Abstract
Crimean-Congo haemorrhagic fever virus (CCHFV) is a deadly human pathogen of the utmost seriousness being highly lethal causing devastating disease symptoms that result in intense and prolonged suffering to those infected. During the past 40 years, this virus has repeatedly caused sporadic outbreaks responsible for relatively low numbers of human casualties, but with an alarming fatality rate of up to 80% in clinically infected patients. CCHFV is transmitted to humans by Hyalomma ticks and contact with the blood of viremic livestock, additionally cases of human-to-human transmission are not uncommon in nosocomial settings. The incidence of CCHF closely matches the geographical range of permissive ticks, which are widespread throughout Africa, Asia, the Middle East and Europe. As such, CCHFV is the most widespread tick-borne virus on earth. It is a concern that recent data shows the geographic distribution of Hyalomma ticks is expanding. Migratory birds are also disseminating Hyalomma ticks into more northerly parts of Europe thus potentially exposing naïve human populations to CCHFV. The virus has been imported into the UK on two occasions in the last five years with the first fatal case being confirmed in 2012. A licensed vaccine to CCHF is not available. In this review, we discuss the background and complications surrounding this limitation and examine the current status and recent advances in the development of vaccines against CCHFV.
Keywords: Crimean-Congo haemorrhagic fever, Vaccine, Review
1. Introduction
1.1. Background
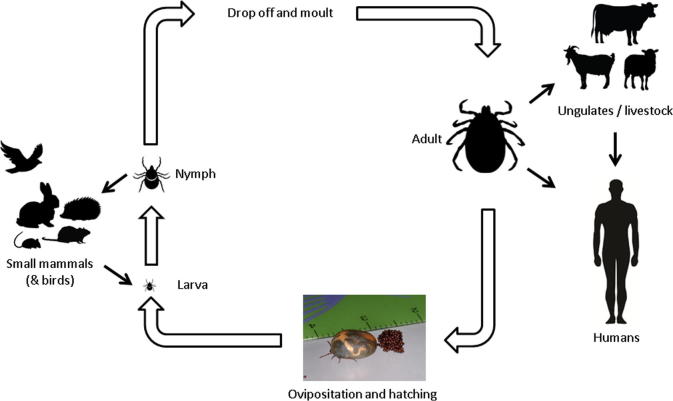
Crimean-Congo haemorrhagic fever (CCHF) is a virulent human disease and the most wide spread tick-borne viral infection of man. It occurs over much of Asia, extending from the XinJiang region of China to the Middle East and Southern Russia, to focal endemic areas over much of Africa, parts of Eastern Europe and most recently the Iberian Peninsula [1], [2]. There are currently no licensed vaccines or therapeutics to treat CCHF. The aetiological agent, CCHF virus (CCHFV), is a single-stranded, negative sense RNA virus classified within the Nairovirus genus of the family Bunyaviridae. It is maintained in an enzoonotic cycle involving tick-mediated transmission between several species of vertebrate including wild and domestic mammals (Fig. 1). While animals develop a transient viremia, they remain asymptomatic, and direct transmission to humans involved in the slaughter and butchering of such domestic animals is a common infection route. Human infections also occur through tick bite, tick crushing in the hand, or exposure to the blood or other body fluids of an infected CCHF patient [1], [2], [3]. CCHF was first brought to modern medical attention in 1945, when it was recognised as an acute febrile illness, accompanied by fever and severe bleeding in over 200 Soviet military personnel and local inhabitants supporting war devastated Crimea [4]. Originally termed Crimean Haemorrhagic Fever, its viral aetiology was identified in 1947 through experiments which included the inoculation of psychiatric patients and human “volunteers” with ultra-filtrates of patient serum and/or extracts of pooled ticks [4], [5]. However, it was not until 1968 that the virus was first isolated [6], which resulted in the international recognition that ‘Crimean haemorrhagic fever’ virus was identical to the ‘Congo’ virus identified in Africa in 1958 [7]. Ultimately, these investigations led to the designation ‘Crimean-Congo Haemorrhagic Fever virus’, a political name conceived during the Cold War [8].
Fig. 1.
Zoonotic cycle of Hyalomma tick species, the main vector for CCHFV (modified from [11]).
1.2. Emergence and growing threat
CCHF is an emerging virus whose incidence and geographic range has been increasing since its early identification. For example, since 2002, the disease has been reported with increasing frequency in Turkey, Iran, India, Greece, the Republic of Georgia and Kosovo. In 2016 it emerged in Spain as two autochthonous cases, including one fatality as the result of a tick bite of the index case, in the province of Ávila, a location ∼300 km from Cáceres where CCHFV was identified in ticks collected in 2010. Modelling of climate change has demonstrated that a rise in temperature and a decrease in rainfall in the Mediterranean region could result in a sharp rise in the distribution of suitable habitats for Hyalomma ticks [9], thus increasing the pool of human populations at risk. Reported cases of CCHF have risen during 2000–2009 (Table 1); it is expected that this trend will continue [10], although specific reports have taken several years to be published and an accurate assessment is not currently possible. It is widely accepted, however, that CCHF cases are under reported. Most endemic countries have poor healthcare systems where diagnosis is not straightforward and often non-existent. Additionally, infection is common in remote rural areas and mild symptoms of the disease are not diagnosed and so not reported.
Table 1.
Cases of CCHFV reported in the medical literature from different regions.
| Year | Region (published CCHF cases/annum)a |
|||
|---|---|---|---|---|
| Africab | Asiac | Europed | Middle Easte | |
| Pre-2000f | 1.1 (since 1956) | 13.5 (since 1965) | 53.3 (since 1944) | 4.9 (since 1979) |
| 2000 | 1 | 48 | 94 | 20 |
| 2001 | 0 | 15 | 107 | 66 |
| 2002 | 0 | 12 | 186 | 111 |
| 2003 | 0 | 9 | 245 | 57 |
| 2004 | 38 | 13 | 364 | 26 |
| 2005 | 0 | 3 | 423 | 18 |
| 2006 | 0 | 10 | 651 | 50 |
| 2007 | 0 | 12 | 951 | 66 |
| 2008 | 8 | 14 | 1333 | 150 |
| 2009 | 2 | 21 | 1312 | 1 |
CCHF cases reported in the medical literature and reported by Bente et al. [11].
Cases since 2000 in Africa from Mauritania, Kenya and Sudan.
Cases since 2000 in Asia from Kazakhstan, Tajikistan, Pakistan and India.
Cases since 2000 in Europe from Bulgaria, Kosovo, Russia, Albania, Turkey and Greece.
Cases since 2000 in the Middle East from Iran and Afghanistan.
Cases per annum averaged from first year figure is available (in brackets) to year 2000.
CCHFV is notorious for causing nosocomial outbreaks, often resulting in severe disease with high mortality rates [12], [13], [14], [15]. Human-to-human transmission via direct contact with contaminated blood and other tissues during surgical procedures, carried out in the absence of a correct diagnosis, is the principle source of infection [16]. Secondary human-to-human transmission in a care setting is frequently associated with exposure to infected blood, however cutaneous contact to non-sanguineous body fluids (e.g., saliva, sweat, vomitus, urine, and faeces) seems not to be linked to human-to-human transmission [17]. Standard barrier nursing methods are sufficient to prevent the transmission of CCHF virus in such circumstances [17], [18]. However, when the risk of infection is generally of less concern, gloves, aprons, goggles etc., are not commonly used. This is a key factor in the zoonotic transmission of CCHFV from viremic animals during slaughter and butchering. Zoonotic transmission as a result of the slaughter and butchering of domestic animals increases during preparations for Eid-al-Adha, an annual religious festival celebrated by Muslims, during which many animals, including goats, sheep, cows, and camels are slaughtered [19], [20]. As the timing of this festival follows the Islamic lunar calendar, it moves approximately 10 days earlier each year. Significantly in the next 10–20 years Eid-al-Adha will occur in the summer and spring months, when sacrificial animals are more likely to be viremic for CCHFV as a result of the transmission of the virus from infected blood-feeding ticks which are more active during these times. There is concern therefore, that the slaughter of viremic animals during these periods may facilitate the spread of CCHFV to humans which may cause secondary and tertiary human-to-human spread and subsequent outbreaks [21], [22].
In addition to the obvious public health threat [23], CCHFV is recognised as a potential agent for maliferous use [24]. It was reported to have been weaponised by the Soviet regime in the 1980s and later in 1990 by scientists linked to Ba’athist Iraq [25]. Despite the lack of conclusive evidence for the transmissibility of CCHFV via the aerosol route, it has been listed as a potential agent of bioterrorism/biowarfare [26].
1.3. Pathogenesis
Vascular dysfunction, resulting in haemorrhage and loss of fluid from the plasma into the interstitial space are the overt symptoms of CCHF disease. Patients display abnormal coagulation parameters from an early stage of illness, characterised by disseminated intravascular coagulation (DIC) [27], [28]. It is not clear, however, whether vascular dysfunction is brought about directly, through viral infection of the endothelium, or indirectly, through the effects of circulating pro-inflammatory mediators.
Interestingly, Ebola haemorrhagic fever shares many features with CCHF [29] and its sobriquet “Asian Ebola” is often used to highlight the nature of disease typified by altered vascular function resulting from host-induced mechanisms, including the induction of pro-inflammatory cytokines, platelet aggregation and degranulation, leukocyte adhesion and activation of the intrinsic coagulation cascade [30]. The importance of pro-inflammatory cytokine responses in the pathogenesis of CCHF is supported by several reports. In a study of mild and severe cases in Turkey, high serum levels of TNF-α were associated with serious illness, whilst interleukin (IL)-6 was elevated in both mild and severe cases [31]. The levels of other mediators were significantly higher in fatal than in nonfatal cases. In a large study in Kosovo, fatally infected CCHF patients had high levels of IFN-gamma, TNF-α and IL-10 [32]; similarly, a study of Albanian CCHF patients showed high serum levels of TNF-α, soluble TNF-R, IL-6 and IL-10, with most significance linked to IL-6, IL-10 and TNF-alpha in relation to CCHF disease [33]. Interestingly the Turkish report [31] showed a strong association of TNF-α and IL-6 with DIC and fatal outcome, however in that study IL-10 levels did not differ significantly. It has been suggested that the major differences between such studies are linked to the many different sampling times, phase of CCHF disease, and the low numbers of tested samples. A more recent study has attempted to address the inconsistencies by adopting a systematic collection of varied patient samples at specific times post symptom onset [34]. Nevertheless, despite demonstrating that many cytokines are released and interact during the acute phase of CCHF disease, the study underlined the complex interrelationships involved in pathogenesis. Thus future studies that can focus on many serial, clinical samples from patients in early-to-late phases of mild and severe CCHF disease are required to provide better insight into pathogenesis including of the development of the human immune response.
Clinical observations are mirrored in laboratory studies which show that CCHFV replicates in human monocyte-derived dendritic cells (DCs) to release pro-inflammatory cytokines [35]. Supernatants of infected DCs could activate cultured endothelial cells, but this could be blocked by antibodies to TNF-α. Additionally, CCHFV infection of primary human macrophages and DCs induced the release of pro-inflammatory cytokines (although levels were higher in cells infected with Dugbe virus and Hazara virus, nairoviruses that produce only a moderate or no illness in humans) [36].
CCHF viral load is an important factor for both the severity and outcome of disease and has been acknowledged in many studies [31], [37], [38], [39], [40], [41], [42]. Recent work correlating levels of the chemokine CXCL10 (also known as IP-10) with CCHF viral load suggests that it is involved in pathogenesis [43].
The involvement of natural killer (NK) cells during pathogenesis has also been investigated and greater numbers are observed in severe than in mild cases [44]. A similar study showed a higher percentage of cytotoxic T cells among circulating lymphocytes in fatal than in non-fatal cases, with the percentage correlated to viral load [45]. Fatally infected CCHF patients typically do not produce detectable IgM or IgG antibodies to the virus, indicating the protective role of the humoral response; however, whether this failure is linked to the loss of lymphocytes through apoptosis is unclear.
While several animal species support CCHFV infection and become viremic, they do not develop disease, and detailed studies examining the pathogenesis and mechanisms of protective immunity in a wild type animal model have not been possible. Nevertheless, interferon-knockout (IFNKO) mice have been shown to be highly susceptible to CCHFV [46], [47] and the lethal infection that results indicates the critical role of interferon pathways in the antiviral response. Nitric oxide (NO), a mediator of innate responses, also suppresses CCHFV replication [48].
1.4. Informative animal models of CCHF
The lack of a susceptible animal model for CCHFV infection has severely hampered work on the development of vaccines; for decades the only lethal animal model was infection of newborn mice [49], [50]. However, in 2010 two reports were published on mouse strains. In the first, mice with deficiencies in their type-I interferon receptor (A129) were shown to be highly susceptible to CCHFV doses as low as 10 focus forming units [47]. It was also demonstrated that the liver was the main target organ [47], which resembles the clinical situation in human CCHFV infection [28]. The second model was the STAT-1 knockout mouse model [46]. Unlike the A129 mice which only lack receptors to type-I interferons, STAT-1 knockout mice exhibit signalling defects for all three types of interferon (type I, α and β; type II, γ; and type III, λ) [51]. These extra deficiencies in the adaptive immune response pathway of STAT-1 knockout mice mean that A129 mice may be considered a more appropriate model for CCHFV vaccine studies.
Many attempts have been made to develop larger animal models for CCHF, including non-human primates (NHPs). In the 1970s, researchers challenged African green monkeys, but apart from a rise in temperature in one animal, no other clinical disease signs were reported [52]. In the same decade, another group challenged two Patas monkeys and a single Guinea baboon; antibodies were detected but no disease was observed apart from a rash and itching in the latter species [53]. More recently, cynomolgus macaques have been used with a challenge dose of around 104 plaque-forming units of CCHFV but they failed to show any signs of disease or evidence of viral replication [54]. Thus, there are no published studies to date that report sustained virus replication and/or pathogenesis in NHPs as a result of CCHFV challenge. This failure may be due to the host’s innate IFN mediated defence mechanism which is difficult to deplete in non-murine species, or the low doses and passage history of viruses used for challenge.
1.5. Current therapeutics
There are currently no approved therapies for CCHF, and supportive care is often the only option [55]. Ribavirin, a broad anti-RNA virus inhibitor, has demonstrated antiviral activity in experimental settings using cell culture [56] including a mouse model [46]. A clinical study conducted between 1999–2001 in Iran demonstrated 80% efficacy [57], which led to the World Health Organisation recommendation for treating CCHF patients with ribavirin [58]. Other studies however, have led to mixed results, with no difference in case fatality rates being reported in the only randomised controlled trial published on this topic so far [59].
2. The case and need for vaccines
With the increasing spread of CCHFV across broader geographical areas and no approved treatments, the virus constitutes a public health risk for many regions [23]. The development of a vaccine to prevent infection in human populations at risk would provide protection against CCHFV. Whilst protection for humans should reduce the number of cases of CCHF, an animal vaccine might also reduce the risk of zoonotic transmission, and the pool of ticks carrying CCHFV.
2.1. Classic Russian/Bulgarian vaccine
A Soviet vaccine developed in 1970 was based on CCHFV cultivated in suckling mouse brain. This virus was subsequently inactivated by chloroform, heated at 58 °C, and absorbed on Al(OH)3 [60]. It was licensed in Bulgaria and has been used in endemic areas for military personnel including medical and agricultural workers since 1974. The current Bulgarian vaccine uses a strain of CCHFV, V42/81, which was isolated from a patient in 1981 [60]. Data from the Bulgarian Ministry of Health suggest a fourfold reduction in reported CCHF cases over a 22 year time period (1953–1974: 1105 cases; 1975–1996, 279 cases) [61]. Unfortunately, however, this was the extent of the efficacy studies at the time and while there has been a reduction in cases, data indicates that this may not necessarily be the result of the vaccine but due to a multitude of factors which have decreased tick exposure in the region per se [62]. In addition, there is a lack of data regarding the incidence of CCHF in the same time frame in neighbouring countries, which also brings into question the analysis of vaccine efficacy at the time [63]. In 2012, the first detailed analysis of the cellular and humoral immune response in healthy individuals following vaccination with the Bulgarian vaccine was carried out [64]. This data showed that although vaccinated individuals developed anti-CCHFV immunity as measured by IFN-γ ELISpot and neutralisation assays, responses were low and required 3 booster vaccinations to improve immune responses [64]. Mouse brain derived vaccines also raise concerns due to possible autoimmune and allergic responses induced by myelin basic protein [65] and they are unlikely to gain widespread international regulatory approval.
2.2. Vaccine targets
CCHFV is a tri-segmented virus, consisting of small (S), medium (M) and large (L) RNA segments which encode the viral nucleoprotein, the envelope spike proteins and RNA polymerase, respectively. As the key structural components of the virus, the nucleoprotein and glycoproteins are identified as potential antigenic targets for inclusion in a vaccine against CCHFV.
2.2.1. Nucleoprotein
The nucleoprotein (NP) is the most conserved protein of CCHFV [11], [66], making it an attractive antigen for consideration as a vaccine target antigen to be effective against different lineages of CCHFV. Its formation and role in the ribonuclear protein complex is essential for virus replication [67] and it is recognised as the predominant antigen inducing an immune response in most bunyavirus infections [68]. Protective effects using NP have been shown against two other viruses of the Bunyaviridae family: Hantavirus [69] and Rift Valley fever virus [70]. Following repeated vaccinations with the CCHFV Bulgarian vaccine it was found that T-cell responses were mounted to peptides making up the entire length of the NP sequence [64]. Additionally, an in silico study suggested that certain regions of NP could act as T-cell epitopes [71].
2.2.2. Viral envelope spike gproteins
The CCHFV spike glycoproteins (Gn and Gc) mature after a complicated series of processing events which take place in the endoplasmic reticulum (ER) and Golgi cellular compartments. The glycoprotein precursor (GPC) (translated from the M segment ORF), contains an N-terminal signal peptide to direct synthesis into the secretory pathway [72], [73]. Upon elongation and translocation into the ER, the signal peptide is removed; GPC is N-glycosylated and folds with the formation of key intra-molecular disulfide bonds and five transmembrane domains which span the ER membrane. Co-translational cleavage in the ER to the Gn precursor (Pre Gn) and Gc precursor (Pre Gc) is followed by transport of each to the Golgi compartment where key glycosylation and further regulated processing events take place, ultimately releasing the 37 kDa mature Gn [74] and 75 kDa mature Gc [72], [75], [76]. In addition to the coordination of glycosylation events the remaining cleavage products of GPC are thought to assist in the maturation and assembly of the final GnGc glycoprotein spike [75].
Studies with monoclonal antibodies have demonstrated that those targeted to Gc, but not to Gn, have neutralisation activity [77]. However, other researchers have demonstrated neutralisation epitopes in both Gn and Gc regions [78].
While the M segment of CCHFV is the most diverse [11], this variation is focused in the N terminal mucin-like domain which is not part of mature GP. Indeed, the amino acid difference between CCHF viruses in the rest of the GP is only 8.4% [79]. Thus it is expected that neutralising epitopes which are part of the mature GP confer protection across the extant clades and sub-types of CCHFV. Furthermore, CCHFV constitutes a single serogroup which was collated using classic virological tools that showed cross reactivity in a range of assays [80].
2.2.3. Diagnostic assays to differentiate infected from vaccinated animals (DIVA)
If animal vaccination for CCHFV becomes an additional control optional in the future, an effective way to differentiate vaccinated from non-vaccinated animals will become an important tool. Classically, vaccines which have DIVA antigens are useful when special restrictions are in place for infected animals which may be relaxed when those animals can be proven to be vaccinated and not infected. Whilst this is applicable for animal pathogens such as bovine tuberculosis [81], avian influenza [82] and foot-and-mouth virus [83], CCHFV is not addressed by the same regulations as it is specifically a human and not an animal pathogen. However, the benefits of distinguishing vaccinated animals from those infected with the wild type CCHFV would be useful to track vaccination and study vaccine efficacy in the field. The CCHFV NP is widely used as an antigen in serological assays [84], [85], [86], like other bunyavirus NP’s is the predominant antigen expressed early after infection inducing in consequence, a specific immune response [87], [88], [89]. Therefore, vaccines based on antigens other than NP will permit the use of existing serological assays which detect antibodies to NP to be used. Alternatively, vaccine candidates may also be developed which contain marker proteins that are absent in natural CCHFV and thus can be used specifically to identify vaccinated subjects.
2.3. Mechanisms of protection
Many reports indicate that viral loads directly correlate with disease outcome and severity: viral loads corresponding to >108 genome copies/ml portend a fatal CCHFV infection while lower viral loads elicit a less severe disease course [32], [38]. It is likely that lower viral loads allow the immune response to have a better chance of fighting infection.
While the robust development of IgM and IgG antibodies to CCHFV infection has long been considered a positive prognostic indicator, most patients seem to develop relatively low levels of neutralising antibodies [90]. Similarly, non-neutralising monoclonal antibodies have been found to be effective at protecting mice from a lethal CCHFV challenge, and interestingly, those specific against Gn were more effective than those against Gc [77]. Additionally, the use of convalescent plasma as a post-exposure prophylactic has not been particularly effective [63]. Recent vaccine studies have also indicated that the induction of neutralisation antibodies does not correspond to vaccine efficacy [91], [92]. Thus cell-mediated immunity looks likely to play a major role in developing effective protection against CCHFV. The interferon pathway has also been shown to play an important role in controlling CCHFV infection [93]. However, while the interferon response may be critical it appears that the kinetics of the response and the activation of multiple pathways may be the most important factors in the outcome of infection [94]. Therefore, to date the correlates of protection remain ill-defined.
There are no reports of patients being re-infected with CCHFV; thus dogma suggests that convalescence results in protective immunity. Detailed studies of the immune responses in recovered patients should help elucidate correlates of protection responses and thus help to define the breadth and repertoire of immunity that a vaccine should deliver.
2.4. Product profile for an ideal CCHF vaccine
A CCHF vaccine that is suitable for the wide range of regions affected and acceptable to regulatory authorities must meet several characteristics. These are outlined in Table 2. Whilst the public health focus is on human vaccination, it is also likely that veterinary vaccines against CCHFV infection will play an important role in preventing spread to humans; controlling infection in livestock will reduce the risk of human exposure to viremic blood from agricultural animals during slaughter. Veterinary vaccines may subsequently reduce the frequencies of naïve ticks acquiring CCHFV infection during blood feeding on viremic livestock. However, control of CCHFV in wild-life and tick populations will remain difficult. Veterinary vaccines may have additional benefits if they have companion DIVA diagnostic tests [95] and are able to confer immunity to off-spring after administration to the mother [96], given that for a significant period of their lives livestock are suckling.
Table 2.
Product profile for a human CCHF vaccine.
| Feature | Reason |
|---|---|
| Induction of humoral and cellular responses | Only vaccine approaches which induce both arms of the adaptive immune system have demonstrated protection against CCHFV challenge |
| Authentic expression of gene product | The antigen exposed to the immune system needs to be identical to the target virus to optimise linear and non-linear epitope recognition |
| Acceptable ‘Costs of Goods’ profile | Cost will be a key factor in uptake by authorities in endemic regions where healthcare finances are limited |
| Manufacturing capability | The vaccine should be able to be produced rapidly in outbreak conditions and in sufficient quantities for immunisation of at risk groups in endemic regions |
| Thermostable | CCHFV is endemic in countries that experience high temperatures. A thermostable vaccine would remove the logistics and extra costs associated with maintaining a cold chain |
| Safety profile | A vaccine based on similar technologies with existing safety data is more likely to be successful in clinical trials and faster to license |
| Few doses required | Vaccine uptake will be increased if multiple boosters are not required |
3. Vaccine progress
Several different vaccination approaches have been used for CCHF. These are summarised (Table 3) and discussed in more detail below.
Table 3.
Approaches for human vaccines against CCHFV.
| Vaccine type | CCHFV antigen | Immunity |
Protection in preclinical model | Clinical evidence |
Refs. | ||
|---|---|---|---|---|---|---|---|
| Antibody | T cell | Safety1 | Manufacturing practicalities | ||||
| Inactivated virus (mouse brain) | Whole virus | ✓ | ✓ | ? | ? | X | [60], [64] |
| Inactivated virus (cell culture) | Whole virus | ✓ | NT | ✓2 | ? | X | [97] |
| Modified Vaccinia Ankara (MVA) | M segment | ✓ | ✓ | ✓3 | ✓ | ✓ | [98] |
| S segment | ✓ | ✓ | X | ✓ | ✓ | [99] | |
| DNA vaccine | M segment | ✓ | NT | NT | ✓ | ✓ | [100] |
| Gc, Gn and NP | ✓ | ✓ | ✓4 | ✓ | ✓ | [91] | |
| Transgenic plant | Glycoprotein | ✓ | NT | NT | ? | ✓ | [79] |
| Protein | Gn glycoprotein | ✓ | NT | X | ? | ✓ | [92] |
| Gc glycoprotein | ✓ | NT | X | ? | ✓ | ||
| Adenovirus | M segment | ✓ | ✓ | X | ✓ | ✓ | [101] |
| Virus-like particles | Gc, Gn and NP | ✓ | ✓ | ✓5 | ✓ | ✓ | [91] |
Key: NT, not tested; ✓, positive results; X, negative results; ?, unknown.
Based on the same technology used for other pathogens.
80% efficacy reported.
100% efficacy reported.
100% efficacy reported.
40% efficacy reported.
3.1. Immunogenicity studies
3.1.1. DNA vaccines
A DNA vaccine expressing the entire M genome segment of CCHFV has been developed [100]. Following vaccination alone or in combination with other DNA vaccines against Rift Valley fever virus, tick-borne encephalitis virus and Hantaan virus, neutralising antibodies were only detected in around half of the treated mice, with immune precipitation demonstrating antibodies recognising both Gn and Gc [100]. Studies with this CCHFV DNA vaccine were limited to immunogenicity assessment by serological assay, as a suitable model of disease was not available at the time. Recently, a DNA vaccine coding for CCHFV Gc, Gn and NP demonstrated induction of both humoral and cell-mediated immune responses which elicited complete protection from lethal disease in a mouse model [91].
3.1.2. Plant expressed vaccines
Another approach to vaccination against CCHFV has been to develop methods to reduce virus levels in animals which play a critical role in the transmission cycle [102], although animals do not show clinical signs of disease [103]. The Gn and Gc coding regions of CCHFV have been manipulated into plant cloning vectors and subsequently expressed in transgenic tobacco plants. The leaves and roots were then fed to mice, leading to oral/mucosal immunisation and the induction of an antigen-specific humoral response [79]. However, serum antibody levels measured by ELISA were relatively low in fed groups (1:256) and required boosting to increase the response (1:32,000) [79]. Unfortunately this study did not have access to an animal model of CCHF disease and no efficacy studies were performed. It is noteworthy that with the transgenic plant vaccine, only the Gn and Gc were included and no other parts of the CCHFV M segment.
3.1.3. Inactivated vaccines
Until recently, data on the immune response to the Bulgarian mouse brain derived vaccine were limited. However, in 2010 a study of eight vaccinees was published, demonstrating T-cell activity and humoral responses [64]. Repeat dosing was required for the antibody to demonstrate neutralisation activity and increase T-cell responses, suggesting that booster vaccinations may be required to confer protective immunity [64]. Whilst this data provides evidence on the immune response, efficacy data on the vaccine is still not published.
In an attempt to avoid the regulatory complications of a mouse brain derived vaccine, a purified, formalin inactivated preparation of CCHFV derived from cell culture was published in 2015 [97]. Vaccination required the use of alum adjuvant and although the data showed that this vaccine was able to induce neutralising antibodies, no studies of T cell immunity were performed. However, efficacy in the IFNAR−/− mouse model of CCHF was carried out showing that 80% of mice were protected from an otherwise lethal challenge.
While inactivated virus vaccines have proven effective for protecting against many viral diseases, those based on highly pathogenic viruses such as CCHFV are difficult to manufacture in bulk within a high containment setting.
3.2. Efficacy studies
Whilst studies on the immunogenicity of CCHFV vaccine candidates can yield interesting findings, results are often difficult to interpret as no defined immune correlate of protection is available. Nevertheless, the availability of a lethal murine model of CCHFV infection has enabled several vaccine candidates to be assessed in protection studies.
3.2.1. Viral vectors
3.2.1.1. Modified Vaccinia virus Ankara (MVA)
The first, and currently only, CCHFV vaccine to demonstrate 100% efficacy in animal studies is a MVA poxvirus vector containing the glycoprotein-encoding M segment ORF (MVA-GP) [98]. MVA has the capacity to express large gene inserts [104], resulting in effective cellular and humoral immunity [105], hence its use as an accommodating vector for the expression of large antigens such as the CCHFV GPC. Despite MVA’s growth deficiency in most mammalian cells, MVA is able to promote high-level gene expression of recombinant genes both in vitro and in vivo, with authentic post-translational modifications in the host cell [106]. MVA also has a proven safety record, having been used in the latter stages of the smallpox eradication campaign [107], and it is thermostable [108] avoiding the requirement to maintain a cold chain and conferring an extra advantage for regions where such a vaccine might be used. The CCHFV MVA-GP encodes the entire ORF of the M segment which translates to the GPC, this includes the non-structural accessory domains (mucin-like domain, GP38 and NSm) in addition to the Gn and Gc coding domains. The accessory domains and cleavage products assist with the complex processing and glycosylation events in the ER [72], [73], [75] including appropriate transport to the Golgi compartment and maturation [77]. Therefore, for vaccines using the glycoprotein as an antigen, these accessory components of GPC may be important to enable effective protein expression of authentic GP. It is known that MVA induces both cellular and humoral immunity without the need for adjuvants [109], [110]. With the CCHFV MVA-GP vaccine, antibody and cellular responses were induced across a range of epitopes [24]. In order to elucidate the relative contribution of the different components of the adaptive immune response, passive and adoptive transfer studies were conducted with results demonstrating that both antibody and T cells are required to exert effective protective benefits against lethal CCHFV infection [111].
Other vaccines tested in vivo have failed to show any protection. Interestingly candidates have included a MVA construct expressing the CCHFV nucleoprotein, which generated antigen-specific immunity but showed no protection against a lethal virus challenge in A129 mice [99].
3.2.1.2. Adenovirus vaccine
Similar to the MVA approach, the entire M segment of CCHFV has been inserted in a human serotype 5 adenovirus (AdHu5) vector. However, despite the induction of both cellular and humoral responses, the vaccine failed to confer protection in a lethal murine model of CCHFV infection [101]. Precise reasons for this failure are unclear, however the use of the STAT-1 knockout mouse model for efficacy studies instead of A129 mice could potentially affect the adaptive immune response to vaccination due to deficiencies in the adaptive immune response in the STAT-1 knockout mice. Additionally, difficulties with adenovirus vectors include high levels of pre-existing immunity, with approximately 45–80% of adults expressing neutralising antibodies against AdHu5 [112], [113]. In order to avoid pre-existing immunity, adenoviruses from different species have been used, and chimpanzee adenovirus (ChAd) is currently a popular choice [114]. However, due to conserved epitopes between adenovirus strains, pre-existing neutralising antibodies against ChAd are still present in humans at levels of up to 4% in Europe and America and up to 20% in developing countries [115], [116], [117].
3.2.2. Subunit vaccine
Insect expression technology has been used to express the structural surface or ectodomains of the Gc and Gn glycoproteins of CCHFV. Using purification tags, adjuvanted Gn or Gc ectodomains were shown to induce neutralising antibodies but they did not protect STAT-1 knockout mice [92] in a subsequent lethal virus challenge. While some insect cells retain similar patterns of glycosylation to mammalian cells, it is possible that the complicated glycosylation processes of CCHF GP may not have been fully supported in insect cells [118], and non-authentic GP may not have been able to induce protective responses.
Transcriptionally-competent virus-like particles (tc-VLPs) have been developed for CCHFV [119]. In mouse efficacy studies using A129 mice, despite the induction of strong neutralising antibody titres, the tc-VLPs only protected 40% of animals [91]. Results with these subunit vaccine approaches therefore demonstrate that neutralisation activity is unlikely alone to be an accurate measure of protection against CCHFV infection.
4. Future directions
With increasing availability of sequence data, several reports have identified potential epitopes for CCHFV which might aid vaccine development. For example data has identified neutralising epitopes in both Gn and Gc [78], [91]. Interestingly, one of these reports [78] studied several strains which segregated into three of the four previously described M segment lineage groups [120]. Despite the genetic diversity in the M segment of CCHFV, neutralising epitopes were found to be conserved among all strains tested. Therefore, vaccines encoding a standard M segment GPC could potentially confer protection across the different virus types without further modification of the insert antigen. In silico molecular docking and immunoinformatics tools have also been used to predict epitopes on the CCHFV nucleoprotein [71] and the RNA-dependent RNA polymerase encoding L segment [121]. Whilst the development of an epitopic vaccine could provide effective and controlled immune responses, reducing the effects of live vaccination, the downside is that it must take into account the binding of epitopes with human leukocyte antigen (HLA), the complexity of which is compounded by extreme HLA polymorphism [122]. Additionally, whilst many of the potential epitopes described refer to neutralisation activity, the important contribution of the T cell immune system in protection is often overlooked.
Live attenuated vaccines may also be developed against CCHFV. For example, it has been suggested that the ovarian tumor domain (OTU)-containing regions on CCHFV may have deubiquitinating enzyme (DUB) activity which can mediate innate immune suppression during infection. The selective inactivation of the DUB function may contribute to the generation of attenuated viruses which may be used for vaccination approaches [123]. Similarly it may be possible to alter or abrogate the OTU activity using a reverse genetic approach, as this domain is dispensable for CCHFV RNA polymerase function [124]. However, given the high pathogenicity of CCHFV infection, its hazard group 4 classification and difficulties associated with testing attenuated vaccines in humans, this approach is unlikely to be accepted.
Recent advances in the development of humanised mice may be beneficial to the CCHFV vaccine field [125]. The current lack of a CCHF model in fully immunocompetent rodents or higher mammals, such as non-human primates, prevents vaccine approval under the Animal Rule devised by the Food and Drug Administration (FDA) in the USA for demonstration of efficacy in place of human clinical trials [126]. The use of more clinically relevant low passage CCHFV strains might support new disease model development [127]. Additionally, the challenge route used in vaccine assessment studies should mimic natural infection by tick bite. Several CCHFV studies use the intraperitoneal route of challenge [97] which is not a normal route and may potentially alter the course of disease progression.
5. Conclusion
Rapid progress is being made in CCHFV vaccine development, accelerated by the availability of an animal model of lethal infection. The outbreak of Ebola virus in West African first recognised in 2014 has also increased attention on viral haemorrhagic fevers, and with the advanced development of several vaccine candidates underway in the filovirus field, attention is expected to turn to other pathogens which cause viral haemorrhagic fever such as CCHFV. The recently published WHO R&D Blue Print highlights the role of international Governments and not for profit agencies to fund the development and stockpiling of new vaccines to emerging high consequence pathogens. The establishment of the Coalition of Epidemic Preparedness Innovations (CEPI) has subsequently been established to fund and develop new emerging disease vaccines with initial funding from the World Economic Forum and international Governments amounting to $500M. The development of a protective vaccine based on the MVA viral vector background is currently being advanced by UK Government funding and will start human clinical trials in late 2017. CCHF vaccines will need to complete early human clinical trials in order to be accepted as safe and rapidly available for use in emergency vaccinations. Recent advances in this field provides hope that in future CCHFV can be controlled in endemic areas and outbreak scenarios.
Acknowledgments
Views expressed in this article are those of the authors and do not necessarily reflect those of Public Health England (PHE).
References
- 1.Leblebicioglu H. Crimean-Congo haemorrhagic fever in Eurasia. Int J of Antimicrobial Agents. 2010;36(Suppl 1):S43–S46. doi: 10.1016/j.ijantimicag.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 2.Vorou R., Pierroutsakos I.N., Maltezou H.C. Crimean-Congo hemorrhagic fever. Curr Opin Infect Dis. 2007;20:495–500. doi: 10.1097/QCO.0b013e3282a56a0a. [DOI] [PubMed] [Google Scholar]
- 3.Whitehouse C.A. Crimean-Congo hemorrhagic fever. Antiv Res. 2004;64:145–160. doi: 10.1016/j.antiviral.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Chumakov M.P. A new tick-borne virus disease-Crimean hemorrhagic fever (acute infectious capillary toxicosis) In: Sokolov A.E., Chumakov M.P., Kolachev A.A., editors. Crimean hemorrhagic fever (acute infectious capillary toxicosis). Izdanie Otdel'noj Primorskoj Armii, Simferopol, USSR. 1945. [Russian] [Google Scholar]
- 5.Chumakov M.P. A short review of the investigation of the virus of Crimean hemorrhagic fever. In: Chumakov M.P., editor. Endemic viral infections: sborn trud inst polio virus encefal, akad med nauk USSR [Russian; NAMRU-3 translation T189] 1965. pp. 193–196. [Google Scholar]
- 6.Chumakov M.P. On 30 years of investigation of Crimean hemorrhagic fever. Trudi Inst Polio Virusn Entsefalitov Akad Med Nauk, USSR. 1974;22:5–18. [Russian; NAMRU-3 translation T950] [Google Scholar]
- 7.Simpson D.I., Knight E.M., Courtois G., Williams M.C., Weinbren M.P., Kibukamusoke J.W. Congo virus: a hitherto undescribed virus occurring in Africa. I. Human isolations–clinical notes. East Afr Med J. 1967;44:86–92. [PubMed] [Google Scholar]
- 8.Woodall J.P. Personal Reflections. In: Ergonul O., Whitehouse C.A., editors. Crimean-congo hemorrhagic fever: a global perspective. Springer; 2007. pp. 23–32. [Google Scholar]
- 9.Estrada-Pena A., Venzal J.M. Climate niches of tick species in the Mediterranean region: modeling of occurrence data, distributional constraints, and impact of climate change. J Med Entomol. 2007;44:1130–1138. doi: 10.1603/0022-2585(2007)44[1130:cnotsi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 10.Randolph S.E., Rogers D.J. The arrival, establishment and spread of exotic diseases: patterns and predictions. Nat Rev Microbiol. 2010;8:361–371. doi: 10.1038/nrmicro2336. [DOI] [PubMed] [Google Scholar]
- 11.Bente D.A., Forrester N.L., Watts D.M., McAuley A.J., Whitehouse C.A., Bray M. Crimean-Congo hemorrhagic fever: history, epidemiology, pathogenesis, clinical syndrome and genetic diversity. Antivir Res. 2013;100:159–189. doi: 10.1016/j.antiviral.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Aradaib I.E., Erickson B.R., Mustafa M.E., Khristova M.L., Saeed N.S., Elageb R.M. Nosocomial outbreak of Crimean-Congo hemorrhagic fever, Sudan. Emerg Inf Dis. 2010;16:837–839. doi: 10.3201/eid1605.091815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ergonul O. Crimean-Congo hemorrhagic fever virus: new outbreaks, new discoveries. Curr Opin Virol. 2012;2:215–220. doi: 10.1016/j.coviro.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Mardani M., Keshtkar-Jahromi M., Ataie B., Adibi P. Crimean-Congo hemorrhagic fever virus as a nosocomial pathogen in Iran. The Am J Trop Med Hyg. 2009;81:675–678. doi: 10.4269/ajtmh.2009.09-0051. [DOI] [PubMed] [Google Scholar]
- 15.Mourya D.T., Yadav P.D., Shete A.M., Gurav Y.K., Raut C.G., Jadi R.S. Detection, isolation and confirmation of Crimean-Congo hemorrhagic fever virus in human, ticks and animals in Ahmadabad, India, 2010–2011. PLoS Negl Trop Dis. 2012;6:e1653. doi: 10.1371/journal.pntd.0001653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hasan Z., Mahmood F., Jamil B., Atkinson B., Mohammed M., Samreen A. Crimean-Congo hemorrhagic fever nosocomial infection in a immunosuppressed patient, Pakistan: case report and virological investigation. J Med Virol. 2013;85:501–504. doi: 10.1002/jmv.23473. [DOI] [PubMed] [Google Scholar]
- 17.Athar M.N., Khalid M.A., Ahmad A.M., Bashir N., Baqai H.Z., Ahmad M. Crimean-Congo hemorrhagic fever outbreak in Rawalpindi, Pakistan, February 2002: contact tracing and risk assessment. The Am J Trop Med Hyg. 2005;72:471–473. [PubMed] [Google Scholar]
- 18.Maltezou H.C., Papa A. Crimean-Congo hemorrhagic fever: risk for emergence of new endemic foci in Europe? Travel Med Infect Dis. 2010;8:139–143. doi: 10.1016/j.tmaid.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Jamil B., Hasan R.S., Sarwari A.R., Burton J., Hewson R., Clegg C. Crimean-Congo hemorrhagic fever: experience at a tertiary care hospital in Karachi, Pakistan. Trans R Soc Trop Med Hyg. 2005;99:577–584. doi: 10.1016/j.trstmh.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Rehman A., Baloch N.U., Awais M. Eid-ul-Azha festival in Pakistan: a vulnerable time for Crimean-Congo hemorrhagic fever outbreak. Am J Inf Control. 2014;42:939–940. doi: 10.1016/j.ajic.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Mallhi T.H., Khan Y.H., Sarriff A., Khan A.H. Crimean-Congo haemorrhagic fever virus and Eid-Ul-Adha festival in Pakistan. The Lancet Infect Dis. 2016;16:1332–1333. doi: 10.1016/S1473-3099(16)30453-4. [DOI] [PubMed] [Google Scholar]
- 22.Leblebicioglu H., Sunbul M., Memish Z.A., Al-Tawfiq J.A., Bodur H., Ozkul A. Consensus report: preventive measures for Crimean-Congo Hemorrhagic Fever during Eid-al-Adha festival. Int J Infect Dis: IJID: Off Publ Int Soc Infect Dis. 2015;38:9–15. doi: 10.1016/j.ijid.2015.06.029. [DOI] [PubMed] [Google Scholar]
- 23.Mertens M., Schmidt K., Ozkul A., Groschup M.H. The impact of Crimean-Congo hemorrhagic fever virus on public health. Antivir Res. 2013;98:248–260. doi: 10.1016/j.antiviral.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 24.Sidwell R.W., Smee D.F. Viruses of the Bunya- and Togaviridae families: potential as bioterrorism agents and means of control. Antivir Res. 2003;57:101–111. doi: 10.1016/s0166-3542(02)00203-6. [DOI] [PubMed] [Google Scholar]
- 25.Zilinskas R.A. Iraq's biological weapons. The past as future? Jama. 1997;278:418–424. [PubMed] [Google Scholar]
- 26.Bronze M.S., Huycke M.M., Machado L.J., Voskuhl G.W., Greenfield R.A. Viral agents as biological weapons and agents of bioterrorism. The Am J Medi Sci. 2002;323:316–325. doi: 10.1097/00000441-200206000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Burt F.J., Swanepoel R., Shieh W.J., Smith J.F., Leman P.A., Greer P.W. Immunohistochemical and in situ localization of Crimean-Congo hemorrhagic fever (CCHF) virus in human tissues and implications for CCHF pathogenesis. Arch Pathol Lab Med. 1997;121:839–846. [PubMed] [Google Scholar]
- 28.Swanepoel R., Gill D.E., Shepherd A.J., Leman P.A., Mynhardt J.H., Harvey S. The clinical pathology of Crimean-Congo hemorrhagic fever. Rev Infect Dis. 1989;11(Suppl 4):S794–S800. doi: 10.1093/clinids/11.supplement_4.s794. [DOI] [PubMed] [Google Scholar]
- 29.Bray M.D. Comparative Pathogenesis of Crimean Congo Hemorrhagic Fever and Ebola Hemorrhagic Fever. In: Ergonul O., Whitehouse C.A., editors. Crimean-Congo hemorrhagic fever: a global perspective. Springer; 2007. pp. 221–231. [Google Scholar]
- 30.Baseler L., Chertow D.S., Johnson K.M., Feldmann H., Morens D.M. The pathogenesis of ebola virus disease. Ann Rev Pathol. 2017;12:387–418. doi: 10.1146/annurev-pathol-052016-100506. [DOI] [PubMed] [Google Scholar]
- 31.Ergonul O., Tuncbilek S., Baykam N., Celikbas A., Dokuzoguz B. Evaluation of serum levels of interleukin (IL)-6, IL-10, and tumor necrosis factor-alpha in patients with Crimean-Congo hemorrhagic fever. The J Infect Dis. 2006;193:941–944. doi: 10.1086/500836. [DOI] [PubMed] [Google Scholar]
- 32.Saksida A., Duh D., Wraber B., Dedushaj I., Ahmeti S., Avsic-Zupanc T. Interacting roles of immune mechanisms and viral load in the pathogenesis of crimean-congo hemorrhagic fever. Clin Vaccine Immunol: CVI. 2010;17:1086–1093. doi: 10.1128/CVI.00530-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Papa A., Bino S., Velo E., Harxhi A., Kota M., Antoniadis A. Cytokine levels in Crimean-Congo hemorrhagic fever. J Clin Virol: Off Publ Pan Am Soc Clin Virol. 2006;36:272–276. doi: 10.1016/j.jcv.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 34.Papa A., Tsergouli K., Caglayik D.Y., Bino S., Como N., Uyar Y. Cytokines as biomarkers of Crimean-Congo hemorrhagic fever. J Med Virol. 2016;88:21–27. doi: 10.1002/jmv.24312. [DOI] [PubMed] [Google Scholar]
- 35.Connolly-Andersen A.M., Douagi I., Kraus A.A., Mirazimi A. Crimean Congo hemorrhagic fever virus infects human monocyte-derived dendritic cells. Virology. 2009;390:157–162. doi: 10.1016/j.virol.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 36.Peyrefitte C.N., Perret M., Garcia S., Rodrigues R., Bagnaud A., Lacote S. Differential activation profiles of Crimean-Congo hemorrhagic fever virus- and Dugbe virus-infected antigen-presenting cells. The J Gen Virol. 2010;91:189–198. doi: 10.1099/vir.0.015701-0. [DOI] [PubMed] [Google Scholar]
- 37.Cevik M.A., Erbay A., Bodur H., Eren S.S., Akinci E., Sener K. Viral load as a predictor of outcome in Crimean-Congo hemorrhagic fever. Clin Infect Dis: Off Publ Infect Dis Soc Am. 2007;45:e96–e100. doi: 10.1086/521244. [DOI] [PubMed] [Google Scholar]
- 38.Duh D., Saksida A., Petrovec M., Ahmeti S., Dedushaj I., Panning M. Viral load as predictor of Crimean-Congo hemorrhagic fever outcome. Emerg Infect Dis. 2007;13:1769–1772. doi: 10.3201/eid1311.070222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Papa A., Drosten C., Bino S., Papadimitriou E., Panning M., Velo E. Viral load and Crimean-Congo hemorrhagic fever. Emerg Infect Dis. 2007;13:805–806. doi: 10.3201/eid1305.061588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Papa A., Dalla V., Papadimitriou E., Kartalis G.N., Antoniadis A. Emergence of Crimean-Congo haemorrhagic fever in Greece. Clin Microbiol Infect: Off Publ Eur Soc Clin Microbiol Infect Dis. 2010;16:843–847. doi: 10.1111/j.1469-0691.2009.02996.x. [DOI] [PubMed] [Google Scholar]
- 41.Ergunay K., Kocak Tufan Z., Bulut C., Kinikli S., Demiroz A.P., Ozkul A. Antibody responses and viral load in patients with Crimean-Congo hemorrhagic fever: a comprehensive analysis during the early stages of the infection. Diagn Microbiol Infect Dis. 2014;79:31–36. doi: 10.1016/j.diagmicrobio.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 42.Kaya A., Engin A., Guven A.S., Icagasioglu F.D., Cevit O., Elaldi N. Crimean-Congo hemorrhagic fever disease due to tick bite with very long incubation periods. Int J Infect Dis: IJID: Off Publ Int Soc Infect Dis. 2011;15:e449–e452. doi: 10.1016/j.ijid.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 43.Papa A., Yagci Caglayik D., Christova I., Tsergouli K., Korukluoglu G., Uyar Y. Crimean-Congo hemorrhagic fever: CXCL10 correlates with the viral load. J Med Virol. 2015;87:899–903. doi: 10.1002/jmv.24141. [DOI] [PubMed] [Google Scholar]
- 44.Yilmaz M., Aydin K., Akdogan E., Sucu N., Sonmez M., Omay S.B. Peripheral blood natural killer cells in Crimean-Congo hemorrhagic fever. J Clin Virol: Off Publ Pan Am Soc Clin Virol. 2008;42:415–417. doi: 10.1016/j.jcv.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 45.Akinci E., Yilmaz M., Bodur H., Onguru P., Bayazit F.N., Erbay A. Analysis of lymphocyte subgroups in Crimean-Congo hemorrhagic fever. Int J Infect Dis: IJID: Off Publ Int Soc Infect Dis. 2009;13:560–563. doi: 10.1016/j.ijid.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 46.Bente D.A., Alimonti J.B., Shieh W.J., Camus G., Stroher U., Zaki S. Pathogenesis and immune response of Crimean-Congo hemorrhagic fever virus in a STAT-1 knockout mouse model. J Virol. 2010;84:11089–11100. doi: 10.1128/JVI.01383-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bereczky S., Lindegren G., Karlberg H., Akerstrom S., Klingstrom J., Mirazimi A. Crimean-Congo hemorrhagic fever virus infection is lethal for adult type I interferon receptor-knockout mice. J Gen Virol. 2010;91:1473–1477. doi: 10.1099/vir.0.019034-0. [DOI] [PubMed] [Google Scholar]
- 48.Simon M., Falk K.I., Lundkvist A., Mirazimi A. Exogenous nitric oxide inhibits Crimean Congo hemorrhagic fever virus. Virus Res. 2006;120:184–190. doi: 10.1016/j.virusres.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 49.Smirnova S.E., Zubri G.L., Savinov A.P., Chumakov M.P. Pathogenesis of experimental Crimean hemorrhagic fever infection in newborn white mice. Acta Virol. 1973;17:409–415. [PubMed] [Google Scholar]
- 50.Gonzalez J.P., Wilson M.L., Cornet J.P., Camicas J.L. Host-passage-induced phenotypic changes in crimean-congo haemorrhagic fever virus. Res Virol. 1995;146:131–140. doi: 10.1016/0923-2516(96)81082-x. [DOI] [PubMed] [Google Scholar]
- 51.Akira S. Functional roles of STAT family proteins: lessons from knockout mice. Stem Cells. 1999;17:138–146. doi: 10.1002/stem.170138. [DOI] [PubMed] [Google Scholar]
- 52.Butenko AM, Chumakov MP, Smirnova SE, Vasilenko SM, Zavodova TI, Tkachenko EA, et al. Isolation of Crimean hemorrhagic fever virus from blood of patients and corpse material (from 1968–1969 investigation data) in Orstov, Astrakhan Oblast, and Bulgaria. Mater 3 Oblast Nauchn Prakt Konf (Rostov-on-Don, May 1970); 1970. p. 6–25. [Russian, translated NAMRU-3 T522].
- 53.Fagbami A.H., Tomori O., Fabiyi A., Isoun T.T. Experimantal Congo virus (Ib-AN 7620) infection in primates. Virologie. 1975;26:33–37. [PubMed] [Google Scholar]
- 54.Garcia-Sastre A. Diversity, replication, pathogenicity and cell biology of Crimean Congo hemorrhagic fever virus. 2010.
- 55.Yilmaz R., Kundak A.A., Ozer S., Esmeray H. Successful treatment of severe Crimean-Congo hemorrhagic fever with supportive measures without ribavirin and hypothermia. J Clin Virol: Off Publ Pan Am Soc Clin Virol. 2009;44:181–182. doi: 10.1016/j.jcv.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 56.Watts D.M., Ussery M.A., Nash D., Peters C.J. Inhibition of Crimean-Congo hemorrhagic fever viral infectivity yields in vitro by ribavirin. The Am J Trop Med Hyg. 1989;41:581–585. doi: 10.4269/ajtmh.1989.41.581. [DOI] [PubMed] [Google Scholar]
- 57.Mardani M., Jahromi M.K., Naieni K.H., Zeinali M. The efficacy of oral ribavirin in the treatment of crimean-congo hemorrhagic fever in Iran. Clin Infect Dis: Off Publ Infect Dis Soc Am. 2003;36:1613–1618. doi: 10.1086/375058. [DOI] [PubMed] [Google Scholar]
- 58.Maltezou H.C., Papa A. Crimean-Congo hemorrhagic fever: epidemiological trends and controversies in treatment. BMC Med. 2011;9:131. doi: 10.1186/1741-7015-9-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koksal I., Yilmaz G., Aksoy F., Aydin H., Yavuz I., Iskender S. The efficacy of ribavirin in the treatment of Crimean-Congo hemorrhagic fever in Eastern Black Sea region in Turkey. J Clin Virol: Off Publ Pan Am Soc Clin Virol. 2010;47:65–68. doi: 10.1016/j.jcv.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 60.Papa A., Papadimitriou E., Christova I. The Bulgarian vaccine Crimean-Congo haemorrhagic fever virus strain. Scandinavian J Infect Dis. 2011;43:225–229. doi: 10.3109/00365548.2010.540036. [DOI] [PubMed] [Google Scholar]
- 61.Christova I., Kovacheva O., Greorgieva G., Ivanova S., Argirov D. Vaccine against Congo-Crimean haemorhagic fever virus-Bulgarian input in fighting the disease. Probl Infect Parasit Dis. 2010;37:7–8. [Google Scholar]
- 62.Papa A., Pappa S., Panayotova E., Papadopoulou E., Christova I. Molecular epidemiology of Crimean-Congo hemorrhagic fever in Bulgaria–An update. J Med Virol. 2016;88:769–773. doi: 10.1002/jmv.24400. [DOI] [PubMed] [Google Scholar]
- 63.Keshtkar-Jahromi M., Kuhn J.H., Christova I., Bradfute S.B., Jahrling P.B., Bavari S. Crimean-Congo hemorrhagic fever: current and future prospects of vaccines and therapies. Antivir Res. 2011;90:85–92. doi: 10.1016/j.antiviral.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 64.Mousavi-Jazi M., Karlberg H., Papa A., Christova I., Mirazimi A. Healthy individuals' immune response to the Bulgarian Crimean-Congo hemorrhagic fever virus vaccine. Vaccine. 2012;30:6225–6229. doi: 10.1016/j.vaccine.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 65.Hemachudha T., Griffin D.E., Giffels J.J., Johnson R.T., Moser A.B., Phanuphak P. Myelin basic protein as an encephalitogen in encephalomyelitis and polyneuritis following rabies vaccination. The New England J Med. 1987;316:369–374. doi: 10.1056/NEJM198702123160703. [DOI] [PubMed] [Google Scholar]
- 66.Hewson R., Chamberlain J., Mioulet V., Lloyd G., Jamil B., Hasan R. Crimean-Congo haemorrhagic fever virus: sequence analysis of the small RNA segments from a collection of viruses world wide. Virus Res. 2004;102:185–189. doi: 10.1016/j.virusres.2003.12.035. [DOI] [PubMed] [Google Scholar]
- 67.Carter S.D., Surtees R., Walter C.T., Ariza A., Bergeron E., Nichol S.T. Structure, function, and evolution of the Crimean-Congo hemorrhagic fever virus nucleocapsid protein. J Virol. 2012;86:10914–10923. doi: 10.1128/JVI.01555-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schmaljohn C.S., Nichol S.T. Bunyaviridae. In: Knipe D.M., Howley P.M., editors. Fields virology. Williams & Wilkins; Philadelphia: 2007. pp. 1741–1789. [Google Scholar]
- 69.Maes P., Clement J., Van Ranst M. Recent approaches in hantavirus vaccine development. Expert Rev Vaccines. 2009;8:67–76. doi: 10.1586/14760584.8.1.67. [DOI] [PubMed] [Google Scholar]
- 70.Boshra H., Lorenzo G., Rodriguez F., Brun A. A DNA vaccine encoding ubiquitinated Rift Valley fever virus nucleoprotein provides consistent immunity and protects IFNAR(-/-) mice upon lethal virus challenge. Vaccine. 2011;29:4469–4475. doi: 10.1016/j.vaccine.2011.04.043. [DOI] [PubMed] [Google Scholar]
- 71.Srinivasan P., Kumar S.P., Karthikeyan M., Jeyakanthan J., Jasrai Y.T., Pandya H.A. Epitope-based immunoinformatics and molecular docking studies of nucleocapsid protein and ovarian tumor domain of crimean-congo hemorrhagic Fever virus. Fron Genet. 2011;2:72. doi: 10.3389/fgene.2011.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sanchez A.J., Vincent M.J., Nichol S.T. Characterization of the glycoproteins of Crimean-Congo hemorrhagic fever virus. J Virol. 2002;76:7263–7275. doi: 10.1128/JVI.76.14.7263-7275.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Altamura L.A., Bertolotti-Ciarlet A., Teigler J., Paragas J., Schmaljohn C.S., Doms R.W. Identification of a novel C-terminal cleavage of Crimean-Congo hemorrhagic fever virus PreGN that leads to generation of an NSM protein. J Virol. 2007;81:6632–6642. doi: 10.1128/JVI.02730-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vincent M.J., Sanchez A.J., Erickson B.R., Basak A., Chretien M., Seidah N.G. Crimean-Congo hemorrhagic fever virus glycoprotein proteolytic processing by subtilase SKI-1. J Virol. 2003;77:8640–8649. doi: 10.1128/JVI.77.16.8640-8649.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sanchez A.J., Vincent M.J., Erickson B.R., Nichol S.T. Crimean-congo hemorrhagic fever virus glycoprotein precursor is cleaved by Furin-like and SKI-1 proteases to generate a novel 38-kilodalton glycoprotein. J Virol. 2006;80:514–525. doi: 10.1128/JVI.80.1.514-525.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Haferkamp S., Fernando L., Schwarz T.F., Feldmann H., Flick R. Intracellular localization of Crimean-Congo Hemorrhagic Fever (CCHF) virus glycoproteins. Virol J. 2005;2:42. doi: 10.1186/1743-422X-2-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bertolotti-Ciarlet A., Smith J., Strecker K., Paragas J., Altamura L.A., McFalls J.M. Cellular localization and antigenic characterization of crimean-congo hemorrhagic fever virus glycoproteins. J Virol. 2005;79:6152–6161. doi: 10.1128/JVI.79.10.6152-6161.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ahmed A.A., McFalls J.M., Hoffmann C., Filone C.M., Stewart S.M., Paragas J. Presence of broadly reactive and group-specific neutralizing epitopes on newly described isolates of Crimean-Congo hemorrhagic fever virus. J Gen Virol. 2005;86:3327–3336. doi: 10.1099/vir.0.81175-0. [DOI] [PubMed] [Google Scholar]
- 79.Ghiasi S.M., Salmanian A.H., Chinikar S., Zakeri S. Mice orally immunized with a transgenic plant expressing the glycoprotein of Crimean-Congo hemorrhagic fever virus. Clin Vaccine Immunol: CVI. 2011;18:2031–2037. doi: 10.1128/CVI.05352-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Casals J., Tignor G.H. Neutralization and hemagglutination-inhibition tests with Crimean hemorrhagic fever-Congo virus. Proc Soc Exp Biol Med Soc Exp Biol Med. 1974;145:960–966. doi: 10.3181/00379727-145-37933. [DOI] [PubMed] [Google Scholar]
- 81.Conlan A.J., Brooks Pollock E., McKinley T.J., Mitchell A.P., Jones G.J., Vordermeier M. Potential benefits of cattle vaccination as a supplementary control for bovine tuberculosis. PLoS Comput Biol. 2015;11:e1004038. doi: 10.1371/journal.pcbi.1004038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hasan N.H., Ignjatovic J., Peaston A., Hemmatzadeh F. Avian influenza virus and DIVA strategies. Viral Immunol. 2016;29:198–211. doi: 10.1089/vim.2015.0127. [DOI] [PubMed] [Google Scholar]
- 83.Barnett P.V., Geale D.W., Clarke G., Davis J., Kasari T.R. A review of OIE country status recovery using vaccinate-to-live versus vaccinate-to-die foot-and-mouth disease response policies i: benefits of higher potency vaccines and associated NSP DIVA test systems in post-outbreak surveillance. Transbound Emerg Dis. 2015;62:367–387. doi: 10.1111/tbed.12166. [DOI] [PubMed] [Google Scholar]
- 84.Dowall S.D., Richards K.S., Graham V.A., Chamberlain J., Hewson R. Development of an indirect ELISA method for the parallel measurement of IgG and IgM antibodies against Crimean-Congo haemorrhagic fever (CCHF) virus using recombinant nucleoprotein as antigen. J Virol Methods. 2012;179:335–341. doi: 10.1016/j.jviromet.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 85.Marriott A.C., Polyzoni T., Antoniadis A., Nuttall P.A. Detection of human antibodies to Crimean-Congo haemorrhagic fever virus using expressed viral nucleocapsid protein. J Gen Virol. 1994;75(Pt 9):2157–2161. doi: 10.1099/0022-1317-75-9-2157. [DOI] [PubMed] [Google Scholar]
- 86.Saijo M., Tang Q., Shimayi B., Han L., Zhang Y., Asiguma M. Recombinant nucleoprotein-based serological diagnosis of Crimean-Congo hemorrhagic fever virus infections. J Med Virol. 2005;75:295–299. doi: 10.1002/jmv.20270. [DOI] [PubMed] [Google Scholar]
- 87.Magurano F., Nicoletti L. Humoral response in Toscana virus acute neurologic disease investigated by viral-protein-specific immunoassays. Clin Diagn Lab Immunol. 1999;6:55–60. doi: 10.1128/cdli.6.1.55-60.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schwarz T.F., Gilch S., Pauli C., Jager G. Immunoblot detection of antibodies to Toscana virus. J Med Virol. 1996;49:83–86. doi: 10.1002/(SICI)1096-9071(199606)49:2<83::AID-JMV2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 89.Vapalahti O., Kallio-Kokko H., Narvanen A., Julkunen I., Lundkvist A., Plyusnin A. Human B-cell epitopes of Puumala virus nucleocapsid protein, the major antigen in early serological response. J Med Virol. 1995;46:293–303. doi: 10.1002/jmv.1890460402. [DOI] [PubMed] [Google Scholar]
- 90.Shepherd A.J., Swanepoel R., Leman P.A. Antibody response in Crimean-Congo hemorrhagic fever. Rev Infect Dis. 1989;11(Suppl 4):S801–S806. doi: 10.1093/clinids/11.supplement_4.s801. [DOI] [PubMed] [Google Scholar]
- 91.Hinkula J., Devignot S., Akerstrom S., Karlberg H., Wattrang E., Bereczky S. Immunization with DNA plasmids coding for crimean-congo hemorrhagic fever virus capsid and envelope proteins and/or virus-like particles induces protection and survival in challenged mice. J Virol. 2017 doi: 10.1128/JVI.02076-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kortekaas J., Vloet R.P., McAuley A.J., Shen X., Bosch B.J., de Vries L. Crimean-Congo hemorrhagic fever virus subunit vaccines induce high levels of neutralizing antibodies but no protection in STAT1 knockout mice. Vector Borne Zoonotic Dis. 2015;15:759–764. doi: 10.1089/vbz.2015.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Andersson I., Lundkvist A., Haller O., Mirazimi A. Type I interferon inhibits Crimean-Congo hemorrhagic fever virus in human target cells. J Med Virol. 2006;78:216–222. doi: 10.1002/jmv.20530. [DOI] [PubMed] [Google Scholar]
- 94.Bowick G.C., Airo A.M., Bente D.A. Expression of interferon-induced antiviral genes is delayed in a STAT1 knockout mouse model of Crimean-Congo hemorrhagic fever. Virol J. 2012;9:122. doi: 10.1186/1743-422X-9-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vannie P., Capua I., Le Potier M.F., Mackay D.K., Muylkens B., Parida S. Marker vaccines and the impact of their use on diagnosis and prophylactic measures. Revue Sci Tech. 2007;26:351–372. [PubMed] [Google Scholar]
- 96.van Oirschot J.T. Present and future of veterinary viral vaccinology: a review. Veter Quart. 2001;23:100–108. doi: 10.1080/01652176.2001.9695094. [DOI] [PubMed] [Google Scholar]
- 97.Canakoglu N., Berber E., Tonbak S., Ertek M., Sozdutmaz I., Aktas M. Immunization of knock-out alpha/beta interferon receptor mice against high lethal dose of Crimean-Congo hemorrhagic fever virus with a cell culture based vaccine. PLoS Neglected Trop Dis. 2015;9:e0003579. doi: 10.1371/journal.pntd.0003579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Buttigieg K.R., Dowall S.D., Findlay-Wilson S., Miloszewska A., Rayner E., Hewson R. A novel vaccine against Crimean-Congo Haemorrhagic Fever protects 100% of animals against lethal challenge in a mouse model. PloS One. 2014;9:e91516. doi: 10.1371/journal.pone.0091516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dowall S.D., Buttigieg K.R., Findlay-Wilson S.J., Rayner E., Pearson G., Miloszewska A. A Crimean-Congo hemorrhagic fever (CCHF) viral vaccine expressing nucleoprotein is immunogenic but fails to confer protection against lethal disease. Hum Vaccines Immunother. 2016;12:519–527. doi: 10.1080/21645515.2015.1078045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Spik K., Shurtleff A., McElroy A.K., Guttieri M.C., Hooper J.W., SchmalJohn C. Immunogenicity of combination DNA vaccines for Rift Valley fever virus, tick-borne encephalitis virus, Hantaan virus, and Crimean Congo hemorrhagic fever virus. Vaccine. 2006;24:4657–4666. doi: 10.1016/j.vaccine.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 101.Sahib M.M. Winnipeg; Canada: 2010. Rapid development of optimized recombinant adenoviral vaccines biosafety level 4 viruses: University of Manitoba. [Google Scholar]
- 102.Ergonul O. Crimean-Congo haemorrhagic fever. Lancet Infect Dis. 2006;6:203–214. doi: 10.1016/S1473-3099(06)70435-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gunes T., Engin A., Poyraz O., Elaldi N., Kaya S., Dokmetas I. Crimean-Congo hemorrhagic fever virus in high-risk population, Turkey. Emerging Infect Dis. 2009;15:461–464. doi: 10.3201/eid1503.080687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Smith G.L., Moss B. Infectious poxvirus vectors have capacity for at least 25 000 base pairs of foreign DNA. Gene. 1983;25:21–28. doi: 10.1016/0378-1119(83)90163-4. [DOI] [PubMed] [Google Scholar]
- 105.Moss B., Carroll M.W., Wyatt L.S., Bennink J.R., Hirsch V.M., Goldstein S. Host range restricted, non-replicating vaccinia virus vectors as vaccine candidates. Adv Exp Med Biol. 1996;397:7–13. doi: 10.1007/978-1-4899-1382-1_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cottingham M.G., Carroll M.W. Recombinant MVA vaccines: dispelling the myths. Vaccine. 2013;31:4247–4251. doi: 10.1016/j.vaccine.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 107.Stickl H., Hochstein-Mintzel V., Mayr A., Huber H.C., Schafer H., Holzner A. [MVA vaccination against smallpox: clinical tests with an attenuated live vaccinia virus strain (MVA) (author's transl)] Deut Med Wochenschr. 1974;99:2386–2392. doi: 10.1055/s-0028-1108143. [DOI] [PubMed] [Google Scholar]
- 108.Alcock R., Cottingham M.G., Rollier C.S., Furze J., De Costa S.D., Hanlon M. Long-term thermostabilization of live poxviral and adenoviral vaccine vectors at supraphysiological temperatures in carbohydrate glass. Sci Translational Med. 2010;2:19ra2. doi: 10.1126/scitranslmed.3000490. [DOI] [PubMed] [Google Scholar]
- 109.Draper S.J., Heeney J.L. Viruses as vaccine vectors for infectious diseases and cancer. Nat Rev Microbiol. 2010;8:62–73. doi: 10.1038/nrmicro2240. [DOI] [PubMed] [Google Scholar]
- 110.Sutter G., Wyatt L.S., Foley P.L., Bennink J.R., Moss B. A recombinant vector derived from the host range-restricted and highly attenuated MVA strain of vaccinia virus stimulates protective immunity in mice to influenza virus. Vaccine. 1994;12:1032–1040. doi: 10.1016/0264-410x(94)90341-7. [DOI] [PubMed] [Google Scholar]
- 111.Dowall S.D., Graham V.A., Rayner E., Hunter L., Watson R., Taylor I. Protective effects of a Modified Vaccinia Ankara-based vaccine candidate against Crimean-Congo Haemorrhagic Fever virus require both cellular and humoral responses. PloS One. 2016;11:e0156637. doi: 10.1371/journal.pone.0156637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Farina S.F., Gao G.P., Xiang Z.Q., Rux J.J., Burnett R.M., Alvira M.R. Replication-defective vector based on a chimpanzee adenovirus. J Virol. 2001;75:11603–11613. doi: 10.1128/JVI.75.23.11603-11613.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vogels R., Zuijdgeest D., van Rijnsoever R., Hartkoorn E., Damen I., de Bethune M.P. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of preexisting adenovirus immunity. J Virol. 2003;77:8263–8271. doi: 10.1128/JVI.77.15.8263-8271.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Capone S., D'Alise A.M., Ammendola V., Colloca S., Cortese R., Nicosia A. Development of chimpanzee adenoviruses as vaccine vectors: challenges and successes emerging from clinical trials. Exp Rev Vaccines. 2013;12:379–393. doi: 10.1586/erv.13.15. [DOI] [PubMed] [Google Scholar]
- 115.Dudareva M., Andrews L., Gilbert S.C., Bejon P., Marsh K., Mwacharo J. Prevalence of serum neutralizing antibodies against chimpanzee adenovirus 63 and human adenovirus 5 in Kenyan children, in the context of vaccine vector efficacy. Vaccine. 2009;27:3501–3504. doi: 10.1016/j.vaccine.2009.03.080. [DOI] [PubMed] [Google Scholar]
- 116.Ersching J., Hernandez M.I., Cezarotto F.S., Ferreira J.D., Martins A.B., Switzer W.M. Neutralizing antibodies to human and simian adenoviruses in humans and New-World monkeys. Virology. 2010;407:1–6. doi: 10.1016/j.virol.2010.07.043. [DOI] [PubMed] [Google Scholar]
- 117.Xiang Z., Li Y., Cun A., Yang W., Ellenberg S., Switzer W.M. Chimpanzee adenovirus antibodies in humans, sub-Saharan Africa. Emerg Infect Dis. 2006;12:1596–1599. doi: 10.3201/eid1210.060078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Harrison R.L., Jarvis D.L. Protein N-glycosylation in the baculovirus-insect cell expression system and engineering of insect cells to produce “mammalianized” recombinant glycoproteins. Adv Virus Res. 2006;68:159–191. doi: 10.1016/S0065-3527(06)68005-6. [DOI] [PubMed] [Google Scholar]
- 119.Devignot S., Bergeron E., Nichol S., Mirazimi A., Weber F. A virus-like particle system identifies the endonuclease domain of Crimean-Congo hemorrhagic fever virus. J Virol. 2015;89:5957–5967. doi: 10.1128/JVI.03691-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hewson R., Gmyl A., Gmyl L., Smirnova S.E., Karganova G., Jamil B. Evidence of segment reassortment in Crimean-Congo haemorrhagic fever virus. J Gen Virol. 2004;85:3059–3070. doi: 10.1099/vir.0.80121-0. [DOI] [PubMed] [Google Scholar]
- 121.Oany A.R., Ahmad S.A., Hossain M.U., Jyoti T.P. Identification of highly conserved regions in L-segment of Crimean-Congo hemorrhagic fever virus and immunoinformatic prediction about potential novel vaccine. Adv Appl Bioinfor Chem: AABC. 2015;8:1–10. doi: 10.2147/AABC.S75250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tipu H.N. Immunoinformatic analysis of crimean congo hemorrhagic fever virus glycoproteins and epitope prediction for synthetic peptide vaccine. J Coll Phys Surg–Pakistan: JCPSP. 2016;26:108–112. [PubMed] [Google Scholar]
- 123.van Kasteren P.B., Bailey-Elkin B.A., James T.W., Ninaber D.K., Beugeling C., Khajehpour M. Deubiquitinase function of arterivirus papain-like protease 2 suppresses the innate immune response in infected host cells. Proc Nat Acad Sci USA. 2013;110:E838–E847. doi: 10.1073/pnas.1218464110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bergeron E., Albarino C.G., Khristova M.L., Nichol S.T. Crimean-Congo hemorrhagic fever virus-encoded ovarian tumor protease activity is dispensable for virus RNA polymerase function. J Virol. 2010;84:216–226. doi: 10.1128/JVI.01859-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Brehm M.A., Shultz L.D., Greiner D.L. Humanized mouse models to study human diseases. Curr Opin Endocrinol Diabetes Obes. 2010;17:120–125. doi: 10.1097/MED.0b013e328337282f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Snoy P.J. Establishing efficacy of human products using animals: the US food and drug administration's “animal rule”. Veter Pathol. 2010;47:774–778. doi: 10.1177/0300985810372506. [DOI] [PubMed] [Google Scholar]
- 127.Papa A., Mirazimi A., Koksal I., Estrada-Pena A., Feldmann H. Recent advances in research on Crimean-Congo hemorrhagic fever. J Clin Virol: Off Publ Pan Am Soc Clin Virol. 2015;64:137–143. doi: 10.1016/j.jcv.2014.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]