Abstract
Tumor cell extravasation is a multistep process preceded by cell rolling and arrest on the vessel wall via the formation of specific receptor–ligand bonds. The strength, availability, and number of receptor–ligand bonds regulate the rate by which tumor cells tether, roll, and adhere to vascular walls. Although the mechanics of selectin-mediated rolling have been extensively studied, little is known regarding how tumor cell rolling on selectins facilitates adhesion to a distinct substrate-bound protein with different kinetic properties. By using multicomponent protein patterning and a microfluidic system, we evaluated how E-selectin-dependent rolling modulates hyaluronic acid (HA) adhesion as a function of fluid shear, contact time, and the spacing between E-selectin and HA regions patterned on the substrate. We show that tumor cells rolling on E-selectin were ∼40-fold more likely to bind to HA than nonrolling cells in shear flow. Furthermore, E-selectin-dependent rolling promotes adhesion to HA by both physically slowing cells and enabling them to position proximal to the surface, thereby increasing the on rate of adhesion. A better understanding of tumor cell adhesion under physiologic shear would lead to the development of new diagnostic assays and pave the way to clinical approaches aimed ultimately to halt metastasis.—Shea, D. J., Li, Y. W., Stebe, K. J., Konstantopoulos, K. E-selectin-mediated rolling facilitates pancreatic cancer cell adhesion to hyaluronic acid.
Keywords: PODXL, CD44v, cell rolling
Metastasis is a multistep process, in which cancer cells separate from the primary tumor and enter the circulatory system where they extensively interact with the endothelial vessel wall before extravasating into the tissue space and eventually colonizing a distal organ. Before extravasation, tumor cells first roll and then arrest on the vessel wall via the formation of distinct receptor–ligands bonds. The probability of binding depends on the frequency of collision between cell membrane–bound ligands and endothelial receptors, the strength of these bonds, and the time scale of these adhesive interactions (1–4).
E-selectin and hyaluronic acid (HA) are vital for the cell–cell interactions pertinent to cancer cell rolling and arrest on the vessel wall. E-selectin is expressed on activated vascular endothelial cells and promotes the tethering and rolling of cancer cells (5–7). Podocalyxin (PODXL) and mucin (MUC)-16 are the major functional ligands of E-selectin that are expressed on pancreatic tumor cells (8, 9). Both MUC16– and PODXL–E-selectin bonds have been demonstrated to facilitate cell rolling on E-selectin at high shear stresses and at relatively low ligand and receptor site densities (1). HA is a major component of the extracellular matrix in most tissues and is upregulated on the surface of endothelial cells in response to inflammatory stimulation (10, 11). CD44, expressed on Pa03c pancreatic cancer cells (Supplemental Fig. S1), is the major counterreceptor for HA (12–14) and has been implicated in pancreatic cancer metastasis (15). HA binding to CD44 has been shown to increase cancer invasion and metastasis (16, 17). Specifically, the HA–CD44 bond can initiate slow cell rolling (12, 18, 19) and mediate stationary (firm) adhesion at low shear stresses (18, 19).
To explore the potential serial nature by which E-selectin-dependent rolling facilitates pancreatic cancer cell adhesion to HA, we used multicomponent micropatterning to coat E-selectin and HA in geometrically defined patterns on a glass substrate. Multicomponent micropatterning has been used to assess cell adhesion in the presence or absence of shear flow (20–22) and to separate circulating tumor cells from leukocytes and other circulating cells (21, 22). However, limitations exist with most multicomponent systems, as typically only simple geometries can be patterned (21, 22) or chemical reactions are essential to patterning the complex geometries (21). Our system uses a flow-based coating method to generate geometrically distinct patterns with different proteins patterned micrometers from one another on a glass substrate (20, 23). This method allowed us to pattern both E-selectin and HA spaced 30–120 µm apart in defined geometric patterns and evaluate how E-selectin-dependent rolling modulates pancreatic cancer cell adhesion to HA.
In the current study, rolling on E-selectin facilitated pancreatic cancer cell adhesion to HA. Rolling cells were ∼40-fold more likely to adhere to HA at both low and high shear stresses than were nonrolling cells. E-selectin-dependent rolling on patches <40 μm in length was sufficient to increase binding to HA, provided that the spacing between the E-selectin and HA patches was ≤60 µm. The knockdown of the major E-selectin receptor PODXL attenuated rolling on E-selectin but did not decrease the rate of adhesion on HA, provided that cells had previously rolled on E-selectin, presumably via MUC16-E-selectin binding. This study uncovered the physical interdependence of the MUC16/PODXL-E-selectin and CD44v-HA bonds and showed how selectin-mediated cancer cell rolling facilitated adhesion to a distinct molecular moiety. The knowledge of tumor cell adhesion under physiologic shear flow can be used for the development of improved diagnostic assays and clinical approaches to stop the metastatic spread of pancreatic tumor cells.
MATERIALS AND METHODS
Cell culture
Human pancreatic adenocarcinoma Pa03c cells were obtained from the American Type Culture Collection (Manassas, VA, USA). Pa03c PODXL-knockdown (KD) and scrambled control (SC) cells were generated as described elsewhere (8, 9). All Pa03c cells were cultured in DMEM with 10% fetal bovine serum with 700 µg/ml G418 and 0.5 µg/ml puromycin added to the PODXL-KD and SC media, respectively (Thermo Fisher Scientific, Waltham, MA, USA).
Cell lysate and Western blot analysis
Whole-cell lysate of Pa03c cells was generated as published (8, 9, 24). Lysates were separated by a 3–8% Tris-acetate SDS-PAGE gel. Proteins were then transferred to an immunoblot PVDF membrane and blocked for 30 min in blocking buffer. Membranes were stained with anti-CD44 mAb (2C5) and rinsed with TBS/0.1% Tween 20, before incubation with horseradish peroxidase–conjugated secondary antibody (25, 26). SuperSignal West Pico chemiluminescent substrate was used to develop the immunoblot.
Patterning device fabrication
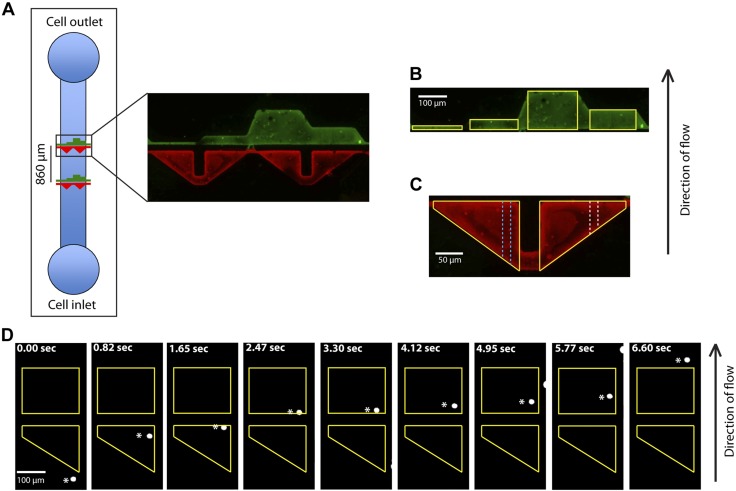
The microfluidic device used for patterning glass slides with E-selectin and HA was fabricated by established methods (2). The polydimethylsiloxane (PDMS) pattern features channels with a height of 10 μm that contain triangular (red) or rectangular (green) regions used to pattern E-selectin and HA, respectively (Fig. 1A). The triangular (E-selectin) regions have a 200-μm base and a 160-μm side, whereas the 4 rectangular (HA) regions have a 10-, 40-, 80-, and 160-μm width and 200-μm base (Fig. 1A). The triangular and rectangular regions align such that in the direction of flow, a cell flowing over E-selectin will next pass over the HA-patterned region. The gap distance between the E-selectin- and HA-patterned regions was set to 30, 60, or 120 μm. The geometries and dimensions of the patterned regions, as well as the gap distances, were selected so that cells have different encounter times with E-selectin or HA. Inlet and outlet holes (3-mm diameter) were punched in both the top and bottom channels to allow the proteins to flow through the device.
Figure 1.
The microfluidic device showing the distinct E-selectin- and HA-patterned regions on the substrate. A) The E-selectin (red)- and HA (green)-patterned regions separated by a gap distance of 30 µm in the direction of flow in the microfluidic device. B) Four HA patterned regions with lengths (in the direction of flow) of 10, 40, 160, and 80 μm are outlined in yellow. C) 2 E-selectin regions are outlined in yellow. Each region is divided laterally into ten 20-µm-wide sections of decreasing lengths in the direction of flow. Two sections of different lengths are depicted by the white and blue dotted lines. E-selectin and HA patterned regions were identified after labeling with Alexa-488 (green) and Alexa-568 (red), respectively. D) Pa03c pancreatic cancer cell (white asterisks) rolling on E-selectin region and adhering to the HA region (yellow).
E-selectin and HA patterning
A precleaned glass slide and the PDMS patterning device were plasma treated for 2 min. After the cleaning, the slide was attached to the patterning device. HA (50 μg/ml) was added to the inlet of the top channel, and PBS was added to the inlet of the bottom channel. The device was incubated overnight at 4°C. Next, the nonbound HA in the top channel was removed via washing with PBS. Concurrently, the PBS in the bottom channel was aspirated, and FITC-conjugated goat anti-human IgG Fc fragment–specific antibody (Millipore-Sigma, St. Louis, MO, USA) was added to the inlet of the bottom channel. The antibody solution was removed via washing with PBS before adding recombinant E-selectin Fc chimera at a concentration of 10 μg/ml. The binding of the goat anti-human IgG Fc antibody to the Fc epitope of E-selectin chimera ensures the correct orientation of the E-selectin molecule (1, 27). After E-selectin incubation, the PDMS device was disassembled, and the slide was washed and blocked in 1% bovine serum albumin (BSA) in DPBS for at least 1 h before the onset of the cell flow experiment.
Microfluidic flow-based adhesion device fabrication
The microfluidic device was fabricated with standard practices using a previously established methodology (2). The dimensions of the device channel (Fig. 1A) used in this experiment were 2 cm × 1000 µm × 22.5 µm (length × width × height).
Microfluidic flow-based adhesion and rolling assays
The microfluidic flow chamber was cleaned in ethanol and dried with air before assembly on top of the patterned glass slide. BSA (0.1%) in PBS was introduced into the inlet channel (Fig. 1A) and allowed to flow to equilibrate the device. The device was placed onto the stage of an inverted microscope (TE300; Nikon, Tokyo, Japan), and 100 μl of Pa03c SC or PODXL-KD cells suspended in 0.1% BSA in PBS (1 × 106 cells/ml) was added to the inlet channel and allowed to flow via gravity. A ×10 field of view (0.69 mm2) was used to obtain images, and each perfusion experiment lasted at least 3 min. Shear stresses of 0.5 and 1 dyn/cm2 correspond to shear rates of 56 and 112 s−1, respectively.
Flow cytometry
The surface expression levels of CD44 and RHAMM on Pa03c SC and PODXL-KD cells were quantified by flow cytometry using an anti-CD44 (Clone 515; BD Biosciences, San Jose, CA, USA) and an anti-RHAMM (clone 2F2C9; Novus, Littleton, CO, USA) monoclonal antibody, respectively, along with matched isotope control antibodies and an appropriate Alexa Fluor-488-conjugated secondary antibody. Flow cytometry was performed with a FACSCalibur (BD Biosciences), and data analysis was performed with FlowJo (Ashland, OR, USA).
Data analysis
To track cells over the course of a 3-min video microscopy experiment, we used the code package kindly provided by Eric M. Furst (University of Delaware, Newark, DE, USA). We set the maximum cell radius to a value of 21 µm, and the size of a pixel in the x and y dimensions was calculated to be 1.51 and 1.32 µm/pixel, respectively. The code output is a matrix of cell position and time data. In our analysis, we excluded incomplete cell tracks, which mainly contained cell fragments. We also eliminated cell aggregates (typically doublets), which enter the field of view within 2 radii of one another. By hand, we also excluded regions of the video where incoming cells contact cells already bound to the substrate.
Data processing
Using MatLab (Natick, MA, USA), we analyzed the fluorescent images of the patterned surfaces to identify the boundaries of E-selectin and HA regions. The triangular E-selectin region was then divided laterally into ten 20-μm-wide sections of decreasing lengths in the direction of flow (Fig. 1C). The 10 sections have a patch length ranging from 10 to 160 μm. The 4 HA patterned regions with lengths (in the direction of flow) of 10, 40, 160, and 80 μm were also identified. The MatLab code analyzes each cell track with respect to these regions and indicates the patch length of E-selectin and HA the cell encounters, whether the cell binds to either region (rolling or stationary adhesion), the overall velocity of the cell, the cell velocity in each region and the hydrodynamic velocity of all noninteracting cells. The gap velocity is defined as the velocity of the cell as it passes over the gap region. Interacting cells were identified if they were located in a binding region (E-selectin or HA) and had an instantaneous velocity <50% of the instantaneous hydrodynamic velocity of unbound cells for at least 5 frames (0.17 s). Stationary or transiently adherent cells on HA were classified with a cutoff threshold of 2 s. Cells adhering to HA were also confirmed via a manual inspection. As a result of this process, data can be compiled easily for a large number of cells, and the different combinations of rolling and stationary adhesion parameters can be easily analyzed.
Data acquisition validation
To validate the automated data analysis, cells from 3 selected videos were tracked both manually and automatically. Both of the data sets were run through data processing, and the results were compared. Matched cell tracks were then compared by using the Bland-Altman analysis to evaluate the 2 measurement methods.
Mathematical model of 2-D selectin-mediated cell adhesion on micropatches
The model for selectin binding to 2-dimensional micropatches used here has been discussed in detail (1, 2, 28). The calculations consider all bonds to break simultaneously (28). The f/τ was set to be 689 pN/dyn-cm2, assuming the cell is behaving more like a neutrophil than a bead (29), and considering the Pa03c cell radius, which was measured to be 10.1 ± 2.6 μm. The kinetic constants for the CD44v-HA bond used were koff° = 0.005 s−1 and xβ = 0.59 nm, determined via single-molecule force spectroscopy (12).
Statistical analysis
Data are expressed as means ± sem or 95% confidence intervals from at least 3 independent experiments. Statistical significance of the differences between means was determined by 1-way ANOVA, followed by the either the Tukey or Sidak test for multiple comparisons, the Student’s t test, or the 2-proportion z test, where appropriate.
RESULTS
Microfluidic device design and cell tracking
Using a microfluidic chamber and the Pa03c pancreatic cancer cell line as a cell model, binding was tracked as cells serially encountered patches of E-selectin (supporting tethering or rolling or both) and HA (slow rolling or stationary adhesion) in shear flow. The triangular E-selectin region as opposed to the discrete HA patches (Fig. 1B, C) was selected to expose cells to a continuum of encounter E-selectin lengths in a single experiment. E-selectin and HA patches varied in length from 10 to 160 μm in geometrically defined regions with a gap distance between the 2 patterned regions ranging from 30 to 60 to 120 μm (Fig. 1A–C). Cells were tracked as they flowed over the E-selectin, gap (BSA-coated) and HA regions at prescribed shear stresses. As a cell passes over the triangular E-selectin region, its path was recorded as it encountered one of the ten 20-μm-wide sections with E-selectin lengths ranging from 10 to 160 μm (Fig. 1C). After exiting the E-selectin region, the gap distance and HA patch length that the individual cell encountered were also recorded. Each cell track was then analyzed with respect to these regions, and the cell position, velocity, and binding to either the E-selectin or HA regions were identified.
To validate this methodology, our automated tracking system was compared directly against manually tracked cells; no differences in cell position or cell speed were found for cells encountering either the E-selectin or HA regions (Supplemental Fig. S2A, C, E). Excellent agreement was found using the two methods, as evidenced by the Bland-Altman analysis (Supplemental Fig. S2B, D, F). Given strong agreement of the two methods (Supplemental Fig. S2G), we tracked cells in our subsequent experiments using the automated tracking system.
E-selectin-dependent rolling modulates cell adhesion to HA
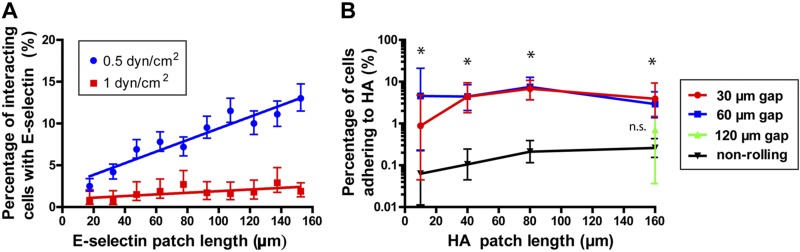
Pa03c cells were tracked as they passed over the E-selectin, gap, and HA regions sequentially. Cells either interacted with E-selectin/HA via the formation of specific receptor–ligand bonds or passed over these regions without binding. Four outcomes were possible: a cell could bind to either the E-selectin or HA regions, to both the E-selectin and HA regions, or to neither of the regions; no binding was detected on the gap (BSA-coated) region. Nonbinding cells passed over the patches at the hydrodynamic cell velocity. Cells interacting with E-selectin displayed a clear rolling behavior (Fig. 1D). The fraction of interacting cells decreased with increasing the shear stress from 0.5 to 1 dyn/cm2 and increased with E-selectin patch length because of increased selectin–ligand contact times (Fig. 2A). These trends are similar to those of MUC16- or PODXL-coated microspheres interacting with E-selectin, as recently described (1). Rolling cells on E-selectin began to increase their average speed once they flowed over the gap region (Supplemental Fig. S3) (30).
Figure 2.
E-selectin-dependent rolling of Pa03c cells increases the frequency of adhesion to HA. A) The percentage of Pa03c cells interacting with E-selectin increases with increasing patch length. B) The proportion of adhesion to HA for cells that roll (red and blue) or that do not roll on E-selectin (black) before their binding to HA for gap distances of 30, 60, or 120 μm and a shear stress of 0.5 dyn/cm2. Data are means ± 95% confidence intervals. Of note, no Pa03c cells adhered to HA patches with lengths less than 160 µm after having encountered a 120-µm gap (green) subsequent to their E-selectin-mediated rolling. *P < 0.01 for both the 30- and 60-μm gaps (red and blue) compared to the nonrolling cells (black).
After passing over the E-selectin and gap regions, cells encountered HA patches with discrete lengths of 10, 40, 80, and 160 μm in the direction of flow (Fig. 1). Cell-HA interactions were typically characterized by prolonged, stationary adhesion ranging from >2 s up to several min, whereas a lower fraction of cells displayed slow rolling on HA. Cells, that previously did not roll on E-selectin, bound infrequently on HA, even though this low binding frequency modestly increased with increasing HA patch lengths (Fig. 2B). It is noteworthy that the HA binding frequency was markedly increased when cells first rolled on E-selectin before encountering HA, provided that the gap distance was ≤60 µm. Thus, E-selectin-dependent rolling cells were ∼40-fold more likely to bind to HA, and the increased binding was nearly constant at all HA patch lengths. When the gap distance increased to 6 cell diameters (120 μm), cells approached their hydrodynamic velocity before flowing over the HA region and behaved like cells that did not first roll on E-selectin. Furthermore, Pa03c cells, pretreated with soluble HA (100 µg/ml) and which rolled on E-selectin first, displayed a marked capacity to subsequently adhere to substrate-immobilized HA because of competitive inhibition of the CD44v receptor function (Supplemental Fig. S4).
Cell velocity over the gap region influences Pa03c cell adhesion to HA
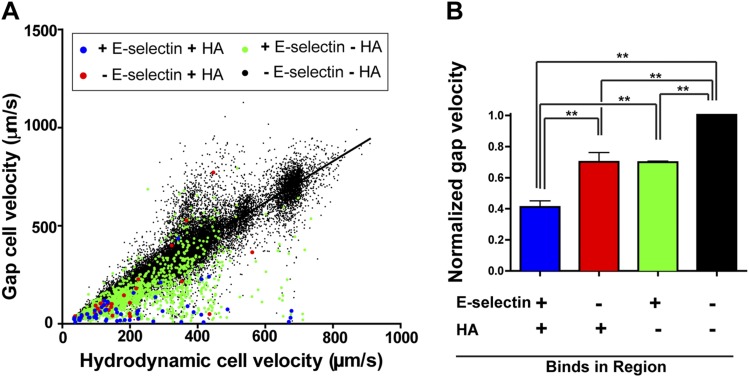
We next examined the binding and velocities of Pa03c pancreatic cancer cells as they passed over and interacted with the E-selectin and HA regions. Clear trends were distinguished for the 4 different binding categories upon plotting the cell velocity over the gap region vs. the hydrodynamic velocity for every cell (Fig. 3). Noninteracting cells (defined as those that failed to bind to E-selectin and HA) have a gap velocity equal to the hydrodynamic velocity, indicating that these cells do not slow down in either of the patterned regions (Fig. 3B). Cells which bind to HA but not E-selectin had a depressed gap velocity when compared to noninteracting cells. E-selectin binding but non-HA binding cells rolled on E-selectin at velocities lower than the hydrodynamic and also featured a decreased gap velocity compared to noninteracting cells. Cells that rolled on E-selectin and subsequently adhered to HA displayed a normalized gap velocity (gap velocity/hydrodynamic velocity) that was significantly lower than the corresponding one of all other binding categories, indicating that this subpopulation of cells slowed considerably because of E-selectin-mediated rolling just before encountering the gap and HA regions.
Figure 3.
Hydrodynamic cell velocity vs. gap velocity for all cells. A) Cells binding to E-selectin and HA display a decreased gap velocity. B) Normalized gap velocity reveals that cells that bind to both E-selectin and HA have a lower normalized gap velocity as compared to those interacting with either E-selectin or HA, as well as nonbinding cells. Data are means ± sem. **P < 0.0001.
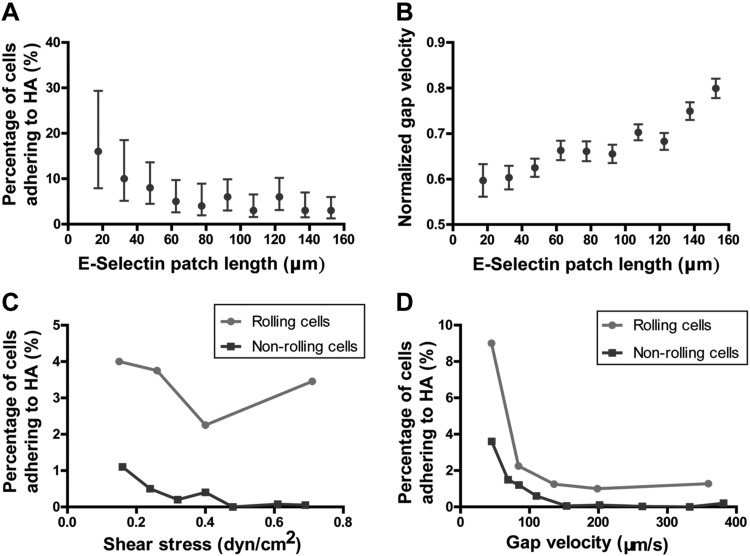
We next sought to explore how E-selectin-dependent rolling facilitates cell adhesion to HA. The percentage of cells interacting with HA after E-selectin-dependent rolling decreased with increasing E-selectin patch length (Fig. 4A). This rather counterintuitive result makes sense in light of our findings showing that the normalized gap velocity of rolling cells increases with increasing the E-selectin patch length (Fig. 4B). This, because tethering and rolling on smaller E-selectin patches occurs immediately before the gap (BSA-coated) region, thereby leading to depressed gap velocities. In contrast, cells rolling on longer patches can break their rolling interactions well before the gap region, thereby allowing these cells to accelerate and approach the hydrodynamic velocity. Taken together, cells that roll on shorter E-selectin patch lengths (up to 40 μm) can successfully facilitate adhesion to HA. Moreover, the velocity of E-selectin-mediated rolling cells over the gap region is a critical determinant of the rate of cell adhesion to HA.
Figure 4.
Factors influencing Pa03c cell binding to HA. A) The percentage of cells adhering to HA after E-selectin-dependent rolling decreases as a function of E-selectin patch length. Data are means ± 95% confidence intervals. B) Normalized gap velocity (gap velocity divided by hydrodynamic velocity) of cells after E-selectin–dependent rolling as a function of E-selectin patch length. Data are means ± sem. C) The percentage of cells adhering to HA for either nonrolling or E-selectin rolling cells as a function of fluid shear stress. D) The percentage of cells adhering to HA for either nonrolling or E-selectin rolling cells as a function of gap velocity.
It is evident that E-selectin-dependent rolling enables cells to bind downstream to HA more frequently when compared to nonrolling cells at all shear stresses (Fig. 4C). This E-selectin-dependent enhancement of cell adhesion to HA is more pronounced at higher shear stresses (≥0.3 dyn/cm2) where the HA binding of nonrolling cells is negligible. When plotting the percentage of cells adhering to HA for either nonrolling or E-selectin rolling cells as a function of gap velocity, rolling compared to nonrolling cells for the same gap velocity are still more likely to adhere to HA (Fig. 4D). This finding suggests that the formation of E-selectin-ligand bonds increases the likelihood of adhesion to HA when compared to nonrolling cells, regardless of the cell speed on the gap region just before the HA region. Furthermore, both nonrolling and rolling cells adhere more frequently to HA at lower gap velocities, again indicating the importance of gap velocity in HA binding.
PODXL-KD attenuates rolling on E-selectin, but does not affect binding with HA once cells have rolled
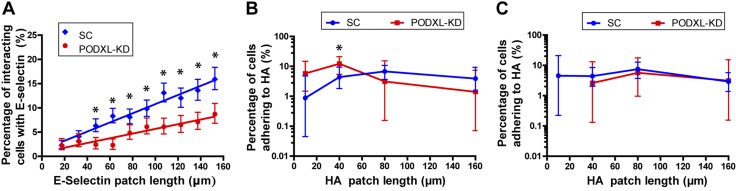
We then evaluated the effect of depleting one of the major E-selectin cell ligands, PODXL, on cell rolling and subsequent adhesion to HA. To this end, we knocked down PODXL from the surface of Pa03c cells and perfused these cells over the E-selectin and HA patches. We found that PODXL-KD cells interacted with E-selectin patches albeit at a significantly lower frequency than SC cells at a shear stress of 0.5 dyn/cm2 and for patch lengths longer than 40 μm (Fig. 5A). Still, PODXL-KD Pa03c cells interacting with E-selectin displayed a rolling behavior, and the fraction of interacting cells increased with increasing E-selectin patch lengths. Remarkably, PODXL-KD cells that first rolled on E-selectin, presumably via the formation of MUC16-E-selectin bonds (1, 9), adhered to HA in a percentage similar to that of SC cells with both the 30- and 60-μm gap lengths (Fig. 5B, C). Of note, PODXL knockdown did not alter the surface expression levels of CD44 on Pa03c cells relative to SC cells (Supplemental Fig. S5A). Moreover, PODXL-KD cells, which rolled first on E-selectin, passed over the gap region with essentially the same velocity as SC cells (Supplemental Fig. S5B). Taken together, these findings reveal that the depletion of PODXL from Pa03c cells primarily impacts cell rolling, but once cells have rolled on E-selectin, the rate of firm adhesion to HA remains unchanged because CD44 expression and cell gap velocity are unaffected by PODXL KD.
Figure 5.
PODXL knockdown decreases cell rolling on E-selectin, but has a minimal effect on cell adhesion to HA provided that PODXL-KD cells had previously rolled on E-selectin. A) The percentage of interacting cells on the E-selectin-patterned region increases with increasing patch lengths for both SC and PODXL-KD Pa03c cells at 0.5 dyn/cm2. B, C) The percentage of cells adhering to HA after E-selectin-dependent rolling for SC vs. PODXL-KD Pa03c cells for gap distances of 30 (B) and 60 μm (C) and for a shear stress level of 0.5 dyn/cm2. Data are means ± 95% confidence intervals. *P < 0.05.
DISCUSSION
Determining the biophysics of receptor–ligand interactions pertinent to metastasis is critical for improving the understanding of tumor cell adhesion to vascular endothelium and determining the critical parameters necessary to design diagnostic tools to interfere with metastasis (31). In this regard, we developed a platform to measure the critical parameters of E-selectin-mediated cell adhesion to HA under physiologically relevant shear flow conditions. Our work uses multicomponent protein patterning of E-selectin and HA in geometrically defined patterns in conjunction with a microfluidic flow chamber to flow and track, in an automated manner, Pa03c pancreatic cancer cells as they passed over the protein-coated patches. We measured how E-selectin-dependent rolling modulates HA adhesion as a function of fluid shear, contact time, and the spacing between E-selectin and HA regions patterned on the substrate. The major findings of this work are: 1) pancreatic cancer cells rolling on E-selectin are ∼40-fold more likely to bind to HA than nonrolling cells in shear flow, provided that the spacing between E-selectin and HA is ≤60 µm; 2) E-selectin-dependent rolling facilitates subsequent adhesion to HA at higher shear stresses compared to nonrolling cells, which are able to bind to HA only at stresses lower than 0.3 dyn/cm2; 3) rolling on E-selectin patches with a length of up to 40 μm is sufficient to increase adhesion to HA; and 4) the knockdown of the major E-selectin ligand, PODXL, attenuates rolling on E-selectin but does not decrease the rate of cell adhesion to HA, provided that cells had previously rolled on E-selectin, presumably via the formation of a MUC16-E-selectin bond.
The adhesive dynamic state of receptor–ligand interactions is determined by the Bell model single-molecule kinetic properties, mainly the unstressed dissociation constant (koff°) and the reactive compliance (xβ) (32–34). The PODXL-E-selectin bond has a koff°of 0.33 s−1 and xβ of 0.33 nm. These kinetic and micromechanical properties of PODXL-E-selectin bond facilitate cell rolling on E-selectin over a range of shear stresses provided that the timescale of the interaction is long enough to allow a sufficient number of bonds to form, overcoming the shear force on the cell (1, 28). In contrast, the CD44v-HA bond has a koff° of 0.005 s−1, indicating that the bond has a longer bond lifetime in the absence of force, and a xβ of 0.59 nm, indicating that the bond is more susceptible to rupture in the presence of force than the PODXL-E-selectin bond (12). Although selectin–ligand binding mediates rolling interactions (34–36), the kinetic properties of the CD44v-HA bond contribute to the slow rolling and stationary adhesion observed for CD44-expressing cells binding to HA at low shear stresses (12, 18, 19). The CD44v-HA bond lifetime decreases exponentially as a force is applied to the bond (12), and therefore we would expect Pa03c cells to adhere to HA only if a sufficient number of bonds form to overcome the hydrodynamic shear force on the cell. Of note, Pa03c cells express minimal levels of receptor for hyaluronan-mediated motility (RHAMM) (Supplemental Fig. S5A), thereby suggesting that Pa03c cell binding to HA is mediated primarily through the formation of CD44v-HA rather than RHAMM–HA bonds.
We observe that cells that do not previously roll on E-selectin adhere to HA primarily at low shear stresses (Fig. 4C). Most of these events occur at shear stresses lower than 0.5 dyn/cm2, which is consistent with prior observations suggesting that CD44-HA-mediated cell binding is initiated only at low hydrodynamic shear (18, 37). However, at all shear stresses, E-selectin-dependent rolling increases the percentage of cells which subsequently adhere to HA when compared to cells that do not first roll on E-selectin (Fig. 4C). This effect likely results in part from the slower velocity in the gap region for cells that first roll on E-selectin. We observe that slower cell velocities in the gap region increase the frequency of adhesion with HA, likely by increasing the contact duration with the HA region (Fig. 4D). Increasing the ligand–receptor contact duration has been demonstrated to increase the frequency of binding to ligand-coated substrates, because longer times allow for more ligand–receptor bonds to form (1, 2).
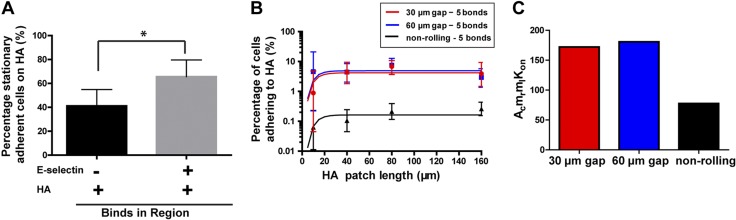
In addition, we determined that the formation of E-selectin-ligand bonds increases the likelihood of adhesion to HA compared to nonrolling cells, regardless of the cell speed in the gap region, indicating that the cell velocity in the gap region is not the only factor that dictates the rate of cell adhesion with HA (Fig. 4D). In light of this observation, we applied the multibond model to the experimental data, to determine the 2D binding parameters: the lumped binding affinity (AcMrMlKon) and the number of bonds needed to facilitate binding (n-bonds) for the CD44v–HA interaction (Fig. 6B) (2, 38, 39). This calculation reveals that both E-selectin rolling and nonrolling cells bind to HA with 5 bonds (Fig. 6B). However, cells that roll first on E-selectin have a higher AcMrMlKon (Fig. 6C). Given that the receptor and ligand densities (MrMl) are the same for both populations, this finding indicates that the 2-dimensional binding affinity (AcKon) is higher for cells that first roll on E-selectin. The higher AcKon for cells that previously rolled on E-selectin likely contributes to the increased frequency of binding and stable adhesion observed for cells adhering to HA (Fig. 6A). Cells that do not roll first on E-selectin can orient anywhere vertically in the 22.5 μm channel and therefore have a lower contact area with HA than cells which previously rolled on E-selectin. As a result, nonrolling cells have a lower AcKon and bind infrequently to HA. On the other hand, cells that roll first on E-selectin orient closer to the ligand interface, have a higher contact area, and thus can more easily form the CD44v–HA bonds necessary for adhesion to HA (Fig. 6C). Larger contact areas have been indicated to increase the frequency of cell binding (40). Therefore, E-selectin-dependent rolling promotes adhesion to HA by both physically slowing cells in the gap and orienting them proximal to the HA surface, thereby increasing the on rate of adhesion. This process allows cells to adhere to HA more frequently at higher shear stresses and to form the necessary bonds, which initiate longer and more stable adhesions (Fig. 6A).
Figure 6.
E-selectin-dependent rolling promotes stationary Pa03c cell adhesion to HA. All cells interacting with HA were tracked immediately after their initial binding interaction and classified as stationary or transiently adherent with a cutoff threshold of 2 s. A) The percentage of stationary adherent cells on HA. Data are means ± 95% confidence intervals. B) The multibond model was fitted to the experimental data (points) and optimized for both AcMrMlKon and n bonds, with the solid lines representing the model prediction for Pa03c cell adhesion to HA. Data are means ± 95% confidence intervals. C) The AcMrMlKon (lumped binding affinity) to HA was determined based on the experimental data for cells, which first rolled on E-selectin before passing over a 30- or 60-µm gap region, as well as nonrolling cells.
In this study, we uncovered the physical interdependence of the E-selectin–ligand and CD44v–HA bonds and determine the critical parameters that mediate pancreatic cancer cell adhesion to HA under physiologically relevant shear flow. This approach allows us to determine how E-selectin-dependent cancer cell rolling facilitates adhesion with HA in shear flow. E-selectin and HA are upregulated on the surface of endothelial cells in response to inflammatory stimulation (10, 11). In vitro flow-based models examining cell binding to activated endothelial cells or purified proteins recapitulate cell rolling and adhesive interactions observed in vivo (41). Although this study is limited to the use of only Pa03c cells, our findings will translate to other metastatic pancreatic cancer cells, given that MUC16 and PODXL are detected in 81.5 and 69%, respectively, of pancreatic ductal adenocarcinomas but they are absent from healthy pancreatic tissue or cells (8, 9, 24, 42). Because CD44 is also highly expressed in pancreatic adenocarcinomas (43), we expect that PODXL- and/or MUC16-dependent pancreatic cancer cell rolling on E-selectin will facilitate subsequent CD44-mediated adhesion to HA. In contrast, noncancerous pancreatic cancer cells, which are devoid of E-selectin ligands, would consequently exhibit minimal binding to immobilized HA. A better understanding of tumor cell adhesion under physiologic shear can be used to improve diagnostic assays and better understand the metastatic spread of pancreatic tumor cells.
ACKNOWLEDGMENTS
This work was supported by National Science Foundation Grant NSF-CBET-1159823 (to K.K. and K.J.S.) and the U.S. National Institutes of Health, National Cancer Institute Grant R01CA186286 (to K.K.). The authors declare no conflicts of interest.
Glossary
- BSA
bovine serum albumin
- DPBS
Dulbecco’s phosphate-buffered saline
- HA
hyaluronic acid
- KD
knockdown
- koff°
unstressed dissociation constant
- MUC
mucin
- n.s.
not significant
- PDMS
polydimethylsiloxane
- PODXL
podocalyxin
- SC
scrambled control
- TBS
Tris-buffered saline
- xβ
reactive compliance
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
D. J. Shea designed experiments, performed experiments and calculations, analyzed and interpreted data, and wrote the manuscript; Y. W. Li wrote the analysis code, performed experiments and calculations, analyzed data, and edited the manuscript; K. J. Stebe designed experiments, interpreted data, and edited the manuscript; and K. Konstantopoulos designed experiments, interpreted data, supervised research, and wrote the manuscript.
REFERENCES
- 1.Shea D. J., Wirtz D., Stebe K. J., Konstantopoulos K. (2015) Distinct kinetic and mechanical properties govern mucin 16- and podocalyxin-mediated tumor cell adhesion to E- and L-selectin in shear flow. Oncotarget 6, 24842–24855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tong Z., Cheung L. S., Stebe K. J., Konstantopoulos K. (2012) Selectin-mediated adhesion in shear flow using micropatterned substrates: multiple-bond interactions govern the critical length for cell binding. Integr. Biol. (Camb). 4, 847–856 [DOI] [PubMed] [Google Scholar]
- 3.Hammer D. A., Apte S. M. (1992) Simulation of cell rolling and adhesion on surfaces in shear flow: general results and analysis of selectin-mediated neutrophil adhesion. Biophys. J. 63, 35–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marshall B. T., Long M., Piper J. W., Yago T., McEver R. P., Zhu C. (2003) Direct observation of catch bonds involving cell-adhesion molecules. Nature 423, 190–193 [DOI] [PubMed] [Google Scholar]
- 5.Konstantopoulos K., Thomas S. N. (2009) Cancer cells in transit: the vascular interactions of tumor cells. Annu. Rev. Biomed. Eng. 11, 177–202 [DOI] [PubMed] [Google Scholar]
- 6.Burdick M. M., McCaffery J. M., Kim Y. S., Bochner B. S., Konstantopoulos K. (2003) Colon carcinoma cell glycolipids, integrins, and other glycoproteins mediate adhesion to HUVECs under flow. Am. J. Physiol. Cell Physiol. 284, C977–C987 [DOI] [PubMed] [Google Scholar]
- 7.Burdick M. M., Konstantopoulos K. (2004) Platelet-induced enhancement of LS174T colon carcinoma and THP-1 monocytoid cell adhesion to vascular endothelium under flow. Am. J. Physiol. Cell Physiol. 287, C539–C547 [DOI] [PubMed] [Google Scholar]
- 8.Chen S. H., Dallas M. R., Balzer E. M., Konstantopoulos K. (2012) Mucin 16 is a functional selectin ligand on pancreatic cancer cells. FASEB J. 26, 1349–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dallas M. R., Chen S.-H., Streppel M. M., Sharma S., Maitra A., Konstantopoulos K. (2012) Sialofucosylated podocalyxin is a functional E- and L-selectin ligand expressed by metastatic pancreatic cancer cells. Am. J. Physiol. Cell Physiol. 303, C616–C624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohamadzadeh M., DeGrendele H., Arizpe H., Estess P., Siegelman M. (1998) Proinflammatory stimuli regulate endothelial hyaluronan expression and CD44/HA-dependent primary adhesion. J. Clin. Invest. 101, 97–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nandi A., Estess P., Siegelman M. H. (2000) Hyaluronan anchoring and regulation on the surface of vascular endothelial cells is mediated through the functionally active form of CD44. J. Biol. Chem. 275, 14939–14948 [DOI] [PubMed] [Google Scholar]
- 12.Raman P. S., Alves C. S., Wirtz D., Konstantopoulos K. (2012) Distinct kinetic and molecular requirements govern CD44 binding to hyaluronan versus fibrin(ogen). Biophys. J. 103, 415–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ponta H., Sherman L., Herrlich P. A. (2003) CD44: from adhesion molecules to signalling regulators. Nat. Rev. Mol. Cell Biol. 4, 33–45 [DOI] [PubMed] [Google Scholar]
- 14.Aruffo A., Stamenkovic I., Melnick M., Underhill C. B., Seed B. (1990) CD44 is the principal cell surface receptor for hyaluronate. Cell 61, 1303–1313 [DOI] [PubMed] [Google Scholar]
- 15.Günthert U., Hofmann M., Rudy W., Reber S., Zöller M., Haussmann I., Matzku S., Wenzel A., Ponta H., Herrlich P. (1991) A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell 65, 13–24 [DOI] [PubMed] [Google Scholar]
- 16.Yu Q., Toole B. P., Stamenkovic I. (1997) Induction of apoptosis of metastatic mammary carcinoma cells in vivo by disruption of tumor cell surface CD44 function. J. Exp. Med. 186, 1985–1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim H.-R., Wheeler M. A., Wilson C. M., Iida J., Eng D., Simpson M. A., McCarthy J. B., Bullard K. M. (2004) Hyaluronan facilitates invasion of colon carcinoma cells in vitro via interaction with CD44. Cancer Res. 64, 4569–4576 [DOI] [PubMed] [Google Scholar]
- 18.Clark R. A., Alon R., Springer T. A. (1996) CD44 and hyaluronan-dependent rolling interactions of lymphocytes on tonsillar stroma. J. Cell Biol. 134, 1075–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogino S., Nishida N., Umemoto R., Suzuki M., Takeda M., Terasawa H., Kitayama J., Matsumoto M., Hayasaka H., Miyasaka M., Shimada I. (2010) Two-state conformations in the hyaluronan-binding domain regulate CD44 adhesiveness under flow condition. Structure 18, 649–656 [DOI] [PubMed] [Google Scholar]
- 20.Ghosh M., Alves C., Tong Z., Tettey K., Konstantopoulos K., Stebe K. J. (2008) Multifunctional surfaces with discrete functionalized regions for biological applications. Langmuir 24, 8134–8142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Launiere C., Gaskill M., Czaplewski G., Myung J. H., Hong S., Eddington D. T. (2012) Channel surface patterning of alternating biomimetic protein combinations for enhanced microfluidic tumor cell isolation. Anal. Chem. 84, 4022–4028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Myung J. H., Gajjar K. A., Chen J., Molokie R. E., Hong S. (2014) Differential detection of tumor cells using a combination of cell rolling, multivalent binding, and multiple antibodies. Anal. Chem. 86, 6088–6094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nalayanda D. D., Kalukanimuttam M., Schmidtke D. W. (2007) Micropatterned surfaces for controlling cell adhesion and rolling under flow. Biomed. Microdevices 9, 207–214 [DOI] [PubMed] [Google Scholar]
- 24.Chen S. H., Hung W. C., Wang P., Paul C., Konstantopoulos K. (2013) Mesothelin binding to CA125/MUC16 promotes pancreatic cancer cell motility and invasion via MMP-7 activation. Sci. Rep. 3, 1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Napier S. L., Healy Z. R., Schnaar R. L., Konstantopoulos K. (2007) Selectin ligand expression regulates the initial vascular interactions of colon carcinoma cells: the roles of CD44v and alternative sialofucosylated selectin ligands. J. Biol. Chem. 282, 3433–3441 [DOI] [PubMed] [Google Scholar]
- 26.Hanley W. D., Napier S. L., Burdick M. M., Schnaar R. L., Sackstein R., Konstantopoulos K. (2006) Variant isoforms of CD44 are P- and L-selectin ligands on colon carcinoma cells. FASEB J. 20, 337–339 [DOI] [PubMed] [Google Scholar]
- 27.Hanley W. D., Wirtz D., Konstantopoulos K. (2004) Distinct kinetic and mechanical properties govern selectin-leukocyte interactions. J. Cell Sci. 117, 2503–2511 [DOI] [PubMed] [Google Scholar]
- 28.Cheung L. S.-L., Konstantopoulos K. (2011) An analytical model for determining two-dimensional receptor-ligand kinetics. Biophys. J. 100, 2338–2346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yago T., Wu J., Wey C. D., Klopocki A. G., Zhu C., McEver R. P. (2004) Catch bonds govern adhesion through L-selectin at threshold shear (published correction in J. Cell Biol. 2005, l68, 975). J. Cell Biol. 166, 913–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidt B. J., Huang P., Breuer K. S., Lawrence M. B. (2008) Catch strip assay for the relative assessment of two-dimensional protein association kinetics. Anal. Chem. 80, 944–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas S. N., Tong Z., Stebe K. J., Konstantopoulos K. (2009) Identification, characterization and utilization of tumor cell selectin ligands in the design of colon cancer diagnostics. Biorheology 46, 207–225 [DOI] [PubMed] [Google Scholar]
- 32.Cheung L. S.-L., Raman P. S., Balzer E. M., Wirtz D., Konstantopoulos K. (2011) Biophysics of selectin-ligand interactions in inflammation and cancer. Phys. Biol. 8, 015013 [DOI] [PubMed] [Google Scholar]
- 33.Chang K.-C., Tees D. F., Hammer D. A. (2000) The state diagram for cell adhesion under flow: leukocyte rolling and firm adhesion. Proc. Natl. Acad. Sci. USA 97, 11262–11267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raman P. S., Alves C. S., Wirtz D., Konstantopoulos K. (2011) Single-molecule binding of CD44 to fibrin versus P-selectin predicts their distinct shear-dependent interactions in cancer. J. Cell Sci. 124, 1903–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCarty O. J., Jadhav S., Burdick M. M., Bell W. R., Konstantopoulos K. (2002) Fluid shear regulates the kinetics and molecular mechanisms of activation-dependent platelet binding to colon carcinoma cells. Biophys. J. 83, 836–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alon R., Chen S., Fuhlbrigge R., Puri K. D., Springer T. A. (1998) The kinetics and shear threshold of transient and rolling interactions of L-selectin with its ligand on leukocytes. Proc. Natl. Acad. Sci. USA 95, 11631–11636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Christophis C., Taubert I., Meseck G. R., Schubert M., Grunze M., Ho A. D., Rosenhahn A. (2011) Shear stress regulates adhesion and rolling of CD44+ leukemic and hematopoietic progenitor cells on hyaluronan. Biophys. J. 101, 585–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu C., Williams T. E. (2000) Modeling concurrent binding of multiple molecular species in cell adhesion. Biophys. J. 79, 1850–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chesla S. E., Selvaraj P., Zhu C. (1998) Measuring two-dimensional receptor-ligand binding kinetics by micropipette. Biophys. J. 75, 1553–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu L., Xiao B., Jia X., Zhang Y., Lü S., Chen J., Long M. (2007) Impact of carrier stiffness and microtopology on two-dimensional kinetics of P-selectin and P-selectin glycoprotein ligand-1 (PSGL-1) interactions. J. Biol. Chem. 282, 9846–9854 [DOI] [PubMed] [Google Scholar]
- 41.Jablonska A., Shea D. J., Cao S., Bulte J. W., Janowski M., Konstantopoulos K., Walczak P. (2017) Overexpression of VLA-4 in glial-restricted precursors enhances their endothelial docking and induces diapedesis in a mouse stroke model [Epub ahead of print]. J. Cereb. Blood Flow Metab. doi:10.1177/0271678X17703888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Streppel M. M., Vincent A., Mukherjee R., Campbell N. R., Chen S.-H., Konstantopoulos K., Goggins M. G., Van Seuningen I., Maitra A., Montgomery E. A. (2012) Mucin 16 (cancer antigen 125) expression in human tissues and cell lines and correlation with clinical outcome in adenocarcinomas of the pancreas, esophagus, stomach, and colon. Hum. Pathol. 43, 1755–1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takada M., Yamamoto M., Saitoh Y. (1994) The significance of CD44 in human pancreatic cancer: I. High expression of CD44 in human pancreatic adenocarcinoma. Pancreas 9, 748–752 [DOI] [PubMed] [Google Scholar]