Abstract
Background
Residents of nursing homes may have low 25-hydroxyvitamin D (25OHD) concentrations. Associations between vitamin D and cognitive performance, dementia and susceptibility to infections are not clearly established. The aim of this study was to investigate the prevalence of vitamin D deficiency and to identify associated factors among residents of nursing homes for elderly.
Methods
In this cross-sectional study blood samples for analysis of 25OHD were collected from all participating residents of Swedish nursing homes for the elderly from January to March 2012.
Exclusion criteria: dementia too severe to collect a blood test, terminally ill or refusing participation. Outcome Measures: Serum 25OHD concentrations. Logistic regression to evaluate factors associated with vitamin D deficiency (25OHD < 25 nmol/L).
Results
Blood samples were obtained from 545 of 901 residents of 22 nursing homes. Mean age 86 years (SD 6.9), 68% were women. Prevalence of vitamin D supplementation 17%, dementia 55%, lack of appetite ≥3 months 45% and any antibiotic treatment during the last 6 months 30%. Serum 25OHD concentrations: mean 34 nmol/L (SD 21, median 27, range 4–125), 82% (448/545) had 25OHD < 50 nmol/L and 41% (224/545) had 25OHD < 25 nmol/L. Adjusted OR (95% CI; p-value) for possible predictors of vitamin D deficiency (25OHD < 25 nmol/L): vitamin D supplementation 0.075 (0.031–0.18; p < 0.001), lack of appetite ≥3 months 0.75 (0.50–1.1; p = 0.15), hours outdoors/week 0.99 (0.96–1.0; p = 0.62), Fitzpatrick skin phototype (4–6) 0.69 (0.44–1.1; p = 0.12); dementia 2.3 (1.5–3.4; p < 0.001) and antibiotics last 6 months 1.6 (1.1–2.6; p < 0.029), adjusted for age and gender.
Conclusions
Vitamin D deficiency was common among nursing home residents and strongly associated with dementia. Regardless of causality or not, it is important to be alert for vitamin D deficiency in nursing homes residents with dementia. As expected vitamin D supplementation was associated with less vitamin D deficiency, however lack of appetite, staying outdoors and skin phototype were not significant predictors. Antibiotic treatments during the last 6 months were associated with vitamin D deficiency, potentially supporting the hypothesis that vitamin D deficiency is associated with infections.
Keywords: Vitamin D, Homes for the aged, Nursing homes, Frail elderly, Dementia, Infectious disease, Antibiotics
Background
Vitamin D refers to a group of fat-soluble molecules, from which the most important in humans are vitamin D3 (cholecalciferol) and vitamin D2 (ergocalciferol) [1]. A small amount of these can be obtained from the diet, but about 90% comes from synthesis of vitamin D in the skin when exposed to ultraviolet B radiation from the sun [2, 3]. This dermal synthesis depends on the area of skin exposed to sunlight, duration of exposure, latitude, season and skin type [4, 5]. In Sweden, latitude 55–69° North, there is no dermal synthesis of vitamin D during the winter months [1, 4]. Vitamin D has a significant role in mineralization of bone, skeletal maturation, regulating the concentration of calcium and phosphate, therefore severe vitamin D deficiency can cause osteomalacia in adults and rickets in pediatric population [6]. Vitamin D deficiency is a known risk factor causing fractures, osteoporosis and muscle weakness in elderly [5, 7, 8].
Vitamin D deficiency might be associated with reduced cognitive functions and dementia, but further research is required regarding this [9–14]. Vitamin D receptors have been discovered throughout the body including the nervous system, the cardiovascular and endocrine system causing biological actions including inflammation and amyloid plaque formation in the brain, potentially related to cognitive decline and Alzheimer’s disease, and inhibition of vascular smooth muscles proliferation potentially related to cardiovascular disease [15]. Vitamin D also affects the immune system [16–20], therefore it is important to study if elderly residents of nursing homes with vitamin D deficiency also have more infections. Several studies during recent years have observed associations between vitamin D deficiency and several medical conditions such as cognitive decline, cancer, diabetes, cardiovascular mortality, autoimmune diseases, metabolic diseases, neurological diseases, lung diseases and increases mortality rate [3, 13–15, 21–27].
There is still a lack of consensus worldwide regarding the optimum serum concentrations of vitamin D [28–30]. A suggestion is to consider 25-hydroxyvitamin D (25OHD) < 12.5 nmol/L as severe deficiency, 12.5–25 nmol/L as moderate deficiency and 25–50 nmol/L as mild deficiency [31]. It was recommended during a meeting among European acknowledged experts in the field of vitamin D that elderly (>65 years) should have 25OHD ≥ 50 nmol/L [29] which corresponds with the Nordic Nutrition Recommendations [32]. Other studies suggest 25OHD ≥ 75 nmol/L as optimum serum concentration [33, 34]. Since there is debate over optimal concentrations of 25OHD we report prevalence of deficiency at a range of values and elected to use an arbitrary cut point of 25 for analysis of predictors.
Several international studies have shown low serum concentrations of vitamin D in elderly individuals and the concentrations have been even lower among those living in nursing homes and long term care facilities [19, 24, 29, 35–43]. Elderly people are at risk for lower concentrations of vitamin D as a result of decreased dietary intake, decreased cutaneous synthesis and less time spent outdoors [15]. A difference is expected in serum concentrations of vitamin D when comparing populations of different countries due to variations in fortification of food, tradition regarding use of supplements, the dominant skin type within the population, amount of sunlight exposure and nutritional status. There is one recently reported study regarding the prevalence of vitamin D deficiency among the elderly residents of Swedish nursing homes [24]. However, in this study the blood samples were collected during different seasons of the year and many of them were collected when the elderly moved into the nursing homes, thus expected to have higher serum concentrations of vitamin D. Therefore, further studies are required in Sweden regarding the prevalence of vitamin D deficiency among elderly residents of nursing homes. It is also unknown whether previously known risk factors for vitamin D deficiency such as less time spent outdoors, lack of appetite and dark skin color are significant risk factors also for elderly residents of Swedish nursing homes. There is a need for further research on possible associations between vitamin D deficiency and dementia and susceptibility to infections.
The aim of this study was to investigate the prevalence of vitamin D deficiency and to identify associated factors among residents of nursing homes for elderly.
Methods
During the first 3 months of 2012, a case report form was completed and blood samples collected from all included residents of 22 nursing homes in south-western Sweden (latitude 57.58° North to 57.82° North). The attending nurses were provided detailed verbal and written information for the study procedure. The study was approved by the Regional ethical review board of Gothenburg University (D-nr 578–11). The data was collected along with another study of interleukin-6 concentrations in the urine and antimicrobial resistance in urinary pathogens among Swedish nursing home residents [44, 45].
Inclusion and exclusion criteria
All residents of the participating nursing homes were invited to participate. Those accepting participation were included if they met the following inclusion criteria:
Permanent residence in nursing homes for the elderly, regardless of gender and for how long time they have been residing there
Presence at a nursing home for the elderly during the study
Participation approval
Residents with dementia were included if cooperative when collecting blood samples
Not terminally ill
The following exclusion criterion was used:
If the resident did not agree to participate or discontinued study participation
Statement of consent
All residents were informed of the study both verbally and in writing. From decision-capable individuals choosing to participate in the study, informed approval was collected. However, a considerable number of participants consisted of residents with dementia of varying degrees. If the resident was incapable of understanding the given information and thereby possessing a reduced decision capability, these residents only participated so long as they both: Did not oppose participation in the study and that the appointed representatives or relatives did not oppose their participation after being provided with information about the study. The Regional ethical review board of Gothenburg University approved this procedure.
Case report form
In addition to collecting the blood sample, the attending nurse made an entry in the case report form for each included resident regarding: age, gender, vitamin D supplementation or not, lack of appetite in the last 3 months, diagnosis of dementia in the medical record and if the resident had received any antibiotic treatment during the last 6 months as a marker of potential bacterial infection. The diagnosis of dementia in the medical records required a previous comprehensive history and physical examination by a physician, laboratory tests, cognitive function test and most often neuroimaging. The average number of hours outdoors per week during April to August was registered, this is the period during which the sun exposure at our northern latitude is sufficient to induce the vitamin D synthesis in the skin. Regarding skin phototype, a simplified Fitzpatrick skin phototype [46] was used; Fitzpatrick skin phototype 1–3 was registered as lighter skin phototype and 4–6 was registered as darker skin phototype.
Laboratory tests
The attending nurse collected blood samples from each participating resident. The blood samples were analysed at the laboratory of clinical chemistry at the Södra Älvsborg Hospital in Borås, Sweden, using routine clinical procedure. The blood samples were chilled before transport and usually arrived at the laboratory within 24 h. Measurements of the concentrations of 25OHD in serum were analyzed by the LIAISON® 25 OH Vitamin D TOTAL Assay (DiaSorin Inc., Stillwater, USA) which uses chemiluminescent immunoassay (CLIA) technology for the quantitative determination of 25-hydroxyvitamin D in human serum. The measuring range of DiaSorin LIAISON® 25 OH Vitamin D assay was 4.0–150 ng/mL. This analysis was accredited at the performing laboratory: the laboratory of clinical chemistry at the Södra Älvsborg Hospital in Borås, Sweden.
Statistical analysis
The first objective was to describe the population by number of individuals, age, gender, 25OHD concentrations, vitamin D supplementation, Fitzpatrick skin phototype, average number of hours outdoors per week during April to August, lack of appetite ≥3 months, antibiotics during the last 6 months and having dementia or not.
The second objective was to calculate the prevalence of vitamin D deficiency defined by four different cut-off values: 25OHD < 12.5 nmol/L, 25OHD < 25 nmol/L, 25OHD < 50 nmol/L and 25OHD < 75 nmol/L. This was calculated for all residents and for residents divided by having vitamin D supplementation or not. The prevalence of vitamin D deficiency (25OHD < 25 nmol/L) was compared between residents with or without dementia using Pearson’s chi-square test.
The third objective was to clarify whether previously known factors for vitamin D deficiency were associated with vitamin D deficiency (25OHD < 25 nmol/L), as well as dementia and antibiotic treatments during the last 6 months, in a nursing home population, adjusted for age and gender. Adjusted and unadjusted logistic regressions were performed with vitamin D deficiency (25OHD < 25 nmol/L) as the dependent variable and the following independent variables: age, gender, vitamin D supplementation, Fitzpatrick skin phototype (4–6), number of hours outdoors per week, lack of appetite ≥3 months, dementia and any antibiotic treatments during the last 6 months. Zero order correlations between independent variables were checked and correlations >0.6 were not allowed.
Statistical significance was considered p < 0.05. IBM SPSS Statistics version 22 was used for statistical analysis (IBM Corporation, Armonk, New York, United States).
Results
Studied population
Inclusion criteria were fulfilled by 836 of 901 residents in 22 nursing homes, and 596/836 (71%) accepted participation (Fig. 1). Blood samples and case report forms were provided from 545 residents, 370 (68%) women and 175 (32%) men. Mean age was 86 years (SD 6.9), range 56–102. Women (mean 87 years, SD 6.7, range 62–100) were slightly older than men (mean 85 years, SD 7.1, range 56–102) (p = 0.0039).
Fig. 1.
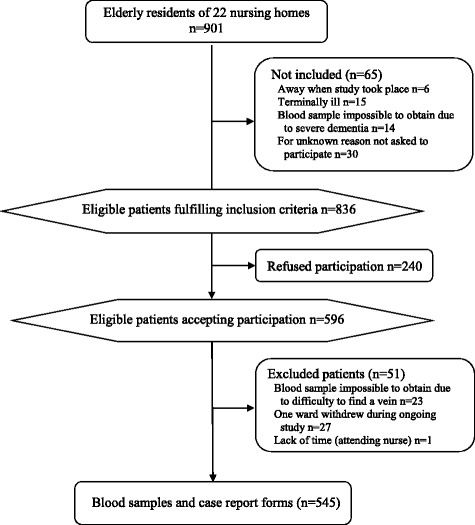
Participant flow chart
Among participating residents 55% (299/545) had dementia, 30% (164/543) had received any antibiotic treatment during the previous 6 months (this was unknown for 2 participants) and 45% (247/545) had lack of appetite ≥3 months. There was no association between lack of appetite ≥3 months and diabetes (p = 0.49), dementia (p = 0.55), rheumatic diseases (p = 0.15), cancer (p = 0.15) and pressure ulcers (p = 0.73). The average number of hours outdoors per week during April to August was known for 489 residents: mean 6.6 h per week (SD 7.1), median 5.0 h per week and range 0–50 h. Twenty percent of the residents spent ≤1.0 h outdoors per week during April to August. Fitzpatrick skin phototype was known for 541 residents, 71% (385/541) had phototype 1–3 and 29% (156/541) had phototype 4–6. Vitamin D supplementation was recorded in 17% (94/545) and of these 85% (80/94) had colecalciferol (vitamin D3) and 15% (14/94) had ergocalciferol (vitamin D2).
Serum 25OHD concentrations
Among all residents, regardless of vitamin D supplementation: 3.7% (20/545) had 25OHD < 12.5 nmol/L, 41% (224/545) had 25OHD < 25 nmol/L, 82% (448/545) had 25OHD < 50 nmol/L and 94% (510/545) had 25OHD < 75 nmol/L. The number of residents in between the different cut-off values are presented in Table 1, as well as residents with and without vitamin D supplementation separate.
Table 1.
Serum 25-hydroxyvitamin D (25OHD) concentrations among nursing home residents
| All residentsa | Residents without vitamin D supplementation | Residents with vitamin D supplementation | |
|---|---|---|---|
| 25OHD < 12.5 nmol/L | 3.7% (20/545) | 4.4% (20/451) | 0% (0/94) |
| 25OHD 12.5 to <25 nmol/L | 37% (204/545) | 44% (198/451) | 6.4% (6/94) |
| 25OHD 25 to <50 nmol/L | 41% (224/545) | 45% (203/451) | 22% (21/94) |
| 25OHD 50 to <75 nmol/L | 11% (62/545) | 4.9% (22/451) | 43% (40/94) |
| 25OHD > 75 nmol/L | 6.4% (35/545) | 1.8% (8/451) | 29% (27/94) |
aIn total 545 residents: 451 residents without vitamin D supplementation and 94 residents with vitamin D supplementation
Residents with dementia had lower 25OHD concentrations (mean 31 nmol/L, SD 19) compared to residents without dementia (mean 38 nmol/L, SD 22) (p < 0.001). Vitamin D deficiency defined as 25OHD < 25 nmol/L was more common (p < 0.001) among residents with dementia; 51% (151/299) versus 30% (73/246) in residents without dementia.
Factors associated with vitamin D deficiency
Adjusted OR (95% CI; p-value) for possible predictors of vitamin D deficiency (25OHD < 25 nmol/L): vitamin D supplementation 0.075 (0.031–0.18; p < 0.001), lack of appetite ≥3 months 0.75 (0.50–1.1; p = 0.15), hours outdoors/week 0.99 (0.96–1.0; p = 0.62), Fitzpatrick skin phototype (4–6) 0.69 (0.44–1.1; p = 0.12), dementia 2.3 (1.5–3.4; p < 0.001) and antibiotics last 6 months 1.6 (1.1–2.6; p < 0.029), adjusted for age and gender (Table 2).
Table 2.
Factors associated with serum 25-hydroxyvitamin D (25OHD) < 25 nmol/L
| Unadjusted odds ratioa(95% CI; p-value) | Adjusted odds ratiob (95% CI; p-value) | |
|---|---|---|
| Age | 0.98 (0.96–1.0; p = 0.12) | 0.98 (0.95–1.0; p = 0.22) |
| Genderc | 1.1 (0.74–1.5; p = 0.72) | 1.4 (0.92–2.2; p = 0.12) |
| Vitamin D supplementation | 0.073 (0.031–0.17; p < 0.001) | 0.075 (0.031–0.18; p < 0.001) |
| Fitzpatrick skin phototype (4–6)d | 0.54 (0.36–0.80; p = 0.0022) | 0.69 (0.44–1.1; p = 0.12) |
| Hours outdoors/weeke | 0.98 (0.96–1.0; p = 0.15) | 0.99 (0.96–1.0; p = 0.62) |
| Lack of appetite ≥3 months | 0.93 (0.66–1.3; p = 0.66) | 0.75 (0.50–1.1; p = 0.15) |
| Dementia | 2.4 (1.7–3.4; p < 0.001) | 2.3 (1.5–3.4; p < 0.001) |
| Antibiotics last 6 months | 1.2 (0.85–1.8; p = 0.28) | 1.6 (1.1–2.6; p = 0.029) |
aN = 545 for age, gender, vitamin D supplementation, lack of appetite and dementia. N = 541 for Fitzpatrick skin phototype. N = 489 for hours outdoors/week. N = 543 for antibiotics last 6 months
bN = 488 included in analysis. Adjusted logistic regressions with 25OHD < 25 nmol/L as the dependent variable and the following independent variables: age, gender, vitamin D supplementation, Fitzpatrick skin phototype, hours outdoors/week, lack of appetite, dementia and antibiotics last 6 months
cReference category: male
dReference category: Fitzpatrick skin phototype 1–3
eAverage number of hours outdoors/week during April to August
Statistically significant findings are bold
Discussion
Vitamin D deficiency was common among nursing home residents and strongly associated with dementia. As expected vitamin D supplementation was associated with lower chance of vitamin D deficiency, however appetite, staying outdoors and skin phototype were not significant predictors in the model. Antibiotic treatments during the last 6 months were associated with vitamin D deficiency.
Strengths and limitations of the study
A strength of this study is that blood samples were collected from every resident accepting participation and of whom it was possible to get a blood sample during three winter months (January to March). However, 240 of the individuals registered at the nursing homes refused participation. We obtained blood samples and study protocols from 60% (545/901) of all individuals registered at the nursing homes, those not fulfilling inclusion criteria are also included in the total number of registered individuals (Fig. 1). This may appear low but it is similar or higher than previously published studies in nursing homes [24, 35, 43]. Still this may be considered acceptable when studying an elderly frail population with a high proportion of residents with dementia as well as the ethical requirement of approval from appointed representative/relatives.
It is a strength that information was collected about appetite, diagnosis of dementia, antibiotic treatments during the last 6 months (as a marker of potential bacterial infection), skin type and the average number of hours outdoors per week during April to August. As the duration of lack of appetite is of importance we have only registered lack of appetite if the duration was ≥3 months. However, it is a weakness that we did not register the total duration of lack of appetite.
There is a lack of consensus regarding the optimum serum concentrations of vitamin D [28, 29], therefore we calculated the prevalence of vitamin D deficiency by four different cut-off values: 25OHD < 12.5 nmol/L, 25OHD < 25 nmol/L, 25OHD < 50 nmol/L and 25OHD < 75 nmol/L. When evaluating factors associated with vitamin D deficiency we used only one cut-off value, 25OHD < 25 nmol/L, representing moderate/severe vitamin D deficiency [31].
According to previous studies vitamin D deficiency might be associated with reduced cognitive functions and dementia [9–14], also supported by our study where 25OHD < 25 nmol/L was strongly associated with dementia (p < 0.001, Table 2). However, with this study design we cannot tell whether vitamin D deficiency caused/worsened dementia, or if it is the other way around, that dementia caused vitamin D deficiency. Although dementia diagnosis was the result of clinical findings and investigation it was not standardised and so precise diagnostic criteria will be subject to some variation.
Antibiotic treatments during the last 6 months, as a marker of potential bacterial infection, were associated with vitamin D deficiency (p = 0.029, Table 2). This potentially supports the hypothesis that vitamin D deficiency is associated with susceptibility to infections [16–20]. A weakness regarding this result is that the unadjusted OR was not significant (p = 0.28, Table 2), therefore the association needs to be interpreted with caution.
Decreased renal function is one factor affecting the vitamin D concentrations, however in this study laboratory tests regarding renal function were not taken [25].
Comparison with existing literature
Vitamin D deficiency was common among Swedish nursing home residents in this study, as well as in previous studies in other countries [19, 24, 29, 35–43]. As expected vitamin D supplementation predicted less vitamin D deficiency. However appetite, staying outdoors and skin phototype were not significant predictors in the model, which differ from previous studies of younger individuals. Possibly these factors are less important in this population since they stay outdoors only for a few hours a week, likely with clothes covering most of their skin or beneath the sunshade. Also the nutritional status and appetite is generally decreased in this population, which might explain why lack of appetite did not influence the vitamin D concentrations in this study. The results are important in clinical practice as it provides knowledge about vitamin D deficiency, since elderly can have vitamin D deficiency regardless of more time spent outdoors and a relatively good appetite.
There is one recent study regarding vitamin D deficiency among elderly residents of Swedish nursing homes. This study also showed high prevalence of vitamin D deficiency, but only 14% of the 333 participating residents had 25OHD < 25 nmol/L at inclusion [24], compared to 41% in our study. However, the aims and study designs differed between these two studies. As the aim of the previous study was to examine whether low levels of 25OHD were associated to an increased risk of mortality, baseline samples were obtained when residents were moving into the nursing homes. These new residents were expected to have higher serum concentrations of vitamin D compared to residents who have lived at the nursing home for a longer time. The previous study also included patients throughout the years 2007 to 2011 and there were seasonal changes in 25OHD concentrations. In our study all blood samples were taken during January to March in a single year.
Implications for research and practice
Clinicians have to be aware of the high prevalence of vitamin D deficiency among elderly residents of nursing homes. Vitamin D deficiency was strongly associated with dementia, therefore there is need for future studies to clarify if there is a causal relationship between vitamin D deficiency and dementia. Regardless of causality or not, it is important to be alert for vitamin D deficiency in residents with dementia as half of them had 25OHD < 25 nmol/L. As antibiotic treatments during the last 6 months were associated with vitamin D deficiency further research is important to clarify whether treatment of vitamin D deficiency can decrease the number of potential antibiotic requiring infections.
Conclusions
Vitamin D deficiency was common among nursing home residents and strongly associated with dementia. Regardless of causality or not, it is important to be alert for vitamin D deficiency in nursing homes residents with dementia. As expected vitamin D supplementation predicted less vitamin D deficiency, however appetite, staying outdoors and skin phototype were not significant predictors in the model. Antibiotic treatments during the last 6 months were associated with vitamin D deficiency, potentially supporting the hypothesis that vitamin D deficiency is associated with susceptibility to infections.
Acknowledgements
We are grateful to all participants at the nursing homes for the elderly, to all the nursing home staff members who assisted in this study and to the laboratory staff of the Bio Imaging and Laboratory Medicine Unit, Södra Älvsborg Hospital. Thanks to Beth Stuart for statistical advice.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- 25OHD
25-hydroxyvitamin D
- CI
confidence interval
- nmol/L
nanomole/l
- OR
odds ratio
Authors’ contributions
PDS and ME designed the study and carried out the data collection. RA, PDS, JT and MM made substantial contributions to analysis and interpretation of data. RA and PDS drafted the manuscript. RA, PDS, JT, ME and MM have been involved in revising the manuscript critically for important intellectual content and they have all given final approval of the version to be published.
Funding
Financial support was obtained from primary health care in Södra Älvsborg County, the Research and Development Council of the Södra Älvsborg County and FoU Sjuhärad Välfärd (a research and development unit in Borås). Sponsors took no part in the design, methods, subject recruitment, data collection, analysis or preparation of the manuscript.
Ethics approval and consent to participate
The study was approved by the Regional ethical review board of Gothenburg University (D-nr 578–11). All residents were informed of the study both verbally and in writing. From decision-capable individuals choosing to participate in the study, informed approval was collected. However, a considerable number of participants consisted of residents with dementia of varying degrees. If the resident was incapable of understanding the given information and thereby possessing a reduced decision capability, these residents only participated so long as they both: Did not oppose participation in the study and that the appointed representatives or relatives did not oppose their participation after being provided with information about the study. The Regional ethical review board of Gothenburg University approved this procedure.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Rebeka Arnljots, Phone: +4673 569 90 43, Email: rebeka.arnljots@vgregion.se.
Jörgen Thorn, Email: jorgen.thorn@vgregion.se.
Marie Elm, Email: marie.elm@boras.se.
Michael Moore, Email: mvm198@soton.ac.uk.
Pär-Daniel Sundvall, Email: par-daniel.sundvall@vgregion.se.
References
- 1.Burgaz A, Akesson A, Oster A, Michaelsson K, Wolk A. Associations of diet, supplement use, and ultraviolet B radiation exposure with vitamin D status in Swedish women during winter. Am J Clin Nutr. 2007;86(5):1399–1404. doi: 10.1093/ajcn/86.5.1399. [DOI] [PubMed] [Google Scholar]
- 2.Webb AR, Holick MF. The role of sunlight in the cutaneous production of vitamin D3. Annu Rev Nutr. 1988;8:375–399. doi: 10.1146/annurev.nu.08.070188.002111. [DOI] [PubMed] [Google Scholar]
- 3.Perez-Lopez FR, Chedraui P, Fernandez-Alonso AM. Vitamin D and aging: beyond calcium and bone metabolism. Maturitas. 2011;69(1):27–36. doi: 10.1016/j.maturitas.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 4.Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab. 1988;67(2):373–378. doi: 10.1210/jcem-67-2-373. [DOI] [PubMed] [Google Scholar]
- 5.Holick MF. Environmental factors that influence the cutaneous production of vitamin D. Am J Clin Nutr. 1995;61(Suppl 3):638s–645s. doi: 10.1093/ajcn/61.3.638S. [DOI] [PubMed] [Google Scholar]
- 6.Munns CF, Shaw N, Kiely M, Specker BL, Thacher TD, Ozono K, Michigami T, Tiosano D, Mughal MZ, Makitie O, et al. Global consensus recommendations on prevention and Management of Nutritional Rickets. J Clin Endocrinol Metab. 2016;101(2):394–415. doi: 10.1210/jc.2015-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bischoff-Ferrari HA. Vitamin D and fracture prevention. Endocrinol Metab Clin N Am. 2010;39(2):347–353. doi: 10.1016/j.ecl.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Bischoff-Ferrari HA, Willett WC, Wong JB, Stuck AE, Staehelin HB, Orav EJ, Thoma A, Kiel DP, Henschkowski J. Prevention of nonvertebral fractures with oral vitamin D and dose dependency: a meta-analysis of randomized controlled trials. Arch Intern Med. 2009;169(6):551–561. doi: 10.1001/archinternmed.2008.600. [DOI] [PubMed] [Google Scholar]
- 9.Karakis I, Pase MP, Beiser A, Booth SL, Jacques PF, Rogers G, DeCarli C, Vasan RS, Wang TJ, Himali JJ, et al. Association of Serum Vitamin D with the risk of incident dementia and subclinical indices of brain aging: the Framingham heart study. J Alzheimers Dis. 2016;51(2):451–461. doi: 10.3233/JAD-150991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Afzal S, Bojesen SE, Nordestgaard BG. Reduced 25-hydroxyvitamin D and risk of Alzheimer's disease and vascular dementia. Alzheimers Dement. 2014;10(3):296–302. doi: 10.1016/j.jalz.2013.05.1765. [DOI] [PubMed] [Google Scholar]
- 11.Shen L, Ji HF. Vitamin D deficiency is associated with increased risk of Alzheimer's disease and dementia: evidence from meta-analysis. Nutr J. 2015;14:76. doi: 10.1186/s12937-015-0063-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Schaft J, Koek HL, Dijkstra E, Verhaar HJ, van der Schouw YT, Emmelot-Vonk MH. The association between vitamin D and cognition: a systematic review. Ageing Res Rev. 2013;12(4):1013–1023. doi: 10.1016/j.arr.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Kubis AM, Piwowar A. The new insight on the regulatory role of the vitamin D3 in metabolic pathways characteristic for cancerogenesis and neurodegenerative diseases. Ageing Res Rev. 2015;24(Pt B):126–137. doi: 10.1016/j.arr.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Pludowski P, Holick MF, Pilz S, Wagner CL, Hollis BW, Grant WB, Shoenfeld Y, Lerchbaum E, Llewellyn DJ, Kienreich K, et al. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality-a review of recent evidence. Autoimmun Rev. 2013;12(10):976–989. doi: 10.1016/j.autrev.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Meehan M, Penckofer S. The role of vitamin D in the aging adult. J Aging Gerontol. 2014;2(2):60–71. doi: 10.12974/2309-6128.2014.02.02.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grant WB, Goldstein M, Mascitelli L. Ample evidence exists from human studies that vitamin D reduces the risk of selected bacterial and viral infections. Exp Biol Med (Maywood) 2010;235(12):1395–1396. doi: 10.1258/ebm.2010.010c01. [DOI] [PubMed] [Google Scholar]
- 17.Schwalfenberg GK. A review of the critical role of vitamin D in the functioning of the immune system and the clinical implications of vitamin D deficiency. Mol Nutr Food Res. 2011;55(1):96–108. doi: 10.1002/mnfr.201000174. [DOI] [PubMed] [Google Scholar]
- 18.Watkins RR, Lemonovich TL, Salata RA. An update on the association of vitamin D deficiency with common infectious diseases. Can J Physiol Pharmacol. 2015;93(5):363–368. doi: 10.1139/cjpp-2014-0352. [DOI] [PubMed] [Google Scholar]
- 19.Zittermann A, Pilz S, Hoffmann H, Marz W. Vitamin D and airway infections: a European perspective. Eur J Med Res. 2016;21:14. doi: 10.1186/s40001-016-0208-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, Bergman P, Dubnov-Raz G, Esposito S, Ganmaa D, Ginde AA, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. doi: 10.1136/bmj.i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hypponen E, Laara E, Reunanen A, Jarvelin MR, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001;358(9292):1500–1503. doi: 10.1016/S0140-6736(01)06580-1. [DOI] [PubMed] [Google Scholar]
- 22.Hejazi ME, Modarresi-Ghazani F, Entezari-Maleki T. A review of vitamin D effects on common respiratory diseases: asthma, chronic obstructive pulmonary disease, and tuberculosis. J Res Pharmacy Practice. 2016;5(1):7–15. doi: 10.4103/2279-042X.176542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brondum-Jacobsen P, Benn M, Jensen GB, Nordestgaard BG. 25-hydroxyvitamin d levels and risk of ischemic heart disease, myocardial infarction, and early death: population-based study and meta-analyses of 18 and 17 studies. Arterioscler Thromb Vasc Biol. 2012;32(11):2794–2802. doi: 10.1161/ATVBAHA.112.248039. [DOI] [PubMed] [Google Scholar]
- 24.Samefors M, Ostgren CJ, Molstad S, Lannering C, Midlov P, Tengblad A. Vitamin D deficiency in elderly people in Swedish nursing homes is associated with increased mortality. Eur J Endocrinol. 2014;170(5):667–675. doi: 10.1530/EJE-13-0855. [DOI] [PubMed] [Google Scholar]
- 25.Nakashima A, Yokoyama K, Yokoo T, Urashima M. Role of vitamin D in diabetes mellitus and chronic kidney disease. World J Diabetes. 2016;7(5):89–100. doi: 10.4239/wjd.v7.i5.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grant WB, Boucher BJ. Randomized controlled trials of vitamin D and cancer incidence: a modeling study. PLoS One. 2017;12(5):e0176448. doi: 10.1371/journal.pone.0176448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garland CF, Kim JJ, Mohr SB, Gorham ED, Grant WB, Giovannucci EL, Baggerly L, Hofflich H, Ramsdell JW, Zeng K, et al. Meta-analysis of all-cause mortality according to serum 25-hydroxyvitamin D. Am J Public Health. 2014;104(8):e43–e50. doi: 10.2105/AJPH.2014.302034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fuleihan Gel H, Bouillon R, Clarke B, Chakhtoura M, Cooper C, McClung M, Singh RJ. Serum 25-Hydroxyvitamin D levels: variability, knowledge gaps, and the concept of a desirable range. J Bone Miner Res. 2015;30(7):1119–1133. doi: 10.1002/jbmr.2536. [DOI] [PubMed] [Google Scholar]
- 29.Brouwer-Brolsma EM, Bischoff-Ferrari HA, Bouillon R, Feskens EJ, Gallagher CJ, Hypponen E, Llewellyn DJ, Stoecklin E, Dierkes J, Kies AK, et al. Vitamin D: do we get enough? A discussion between vitamin D experts in order to make a step towards the harmonisation of dietary reference intakes for vitamin D across Europe. Osteoporos Int. 2013;24(5):1567–1577. doi: 10.1007/s00198-012-2231-3. [DOI] [PubMed] [Google Scholar]
- 30.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 31.Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev. 2001;22(4):477–501. doi: 10.1210/edrv.22.4.0437. [DOI] [PubMed] [Google Scholar]
- 32.Nordic Council of Ministers. Nordic Nutrition Recommendations 2012 : Integrating nutrition and physical activity. Copenhagen: Nordic Council of Ministers; 2014.
- 33.Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int. 2005;16(7):713–716. doi: 10.1007/s00198-005-1867-7. [DOI] [PubMed] [Google Scholar]
- 34.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84(1):18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 35.Pilz S, Dobnig H, Tomaschitz A, Kienreich K, Meinitzer A, Friedl C, Wagner D, Piswanger-Solkner C, Marz W, Fahrleitner-Pammer A. Low 25-hydroxyvitamin D is associated with increased mortality in female nursing home residents. J Clin Endocrinol Metab. 2012;97(4):E653–E657. doi: 10.1210/jc.2011-3043. [DOI] [PubMed] [Google Scholar]
- 36.Jacques PF, Felson DT, Tucker KL, Mahnken B, Wilson PW, Rosenberg IH, Rush D. Plasma 25-hydroxyvitamin D and its determinants in an elderly population sample. Am J Clin Nutr. 1997;66(4):929–936. doi: 10.1093/ajcn/66.4.929. [DOI] [PubMed] [Google Scholar]
- 37.Sem SW, Sjoen RJ, Trygg K, Pedersen JI. Vitamin D status of two groups of elderly in Oslo: living in old people’s homes and living in own homes. Compr Gerontol A. 1987;1(3):126–130. [PubMed] [Google Scholar]
- 38.Egsmose C, Lund B, McNair P, Lund B, Storm T, Sorensen OH. Low serum levels of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D in institutionalized old people: influence of solar exposure and vitamin D supplementation. Age Ageing. 1987;16(1):35–40. doi: 10.1093/ageing/16.1.35. [DOI] [PubMed] [Google Scholar]
- 39.Bruyere O, Decock C, Delhez M, Collette J, Reginster JY. Highest prevalence of vitamin D inadequacy in institutionalized women compared with noninstitutionalized women: a case-control study. Womens Health (Lond) 2009;5(1):49–54. doi: 10.2217/17455057.5.1.49. [DOI] [PubMed] [Google Scholar]
- 40.Braddy KK, Imam SN, Palla KR, Lee TA. Vitamin d deficiency/insufficiency practice patterns in a veterans health administration long-term care population: a retrospective analysis. J Am Med Dir Assoc. 2009;10(9):653–657. doi: 10.1016/j.jamda.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 41.Gloth FM, 3rd, Gundberg CM, Hollis BW, Haddad JG, Jr, Tobin JD. Vitamin D deficiency in homebound elderly persons. JAMA. 1995;274(21):1683–1686. doi: 10.1001/jama.1995.03530210037027. [DOI] [PubMed] [Google Scholar]
- 42.Papapetrou PD, Triantafyllopoulou M, Korakovouni A. Severe vitamin D deficiency in the institutionalized elderly. J Endocrinol Investig. 2008;31(9):784–787. doi: 10.1007/BF03349258. [DOI] [PubMed] [Google Scholar]
- 43.Perez-Llamas F, Lopez-Contreras MJ, Blanco MJ, Lopez-Azorin F, Zamora S, Moreiras O. Seemingly paradoxical seasonal influences on vitamin D status in nursing-home elderly people from a Mediterranean area. Nutrition. 2008;24(5):414–420. doi: 10.1016/j.nut.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 44.Sundvall PD, Elm M, Ulleryd P, Molstad S, Rodhe N, Jonsson L, Andersson B, Hahn-Zoric M, Gunnarsson R. Interleukin-6 concentrations in the urine and dipstick analyses were related to bacteriuria but not symptoms in the elderly: a cross sectional study of 421 nursing home residents. BMC Geriatr. 2014;14:88. doi: 10.1186/1471-2318-14-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sundvall PD, Elm M, Gunnarsson R, Molstad S, Rodhe N, Jonsson L, Ulleryd P. Antimicrobial resistance in urinary pathogens among Swedish nursing home residents remains low: a cross-sectional study comparing antimicrobial resistance from 2003 to 2012. BMC Geriatr. 2014;14:30. doi: 10.1186/1471-2318-14-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fitzpatrick DE. Soleil et peau (Sun and Skin) J Med Esthetique. 1975;2:33–34. doi: 10.1136/bmj.2.5961.33. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


