Abstract
Previous studies confirmed that dietary supplements of fish oil and krill oil can alleviate obesity in mice, but the underlying mechanism remains unclear. This study aims to discern whether oil treatment change the structure of the gut microbiota during the obesity alleviation. The ICR mice received high-fat diet (HFD) continuously for 12 weeks after two weeks of acclimatization with a standard chow diet, and the mice fed with a standard chow diet were used as the control. In the groups that received HFD with oil supplementation, the weight gains were attenuated and the liver index, total cholesterol, triglyceride and low-density lipoprotein cholesterol were reduced stepwise compared with the HFD group, and the overall structure of the gut microbiota, which was modulated in the HFD group, was shifted toward the structure found in the control group. Moreover, eighty-two altered operational taxonomic units responsive to oil treatment were identified and nineteen of them differing in one or more parameters associated with obesity. In conclusion, this study confirmed the effect of oil treatment on obesity alleviation, as well as on the microbiota structure alterations. We proposed that further researches are needed to elucidate the causal relationship between obesity alleviation and gut microbiota modulation.
Introduction
In the last three decades, overweight and obesity levels have more than doubled worldwide. In 2014, 39% of 18-year-olds in the world were overweight, and 13% were obese [1]. Accumulating evidence suggests that lipocytes not only provide energy storage but also act as an integral part of the endocrine function of superfluous fat tissue, with diverse health consequences [2]. Obesity often contributes to the pathogenesis, complicates the course, and increases the risk of several other life-threatening diseases [3]. Obesity-related metabolic diseases have attracted great attention all over the world. The etiology, prevention and treatment of such problems has become a global research interest in the past few decades [4].
The development of obesity is a complicated process involving genetic and environmental factors. High energy intake and low physical activity are the typical environmental factors associated with obesity. Obesity usually accompanied with increase of total cholesterol (TC), triglyceride (TG) and low-density lipoprotein cholesterol (LDL-C) and a decrease of high-density lipoprotein cholesterol (HDL-C) in serum [5]. These four indicators had been widely used to validate obesity symptoms in various studies related to obesity [6–9]. Several pharmacotherapeutic agents, such as phentermine, have been employed to alleviate this complex disorder in recent decades. They mainly exert their effects by restraining appetite, controlling lipometabolism, and restricting energy intake [10–12]. However, the development of pharmacological drugs has been seriously challenged because candidate drugs tended to lack efficacy and have various side effects, such as depression, suicide and apoplexy [13]. The regulatory agencies has limited the use of pharmacological agents and removed some drugs from the market [14]. Therefore, intervention strategies with new targets are needed to advance the treatment of obesity.
Accumulating evidence suggests that the obesity and its complications such as insulin resistance, hyperlipidemia and atherosclerosis caused by high-fat, high calorie diet are often accompanied with the alteration in the gut microbiota [15, 16]. The ratio of Firmicutes to Bacteroidetes is the best known example at the phylum level, which increased in obese mice when compared with lean ones [17]. Furthermore, the development of obesity, adipose tissue and systemic inflammation and metabolic comorbidities in humans are also associated with the decreased in probiotics (such as Bifidobacterium spp. and Pediococcus pentosaceus) and increased in pro-inflammatory/pathogenic bacteria (such as Desulfovibrionaceae) [18, 19]. As special species in the gut microbiota, such as Enterobacter cloacae, Akkermansia muciniphila, Clostridium bolteae and Desulfovibrio sp, were proved to be involved in the development of obesity and its-related metabolic diseases, such as type 2 diabetes [18, 20]. Up to now, there is still lack of studies showed that the gut microbiota acted as a pivotal contributing factor in the development of obesity, whereas some studies found that fat deposits was increased in germ-free mouse recipients after transplantation of the gut microbiota from obese humans or mice [21, 22]. These studies suggest that intestinal microbiota might make contribution to metabolic syndrome therapy, and it may be a potential target for disease controlling.
Omega-3 (Ω-3) fatty acids, which are abundant in fatty fish, marine plankton, walnut oil and linseed (flaxseed) oil, rank among the most important essential nutrients [23]. The consumption of Ω-3 fatty acids, especially docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), is strongly correlated with health benefits. A previous study showed that fish oil supplementation (1 g/kg/day) was able to reduce TC and TG levels, in addition to improved systemic and muscle insulin sensitivity in rat [24]. Furthermore, fish oil supplementation ameliorated some inflammatory diseases such as asthma and Crohn’s disease [25, 26]. Krill oil is extracted from Antarctic krill. It contains long-chain Ω-3 polyunsaturated fatty acids [27]. The fatty acids in fish oil are stored as triglyceride, whereas in krill oil approximately 30–65% of the fatty acids are incorporated into phospholipids [28]. In addition, krill oil contains astaxanthin, and it may maintain the stability of EPA and DHA against oxidative damage [29]. Various studies have confirmed that supplementation with krill oil alleviated chronic disorders, such as cardiovascular diseases, endocannabinoid dysregulation, poor infant development, non-alcoholic fatty liver disease, premenstrual syndrome, inflammation and certain cancers [28, 30]. The beneficial effects of krill oil and fish oil on human health have been confirmed. However, there is no direct evidence that krill oil and fish oil alleviate obesity or other metabolic syndromes by modulating the gut microbiota, and the efficacy of mixtures of the two oils against obesity remains unclear.
For the study, we used a randomized approach to assess the efficacy of pure oils or various mixtures thereof against obesity in mice. The blood biochemical indices, the liver index, and the shift in the structure of the gut microbiota in response to different ratios of oil supplementation in obese mice were examined. Our results provide new insight into the alteration of gut microbiota after the oil treatment in the high fat diet induced obesity mice, and this study may help to identify alternative strategies to ameliorate obesity.
Materials and methods
Ethics statement
All experimental and animal care procedures were performed in accordance with the guideline prepared by the Ningbo University Laboratory Animal Center (affiliated with the Zhejiang Laboratory Animal Common Service Platform), and all of the protocols were approved by the Ningbo University Laboratory Animal Center under permit number SYXK (ZHE 2008–0110).
Animal trial
All experimental procedures and animal care were in accordance with the experimental animal care and use guidelines prepared by the Ningbo University Experimental Animal Center (affiliated with the Zhejiang Laboratory Animal Common Service Platform), and all animal programs have received the approval of the Ningbo University Laboratory Animal Center under permit number No. SCXK (ZHE 2014–0001).
After 2 weeks of acclimatization with a standard chow diet, we randomly divided 96 10-week-old male ICR mice (23.33±2.17 g, purchased from Laboratory Animal Center of Zhejiang province (Hangzhou, China), SCXK (Zhejiang) 2014–0001) into 8 groups (12 mice per group): (1) control group, the mice in control group was fed normal chow (protein with 20% Kcal, carbohydrate with 70% kcal, fat with 10% kcal, purchased from Laboratory Animal Center of Ningbo University, Ningbo, China); (2) HFD group, The mice in high-fat diet (HFD) group was fed with high-fat diet (protein with 20% Kcal, carbohydrate with 35% kcal, fat with 45% kcal purchased from Laboratory Animal Center of Ningbo University, Ningbo, China); (3) HFD+M group, fed with HFD and received 1 μg/g metformin by gavage once daily; (4) HFD+FO600 group, fed with HFD and received 600 μg/g fish oil by gavage once daily; (5) HFD+KO600 group, fed with HFD and received 600 μg/g krill oil by gavage once daily; (6) HFD+FO300KO300 group, fed with HFD and received 600 μg/g mixture of fish oil and krill oil in a ratio of 1:1 by gavage once daily; (7) HFD+FO400KO200 group, fed with HFD and received 600 μg/g mixture of fish oil and krill oil in a ratio of 2:1 by gavage once daily; (8) HFD+FO450KO150 group, fed with HFD and received 600 μg/g mixture of fish oil and krill oil in a ratio of 3:1 by gavage once daily. All oil and drug were given to mice by gavage in 0.9% stroke-physiological saline solution. Non-treatment groups were given the same volume of 0.9% stroke-physiological saline solution to minimize the effects of gavage procedure. Within each group, the twelve mice were divided into three cages (4 mice in a cage). The mice were kept in the same house during the experiments simultaneously for 12 weeks, and none of the mice died.
Throughout the duration of the trial, the body weight of each mouse was monitored weekly. At the end of the trial, fecal samples were collected and placed in liquid nitrogen immediately after sampling and stored at -80°C. After 12 h of food deprivation, fasting body weight was precisely examined and then all the animals were anaesthetized with ether. Ether was used as the anesthetic due to its high efficiency, low cost and easy manipulation, thus it was still widely used in the animal experiments [7, 31, 32]. In consideration that the use of ether as an anesthetic might pose a safety hazard to researchers, a similar small equipment as previously described had been made and used to reduce the safety hazard [33]. Blood was collected from the orbital plexus, and serum was isolated by centrifugation at 3000 rpm at 4°C for 15 min and stored at -80°C for subsequent biochemical testing. Then, mice were sacrificed by cervical dislocation, and the tissues, including adipose tissues (epididymal, subcutaneous, visceral, interscapular) and liver were excised, weighed, and frozen in liquid nitrogen immediately for further analysis. Adiposity index was then calculated according to the following formula: adiposity weight/body weight (mg/g). The liver index was calculated using this formula: liver weight/body weight (mg/g).
Analysis of physiological and biochemical indices in plasma
TC, TG, LDL-C and HDL-C levels in the plasma were measured using commercial enzymatic kits purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China) according to the manufacturer's instructions.
Measurement of the composition of fish oil and krill oil
The fatty acid composition of krill oil and fish oil was measured via Gas Chromatography-Mass Spectrometer (GC-MS) as previously described [34]. The concentration of astaxanthin in krill oil was measured via High Performance Liquid Chromatography (HPLC) as previously described [35] and the astaxanthin standard was purchased from Sigma-Aldrich Co., LLC(St. Louis, MO, USA).
Total DNA extraction, PCR and sequencing
The total DNA was isolated from each sample (feces from a shared cage) using a previously described method [36]. The quantity of extracted DNA was measured using a Thermo NanoDrop 2000C (Thermo Fisher Scientific, USA).
PCR primers were designed in the V3 and V4 hypervariable regions of the bacterial 16S rRNA gene. The primers 319F 5′-ACTCCTACGGGAGGCAGCAG-3′ and 806R 5′-GGACTACHVGGGTWTCTAAT-3′ were designed with a barcode sequence that was unique to each sample. PCR products which satisfied the quality demands were used for further sequencing. Amplification reactions were performed in 25 μL volume containing 20 ng of template, 0.1 μM of each primer and 12.5 μL Premix Ex TaqTM Hot Start Version (Takara Biotechnology Co. Ltd, Dalian, China). Amplification was initiated at 98°C for 30 s, followed by 35 cycles of denaturation at 98°C for 10 s, primer annealing at 54°C for 30 s, extension at 72°C for 45 s, and final extension for 10 min. PCR reactions for each sample were performed with a negative control in each run. The presence of amplicons was confirmed using gel electrophoresis, and the PCR products were normalized using AxyPrepTM Mag PCR Normalizer and then sequenced using the MiSeq system constructed with the Illumina Nextera XT Index kit in Sangon Biotech Co., Ltd. (Shanghai, China). Sequencing was performed on an Illumina MiSeq (Illumina, San Diego, CA, USA) using 2×300 bp paired-end sequencing and multiple sequencing runs in accordance with the manufacturer’s instructions.
Data and statistical analysis
Raw FASTQ files were multiplexed and filtered using QIIME[37] (version 1.8.0) according to the following steps: (1) the reads that overlapped more than 10 bp were merged via software FLASH [38], and the maximum of overlap length was 70 bp and the ratio of sequence mismatches was 0.25. Reads that could not be merged were removed. (2) The data belonging to each sample were identified using the barcode sequence. (3) The reads were truncated at any position with an average quality score below 20 in a 10 bp sliding window. (4) Reads that contained undetected nucleotides (N) or were shorter than 200 bp were removed. UCHIME (version 4.2.40) [39] was used to identify and remove the chimeric sequences. Operational taxonomic units (OTUs) were clustered using USEARCH (version 7.1) [40], and 97% was chosen as the similarity level in OTU analysis. The community richness and community diversity indices were calculated using QIIME (version 1.8.0) and sequence normalized to the smallest number to reduce biases in sequencing depth for alpha and beta diversity analysis [41]. Taxonomic classifications were identified via the Ribosomal Database Project Classifier [42].
All data are represented as the means ± S.D. ANOVA and Tukey's post hoc test (SPSS, veision 19.0, Chicago, IL, USA) were used to analyze the normally distributed data, and the Mann-Whitney test (MATLAB R2012a, Natick, MA, USA) was used to analyze the data that did not meet the assumptions of ANOVA. P<0.05 was defined as a standard criterion of statistical significance. Correlations between the intestinal microbial composition and the phenotype of hyperlipidemia were calculated via Spearman’s correlation (SPSS) with Spearman’s correlation coefficient (R) and P-value. Correlations were considered as significant when P<0.05, and correlations were significant at false discovery rate<0.25.
Data availability statement
The sequences have been deposited in the NCBI Sequence Read Archive database under the accession number SRP101547.
Results
Measurement of the composition of krill oil and fish oil
The fatty acid compositions of the krill oil and fish oil are presented in S1 Table. The concentrations of unsaturated fatty acids (FA) in krill oil and fish oil are 69.46% and 64.98%, respectively. The fish oil contained 25.76% DHA and 5.67% EPA, while the krill oil contained 3.90% DHA and 17.82% EPA. The astaxanthin concentration was 44.98 mg/100 g in the krill oil.
Effect of oil supplementation on body weight gain, liver index and fat pad weights
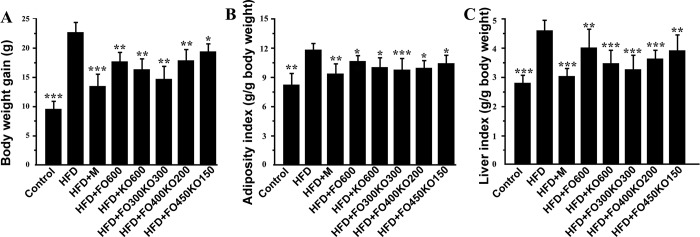
A high-fat (HFD) diet induced obesity in mice after 12 weeks of feeding (S2 Table). Fish oil, krill oil or their mixture was gavaged for 12 weeks while the animals received HFD feed. All of these oil treatments attenuated the increase of body weight. Likewise, adiposity index and liver index were significantly enhanced in the HFD group compared with the control (P<0.01). When compared to the HFD group, oil supplementation reduced the adiposity index and liver index of all the groups (Fig 1). The HFD+M group showed the same pattern as the oil groups in terms of the body weight gain, adiposity index and liver index. In addition, HFD+FO300KO300 showed significant efficacy in alleviating body weight gain and decreasing the adiposity index and liver index (P<0.01).
Fig 1. Effect of oil supplementation on physical features in HFD-induced mice.
(A) Relative body weight gain. (B) Adiposity index. (C) Liver index. Data are represented as the means ± S.D. ***P<0.001, **P<0.01 and *P<0.05 vs the HFD group by ANOVA followed by Tukey post hoc test.
Effect of oil supplementation on plasma biochemical indices
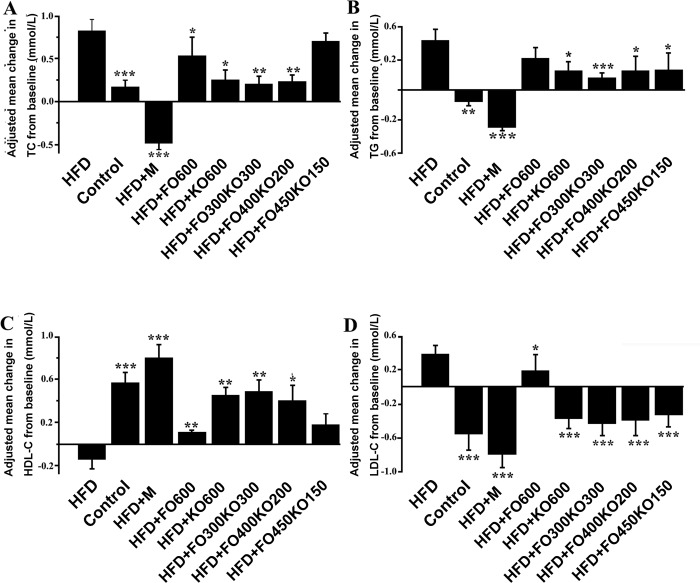
HFD-fed mice developed the hallmark features of obesity and revealed significant increases from baseline in the adjusted means of LDL-C (P<0.001), TC (P<0.001) and TG (P<0.001) compared with the control group. Compared with the control group, a decrease in HDL-C (P<0.001) was also observed in HFD-fed mice. Oil treatment significantly attenuated obesity in HFD-fed mice. The HFD+FO300KO300 group showed significant reductions in LDL-C (P<0.001), TC (P<0.01) and TG (P<0.001) and a significant enhancement in HDL-C (P<0.01) compared with the HFD group (Fig 2 and S3 Table).
Fig 2. Effect of supplementation with oil on plasma biochemical indicators of mice fed an HFD.
(A) The level of TC. (B) The level of TG. (C) The level of HDL-C. (D) The level of LDL-C. TC: total cholesterol, TG: triglyceride, HDL-C: high-density lipoprotein cholesterol, LDL-C: low-density lipoprotein cholesterol. Data are represented as the means ± S.D. ***P<0.001, **P<0.01 and *P<0.05 vs the HFD group by ANOVA followed by Tukey post hoc test.
Quality control and microbial diversity
To elucidate the effects of the oil treatment on the gut microbiota, we collected fecal samples from 96 mice after 12 weeks and performed 16S amplicon sequencing analyses of the V3 and V4 regions (S4 Table). The Simpson and Shannon diversity indices were used to measure the diversity of the microbial communities in each group. A diversity index is used to represent the number of different taxa in the sample, a higher Shannon index indicates greater diversity. According to these metrics, an HFD significantly reduced the diversity of the gut microbiota in mice, while oil treatment restored the index (S5 Table).
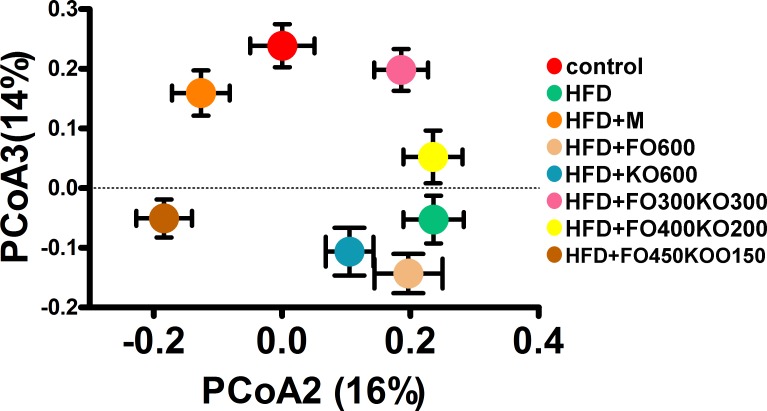
The overall compositions of the gut microbiota in the six groups were analyzed via PCoA (Fig 3, S1 Fig and S2 Fig). The mixtures of fish oil and krill oil shifted the overall composition of the intestinal microbiota in the HFD group towards that of the control, while the FO300KO300 group showed the closest relationship with the control, corresponding to the most significant effect on obesity alleviation. However, compared with the mixture oil treatment, the HFD+FO600 and HFD+KO600 groups showed relatively more similar structures with the HFD group. Interestingly, a close relationship was identified between the HFD+M and control groups in the structure of the gut microbiota, corresponding to significant reductions in LDL-C, TC and TG and attenuation of the features of hyperlipidemia in the HFD+M group.
Fig 3. Change in the gut microbiota structure of mice fed the HFD and treated with different ratio of oil via Weighted Unifrac PCoA analysis.
Data are presented as the means±S.D.
Microbial shifts based on taxon-based analysis
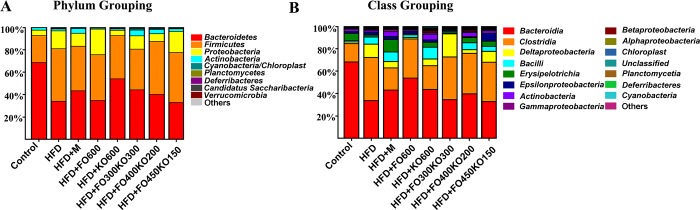
The RDP classifier was used to analyze the variation of intestinal microbial composition in animals receiving different oil supplements. The most abundant phyla included Bacteroidetes, Firmicutes, Proteobacteria and Verrucomicrobia. Two phyla, Firmicutes and Bacteroidetes, accounted for more than 77.45% of the total sequences in each group. The intestinal microorganism community structure had changed at the phylum level after 12 weeks of HFD feeding. Compared with the control group, the abundance of Actinobacteria (P>0.05) and Bacteroidetes (P<0.001) decreased, and the abundance of Proteobacteria (P<0.01) and Firmicutes (P<0.001) increased in the HFD group (S6 Table). The ratio of Firmicutes to Bacteroidetes was increased (P<0.05) after HFD treatment. Supplementation with oil decreased (P<0.05) this ratio compared with the HFD group. HFD+M and the other six experimental treatments mitigated the HFD-induced increase in Firmicutes (P<0.05 HFD+KO600, HFD+FO600 and HFD+FO300KO300 vs the HFD group; P>0.05 HFD+F0400KO200 and HFD+FO450KO150 vs the HFD group) and reduction in Bacteroidetes (Fig 4A and S6 Table, P<0.01 HFD+FO400KO200, HFD+KO600 and HFD+FO300KO300 vs the HFD group; P>0.05 HFD+F0600 and HFD+FO450KO150 vs the HFD group).
Fig 4. RDP classification of the sequences.
(A) the phylum levels. (B) the class levels.
Within the phylum Firmicutes, Bacilli and Clostridia were the most common classes. The proportions of Clostridia (P<0.001) and Bacilli (P<0.01) increased after HFD treatment, while the ratio of Bacteroidia (P<0.001), the most abundant class of Bacteroides, reduced. The extra addition of krill oil, fish oil or their mixture partly restored the abundance of these three classes (Fig 4B and S7 Table).
Eight abundant genera (total DNA sequences with more than 1%) were identified in this study, of which four genera belonged to the phylum Bacteroidetes, two genera belonged to Firmicutes and two genera belonged to Proteobacteria. Among the 8 abundant genera, the frequencies of five genera increased after the HFD treatment (P<0.05), whereas the other three genera decreased (P<0.001). Furthermore, the different kinds of oil led to various effects on the genus abundance; detailed information is listed in S8 Table.
Key phenotypes responding to the oil treatment in HFD fed mice
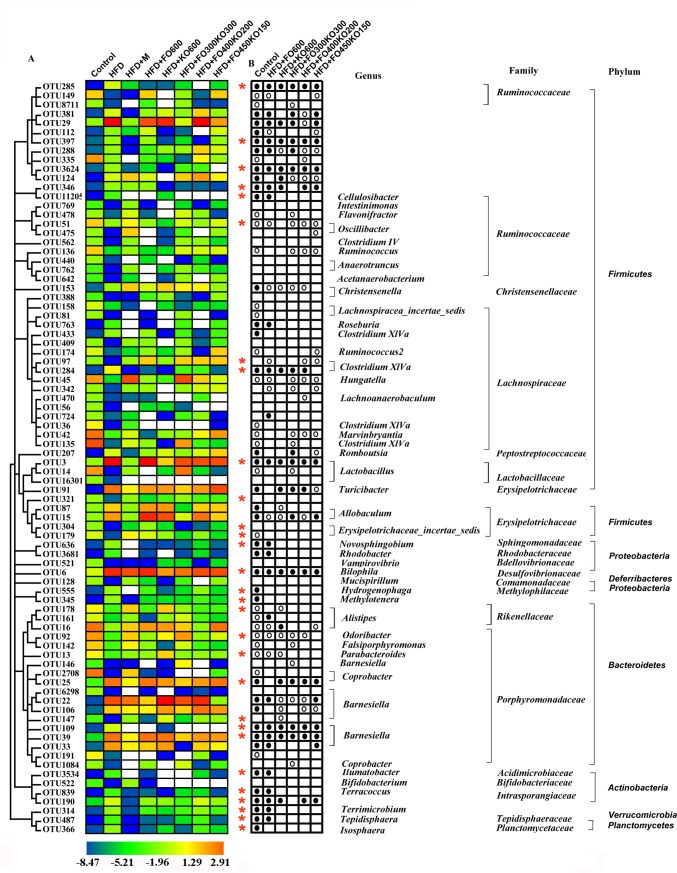
Next, we wanted to identify specific intestinal bacteria. The gut microbiota might mediate the beneficial effects of oil supplementation on HFD-induced obesity. We used redundancy analysis models to appraise specially designated bacterial phylotypes. Oil treatment (HFD group vs each HFD+oil group) and HFD feeding (HFD group vs control group) changed the abundance of these specific bacterial phylotypes. Altogether, the abundance levels of 82 OTUs were changed by HFD feeding (S9 and S10 Tables). Supplementation with FO600, KO600, FO300KO300, FO400KO200 and FO450KO250 altered the abundance of 25, 36, 31, 28 and 31 OTUs, respectively (Fig 5 and S10 Table). Notably, oil supplementation altered the changes of 29 (out of 82) OTUs caused by an HFD (Fig 5 and S10 Table). Eleven OTUs were altered in all five oil treatment groups among the 82 OTUs changed by HFD feeding. Spearman’s correlation analysis was conducted between these 82 OTUs and specific plasma biochemical indicators. In total, 19 OTUs were significantly correlated with at least one plasma biochemical indicator. Eleven OTUs were negatively correlated with obesity disease phenotypes, because they were associated with decreased TC, TG and LDL-C and increased HDL-C (Fig 6), while eight of the OTUs were positively correlated with obesity phenotypes. The abundance levels of 5 OTUs were altered by all the oil groups compared with the HFD group (Figs 5 and 6).
Fig 5. Eighty-two OTUs that were altered in abundance by an HFD and oil treatment based on redundancy analysis (RDA).
(A) The color of the heatmap represents the normalized and log-transformed relative abundance of 82 OTUs. Undetected OTUs are represented in white. According to Mann-Whitney test, the rows correspond to 36 OTUs that were reduced and 46 OTUs that were enriched in HFD group compared with control group. (B) The directional changes of the 82 OTUs under the HFD and oil treatment. Dots represent reduced and circles represent enriched abundance of OTUs in the control group and oil groups compared with the HFD group. A description of the taxonomy (genus, family and phylum) of the OTUs is on the right. The red asterisks (*) represent the OTUs whose abundance was changed by HFD (P<0.05) and then restored by oil treatment (P<0.05).
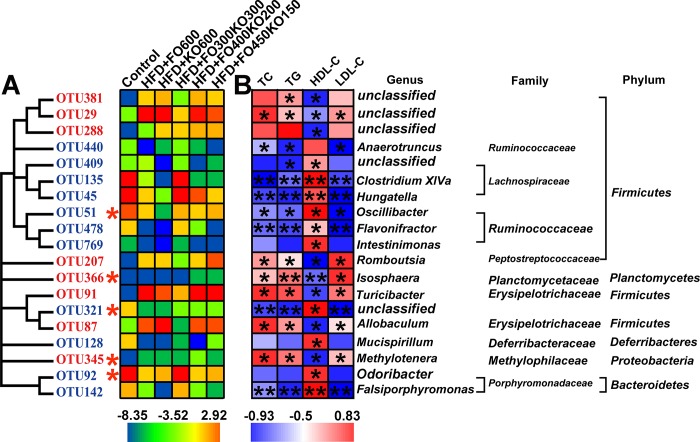
Fig 6. Nineteen oil-altered OTUs that were significantly correlated with host obesity parameters.
(A) The color of the heatmap represents the normalized and log-transformed relative abundance of 19 OTUs. The rows corresponding to the OTUs are labeled on the left, blue represents the OTUs were negative correlated with obesity phenotype, and red represents the OTUs were positive correlated with obesity. The red asterisks (*) represents the OTUs whose abundance was changed by HFD and then restored by oil treatment (P<0.05). (B) The correlation between host obesity parameters and 19 OTUs. The degree of association between host parameters and OTU abundance, as computed by Spearman correlation analysis, is expressed by the intensity of colors. In red/blue squares, asterisks show that this correlation is significant, **P<0.01 and *P<0.05. The right shows the taxonomy of the OTUs.
Notably, three of the eleven OTUs negatively correlated with obesity phenotypes were increased by oil treatments (Fig 6). These three OTUs included bacteria belonging to Hungatella (P<0.05), Oscillibacter (P<0.05) and Odoribacter (P<0.01). Similarly, two of the eight OTUs that were positively correlated with obesity phenotypes were decreased by all oil treatment groups (Fig 6). These OTUs included Isosphaera (P<0.05) and Methylotenera (P<0.05).
Discussion
This study examined the impact of dietary supplementation with fish oil, krill oil, or mixtures of the two on changes in body weight, adiposity index, liver index and gut microbiota in HFD-induced obese mice. A high-fat diet led to obesity in mice, with their body weight increasing 2.1-fold in 12 weeks (21.16±2.65 g at the starting point, 44.93±4.21 g at the endpoint). Dietary supplementation with FO300KO300 significantly reduced body weight gain, adiposity index and liver index. Just as we mentioned, restraining appetite is one of the mechanism for obesity management. In this study, the amount of food intake in different treatments had not been measured due to the actions like chew and molar generate a variable degree of food loss and waste, which make it difficult to accurately quantify the food intake. However, almost equal amounts of food surplus were observed in different treatments every day. On the other hand, previous studies found that dietary fish oil had no effect on appetite suppression [43], as well as the krill oil [44]. Appetite suppressed drugs might increase tolerance and dependency in a long-term clinical trials [11]. Therefore, we proposed that dietary supplementation of fish oil and/or krill oil is a much safer way to obesity alleviation.
In a previous study, Bashir et al suggested that fish oil, an oil rich in n-3 PUFA, can be used as an insulin sensitizer in some reports and can reduce the inflammatory state of adipose tissue and decrease insulin resistance in obese mice [45]. The latent capacity of long-chain n-3 PUFA against obesity-associated inflammation has been investigated in many studies [46]. Supplementation with DHA and EPA both revealed an anti-inflammatory effect with reduced high-sensitivity C-reactive protein, monocyte chemoattractant protein-1 and inflammatory mediators [47–50]. We observed that the body weight gain, liver index and adiposity index of the HFD+KO600 group were lower than those of the HFD+FO600 group. The content of EPA is higher in krill oil than in fish oil. EPA can increase the thromboxanes and 3-series prostaglandins, both of which play important anti-inflammatory roles [51]. However, in the HFD+FO300KO300 group, the ratio of EPA to DHA is closer to 1:1 compared with the other groups. Gabriel et al. proposed that, through lowering the production of the strong pro-inflammatory omega-6 arachidic acid, 2:1 and 1:1 EPA to DHA have more significant health benefits than 1:2 EPA to DHA [52].
Oil treatment was related to significant reductions in plasma TC, TG and HDL-C levels (Fig 2). The ratio of LDL-C to HDL-C is often used to assess the risk of coronary heart disease. In the HFD+FO300KO300 group, this ratio was significantly lower. Moreover, TC, TG and HDL-C levels in plasma were also significantly reduced by FO300KO300. These findings were consistent with a previous study of dietary krill oil and fish oil supplementation in high-fat-fed mice and overweight humans [53, 54]. A vast literature shows that fish oil and krill oil have potential benefits against chronic disorders such as cardiovascular diseases, endocannabinoid dysregulation, poor infant development, non-alcoholic fatty liver disease, premenstrual syndrome, inflammation and certain cancers [28, 55–57]. De Boer et al., suggest that n-3 polyunsaturated fatty acids reduce inflammation, partly by increasing adiponectin and reducing the intensity of adipocyte-macrophage cross-linking, to reduce obesity related diseases [58]. Haider et al., suggested that the preventive effect of krill oil was attributable to the synergistic action among n-3 PUFAs, phospholipids and astaxanthin [28]. However, several clinical studies reported that insulin resistance and glucose tolerance in patients with type 2 diabetes may be aggravated by high concentrations of n-3 PUFAs [59]. Mixtures of the two oils contain a more balanced concentration of n-3 polyunsaturated fatty acids (n-3 PUFA), which might be the reason for the prominent effect of HFD+FO300KO300 in controlling obesity. These data show that the krill oil and fish oil mixtures are effective in controlling blood lipids, making them promising candidates for hyperlipidemia treatment.
The oil treatment also altered the structures of the gut microbiota in mice and eight abundant genera (include some acetate-producing bacteria) were identified in this study (S8 Table). Acetate had been reported to inhibit obesity-related inflammation and body fat accumulation or rodent diabetes through a variety of mechanisms [60, 61]. The majority of acetate-producing bacteria, such as Barnesiella [62], decreased (P<0.001) in the HFD group compared with the control and restored (P<0.01) after oil treatment in the HFD+FO300KO300 and HFD+FO400KO200 groups. Oil also has a significant effect on a number of well-known probiotics, such as Lactobacillus [63, 64]. HFD treatment significantly increased the ratio of Lactobacillus (P<0.001), and the subsequently oil treatment restored the abundance of this bacteria (S8 Table), which is in agreement with previous studies that Lactobacillus increased in obese patients [65]. It is interesting to find that the abundance of the Helicobacter was reduced after the oil treatment, which is known as a pathogenic bacteria for peptic ulcer disease [66].
Consistent with previous individual studies of fish oil, we found that supplementation with krill oil and fish oil apparently changed the gut microbiota of HFD-induced obese mice [67]. Changes in the composition of the gut microbiota can be caused by various environmental disturbances, whereas the same environmental stress factors may elicit different responses from different bacterial species in the same genus [68]. Thus, identifications of the changes in the species-level is very important. In this study, the abundance change profiles of 19 OTUs showed correlations with the obesity phenotype. Eight OTUs showed positive correlations with the obesity phenotype, including OTU381, OTU29, OTU288, OTU207, OTU366, OTU91, OTU87 and OTU345. Among them, Allobaculum (OTU87) is one of the short-chain fatty acid (SCFA)-producing bacteria in the gut. SCFA-producing bacteria can benefit the host through mitigating inflammation, protecting the mucosa from damage induced by pathogens and may contribute to obesity, insulin resistance and the alleviation of inflammation by reducing the intestinal endotoxins into the blood [69, 70]. On the other hand, 11 out of 19 OTUs showed negative correlations with the obesity phenotype, including OTU440, OTU409, OTU2135, OTU45, OTU51, OTU478, OTU769, OTU321, OTU128, OTU92 and OTU142. In addition, we found that Oscillibacter (OTU51, P<0.05), which is involved in the development of metabolic disorders or pro-inflammatory processes associated with obesity, enriched by almost all of the oil groups [71, 72]. For other key variables that were altered by oil treatment such as Odoribacter (OTU92, P<0.05 vs the HFD group), Clostridium XlVa (OTU135, P<0.05 vs HFD group), and Flavonifractor (OTU478, P<0.05, HFD+FO600, HGD+FO300KO300 and HFD+FO400KO200 group vs HFD group). Odoribacter was significantly increased in BALB/c mice in response to grid floor stress [73]. Clostridium XlVa is proved to affect various aspects of host biology, including intestinal epithelial barrier maintenance and food decomposition [74]. Flavonifractor has been found significantly enriched in fecal samples from non-obese compared with the obese subjects [75]. However, the mechanism whereby they may alleviate obesity remains unknown.
In conclusion, our studies showed that the treatment of fish oil, krill oil and their mixtures lead to the obesity alleviation, as well as the gut microbiota modulation. Fish oil and krill oil supplementation decreased the body weight gain, adiposity index and liver index, increased the abundance of the genera Allobaculum, Odoribacter, Oscillibacter and Barnesiella in the gut microbiota and decreased the proportion of Lactobacillus. Obesity development and alleviation showed differences in the gut microbiota in this study, and further studies are needed to address the causality between them.
Supporting information
(PDF)
Data are represented as the means ± S.D and analyzed by ANOVA followed by Tukey post hoc test. *P<0.05, ** P<0.01 and ***P<0.001 vs the HFD group.
(PDF)
Data are expressed in terms of mean ± S.D and analyzed by ANOVA followed by Tukey post hoc test. ***P<0.001, **P<0.01 and *P<0.05 all group compared with the Control group.
(PDF)
(PDF)
Data are represented as the means ± S.D. and analyzed by Mann-Whitney test, **P<0.01 and *P<0.05 vs the HFD group.
(PDF)
Data are represented as the means ± S.D and analysed by Mann-Whitney test. ***P<0.001, **P<0.01 and *P<0.05 vs the HFD group.
(PDF)
Data are presented as the means ± S.D and analyzed by Mann-Whitney test, ***P<0.001, **P<0.01 and *P<0.05 vs the HFD group.
(PDF)
Data are presented as the means ± S.D and analyzed by Mann-Whitney test, ***P<0.001, **P<0.01 and *P<0.05 vs the HFD group.
(PDF)
(PDF)
Data are presented as the means ± S.D and analyzed by Mann-Whitney test, ***P<0.001, **P<0.01 and *P<0.05 vs the HFD group.
(PDF)
Data are presented as the means ± S.D. Each point represents the mean principal coordinate (PC) score of all mice in a group, and the error bar represents the S.D.
(TIF)
Data are presented as the means ± S.D. Each point represents the mean principal coordinate (PC) score of all mice in a group, and the error bar represents the S.D.
(TIF)
Acknowledgments
We thank Nature Research Editing Service for English language editing.
Data Availability
The sequences have been deposited in the NCBI Sequence Read Archive database under the accession number SRP101547.
Funding Statement
This work was supported by the the Key Specialty of Ningbo City (http://www.nbu.edu.cn) to XRS, the Talent project in Ningbo University (http://www.nbu.edu.cn) to XRS, the K.C.Wong Magna Fund in Ningbo University (http://www.nbu.edu.cn) to CYL and XRS.
References
- 1.Antelo M, Magdalena P, Reboredo JC. Obesity: A major problem for Spanish minors. Econ Hum Biol. 2017;24:61–73. doi: 10.1016/j.ehb.2016.11.002 . [DOI] [PubMed] [Google Scholar]
- 2.Eknoyan G. Obesity, diabetes, and chronic kidney disease. Curr Diab Rep. 2007;7(6):449–53. . [DOI] [PubMed] [Google Scholar]
- 3.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and Trends in Obesity Among US Adults, 1999–2008. Jama-J Am Med Assoc. 2010;303(3):235–41. doi: 10.1001/jama.2009;2014 [DOI] [PubMed] [Google Scholar]
- 4.Zhang X, Zhao Y, Xu J, Xue Z, Zhang M, Pang X, et al. Modulation of gut microbiota by berberine and metformin during the treatment of high-fat diet-induced obesity in rats. Sci Rep. 2015;5(6):14005 doi: 10.1038/srep14405 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maraninchi M, Padilla N, Béliard S, Berthet B, Nogueira JP, Dupont-Roussel J, et al. Impact of bariatric surgery on apolipoprotein C-III levels and lipoprotein distribution in obese human subjects. J Clin Lipidol. 2017;11(2):495–506. doi: 10.1016/j.jacl.2017.02.012 . [DOI] [PubMed] [Google Scholar]
- 6.Dangol M, Kim S, Li CG, Fakhraei LS, Jang M, Ma Y, et al. Anti-obesity effect of a novel caffeine-loaded dissolving microneedle patch in high-fat diet-induced obese C57BL/6J mice. J. Controlled Release. 2017. doi: 10.1016/j.jconrel.2017.03.400 . [DOI] [PubMed] [Google Scholar]
- 7.Wang B, Zhang S, Wang X, Yang S, Jiang Q, Xu Y, et al. Transcriptome analysis of the effects of chitosan on the hyperlipidemia and oxidative stress in high-fat diet fed mice. Int J Biol Macromol. 2017;102:104–10. doi: 10.1016/j.ijbiomac.2017.03.187 . [DOI] [PubMed] [Google Scholar]
- 8.Zhao JL, Zhao YY, Zhu WJ. A high-fat, high-protein diet attenuates the negative impact of casein-induced chronic inflammation on testicular steroidogenesis and sperm parameters in adult mice. Gen Comp Endocrinol. 2017;252:48–59. doi: 10.1016/j.ygcen.2017.07.013 . [DOI] [PubMed] [Google Scholar]
- 9.Cho IJ, Choung SY, Hwang YC, Ahn KJ, Chung HY, Jeong IK. Aster spathulifolius Maxim extract reduces body weight and fat mass in obese humans. Nutr Res. 2016;36(7):671–8. doi: 10.1016/j.nutres.2016.03.001 . [DOI] [PubMed] [Google Scholar]
- 10.Silventoinen K, Rokholm B, Kaprio J, Sørensen TI, editors. The genetic and environmental influences on childhood obesity: a systematic review of twin and adoption studies. Int J Obes. 2010;34(1): 29–40. doi: 10.1038/ijo.2009.177 . [DOI] [PubMed] [Google Scholar]
- 11.Torres-Fuentes C, Schellekens H, Dinan TG, Cryan JF. A natural solution for obesity: bioactives for the prevention and treatment of weight gain. A review. Nutr Neurosci. 2015;18(2):49–65. doi: 10.1179/1476830513Y.0000000099 . [DOI] [PubMed] [Google Scholar]
- 12.Albuquerque D, Stice E, Rodriguez-Lopez R, Manco L, Nobrega C. Current review of genetics of human obesity: from molecular mechanisms to an evolutionary perspective. Mol Genet Genomics. 2015;290(4):1191–221. doi: 10.1007/s00438-015-1015-9 . [DOI] [PubMed] [Google Scholar]
- 13.Connolly HM, Crary JL, Mcgoon MD, Hensrud DD, Edwards BS, Edwards WD, et al. Valvular heart disease associated with fenfluramine-phentermine. N Engl J Med. 1997;337(24):1775–6. doi: 10.1056/NEJM199708283370901 . [DOI] [PubMed] [Google Scholar]
- 14.Zhang WL, Zhu L, Jiang JG. Active ingredients from natural botanicals in the treatment of obesity. Obes Rev. 2014;15(12):957–67. doi: 10.1111/obr.12228 . [DOI] [PubMed] [Google Scholar]
- 15.Fei N, Zhao L. An opportunistic pathogen isolated from the gut of an obese human causes obesity in germfree mice. ISME J. 2013;7(4):880–4. doi: 10.1038/ismej.2012.153 ; PubMed Central PMCID: PMC3603399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norris GH, Jiang C, Ryan J, Porter CM, Blesso CN. Milk sphingomyelin improves lipid metabolism and alters gut microbiota in high fat diet-fed mice. J Nutr Biochem. 2016;30(38):93–101. doi: 10.1016/j.jnutbio.2015.12.003 . [DOI] [PubMed] [Google Scholar]
- 17.Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102(31):11070–5. doi: 10.1073/pnas.0504978102 ; PubMed Central PMCID: PMC1176910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55–60. doi: 10.1038/nature11450 . [DOI] [PubMed] [Google Scholar]
- 19.Vrieze A, Van NE, Holleman F, Salojärvi J, Kootte RS, Bartelsman JF, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143(4):913–6. doi: 10.1053/j.gastro.2012.06.031 . [DOI] [PubMed] [Google Scholar]
- 20.Shang QS, Song GR, Zhang MF, Shi JJ, Xu CY, Hao JJ, et al. Dietary fucoidan improves metabolic syndrome in association with increased Akkermansia population in the gut microbiota of high-fat diet-fed mice. J Funct Foods. 2017;28:138–46. doi: 10.1016/j.jff.2016.11.002 [Google Scholar]
- 21.Zhang C, Yin A, Li H, Wang R, Wu G, Shen J, et al. Dietary modulation of gut microbiota contributes to alleviation of both genetic and simple obesity in children ☆. Ebiomedicine. 2015;2(8):968–84. doi: 10.1016/j.ebiom.2015.07.007 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao L. The gut microbiota and obesity: from correlation to causality. Nature Reviews Microbiology. 2013;11(9):639–47. doi: 10.1038/nrmicro3089 . [DOI] [PubMed] [Google Scholar]
- 23.Li BZ, Truong T, Bhandari B. Crystallization and melting properties of mixtures of milk fat stearin and omega-3 rich oils. Food Chem. 2017;218:199–206. doi: 10.1016/j.foodchem.2016.09.059 . [DOI] [PubMed] [Google Scholar]
- 24.Yamazaki RK, Brito GA, Coelho I, Pequitto DC, Yamaguchi AA, Borghetti G, et al. Low fish oil intake improves insulin sensitivity, lipid profile and muscle metabolism on insulin resistant MSG-obese rats. Lipids Health Dis. 2011;10(1):66 doi: 10.1186/1476-511X-10-66 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bargut TC, Mandarim-de-Lacerda CA, Aguila MB. A high-fish-oil diet prevents adiposity and modulates white adipose tissue inflammation pathways in mice. J Nutr Biochem. 2015;26(9):960–9. doi: 10.1016/j.jnutbio.2015.04.002 . [DOI] [PubMed] [Google Scholar]
- 26.Todoric J, Loffler M, Huber J, Bilban M, Reimers M, Kadl A, et al. Adipose tissue inflammation induced by high-fat diet in obese diabetic mice is prevented by n-3 polyunsaturated fatty acids. Diabetologia. 2006;49(9):2109–19. doi: 10.1007/s00125-006-0300-x . [DOI] [PubMed] [Google Scholar]
- 27.Le Grandois J, Marchioni E, Zhao M, Giuffrida F, Ennahar S, Bindler F. Investigation of natural phosphatidylcholine sources: separation and identification by liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS2) of molecular species. J Agric Food Chem. 2009;57(14):6014–20. doi: 10.1021/jf900903e . [DOI] [PubMed] [Google Scholar]
- 28.Haider J, Majeed H, Williams PA, Safdar W, Zhong F. Formation of chitosan nanoparticles to encapsulate krill oil (Euphausia superba) for application as a dietary supplement. Food Hydrocolloids. 2016;63:27–34. [Google Scholar]
- 29.Lee MF, Lai CS, Cheng AC, Hou JS, Badmaev V, Ho CT, et al. Krill oil and xanthigen separately inhibit high fat diet induced obesity and hepatic triacylglycerol accumulation in mice. J Funct Foods. 2014;19:913–21. [Google Scholar]
- 30.Burri L, Berge K, Wibrand K, Berge RK, Barger JL. Differential effects of krill oil and fish oil on the hepatic transcriptome in mice. Front Genet. 2011;2:45 doi: 10.3389/fgene.2011.00045 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hasan M, Seo JE, Asiqur RK, Min H, Hun KK, Park JH, et al. Novel genes in brain tissues of EAE-induced normal and obese mice: Upregulation of metal ion-binding protein genes in obese-EAE mice. Neuroscience. 2016;343:322–36. doi: 10.1016/j.neuroscience.2016.12.002 . [DOI] [PubMed] [Google Scholar]
- 32.Choi BK, Park SB, Lee DR, Lee HJ, Jin YY, Yang SH, et al. Green coffee bean extract improves obesity by decreasing body fat in high-fat diet-induced obese mice. Asian Pac J Trop Med 2016;9(7):635–43. doi: 10.1016/j.apjtm.2016.05.017 . [DOI] [PubMed] [Google Scholar]
- 33.Qinfang Luo, Ling Chen, Nanshan Zhong, The First Affiliated Hospital of Guangzhou Medical University. Mouse anesthesia apparatus. China patent CN103976804A. 2014 Aug 13.
- 34.Satil F, Azcan N, Baser KHC. Fatty acid composition of pistachio nuts in Turkey. Chen Nat Compd. 2003;39(4):322–4. doi: 10.1023/B:Conc.0000003408.63300.B5 [Google Scholar]
- 35.Lópezcervantes J, Sánchezmachado DI, Gutiérrezcoronado MA, Ríosvázquez NJ. Quantification of astaxanthin in shrimp waste hydrolysate by HPLC. Biomed Chromatogr. 2006;20(10):981–4. doi: 10.1002/bmc.676 . [DOI] [PubMed] [Google Scholar]
- 36.Z Y M M. Improved extraction of PCR-quality community DNA from digesta and fecal samples. Biotechniques. 2004;36(5):808–12. [DOI] [PubMed] [Google Scholar]
- 37.Kuczynski J, Stombaugh J, Walters WA, González A, Caporaso JG, Knight R. Using QIIME to analyze 16S rRNA gene sequences from microbial communities. Curr Protoc Bioinformatics. 2011;Chapter 10:Unit 10.7. doi: 10.1002/0471250953.bi1007s36 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Magoč T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27(21):2957–63. doi: 10.1093/bioinformatics/btr507 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27(16):2194–200. doi: 10.1093/bioinformatics/btr381 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26(19):2460–1. doi: 10.1093/bioinformatics/btq461 [DOI] [PubMed] [Google Scholar]
- 41.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75(23):7537–41. doi: 10.1128/AEM.01541-09 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Appl Environ Microbiol. 2007;73(16):5261–7. doi: 10.1128/AEM.00062-07 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iizuka Y, Kim H, Nakasatomi M, Izawa T, Hirako S, Matsumoto A. Fish oil prevents excessive accumulation of subcutaneous fat caused by an adverse effect of pioglitazone treatment and positively changes adipocytes in KK mice. Toxicology Reports. 2016;3(C):4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu C, Sun T, Li Y, Zhang D, Zhou J, Su X. Modulation of the Gut Microbiota by Krill Oil in Mice Fed a High-Sugar High-Fat Diet. Front Microbiol. 2017;8:905 doi: 10.3389/fmicb.2017.00905 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bashir S, Sharma Y, Elahi A, Khan F. Amelioration of obesity-associated inflammation and insulin resistance in c57bl/6 mice via macrophage polarization by fish oil supplementation. J Nutr Biochem. 2016;33:82–90. doi: 10.1016/j.jnutbio.2016.02.011 . [DOI] [PubMed] [Google Scholar]
- 46.Mehra MR, Lavie CJ, Ventura HO, Milani RV. Fish oils produce anti-inflammatory effects and improve body weight in severe heart failure. J Heart Lung Transplant. 2006;25(7):834–8. doi: 10.1016/j.healun.2006.03.005 . [DOI] [PubMed] [Google Scholar]
- 47.Kalupahana NS, Claycombe K, Newman SJ, Stewart T, Siriwardhana N, Matthan N, et al. Eicosapentaenoic acid prevents and reverses insulin resistance in high-fat diet-induced obese mice via modulation of adipose tissue inflammation. J Nutr. 2010;140(11):1915–22. doi: 10.3945/jn.110.125732 . [DOI] [PubMed] [Google Scholar]
- 48.Micallef MA, Garg ML. Anti-inflammatory and cardioprotective effects of n-3 polyunsaturated fatty acids and plant sterols in hyperlipidemic individuals. Atherosclerosis. 2009;204(2):476–82. doi: 10.1016/j.atherosclerosis.2008.09.020 . [DOI] [PubMed] [Google Scholar]
- 49.Ibrahim A, Mbodji K, Hassan A, Aziz M, Boukhettala N, Coeffier M, et al. Anti-inflammatory and anti-angiogenic effect of long chain n-3 polyunsaturated fatty acids in intestinal microvascular endothelium. Clin Nutr. 2011;30(5):678–87. doi: 10.1016/j.clnu.2011.05.002 . [DOI] [PubMed] [Google Scholar]
- 50.Serini S, Bizzarro A, Piccioni E, Fasano E, Rossi C, Lauria A, et al. EPA and DHA differentially affect in vitro inflammatory cytokine release by peripheral blood mononuclear cells from Alzheimer's patients. Curr Alzheimer Res. 2012;9(8):913–23. . [DOI] [PubMed] [Google Scholar]
- 51.Bagga D, Wang L, Farias-Eisner R, Glaspy JA, Reddy ST. Differential effects of prostaglandin derived from omega-6 and omega-3 polyunsaturated fatty acids on COX-2 expression and IL-6 secretion. Proc Natl Acad Sci U S A. 2003;100(4):1751–6. doi: 10.1073/pnas.0334211100 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dasilva G, Pazos M, Garcia-Egido E, Perez-Jimenez J, Torres JL, Giralt M, et al. Lipidomics to analyze the influence of diets with different EPA: DHA ratios in the progression of Metabolic Syndrome using SHROB rats as a model. Food Chem. 2016;205:196–203. doi: 10.1016/j.foodchem.2016.03.020 . [DOI] [PubMed] [Google Scholar]
- 53.Nascimento FA, Barbosa-da-Silva S, Fernandes-Santos C, Mandarim-de-Lacerda CA, Aguila MB. Adipose tissue, liver and pancreas structural alterations in C57BL/6 mice fed high-fat-high-sucrose diet supplemented with fish oil (n-3 fatty acid rich oil). Exp Toxicol Pathol. 2010;62(1):17–25. doi: 10.1016/j.etp.2008.12.008 . [DOI] [PubMed] [Google Scholar]
- 54.Burri L, Berge K, Wibrand K, Berge RK, Barger JL. Differential Effects of Krill Oil and Fish Oil on the Hepatic Transcriptome in Mice. Front Genet. 2011;2:45 doi: 10.3389/fgene.2011.00045 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruiz-Núñez B, Pruimboom L, Dijck-Brouwer DA, Muskiet FA. Lifestyle and nutritional imbalances associated with Western diseases: causes and consequences of chronic systemic low-grade inflammation in an evolutionary context. J Nutr Biochem. 2013;24(7):1183–201. doi: 10.1016/j.jnutbio.2013.02.009 . [DOI] [PubMed] [Google Scholar]
- 56.Calder PC. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim Biophys Acta. 2015;1851(4):469–84. doi: 10.1016/j.bbalip.2014.08.010 . [DOI] [PubMed] [Google Scholar]
- 57.Tur JA, Bibiloni MM, Sureda A, Pons A. Dietary sources of omega 3 fatty acids: public health risks and benefits. Br J Nutr. 2012;107 Suppl 2(S2):S23–52. doi: 10.1017/S0007114512001456 . [DOI] [PubMed] [Google Scholar]
- 58.De Boer AA, Monk JM, Liddle DM, Hutchinson AL, Power KA, Ma DW, et al. Fish-oil-derived n-3 polyunsaturated fatty acids reduce NLRP3 inflammasome activity and obesity-related inflammatory cross-talk between adipocytes and CD11b(+) macrophages. J Nutr Biochem. 2016;34:61–72. doi: 10.1016/j.jnutbio.2016.04.004 . [DOI] [PubMed] [Google Scholar]
- 59.Friday KE, Childs MT, Tsunehara CH, Fujimoto WY, Bierman EL, Ensinck JW. Elevated plasma glucose and lowered triglyceride levels from omega-3 fatty acid supplementation in type II diabetes. Diabetes Care. 1989;12(4):276–81. . [DOI] [PubMed] [Google Scholar]
- 60.Motoshima H, Hanatani S, Takaki Y, Kawasaki S, Igata M, Senokuchi T, et al. Potent anti-obesity effect of acetate; acetate may alter the expression of genes involved in beige adipogenesis in obese KK-Ay mice. Diabetes Res Clin Pract. 2016;120:S83–S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carvalho BM, Guadagnini D, Tsukumo DM, Schenka AA, Latuf-Filho P, Vassallo J, et al. Modulation of gut microbiota by antibiotics improves insulin signalling in high-fat fed mice. Diabetologia. 2012;55(10):2823–34. doi: 10.1007/s00125-012-2648-4 . [DOI] [PubMed] [Google Scholar]
- 62.Sakamoto M, Lan PT, Benno Y. Barnesiella viscericola gen. nov., sp. nov., a novel member of the family Porphyromonadaceae isolated from chicken caecum. Int J Syst Evol Microbiol. 2007;57(Pt 2):342–6. doi: 10.1099/ijs.0.64709-0 . [DOI] [PubMed] [Google Scholar]
- 63.Everard A, Lazarevic V, Gaïa N, Johansson M, Ståhlman M, Backhed F, et al. Microbiome of prebiotic-treated mice reveals novel targets involved in host response during obesity. ISME J. 2014;8(10):2116–30. doi: 10.1038/ismej.2014.45 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang X, Zhao Y, Zhang M, Pang X, Xu J, Kang C, et al. Structural Changes of Gut Microbiota during Berberine-Mediated Prevention of Obesity and Insulin Resistance in High-Fat Diet-Fed Rats. PLoS One. 2012;7(8):e42529 doi: 10.1371/journal.pone.0042529 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Armougom F, Henry M, Vialettes B, Raccah D, Raoult D. Monitoring Bacterial Community of Human Gut Microbiota Reveals an Increase in Lactobacillus in Obese Patients and Methanogens in Anorexic Patients. PLoS One. 2009;4(9):e7125 doi: 10.1371/journal.pone.0007125 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sipponen P, Hyvarinen H. Role of Helicobacter pylori in the pathogenesis of gastritis, peptic ulcer and gastric cancer. Scand J Gastroenterol Suppl. 1993;196(Supp 196):3–6. . [DOI] [PubMed] [Google Scholar]
- 67.Yu HN, Zhu J, Pan WS, Shen SR, Shan WG, Das UN. Effects of fish oil with a high content of n-3 polyunsaturated fatty acids on mouse gut microbiota. Arch Med Res. 2014;45(3):195–202. doi: 10.1016/j.arcmed.2014.03.008 . [DOI] [PubMed] [Google Scholar]
- 68.Wang J, Tang H, Zhang C, Zhao Y, Derrien M, Rocher E, et al. Modulation of gut microbiota during probiotic-mediated attenuation of metabolic syndrome in high fat diet-fed mice. ISME J. 2015;9(1):1–15. doi: 10.1038/ismej.2014.99 ; PubMed Central PMCID: PMC4274436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ng A. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461(7268):1282–6. doi: 10.1038/nature08530 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Filippo CD, Cavalieri D, Paola MD, Ramazzotti M, Poullet JB, Massart S, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010;107(33):14691–6. doi: 10.1073/pnas.1005963107 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yan YL, Ha CWY, Campbell CR, Mitchell AJ, Dinudom A, Oscarsson J, et al. Increased Gut Permeability and Microbiota Change Associate with Mesenteric Fat Inflammation and Metabolic Dysfunction in Diet-Induced Obese Mice. PLoS One.2012;7(3):e34233 doi: 10.1371/journal.pone.0034233 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tuovinen E, Keto J, Nikkila J, Matto J, Lahteenmaki K. Cytokine response of human mononuclear cells induced by intestinal Clostridium species. Anaerobe. 2013;19(1):70–6. doi: 10.1016/j.anaerobe.2012.11.002 . [DOI] [PubMed] [Google Scholar]
- 73.Maria BBK, Lukasz K, Bratbo SD, Pang W, Sandris ND, Knud J, et al. Gut Microbiota Composition Is Correlated to Grid Floor Induced Stress and Behavior in the BALB/c Mouse. PLoS One. 2012;7(10):e46231 doi: 10.1371/journal.pone.0046231 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Honda K, Tanoue T, Nagano Y, Atarashi K. Microbiota's Influence on Immunity. Else Kroner-Fresenius Symposia. 2013;4(2):43–7. [Google Scholar]
- 75.Kläring K, Hanske L, Bui N, Charrier C, Blaut M, Haller D, et al. Intestinimonas butyriciproducens gen. nov., sp. nov., a butyrate-producing bacterium from the mouse intestine. Int J Syst Evol Microbiol. 2013;63(12):4606–12. doi: 10.1099/ijs.0.051441–0 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data are represented as the means ± S.D and analyzed by ANOVA followed by Tukey post hoc test. *P<0.05, ** P<0.01 and ***P<0.001 vs the HFD group.
(PDF)
Data are expressed in terms of mean ± S.D and analyzed by ANOVA followed by Tukey post hoc test. ***P<0.001, **P<0.01 and *P<0.05 all group compared with the Control group.
(PDF)
(PDF)
Data are represented as the means ± S.D. and analyzed by Mann-Whitney test, **P<0.01 and *P<0.05 vs the HFD group.
(PDF)
Data are represented as the means ± S.D and analysed by Mann-Whitney test. ***P<0.001, **P<0.01 and *P<0.05 vs the HFD group.
(PDF)
Data are presented as the means ± S.D and analyzed by Mann-Whitney test, ***P<0.001, **P<0.01 and *P<0.05 vs the HFD group.
(PDF)
Data are presented as the means ± S.D and analyzed by Mann-Whitney test, ***P<0.001, **P<0.01 and *P<0.05 vs the HFD group.
(PDF)
(PDF)
Data are presented as the means ± S.D and analyzed by Mann-Whitney test, ***P<0.001, **P<0.01 and *P<0.05 vs the HFD group.
(PDF)
Data are presented as the means ± S.D. Each point represents the mean principal coordinate (PC) score of all mice in a group, and the error bar represents the S.D.
(TIF)
Data are presented as the means ± S.D. Each point represents the mean principal coordinate (PC) score of all mice in a group, and the error bar represents the S.D.
(TIF)
Data Availability Statement
The sequences have been deposited in the NCBI Sequence Read Archive database under the accession number SRP101547.
The sequences have been deposited in the NCBI Sequence Read Archive database under the accession number SRP101547.