Significance
B cells produce antibodies in the context of immunoglobulin heavy chain (IgH) isotypes (e.g., IgM, IgG, and IgE). Each of these is generated either as secreted proteins or as membrane-bound B cell antigen receptors (BCRs). While much is known about how IgH isotype dictates effector function of soluble antibodies, the role of antibody isotype in the context of BCRs is not well defined. Here we demonstrate that the membrane-bound versions (mIg) of IgM, IgG1, and IgE are produced from their natural genomic loci in a hierarchal fashion, where mRNA transcripts for mIgM are always more dominant than mIgG1, which are always more dominant than mIgE, regardless of cell stage. These isotype-specific expression differences contribute to B cell regulation.
Keywords: B cell, antibody, IgE, BCR, memory
Abstract
Ig heavy chain (IgH) isotypes (e.g., IgM, IgG, and IgE) are generated as secreted/soluble antibodies (sIg) or as membrane-bound (mIg) B cell receptors (BCRs) through alternative RNA splicing. IgH isotype dictates soluble antibody function, but how mIg isotype influences B cell behavior is not well defined. We examined IgH isotype-specific BCR function by analyzing naturally switched B cells from wild-type mice, as well as by engineering polyclonal Ighγ1/γ1 and Ighε/ε mice, which initially produce IgG1 or IgE from their respective native genomic configurations. We found that B cells from wild-type mice, as well as Ighγ1/γ1 and Ighε/ε mice, produce transcripts that generate IgM, IgG1, and IgE in an alternative splice form bias hierarchy, regardless of cell stage. In this regard, we found that mIgμ > mIgγ1 > mIgε, and that these BCR expression differences influence respective developmental fitness. Restrained B cell development from Ighγ1/γ1 and Ighε/ε mice was proportional to sIg/mIg ratios and was rescued by enforced expression of the respective mIgs. In addition, artificially enhancing BCR signal strength permitted IgE+ memory B cells—which essentially do not exist under normal conditions—to provide long-lived memory function, suggesting that quantitative BCR signal weakness contributes to restraint of IgE B cell responses. Our results indicate that IgH isotype-specific mIg/BCR dosage may play a larger role in B cell fate than previously anticipated.
Ig heavy chain (IgH) constant region (CH) isotypes enable coupling of antigen-binding Ig variable regions (V) to diverse functional contexts. The CH exons are arranged in tandem, with Cμ (encoding the IgM constant region) initially located most proximal to the Ig VH exon, followed by a number of alternative CH isotypes (e.g., Cγ, Cε, and Cα). Each CH is supplied with terminal exon(s) encoding transmembrane and cytoplasmic tail moieties enabling expression of membrane Ig (mIg), which, together with the CD79A and CD79B signaling accessory proteins, form the antigen-binding part of the B cell receptor (BCR) (1). Mutually exclusive alternative splicing can exclude membrane exons to produce secreted Ig (sIg) (2).
Ig V exons of IgH and Ig light (IgL) chains are assembled in bone marrow (BM) progenitor (pro) and precursor (pre) B cells, respectively (3). Productive VH and VL assembly results in IgM expression on the surface of immature B cells, which further develop to mature naïve IgM+ IgD+ B cells upon emigration from the BM to the periphery, where they can participate in immune responses. Activated B cells can undergo Igh class switch recombination (CSR), mediated by activation-induced cytidine deaminase (AID). CSR replaces initially expressed IgM with IgG, IgE, or IgA by targeted repositioning of the alternative Igh locus CHs, resulting in permanent deletion of intervening CHs (4). Following activation, B cells can maintain a general B cell transcriptional program to support the production of long-lived memory B cells, which continue to be dependent upon BCR signals. An alternate fate results from a large shift in the general B cell transcriptional program toward specialization as antibody secreting cells (ASCs) (5).
IgH CSR is associated with different IgH isotype-specific B cell fates following activation (6–12). Investigations into mechanisms underlying how IgH isotype influences BCR/mIg function to date have largely relied upon overexpression and transgenic experiments of monoclonal Ig to identify how differences in protein sequence between IgH isotypes influence BCR signaling (13–18). However, whether differences of endogenous BCR expression from the Igh locus occur between isotypes is not fully defined. To address this, we generated preswitched Ighε/ε and Ighγ1/γ1 mice engineered to produce polyclonal IgE and IgG1 B cells, respectively, to explore the role of IgH isotype on BCR function from native genomic contexts. We identified an isotype-specific BCR expression hierarchy in naïve IgG1 and IgE B cells from preswitched mice that is preserved in B cells after regular activation-induced CSR. We report that Igh isotype-specific BCR expression is an underlying feature that contributes to isotype-specific B cell behaviors.
Results
Generation of Ighε/ε and Ighγ1/γ1 Mice.
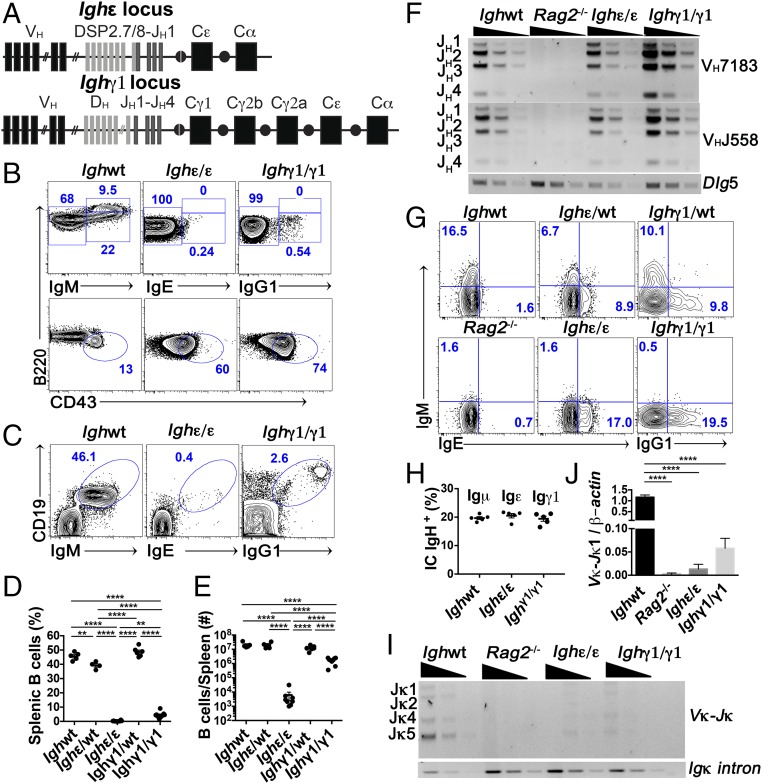
To explore the degree to which IgH isotype regulates BCR function, we generated Ighε/ε and Ighγ1/γ1 mice in an effort to produce native polyclonal IgE+ and IgG1+ B cells, respectively, from natural genomic contexts. The Ighε/ε mice were derived from induced pluripotent stem cells (iPSCs) generated from B-lineage cells that had previously undergone IgH CSR to IgE on an allele that had rearranged a DH to JH1, but that had not yet undergone VH to DHJH assembly (Fig. 1A and SI Appendix, Fig. S1A). The Ighγ1/γ1 mice were generated by deletion of a portion of the CH locus such that the resulting arrangement would be identical to a natural CSR event to Cγ1. This was achieved by CRISPR-mediated embryonic stem cell (ESC) targeting of the same DNA regions targeted for DNA cleavage events during CSR from Cμ to Cγ1 (Fig. 1A and SI Appendix, Fig. S1B).
Fig. 1.
B cell development in Ighε/ε and Ighγ1/γ1 mice is impaired. (A) Schematic representations of the Ighε (Top) and Ighγ1 (Bottom) alleles. (B) FACS plots of live CD19+ and B220+ bone marrow cells (Top plots) as well as live B220+ CD19+ and BCR− (Bottom plots). Mature recirculating B cells (B220hi BCR+), immature B cells (B220int BCR+), and pro–B cell (B220lo BCR− CD43+) frequencies are indicated (n = 6). (C) FACS plots of splenic lymphocytes showing CD19 expression versus IgM, IgE, or IgG1 from the indicated mice (n = 6). (D and E) Dot graph showing summary statistics of percentages (D), and total number (E), of splenic B cells from the indicated mice. Each dot represents one mouse (n = 4–9). (F) Semiquantitative PCR analyses of JH-proximal VH7183 and JH-distal VHJ558 family rearrangements in sorted bone marrow pro-B cells from indicated mice. Dlg5 was amplified as a loading control. Threefold serial dilutions are shown. Results are typical of three experiments. Bands corresponding to rearrangements to various JH segments are indicated. (G) FACS plots showing intracellular Igμ, Igε, and Igγ1 heavy chain in pro-B cells (CD19+ B220lo Igκ− CD43+) from bone marrows of the indicated mice. Results are typical of at least four experiments. (H) Percentage of intracellular Igμ, Igε, and Igγ1 heavy chain in BM pro-B cells. Each dot represents one mouse (n = 5). (I) Semiquantitative PCR analyses of Vκ Igl chain rearrangements in magnetically separated bone marrow B220+ cells from indicated mice. Intronic Igκ was amplified as a loading control. Threefold serial dilutions are shown. Bands corresponding to rearrangements to various Jκ segments are indicated on the Left. Results are typical of four experiments. (J) Quantitative PCR analyses of Vκ to Jκ1 Igl chain rearrangement relative to β-actin DNA in purified B220+ BM cells from the indicated mice. Expression is shown as fold change relative to wild-type levels. **P < 0.01, ****P < 0.0001; one-way ANOVA followed by Tukey’s post hoc test. Summary data are mean values ± SEM. See also SI Appendix, Figs. S1 and S2.
Differential B-Lineage Developmental Competence in Ighε/ε and Ighγ1/γ1 Mice.
Early B cell maturation requires BCR signals to license maturation through developmental stages. Productive assembly of Igμ in the pro–B cell stage leads to surface assembly of the pre-BCR, containing mIgμ, a surrogate light chain complex (SLC), and the CD79A/B proteins. Pre-BCR signaling stimulates Igl (Igκ or Iglλ) V exon assembly in pre-B cells. Productively assembled Igl produces Igκ or Iglλ, which complexes with mIgμ to form IgM, which together with CD79A/B, form the BCR on the surface of immature B cells that provided signals for continued B cell development (19).
We examined the competence of IgE and IgG1 as BCRs to support BCR-dependent developmental steps during early B-lineage cell maturation. We found that Ighε/ε mouse BM contains abundant B220lo CD43+ pro-B cells; however, CD43− B220int pre-B, BCR+ immature, and B220hi BCR+ recirculating B cells, were severely reduced in amount (Fig. 1B). In addition, IgE+ B cells in the spleen were nearly undetectable (Fig. 1 C and D). Developmental blockade in Ighγ1/γ1 mice was not as severe, with detectable BM immature and recirculating B cells (Fig. 1B) and a clear splenic B cell population numbering over 10-fold less compared with wild-type and heterozygous mice (Fig. 1 C–E). BM pro-B cells from each mouse expressed levels of IL-7 receptor similar to wild type (SI Appendix, Fig. S2A) and both had a similar mild reduction of proliferation capacities when stimulated ex vivo with low doses of IL-7, although this did not reach statistical significance (SI Appendix, Fig. S2B). These data suggest that endogenously produced, polyclonal IgE and IgG1 have different levels of BCR fitness in a model of early B cell development, with IgE being the most severely restricted.
Igγ1 and Igε Proteins Are Produced in Pro-B Cells, but Provide Insufficient Stimuli to Induce Igκ Assembly.
To determine whether blockade of B cell development is due to inhibition of VDJH recombination, we assessed the level of Igh VDJH recombination of the two main VH families (proximally positioned 7183, and distally positioned J558 families) on sorted BM B cell progenitors by semiquantitative PCR. Ighε/ε and Ighγ1/γ1 mice showed similar levels of assembled VDJH compared with wild-type progenitor B cells (Fig. 1F). Despite the preassembled DH to JH1 assembly in Ighε/ε mice, all JHs were used (Fig. 1F). In addition, flow cytometric analysis of intracytoplasmic IgH expression in pro-B cells demonstrated similar levels of intracellular Igε and Igγ1 compared with wild-type pro-B cells expressing Igμ (Fig. 1 G and H). In addition, heterozygous Ighε/WT, as well as Ighγ1/WT heterozygote B cell progenitors show Igε:Igμ and Igγ1:Igμ ratios of 1:1 for each (Fig. 1 G and H and SI Appendix, Fig. S2 C and D). These data demonstrate both Igε and Igγ1 heavy chains are expressed intracytoplasmically at levels very comparable to Igμ. Despite this, mature B cells from both Ighε/WT and Ighγ1/WT mice are essentially all IgM+, suggesting a strong competitive advantage for Igμ over Igγ1 or Igε in later stages of development.
To determine the degree to which allelic exclusion is affected, we performed quantitative analysis of cells expressing both Igh alleles in Ighε/WT and Ighγ1/WT heterozygous mice from developing BM and splenic B cells. While intact allelic exclusion makes IgH production from both alleles scarce (less than 1%), a full break in allelic exclusion would theoretically be indicated by 12.2% of double producers (20), although in practice this may be less due to the ability of IgH mRNA from productively assembled Igh loci to mediate allelic exclusion of homologous loci in the absence of IgH protein (21). Within the pool of IgH-expressing B220lo CD43+ BM B-lineage cells, we found 5–8% double IgH producers in both Ighε/WT and Ighγ1/WT heterozygous mice (SI Appendix, Fig. S2 C and D). For splenic B cells, 2–3% express both IgM and IgG1 in Ighγ1/WT mice, whereas 5–7% are positive for both IgM and IgE in Ighε/WT mice (SI Appendix, Fig. S2 E and F). Of note, cells heterozygous for an allele that can only produce the secreted version of IgM (μMT allele) (22), also contain ∼6% of double producers in the spleen (20). This analysis suggests that IgG1, and to a greater extent, IgE, are unable to mediate normal allelic exclusion.
To determine the degree of Igl rearrangement in Ighε/WT and Ighγ1/WT mice, we assessed Igκ V-J rearrangement by semiquantitative PCR as well as the level of rearrangement to Jκ1 by qPCR. We found that Ighε/ε BM B cell Igκ rearrangement is very near the Rag2−/− background control with Ighγ1/γ1 Igκ rearrangement slightly higher (Fig. 1 I and J). Together, these results indicate that, while IgH intracytoplasmic expression reaches wild-type Igμ levels, BCR signaling in the context of endogenously produced Igγ1 and Igε provides insufficient signaling to stimulate entry into subsequent BCR-dependent cell stages.
Ighγ1/γ1 and Ighε/ε B Cell Development Is Partially Rescued by a Pre-Assembled Igκ (VJκ5).
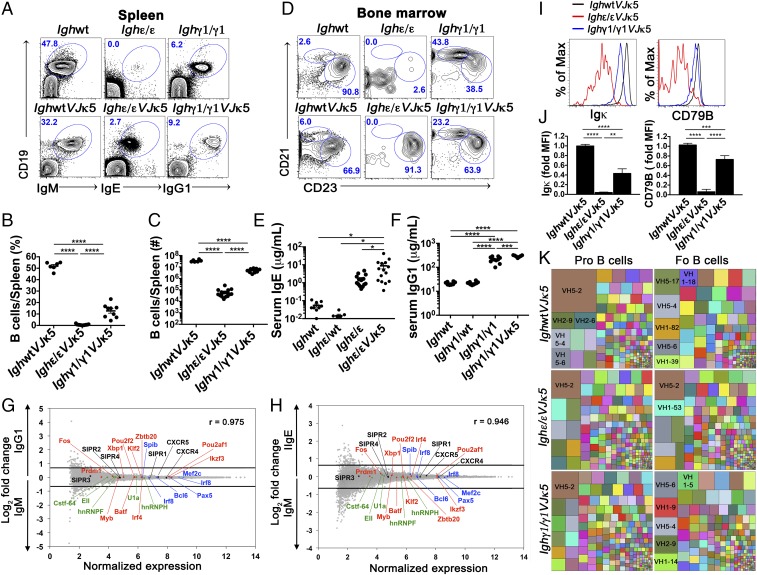
To determine the extent to which introduction of a preassembled Igκ can rescue development in Ighγ1/γ1 and Ighε/ε B cells, we crossed both Ighγ1/γ1 and Ighε/ε mice to the VJκ5 allele, a natural productive Igκ assemblage, also produced via B cell to iPSC reprogramming (SI Appendix, Fig. S1C). The presence of the preassembled VJκ5 resulted in a partial rescue of the developmental blockade observed in Ighε/ε mice. IgE+ B cells in Ighε/εVJκ5 mice increased in percentage (Fig. 2 A and B), with an ∼10-fold increase in splenic B cell number (compare Fig. 1E to Fig. 2C). IgG1+ cells increased ∼5-fold in Ighγ1/γ1 mice (compare Fig. 1E to Fig. 2C). We found that mature IgE+ B cells from Ighε/εVJκ5 mice are nearly all of the follicular B cell phenotype (CD19+ B220+ CD93− CD23hi CD21int), whereas splenic IgG1+ cells from Ighγ1/γ1 and Ighγ1/γ1VJκ5 mice have both follicular and marginal zone (CD19+ B220+ CD93− CD23lo CD21hi) populations in the splenic B cell compartment, with an increased percentage of marginal zone B cells compared with wild-type mice (Fig. 2D). The CD19+ IgG1− cells observed in Ighγ1/γ1 mice spleens (Fig. 1C) expressed CD43 and CD93, consistent with pro-B cells (SI Appendix, Fig. S3A). In addition, despite significant B lymphopenia, Ighε/ε mice and Ighε/εVJκ5 mice have 100- to 1,000-fold higher serum IgE levels (Fig. 2E) compared with wild-type controls. IgG1 levels in both Ighγ1/γ1 and Ighγ1/γ1VJκ5 mice are ∼10-fold higher than wild type (Fig. 2F).
Fig. 2.
Development and characteristics of IgE+ and IgG1+ mature B cells with the introduction of a prerearranged Igκ (VJκ5). (A) FACS plots show splenic lymphocytes of the indicated mice. Numbers in the plots indicate percentage of gated live CD19+ BCR+ cells (n = 6). (B and C) Dot graphs showing percentage (B) and absolute number (C) of splenic B cells of indicated mice. (D) FACS plots of live CD19+ B220+ CD93− gated lymphocytes from spleens of the indicated mice to identify splenic marginal zone (CD21hi CD23lo) and follicular (CD21int CD23hi) B cells (n = 6). Because Ighε/εVJκ5 and Ighγ1/γ1VJκ5 mice appear to express higher levels of CD23, the gating is relative within each mouse to identify CD23hi CD21int follicular B cells. Numbers in the plots indicate percentages. (E and F) Total serum IgE (E) and IgG1 (F) concentration measured by ELISA from the indicated mice. Each dot represents individual mice. (G and H) Naïve splenic IgE+ and IgG1+ B cells show similar gene expression pattern to WT naïve IgM B cells. Microarray analysis of sorted B220+ CD93− CD23hi CD21int (follicular) splenic B cells from IghWTVJκ5 (IgM) versus Ighγ1/γ1VJκ5 (IgG1) mice (G), and IghWTVJκ5 (IgM) versus Ighε/εVJκ5 (IgE) mice (H). Selected chemokine receptor genes (black), splicing factors (green), as well as positive (red) and negative (blue) regulators of plasma cell differentiation are shown. Lines represent cutoffs for genes up- or down-regulated by a fold-change of at least 0.67 (log2). The Pearson correlation coefficient (r) between gene expression levels is given for respective plots (n = 3). (I and J) Flow cytometric histogram plots (I) and summary bar graphs (J) of live BCR+ follicular B cells from the indicated mice analyzed for surface Igκ expression (Left of I and J) and CD79B expression (Right of I and J). Fold median fluorescence intensity (MFI) was calculated by dividing MFI values by the average MFI from IgM+ from IghWTVJκ5 mice for each given subset (n = 4–5). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; one-way ANOVA followed by Tukey’s post hoc test. Data are mean values ± SEM. (K) Treemaps showing VH gene segment frequencies in pro- and follicular (Fo) B cells from IghWTVJκ5, Ighε/εVJκ5, and Ighγ1/γ1VJκ5 mice. Each block represents combined data from all biologic repeats (n = 5–6). Within a block, each colored box represents one VH segment. The size of the box is directly proportional to the percentage of sequences, which belongs to the VH segment. The same VH segment has the same color in all of the treemaps and the largest boxes contain the VH name. PCR repeats were removed via unique molecular indexing. See also SI Appendix, Figs. S3–S6.
Analysis of BM and transitional cell populations demonstrated that the VJκ5 allele had a large effect increasing the BM populations for Ighγ1/γ1VJκ5 mice, particularly immature B cells (SI Appendix, Fig. S3 B–F), whereas in Ighε/εVJκ5 mice, the largest effect appeared to be an increase in CD93+ transitional B cells (SI Appendix, Fig. S3 G–I). A 26-d pulse of BrdU in drinking water followed by over a month of normal water (chase) showed that the VJκ5 rescue had no significant effect on the maintenance of the circulating B cell pool from IghWT, Ighε/ε, and Ighγ1/γ1 mice, but did show that both Ighε/ε and Ighε/εVJκ5 B cells had shorter half lives than the others by about a week (SI Appendix, Fig. S4A). Together, these data indicate that the developmental arrest in Ighγ1/γ1 and Ighε/ε mice can be partially rescued by a preassembled Igl, but that the amount of IgG1+ and IgE+ B cell levels continue to be moderately and severely restricted, respectively, in the periphery, despite both having productive IgH and IgL expression. Enhanced peripheral B cell numbers with preassembled Igl also suggests that, in general, BCR signal weakness, rather than too much signal strength, contributes to the developmental blockade in Ighγ1/γ1 and Ighε/ε mice.
Mature Naïve IgE+ and IgG1+ B Cells from Ighε/εVJκ5 and Ighγ1/γ1VJκ5 Mice, Respectively, Are Transcriptionally Similar to Mature Naïve IgM+ B Cells.
We considered the possibility that IgG1 and IgE may direct a plasma cell fate by autonomous signaling (9, 11, 12, 15, 16). To address this, we sorted IgM+ follicular B cells from wild-type mice and IgE+ and IgG1+ cells with the same surface phenotype from Ighε/εVJκ5 and Ighγ1/γ1VJκ5 mice, respectively (SI Appendix, Fig. S4B), to measure gene expression profiles. We found that both IgG1 and IgE B cells align closely to IgM-expressing follicular B cells, with r values of 0.975 and 0.946, respectively (Fig. 2 G and H). We find no differences in genes important for plasma cell differentiation (23) or in core B cell regulatory genes and splicing regulators between IgM, IgE, and IgG1 cells (Fig. 2 G and H). These data indicate that expression of polyclonal IgG1 and IgE from native loci is not sufficient to directly instruct B cells to become plasma cells when expressed from endogenous genomic context.
IgE and IgG1 B Cells Express Low Cell Surface BCR Density.
Previous reports have indicated that BCR density influences B cell fate (8, 24–27). In addition, limited BCR density has been proposed to underlie restriction of IgE B cell numbers (8, 27). To explore the mechanism for the differential insufficiencies of endogenously produced, polyclonal IgG1 and IgE in supporting early B cell development and peripheral B cell numbers, we hypothesized that different BCR dosages may play a role in differential IgG1 and IgE B cell behaviors.
To test this, we measured BCR density by cytometrically assessing Igκ expression on the cell surface of IgM-expressing B cells from wild-type mice and compared them to IgG1+ and IgE+ B cells from Ighγ1/γ1VJκ5 and Ighε/εVJκ5 mice, respectively. Because the Ighε/εVJκ5 mice only make B cells with the follicular phenotype, we compared the IgE+ follicular B cells to IgG1+ and IgM+ follicular B cells from the other mice. We also assessed BCR density by staining cells for CD79B (also known as Igβ), as this forms a part of the BCR for all IgH isotypes (28). We found that both Igκ as well as CD79B median fluorescence intensity (MFI) was highest for IgM-expressing naïve follicular B cells, and lowest in IgE+ cells, with IgG1-expressing B cells falling in between the two (Fig. 2 I and J). These data suggest that BCR dosage may influence B cell numbers by regulating integrated BCR signaling strength.
BCR-Related Phosphoprotein Analysis in IgM, IgG1, and IgE Cells.
We also examined baseline phosphorylation levels of Erk, Syk, and Akt, which are related to BCR signaling (29), by flow cytometry. We found that basal phosphorylation levels of Akt were similar, whereas phospho (p)-Erk, and p-Syk were modestly higher in splenic cells from Ighγ1/γ1VJκ5 and Ighε/εVJκ5 mice compared with IghWTVJκ5 mice (SI Appendix, Fig. S4C). These results are consistent with the concept that intrinsic BCR signaling differences exist between IgH isotypes and may be playing a role in the phenotypic differences. However, the degree to which the phenotypic differences are directly related to the BCR is not clear. Also unclear is whether or not these differences are a cause or an effect of the developmental blockade.
Given the BCR density differences between IgM, IgG1, and IgE, we assessed levels of BCR-related phosphoproteins in a system where expression levels of BCR isotypes from endogenous loci are similar to each other. For this we generated CH12 cell lines expressing IgM, IgG1, or IgE from endogenous loci (SI Appendix, Fig. S4D). We find similar levels of baseline p-Syk, p-Erk, and p-Akt in all three in CH12-IgM and CH12-IgE cells, with higher levels of each in CH12-IgG1 cells (SI Appendix, Fig. S4E). These results suggest that intrinsic differences in isotype-specific BCR signaling likely contribute to B cell behaviors.
Weaker Developmental Ig Repertoire Selection in IgG and IgE B Cells.
To explore the degree to which IgG1 and IgE may influence selection of preimmune Ig repertoires, we sorted pro-B and follicular B cells from IghWTVJκ5, Ighγ1/γ1VJκ5, and Ighε/εVJκ5 mice for Ig repertoire sequencing (SI Appendix, Fig. S5 A and B). Analysis showed grossly similar VH segment use patterns in pro-B cells from each of the genotypes (Fig. 2K and SI Appendix, Fig. S5C). However, the selection patterns between pro-B and follicular B cells varied. In this regard, the VH segment VH5–2 (also known as 81x), is known to be highly used in pro–B cell VDJH assemblies, but is strongly selected against during B cell development (30). This results in more rare VH5–2 use in mature B cells, presumably due to its autoreactivity (31, 32). We see VH5–2 highly represented in pro-B cells from all genotypes (Fig. 2K and SI Appendix, Figs. S5C and S6 A–C, brown box in Upper Left corner of the treemap plots), and it is selected against in follicular B cells from IghWTVJκ5 and Ighγ1/γ1VJκ5 mice, indicating that this aspect of selection is grossly intact in Ighγ1/γ1VJκ5 mice. However, the VH5–2 gene segment is still the highest used in Ighε/εVJκ5 follicular B cells (Fig. 2K and SI Appendix, S5C and S6 A–C). This may indicate selection of autoreactive cells capable of overcoming developmental blockade due to otherwise weak signaling. An alternative possibility is that BCR expression may be insufficient to arbitrate BCR-mediated and/or ligand-mediated developmental selection. The fact that overall VH use patterns between pro-B and follicular B cells in Ighε/εVJκ5 is significantly more highly correlated than what is seen in the others suggests the latter is more likely (SI Appendix, Fig. S6 D and E). Analysis of BM pro-B and splenic follicular B cell VH gene segment use in Ighγ1/γ1VJκ5 mice also showed a nonsignificant trend toward preservation of the pro–B cell repertoire profile compared to IghWTVJκ5 mice. Postsort cell analysis indicates the preservation of pro–B cell repertoires is not due to early B-lineage cell contamination (SI Appendix, Fig. S5A).
IgG1 and IgE B Cells Are Moderately and Severely Biased, Respectively, Against the mIg Alternatively Spliced mRNA Variant.
We asked whether differences in alternatively spliced mRNA might contribute to the different BCR density rankings observed in IgG1, IgE, and IgM B cells. Because alternative promoter use can influence alternative splicing (33), as well as mRNA stability (34), we developed an absolute qPCR assay to measure absolute amounts of productive sIg and mIg mRNA splice variants by comparing to a known amount of a standard. Productive mRNA transcripts encoding the VDJH exon together with the CH exons are generated from VH promoters 5′ to the VDJH exon. Germline (GL) CH transcripts are initiated downstream of the VDJH exon from a noncoding exon 5′ to each CH region (called the IH exon) present at each Igh isotype. B cells constitutively produce transcripts from both VH and Iμ promoters, while the other IH region promoters (e.g., Iγ1, Iε) are induced upon activation. GL transcripts from IH promoters are capped, polyadenylated, and spliced. Previous measurements of sIg and mIg splice variants using relative qPCR assessments of 3′ ends of sIg and mIg mRNA have not accounted for possible differences of these different promoters on splicing bias (8, 27).
We amplified either VH or IH promoter-driven transcripts from wild-type B cells activated for IgG1 and IgE CSR in vitro before using absolute qPCR to measure sIg and mIg variants from each pool against their respective standards (SI Appendix, Fig. S7). RNA transcripts produced from Iμ and Iγ1 promoters were relatively more biased toward mIg compared with their VH promoter counterparts (SI Appendix, Fig. S8 B–D). In contrast, the sIg/mIg ratio was similar between Iε and its VH promoter counterpart (SI Appendix, Fig. S8C). CSR to IgE and IgG1 results in the juxtaposition of Iμ to Cε or Cγ1, respectively. In addition, downstream CSR can juxtapose Iγ1 to Cε (35). We found that Iμ-driven transcripts were also relatively biased toward mIg for Iμ-Cγ1 and Iμ-Cε transcripts compared with corresponding VH transcripts, and that mIg was also relatively favored in the context of Iγ1-Cε transcripts (SI Appendix, Fig. S8 B–D). These results suggest that promoter use influences alternative splicing biases in the Igh locus, thus supporting a need to isolate and assess transcripts from VH promoters to quantify mRNA variants relevant for protein production.
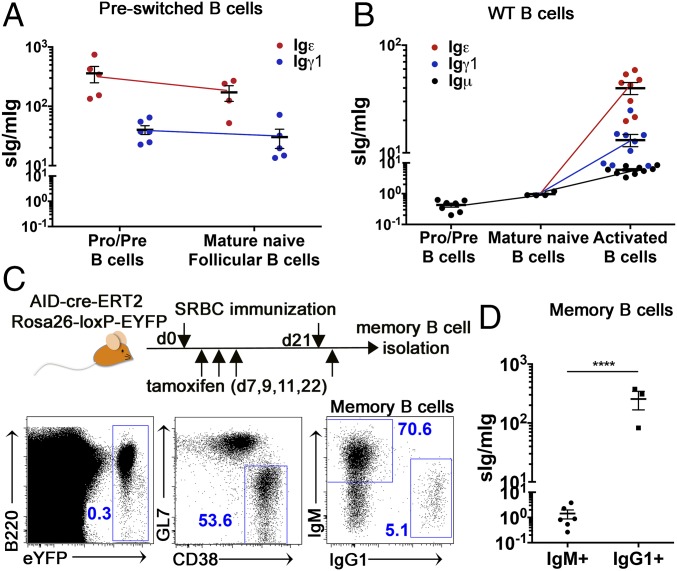
We used VH promoter-driven transcript amplification and absolute qPCR assay to assess sIg/mIg mRNA ratios in B cell subsets from Ighε/εVJκ5 and Ighγ1/γ1VJκ5 mice. We found that pro-B cells from Ighε/ε mice make several hundred-fold higher sIgε than mIgε (Fig. 3A and SI Appendix, Fig. S9A). Pro-B cells from Ighγ1/γ1VJκ5 mice are also biased to the sIgγ1 mRNA variant at a level of ∼40-fold over mIgγ1 mRNA (Fig. 3A and SI Appendix, Fig. S9A). The sIg/mIg ratios from Ighγ1/γ1VJκ5 and Ighε/εVJκ5 splenic follicular IgG1 and IgE B cells, respectively, showed similar levels to those found in pro-B cells from the respective mice (Fig. 3A). Igμ mRNA in wild-type pro-B cells is biased toward the mIgμ splice variant, with an sIgμ/mIgμ of ∼0.4, while the sIgμ/mIgμ increased to an ∼1:1 ratio at the mature naïve B cell stage (Fig. 3B).
Fig. 3.
Moderate and severe bias to sIg in IgG1 and IgE cells, respectively. (A) Dot graph showing the ratio of sIg/mIg mRNA expression for Igε (red) and Igγ1 (blue) from B220+ BCR− bone marrow (pro/pre) and mature follicular (B220+ CD93− BCR+ CD21int CD23hi) B cells from Ighε/ε (red) and Ighγ1/γ1 (blue) mouse spleens (n = 4–5). The sIg and mIg levels, as well as total Ig mRNA levels were determined by absolute qPCR using known levels of standards. (B) Dot graph showing the ratio of sIg/mIg for Igμ (black), Igε (red), and Igγ1 (blue) from the indicated B cells isolated from wild-type (WT) mice. Pro/pre B cells are from B220+ BCR− bone marrow. Mature B cells are from magnetically purified B220+ splenic cells. Activated B cells were derived by stimulation of magnetically purified B220+ splenic cells with anti-CD40 antibody plus IL-4 for 4 d (n = 4–9). (C) Schematic outline (above) of AID-cre-ERT2 Rosa26-loxP-EYFP mice immunized with sheep red blood cells (SRBCs) and induced with tamoxifen as outlined. FACS plots (Below) show gating strategy for flow cytometric sorting of IgM+ and IgG1+ memory (EYFP+ CD38+ GF7−) B cells (n = 6). (D) Graph showing sIg/mIg mRNA ratios of IgG1+ and IgM+ of memory B cells shown in C by the absolute qPCR method described in A. Dots represent individual mice (n = 6). The mIgG1 mRNA level was below detection in three IgG1+ memory cell samples. ****P < 0.000, two-tailed t test. Summary data are means ± SEM. See also SI Appendix, Figs. S7–S9.
To evaluate IgH isotype mRNA splicing bias in wild-type cells, we stimulated wild-type B cells to undergo CSR to IgE and IgG1 in vitro (SI Appendix, Fig. S9B). We found that activated, switched B cells are heavily biased toward the sIgε and sIgγ1 splice variants, with Igε mRNA being the most biased toward sIg and Igμ being the least (Fig. 3B). To determine if similar splicing bias can be detected in memory B cells, we immunized the AID-cre-ERT2 Rosa26-loxP-EYFP memory cell reporter mice (6) with sheep red blood cells and sorted IgM+ and IgG1+ memory (EYFP+ CD38+GL7−) B cells to measure splice bias (Fig. 3C) (no IgE+ B cells detected). We found an ∼100-fold higher sIg/mIg ratio in IgG1+ B cells compared with IgM+ memory B cells (Fig. 3D), which remains at an approximate 1:1 ratio (Fig. 3D). Together, these data indicate that the general splicing bias hierarchy found in Ighγ1/γ1VJκ5 and Ighε/εVJκ5 mice is also found in IgE and IgG1 cells from wild-type B cells.
Ectopic mIgγ1 or mIgε Rescues B Cell Development in Ighε/ε, Ighγ1/γ1, and μMT Pro-B Cells.
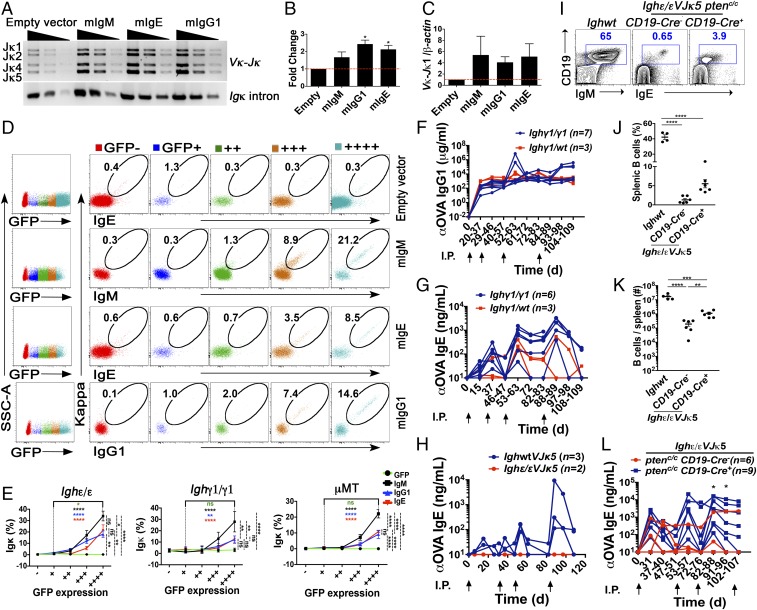
To determine the degree to which developmental blockage at pro–B cell stage is a result of low density of mIgε and mIγ1 in Ighε/ε and Ighγ1/γ1 mice, we retrovirally transduced the membrane form of IgE and IgG1, or empty vector, in developing B-lineage cells ex vivo from Ighε/ε and Ighγ1/γ1 mice, and measured Igκ rearrangement. We included developing B-lineage cells from μMT mice, which have a similar B cell developmental blockade due to inability to produce mIgμ (22). Pro-B cells were transduced with vectors expressing GFP alone, or with mIgM, mIgG1, or mIgE followed by measurement of Igκ assembly and Igκ protein expression. Ighε/ε, Ighγ1/γ1, and μMT pro-B cells transduced with GFP alone had minimal Igκ assembly and protein expression; however, enforced expression of mIgE and mIgG1 was able to induce Igκ gene assembly (Fig. 4 A–C). This resulted in surface Igκ expression in pro-B cells from all three genotypes, reaching significance in Ighε/ε, and μMT pro-B cells, but not in Ighγ1/γ1 pro-B cells, likely due to the higher background from natural assembly and expression of Igκ in these mice (SI Appendix, Fig. S10A).
Fig. 4.
Overexpression of mIgH rescues B cell development in Ighε/ε, Ighγ1/γ1, and μMT pro-B cells, and Pten deletion can generate a memory response mediated by IgE+ B cells. (A) Image of semiquantitative PCR results to detect Vκ to Jκ1 assemblages in Ighε/ε pro/pre-B cells transduced with the indicated retroviral vectors. Threefold dilutions are shown. Amplification of an intronic Igκ sequence was used as a loading control. Result is representative of three experiments. Bands corresponding to rearrangements to various Jκ segments are indicated on the Left. (B) Densitometry analysis of the semiquantitative PCR data in A with ImageJ for three repeated experiments. Shown are fold changes relative to the empty vector control (n = 3), *P < 0.05, one sample t test. Summary data are means ± SEM. (C) Quantitative PCR analyses of Vκ to Jκ1 rearrangement relative to intronic β-actin DNA in Ighε/ε pro/pre-B cells transduced with the indicated retroviral vectors. Shown are fold changes relative to the empty vector control. Summary data are means ± SEM. While each mIgH expression vector was at least twofold higher than the empty vector control, one-sample t tests showed no significant differences. (D) Representative FACS analysis of Igκ and IgE, IgM, or IgG1 surface expression in Ighε/ε pro/pre-B cells transduced with the indicated retroviral vectors. Numbers indicate percentage of surface Igκ+ IgH+ on live CD19+ B cells, which express from no GFP to highest GFP, indicated by increasing number of “+” signs (n = 6). (E) Quantification of surface Igκ+ on live CD19+ B cells which express from no GFP to highest GFP after retroviral transduction in Ighε/ε, Ighγ1/γ1, and μMT pro/pre-B cells (n = 4–6). *P < 0.05, **P < 0.01, ****P < 0.0001; one-way ANOVA followed by Tukey’s post hoc test. Data are mean values ± SEM. (F and G) Graph of ELISA data showing ovalbumin (OVA)-reactive IgG1 (F) and IgE (G) in sera from Ighγ1/γ1 (blue) and heterozygous Ighγ1/WT (red) mice immunized and boosted intraperitoneally with OVA at the time intervals shown by the upward arrows. Fisher’s exact test showed no significant differences. (H) Graph of ELISA data showing OVA-reactive IgE in sera from WT (blue) and Ighε/εVJκ5 (red) mice immunized and boosted intraperitoneally with OVA at the times shown by the upward arrows. No response was observed in two Ighε/εVJκ5 mice, whereas all three WT mice responded. (I) FACS plots showing splenic lymphocytes analyzed for CD19 and IgE expression from the indicated mice (n = 4–6). (J and K) Summary dot graphs showing percentages (J) and total number (K) of splenic B cells from the indicated mice. Dots represent individual mice (n = 4–6). **P < 0.01, ***P < 0.001, ****P < 0.0001; one-way ANOVA followed by Tukey’s post hoc test. Data are mean values ± SEM. (L) Graph of ELISA data showing OVA-reactive IgE in sera from Ighε/εVJκ5 Ptenc/cCd19cre− (blue) and Ighε/εVJκ5 Ptenc/cCd19cre+ (red) mice immunized and boosted intraperitoneally with OVA at the time intervals shown by the upward arrows. The y axis shows apparent binding in milligrams/milliliters or nanograms/milliliters as indicated based on comparisons to a standard anti-ovalbumin IgG1 or anti-ovalbumin IgE antibody. Number of mice used in each immunization is shown. *P < 0.05, Fisher’s exact test. See also SI Appendix, Fig. S10.
As the mIgH constructs contained a GFP reporter controlled by an internal ribosomal entry site (IRES), GFP expression can provide an indicator of amount of mIgH RNA. Cells gated based on low to high expression of GFP showed an expression-dependent increase of the percentage of cells expressing Igκ. (Fig. 4 D and E). These results indicate that both IgE and IgG1 can compensate for IgM in delivering a BCR signal sufficient to induce B-lineage maturation in a density-dependent fashion. Because SLC interaction with IgH is known to be functionally required for Igκ assembly (36), these results also suggest that IgG1 and IgE can functionally interact with SLC. These findings suggest that insufficient BCR density is likely a contributing factor in the BM blockade seen in Ighε/ε and Ighγ1/γ1 mice.
While mIgE and mIgG1 could both rescue Igκ production, mIgM appeared to have an advantage in producing a higher percentage of Igκ+ cells, which was more apparent when tested in the context of Ighγ1/γ1 and μMT pro-B cells (Fig. 4E). We found that this mIgM advantage may be related to the ability of mIgM to induce proliferation to a greater extent than mIgG1 or mIgE in the context of pro-B cells from Ighγ1/γ1 or Ighε/ε mice retrovirally transduced with mIgH (SI Appendix, Fig. S10 B and C). The advantage seen by mIgM in inducing more proliferation suggests that isotype-intrinsic BCR signaling may contribute as well to the BM blockade seen in Ighε/ε and Ighγ1/γ1 mice.
Strengthened PI3K Signaling Can Generate a Memory Response Mediated by IgE+ B Cells.
Immunologic IgE memory responses are unlikely to be housed in IgE B cells themselves, whereas IgG1 B cells can function in both IgG1 and IgE memory (8, 12). To determine the degree to which IgG1 and IgE B cells in Ighγ1/γ1 and Ighε/εVJκ5 mice can participate in a conventional immune response to produce antigen-specific antibodies to immunization, we immunized them with ovalbumin (OVA) and assessed IgH isotype-specific serum anti-OVA antibodies. We rechallenged the mice as well to test whether a functional memory response could be detected. For Ighγ1/γ1 mice, we detected IgG1 and IgE anti-OVA responses similar to Ighγ1/WT heterozygotes with immunization, with levels increasing in magnitude after rechallenge for IgE responses (Fig. 4 F and G), indicating that the IgG1 cells are competent for germinal center entry and selection as well as CSR to IgE upon activation as expected. In contrast, immunization of Ighε/εVJκ5 mice did not induce any detectable anti-OVA IgE response (Fig. 4H), consistent with previous reports of rapid death of IgE+ cells upon activation (8, 9).
We crossed Ighε/εVJκ5 mice to Cd19cre Ptenc/c mice for conditional PTEN deletion in B cells with the goal to examine the degree to which strengthened BCR signaling could rescue a functional memory response for IgE+ B cells. PTEN is a negative regulator of PI3K signaling, which is downstream of BCR. When Pten is conditionally deleted in B cells, BCR signaling is bypassed and B cells act as though they are receiving stronger BCR signals (37). CD19 has also been shown to interact with IgE and negatively regulate IgE responses (16). Ighε/εVJκ5 Cd19cre+ Ptenc/c mice have over fivefold more splenic B cells compared with controls at baseline (Fig. 4 I–K), consistent with previous reports of a role of B cell Pten deletion rescuing B cell numbers in the setting of insufficient BCR expression (37). After immunization, clear anti-OVA IgE responses were observed in several Ighε/εVJκ5 mice with B cell Pten deletion that increases in magnitude upon rechallenge, while five out of six Ighε/εVJκ5 cre-negative control mice showed no sustained response (Fig. 4L). IgE responses have recently been shown to be increased in the setting of CD19 haploinsufficiency via an unclear mechanism (16). Since Cd19cre mice are also haploinsufficient for CD19, this may also contribute to the increased IgE responses in Ighε/εVJκ5 Cd19cre+ Ptenc/c mice. Because the magnitude of the Cd19 haploinsufficiency is mild (16), lower CD19 expression alone in Cd19cre mice is unlikely to explain the large effects observed (Fig. 4L). These results suggest that the lack of functional memory cell capabilities within IgE+ cells may be restored by B cell Pten deletion, implying that weak BCR signaling by IgE, potentially provided by minimal BCR density, plays a key role in limiting IgE+ activation and memory cell functional capacity.
Discussion
IgH isotype plays a major role in defining function of secreted antibodies and can influence BCR function to regulate B cell fate when expressed as mIg. Our data are consistent with a concept that individual IgH isotypes are linked to CH-specific control elements that contribute to isotype-specific BCR dosage regulation.
Alternative RNA splicing was first identified in Igμ transcripts (38–40) and was later shown to be a widespread mechanism of gene regulation (41). Alternative splicing for Igμ favors mIg during early B cell development and in naïve B cells, whereas sIg is favored upon activation (2, 42). The sIg/mIg mRNA ratios of Cε and Cγ1 do not appear to be susceptible to this regulation as they favor the sIg splice variant at the expense of mIg, regardless of cell stage. For Cε, nonconsensus polyadenylation sequences downstream of membrane exons have been shown to underlie bias against mIg splice forms due to higher efficiency polyadenylation at a consensus site upstream of the membrane exons (27). The Cγ1 locus has consensus polyadenylation signals both upstream and downstream of its membrane exons, implicating other regulatory mechanisms involved in producing sIg bias observed in IgG1 B cells. While splicing plays a role in Igh gene expression, other features may contribute to IgH isotype-specific BCR density regulation, such as mRNA turn over, translation, surface protein stability, intrinsic signaling differences, as well as endocytosis and BCR recycling. In addition, while IgM appears to be produced with relatively higher mIg/sIg compared with other IgH isotypes, BCR density has the potential to be even greater when IgD is expressed in addition to IgM, as occurs in mature naïve B cells and in some IgM+ memory B cells.
Our finding that retrovirally transduced mIgE and mIgG1 can rescue B cell developmental progression in Ighε/ε, Ighγ1/γ1, as well as μMT mice in an expression density-dependent fashion suggests that there may be more functional overlap of autonomous signaling between IgH isotypes than previously anticipated. However, our data are not inconsistent with the concept that differences in autonomous signaling between IgH isotypes contribute to isotype-specific differences in BCR function. BCR dosage levels and autonomous signaling differences likely act together to influence BCR function. Isotype-specific BCR dosage can impact B cell fate by defining signaling needs required to reach functional thresholds through ligand engagement (43). For example, B cells with more dilute BCR isotypes may require higher affinity and/or more cognate antigen availability to reach an integrated BCR signal strength that is similar to cells endowed with higher BCR density (24). The observation that autoreactive VH5–2 was found to be dominant in Ighε/εVJκ5 follicular B cells is consistent with this concept, in that rare IgE cells may have been allowed to develop due to continuous recognition of a putative self-antigen. However, because VH use frequencies between Ighε/εVJκ5 pro-B cells and follicular IgE B cells are so highly correlated (SI Appendix, Fig. S6E), with VH5–2 favored in both, an alternative explanation is that dilute IgE (and perhaps to a lesser extent, IgG1) BCR may render cells relatively deaf to the normal ligand-mediated signals that usually accompany early B cell maturation, resulting in preservation of the pro–B cell VH use pattern (SI Appendix, Fig. S6).
While we know of no other reports of IgE preswitched models, other work has generated preswitched models for IgG (44–47), most with monoclonal Ig specificities. Since Ig specificity alone can substantially impact B cell development (48, 49), it is not clear what aspects of development are due to IgH isotype versus Ig specificity in monoclonal models (44–46, 50). A polyclonal IgG1 preswitched model, called IgHγ1μ mice, was produced wherein the intronic polyA site (that regulates splicing to the secreted IgG variant) was deleted to enhance mIgG1 over sIgG1 production (47). In this setting, the IgHγ1μ mice have normal peripheral B cell numbers compared with wild-type controls (47), whereas our Ighγ1/γ1 mice are B cell lymphopenic, harboring over 10-fold less peripheral B cells than wild-type controls (Fig. 1E). This comparison is consistent with the concept that BCR expression, at least at the mRNA level, plays a role in influencing B cell numbers. The observation of an early BM developing B-lineage cell blockade in IgHγ1μ mice suggests that aspects of IgG1 intrinsic signaling contribute to similar blockage in Ighγ1/γ1 mice, consistent with our finding that mIgM provides a pre–B cell proliferative advantage (SI Appendix, Fig. S10 B and C). The fact that the blockade in IgHγ1μ mice is much less severe (47) suggests that BCR expression plays a role as well at this stage of development.
Isotype-specific BCR density limits may contribute to functional differences observed in IgG1 and IgE B cells under normal settings, such as limited entry into the memory B cell compartments. The moderate and severe limitations of mIg production for IgG1 and IgE, respectively, are in line with findings showing that IgM-expressing B cells appear to enter the memory compartment at a higher level compared with IgG1-expressing B cells (6, 7, 10), and that IgE-expressing memory B cells are essentially nonexistent (8, 9, 12). In addition, our finding that functional antigen-specific IgE memory carried out by IgE-expressing B cells in the setting of Cd19 hemizygosity and Pten deficiency, is consistent with the concept that weak signaling from IgE BCRs limits IgE memory B cell formation. We conclude that IgH isotype-specific BCR dosage control is a regulatory mechanism in the B cell system.
Materials and Methods
Mice.
The Children’s Hospital Boston Animal Care and Use Committee (IACUC) and the Warren Alpert Building, Boston, IACUC approved all experiments. Doxycycline-inducible reprogrammable mice used in these experiments have been described previously (46, 51). Splenic B cells from reprogrammable mice were isolated and CSR to IgG1 and IgE was induced as described previously (46). Details can be found in SI Appendix. The Ighγ/γ1, IghWT, Ighε/ε, and VJκ5 variants were all maintained on a mixed 129.B6 background. The μMT mice (B6.129S2-Ighmtm1Cgn/J), Ptenc/c (B6.129S4-Ptentm1Hwu/J) mice and Cd19cre (B6.129P2(C)-Cd19tm1(cre)Cgn/J) mice were purchased from The Jackson Laboratory. The Rag2−/−, described previously (52), was provided by Frederick Alt, Boston Children’s Hospital, Boston. Aid−/− mice were provided by Honjo and colleagues (53). Unless otherwise noted, all mice were housed at the Boston Children’s Hospital animal facility under specific pathogen-free (SPF) conditions. AID-cre-ERT2 Rosa26-loxp-EYFP mice were housed in the Warren Alpert Building under SPF conditions. See SI Appendix for further details.
Cell Isolation and Flow Cytometry.
BM cells were flushed from femurs and tibias with ice-cooled staining buffer (PBS supplemented with 2% FBS). Spleen cell suspensions were obtained by gently teasing spleens onto a 70-μm cell strainer. Erythrocytes were depleted using red blood cell lysis buffer (Sigma). Cells were counted using a hemocytometer with exclusion of dead cells with Trypan blue dye. Cells were stained with fluorophore or biotin-conjugated antibodies as described (54, 55) where indicated. The cell sorting was performed on a FACSAria II flow cytometer (BD Biosciences). For the in vitro CSR experiments and the allelic exclusion experiments, staining for IgM, IgG1, and IgE for intracytoplasmic IgH expression was done by using trypsinization followed by fixation/permeabilization as described (54, 55). Data analysis was performed with FlowJo software (v9.9.4). See SI Appendix for further details.
Cell Culture and CSR Assay.
Splenic and BM cells were isolated by B220 positive selection via magnetic columns (Miltenyi Biotech) according to manufacture instructions. The CH12 B cell line was previously described (56) and provided by Frederick Alt. Cells were cultured in RPMI (Corning/Cellgro) supplemented with 2 mM l-glutamine (Gibco), 100 units/mL penicillin and 100 μg/mL streptomycin (Gibco), 0.1 mM nonessential amino acids (Gibco), 20 mM Hepes (Gibco), 0.1 mM β-mercaptoethanol (Sigma-Aldrich) and 15% FBS (HyClone). CSR to IgG1 and IgE was performed as described (46). For pro/pre–B cell enrichment, B220+ BM cell cultures were stimulated with 20 ng/mL IL-7 (R&D Systems) for 3–4 d. Immature B cells were excluded via BCR negative selection using biotinylated anti-mouse IgM, IgG1, or IgE followed by anti-biotin magnetic columns (Miltenyi Biotech) according manufacture instructions. Pro/pre–B cell populations showed purity >95%.
Immunization.
Male and female Ighγ1/γ1, Ighγ1/WT, Ighε/εVJκ5, Ighε/εVJκ5 Ptenc/cCd19cre−, and Ighε/εVJκ5 Ptenc/cCd19cre+ mice at 6–8 wk were immunized with 50 μg per mouse chicken OVA (Sigma-Aldrich) at days 0, 21, 47, and 73. AID-cre-ERT2 Rosa26-loxp-EYFP mice were immunized with 2 × 108 sheep red blood cells (Colorado Serum Company) at days 0 and 21. AID expression is induced by oral administration with 15 mg tamoxifen per mouse per time point at days 7, 9, 11, and 22.
ELISA.
Total serum IgE and IgG1 were quantified by sandwich ELISA with the following antibodies: purified anti-mouse IgE (R35-72, BD Biosciences) and alkaline phosphatase conjugated anti-mouse IgE (23G3, Southern Biotech); purified anti-mouse IgG1 (SB77e, Southern Biotech), and alkaline phosphatase conjugated anti-mouse IgG1 (X56, BD Biosciences). To measure serum OVA-specific IgE and IgG1 by ELISA, plates were coated with 20 μg/mL of chicken OVA (Sigma-Aldrich), followed by blocking and incubation with mouse serum dilutions. The same detection antibodies described earlier were used. In both assays, standard curves were generated with serial two- or fourfold dilutions of OVA-specific mouse IgE (Cayman Chemical) or OVA-specific mouse IgG1 (TOSG1C6, Biolegend). Phosphatase substrate tablets (Sigma-Aldrich) were used according to manufacture instructions.
Overexpression of mIgH in Pro-B Cells.
The cDNAs for mIgM, mIgG1, and mIgE were prepared from the CH12 B cell line (mIgM) or CH12-derived B cell lines induced to undergo IgH CSR to IgG1 and IgE and cloned into the pMIG vector (Addgene). BM cells from Ighε/ε, Ighγ1/γ1, and μMT mice were cultured in the presence of IL-7 (20 ng/mL). After 2–3 d, cultured BM cells were infected with GFP or mIgH-encoding retroviruses. After two additional days, culture medium was removed and cells were cultured in the presence of BAFF and IL-4 for 3 d. Kappa chain rearrangement was analyzed by flow cytometry. See SI Appendix for further details.
Ig VH Assembly Analysis.
Threefold serial dilutions of genomic DNA (≈100 ng, 30 ng, and 10 ng) were used to perform PCR to analyze Ig heavy chain VDJH and Ig light chain Vκ-Jκ rearrangements. Two main VH families were analyzed (7183 and J558) using primers described previously (57). Primers flanking exon 6 of the Dlg5 gene were used as a loading control (SI Appendix, Table S1). Vκ-Jκ rearrangement products were PCR amplified using a degenerate Vκ and the Mar35 primers described previously (58). Primers in the Igκ intron were used as a loading control (SI Appendix, Table S1). Vκ-Jκ1 rearrangement was also determined by quantitative PCR assay using the degenerate Vκ forward primer and a reverse primer complementary to sequences downstream of Jκ1 (Jκ1–2R) as described previously (59, 60). Rearrangement levels measured by qPCR were normalized to the levels of β-actin DNA.
Total RNA Isolation and Gene Expression Analysis.
Total RNA was extracted using the TRIzol method (Invitrogen), followed by treatment with RNase-free DNase (Qiagen) and RNeasy columns cleanup (Qiagen). Affymetrix Mouse 2.0 ST GeneChips microarray gene expression data were done with the Bioconductor package (R version 3.3.1, Bioconductor version 3.4). The raw data from the .CEL files were normalized and expression matrix was log transformed. Pearson’s correlation coefficient (r) and fold change in expression level of various genes under different conditions were calculated based on the biweight average of three biological replicates.
Isolation and Measurement of Membrane Secretory IgH mRNA.
Cell mRNA was isolated using Dynabeads mRNA DIRECT Micro Purification Kit (ambion/Life technologies), followed by DNA elimination with gDNA wipeout (Qiagen). cDNA was obtained using SuperScript III reverse transcription system (Invitrogen) using anchored Oligo(dT)20 primers (Invitrogen). When VH and IH promoter-driven transcripts were analyzed, the RT step was performed with anchored oligo dT coupled to an universal sequence (SI Appendix, Table S1), followed by a PCR step to amplify either VH or IH promoter-driven transcripts. For VH promoter transcripts, the VH segment leader sequence (VH leader Fw) was used as a forward primer. A mixture of forward primers against all four JH regions (JH1–4 Fw) were also used (SI Appendix, Table S1). For IH promoter transcript amplification, Primers complementary to Iμ, Iε, or Iγ1 (Iμ2 Fw, Iε Fw or Iγ1 Fw) were used as forward primers; the reverse primer (UNV Rv) was the universal sequence that was appended to the poly T primer on the RT step. Levels were normalized by total μ, ε or γ1 transcripts using primers located in the shared constant exon. The following primers set were used: mμ, sμ and tμ; mε, sε and tε; mγ1, sγ1 and tγ1 (SI Appendix, Table S1). Absolute quantification was determined by standard curves with linearized pGEM plasmids expressing mIgM, sIgM, mIgE, sIgE, mIgG1 or sIgG1 genes cloned from CH12 cells (for IgM), as well as CH12s isolated after in vitro CSR to IgE (CH12-IgE) and IgG1 (CH12-IgG1). See SI Appendix information for further details.
Deep Sequencing.
Pro-B and follicular B cell RNA was reverse-transcribed before heat inactivation of reverse transcriptase and primer removal using Uracyl DNA glycosylase. The glycosylase was then heat inactivated and the first cDNA strand was tagged with a unique molecular identifier (UMI) with one cycle of second strand DNA synthesis before removing residual primers with RNAClean XP beads. Nine cycles of preamplification were used to enrich the samples from Ighγ1/γ1VJκ5 and Ighε/εVJκ5 mice (due to low cell count) before first round PCR. Qiaquick PCR purification kit was used to purify the preamplification products. Subsequently, first round PCR added 5′ and 3′ adaptors to both ends of the amplicon and resulted in amplification of heavy chains using 25 cycles. For samples from IghWTVJκ5 mice, purified barcoding products go directly to first round PCR without preamplification step. Second round PCR added Illumina linkers (P5 and P7) and sample barcodes in separate reactions using 17 cycles. All of the primers used for the deep sequencing are listed in SI Appendix, Table S1. Products were quantified on a Qubit fluorimeter, run on a fragment analyzer with amplicon regions and expected sizes confirmed. Samples were then pooled in equal amounts according to product concentration. The pooled products were then size selected on an agarose gel and purified by gel extraction. Purified, size-selected products were run on an Agilent Bioanalyzer to confirm appropriate profile and determination of average size. The final pools were quantitated using Qubit and diluted to 5 nM before further quantification by qPCR on a BioRad CFX Connect Real-Time System and pooled evenly. The pool was denatured and spiked with 15% nonindexed PhiX control library provided by Illumina and loaded onto the MiSeq V2 Nano flowcell at a concentration of 7 pM for cluster formation and sequencing. The PhiX control library provides a balanced genome for calculation of matrix, phasing and prephasing, which are essential for accurate basecalling. The libraries were sequenced from both ends of the molecules to a total read length of 250 nt from each end.
Sequencing Data Analysis.
The sequences obtained from Illumina MiSeq deep sequencing (nano-run) were run through the standalone IgBlast software (version 1.4.0) to identify the VH segment using reference sequences from IMGT. PCR repeats were filtered using unique molecular identifies (UMIs) applied during cDNA synthesis. Sequences with the same VH and the same UMI were considered PCR repeats of the same mRNA. Only forward reads (R1) are used as the quality of R2 sequences was poor leading to low merge efficiency. Also, the CDR3 regions predicted were not reliable due to low quality intermittent nucleotides, which in-turn was required to rule out PCR repeats using the UMIs. By using only the UMIs and the VH, we risk loosing some unique sequences attached to the same UMI at the cost of excluding all PCR repeats. The frequency of use of each VH was calculated and compared for IghWTVJκ5, Ighε/εVJκ5, and Ighγ1/γ1VJκ5 mice. All preprocessing and analysis was done using Bioconductor package v 3.4 (R version 3.3.1).
Data Availability.
The raw sequence data for this study are accessible at the NCBI Sequence Read Archive (SRA) under BioProject accession number PRJNA394007 with BioSample accession numbers SAMN07347175–SAMN07347206.
Statistical Analysis.
The n values in figures and figure legends indicate the number of individual mice, representing biologic replicates. Statistical analysis is described in the figure legends. Studies were not conducted blinded.
Supplementary Material
Acknowledgments
We thank Christine Milcarek and Hans Oettgen for critical review of the manuscript and Soren Degn, Elisabeth Carroll, Rupa Kumari, Yuezhou Chen, Jiaxin Li, Colby Devereaux, John Manis, and Thomas Kepler for technical help and advice. This work is supported by the National Institutes of Health Grants AI121394 and AI1113217 (to D.R.W.), and National Council for Scientific and Technological Development (CNPq)/Science Without Borders Program, Brazil (to A.G.). D.R.W. holds a Career Award for Medical Scientists from the Burroughs Wellcome Fund and is supported by a New Investigator Award from Food Allergy Research & Education (FARE). This work was also supported by an anonymous gift.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The raw sequence data for this study are accessible at the NCBI Sequence Read Archive (SRA) under BioProject accession no. PRJNA394007 with BioSample accession nos. SAMN07347175–SAMN07347206.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1704962114/-/DCSupplemental.
References
- 1.Reth M. Antigen receptors on B lymphocytes. Annu Rev Immunol. 1992;10:97–121. doi: 10.1146/annurev.iy.10.040192.000525. [DOI] [PubMed] [Google Scholar]
- 2.Peterson ML. Immunoglobulin heavy chain gene regulation through polyadenylation and splicing competition. Wiley Interdiscip Rev RNA. 2011;2:92–105. doi: 10.1002/wrna.36. [DOI] [PubMed] [Google Scholar]
- 3.Jung D, Giallourakis C, Mostoslavsky R, Alt FW. Mechanism and control of V(D)J recombination at the immunoglobulin heavy chain locus. Annu Rev Immunol. 2006;24:541–570. doi: 10.1146/annurev.immunol.23.021704.115830. [DOI] [PubMed] [Google Scholar]
- 4.Tong P, Wesemann DR. Molecular mechanisms of IgE class switch recombination. Curr Top Microbiol Immunol. 2015;388:21–37. doi: 10.1007/978-3-319-13725-4_2. [DOI] [PubMed] [Google Scholar]
- 5.Nutt SL, Hodgkin PD, Tarlinton DM, Corcoran LM. The generation of antibody-secreting plasma cells. Nat Rev Immunol. 2015;15:160–171. doi: 10.1038/nri3795. [DOI] [PubMed] [Google Scholar]
- 6.Dogan I, et al. Multiple layers of B cell memory with different effector functions. Nat Immunol. 2009;10:1292–1299. doi: 10.1038/ni.1814. [DOI] [PubMed] [Google Scholar]
- 7.Pape KA, Taylor JJ, Maul RW, Gearhart PJ, Jenkins MK. Different B cell populations mediate early and late memory during an endogenous immune response. Science. 2011;331:1203–1207. doi: 10.1126/science.1201730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He JS, et al. The distinctive germinal center phase of IgE+ B lymphocytes limits their contribution to the classical memory response. J Exp Med. 2013;210:2755–2771. doi: 10.1084/jem.20131539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Z, Sullivan BM, Allen CD. Fluorescent in vivo detection reveals that IgE(+) B cells are restrained by an intrinsic cell fate predisposition. Immunity. 2012;36:857–872. doi: 10.1016/j.immuni.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 10.Gitlin AD, et al. Independent roles of switching and hypermutation in the development and persistence of B lymphocyte memory. Immunity. 2016;44:769–781. doi: 10.1016/j.immuni.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kometani K, et al. Repression of the transcription factor Bach2 contributes to predisposition of IgG1 memory B cells toward plasma cell differentiation. Immunity. 2013;39:136–147. doi: 10.1016/j.immuni.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 12.Erazo A, et al. Unique maturation program of the IgE response in vivo. Immunity. 2007;26:191–203. doi: 10.1016/j.immuni.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pogue SL, Goodnow CC. Gene dose-dependent maturation and receptor editing of B cells expressing immunoglobulin (Ig)G1 or IgM/IgG1 tail antigen receptors. J Exp Med. 2000;191:1031–1044. doi: 10.1084/jem.191.6.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roth PE, et al. Immunoglobulin gamma 2b transgenes inhibit heavy chain gene rearrangement, but cannot promote B cell development. J Exp Med. 1993;178:2007–2021. doi: 10.1084/jem.178.6.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin SW, Goodnow CC. Burst-enhancing role of the IgG membrane tail as a molecular determinant of memory. Nat Immunol. 2002;3:182–188. doi: 10.1038/ni752. [DOI] [PubMed] [Google Scholar]
- 16.Haniuda K, Fukao S, Kodama T, Hasegawa H, Kitamura D. Autonomous membrane IgE signaling prevents IgE-memory formation. Nat Immunol. 2016;17:1109–1117. doi: 10.1038/ni.3508. [DOI] [PubMed] [Google Scholar]
- 17.Laffleur B, et al. 2015. Self-restrained B cells arise following membrane IgE expression. Cell Rep S2211-1247(15)00048-0.
- 18.Poggianella M, Bestagno M, Burrone OR. The extracellular membrane-proximal domain of human membrane IgE controls apoptotic signaling of the B cell receptor in the mature B cell line A20. J Immunol. 2006;177:3597–3605. doi: 10.4049/jimmunol.177.6.3597. [DOI] [PubMed] [Google Scholar]
- 19.Dal Porto JM, et al. B cell antigen receptor signaling 101. Mol Immunol. 2004;41:599–613. doi: 10.1016/j.molimm.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Kitamura D, Rajewsky K. Targeted disruption of mu chain membrane exon causes loss of heavy-chain allelic exclusion. Nature. 1992;356:154–156. doi: 10.1038/356154a0. [DOI] [PubMed] [Google Scholar]
- 21.Lutz J, et al. Pro-B cells sense productive immunoglobulin heavy chain rearrangement irrespective of polypeptide production. Proc Natl Acad Sci USA. 2011;108:10644–10649. doi: 10.1073/pnas.1019224108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitamura D, Roes J, Kühn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 23.Tellier J, et al. Blimp-1 controls plasma cell function through the regulation of immunoglobulin secretion and the unfolded protein response. Nat Immunol. 2016;17:323–330. doi: 10.1038/ni.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kouskoff V, Lacaud G, Pape K, Retter M, Nemazee D. B cell receptor expression level determines the fate of developing B lymphocytes: Receptor editing versus selection. Proc Natl Acad Sci USA. 2000;97:7435–7439. doi: 10.1073/pnas.130182597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang LD, et al. Selection of B lymphocytes in the periphery is determined by the functional capacity of the B cell antigen receptor. Proc Natl Acad Sci USA. 2004;101:1027–1032. doi: 10.1073/pnas.0307040101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaudin E, et al. Positive selection of B cells expressing low densities of self-reactive BCRs. J Exp Med. 2004;199:843–853. doi: 10.1084/jem.20030955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karnowski A, Achatz-Straussberger G, Klockenbusch C, Achatz G, Lamers MC. Inefficient processing of mRNA for the membrane form of IgE is a genetic mechanism to limit recruitment of IgE-secreting cells. Eur J Immunol. 2006;36:1917–1925. doi: 10.1002/eji.200535495. [DOI] [PubMed] [Google Scholar]
- 28.Venkitaraman AR, Williams GT, Dariavach P, Neuberger MS. The B-cell antigen receptor of the five immunoglobulin classes. Nature. 1991;352:777–781. doi: 10.1038/352777a0. [DOI] [PubMed] [Google Scholar]
- 29.Rickert RC. New insights into pre-BCR and BCR signalling with relevance to B cell malignancies. Nat Rev Immunol. 2013;13:578–591. doi: 10.1038/nri3487. [DOI] [PubMed] [Google Scholar]
- 30.Huetz F, Carlsson L, Tornberg UC, Holmberg D. V-region directed selection in differentiating B lymphocytes. EMBO J. 1993;12:1819–1826. doi: 10.1002/j.1460-2075.1993.tb05830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bellon B, et al. High frequency of autoantibodies bearing cross-reactive idiotopes among hybridomas using VH7183 genes prepared from normal and autoimmune murine strains. J Clin Invest. 1987;79:1044–1053. doi: 10.1172/JCI112917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen X, Kearney JF. Generation and function of natural self-reactive B lymphocytes. Semin Immunol. 1996;8:19–27. doi: 10.1006/smim.1996.0004. [DOI] [PubMed] [Google Scholar]
- 33.Xin D, Hu L, Kong X. Alternative promoters influence alternative splicing at the genomic level. PLoS One. 2008;3:e2377. doi: 10.1371/journal.pone.0002377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ayoubi TA, Van De Ven WJ. Regulation of gene expression by alternative promoters. FASEB J. 1996;10:453–460. [PubMed] [Google Scholar]
- 35.Zhang T, et al. Downstream class switching leads to IgE antibody production by B lymphocytes lacking IgM switch regions. Proc Natl Acad Sci USA. 2010;107:3040–3045. doi: 10.1073/pnas.0915072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miyazaki T, Kato I, Takeshita S, Karasuyama H, Kudo A. Lambda5 is required for rearrangement of the Ig kappa light chain gene in pro-B cell lines. Int Immunol. 1999;11:1195–1202. doi: 10.1093/intimm/11.8.1195. [DOI] [PubMed] [Google Scholar]
- 37.Srinivasan L, et al. PI3 kinase signals BCR-dependent mature B cell survival. Cell. 2009;139:573–586. doi: 10.1016/j.cell.2009.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cushley W, Coupar BE, Mickelson CA, Williamson AR. A common mechanism for the synthesis of membrane and secreted immunoglobulin alpha, gamma and mu chains. Nature. 1982;298:77–79. doi: 10.1038/298077a0. [DOI] [PubMed] [Google Scholar]
- 39.Early P, et al. Two mRNAs can be produced from a single immunoglobulin mu gene by alternative RNA processing pathways. Cell. 1980;20:313–319. doi: 10.1016/0092-8674(80)90617-0. [DOI] [PubMed] [Google Scholar]
- 40.Alt FW, et al. Synthesis of secreted and membrane-bound immunoglobulin mu heavy chains is directed by mRNAs that differ at their 3′ ends. Cell. 1980;20:293–301. doi: 10.1016/0092-8674(80)90615-7. [DOI] [PubMed] [Google Scholar]
- 41.Smith CW, Patton JG, Nadal-Ginard B. Alternative splicing in the control of gene expression. Annu Rev Genet. 1989;23:527–577. doi: 10.1146/annurev.ge.23.120189.002523. [DOI] [PubMed] [Google Scholar]
- 42.Park KS, et al. Transcription elongation factor ELL2 drives Ig secretory-specific mRNA production and the unfolded protein response. J Immunol. 2014;193:4663–4674. doi: 10.4049/jimmunol.1401608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Geisberger R, Lamers M, Achatz G. The riddle of the dual expression of IgM and IgD. Immunology. 2006;118:429–437. doi: 10.1111/j.1365-2567.2006.02386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dougan SK, et al. IgG1+ ovalbumin-specific B-cell transnuclear mice show class switch recombination in rare allelically included B cells. Proc Natl Acad Sci USA. 2012;109:13739–13744. doi: 10.1073/pnas.1210273109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dougan SK, et al. Antigen-specific B-cell receptor sensitizes B cells to infection by influenza virus. Nature. 2013;503:406–409. doi: 10.1038/nature12637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wesemann DR, et al. Reprogramming IgH isotype-switched B cells to functional-grade induced pluripotent stem cells. Proc Natl Acad Sci USA. 2012;109:13745–13750. doi: 10.1073/pnas.1210286109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Waisman A, et al. IgG1 B cell receptor signaling is inhibited by CD22 and promotes the development of B cells whose survival is less dependent on Ig alpha/beta. J Exp Med. 2007;204:747–758. doi: 10.1084/jem.20062024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pelanda R, et al. Receptor editing in a transgenic mouse model: Site, efficiency, and role in B cell tolerance and antibody diversification. Immunity. 1997;7:765–775. doi: 10.1016/s1074-7613(00)80395-7. [DOI] [PubMed] [Google Scholar]
- 49.Lam KP, Rajewsky K. B cell antigen receptor specificity and surface density together determine B-1 versus B-2 cell development. J Exp Med. 1999;190:471–477. doi: 10.1084/jem.190.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kumar R, et al. Antibody repertoire diversification through VH gene replacement in mice cloned from an IgA plasma cell. Proc Natl Acad Sci USA. 2015;112:E450–E457. doi: 10.1073/pnas.1417988112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stadtfeld M, et al. Aberrant silencing of imprinted genes on chromosome 12qF1 in mouse induced pluripotent stem cells. Nature. 2010;465:175–181. doi: 10.1038/nature09017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shinkai Y, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 53.Muramatsu M, et al. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 54.Gallagher MP, Shrestha A, Magee JM, Wesemann DR. Detection of true IgE-expressing mouse B lineage cells. J Vis Exp. 2014 doi: 10.3791/52264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wesemann DR, et al. Immature B cells preferentially switch to IgE with increased direct Sμ to Sε recombination. J Exp Med. 2011;208:2733–2746. doi: 10.1084/jem.20111155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nakamura M, et al. High frequency class switching of an IgM+ B lymphoma clone CH12F3 to IgA+ cells. Int Immunol. 1996;8:193–201. doi: 10.1093/intimm/8.2.193. [DOI] [PubMed] [Google Scholar]
- 57.Guo C, et al. CTCF-binding elements mediate control of V(D)J recombination. Nature. 2011;477:424–430. doi: 10.1038/nature10495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schlissel MS, Baltimore D. Activation of immunoglobulin kappa gene rearrangement correlates with induction of germline kappa gene transcription. Cell. 1989;58:1001–1007. doi: 10.1016/0092-8674(89)90951-3. [DOI] [PubMed] [Google Scholar]
- 59.Inlay MA, Tian H, Lin T, Xu Y. Important roles for E protein binding sites within the immunoglobulin kappa chain intronic enhancer in activating Vkappa Jkappa rearrangement. J Exp Med. 2004;200:1205–1211. doi: 10.1084/jem.20041135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Inlay MA, Lin T, Gao HH, Xu Y. Critical roles of the immunoglobulin intronic enhancers in maintaining the sequential rearrangement of IgH and Igk loci. J Exp Med. 2006;203:1721–1732. doi: 10.1084/jem.20052310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw sequence data for this study are accessible at the NCBI Sequence Read Archive (SRA) under BioProject accession number PRJNA394007 with BioSample accession numbers SAMN07347175–SAMN07347206.