Abstract
Purpose
Prenatal exposure to cocaine (PCE) may alter areas of the brain dense with monoamine receptors such as the prefrontal cortex and negatively affect cognitive processes implicated in executive function (EF). This study investigated the effects of PCE on EF at 12 and 15 years.
Methods
EF was examined in 189 PCE and 183 non-cocaine exposed (NCE) children who were primarily African American and of low socioeconomic status. Caregivers rated their child on the Behavior Rating Inventory of Executive Function (BRIEF) at ages 12 and 15. The BRIEF includes two summary scales and eight subscales: Behavioral Regulation Index (BRI) (Inhibit, Shift, and Emotion) and Metacognition Index (MI) (Monitor, Working Memory, Plan/Organize, Organization of Materials and Task Completion). Two additional measures were included at age 15 (BRIEF Self-Report and the CANTAB Stockings of Cambridge (SOC)).
Results
Girls with PCE were perceived by caregivers to have more behavioral regulation problems at age 12 (p < 0.005) and more metacognitive problems at age 12 (p < 0.003) than NCE females, but there was no association for males. PCE girls improved in behavioral regulation (p < 0.05) and metacognition (p < 0.04) from 12 to 15 years compared to NCE girls based on caregiver report. By self-report PCE was associated with problems of inhibition (p < 0.006). Girls with PCE performed more poorly on number of moves to complete the SOC, requiring planning and problem solving, than NCE girls.
Conclusion
Prenatally cocaine exposed girls were perceived by caregivers as having problems of behavioral regulation, and by self-report, inhibitory control problems. Girls with PCE also performed more poorly on a task of planning and problem solving at age 15 which corresponded to caregiver report at age 12. Early assessment and remediation of these weaknesses in girls may improve school performance and behavior associated with poor EF.
Keywords: Executive function, Cocaine, Prenatal, Behavioral regulation, Metacognition
1. Introduction
During the late 1980’s to mid-1990’s, approximately 18% of live births, particularly in urban low socioeconomic status areas, were affected by prenatal cocaine exposure (PCE) (Kandel et al., 1998; Ostrea et al., 1992). This level decreased to a relatively steady rate of 5.4% of pregnant US women continuing to report illicit drug use during 2012–2013 (SAMSHA, 2013). Cocaine permeates the fetal blood brain barrier and acts as a powerful central nervous system stimulant affecting areas of the brain dense with monoamine receptors (e.g. dopamine, serotonin & norepinephrine). These neurotransmitters, in high concentrations in the frontal cortex, play a critical role in fetal brain development including neuronal organization, cell growth and differentiation (Malanga & Kosofsky, 1999). PCE may also act indirectly causing additional CNS alterations related to poor maternal nutrition, fetal growth restriction, and hypoxic states due to cocaine induced vasoconstriction (Volpe, 1992), Mounting evidence indicates that these combined factors will have negative effects on frontal brain circuitry and subsequently early arousal regulatory systems which include regulation of attentional states, executive function, information processing and learning (Mayes, 2002) (Minnes et al., 2014; Singer et al., 2004; Singer et al., 2008) (McLaughlin et al., 2011; Min et al., 2014a, b; Minnes et al., 2010).
Executive functions (EF) are a group of higher order cognitive processes primarily regulated by the prefrontal cortex of the brain. EF includes regulatory processes necessary for goal directed behavior such as attending, selecting, initiating, implementing and overseeing thoughts, emotions and certain facets of motor and sensory function (Roth et al., 2005b). EF is a component of self-regulatory behavior, the adaptive regulation of behavior, emotion and cognition that contributes to both adaptive and non-adaptive outcomes in children, adolescents and adults (Bridgett et al., 2013, 2015; Zhou et al., 2012). Two conceptualizations of EF have developed. One conceptualization is as a single construct emphasizing a central executive control system that guide’s behavior and directs attention (Zelazo et al., 2003; Zhou et al., 2012). The other conceptualization is based largely on factor analytic work and indicates that EF is comprised of distinct but interrelated processes (Bridgett et al., 2013). The three distinct processes identified are working memory, response inhibition and cognitive flexibility/attentional set-shifting. Problems of EF, although not always readily apparent, can compromise classroom behavior, academic achievement, and social-emotional competence via negative affective expression (Bridgett et al., 2015), and increase the risk for delinquency, externalizing behaviors and substance use (Langberg et al., 2013; McDermott et al., 2013; Pentz & Riggs, 2013).
Negative PCE effects on early arousal regulatory systems, the foundation for the development of executive function (Mayes, 2002), include infant excitability, poorer state regulation, physiological lability, information processing, poor impulse control, diminished sustained attention and more emotional lability and or behavioral disorganization. Effects of PCE on EF more specifically have been found via standard neuropsychological assessment during infancy (Noland et al., 2003b), pre-school (Noland et al., 2003a) and at elementary school ages (Eyler et al., 2009; Rose-Jacobs et al., 2009; Singer et al., 2008). However, findings from longer term follow-up during adolescent development and an understanding of the role of EF in adaptive functioning of children with PCE are still evolving and inconclusive. For example, in a study of the EF component of inhibitory control in 7 to 12 year olds (Bridgett & Mayes, 2011), children with PCE made more errors on the Stroop task than non-cocaine exposed (NCE) children at 7 years. PCE children had had more age related improvements between ages 7 and 12. There were no differences in performance among boys at age 12 but PCE girls performed more poorly than NCE girls. These findings underscore the need to evaluate EF longitudinally as outcomes may differ depending on the stage of brain development, assessment type, gender and risks and/or protective factors. Parental ratings of EF that assess functional aspects of EF during school and home life indicated that 12 year old girls with PCE had more EF problems in metacognition (planning/organizing and self-monitoring) but not behavioral regulation (Minnes et al., 2014). In contrast, (Rose-Jacobs et al., 2011) did not find a specific PCE effect on EF, but rather that both prenatal marijuana and alcohol exposure and concomitant teen drug use had negative effects on EF. Taken together, these studies suggest the need to broadly examine prenatal drug exposure and current substance use as a predictor of EF problems in adolescence.
Increasing physiologic evidence has been offered for the negative effects of PCE on frontal brain development and function using MRI. Studies suggest negative effects from prenatal cocaine exposure on both grey and white matter areas of the brain that may underlie problems of EF in children (Dow-Edwards et al., 2006, Grewen et al., 2014, Warner et al., 2006). In late adolescence a significant interaction between PCE, tobacco exposure and greater cortical thickness indicates the importance of evaluating drug interaction effects (Gautam et al., 2015). Research by Lebel and colleagues (Lebel et al., 2013) demonstrated an association between structural and functional brain abnormalities, white matter tract integrity, and behavioral performance on EF tasks compromised by PCE.
Environmental and behavioral factors also known to adversely affect EF development frequently co-occur with PCE. Adverse rearing environments, particularly low income, low maternal education and insensitive/unresponsive parenting, may negatively affect development of EF in early childhood (Blair & Ursache, 2011) and adolescence (Evans & Schamberg, 2009) raising important questions about the potential intergenerational transmission of self-regulatory behavior more broadly and executive function more specifically (Bridgett et al., 2015). Several studies have also found an association between early exposure to violence, including witnessing parent’s interpersonal violence and deficits in EF (Edalati & Krank, 2015). The negative impact that adverse environments and exposure to violence can have on cognition and EF may largely be attributable to the effect of stress physiology on ongoing brain development (Edalati & Krank, 2015).
Pregnant cocaine using mothers ingest higher rates of other recreational drugs (Singer et al., 2002b; Singer et al., 2004) that also have negative effects on EF such as prenatal alcohol (Burden et al., 2005) and marijuana (Fried & Smith, 2001). In addition, adolescents’ current drug use, including heavy alcohol use (Squeglia et al., 2009) and marijuana use (Jacobus et al., 2009; Schweinsburg et al., 2008) can also independently affect EF performance. Elevated blood lead levels are also associated with impairments in EF (Chiodo et al., 2004; Singer et al., 2008; Trope et al., 2001) and occur at high rates among high risk prenatally drug exposed children.
Despite the research to date, it remains unclear how EF skills change over the course of adolescent development, particularly when compromised early by PCE and later environmental influences, and how EF deficits may impact daily functioning of affected children. Studies to date have typically used few sources of information to assess EF, limiting an in-depth understanding of how EF deficits may interfere with learning and social interaction. More importantly, most studies are cross-sectional in nature and therefore do not capture the developmental changes that emerge over time and how individuals respond when increased EF challenges are encountered in the later grades in school (Taylor et al., 2013). Also, studies rarely assess a full range of potentially important confounders including exposure violence.
The current study examined not only developmental differences in caregiver perceptions of EF performance at ages 12 and 15 years in youth with and without PCE, but also examined adolescent self-perception of EF and neuropsychological assessment of EF at 15 years. Based on our findings at 12 years (Minnes et al., 2014), we hypothesized that girls with PCE would continue to show more impaired EF on all parameters at 15 years and that their own drug use at age 12 (e.g. tobacco, alcohol & marijuana) would be associated with poorer EF at age 15. The effects of early blood lead and exposure to violence were hypothesized to also compromise EF. Since our studies have previously found that foster or adoptive caregivers rate their children with PCE as having more behavioral problems, we expect that this will also be reflected in ratings of executive function.
2. Method
2.1. Participants
Ninety two percent of the original sample, 372 children (189 PCE & 183 NCE) were assessed at age 12 or 15 on any of three EF measures (Singer et al., 2004; Singer et al., 2008). To investigate the developmental effects of PCE, mothers and their newborn infants were recruited at birth from a large, urban, county teaching hospital. A nurse recruiter approached women screened for prenatal substance use at delivery. Mothers received a urine drug screen if they self-reported drug use, appeared intoxicated at delivery, received no prenatal care or had previous involvement with the Department of Children and Family Services. Women with major depression, schizophrenia, bipolar disorder or intellectual disability (per medical chart review), or who were age < 19 years, positive for HIV or non-English speaking were excluded (54 mother/infant pairs). Subsequently, 155 of these 647 women refused to participate (49 PCE & 106 NCE), with an additional 23 women (9 PCE &14 NCE) not coming to their newborn enrollment visit. Four hundred fifteen women (218 PCE & 197 NCE) of the 647 mothers were enrolled in the study. Since enrollment, 12 children died (9 PCE & 3 NCE). The 31 adolescents not included in the study were more likely to be white, male and to have birth mothers who were older, had higher WAIS-R picture completion scale scores and higher average cigarette use per day compared to the 372 participating adolescents.
Children were assigned to PCE or NCE groups based on maternal self-report of cocaine use during pregnancy, maternal and infant biologic data (urine), and infant meconium screening. The Syva Emit method (Syva Co, Palo Alto, California) was used for urine analyses with follow-up gas chromatography to confirm positive analyses. Infant meconium was collected in the newborn nursery to further identify cocaine and other drug metabolites, including the presence of benzoylecgonine (BZE), meta-hydroxybenzoylecgonine (m-OH-bze), and cocaethylene (major metabolites for cocaine). PCE status was assigned based on positive results on any one of the assessments (e.g. maternal or child). NCE status was assigned if all assessments on cocaine were negative.
2.2. Procedures
Informed consent approved by the hospital’s Institutional Review Board (IRB), were reviewed and signed by mothers and teens to indicate their assent at the time of this study. Their decision to assent or not was respected in all cases regardless of their caregiver’s agreement to participate. Medical records were reviewed to extract maternal and infant birth and demographic data. A research assistant interviewed mothers regarding prenatal drug use at post-natal follow-up. Frequency and amount of drug use during the month prior to and during each trimester of pregnancy were assessed. The number of tobacco cigarettes, marijuana “joints” smoked and the number of drinks of beer, wine, or hard liquor consumed per day were computed, with each drink equivalent to 0.5 oz. of absolute alcohol. For cocaine, the number of “rocks” consumed and/or the amount of money spent per day were noted and converted to a standard “unit” of cocaine. Frequency of use ranging from 0 (not at all) to 7 (daily use), was multiplied by the amount used per day to compute an average use per week (per day for cigarettes) score for the month prior to pregnancy and each trimester. A total average pregnancy use variable for each drug was computed.
Continued drug use by caregivers was updated at each follow-up visit. Caregiver measures of receptive vocabulary and non-verbal reasoning were administered during the first year of the study and any time there was a change in caregiver until age 15 years.
At the 12- and 15-year assessments, a trained research assistant interviewed the child’s current caregiver, who completed a rating of their child’s EF and other self-report measures. Children completed neuropsychological assessments and questionnaires about their substance use and behavior administered by a second research assistant. Subjects received $100 ($50 each for caregiver & child) at 12 years and $150 ($100 for child & $50 for the caregiver) at 15 years.
2.3. Primary child outcome measures
The Behavior Rating Inventory of Executive Function (BRIEF) (Roth et al., 2005a) and BRIEF Self-Report Version (BRIEF-SR) (Gioia et al., 2000). The BRIEF contains 86 items (80 items for BRIEF-SR) which assesses ecologically valid EF behaviors in home and school environments. Three clinical scales, Inhibit, Shift and Emotional Control, comprise the Behavioral Regulation Index (BRI) (α = 0.95 at 12 years, 0.96 at 15 years). The remaining clinical scales, Initiate, Working Memory, Plan/Organize, Organization of Materials, and Monitor, form the Meta-cognition Index (MI) (α =0.97 at 12 and 15 years). t-Scores, adjusted for age and gender, greater than or equal to 65 are indicative of EF problems which interfere with daily functioning. The BRIEF was completed by caregivers at ages 12 and 15, with the BRIEF-SR completed by youth at age 15. Subjects with IQ scores <70 were excluded due to potentially low reading levels.
The Cambridge Neuropsychological Test Automated Battery, Stockings of Cambridge (CANTAB eclipse, Version 3) (Fray & Robbins, 1996) was administered at 15 years. The Stockings of Cambridge (SOC) is a spatial planning test that measures frontal lobe function, including working memory and planning skills. SOC Score used in analysis was the number of problems solved in minimum moves (the number of occasion which the subject has successfully completed a test problem in the minimum possible number of moves) (range 0–12). This is a measure of succinct overall planning accuracy. SOC mean initial thinking time and mean subsequent thinking time were also analyzed. Subjects with IQ scores <70 were not given the clinical EF assessment.
2.4. Potential covariates and demographic characteristics
Demographic and medical characteristics, including birth weight (grams), height (cm), head circumference (cm), gestational age, race, maternal age, parity, and number of prenatal care visits, were collected from the birth records. Occupation, years of education and number in family were collected via interview. The Maternal Post-Partum Interview and Update assessed drug use during pregnancy and during the last 30 days at follow-up visits (ages 12 and 15). It included summary measures of average cigarette (per day), alcohol, marijuana and cocaine (per week) use over the pregnancy (Singer et al., 2002a; Streissguth, 1986).
Maternal and/or current caregiver receptive vocabulary was measured using the Peabody Picture Vocabulary Test-Revised (PPVT-R) (Dunn & Dunn, 1981). Two subtests (Block Design & Picture Completion) of the Wechsler Adult Intelligence Scale-Revised (WAIS-R) (Wechsler, 1981) were used to estimate non-verbal intelligence. The Global Severity Index (GSI) of the Brief Symptom Inventory (BSI) (Derogatis, 1992) was used as a measure of caregiver psychological distress. The BSI is a 53-item self-report questionnaire with scores ranging from 0 to 4. Reliability (α) of the BSI Global Severity Index (GSI) (Min et al., 2013) was 0.95. The Home Observation for Measurement of the Environment (HOME) - Early Adolescent version was employed as an interview with caregivers to assess quality of the home environment (Caldwell & Bradley, 1984).
The Wechsler Intelligence Scale for Children-Fourth Edition (WISC-IV) (Wechsler, 2003) Full Scale IQ obtained at age 11 was used to evaluate the effect of PCE on EF independent of IQ. The Assessment of Liability and Exposure to Substance Use and Antisocial Behavior© (ALEXSA©) (Ridenour et al., 2009), an illustration-based, computerized, audio, self-report of substance use risk factors, was completed by children at 9, 10, 11, and 12 years of age, with high levels of reliability and validity (Ridenour et al., 2009; Ridenour et al., 2011; Ridenour et al., 2012). The ALEXSA violence exposure subscale has 8-items: ever witnessing/ experiencing a beating, being chased or threatened, a robbing or mugging, and a stabbing or shooting (∝ = 0.74), each rated on a 5-point scale (0 times to 5 times) with higher scores representing more violence exposure. Average total scores were used. Blood lead level was examined as a covariate related to EF outcomes in a subset of this sample (see Min et al., 2009 for a detailed description of the blood lead assessment) (Min et al., 2009).
2.5. Statistical analysis
Positively skewed data were normalized using log transformation (prenatal drug exposure, GSI/BSI, & lead). Continuous data were compared by cocaine group status using t-tests or the Wilcoxon-Mann-Whitney test. Pearson Chi-Square test or Fisher’s Exact test were used for categorical variables. Pearson’s and Spearman’s correlations were completed to examine the correlations between key independent and outcome variables. The effects of PCE on caregiver rating of EF were evaluated using a mixed linear model with maximum likelihood estimation procedures. Unstructured covariance matrix was used to account for correlated responses within the subject. Missing data were modeled with full-information maximum likelihood estimation which uses all available information from the observed data. Multiple regression analyses were used to assess group differences in adolescent self-reported EF and performance on the CANTAB SOC at age 15.
Covariates correlated with each of the two summary scores of the BRIEF (caregiver and self-report), the BRIEF subscale scores and the CANTAB outcome variables at p < 0.2 and different by cocaine group status at p < 0.2, at either 12 or 15 years were evaluated in the model stepwise. Covariates were retained in the model if they were significant at p < 0.10 or caused substantial change (>10%) in the PCE coefficient. Cocaine status, time, and cocaine by time interaction were considered predictors if they were significant in the final model at ≤ 0.05, two tailed. Based on our previous findings (Minnes et al., 2014), longitudinal analyses were conducted separately for males and females and an interaction term of PCE by child gender was tested for cross-sectional analyses.
When main effects for PCE were noted, follow-up analyses were conducted including current caregiver type (PCE biologic/relative, PCE foster/adoptive care and NCE). Head circumference (used as a continuous variable) and 11 year Full Scale IQ, due to their potential relationship with EF, were evaluated as mediators using the criteria of Baron and Kenny (Baron & Kenny, 1986). The effects of blood lead level at 2 or 4 years, if no 2 year data existed, were evaluated last to examine additive or confounding effects of lead exposure.
3. Results
3.1. Characteristics of the sample
Table 1 indicates that at birth, children with PCE had smaller head circumferences, birth weights and birth lengths corrected for gestational age, had higher incidence of head circumference <10% and were more likely to be small for gestational age than NCE children. PCE children were more likely to be placed in non-relative foster or adoptive care at both 12 and 15 years, and to have lower blood lead levels at 2 and 4 years than NCE controls. Table 2 presents demographic, cognitive and drug use data for birth mothers and current caregivers. Women who used cocaine were older, had more children, higher levels of psychological stress and fewer prenatal care visits and years of education at infant birth than women who did not use cocaine. They were also less likely to be married. Women who used cocaine also consumed greater amounts of other drugs during pregnancy including tobacco, alcohol and marijuana. Current caregivers of NCE children had more years of education and smoked fewer cigarettes per day than caregivers of PCE children. Table 3 displays the correlations between PCE, the BRIEF at age 12 and other potential confounders for boys (above the diagonal) and girls (below the diagonal). Of note are the significant correlations between PCE and the BRI, MRI and SOC for girls but not boys (all p’s < 0.01).
Table 1.
Child demographics.
| Cocaine (n = 189)
|
Non-cocaine (n = 183)
|
p | |||
|---|---|---|---|---|---|
| M | SD | M | SD | ||
| Gestational age | 37.81 | 2.86 | 38.46 | 2.86 | 0.03 |
| Hobel neonatal risk score | 7.49 | 16.55 | 5.83 | 15.80 | 0.32 |
| Birth length (cm)* | 47.32 | 3.97 | 49.14 | 3.73 | <0.0001 |
| Head circumference (cm)* | 32.29 | 2.15 | 33.48 | 2.38 | <0.0001 |
| Birth weight (grams)* | 2712 | 649 | 3106 | 700 | <0.0001 |
| Male, n (%) | 85 | 44.97 | 89 | 48.63 | 0.48 |
| African-American, n (%) | 155 | 82.01 | 147 | 80.33 | 0.68 |
| Head circumference < 10th%, n (%) | 28 | 15.05 | 8 | 4.42 | 0.0006 |
| Adopted/foster care at 12 years, n (%) | 45 | 26.63 | 7 | 4.14 | <0.0001 |
| Adopted/foster care at 15 years, n (%) | 47 | 27.01 | 6 | 3.49 | <0.0001 |
| 2 years and/or 4 years lead levela | 7.00 | 4.13 | 8.02 | 4.60 | 0.04 |
| IQ at 11 years | 81.39 | 11.67 | 83.82 | 14.41 | 0.07 |
| Violence exposure at 12 years | 0.63 | 0.76 | 0.57 | 0.80 | 0.50 |
| Early (age ≤ 12) drug use, n (%) | 61 | 33.15 | 57 | 32.20 | 0.85 |
| Age at assessment 12 years | 12.07 | 0.27 | 12.11 | 0.24 | 0.18 |
| Age at assessment 15 years | 15.67 | 0.24 | 15.68 | 0.29 | 0.77 |
p-value is adjusted for prematurity.
Sub-sample size of lead is 290, included 147 CE and 143 NCE.
Table 2.
Maternal and current caregiver characteristics.
| PCE (n = 189)
|
NCE (n = 183)
|
p | |||
|---|---|---|---|---|---|
| M | SD | M | SD | ||
| Maternal | |||||
| Mother’s age at birth | 29.65 | 4.98 | 25.50 | 4.73 | <0.0001 |
| Parity | 3.50 | 1.88 | 2.74 | 1.85 | <0.0001 |
| Number of prenatal visits | 5.27 | 4.62 | 8.70 | 4.91 | <0.0001 |
| Maternal years of education | 11.54 | 1.65 | 11.97 | 1.41 | 0.008 |
| Completion of high school, n (%) | 97 | 51.32 | 124 | 67.76 | 0.001 |
| PPVT standard score | 73.34 | 14.19 | 77.88 | 14.75 | 0.003 |
| WAIS-R block design scale | 6.88 | 2.09 | 7.17 | 2.08 | 0.18 |
| WAIS-R picture completion | 6.71 | 2.15 | 6.99 | 2.37 | 0.24 |
| BSI Global Severity Index | 0.83 | 0.75 | 0.50 | 0.53 | <0.0001 |
| Married, n (%) | 14 | 7.41 | 29 | 15.85 | 0.01 |
| Low SES, n (%) | 184 | 97.87 | 179 | 97.81 | 0.97 |
| African-American, n (%) | 156 | 82.54 | 148 | 80.87 | 0.68 |
| Substance use during pregnancy | |||||
| Cigarettes per daya | 11.61 | 11.20 | 3.88 | 7.17 | <0.0001 |
| Drinks per weekb | 9.81 | 17.52 | 1.37 | 4.58 | <0.0001 |
| Marijuana per weekc | 1.33 | 3.46 | 0.60 | 3.51 | <0.0001 |
| Cocaine per weekd | 22.55 | 37.88 | – | – | – |
| Current caregiver | |||||
| Years of education | 12.50 | 2.27 | 12.91 | 1.95 | 0.007 |
| Completion of high school, n (%) | 127 | 68.65 | 140 | 80.00 | 0.01 |
| PPVT standard score | 79.84 | 14.90 | 79.26 | 15.06 | 0.72 |
| WAIS-R block design scale | 7.12 | 2.10 | 7.26 | 1.93 | 0.50 |
| WAIS-R picture completion | 7.56 | 2.52 | 7.19 | 2.35 | 0.16 |
| BSI Global Severity Index | 0.35 | 0.42 | 0.34 | 0.46 | 0.50 |
| Substance use in the past 30 days | |||||
| Cigarettes per daya | 4.97 | 7.35 | 3.73 | 6.98 | 0.02 |
| Drinks per weekb | 1.77 | 4.49 | 1.99 | 4.58 | 0.34 |
| Marijuana per weekc | 0.52 | 5.37 | 0.44 | 3.02 | 0.62 |
| Cocaine per weekd | 0 | 0 | 0 | 0 | – |
| HOME score at 12 years | 48.00 | 6.71 | 49.17 | 6.21 | 0.10 |
| HOME score at 15 years | 47.77 | 6.71 | 48.89 | 6.10 | 0.11 |
Average number of cigarettes smoked per day.
Average drinks of beer, wine, or hard liquor per week, each equivalent to 0.5 mL absolute alcohol.
Average joints of marijuana per week.
Average rocks of cocaine per week.
Table 3.
Correlations between selected variables by sex with boys above the diagonal (n = 174); girls below the diagonal (n = 198).
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. PCE | – | 0.46 | 0.47 | 0.23 | 0.07 | −0.08 | −0.14 | 0.05 | −0.09 | −0.06 | 0.06 | −0.05 | −0.21 | 0.28 | 0.08 | 0.05 | 0.06 |
| 2. Prenatal alcohol, drinks per week | 0.52 | – | 0.32 | 0.13 | −0.03 | −0.09 | −0.12 | −0.05 | 0.00 | −0.04 | −0.03 | −0.01 | −0.16 | 0.24 | 0.11 | 0.14 | −0.01 |
| 3. Prenatal cigarette per day | 0.51 | 0.53 | – | 0.19 | −0.28 | −0.22 | −0.01 | −0.02 | −0.08 | 0.07 | −0.02 | −0.03 | −0.06 | 0.30 | 0.19 | 0.13 | 0.12 |
| 4. Prenatal marijuana per week | 0.21 | 0.09 | 0.20 | – | −0.01 | −0.03 | 0.02 | 0.10 | −0.10 | −0.13 | −0.08 | −0.10 | −0.05 | 0.05 | 0.00 | 0.03 | 0.09 |
| 5. Child race | −0.02 | 0.03 | −0.26 | −0.13 | – | 0.16 | −0.22 | −0.23 | 0.06 | −0.13 | 0.19 | −0.02 | 0.19 | −0.08 | −0.16 | −0.15 | −0.24 |
| 6. Maternal education | −0.19 | −0.05 | −0.21 | 0.03 | 0.04 | – | 0.24 | 0.15 | 0.12 | −0.11 | −0.03 | 0.10 | −0.02 | −0.15 | −0.14 | −0.07 | −0.04 |
| 7. Maternal PPVT | −0.17 | −0.08 | −0.03 | 0.10 | −0.21 | 0.44 | – | 0.34 | 0.03 | 0.04 | −0.19 | −0.03 | −0.04 | −0.09 | −0.04 | 0.08 | 0.20 |
| 8. Full scale IQ at 11 | −0.17 | −0.07 | −0.04 | 0.08 | −0.08 | 0.20 | 0.30 | – | 0.10 | −0.10 | −0.28 | −0.08 | −0.21 | −0.05 | −0.22 | −0.08 | 0.36 |
| 9. HOME at 12 | −0.12 | −0.08 | −0.10 | −0.01 | 0.05 | 0.19 | 0.13 | 0.17 | – | −0.17 | −0.07 | −0.03 | −0.05 | 0.16 | −0.08 | 0.00 | 0.03 |
| 10. Current caregiver’s GSI | 0.07 | 0.02 | 0.15 | 0.22 | −0.06 | −0.02 | 0.09 | −0.06 | −0.17 | – | 0.04 | 0.01 | 0.05 | −0.07 | 0.39 | 0.28 | −0.04 |
| 11. Violence exposure | 0.01 | 0.07 | 0.04 | 0.00 | 0.18 | −0.05 | 0.04 | −0.02 | −0.02 | 0.16 | – | 0.20 | 0.14 | −0.03 | 0.18 | 0.04 | −0.14 |
| 12. Early (≤age 12) drug use | 0.08 | −0.03 | 0.05 | −0.03 | 0.05 | 0.05 | −0.06 | −0.09 | −0.06 | 0.07 | 0.27 | – | −0.07 | 0.14 | −0.05 | −0.06 | 0.01 |
| 13. Lead level | −0.03 | 0.21 | 0.08 | −0.10 | 0.24 | −0.15 | −0.14 | −0.12 | −0.28 | 0.06 | 0.11 | 0.12 | – | −0.17 | −0.03 | −0.12 | −0.14 |
| 14. Adoptive/foster care at 12 | 0.32 | 0.17 | 0.17 | 0.00 | −0.01 | −0.11 | −0.24 | −0.07 | 0.11 | −0.20 | −0.07 | 0.04 | −0.12 | – | 0.18 | 0.20 | 0.10 |
| 15. BRIEF behavioral regulation at 12a | 0.28 | 0.11 | 0.18 | 0.17 | −0.04 | −0.11 | 0.04 | −0.20 | −0.15 | 0.47 | 0.17 | 0.14 | 0.06 | 0.13 | – | 0.78 | −0.20 |
| 16. BRIEF Metacognition at 12a | 0.30 | 0.08 | 0.23 | 0.19 | −0.08 | −0.09 | 0.02 | −0.22 | −0.12 | 0.41 | 0.08 | 0.13 | −0.07 | 0.24 | 0.84 | – | −0.10 |
| 17. SOC at 15b | −0.21 | −0.09 | −0.13 | −0.07 | −0.10 | 0.11 | 0.04 | 0.27 | 0.01 | −0.15 | −0.09 | −0.04 | 0.16 | −0.06 | −0.15 | −0.18 | – |
Note. p < 0.05 when r ≥ |0.14|; p < 0.01 when r ≥ |0.19|; p < 0.001 when r ≥ |0.24|.
Parent-reported.
SOC problem solved in minimum moves.
3.2. Longitudinal outcome (BRIEF caregiver report)
3.2.1. Effects of cocaine and time on EF
Fig. 1 depicts adjusted mean BRIEF Behavioral Regulation Index (BRI) scores for male and female PCE and NCE subjects at 12 and 15 years. PCE was associated with more behavioral regulation problems at 12 (p < 0.005) years for girls but not boys. There was a trend for PCE to be associated with more behavioral regulation problems at 15 (p < 0.053) for girls as well. PCE girls improved in behavioral regulation from 12 to 15 years (p < 0.05). NCE males also made significant improvements in behavioral regulation over time (p < 0.002) but PCE males (p < 0.09) and NCE females (p < 0.88) did not improve over time. Among the girls with PCE, those placed in non-relative foster or adoptive care were rated as having poorer behavioral regulation than those in maternal or relative care at both 12 (p < 0.05) and 15 years (p < 0.04). Analyses of the BRIEF BRI subscales revealed main effects of cocaine on lower Inhibit (p < 0.006) and Shift subscales scores of the BRI for girls only. PCE was not associated with Emotional Control for boys or girls.
Fig. 1.
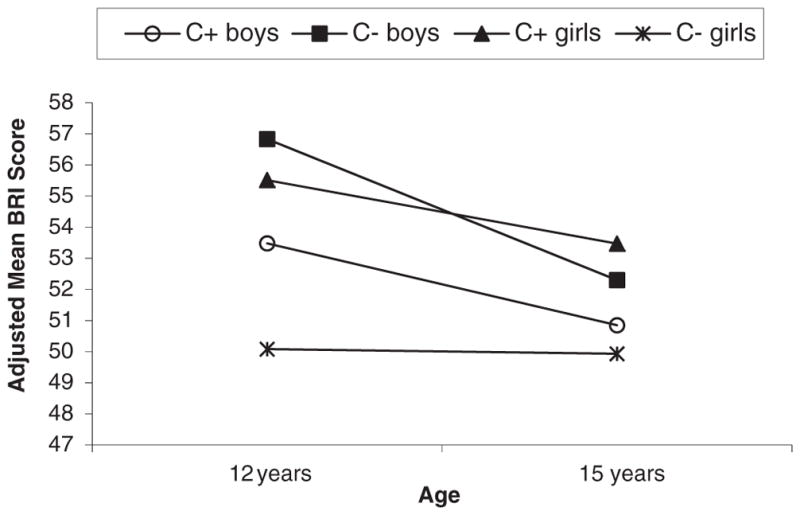
Behavioral Regulation Index (BRI) mean scores by cocaine status and gender at 12 and 15 years. Adjusted for cocaine status *time, HOME score, current caregiver’s Global Severity Index (GSI), average prenatal cigarette†, average prenatal alcohol, 12 year drug use and violence exposure†. A significant cocaine effect (PCE vs. NCE) among girls at 12 years (p = 0.004), and a significant time effect for PCE girls (p = 0.047). No cocaine effect among boys, but a significant time effect for NCE boys (p = 0.002). Note: The significant covariates were italicized; † was only significant for boys.
PCE was associated with more metacognitive problems (MI) for girls at age 12 (p < 0.003) (see Fig. 2) and there was a non-significant trend for more metacognitive problems for PCE girls at age 15 (p < 0.08). No associations between PCE and metacognition were obtained for boys. Only girls with PCE improved in metacognitive skills between ages 12 and 15 years (p < 0.04). Examination of the BRIEF MI subscales reveals a similar pattern as the MI summary score with PCE significant or trend effects for girls only at age 12. This effect on BRIEF MI subscales was not seen at age 15 as the PCE girls performance improved over time.
Fig. 2.
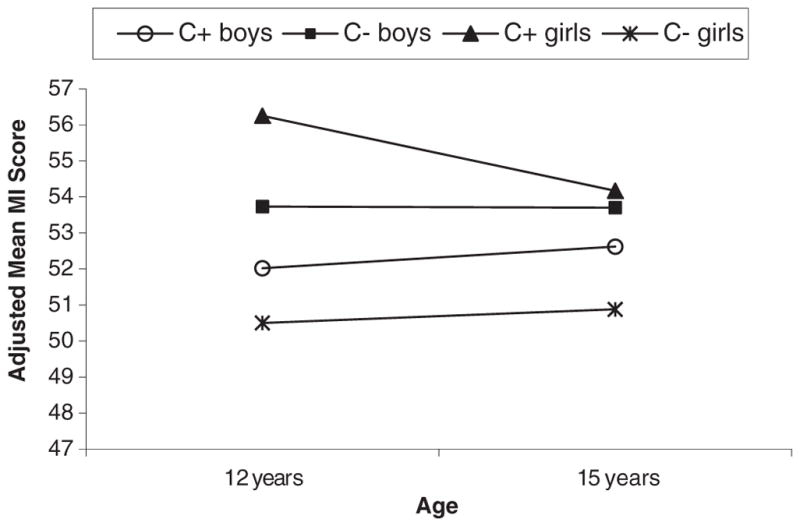
Mean Metacognition Index (MI) by cocaine status and gender at 12 and 15 years. Adjusted for cocaine-status*time, HOME score, current caregiver’s Global Severity Index (GSI), average prenatal cigarette, average prenatal alcohol†, and 12 year drug use. A significant cocaine effect (PCE vs. NCE) for girls at 12 years (p = 0.003), and a significant time effect for PCE girls (p = 0.04). No cocaine effect, time effect, or cocaine*time effect for boys. Note: The significant covariates were italicized; † was only significant for boys.
PCE girls placed in non-relative foster or adoptive care were rated as having poorer metacognition than PCE girls in maternal or relative care at 12 (p < 0.0001) and 15 years (p = 0.001).
3.2.2. Association of other prenatal exposures and environmental factors with caregiver report of EF
As expected, many environmental and prenatal exposure variables were associated with BRIEF caregiver ratings. Maternal caregiver distress was positively associated with more problems of behavioral regulation (b = 5.29, se = 0.96; p < 0.0001 for boys, b = 6.51, se = 0.68; p < 0.0001 for girls) and metacognition (b = 3.14, se = 0.70; p < 0.0001 for boys, b = 6.00, se = 0.68; p < 0.0001 for girls). Greater exposure to violence was associated with caregiver reported behavioral regulation problems for boys only (b = 2.02, se = 0.76; p < 0.009). Higher prenatal exposure to cigarettes was associated with more problems of behavioral regulation (b = 2.24, se = 0.79, p < 0.006) in boys and higher levels of prenatal alcohol exposure were associated with metacognition problems (b = 1.62, se = −0.64, p < 0.02) for boys only (b = 1.80, se =0.74, p < 0.02). Twelve year substance use was not associated with BRIEF scales.
3.2.3. Effects of blood lead level, IQ and head circumference on EF
Preschool blood lead levels were not associated with EF outcomes. Full Scale IQ at age 11 was associated with girls’ BRIEF ratings but did not mediate effects of PCE. Lower Full Scale IQ was associated with more behavioral regulation problems in boys and more metacognitive problems in girls. Head circumference was not associated with BRIEF ratings.
3.3. Fifteen year cross sectional EF data
3.3.1. BRIEF self-report
There were no cocaine effects on either broadband score of the BREIF-SR (BRI and MI). However, follow-up analysis of the BRI subscales, because there was a non-significant trend for a cocaine effect in behavioral regulation (p < 0.10), indicated that the BRI component scale Inhibit (b = 3.92, se = 1.40, β = 0.17, p < 0.006) was significantly associated and there was a trend for significance on the Cognitive Shift (b = 0.46, se = 0.24, β = 0.12, p < 0.06) scale. Maternal psychological distress was associated with more behavioral regulation problems (b = 6.24, se = 1.95, β = 0.19, p < 0.002). Higher levels of violence exposure was a significant predictor of both behavioral regulation (b = 0.78, se = 0.21, β = 0.21, p < 0.0002) and metacognitive (b = 0.44, se = 0.20, β = 0.13, p < 0.03) problems. While child Full Scale IQ was associated with BRI, MI and all subscales except Organization of Materials, it did not change the effect of cocaine. Blood lead level was not associated with BRI or MI.
3.3.2. CANTAB - SOC
Cross sectional analyses of SOC (problems solved–minimum moves) at age 15 indicated that girls with PCE performed more poorly than NCE girls after control for confounders (Table 4). Initial or mean subsequent thinking time were not associated with PCE. PCE and NCE boys did not perform differently. Full Scale IQ at age 11 was associated with girls’ SOC efficiency but did not mediate cocaine’s effect. Twelve year substance use was not associated with SOC mean initial thinking time or mean subsequent thinking time (Table 5).
Table 4.
Association of BRIEF-self-report with cocaine at 15 years.
| β | b (SE) | t | p | |
|---|---|---|---|---|
| Behavioral regulationa | 0.11 | 2.52 (1.54) | 1.64 | 0.10 |
| Inhibitb | 0.17 | 3.92 (1.40) | 2.80 | 0.006 |
| Shiftc | 0.10 | 2.03 (1.43) | 1.43 | 0.16 |
| Behavioral shiftd | −0.02 | −0.06 (0.29) | −0.23 | 0.82 |
| Cognitive shifte | 0.12 | 0.46 (0.24) | 1.89 | 0.06 |
| Emotionf | 0.07 | 1.42 (1.27) | 1.11 | 0.27 |
| Metacognitiong | 0.05 | 1.01 (1.41) | 0.71 | 0.48 |
| Monitorh | 0.03 | 0.65 (1.35) | 0.48 | 0.63 |
| Work memoryi | 0.07 | 1.48 (1.40) | 1.06 | 0.29 |
| Plan/organizej | 0.05 | 0.97 (1.35) | 0.72 | 0.47 |
| Organization of materialk | 0.04 | 0.81 (1.08) | 0.75 | 0.46 |
| Task completionl | −0.03 | −0.69 (1.33) | −0.52 | 0.60 |
| Global executivem | 0.09 | 2.07 (1.57) | 1.32 | 0.19 |
Note: The significant covariates were italicized.
Adjusted for maternal GSI score, HOME score, prenatal cigarettes use month prior, prenatal average alcohol exposure, gender, violence exposure and IQ.
Adjusted for maternal GSI score, mother’s age at birth, 3rd trimester maternal marijuana exposure, gender, violence exposure and IQ.
Adjusted for maternal GSI score, HOME score, maternal PPVT score, current caregiver cigarette use, prenatal average alcohol exposure, gender, violence exposure and IQ.
Adjusted for maternal GSI score, maternal PPVT score, current caregiver picture completion scale, current caregiver cigarette use, prenatal average alcohol exposure, gender, violence exposure and IQ.
Adjusted for maternal GSI score, HOME score, current caregiver cigarette use, 2nd trimester prenatal alcohol exposure, violence exposure and IQ.
Adjusted for maternal GSI score, HOME score, mother’s age at birth, prenatal average marijuana exposure, violence exposure and IQ.
Adjusted for maternal GSI score, 1st trimester prenatal cigarettes use, gender, violence exposure and IQ.
Adjusted for maternal GSI score, 3rd trimester prenatal average cigarettes exposure, gender, violence exposure and IQ.
Adjusted for maternal GSI score, 3rd trimester prenatal average cigarettes exposure, violence exposure and IQ.
Adjusted for maternal GSI score, HOME score, parity, maternal block design scale, gender, violence exposure and IQ.
Adjusted for maternal GSI score, HOME score, maternal block design scale, current caregiver cigarette use, 2nd trimester prenatal average alcohol exposure, violence exposure and IQ.
Adjusted for maternal GSI score, HOME score, prenatal average cigarettes exposure, prenatal average alcohol exposure, gender, violence exposure and IQ.
Adjusted for maternal GSI score, current caregiver picture completion scale, 2nd trimester prenatal alcohol exposure, violence exposure and IQ.
Table 5.
Adjusted means of SOC problem solved in minimum moves by cocaine and gender at 15 years.
| Gender | Cocaine (n = 188)
|
Non-Cocaine (n = 182)
|
p | ||
|---|---|---|---|---|---|
| M | SE | M | SE | ||
| Male | 7.63 | 0.21 | 7.35 | 0.20 | 0.33 |
| Female | 7.28 | 0.19 | 7.93 | 0.19 | 0.02 |
Adjusted for HOME, parity, maternal PPVT standard score, maternal 3rd trimester alcohol use and violence exposure.
4. Discussion
Prenatal cocaine exposure was associated with increased caregiver reported problems of behavioral regulation at 12 and a non-significant trend at 15 years and metacognitive problems at 12 years of age in girls but not boys. Behavioral regulation problems include difficulties in inhibiting behavior and shifting from one type of activity to another. Girls with PCE improved in behavioral regulation and metacognition from ages 12 to 15, whereas NCE girls did not. NCE boys also improved in behavioral regulation from 12 to 15 years but PCE boys did not. Neither PCE nor NCE boys changed in metacognitive abilities from 12 to 15 years.
By self-report, cross sectional analysis at age 15 yielded no effects of cocaine on BRIEF-SR BRI and MI broadband scores in either girls or boys. However, there was an effect of cocaine, not specific for girls, on one of the subscale scores of the BRI, inhibiting behavior, and a trend for another subscale, shifting cognitive sets, in a follow-up analysis. Girls with PCE performed more poorly than NCE girls and both PCE and NCE boys on the CANTAB SOC, a task that requires working memory and planning, skills consistent with the BRIEF MI finding at age 12. Given that the SOC is a clinical exam it is free of rater bias substantiating the finding of a PCE effect on working memory and planning. Taken together the findings provide evidence of EF problems involving behavioral regulation and planning and organizing, particularly among girls with PCE. However, associations of PCE and types of EF problems are not completely consistent across methods of assessment, with caregivers indicating behavioral regulation problems and teens less consistently indicating behavioral regulation problems. While metacognitive problems were reported only by caregivers at age twelve teens did not indicate metacognitive difficulties at 12 or 15. However, clinical assessment suggest metacognitive, or problem solving difficulty, at 15 among PCE girls. Caregivers report that girls with PCE make EF gains in both behavioral regulation and metacognition between 12 and 15 years. Further verification of this via repeated measures across adolescence using a neuropsychological assessment would be of value.
These results extend findings of other researchers by using a multi-method assessment of EF and a longitudinal design to study the effects on EF, with control for early blood lead level, out-of-home placement, violence exposure and current use of drugs provides a clearer picture of those effects due to prenatal cocaine exposure versus other factors related to the development of EF. Cross sectional studies finding negative effects of PCE on EF at various ages (Eyler et al., 2009; Noland et al., 2003a; Rose-Jacobs et al., 2009; Singer et al., 2008) are consistent with our findings. Our data introduces small but growing support for an interaction of PCE and gender, with girls having more EF problems than their male counterparts, as did Mayes’ study (2002) on executive function at age 7. The current findings are consistent with caregiver rated data on externalizing behavior in PCE children (McLaughlin et al., 2011; Minnes et al., 2011) that indicate a specific behavioral risk for girls with PCE. However, teen rated data from this cohort did not indict a PCE by gender interaction (Min et al., 2014b). The present findings also extend our previous research by indicating that PCE girls, while having the greatest deficit in EF skills at age 12, catch up to the other groups in performance by age 15. Further study would indicate if boys would make more EF improvements if they were further along in adolescent development.
Compared to PCE girls living with biologic mothers/relatives, those living in foster/adoptive care were rated as having more behavioral regulation and metacognitive EF problems at 12 and 15 years. This finding of more behavioral problems among girls is similar to other behavioral assessments completed by caregivers in this cohort (Linares et al., 2006; McLaughlin et al., 2011; Minnes et al., 2014). It is not clear why foster/adoptive caregivers of girls with PCE indicate more EF problems. It is possible that foster/adoptive caregivers are more aware of behavioral difficulties and/or have higher behavioral expectations for their foster/adopted children, especially girls, due to having higher verbal abilities and socioeconomic status than birth mothers or relative caregivers of PCE children in the study. However, it is also possible that EF problems may actually be more prevalent among girls who have been separated from their biologic mothers or relatives than boys. Evidence from the SOC findings, which have the benefit of not incorporating such bias, indicate that cocaine may exert a specific behavioral effect in PCE girls. Additional research that includes NCE children in foster/ adoptive care for comparison is required to fully understand this finding.
Caregiver psychological distress was a consistent predictor of BRIEF caregiver ratings (BRI & MI) and child self-report (BRI) but not SOC performance. These results were not unexpected as maternal distress is often reflected in perceptions of children’s behavior (Youngstrom et al., 1999). The reason why maternal psychological distress is also reflected in child self-report of behavioral regulation, and not metacognition, is not clear. It may be that greater maternal psychological distress is communicated via disciplinary practices related to teen problems of inhibitory and emotional control. Effects of prenatal alcohol and marijuana on BRIEF ratings for boys are consistent with multiple studies indicating that prenatal alcohol exposure is associated with EF problems (Rasmussen et al., 2011). Not surprisingly, violence exposure was associated with poorer BRI and MI caregiver ratings and self-reported behavioral regulation in boys (Edalati & Krank, 2015).
4.1. Strengths and limitations
A potential limitation of the longitudinal data presented in this study was the reliance on caregiver report which can be influenced by the rater’s psychological well-being and education. In addition, we did not look specifically at protective factors that might have accounted for improvement. However, by evaluating a large number of potentially confounding factors, some of which might be considered protective factors like the presence of a high quality home environment, the effects of this weakness were diminished. Additionally, the addition of an objective, clinical measure of EF and self-report rating at 15 years strengthened our conclusions that PCE is associated with problems of EF. If CANTAB and BRIEF child self-report data had been available at 12 years as well, a more complete picture of how PCE and time may interact differently for boys and girls would have been possible. Finally, the nature of the sample, urban, primarily African American and of low socioeconomic status, limits the generalizability of the results.
Among the study strengths, participants were recruited prospectively at birth and the 12 and 15 year retention rates were exceptional. Multi-method assessment of gestational cocaine exposure (interview, urine, meconium) assured that correct group status was assigned. A full range of covariates was controlled, reducing the possibility of erroneously attributing a PCE effect to EF. The BRIEF is ecologically sensitive, and was completed by caregivers who observe the child under naturally occurring situations and by the teens themselves. This type of assessment can also provide useful clinical information regarding the developmental nature of behavioral problems related to PCE. Child self-report data complement findings from caregiver report. The association of PCE with CANTAB SOC corroborates caregiver report of EF metacognitive problems but only for girls.
4.2. Implications and contributions
As EF deficits have been associated with school failure, behavioral problems and substance use, serious attention to this risk group of children with PCE is imperative. Focus on developing therapeutic interventions and earlier remediation in the developmental trajectory could have substantial impact in reducing or preventing negative outcomes for children with PCE.
Acknowledgments
This research was funded by the National Institute on Drug Abuse grant R01 07957. Portions of this work were previously presented at the 75th Annual Meeting of the College for Problems of Drug Dependence, San Diego CA, June 2013 and the Neurobehavioral Teratology Society Annual Meeting, Seattle WA, June 2014.
Footnotes
Transparency document
The Transparency document associated with this article can be found, in the online version.
References
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Blair C, Ursache A. A bidirectional model of executive functions and self-regulation. In: Vohs KD, Baumeister RF, editors. Handbook of Self-Regulation: Research, Theory, and Applications. Guilford Press; New York: 2011. pp. 300–320. [Google Scholar]
- Bridgett D, Mayes LC. Development of inhibitory control among prenatally cocaine exposed and non-cocaine exposed youths from late childhood to early adolescence: the effects of gender and risk and subsequent aggressive behavior. Neurotoxicol Teratol. 2011;33(1):47–60. doi: 10.1016/j.ntt.2010.08.002. http://dx.doi.org/10.1016/j.ntt.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgett DJ, Oddi KB, Laake LM, Murdock KW, Bachmann MN. Integrating and differentiating aspects of self-regulation: Effortful control, executive functioning, and links to negative affectivity. Emotion (Washington, DC) 2013;13(1):47–63. doi: 10.1037/a0029536. http://dx.doi.org/10.1037/a0029536. [DOI] [PubMed] [Google Scholar]
- Bridgett DJ, Burt NM, Edwards ES, Deater-Deckard K. Intergenerational transmission of self-regulation: a multidisciplinary review and integrative conceptual framework. Psychol Bull. 2015;141(3):602–654. doi: 10.1037/a0038662. http://dx.doi.org/10.1037/a0038662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burden MJ, Jacobson SW, Sokol RJ, Jacobson JL. Effects of prenatal alcohol exposure on attention and working memory at 7.5 years of age. Alcohol Clin Exp Res. 2005;29(3):443–452. doi: 10.1097/01.alc.0000156125.50577.ec. [DOI] [PubMed] [Google Scholar]
- Caldwell B, Bradley R. Home Observation for Measurement of the Environment (HOME-Revised Edition) University of Arkansas at Little Rock; Little Rock, AR: 1984. [Google Scholar]
- Chiodo LM, Jacobson SW, Jacobson JL. Neurodevelopmental effects of postnatal lead exposure at very low levels. Neurotoxicol Teratol. 2004;26(3):359–371. doi: 10.1016/j.ntt.2004.01.010. http://dx.doi.org/10.1016/j.ntt.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Derogatis LR. The Brief Symptom Inventory (BSI): Administration, Scoring, and Procedures Manual—II. Clinical Psychometric Research; Towson, MD: 1992. [Google Scholar]
- Dow-Edwards DL, Benveniste H, Behnke M, Bandstra ES, Singer LT, Hurd YL, Stanford LR. Neuroimaging of prenatal drug exposure. Neurotoxicol Teratol. 2006;28(3):386–402. doi: 10.1016/j.ntt.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn L, Dunn L. Peabody Picture Vocabulary Test - Revised. American Guidance Service; Circle Pines, MN: 1981. [Google Scholar]
- Edalati H, Krank MD. Childhood maltreatment and development of substance use disorders: a review and a model of cognitive pathways. Trauma Violence Abuse. 2015:1–14. doi: 10.1177/1524838015584370. 1524838015584370 [pii]) [DOI] [PubMed] [Google Scholar]
- Evans GW, Schamberg MA. Childhood poverty, chronic stress, and adult working memory. Proc Natl Acad Sci U S A. 2009;106(16):6545–6549. doi: 10.1073/pnas.0811910106. http://dx.doi.org/10.1073/pnas.0811910106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyler FD, Warner TD, Behnke M, Hou W, Wobie K, Garvan CW. Executive functioning at ages 5 and 7 years in children with prenatal cocaine exposure. Dev Neurosci. 2009;31(1–2):121–136. doi: 10.1159/000207500. http://dx.doi.org/10.1159/000207500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fray PJ, Robbins TW. CANTAB battery: proposed utility in neurotoxicology. Neurotoxicol Teratol. 1996;18(4):499–504. doi: 10.1016/0892-0362(96)00027-x. [DOI] [PubMed] [Google Scholar]
- Fried PA, Smith AM. A literature review of the consequences of prenatal marihuana exposure. An emerging theme of a deficiency in aspects of executive function. Neurotoxicol Teratol. 2001;23(1):1–11. doi: 10.1016/s0892-0362(00)00119-7. [DOI] [PubMed] [Google Scholar]
- Gautam P, Warner TD, Kan EC, ERS Executive function and cortical thickness in youths prenatally exposed to cocaine, alcohol and tobacco. Dev Cogn Neurosci. 2015 doi: 10.1016/j.dcn.2015.01.010. S1878-9293(15)00024-9 [pii] In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioia GA, Isquith PK, Guy SC, Kenworthy L. BRIEF: Behavior Rating Inventory of Executive Function. Psychological Assessment Resources; Lutz, FL: 2000. [Google Scholar]
- Grewen K, Burchinal M, Vachet C, Gouttard S, Gilmore JH, Lin W, … Gerig G. Prenatal cocaine effects on brain structure in early infancy. NeuroImage. 2014;101:114–123. doi: 10.1016/j.neuroimage.2014.06.070. http://dx.doi.org/10.1016/j.neuroimage.2014.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Bava S, Cohen-Zion M, Mahmood O, Tapert SF. Functional consequences of marijuana use in adolescents. Pharmacol Biochem Behav. 2009;92(4):559–565. doi: 10.1016/j.pbb.2009.04.001. http://dx.doi.org/10.1016/j.pbb.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel DB, Warner LA, Kessler RC. The epidemiology of substance use and dependence among women. In: Wetherington CL, Roman AB, editors. Drug Addiction Research and the Health of Women. USDHHS; Rockville, MD: 1998. pp. 105–130. [Google Scholar]
- Langberg JM, Dvorsky MR, Evans SW. What specific facets of executive function are associated with academic functioning in youth with attention-deficit/hyperactivity disorder? J Abnorm Child Psychol. 2013 doi: 10.1007/s10802-013-9750-z. http://dx.doi.org/10.1007/s10802-013-9750-z. [DOI] [PMC free article] [PubMed]
- Lebel C, Warner T, Colby J, Soderberg L, Roussotte F, Behnke M, Sowell ER. White matter microstructure abnormalities and executive function in adolescents with prenatal cocaine exposure. Psychiatry Res. 2013;213(2):161–168. doi: 10.1016/j.pscychresns.2013.04.002. http://dx.doi.org/10.1016/j.pscychresns.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linares TJ, Singer LT, Kirchner HL, Short EJ, Min MO, Hussey P, Minnes S. Mental health outcomes of cocaine-exposed children at 6 years of age. J Pediatr Psychol. 2006;31(1):85–97. doi: 10.1093/jpepsy/jsj020. http://dx.doi.org/10.1093/jpepsy/jsj020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malanga CJ, Kosofsky BE. Mechanisms of action of drugs of abuse on the developing fetal brain. Clin Perinatol. 1999;26(1):17–37. v–vi. [PubMed] [Google Scholar]
- Mayes LC. A behavioral teratogenic model of the impact of prenatal cocaine exposure on arousal regulatory systems. Neurotoxicol Teratol. 2002;24(3):385–395. doi: 10.1016/s0892-0362(02)00200-3. http://dx.doi.org/10.1016/S0892-0362(02)00200-3. [DOI] [PubMed] [Google Scholar]
- McDermott JM, Troller-Renfree S, Vanderwert R, Nelson CA, Zeanah CH, Fox NA. Psychosocial deprivation, executive functions, and the emergence of socio-emotional behavior problems. Front Hum Neurosci. 2013;7:167. doi: 10.3389/fnhum.2013.00167. http://dx.doi.org/10.3389/fnhum.2013.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin AA, Minnes S, Singer LT, Min M, Short EJ, Scott TL, Satayathum S. Caregiver and self-report of mental health symptoms in 9-year old children with prenatal cocaine exposure. Neurotoxicol Teratol. 2011;33(5):582–591. doi: 10.1016/j.ntt.2011.03.002. http://dx.doi.org/10.1016/j.ntt.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min MO, Singer LT, Kirchner HL, Minnes S, Short E, Hussain Z, Nelson S. Cognitive development and low-level lead exposure in poly-drug exposed children. Neurotoxicol Teratol. 2009;31(4):225–231. doi: 10.1016/j.ntt.2009.03.002. http://dx.doi.org/10.1016/j.ntt.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min MO, Singer LT, Minnes S, Kim H, Short E. Mediating links between maternal childhood trauma and preadolescent behavioral adjustment. J Interpers Violence. 2013;28(4):831–851. doi: 10.1177/0886260512455868. http://dx.doi.org/10.1177/0886260512455868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min MO, Minnes S, Lang A, Weishampel P, Short EJ, Yoon S, Singer LT. Externalizing behavior and substance use related problems at 15 years in prenatally cocaine exposed adolescents. J Adolesc. 2014a;37(3):269–279. doi: 10.1016/j.adolescence.2014.01.004. http://dx.doi.org/10.1016/j.adolescence.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min MO, Minnes S, Short EJ, Yoon S, Singer LT. Self-reported adolescent behavioral adjustment: effects of prenatal cocaine exposure. J Adolesc Health. 2014b;55(2):167–174. doi: 10.1016/j.jadohealth.2013.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnes S, Singer LT, Kirchner HL, Short E, Lewis B, Satayathum S, Queh D. The effects of prenatal cocaine exposure on problem behavior in children 4–10 years. Neurotoxicol Teratol. 2010;32(4):443–451. doi: 10.1016/j.ntt.2010.03.005. http://dx.doi.org/10.1016/j.ntt.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnes S, Lang A, Singer L. Prenatal tobacco, marijuana, stimulant, and opiate exposure: outcomes and practice implications. Addict Sci Clin Pract. 2011;6(1):57–70. [PMC free article] [PubMed] [Google Scholar]
- Minnes S, Singer LT, Min MO, Lang AM, Ben-Harush A, Short E, Wu M. Comparison of 12-year-old children with prenatal exposure to cocaine and non-exposed controls on caregiver ratings of executive function. J Youth Adolesc. 2014;43(1):53–69. doi: 10.1007/s10964-013-9927-3. http://dx.doi.org/10.1007/s10964-013-9927-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noland JS, Singer LT, Arendt RE, Minnes S, Short EJ, Bearer CF. Executive functioning in preschool-age children prenatally exposed to alcohol, cocaine, and marijuana. Alcohol Clin Exp Res. 2003a;27(4):647–656. doi: 10.1097/01.ALC.0000060525.10536.F6. http://dx.doi.org/10.1097/01.ALC.0000060525.10536.F6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noland JS, Singer LT, Mehta SK, Super DM. Prenatal cocaine/polydrug exposure and infant performance on an executive functioning task. Dev Neuropsychol. 2003b;24(1):499–517. doi: 10.1207/S15326942DN2401_05. http://dx.doi.org/10.1207/S15326942DN2401_05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrea EM, Jr, Brady M, Gause S, Raymundo AL, Stevens M. Drug screening of newborns by meconium analysis: a large-scale, prospective, epidemiologic study. Pediatrics. 1992;89(1):107–113. [PubMed] [Google Scholar]
- Pentz MA, Riggs NR. Longitudinal relationships of executive cognitive function and parent influence to child substance use and physical activity. Prev Sci. 2013;14(3):229–237. doi: 10.1007/s11121-012-0312-3. http://dx.doi.org/10.1007/s11121-012-0312-3. [DOI] [PubMed] [Google Scholar]
- Rasmussen C, Soleimani M, Pei J. Executive functioning and working memory deficits on the CANTAB among children with prenatal alcohol exposure. J Popul Ther Clin Pharmacol. 2011;18(1):e44–e53. [PubMed] [Google Scholar]
- Ridenour TA, Clark DB, Cottler LB. The illustration-based assessment of liability and EXposure to substance use and antisocial behavior for children. Am J Drug Alcohol Abuse. 2009;35(4):242–252. doi: 10.1080/00952990902998715. http://dx.doi.org/10.1080/00952990902998715. [DOI] [PubMed] [Google Scholar]
- Ridenour TA, Minnes S, Maldonado-Molina MM, Reynolds MD, Tarter RE, Clark DB. Psychometrics and cross-cultural comparisons of the illustration-based assessment of liability and exposure to substance use and antisocial behavior(c) for children. Open Fam Stud J. 2011;4(Suppl 1-M2):17–26. doi: 10.2174/1874922401104010017. http://dx.doi.org/10.2174/1874922401104010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridenour TA, Meyer-Chilenski S, Reid EE. Developmental momentum toward substance dependence: natural histories and pliability of risk factors in youth experiencing chronic stress. Drug Alcohol Depend. 2012;123(Suppl 1):S87–S98. doi: 10.1016/j.drugalcdep.2011.12.016. http://dx.doi.org/10.1016/j.drugalcdep.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose-Jacobs R, Waber D, Beeghly M, Cabral H, Appugleise D, Heeren T, … Frank DA. Intrauterine cocaine exposure and executive functioning in middle childhood. Neurotoxicol Teratol. 2009;31(3):159. doi: 10.1016/j.ntt.2008.12.002. http://dx.doi.org/10.1016/j.ntt.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose-Jacobs R, Soenksen S, Appugliese DP, Cabral HJ, Richardson MA, Beeghly M, Frank DA. Early adolescent executive functioning, intrauterine exposures and own drug use. Neurotoxicol Teratol. 2011;33(3):379–392. doi: 10.1016/j.ntt.2011.02.013. http://dx.doi.org/10.1016/j.ntt.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth RM, Isquith PK, Gioia GA. Behavior Rating Inventory of Executive Function - Adult Version(BRIEF-A) Professional Manual. Psychological Assessment Resources; Lutz, FL: 2005a. [Google Scholar]
- Roth RM, Isquith PK, Gioia GA. Executive function: concepts, assessment and intervention. In: Koocher GP, Norcross JC, Hill SS, editors. Psychologists’ desk reference. 2. Oxford University Press; Oxford; New York: 2005b. pp. 38–41. [Google Scholar]
- SAMSHA. Substance Abuse and Mental Health Services Administration Results From the 2012 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-46, HHS Publication No (SMA) 13-4795. Substance abuse and mental health services administration; Rockville, MD: 2013. [Google Scholar]
- Schweinsburg AD, Brown SA, Tapert SF. The influence of marijuana use on neurocognitive functioning in adolescents. Curr Drug Abuse Rev. 2008;1(1):99–111. doi: 10.2174/1874473710801010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer LT, Arendt R, Minnes S, Farkas K, Salvator A, Kirchner HL, Kliegman R. Cognitive and motor outcomes of cocaine-exposed infants. JAMA. 2002a;287(15):1952–1960. doi: 10.1001/jama.287.15.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer LT, Salvator A, Arendt R, Minnes S, Farkas K, Kliegman R. Effects of cocaine/polydrug exposure and maternal psychological distress on infant birth outcomes. Neurotoxicol Teratol. 2002b;24(2):127–135. doi: 10.1016/s0892-0362(01)00208-2. http://dx.doi.org/10.1016/S0892-0362(01)00208-2. [DOI] [PubMed] [Google Scholar]
- Singer LT, Minnes S, Short E, Arendt R, Farkas K, Lewis B, … Kirchner HL. Cognitive outcomes of preschool children with prenatal cocaine exposure. JAMA. 2004;291(20):2448–2456. doi: 10.1001/jama.291.20.2448. http://dx.doi.org/10.1001/jama.291.20.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer LT, Nelson S, Short E, Min MO, Lewis B, Russ S, Minnes S. Prenatal cocaine exposure: drug and environmental effects at 9 years. J Pediatr. 2008;153(1):105–111. doi: 10.1016/j.jpeds.2008.01.001. http://dx.doi.org/10.1016/j.jpeds.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Jacobus J, Tapert SF. The influence of substance use on adolescent brain development. Clin EEG Neurosci. 2009;40(1):31–38. doi: 10.1177/155005940904000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streissguth A. The behavioral teratology of alcohol: performance, behavioral, and intellectual deficits in prenatally exposed children. In: West JR, editor. Alcohol and Brain Development. Oxford University Press; New York, NY: 1986. pp. 3–44. [Google Scholar]
- Taylor SJ, Barker LA, Heavey L, McHale S. The typical developmental trajectory of social and executive functions in late adolescence and early adulthood. Dev Psychol. 2013;49(7):1253–1265. doi: 10.1037/a0029871. http://dx.doi.org/10.1037/a0029871. [DOI] [PubMed] [Google Scholar]
- Trope I, Lopez-Villegas D, Cecil KM, Lenkinski RE. Exposure to lead appears to selectively alter metabolism of cortical gray matter. Pediatrics. 2001;107(6):1437–1442. doi: 10.1542/peds.107.6.1437. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. Effect of cocaine use on the fetus. N Engl J Med. 1992;327(6):399–407. doi: 10.1056/NEJM199208063270607. http://dx.doi.org/10.1056/NEJM199208063270607. [DOI] [PubMed] [Google Scholar]
- Warner TD, Behnke M, Eyler FD, Padgett K, Leonard C, Hou W, et al. Diffusion tensor imaging of frontal white matter and executive functioning in cocaine-exposed children. Pediatrics. 2006;118(5):2014–2024. doi: 10.1542/peds.2006-0003. http://dx.doi.org/10.1542/peds.2006-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-Revised. The Psychological Corporation; San Antonio, TX: 1981. [Google Scholar]
- Wechsler D. WISC-IV: Administration and Scoring Manual. Psychological Corp; San Antonio, TX: 2003. [Google Scholar]
- Youngstrom E, Izard C, Ackerman B. Dysphoria-related bias in maternal ratings of children. J Consult Clin Psychol. 1999;67(6):905–916. doi: 10.1037//0022-006x.67.6.905. [DOI] [PubMed] [Google Scholar]
- Zelazo PD, Muiller U, Frye D, Marcovitch S. The development of executive function in early childhood. Monogr Soc Res Child Dev. 2003;68(3):1–27. doi: 10.1111/j.0037-976x.2003.00260.x. http://dx.doi.org/10.1111/j.0037-976X.2003.00261.x. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Chen SH, Main A. Commonalities and differences in the research on children’s effortful control and executive function: a call for an integrated model of self-regulation. Child Dev Perspect. 2012;6(2):112–121. [Google Scholar]


