet 75: 639–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrault I, Delphin N, Hanein S, Gerber S, Dufier JL, Roche O, Defoort-Dhellemmes S, Dollfus H, Fazzi E, Munnich A, et al. 2007. Spectrum of NPHP6/CEP290 mutations in Leber congenital amaurosis and delineation of the associated phenotype. Hum Mutat 28: 416. [DOI] [PubMed] [Google Scholar]
- Perrault I, Saunier S, Hanein S, Filhol E, Bizet AA, Collins F, Salih MAM, Gerber S, Delphin N, Bigot K, et al. 2012. Mainzer–Saldino syndrome is a ciliopathy caused by IFT140 mutations. Am J Hum Genet 90: 864–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce EA, Quinn T, Meehan T, McGee TL, Berson EL, Dryja TP. 1999. Mutations in a gene encoding a new oxygen-regulated photoreceptor protein cause dominant retinitis pigmentosa. Nat Genet 22: 248–254. [DOI] [PubMed] [Google Scholar]
- Poloschek CM, Bach M, Lagrèze WA, Glaus E, Lemke JR, Berger W, Neidhardt J, Lagreze WA, Glaus E, Lemke JR, et al. 2010. ABCA4 and ROM1: Implications for modification of the PRPH2-associated macular dystrophy phenotype. Invest Ophthalmol Vis Sci 51: 4253–4265. [DOI] [PubMed] [Google Scholar]
- Pretorius PR, Baye LM, Nishimura DY, Searby CC, Bugge K, Yang B, Mullins RF, Stone EM, Sheffield VC, Slusarski DC. 2010. Identification and functional analysis of the vision-specific BBS3 (ARL6) long isoform. PLoS Genet 6: e1000884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puffenberger EG, Jinks RN, Sougnez C, Cibulskis K, Willert RA, Achilly NP, Cassidy RP, Fiorentini CJ, Heiken KF, Lawrence JJ, et al. 2012. Genetic mapping and exome sequencing identify variants associated with five novel diseases. PLoS ONE 7: e28936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh EN. 2015. Photoreceptor disc morphogenesis: The classical evagination model prevails. J Cell Biol 211: 2–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamurthy V, Cayouette M. 2009. Development and disease of the photoreceptor cilium. Clin Genet 76: 137–145. [DOI] [PubMed] [Google Scholar]
- Rattner A, Smallwood PM, Williams J, Cooke C, Savchenko A, Lyubarsky A, Pugh EN, Nathans J. 2001. A photoreceptor-specific cadherin is essential for the structural integrity of the outer segment and for photoreceptor survival. Neuron 32: 775–786. [DOI] [PubMed] [Google Scholar]
- Riazuddin SA, Iqbal M, Wang Y, Masuda T, Chen Y, Bowne S, Sullivan LS, Waseem NH, Bhattacharya S, Daiger SP, et al. 2010. A splice-site mutation in a retina-specific exon of BBS8 causes nonsyndromic retinitis pigmentosa. Am J Hum Genet 86: 805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riazuddin S, Belyantseva IA, Giese APJ, Lee K, Indzhykulian AA, Nandamuri SP, Yousaf R, Sinha GP, Lee S, Terrell D, et al. 2012. Alterations of the CIB2 calcium- and integrin-binding protein cause Usher syndrome type 1J and nonsyndromic deafness DFNB48. Nat Genet 44: 1265–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivolta C, Sweklo EA, Berson EL, Dryja TP. 2000. Missense mutation in the USH2A gene: Association with recessive retinitis pigmentosa without hearing loss. Am J Hum Genet 66: 1975–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepman R, van Duijnhoven G, Rosenberg T, Pinckers AJ, Bleeker-Wagemakers LM, Bergen AA, Post J, Beck A, Reinhardt R, Ropers HH, et al. 1996. Positional cloning of the gene for X-linked retinitis pigmentosa 3: Homology with the guanine-nucleotide-exchange factor RCC1. Hum Mol Genet 5: 1035–1041. [DOI] [PubMed] [Google Scholar]
- Romani M, Micalizzi A, Kraoua I, Dotti MT, Cavallin M, Sztriha L, Ruta R, Mancini F, Mazza T, Castellana S, et al. 2014. Mutations in B9D1 and MKS1 cause mild Joubert syndrome: Expanding the genetic overlap with the lethal ciliopathy Meckel syndrome. Orphanet J Rare Dis 9: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronquillo CC, Bernstein PS, Baehr W. 2012. Senior-Løken syndrome: A syndromic form of retinal dystrophy associated with nephronophthisis. Vision Res 75: 88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roosing S, Rohrschneider K, Beryozkin A, Sharon D, Weisschuh N, Staller J, Kohl S, Zelinger L, Peters TA, Neveling K, et al. 2013. Mutations in RAB28, encoding a farnesylated small GTPase, are associated with autosomal-recessive cone–rod dystrophy. Am J Hum Genet 93: 110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AM, Shah AZ, Venturini G, Krishna A, Chakravarti A, Rivolta C, Bhattacharya SS. 2016. Transcriptional regulation of PRPF31 gene expression by MSR1 repeat elements causes incomplete penetrance in retinitis pigmentosa. Sci Rep 6: 19450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum JL, Witman GB. 2002. Intraflagellar transport. Nat Rev Mol Cell Biol 3: 813–825. [DOI] [PubMed] [Google Scholar]
- Rosenfeld PJ, Cowley GS, McGee TL, Sandberg MA, Berson EL, Dryja TP. 1992. A null mutation in the rhodopsin gene causes rod photoreceptor dysfunction and autosomal recessive retinitis pigmentosa. Nat Genet 1: 209–213. [DOI] [PubMed] [Google Scholar]
- Ruiz-Perez VL, Ide SE, Strom TM, Lorenz B, Wilson D, Woods K, King L, Francomano C, Freisinger P, Spranger S, et al. 2000. Mutations in a new gene in Ellis–van Creveld syndrome and Weyers acrodental dysostosis. Nat Genet 24: 283–286. [DOI] [PubMed] [Google Scholar]
- Sahly I, Dufour E, Schietroma C, Michel V, Bahloul A, Perfettini I, Pepermans E, Estivalet A, Carette D, Aghaie A, et al. 2012. Localization of Usher 1 proteins to the photoreceptor calyceal processes, which are absent from mice. J Cell Biol 199: 381–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders AAWM, de Vrieze E, Alazami AM, Alzahrani F, Malarkey EB, Sorusch N, Tebbe L, Kuhns S, van Dam TJP, Alhashem A, et al. 2015. KIAA0556 is a novel ciliary basal body component mutated in Joubert syndrome. Genome Biol 16: 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal S, De Ruiter A, Hawkins R. 1980. Development and degeneration of retina in rds mutant mice: Light microscopy. J Comp Neurol 194: 193–207. [DOI] [PubMed] [Google Scholar]
- Sato M, Nakazawa M, Usui T, Tanimoto N, Abe H, Ohguro H. 2005. Mutations in the gene coding for guanylate cyclase-activating protein 2 (GUCA1B gene) in patients with autosomal dominant retinal dystrophies. Graefes Arch Clin Exp Ophthalmol 243: 235–242. [DOI] [PubMed] [Google Scholar]
- Sayer JA, Otto EA, O’Toole JF, Nurnberg G, Kennedy MA, Becker C, Hennies HC, Helou J, Attanasio M, Fausett BV, et al. 2006. The centrosomal protein nephrocystin-6 is mutated in Joubert syndrome and activates transcription factor ATF4. Nat Genet 38: 674–681. [DOI] [PubMed] [Google Scholar]
- Scheidecker S, Etard C, Pierce NW, Geoffroy V, Schaefer E, Muller J, Chennen K, Flori E, Pelletier V, Poch O, et al. 2014. Exome sequencing of Bardet–Biedl syndrome patient identifies a null mutation in the BBSome subunit BBIP1 (BBS18). J Med Genet 51: 132–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidts M, Frank V, Eisenberger T, Al Turki S, Bizet AA, Antony D, Rix S, Decker C, Bachmann N, Bald M, et al. 2013a. Combined NGS approaches identify mutations in the intraflagellar transport gene IFT140 in skeletal ciliopathies with early progressive kidney disease. Hum Mutat 34: 714–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidts M, Vodopiutz J, Christou-Savina S, Cortés CR, McInerney-Leo AM, Emes RD, Arts HH, Tüysüz B, D’Silva J, Leo PJ, et al. 2013b. Mutations in the gene encoding IFT dynein complex component WDR34 cause Jeune asphyxiating thoracic dystrophy. Am J Hum Genet 93: 932–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior B, Friedmann AI, Braudo JL. 1961. Juvenile familial nephropathy with tapetoretinal degeneration. A new oculorenal dystrophy. Am J Ophthalmol 52: 625–633. [DOI] [PubMed] [Google Scholar]
- Shaheen R, Faqeih E, Seidahmed MZ, Sunker A, Alali FE, AlQahtani K, Alkuraya FS. 2011. A TCTN2 mutation defines a novel Meckel–Gruber syndrome locus. Hum Mutat 32: 573–578. [DOI] [PubMed] [Google Scholar]
- Shaheen R, Ansari S, Mardawi EAl, Alshammari MJ, Alkuraya FS. 2013. Mutations in TMEM231 cause Meckel–Gruber syndrome. J Med Genet 50: 160–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen R, Shamseldin HE, Loucks CM, Seidahmed MZ, Ansari S, Ibrahim Khalil M, Al-Yacoub N, Davis EE, Mola NA, Szymanska K, et al. 2014. Mutations in CSPP1, encoding a core centrosomal protein, cause a range of ciliopathy phenotypes in humans. Am J Hum Genet 94: 73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen R, Schmidts M, Faqeih E, Hashem A, Lausch E, Holder I, Superti-Furga A, Mitchison HM, Almoisheer A, Alamro R, et al. 2015. A founder CEP120 mutation in Jeune asphyxiating thoracic dystrophy expands the role of centriolar proteins in skeletal ciliopathies. Hum Mol Genet 24: 1410–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar SP, Hughbanks-Wheaton DK, Birch DG, Sullivan LS, Conneely KN, Bowne SJ, Stone EM, Daiger SP. 2016. Autosomal dominant retinal dystrophies caused by a founder splice site mutation, c.828+3A>T, in PRPH2 and protein haplotypes in trans as modifiers. Investig Opthalmology Vis Sci 57: 349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon D, Namburi P, Ratnapriya R, Lazar C, Obolensky A, Ben-Yosef T, Pras E, Gross M, Banin E, Swaroop A. 2016. Whole exome sequencing reveals a homozygous splicing mutation in CEP78 as the cause of atypical Usher syndrome in Eastern Jewish patients. ARVO Annual Meeting, Abstract B0253 Seattle, WA, May 1–6. [Google Scholar]
- Shevach E, Ali M, Mizrahi-Meissonnier L, McKibbin M, El-Asrag M, Watson CM, Inglehearn CF, Ben-Yosef T, Blumenfeld A, Jalas C, et al. 2015. Association between missense mutations in the BBS2 gene and nonsyndromic retinitis pigmentosa. JAMA Ophthalmol 133: 312. [DOI] [PubMed] [Google Scholar]
- Shin JB, Longo-Guess CM, Gagnon LH, Saylor KW, Dumont RA, Spinelli KJ, Pagana JM, Wilmarth PA, David LL, Gillespie PG, et al. 2010. The R109H variant of fascin-2, a developmentally regulated actin crosslinker in hair-cell stereocilia, underlies early-onset hearing loss of DBA/2J mice. J Neurosci 30: 9683–9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva E, Dharmaraj S, Li YY, Pina AL, Carter RC, Loyer M, Traboulsi E, Theodossiadis G, Koenekoop R, Sundin O, et al. 2004. A missense mutation in GUCY2D acts as a genetic modifier in RPE65-related Leber congenital amaurosis. Ophthalmic Genet 25: 205–217. [DOI] [PubMed] [Google Scholar]
- Sjostrand FS. 1953. The ultrastructure of the outer segments of rods and cones of the eye as revealed by the electron microscope. J Cell Physiol 42: 15–44. [DOI] [PubMed] [Google Scholar]
- Slavotinek AM, Stone EM, Mykytyn K, Heckenlively JR, Green JS, Heon E, Musarella MA, Parfrey PS, Sheffield VC, Biesecker LG. 2000. Mutations in MKKS cause Bardet–Biedl syndrome. Nat Genet 26: 15–16. [DOI] [PubMed] [Google Scholar]
- Smith UM, Consugar M, Tee LJ, McKee BM, Maina EN, Whelan S, Morgan NV, Goranson E, Gissen P, Lilliquist S, et al. 2006. The transmembrane protein meckelin (MKS3) is mutated in Meckel–Gruber syndrome and the wpk rat. Nat Genet 38: 191–196. [DOI] [PubMed] [Google Scholar]
- Sokal I, Li N, Surgucheva I, Warren MJ, Payne AM, Bhattacharya SS, Baehr W, Palczewski K. 1998. GCAP1 (Y99C) mutant is constitutively active in autosomal dominant cone dystrophy. Mol Cell 2: 129–133. [DOI] [PubMed] [Google Scholar]
- Sokolov M, Lyubarsky AL, Strissel KJ, Savchenko AB, Govardovskii VI, Pugh EN, Arshavsky VY. 2002. Massive light-driven translocation of transducin between the two major compartments of rod cells: A novel mechanism of light adaptation. Neuron 34: 95–106. [DOI] [PubMed] [Google Scholar]
- Srour M, Hamdan FF, Schwartzentruber JA, Patry L, Ospina LH, Shevell MI, Désilets V, Dobrzeniecka S, Mathonnet G, Lemyre E, et al. 2012a. Mutations in TMEM231 cause Joubert syndrome in French Canadians. J Med Genet 49: 636–641. [DOI] [PubMed] [Google Scholar]
- Srour M, Schwartzentruber J, Hamdan FF, Ospina LH, Patry L, Labuda D, Massicotte C, Dobrzeniecka S, Capo-Chichi J-M, Papillon-Cavanagh S, et al. 2012b. Mutations in C5ORF42 cause Joubert syndrome in the French Canadian population. Am J Hum Genet 90: 693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg RH, Fisher SK, Anderson DH. 1980. Disc morphogenesis in vertebrate photoreceptors. J Comp Neurol 190: 501–518. [DOI] [PubMed] [Google Scholar]
- Stoetzel C, Laurier V, Davis EE, Muller J, Rix S, Badano JL, Leitch CC, Salem N, Chouery E, Corbani S, et al. 2006. BBS10 encodes a vertebrate-specific chaperonin-like protein and is a major BBS locus. Nat Genet 38: 521–524. [DOI] [PubMed] [Google Scholar]
- Stoetzel C, Muller J, Laurier V, Davis EE, Zaghloul NA, Vicaire S, Jacquelin C, Plewniak F, Leitch CC, Sarda P, et al. 2007. Identification of a novel BBS gene (BBS12) highlights the major role of a vertebrate-specific branch of chaperonin-related proteins in Bardet–Biedl syndrome. Am J Hum Genet 80: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone EM, Cideciyan AV, Aleman TS, Scheetz TE, Sumaroka A, Ehlinger MA, Schwartz SB, Fishman GA, Traboulsi EI, Lam BL, et al. 2011. Variations in NPHP5 in patients with nonsyndromic leber congenital amaurosis and Senior–Løken syndrome. Arch Ophthalmol 129: 81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Nathans J. 1997. Stargardt’s ABCR is localized to the disc membrane of retinal rod outer segments. Nat Genet 17: 15–16. [DOI] [PubMed] [Google Scholar]
- Tallila J, Jakkula E, Peltonen L, Salonen R, Kestilä M. 2008. Identification of CC2D2A as a Meckel syndrome gene adds an important piece to the ciliopathy puzzle. Am J Hum Genet 82: 1361–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taschner M, Bhogaraju S, Lorentzen E. 2012. Architecture and function of IFT complex proteins in ciliogenesis. Differentiation 83: S12–S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiadens AAHJ, den Hollander AI, Roosing S, Nabuurs SB, Zekveld-Vroon RC, Collin RWJ, De Baere E, Koenekoop RK, van Schooneveld MJ, Strom TM, et al. 2009. Homozygosity mapping reveals PDE6C mutations in patients with early-onset cone photoreceptor disorders. Am J Hum Genet 85: 240–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel C, Kessler K, Giessl A, Dimmler A, Shalev SA, von der Haar S, Zenker M, Zahnleiter D, Stöss H, Beinder E, et al. 2011. NEK1 mutations cause short-rib polydactyly syndrome type majewski. Am J Hum Genet 88: 106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S, Legendre M, Saunier S, Bessières B, Alby C, Bonnière M, Toutain A, Loeuillet L, Szymanska K, Jossic F, et al. 2012. TCTN3 mutations cause Mohr–Majewski syndrome. Am J Hum Genet 91: 372–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S, Wright KJ, Le Corre S, Micalizzi A, Romani M, Abhyankar A, Saada J, Perrault I, Amiel J, Litzler J, et al. 2014. A homozygous PDE6D mutation in Joubert syndrome impairs targeting of farnesylated INPP5E protein to the primary cilium. Hum Mutat 35: 137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis GH, Sutcliffe JG, Bok D. 1991. The retinal degeneration slow (rds) gene product is a photoreceptor disc membrane-associated glycoprotein. Neuron 6: 61–70. [DOI] [PubMed] [Google Scholar]
- Tucker BA, Scheetz TE, Mullins RF, DeLuca AP, Hoffmann JM, Johnston RM, Jacobson SG, Sheffield VC, Stone EM. 2011. Exome sequencing and analysis of induced pluripotent stem cells identify the cilia-related gene male germ cell-associated kinase (MAK) as a cause of retinitis pigmentosa. Proc Natl Acad Sci 108: E569–E576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuz K, Bachmann-Gagescu R, O’Day DR, Hua K, Isabella CR, Phelps IG, Stolarski AE, O’Roak BJ, Dempsey JC, Lourenco C, et al. 2014. Mutations in CSPP1 cause primary cilia abnormalities and Joubert syndrome with or without Jeune asphyxiating thoracic dystrophy. Am J Hum Genet 94: 62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente EM, Silhavy JL, Brancati F, Barrano G, Krishnaswami SR, Castori M, Lancaster MA, Boltshauser E, Boccone L, Al-Gazali L, et al. 2006. Mutations in CEP290, which encodes a centrosomal protein, cause pleiotropic forms of Joubert syndrome. Nat Genet 38: 623–625. [DOI] [PubMed] [Google Scholar]
- Valente EM, Logan CV, Mougou-Zerelli S, Lee JH, Silhavy JL, Brancati F, Iannicelli M, Travaglini L, Romani S, Illi B, et al. 2010. Mutations in TMEM216 perturb ciliogenesis and cause Joubert, Meckel and related syndromes. Nat Genet 42: 619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente EM, Dallapiccola B, Bertini E. 2013. Joubert syndrome and related disorders. Handb Clin Neurol 113: 1879–1888. [DOI] [PubMed] [Google Scholar]
- Valente EM, Rosti RO, Gibbs E, Gleeson JG. 2014. Primary cilia in neurodevelopmental disorders. Nat Rev Neurol 10: 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verpy E, Leibovici M, Zwaenepoel I, Liu XZ, Gal A, Salem N, Mansour A, Blanchard S, Kobayashi I, Keats BJ, et al. 2000. A defect in harmonin, a PDZ domain-containing protein expressed in the inner ear sensory hair cells, underlies Usher syndrome type 1C. Nat Genet 26: 51–55. [DOI] [PubMed] [Google Scholar]
- Vithana EN, Safieh LA, Pelosini L, Winchester E, Hornan D, Bird AC, Hunt DM, Bustin SA, Bhattacharya SS. 2003. Expression of PRPF31 mRNA in patients with autosomal dominant retinitis pigmentosa: A molecular clue for incomplete penetrance? Invest Ophthalmol Vis Sci 44: 4204–4209. [DOI] [PubMed] [Google Scholar]
- Wada Y, Abe T, Takeshita T, Sato H, Yanashima K, Tamai M. 2001. Mutation of human retinal fascin gene (FSCN2) causes autosomal dominant retinitis pigmentosa. Invest Ophthalmol Vis Sci 42: 2395–2400. [PubMed] [Google Scholar]
- Wada Y, Abe T, Itabashi T, Sato H, Kawamura M, Tamai M. 2003. Autosomal dominant macular degeneration associated with 208delG mutation in the FSCN2 gene. Arch Ophthalmol 121: 1613–1620. [DOI] [PubMed] [Google Scholar]
- Walczak-Sztulpa J, Eggenschwiler J, Osborn D, Brown DA, Emma F, Klingenberg C, Hennekam RC, Torre G, Garshasbi M, Tzschach A, et al. 2010. Cranioectodermal Dysplasia, Sensenbrenner syndrome, is a ciliopathy caused by mutations in the IFT122 gene. Am J Hum Genet 86: 949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Deretic D. 2014. Molecular complexes that direct rhodopsin transport to primary cilia. Prog Retin Eye Res 38: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, den Hollander AI, Moayedi Y, Abulimiti A, Li Y, Collin RWJ, Hoyng CB, Lopez I, Abboud EB, Al-Rajhi AA, et al. 2009. Mutations in SPATA7 cause Leber congenital amaurosis and juvenile retinitis pigmentosa. Am J Hum Genet 84: 380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AM, Beales PL. 2011. Ciliopathies: An expanding disease spectrum. Pediatr Nephrol 26: 1039–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb TR, Parfitt DA, Gardner JC, Martinez A, Bevilacqua D, Davidson AE, Zito I, Thiselton DL, Ressa JHC, Apergi M, et al. 2012. Deep intronic mutation in OFD1, identified by targeted genomic next-generation sequencing, causes a severe form of X-linked retinitis pigmentosa (RP23). Hum Mol Genet 21: 3647–3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil D, Blanchard S, Kaplan J, Guilford P, Gibson G, Walsh J, Mburu P, Varela A, Levilliers J, Weston M, et al. 1995. Defective myosin VIIA gene responsible for Usher syndrome type 1B. Nature 374: 60–61. [DOI] [PubMed] [Google Scholar]
- Weil D, El-Amraoui A, Masmoudi S, Mustapha M, Kikkawa Y, Laine S, Delmaghani S, Adato A, Nadifi S, Ben Zina Z, et al. 2003. Usher syndrome type I G (USH1G) is caused by mutations in the gene encoding SANS, a protein that associates with the USH1C protein, harmonin. Hum Mol Genet 12: 463–471. [DOI] [PubMed] [Google Scholar]
- Weitz CJ, Miyake Y, Shinzato K, Montag E, Zrenner E, Went LN, Nathans J. 1992a. Human tritanopia associated with two amino acid substitutions in the blue-sensitive opsin. Am J Hum Genet 50: 498–507. [PMC free article] [PubMed] [Google Scholar]
- Weitz CJ, Went LN, Nathans J. 1992b. Human tritanopia associated with a third amino acid substitution in the blue-sensitive visual pigment. Am J Hum Genet 51: 444–446. [PMC free article] [PubMed] [Google Scholar]
- Weleber RG. 2002. Infantile and childhood retinal blindness: A molecular perspective (The Franceschetti Lecture). Ophthalmic Genet 23: 71–97. [DOI] [PubMed] [Google Scholar]
- Weleber RG, Carr RE, Murphey WH, Sheffield VC, Stone EM. 1993. Phenotypic variation including retinitis pigmentosa, pattern dystrophy, and fundus flavimaculatus in a single family with a deletion of codon 153 or 154 of the peripherin/RDS gene. Arch Ophthalmol 111: 1531–1542. [DOI] [PubMed] [Google Scholar]
- Weston MD, Luijendijk MWJ, Humphrey KD, Möller C, Kimberling WJ. 2004. Mutations in the VLGR1 gene implicate G-protein signaling in the pathogenesis of Usher syndrome type II. Am J Hum Genet 74: 357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DS. 2002. Transport to the photoreceptor outer segment by myosin VIIa and kinesin II. Vision Res 42: 455–462. [DOI] [PubMed] [Google Scholar]
- Williams D. 2008. Usher syndrome: Animal models, retinal function of Usher proteins, and prospects for gene therapy. Vision Res 48: 433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CL, McIntyre JC, Norris SR, Jenkins PM, Zhang L, Pei Q, Verhey K, Martens JR. 2014. Direct evidence for BBSome-associated intraflagellar transport reveals distinct properties of native mammalian cilia. Nat Commun 5: 5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winderickx J, Sanocki E, Lindsey DT, Teller DY, Motulsky AG, Deeb SS. 1992. Defective colour vision associated with a missense mutation in the human green visual pigment gene. Nat Genet 1: 251–256. [DOI] [PubMed] [Google Scholar]
- Wright C, Healicon R, English C, Burn J. 1994. Meckel syndrome: What are the minimum diagnostic criteria? J Med Genet 31: 482–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Yang L, Wang F, Li HH, Wang X, Wang W, Ge Z, Wang K, Zhao L, Li HH, et al. 2015. Mutations in human IFT140 cause non-syndromic retinal degeneration. Hum Genet 134: 1069–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Sippel KC, Berson EL, Dryja TP. 1997. Defects in the rhodopsin kinase gene in the Oguchi form of stationary night blindness. Nat Genet 15: 175–178. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Liu J, Gao J, LeNoue S, Wang C, Kaminoh J, Bowne SJ, Sullivan LS, Daiger SP, Zhang K, et al. 2009. Essential and synergistic roles of RP1 and RP1L1 in rod photoreceptor axoneme and retinitis pigmentosa. J Neurosci 29: 9748–9760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan D, Liu XZ. 2010. Genetics and pathological mechanisms of Usher syndrome. J Hum Genet 55: 327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Peachey NS, Moshfeghi DM, Thirumalaichary S, Chorich L, Shugart YY, Fan K, Zhang K. 2002. Mutations in the RPGR gene cause X-linked cone dystrophy. Hum Mol Genet 11: 605–611. [DOI] [PubMed] [Google Scholar]
- Yang J, Gao J, Adamian M, Wen XH, Pawlyk B, Zhang L, Sanderson MJ, Zuo J, Makino CL, Li T. 2005. The ciliary rootlet maintains long-term stability of sensory cilia. Mol Cell Biol 25: 4129–4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Chen Y, Lillo C, Chien J, Yu Z, Michaelides M, Klein M, Howes KA, Li Y, Kaminoh Y, et al. 2008. Mutant prominin 1 found in patients with macular degeneration disrupts photoreceptor disk morphogenesis in mice. J Clin Invest 118: 2908–2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RW. 1967. The renewal of photoreceptor cell outer segments. J Cell Biol 33: 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki MS, Sattar S, Massoudi RA, Gleeson JG. 2011. Co-occurrence of distinct ciliopathy diseases in single families suggests genetic modifiers. Am J Med Genet Part A 155: 3042–3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallocchi M, Meehan DT, Delimont D, Askew C, Garige S, Gratton MA, Rothermund-Franklin CA, Cosgrove D. 2009. Localization and expression of clarin-1, the Clrn1 gene product, in auditory hair cells and photoreceptors. Hear Res 255: 109–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Li S, Xiao X, Jia X, Guo X. 2007. The 208delG mutation in FSCN2 does not associate with retinal degeneration in Chinese individuals. Invest Ophthalmol Vis Sci 48: 530–533. [DOI] [PubMed] [Google Scholar]
- Zito I, Downes SM, Patel RJ, Cheetham ME, Ebenezer ND, Jenkins SA, Bhattacharya SS, Webster AR, Holder GE, Bird AC, et al. 2003. RPGR mutation associated with retinitis pigmentosa, impaired hearing, and sinorespiratory infections. J Med Genet 40: 609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]