Abstract
CD4+ T cells have the capacity to differentiate into various T helper (Th) cell subsets after activation, and by acquiring distinct cytokine profiles and effector functions, they regulate the nature as well as the outcomes of immune responses. Th9 cells are a relatively new member in the Th cell family. The signature cytokine for Th9 cells is IL-9, a cytokine in the IL-2Rγc-chain family. Over the past few years, there has been an explosion of knowledge on the roles of Th9 cells in immunity and immunopathology, but the exact mechanisms in the control of Th9 cells remain poorly defined. This apparent paradox presents both challenges and opportunities. Here we review recent advances in our understanding of the fundamental biology of IL-9 and Th9 cells, highlighting the challenges and unanswered questions in the field. We also discuss potential opportunities in targeting Th9 cells for therapeutic purposes in the clinic.
Keywords: Th9, transcription mechanism, immunity and immunopathology, therapeutic purposes
Graphical abstract
Introduction
T cells are hardwired to protective immunity, immune surveillance, and autoimmune diseases. One of the remarkable features of T cells is their ability to differentiate into functionally diverse subsets, and in the case of CD4+ T cells, such subsets include Th1, Th2, Th9, Th17, Th22, Tfh and Foxp3+Tregs[1–5]. These subsets, by acquiring distinct cytokine profiles and functional attributes, define the nature as well as the outcomes of immune responses. Among all the T helper subsets, Th9 cells are a relative new and less well-characterized one[6]. It is only recently do we witness a rapid expansion of knowledge on the induction and functions of Th9 cells [6–15]. In essence, the signature cytokine for Th9 cells is IL-9, a pleiotropic cytokine with diverse functions [7, 8, 10, 12, 14–16]. Functionally, Th9 cells promote immune tolerance in certain models [17], and protect against parasitic infections [18, 19]. They also exert strong anti-tumor immunity, thus providing a promising strategy in cancer immunotherapy [20–23]. However, Th9 cells also trigger prominent allergic inflammation, asthma, and autoimmune diseases, highlighting their pathological roles in the immune system [24–26].
There remain significant challenges in the Th9 field and further studies in this area are warranted to overcome such challenges, so that therapeutic manipulation of Th9 cells could one day be possible for the benefit of patients. The Il9 locus per se has been well characterized and the transcription network involved in IL-9 expression has been extensively reviewed[9]. However, we remain poorly informed about how Th9 cells are induced and sustained, especially under in vivo conditions[27]. In most in vitro studies, Th9 cells are best induced by TGF-β and IL-4, but this induction is confined to a very small fraction of CD4+ T cells, and most T cells are refractory to Th9 induction [27, 28]. In contrast to other T helper subsets whose induction is controlled by lineage-specific transcription factors [29], numerous transcription factors are involved in the generation of Th9 cells, and a Th9 specific transcription factor has not been identified thus far[9]. Furthermore, costimulatory molecules in the TNFR super family (TNFRSF), such as OX40, glucocorticoid-induced tumor necrosis factor receptor (GITR), and death receptor 3 (DR3), have been consistently shown to promote Th9 induction[11]. These findings suggest that Th9 cells may be controlled by different mechanisms, but such mechanisms remain to be uncovered.
Besides Th9 cells, other cell types, such as innate lymphoid cells and mast cells, can also produce IL-9, but little is known about potential interactions among such cell types, either under physiological conditions or in disease settings. Additionally, Th9 cells are extremely unstable, at least under in vitro conditions. Once induced, they tend to lose IL-9 expression within days [27, 28]. Thus, mechanisms involved in Th9 induction and stability are likely to be very different, and further studies are required in understanding exactly what sustains Th9 cells both in vitro and in vivo. Importantly, more translational studies are needed in clarifying whether human Th9 cells behave in the same way as mouse Th9 cells, and how such information can be translated into better therapies in the clinic.
In this review, we provide a broad overview on the unique features of Th9 cells, highlighting recent development in the field and some of the challenges in defining Th9 cells. We also discuss the evolving role of Th9 cells in vivo, both under physiological conditions and in disease settings. We conclude by highlighting the emerging strategies in therapeutically manipulating Th9 cells in treatment of human diseases.
A historic perspective
IL-9 was discovered more than two decades ago from mouse T-cell clones, and classified as a common IL-2Rγc-chain family cytokine. Similar to the other members in the family (i.e., IL-2, IL-4, IL-7, IL-15, IL-21), IL-9 was initially thought to be a T cell growth factor, and its chief function was driving T cell proliferation[30]. Because of the sharing of receptor component with other γc-chain family cytokines, IL-9 was also thought to be redundant in T cell proliferation [31]. Subsequent studies revealed that IL-9 had marginal effects on proliferation of primary T cells, despite the fact that it could drive vigorous proliferation of T cell clones [30]. In most cases, IL-9 was induced under Th2 conditions in vitro or in a Th2-type of immunity in vivo. Thus, for a long time IL-9 was believed to be just a Th2 cytokine. In the mouse, transgenic expression of IL-9 in the lungs resulted in extensive airway inflammation, characterized by airway epithelial hyperplasia, proliferation of mucin-producing cells, and eosinophilia[24, 26, 32], features that are also seen in Th2 responses. These mice exhibited severe airway hypersensitivity to Ag challenges. Studies using IL-9 knockout mice further established the role of IL-9 in proliferation of mucin-producing cells, as well as mast cells and eosinophils in the lungs [24]. Thus, IL-9 has unexpected effects on cells other than T cells. Moreover, besides Th2 cells, other cell types, including mast cells, innate lymphoid cells (ILCs), NKT cells and even Foxp3+ Tregs, could also produce IL-9[33]. For various reasons, interest in IL-9 and its clinical significance was very much limited at that time.
In 1994, Schmidt et al reported that naive CD4+ T cells could be converted to IL-9-producing cells when activated in the presence of IL-2, IL-4, and TGF-β [34]. Although IL-4 alone was insufficient to promote the production of IL-9, it strongly enhanced IL-9 production in the presence of TGF-β and IL-2. Interestingly, IL-9 expression by CD4+ T cells was strongly inhibited by IFN-γ [34]. This is a landmark study, and for the first time revealed the cytokine milieu that either promotes or inhibits IL-9 production by CD4+ T cells. Intriguingly, later studies demonstrated that a combination of TGF-β and IL-2 had remarkable potency in the induction of Foxp3+ Tregs [35], but IL-4 alone was known to promote the induction of Th2 cells. How Th9 cells would fit in this cellular dynamics was entirely unknown. In 2008, a pair of papers published in Nature Immunology provide substantial insights into this issue [36, 37]. At a cellular level, CD4+ T cells that produced IL-4 are segregated from those that produced IL-9, and only cells that turned off IL-4 expression do they become IL-9 producing cells [36, 37]. At a molecular level, it appears that IL-4 shuts down Foxp3 expression in CD4+ T cells, and together with TGF-β, they convert activated CD4+ T cells into Th9 cells [36, 37]. In those in vitro cultures, TGF-β is a potent inhibitor of Th2 cells, but is required for Th9 induction [36, 37]. IL-4 promotes Th9 cells with TGF-β, but supports Th2 cells without TGF-β. Collectively, these studies provide strong evidence that Th2 cells and Th9 cells are distinct cell types. However, Th9 cells induced by TGF- β and IL-4 are low in cell numbers (~5% activated CD4+T cells), critically dependent on STAT6 and GATA3, and also express low levels of IL-10 [36, 37]. Thus, the possibility that Th2 and Th9 cells are somehow related remains.
In the time that followed, studies from several labs identified PU.1, IRF4, and Batf as key transcription factors that are involved in the induction of Th9 cells in vitro and Th9-mediated airway inflammation in vivo [38–41]. Moreover, expression of PU.1 and IRF4 in Th9 cells are induced by TGF-β and IL-4, respectively [38, 41]. These studies place Th9 cells under the same conceptual framework as other Th subsets in which Th9 induction is transcriptionally regulated and driven by polarizing cytokines in the local milieu. This allows the generation of renewed interest in the field, and this interest led to remarkable advances in our understanding of Th9 cells.
Key features of Th9 cells
What’s unique about Th9 cells? Historically, IL-9 was thought to be a Th2 cytokine and its re-assignment to Th9 cells put Th2 cells and Th9 cells at odds. It remains unclear what the exact relationships are between Th2 cells and Th9 cells. They clearly share certain features. For example, both subsets depend on IL-4 for their induction, and possibly differentiation; transcription factors that are central to Th2 induction (e.g., STAT6, GATA3) are also important for the induction of Th9 cells[9]. However, Th2 and Th9 subsets are also very different, especially the cytokine milieu required for their induction. As mentioned above, TGF-β is critical to Th9 induction, but it inhibits Th2 cells. Functionally, evidence supporting Th9 cells as a distinct subset is strong[13]. Some studies suggest that Th9 cells might be a subset of Th2 cells, derived in a Th2 environment in which IL-4 expression in Th2 cells is turned off [37]. In earlier studies, Th9 cells were found to express other Th2 cytokines also such as IL-10[36, 37], but in other studies, IL-10 was clearly absent in Th9 cells.
Th9 cells are closely associated with other cell types in the immune system, and their interactions are involved in mounting protective immune responses, as well as induction of immunopathologies. For example, Th9 cells are closely associated with mast cells, type II innate lymph cells (ILC2), and Foxp3+Tregs [33]. Their crosstalks can have profound impact on the outcomes of immune responses, and therefore, attracting much attention recently. The interaction between Th9 cells and mast cells was studied in multiple models. Th9 cells, by producing IL-9, promote the proliferation and survival of mast cells, which play a key role in allergic inflammation[42]. IL-9 is not required for the generation of mast cell precursors, but IL-9 acts as a crucial growth factor of mast cells. Indeed, neutralization of IL-9 or deficiency of IL-9 receptor resulted in defective expansion and recruitment of mast cells in different disease models [43, 44]. Moreover, IL-9 was shown to induce mast cell to produce multiple other cytokines, including IL-5, IL-6, IL-9, IL-10 and IL-13, which mediate allergic inflammation. IL-9 also facilitates the expansion of Th17 cells in certain models, thus promoting autoimmune inflammation [45, 46]. Besides Th17 cells, IL-9 also targets Foxp3+Tregs. It has been shown that IL-9 is required for the suppressive function of Tregs, as IL-9 receptor deficient Foxp3+Tregs exhibit impaired ability to suppress effector CD4+ T cells in vitro and in vivo, resulting in much more severe experimental autoimmune encephalomyelitis (EAE) [46].
Another cell type that often associates with Th9 cells is ILC2. ILC2 cells are prominently featured in the pathogenesis of asthma; they produce copious amount of cytokines, especially IL-5 and IL-13, and act as key drivers of type 2 immunity[47]. ILC2 cells also serve as a source of IL-9 in allergic and autoimmune inflammation. In a model of papain-induced lung inflammation, IL-9 from ILC2 cells was clearly involved in lung inflammation [48]. Additionally, IL-9 is required for ILC2 cell functions, as neutralization of IL-9 leads to reduced expression of IL-5 and IL-13 by ILC2 cells [48]. Other studies also showed that reduced IL-9 and Th9 cells were correlated with decreased IL-9+ ILC2 and allergic responses in itk-deficient mice[49]. More recently, Moretti and colleagues reported that in cystic fibrosis, mast cells, ILC2, and Th9 cells form an intricate amplification loop, driving severe lung inflammation [50]. Clearly, Th9 cells, together with the above-mentioned cell types, can form a network that drives productive type 2 immunity, as well as allergic and autoimmune inflammations.
Inducing and defining Th9 cells
The Il9 locus and its transcriptional network
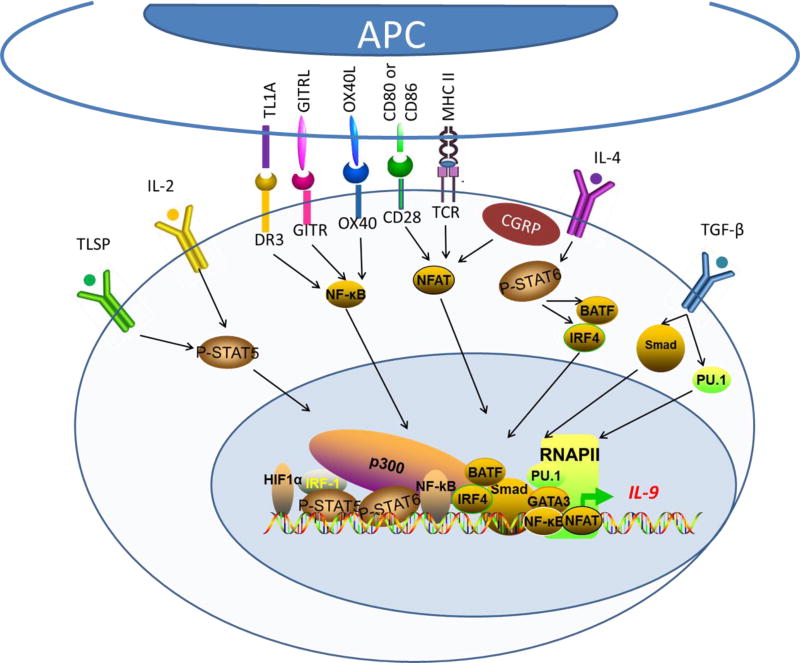
The genomic structure of Il9 locus has been extensive characterized[9]. IL-9 gene is located at chromosome 5 in the mouse. The Il9 locus is about 11kb in size, and the coding region of the Il9 locus consists of 5 exons, with additional 3 conserved non-coding sequences (CNS0–2). CNS0 is located ~6kb upstream of the Il9 transcription start site (TSS) (−6kb), while CNS2 is about 5.4kb downstream of the TSS site (+5.4kb). CNS1 denotes the promoter region, which contains binding sites for multiple transcription factors [24, 41, 51, 52]. Consistent with the complex cytokine milieu in which Th9 cells are induced, the transcription factors that are shown to promote Th9 induction are numerous. For example, those that can engage the Il9 promoter region include PU.1, IRF1, IRF4, STAT5, STAT6, NFAT, GATA1, GATA3, Smads, Etv5 and Notch, as well as NF-κB, BATF, AP-1[9]. Etv5 can also bind the CNS0 and CNS2 regions and mediate chromatin remodeling by recruiting the histone acetyltransferase p300 [53]. TCR stimulation, polarizing cytokines, and costimulatory molecules, especially those in the TNFR superfamily, are all involved in the expression of such transcription factors. It should be noted that none of those transcription factors are Th9 specific, thus making a detailed study of Th9 cells a challenging task. In fact, as compared to other Th subsets (Th1, Th2, Th17), a “lineage-specific” or a “lineage-defining” transcription factor has not been identified thus far for Th9 cells, despite enormous efforts devoted to this area[9]. It remains contentious whether Th9 cells are truly a distinct subset or simply intermediaries of other Th subsets.
Mechanisms driving the transcriptional network
Because of the complexity of transcription factors in regulating Th9 differentiation, induction of Th9 cells remains conditional, relying on a combination of multiple signals during T cell activation, which are summarized below.
T cell receptor signaling
T cell receptor (TCR) stimulation by cognate antigens is a key prerequisite for the development of T helper subsets. Thus, TCR-derived signaling is indispensable for induction of Th9 cells. IL-9 gene expression is positively correlated with the strength of TCR signals [49, 54]. TCR stimulation activates the transcription factor NFAT1 and NF-κB (p65), promoting their nuclear localization to stimulate transcription of IL-9 [52]. NFAT1 also cooperates with p300 to induce permissive chromatin modification at Il9 locus, while NF-κB (p65) induces transactivation of IL-9 gene. In fact, both deficiency of NFAT1 and knockdown of NF-κB (p65) impaired the expression of IL-9[52]. In addition, TCR activation can induce the expression of the IFN-regulation factor 4 (IRF4), which is essential for the development of Th9 cells, as well as differentiation of Th2 and Th17 cells [41, 55, 56]. IRF4 was critical for Th9 differentiation, as either IRF4-deficiency or IRF4 knockdown by IRF4-specific siRNA impaired IL-9 expression under Th9 conditions [41]. Besides, overexpression of IRF4 enhanced the differentiation of Th9 cells [40]. Furthermore, recent studies suggest that TCR signaling induces Itk, which regulates IL-2 and IRF4 in the control of Th9 differentiation and IL-9 expression [49].
Cytokines
Cytokines in the local environment where naive CD4+ T cells are activated play a critical role in the induction of T helper subsets. In the case of Th9 cells, a combination of TGF-β and IL-4 is required for Th9 induction [34, 36]. TGF-β and IL-4 individually exhibits strikingly different functions, but integration of both cytokine signals activates the Th9 inducing program. Deficiency of IL-4 or TGF-β signaling dramatically impairs Th9 differentiation. However, induction of Th9 cells is complex as IL-4 alone without TGF-β promotes Th2 induction, while TGF-β without IL-4 induces Foxp3+ Tregs [36, 37]. Certain evidence suggests that IL-4 inhibits Foxp3 induction by TGF-β and TGF-β prevents IL-4 from inducing Th2 cells. But together, they induce PU.1 and histone acetyltransferases to program differentiation of activated CD4+ T cells toward Th9 cells.
TGF- β signals through the Smad proteins Smad2, Smad3 as well as Smad4 for differentiation of Th9 cells, as deficiency of Smad2, Smad3 or Smad4 all leads to diminished Th9 induction [39, 57]. The Smad proteins can bind to the CNS regions of Il9 gene, and the induction of IL-9 is partially mediated by displacement of EZH2, a histone methyltransferase that represses gene expression [57]. Additionally, Smad3 also cooperates with RBP-Jk and the Notch intracellular domain (NICD) to mediate Th9 differentiation [51]. Furthermore, PU.1, an ETS-family transcription factor, also plays a critical role in TGF-β-mediated Th9 polarization. It has been shown that reduced expression of PU.1 resulted in impaired IL-9 production, whereas overexpression of PU.1 enhanced IL-9 production. Moreover, mice with PU.1-deficient T cells exhibited attenuated lung inflammation with reduced expression of IL-9 [38]. Mechanistically, PU.1 serves as transcription factor to bind to the IL-9 promoter; PU.1 also regulates permissive chromatin remodeling at the IL-9 locus via recruiting histone acetyltransferases [38, 58]. In addition, PU.1 promotes Th9 differentiation by inhibiting the expression of Th2 cytokines primarily through modulating the DNA binding activities of GATA3 and IRF4 [59–61].
Besides TGF-β, IL-4 signaling is mandatory for Th9 differentiation. STAT6 is activated downstream of IL-4 receptor and is required for induction of IL-9. STAT6 deficiency in CD4+ T cells leads to impaired IL-9 differentiation, which is associated with increased expression of T-bet and Foxp3 transcription factors that suppress IL-9 expression [36, 37, 62]. In addition, STAT6 promotes the expression of BATF, GATA3 and IRF4, which are essential transcription factors for Th9 differentiation. However, recent studies suggest that IL-4 signaling can attenuate Th9 differentiation via negative feedback mechanisms. IL-4 induces cytokine-induced SH-2 protein (CIS), a member of the suppressor of cytokine signaling (SOCS) family, which inhibits activation of STAT3, STAT5 and STAT6 in T cells [32]. CIS deficiency in T cells results in enhanced Th9 differentiation, which is associated with increased binding of STAT5 and STAT6 to the IL-9 promoter. Moreover, CIS-deficient mice spontaneously develop airway inflammation in which Th9 cells are involved [32], demonstrating a role for CIS in the control of Th9 differentiation.
Among other cytokines, IL-1 and IL-25 enhance the induction of Th9 cells. Moreover, IL-33, an IL-1 family member cytokine, favors IL-9 expression in activated CD4+ T cells [63]. However, some cytokines, including IFN-γ, IL-12, IL-18 and IL-27, suppress Th9 differentiation by upregulating T-bet expression.
Costimulatory signals
Emerging evidence suggests that T-cell costimulatory signals are not only critical to T-cell activation, but also to T helper cell differentiation. CD28 engagement leads to enhanced induction of IL-9, which is correlated with increased IL-4 production as well as expression and phosphorylation of FoxO3a [64]. Costimulatory molecules in the TNFRSF (OX40, GITR, DR3) are known to stimulate T cell proliferation and survival[11]. But recent studies have identified exciting new roles for such costimulatory molecules in regulating Th9 differentiation[11]. Our group first reported that OX40 is surprisingly potent in inducing IL-9 production. OX40 engagement on CD4+ T cells under Th9-inducing conditions dramatically increased the generation of Th9 cells, while induction of Foxp3+ Tregs and Th17 cells was strongly inhibited by OX40 costimulation[24]. Enhanced Th9 differentiation was observed in OX40L transgenic mice or administration of agonist anti-OX40 antibody in vivo. However, the role of OX40 in robust Th9 induction is neither through modification of cytokine-signaling pathways, nor through induction of PU.1 or IRF4 [24]. Instead, OX40 costimulation activated the NF-kB inducing kinase (NIK) in CD4+ T cells, which resulted in activation of the non-canonical NF-kB pathway to drive robust Th9 generation [24].
Besides OX40, we and other groups found that GITR can also promote Th9 induction [22, 23]. Interestingly, GITR ligation not only induces Th9 cells under Th9-inducing conditions but also converts Foxp3+ Tregs to Th9 cells. Mechanistically, GITR activates the NF-kB family member p50, which closes the Foxp3 locus via recruiting histone deacetylases. Moreover, GITR ligation activates STAT6, which recruits histone acetyltransferase to mediate 'open' chromatin remodeling, thus resulting in strong Th9 induction [22]. The DR3 ligand TL1A (TNFSF15) was recently reported to enhance Th9 induction. TL1A strongly repressed the generation of iTregs under iTreg polarization conditions, meanwhile promoted the expression of IL-9[65]. Under Th9 inducing conditions (TGF-β and IL-4), however, TL1A exhibits strong effects in supporting Th9 generation[65].
Other costimulatory molecules capable of regulating Th9 cells include the Notch pathway and PD-L2 [51, 66]. Conditional deletion of Notch1 and Notch2 significantly impaired IL-9 production in Th9 cultures [51]. And among the multiple ligands for the Notch receptors, it is Jagged2 but not Delta-like 1 that induced IL-9 expression under Th9 inducing conditions. On the other hand, PD-L2 was shown to negatively regulate Th9 cell development, as blockade of PD-L2 leads to increased numbers of IL-9-secreting Th9 cells [66]. Clearly, these studies highlight the complex roles of costimulatory pathways, in addition to cytokines, in regulation of Th9 cells.
IL-9 and Th9 cells in immunity and immunopathology
IL-9 is a pleiotropic cytokine that is involved in both protective immunity and immunopathologies. Thus, the role of Th9 cells in vivo can be multi-faceted, depending on the context and specific disease settings. Here we highlight our current understanding of Th9 cells in various disease models.
Th9 cells in parasitic infections
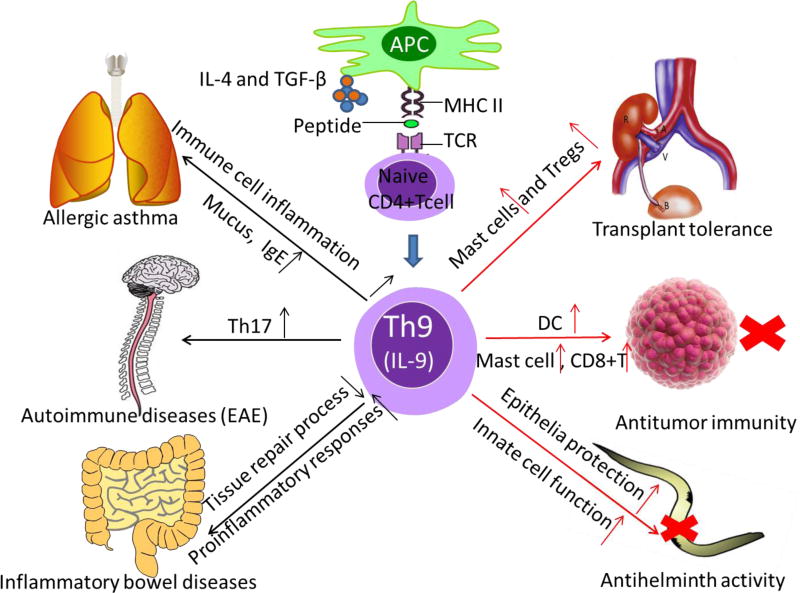
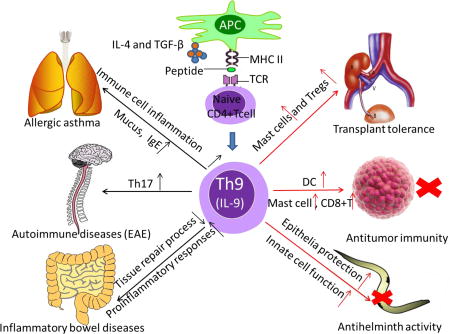
Besides Th2 cells, Th9 cells also exhibit potent effects against parasitic infections [10]. Ag-specific expansion of Th9 cells was observed in patients with lymphatic filariasis, a chronic helminth infection. Moreover, positive correlation was found between the frequency of Th9 cells and the severity of lymphedema in filarial-infected patients, suggesting the involvement of Th9 cells in chronic lymphatic filariasis and other inflammatory disorders [67]. In a Trichuris muris infection model, it has been shown that CD4dnTGF-βRII mice with defective TGF-β signaling showed decreased IL-9 expression and increased parasite burden [37]. Thus, an impaired Th9 development often results in severe helminth infection, demonstrating the importance of Th9 cells in anti-parasitic immunity. Indeed, adoptive transfer of Th9 cells into Rag-1 deficient mice induced rapid expulsion of Nippostrongylus brasiliensis[19], further demonstrating a critical role for Th9 cells in immunity against parasitic infection. These studies clearly highlight the protective effects of Th9 cells in resistance to helminth infections.
Th9 cells in allergic inflammation
Studies in multiple mouse models demonstrate a critical role for Th9 cells in allergic inflammation, including atopic dermatitis and asthma [7, 16]. In mouse models of asthma, high levels of Th9 cells were detected. Adoptive transfer of OVA specific Th9 cells caused allergic airway diseases following OVA challenge. And constitutive expression of IL-9 in the lungs of IL-9 transgenic mice results in prominent asthma symptoms [68–70]. Furthermore, neutralization of IL-9 markedly reduced asthma symptoms in the mouse, such as airway hyper-responsiveness, eosinophil recruitment, mucus production, and goblet cell metaplasia [40, 41].
Patients with allergic diseases have higher levels of IL-9-secreting T cells, suggesting that these cells may contribute to allergic diseases. And there is a strong correlation between elevated IgE levels and increased Th9 cells in those allergic patients. Besides, preclinical and certain clinical studies provide strong support that modulation of Th9 cells may be therapeutically valuable. Among the translational studies of IL-9 or Th9 cells in different diseases, asthma studies are leading the way. The humanized anti-IL-9, MEDI-528 (also known as enokizumab) has entered clinic trials [76–78]. However, the clinic trials on the efficacy of anti-IL-9 in asthma patients did not meet the expected endpoints. At phase 1 trial, MEDI-528 showed an acceptable safety profile, and MEDI-528 treatment displayed positive trends for improvement in asthma symptoms, as well as a reduction in the number of asthma attacks in patients with mild or mild-to-moderate asthma [77]. This suggests potential benefits of MEDI-528 in asthma treatment. Nonetheless, no positive effects were noted in adults with moderate-to-severe, persistent asthma [78]. Collectively, clinical effects of neutralizing IL-9 remains promising, but better designed trials are needed in future studies in the clinic.
Th9 cells in transplant tolerance
Earlier studies suggest a role for IL-9 in the induction of transplant tolerance. In a mouse model of skin transplantation, Noelle’s group reported that mast cells produced high levels of IL-9 in the tolerant grafts, which boosted the suppressive activities of Foxp3+ Tregs and consequently allograft tolerance [17]. Thus, mice those are genetically deficient in mast cells are resistant to tolerance induction. They also showed that neutralizing IL-9 led to accelerated allograft rejection [17]. Though the role of Th9 cells was not directly addressed in those studies, the cytokine IL-9 seems to act as a linchpin in Treg functions and donor specific transplant tolerance [17]. However, subsequent studies showed that IL-9 doesn’t appear to promote the survival or proliferation of Foxp3+ Treg cells, but IL-9 may facilitate the suppressive functions of Tregs[46], which inhibited ongoing immune responses against the allografts. Furthermore, IL-9 seems to have a protective role in renal ischemia and reperfusion injury, as neutralization of IL-9 aggravated kidney damage[71]. However, considering the pro-inflammatory nature of mast cells and Th9 cells, the context in which allograft tolerance is induced by IL-9 deserves further clarification. Importantly, the exact role of Th9 cells in transplant outcomes remains to be elucidated.
Th9 cells in tumor immunity
Th9 cells show unexpected potency in anti-cancer immune responses. It has been shown that that RORγt-deficient mice are resistant to tumor growth, partly because of induction of Th9 cells in vivo [20]. Moreover, adoptive transfer of Th9 cells into mice bearing B16 melanoma or lung carcinoma significantly reduced tumor cell growth. Other studies reported that administration of recombinant IL-9 protein also markedly restricted tumor growth, while neutralizing anti-IL-9 antibody abolished the beneficial effect of adoptively transferred Th9 cells in vivo[20, 21]. Mechanistically, the anti-tumor effects of Th9 cells are mediated by promoting activation of innate and adaptive immune cells. Anti-tumor effects of IL-9 or adoptive transfer of Th9 cell were observed in tumor bearing Rag-1 deficient mice, suggesting innate immune cells may be critically involved in the anti-tumor effects. As IL-9 strongly promotes proliferation and functions of mast cells, mast cells are a key component of Th9-mediated anti-tumor immunity [20]. In fact, mast-cell-deficient mice failed to mount effective anti-tumor immunity upon administration of recombinant IL-9. Additionally, inhibition of mast cell activities or depleting mast cells in vivo abrogated tumor-specific Th9 mediated suppression of tumor cell engraftment [72], which further confirmed the role of mast cells in Th9-mediated anti-tumor immunity.
In other models, the anti-tumor activity of Th9 is mediated by activation of the adaptive immune responses. Specifically, adoptive transfer of Th9 triggered intense leukocyte infiltration in the tumor tissue by CD4+ T cells and CD8+ T cells, as well as dendritic cells [21]. Furthermore, Th9 cells mediated recruitment of dendritic cells and lymphocytes to the tumor sites via the CCL20/CCR6 pathway, resulting in CD8+ T cell activation and strong tumor immunity [21].
The direct involvement of Th9 cells in human in malignancy remains unclear. But some studies indicated the correlations between Th9 cells and anti-tumor responses. It has been reported that the markedly decreased Th9 cells were detected in the blood and skin of patients with aggressive melanoma [20]. Some studies suggest that the IL-9 single-nucleotide polymorphism is associated with an increased risk of cutaneous malignant melanoma.[73]
Th9 cells in autoimmune diseases
There are multiple autoimmune diseases where Th9 cells are potentially involved. Inflammatory bowel diseases (IBD) are characterized by chronic relapsing inflammation of the gastrointestinal tract. Current studies indicate that Th9 cells are involved in the pathogenesis of IBD[15]. High expression of IL-9 and increased IL-9+ T cells were detected in patients with IBD, especially in those with ulcerative colitis. Moreover, induction of Th9 cells is associated with severity of gut pathology. In mouse models, increased IL-9 and IL-9R were also observed during inflammation in the gut. Importantly, adoptive transfer of Th9 cells into Rag1-deficient mice induced severe colitis, and treatment with neutralizing anti-IL-9 monoclonal antibody (mAb) markedly suppressed the development of colitis [74]. Furthermore, in IL-9 deficient mice, the progression of colitis was attenuated. Thus, Th9 may contribute to the pathogenesis of IBD.
Th9 cells are also involved in other autoimmune diseases, such as multiple sclerosis (MS), systemic lupus erythematosus (SLE) and rheumatic arthritis (RA). In the case of MS, which is an autoimmune disease targeting the central nerve system (CNS), Th1, Th17 and Th2 cells play important roles in the pathogenesis of MS, but Th9 cells are increasing appreciated in MS. In animal models of MS (e.g., EAE), which is characterized by T cell-dependent demyelination, Th9 cell infiltration in CNS was observed [75], and adoptive transfer of myelin oligodendrocyte glycoprotein (MOG)-specific Th9 cells resulted in EAE [45]. IL-9 signaling deficiency mice displayed reduced T cell infiltration in CNS and reduced IL-17 and IFN-g, which suggest that IL-9 plays a critical role in EAE. This effect was thought due to its ability to enhance Th1 and Th17 cell responses. Nevertheless, the connection between Th9 cells and the pathogenesis of EAE is still contentious. Some studies suggest a protective role of IL-9 in EAE, via strengthening the suppressive function of Tregs[46]. Additionally, in EAE model, whether IL-9 is derived from Th9 or Th17 cells remains to be elucidated. Thus, further study is needed to validate the role of Th9 in EAE and ultimately its role in MS.
Unanswered questions and path forward
In the past few years, significant progress has been made in our understanding of the fundamental biology of IL-9 and Th9 cells, as well as the role of Th9 cells in various disease settings. It is widely accepted that development of Th9 cells, including the expression of IL-9, requires integration of multiple signaling pathways triggered by the TCR, cytokine receptors (IL-4R, TGF-βR and IL-2R), and the T cell costimulatory molecules. Although findings in preclinical models of allergic inflammation, autoimmunity, parasitic infections and tumors demonstrate the involvement of Th9 cells and the therapeutic potentials of targeting Th9 cells in the clinic, many questions remain, especially about the transcriptional control of Th9 cells, Th9 stability, and the role of Th9 cells in human diseases.
The current paradigm suggests that the Th cell differentiation is specified by the lineage-defining transcription factors, such as T-bet for Th1, GATA3 for Th2 or RORγt for Th17 cells. However, Th9 cells appear to be an exception, as a lineage-defining factor for Th9 cells has not been identified. Meanwhile, the stability and flexibility of Th9 are not fully understood. Clearly, resolving those questions is critical in therapeutically manipulating Th9 cells in the clinic to treat Th9-mediated diseases.
Current studies demonstrate the beneficial and detrimental functions of Th9 and IL-9 in multiple models. Substantial data suggest that Th9 and IL-9 are pathogenic in asthma, IBD, EAE and other allergic and autoimmune diseases, while neutralization of IL-9 or deficiency of IL-9 signaling resulted in attenuated diseases. It is also reported that Th9 and IL-9 have beneficial effects in initiating immunity against helminth infection and tumor, and high levels of IL-9 lead to better outcome of these diseases. Thus, Th9-targeted therapies have tremendous therapeutic potential in the clinic and deserve further investigation. However, it remains unclear whether Th9 development and functions in the mouse are the same as those in humans. Our knowledge about human Th9 cells is very much limited, and much remains to be studied to move the field forward.
Fig 1.
Transcriptional regulation of Th9 cell generation. The generation of Th9 cells from naive CD4+ T cells requires T cell receptor (TCR) signaling, IL-2 and stimulation by a combination of TGF-β and IL-4. Thus, TCR-nuclear factor of activated T cells (NFAT), IL-2–signal transducer and activator of transcription 5 (STAT5) and IL-4–STAT6 signaling, as well as TGFβ-mediated diversion from Th2 cell differentiation pathway, synergistically induce Th9 differentiation. Though these signaling was sufficient for the development of Th9 cells, costimulation signaling pathways have been identified that drive robust Th9 generation. Costimulatory molecules in TNF receptor superfamily, including OX40, GITR and DR3, can activate NF-kB which will translocate to the nucleus to induce gene expression. IRF4, interferon (IFN)-regulatory factor 4; BATF, basic leucine zipper transcription factor ATF-like; CGRP, calcitonin gene-related peptide; DLL, Delta-like ligand; GATA3, GATA-binding protein 3; OX40L, OX40 ligand; TSLP, thymic stromal lymphopoietin.
Fig 2.
Th9 cells and IL-9 in immunity and immunopathology. Th9 cells were demonstrated to have protect effects in resistance to certain parasites infections. This anti-helminth activity was mediated by IL-9, which provided epithelial cell protection and augmented innate immune cell infiltration and function at infected location. Moreover, Th9 cells were involved in antitumor immunity. Th9 cells can inhibit the growth of melanoma cells by promoting proliferation and function of mast cells, and by recruitment of CD8+ T cells and dendritic cells (DCs). Besides, IL-9 was found can contribute to transplant tolerance via mast cells and suppressive Foxp3+Tregs. On the contrast, Th9 cell was shown to favor allergic asthma, especially upon induction of immune cell inflammation and mucus and IgE. MOG-specific Th9 cells can induce experimental autoimmune encephalomyelitis (EAE), through IL-9-mediated recruitment of autoimmune Th17 cells. Furthermore, IBD can be induced by Th9 cells, through IL-9 mediated impaired tissue repair processes and enhanced pro-inflammatory responses.
Highlights.
Th9 cells are a relatively new subset of T helper cells; they are induced by a combination of TGF-β and IL-4 and are characterized by producing IL-9.
IL-9 is a pleiotropic cytokine, exerting beneficial effects in graft tolerance, parasitic infections and tumor immunity, but also inducing allergic and autoimmune diseases.
IL-9 and Th9 cells are considered as a promising target for clinical intervention, though the mechanisms of inducing and maintaining Th9 cells are still poorly defined, especially in vivo.
Fundamental research is required in the understanding Th9 cells, aimed at therapeutically targeting Th9 cells to improve disease outcomes and patient wellbeing.
Acknowledgments
We are grateful for colleagues at Immunology and Transplant Science Center for their critical reading and providing valuable suggestions on the manuscript.
Abbreviation
- TNFRSF
Tumor necrosis factor receptor super family
- GITR
Glucocorticoid-induced tumor necrosis factor receptor
- DR3
Death receptor 3
- ILC2
Type II innate lymph cells
- CNS
Conserved non-coding sequences
- TSS
Transcription start site
- TCR
T cell receptor
- NICD
Notch intracellular domain
- CIS
Cytokine-induced SH-2
- SOCS
Suppressor of cytokine signaling
- NIK
NF-Kb inducing kinase
- TL1A
TNF-like ligand 1A
- IBD
Inflammatory bowel diseases
- MS
Multiple sclerosis
- SLE
Systemic lupus erythematosus
- RA
Rheumatic arthritis
- EAE
Experimental autoimmune encephalomyelitis
- MOG
Myelin oligodendrocyte glycoprotein
- IRF4
IFN-regulation factor 4
- mAb
Monoclonal antibody
Biographies

Junhui Li is a graduate research fellow at Immunobiology and Transplant Science Center in Houston Methodist Hospital. He is a medical student at Xiangya School of Medicine, Central South University, China. Transplant immunology is his major research interest.

Shuqiu Chen is a research fellow at Immunobiology and Transplant Science Center in Houston Methodist Hospital. He is an attending surgeon at central hospital, Southeast University in China. His research interests are related to tolerance in organ transplantation.

Xiang Xiao is an Assistant Research Professor of Transplant Immunology in Surgery at Houston Methodist Hospital Research Institute. He obtained his PhD in 2005 in China. Key research activities are in the fields of costimulatory molecules and T cell fate decisions.

Yong Zhao is a professor of Transplantation Biology Research Division, Institute of Zoology at Chinese Academy of Sciences. His research interests are understanding the molecular and cellular mechanisms for immune tolerance and thymus development.

Wenjun Ding is a professor in College of Life Sciences at University of the Chinese Academy of Sciences. He received his Ph.D. at Chinese Academy of Sciences. His research interests are biochemistry and molecular biology, especially environment and health, diabetes mellitus and obesity.

Xian C. Li is a professor of Immunology at Cornell University and the director of the Immunobiology & Transplant Science Center at Houston Methodist Research Institute. His interest focuses on the fundamental mechanisms of Immunobiology, transplant rejection and transplant tolerance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None.
References
- 1.Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nature reviews Immunology. 2002;2:933–44. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 2.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 3.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–32. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 4.Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nature immunology. 2009;10:857–63. doi: 10.1038/ni.1767. [DOI] [PubMed] [Google Scholar]
- 5.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–63. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 6.Neurath MF, Kaplan MH. Th9 cells in immunity and immunopathological diseases. Semin Immunopathol. 2017;39:1–4. doi: 10.1007/s00281-016-0611-z. [DOI] [PubMed] [Google Scholar]
- 7.Clark RA, Schlapbach C. TH9 cells in skin disorders. Semin Immunopathol. 2017;39:47–54. doi: 10.1007/s00281-016-0607-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elyaman W, Khoury SJ. Th9 cells in the pathogenesis of EAE and multiple sclerosis. Semin Immunopathol. 2017;39:79–87. doi: 10.1007/s00281-016-0604-y. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan MH. The transcription factor network in Th9 cells. Seminars in immunopathology. 2017;39:11–20. doi: 10.1007/s00281-016-0600-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Licona-Limon P, Arias-Rojas A, Olguin-Martinez E. IL-9 and Th9 in parasite immunity. Semin Immunopathol. 2017;39:29–38. doi: 10.1007/s00281-016-0606-9. [DOI] [PubMed] [Google Scholar]
- 11.Meylan F, Siegel RM. TNF superfamily cytokines in the promotion of Th9 differentiation and immunopathology. Seminars in immunopathology. 2017;39:21–8. doi: 10.1007/s00281-016-0612-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rivera Vargas T, Humblin E, Vegran F, Ghiringhelli F, Apetoh L. TH9 cells in anti-tumor immunity. Semin Immunopathol. 2017;39:39–46. doi: 10.1007/s00281-016-0599-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmitt E, Bopp T. Discovery and initial characterization of Th9 cells: the early years. Semin Immunopathol. 2017;39:5–10. doi: 10.1007/s00281-016-0610-0. [DOI] [PubMed] [Google Scholar]
- 14.Shik D, Tomar S, Lee JB, Chen CY, Smith A, Wang YH. IL-9-producing cells in the development of IgE-mediated food allergy. Semin Immunopathol. 2017;39:69–77. doi: 10.1007/s00281-016-0605-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weigmann B, Neurath MF. Th9 cells in inflammatory bowel diseases. Semin Immunopathol. 2017;39:89–95. doi: 10.1007/s00281-016-0603-z. [DOI] [PubMed] [Google Scholar]
- 16.Koch S, Sopel N, Finotto S. Th9 and other IL-9-producing cells in allergic asthma. Semin Immunopathol. 2017;39:55–68. doi: 10.1007/s00281-016-0601-1. [DOI] [PubMed] [Google Scholar]
- 17.Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442:997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- 18.Richard M, Grencis RK, Humphreys NE, Renauld JC, Van Snick J. Anti-IL-9 vaccination prevents worm expulsion and blood eosinophilia in Trichuris muris-infected mice. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:767–72. doi: 10.1073/pnas.97.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Licona-Limon P, Henao-Mejia J, Temann AU, Gagliani N, Licona-Limon I, Ishigame H, et al. Th9 Cells Drive Host Immunity against Gastrointestinal Worm Infection. Immunity. 2013;39:744–57. doi: 10.1016/j.immuni.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Purwar R, Schlapbach C, Xiao S, Kang HS, Elyaman W, Jiang X, et al. Robust tumor immunity to melanoma mediated by interleukin-9-producing T cells. Nat Med. 2012;18:1248–53. doi: 10.1038/nm.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu Y, Hong S, Li H, Park J, Hong B, Wang L, et al. Th9 cells promote antitumor immune responses in vivo. J Clin Invest. 2012;122:4160–71. doi: 10.1172/JCI65459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao X, Shi X, Fan Y, Zhang X, Wu M, Lan P, et al. GITR subverts Foxp3(+) Tregs to boost Th9 immunity through regulation of histone acetylation. Nature communications. 2015;6:8266. doi: 10.1038/ncomms9266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim IK, Kim BS, Koh CH, Seok JW, Park JS, Shin KS, et al. Glucocorticoid-induced tumor necrosis factor receptor-related protein co-stimulation facilitates tumor regression by inducing IL-9-producing helper T cells. Nature medicine. 2015;21:1010–7. doi: 10.1038/nm.3922. [DOI] [PubMed] [Google Scholar]
- 24.Xiao X, Balasubramanian S, Liu W, Chu X, Wang H, Taparowsky EJ, et al. OX40 signaling favors the induction of T(H)9 cells and airway inflammation. Nat Immunol. 2012;13:981–90. doi: 10.1038/ni.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones CP, Gregory LG, Causton B, Campbell GA, Lloyd CM. Activin A and TGF-beta promote T(H)9 cell-mediated pulmonary allergic pathology. The Journal of allergy and clinical immunology. 2012;129:1000–10. e3. doi: 10.1016/j.jaci.2011.12.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicolaides NC, Holroyd KJ, Ewart SL, Eleff SM, Kiser MB, Dragwa CR, et al. Interleukin 9: a candidate gene for asthma. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:13175–80. doi: 10.1073/pnas.94.24.13175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan C, Gery I. The unique features of Th9 cells and their products. Critical reviews in immunology. 2012;32:1–10. doi: 10.1615/critrevimmunol.v32.i1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan C, Aziz MK, Lovaas JD, Vistica BP, Shi G, Wawrousek EF, et al. Antigen-specific Th9 cells exhibit uniqueness in their kinetics of cytokine production and short retention at the inflammatory site. Journal of immunology. 2010;185:6795–801. doi: 10.4049/jimmunol.1001676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010;327:1098–102. doi: 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li XC, Schachter AD, Zand MS, Li Y, Zheng XX, Harmon WE, et al. Differential expression of T-cell growth factors in rejecting murine islet and human renal allografts: conspicuous absence of interleukin (IL)-9 despite expression of IL-2, IL-4, IL-7, and IL-15. Transplantation. 1998;66:265–8. doi: 10.1097/00007890-199807270-00022. [DOI] [PubMed] [Google Scholar]
- 31.Zhao P, Xiao X, Ghobrial RM, Li XC. IL-9 and Th9 cells: progress and challenges. Int Immunol. 2013;25:547–51. doi: 10.1093/intimm/dxt039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang XO, Zhang H, Kim BS, Niu X, Peng J, Chen Y, et al. The signaling suppressor CIS controls proallergic T cell development and allergic airway inflammation. Nat Immunol. 2013;14:732–40. doi: 10.1038/ni.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noelle RJ, Nowak EC. Cellular sources and immune functions of interleukin-9. Nature reviews Immunology. 2010;10:683–7. doi: 10.1038/nri2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmitt E, Germann T, Goedert S, Hoehn P, Huels C, Koelsch S, et al. IL-9 production of naive CD4+ T cells depends on IL-2, is synergistically enhanced by a combination of TGF-beta and IL-4, and is inhibited by IFN-gamma. Journal of immunology. 1994;153:3989–96. [PubMed] [Google Scholar]
- 35.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–64. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(−) effector T cells. Nature immunology. 2008;9:1347–55. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Veldhoen M, Uyttenhove C, van Snick J, Helmby H, Westendorf A, Buer J, et al. Transforming growth factor-beta 'reprograms' the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nature immunology. 2008;9:1341–6. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 38.Chang HC, Sehra S, Goswami R, Yao W, Yu Q, Stritesky GL, et al. The transcription factor PU.1 is required for the development of IL-9-producing T cells and allergic inflammation. Nat Immunol. 2010;11:527–34. doi: 10.1038/ni.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tamiya T, Ichiyama K, Kotani H, Fukaya T, Sekiya T, Shichita T, et al. Smad2/3 and IRF4 play a cooperative role in IL-9-producing T cell induction. J Immunol. 2013;191:2360–71. doi: 10.4049/jimmunol.1301276. [DOI] [PubMed] [Google Scholar]
- 40.Jabeen R, Goswami R, Awe O, Kulkarni A, Nguyen ET, Attenasio A, et al. Th9 cell development requires a BATF-regulated transcriptional network. J Clin Invest. 2013;123:4641–53. doi: 10.1172/JCI69489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Staudt V, Bothur E, Klein M, Lingnau K, Reuter S, Grebe N, et al. Interferon-regulatory factor 4 is essential for the developmental program of T helper 9 cells. Immunity. 2010;33:192–202. doi: 10.1016/j.immuni.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 42.Renauld JC, Goethals A, Houssiau F, Van Roost E, Van Snick J. Cloning and expression of a cDNA for the human homolog of mouse T cell and mast cell growth factor P40. Cytokine. 1990;2:9–12. doi: 10.1016/1043-4666(90)90037-t. [DOI] [PubMed] [Google Scholar]
- 43.Nowak EC, Weaver CT, Turner H, Begum-Haque S, Becher B, Schreiner B, et al. IL-9 as a mediator of Th17-driven inflammatory disease. The Journal of experimental medicine. 2009;206:1653–60. doi: 10.1084/jem.20090246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Townsend JM, Fallon GP, Matthews JD, Smith P, Jolin EH, McKenzie NA. IL-9-deficient mice establish fundamental roles for IL-9 in pulmonary mastocytosis and goblet cell hyperplasia but not T cell development. Immunity. 2000;13:573–83. doi: 10.1016/s1074-7613(00)00056-x. [DOI] [PubMed] [Google Scholar]
- 45.Jager A, Dardalhon V, Sobel RA, Bettelli E, Kuchroo VK. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. Journal of immunology. 2009;183:7169–77. doi: 10.4049/jimmunol.0901906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elyaman W, Bradshaw EM, Uyttenhove C, Dardalhon V, Awasthi A, Imitola J, et al. IL-9 induces differentiation of TH17 cells and enhances function of FoxP3+ natural regulatory T cells. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:12885–90. doi: 10.1073/pnas.0812530106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Licona-Limon P, Kim LK, Palm NW, Flavell RA. TH2, allergy and group 2 innate lymphoid cells. Nature immunology. 2013;14:536–42. doi: 10.1038/ni.2617. [DOI] [PubMed] [Google Scholar]
- 48.Wilhelm C, Hirota K, Stieglitz B, Van Snick J, Tolaini M, Lahl K, et al. An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation. Nature immunology. 2011;12:1071–7. doi: 10.1038/ni.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gomez-Rodriguez J, Meylan F, Handon R, Hayes ET, Anderson SM, Kirby MR, et al. Itk is required for Th9 differentiation via TCR-mediated induction of IL-2 and IRF4. Nature communications. 2016;7:10857. doi: 10.1038/ncomms10857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moretti S, Renga G, Oikonomou V, Galosi C, Pariano M, Iannitti RG, et al. A mast cell-ILC2-Th9 pathway promotes lung inflammation in cystic fibrosis. Nature communications. 2017;8:14017. doi: 10.1038/ncomms14017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Elyaman W, Bassil R, Bradshaw EM, Orent W, Lahoud Y, Zhu B, et al. Notch receptors and Smad3 signaling cooperate in the induction of interleukin-9-producing T cells. Immunity. 2012;36:623–34. doi: 10.1016/j.immuni.2012.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jash A, Sahoo A, Kim GC, Chae CS, Hwang JS, Kim JE, et al. Nuclear factor of activated T cells 1 (NFAT1)-induced permissive chromatin modification facilitates nuclear factor-kappaB (NF-kappaB)-mediated interleukin-9 (IL-9) transactivation. The Journal of biological chemistry. 2012;287:15445–57. doi: 10.1074/jbc.M112.340356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koh B, Hufford MM, Pham D, Olson MR, Wu T, Jabeen R, et al. The ETS Family Transcription Factors Etv5 and PU.1 Function in Parallel To Promote Th9 Cell Development. Journal of immunology. 2016;197:2465–72. doi: 10.4049/jimmunol.1502383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tan C, Wei L, Vistica BP, Shi G, Wawrousek EF, Gery I. Phenotypes of Th lineages generated by the commonly used activation with anti-CD3/CD28 antibodies differ from those generated by the physiological activation with the specific antigen. Cellular & molecular immunology. 2014;11:305–13. doi: 10.1038/cmi.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lohoff M, Mittrucker HW, Prechtl S, Bischof S, Sommer F, Kock S, et al. Dysregulated T helper cell differentiation in the absence of interferon regulatory factor 4. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:11808–12. doi: 10.1073/pnas.182425099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brustle A, Heink S, Huber M, Rosenplanter C, Stadelmann C, Yu P, et al. The development of inflammatory T(H)-17 cells requires interferon-regulatory factor 4. Nature immunology. 2007;8:958–66. doi: 10.1038/ni1500. [DOI] [PubMed] [Google Scholar]
- 57.Wang A, Pan D, Lee YH, Martinez GJ, Feng XH, Dong C. Cutting edge: Smad2 and Smad4 regulate TGF-beta-mediated Il9 gene expression via EZH2 displacement. J Immunol. 2013;191:4908–12. doi: 10.4049/jimmunol.1300433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goswami R, Kaplan MH. Gcn5 is required for PU.1-dependent IL-9 induction in Th9 cells. Journal of immunology. 2012;189:3026–33. doi: 10.4049/jimmunol.1201496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chang HC, Han L, Jabeen R, Carotta S, Nutt SL, Kaplan MH. PU.1 regulates TCR expression by modulating GATA-3 activity. Journal of immunology. 2009;183:4887–94. doi: 10.4049/jimmunol.0900363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chang HC, Zhang S, Thieu VT, Slee RB, Bruns HA, Laribee RN, et al. PU.1 expression delineates heterogeneity in primary Th2 cells. Immunity. 2005;22:693–703. doi: 10.1016/j.immuni.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 61.Ahyi AN, Chang HC, Dent AL, Nutt SL, Kaplan MH. IFN regulatory factor 4 regulates the expression of a subset of Th2 cytokines. Journal of immunology. 2009;183:1598–606. doi: 10.4049/jimmunol.0803302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goswami R, Jabeen R, Yagi R, Pham D, Zhu J, Goenka S, et al. STAT6-dependent regulation of Th9 development. J Immunol. 2012;188:968–75. doi: 10.4049/jimmunol.1102840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blom L, Poulsen BC, Jensen BM, Hansen A, Poulsen LK. IL-33 induces IL-9 production in human CD4+ T cells and basophils. PloS one. 2011;6:e21695. doi: 10.1371/journal.pone.0021695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takami M, Love RB, Iwashima M. TGF-beta converts apoptotic stimuli into the signal for Th9 differentiation. Journal of immunology. 2012;188:4369–75. doi: 10.4049/jimmunol.1102698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Richard AC, Tan C, Hawley ET, Gomez-Rodriguez J, Goswami R, Yang XP, et al. Correction: The TNF-Family Ligand TL1A and Its Receptor DR3 Promote T Cell-Mediated Allergic Immunopathology by Enhancing Differentiation and Pathogenicity of IL-9-Producing T Cells. Journal of immunology. 2015;195:5839–40. doi: 10.4049/jimmunol.1502026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kerzerho J, Maazi H, Speak AO, Szely N, Lombardi V, Khoo B, et al. Programmed cell death ligand 2 regulates TH9 differentiation and induction of chronic airway hyperreactivity. The Journal of allergy and clinical immunology. 2013;131:1048–57. 57 e1–2. doi: 10.1016/j.jaci.2012.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Anuradha R, George PJ, Hanna LE, Chandrasekaran V, Kumaran P, Nutman TB, et al. IL-4-, TGF-beta-, and IL-1-dependent expansion of parasite antigen-specific Th9 cells is associated with clinical pathology in human lymphatic filariasis. Journal of immunology. 2013;191:2466–73. doi: 10.4049/jimmunol.1300911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Temann UA, Geba GP, Rankin JA, Flavell RA. Expression of interleukin 9 in the lungs of transgenic mice causes airway inflammation, mast cell hyperplasia, and bronchial hyperresponsiveness. The Journal of experimental medicine. 1998;188:1307–20. doi: 10.1084/jem.188.7.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Temann UA, Ray P, Flavell RA. Pulmonary overexpression of IL-9 induces Th2 cytokine expression, leading to immune pathology. The Journal of clinical investigation. 2002;109:29–39. doi: 10.1172/JCI13696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Temann UA, Laouar Y, Eynon EE, Homer R, Flavell RA. IL9 leads to airway inflammation by inducing IL13 expression in airway epithelial cells. International immunology. 2007;19:1–10. doi: 10.1093/intimm/dxl117. [DOI] [PubMed] [Google Scholar]
- 71.Kortekaas KA, de Vries DK, Reinders ME, Lievers E, Ringers J, Lindeman JH, et al. Interleukin-9 release from human kidney grafts and its potential protective role in renal ischemia/reperfusion injury. Inflamm Res. 2013;62:53–9. doi: 10.1007/s00011-012-0550-7. [DOI] [PubMed] [Google Scholar]
- 72.Abdul-Wahid A, Cydzik M, Prodeus A, Alwash M, Stanojcic M, Thompson M, et al. Induction of antigen-specific TH 9 immunity accompanied by mast cell activation blocks tumor cell engraftment. Int J Cancer. 2016;139:841–53. doi: 10.1002/ijc.30121. [DOI] [PubMed] [Google Scholar]
- 73.Yang XR, Pfeiffer RM, Wheeler W, Yeager M, Chanock S, Tucker MA, et al. Identification of modifier genes for cutaneous malignant melanoma in melanoma-prone families with and without CDKN2A mutations. Int J Cancer. 2009;125:2912–7. doi: 10.1002/ijc.24622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gerlach K, Hwang Y, Nikolaev A, Atreya R, Dornhoff H, Steiner S, et al. TH9 cells that express the transcription factor PU.1 drive T cell-mediated colitis via IL-9 receptor signaling in intestinal epithelial cells. Nat Immunol. 2014;15:676–86. doi: 10.1038/ni.2920. [DOI] [PubMed] [Google Scholar]
- 75.Kara EE, Comerford I, Bastow CR, Fenix KA, Litchfield W, Handel TM, et al. Distinct chemokine receptor axes regulate Th9 cell trafficking to allergic and autoimmune inflammatory sites. Journal of immunology. 2013;191:1110–7. doi: 10.4049/jimmunol.1203089. [DOI] [PMC free article] [PubMed] [Google Scholar]