Abstract
Here we examined how intravenous heroin at a dose that maintains self-administration (0.1 mg/kg) affects brain temperature homeostasis in freely moving rats under conditions that seek to mimic some aspects of human drug use. When administered under standard laboratory conditions (quiet rest at 22°C ambient temperature), heroin induced moderate temperature increases (1.0–1.5°C) in the nucleus accumbens (NAc), a critical structure of the brain motivation-reinforcement circuit. By simultaneously recording temperatures in the temporal muscle and skin, we demonstrate that the hyperthermic effects of heroin results primarily from inhibition of heat loss due to strong and prolonged skin vasoconstriction. Heroin-induced brain temperature increases were enhanced during behavioral activation (i.e., social interaction) and in a moderately warm environment (29°C). By calculating the “net” effects of the drug in these two conditions, we found that this enhancement results from the summation of the hyperthermic effects of heroin with similar effects induced by either social interaction or a warmer environment. When the dose of heroin was increased (to 0.2, 0.4, 0.8, 1.6, 3.2, and 6.4 mg/kg), brain temperature showed a biphasic down-up response. The initial temperature decrease was dose-dependent and resulted from a transient inhibition of intra-brain heat production coupled with increased heat loss via skin surfaces—the effects typically induced by general anesthetics. These initial inhibitory effects induced by large-dose heroin injections could be related to profound CNS depression—the most serious health complications typical of heroin overdose in humans.
Keywords: opiates, brain and body hypothermia, metabolic brain activation, metabolic brain inhibition, vasoconstriction, vasodilation, nucleus accumbens, rats
Graphical abstract
1. Introduction
Brain temperature is an important physiological parameter that depends on metabolic neural activity and affects multiple neural functions (Kiyatkin, 2010). Our previous thermorecording studies (see Kiyatkin, 2013 for review) revealed that psychomotor stimulant drugs, such as methamphetamine (METH) and 3,4-methylenedioxymethamphetamine (MDMA), induce dose-dependent increases in brain temperature. By combining brain temperature measurements with recordings from the temporal muscle and skin, we clarified that the brain hyperthermic effects of these drugs result from two physiological mechanisms: increased intra-cerebral heat production due to metabolic brain activation and diminished heat loss due to sustained skin vasoconstriction. We also found that the hyperthermic effects of MDMA and METH are strongly enhanced when these drugs are administered during behavioral activation (i.e., social interaction) and at a moderately warm environment—conditions that often accompany drug use in humans. Under these conditions, METH and MDMA at doses much lower than LD50 induced pathologic hyperthermia, and even lethality in some rats. In the present study we extended this research line to examine how heroin affects brain temperature homeostasis in awake, freely moving rats.
In contrast to psychomotor stimulants, which induce sympathetic activation and locomotor hyperactivity, heroin causes profound sedation, locomotor hypoactivity (freezing) and, at higher doses, a comatose state that may result in lethality (see Simon, 1997; Jaffe et al, 1997). Based on these differences, it could be assumed that intravenous heroin could induce brain hypothermia—a response opposite to that induced by psychostimulants. However, direct data on the effects of heroin on brain temperature are absent and existing data on the effects of heroin on body temperature are limited and controversial (Martin et al, 1977; Geller et al, 1983).
The primary goal of this study was to examine how intravenous (iv) heroin at a well-studied self-administering dose (0.1 mg/kg) affects brain temperature homeostasis in freely moving rats under conditions that aimed to mimic some aspects of human drug use. While heroin in rats is self-administered within a wide range of doses (0.025–0.2 mg/kg), a 0.1 mg/kg dose appears to be optimal for maintaining consistent behavioral performance with stable inter-injection intervals (Gerber and Wise, 1989). This dose is much lower than the estimated LD50 for iv administration in rats (15–20 mg/kg; Jackson, 1952; Strandberg et al, 2006; Gable, 2004) and heroin at this dose is self-administered by rats during multiple sessions without any significant health complications (Bozarth and Wise, 1985; Kiyatkin et al, 1993; Kiyatkin and Wise, 2002). This dose is also close to typical human doses in terms of drug amount per body weight (5–10 mg/70 kg; Goldstein, 1994; see also www.erowid.org). Temperatures in this study were simultaneously recorded with high temporal resolution from three locations: a brain site, the temporal muscle, and the skin. As a brain recording site, we chose the NAc, a deep brain structure critically involved in sensorimotor integration (Mogenson et al, 1980; Wise, 1989). Simultaneous recording temperatures from temporal muscle and skin allowed us to examine the mechanisms underlying the brain temperature effects of heroin, specifically its effects on intra-cerebral heat production and heat loss via skin surfaces (Kiyatkin, 2010).
First, we examined the effects of heroin on brain, muscle, and skin temperatures when the drug was used under standard laboratory conditions (quiet rest at 22°C ambient temperatures). Second, we examined how the temperature effects of heroin are modulated during behavioral activation modeled in rats by social interaction between two animals. Third, we examined how the temperature effects of heroin are modulated at a moderately warm ambient temperature (29.0±0.5°C, i.e. at the levels corresponding to normothermy [Romanovsky et al, 2002]) that prevents normal heat dissipation to the external environment. Finally, we examined the temperature effects of heroin at doses well above a typical self-administering range--doses that may result in serious health complications (Louria et al., 1967; Compton et al, 2016).
2. Materials and Methods
2.1. Subjects, Surgery, and Thermocouple Sensors
We used 25 Long-Evans male rats (Charles River Laboratories, Greensboro, NC) 3–4 months in age and 440±40 g in weight, that were housed individually in a temperature-, humidity-, and light-controlled room (12/12 h light/dark cycle, lights on at 07:00) with free access to food and water. Protocols were performed in compliance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, Publication 865-23) and were approved by the NIDA-IRP Animal Care and Use Committee.
Each rat underwent the same three-point thermocouple probe implantation procedure described in detail elsewhere (Kiyatkin et al, 2014). Briefly, under general anesthesia (sodium pentobarbital + chloral hydrate), rats were implanted with miniature copper-constantan thermocouple probes (125 μm in diameter) in the NAc shell (AP=1.2 mm; L=0.9 mm; DV=7.2–7.4 mm according to the atlas of Paxinos and Watson, 1998), deep temporal muscle and subcutaneously, along the nasal ridge with the tip approximately 15 mm anterior to the bregma. Using dental cement, we secured the probes to three stainless steel screws threaded into the skull. During the same surgical procedure, rats were implanted with a chronic jugular catheter, which ran subcutaneously to an injection port secured with the head assembly. Rats were allowed a minimum of 4 days of post-operative recovery; jugular catheters were flushed daily with 0.2 ml heparinized saline (10 units/ml) to maintain patency. At the beginning of each experiment, the injection port of the jugular catheter was connected to a plastic catheter extension that allowed for stress- and cue-free delivery of tested substances from outside the chamber, thus minimizing possible detection of the injection procedure by the rat.
While our primary focus was on brain temperature, two other recording locations were used to evaluate basic physiological mechanisms underlying changes in brain temperature. Since the brain and temporal muscle receive arterial blood from the same common carotid artery and thus are equally exposed to blood-delivered heat from the body, the change in NAc-Muscle temperature differentials shows the source of heat production and serves as a measure of drug-induced metabolic brain response (Kiyatkin, 2010). Specifically, increases in NAc-Muscle differentials suggest increased metabolic brain activity, while their decreases suggest metabolic brain inhibition. Skin temperature is determined by the state of peripheral vessels and its decreases suggest vasoconstriction, however it also depends on the temperature of arterial blood inflow. Therefore, Skin-Muscle temperature differentials exclude this latter contribution, providing an accurate measure of changes in skin vascular tone (Kiyatkin, 2010). Specifically, increases in Skin-Muscle differential indicate enhanced heat loss due to vasodilation whereas decreases indicate diminished heat loss due to vasoconstriction.
2.2. Experimental Protocol
All recordings took place during the light phase of the rat’s cycle in a light- and sound-attenuated chamber under a dim white light. Each morning, we brought the rats from their housing facility, placed them in the chambers, and connected them to the recording instrument (Thermes-16, Physitemp Instruments; Clifton, NJ) via individual sockets attached to a common cord and an electric swivel commutator. We exposed each rat to two habituation sessions (~6 hours each) prior to surgeries and one habituation session (6 hours) thereafter, which preceded heroin or saline injections. During each recording session, drug or saline injections began at least two hours after placement in the chamber, thus allowing the rats to habituate to the environment and their temperatures to return to baseline levels. We recorded temperatures continuously and collected the data at 10-s intervals. We maintained room temperature at either 22.5±0.5°C (“standard laboratory conditions”) or 29.0±0.5°C (“warm environmental conditions”). Additionally, we measured locomotor activity using four photobeams located at the chamber’s walls as previously described (Kiyatkin et al, 2014).
In Experiment I (n=6 rats), we examined the temperature and locomotor effects of heroin delivered iv at a standard self-administering dose (0.1 mg/kg in 0.3 ml saline over 30 s) to quietly resting rats at standard ambient temperature (22°C). Each rat in this experiment was exposed to three drug sessions, in which rats received three iv heroin injections with an inter-injection interval of at least 120 min that is sufficient for restoring baseline temperatures. In Experiment I we did not employ a saline control. As shown previously (Kiyatkin and Brown, 2004), iv saline injected under cue- and stress-free conditions induces no changes in temperatures.
In Experiment II (n=5 rats), we examined the temperature and locomotor effects of heroin delivered iv at the same dose (0.1 mg/kg) during social interaction. We placed a novel, drug-naive conspecific male in the experimental rat’s chamber 10 min before the experimental rat received a heroin or saline injection. This guest rat was kept in the chamber with the experimental rat for 50 min following the injection. During this time period (a total of 60 min), we allowed the two rats to freely interact with each other. This procedure was used extensively in our previous studies and it results in consistent temperature responses. 10 min from the onset of interaction was selected as the injection time as it corresponds to the most robust temperature responses caused by social interaction (Kiyatkin et al, 2014). The recordings continued for at least four hours after heroin or saline injections.
In Experiment III (n=6 rats), we examined the temperature and locomotor effects of heroin delivered iv at the same dose (0.1 mg/kg) in quietly resting rats maintained at a moderately warm ambient temperature (29.0±0.5°C). This temperature is 7°C higher than standard housing conditions (22–23°C) and it corresponds to the thermoneutral zone in rats, when endogenous heat production is minimal and balanced with heat loss (Romanovsky et al, 2002). The recordings continued for at least four hours after heroin or saline injections.
In Experiment IV (n=8 rats), we examined the temperature and locomotor effects of heroin delivered to quietly resting rats iv at increasing doses, which exceed (0.2, 0.4, 0.8, and 1.6 mg/kg) and greatly exceed (3.2, and 6.4 mg/kg) the range of self-administration. In this case, each rat received only one drug injection per session; each drug session followed by one drug free day.
2.3. Data Analysis
All temperature data were collected with 10-s time resolution and later averaged for 1- or 2-min bins depending on the analysis duration. We present heroin-induced temperature changes in four ways: as absolute changes in each recording location, as relative changes, as NAc-Muscle and Skin-Muscle temperature differentials (i.e., the difference between relative temperature changes between the corresponding recording locations), and as a difference from the saline control condition. The first two parameters show the pattern and time-course of temperature changes in each recording location, and we use the NAc-Muscle and Skin-Muscle temperature differences to determine the source of the brain temperature response and the strength of stimulus- or drug-induced vasoconstriction, respectively (Kiyatkin, 2010). We also determined “pure” or “net” effects of heroin administered during social interaction by subtracting the values obtained in control conditions (social interaction + saline). We analyzed the temperature and locomotor responses using one-way ANOVA with repeated measures; we follow up on significant ANOVA results with Fisher’s LSD post-hoc tests. We also performed ANOVA analysis for between-condition comparisons after calculating the mean effects of heroin on individual parameters as the area under the curve over time.
3. Results
Data obtained in this study were collected from 25 rats with histologically verified locations of thermocouple sensors in the NAc.
3.1. Temperature Effects of Heroin Under Standard Laboratory Conditions
When tested at standard laboratory conditions (quiet rest at 22–23°C ambient temperatures), iv heroin induced very consistent temperature and locomotor responses. At the initial stage of our analysis, the data were combined for all six rats and all injections conducted in three repeated sessions (n=51; 3 temperature responses were excluded due to recording artifacts).
As can be seen in Fig. 1A–B, heroin moderately increased brain and muscle temperatures (F61, 3050 =80.89 and 70.12, respectively; p<0.0001). These increases were generally correlative, became significant at the second data point (2–4 min), peaked at ~45 min (~1.4°C) and returned to the baseline at ~100 min post-injection. Heroin also increased skin temperature (F61, 3050 = 28.63; p<0.0001), but this increase was much weaker (~0.6°C) and was preceded by a transient temperature drop immediately after injection.
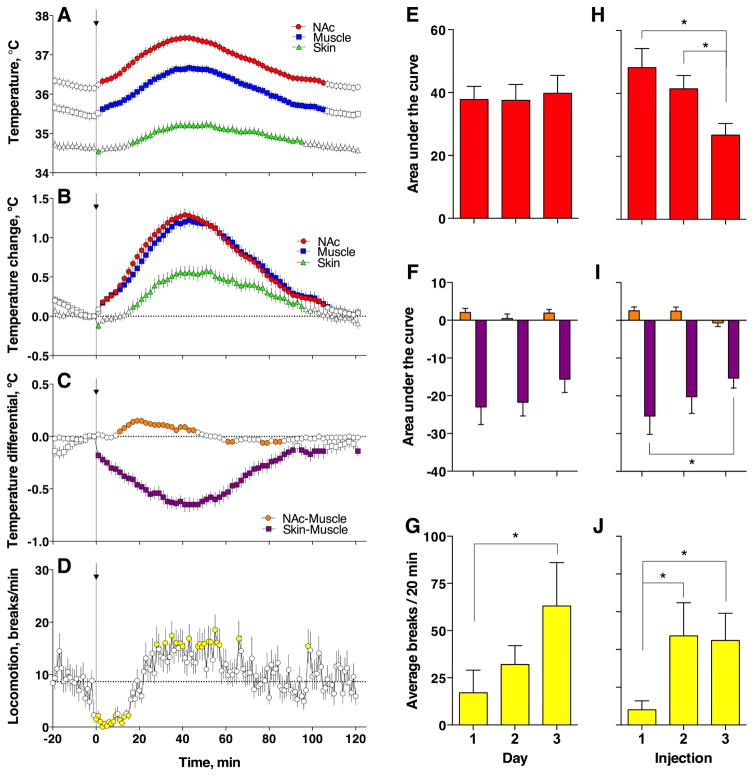
Figure 1. Temperature and locomotor responses induced by iv heroin (0.1 mg/kg) in freely-moving rats under standard laboratory conditions.
Left panel shows the time-course of different temperature parameters (A–C) and locomotor activity (D) assessed for all heroin injections made during three consecutive sessions (n=51 in 6 rats during 18 sessions). Data are shown as mean±sem with 1-min time resolution. Filled symbols show values significantly different from pre-injection baseline (Fisher test, p<0.05). Two right panels show mean changes of different temperature parameters and locomotor activity for different sessions (E–G) and different injections within a session (H–J). Temperature parameters were assessed as an area under the curve for the duration of the effect (E, F, H, I) and locomotion was assessed as the average number of beam breaks for 20 min post injection (G, J). Asterisks show significant differences between groups indicated (p<0.05; Fisher test).
Although changes in brain and muscle temperatures were parallel, their difference, the NAc-Muscle differential, significantly but moderately increased from ~10 to ~50 min post injection, with a peak at ~20 min (F61, 3050 = 12.76, p<0.0001; Fig. 1C). Due to different temperature dynamics in temporal muscle and skin, the Skin-Muscle differential rapidly and strongly decreased for ~100 min post-injection (F61, 3050 = 25.63, p<0.0001; Fig. 1C). This change occurred very rapidly (0–2 min) and peaked at 40–50 min. Consistent with known behavioral effects of heroin, locomotor activity rapidly dropped immediately after the injection (F121, 6050 = 3.85; p<0.0001), maintained at low levels for ~20 min, and then slightly increased above baseline from ~20 to 60 min post-injection (Fig. 1D). Heroin-induced changes in locomotor activity have complex relationships with changes in brain temperature (Fig. 1B and D). Within the first 10 min, changes in these parameters did not correlate and a rapid drop in motor activity was associated with gradual increase in brain temperature. However, these parameters strongly correlated (r=0.96, p<0.0001) within the next 30 min, when both locomotor activity and brain temperature increased to their peaks. A much weaker, but significant correlation (r=0.67, p<0.001) was seen from 40 to 120 min post-injection, when both parameters returned to their baseline.
We next examined whether and how temperature and locomotor responses induced by repeated heroin injections differ within sessions and between sessions. As shown in Fig. 1E and F, changes in NAc temperatures and both NAc-Muscle and Skin-Muscle differentials were identical in each of the three drug sessions, but locomotor inhibition significantly weakened depending on session number (F2,22 = 4.21, p<0.01; Fig. 1G). However, increases in the NAc temperature and locomotor inhibition became weaker following repeated injections within a session (F2,22=4.91 and =3.15, respectively; both p<0.05; Fig. 1H and J). The NAc-Muscle and Skin-Muscle differentials also decreased with each subsequent injection within a session, but the effect was significant only for the latter parameter (F2,22=4.09, p<0.05; Fig. 1I).
3.2. Temperature Effects of Heroin During Social Interaction
To examine how the effects of iv heroin are modulated during behavioral activation, we compared the temperature and locomotor effects of saline and heroin (0.1 mg/kg) administered during social interaction between two rats. As shown in Fig. 2A, B and D, the presentation of another male rat to the rat being recorded induces relatively large increases in NAc and muscle temperatures (F91,819 = 9.40 and 7.75, respectively; both p<0.001) coupled with strong locomotor activation (F181,1629 = 14.53; p<0.001). In control rats that received saline, both temperatures rapidly increased within 2–4 min of social interaction, with increases peaking at ~1°C at 10–12 min and gradually decreasing in parallel with a weakening in locomotor activity, despite the presence of another rat in the chamber. Skin temperature also increased (F91,819=9.40; p<0.001) but the change was weaker and it was preceded by a small, transient temperature decrease within the first two minutes of social interaction.
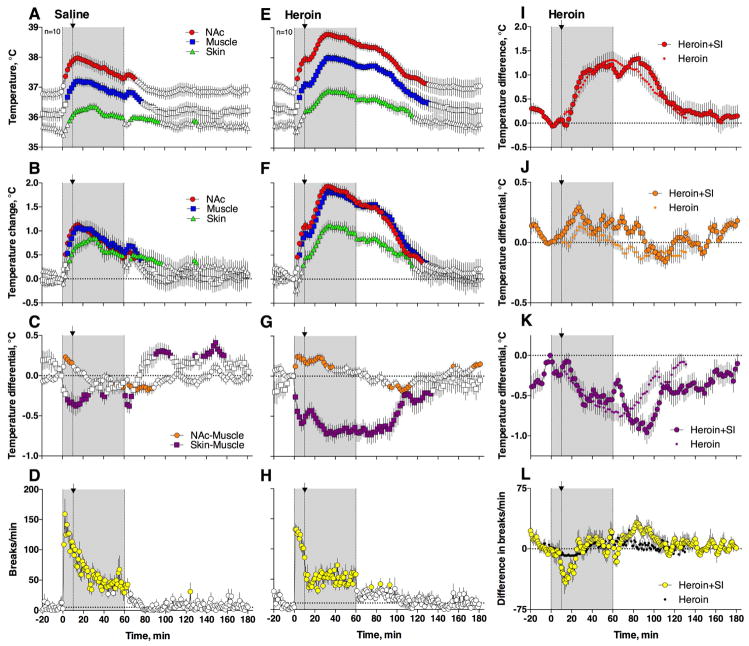
Figure 2. Temperature and locomotor responses induced by iv heroin (0.1 mg/kg) administered during social interaction.
Left panel (A–D) shows temperature and locomotor changes during social interaction with saline injection (+10 min); middle panel (E–H) shows changes during social interaction with heroin injection (+10 min); left column (I–L) shows “pure” or “net” effects of heroin assessed during social interaction (Heroin –Saline) and in standard laboratory conditions. Data are shown as mean (±sem) with 2-min time resolution. Filled symbols in A–H show values significantly different from baseline (at least p<0.05, Fisher test after one-way RM ANOVA). In D, H, and L, graphs show locomotor activity for two rats from 0 to 60 min, n is the number of analyzed tests in the sample.
Exposure to another rat was also associated with a rapid rise in the NAc-Muscle temperature differential (F91,819=3.78, p<0.001; Fig. 2C), suggesting metabolic brain activation. This change was evident only for the first 10 min of social interaction and later disappeared despite increased temperatures and motor activity. In contrast, the Skin-Muscle temperature differential rapidly dropped (F91,819=7.27, p<0.001) but later slowly returned to baseline with a second, more transient drop after the guest-rat was removed from the chamber (Fig. 2C).
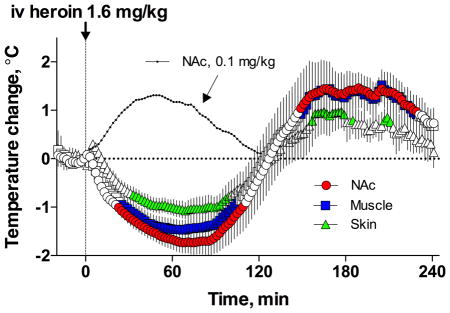
When rats were injected with heroin instead of saline, temperature changes drastically differed from those observed under control conditions (Fig. 2E–G). In this case, both brain and muscle temperatures further increased (F91,819 = 34.93 and 29.37, respectively; both p<0.001), peaking at higher levels (NAc: 38.78°C or 1.93°C higher than the initial baseline) and descending toward baseline for a longer time (NAc: 125 min vs. 71 min in saline control). Heroin injection during social interaction also induced stronger and more prolonged changes in both NAc-Muscle and Skin-Muscle differentials (F91,819 = 7.14 and 11.91, respectively; both p<0.001) and a stronger drop in motor activity observed after drug injection (Fig. 2H).
While the temperature effects of heroin during social interaction were clearly larger than those in standard laboratory conditions (compare with Fig. 1), this change could be determined by the summation of two independent effects. To explore this possibility, we compared the “pure” or “net” effects of heroin: the difference between heroin and saline effects during social interaction and its effects under quiet resting conditions (Fig. 2I–L). During this analysis, we found that under both conditions heroin has virtually identical effects on NAc temperature (Fig. 2I). Therefore, the seemingly larger effects of heroin on brain temperature observed during social interaction could be explained by the summation of two effects: that of social interaction and that of the drug. While the pure effects of heroin on NAc-Muscle and Skin-Muscle differentials appear qualitatively similar in both conditions, the changes during social interaction were slightly larger and more prolonged (Fig. 2J and K). However, a comparison of the areas under the curves revealed no significant between-group differences for both these parameters. A similar difference was found for locomotor activity, where the heroin-induced drop was stronger when the drug was administered during social interaction (Fig. 2L).
3.3. Temperature Effects of Heroin at Warm Ambient Temperatures
To examine how the effects of iv heroin are modulated in warm environment, we examined temperature and locomotor effects of heroin (0.1 mg/kg) administered at a 29°C and compared these effects with those seen at a standard ambient temperature (Fig. 3).
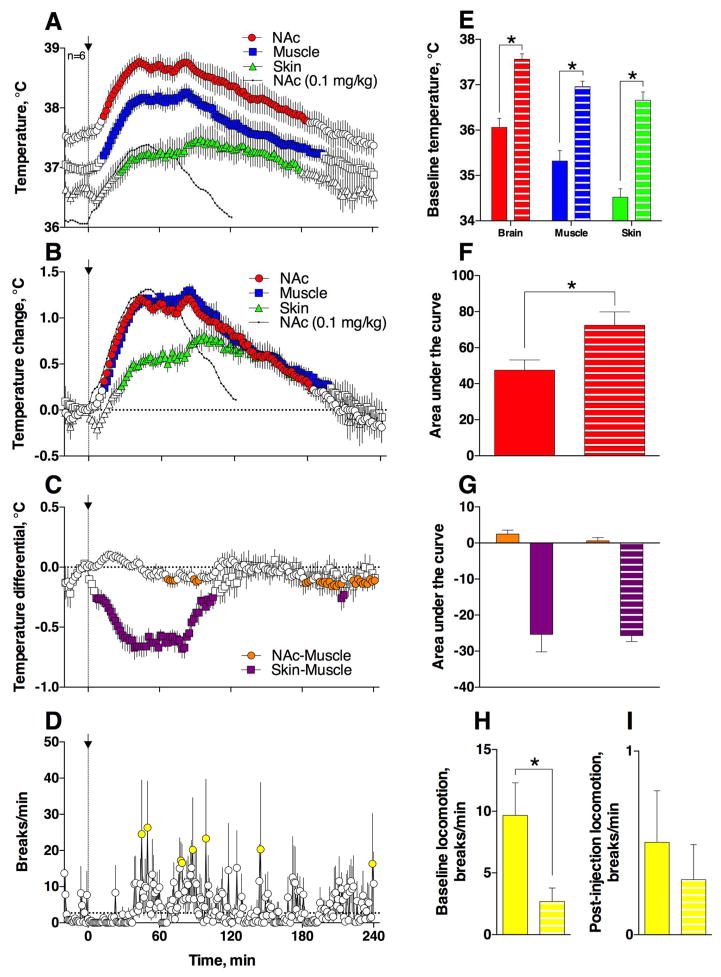
Figure 3. Temperature and locomotor responses induced by iv heroin (0.1 mg/kg) administered at 29°C ambient temperatures.
Left panel (A–D) shows the time-course of changes in temperatures and locomotion. Filled symbols show values significantly different from baseline (at least p<0.05, Fisher test). Data are shown as mean (±sem) with 2-min time resolution. Right panel (E–I) shows between-group (22 vs. 29°C) differences in basal values of temperature and locomotion and their changes induced by heroin. Solid bars indicate the 22°C group, and striped bars indicate the 29°C group. Asterisks show significant differences between groups indicated (p<0.05; Fisher test). Black curves in A and B show absolute (A) and relative (B) changes in NAc temperature induced by heroin at 22°C.
As can be seen in Fig. 3A–B, heroin injected at 29°C induced larger and more prolonged changes in temperatures. In this case, NAc and muscle temperatures increased significantly (F121,605 = 30.52 and 25.31, respectively; p<0.0001), peaking at 38.77 and 38.26°C (50–80 min) and maintaining above baseline for ~190 min. Although these increases in absolute terms were clearly larger and more prolonged than those in the 22°C control group (compare with Fig. 1A–B), the amplitude of increases in relative terms were similar in both conditions (NAc: 1.21±0.10°C vs. 1.31±0.16°C in control). These differences between absolute and relative temperature changes originate from significant differences in basal temperature values, which were 1.50, 1.64 and 2.14°C larger in warmer conditions for NAc, muscle, and skin, respectively (Fig. 3E). Despite similar response magnitudes, the duration of increases was much longer at 29°C (NAc: 184 min vs. 110 min in control) and the hyperthermic response, assessed as the area under the curve, was significantly stronger (Fig. 3B and F).
Despite larger temperature increases observed at elevated temperatures, changes in NAc-Muscle and Skin-Muscle differentials were similar in both groups (Fig. 3C and G). The post-injection locomotor freezing was not as evident at 29°C (Fig. 3I) possibly due to significantly lower basal locomotion (Fig. 3H) seen in rats of this group.
3.4. Temperature Effects of Heroin at High Doses
As shown in Figure 4, increase in heroin doses results in qualitative and quantitative changes in temperature and locomotor responses. In contrast to monotonic increases in brain and muscle temperatures induced by heroin at 0.1 mg/kg dose, both temperatures initially slightly decreased below baseline following 0.2 mg/kg injection but then slowly increased to the levels similar or even smaller than those observed with a standard 0.1 mg/kg dose. In contrast to the monophasic decrease in the Skin-Muscle differential seen with a 0.1 mg/kg dose, this parameter rapidly and transiently increased for ~20 min post-injection following double-dose heroin before decreasing to the levels seen with a 0.1 mg/kg dose. The NAc-Muscle differential, which had a transient rise after injection with a 0.1 mg/kg dose, did not show increases and even transiently decreased after heroin injection at a doubled dose. These two parameters negatively correlated (r = −0.86; p <0.0001). Doubling of heroin dose also resulted in clear increase in locomotor activity, which approximately doubled in its duration.
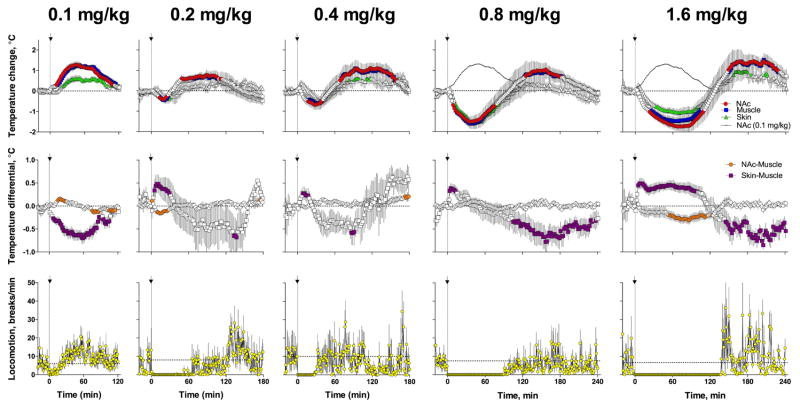
Figure 4. Temperature responses induced by iv heroin at high doses.
Top panels show relative temperature changes induced by heroin at different doses; middle panels show changes in temperature differentials; bottom panels show changes in locomotor activity. Data are shown as mean (±sem) with 2-min time resolution. Black curves on top graphs for 0.8 and 1.6 mg/kg heroin dose show brain temperature response induced by heroin at a standard dose (0.1 mg/kg). Filled symbols show values significantly different from pre-injection baseline.
These qualitative changes became more pronounced when heroin was injected at larger doses. In this case, the initial drop in temperatures became more pronounced, the Skin-Muscle differential showed a larger increase, and the NAc-Muscle differential significantly decreased below baseline. Similar to what was observed with a 0.2 mg/kg dose, both differentials tightly and negatively correlated with each other (r = −0.89; p<0.0001) at 0.4 mg/kg dose. Additionally, the doubling and quadrupling of heroin doses resulted in strong prolongation of drug-induced freezing; mean duration of a significant decrease in locomotion was 15, 32, and 67 min for 0.1, 0.2, and 0.4 mg/kg, respectively. Despite prolongation of motor hypoactivity and shallow breathing, all rats tolerated heroin at 0.2 and 0.4 mg/kg doses, returning to normal behavior 4–5 hrs post-injection and showing normal activity during subsequent days.
When the doses of heroin were further increased above the typical self-administration range (0.8 and 1.6 mg/kg), biphasic, down-up brain temperature fluctuations became more pronounced and prolonged, with a significant prolongation of the initial temperature decrease. In contrast to the rapid rise seen with a 0.1 mg/kg heroin dose, temperatures rapidly decreased after the injection of larger doses and reached a nadir (~2°C below baseline with progressively increasing durations) before increasing well above the baseline. Despite differences in dose, the amplitudes of brain temperature increases were similar, peaking at progressively later post-injection times. In contrast to what was observed with a 0.1 mg/kg dose, the NAc-Muscle differential showed progressively larger decreases with larger doses. Additionally, the Skin-Muscle differential showed biphasic changes with initial increases followed by a decrease below baseline. This decrease appeared at a later time with each dose increase, correlating with the restoration of locomotion after post-injection immobility, which became progressively longer with dose increases. Despite the dramatic prolongation of locomotor inactivity and superficial breathing, rats tolerated large doses and showed normal behavior days after heroin treatment.
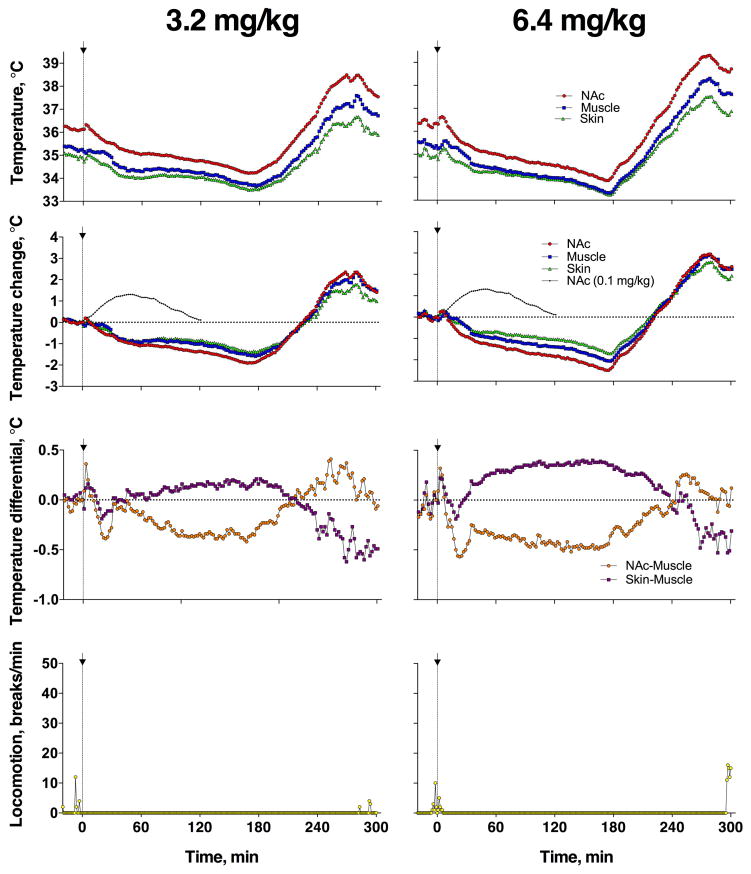
Due to animal safety concerns, only two rats were tested with extra-large heroin doses (3.2 and 6.4 mg/kg). Figure 5 shows raw, unaveraged changes in temperature and locomotion observed in two different rats. As can be seen, heroin at these super-large doses virtually fully blocked locomotor activity for 300 min post-injection. Similar to a 0.2 mg/kg dose, brain and muscle temperature responses were biphasic, with a strong (−1.5–2.0°C) and prolonged (3–4 hrs) decrease followed by increase that was cut out by the end of 5-hr post-injection recording. In contrast to low doses, which induced skin vasoconstriction and weak metabolic activation, heroin at ultra-high doses induced strong vasodilation and metabolic inhibition that could be both responsible for brain and body hypothermia. Both rats that received a 6.4 mg/kg dose displayed convulsions during and immediately after injection. As such, no further doses were tested.
Figure 5. Changes in temperature and locomotion induced in individual rats by iv heroin at ultra-high doses (3.2 and 6.4 mg/kg).
Graphs show changes in absolute temperatures (top row), relative temperatures (middle row), temperature differentials (second middle row), and locomotor activity (bottom row).
4. Discussion
Humans self-administer heroin within a specific dose range and under specific environmental conditions. Therefore, we first examined the effects of heroin delivered iv at a 0.1 mg/kg dose, which is a well-studied, low dose self-administered by rats (Gerber and Wise, 1989) and well within the dose range (5–20 mg/70 kg) used by humans with limited heroin experience (see Goldstein, 1994 and www.erowid.org). To mimic the conditions often associated with human drug use, we next examined the effects of heroin at this low dose during social interaction and at a warm ambient temperature (29°C). This approach allowed us to examine how the effects of heroin are modulated by behavioral activation and under conditions of diminished heat dissipation to the external environment. Since heroin could be taken at much higher doses, we also examined its effects when the dose was above (2- and 4-fold) and greatly above (8-, 16-, 32-, and 64-fold) the self-administered dose. The 2- to 8-fold dose increases are within the limits that could be taken as a single injection by heavy addicts (20–40 mg/70 kg; see www.erowid.org), and 32- to 64-fold increases could be viewed as overdose-inducing but they are still lower than the traditional estimates of LD50 for iv heroin in drug-naïve rats (20–23 mg/kg; Jackson, 1952; Strandberg et al, 2006; Gable, 2004). These estimates, however, should be viewed with caution because mortality strongly depends on age, weight and sex of rats as well as physical and social environment of drug testing. In one study, 96% of Wistar rats died following iv heroin injection at a 15 mg/kg dose (Siegel et al., 1982).
When tested under standard laboratory conditions, iv heroin induces moderate, monophasic temperature increases in the NAc, which correlate with temperature increases in temporal muscle (see Fig. 1). While generally consistent with earlier work suggesting moderate hyperthermic effects of heroin administered subcutaneously or intraperitoneally at low or moderate doses (Martin et al, 1977; Geller et al, 1983; Rosow et al, 1980), we found that increases in NAc temperature are more rapid and stronger than in the muscle (i.e., increase in NAc-Muscle differential), suggesting the enhanced intra-brain heat production resulting from metabolic brain activation. This effect, however, was weak, developed with a definite latency (~8 min) and did not correlate with the rapid block of locomotor activity, which began within the first post-injection minute. Moreover, the maximal increase in the NAc-Muscle differential was at ~20 min, the time interval when locomotor hypoactivity is inverted into hyperactivity. Interestingly, at this time the levels of the primary heroin metabolite (6-MAM), which has high affinity to μ-opioid receptors (Boix et al, 2013; Gottas et al, 2013), are on descending curve after their peaks at ~5 min but levels of morphine, another heroin’s metabolite, are at their peaks (Gottas et al, 2013).
While heroin slightly enhances intra-cerebral heat production, suggesting its ability to induce metabolic brain activation, the rapid, strong, and prolonged drop in Skin-Muscle differential indicates skin vasoconstriction as another, more powerful contributor to brain and body temperature increases. This effect occurred equally as rapid as motor freezing, but peaked at ~40 min, when rats increased their locomotion. Therefore, by increasing intra-brain heat production and limiting heat dissipation, heroin at low, self-administering doses mimics the general pattern of brain thermoregulatory response induced by both natural salient stimuli (i.e., tail-pinch, social interaction) and psychomotor stimulant drugs (METH, MDMA, cocaine) (Brown et al, 2003; Kiyatkin and Brown, 2005; Kiyatkin et al, 2014). However, while all these physiological and pharmacological stimuli induce locomotor activation, heroin inhibits locomotor activity, suggesting the absence of direct relationships between changes in temperature and behavior.
It is known that the effects of opioids, including heroin, show tolerance, or weakening in effect, following repeated drug use (Jaffe et al, 1997; Simon, 1997). However, temperature effects of iv heroin at low doses remained very consistent during repeated injections (see Fig. 1). Within the three testing sessions, there were no significant changes in any temperature measure, while the blockade of locomotion slightly decreased. However, we observed relatively weak but significant decreases in temperature and behavioral effects of heroin between the three injections within the session, suggesting within-session tolerance.
Previously, we showed that the hyperthermic effects of METH (Brown et al, 2003) and MDMA (Kiyatkin et al, 2014) are strongly enhanced during behavioral activation and at warm ambient temperatures. In contrast, our present results suggest that environmental conditions and a high-arousal state have only weak enhancing effects on the hyperthermic effects of iv heroin at low, self-administering doses. When iv heroin was administered during social interaction, the absolute temperature increases became larger, but the effects of heroin with and without social interaction were virtually identical when control data (social interaction + saline) were subtracted from data from the latter condition (see Fig. 2I–L). Therefore, the hyperthermic effects of heroin in this case are summated with similar effects occurring during social interaction. A similar summation of two effects was also found when heroin was administered at 29°C (see Fig. 3). In this condition, the hyperthermic response increased in absolute terms due to higher basal levels of brain and muscle temperatures in rats exposed to a warmer environment. However, the hyperthermic effect of heroin at 29°C became more prolonged (~180 vs. ~100 min in 22°C) reflecting diminished heat dissipation under these conditions.
The temperature effects of iv heroin drastically changed when doses exceeded the self-administering range (Fig. 4 and 5). In this case, brain and muscle temperatures decreased immediately after injection and these new effects were associated with a decrease in NAc-Muscle and increase in Skin-Muscle differentials—the effects opposite to those induced by heroin at the typical self-administering dose. These two effects, which suggest inhibition of brain metabolic activity and skin vasodilation, tightly correlated with each other and occurred when the rat was frozen. This pattern of changes is similar to that seen during general anesthesia (Kiyatkin and Brown, 2005; Bola and Kiyatkin, 2016)—a condition associated with metabolic inhibition and intense heat loss to the external environment due to skin vasodilation. While weaker in amplitude and relatively transient in duration (~30 min), these effects are consistent with the CNS depressive action of heroin—a presumed cause of acute, overdose-induced health complications. This decreased metabolic activity coupled with the enhanced heat loss from skin surfaces makes the rat exceptionally susceptible to more dramatic decreases in brain and body temperatures if large-dose heroin use is combined with other drugs with CNS inhibiting and vasodilatory actions such as alcohol (Gillespie, 1967; Volkow et al., 1990) and benzodiazepines (Sari et al., 1975; Abel et al., 1970; Kiyatkin and Bae, 2008).
4.1. Conclusions
Heroin use is on the rise in recent years due to a drop in drug prices and increased use of prescription opioid analgesics (Compton et al., 2016). This consumption trend runs parallel with the rise in serious medical complications, including comatose state and death due to overdose. While the focus of our current study was on the temperature effects of heroin used at a typical self-administering dose, we found that healthy rats tolerate much higher doses of iv heroin, despite a powerful and prolonged inhibition of locomotor activity coupled with ~2°C brain and body temperature decreases. If similar changes, which suggest CNS depression, occur in humans after high-dose heroin intake, they could contribute to the development of a comatose state and perhaps to overdose-induced lethality. While in this study the effects of heroin were assessed in quietly resting rats with no or limited drug experience, human heroin users usually have significant drug experience and often inject this drug in much higher doses, following highly activating drug-seeking activity. While these factors and presumed tolerance to the psychoactive and physiological effects of heroin could significantly affect the outcome of high-dose drug intake, these important issues were out of the scope of the present study and require special investigation.
Highlights.
Iv heroin at low, self-administering doses moderately increases brain temperature
Brain hyperthermic effects result primarily from peripheral vasoconstriction
Brain hyperthermia is potentiated during social interaction
Brain hyperthermia is potentiated at warm ambient temperatures
At higher doses, heroin induces a biphasic, down-up brain temperature response
Acknowledgments
Supported by the National Institute on Drug Abuse - Intramural Research Program, NIH (1ZIADA000566-05). There is no conflict of interest defined by the Authors. The Authors thank Keaton T. Cameron-Burr for intellectual and editorial assistance during preparation and revision of this manuscript.
Footnotes
Conflict of interest: The Authors report no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel RM, Reis RL, Staroscik RN. The pharmacological basis of coronary and systemic vasodilator actions of diazepam (Valium) Br J Pharmacol. 1970;39:261–274. doi: 10.1111/j.1476-5381.1970.tb12890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boix F, Anersen JM, Morland J. Pharmacokinetic modeling of subcutaneous heroin and its metabolites in blood and brain of mice. Addiction Biol. 2013;18:1–7. doi: 10.1111/j.1369-1600.2010.00298.x. [DOI] [PubMed] [Google Scholar]
- Bola RA, Kiyatkin EA. Robust brain hyperglycemia during general anesthesia: Relationships with metabolic brain activation and vasodilation. Front Physiol. 2016;7:39. doi: 10.3389/fphys.2016.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozarth MA, Wise RA. Toxicity associated with long-term intravenous heroin and cocaine self-administration in the rat. JAMA. 1985;254:81–83. [PubMed] [Google Scholar]
- Brown PL, Wise RA, Kiyatkin EA. Brain hyperthermia is induced by methamphetamine and exacerbated by social interaction. J Neurosci. 2003;23:3924–3929. doi: 10.1523/JNEUROSCI.23-09-03924.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton WM, Jones CM, Baldwin GT. Relationships between nonmedical prescription-opioid use and heroin use. New England J Med. 2016;374:154–162. doi: 10.1056/NEJMra1508490. [DOI] [PubMed] [Google Scholar]
- Gable RS. Comparison of acute lethal toxicity of commonly abused psychoactive substances. Addiction. 2004;99:686–696. doi: 10.1111/j.1360-0443.2004.00744.x. [DOI] [PubMed] [Google Scholar]
- Geller EB, Hawk C, Keinath H, Tallarida RJ, Adler MW. Subclasses of opioids based on body temperature changes in rats: acute subcutaneous administration. J Pharmacol Exp Ther. 1983;225:391–398. [PubMed] [Google Scholar]
- Gerber GJ, Wise RA. Pharmacological regulation of intravenous cocaine and heroin self-administration in rats: a variable dose paradigm. Pharmacol Biochem Behav. 1989;32:527–531. doi: 10.1016/0091-3057(89)90192-5. [DOI] [PubMed] [Google Scholar]
- Gillespie JA. Vasodilator properties of alcohol. Br Med J. 1967;2:274–277. doi: 10.1136/bmj.2.5547.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein A. Addiction: From biology to drug policy. New York: W.H. Freeman; 1994. [Google Scholar]
- Gottas A, Oiestad EL, Boix F, Vindenes V, Ripel A, Thaulow CH, Morland J. Levels of heroin and its metabolites in blood and brain extracellular fluid after i.v. heroin administration to freely moving rats. Br J Pharmacol. 2013;170:546–556. doi: 10.1111/bph.12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe JH, Knapp CM, Ciraulo DA. Opiates: Clinical Aspects. In: Lowinson JH, Ruis P, Millman RB, Langrod JG, editors. Substance Abuse. A comprehensive Textbook. 3. Baltimore et al: Williams & Wilkins; 1997. pp. 148–158. [Google Scholar]
- Jackson H. The evaluation if analgesic potency of drugs using thermal stimulation in the rat. Br J Pharmacol Chemother. 1952;7:196–203. doi: 10.1111/j.1476-5381.1952.tb01313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin EA. Brain temperature homeostasis: physiological fluctuations and pathological shifts. Front Biosci. 2010;15:73–92. doi: 10.2741/3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin EA. The hidden side of drug action: brain temperature changes induced by neuroactive drugs. Psychopharmacology (Berlin) 2013;225:765–780. doi: 10.1007/s00213-012-2957-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin EA, Bae D. Behavioral and brain temperature responses to salient environmental stimuli and intravenous cocaine in rats: effects of diazepam. Psychopharmacology. 2008;196:343–356. doi: 10.1007/s00213-007-0965-y. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Brown PL. Modulation of physiological brain hyperthermia by environmental temperature and impaired blood outflow in rats. Physiol Behav. 2004;83:467–474. doi: 10.1016/j.physbeh.2004.08.032. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Brown PL. Dopamine-dependent and dopamine-independent actions of cocaine as revealed by brain thermorecording in freely moving rats. Eur J Neurosci. 2005;22:9430–9438. doi: 10.1111/j.1460-9568.2005.04269.x. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Brown PL. Brain and body temperature homeostasis during sodium pentobarbital anesthesia with and without body warming. Physiol Behav. 2005;84:563–570. doi: 10.1016/j.physbeh.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Kim AH, Wakabayashi KT, Baumann MH, Shaham Y. Critical role of peripheral vasoconstriction in fatal brain hyperthermia induced by MDMA (Ecstasy) under conditions that mimic human drug use. J Neurosci. 2014;34:7754–7762. doi: 10.1523/JNEUROSCI.0506-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin EA, Wise RA, Gratton A. Drug- and behavior-associated changes in dopamine-related electrochemical signals during intravenous heroin self-administration in rats. Synapse. 1993;14:60–72. doi: 10.1002/syn.890140109. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Wise RA. Brain and body hyperthermia associated with heroin self-administration in rats. J Neurosci. 2002;22:1072–1080. doi: 10.1523/JNEUROSCI.22-03-01072.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louria DE, Hensle T, Rose J. The major medical complications of heroin addiction. Ann Intern Med. 1967;76:1–22. doi: 10.7326/0003-4819-67-1-1. [DOI] [PubMed] [Google Scholar]
- Martin GE, Pryzbylik AT, Spector H. Restraint alters the effects of morphine and heroin on core temperature in the rat. Pharmacol Biochem Behav. 1977;7:463–469. doi: 10.1016/0091-3057(77)90215-5. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. San Diego: Academic Press; 1998. [Google Scholar]
- Romanovsky AA, Ivanov AI, Shimansky YP. Ambient temperature for experiments in rats: a new method for determining the zone of thermal neutrality. J Appl Physiol. 2002;92:2667–2679. doi: 10.1152/japplphysiol.01173.2001. [DOI] [PubMed] [Google Scholar]
- Rosow CE, Miller JM, Poulsen-Burke J, Cochin J. Opiates and thermoregulation in mice. I. Agonists. J Pharmacol Exper Ther. 1980;213:273–283. [PubMed] [Google Scholar]
- Sari A, Fukuda Y, Sakabe T, Maekawa T, Ishikawa T. Effects of psychotropic drugs on canine cerebral metabolism and circulation related to EEG—diazepam, clomipramine, and chlorpromazine. J Neurol Neurosurg Psychiatry. 1975;38:838–844. doi: 10.1136/jnnp.38.9.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel S, Hinson RE, Krank MD, McCully J. Heroin “over-dose” death: contribution of drug-associated environmental cues. Science. 1982;216:436–437. doi: 10.1126/science.7200260. [DOI] [PubMed] [Google Scholar]
- Simon EJ. Opiates: neurobiology. In: Lowinson JH, Ruis P, Millman RB, Langrod JG, editors. Substance Abuse. A comprehensive Textbook. 3. Baltimore et al: Williams & Wilkins; 1997. pp. 148–158. [Google Scholar]
- Strandberg JJ, Kugelberg FC, Alkass K, Gustavsson A, Zahisen K, Spigset O, Druid H. Toxicological analysis in rats subjected to heroin and morphine overdose. Toxicol Lett. 2006;166:11–18. doi: 10.1016/j.toxlet.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Hitzemann R, Wolf AP, Logan J, Fowler JS, Christman D, Dewey SL, Chhlyer D, Burr G, Vitkin S, Hirschowitz J. Acute effects of ethanol on regional brain glucose metabolism and transport. Psych Res Neuroimaging. 1990;35:39–48. doi: 10.1016/0925-4927(90)90007-s. [DOI] [PubMed] [Google Scholar]
- Wise RA. The brain and reward. In: Liebman JM, Kooper SJ, editors. The neuropharmacological basis of reward. Oxford University Press; Oxford: 1989. pp. 377–424. [Google Scholar]