Significance
Knowledge of plant rooting depth is critical to understanding plant-mediated global change. Earth system models are highly sensitive to this particular parameter with large consequences for modeled plant productivity, water–energy–carbon exchange between the land and the atmosphere, and silicate weathering regulating multimillion-year-timescale carbon cycle. However, we know little about how deep roots go and why. Accidental discoveries of >70-m-deep roots in wells and >20-m-deep roots in caves offer glimpses of the enormous plasticity of root response to its environment, but the drivers and the global significance of such deep roots are not clear. Through observations and modeling, we demonstrate that soil hydrology is a globally prevalent force driving landscape to global patterns of plant rooting depth.
Keywords: plant rooting depth, soil hydrology, global change biology, infiltration depth, water table depth
Abstract
Plant rooting depth affects ecosystem resilience to environmental stress such as drought. Deep roots connect deep soil/groundwater to the atmosphere, thus influencing the hydrologic cycle and climate. Deep roots enhance bedrock weathering, thus regulating the long-term carbon cycle. However, we know little about how deep roots go and why. Here, we present a global synthesis of 2,200 root observations of >1,000 species along biotic (life form, genus) and abiotic (precipitation, soil, drainage) gradients. Results reveal strong sensitivities of rooting depth to local soil water profiles determined by precipitation infiltration depth from the top (reflecting climate and soil), and groundwater table depth from below (reflecting topography-driven land drainage). In well-drained uplands, rooting depth follows infiltration depth; in waterlogged lowlands, roots stay shallow, avoiding oxygen stress below the water table; in between, high productivity and drought can send roots many meters down to the groundwater capillary fringe. This framework explains the contrasting rooting depths observed under the same climate for the same species but at distinct topographic positions. We assess the global significance of these hydrologic mechanisms by estimating root water-uptake depths using an inverse model, based on observed productivity and atmosphere, at 30″ (∼1-km) global grids to capture the topography critical to soil hydrology. The resulting patterns of plant rooting depth bear a strong topographic and hydrologic signature at landscape to global scales. They underscore a fundamental plant–water feedback pathway that may be critical to understanding plant-mediated global change.
Plant rooting depth is a sensitive parameter in Earth system models for understanding past and predicting future global change (1–3) because it is a basic plant functional trait determining ecosystem resilience (4–6), plant biogeography (7, 8), pedogenesis (9, 10), and long-term carbon cycle (1, 10, 11). Unlike their aboveground counterparts, roots are difficult to observe, and basic knowledge such as their vertical extent remains limited, hindering mechanistic understanding and prediction of plant-mediated global change. Observation syntheses in the 1990s (12–14) revealed widely varying rooting depths (0.3–68 m; SI Appendix, Fig. S1) and broad associations with biome types; roots are shallow in boreal biomes on thinly thawed soils; roots of annual crops start from seeds each season reaching only shallow depths; and deep roots are found in arid, semiarid, and season-arid climates. In fact, a key finding from past syntheses is that both the shallowest and the deepest roots are found in dry biomes (3, 15). Because biomes are closely associated with climate, subclimate-scale factors likely explain the large within-biome spread. Candidates include species and age, but the same species/age displayed contrasting rooting depths in monoculture crops and plantations (16, 17), because roots are highly adaptive to local soil environments (18).
A frequently evoked subclimate-scale environmental factor is soil water status. Near the surface, coarse-grained soils with low water holding capacity allow deep infiltration encouraging deep roots (19–21); at the base, waterlogging and associated oxygen stress in topographic depressions inhibit deep roots (16, 17, 22, 23). Thus, variations in local soil water profile, driven by infiltration above and drainage below, are known causes for rooting depth variations (12). While climate and soil regulate infiltration, topography drives drainage; water flows toward topographic depressions creating shallow water tables in the latter. This is why the water table depth (WTD) reflects local topography as much as or more than regional climate (SI Appendix, Fig. S2); one finds deep and shallow water tables under most climates (24).
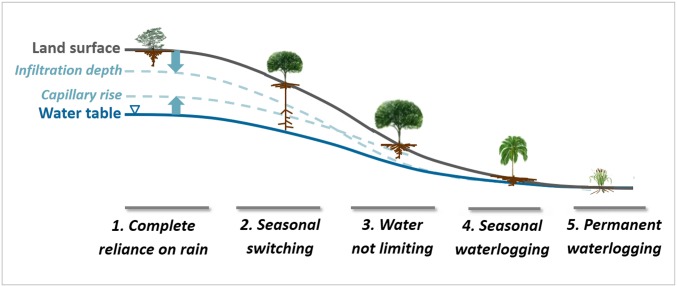
We hypothesize that along a topographic gradient, root–water relation shifts systematically (Fig. 1); on excessively drained uplands (position 1), the water table is deep or absent and rooting depth is limited to infiltration depth/frequency (25) (example in SI Appendix, Fig. S3B). At a lower position 2, roots may sense groundwater capillary rise; in climates with dry seasons, dimorphic roots (SI Appendix, Fig. S3C) are observed, with a shallow cluster using rain and a deep cluster using groundwater in dry seasons (26–28). At position 3, infiltration meets capillary rise and water is not limiting. At position 4, seasonal waterlogging limits roots to the oxygenated soils above the water table (SI Appendix, Fig. S3D); shallow or aerial roots are common in lowland forests (12, 29, 30). At position 5, permanent waterlogging selects wetland species insensitive to WTD.
Fig. 1.
Schematic of soil water profiles along a drainage gradient, wetted from above by rain infiltration and from below by groundwater capillary rise, with a dry gap that diminishes downslope. Along this gradient, plant rooting depths vary systematically (see text). SI Appendix, Fig. S3 gives examples of published root images at different drainage positions.
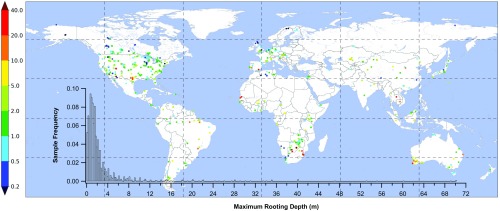
To test this hypothesis, we compiled rooting depth observations (SI Appendix and Dataset S1), recording local drainage conditions neglected in earlier syntheses. The 2,200 observations (Fig. 2) represent >1,000 species under a range of climate, soil, and drainage conditions. Rooting depth varied from <0.01 to >70 m with a distribution peak at 1 m. Because the deepest roots were discovered accidentally, they are likely undersampled.
Fig. 2.
Maximum observed rooting depth (in meters). Points overlap due to multiple samples in a small area. The deepest root is shown at sites with multiple samples. Inset gives frequency distribution (0.2-m bin width).
We plot (log) rooting depth (Fig. 3) vs. several potential drivers (SI Appendix, Table S1). A wide range of rooting depths are observed at any given annual rainfall (Fig. 3A) and soil texture (Fig. 3B). Many roots penetrated soil concretions (27, 28) or into bedrock fractures (31–34) (Fig. 3C, below 1:1 line). Across growth forms (Fig. 3D), shallow roots are found in small, annual herbs and deep roots in large, broadleaf trees. Among the 30 best-observed genera (Fig. 3E), shallower roots occur in wetland, desert, and boreal taxa and deeper roots in those of warm-dry regions. The wide spread within a growth form or genera suggests large plasticity in root response to local environments.
Fig. 3.
Rooting depth (log-scale) vs. (A) mean annual rainfall, (B) soil texture (SI Appendix, Table S1A), (C) depth of soil barriers, (D) growth form (SI Appendix, Table S1B), (E) genera (SI Appendix, Table S1C), and (F) WTD, giving Pearson correlation coefficient r (on original data) and sample size N out of 2,200 observations.
Drainage, quantified by WTD, emerged as a strong environmental factor (Fig. 3F). On one hand, it restricts roots to the oxygenated soils above the water table (above 1:1 line); points below are attributable to water table rise/fall outpacing root response, temporary perched saturation, or wetland plants insensitive to waterlogging. On the other hand, the presence of a water table can draw roots deeper to tap its capillary rise; roots terminating in the capillary fringe and root elongation following a declining water table are observed in laboratories and the field (27, 35). Points far above the 1:1 line are from upland plants disconnected from deep groundwater. These two functions of the water table, pushing roots shallower to avoid oxygen stress, and pulling them deeper to tap the capillary rise, result in a narrower range of rooting depth at a given WTD.
This groundwater push–pull is illustrated in an unintended experiment: the 46 mature trees of 37 species planted as windbreaks in eastern Nebraska (36). The sites have similar rainfall (∼735 mm/y) and soil (silt loam), but WTD varied (1–25 m) (SI Appendix, Fig. S4 A and B). The same species displayed contrasting rooting depths (SI Appendix, Fig. S4 C and D, and E and F) but all terminating near the water table. Plotting rooting depth vs. WTD (SI Appendix, Fig. S5) reveals three distinct root–WTD relations: roots independent of WTD, roots tapping groundwater (pulled deeper), and roots restricted by WTD (pushed shallower). Here, the climate and soil varied little, bringing out the WTD influence.
An important question is the relative control of genetic vs. environmental factors. Besides the Nebraska example, we plot the six most-observed genera (named in Fig. 3E) against mean annual rainfall (SI Appendix, Fig. S6, Top) and WTD (Bottom). The latter better explains the spread, as reported in many single-species studies [e.g., spruce (16) and maize (17)].
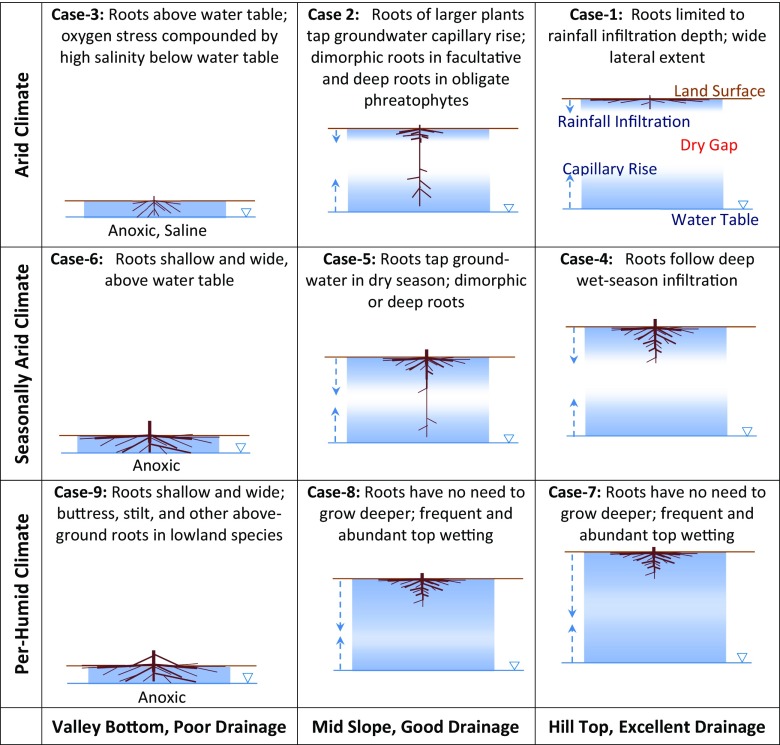
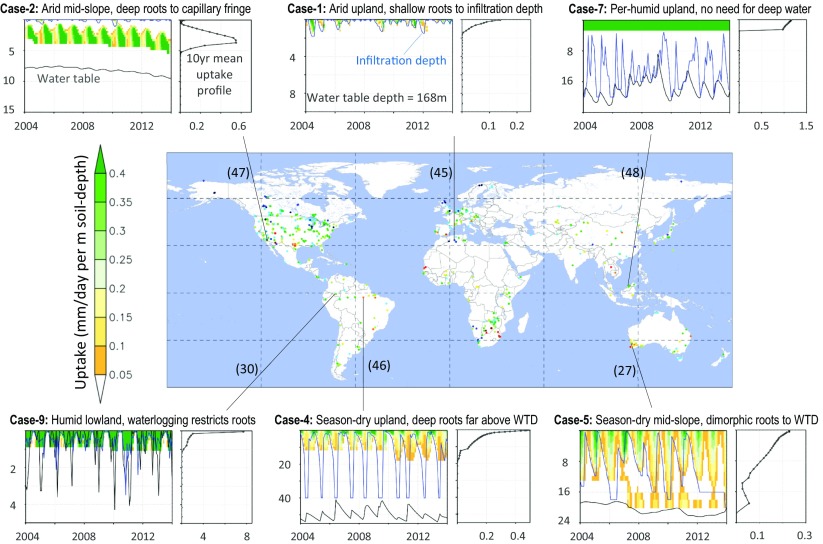
These results point to land drainage as a powerful environmental axis at the subclimate scale, a main distinction of this study from past syntheses and modeling that evoked climate and soil as primary abiotic drivers (3, 13, 20). We suggest that drainage gradient be considered along with climate and soil gradients to fully describe the soil water conditions for roots. Such an attempt is made in Fig. 4; from Top to Bottom is rainfall gradient driving regional-scale but episodic infiltration events (top wetting); from Left to Right is drainage gradient driving local-scale but more stable water-oxygen status (bottom wetting). Increasing rainfall (Top to Bottom) wets a deeper profile. Deteriorating drainage (Right to Left) increases groundwater access but also oxygen stress. At any point in the landscape, the soil water profile reflects both wetting mechanisms, with a dry gap that diminishes toward wetter climates or lower grounds. For example, in an arid climate on uplands (case 1), shallow infiltration leads to shallow rooting (25). Downslope (case 2), occasional deep infiltration (35) leads roots across the dry gap (25, 26). This explains why both the shallowest (case 1) and deepest (case 2) roots are found in dry climates; the polarizing root behavior reflects the polarizing soil hydrology with and without groundwater access (15, 37). In a season-dry climate on well-drained uplands (case 4), ample wet-season rain wets the soil deeply, enabling deeper roots necessitated by higher productivity in season-wet biomes, but groundwater is out of reach (4, 5). Down gradient (case 5), groundwater becomes accessible; deep roots are frequently observed in upland trees in season-dry climates (26–28). In a per-humid climate on well-drained slopes (cases 7 and 8), the ample rain wets the soils completely, but with frequent surface wetting roots do not need deep water. Across all climates, waterlogging in lowlands (cases 3, 6, and 9) plus high salinity in arid climates restrict roots; observations reveal ubiquitously shallow and wide roots, aerial roots, or short ephemeral roots tracking seasonal water table rise/fall (12, 16, 22, 23, 30).
Fig. 4.
A hydrologic framework for interpreting plant rooting depth along the climate gradient (vertical axis) defining regional patterns in infiltration depth and frequency, and land drainage gradient (horizontal axis) defining local patterns in groundwater accessibility and oxygen stress. See text for discussions of the cases.
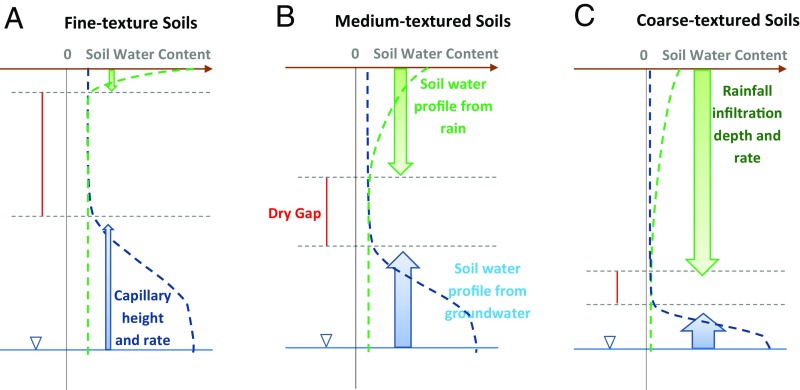
Within this framework, the role of soil texture can be understood by how it regulates the downward infiltration and the upward capillary rise (Fig. 5). From the top, infiltration is stronger–deeper in coarse, and weaker/shallower in fine, textured soils, leading to systematic shifts in rooting depth (19–21, 38). However, the presence of a water table can confound such a simple relationship; a shallow water table in a coarse soil can push roots shallower, and a deep water table in a finer soil can pull roots deeper, contributing to the poor correlation between rooting depth and soil texture (Fig. 3B).
Fig. 5.
Schematic of how soil texture regulates the two soil water fluxes and profiles: from the top, precipitation infiltration flux (green arrows) and the resulting soil water profile (green dashed line), and from below, the groundwater capillary rise (blue arrows) and resulting soil water profile (blue dashed line), in (A) fine-textured, for example, clay, (B) medium-textured, for example, silt, and (C) coarse-textured, for example, sandy, soils. The width of the arrow indicates flux rate, and the length indicates flux reach, with equal precipitation and WTD.
This framework accounts for the influence of regional-scale, fast-changing atmosphere from the top, and landscape-scale, slower-changing topography from below, in jointly shaping soil water–oxygen profiles that roots sense and exploit. The following question arises: what is the global significance of such landscape-scale root–water relations?
We use inverse modeling to estimate the necessary depths of root–water uptake (SI Appendix). First, we calculate the soil water supply profile driven by the observed atmosphere, soil texture, and topography using a hydrology model that tracks the store and fluxes in the soil water, groundwater, rivers, and wetlands (39, 40). This gives the soil water profile as shown in Figs. 4 and 5 at each grid cell at each time step. To capture the local drainage emphasized here with computational feasibility, we use 30-arcsecond global grids (<1-km) at hourly intervals over eleven years (2003–2013). Second, we calculate the ecosystem water demand from satellite-observed leaf area index and observed-reanalysis atmosphere, based on the Shuttleworth and Wallace (41) formula of the Penman–Monteith equation separating plant transpiration from soil evaporation. Third, given plant water demand and the soil water supply profile, we estimate the necessary depths of water uptake to meet the demand, based on Ohm’s law for parallel-connected conductors (details in SI Appendix). We assume that the many individual plants within a model grid cell behave collectively to withdraw soil water to meet the transpiration demand of grid-level productivity observed from space.
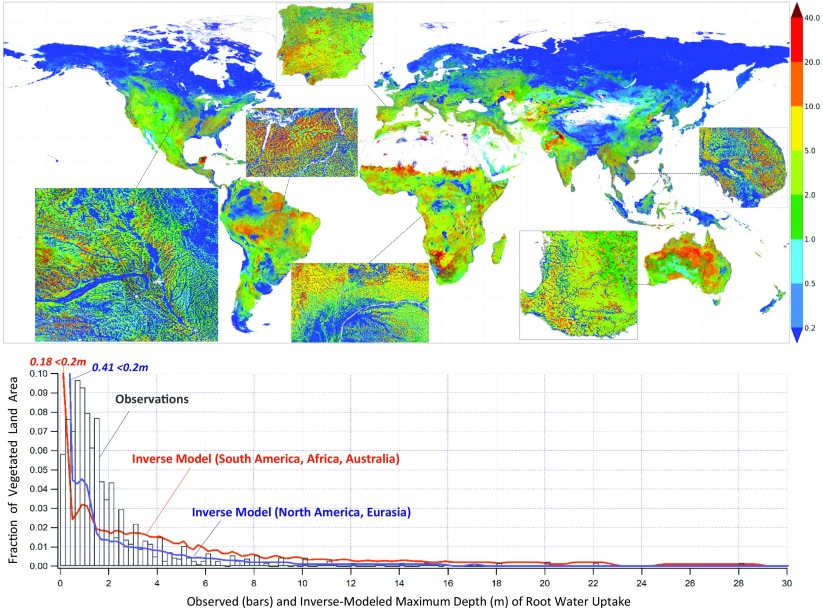
Fig. 6 gives the model maximum depth of root uptake averaged over ten years (2004–2013). Continent maps are in SI Appendix, Figs. S11–S15. At the global scale, climate and major biomes are visible (e.g., shallow in boreal, deep in seasonal forests), consistent with earlier syntheses and models (3, 13, 14, 20), but under a given climate or within a biome, the topographic structure emerges as a powerful force (Fig. 6, Insets and SI Appendix, Figs. S11–S15) as it is the primary driver of land drainage. Topography is visible in both the infiltration depth (SI Appendix, Fig. S16) and the WTD (SI Appendix, Fig. S17) because deep infiltration terminates at the WTD, the latter strongly reflecting the topography. Fig. 7 gives the monthly time series and the 10-y mean uptake profile at six sites with observations, representing six cases in Fig. 4. The inverse model gives the same behavior as observed in the given environment; for example, shallow roots occur in both arid uplands (case 1) and humid lowlands (case 9), deeper roots are initiated in drought years (cases 4, 5, and 7), and dimorphic roots develop in seasonal climates (case 5).
Fig. 6.
Inverse-model results of 10-y mean maximum depth (in meters) of root water uptake (Upper). Insets reveal strong local topographic influence. The frequency distribution (Lower, 0.2-m bin width), over vegetated surface only, suggests large model–observation discrepancy, which may imply observation bias (undersampling of very shallow and very deep roots). The oscillations in the model distribution are due to soil water uptake crossing discrete soil layers.
Fig. 7.
Modeled uptake profiles at six grid cells with rooting depth observations (27, 30, 45–48), corresponding to six cases in the conceptual model of Fig. 4. The colored panels plot monthly root water uptake (in millimeters per day per meter soil depth) over the 10 y of simulation, at different soil depth (in meters), with monthly mean infiltration depth (blue line) WTD (gray line). The 10-y mean uptake profile is shown to the Right. The map at Center is a reduced version of Fig. 2. The model exhibits the same behavior as observations under different climate/drainage combinations. In the desert, observations are of single plants not detectable by satellites, so model results of a nearby grid are shown, which have higher leaf area index and thus deeper uptake than observed.
The distribution of maximum uptake depth from the inverse model (Fig. 6) suggests a much higher occurrence of very shallow (especially northern continents) and very deep (especially southern continents) uptake than observed (bars). It may be that current field sampling of roots is biased toward moderate climates, and that it may have stopped too soon in seasonal and arid climates (6, 42, 43), missing a vital part of the plant that may be small in biomass but essential for survival and functioning in the absence of rain (4, 5).
The insights gained here are highly relevant to Earth System Modeling, an essential tool for understanding past and predicting future global change that regulates and is regulated by land plants. Current models prescribe rooting depth according to plant functional types, and neglect landscape-scale topography and the resulting hydrologic convergence from high to low grounds, a fundamental hydrologic process that creates a strongly articulated, fine-scaled spatial structure in water availability regardless of the climate (24, 44). Here, we demonstrate that this hydrologic structure is a potent force in shaping plant rooting depths, a biological response that may further differentiate plant–water relations from uplands to lowlands. The adaptive ability of plant roots to reach deep soil and groundwater has important consequences for the hydrologic cycle and regional climate through land–atmosphere interactions, and it may have expanded the hydrologic habitats for land plants by allowing them to survive water-stressed periods. Therefore, accounting for landscape-scale topography, the resulting hydrology, and the consequent plant root–water interactions in Earth System Models may enable critical mechanistic pathways in these models for exploring and predicting global change that are strongly mediated by land plants.
Supplementary Material
Acknowledgments
We thank those who conducted painstaking root investigations in the field and the Rutgers University Libraries who brought these observations to our desktop, making the synthesis possible. We thank Gabriel Tucker for the Japanese monograph on roots and Dai Yamazaki for providing global high-resolution terrain analyses. Funding comes from US Environmental Protection Agency (STAR-RD834190) and National Science Foundation (AGS-1045110, EAR-1528298) (to Y.F.), the European Commission Seventh Framework Programme (EartH2Observe 603608) (to G.M.-M.), and US Department of Agriculture National Institute of Food and Agriculture (2012-68002-19795) (to R.B.J.). Computation is at the Centro de Supercomputación de Galicia (CESGA) at Universidade de Santiago de Compostela, Galicia, Spain.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The model simulation results reported in this paper have been deposited in European Commission EartH2Observe (https://wci.earth2observe.eu/thredds/catalog/usc/root-depth/catalog.html).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1712381114/-/DCSupplemental.
References
- 1.Berner RA. The rise of trees and how they changed paleozoic atmospheric CO2, climate, and geology. In: Ehleringer JR, Cerling T, Dearing MD, editors. A History of Atmospheric CO2 and Its Effects on Plants, Animals, and Ecosystems. Springer; New York: 2005. [Google Scholar]
- 2.Feddes R, et al. Modeling root water uptake in hydrological and climate models. Bull Am Meteorol Soc. 2001;82:2797–2809. [Google Scholar]
- 3.Kleidon A, Heimann M. A method of determining rooting depth from a terrestrial biosphere model and its impacts on the global water and carbon cycle. Glob Change Biol. 1998;4:275–286. [Google Scholar]
- 4.Nepstad DC, et al. The role of deep roots in the hydrological and carbon cycles of Amazonian forests and pastures. Nature. 1994;372:666–669. [Google Scholar]
- 5.Oliveira RS, et al. Deep root function in soil water dynamics in cerrado savannas of central Brazil. Funct Ecol. 2005;19:574–581. [Google Scholar]
- 6.Maeght J-L, Rewald B, Pierret A. How to study deep roots—and why it matters. Front Plant Sci. 2013;4:299. doi: 10.3389/fpls.2013.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weaver JE. The Ecological Relations of Roots. Digital Commons @ University of Nebraska–Lincoln; Lincoln, NE: 1919. pp. 122–127. [Google Scholar]
- 8.Bowman DMJS, Prior DL. Why do evergreen trees dominate the Australian seasonal tropics? Aust J Bot. 2016;53:379–399. [Google Scholar]
- 9.Reubens B, Poesen J, Danjon F, Geudens G, Muys B. The role of fine and coarse roots in shallow slope stability and soil erosion control with a focus on root system architecture: A review. Trees (Berl) 2007;21:385–402. [Google Scholar]
- 10.Algeo TJ, Scheckler SE. Terrestrial-marine teleconnections in the Devonian: Links between the evolution of land plants, weathering processes, and marine anoxic events. Philos Trans R Soc Lond B Biol Sci. 1998;353:113–130. [Google Scholar]
- 11.Beerling DJ, Berner RA. Feedbacks and the coevolution of plants and atmospheric CO2. Proc Natl Acad Sci USA. 2005;102:1302–1305. doi: 10.1073/pnas.0408724102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stone EL, Kalisz PJ. On the maximum extent of tree roots. For Ecol Manage. 1991;46:59–102. [Google Scholar]
- 13.Jackson RB, et al. A global analysis of root distributions for terrestrial biomes. Oecologia. 1996;108:389–411. doi: 10.1007/BF00333714. [DOI] [PubMed] [Google Scholar]
- 14.Canadell J, et al. Maximum rooting depth of vegetation types at the global scale. Oecologia. 1996;108:583–595. doi: 10.1007/BF00329030. [DOI] [PubMed] [Google Scholar]
- 15.Cannon WA. Some relations between root characters, ground water and species distribution. Science. 1913;37:420–423. doi: 10.1126/science.37.950.420. [DOI] [PubMed] [Google Scholar]
- 16.Wagg JWB. 1967. Origin and Development of White Spruce Root-Forms (Queen’s Printer and Controller of Stationery, Ottawa, Ontario, Canada), Forestry Branch Departmental Publication No. 1192.
- 17.Follett R, Allmaras R, Reichman G. Distribution of corn roots in sandy soil with a declining water table. Agron J. 1974;66:288–292. [Google Scholar]
- 18.Hodge A. The plastic plant: Root responses to heterogeneous supplies of nutrients. New Phytol. 2004;162:9–24. [Google Scholar]
- 19.Sperry J, Hacke U. Desert shrub water relations with respect to soil characteristics and plant functional type. Funct Ecol. 2002;16:367–378. [Google Scholar]
- 20.Schenk HJ, Jackson RB. Mapping the global distribution of deep roots in relation to climate and soil characteristics. Geoderma. 2005;126:129–140. [Google Scholar]
- 21.Xu GQ, Li Y. Rooting depth and leaf hydraulic conductance in the xeric tree Haloxyolon ammodendron growing at sites of contrasting soil texture. Funct Plant Biol. 2008;35:1234–1242. doi: 10.1071/FP08175. [DOI] [PubMed] [Google Scholar]
- 22.Martin MH. Conditions affecting the distribution of Mercurialis perennis L. in certain Cambridgeshire woodlands. J Ecol. 1968;56:777–793. [Google Scholar]
- 23.Armstrong W, Booth TC, Priestley P, Read DJ. The relationship between soil aeration, stability and growth of Sitka spruce (Picea sitchensis (Bong.) Carr.) on upland peaty gleys. J Appl Ecol. 1976;13:585–591. [Google Scholar]
- 24.Fan Y, Li H, Miguez-Macho G. Global patterns of groundwater table depth. Science. 2013;339:940–943. doi: 10.1126/science.1229881. [DOI] [PubMed] [Google Scholar]
- 25.Cannon WA. The Root Habits of Desert Plants. Carnegie Institution of Washington; Washington, DC: 1911. [Google Scholar]
- 26.Howard A. The effects of grass on trees. Proc R Soc Lond B. 1925;97:284–321. [Google Scholar]
- 27.Kimber PC. 1974. The Root System of Jarrah (Eucalyptus marginata) (Forests Department of Western Australia, Perth, WA, Australia), Research Papers No. 10.
- 28.Dawson TE, Pate JS. Seasonal water uptake and movement in root systems of Australian phraeatophytic plants of dimorphic root morphology: A stable isotope investigation. Oecologia. 1996;107:13–20. doi: 10.1007/BF00582230. [DOI] [PubMed] [Google Scholar]
- 29.Smith AP. Butteressing of tropical trees: A descriptive model and new hypotheses. Am Nat. 1972;106:32–46. [Google Scholar]
- 30.Pavlis J, Jenik J. Roots of pioneer trees in Amazonian rain forest. Trees (Berl) 2000;14:442–455. [Google Scholar]
- 31.Hellmers H, Horton JS, Juhren G, O’Keefe J. Root systems of some chaparral plants in southern California. Ecology. 1955;36:667–678. [Google Scholar]
- 32.Estrada-Medina H, Graham RC, Allen MF, Jimenez-Osornio JJ, Robles-Casolco S. The importance of limestone bedrock and dissolution karst features on tree root distribution in northern Yucatan, Mexico. Plant Soil. 2013;362:37–50. [Google Scholar]
- 33.Jackson RB, Moore LA, Hoffmann WA, Pockman WT, Linder CR. Ecosystem rooting depth determined with caves and DNA. Proc Natl Acad Sci USA. 1999;96:11387–11392. doi: 10.1073/pnas.96.20.11387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salve R, Rempe DM, Dietrich WE. Rain, rock moisture dynamics, and the rapid response of perched groundwater in weathered, fractured argillite underlying a steep hillslope. Water Resour Res. 2012;48:1–25. [Google Scholar]
- 35.Naumburg E, Mata-Gonzalez R, Hunter RG, McLendon T, Martin DW. Phreatophytic vegetation and groundwater fluctuations: A review of current research and application of ecosystem response modeling with an emphasis on great basin vegetation. Environ Manage. 2005;35:726–740. doi: 10.1007/s00267-004-0194-7. [DOI] [PubMed] [Google Scholar]
- 36.Sprackling JA, Read RA. 1979. Tree Root Systems in Eastern Nebraska (The Conservation and Survey Division, Institute of Agriculture and Natural Resources, University of Nebraska–Lincoln, Lincoln, NE), Nebraska Conservation Bulletin 37.
- 37.Meinzer OE. 1927. Plants as Indicators of Ground Water (US Geological Survey, Washington, DC), USGS Water-Supply Paper, Vol 577.
- 38.Zipper SC, Evren Soylu M, Booth EG, Loheide SP. Untangling the effect of shallow grounwater and soil texture as drivers of subfield-scale yield variability. Water Resour Res. 2015;51:6338–6358. [Google Scholar]
- 39.Miguez-Macho G, Fan Y. The role of groundwater in the Amazon water cycle: 1. Influence on seasonal streamflow, flooding and wetlands. J Geophys Res. 2012;117:D15113. [Google Scholar]
- 40.Miguez-Macho G, Fan Y. The role of groundwater in the Amazon water cycle: 2. Influence on seasonal soil moisture and evapotranspiration. J Geophys Res Atmos. 2012;117:D15114. [Google Scholar]
- 41.Shuttleworth WJ, Wallace JS. Evaporation from sparse crops–An energy combination theory. Q J R Meteorol Soc. 1985;111:839–855. [Google Scholar]
- 42.Richter Dd, Billings SA. “One physical system”: Tansley’s ecosystem as Earth’s critical zone. New Phytol. 2015;206:900–912. doi: 10.1111/nph.13338. [DOI] [PubMed] [Google Scholar]
- 43.Harper RJ, Tibbett M. The hidden organic carbon in deep mineral soils. Plant Soil. 2013;368:641–648. [Google Scholar]
- 44.Fan Y. Groundwater in the Earth’s critical zone: Relevance to large-scale patterns and processes. Water Resour Res. 2015;51:3052–3069. [Google Scholar]
- 45.Cannon WA. Botanical Features of the Algerian Sahara. Carnegie Institution of Washington; Washington, DC: 1913. [Google Scholar]
- 46.Davidson E, et al. Carbon inputs and water uptake in deep soils of an eastern Amazon forest. For Sci. 2011;57:51–58. [Google Scholar]
- 47.Nilsen ET, Sharifi MR, Rundel PW, Jarrell WM, Ross A. Diurnal and seasonal water relations of the desert phreatophyte Prosopis glandulosa (honey mesquite) in the Sonoran Desert of California. Ecology. 1983;64:1381–1393. [Google Scholar]
- 48.Kenzo T, et al. Development of allometric relationships for accurate estimation of above- and below-ground biomass in tropical secondary forests in Sarawak, Malaysia. J Trop Ecol. 2009;25:371–386. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.