Abstract
The basolateral amygdala (BLA) is a key site for crossmodal association of sensory stimuli and an important relay in the neural circuitry of emotion. Indeed, the BLA receives substantial glutamatergic inputs from multiple brain regions including the prefrontal cortex and thalamic nuclei. Modulation of glutamatergic transmission in the BLA regulates stress- and anxiety-related behaviors. Serotonin (5-HT) also plays an important role in regulating stress-related behavior through activation of both pre- and postsynaptic 5-HT receptors. Multiple 5-HT receptors are expressed in the BLA, where 5-HT has been reported to modulate glutamatergic transmission. However, the 5-HT receptor subtype mediating this effect is not yet clear. The aim of this study was to use patch-clamp recordings from BLA neurons in an ex vivo slice preparation to examine 1) the effect of 5-HT on extrinsic sensory inputs, and 2) to determine if any pathway specificity exists in 5-HT regulation of glutamatergic transmission. Two independent input pathways into the BLA were stimulated: the external capsule to mimic cortical input, and the internal capsule to mimic thalamic input. Bath application of 5-HT reversibly reduced the amplitude of evoked excitatory postsynaptic currents (eEPSCs) induced by stimulation of both pathways. The decrease was associated with an increase in the paired-pulse ratio and coefficient of variation of eEPSC amplitude, suggesting 5-HT acts presynaptically. Moreover, the effect of 5-HT in both pathways was mimicked by the selective 5-HT1B receptor agonist CP93129, but not by the 5-HT1A receptor agonist 8-OH DPAT. Similarly the effect of exogenous 5-HT was blocked by the 5-HT1B receptor antagonist GR55562, but not affected by the 5-HT1A receptor antagonist WAY 100635 or the 5-HT2 receptor antagonists pirenperone and MDL 100907. Together these data suggest 5-HT gates cortical and thalamic glutamatergic inputs into the BLA by activating presynaptic 5-HT1B receptors.
Keywords: serotonin receptor, glutamatergic transmission, basolateral amygdala
Introduction
The basolateral amygdala (BLA) is a key relay structure in emotional circuitry. The BLA receives substantial glutamatergic input from both the somatosensory cortex through the external capsule, and from the thalamic nuclei through the internal capsule. The BLA in turn projects to multiple downstream targets critically involved in the regulation of stress and anxiety-like behavior, including the central nucleus of amygdala (CeA) and the bed nucleus of the stria terminalis (BNST) (Walker et al., 2003). Significantly, abnormal hyperactivity of the BLA has been implicated in the etiology of several emotional disorders including depression and anxiety. Consistent with this observation, repeated stress increases the activity of BLA neurons in vivo (Zhang and Rosenkranz, 2012, Padival et al., 2013). Furthermore, activation or inhibition of neural activity in the BLA respectively enhances or reduces anxiety-like behavior (Davis, 2002, Tye et al., 2011). Additionally, stressful stimuli increase glutamate release in the BLA (Reznikov et al., 2007), and microinjection of glutamate antagonists into the BLA abolishes the expression of conditioned fear (Kim et al., 1993, Lee et al., 2001). Hence, glutamatergic transmission is essential for normal BLA function.
Importantly, glutamatergic input into the BLA can be regulated by neurotransmitters and/or neuromodulators such as serotonin (5-HT) (Bocchio et al., 2016). Indeed, dysregulation of 5-HT transmission is thought to play a major role in the etiology of emotional disorders (Lowry et al., 2005, Asan et al., 2013). For example, the BLA hyperactivity seen in major depressive disorder is normalized after successful pharmacotherapy using selective serotonin reuptake inhibitors (SSRIs). The BLA is heavily innervated by 5-HT terminals originating from the dorsal raphe nucleus (Abrams et al., 2004, Muller et al., 2007), and multiple 5-HT receptor subtypes are expressed by both BLA principal neurons and interneurons (Asan et al., 2013). Indeed, local activation of 5-HT2A or 5-HT2C receptors induces anxiety-like behavior in rodents (Campbell and Merchant, 2003, de Mello Cruz et al., 2005, Cornelio and Nunes-de-Souza, 2007, Christianson et al., 2010), an effect thought to be mediated by activating postsynaptic 5-HT receptors. Far fewer studies have examined 5-HT receptor-mediated modulation of glutamatergic transmission in the BLA. Previous studies reported that activating presynaptic 5-HT receptors inhibits glutamatergic transmission in the BLA (Cheng et al., 1998, Rainnie, 1999, Daftary et al., 2012, Yamamoto et al., 2012). However, the receptor subtype(s) mediating this response have not been clearly established. In this study we used in vitro patch clamp recording from BLA slices to 1) determine the identity of the presynaptic 5-HT receptor(s) modulating BLA glutamatergic transmission, and 2) determine if presynaptic 5-HT differentially regulated cortical and thalamic glutamatergic inputs into the BLA.
Methods
Animals
Adolescent male Sprague-Dawley rats (35–49 days old, Charles River, Raleigh, NC) were used throughout this study. Animals were group-housed with 4–5 rats per cage and had access to food and water ad libitum. Efforts were taken to minimize both animal suffering and the number of animals used in experiments. Animal care and all procedures used in this study were performed in accordance with the National Institutes for Health Guide for the Care and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use Committee of Emory University.
Slice preparation
Coronal slices (350 µm) containing the BLA were obtained as previously reported (Li et al., 2011). Briefly, under deep anesthesia (isofluorane, Henry Schein Inc, Melville, NY, USA), the brains of anesthetized rats were rapidly dissected and immersed in a cold (4°C) oxygenated artificial cerebrospinal fluid (ACSF) “cutting solution”, of the following composition (in mM): NaCl (130), NaHCO3 (30), KCl (3.50), KH2PO4 (1.10), MgCl2 (6.0), CaCl2 (1.0), glucose (10), ascorbate (0.4), thiourea (0.8), sodium pyruvate (2.0), and kynurenic acid (2.0). BLA slices were cut using a Leica VTS-1000 vibratome (Leica Microsystems Inc., Bannockburn, IL, USA). After sectioning, slices were maintained at 32°C in “cutting solution” oxygenated with a mixture of 95% oxygen and 5% carbon dioxide for 1 h prior to recording. Slices were then transferred to a holding chamber containing “regular” ACSF maintained at room temperature, with the following composition (in mM): NaCl (130), NaHCO3 (30), KCl (3.50), KH2PO4 (1.10), MgCl2 (1.30), CaCl2 (2.50), and glucose (10), ascorbate (0.4), thiourea (0.8), and sodium pyruvate (2.0).
Patch clamp recording
For whole-cell patch clamp recording, slices were continuously perfused by gravity-fed oxygenated “regular” ACSF heated to 32°C (2–3 ml per min) in a Warner Series 20 submersion-type slice chamber (0.5 ml volume; Warner Instruments, Hamden, CT). Slices were viewed using differential interference contrast (DIC) optics and infrared (IR) illumination with an IR sensitive CCD camera (Orca ER, Hamamatsu, Tokyo, Japan) mounted on a Leica DMF6000 microscope (Leica Microsystems Inc., Bannockburn, IL). Patch pipettes were fabricated from borosilicate glass (resistance 4–6 MΩ) and filled with a recording solution of the following composition (in mM): K-Gluconate (130), KCl (2), HEPES (10), MgCl2 (3), phosphocreatine (5), K-ATP (2), and NaGTP (0.2). The patch solution was adjusted to pH 7.3 with KOH and had an osmolarity of 280–290 mOsm. Whole-cell recordings were made with a Multiclamp 700B amplifier (Molecular Devices Corporation, Sunnyvale, CA) using pClamp 10.4 software and an Axon Digidata 1550 A-D interface (Molecular Devices Corporation). BLA principal neurons were identified visually by their pyramidal shape and confirmed physiologically by their membrane properties. Whole-cell access resistances measured in voltage clamp were in the range 5 – 20 MΩ and were routinely monitored throughout each experiment; a change of <15% was deemed acceptable.
Evoked EPSCs
Postsynaptic currents onto BLA neurons were evoked by stimulating the external capsule (cortical input) or the internal capsule (thalamic input) with a concentric bipolar stimulation electrode (FHC, Bowdoinham, ME) as previously reported (Li et al., 2011). To isolate evoked excitatory postsynaptic currents (eEPSCs), the GABAA receptor antagonist picrotoxin (100 µM) was included in the patch solution to block inhibitory postsynaptic currents (IPSCs). Furthermore, the membrane potential was held at −65 mV, which is close to chloride equilibrium potential, to minimize contamination by residual GABAA receptor-mediated evoked IPSCs. The selective GABAB receptor antagonist CGP 53432 (1 µM) was also bath applied to block the slow component of evoked IPSCs. Two stimulation paradigms were used in this study to induce eEPSCs: 1) one train of five single square wave pulses (150 µs, 0.2 Hz) delivered every 2 min, and 2) consecutive single pulse stimulations (150 µs, 0.1 Hz) were delivered throughout the experiment. Baseline amplitude of eEPSCs was adjusted to half maximal stimulation response. For analysis, all eEPSCs values were normalized to the baseline amplitude and expressed as the percentage of baseline.
To examine the potential involvement of presynaptic 5-HT receptors in the attenuation of glutamate release, we employed a paired-pulse paradigm in conjunction with an analysis of the coefficient of variation (CV) of eEPSC amplitude, as previously reported (Guo et al., 2012). Alterations in the paired-pulse ratio (PPR) are thought to represent changes in release probability in the presynaptic terminal (Hess and Ludin, 1987; Manabe et al., 1993). A change of CV is associated with either a change of release probability or the number of release sites (Choi and Lovinger, 1997). For the paired-pulse paradigm, two electrical stimuli were delivered with an inter-stimulus-interval of 50 ms. The PPR was calculated as the mean peak amplitude of the second eEPSC (P2) divided by the first eEPSC (P1). CV was calculated as δ/µ, where δ is the stand deviation of the peak eEPSC amplitude and µ is the mean eEPSC amplitude. Here, we used 10 eEPSCs immediately before drug application and 10 eEPSCs during the maximal drug effect to calculate CV in the baseline and drug application respectively. The selection of 10 sweeps was adequate for analysis based on the low variation of eEPSCs amplitude and ensured that they were captured at the time of the maximum drug response. Including more sweeps would increase the chance that some sweeps may not have reached maximal 5-HT effect and if included in the analysis would erroneously increase CV value.
Drug application
The following drugs were obtained from 1) Sigma-Aldrich (St. Louis, MO): 5-HT, 8-OH-DPAT, and WAY 100635; 2) Tocris Bioscience (Ellisville, MO): serotonin, CP93129, GR55562, picrotoxin, and CGP 53432. Picrotoxin was included in the patch solution with final concentration of 100 µM. All other drugs were applied in the ACSF using a continuous gravity-fed bath application. Agonists were applied for 6 min and antagonists were continuously applied throughout whole recording duration.
Statistical analysis
Statistical analysis was performed using Graphpad Prism 4.0 (Graphpad Software Inc, La Jolla, CA) or Microsoft Excel software (Office 2010). For evoked EPSCs, the amplitudes were normalized and expressed as the percentage of the baseline eEPSC amplitude. Data are presented as mean ± S.E.M. Sample size n refers to numbers of neurons; no more than 2 neurons were sampled per rat. Changes before and during drug applications were analyzed using paired Student’s t-test. Two-way repeated measures ANOVA (two-way RM ANOVA) was used to determine the difference between drug treatments during application. For all comparisons, a p value < 0.05 was considered statistically significant.
Results
Monosynaptic evoked postsynaptic currents were elicited in BLA principal neurons by stimulation of either the external- or internal capsule. Evoked excitatory post-synaptic currents (eEPSCs) were isolated by inclusion of picrotoxin (100 µM) in the intracellular solution, and CGP 54532 (1 µM) in the ACSF (see Methods). Any apparent polysynaptic events were excluded from the analysis.
5-HT suppresses glutamatergic transmission in afferent pathways from the cortex and thalamus onto BLA principal neurons
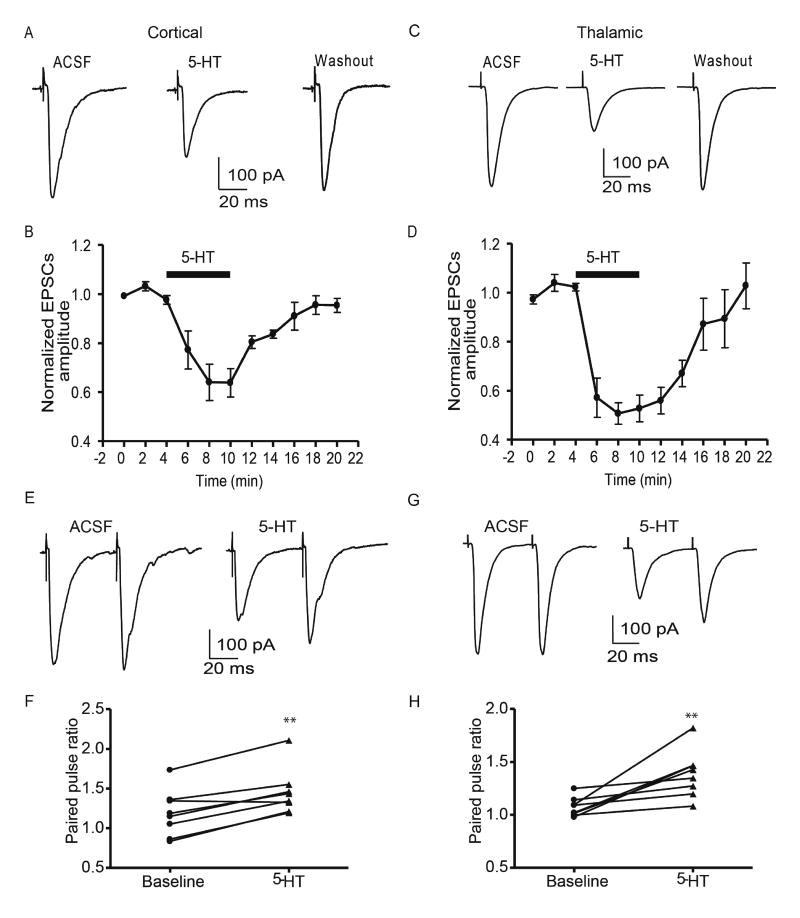
Bath application of 5-HT (10 µM) caused a time-dependent decrease in the amplitude of cortical eEPSCs, with a peak inhibition of 63.8±5.8% of baseline reached 6 min after drug application (baseline 335±29 pA, 5-HT 203±21 pA, p<0.01, paired t-test, n=6 from 3 rats, Fig 1A, B). Similarly, bath application of 5-HT (10 µM) decreased the amplitude of thalamic eEPSCs to 52.8±5.4% of baseline after 6 min (baseline 391±47 pA, 5-HT 206±26 pA; n=7 from 4 rats, p<0.01, paired t-test, Fig 1C, D). The inhibitory effect of 5-HT could be reversed after a 10-minute washout with ACSF for both cortical (314±32 pA, 94±8.9% of baseline, p<0.01, paired t-test, n=6 from 3 rats) and thalamic pathways (374±68 pA; 102.8%±9.3% of baseline, p<0.01 vs 5-HT, paired t-test, n=7 from 4 rats). No significant difference was observed in the effects of 5-HT on the cortical versus thalamic pathway (two-way RM ANOVA, F(1,55)=2.62, p>0.05). Notably, increasing the concentration of 5-HT to 50 µM failed to further reduce the amplitude of cortical eEPSCs (baseline 251±45 pA; 5-HT 151±29 pA; 65±5% of baseline, n=5 from 3 rats; paired t-test p<0.05 vs baseline; p>0.05 vs 5-HT 10 µM, unpaired t-test), suggesting that 10 µM 5-HT was close to the asymptote of the dose-response curve. Hence, in subsequent studies, we used 10 µM 5-HT to determine 1) the site of action of 5-HT and, 2) the receptor subtype mediating the 5-HT response.
Figure 1. Effects of 5-HT on evoked EPSCs in cortical and thalamic glutamate inputs onto BLA pyramidal neurons.
5-HT (10 µM) reversibly decreased the amplitude of eEPSCs in either cortical (A, B) or thalamic pathway (C,D). The decrease of eEPSCs amplitude during 5-HT application was associated with an increase of paired pulse ratio in either cortical (E, F) or thalamic pathway (G, H). **, p<0.01 vs baseline.
A change of the eEPSC amplitude induced by 5-HT could be due to either pre- or postsynaptic actions. To identify the locus of action of 5-HT, we examined the paired-pulse ratio (PPR, interval 50 ms) of eEPSCs as well as the coefficient of variation (CV) before and during 5-HT application. In both cortical and thalamic pathways, application of 5-HT decreased the amplitude of both the first and second eEPSC, with the first eEPSC reduced more than the second. At baseline, the cortical PPR of eEPSCs was 1.22±0.10, whereas during 5-HT application the PPR increased to 1.52±0.10 (n=8 from 4 rats, paried t-test, p<0.01, Fig 1E,F). Similarly, 5-HT application increased the thalamic PPR compared to baseline (1.10±0.03 baseline; 1.42±0.08 5-HT; n=8 from 4 rats, paried t-test, p<0.01, Fig 1 G, H). Together the reduced eEPSC amplitude and the increase in the PPR strongly suggested 5-HT acted presynaptically in both cortical and thalamic pathways.
Consistent with this observation, 5-HT application also increased the CV of cortical and thalamic eEPSCs compared to baseline. Cortical CV increased from 0.15±0.03 to 0.23±0.05 during 5-HT application (n=6 from 3 rats, paired t-test, p<0.05), and thalamic CV increased from 0.12±0.02 to 0.22±0.06 (n=7 from 4 rats, paried t-test, p<0.05). The increase in CV in both pathways further suggested that presynaptic 5-HT receptors changed the probability of release and/or the number of release sites in glutamatergic terminals. We next investigated whether the two pathways were regulated by the same, or different, presynaptic 5-HT receptor subtypes.
5-HT1B agonists mimicked the 5-HT effect
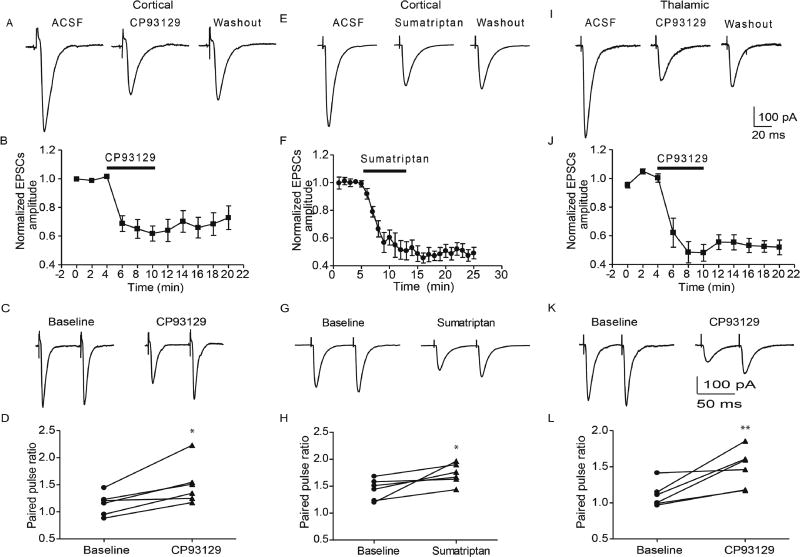
Out of the seven 5-HT receptor subfamilies, the Gi-coupled 5-HT1 receptor family has been most widely examined for its inhibitory action on glutamatergic transmission (Pehrson and Sanchez, 2014). Indeed, the 5-HT1B receptor is predominantly expressed on presynaptic terminals (Boschert et al., 1994), and has been shown to decrease glutamate release in multiple brain regions, including the extended amygdala (Guo and Rainnie, 2010). Hence, we first tested whether a selective 5-HT1B receptor agonist CP93129 could mimic the effect of 5-HT on eEPSCs. Bath application of CP93129 (10 µM) significantly decreased cortical eEPSC amplitude to 61.8 ± 5.3% of baseline (baseline 415±33 pA, CP93129 272±36 pA; paried t-test, p<0.01, n=6 from 3 rats, Fig 2A,B), and thalamic eEPSC amplitude to 48.2±5.2% of baseline (baseline 295±42 pA, CP93129 163±34 pA; paired t-test, p<0.01, n=7 from 4 rats, Fig 2I,J). Similar to the effect of 5-HT on cortical and thalamic pathways, the effect of CP93129 in the thalamic pathway showed no significant difference from that of cortical pathway (two way RM ANOVA, F(1,55)=2.62, p>0.05). In contrast to 5-HT, the effect of CP93129 on eEPSC amplitude showed little to no reversal after a 10 min washout in ACSF for both the cortical (288±46 pA; 73±8% of baseline, paired t-test, p>0.05 vs CP93129, n=6 from 3 rats) and thalamic pathways (201±49 pA, 53±5.2% of baseline, paired t-test, p>0.05 vs 5-HT, n=7 from 4 rats). Similar to the 5-HT response, the CP93129-induced decrease of eEPSCs amplitude was associated with an increase of PPR for eEPSCs in both the cortical (baseline 1.15±0.08; CP93129 1.51±0.16, n=7 from 4 rats, paired t-test, p<0.05, Fig 2E, F) and thalamic pathways (baseline 1.13±0.06; CP93129 1.44±0.13, paired t-test, p<0.05, n=6 from 3 rats, Fig 2K, L). Application of CP93129 also increased the CV of cortical (0.11±0.022 baseline; 0.16±0.024 CP93129, n=6 from 3 rats, paired t-test, p<0.05) and thalamic eEPSCs (baseline 0.13±0.02, CP93129 0.25±0.02, n=6 from 3 rats, paired t-test, p<0.05). Taken together, these results strongly suggest that presynaptic 5-HT1B receptor activation decreases glutamate release in both cortical and thalamic pathways.
Figure 2. 5-HT1B receptor agonist mimicked the effect of 5-HT on eEPSCs in BLA.
A, B) 5-HT1B receptor agonist CP93129 (10 µM) reduced the amplitude of eEPSCs in cortical pathway, which effect was accompanied by an increase of paired pulse ratio (C, D). E,F) Mixed 5-HT1B/D agonist sumatriptan (10 µM) reduced the amplitude of eEPSCs in cortical pathway. The decrease of eEPSCs amplitude was associated with increases of PPR (G,H). I,J) In thalamic pathway CP 93129 (10 µM) decreased the amplitude of eEPSCs, which associated with an increase of paired pulse ratio. *,**, p<0.05 and 0.01 respectively vs baseline.
We also examined the effect of the mixed 5-HT1B/D agonist, sumatriptan, on eEPSCs in cortical pathway. Similar to CP93129, sumatriptan (10 µM) decreased the amplitude of eEPSCs (peak inhibition to 45.5±4% of baseline; baseline 361±47 pA; sumatriptan 159±22 pA; n=6 from 3 rats, p<0.01 paired t-test, Fig 2E,F). The decreased eEPSCs amplitude was also accompanied by a significant increase of PPR (Baseline 1.44±0.08; sumatriptan 1.73±0.08; n=6 from 3 rats, paried t-test, p<0.05, Fig 2 G,H), suggesting this effect was mediated by a presynaptic inhibition of glutamate release. Consistent with the action of CP93129, the sumatriptan-induced inhibition of eEPSCs persisted after 10 min of washout as well.
As there is no apparent difference in serotoninergic inhibition of EPSCs between cortical and thalamic pathways, in following experiments further justifying underlying receptors we only examined drug actions in the cortical pathway.
The inhibitory effect of 5-HT receptor activation on EPSC amplitude was blocked by 5-HT1B receptor antagonists
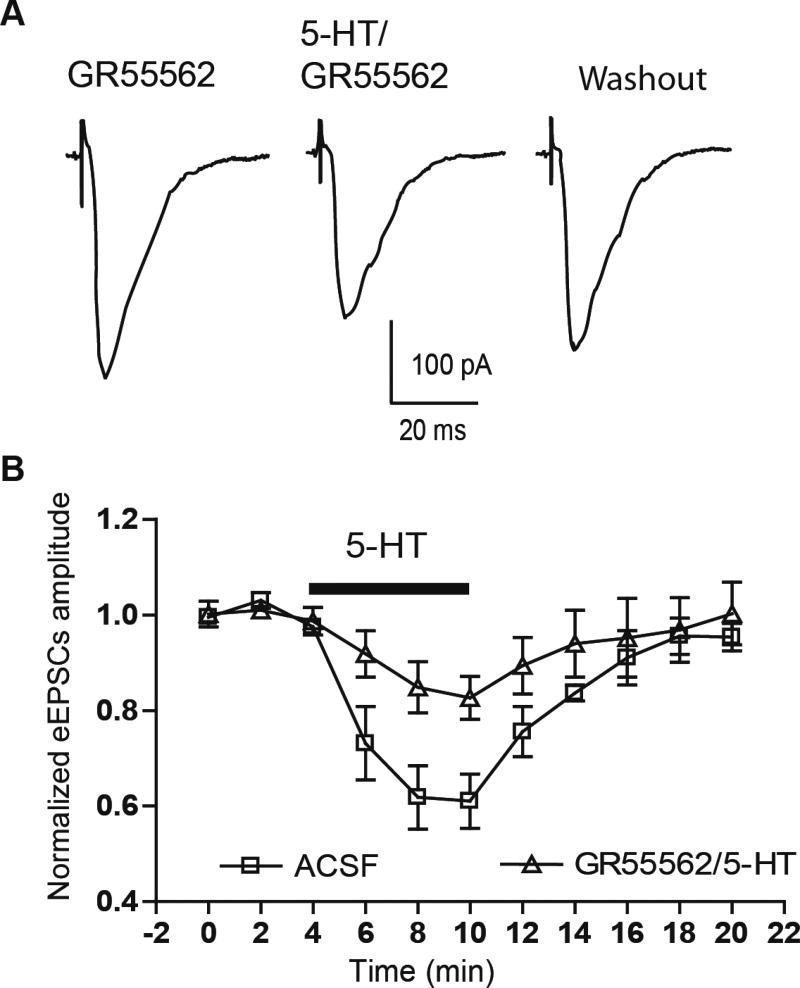
To verify that presynaptic 5-HT1B receptors mediated the effect of 5-HT on eEPSCs, we tested whether prior application of the selective 5-HT1B receptor antagonist GR55562 could block the effect of exogenous 5-HT. Here, application of GR55562 (20 µM), which had no effect on the amplitude of eEPSCs by itself (baseline 354±55 pA, GR55562 360±53 pA, n=5 from 3 rats, p>0.05, paired t-test), markedly attenuated the inhibitory effect of 5-HT (10 µM) on cortical eEPSC amplitude, with a reduction to 82.6±4.5% of baseline (GR55562 alone 334±47 pA, 5-HT in GR55562 276±44 pA, paired t-test, p<0.05, n=6 from 3 rats, Fig 3A, B). ANOVA showed that GR55562 caused a significant reduction in the effect of 5-HT application compared to that in ACSF alone (61.2±5.2% of baseline, two-way RM ANOVA, F(1,66)=7.06, p<0.05).
Figure 3. 5-HT1B antagonist blocked the effect of 5-HT on eEPSCs.
Selective 5-HT1B antagonist GR55562 (20 µM) significantly attenuated the effect of 5-HT (10 µM) on eEPSCs in cortical pathway(A,B).
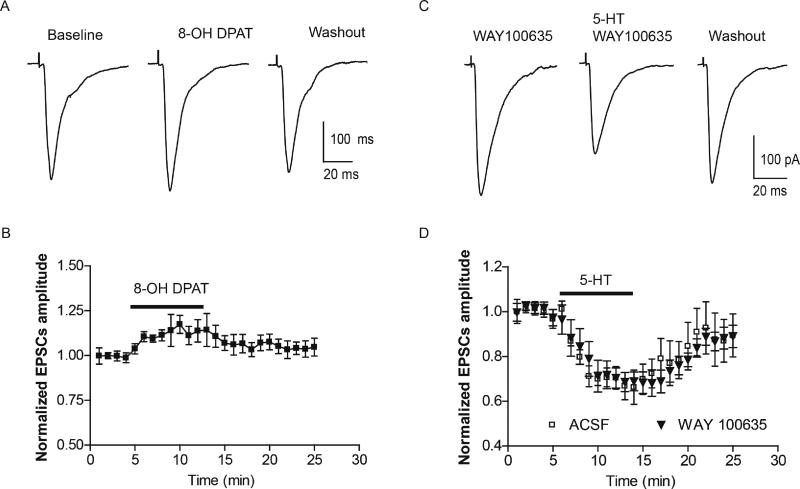
Because application of GR55562 did not fully block the effects of 5-HT we next examined if additional 5-HT receptor subtypes contribute to the presynaptic 5-HT response. Indeed, presynaptic 5-HT1A receptor activation was reported to attenuate glutamatergic transmission from the lateral amygdala (LA) to the BLA (Cheng et al., 1998). We next tested whether presynaptic 5-HT1A receptor activation contributed to the 5-HT-induced attenuation of glutamate inputs to the BLA. Unlike CP93129, application of the selective 5-HT1A receptor agonist 8-OH DPAT (10 µM) failed to decrease the amplitude of cortical eEPSCs in any neuron tested. Instead, a slight yet non-significant increase was noticed (baseline 260±30 pA, 8-OH DPAT 291±40 pA; 110±7% of baseline, n=7 from 4 rats, paired t-test, p>0.05, Fig 4A,B). To further exclude the possibility that 5-HT1A receptor activation contributed to the 5-HT response, we examined the effects of prior application of the 5-HT1A receptor antagonist, WAY100635. Application of WAY 100635 (1 µM) alone had no effect on eEPSC amplitude compared to baseline, and had no effect on the 5-HT(10 µM) induced attenuation of the eEPSC amplitude (WAY 100635 324±60 pA, 5-HT in WAY 100635 237±57 pA, paired t-test, p<0.01 vs baseline, n=7 from 4 rats, Fig 4C,D), a response that was not significantly different from the effect of 5-HT application in ACSF alone (two way RM ANOVA, F(1,288)=0.54, p>0.05).
Figure 4. Effect of 5-HT1A activation on cortical eEPSCs.
A,B) 5-HT1A agonist 8-OH DPAT slightly increased the amplitude of eEPSCs in cortical pathway. C,D) Blockade of 5-HT1A receptor with WAY 100635 (1 µM) has no effect on 5-HT (10 µM) inhibition of cortical eEPSCs.
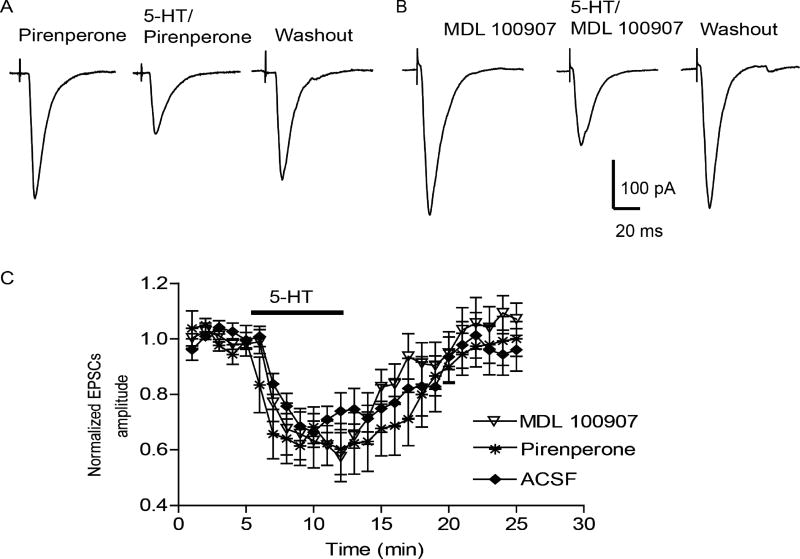
Finally, a recent study reported that 5-HT2 receptor activation also contributes to the 5-HT-induced suppression of glutamatergic transmission in the BLA (Yamamoto et al., 2012). To determine whether 5-HT2 receptor activation contributed to the residual 5-HT-induced EPSCs decrease observed in this study, we examined the effect of 5-HT (10 µM) in the presence of two 5-HT2 receptor antagonists. First, we tested the effect of the non-selective 5-HT2 receptor antagonist pirenperone. In the presence of 10 µM pirenperone, a concentration known to fully block 5-HT2-mediated postsynaptic actions in the BNST (Guo et al., 2009), 5-HT(10 µM) significantly reduced the amplitude of eEPSCs to 60± 9% of baseline (peak inhibition, baseline 256±11 pA, 5-HT 167±17 pA, n=6 from 3 rats, paired t-test, p<0.05), which was not different from the 5-HT effect in ACSF alone (two-way RM ANOVA, F(1,264)=0.50, p>0.05, Fig 5A,C).
Figure 5. 5-HT2 receptor antagonists have no effect on 5-HT inhibition of EPSCs in cortical pathway.
In the presence of 5-HT2A antagonist MDL 100907 (10 µM) (A) or non-selective 5-HT2 antagonist pirenperone (10 µM)(B), 5-HT (10 µM) significantly decreased the amplitude of cortical eEPSCs, which is not different from 5-HT effect in control ACSF (C).
Lastly, 5-HT2A receptors are highly expressed in the BLA (Bombardi, 2011). Despite the fact that 5-HT2A receptor activation has been reported to facilitate glutamatergic transmission elsewhere in the CNS (Hasuo et al., 2002), we tested whether the selective 5-HT2A receptor antagonist MDL 100907 could block the effect of 5-HT on glutamatergic transmission. Prior application of MDL 100907 (10 µM) had no significant effect on the 5-HT(10 µM) induced attenuation of eEPSC amplitude (MDL 100907 293±29 pA, 5-HT 171±27 pA, maximal inhibition to 60±2% of baseline, paired t-test, p<0.05, n=6 from 3 rats, Fig 5B, C), which was not different from 5-HT effect in ACSF alone (two-way RM ANOVA, F(1,264)=0.03, p>0.05).
Taken together, our results strongly suggest that presynaptic 5-HT1B receptors are the predominant 5-HT receptor subtype mediating presynaptic inhibition of glutamatergic input onto BLA principal neurons. Moreover, our data further suggest that the response to 5-HT is common to both cortical and subcortical pathways into the BLA.
Discussion
In this study, we extended earlier observations that reported serotonergic modulation of glutamatergic transmission onto BLA principal neurons. We conclude this effect is consistent across afferent pathways and predominantly mediated by activation of presynaptic 5-HT1B receptors and not by activation of 5-HT1A or 5-HT2 receptors.
Serotonin is known to play a central role in early brain development, regulation of mood, aggression, stress reactivity, and the risk of developing psychiatric diseases (Lesch et al., 2012; Azmitia, 2007; Sodhi & Sanders-Bush, 2004). However, the exact role of 5-HT in regulating such a diverse array of functions has been complicated due to the existence of 14 receptor subtypes (Palacios, 2016). Moreover, not only does 5-HT regulate neuronal function by direct actions at postsynaptic 5-HT receptors, it also acts as a presynaptic neuromodulator to regulate release of many other neurotransmitters. Of the 14 5-HT receptor subtypes, 5-HT1A/B/D receptors are believed to be the predominant presynaptic 5-HT receptors (Hoyer et al., 2002, Fink and Gothert, 2007). Whereas, 5-HT1A receptors are located both pre- and postsynaptically, mRNA expression studies, binding studies, and electron microscopy studies suggest that 5-HT1B receptors are mainly presynaptic (Boschert et al., 1994, Sari et al., 1997). Similar to 5-HT1B receptors, 5-HT1D receptors are thought to act predominantly as presynaptic receptors, but are only expressed at low levels in the rodent brain (Lanfumey and Hamon, 2004). In this study we showed that the 5-HT1B receptor agonist CP93129 and the mixed 5-HT1B/D receptor agonist sumatriptan had similar effects on eEPSC amplitude, and that the 5-HT1B receptor antagonist GR55562 blocked the effect of 5-HT. Taken together we propose that the 5-HT1B receptor, and not the 5-HT1D receptor, is the major 5-HT receptor subtype acting to reduce glutamate release in the BLA.
In rats the amygdala receives dense and topographically organized serotonergic inputs from the raphe nucleus, with the BLA showing the highest density of 5-HT terminal innervation (Vertes et al., 1999, Muller et al., 2007, Bonn et al., 2013, Linley et al., 2017). The amygdala also shows moderate to high levels of ligand binding for multiple 5-HT receptor subtypes, including 5-HT1A, 1B, 2A, 2C,3 and 5-HT7 receptors (Gustafson et al., 1996, Saha et al., 2010, Bombardi, 2011, Bocchio et al., 2016). As mentioned earlier, 5-HT1B receptors are mainly expressed on axon terminals. Hence, it is possible that the 5-HT1B binding observed in the amygdala (Bonaventure et al., 1998) represents heteroreceptors on axon terminals from upstream input regions, including cerebral cortex and thalamus, which also express mRNA for 5-HT1B receptors (Bruinvels et al., 1994). These studies offer morphological support for 5-HT1B receptor modulation of glutamate release in the BLA.
Indeed, we have shown that the decreased eEPSC amplitude during 5-HT application was associated with an increase of both the paired-pulse ratio and the coefficient of variation, strongly suggesting a presynaptic mechanism of 5-HT action. Our results are consistent with previous studies showing that 5-HT decreased glutamatergic transmission in multiple brain regions including the BLA (Cheng et al., 1998, Rainnie, 1999, Yamamoto et al., 2012). However depending on the brain region examined, the inhibitory effect of 5-HT was reported to be mediated by 5-HT1A receptors (Costa et al., 2012, Ostrowski et al., 2014), 5-HT1B receptors (Singer et al., 1996, Laurent et al., 2002, Lemos et al., 2006, Choi et al., 2012), or both (Bouryi and Lewis, 2003).
In the current study we found the selective 5-HT1B receptor agonist and antagonist could respectively mimic or block the effect of 5-HT, but the selective 5-HT1A receptor agonist or antagonist had no effect. The latter result is in conflict with an earlier study that reported 5-HT1A receptor-mediated inhibition of glutamate release in the BLA (Cheng et al., 1998). However consistent with our observation, Yamamoto and colleagues reported that application of 8-OH DPAT had no inhibitory effect on eEPSCs amplitude in the BLA (Yamamoto et al., 2012). The discrepancy between our results and that of Cheng and colleagues may result from activation of different afferent pathways. However, this cannot explain the discrepancy between the Cheng and Yamamoto studies in which both groups induced eEPSCs in the BLA by stimulating the LA. Interestingly, in this study application of 8-OH DPAT induced a slight yet non-significant increase of amplitude of eEPSCs. This effect may be due to an indirect activation of 5-HT1A receptors located on NPY-expressing GABAergic interneurons (Bonn et al., 2013). Activation of 5-HT1A receptors could in turn hyperpolarize GABA interneurons and lead to disinhibition of glutamate release. However, to avoid complications arising from bath application of picrotoxin, we isolated eEPSCs using intracellular picrotoxin application. Hence, this indirect gating of glutamatergic transmission mediated by 5-HT1A receptor cannot be excluded.
Interestingly, Yamamoto and colleagues further suggested that 5-HT2 receptors may contribute to the inhibitory effect of 5-HT (Yamamoto et al., 2012) as the non-selective 5-HT2 receptor agonist α-methyl-5-HT mimicked the effect of 5-HT. However, we think this conclusion needs reevaluation. Firstly, 5-HT2 receptors are Gq/11 coupled and are consistently reported to increase excitability (Hasuo et al., 2002, Campbell and Merchant, 2003). Indeed, Daftary and colleagues have reported that 5-HT facilitated glutamatergic transmission in BLA pyramidal neurons through activation of 5-HT2A receptors (Daftary et al., 2012). Secondly, α-methyl-5-HT has a high affinity for 5-HT1A (Ki 42 nM) and 5-HT1B receptors (Ki 85 nM) (Ismaiel et al., 1990). At the agonist concentration used in Yamamoto study, 5 µM, α-methyl-5-HT most likely also activated presynaptic 5-HT1B receptors. Hence, the effect of α-methyl-5-HT on glutamatergic transmission might be due to a spillover effect on presynaptic 5-HT1B receptors rather than through 5-HT2 receptor activation. Finally, we saw no effects of prior application of the non-selective 5-HT2 receptor antagonist pirenperone on the inhibitory 5-HT response. While we never observed a facilitatory effect of 5-HT on the eEPSC amplitude, it is possible that residual 5-HT2A receptor activation is insufficient to overcome the inhibitory effect of 5-HT1B activation. In future studies we will selectively examine the role of 5-HT2 receptors in modulating glutamatergic transmission in the BLA.
Functional MRI studies have revealed that the amygdala is abnormally active in anxiety disorders, post-traumatic stress disorder (PTSD), and depression (Fredrikson and Faria, 2013). Significantly, treatment of patients with depression using selective serotonin reuptake inhibitors (SSRIs) normalizes amygdala hyper-activation, and this response positively correlates with reduced symptom severity (Sheline et al., 2001, Langenecker et al., 2007). Notably, decreased 5-HT1B receptor binding has been observed in patients with PTSD, which was closely associated with the age of first trauma (Murrough et al., 2011a). A similar decrease of 5-HT1B receptor binding has also been found in patients with MDD (Murrough et al., 2011b). Moreover, a polymorphism of the HTR1B gene has been associated with the antidepressant effect of SSRIs (Villafuerte et al., 2009), and the methylation level of the HTR1B gene is negatively correlated with clinical improvement after fluoxetine (Gasso et al., 2016). As shown in this study, 5-HT1B acts to decrease glutamate release from cortical and thalamic inputs onto BLA projection neurons. Together the evidence presented above suggests 5-HT1B receptors in the BLA may be a unique target for the treatment of mood and anxiety disorders.
Highlights.
5-HT attenuates glutamatergic cortical and thalamic inputs onto BLA principal neurons
Presynaptic 5-HT receptors gate cortical and thalamic glutamate inputs into the BLA
Presynaptic gating is mediated by 5-HT1B, but not 5-HT1A or 5-HT2 receptors
Acknowledgments
This work was supported by NIH MH069852 and MH069056 Sub-Project 6569 to DGR, National Primate Research Center base grant #RR-00165, Animal Resource Program at NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrams JK, Johnson PL, Hollis JH, Lowry CA. Anatomic and functional topography of the dorsal raphe nucleus. Ann N Y Acad Sci. 2004;1018:46–57. doi: 10.1196/annals.1296.005. [DOI] [PubMed] [Google Scholar]
- Asan E, Steinke M, Lesch KP. Serotonergic innervation of the amygdala: targets, receptors, and implications for stress and anxiety. Histochem Cell Biol. 2013;139:785–813. doi: 10.1007/s00418-013-1081-1. [DOI] [PubMed] [Google Scholar]
- Bocchio M, McHugh SB, Bannerman DM, Sharp T, Capogna M. Serotonin, Amygdala and Fear: Assembling the Puzzle. Front Neural Circuits. 2016;10:24. doi: 10.3389/fncir.2016.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bombardi C. Distribution of 5-HT2A receptor immunoreactivity in the rat amygdaloid complex and colocalization with gamma-aminobutyric acid. Brain Res. 2011;1370:112–128. doi: 10.1016/j.brainres.2010.11.055. [DOI] [PubMed] [Google Scholar]
- Bonaventure P, Voorn P, Luyten WH, Jurzak M, Schotte A, Leysen JE. Detailed mapping of serotonin 5-HT1B and 5-HT1D receptor messenger RNA and ligand binding sites in guinea-pig brain and trigeminal ganglion: clues for function. Neuroscience. 1998;82:469–484. doi: 10.1016/s0306-4522(97)00302-3. [DOI] [PubMed] [Google Scholar]
- Bonn M, Schmitt A, Lesch KP, Van Bockstaele EJ, Asan E. Serotonergic innervation and serotonin receptor expression of NPY-producing neurons in the rat lateral and basolateral amygdaloid nuclei. Brain Struct Funct. 2013;218:421–435. doi: 10.1007/s00429-012-0406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boschert U, Amara DA, Segu L, Hen R. The mouse 5-hydroxytryptamine1B receptor is localized predominantly on axon terminals. Neuroscience. 1994;58:167–182. doi: 10.1016/0306-4522(94)90164-3. [DOI] [PubMed] [Google Scholar]
- Bouryi VA, Lewis DI. The modulation by 5-HT of glutamatergic inputs from the raphe pallidus to rat hypoglossal motoneurones, in vitro. J Physiol. 2003;553:1019–1031. doi: 10.1113/jphysiol.2003.053843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruinvels AT, Landwehrmeyer B, Gustafson EL, Durkin MM, Mengod G, Branchek TA, Hoyer D, Palacios JM. Localization of 5-HT1B, 5-HT1D alpha, 5-HT1E and 5-HT1F receptor messenger RNA in rodent and primate brain. Neuropharmacology. 1994;33:367–386. doi: 10.1016/0028-3908(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Campbell BM, Merchant KM. Serotonin 2C receptors within the basolateral amygdala induce acute fear-like responses in an open-field environment. Brain Res. 2003;993:1–9. doi: 10.1016/s0006-8993(03)03384-5. [DOI] [PubMed] [Google Scholar]
- Cheng LL, Wang SJ, Gean PW. Serotonin depresses excitatory synaptic transmission and depolarization-evoked Ca2+ influx in rat basolateral amygdala via 5-HT1A receptors. Eur J Neurosci. 1998;10:2163–2172. doi: 10.1046/j.1460-9568.1998.00229.x. [DOI] [PubMed] [Google Scholar]
- Choi IS, Cho JH, An CH, Jung JK, Hur YK, Choi JK, Jang IS. 5-HT(1B) receptors inhibit glutamate release from primary afferent terminals in rat medullary dorsal horn neurons. Br J Pharmacol. 2012;167:356–367. doi: 10.1111/j.1476-5381.2012.01964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JP, Ragole T, Amat J, Greenwood BN, Strong PV, Paul ED, Fleshner M, Watkins LR, Maier SF. 5-hydroxytryptamine 2C receptors in the basolateral amygdala are involved in the expression of anxiety after uncontrollable traumatic stress. Biol Psychiatry. 2010;67:339–345. doi: 10.1016/j.biopsych.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelio AM, Nunes-de-Souza RL. Anxiogenic-like effects of mCPP microinfusions into the amygdala (but not dorsal or ventral hippocampus) in mice exposed to elevated plus-maze. Behav Brain Res. 2007;178:82–89. doi: 10.1016/j.bbr.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Costa L, Trovato C, Musumeci SA, Catania MV, Ciranna L. 5-HT(1A) and 5-HT(7) receptors differently modulate AMPA receptor-mediated hippocampal synaptic transmission. Hippocampus. 2012;22:790–801. doi: 10.1002/hipo.20940. [DOI] [PubMed] [Google Scholar]
- Daftary SS, Calderon G, Rios M. Essential role of brain-derived neurotrophic factor in the regulation of serotonin transmission in the basolateral amygdala. Neuroscience. 2012;224:125–134. doi: 10.1016/j.neuroscience.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. Neural circuitry of anxiety and stress disorders. In: Davis Kenneth L, Joseph T Coyle DC, Nemeroff Charles., editors. Neuropsychopharmacology: The Fifth Generation of Progress. Philadelphia: American College of Neuropsychopharmacology; 2002. pp. 931–951. [Google Scholar]
- de Mello Cruz AP, Pinheiro G, Alves SH, Ferreira G, Mendes M, Faria L, Macedo CE, Motta V, Landeira-Fernandez J. Behavioral effects of systemically administered MK-212 are prevented by ritanserin microinfusion into the basolateral amygdala of rats exposed to the elevated plus-maze. Psychopharmacology (Berl) 2005;182:345–354. doi: 10.1007/s00213-005-0108-2. [DOI] [PubMed] [Google Scholar]
- Fink KB, Gothert M. 5-HT receptor regulation of neurotransmitter release. Pharmacol Rev. 2007;59:360–417. doi: 10.1124/pr.107.07103. [DOI] [PubMed] [Google Scholar]
- Fredrikson M, Faria V. Neuroimaging in anxiety disorders. Mod Trends Pharmacopsychiatri. 2013;29:47–66. doi: 10.1159/000351938. [DOI] [PubMed] [Google Scholar]
- Gasso P, Rodriguez N, Blazquez A, Monteagudo A, Boloc D, Plana MT, Lafuente A, Lazaro L, Arnaiz JA, Mas S. Epigenetic and genetic variants in the HTR1B gene and clinical improvement in children and adolescents treated with fluoxetine. Prog Neuropsychopharmacol Biol Psychiatry. 2016;75:28–34. doi: 10.1016/j.pnpbp.2016.12.003. [DOI] [PubMed] [Google Scholar]
- Guo JD, Hammack SE, Hazra R, Levita L, Rainnie DG. Bi-directional modulation of bed nucleus of stria terminalis neurons by 5-HT: molecular expression and functional properties of excitatory 5-HT receptor subtypes. Neuroscience. 2009;164:1776–1793. doi: 10.1016/j.neuroscience.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JD, Hazra R, Dabrowska J, Muly EC, Wess J, Rainnie DG. Presynaptic muscarinic M(2) receptors modulate glutamatergic transmission in the bed nucleus of the stria terminalis. Neuropharmacology. 2012;62:1671–1683. doi: 10.1016/j.neuropharm.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JD, Rainnie DG. Presynaptic 5-HT(1B) receptor-mediated serotonergic inhibition of glutamate transmission in the bed nucleus of the stria terminalis. Neuroscience. 2010;165:1390–1401. doi: 10.1016/j.neuroscience.2009.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasuo H, Matsuoka T, Akasu T. Activation of presynaptic 5-hydroxytryptamine 2A receptors facilitates excitatory synaptic transmission via protein kinase C in the dorsolateral septal nucleus. J Neurosci. 2002;22:7509–7517. doi: 10.1523/JNEUROSCI.22-17-07509.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- Ismaiel AM, Titeler M, Miller KJ, Smith TS, Glennon RA. 5-HT1 and 5-HT2 binding profiles of the serotonergic agents alpha-methylserotonin and 2-methylserotonin. J Med Chem. 1990;33:755–758. doi: 10.1021/jm00164a046. [DOI] [PubMed] [Google Scholar]
- Kim M, Campeau S, Falls WA, Davis M. Infusion of the non-NMDA receptor antagonist CNQX into the amygdala blocks the expression of fear-potentiated startle. Behav Neural Biol. 1993;59:5–8. doi: 10.1016/0163-1047(93)91075-x. [DOI] [PubMed] [Google Scholar]
- Lanfumey L, Hamon M. 5-HT1 receptors. Curr Drug Targets CNS Neurol Disord. 2004;3:1–10. doi: 10.2174/1568007043482570. [DOI] [PubMed] [Google Scholar]
- Langenecker SA, Kennedy SE, Guidotti LM, Briceno EM, Own LS, Hooven T, Young EA, Akil H, Noll DC, Zubieta JK. Frontal and limbic activation during inhibitory control predicts treatment response in major depressive disorder. Biol Psychiatry. 2007;62:1272–1280. doi: 10.1016/j.biopsych.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent A, Goaillard JM, Cases O, Lebrand C, Gaspar P, Ropert N. Activity-dependent presynaptic effect of serotonin 1B receptors on the somatosensory thalamocortical transmission in neonatal mice. J Neurosci. 2002;22:886–900. doi: 10.1523/JNEUROSCI.22-03-00886.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Choi JS, Brown TH, Kim JJ. Amygdalar nmda receptors are critical for the expression of multiple conditioned fear responses. J Neurosci. 2001;21:4116–4124. doi: 10.1523/JNEUROSCI.21-11-04116.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos JC, Pan YZ, Ma X, Lamy C, Akanwa AC, Beck SG. Selective 5-HT receptor inhibition of glutamatergic and GABAergic synaptic activity in the rat dorsal and median raphe. Eur J Neurosci. 2006;24:3415–3430. doi: 10.1111/j.1460-9568.2006.05222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Dabrowska J, Hazra R, Rainnie DG. Synergistic activation of dopamine D1 and TrkB receptors mediate gain control of synaptic plasticity in the basolateral amygdala. PLoS One. 2011;6:e26065. doi: 10.1371/journal.pone.0026065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linley SB, Olucha-Bordonau F, Vertes RP. Pattern of distribution of serotonergic fibers to the amygdala and extended amygdala in the rat. J Comp Neurol. 2017;525:116–139. doi: 10.1002/cne.24044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry CA, Johnson PL, Hay-Schmidt A, Mikkelsen J, Shekhar A. Modulation of anxiety circuits by serotonergic systems. Stress. 2005;8:233–246. doi: 10.1080/10253890500492787. [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Serotonin-immunoreactive axon terminals innervate pyramidal cells and interneurons in the rat basolateral amygdala. J Comp Neurol. 2007;505:314–335. doi: 10.1002/cne.21486. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Czermak C, Henry S, Nabulsi N, Gallezot JD, Gueorguieva R, Planeta-Wilson B, Krystal JH, Neumaier JF, Huang Y, Ding YS, Carson RE, Neumeister A. The effect of early trauma exposure on serotonin type 1B receptor expression revealed by reduced selective radioligand binding. Arch Gen Psychiatry. 2011a;68:892–900. doi: 10.1001/archgenpsychiatry.2011.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Henry S, Hu J, Gallezot JD, Planeta-Wilson B, Neumaier JF, Neumeister A. Reduced ventral striatal/ventral pallidal serotonin1B receptor binding potential in major depressive disorder. Psychopharmacology (Berl) 2011b;213:547–553. doi: 10.1007/s00213-010-1881-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski TD, Ostrowski D, Hasser EM, Kline DD. Depressed GABA and glutamate synaptic signaling by 5-HT1A receptors in the nucleus tractus solitarii and their role in cardiorespiratory function. J Neurophysiol. 2014;111:2493–2504. doi: 10.1152/jn.00764.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padival M, Quinette D, Rosenkranz JA. Effects of repeated stress on excitatory drive of basal amygdala neurons in vivo. Neuropsychopharmacology. 2013;38:1748–1762. doi: 10.1038/npp.2013.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pehrson AL, Sanchez C. Serotonergic modulation of glutamate neurotransmission as a strategy for treating depression and cognitive dysfunction. CNS Spectr. 2014;19:121–133. doi: 10.1017/S1092852913000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainnie DG. Serotonergic modulation of neurotransmission in the rat basolateral amygdala. J Neurophysiol. 1999;82:69–85. doi: 10.1152/jn.1999.82.1.69. [DOI] [PubMed] [Google Scholar]
- Reznikov LR, Grillo CA, Piroli GG, Pasumarthi RK, Reagan LP, Fadel J. Acute stress-mediated increases in extracellular glutamate levels in the rat amygdala: differential effects of antidepressant treatment. Eur J Neurosci. 2007;25:3109–3114. doi: 10.1111/j.1460-9568.2007.05560.x. [DOI] [PubMed] [Google Scholar]
- Sari Y, Lefevre K, Bancila M, Quignon M, Miquel MC, Langlois X, Hamon M, Verge D. Light and electron microscopic immunocytochemical visualization of 5-HT1B receptors in the rat brain. Brain Res. 1997;760:281–286. doi: 10.1016/s0006-8993(97)00400-9. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Singer JH, Bellingham MC, Berger AJ. Presynaptic inhibition of glutamatergic synaptic transmission to rat motoneurons by serotonin. J Neurophysiol. 1996;76:799–807. doi: 10.1152/jn.1996.76.2.799. [DOI] [PubMed] [Google Scholar]
- Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP, Fortin WJ, Crane AM. Projections of the median raphe nucleus in the rat. J Comp Neurol. 1999;407:555–582. [PubMed] [Google Scholar]
- Villafuerte SM, Vallabhaneni K, Sliwerska E, McMahon FJ, Young EA, Burmeister M. SSRI response in depression may be influenced by SNPs in HTR1B and HTR1A. Psychiatr Genet. 2009;19:281–291. doi: 10.1097/YPG.0b013e32832a506e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Yamamoto R, Ueta Y, Sugai T, Kato N. A serotonergic discrimination favoring synaptic inputs that accompany robust spike firing in lateral amygdala neurons. Neuroscience. 2012;220:119–130. doi: 10.1016/j.neuroscience.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Zhang W, Rosenkranz JA. Repeated restraint stress increases basolateral amygdala neuronal activity in an age-dependent manner. Neuroscience. 2012;226:459–474. doi: 10.1016/j.neuroscience.2012.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]