Abstract
Introduction
Previous studies have found associations between respiratory morbidity and high temperatures; however, few studies have explored associations in potentially sensitive sub-populations.
Methods
We evaluated individual and area-level factors as modifiers of the association between warm-season (May–Sept.) temperature and pediatric respiratory morbidity in Atlanta. Emergency department (ED) visit data were obtained for children, 5–18 years old, with primary diagnoses of asthma or respiratory disease (diagnoses of upper respiratory infections, bronchiolitis, pneumonia, chronic obstructive pulmonary disease, asthma, or wheeze) in 20-county Atlanta during 1993–2012. Daily maximum temperature (Tmax) was acquired from the automated surface observing station at Atlanta Hartsfield International Airport. Poisson generalized linear models were used to estimate rate ratios (RR) between daily Tmax and asthma or respiratory disease ED visits, controlling for time and meteorology. Tmax effects were estimated for single-day lags of 0–6 days, for 3-, 5-, and 7-day moving averages and modeled with cubic terms to allow for non-linear relationships. Effect modification by individual factors (sex, race, insurance status) and area-level socioeconomic status (SES; ZIP code levels of poverty, education, and the neighborhood deprivation index) was examined via stratification.
Results
Estimated RRs for Tmax and pediatric asthma ED visits were positive and significant for lag days 1–5, with the strongest single day association observed on lag day 2 (RR=1.06, 95% CI: 1.03, 1.09) for a change in Tmax from 27 °C to 32 °C (25th to 75th percentile). For the moving average exposure periods, associations increased as moving average periods increased. We observed stronger RRs between Tmax and asthma among males compared to females, non-white children compared to white children, children with private insurance compared to children with Medicaid, and among children living in high compared to low SES areas. Associations between Tmax and respiratory disease ED visits were weak and non-significant (p-value > 0.05).
Conclusions
Results suggest socio-demographic factors (race/ethnicity, insurance status, and area-level SES) may confer vulnerability to temperature-related pediatric asthma morbidity. Our findings of weaker associations among children with Medicaid compared to other health insurance types and among children living in low compared to high SES areas run counter to our belief that children from disadvantaged households or ZIP codes would be more vulnerable to the respiratory effects of temperature. The potential reasons for these unexpected results are explored in the discussion.
Keywords: climate change, temperature, childhood asthma, time-series, effect modification
1. INTRODUCTION
Global surface temperatures have risen steadily and rapidly for the past several decades, resulting in location-specific variation in ambient temperatures and more frequent episodes of extreme heat (McMichael et al., 2011; Meehl et al., 2004). Emerging research has shown that the annual number of extreme heat events is increasing more rapidly in sprawling cities compared to compact cities (Stone et al., 2010), and climate change is expected to cause higher warm-season ambient temperatures in large metropolitan areas where temperatures are amplified by the urban heat island effect (Conlon et al., 2016; Luber et al., 2008; Stone et al., 2010; Winquist et al., 2016; Zhou et al., 2009). Although high ambient temperature is a well-documented cause of cardiorespiratory mortality, particularly among the elderly (Basu, 2002; Basu, 2009; Basu et al., 2011; Benmarhnia et al., 2015; Braga et al., 2002; Cheng et al., 2014; Laaidi et al., 2012), much less is known about the effects of high ambient temperature on respiratory morbidity, and the influence of modifying factors among sensitive subpopulations remains largely unexplored.
Among the studies that have investigated high temperature-related respiratory morbidity, there is mounting epidemiologic evidence for a lagged effect of temperature (Bunker et al., 2016; Cheng et al., 2014; Li et al., 2014b; Winquist et al., 2016; Xu et al., 2013; Ye et al., 2012), and several studies have found that the effects of temperature on respiratory morbidity remain after controlling for ambient air pollution (Anderson et al., 2013; Cheng et al., 2014; Li et al., 2014a; Lin et al., 2009; Winquist et al., 2016); these findings suggest a strong, independent effect of high temperature in addition to that potentially mediated through the effect of air pollution. There is less agreement on whether thresholds or non-linear exposure-response functions exist regarding the effects of high temperature on respiratory outcomes (Carreras et al., 2015; Green et al., 2010; Kovats et al., 2004; Li et al., 2014a; Li et al., 2014b; Lin et al., 2009; Michelozzi et al., 2009; Xu et al., 2013), and multi-city studies have reported heterogeneity in the exposure-response function between study locations (Anderson et al., 2013; Michelozzi et al., 2009). Inconsistencies between studies may be due to a variety of factors including differences in the climate of the study area, different adaptive strategies employed in cities (e.g. high utilization of air conditioning, cooling centers, early warning systems), differences in population-level acclimation to climate, and the use of disparate temperature metrics to capture exposure to high ambient temperature (e.g. daily minimum temperature, daily mean temperature, daily maximum temperature, diurnal temperature ranges, heat waves, and heat stress indices) (Davis et al., 2016; Turner et al., 2012). Certain populations may also be more or less responsive to high temperature and studies that have examined age as a modifying factor have reported stronger associations among children and the elderly compared to other age groups (Bunker et al., 2016; Cheng et al., 2014; Kovats et al., 2004; Michelozzi et al., 2009; Xu et al., 2013).
Children may be inherently more susceptible than adults to temperature-related respiratory morbidity due to higher ventilation rates, developing respiratory and immunological systems, and anatomically smaller peripheral airways that predispose children to airway inflammation and obstruction (Bateson et al., 2007; Makri et al., 2008; Selgrade et al., 2008; Sharma et al., 2006). They may also spend more time outdoors during the warm-season compared to adults, thus experiencing greater exposures to high ambient temperatures. Additionally, children have an underdeveloped thermoregulatory system that results in a diminished capacity to maintain optimal internal temperatures under heat stress (Hanna et al., 2015). Impaired thermoregulation and prolonged heat exposure can result in hyperthermia and lead to increases in core body temperature, systemic inflammation, increased cardiac output, and increases in tidal volume, respiratory rate, and pulmonary ventilation (Anderson et al., 2013; Hanna et al., 2015; Leon et al., 2010; White, 2006). Finally, individual-level factors (e.g. sex, race, health care access, time-activity patterns) and area-level socioeconomic status (SES) may further confer susceptibility and vulnerability among children to temperature related respiratory disease; however, few studies to date provide estimates of temperature-related respiratory morbidity for such susceptible and/or vulnerable groups.
To address this gap, we built on an extensive previous assessment of heat-related morbidity in Atlanta, in which we observed a positive and significant association between high warm-season temperature and asthma ED visits among children (5–18 years old), but not in other age groups (Winquist et al., 2016). Here, using a similar methodology, we focus on this previously observed association between high temperature and respiratory morbidity among children (5–18 years) to specifically examine the degree to which individual-level factors (sex, race/ethnicity, insurance status) and area-level SES modify associations, and to examine the non-linear effects of temperature across these different modifying factors.
2. METHODS
2.1. Data sources
Hourly meteorological data from January 1, 1993 through December 31, 2012 were obtained from the Automated Surface Observing Station (ASOS) located at Atlanta Hartsfield International Airport. Hourly observations were used to create daily ambient meteorological metrics including daily maximum temperature and daily maximum dew-point temperature. We selected daily maximum temperature (in degrees Celsius, °C) as our primary exposure of interest based on our previous work (Winquist et al., 2016) and use of this metric by others in related health studies (Davis et al., 2016; Ebi et al., 2004; Hondula et al., 2014; Linares et al., 2008; Saha et al., 2015; Ye et al., 2001). Additionally, maximum temperature values may coincide with a time of day when children may be most active and exposed to outdoor temperatures (Barnett et al., 2010), and may represent an exposure that could cause the greatest amount of physiological stress due to temperature on a given day. For sensitivity analyses, we also acquired daily maximum and daily minimum temperature data from 13 cooperative meteorological stations located within the 20-county Atlanta study area. Cooperative stations are part of the National Weather Service (NWS) Cooperative Observer Program (COOP), and daily weather observations at COOP stations are recorded by volunteers (National Oceanic and Atmospheric Administration). Because COOP stations are maintained by volunteers, different stations may use different analytical instruments to measure ambient temperature, and some station volunteers may not be able to take daily measurements. In our sensitivity analyses, 12 of the 13 cooperative stations did not provide complete daily temperature records during the 1993–2012 study period. The names and locations of the cooperative stations used in sensitivity analyses are discussed in greater detail in Section 3.1, Descriptive Results.
Patient-level emergency department (ED) visit data from January 1, 1993 to December 31, 2012 were acquired from 41 hospitals located within the 20-county metropolitan area of Atlanta. The 20-county study area reflects the counties included in the Atlanta metropolitan statistical area definition of 1999 (United States Census Bureau), the year that our studies of ED visits and air quality in Atlanta began (Tolbert et al., 2000). All acute care hospitals in the 20-county Atlanta area were included, except for the Veterans Affairs hospital. Data from these hospitals were included if they were able to provide electronic billing records for at least part of the study period. In 1993, only 7 hospitals were contributing data. Additional hospitals were added over time as more facilities moved towards electronic records; by 2005, 40 hospitals were participating. ED visit data from 1993–2004 were acquired directly from individual hospitals and ED data from 2005–2012 were acquired from the Georgia Hospital Association (GHA). The ED visit data collected by GHA are from the same hospital billing records as those collected from individual hospitals. In order to compare between hospital data sources we obtained 3 years of overlap data (2002–2005) and found no considerable differences at the daily count level that would impact assessment of acute effects.
Relevant data elements reported on ED visit records included admission date, age, sex, race/ethnicity, method of ED visit payment, ZIP code of patient residence, and International Classification of Diseases, 9th Revision (ICD-9) diagnosis codes. ED visits for asthma were identified using primary ICD-9 diagnosis codes for asthma (493.0–493.9) or wheeze (786.07) and ED visits for respiratory disease were identified using primary ICD-9 codes 460–466, 477, 480–486, 491–493, 496, 786.07, which indicated diagnoses of upper respiratory infections, bronchiolitis, pneumonia, chronic obstructive pulmonary disease, asthma, and wheeze. Data were restricted to the pediatric population (5–18 years old) and to patients with a residential ZIP code located wholly or partially in 20-county Atlanta; the final study area was 20,627 square kilometers. Data were aggregated to daily counts of asthma and respiratory ED visits by strata defined by individual factors [sex, race/ethnicity, insurance status (a proxy for individual-level SES)] and area-level SES. The Emory University Institutional Review Board approved this study and granted exemption from informed consent requirements.
Area-level socioeconomic composition was evaluated using census data at the ZIP Code Tabulation Area (ZCTA, Census Bureau boundaries, created from census blocks to approximate ZIP codes) level. Over the 20-year study period, we identified 31 ZCTAs with changes in borders. In order to address such boundary changes, we fixed the spatial scale for our analysis at the 2010 ZCTA level. To create spatial scales compatible with Census Bureau data, each ZIP code in the ED visit database was assigned to a 2010 ZCTA. Assignments were accomplished by matching each ZIP code to a 2010 ZCTA based on 5-digit ZCTA ID numbers. ZIP code change reports helped facilitate ZCTA assignments for 31 ZIP codes that were altered or eliminated during the study period. ZCTAs that represented locations of businesses, P.O. boxes, and university campuses were excluded from the study. The resulting study area included 191 ZCTAs in Atlanta.
ZCTA-level (area-level) SES was estimated from the 1990 US Census long form, the 2000 US Census long form, and the American Community Survey (ACS) 5-year (2007–2011) summary file using a data aggregation product through Geolytics that normalized data to 2010 ZCTA borders (“The Time-Series Research Package”, GeoLytics, Inc., East Brunswick, NJ, 2013). The data aggregation product through Geolytics normalized all Census and American Community Survey data to 2010 ZCTA borders while accounting for changes to ZCTA boundaries over time. We estimated annual values of area-level SES between 1993 and 2006 by linear interpolation of Census 1990, Census 2000, and ACS 2007–2011 data. Area-level SES from 2007–2012 was estimated using only ACS 2007–2011 data. To represent area-level SES we chose two single SES indicators: percentage of the population (≥25 years old) with less than a 12th grade education (% < 12th grade), and percentage of households living below the poverty line (% below poverty). We chose these single indicators based on the frequency of their use in the literature and to facilitate comparisons between this study and similar analyses. To capture the multifaceted nature of area-level SES, we chose the Neighborhood Deprivation Index (NDI), a composite index comprised of 8 variables (i.e. % households with low income (<$30,000), % males not in management, % <12th grade, % below poverty line, % female headed households, % living in crowding, % households on public assistance, and % unemployed civilian population) summarized by principle components analysis (Messer et al., 2006). The Geolytics 1990 Census product did not include all of the variables necessary to create the NDI, a result of substantial changes to occupational classification systems that occurred only after the 1990 Census; therefore, ED visit data from 1993–1999 were assigned NDI values based on data from the 2000 Census.
2.2. Statistical analyses
Poisson generalized linear models were used to estimate associations between daily maximum temperature and daily counts of pediatric asthma or pediatric respiratory disease ED visits. All analyses were restricted to the warm-season, defined here as May through September. Poisson models were fitted with cubic functions of maximum temperature (using linear, squared, and cubic terms) to allow for non-linear relationships. Associations between maximum temperature and ED visits were assessed for lag days 0 to 6 and for 3-, 5-, and 7-day moving averages (i.e. averages across lags 0–2, lags 0–4, and lags 0–6). Separate models were fitted for each lag day/moving average and for each health outcome (asthma or respiratory disease). Distributed lag models were not considered in this analysis given that non-linear modeling of temperature effects would result in overly complex models that would be difficult to interpret.
Models accounted for Poisson overdispersion, and included additional control for atmospheric moisture by including terms for maximum dew-point temperature using cubic functions at the same lag period as maximum temperature. Dew-point temperature directly measures atmospheric moisture; by including dew-point temperature as a covariate we controlled for the effect that atmospheric moisture may have had on respiratory outcomes and thus only estimated the effect of maximum ambient temperature on pediatric respiratory outcomes. We did not consider controlling for relatively humidity because it is a function of moisture and temperature. Time-varying factors were controlled for using indicator variables for day of the week, holidays, periods of hospital participation, year of the study period, a cubic spline for day of the warm season with monthly knots to control smoothly for time-trends, and terms for the interaction between the linear term for day of the warm season and the indicator variable for each year of the study period.
In stratified analyses, we evaluated whether individual factors (sex, race/ethnicity, and insurance status) or area-level SES modified associations between maximum temperature and pediatric asthma or respiratory disease ED visits. For the individual factors, daily ED visit counts were aggregated for the following strata: male or female sex; white or non-white race (a consolidated category that included African American, Hispanic, and other race/ethnicity); and private insurance or Medicaid insurance. ED visit records with missing information on sex, race/ethnicity, or insurance status were excluded from analyses that examined the respective individual-level factors as effect modifiers, and ED visits paid by worker’s compensation or paid for directly by the patient were also excluded from analyses examining modification by insurance status. For area-level SES, daily ED visit counts were aggregated for strata based on several a priori cut-points of continuous ZCTA-level education, poverty, and NDI values including median, tertile, quartile, and 90th percentile cut-points.
For all models, we estimated rate ratios (RR) and 95% confidence intervals (CI) for changes in maximum temperature. The primary temperature increment evaluated was 27–32 °C, representing an interquartile range increase in maximum temperature from the 25th to 75th percentile; other temperature increments relative to a maximum temperature value of 27°C were considered for evaluating non-linearity in effects. RRs for a given temperature relative to 27°C were estimated by contrasting linear, squared, and cubic terms of the chosen maximum temperature value to the referent value. Evidence of significant effect modification by individual factors and area-level SES was assessed by estimating the degree of heterogeneity between stratum-specific RRs in pairwise comparisons for the primary temperature increment (27–32 °C) (Kaufman et al., 2013). All analyses were performed using SAS 9.4 (SAS Institute, Cary, NC) and R version 3.2.2 (R Foundation for Statistical Computing, Vienna, Austria).
2.3. Sensitivity analyses
Given the large study area, using temperature data from a central monitoring site is a limitation of this study; therefore, we acquired data from 13 cooperative meteorological stations and performed sensitivity analyses to demonstrate how temperatures vary temporally and spatially across our study area. Use of a central monitoring site could also induce differential exposure measurement error across our study area. To investigate possible differences in exposure measurement error between more urban and less urban areas we examined the effect of temperature on respiratory outcomes in urban core and non-urban core areas. Counties were identified as part of the urban core if the population of the county in 2010 was greater than 250,000 and the population density was greater than 1000 people per square mile. Based on these criteria, we identified Clayton, Cobb, Dekalb, Fulton, and Gwinnett counties as urban core counties. The 15 other counties in our study area (Barrow, Bartow, Carroll, Cherokee, Coweta, Douglas, Fayette, Forsyth, Henry, Newton, Paulding, Pickens, Rockdale, Spalding, and Walton) were designated as non-urban core counties. Daily ED visit counts were aggregated into urban core and non-urban core strata.
Additional sensitivity analyses were performed to ensure that patterns of effect modification by race/ethnicity, observed across the entire study period, were not influenced by missingness nor due to a priori categorization of race/ethnicity strata (i.e., white children versus non-white children). In these analyses, we further disaggregated our ED population into White, African American, and Hispanic strata, and analyses were performed for a restricted time-period (4 years) where race/ethnicity was reported for > 97% of all respiratory ED visits.
3. RESULTS
3.1. Descriptive results
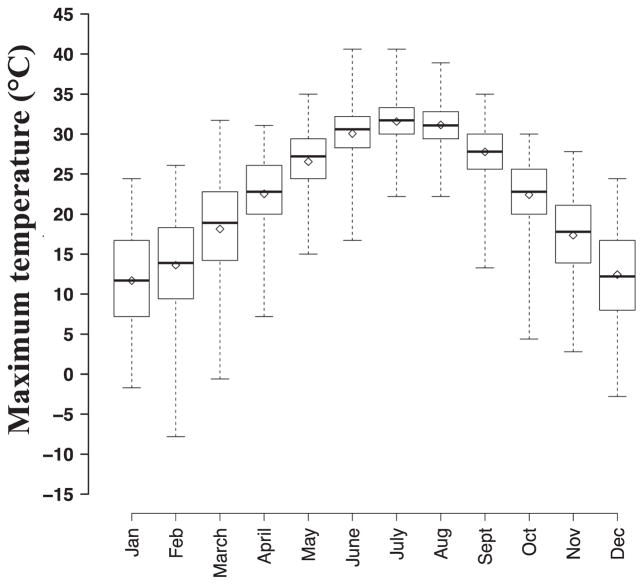
During 1993–2012, year-round daily maximum temperatures from the ASOS at Atlanta Hartsfield International Airport ranged from −7.80 °C to 40.6°C and were highly correlated with maximum dew-point temperature (Spearman’s ϱ of 0.89, Table 1). Monthly average distributions of maximum temperature from 1993 to 2012 indicated that the 5 warmest months in Atlanta were May to September, defined here as the warm-season, with peak temperatures observed in July and August (Figure 1). During the warm-season, maximum temperature values ranged from 13.3 °C to 40.6 °C (Table 1); previous findings in Atlanta reported no apparent trend in daily warm-season maximum temperature from year to year between 1993 and 2012 (Winquist et al., 2016).
Table 1.
Descriptive statistics for relevant meteorological metrics, Atlanta 1993–2012
| Meteorological Metrics | # days* | Mean | SD | Min | p25 | p50 | p75 | Max | Spearman Correlations
|
|
|---|---|---|---|---|---|---|---|---|---|---|
| Tmax | Tdmx | |||||||||
| Max temperature, °C (year-round) | 7,305 | 22.1 | 8.35 | −7.80 | 16.1 | 23.3 | 28.9 | 40.6 | 1.00 | 0.84 |
| Max dew-point temperature, °C (year-round) | 7,305 | 12.8 | 8.47 | −17.2 | 7.20 | 15.0 | 20.0 | 28.0 | 1.00 | |
| Max temperature, °C (warm season) | 3,060 | 29.4 | 3.71 | 13.3 | 27.2 | 30 | 32.0 | 40.6 | 1.00 | 0.47 |
| Max dew-point temperature, °C (warm season) | 3,060 | 19.6 | 3.57 | 2.20 | 17.8 | 20.6 | 22.2 | 28.0 | 1.00 | |
Number of days for which temperature data was available
Abbreviations: °C, degrees Celsius; #, number; max, maximum; min, minimum; mm, millimeters; p25, 25th percentile; p50, 50th percentile; p75, 75th percentile; SD, standard deviation; Tdmax, daily maximum dew-point temperature °C; Tmax, daily maximum temperature °C
Figure 1.
Monthly distribution of maximum temperature values during 1993–2012. Each box represents the distribution of daily maximum temperature values by month across all years. Solid black lines represent 50th percentile values and hollow diamonds represent monthly means. Boxes represent the 25th to 75th percentile values of maximum temperature and whiskers range from minimum to maximum values for each month.
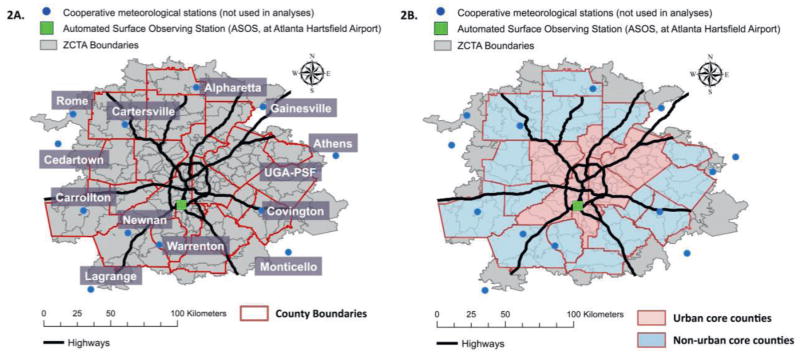
Within our study area, 13 cooperative meteorological stations were identified through the National Weather Service’s Cooperative Observer Program. Figure 2 illustrates the entire study-area, the counties identified as urban core and non-urban core areas, the location of the ASOS (Atlanta meteorological station) at Atlanta Hartsfield International Airport used in epidemiologic analyses, and the location of the 13 cooperative meteorological stations within our study area. We compared temperature data from the ASOS in Atlanta (central monitoring site) to temperature data from the cooperative monitoring stations across our study period (Table 2). On average, daily maximum and daily minimum temperatures were similar at each monitoring location. However, the daily minimum temperature values from the ASOS in Atlanta were higher than the other study locations (Table 2), indicating possible capture of urban heat island effects on minimum temperature by the ASOS in Atlanta. Spearman correlation tests demonstrated that daily maximum temperature values from the central monitoring site used in epidemiological analyses and data from monitors in outlying counties had strong temporal correlations (e.g., Spearman’s ϱ ranging from 0.73 to 0.92). The observation of strong temporal correlations indicates that temperatures rise and fall together across our entire 20-county study area and suggest that we can capture day-to-day variation in ambient temperature for the entire study population by using data from the ASOS central monitor in Atlanta. Daily temperature data from the ASOS central monitor in Atlanta and the Athens monitoring station had the strongest temporal correlations (Spearman’s ϱ= 0.92) and were the only stations with complete daily records of temperature data during our study period (Table 2). The monitoring station in Athens is also an automated surface observing station (ASOS), meaning that data collection methods are most similar between the Atlanta central station and the Athens station.
Figure 2.
Study area maps for 20-County Atlanta. Figure 2A presents the names and locations of the 14 meteorological monitoring stations across 20-county Atlanta. Figure 2B presents the spatial distribution of urban core and non-urban core counties and the cooperative monitoring stations within these areas. Main epidemiological analyses used temperature data collected from the Automated Surface Observing Station (ASOS) at Atlanta Hartsfield International Airport. Temperature data from the cooperative meteorological stations were used in sensitivity analyses. Urban counties (> 250,000 population and 1000 people/square mile): Clayton, Cobb, Dekalb, Fulton and Gwinnett Counties. Non-urban core counties: Barrow, Bartow, Carroll, Cherokee, Coweta, Douglas, Fayette, Forsyth, Henry, Newton, Paulding, Pickens, Rockdale, Spalding, and Walton. Abbreviations: UGA-PSF, Plant Sciences Farm associated with the University of Georgia (UGA), Bogart, GA; ZCTA, ZIP code Tabulation Area.
Table 2.
Descriptive statistics and Spearman correlations for relevant meteorological metrics by monitoring station, warm season (May–Sept.), Atlanta 1993–2012
| Monitoring Stations | # days* | # days missing | Daily Maximum Temperature (Tmax)# | Daily Minimum Temperature (Tmin)@ | Spearman Correlations with ASOS (Atlanta)** | |
|---|---|---|---|---|---|---|
|
| ||||||
| Mean (SD) | Mean (SD) | Tmax | Tmin | |||
| ASOS (Atlanta) | 3,060 | 0 | 29.8 (3.8) | 19.6 (3.4) | 1.00 | 1.00 |
| Alpharetta | 1,341 | 1719 | 28.7 (3.9) | 17.2 (4.0) | 0.78 | 0.87 |
| Athens | 3,060 | 0 | 30.5 (4.0) | 18.5 (3.6) | 0.92 | 0.90 |
| Carrollton | 3,018 | 42 | 29.4 (3.5) | 17.5 (4.2) | 0.81 | 0.80 |
| Cartersville | 2,719 | 341 | 30.2 (3.9) | 17.6 (4.0) | 0.78 | 0.79 |
| Cedartown | 2,970 | 90 | 30.3 (3.8) | 16.9 (4.4) | 0.80 | 0.78 |
| Covington | 2,779 | 281 | 30.3 (3.8) | 17.9 (3.9) | 0.76 | 0.82 |
| Gainesville | 3,038 | 22 | 29.0 (3.9) | 17.8 (3.7) | 0.77 | 0.86 |
| Lagrange | 1,706 | 1354 | 30.6 (3.6) | 18.0 (3.9) | 0.80 | 0.79 |
| Monticello | 3,007 | 53 | 30.6 (3.6) | 17.2 (4.2) | 0.78 | 0.76 |
| Newnan | 2,626 | 434 | 29.8 (3.6) | 17.7 (3.8) | 0.80 | 0.83 |
| Rome | 2,683 | 377 | 29.7 (3.9) | 17.7 (4.1) | 0.73 | 0.81 |
| UGA-PSF | 2,999 | 61 | 30.2 (3.8) | 17.9 (3.7) | 0.77 | 0.87 |
| Warrenton | 2,893 | 167 | 30.7 (3.8) | 17.8 (3.9) | 0.74 | 0.76 |
Number of days for which temperature data were available during the warm season (May–Sept.).
Descriptive statistics for daily maximum and daily minimum temperatures estimated for the warm season (May–Sept.)
Spearman rank correlations between the ASOS (Atlanta) central monitoring site and 13 cooperative meteorological stations.
Abbreviations: #, number; ASOS, Automated Surface Observing Station (located at Atlanta Hartsfield International Airport); UGA-PSF, Plant Sciences Farm associated with the University of Georgia (UGA), Bogart, GA; standard deviation; Tmax, daily maximum temperature °C; Tmin, daily minimum temperature °C
Our warm-season health outcome database included 1,528,145 total ED visits among children aged 5–18 years with 51,360 ED visits for asthma and 161,301 ED visits for respiratory disease during the years 1993–2012 in 20-county Atlanta (191 ZCTAs). A greater number of ED visits were made by children living in urban core counties compared to non-urban core counties, male compared to female children, non-white compared to white children, and children paying for their visit using Medicaid compared to private insurance (Table 2). Nearly all ED visits recorded information on patient sex and insurance status (missing for <1% and 5% of ED visits, respectively); however, race/ethnicity information was missing for a majority of ED records, and was completely missing during 2007–2009 (Supplemental Table S2). However, ED records with information on race/ethnicity were nearly complete (< 3% missingness) for 2005, 2006, 2010, and 2011. ED data from these years were used in sensitivity analyses to evaluate the impact of data missingness on observed effect modification by race/ethnicity.
During our study period, socioeconomic composition of the population varied widely across the 191 Atlanta ZCTAs (e.g., in 2010, % below poverty varied from 1.66% to 45.5%) and large variability was observed consistently during each year of our study period (Supplemental Figures S1a-S1c). Between 1993 and 2012, maximum and mean values of % <12th grade education declined, indicating an increase in educational attainment for Atlanta ZCTAs during our study period (Supplemental Figure S1a). Conversely, mean values of ZCTA % below poverty increased from 1993 to 2012, suggesting, on average, an increase over time in the number of households in Atlanta ZCTAs that were living in poverty (Supplemental Figure S1b). Across single indicators of ZCTA SES, we observed weak-correlations between % <12th grade and % below poverty (Spearman’s ϱ 0.49), suggesting that these indicators describe disparate SES constructs and/or have dissimilar spatial patterning (Supplemental Figure S2). We observed moderate to strong correlations between the NDI and the other SES indicators (Spearman’s ϱ 0.72 to 0.87, Supplemental Table S1), reflecting the use of % < 12th grade and % below poverty in the calculation of the NDI.
3.2. Overall associations
Associations between pediatric respiratory outcomes and maximum temperature for lag days 0 to 6 and 3-, 5-, and 7-day moving averages are reported in Table 4. Significant associations between maximum temperature and pediatric asthma ED visits were observed across lag days 1–5, with the strongest single-day association observed on lag day 2 [RR = 1.059 (95% CI: 1.030, 1.088) for an increase in maximum temperature from 27 °C to 32 °C]. For the moving average exposure periods, associations increased as moving average periods increased. Confidence intervals around effect estimates were also wider when using moving averages due to the reduction in the temporal variability of these exposure metrics. Associations between maximum temperature and pediatric respiratory disease ED visits were weak and non-significant for all lag days and moving averages examined.
Table 4.
Associations between maximum temperature and pediatric respiratory outcomes on lag days 0 to 6 and for 3-, 5-, and 7-day moving averages per increase in maximum temperature from 27 °C to 32 °C
| Tmax | Asthmaa ED | Respiratory Disease b ED | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Lag day | Rate Ratio | 95% CI | Rate Ratio | 95% CI | ||
| 0 | 1.023 | 0.994 | 1.053 | 0.998 | 0.979 | 1.018 |
| 1 | 1.042 | 1.013 | 1.071 | 0.996 | 0.977 | 1.015 |
| 2 | 1.059 | 1.030 | 1.088 | 1.008 | 0.990 | 1.027 |
| 3 | 1.058 | 1.030 | 1.087 | 1.006 | 0.988 | 1.024 |
| 4 | 1.038 | 1.010 | 1.067 | 0.987 | 0.970 | 1.006 |
| 5 | 1.037 | 1.009 | 1.065 | 0.988 | 0.970 | 1.006 |
| 6 | 1.024 | 0.997 | 1.053 | 0.989 | 0.972 | 1.008 |
| 3-day avg. | 1.072 | 1.036 | 1.109 | 1.006 | 0.983 | 1.029 |
| 5-day avg. | 1.123 | 1.079 | 1.170 | 1.008 | 0.982 | 1.036 |
| 7-day avg. | 1.147 | 1.097 | 1.199 | 1.004 | 0.975 | 1.035 |
primary diagnosis of asthma/wheeze (ICD-9 codes 493.0–493.9/786.07)
primary diagnosis of respiratory disease (ICD-9 codes 460–486, 493.0–493.9,786.07)
Abbreviations: °C, degrees Celsius; CI, confidence interval; ED, emergency department; ICD-9, International Classification of Diseases, 9th Revision; RR, rate ratio; Tmax, daily maximum temperature °C
3.3. Effect modification
For all analyses examining effect modification, we focused on results for lag day 2 (Figures 3–6); effect modification results for lag days 0 to 6 and 3-, 5-, and 7-day moving averages are presented in the Supplemental Material. Unless otherwise noted, stratum-specific RRs are scaled to an increase in maximum temperature from 27°C to 32°C.
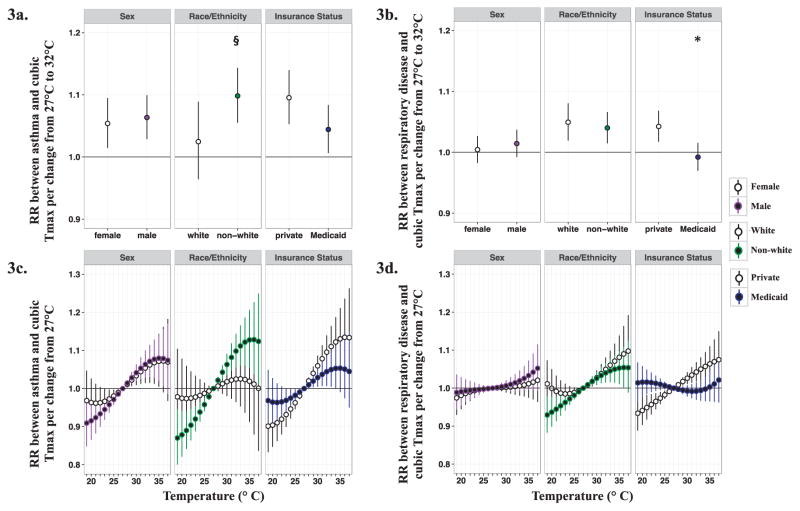
Figure 3.
Estimated RRs and 95% CIs between maximum temperature at lag 2 and pediatric respiratory outcomes stratified by individual factors. Figure 3a represents associations between maximum temperature (Tmax) at lag 2 and pediatric asthma emergency department (ED) visits across individual factors for a change from 27°C to 32°C. Figure 3c shows the non-linear effects of Tmax at lag 2 on pediatric asthma ED visits across individual factors by graphing associations for a given temperature relative to 27°C. Figure 3b and 3d represent associations between Tmax at lag 2 and pediatric respiratory disease ED visits across individual factors for a given change in Tmax relative to 27°C. * indicates significant statistical difference (two-sided P < 0.05) from referent group (i.e. female sex, white race, or private insurance) and § indicates P = 0.06 for test of significant difference from referent group.
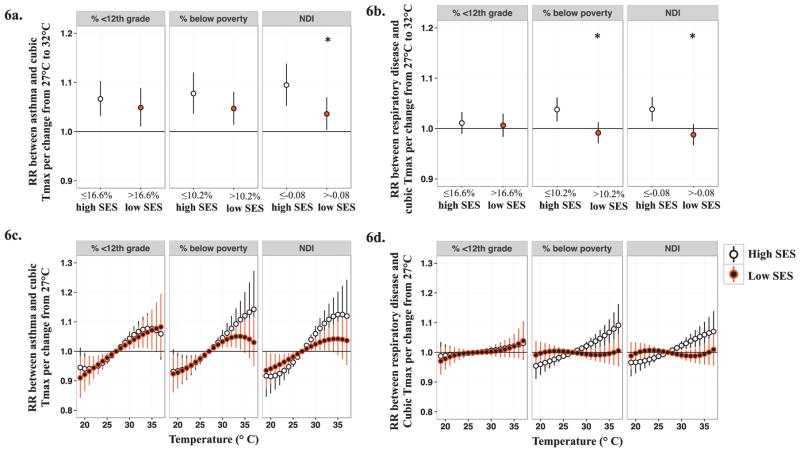
Figure 6.
Associations and 95% CIs between maximum temperature at lag 2 and pediatric respiratory outcomes stratified by median values of area-level socioeconomic status. Figure 6a represents associations between maximum temperature (Tmax) at lag 2 and pediatric asthma emergency department (ED) visits across SES strata for a change from 27°C to 32°C. Figure 6c shows the non-linear effects of Tmax at lag 2 on pediatric asthma ED visits across SES strata by graphing associations for a given temperature relative to 27°C. Figure 6b and 6d represent associations between Tmax at lag 2 and pediatric respiratory disease ED visits across SES strata for a given change in temperature relative to 27°C. * indicates significant statistical difference (two-sided P < 0.05) from referent group (‘high SES’)
3.3.1. Effect modification: Individual factors
Analyses stratified by sex (male verses female) suggested somewhat stronger associations between maximum temperature and asthma ED visits among males compared to females for lag days 1 to 4 and for 3-, 5-, and 7-day moving averages [e.g. lag 2 RR among males = 1.064 (95% CI: 1.029, 1.100); lag 2 RR among females = 1.054 (95% CI: 1.015,1.095)] (Figure 3a, Supplemental Figures S3a and S4a); however, differences in RRs between strata were not significant at the 0.05 level. Associations between maximum temperature and pediatric respiratory disease ED visits did not differ by patient sex, and associations among both males and females were non-significant for all lag days and moving averages (Figure 3b, Supplemental Figures S3b and S4b).
In analyses stratified by race/ethnicity (white verses non-white), associations between maximum temperature and asthma ED visits were somewhat stronger among non-white children compared to white children for lag days 1 to 3 and for 3-, 5-, and 7-day moving averages (Supplemental Figure S3a, S4a). While differences in RRs between white and non-white strata were small and not statistically significant at the 0.05 level, there was suggestive evidence that non-white children are at a greater risk of temperature-related asthma exacerbation (i.e. lag day 2 test for heterogeneity, P=0.06, Figure 3a). We did not find evidence that race/ethnicity modified the association between maximum temperature and respiratory disease ED visits. Note that significant associations between temperature and respiratory disease ED visits were observed within each race stratum for lag days 0–3 and for all moving averages (Supplemental Figures S3b and S4b); these observations were different from the weak overall associations we observed between maximum temperature and pediatric respiratory disease ED visits (Table 4) and may be due to the subset of ED visits with race data.
Additional sensitivity analyses examined associations between daily maximum temperature and respiratory outcomes among White, African American, and Hispanic children during years when nearly all ED data recorded information on patient race/ethnicity (< 3% missingness in 2005< 3% missingness in 2006< 3% missingness in 2010, and 2011). Results from these sensitivity analyses for lag day 2 are presented in Figure 4. For single-day lags 1–5 and for all moving average periods, we observed stronger associations between maximum temperature and asthma ED visits among African American children compared to White children (e.g. lag day 2 RR for African Americans = 1.103 (95% CI: 1.030, 1.180); lag day 2 RR for Whites = 0.965 (95% CI: 0.866, 1.076), test for heterogeneity, P < 0.05). For respiratory disease outcomes, rate ratios were similar across strata (Figure 4). Results from sensitivity analyses suggest potential effect modification by race/ethnicity and demonstrate that the stronger associations among non-white children (observed in main analyses) appear to be largely driven by associations among African Americans. Hispanic children may also be a vulnerable population (rate ratios were positive), however, the small number of ED visits by Hispanic children resulted in wide confidence intervals around rate ratios (Figure 4). Patterns of effect modification during the restricted years were similar to patterns across the entire study period suggesting that despite substantial missingness, findings from our main analyses (i.e. the potential for greater vulnerability among non-white children) may be representative for 20-county Atlanta.
Figure 4.
Estimated RRs and 95% CIs between maximum temperature at lag 2 and pediatric respiratory outcomes stratified by race/ethnicity. Figure 4 represents associations between maximum temperature (Tmax) at lag 2 and pediatric respiratory outcomes (asthma and respiratory disease emergency department visits) by White, Hispanic, and African American race/ethnicity. RRs and 95% CIs are scaled to a change in Tmax from 27°C to 32°C. * indicates significant statistical difference (two-sided P < 0.05) from referent group (i.e. white race).
Results from analyses stratified by insurance status (private insurance verses Medicaid insurance) showed consistently stronger associations between maximum temperature and respiratory outcomes in children whose ED visit was paid by private insurance compared to those who used Medicaid insurance [e.g. for asthma, lag day 2 RR by private insurance = 1.095 (95% CI: 1.053, 1.140); lag day 2 RRs by Medicaid = 1.044 (95% CI: 1.007, 1.084)] (Figures 3a–3b). This pattern was observed consistently across all lag days and all moving average periods (Supplemental Figures S3 and S4); significant differences between strata were detected, suggesting insurance status, or other conditions represented by insurance status, modify the effect of maximum temperature on asthma and respiratory disease among children.
To visualize the non-linear effects of maximum temperature and assess differences in the shape of maximum temperature effects across individual factors, we also estimated RRs and 95% CIs for several temperature changes from a reference maximum temperature of 27 °C for lags days 0–6. Maximum temperature changes of −8 °C to +10 °C relative to 27 °C were chosen to capture values between the 1st and 99th percentiles of the maximum temperature distribution during our study period and represent a temperature range of 19 °C to 37 °C. Through this assessment we observed differences in the shape of the effect of maximum temperature on asthma and respiratory disease, and these graphs illustrate distinct, stratum-specific relationships with maximum temperature, particularly when stratifying by race/ethnicity and insurance status (Figures 3c–3d; Supplemental Figure S5).
3.3.2. Effect modification: Area-level SES
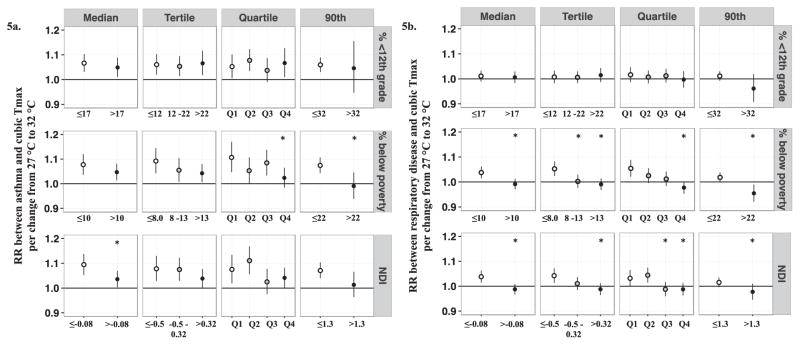
Area-level SES was examined for its potential to modify associations between maximum temperature and pediatric respiratory outcomes. In general, observed patterns of effect modification (determined by differences between strata per change in maximum temperature from 27°C to 32°C) were consistent across stratification criteria (median, tertile, quartile, or 90th percentiles values of continuous area-level SES), indicating that models stratified by median SES values sufficiently captured patterns of effect modification observed across other stratification approaches (lag day 2 results presented in Figure 5; results for all lag days /moving averages presented in Supplemental Figures S6–S11).
Figure 5.
Associations and 95% CIs between maximum temperature at lag 2 and pediatric respiratory outcomes stratified by categories of area-level socioeconomic status. Figure 5a represents associations between maximum temperature (Tmax) at lag 2 and pediatric asthma emergency department (ED) visits across SES strata for a change from 27°C to 32°C. Figure 5b represents associations between maximum temperature (Tmax) at lag 2 and pediatric respiratory disease ED visits across SES strata for a change from 27°C to 32°C. * indicates significant statistical difference (two-sided P < 0.05) from referent group (i.e. the highest SES strata). Quartiles values: % <12th grade quartiles: Q1: < 10.1; Q2: ≥ 10.1 - < 16.6; Q3: ≥ 16.6 - <25; Q4: > 25; % below poverty quartiles: Q1: < 6.59; Q2: ≥ 6.59 - < 10.2; Q3: ≥ 10.2 - <14.9; Q4: > 14.9; NDI quartiles: Q1: < -0.76; Q2: ≥ -0.76 - < -0.08; Q3: ≥ -0.08- <0.55; Q4: > 0.55.
However, effect modification was inconsistently observed depending on the choice of SES indicator. For example, when examining effect modification by gradations of % < 12th grade as an indicator of area-level SES, magnitudes of associations between maximum temperature and pediatric respiratory outcomes (asthma and respiratory disease ED visits) were very similar across all strata, demonstrating no modification by % <12th grade (Figures 5 and 6; Supplemental Figures S6 and S7). Conversely, when area-level SES was characterized by % below poverty or the NDI, we observed weaker associations between maximum temperature and respiratory outcomes among children living in low SES areas compared to children living in areas of higher SES (Figures 5 and 6; Supplemental Figures S8–S11). This pattern was observed for both asthma and respiratory disease ED visits and across several lag days/moving averages (Supplemental Figures S8–S11). In sensitivity analyses, we examined the impact of assigning NDI values from the Census 2000 to 1993–1999 data by performing analyses for a restricted time period, 2000–2012. Estimated RRs for the full time period (1993–2012) and the restricted time period (2000–2012) were very similar (less than 10% different, results not shown), indicating that the use of 2000 Census data for earlier years of the study period did not heavily impact the estimated average associations or interpretation of results.
In models stratified by median values of area-level SES, we graphed the non-linear effects of maximum temperature by plotting RRs and 95% CIs per maximum temperature changes of −8°C to +10°C relative to 27°C for each strata (maximum temperature range of 19°C to 37°C). The shape of the maximum temperature-response function differed between high and low SES strata when area-level SES was defined by % below poverty or the NDI, but not when defined by % 12th grade. In general, differences in RRs between strata increased as maximum temperatures increased compared to the 27 °C reference for lag day 2 (Figure 6).
3.3.3. Sensitivity Analyses: Urban core versus non-urban core areas
Given our large, 20-county study area, using temperature data from a central monitoring site is a limitation of this study because ambient temperatures may vary between more urban and less urban areas due to urban heat island effects. To explore potential exposure measurement error between more urban and less urban areas, we estimated associations between temperature and pediatric respiratory outcomes in 5 urban core (i.e. Clayton, Dekalb, Fulton, Gwinnett, Cobb) and 15 non-urban core counties. Figure 2 presents the urban core and non-urban core counties on a map of our entire study area. Supplemental Figure S12 presents associations between temperature and respiratory outcomes by urban core and non-urban core areas for lag days 0–6 and 3-, 5-, and 7-day moving averages. During earlier lag periods (lag days 0–2 and 3-day moving averages) we observed stronger associations among non-urban core areas; these differences were not statistically significant (Figure S12). As single day lags and moving average periods increased, there were no discernable differences in RRs between temperature and respiratory outcomes in urban core and non-urban core areas (Figure S12), suggesting no real differences in exposure measurement error between central and more outlying populations.
4. DISCUSSION
In this 20-year time-series study, we evaluated the short-term effects of maximum temperature on respiratory ED visits among children in Atlanta, and assessed the degree to which individual or area-level factors act as effect modifiers. Few studies have examined vulnerability to temperature-related respiratory outcomes among children and our findings add to the small, yet growing body of literature on climate-related health effects among sensitive subpopulations.
In overall analyses, we found significant associations between maximum temperature and pediatric asthma ED visits across several lag days and 3-, 5-, and 7-day moving average periods. Conversely, we observed weak, non-significant associations between maximum temperature and pediatric respiratory disease ED visits. Observed differences in effect across these health outcomes may be due in part to greater specificity of the asthma/wheeze health outcome compared to general respiratory disease, which included upper respiratory infections, bronchiolitis, pneumonia, chronic obstructive pulmonary disease, asthma, and wheeze. Previous studies have also reported larger magnitudes of associations between temperature and asthma/wheeze compared to other respiratory outcomes (Anderson et al., 2013; Li et al., 2014a; Winquist et al., 2016). Studies exploring the mechanistic causes of temperature-related respiratory morbidity suggest that inhalation of hot air activates airway sensory nerves, the cholinergic reflex pathway, and transient bronchoconstriction (Hayes et al., 2012; Khosravi et al., 2014); therefore, it is possible that asthma/wheeze and related cough disorders may have a more direct relationship with high temperature compared to other respiratory illnesses such as upper and lower respiratory infections.
We found a lagged effect of high ambient temperature on asthma, with the strongest associations observed on single day lags 2 and 3. Previous studies have also reported lagged effects for temperature-related respiratory morbidity (Bunker et al., 2016; Cheng et al., 2014; Li et al., 2014a; Li et al., 2014b; Winquist et al., 2016; Xu et al., 2013; Ye et al., 2012); plausible reasons for a lagged effect include delayed onset of respiratory symptoms, failed attempts at personal management of respiratory symptoms, reduced access to health care for some of our ED population, and/or the concurrence of high maximum temperatures during the day with relatively high ambient minimum temperatures at night, (i.e. a narrow diurnal temperature range), preventing adequate physiological thermoregulation and recovery from high daytime temperatures (Cheng et al., 2014; Hanna et al., 2015). While estimates from single day lags likely correlate with one another, there also appears to be a robust cumulative effect of high temperature on asthma as evidenced by the increasing strength of association for 3-, 5- and 7-day moving average periods. These findings indicate that children are at risk for increased asthma morbidity when temperatures are high, on average, for several days.
4.1. Effect modification by individual-level factors
A primary objective of this study was to identify susceptibility and vulnerability factors among children in relation to temperature and respiratory health. Results from all effect modification analyses should be interpreted with care, as differences among strata–even statistically significant differences–were relatively small (i.e. changes in association were generally observed at the hundredth decimal place). In stratified analyses, we examined potential effect modification by sex, race/ethnicity, and insurance status (a proxy for individual-level socioeconomic status). When assessing modification of temperature-related asthma by sex, we observed significant RRs among males for lag days 1–4, while RRs were typically weaker and non-significant among females on the same lag days; however, differences in RRs between males and females were not statistically significant. Previous studies focusing on temperature-related pediatric respiratory health have also reported slightly stronger effects between temperature and respiratory symptoms among male children compared to female children (Li et al., 2014a; Xu et al., 2013). Male children may be more susceptible to respiratory morbidity due to sex differences in airway maturation and function (young males tend to have greater airway resistance), a greater propensity for atopy, differences in immunological function, and time-activity patterns that increase their exposure to environmental triggers (Becklake et al., 1999; Clougherty, 2010; Sheffield et al., 2015). While physiological differences and gendered behaviors may confer vulnerability among young males, distinguishing sex effects among children is challenging due to changes in lung development and pubertal maturation that occur with age and differ by sex (Clougherty, 2010; Sheffield et al., 2015). For example, young males have more physiological disadvantages than young females, but these disadvantages are less apparent by adolescence and by adulthood women may have greater physiological susceptibility (Becklake et al., 1999; Clougherty, 2010). Given the analytical difficulties of discerning a sex effect among children when using broad age categories, as used here, additional studies examining modification by sex are needed.
When we stratified models based on patient race/ethnicity (i.e. white or non-white race/ethnicity in main analyses; White, African American, and Hispanic race/ethnicity in sensitivity analyses), we found suggestive evidence that race/ethnicity modifies associations between temperature and asthma ED visits, with stronger RRs observed among non-white compared to white children. Similar findings have been observed in other temperature-health studies (Gronlund, 2014; Lin et al., 2009; O'Neill, 2003; Schwartz, 2005; Uejio et al., 2011) and previous research in Atlanta reported similar findings concerning modification by race/ethnicity of air pollution related-health effects (Alhanti et al., 2016). Although the underlying etiology of effect modification by race/ethnicity is unclear, potential racial/ethnic differences in exposure and potential racial/ethnic differences in adherence to medication and/or asthma control may be contributing factors to the observed vulnerability to temperature-related asthma morbidity in this study (Alhanti et al., 2016; Crocker et al., 2009; Law et al., 2011; Roy et al., 2010).
To examine effect modification by individual-level SES, we used the patient’s insurance status as a proxy for household SES. In stratified analyses, we observed statistically stronger associations between temperature and respiratory outcomes (asthma and respiratory disease ED visits) among children whose ED visits were paid through private insurance (high SES) compared to children whose ED visits were paid through Medicaid (low SES), indicating evidence of effect modification by insurance status or other conditions indicated by insurance status. Because Medicaid eligibility in Georgia is based on family size and income limits at or below the Federal Poverty Line (Georgia Department of Community Health, 2016), we assumed that individual-level health insurance status is a meaningful indicator of low household socioeconomic status for children, and we assumed it would have been directly related to a child’s vulnerability through reduced access to health care, preventative medications, or through risk factors associated with low SES. However, our findings did not support these assumptions, and the reasons for observing weaker effects among children on Medicaid insurance compared to other insurance types are unclear.
Interestingly, similar findings were reported by Grineski et al. in a comprehensive study examining effect modification of nitrogen dioxide (NO2)-related asthma exacerbation by insurance status and race (Grineski et al., 2010). In that study, Grineski et al. disaggregated insurance status into three categories: private insurance, Medicaid, and no insurance and reported significantly lower relative risks to NO2-related asthma among children on Medicaid compared to children on private insurance (Grineski et al., 2010), and significantly greater relative risks to NO2-related asthma among uninsured children compared to those using Medicaid or private insurance. In light of the findings by Grineski et al., our results may have been more interpretable had we been able to disaggregate our ED data further, exploring associations among the uninsured population.
In our patient population, Medicaid insurance status was associated with non-white race/ethnicity; therefore, we also expected results from these stratified analyses to be similar. However, the race/ethnicity effect seemed to be in conflict with that of the insurance status effect [i.e. associations between high temperature and asthma ED visits were stronger among non-white children compared to white children, while associations between high temperature and asthma ED visits were stronger among children on private insurance (high SES children), compared to children on Medicaid insurance (low SES children)]. In sensitivity analyses we examined whether this disagreement was due to missingness among the race/ethnicity data by restricting our asthma ED data to that which included information on both race/ethnicity and insurance status. Results from sensitivity analyses were similar to those from our main analyses and did not suggest missingness was a cause of the disagreement between the race/ethnicity and the insurance status effect (Supplemental Table S3).
Although our study cannot directly address this discrepancy, the racial composition of our ED data may be partially responsible for the observed patterns of effect modification: non-white children accounted for approximately 84% of asthma ED visits paid by Medicaid insurance and approximately 60% of asthma ED visits paid by private insurance (Supplemental Table S4). Grineski et al. also examined the combined modifying effect of race and insurance status on NO2-related asthma exacerbation and reported no racial disparities in the effect of NO2 on asthma among children on Medicaid, but did find racial disparities in the effect of NO2 on asthma among children on private insurance. Their findings may be due to differences by race in asthma control among children with private insurance, and/or due to the possibility that despite being privately insured, non-white children may have more barriers to adequate health care or inferior coverage plans compared to white children on private insurance. It is also possible that among insured children, race may be associated with additional factors that lead to increased exposures to ambient heat or to increased vulnerability to heat effects. If racial disparities in exposure-response relationships are not apparent among children on Medicaid insurance, but are apparent among children on private insurance, as suggested by Grineski et. al., then having a majority of non-white children among our private insurance subgroup could have driven the stronger associations between temperature and asthma ED visits observed within this strata compared to the Medicaid strata. Note, that we were only able to assess racial composition across insurance status categories for ED visits with information on race/ethnicity and insurance status (about 50% of asthma ED visits).
4.2. Effect modification by area-level SES
We also explored the influence of socioeconomic composition at the area-level by characterizing ZCTA of patient residence by gradations of neighborhood % <12th grade education, % below poverty, and the NDI. Although the NDI is highly correlated with % <12th grade and % below poverty (reflecting their contribution to the index), the NDI conceptualizes area-level SES as multidimensional, potentially providing a more complete picture than can be gained from any single-indicator of area-level SES. However, the NDI (and other composite indices) do not reveal which of the constituent variables or processes matter to temperature-health associations. Thus, by comparing results from individual indicators (i.e. % <12th grade and % below poverty) alongside results from a composite index, we may gain a better understanding of area-level social composition and the relevant social processes driving temperature-health associations in our study area.
Using these complementary indicators, there was no evidence that associations varied across area-level SES strata based on gradations of % <12th grade. However, when using % below poverty and the NDI to indicate neighborhood SES, we observed weaker associations among children living in low SES compared to high SES areas, regardless of the stratification approach used (e.g. median, tertile, quartile, and 90th percentiles). These results echoed our findings of effect modification by insurance status, an indicator of individual-level SES. With regard to data source, indicators of individual SES and area-level SES were completely independent in these analyses and yet the same patterns of effect modification were observed. If being insured through Medicaid is a good proxy for individual low SES and if the ZCTA-level is a suitable scale to assess the social influences of one’s neighborhood then the similarities in effect modification (i.e. weaker associations among low SES populations) for both individual and area-level SES effects lend strength to our findings, especially given that observed modification was not in the expected direction (i.e. we would have expected to observe stronger associations in low SES groups). We did not have sufficient power to stratify on both individual and neighborhood SES, and as such it is unclear whether neighborhood SES, individual SES, or a joint effect of both were drivers of the observed modification.
Although weaker associations in low SES populations run counter to our belief that children from disadvantaged households or ZCTAs would be more vulnerable to the respiratory effects of temperature, similar patterns of effect modification (or no evidence of effect modification) have been observed in air pollution-health studies (Burra et al., 2009; Delfino et al., 2009; Laurent et al., 2009; Laurent et al., 2008; Norris et al., 1999; O'Lenick et al., 2016; O’Lenick et al., In Revision; Sacks et al., 2014; Sarnat et al., 2013; Wilhelm et al., 2009; Winquist et al., 2012; Yang et al., 2003). Plausible reasons for observing weaker associations among low SES populations compared to high SES populations include 1) possible misclassification error of individual or area-level SES depending on how well ZIP code areas reflect relevant social environments, and/or how well we were able to measure and classify household or area-level SES with our choice of SES indicators; 2) the possibility that single measures of SES are poor proxies for nuanced home or area-level socioeconomic environments; 3) the use of multiplicative models to assess environmental determinants of health outcomes that tend to have differing baseline risks across socioeconomic subpopulations (i.e. asthma); and 4) children from wealthier households or living in wealthier neighborhoods may have few component causes of respiratory morbidity; therefore, temperature would have a substantial relative influence (i.e. a large piece of the ‘causal pie’) (Rothman, 1976). Whereas, children from low SES households or living in low SES neighborhoods may have many different exposures that could exacerbate respiratory disease; in this context, high ambient temperatures may only be one of many contributing factors, thus exerting little relative influence on respiratory morbidity (i.e. a small piece of the ‘causal pie’). While these myriad considerations may limit the interpretability of our results on SES effect modification, acknowledging them can be useful for informing future research and understanding inconsistent findings already reported in the literature.
4.3. Limitations
Our ability to examine modification by individual and neighborhood SES factors was facilitated by the long 20-year study period, and rich, patient-level data. However, there are additional considerations to acknowledge when interpreting results. First, daily maximum temperature data were only available at the city-level and may have induced exposure misclassification error. We expect this error to be minimal and non-differential across our study population as our time-series analysis relates daily changes in maximum temperature to health outcomes and results from our sensitivity analyses suggest that the central monitoring station can capture day-to-day variation in ambient temperature for the entire study area. While more spatially resolved temperature estimates would have enabled us to better approximate absolute temperature levels within neighborhoods, which may vary with urban heat island (UHI) effects, researchers studying UHI conditions specifically in Atlanta have observed the smallest UHI magnitudes during the summer months and have demonstrated that temperature differences between urban and non-urban areas are primarily driven by higher minimum temperatures in urban areas (Zhou et al., 2009). Thus, findings from Zhou and Shepherd suggest that UHI effects may be less of a concern in this analysis because we focused on temperature effects during the warm-season and because we used daily maximum temperature as our exposure metric, which does not appear to be as sensitive to UHI effects as minimum temperatures in Atlanta. Second, our models did not examine temperature exposure metrics that took into account the effect of relative humidity on respiratory outcomes nor did we control for air pollution; these analytical decisions were informed by findings from our previous assessment of heat-related respiratory morbidity in Atlanta (Winquist et al., 2016). In Winquist et al., we demonstrated that rate ratios from models using maximum apparent temperature as the exposure metric, which takes into account relative humidity, were within 5% of rate ratios from models using maximum temperature. Similarly, Winquist et al. also demonstrated that rate ratios from models controlling for air pollution (i.e. ozone, nitrogen dioxide, coarse particulate matter, and sulfur dioxide) were within 10% of rate ratios from models that did not control for air pollution, with effect estimates for maximum temperature typically larger when models controlled for pollutants. Previous studies by others have also shown that control for air pollution made a nominal impact on temperature effect estimates (Anderson et al., 2013; Cheng et al., 2014; Li et al., 2014a; Lin et al., 2009). Although air pollution is associated with respiratory outcomes among children, air pollutants are not considered confounders of the effect of temperature in our analysis (Buckley et al., 2012; Buckley et al., 2014). By not including control for ozone or other pollutants, our effect estimates reflect the total effect of maximum temperature on respiratory health, including that which may be mediated by air pollution. Third, 50% of our ED visit data were missing information on race/ethnicity which may have severely limited the interpretability and generalizability of the observed pattern of effect modification by race. Fourth, by assessing area-level SES effects at the ZCTA level, we assumed that ZCTA boundaries were a suitable scale to capture the social influences of one’s local area. Fifth, SES variables were not available for every year of our study period and in some cases we had to impute SES data from later time periods; these imputed data would not be able to capture important shifts in socioeconomic composition that may have occurred during the study period. Finally, although we had large numbers of daily ED visits during our 20-year study period, we had limited power to assess individual and neighborhood factors in models together.
5. CONCLUSION
As warm season temperatures rise due to climate change, populations living within large, sprawling metropolitan areas, like Atlanta, will become exceedingly exposed and vulnerable to high temperatures. Our results demonstrate that short-term exposures to maximum temperature significantly increase pediatric asthma ED visits in Atlanta, and observed lagged (delayed) effects suggest that health impacts can be observed for several days after exposure to high temperatures. When temperatures are high for several days, health effects appear cumulative, resulting in a possibly substantial health burden among Atlanta’s pediatric population. We also identified race/ethnicity and insurance status as potential vulnerability factors at the individual-level. At the area-level, results suggest that area-level SES (specifically poverty-related SES) is a factor contributing short-term vulnerability to temperature-related pediatric asthma in Atlanta. However, it is unclear whether neighborhood effects were influenced by individual-level SES effects (indicated by insurance status) or were independent of them. While, some of our results were counterintuitive (i.e. weaker associations among children with Medicaid insurance and among children living in low SES areas), our findings on vulnerability factors contribute new insights to the growing knowledge base on climate-related health effects and can be used to help tailor climate change adaptation and public health interventions strategies.
Supplementary Material
Table 3.
Summary of Emergency Department Visit Data and Visits Stratified by Modifying Factors, Warm Season, Atlanta, GA, 1993–2012
|
|
Asthma/wheezea
|
Respiratory diseaseb
|
All ED visits
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Stratification Criteria | SES category | # Days | Total # ED visits | Mean # ED visits/day | Total # ED visits | Mean # ED visits/day | Total # ED visits | Mean # ED visits/day | |
|
|
|
|
|
||||||
| Overall | N/A | - | 3,060 | 51,360 | 16.8 | 161,301 | 52.7 | 1,528,145 | 499 |
| Urbanization | Urban corec | - | 3,060 | 43,197 | 14.1 | 122,215 | 39.9 | 1,113,091 | 364 |
| Non-urban core | - | 3,060 | 8,163 | 2.67 | 39,086 | 12.8 | 415,054 | 136 | |
| Sex | Female | - | 3,060 | 20,000 | 6.54 | 78,057 | 25.5 | 726,290 | 237 |
| Male | - | 3,060 | 30,929 | 10.1 | 81,601 | 26.7 | 785,842 | 257 | |
| White | - | 2,601d | 6,552 | 2.52 | 33,125 | 12.7 | 445,333 | 171 | |
| African American | - | 2,601d | 17,061 | 6.56 | 48,304 | 18.6 | 367,600 | 141 | |
| Race /Ethnicity | Hispanic | - | 2,601d | 1,740 | 0.67 | 6,467 | 2.49 | 60,997 | 23.5 |
| Other | - | 2,601d | 970 | 0.37 | 3,595 | 1.38 | 37,759 | 14.5 | |
| Non-whitee (Total) | - | 2,601 d | 19,771 | 7.60 | 58,366 | 22.4 | 466,356 | 179 | |
| Insurance status | Private | High SES | 3,060 | 18,489 | 6.04 | 54,767 | 17.9 | 658,815 | 215 |
| Medicaidf | Low SES | 3,060 | 23,536 | 9.94 | 75,049 | 32.3 | 564,737 | 260 | |
| % < 12th grade * | ≤16.6% | High SES | 3,060 | 30,258 | 9.89 | 91,620 | 29.9 | 923,210 | 302 |
| <16.6% | Low SES | 3,060 | 21,102 | 6.90 | 69,681 | 22.8 | 604,935 | 198 | |
| % below poverty* | ≤10.2% | High SES | 3,060 | 18,150 | 5.93 | 59,631 | 19.5 | 684,636 | 224 |
| <10.2% | Low SES | 3,060 | 33,210 | 10.9 | 101,670 | 33.2 | 843,509 | 276 | |
| NDI* | ≤ −0.08 | High SES | 3,060 | 19,955 | 6.52 | 66,050 | 21.6 | 751,075 | 245 |
| < −0.08 | Low SES | 3,060 | 31,405 | 10.3 | 95,251 | 31.1 | 777,070 | 254 | |
primary diagnosis of asthma/wheeze (ICD-9 codes 493.0–493.9/786.07)
primary diagnosis of respiratory disease (ICD-9 codes 460–486, 493.0–493.9,786.07)
A county was considered part of the urban core if the county’s 2010 population was > 250,000 and its population density was >1000 people/square mile
Race/ethnicity data completely missing during 2007–2009
Non-white strata combined ED visit data from African American, Hispanic, and Other race/ethnicity categories
Medicaid category included ED visits paid for by Medicaid insurance
Categorized by median values of continuous ZCTA-level SES
Abbreviations: % <12th grade, percentage of the adult population (≥25 years old) with less than a 12th grade education; % below poverty, percentage of households living below the Federal Poverty Line; #, number; ED, emergency department; ICD-9, International Classification of Diseases, 9th Revision; NDI, Neighborhood Deprivation Index; SES, socioeconomic status
Highlights.
Warm-season maximum temperature was associated with asthma ED visits among children
Observed lagged and cumulative effects of maximum temperature on asthma ED visits
Effect modification by individual and area-level factors was examined
Acknowledgments
Funding
This research was supported by the National Institute of Environmental Health Sciences (NIEHS) of the National Institutes of Health (NIH) under Award # R21ES023763. This research was also made possible by grants to Emory University from the US Environmental Protection Agency (USEPA; R82921301, RD834799), NIEHS (R01ES11294), and the Electric Power Research Institute (EP-P27723/C13172 and EP-P4353/C2124). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the USEPA. Further, USEPA does not endorse the purchase of any commercial products or services mentioned in this manuscript. This publication is based in part upon information obtained from hospitals and the Georgia Hospital Association; we are grateful for the support of all participating hospitals.
Human Subjects
Ethics approval for human subjects research was obtained by the Emory University Institutional Review Board (IRB: IRB00046509). The Emory University Institutional Review Board also granted exemption from informed consent requirements given the minimal risk nature of the study and the infeasibility of obtaining informed consent from individual patients for the large number of ED visit records examined in this study.
The authors would like to acknowledge the contributions of members of the Southeastern Center for Air Pollution and Epidemiology (SCAPE) research group for their thoughtful feedback on data analysis approaches and results interpretation.
List of abbreviations
- ACS
American Community Survey
- CI
confidence interval
- ED
emergency department
- ICD-9
International Classification of Diseases, 9th Revision
- NDI
Neighborhood Deprivation Index
- NO2
nitrogen dioxide
- RR
rate ratio
- SES
socioeconomic status
- ZCTA
ZIP Code Tabulation Area
Footnotes
DECLARATIONS
Competing financial interests: None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alhanti BA, Chang HH, Winquist A, Mulholland JA, et al. Ambient air pollution and emergency department visits for asthma: A multi-city assessment of effect modification by age. J Expo Sci Environ Epidemiol. 2016;26:180–188. doi: 10.1038/jes.2015.57. [DOI] [PubMed] [Google Scholar]
- Anderson GB, Dominici F, Wang Y, McCormack MC, et al. Heat-related emergency hospitalizations for respiratory diseases in the medicare population. Am J Respir Crit Care Med. 2013;187:1098–1103. doi: 10.1164/rccm.201211-1969OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett AG, Tong S, Clements ACA. What measure of temperature is the best predictor of mortality? Environ Res. 2010;110:604–611. doi: 10.1016/j.envres.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Basu R. Relation between elevated ambient temperature and mortality: A review of the epidemiologic evidence. Epidemiol Rev. 2002;24:190–202. doi: 10.1093/epirev/mxf007. [DOI] [PubMed] [Google Scholar]
- Basu R. High ambient temperature and mortality: A review of epidemiologic studies from 2001 to 2008. Environ Health. 2009;8:40. doi: 10.1186/1476-069X-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu R, Malig B. High ambient temperature and mortality in california: Exploring the roles of age, disease, and mortality displacement. Environ Res. 2011;111:1286–1292. doi: 10.1016/j.envres.2011.09.006. [DOI] [PubMed] [Google Scholar]
- Bateson TF, Schwartz J. Children's response to air pollutants. J Toxicol Environ Health A. 2007;71:238–243. doi: 10.1080/15287390701598234. [DOI] [PubMed] [Google Scholar]
- Becklake MR, KauVmann F. Gender diverences in airway behaviour over the human life span. Thorax. 1999;54:1119–1138. doi: 10.1136/thx.54.12.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benmarhnia T, Deguen Sv, Kaufman JS, Smargiassi A. Vulnerability to heat-related mortality: A systematic review, meta-analysis, and meta-regression analysis. Epidemiology. 2015;26:781–793. doi: 10.1097/EDE.0000000000000375. [DOI] [PubMed] [Google Scholar]
- Braga AsLF, Zanobetti A, Schwartz J. The effect of weather on respiratory and cardiovascular deaths in 12 u. S Cities Environ Health Perspect. 2002;110:859–863. doi: 10.1289/ehp.02110859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley JP, Richardson DB. Seasonal modification of the association between temperature and adult emergency department visits for asthma: A case-crossover study. Environ Health. 2012:11. doi: 10.1186/1476-069X-11-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley JP, Samet JM, Richardson DB. Commentary: Does air pollution confound studies of temperature? Epidemiology. 2014;25:242–245. doi: 10.1097/EDE.0000000000000051. [DOI] [PubMed] [Google Scholar]
- Bunker A, Wildenhain J, Vandenbergh A, Henschke N, et al. Effects of air temperature on climate-sensitive mortality and morbidity outcomes in the elderly; a systematic review and meta-analysis of epidemiological evidence. EBioMedicine. 2016 doi: 10.1016/j.ebiom.2016.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burra TA, Moineddin R, Agha MM, Glazier RH. Social disadvantage, air pollution, and asthma physician visits in toronto, canada. Environ Res. 2009;109:567–574. doi: 10.1016/j.envres.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Carreras H, Zanobetti A, Koutrakis P. Effect of daily temperature range on respiratory health in argentina and its modification by impaired socio-economic conditions and pm10 exposures. Environ Pollut. 2015;206:175–182. doi: 10.1016/j.envpol.2015.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Xu Z, Zhu R, Wang X, et al. Impact of diurnal temperature range on human health: A systematic review. Int J Biometeorol. 2014;58:2011–2024. doi: 10.1007/s00484-014-0797-5. [DOI] [PubMed] [Google Scholar]
- Clougherty JE. A growing role for gender analysis in air pollution epidemiology. Environ Health Perspect. 2010;118:167–176. doi: 10.1289/ehp.0900994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon K, Monaghan A, Hayden M, Wilhelmi O. Potential impacts of future warming and land use changes on intra-urban heat exposure in houston, texas. PLoS One. 2016;11:e0148890. doi: 10.1371/journal.pone.0148890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker D, Brown C, Moolenaar R, Moorman J, et al. Racial and ethnic disparities in asthma medication usage and health-care utilization: Data from the national asthma survey. Chest. 2009;136:1063–1071. doi: 10.1378/chest.09-0013. [DOI] [PubMed] [Google Scholar]
- Davis RE, Hondula DM, Patel AP. Temperature observation time and type influence estimates of heat-related mortality in seven u.S Cities. Environ Health Perspect. 2016;124:795–804. doi: 10.1289/ehp.1509946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfino RJ, Chang J, Wu J, Ren C, et al. Repeated hospital encounters for asthma in children and exposure to traffic-related air pollution near the home. Annals of Allergy, Asthma & Immunology. 2009;102:138–144. doi: 10.1016/S1081-1206(10)60244-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebi KL, Exuzides KA, Lau E, Kelsh M, et al. Weather changes associated with hospitalizations for cardiovascular diseases and stroke in california, 1983–1998. Int J Biometeorol. 2004;49:48–58. doi: 10.1007/s00484-004-0207-5. [DOI] [PubMed] [Google Scholar]
- Georgia Department of Community Health. Medicaid eligibility criteria chart. 2016 Sep 12 [ https://dch.georgia.gov/eligibility-criteria-chart]
- Green RS, Basu R, Malig B, Broadwin R, et al. The effect of temperature on hospital admissions in nine california counties. Int J Public Health. 2010;55:113–121. doi: 10.1007/s00038-009-0076-0. [DOI] [PubMed] [Google Scholar]
- Grineski SE, Staniswalis JG, Peng YL, Atkinson-Palombo C. Children's asthma hospitalizations and relative risk due to nitrogen dioxide: Effect modification by race, ethnicity, and insurance status. Environ Res. 2010;110:178–188. doi: 10.1016/j.envres.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronlund CJ. Racial and socioeconomic disparities in heat-related health effects and their mechanisms: A review. Curr Epidemiol Rep. 2014;1:165–173. doi: 10.1007/s40471-014-0014-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna EG, Tait PW. Limitations to thermoregulation and acclimatization challenge human adaptation to global warming. Int J Environ Res Public Health. 2015;12:8034–8074. doi: 10.3390/ijerph120708034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes D, Jr, Collins PB, Khosravi M, Lin RL, et al. Bronchoconstriction triggered by breathing hot humid air in patients with asthma: Role of cholinergic reflex. Am J Respir Crit Care Med. 2012;185:1190–1196. doi: 10.1164/rccm.201201-0088OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hondula DM, Barnett AG. Heat-related morbidity in brisbane, australia: Spatial variation and area-level predictors. Environ Health Perspect. 2014;122:831–836. doi: 10.1289/ehp.1307496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman JS, MacLehose RF. Which of these things is not like the others? Cancer. 2013;119:4216–4222. doi: 10.1002/cncr.28359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosravi M, Collins PB, Lin RL, Hayes D, Jr, et al. Breathing hot humid air induces airway irritation and cough in patients with allergic rhinitis. Respir Physiol Neurobiol. 2014;198:13–19. doi: 10.1016/j.resp.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovats RS, Hajat S, Wilkinson P. Contrasting patterns of mortality and hospital admissions during hot weather and heat waves in greater london, uk. Occup Environ Med. 2004;61:893–898. doi: 10.1136/oem.2003.012047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laaidi K, Zeghnoun A, Dousset B, Bretin P, et al. The impact of heat islands on mortality in paris during the august 2003 heat wave. Environ Health Perspect. 2012;120:254–259. doi: 10.1289/ehp.1103532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent O, Pedrono G, Filleul L, Segala C, et al. Influence of socioeconomic deprivation on the relation between air pollution and beta-agonist sales for asthma. Chest. 2009;135:717–723. doi: 10.1378/chest.08-1604. [DOI] [PubMed] [Google Scholar]
- Laurent O, Pedrono G, Segala C, Filleul L, et al. Air pollution, asthma attacks, and socioeconomic deprivation: A small-area case-crossover study. Am J Epidemiol. 2008;168:58–65. doi: 10.1093/aje/kwn087. [DOI] [PubMed] [Google Scholar]
- Law HZ, Oraka E, Mannino DM. The role of income in reducing racial and ethnic disparities in emergency room and urgent care center visits for asthma-united states, 2001–2009. J Asthma. 2011;48:405–413. doi: 10.3109/02770903.2011.565849. [DOI] [PubMed] [Google Scholar]
- Leon LR, Helwig BG. Heat stroke: Role of the systemic inflammatory response. J Appl Physiol. 2010;109:1980–1988. doi: 10.1152/japplphysiol.00301.2010. [DOI] [PubMed] [Google Scholar]
- Li S, Baker PJ, Jalaludin BB, Guo Y, et al. Are childrens asthmatic symptoms related to ambient temperature? A panel study in australia. Environ Res. 2014a;133:239–245. doi: 10.1016/j.envres.2014.05.032. [DOI] [PubMed] [Google Scholar]
- Li S, Baker PJ, Jalaludin BB, Marks GB, et al. Ambient temperature and lung function in children with asthma in australia. Eur Respir J. 2014b;43:1059–1066. doi: 10.1183/09031936.00079313. [DOI] [PubMed] [Google Scholar]
- Lin S, Luo M, Walker RJ, Liu X, et al. Extreme high temperatures and hospital admissions for respiratory and cardiovascular diseases. Epidemiology. 2009;20:738–746. doi: 10.1097/EDE.0b013e3181ad5522. [DOI] [PubMed] [Google Scholar]
- Linares C, Diaz J. Impact of high temperatures on hospital admissions: Comparative analysis with previous studies about mortality (madrid) Eur J Public Health. 2008;18:317–322. doi: 10.1093/eurpub/ckm108. [DOI] [PubMed] [Google Scholar]
- Luber G, McGeehin M. Climate change and extreme heat events. Am J Prev Med. 2008;35:429–435. doi: 10.1016/j.amepre.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Makri A, Stilianakis NI. Vulnerability to air pollution health effects. Int J Hyg Environ Health. 2008;211:326–336. doi: 10.1016/j.ijheh.2007.06.005. [DOI] [PubMed] [Google Scholar]
- McMichael AJ, Lindgren E. Climate change: Present and future risks to health, and necessary responses. J Intern Med. 2011;270:401–413. doi: 10.1111/j.1365-2796.2011.02415.x. [DOI] [PubMed] [Google Scholar]
- Meehl GA, Tebaldi C. More intense, more frequent, and longer lasting heat waves in the 21st century. Science. 2004:305. doi: 10.1126/science.1098704. [DOI] [PubMed] [Google Scholar]
- Messer LC, Laraia BA, Kaufman JS, Eyster J, et al. The development of a standardized neighborhood deprivation index. J Urban Health. 2006;83:1041–1062. doi: 10.1007/s11524-006-9094-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelozzi P, Accetta G, De Sario M, D'Ippoliti D, et al. High temperature and hospitalizations for cardiovascular and respiratory causes in 12 european cities. Am J Respir Crit Care Med. 2009;179:383–389. doi: 10.1164/rccm.200802-217OC. [DOI] [PubMed] [Google Scholar]
- National Oceanic and Atmospheric Administration. National centers for environmental information: Cooperative observer network (coop) 2016 Dec 23; [ https://www.ncdc.noaa.gov/data-access/land-based-station-data/land-based-datasets/cooperative-observer-network-coop]
- Norris G, YoungPong SN, Koenig JQ, Larson TV, et al. An association between fine particles and asthma emergency department visits for children in seattle. Environ Health Perspect. 1999;107:489–493. doi: 10.1289/ehp.99107489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Lenick CR, Winquist A, Mulholland JA, Friberg MD, et al. Assessment of neighborhood socioeconomic status as a modifier of air pollution-asthma associations among children in atlanta. J Epidemiol Community Health. 2016 doi: 10.1136/jech-2015-206530. Online first: 15 July 2016; 0:1–8. doi: 10.1136/jech-2015-206530. [DOI] [PubMed] [Google Scholar]
- O'Neill MS. Modifiers of the temperature and mortality association in seven us cities. American Journal of Epidemiology. 2003;157:1074–1082. doi: 10.1093/aje/kwg096. [DOI] [PubMed] [Google Scholar]
- O’Lenick CR, Chang HH, Kramer MR, Winquist A, et al. Ozone and childhood respiratory disease in three us cities: Evaluation of effect measure modification by neighborhood socioeconomic status using a bayesian hierarchal approach. Environ Health. doi: 10.1186/s12940-017-0244-2. In Revision. Under Revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman KJ. Causes. Am J Epidemiol. 1976;104:587–592. doi: 10.1093/oxfordjournals.aje.a112335. [DOI] [PubMed] [Google Scholar]
- Roy A, Wisnivesky JP. Racial and ethnic differences in the use of environmental control practices among children with asthma. J Asthma. 2010;47:507–512. doi: 10.3109/02770901003734314. [DOI] [PubMed] [Google Scholar]
- Sacks JD, Rappold AG, Davis JA, Jr, Richardson DB, et al. Influence of urbanicity and county characteristics on the association between ozone and asthma emergency department visits in north carolina. Environ Health Perspect. 2014;122:506–512. doi: 10.1289/ehp.1306940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S, Brock JW, Vaidyanathan A, Easterling DR, et al. Spatial variation in hyperthermia emergency department visits among those with employer-based insurance in the united states - a case-crossover analysis. Environ Health. 2015;14:20. doi: 10.1186/s12940-015-0005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnat SE, Sarnat JA, Mulholland J, Isakov V, et al. Application of alternative spatiotemporal metrics of ambient air pollution exposure in a time-series epidemiological study in atlanta. J Expo Sci Environ Epidemiol. 2013;23:593–605. doi: 10.1038/jes.2013.41. [DOI] [PubMed] [Google Scholar]
- Schwartz J. Who is sensitive to extremes of temperature?: A case-only analysis. Epidemiology. 2005;16:67–72. doi: 10.1097/01.ede.0000147114.25957.71. [DOI] [PubMed] [Google Scholar]
- Selgrade MK, Plopper CG, Gilmour MI, Conolly RB, et al. Assessing the health effects and risks associated with children's inhalation exposures--asthma and allergy. J Toxicol Environ Health A. 2008;71:196–207. doi: 10.1080/15287390701597897. [DOI] [PubMed] [Google Scholar]
- Sharma G, Goodwin J. Effect of aging on respiratory system physiology and immunology. Clin Interv Aging. 2006;1:253–260. doi: 10.2147/ciia.2006.1.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield PE, Zhou J, Shmool JL, Clougherty JE. Ambient ozone exposure and children's acute asthma in new york city: A case-crossover analysis. Environ Health. 2015;14:25. doi: 10.1186/s12940-015-0010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone B, Hess JJ, Frumkin H. Urban form and extreme heat events: Are sprawling cities more vulnerable to climate change than compact cities? Environ Health Perspect. 2010;118:1425–1428. doi: 10.1289/ehp.0901879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert P, Klein M, Metzger K, Peel J, et al. Interim results of the study of particulates and health in atlanta (sophia) J Expo Anal Environ Epidemiol. 2000;10:446–460. doi: 10.1038/sj.jea.7500106. [DOI] [PubMed] [Google Scholar]
- Turner LR, Barnett AG, Connell D, Tong S. Ambient temperature and cardiorespiratory morbidity: A systematic review and meta-analysis. Epidemiology. 2012;23:594–606. doi: 10.1097/EDE.0b013e3182572795. [DOI] [PubMed] [Google Scholar]
- Uejio CK, Wilhelmi OV, Golden JS, Mills DM, et al. Intra-urban societal vulnerability to extreme heat: The role of heat exposure and the built environment, socioeconomics, and neighborhood stability. Health Place. 2011;17:498–507. doi: 10.1016/j.healthplace.2010.12.005. [DOI] [PubMed] [Google Scholar]
- United States Census Bureau. Metropolitan areas components. 1999 Dec 5; https://www.census.gov/population/metro/files/lists/historical/99mfips.txt. 2016.
- White MD. Components and mechanisms of thermal hyperpnea. J Appl Physiol. 2006;101:655–663. doi: 10.1152/japplphysiol.00210.2006. [DOI] [PubMed] [Google Scholar]
- Wilhelm M, Qian L, Ritz B. Outdoor air pollution, family and neighborhood environment, and asthma in la fans children. Health Place. 2009;15:25–36. doi: 10.1016/j.healthplace.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winquist A, Grundstein A, Chang HH, Hess J, et al. Warm season temperatures and emergency department visits in atlanta, georgia. Environ Res. 2016;147:314–323. doi: 10.1016/j.envres.2016.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winquist A, Klein M, Tolbert P, Flanders WD, et al. Comparison of emergency department and hospital admissions data for air pollution time-series studies. Environ Health. 2012;11:70. doi: 10.1186/1476-069X-11-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Huang C, Su H, Turner LR, et al. Diurnal temperature range and childhood asthma: A time-series study. Environ Health. 2013;12:2–5. doi: 10.1186/1476-069X-12-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Chen Y, Shi Y, Burnett RT, et al. Association between ozone and respiratory admissions among children and the elderly in vancouver, canada. Inhal Toxicol. 2003;15:1297–1308. doi: 10.1080/08958370390241768. [DOI] [PubMed] [Google Scholar]
- Ye F, Piver WT, Ando M, Portier CJ. Effects of temperature and air pollutants on cardiovascular and respiratory diseases for males and females older than 65 years of age in tokyo, july and august 1980–1995. Environ Health Perspect. 2001;109:355–359. doi: 10.1289/ehp.01109355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Wolff R, Yu W, Vaneckova P, et al. Ambient temperature and morbidity: A review of epidemiological evidence. Environ Health Perspect. 2012;120:19–28. doi: 10.1289/ehp.1003198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Shepherd JM. Atlanta’s urban heat island under extreme heat conditions and potential mitigation strategies. Nat Hazards. 2009;52:639–668. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.