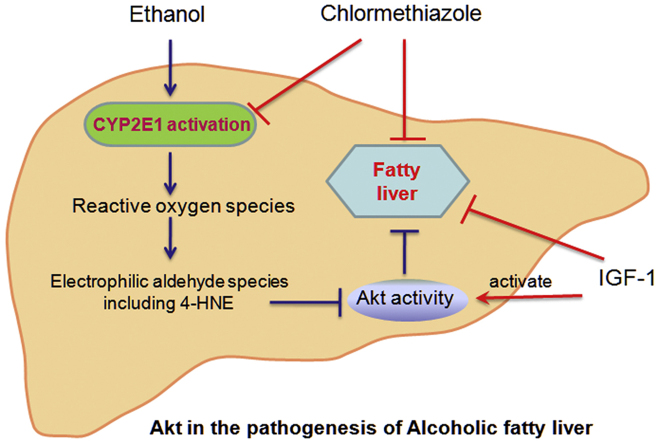
Abstract
Protein kinase B (PKB/Akt) plays important roles in the regulation of lipid homeostasis, and impairment of Akt activity has been demonstrated to be involved in the development of non-alcoholic fatty liver disease (NAFLD). Previous studies suggest that cytochrome P4502E1 (CYP2E1) plays causal roles in the pathogenesis of alcoholic fatty liver (AFL). We hypothesized that Akt activity might be impaired due to CYP2E1-induced oxidative stress in chronic ethanol-induced hepatic steatosis. In this study, we found that chronic ethanol-induced hepatic steatosis was accompanied with reduced phosphorylation of Akt at Thr308 in mice liver. Chronic ethanol exposure had no effects on the protein levels of phosphatidylinositol 3 kinase (PI3K) and phosphatase and tensin homologue deleted on chromosome ten (PTEN), and led to a slight decrease of phosphoinositide-dependent protein kinase 1 (PDK-1) protein level. Ethanol exposure resulted in increased levels of malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE)-Akt adducts, which was significantly inhibited by chlormethiazole (CMZ), an efficient CYP2E1 inhibitor. Interestingly, N-acetyl-L-cysteine (NAC) significantly attenuated chronic ethanol-induced hepatic fat accumulation and the decline of Akt phosphorylation at Thr308. In the in vitro studies, Akt phosphorylation was suppressed in CYP2E1-expressing HepG2 (CYP2E1-HepG2) cells compared with the negative control HepG2 (NC-HepG2) cells, and 4-HNE treatment led to significant decrease of Akt phosphorylation at Thr308 in wild type HepG2 cells. Lastly, pharmacological activation of Akt by insulin-like growth factor-1 (IGF-1) significantly alleviated chronic ethanol-induced fatty liver in mice. Collectively, these results indicate that CYP2E1-induced oxidative stress may be responsible for ethanol-induced suppression of Akt phosphorylation and pharmacological modulation of Akt in liver may be an effective strategy for the treatment of ethanol-induced fatty liver.
Abbreviations: AFL, alcoholic fatty liver; ALD, alcoholic liver disease; ALT, alanine transaminase; AMPK, AMP-activated protein kinase; AST, aspartate transaminase; CMZ, chlormethiazole; CYP2E1, cytochrome P4502E1; 4-HNE, 4-hydroxynonenal; IGF-1, insulin-like growth factor-1; LPS, lipopolysaccharide; MDA, malondialdehyde; NAC, N-acetyl-L-cysteine; NAFLD, non-alcoholic liver disease; PDK-1, phosphoinositide- dependent protein kinase 1; PI3K, phosphatidylinositol 3 kinase; PKB/Akt, protein kinase B; PPAR-α, peroxisome proliferators-activated receptor α; PPAR-γ, peroxisome proliferators- activated receptor γ; PTEN, phosphatase and tensin homologue deleted on chromosome ten; ROS, reactive oxygen species; SIRT-1, sirtuin 1; SREBP-1c, sterol regulatory element-binding protein 1c; TG, triglyceride
Keywords: Alcoholic fatty liver, Protein kinase B, Cytochrome P4502E1, Oxidative stress, Insulin-like growth factor-1
Graphical abstract
1. Introduction
Alcoholic liver disease (ALD) is a progressively aggravated liver disease, which ranges from steatosis to hepatitis, fibrosis, and finally cirrhosis [1]. Alcoholic fatty liver (AFL) is the earliest and most common phenotype of ALD, and continued drinking of excessive amounts of alcohol can subsequently lead to severe forms of ALD. AFL has been considered as a benign condition for a long time due to the asymptomatic and reversible characteristics. However, increasing evidences suggest that AFL is a potentially pathologic condition [2], [3]. AFL could progress to fibrosis and cirrhosis in about 5–15% of AFL patients despite abstinence; and the severity of steatosis on the initial liver biopsy predicted the development of cirrhosis on the subsequent biopsy 10 year later [4], [5]. Animal studies demonstrated that fatty liver was more vulnerable to hepatotoxins such as lipopolysaccharide [6]. Now, it is generally recognized that AFL is the optimal phase to block or delay the progress to advanced ALD [7], [8].
Protein kinase B (PKB/Akt) is a central player in the signal transduction pathways activated in response to many growth factors, hormones, cytokines, and nutrients [9]. Akt has been described as one of the most important and versatile protein kinases at the core of human physiology and disease [10]. Dysregulated Akt activity is implicated in the pathogenesis of a growing number of disorders [9]. The roles of Akt on lipid homeostasis have been investigated in several studies. Although results of in vitro studies showed that Akt activation promoted fat accumulation by activating sterol regulatory element binding protein 1c (SREBP-1c) [11], [12], in vivo studies revealed that Akt played protective roles against fatty liver. For example, hepatic fat accumulation in rats with high-fat diet-induced nonalcoholic fatty liver disease (NAFLD) was accompanied with reduced phosphorylation of Akt, and pharmacological inhibitor of Akt led to significant fat accumulation in rat liver [13]. Some other studies demonstrated that Akt activation could ameliorate hepatic steatosis in lean mice, ob/ob mice, NAFLD mice, and diabetic mice [14], [15], [16]. Results of these studies clearly demonstrate that Akt plays important roles in regulating the lipid homeostasis in the liver. Thus, it is necessary to investigate the roles of Akt in the pathogenesis of AFL.
Microsomal cytochrome P4502E1 (CYP2E1) is a member of the cytochrome P450 mixed-function oxidase system, which is responsible for the metabolism of many endogenous and xenobiotic substrates. Previous studies have demonstrated that CYP2E1 plays etiological roles in the development of AFL [17], [18], [19]. However, the underlying mechanisms for CYP2E1 activation and subsequent fat accumulation in the liver are not fully elucidated. It has been well documented that CYP2E1 activation leads to the generation of large amounts of reactive oxygen species (ROS), which can initiate the autocatalytic degradation of polyunsaturated fatty acids to yield electrophilic aldehyde species including 4-hydroxynonenal (4-HNE), which can preferentially modify cysteine, histidine, and lysine residues via Michael addition and for lysine, Schiff base products [20], [21]. Indeed, some previous studies have demonstrated that ethanol-induced 4-HNE modification of hepatocellular proteins resulted in the inhibition of normal enzyme function [22], [23]. However, the relationship between CYP2E1 and Akt has not been investigated.
The current study was designed to investigate the roles of Akt in the pathogenesis of AFL. We aimed to explore: 1) whether the hepatic Akt activity was impaired in chronic ethanol-exposed mice? 2) if so, whether pharmacological activation of Akt could attenuate chronic ethanol-induced fat accumulation in mice? And 3) the potential links between Akt suppression and ethanol-induced activation of CYP2E1.
2. Materials and methods
2.1. Materials
Ethanol was purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). Chlormethiazole (CMZ) and N-acetyl-L-cysteine (NAC) were bought from Sigma (St. Louis, MO, USA). Specific primary antibodies against Akt, p-Aktser473, p-Aktthr308, acyl-CoA carboxylase (ACC), p-ACCser79, glycogen synthase kinase-3β (GSK-3β), p-GSK-3βser9, phosphatidylinositol 3 kinase (PI3K)-p110α and PI3K-p85 were bought from Cell Signaling Technology (Beverly, MA, USA). Primary antibodies against CYP2E1, liver fatty acid-binding protein (LFABP), acyl-CoA oxidase (ACOX) and 4-HNE were provided by Abcam (Cambridge, UK). Primary antibodies against SREBP-1c, peroxisome proliferator-activated receptor a (PPAR-a), peroxisome proliferator-activated receptor γ (PPAR-γ), and fatty acid synthase (FAS) were obtained from Santa Cruz (Santa Cruz, CA, USA). Insulin-like growth factor (IGF-1) was obtained from Sino Biological Inc. (Beijing, China). Specific primary antibodies against phosphatase and tensin homologue deleted on chromosome ten (PTEN) and phosphoinositide-dependent protein kinase 1 (PDK-1) were bought from Proteintech (Chicago, IL, USA). 4-HNE was purchased from Calbiochem (San Diego, CA, USA). Malondialdehyde (MDA) and triglyceride (TG) assay kits were supplied by Nanjing Jiancheng Bioengineering Institute (Nanjing, China) and Applygen Technologies Inc. (Beijing, China), respectively. All other reagents were purchased from Sigma unless indicated otherwise.
2.2. Animal treatment
Specific pathogen free (SPF) KM mice (male, 8 weeks old) were provided by Laboratory Animal Center of Shandong University (Jinan, China). The mice were maintained in a temperature-controlled environment (20–22 °C) with a 12-h light: 12-h dark cycle and 50–60% humidity. Chronic AFL was induced by feeding mice with regular Lieber-DeCarli liquid diet containing 5% (w/v) ethanol for 4 weeks. To explore the potential links between ethanol-induced CYP2E1 activation and Akt suppression, chlormethiazole (CMZ, 50 mg/kg body weight), an efficient inhibitor of CYP2E1, was injected intraperitoneally to mice every other day as previously reported [17]. Pharmacological activation of Akt was achieved by intraperitoneal administration of recombinant human insulin-like growth factor-1 (IGF-1, 100 μg/kg body weight) [24]. NAC was administered to mice (100 mg/kg bw) to evaluate the effects of antioxidant on chronic ethanol-induced steatosis. All animal experiments were carried out in accordance with the Guide for the Care and Use of Laboratory Animals as adopted by the U.S. National Institutes of Health and were approved by the Ethics Committee of Shandong University Institute of Preventive Medicine.
2.3. Cell culture and treatment
Human hepatocarcinoma cell line (HepG2) was obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA). A CYP2E1 cDNA plasmid was kindly provided by Dr. F.J. Gonzalez (National Cancer Institute, Bethesda, MD, USA). A monoclonal cell line stably expressing CYP2E1 (CYP2E1-HepG2) was established by transfecting HepG2 cells with recombinant lentiviral vector (Shanghai Genechem Co., Shanghai, China), while a negative control cell line (NC-HepG2) was also obtained by transfecting HepG2 cells with GFP lentiviral vector. The preparation of the recombinant lentiviral vectors and the transfection procedure were performed as previously reported [25].
The wild type HepG2 cells were grown in DMEM medium (GIBCO BRL, NY, USA) supplemented with 10% fetal bovine serum (FBS, Hyclone, Logan, UT, USA) in a humidified incubator set with 5% CO2/95% air atmosphere at 37 °C. The CYP2E1-HepG2 and NC-HepG2 cells were cultured in the same medium supplemented with 2 μg/mL puromycin. HepG2 cells were exposed to 25 μΜ 4-hydroxynonenal (4-HNE) for a time course from 1 h to 8 h. CYP2E1-HepG2 and NC-HepG2 cells were treated with 0, 25, 50, 100, and 200 mM ethanol for 5 d. To minimize the evaporation of ethanol in the medium, fresh medium containing ethanol was replaced every 24 h. Cytotoxicity of ethanol and 4-HNE was tested using CCK-8 kits (Dojin Laboratories, Kumamoto, Japan). The doses of ethanol and 4-HNE used in this study did not induce significant cytotoxicity.
2.4. Biochemical analyses
The levels of alanine transaminase (ALT), aspartate transaminase (AST) and triglyceride (TG) in serum were measured using GLAMOUR 1600 automatic biochemistry analyzer with commercial assay kits provided by BioSino Biotechnology and Science, Inc (Beijing, China). TG levels in mice liver and cultured cells were determined using TG assay kits obtained from Applygen Technologies Inc. (Beijing, China).
2.5. Determination of hepatic lipid peroxidation
Hepatic lipid peroxidation was evaluated using the thiobarbituric acid reactive substances method (TBARS) and was expressed as malondialdehyde (MDA) levels [26]. To determine the hepatic MDA level, liver tissues were homogenized in 9 volumes of cold buffer (10 mM Tris, 100 μM EDTA, 10 mM saccharose, 0.8% saline, pH 7.4). The homogenates were centrifuged at 1000×g for 15 min at 4 °C, and the supernatant were collected for the detection of MDA using a commercial assay kit provided by Nanjing Jiancheng Bioengineering Institute (Nanjing, China) [27].
2.6. Histopathological examination and immunohistochemical staining
Liver frozen sections (10 µm) were fixed in 10% neutral formalin for 5 min, stained in Sudan Ⅲ or Oil red O dyes, and then counterstained with hematoxylin [8]. Immunohistochemical staining was performed according to the instruction of a commercial immunohistochemical staining kit (PV-9000, ZSGB-BIO, China). The sections were viewed and the representative photographs were captured using a Nikon microscope (Nikon, Melville, NY, USA).
2.7. Immunoprecipitation assay
Immunoprecipitation was performed to examine the levels of 4-HNE-Akt adduct. Briefly, protein extracts (1 mg/mL) were incubated with 5 μl primary Akt antibody at 4 °C overnight with gentle agitation. After that, 20 μl of protein A/G-Sepharose bead suspension (Santa Cruz, CA, USA) was added and gently mixed for 1 h at 4 °C. Samples were centrifuged at 1000×g for 30 s, and the pellet was washed in RIPA buffer for 4 times. Finally, the pellet was suspended in 40 μl 2×SDS-PAGE buffer, heated to 100 °C for 5 min, and was used for western blotting analysis.
2.8. Western blotting analysis
Total protein lysates were prepared using RIPA buffer (50 mM Tris, 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 1 mM PMSF, 1 mM Na3VO4, 5 mM NaF, and 1% cocktail protein protease inhibitors (Sigma), pH 7.4). The protein samples were separated on a 4–15% denatured SDS-PAGE electrophoresis and transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). The membranes were blocked in 5% (w/v) nonfat milk solution for 1 h, and then incubated with specific primary antibodies overnight at 4 °C followed by incubation with horseradish peroxidase (HRP)-conjugated anti-rabbit or anti-mouse antibodies for 1 h at room temperature. After washing, chemiluminescent detection was performed using an enhanced chemiluminescence (ECL) western blotting detection reagent (Kibbutz Beit Haemek, Israel). The immunoreactive bands of proteins were scanned using Agfa Duoscan T1200 scanner, and the digitized data were quantified as integrated optical density (IOD) using Kodak Imaging Program [8].
2.9. Statistical analyses
Data were expressed as means ± standard deviations. Statistical significance of difference was analyzed by way of Student t-test or one-way analysis of variance (ANOVA). Differences between groups were considered statistically significant if P < 0.05. The statistical analyses were performed using SPSS16.0 statistical software.
3. Results
3.1. Chronic ethanol-induced hepatic steatosis in mice was accompanied with the suppression of Akt phosphorylation
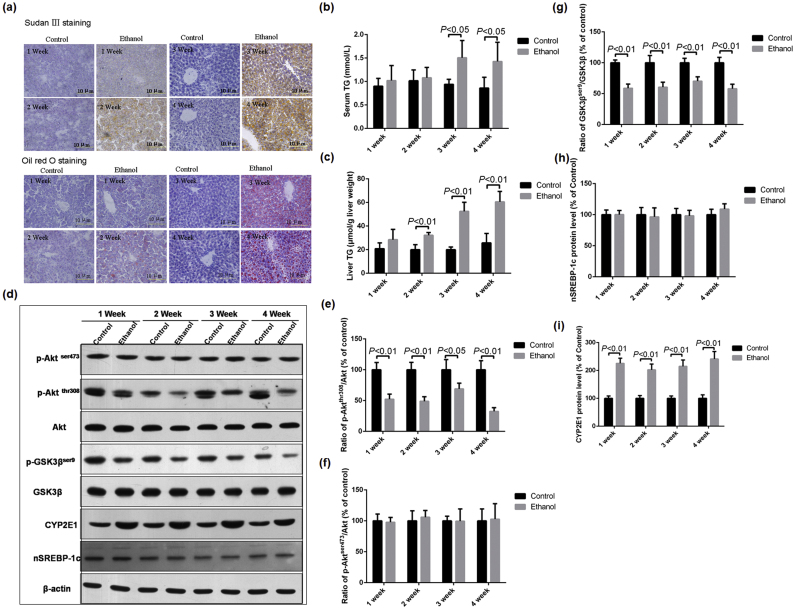
Histopathological examination showed that liver section of mice exposed to 3 and 4 weeks of ethanol were filled with massive lipid droplets (Fig. 1a). Biochemical assay revealed that hepatic TG levels increased significantly after 2 weeks of ethanol exposure compared with the control mice, while serum TG levels increased significantly after 3 weeks of ethanol exposure (Fig. 1b and c). Results of western blotting showed that the protein levels of Akt and p-Aktser473 did not significantly changed after ethanol intoxication; however, the protein levels of p-Aktthr308 in mice of ethanol group dramatically decreased compared with those in mice of control group (Fig. 1d–f). The phosphorylation of GSK3β at Ser9, a downstream target of Akt, also significantly decreased in liver of ethanol group mice (Fig. 1g). In addition, the protein level of mature form of SREBP-1c (nSREBP-1c, 68 kD) was not affected by ethanol (Fig. 1h). However, chronic ethanol exposure resulted in significant increase of hepatic CYP2E1 protein levels (Fig. 1i).
Fig. 1.
Effects of chronic ethanol feeding on hepatic fat contents and the protein levels of Akt, GSK3β, SREBP-1c, and CYP2E1 in mice. Mice were exposed to control or ethanol-containing (5%, w/v) liquid diets for 1, 2, 3, and 4 weeks, respectively. (a) Histopathological examination was performed using Sudan Ⅲ and Oil Red O staining as described in the materials and methods section (Scale bar = 10 µm); (b) Serum TG levels; (c) hepatic TG levels; (d) Representative western blotting bands of p-Aktser473, p-Aktthr308, Akt, p-GSK3βser9, GSK3β, mature form of SREBP-1c (n-SREBP-1c), and CYP2E1; (e)-(i) Quantitative data analyses of and p-Aktthr308, p-Aktser473, p-GSK3βser9, and CYP2E1. Data were presented as mean ± SD (n = 5), and expressed as the percentage of the control.
3.2. CYP2E1-induced oxidative stress might account for chronic ethanol-induced suppression of Akt phosphorylation and activation
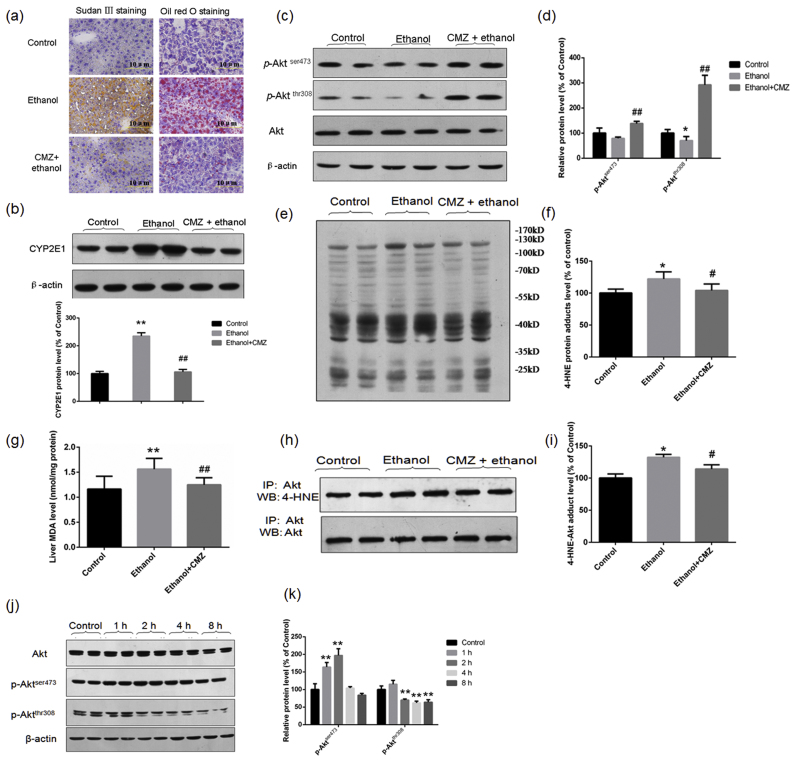
CYP2E1 has been demonstrated to play causal roles in the pathogenesis of ethanol-induced fatty liver [17], [28]. We investigated the roles of CMZ, a specific inhibitor of CYP2E1, on chronic ethanol-induced suppression of Akt pathway. As reported in previous studies, CMZ efficiently blocked chronic ethanol-induced increase of CYP2E1 protein level and hepatic fat accumulation in mice (Fig. 2a–b). Interestingly, the protein levels of p-Aktser473 and p-Aktthr308 all significantly increased in the liver of CMZ/ethanol group mice compared with those of ethanol group mice (Fig. 2c–d).
Fig. 2.
CYP2E1-induced oxidative stress might account for chronic ethanol-induced suppression of Akt phosphorylation and activation. Mice in were treated with a specific CYP2E1 inhibitor CMZ (50 mg/kg body weight, by intraperitoneal injection) or same volume of sterile saline every other day, and were fed with 5% (w/v) ethanol-containing liquid diet or control diet for 4 weeks. (a) Representative photos of Sudan Ⅲ staining and Oil Red O staining (Scale bar = 10 µm); (b) Representative western blotting bands of CYP2E1 and the quantitative data; (c)&(d) Representative western blotting bands of Akt, p-Aktser473 and p-Aktthr308 and the quantitative data; (e)&(f) Representative western blotting bands and the quantitative data of 4-HNE-protein adducts; (g) Hepatic MDA levels determined by TBARS methods; (h) &(i) Levels of 4-HNE-Akt adducts determined by immunoprecipitation assay; (j)&(k) In vitro study showed that 4-HNE treatment led to decreased phosphorylation of Akt at Thr308 in HepG2 cells. *P < 0.05, **P < 0.01, compared with the control group mice; #P < 0.05, ##P < 0.01, compared with the ethanol group mice in (a)–(i). **P < 0.01, compared with the control group cells in (k).
PI3K is the upstream kinase of Akt, which can induce the phosphorylation of Akt at Thr308 by PDK-1 and at Ser473 by mTOR complex 2 (mTORC-2) [10], [29], [30]. In contrast, PTEN is a major negative regulator of Akt activity [31]. To investigate whether the decreased phosphorylation of Akt at Thr308 was associated with the above modulators, the protein levels of PI3K, PTEN, and PDK-1 were detected by western blot. However, the protein levels of the catalytic subunit of PI3K (p110 α), the regulatory subunits of PI3K (p85 and p50), and PTNE were all not significantly affected by ethanol or CMZ, although chronic ethanol exposure led to a slight decrease of PDK-1 protein level (Fig. S1). These results suggest that chronic ethanol exposure may lead to the inhibition of Akt phosphorylation by other mechanisms.
Activated CYP2E1 can produce a large amount of ROS, which has been suggested to be responsible for ethanol-induced liver injury [18], [32], [33]. ROS can lead to the autocatalytic degradation of polyunsaturated fatty acids to yield electrophilic aldehyde species including 4-HNE, which can modify many hepatocellular proteins resulting in the inhibition of normal enzyme function [22], [23]. To test whether 4-HNE linked the activation of CYP2E1 and the suppression of Akt phosphorylation, we examined the levels of hepatic MDA, 4-HNE adducts, and 4-HNE-Akt adduct. CMZ treatment almost completely abrogated chronic ethanol-induced increase of hepatic MDA level and the 4-HNE modified protein level (Fig. 2e–g). Furthermore, chronic ethanol led to significant increase of the 4-HNE-Akt adduct level in mice liver, which was significantly inhibited by CMZ treatment (Fig. 2h–i).
To investigate whether 4-HNE could affect Akt activity, HepG2 cells were exposed to a non-toxic dose of 4-HNE (25 μM) for 1, 2, 4, and 8 h, respectively, and the protein levels of Akt, p-Aktser473 and p-Aktthr308 were determined. This dose of 4-HNE showed no toxicity to HepG2 cells determined by CCK-8 test (Fig. S2). Compared with the control group, the protein levels of p-Aktser473 in HepG2 cells exposed to 4-HNE were significantly increased at the 1 h and 2 h time points, and then decreased to the control value. However, the protein levels of p-Aktthr308 in 4-HNE-treated HepG2 cells significantly decreased at the 2 h, 4 h, and 8 h time points compared with the control cells (Fig. 2j–k).
3.3. Akt phosphorylation was suppressed in CYP2E1-HepG2 cells compared with the NC-HepG2 cells
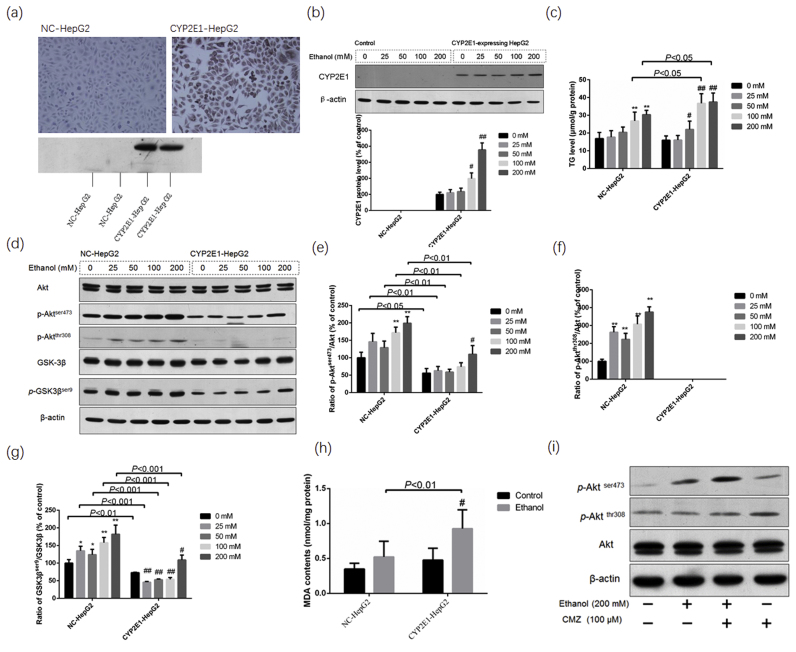
To confirm the interaction between CYP2E1 and Akt phosphorylation in ethanol-induced fatty liver, we examined the cellular TG levels and protein levels of p-Aktser473, p-Aktthr308 and the total Akt in CYP2E1-HepG2 cells and NC-HepG2 cells. Immunohistochemical staining and western blotting analyses showed that NC-HepG2 cells did not express CYP2E1, while CYP2E1 was significantly induced by ethanol in CYP2E1-HepG2 cells (Fig. 3a and b). The TG levels in CYP2E1-HepG2 cells exposed to 100 mM and 200 mM ethanol for 5 d were significantly higher than those in NC-HepG2 cells (Fig. 3c). Ethanol exposure led to increase of Akt phosphorylation at Ser473 and Thr308 in NC-HepG2 cells. However, the protein level of p-Aktser473 and p-Aktthr308 in CYP2E1-HepG2 cells significantly decreased compared with that in NC-HepG2 cells (Fig. 3d–f). In addition, the protein levels of p-GSK3βser9 in CYP2E1-HepG2 cells also significantly decreased compared with that in NC-HepG2 cells (Fig. 3d and g). Furthermore, the cellular MDA level of ethanol-exposed CYP2E1-HepG2 cells was significantly higher than that of the ethanol-exposed NC-HepG2 cells, and CMZ (100 µm) could increase the phosphorylation of Akt in CYP2E1-HepG2 cells (Fig. 3h and i). These in vitro results further demonstrated that CYP2E1 overexpression could impair the phosphorylation and activation of Akt.
Fig. 3.
Akt phosphorylation was suppressed in CYP2E1-HepG2 cells compared with the NC-HepG2 cells. Monoclonal cell lines stably expressing CYP2E1 (CYP2E1-HepG2) and the control HepG2 (NC-HepG2) were established as described in the materials and methods sections. CYP2E1-HepG2 and NC-HepG2 were exposed to different doses of ethanol for 5 d, and the cellular TG levels and protein levels of Akt, p-Aktser473, p-Aktthr308, GSK3β and p-GSK3βser9 were determined by western blotting. (a) Immunohistochemical staining and western blotting analysis showed that CYP2E1 was expressed in CYP2E1-HepG2 cells, but not in NC-HepG2 cells; (b) ethanol exposure led to increased protein levels of CYP2E1; (c) TG levels in CYP2E1-HepG2 were much higher than those in NC-HepG2 cells; (d)–(g) CYP2E1 expression suppressed the phosphorylation of Akt at Ser473 and Thr308, and inhibited the GSK3β at Ser9; (h) the cellular MDA levels in NC-HepG2 and CYP2E1-HepG2 cells exposed to ethanol (200 Mm) for 5 d; (i) CMZ treatment led to increased phosphorylation of Akt in CYP2E1-HepG2 cells. Data were presented as mean ± SD from at least 3 independent experiments, and expressed as the percentage of the control. *P < 0.05, **P < 0.01, compared with the control NC-HepG2 cells (0 mM group); #P < 0.05, ##P < 0.01, compared with the control CYP2E1-HepG2 cells (0 mM group).
3.4. NAC attenuated chronic ethanol-induced hepatic TG accumulation and the decline of Akt phosphorylation at Thr308
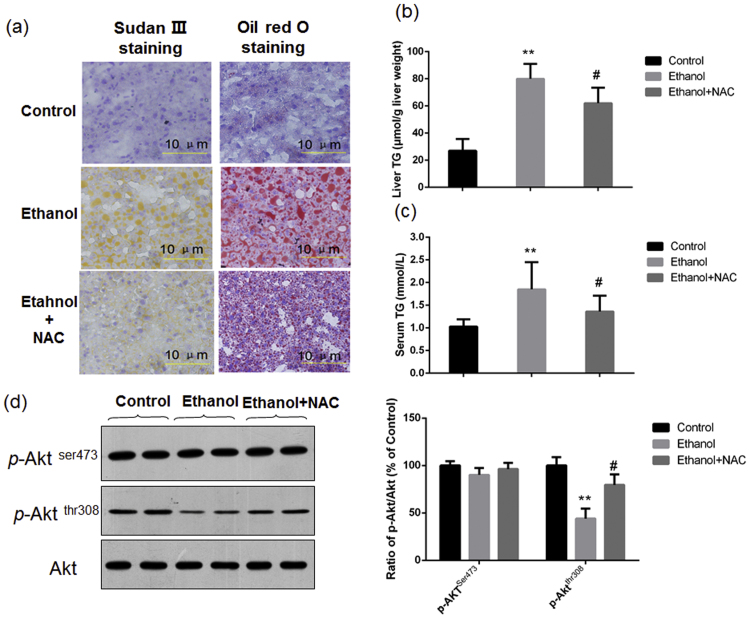
If the decline of Akt phosphorylation at Thr308 after ethanol exposure was mediated by oxidative stress, antioxidant could theoretically suppress ethanol-induced inhibition of Akt phosphorylation. Thus, we investigated the roles of NAC on chronic ethanol-induced fatty liver and the decline of Akt phosphorylation. As shown in Fig. 4a–c, NAC co-treatment indeed significantly attenuated chronic ethanol-induced fatty liver, shown as the reduction of fat droplets in the liver sections and the decrease of hepatic TG level. Furthermore, NAC treatment also suppressed chronic ethanol-induced decline of Akt phosphorylation at Thr308 (Fig. 4d).
Fig. 4.
NAC attenuated chronic ethanol-induced hepatic TG accumulation and the decline of Akt phosphorylation at Thr308 in mice. Mice were treated with NAC (100 mg/kg body weight/d) or equal volume of sterile saline, and exposed to ethanol-containing liquid diet or control diet for 4 weeks. Histological examination and the determination of liver TG levels showed that NAC significantly suppressed chronic ethanol-induced fat accumulation in liver sections and the increase of hepatic TG levels (a)&(b); (c) Serum TG levels; (d) Results of western blotting showed that IGF-1 treatment increased the phosphorylation of Akt at ser473 and thr308. **P<0.01, compared with the Control group mice; #P<0.01, compared with the ethanol group mice.
3.5. Pharmacological activation of Akt by IGF-1 significantly attenuated chronic ethanol-induced fatty liver in mice
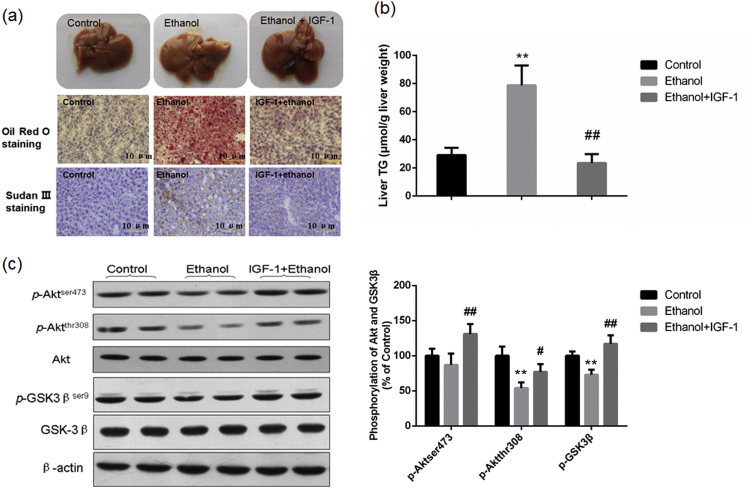
IGF-1 has been widely used as a strong activator of Akt pathway [12], [24]. To investigate whether pharmacological activation of Akt could ameliorate chronic ethanol-induced hepatic steatosis, mice were treated with IGF-1 (100 μg/kg body weight/d) by intraperitoneal injection to induce Akt activation. As expected, IGF-1 treatment significantly ameliorated chronic ethanol-induced hepatic fat accumulation (Fig. 5a and b), although it did not inhibit chronic ethanol-induced increase of the ratio of liver weight to body weight and the serum aminotransferase activity (data not shown). Results of western blotting showed that both the protein levels of hepatic p-Aktser473, p-Aktthr308 and p-GSK3βser9 in ethanol/IGF-1 group mice were all dramatically increased compared with those of ethanol group mice Fig. 5c.
Fig. 5.
Pharmacological activation of Akt by IGF-1 alleviated chronic ethanol-induced fatty liver in mice. Mice were treated with recombinant IGF-1 (100 μg/kg body weight, in IGF/ethanol group) or equal volume of sterile saline (in Control and Ethanol groups), and exposed to ethanol-containing liquid diet or control diet for 4 weeks. Histological examination and the determination of liver TG levels showed that IGF-1 significantly suppressed chronic ethanol-induced fat accumulation in liver sections and the increase of hepatic TG level (a) & (b); Results of western blotting showed that IGF-1 treatment increased the phosphorylation of Akt at ser473 and thr308, and also increased the phosphorylation of GSK3β at ser9 (c). **P<0.01, compared with the Control group mice; #P<0.05, ##P<0.01, compared with the ethanol group mice.
To investigate how Akt activation ameliorated the lipid accumulation in mice liver, we detected the protein levels of several important lipid metabolism-associated factors by western blot. IGF-1 treatment led to a slight decrease of the CYP2E1 protein level, but did not resulted in significant reduction of hepatic MDA and 4-HNE adducts levels (Fig. S3). In consistency with previous studies [34], [35], [36], chronic ethanol exposure led to significant inhibition of PPAR-α pathway shown as the decrease of the protein levels of PPAR-α, LFABP and ACOX. Although no significant difference in the protein levels of nSREBP-1c among three groups were detected, the protein levels of ACC and FAS in ethanol-group mice all significantly decreased compared with those of control group mice. IGF-1 treatment had no effects on the protein levels of the above factors. Interestingly, IGF-1 treatment significantly blocked chronic ethanol-induced decrease of the hepatic PPAR-γ protein level (Fig. S4).
4. Discussion
Akt is a 56-kD member of the AGC serine/threonine protein kinase family, which has been demonstrated to play important roles in the regulation of cell survival, glycogen synthesis, cell cycle, and lipid homeostasis. Akt can be activated by phosphorylation at thr308 by PDK-1 and at ser473 by mTORC2 [29], [30]. Akt activation could alleviate lipid accumulation in NAFLD models and diabetic models [13], [14], [15], [16], [37]. In the current study, we found that chronic ethanol-induced hepatic steatosis was accompanied with decreased phosphorylation of Akt at Thr308. Although the protein level of p-Aktser473 was not affected, the protein level of downstream p-GSK3βser9 in ethanol group mice liver was significantly decreased compared with that in control group mice (Fig. 1d–i). These results were consistent with the previous report that the phosphorylation of both kinase domains of Akt was required for its full activation [38]. As the impairment of Akt activity occurred earlier than the onset of fatty liver, it was possible that Akt impairment might be responsible for ethanol-induced fatty liver in mice. Furthermore, pharmacological activation of Akt by IGF-1 significantly alleviated chronic ethanol-induced fatty liver (Fig. 5a and b). These results support the hypothesis that chronic ethanol-induced impairment of Akt activity may be involved in chronic ethanol-induced fatty liver.
Accumulating evidences have demonstrated the critical roles of CYP2E1 in the pathogenesis of chronic ethanol-induced fatty liver [17], [18]. CYP2E1 is the major member of the microsomal ethanol oxidizing system (MEOS), and is believed to be a major contributor to ethanol-induced oxidative stress [32], [39], [40]. The central roles of CYP2E1 in the pathogenesis of ethanol-induced toxicity have been highlighted by a battery of studies. CYP2E1 (-/-) mice showed reduced live injury compared with the wild type animals [17], [41], [42], and CYP2E1 inhibitors alleviated ethanol-induced liver damage [43], [44]. Furthermore, mice infected with an adenovirus to overexpress CYP2E1 exacerbated oxidant stress and liver injury [45]. In parallel well with previous studies, the current study demonstrated that CYP2E1 suppression by CMZ significantly attenuated chronic ethanol-induced fat accumulation in the liver [8], [17]. Interestingly, CMZ also led to significant increase of Akt phosphorylation at Thr308 and Ser473 (Fig. 2c and d). In the in vitro study, ethanol exposure for 5 d led to a dose-dependent increased of p-Aktser473 and p-Aktthr308 protein levels in NC-HepG2 cells, which was apparently reduced in CYP2E1-HepG2 cells. The phosphorylation of GSK3β at Ser9 was also significantly decreased in CYP2E1-HepG2 cells compared with the NC-HepG2 cells (Fig. 3d–g). These results suggest that chronic ethanol-induced Akt suppression may be associated with CYP2E1 activation.
Akt phosphorylation and activation can be controlled by PI3K and PTEN [10], [22]. PI3K are heterodimers composed of a catalytic and a regulatory subunit, and is responsible for the production of phosphatidylinositol (3,4,5)-trisphosphate (PIP3), which could bind to the pleckstrin homology (PH) domain of Akt and PDK1, leading to the phosphorylation and activation of Akt [46], [47]. In contrast, PTEN negatively regulates intracellular PIP3 level and functions as a tumor suppressor by negatively regulating the Akt pathway [22]. In this study, we found that chronic ethanol exposure had no effects on the protein levels of PI3K and PTEN in mice liver and led to slight decrease in the protein levels of PDK-1. However, CMZ treatment had no significant effects on the protein levels of PI3K, PTEN, and PDK-1. These results suggest that there might be other mechanisms responsible for the chronic ethanol-induced inactivation of Akt.
As CYP2E1 activation could lead to generation of significant amounts of ROS, it would be plausible that oxidative stress promotes the fat accumulation in the liver [17]. Previous studies have suggested that CYP2E1-induced oxidative stress could lead to the inhibition of PPAR-α and the suppression of autophagy [17], [33], [39]; however, oxidative stress may also disturb the lipid homeostasis by other mechanisms. 4-HNE is one of the major lipid peroxidation products, which can preferentially modify cysteine, histidine, and lysine residues via Michael addition and for lysine, Schiff base products [20], [21]. Indeed, some previous studies have demonstrated that ethanol-induced 4-HNE modification of hepatocellular proteins resulted in the inhibition of normal enzyme function [22], [23]. Thus, it might be reasonable that ethanol-induced oxidative stress resulted in the inhibition of Akt phosphorylation by covalent binding to 4-HNE. In the current study, we found that the chronic ethanol exposure led to significant increase of hepatic MDA and 4-HNE-adducts levels, which was inhibited by CMZ co-treatment. To evaluate the interactions between 4-HNE and Akt, Akt was immunoprecipitated from the liver protein extracts and the levels of 4-HNE-Akt adduct were detected using immunoblotting analysis. The results showed CMZ treatment also significantly inhibited chronic ethanol-induced increase of the 4-HNE-Akt adduct level. The in vitro study showed that phosphorylation of Akt at Ser473 initially increased (at 1 and 2 h after 4-HNE exposure) and then decreased to normal values, while the phosphorylation of Akt at Thr308 significantly decreased at 2–8 h after 4-HNE exposure. These results indicate that ethanol-induced suppression of Akt activation might be, at least partially, attributed to the increased covalent binding with 4-HEN.
Shearn et al. have conducted a couple of elegant studies to investigate the interactions between Akt and 4-HNE. They found that 4-HNE (50–100 μM) exposure resulted in the phosphorylation of Akt at Ser473 and Thr308 by inhibiting the activity of PTEN in HepG2 cells [48]. The contradictory results about the phosphorylation of Akt at Thr308 2 h after 4-HNE exposure, i.e. decreased phosphorylation observed in our study and increased phosphorylation in studies by Shearn et al., may be attributed to several reasons. Firstly, the dose of 4-HNE used in our study (25 μM) is much lower than that use in the studies by Shearn et al. (100 μM). In fact, the protein band of p-Aktthr308 in HepG2 cell exposed to 0–25 μM 4-HEN was not detected in the study by Shearn et al. [49]. It might be possible that lower doses of 4-HNE leads to decreased phosphorylation of Akt at Thr308, while higher doses of 4-HNE might result in increased phosphorylation of Akt at Thr308. Secondly, the studies by Shearn et al. used serum-free medium, while serum-containing medium was used in our study. Interestingly, although 4-HNE (100 μM) selectively led to the phosphorylation of Akt, the Akt activity was inhibited as shown by the decreased phosphorylation of GSK3β [48], [49]. More importantly, the impaired Akt activity by 4-HNE led to the accumulation of neutral lipid as observed in our study.
The current study and some previous studies suggested that Akt activation had protective effects against fatty liver diseases; however, Akt activation was found to promote fatty liver in some in vitro studies and in binge drinking-induced acute fatty liver in mice. In the in vitro studies, Akt activation induced lipogenesis by activating SREBP-1 pathway [12], [50]. However, in the current study as well as in some previous studies, the protein levels of nSREBP-1c were not affected by ethanol, while the protein levels of ACC and FAS were decreased (Fig. 1h and Fig. S4) [8], [51]. The decreased activity of SREBP-1c-regulated lipogenesis was paralleled with the impaired Akt activation. These results also suggest that the hepatic TG accumulation in mice received 4 weeks of regular Lieber-DeCarli liquid diet with 5% ethanol (w/v) could not be due to increased fatty acid synthesis [51], [52], [53]. Furthermore, the pharmacological activation of Akt did not lead to the activation of SREBP-1c-regulated lipogenesis pathway (Fig. S4). Thus, it could be speculated that the modulation of Akt on lipid homeostasis in chronic AFL might be not mainly associated with SREBP-1c-mediated lipogenesis. In acute AFL mice model, Wu et al. found that binge drinking-induced fatty liver was accompanied with the increased activity of Akt, which might account for the impairment of autophagy [54]. However, some researchers have emphasized that acute ethanol models by no means resemble all effects of chronic ethanol exposure on the liver, although acute and chronic ethanol-induced liver injury may share some similar mechanisms [55]. In deed, it has been demonstrated that liver TG in mice exposed to binge drinking resembles to adipose tissues, whereas the fat accumulated in the liver induced by chronic ethanol consumption differed markedly from adipose tissue lipids [56]. Results of these studied suggest that Akt may play different roles in acute and chronic ethanol-induced fatty liver. The activation of Akt by IGF-1 can lead to the activation of medium chain acyl-CoA dehydrogenase (MCAD) and carnitine palmitoyl transferase 1 (CPT-1) by way of the inactivation of PGC-1 α and forkhead box protein A2 (FoxA2), thus increasing the β-oxidation of fatty acids [57], [58]. In addition, IGF-1 may improve chronic ethanol-induced fatty liver by blocking chronic ethanol-induced decline of hepatic PPAR-γ (Fig. S4). A line of studies demonstrated that PPAR-γ activation could ameliorate chronic ethanol-induced liver injury [59], [60], [61]. The protective effects of PPAR-γ against AFL might be due to increased insulin sensitivity in adipose tissues and skeletal muscle leading to decreased FFA deposition in the liver [62]. Additionally, PPAR-γ activation may also suppress Kupffer cell-sourced TNF-α production, which has been demonstrated to play critical roles in the pathogenesis of ethanol-induced liver disease [63], [64]. It is needed to further investigate whether the protective effects of Akt against AFL is associated with the activation of PPAR-γ.
In summary, the current study demonstrated that chronic ethanol exposure led to reduced phosphorylation of Akt at Thr308, which might be associated with CYP2E1-induced oxidative stress. Pharmacological activation of Akt by IGF-1 ameliorated ethanol-induced steatosis. These results suggest that impaired Akt activity may play important roles in the pathogenesis of chronic ethanol-induced fatty liver, and pharmacological modulation of Akt in liver may be an effective strategy for the treatment of ethanol-induced fatty liver.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Acknowledgement
This work was supported by the National Natural Science Foundation of China (NSFC Grant nos. 81473004 and 81102153) and the Young Scholars Program of Shandong University (Grant no. 2015WLJH52).
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2017.09.018.
Contributor Information
Tao Zeng, Email: zengtao@sdu.edu.cn.
Ke-Qin Xie, Email: keqinx@sdu.edu.cn.
Appendix A. Supplementary material
Supplementary material
References
- 1.Zeng T., Zhang C.L., Song F.Y., Zhao X.L., Yu L.H., Zhu Z.P., Xie K.Q. The activation of HO-1/Nrf-2 contributes to the protective effects of diallyl disulfide (DADS) against ethanol-induced oxidative stress. Biochim. Biophys. Acta. 2013;1830(10):4848–4859. doi: 10.1016/j.bbagen.2013.06.028. [DOI] [PubMed] [Google Scholar]
- 2.Donohue T.M., Jr. Alcohol-induced steatosis in liver cells. World J. Gastroenterol. 2007;13(37):4974–4978. doi: 10.3748/wjg.v13.i37.4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Purohit V., Gao B., Song B.J. Molecular mechanisms of alcoholic fatty liver. Alcohol. Clin. Exp. Res. 2009;33(2):191–205. doi: 10.1111/j.1530-0277.2008.00827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sorensen T.I., Orholm M., Bentsen K.D., Hoybye G., Eghoje K., Christoffersen P. Prospective evaluation of alcohol abuse and alcoholic liver injury in men as predictors of development of cirrhosis. Lancet. 1984;2(8397):241–244. doi: 10.1016/s0140-6736(84)90295-2. [DOI] [PubMed] [Google Scholar]
- 5.Leevy C.M. Fatty liver: a study of 270 patients with biopsy proven fatty liver and review of the literature. Medicine. 1962;41:249–276. doi: 10.1097/00005792-196209000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Yang S., Lin H., Diehl A.M. Fatty liver vulnerability to endotoxin-induced damage despite NF-kappaB induction and inhibited caspase 3 activation. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;281(2):G382–G392. doi: 10.1152/ajpgi.2001.281.2.G382. [DOI] [PubMed] [Google Scholar]
- 7.Shin S., Park J., Li Y., Min K.N., Kong G., Hur G.M., Kim J.M., Shong M., Jung M.S., Park J.K., Jeong K.H., Park M.G., Kwak T.H., Brazil D.P. beta-Lapachone alleviates alcoholic fatty liver disease in rats. Cell. Signal. 2014;26(2):295–305. doi: 10.1016/j.cellsig.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 8.Zeng T., Zhang C.L., Song F.Y., Zhao X.L., Xie K.Q. CMZ reversed chronic ethanol-induced disturbance of PPAR-alpha possibly by suppressing oxidative stress and PGC-1alpha acetylation, and activating the MAPK and GSK3beta pathway. PLoS One. 2014;9(6):e98658. doi: 10.1371/journal.pone.0098658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martelli A.M., Tabellini G., Bressanin D., Ognibene A., Goto K., Cocco L., Evangelisti C. The emerging multiple roles of nuclear Akt. Biochim. Biophys. Acta. 2012;1823(12):2168–2178. doi: 10.1016/j.bbamcr.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 10.Krycer J.R., Sharpe L.J., Luu W., Brown A.J. The Akt-SREBP nexus: cell signaling meets lipid metabolism. Trends Endocrinol. Metab. 2010;21(5):268–276. doi: 10.1016/j.tem.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Porstmann T., Griffiths B., Chung Y.L., Delpuech O., Griffiths J.R., Downward J., Schulze A. PKB/Akt induces transcription of enzymes involved in cholesterol and fatty acid biosynthesis via activation of SREBP. Oncogene. 2005;24(43):6465–6481. doi: 10.1038/sj.onc.1208802. [DOI] [PubMed] [Google Scholar]
- 12.Smith T.M., Gilliland K., Clawson G.A., Thiboutot D. IGF-1 induces SREBP-1 expression and lipogenesis in SEB-1 sebocytes via activation of the phosphoinositide 3-kinase/Akt pathway. J. Investig. Dermatol. 2008;128:1286–1293. doi: 10.1038/sj.jid.5701155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han J.W., Zhan X.R., Li X.Y., Xia B., Wang Y.Y., Zhang J., Li B.X. Impaired PI3K/Akt signal pathway and hepatocellular injury in high-fat fed rats. World J. Gastroenterol. 2010;16(48):6111–6118. doi: 10.3748/wjg.v16.i48.6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang C., Chi Y., Li J., Miao Y., Li S., Su W., Jia S., Chen Z., Du S., Zhang X., Zhou Y., Wu W., Zhu M., Wang Z., Yang H., Xu G., Wang S., Yang J., Guan Y. FAM3A activates PI3K p110alpha/Akt signaling to ameliorate hepatic gluconeogenesis and lipogenesis. Hepatology. 2014;59(5):1779–1790. doi: 10.1002/hep.26945. [DOI] [PubMed] [Google Scholar]
- 15.Bijl N., Sokolovic M., Vrins C., Langeveld M., Moerland P.D., Ottenhoff R., van Roomen C.P., Claessen N., Boot R.G., Aten J., Groen A.K., Aerts J.M., van Eijk M. Modulation of glycosphingolipid metabolism significantly improves hepatic insulin sensitivity and reverses hepatic steatosis in mice. Hepatology. 2009;50(5):1431–1441. doi: 10.1002/hep.23175. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y., Hai J., Cao M., Pei S., Wang J., Zhang Q. Silibinin ameliorates steatosis and insulin resistance during non-alcoholic fatty liver disease development partly through targeting IRS-1/PI3K/Akt pathway. Int. Immunopharmacol. 2013;17(3):714–720. doi: 10.1016/j.intimp.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 17.Lu Y., Zhuge J., Wang X., Bai J., Cederbaum A.I. Cytochrome P450 2E1 contributes to ethanol-induced fatty liver in mice. Hepatology. 2008;47(5):1483–1494. doi: 10.1002/hep.22222. [DOI] [PubMed] [Google Scholar]
- 18.Abdelmegeed M.A., Banerjee A., Jang S., Yoo S.H., Yun J.W., Gonzalez F.J., Keshavarzian A., Song B.J. CYP2E1 potentiates binge alcohol-induced gut leakiness, steatohepatitis, and apoptosis. Free Radic. Biol. Med. 2013;65:1238–1245. doi: 10.1016/j.freeradbiomed.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y.Y., Zhang C.L., Zhao X.L., Xie K.Q., Zeng T. Inhibition of cytochrome P4502E1 by chlormethiazole attenuated acute ethanol-induced fatty liver. Chem. Biol. Interact. 2014;222C:18–26. doi: 10.1016/j.cbi.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Doorn J.A., Petersen D.R. Covalent modification of amino acid nucleophiles by the lipid peroxidation products 4-hydroxy-2-nonenal and 4-oxo-2-nonenal. Chem. Res. Toxicol. 2002;15(11):1445–1450. doi: 10.1021/tx025590o. [DOI] [PubMed] [Google Scholar]
- 21.Ishii T., Tatsuda E., Kumazawa S., Nakayama T., Uchida K. Molecular basis of enzyme inactivation by an endogenous electrophile 4-hydroxy-2-nonenal: identification of modification sites in glyceraldehyde-3-phosphate dehydrogenase. Biochemistry. 2003;42(12):3474–3480. doi: 10.1021/bi027172o. [DOI] [PubMed] [Google Scholar]
- 22.Carbone D.L., Doorn J.A., Kiebler Z., Ickes B.R., Petersen D.R. Modification of heat shock protein 90 by 4-hydroxynonenal in a rat model of chronic alcoholic liver disease. J. Pharmacol. Exp. Ther. 2005;315(1):8–15. doi: 10.1124/jpet.105.088088. [DOI] [PubMed] [Google Scholar]
- 23.Sampey B.P., Stewart B.J., Petersen D.R. Ethanol-induced modulation of hepatocellular extracellular signal-regulated kinase-1/2 activity via 4-hydroxynonenal. J. Biol. Chem. 2007;282(3):1925–1937. doi: 10.1074/jbc.M610602200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stitt T.N., Drujan D., Clarke B.A., Panaro F., Timofeyva Y., Kline W.O., Gonzalez M., Yancopoulos G.D., Glass D.J. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol. Cell. 2004;14(3):395–403. doi: 10.1016/s1097-2765(04)00211-4. [DOI] [PubMed] [Google Scholar]
- 25.Qin W.Z., Li Q.L., Chen W.F., Xu M.D., Zhang Y.Q., Zhong Y.S., Ma L.L., Hu J.W., Cai M.Y., He M.J., Yao L.Q., Zhou P.H. Overexpression of fibrinogen-like protein 2 induces epithelial-to-mesenchymal transition and promotes tumor progression in colorectal carcinoma. Med. Oncol. 2014;31(9):181. doi: 10.1007/s12032-014-0181-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lykkesfeldt J. Malondialdehyde as biomarker of oxidative damage to lipids caused by smoking. Clin. Chim. Acta. 2007;380(1–2):50–58. doi: 10.1016/j.cca.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 27.Zeng T., Zhang C.L., Zhu Z.P., Yu L.H., Zhao X.L., Xie K.Q. Diallyl trisulfide (DATS) effectively attenuated oxidative stress-mediated liver injury and hepatic mitochondrial dysfunction in acute ethanol-exposed mice. Toxicology. 2008;252(1–3):86–91. doi: 10.1016/j.tox.2008.07.062. [DOI] [PubMed] [Google Scholar]
- 28.Cederbaum A.I. Role of CYP2E1 in ethanol-induced oxidant stress, fatty liver and hepatotoxicity. Dig. Dis. 2010;28(6):802–811. doi: 10.1159/000324289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bayascas J.R. PDK1: the major transducer of PI 3-kinase actions. Curr. Top. Microbiol. Immunol. 2010;346:9–29. doi: 10.1007/82_2010_43. [DOI] [PubMed] [Google Scholar]
- 30.Zoncu R., Efeyan A., Sabatini D.M. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 2011;12(1):21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacinto E., Facchinetti V., Liu D., Soto N., Wei S., Jung S.Y., Huang Q., Qin J., Su B. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127(1):125–137. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 32.Leung T.M., Nieto N. CYP2E1 and oxidant stress in alcoholic and non-alcoholic fatty liver disease. J. Hepatol. 2013;58(2):395–398. doi: 10.1016/j.jhep.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 33.Wu D., Wang X., Zhou R., Cederbaum A. CYP2E1 enhances ethanol-induced lipid accumulation but impairs autophagy in HepG2 E47 cells. Biochem. Biophys. Res. Commun. 2010;402(1):116–122. doi: 10.1016/j.bbrc.2010.09.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kong L., Ren W., Li W., Zhao S., Mi H., Wang R., Zhang Y., Wu W., Nan Y., Yu J. Activation of peroxisome proliferator activated receptor alpha ameliorates ethanol induced steatohepatitis in mice. Lipids Health Dis. 2011;10:246. doi: 10.1186/1476-511X-10-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stienstra R., Saudale F., Duval C., Keshtkar S., Groener J.E., van Rooijen N., Staels B., Kersten S., Muller M. Kupffer cells promote hepatic steatosis via interleukin-1beta-dependent suppression of peroxisome proliferator-activated receptor alpha activity. Hepatology. 2010;51(2):511–522. doi: 10.1002/hep.23337. [DOI] [PubMed] [Google Scholar]
- 36.Kang X., Zhong W., Liu J., Song Z., McClain C.J., Kang Y.J., Zhou Z. Zinc supplementation reverses alcohol-induced steatosis in mice through reactivating hepatocyte nuclear factor-4alpha and peroxisome proliferator-activated receptor-alpha. Hepatology. 2009;50(4):1241–1250. doi: 10.1002/hep.23090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chou I.P., Lin Y.Y., Ding S.T., Chen C.Y. Adiponectin receptor 1 enhances fatty acid metabolism and cell survival in palmitate-treated HepG2 cells through the PI3 K/AKT pathway. Eur. J. Nutr. 2014;53(3):907–917. doi: 10.1007/s00394-013-0594-7. [DOI] [PubMed] [Google Scholar]
- 38.Bellacosa A., Chan T.O., Ahmed N.N., Datta K., Malstrom S., Stokoe D., McCormick F., Feng J., Tsichlis P. Akt activation by growth factors is a multiple-step process: the role of the PH domain. Oncogene. 1998;17(3):313–325. doi: 10.1038/sj.onc.1201947. [DOI] [PubMed] [Google Scholar]
- 39.Lu Y., Cederbaum A.I. CYP2E1 and oxidative liver injury by alcohol. Free Radic. Biol. Med. 2008;44(5):723–738. doi: 10.1016/j.freeradbiomed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang L., Wu D., Wang X., Cederbaum A.I. Cytochrome P4502E1, oxidative stress, JNK, and autophagy in acute alcohol-induced fatty liver. Free Radic. Biol. Med. 2012;53(5):1170–1180. doi: 10.1016/j.freeradbiomed.2012.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu Y., Wu D., Wang X., Ward S.C., Cederbaum A.I. Chronic alcohol-induced liver injury and oxidant stress are decreased in cytochrome P4502E1 knockout mice and restored in humanized cytochrome P4502E1 knock-in mice. Free Radic. Biol. Med. 2010;49(9):1406–1416. doi: 10.1016/j.freeradbiomed.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bradford B.U., Kono H., Isayama F., Kosyk O., Wheeler M.D., Akiyama T.E., Bleye L., Krausz K.W., Gonzalez F.J., Koop D.R., Rusyn I. Cytochrome P450 CYP2E1, but not nicotinamide adenine dinucleotide phosphate oxidase, is required for ethanol-induced oxidative DNA damage in rodent liver. Hepatology. 2005;41(2):336–344. doi: 10.1002/hep.20532. [DOI] [PubMed] [Google Scholar]
- 43.Shimada M., Liu L., Nussler N., Jonas S., Langrehr J.M., Ogawa T., Kaminishi M., Neuhaus P., Nussler A.K. Human hepatocytes are protected from ethanol-induced cytotoxicity by DADS via CYP2E1 inhibition. Toxicol. Lett. 2006;163(3):242–249. doi: 10.1016/j.toxlet.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 44.Ronis M.J., Korourian S., Blackburn M.L., Badeaux J., Badger T.M. The role of ethanol metabolism in development of alcoholic steatohepatitis in the rat. Alcohol. 2010;44(2):157–169. doi: 10.1016/j.alcohol.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bai J., Cederbaum A.I. Adenovirus-mediated expression of CYP2E1 produces liver toxicity in mice. Toxicol. Sci. 2006;91(2):365–371. doi: 10.1093/toxsci/kfj165. [DOI] [PubMed] [Google Scholar]
- 46.Martelli A.M., Evangelisti C., Chiarini F., McCubrey J.A. The phosphatidylinositol 3-kinase/Akt/mTOR signaling network as a therapeutic target in acute myelogenous leukemia patients. Oncotarget. 2010;1(2):89–103. doi: 10.18632/oncotarget.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ogita S., Lorusso P. Targeting phosphatidylinositol 3 kinase (PI3K)-Akt beyond rapalogs. Target Oncol. 2011;6(2):103–117. doi: 10.1007/s11523-011-0176-7. [DOI] [PubMed] [Google Scholar]
- 48.Shearn C.T., Smathers R.L., Stewart B.J., Fritz K.S., Galligan J.J., Hail N., Jr., Petersen D.R. Phosphatase and tensin homolog deleted on chromosome 10 (PTEN) inhibition by 4-hydroxynonenal leads to increased Akt activation in hepatocytes. Mol. Pharmacol. 2011;79(6):941–952. doi: 10.1124/mol.110.069534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shearn C.T., Fritz K.S., Reigan P., Petersen D.R. Modification of Akt2 by 4-hydroxynonenal inhibits insulin-dependent Akt signaling in HepG2 cells. Biochemistry. 2011;50(19):3984–3996. doi: 10.1021/bi200029w. [DOI] [PubMed] [Google Scholar]
- 50.Porstmann T., Griffiths B., Chung Y.L., Delpuech O., Griffiths J.R., Downward J., Schulze A. PKB/Akt induces transcription of enzymes involved in cholesterol and fatty acid biosynthesis via activation of SREBP. Oncogene. 2005;24(43):6465–6481. doi: 10.1038/sj.onc.1208802. [DOI] [PubMed] [Google Scholar]
- 51.Shearn C.T., Smathers R.L., Jiang H., Orlicky D.J., Maclean K.N., Petersen D.R. Increased dietary fat contributes to dysregulation of the LKB1/AMPK pathway and increased damage in a mouse model of early-stage ethanol-mediated steatosis. J. Nutr. Biochem. 2013;24(8):1436–1445. doi: 10.1016/j.jnutbio.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simpson K.J., Venkatesan S., Peters T.J. Fatty acid synthesis by rat liver after chronic ethanol feeding with a low-fat diet. Clin. Sci. 1994;87(4):441–446. doi: 10.1042/cs0870441. [DOI] [PubMed] [Google Scholar]
- 53.Venkatesan S., Ward R.J., Peters T.J. Fatty acid synthesis and triacylglycerol accumulation in rat liver after chronic ethanol consumption. Clin. Sci. 1987;73(2):159–163. doi: 10.1042/cs0730159. [DOI] [PubMed] [Google Scholar]
- 54.Wu D., Wang X., Zhou R., Yang L., Cederbaum A.I. Alcohol steatosis and cytotoxicity: the role of cytochrome P4502E1 and autophagy. Free Radic. Biol. Med. 2012;53(6):1346–1357. doi: 10.1016/j.freeradbiomed.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kanuri G., Weber S., Volynets V., Spruss A., Bischoff S.C., Bergheim I. Cinnamon extract protects against acute alcohol-induced liver steatosis in mice. J. Nutr. 2009;139(3):482–487. doi: 10.3945/jn.108.100495. [DOI] [PubMed] [Google Scholar]
- 56.Lieber C.S., Spritz N. Effects of prolonged ethanol intake in man: role of dietary adipose, and endogenously synthesized fatty acids in the pathogenesis of the alcoholic fatty liver. J. Clin. Investig. 1966;45(9):1400–1411. doi: 10.1172/JCI105448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fritsche L., Weigert C., Haring H.-U., Lehmann R. How insulin receptor substrate proteins regulate the metabolic capacity of the liver-implications for health and disease. Curr. Med. Chem. 2008;15(13):1316–1329. doi: 10.2174/092986708784534956. [DOI] [PubMed] [Google Scholar]
- 58.Wang C., Chi Y., Li J., Miao Y., Li S., Su W., Jia S., Chen Z., Du S., Zhang X. FAM3A activates PI3Kp110α/Akt signaling to ameliorate hepatic gluconeogenesis and lipogenesis. Hepatology. 2013 doi: 10.1002/hep.26945. [DOI] [PubMed] [Google Scholar]
- 59.Sun X., Tang Y., Tan X., Li Q., Zhong W., Jia W., McClain C.J., Zhou Z. Activation of peroxisome proliferator-activated receptor-gamma by rosiglitazone improves lipid homeostasis at the adipose tissue-liver axis in ethanol-fed mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2012;302(5):G548–G557. doi: 10.1152/ajpgi.00342.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shen Z., Liang X., Rogers C.Q., Rideout D., You M. Involvement of adiponectin-SIRT1-AMPK signaling in the protective action of rosiglitazone against alcoholic fatty liver in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2009 doi: 10.1152/ajpgi.00456.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Enomoto N., Takei Y., Hirose M., Konno A., Shibuya T., Matsuyama S., Suzuki S., Kitamura K.I., Sato N. Prevention of ethanol-induced liver injury in rats by an agonist of peroxisome proliferator-activated receptor-gamma, pioglitazone. J. Pharmacol. Exp. Ther. 2003;306(3):846–854. doi: 10.1124/jpet.102.047217. [DOI] [PubMed] [Google Scholar]
- 62.Huang Y.Y., Gusdon A.M., Qu S. Nonalcoholic fatty liver disease: molecular pathways and therapeutic strategies. Lipids Health Dis. 2013;12:171. doi: 10.1186/1476-511X-12-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mitra S.K., Varma S.R., Godavarthi A., Nandakumar K.S. Liv.52 regulates ethanol induced PPARgamma and TNF alpha expression in HepG2 cells. Mol. Cell. Biochem. 2008;315(1–2):9–15. doi: 10.1007/s11010-008-9782-9. [DOI] [PubMed] [Google Scholar]
- 64.Ohata M., Suzuki H., Sakamoto K., Hashimoto K., Nakajima H., Yamauchi M., Hokkyo K., Yamada H., Toda G. Pioglitazone prevents acute liver injury induced by ethanol and lipopolysaccharide through the suppression of tumor necrosis factor-alpha. Alcohol. Clin. Exp. Res. 2004;28(8):139S–144S. doi: 10.1097/01.alc.0000134412.38510.f7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material