Abstract
The development of an effective HIV-1 eradication strategy relies upon a clear understanding of the cellular mechanisms involved in HIV-1 latency. Among such cellular processes, microRNA activities affect HIV-1 production by regulating viral transcripts as well as host cell HIV-1 dependency factors. miR-29a stands apart from other relevant microRNAs as a potential therapeutic target in HIV-1 eradication. In vitro experiments have shown that miR-29a binds to a sequence in the 3′UTR of viral transcripts and inhibits their expression. In vivo data revealed the existence of a cytokine-microRNA (i.e. IL-21/miR-29a) pathway that significantly impacts HIV-1 replication. Here we present and discuss evidence supporting the role of miR-29a in HIV-1 replication and latency. We also discuss potential clinical applications of miR-29a inhibitors and enhancers in HIV-1 eradication strategies.
Keywords: shock and kill, lock and block, microRNAs, HIV eradication
Introduction
Combined antiretroviral therapy (cART), a combination of antiretroviral drugs targeting the different stages of the viral life cycle, suppresses HIV-1 viraemia. However, cART cannot eradicate the infection because it targets actively replicating viruses, not viruses persisting in a latent form. HIV-1 latency has been defined as a non-productive state of infection and has been observed in resting CD4 T cells [1]. The discovery that microRNAs (miRNAs) are involved in HIV-1 latency is relatively recent [2], yet there is a growing body of evidence suggesting that miRNAs could be important components of successful HIV-1 eradication approaches. While the breadth of miRNAs identified as potentially useful in HIV-1 eradication continues to grow, the depth of analyses on particular miRNAs in this context is somewhat lacking for most candidates. Of the few miRNAs that have been studied in depth for their impact on HIV-1, miR-29a has repeatedly been shown to inhibit HIV-1 replication and infection in vitro [3–6]. Furthermore, recent in vivo data support the existence of a substantial interaction between miR-29a and HIV-1 [7]. Therefore, miR-29a stands out as a promising candidate for successful incorporation into an HIV-1 eradication strategy. Besides cellular miRNAs, the existence of HIV-1-encoded miRNAs has also been reported [8–10]; however, this topic is not addressed herein. This study reviews the literature discussing miRNA regulation of HIV-1 replication; highlights and discusses the evidence for and against the role of miR-29a in HIV-1 replication and latency; and discusses this information in an HIV-1 eradication context.
Biogenesis and silencing activity of miRNAs
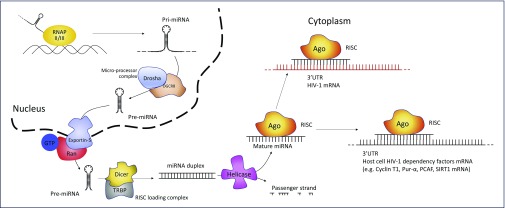
miRNAs are 20–23 nucleotides (nt) long, single-stranded, non-coding RNAs that mediate post-transcriptional regulation of gene expression [11]. miRNAs are originally transcribed as a part of a hairpin structure embedded within a longer transcript – the primary miRNA (pri-miRNA). The pri-miRNA is processed inside the nucleus to form the precursor miRNA (pre-miRNA), which is exported to the cytoplasm and then cleaved to an miRNA duplex. Helicase activity then results in the unwinding of the duplex into a passenger strand and a functional mature miRNA. The passenger strand is degraded whereas the functional strand is loaded into a RISC (RNA-induced silencing complex) that contains Argonaute (Ago) proteins [11]. The functional strand is complementary to a sequence on the targeted mRNA, usually in the 3′UTR, and guides the RISC to its target transcripts. Although full complementarity along the entire sequence is not required, Watson–Crick base pairing of miRNA nucleotides 2–8, known as the seed region, plays a critical role in mRNA binding and target recognition [12]. Furthermore, the degree of miRNA–mRNA complementarity has a functional consequence. Specifically, near-perfect matching of these strands leads to Ago2-catalysed cleavage whereas multiple mismatches and nucleotide bulges promote repression of mRNA translation as well as mRNA decay [12]. The biogenesis of miRNAs and the major proteins involved in the process are depicted in Figure 1.
Figure 1.
miRNA biogenesis and HIV-1 regulation. Primary miRNA (pri-miRNA) is transcribed by RNA polymerase (RNAP) II or III as a several-hundred-nucleotides-long transcript with a ~33-base pair hairpin loop. Cleavage of the pri-miRNA by the micro-processor complex (composed by Drosha and DGCR8) yields a ~60-nt precursor miRNA (pre-miRNA). Transfer of the pre-miRNA from the nucleus to the cytoplasm is mediated by exportin-5 and Ran-GTP. In the cytoplasm, the RISC loading complex – composed by Dicer and TRBP – cleaves the pre-miRNA to a ~22 nt miRNA duplex. Helicase activity unwinds the duplex into a passenger strand, which is degraded, and a mature miRNA, which is then loaded into the RISC (RNA-induced silencing complex). Here, mature miRNA guides cleaving enzymes (e.g. Ago-proteins) to matching target transcripts [11]. Through this posttranscriptional repression pathway, miRNAs can regulate HIV-1 replication by targeting either HIV-1 transcripts or mRNAs coding for host factors that regulate HIV-1 (host cell HIV-1 dependency factors)
HIV-1 replication and miRNAs
miRNAs affect HIV-1 replication indirectly (e.g. affecting host cell HIV-1 dependency factors that regulate HIV-1 integration and transcription) and directly (e.g. binding to viral transcripts and inhibiting translation) (Figure 1; summarised in Table 1; also reviewed in [13]). An example of how miRNAs can indirectly affect HIV-1 integration is miR-155 [14]. Specifically, miR-155 targets cellular LEDGF/p75, which plays a critical role in guiding reverse-transcribed genomes to the intronic regions of highly expressed genes [15]. At the transcriptional level, miRNAs can reinforce HIV-1 latency through their regulation of constitutively expressed factors that control cellular proliferation regardless of the cell's infection status. One of the most studied of these is cyclin T1, whose association with CDK9 in the P-TEFb complex is essential for HIV-1 transcription. Therefore, miRNAs known to target cyclin T1 (e.g. miR-27b, miR-29b, miR-150, miR-198 and miR-223) might play a key role in HIV-1 latency regulation [16]. MiR-17-5p and miR-20a are, instead, involved in the epigenetic control of HIV-1 replication. These miRNAs affect the cellular levels of p300/CBP-associated factor (PCAF) [17], a histone acetyltransferase that enhances HIV-1 transcription by acetylating both the histone proteins and the p65 component of NF-κB [15]. Another regulator of NF-κB activity is, again, miR-155, which inhibits the ubiquitinating effect of TRIM-32 on IκBα, thus enhancing the availability of IκB and the following sequestration of NF-κB in the cytoplasm [18]. Conversely, some miRNAs can enhance HIV-1 infection by inhibiting cellular repressors of viral replication. For example, miR-34a and miR-217 downregulate SIRT-1, a p65 and Tat deacetylase, thus enhancing their efficiency under HIV-1 transcription [19,20]. This group of miRNAs could therefore be responsible for boosting viral replication when latency is interrupted.
Table 1.
The predominant human miRNAs involved in the regulation of HIV-1 replication are reported together with their target and the resulting effect
| Classification | MicroRNA | Target | Effect on HIV-1 replication | Ref. |
|---|---|---|---|---|
| Human miRNAs targeting host cell HIV-1 dependency factors | miR-16 | Pur-α | (-) | [42] |
| miR-17-5p | PCAF | (-) | [17] | |
| miR-20a | PCAF | (-) | [17] | |
| Pur-α | (-) | [42] | ||
| miR-27b | Cyclin T1 | (-) | [16] | |
| miR-29b | Cyclin T1 | (-) | [16] | |
| miR-34a | SIRT1 | (+) | [19] | |
| PNUTS/PPP1R10 | (+) | [43] | ||
| TASK1 | (+) | [44] | ||
| miR-93 | Pur-α | (-) | [42] | |
| miR-106b | Pur-α | (-) | [42] | |
| miR-124a | TASK1 | (+) | [44] | |
| miR-132 | MeCP2 | (+) | [45] | |
| miR-150 | Cyclin T1 – indirect | (-) | [16] | |
| miR-155 | LEDGF/p75 | (-) | [14] | |
| ADAM10 | (-) | [14] | ||
| NUP153 | (-) | [14] | ||
| TRIM32 | (-) | [18] | ||
| miR-182 | NAMPT, which then alters SIRT1 | (+) | [46] | |
| miR-198 | CyclinT1 | (-) | [47] | |
| miR-217 | SIRT1 | (+) | [20] | |
| miR-223 | Cyclin T1 – indirect | (-) | [16] | |
| miR-1236 | VprBP | (-) | [48] | |
| Let-7c | P21 | (+) | [44] | |
| Human miRNAs targeting HIV-1 mRNA | miR-28 | HIV-1 mRNA – 3'UTR | (-) | [2] |
| miR-29a | HIV-1 mRNA-3'UTR Nef | (-) | [3–5] | |
| miR-29b | HIV-1 mRNA-3'UTR Nef | (-) | [3,4] | |
| miR-125b | HIV-1 mRNA-3'UTR | (-) | [2] | |
| miR-133b | HIV-1 mRNA | (-) | [49] | |
| miR-138 | HIV-1 mRNA | (-) | [49] | |
| miR-149 | HIV-1 mRNA | (-) | [49] | |
| miR-150 | HIV-1 mRNA-3'UTR | (-) | [2] | |
| miR-196b | HIV-1 mRNA-3'UTR | (-) | [50] | |
| miR-223 | HIV-1 mRNA-3'UTR | (-) | [2] | |
| miR-326 | HIV-1 mRNA-3'UTR | (-) | [49] | |
| miR-382 | HIV-1 mRNA-3'UTR | (-) | [2] | |
| miR-1290 | HIV-1 mRNA-3'UTR | (-) | [50] |
+: enhances; -: inhibits
Host miRNAs can also bind directly to viral transcripts, which opens the door to more HIV-1-specific miRNA-based therapies. In a seminal paper, Huang et al. showed that miR-28, miR-125b, miR-150, miR-223 and miR-382 could downregulate transcripts containing a 1.2-kb fragment from HIV-1 3′UTR harbouring target sequences for these miRNAs and that mutations in these putative miRNA targets relieved the inhibition [2]. In addition, they reported that virus transcript downregulation takes place in resting, but not stimulated, CD4 T cells. They also reported that inhibition of the five miRNAs simultaneously caused reactivation of HIV-1 infection in CD4 T cells from HIV-1 patients on cART [2]. miR-29a was not included in these experiments; however, it is noteworthy that qualitatively miR-29a alone has shown the same effects as the five combined miRNAs described by Huang et al. [3–6]. A quantitative head-to-head comparison has not yet been performed. The multiple interactions between miR-29a and HIV-1 replication (both in vitro and in vivo) are summarised in Table 2 and elaborated upon below.
Table 2.
Overview of selected experiments and findings of the six main articles describing the interaction of miR-29a with HIV-1
| Study, year | Method | Cell line | miR-29a source | Plasmid (p)/infectious virus | Observations | Conclusion |
|---|---|---|---|---|---|---|
| Ahluwalia et al. 2008 [3] | Luciferase assay
Northern blot for luciferase transcripts |
HeLa | Endogenous | pMIR-REPORT with Nef | Lower luciferase signal compared to luciferase vectors with no Nef
No differences in RNA levels compared to luciferase vectors with no Nef |
miR-29a targets Nef post-transcriptionally |
| Immunoblotting for Nef
ELISA for p24 |
293T
Jurkat |
Exogenous | pNL4-3 | Reduced Nef signal in immunoblotting
Lower p24 levels in supernatant of miR-29a-co-transfected cells |
Overexpression of miR-29a inhibits viral production and Nef expression | |
| ELISA for p24 | 293 T | Endogenous – inhibited | pNL4-3 | Increased p24 levels in supernatant of anti-miR-29a-cotransfected cells | Inhibition of miR-29a enhances HIV-1 production | |
| Nathans et al. 2009 [4] | miRNA microarray | H9
293T |
Endogenous | NL4-3 | miR-29a is the most expressed miRNA among 11 miRNAs predicted to match HIV-1 3'UTR | miR-29a is highly expressed in HIV-1-infected H9 and 293T cells |
| ELISA for p24 | 293T | Endogenous – inhibited | pNL4-3LucR-E | Increased p24 levels in supernatant of anti-miR-29a-co-transfected cells | Inhibition of miR-29a enhances HIV-1 production | |
| ELISA for p24 | 293T | Exogenous | pNL4-3LucR-E | Lower p24 levels in supernatant of miR-29a-co-transfected cells | miR-29a mimic insert decreases HIV-1 production | |
| Luciferase assay | H9 | Endogenous – inhibited | NL4-3LucR-E-virus | Increased luciferase activity in total lysates of anti-miR-29a-transfected cells | miR-29a inhibition enhances viral infectivity | |
| ELISA for p24 | 293T | Exogenous
Exogenous mutant miR-29a-mt |
pNL4-3LucR-E WT
pNL4-3LucR-E-m29t (4 mutations in 3'UTR target region) pNL4-3LucR-E-Δ29t (20 nt deletion at the miR-29a target site in the HIV-1 3'UTR region) |
miR-29a inhibits WT viral production, but does not inhibit mutant plasmid
miR-29a-mt inhibits mutant plasmid, but does not inhibit WT plasmid miR-29a does not inhibit the seed-deleted plasmid |
miR-29a targets the predicted site in HIV-1 3'UTR directly, no intermediary factor is required | |
| ELISA for p24 | 293T | Exogenous
(+ siRNA to inhibit Dicer) |
pNL4-3LucR-E | Increased p24 levels in supernatant, when Dicer is knocked down | Dicer depletion enhances HIV-1 production | |
| Immunoprecipitation with anti-Myc-antibodies
Immunoblotting RT-qPCR for mRNA associated with Ago2 |
293T | Exogenous
Exogenous mutant miR-29a-mt |
pNL4-3LucR-E WT
pNL4-3LucR-E-m29t |
Gag-mRNA from WT-HIV-1 associates with Ago2 immuno-precipitate, Gag-mRNA with mutated miR-29a target does not | miR-29a specifically enhances the association of HIV-1 mRNA with RISC complex | |
| Immunoprecipitation with anti-RCK/p54 antibodies
Immunoblotting RT-qPCR for mRNA associated with RCK/p54 |
293T | Exogenous
Exogenous mutant miR-29a-mt |
pNL4-3LucR-E WT
pNL4-3LucR-E-m29t |
Gag-mRNA from WT-HIV-1 associates with RCK/p54 immuno-precipitate, Gag-mRNA with mutated miR-29a target does not | miR-29a specifically enhances the association of HIV-1 mRNA with endogenous P-body proteins | |
| Sun et al. 2012 [6] | miRNA-array followed by TaqMan miRNA RT-PCR | CD4+CD8- PBMCs
CEM Jurkat |
Endogenous | III B | miR-29a is downregulated, as well as miR-21, 26a, 155, 29a, 29b, 29c | miR-29a is downregulated in HIV-1-infected T cells, CEM and Jurkat cells |
| ELISA for p24 | HeLa-T4 | Exogenous | pNL4-3 | Lower p24 levels in supernatant of miR-29a-co-transfected cells | miR-29a mimic insert decreases HIV-1 production | |
| ELISA for p24 | HeLa-T4 | Exogenous – inhibited | pNL4-3 | Increased p24 levels in supernatant of anti-miR-29a-cotransfected cells | Inhibition of miR-29a enhances HIV-1 production | |
| Dual-luciferase reporter assay | HEK293 | Exogenous | 39 nt target sequence on a psiCheck plasmid as 3'UTR | Rluc/Fluc ratio is lowered following miR-29a cotransfection | miR-29a target prediction is correct | |
| Dual-luciferase reporter assay | HEK293 | Exogenous | 56 nt target sequence on a psiCheck plasmid as 3'UTR | Rluc/Fluc ratio is not lowered despite miR-29a cotransfection | Three-dimensional structure inhibits miR-29a binding | |
| Whisnant et al. 2013 [22] | Total small RNA sequencing | TZM-bl
C8166 CD4+ PBMCs |
Endogenous | NL4-3
BaL |
Few differences between infected and non-infected cells: no differences resulted in an increase (≥2-fold) in miR-levels | HIV-1 infection does not alter miRNA profile in CD4 cells |
| Dual-luciferase reporter assay | 293T | Exogenous | PCR-cloned 300 bp segment from NL4-3 fused with Rluc gene | Rluc/Fluc ratio is not lowered despite miR-29a co-transfection | miR-29a does not downregulate HIV-1 transcripts | |
| Patel et al. 2014 [5] | RT-qPCR for miR-29a
Western blot for p24 |
U1
J1.1 (PMA activated) |
Endogenous | Latent LAV | Lower miR-29a levels, high p24 signal in western blot following PMA-activation
Control for bias: PMA activation of U937 and Jurkat does not downregulate miR-29a, rather enhances miR-29a expression |
miR-29a expression is downregulated once HIV-1 transcription is triggered |
| RT-qPCR for miRNA
Western blot for Nef |
U937 | Endogenous | Nef protein from latent LAV | Lower miR-29a levels in U937 cells stably expressing Nef protein | Nef expression downregulates miR-29a | |
| ELISA for p24 | J1.1
(PMA activated) |
Exogenous | Latent LAV | Lower p24 levels in supernatant of miR-29a-co-transfected cells | miR-29a inhibits viral production | |
| ELISA for p24
Western blot for intracellular Gag |
U1 | Endogenous – inhibited | Latent LAV | Increased p24 levels in supernatant of anti-miR-29a-co-transfected cells
Increased intracellular Gag expression in anti-miR-29a-cotransfected cells |
miR-29a-inhibition induces HIV-1 production in latently infected cells | |
| Adoro et al. 2015 [7] | RT-qPCR for miRNAs | Splenic CD4 T cells from HIV-1-negative donors
(IL-21-treated ex vivo) |
Endogenous | Increased expression of all mature miR-29 species in both memory and naïve CD4 T cells
No change in miR-142-5p expression |
IL-21 upregulates miR-29 species expression, but does not affect other miRNAs | |
| RT-qPCR for pri-miRNA and miRNA | Splenic CD4 T cells from HIV-1-negative donors
(IL-21-treated ex vivo) |
Endogenous | Increased expression of pri-miR-29 transcripts peaking at 4 h
Increased expression of mature miR-29a peaking at 12 h |
IL-21 upregulates mIR-29a biogenesis | ||
| Chromatin immunoprecipitation and PCR | Splenic CD4 T cells from HIV-1-negative donors
(IL-21-treated ex vivo) |
Significantly enriched STAT3 binding to two putative regulatory regions upstream of MIR29B1/29A after IL-21 treatment | IL-21 induces miR-29a through STAT3 | |||
| RT-qPCR for miR-29 species | Splenic CD4 T cells from HIV-1-negative donors
(infected ex vivo) |
Endogenous | NL4-3 | Lower miR-29a levels following infection | HIV-1 infection induces miR-29a downregulation | |
| RT-qPCR for miR-29 species | Splenic CD4 T cells from HIV-1-negative donors
(infected + IL-21-treated ex vivo) |
Endogenous | NL4-3 | Increased miR-29a levels in IL-21-treated infected cells compared to untreated | IL-21 reverses HIV-1 induced miR-29a downregulation | |
| ELISA for IL-21
PCR for IL-21 mRNA RT-qPCR for miR-29a |
Splenic CD4 T cells from BLT humanised mice
(infected + IL-21-treated in vivo – plasmid injection to induce systemic IL-21 expression 72 h prior infection) |
Endogenous | JR-CSF | 2 weeks after infection: HIV-1 viral titres inversely correlate with plasma IL-21 levels;
IL-21 mRNA levels correlate with miRNA; significant inverse correlation between miR-29a expression and plasma HIV-1 titres |
IL-21–miR29a axis suppresses HIV-1 directly in CD4 T cells in vivo |
nt: nucleotides; PBMC: peripheral blood mononuclear cells; WT: wild type
miR-29a and HIV-1 protein expression are inversely related
Experiments conducted in cell lines transfected with HIV-1 plasmids, mainly pNL4-3 or its luciferase variant pNL4-3LucR-E, show that overexpression of miR-29a downregulates HIV-1 virus production [3,4,6] and reduces the infectivity of resulting viruses [4]. In a similar experiment, cells harbouring artificial constructs that mimic miR-29a in structure and function show decreased HIV-1 production [4]. In a reciprocal experiment, inhibition of the endogenous miR-29a enhanced viral production [3,4,6]. Furthermore, inhibition of endogenous miR-29a can reactivate latent provirus in HIV-1 latently infected Jurkat E6 cells (J1.1 cells) [5]. These observations strongly suggest an existing association between cellular miR-29a levels and HIV-1 protein expression, although these data do not specify the mechanism or prove a direct interaction of miR-29a with the HIV-1 transcripts.
The role of miR-29a in latency: compelling evidence
A 2005 study that used four target-prediction software platforms reported the existence of a putative binding site for miR-29a in the HIV-1 genome. Specifically, this site is located in a highly conserved region of the Nef gene that also serves as 3′UTR for the HIV-1 transcripts [21]. The finding was confirmed by the experiments of Nathans et al. from 2009 [4], which are based on a mutant pNL4-3 created by inserting four mutations in the putative target located on the wild type pNL4-3. At the same time, this group also created a mutant miR-29a that matched the new sequence, and they designed a third viral plasmid with a 20 nt deletion at the target region. Using different combinations of these plasmids and miRNAs, they demonstrated that miR-29a could downregulate the production of the wild type virus, as well as infectivity of the wild type virus. Importantly, miR-29a had no effect on mutated or deleted sequences, whereas mutant miRNAs inhibited the concordantly mutated viral plasmids. Taken together, these findings provide strong evidence of a specific and direct interaction between miR-29a and the previously identified region in the 3′UTR. While the modification of the target itself might have influenced replication in other ways, the fact that a matching mutation of miR-29a can re-establish the inhibition of the mutant plasmids suggests that an intermediary factor is not required for miR-29a inhibition.
Another important aspect of the study by Nathans et al. is their investigation into the interaction of miR-29a and HIV-1 inside mRNA processing bodies (P-bodies). P-bodies are the cytoplasmic substructures where Ago-proteins, miRNAs and untranslated mRNAs accumulate, together with other enzymes involved in mRNA turnover and translational repression [11]. Here, HIV-1 gag mRNA was found associated with immuno-purified Ago2 proteins from the RISC and RCK/p54 from P-bodies, but only if the HIV-1-transfected cells had also been co-transfected with miR-29a [4]. Again, co-transfection with miR-29a and mutant plasmid showed no inhibition, whereas introduction of the concordantly mutated miR-29a reproduced the wild type inhibitory effect. Taken together, all these findings suggest that miR-29a allows the RISC to bind HIV-1 mRNA, and that the miR-29a–HIV-1 mRNA–RISC complex then associates with P-bodies, where mRNA translational repression takes place.
The evidence from different studies in vitro [3–5] strongly indicates that miR-29a interacts with HIV-1 transcripts, silencing viral production and infectivity. Yet results from two other studies have questioned whether this interaction may exist in vivo [6,22]. Specifically, these studies suggest that the virus can escape miR-29a-mediated restriction when cellular miR-29a expression is at physiological concentrations. Whether overexpression of miR-29a drives the results remains an open question. Nevertheless, there is strong evidence that miR-29a downregulates Nef protein levels in infected cells [3,5]. The ability to target Nef mRNA is common for the miR-29 family but stronger for miR-29a [3], and such targeting might play a role in regulating HIV-1 pathogenesis given that functional Nef is essential for in vivo viral pathogenesis [23]. It should be noted that miR-29a-silencing activity is not limited to Nef, since the mRNA sequence that harbours the target is in the 3′UTR that is shared by all HIV-1 transcripts [2]. Indeed, Nathans et al. showed that miR-29a also mediated HIV-1 gag mRNA association with Ago2 proteins [4]. However, the available evidence does not provide information regarding whether Nef downregulation is essential to cause the observed effects on virus production and infectivity. This is because direct 3′UTR targeting of whole length HIV-1 mRNAs by miR-29a could be sufficient to cause these inhibitory effects.
HIV-1 possible defences against miR-29a
Much as HIV-1 has evolved defences against other innate immune effectors (e.g. Vif to counteract APOBEC3G and Vpu to counteract tetherin [24]), it is conceivable that HIV-1 has evolved defences against miRNAs. To date, no extensive or effective virus-encoded defences against miRNAs have been described. Nevertheless, specific HIV-1 encoded mechanisms could play a central role in avoiding miRNA silencing. These proposed activities are beyond the likely inhibitor effects of RNA secondary structures at the miRNA target site [6,25]. For example, HIV-1 Tat has RNAi silencing suppressor activity as it inhibits Dicer [26]. Also, Nef has been shown to directly bind to Ago2, inhibiting its cleaving activity [27], and to downregulate miR-29a expression [5]. Moreover, HIV-1 trans-activation response element (TAR)-mimic constructs have been reported to interact with TRBP and alter miRNA activity at the RISC loading complex level [28]. Mutations due to the error prone reverse transcriptase could theoretically protect HIV-1 from miR-29a, since miR-29a cannot bind to sequences where the seed is sufficiently mutated or has been deleted [4]. However, the region harbouring the seed sequence appears to be highly conserved among different HIV-1 clades [4,6,21]. This could be because this sequence is inside the Nef gene and mutations in this region can impair HIV-1 infectivity.
From in vitro to in vivo
In vitro studies provide considerable evidence regarding the impact of miR-29a on HIV-1, but tissue culture and/or animal models are necessary to conclude whether miR-29a affects HIV-1 replication in vivo. Towards this goal, Adoro et al. found that interactions between miR-29a and HIV-1 can take place in a human lymphoid organ aggregate culture [7]. This ex vivo model allows the study of early events during HIV-1 exposure in more physiological settings and without the need for mitogen stimulation. Using this setup, Adoro et al. described the existence of an IL-21–STAT3–miR-29a pathway suppressing HIV-1 replication: IL-21-stimulated CD4 T cells upregulate miR-29a production, showing an increase of pri-miR-29 transcripts at about 4 h post stimulation, whereas the expression of other miRNAs (e.g. miR-142-5p) does not change. Moreover, IL-21 reverses HIV-1-induced miR-29a downregulation. Finally, exogenous IL-21 treatment in HIV-1-infected humanised mice showed: (i) a direct correlation between IL-21 and miR-29a levels in splenic CD4 T cells; and (ii) a significant inverse correlation between miR-29a expression and plasma HIV-1 titre. Taken together, these results support the hypothesis that miR-29 affects HIV-1 replication in vivo. They also confirm that HIV-1 infection causes downregulation of miR-29a and that upregulation of miR-29a (e.g. due to cytokine stimulation) can efficiently limit HIV-1 infection in vivo. Furthermore, the results obtained in the IL-21-treated mice highlight the need for a better understanding of how cytokines and other factors interact with miR-29a expression and activity in vivo.
Several studies have measured altered miRNA profiles in peripheral blood mononuclear cells (PBMC) of chronically infected HIV-1 patients [29–32]. One study shows downregulation of miR-29a, especially in viraemic individuals [29], whereas others show no change in expression of miR-29a compared to healthy controls [30,31]. Substantial differences in the methods make a proper comparison of these studies difficult. Moreover, screening total PBMCs may not be useful to understand variations in miRNA expression pattern correlates to HIV-1 replication and/or persistence in different cell subsets (e.g. memory vs. naïve T cells; infected vs. non-infected cells). This is because different cells within the total PBMC pool will contribute differentially to the miRNA profiles [29]. Therefore, to assess whether miR-29a directly affects HIV-1 latency in humans, future studies should focus on defining the role of this miRNA in different subsets of cells known to harbour latent HIV-1 (e.g. resting CD4 T cells) [33]. Furthermore, the in vivo cytokine (e.g. IL-21) environment and receptor (e.g. TLR) signalling activities will affect miRNA expression. Therefore, future experiments should consider cytokines and other inflammation biomarkers when comparing HIV-infected individuals.
Also, the relationship between the IL-21–miR-29a axis and HIV-1 replication upon viral reactivation could be further dissected in humanised mice. A first step could be to test the effects of IL-21 on viraemia in HIV-1-infected humanised BLT mice on ART that harbour latently infected resting CD4 T cells [34]. The setup could be further improved by knocking miR-29a out in the human hematopoietic stem cells used as the bone marrow transplant to generate the humanised mice. Such a setup would facilitate the testing of whether IL-21 still limits viral replication through other pathways.
Potential clinical applications of miR-29a in HIV-1 eradication approaches
cART can achieve durable viral suppression, but the therapy requires constant access to the medicines, high compliance and has associated toxicities. To overcome these challenges, different approaches for achieving HIV-1 eradication have been proposed and are being pursued experimentally. Some approaches aim to eradicate all replication-competent virus in the body while others attempt to reduce the reservoir to a size that is thereafter controlled by the immune system [35]. The present knowledge about miR-29a, as well as miRNAs more generally, could be used to enhance the efficacy of eradication strategies.
Both the inhibition and overexpression of miR-29a are readily achieved in vitro. Furthermore, immunotherapies based on these have been proposed and are being tested against different cancers [36]. Therefore, there is a strong rationale for considering similar application in HIV-1 therapies. Therapeutic developments from the cancer field are already crossing over into HIV-1 eradication regimens, including vorinostat, panobinostat and romidepsin as ‘shock’ agents in ‘shock and kill’ eradication approaches [37]. Since inhibition of endogenous miR-29a can enhance HIV-1 replication [3,4,6], and even reactivate latent virus [5], anti-miR-29a constructs may successfully augment such ‘shock and kill’ therapies. Alone or combined with latency reversal agents, miR-29a-inhibitors might boost the reactivation of the viral reservoir, allow better immune-mediated killing and raise the odds for successful eradication. Adverse effects of miR-29a inhibitors are possible given that miR-29a also targets host factors. Specifically, miR-29a is involved in cellular homeostasis and deregulation of miR-29a may be involved in oncogenesis [38]. Constant suppression of miR-29a in uninfected cells could increase the risk of oncogene expression. Therefore, a potential strategy for delivering an miR-29a-inhibitor would be in short-course dosing, in a similar manner to that already being utilised for latency reversal agent administration [37].
Since miR-29a can directly target any HIV-1 transcript, enhancement of endogenous miR-29a levels (e.g. with exogenous IL-21 therapy [7]) or treatment with miR-29a-mimic constructs could be essential elements in recently proposed ‘lock and block’ HIV-1 eradication strategies that aim to permanently silence the virus and eliminate the adverse effects associated with viral replication [39]. Induction of deep latency through miR-29a agonists would block virus production and infectivity, reducing chronic inflammation and immune activation that represent a considerable burden in HIV-1 infection. Enhancement of miR-29a expression would likely be used to complement ongoing strategies (e.g. those aiming to induce deep latency through Tat inhibitors [40]). As some miRNAs are known to play an immuno-regulatory role in inflammation [41], the anti-inflammatory effect of this strategy could also be harnessed by other miRNAs simultaneously. A ‘lock and block’ enhancement based on miR-29a agonists could also work as a safety net in more advanced cellular strategies such as genome editing of autologous CD4 T cells or hematopoietic stem cells [35]. Given that overexpression of miR-29a may promote epithelial-to-mesenchymal transition as well as metastasis in breast and colon cancer [38], it is essential that the miR-29a agonists be shown to have a broad therapeutic index to ensure safety before incorporating such molecules into an HIV-1 eradication strategy.
Conclusions
Multiple observations confirm that miR-29a can directly bind to a sequence in Nef within the HIV-1 3′UTR. This allows miR-29a to regulate the expression of all the HIV-1 transcripts, including those for Nef. In this way, miR-29a suppresses both HIV-1 production, viral infectivity and viral pathogenesis. There is also evidence that miR-29a contributes to HIV-1 latency, although overexpression of miR-29a may be needed for this phenotype. Given that miR-29a impacts HIV-1 replication and infectivity, miR-29a agonists or inhibitors could be valuable elements in different HIV-1 eradication therapies.
Acknowledgements
This study was funded by Danish Council for Independent Research (DFF grant #6120-00020), Aarhus University Research Foundation (AUFF-E-2016-FLS-8-9) and Aarhus University, Denmark.
References
- 1. Eisele E, Siliciano RF. Redefining the viral reservoirs that prevent HIV-1 eradication. Immunity 2012; 37: 377– 388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang J, Wang F, Argyris E et al. . Cellular microRNAs contribute to HIV-1 latency in resting primary CD4+ T lymphocytes. Nat Med 2007; 13: 1241– 1247. [DOI] [PubMed] [Google Scholar]
- 3. Ahluwalia JK, Khan SZ, Soni K et al. . Human cellular microRNA hsa-miR-29a interferes with viral nef protein expression and HIV-1 replication. Retrovirology 2008; 5: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nathans R, Chu CY, Serquina AK et al. . Cellular microRNA and P bodies modulate host-HIV-1 interactions. Mol Cell 2009; 34: 696– 709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Patel P, Ansari MY, Bapat S et al. . The microRNA miR-29a is associated with human immunodeficiency virus latency. Retrovirology 2014; 11: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sun G, Li H, Wu X et al. . Interplay between HIV-1 infection and host microRNAs. Nucleic Acids Res 2012; 40: 2181– 2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Adoro S, Cubillos-Ruiz JR, Chen X et al. . IL-21 induces antiviral microRNA-29 in CD4 T cells to limit HIV-1 infection. Nat Commun 2015; 6: 7562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Harwig A, Jongejan A, Kampen AH et al. . Tat-dependent production of an HIV-1 TAR-encoded miRNA-like small RNA. Nucleic Acids Res 2016; 44: 4340– 4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang Y, Fan M, Geng G et al. . A novel HIV-1-encoded microRNA enhances its viral replication by targeting the TATA box region. Retrovirology 2014; 11: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kaul D, Ahlawat A, Gupta SD. HIV-1 genome-encoded hiv1-mir-H1 impairs cellular responses to infection. Mol Cell Biochem 2009; 323: 143– 148. [DOI] [PubMed] [Google Scholar]
- 11. Winter J, Jung S, Keller S et al. . Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol 2009; 11: 228– 234. [DOI] [PubMed] [Google Scholar]
- 12. Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell 2009; 136: 642– 655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Swaminathan G, Navas-Martin S, Martin-Garcia J. MicroRNAs and HIV-1 infection: antiviral activities and beyond. J Mol Biol 2014; 426: 1178– 1197. [DOI] [PubMed] [Google Scholar]
- 14. Swaminathan G, Rossi F, Sierra LJ et al. . A role for microRNA-155 modulation in the anti-HIV-1 effects of Toll-like receptor 3 stimulation in macrophages. PLoS Pathog 2012; 8: e1002937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ruelas DS, Greene WC. An integrated overview of HIV-1 latency. Cell 2013; 155: 519– 529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chiang K, Sung TL, Rice AP. Regulation of cyclin T1 and HIV-1 Replication by microRNAs in resting CD4+ T lymphocytes. J Virol 2012; 86: 3244– 3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Triboulet R, Mari B, Lin YL et al. . Suppression of microRNA-silencing pathway by HIV-1 during virus replication. Science 2007; 315: 1579– 1582. [DOI] [PubMed] [Google Scholar]
- 18. Ruelas DS, Chan JK, Oh E et al. . MicroRNA-155 Reinforces HIV Latency. J Biol Chem 2015; 290: 13736– 13748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang HS, Chen XY, Wu TC et al. . MiR-34a is involved in Tat-induced HIV-1 long terminal repeat (LTR) transactivation through the SIRT1/NFkappaB pathway. FEBS Lett 2012; 586: 4203– 4207. [DOI] [PubMed] [Google Scholar]
- 20. Zhang HS, Wu TC, Sang WW, Ruan Z. MiR-217 is involved in Tat-induced HIV-1 long terminal repeat (LTR) transactivation by down-regulation of SIRT1. Biochim Biophys Acta 2012; 1823: 1017– 1023. [DOI] [PubMed] [Google Scholar]
- 21. Hariharan M, Scaria V, Pillai B, Brahmachari SK. Targets for human encoded microRNAs in HIV genes. Biochem Biophys Res Commun 2005; 337: 1214– 1218. [DOI] [PubMed] [Google Scholar]
- 22. Whisnant AW, Bogerd HP, Flores O et al. . In-depth analysis of the interaction of HIV-1 with cellular microRNA biogenesis and effector mechanisms. MBio 2013; 4: e000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zou W, Denton PW, Watkins RL et al. . Nef functions in BLT mice to enhance HIV-1 replication and deplete CD4+CD8+ thymocytes. Retrovirology 2012; 9: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Simon V, Bloch N, Landau NR. Intrinsic host restrictions to HIV-1 and mechanisms of viral escape. Nat Immunol 2015; 16: 546– 553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Watts JM, Dang KK, Gorelick RJ et al. . Architecture and secondary structure of an entire HIV-1 RNA genome. Nature 2009; 460: 711– 716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bennasser Y, Le SY, Benkirane M, Jeang KT. Evidence that HIV-1 encodes an siRNA and a suppressor of RNA silencing. Immunity 2005; 22: 607– 619. [DOI] [PubMed] [Google Scholar]
- 27. Aqil M, Naqvi AR, Bano AS, Jameel S. The HIV-1 Nef protein binds argonaute-2 and functions as a viral suppressor of RNA interference. PLoS One 2013; 8: e74472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bennasser Y, Yeung ML, Jeang KT. HIV-1 TAR RNA subverts RNA interference in transfected cells through sequestration of TAR RNA-binding protein, TRBP. J Biol Chem 2006; 281: 27674– 27678. [DOI] [PubMed] [Google Scholar]
- 29. Houzet L, Yeung ML, Lame V et al. . MicroRNA profile changes in human immunodeficiency virus type 1 (HIV-1) seropositive individuals. Retrovirology 2008; 5: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Monteleone K, Selvaggi C, Cacciotti G et al. . MicroRNA-29 family expression and its relation to antiviral immune response and viro-immunological markers in HIV-1-infected patients. BMC Infect Dis 2015; 15: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Duskova K, Nagilla P, Le HS et al. . MicroRNA regulation and its effects on cellular transcriptome in human immunodeficiency virus-1 (HIV-1) infected individuals with distinct viral load and CD4 cell counts. BMC Infect Dis 2013; 13: 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bignami F, Pilotti E, Bertoncelli L et al. . Stable changes in CD4+ T lymphocyte miRNA expression after exposure to HIV-1. Blood 2012; 119: 6259– 6267. [DOI] [PubMed] [Google Scholar]
- 33. Olesen R, Leth S, Nymann R et al. . Immune checkpoints and the HIV-1 reservoir: proceed with caution. J Virus Erad 2016; 2: 183– 186. [PMC free article] [PubMed] [Google Scholar]
- 34. Denton PW, Olesen R, Choudhary SK et al. . Generation of HIV latency in humanized BLT mice. J Virol 2012; 86: 630– 634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cillo AR, Mellors JW. Which therapeutic strategy will achieve a cure for HIV-1? Curr Opin Virol 2016; 18: 14– 19. [DOI] [PubMed] [Google Scholar]
- 36. Paladini L, Fabris L, Bottai G et al. . Targeting microRNAs as key modulators of tumor immune response. J Exp Clin Cancer Res 2016; 35: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Deeks SG, Lewin SR, Ross AL et al. . International AIDS Society global scientific strategy: towards an HIV cure 2016. Nat Med 2016; 22: 839– 850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jiang H, Zhang G, Wu JH, Jiang CP. Diverse roles of miR-29 in cancer (review). Oncol Rep 2014; 31: 1509– 1516. [DOI] [PubMed] [Google Scholar]
- 39. Darcis G, Van Driessche B, Van Lint C. HIV latency: should we shock or lock? Trends Immunol 2017; 38: 217– 228. [DOI] [PubMed] [Google Scholar]
- 40. Mousseau G, Kessing CF, Fromentin R et al. . The Tat inhibitor didehydro-cortistatin A prevents HIV-1 reactivation from latency. MBio 2015; 6: e00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu G, Friggeri A, Yang Y et al. . miR-147, a microRNA that is induced upon Toll-like receptor stimulation, regulates murine macrophage inflammatory responses. Proc Natl Acad Sci U S A 2009; 106: 15819– 15824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shen CJ, Jia YH, Tian RR et al. . Translation of Pur-alpha is targeted by cellular miRNAs to modulate the differentiation-dependent susceptibility of monocytes to HIV-1 infection. FASEB J 2012; 26: 4755– 4764. [DOI] [PubMed] [Google Scholar]
- 43. Kapoor R, Arora S, Ponia SS et al. . The miRNA miR-34a enhances HIV-1 replication by targeting PNUTS/PPP1R10, which negatively regulates HIV-1 transcriptional complex formation. Biochem J 2015; 470: 293– 302. [DOI] [PubMed] [Google Scholar]
- 44. Farberov L, Herzig E, Modai S et al. . MicroRNA-mediated regulation of p21 and TASK1 cellular restriction factors enhances HIV-1 infection. J Cell Sci 2015; 128: 1607– 1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chiang K, Liu H, Rice AP. miR-132 enhances HIV-1 replication. Virology 2013; 438: 1– 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chen XY, Zhang HS, Wu TC et al. . Down-regulation of NAMPT expression by miR-182 is involved in Tat-induced HIV-1 long terminal repeat (LTR) transactivation. Int J Biochem Cell Biol 2013; 45: 292– 298. [DOI] [PubMed] [Google Scholar]
- 47. Sung TL, Rice AP. miR-198 inhibits HIV-1 gene expression and replication in monocytes and its mechanism of action appears to involve repression of cyclin T1. PLoS Pathog 2009; 5: e1000263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ma L, Shen CJ, Cohen EA et al. . miRNA-1236 inhibits HIV-1 infection of monocytes by repressing translation of cellular factor VprBP. PLoS One 2014; 9: e99535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Houzet L, Klase Z, Yeung ML et al. . The extent of sequence complementarity correlates with the potency of cellular miRNA-mediated restriction of HIV-1. Nucleic Acids Res 2012; 40: 11684– 11696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang P, Qu X, Zhou X et al. . Two cellular microRNAs, miR-196b and miR-1290, contribute to HIV-1 latency. Virology 2015; 486: 228– 238. [DOI] [PubMed] [Google Scholar]