Abstract
Infants and young children with acute onset of wheezing and reduced respiratory airflows are often diagnosed with obstruction and inflammation of the small bronchiolar airways, ie bronchiolitis. The most common aetological agents causing bronchiolitis in young children are the respiratory viruses, and of the commonly encountered respiratory viruses, respiratory syncytial virus (RSV) has a propensity for causing bronchiolitis. Indeed, RSV bronchiolitis remains the major reason why previously healthy infants are admitted to hospital. Why RSV infection is such a predominant cause of bronchiolitis is the subject of this review. By reviewing the available histopathology of RSV bronchiolitis, both in humans and relevant animal models, we identify hallmark features of RSV infection of the distal airways and focus attention on the consequences of columnar cell cytopathology occurring in the bronchioles, which directly impacts the development of bronchiolar obstruction, inflammation and disease. Copyright © 2014 Pathological Society of Great Britain and Ireland. Published by John Wiley & Sons, Ltd.
Keywords: respiratory syncytial virus, airway obstruction, bronchiolitis, acute lung disease
Severity of pulmonary disease caused by respiratory viruses often depends on the lung region infected
Rhinoviruses, paramyxoviruses, coronaviruses and influenza viruses are commonly encountered respiratory viruses responsible for causing most respiratory tract disease in human populations 1, 2. Viruses from these families infect, replicate and spread in the epithelial cells of the respiratory tract mucosa. Infection of upper respiratory tract regions, such as the nasopharynx, the paranasal sinuses and the Eustachian tubes of the inner ear, can cause characteristic symptoms of the common cold, including rhinorrhea, coryza and otitis media. Virus infection of the lower respiratory tract results in the worsening of pulmonary symptoms, including coughing, tachypnoea, laboured breathing and an audible wheeze. However, it is bronchiolitis and pneumonia (infection and inflammation of the distal bronchiolar airways and alveolar lung regions, respectively) that results in the most severe and potentially life‐threatening pulmonary disease. Bronchiolitis causes obstruction of the already narrow diameter lumens of the bronchiolar airways, reducing normal airflow through the bronchioles. Airway constriction, in addition to the obstructive event, may also be a contributing factor to functional narrowing of the small airways, and occurs in subgroups of older infected infants. Airway obstruction causes a reduced capacity for exhalation, leading to lung gas trapping, lung hyperexpansion, increased respiratory rates and rapid decline in lung function. Additionally, the trapped air is resorbed, causing micro‐ and subsegmental atelectasis, which results in worsening lung disease, ventilation–perfusion mismatch and further hypoxaemia due to intrapulmonary shunting. Respiratory viruses can also cause pneumonia, either directly, by infecting the alveolar epithelium, or by spillover of distal airway inflammation into the alveolus. Resulting alveolar inflammation, injury and oedema hinders efficient alveolar gas‐exchange processes, resulting in severe pulmonary disease, including hypoxia, respiratory failure and death.
Susceptibility of the bronchiolar airways to virus infection
Respiratory virus infections in infants and young children often progress to the distal airways, resulting in the diagnosis of acute bronchiolitis. Indeed, acute bronchiolitis is so common in early life that it remains the single most common reason for infants and young children to be admitted to hospital 3, 4, 5, 6, 7, 8, 9. Why respiratory virus infection so frequently progresses from clinically manageable involvement of the upper respiratory tract to the more severe involvement of the lower respiratory tract is largely attributed to the immature immune systems of young naive hosts and the general lack of functionally RSV‐protective maternal transplacental antibody. Another explanation for why infant lungs are more likely to develop severe distal airway disease is the smaller dimensions of the airways of the infant lung. Specifically, the average diameter of an adult respiratory bronchiole is approximately 250 µm, compared to 120 µm for those of a 2–4 month‐old infant, thus greatly increasing the potential impact of obstruction on the infant bronchiolar lumen during virus‐induced cytopathology and inflammation 10, 11. The infant lung also has poor development of collateral ventilation of alveolar regions, which in adult lungs enables the ventilation of lung regions experiencing obstructed airflow 11. Therefore, the immature immune system, combined with the smaller physical dimensions of the airways, is a logical explanation of why infants are more likely to develop severe distal airway disease during respiratory virus infection than older children or adults.
Although infants are more likely to develop severe disease during respiratory virus infections, overwhelming clinical and epidemiological evidence indicates that specific respiratory viruses are responsible for causing the majority of severe airway disease in infants. Respiratory syncytial virus (RSV) is a notorious cause of infant bronchiolitis, so much so that young children with symptoms of severe airway disease occurring during a predictable winter epidemic will often be assumed by clinicians to be infected by RSV, even before identity of the aetiological agent has been confirmed. Of infants and young children hospitalized with bronchiolitis, 60–80% will be infected by RSV 4, 5, 6. RSV also causes more severe and prolonged bronchiolitis compared to that caused by other aetiologies, including rhinoviruses or the closely related human metapneumoviruses or parainfluenza viruses (PIV) 12, 13. A recent study of young children with acute respiratory illness found that those infected with RSV had twice as many emergency room visits and six times more hospitalizations than those with seasonal influenza virus infections 14. The importance of RSV is further highlighted by the observations that when multiple potentially pathogenic viruses are identified along with RSV in an infant with bronchiolitis, the disease course and severity are indistinguishable from those caused by infection by RSV alone.
Risk factors for an upper respiratory tract RSV infection progressing to severe distal airway disease have been identified. Very young age (<3 months) at the time of infection, premature birth, underlying immunodeficiency or underlying cardiopulmonary disease are important risk factors for severe RSV disease. Of these, very young age at the time of infection is the most significant, with 80% of hospitalized RSV‐infected infants under the age of 2 months being previously healthy 5, 12, 15. Why RSV has an increased propensity to cause more frequent and more severe bronchiolitis in previously healthy infants is due to both increased population exposure to infection from the virus at early ages and also to increased likelihood of severe disease once infected. The underlying reason for this increased severity of individual infections with RSV is unclear. Epidemiological studies suggest that environmental exposure of infants and young children to respiratory viruses is no more common for RSV than for other viruses, especially considering the high frequency of exposure to rhinoviruses 2, 16.
The propensity of RSV to cause more severe bronchiolitis suggests there is something unusual about RSV and its ability to infect and cause disease in the distal airways of young infants. Significant understanding of the genetic and biological properties of RSV, and how infection impacts host cells, has been achieved since the virus was first isolated 50 years ago 17. However, the precise details of how RSV infection causes bronchiolitis are poorly understood. Here, we focus on the current knowledge of RSV bronchiolitis and describe animal models which may provide further understanding of how RSV causes distal airway disease. By describing pathological outcomes of RSV infection of bronchiolar airways obtained from natural RSV infection of humans and experimental models, we aim to define hallmark features of RSV bronchiolitis. Finally, we discuss recent data from our own laboratory suggesting that expression of specific RSV‐encoded genes may, in part, be responsible for the increased propensity of RSV to infect, spread and cause severe obstructive bronchiolar airway disease. We focus our discussions of RSV infection on distal bronchiolar airways, as this pathology is responsible for the most severe and life‐threatening aspects of disease associated with RSV infection.
RSV bronchiolitis: significance and clinical disease
Identified over 50 years ago from airway samples of young children with severe airway disease 17, RSV is now known to be responsible for significant global morbidity and mortality, with more children aged < 1 year dying from RSV infection than from any other single pathogen besides malaria 6, 18. Although RSV infects humans of all ages, it is the very young, the immunocompromised and the elderly who experience the most severe consequences, and RSV‐associated severe airway disease remains the most common reason why previously healthy infants and young children will require hospitalization. Of the US birth cohort, 2–3% become hospitalized for RSV within their first year of life. One‐third of infants, likely by avoiding exposure, avoid infection during their first winter, only to become infected in their second winter, but by the age of 3 years all children will have experienced at least one RSV infection 3. World‐wide, RSV is estimated to be responsible for 34 million new paediatric cases of distal airway disease annually, resulting in approximately 200 000 paediatric deaths/year 5. While such RSV‐associated mortality rates are observed in countries with underdeveloped health care, in the USA and other developed countries, RSV mortality rates are significantly lower, due to the availability of appropriate hospital‐based supportive care and the safety net of mechanical ventilation. Nevertheless, RSV infection maintains a significant health burden on the US population, with an estimated 1.5 million outpatient visits/year attributable to RSV infections in the under‐5 year‐olds, and 75 000–125 000 hospitalizations, of which 1.5% (1500) require admission to paediatric intensive care units 5, 7, 8, 19. Appropriate supportive care for hospitalized RSV‐infected infants includes administration of supplemental oxygen and intravenous fluids, after which most RSV‐infected infants can be discharged, with an average hospital stay of 3.5 days 20, 21, 22. Immunocompromised individuals, or those with underlying cardiopulmonary disorders, suffer prolonged and more severe RSV infections.
The clinical presentation of acute symptoms of lower respiratory tract infection in infants, especially during the winter months, is a tell‐tale sign of RSV infection. However, diagnosis of acute RSV bronchiolitis, while often assumed, cannot be confirmed until a positive identification of RSV in patient‐derived samples is obtained, since lower respiratory tract symptoms indicative of RSV infection can also be due a number of other respiratory viruses, including parainfluenza viruses, influenza viruses, coronaviruses, human metapneumoviruses and, in some cases, rhinoviruses. Common upper airway symptoms of RSV infection include nasal congestion, voluminous rhinorrhea, otis media and intermittent fevers. The progression to RSV severe lower respiratory disease occurs quickly, with the mean duration of symptoms prior to hospitalization and requirement for mechanical ventilation being only 4 days. Progression of RSV infection from the upper airways into the lower airways is presumed, but not proven, to be via mechanical aspiration of infectious material, resulting in a worsening of disease symptoms, including onset of moderate tachypnoea, diffuse rhonchi, fine rales and wheezing. At this point in infection, chest X‐rays are normal and the disease can resolve within 1–2 weeks. However, further spread of infection into distal airway regions results in exacerbation of pulmonary disease. Respiratory rates increase and coughing and wheezing become more significant, with the development of severe tachypnoea and chest hyperexpansion. At this point, radiological evidence of gas trapping and peribronchial thickening are common, in combination with interstitial pneumonia. The clinical diagnosis for these severe symptoms is acute bronchiolitis, with or without evidence of pneumonia. Such symptoms of acute bronchiolitis and pneumonia can also be attributed to distal airway infection by several different respiratory virus families, requiring RSV detection to confirm diagnosis of RSV bronchiolitis. Standard radiological techniques are unable to distinguish between acute bronchiolitis caused by RSV versus that caused by infection by other respiratory viruses. Indeed, a recent computed tomography (CT)‐based study, designed to compare and contrast scans of patients exhibiting acute, infectious, distal airway disease, was unable to discriminate between distal airway disease caused by several respiratory viruses, including RSV 23, 24.
Histopathology of RSV bronchiolitis
Although currently available imaging techniques are unable to discriminate between RSV distal airway disease and that caused other respiratory viruses, histopathological evidence from infants who died from respiratory infections reveal more robust pathology in RSV‐infected bronchiolar airways. Common post‐mortem findings in RSV‐infected infant lungs are robust infection and cytopathology of the bronchiolar airway epithelium, with inflammatory cell infiltrates sufficient to constrict and obstruct the narrow‐diameter bronchiolar airway lumens (Figure 1). As early as 1965, Shedden and Emery 25 provided one of the first reports combining histopathology and RSV antigen localization in lung tissue from infants who had died from severe RSV airway disease. They showed that RSV antigen was localized predominately in the epithelial cells of the bronchiolar airways and the alveolar regions. Most notably, RSV infection of bronchiolar airway epithelium severely disrupted epithelial cell morphology, and large, multinucleated, polypoid epithelial cell masses were seen to protrude and slough into the infected bronchiolar lumen (Figure 1A). The authors described this observation as RSV infection causing 'bizarre giant cells to be cast off into the lumen of the bronchioles and alveoli'. Furthermore, the cast‐off giant cells retained RSV immunoreactivity and accumulated in the bronchiolar airway lumen, raising the possibility that this loose and infected cellular material may influence further spread of RSV infection.
Figure 1.
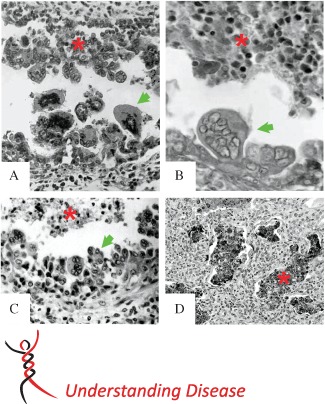
Histopathology of RSV‐infected small airways. Previously published histology images of common small airway lesions identified post mortem in human infant lungs infected with RSV. All examples describe disruption of the distal airway epithelium, with giant cell or polypoid formation (green arrows) and sloughed or protruding RSV antigen‐positive epithelial cells retained in the bronchiolar lumen (red asterisk). (A) A 1959 RSV case, showing bizarre dearrangement of the small airway epithelium, with multi‐nucleated, polypoid epithelial cells casting off into the airway lumen. Figure reproduced from 25. ‘Immunofluorescent evidence of respiratory syncytial virus infection in cases of giant‐cell bronchiolitis in children’, Vol. 89, Pages 343–347. Copyright © 1965 The Pathological Society of Great Britain and Ireland. This material is reproduced with permission of John Wiley & Sons, Inc. (B) A 1949 RSV case, demonstrating papilliary projections and intrabronchiolar syncytia contributing to the intraluminal cellular debris. Figure reproduced from 27. Reprinted by permission from Macmillan Publishers Ltd: Modern Pathology, ‘The histopathology of fatal untreated human respiratory syncytial virus infection’, Copyright 2007. (C) A pre‐1988 RSV case, showing virus‐induced airway epithelium injury, characterized by uneven proliferation of epithelial cells with protrusions entering into the bronchiolar lumen, creating a polypoid appearance. Figure reproduced from 30: KA Neilson and EJ Yunis, Fetal & Pediatric Pathology, [1990; 10 (4): 491–502], Copyright © 1990, Informa Healthcare. Reproduced with permission of Informa Healthcare. (D) Immunohistochemical detection of RSV antigen in exfoliated bronchiolar epithelial cells clogging the lumens of small airways. Reproduced from 28. Welliver et al, ‘Respiratory syncytial virus and influenza virus infections: observations from tissues of fatal infant cases’, The Pediatric Infectious Disease Journal, Vol. 27, Suppl. 10, pages S92–S96, with permission. Original magnifications = (A) × 150; (B) × 250; (C) × 650; (D) × 40
Whether the unusual epithelial cell cytopathology seen during RSV infection was a specific feature of RSV infection, or a general response of the epithelium to infection and inflammation common to other respiratory viruses, was addressed several years later by Zinserling 26, who compared lung histopathology from 250 autopsy cases of young children with acute respiratory infections, including RSV, influenza viruses, parainfluenza viruses and adenoviruses. The conclusion was that RSV caused the greatest degree of bronchiolar involvement and that RSV‐infected bronchiolar airway epithelial cells exhibited a peculiar morphology, with ‘RSV‐infected epithelial cells increasing in size and proliferating to result in nipple‐like outgrowths which occupy a considerable part of the bronchiolar lumen’. Both studies also provided clear evidence of RSV infection of the alveolar epithelium causing epithelial cell damage, and an associated polymorphonuclear cellular accumulation within the airway lumen. Alveolar macrophages were also shown to contain RSV antigen, although whether these macrophages were productively infected by RSV or simply contained ingested RSV protein remains unknown. It also remains unknown whether a true infection of macrophages by RSV might contribute to disease progression via promotion of inappropriate pro‐inflammatory responses. Together, these two early histopathology reports provided the first evidence that RSV infection significantly disrupted the bronchiolar and alveolar epithelium and altered, perhaps uniquely, the morphology of the epithelial cells in a manner which could potentially interfere with airflow to and from the lungs.
The early observations of unusual morphological consequences of RSV infection of airway epithelium have largely been confirmed by more detailed characterization 27, 28, 29. RSV antigens localize almost exclusively to the ciliated and non‐ciliated columnar cells of the proximal and distal airways 27, 29, 30. In the larger cartilaginous airways, ciliated columnar cells were the predominant target of RSV infection, with mucin‐containing goblet cells and underlying basal epithelial cells not expressing RSV antigens. In the non‐cartilaginous distal airways, ciliated and non‐ciliated epithelial cells were infected by RSV, with more extensive infection of the epithelium often encompassing the entire circumference of the bronchiolar airway. As in earlier reports, these studies also noted airway epithelial cells positive for RSV antigen being sloughed or shed into the lumens of the infected airways, suggesting that infected and necrotic epithelial cells may contribute to the airway obstruction and inflammation during RSV infection 27, 28, 29. The infection of ciliated cells also implied that RSV‐induced ciliated cell cytopathology may negatively impact ‘mechanical airway clearance’ mechanisms, due to the loss of beating cilia on the apical surface. It was proposed that the loss of mechanical clearance in the very narrow distal airways of infants may lead to the accumulation and retention of sloughed necrotic cellular material in the bronchiolar airway lumens, thus providing a mechanism for acute obstruction of the distal airways.
In sum, the pathology of RSV bronchiolitis is most commonly described as plugging or occlusion of bronchiolar airway lumens by sloughed necrotic and irregular epithelium, combined with peribronchiolar infiltration and submucosal oedema. The precise composition of the material occluding the bronchiolar lumens is likely heterogeneous, even within bronchioles of the same patient, but most histopathology suggests that the obstructive material is largely cellular in nature, and describes accumulations of papilliary epithelial projections that protrude into the airway lumen, detached and necrotic epithelial cell debris, and intraluminal inflammatory cells, which are overwhelmingly neutrophils. Multinucleated cells or syncytial giant cells are another feature of RSV infection likely also to contribute to lumenal obstruction (Figure 1). Excessive secretions of mucus, fibrin deposits and other non‐cellular material are also described as components of the obstructive material 11 but are likely more minor components 27, 29. Although increased mucus secretion is a common consequence of RSV and other respiratory virus infections of the larger airway regions, the relative absence of secreted mucins in the obstructed bronchiolar airways infected by RSV likely reflects the limited cellular sources of secreted mucins, ie submucosal glands and mucin‐containing goblet cells, in distal airway regions especially those of infants.
Within examined human tissues, inflammatory cell infiltrates appear to be abundant at the specific locations where RSV antigen is seen, and this inflammatory infiltrate appears to contribute to the narrowing and obstruction of the bronchiolar airway lumen 26, 27, 31, 32. In particular, RSV infection appears highly chemotactic for neutrophils, and large numbers of neutrophils are detected entering the airway submucosa, transiting through the epithelium and intermingling with epithelial cell debris in the airway lumen 27, 31, 32, 33, 34, 35. Precisely how RSV interacts with neutrophils and affects their function and its ultimate impact on disease severity is poorly defined.
Lung tissues obtained post mortem from RSV‐infected patients continue to provide insights into sentinel features of RSV bronchiolitis and have significantly contributed to our understanding of RSV pathogenesis. However, they do little to inform us about the early events of RSV infection in vivo which subsequently lead to bronchiolitis. Most available post‐mortem lungs are from patients with end‐stage lung disease, and very few are available from patients exhibiting mild symptoms of RSV bronchiolitis, ie early‐stage disease. End‐stage disease lungs have likely been ravaged by many days of virus infection and inflammation plus the superimposed effects of therapeutic interventions, such as oxidative stress from hyperoxia, positive‐pressure mechanical ventilation and ventilator‐associated bacterial colonization and/or pneumonia, all of which complicate interpretation of the pathological findings. Fortunately, in the studies of Johnson et al 27 and Welliver et al 28, lung histopathology was obtained from deceased RSV‐infected infants who, although experiencing severe disease, had received no mechanical ventilation. The findings were remarkably similar to the other previously published reports. Another valuable source of RSV‐infected lung tissues are those obtained from immunocompromised individuals who have succumbed to RSV infection. Although these specimens are more frequently available than those from immunocompetent patients, airway histopathology should be examined with the knowledge that immune cell dysfunction may further complicate interpretation of the findings in addition to the effects of therapies mentioned above.
How can we better understand the pathogenesis of RSV bronchiolitis?
Several experimental model systems are available for investigating how RSV infection causes bronchiolitis. These include obtaining samples from naturally and experimentally RSV‐infected humans, infection of appropriate animal models and RSV infection of the airway epithelium in vitro. However, as in most human viral infections, a truer understanding of pathogenesis requires the development of antiviral interventions blocking viral pathways, applied at various times during acute illness.
Natural and experimental RSV infections of humans
Airway washes obtained from human infants diagnosed with RSV bronchiolitis have provided important information on how RSV loads and inflammatory responses progress, plateau and regress dynamically during the disease. For example, in previously healthy infants with naturally occurring RSV infection, higher viral loads in upper airway washes were associated with prolonged hospitalization, increased disease severity and increased requirement for intensive care 36, 37. Additionally a faster rate of decline in viral load within infants is associated with more rapid resolution of disease and a shorter hospitalization. Similar studies have confirmed that neutrophils dominate the inflammatory cell infiltrate, representing 80–93% of cells in upper airway washes of RSV‐infected infants and 76–83% of cells in lower airway washes (non‐bronchoscopic lavage) 31, 32, 38. Caveats with using samples obtained from naturally infected infants are largely related to the significant heterogeneity between samples obtained from individual patients who may be at different stages of the infection and disease process, and the variations in concentrations produced by the collection processes themselves.
Experimental infection of humans enables more controlled analysis and an analysis of early time points in infection. Human RSV challenge models inoculate the nasal epithelium of adult volunteers with a low‐passage virus (RSV Memphis 37 from an infant hospitalized with RSV bronchiolitis). In these experimentally infected adult volunteers, RSV load correlated directly with nasal symptom severity, nasal cytokine secretion and nasal mucus output, highlighting the usefulness of this model for testing the in vivo efficacy of RSV specific anti‐virals, immune modulators or the effectiveness of RSV vaccine candidates 39. However, these studies induce an infection limited to the nasal epithelium of healthy adult volunteers, and therefore information on RSV infection of the distal airways, especially of infants, must be extrapolated from evidence generated from the upper respiratory tract. Human RSV challenge studies are also costly, as they require a specially manufactured, quantified and regulatory approved RSV challenge material, specialized facilities, personnel and appropriate review by regulatory agencies.
Animal models of distal airway RSV disease
Despite intense efforts, there remains no consensus in vivo small animal model that reproducibly recapitulates the histopathology and clinical disease of RSV bronchiolitis in human infants. Several large animal models have been shown to reproduce bronchiolar airway histopathology similar to that in RSV‐infected infants. Neonatal and preterm lambs experimentally infected with ovine or human RSV strains by directly inoculating the lower airway regions results in robust bronchiolar airway infection and inflammation, with significant clogging of distal small airways by atypical epithelial cells and neutrophil‐rich inflammatory infiltrates 40, 41, 42, 43. Similar bronchiolar pathology has been reported for natural and experimental infection of calves with bovine RSV 44, 45, 46, 47, 48. More recently, baboons inoculated with RSV have been shown to develop severe bronchiolar airway infection and inflammation 49. Sloughed RSV antigen‐positive cells accumulating in bronchiolar airway lumens is a common histological feature of all these models, emphasizing the significance of epithelium cytopathology to the functional narrowing of the distal airways. Detailed morphological analysis of distal airways of calves infected with bovine RSV specifically noted disruption of ciliated cell morphology, loss of cilia, basal body disorganization and ciliary fragment accumulation in the airway lumen 47. Neutrophil‐rich inflammatory cell infiltrates were also a dominant feature in these animal models 40, 42, 45, 48. Preterm lambs infected with human RSV, or calves infected with bovine RSV, demonstrate robust neutrophil infiltration into sites of infection with neutrophils associated with, and occasionally fused to, infected epithelial cells 27, 40, 42, 45. Large animal species, although likely offering authentic models, rarely recapitulate the severity of clinical disease seen in infants infected by RSV, do not model the progression from upper to lower respiratory tract infection and suffer from a limited reagent‐base for mechanistic studies. Large animal models are also impractical for most RSV researchers, due to limited availability, expense of these large animals and the requirement of specialized infectious disease housing and staff.
Mice are attractive models for infectious disease research, due to their broad use in research, the ease of genetic manipulation and the broad availability of immunological reagents. However, RSV is poorly infectious for the murine airway epithelium and requires extremely large quantities of inoculating virus for generating significant infection outcomes. Histopathology studies on mouse lungs inoculated with RSV do not reveal airway pathology that recapitulates that of humans. Strategies to improve RSV infection of mouse airways by genetic optimization of the genome of either RSV or the mouse are actively being pursued 50, 51. The cotton rat, known to be naturally interferon‐deficient, is often touted as the 'gold standard' model for RSV infection amongst the rodent species. While a useful model, the cotton rat fails to faithfully recapitulate the histopathology and natural progression of RSV infection or the clinical disease of RSV bronchiolitis in humans 52, 53.
RSV infection of in vitro models of airway epithelium
Investigation of the consequences of RSV infection of the airway epithelium in vitro has traditionally relied on studies with non‐polarized epithelial cell lines, such as HEp‐2 and A549 cells. Although informative, these cells do not recapitulate the morphology, biology or structural properties of differentiated columnar airway epithelial cells – the primary target of RSV infection in vivo. Over the last decade there has been a significant increase in the availability of human differentiated airway epithelial cell culture models (HAE) used to investigate specific functions of the airway epithelium, such as mucociliary transport and how respiratory pathogens affect these functions 54, 55, 56, 57, 58, 59. To generate these culture models, nasal or tracheobronchial airway epithelial cells are isolated from airway tissues excised from deceased donors, or from airway scrapings from living donors. These epithelial cells are cultured on semi‐permeable supports to generate a differentiated pseudostratified mucociliary epithelium similar in morphology to human cartilaginous airways in vivo 60, 61. Differentiated culture models of human airway epithelium are easily infected by RSV, and several groups have shown that RSV infection is robust and restricted to the ciliated columnar epithelial cells in these models 54, 57. Ciliated cell tropism in HAE is, however, not unique to RSV, since parainfluenza viruses (human PIV1‐5, Sendai virus), human/avian influenza viruses and most coronaviruses also preferentially infect these cells 55, 58, 62, 63, 64, 65, 66, 67, 68. Non‐ciliated columnar cells, ie mucin‐secreting goblet cells, account for 10–30% of the columnar cells present in HAE and are resistant to infection by RSV, reproducing the known tropism of RSV in human airways in vivo 54, 57.
Ciliated cells are also abundant in the human non‐cartilaginous bronchiolar airway epithelium, but the transition from the terminal to the respiratory bronchioles results in a decline in ciliated cell density in favour of club columnar epithelial cells (Clara cells). Histopathology of human bronchiolar regions naturally infected by RSV indicates that both ciliated cells and club cells are infected by RSV, suggesting expanded RSV tropism for both ciliated and non‐ciliated cells in the bronchiolar airway regions 27. The significance of expanded RSV tropism in the distal airways is unknown. Club cells, known to possess xenobiotic and anti‐inflammatory properties, have been difficult to study in isolation, since differentiated culture models recapitulating club cell or bronchiolar airway epithelium morphology and function have not been reported.
In the absence of in vitro models of differentiated club cells, studies have focused on ciliated cells and their key roles in providing ‘mechanical’ airway clearance facilitated by coordinated ciliary beating. HAE models have been used to show that RSV infection causes cilia dyskinesia, loss of cilia structure and loss of ciliated cells themselves 54, 69, 70. We have recently shown that RSV infection inhibits the unidirectional transport of mucus secretions across the lumenal surface of HAE, with abnormal ciliary beating patterns observed as early as 24 h after infection and complete ablation of mucus transport occurring 3 days later 71. The ciliated cell cytopathology observed after RSV infection in vitro is remarkably similar to that seen in histopathological studies in the bronchiolar airways of humans, calves and lambs infected by RSV 27, 42, 45, 48. Nasal biopsies from infants with RSV‐associated bronchiolitis also showed decreased numbers of ciliated cells within the epithelium, increased numbers of cells detached from the epithelium, and abnormalities in ultrastructural features of cilia compared to uninfected controls 72. Combined, these studies suggest that ciliated cell cytopathology caused by RSV infection is predominately a direct consequence of virus replication, and not due to inflammation‐mediated injury, as inflammatory cell infiltrates are not present in HAE models.
The dysfunction of ciliary beating and loss of effective mechanical clearance is likely an important and early consequence of RSV infection which, over time and dependent on the extent of infection, will impair the ability of affected airways to clear occluding debris, infection and inflammation. RSV infection is often more robust and extensive in the distal than proximal airways, suggesting that virus‐induced disruption of mechanical airway clearance mechanisms may be exaggerated in the bronchiolar airways. Effective mechanical clearance mechanisms are critical for the clearance of airway‐obstructive material, and impairment of these mechanisms may contribute to the prolonged distal airway obstruction noted in human infants infected by RSV 73.
Is there something special about RSV infection that may account for exaggerated bronchiolar airway pathology?
Since a number of different respiratory viruses, including RSV, exhibit ciliated cell tropism, HAE models provide unique biological tools for comparing infection outcomes after ciliated cell infection. For example, the extent of ciliated cell cytopathology during RSV or Sendai virus infection was recently reported to be different 61. We have also shown striking differences in ciliated cell cytopathology after RSV infection compared to other respiratory viruses, including PIV3 71. Despite similar percentages of cells becoming infected, ciliated cell cytopathology was more rapid and extensive during RSV than during PIV3 infection. Strikingly, ciliated cells infected by RSV, but not PIV3, exhibited unusual morphology, with infected cells becoming rounded during the extrusion of the infected cells from the plane of the epithelium, resulting in significant numbers of shed cells accumulating in the lumenal surface secretions (Figure 2). As RSV and PIV3 replicate with similar life cycles, we predicted that these differences in ciliated cell morphology may be due to the expression of specific RSV genes. Infecting ciliated cells with RSV gene deletion mutants revealed that the unusual morphology was due to expression of a single RSV gene encoding the non‐structural protein 2 (NS2). Ciliated cell rounding and extrusion as a consequence of RSV NS2 expression was confirmed by infecting ciliated cells with PIV3 engineered to express RSV NS2 (PIV3–NS2). Ciliated cells infected by PIV3–NS2 were morphologically indistinguishable from those infected by RSV.
Figure 2.
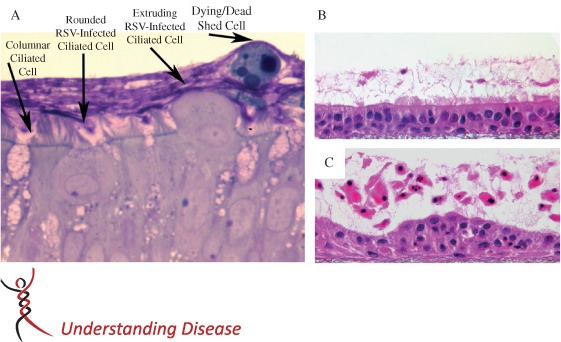
RSV infection of differentiated cultures of human airway epithelium. (A) Histological cross‐section of HAE cultures, with ciliated columnar cells transitioning from columnar to rounded cell morphology during RSV infection and followed by extrusion of the infected cells from the epithelium and into lumenal surface secretions. After shedding, detached epithelial cells rapidly become apoptotic, while being transported across the culture surface by beating cilia located on underlying and non‐infected ciliated cells. Cultures were fixed in perfluorocarbon impregnated with osmium tetroxide and embedded in plastic. Section shown was counterstained with Richardson's. (B, C) Histological cross‐sections of HAE, 3 days after inoculation of UV‐inactivated RSV (B) or RSV (C), demonstrating how RSV infection of ciliated cells results in disruption of the epithelium, atypical epithelial cell morphology and robust shedding of epithelial cells into lumenal surface secretions. Detached epithelial cells showed morphological evidence of pyknosis, karyorrhexis and karyolysis, indicative of an apoptosis‐like cell death. Cultures were fixed in Omnifix to preserve lumenal secretions, embedded in paraffin and the sections counterstained with haematoxylin and eosin (H&E) 71. Republished with permission of the American Society for Clinical Investigation, from ‘RSV‐encoded NS2 promotes epithelial cell shedding and distal airway obstruction’, The Journal of Clinical Investigation, RM Liesman et al, Vol. 124, Iss. 5, Copyright 2014; permission conveyed through Copyright Clearance Center Inc.
The in vivo significance of RSV NS2 expression was revealed by comparing infection of hamster airways with PIV3–NS2 or PIV3. Throughout the airways, columnar epithelial cells infected by PIV3 were abundant and remained embedded in the airway epithelium until being cleared, presumably by inflammatory cell infiltrates. In contrast, airway epithelial cells infected by PIV3–NS2 were most commonly observed shedding from the airway epithelium and, in the bronchiolar airways, the shedding cells accumulated in the narrow airway lumens. Shed cell accumulations consisted of intact and necrotic virus‐infected epithelial cells and, at later time points, abundant infiltrating neutrophils. Several histopathological features of this in vivo model were reminiscent of humans and large animal models (Figure 3). Epithelial cells infected by PIV3–NS2 exhibited unusual and peculiar morphology as the infected cells were shed from the epithelium. Accumulations of shed and pleomorphic epithelial cells and infiltrated neutrophils in the bronchiolar airways were sufficient to partially or fully occlude the distal airway lumens. Also, as described for human bronchioles infected by RSV, secreted mucins were not detected in the obstructive material in PIV3–NS2‐infected hamster bronchioles, at least as determined by AB–PAS staining. Whether the lack of mucin in these bronchiolar accumulations reflects an absence of goblet cells in the hamster bronchioles, or whether it is a species‐dependent phenomenon, remains to be determined. Syncytia formation was also a common finding in PIV3–NS2‐infected hamsters, while distinctly absent in PIV3‐infected animals. Shedding cells could often be seen fusing to other cells shedding in close proximity to each other, and forming elongated syncytia‐like cellular masses resembling the papillary projections described in humans, calves and lambs infected by RSV 27, 42, 45. Overall, these studies identified RSV NS2 as an important viral genetic determinant for much of the unusual histopathology observed in infants and animal models infected by RSV, and suggest that RSV NS2 expression may be one reason why RSV has an increased propensity for causing acute bronchiolar airway disease. Since cell‐associated RSV likely remains infectious, increased sloughing of columnar cells mediated by RSV NS2 expression may also increase the person‐to‐person spread of RSV within crowded human populations.
Figure 3.

Early epithelial cell cytopathology and inflammation in hamster bronchioles infected by PIV3 or PIV3–NS2, compared to neonatal lamb bronchioles infected by RSV. Histological cross‐sections of bronchiolar airways from hamsters (A, B) infected by PIV3 (A) or PIV3–NS2 (B), and bronchiolar airways from neonatal lambs infected by RSV. Reproduced from 42, ‘Perinatal Lamb Model of Respiratory Syncytial Virus (RSV) Infection’, by Derscheid and Ackermann, 2012, licensed under CC‐BY 3.0. (C). PIV infection results in modest epithelium cytopathology, with robust neutrophil‐rich inflammatory cell infiltration into peribronchiolar (green arrow) and intralumenal (white arrow) compartments, resulting in moderate loss of airway patency. In contrast, hamster and lamb bronchioles infected by PIV3–NS2 or RSV, respectively, show strikingly similar consequences of infection: disruption and dearrangement of the epithelium, with epithelial cells protruding and shedding into the airway lumen (yellow arrows), some syncytia formation and robust peribronchiolar (green arrow) and intralumenal (white arrow), neutrophil‐rich inflammatory infiltrates. The distinctive epithelium cytopathology, likely a consequence of RSV NS2 expression, combined with inflammatory cell infiltrates, significantly contribute to the obstruction of the bronchiolar airway lumen
Conclusions
Studies of RSV pathogenesis have been historically limited because of the inability of animal models to recapitulate characteristics of human RSV infection, pathology and disease. Recent studies performed directly in naturally and experimentally infected infants and adults have helped uncover a more prominent role of direct viral‐induced cytopathology. Recent in‐depth in vitro studies of human airway epithelial cells have elucidated mechanisms of this cytopathic effect which appear unique to RSV. The capacity for RSV to cause exaggerated cytopathology in the bronchiolar airways of infants sheds light on why RSV may have increased propensity for causing more frequent and severe acute bronchiolitis. Increased RSV cytopathology, promoted largely by RSV NS2 expression, causes increased sloughing or shedding of infected epithelium, which accumulates in the narrow lumens of the bronchiolar airways. These cellular accumulations are likely to result in acute obstruction of the distal airways; an outcome much more likely to occur in the extremely narrow bronchioles of infants. The retention of accumulations of shed and infected epithelial cells due to the narrow lumenal diameter, combined with loss of mechanical clearance of these airways, likely leads to increased spread of infection, increased inflammation and increased disease symptoms. Much remains to be determined about how early RSV bronchiolar cytopathology manifests into severe enough bronchiolitis that the affected infant will require hospitalization. For example, it is unknown which factors determine whether acute RSV bronchiolitis will either spontaneously resolve or develop into more severe airway disease requiring hospitalization. Understanding why some RSV‐infected infants more effectively clear infection and inflammation from the distal airways to avoid more severe and prolonged disease will enable paediatricians to better identify those infants who would likely benefit more from hospitalization, and may provide clues for new therapeutic interventions for limiting the severity of RSV‐associated distal airway disease and the associated long‐term sequelae.
Author contributions
The two authors on this review contributed equally.
Acknowledgements
The authors thank Dr Peter Collins and Dr Ulla Buchholz for many years of enjoyable and fruitful collaboration, and members of the laboratory who contributed to the thoughts and experiments discussed here, in particular Dr Liqun Zhang, Dr Meg Hennessey and Dr Racheal Liesman. Original studies described here were supported by the UNC University Research Council, the Cystic Fibrosis Foundation and the National Institutes of Health (NIH; Grant Nos R01HL103940, R01 HL77844, P50HL084934).
Conflicts of interest: RJP is Chief Scientific Officer for Spirovation Inc., a non‐profit contract research organization based on technology and expertise, located in the UNC Marsico Lung Institute. JdV reports support from Gilead Sciences, Alnylam Pharmaceuticals, Alios Pharmaceuticals and Microdose Therapeutx/Teva Pharmaceutical for the development of anti‐virals specific to RSV treatment.
References
- 1. Wright AL, Taussig LM, Ray CG, et al. The Tucson Children's Respiratory Study. II. Lower respiratory tract illness in the first year of life. Am J Epidemiol 1989; 129: 1232–1246. [DOI] [PubMed] [Google Scholar]
- 2. Edwards KM, Zhu Y, Griffin MR, et al. Burden of human metapneumovirus infection in young children. N Engl J Med 368: 633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Glezen WP, Taber LH, Frank AL, et al. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child 1986; 140: 543–546. [DOI] [PubMed] [Google Scholar]
- 4. Hall CB. Respiratory syncytial virus and parainfluenza virus. N Engl J Med 2001; 344: 1917–1928. [DOI] [PubMed] [Google Scholar]
- 5. Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med 2009; 360: 588–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nair H, Nokes DJ, Gessner BD, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta‐analysis. Lancet 375: 1545–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shay DK, Holman RC, Newman RD, et al. Bronchiolitis‐associated hospitalizations among US children, 1980–1996. J Am Med Assoc 1999; 282: 1440–1446. [DOI] [PubMed] [Google Scholar]
- 8. Shay DK, Holman RC, Roosevelt GE, et al. Bronchiolitis‐associated mortality and estimates of respiratory syncytial virus‐associated deaths among US children, 1979–1997. J Infect Dis 2001; 183: 16–22. [DOI] [PubMed] [Google Scholar]
- 9. Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. J Am Med Assoc 2003; 289: 179–186. [DOI] [PubMed] [Google Scholar]
- 10. Hislop AA, Haworth SG. Airway size and structure in the normal fetal and infant lung and the effect of premature delivery and artificial ventilation. Am Rev Respir Dis 1989; 140: 1717–1726. [DOI] [PubMed] [Google Scholar]
- 11. Aherne W, Bird T, Court S, et al. Pathological changes in virus infections of the lower respiratory tract in children. J Clin Pathol 1970; 23: 7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garcia CG, Bhore R, Soriano‐Fallas A, et al. Risk factors in children hospitalized with RSV bronchiolitis versus non‐RSV bronchiolitis. Pediatrics 126: e1453–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hervas D, Reina J, Yanez A, et al. Epidemiology of hospitalization for acute bronchiolitis in children: differences between RSV and non‐RSV bronchiolitis. Eur J Clin Microbiol Infect Dis 31: 1975–1981. [DOI] [PubMed] [Google Scholar]
- 14. Bourgeois FT, Valim C, McAdam AJ, et al. Relative impact of influenza and respiratory syncytial virus in young children. Pediatrics 2009; 124: e1072–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Collins PL, Chanock RM, Murphy BR. Respiratory syncytial virus In Field's Virology, 4th edn, Knipe DM. and Howley PM. (eds.). Lippincott Williams and Wilkins: Philadelphia, 2001; 1443–1485. [Google Scholar]
- 16. Monto AS, Malosh RE, Petrie JG, et al. Frequency of acute respiratory illnesses and circulation of respiratory viruses in households with children over 3 surveillance seasons. J Infect Dis 2014; 210: 1792–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chanock RM, Finberg L. Recovery from infants with respiratory illness of a virus related to chimpanzee coryza agent (CCA) II. Epidermological aspect s of infection in infants and young children. Am J Hyg 1957; 66: 291–300. [DOI] [PubMed] [Google Scholar]
- 18. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study, 2010. Lancet 380: 2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hon KL, Leung TF, Cheng WY, et al. Respiratory syncytial virus morbidity, premorbid factors, seasonality, and implications for prophylaxis. J Crit Care 27: 464–468. [DOI] [PubMed] [Google Scholar]
- 20. Stang P, Brandenburg N, Carter B. The economic burden of respiratory syncytial virus‐associated bronchiolitis hospitalizations. Arch Pediatr Adolesc Med 2001; 155: 95–96. [DOI] [PubMed] [Google Scholar]
- 21. Leader S, Kohlhase K. Recent trends in severe respiratory syncytial virus (RSV) among US infants, 1997 to 2000. J Pediatr 2003; 143: S127–132. [DOI] [PubMed] [Google Scholar]
- 22. Leader S, Kohlhase K. Respiratory syncytial virus‐coded pediatric hospitalizations, 1997 to 1999. Pediatr Infect Dis J 2002; 21: 629–632. [DOI] [PubMed] [Google Scholar]
- 23. Miller WT, Jr ., Barbosa E, Jr ., Mickus TJ, et al. Chest computed tomographic imaging characteristics of viral acute lower respiratory tract illnesses: a case‐control study. J Comput Assist Tomogr 35: 524–530. [DOI] [PubMed] [Google Scholar]
- 24. Miller WT Jr, Mickus TJ, Barbosa E Jr, et al. CT of viral lower respiratory tract infections in adults: comparison among viral organisms and between viral and bacterial infections. Am J Roentgenol 197: 1088–1095. [DOI] [PubMed] [Google Scholar]
- 25. Shedden WI, Emery JL. Immunofluorescent evidence of respiratory syncytial virus infection in cases of giant cell bronchiolitis in children. J Pathol Bacteriol 1965; 89: 343–347. [PubMed] [Google Scholar]
- 26. Zinserling A. Pecularities of lesions in viral and mycoplasma infections of the respiratory tract. Virchows Arch A Pathol Pathol Anat 1972; 356: 259–273. [DOI] [PubMed] [Google Scholar]
- 27. Johnson JE, Gonzales RA, Olson SJ, et al. The histopathology of fatal untreated human respiratory syncytial virus infection. Mod Pathol 2007; 20: 108–119. [DOI] [PubMed] [Google Scholar]
- 28. Welliver TP, Reed JL, Welliver RC, Sr. Respiratory syncytial virus and influenza virus infections: observations from tissues of fatal infant cases. Pediatr Infect Dis J 2008; 27: S92–96. [DOI] [PubMed] [Google Scholar]
- 29. Welliver TP, Garofalo RP, Hosakote Y, et al. Severe human lower respiratory tract illness caused by respiratory syncytial virus and influenza virus is characterized by the absence of pulmonary cytotoxic lymphocyte responses. J Infect Dis 2007; 195: 1126–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Neilson KA, Yunis EJ. Demonstration of respiratory syncytial virus in an autopsy series. Pediatr Pathol 1990; 10: 491–502. [DOI] [PubMed] [Google Scholar]
- 31. Smith PK, Wang SZ, Dowling KD, et al. Leucocyte populations in respiratory syncytial virus‐induced bronchiolitis. J Paediatr Child Health 2001; 37: 146–151. [DOI] [PubMed] [Google Scholar]
- 32. McNamara PS, Ritson P, Selby A, et al. Bronchoalveolar lavage cellularity in infants with severe respiratory syncytial virus bronchiolitis. Arch Dis Child 2003; 88: 922–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McNamara PS, Flanagan BF, Baldwin LM, et al. Interleukin 9 production in the lungs of infants with severe respiratory syncytial virus bronchiolitis. Lancet 2004; 363: 1031–1037. [DOI] [PubMed] [Google Scholar]
- 34. McNamara PS, Flanagan BF, Selby AM, et al. Pro‐ and anti‐inflammatory responses in respiratory syncytial virus bronchiolitis. Eur Respir J 2004; 23: 106–112. [DOI] [PubMed] [Google Scholar]
- 35. McNamara PS, Smyth RL. The pathogenesis of respiratory syncytial virus disease in childhood. Br Med Bull 2002; 61: 13–28. [DOI] [PubMed] [Google Scholar]
- 36. DeVincenzo JP, Wilkinson T, Vaishnaw A, et al. Viral load drives disease in humans experimentally infected with respiratory syncytial virus. Am J Respir Crit Care Med 182: 1305–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Buckingham SC, Bush AJ, Devincenzo JP. Nasal quantity of respiratory syncytical virus correlates with disease severity in hospitalized infants. Pediatr Infect Dis J 2000; 19: 113–117. [DOI] [PubMed] [Google Scholar]
- 38. Everard ML, Swarbrick A, Wrightham M, et al. Analysis of cells obtained by bronchial lavage of infants with respiratory syncytial virus infection. Arch Dis Child 1994; 71: 428–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. DeVincenzo JP, Whitley RJ, Mackman RL, et al. Oral GS‐5806 activity in a respiratory syncytial virus challenge study. N Engl J Med 371: 711–722. [DOI] [PubMed] [Google Scholar]
- 40. Olivier A, Gallup J, de Macedo MM, et al. Human respiratory syncytial virus A2 strain replicates and induces innate immune responses by respiratory epithelia of neonatal lambs. Int J Exp Pathol 2009; 90: 431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Olivier AK, Gallup JM, van Geelen A, et al. Exogenous administration of vascular endothelial growth factor prior to human respiratory syncytial virus a2 infection reduces pulmonary pathology in neonatal lambs and alters epithelial innate immune responses. Exp Lung Res 37: 131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Derscheid RJ, Ackermann MR. Perinatal lamb model of respiratory syncytial virus (RSV) infection. Viruses 4: 2359–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sow FB, Gallup JM, Krishnan S, et al. Respiratory syncytial virus infection is associated with an altered innate immunity and a heightened pro‐inflammatory response in the lungs of preterm lambs. Respir Res 12: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bryson DG, McConnell S, McAliskey M, et al. Ultrastructural features of alveolar lesions in induced respiratory syncytial virus pneumonia of calves. Vet Pathol 1991; 28: 286–292. [DOI] [PubMed] [Google Scholar]
- 45. Bryson DG, Platten MF, McConnell S, et al. Ultrastructural features of lesions in bronchiolar epithelium in induced respiratory syncytial virus pneumonia of calves. Vet Pathol 1991; 28: 293–299. [DOI] [PubMed] [Google Scholar]
- 46. Valarcher JF, Taylor G. Bovine respiratory syncytial virus infection. Vet Res 2007; 38: 153–180. [DOI] [PubMed] [Google Scholar]
- 47. Philippou S, Otto P, Reinhold P, et al. Respiratory syncytial virus‐induced chronic bronchiolitis in experimentally infected calves. Virchows Arch 2000; 436: 617–621. [DOI] [PubMed] [Google Scholar]
- 48. Viuff B, Tjornehoj K, Larsen LE, et al. Replication and clearance of respiratory syncytial virus: apoptosis is an important pathway of virus clearance after experimental infection with bovine respiratory syncytial virus. Am J Pathol 2002; 161: 2195–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Papin JF, Wolf RF, Kosanke SD, et al. Infant baboons infected with respiratory syncytial virus develop clinical and pathological changes that parallel those of human infants. Am J Physiol Lung Cell Mol Physiol 304: L530–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Moore ML, Chi MH, Luongo C, et al. A chimeric A2 strain of respiratory syncytial virus (RSV) with the fusion protein of RSV strain line 19 exhibits enhanced viral load, mucus, and airway dysfunction. J Virol 2009; 83: 4185–4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Stokes KL, Chi MH, Sakamoto K, et al. Differential pathogenesis of respiratory syncytial virus clinical isolates in BALB/c mice. J Virol 85: 5782–5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Moore ML, Peebles RS Jr. Respiratory syncytial virus disease mechanisms implicated by human, animal model, and in vitro data facilitate vaccine strategies and new therapeutics. Pharmacol Ther 2006; 112: 405–424. [DOI] [PubMed] [Google Scholar]
- 53. Bem RA, Domachowske JB, Rosenberg HF. Animal models of human respiratory syncytial virus disease. Am J Physiol Lung Cell Mol Physiol 301: L148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhang L, Peeples ME, Boucher RC, et al. Respiratory syncytial virus infection of human airway epithelial cells is polarized, specific to ciliated cells, and without obvious cytopathology. J Virol 2002; 76: 5654–5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhang L, Bukreyev A, Thompson CI, et al. Infection of ciliated cells by human parainfluenza virus type 3 in an in vitro model of human airway epithelium. J Virol 2005; 79: 1113–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhang L, Button B, Gabriel SE, et al. CFTR delivery to 25% of surface epithelial cells restores normal rates of mucus transport to human cystic fibrosis airway epithelium. PLoS Biol 2009; 7: e1000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Villenave R, Thavagnanam S, Sarlang S, et al. In vitro modeling of respiratory syncytial virus infection of pediatric bronchial epithelium, the primary target of infection in vivo . Proc Natl Acad Sci USA 109: 5040–5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sims AC, Baric RS, Yount B, et al. Severe acute respiratory syndrome coronavirus infection of human ciliated airway epithelia: role of ciliated cells in viral spread in the conducting airways of the lungs. J Virol 2005; 79: 15511–15524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pyrc K, Sims AC, Dijkman R, et al. Culturing the unculturable: human coronavirus HKU1 infects, replicates, and produces progeny virions in human ciliated airway epithelial cell cultures. J Virol 84: 11255–11263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fulcher ML, Gabriel S, Burns KA, et al. Well‐differentiated human airway epithelial cell cultures. Methods Mol Med 2005; 107: 183–206. [DOI] [PubMed] [Google Scholar]
- 61. Villenave R, O'Donoghue D, Thavagnanam S, et al. Differential cytopathogenesis of respiratory syncytial virus prototypic and clinical isolates in primary pediatric bronchial epithelial cells. Virol J 8: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhang L, Collins PL, Lamb RA, et al. Comparison of differing cytopathic effects in human airway epithelium of parainfluenza virus 5 (W3A), parainfluenza virus type 3, and respiratory syncytial virus. Virology 421: 67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Villenave R, Touzelet O, Thavagnanam S, et al. Cytopathogenesis of Sendai virus in well‐differentiated primary pediatric bronchial epithelial cells. J Virol 84: 11718–11728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Scull MA, Gillim‐Ross L, Santos C, et al. Avian influenza virus glycoproteins restrict virus replication and spread through human airway epithelium at temperatures of the proximal airways. PLoS Pathog 2009; 5: e1000424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Thompson CI, Barclay WS, Zambon MC, et al. Infection of human airway epithelium by human and avian strains of influenza a virus. J Virol 2006; 80: 8060–8068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Matrosovich MN, Matrosovich TY, Gray T, et al. Human and avian influenza viruses target different cell types in cultures of human airway epithelium. Proc Natl Acad Sci USA 2004; 101: 4620–4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bartlett EJ, Hennessey M, Skiadopoulos MH, et al. Role of interferon in the replication of human parainfluenza virus type 1 wild type and mutant viruses in human ciliated airway epithelium. J Virol 2008; 82: 8059–8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Schaap‐Nutt A, Liesman R, Bartlett EJ, et al. Human parainfluenza virus serotypes differ in their kinetics of replication and cytokine secretion in human tracheobronchial airway epithelium. Virology 433: 320–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Smith CM, Fadaee‐Shohada MJ, Sawhney R, et al. Ciliated cultures from patients with primary ciliary dyskinesia do not produce nitric oxide or inducible nitric oxide synthase during early infection. Chest 144: 1671–1676. [DOI] [PubMed] [Google Scholar]
- 70. Mata M, Martinez I, Melero JA, et al. Roflumilast inhibits respiratory syncytial virus infection in human differentiated bronchial epithelial cells. PLoS One 8: e69670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Liesman RM, Buchholz UJ, Luongo CL, et al. RSV‐encoded NS2 promotes epithelial cell shedding and distal airway obstruction. J Clin Invest 124: 2219–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wong JY, Rutman A, O'Callaghan C. Recovery of the ciliated epithelium following acute bronchiolitis in infancy. Thorax 2005; 60: 582–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Randell SH, Boucher RC. Effective mucus clearance is essential for respiratory health. Am J Respir Cell Mol Biol 2006; 35: 20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]


