Abstract
Background
Some of the country's highest rates of morbidity and mortality from cardiovascular disease are found in lower‐income black communities in the rural Southeast. Research suggests these disparities originate in the early decades of life, and partly reflect the influence of broader socioeconomic forces acting on behavioral and biological processes that accelerate cardiovascular disease progression. However, this hypothesis has not been tested explicitly. Here, we examine metabolic syndrome (MetS) in rural black young adults as a function of their family's economic conditions before and after the Great Recession.
Methods and Results
In an ongoing prospective study, we followed 328 black youth from rural Georgia, who were 16 to 17 years old when the Great Recession began. When youth were 25, we assessed MetS prevalence using the International Diabetes Federation's guidelines. The sample's overall MetS prevalence was 18.6%, but rates varied depending on family economic trajectory from before to after the Great Recession. MetS prevalence was lowest (10.4%) among youth whose families maintained stable low‐income conditions across the Recession. It was intermediate (21.8%) among downwardly mobile youth (ie, those whose families were lower income before the Recession, but slipped into poverty). The highest MetS rates (27.5%) were among youth whose families began the Recession in poverty, and sank into more meager conditions afterwards. The same patterns were observed with 3 alternative MetS definitions.
Conclusions
These patterns suggest that broader economic forces shape cardiometabolic risk in young blacks, and may exacerbate disparities already present in this community.
Keywords: metabolic syndrome, race and ethnicity, socioeconomic position
Subject Categories: Race and Ethnicity, Pediatrics, Secondary Prevention
Clinical Perspective
What Is New?
Black teenagers whose families experienced a financial downturn following the Great Recession showed higher rates of metabolic syndrome 5 years later.
These results suggest that larger macroeconomic conditions have implications for the cardiometabolic health of young people making the transition to adulthood.
What Are the Clinical Implications?
Youth who experience financial downturns and other major stressors may benefit from secondary prevention efforts focused around lifestyle modification.
Introduction
Morbidity and mortality from cardiovascular disease (CVD) have declined markedly during the past half‐century in the United States.1 However, the strength of these trends varies substantially across demographic groups, with the largest improvements seen in educated, affluent Americans in metropolitan areas.2, 3 As a result, inequalities in CVD have actually widened over the same period, as have gaps in overall longevity.4, 5 Some of the highest rates of morbidity and mortality from CVD are found in lower‐income black communities in the rural Southeast of the country.6, 7 Developmental precursors of these inequities, such as raised blood pressure and higher circadian variability, are evident by adolescence,8, 9 suggesting that disparities originate in the earlier decades of life,10, 11 long before CVD manifests clinically.
From late 2007 to mid 2009, the United States experienced the largest economic contraction since the Great Depression. Rural black communities in the Southeast, already in financially precarious situations, were among the hardest hit, and many have yet to recover the lost jobs, social services, and wealth.12 During previous economic downturns, rates of stroke and myocardial infarction spiked among older adults, particularly those who lost jobs in recessionary labor markets.13, 14 On the basis of these patterns, we predicted the economic turmoil generated by the Great Recession would exacerbate young people's CVD risks, widening the disparities outlined above. We tested this hypothesis in a longitudinal study that has followed black youth in rural Georgia from the ages of 11 to 25.
The Recession began when these youths were 16 to 17 years old. Over the next few years, 60% of their families experienced a significant financial downturn. One group of families, who began the Recession in poverty, sunk into even more meager conditions. Another group, mostly lower‐income before the downturn, dropped into poverty. (In 2016, the federal government defined poverty as household income below $24 600 for a family of 4. The term “lower income” typically refers to families whose income is 1.00–1.99 times that value.) About 40% of the sample was unaffected by the Recession; their families maintained conditions best described as stably lower income. We predicted that a decade later, when youth were 25, their CVD risks would vary according to their family's economic trajectory across the Recession. To evaluate this hypothesis, we assessed rates of metabolic syndrome (MetS), a complex of interrelated risk factors that has become increasingly common in young Americans,15 and whose presence forecasts higher risks of diabetes mellitus, myocardial infarction, and stroke later in the life course. We expected the highest rates of MetS among those subjects whose trajectories were characterized by deepening poverty, followed by those who experienced downward mobility, and finally subjects in stable lower‐income conditions.
Methods
Sample and Design
Data are from the SHAPE (Strong African American Families Healthy Adult Panel) study.16 Starting in 2001, SHAPE enrolled 667 black children in fifth grade (mean age=11.2 years; range 11–13) and their primary caregiver. The families resided in 9 rural counties of Georgia, where poverty rates are among the highest in the nation. In 2009–2010, when youth had reached ages 19 to 20, a subgroup of 500 was randomly selected for a substudy of stress hormones and blood pressure. In 2015–2016, when subjects had a mean age of 25, we re‐assessed all members of this health subcohort who were available and consented to participate, resulting in MetS data on 391 participants. In the current article, the analytic sample consists of 328 of those individuals. (The outstanding 63 subjects were missing economic hardship data in 2007 and/or 2010, so we excluded them from the analysis. These subjects were similar to the health subcohort on all 3 MetS outcomes, Ps from 0.09 to 0.30). Compared with the original cohort, the analytic sample had a higher percentage of female (59.5% versus 52.8%) and single‐parent families (60.9% versus 56.3%), but were otherwise similar demographically. The University of Georgia's Institutional Review Board approved the protocol, and written consent was obtained from subjects and/or their caregivers at all assessments.
Economic Hardship Trajectories
To characterize the Recession's impact on families, we developed a composite hardship indicator based on caregiver self‐reports obtained in 2007 and 2010.17 At each time point, families were assigned 1 point for each of the following indicators of hardship: (1) income below the federal poverty threshold (ie, income‐to‐needs ratio <1.00); (2) receipt of Temporary Assistance for Needy Families; and (3) the primary caregiver was unemployed. Because hardship is not simply an objective condition,18 the composite also included 3 subjective indicators of the family's economic circumstances, as reported by the caregiver. These indicators were adequacy of income, unmet material needs, and inability to make ends meet. Adequacy of income was measured on a 5‐point scale with anchors of 1 (much less than adequate to meet our needs), 3 (adequate to meet our needs), and 5 (much more than adequate to meet our needs). Scores below 3 were assigned 1 point towards the hardship composite. For unmet material needs, caregivers completed a 4‐item scale,18 indicating agreement with statements like “My family has enough money to afford the kind of home we need.” Cronbach αs were 0.89 in 2007 and 0.85 in 2010. For inability to make ends meet, caregivers completed a 2‐item scale18 about their difficulties paying bills during the past 12 months. Cronbach αs were 0.89 in 2007 and 0.70 in 2010. For both of these scales, scores above the sample mean were assigned 1 point towards the hardship composite.17
Scores on the economic hardship composite ranged from 0 to 6 at each time point. In 2007, before the Recession, the sample mean was 1.64 (SD=1.49). In 2010, it climbed to 2.66 (SD=1.75). At both times, the median composite score was 3.0, which we used as a cutoff to define groups with lower versus higher degrees of hardship. As detailed in Results, this approach led to the formation of 3 groups of subjects, whose economic trajectories across the Recession were characterized as Stable Low Income (low hardship in both 2007 and 2010), Downward Mobility (low hardship in 2007 followed by high hardship in 2010), and Deepening Poverty (high hardship in 2007, followed by even greater hardship in 2010). We should note that in an earlier article, we examined these groups' health in 2010, when subjects were age 19, with a focus on cellular aging.17 Because the sample composition changed somewhat in the ensuing 6 years, we updated 2 of the group's names to better reflect the economic trajectories of their members. Specifically, the group earlier referred to as Stable Low Hardship is now Stable Low Income, and the group earlier referred to as Stable High Hardship is now Deepening Poverty.
Metabolic Syndrome Assessment
At the age 25 assessments, a phlebotomist visited each participant's home in the morning hours to draw an overnight fasting blood sample. Blood was drawn into Serum Separator Tubes (Becton‐Dickinson). Specimens were centrifuged on site, and the serum was harvested, divided into aliquots, and frozen immediately on dry ice. At the end of the study, glucose was measured photometrically on a Roche/Hitachi cobas c502 analyzer. The average intra‐ and interassay coefficients of variation were 0.7% and 1.8%, respectively. High‐density lipoproteins and triglycerides were measured on a Roche/Hitachi cobas c701 analyzer. The average intra‐ and interassay coefficients of variation for these assays were below 1.6% and 2.4%, respectively. Resting blood pressure was monitored with a Critikon Dinamap Pro 100 (Critikon) while the youth sat reading quietly. Three readings were taken every 2 minutes, and the average of the last 2 readings was used as the resting index. The field researcher measured waist circumference twice at the midpoint of the upper iliac crest and lower costal margin, at the midaxillary line. If readings differed by 1 cm, they were repeated, and the closest 2 values were averaged.
MetS was diagnosed according to the International Diabetes Federation (IDF) guidelines.19 These criteria specify that in adults, a MetS diagnosis requires central adiposity, which for the black subjects in this sample is defined as waist circumference ≥94 cm for males and ≥80 cm for females. At least 2 of 4 additional components must also be present. They include (1) signs of early hypertension (systolic pressure ≥130 or diastolic pressure ≥85), (2) elevated triglycerides (>150 mg/dL), (3) raised fasting glucose (≥100 mg/dL), or (4) lowered high‐density lipoprotein levels (<40 mg/dL in men and <50 mg/dL in women).
There are multiple approaches to defining MetS. Some question the validity of IDF's clinical thresholds, and/or whether a categorical diagnostic scheme is appropriate.19, 20, 21 To address these concerns, we considered 2 alternate definitions of MetS here. One was a count variable reflecting the number of MetS components for which a participant met IDF criteria; values could range from 0 to 5. The other definition was a continuously distributed composite formed using weighted scores derived from a factor analysis of MetS components. The factors weights were as follows: waist circumference (0.63), fasting glucose (0.46), triglycerides (0.42), high‐density lipoproteins (0.46), and systolic blood pressure (0.78).
Covariates
The SHAPE cohort was initially recruited for a randomized controlled trial of a family‐oriented intervention to prevent behavior problems and substance abuse.16 Participation in the intervention was not associated with any of the MetS outcomes (P=0.24 for diagnosis; P=0.94 for counts; P=0.21 for composite). However, to minimize any residual confounding, we included a dichotomous covariate reflecting intervention arm (treatment versus control) in all models. Because of established sex differences in MetS prevalence,15 we also included a dichotomous covariate reflecting male/female status. Finally, preliminary analyses of the economic trajectory groups revealed differences in caregiver age (P=0.045), so this variable was included as a covariate in all models. Neither age nor racial/ethnic group was included as a covariate, because all of the participants self‐identified as black, and were the same age within 1 year.
Statistical Analysis
In the first wave of analyses, we compared the economic trajectory groups using univariate ANCOVA. All models included the covariates intervention arm, sex, and parental age, and assumptions of normality and homogeneity of variance were confirmed. When an omnibus comparison was statistically significant, we followed up with planned contrasts to identify which groups differed from each other. In the next wave of analyses, we conducted orthogonal polynomial contrasts testing for linearity; these analyses asked whether there was evidence for a dose–response association between economic hardship and MetS outcomes. All statistical tests were 2‐tailed with α set to 0.05.
Results
Economic Hardship Trajectories
According to our composite, 40.9% (n=134) of the sample experienced relatively little hardship over the study period of 2007–2010. These families' economic conditions were mostly unaffected by the Recession; based on their stable, modest, incomes (Table 1), we refer to them as “Stable Low Income.” By contrast, roughly a third of the families (30.8%, n=101) experienced a significant downturn between 2007 and 2010, moving from the lower‐to‐higher hardship category. As Table 1 shows, before the Recession, these were typically lower‐income, working families, fairly similar to the Stable Low Income group on both objective and subjective indicators. But after the Recession, their average income dropped below the poverty threshold, rates of unemployment and government assistance climbed markedly, and subjective experience of hardship grew. We characterize their experience as “Downward Mobility.” Another group of families (24.4%, n=80) experienced consistently higher amounts of hardship according to scores on the composite, but as Table 1 shows, their already precarious financial conditions worsened markedly from 2007 to 2010, a change we characterize as “Deepening Poverty.” A small group of families (n=13, 4.0%) experienced upward mobility, going from higher to lower hardship categories between 2007 and 2010, but the small size of this group precluded statistical analyses, so we did not consider them further.
Table 1.
Sample Characteristics by Family Economic Trajectory
| Characteristic | Whole Sample (N=328) | Stable Low Income (n=134) | Downward Mobility (n=101) | Deepening Poverty (n=80) |
|---|---|---|---|---|
| % or M (SD) | % or M (SD) | % or M (SD) | % or M (SD) | |
| Subject age in 2007 (y) | 16.60 (0.51) | 16.55 (0.51) | 16.61 (0.51) | 16.65 (0.51) |
| Subject sex (female) | 59.5% | 59.0% | 59.4% | 58.7% |
| Parent age in 2007 (y) | 43.38 (7.61) | 42.14 (6.35) | 43.84 (8.56) | 44.30 (7.73) |
| Parent education (<high school) | 22.3% | 14.2% | 19.0% | 41.2% |
| Parent education (high school or GED) | 30.6% | 22.4% | 39.0% | 36.2% |
| Parent education (≥college graduate) | 6.7% | 11.2% | 3.0% | 3.8% |
| Income‐to‐needs ratio in 2007 | 1.73 (2.98) | 2.23 (3.05) | 1.96 (3.88) | 0.79 (0.73) |
| Income‐to‐needs ratio in 2010 | 1.15 (1.10) | 1.78 (1.28) | 0.77 (0.71) | 0.51 (0.50) |
| Receipt of TANF in 2007 | 18 (5.5%) | 4 (3.0%) | 2 (2.0%) | 10 (12.5%) |
| Receipt of TANF in 2010 | 91 (27.7%) | 16 (11.9%) | 36 (35.6%) | 35 (43.8%) |
| Unemployment in 2007 | 80 (24.4%) | 12 (9.0%) | 20 (20.0%) | 40 (50.0%) |
| Unemployment in 2010 | 110 (33.5%) | 10 (7.5%) | 48 (47.5%) | 50 (62.5%) |
| Adequacy of income in 2007 (1–5) | 3.01 (1.20) | 3.58 (1.04) | 3.18 (1.04) | 1.91 (0.85) |
| Adequacy of income in 2010 (1–5) | 2.18 (0.98) | 2.78 (0.77) | 1.74 (0.76) | 1.66 (0.98) |
| Unmet material needs in 2007 (4–16) | 9.16 (3.01) | 7.68 (2.32) | 8.74 (2.68) | 11.76 (2.44) |
| Unmet material needs in 2010 (4–16) | 10.46 (2.84) | 8.60 (2.14) | 11.27 (2.42) | 12.73 (2.17) |
| Cannot make ends meet in 2007 (2–10) | 5.14 (2.25) | 4.01 (1.78) | 4.78 (1.68) | 7.44 (1.89) |
| Cannot make ends meet in 2010 (2–10) | 6.31 (2.11) | 4.93 (1.58) | 7.18 (1.89) | 7.70 (1.75) |
Whole sample column includes values for 13 families who experienced upward mobility, but were not analyzed further. TANF indicates Temporary Assistance for Needy Families, a federal program that provides financial assistance for families with dependent children.
MetS Outcomes
Table 2 presents MetS outcomes according to economic trajectory. At the most recent assessment, when subjects were 25, the sample prevalence of MetS was 18.6%. Rates followed a linear trend according to hardship trajectory (Figure 1). The crude rates were 27.5% for the Deepening Poverty group, 21.8% for the Downward Mobility group, and 10.4% for the Stable Low‐Income group. This linear trend was significant in covariate‐adjusted models (contrast estimate=0.117, SE=0.038, P=0.003). Planned comparisons revealed that MetS prevalence was higher in the Deepening Poverty and Downward Mobility groups versus the Stable Low‐Income group (Ps=0.003 and 0.037 for respective contrasts). However, subjects in the Deepening Poverty and Downward Mobility groups did not differ (P=0.305).
Table 2.
Metabolic Syndrome Outcomes and Components at 25 Years of Age by Family Economic Trajectory
| Outcome | Whole Sample (N=328) | Stable Low Income (n=134) | Downward Mobility (n=101) | Deepening Poverty (n=80) |
|---|---|---|---|---|
| n (%) or M (SE) | n (%) or M (SE) | n (%) or M (SE) | n (%) or M (SE) | |
| Meets MetS diagnosis by IDF criteria | 61 (18.6%) | 14 (10.4%)a | 22 (21.8%)b | 22 (27.5%)b |
| Count, no. of signs meeting IDF criteria | 1.58 (0.06) | 1.28 (0.09)a | 1.65 (0.11)b | 1.91 (0.12)b |
| Central adiposity | 225 (68.6%) | 81 (60.4%)a | 75 (74.3%)b | 57 (71.3%) |
| Raised fasting glucose | 49 (14.9%) | 11 (8.2%)a | 19 (18.8%)b | 16 (20.0%)b |
| Raised triglycerides | 25 (7.6%) | 9 (6.7%) | 6 (5.9%) | 8 (10.0%) |
| Low high‐density lipoproteins | 131 (39.9%) | 44 (32.8%)a | 44 (43.6%)b | 39 (48.8%)b |
| High blood pressure | 88 (26.8%) | 28 (20.9%)a | 22 (21.8%)a | 32 (40.0%)b |
| Composite, weighted component score | 0.02 (0.06) | −0.21 (0.08)a | −0.01 (0.10)a | 0.40 (0.11)b |
| Waist circumference, cm | 99.74 (1.11) | 94.97 (1.72)a | 101.55 (1.98)b | 105.09 (2.22)b |
| Fasting glucose, mg/dL | 92.03 (1.18) | 90.15 (1.44) | 90.94 (1.67) | 94.25 (1.87) |
| Triglyceride levels, mg/dL | 87.83 (2.66) | 82.88 (4.10) | 87.51 (4.70) | 93.73 (5.27) |
| High‐density lipoproteins, mg/dL | 51.12 (0.77) | 53.15 (1.20)a | 48.44 (1.38)b | 50.88 (1.55) |
| Systolic blood pressure, mm Hg | 115.74 (0.78) | 113.83 (1.08)a | 114.48 (1.24)a | 120.11 (1.39)b |
| Diastolic blood pressure, mm Hg | 75.71 (0.61) | 74.26 (0.94)a | 74.17 (1.08)a | 79.75 (1.22)b |
Whole sample column includes values for 13 families who experienced upward mobility, but were not analyzed further. Continuous outcomes are adjusted for sex, intervention, and parent age. Cells with different superscripts are significantly different from each other (P<0.05). IDF indicates International Diabetes Foundation; MetS, metabolic syndrome.
Figure 1.
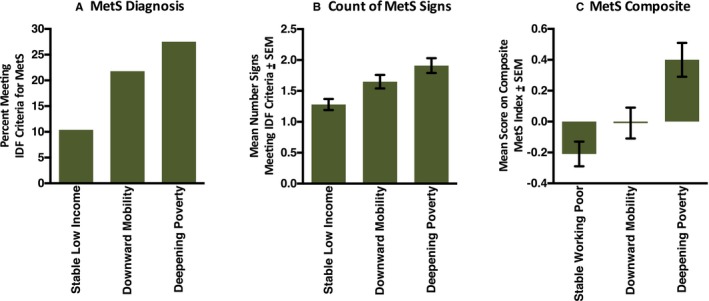
Metabolic syndrome (MetS) by economic hardship. Using prospectively collected data, subjects' economic trajectories across the years of the Great Recession (2007–2010) were classified as Stable Low Income, Downward Mobility, or Deepening Poverty. In 2015, when subjects were 25 years of age, components of the MetS were assessed. For each group, the figure shows (A) rates of MetS by International Diabetes Federation (IDF) criteria, (B) a count reflecting the number of MetS components that met IDF clinical thresholds, and (C) a composite formed using weighted scores derived from a factor analysis of MetS components.
As Figure 1 shows, the same linear pattern was observed with 2 alternate definitions of MetS (Table 3). One definition was based on the number of MetS signs for which a subject met IDF criteria (second row of Table 3; adjusted contrast estimate=0.443, SE=0.109, P<0.0001). Planned comparisons of this outcome revealed higher MetS counts in the Deepening Poverty and Downward Mobility versus Stable Low‐Income group (Ps<0.0001 and 0.011 for respective contrasts). Count scores were higher in the Deepening poverty versus Downward mobility group, but not significantly (P=0.114).
Table 3.
Results of Linear Polynomial Contrasts for MetS Outcomes at 25 Years of Age by Family Economic Trajectory
| Outcome | Linear Polynomial Contrasts | ||
|---|---|---|---|
| Estimate | SE | P Value | |
| Meets MetS diagnosis by IDF criteria | 0.117 | 0.038 | 0.003 |
| Count, no. of signs meeting IDF threshold | 0.443 | 0.109 | <0.0001 |
| Central adiposity | 0.079 | 0.044 | 0.071 |
| Raised fasting‐glucose | 0.081 | 0.035 | 0.022 |
| Raised triglycerides | 0.021 | 0.026 | 0.434 |
| Low high‐density lipoprotein | 0.125 | 0.047 | 0.008 |
| High blood pressure | 0.137 | 0.044 | 0.002 |
| Composite, weighted factor score | 0.431 | 0.097 | <0.001 |
| Waist circumference, cm | 2.817 | 0.785 | <0.001 |
| Fasting glucose, mg/dL | 2.895 | 1.685 | 0.087 |
| Triglyceride levels, mg/dL | 7.673 | 4.739 | 0.106 |
| High‐density lipoproteins, mg/dL | −1.605 | 1.390 | 0.249 |
| Systolic blood pressure, mm Hg | 4.438 | 1.254 | <0.001 |
| Diastolic blood pressure, mm Hg | 3.884 | 1.093 | <0.001 |
All coefficients are from models adjusted for sex, intervention status, and parent age. For the composite, the factor loadings were as follows: waist circumference (0.629), fasting glucose (0.455), triglycerides (0.422), high‐density lipoproteins (−0.459), systolic blood pressure (0.781), and diastolic blood pressure (0.792). IDF indicates International Diabetes Foundation; MetS, metabolic syndrome.
The other definition was a continuously distributed composite derived using weighted scores from factor analysis. It showed a linear trend by economic trajectory (eighth row of Table 3; adjusted contrast estimate=0.431, SE=0.097, P<0.0001). Planned comparisons revealed significantly higher composite scores in the Deepening Poverty versus other groups (P=0.005 versus Downward Mobility group and P<0.0001 versus Stable Low‐Income group). Again, the Downward Mobility group scored higher on this composite relative to the Stable Low‐Income group, but the difference was nonsignificant, P=0.119.
We also re‐analyzed data using the Harmonized MetS Criteria.19 This definition is quite similar to IDF's, except that central adiposity is not obligatory for diagnosis. Under this definition, just 1 subject changed status, now meeting diagnostic criteria. However, the overall pattern of findings related to economic trajectories was identical.
To clarify the metabolic abnormalities underlying the observed associations, we repeated the analyses for individual components of MetS. Binary outcome variables were used to indicate whether subjects' values exceeded IDF thresholds on each component. Linear trends by hardship trajectory were apparent for raised fasting glucose, lowered high‐density lipoprotein cholesterol, and raised blood pressure (see Figure 2 and rows 3–7 of Table 3). By contrast, neither central adiposity nor triglyceride levels patterned in a linear fashion by hardship trajectory (Ps=0.071 and 0.434, respectively). By contrast, when components were analyzed as continuous variables, linear trends by hardship trajectory were observed for central adiposity and blood pressure, but not fasting glucose, high‐density lipoprotein cholesterol, or triglycerides (see rows 9–13 of Table 3).
Figure 2.
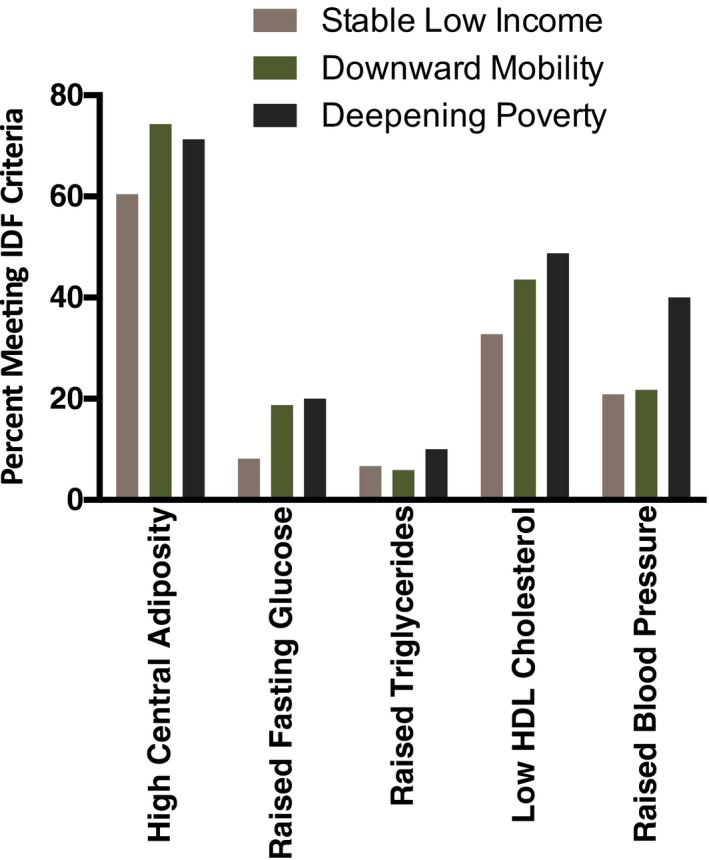
Metabolic syndrome (MetS) components by economic hardship. Using prospectively collected data, subjects' economic trajectories across the years of the Great Recession (2007–2010) were classified as Stable Low Income, Downward Mobility, or Deepening Poverty. In 2015, when subjects were 25 years of age, components of MetS were assessed. Values are percent of subgroup who met clinical threshold for each MetS component based on IDF criteria. HDL indicates high‐density lipoprotein; IDF, International Diabetes Federation.
Considering Alternative Explanations
There are several alternative explanations for these observations. First, as Table 1 shows, the hardship trajectory groups varied on parental education before the Recession. Thus, any MetS disparities could simply be a reflection of long‐standing familial differences in schooling, rather than hardship because of the Recession. To evaluate this possibility, we re‐estimated the models above while including parental educational attainment in 2007 as an additional covariate. However, the pattern of results did not change (P values for omnibus group comparisons were 0.007, 0.001, and 0.0002 for MetS diagnosis, count variable, and MetS composite, respectively), suggesting that pre‐existing variations on parental education were not responsible for the observed patterns.
A second possibility is that between 2010 and 2016, the hardship trajectory groups reached positive developmental milestones at different rates. To evaluate this hypothesis, we used Hardship×Time ANOVAs to compare the groups on education level, marital status, and living situation from 2010 to 2016. Not surprisingly, there were main effects of Time for each of these variables, showing that as subjects matured they were more likely to get married, obtain college degrees, and leave their parents' homes (all Ps<0.0001). However, in no case was there a Hardship×Time interaction (all Ps ranged from 0.24 to 0.98), suggesting the groups achieved these milestones at similar rates.
A last alternative is that MetS disparities predated the Recession, as opposed to being consequences of its fallout. Unfortunately, the study did not assess MetS before age 25, so we cannot directly test this possibility. However, blood pressure was measured in 2010, when subjects were 19, and the Recession had officially ended. Including these values as covariates did not change the pattern of results. Linear trends for hardship trajectories continued to be significant for the individual MetS components of raised blood pressure (P=0.006) and raised fasting glucose (P=0.019), as well as lowered high‐density lipoprotein cholesterol (P=0.012). Similarly, reanalysis of aggregate outcomes continued to show hardship‐related linear trends for all 3 MetS definitions, including diagnosis (adjusted contrast estimate=0.105, SE=0.038, P=0.006), counts (adjusted contrast estimate=0.400, SE=0.107, P=0.0002), and the composite (adjusted contrast estimate=0.354, SE=0.086, P<0.0001). These findings suggest that MetS patterns at 25 were not simply a reflection of cardiometabolic disparities that predated the Recession.
Consistent with this view, the groups had diverging trajectories of blood pressure between 2009/2010 (when they were 19) and 2015/2016 (when they were 25). As Figure 3 shows, subjects in the Deepening Poverty group gained 7 mm Hg in systolic (P<0.0001) and 5.5 mm Hg in diastolic pressure (P<0.0001) over this period. By contrast, the Stable Low‐Income group increased 2.5 (P=0.018 for systolic) and 2.0 (P=0.057 for diastolic) mm Hg. For subjects in the Downward Mobility group, pressure remained stable over this period (P=0.789 for systolic; P=0.821 for diastolic).
Figure 3.
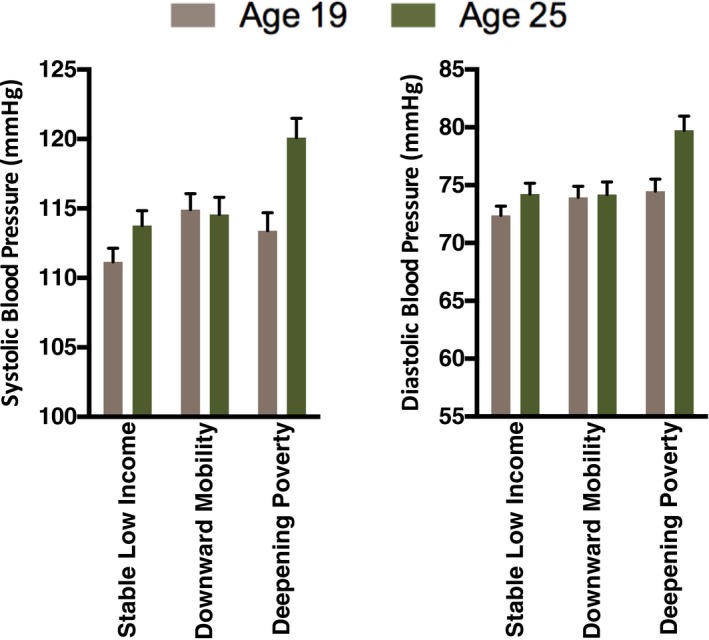
Blood pressure by economic hardship. Using prospectively collected data, subjects' economic trajectories across the years of the Great Recession (2007–2010) were classified as Stable Low Income, Downward Mobility, or Deepening Poverty. The figure shows blood pressure readings collected in 2009–2010 and 2015–2016, when subjects were 19 and 25 years of age.
Discussion
Building on previous studies of CVD following economic downturns,13, 14 we found that financial hardship associated with the Great Recession presaged higher risks of MetS 5 years later. This association was evident with 3 separate definitions of MetS, and was linear in nature, meaning that cardiometabolic risk increased in tandem with financial hardship. Previous research demonstrates that during economic downturns, older adults experience higher rates of acute coronary events.13, 14 This article's findings are unique in suggesting that larger macroeconomic conditions also have implications for the cardiometabolic health of young people making the transition to adulthood. Of particular note is the 27.5% prevalence of MetS among subjects who experienced Deepening Poverty across the Recession. According to National Health and Nutrition Examination Survey, this rate is roughly 50% higher than the broader population of 20‐ to 29‐year‐old Americans.22 If these disparities in cardiometabolic risk persist, these young adults will likely show disproportionately high rates of CVD morbidity and mortality in the coming decades,23 accentuating the already pronounced health inequities present in disadvantaged black communities in the rural Southeast.6, 7
There are several potential explanations for this study's findings. First, the MetS disparities we observed could predate the Great Recession, and be secondary to genetic liabilities, perinatal complications, toxicant exposures, family demographics, or other confounders associated with economic hardship trajectories. Though plausible, we view this scenario as unlikely, especially given the findings in models adjusted for parental education, and the groups' diverging blood pressure trajectories in the 5 years following the Recession. During this period, subjects in the Deepening Poverty group had a 7‐point increase in systolic pressure and 5‐point increase in diastolic pressure. The comparable values in the Stable Low‐Income group were 2.5 and 2.0 points, respectively. If gaps in cardiometabolic risk predated the Recession, it seems unlikely these groups' trajectories would be disparate. Nevertheless, follow‐up studies with more detailed assessments of potential third variables are necessary to evaluate the possibility of confounding; even more definitive would be natural experiments where MetS is assessed as a function of income shocks or policy changes that are distributed to populations in a presumptively random manner.24 A second possibility is that financial hardship arising from the Recession altered subjects' lifestyles in a manner that promoted weight gain and/or decreased physical activity,25 accelerating the development of MetS. Many of our subjects' parents lost jobs during the Recession, which could have reduced the family's ability to purchase healthy foods, seek preventive medical care, and access facilities for physical activity. The Recession also forced many rural communities to scale back spending on resources (food‐assistance programs, recreational facilities, public health programs), which in other circumstances might have functioned as buffers for economically struggling families. Finally, the unemployment, financial insecurity, and uncertainty associated with the Recession may have contributed to MetS through stress‐related mechanisms (eg, sleep loss, circadian disruption, increased adrenocortical and sympathetic outflow, or inflammatory activity).26, 27, 28 In a previous analysis of this sample's health the year after the Recession ended (mean age 19), we found that economic hardship was related to higher adrenocortical, sympathetic, and inflammatory activity.17 However, evidence did not suggest a mediating role for these processes in the MetS findings presented here. Nevertheless, youth in this sample experienced the Recession and its repercussions as they were transitioning into adulthood. Even in better economic circumstances, this can be a stressful developmental period for young people, who are trying to establish careers, families of their own, and financial independence from their parents. Particularly in the Deepening Poverty group, the Recession may have accentuated the severity and consequences of these stressors in a manner that heightened MetS risk.
Although we observed high rates of MetS in the Deepening Poverty group, it is important to keep in mind that the cardiometabolic risks associated with the Recession were not universal. Consistent with previous research,14 the outcomes we found differed by the family's economic context. Indeed, subjects in the Stable Low‐Income group seemed to be insulated from health‐related consequences of the Recession; their rate of MetS (10.4%) was considerably below National Health and Nutrition Examination Survey estimates for 20‐ to 29‐year‐olds (18.5%; see22). Also, those in the Downward Mobility group had a MetS prevalence (21.8%) only slightly higher than the National Health and Nutrition Examination Survey population. These patterns underscore the heterogeneity in rural black communities. When considered in the aggregate, these communities show increased rates of morbidity and mortality from CVD.7, 29, 30 However, as the findings here illustrate, a sizeable minority of individuals in these communities are resilient, meaning their health is better than expected given overall demographic trends. These patterns suggest the existence of protective factors (eg, social support, family wealth, community resources) that operated as buffers against broader macroeconomic stressors. If future research can identify these protective factors, interventions could attempt to cultivate them in at‐risk individuals, families, and neighborhoods.31, 32 Doing so might provide an alternative strategy for mitigating CVD disparities at a time when federal resources to support the health and well‐being of lower‐income Americans are likely to be diminishing.33, 34
Sources of Funding
This research was supported by grants from the National Institute of Child Health and Human Development (R01 HD030588), the National Heart, Lung, and Blood Institute (R01 HL108723, HL122328), and the National Institute on Drug Abuse (P30 DA027827).
Disclosures
None.
(J Am Heart Assoc. 2017;6:e006052 DOI: 10.1161/JAHA.117.006052.)28877875
References
- 1. Ford ES, Capewell S. Proportion of the decline in cardiovascular mortality disease due to prevention versus treatment: public health versus clinical care. Annu Rev Public Health. 2011;32:5–22. [DOI] [PubMed] [Google Scholar]
- 2. Vaughan AS, Quick H, Pathak EB, Kramer MR, Casper M. Disparities in temporal and geographic patterns of declining heart disease mortality by race and sex in the United States, 1973–2010. J Am Heart Assoc. 2015;4:e002567 DOI: 10.1161/JAHA.115.002567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kramer MR, Valderrama AL, Casper ML. Decomposing Black‐White disparities in heart disease mortality in the United States, 1973–2010: an age‐period‐cohort analysis. Am J Epidemiol. 2015;182:302–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Meara ER, Richards S, Cutler DM. The gap gets bigger: changes in mortality and life expectancy, by education, 1981–2000. Health Aff (Millwood). 2008;27:350–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Olshansky SJ, Antonucci T, Berkman L, Binstock RH, Boersch‐Supan A, Cacioppo JT, Carnes BA, Carstensen LL, Fried LP, Goldman DP, Jackson J, Kohli M, Rother J, Zheng Y, Rowe J. Differences in life expectancy due to race and educational differences are widening, and many may not catch up. Health Aff (Millwood). 2012;31:1803–1813. [DOI] [PubMed] [Google Scholar]
- 6. Singh GK, Siahpush M, Azuine RE, Williams SD. Widening socioeconomic and racial disparities in cardiovascular disease mortality in the United States, 1969–2013. Int J MCH AIDS. 2015;3:106–118. [PMC free article] [PubMed] [Google Scholar]
- 7. Dwyer‐Lindgren L, Bertozzi‐Villa A, Stubbs RW, Morozoff C, Kutz MJ, Huynh C, Barber RM, Shackelford KA, Mackenbach JP, van Lenthe FJ, Flaxman AD, Naghavi M, Mokdad AH, Murray CJ. US county‐level trends in mortality rates for major causes of death, 1980–2014. JAMA. 2016;316:2385–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang X, Poole JC, Treiber FA, Harshfield GA, Hanevold CD, Snieder H. Ethnic and gender differences in ambulatory blood pressure trajectories: results from a 15‐year longitudinal study in youth and young adults. Circulation. 2006;114:2780–2787. [DOI] [PubMed] [Google Scholar]
- 9. Li Z, Snieder H, Su S, Harshfield GA, Treiber FA, Wang X. A longitudinal study of blood pressure variability in African‐American and European American youth. J Hypertens. 2010;28:715–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Matthews KA, Gallo LC. Psychological perspectives on pathways linking socioeconomic status and physical health. Annu Rev Psychol. 2011;62:501–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Clark AM, DesMeules M, Luo W, Duncan AS, Wielgosz A. Socioeconomic status and cardiovascular disease: risks and implications for care. Nat Rev Cardiol. 2009;6:712–722. [DOI] [PubMed] [Google Scholar]
- 12. Hertz T, Kusmin L, Marre A, Parker T. Rural Employment Trends in Recession and Recovery. Washington, DC: US Department of Agriculture, Economic Research Service; 2014. [Google Scholar]
- 13. van den Berg GJ, Doblhammer‐Reiter G, Christensen K. Being born under adverse economic conditions leads to a higher cardiovascular mortality rate later in life: evidence based on individuals born at different stages of the business cycle. Demography. 2011;48:507–530. [DOI] [PubMed] [Google Scholar]
- 14. Noelke C, Avendano M. Who suffers during recessions? Economic downturns, job loss, and cardiovascular disease in older Americans. Am J Epidemiol. 2015;182:873–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the United States, 2003‐–2012. JAMA. 2015;313:1973–1974. [DOI] [PubMed] [Google Scholar]
- 16. Brody GH, Murry VM, Gerrard M, Gibbons FX, Molgaard V, McNair L, Brown AC, Wills TA, Spoth RL, Luo Z, Chen YF, Neubaum‐Carlan E. The Strong African American Families Program: translating research into prevention programming. Child Dev. 2004;75:900–917. [DOI] [PubMed] [Google Scholar]
- 17. Chen E, Miller GE, Yu T, Brody GH. The Great Recession and health risks in African American youth. Brain Behav Immun. 2016;53:234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Conger RD, Elder GH. Families in Troubled Times: Adapting to Change in Rural America. New York, NY: Aldine de Gruyter; 1994. [Google Scholar]
- 19. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC; International DFTFOEAP, National Heart L, and Blood Institute, American HA, World HF, International AS, International AFTSOO . Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. [DOI] [PubMed] [Google Scholar]
- 20. Cornier MA, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, Van Pelt RE, Wang H, Eckel RH. The metabolic syndrome. Endocr Rev. 2008;29:777–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goodman E. Metabolic syndrome and the mismeasure of risk. J Adolesc Health. 2008;42:538–540. [DOI] [PubMed] [Google Scholar]
- 22. Ford ES. Prevalence of the metabolic syndrome defined by the International Diabetes Federation among adults in the U.S. Diabetes Care. 2005;28:2745–2749. [DOI] [PubMed] [Google Scholar]
- 23. Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, Rinfret S, Schiffrin EL, Eisenberg MJ. The metabolic syndrome and cardiovascular risk a systematic review and meta‐analysis. J Am Coll Cardiol. 2010;56:1113–1132. [DOI] [PubMed] [Google Scholar]
- 24. Duncan GJ, Magnuson K, Votruba‐Drzal E. Moving beyond correlations in assessing the consequences of poverty. Annu Rev Psychol. 2017;68:413–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hanson MD, Chen E. Socioeconomic status and health behaviors in adolescence: a review of the literature. J Behav Med. 2007;30:263–285. [DOI] [PubMed] [Google Scholar]
- 26. Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annu Rev Physiol. 2005;67:259–284. [DOI] [PubMed] [Google Scholar]
- 27. Scrivo R, Vasile M, Bartosiewicz I, Valesini G. Inflammation as “common soil” of the multifactorial diseases. Autoimmun Rev. 2011;10:369–374. [DOI] [PubMed] [Google Scholar]
- 28. Koren D, O'Sullivan KL, Mokhlesi B. Metabolic and glycemic sequelae of sleep disturbances in children and adults. Curr Diab Rep. 2015;15:562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Singh GK, Azuine RE, Siahpush M, Williams SD. Widening geographical disparities in cardiovascular disease mortality in the United States, 1969–2011. Int J MCH AIDS. 2015;3:134–149. [PMC free article] [PubMed] [Google Scholar]
- 30. Chetty R, Stepner M, Abraham S, Lin S, Scuderi B, Turner N, Bergeron A, Cutler D. The association between income and life expectancy in the United States, 2001–2014. JAMA. 2016;315:1750–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Masten AS. Ordinary magic. Resilience processes in development. Am Psychol. 2001;56:227–238. [DOI] [PubMed] [Google Scholar]
- 32. Cicchetti D, Blender JA. A multiple‐levels‐of‐analysis perspective on resilience: implications for the developing brain, neural plasticity, and preventive interventions. Ann N Y Acad Sci. 2006;1094:248–258. [DOI] [PubMed] [Google Scholar]
- 33. Chen E, Miller GE. Shift and persist strategies: why being low in socioeconomic status isn't always bad for your health. Perspect Psychol Sci. 2012;7:135–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Williams DR. Miles to go before we sleep: racial inequities in health. J Health Soc Behav. 2012;53:279–295. [DOI] [PMC free article] [PubMed] [Google Scholar]


