Abstract
Background
The objective of the present study was to investigate whether the analysis of magnesium (Mg), high-sensitivity C-reactive protein (hsCRP), and ischemia-modified albumin (IMA) concentrations can be used as a non-invasive and convenient method for diagnosing obstructive sleep apnea syndrome (OSAS).
Material/Methods
After polysomnography, venous blood was collected from 33 patients with OSAS and 30 control individuals. Serum levels of Mg, hsCRP, and IMA were investigated. The relationship between these factors and apnea–hypopnea index (AHI) was analyzed using the Pearson correlation coefficient. The role of the factors was determined using a receiver operating characteristic (ROC) curve and multivariate logistic regression analysis.
Results
The levels of hsCRP and IMA were significantly higher in patients with OSAS than in control subjects, while the levels of Mg were lower (P<0.05 for all). A significant correlation was noted between serum IMA (r=0.614; P<0.001) and hsCRP (r=0.453; P<0.001) levels and the AHI. The ROC showed that serum Mg (AUC=0.74(0.62–0.85)), hsCRP (AUC=0.77(0.65–0.87)), and IMA (AUC=0.78(0.66–0.87)) levels could be used as markers to diagnose OSAS. Moreover, our new model, MIh, which is obtained by multivariate analysis, yielded an AUC value of 0.93 (0.83–0.98). Continuous positive airway pressure (CPAP) treatment reversed the changes in the serum levels of Mg, hsCRP, and IMA.
Conclusions
Patients with OSAS show reduced serum Mg levels and elevated serum hsCRP and IMA levels. These observed alterations can be reversed by CPAP treatment. A novel model, named MIh, may be a promising tool for OSAS diagnosis.
MeSH Keywords: C-Reactive Protein; Diagnosis; Magnesium Deficiency; Sleep Apnea, Obstructive
Background
Obstructive sleep apnea syndrome (OSAS) commonly develops secondary to recurrent obstruction of the upper respiratory tract during sleep. The disease is characterized by recurrent obstructions of the upper airway associated with snoring, disrupted sleep, and intermittent hypoxia [1,2]. Epidemiological studies have shown that the prevalence of OSAS in the population has increased over time [3]. However, the current criterion standard for diagnosis of OSAS is polysomnography (PSG), which is expensive, technically demanding, and time-consuming [4]. Thus, clinicians require alternative, non-invasive techniques for OSAS diagnosis.
Generally, repeated oxygen desaturation and re-saturation lead to increased oxidative stress, and previous studies have reported that OSAS is associated with increased levels of inflammatory mediators and oxidative stress [5–7]. More specifically, recent studies have suggested that the levels of systemic inflammatory markers, such as the known cardiovascular risk marker C-reactive protein (CRP), are increased in patients with OSAS, and that these markers are significantly reduced after treatment [8,9]. Ischemia-modified albumin (IMA) is a promising biomarker whose levels have been shown to be elevated in various diseases associated with ischemia and oxidative stress, such as myocardial infarction and pulmonary embolism [10,11]. Interestingly, IMA may serve as a biomarker for OSAS severity in cases that are associated with obesity [12].
On a related note, magnesium (Mg) acts as an antioxidant and co-factor for several enzymes involved in cell membrane stabilization, and it mitigates the effects of oxidative stress [13]. Hypomagnesemia contributes to a reduction in the expression and activity of antioxidant enzymes [14]. Lower serum Mg concentrations were recently reported bee inversely correlated with elevated CRP levels in patients with obstructive sleep apnea [15].
The present study was designed to investigate a novel, non-invasive biomarker to diagnose OSAS, as technological advances have allowed multiple inflammatory cytokines to be tested rapidly and sensitively using only small amounts of serum. We found that serum high-sensitivity (hsCRP) and IMA concentrations were significantly higher in the OSAS group than in the control group. Serum Mg levels showed the opposite trend and were found to decrease. The findings demonstrate that OSAS is strongly associated with elevated concentrations of hsCRP and IMA and decreased Mg levels, which can be reversed by effective CPAP treatment. Subsequently, we designed a new scoring model and named it the “MIh score” (MIh score=−8.68–2.03*Mg+19.28*IMA+1.05*hsCRP) based on the above 3 biomarkers; we investigated this model as a potential biomarker for diagnosing OSAS using receiver operating characteristic (ROC) curve analyses. Our data suggest that the MIh model is a promising biomarker for diagnosing OSAS.
Material and Methods
Study design and population
The procedures followed in this study are depicted in Figure 1. Patients admitted to the Sleep Center of the Hangzhou Fuyang First People’s Hospital between 2014 and 2015 were evaluated. The study population comprised 102 patients suspected of having obstructive sleep apnea syndrome; they were matched for age, sex, body mass index (BMI), and smoking habits. Patients were excluded if they: (1) had a history of ventilatory failure or were currently being treated with continuous positive airway pressure (CPAP), (2) were aged ≤20 years or ≥60 years, (3) had an acute upper-respiratory tract infection, (4) had an unstable coronary artery or other severe disease, or (5) did not complete the questionnaire and informed consent form. Patients were then assigned to the control group (apnea-hypopnea index [AHI] <5) or the OSAS group (AHI ≥5 with associated symptoms, or AHI ≥15). The study was approved by the Research Ethics Committee of the Hangzhou Fuyang First People’s Hospital, and informed consent was obtained from all patients.
Figure 1.
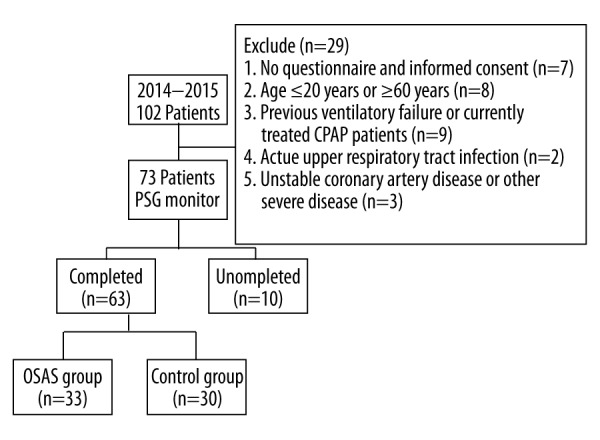
Flow diagram of the phases of the study.
Overnight polysomnography
All subjects underwent overnight PSG monitoring (Embletta X100; Natus Medical Inc., Pleasanton, CA, USA) at the Sleep Center; the PSG was carried out by technicians using Embla Sleepware Software, which comprises an electroencephalogram, an electrooculogram, a genioglossus electromyogram, oral and nasal flow monitoring using a thermistor, an electrocardiogram, recording of thoracoabdominal respiratory movements (with a tightness measure), and measurement of oxygen saturation. A minimum of 7 h of PSG data were recorded. Respiratory incidents were scored using standard criteria by a physician blinded to both the purpose of the research and the subjects’ identity. After PSG recording, the following parameters were scored manually: changes in heart rate and rhythm, sleep stage, changes in breathing patterns (hypopnea, arousal, and apnea), and periodic leg movements during sleep. Apnea was defined as airflow cessation for at least 10 s, and hypopnea was defined as an airflow reduction of ≥30% for at least 10 s accompanied by either an oxygen desaturation of >3% or an arousal. The AHI comprised the apnea score plus the hypopnea score per hour of sleep. The apnea severity indices used were AHI and mean arterial oxygen saturation.
Blood tests
All subjects provided peripheral whole venous blood samples (≥7 mL), which were drawn into test tubes containing heparin between 7: 00 and 7: 30 AM on the morning after finishing overnight PSG monitoring. The sera were separated by centrifugation at 3000 rpm for 10 min and immediately stored at −80°C.
Serum levels of Mg were determined using a Cobas Integra 800 analyzer (Roche Diagnostics, Mannheim, Germany) and the colorimetric method, which uses the original Cobas reagents. The results are expressed in milligrams per deciliter. Serum levels of hsCRP were measured using an IMMULITE 2000 system (Diagnostic Products Corporation, Los Angeles, CA, USA); serum levels of IMA were measured using the albumin cobalt binding assay, as described by Bar-Or et al. [16]. The results are expressed in absorbance units (ABSU).
Continuous positive airway pressure treatment
The CPAP titration was performed manually by a technician using 2 devices (Respironics, PA, USA and Weinmann, Hamburg, Germany) under complete PSG monitoring. The CPAP titration was started at an initial pressure of 4 cm H2O under overnight PSG; the pressure was then increased incrementally until apnea-hypopnea events had disappeared. Each titration study lasted at least 6 h. All participants demonstrated adequate sleep efficiency (70%). After 3 months of treatment using nasal CPAP, these patients underwent clinical reassessment and biochemical analysis.
Statistical analysis
The data were analyzed using SPSS version 20.0 (SPSS Inc., Chicago, IL, USA). Continuous variables are presented as medians ± standard deviation. The 2 groups were compared using the Student’s t-test or non-parametric ANOVA (Mann-Whitney test). The association between serum marker concentrations and PSG study results was assessed using Pearson’s correlation test. To evaluate the diagnostic accuracy of the potential OSAS biomarkers (Mg, hsCRP, and IMA), ROC curves were created, and the area under the curve (AUC) was used. Multivariate analysis of these markers was performed by constructing a logistic regression model, as well as a new scoring model named the MIh score (MIh score=−8.68–2.03*Mg+19.28*IMA+1.05*hsCRP). The 95% confidence intervals (CIs) were calculated, and a P-value of <0.05 was considered statistically significant.
Results
Clinical characteristics of study subjects
The final 63 participants enrolled in this study comprised 44 men and 19 women with a mean age of 52.1±10.9 years. The study group consisted of 33 participants (23 men and 10 women) aged 51.6±9.8 years. The AHI of the OSAS group was 19.6±4.7 (range: 5.4–78.1), which was significantly higher than that of the control group (2.2±1.5), while the mean arterial oxygen saturation of the OSAS group was 90.9±4.0% (range: 6.5–130.0%), which was lower than that of the control group (P<0.05 in all cases). There were no significant differences between the patients with OSAS and the controls in terms of age, sex, BMI, smoking status, hypertension, or diabetes mellitus status. Table 1 shows the demographic characteristics and parameters under investigation in both groups.
Table 1.
Demographic, clinical characteristics and polysomnographic evaluation of the study groups.
| Clinical characteristics | Control group (n=30) | OSAS group (n=33) | P value |
|---|---|---|---|
| Age (years) | 49.2±13.1 | 51.6±9.8 | 0.570 |
| Male/Female gender | 21/9 | 23/10 | 0.312 |
| BMI (kg/m2) | 28.9±4.4 | 30.1±3.5 | 0.099 |
| Smoker/nonsmoker | 14/16 | 19/14 | 0.137 |
| Comorbidities | |||
| Hypertension | 6 (20%) | 8 (24%) | 0.537 |
| Diabetes mellitus | 3 (10%) | 5 (17%) | 0.141 |
| Hyperlipidemia | 6 (20%) | 6 (18% | 0.834 |
| Polysomnographic evaluation | |||
| Total sleep time (TST), h | 6.8±1.2 | 6.2±1.3 | 0.681 |
| Sleep efficiency (%) | 83.7±10.1 | 82.8±10.5 | 0.730 |
| Stages 3,% of TST | 20.4±6.4 | 12.3±7.4 | 0.032* |
| REM,% of TST | 22.7±4.1 | 16.0±5.2 | 0.014* |
| Mean SaO2 (%) | 93.9±2.8 | 90.2±4.1 | <0.001* |
| AHI (events/h of sleep) | 2.2±1.5 | 19.6±4.7 | <0.001* |
Data are numbers or medians ±SD. P<0.05, Mann-Whitney U-test. BMI – body mass index; REM – rapid eye movement; Mean SaO2 – mean of arterial oxygen saturation; AHI – Apnea-Hypopnea Index.
The impact of OSAS on serum Mg, hsCRP, and IMA concentrations
As illustrated in Table 2, serum Mg levels were significantly lower in the OSAS group than in the control group (1.71±0.21 mg/dL vs. 2.19±0.36 mg/dL; P=0.021). Moreover, in the OSAS group, 23 patients (69.7%) demonstrated hypomagnesemia, and patients with OSAS showed higher hsCRP concentrations than those without (1.47±1.60 mg/L vs. 0.97±1.22 mg/L; P<0.05). In addition, serum IMA occurred at higher levels in the OSAS group than in the control group (P<0.05).
Table 2.
The Impact of OSAS on serum Mg, hsCRP and IMA concentrations.
| Marker | Control group (n=30) | OSAS group (n=33) | P value |
|---|---|---|---|
| Mg (mg/dL) | 2.19±0.36 | 1.71±0.21 | 0.021 |
| hsCRP (mg/L) | 0.97±1.22 | 1.47±1.60 | <0.001 |
| IMA (ABSU) | 0.43±0.09 | 0.58±0.11 | <0.001 |
hsCRP – high-sensitivity C-reactive protein; IMA – ischemia-modified albumin.
Next, we performed Pearson’s correlation test to evaluate the association between these biomarkers and the PSG study results. Table 3 documents the Pearson correlation coefficients (r-values); the results revealed a significant correlation between serum IMA concentrations and the AHI (r=0.61; P<0.001) and mean SaO2 (r=−0.560; P<0.001). Serum hsCRP concentrations were significantly correlated with both AHI (r=0.453; P<0.001) and mean SaO2 (r=−0.378; P=0.009). Based on the above findings, a multivariate logistic regression model was created to determine risk factors in OSAS patients. Notably, the model indicated that serum hsCRP and IMA concentrations were risk factors for OSAS, while serum Mg levels were a protective factor (Table 4; P<0.05).
Table 3.
Correlation between marker concentrations and polysomnographic study results in all groups.+
| Marker | AHI (events/h of sleep) | Mean SaO2 (%) | |
|---|---|---|---|
| Mg (mg/dL) | r | −0.302 | 0.191 |
| P | 0.056 | 0.140 | |
| IMA (ABSU) | r | 0.614 | −0.560 |
| P | <0.001 | <0.001 | |
| hsCRP (mg/dL) | r | 0.453 | −0.378 |
| P | <0.001 | 0.009 | |
hsCRP – high-sensitivity C-reactive protein; IMA – ischemia-modified albumin; AHI – Apnea-Hypopnea Index; Mean SaO2 – mean of arterial oxygen saturation.
Table 4.
Independent predictors of obstructive sleep apnoea syndrome in multivariate logistic regression analysis.
| Parameter | Wald | OR (95% CI) | P values |
|---|---|---|---|
| Age | 1.324 | 0.91 (0.62–1.14) | 0.621 |
| BMI | 1.435 | 1.07 (0.50–1.41) | 0.453 |
| Hypertension | 2.016 | 1.33 (0.42–2.77) | 0.174 |
| Mg+ | 4.363 | 0.54 (0.38–0.88) | 0.006 |
| hsCRP | 6.114 | 3.58 (2.04–7.21) | 0.001 |
| IMA positive group | 7.998 | 8.05 (4.89–24.55) | 0.002 |
Status (control=0, OSAS=1); Age (female=0, male=1); BMI (<25.0=0, ≥25.0=1); Hypertension (no=0, yes=1); Mg+ (<1.98=0, ≥1.98=1); hsCRP (<1.20=0, ≥1.20=1); IMA (<0.49=0, ≥0.49=1).
The value of serum Mg, hsCRP, and IMA levels for diagnosing OSAS
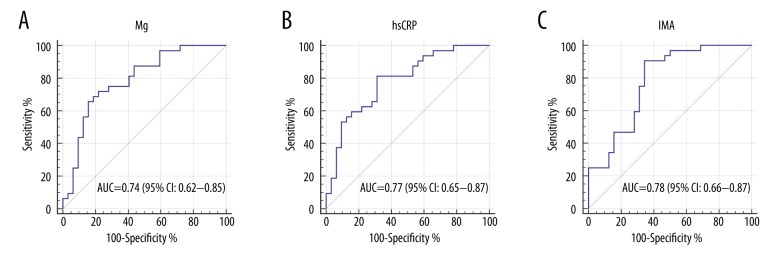
To ascertain the diagnostic performance of Mg, hsCRP, and IMA serum biomarkers in differentiating OSAS patients from non-OSAS patients, ROC curves were constructed, and the area under the curve (AUC) was generated. As shown in Figure 2A, 2B, Mg and hsCRP yielded AUC values of 0.74 (95% CI: 0.62–0.85) and 0.77 (95% CI: 0.65–0.87), respectively. The results showed that IMA has a stronger ability to diagnose OSAS (Figure 2C) than Mg and hsCRP, with an AUC value of 0.78 (95% CI: 0.66–0.87). However, these biomarkers had low sensitivity or specificity when used alone, so they would be of limited clinical use. Thus, we used the optimal equation from the multivariate logistic regression model to generate a new score named the MIh score. Subsequent ROC analysis of the MIh score yielded an AUC value of up to 0.93 (95% CI: 0.83–0.98), with ideal sensitivity (84.37%) and specificity (96.87%) (Figure 3), indicating that the MIh score is a promising biomarker in the diagnosis of OSAS. After 3 months of CPAP treatment, 22 patients had their serum Mg, hsCRP, and IMA concentrations reassessed. The CPAP titration pressure ranged from 5 to 12 cmH2O (mean=7.2±1.49 cmH2O). The results showed a significant increase in serum Mg concentrations (from 1.71±0.21 mg/dL to 2.02±0.25 mg/dL; P<0.001) and decreases in serum IMA concentrations (from 0.58±0.11 ABSU to 0.45±0.10 ABSU; P<0.001) and hsCRP concentrations (from 1.47±1.60 mg/L to 1.13±1.10 mg/L; P<0.001) after 3 months of CPAP treatment (Table 5).
Figure 2.
ROC analysis of serum magnesium (Mg), high-sensitivity C-reactive protein (hsCRP), and ischemia-modified albumin (IMA) in the diagnosis of obstructive sleep apnea syndrome. (A) In the ROC analysis of Mg, the AUC value is 0.74 (95% CI: 0.62–0.85) with a sensitivity and specificity of 61.62% and 80.37%, respectively. The cutoff value is 1.62 mg/dL. (B) In the ROC analysis of hsCRP, the AUC value is 0.77 (95% CI: 0.65–0.87) with a sensitivity and specificity of 81.25% and 58.75%, respectively. The cutoff value is 1.20 mg/L. (C) In the ROC analysis of IMA, the AUC value is 0.78 (95% CI: 0.66–0.87) with a sensitivity and specificity of 87.62% and 62.62%, respectively. The cutoff value is 0.53 ABSU.
Figure 3.
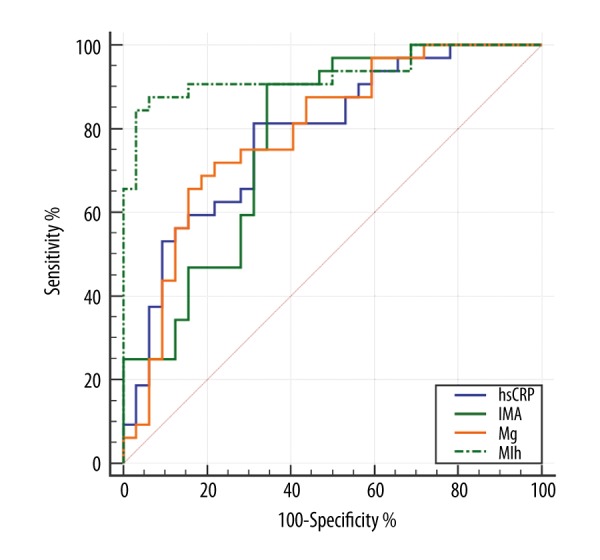
ROC analysis of the MIh score (combination of serum magnesium, high-sensitivity C-reactive protein, and ischemia-modified albumin) in diagnosing obstructive sleep apnea syndrome. The AUC value of MIh is 0.93 (95% CI: 0.83–0.98) with a sensitivity and specificity of 84.37% and 96.87%, respectively. The cutoff value is −7.35. MIh=−8.68–2.03*Mg+19.28*IMA+1.05*hsCRP.
Table 5.
Alterations in demographic information and serum Mg, IMA and hsCRP concentrations after 3 months of CPAP therapy.
| Marker | Pre-CPAP (n=22) | Post-CPAP | P value |
|---|---|---|---|
| BMI (kg/m2) | 31.03±3.7 | 30.4±3.9 | 0.553 |
| Mg (mg/dL) | 1.71±0.21 | 2.02±0.25 | <0.001 |
| IMA (ABSU) | 0.58±0.11 | 0.45±0.10 | <0.001 |
| hsCRP (mg/L) | 1.47±1.60 | 1.13±1.10 | <0.001 |
Discussion
Our results demonstrated that serum Mg, hsCRP, and IMA concentrations differed between the OSAS and control groups. Furthermore, AHI was significantly positively correlated with hsCRP and IMA concentrations, and negatively correlated with Mg levels. Each of these parameters could serve as a potential diagnostic biomarker for OSAS. In our study, there was no significant difference between the groups in terms of age, sex, or BMI, nor were there significant differences in the number of patients with hypertension, hyperlipidemia, or diabetes. This discrepancy with other findings may be due to the small sample size in this study.
Previous studies have indicated that oxidative stress and inflammation are important mechanisms underlying the pathophysiology of OSAS [17,18]. Furthermore, Mg plays a role in several enzymatic reactions that maintain cellular homeostasis [19], and hypomagnesemia appears to be associated with a higher degree of oxidative stress in obese individuals [20]. Several clinical trials have indicated that Mg stimulates the synthesis of nitric oxide and prostacyclin, both of which may reduce asthma severity in adults and children [21,22]. Interestingly, hypomagnesemia elevates the risk of high blood pressure, atherogenic lipid profile, metabolic syndrome, and type 2 diabetes [23–25]. Moreover, in a recent study, patients with OSAS showed a significant decrease in plasma levels of Mg, and there was a negative correlation between Mg concentrations and OSAS severity [15]. Similarly, in the present study, serum Mg levels were significantly lower in the OSAS group than in the control group. Specifically, Mg yielded an AUC value of 0.74 (95% CI: 0.62–0.85) for diagnosing OSAS, and CPAP treatment reversed the downregulated serum Mg levels. Of note, many OSAS patients use magnesium in their therapies, especially cardiac patients and those with chronic headaches or migraines. These may lead to false-negative results during the follow-up. Thus, further research is needed to reveal the exact relationship between Mg and OSAS and its molecular mechanisms.
On a different note, CRP is an important marker of endothelial dysfunction in the pathogenesis of coronary artery disease, and CRP level is a strong independent predictor of future myocardial infarction, stroke, and peripheral arterial disease [26]. For instance, Yokoe et al. found that plasma levels of CRP are elevated in patients with OSAS and that they are decreased by nasal CPAP in these patients [27]. In another study, Guven et al. reported similar results; they also found that hsCRP levels were positively correlated with both BMI and AHI [28]. In the present study, serum hsCRP concentrations were positively correlated with the AHI (which could also be regarded as indicating OSAS severity); in addition, multivariate logistic regression analyses indicated that hsCRP is a risk factor.
Excess production of free radicals may modify the N-terminal region of human serum albumin, generating ischemia-modified albumin (IMA), a sensitive marker of ischemia whose levels are also increased in diseases associated with obesity [12]. The potential role of IMA in several diseases has been reported previously [29–31]. However, few investigations have addressed the role of serum IMA in OSAS. Dogan et al. demonstrated that serum IMA may act as a valuable oxidative stress indicator in OSAS, and that it may be a biomarker reflecting the presence and severity of OSAS [32]. A study by Sunnetcioglu et al. found that IMA levels were significantly higher in patients with OSAS, and that they were positively correlated with AHI score. Similarly, Karamanli et al. found a mean IMA level of 1.231±0.102 ABSU among 61 study subjects; this was significantly higher than the mean level of 24 control participants (1.088±0.156 ABSU), and the authors suggested using serum IMA levels as a novel marker of oxidative stress in OSAS. The present study confirms this previous finding that serum IMA levels are higher in subjects with OSAS than in the control group, despite similar BMIs and mean ages [33]. OSAS was more reliably diagnosed by serum IMA than by Mg and hsCRP. In addition, multivariate analyses indicated that high levels of IMA are a risk factor for OSAS.
Unfortunately, the low sensitivity and specificity of these biomarkers may limit their clinical usefulness. For this reason, we calculated a new biomarker using a logistic regression model that was based both on previous research and our own findings. Surprisingly, the new model, which we named MIh, showed outstanding power to diagnose OSAS. Its AUC value was 0.93 (95% CI: 0.83–0.98), with high sensitivity (84.37%) and specificity (96.87%), indicating that it is a promising biomarker for OSAS that could be applied in clinical practice.
Certain limitations of our study should be mentioned. As a preliminary study, we explored the diagnostic value of Mg, hsCRP, and IMA in a small cohort only; this may have caused weak correlations among some of the factors, and we did not confirm our findings in a larger group. Furthermore, we could not consider the effects of alterations in glucose metabolism or of insulin resistance, which are 2 processes that may also play important roles in the elevation of hsCRP and IMA levels in OSAS. Finally, we did not take into account whether the 3 factors analyzed were correlated; a correlation may have affected their concentration values. Thus, further studies are required to understand the role of these factors in OSAS, and long-term prospective studies are needed to confirm these results.
Conclusions
In conclusion, we demonstrated that serum levels of both hsCRP and IMA increased with the presence and severity of OSAS, and that serum Mg levels changed with the presence of OSAS. Each of these changes was reversed after CPAP treatment and could be individually used as promising biomarker in OSAS diagnosis. However, the results obtained were inferior to those recorded using a diagnostic tool that combined the results of the 3 individual markers in the current study. Therefore, the MIh scoring system is a promising tool to diagnose OSAS in clinical settings.
Footnotes
Source of support: This study was supported by the Science and Technology Bureau of Fuyang District, Hangzhou (2013SF023)
Conflicts of interest
None to disclose.
References
- 1.Fava C, Montagnana M, Favaloro EJ, et al. Obstructive sleep apnea syndrome and cardiovascular diseases. Semin Thromb Hemost. 2011;37:280–97. doi: 10.1055/s-0031-1273092. [DOI] [PubMed] [Google Scholar]
- 2.Lindberg E, Berne C, Franklin KA, et al. Snoring and daytime sleepiness as risk factors for hypertension and diabetes in women – a population-based study. Respir Med. 2007;101:1283–90. doi: 10.1016/j.rmed.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 3.Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population – a review on the epidemiology of sleep apnea. J Thorac Dis. 2015;7:1311–22. doi: 10.3978/j.issn.2072-1439.2015.06.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paruthi S, Rosen CL, Wang R, et al. End-tidal carbon dioxide measurement during pediatric polysomnography: Signal quality, association with apnea severity, and prediction of neurobehavioral outcomes. Sleep. 2015;38:1719–26. doi: 10.5665/sleep.5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lavie L, Lavie P. Molecular mechanisms of cardiovascular disease in OSAHS: The oxidative stress link. Eur Respir J. 2009;33:1467–84. doi: 10.1183/09031936.00086608. [DOI] [PubMed] [Google Scholar]
- 6.Tamaki S, Yamauchi M, Fukuoka A, et al. Production of inflammatory mediators by monocytes in patients with obstructive sleep apnea syndrome. Intern Med. 2009;48:1255–62. doi: 10.2169/internalmedicine.48.2366. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, Yu Z, Hua D, et al. Association of serum hepcidin levels with the presence and severity of obstructive sleep apnea syndrome. Med Sci Monit. 2015;21:27–31. doi: 10.12659/MSM.891297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Israel LP, Benharoch D, Gopas J, Goldbart AD. A pro-inflammatory role for nuclear factor kappa B in childhood obstructive sleep apnea syndrome. Sleep. 2013;36:1947–55. doi: 10.5665/sleep.3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tauman R, O’Brien LM, Gozal D. Hypoxemia and obesity modulate plasma C-reactive protein and interleukin-6 levels in sleep-disordered breathing. Sleep Breath. 2007;11:77–84. doi: 10.1007/s11325-006-0085-7. [DOI] [PubMed] [Google Scholar]
- 10.Christenson RH, Duh SH, Sanhai WR, et al. Characteristics of an Albumin Cobalt Binding Test for assessment of acute coronary syndrome patients: A multicenter study. Clin Chem. 2001;47:464–70. [PubMed] [Google Scholar]
- 11.Kaya Z, Kayrak M, Gul EE, et al. The role of ischemia modified albumin in acute pulmonary embolism. Heart Views. 2014;15:106–10. doi: 10.4103/1995-705X.151083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piva SJ, Duarte MM, Da Cruz IB, et al. Ischemia-modified albumin as an oxidative stress biomarker in obesity. Clin Biochem. 2011;44:345–47. doi: 10.1016/j.clinbiochem.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 13.de Baaij JH, Hoenderop JG, Bindels RJ. Magnesium in man: implications for health and disease. Physiol Rev. 2015;95:1–46. doi: 10.1152/physrev.00012.2014. [DOI] [PubMed] [Google Scholar]
- 14.Belin RJ, He K. Magnesium physiology and pathogenic mechanisms that contribute to the development of the metabolic syndrome. Magnes Res. 2007;20:107–29. [PubMed] [Google Scholar]
- 15.Karamanli H, Kizilirmak D, Akgedik R, Bilgi M. Serum levels of magnesium and their relationship with CRP in patients with OSA. Sleep Breath. 2016 doi: 10.1007/s11325-016-1402-4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Bar-Or D, Lau E, Winkler JV. A novel assay for cobalt-albumin binding and its potential as a marker for myocardial ischemia – a preliminary report. J Emerg Med. 2000;19:311–15. doi: 10.1016/s0736-4679(00)00255-9. [DOI] [PubMed] [Google Scholar]
- 17.Driessen C, Plomp RG, van der Spek PJ, et al. Is there an effect of obstructive sleep apnea syndrome on oxidative stress and inflammatory parameters in patients with craniofacial anomalies? J Craniofac Surg. 2013;24:1908–13. doi: 10.1097/SCS.0b013e3182a41c05. [DOI] [PubMed] [Google Scholar]
- 18.Karamanli H, Ozol D, Ugur KS, et al. Influence of CPAP treatment on airway and systemic inflammation in OSAS patients. Sleep Breath. 2014;18:251–56. doi: 10.1007/s11325-012-0761-8. [DOI] [PubMed] [Google Scholar]
- 19.Rowe BH, Bretzlaff JA, Bourdon C, et al. Magnesium sulfate for treating exacerbations of acute asthma in the emergency department. Cochrane Database Syst Rev. 2000;(2):Cd001490. doi: 10.1002/14651858.CD001490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morais JB, Severo JS, Santos LR, et al. Role of magnesium in oxidative stress in individuals with obesity. Biol Trace Elem Res. 2017;176(1):20–26. doi: 10.1007/s12011-016-0793-1. [DOI] [PubMed] [Google Scholar]
- 21.Davalos Bichara M, Goldman RD. Magnesium for treatment of asthma in children. Can Fam Physician. 2009;55:887–89. [PMC free article] [PubMed] [Google Scholar]
- 22.Rowe BH, Bretzlaff JA, Bourdon C, et al. Intravenous magnesium sulfate treatment for acute asthma in the emergency department: A systematic review of the literature. Ann Emerg Med. 2000;36:181–90. doi: 10.1067/mem.2000.105659. [DOI] [PubMed] [Google Scholar]
- 23.Kisters K, Spieker C, Tepel M, Zidek W. New data about the effects of oral physiological magnesium supplementation on several cardiovascular risk factors (lipids and blood pressure) Magnes Res. 1993;6:355–60. [PubMed] [Google Scholar]
- 24.Guerrero-Romero F, Rodriguez-Moran M. Low serum magnesium levels and metabolic syndrome. Acta Diabetol. 2002;39:209–13. doi: 10.1007/s005920200036. [DOI] [PubMed] [Google Scholar]
- 25.Kao WH, Folsom AR, Nieto FJ, et al. Serum and dietary magnesium and the risk for type 2 diabetes mellitus: The Atherosclerosis Risk in Communities Study. Arch Intern Med. 1999;159:2151–59. doi: 10.1001/archinte.159.18.2151. [DOI] [PubMed] [Google Scholar]
- 26.Lindahl B, Toss H, Siegbahn A, et al. Markers of myocardial damage and inflammation in relation to long-term mortality in unstable coronary artery disease. FRISC Study Group. Fragmin during Instability in Coronary Artery Disease. N Engl J Med. 2000;343:1139–47. doi: 10.1056/NEJM200010193431602. [DOI] [PubMed] [Google Scholar]
- 27.Yokoe T, Minoguchi K, Matsuo H, et al. Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation. 2003;107:1129–34. doi: 10.1161/01.cir.0000052627.99976.18. [DOI] [PubMed] [Google Scholar]
- 28.Guven SF, Turkkani MH, Ciftci B, et al. The relationship between high-sensitivity C-reactive protein levels and the severity of obstructive sleep apnea. Sleep Breath. 2012;16:217–21. doi: 10.1007/s11325-011-0492-2. [DOI] [PubMed] [Google Scholar]
- 29.Shen XL, Lin CJ, Han LL, et al. Assessment of ischemia-modified albumin levels for emergency room diagnosis of acute coronary syndrome. Int J Cardiol. 2011;149:296–98. doi: 10.1016/j.ijcard.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 30.Pantazopoulos I, Papadimitriou L, Dontas I, et al. Ischaemia modified albumin in the diagnosis of acute coronary syndromes. Resuscitation. 2009;80:306–10. doi: 10.1016/j.resuscitation.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 31.Zhong Y, Wang N, Xu H, et al. Ischemia-modified albumin in stable coronary atherosclerotic heart disease: Clinical diagnosis and risk stratification. Coron Artery Dis. 2012;23:538–41. doi: 10.1097/MCA.0b013e328358a5e9. [DOI] [PubMed] [Google Scholar]
- 32.Dogan D, Ocal N, Aydogan M, et al. Assessment of the role of serum ischemia-modified albumin in obstructive sleep apnea in comparison with interleukin-6. Postgrad Med. 2016;128:603–8. doi: 10.1080/00325481.2016.1203237. [DOI] [PubMed] [Google Scholar]
- 33.Uygur F, Tanriverdi H, Can M, et al. The impact of obstructive sleep apnoea and nasal continuous positive airway pressure on circulating ischaemia-modified albumin concentrations. Mediators Inflamm. 2016;2016:8907314. doi: 10.1155/2016/8907314. [DOI] [PMC free article] [PubMed] [Google Scholar]