Abstract
Cell- and tissue-specific actions of glucocorticoids are mediated by the glucocorticoid receptor. Here, we demonstrate that the glucocorticoid receptor (GR) in macrophages is essential for cardiac healing after myocardial infarction. Compared with GRflox (wild-type controls), GRLysMCre mice that lacked GR in myeloid cells showed increased acute mortality as a result of cardiac rupture. Seven days after left coronary artery ligation, GRLysMCre mice exhibited worse cardiac function and adverse remodeling associated with impaired scar formation and angiogenic response to ischemic injury. Inactivation of GR altered the functional differentiation/maturation of monocyte-derived macrophages in the infarcted myocardium. Mechanistically, CD45+/CD11b+/Ly6G−/F4/80+ macrophages isolated from GRLysMCre infarcts showed deregulation of factors that control inflammation, neovascularization, collagen degradation, and scar tissue formation. Moreover, we demonstrate that cardiac fibroblasts sorted from the ischemic myocardium of GRLysMCre mice compared with cells isolated from injured GRflox hearts displayed higher matrix metalloproteinase 2 expression, and we provide evidence that the macrophage GR regulates myofibroblast differentiation in the infarct microenvironment during the early phase of wound healing. In summary, GR signaling in macrophages, playing a crucial role in tissue-repairing mechanisms, could be a potential therapeutic target during wound healing after ischemic myocardial injury.—Galuppo, P., Vettorazzi, S., Hövelmann, J., Scholz, C.-J., Tuckermann, J. P., Bauersachs, J., Fraccarollo, D. The glucocorticoid receptor in monocyte-derived macrophages is critical for cardiac infarct repair and remodeling.
Keywords: fibroblast, myeloid cells, myocardial infarction, wound healing
Myocardial infarction (MI) remains one of the major causes of morbidity and mortality worldwide. Cell death after ischemic injury induces a cascade of cellular events that promote wound healing and complex structural and functional changes that involve the infarcted region and the residual viable myocardium (1, 2). The pivotal role of monocytes/macrophages as regulators and effectors of myocardial wound healing and remodeling has been recognized, identifying an important therapeutic target for improving the repair of the injured myocardium and for preventing progressive functional deterioration, life-threatening arrhythmia, and heart failure (1, 3–6).
Controlled recruitment and coordinated differentiation/maturation of blood monocyte–derived macrophages at the site of ischemic injury are required for efficient wound healing after MI. Accumulation of monocytes/macrophages in the infarcted myocardium involves an early lymphocyte antigen 6 complex, locus C (Ly6C)high inflammatory phase, and a later Ly6Clow phase linked to angiogenesis and the formation of an extracellular matrix (4, 7). A better understanding of the mechanisms that regulate the differentiation of monocyte-derived macrophages in the ischemic microenvironment may lead to the development of novel approaches to promote myocardial repair and minimize remodeling postinfarction.
Synthetic glucocorticoids are widely used for the treatment of inflammatory and immune disorders and hematologic cancers. Glucocorticoids exert profound regulatory effects on components of immunity via different and complex mechanisms and by interfering with several signaling pathways (8). Cell- and tissue-specific and stress-dependent transcriptional responses to glucocorticoids are mediated by the glucocorticoid receptor (GR) (9–11).
Glucocorticoids have been shown to impair infarct healing; however, there are also reports that claim protective effects of glucocorticoids after myocardial ischemia (12–16). Conflicting results in experimental and clinical studies highlight possibly strict cell-specific and microenvironment-dependent effects of glucocorticoids during wound healing after myocardial infarction.
Here, we demonstrate a crucial role of the myelomonocytic GR in promoting wound healing after myocardial infarction. GRLysMCre mice that lack GR in myeloid cells exhibit exacerbated cardiac remodeling and function, impaired collagen scar formation, and angiogenic response to ischemic injury. Deletion of the GR alters the differentiation of monocyte-derived macrophages in the infarct microenvironment during the early phase of wound healing, which results in the deregulation of factors that control inflammation, neovascularization, collagen degradation, and scar tissue formation.
MATERIALS AND METHODS
Study protocol
All animal experiments were in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD, USA; publication no. 85–23, revised 1985). All procedures were approved by the Niedersächsisches Landesamt für Verbraucherschutz und Lebensmittelsicherheit (Oldenburg, Germany; permit no. 33.12-42502-04-11/0644). Adult Nr3c1tm2Gsc (GRflox, wild-type controls) and Nr3c1tm2GscLyz2tm1(cre)lfo/J (GRLysMCre) mice of both sexes were used in this study. All mice that were bred as homozygous for GR are referred to as flox/flox, whereas mice heterozygous for the Cre recombinase are referred to as tg/+ bred with +/+ animals, meaning that all wild-type mice in the experiments are GR flox/flox; +/+ and all mutant mice are GR flox/flox; tg/+.
Mice that were subjected to coronary artery ligation were euthanized at d 1, 3, or 7 after surgery. Mice were excluded from the analyses for two reasons: perioperative death—within the first 12 h after surgery—and MI size <40%.
MI
MI was induced by permanent left coronary artery ligation (17, 18) in age- and gender-matched mice. In brief, mice were anesthetized with 1.8% isoflurane in a 100% oxygen mix, intubated, and ventilated using a ventilator (MiniVent mouse ventilator model 845; Harvard Apparatus, Holliston, MA, USA) with the tidal volume adjusted on the basis of body weight (10 µl/g body weight). Buprenorphine (0.1 mg/kg body weight) was intraperitoneally administered for postoperative pain relief. The thoracic cavity was opened via an incision between the third and fourth intercostal space. A rib spreader was introduced to allow for visualization of the heart. The left coronary artery was ligated with 6-0 silk suture just below the left auricular level. In sham-treated mice, the suture passing around the left anterior descending coronary artery was not tied.
Hemodynamic and volume measurements
Hemodynamic and volume measurements were performed 7 d after coronary artery ligation under light isoflurane anesthesia and spontaneous respiration by using conductance catheter (SPR-839; Millar Instruments, Houston, TX, USA) connected to a data acquisition system (Chart 5; ADInstruments PowerLab, Lahore, Pakistan). Pvan software (Millar Instruments) was used to analyze all pressure-volume loop data recorded at steady state and during the injection of hypertonic saline for the calibration of parallel conductance volume (Vp). Left ventricle (LV) volume was calculated for each mouse from conductance volume corrected by the relative Vp (17, 18).
Infarct size and scar collagen content
The heart was arrested in diastole with potassium chloride. Thin sections (5 μm) were serially cut from apex to the base and stained with 0.1% Sirius red F3B in saturated picric acid. Infarct size—fraction of the infarcted LV—was quantified histologically by planimetry and expressed as a percentage of length. For scar collagen content, LV sections were examined by using a Nikon ECLIPSE 50i microscope (Nikon, Tokyo, Japan) equipped with filters to provide circularly polarized illumination. Tissue images were recorded with a cooled digital camera (DS-5Mc; Nikon) with a magnification ×200 and analyzed using SigmaScan Pro 5.0 image analysis software (Systat Software, San Jose, CA, USA). Collagen content was expressed as a percentage of the area of each image (17, 18).
Immunohistochemistry
For immunohistochemical analysis, LV frozen 5-μm sections were stained by using primary Abs against CD31 (MCA2388; Bio-Rad, Hercules, CA, USA) and α-smooth muscle actin (α-SMA; VPS281; Vector Laboratories, Burlingame, CA, USA), a biotinylated goat anti-rat Ab, mouse adsorbed (BA-9401; Vector Laboratories), and Mouse on Mouse Kit (PK-2200; Vector Laboratories). Dual immunohistochemical staining was performed by using 3,3’-diaminobenzidine (DAB) Substrate Kit (550880; BD Biosciences, San Jose, CA, USA) for CD31 and the HistoGreen horseradish peroxidase substrate kit (E109; Linaris GmbH, Mannheim, Germany) for α-SMA. Sections were counterstained with Hematoxylin QS (H-3404; Vector Laboratories).
For immunofluorescence double staining, after avidin/biotin blocking (SP-2001; Vector Laboratories), sections were blocked with 2% donkey serum and incubated overnight with primary Ab against CD68 (MCA1957; Bio-Rad) followed by incubation with Cy3 AffiniPure Donkey Anti-Rat (712-165-153; Jackson ImmunoResearch Labs, West Grove, PA, USA). Subsequently, sections were stained by using the Mouse on Mouse Kit, primary Abs against TGF-β1 (MCA797; Bio-Rad) or matrix metalloproteinase 13 (MMP-13; MA5-14247; Thermo Fisher Scientific, Waltham, MA, USA), and DyLight488 Streptavidin (SA-5488; Vector Laboratories). Nuclei were stained with NucBlue Live Cell Stain (R37605; Molecular Probes, Eugene, OR, USA).
Isolation of cardiac monocytes and macrophages and fibroblasts and myofibroblasts
A method aimed at preserving antigens and morphology was developed to obtain a single-cell suspension from mouse heart after MI (19). In brief, hearts were perfused for 5 min with the perfusion buffer (113 mM NaCl, 4.7 mM KCl, 0.6 mM KH2PO4, 0.6 mM Na2HPO4, 1.2 mM MgSO4, 12 mM NaHCO3, 10 mM KHCO3, 10 mM HEPES, 30 mM taurine, 5.5 mM glucose, 10 mM 2,3-butanedione monoxime, pH 7.46), and subsequently digested for 8 min with the digestion buffer (0.2 mg/ml liberase DL research grade and 400 µM calcium chloride in perfusion buffer) using a modified Langendorff perfusion system. The last 5 ml of the digestion buffer running through the heart were collected in a Petri dish, the heart was transferred, and the ischemic area and surviving myocardium were separated by using a dissecting microscope. Subsequently, heart tissue was pipetted smoothly several times through a sterile low-waste syringe to obtain a cell suspension, and the enzyme activity was inhibited with 5 ml of stop buffer [perfusion buffer supplemented with 10% (v/v) heat-inactivated fetal calf serum]. Cell suspension was carefully filtered through a 100-µm cell strainer in a 50-ml conical tube, and the cell strainer was washed with 30 ml of perfusion buffer. Cell suspension was then carefully centrifuged at 10 g for 4 min and supernatant was filtered through a 70-µm cell strainer followed by centrifugation at 400 g for 20 min. Pelleted cells were washed and resuspended in fluorescence-activated cell sorting (FACS) staining buffer (PBS, pH 7.2, supplemented with 0.5% bovine serum albumin and 2 mM EDTA).
FACS
To prevent capping of Abs on the cell surface and nonspecific cell labeling, all steps were performed on ice and were protected from light. Cells were resuspended in FACS staining buffer and preincubated with Fc Block (Mouse BD Fc Block; BD Biosciences) for 10 min. Subsequently, fluorochrome-conjugated Abs (Supplemental Table 1) were added and incubated for 30 min. Finally, cells were washed twice with ice-cold FACS staining buffer. After preselection in side scatter vs. forward scatter dot plot to exclude debris and forward scatter vs. time-of-flight dot plot to discriminate doublets by gating single cells, monocyte-derived macrophages were identified as CD45+/CD11b+/Ly6G−/F4/80+ cells, then further stratified by Ly6C and major histocompatibility complex class II expression. Fibroblasts and myofibroblasts were identified as CD31−/TER-119−/CD45−/CD11b−/NG2−/PDGFRβ− and MEFSK4+ cells (19).
Cells were sorted in sterile sorting medium [DMEM supplemented with 5% (v/v) HI-FCS] or lysis buffer (PN78766, USB, PreEase RNA Spin Kit; Affymetrix, Santa Clara, CA, USA). Our approach allowed for the preservation of cell surface antigens along with cell viability, which allowed us to obtain highly purified monocytes and macrophages as well as fibroblasts from ischemic mouse myocardium, with maintenance of the phenotype. Fluorescence minus one controls were included during acquisition for gating analyses to distinguish positively from negatively staining cell populations. FACS data were acquired on a Gallios flow cytometer and analyzed with Gallios software (Beckman Coulter, Brea, CA, USA). Cell sorting was performed using a FACS-Aria Fusion cell sorter (BD Biosciences) at the Research Facility Cell Sorting of the Hannover Medical School. Peripheral leukocytes were examined by using hematology analyzer (XT 2000i; Sysmex, Kobe, Japan).
Immunocytofluorescence
For immunocytochemical staining, sorted MEFSK4+ cells were seeded in chamber slides (80381; Ibidi, Martinsried, Germany) and allowed to attach in DMEM that was supplemented with 5% HI-FCS for 12 h. Cells were subsequently fixed for 10 min with BD Cytofix (5546555; BD Biosciences) and permeabilized by using 0.1% Triton-X in PBS. Abs included Alexa Fluor 594–labeled anti-vimentin (7675; Cell Signaling Technology, Danvers, MA, USA) and FITC-labeled anti–α-SMA (F3777; Sigma-Aldrich, St. Louis, MO, USA). For morphologic characterization of sorted monocyte-derived macrophages, cells were cytocentrifuged onto slides, air-dried, and fixed for 10 min with BD Cytofix. Abs included Alexa Fluor 594–labeled anti-CD68 (137020; BioLegend, San Diego, CA, USA) and Alexa Fluor 488–labeled anti-Ly6C (128022; BioLegend). Nuclei were stained with NucBlue Live Cell Stain.
Zymography and reverse zymography
Sorted MEFSK4+ cells were cultured in DMEM that was supplemented with 5% HI-FCS for 12 h, then incubated in serum-free medium for 12 h. Conditioned medium was centrifuged at 2000 g for 10 min, mixed with loading buffer, and electrophoresed on SDS polyacrylamide gel that contained 2 mg/ml of gelatin under nonreducing conditions. Gelatinolytic bands were quantified by using image software Quantity One (Bio-Rad). Reverse zymography was performed in a similar manner, except that purified MMP-2 (30 ng/ml, PF023; Calbiochem, San Diego, CA, USA) was incorporated into a 12% SDS polyacrylamide gel with 2 mg/ml of gelatin (6).
RNA sequencing
Total RNA was isolated by using PrepEase RNA Spin Kit (PN78766, USB; Affymetrix) according to manufacturer instructions with minor modifications. Sorted cells were directly collected in lysis buffer and immediately processed. RNA quantification and quality testing were assessed by NanoDrop 2000 (Thermo Fisher Scientific) and Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA, USA).
Libraries for RNA sequencing were prepared from 20 ng of total RNA. From each sample, poly-A RNA was purified, converted to cDNA, and linked to Illumina adapters by using the Illumina TruSeq stranded mRNA Kit according to manufacturer instructions (Illumina, San Diego, CA, USA). Samples were multiplexed and sequenced on an Illumina NextSeq 500 in a 75-nt single-end setting using a high-output run mode. Raw BCL files were demultiplexed and converted to sample-specific FASTQ files using bcl2fastq v.1.8.4 (Illumina). Residual adapter sequences that were present in the sequencing reads were removed with Cutadapt v.1.12 (https://cutadapt.readthedocs.org/). Reads were aligned to the mouse reference sequence GenCode vM8 (https://www.gencodegenes.org/) using Star v.2.5.2b (https://code.google.com/archive/p/rna-star/). RNA sequencing data analysis was undertaken with the statistical programming language, R (The R Foundation, Vienna, Austria). The R package DeSeq2 (v.1.14.1) was used to evaluate differential gene expression.
Quantitative RT-PCR
cDNA synthesis was performed by using 10 ng of total RNA and iScript Reverse Transcription Supermix (Bio-Rad). Relative quantitation of mRNA expression levels was determined with CFX96 Touch Real Time PCR by using SsoAdvanced Universal SYBR Green Supermix, PrimePCR Primers, or Assays [Wound healing (SAB target list); Bio-Rad]. Glyceraldehyde-3-phosphate dehydrogenase was chosen as endogenous control. PCR amplification was performed initially at 95°C for 2 min followed by 40 cycles at 95°C for 5 s and terminated by 60°C for 30 s. The δ-δ Ct method was used for data analysis.
Statistical analysis
Results are reported as means ± sem. An unpaired Student’s t test was applied to evaluate differences between 2 study groups. Analysis of survival rate was performed by using the Kaplan-Meier method followed by log-rank test. Values of P < 0.05 were considered statistically significant.
RESULTS
GR DNA expression is significant reduced in bone marrow–derived macrophages and alveolar macrophages from GRLysMCre animals (Supplemental Fig. 1A). Furthermore, GR protein expression is significantly down-regulated in bone marrow–derived macrophages from GRLysMCre animals compared with GRflox mice (Supplemental Fig. 1B).
We did not detect differences in the number of peripheral blood monocytes and circulating monocyte subsets Ly6Chigh and Ly6Clow between GRflox and GRLysMCre mice (Supplemental Fig. 1C, D).
Mice that lack GR in myeloid cells showed increased acute mortality and exhibited worse cardiac function and remodeling after MI
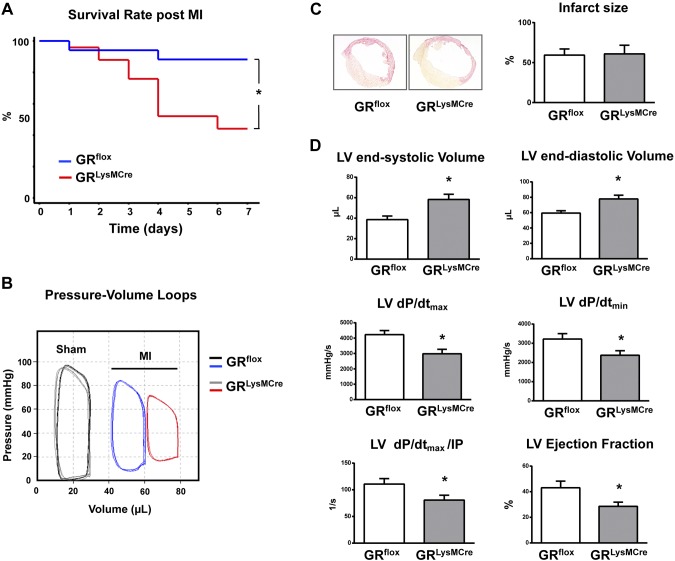
GRLysMCre mice that were subjected to permanent occlusion of the left coronary artery demonstrated increased acute mortality mainly as a result of cardiac rupture: 56% of GRLysMCre mice (males, 11 of 14; females, 3 of 11) compared with 12% of GRflox mice (males, 2 of 7; females, 0 of 10) died until d 7 after MI. Rupture occurred primarily between 3 and 4 d post-MI (Fig. 1A).
Figure 1.
GRLysMCre mice demonstrated increased mortality rates as a result of LV rupture and exacerbated cardiac remodeling and function after myocardial infarction. A) Kaplan-Meier survival curve. B) Representative LV pressure–volume loops measured in vivo with conductance catheter in GRflox and GRLysMCre mice 7 d after sham operation (sham) or MI. C) Representative sections from GRflox and GRLysMCre infarcted hearts and infarct size. D) LV end-systolic and end-diastolic volumes, LV maximal rate of pressure rise (LV dP/dtmax), maximal rate of pressure decline (LV dP/dtmin), and LV dP/dtmax divided by instantaneous pressure (IP) and LV ejection fraction. Means ± sem (n = 10; male/female: 3/7). *P < 0.01 vs. GRflox.
We did not detect differences in LV systolic or diastolic pressure or cardiac volume or function between GRflox and GRLysMCre sham-treated mice (Fig. 1B and Supplemental Table 2).
MI size was similar among GRflox and GRLysMCre mice (Fig. 1C). At 7 d, conductance catheter measurements revealed enhanced adverse remodeling in GRLysMCre mice, as shown by the marked rightward shift of the pressure-volume curve (Fig. 1B). Correspondingly, GRLysMCre mice exhibited significantly increased LV end-systolic and end-diastolic volumes compared with control GRflox mice. Aggravation of early LV remodeling in infarcted GRLysMCre mice was associated with more severely impaired LV ejection fraction, LV dP/dtmax, LV dP/dtmin, and LV dP/dtmax divided by instantaneous pressure, a load-independent measure of contractile function (Fig. 1D).
GR deficiency in GRLysMCre mice targets monocytes and neutrophils (20). To exclude a major role for GR in neutrophils during MI healing, GRloxP mice were crossed with Nr3c1tm2GscTg(Itgax-cre)1-1Reiz (CD11cCre) mice. GRCD11cCre mice demonstrated efficient recombination of GRloxP allele in monocytes, but did not exhibit any recombination in neutrophils (9). Of note, compared with wild-type littermates, infarcted GRCD11cCre animals showed increased acute mortality as a result of cardiac rupture and exacerbated cardiac function 7 d after MI. Mortality occurred primarily between 3 and 4 d after coronary artery ligation, as in GRLysMCre mice (Supplemental Fig. 2A–C). These data suggest that ablation of GR in monocytes and macrophages is sufficient to adversely affect cardiac infarct repair.
Impaired collagen scar formation and angiogenic response to ischemic injury in GRLysMCre mice
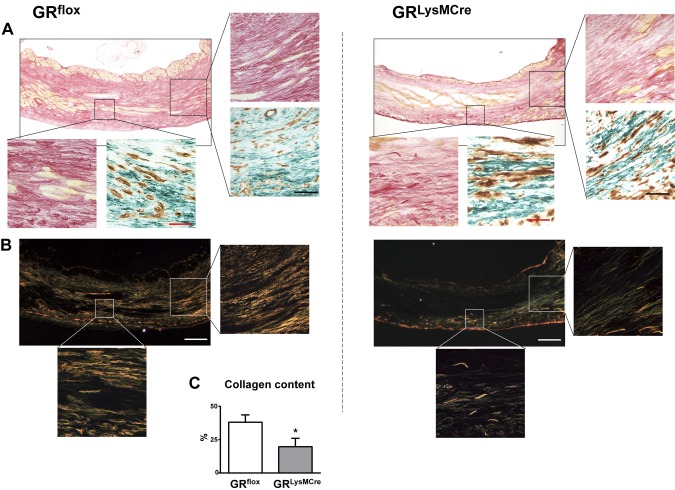
Compared with GRflox mice, GRLysMCre animals exhibited reduced collagen content in scars 7 d after MI (Fig. 2A–C). Sirius red polarization microscopy revealed an impaired collagen network (Fig. 2B) with disarrayed, loosely arranged, and fragmented collagen fibers. As shown in Figs. 2A and 3A, both GRflox and GRLysMCre infarcts were infiltrated with myofibroblasts, identified as extramural spindle-shaped, α-SMA–positive cells. Considerably larger myofibroblasts were found in the infarcted myocardium of GRLysMCre mice (Figs. 2A and 3A, enlarged insets).
Figure 2.
Impaired collagen scar formation in GRLysMCre mice. A) Sirius red staining of scar sections and immunohistochemical staining for α-SMA (green) and CD31 (brown) showing myofibroblasts (spindle-shaped α-SMA–positive cells) and neovessel (enlarged insets) in GRflox and GRLysMCre mice. B, C) Sirius red polarization microscopy of scar sections revealed a disorganized collagen matrix (B) and less scar collagen content in GRLysMCre compared with GRflox mice (C) 7 d after MI. Red bars, 50 μm; black bars, 100 µm; white bars, 250 µm. Means ± sem (n = 6). *P < 0.05 vs. GRflox.
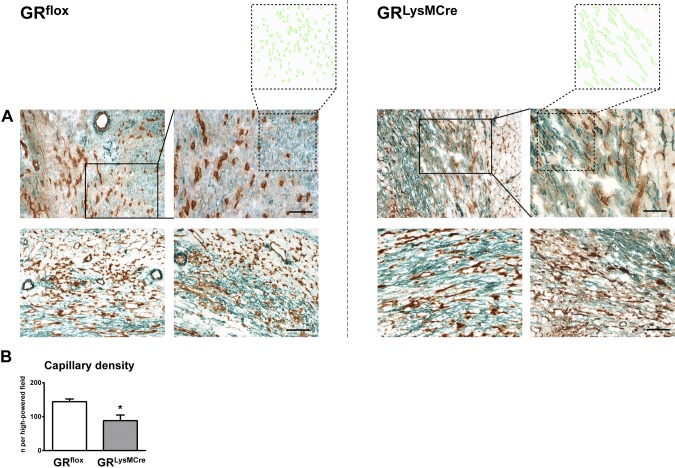
Figure 3.
Impaired angiogenic response to ischemic injury in GRLysMCre mice. A) Immunohistochemical staining (CD31, brown; α-SMA, green) showing capillaries, coated vessels, and myofibroblasts in the healing myocardium of GRflox and GRLysMCre mice 7 d after MI. Neovasculature in GRLysMCre mice seems to be immature and collapsed, as indicated by a lack of open lumen in the CD31+ vessels. Myofibroblasts appear considerably larger compared with those in GRflox mice (enlarged insets). B) Capillary density quantification in GRflox and GRLysMCre mice 7 d after MI. Scale bars, 100 µm; enlarged insets, 50 µm. Means ± sem (n = 6). *P < 0.05 vs. GRflox.
Moreover, we observed impaired neovascularization in the healing myocardium of GRLysMCre mice 7 d after MI (Fig. 3). Immunohistochemical studies revealed decreased capillaries, identified as small lumen vessels that lacked smooth muscle layers and that stained positively for CD31 (Fig. 3A, B). As the neovasculature matures, many vessels acquire a muscular coat, which leads to the stabilization of the infarct scar. Of note, fewer α-SMA–positive vessels were found in the infarcted myocardium of GRLysMCre mice, and immunohistochemical staining with CD31 and α-SMA disclosed mostly collapsed vessels. These results indicate that GR in myeloid cells plays a critical role in promoting scar tissue and neovessel formation at the site of ischemic injury.
Inactivation of GR alters the differentiation/maturation of monocyte-derived macrophages in infarcted myocardium
To identify monocyte/macrophage-specific mechanisms that lead to impaired healing in GRLysMCre mice, we investigated whether the deletion of GR resulted in alterations in monocyte/macrophage subsets.
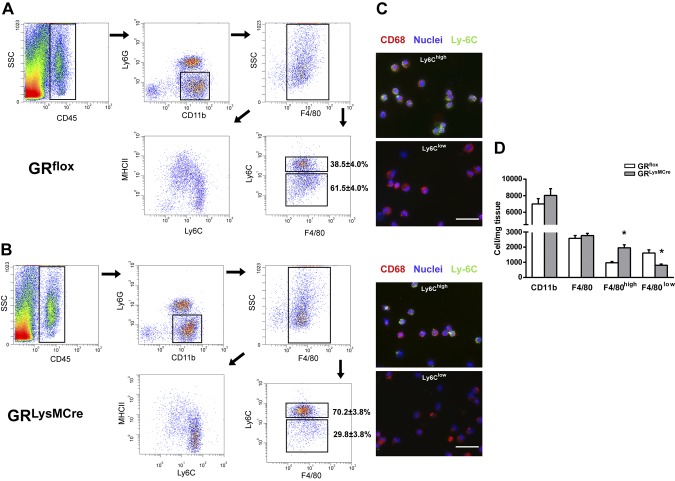
By using FACS analysis, we demonstrate that resident CD45+/CD11b+/Ly6G−/F4/80+ macrophages were entirely Ly6Clow before MI (Supplemental Fig. 3). In contrast, F4/80+ monocytes/macrophages were mostly Ly6Chigh at the site of ischemic injury on day 1 post-MI (Supplemental Fig. 3). Differentiation/maturation of monocyte-derived macrophages is characterized by loss of Ly6C expression (7, 21, 22). At d 3, corresponding to the transition from the inflammatory to the reparative/proliferative phase of infarct wound healing, the number of CD45+/CD11b+ cells as well as CD45+/CD11b+/Ly6G−/F4/80+ macrophages was similar in GRLysMCre compared with GRflox controls (Fig. 4D); however, the number of Ly6Chigh and Ly6Clow macrophages was significantly different. F4/80+ Ly6Chigh cells were the predominant subset in the infarct area of GRLysMCre mice (Fig. 4B, D).
Figure 4.
Deletion of GR affects the differentiation/maturation of monocyte-derived macrophages in infarcted myocardium. A, B) Cells were sorted from GRflox (A) and GRLysMCre (B) infarcts 3 d after coronary artery ligation. Monocyte-derived macrophages were identified as CD45+/CD11b+/Ly6G−/F4/80+ cells, then further stratified by Ly6C and major histocompatibility complex class II expression. C) Cytospin preparation of sorted F4/80+/Ly6Chigh and F4/80+/Ly6Clow cells. Scale bars, 50 µm. D) FACS-based quantification of cells per milligram tissue. Means ± sem (n = 5). *P < 0.05 vs. GRflox.
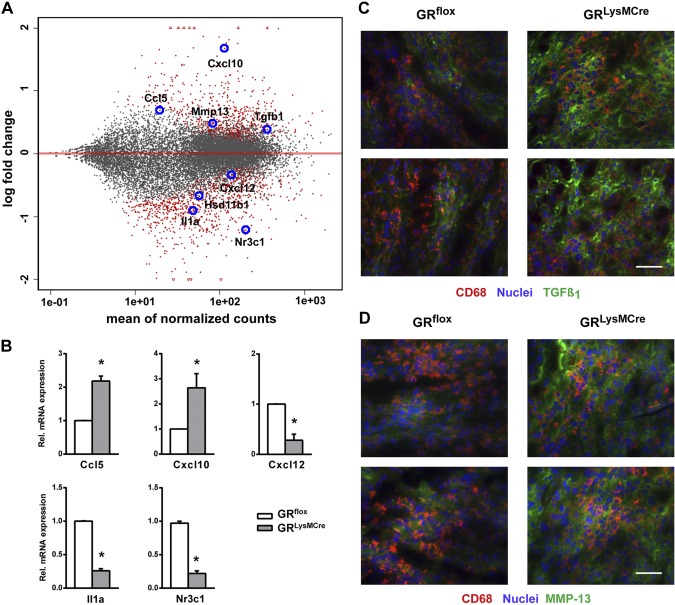
Furthermore, we sought to identify phenotypic differences between monocyte-derived macrophages that were sorted from ischemic myocardium of GRLysMCre and GRflox mice 3 d after MI. Gene expression analysis revealed that GR was significantly down-regulated in CD45+/CD11b+/Ly6G−/F4/80+ macrophages that were sorted from GRLysMCre infarcts vs. GRflox (Fig. 5A, B). In addition, we found that monocyte-derived macrophages lacking GR showed deregulation of factors that control inflammation, neovascularization, collagen degradation, and scar tissue formation (Fig. 5A). Macrophages that were sorted from GRLysMCre infarcts demonstrated up-regulation of the proinflammatory chemokine CCL5 (Fig. 5A, B). Of interest, neutralization of CCL5 bioactivity during the early phases of ischemia has been shown to improve cardiac function, postinfarction heart failure, and survival (23).
Figure 5.
Monocyte-derived macrophages that were sorted from GRLysMCre infarcts showed deregulation of inflammatory and wound healing–related factors. A) MA plot of the contrast GRLysMCre vs. GRflox monocyte-derived macrophages sorted from GRflox and GRLysMCre infarcts 3 d after coronary artery ligation. Differential gene expression was derived from RNA sequencing analysis; significantly regulated genes (red) are shown. B) RT-PCR was used to detect the relative gene expression of CCL5, CXCL10, CXCL12, interleukin-1α (IL-1α), and GR (Nr3c1). Immunofluorescence double-staining for CD68 and TGF-β1 (C) and CD68 and the collagenase-3 (MMP-13; D). Intense immunoreactivity for TGF-β1 and MMP-13 was identified in the infarcted myocardium of GRLysMCre mice 3 d after MI. Scale bars, 50 µm. Means ± sem (n = 4–5). *P < 0.05 vs. GRflox.
Of note, expression levels of the angiostatic chemokine, CXCL10, were significantly enhanced, whereas the proangiogenic chemokine, CXCL12, was down-regulated in CD45+/CD11b+/Ly6G−/F4/80+ macrophages that were isolated from GRLysMCre infarcts (Fig. 5A, B).
Moreover, expression of IL-1α, a cytokine that plays a central role in delaying myofibroblast differentiation during the inflammatory phase of healing (2, 24–26), was substantially reduced in GR-knockout monocyte-derived macrophages (Fig. 5A, B). Of interest, monocyte-derived macrophages that were sorted from the ischemic myocardium of GRLysMCre mice showed up-regulation of TGF-β1 and MMP-13. Furthermore, we demonstrated increased immunoreactivity for TGF-β1 and MMP-13 as well as MMP-13 and TGF-β1–positive macrophages in the infarcted myocardium of GRLysMCre mice 3 d after MI (Fig. 5C, D).
Gene expression data analysis further revealed that, at d 3, the expression of 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) was up-regulated in monocyte-derived macrophages (3.8-fold vs. resident macrophages, d 0; P < 0.01), and was significantly down-regulated in CD45+/CD11b+/Ly6G−/F4/80+ macrophages that were sorted from GRLysMCre infarcts vs. GRflox.
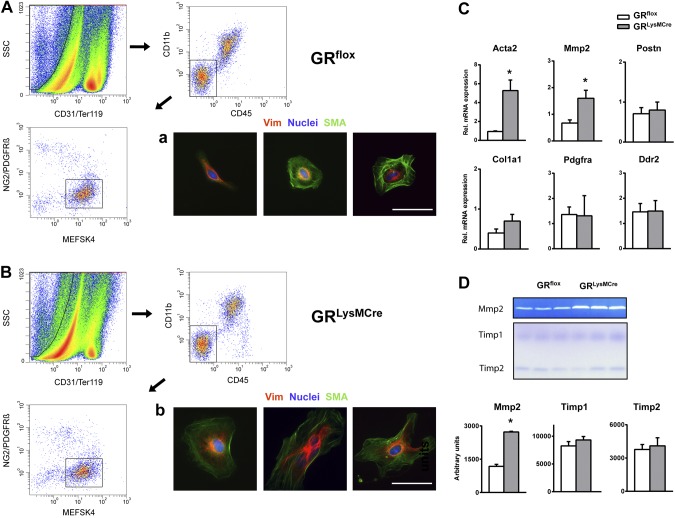
Macrophage GR controls myofibroblast differentiation during the early phase of infarct healing
Cardiac fibroblasts sense microenvironmental signals and phenotypically differentiate into activated α-SMA–positive myofibroblasts. Accordingly, we investigated whether GR in monocytes/macrophages controls fibroblast activation in the infarct microenvironment during the early phase of healing. We developed an ad hoc protocol for the efficient isolation of cardiac fibroblasts/myofibroblasts from the ischemic mouse myocardium, with preservation of the phenotype, 3 d post-MI (Fig. 6A, B). Quantitative RT-PCR indicated that cardiac fibroblasts that were sorted from GRLysMCre infarcts compared with cells that were isolated from injured GRflox hearts displayed substantially higher α-SMA and MMP-2 gene expression (Fig. 6C). Immunocytochemical staining of isolated fibroblasts for α-SMA supported the RT-PCR findings (Fig. 6A, B). Furthermore, we found that fibroblasts that were isolated from GRLysMCre ischemic myocardium secreted more MMP-2 compared with cells from injured GRflox hearts. In contrast, reverse zymograms demonstrated that tissue inhibitor of metalloproteinase 1 and 2 inhibitory activity was not significantly different (Fig. 6D). Thus, inactivation of GR signaling affecting the differentiation/maturation of monocyte-derived macrophages in the infarct microenvironment likely resulted in altered myofibroblast differentiation.
Figure 6.
Macrophage GR as a crucial regulator of myofibroblast differentiation in the infarct microenvironment. A, B) FACS strategy to obtain highly purified fibroblasts and myofibroblasts from GRflox (A) and GRLysMCre (B) ischemic myocardium 3 d after coronary artery ligation. Endothelial, hematopoietic, and vascular cells were excluded by selecting cells that are CD31−/TER-119−, CD45−/CD11b−, and NG2−/PDGFRβ−. Immunocytochemical staining of sorted MEFSK4+ cells showing vimentin (Vim) and α-SMA. Scale bars = 50 µm. C) RT-PCR was used to detect the relative gene expression of α-SMA (Acta2), MMP-2, periostin (Postn), collagen I α1 (Col1a1), platelet-derived growth factor receptor α (Pdgfra), and discoidin domain-containing receptor 2 (Ddr2). D) Zymography and reverse zymography of conditioned medium from cardiac fibroblasts isolated from GRflox/GRLysMCre infarcts. Means ± sem (n = 3–4). *P < 0.05 vs. GRflox.
DISCUSSION
Our current study highlights a pivotal role of the myelomonocytic GR in promoting wound healing after MI. Ablation of GR alters the functional differentiation/maturation of monocyte-derived macrophages in the infarcted myocardium, which results in an impaired postischemic angiogenic response and scar formation. GRLysMCre mice demonstrated increased acute mortality and exhibited worse cardiac function and exacerbated cardiac remodeling after MI.
Several monocyte/macrophage-specific mechanisms may account for the impaired wound healing in GRLysMCre mice.
GR deficiency resulted in the compromised differentiation/maturation of Ly6Chigh monocytes into Ly6Clow macrophages. Optimal healing after MI requires coordinated differentiation/maturation of blood monocyte–derived macrophages at the site of ischemic injury. The biphasic macrophage response involves an early F4/80+ Ly6Chigh inflammatory phase and a later F4/80+ Ly6Clow phase, linked to inflammation resolution, neovascularization, and the formation of a collagen scar (2, 4, 5, 7). We showed that F4/80+ Ly6Chigh cells were the predominant subset in the ischemic myocardium of GRLysMCre mice. Strikingly, gene expression analysis revealed that GR deletion promoted macrophage differentiation toward a proinflammatory phenotype linked to the deregulation of healing-related factors that critically control angiogenesis and scar tissue formation.
Formation and maturation of new vessels is a key event in infarct wound healing and fibrous tissue formation. Up-regulation of the proinflammatory and angiostatic chemokine, CXCL10, together with the down-regulation of the proangiogenic chemokine, CXCL12, in GR knockout monocyte-derived macrophages, likely contributed to impaired neovascularization and wound healing in GRLysMCre mice. The angiogenic response is tightly regulated by a balance of anti- and proangiogenic factors within the infarct microenvironment and requires well-coordinated interactions between immune cells, endothelial cells, pericytes, and the extracellular matrix (1, 2). Transient induction of CXCL10 during the first hours after ischemic injury is important in the inhibition of premature angiogenesis until a provisional matrix is formed, whereas subsequent CXCL10 down-regulation plays an essential role for neovessel and granulation tissue formation (27). In contrast, CXCL12 regulates monocyte-derived macrophage differentiation toward cells with proangiogenic functions (28) and plays an essential role in ischemia-mediated physiologic angiogenesis (29, 30).
Infarct wound healing is a complex process that involves a coordinated cascade of sequential cellular events. During the proliferative phase, fibroblasts phenotypically differentiate to myofibroblasts that are responsible for producing the extracellular matrix in the wound microenvironment (31). Macrophages provide regulatory factors and mediators that orchestrate the phenotypic and functional changes of fibroblasts, and critically regulate the synthesis and organization of collagen deposition in wound healing (32, 33). We found that monocyte-derived macrophages that were sorted from GRLysMCre infarcts showed increased expression of TGF-β1, a key mediator of fibroblast activation. Moreover, IL-1α, a pleiotropic cytokine that critically regulates scar tissue formation (24, 25), was down-regulated in GR-knockout monocyte-derived macrophages. Of note, IL-1α release, which inhibits α-SMA expression, prevents premature differentiation of fibroblasts into matrix-synthetic myofibroblasts during the inflammatory phase of wounding after MI (2, 26). Our findings suggest that in the absence of GR, impaired macrophage differentiation associated with up-regulation of TGF-β1 and decreased IL-1α expression may result in perturbed myofibroblast differentiation, finally culminating in adverse cardiac repair.
Current knowledge of fibroblast and myofibroblast biology and physiology has been hampered by the absence of specific cardiac fibroblast markers (34–36). Pinto et al. (35) recently demonstrated that the Ab, mEF-SK4, labeled cardiac fibroblasts with high sensitivity and specificity. Our optimized FACS protocol using the mEF-SK4 Ab allowed for the preservation of cell surface antigens along with cell viability, which permitted us to obtain highly purified fibroblasts from the ischemic myocardium, with maintenance of the phenotype. Of interest, we observed that cardiac fibroblasts and myofibroblasts that were sorted from GRLysMCre infarcts compared with cells that were isolated from injured WT hearts displayed higher α-SMA and MMP-2 expression, which was indicative of a matrix-degrading fibroblast phenotype. Overall, our results provide in vivo evidence that the macrophage GR regulates myofibroblast differentiation in the infarct microenvironment during the early phase of wound healing.
Increased expression of the collagenase, MMP-13, in GR-deficient macrophages, associated with enhanced secretion of MMP-2 by myofibroblasts, likely contributed to the impaired collagen network in GRLysMCre mice. Formation of well-organized scar tissue is a crucial stage of infarct repair. A poorly structured matrix renders the scar less resistant to distension during overload, which leads to ventricular dilation and cardiac dysfunction.
Glucocorticoid concentrations are regulated by the hypothalamic-pituitary-adrenal axis, but 11β-HSD1 is the major regulator of the tissue-specific action of glucocorticoids, increasing active glucocorticoid levels within cells and tissues (13). 11β-HSD1 is expressed in cardiomyocytes, vascular smooth muscle cells, fibroblasts, myofibroblasts, and recruited inflammatory cells. Increased 11β-HSD1 expression in monocyte-derived macrophages might be a significant contributor to intracellular glucocorticoid levels and activation of GR in the infarcted myocardium; however, the specific source of glucocorticoids that activate the myelomonocytic GR during the early phase of infarct healing must be investigated in future studies.
Ultimately, GR signaling in macrophages—playing an important role in tissue-repairing mechanisms—may be a potential therapeutic target for the prevention of adverse cardiac dilation and functional deterioration after MI. Thus, targeting of glucocorticoids to macrophages using surface-engineered liposomes or nanoparticles might allow novel treatment paradigms. Liposome-encapsulated glucocorticoids selectively repress proinflammatory macrophage responses under inflammatory conditions, and, of interest, glucocorticoid-loaded liposomes are currently being evaluated in patients who suffer from autoinflammatory diseases and cancer (37, 38).
ACKNOWLEDGMENTS
The authors thank Margarete Göbel from the Core Unit Systems Medicine (University of Würzburg) for excellent technical assistance. J.H. was supported by a research grant from the German Cardiac Society. The authors declare no conflicts of interest.
Glossary
- 11β-HSD1
11β-hydroxysteroid dehydrogenase type 1
- FACS
fluorescence-activated cell sorting
- GR
glucocorticoid receptor
- Ly6C
lymphocyte antigen 6 complex, locus C
- Ly6G
lymphocyte antigen 6 complex, locus G
- LV
left ventricle
- MI
myocardial infarction
- MMP
matrix metalloproteinase
- SMA
smooth muscle actin
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
P. Galuppo, J. Bauersachs, and D. Fraccarollo conceived and designed the study; P. Galuppo, C.-J. Scholz, and D. Fraccarollo analyzed the data; P. Galuppo, S. Vettorazzi, J. Hövelmann, C.-J. Scholz, and D. Fraccarollo performed the experiments; S. Vettorazzi and J. P. Tuckermann gave suggestions for the study and critically revised the manuscript; J. P. Tuckermann and J. Bauersachs obtained funding; P. Galuppo and D. Fraccarollo wrote the manuscript; and J. Bauersachs critically revised the manuscript.
REFERENCES
- 1.Fraccarollo D., Galuppo P., Bauersachs J. (2012) Novel therapeutic approaches to post-infarction remodelling. Cardiovasc. Res. 94, 293–303 [DOI] [PubMed] [Google Scholar]
- 2.Prabhu S. D., Frangogiannis N. G. (2016) The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ. Res. 119, 91–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frantz S., Hofmann U., Fraccarollo D., Schäfer A., Kranepuhl S., Hagedorn I., Nieswandt B., Nahrendorf M., Wagner H., Bayer B., Pachel C., Schön M. P., Kneitz S., Bobinger T., Weidemann F., Ertl G., Bauersachs J. (2013) Monocytes/macrophages prevent healing defects and left ventricular thrombus formation after myocardial infarction. FASEB J. 27, 871–881 [DOI] [PubMed] [Google Scholar]
- 4.Dutta P., Nahrendorf M. (2015) Monocytes in myocardial infarction. Arterioscler. Thromb. Vasc. Biol. 35, 1066–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinberger T., Schulz C. (2015) Myocardial infarction: a critical role of macrophages in cardiac remodeling. Front. Physiol. 6, 107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fraccarollo D., Galuppo P., Schraut S., Kneitz S., van Rooijen N., Ertl G., Bauersachs J. (2008) Immediate mineralocorticoid receptor blockade improves myocardial infarct healing by modulation of the inflammatory response. Hypertension 51, 905–914 [DOI] [PubMed] [Google Scholar]
- 7.Hilgendorf I., Gerhardt L. M., Tan T. C., Winter C., Holderried T. A., Chousterman B. G., Iwamoto Y., Liao R., Zirlik A., Scherer-Crosbie M., Hedrick C. C., Libby P., Nahrendorf M., Weissleder R., Swirski F. K. (2014) Ly-6Chigh monocytes depend on Nr4a1 to balance both inflammatory and reparative phases in the infarcted myocardium. Circ. Res. 114, 1611–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cain D. W., Cidlowski J. A. (2017) Immune regulation by glucocorticoids. Nat. Rev. Immunol. 17, 233–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vettorazzi S., Bode C., Dejager L., Frappart L., Shelest E., Klaßen C., Tasdogan A., Reichardt H. M., Libert C., Schneider M., Weih F., Henriette Uhlenhaut N., David J. P., Gräler M., Kleiman A., Tuckermann J. P. (2015) Glucocorticoids limit acute lung inflammation in concert with inflammatory stimuli by induction of SphK1. Nat. Commun. 6, 7796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hübner S., Dejager L., Libert C., Tuckermann J. P. (2015) The glucocorticoid receptor in inflammatory processes: transrepression is not enough. Biol. Chem. 396, 1223–1231 [DOI] [PubMed] [Google Scholar]
- 11.Desmet S. J., De Bosscher K. (2017) Glucocorticoid receptors: finding the middle ground. J. Clin. Invest. 127, 1136–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mannisi J. A., Weisman H. F., Bush D. E., Dudeck P., Healy B. (1987) Steroid administration after myocardial infarction promotes early infarct expansion. A study in the rat. J. Clin. Invest. 79, 1431–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gray G. A., White C. I., Castellan R. F., McSweeney S. J., Chapman K. E. (2017) Getting to the heart of intracellular glucocorticoid regeneration: 11β-HSD1 in the myocardium. J. Mol. Endocrinol. 58, R1–R13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hafezi-Moghadam A., Simoncini T., Yang Z., Limbourg F. P., Plumier J. C., Rebsamen M. C., Hsieh C. M., Chui D. S., Thomas K. L., Prorock A. J., Laubach V. E., Moskowitz M. A., French B. A., Ley K., Liao J. K. (2002) Acute cardiovascular protective effects of corticosteroids are mediated by non-transcriptional activation of endothelial nitric oxide synthase. Nat. Med. 8, 473–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giugliano G. R., Giugliano R. P., Gibson C. M., Kuntz R. E. (2003) Meta-analysis of corticosteroid treatment in acute myocardial infarction. Am. J. Cardiol. 91, 1055–1059 [DOI] [PubMed] [Google Scholar]
- 16.Seropian I. M., Toldo S., Van Tassell B. W., Abbate A. (2014) Anti-inflammatory strategies for ventricular remodeling following ST-segment elevation acute myocardial infarction. J. Am. Coll. Cardiol. 63, 1593–1603 [DOI] [PubMed] [Google Scholar]
- 17.Fraccarollo D., Berger S., Galuppo P., Kneitz S., Hein L., Schütz G., Frantz S., Ertl G., Bauersachs J. (2011) Deletion of cardiomyocyte mineralocorticoid receptor ameliorates adverse remodeling after myocardial infarction. Circulation 123, 400–408 [DOI] [PubMed] [Google Scholar]
- 18.Fraccarollo D., Galuppo P., Sieweke J. T., Napp L. C., Grobbecker P., Bauersachs J. (2015) Efficacy of mineralocorticoid receptor antagonism in the acute myocardial infarction phase: eplerenone versus spironolactone. ESC Heart Fail. 2, 150–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fraccarollo D., Galuppo P., Bauersachs J. (2017) Modeling cardiac fibrosis in mice: (myo)fibroblast phenotype after ischemia. In Methods in Molecular Biology, Fibrosis: Methods and Protocols (Rittié L., ed.), Springer Nature, South Yarra, VIC, Australia: [DOI] [PubMed] [Google Scholar]
- 20.Tuckermann J. P., Kleiman A., Moriggl R., Spanbroek R., Neumann A., Illing A., Clausen B. E., Stride B., Förster I., Habenicht A. J., Reichardt H. M., Tronche F., Schmid W., Schütz G. (2007) Macrophages and neutrophils are the targets for immune suppression by glucocorticoids in contact allergy. J. Clin. Invest. 117, 1381–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sunderkötter C., Nikolic T., Dillon M. J., Van Rooijen N., Stehling M., Drevets D. A., Leenen P. J. (2004) Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J. Immunol. 172, 4410–4417 [DOI] [PubMed] [Google Scholar]
- 22.Geissmann F., Manz M. G., Jung S., Sieweke M. H., Merad M., Ley K. (2010) Development of monocytes, macrophages, and dendritic cells. Science 327, 656–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montecucco F., Braunersreuther V., Lenglet S., Delattre B. M., Pelli G., Buatois V., Guilhot F., Galan K., Vuilleumier N., Ferlin W., Fischer N., Vallée J. P., Kosco-Vilbois M., Mach F. (2012) CC chemokine CCL5 plays a central role impacting infarct size and post-infarction heart failure in mice. Eur. Heart J. 33, 1964–1974 [DOI] [PubMed] [Google Scholar]
- 24.Saxena A., Chen W., Su Y., Rai V., Uche O. U., Li N., Frangogiannis N. G. (2013) IL-1 induces proinflammatory leukocyte infiltration and regulates fibroblast phenotype in the infarcted myocardium. J. Immunol. 191, 4838–4848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bujak M., Dobaczewski M., Chatila K., Mendoza L. H., Li N., Reddy A., Frangogiannis N. G. (2008) Interleukin-1 receptor type I signaling critically regulates infarct healing and cardiac remodeling. Am. J. Pathol. 173, 57–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frangogiannis N. G. (2016) The functional pluralism of fibroblasts in the infarcted myocardium. Circ. Res. 119, 1049–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frangogiannis N. G., Mendoza L. H., Lewallen M., Michael L. H., Smith C. W., Entman M. L. (2001) Induction and suppression of interferon-inducible protein 10 in reperfused myocardial infarcts may regulate angiogenesis. FASEB J. 15, 1428–1430 [DOI] [PubMed] [Google Scholar]
- 28.Sánchez-Martín L., Estecha A., Samaniego R., Sánchez-Ramón S., Vega M. A., Sánchez-Mateos P. (2011) The chemokine CXCL12 regulates monocyte-macrophage differentiation and RUNX3 expression. Blood 117, 88–97 [DOI] [PubMed] [Google Scholar]
- 29.Ridiandries A., Tan J. T. M., Bursill C. A. (2016) The role of CC-chemokines in the regulation of angiogenesis. Int. J. Mol. Sci. 17, 1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ridiandries A., Tan J. T., Ravindran D., Williams H., Medbury H. J., Lindsay L., Hawkins C., Prosser H. C., Bursill C. A. (2017) CC-chemokine class inhibition attenuates pathological angiogenesis while preserving physiological angiogenesis. FASEB J. 31, 1179–1192 [DOI] [PubMed] [Google Scholar]
- 31.Chen B., Frangogiannis N. G. (2017) Immune cells in repair of the infarcted myocardium. [E-pub ahead of print] Microcirculation doi:10.1111/micc.12305 [DOI] [PubMed] [Google Scholar]
- 32.Minutti C. M., Knipper J. A., Allen J. E., Zaiss D. M. (2017) Tissue-specific contribution of macrophages to wound healing. Semin. Cell Dev. Biol. 61, 3–11 [DOI] [PubMed] [Google Scholar]
- 33.Snyder R. J., Lantis J., Kirsner R. S., Shah V., Molyneaux M., Carter M. J. (2016) Macrophages: a review of their role in wound healing and their therapeutic use. Wound Repair Regen. 24, 613–629 [DOI] [PubMed] [Google Scholar]
- 34.Zhou P., Pu W. T. (2016) Recounting cardiac cellular composition. Circ. Res. 118, 368–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinto A. R., Ilinykh A., Ivey M. J., Kuwabara J. T., D’Antoni M. L., Debuque R., Chandran A., Wang L., Arora K., Rosenthal N. A., Tallquist M. D. (2016) Revisiting cardiac cellular composition. Circ. Res. 118, 400–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tillmanns J., Hoffmann D., Habbaba Y., Schmitto J. D., Sedding D., Fraccarollo D., Galuppo P., Bauersachs J. (2015) Fibroblast activation protein alpha expression identifies activated fibroblasts after myocardial infarction. J. Mol. Cell. Cardiol. 87, 194–203 [DOI] [PubMed] [Google Scholar]
- 37.Cao J., Naeem M., Noh J.-K., Lee E. H., Yoo J.-W. (2015) Dexamethasone phosphate-loaded folate-conjugated polymeric nanoparticles for selective delivery to activated macrophages and suppression of inflammatory responses. Macromol. Res. 23, 485–492 [Google Scholar]
- 38.Pentecost A. E., Lurier E. B., Spiller K. L. (2016) Nanoparticulate systems for controlling monocyte/macrophage behavior. In Microscale Technologies for Cell Engineering (Singh A., Gaharwar A. K., ed.), pp. 292–302, Springer International Publishing, Cham, Switzerland: [Google Scholar]