Abstract.
Safe and sufficient water, sanitation, and hygiene (WaSH) prevent the spread of disease in health-care facilities (HCFs). Little research has been conducted on WaSH in HCF in sub-Saharan Africa. We carried out a cross-sectional study of WaSH in 1,318 randomly selected rural HCF (hospitals, health centers, health posts, and clinics) in regions throughout Ethiopia, Kenya, Mozambique, Rwanda, Uganda, and Zambia. Methods included questionnaires with head doctors and nurses to document WaSH access, continuity, quality, quantity and reliability, and analysis of drinking water samples for Escherichia coli. We found that fewer than 50% of rural HCFs had access to improved water sources on premises, improved sanitation, and consistent access to water and soap for handwashing (Ethiopia [7%), Kenya [30%], Mozambique [29%], Rwanda [50%], Uganda [30%], and Zambia [21%]). Adequate hand hygiene reduces disease transmission and health-care-acquired infections, but fewer than 25% of HCF in each country reported that a combination of water, soap, and hand-drying materials were always available. Our research points to a lack of basic WaSH services in rural HCFs in regions of sub-Saharan Africa, which poses a threat to the health of patients and health-care workers in these settings.
INTRODUCTION
In 2012, an estimated 842,000 deaths occurred due to diarrheal disease from inadequate water, sanitation, and hygiene; this disease burden can be reduced by increasing access to safe water and sanitation and promoting basic hygiene behaviors (WaSH).1 A well-targeted and well-executed intervention to improve water or sanitation can reduce the prevalence of diarrheal disease by about a third.2 The provision of a piped or on-premises water service can further reduce the disease burden.3 Availability of clean water and soap enables and encourages people to wash their hands, reducing the likelihood of disease transmission.4 Safely managed sanitation can control flies and other insects that spread disease, and prevent contact with infectious organisms shed in feces, such as helminth ova and diarrheagenic pathogens.5 An adequate and continuous supply of safe drinking-water on-premises can interrupt waterborne disease transmission.6,7 Together these factors reduce rates of diarrhea, malnutrition, and dehydration, leading causes of death in low- and middle-income countries.8
There is increasing interest in understanding and monitoring WaSH services in non-household settings, such as schools, workplaces, and health-care facilities (HCFs).9 WaSH is particularly important in HCF, sites where medical services are provided. Adequate WaSH in these settings are essential for realizing the human right to health as described by the United Nations Committee on Economic, Social, and Cultural Rights in General Comment 14,10 as well as the human right to water described in General Comment 15.11 Health practitioners in HCF are tasked with treating disease, but their first edict is to “do no harm.” This is only achievable with adequate WaSH services, since unsafe water and sanitation and poor hygiene practices facilitate health-care-acquired infections.
There are few peer-reviewed publications on WaSH and WaSH interventions in HCF within low-income countries, specifically in sub-Saharan Africa, and no multicountry studies. One study, conducted in 12 HCF in the rural Pune District of India, found limited availability and satisfactoriness of latrines and handwashing stations.12 A 2014 qualitative study of 10 HCFs in a rural province in Cambodia documented suboptimal hand hygiene, unsafe water storage practices, and a lack of sanitation facilities in health centers where women give birth.13 A 2011 study in Tanzania analyzed the Service Provision Assessment survey, documenting that 44% of health facilities that conduct deliveries have delivery rooms that are WATSAN-safe14; a similar analysis of the Service Provision Assessment survey in Malawi revealed that 59.3% of HCF conducting deliveries had “safe, reliable, and accessible” water sources.15 These studies are limited in scope and have focused on HCF in one country or a few HCF in one district or province. One 2015 World Health Organization (WHO) and the United Nations Children’s Fund (UNICEF) report offers a broader perspective: it estimates the proportion of HCF with access to improved water within 54 low- and middle-income countries across the world, as well as the proportion of HCF with improved sanitation in 36 countries and the proportion with water and soap in 35 countries.16 The report notes that different definitions and survey instruments were used for measuring the same indicators across countries, and that increased, more consistent monitoring in HCF across countries is needed.16
WaSH indicators have been monitored to track progress toward international WaSH targets; yet, monitoring efforts have focused mainly on households and have not included HCF. Indicators commonly used to monitor water services with substantial health-, economic-, and rights-based evidences are accessibility (distance to source), continuity, quantity, safety, and service type.17,18 Service types have also been categorized as improved and unimproved, where improved sources “protect the source [water] from outside contamination, particularly fecal matter” and unimproved sources do not.19 Although an improved water source typically reduces risk of contamination, it does not guarantee safety. One meta-analysis of water in households, schools, and HCFs found that in more than 38% of studies, fecal indicator levels exceeded guidelines from the WHO in over a quarter of samples from improved sources, with contamination particularly likely in rural areas and low-income countries.20 Few studies measure water quality in HCF in low-income countries, especially in sub-Saharan Africa, but fecally contaminated water has been identified in hospitals in Brazil and Fiji.21,22 Treatment and safe storage are critical for maintaining water safety if water quality is compromised at the source, water is intermittently available (continuity), or water is located some distance from the point of consumption (accessibility).6,23
Indicators such as service type, functionality, and safety have been used to categorize sanitation services.24 Poor sanitation within HCF can contribute to the spread of disease16; for instance, improper management of waste from cholera-infected patients or staff can lead to inadvertent fecal contamination of medical equipment and eventual cholera outbreaks, especially under crowded conditions.25,26 Inadequate quality sanitation in HCF can also lead to embarrassment and discomfort in patients seeking medical assistance. In addition to the previously mentioned study which found that 44% of Tanzanian HCF performing deliveries were WATSAN-safe,14 a 2014 assessment of Service Provision Assessment survey data from Ethiopia found that more than 26% of HCF across the country did not have a functioning latrine for patient use.27 The previously mentioned Steinmann and others study of 12 HCF in India found that women consider sanitation and hand hygiene installations a vital part of HCFs; a lack of these amenities can detract from a facility’s appeal and reputation, and can affect the consistency and timeliness of care seeking.12
Finally, hand hygiene monitoring efforts have focused on indicators that track access to water and soap, and the continuity of that access, and use.28 Hand hygiene, the use of water, soap, and hand-drying materials, decreases the likelihood that a health-care professional will pass pathogens between patients.29 Thousands of deaths per year are attributable to health-care-acquired infections,30 but consistent use of soap, water, and hand-drying materials for hand hygiene could reduce this number: one review suggests that hand hygiene interventions reduce health-care-acquired infections by 23–57%.31 Improved hand hygiene could significantly reduce neonatal mortality for births within HCF in sub-Saharan Africa: one review estimated that increased handwashing among birth attendants could reduce neonatal mortality rates by 19%.32
The benefits from universal access to sufficient WaSH in HCF (better health outcomes, increased dignity for patients and health-care professionals, and increased trust in the health-care system) are substantial, especially in sub-Saharan Africa, a region with the highest rates of neonatal and maternal mortality in the world, with infections causing 39% of neonatal deaths.33,34
We conducted a cross-sectional study of WaSH infrastructure, supplies, and behaviors in 1,318 randomly selected rural HCF in regions of Ethiopia, Kenya, Mozambique, Rwanda, Uganda, and Zambia. We focused on HCF in rural areas. The majority of the population in sub-Saharan Africa live in rural areas.35 Furthermore, rural HCF in middle- and low-income countries tend to offer lower levels of care than their urban counterparts, because of problems such as inadequate staffing and infrastructure deficiencies.36 This is the first known cross-sectional multicountry study of WaSH in HCF within sub-Saharan Africa. It adds to efforts to track progress toward the Sustainable Development Goal of universal access to WaSH coverage, including a WHO/UNICEF commitment to monitoring WaSH improvements in extrahousehold settings such as schools and HCF9,37 with proposed core indicators such as the percentage of HCF with basic drinking water service, basic sanitation services, basic hand hygiene facilities, and basic health-care waste management.38,39 This study also allows health sectors in the countries of study to identify areas that need to be addressed as they work toward the goal of universal coverage of basic WaSH in HCFs and aim to reduce infant and maternal mortality in sub-Saharan Africa.
METHODS
Study population and design.
A stratified random sample of rural HCFs was taken in Ethiopia, Kenya, Mozambique, Rwanda, Uganda, and Zambia. Lists of HCF within selected rural districts or subcounties were obtained from each country’s Ministry of Health and comprised the sample frame. As this study was funded by a nongovernmental organization (NGO), World Vision, and was part of a larger study to evaluate WaSH in HCF for this organization, rural HCF were randomly selected from two strata—HCF where the NGO worked and HCF where the NGO did not work. In the analysis, the two groups were collapsed and weights were computed to account for unequal probabilities of selection (Table 1).
Table 1.
Sample sizes and specific regions sampled within six African countries (Ethiopia, Kenya, Mozambique, Rwanda, Uganda, and Zambia)
| Country | Region type | Sampled regions | Total sample | Total frame |
|---|---|---|---|---|
| Ethiopia | District (woreda) | Wonchi, Muher ena Aklil, Omo Nada, Gechi, Gewata, Abaya, Hulla, Quachabirra, Banja, Dembia, Angolela, Mersa, Jille, Samre, Jarso, Melka Belo, Ilu, Meskan, Tiro Afeta, Dedessa, Gimbo, Gelana, Aleta Wundo, Angacha, Dangila, West Belesa, Basona Worena, Kobo, Dewa Harewa, Hintalo Wajirat, Haro Maya, and Kersa | 534 | 938 |
| Kenya | Subcounty | Ganze, Turkana South, Nyakach, Transmara East, Transmara West, Magarini, Pokot South, Pokot West, Malava, Matete, Kibwezi, Kitui South, Kajiado Central, Tseikuru, Ronga, and Subukia | 126 | 126 |
| Mozambique | District | Manjacaze, Guija, Xai-Xai, Chibuto, Murrumbala, Mocuba, Namacurra, Cahora Bassa, Angonia, Changara, Murrupula, Nacaroa, Muecate, Guija, Meconta, Chire, and Nicodale | 198 | 202 |
| Rwanda | District (akarere) | Bugesera, Gakenke, Gatsibo, Huye, Kicukiro, Munini, Gicumbi, Rulindo, Gisagara, Kicukiro, Nyagatare, Nyamagabe, Nyaruguru, Rutsiro, Ngororero, Kayonza, and Karongi | 74 | 109 |
| Uganda | District | Amuru, Buliisa, Gulu, Hoima, Kaliro, Kibaale, Kiryandongo, Lamwo, Luweero, Masindi, Nakaseke, and Nwoya | 182 | 243 |
| Zambia | District | Mazabuka, Monze, Mpulungu, Mbala, Pemba, Rufunsa, Chongwe, Solwezi, Kalomo, Sinazongwe, Kasama, and Chipata | 204 | 204 |
| Total | 1,318 | 1,822 | ||
The number of HCF sampled was equal to the number of HCF on the entire sample frame in two countries, Kenya and Zambia: in these countries, all HCF in the sample frame were contacted for interviews. We were, therefore, able to calculate point estimates for our results in these countries. In Ethiopia, Mozambique, Rwanda, and Uganda, we calculated 95% confidence intervals because we sampled a portion of the HCF in the sample frame. Although the original study design called for equal sample sizes in all countries, this was part of a larger study and sample sizes were restricted by limited resources (time, money, and personnel available to interview in each country).
Survey instrument.
The HCF WaSH questionnaire, designed for this study, included WaSH indicators based on international standards—WHO/UNICEF core questions for water and sanitation,40 WHO/UNICEF Joint Monitoring Program (JMP) water and sanitation definitions,19 the WaSH portion of USAID Demographic and Health Surveys,40,41 and reviews of WaSH indicators and frameworks.17 The HCF questionnaire contained sections on HCF demographics; water source and service (access, quality, quantity, continuity, and reliability); sanitation facilities (type, quantity, and quality); and hygiene supplies (access and continuity). All questionnaires were translated into local languages, and translation verified with back translation into English. Questionnaires were pretested with local nationals from each country and revised based on their comments. Local nationals included those with survey expertise and those with and without WaSH expertise.
Data collection.
In each county, enumerators, supervisors, and statisticians were selected based on their knowledge of the local languages, survey experience, and WaSH expertise. Study personnel were trained by UNC Water Institute staff in study design, interviewing techniques and ethics, sampling procedures, WaSH questionnaires and indicators, data collection and entry, and water quality testing. From June 2014 to January 2015, enumerators traveled to the selected HCFs to conduct interviews. This was during the dry season in all countries except Ethiopia; in Ethiopia, interviews began in the rainy season and stretched into the dry season. After establishing informed consent, enumerators administered questionnaires in the local language with a health professional at the HCF, preferably the head doctor; if unavailable, the head nurse; and if neither head doctor nor head nurse were available, a nurse who had worked at the HCF for more than 2 years. A minimum of four attempts were made to contact each HCF to ensure the highest possible response rate. Though the research teams asked all questions from the questionnaire at each HCF, the interviewees could decline to respond to any question. In Ethiopia, Rwanda, Uganda, and Zambia, responses were recorded on paper by hand, whereas in Kenya and Mozambique, results were recorded on a handheld electronic device at the time of interview.
Designated supervisors in each country were responsible for data checks. These included reviewing questionnaires for completion; randomly verifying 10% of interviews by revisiting HCF and readministering the survey; and directly observing interviews.
Water sampling and analysis.
In two countries, Mozambique and Uganda, enumerators took water samples from stored water in HCF. When collecting water samples, researchers disinfected their hands with hand sanitizer and put on sterile gloves before using a 500-mL Whirlpak® (Nasco, Fort Atkinson, WI) bag to collect water from the source. The controls and samples were stored in a cooler, chilled with ice, and transferred to a laboratory for analysis, after confirmation that the samples had been collected in the past 24 hours and that the temperature of the samples had not exceeded 5°C for more than 1 hour based on readings from cold chain indicator strips.
Samples were tested using the Aquagenx (Chapel Hill, NC) compartment bag test, a method comparable to membrane filtration techniques, to determine the most probable number (MPN) of Escherichia coli42 according to instructions from the test manufacturer.43 Results were recorded and categorized as low, intermediate, high, or very high risk according to WHO risk levels (< 1, 1–10, 10–100, and > 100 MPN/100 mL, respectively).44
Data entry, storage, and analysis.
Survey results were entered into a Microsoft Access database and checked via double data entry in Ethiopia, Rwanda, Uganda, and Zambia. In Kenya, data were directly uploaded into a Microsoft Excel database from the handheld devices every day. In Mozambique, data were directly uploaded to a Microsoft Access database every day. These data were converted to SAS files using Stat/Transfer 12.0 (Circle Systems Inc., Seattle, WA).
All analysis was performed in SAS 9.4 (SAS Institute Inc., Cary, NC) using the PROC SURVEYFREQ and PROC SURVEYMEANS procedures, where data were weighted to account for unequal probabilities of selection within strata. Categorical outcomes were analyzed as proportions, whereas numerical outcomes were analyzed as means.
Ethics.
Free and informed participant consent was obtained from all health-care professionals surveyed. The Institutional Review Board (IRB) of the University of North Carolina at Chapel Hill approved this study protocol on June 3, 2014 (IRB Reference ID: 135667).
RESULTS
Health centers were the primary sites of data collection within each country, except for Ethiopia, where 77% of facilities contacted were health posts (Table 2). These typically have fewer health-care professionals and are in more remote locations than health centers. In Kenya, 29 respondents indicated that the HCF was a dispensary; these are small outpatient HCFs which provide care for basic illnesses such as the common cold, and are analogous to health posts. Dispensaries are categorized as “other” in the table of HCF types (Table 2).
Table 2.
Numbers of health-care facilities where interviews were conducted
| Ethiopia | Kenya | Mozambique | Rwanda | Uganda | Zambia | |
|---|---|---|---|---|---|---|
| Health-care facility type | ||||||
| Health post | 397 | 8 | 5 | 1 | 0 | 32 |
| Health center | 115 | 73 | 151 | 71 | 139 | 169 |
| Hospital | 0 | 11 | 6 | 2 | 2 | 3 |
| Private clinic | 2 | 2 | 0 | 0 | 0 | 0 |
| Other | 1 | 31 | 10 | 0 | 0 | 0 |
The response rates, as calculated from American Association for Public Opinion Research guidelines,45 were 97.8% in Ethiopia; 100% in Kenya; 86.9% in Mozambique; 100% in Rwanda; 99.3% in Uganda, and 100% in Zambia.
Water source.
In over 74% of the HCF studied in each country, interviewees reported that the water used at the HCF came from an improved water source, most commonly boreholes (Ethiopia, Uganda, and Zambia), rainwater (Kenya), and piped water into the yard (Rwanda and Mozambique). Although 26% (Zambia) to 54% (Rwanda) reported use of a secondary water source in addition to their primary (most frequently used) water source, 4% (Uganda) to 52% (Ethiopia) of these secondary water sources were not improved. Overall, fewer than 45% reported that they used improved primary and secondary sources. Only 32% of HCF in Ethiopia and 62% of HCF in Mozambique had access to an improved source of water within a 5-minute walk, in contrast to 78–90% in Kenya, Rwanda, Uganda, and Zambia. Furthermore, only 16% of HCF in Ethiopia and 59% of HCF in Mozambique used water from an improved source directly on the premises, as compared with 76–89% in the other four countries.
Most HCF had a continuous water supply (24 hours per day of water supply), ranging from 62% in Ethiopia to 84% in Zambia. Overall, the mean number of hours per week of water supply ranged from 108 hours in Kenya to 153 hours in Uganda; this translates to an average of 15–22 hours per 24-hour period. Some facilities in each country (from 7% in Mozambique to 22% in Ethiopia) reported that a breakdown in the water source or system had occurred in the past 2 weeks at the time of survey. The median duration of the most recent breakdown ranged between 24 hours in Mozambique and Rwanda to 168 hours (1 week) in Zambia (Table 3).
Table 3.
Water source type, access, continuity, and reliability in surveyed health-care facilities
| Ethiopia | Kenya | Mozambique | Rwanda | Uganda | Zambia | |
|---|---|---|---|---|---|---|
| Improved primary source of water | 0.74 (0.71, 0.76) | 0.88 | 0.94 (0.93, 0.95) | 0.97 (0.95, 0.99) | 0.97 (0.95, 0.99) | 0.95 |
| Presence of secondary source(s) of water | 0.36 (0.33, 0.39) | 0.54 | 0.37 (0.36, 0.38) | 0.54 (0.47, 0.60) | 0.44 (0.39, 0.50) | 0.26 |
| Secondary source type | ||||||
| Improved | 0.48 (0.42, 0.53) | 0.73 | 0.70 (0.69, 0.71) | 0.81 (0.74, 0.89) | 0.96 (0.92, 0.99) | 0.87 |
| Unimproved | 0.52 (0.47, 0.58) | 0.27 | 0.30 (0.29, 0.31) | 0.19 (0.11, 0.26) | 0.04 (0.01, 0.08) | 0.13 |
| Improved primary and secondary sources | 0.14 (0.12, 0.16) | 0.27 | 0.22 (0.21, 0.23) | 0.45 (0.39, 0.52) | 0.35 (0.29, 0.40) | 0.23 |
| Continuous (24-hour) water supply | 0.62 (0.59, 0.65) | 0.73 | 0.70 (0.69, 0.71) | 0.68 (0.62, 0.74) | 0.81 (0.77, 0.85) | 0.84 |
| Improved source of water within 5-minute time to source | 0.32 (0.30, 0.35) | 0.80 | 0.62 (0.61, 0.63) | 0.90 (0.86, 0.94) | 0.78 (0.73, 0.82) | 0.85 |
| Improved source of water on premises | 0.16 (0.14, 0.18) | 0.80 | 0.59 (0.58, 0.60) | 0.89 (0.84, 0.93) | 0.76 (0.72, 0.81) | 0.84 |
| 2-week breakdown | 0.22 (0.19, 0.24) | 0.11 | 0.07 (0.06, 0.07) | 0.19 (0.13, 0.24) | 0.14 (0.10, 0.18) | 0.09 |
| Median length of last breakdown | 72 hours | 144 hours | 24 hours | 24 hours | 72 hours | 168 hours |
| Mean hours per week of water service | 133 ± 3 hours | 109 ± 7 hours | 136 ± 5 hours | 125 ± 8 hours | 153 ± 4 hours | – |
Water storage and treatment.
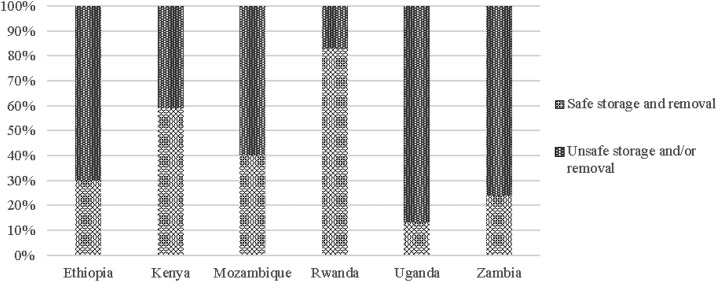
The majority of HCFs, from 87% in Mozambique to 100% in Kenya, Uganda, and Zambia, reported that water in their HCF was stored in a container. Of these, at least 84% in each country reported that the storage container had a cover. However, water was not always removed safely from safe storage containers: instead of using a tap, spigot, or long-handled dipper, many HCF reported unsafe water removal methods such as scooping with cups, bowls, or hands: 67% (Ethiopia), 41% (Kenya), 58% (Mozambique), 17% (Rwanda), 87% (Uganda), and 75% (Zambia). Except for Rwanda, both safe water storage and safe removal were practiced by only 13% (Uganda) to 59% (Kenya) of HCF. Many more HCF in Rwanda practiced safe water storage and safe water removal (83%) (Figure 1).
Figure 1.
Safe water storage rates in surveyed health-care facilities.
Water treatment was used in 51% (Mozambique), 52% (Ethiopia), 62% (Uganda), 64% (Kenya), 67% (Zambia), and 97% (Rwanda) of the surveyed HCF. Chlorine was the most frequent form of treatment in Ethiopia, Kenya, Mozambique, Rwanda, and Zambia, with multiple respondents in Ethiopia specifying the use of Wuha Agar™ (chlorine-based treatment). In Uganda, even though a chlorine-based treatment was used in one HCF in the form of WaterGuard™ (PSI/PACE, Kampala, Uganda), AquaSafe™ (Kampala, Uganda) (sodium dichloroisocyanurate tablet) was much more commonly used for treatment (Table 4).
Table 4.
Water storage and treatment practices in surveyed health-care facilities
| Ethiopia | Kenya | Mozambique | Rwanda | Uganda | Zambia | |
|---|---|---|---|---|---|---|
| % Storing water | 0.95 (0.94, 0.96) | 1.00 | 0.87 (0.86, 0.88) | 0.98 (0.97, 1.00) | 1.00 | 1.00 |
| Presence of cover on water storage container | 0.86 (0.84, 0.88) | 0.98 | 0.84 (0.83, 0.84) | 1.00 | 0.92 (0.89, 0.95) | 0.90 |
| Safe removal of water from container | ||||||
| Safe | 0.33 (0.30, 0.36) | 0.59 | 0.41 (0.40, 0.42) | 0.83 (0.78, 0.88) | 0.13 (0.07, 0.18) | 0.25 |
| Unsafe | 0.67 (0.64, 0.70) | 0.41 | 0.59 (0.58, 0.60) | 0.17 (0.12, 0.22) | 0.87 (0.82, 0.93) | 0.75 |
| Practices water treatment | 0.52 (0.50, 0.55) | 0.64 | 0.51 (0.50, 0.52) | 0.97 (0.95, 0.99) | 0.62 (0.57, 0.67) | 0.67 |
| Treatment type | ||||||
| Boiling | 0.08 (0.06, 0.11) | 0.04 | 0.06 (0.05, 0.06) | 0.33 (0.27, 0.39) | 0.00 | 0.00 |
| Chlorine | 0.79 (0.75, 0.82) | 0.67 | 0.90 (0.89, 0.91) | 0.36 (0.30, 0.43) | 0.00 | 1.00 |
| Filtration with cloth | 0.05 (0.04, 0.07) | 0.03 | 0.01 (0.01, 0.02) | 0.14 (0.09, 0.19) | 0.00 | 0.00 |
| Other treatment | 0.01 (0.003, 0.02) | 0.10 | 0.00 | 0.03 (0.01, 0.05) | 0.93 (0.82, 1.00) | 0.00 |
| Multiple types | 0.07 (0.01, 0.09) | 0.16 | 0.03 (0.03, 0.04) | 0.14 (0.09, 0.18) | 0.07 (0.00, 0.18) | 0.00 |
Water quality.
Of the HCF where water quality was tested for microbiological contamination (in Uganda and Mozambique), the majority had low-risk water; however, 15.3% of facilities in Uganda and 29.6% in Mozambique had intermediate or high-risk water quality (Table 5).
Table 5.
Water samples with E. coli in surveyed rural health-care facilities in Uganda and Mozambique
| Uganda (N = 144) | Mozambique (N = 172) | |
|---|---|---|
| Low risk (< 1 MPN) (%) | 84.7 | 70.4 |
| Intermediate risk (1–10 MPN) (%) | 7.3 | 10.7 |
| High risk (10–100 MPN) (%) | 8.0 | 18.9 |
| Very high risk (> 100 MPN) (%) | 0.0 | 0.0 |
| Total (%) | 100.0 | 100.0 |
MPN = most probable number.
There was no significant difference in water quality between Ugandan HCF that did and did not report water treatment (P = 0.1507). However, in Mozambique, HCF where water was treated reported worse water quality than those where no treatment was reported: 80% of HCF reporting water treatment had low-risk water samples in comparison to 97% of HCF reporting no water treatment (P = 0.0039, significant at α = 0.05).
Sanitation.
Sixty-six percent of surveyed HCF in Ethiopia to 96% in Zambia had improved sanitation facilities, which when functioning properly should “hygienically separate human excreta from human contact.”19 The most common sanitation facilities were pit latrines with slabs (Ethiopia, Kenya, Rwanda, and Mozambique) and ventilated improved pit latrines (Uganda and Zambia). However, 3% (Uganda and Zambia) to 31% (Ethiopia) of HCF reported that their main sanitation facility was unimproved. More than 87% of HCF report their sanitation facilities were in use; however, problems with privacy (e.g., lack of locking doors), cleanliness, and regular repair were reported across multiple countries (Table 6). Overall, 61% (Ethiopia) to 95% (Zambia) of HCF had improved and functioning sanitation facilities available for use.
Table 6.
Sanitation facility type, access, functionality and condition in surveyed health-care facilities
| Ethiopia | Kenya | Mozambique | Rwanda | Uganda | Zambia | |
|---|---|---|---|---|---|---|
| Sanitation type | ||||||
| Improved | 0.66 (0.64, 0.69) | 0.86 | 0.79 (0.78, 0.80) | 0.93 (0.89, 0.96) | 0.93 (0.90, 0.96) | 0.96 |
| Unimproved | 0.31 (0.29, 0.34) | 0.10 | 0.21 (0.20, 0.22) | 0.04 (0.01, 0.07) | 0.03 (0.01, 0.05) | 0.03 |
| Multiple (both improved and unimproved sources reported) | 0.02 (0.01, 0.03) | 0.04 | – | 0.03 (0.01, 0.05) | 0.04 (0.02, 0.06) | 0.01 |
| Sanitation facilities functioning properly | 0.88 (0.86, 0.90) | 0.97 | 0.93 (0.93, 0.94) | 0.95 (0.91, 0.98) | 0.98 (0.96, 0.99) | 0.98 |
| Improved and functioning sanitation facilities | 0.61 (0.58, 0.63) | 0.83 | 0.85 (0.84, 0.86) | 0.87 (0.83, 0.92) | 0.91 (0.88, 0.94) | 0.95 |
| Sanitation facilities currently in use | 0.87 (0.86, 0.89) | 0.98 | 0.92 (0.91, 0.92) | 1.00 | 0.97 (0.95, 0.99) | 0.97 |
| % Reporting problems | 0.22 (0.19, 0.24) | 0.39 | 0.35 (0.35, 0.36) | 0.22 (0.17, 0.28) | 0.52 (0.47, 0.57) | 0.93 |
| Problems | ||||||
| Privacy | 0.04 (0.03, 0.05) | 0.06 | 0.01 (0.01, 0.02) | 0.04 (0.01, 0.07) | 0.13 (0.10, 0.17) | 0.76 |
| Cleanliness | 0.02 (0.01, 0.03) | 0.08 | 0.15 (0.14, 0.16) | 0.00 | 0.14 (0.10, 0.18) | 0.61 |
| Repair | 0.10 (0.08, 0.12) | 0.19 | 0.15 (0.15, 0.16) | 0.01 (0.01, 0.06) | 0.22 (0.18, 0.27) | 0.52 |
| No/inadequate facilities | 0.02 (0.01, 0.03) | 0.02 | 0.08 (0.08, 0.09) | 0.07 (0.03, 0.10) | 0.05 (0.02, 0.07) | 0.29 |
Hygiene.
Between 35% (Mozambique) and 96% (Rwanda) of HCF reported at least one designated handwashing station for hand hygiene, but these stations did not always have the necessary supplies. The proportion of HCF that always had water and soap is between 25% (Zambia) and 63% (Rwanda). The percentage of HCF that reported always having water, soap, and drying materials available ranged from 6% (Uganda) to 25% (Zambia) (Table 7).
Table 7.
Accessibility of hand hygiene materials in surveyed health-care facilities
| Ethiopia | Kenya | Mozambique | Rwanda | Uganda | Zambia | |
|---|---|---|---|---|---|---|
| Presence of handwashing station | 0.62 (0.59, 0.64) | 0.74 | 0.35 (0.34, 0.36) | 0.96 (0.94, 0.98) | 0.82 (0.78, 0.86) | 0.78 |
| Presence of water for handwashing | ||||||
| Always | 0.58 (0.54, 0.61) | 0.86 | 0.65 (0.64, 0.66) | 0.88 (0.84, 0.93) | 0.60 (0.54, 0.65) | 0.72 |
| Sometimes | 0.42 (0.39, 0.46) | 0.14 | 0.33 (0.32, 0.34) | 0.12 (0.07, 0.16) | 0.28 (0.33, 0.45) | 0.28 |
| Never | 0.00 | 0.00 | 0.01 (0.01, 0.02) | 0.00 | 0.01 (0.01, 0.02) | 0.00 |
| Presence of soap or ash for handwashing | ||||||
| Always | 0.50 (0.46, 0.53) | 0.44 | 0.61 (0.59, 0.62) | 0.71 (0.64, 0.77) | 0.48 (0.42, 0.54) | 0.27 |
| Sometimes | 0.47 (0.44, 0.51) | 0.43 | 0.29 (0.28, 0.30) | 0.22 (0.17, 0.28) | 0.28 (0.23, 0.34) | 0.60 |
| Never | 0.03 (0.02, 0.04) | 0.13 | 0.10 (0.09, 0.11) | 0.07 (0.04, 0.11) | 0.23 (0.19, 0.28) | 0.14 |
| Presence of hygienic hand-drying materials | ||||||
| Always | 0.22 (0.19, 0.25) | 0.18 | 0.23 (0.22, 0.24) | 0.08 (0.05, 0.12) | 0.08 (0.05, 0.12) | 0.27 |
| Sometimes | 0.24 (0.21, 0.27) | 0.14 | 0.04 (0.04, 0.05) | 0.14 (0.10, 0.19) | 0.08 (0.05, 0.12) | 0.60 |
| Never | 0.53 (0.50, 0.57) | 0.68 | 0.73 (0.72, 0.74) | 0.77 (0.72, 0.83) | 0.83 (0.79, 0.88) | 0.13 |
| Water and soap or ash, sometimes present | 0.97 (0.89, 1.00) | 0.87 | 0.90 (0.89, 0.91) | 0.93 (0.89, 0.96) | 0.81 (0.77, 0.85) | 0.87 |
| Water and soap or ash, always present | 0.45 (0.41, 0.48) | 0.43 | 0.43 (0.42, 0.45) | 0.63 (0.57, 0.70) | 0.34 (0.29, 0.40) | 0.25 |
| Water and soap or ash and drying materials, sometimes present | 0.45 (0.38, 0.51) | 0.32 | 0.26 (0.24, 0.27) | 0.22 (0.17, 0.28) | 0.17 (0.12, 0.22) | 0.87 |
| Water and soap or ash and drying materials, always present | 0.18 (0.15, 0.20) | 0.15 | 0.19 (0.18, 0.20) | 0.08 (0.05, 0.12) | 0.06 (0.03, 0.09) | 0.25 |
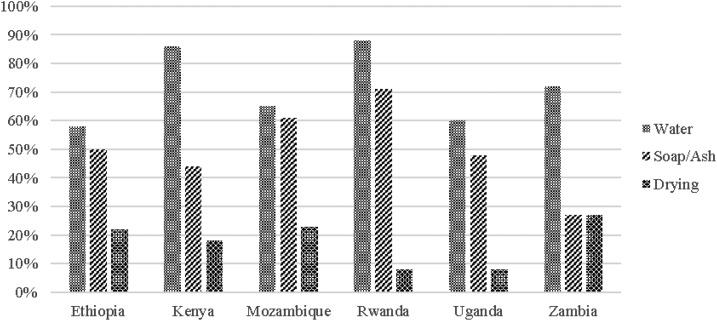
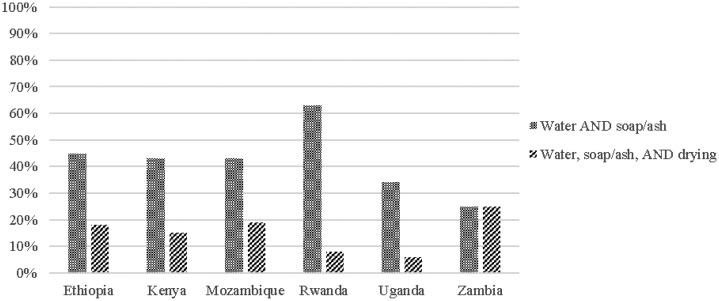
The majority of HCF reported having water for handwashing, but soap and drying materials were far less common. When presence rates of these three types of supplies are considered together, Rwanda is the only country where a majority (63%) of HCF reported that both water and soap or ash for handwashing are always available. Fewer than 45% of HCF in Ethiopia, Kenya, Mozambique, Uganda, and Zambia always had water and soap or ash for handwashing. Fewer than 25% of HCF had continuous access to all three hygiene amenities—water, soap or ash, and drying materials for handwashing (Figures 2 and 3).
Figure 2.
Availability of individual hand hygiene in surveyed health-care facilities.
Figure 3.
Access to hand hygiene supplies in surveyed health-care facilities.
Combined access to water, sanitation, and hygiene.
We found that fewer than 50% of rural HCF in all countries studied had access to the most basic WaSH services in HCF—improved water on premises, functional and improved sanitation, and continuous access to soap and water for handwashing (Table 8). In Ethiopia, only 7% of HCF had access to these three WaSH services.
Table 8.
Levels of basic WaSH services across surveyed health-care facilities
| Ethiopia | Kenya | Mozambique | Rwanda | Uganda | Zambia | |
|---|---|---|---|---|---|---|
| Access to improved water on premises, functional improved sanitation, and soap and water always | 0.07 (0.05, 0.09) | 0.30 | 0.29 (0.27, 0.30) | 0.50 (0.43, 0.57) | 0.30 (0.24, 0.35) | 0.21 |
WaSH = water, sanitation, and hygiene.
DISCUSSION
In this cross-sectional study of WaSH in HCFs in rural regions of Ethiopia, Kenya, Mozambique, Rwanda, Uganda, and Zambia, we found that fewer than half of surveyed HCF in each country had an improved water source on premises, functional improved sanitation services, and handwashing supplies (water and soap) always present. In Ethiopia and Mozambique, only 16% and 59% of HCF, respectively, had an improved water source on premises. Water sources which are off premises limit the amount of water available to the HCF as it must be carried, increase the risk of contamination as water must be stored and carried, and increase time lost if health workers must travel to the water source to collect and carry water to the HCF.46 Although most HCF store their water in a covered storage container, most interviewees, except for those in Rwanda, reported that HCF staff members failed to extract water from storage containers safely. Instead, they scooped water from the container with a cup, bowl, or hands, which can introduce contaminants.
Consistent availability of water and soap was not common in most HCF. This is critical for public health; handwashing with soap is one of the most important infection control interventions to prevent disease transmission in HCF.47 Low rates of access to hygiene materials in rural HCF in the surveyed countries limits the ability of health-care professionals to routinely conduct hygienic handwashing.29 Numerous studies document the importance of washing with soap, to bind with dirt and grime; clean running water, to effectively rinse the hands without contaminating them; and drying materials, such as paper towels, to reduce germ transfer from wet hands.48,49 But despite campaigns such as the WHO “Clean Care is Safer Care” program (launched in October 2005 to promote proper hand hygiene at critical times within health-care settings) and the “SAVE LIVES: Clean Your Hands” campaign (a day dedicated to hand hygiene awareness among health workers, first observed in 2009), our results suggest that routine handwashing is not occurring in up to 75% of the surveyed HCF because of a lack of supplies (water, soap, and drying materials). Apart from Rwanda, fewer than 45% of HCF in all countries always had water and soap available for handwashing. In areas lacking one or more of the three handwashing materials, it would be impossible for patients or personnel to wash their hands according to the WHO guidelines, mentioned earlier. The number of HCF where routine and appropriate handwashing is practiced is most likely much lower. According to one review of intensive care units in high-income countries, where health-care professionals have access to multiple piped water sources and consistent supplies of handwashing materials, handwashing compliance is typically 50–60%.50 Another review in 2010 estimated that the median compliance rate for handwashing in HCF among industrialized countries was closer to 40%.51 Consistent access to handwashing materials is an urgent public health problem in HCF.
There are limitations to this study. Results within each country are not nationally representative of all rural areas; they are representative of rural areas in certain districts in each country. Water samples were collected during the dry season in Uganda and in the wet season in Ethiopia, and collection in the dry season may lead to overestimation of year-round water quality because fecal contamination is less frequent during the dry season.52 Most data were collected through interviews; therefore, inaccurate or biased responses are possible. This might be mitigated in future studies by introducing additional mechanisms for verifying responses, such as photographing water sources, sanitation facilities, handwashing stations, and so on, and comparing their condition to that reported within the questionnaire. Though overall response rates were high, with 95–100% of contacted HCF agreeing to be interviewed, item nonresponse rates were high for two questions in the questionnaire on secondary source type and water treatment type. (In Uganda, 62% of HCF reported that they treated their water, but only 11% responded to a follow-up question on the type of treatment). Even though our overall sample sizes within each country were high, we had limited numbers of certain types of HCF, like hospitals; thus, we were unable to stratify analysis by HCF type. Disaggregating by HCF type may have provided greater insights into the WaSH circumstances of different types of HCF, and it may be beneficial for future research to distinguish between inpatient or outpatient HCF setting types and their specific WaSH needs.
Finally, our estimates of “basic” service are likely overestimates. The WHO/UNICEF JMP’s proposed definitions of “basic” water, sanitation, and hygiene services in HCF encompass dimensions beyond “improved” and “unimproved” facilities; however, since these definitions had been neither proposed nor developed at the time we designed this study, we did not collect data on some of the additional requirements for basic service mentioned in the new HCF-specific definitions. For reference, the requirements for basic water service in HCF are access to an improved (piped or protected) source of water on the premises.39 Basic sanitation in HCF is defined as availability of usable improved sanitation facilities which are accessible by people with limited mobility, equipped for menstrual hygiene management, separated by sex, and separated for patients and staff.39 Finally, basic hand hygiene service is achieved when water and soap are available at all points of care and at all toilets.39 Some of the new requirements should be collected in future WaSH in HCF studies, including menstrual hygiene management, separate-sex latrines, and sanitation accessibility by people with limited mobility, as well as availability of water and soap on a handwashing station–specific rather than HCF-wide basis.
Access to water on premises is critical if a sufficient quantity of the water necessary in all HCF is to be provided. Although more than 75% of HCF in Kenya, Rwanda, Uganda, and Zambia had access to water on premises, only 14% of HCF in Ethiopia had access to water on premises. To improve quality of care in HCF, future installations of improved water sources on premises are needed, especially in Ethiopia. Piped water connections into the building or into the yard of the HCF could improve the quantity of water available in these HCF, decrease water collection time, and reduce the risk of contamination during water collection and storage.
Although point of use water treatment can improve the quality of water consumed, water quality results from Mozambique surprisingly reveal that HCF that treated their water had higher levels of contamination than those that did not report treatment of their water. Potential explanations for this result may be nonsampling error, inadequate treatment practices in HCF in rural areas in Mozambique because of lack of knowledge of treatment methods, inconsistent treatment practices, and recontamination during water use and extraction from storage after treatment.
A reliable supply of soap and clean hand-drying materials should be available in all HCF. Past HCF interventions in Kenya, which combined installation of water stations, provision of soap, and provision of chlorine-based treatment with a 4-hour training of trainers in water treatment and hygiene behaviors showed promising results—during a surprise visit, 97% of targeted facilities were using their water stations, and about half of all water stations had detectable chlorine residuals.53 Importantly, 33% of these facilities did not have soap available near the water station,53 the reasons for which were not explored. In addition, future research could benefit from additional questions on which alternative materials, if any, are being used for handwashing and hand-drying if soap, ash, or drying materials such as paper towels are unavailable, and could discern if soap or ash is being used at each health facility.
Our research points to a need for regularly occurring WaSH in HCF-monitoring so that policies and programming can be targeted and designed to improve WaSH in rural HCF. Although we found some national-level analyses of WaSH in HCF, their estimates of WaSH access were difficult to compare across countries because of discrepancies in definitions used to assess “access.”16 The WHO/UNICEF JMP has taken steps to address this by developing new definitions of water service, sanitation service, handwashing, and handling of health-care waste; these can be incorporated into research on WaSH service levels in HCF. Qualitative research is needed on the reasons for low rates of continuous access to soap, water, and drying materials; the challenges associated with safe water removal from water storage containers; and the obstacles that prevent the repair and maintenance of sanitation facilities in HCFs to improve health and well-being and decrease the spread of disease in HCF.
CONCLUSION
Deficiencies in WaSH in HCF in the countries of study pose immediate health risks to patients who depend on these HCFs for medical care and the medical personnel who work in these HCF. Although it is important to continue efforts in expanding future monitoring and research on WaSH in HCF, we document low levels of access to WaSH services, especially water on premises and hand hygiene materials, in rural HCF in Ethiopia, Kenya, Mozambique, Rwanda, Uganda, and Zambia. The conditions we found in rural HCF in these countries are inimical to effective health care and threaten patient health in these settings. Our research points to a desperate need for improvements in WaSH services in rural HCF in regions of Ethiopia, Kenya, Mozambique, Rwanda, Uganda, and Zambia so that patient health is not compromised and the human right to health is upheld.
Acknowledgments:
This study was a project of the Water Institute at the University of North Carolina under Principal Investigator Pete Kolsky. We thank team members Camille Morgan and Ronna Chan for their support and guidance; the Odum Institute at UNC for technical support; and all in-country research teams who helped collect and record responses. We also thank Ryan Cronk for his valuable suggestions and comments on this manuscript. Finally, we are very grateful to the doctors, nurses, and health-care professionals who took the time to participate in this study.
REFERENCES
- 1.Pruss-Ustun A, et al. , 2014. Burden of disease from inadequate water, sanitation and hygiene in low- and middle-income settings: a retrospective analysis of data from 145 countries. Trop Med Int Health 19: 894–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fewtrell L, Kaufmann RB, Kay D, Enanoria W, Haller L, Colford JMC, 2005. Water, sanitation, and hygiene interventions to reduce diarrhoea in less developed countries: a systematic review and meta-analysis. Lancet Infect Dis 5: 42–52. [DOI] [PubMed] [Google Scholar]
- 3.Waddington H, Snilstveit B, 2009. Effectiveness and sustainability of water, sanitation, and hygiene interventions in combating diarrhoea. J Dev Effect 1: 295–335. [Google Scholar]
- 4.Bartram J, Cairncross S, 2010. Hygiene, sanitation, and water: forgotten foundations of health. PLoS Med 7: e1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Emerson PM, et al. , 2004. Role of flies and provision of latrines in trachoma control: cluster-randomised controlled trial. Lancet 363: 1093–1098. [DOI] [PubMed] [Google Scholar]
- 6.Brown J, Hien VT, McMahan L, Jenkins MW, Thie L, Liang K, Printy E, Sobsey MD, 2012. Relative benefits of on-plot water supply over other ‘improved’ sources in rural vietnam. Trop Med Int Health 18: 65–74. [DOI] [PubMed] [Google Scholar]
- 7.Kumpel E, Nelson KL, 2014. Mechanisms affecting water quality in an intermittent piped water supply. Environ Sci Technol Lett 48: 2766–2775. [DOI] [PubMed] [Google Scholar]
- 8.Howard G, Bartram J, 2003. Domestic Water Quantity, Service Level and Health. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 9.Cronk R, Slaymaker T, Bartram J, 2015. Monitoring drinking water, sanitation, and hygiene in non-household settings: priorities for policy and practice. Int J Hyg Environ Health 218: 694–703. [DOI] [PubMed] [Google Scholar]
- 10.UN Committee on Economic, Social and Cultural Rights. General Comment No. 14: The Right to the Highest Attainable Standard of Health (Art. 12 of the covenant). 2011; E/C. 12/2000/4.
- 11.Meier B, Kayser GL, Amjad U, Bartram J, 2013. Implementing an evolving human right through water and sanitation policy. Water Policy 15: 116–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steinmann P, Bratschi MW, Lele P, Chavan U, Sundaram N, Weiss M, Juvekar SK, Hirve S, 2015. Availability and satisfactoriness of latrines and hand washing stations in health facilities, and role in health seeking behavior of women: evidence from rural Pune district, India. J Water Sanit Hyg Dev 5: 474–482. [Google Scholar]
- 13.Bazzano AN, Oberhelman RA, Potts KS, Gordon A, Var C, 2015. Environmental factors and WASH practices in the perinatal period in Cambodia: implications for newborn health. Int J Environ Res Public Health 12: 2392–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benova L, Cumming O, Gordon BA, Magoma M, Campbell OMR, 2014. Where there is no toilet: water and sanitation environments of domestic and facility births in Tanzania. PLoS One 9: e106738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leslie HH, Fink G, Nsona H, Kruk ME, 2016. Obstetric facility quality and newborn mortality in Malawi: a cross-sectional study. PLoS Med 13: e1002151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartram J, Cronk R, 2015. Water, sanitation and hygiene in health care facilities: status in low- and middle-income countries and way forward. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 17.Kayser G, Moriarty P, Fonseca C, Bartram J, 2013. Domestic water service delivery indicators and frameworks for monitoring, evaluation, policy and planning: a review. Int J Hyg Environ Health 10: 4812–4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization, 2017. Safely Managed Drinking Water: Thematic Report on Drinking Water 2017. Geneva, Switzerland: WHO. [Google Scholar]
- 19.WHO/UNICEF Joint Monitoring Programme, 2016. Improved and Unimproved Water Sources and Sanitation Facilities Available at: https://www.wssinfo.org/definitions-methods/watsan-categories/2016. Accessed July 12, 2017.
- 20.Bain R, Cronk R, Wright J, Yang H, Slaymaker T, Bartram J, 2014. Fecal contamination of drinking-water in low- and middle-income countries: a systematic review and meta-analysis. PLoS Med 11: e1001644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guedes ZBL, Oriá HF, Britto NPB, Neto JWS, Silveira JW, Lopes AEC, 2004. Controle sanitario da agua consumida nas unidades de saude do municipio de fortaleza, CE. Higiene Alimentar 18: 28–31. [Google Scholar]
- 22.Mosley LM, Sharp DS, Singh S, 2004. Effects of a tropical cyclone on the drinking-water quality of a remote pacific island. Disasters 28: 405–417. [DOI] [PubMed] [Google Scholar]
- 23.Lee EJ, Schwab KJ, 2005. Deficiencies in drinking water distribution systems in developing countries. J Water Health 3: 109–127. [PubMed] [Google Scholar]
- 24.Potter A, Klutse A, Snehalatha M, Batchelor C, Uandela A, Naafs A, Fonseca C, Moriarty P, 2010. Assessing Sanitation Service Levels. The Hague, The Netherlands: IRC, International Water and Sanitation Centre.
- 25.Hernández JE, Mejía CR, Cazali IL, Arathoon EG, 1996. Nosocomial infection due to vibrio cholerae in two referral hospitals in guatemala. Infect Control Hosp Epidemiol 17: 371–372. [DOI] [PubMed] [Google Scholar]
- 26.Swaddiwudhipong W, Peanumlom P, 2010. A case of nosocomial cholera during a community outbreak in a thai-myanmar border area. J Med Assoc Thai 93: 1112–1114. [PubMed] [Google Scholar]
- 27.Ethiopian Public Health Institute, Federal Ministry of Health Ethiopia, ICF International, 2014. Ethiopia Service Provision Assessment Plus (SPA+) Survey 2014 Key Findings. Addis Ababa, Ethiopia: Ethiopian Public Health Institute.
- 28.Boyce JM, Pittet D, 2002. Guideline for hand hygiene in health-care settings: Recommendations of the healthcare infection control practices advisory committee and the hicpac/shea/apic/idsa hand hygiene task force. Am J Infect Control 30: S1–S46. [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization, 2009. WHO Guidelines on Hand Hygiene in Health Care: First Global Patient Safety Challenge, Clean Care is Safer Care. Geneva, Switzerland: WHO. [PubMed] [Google Scholar]
- 30.Allegranzi B, Nejad S, Combescure C, Graafmans W, Attar H, Donaldson L, 2011. Burden of endemic health-careassociated infection in developing countries: systematic review and meta-analysis. Lancet 377: 228–241. [DOI] [PubMed] [Google Scholar]
- 31.Allegranzi B, Pittet D, 2009. Role of hand hygiene in healthcare-associated infection prevention. J Hosp Infect 73: 305–315. [DOI] [PubMed] [Google Scholar]
- 32.Blencowe H, Cousens S, Mullany LC, Lee AC, Kerber K, Wall S, Darmstadt GL, Lawn JE, 2011. Clean birth and postnatal care practices to reduce neonatal deaths from sepsis and tetanus: a systematic review and delphi estimation of mortality effect. BMC Public Health 11 (Suppl 3): S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bartram J, Cronk R, Montgomery M, Gordon B, Neira M, Kelley E, Velleman Y, 2015. Lack of toilets and safe water in health-care facilities. Bull World Health Organ 93: 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lawn J, Mongi P, Cousens S, 2006. Africa’s newborns–counting them and making them count. In: Opportunities for Africa’s Newborns: Practical Data, Policy and Programmatic Support for Newborn Care in Africa. Geneva, Switzerland: World Health Organization, 11–22. Available at: http://www.who.int/pmnch/media/publications/aonsection_I.pdf. Accessed July 10, 2017. [Google Scholar]
- 35.UN Department of Economic and Social Affairs, 2014. World Urbanization Prospects, the 2014 Revision: Percentage Urban and Urban Agglomerations by Size Class Available at: https://esa.un.org/unpd/wup/Maps/CityDistribution/CityPopulation/CityPop.aspx. Accessed July 13, 2017
- 36.Lehmann U, Dieleman M, Martineau T, 2008. Staffing remote rural areas in middle- and low-income countries: a literature review of attraction and retention. BMC Health Serv Res 8: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.WHO/UNICEF Joint Monitoring Programme, 2014. Progress on Drinking Water and Sanitation: 2014 Update. Geneva, Switzerland: WHO. [Google Scholar]
- 38.WHO/UNICEF Joint Monitoring Programme, 2015. JMP Green Paper: Global Monitoring of Water, Sanitation and Hygiene Post-2015. Geneva, Switzerland: WHO. [Google Scholar]
- 39.WHO/UNICEF Joint Monitoring Programme, 2016. Monitoring WASH in Health Care Facilities: Core Indicators and QuestionsAvailable at: http://www.who.int/water_sanitation_health/monitoring/coverage/wash-in-hcf-core-questions.pdf?ua=1. Accessed July 13, 2017. [Google Scholar]
- 40.WHO/UNICEF Joint Monitoring Programme, 2006. Core Questions on Drinking-Water and Sanitation for Household Surveys. Geneva, Switzerland: WHO. [Google Scholar]
- 41.MEASURE DHS, 2012. DHS Model Questionnaires Available at: http://www.measuredhs.com/What-We-Do/Questionnaires.cfm. Accessed July 13, 2017.
- 42.Stauber C, Miller C, Cantrell B, Kroell K, 2014. Evaluation of the compartment bag test for the detection of Escherichia coli in water. J Microbiol Methods 99: 66–70. [DOI] [PubMed] [Google Scholar]
- 43.Aquagenx LLC, 2013. Compartment bag test: instructions for use.
- 44.World Health Organization, 2011. Guidelines for Drinking-Water Quality, 4th edition. Geneva, Switzerland: WHO. [Google Scholar]
- 45.The American Association for Public Opinion Research, 2008. Standard Definitions: Final Dispositions of Case Codes and Outcome Rates for Surveys.
- 46.Shields K, Bain R, Cronk R, Wright J, Bartram J, 2015. Association of supply type with fecal contamination of source water and household stored drinking water in developing countries: a bivariate meta-analysis. Environ Health Perspect 123: 1222–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Langley J, 2002. From soap and water, to waterless agents: update on hand hygiene in health care settings. Can J Infect Dis 13: 285–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jumaa P, 2005. Hand hygiene: simple and complex. Int J Infect Dis 9: 3–14. [DOI] [PubMed] [Google Scholar]
- 49.Patrick D, Findon G, Miller T, 1997. Residual moisture determines the level of touch-contact-associated bacterial transfer following hand washing. Epidemiol Infect 119: 319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Wandel D, Maes L, Labeau S, Vereecken C, Blot S, 2010. Behavioral determinants of hand hygiene compliance in intensive care units. Am J Crit Care 19: 230–239. [DOI] [PubMed] [Google Scholar]
- 51.Erasmus V, Daha T, Brug H, Richardus JH, Behrendt MD, Vos MC, van Beeck EF, 2010. Systematic review of studies on compliance with hand hygiene guidelines in hospital care. Infect Control Hosp Epidemiol 31: 283–294. [DOI] [PubMed] [Google Scholar]
- 52.Kostyla C, Bain R, Cronk R, Bartram J, 2015. Seasonal variation of fecal contamination in drinking water sources in developing countries: a systematic review. Sci Total Environ 514: 333–343. [DOI] [PubMed] [Google Scholar]
- 53.Sreenivasan N, Gotestrand SA, Ombeki S, Oluoch G, Fischer TK, Quick R, 2015. Evaluation of the impact of a simple hand-washing and water-treatment intervention in rural health facilities on hygiene knowledge and reported behaviours of health workers and their clients, Nyanza province, Kenya, 2008. Epidemiol Infect 143: 873–880. [DOI] [PMC free article] [PubMed] [Google Scholar]