Abstract
Statement of the Problem:
Evidence shows thiabendazole has the potential to inhibit angiogenesis in melanoma and fibrosarcoma; however, its effect on oral squamous cell carcinoma has not been previously studied.
Purpose:
This study sought to assess the cytotoxic effects of thiabendazole on HN5 head and neck squamous carcinoma cell line.
Materials and Method:
HN5 cell lines were exposed to different concentrations of thiabendazole (prepared from 99% pure powder) for 24, 48 and 72 hours. Cell viability was assessed by the methyl thiazol tetrazolium assay, and IC50 of thiabendazole was calculated. Cells were also exposed to different concentrations of thiabendazole for 48 hours to determine its effect on expression and transcription of vascular endothelial growth factor gene. Expression of vascular endothelial growth factor mRNA was assessed by real-time polymerase chain reaction. The vascular endothelial growth factor release was assessed by the enzyme-linked immunosorbent assay test.
Results:
In all concentrations of thiabendazole except for 200 and 550μM, cell viability was significantly different at different time points (p< 0.05). At 48 and 72 hours, cell viability at all concentrations of thiabendazole (100-650μM) significantly decreased compared to the control group (zero concentration). In addition, cell viability significantly decreased with an increase in thiabendazole concentration. At 48 hours, expression of vascular endothelial growth factor mRNA was significantly lower in presence of 500μM thiabendazole compared to the control group (p< 0.001) and release of vascular endothelial growth factor was inhibited in a dose-dependent manner.
Conclusion:
Thiabendazole inhibited the proliferation of HN5 cells in a dose-dependent and time-dependent manner. It also inhibited the expression of vascular endothelial growth factor gene.
Keywords: Carcinoma , Squamous Cell , Vascular Endothelial Growth Factor , Angiogenesis Inhibitors , Thiabendazole
Introduction
Oral squamous cell carcinoma (OSCC) is a subgroup of oral and or pharyngeal squamous cell carcinomas (SCCs) and ranks the 11th most common cancer in humans. It accounts for 2-3% of all malignancies and 94% of oral malignancies. It is characterized by poor prognosis and low survival rate. Despite recent advances in treatment of OSCC, survival rate of patients has only slightly increased in the past couple of decades (40% in the 1950s to 59% at present); thus, OSCC is hard to treat.[1-5]
Angiogenesis plays a pivotal role in proliferation of tumoral cells and growth and metastasis of tumors.[6] The association of angiogenesis and tumor growth has long been acknowledged, and it is believed that tumors derive their blood supply from the preexisting blood vessels.[7] Vascular endothelial growth factor is an important signaling protein that stimulates angiogenesis.[8] Evidence shows that the level of vascular endothelial growth factor (VEGF) in OSCC is much higher than that in normal tissues.[9] Moreover, it has been documented that level of VEGF in metastatic tumors is much higher than that in primary tumors. Therefore, VEGF appears to be a critical factor for tumor growth and metastasis, and level of VEGF has a significant correlation with the stage of tumor and its progression.[9]
Considering all the above, targeting an angiogenic factor such as VEGF may prevent tumor growth. Thiabendazole (TBZ) (C10H7N3S) with a molecular weight of 201.24 g/mol was granted Food and Drug Administration (FDA) approval in 1967 as an anti-fungal and anti-helminthic drug. It is supplied in 500mg chewable tablets and is in clinical use for 40 years. Cha et al.[10] showed that TBZ decreased angiogenesis and vascular density by 50% in fibrosarcoma xenografts. Furthermore, they found that TBZ reversibly disassembled the newly formed blood vessels. Therefore, it may be suitable for use as a potential adjunct to the currently administered anti-angiogenic medications and chemotherapy drugs. Zhang et al.[9] also showed that TBZ inhibited angiogenesis in murine metastatic melanoma cell line B16F10 in vitro and in vivo via inhibiting the expression of vascular endothelial growth factor (VEGF) and inducing angiogenesis. In a recent study, Zhang et al.[11] optimized the structure of the lead compound of TBZ and obtained a new derivative, which they claimed to be over 100-fold more potent than the lead compound for inhibition of tumoral cell growth.
However, OSCC tumoral cells are more resistant to treatment and are less angiogenesis-dependent compared to other tumors.[12-13] thus, the efficacy of TBZ for inhibition of growth of OSCC is a matter of question. To the best of authors’ knowledge, the anti-angiogenic effects of TBZ on OSCC cell line have not been previously investigated. Considering the availability and affordability of this drug, this study sought to assess the cytotoxic effects of different concentrations of TBZ on HN5 head and neck SCC cell line. The effects of different concentrations of TBZ on expression and transcription of VEGF mRNA were also evaluated.
Materials and Method
This in vitro experimental study was conducted on HN5 SCC cell line.
Cell culture
The HN5 SCC cell line (code CI96) was obtained from the Pasture Institute of Iran and transferred to the cell laboratory of Tehran University of Medical Sciences for culture. The HN5 cells had been isolated from SCC of the tongue of a 73 year-old man with tumor stage T2N0M0 and moderate level of differentiation. [14] The HN5 cells were cultured in RPMI-1640 (Gibco, Germany) culture medium supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin and 100 U/mL streptomycin and incubated at 37°C and 5% CO2. Thiabendazole was obtained as 99% pure powder (Sigma Aldrich, St. Louis, MO, USA).
The MTT assay
The cytotoxic effects of different concentrations of TBZ (0-650μM) on HN5 cells were assessed using the MTT assay. The HN5 cells in exponential growth phase were seeded on a 96-well plate at a concentration of 5×103 cells per well in RPMI-1640 medium supplemented with 10% FBS. Different concentrations of TBZ (0-650μM; selected based on a pilot study and witnessing reduction in cell viability by 50%)were dissolved in 1% dimethyl sulfoxide, added to HN5 cells, and incubated at 37°C and 5% CO2 for 24, 48 and 72 hours. After completion of incubation, the overlaying medium was removed and HN5 cells were washed with phosphate buffered saline; 200μL of the culture medium containing 0.5 mg/mL MTT was added to the cells and they were incubated at 37°C and 5% CO2 for four hours. The cells were lysed after the completion of the afore-mentioned time period and purple formazan crystals were dissolved and read at 570nm wavelength using an enzyme-linked immunosorbent assay (ELISA) reader (Epoch/SN: 140416B/ Read speed: Normal, Delay: 100msec., Measurements/ Data point:8) (Figure 1).
Figure1.

Cell lysis after 48 hours
The percentage of cell viability was calculated using the formula below:
Cell viability (%)= ODA/ODB×100
Where ODA is the absorbance of the experimental groups at 570nm and ODB is the absorbance of the control group.
ELISA test
Standard solution was diluted. Bioassay Technology Laboratory kit, containing standard original concentrations, was used and five dilutions were prepared of the standard (6400 ng/L) concentration as follows: S5: 3200ng/L, S4: 1600ng/L, S3: 800ng/L, S2:400ng/L and S1:200ng/L.Three wells were allocated to each standard solution. Three blank wells were also considered. Only A and B chromogens and then the stop solution were added to blank wells. First, 50μL of the standard solution and then 50μL of streptavidin-HRP were added to the standard solution wells.
For the experimental sample wells, first 40μL of the sample and then 10μL of the VEGF antibody and 50μL of streptavidin-HRP were added. Plates were then sealed by a wrap and gently shaken; they were then incubated at 37°C for 60 minutes. After incubation, the wrap was removed, the overlaying medium was removed and the remaining solution was gently shaken. Each well was then filled with irrigating solution (30X diluted with distilled water), 30 seconds time was allowed and then the solution was extracted; this process was repeated for five times. Next, 50μL of chromogen A was added to each well and then 50μL of chromogen B was added. The wells were gently shaken and incubated at 37°C for 10 minutes. Afterwards, 50μL of the stop solution was added to each well to stop the reactions (the blue color immediately turned yellow). For the assay, blank well was considered as zero and optical density (OD) of each well at 450μM wavelength was measured. This was done 10 minutes after using the stop solution. According to the standard concentrations and the corresponding ODs, linear regression equation was calculated using the standard curve. Then the concentrations of the samples were calculated based on the OD values.
RNA extraction
The RNeasy Mini Kit (Qiagen Cat No. 52304, Germany) was used for RNA extraction. The cell culture content was centrifuged and the cell mass was rinsed with phosphate buffered saline twice. Extraction was done as instructed in the kit. The total RNA obtained of each well was dissolved in the buffer provided in the kit. The cell culture content included cancer cells without the drug, cancer cells exposed to 100μm TBZ, cancer cells exposed to 200μM TBZ, cancer cells exposed to 300μM TBZ, cancer cells exposed to 400μM TBZ and cancer cells exposed to 550μM TBZ. Thus, extraction was performed separately for each group. Briefly, cell mass was lysed by the solution provided in the kit. The lysate was homogenized using a 5cc syringe, ethanol was added and the lysate and possibly the precipitate were transferred to the column for RNA adhesion to the column. The column was washed to exclude excess material. The extracted RNA was eluted and quantified qualitatively and qualitatively using NanoDrop (Thermo Fisher Scientific, MA, USA). This method provides OD at 260nm and 280nm and the ratio of OD at 260/280nm wavelength must be approximately 1.8 to 2.
cDNA synthesis
cDNA was synthesized using Viva2-step RT-PCR kit (Cat No. RTPL12; Qiagen, Germany). The RT Primer Mix provided in the kit contained the components required for reverse transcription.
Designing specific primers
Specific primers for each marker were designed and ordered for synthesis using AlleleID7 software. The synthesized primers (Gene Fanavaran Company; Tehran, Iran) in the form of lyophilized powder were dissolved in autoclave-sterilized distilled water and entered into the final reaction. Table 1 shows the characteristics of the primers used in real-time RT-PCR reaction.
Table 1.
Characteristics of the primers used in real-time RT-PCR
| Gene | ||
|---|---|---|
| VEGF | 18s RNA | |
| NCBI number | NM_001025370 | X03205 |
| Initiator F | AAGGAGGAGGGCAGAATCAT | GTAACCCGTTGAACCCCATT |
| Primer length | 20 | 20 |
| Initiator R | ATCTGCATGGTGATG TTGGA | CCATCCAATCGGTAGTAGCG |
| Primer length | 20 | 20 |
| Segment length | 226 | 152 |
| Annealing temperature | 60 ℃ | 56.5℃ |
Conduction of real-time RT-PCR using EvaGreenHotTaqqPCR Mix Sina clone kit (Cat No. BT11101; Qiagen, Germany) was used for real time RT-PCR. EvaGreen fluorescent dye bonds to double-strand DNA and emits florescent light quantifiable by real-time device. Temperatures and reaction times were adjusted according to the manufacturer’s instructions. To find possible differences in relative expression of VEGF gene in the two groups, the ΔΔCt method was used, and 2 squared- ΔΔCt was applied to find possible differences in expression of VEGF mRNA.
Statistical analysis
Two-way ANOVA was applied to assess the effect of concentration of TBZ and exposure time on the viability of cells. Since the interaction effect of concentration and time on the viability of cells was significant (p< 0.001), one-way ANOVA was used to assess the effect of each factor on cell viability. Tukey’s test was used for pairwise comparison of the groups wherever the homogeneity of variances was met; otherwise, Dunnett’s T3 test was applied for this purpose. p< 0.05 was considered statistically significant.
Results
Comparison of cell viability at zero concentration of TBZ revealed significant differences between the three time points, and cell proliferation increased over time (p= 0.01 between 24 and 48 hours, p< 0.001 between 24 and 72 hours and p< 0.001 between 48 and 72 hours).
In 100μm concentration of TBZ, cell viability was significantly different at different time points (p= 0.02). Viability of HN5 cells at 72 hours was significantly higher than that at 48 hours (p= 0.002). However, no significant differences were noted in this regard between 72 and 24 hours (p= 0.17) or 48 and 24 hours (p= 0.54). In 200μm concentration of TBZ, cell viability was not significantly different at different time points (p= 0.12). In 300μm concentration of TBZ, cell viability was significantly different at different time points (p= 0.001). Viability of HN5 cells at 48 hours was significantly higher than that at 24 hours (p= 0.002) but no significant differences were noted at 72 hours compared to the values at 24 (p= 0.08) and 48 (p= 1.00) hours.
In 400μm concentration of TBZ, cell viability was significantly different at different time points (p= 0.002). Viability of HN5 cells at 48 hours was significantly higher than that at 24 hours (p< 0.001) but no significant differences were noted at 72 hours compared to the values at 24 (p= 0.13) and 48 (p= 0.42) hours.
In 500μm concentration of TBZ, cell viability was significantly different at different time points (p= 0.005). Viability of HN5 cells at 48 hours was significantly higher than that at 24 hours (p< 0.001) but no significant differences were noted at 72 hours compared to the value at 24 (p= 0.32) and 48 (p= 0.09) hours. In 550μm concentration of TBZ, cell viability was not significantly different at different time points (p= 0.30).
In 600μm concentration of TBZ, cell viability was significantly different at different time points (p= 0.006). Cell viability at 72 hours was significantly lower than that at 24 (p= 0.005) and 48 (p= 0.05) hours but no significant difference was noted in this regard at 24 and 48 hours (p= 0.16). At 650μm concentration of TBZ, cell viability was significantly different at different time points (p< 0.001) and cell viability decreased over time (p= 0.007 between 24 and 48 hours, p< 0.001 between 24 and 72 hours and p= 0.003 between 48 and 72 hours).
The mean percentage of cell viability at different concentrations in the three time points is presented in Table 2.
Table 2.
The mean and standard deviation (SD) of the percentage of cell viability in presence of different concentrations of TBZ at different time points
| Cell viability (%) | |||
|---|---|---|---|
| 24 hours (Mean±SD) | 48 hours (Mean±SD) | 72 hours (Mean±SD) | |
| Concentration of thiabendazole | |||
| 100mcM | 92.1±9.65 | 90.89±1.17 | 83.24±2.01 |
| 200mcM | 83.87±4.49 | 87.49±1.98 | 72.49±7.5 |
| 300mcM | 75.56±0.97 | 84.1±2.25 | 69.2±5.76 |
| 400mcM | 68.53±1.05 | 81.36±0.25 | 62.5±3 |
| 500mcM | 67.9±1.91 | 77.29±1.88 | 39.67±11.13 |
| 550mcM | 60.75±7.6 | 47.79±6.95 | 38.48±3.21 |
| 600mcM | 45.86±7.59 | 34.33±3.58 | 21.41±1.46 |
| 650mcM | 43.28±1.15 | 30.58±1.98 | 17.59±1.02 |
After 24 hours, 48 hours and 72 hours of incubation of HN5 cells with TBZ, the viability of cells exposed to different concentrations of TBZ was variable (all p< 0.001). The changes in cell viability in presence of different concentrations of TBZ are shown in Figure 2.
Figure2.
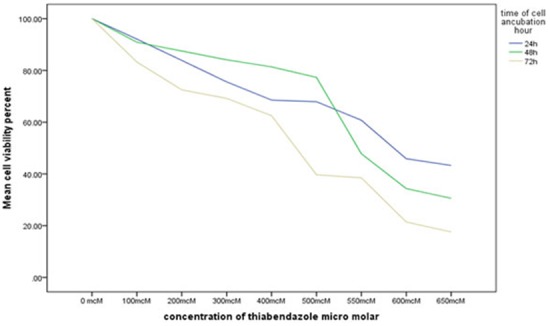
Comparison of the percentage of cell viability at different concentrations of TBZ at 24, 48 and 72 hours
The mean and SD of fold change for expression of VEGF mRNA in HN5 cells after 48 hours of incubation in presence of 0-650μM concentrations of TBZ are presented in Table 3.
Table 3.
The mean difference in VEGF mRNA expression in different concentrations of TBZ after real-time PCR at 48 hours
| Concentration of TBZ | Mean difference (fold change) | Standard error | p value | |
|---|---|---|---|---|
| 0 | 100 μM | 3.54 | 0.76 | 0.18 |
| μM | 4.29 | 0.89 | 0.17 | |
| 300 μM | 5.92 | 0.68 | 0.06 | |
| 400 μM | 7.31 | 0.30 | 0.01 | |
| 550 μM | 8.36 | 0.06 | <0.001 | |
| 100μM | 0 μM | -3.54 | 0.76 | 0.18 |
| 200 μM | .74 | 1.17 | 1.00 | |
| 300 μM | 2.37 | 1.02 | 0.46 | |
| 400 μM | 3.77 | 0.82 | 0.14 | |
| 550 μM | 4.82 | 0.76 | 0.10 | |
| 200μM | 0 μM | -4.29 | 0.89 | 0.17 |
| 100 μM | -.74 | 1.17 | 1.00 | |
| 300 μM | 1.63 | 1.11 | 0.84 | |
| 400 μM | 3.03 | 0.94 | 0.29 | |
| 550 μM | 4.08 | 0.89 | 0.18 | |
| 300μM | 0 μM | -5.92 | 0.68 | 0.06 |
| 100 μM | -2.37 | 1.02 | 0.46 | |
| 200 μM | -1.63 | 1.12 | 0.84 | |
| 400 μM | 1.40 | 0.74 | 0.66 | |
| 550 μM | 2.45 | 0.68 | 0.28 | |
| 400μM | 0 μM | -7.32 | 0.30 | 0.01 |
| 100 μM | -3.77 | 0.82 | 0.14 | |
| 200 μM | -3.03 | 0.94 | 0.29 | |
| 300 μM | -1.40 | 0.74 | 0.66 | |
| 550 μM | 1.05 | 0.30 | 0.30 | |
| 550μM | 0 μM | -8.36 | 0.06 | <0.001 |
| 100 μM | -4.82 | 0.76 | 0.10 | |
| 200 μM | -4.08 | 0.89 | 0.18 | |
| 300 μM | -2.45 | 0.68 | 0.28 | |
| 400 μM | -1.05 | 0.31 | 0.30 |
Real-time PCR conducted after 48 hours of incubation of cells with different concentrations of TBZ revealed variable expressions of VEGF mRNA (p< 0.001), and significant differences were noted between 0 and 400 (p= 0.01) and 0 and 550μM (p< 0.001) concentrations. No other significant differences were noted (all p> 0.05) (Figure 3a). Assessment of VEGF production by HN5 cells after 48 hours of incubation with different concentrations of TBZ revealed that by an increase in concentration of TBZ, production of VEGF decreased (Figure 3b).
Figure3.
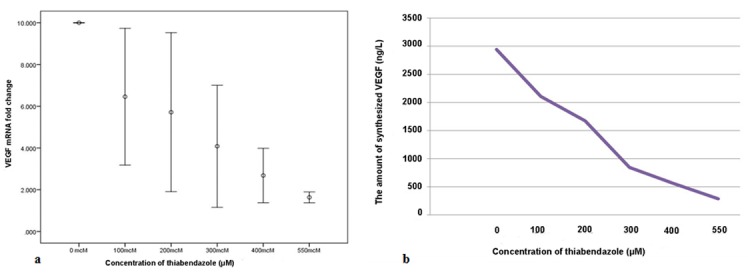
a: Comparison of VEGF mRNA expression in presence of different concentrations of TBZ at 48 hours, b: Comparison of VEGF production by HN5 cells in presence of different concentrations of TBZ
Discussion
The anti-angiogenic and growth inhibition effects of TBZ on OSCC have not been well investigated. Thus, we hypothesized that VEGF in HN5 SCC cell line may be targeted by TBZ. Our results showed that TBZ inhibited the proliferation of HN5 cell line in a dose- and time-dependent manner. At 72 hours, in presence of 500μM concentration of TBZ, the percentage of cell viability decreased to 39% of that in the control group (IC50). At 48 hours, in presence of 550μM concentration of TBZ, the percentage of viable cells decreased by 47% of the baseline concentration, this value was 45% in presence of 600μM TBZ at 24 hours.
Zhang et al.[9] evaluated the effect of TBZ on B16 and B16F10 (malignant melanoma) cell lines revealed that HN5 cells were more resistant to TBZ. In presence of 300μM TBZ, both malignant melanoma cell lines (B16 and B16F10) reached IC50 concentration at 72 hours. However, HN5 cell line in presence of 500μM concentration was decreased by 39.6% of its baseline concentration at 72 hours. This may indicate higher resistance of OSCC cells to treatment with TBZ and consequently poor prognosis of OSCC. In addition, Zhang et al.[9] showed that increase in drug concentration and time lapse decreased the proliferation of B16 and B16F10 cell lines. They also noticed that TBZ inhibited tumor growth in the B16F10-bearing mice model. In our study, in presence of 200, 300, 400 and 500μM concentrations of TBZ, HN5 cell line showed increased proliferation at 48 hours compared to 24 hours; while in 550, 600 and 650μM concentrations of TBZ, proliferation of HN5 cells decreased with time. Evidence shows that the doubling time of HN5 cells is 34 hours;[15] thus, cells at 24 hours have yet to reach their doubling time. At 48 hours, cells are in logarithmic phase while at 72 hours, cells gradually reach their death phase. Thus, among the afore-mentioned three time points, 48 hours would be a more appropriate time for testing.[14-15]
In a study by Cha et al.,[10] TBZ in 250μM concentration inhibited angiogenesis. Proliferation of human umbilical vein endothelial cells was also inhibited by 100-250μM concentrations of TBZ (equal to 20-50mg/ kg). Effect of TBZ on implanted fibrosarcoma in mice (as a highly angiogenesis-dependent tumor) was evaluated in another study[16] and it was shown that after 27 days of treatment with 50mg/kg TBZ (equal to 250mg/kg), size of fibrosarcoma tumor in the test group decreased by more than 50% compared to that in controls.
Zhang et al.[9] showed that in the B16F10-bearing mice model, 100-400μM concentrations of TBZ (20, 40, 80mg/kg) caused 16.5%, 35.4% and 48.7% reduction in tumor growth, respectively. The concentrations of TBZ used by Zhang et al.[9] on melanoma were higher than concentrations used by Cha et al.[10] on fibrosarcoma. This increase in concentration can be explained by the fact that fibrosarcoma is a mesenchymal tumor that contains numerous blood vessels.[17] Thus, it is more dependent on angiogenesis than melanoma and consequently, fibrosarcoma is more susceptible to anti-angiogenic drugs like TBZ. Based on this theory, it is speculated that since OSCC is resistant to treatment and less dependent on angiogenesis, [18] higher concentrations of TBZ will be probably required for inhibition of tumor growth and proliferation of tumoral cells in animal models. The mechanism of action of TBZ is not well understood. However, it has been shown that this drug inhibits fumarate reductase and results in cell death as such.[10]
Comparison of the results of real time PCR of B16F10 cells in the study by Zhang et al. [9] and HN5 cells in the current study reveals that VEGF mRNA expression in B16F10 cells significantly decreased in presence of 300 and 500μM concentrations of TBZ compared to the control group. However, in our study, significant reduction in VEGF mRNA expression in HN5 cells was noted in presence of 400 and 550μM concentrations of TBZ compared to the control group. Inhibition of VEGF mRNA expression by higher concentrations of TBZ in our study compared to that of Zhang et al.[9] further confirms that head and neck tumors are less dependent on angiogenesis compared to tumors like melanoma, which are highly angiogenesis-dependent. This finding has been reported in previous studies as well.[19-20]
The results of ELISA showed that TBZ inhibited the production of VEGF protein in a dose-dependent manner. In presence of 300μM concentration of TBZ, level of VEGF decreased by more than 50% (840ng/L) of its level in zero concentration of TBZ (2942mg/L). In presence of 550μM, level of VEGF reached to 283ng/L, which was one-tenth of its baseline level. Real-time PCR revealed that level of VEGF mRNA expression in presence of 300μM concentration of TBZ decreased by more than 50% of its value in the control group, which was equal to 10-fold change in its level.
Maximum safe dose of TBZ for administration in humans is 3g/day, which is equal to 50mg/kg (250μM) in a patient weighing 60kg. However, in our study, TBZ in 550μM concentration decreased the proliferation of HN5 cells by 50% at 48 hours. But, it should be noted that our study had an in vitro design and significant differences exist in drug pharmacokinetics in the human body and animals and in the culture medium with regard to protein-drug interactions, level of free drug, drug excretion, site of administration, and dose of administered drug at the target site. Thus, it should be kept in mind that effective concentration of drug in vitro is not equal to that in vivo since in the human body, part of the administered drug binds to plasma proteins and only free drug can affect cells.
To convert the IC50 dose of a drug in vitro to its equivalent in vivo, the above-mentioned pharmacokinetic properties must be evaluated in animal studies and then the required dose to reach the desired concentration of drug in vivo can be estimated. Another technique, which is based on try and error, is that the IC50 concentration in vitro can be injected to animal models to assess the magnitude of its inhibitory effect on tumor growth and then the dosage can be modified based on the observed results.
Considering the observed inhibitory effect of TBZ on HN5 cells and VEGF mRNA expression, it may be effective for inhibition of OSCC growth in the clinical setting. Future studies on animal models are required to determine the effective drug dosage for potential use as an adjunct to other chemotherapy drugs. In addition, the efficacy of TBZ in conjunction with other medications with confirmed anti-angiogenic properties such as Avastin should be evaluated in future studies.
Conclusion
Within the limitations of this study, the results showed that TBZ inhibited the proliferation of HN5 cells in a dose- and time-dependent manner. Expression of VEGF mRNA and production of VEGF protein were also inhibited by TBZ in a dose-dependent manner. Such promising findings can pave the way towards the use of TBZ as an efficient anti-cancer drug in the clinical setting.
Footnotes
Conflict of interests: The authors disclose no potential conflicts of interest.
References
- 1.Chen JK, Katz RV, Krutchkoff DJ. Intraoral squamous cell carcinoma. Epidemiologic patterns in Connecticut from 1935 to 1985. Cancer 1990; 66: 1288–1296. doi: 10.1002/1097-0142(19900915)66:6<1288::aid-cncr2820660632>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 2.Llewellyn CD, Johnson NW, Warnakulasuriya KA. Risk factors for squamous cell carcinoma of the oral cavity in young people--a comprehensive literature review. Oral Oncol. 2001; 37: 401–418. doi: 10.1016/s1368-8375(00)00135-4. [DOI] [PubMed] [Google Scholar]
- 3.Silverman S Jr. Demographics and occurrence of oral and pharyngeal cancers. The outcomes, the trends, the challenge. J Am Dent Assoc. 2001; 132 Suppl: 7S–11S. doi: 10.14219/jada.archive.2001.0382. [DOI] [PubMed] [Google Scholar]
- 4.Silverman S Jr, Gorsky M. Epidemiologic and demographic update in oral cancer: California and national data--1973 to 1985. J Am Dent Assoc. 1990; 120: 495–499. doi: 10.14219/jada.archive.1990.0082. [DOI] [PubMed] [Google Scholar]
- 5.Swango PA. Cancers of the oral cavity and pharynx in the United States: an epidemiologic overview. J Public Health Dent. 1996; 56: 309–318. doi: 10.1111/j.1752-7325.1996.tb02458.x. [DOI] [PubMed] [Google Scholar]
- 6.Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dyeprocedure giving improved sensitivity and reliability. J Immunol Methods. 1986; 89: 271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- 7.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004; 25: 581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 8.Roskoski R Jr. Vascular endothelial growth factor (VEGF) signaling in tumor progression. Crit Rev Oncol Hematol. 2007; 62: 179–213. doi: 10.1016/j.critrevonc.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, Zhao C, Gao Y, Jiang Y, Liang H, Zhao G. Thiabendazole, a well-known antifungal drug, exhibits anti-metastatic melanoma B16F10activity via inhibiting VEGF expression and inducing apoptosis. Pharmazie. 2013; 68: 962–968. [PubMed] [Google Scholar]
- 10.Cha HJ, Byrom M, Mead PE, Ellington AD, Wallingford JB, Marcotte EM. Evolutionarily repurposed networks reveal the well-known antifungal drug thiabendazole to be a novel vascular disrupting agent. PLoS Biol. 2012; 10: e1001379. doi: 10.1371/journal.pbio.1001379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang C, Zhong B, Yang S, Pan L, Yu S, Li Z, et al. Synthesis and biological evaluation of thiabendazole derivatives as anti-angiogenesis and vascular disrupting agents. Bioorg Med Chem. 2015; 23: 3774–3780. doi: 10.1016/j.bmc.2015.03.085. [DOI] [PubMed] [Google Scholar]
- 12.Shieh YS, Lee HS, Shiah SG, Chu YW, Wu CW, Chang LC. Role of angiogenic and non-angiogenic mechanisms in oral squamous cell carcinoma: correlation with histologic differentiation and tumor progression. J Oral Pathol Med. 2004; 33: 601–606. doi: 10.1111/j.1600-0714.2004.00252.x. [DOI] [PubMed] [Google Scholar]
- 13.Kukreja I, Kapoor P, Deshmukh R, Kulkarni V. VEGF and CD 34: A correlation between tumor angiogenesis and microvessel densityan immunohistochemical study. J Oral Maxillofac Pathol. 2013; 17: 367–373. doi: 10.4103/0973-029X.125200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Easty DM, Easty GC, Carter RL, Monaghan P, Butler LJ. Ten human carcinoma cell lines derived from squamous carcinomas of the head and neck. Br J Cancer. 1981; 43: 772–785. doi: 10.1038/bjc.1981.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin CJ, Grandis JR, Carey TE, Gollin SM, Whiteside TL, Koch WM, et al. Head and neck squamous cell carcinoma cell lines: established models and rationale for selection. Head Neck. 2007; 29: 163–188. doi: 10.1002/hed.20478. [DOI] [PubMed] [Google Scholar]
- 16.Goldmann E. The Growth of Malignant Disease in Man and the Lower Animals, with special reference to the Vascular System. Proc R Soc Med. 1908; 1(Surg Sect): 1–13. [PMC free article] [PubMed] [Google Scholar]
- 17.McDonough SP, MacLachlan NJ, Tobias AH. Canine pericardial mesothelioma. Vet Pathol. 1992; 29: 256–260. doi: 10.1177/030098589202900312. [DOI] [PubMed] [Google Scholar]
- 18.Curry JM, Sprandio J, Cognetti D, Luginbuhl A, Barad V, Pribitkin E, et al. Tumor microenvironment in head and neck squamous cell carcinoma. Semin Oncol. 2014; 41: 217–234. doi: 10.1053/j.seminoncol.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Gleich LL, Biddinger PW, Duperier FD, Gluckman JL. Tumor angiogenesis as a prognostic indicator in T2-T4 oral cavity squamous cell carcinoma: a clinical-pathologic correlation. Head Neck. 1997; 19: 276–280. doi: 10.1002/(sici)1097-0347(199707)19:4<276::aid-hed5>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 20.Streit M, Detmar M. Angiogenesis, lymphangiogenesis, and melanoma metastasis. Oncogene. 2003; 22: 3172–3179. doi: 10.1038/sj.onc.1206457. [DOI] [PubMed] [Google Scholar]


