Abstract
Drosophila segmentation is a well-established paradigm for developmental pattern formation. However, the later stages of segment patterning, regulated by the “pair-rule” genes, are still not well understood at the system level. Building on established genetic interactions, I construct a logical model of the Drosophila pair-rule system that takes into account the demonstrated stage-specific architecture of the pair-rule gene network. Simulation of this model can accurately recapitulate the observed spatiotemporal expression of the pair-rule genes, but only when the system is provided with dynamic “gap” inputs. This result suggests that dynamic shifts of pair-rule stripes are essential for segment patterning in the trunk and provides a functional role for observed posterior-to-anterior gap domain shifts that occur during cellularisation. The model also suggests revised patterning mechanisms for the parasegment boundaries and explains the aetiology of the even-skipped null mutant phenotype. Strikingly, a slightly modified version of the model is able to pattern segments in either simultaneous or sequential modes, depending only on initial conditions. This suggests that fundamentally similar mechanisms may underlie segmentation in short-germ and long-germ arthropods.
Author summary
Segmentation in insects involves the division of the body into several repetitive units. In Drosophila embryos, all segments are patterned rapidly and simultaneously during early development, in a process known as “long-germ” embryogenesis. In contrast, many insect embryos retain an ancestral or “short-germ” mode of development, in which segments are patterned sequentially, from head to tail, over a period of time. In both types of embryo, the patterning of segment boundaries is regulated by a network of so-called “pair-rule” genes. These networks are thought to be quite divergent due to the different expression patterns observed for the pair-rule genes in each case: regularly spaced arrays of transient stripes in Drosophila, and dynamic expression within a posterior “segment addition zone” in short-germ insects. However, even in Drosophila, a clear understanding of pair-rule patterning has been lacking. Here, I make a computational model of the Drosophila pair-rule network and use simulations to explore how segmentation works. Surprisingly, I find that Drosophila segment patterning relies on pair-rule gene expression moving across cells over time. This conclusion differs from older models of pair-rule patterning but is consistent with the subtly dynamic nature of pair-rule stripes in real embryos, previously described in quantitative studies. I conclude that long-germ and short-germ segmentation involve similar expression dynamics at the level of individual cells, even though they look very different at the level of whole tissues. This suggests that the gene networks involved may be much more conserved than previously thought.
Introduction
Like other arthropods, the fruit fly Drosophila melanogaster has a segmented body plan. This segmental pattern is laid down in the embryo during the first 3 hours of development. During this time, the anteroposterior (AP) axis of the blastoderm is progressively patterned down to cellular-level resolution by an elaborate, multi-tiered network of genes and their encoded transcription factors [1,2]. These genes were first identified in a landmark genetic screen [3,4], and their regulatory interactions have subsequently been dissected by 3 decades of genetic experiments. Along the way, this body of research has revealed many fundamental principles of transcriptional regulation [5], and Drosophila segmentation remains a central model for developmental systems biology today.
Much of the “heavy lifting” of segment patterning is carried out by the so-called “pair-rule” genes, which make up the penultimate tier of the Drosophila segmentation cascade. The pair-rule genes are the first genes to be expressed in spatially periodic patterns in the Drosophila embryo and are collectively responsible for patterning the expression of the “segment-polarity” genes, which organise and maintain segmentally reiterated compartment boundaries termed “parasegment boundaries”. Notably, this involves transducing a double segment pattern of early pair-rule gene expression, in which each set of stripes is offset slightly from the others, into a single-segment pattern of segment-polarity gene expression, in which most genes are expressed in discrete, non-overlapping domains [6–8].
There are 7 canonical pair-rule genes: hairy [9], even-skipped (eve) [10], runt [11], fushi tarazu (ftz) [12], odd-skipped (odd) [13], paired (prd) [14], and sloppy-paired (slp) [15]. 5 of these genes (hairy, eve, runt, ftz, and odd) are known as the “primary” pair-rule genes because they are expressed earlier than the 2 “secondary” pair-rule genes, prd and slp [16]. (Note that these terms have a somewhat tortuous history, and older literature will classify the genes differently.)
Each of the primary pair-rule genes is initially patterned by spatial inputs from the upstream tier of transcription factors, encoded by the “gap” genes, which are expressed in broad, overlapping AP domains during cellularisation [17]. This patterning occurs in an ad hoc manner, with specific combinations of gap factors regulating the expression of particular pair-rule stripes through discrete “stripe-specific” enhancer elements [18–21], which act additively with one another. For certain pair-rule genes, such as eve, this regulation is sufficient to generate an overall pattern of 7 equally spaced stripes along the future trunk of the embryo [16,22–24]. For other pair-rule genes, such as odd, the gap-driven pattern is irregular and may have missing stripes [16]. In these cases, the initial patterns are regularised by cross-regulatory “zebra” enhancer elements [25–27], which take periodic inputs from other pair-rule factors and yield periodic outputs. Similar zebra elements are responsible for driving the periodic expression of the secondary pair-rule genes, which turn on after the primary pair-rule patterns have refined [16,28].
At gastrulation, the segment-polarity genes turn on, activated by a broadly expressed transcription factor, Odd-paired (Opa) [29], and spatially regulated by the pair-rule genes [6,7,30,31]. Opa activity also “rewires” the regulatory interactions between the pair-rule genes, causing several of their expression patterns to transition dynamically from double- to single-segment periodicity (i.e., from 7 stripes to 14 stripes) [32]. These pair-rule factors (and/or their paralogs) then play roles in the segment-polarity network, which also contains several components of the Wingless (Wg) and Hedgehog signalling pathways [33–37].
The Drosophila gap gene network has been used frequently in recent years as a case study for the application of dynamical systems [38–40] and information theory [41–43] approaches to developmental patterning, but the pair-rule network has received little system-level attention. Indeed, the most recent models of pair-rule patterning [8,44] date from more than 10 years ago. Since these were published, 3 important discoveries have been made about segment patterning, all of which challenge established assumptions about the Drosophila segmentation cascade and all of which concern the pair-rule genes in some way. So long as the pair-rule network remains poorly understood, key questions raised by these findings will go unanswered.
The first discovery is from comparative studies in other arthropod embryos. Drosophila is a “long-germ” embryo, patterning almost all of its segments simultaneously in the blastoderm prior to germ-band extension [45]. However, the ancestral mode of arthropod development is “short-germ” embryogenesis, in which segmentation is sequential and coordinated with germ-band elongation [46–48]. Orthologs of the pair-rule genes play a key role in segment patterning in all arthropods studied thus far (for example, [49–52]), but in short-germ embryos, their expression has been shown to oscillate in a posterior “segment addition zone” throughout germ-band extension [53–55]. This periodic dynamic expression indicates that in these organisms, they are either components of or entrained by a segmentation “clock” [56]. How the expression of the pair-rule genes in long-germ embryos such as Drosophila relates to their expression in short-germ embryos (for example, the model beetle, Tribolium castaneum) is unclear. It is thus not understood how long-germ segmentation was derived from short-germ segmentation, an important evolutionary transition that has occurred multiple times independently within the higher insects [57].
The second discovery stems from quantitative studies of Drosophila segmentation gene dynamics. These studies have revealed that the domains of gap gene expression in the trunk of the embryo shift anteriorly across the blastoderm over the course of nuclear division cycle 14 (cellularisation) [58–60]. The shifts are mirrored downstream in similarly shifting expression of the pair-rule stripes [59,61], a finding that is at odds with existing models of segment patterning, which assume these stripes to be static domains [6,8,44,62–64]. While we know that these subtle shifts are ultimately driven by feedback interactions within the gap gene network [38,39,65–67], their functional role (if any) remains unclear.
The final key finding relates to the structure of the pair-rule network itself. In a recent paper on the pair-rule network [32], Michael Akam and I showed that many of the regulatory interactions between the pair-rule genes are temporally regulated (by Opa, as described above). We argued that the pair-rule network is best viewed as 2 distinct networks, 1 operating during cellularisation and 1 during gastrulation, each with a specific topology and resultant dynamics. Analysing the “early” (cellularisation-stage) and “late” (gastrulation-stage) pair-rule networks separately should lead to a better understanding of pair-rule patterning and might also reveal why the network shows this bipartite organisation in the first place.
In this paper, I present a new model of the pair-rule system, which incorporates the stage-specific architecture of the pair-rule network. I take the set of identified genetic interactions between the pair-rule genes as a starting assumption, formalise them in a Boolean logical model, and use dynamical simulations to analyse how they collectively lead to complex pattern formation. I find that gap-mediated kinematic shifts are required for correctly phasing the pair-rule stripes, something that proves crucial for downstream segment patterning. I also find that graded Eve activity is not strictly necessary for pair-rule patterning, and I explain the aetiology of the surprisingly severe eve null mutant phenotype. Finally, I show that a slightly modified version of the Drosophila pair-rule network gains the capacity to pattern in both simultaneous and sequential modes, conceptually reconciling long- and short-germ segmentation.
Results
Topology of the pair-rule network
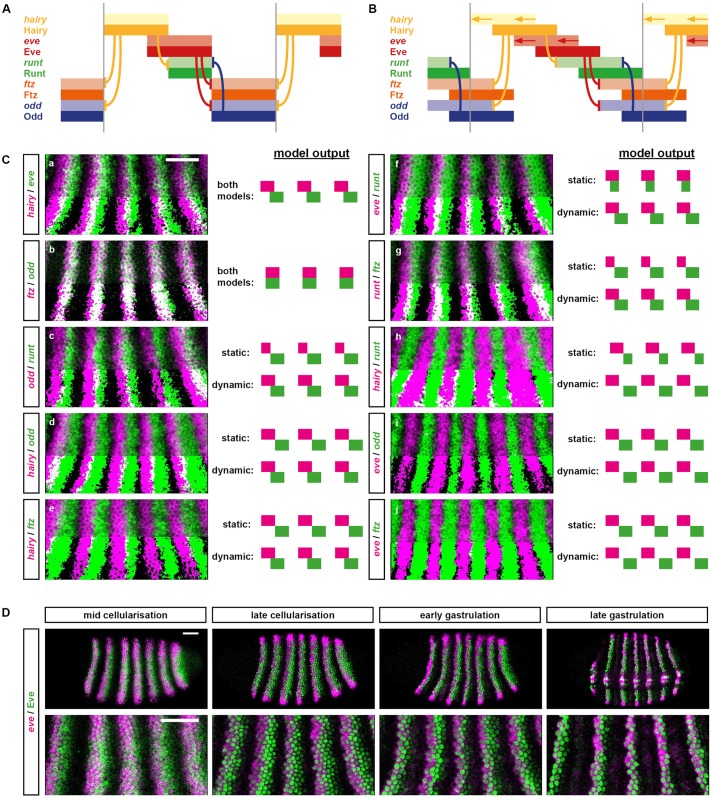
Fig 1A summarises the inferred regulatory interactions between the pair-rule genes. Following Clark and Akam (2016) [32], individual interactions are assigned to distinct “early” and “late” networks, which operate during mid-cellularisation or late cellularisation/gastrulation, respectively. Note that a few regulatory interactions (e.g., repression of ftz by Eve) are common to both networks, but the majority are restricted to a single phase of patterning.
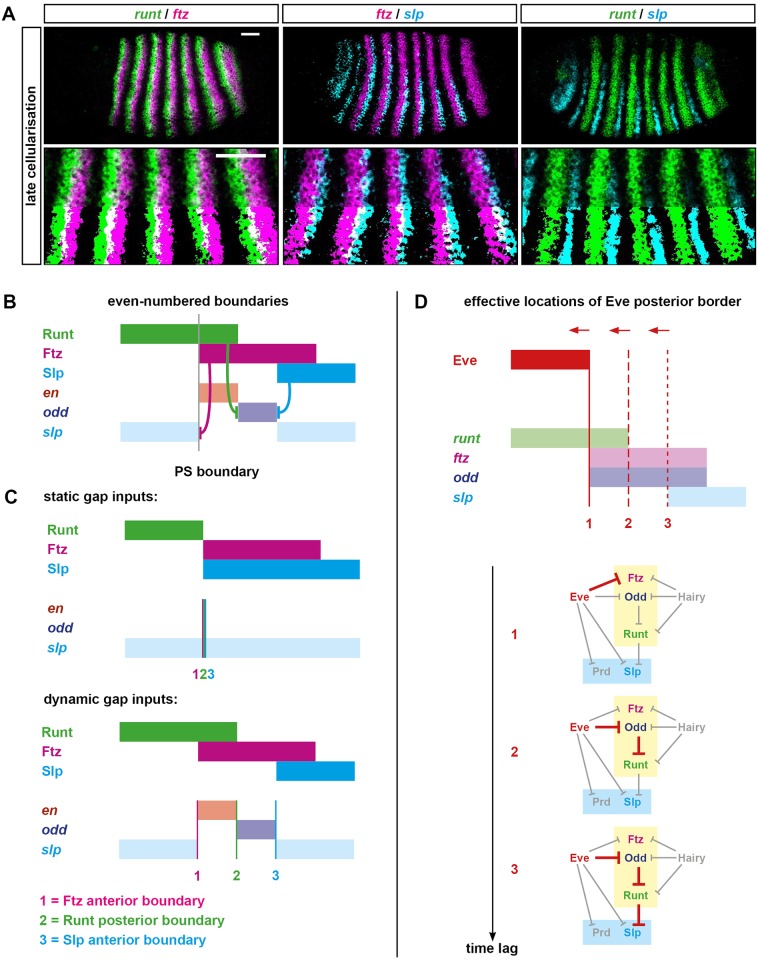
Fig 1. Structure and patterning function of the pair-rule gene regulatory network.
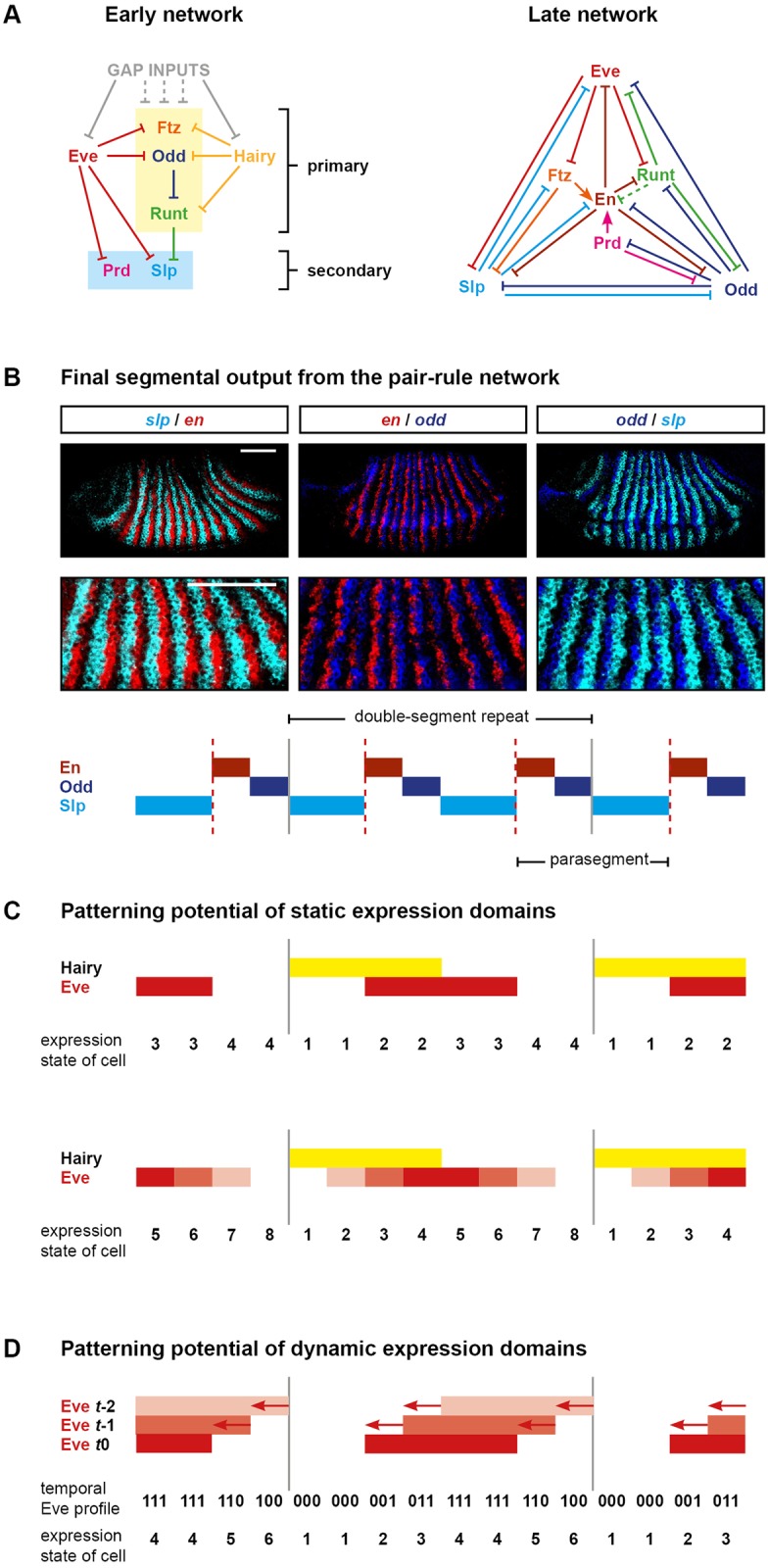
(A) Cross-regulatory interactions between pair-rule genes during cellularisation (left) and gastrulation (right). Hammerhead arrows represent repression; pointed arrows represent activation. The zebra elements of ftz, odd, and runt (yellow box) turn on earlier than those of prd and slp (blue box). ftz, odd, and runt are also regulated by gap inputs through their stripe-specific elements (noted by grey dotted lines), but pair-rule inputs are the dominant influence on their patterns by mid-cellularisation (see S1 Text). The repression of En by Runt in the late network is dotted because, while the odd-numbered en stripes are sensitive to Runt, the even-numbered stripes are regulated differently [68]. Note that the regulation of runt in the late network reflects the regulatory logic of the “7-stripe” element rather than that of the “6-stripe” element [32,69]. (B) The template for polarised parasegment boundaries is formed by a repeating pattern of En, Odd, and Slp stripes. Top: whole mount double FISH images (anterior left, dorsal top) showing that en, odd, and slp are expressed in abutting, mutually exclusive domains. Middle: enlarged views of the stripes. Bottom: schematic of the overall pattern (anterior left). The grey vertical lines indicate the span of an initial pair-rule repeat relative to the final output pattern. Parasegment boundaries (dotted red lines) will form at the interface between En and Slp domains. Scale bars = 100μm. (C) Schematics indicating the number of distinct states that can be specified by static domains of Hairy and Eve expression. Top: Hairy and Eve are both Boolean variables. There are only 4 possible expression states (1: Hairy on, Eve off; 2: Hairy on, Eve on; 3: Hairy off, Eve on; 4: Hairy off, Eve off). Bottom: Hairy is still Boolean, but Eve is now a multilevel variable. Different shades of red represent different levels of Eve activity: low (lightest), medium, or high (darkest). There are now 8 different possible combined expression states of Hairy and Eve. Grey vertical lines are as in (B). (D) Rich positional information can be conveyed by a dynamic signal. Top: Boolean Eve stripes are depicted travelling from posterior to anterior over time (darker red represents a more recent time point). Middle: The Eve profile over time is recorded in binary digits. Bottom: The Eve signal can be decoded into 6 distinct expression domains, aligned with those in (B). Grey lines are as in (B). Abbreviations: en, engrailed; FISH, fluorescent in situ hybridization; ftz, fushi tarazu; odd, odd-skipped; prd, paired; slp, sloppy-paired.
Gene regulatory network models represent “intellectual syntheses” of diverse experimental data [70]. I arrived at the topologies in Fig 1A by carefully analysing relative expression data in tightly staged wild-type embryos and cross-referencing these observations with the large number of mutant and misexpression experiments recorded in the genetic literature (for example, [8,30,31,64,71–78]). In places where the data were particularly ambiguous, I also re-characterised pair-rule gene expression in select pair-rule mutants in order to pick apart direct versus indirect regulatory interactions. In almost all cases, the interactions in the network diagrams have been previously inferred by multiple sets of researchers; my contribution has been (1) to bring this body of work together into something consistent and relatively complete and (2) to recognise the distinction between the early and late phases of regulation, rather than pooling all interactions into a single network. Most of the evidence and reasoning behind the inferred interactions (and interactions inferred to be absent) in Fig 1A are described in Appendix 1 of Clark and Akam (2016) [32]. Additional evidence in favour of the “early” cross-regulatory interactions between the primary pair-rule genes (boxed yellow area in Fig 1A, left) is presented in S1 Text, based on patterns of pair-rule gene expression in hairy, eve, and runt mutants.
Two things are immediately clear from the network diagrams. First, the direct regulatory interactions between the pair-rule genes are overwhelmingly repressive. This is consistent with a mode of patterning consisting of spatially ubiquitous activation (by maternally provided factors, for example) combined with precisely positioned repression from other segmentation genes [76,79–81]. While certain of the pair-rule factors (e.g., Ftz and Prd) have been shown to quantitatively up-regulate the expression of other pair-rule genes and thus contribute to this background activation in a spatially modulated way [82–84], these effects do not, for the most part, seem to be important for qualitatively determining the spatial pattern of pair-rule gene expression and so have been omitted from the diagram. Most described incidences of one pair-rule gene genetically activating another pair-rule gene are instead indirect (i.e., mediated by the direct repression of another repressor).
Second, the 2 networks have very different structures, presumably reflecting the different patterning function each must perform. During mid-cellularisation, pair-rule gene cross-regulation is responsible for refining many of the pair-rule stripes and standardising their phasing relative to other pair-rule stripes, resulting in a regular repeating pattern of double-segment periodicity. This is carried out by the early network, which is sparse, composed of unidirectional regulatory interactions, and has no feedback loops. Two of the pair-rule genes, eve and hairy, are patterned by gap factors rather than other pair-rule factors and so represent “input-only” factors to the network [16]. The remaining primary pair-rule genes (runt, ftz, and odd) do receive extensive gap inputs at early stages of cellularisation, but, by mid-cellularisation, their patterns are largely specified by other pair-rule genes (see S1 Text). (Note, however, that some aspects of the ftz pattern cannot be explained by pair-rule inputs alone, see Appendix 2 of [32].) The secondary pair-rule genes turn on later (prd at mid-cellularisation and slp towards the end of cellularisation) and are patterned by primary pair-rule genes. Overall, the early network has a hierarchical structure, in which Eve and Hairy convey positional information derived from the gap factors to the remaining primary pair-rule genes and eventually to the secondary pair-rule genes.
The late network, on the other hand, is extremely dense and consists largely of mutually repressive pairs of interactions. It is responsible for converting a double-segmental pattern of overlapping stripes into a segmental pattern of discrete segment-polarity fates. This is the final step in the Drosophila “segmentation cascade” and completes the transition from the analog (graded) positional information carried by the maternal and gap gene products to the essentially digital positional information carried by the segment-polarity genes [64]. The numerous positive (i.e., double-negative) feedback loops within the late network are consistent with it acting like a multi-stable switch, individual segment-polarity fates representing attractor states towards which the system will rapidly converge.
Pair-rule patterning and positional information
As described above, gap inputs and the early pair-rule network combine to establish a repeating double-segmental pattern of pair-rule gene expression. The positional information within this pattern is then converted into a stable output pattern of segment-polarity states by the late network. Each initial double-segment repeat is about 7–8 nuclei wide, and each specified segment will consist of at least 3 distinct states characterised by the expression of engrailed (en), odd, and slp, respectively (Fig 1B). The en and odd stripes are about 1 nucleus wide, while the slp stripes are about 1–2 nuclei wide. Parasegment boundaries form wherever En and Slp domains abut, while Odd provides a buffer zone that preserves the AP polarity of each segment. (This tripartite segment pattern conforms to prescient theoretical predictions made by Hans Meinhardt in the early 1980s [62,85,86].) It is crucial that all 3 domains are specified within each segment—and that they are in the correct order—because patterning defects such as boundary losses, ectopic boundaries, and/or polarity reversals arise when the pattern is perturbed [8,31,33,76,87].
The extremely high resolution of the final segmental output pattern implies that the initial double segment pattern established by the early pair-rule network must contain sufficient positional information to allow almost every nucleus to be distinguished from its immediate neighbours. We are thus left with 2 questions. First, how does the early network establish a situation in which the different nuclei within a double-segment repeat each expresses a unique combination of pair-rule factors? Second, how exactly is this code “read” by the late network? (Or, in other words, which sets of initial conditions will result in a cell following an expression trajectory that ends at, for example, stable en expression, rather than stable odd or stable slp?)
In later sections, I address these questions by simulating and analysing the networks shown in Fig 1A. However, before getting into specifics of how particular genes are regulated and expressed, it is worth considering a more fundamental question: where is the positional information coming from in the first place? The topology of the early network (Fig 1A, left) implies that, to a first approximation, all the positional information in the final pattern must trace back to the expression patterns of just 2 factors, Eve and Hairy. Boolean (ON/OFF) combinations of Eve and Hairy would only be sufficient to specify 4 different domains within each double-segment repeat (Fig 1C, top), whereas the real output pattern consists of at least 6 distinct domains (i.e., En, Odd, Slp, En, Odd, Slp). How is it possible that just 2 independent spatial signals are able to give rise to such a high-resolution final output?
One potential answer is that stripes of Eve and/or Hairy might carry quantitative information that permits them to convey more than 2 “states” within the positional code. Since the early 1990s, this idea has been applied to the graded margins of the early Eve stripes [30,75,88]. These stripes have been proposed to act as local morphogen gradients, repressing different target genes at different concentration thresholds and thus differentially positioning their respective expression boundaries. Current models of pair-rule patterning rely on the assumption that there are 4 functionally distinct levels of Eve activity across an Eve stripe (from the centre to the edge: HIGH, MEDIUM, LOW, and OFF) [8,44]. These different levels would provide cellular-level resolution within each double-segment repeat and, combined with information from the Hairy stripe, allow each nucleus to be uniquely specified (Fig 1C, bottom).
While a given concentration of Eve protein may well repress its various targets with different efficacies, it is unlikely that segment patterning relies significantly upon this mechanism, for 3 main reasons. First, for the model to be viable, the Eve stripes would need to provide an accurate and precise set of positional signals within each double-parasegment repeat, i.e., the Eve stripes would have to be extremely regular and all share the same shape and amplitude. However, more posterior Eve stripes show significantly lower expression levels than more anterior Eve stripes throughout most of cellularisation [59]. Furthermore, pair-rule transcripts are apically localised, and therefore pair-rule gene expression becomes effectively cell autonomous soon after membrane invagination begins [89,90]. This means that, unlike for the gap genes (whose transcripts remain free to diffuse between neighbouring nuclei), for eve, there is little or no spatial averaging to buffer the high intrinsic noise of transcription [91,92]. This reduces the precision of the Eve signal and thus its capacity to reliably convey analog information.
Second, the morphogen model also requires the readout of the Eve signal to be very sensitive; i.e., eve target genes would have to reliably discriminate between different Eve expression levels and pattern their expression boundaries accordingly. However, it is not clear that this actually occurs within the embryo—for example, the model proposes that graded Eve stripes result in offset boundaries of the Eve targets odd and ftz, but recent observations indicate that these offsets are in fact produced by other mechanisms [32].
Third, the morphogen model does not explain the full severity of the eve null mutant phenotype, in which aberrant expression patterns are seen even in regions that would be outside the Eve stripes in wild-type embryos. Neither does the morphogen model explain the patterning robustness of eve heterozygotes, in which halving Eve expression levels fails to perturb the overall pattern of segment-polarity domains.
How, then, might the spatial resolution of the segment pattern be explained if not by an Eve morphogen gradient? Traditional models of Drosophila segmentation are essentially static: each tier of segmentation gene expression provides a single set of spatial signals, which is transduced into a new set of spatial signals by the tier below. This simplifies the real situation in the embryo, in which both gap and pair-rule expression domains shift subtly from posterior to anterior over time [59,93]. Explicitly considering these temporal aspects of segmentation gene expression suggests an alternative segment patterning mechanism: using the temporal dynamics of a relatively coarse pair-rule signal to provide high-resolution spatial information across each pattern repeat.
A signal that varies over time can be used to convey an arbitrary quantity of information, even if each reading of that signal provides very little (think of Morse code or binary storage). The eve and hairy stripes continue to be regulated by gap inputs throughout most of cellularisation and therefore shift across nuclei in concert with the gap domains. This means that, rather than each nucleus having to deduce its position from a single level of, e.g., Eve protein (as in the morphogen model), the nucleus actually experiences a temporal sequence of Eve protein levels. Strikingly, an overall shift of just 2 nuclei would be theoretically sufficient for a Boolean Eve stripe to, on its own, specify the positions of all 6 segment-polarity domains within a double-segment repeat (Fig 1D).
This kind of mechanism would, however, rely on the downstream targets of Eve and Hairy being able to decode a temporal sequence of Eve/Hairy expression and convert it into an appropriate segment-polarity fate. In the following sections, I carry out simulations and analysis of the network shown in Fig 1A and, based on the results, argue that the cross-regulatory interactions between the pair-rule genes function to achieve exactly this task.
A simple model of the pair-rule system
In order to investigate how pair-rule patterning works, I used the networks shown in Fig 1A to create a toy model of the pair-rule system and then simulated pair-rule gene expression across an idealised 1-dimensional tissue. In this section, I briefly describe the structure and assumptions of the modelling approach; a full description of the model plus details of all simulations are given in S2 Text. (Source code for running the simulations is available in S1 and S2 Files, while pair-rule networks in SBML-qual format are available in S3 and S4 Files).
The genes whose regulation I model explicitly are the 7 pair-rule genes, plus en, whose product plays a key role in regulating late pair-rule gene expression. I have also included 4 inputs that are extrinsic to the system: 2 temporal signals, Caudal (Cad) [94] and Opa, and 2 signals to represent the positional information provided by the gap system, “G1” and “G2”. Cad represses the secondary pair-rule genes during early stages of patterning [87], while Opa turns on midway through patterning and triggers the switch from the early network to the late network [32]. G1 is responsible for patterning the hairy pair-rule stripes while G2 is responsible for patterning the eve pair-rule stripes. G1 and G2 do not represent specific gap factors but are instead an abstraction of the spatial inputs (i.e., stripe boundary locations) provided by the gap system as a whole.
Each gene in the system is represented by a Boolean variable, and its control logic is formalised using logical rules (essentially equivalent to the “logical equations” used in Sanchez and Thieffry [2003] [44] or the “vector equations” used in Peter et al. [2012] [70]). For example, if Opa is OFF (early network), odd is expressed only if both Hairy and Eve are also OFF, while if Opa is ON (late network), odd expression relies on all of Runt, En, and Slp being OFF (compare Fig 1A). In most cases (apart from, e.g., activation of en by Ftz or Prd), gene activation is assumed to be driven by some ubiquitous background factor(s) and is not explicitly included in the model.
The network simulation proceeds by discrete time steps, with expression output at time point t + 1 calculated from the state of the system at time point t. Because of the speed and dynamicity of segment patterning, time delays associated with protein synthesis and protein decay imply that protein and transcript expression domains for a given gene will often be non-congruent within the Drosophila embryo. This is likely to be significant for patterning, and I therefore approximate this effect by adding simple time delay rules into the simulation. Each gene has associated “synthesis delay” and “decay delay” parameter values s and d, both of which are integers representing a certain number of time steps. (Once a gene turns on, transcript will be present immediately, but protein will only appear s time steps later. Similarly, once a gene turns off, transcript will disappear immediately, but protein will only disappear after another d timesteps have elapsed.) For parsimony (and consistent with real expression kinetics [82]) all pair-rule genes and en are assigned the same delay value, which applies to both s and d. The specific value of this delay is fairly arbitrary, because the ratio between different delays is what affects how the system behaves, but I have chosen this value to be 6. Given that the half-life of ftz RNA during cellularisation is 7 minutes [79], this means that each time step in the simulation can be thought of as representing on the order of 1 minute of real developmental time. The time delays of the other components in the network (Cad, Opa, G1, and G2) are assigned appropriate values relative to this timescale, so that their simulated behaviour roughly approximates their spatiotemporal expression in a real embryo.
The simulation is set up to occur across a row of 20 “cells”, an idealised representation of the AP axis. This row of cells is not meant to correspond to a specific region of the Drosophila embryo but rather to be generally representative of patterning within the main trunk (i.e., pair-rule stripes 3–6), in which pair-rule genes are not additionally affected by cephalic or terminal factors. Each cell within this “tissue” is simulated independently, starting from a specific set of initial conditions. (As mentioned above, pair-rule transcripts are apically localised, and therefore the cross-regulation between the pair-rule genes is likely to be effectively cell autonomous from roughly mid-cellularisation onwards.)
The starting conditions for each cell usually involve specifying the appropriate expression of G1 and G2, setting Cad to ON, and setting all other genes to OFF. G1 and G2 are initialised with patterns that are offset by 2 cells and repeat every 8 cells, meaning that the hairy and eve stripes specified by these inputs will partially overlap and exhibit a double-segment periodicity, as in real embryos. Gap inputs into runt, ftz, and odd are omitted, meaning that their early expression is organised entirely by the spatial inputs from Hairy and Eve. As the simulation proceeds, Cad protein will disappear, allowing prd to turn on [87], followed by slp. (Note that we do not currently know how exactly the timing of slp expression is controlled, so in order to reproduce the timing observed in the embryo, slp expression in the simulation requires Prd expression to already be present.) Shortly afterwards, Opa protein will turn on, switching the control logic of pair-rule gene expression to the late network and causing pair-rule gene expression to eventually reach a final, stable state. After the switch to the late network, the gap factors and Hairy cease to regulate the pair-rule genes and then fade away, as in real embryos.
Note that the model just described, which is Boolean, deterministic, and uses discrete time steps, is not designed to capture the full complexity of the embryo (in which gene expression is, of course, quantitative, stochastic, and continuous). Rather, it represents a tool to expose the key mechanisms of patterning—and to delineate how much of what is observed in the embryo follows simply from the qualitative structure of the regulatory network. It also provides an important sanity check of the inferences that led to that structure being proposed in the first place.
Posterior-to-anterior expression shifts are crucial for pair-rule patterning
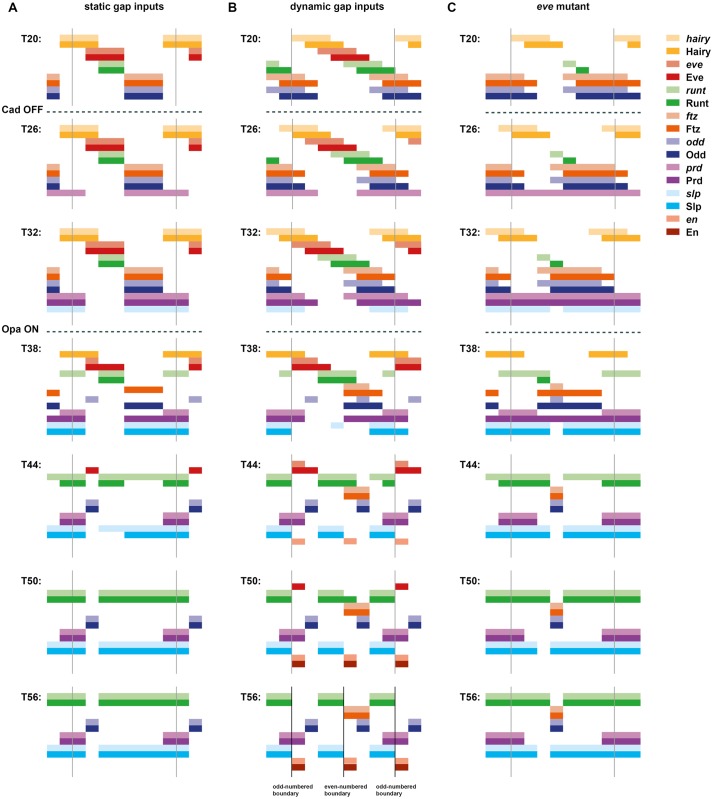
Using the model described above, I first simulated a scenario in which gap gene inputs and hence the pair-rule stripes of Hairy and Eve are completely static. The results are shown in S9 Movie and are summarised in Fig 2A. Under these conditions, the positional information provided by Hairy and Eve is essentially equivalent to the situation diagrammed in Fig 1C (bottom) and thus has no possibility of generating the correct segmental output. Unsurprisingly, the simulation does a bad job of recapitulating the patterns of pair-rule gene expression seen in real embryos (see below). In particular, at the end of the simulation, there is no en expression anywhere at all, and neither odd nor slp is expressed in a segmental pattern.
Fig 2. Simulation output from a Boolean model of the pair-rule network.
Pair-rule gene expression patterns generated by simulating a Boolean model of the pair-rule network, assuming static “gap” inputs (A), dynamic “gap” inputs (B), or dynamic “gap” inputs and no Eve expression (C). Original simulations are shown in S9–S11 Movies. In each panel, the horizontal axis represents the anteroposterior (AP) axis (anterior left), while the vertical axis represents the different gene products that might be expressed in a given “cell” (column). Pale colours represent active transcription; dark colours represent active protein (see colour key at top right). Grey vertical lines indicate the span of an idealised double-segment repeat of 8 “cells”. The same 7 time points (T20–T56) are shown for each simulation. Cad turns off between the first (T20) and second (T26) panels (dashed lines), derepressing prd and slp. Opa turns on between the third (T32) and fourth (T38) panels (dashed lines), causing a switch from the early to the late network logic. Parasegment boundaries (black vertical lines at T56, located between abutting domains of Slp and En) are only produced by the simulation with dynamic gap inputs (B). Abbreviations: Cad, Caudal; Opa, Odd-paired; prd, paired; slp, sloppy-paired.
I then simulated a scenario in which the gap gene inputs and hence the Hairy and Eve stripes shift anteriorly over time. Given that the shift rate in real embryos is of the same order as the synthesis and decay rates of the segmentation gene products, I set the rate of these shifts to be such that the time taken for an expression domain to shift anteriorly by 1 cell is equal to the synthesis/decay delay parameter value of the pair-rule genes, i.e., 6 time steps (see S2 Text).
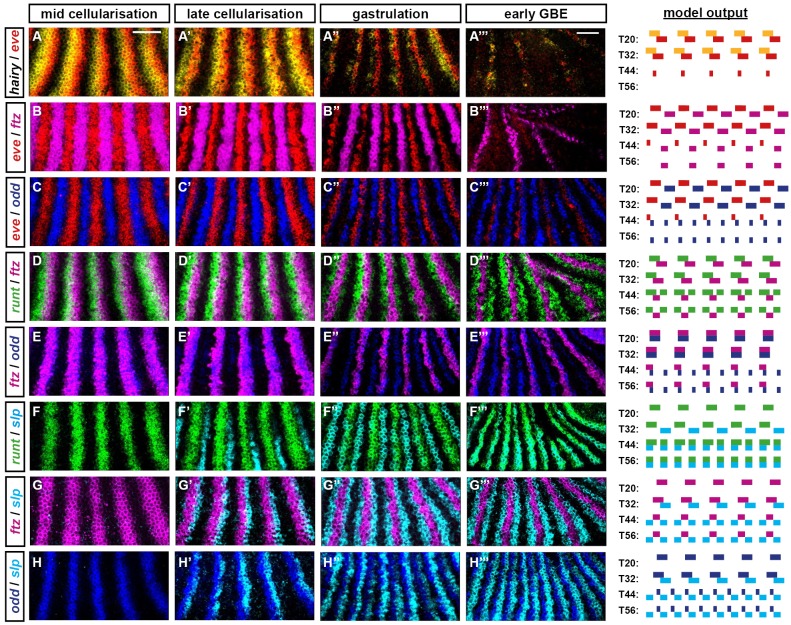
The simulation output for this scenario is shown in S10 Movie and summarised in Fig 2B. Even though the pair-rule network is unchanged and the Hairy and Eve stripes retain the same pattern and relative phasing as for the static simulation, the model now performs completely differently. Qualitative aspects of actual pair-rule gene expression (i.e., whether the expression domains of each pair of genes are congruent, overlapping, abutting, or separate, and the way this changes over time) are recapitulated remarkably well. For all pair-rule genes except prd, the match between the model output and the real spatiotemporal dynamics of gene expression is about as close as could be achieved by a simple, Boolean model—a few examples are highlighted in Fig 3, and the full set of comparisons is shown in S1 Fig. For prd, the real spatiotemporal expression profile is only partially recovered: the early pair-rule stripes are positioned correctly but do not refine correctly at later stages—they narrow rather than split, meaning that alternate segmental stripes are missing from the final pattern (S3 Fig). However, the prd domains missing from the simulation are not actually required for segment boundary patterning in real embryos (they are not reflected in the larval cuticles of prd mutants, although they do have minor effects on wg expression [6,31,95]). Accordingly, the simulation still generates the correct final segmental output: a repeating pattern of En, Odd, Slp x2, En, Odd, Slp x2.
Fig 3. The “dynamic” simulation accurately recapitulates the spatiotemporal expression of the pair-rule genes.
Left: false-coloured double FISH images for selected combinations of pair-rule gene transcripts at 4 different stages. Each panel shows a lateral view of stripes 2–6 (anterior left, dorsal top). Scale bars = 50 μm (rightmost panels use a slightly lower magnification due to tissue rearrangements). Additional expression combinations are shown in S1 Fig, while uncropped views of the embryos are shown in S2 Fig. Right: simulated transcriptional output for these pairs of genes at 4 different time points (see Fig 2B). The earliest time point of the simulated expression (T20) is representative of the leftmost panels of real expression (mid-cellularisation), and so on. The simulation output is generally very similar to the real expression patterns, with 2 main differences related to the discrete nature of the simulations. (1) Real gene expression domains fade over time rather than turning off instantaneously (e.g., compare hairy in A′–A″ to the simulated hairy expression at T32/T44/T56). (2) Qualitative expression pattern changes may occur gradually between late cellularisation and early gastrulation (e.g., eve expression in B–B″/C–C″ or odd expression in C–C″/E–E″) rather than instantaneously, as between T32 and T44. (A) hairy and eve pair-rule stripes partially overlap during cellularisation. At gastrulation, hairy expression fades away, while the eve stripes narrow from the posterior and then also fade. (B) eve and ftz pair-rule stripes are at first expressed in complementary patterns. Starting from late cellularisation, they both narrow from the posterior (eve more than ftz). eve expression later fades away, while ftz persists. (C) eve and odd pair-rule stripes are at first expressed in complementary patterns before both narrowing. odd secondary stripes emerge at the posterior of the narrowing eve domains, which then fade away, leaving segmental stripes of odd. (D) runt and ftz pair-rule stripes partially overlap throughout cellularisation. At gastrulation, runt secondary stripes emerge to the posterior of the narrowing ftz stripes. Later, the runt primary stripes refine from the posterior, and the overlaps with ftz are lost. (E) The ftz and odd stripes are fairly congruent during cellularisation. At gastrulation, both narrow from the posterior, and the odd secondary stripes intercalate between them. Over the course of patterning, their anterior boundaries also become offset from one another. (F) The slp primary stripes emerge later than the runt primary stripes and are offset slightly from their posterior boundaries. (The simulated slp domains are wider than the real slp domains.) At gastrulation, secondary stripes of both genes emerge between the primary stripes (the widths of the simulated slp stripes are now appropriate). The expression patterns become largely congruent, except at the posteriors of the runt primary stripes. These differences resolve later, when the runt primary stripes narrow. (G) The slp primary stripes emerge later than the ftz primary stripes and partially overlap with them. At gastrulation, the secondary slp stripes emerge just anterior to the ftz domains, which narrow from the posterior, losing the overlaps with the slp primary stripes. (H) As for (G), the slp primary stripes partially overlap the odd primary stripes, and these overlaps are later lost by the odd stripes narrowing from the posterior. The secondary stripes of odd and slp intercalate between the primary stripes and abut one another. Abbreviations: eve, even-skipped; FISH, fluorescent in situ hybridization; ftz, fushi tarazu; odd, odd-skipped; slp, sloppy-paired.
These results tell us a number of things. First, it is not strictly necessary to invoke morphogen gradients in order to account for Drosophila pair-rule patterning. Second, posterior-to-anterior shifts of the Hairy and/or Eve stripes appear to be crucial for properly patterning the other pair-rule genes, and analysing the different behaviour of the static and shifting simulations should reveal exactly why. Third, the model as formulated is too simple to explain important aspects of the prd expression profile. Additional complexities that influence prd expression in real embryos could include (1) additional spatial or temporal regulatory inputs missing from the model, (2) quantitative information from existing spatial or temporal inputs that is not captured by the use of Boolean variables, or (3) differential synthesis/degradation rates of particular segmentation gene products not accounted for by the equal time delays assumed by the model. At least the first option seems to apply, as I have recently discovered that the Sox transcription factor Dichaete [96,97] also affects prd regulation [87].
How and why do the shifts affect patterning?
Dynamic patterning of the early pair-rule stripes
First, let us consider the earliest phase of the simulations (time points 0–24, corresponding to mid-cellularisation in a real embryo), in which double-segmental stripes of the primary pair-rule genes runt, ftz, and odd are patterned by the early pair-rule network. Each of these genes is repressed by 2 other pair-rule factors: ftz and odd by Hairy and Eve, and runt by Hairy and Odd (Fig 1A, left). In the static simulation, this system rapidly reaches steady state (Fig 4A): ftz and odd are expressed in cells that express neither Eve nor Hairy, while runt is restricted to cells that don’t express Hairy but do express Eve (and hence don’t express Odd). This scenario is significantly different from the expression patterns of these genes in a real embryo, in which there are more overlaps between the various stripes (Fig 4C). For example, runt expression overlaps with ftz (Fig 4Cg) and odd (Fig 4Cc) as well as eve, while ftz and odd overlap slightly with hairy (Fig 4Cd and 4Ce).
Fig 4. Dynamic patterning of the primary pair-rule genes.
(A) Regulatory schematic showing the predicted phasing of the primary pair-rule stripes during cellularisation, assuming static, partially overlapping domains of Hairy and Eve. Pale colours represent transcript domains; intense colours represent protein domains; hammerhead arrows represent repressive interactions; grey vertical lines indicate the span of a double-segment pattern repeat. Cross-regulatory interactions are from the early network (compare Fig 1A, left). For simulation output, see S1 Movie. (B) Regulatory schematic showing the predicted phasing of the pair-rule stripes during cellularisation, assuming dynamic, partially overlapping domains of Hairy and Eve. hairy and eve domains shift anteriorly over time, resulting in offsets between transcript and protein domains. Colours, etc., as for (A). For simulation output, see S2 Movie. (C) Comparisons between real and predicted phasings of the primary pair-rule stripes. Double FISH images show lateral views of stripes 2–6 (anterior left, dorsal top) in mid-cellularisation stage embryos. In the bottom half of each image, the 2 channels have been thresholded, making regions of overlap easier to see. Scale = 50 μm. Diagrams to the right of each image show the stripe phasings predicted by static (top) or shifting (bottom) gap inputs, respectively (compare A and B). For panels (A) and (B), the 2 models predict the same relative pattern. In all other panels, the models predict different relative patterns. (D) Simultaneous visualisation of eve transcript (magenta) and Eve protein (green) in embryos at 4 different stages. Rightmost panels show a ventrolateral view; all other panels show lateral views. Upper panels show whole embryo views; lower panels show enlarged views of stripes 2–6. In stripes 3 onwards, the protein domains lag behind the transcript domains until late gastrulation, indicating that the anterior boundaries of the eve stripes shift anteriorly until early gastrulation. In stripe 2, the anterior boundary stabilises significantly earlier. Scale = 50 μm. Abbreviations: eve, even-skipped; FISH, fluorescent in situ hybridization.
These overlaps represent unstable combinations of gene expression (Hairy represses ftz and odd while Odd represses runt), but they are nevertheless present throughout the “early” phase of the second simulation, which incorporates stripe shifts. This is because the dynamic inputs from Hairy and Eve mean that primary pair-rule gene expression is not permitted to reach stable state (Fig 4B), and therefore a fraction of cells within the tissue will be in these transient states at any given point in time. Note that although the gene expression within each cell is constantly changing over the course of the simulation, the overall pattern of stripes remains constant once established, moving across the tissue like an elaborate Mexican wave.
Specifically, the additional overlaps seen in the second simulation (Fig 4B) arise because the combination of shifting dynamics with time delays for protein synthesis/decay means that domains of active transcription do not necessarily reflect domains of protein activity, and vice versa. If a pair-rule stripe is shifting anteriorly, the protein distribution of that stripe should lag behind the transcript distribution, a prediction I have verified by comparing eve transcript and Eve protein expression patterns within individual embryos (Fig 4D). After a gene turns on at the anterior edge of a stripe, there will be a delay before the protein appears and represses its targets, resulting in small transcriptional overlaps between repressors and their targets (i.e., anterior hairy/posterior ftz, anterior hairy/posterior odd, and anterior odd/posterior runt). Similarly, after a gene turns off at the posterior edge of a stripe, there will be a delay before the protein disappears and its targets are derepressed, resulting in small transcriptional gaps between repressors and their targets (i.e., posterior eve/anterior odd, posterior hairy/anterior runt, and posterior eve/anterior ftz). The exact size of the gaps and overlaps depends on the relationship between the speed of the expression shifts and the length of the time delays involved in protein synthesis and decay: faster relative shift rates will result in larger gaps and overlaps, while slower relative shift rates will result in a pattern closer to the static scenario (S6 Fig; S1–S5 Movies).
Dynamic patterning of the odd-numbered parasegment boundaries
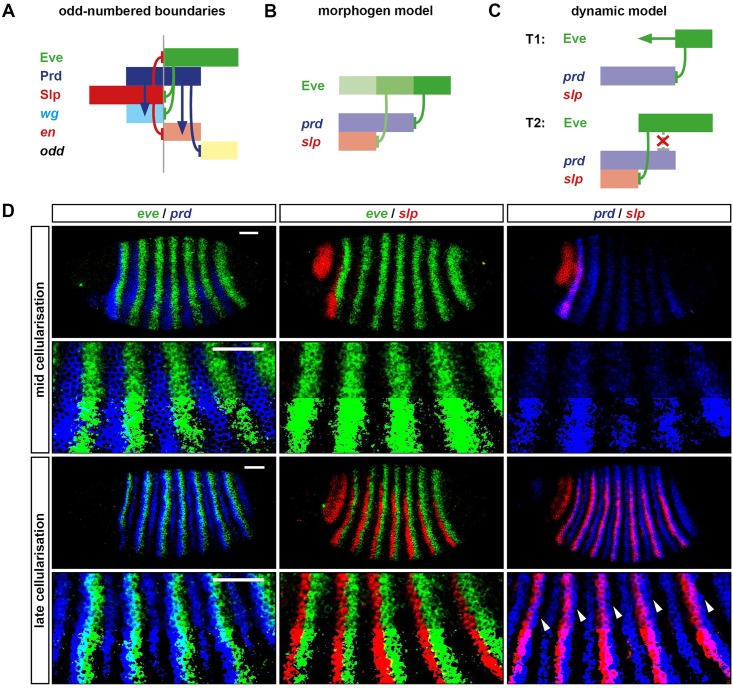
The next phase of the simulations (time points 25–36, corresponding to late cellularisation in a real embryo) is when the secondary pair-rule genes turn on in double-segment patterns and prepattern the odd-numbered (Prd-dependent) parasegment boundaries. These boundaries rely on setting up offset posterior boundaries of prd and slp expression: en is activated by Prd but repressed by Slp, thus resulting in narrow stripes of en expression posteriorly abutting each of the slp pair-rule stripes (Fig 5A; S4 Fig).
Fig 5. Dynamic patterning of the odd-numbered parasegment boundaries.
(A) Regulatory schematic of gene expression at the odd-numbered parasegment boundaries. Domains of Slp, Prd, and Eve expression (dark colours) pattern segment-polarity stripes of wg, en, and odd (pale colours). Anterior left. Hammerhead arrows represent repressive interactions; pointed arrows represent activatory interactions; grey vertical line represents a prospective parasegment boundary. See S4 Fig for relevant in situ data. (B) Static, “morphogen gradient” model for the patterning of the prd and slp posterior borders by Eve. The anterior margin of the Eve stripe is graded, with higher levels of Eve protein (darker green) present more posteriorly. High Eve (dark green) is required to repress prd, but only medium Eve (medium green) is required to repress slp. Based on Fujioka et al. (1995) [30]. (C) Dynamic model for the patterning of the prd and slp posterior borders by Eve. prd is activated earlier (T1, mid-cellularisation) than slp (T2, late cellularisation). In between these time points, the anterior border of the Eve domain shifts anteriorly. The posterior border of the prd domain is patterned by Eve at T1, but the posterior border of the slp domain is patterned by Eve at T2, resulting in a more anterior location. prd is no longer repressed by Eve at T2, resulting in stable, overlapping expression of Eve and prd. (D) Double FISH images showing the relative phasing of eve (green), prd (blue), and slp (red) expression domains at mid-cellularisation and late cellularisation. Enlarged views of stripes 2–6 are shown below the whole embryo lateral views (anterior left, dorsal top). In the bottom half of each image, the 2 channels have been thresholded, making regions of overlap easier to see. At mid-cellularisation, slp is not expressed and the posterior borders of the prd stripes abut the anterior borders of the eve stripes. At late cellularisation, the posterior borders of the prd stripes overlap the anterior borders of eve stripes (note the regions that appear cyan), the posterior borders of the slp stripes sharply abut the anterior borders of the eve stripes, and the posterior borders of the slp stripes are offset anteriorly from the posterior borders of the prd stripes (arrowheads). These expression patterns are more consistent with the dynamic model (C) than the static morphogen model (B). Scale bars = 50 μm. Abbreviations: en, engrailed; eve, even-skipped; FISH, fluorescent in situ hybridization; odd, odd-skipped; prd, paired; slp, sloppy-paired; wg, wingless.
The posterior boundaries of prd and slp are patterned by Eve, which represses both genes during cellularisation (Fig 1A, left). While slp continues to be repressed by Eve throughout later development [88], prd becomes insensitive to Eve towards the end of cellularisation [30,32] for reasons that are not entirely clear but may relate to the loss of Dichaete expression from the blastoderm [87]. The current model for how the offsets are set up is that the anterior edge of each Eve stripe acts as a morphogen gradient, which represses slp at a lower Eve concentration than is required to repress prd, thus differentially positioning their expression boundaries (Fig 5B) [30]. The reason that patterning fails in the first simulation (Fig 2A) is that the static, Boolean stripes of Eve position the boundaries of both prd and slp at the same place, resulting in no offsets and hence no en expression. In the second simulation (Fig 2B), however, the prd/slp offsets do emerge, even though Eve expression remains Boolean. This is because slp expression turns on significantly later than prd expression (about 10 minutes later in real embryos [59]), by which time, the Eve boundary has shifted to a more anterior position than where it was when it patterned the prd stripes. Thus, whereas under the morphogen model, the distance between the prd and slp boundaries is measured using Eve concentration, an alternative model is that this distance is instead measured using time (Fig 5C).
These 2 mechanisms are not mutually exclusive, and it may be that they both contribute to patterning in the embryo. However, in their basic forms, they make different predictions about the relative expression dynamics of the prd and Eve domains. Under the static morphogen model, the 2 sets of domains should show significant overlaps throughout patterning (Fig 5B). Under the dynamic model, these overlaps should be absent when the prd stripes are first patterned and emerge later, once prd becomes resistant to Eve (Fig 5C). Real pair-rule gene expression in the embryo is more consistent with the dynamic model: the posterior boundaries of the prd stripes abut the anterior boundaries of the eve stripes when they first emerge, and it is only later, when slp starts to be expressed, that overlaps between prd and eve become obvious (Fig 5D).
Dynamic patterning of the even-numbered parasegment boundaries
The final phase of the simulations (time points 37–60, corresponding to gastrulation in real embryos) is when the system switches over to the late network, and the even-numbered (Ftz-dependent) parasegment boundaries are patterned. These boundaries are patterned by the pair-rule stripes of Runt, Ftz, and Slp, which together pattern segmental stripes of en, odd, and slp (Fig 6A and 6B) [32]. Correctly patterning these latter domains therefore relies on the early network being able to set up the correct relative pattern of runt, ftz, and slp expression. Crucially, this pattern includes narrow overlaps between runt and ftz (which will later give rise to en expression) and narrow gaps between runt and slp (which will later allow odd expression to be maintained). This means that the 3 relevant sets of expression boundaries (ftz anterior boundaries, runt posterior boundaries, and slp anterior boundaries) each need to fall in different positions.
Fig 6. Dynamic patterning of the even-numbered parasegment boundaries.
(A) Double FISH images showing the relative expression patterns of runt, ftz, and slp at late cellularisation. Enlarged views of stripes 2–6 are shown below whole embryo lateral views (anterior left, dorsal top). In the bottom half of each enlarged image, the 2 channels have been thresholded, making regions of overlap easier to see. Scale bars = 50 μm. (B) Regulatory schematic showing the patterning of the even-numbered parasegment boundaries. At gastrulation, Runt, Ftz, and Slp are expressed in partially overlapping domains similar to their transcript expression at late cellularisation (see A). These overlapping domains provide a template for the segment-polarity stripes of en, odd, and slp: the anterior borders of the Ftz stripes define the posterior borders of the slp secondary stripes, the posterior borders of the Runt stripes define the anterior borders of the odd primary stripes, and the Slp anterior borders define the posterior borders of the odd primary stripes. The even-numbered en stripes are activated by Ftz but repressed by Odd and Slp and so are restricted to the region of overlap between Runt and Ftz, in which both odd and slp are repressed [32]. Anterior left. Hammerhead arrows represent repressive interactions; grey vertical line represents a prospective parasegment boundary. (C) Schematic explaining why the even-numbered parasegment boundaries require dynamic gap inputs in order to be patterned. Given static inputs (top panel, compare Fig 2A), the Ftz anterior boundary (1, pink vertical line), the Runt posterior boundary (2, green vertical line), and the Slp anterior boundary (3, blue vertical line) all coincide, resulting only in broad slp expression. Given dynamic inputs (bottom panel, compare Fig 2B), the 3 boundaries are each located at different anteroposterior (AP) positions (as in B), resulting in the segment-polarity pattern: slp, en, odd, slp. (D) Schematic explaining the origin of the offset boundaries of ftz, runt, and slp. Top: diagram of the relative expression of Eve, runt, ftz, odd, and slp at late cellularisation (compare A, and see T32 in Fig 2B). The solid red vertical line indicates the current position of the Eve posterior border, which coincides with the ftz anterior border (1). Dotted red vertical lines indicate previous positions of the dynamic Eve posterior border, coinciding with the runt posterior border (2) or the slp anterior border (3). Bottom: the regulatory chains responsible for patterning each of the 3 expression boundaries are highlighted in red on the early pair-rule network. All 3 boundaries trace back to Eve, but more posterior boundaries correspond to longer regulatory chains and so incur a longer time lag to resolve, given a change in Eve expression. The 3 different genes (ftz, runt, and slp) are effectively patterned by increasingly earlier incarnations of the Eve stripes, and therefore the existence of spatial offsets between boundaries 1, 2, and 3 relies on the Eve posterior border shifting anteriorly over time. Abbreviations: en, engrailed; FISH, fluorescent in situ hybridization; ftz, fushi tarazu; odd, odd-skipped; slp, sloppy-paired.
The patterning of each of these sets of boundaries eventually traces back to the posteriors of the Eve stripes (see Fig 4B). ftz expression is directly patterned by Eve; runt is patterned by Odd, which is itself patterned by Eve; and slp is patterned by Runt, which, as just mentioned, is indirectly downstream of Eve. In the first simulation (Fig 6C, top), these Eve boundaries are static, and thus the boundaries of Runt, Ftz, and Slp end up coinciding exactly. This explains why the en stripes fail to emerge and odd expression is lost: the expression states that give rise to them are missing from the positional code.
However, the correct boundary positions do emerge from the second simulation (Fig 6C, bottom), even though the network is unchanged. Again, this result is caused by the dynamic nature of the Eve stripes. Each of the 3 boundaries is patterned by a regulatory chain of a different length (1, 2, or 3 interactions, respectively) and so will take different lengths of time to adjust to a given change in Eve expression (Fig 6D). This means that when the Eve stripes shift anteriorly over time, the 3 sets of boundaries each lag behind the Eve domains by different distances, thereby providing the necessary positional framework for the final segment pattern.
Patterning dynamics explain the severity of the eve mutant phenotype
Above, I described how the final segmental output consists of the pattern [En, Odd, Slp, En, Odd, Slp] across each double-parasegment repeat. I then used a dynamical model of the pair-rule system to show how the Eve stripes are directly or indirectly responsible for patterning most of the expression boundaries in this pattern, including both sets of parasegment boundaries. In this section, I use the same model to simulate and dissect the eve mutant phenotype, which has proved hard to account for using traditional patterning models.
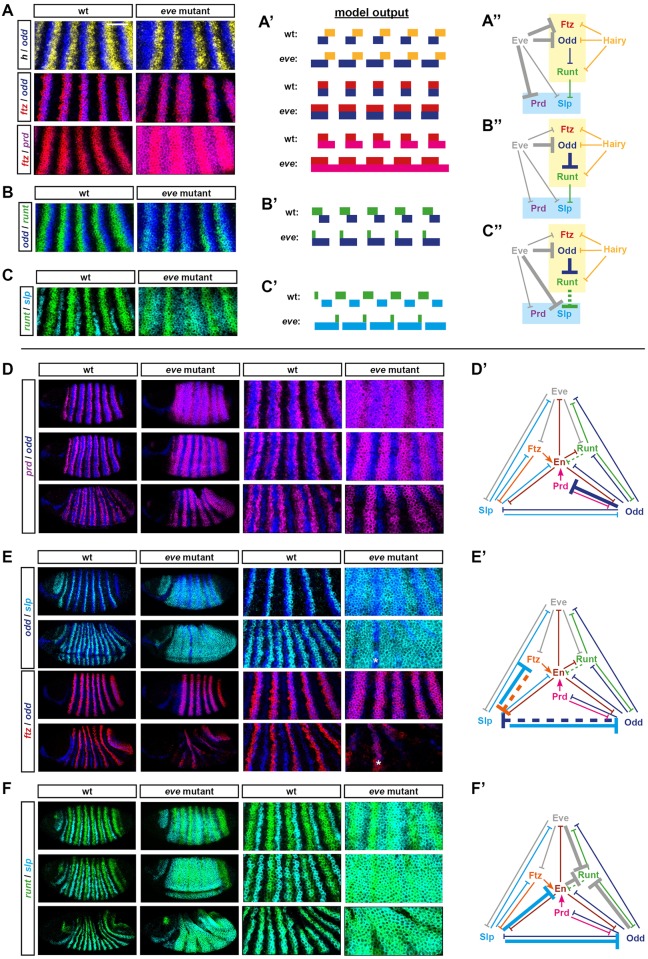
Although eve was originally identified as a pair-rule gene on the basis of a pair-rule cuticle phenotype [3], it turned out that this particular mutant allele was an eve hypomorph, while eve null mutants yield an aperiodic denticle lawn phenotype instead [98]. Both odd-numbered and even-numbered en stripes are absent from eve null mutant embryos [71], indicating severe mispatterning of upstream pair-rule gene expression. To investigate the aetiology of these effects, I characterised pair-rule gene expression patterns in precisely staged eve mutant embryos using double fluorescent in situ hybridisation (FISH) (Fig 7) and then cross-referenced these observations with the patterning output of an “in silico” eve mutant (Fig 2C; S11 Movie), simulated by starting with the dynamic model and then setting eve transcription to remain off (see S2 Text).
Fig 7. Aetiology of the eve mutant phenotype.
(A–C) “Early” effects. (A–C) Double FISH images of pair-rule gene expression in cellularisation stage wild-type and eve mutant embryos. Enlarged views of stripes 2–6 are shown (anterior left, dorsal top). Scale = 50 μm. For whole embryo views and single channel views, see S5 Fig. (A′–C′) Predicted transcriptional output of these genes from “wild-type” and “eve mutant” simulations (compare T32 in Fig 2B and 2C). (A″–C″) Regulatory interactions relevant to the aberrant expression patterns in eve mutants are highlighted on the early pair-rule network (bold arrows). Eve and its regulatory effects, which are absent from the mutant embryos, are shown in grey. (A) Eve normally represses ftz, odd, and prd. In eve mutant embryos, all 3 genes are ectopically expressed: the ftz and odd stripes expand anteriorly, and prd is expressed ubiquitously rather than in stripes. These expression changes are recapitulated by the simulation. (B) Eve normally indirectly regulates runt expression by repressing its repressor, Odd. In eve mutant embryos, odd expression expands anteriorly (see A), resulting in a down-regulation of the runt stripes, except at their anterior margins (S5 Fig). This effect is recapitulated in a discrete manner by the simulation. (C) Eve normally regulates slp in 2 ways: (1) by repressing it directly, and (2) by repressing it indirectly via indirectly maintaining the expression of its repressor, Runt (see B), via direct repression of odd (see A). In eve mutant embryos, slp is expressed fairly ubiquitously rather than in narrow stripes. This expansion is evident in the simulated expression, but see legend of S5 Fig for discussion of differences between the real and simulated patterns. (D–F) “Late” effects. Double FISH images of pair-rule gene expression in wild-type and eve mutant embryos over the course of gastrulation. For each set of images, each row compares a wild-type and a mutant embryo of roughly equal age (age increases from top to bottom). Both whole embryo views (anterior left, dorsal top) and enlarged views of stripes 2–6 are shown. For single channel views, see S5 Fig. (D′–F′) Regulatory interactions that explain the observed pattern maturation are highlighted on the late network (bold arrows). (D) Odd represses prd in the late network, and so prd expression is lost from cells in which odd and prd expression initially overlap. In wild-type embryos, the odd primary stripes overlap the centres of the prd pair-rule stripes, which therefore split in two. In eve mutant embryos, broad odd stripes are overlain on initially aperiodic prd expression, which therefore resolves into a pair-rule pattern. (E) There is mutual repression between Slp and Ftz/Odd in the late network (E′). (Note that the repression from Slp appears to be stronger than the reciprocal repression from Ftz and Odd.) In wild-type embryos, Slp causes the primary stripes of both odd and ftz to narrow from the posterior (where they overlap the slp primary stripes). In eve mutant embryos, Slp is broadly expressed, causing general repression of odd and ftz. Note that expression of both odd and ftz persists in stripe 3 (asterisks), in which there is a corresponding gap in the slp expression domain. (F) In the late network, slp and runt are regulated similarly, and Slp represses all of the repressors of runt (i.e., eve, odd, and en). Consequently, runt and slp take on almost identical expression patterns. In wild-type embryos, the 2 genes become expressed in coincident segmental stripes. In eve mutant embryos, early broad expression of slp allows runt to also become ubiquitously expressed. Note that the slp domain later resolves into a pair-rule pattern (perhaps due to repression from residual Ftz protein). Abbreviations: en, engrailed; eve, even-skipped; FISH, fluorescent in situ hybridization; ftz, fushi tarazu; odd, odd-skipped; prd, paired; slp, sloppy-paired.
The experimental results are in accordance with earlier, more fragmentary characterisations of eve mutants [30,64,73,74,99–101] and reveal a number of significant changes to pair-rule gene expression patterns. The odd and ftz primary stripes are broader than usual (Fig 7A), and the early expression of prd and slp is largely aperiodic rather than pair rule (Fig 7A and 7C). Then, at gastrulation, segmental patterns of pair-rule gene expression fail to emerge; instead, prd resolves into broad pair-rule stripes (Fig 7D), runt and slp become expressed fairly ubiquitously (Fig 7F), and odd and ftz expression largely disappears (Fig 7E). These changes are also seen in the eve mutant simulation (Fig 2C) and, as described below, follow logically from the structure of the pair-rule network.
Because Eve expression plays a relatively minor role in late patterning, most of the expression changes just described result from the loss of Eve activity during cellularisation (time points 0–36 in Fig 2C and S11 Movie). First, odd, ftz, and prd, all of which are direct targets of Eve repression (Fig 7A′), are expressed ectopically: the odd and ftz primary stripes expand anteriorly (judged relative to hairy, Fig 7A, top/middle rows), while the prd interstripes (i.e., the gaps between the early broad stripes) are derepressed (Fig 7A, bottom row). The ectopic Odd expression has a knock-on effect on runt expression, which becomes down-regulated (Fig 7B and 7B′). (Note that while runt expression is almost entirely lost at this stage of the simulation, in real embryos runt remains fairly widely expressed, although at significantly lower levels—see S5 Fig. This difference may be partially due to expression from the runt stripe-specific elements, which are not included in the model.) The loss of Runt activity then contributes to the misexpression of the slp primary stripes, which turn on at the end of cellularisation and would normally be patterned by both Eve and Runt (Fig 7C′). Given the absence of Eve activity and the loss/weakening of Runt activity, slp becomes expressed almost ubiquitously within the trunk of the mutant embryos (Fig 7C).
Finally, the downstream effects of these aberrant patterns play out over the course of gastrulation, after the switch to the late network (time points 37–60 in Fig 2C / S11 Movie). Odd represses prd, causing the aperiodic domain of prd to resolve into a pair-rule pattern (Fig 7D and 7D′). Odd and Ftz also repress slp, causing some small gaps to appear in the slp pattern (Fig 7E and 7F). However, odd and ftz are themselves repressed by Slp very strongly (Fig 7E′), and the ectopic Slp expression in the embryo causes their expression to be almost completely lost (Fig 7E). In, contrast, new runt expression emerges throughout most of the trunk, due to the absence of its repressors, Eve and Odd (Fig 7F and 7F′).
As a consequence of all this mispatterning, en expression is completely repressed, and parasegment boundaries never form. The odd-numbered en stripes are specifically blocked by the ectopic Slp expression that replaces the Eve stripes. Above, we saw that these en domains are specified by the short regulatory chain [Eve––| Slp––| En], thus explaining why they have been observed to reappear in eve, slp double mutants [8,101]. On the other hand, the even-numbered en stripes are redundantly repressed in eve mutants, by both ectopic Odd and ectopic Slp, as a result of the regulatory chains [Eve––| Odd––| En] and [Eve––| Odd––| Runt––| Slp––| En], respectively. The model therefore explains why these stripes reappear in eve, odd double mutants [102] but not in eve, slp double mutants [8].
The Drosophila pair-rule network is compatible with both simultaneous and sequential segmentation
By simulating the Drosophila pair-rule network, I have shown that dynamic spatial inputs from just 2 factors, Hairy and Eve, are sufficient to organise the expression of the system as a whole. In the Drosophila blastoderm, these inputs are driven by the dynamic output of the posterior gap system. However, the elaborate control of pair-rule gene expression by gap factors appears to be a relatively recent novelty in arthropod segment patterning, originating during the evolutionary transition from short-germ to long-germ embryogenesis [103,104]. It is currently not clear how much of the cross-regulation between the pair-rule genes seen in Drosophila is a new adaptation to long-germ development and how much is retained from a short-germ ancestral state.
To explore this question, I determined how many changes to the “wild-type” Drosophila simulation it would take in order to produce something resembling a sequential, “clock-and-wave-front” mode of segmentation. I managed to achieve this transition by way of a few plausible alterations, leaving the bulk of the network untouched (Fig 8C).
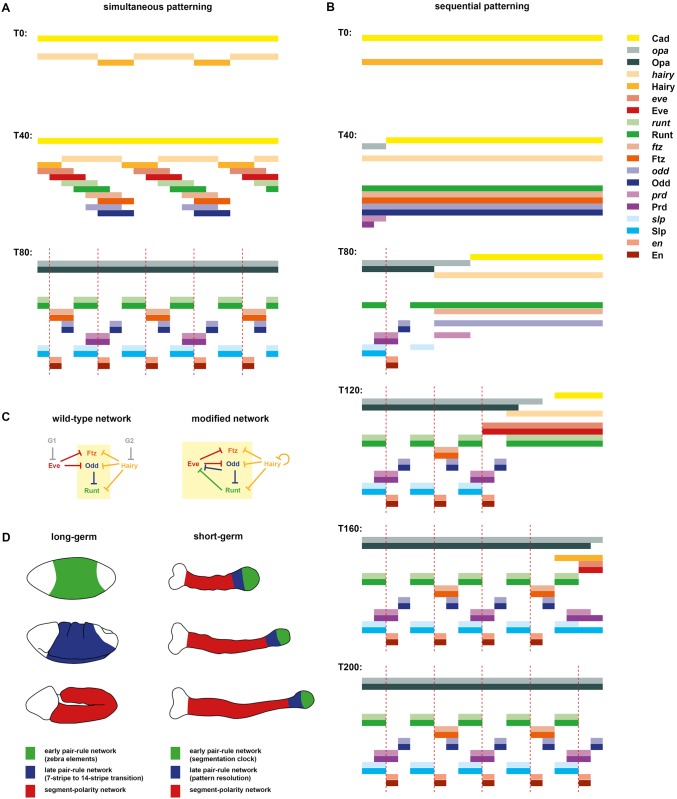
Fig 8. A slightly modified pair-rule network can pattern segments in both simultaneous and sequential modes.
(A,B) Simulation output for the modified network. Each panel shows the system state at a specific time point between T0 and T200 (see S12 and S13 Movies for complete output). In each panel, the horizontal axis represents a region of the anteroposterior (AP) axis (anterior left) and the vertical axis represents the different gene products that might be expressed in a given “cell” (individual columns C1 to C20). Pale colours represent active transcription; dark colours represent protein activity (see colour key at top right). Dotted red lines indicate parasegment boundaries. (A) The system is initialised with uniform expression of Cad and a periodic phase gradient of Hairy expression (protein or transcript “age” increases from posterior to anterior, and the pattern repeats every 8 cells—see S2 Text for details). Given these starting conditions, the dynamical behaviour of the system is almost identical to the unmodified network (compare Fig 2B or S10 Movie). Note, however, that hairy transcript is always out of phase with Hairy protein. (B) The system is initialised with uniform expression of Hairy but a decay gradient of Cad (protein disappears from anterior to posterior over time). Given these altered starting conditions, the system behaves differently and patterning takes longer, but the same stable segmental pattern eventually emerges. Note that in this simulation, the primary pair-rule genes continuously oscillate within cells that do not express Opa, and segmental stripes emerge progressively from anterior to posterior as Opa turns on along the AP axis. (C) Comparison of the original (left) and modified (right) early networks. For simplicity, the secondary pair-rule genes are not shown. See text for details. (D) Proposed regulatory homology between phases of segmentation gene expression in long-germ embryos (left) and short-germ embryos (right). In Drosophila, the quintessential long-germ insect, there are 3 main phases of segment patterning during embryogenesis. During cellularisation (top), most pair-rule genes are regulated via zebra elements and become expressed in periodic patterns throughout the trunk of the embryo (green region). During gastrulation (middle), segmental patterns of pair-rule genes and segment-polarity genes emerge throughout the trunk (blue region) fairly simultaneously. Finally, during germ band extension (bottom), the segment-polarity network maintains the segment pattern via intercellular signalling throughout the trunk (red region). In short-germ embryos, for example, those of the red flour beetle Tribolium castaneum, segment patterning occurs continuously throughout germ band extension. Three germ bands of increasing age are depicted: the trunk elongates from the posterior as embryogenesis proceeds. Throughout the whole process, primary pair-rule genes exhibit oscillatory expression in the posterior segment addition zone (green regions). Pair-rule stripes then resolve (and may undergo frequency doubling) in the anterior segment addition zone (blue region). Finally, segment-polarity genes are expressed in segmental stripes starting just anterior to the segment addition zone (red regions). This phase of expression is thought to be regulated by a conserved signalling network [105,106]. Abbreviations: Cad, Caudal; Opa, Odd-paired.
The only explicit regulatory changes that I made to the simulation were to the control logic of eve, hairy, and opa. First, I removed the regulation of eve by gap inputs (G2 in the model) and replaced it with early repression from Runt and Odd, both of which repress the eve late element in wild-type embryos (Fig 1A, right). Second, I removed the regulation of hairy by gap inputs (G1 in the model) and replaced it with autorepression, which is a common phenomenon for her/hes family genes [107,108]. Third, I tied the onset of opa expression to the decay of Cad by making Cad repress opa. These changes allowed the gap inputs G1 and G2 to be removed from the model and simplified the temporal control of the patterning process, putting everything downstream of Cad and Hairy.
I took this modified version of the Drosophila network and explored how it behaved at the tissue level, given various sets of initial conditions. I found that if I set up a repeating initial phase gradient of Hairy expression (i.e., for each cell along a double-segment repeat, Hairy expression begins at a slightly different point in its feedback cycle) and left initial Cad expression unchanged, the simulation output was essentially identical to before (Fig 8A and S12 Movie). This is because the waves of oscillating Hairy expression that sweep through the tissue while the early network is active are sufficient to correctly organise the pair-rule stripes of all the remaining primary pair-rule genes (including Eve), and therefore downstream patterning proceeds unperturbed.
However, I also discovered that if I removed all spatial patterning of Hairy from the initial conditions and instead established an anterior-to-posterior decay gradient of Cad (mimicking the retracting Cad domain seen in short-germ insects [87,109]), the simulation still produced the correct segmental output except that, now, this pattern emerged sequentially instead of simultaneously (Fig 8B and S13 Movie). As Cad decays and disappears from progressively more posterior cells over time, Opa turns on in an anterior-to-posterior wave, switching cells from the early network over to the late network. Cells in the posterior portion of the tissue synchronously passage through a repeating sequence of pair-rule gene expression (Hairy → Eve → Runt → Ftz/Odd → Hairy) until this point and subsequently differentiate into particular segment-polarity fates.
The modified network (Fig 8C, right) is thus compatible both with Drosophila-like pair-rule gene expression and with “clock-and-wave-front” pair-rule gene expression similar to that seen in, e.g., Tribolium. This finding suggests (1) that many of the regulatory interactions within the Drosophila pair-rule network might be conserved in short-germ arthropod species and (2) that long-germ and short-germ segmentation involve essentially similar patterning mechanisms and dynamics. Specifically, segment-polarity fate appears to be determined in both cases by the intersection of 2 sets of temporal signals, those carried by the pair-rule genes and those carried by broadly expressed extrinsic timing factors. In long-germ embryos, a periodic pattern of segment-polarity fates is achieved by directly imparting spatial information to the pair-rule genes using the gap system and stripe-specific elements. In short-germ embryos, however, retracting wave fronts of the timing factors provide this spatial information, and the gap genes play other roles.
Discussion
A revised view of the Drosophila pair-rule network reconciles long-germ and short-germ segmentation
In this manuscript, I have analysed the structure and dynamics of the Drosophila pair-rule network using a combination of simulated and experimental data to reveal how segment patterning is achieved. I have discovered a functional role for dynamic gap inputs in correctly phasing the pair-rule stripes and propose revised mechanisms for the patterning of the odd-numbered and even-numbered parasegment boundaries. In contrast to previous models based around the principle of static morphogen gradients, these mechanisms involve a coordinated interplay between intrinsic network dynamics and extrinsic spatiotemporal signals and do not necessarily require graded pair-rule activity.
These findings contribute to the evolving view of the role of Even-skipped, perhaps the best known of the pair-rule factors. Eve has long been known to be required for the expression of both sets of en stripes and hence both sets of parasegment boundaries [71,98]. Originally, Eve was thought to achieve this directly by activating en. Later, it was recognised that Eve does not regulate en directly but instead represses several other pair-rule factors that themselves repress en [75,76]; however, quantitative information inherent within the Eve stripes was still believed to establish the template for the en stripes [8,30]. This conclusion is challenged by the new model presented here, which suggests that static domains of Eve expression would cause a similar degree of pattern loss to that seen in eve mutant embryos. Instead, I propose that Eve conveys positional information largely via its expression dynamics, which are decoded downstream by the rest of the pair-rule gene network. (Interestingly, while “French flag” type morphogen gradient mechanisms have been proposed to underpin many developmental patterning systems, several modern studies have found that the reality often involves complex dynamics [58,110–113].)
The findings also clarify the relationship between long-germ and short-germ segmentation. I have shown that pair-rule patterning is fundamentally a temporal process and that it is therefore straightforward to convert the Drosophila pair-rule network into a clock-and-wave-front system. Under this scenario, the dynamic “early” network regulates oscillatory expression in the posterior of the tissue, while the switch-like “late” network stabilises these outputs into segmental patterns in the anterior. Given that long-germ insects such as Drosophila are derived from short-germ ancestors, it seems unlikely that this fluidity between simultaneous and sequential patterning modes is a coincidence. I therefore propose that output from the Drosophila early pair-rule network is developmentally homologous to the oscillatory pair-rule gene expression seen in the segment addition zone of short-germ insects such as Tribolium, while the output from the late pair-rule network is developmentally homologous to the stripe refinement and frequency doubling that occurs just anterior to the segment addition zone (Fig 8D). This hypothesis is strongly supported by a recent comparative study carried out by Andrew Peel and myself, which found that different phases of pair-rule gene expression in Tribolium exhibit the same correlations with cad and opa expression as seen in Drosophila [87]. Strikingly, the hypothesis implies that the “zebra” elements of runt and odd (which drive pair-rule patterns in the Drosophila blastoderm) represent the relictual components of some ancestral segmentation clock that drove oscillations of these genes with double-segment periodicity [50].
How did long-germ segmentation evolve?
The evolutionary transition from short-germ segmentation to long-germ segmentation is thought to have been driven largely by selection for faster development. A segmentation clock can only tick so fast, and therefore represents a tight production bottleneck when generating segments: a longer body requires a longer time (S7A Fig). (Selection for quicker development may also underlie the evolutionary success of “pair-rule” patterning, seen in centipedes [114] as well as insects [115,116], which effectively halves the number of clock cycles required to produce a body of a given length.) In contrast, segmentation can be accomplished remarkably quickly when pair-rule gene expression is initialised with a periodic spatial pattern, as in Drosophila.
Mechanistically, the transition to long-germ segmentation seems to have involved 2 main changes: (1) a heterochronic shift in the deployment of the pair-rule network from posterior segment addition zone to blastoderm and (2) the elaboration of the posterior gap network and associated stripe-specific elements. These latter processes are likely to have occurred progressively along the AP axis, with intermediate forms exhibiting a composite mode of development: simultaneous for more anterior segments, sequential for more posterior segments [103]. The process also seems to have been facilitated by the re-use of anterior stripe-specific elements to also drive more posterior pair-rule stripes [23,117,118]. However, given the complexity of Drosophila pair-rule patterning, it has not been clear how this process could have evolved gradually and seamlessly, all the while maintaining a perfect segmental output pattern.
The findings in this paper significantly mitigate this problem. In my model of the Drosophila pair-rule network, 2 of the primary pair-rule genes are patterned by gap inputs while 3 are patterned by cross-regulation. However, the system works equally well if only 1 of these genes is explicitly patterned and the rest are cross-regulated (S12 Movie) or if all 5 are patterned by gap inputs and there is no cross-regulation (S8 Movie). Therefore, direct stripe patterning through stripe-specific elements and indirect stripe patterning through zebra elements can stand in for one another without affecting events downstream. (Similar conclusions, including a potential role for stripe shifts, were reached by a recent in silico evolution study focused on the Drosophila pair-rule genes [119].) Consequently, there is no need for multiple stripe-specific elements to have evolved simultaneously during the transition to long-germ segmentation: new stripe-specific elements could have evolved one by one, each time slotting into or replacing parts of the existing patterning machinery. Retention of an intact segmentation clock could also have buttressed gap-driven segment patterning during the transition, and such a scenario may apply today to insects such as Nasonia and Bombyx, which exhibit some evidence of both patterning modes [120–122]. The corollary of such flexibility between gap-driven and cross-regulated pair-rule gene expression is, however, that the dynamics of the gap network need to be highly constrained and should resemble those of the original segmentation clock. This appears to be the case [67].
A trade-off between patterning speed and patterning robustness
Above, I stated that stripe-specific elements and cross-regulation are theoretically interchangeable for positioning pair-rule stripes. In the Drosophila blastoderm, the regulatory situation appears to be partially redundant: at least 3 of the primary pair-rule genes possess stripe-specific elements in any given pair-rule repeat, but only 2 of these genes lack zebra elements [16]. Why then have multiple pair-rule genes evolved stripe-specific elements, when patterning just a single gene in this way is theoretically sufficient for making segments? Additionally, why do genes such as runt retain a zebra element when they already have a full set of stripe-specific elements?
The first question has 2 likely answers. Using gap inputs to directly pattern multiple genes speeds up the first emergence of a correctly phased pair-rule repeat and hence reduces the total time required for segmentation as well as the minimum magnitude of the stripe shifts (S7B Fig; S6–S8 Movies). (The extreme scenario is that gap shifts are dispensed with entirely, as seems to have occurred in the anterior of the Drosophila trunk, based on comparisons with the scuttle fly Megaselia abdita [123,124].) The acquisition of extra stripe-specific elements could therefore have been driven by selection for marginal decreases to the length of development. Alternatively, these elements could have been selected for in order to mitigate irregularities in the patterns of upstream pair-rule genes. For example, runt, ftz, and odd all possess a stripe-specific element for stripe 3 [16], corresponding to a region of the blastoderm in which their repressor, Hairy, is inappropriately expressed early on [59].
While the stripe-specific elements of runt, ftz, and odd may therefore increase the speed of patterning, their output patterns are not as regular as those of hairy and eve, probably answering the second question. Irregular gap-driven pair-rule stripes, if not later refined by cross-regulation, would result in aberrant segment-polarity patterns. For example, cross-regulation of the runt pair-rule stripes appears to be absent in Dichaete mutant embryos, and this leads to severe and variable segmentation defects [87].
However, even if these runt stripes were as regular as those of eve, cross-regulation might still be required. The eve stripes are patterned with a precision of about 1% AP length, i.e., differing in position from embryo to embryo by roughly 1 nucleus [41]. This positional noise is equivalent to the spatial resolution of the final segment-polarity pattern, in which most stripes are only 1 cell wide (Fig 1B). Therefore, if the eve and runt stripes both showed positional variation of this magnitude, and this noise was uncorrelated, segmentation defects would likely occur. The benefit of the dynamic patterning carried out by the pair-rule network is that the influence of Eve activity on gene expression is not restricted to the cells currently within each Eve stripe but also extends to cells posterior to these stripes, like wakes stretching out behind moving ships. This means that entire pair-rule repeats become coordinated with the Eve stripes, regardless of where exactly these stripes are located in the blastoderm. Thus, spending extra time to cross-regulate is likely to minimise the consequences of noise in the original spatial signals, a trade-off that is characteristic of many developmental patterning systems [125].
Assumptions, unexplained phenomena, and model limitations
I have presented theoretical evidence that posterior-to-anterior shifts of pair-rule stripes, driven by dynamic gap inputs, play a functional role in segment patterning. The plausibility of my model therefore rests on these shifts existing and being of an appropriate speed and magnitude. The basic model (only hairy and eve patterned by gap inputs) requires a minimum shift of 3 nuclei, while versions including greater control of pair-rule gene expression by gap inputs reduce this minimum to 1 or 2 nuclei (S6–S8 Movies). As yet, these shifts have only been measured in real embryos by comparing population estimates of absolute stripe position (in percent egg length) in fixed material of different ages [59,61]. Interpreting these measurements is not straightforward, but adjustments for nuclear migration suggest shifts of at least 2–3 nuclei for eve stripes 3–7, consistent with the requirements of the model. Recent developments in live imaging [126–128] mean that it should now be possible to test these estimates directly in individual embryos.
While the new model clarifies many aspects of segment patterning, it also highlights certain regulatory phenomena that are currently unexplained and require further study. For example, it is not clear how the timing of slp transcription is controlled, how exactly prd is spatially regulated, or which factors are responsible for activating the pair-rule genes.
Finally, the model is obviously simplistic and represents only the first step towards a modern, system-level understanding of the pair-rule network. For example, while the model demonstrates that morphogen-like effects are not necessary to account for pair-rule patterning, it does not rule them out. (Indeed, anterior-to-posterior expression shifts might well contribute to concentration-dependent spatial patterning of target genes, by helping shape the contours of the pair-rule stripes at the protein level.) Careful experimental characterisation of the quantitative relationship between the protein concentrations of input factors and the transcription rate of output factors could form the basis for future, more sophisticated models of Drosophila pair-rule patterning, which explore the roles of quantitative and stochastic effects. Future models of short-germ segmentation, on the other hand, could account for morphogenetic processes such as cell division and rearrangement [129–131] and/or incorporate known intercellular communication processes that occur both upstream (e.g., Notch-Delta signalling [132–134]) and downstream (e.g., Toll receptor codes [135,136]) of the pair-rule genes.
Concluding remarks
In the introduction to this paper, I highlighted 3 recent findings related to pair-rule patterning: (1) that pair-rule orthologs exhibit oscillating expression in the segment addition zones of short-germ arthropods, (2) that pair-rule gene expression in long-germ insects is patterned by dynamic gap gene expression, and (3) that the pair-rule network in Drosophila undergoes an extensive topology change at gastrulation. I then presented evidence that dynamic Eve expression is integral to segment patterning and also that the Drosophila pair-rule network is broadly compatible with both simultaneous and sequential modes of segmentation.
I therefore propose the following evolutionary hypothesis, which ties together and potentially explains the 3 original findings. (1) Long-germ and short-germ segmentation are not dichotomous modes of patterning but rather alternative behaviours of a largely conserved pair-rule network. (2) In long-germ insects, ad hoc patterning of stripes by gap inputs stands in for the expression of particular pair-rule gene “clock enhancers”, meaning that gap gene networks are under strong selection to preserve dynamic behaviour. (3) In Drosophila, the “early” pair-rule network is derived from the “clock” part of an ancestral short-germ segmentation mechanism, while the “late” network is derived from the stabilising genetic interactions that would have originally established parasegment boundaries anterior to the segment addition zone.
These predictions can be tested in the future by comparative studies in emerging arthropod model species.
Materials and methods
Wild-type in situ images are from a previously published data set deposited in the Dryad repository: http://dx.doi.org/10.5061/dryad.cg35k [137]. The eve mutation used was eve3 (gift of Bénédicte Sanson) and was balanced over CyO hb::lacZ (Boomington stock no. 6650) in order to easily distinguish homozygous mutant embryos. Whole mount double FISH, microscopy, and image analysis were carried out as described previously [32]. The eve/Eve doubles in Fig 4D were generated by incubating embryos with 1:1,000 rabbit anti-Eve [138] and 1:500 anti-rabbit 488 following in situ hybridisation to eve. Images were analysed using Fiji [139,140]: intensity profiles were produced using the “multi plot” function, while thresholded images were produced using the “make binary” tool. Simulations were coded in Python (www.python.org) using the libraries NumPy [141] and Matplotlib [142]. SBML-qual files were generated using the GINsim software (http://ginsim.org) [143]. All models and simulations are described in detail in S2 Text.
Supporting information
Expanded version of Fig 3 from the main text, showing all pairwise combinations between hairy, eve, runt, ftz, odd, and slp. (A) hairy and eve pair-rule stripes partially overlap during cellularisation. At gastrulation, hairy expression fades away, while the eve stripes narrow from the posterior and then also fade. (B) eve and runt pair-rule stripes partially overlap during cellularisation. The eve stripes become increasingly narrow at gastrulation, and the runt secondary stripes emerge anterior to the eve stripes. By early GBE, eve expression has faded away and runt expression has resolved into a regular, segmental pattern. The refinement of the eve stripes occurs more gradually in real embryos than in the simulation. Arrowheads in B”‘ indicate new runt expression related to the developing nervous system. (C) eve and ftz pair-rule stripes are at first expressed in complementary patterns. Starting from late cellularisation, they both narrow from the posterior (eve more than ftz). eve expression later fades away, while ftz persists. (D) eve and odd pair-rule stripes are at first expressed in complementary patterns, before both narrowing. odd secondary stripes emerge at the posterior of the narrowing eve domains, which then fade away, leaving segmental stripes of odd. (E) hairy and runt pair-rule stripes are expressed in complementary patterns during cellularisation. hairy expression then fades away, while runt transitions to segmental stripes. (F) hairy and ftz pair-rule stripes slightly overlap during cellularisation. hairy expression then fades away, while the ftz stripes narrow. (G) hairy and odd pair-rule stripes slightly overlap during cellularisation. hairy expression then fades away, while odd transitions to narrow segmental stripes. (H) runt and ftz pair-rule stripes partially overlap throughout cellularisation. At gastrulation, runt secondary stripes emerge to the posterior of the narrowing ftz stripes. Later, the runt primary stripes refine from the posterior, and the overlaps with ftz are lost. (I) runt and odd pair-rule stripes slightly overlap during cellularisation. These overlaps resolve at late cellularisation (slightly earlier than in the simulation). At gastrulation, new odd expression emerges just anterior to the runt primary stripes, while new runt expression emerges just posterior to the refining odd primary stripes. By early GBE there is a regular segmental pattern of abutting odd and runt stripes, separated by gaps. Arrowheads in I”‘ indicate new runt expression related to the developing nervous system. (J) The ftz and odd stripes are fairly congruent during cellularisation. At gastrulation, both narrow from the posterior, and the odd secondary stripes intercalate between them. Over the course of patterning, their anterior boundaries also become offset from one another. (K) The slp primary stripes emerge later than the hairy stripes, but share an anterior border with them. (The slp stripes in the simulation are too broad at this stage—they should be narrower than the hairy stripes.) hairy expression then fades away, while slp transitions to a segmental pattern. (L) The slp primary stripes emerge later than the eve stripes, and abut their anterior borders. (In the simulation, these slp stripes extend too far posteriorly, and overlap with eve. The reason is that the eve anterior borders would have stabilised by this point in real embryos, but they are still shifting in the simulation.) At gastrulation, the eve stripes narrow from the posterior and then fade, while slp transitions to segmental stripes. (M) The slp primary stripes emerge later than the runt primary stripes, and are offset slightly from their posterior boundaries. (The simulated slp domains are wider than the real slp domains.) At gastrulation, secondary stripes of both genes emerge between the primary stripes (the widths of the simulated slp stripes are now appropriate). The expression patterns become largely congruent, except at the posteriors of the runt primary stripes. These differences resolve later, when the runt primary stripes narrow. (N) The slp primary stripes emerge later than the ftz primary stripes, and partially overlap with them. At gastrulation, the secondary slp stripes emerge just anterior to the ftz domains, which narrow from the posterior, losing the overlaps with the slp primary stripes. (O) As for (N), the slp primary stripes partially overlap the odd primary stripes, and these overlaps are later lost by the odd stripes narrowing from the posterior. The secondary stripes of odd and slp intercalate between the primary stripes, and abut one other.
(DOCX)
Each panel shows an uncropped version of the corresponding double fluorescent in situ image in S1 Fig. All panels show a whole embryo lateral view, anterior left, dorsal top. Embryos within each column are of approximately equal age. Scale = 100 μm.
(DOCX)
This figure compares double FISH data of pair-rule gene expression with simulated transcript expression, as in Fig 3 and S2 Fig. (A) prd transcripts (magenta) are shown relative to eve (green), odd (blue) or slp (cyan) transcripts, at four different embryo ages (left) or simulation timepoints (right). Note that the figure presents a different set of developmental ages / simulated timepoints from those in Fig 3 and Supplementary Figure 3, in order to show cellularisation in greater temporal detail. At “early cellularisation”, the primary pair-rule genes are expressed but prd and slp are not; at “mid cellularisation”, prd is expressed but slp is not; at “late cellularisation”, slp is additionally expressed. While the simulated expression of prd is broadly appropriate early on (e.g. compare prd and odd expression at mid-cellularisation / T26), several aspects of prd expression are not recapitulated by the model. First, the changing phasing of prd posterior borders and eve anterior borders between mid-cellularisation and late cellularisation (see also Fig 5). Second, the splitting of the prd stripes at late cellularisation, slightly prior to the appearance of the secondary stripes of odd and slp. Third, the patterning of the prd “A” stripes (i.e. the narrow stripes formed from anterior portions of the early broad stripes, asterisks in the in situ images) and the consequent emergence of single-segment periodicity. (B) Uncropped views of the embryos shown in (A). Scale bars 100 μm.
(DOCX)
(A) Double FISH images of segmentation gene expression in gastrulation stage embryos (lateral view, anterior left, dorsal top). Each row shows a different pairwise comparison of the transcripts of the genes eve (magenta), prd (blue), slp (green), en (red), odd (yellow), and wg (cyan). From left to right, the panels in each row show: 1) a whole embryo view; 2) an enlarged view of pair-rule repeats 2–6; 3) the individual channel for the first gene listed in the row label; 4) the individual channel for the second gene listed in the row label. Arrowheads mark the locations of prospective odd-numbered parasegment boundaries. Scale bars 100 μm. (B) Summary schematic showing the relative expression of the Eve, Prd, and Slp protein domains, and the en, wg, and odd transcript domains, at the odd-numbered parasegment boundaries. (C) The same schematic as in B, with the addition of the regulatory interactions between Eve, Prd, and Slp and their target genes. Aside from the colours, which are changed to match the in situ images, this schematic is identical to Fig 5A.
(DOCX)
This figure is an expansion of Fig 7 in the main text, showing additional whole embryo and single channel views. Enlarged views show stripes 2–6. Asterisks in (E) indicate the stripe 3 region. Scale = 50 μm. Note the quantitative traces below the images in (C). These plots show the AP intensity profiles of runt (green) and slp (blue) along a narrow ventral strip of the trunk of the two embryos pictured. In the wild-type embryo, the runt stripes are all (except stripe 7) roughly symmetrical and strongly expressed. In the eve mutant embryo, runt stripes 1–6 (which overlap with odd expression, see B), have much lower intensity than runt stripe 7 (which doesn’t overlap with odd expression, see B) and exhibit a sawtooth pattern, in which expression intensity decreases from anterior to posterior. The slp expression in the eve mutant embryo, while broad, does display a pair-rule modulation, which is in opposite phase to the downregulated runt stripes. Therefore, the same two regulatory interactions (repression of runt by Odd, and of slp by Runt) are evident in both the in situ data and the simulated data (Fig 7A’), but lead to slightly different expression patterns in each case, one quantitative and one qualitative.
(DOCX)
Simulation output showing the expression patterns generated by the early pair-rule network, assuming various anterior-to-posterior shifts speeds of the gap inputs (shown in black). See supplementary movies for full simulation output (S1 Movie = 0x; S2 Movie = 1x; S3 Movie = 0.5x; S4 Movie = 2x; S5 Movie = 3x). Gap domain shift speeds are relative to the time delay for pair-rule protein synthesis / decay: a speed of 1x leads to a one nucleus offset between the anterior borders of transcript and protein domains, a speed of 2x leads to a two nucleus offset, and so on. See S2 Text for further details about the simulations.
(DOCX)
(A,B) Minimum duration required for generating a correct segment pattern (En, Odd, Slp repeats), under various simulation scenarios. Minimum simulation duration (Y-axis) is given as a multiple of the synthesis / decay delay parameter value of the pair-rule gene products (corresponding to 6 timesteps for all simulations shown in this manuscript). See S2 Text for further details on the various simulations. (A) Minimum time required to pattern a given number of segments by either simultaneous or sequential patterning, using the same network model as S12 and S13 Movies. Sequential patterning requires 12x the delay for the initial two segments, plus 8x the delay for every two additional segments. Simultaneous patterning requires a constant minimum time of 10x the delay, no matter the number of segments. (B) Minimum time (in multiples of the synthesis / decay delay parameter value) required for simultaneous patterning, for systems with increasingly elaborate gap patterning. If only hairy receives gap inputs (1), a minimum of time 10x the delay is required (network model as in simulation 12). If both hairy and eve receive gap inputs (2), the minimum is 7x the delay (network model as in simulation 10). If runt or ftz/odd additionally receive gap inputs (3), the minimum is 6x the delay (early network model as in simulations 6 or 7, respectively, plus late network model as for simulation 10). Finally, if all the primary pair-rule genes receive gap inputs (4), the minimum is 5x the delay (early network model as in simulation 8, plus late network model as for simulation 10). Note that the stripes of ftz and odd are considered a single pattern, because of their identical regulation in the simulations.
(DOCX)
(PDF)
(PDF)
(PY)
Corresponds to the network in Simulation 10 and Fig 2B.
(PY)
Corresponds to the network in Simulation 10 and Fig 2B.
(SBML)
Corresponds to the network in Simulations 12 and 13, and Fig 8.
(SBML)
Simulated expression of the primary pair-rule genes, assuming static gap inputs.
(MP4)
Simulated expression of the primary pair-rule genes, assuming dynamic gap inputs.
(MP4)
Simulated expression of the primary pair-rule genes, assuming slow gap shifts.
(MP4)
Simulated expression of the primary pair-rule genes, assuming fast gap shifts.
(MP4)
Simulated expression of the primary pair-rule genes, assuming very fast gap shifts.
(MP4)
Pattern establishment when hairy, eve, and runt take gap inputs.
(MP4)
Pattern establishment when hairy, eve, and ftz/odd take gap inputs.
(MP4)
Pattern establishment when all primary pair-rule genes take gap inputs.
(MP4)
Simulated behaviour of the whole pair-rule system, assuming static gap inputs.
(MP4)
Simulated behaviour of the whole pair-rule system, assuming dynamic gap inputs.
(MP4)
Pair-rule gene expression in a simulated eve mutant.
(MP4)
Simultaneous patterning by the modified pair-rule network.
(MP4)
Sequential patterning by the modified pair-rule network.
(MP4)
Acknowledgments
I am very grateful to Michael Akam, who advised and encouraged me throughout the course of this project and commented on the manuscript, and to Tim Weil, who contributed lab space and taught me to work with flies. Michalis Averof, Matt Benton, Berta Verd, Olivia Tidswell, and Ezzat El-Sherif commented on a draft of the manuscript. I thank Andrew Peel, Andy Oates, Johannes Jaeger, Siegfried Roth, Bénédicte Sanson, James Briscoe, and Alfonso Martinez-Arias for useful discussions.
Abbreviations
- AP
anteroposterior
- Cad
Caudal
- en
engrailed
- eve
even-skipped
- FISH
fluorescent in situ hybridisation
- ftz
fushi tarazu
- odd
odd-skipped
- Opa
Odd-paired
- prd
paired
- SBML
Systems Biology Markup Language
- slp
sloppy-paired
- wg
wingless
Data Availability
All relevant data are within the paper and its Supporting Information files. Additional expression data from wild-type embryos are held in the Dryad Digital Repository (DOI: http://dx.doi.org/10.5061/dryad.cg35k).
Funding Statement
Isaac Newton Trust www.newtontrust.cam.ac.uk. Research grant. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Biotechnology and Biological Sciences Research Council www.bbsrc.ac.uk. Genes to Organisms PhD studentship. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Akam M. The molecular basis for metameric pattern in the Drosophila embryo. Development. 1987;101:1–22. [PubMed] [Google Scholar]
- 2.Nasiadka A, Dietrich BH, Krause HM. Anterior—posterior patterning in the Drosophila embryo. Adv Dev Biol Biochem. 2002;12:155–204. [Google Scholar]
- 3.Nüsslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801. [DOI] [PubMed] [Google Scholar]
- 4.Wieschaus E, Nüsslein-Volhard C. The Heidelberg Screen for Pattern Mutants of Drosophila : A Personal Account. Annu Rev Cell Dev Biol. 2016;32:1–46. doi: 10.1146/annurev-cellbio-113015-023138 [DOI] [PubMed] [Google Scholar]
- 5.Arnosti DN. Analysis and function of transcriptional regulatory elements: insights from Drosophila. Annu Rev Entomol. 2003;48:579–602. doi: 10.1146/annurev.ento.48.091801.112749 [DOI] [PubMed] [Google Scholar]
- 6.DiNardo S, O’Farrell PH. Establishment and refinement of segmental pattern in the Drosophila embryo: spatial control of engrailed expression by pair-rule genes. Genes Dev. 1987;1:1212–25. [DOI] [PubMed] [Google Scholar]
- 7.Ingham PW, Baker NE, Martinez-Arias A. Regulation of segment polarity genes in the Drosophila blastoderm by fushi tarazu and even skipped. Nature. 1988;331:73–5. doi: 10.1038/331073a0 [DOI] [PubMed] [Google Scholar]
- 8.Jaynes JB, Fujioka M. Drawing lines in the sand: even skipped et al. and parasegment boundaries. Dev Biol. 2004;269:609–22. doi: 10.1016/j.ydbio.2004.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ingham P, Howard K, Ish-Horowicz D. Transcription pattern of the Drosophila segmentation gene hairy. Nature. 1985;318:162–3. [Google Scholar]
- 10.Macdonald PM, Ingham P, Struhl G. Isolation, structure, and expression of even-skipped: a second pair-rule gene of Drosophila containing a homeo box. Cell. 1986;47:721–34. [DOI] [PubMed] [Google Scholar]
- 11.Gergen JP, Butler BA. Isolation of the Drosophila segmentation gene runt and analysis of its expression during embryogenesis. Genes Dev. 1988;2:1179–93. [DOI] [PubMed] [Google Scholar]
- 12.Hafen E, Kuroiwa A, Gehring WJ. Spatial distribution of transcripts from the segmentation gene fushi tarazu during Drosophila embryonic development. Cell. 1984;37:833–41. [DOI] [PubMed] [Google Scholar]
- 13.Coulter DE, Swaykus EA, Beran-Koehn MA, Goldberg D, Wieschaus E, Schedl P. Molecular analysis of odd-skipped, a zinc finger encoding segmentation gene with a novel pair-rule expression pattern. Embo J. 1990;8:3795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kilchherr F, Baumgartner S, Bopp D, Frei E, Noll M. Isolation of the paired gene of Drosophila and its spatial expression during early embryogenesis. 1986;321:493–9. [Google Scholar]
- 15.Grossniklaus U, Pearson RK, Gehring WJ. The Drosophila Sloppy Paired Locus Encodes Two Proteins Involved in Segmentation That Show Homology To Mammalian Transcription Factors. Genes Dev. 1992;6:1030–51. [DOI] [PubMed] [Google Scholar]
- 16.Schroeder MD, Greer C, Gaul U. How to make stripes: deciphering the transition from non-periodic to periodic patterns in Drosophila segmentation. Development. 2011;138:3067–78. doi: 10.1242/dev.062141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaeger J. The gap gene network. Cell Mol Life Sci. 2011;68:243–74. doi: 10.1007/s00018-010-0536-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howard K, Ingham P, Rushlow C. Region-specific alleles of the Drosophila segmentation gene hairy. Genes Dev. 1988;2:1037–46. [DOI] [PubMed] [Google Scholar]
- 19.Goto T, Macdonald P, Maniatis T. Early and late periodic patterns of even skipped expression are controlled by distinct regulatory elements that respond to different spatial cues. Cell. 1989;57:413–22. [DOI] [PubMed] [Google Scholar]
- 20.Harding K, Hoey T, Warrior R, Levine M. Autoregulatory and gap gene response elements of the even-skipped promoter of Drosophila. EMBO J. 1989;8:1205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pankratz MJ, Jäckle H. Making stripes in the Drosophila embryo. Trends Genet. 1990;6:287–92. [DOI] [PubMed] [Google Scholar]
- 22.Small S, Kraut R, Hoey T, Warrior R, Levine M. Transcriptional regulation of a pair-rule stripe in Drosophila. Genes Dev. 1991;5:827–39. [DOI] [PubMed] [Google Scholar]
- 23.Small S, Blair A, Levine M. Regulation of two pair-rule stripes by a single enhancer in the Drosophila embryo. Dev Biol. 1996;175:314–24. doi: 10.1006/dbio.1996.0117 [DOI] [PubMed] [Google Scholar]
- 24.Fujioka M, Emi-Sarker Y, Yusibova GL, Goto T, Jaynes JB. Analysis of an even-skipped rescue transgene reveals both composite and discrete neuronal and early blastoderm enhancers, and multi-stripe positioning by gap gene repressor gradients. Development. 1999;126:2527–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hiromi Y, Kuroiwa A, Gehring WJ. Control elements of the Drosophila segmentation gene fushi tarazu. Cell. 1985;43:603–13. [DOI] [PubMed] [Google Scholar]
- 26.Dearolf CR, Topol J, Parker CS. Transcriptional control of Drosophila fushi tarazu zebra stripe expression. Genes Dev. 1989;3:384–98. [DOI] [PubMed] [Google Scholar]
- 27.Butler BA, Soong J, Gergen JP. The Drosophila segmentation gene runt has an extended cis-regulatory region that is required for vital expression at other stages of development. Mech Dev. 1992;39:17–28. [DOI] [PubMed] [Google Scholar]
- 28.Gutjahr T, Vanario-Alonso CE, Pick L, Noll M. Multiple regulatory elements direct the complex expression pattern of the Drosophila segmentation gene paired. Mech Dev. 1994;48:119–28. [DOI] [PubMed] [Google Scholar]
- 29.Benedyk MJ, Mullen JR, DiNardo S. Odd-paired: A zinc finger pair-rule protein required for the timely activation of engrailed and wingless in Drosophila embryos. Genes Dev. 1994;8:105–17. [DOI] [PubMed] [Google Scholar]
- 30.Fujioka M, Jaynes JB, Goto T. Early even-skipped stripes act as morphogenetic gradients at the single cell level to establish engrailed expression. Development. 1995;121:4371–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mullen JR, DiNardo S. Establishing parasegments in Drosophila embryos: roles of the odd-skipped and naked genes. Dev Biol. 1995;169:295–308. doi: 10.1006/dbio.1995.1145 [DOI] [PubMed] [Google Scholar]
- 32.Clark E, Akam M. Odd-paired controls frequency doubling in Drosophila segmentation by altering the pair-rule gene regulatory network. Elife. 2016;5:e18215 doi: 10.7554/eLife.18215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cadigan KM, Grossniklaus U, Gehring WJ. Localized Expression of Sloppy Paired Protein Maintains the Polarity of Drosophila Parasegments. Genes Dev. 1994;8:899–913. [DOI] [PubMed] [Google Scholar]
- 34.Li X, Noll M. Role of the gooseberry gene in Drosophila embryos: maintenance of wingless expression by a wingless—gooseberry autoregulatory loop. EMBO J. 1993;12:4499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DiNardo S, Heemskerk J, Dougan S, O’Farrell PH. The making of a maggot: patterning the Drosophila embryonic epidermis. Curr Opin Genet Dev. 1994;4:529–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perrimon N. The genetic basis of patterned baldness in Drosophila. Cell. 1994;76:781–4. [DOI] [PubMed] [Google Scholar]
- 37.Sanson B. Generating patterns from fields of cells: Examples from Drosophila segmentation. EMBO Rep. 2001;2:1083–8. doi: 10.1093/embo-reports/kve255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jaeger J, Blagov M, Kosman D, Kozlov KN, Manu, Myasnikova E, et al. Dynamical analysis of regulatory interactions in the gap gene system of Drosophila melanogaster. Genetics. 2004;167:1721–37. doi: 10.1534/genetics.104.027334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manu, Surkova S, Spirov AV, Gursky VV, Janssens H, Kim A-R, et al. Canalization of Gene Expression and Domain Shifts in the Drosophila Blastoderm by Dynamical Attractors. PLoS Comput Biol. 2009;5(3):e1000303 doi: 10.1371/journal.pcbi.1000303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gursky VV, Panok L, Myasnikova EM, Manu, Samsonova MG, Reinitz J, et al. Mechanisms of gap gene expression canalization in the Drosophila blastoderm. BMC Syst Biol. 2011;5:118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dubuis JO, Tkacik G, Wieschaus EF, Gregor T, Bialek W. Positional information, in bits. Proc Natl Acad Sci U S A. 2013;110:16301–8. doi: 10.1073/pnas.1315642110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tkačik G, Dubuis JO, Petkova MD, Gregor T. Positional information, Positional error, and readout precision in morphogenesis: A mathematical framework. Genetics. 2015;199:39–59. doi: 10.1534/genetics.114.171850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petkova MD, Tkačik G, Bialek W, Wieschaus EF, Gregor T. Optimal decoding of information from a genetic network. 2016. Preprint. Available from: arXiv:1612.08084v1.
- 44.Sánchez L, Thieffry D. Segmenting the fly embryo: a logical analysis of the pair-rule cross-regulatory module. J Theor Biol. 2003;224:517–37. [DOI] [PubMed] [Google Scholar]
- 45.Campos-Ortega JA, Hartenstein V. The Embryonic Development of Drosophila melanogaster. Springer-Verlag; 1985. [Google Scholar]
- 46.Krause G. Die Eytipen der Insekten. Biol Zbl. 1939;59:495–536. [Google Scholar]
- 47.Sander K. Specification of the Basic Body Pattern in Insect Embryogenesis. Adv In Insect Phys. 1976;12:125–238. [Google Scholar]
- 48.Davis GK, Patel NH. Short, Long, and Beyond: Molecular and Embryological Approaches to Insect Segmentation. Annu Rev Entomol. 2002;669–99. doi: 10.1146/annurev.ento.47.091201.145251 [DOI] [PubMed] [Google Scholar]
- 49.Damen WGM, Janssen R, Prpic NM. Pair rule gene orthologs in spider segmentation. Evol Dev. 2005;7:618–28. doi: 10.1111/j.1525-142X.2005.05065.x [DOI] [PubMed] [Google Scholar]
- 50.Choe CP, Miller SC, Brown SJ. A pair-rule gene circuit defines segments sequentially in the short-germ insect Tribolium castaneum. Proc Natl Acad Sci U S A. 2006;103:6560–4. doi: 10.1073/pnas.0510440103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Janssen R, Budd GE, Prpic NM, Damen WG. Expression of myriapod pair rule gene orthologs. Evodevo. 2011;2:5 doi: 10.1186/2041-9139-2-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Green J, Akam M. Evolution of the pair rule gene network: Insights from a centipede. Dev Biol. 2013;382:235–45. doi: 10.1016/j.ydbio.2013.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sarrazin AF, Peel AD, Averof M. A segmentation clock with two-segment periodicity in insects. Science. 2012;336:338–41. doi: 10.1126/science.1218256 [DOI] [PubMed] [Google Scholar]
- 54.El-Sherif E, Averof M, Brown SJ. A segmentation clock operating in blastoderm and germband stages of Tribolium development. Development. 2012;139:4341–6. doi: 10.1242/dev.085126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brena C, Akam M. An analysis of segmentation dynamics throughout embryogenesis in the centipede Strigamia maritima. BMC Biol. 2013;11:112 doi: 10.1186/1741-7007-11-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cooke J, Zeeman EC. A clock and wavefront model for control of the number of repeated structures during animal morphogenesis. J Theor Biol. 1976;58:455–76. [DOI] [PubMed] [Google Scholar]
- 57.Liu PZ, Kaufman TC. Short and long germ segmentation: Unanswered questions in the evolution of a developmental mode. Evol Dev. 2005;7:629–46. doi: 10.1111/j.1525-142X.2005.05066.x [DOI] [PubMed] [Google Scholar]
- 58.Jaeger J, Surkova S, Blagov M, Janssens H, Kosman D, Kozlov KN, et al. Dynamic control of positional information in the early Drosophila embryo. Nature. 2004;430:368–71. doi: 10.1038/nature02678 [DOI] [PubMed] [Google Scholar]
- 59.Surkova S, Kosman D, Kozlov K, Manu, Myasnikova E, Samsonova AA, et al. Characterization of the Drosophila segment determination morphome. Dev Biol. 2008;313:844–62. doi: 10.1016/j.ydbio.2007.10.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.El-Sherif E, Levine M. Shadow Enhancers Mediate Dynamic Shifts of Gap Gene Expression in the Drosophila Embryo. Curr Biol. 2016;26:1164–9. doi: 10.1016/j.cub.2016.02.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Keränen SVE, Fowlkes CC, Luengo Hendriks CL, Sudar D, Knowles DW, Malik J, et al. Three-dimensional morphology and gene expression in the Drosophila blastoderm at cellular resolution II: dynamics. Genome Biol. 2006;7:R124 doi: 10.1186/gb-2006-7-12-r124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meinhardt H. Hierarchical inductions of cell states: a model for segmentation in Drosophila. J Cell Sci Suppl. 1986;4:357–81. [DOI] [PubMed] [Google Scholar]
- 63.Edgar BA, Odell GM, Schubiger G. A genetic switch, based on negative regulation, sharpens stripes in Drosophila embryos. Dev Genet. 1989;10:124–42. doi: 10.1002/dvg.1020100303 [DOI] [PubMed] [Google Scholar]
- 64.Baumgartner S, Noll M. Network of interactions among pair-rule genes regulating paired expression during primordial segmentation of Drosophila. Mech Dev. 1990;33:1–18. [DOI] [PubMed] [Google Scholar]
- 65.Perkins TJ, Jaeger J, Reinitz J, Glass L. Reverse engineering the gap gene network of Drosophila melanogaster. PLoS Comput Biol. 2006;2(5):e51 doi: 10.1371/journal.pcbi.0020051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Manu, Surkova S, Spirov AV, Gursky VV, Janssens H, Kim A-R, et al. Canalization of Gene Expression in the Drosophila Blastoderm by Gap Gene Cross Regulation. PLoS Biol. 2009;7(3):e1000049 doi: 10.1371/journal.pbio.1000049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Verd B, Clark E, Crombach A, Jaeger J. A damped oscillator imposes temporal order on posterior gap gene expression in Drosophila. 2017. Preprint. Available from: bioRxiv doi: 10.1101/068072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tracey WD, Ning X, Klingler M, Kramer SG, Gergen JP. Quantitative analysis of gene function in the Drosophila embryo. Genetics. 2000;154:273–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Klingler M, Soong J, Butler B, Gergen JP. Disperse versus compact elements for the regulation of runt stripes in Drosophila. Dev Biol. 1996;177:73–84. doi: 10.1006/dbio.1996.0146 [DOI] [PubMed] [Google Scholar]
- 70.Peter IS, Faure E, Davidson EH. Predictive computation of genomic logic processing functions in embryonic development. Proc Natl Acad Sci U S A. 2012;109:16434–42. doi: 10.1073/pnas.1207852109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Harding K, Rushlow C, Doyle H, Hoey T, Levine M. Cross-regulatory interactions among pair-rule genes in Drosophila. Science. 1986;233:953–9. [DOI] [PubMed] [Google Scholar]
- 72.Frasch M, Levine M. Complementary patterns of even-skipped and fushi tarazu expression involve their differential regulation by a common set of segmentation genes in Drosophila. 1987;1:981–95. [DOI] [PubMed] [Google Scholar]
- 73.Ingham P, Gergen P. Interactions between the pair-rule genes runt, hairy, even-skipped and fushi tarazu and the establishment of periodic pattern in the Drosophila embryo. Development. 1988;104:51–60.3075544 [Google Scholar]
- 74.Vavra SH, Carroll SB. The zygotic control of Drosophila pair-rule gene expression: II. Spatial repression by gap and pair-rule gene products. Development. 1989;107:663–72. [DOI] [PubMed] [Google Scholar]
- 75.Manoukian AS, Krause HM. Concentration-dependent activities of the even-skipped protein in Drosophila embryos. Genes Dev. 1992;6:1740–51. [DOI] [PubMed] [Google Scholar]
- 76.Manoukian AS, Krause HM. Control of segmental asymetry in Drosophila embryos. Development. 1993;118:785–96. [DOI] [PubMed] [Google Scholar]
- 77.Saulier-Le Dréan B, Nasiadka A, Dong J, Krause HM. Dynamic changes in the functions of Odd-skipped during early Drosophila embryogenesis. Development. 1998;125:4851–61. [DOI] [PubMed] [Google Scholar]
- 78.Swantek D, Gergen JP. Ftz modulates Runt-dependent activation and repression of segment-polarity gene transcription. Development. 2004;131:2281–90. doi: 10.1242/dev.01109 [DOI] [PubMed] [Google Scholar]
- 79.Edgar BA, Weir MP, Schubiger G, Kornberg T. Repression and turnover pattern fushi tarazu RNA in the early Drosophila embryo. Cell. 1986;47:747–54. [DOI] [PubMed] [Google Scholar]
- 80.Weir MP, Edgar BA, Kornberg T, Schubiger G. Spatial regulation of engrailed expression in the Drosophila embryo. Genes Dev. 1988;2:1194–203. [DOI] [PubMed] [Google Scholar]
- 81.Vavra SH, Carroll SB. The zygotic control of Drosophila pair-rule gene expression: I. A search for new pair-rule regulatory loci. Development. 1989;107:663–72. [DOI] [PubMed] [Google Scholar]
- 82.Nasiadka A, Krause HM. Kinetic analysis of segmentation gene interactions in Drosophila embryos. Development. 1999;126:1515–26. [DOI] [PubMed] [Google Scholar]
- 83.Hiromi Y, Gehring WJ. Regulation and Function of the Drosophila Segmentation Gene fushi tarazu. Cell. 1987;50:963–74. [DOI] [PubMed] [Google Scholar]
- 84.Schier A, Gehring W. Direct homeodomain—DNA interaction in the autoregulation of the fushi tarazu gene. Nature. 1992;356:804–7. doi: 10.1038/356804a0 [DOI] [PubMed] [Google Scholar]
- 85.Meinhardt H. Models of biological pattern formation. London: Academic Press; 1982. [Google Scholar]
- 86.Meinhardt H. Models for positional signalling, the threefold subdivision of segments and the pigmentation pattern of molluscs. J Embryol Exp Morphol. 1984;83 Suppl:289–311. [PubMed] [Google Scholar]
- 87.Clark E, Peel AD. Evidence for the temporal regulation of insect segmentation by a conserved set of developmental transcription factors. 2017. Preprint. Available from: bioRxiv doi: 10.1101/145151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fujioka M, Yusibova GL, Patel NH, Brown SJ, Jaynes JB. The repressor activity of Even-skipped is highly conserved, and is sufficient to activate engrailed and to regulate both the spacing and stability of parasegment boundaries. Development. 2002;129:4411–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Edgar BA, Odell GM, Schubiger G. Cytoarchitecture and the patterning of fushi tarazu expression in the Drosophila blastoderm. Genes Dev. 1987;1:1226–37. [DOI] [PubMed] [Google Scholar]
- 90.Davis I, Ish-Horowicz D. Apical localization of pair-rule transcripts requires 3’ sequences and limits protein diffusion in the Drosophila blastoderm embryo. Cell. 1991;67:927–40. [DOI] [PubMed] [Google Scholar]
- 91.Little SC, Tikhonov M, Gregor T. Precise developmental gene expression arises from globally stochastic transcriptional activity. Cell. 2013;154:789–800. doi: 10.1016/j.cell.2013.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Desponds J, Tran H, Ferraro T, Lucas T, Perez Romero C, Guillou A, et al. Precision of readout at the hunchback gene: analyzing short transcription time traces in living fly embryos. PLoS Comput Biol. 2016;12(12):e1005256 doi: 10.1371/journal.pcbi.1005256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jaeger J, Reinitz J. On the dynamic nature of positional information. BioEssays. 2006;28:1102–11. doi: 10.1002/bies.20494 [DOI] [PubMed] [Google Scholar]
- 94.Macdonald PM, Struhl G. A molecular gradient in early Drosophila embryos and its role in specifying the body pattern. Nature. 1986;324:537–45. doi: 10.1038/324537a0 [DOI] [PubMed] [Google Scholar]
- 95.Copeland JW, Nasiadka A, Dietrich BH, Krause HM. Patterning of the Drosophila embryo by a homeodomain-deleted Ftz polypeptide. Nature. 1996;379:162–5. doi: 10.1038/379162a0 [DOI] [PubMed] [Google Scholar]
- 96.Russell SR, Sanchez-Soriano N, Wright CR, Ashburner M. The Dichaete gene of Drosophila melanogaster encodes a SOX-domain protein required for embryonic segmentation. Development. 1996;122:3669–76. [DOI] [PubMed] [Google Scholar]
- 97.Nambu PA, Nambu JR. The Drosophila fish-hook gene encodes a HMG domain protein essential for segmentation and CNS development. Development. 1996;122:3467–75. [DOI] [PubMed] [Google Scholar]
- 98.Nusslein-Volhard C, Kluding H, Jurgens G. Genes affecting the segmental subdivision of the Drosophila embryo. Cold Spring Harb Symp Quant Biol. 1985;50:145–54. [DOI] [PubMed] [Google Scholar]
- 99.Frasch M, Warrior R, Tugwood J, Levine M. Molecular analysis of even-skipped mutants in Drosophila development. Genes Dev. 1988;2:1824–38. [DOI] [PubMed] [Google Scholar]
- 100.Klingler M, Gergen JP. Regulation of runt transcription by Drosophila segmentation genes. Mech Dev. 1993;43:3–19. [DOI] [PubMed] [Google Scholar]
- 101.Riechmann V, Irion U, Wilson R, Grosskortenhaus R, Leptin M. Control of cell fates and segmentation in the Drosophila mesoderm. Development. 1997;124:2915–22. [DOI] [PubMed] [Google Scholar]
- 102.Coulter DE, Wieschaus E. Gene activities and segmental patterning in Drosophila: analysis of odd-skipped and pair-rule double mutants. Genes Dev. 1988;2:1812–23. [DOI] [PubMed] [Google Scholar]
- 103.Peel A, Akam M. Evolution of segmentation: Rolling back the clock. Curr Biol. 2003;13:708–10. [DOI] [PubMed] [Google Scholar]
- 104.Peel AD, Chipman AD, Akam M. Arthropod segmentation: beyond the Drosophila paradigm. Nat Rev Genet. 2005;6:905–16. doi: 10.1038/nrg1724 [DOI] [PubMed] [Google Scholar]
- 105.Farzana L, Brown SJ. Hedgehog signaling pathway function conserved in Tribolium segmentation. 2008;10:181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Janssen R, Budd GE. Deciphering the onychophoran “segmentation gene cascade”: Gene expression reveals limited involvement of pair rule gene orthologs in segmentation, but a highly conserved segment polarity gene network. Dev Biol. 2013;382:224–34. doi: 10.1016/j.ydbio.2013.07.010 [DOI] [PubMed] [Google Scholar]
- 107.Lewis J. Autoinhibition with Transcriptional Delay. Curr Biol. 2003;13:1398–408. [DOI] [PubMed] [Google Scholar]
- 108.Schröter C, Ares S, Morelli LG, Isakova A, Hens K, Soroldoni D, et al. Topology and dynamics of the zebrafish segmentation clock core circuit. PLoS Biol. 2012;10(7):e1001364 doi: 10.1371/journal.pbio.1001364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schulz C, Schröder R, Hausdorf B, Wolff C, Tautz D. A caudal homologue in the short germ band beetle Tribolium shows similarities to both, the Drosophila and the vertebrate caudal expression patterns. Dev Genes Evol. 1998;208:283–9. [DOI] [PubMed] [Google Scholar]
- 110.Dessaud E, Yang LL, Hill K, Cox B, Ulloa F, Ribeiro A, et al. Interpretation of the sonic hedgehog morphogen gradient by a temporal adaptation mechanism. Nature. 2007;450:717–21. doi: 10.1038/nature06347 [DOI] [PubMed] [Google Scholar]
- 111.Chen H, Xu Z, Mei C, Yu D, Small S. A System of Repressor Gradients Spatially Organizes the Boundaries of Bicoid-Dependent Target Genes. Cell. 2012;149:618–29. doi: 10.1016/j.cell.2012.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu F, Morrison AH, Gregor T. Dynamic interpretation of maternal inputs by the Drosophila segmentation gene network. Proc Natl Acad Sci U S A. 2013;110:6724–9. doi: 10.1073/pnas.1220912110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Alexandre C, Baena-lopez A, Vincent J. Patterning and growth control by membrane-tethered Wingless. Nature. 2014;505:180–5. doi: 10.1038/nature12879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chipman AD, Arthur W, Akam M. A double segment periodicity underlies segment generation in centipede development. Curr Biol. 2004;14:1250–5. doi: 10.1016/j.cub.2004.07.026 [DOI] [PubMed] [Google Scholar]
- 115.Davis GK, Jaramillo CA, Patel NH. Pax group III genes and the evolution of insect pair-rule patterning. Development. 2001;128:3445–3458. [DOI] [PubMed] [Google Scholar]
- 116.Davis GK, D’Alessio JA, Patel NH. Pax3/7 genes reveal conservation and divergence in the arthropod segmentation hierarchy. Dev Biol. 2005;285:169–84. doi: 10.1016/j.ydbio.2005.06.014 [DOI] [PubMed] [Google Scholar]
- 117.Clyde DE, Corado MSG, Wu X, Paré A, Papatsenko D, Small S. A self-organizing system of repressor gradients establishes segmental complexity in Drosophila. Nature. 2003;426:849–53. doi: 10.1038/nature02189 [DOI] [PubMed] [Google Scholar]
- 118.Goltsev Y, Hsiong W, Lanzaro G, Levine M. Different combinations of gap repressors for common stripes in Anopheles and Drosophila embryos. Dev Biol. 2004;275:435–46. doi: 10.1016/j.ydbio.2004.08.021 [DOI] [PubMed] [Google Scholar]
- 119.Rothschild JB, Tsimiklis P, Siggia ED, François P. Predicting Ancestral Segmentation Phenotypes from Drosophila to Anopheles Using In Silico Evolution. PLoS Genet. 2016;12(5):e1006052 doi: 10.1371/journal.pgen.1006052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rosenberg MI, Brent AE, Payre F, Desplan C. Dual mode of embryonic development is highlighted by expression and function of Nasonia pair-rule genes. Elife. 2014;3:e01440 doi: 10.7554/eLife.01440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nakao H. Analyses of interactions among pair-rule genes and the gap gene Krüppel in Bombyx segmentation. Dev Biol. 2015;405:1–9. doi: 10.1016/j.ydbio.2015.06.005 [DOI] [PubMed] [Google Scholar]
- 122.Nakao H. Hunchback knockdown induces supernumerary segment formation in Bombyx. Dev Biol. 2016;413:207–16. doi: 10.1016/j.ydbio.2016.03.024 [DOI] [PubMed] [Google Scholar]
- 123.Wotton KR, Jimenez-Guri E, Crombach A, Janssens H, Alcaine-Colet A, Lemke S, et al. Quantitative system drift compensates for altered maternal inputs to the gap gene network of the scuttle fly megaselia abdita. Elife. 2015;4:e04785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Crombach A, Wotton KR, Jiménez-Guri E, Jaeger J. Gap Gene Regulatory Dynamics Evolve along a Genotype Network. Mol Biol Evol. 2016;33:1293–307. doi: 10.1093/molbev/msw013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lander AD. How Cells Know Where They Are. Science. 2013;339:923–8. doi: 10.1126/science.1224186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bertrand E, Chartrand P, Schaefer M, Shenoy SM, Singer RH, Long RM. Localization of ASH1 mRNA Particles in Living Yeast. Mol Cell. 1998;2:437–45. [DOI] [PubMed] [Google Scholar]
- 127.Forrest KM, Gavis ER. Live Imaging of Endogenous RNA Reveals a Diffusion and Entrapment Mechanism for nanos mRNA Localization in Drosophila. Curr Biol. 2003;13:1159–68. [DOI] [PubMed] [Google Scholar]
- 128.Garcia HG, Tikhonov M, Lin A, Gregor T. Quantitative Imaging of Transcription in Living Drosophila Embryos Links Polymerase Activity to Patterning. Curr Biol. 2013;23:2140–5. doi: 10.1016/j.cub.2013.08.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Benton MA, Akam M, Pavlopoulos A. Cell and tissue dynamics during Tribolium embryogenesis revealed by versatile fluorescence labeling approaches. Development. 2013;140:3210–20. doi: 10.1242/dev.096271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nakamoto A, Hester SD, Constantinou SJ, Blaine WG, Tewksbury AB, Matei MT, et al. Changing cell behaviours during beetle embryogenesis correlates with slowing of segmentation. Nat Commun. 2015;6:6635 doi: 10.1038/ncomms7635 [DOI] [PubMed] [Google Scholar]
- 131.Williams TA, Nagy LM. Linking gene regulation to cell behaviors in the posterior growth zone of sequentially segmenting arthropods. Arthropod Struct Dev. 2016;46:380–94. [DOI] [PubMed] [Google Scholar]
- 132.Stollewerk A, Schoppmeier M, Damen WGM. Involvement of Notch and Delta genes in spider segmentation. Nature. 2003;423:863–5. doi: 10.1038/nature01682 [DOI] [PubMed] [Google Scholar]
- 133.Pueyo JI, Lanfear R, Couso JP. Ancestral Notch-mediated segmentation revealed in the cockroach Periplaneta americana. Proc Natl Acad Sci U S A. 2008;105:16614–9. doi: 10.1073/pnas.0804093105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Eriksson BJ, Ungerer P, Stollewerk A. The function of Notch signalling in segment formation in the crustacean Daphnia magna (Branchiopoda). Dev Biol. 2013;383:321–30. [DOI] [PubMed] [Google Scholar]
- 135.Paré AC, Vichas A, Fincher CT, Mirman Z, Farrell DL, Mainieri A, et al. A positional Toll receptor code directs convergent extension in Drosophila. Nature. 2014;515:523–7. doi: 10.1038/nature13953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Benton MA, Pechmann M, Frey N, Stappert D, Conrads KH, Chen Y-T, et al. Toll Genes Have an Ancestral Role in Axis Elongation. Curr Biol. 2016;26. [DOI] [PubMed] [Google Scholar]
- 137.Clark E, Akam M. 2016. Data from: Odd-paired controls frequency doubling in Drosophila segmentation by altering the pair-rule gene regulatory network. Dryad Digital Repository. Openly available via http://dx.doi.org/10.5061/dryad.cg35k [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Frasch M, Hoey T, Rushlow C, Doyle H, Levine M. Characterization and localization of the even-skipped protein of Drosophila. EMBO J. 1987;6:749–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–82. doi: 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.van der Walt S, Colbert SC, Varoquaux G. The NumPy Array: A Structure for Efficient Numerical Computation. Comput Sci Eng. 2011;13:22–30. [Google Scholar]
- 142.Hunter JD. Matplotlib: A 2D Graphics Environment. Comput Sci Eng. 2007;9:90–5. [Google Scholar]
- 143.Gonzalez AG, Naldi A, Sánchez L, Thieffry D, Chaouiya C. GINsim: A software suite for the qualitative modelling, simulation and analysis of regulatory networks. BioSystems. 2006;84:91–100. doi: 10.1016/j.biosystems.2005.10.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expanded version of Fig 3 from the main text, showing all pairwise combinations between hairy, eve, runt, ftz, odd, and slp. (A) hairy and eve pair-rule stripes partially overlap during cellularisation. At gastrulation, hairy expression fades away, while the eve stripes narrow from the posterior and then also fade. (B) eve and runt pair-rule stripes partially overlap during cellularisation. The eve stripes become increasingly narrow at gastrulation, and the runt secondary stripes emerge anterior to the eve stripes. By early GBE, eve expression has faded away and runt expression has resolved into a regular, segmental pattern. The refinement of the eve stripes occurs more gradually in real embryos than in the simulation. Arrowheads in B”‘ indicate new runt expression related to the developing nervous system. (C) eve and ftz pair-rule stripes are at first expressed in complementary patterns. Starting from late cellularisation, they both narrow from the posterior (eve more than ftz). eve expression later fades away, while ftz persists. (D) eve and odd pair-rule stripes are at first expressed in complementary patterns, before both narrowing. odd secondary stripes emerge at the posterior of the narrowing eve domains, which then fade away, leaving segmental stripes of odd. (E) hairy and runt pair-rule stripes are expressed in complementary patterns during cellularisation. hairy expression then fades away, while runt transitions to segmental stripes. (F) hairy and ftz pair-rule stripes slightly overlap during cellularisation. hairy expression then fades away, while the ftz stripes narrow. (G) hairy and odd pair-rule stripes slightly overlap during cellularisation. hairy expression then fades away, while odd transitions to narrow segmental stripes. (H) runt and ftz pair-rule stripes partially overlap throughout cellularisation. At gastrulation, runt secondary stripes emerge to the posterior of the narrowing ftz stripes. Later, the runt primary stripes refine from the posterior, and the overlaps with ftz are lost. (I) runt and odd pair-rule stripes slightly overlap during cellularisation. These overlaps resolve at late cellularisation (slightly earlier than in the simulation). At gastrulation, new odd expression emerges just anterior to the runt primary stripes, while new runt expression emerges just posterior to the refining odd primary stripes. By early GBE there is a regular segmental pattern of abutting odd and runt stripes, separated by gaps. Arrowheads in I”‘ indicate new runt expression related to the developing nervous system. (J) The ftz and odd stripes are fairly congruent during cellularisation. At gastrulation, both narrow from the posterior, and the odd secondary stripes intercalate between them. Over the course of patterning, their anterior boundaries also become offset from one another. (K) The slp primary stripes emerge later than the hairy stripes, but share an anterior border with them. (The slp stripes in the simulation are too broad at this stage—they should be narrower than the hairy stripes.) hairy expression then fades away, while slp transitions to a segmental pattern. (L) The slp primary stripes emerge later than the eve stripes, and abut their anterior borders. (In the simulation, these slp stripes extend too far posteriorly, and overlap with eve. The reason is that the eve anterior borders would have stabilised by this point in real embryos, but they are still shifting in the simulation.) At gastrulation, the eve stripes narrow from the posterior and then fade, while slp transitions to segmental stripes. (M) The slp primary stripes emerge later than the runt primary stripes, and are offset slightly from their posterior boundaries. (The simulated slp domains are wider than the real slp domains.) At gastrulation, secondary stripes of both genes emerge between the primary stripes (the widths of the simulated slp stripes are now appropriate). The expression patterns become largely congruent, except at the posteriors of the runt primary stripes. These differences resolve later, when the runt primary stripes narrow. (N) The slp primary stripes emerge later than the ftz primary stripes, and partially overlap with them. At gastrulation, the secondary slp stripes emerge just anterior to the ftz domains, which narrow from the posterior, losing the overlaps with the slp primary stripes. (O) As for (N), the slp primary stripes partially overlap the odd primary stripes, and these overlaps are later lost by the odd stripes narrowing from the posterior. The secondary stripes of odd and slp intercalate between the primary stripes, and abut one other.
(DOCX)
Each panel shows an uncropped version of the corresponding double fluorescent in situ image in S1 Fig. All panels show a whole embryo lateral view, anterior left, dorsal top. Embryos within each column are of approximately equal age. Scale = 100 μm.
(DOCX)
This figure compares double FISH data of pair-rule gene expression with simulated transcript expression, as in Fig 3 and S2 Fig. (A) prd transcripts (magenta) are shown relative to eve (green), odd (blue) or slp (cyan) transcripts, at four different embryo ages (left) or simulation timepoints (right). Note that the figure presents a different set of developmental ages / simulated timepoints from those in Fig 3 and Supplementary Figure 3, in order to show cellularisation in greater temporal detail. At “early cellularisation”, the primary pair-rule genes are expressed but prd and slp are not; at “mid cellularisation”, prd is expressed but slp is not; at “late cellularisation”, slp is additionally expressed. While the simulated expression of prd is broadly appropriate early on (e.g. compare prd and odd expression at mid-cellularisation / T26), several aspects of prd expression are not recapitulated by the model. First, the changing phasing of prd posterior borders and eve anterior borders between mid-cellularisation and late cellularisation (see also Fig 5). Second, the splitting of the prd stripes at late cellularisation, slightly prior to the appearance of the secondary stripes of odd and slp. Third, the patterning of the prd “A” stripes (i.e. the narrow stripes formed from anterior portions of the early broad stripes, asterisks in the in situ images) and the consequent emergence of single-segment periodicity. (B) Uncropped views of the embryos shown in (A). Scale bars 100 μm.
(DOCX)
(A) Double FISH images of segmentation gene expression in gastrulation stage embryos (lateral view, anterior left, dorsal top). Each row shows a different pairwise comparison of the transcripts of the genes eve (magenta), prd (blue), slp (green), en (red), odd (yellow), and wg (cyan). From left to right, the panels in each row show: 1) a whole embryo view; 2) an enlarged view of pair-rule repeats 2–6; 3) the individual channel for the first gene listed in the row label; 4) the individual channel for the second gene listed in the row label. Arrowheads mark the locations of prospective odd-numbered parasegment boundaries. Scale bars 100 μm. (B) Summary schematic showing the relative expression of the Eve, Prd, and Slp protein domains, and the en, wg, and odd transcript domains, at the odd-numbered parasegment boundaries. (C) The same schematic as in B, with the addition of the regulatory interactions between Eve, Prd, and Slp and their target genes. Aside from the colours, which are changed to match the in situ images, this schematic is identical to Fig 5A.
(DOCX)
This figure is an expansion of Fig 7 in the main text, showing additional whole embryo and single channel views. Enlarged views show stripes 2–6. Asterisks in (E) indicate the stripe 3 region. Scale = 50 μm. Note the quantitative traces below the images in (C). These plots show the AP intensity profiles of runt (green) and slp (blue) along a narrow ventral strip of the trunk of the two embryos pictured. In the wild-type embryo, the runt stripes are all (except stripe 7) roughly symmetrical and strongly expressed. In the eve mutant embryo, runt stripes 1–6 (which overlap with odd expression, see B), have much lower intensity than runt stripe 7 (which doesn’t overlap with odd expression, see B) and exhibit a sawtooth pattern, in which expression intensity decreases from anterior to posterior. The slp expression in the eve mutant embryo, while broad, does display a pair-rule modulation, which is in opposite phase to the downregulated runt stripes. Therefore, the same two regulatory interactions (repression of runt by Odd, and of slp by Runt) are evident in both the in situ data and the simulated data (Fig 7A’), but lead to slightly different expression patterns in each case, one quantitative and one qualitative.
(DOCX)
Simulation output showing the expression patterns generated by the early pair-rule network, assuming various anterior-to-posterior shifts speeds of the gap inputs (shown in black). See supplementary movies for full simulation output (S1 Movie = 0x; S2 Movie = 1x; S3 Movie = 0.5x; S4 Movie = 2x; S5 Movie = 3x). Gap domain shift speeds are relative to the time delay for pair-rule protein synthesis / decay: a speed of 1x leads to a one nucleus offset between the anterior borders of transcript and protein domains, a speed of 2x leads to a two nucleus offset, and so on. See S2 Text for further details about the simulations.
(DOCX)
(A,B) Minimum duration required for generating a correct segment pattern (En, Odd, Slp repeats), under various simulation scenarios. Minimum simulation duration (Y-axis) is given as a multiple of the synthesis / decay delay parameter value of the pair-rule gene products (corresponding to 6 timesteps for all simulations shown in this manuscript). See S2 Text for further details on the various simulations. (A) Minimum time required to pattern a given number of segments by either simultaneous or sequential patterning, using the same network model as S12 and S13 Movies. Sequential patterning requires 12x the delay for the initial two segments, plus 8x the delay for every two additional segments. Simultaneous patterning requires a constant minimum time of 10x the delay, no matter the number of segments. (B) Minimum time (in multiples of the synthesis / decay delay parameter value) required for simultaneous patterning, for systems with increasingly elaborate gap patterning. If only hairy receives gap inputs (1), a minimum of time 10x the delay is required (network model as in simulation 12). If both hairy and eve receive gap inputs (2), the minimum is 7x the delay (network model as in simulation 10). If runt or ftz/odd additionally receive gap inputs (3), the minimum is 6x the delay (early network model as in simulations 6 or 7, respectively, plus late network model as for simulation 10). Finally, if all the primary pair-rule genes receive gap inputs (4), the minimum is 5x the delay (early network model as in simulation 8, plus late network model as for simulation 10). Note that the stripes of ftz and odd are considered a single pattern, because of their identical regulation in the simulations.
(DOCX)
(PDF)
(PDF)
(PY)
Corresponds to the network in Simulation 10 and Fig 2B.
(PY)
Corresponds to the network in Simulation 10 and Fig 2B.
(SBML)
Corresponds to the network in Simulations 12 and 13, and Fig 8.
(SBML)
Simulated expression of the primary pair-rule genes, assuming static gap inputs.
(MP4)
Simulated expression of the primary pair-rule genes, assuming dynamic gap inputs.
(MP4)
Simulated expression of the primary pair-rule genes, assuming slow gap shifts.
(MP4)
Simulated expression of the primary pair-rule genes, assuming fast gap shifts.
(MP4)
Simulated expression of the primary pair-rule genes, assuming very fast gap shifts.
(MP4)
Pattern establishment when hairy, eve, and runt take gap inputs.
(MP4)
Pattern establishment when hairy, eve, and ftz/odd take gap inputs.
(MP4)
Pattern establishment when all primary pair-rule genes take gap inputs.
(MP4)
Simulated behaviour of the whole pair-rule system, assuming static gap inputs.
(MP4)
Simulated behaviour of the whole pair-rule system, assuming dynamic gap inputs.
(MP4)
Pair-rule gene expression in a simulated eve mutant.
(MP4)
Simultaneous patterning by the modified pair-rule network.
(MP4)
Sequential patterning by the modified pair-rule network.
(MP4)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files. Additional expression data from wild-type embryos are held in the Dryad Digital Repository (DOI: http://dx.doi.org/10.5061/dryad.cg35k).