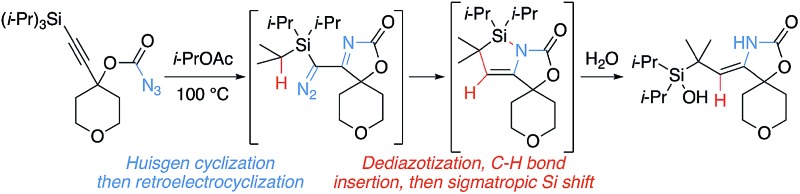
 Alkynyl carbonazidates form azasilacyclopentenes via a series of cyclizations, rearrangements, and an α-sily1 C–H bond insertion as determined by mechanistic investigations.
Alkynyl carbonazidates form azasilacyclopentenes via a series of cyclizations, rearrangements, and an α-sily1 C–H bond insertion as determined by mechanistic investigations.
Abstract
An unusual transition metal-free cascade reaction of alkynyl carbonazidates was discovered to form azasilacyclopentenes. Mild thermolysis afforded the products via a series of cyclizations, rearrangements, and an α-silyl C–H bond insertion (rather than the more common Wolff rearrangement, 1,2-shift, or β-silyl C–H insertion) to form silacyclopropanes. A mechanistic proposal for the sequence was informed by control experiments and the characterization of reaction intermediates. The substrate scope and post-cascade transformations were also explored.
Introduction
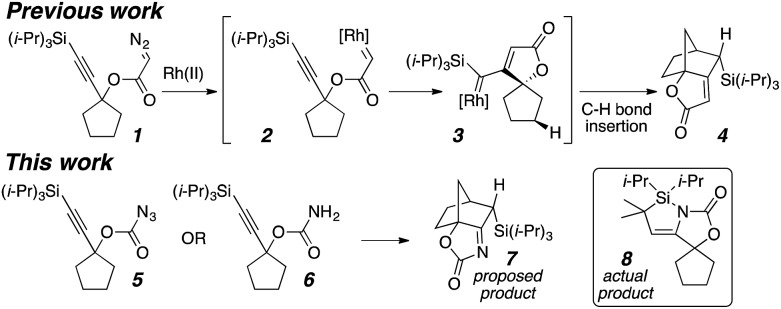
Carbenes have been employed in cyclopropanations,1 Büchner ring expansion,2 ylide formation,3 X–H bond insertion,4 and especially C–H bond insertions,5 which have provided a powerful tool for synthetic chemistry.6 Cascade reactions using highly reactive carbenes allow the efficient synthesis of complex molecules.7 Our research group recently reported the formation of bridged polycycles via carbene/alkyne cascade reactions terminated in C–H bond insertion (Scheme 1).8 We were also interested in utilizing this cascade reaction to synthesize nitrogen-containing polycyclic compounds like 7. Instead, a complex sequence with an unusual α-silyl C–H bond insertion formed azasilacyclopentene 8 from carbonazidate 5.
Scheme 1. Carbene- and nitrene-based cascade reactions.
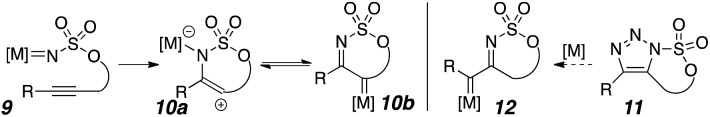
Nitrenes have been used for the aziridination of olefins,9 the C–H amination of alkanes,10 and the synthesis of bioactive molecules.11 Pioneering work from Blakey showed nitrene/alkyne cascades proceeded through an endo-cyclization with the alkyne to the postulated vinyl cation/metalloenamine 10a, which potentially could rearrange to the α-iminometallocarbene 10b or react directly (Scheme 2).12 Importantly, metallocarbene 12 could not be accessed via that approach, and α-diazoimines are difficult to synthesize. Carbene 12 could be directly produced from a transition metal catalyzed triazole ring opening.13 The incorporation of C–H bond insertions in these cascade reactions is still rare.14,15 An investigation to generate intermediate 12 and use that highly reactive species and its high potential for complex and interesting transformations has unveiled surprising and novel reactivity. Starting with carbonazidates like 5, carbene 12 was accessed via a metal-free Huisgen cyclization and dediazatization. From this reactive intermediate, C–H bond insertion, bond fragmentations, and pseudopericyclic rearrangements have been identified, and mechanistic intermediates isolated. Reactivity patterns suggest the intermediacy of imino-conjugated triplet carbenes.
Scheme 2. Nitrene and triazole carbene formation.
Results and discussion
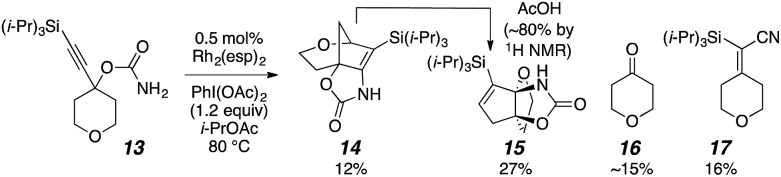
The initial goal was to establish a nitrene/alkyne cascade reaction to synthesize nitrogen-containing polycycles like 7. Treating carbamate 13 with Rh2(esp)2 and PhI(OAc)2 resulted in the formation of multiple products: the predicted bridged tricycle 14, propellane 15,16 tetrahydropyranone 16, and silyl vinyl nitrile 17 (Scheme 3).16 A control experiment revealed that acetic acid, produced during the carbamate oxidation, slowly promoted the rearrangement of 14 to propellane 15 in situ. This exploratory reaction demonstrated a proof-of-principle for a nitrene/alkyne cascade that results in C–H insertion to generate a C–C bond; however, controlling product selectivity was problematic. Consequently, other nitrene precursors were sought to achieve a more selective outcome.
Scheme 3. Cascade reaction of a carbamate.
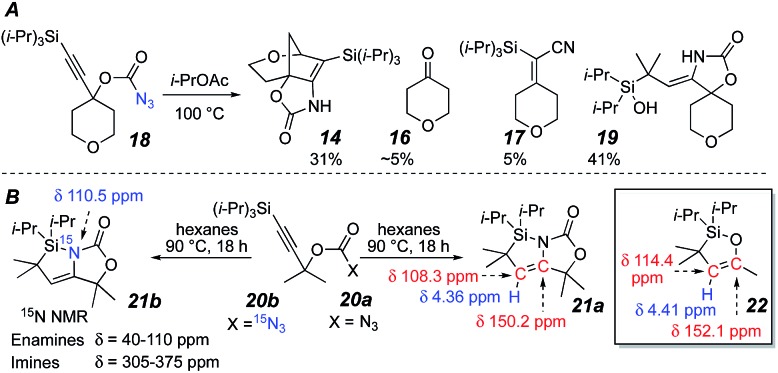
Carbonazidate17 18 allowed an improved yield (31%) of tricycle 14 without a catalyst (Scheme 4A). Surprisingly, silanol 19 was also isolated, which was thought to be the result of silica gel-mediated hydrolysis of a labile product during purification since it was not observed in the NMR of the crude reaction material. Dimethyl carbonazidate 20a was prepared to minimize overlapping NMR signals in order to identify the immediate product of the reaction. After 1H and 13C NMR analysis, its structure was determined to be azasilacyclopentene18 21a (Scheme 4B). The NMR signals of 21a (vinyl proton: 4.36 ppm; enamine carbons: 108.3 and 150.2 ppm) correlated well to those of oxasilacyclopentene 22.19 In addition, 15N NMR analysis of 15N-enriched azasilacyclopentene 21b (110.5 ppm, no signals from 305 to 375 ppm)20 suggested that the nitrogen was involved in an enamine motif, corroborating the 1H and 13C NMR data. Hydrolysis of 21a to the silanol proved to be facile with a small amount of water.
Scheme 4. Cascade reaction of carbonazidates.
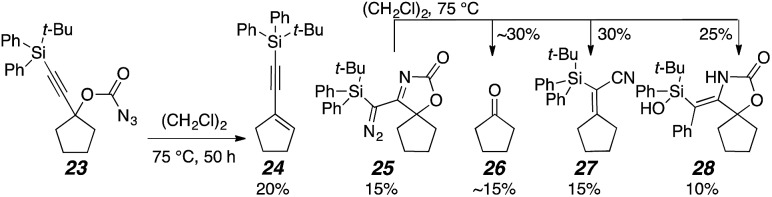
The products 19 and 21 appeared to be formed via C–H bond insertion at one of the isopropyl groups. In order to bias product formation toward the bridged tricycle by removing all α-silyl C–H bonds, tert-butyldiphenyl silylacetylene carbonazidate 23 was prepared (Scheme 5). The reaction produced five compounds, 24–28. The α-diazo oxazolone 25 and the silanol 28 were crystallized and analyzed by X-ray diffraction to confirm the structures shown.21 In a control experiment, diazoimine 25 was heated again to form 26–28, indicating that it is a viable reaction intermediate and that the vinyl nitrile and ketone products are derived from it. Enyne 24, however, was not observed, demonstrating that it likely formed by elimination from 23.
Scheme 5. Avoiding α-silyl C–H bond insertion.
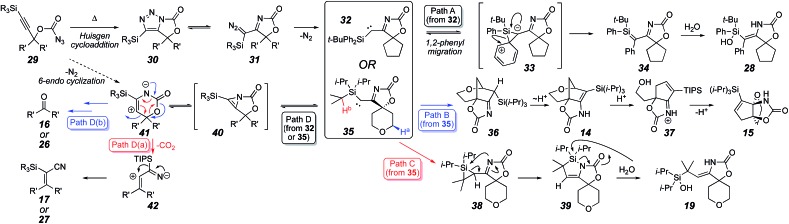
These results have been incorporated in a proposed mechanism for the cascade reaction (Scheme 6). Identification of α-diazo imine 25 suggests that a Huisgen cycloaddition22 generated triazole 30, followed by ring opening to 31.13,23 The α-imino carbene 32 (for R3Si = Ph2 t-BuSi) could be directly produced via dinitrogen extrusion. Silanol 28 would be produced from 32 by 1,2-phenyl migration (see 33 to 34, Path A)14b and hydration. When R3Si = i-Pr3Si, loss of N2 would form carbene 35, which could proceed through either a transannular ring insertion into the ether-activated methylene (Ha, see Path B), or insertion into the isopropyl C–H bond (Hb, see Path C). The former leads to the bridged tricycles 36 and 14, which can rearrange in the presence of acid to propellane 15 through an elimination/addition sequence.8 The latter pathway would form silacyclopropane 38. Silacyclopropanes are usually formed through silylene transfer,24 but there are a few cases of formation via intramolecular C–H bond insertion, though these intermediates were unstable and were not isolated.25 Likewise, intermediate 38 was not stable, but rearranged26 to azasilacyclopentene 39, which could be observed and characterized spectroscopically (vide supra). Exposure to water rapidly hydrolyzed the weak N–Si bond to give silanol 19.
Scheme 6. Proposed mechanism.
The isolation of ketone 26 and vinyl nitrile 27 suggested either a 6-endo reaction of a nitrene derived from 29 to form zwitterion 41, or else 41 would be derived from carbenes 32 or 35 (Path D).10–15 Since 26 and 27 were formed from heating diazoimine 25 (Scheme 5), it is likely that the latter route was operative. Therefore, a carbene electrocyclization of 32 (likely pseudopericyclic) would allow the highly strained azirine 40 to be transiently formed.26 Either of the C–N bonds could fragment, with an internal fragmentation forming the ring expanded product 41. A retro-hetero Diels–Alder reaction allows for decarboxylation (Path D(a)), and 1,2-silyl migration in zwitterion 42 would give the vinyl nitriles 17 or 27. Alternatively, C–C bond fragmentation would provide ketones 16 or 26 (Path D(b)). These pathways account for all the observed products and intermediates.
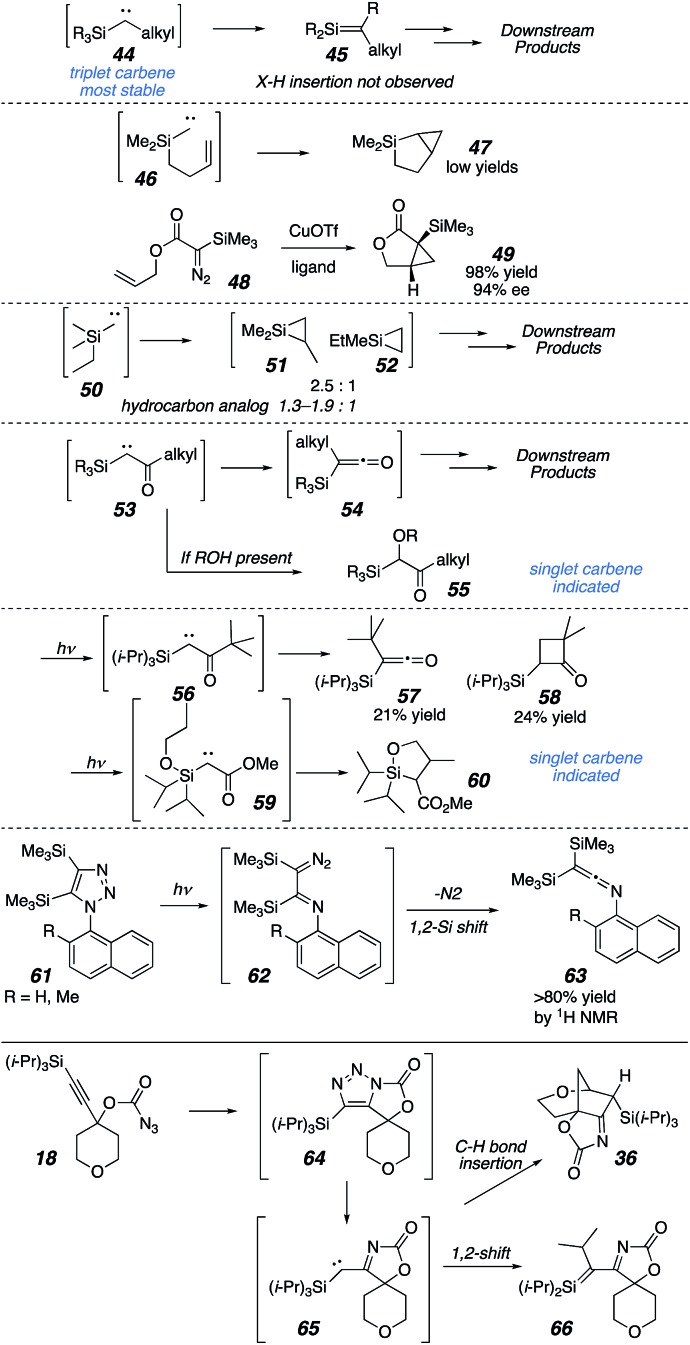
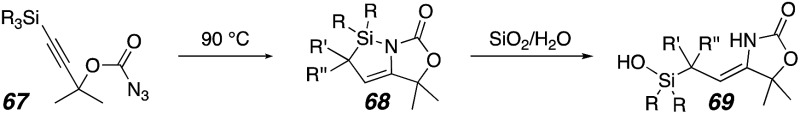
Only a few examples of intramolecular C–H bond insertion to form silacyclopropanes have been reported, and in those cases these intermediates were also fleeting and inferred from downstream products rather than observed directly.25 For simple silicon-substituted carbenes like 44, the triplet state is significantly stabilized relative to the singlet state.27,28 1,2-Rearrangements to silenes are the most common outcomes for these compounds,29 though cyclopropanations and C–H insertions have also been observed in suitable substrates (see 47 and 51). Intramolecular cyclopropanations appear to be possible with silyl carbenes of either a triplet or singlet nature if pendant olefins are present (47 and 49, respectively). On the other hand, the presence of a carbonyl induces more singlet-like reactivity,28,30 including Wolff rearrangements and X–H insertion reactions.31 Intramolecular C–H bond insertions may also occur for these cases, but the preference for insertion is typically at the β- or γ-position relative to silicon (see 60).32 A single report exists for imine-adjacent silyl carbene formation, which is also derived from a triazole precursor (61). In this case, the sole product was a 1,2-silyl shift to give ketene imine 63. Based on these precedents, products 36 (intramolecular C–H bond insertion) or 66 (1,2-shift: see 34, Scheme 6) would have been expected to dominate this reaction, but instead outcomes derived from silacyclopropane formation (see Schemes 3 and 4A) dominated when C–H bond insertion was possible and the formation of 66 and derivatives thereof were not observed.
The unanticipated formation of the azasilacyclopentenes from the reaction cascade prompted further investigation. The acyclic carbonazidate 20a was selected to bias the reaction for azasilacyclopentene formation by eliminating competitive C–H insertion in an appended ring (Table 1, entries 1–5). A lower yield of 21a resulted from the use of a halogenated solvent, dichloroethane, but the use of hexanes resulted in fewer byproducts and a slightly higher yield (entry 3). However, the use of Rh2(esp)2 or Cp*RuCl(cod)14 was deleterious (entries 4 and 5).
Table 1. Variation of the silyl alkyl group34 .
|
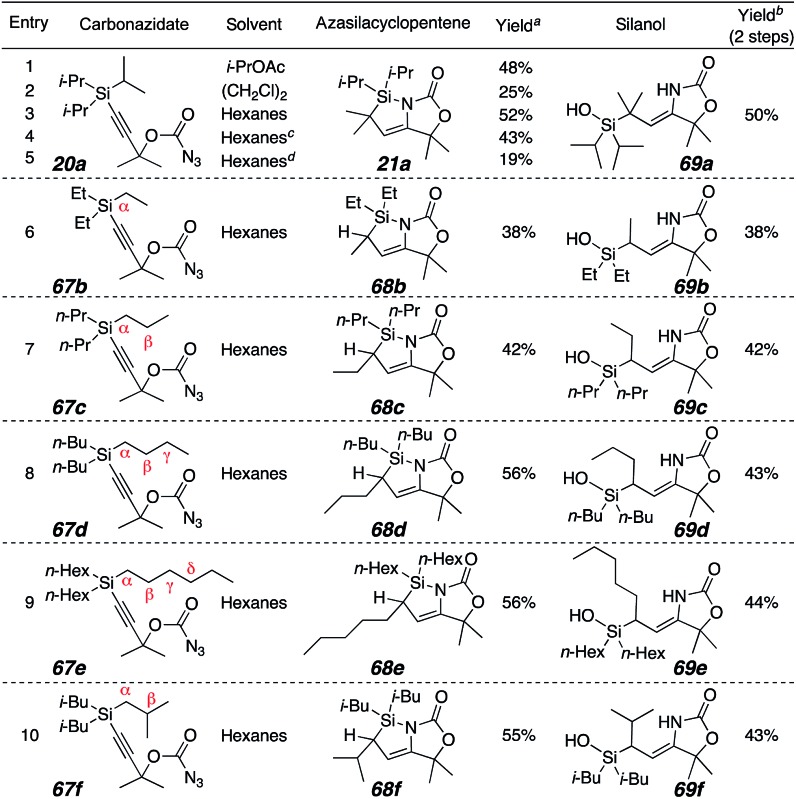
|
aYield based on 1H NMR peak integration relative to methyl-4-nitro-benzoate.
bIsolated yields (average of 2 trials).
c1 mol% Rh2(esp)2.
d2 mol% Cp*RuCl(cod).
We were curious if C–H bond insertion would take place at different positions on larger silyl alkanes, or the α-position was preferred. However, all of the alkyl groups only generated the azasilacyclopentenes corresponding to insertion into the C–H bond alpha to silicon (entries 6–9).19b These results contradict literature examples of singlet or metal carbene insertion into alkylsilanes, which show insertion primarily at the methylene or methine beta to silicon,33 presumably due to the “β-silicon effect”. In the examples in Table 1, none of the available β, γ, or δ-C–H bonds reacted. High regioselectivity was shown even in the isobutyl substrate 69f, where the electron-rich β-methine was untouched.
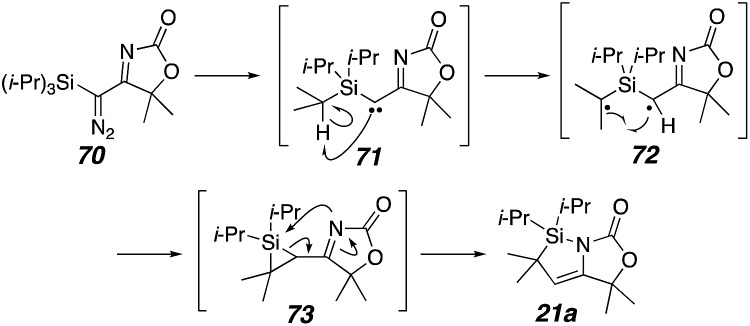
It appears that unlike the other acyl silyl carbenes in Scheme 7, the carbenes 32 and 35 are triplet carbenes and therefore exhibit diradical character.35 The C–C bond formation would then proceed via hydrogen atom abstraction from the carbon attached to the silicon in 71 to generate an α-silyl radical36 72 (Scheme 8), which would be stabilized by backbonding from silicon δ-orbitals.37 Then, the silacyclopropane 73 would be formed from radical combination.
Scheme 7. Typical outcomes for silyl-substituted carbenes.
Scheme 8. Proposed triplet carbene/diradical pathway.
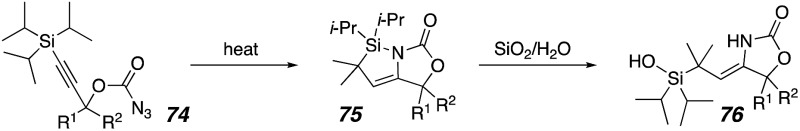
Next, a series of acyclic, cyclic, and heterocyclic carbonazidates was examined (Table 2). Varying the alkyl groups at R1 and R2 of 74 does not significantly affect the formation of the azasilacyclopentene. Although an allyl group could potentially undergo cyclopropanation with an intermediate silyl carbene,32a,38 only the azasilacyclopentene 75e is seen (entry 5). Cyclic substrates generally provided spiro-oxazolones (entry 6–10). Bridged polycycles were not observed, even for pyrrolidine 74i and thiolane 74j, where the heteroatoms could activate α-C–H bonds for carbene insertion.39 Two substrates with six-membered rings provided a different result, presumably due to greater conformational flexibility to react with the activated C–H bonds compared to the 5-membered rings (entries 11 and 12). For the 3-methyl cyclohexane 74k, the reaction produced a 1 : 1 ratio of azasilacyclopentene 75k and bridged tricycle 77. For the tosyl piperidine 74l, the major isolated product was the silanol 76l, but some bridged bicycle was observed.40
Table 2. Propargylic variation in the substrates34 .
|

|
aYield based on 1H NMR peak integration relative to methyl-4-nitro-benzoate.
bIsolated yields (average of 2 trials).
cHexanes, 90 °C.
dIsopropyl acetate, 100 °C.
eHexanes, 100 °C.
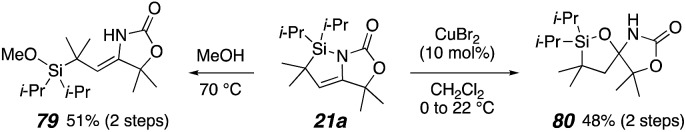
Preliminary tests were made for transformations of the azasilacyclopentene products. Methyl silyl ether 79 is obtained in good yield by ring opening of 21a in methanol. Treatment of 21a with CuBr2 generated spiro-hemiaminal 80.16 Silanol 69a also produced 80 with CuBr2, suggesting that 21a is first hydrolyzed by adventitious water before spirocycle formation (Scheme 9).
Scheme 9. Reactions of azasilacyclopentene.
Conclusions
A novel approach for the synthesis of azasilacyclopentenes via a Huisgen cycloaddition-initiated cascade reaction terminating in an α-silyl C–H bond insertion has been discovered. Control experiments suggest a likely mechanism with triplet silylcarbene intermediates undergoing C–H bond insertion at the carbon attached to silicon to form transient silacyclopropanes. Further reaction of the azasilacyclopentene showed the formation of interesting products. Ongoing efforts are seeking selective synthesis of bridged polycycles and vinyl nitriles from the same precursors.
Conflicts of interest
There are no conflicts of interest to declare.
Acknowledgments
The authors thank the Welch Foundation (grant E-1744) and the NSF (grant CHE-1352439) for funding. J.-L. S. is grateful to CBIP (UH) and the Studying Abroad Scholarship of Taiwan for fellowships. They are also grateful to Dr Ilja Popovs for discussion of the spectroscopic characterization of mechanistic intermediates.
Footnotes
References
- (a) Qin C., Boyarskikh V., Hansen J. H., Hardcastle K. I., Musaev D. G., Davies H. M. L. J. Am. Chem. Soc. 2011;133:19198. doi: 10.1021/ja2074104. [DOI] [PubMed] [Google Scholar]; (b) Parr B. T., Davies H. M. L. Angew. Chem., Int. Ed. 2013;52:10044. doi: 10.1002/anie.201304310. [DOI] [PubMed] [Google Scholar]; (c) Hou S.-H., Tu Y.-Q., Liu L., Zhang F.-M., Wang S.-H., Zhang X.-M. Angew. Chem., Int. Ed. 2013;52:11373. doi: 10.1002/anie.201306369. [DOI] [PubMed] [Google Scholar]; (d) Lu H., Dzik W. I., Xu X., Wojtas L., de Bruin B., Zhang X. P. J. Am. Chem. Soc. 2011;133:8518. doi: 10.1021/ja203434c. [DOI] [PubMed] [Google Scholar]; (e) Lindsay V. N. G., Nicolas C., Charette A. B. J. Am. Chem. Soc. 2011;133:8972. doi: 10.1021/ja201237j. [DOI] [PubMed] [Google Scholar]; (f) DeAngelis A., Panish R., Fox J. M. Acc. Chem. Res. 2016;49:115. doi: 10.1021/acs.accounts.5b00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Panne P., Fox J. M. J. Am. Chem. Soc. 2007;129:22. doi: 10.1021/ja0660195. [DOI] [PubMed] [Google Scholar]; (b) Crombie A. L., Kane J. L., Shea K. M., Danheiser R. L. J. Org. Chem. 2004;69:8652. doi: 10.1021/jo048698c. [DOI] [PubMed] [Google Scholar]
- (a) Xu X., Zavalij P. Y., Doyle M. P. Angew. Chem., Int. Ed. 2012;51:9829. doi: 10.1002/anie.201203962. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Barluenga J., Lonzi G., Riesgo L., López L. A., Tomás M. J. Am. Chem. Soc. 2010;132:13200. doi: 10.1021/ja106751t. [DOI] [PubMed] [Google Scholar]; (c) Wang X., Xu X., Zavalij P. Y., Doyle M. P. J. Am. Chem. Soc. 2011;133:16402. doi: 10.1021/ja207664r. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Xu X., Zavalij P. Y., Doyle M. P. J. Am. Chem. Soc. 2013;135:12439. doi: 10.1021/ja406482q. [DOI] [PubMed] [Google Scholar]; (e) Qin C., Davies H. M. L. J. Am. Chem. Soc. 2013;135:14516. doi: 10.1021/ja4069003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Qu Z., Shi W., Wang J. J. Org. Chem. 2004;69:217. doi: 10.1021/jo0350312. [DOI] [PubMed] [Google Scholar]; (b) DeAngelis A., Dmitrenko O., Fox J. M. J. Am. Chem. Soc. 2012;134:11035. doi: 10.1021/ja3046712. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Maier T. C., Fu G. C. J. Am. Chem. Soc. 2006;128:4594. doi: 10.1021/ja0607739. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Zhu S.-F., Xu B., Wang G.-P., Zhou Q.-L. J. Am. Chem. Soc. 2012;134:436. doi: 10.1021/ja2084493. [DOI] [PubMed] [Google Scholar]; (e) Liu B., Zhu S.-F., Zhang W., Chen C., Zhou Q.-L. J. Am. Chem. Soc. 2007;129:5834. doi: 10.1021/ja0711765. [DOI] [PubMed] [Google Scholar]; (f) Sambasivan R., Ball Z. T. J. Am. Chem. Soc. 2010;132:9289. doi: 10.1021/ja103747h. [DOI] [PubMed] [Google Scholar]
- (a) Herrmann P., Bach T. Chem. Soc. Rev. 2011;40:2022. doi: 10.1039/c0cs00027b. [DOI] [PubMed] [Google Scholar]; (b) Doyle M. P., Duffy R., Ratnikov M., Zhou L. Chem. Rev. 2010;110:704. doi: 10.1021/cr900239n. [DOI] [PubMed] [Google Scholar]; (c) Hansen J. H., Gregg T. M., Ovalles S. R., Lian Y., Autschbach J., Davies H. M. L. J. Am. Chem. Soc. 2011;133:5076. doi: 10.1021/ja111408v. [DOI] [PubMed] [Google Scholar]; (d) Wang H., Li G., Engle K. M., Yu J.-Q., Davies H. M. L. J. Am. Chem. Soc. 2013;135:6774. doi: 10.1021/ja401731d. [DOI] [PubMed] [Google Scholar]; (e) Davies H. M. L., Morton D. Chem. Soc. Rev. 2011;40:1857. doi: 10.1039/c0cs00217h. [DOI] [PubMed] [Google Scholar]; (f) Lian Y., Davies H. M. L. J. Am. Chem. Soc. 2011;133:11940. doi: 10.1021/ja2051155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Davies H. M. L., Denton J. R. Chem. Soc. Rev. 2009;38:3061. doi: 10.1039/b901170f. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Yamaguchi J., Yamaguchi A. D., Itami K. Angew. Chem., Int. Ed. 2012;51:8960. doi: 10.1002/anie.201201666. [DOI] [PubMed] [Google Scholar]; (c) Lu P., Gu Z., Zakarian A. J. Am. Chem. Soc. 2013;135:14552. doi: 10.1021/ja408231t. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Lu P., Mailyan A., Gu Z., Guptill D. M., Wang H., Davies H. M. L., Zakarian A. J. Am. Chem. Soc. 2014;136:17738. doi: 10.1021/ja510573v. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Daugulis O., Roane J., Tran L. D. Acc. Chem. Res. 2015;48:1053. doi: 10.1021/ar5004626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Padwa A., Weingarten M. D. Chem. Rev. 1996;96:223. doi: 10.1021/cr950022h. [DOI] [PubMed] [Google Scholar]; (b) Hoye T. R. J. Am. Chem. Soc. 1991;113:4343. [Google Scholar]; (c) Zheng Z., Zheng L. Org. Chem. Front. 2015;2:1556. [Google Scholar]; (d) Padwa A. Chem. Soc. Rev. 2009;38:3072. doi: 10.1039/b816701j. [DOI] [PubMed] [Google Scholar]
- (a) Jansone-Popova S., May J. A. J. Am. Chem. Soc. 2012;134:17877. doi: 10.1021/ja308305z. [DOI] [PubMed] [Google Scholar]; (b) Jansone-Popova S., Le P. Q., May J. A. Tetrahedron. 2014;70:4118. [Google Scholar]; (c) Le P. Q., May J. A. J. Am. Chem. Soc. 2015;137:12219. doi: 10.1021/jacs.5b08157. [DOI] [PubMed] [Google Scholar]
- (a) Rigoli J. W., Weatherly C. D., Alderson J. M., Vo B. T., Schomaker J. M. J. Am. Chem. Soc. 2013;135:17238. doi: 10.1021/ja406654y. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Desai A. A., Wulff W. D. J. Am. Chem. Soc. 2010;132:13100. doi: 10.1021/ja1038648. [DOI] [PubMed] [Google Scholar]; (c) Fructos M. R., Álvarez E., Díaz-Requejo M. M., Pérez P. J. J. Am. Chem. Soc. 2010;132:4600. doi: 10.1021/ja1006614. [DOI] [PubMed] [Google Scholar]; (d) Maestre L., Sameera W. M. C., Díaz-Requejo M. M., Maseras F., Pérez P. J. A. J. Am. Chem. Soc. 2013;135:1338. doi: 10.1021/ja307229e. [DOI] [PubMed] [Google Scholar]; (e) Guthikonda K., Du Bois J. J. Am. Chem. Soc. 2002;124:13672. doi: 10.1021/ja028253a. [DOI] [PubMed] [Google Scholar]
- (a) Milczek E., Boudet N., Blakey S. Angew. Chem., Int. Ed. 2008;47:6825. doi: 10.1002/anie.200801445. [DOI] [PubMed] [Google Scholar]; (b) Zalatan D. N., Du Bois J. J. Am. Chem. Soc. 2008;130:9220. doi: 10.1021/ja8031955. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Nguyen Q., Nguyen T., Driver T. G. J. Am. Chem. Soc. 2013;135:620. doi: 10.1021/ja3113565. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Nörder A., Warren S. A., Herdtweck E., Huber S. M., Bach T. J. Am. Chem. Soc. 2012;134:13524. doi: 10.1021/ja3066682. [DOI] [PubMed] [Google Scholar]; (e) Zalatan D. N., Du Bois J. J. Am. Chem. Soc. 2009;131:7558. doi: 10.1021/ja902893u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Dequirez G., Pons V., Dauban P. Angew. Chem., Int. Ed. 2012;51:7384. doi: 10.1002/anie.201201945. [DOI] [PubMed] [Google Scholar]; (b) Sloan Devlin A., Du Bois J. Chem. Sci. 2013;4:1059. doi: 10.1039/C2SC21723F. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Fleming J. J., McReynolds M. D., Du Bois J. J. Am. Chem. Soc. 2007;129:9964. doi: 10.1021/ja071501o. [DOI] [PubMed] [Google Scholar]; (d) Hinman A., Du Bois J. J. Am. Chem. Soc. 2003;125:11510. doi: 10.1021/ja0368305. [DOI] [PubMed] [Google Scholar]; (e) Mulcahy J. V., Du Bois J. J. Am. Chem. Soc. 2008;130:12630. doi: 10.1021/ja805651g. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Quasdorf K. W., Huters A. D., Lodewyk M. W., Tantillo D. J., Garg N. K. J. Am. Chem. Soc. 2012;134:1396. doi: 10.1021/ja210837b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Thornton A. R., Blakey S. B. J. Am. Chem. Soc. 2008;130:5020. doi: 10.1021/ja7111788. [DOI] [PubMed] [Google Scholar]; (b) Thornton A. R., Martin V. I., Blakey S. B. J. Am. Chem. Soc. 2009;131:2434. doi: 10.1021/ja809078d. [DOI] [PubMed] [Google Scholar]; (c) Stoll A. H., Blakey S. B. J. Am. Chem. Soc. 2010;132:2108. doi: 10.1021/ja908538t. [DOI] [PubMed] [Google Scholar]; (d) Stoll A. H., Blakey S. B. Chem. Sci. 2011;2:112. doi: 10.1039/C0SC00577K. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Mace N., Thornton A. R., Blakey S. B. Angew. Chem., Int. Ed. 2013;52:5836. doi: 10.1002/anie.201301087. [DOI] [PubMed] [Google Scholar]; (f) Boyer A. J. Org. Chem. 2015;80:4771. doi: 10.1021/acs.joc.5b00399. [DOI] [PubMed] [Google Scholar]
- (a) Spangler J. E., Davies H. M. L. J. Am. Chem. Soc. 2013;135:6802. doi: 10.1021/ja4025337. [DOI] [PubMed] [Google Scholar]; (b) Alford J. S., Spangler J. E., Davies H. M. L. J. Am. Chem. Soc. 2013;135:11712. doi: 10.1021/ja405043g. [DOI] [PubMed] [Google Scholar]; (c) Parr B. T., Green S. A., Davies H. M. L. J. Am. Chem. Soc. 2013;135:4716. doi: 10.1021/ja401386z. [DOI] [PubMed] [Google Scholar]; (d) Miura T., Funakoshi Y., Murakami M. J. Am. Chem. Soc. 2014;136:2272. doi: 10.1021/ja412663a. [DOI] [PubMed] [Google Scholar]; (e) Miura T., Tanaka T., Hiraga K., Stewart S. G., Murakami M. J. Am. Chem. Soc. 2013;135:13652. doi: 10.1021/ja407166r. [DOI] [PubMed] [Google Scholar]; (f) Horneff T., Chuprakov S., Chernyak N., Gevorgyan V., Fokin V. V. J. Am. Chem. Soc. 2008;130:14972. doi: 10.1021/ja805079v. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Alford J. S., Davies H. M. L. J. Am. Chem. Soc. 2014;136:10266. doi: 10.1021/ja5058967. [DOI] [PubMed] [Google Scholar]
- (a) Chuprakov S., Malik J. A., Zibinsky M., Fokin V. V. J. Am. Chem. Soc. 2011;133:10352. doi: 10.1021/ja202969z. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Boren B. C., Narayan S., Rasmussen L. K., Zhang L., Zhao H., Lin Z., Jia G., Fokin V. V. J. Am. Chem. Soc. 2008;130:8923. doi: 10.1021/ja0749993. [DOI] [PubMed] [Google Scholar]
- Lindsay V. N. G., Viart H. M. F., Sarpong R. J. Am. Chem. Soc. 2015;137:8368. doi: 10.1021/jacs.5b04295. [DOI] [PubMed] [Google Scholar]
- Structure confirmed via single crystal X-ray diffraction
- (a) Singh R., Kolev J. N., Sutera P. A., Fasan R. ACS Catal. 2015;5:1685. doi: 10.1021/cs5018612. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Heinisch T., Ward T. R. Eur. J. Inorg. Chem. 2015;2015:3406. [Google Scholar]; (c) Bach T., Schlummer B., Harms K. Chem.–Eur. J. 2001;7:2581. doi: 10.1002/1521-3765(20010618)7:12<2581::aid-chem25810>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]; (d) Uccello D. P., Miller S. M., Dieterich N. A., Stepan A. F., Chung S., Farley K. A., Samas B., Chen J., Montgomery J. I. Tetrahedron Lett. 2011;52:4247. [Google Scholar]
- Nevárez Z., Woerpel K. A. Org. Lett. 2007;9:3773. doi: 10.1021/ol701424a. [DOI] [PubMed] [Google Scholar]
- (a) Calad S. A., Woerpel K. A. J. Am. Chem. Soc. 2005;127:2046. doi: 10.1021/ja042893r. [DOI] [PubMed] [Google Scholar]; (b) Bruckmann R., Maas G. Chem. Ber. 1987;120:635. [Google Scholar]
- Kevy G. C. and Lichter R. L., Nitrogen-15 Nuclear Magnetic Resonance Spectroscopy, J. Wiley, New York, NY, 1979. [Google Scholar]
- Crystal structures have been deposited in the Cambridge Crystallographic Data Centre, reference numbers 1477662–1477666 and ; 1477728.
- (a) Bräse S., Gil C., Knepper K., Zimmermann V. Angew. Chem., Int. Ed. 2005;44:5188. doi: 10.1002/anie.200400657. [DOI] [PubMed] [Google Scholar]; (b) Brisbois R. G., Bergan A. M., Ellison A. J., Griffin P. Y., Hackbarth K. C., Larson S. R. Tetrahedron Lett. 2013;54:272. [Google Scholar]
- Mitchell G., Rees C. W. J. Chem. Soc., Perkin Trans. 1. 1987:413. [Google Scholar]
- (a) Franz A. K., Woerpel K. A. Acc. Chem. Res. 2000;33:813. doi: 10.1021/ar9900562. [DOI] [PubMed] [Google Scholar]; (b) Driver T. G., Franz A. K., Woerpel K. A. J. Am. Chem. Soc. 2002;124:6524. doi: 10.1021/ja020183k. [DOI] [PubMed] [Google Scholar]; (c) Ćiraković J., Driver T. G., Woerpel K. A. J. Am. Chem. Soc. 2002;124:9370. doi: 10.1021/ja020566i. [DOI] [PubMed] [Google Scholar]; (d) Driver T. G., Woerpel K. A. J. Am. Chem. Soc. 2003;125:10659. doi: 10.1021/ja0301370. [DOI] [PubMed] [Google Scholar]
- (a) Connolly J. W., Urry G. J. J. Org. Chem. 1964;29:619. [Google Scholar]; (b) Connolly J. W. J. Organomet. Chem. 1968;11:429. [Google Scholar]
- (a) Huisgen R. Angew. Chem., Int. Ed. Engl. 1980;19:947. [Google Scholar]; (b) Ji H., Li L., Xu X., Ham S., Hammad L. A., Birney D. M. J. Am. Chem. Soc. 2009;131:528. doi: 10.1021/ja804812c. [DOI] [PubMed] [Google Scholar]
- Maas G., Silicon-Substituted Carbenes, John Wiley & Sons, Ltd, Chichester, UK, 2nd edn, 2009, vol. I. [Google Scholar]
- Nemirowski A., Schreiner P. R. J. Org. Chem. 2007;72:9533. doi: 10.1021/jo701615x. [DOI] [PubMed] [Google Scholar]
- (a) Ando W., Hagiwara T., Migita T. J. Am. Chem. Soc. 1973;95:7518. [Google Scholar]; (b) Ando W., Sekiguchi A., Ogiwara J., Migita T. J. Chem. Soc., Chem. Commun. 1975:145. [Google Scholar]
- Hirai K., Itoh T., Tomioka H. Chem. Rev. 2009;109:3275. doi: 10.1021/cr800518t. [DOI] [PubMed] [Google Scholar]
- (a) Ando W., Sekiguchi A., Hagiwara T., Migita T., Chowdhry V., Westheimer F. H., Kammula S. L., Green M., Jones M. J. Am. Chem. Soc. 1979;101:6393. [Google Scholar]; (b) Brückmann R., Schneider K., Maas G. Tetrahedron. 1989;45:5517. [Google Scholar]; (c) Davies M. J., Moody C. J., Taylor R. J. J. Chem. Soc., Perkin Trans. 1. 1991:1. [Google Scholar]; (d) Alt M., Maas G. Tetrahedron. 1994;50:7435. [Google Scholar]; (e) Marsden S. P., Pang W. K. Tetrahedron Lett. 1998;39:6077. [Google Scholar]; (f) Braddock D. C., Badine D. M., Gottschalk T., Matsuno A., Rodriguez-Lens M. Synlett. 2003:345. [Google Scholar]
- (a) Schöllkopf U., Hoppe D., Rieber N., Jacobi V. Eur. J. Org. Chem. 1969;730:1. [Google Scholar]; (b) Brückmann R., Schneider K., Maas G. Tetrahedron. 1989;45:5517. [Google Scholar]
- (a) Seyferth D., Washburne S. S., Attridge C. J. J. Am. Chem. Soc. 1970;92:4405. [Google Scholar]; (b) Wang P., Adams J. J. Am. Chem. Soc. 1994;116:3296. [Google Scholar]; (c) Hatanaka Y., Watanabe M., Onozawa S.-Y., Tanaka M., Sakurai H. J. Org. Chem. 1998;63:422. doi: 10.1021/jo971910a. [DOI] [PubMed] [Google Scholar]
- Although azasilacyclopentenes can be observed in the NMR and GC/MS of the crude reaction mixture, they do not survive silica gel chromatography. Thus, NMR-based yields are reported for the azasilacyclopentenes and isolated yields are reported for the corresponding silanols
- (a) Ellis M. J., Stevens M. F. G. J. Chem. Soc., Perkin Trans. 1. 2001:3174. [Google Scholar]; (b) Hagan D. J., Chan D., Schwalbe C. H., Stevens M. F. G. J. Chem. Soc., Perkin Trans. 1. 1998:915. [Google Scholar]
- Walsh R. Acc. Chem. Res. 1981;14:246. [Google Scholar]
- (a) Wilt J. W., Kolewe O., Kraemer J. F. J. Am. Chem. Soc. 1969;91:2624. [Google Scholar]; (b) Manabe T., Yanagi S.-I., Ohe K., Uemura S. Organometallics. 1998;17:2942. [Google Scholar]; (c) Lee Y. J., Linf R., Mariano P. S. J. Org. Chem. 1996;61:3304. [Google Scholar]; (d) Bogen S., Fensterbank L., Malacria M. J. Org. Chem. 1999;64:819. doi: 10.1021/jo981622u. [DOI] [PubMed] [Google Scholar]; (e) Grieco P., Yokoyama Y., Williams E. J. Org. Chem. 1978;43:1285. [Google Scholar]; (f) Cocivera M., Grunwald E. J. Am. Chem. Soc. 1965;87:2071. [Google Scholar]; (g) Krusic P. J., Kochi J. K. J. Am. Chem. Soc. 1969;91:6161. [Google Scholar]
- (a) Ando W., Sekiguchi A., Hagiwara T., Migita T. J. Chem. Soc., Chem. Commun. 1974:372. [Google Scholar]; (b) Barton T. J., Kilgour J. A., Gallucci R. G., Rothschild A. J., Slutsky J., Wolf A. D., Jones M. J. Am. Chem. Soc. 1975;97:657. [Google Scholar]; (c) Maas G., Daucher B., Maier A., Gettwert V. Chem. Commun. 2004;26:238. doi: 10.1039/b312110k. [DOI] [PubMed] [Google Scholar]; (d) Inoue S., Nagatani K., Tezuka H., Hoshino Y., Nakada M. Synlett. 2017;28:1065. [Google Scholar]
- i-PrOAc was used for solubility
- The azasilylcyclopentene intermediate could not accurately be quantified
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.