Abstract
In pancreatic β cells, muscarinic cholinergic receptor M3 (M3R) stimulates glucose-induced secretion of insulin. Regulator of G-protein signaling (RGS) proteins are critical modulators of GPCR activity, yet their role in β cells remains largely unknown. R7 subfamily RGS proteins are stabilized by the G-protein subunit Gβ5, such that the knockout of the Gnb5 gene results in degradation of all R7 subunits. We found that Gnb5 knockout in mice or in the insulin-secreting MIN6 cell line almost completely eliminates insulinotropic activity of M3R. Moreover, overexpression of Gβ5-RGS7 strongly promotes M3R-stimulated insulin secretion. Examination of this noncanonical mechanism in Gnb5−/− MIN6 cells showed that cAMP, diacylglycerol, or Ca2+ levels were not significantly affected. There was no reduction in the amplitude of free Ca2+ responses in islets from the Gnb5−/− mice, but the frequency of Ca2+ oscillations induced by cholinergic agonist was lowered by more than 30%. Ablation of Gnb5 impaired M3R-stimulated phosphorylation of ERK1/2. Stimulation of the ERK pathway in Gnb5−/− cells by epidermal growth factor restored M3R-stimulated insulin release to near normal levels. Identification of the novel role of Gβ5-R7 in insulin secretion may lead to a new therapeutic approach for improving pancreatic β-cell function.—Wang, Q., Pronin, A. N., Levay, K., Almaca, J., Fornoni, A., Caicedo, A., Slepak, V. Z. Regulator of G-protein signaling Gβ5-R7 is a crucial activator of muscarinic M3 receptor-stimulated insulin secretion.
Keywords: islet of Langerhans, pupil sphincter, CRISPR-Cas9, UBO-QIC, fluorescent biosensor
Pancreatic β cells respond to rising levels of glucose by secreting insulin. Because insulin is the only hormone that stimulates glucose uptake, this process has a vital role in homeostasis. Secretory activity of the endocrine pancreas is tuned by various hormones and neurotransmitters, many of which signal through GPCRs. Gq/11- and Gs-coupled receptors promote glucose-stimulated insulin release by β cells, whereas Gi-coupled receptors exert an inhibitory effect (1). Regulation of β cells also involves ion channels, protein kinases, the cytoskeleton, and secretory machinery, and this intricate network is still far from being understood.
Neurotransmitter acetylcholine, which is thought to cause prefeeding (cephalic phase) increase in serum insulin, is one of the most essential inputs for the pancreas. A study has established that the receptor responsible for the acetylcholine sensitivity of β cells is muscarinic receptor type 3 (M3R) (2). M3R is a Gq-coupled GPCR that stimulates PLC, an effector enzyme that influences levels of phosphoinositides and Ca2+, second messengers that regulate activity of proteins that are essential for trafficking, docking, and release of insulin granules (3–5).
A critical mechanism responsible for the appropriate kinetics of GPCR signaling involves acceleration of guanosine triphosphate hydrolysis by the G proteins. This function is fulfilled by regulators of G-protein signaling (RGS) proteins, a family discovered as G-protein inhibitors acting as GTPase activating proteins (GAPs) for Gα subunits. All of the ∼30 diverse RGS protein family members contain an ∼100 aa “RGS box” responsible for the interaction with Gα and the GAP activity (6). Through distinct domains, RGS proteins interact with other molecules, including GPCRs, effectors, and adapter proteins. RGS proteins play central roles in cardiovascular and immune systems and particularly in the nervous system (7–10). Surprisingly, their function in the pancreas has only begun to be investigated. It has been shown that the knockout of RGS4 enhances insulin secretion by M3R-stimulated β cells, which is consistent with the canonical RGS function as an inhibitor of G-protein signaling (11).
In this article, we investigate the role of the R7 subfamily of RGS proteins in the signaling pathway that couples M3R to insulin release. The R7 subfamily includes RGS-6, -7, -9, and -11. In addition to the RGS box, the proteins have 3 unique domains: one originally found in Disheveled, Egl10 and Pleckstrin (DEP) and two others, DEP helical extension (DHEX) and G γ-like (GGL) (12, 13). The GGL domain is responsible for dimerization with the atypical G-protein β subunit Gβ5 (14, 15). This association is obligatory and neither Gβ5 nor R7 proteins have been found apart from each other in native tissues (16). Ectopically expressed monomers degrade 10–100 times faster than their complex (16) and, consequently, gene knockouts show that all R7 proteins disappear in the absence of Gβ5 (17).
The knockout of the Gβ5 gene in mice produces a pleiotropic phenotype affecting sensory transduction, neuronal development, locomotor activity, metabolism, and other functions (17–21). Gβ5 and R7 proteins are highly expressed in the nervous system (12, 13) and at much lower levels in some other organs and tissues [e.g., the heart (22, 23) and glands, including the pancreas (21)]. One of the changes detected in the Gnb5-null mice is a 2-fold reduction in serum insulin level (21). We asked if this reduction occurs autonomously within the pancreas or results from global changes in the neuroendocrine system. Our experiments showed that Gβ5 ablation has a profound negative effect on M3R-induced insulin secretion at the cellular level. This unexpected result implies that the function of the Gβ5-R7 complex in this pathway is opposite that of inhibiting G-protein signaling.
MATERIALS AND METHODS
Animals
Animal procedures were performed according to the Guidelines for the Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD, USA) and protocols were approved by the University of Miami Committee on Use and Care of Animals. Gβ5-knockout (Gnb5−/−) mice (21) were back-crossed into the C57Bl6/6J background for at least 10 generations. Age-matched (12–18 wk old) males were used for tissues collection.
Preparation and perifusion of mouse islets
Pancreatic islets were isolated by using a combined enzymatic and mechanical dissociation of the tissue (21) and cultured in Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 10% fetal bovine serum (VWR International, West Chester, PA, USA), 100 IU/ml penicillin and 100 μg/ml streptomycin. Islets were perifused with Krebs Ringer Bicarbonate containing glucose and agonists; eluted fractions were analyzed by insulin ELISA (21).
MIN6 culture, transfection, and stimulation
Cells were cultured in DMEM (Thermo Fisher Scientific, Waltham, MA, USA) with 25 mM glucose and 4 mM l-glutamine. They were transfected with Lipofectamine 2000 (Thermo Fisher Scientific) and cultured for 48 h; before application of stimulants, cells were preincubated with serum- and glucose-free DMEM for 1 h. After 30 min stimulation at 37°C, the supernatant was collected and stored frozen until measurement of insulin. The cells were then harvested for immunoblot analysis.
CRISPR-Cas9-mediated knockout of Gnb5 in MIN6 cells
To find the guide sequence 5′-GCTGCACGAGAACGAGACGC-3′ that targets exon-1 of the mouse Gnb5 gene, we used the CRISPR optimized design tool (crispr.mit.edu; Massachusetts Institute of Technology, Cambridge, MA, USA). Two oligonucleotides 5′-CACCGGCTGCACGAGAACGAGACGC-3′ (sense) and 5′-AAACGCGTCTCGTTCTCGTGCAGCC-3′ (antisense) were annealed, cloned into BsmBI digested lentiCRISPRv2 plasmid (Addgene, Cambridge, MA, USA), and transformed into C3040I stable competent Escherichia coli to generate the lentivirus backbone plasmid pLenti-MmGnb5. For the negative control pLenti-lacZ we used the guide sequence 5′-TGCGAATACGCCCACGCGAT-3′ targeting the E. coli lacZ gene. The constructs were verified by sequencing.
The pLenti-MmGnb5 or pLenti-lacZ plasmids carrying the CRISPR-Cas9 guide sequences were cotransfected with pCMVdR8.74 and pMD2VSVG (plasmid DNA ratio of 20:13:7) into the HEK293 packaging cells by using the TransIT-LT1 transfection reagent. Medium was replaced 16 h later with DMEM containing 2% fetal bovine serum. After a 48-h incubation, lentivirus-containing supernatants were harvested, supplemented with polybrene to the final concentration of 8 mg/ml, fetal bovine serum to 10%, and 2-ΜΕ to 50 μM, and passed through a 0.45-μm filter. For transduction, MIN6 cells were washed with PBS, treated with trypsin/EDTA for 5 min, and resuspended in complete culture medium. After centrifugation, they were resuspended in the lentivirus-containing medium, seeded into 10-cm plates, and incubated overnight. The lentivirus-containing medium was aspirated, cells were washed 3 times with PBS before the addition of complete MIN6 culture medium. Cells were detached by trypsin/EDTA after 48 h, diluted 10-fold in the selective medium containing 2 mg/ml puromycin, plated, and grown for ∼14 d until single colonies were formed. The selective medium was replaced every 3 d. Isolated single colonies of transduced MIN6 cells were selected under a microscope and expanded into 24-well plates.
Genomic DNA was extracted from the selected MIN6 clones by using the Quick gDNA micro prep kit (Zymo Research, Irvine, CA, USA), according to the manufacturer’s protocol. Genotyping was performed by PCR with 2 forward (fwd) primers corresponding to Gnb5: 5′-CCAGCAGAGTCCGTCACC-3′ (fwd_1), 5′-GCTGCACGAGAACGAGAC-3′ (fwd_2), and common reverse (rev) primer 5′-ATCCACGTCATGCAGCTTT-3′ (rev). PCR products obtained with phosphorylated fwd_1 and rev primers were blunt ended and cloned into pcDNA3 vector (Promega, Madison, WI, USA) at the EcoRV site. After transformation, DNA from individual clones was sequenced across the insert with a custom primer, to determine the nature of the mutation (e.g., frame shift). Elimination of Gβ5 was then confirmed by immunoblot.
Insulin ELISA
Insulin was measured with a sandwich ELISA kit (Mercodia, Uppsala, Sweden) (21).
Free Ca2+ assays
MIN6 cells were grown on 12-mm glass coverslips, loaded with fura-2 AM, and imaged in a flow chamber on the stage of an inverted fluorescence microscope with a ×20 UV objective lens (24, 25). To stimulate the cells, the flow was switched to agonist-containing HBSS and then back to the agonist-free buffer. Clusters of 10–20 cells were selected as regions of interest for signal quantification with MetaFluor software (Molecular Devices, Sunnyvale, CA, USA). Islets isolated from the wild-type and knockout mice were loaded with fura-2 AM and allowed to adhere to a polylysine-coated glass coverslip for 30 min before perifusion and imaging. The presented traces are averages of several independent experiments with several replicate coverslips per experiment.
cAMP and diacylglycerol biosensor assays
MIN6 cells grown on coverslips were infected with BacMam viruses encoding the green “up” cAMP sensor or green “down” diacylglycerol (DAG) sensor (Montana Molecular, Bozeman, MT, USA) (26), and grown for 24 h before live imaging under a ×20 objective. Images were taken every 5 s. In a typical experiment, we averaged responses from 30–50 cells selected from a visual field.
Western blot
Cell lysates were subjected to immunoblot analysis with antibodies against RGS7, Gβ5, Gβ1, Gαq, and Gαo (16, 21). Antibodies against actin, pan-Gβ, p-ERK, and ERK were purchased from Cell Signaling Technology (Danvers, MA, USA). The immune complexes were visualized using the Odyssey (Li-Cor Biosciences, Lincoln, NE, USA) infrared fluorescence system. For quantitative analysis, the signal in the band of interest (i.e., p-ERK) was normalized to that for actin in the same lane.
In situ mRNA hybridization
Localization of mRNA in paraffin-embedded slices of the mouse pancreata and eyes was performed with custom fluorescence RNAscope probes (Advanced Cell Diagnostics, Newark, CA, USA), according to the manufacturer’s instructions, with minor modifications described earlier (27).
Mouse eye pupil constriction
Enucleated eyes were secured in a chamber with 100 μl HBSS, and an image was taken to record the pupil diameter at time 0. After addition of 1 mM pilocarpine, additional images were taken and pupil diameter at each time point was compared to the value at time 0.
Statistics
Data are reported as means ± sd. Groups of data were compared using 2-tailed, unpaired Student’s t test or ANOVA (Prism software; GraphPad, La Jolla, CA, USA), with P < 0.05 considered statistically significant.
RESULTS
The knockout of Gβ5 abrogates cholinergic stimulation of insulin secretion
To study the role of the Gβ5-R7 complex in regulation of insulinotropic activity of M3R, we examined pancreatic islets isolated from Gnb5−/− mice (Fig. 1). Our data showed no significant difference in insulin secretion between the islets from Gnb5−/− and wild-type mice induced by 16.7 mM glucose. In contrast, the robust potentiation of insulin release caused by the muscarinic agonist oxotremorine-M (Oxo-M; 10 μM) observed in the wild-type islets was almost completely eliminated by the Gnb5 knockout (Fig. 1A, B). This effect is surprising because the canonical function of RGS proteins is to inhibit GPCR signaling. If Gβ5-R7 were an inhibitor, its elimination would have enhanced M3R-evoked insulin secretion, similar to the knockout of RGS4 (11).
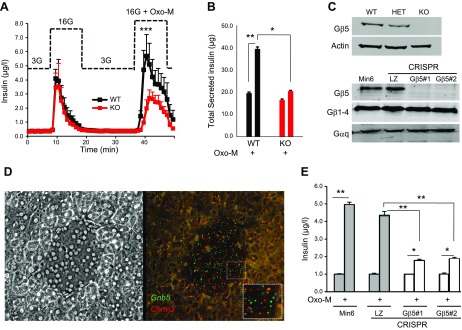
Figure 1.
Gβ5 knockout inhibits M3R-stimulated insulin secretion. Cholinergic stimulation of insulin secretion was tested in primary islets (A, B) or MIN6 cells lacking Gβ5 (E). A) Islets from Gnb5−/− (KO, red) and wild-type (WT, black) mice were perifused. After equilibration with media containing 3 mM glucose (3G), islets were challenged with 16.7 mM glucose (16G), with or without 10 μM Oxo-M. The data points are the mean ± sd of insulin concentrations measured in collected fractions from experiments on 4 independent animal cohorts (3–5 animals/genotype). ***P < 0.001 in the peak; for other data points, P < 0.05. B) Quantification of total secreted insulin (area under the curve shown in A). C) Immunoblot analysis of islets from Gnb5−/− mice (top) and CRISPR-Cas9-knockout MIN6 clones (bottom). Total cell lysates were probed for Gβ5, Gβ1, Gαq, and actin (representative of at least 2 experiments). D) In situ RNA hybridization of mouse pancreatic slices using RNAscope probes against Gnb5 (green) and Chrm3 (red) gene products was performed. Shown are phase-contrast (left) and fluorescence (right) images of a representative islet. The inset shows magnification of the fluorescent dots representing the single target mRNA molecules. E) Wild-type MIN6 cells, control CRISPR (LZ), and 2 stable clones after CRISPR-Cas9-mediated knockout of the Gnb5 gene (#1 and #2) were stimulated with 16.7 mM glucose or 16.7 mM glucose plus 100 μM Oxo-M. Data are means ± sd of insulin concentration in the supernatant from the cultured cells. *P < 0.05; **P < 0.01.
Our results also indicate that the previously reported decrease in serum insulin in the Gnb5−/− mice (21) can be attributed to the absence of Gβ5-R7 within the islets rather than the global effect of this knockout on neuroendocrine regulation. In support of this notion, genes encoding both Gβ5 and M3R were expressed in β cells (Fig. 1C, D). To rule out a contribution of other cell types within the islets, we performed a series of experiments in the mouse insulinoma cell line MIN6. These cells express endogenous M3R, can secrete insulin, and are amenable to transfection and other experimentation (5, 11, 28–30).
We deleted the Gnb5 gene in MIN6 cells with the CRISPR-Cas9 system and selected 2 stable knockout clones (Gβ5−/− 1 and 2) and a control clone where the CRISPR-Cas9 system targeted the sequence of the bacterial gene lacZ. These clones were compared to our original wild-type MIN6 cells. The knockout resulted in disappearance of the Gβ5 protein (Fig. 1C) and a dramatic reduction of Oxo-M-stimulated insulin secretion (Fig. 1E). This effect is consistent with the absence of Oxo-M potentiation in primary Gnb5−/− islets (Fig. 1A, B). Moreover, as shown in Fig. 2, overexpression of Gβ5-RGS7 has exactly the opposite effect.
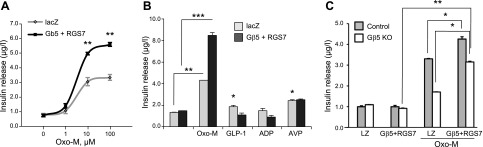
Figure 2.
Overexpression of the Gβ5-RGS7 complex promotes coupling of M3R to insulin secretion in MIN6 cells. A) MIN6 cells were transfected with plasmids encoding Gβ5 and RGS7 or lacZ and stimulated with the indicated concentrations of Oxo-M in the presence of 16.7 mM glucose. Insulin in the supernatants was measured by ELISA. Data are means ± sd from 3 independent transfections, each performed in triplicate. B) Transfected MIN6 cells were incubated with 100 μM Oxo-M or 0.1 μM GLP-1, 100 μM ADP, 0.1 μM AVP, all added in the presence of 16.7 mM glucose. For GLP-1 and AVP, there was a significant increase in insulin secretion compared to control; ADP did not cause significant change. C) The Gβ5−/− MIN6 cells (clone #1) or control CRISPR cells (Gβ5 +/+) were transfected with Gβ5 and RGS7 or control (LZ) plasmid, and then stimulated with 16.7 mM glucose or 16.7 mM glucose plus 100 μM Oxo-M (n = 3). Data are means ± sd. ***P < 0.001; **P < 0.01; *P < 0.05.
Gβ5-RGS7 complex enhances M3R-stimulated insulin secretion in MIN6 cells
Transient transfection of MIN6 cells with Gβ5- and RGS7-expressing plasmids increased Oxo-M-evoked insulin secretion by nearly 100% at 100 μM Oxo-M (Fig. 2A). In contrast, Gβ5- and RGS7-overexpressing MIN6 cells did not exhibit a significant difference in insulin secretion in response to other insulin secretagogues, including glucagon-like peptide (GLP)-1, AVP, and ADP, an inhibitor of insulin secretion (Fig. 2B).
Next, we tested whether the loss of cholinergic sensitivity was indeed caused by the absence of Gβ5 and not by clonal variation or an off-target effect of the CRISPR-Cas9 procedure. To this end, we transfected Gnb5−/− cells with plasmids encoding Gβ5 and RGS7 and found that this rescued responsiveness of the cells to Oxo-M in the presence of high glucose (Fig. 2C). The amount of secreted insulin increased from ∼25 to 70% of that observed in the wild-type or Gβ5+/+ MIN6 cells.
Together, our gene ablation and overexpression experiments demonstrate that in β cells, the Gβ5-R7 complex promotes M3R-stimulated insulin secretion. We set out to investigate the molecular mechanism underlying this paradoxical effect. Our approach was to restore the insulinotropic function of M3R in the Gnb5−/− cells by using a pharmacologic agent or another treatment. We reasoned that the reversal of the Gnb5−/− phenotype would point to the specific molecular event disabled by the absence of Gβ5-R7.
Gβ5 knockout does not compromise M3R-stimulated Ca2+ signaling
First, we characterized the signaling pathway that links M3R to insulin secretion in control MIN6 cells. Our data show that the Gq/11 inhibitor FR900359 (31) (abbreviated as FR) at 0.1 μM concentration blocked insulinotropic activity of Oxo-M (Fig. 3A). This plant-derived depsipeptide is also known as UBO-QIC and is very similar to YM-254890 (25, 32). A recent comprehensive study demonstrated that FR is highly selective for Gαq, -11, and -14, but has no effect on other G proteins (33). In contrast to FR, pertussis toxin did not influence cholinergic stimulation of insulin secretion, strongly indicating that this pathway is mediated by Gq, but not Gi. Accordingly, there was no detectable effect of 100 μM Oxo-M on the cAMP level (Fig. 3B), whereas in the same conditions 1 μM forskolin or 0.1 μM GLP-1 caused a robust increase of the cAMP signal. Compound U73122, commonly [and incorrectly because of its poor specificity (34)] referred to as a PLC inhibitor, blocked insulin release stimulated by 100 μM Oxo M (Fig. 3A). Insulin secretion did not occur in Ca2+-free medium, and 10 μM nifedipine, a Ca2+ channel blocker, strongly suppressed Oxo-M-stimulated insulin release, showing the necessity of extracellular Ca2+. As expected, muscarinic agonists induced increases in free cytoplasmic Ca2+ (Fig. 3C) and generation of DAG (Fig. 3D), both pointing to activation of PLC. Like insulin secretion, Ca2+ mobilization was completely blocked by FR (data not shown). Overall, these data are in agreement with the current view that cholinergic activation of β cells is mediated by Gq, but not Gi or Gs and requires an increase in the intracellular free Ca2+ concentration (3).
Figure 3.
M3R-stimulated insulin secretion and second-messenger responses in Gnb5−/− and Gnb5+/+ Min6 cells. A) Wild-type Min6 cells were cultured and treated with 20 ng/ml pertussis toxin (PTX), 0.1 μM FR, 10 μM U73122, Ca2+-free medium, or 10 μM nifedipine (Nif), and released insulin was measured after stimulation with 100 μM Oxo-M. Data are means ± sd from at least 3 independent experiments. There was no statistically significant effect of PTX. B) MIN6 cells were transduced with the cAMP sensor and imaged in a flow cell on a fluorescence microscope in real time. After 200 s, solutions of 1 μM forskolin, 100 μM Oxo-M, or 100 nM GLP-1 were added for 200 s. Shown is representative results of 3 independent experiments. C) Free Ca2+ responses to 100 μM Oxo-M in the knockout (Gβ5 KO) and control MIN6 cells were recorded by using fura-2 with microscopy. Data points are averages from the total of 100–200 cells. Shown is a representative of 3 independent experiments. D) Cells were transduced with the fluorescence sensor for DAG. Data are representative of 2 experiments. E) Effect of ionomycin (Ion) on insulin secretion from the Gnb5−/− MIN6 cells. Oxo-M (100 μM) and ionomycin (4 μM) were added in the presence of 16.7 mM glucose. Data are means ± sd from at least 3 independent experiments. *P < 0.05; **P < 0.01.
The knockout of Gnb5 did not reduce, but rather enhanced Ca2+ responses of MIN6 cells to cholinergic stimulation (Fig. 3C). Similarly, there was a trend to increase Oxo-M stimulated production of DAG in the Gnb5−/− MIN6 cells (Fig. 3D). These effects of the Gnb5 knockout are in agreement with our previous reports showing that overexpression of Gβ5-RGS7 suppresses M3R-induced Ca2+ signaling in COS7 and CHO-K1 cells (16, 35). These findings also indicate that elimination of the Gβ5-R7 complex does not limit availability of Ca2+ required for insulin secretion. Furthermore, our experiment with ionomycin showed that application of this Ca2+ ionophore at a concentration of 4 μM did not restore the Oxo-M-evoked insulin release in the Gnb5−/− cells (Fig. 3E). Thus, Gβ5-R7 knockout inhibits insulin secretion via a mechanism that does not involve impairment of PLC activity or Ca2+ influx.
Gnb5 knockout reduces M3R-stimulated phosphorylation of ERK1/2
Consistent with another report (5), we found that Oxo-M stimulates a rapid and transient phosphorylation of ERK1/2 in MIN6 cells. In the Gβ5-knockout cells, M3R-induced phosphorylation of ERK1/2 was significantly lower than in the control (Fig. 4A, B). This reduction was detected with Oxo-M and other muscarinic agonists (data not shown) and the difference was stronger at later time points. Since protein phosphorylation is involved in insulin secretion, we hypothesized that changes in the kinase activity downstream of M3R could explain the loss of insulinotropic function in Gnb5−/− cells. To investigate this possibility, we tested whether activation of ERK via an M3R-independent mechanism could rescue this function.
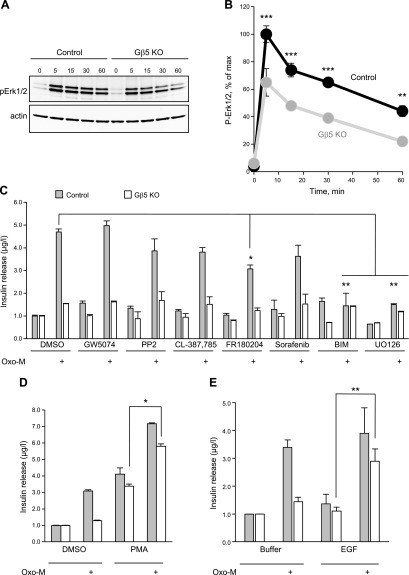
Figure 4.
Gβ5 knockout impairs M3R-stimulated ERK1/2 phosphorylation in MIN6 cells. A) Gnb5+/+ (control) or knockout (Gβ5-KO) cells were stimulated with 100 μM Oxo-M for the indicated periods (minutes) and subjected to Western blot analysis with anti-phospho-ERK1/2 and anti-actin antibodies. Shown is a representative immunoblot. B) Quantitative analysis of Western blots (n = 3). Mean ± sd. At least 5 additional experiments with individual time points had a similar reduction of ERK1/2 phosphorylation in Gβ5-KO (data not shown). C) The control and knockout cells were stimulated with Oxo-M in the presence of inhibitors of protein kinases PKC (BIM, bisindolylmaleimide I), Src (GW5074 and PP2), EGFR (CL-387785), Erk1/2 (FR180204), MKK1/2 (U0126), and sorafenib (Raf and other Tyr-kinases), and insulin secretion was measured. D) The Gβ5-knockout and control MIN6 cells were stimulated with Oxo-M in the presence or absence of 100 ng/ml PMA, which was added with Oxo-M. E) Cells were treated as in D with 50 ng/ml EGF. Data in C–E are means ± sd from 5 independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001.
To begin identification of specific kinases, we tested a panel of kinase inhibitors. As shown in Fig. 4C, inhibitors of MEK2, epidermal growth factor receptor (EGFR), Raf, and Src kinases had no effect, whereas inhibition of MEK1 and PKC blocked the M3R-stimulated insulin secretion by MIN6 cells. We also treated cells with the PKC activator phorbol myristate acetate (PMA) and found that it promoted insulin secretion in both Gnb5+/+ and Gnb5−/− cells (Fig. 4D). Upon application of Oxo-M together with PMA, secretion of insulin from the knockout cells was almost as high as in the control. One of the ways to stimulate the ERK cascade is via a receptor tyrosine kinase such as EGFR. Our results showed that application of EGF by itself did not stimulate insulin release from Gnb5+/+ or Gnb5−/− MIN6 cells. However, when the Gnb5−/− cells were treated with EGF together with Oxo-M, they secreted almost as much insulin as the Gnb5+/+ control or wild-type cells (Fig. 4E). This rescue effect suggests that the Gβ5-R7 complex is necessary for the appropriate function of the kinase network involved in activation of insulin secretion.
Gβ5 knockout decreases frequency of Oxo-M-induced Ca2+ oscillations in pancreatic islets
It is known that free cytoplasmic Ca2+ in mouse pancreatic islets displays oscillations in high glucose, and this behavior is synchronous between the individual cells in the islet (Fig. 5A). In the presence of 3 mM glucose, 100 μM Oxo-M induced a similar rise and fall in free cytoplasmic Ca2+ in the islets isolated from the Gnb5−/− and control mice (Fig. 5C). After the increase of glucose concentration from 3 to 16 mM, cytosolic Ca2+ began to oscillate. These oscillations began with an ∼5 min delay after switching to high glucose, which reflects the need for glucose uptake, its metabolism, and the increase in the ATP:ADP ratio in the cells. Analysis of these oscillations revealed an ∼33% reduction in frequency in the Gnb5-knockout islets compared with the control (Fig. 5B, E), but no statistically significant change in the amplitude (Fig. 5D). When islets were challenged with 16 mM glucose for 10 min in the absence of cholinergic stimulation, we did not observe oscillations, and the responses were similar between the control and knockout islets. These experiments show that the Gβ5-R7 complex promotes high frequency of free Ca2+ oscillations, which may, in part, explain its robust stimulatory effect on the insulinotropic function of M3R (Figs. 1 and 2).
Figure 5.
Effects of Gβ5 knockout on cholinergic stimulation of pancreatic islets and pupillary constrictor muscle. A) Islets from wild-type (control) and Gnb5−/− mice were loaded with fura-2. The series of 13 traces show raw data from selected regions of interest within a representative control islet. The islet was perifused with 3 mM glucose (3G) to establish a baseline, then challenged with 100 μM Oxo-M in 3G, followed up by 100 μM Oxo-M in 16 mM (high) glucose (16G) for 10 min. It was then perifused with 3G for 20 min (gap in the traces) and challenged again with 16G for 10 min. Oscillations of free Ca2+ occurred only in the presence of Oxo-M. B) Ca2+ oscillations in wild-type and Gnb5−/− islets. The data show an average of >10 areas of interest selected within 1 islet of each genotype. C) Changes in [Ca2+]i induced by Oxo-M in 3G. Each dot represents the response of 1 islet (region of interest placed around the whole islet). D) Average amplitude of Ca2+ oscillations induced by Oxo-M in 16G. E) Frequency of Ca2+ oscillations induced by Oxo-M in 16G quantified as the number of peaks per minute. C–E) Each dot represents 1 islet (3–4 mice per genotype). F) Enucleated eyes from WT and Gnb5−/− (KO) mice were incubated in HBSS and photographed 60 min after addition of 1 mM pilocarpine. G) Time-course of pilocarpine-induced pupil constriction recorded in eyes from control and Gnb5−/− mice. After stimulation, images were taken at the indicated times, and pupil diameter was measured. Data points are means ± sd from 6 independent experiments on eyes from 3 individual animals of each genotype. H) In situ RNA hybridization of a mouse eye section using probes for mRNAs encoding Gβ5 (Gnb5) and M3R (Chrm3). A representative image shows the sphincter smooth muscle, iris, lens, and lens epithelium (LE).
Impaired pupil constriction in the Gnb5−/− mice
In the course of our studies, we also analyzed the influence of Gβ5 knockout in another physiologic system regulated by endogenous M3R, the pupil constrictor muscle. For this purpose, we compared the rate of pilocarpine-induced pupil constriction in enucleated eyes from wild-type and Gnb5−/− mice (Fig. 5F). The time required for pilocarpine response was at least 2 times shorter in the knockout, indicating that the pathway works more effectively (Fig. 5G). In other words, in the pupil constrictor smooth muscle, the Gβ5 complex works as a negative regulator of M3R-evoked signaling, similar to the M3R/Gβ5-R7 system reconstituted in CHO-K1 cells (25, 35). In situ mRNA hybridization (RNAscope) confirmed the expression of the Gnb5 and Chrm3 genes in the sphincter muscle cells (Fig. 5H). Along with the de facto denervation of the eye in these experiments, the presence of Gβ5 and M3R indicates that this mechanism takes place autonomously within the muscle cell.
Thus, the Gβ5-R7 complex can act as either a positive or a negative regulator of M3R signaling, depending on the biologic system where this receptor is expressed.
DISCUSSION
G-protein signaling plays a crucial role in regulation of glucose-induced insulin secretion. Of note, studies of pancreatic islets led to one of the fundamental findings in G-protein signaling, the discovery of Gi (36). Many novel antidiabetes therapeutic strategies target GPCRs, underscoring the significance of research in this area (37–39). Considering the importance of G-protein signaling, it is surprising how little research has been undertaken on RGS proteins in the β-cell system. One study showed that RGS16 and -8 are expressed and developmentally regulated in the pancreas (40). Mechanistic investigations have thus far been limited to the findings that RGS4 serves as an inhibitor of M3R signaling and forms a complex with M3R and a scaffold protein spinophilin (11, 41).
While investigating the role of the Gβ5-R7 RGS complex, we unexpectedly found that it is essentially as important for cholinergic stimulation of insulin secretion as M3R itself. Indeed, alteration of Gβ5-R7 levels leads to phenotypes resembling previously reported β-cell-selective knockout and overexpression of M3R (42). The Chrm3−/− animals had reduced insulin release, whereas M3R overexpression greatly increased insulin release and improved glucose tolerance. Like the M3R knockout, the knockout of Gβ5 almost completely uncouples cholinergic stimulation from insulin secretion (Fig. 1), and overexpression of Gβ5-RGS7 boosts insulin output (Fig. 2). Using green fluorescent protein as a tracer, we estimated transfection efficiency of MIN6 cells to be ∼20%. In other words, only these 20% of cells can contribute to the increase of the Oxo-M stimulated insulin release (Fig. 2). This finding means that in cells overexpressing Gβ5-RGS7, the magnitude of M3R-stimulated insulin release is considerably higher than in cells that have endogenous levels of Gβ5-R7. The robust effect of Gβ5-R7 overexpression leads us to speculate that the activity of this protein complex may in fact be a limiting factor in coupling M3R to insulin secretion.
Our finding that the Gβ5-R7 complex is a strong stimulator of the insulinotropic activity of M3R (Figs. 1–3) contradicts the general concept of RGS proteins acting as G-protein inhibitors. This result is also at odds with the inhibitory role that the R7 family plays in other biologic systems (13, 18, 22). However, the reduction in M3R-dependent insulin secretion in the absence of Gβ5-R7 is consistent with the decrease in the serum insulin level in the Gβ5-knockout mice (21). Obviously, there are many possible explanations of the Gnb5−/− phenotype, both at the molecular and physiologic levels. For example, because Gβ5-R7 is a GAP for the Gi family, the knockout could have enhanced Gi activity, inhibition of adenylate cyclase and a drop in cAMP, a stimulator of insulin secretion. Alternatively, because Gβ5 is highly expressed in neurons, its knockout could impair neuronal and endocrine inputs to the pancreas. To test which of these possibilities is correct, we performed ex vivo analysis of isolated pancreatic islets (Figs. 1 and 5) and then further reduced the system by investigating the MIN6 cell line (Figs. 2–4).
The loss of the response to cholinergic stimulation in Gnb5-knockout cells and islets indicates that the effect of the Gnb5 ablation in mice can be, at least in part, autonomous. We cannot yet rule out the possibility that this knockout also alters regulation of the pancreas at the physiologic level; analysis of tissue-specific knockouts will determine the relative contribution of local and systemic effects. What we know is that the Gnb5−/− islets respond to glucose normally (Figs. 1A and 5A), which is consistent with the fact that despite the lower insulin level, Gnb5−/− mice can clear glucose (21). Because the effect of Gβ5-R7 is selective for M3R (35; Fig. 2B), Gnb5−/− mice are likely to retain normal responsiveness to insulinotropic inputs via other receptors. Yet, the phenotype of the Gβ5-knockout mice (21) underscores the importance of cholinergic signaling in maintaining the tone of the endocrine pancreas.
Having established that Gβ5 in pancreatic β cells promotes M3R signaling, we began investigating the mechanism underlying this unusual effect (Fig. 3). As mentioned above, one obvious possibility could be that Gβ5-R7 acts via the canonical GAP mechanism inhibiting Gi. However, it is well established that M3R is the only muscarinic receptor in β cells and cholinergic agonists act through M3R and Gq. Accordingly, our data with pertussis toxin and the Gq inhibitor FR support the involvement of Gq, but not Gi (Fig. 3). Even if M3R could activate Gi via some bizarre mechanism, we were unable to detect a change in cAMP that could explain the profound effect of Gβ5-R7 on M3R-stimulated insulin secretion.
Intracellular Ca2+ is essential for insulin secretion, and it is known that Ca2+ is upregulated downstream of M3R; our results are consistent with this concept. Earlier, the Gβ5-R7 complex was shown to regulate M3R-induced Ca2+ upregulation, but this mechanism is nuanced. Overexpression of Gβ5-RGS7 attenuates M3R-induced Ca2+ signaling in transfected cells (16), which is at odds with the evidence that the R7 family does not have GAP activity for Gq/11 (43). This contradiction was resolved by the demonstration that the inhibitory effect of Gβ5-RGS7 is not based on GAP activity, but rather on occlusion of M3R via the direct interaction between M3R and Gβ5-RGS7. The DEP domain of RGS7 binds to a unique region within the third intracellular loop of the receptor, which explains why Gβ5-R7 can attenuate M3R but does not influence other Gq-coupled receptors (24, 35). In this study, we showed for the first time to our knowledge that attenuation of M3R signaling by Gβ5-R7 takes place in a native system, the pupillary sphincter muscle (Fig. 5).
In pancreatic β cells, however, the mechanism of M3R regulation by Gβ5-R7 should be different, because Gβ5-R7 acts as a strong stimulator, rather than an inhibitor of the biologic response. An attractive hypothesis to explain this unexpected effect can be inferred from our earlier study showing that Gβ5-RGS7 can have a dual effect on M3R Ca2+ signaling. While Gβ5-RGS7 suppresses the release of Ca2+ from intracellular stores, it can also promote influx from the extracellular space (25). Stimulation of Ca2+ entry can be a rather straightforward explanation for the proinsulinotropic activity of Gβ5-RGS7 in the M3R pathway. Indeed, extracellular Ca2+ is essential for cholinergic stimulation of insulin secretion (Fig. 3). However, our data showed that the effect on Ca2+ cannot fully explain the insulinotropic action of Gβ5-R7. First, the knockout of Gβ5 did not inhibit M3R-stimulated transients of total Ca2+ level in MIN6 and islets (Figs. 3 and 5). To the contrary, there was a slight increase, an effect consistent with the notion that Gβ5-R7 is inhibitor of M3R. Second, supplying Ca2+ to Gnb5−/− cells by ionomycin did not restore the insulin response to Oxo-M. Third, nifedipine had only a partial inhibitory effect on M3R-stimulated insulin secretion in MIN6 cells, indicating that there should be a substantial source of Ca2+ in addition to its influx from the extracellular space.
Our analysis of Ca2+ in intact islets (Fig. 5A–E) may shed light on how the Gβ5 complex promotes insulin secretion. As in MIN6 cells, the amplitude of Ca2+ responses was not significantly affected by elimination of Gβ5. The level of Chrm3 gene expression in the knockout islets was the same as in WT (data not shown), also supporting the notion that Gβ5 ablation does not impair the overall activity of M3R. However, there was a clear decrease in frequency of Ca2+ oscillations in the presence of Oxo-M and high glucose. It is thought that oscillations protect cells from the toxicity of a sustained elevation of free intracellular Ca2+, while allowing peak Ca2+ to trigger secretory events. Even though it is established that frequency of Ca2+ oscillations is directly related to insulin release, molecular mechanisms supporting the oscillations are unknown (44, 45). Oscillatory behavior of free Ca2+ involves mutually regulating phenomena, such as phosphorylation of ion channels and activation of kinases by Ca2+. Discerning the exact role of Gβ5-R7 in this network will be challenging, but our data showed correlation of the Gnb5 knockout with the changes in M3R-initiated kinase activity. We think that reduced ERK phosphorylation (Fig. 4) is one of the results of this change and that it is more likely that an upstream kinase rather than ERK itself is responsible for the dramatic reduction in M3R-stimulated insulin secretion. Our data implicate PKC in the regulation of M3R signaling by the Gβ5 complex. It has been shown that PKC is responsible for phosphorylation of proteins involved in the secretory machinery (46, 47), and we speculate that the Gβ5-R7 complex can play a role in this mechanism. This idea may be attractive considering the direct interaction between the DEP domain of RGS7 and snapin, a SNARE complex-associated protein (48). Our experiments indicate that activation of kinases such as PKC and EGFR can restore insulinotropic activity of M3R in Gβ5-knockout cells. In contrast, supplying the cells with additional Ca2+ does not provide such rescue, which hints that Gβ5-R7 is more important for kinase activity than for Ca2+ signaling.
In summary, this study showed that in pancreatic β cells, the Gβ5-R7 complex strongly facilitates cholinergic stimulation of insulin secretion. This unique effect is opposite that described in transfected cells or observed in this study in another native system controlled by M3R, the pupillary sphincter muscle. The stimulatory effect of Gβ5-R7 in β cells correlates with an increased ERK1/2 phosphorylation and frequency of Ca2+ oscillations but not with changes in global level of Ca2+ or cAMP. Our findings highlight the importance of Gβ5-R7 in M3R signaling and suggest that tuning the levels of the Gβ5-R7 complex may present a novel therapeutic approach to improving β-cell function.
ACKNOWLEDGMENTS
The authors thank Jongmin Jeon and Junior Tayou (Leonard M. Miller School of Medicine, University of Miami) for sharing technical expertise. This work was supported by U.S. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Grants R01DK105427 (to V.Z.S.), R01DK0090316 and R01DK104753 (to A.F.), and R01DK111538 (to A.C.). The authors declare no conflicts of interest.
Glossary
- AVP
arginine vasopressin
- CCh
carbachol, cholinergic agonist
- DAG
diacylglycerol
- DEP
Disheveled, Egl10, and Pleckstrin
- EGF
epidermal growth factor
- EGFR
EGR receptor
- GAP
GTPase-activating protein
- GGL
G γ-like
- GLP
glucagon-like peptide
- M3R
muscarinic cholinergic receptor type 3
- Oxo-M
oxotremorine-M, a muscarinic cholinergic agonist
- PMA
phorbol myristate acetate
- RGS
regulator of G-protein signaling
AUTHOR CONTRIBUTIONS
All authors participated in designing the research; Q. Wang, A. N. Pronin, K. Levay, and J. Almaca performed experiments; Q. Wang, A. N. Pronin, and J. Almaca analyzed data; and Q. Wang and V. Z. Slepak wrote the paper.
REFERENCES
- 1.Moran B. M., Flatt P. R., McKillop A. M. (2016) G protein-coupled receptors: signalling and regulation by lipid agonists for improved glucose homoeostasis. Acta Diabetol. 53, 177–188 [DOI] [PubMed] [Google Scholar]
- 2.Ruiz de Azua I., Gautam D., Jain S., Guettier J. M., Wess J. (2012) Critical metabolic roles of β-cell M3 muscarinic acetylcholine receptors. Life Sci. 91, 986–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kong K. C., Tobin A. B. (2011) The role of M(3)-muscarinic receptor signaling in insulin secretion. Commun. Integr. Biol. 4, 489–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Selway J., Rigatti R., Storey N., Lu J., Willars G. B., Herbert T. P. (2012) Evidence that Ca2+ within the microdomain of the L-type voltage gated Ca2+ channel activates ERK in MIN6 cells in response to glucagon-like peptide-1. PLoS One 7, e33004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Selway J. L., Moore C. E., Mistry R., John Challiss R. A., Herbert T. P. (2011) Molecular mechanisms of muscarinic acetylcholine receptor-stimulated increase in cytosolic free Ca(2+) concentration and ERK1/2 activation in the MIN6 pancreatic β-cell line. Acta Diabetol. 49, 277–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ross E. M., Wilkie T. M. (2000) GTPase-activating proteins for heterotrimeric G proteins: regulators of G protein signaling (RGS) and RGS-like proteins. Annu. Rev. Biochem. 69, 795–827 [DOI] [PubMed] [Google Scholar]
- 7.Abramow-Newerly M., Roy A. A., Nunn C., Chidiac P. (2006) RGS proteins have a signalling complex: interactions between RGS proteins and GPCRs, effectors, and auxiliary proteins. Cell. Signal. 18, 579–591 [DOI] [PubMed] [Google Scholar]
- 8.Osei-Owusu P., Blumer K. J. (2015) Regulator of G protein signaling 2: a versatile regulator of vascular function. Prog. Mol. Biol. Transl. Sci. 133, 77–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie Z., Chan E. C., Druey K. M. (2016) R4 regulator of G protein signaling (RGS) proteins in inflammation and immunity. AAPS J. 18, 294–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerber K. J., Squires K. E., Hepler J. R. (2016) Roles for regulator of G protein signaling proteins in synaptic signaling and plasticity. Mol. Pharmacol. 89, 273–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruiz de Azua I., Scarselli M., Rosemond E., Gautam D., Jou W., Gavrilova O., Ebert P. J., Levitt P., Wess J. (2010) RGS4 is a negative regulator of insulin release from pancreatic beta-cells in vitro and in vivo. Proc. Natl. Acad. Sci. USA 107, 7999–8004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slepak V. Z. (2009) Structure, function, and localization of Gβ5-RGS complexes. Prog. Mol. Biol. Transl. Sci. 86, 157–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson G. R., Posokhova E., Martemyanov K. A. (2009) The R7 RGS protein family: multi-subunit regulators of neuronal G protein signaling. Cell Biochem. Biophys. 54, 33–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levay K., Cabrera J. L., Satpaev D. K., Slepak V. Z. (1999) Gbeta5 prevents the RGS7-Galphao interaction through binding to a distinct Ggamma-like domain found in RGS7 and other RGS proteins. Proc. Natl. Acad. Sci. USA 96, 2503–2507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snow B. E., Krumins A. M., Brothers G. M., Lee S. F., Wall M. A., Chung S., Mangion J., Arya S., Gilman A. G., Siderovski D. P. (1998) A G protein gamma subunit-like domain shared between RGS11 and other RGS proteins specifies binding to Gbeta5 subunits. Proc. Natl. Acad. Sci. USA 95, 13307–13312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Witherow D. S., Wang Q., Levay K., Cabrera J. L., Chen J., Willars G. B., Slepak V. Z. (2000) Complexes of the G protein subunit gbeta 5 with the regulators of G protein signaling RGS7 and RGS9: characterization in native tissues and in transfected cells. J. Biol. Chem. 275, 24872–24880 [DOI] [PubMed] [Google Scholar]
- 17.Chen C. K., Eversole-Cire P., Zhang H., Mancino V., Chen Y. J., He W., Wensel T. G., Simon M. I. (2003) Instability of GGL domain-containing RGS proteins in mice lacking the G protein beta-subunit Gbeta5. Proc. Natl. Acad. Sci. USA 100, 6604–6609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie K., Ge S., Collins V. E., Haynes C. L., Renner K. J., Meisel R. L., Lujan R., Martemyanov K. A. (2012) Gβ5-RGS complexes are gatekeepers of hyperactivity involved in control of multiple neurotransmitter systems. Psychopharmacology 219, 823–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hooks S. B., Martemyanov K., Zachariou V. (2008) A role of RGS proteins in drug addiction. Biochem. Pharmacol. 75, 76–84 [DOI] [PubMed] [Google Scholar]
- 20.Krispel C. M., Chen D., Melling N., Chen Y. J., Martemyanov K. A., Quillinan N., Arshavsky V. Y., Wensel T. G., Chen C. K., Burns M. E. (2006) RGS expression rate-limits recovery of rod photoresponses. Neuron 51, 409–416 [DOI] [PubMed] [Google Scholar]
- 21.Wang Q., Levay K., Chanturiya T., Dvoriantchikova G., Anderson K. L., Bianco S. D., Ueta C. B., Molano R. D., Pileggi A., Gurevich E. V., Gavrilova O., Slepak V. Z. (2011) Targeted deletion of one or two copies of the G protein β subunit Gβ5 gene has distinct effects on body weight and behavior in mice. FASEB J. 25, 3949–3957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Posokhova E., Wydeven N., Allen K. L., Wickman K., Martemyanov K. A. (2010) RGS6/Gβ5 complex accelerates IKACh gating kinetics in atrial myocytes and modulates parasympathetic regulation of heart rate. Circ. Res. 107, 1350–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang J., Huang J., Maity B., Gao Z., Lorca R. A., Gudmundsson H., Li J., Stewart A., Swaminathan P. D., Ibeawuchi S. R., Shepherd A., Chen C. K., Kutschke W., Mohler P. J., Mohapatra D. P., Anderson M. E., Fisher R. A. (2010) RGS6, a modulator of parasympathetic activation in heart. Circ. Res. 107, 1345–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karpinsky-Semper D., Tayou J., Levay K., Schuchardt B. J., Bhat V., Volmar C. H., Farooq A., Slepak V. Z. (2015) Helix 8 and the i3 loop of the muscarinic M3 receptor are crucial sites for its regulation by the Gβ5-RGS7 complex. Biochemistry 54, 1077–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karpinsky-Semper D., Volmar C. H., Brothers S. P., Slepak V. Z. (2014) Differential effects of the Gβ5-RGS7 complex on muscarinic M3 receptor-induced Ca2+ influx and release. Mol. Pharmacol. 85, 758–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tewson P. H., Martinka S., Shaner N. C., Hughes T. E., Quinn A. M. (2016) New DAG and cAMP sensors optimized for live-cell assays in automated laboratories. J. Biomol. Screen. 21, 298–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pronin A., Levay K., Velmeshev D., Faghihi M., Shestopalov V. I., Slepak V. Z. (2014) Expression of olfactory signaling genes in the eye. PLoS One 9, e96435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishihara H., Asano T., Tsukuda K., Katagiri H., Inukai K., Anai M., Kikuchi M., Yazaki Y., Miyazaki J. I., Oka Y. (1993) Pancreatic beta cell line MIN6 exhibits characteristics of glucose metabolism and glucose-stimulated insulin secretion similar to those of normal islets. Diabetologia 36, 1139–1145 [DOI] [PubMed] [Google Scholar]
- 29.Fridlyand L. E., Harbeck M. C., Roe M. W., Philipson L. H. (2007) Regulation of cAMP dynamics by Ca2+ and G protein-coupled receptors in the pancreatic beta-cell: a computational approach. Am. J. Physiol. Cell Physiol. 293, C1924–C1933 [DOI] [PubMed] [Google Scholar]
- 30.Alejandro E. U., Lim G. E., Mehran A. E., Hu X., Taghizadeh F., Pelipeychenko D., Baccarini M., Johnson J. D. (2011) Pancreatic β-cell Raf-1 is required for glucose tolerance, insulin secretion, and insulin 2 transcription. FASEB J. 25, 3884–3895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zaima K., Deguchi J., Matsuno Y., Kaneda T., Hirasawa Y., Morita H. (2013) Vasorelaxant effect of FR900359 from Ardisia crenata on rat aortic artery. J. Nat. Med. 67, 196–201 [DOI] [PubMed] [Google Scholar]
- 32.Nishimura A., Kitano K., Takasaki J., Taniguchi M., Mizuno N., Tago K., Hakoshima T., Itoh H. (2010) Structural basis for the specific inhibition of heterotrimeric Gq protein by a small molecule. Proc. Natl. Acad. Sci. USA 107, 13666–13671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schrage R., Schmitz A. L., Gaffal E., Annala S., Kehraus S., Wenzel D., Büllesbach K. M., Bald T., Inoue A., Shinjo Y., Galandrin S., Shridhar N., Hesse M., Grundmann M., Merten N., Charpentier T. H., Martz M., Butcher A. J., Slodczyk T., Armando S., Effern M., Namkung Y., Jenkins L., Horn V., Stößel A., Dargatz H., Tietze D., Imhof D., Galés C., Drewke C., Müller C. E., Hölzel M., Milligan G., Tobin A. B., Gomeza J., Dohlman H. G., Sondek J., Harden T. K., Bouvier M., Laporte S. A., Aoki J., Fleischmann B. K., Mohr K., König G. M., Tüting T., Kostenis E. (2015) The experimental power of FR900359 to study Gq-regulated biological processes. Nat. Commun. 6, 10156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leitner M. G., Michel N., Behrendt M., Dierich M., Dembla S., Wilke B. U., Konrad M., Lindner M., Oberwinkler J., Oliver D. (2016) Direct modulation of TRPM4 and TRPM3 channels by the phospholipase C inhibitor U73122. Br. J. Pharmacol. 173, 2555–2569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sandiford S. L., Slepak V. Z. (2009) The Gbeta5-RGS7 complex selectively inhibits muscarinic M3 receptor signaling via the interaction between the third intracellular loop of the receptor and the DEP domain of RGS7. Biochemistry 48, 2282–2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katada T., Bokoch G. M., Northup J. K., Ui M., Gilman A. G. (1984) The inhibitory guanine nucleotide-binding regulatory component of adenylate cyclase. Properties and function of the purified protein. J. Biol. Chem. 259, 3568–3577 [PubMed] [Google Scholar]
- 37.Ahrén B. (2009) Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nat. Rev. Drug Discov. 8, 369–385 [DOI] [PubMed] [Google Scholar]
- 38.Kimple M. E., Neuman J. C., Linnemann A. K., Casey P. J. (2014) Inhibitory G proteins and their receptors: emerging therapeutic targets for obesity and diabetes. Exp. Mol. Med. 46, e102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jain S., Ruiz de Azua I., Lu H., White M. F., Guettier J. M., Wess J. (2013) Chronic activation of a designer G(q)-coupled receptor improves β cell function. J. Clin. Invest. 123, 1750–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villasenor A., Wang Z. V., Rivera L. B., Ocal O., Asterholm I. W., Scherer P. E., Brekken R. A., Cleaver O., Wilkie T. M. (2010) Rgs16 and Rgs8 in embryonic endocrine pancreas and mouse models of diabetes. Dis. Model. Mech. 3, 567–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruiz de Azua I., Nakajima K., Rossi M., Cui Y., Jou W., Gavrilova O., Wess J. (2012) Spinophilin as a novel regulator of M3 muscarinic receptor-mediated insulin release in vitro and in vivo. FASEB J. 26, 4275–4286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gautam D., Gavrilova O., Jeon J., Pack S., Jou W., Cui Y., Li J. H., Wess J. (2006) Beneficial metabolic effects of M3 muscarinic acetylcholine receptor deficiency. Cell Metab. 4, 363–375 [DOI] [PubMed] [Google Scholar]
- 43.Hooks S. B., Waldo G. L., Corbitt J., Bodor E. T., Krumins A. M., Harden T. K. (2003) RGS6, RGS7, RGS9, and RGS11 stimulate GTPase activity of Gi family G-proteins with differential selectivity and maximal activity. J. Biol. Chem. 278, 10087–10093 [DOI] [PubMed] [Google Scholar]
- 44.Tengholm A., Gylfe E. (2009) Oscillatory control of insulin secretion. Mol. Cell. Endocrinol. 297, 58–72 [DOI] [PubMed] [Google Scholar]
- 45.Gylfe E., Tengholm A. (2014) Neurotransmitter control of islet hormone pulsatility. Diabetes Obes. Metab. 16(Suppl 1), 102–110 [DOI] [PubMed] [Google Scholar]
- 46.Shu Y., Liu X., Yang Y., Takahashi M., Gillis K. D. (2008) Phosphorylation of SNAP-25 at Ser187 mediates enhancement of exocytosis by a phorbol ester in INS-1 cells. J. Neurosci. 28, 21–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Malmersjö S., Di Palma S., Diao J., Lai Y., Pfuetzner R. A., Wang A. L., McMahon M. A., Hayer A., Porteus M., Bodenmiller B., Brunger A. T., Meyer T. (2016) Phosphorylation of residues inside the SNARE complex suppresses secretory vesicle fusion. EMBO J. 35, 1810–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hunt R. A., Edris W., Chanda P. K., Nieuwenhuijsen B., Young K. H. (2003) Snapin interacts with the N-terminus of regulator of G protein signaling 7. Biochem. Biophys. Res. Commun. 303, 594–599 [DOI] [PubMed] [Google Scholar]