Abstract
Predators play a crucial role in the structure and function of ecosystems. However, the magnitude of this role is often unclear, particularly for large marine predators, as predation rates are difficult to measure directly. If relevant biotic and abiotic parameters can be obtained, then bioenergetics modelling offers an alternative approach to estimating predation rates, and can provide new insights into ecological processes. We integrate demographic and ecological data for a marine apex predator, the broadnose sevengill shark Notorynchus cepedianus, with energetics data from the literature, to construct a bioenergetics model to quantify predation rates on key fisheries species in Norfolk Bay, Australia. We account for the uncertainty in model parameters by incorporating parameter confidence through Monte Carlo simulations and running alternative variants of the model. Model and parameter variants provide alternative estimates of predation rates. Our simplest model estimates that ca. 1130 ± 137 N. cepedianus individuals consume 11,379 (95% CI: 11,111–11,648) gummy sharks Mustelus antarcticus (~21 tonnes) over a 36-week period in Norfolk Bay, which represents a considerable contribution to total predation mortality on this key fishery species. This study demonstrates how the integration of ecology and fisheries science can provide information for ecosystem and fisheries management.
Introduction
It is well-accepted that predators play crucial roles in the structure and function of ecosystems, but quantifying rates of predation remains difficult1–4. Predation pressure is often inferred5–7, and a number of studies have quantified non-consumptive effects (risk effects) on prey8. Quantifying predation rates provides information for better detecting ecological processes, defining predators’ roles in different systems, and determining the strength of species interactions1,9. It can also assist applications such as providing more precise data for ecosystem models, ensuring sustainable harvests of prey species, and improving estimates of natural mortality in commercially fished populations10–12. In fisheries research and management, natural mortality is a difficult parameter to quantify, yet often one of the most important10,13. For example, mortality from predation can exceed that from fisheries, and so estimates of mortality from predation can add important information to stock assessment models10,14.
Determining the direct effects of predation requires information that is often difficult to obtain, including the rate of prey consumption, which is dictated by the predator’s metabolic rate and influenced by the energetic value of its prey1,15. Another obstacle is obtaining reliable estimates of absolute abundance of predators (as opposed to relative abundance indices), so that individual prey consumption rates can be scaled-up to the population level15. For example, several studies use different methods to assess the rates of prey consumption for killer whales Orcinus orca, but none of these studies quantify O. orca population sizes (see Noren15 and references therein). Studies that estimate marine predator abundance for use with bioenergetics models not only quantify predation at the population level, but also contribute significantly to our understanding of the role of marine predators in the structure and function of ecosystems1,16–19. For example, incorporating abundance estimates into a bioenergetics model for adult grey reef sharks Carcharhinus amblyrhynchos demonstrates the significant contribution of fish spawning aggregations to meeting the energetic demands of a population in Fakarava Pass, French Polynesia19.
Given the increasing acceptance of ecosystem and multi-species approaches to fisheries management, integrating ecological methods, such as identifying and quantifying key species interactions, is believed to be a primary direction for fisheries science12,20. However, the general uncertainty of species interactions and the poor understanding of the ecology of many species (e.g. diet, population dynamics, spatial distribution, habitat use, etc.) can hinder multi-species approaches21.
A suite of relevant studies on the broadnose sevengill shark Notorynchus cepedianus in Norfolk Bay, Tasmania, southern Australia (Fig. 1), provides a rare opportunity to estimate predation rates by a seasonal population of a marine apex predator. This shark is a fishery-associated species with a broad global distribution22. It is a common bycatch species (with a low commercial value) in the southern shark fishery of Australia, and is the most significant predator of commercially important juvenile shark species23–25. Notorynchus cepedianus occurs in high numbers in coastal Tasmania over the warmer months of the year (spring to autumn), where it exerts significant predation pressure on prey inhabiting those waters6,24–26, and likely plays a crucial role as one of the key apex predators in temperate waters6. This study integrates a suite of available information on the demography, activity, and diet of N. cepedianus with information on energetics from the literature into a bioenergetics model. Uncertainty in model input parameters is accounted for by incorporating parameter confidence through Monte Carlo simulations and running alternative variants of the model. The model variants are used (1) to quantify the overall role of N. cepedianus as the apex predator in Norfolk Bay and (2) to specifically estimate predation mortality of a key fisheries species, the gummy shark Mustelus antarcticus, in an elasmobranch protected area. Model and parameter variants provide alternative estimates of predation rates for all prey species. The strengths and weaknesses of model variants and parameter uncertainty are discussed.
Figure 1.
Map showing study area Norfolk Bay in southern Tasmania, Australia. Grey and black lines represents gummy shark Mustelus antarcticus (grey) and sevengill shark Notorynchus cepedianus (black) distributions in southern Australia (approximately from line into coast). Figure generated in Powerpoint (Microsoft Office 2013).
Results
A bioenergetics model was constructed to estimate predation rates by N. cepedianus on the gummy shark Mustelus antarcticus and on other key prey species, in Norfolk Bay, Tasmania (Fig. 1). The model was applied over a 36 week period from September to May (spring-summer-autumn seasons) during which N. cepedianus are known to aggregate in the bay. The model incorporates (1) routine energy expenditure of free-swimming N. cepedianus in Norfolk Bay, (2) population size of N. cepedianus in Norfolk Bay, (3) demographic information for N. cepedianus and for prey species, (4) energy content of various prey species inhabiting Norfolk Bay, and the (5) relative importance of these prey species to the diet of N. cepedianus in Norfolk Bay. To account for uncertainty in model structure and input parameters, we built variants of the model based on three alternative techniques for measuring diet composition (see Methods). Separate models were run for each of the three diet variants taking into account two estimates of N. cepedianus population sizes in Norfolk Bay during the 36 week period: 562 ± 71 and 1130 ± 137 sharks. For each of the six scenarios considered, 1000 Monte Carlo simulations were performed to account for uncertainty in model parameters (see Methods).
Our sampling data indicate that N. cepedianus occurring in Norfolk Bay have an average body mass of 42 kg (Table 1). Accordingly, we estimate that the average free-swimming routine energy expenditure of N. cepedianus in Norfolk Bay is approximately 1150 kJ day−1 at a water temperature of 16.7 °C (assuming Q10 = 2.2 and RQ = 0.88). During spring, when water temperature drops to 14.0 °C, routine energy expenditure is predicted to decrease to 930 kJ day−1, and during summer, when water temperature increases to 19.1 °C, routine energy expenditure is predicted to increase to 1390 kJ day−1 (Q10 = 2.2 and RQ = 0.88).
Table 1.
Parameter used in the bioenergetics model.
| Species (or group) | TL or DW (cm) (mean ± SD; range) | Mb (kg) (mean ± SD; range) | TL or DW to Mbrelationship | Reference for TL or DW to Mb conversion | Tissue energy-density (kcal/g) | Reference for tissue energy-density |
|---|---|---|---|---|---|---|
| Sevengill shark Notorynchus cepedianus | 208 ± 35; 105–270 | 42.0 ± 22.0; 2.8–88.0 | Females: Mb = 0.003TL2–0.42TL + 19.501 (R² = 0.996; n = 216) Males: Mb = 0.002TL2–0.22TL + 8.803 (R² = 0.98, n = 78) | 42 | ||
| Fur seal (FS) Arctocephalus pusillus; Other mammals (M) (undigested contents only) | 2.1 ± 1.3; 0.7–4.0 | Barnett unpub. data | 2.5 (FS) 2.4 (M) | FS based on fur seal species; M based on the average of pinniped & whale estimates1,18,58 | ||
| Gummy shark Mustelus antarcticus | 74 ± 20; 28–143 | 1.8 ± 1.6; 0.1–11.5 | Females: Mb = 0.93 × 10−29 × 1.07 × (TL × 10)3.21 (R2 = 0.95; n = 1077) Males: Mb = 4.210.10−9 × 1.016 × (TL × 10)2.976 (R2 = 0.93; n = 862) | 59 | 1.5 | Based on Squalus acanthias 60 |
| School shark Galeorhinus galeus | 66 ± 16; 31–113 | 1.2 ± 0.8; 0.1–5.2 | Same as gummy shark | 59 | 1.5 | 60 |
| Dogshark Squalus acanthias | 54 ± 11; 19–94 | 0.73 ± 0.5; 0.03–4.2 | Mb = 0.05TL2.6 × 1000 (R2 = 0.96; n = 32) | 61 | 1.5 | 60 |
| Eagle rays Myliobatis tenuicaudatus. | 81 ± 14; 70–110 | 9.1 ± 5.8; 0.9–48.6 | Mb = 2.76 × 10−05 × DW2.9 (R2 = 0.95; n = 393) | 62 | 1.1 | Based on batoid species in (60, 16) |
| Melbourne skate Spiniraja whitleyi | 87 ± 29; 33–196 | 18.2 ± 17.7; 0.1–124.6 | Mb = 0.005DW2–0.29DW + 4.65 (R2 = 0.96; n = 72) | Treloar unpub. data | 1.1 | 60 |
| Banded stingaree Urolophus cruciatus | 18 ± 4; 9–30 | 0.3 ± 0.2; 0.03–1.1 | Mb = 0.002DW2–0.03DW + 0.14 (R² = 0.96; n = 75) | Yick unpub. data | 1.1 | 60 |
| Elephantfish Callorhynchus milii | 73 ± 9; 45–100 | 2.5 ± 1.2; 0.4–7.3 | Females: Mb = 7.54e−10 × (TL × 10)3.3 Males: Mb = 6.3e−11 × (TL × 10)3.7 | Braccini unpub. data | 1.0 | Based on Callorhynchus callorhynchus 60 |
| Teleosts | 0.8 | Estimated average weight for multiple species combined | 1.5 | Average of all teleosts in60 | ||
| Cephalopods (mainly arrow squid) | 0.7 | Estimated weight of squid63,64 | 1.5 | 60 |
Because entire seals/mammals were not consumed by an individual N. cepedianus, Only weight of undigested mammal occurring in stomach samples was used to obtain average weight of mammal consumed. TL = total length, DW = disc width for batoids, Mb = body mass.
The number of prey consumed over the 36 week period, along with the relative importance of the different prey species (or groups), varied among the three models and six scenarios (Table 2, Fig. 2). For example, the consumption rate of M. antarcticus differed among models (Fig. 2, Table 2). Moreover, uncertainties and variations in model parameters that likely fluctuate within and between years (i.e. water temperature and number of N. cepedianus) or parameters lacking specific data for N. cepedianus (Q10) produced differences in modelled prey consumption rates (Fig. 3). Changes in all three parameters led to differences in modelled consumption rates of M. antarcticus (Fig. 3).
Table 2.
Estimated number (with 95% confidence interval range) of each prey type consumed by N. cepedianus over the 36-week sampling year in Norfolk Bay based on three model variants and two scenarios of N. cepedianus population size26.
| Species (or groups) | M1 N1 | M1 N2 | M2 N1 | M2 N2 | M3 N1 | M3 N2 |
|---|---|---|---|---|---|---|
| Fur seal Arctocephalus pusillus | 49 (47–50) | 98 (95–102) | 10 (10–11) | 20 (19–21) | ||
| Other mammal | 15 (14–15) | 29 (28–31) | 6 (6–7) | 13 (12–13) | ||
| Gummy shark Mustelus antarcticus | 5656 (5523–5789) | 11379 (11111–11648) | 4085 (3897–4273) | 8294 (7913–8675) | 2241 (2119–2364) | 4653 (4396–4911) |
| School shark Galeorhinus galeus | 499 (473–524) | 1009 (958–1061) | 131 (123–139) | 266 (252–281) | ||
| Dogshark Squalus acanthias | 1061 (1005–1118) | 2179 (2052–2305) | 815 (770–860) | 1616 (1527–1705) | ||
| Unidentified shark | 4657 (4403–4910) | 9357 (8842–9872) | 946 (880–1012) | 1991 (1859–2123) | ||
| Eagle rays Myliobatis tenuicaudatus | 495 (471–520) | 1006 (957–1056) | 882 (843–922) | 1862 (1778–1947) | ||
| Melbourne skate Spiniraja whitleyi | 317 (299–334) | 654 (617–690) | 1133 (1086–1179) | 2297 (2206–2387) | ||
| Banded stingaree Urolophus cruciatus | 3359 (3171–3548) | 6775 (6407–7143) | 744 (701–787) | 1406 (1328–1484) | ||
| Unidentified batoid | 88 (80–97) | 177 (160–193) | 611 (569–652) | 1195 (1101–1289) | ||
| Elephantfish Callorhynchus milii | 195 (187–204) | 396 (378–414) | 308 (294–321) | 610 (583–636) | ||
| Teleosts | 2404 (2335–2473) | 4858 (4718–4998) | 3429 (3161–3698) | 6554 (6041–7066) | ||
| Cephalopods (mainly arrow squid) | 136 (132–140) | 275 (267–283) | 536 (518–554) | 1083 (1048–1118) |
M1–3 = model variant 1–3, N1 = population of 562, and N2 = population of 1130.
Figure 2.
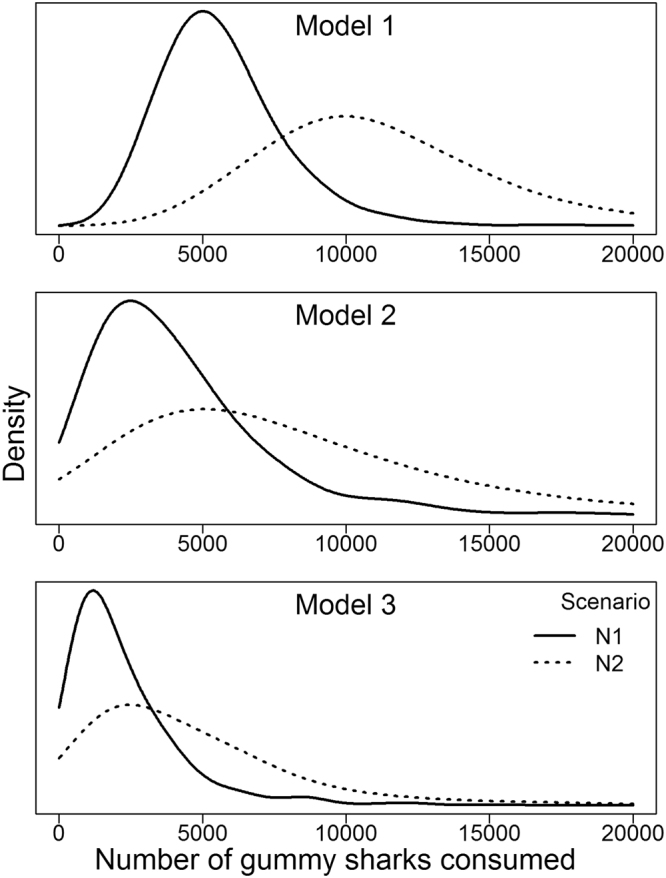
Probability of N. cepedianus predation on gummy shark M. antarcticus based on the outputs of the three variants of the bioenergetics model, fitted by a log normal distribution.
Figure 3.
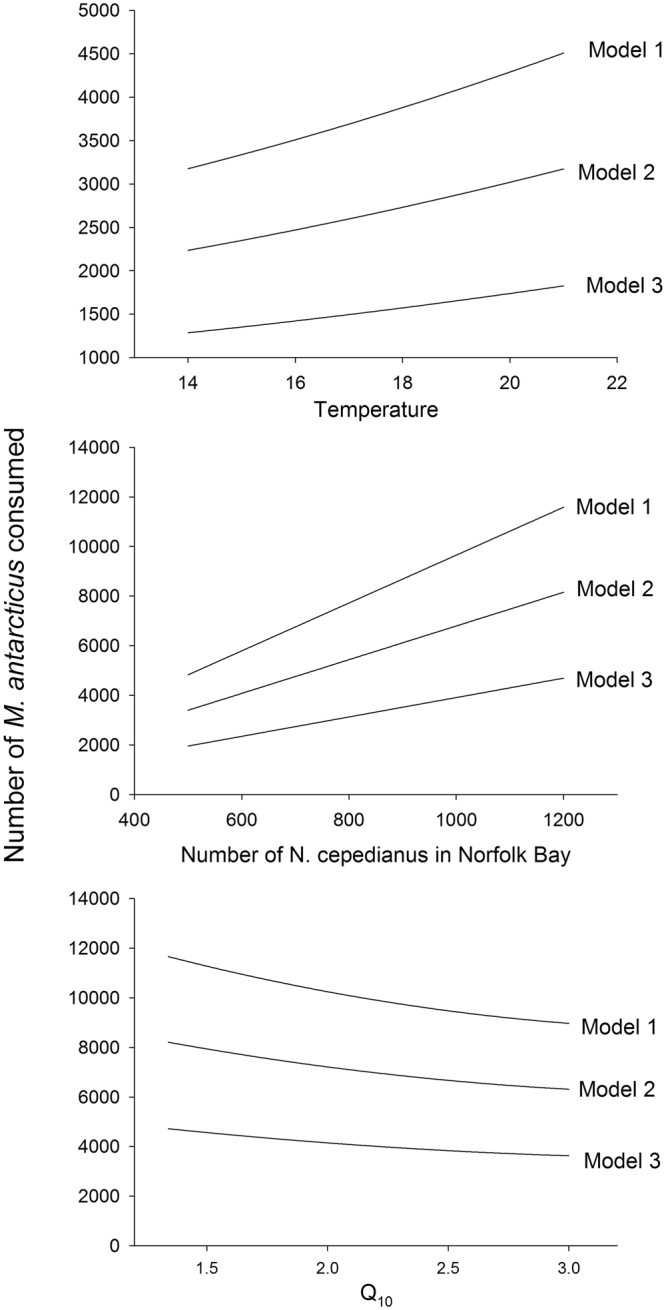
Variation in the predicted N. cepedianus predation on gummy shark M. antarcticus for the three model variants, and the effect of temperature, Q10 and population size on the model outputs. Temperature and Q10 are based on the population estimates of 1130 N. cepedianus occurring in Norfolk Bay.
Model variant 1
Assuming that 25% of N. cepedianus population in Norfolk Bay contains M. antarcticus at any given time25, and that N. cepedianus abundance in the bay varies between 562 and 1130 individuals throughout spring to autumn22, this model estimates that between 5656 (95% CI: 5523–5789) and 11,379 (95% CI: 11111–11648) M. antarcticus are consumed over the 36 week period (Fig. 2, Table 2).
Model variant 2
The high relative weight of mammal in the diet25 influences this model’s output, with up to 127 mammals (98 fur seals) consumed over the 36-week period (Table 2). This model predicts that between 4085 (95% CI: 3897–4273) and 8294 (95% CI: 7913–8675) M. antarcticus are consumed over the 36 week period annually in Norfolk Bay (Table 2).
Model variant 3
The influence of marine mammal is reduced considerably in this model compared to model variant 2, with a maximum of 33 mammals (20 fur seals) consumed over the 36-week period (Table 2). Batoids, most notably Myliobatis tenuicaudatus and Spiniraja whitleyi, have a stronger influence on this model’s output (Table 2). This is influenced by the large average weight of skates and eagle rays (Table 1), resulting in those species having the highest average relative weight, i.e. skates 43% and eagle rays 16% (Appendix S1). All shark species (or groups) were consumed in lower numbers compared to model variant 2, including M. antarcticus (Table 2). Between 2241 (95% CI: 2119–2364) and 4653 (95% CI: 4396–4911) M. antarcticus individuals were estimated to be consumed over the 36-week period annually in Norfolk Bay. Estimates of teleost and cephalopod consumption were also higher in model variant 3 than in model variant 2 (Table 2).
Discussion
Bioenergetics model
Quantifying predation rates by marine predator populations is a significant challenge in ecology and fisheries science. Previous studies that have incorporated predator abundance estimates with bioenergetics models to quantify predation mainly includes marine mammals17,18,27, and fisheries species, such as tunas28,29. Although previous studies have constructed bioenergetics models for sharks (e.g.30–32), few have incorporated abundance estimates that allow consumption rates to be scaled up to the population level16,19. The paucity of marine studies is not surprising given the difficulties associated with observing predators in general, and obtaining the suite of relevant parameters required for bioenergetics models. To compensate for this, these models are often based on best available assumptions, such as published data from similar species. Here, we present three model simulations using a combination of field data for predator and prey demographics and ecology, and information on energetics from the literature. Model 1 centres on the main strengths of available data, which is a very good understanding of N. cepedianus and M. antarcticus demographics and ecology in Norfolk Bay. This model provides estimates of predation mortality for M. antarcticus, a key fisheries species, in an area closed to commercial and recreational fishing to protect the population (see below). The second and third models take a broader system approach, and were populated with as much information that could be obtained for other prey species.
For fisheries applications, model 1 is the simplest to obtain data for as it only requires data for the predator and the target fisheries species. However, model 1 does not consider the input of other prey species (number or energetic value) to the diets of N. cepedianus, and this could be driving the higher estimates of M. antarcticus consumed compared to the other models. Still, higher estimates of M. antarcticus consumption may be the most appropriate if a conservative approach is considered best for fisheries management10. For ecosystem studies, model 3 is likely more accurate than model 2. Model 2 (based on % weight) overestimates mammal consumption. Marine mammals are not seen in Norfolk Bay in large numbers (Barnett pers. obs.), and the closest haul-out site is over 50 km away25. Either predation is significantly less, as predicted in model 3, or N. cepedianus are feeding on mammals elsewhere before entering Norfolk Bay. In general, % weight alone is not a good indicator of prey value as issues such as partial and differential digestion can provide ambiguous interpretations33. Furthermore, as digestion proceeds, only the components that are indigestible, or slow to digest, remain identifiable and potentially measureable33. For example, cephalopod beaks and fish otoliths are often all that remains of these animals in stomach samples. In model 3, the increase in the importance of batoids is primarily driven by the large average size of S. whitleyi and M. tenuicaudatus caught in Norfolk Bay, which influences their average % weight in the model (Appendix S1). The importance of batoids is likely overestimated in this model because smaller size classes of skates and eagle rays were not caught in the fishing gears used (inflating average size of batoids), and the likelihood that larger batoids are consumed by multiple N. cepedianus. This had some effect on the model output, such as lowering the estimates of M. antarcticus consumed (Table 2).
A major challenge with bioenergetics models is the level of uncertainty associated with input parameter values27. A few of the parameters in our model undoubtedly introduce some uncertainty. For example, Q10 for N. cepedianus is unknown, and available literature suggests it could be between 1.3 and 3.0. This difference in Q10 can lead to estimates that differ by ~2000 individual M. antarcticus consumed over the sampling year (Fig. 3).There could be some uncertainty regarding dietary composition, as stomach content analysis only provides information for a snapshot in time. However, stomach data were collected over three years and studies show that N. cepedianus diet composition can be linked to prey abundance25, as discussed in fisheries section below. Thus, uncertainty associated with parameters such as Q10, water temperature, N. cepedianus body mass (which are all related to uncertainty in metabolic rate), other metabolic rate parameters (power equation coefficient and power equation exponent) and prey composition, were factored into our analysis by running Monte Carlo simulations. Furthermore, uncertainty in N. cepedianus abundance was also factored in by using two different scenarios for each of the three versions of the model. Models can be updated when improved estimates for the different parameters become available. For instance, when technology becomes available for sharks of this size, conducting respirometry experiments and integrating field-derived activity data for N. cepedianus to determine species-specific metabolic rate may improve estimates of energetics and prey consumption34, as it addresses the most likely variable component of our bioenergetics model. Considering the best available information, we assumed similar activity for day and night based on N. cepedianus cruising speeds calculated from acoustic telemetry not being significantly different35. However, given that N. cepedianus appear to move over a larger area at night and the significant increase in movement rates between cruising and burst speeds35, field activity studies may also elucidate diel patterns in active metabolic rates.
Fisheries implications
A number of coastal areas in southern Australia prohibit the taking of any elasmobranchs to protect neonate and juvenile M. antarcticus 23. Based on the average weight of M. antarcticus in Norfolk Bay (1.8 ± 1.6 kg), the consumption of 2241 to 11,379 individuals by N. cepedianus equates to an annual consumption of between 4 and 21 tonnes. This is the first estimate of the predation component of natural mortality for M. antarcticus. Norfolk Bay is a relatively small area (~180 km2) within the spatial distribution of M. antarcticus and N. cepedianus (Fig. 1) so, at the broader population level (distribution in Australian temperate waters), the total annual consumption of M. antarcticus by N. cepedianus could be at the same order of magnitude of the current catch quota for M. antarcticus, in the Southern and Eastern Scalefish and Shark Fishery (1836 tonnes)36.
It is however important to recognise that predation rates on each species by N. cepedianus likely varies between locations, depending on differences in prey availability, water temperature and N. cepedianus abundance. For example, the high consumption of M. antarcticus coincides with it being one of the most relative abundant prey in Norfolk Bay and neighbouring bays6,25 (Appendix S2). Similarly, increases in consumption of S. acanthias that coincide with high relative abundance in the neighbouring Derwent Estuary have been reported25. Likewise, in Tasmania and southern Africa, N. cepedianus consumed more marine mammals in the region with the highest concentration of seal rookeries, while chondrichthyans were the most important prey in the other regions25,37,38. Catches of some other species are high in neighbouring bays compared to Norfolk Bay. For example, S. acanthias and C. milii are caught in higher numbers in the neighbouring Fredrick Henry Bay and Pittwater, respectively. The greater presence of these species would likely result in an increase in their occurrence in the diet of N. cepedianus at those locations (Appendix S2). In general, N. cepedianus target other elasmobranchs and marine mammals globally, but the main species consumed within these groups can vary25. However, sharks from the genus Mustelus (family Triakidae) and other triakid species are the most common prey consumed by N. cepedianus in all regions globally25, suggesting that, when they are abundant, triakids are the main prey.
Besides the aforementioned links to fisheries, N. cepedianus is also linked to fisheries by being an important predator of elasmobranchs and pinnipeds that compete with fisheries39,40. In particular, fur seal Arctocephalus pusillus numbers have recovered significantly in Australia since their protection in 1975 and many in the fishing industry deem them as competitors for diminishing resources40. In areas of southern Australia with greater pinniped abundance, N. cepedianus likely consume more pinnipeds, and probably play a role in reducing pinniped competition with fisheries.
Role of Notorynchus cepedianus
Previous work has inferred high predation pressure by N. cepedianus in coastal areas, as reviewed by Barnett and colleagues22. The current study shows that in areas of high abundance, N. cepedianus have significant impacts on the various prey species and very likely play an important role in ecosystem dynamics, e.g. top-down control of ecosystems. Notorynchus cepedianus consume the same prey as white sharks Carcharodon carcharias, including marine mammals, teleosts and elasmobranchs22,37,41. However, despite rivalling C. carcharias as the dominant apex predator in temperate waters, the ecosystem importance of N. cepedianus has been largely overlooked. Indeed, N. cepedianus arguably have a greater influence on top-down effects, such as ecosystem structure and controlling mesopredator numbers, as available information suggests they are much more abundant across temperate systems than C. carcharias 26,42.
Given that water temperature plays an important role in predation rates (Fig. 3), increases in water temperature due to climate change could change the dynamics in shallow coastal bays such as Norfolk Bay. For example, model 1 predicts that 1130 N. cepedianus would consume ~1000 more M. antarcticus in summer compared to spring (Fig. 3). Tasmania is considered particularly susceptible to climate change, with warmer waters extending the southern range of some species along the east coast of Australia43. However, Tasmania is the most southern coastal area, and there is nowhere further south for N. cepedianus to move, and so if temperatures increase, they will need to adapt by increasing predation rates to meet the increasing energetic demands, or by spending more time in cooler deeper waters, which may affect their diet.
In conclusion, the integration of multiple types of information from a comprehensive suite of studies on N. cepedianus and its prey in Norfolk Bay has culminated in one of the first quantified estimates of predation for an apex predator shark species. Notorynchus cepedianus is an undervalued predator in coastal systems that competes directly with fisheries for common food resources. Given the wide distribution of N. cepedianus, they likely play an important role in ecosystem dynamics in temperate systems globally. Furthermore, N. cepedianus are intrinsically linked to fisheries, making them a good case study to show how the integration of ecology into fisheries science, i.e. “fisheries ecology”, can provide data that can be used for applied outcomes in ecosystem and fisheries management.
Methods
Study site
Norfolk Bay is a relatively shallow (maximum depth of ~20 m), semi-enclosed bay, covering an area of ~180 km2, off the southeast coast of Tasmania, Australia (Fig. 1). Norfolk Bay is located within a shark refuge area, and as such, commercial and recreational fishing for elasmobranchs is not permitted. The bay provides an important feeding site for the broadnose sevengill shark Notorynchus cepedianus and aggregations occur in the bay from September to May26,44. In this study, the energetics and predation habits of N. cepedianus in Norfolk Bay were analysed over this 36-week period, encompassing the spring-summer-autumn seasons. All field work was conducted under an Australian Fisheries Management Authority Scientific Permit (#901193) and the methods were approved by the University of Tasmania Animal Ethics Committee (#A0012578).
Routine energy expenditure of N. cepedianus
The bioenergetics model constructed for this study estimates predation rates by N. cepedianus on the gummy shark Mustelus antarcticus, as well as other prey species, in Norfolk Bay from spring to autumn. To achieve this aim, an estimate of the routine energy expenditure of free-swimming N. cepedianus in Norfolk Bay was required. Over a period of two years and 3 months (to include 3 summers), 294 N. cepedianus individuals were caught in Norfolk Bay using longline fishing methods24. For each of these sharks, length measurements, sex and stomach contents (from stomach flushing) were recorded45. Since N. cepedianus is an ectotherm, the routine energy expenditure (MR; mg O2 h−1) was calculated for each of these 294 individuals using the allometric power equation for a group of free-swimming ectothermic sharks species, MR = 214M b 0.79, correct to 20 °C46, where M b is body mass in kg, which was estimated for each individual using sex-specific N. cepedianus length-weight data47 (Table 1). This estimate of overall mean routine energy expenditure is unlikely to vary across a 24-h cycle owing to activity measurements that indicate the rate of movement by N. cepedianus in Norfolk Bay is relatively constant during the day and night35. The routine energy expenditure of each individual N. cepedianus was, however, adjusted according to seasonal mean variation in water temperature (measured in the adjoining Fredrick Henry Bay: spring 14.0 °C, summer 19.1 °C, autumn 16.9 °C, overall mean 16.7 °C) using a uniform distribution Q10 between 1.3 and 3.0. This Q10 range was applied because it represents the temperature sensitivity of metabolism reported across nine species of elasmobranchs48,49. We also allocated an additional 5% energy expenditure to account for the cost of growth, which is estimated at approximately 8.7–14.6 cm year−1 given the size range of N. cepedianus in Norfolk Bay50, and is consistent with the little available literature that suggests between 3.5 and 7.2% of metabolic rate is invested in growth in sharks30,51. We also allocated another 5% increase in energy expenditure to account for the cost of reproduction, but only in one-third of the mature females, which was based on N. cepedianus probably having a three-year reproductive cycle52. Some mature females in Norfolk Bay have been found to be ovulating, in the initial stages of pregnancy, or starting a new vitellogenic cycle52. The cost of reproduction is unlikely to be much higher because Norfolk Bay and its neighbouring coastal areas are not used as pupping grounds, mating rarely occurs there, and most female N. cepedianus are non-gravid while in Norfolk Bay52.
After accounting for the effect of temperature, growth and reproduction on the estimated energy costs for each individual N. cepedianus, we then averaged energy expenditure across all individuals, to derive the mean routine energy expenditure of N. cepedianus in Norfolk Bay, assuming that our sample of 294 individuals is a reasonable representation of the population demographics at any given time. We then converted the units of routine energy expenditure from mg O2 h−1 to kJ sampling year−1, given there are 6048 h in a 36-week-period, and there is 68.3 mg O2 kJ−1 given a respiratory exchange ratio of 0.8853. We then used population estimates of N. cepedianus in Norfolk Bay at any given time26 to obtain the routine energy expenditure of the entire population while in Norfolk Bay from spring to autumn.
Energy content of prey
The bioenergetics model constructed required an estimate of energy content for the key prey species of N. cepedianus in Norfolk Bay. Previous work identified the key prey species of N. cepedianus in Norfolk Bay25. The key prey species were categorized into fur seal Arctocephalus pusillus, other mammals, M. antarcticus, school shark Galeorhinus galeus, dogshark Squalus acanthias, unidentified sharks, eagle ray Myliobatis tenuicaudatus, Melbourne skate Spiniraja whitleyi, banded stingaree Urolophus cruciatus, unidentified batoids, elephantfish Callorhinchus milii, teleosts, and cephalopods (Table 1). The average available energy content (kJ) of each of these prey species or groups was calculated as the product of the energy-density of the tissue and their average body mass, multiplied by a factor of 0.73 to account for energy assimilation efficiency54. Tissue energy-density values were obtained for the various prey species or groups from bomb calorimetry measurements published in the literature, and where such data were unavailable we substituted for closely related species (Table 1). The body masses of the various chondrichthyan prey species were calculated by applying length-weight conversions derived using published and unpublished data (Table 1). The body lengths used in these length-weight conversions were recorded during long line sampling and gill-net surveys in Norfolk Bay and adjoining Frederick Henry Bay24,55; McAllister unpublished data; CSIRO, Australia, unpub. data. Average body mass for cephalopods was based on arrow squid Nototodarus gouldi, as it is abundant in the bay, and the most commonly consumed cephalopod species25. Average body mass of marine mammal was based on fur seal adult males and sub-adult of both sexes, which are the most common marine mammal in the Norfolk Bay region56. We assume that the whole-body of the prey is consumed by N. cepedianus, even if it is consumed by several individual N. cepedianus, as is likely the case for large prey items, such as mammals.
Model simulations, variants and sensitivity analyses
To account for uncertainty in model structure and input parameters, we built variants of the bioenergetics model by incorporating parameter confidence through Monte Carlo simulations. Using our calculated value for the routine energy expenditure of the entire population of N. cepedianus in Norfolk Bay across the 36-week period each year, and the total energy available from each prey species (or group), we ran three model variants to estimate local predation rates by N. cepedianus on the various prey in Norfolk Bay. The three model variants provide estimates of N. cepedianus predation rates depending on the relative fraction that each prey species (or group) contributes to supporting the energy expenditure of N. cepedianus, which we based on three alternative techniques that are commonly used for measuring diet composition: (1) the frequency a prey species occurs in the diet, (2) the weight of each prey species (or group) as a fraction of the total weight of all prey consumed, and (3) the number of each prey consumed as a fraction of the total number of all prey consumed33.
The model estimates predation rate (Px; sampling year−1) on species x (or group x) over a sampling year following the equation, Px = MR × Fx/Ex, where MR (kJ sampling year−1) is the routine energy expenditure of N. cepedianus, Ex is the available energy content (kJ) of species x (or group x), and Fx is the fraction of the diet of N. cepedianus represented by species x (or group x). Thus, the three model variants provide alternative estimates of Fx, therefore leading to different estimates of Px. In model variant 1, which focuses only on the predation rate of M. antarcticus, Fx was set as 0.25 based on stomach flushing data that showed 25% of N. cepedianus sampled in Norfolk Bay had consumed M. antarcticus 25. In model variant 2, Fx is set as equal to the partly digested weight of each prey species (or group) in the stomach of N. cepedianus, divided by the total partly digested weight of all prey items present in the stomach. The weight of prey items was measured from regurgitated stomach contents at varying stages of digestion, obtained from stomach flushing N. cepedianus sampled in Norfolk Bay25,45. In model variant 3, Fx is the proportion that each prey contributes to the overall diet, calculated by multiplying the average weight of each prey (Table 1) by the number of that prey present in the stomach (Appendix S1), as determined from stomach flushing of N. cepedianus sampled in Norfolk Bay25,45. For the much larger mammalian prey species, ingestion weight was calculated as the average weight of the ingested pieces of mammal (Table 1). We only included pieces of mammal in the weight calculations that minimal digestion had occurred.
The three model variants were run using two alternative variations in the population size estimate of N. cepedianus in Norfolk Bay (Table 2). For the two population scenarios, we considered a log-normal distribution with mean of 562 ± 71 sharks, and another scenario with mean of 1130 ± 137 sharks26. These means are based on mark-recapture estimates spanning 20 and 44 weeks sampling, respectively. Given the potential temporal fluctuations in abundance in Norfolk Bay over the study period, both mean values are included in the model to span the potential range of abundance values for Norfolk Bay in this study. Natural fluctuations in abundance occur over days or weeks, as evident by tracking data that shows individual N. cepedianus move in and out of the bay during a season26. All simulations were done in the statistical package R57. For each of the six scenarios considered, 1000 Monte Carlo simulations were performed to account for uncertainty in N. cepedianus prey composition, body mass, and routine energy expenditure parameters (Q10, water temperature, the allometric power equation coefficient and the allometric power equation exponent) by drawing samples from a log-normal distribution with mean and standard deviation as presented in Table 1.
Electronic supplementary material
Acknowledgements
We thank Michelle Treloar and Jonah Yick for length-to-weight data. We also acknowledge the CSIRO, Australia, in particular John Stevens for length measurements of prey. This study was supported by grants to A.B. from the Save Our Seas Foundation, Winifred Violet Scott Foundation and the Holsworth Wildlife Research Endowment. E.P.S. holds a South African Claude Leon Foundation Postdoctoral Fellowship.
Author Contributions
A.B. formulated the idea and secured the funding. A.B., E.P.S. and N.L.P. developed the concept. A.B. and K.A. conducted the field work. E.P.S., A.B., M.B., N.L.P. and C.L.D. conducted the analyses. A.B. wrote the manuscript with contributions from all authors.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-13388-y.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Williams TM, Estes JA, Doak DF, Springer AM. Killer appetites: assessing the role of predators in ecological communities. Ecology. 2004;85:3373–3384. doi: 10.1890/03-0696. [DOI] [Google Scholar]
- 2.Laundré J. Summer predation rates on ungulate prey by a large keystone predator: how many ungulates does a large predator kill? Journal of Zoology. 2008;275:341–348. doi: 10.1111/j.1469-7998.2008.00443.x. [DOI] [Google Scholar]
- 3.Sand H, et al. Summer kill rates and predation pattern in a wolf–moose system: can we rely on winter estimates? Oecologia. 2008;156:53–64. doi: 10.1007/s00442-008-0969-2. [DOI] [PubMed] [Google Scholar]
- 4.Elbroch LM, Allen ML, Lowrey BH, Wittmer HU. The difference between killing and eating: ecological shortcomings of puma energetic models. Ecosphere. 2014;5:1–16. doi: 10.1890/ES13-00373.1. [DOI] [Google Scholar]
- 5.Laundré JW. Behavioral response races, predator-prey shell games, ecology of fear, and patch use of pumas and their ungulate prey. Ecology. 2010;91:2995–3007. doi: 10.1890/08-2345.1. [DOI] [PubMed] [Google Scholar]
- 6.Barnett A, Semmens JM. Sequential movement into coastal habitats and high spatial overlap of predator and prey suggest high predation pressure in protected areas. Oikos. 2012;121:882–890. doi: 10.1111/j.1600-0706.2011.20000.x. [DOI] [Google Scholar]
- 7.Terborgh J, et al. Ecological meltdown in predator-free forest fragments. Science. 2001;294:1923–1926. doi: 10.1126/science.1064397. [DOI] [PubMed] [Google Scholar]
- 8.Wirsing AJ, Ripple WJ. A comparison of shark and wolf research reveals similar behavioral responses by prey. Frontiers in Ecology and the Environment. 2011;9:335–341. doi: 10.1890/090226. [DOI] [Google Scholar]
- 9.Power ME, et al. Challenges in the quest for keystones. BioScience. 1996;46:609–620. doi: 10.2307/1312990. [DOI] [Google Scholar]
- 10.Tyrrell M, Link J, Moustahfid H. The importance of including predation in fish population models: implications for biological reference points. Fish Res. 2011;108:1–8. doi: 10.1016/j.fishres.2010.12.025. [DOI] [Google Scholar]
- 11.Plagányi ÉE. Fitting the puzzle—modeling species interactions in marine ecosystems. Bull Mar Sci. 2013;89:397–417. doi: 10.5343/bms.2011.1126. [DOI] [Google Scholar]
- 12.Hunsicker ME, et al. Functional responses and scaling in predator-prey interactions of marine fishes: contemporary issues and emerging concepts. Ecology Letters. 2011;14:1288–1299. doi: 10.1111/j.1461-0248.2011.01696.x. [DOI] [PubMed] [Google Scholar]
- 13.Hewitt DA, Hoenig JM. Comparison of two approaches for estimating natural mortality based on longevity. Fishery Bulletin. 2005;103:433–437. [Google Scholar]
- 14.Bax N. The significance and prediction of predation in marine fisheries. ICES J Mar Sci. 1998;55:997–1030. doi: 10.1006/jmsc.1998.0350. [DOI] [Google Scholar]
- 15.Noren DP. Estimated field metabolic rates and prey requirements of resident killer whales. Marine Mammal Science. 2011;27:60–77. doi: 10.1111/j.1748-7692.2010.00386.x. [DOI] [Google Scholar]
- 16.Dowd W, Brill RW, Bushnell PG, Musick JA. Estimating consumption rates of juvenile sandbar sharks (Carcharhinus plumbeus) in Chesapeake Bay, Virginia, using a bioenergetics model. Fishery Bulletin. 2006;104:332–342. [Google Scholar]
- 17.Forcada J, Malone D, Royle JA, Staniland IJ. Modelling predation by transient leopard seals for an ecosystem-based management of Southern Ocean fisheries. Ecological Modelling. 2009;220:1513–1521. doi: 10.1016/j.ecolmodel.2009.03.020. [DOI] [Google Scholar]
- 18.Reisinger RR, de Bruyn PJN, Bester MN. Predatory impact of killer whales on pinniped and penguin populations at the Subantarctic Prince Edward Islands: fact and fiction. Journal of Zoology. 2011;285:1–10. [Google Scholar]
- 19.Mourier J, et al. Extreme inverted trophic pyramid of reef sharks supported by spawning groupers. Current Biology. 2016;26:2011–2016. doi: 10.1016/j.cub.2016.05.058. [DOI] [PubMed] [Google Scholar]
- 20.Travis J, et al. Integrating the invisible fabric of nature into fisheries management. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:581–584. doi: 10.1073/pnas.1305853111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hunsicker ME, et al. Potential for top-down control on tropical tunas based on size structure of predator-prey interactions. Marine Ecology Progress Series. 2012;445:263–U535. doi: 10.3354/meps09494. [DOI] [Google Scholar]
- 22.Barnett A, Braccini JM, Awruch CA, Ebert DA. An overview on the role of Hexanchiformes in marine ecosystems: biology, ecology and conservation status of a primitive order of modern sharks. Journal of Fish Biology. 2012;80:966–990. doi: 10.1111/j.1095-8649.2012.03242.x. [DOI] [PubMed] [Google Scholar]
- 23.Stevens, J. D. & West, G. J. Investigation of school and gummy shark nursery areas in south eastern Australia Fisheries Research and Development Corporation Project 93/061 final report (1997).
- 24.Barnett A, Stevens JD, Frusher SD, Semmens JM. Seasonal occurrence and population structure of the broadnose sevengill shark Notorynchus cepedianus in coastal habitats of south-east Tasmania. Journal of Fish Biology. 2010;77:1688–1701. doi: 10.1111/j.1095-8649.2010.02810.x. [DOI] [PubMed] [Google Scholar]
- 25.Barnett A, et al. Predator-prey relationships and foraging ecology of a marine apex predator with a wide temperate distribution. Marine Ecology Progress Series. 2010;416:189–200. doi: 10.3354/meps08778. [DOI] [Google Scholar]
- 26.Dudgeon CL, Pollock KH, Braccini JM, Semmens JM, Barnett A. Integrating acoustic telemetry into mark-recapture models to improve the precision of apparent survival and abundance estimates. Oecologia. 2015;178:761–772. doi: 10.1007/s00442-015-3280-z. [DOI] [PubMed] [Google Scholar]
- 27.Winship AJ, Trites AW, Rosen DAS. A bioenergetic model for estimating the food requirements of Steller sea lions Eumetopias jubatus in Alaska, USA. Marine Ecology Progress Series. 2002;229:291–312. doi: 10.3354/meps229291. [DOI] [Google Scholar]
- 28.Essington TE, et al. Alternative fisheries and the predation rate of yellowfin tuna in the Eastern Pacific Ocean. Ecological Applications. 2002;12:724–734. doi: 10.1890/1051-0761(2002)012[0724:AFATPR]2.0.CO;2. [DOI] [Google Scholar]
- 29.Glaser SM, Waechter KE, Bransome NC. Through the stomach of a predator: Regional patterns of forage in the diet of albacore tuna in the California Current System and metrics needed for ecosystem-based management. Journal of Marine Systems. 2015;146:38–49. doi: 10.1016/j.jmarsys.2014.07.019. [DOI] [Google Scholar]
- 30.Sundstrom LF, Gruber SH. Using speed-sensing transmitters to construct a bioenergetics model for subadult lemon sharks, Negaprion brevirostris (Poey), in the field. Hydrobiologia. 1998;371–372:241–247. doi: 10.1023/A:1017031406947. [DOI] [Google Scholar]
- 31.Lowe CG. Bioenergetics of free-ranging juvenile scalloped hammerhead sharks (Sphyrna lewini) in Kane’ohe Bay, O’ahu, HI. J Mar Biol Ecol. 2002;278:141–156. doi: 10.1016/S0022-0981(02)00331-3. [DOI] [Google Scholar]
- 32.Bethea DM, et al. Geographic and ontogenetic variation in the diet and daily ration of the bonnethead shark, Sphyrna tiburo, from the eastern Gulf of Mexico. Mar Biol. 2007;152:1009–1020. doi: 10.1007/s00227-007-0728-7. [DOI] [Google Scholar]
- 33.Baker R, Buckland A, Sheaves M. Fish gut content analysis: robust measures of diet composition. Fish and Fisheries. 2014;15:170–177. doi: 10.1111/faf.12026. [DOI] [Google Scholar]
- 34.Brodie S, et al. Improving consumption rate estimates by incorporating wild activity into a bioenergetics model. Ecology and Evolution. 2016;6:2262–2274. doi: 10.1002/ece3.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barnett A, Abrantes KG, Stevens JD, Bruce BD, Semmens JM. Fine-scale movements of the broadnose sevengill shark and its main prey, the gummy shark. Plos One. 2010;5:e15464. doi: 10.1371/journal.pone.0015464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.AFMA. http://www.afma.gov.au/fisheries/southern-eastern-scalefish-shark-fishery/ Accessed on the 8 December 2016 (2016).
- 37.Abrantes KG, Barnett A. Intrapopulation variations in diet and habitat use in a marine apex predator, the broadnose sevengill shark Notorynchus cepedianus. Marine Ecology Progress Series. 2011;442:133–148. doi: 10.3354/meps09395. [DOI] [Google Scholar]
- 38.Ebert DA. Observations on the predatory behaviour of the sevengill shark Notorynchus cepedianus. South African Journal of Marine Science. 1991;11:455–465. doi: 10.2989/025776191784287637. [DOI] [Google Scholar]
- 39.Barnett A, Yick JL, Abrantes KG, Awruch CA. Trophic ecology of an abundant predator and its relationship with fisheries. Marine Ecology Progress Series. 2013;494:241–248. doi: 10.3354/meps10577. [DOI] [Google Scholar]
- 40.Kirkwood R, et al. Continued population recovery by Australian fur seals. Mar. Freshw. Res. 2010;61:695–701. doi: 10.1071/MF09213. [DOI] [Google Scholar]
- 41.Hussey, N. E., McCann, H. M., Cliff, G., Dudley, S. F. & Wintner, S. P. In Global Perspectives on the Biology and Life History of the White Shark (ed. Michael L. Domeier), CRC Press, Boca Raton FL (2012).
- 42.Blower DC, Pandolfi JM, Bruce BD, Gomez-Cabrera MD, Ovenden JR. Population genetics of Australian white sharks reveals fine-scale spatial structure, transoceanic dispersal events and low effective population sizes. Marine Ecology Progress Series. 2012;455:229–244. doi: 10.3354/meps09659. [DOI] [Google Scholar]
- 43.Last PR, et al. Long-term shifts in abundance and distribution of a temperate fish fauna: a response to climate change and fishing practices. Global Ecology and Biogeography. 2011;20:58–72. doi: 10.1111/j.1466-8238.2010.00575.x. [DOI] [Google Scholar]
- 44.Barnett A, Abrantes KG, Stevens JD, Semmens JM. Site fidelity and sex-specific migration in a mobile apex predator: implications for conservation and ecosystem dynamics. Animal Behaviour. 2011;81:1039–1048. doi: 10.1016/j.anbehav.2011.02.011. [DOI] [Google Scholar]
- 45.Barnett A, Redd KS, Frusher SD, Stevens JD, Semmens JM. Non-lethal method to obtain stomach samples from a large marine predator and the use of DNA analysis to improve dietary information. J Mar Biol Ecol. 2010;393:188–192. doi: 10.1016/j.jembe.2010.07.022. [DOI] [Google Scholar]
- 46.Payne NL, et al. A new method for resolving uncertainty of energy requirements in large water breathers: the “mega-flume’ seagoing swim-tunnel respirometer. Methods in Ecology and Evolution. 2015;6:668–677. doi: 10.1111/2041-210X.12358. [DOI] [Google Scholar]
- 47.Braccini, J. M., Walker, T. I. & Gason, A. S. GHATF shark survey of population abundance and population size composition for target, byproduct and bycatch species. (Department of Primary Industries, Fisheries Research Branch, 2009).
- 48.Chen WK, Liu KM, Liao YY. Bioenergetics of juvenile whitespotted bamboo shark Chiloscyllium plagiosum [Anonymous (Bennett)] Journal of Fish Biology. 2008;72:1245–1258. doi: 10.1111/j.1095-8649.2008.01766.x. [DOI] [Google Scholar]
- 49.Whitney NM, Lear KO, Gaskins LC, Gleiss AC. The effects of temperature and swimming speed on the metabolic rate of the nurse shark (Ginglymostoma cirratum, Bonaterre) J Mar Biol Ecol. 2016;477:40–46. doi: 10.1016/j.jembe.2015.12.009. [DOI] [Google Scholar]
- 50.Braccini JM, et al. Incorporating heterogeneity into growth analyses of wild and captive broadnose sevengill sharks Notorynchus cepedianus. Aquatic Biology. 2010;9:131–138. doi: 10.3354/ab00246. [DOI] [Google Scholar]
- 51.Parsons GR. Geographic variation in reproduction between two populations of the bonnethead shark, Sphyrna tiburo. Enviromental Biology of Fishes. 1993;38:25–35. doi: 10.1007/BF00842901. [DOI] [Google Scholar]
- 52.Awruch, C. A., Jones, S. M., Asorey, M. G. & Barnett, A. Non-lethal assessment of the reproductive status of broadnose sevengill sharks (Notorynchus cepedianus) to determine the significance of habitat use in coastal areas. Conservation Physiology2, doi:10.1093/conphys/cou013 (2014). [DOI] [PMC free article] [PubMed]
- 53.Dejours, P. Principles of comparative respiratory physiology. (sole distributors for the USA and Canada, Elsevier North-Holland, 1981).
- 54.Brett, J. R. & Groves, D. D. In Fish Physiology (eds William S. Hoar, David J. Randall & Edward M. Donaldson) (Elsevier Science & Technology, 1979).
- 55.Yick JL, Barnett A, Tracey SR. The trophic ecology of two abundant mesopredators in south-east coastal waters of Tasmania, Australia. Mar Biol. 2012;159:1183–1196. doi: 10.1007/s00227-012-1899-4. [DOI] [Google Scholar]
- 56.Arnould J, Warneke R. Growth and condition in Australian fur seals (Arctocephalus pusillus doriferus)(Carnivora: Pinnipedia) Australian Journal of Zoology. 2002;50:53–66. doi: 10.1071/ZO01077. [DOI] [Google Scholar]
- 57.R Development Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. (2016).
- 58.Arnould JPY, Boyd IL, Speakman JR. Measuring the body composition of Antarctic fur seals (Arctocephalus gazella): Validation of hydrogen isotope dilution. Physiol Zool. 1996;69:93–116. doi: 10.1086/physzool.69.1.30164202. [DOI] [Google Scholar]
- 59.Walker TI. Spatial and temporal variation in the reproductive biology of gummy shark Mustelus antarcticus (Chondrichthyes: Triakidae) harvested off southern Australia. Mar. Freshw. Res. 2007;58:67–97. doi: 10.1071/MF06074. [DOI] [Google Scholar]
- 60.Eder E, Lewis M. Proximate composition and energetic value of demersal and pelagic prey species from the SW Atlantic Ocean. Mar. Ecol. Prog. Ser. 2005;291:43–52. doi: 10.3354/meps291043. [DOI] [Google Scholar]
- 61.Filiz H, Mater S. A preliminary study on length-weight relationships for seven elasmobranch species from North Aegean Sea, Turkey. EÜ Su Ürünleri Dergisi. 2002;19:401–409. [Google Scholar]
- 62.Bassos-Hull K, et al. Life history and seasonal occurrence of the spotted eagle ray, Aetobatus narinari, in the eastern Gulf of Mexico. Environ. Biol. Fish. 2014;97:1039–1056. doi: 10.1007/s10641-014-0294-z. [DOI] [Google Scholar]
- 63.Willcox, S., Lyle, J. & Steer, M. Tasmanian arrow squid fishery—status report 2001. Tasmanian Aquaculture and Fisheries Institute, Hobart (2001).
- 64.Pecl G, Moltschaniwskyj N, Tracey S, Jordan A. Inter-annual plasticity of squid life history and population structure: ecological and management implications. Oecologia. 2004;139:515–524. doi: 10.1007/s00442-004-1537-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


