Abstract
Statement of the Problem:
Aloe vera gel contains various components with antibiotic and anti-inflammatory characteristics, which may have potential advantages to treat periodontal diseases.
Purpose:
The aim of this study was to evaluate the effects of local application of aloe vera gel as an adjunct to scaling and root planning in the treatment of patients with chronic periodontitis.
Materials and Method:
This single-blind clinical trial, performed in a split mouth design, was conducted on 20 patients with moderate to severe chronic periodontitis. Following a baseline examination at first day which included the assessments of plaque index (PI), gingival index (GI), and probing depth (PD); patients randomly received either SRP in one quadrant (control group), or SRP combined with aloe vera gel in another quadrant (experimental group). All cases were examined again, assessing PI, GI, and PD at 30th and 60th day.
Results:
There was no significant difference in PI in the three stages between control and experimental groups. In all patients, there was a significant improvement in the three stages in GI and PD for both quadrants treated only with SRP or combination of SRP and aloe vera. However, experimental group presented significantly lower GI (p= 0.0001) and PD (p= 0.009) than the control group at the end of study period.
Conclusion:
This study revealed that local application of aloe vera gel could be considered as an adjunctive treatment with scaling and root planning for chronic periodontitis.
Keywords: Aloe vera gel , Scaling and root planning , Chronic periodontitis
Introduction
Periodontal problems, the most common inflammatory disease, result from the interaction between invasion of biofilm microorganisms and periodontal immune response in a susceptible host.[1-3] Gingivitis as the early stage of this disease may turn into more severe periodontitis characterized by pocket formation, alveolar bone destruction, and loss of clinical attachment level.[1]
The first treatment goal in periodontal disease is changing or removing microbial origin and risk factors to prevent disease progression and maintain periodontal tissue health.[2] Then, the recurrence of the disease must be prevented and finally, restructuring and reorganizing attachment has to be done.[2]
Periodontal diseases are treated by the means of surgical or non-surgical methods.[4] The first non-surgical treatment is the mechanical debridement of the tooth surface with scaling and root planning (SRP).[4] Non-surgical SRP remove bacterial plaque and calculus from supra gingival and sub gingival areas, can cause restoration and preservation of periodontal health.[4-5] However, in some cases with deep periodontal pocket or in areas of furcation involvements, mechanical treatment is not effective.[6-9] Due to the access restrictions of deep sub-gingival areas, some pathogens such as Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis, Prevotella Intermedia, Bacteroides forsytus, peptostreptococci micros cannot be eradicated and consequently increase the risk of treatment failure.[6-9] Surgical intervention is necessary if non-surgical treatments could not reduce the pocket depth.[2] Periodontists can perform SRP in difficult access areas by harvesting inflamed tissue during surgery. This procedure removes bacterial concentrations and could reduce alveolar bone destruction around the infected area and then reconstruct and reduce pocket depth.[2-3] If necessary, other treatments such as bone graft, bone reconstruction and soft tissue graft can be done to reduce bone and gingival recession.[2-4] Since bacterial products, immunological and inflammatory factors are involved in the pathogenesis of periodontal disease, some products as adjunctive therapy can be used to help the healing process or controlling bacterial infection, and they can overcome the disadvantages of mechanical methods of plaque control.[2,9-11] A variety of non-chemical alternative products such as lasers,[12] topical and systemic antibiotics like azithromycin[13] and clarithromycin,[14] and chemicals such as hyaluronic acid,[15] antiseptic and anti-inflammatory agents with positive effects on immune system[12,16] are reported to be beneficial.[10] Due to the fewer side effects of medicinal herbs, some studies showed green tea, Cordiaverbenacea (a native plant of Brazil coasts) and Mikanialaevigata, per se or in combination with SRP, can be beneficial in periodontitis treatment.[17-22] Aloe vera is a cactus-like plant of Liliaceae family with about 360 species containing 75 active ingredients such as vitamins, enzymes, minerals, sugars, lignins, saponins, salicylic acids, and amino acids.[23] Aloe vera medicinal effect in vitro or on animal models has shown to have anti-inflammatory, anti-arthritic and anti-bacterial effects.[23-24] Furthermore, the treatment effects of aloe vera on systemic disease, cancers, regulation of blood glucose levels, wound and infection healing have been evaluated in some studies.[23-27]There are some published studies that reported the use of aloe vera in dentistry for various purposes such as disinfecting dental unit water network,[28] gutta-percha sterilization,[29] antiseptic effect on candida albicans,[30] aphthous stomatitis treatment,[31] as an ingredient in toothpastes[32-33] and mouthwash,[23-34] and for gingivitis treatment.
Up to date, few studies on the effect of topical application of aloe vera in the periodontal pocket for periodontal disease treatment have been reported.[35] Thus, this study intended to clinically evaluate the effects of local application of aloe vera gel as an adjunct to SRP in patients with chronic periodontitis in the form of a single-blind, split-mouth trial and ultimately, observe the possibility of using aloe vera gel or its extract as an adjunctive therapy to improve the mechanical treatment of periodontal disease.
Materials and Method
This split-mouth, single-blind clinical trial was conducted in the city of Rasht, Iran, under the supervision of Committee of Research, and Ethics Committee of Guilan University of Medical Sciences. Populations of the study were the patients referring to the periodontology department of dental school of Guilan University of Medical Sciences who suffered from moderate to severe chronic periodontitis. Research was registered with the Iranian Registry of Clinical Trials (IRCT), an approved member World Health Organization with acceptance code of IRCT2015010318054N2. Sample size was in a group of 18 people (with 95% confidence interval, 90% test placing, and consideration of the comparative formula); finally, 20 patients with at least one tooth or one area in each quadrant (upper or lower jaw) with pocket probing depth of 4 to 5mm and positive BOP tests were chosen. As an inclusion criterion for the study, all patients were candidates for nonsurgical treatment phase. Exclusion criteria were history of allergy to aloe vera or its products, tobacco use, habits like mouth breathing or tongue trusting, endodontic and periodontal combined lesions, periapical lesion, severe decay of teeth, partially impacted teeth, patients undergoing orthodontic treatment, systemic diseases, systemic or topical (oral) antibiotics use six months prior to the study, any periodontal treatment six months prior to the study, and pocket depth more than 5mm. The project was explained to the patients, consent forms were obtained, and participants were allowed to leave the study at any moment their convenient.
Aloe vera gel preparation method
Aloe vera gel used in this study was %98 aloe vera® gel concentration (Avivir; Denmark) and 2% normal saline.
Commercial preparation method of %98 concentration of aloe vera gel was washing the ripe aloe vera leaves thoroughly under water and cutting off their skin. Each leaf was cut into several pieces and seeping gel from the pieces were collected in a sterile container and stored at 4°C until the time of application. At first day as the baseline, before SRP, patients’ clinical parameters including plaque index (PI), gingival index (GI) and probing depth (PD) were measured in two examined quadrants and four dental surfaces, except occlusal surface, were examined .To determine PI, O’Leary Index (1972) and to measure GI, Loe & Sillness index (1963) were employed. For each of 4 sections of surrounding soft tissues of teeth, a score of 0 to 3 was assigned to measure GI. If this number was 1 or less, it indicated a mild gingivitis, 1.1 to 2 indicated a moderate gingivitis, and finally, 2.1 and more was considered as severe gingivitis. PD was measured from the gingival margin to the bottom of periodontal pocket using William’s probe, with an accuracy of 0.5mm, at 6 levels of mesiobuccal, distobuccal, mesiolingual, distolingual, midbuccal and midlingual. Then, the PD mean was calculated.
Then, SRP was performed for all teeth, and all patients were instructed not to change their oral health habits, and to use the Signal®Family Protection toothpaste (Unilever; Slovakia) and not to use any other chemical adjunctive hygiene agents.
Mouth area was divided into two quadrants of case and control by using a randomized block for each patient. In the case quadrant, SRP treatment was performed routinely. Then, aloe vera gel was injected into the periodontal pocket by means of an insulin syringe inserted up to the base of the pocket until the space was filled and the gel leaked out of the gingival margin. In the control quadrant, entire process was done similar to the other group, but the aloe vera gel was replaced by distilled water. Then, for a longer lasting effect of aloe vera gel, the treated area and the contralateral area were dressed for 24 hours.
Clinical parameters of PI, GI, and PD were measured again on 30th and 60th days after initial treatment. Gel application was carried out by a dentist and measuring process was performed by another dentist 1 and 2 months after the treatment by a single-blind intra-calibration method. Intra-examiner reliability was done by choosing 5 randomized patients, and measurement of PI, GI, and PD within a week; then reliability measured by kappa. Demographic information (such as age and gender) and the findings of the clinical examination were recorded in a pre-prepared form. Statistical analysis was performed using SPSS version 19. Changes in PI, GI, and PD in each quadrant between 30 and 60 days were compared to the baseline using ANOVA repeated measure test. The differences of PI, GI, and PD between case and control quadrants were analyzed using Independent t-test at any point in time on days 30 and 60 .Statistical significance level was set as p < 0.05. At the end, Kolmogorov-Smirnov test was employed to evaluate the variables normality.
Results
Statistical tests showed no significant differences between PI of case and control groups at three study times after gel application on periodontal pocket (p= 0.244), while there was a statistically significant difference between GI and PD of case and control groups at three study times after gel placement on periodontal pocket (p= 0.001 and p= 0.026).
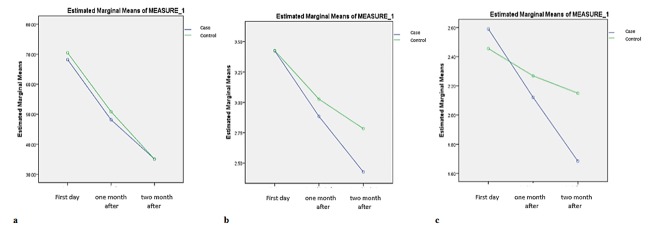
Table 1 shows in the case group per se, there was a statistically significant difference between the percentage of PI in the first day compared to one month after using aloe vera gel and also between the first day compared to two months after the consumption (p= 0.000 and p= 0.005).However, compared to the percentage of PI one and two months afterwards, there was no statistically significant difference (p= 0.108). Table 2 shows in the control group per se, there was a statistically significant difference between the percentage of PI at first (first day) compared to one and two month after using aloe vera gel consumption consequently (p= 0.000 and p= 0.015). However, compared to the percentage of PI one and two months afterwards, there was no statistically significant difference (p= 0.065). The comparison of PI in case and control groups is illustrated in Figure 1a.
Table 1.
Pairwise comparison of PI, GI and PD in case group
| Mean differences | CI 95% | p Value | ||||
|---|---|---|---|---|---|---|
| Lower limit | Upper limit | |||||
| PI | Baseline (first day) | After 1month | 20.02 | 5.005 | 35.26 | 0.005 |
| After 2months | 33.07 | 18.067 | 48.089 | 0.000 | ||
| After 1 month | After 2months | 13.06 | -1.947 | 28.073 | 0.108 | |
| GI | Baseline (first day) | After 1month | 0.46 | 0.770 | 0.859 | 0.140 |
| After 2months | 0.91 | 0.514 | 1.296 | 0.000 | ||
| After one month | After 2months | 0.44 | 0.045 | 0.828 | 0.024 | |
| PD | Baseline (first day) | After 1month | 0.54 | 0.570 | 1.23 | 0.023 |
| After 2months | 0.99 | 0.513 | 1.47 | 0.000 | ||
| After one month | After 2months | 0.46 | -0.026 | 0.939 | 0.070 | |
Table 2.
Pairwise comparison of PI, GI and PD in control group
| Mean differences | CI 95% | p Value | ||||
|---|---|---|---|---|---|---|
| Lower limit | Upper limit | |||||
| PI | Baseline (first day) | After 1 month | 19.58 | 3.061 | 36.103 | 0.015 |
| After 2 months | 35.39 | 18.867 | 51.911 | 0.00 | ||
| After 1 month | After 2 months | 15.81 | -0.714 | 32.328 | 0.065 | |
| GI | Baseline (first day) | After 1 month | 0.18 | -0.158 | 0.532 | 0.560 |
| After 2 months | 0.30 | -0.039 | 0.650 | 0.100 | ||
| After one month | After 2 months | 0.12 | -0.226 | 0.463 | .0998 | |
| PD | Baseline (First day) | After 1 month | 0.40 | 0.012 | 0.787 | 0.041 |
| After 2 months | 0.64 | 0.254 | 1.029 | 0.000 | ||
| After one month | After 2 months | 0.24 | 0.145 | 0.629 | 0.386 | |
Figure1.
a: Comparison of PI in case and control groups, b: Comparison of GI in case and control groups, c: Comparison of PD in case and control group
Table 1 shows in the case group per se, there was a statistically significant difference between the percentage of PI in the first day compared to one month after using aloe vera gel and also between the first day compared to two months after the consumption (p= 0.000 and p= 0.005).However, compared to the percentage of PI one and two months afterwards, there was no statistically significant difference (p= 0.108). Table 2 shows in the control group per se, there was a statistically significant difference between the percentage of PI at first (first day) compared to one and two month after using aloe vera gel consumption consequently (p= 0.000 and p= 0.015). However, compared to the percentage of PI one and two months afterwards, there was no statistically significant difference (p= 0.065). The comparison of PI in case and control groups is illustrated in Figure 1a.
Table 1 shows, in the case group per se, there was statistically a significant difference between the percentage of GI at first day compared to one month after using aloe vera gel and also between the baseline (first day) compared to two months of consumption, as well as the percentage of GI one and two months afterwards (p= 0.000, p= 0.024, and p= 0.014). However, there was not a statistically significant difference in the control group per se (Table 2). The comparison of GI in case and control groups is illustrated in Figure 1b.
Table 1 shows that, in the case group per se, there was a statistically significant difference between the percentage of PD on first day compared to one month after using aloe vera gel (p= 0.070) and between the baseline (first day) compared to two months of consumption, and compared to the percentage of PI one and two months afterwards (p= 0.000 and 0.023). But, in the control group per se, (Table 2) there was no statistically significant difference between the percentage of PD in one month compared to two month after using aloe vera gel (p= 0.386). On the other hand, between the percentage of PD at first day compared to one month of consumption and also compared to the percentage of PI on first day and two months after, there were statistically significant differences (p= 0.000 and p= 0.041 respectively). Comparison of PD in case and control groups is presented in Figure 1c.
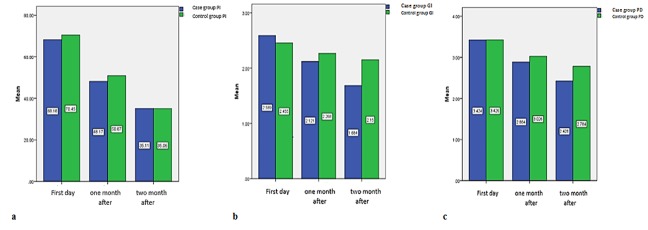
The differences of PI, GI, and PD between case and control quadrants on 30th and 60th days after consumption were assessed using independent t-test and the statistical significance level of p< 0.05 was achieved. Figure 2a, 2b and 2c show the independent t-test analysis results.
Figure2.
a: Comparison of mean PI percentage in case and control groups, b: Comparison of mean GI percentage in case and control groups, c: Comparison of mean PD percentage in case and control groups
Discussion
Periodontitis is a multi-factorial disturbance between bacterial biofilm and susceptible host.[1,2,4-5] SRP is the most common treatment plan for controlling inflammation in non-surgical treatment method; however, since this modality is not successful in many cases, the auxiliary agents are used in combination with SRP.[4] The effect of some non-chemical, chemical, antibiotics, and herbal factors, employed as auxil iary agents, have been evaluated in various studies.[4,12-19,36] In this study, the effects of local application of aloe vera gel as an adjunct to scaling and root planning in patients with chronic periodontitis were evaluated and the clinical improvement, GI and PD were considered.
A correct identification and evaluation of the number, type, depth, and development of periodontal pocket, is the basis for diagnosis of periodontitis. Moreover, assessing changes in pocket depth are of great importance for evaluating the severity and progress of the disease as well as effectiveness of the treatment methods. Beside clinical researches, pocket assessment is essential in daily treatment of the patients.[37]
This research analyzed and compared the periodontal index of both the case and the control groups. The results showed that, despite reduction of PI in 60 days of study, there were no statistically significant differences. Therefore, it can be concluded that aloe vera gel had no effect on reducing PI, and the PI reduction may be due to effect of primary SRP and Hawthorn effect of motivating patients to brush.[1,8]
According to this study, clinical improvement of PI was observed in both case and control groups during the study. Although based on the GI and PD, the case group showed a considerably better improvement rate than the control group.
Bhat et al.,[34] with similar findings in patients with chronic periodontitis, reported a considerable reduction of pocket depth in areas of aloe vera gel treatment combined with SRP, which was statistically significant. Verdi et al.[35] have also published similar results on the effectiveness of aloe vera gel in patients with chronic periodontitis.
Aloe vera has been shown to be effective on gingivitis.[38] Chandrahas et al.[33] showed aloe vera mouthwash to be similar to chlorhexidine 0.2% in recovery of gingivitis. Also compared to experimental group (using distilled water), greatly decreased modified gingival index and bleeding index. Pradeep et al.[13] showed healing effects of aloe vera toothpaste on gingivitis and observed a reduction in gingival inflammation. They reported higher therapeutic effects compared to toothpastes without such compounds and similar to the ones with fluoride and triclosan.[13] Ajmera et al.[22] showed aloe vera, as an auxiliary treatment with mechanical cleaning can be a beneficiary treatment for gingivitis, although, aloe vera alone cannot greatly heal gingivitis.
In this study, gingival index was used to assess periodontal tissue status. This criterion was determined based on the inflammation symptoms including swelling, redness, and bleeding.[22] Thus, significant reduction in the GI reduces inflammation markers in case group.
It has been shown that aloe vera gel contains biologically active compounds such as mannose-6-phosphate, carboxypeptidase, glutathione peroxidase, and superoxide dismutase.[39] These compounds possess anti-inflammatory, antioxidant, and anti-bacterial properties and regulate the immune system and help in healing wounds.[40]
Biju et al.[40] found out that glutathione peroxidase and superoxide dismutase are associated with periodontal disease outbreak. The results of their study indicated an increase in the serum levels of these two factors after treatment of gingivitis and periodontitis.[40] Results of this study showed a significant reduction of PD in case group. Some parts of the improvement can be attributed to oxidant-antioxidant system performance. Ellis et al.[39] found out that decreased performance of dismutase is associated with an increase in probing depth. A decrease in dismutase and an increase in leukocytes can lead to an increase in active oxidizing agents, resulting in the destruction of the tissue.[39]
Improvement of periodontitis is considered to be the result aloe vera’s effect on Matrix metalloproteinase (MMPs). Makela et al.[41] showed that two types of gelatinase, MMP-2 and MMP-9, can be effective on tissue destruction related to periodontitis.[41] Kudalkar et al.[42] on the other hand, found out that aloe vera caused a reduction of the mentioned gelatinase in the gingival tissue samples; the results could be improved by increasing aloe vera’s density. For evaluating the effect of alo vera on the MMP’s, immunologically, comprehensive study with different density of alo vera could be done in future.
Conclusion
According to this study, SRP combined with aloe vera as adjunctive therapy resulted in significant improvements of severe periodontitis. Significant reduction of GI and PD clinical criteria explained significant effectiveness of aloe vera gel on improvement of disease.
Acknowledgement
This article is had been written based on the thesis that done in Guilan University of Medical Sciences and hereby we thank them.
Conflict of Interest:We confident that author judgments have not been influenced in preparing the manuscript. We conflicts that, during this research have not any financial support or private connections to pharmaceutical companies, political pressure from interest groups, or academic problems; and sole purpose of this study was to spread and development of knowledge and science.
References
- 1.Tariq M, Iqbal Z, Ali J, Baboota S, Talegaonkar S, Ahmad Z, Sahni JK. Treatment modalities and evaluation models for periodontitis. Int J Pharm Investig. 2012; 2:106–122. doi: 10.4103/2230-973X.104394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim J, Amar S. Periodontal disease and systemic conditions: a bidirectional relationship. Odontology. 2006; 94: 10–21. doi: 10.1007/s10266-006-0060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curtis MA, Zenobia C, Darveau RP. The relationship of the oral microbiotia to periodontal health and disease. Cell Host Microbe. 2011; 10: 302–306. doi: 10.1016/j.chom.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ehizele A, Akhionbare O. Effect of non-surgical periodontal therapy on the concentration of volatile sulfur com-pound in mouth air of a group of nigerian young adults. Ann Med Health Sci Res. 2013; 3: 433–437. doi: 10.4103/2141-9248.117951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohan R, Agrawal S, Gundappa M. Atomic force mi-croscopy and scanning electron microscopy evaluation of efficacy of scaling and root planing using magnification: A randomized controlled clinical study. Contemp Clin Dent. 2013; 4: 286–294. doi: 10.4103/0976-237X.118347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morozumi T, Kubota T, Abe D, Shimizu T, Nohno K, Yoshie H. Microbiological effect of essential oils in combination with subgingival ultrasonic instrumentation and mouth rinsing in chronic periodontitis patients. Int J Dent. 2013; 2013: 146479. doi: 10.1155/2013/146479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jain M, Dave D, Jain P, Manohar B, Yadav B, Shetty N. Efficacy of xanthan based chlorhexidine gel as an adjunct to scaling and root planing in treatment of the chronic periodontitis. J Indian Soc Periodontol. 2013; 17: 439–443. doi: 10.4103/0972-124X.118313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jagadish Pai BS, Rajan SA, Srinivas M, Padma R, Suragimath G, Walvekar A, Goel S. Comparison of the efficacy of chlorhexidine varnish and chip in the treatment of chronic periodontitis. Contemp Clin Dent. 2013; 4: 156–161. doi: 10.4103/0976-237X.114848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rams TE, Slots J. Local delivery of antimicrobial agents in the periodontal pocket. Periodontol 2000. 1996; 10: 139–159. doi: 10.1111/j.1600-0757.1996.tb00072.x. [DOI] [PubMed] [Google Scholar]
- 10.Krayer JW, Leite RS, Kirkwood KL. Non-surgical chemotherapeutic treatment strategies for the management of periodontal diseases. Dent Clin North Am. 2010; 54: 13–33. doi: 10.1016/j.cden.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zare D, Haerian A, Molla R, Vaziri F. Evaluation of the effects of diode (980 nm) laser on gingivalinflammation after nonsurgical periodontal therapy. J Lasers Med Sci. 2014; 5: 27–31. [PMC free article] [PubMed] [Google Scholar]
- 12.Saglam M, Kantarci A, Dundar N, Hakki SS. Clinical and biochemical effects of diode laser as an adjunct to nonsurgical treatment of chronic periodonti-tis: a randomized, controlled clinical trial. Lasers Med Sci. 2014; 29: 37–46. doi: 10.1007/s10103-012-1230-0. [DOI] [PubMed] [Google Scholar]
- 13.Pradeep AR, Bajaj P, Agarwal E, Rao NS, Naik SB, Kalra N, et al. Local drug delivery of 0. 5% azithromycin in the treatment of chronic periodontitis among smokers. Aust Dent J 2013; 58: 34–40. doi: 10.1111/adj.12019. [DOI] [PubMed] [Google Scholar]
- 14.Agarwal E, Pradeep AR, Bajaj P, Naik SB. Efficacy of local drug delivery of 0. 5% clarithromycin gel as an adjunct to non-surgical periodontal therapy in the treatment of current smokers with chronic periodontitis: a randomized controlled clinical trial. J Periodontol 2012; 83: 1155–1163. doi: 10.1902/jop.2012.110600. [DOI] [PubMed] [Google Scholar]
- 15.Eick S, Renatus A, Heinicke M, Pfister W, Stratul SI, Jentsch H. Hyaluronic Acid as an adjunct after scaling and root planing: a prospective randomized clinical trial. J Periodontol. 2013; 84: 941–949. doi: 10.1902/jop.2012.120269. [DOI] [PubMed] [Google Scholar]
- 16.Prasad D, Kunnaiah R. Punica granatum: A review on its potential role in treating periodontal disease. J Indian Soc Periodontol. 2014; 18: 428–432. doi: 10.4103/0972-124X.138678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suchetha A, Bharwani AG. Efficacy of a commercially available multi-herbal formulation in periodontal therapy. J Indian Soc Periodontol. 2013; 17: 193–197. doi: 10.4103/0972-124X.113068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hattarki SA, Pushpa SP, Bhat K. Evaluation of the efficacy of green tea catechins as an adjunct to scaling and root planing in the management of chronic periodontitis using PCR analysis: A clinical and microbiological study. J Indian Soc Periodontol. 2013; 17: 204–209. doi: 10.4103/0972-124X.113071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hrishi TS, Kundapur PP, Naha A, Thomas BS, Kamath S, Bhat GS. Effect of adjunctive use of green tea dentifrice in periodontitis patients - A Randomized Controlled Pilot Study. Int J Dent Hyg. 2016; 14: 178–183. doi: 10.1111/idh.12131. [DOI] [PubMed] [Google Scholar]
- 20.Pimentel SP, Barrella GE, Casarin RC, Cirano FR, Casati MZ, Foglio MA, et al. Protective effect of top-ical Cordia verbenacea in a rat periodontitis model: im-mune-inflammatory, antibacterial and morphometric assays. BMC Complement Altern Med. 2012; 12: 224. doi: 10.1186/1472-6882-12-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benatti BB, Campos-Júnior JC, Silva-Filho VJ, Alves PM, Rodrigues IR, Uber-Bucek E, et al. Effects of a Mikania laevigata extract on bone resorption and RANKLexpression during experimental periodontitis in rats. J Appl Oral Sci. 2012; 20: 340–346. doi: 10.1590/S1678-77572012000300008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ajmera N, Chatterjee A, Goyal V. Aloe vera: It's effect on gingivitis. J Indian Soc Periodontol. 2013; 17: 435–438. doi: 10.4103/0972-124X.118312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vogler BK, Ernst E. Aloe vera: a systematic review of its clinical effectiveness. Br J Gen Pract. 1999; 49: 823–828. [PMC free article] [PubMed] [Google Scholar]
- 24.Harlev E, Nevo E, Lansky EP, Ofir R, Bishayee A. Anticancer potential of aloes: antioxidant, antiproliferative, and im-munostimulatory attributes. Planta Med. 2012; 78: 843–852. doi: 10.1055/s-0031-1298453. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka M, Misawa E, Ito Y, Habara N, Nomaguchi K, Yamada M, et al. Identification of five phytosterols from Aloe vera gel as anti-diabetic compounds. Biol Pharm Bull. 2006; 29: 1418–1422. doi: 10.1248/bpb.29.1418. [DOI] [PubMed] [Google Scholar]
- 26.Khan AW, Kotta S, Ansari SH, Sharma RK, Kumar A, Ali J. Formulation development, optimization and evaluation of aloe vera gel for wound healing. Pharmacogn Mag. 2013; 9(Suppl 1): S6–S10. doi: 10.4103/0973-1296.117849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pareek S, Nagaraj A, Sharma P, Atri M, Walia S, Naidu S, et al. Disinfection of dental unit water line using aloe vera: in vitro study. Int J Dent. 2013; 2013: 618962. doi: 10.1155/2013/618962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Athiban PP, Borthakur BJ, Ganesan S, Swathika B. Evaluation of antimicrobial efficacy of Aloe vera and its effectiveness in decontaminating gutta percha cones. J Conserv Dent. 2012; 15: 246–248. doi: 10.4103/0972-0707.97949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doddanna SJ, Patel S, Sundarrao MA, Veerabhadrappa RS. Antimicrobial activity of plant extracts on Candida albicans: an in vitro study. Indian J Dent Res. 2013; 24: 401–405. doi: 10.4103/0970-9290.118358. [DOI] [PubMed] [Google Scholar]
- 30.Babaee N, Zabihi E, Mohseni S, Moghadamnia AA. Evaluation of the therapeutic effects of Aloe vera gel on minor recurrent aphthous stomatitis. Dent Res J (Isfahan) 2012; 9: 381–385. [PMC free article] [PubMed] [Google Scholar]
- 31.Pradeep AR, Agarwal E, Naik SB. Clinical and microbiologic effects of commercially available denti-frice containing aloe vera: a randomized controlled clinical trial. J Periodontol. 2012; 83: 797–804. doi: 10.1902/jop.2011.110371. [DOI] [PubMed] [Google Scholar]
- 32.Namiranian H, Serino G. The effect of a toothpaste containing aloe vera on established gingivitis. Swed Dent J. 2012; 36: 179–185. [PubMed] [Google Scholar]
- 33.Chandrahas B, Jayakumar A, Naveen A, Butchibabu K, Reddy PK, Muralikrishna T. A randomized, double-blind clinical study to assess the antiplaque and antigingivitis efficacy of Aloe vera mouth rinse. J Indian Soc Periodontol. 2012; 16: 543–548. doi: 10.4103/0972-124X.106905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhat G, Kudva P, Dodwad V. Aloe vera: Nature's soothing healer to periodontal disease. J Indian Soc Periodontol. 2011; 15: 205–209. doi: 10.4103/0972-124X.85661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Virdi HK, Jain S, Sharma S. Effect of locally delivered aloe vera gel as an adjunct to scaling and root planing in the treatment of chronic periodontitis: A clinical study. Indian J Oral Sci. 2012; 3: 84–89. [Google Scholar]
- 36.Benamghar L, Penaud J, Kaminsky P, Abt F, Martin J. Comparison of gingival index and sulcus bleeding index as indicators of periodontal status. Bull World Health Organ. 1982; 60: 147–151. [PMC free article] [PubMed] [Google Scholar]
- 37.Hefti AF. Periodontal probing. Crit Rev Oral Biol Med. 1997; 8: 336–356. doi: 10.1177/10454411970080030601. [DOI] [PubMed] [Google Scholar]
- 38.Altincik A, Sönmez F, Yenisey C, Duman S, Can A, Akev N, et al. Effects of Aloe vera leaf gel extract on rat peritonitis model. Indian J Pharmacol. 2014; 46: 322–327. doi: 10.4103/0253-7613.132184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ellis SD, Tucci MA, Serio FG, Johnson RB. Factors for progression of periodontal diseases. J Oral Pathol Med. 1998; 27: 101–105. doi: 10.1111/j.1600-0714.1998.tb01923.x. [DOI] [PubMed] [Google Scholar]
- 40.Biju T, Shabeer MM, Amitha R, Rajendra BP, Suchetha K. Comparative evaluation of serum superoxide dismutase and glutathione levels in periodontally diseased patients: an inter-ventional study. Indian J Dent Res. 2014; 25: 613–616. doi: 10.4103/0970-9290.147105. [DOI] [PubMed] [Google Scholar]
- 41.Mäkelä M, Salo T, Uitto VJ, Larjava H. Matrix metallopro-teinases (MMP-2 and MMP-9) of the oral cavity: cellular origin and relationship to periodontal status. J Dent Res. 1994; 73: 1397–1406. doi: 10.1177/00220345940730080201. [DOI] [PubMed] [Google Scholar]
- 42.Kudalkar MD, Nayak A, Bhat KS, Nayak RN. Effect of Azadirachta indica (Neem) and Aloe vera as compared to subantimicrobial dose doxycycline on matrix metallopro-teinases (MMP)-2 and MMP-9: An in-vitro study. Ayu. 2014; 35: 85–89. doi: 10.4103/0974-8520.141947. [DOI] [PMC free article] [PubMed] [Google Scholar]