Abstract
Background
Pancreatic cancer statistics are dismal, with a five-year survival of less than 10%, and over 50% of patients presenting with metastatic disease. Metabolic reprogramming is an emerging hallmark of pancreatic adenocarcinoma, including aerobic glycolysis, oxidative phosphorylation, glutaminolysis, lipogenesis and lipolysis, autophagic status, and anti-oxidative stress. CPI-613 is a novel anti-cancer agent that selectively targets the altered form of mitochondrial energy metabolism in tumor cells, causing changes in mitochondrial enzyme activities and redox status which lead to apoptosis, necrosis and autophagy of tumor cells.
Methods
This is a phase 1 study to determine the maximum-tolerated dose (MTD) of CPI-613 when used in combination with modified FOLFIRINOX (oxaliplatin at 65 mg/m2 and irinotecan at 140 mg/m2, and fluorouracil 400 mg/m2 bolus and 2400 mg/m2 over 46 h) in combination with CPI-613 in patients with newly diagnosed metastatic pancreatic adenocarcinoma with good bone marrow, liver and kidney function and good performance status (NCT01835041 – closed to recruitment). A two-stage dose-escalation scheme (single patient and traditional 3+3 design) was applied. In the single patient stage, one patient was accrued per dose level. The starting dose of CPI-613 was 500 mg/m2/day; the dose level was then escalated by doubling the previous dose if there was no toxicity greater than Grade 2 within 4 weeks attributed as probably or definitely related to CPI-613. The traditional 3+3 dose-escalation stage was triggered if toxicity attributed as probably or definitely related to CPI-613 was ≥ Grade 2. The dose level for CPI-613 for the first cohort in the traditional dose-escalation stage was the same as used in the last cohort of the single patient dose-escalation stage. Secondary objectives were safety, preliminary efficacy, and tissue collection for future analyses. Response rates, progression-free survival and overall survival data were assessed in the patients treated at the MTD.
Findings
Twenty patients were enrolled April 22, 2013 – January 8, 2016. The MTD of CPI-613 was 500 mg/m2. The median number of treatment cycles administered at the MTD was 11 (interquartile range, 4–19). Two patients enrolled at a higher dose (1000 mg/m2) both experienced a DLT (dose limiting toxicity). There were 2 unexpected serious adverse events (SAEs), both for the first patient enrolled: 1) possible leaching due to infusion of CPI-613 via non-PVC tubing, and 2) the patient re- accessed her port at home after accidental de-access. Neither incident resulted in a negative clinical outcome. Expected SAEs were: thrombocytopenia, anemia and lymphopenia (all for Patient #2, with anemia and lymphopenia being a DLT); hyperglycemia (Patient #7); hypokalemia, hypoalbuminemia and sepsis (Patient #11); and neutropenia (Patient #20). There was no grade 5 toxicity. For the 18 patients treated at the MTD, the most common Grade 3–4 toxicities were hypokalemia (6/18, 33%), diarrhea (5/18, 28%) and abdominal pain (4/18, 22%). Sensorial neuropathy (17/18, 94%) was managed with dose de-escalation or discontinuation per standard of care. None of the patients experienced grade 4 or 5 neuropathy. No patients died while on active treatment; 11 study participants died, with cause of death as terminal pancreatic cancer.
Among the 18 patients treated with the MTD, there were 3 patients with a complete response (CR), 1 with a non-CR/non-progressive disease, 7 with a partial response (PR), 3 with stable disease, and 4 with PD. The partial + complete response rate was 61% (11/18).
Interpretation
The treatment was well tolerated and all end points were met. The intriguing signal of efficacy will require validation in a phase 2 study.
Funding
Comprehensive Cancer Center of Wake Forest Baptist Medical Center
Introduction
Pancreatic cancer is the third leading cause of cancer death. Its prognosis is grim, with a 5-year survival rate of 7.2%.1 Over 50% of pancreatic cancer patients present with metastatic disease, when treatment is considered to be only palliative. The most efficacious treatments are FOLFIRINOX (a four-drug combination of 5-fluorouracil, leucovorin, irinotecan and oxaliplatin) and gemcitabine plus nab-paclitaxel, which provide a median overall survival of 11.1 months and 8.5 months, respectively.2,3 However, these drugs have moderate toxicity and are usually restricted to patients with good performance status and long term survival is rarely achieved. Safer and more effective therapies are sorely needed. CPI-613 is a novel anti-cancer agent that selectively targets the altered form of mitochondrial energy metabolism in tumor cells, causing changes in mitochondrial enzyme activities and redox status which lead to apoptosis, necrosis and autophagy of tumor cells.4 Rationale for targeting mitochondrial metabolism in pancreatic cancer is provided in Suppl, p. 1. These activities of CPI-613 (Suppl, p. 1) involve the catalytic and regulatory functions of the pyruvate dehydrogenase complex, its regulatory kinases, and the α-ketoglutarate dehydrogenase complex.4,5 The anti-tumor activity of CPI-613 in cell culture of multiple cancer cell lines, animal tumor models and clinical trials against diverse cancers have been documented, particularly against pancreatic cancer and leukemic cells.6–11 CPI-613 has been shown to be well-tolerated at doses up to 3,000 mg/m2 in single agent phase 1 trials of patients with solid tumors and patients with hematologic malignancies.11
In vitro data using two different pancreatic cell lines (PANC-1 and BxPC-3) shows that CPI-613 enhances FOLFIRINOX cytotoxicity in both PANC-1 and BxPC-3 cell lines (unpublished data). Due to the safety profile and anti-cancer activities as well as the preclinical data described above, it was hypothesized that CPI-613, when used in combination with FOLFIRINOX, would enhance therapeutic efficacy with little to no additional toxicity. A phase 1, open-label, dose-escalation clinical trial was conducted to determine the maximum tolerated dose (MTD) of CPI-613, when used in combination with modified FOLFIRINOX, as well as the safety and efficacy of this regimen for the treatment of metastatic pancreatic cancer. We chose modified FOLFIRINOX as a dose reduced FOLFIRINOX regimen appears to be equally effective and better tolerated than the original FOLFINIROX.12,13
Methods
Study Design and Participants
Patients were eligible for this single center 3+3 dose escalation phase 1 study if they: were ≥18 years of age; had histologically or cytologically confirmed metastatic pancreatic adenocarcinoma (pancreatic neuroendocrine tumors were excluded); had an Eastern Cooperative Group (ECOG) performance status of 0 or 1; had adequate hematologic function (granulocyte count ≥1500/mm3; white blood cell count ≥3500 cells/mm3 or ≥3.5 bil/L; platelet count ≥100,000 cells/mm3 or ≥100 bil/L; absolute neutrophil count ≥1500 cells/mm3 or ≥1.5 bil/L; and hemoglobin ≥9 g/dL or ≥90 g/L), hepatic function (aspartate aminotransferase ≤3× upper normal limit [UNL], alanine aminotransferase ≥3× UNL (≤ 5× UNL if liver metastases present), bilirubin ≤1.5× UNL), renal function (serum creatinine ≤2.0 mg/dL or 177 µmol/L), and coagulation (International Normalized Ratio ≤1.5) unless on therapeutic blood thinners. Exclusion criteria included: history of radiotherapy for cerebral metastases, central nervous system or epidural tumor; prior chemotherapy for metastatic pancreatic cancer; receipt of any other standard or investigational treatment for cancer, or any investigational agent for any indication within 2 weeks prior to initiation of CPI-613; active, uncontrolled bleeding, active heart disease, myocardial infarction within 3 months prior to study registration, or active infection or serious infection within the past month. By inclusion/exclusion criteria for the study, the life expectancy of eligible patients was more than 2 months. This study was reviewed and approved by the Wake Forest Health Sciences Institutional Review Board. All patients gave written informed consent prior to undergoing any study-related procedures or testing. A data and safety monitoring committee supervised the collection of efficacy and safety data.
Procedures
Assessments
Patients were assessed at the start of each cycle (medical history, physical examination by a physician, ECOG performance status, and complete blood counts and blood chemistry tests). Baseline evaluation also included serum carbohydrate antigen 19–9 (CA 19–9) level, computed tomographic (CT) evaluation of the chest, and magnetic resonance imaging (MRI) of the abdomen and pelvis. Tumors were evaluated radiologically every four cycles using CT and MRI. Tumor response was determined according to the Response Evaluation Criteria in Solid Tumors.14
Treatment and Dose Escalation
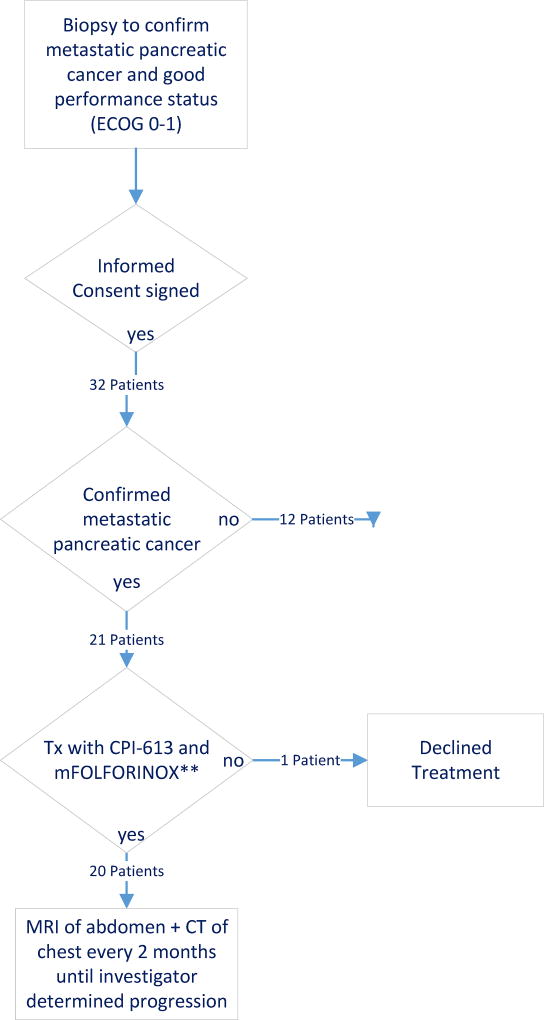
A trial profile showing the flow of participants is depicted in Fig. 1. The standard FOLFIRINOX regimen was modified (mFOLFIRINOX) with reduction of oxaliplatin at 65 mg/m2 and irinotecan at 140 mg/m2 in combination with CPI-613. The fluorouracil dose was 400 mg/m2 bolus and 2400 mg/m2 over 46 h continuous infusion. These modifications were incorporated to reduce the confounding toxicity profile of the combination. Treatment was given in two-week cycles, with CPI-613 administered on Days 1 and 3, and FOLFIRINOX administered on Days 1–3 with growth factor support (Neulasta) on day 4. In the event of pre-defined toxic events, protocol-specific treatment modifications were permitted.
Figure 1.
This phase 1 dose-escalation trial followed a two-stage dose-escalation scheme (single patient and traditional 3+3 design).15 In the single patient stage, one patient was accrued per dose level. The starting dose of CPI-613 was 500 mg/m2/day given at a rate of 4 mL/min. CPI-613 dose level was then escalated by doubling the previous dose if there was no toxicity greater than Grade 2 within 4 weeks attributed as probably or definitely related to CPI-613. The traditional 3+3 dose-escalation stage was triggered if toxicity attributed as probably or definitely related to CPI-613 was ≥ Grade 2. All CPI-613 dose escalations conducted in this traditional dose-escalation stage were escalated according to the modified Fibonacci Dose-Escalation scheme. The maximum allowable dose was 3,000mg/m2/day. The dose level for CPI-613 for the first cohort in the traditional dose-escalation stage was the same as used in the last cohort of the single patient dose-escalation stage. The number of patients in each cohort at this stage was initially three, including the first patient in which a > Grade l toxicity that was probably or definitely attributable to CPI-613 was observed in the single patient dose-escalation stage. If no patients in any cohort developed a dose-limiting toxicity (DLT), dose escalation continued in cohorts of three patients. A DLT was defined as the occurrence of any clinically relevant ≥ Grade 3 toxicity at least possibly related to the combination regimen. The following toxicities of any source were excluded from defining a DLT: Grade 3 nausea and vomiting responsive to anti-emetics or Grade 3 diarrhea responsive to anti-diarrheal therapy unless persistent >7 consecutive days in spite of treatment; Grade 3 or 4 neutropenia lasting >7 days; Grade 3 thrombocytopenia; Grade 3 or 4 metabolic derangements attributed to tumor lysis syndrome unless metabolic derangement is >7 days. Once the MTD was found using this design, additional patients at that dose level were enrolled until a total sample size of 6 patients were treated. If no DLTs were identified then the cohort was then expanded to 18 total patients to further characterize activity. The trial did not have a prespecified number of treatment cycles. Patients discontinued the study in the event of unacceptable toxic effects, evidence of disease progression, or patient request. The primary outcome was investigator-assessed.
Pharmacokinetics
Blood samples for post-hoc exploratory pharmacokinetic (PK) analysis were collected pre-dose and at approximately 5, 30, 60, 90 mins, 2, 4, 6, 8, 24 and 72 hrs post infusion. Concentrations of CPI-613 (6,8-bis-benzylsulfanyloctanoic acid) and its major active metabolite CPI-2850 (4,6-bis-benzylsulfanyloctanoic acid) were quantitated in plasma using authentic reference standards. PK parameters (Cmax, Tmax, AUC, t1/2, CL and Vd) were estimated by Non-Compartmental Analysis (NCA) using a validated installation of Phoenix WinNonLin v6.4 (PKPD Bioscience Inc) and actual sampling times. (See detailed analysis: Suppl, p. 1–4).
Outcomes
The primary objective was to determine the MTD; thus, DLTs represent the primary endpoint for the phase 1 analysis. Secondary objectives were to assess the safety of CPI-613 and mFOLFIRINOX in combination in patients with metastatic pancreatic cancer and to obtain preliminary data on efficacy of treatment with CPI-613 and mFOLFIRINOX. Safety was assessed per National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0)16 before each cycle. Toxicity was calculated and tabulated both overall by patient (i.e., highest grade toxicity observed) and by event (i.e., total number of toxicities observed over the trial, allowing for each patient to have possibly multiple observed toxicities). Collection of tissue was performed for future genomic analyses.
Statistical Analyses
This study hypothesized that the combination of CPI-613 and mFOLFIRINOX in patients with metastatic pancreatic cancer will be safe and acceptable. There were no formal power calculations performed for this Phase 1 study – we used a standard 3+3 design with an expansion cohort at the proposed MTD. Descriptive statistics were calculated for all patients. All patients were evaluable for toxicity, which is the primary goal of the Phase 1 study. All the patients treated at the MTD underwent at least one restaging scan to determine their response to treatment. The only two patients that did not have a restaging scan were the patients treated at the 1000mg/m2 that had the DLTs.
Using the expanded cohort (only patients at the MTD), descriptive statistics for response rate data (complete response [CR], partial response [PR], stable disease [SD], and progressive disease [PD]) were calculated (counts/percents). Additionally, response (CR+PR) rate was estimated with 95% Copper-Pearson. Next, preliminary time-to-event data was calculated. This included preliminary estimates of median progression-free survival (PFS). Overall survival (OS) and progression-free survival were calculated from the date of enrollment until the date of death and the date of documentation of disease progression or death in patients with disease progression, respectively. As of January 2017 less than half (n=8) of patients had died so accurate median OS estimates could not be obtained; however, minimum median OS at the time of submission was determined. SAS version 9.3 was used for these analyses (Cary, NC). Individual PK parameters were estimated by NCA using the statistical analysis module in WinNonLin and median, min, max, mean, standard deviation and %CV values were summarized in tabular format and graphically using semi-logarithmic plots. The study was registered with clinicaltrials.gov (NCT01835041).
Role of the Funding Source
The funding source did not play a role in this study. Oxaliplatin, fluorouracil, irinotecan and leucovorin were provided by patient’s private insurance, as per standard of care. CPI-613 was provided by Cornerstone Pharmaceutical. Cornerstone Pharmaceutical had no role in the design, collection, analysis, or interpretation of the clinical trial data, or writing of the report. The corresponding author had full access to all of the data and the final responsibility to submit for publication. Co-authors AA, RD, BP, UT, GH, WZ, GJ, and LM had access to the raw data.
Results
Twenty patients were enrolled April 22, 2013 – January 8, 2016. The database was closed for interim analysis in January 2017. There were no violations of eligibility criteria. Demographic and baseline disease characteristics of the patients are described in Table 1. The median number of treatment cycles administered at the MTD dose was 11 (interquartile range, 4–19). The median relative dose intensities were 92% for fluorouracil, 85% for irinotecan and 76.9 % for oxaliplatin. Thirteen patients underwent oxaliplatin reduction, 2 patients underwent irinotecan reduction, and 7 patients underwent fluorouracil reduction. Nine patients underwent more than 12 cycles and 7 patients underwent more than 24 cycles. A DLT was not observed in the first patient enrolled during the single patient dose escalation stage, and the dose was increased. Two patients were enrolled at this higher dose (1000 mg/m2) and both experienced a DLT. The DLTs for Patient 2 were anemia, lymphopenia, pulmonary embolus, hyponatremia and dehydration. This patient refused further treatment and opted for hospice. The DLTs for Patient 3 were hyponatremia, hypotension and lymphopenia. This patient came off the study due to drug-related toxicity. Thus, the dose was lowered to the original dose. Three patients were enrolled at this lower dose and none experienced a DLT. An additional three patients were enrolled, and none experienced a DLT. At this point 500 mg/m2/day given at a rate of 4 mL/min on day 1 and day 3 of each cycle was considered the MTD. An additional 11 patients (for a total of 18) were then enrolled at this dose to further evaluate toxicity and preliminary efficacy. None of these additional 11 patients experienced a DLT.
Table 1.
Demographic and Baseline Patient Characteristics (N = 20)
| Characteristic | N (%) |
|---|---|
| Sex | |
| Male | 11 (55.0) |
| Female | 9 (45.0) |
| ECOG performance status score | |
| 0 | 3 (15.0) |
| 1 | 17 (85.0) |
| Pancreatic tumor location | |
| Head | 12 (60.0) |
| Body | 2 (10.0) |
| Tail | 6 (30.0) |
| Biliary stent | |
| Yes | 2 (10.0) |
| No | 18 (90.0) |
| Level of carbohydrate antigen 19–9 | |
| Normal | 2 (10.0) |
| Elevated, <59xULN | 6 (30.0) |
| Elevated, ≥59xULN | 12 (60.0) |
| No. of metastatic sites involved | |
| 1 site | 12 (60.0) |
| 2 or more sites | 8 (40.0) |
| Type of metastatic disease | |
| Synchronous | 14 (70.0) |
| Metachronous | 6 (30.0) |
| Characteristic | Median (Interquartile Range) |
|
| |
| Age in years | 65 (55–68) |
| No. of metastatic sites involved | 1 (1–2.5) |
There were 2 unexpected serious adverse events (SAEs), both for the first patient enrolled: 1) possible leaching due to infusion of CPI-613 via non-PVC tubing, and 2) the patient re- accessed her port at home after accidental de-access. Neither incident resulted in a negative clinical outcome. Expected SAEs were: thrombocytopenia, anemia and lymphopenia (all for Patient #2, with anemia and lymphopenia being a DLT); hyperglycemia (Patient #7); hypokalemia, hypoalbuminemia and sepsis (Patient #11); and neutropenia (Patient #20). There was no grade 5 toxicity. Treatment-related grade 1–4 adverse events are summarized in Table 2. For the 18 patients treated at the MTD, the most common Grade 3–4 toxicities were hypokalemia (6/18, 33%), diarrhea (5/18, 28%) and abdominal pain (4/18, 22%). Hematologic toxicity was comparable with the historical data reported in the PRODIGE trial of FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. Likely due to Neulasta support, Grade 3–4 (no grade 5 experienced) neutropenia occurred in 28% of patients compared with 45.7% in the PRODIGE study where Neulasta was used as secondary prophylaxis. Grade 3–4 anemia (4/18, 22%) and thrombocytopenia (3/18, 17%) were higher than reported historical data. Sensorial neuropathy (17/18, 94%) was higher than historical data, developed late and was mainly grade 1–2 (Suppl, p. 5). Cumulative toxicity data is reported in Suppl, p. 9.
Table 2.
Summary of Worst Toxicities by Patient Grade1, n=18 Participants
| Toxicity | Grades 1–22 | Grade 3 | Grade 4 | |||
|---|---|---|---|---|---|---|
| Anemia | 14 | 78% | 4 | 22% | 0 | 0% |
| Hyperglycemia | 8 | 44% | 9 | 50% | 1 | 6% |
| Alkaline phosphatase increased | 17 | 94% | 0 | 0% | 0 | 0% |
| Fatigue | 14 | 78% | 3 | 17% | 0 | 0% |
| Peripheral sensory neuropathy | 12 | 67% | 5 | 28% | 0 | 0% |
| Platelet count decreased | 14 | 78% | 3 | 17% | 0 | 0% |
| Diarrhea | 11 | 61% | 5 | 28% | 0 | 0% |
| Hypoalbuminemia | 15 | 83% | 0 | 0% | 1 | 6% |
| Hyponatremia | 11 | 61% | 3 | 17% | 0 | 0% |
| Nausea | 12 | 67% | 2 | 11% | 0 | 0% |
| Hypomagnesemia | 13 | 72% | 0 | 0% | 0 | 0% |
| Lymphocyte count decreased | 8 | 44% | 5 | 28% | 0 | 0% |
| Pain | 13 | 72% | 0 | 0% | 0 | 0% |
| Alanine aminotransferase increased | 12 | 67% | 0 | 0% | 0 | 0% |
| Anorexia | 11 | 61% | 1 | 6% | 0 | 0% |
| Hypokalemia | 6 | 33% | 5 | 28% | 1 | 6% |
| Abdominal pain | 7 | 39% | 4 | 22% | 0 | 0% |
| Anxiety | 11 | 61% | 0 | 0% | 0 | 0% |
| Aspartate aminotransferase increased | 11 | 61% | 0 | 0% | 0 | 0% |
| Dysgeusia | 11 | 61% | 0 | 0% | 0 | 0% |
| Hypertension | 10 | 56% | 1 | 6% | 0 | 0% |
| Hypocalcemia | 10 | 56% | 0 | 0% | 0 | 0% |
| Weight loss | 9 | 50% | 1 | 6% | 0 | 0% |
| White blood cell decreased | 9 | 50% | 0 | 0% | 0 | 0% |
| Neutrophil count decreased | 3 | 17% | 4 | 22% | 1 | 6% |
| Constipation | 7 | 39% | 0 | 0% | 0 | 0% |
| Cough | 7 | 39% | 0 | 0% | 0 | 0% |
| Insomnia | 7 | 39% | 0 | 0% | 0 | 0% |
| Vomiting | 4 | 22% | 3 | 17% | 0 | 0% |
| Back pain | 4 | 22% | 1 | 6% | 0 | 0% |
| Chronic kidney disease | 5 | 28% | 0 | 0% | 0 | 0% |
| Dehydration | 3 | 17% | 2 | 11% | 0 | 0% |
| Dizziness | 5 | 28% | 0 | 0% | 0 | 0% |
| Dyspnea | 4 | 22% | 1 | 6% | 0 | 0% |
| Edema limbs | 5 | 28% | 0 | 0% | 0 | 0% |
| Fever | 4 | 22% | 1 | 6% | 0 | 0% |
| Cardiac disorders - Other | 2 | 11% | 2 | 11% | 0 | 0% |
| Chills | 4 | 22% | 0 | 0% | 0 | 0% |
| Gastroesophageal reflux disease | 4 | 22% | 0 | 0% | 0 | 0% |
| General disorders and administration site conditions - Other | 3 | 17% | 1 | 6% | 0 | 0% |
| Hypophosphatemia | 2 | 11% | 2 | 11% | 0 | 0% |
| Sinus tachycardia | 4 | 22% | 0 | 0% | 0 | 0% |
| Urinary frequency | 4 | 22% | 0 | 0% | 0 | 0% |
| Activated partial thromboplastin time prolonged | 3 | 17% | 0 | 0% | 0 | 0% |
| Atelectasis | 3 | 17% | 0 | 0% | 0 | 0% |
| Blood bilirubin increased | 3 | 17% | 0 | 0% | 0 | 0% |
| Depression | 2 | 11% | 1 | 6% | 0 | 0% |
| Flatulence | 3 | 17% | 0 | 0% | 0 | 0% |
| Gastrointestinal disorders - Other | 3 | 17% | 0 | 0% | 0 | 0% |
| Leukocytosis | 0 | 0% | 3 | 17% | 0 | 0% |
| Pleural effusion | 3 | 17% | 0 | 0% | 0 | 0% |
| Thromboembolic event | 0 | 0% | 3 | 17% | 0 | 0% |
| Urinary tract infection | 3 | 17% | 0 | 0% | 0 | 0% |
| Ventricular arrhythmia | 2 | 11% | 1 | 6% | 0 | 0% |
| Ascites | 2 | 11% | 0 | 0% | 0 | 0% |
| Blurred vision | 2 | 11% | 0 | 0% | 0 | 0% |
| Dysphagia | 1 | 6% | 1 | 6% | 0 | 0% |
| Edema face | 2 | 11% | 0 | 0% | 0 | 0% |
| Fall | 2 | 11% | 0 | 0% | 0 | 0% |
| Generalized muscle weakness | 2 | 11% | 0 | 0% | 0 | 0% |
| Headache | 2 | 11% | 0 | 0% | 0 | 0% |
| Hypernatremia | 2 | 11% | 0 | 0% | 0 | 0% |
| Hypoxia | 1 | 6% | 1 | 6% | 0 | 0% |
| Nasal congestion | 2 | 11% | 0 | 0% | 0 | 0% |
| Palpitations | 2 | 11% | 0 | 0% | 0 | 0% |
| Pericardial effusion | 2 | 11% | 0 | 0% | 0 | 0% |
| Productive cough | 2 | 11% | 0 | 0% | 0 | 0% |
| Proteinuria | 2 | 11% | 0 | 0% | 0 | 0% |
| Rash maculo-papular | 2 | 11% | 0 | 0% | 0 | 0% |
| Respiratory, thoracic and mediastinal disorders - Other | 2 | 11% | 0 | 0% | 0 | 0% |
| Skin and subcutaneous tissue disorders - Other | 2 | 11% | 0 | 0% | 0 | 0% |
| Weight gain | 2 | 11% | 0 | 0% | 0 | 0% |
| Enterocolitis infectious | 0 | 0% | 1 | 6% | 0 | 0% |
| Esophageal infection | 0 | 0% | 1 | 6% | 0 | 0% |
| GGT increased | 0 | 0% | 1 | 6% | 0 | 0% |
| Glucose intolerance | 0 | 0% | 1 | 6% | 0 | 0% |
| Infections and infestations - Other | 0 | 0% | 1 | 6% | 0 | 0% |
| Lung infection | 0 | 0% | 1 | 6% | 0 | 0% |
| Pancreatitis | 0 | 0% | 1 | 6% | 0 | 0% |
| Sepsis | 0 | 0% | 0 | 0% | 1 | 6% |
There were no grade 5 toxicities
For incidence greater than 10%
As anticipated, electrolytes imbalance was relatively more frequent and managed with supportive care. No patients died while on active treatment; 11 study participants died, with cause of death as terminal pancreatic cancer.
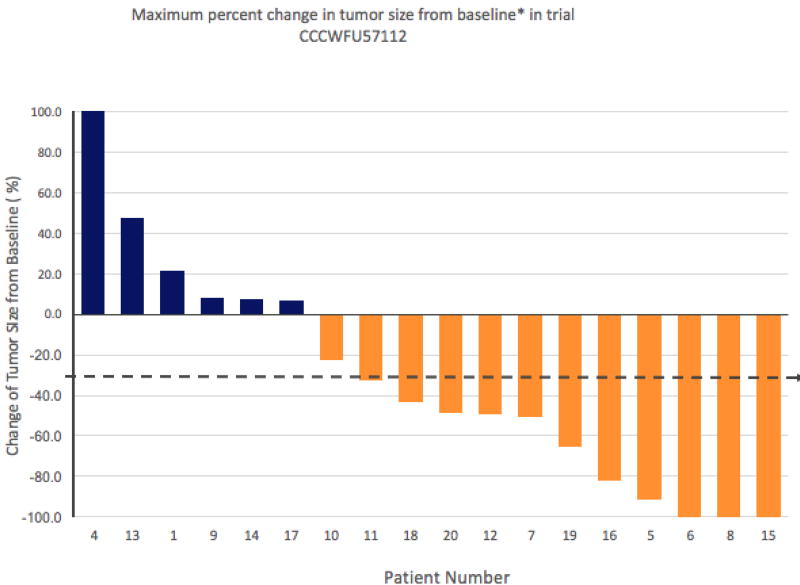
Among the 18 patients enrolled at MTD, there were 3 patients with a CR, 8 with a PR, 3 with SD and 4 with PD. Thus the PR/CR rate was 61% with a 95% Clopper-Pearson (exact) confidence interval of 36% to 83%. The median PFS was 11.5 months (95% CI 133– 560). The radiologic response is captured on the waterfall plot (Fig 2). The two patients treated above the MTD were not included in efficacy analyses. Patient #2 was admitted with sepsis to the ICU and was discharged home with hospice due to significant decline in performance status. Patient #3 was admitted to the hospital with nausea and failure to thrive, developed esophageal variceal bleeding and aspirated. She deferred aggressive management and was discharged home with hospice. Four patients received chemotherapy or targeted treatment prior to the present study. Patient #7 was stage III status post neoadjuvant chemoradiation followed by Whipple surgery and adjuvant therapy with gemcitabine based therapy. He relapsed post adjuvant treatment with liver metastases and enrolled in the present study. His best response was PR. Patient #8 was stage III status post whipple with short interval post-operative recurrence with liver metastases and a best response of CR on the present study. Patient #13 was stage II status post pylorus-sparing whipple followed by adjuvant chemotherapy and chemoradiation with relapse post adjuvant therapy. On the present study her best response POD. Patient #17 had locally advanced disease treated with neoadjuvant FOLFIRINOX followed by chemoradiation and then enrolled in the present study for newly diagnosed metastatic disease. Her best response was SD.
Figure 2.
Of the three patients with a CR, one maintained CR 6 months after treatment discontinuation. The other two patients with CR relapsed 2 months after treatment discontinuation with small tumor burden. Due to their excellent performance status, all three patients that achieved a CR were re-challenged with the same therapy as a compassionate use program approved by the FDA. One of the patients with a PR had a prolonged non-CR/non-PD response. At diagnosis this patient had innumerable metastatic liver and peritoneal lesions. After 32 cycles his treatment was discontinued per patient’s preference. He now has sub-centimeter residual disease that was not amenable to biopsy and below PET scan detection. His disease status remained unchanged at 12 months off therapy. Among the 18 patients treated at the MTD, in addition to the 3 patients with CR, there were 1 with a non-CR/non-PD, 7 with PR, 3 with stable disease, and 4 with PD. Four of the patients who progressed on the clinical trial did not receive 2nd line therapies and were enrolled in hospice. Five of the patients that were treated with second line therapies experienced progression of disease and did not receive third line therapies. Of the three patients with CR that came off treatment and were re-challenged with the same combination as compassionate care under FDA approval two experienced SD and one PD.
Tumor and germline tissues from the three exceptional responders underwent whole exome sequencing to gain insight into their genomic characteristics. All had KRAS mutations and two had TP53 mutations. None had SMAD4 mutation. Recurring mutations among all three exceptional responders were observed in the mucin gene family (Suppl, p. 5–6). As of January 2017, 9 patients were still alive, which means that the median OS has not yet been determined. Among the nine patients who have died, the 8th patient died at 284 days and the 9th patient died at 602 days. This fact, coupled with the fact that all 9 patients who are still alive have now survived longer than 374 days, means that the smallest median OS value is 374 days (12.4 months). We assessed the outcome of patients treated with FOLFIRINOX during the same period of time at our institution (Suppl, p. 7).
The biotransformation of CPI-613 occurred rapidly following infusion, with the active metabolite CPI-2850 becoming the major circulating species in plasma over time. CPI-613 and CPI-2850 followed a biexponential disposition profile, with the emergence of secondary peaks during the elimination phase indicative of enterohepatic recirculation. The median terminal half-life (t1/2) of CPI-613 following a 2 hr IV infusion at 500 mg/m2 was approximately 2.0 hrs, whereas the active metabolite, CPI-2850, was cleared at a markedly slower rate with a t1/2 of approximately 54 hrs.
Discussion
In this study, CPI-613 in combination with mFOLFIRINOX was well tolerated. The MTD for the investigational agent CPI-613 was identified at 500 mg/m2. The current first line standard of care treatment for patients with stage IV pancreatic adenocarcinoma is FOLFIRINOX or gemcitabine with nab-paclitaxel. The current study sought to explore the feasibility of combining FOLFIRINOX with a novel agent, CPI–613. The dosing of FOLFIRINOX was modified based on clinical experience and published reports with FOLFIRINOX alone.2,13 In standard clinical practice most patients require dose de-escalation of either irinotecan, oxaliplatin, fluorouracil or all three early in their treatments as well as growth factor support. The study was designed to include patients that would have met criteria for the PRODIGE study2 (i.e., fit patients with good performance status [ECOG 0–1]), thus limiting the generalizability of this study and resulting in a potential study bias. Additionally, this was a small pilot study, and tumor burden and number of disease sites were not taken into account. The authors acknowledge these potential study limitations.
This phase 1 clinical trial met its first end point, identifying the MTD for CPI-613. It also demonstrates that the combination of CPI-613 with FOLFIRINOX is feasible and tolerable. The most common hematologic toxicities were anemia, thrombocytopenia and lymphopenia. The most common non-hematologic toxicities were diarrhea, fatigue, electrolytes imbalance mostly grade 3 or less. Although this seems higher than the reported toxicity by Stein et.al,13 we observed few grade 4 toxicities. Likely due to Neulasta support, incidence of neutropenia was lower compared with 45.7% in the PRODIGE study, where Neulasta was used as secondary prophylaxis, but higher than data reported by Stein et.al22 (16.3%). Anemia and thrombocytopenia were higher than reported historical data. Most of the studies exploring modified FOLFIRINOX include patients with locally advanced pancreatic cancer hence the comparison with our cohort is difficult. A meta-analysis of these studies reflects consistently the most common toxicities such as neutropenia, fatigue, thrombocytopenia, vomiting and diarrhea.17,18 A comparison with a contemporary cohort of patients treated at our institution suggests a favorable profile for the CPI-613 FOLFIRINOX combination. The small sample size and the duration of responders’ follow-up precludes a formal toxicity comparison with other phase 2 or 3 studies. Some of the AEs may have higher incidence in our trial or they could be an artifact of longer exposure to treatment. Further studies are needed to thoroughly assess the toxicity profile of this regimen.
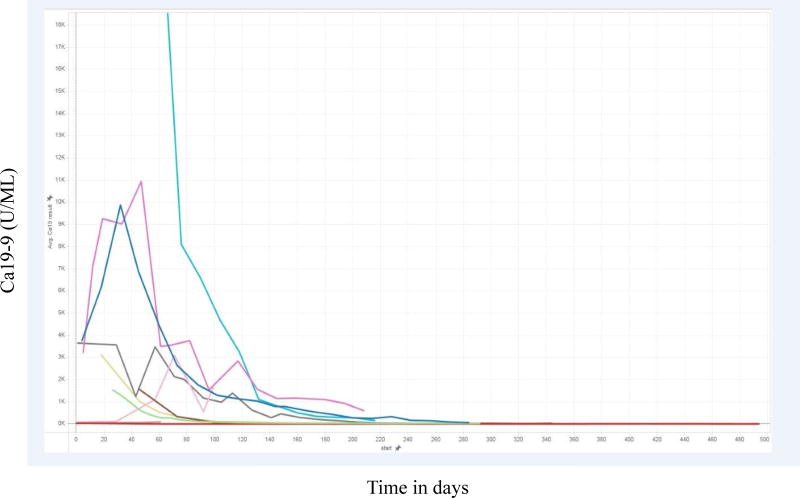
Although efficacy data was not the main endpoint of the study, we observed an encouraging signal for possible synergy with standard of care chemotherapy without significant additional toxicity. Substantial changes in mitochondrial metabolism regulation occur during disease progression. Among these are alterations in lipoate-sensitive control of TCA cycle enzymes pyruvate dehydrogenase (PDH) and alpha-ketoglutarate dehydrogenase (KGDH). CPI-613, a stable analog of lipoate catalytic intermediates, activates repressive components of this machinery, inactivating PDH4 and KGDH5 selectively in tumor cells. The resulting decrease in mitochondrial metabolism is expected to compromise the DNA damage response induced by FOLFIRINOX components, leading to synergistic anti-tumor effects. Moreover, the selectivity of CPI-613 for tumor cells and the prior phase 1 experience7 suggested that this drug will add little to FOLFIRINOX side effect toxicity. Biologic response identified as decline in tumor marker (CA 19–9) was consistent among all responders and will likely be a reliable predictive biomarker for future investigation. See Figure 3.
Figure 3.
These encouraging results inform the next step of development for the drug combination. A randomized phase 2–3 study of FOLFIRINOX vs. mFOLFIRINOX + CPI613 is scheduled to be initiated in early 2017. Questions that remain un-answered at this point in investigation include:
What is the role of maintenance therapy for patients that achieve a radiologic CR?
How does the combination of CPI-613 and mFOLFIRINOX affect quality of life? The clinical observation noted in this trial suggests that CPI-613 may have protective benefits and mitigate some of the chemotherapy-induced toxicity. This is highly relevant for patients with pancreatic cancer that have typically a high symptom burden. The planned randomized phase 2–3 clinical trial will include a quality of life analysis to further explore its effect under this treatment schema.
Research in context
Evidence before this study
For about two decades, 5-Fluorouracil (5-FU) was the only chemotherapeutic option for advanced pancreatic cancer patients. The introduction of gemcitabine in the 1990s demonstrated both improved survival and fewer side effects compared with 5-FU, and in 1997 supplanted 5-FU as the first-line drug of choice. The PRODIGE phase II/III trial later demonstrated superiority of FOLFIRINOX over gemcitabine (median survival and PFS of 11.1 months and 6.4 months in FOLFIRINOX arm vs 6.8 months and 3.3 months in the gemcitabine arm, respectively), albeit strict eligibility criteria limit its use in this patient population. In 2013, the MPACT trial presented an additional intensified combination chemotherapy option, gemcitabine plus nab-paclitaxel. This study demonstrated improved results over gemcitabine monotherapy, including a median OS of 8.5 months and median PFS of 5.5 months, yet was less restrictive than the PRODIGE trial for patient eligibility, notably including ECOG 2 patients. Both FOLFIRINOX and gemcitabine + nab-paclitaxel are first-line therapy options for metastatic pancreatic cancer, with the acknowledgment that OS rates may be overestimated due to the stringent enrollment criteria for the sentinel trials.
Added value of this study
This study provides evidence for a novel, potentially more effective combination chemotherapy regimen – mFOLFIRINOX + CPI-613 – that was safe and well tolerated for patients with metastatic pancreatic cancer.
Implications of all the available evidence
The response rate to the CPI-613 combination regimen was 61% in this small cohort of patients. The patients eligible for the current study met the same criteria as the patients enrolled in the PRODIGE study. While we recognize that the experience with this small cohort of patients is not reflective of what we may find in a phase 2, 3 study, we are encouraged to further explore this novel therapeutic combination.
Supplementary Material
Acknowledgments
The Biostatistics and Bioinformatics as well as the Cancer Genomics Shared Resource Shared Resources services were supported by the Wake Forest Baptist Comprehensive Cancer Center’s NCI Cancer Center Support Grant P30CA012197
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
The study was designed and conducted by investigators at the Comprehensive Cancer Center of Wake Forest Baptist Medical Center. AA: generated the hypothesis and the clinical design of the study; conducted the trial and supervised it from start to finish; wrote the first draft of the manuscript and worked closely with the co-investigators in conducting the clinical trial and interpretation of the data. BP: contributed to the development of the manuscript through analysis and interpretation of data, revisions of the manuscript, and final approval of the version submitted. BM: assisted AA in drafting the manuscript and its revisions. RD: contributed to patient enrollment and the manuscript. HK: data collection, data interpretation, manuscript preparation and approval. KH: radiologic assessment of scan and manuscript review and revisions. CC: manuscript review and revisions. AC: manuscript review and revisions and active management of patients on the clinical trial. JL: radiologic reading on the clinical trial. RD, Jr.: performed the clinical trial design, analysis of the clinical data, and contributed to manuscript revisions. UT: analysis of cumulative chemotherapy dosing and biomarker analysis. LWB: Pharmacokinetic and pharmacodynamics data evaluation and documentation. ARB: pharmacokinetics and pharmacodynamics laboratory analysis of CPI-613 (data collection). RS: the design, implementation, and interpretation of studies and results related to the determination of elimination pharmacokinetics of drug as measured in blood samples drawn from patients and the description of these results. ZZ and PB: conceived and designed the in vitro study and Fig 1 in supplemental material; ZZ carried out and analyzed the experiments. TA, SC and RS: collection of historic data and chart review for pancreatic cancer patients treated with FOLFIRINOX 2011 to 2016 at Wake Forest Baptist Medical Center. JJM: collection and analysis of the historical data and comparison with patients in study. TSP: genomic analysis, description of mechanism of action, manuscript review. LM: genomic analysis and manuscript review. GAH: data collection and interpretation. GJ: data analysis, data interpretation, writing, and tables. WZ: literature search, study design, data interpretation, writing. BP: manuscript revisions and data analysis. All authors vouch for the accuracy and completeness of the data and analyses and for the fidelity of the study to the protocol. All the authors reviewed and revised the manuscript, approved the final version, and made the decision to submit the manuscript for publication.
Declarations of Interests
LWB, ARB, and RS are employees of Cornerstone Pharmaceuticals. RS has ownership interest (including patents) in Cornerstone Pharmaceuticals. TSP is a paid consultant for Cornerstone pharmaceuticals. The other authors declared no conflicts of interest.
References
- 1.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2012. National Cancer Institute; Bethesda, MD: http://seer.cancer.gov/csr/1975_2012/, based on November 2014 SEER data submission, posted to the SEER web site, April 2015. [Google Scholar]
- 2.Conroy T, Desseigne F, Ychou M, et al. Intergroup, P. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 3.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zachar Z, Marecek J, Maturo C, et al. Non-redox-active lipoate derivates disrupt cancer cell mitochondrial metabolism and are potent anti-cancer agents in vivo. J Mol Med. 2011;89:1137–48. doi: 10.1007/s00109-011-0785-8. [DOI] [PubMed] [Google Scholar]
- 5.Stuart SD, Schauble A, Gupta S, et al. A strategically designed small molecule attacks alpha-ketoglutarate dehydrogenase in tumor cells through a redox process. Cancer Metab. 2014 Mar;2(1):4. doi: 10.1186/2049-3002-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pardee TS, Levitan DA, Hurd DD. Altered mitochondrial metabolism as a target in acute myeloid leukemia [abstract] J Clin Oncol. 2011;29(suppl) abstr 6590. [Google Scholar]
- 7.Pardee TS, DeFord-Watts LM, Peronto E, et al. The 1st in class tumor specific anti-mitochondrial metabolism agent CPI-613 is well tolerated and has activity in several hematologic malignancies [abstract] J Clin Oncol. 2012;20(suppl) [Google Scholar]
- 8.Lee K, Khaira D, Rodriguez R, et al. Long-term stable disease of stage IV pancreatic neuroendocrine tumors and without significant adverse effect by CPI-613, an investigational novel anti-cancer agent. Case Study and Case Report. 2011;1(3):137–145. [Google Scholar]
- 9.Lee K, Maturo C, Luddy J, et al. Pseudo-progression of metastatic pancreatic cancer assessed by imaging studies - a case report. Case Study and Case Report. 2012;2(3):95–101. [Google Scholar]
- 10.Senzer N, Bedell C, Maturo C, et al. CPI-613, an investigational novel anti-cancer agent, provides long-term stable disease without significant adverse effects in a patient with stage IV relapsed hepatocellular carcinoma. Case Study and Case report. 2012;2(2):38–45. 2012. [Google Scholar]
- 11.Pardee TS, Lee K, Luddy J, et al. A phase I study of the first-in-class antimitochondrial metabolism agent, CPI-613, in patients with advanced hematologic malignancies. Clin Cancer Res. 2014 Oct 15;20(20):5255–64. doi: 10.1158/1078-0432.CCR-14-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghorani E, Wong HH, Hewitt C, et al. Safety and Efficacy of Modified FOLFIRINOX for Advanced Pancreatic Adenocarcinoma: A UK Single-Centre Experience. Oncology. 2015;89(5):281–287. doi: 10.1159/000439171. [DOI] [PubMed] [Google Scholar]
- 13.Stein SM, James ES, Deng Y, et al. Final analysis of a phase II study of modified FOLFIRINOX in locally advanced and metastatic pancreatic cancer. Br J Cancer. 2016;114(7):809–812. doi: 10.1038/bjc.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) European journal of cancer (Oxford, England: 1990) 2009 Jan;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 15.Le Tourneau C, Lee JJ, Siu LL. Dose escalation methods in phase I cancer clinical trials. J Natl Cancer Inst. 2009;101(10):708–720. doi: 10.1093/jnci/djp079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE), version 4.0. 2009 May; http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.
- 17.Suker M, Beumer BR, Sadot E, et al. FOLFIRINOX for locally-advanced pancreatic cancer: a systematic review and patient-level meta-analysis. Lancet Oncol. 2016 Jun;17(6):801–10. doi: 10.1016/S1470-2045(16)00172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chllamma MK, Cook N, Dhani NC, et al. FOLFIRINOX for advanced pancreatic cancer: the Princess Margaret Cancer Centre experience. Br J Cancer. 2016 Sep;115(6):649–54. doi: 10.1038/bjc.2016.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.