Abstract
IMPORTANCE
Professionally delivered integrated pest management (IPM) interventions can reduce home mouse allergen concentrations, but whether they reduce asthma morbidity among mouse-sensitized and exposed children and adolescents is unknown.
OBJECTIVE
To determine the effect of an IPM intervention on asthma morbidity among mouse-sensitized and exposed children and adolescents with asthma.
DESIGN, SETTING, AND PARTICIPANTS
Randomized clinical trial conducted in Baltimore, Maryland, and Boston, Massachusetts. Participants were mouse-sensitized and exposed children and adolescents (aged 5–17 years) with asthma randomized to receive professionally delivered IPM plus pest management education or pest management education alone. Enrollment occurred between May 2010 and August 2014; the final follow-up visit occurred on September 25, 2015.
INTERVENTIONS
Integrated pest management consisted of application of rodenticide, sealing of holes that could serve as entry points for mice, trap placement, targeted cleaning, allergen-proof mattress and pillow encasements, and portable air purifiers. Infestation was assessed every 3 months, and if infestation persisted or recurred, additional treatments were delivered. All participants received pest management education, which consisted of written material and demonstration of the materials needed to set traps and seal holes.
MAIN OUTCOMES AND MEASURES
The primary outcome was maximal symptom days defined as the highest number of days of symptoms in the previous 2 weeks among 3 types of symptoms (days of slowed activity due to asthma; number of nights of waking with asthma symptoms; and days of coughing, wheezing, or chest tightness) across 6, 9, and 12 months.
RESULTS
Of 361 children and adolescents who were randomized (mean [SD] age, 9.8 [3.2] years; 38% female; 181 in IPM plus pest management education group and 180 in pest management education alone group), 334 were included in the primary analysis. For the primary outcome, there was no statistically significant between-group difference for maximal symptom days across 6, 9, and 12 months with a median of 2.0 (interquartile range, 0.7–4.7) maximal symptom days in the IPM plus pest management education group and 2.7 (interquartile range, 1.3–5.0) maximal symptom days in the pest management education alone group (P = .16) and a ratio of symptom frequencies of 0.86 (95% CI, 0.69–1.06).
CONCLUSIONS AND RELEVANCE
Among mouse-sensitized and exposed children and adolescents with asthma, an intensive year-long integrated pest management intervention plus pest management education vs pest management education alone resulted in no significant difference in maximal symptom days from 6 to 12 months.
TRIAL REGISTRATION
clinicaltrials.gov Identifier: NCT01251224
Mouse infestation is endemic in many low-income, urban neighborhoods in the United States.1–3 Previous studies found that mouse-sensitized children and adolescents with asthma who are exposed to mouse allergen have greater asthma morbidity than similar children and adolescents who either are not sensitized or not exposed to mouse allergen,4–6 but it is unclear if reducing mouse allergen exposure results in a reduction in asthma morbidity among mouse-sensitized children and adolescents.4, 7
The Mouse Allergen and Asthma Intervention Trial (MAAIT) was designed to test the hypothesis that an intensive, professionally delivered, integrated pest management (IPM) home intervention that included education about pest management would result in improvements in asthma symptoms compared with pest management educational one among mouse-sensitized and exposed children and adolescents with persistent asthma.
Methods
Study Participants
Individuals were recruited from emergency departments, primary care and subspecialty clinics, and databases of previous study participants who had given permission to be contacted. Children and adolescents aged 5 to 17 years living in the Boston, Massachusetts, or Baltimore, Maryland, metropolitan areas with persistent asthma and an exacerbation in the previous year were eligible for the clinic screening visit. Children and adolescents with mouse sensitization, which was defined as either a positive skin test to mouse epithelial extract (defined as an orthogonal wheal diameter ≥3 mm larger than the negative control) or a mouse urine-specific IgE of 0.10 kU/L or greater, were eligible for a home visit to assess mouse allergen levels.
Allergy skin testing was performed using a multitester device (Multitest II, Lincoln Diagnostics) on a panel of 14 common aeroallergens, including mouse epithelium (Stallergenes Greer). Participants who were wheezing at the screening visit did not undergo skin testing; and for these participants, mouse sensitization was determined by mouse-specific IgE testing.
Children and adolescents who had a bed dust mouse allergen concentration of 0.4 µg/g or greater or a bedroom floor dust mouse allergen concentration of 0.5 µg/g or greater were eligible for randomization. Children and adolescents also had to spend at least 4 nights per week in the primary home to be eligible.
The trial protocol (appears in Supplement 1) was approved by the institutional review boards of Johns Hopkins University School of Medicine, Boston Children’s Hospital, and Columbia University School of Public Health and by the data and safety monitoring committee of the National Institute of Allergy and Infectious Diseases. Written informed consent was obtained from the parent and the head of household if the parent was not also the head of household, and assent was obtained from children and adolescents.
Study Design and Treatment
MAAIT was a 1 year, parallel-group, randomized clinical trial of an intensive, professionally delivered IPM intervention. Participants were randomized by the site coordinator in a ratio of 1 to 1 (using random block sizes of 4–6) to either IPM plus pest management education or pest management education alone (using a web-based application developed by the data management and analysis core staff). Randomization was stratified by study site.
Participants were enrolled and randomized between May 2010 and August 2014 (date when the prespecified sample size was met), and the last data point was collected on September 25, 2015. Participants continued to receive their usual care from their regular asthma clinician during the study. Pest management education was delivered at a home visit approximately 1 month after randomization and included written material about and demonstration of setting mouse traps, sealing of holes and cracks that may serve as entry points for mice, and housekeeping practices.
The IPM intervention was adapted from previous work8 in collaboration with licensed pest management experts and was delivered in treatments, with each treatment consisting of a 2- to 2.5-hour primary visit followed by a 1-hour booster visit 1 to 2 weeks later to reset traps and complete any work remaining from the primary visit. The first treatment included targeted cleaning to remove allergen reservoirs, placement of traps, application of rodenticide, sealing of holes and cracks, installation of allergen-proof mattress and pillow encasements (CleanBrands LLC), and 2 portable air purifiers (Filtrete Room Air Purifier, 3M). Further details about the intervention can be found in the eMethods in Supplement 2.
Infestation was assessed every 3 months; however, if infestation was persistent or recurred, an additional treatment was delivered. Participants in the IPM plus pest management education group could receive as few as 1 or as many as 4 treatments. Participants randomized to the pest management education alone group were offered an IPM intervention home visit after completing the study. Self-reported socioeconomic and demographic information, including race/ethnicity, was collected because low-income, minority children are disproportionately affected by asthma.
Outcome Measures
Asthma-related outcomes were assessed at clinic visits at 6 and 12 months and by telephone calls at 3 and 9 months. Asthma symptoms, asthma-related health care use, and medication use were captured during clinic visits and telephone calls. The primary outcome of maximal symptom days was the maximal number of days with symptoms in the 2 weeks prior to the visit or telephone call, and was defined as the largest value among the following 3 symptom variables: days of slowed activity due to asthma; number of nights of waking with asthma symptoms; and days of coughing, wheezing, or chest tightness.9 This outcome variable has been used in a previous multicenter randomized clinical trial of an environmental intervention among inner-city children with asthma as well as other similar studies.9 Maximal symptom days across the 6-, 9-, and 12-month time points was chosen a priori as the primary outcome because previous studies suggested that mouse allergen levels would not substantially decrease for several months after starting the IPM intervention.8
Secondary outcomes included individual symptoms (cough without a cold, slowed activity due to asthma, nocturnal awakening due to asthma, and inability to speak in full sentences due to asthma); rescue medication use (days of short-acting β-agonist use over 2 weeks); health care use (urgent physician visits, emergency department visits, and hospitalizations in the previous 3 months); assessment of mouse allergen exposure (bedroom floor, bed, and airborne mouse allergen levels); biomarkers of mouse allergen exposure (serum mouse-specific IgE levels collected at baseline and 12 months); and pulmonary function (forced expiratory volume in the first second of expiration [FEV1] predicted percentage, ratio of FEV1 to percentage of forced vital capacity and bronchodilator reversibility collected at baseline, 6 months, and 12 months).
Three other prespecified secondary outcomes of pulmonary inflammation, serum mouse-specific IgG levels, and kitchen mouse allergen levels are not reported in this article. A total of 21 secondary outcomes were measured and 18 are reported herein. Use of a short course of oral corticosteroids over 3 months was a post hoc exploratory outcome. A prespecified exploratory analysis was performed to understand whether reductions in mouse allergen were associated with improvements in asthma symptoms and morbidity. Further details can be found in the eMethods in Supplement 2.
Adverse Events
Adverse events were assessed during clinic visits and telephone calls by asking participants if they had any problems since the last clinic visit or telephone call. Severity and relatedness to study procedures were determined and recorded.
Statistical Analysis
Sample size estimates indicated that 150 participants in each group (total sample size of 300) would provide approximately 90% power to detect a difference of at least 0.7 days in maximal symptom days per 2-week periods across the 6-, 9-, and 12-month time points with an α level of .05. This effect size was chosen because a similar effect size was associated with an individually tailored, multifaceted environmental intervention in a similar population and because it is similar to the effect associated with inhaled corticosteroids.9
To account for a projected 15% dropout rate, a total of 361 participants were enrolled. Five participants in the IPM plus pest management education group and 6 participants in the pest management education alone group never received the first intervention or education visit, respectively, and were replaced according to the protocol.
For the primary outcome, maximal symptom days in the past 2 weeks assessed across 6, 9, and 12 months were related to group assignment with generalized estimating equation models assuming a log-link function, an exchangeable correlation, and a robust variance estimator. Using this model, the exponentiated coefficient associated with the group assignment is interpreted as the ratio of frequencies of symptoms. The analysis was performed on the prespecified population of all randomized participants who received at least 1 intervention or education visit.
Missing data were evaluated to determine if there were differences between participants who were lost to follow-up and the overall study population. Because there was no evidence that the missing data significantly modified the study result, missing data were not imputed (eMethods in Supplement 2). Secondary symptom-day outcomes and health care use outcomes were analyzed similarly, and for dichotomous outcomes (short course of oral corticosteroids and bronchodilator reversibility), a binomial link function was used. For mouse-specific IgE, a generalized linear model was used. No adjustments for analysis of multiple outcomes were made; therefore, the secondary outcomes should be considered exploratory.
For the prespecified exploratory analysis of the association of reductions in mouse allergen with improvements in asthma symptoms and morbidity, random-effects models were used to relate longitudinal changes in mouse allergen levels to health outcomes. Log2 (mouse allergen) was used as the predictor so that the models would provide an estimate of the within-person effect of each log2-unit decrease or each 50% decrease in mouse allergen. Similar to the analyses of the primary and secondary outcomes, a Poisson link was used to estimate rate changes for the outcomes except for the dichotomous outcomes for which a binomial link function was used. For these random-effects models, data were used from all available visits.
Estimates of the expected change in maximal symptom days and other outcomes for prespecified reductions of 50%, 75%, and 90% in bedroom floor mouse allergen were calculated using the coefficients from the random-effects models (eMethods in Supplement 2). Descriptive analyses were performed using R version 3.2.1 (R Foundation for Statistical Computing) and all other analyses were performed using Stata version 14.0 (StataCorp). A 2-tailed P value of less than .05 was considered statistically significant.
Results
Enrollment and Study Completion
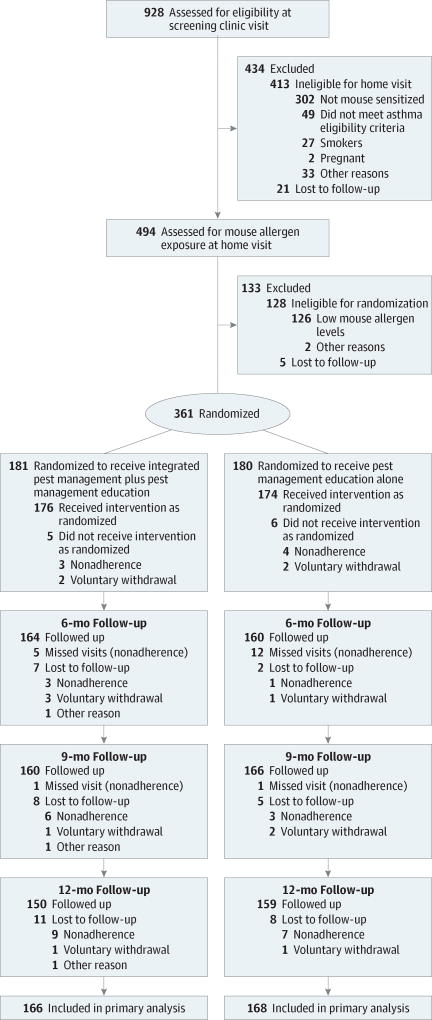
Nine hundred twenty-eight participants were assessed for eligibility and 361 were randomized (181 to the IPM plus pest management education group and 180 to the pest management education alone group). One hundred sixty-six participants in the IPM plus pest management education group (94%) and 168 participants in the pest management education alone group (97%) were included in the primary analysis because they completed at least 1 study visit at 6, 9, or 12 months and therefore contributed outcome data to the primary analysis (Figure). A comparison of baseline characteristics of participants excluded from the primary analysis with the overall study population appears in eTable 1 in Supplement 2.
Figure.
Flow Diagram for Treatment Effect of an Intensive Integrated Pest Management Intervention on Maximal Symptom Days
Study Population Characteristics
The mean (SD) age of the population was 9.8 (3.2) years and 132 (38%) were female. The population was predominantly low-income and minority (79% black and 21% Hispanic), had a median maximal symptom days of 3.0 days in the previous 2 weeks, and a median of 2 acute visits for asthma in the previous year (Table 1). Twenty-three percent of participants in the IPM plus pest management education group and 27% in the pest management education alone group reported being hospitalized for asthma in the previous year.
Table 1.
Baseline Characteristics
| IPM Plus Pest Management Education (n = 176) |
Pest Management Education Alone (n = 174) |
|
|---|---|---|
| Demographic Characteristics | ||
| Age, mean (SD), y | 9.7 (3.2) | 9.9 (3.2) |
| Sex, No. (%) | ||
| Male | 111 (63) | 107 (61) |
| Female | 65 (37) | 67 (39) |
| Race, No. (%) | ||
| Black | 142 (81) | 136 (78) |
| White | 19 (11) | 17 (10) |
| Other or unknown | 15 (8) | 21 (12) |
| Hispanic ethnicity, No. (%) | 37 (21) | 38 (22) |
| Income, No. (%) | ||
| <$30 000 | 113 (64) | 119 (68) |
| $30 000-$50 000 | 34 (19) | 23 (13) |
| >$50 000 | 16 (9) | 15 (9) |
| Refused or unknown | 13 (7) | 17 (10) |
| Site, No. (%) | ||
| Baltimore, Maryland | 120 (68) | 119 (68) |
| Boston, Massachusetts | 56 (32) | 55 (32) |
| Asthma Symptoms and Medication Use | ||
| Maximal symptom days, median (IQR)a | 2.5 (1.0–5.3) | 3.0 (0–7.0) |
| Short-acting β-agonist use, median (IQR), d/2 wk | 3.0 (0.3–7.0) | 3.0 (1.0–10.0) |
| Asthma controller medication | ||
| Use, No. (%) | 162 (92) | 147 (84) |
| Treatment step, No./total (%)b | ||
| 1: Short-acting β-agonist only | 14/165 (8) | 24/161 (15) |
| 2: Low-dose inhaled corticosteroid or leukotriene modifier | 32/165 (19) | 29/161 (18) |
| 3: Low-dose inhaled corticosteroid plus long-acting β-agonist or medium-dose inhaled corticosteroid | 23/165 (14) | 26/161 (16) |
| 4: Medium-dose inhaled corticosteroid plus long-acting β-agonist | 10/165 (6) | 6/161 (4) |
| 5: High-dose inhaled corticosteroid with or without long-acting β-agonist | 86/165 (52) | 76/161 (47) |
| Asthma-Related Health Care Use | ||
| Acute visits in prior year, median (IQR) | 2 (1–5) | 2 (1–4) |
| Emergency department visits in prior year, median (IQR) | 1 (1–3) | 1 (0–2) |
| Hospitalizations in prior year, median (IQR) | 0 (0–0) | 0 (0–1) |
| Short course of oral corticosteroids in prior 3 mo, No. (%) | 87 (49) | 78 (45) |
| Lung Functionc | ||
| Prebronchodilator, No. of participants | 97 | 108 |
| FEV1, mean (SD), % predicted | 89.2 (13.9) | 86.4 (19.0) |
| FVC, mean (SD), % predicted | 99.5 (13.3) | 95.9 (16.9) |
| Ratio of FEV1 to FVC, mean (SD), % | 78.5 (9.1) | 78.3 (8.7) |
| Bronchodilator reversibility, No./total (%)d | 37/96 (39) | 41/103 (40) |
| Allergic Sensitization Characteristics | ||
| Skin test sensitivity, No./total (%) | ||
| Cockroach | 84/152 (55) | 78/145 (54) |
| Cat | 92/152 (61) | 67/145 (46) |
| Dog | 37/152 (24) | 30/145 (21) |
| Dust mite | 65/152 (43) | 67/145 (46) |
| Molde | 48/152 (32) | 52/145 (36) |
| Mouse-specific IgE, median (IQR), kU/L | 13 (2–35) | 10 (3–46) |
| Mouse Allergen Exposure | ||
| Bed dust, geometric mean (SD), µg/g | 1.1 (5.4) | 1.1 (5.2) |
| Bedroom floor dust, geometric mean (SD), µg/g | 6.1 (5.6) | 6.6 (5.6) |
| Air, No. of participants | 141 | 146 |
| Geometric mean (SD), pg/m3 | 4.8 (5.3) | 5.4 (6.0) |
| Detectable, No. (%) | 109 (77) | 115 (79) |
| Type of Pest Infestationf | ||
| Mouse, No. (%) | 155 (88) | 146 (84) |
| Cockroach, No. (%) | 40 (23) | 41 (24) |
| Other Indoor Allergens | ||
| Detectable, No./total (%) | ||
| Cockroach | 41/176 (23) | 37/174 (21) |
| Dust mite | 33/173 (19) | 42/171 (25) |
| Rat | 5/173 (3) | 10/171 (6) |
| Dog | ||
| Detectable, No./total (%) | 94/173 (54) | 99/171 (58) |
| Geometric mean (SD), µg/g | 0.05 (31.7) | 0.05 (29.0) |
| Cat | ||
| Detectable, No./total (%) | 132/173 (76) | 125/172 (73) |
| Geometric mean (SD), µg/g | 0.13 (13.7) | 0.11 (13.2) |
| Housing Characteristics | ||
| Type of home, No. (%) | ||
| Row house | 96 (55) | 94 (54) |
| Apartment | 53 (30) | 49 (28) |
| Detached house | 18 (10) | 15 (9) |
| Duplex, semi-detached home, or other | 9 (5) | 16 (9) |
| No. of floors in building, median (range)g | 2 (1–4) | 2 (1–6) |
| Adjacent vacant building, No. (%) | 31 (18) | 21 (12) |
Abbreviations: FEV1, forced expiratory volume in the first second of expiration; FVC, forced vital capacity; IPM, integrated pest management; IQR, interquartile range.
During the previous 2 weeks across 3 types of symptoms (slowed activity due to asthma; nocturnal wakening with asthma symptoms; and coughing, wheezing, or chest tightness).
Based on the Composite Asthma Severity Index.10
Reported for participants aged 8 years or older at baseline.
Indicates increase in FEV1 of 12% or greater following treatment with a short-acting β-agonist.
Included Aspergillus mix and Alternaria tenuis.
Based on observed rodent droppings, holes that could be due to rodents, or signs of rodent gnawing at home visit; parent report of mouse sighting, droppings, evidence of chewing by mice, or mice caught in previous 2 weeks; or cockroach bodies or excrement in kitchen, bedroom, or family room at home visit.
There were 163 participants in the IPM plus pest management education group and 156 in the pest management education alone group.
The median mouse-specific IgE level was 13 kU/L in the IPM plus pest management education group and 10 kU/L in the pest management education alone group. Mouse allergen concentrations in home environmental samples were high, with geometric mean concentrations for bedroom floor dust of 6.1 µg/g in the IPM plus pest management education group and 6.6 µg/g in the pest management education alone group and 1.1 µg/g and 1.1 µg/g, respectively, for bed dust (Table 1).
Primary and Secondary Clinical Outcomes
The IPM plus pest management education group had a median of 2.0 (interquartile range [IQR], 0.7–4.7) maximal symptom days across 6, 9, and 12 months vs 2.7 (IQR, 1.3–5.0) maximal symptom days in the pest management education alone group (P = .16). The ratio of symptom frequencies between the 2 groups was 0.86 (95% CI, 0.69–1.06) (Table 2 and eFigure in Supplement 2).
Table 2.
Asthma Outcomes at Follow-up
| IPM Plus Pest Management Education |
Pest Management Education Alone |
Ratio of Symptom Frequencies (95% CI)a |
|
|---|---|---|---|
| Primary Outcome, Median (IQR)b | |||
| Maximal symptom days/2 wkc | 2.0 (0.7–4.7) | 2.7 (1.3–5.0) | 0.86 (0.69 to 1.06) |
| Secondary Outcomes, Median (IQR), Symptom Days/2 wkb | |||
| Coughing, wheezing, or chest tightness | 1.7 (0.7–3.7) | 2.3 (1.0–4.7) | 0.87 (0.70 to 1.09) |
| Slowed activity due to asthma | 0.7 (0–2.0) | 1.3 (0–3.0) | 0.76 (0.57 to 1.02) |
| Nocturnal wakening with asthma symptoms | 0.4 (0–1.7) | 0.8 (0–2.0) | 0.86 (0.62 to 1.20) |
| Cough without a cold | 0.7 (0–2.0) | 0.7 (0–2.1) | 0.77 (0.56 to 1.07) |
| Exercise-related symptoms | 0.7 (0–2.0) | 1.0 (0–2.3) | 0.80 (0.59 to 1.08) |
| Inability to speak in full sentences due to asthma | 0 (0–0.3) | 0 (0–0.7) | 0.74 (0.46 to 1.20) |
| Short-acting β-agonist use | 2.0 (0.7–4.5) | 2.3 (1.0–5.7) | 0.86 (0.69 to 1.08) |
| Health Care Use During Past 6 mo, Median (IQR)d | Relative Risk (95% CI) | ||
| Acute visits | 0 (0–1) | 0 (0–1) | 0.93 (0.65 to 1.32) |
| Hospitalizations | 0 (0–0) | 0 (0–0) | 1.28 (0.50 to 3.31) |
| ED visits | 0 (0–1) | 0 (0–1) | 1.15 (0.72 to 1.83) |
| Lung Function, Mean (SD)e | β Coefficient (95% CI)f | ||
| FEV1, % predicted | 87.9 (14.0) | 85.9 (14.2) | 2.29 (−1.63 to 6.22) |
| FVC, % predicted | 97.4 (13.8) | 96.6 (12.6) | 0.99 (−2.70 to 4.67) |
| Ratio of FEV1 to FVC, % | 78.3 (7.7) | 77.8 (7.9) | 0.52 (−1.66 to 2.70) |
| Other Outcomes, No./Total (%) | Odds Ratio (95% CI) | ||
| Bronchodilator reversibilityg | 27/80 (34) | 45/98 (46) | 0.72 (0.44 to 1.17) |
| Short course of oral corticosteroidsh | 48/160 (30) | 57/168 (34) | 0.90 (0.58 to 1.38) |
Abbreviations: FEV1, forced expiratory volume in the first second of expiration; FVC, forced vital capacity; IPM, integrated pest management; IQR, interquartile range.
Calculated as (IPM plus pest management education group)/(pest management education alone group). The 95% CIs created from generalized estimating equation models of outcomes measured during the last 6 months of the study vs group as the predictor.
Assessed at the 6-, 9-, and 12-month visits for each participant.
Across 3 types of symptoms (slowed activity due to asthma; nocturnal wakening with asthma symptoms; and coughing, wheezing, or chest tightness).
Captured at 9- and 12-month visits and analyzed as number of health care use events in the final 6 months of the study.
Reported for participants who were aged 8 years or older at the baseline visit (n = 94in IPM plus pest management education group and n = 103 in pest management education alone group). Measured at 6- and 12-month visits and analyzed as a continuous variable.
Represent the between-group differences in the mean of the corresponding lung function index across 6 and 12 months.
Indicates increase in FEV1 of 12% or greater following short-acting β-agonist use and measured at 12 months.
Measured at 9 and 12 months.
There were no statistically significant differences in secondary outcomes, including mouse-specific IgE, with a median of 6.0 kU/L (IQR, 1.0 to 22.0 kU/L) in the IPM plus pest management education group and 9.0 kU/L (IQR, 1.0 to 26.0 kU/L) in the pest management education alone group (β coefficient, −0.29; 95% CI, −1.04 to 0.46). There were a total of 4 probable or definitely related adverse events in the IPM plus pest management education group and 2 in the pest management education alone group, all of which were associated with data collection procedures (eTable 2 in Supplement 2).
Intervention and Mouse Allergen
Thirty-two participants (18%) in the IPM plus pest management education group received 1 IPM treatment, 50 (28%) received 2 treatments, 42 (24%) received 3 treatments, and 52 (30%) received 4 treatments. At 6 months, 11%of the pest management education alone group reported engaging the services of an exterminator and 8% at 12 months. A total of 311 treatments were indicated due to persistent or recurring mouse infestation, and 290 (93%) were delivered.
The geometric mean was 2.0 µg/g for bedroom floor mouse allergen level across all follow-up visits in the IPM plus pest management education group and 2.5 µg/g in the pest management education alone group, which was not statistically different between groups (β coefficient, −0.42; 95% CI, −0.91 to 0.07). In prespecified analyses, 63% of participants in the IPM plus pest management education group and 58% of participants in the pest management education alone group had a reduction of bedroom floor mouse allergen of at least 75% at some point during the study (P = .45) and 46% and 41%, respectively, had a reduction of at least 90% during some point of the study (P = .44; Table 3).
Table 3.
Mouse Allergen Concentration Levels
| Type of Mouse Allergen |
Follow-up Time Points | No./Total (%)a | β Coefficient (95% CI)b |
P Valuec |
|
|---|---|---|---|---|---|
| IPM Plus Pest Management Education |
Pest Management Education Alone |
||||
| Bedroom floor | n = 174 | n = 172 | |||
| Geometric mean (95% CI), µg/g | Across 6, 9, and 12 mo | 2.0 (1.6 to 2.5) | 2.5 (2.0 to 3.3) | −0.42 (−0.91 to 0.07) | |
| Reduction in mouse allergen ≥50% | At any | 144/174 (83) | 137/172 (80) | .55 | |
| Reduction in mouse allergen ≥75% | At any | 109/174 (63) | 100/172 (58) | .45 | |
| Reduction in mouse allergen ≥90% | At any | 80/174 (46) | 71/172 (41) | .44 | |
| Airborned | n = 155 | n = 156 | |||
| Geometric mean (95% CI), pg/m3 | Across 6, 9, and 12 mo | 2.19 (1.77 to 2.71) | 4.68 (3.72 to 5.90) | −1.08 (−1.51 to −0.65) | |
| Reduction in mouse allergen ≥50% | At any | 77/126 (61) | 60/132 (45) | .02 | |
| Reduction in mouse allergen ≥75% | At any | 51/126 (40) | 45/132 (34) | .35 | |
| Reduction in mouse allergen ≥90% | At any | 36/126 (29) | 24/132 (18) | .07 | |
| Bed dust | n = 174 | n = 172 | |||
| Geometric mean (95% CI), µg/g | Across 6, 9, and 12 mo | 0.58 (0.47 to 0.72) | 0.75 (0.61 to 0.92) | −0.43 (−0.84 to −0.02) | |
| Reduction in mouse allergen ≥50% | At any | 136/174 (78) | 124/172 (72) | .24 | |
| Reduction in mouse allergen ≥75% | At any | 90/174 (52) | 89/172 (52) | >.99 | |
| Reduction in mouse allergen ≥90% | At any | 53/174 (30) | 44/172 (26) | .37 | |
Unless otherwise indicated.
Determined from generalized estimating equation models for between-group differences and log2 (mouse allergen) concentrations at all follow-up time points.
Calculated using the χ2 test.
Collected at 6- and 12-month follow-up time points.
In a post hoc analysis, 47% in the IPM plus pest management education group and 40% in the pest management education alone group had a reduction below a threshold previously associated with morbidity (0.5 µg/g in bedroom floor dust).5 The geometric mean airborne mouse allergen levels across all follow-up visits were 2.19 pg/m3 in the IPM plus pest management education group and 4.68pg/m3 in the pest management education alone group, which was statistically significantly different between groups (β coefficient, −1.08; 95% CI, −1.51 to −0.65). Similar results were found for bed dust mouse allergen.
However, there were no statistically significant differences for either airborne or bed dust mouse allergen in the percentage of participants with reductions of at least 75% or 90% in allergen levels. The geometric mean bed cockroach allergen level was 0.004 µg/g at baseline and 0.002 µg/g at 12 months in the IPM plus pest management education group (P = .13) and 0.003 µg/g and 0.002 µg/g, respectively, in the pest management education alone group (P = .24). There was no evidence of change in the performance of the mouse allergen assay over time.
Effects of Mouse Allergen Reduction on Asthma
Prespecified exploratory analyses examining the effect of reducing mouse allergen levels on asthma-related outcomes were performed across the entire study population (eTable 3 in Supplement 2). Each log2 decrease, or 50% decrease, in bedroom floor mouse allergen levels was associated with statistically significant reductions in the frequencies of asthma symptoms and short-acting β-agonist use, acute visits, and ED visits for asthma. For example, every 50% reduction in mouse allergen was associated with a 4% decrease in days of short-acting β-agonist use in the previous 2 weeks (ratio of frequencies, 0.96 [95% CI, 0.95–0.98]; P < .001).
There was no statistically significant association between reduction in bedroom floor mouse allergen and hospitalizations, short course of oral corticosteroids, or lung function. Reductions in bedroom floor mouse allergen were associated with reductions in mouse-specific IgE levels. The effects of reducing bed dust and airborne mouse allergen levels on asthma-related outcomes and mouse-specific IgE levels were similar (eTables 4 and 5 in Supplement 2).
The with in-person effects of a 50%, 75%, and 90% reduction in bedroom floor mouse allergen on asthma outcomes were estimated, and the magnitude of the clinical benefit increased with greater reduction in mouse allergen (eTable 6 in Supplement 2).For example, an individual experiencing a 90% reduction in bedroom floor mouse allergen levels was estimated to have 14.1 fewer maximal symptom days (P < .001), 16.4 fewer days of short-acting β-agonist use (P < .001), 16.7 fewer days of exercise-related symptoms (P < .001), and 0.8 less acute visits (P < .001) per year compared with baseline.
Discussion
A year-long, professionally delivered IPM plus education intervention targeting mouse allergen was no more effective than pest management education alone in reducing asthma symptoms among mouse-sensitized and exposed children and adolescents with persistent asthma. Similarly, there were no statistically significant differences between the IPM plus pest management education group and the pest management education alone group in any of the secondary asthma outcomes, including short-acting β-agonist use, health care use, and lung function. In addition, there were no statistically significant between-group differences in bedroom floor mouse allergen levels or serum mouse-specific IgE levels, which is a biomarker of exposure. Although there were some statistically significant between-group differences in bed and airborne mouse allergen, the differences were small and there was no statistically significant between-group difference in the proportion of participants with large decreases in any measure of mouse allergen.
It is possible that the IPM intervention was not superior to pest management education alone in reducing asthma symptoms and other markers of asthma morbidity because it was not associated with substantially larger decreases in home mouse allergen levels. The pest management education alone group had decreases in home mouse allergen levels of approximately 65%, which is larger than that observed in previous home intervention studies among the active intervention group,4, 7 and approximately 40% hadatleasta90% decrease in mouse allergen levels. This degree of reduction does not appear to be explained by changes in allergen assay performance over time. In the mouse allergen intervention subgroup in the Inner-city Asthma Study,4 education and research staff were provided to facilitate the family’s IPM activities, which resulted in a 27% reduction in bedroom floor mouse allergen concentrations, and no reduction in bed mouse allergen concentrations.
DiMango et al7 conducted a home environmental intervention that was delivered by intervention counselors, but not pest management professionals, and included IPM targeting mouse infestation and observed 30% and 50% reductions in home mouse allergen concentrations in the control and active treatment groups, respectively, which are modest reductions that may not be sufficient to achieve a clinical benefit.11 The interventions in both of these previous studies aimed to reduce home environmental exposures generally, whereas in MAAIT, the interventions aimed to reduce mouse allergen specifically.
Therefore, the MAAIT population may have been primed for behavior change related to reducing mouse infestation, and thus more responsive to education about pest management than participants in these other studies. It is also possible that certain aspects of the pest management education, such as delivery of the education at a home visit, contributed to the better than expected effects on mouse allergen levels in the pest management education alone group.
Mouse allergen concentrations were unlikely to have decreased because of regression to the mean because a prior study that also enrolled participants based on home mouse exposure did not observe reductions in mouse allergen in the control group, suggesting that mouse allergen exposure is generally stable over time, even when used as a study inclusion criterion.8 A crossover effect might explain the large reduction in mouse allergen in the pest management education alone group; however, only a few participants in this group reported engaging professional pest management services.
Although the large reduction in allergen in the pest management education alone group provides some evidence that education alone may be effective in reducing mouse allergen levels, a randomized clinical trial comparing pest management education with no intervention would be the optimal study design to determine the efficacy of education alone. However, it may be difficult to design a clinical trial that adequately addresses the ethical concerns related to randomizing children who are exposed to a potentially harmful allergen to a group that receives no education about reducing exposure to the allergen.12
There are several limitations to consider in addition to the unexpectedly large reductions in mouse allergen and the lack of a comparison group that received no pest management education. The study was not blinded so it is possible that outcome data were biased by knowledge of group assignment by the participant, family, or the study team. However, environmental interventions are difficult to blind, and the findings with respect to outcomes that are less subject to bias, such as lung function, mouse allergen levels, and mouse-specific IgE levels, were consistent with self-reported outcomes.
As with all clinical trials, the MAAIT population is not likely representative of the overall population of US children and adolescents with similar asthma, allergic sensitization, and exposure characteristics.13–15 Even in the setting of an intensive yearlong IPM intervention, mouse allergen concentrations in the majority of homes never fell below a level previously associated with increased risk of morbidity,5 so many of the children and adolescents who experienced some reduction in mouse allergen and clinical benefit continued to have exposure to potentially harmful concentrations of mouse allergen. Another potential limitation is that medical management for asthma was not a component of the study protocol; therefore, the results of the study are applicable to a population receiving only typical care in a community setting.
Prespecified exploratory analyses showed that both groups experienced substantial reductions in home mouse allergen of a magnitude not observed in other studies,4, 7 which were associated with marked improvement in asthma symptoms and morbidity. Individuals who experienced a 90% reduction in home mouse allergen levels had improvements in asthma that were larger in magnitude than those observed with inhaled corticosteroids. Although inhaled corticosteroid effects have not been estimated for a population similar to the one enrolled in MAAIT, in the Childhood Asthma Management Program, which enrolled children with mild to moderate asthma, budesonide was associated with 0.1 fewer urgent care visits and 0.02 fewer hospitalizations per person-year.16
In the MAAIT population, a 90% reduction in mouse allergen was estimated to result in 0.8 fewer acute care visits and 0.07 fewer hospitalizations per person-year. Although the differences in clinical benefit between the 2 studies may be entirely explained by the differences between the study populations, this observation supports the conduct of studies to understand how the effect of mouse allergen reduction compares with that of asthma controller medication.
Conclusions
Among mouse-sensitized and exposed children and adolescents with asthma, an intensive year-long integrated pest management intervention plus pest management education vs pest management education alone resulted in no significant difference in maximal symptom days from 6 to 12 months.
Supplementary Material
Key Points.
Question
Does a professionally delivered intervention plus education aimed at mouse infestation reduce asthma symptoms among mouse-sensitized children and adolescents with persistent asthma compared with education alone?
Findings
In this randomized clinical trial, the median number of maximal symptom days, defined as the highest number of days of symptoms among 3 types of symptoms (days of slowed activity due to asthma; number of nights of waking with asthma symptoms; and days of coughing, wheezing, or chest tightness), within a 2-week period across 6, 9, and 12 months was 2.0 days with an intensive integrated pest management intervention and 2.7 days with education alone, with no statistically significant difference between groups.
Meaning
A year-long intensive integrated pest management intervention was no more effective than education in improving symptoms among mouse-sensitized children and adolescents with asthma.
Acknowledgments
Funding/Support: This work was supported by grants U01Al083238, K24 AI114769, and K24 AI106822 from the National Institute of Allergy and Infectious Diseases and grants R01 ES023447 and R01 ES026170 from the National Institute of Environmental Health Sciences. The 3M Corporation donated air purifiers and Clean Brands LLC donated allergen-proof mattress and pillow encasements for the study.
Role of the Funder/Sponsor: The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
ARTICLE INFORMATION
Author Contributions: Drs Matsui and Peng had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Matsui, Perzanowski, Wise, Chew, Phipatanakul.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Matsui, Peng.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Matsui, Peng, Zhai.
Obtained funding: Matsui.
Administrative, technical, or material support: Matsui, Perzanowski, Balcer-Whaley, Newman, Cunningham, Divjan, Chew, Miller, Phipatanakul.
Supervision: Matsui, Balcer-Whaley, Phipatanakul.
Additional Contributions: We thank the pest management teams from Innovative Pest Management and Buono Pest Control Co Inc. We also thank the following pest management consultants, who received compensation for their services: Donald Rivard (Rivard’s Resources) and Harold J. Harlan, PhD, and the IPM Environmental Management Consultants. In addition, we thank the community advisory boards for guidance.
Conflict of Interest Disclosures: The authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Matsui reported receiving grant support from the National Institutes of Health and Inspirotec LLC; travel reimbursment and research funding from ThermoFisher Scientific; travel reimbursment and honoraria from Indoor Biotechnologies Inc; and personal fees from Dwight & Church and the Environmental Defense Fund. Dr Perzanowski reported receiving honoraria from Indoor Biotechnologies Inc. Dr Peng reported receiving personal fees from the Health Effects Institute. Dr Wise reported receiving grants from Pearl Therapeutics; grants and personal fees from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, and GlaxoSmithKline; and personal fees from Contrafect, Janssen, Mylan, Novartis, Pfizer, Pulmonx, Roche, Sarepta, Spiration, Sunovion, Teva, Theravance, Verona, and Vertex. Ms Balcer-Whaley reported receiving personal fees from Johns Hopkins University. Dr Bollinger reported receiving grants from the National Institutes of Health, National Institute of Allergy and Infectious Diseases. Dr Zhai reported receiving grants from the National Institute of Allergy and Infectious Diseases and the National Institute of Environmental Health Sciences. Dr Phipatanakul reported receiving grants from the National Institutes of Health. No other disclosures were reported.
Meeting Presentation: Presented at the 2017 Annual Meeting of the American Academy of Allergy, Asthma & Immunology; March 6, 2017; Los Angeles, California.
Reproducible Research Statement: The data set and related documentation are available at https://rdpeng.github.io/MAAIT/.
References
- 1.Phipatanakul W, Eggleston PA, Wright EC, Wood RA. Mouse allergen, I: the prevalence of mouse allergen in inner-city homes: the National Cooperative Inner-City Asthma Study. J Allergy Clin Immunol. 2000;106(6):1070–1074. doi: 10.1067/mai.2000.110796. [DOI] [PubMed] [Google Scholar]
- 2.Matsui EC, Simons E, Rand C, et al. Airborne mouse allergen in the homes of inner-city children with asthma. J Allergy Clin Immunol. 2005;115(2):358–363. doi: 10.1016/j.jaci.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Chew GL, Perzanowski MS, Miller RL, et al. Distribution and determinants of mouse allergen exposure in low-income New York City apartments. Environ Health Perspect. 2003;111(10):1348–1351. doi: 10.1289/ehp.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pongracic JA, Visness CM, Gruchalla RS, Evans RIII, Mitchell HE. Effect of mouse allergen and rodent environmental intervention on asthma in inner-city children. Ann Allergy Asthma Immunol. 2008;101(1):35–41. doi: 10.1016/S1081-1206(10)60832-0. [DOI] [PubMed] [Google Scholar]
- 5.Matsui EC, Eggleston PA, Buckley TJ, et al. Household mouse allergen exposure and asthma morbidity in inner-city preschool children. Ann Allergy Asthma Immunol. 2006;97(4):514–520. doi: 10.1016/S1081-1206(10)60943-X. [DOI] [PubMed] [Google Scholar]
- 6.Ahluwalia SK, Peng RD, Breysse PN, et al. Mouse allergen is the major allergen of public health relevance in Baltimore City. J Allergy Clin Immunol. 2013;132(4):830–835. doi: 10.1016/j.jaci.2013.05.005. e1, e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DiMango E, Serebrisky D, Narula S, et al. Individualized household allergen intervention lowers allergen level but not asthma medication use: a randomized controlled trial. J Allergy Clin Immunol Pract. 2016;4(4):671–679. doi: 10.1016/j.jaip.2016.01.016. e4, e674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phipatanakul W, Cronin B, Wood RA, et al. Effect of environmental intervention on mouse allergen levels in homes of inner-city Boston children with asthma. Ann Allergy Asthma Immunol. 2004;92(4):420–425. doi: 10.1016/S1081-1206(10)61777-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morgan WJ, Crain EF, Gruchalla RS, et al. Inner-City Asthma Study Group. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;351(11):1068–1080. doi: 10.1056/NEJMoa032097. [DOI] [PubMed] [Google Scholar]
- 10.Wildfire JJ, Gergen PJ, Sorkness CA, et al. Development and validation of the Composite Asthma Severity Index—an outcome measure for use in children and adolescents. J Allergy Clin Immunol. 2012;129(3):694–701. doi: 10.1016/j.jaci.2011.12.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eggleston PA. Can we clear the air? J Allergy Clin Immunol Pract. 2016;4(4):680–681. doi: 10.1016/j.jaip.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 12.Paulson JA. An exploration of ethical issues in research in children’s health and the environment. Environ Health Perspect. 2006;114(10):1603–1608. doi: 10.1289/ehp.9000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Permaul P, Hoffman E, Fu C, et al. Allergens in urban schools and homes of children with asthma. Pediatr Allergy Immunol. 2012;23(6):543–549. doi: 10.1111/j.1399-3038.2012.01327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perry TT, Vargas PA, Bufford J, et al. Classroom aeroallergen exposure in Arkansas head start centers. Ann Allergy Asthma Immunol. 2008;100(4):358–363. doi: 10.1016/S1081-1206(10)60599-6. [DOI] [PubMed] [Google Scholar]
- 15.Chew GL, Correa JC, Perzanowski MS. Mouse and cockroach allergens in the dust and air in northeastern United States inner-city public high schools. Indoor Air. 2005;15(4):228–234. doi: 10.1111/j.1600-0668.2005.00363.x. [DOI] [PubMed] [Google Scholar]
- 16.Childhood Asthma Management Program Research Group. Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med. 2000;343(15):1054–1063. doi: 10.1056/NEJM200010123431501. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.