Abstract
Introduction
Low body temperatures following prehospital transport are associated with poor outcomes in patients with traumatic brain injury (TBI). However, little is known about potential associations across a range of temperatures obtained immediately after prehospital transport. Furthermore, little is known about the influence of body temperature on non-mortality outcomes. The purpose of this study was to assess the correlation between temperatures obtained immediately following prehospital transport and TBI outcomes across the entire range of temperatures.
Methods
This retrospective observational study included all moderate/severe TBI cases (CDC Barell Matrix Type 1) in the pre-implementation cohort of the Excellence in Prehospital Injury Care (EPIC) TBI Study (NIH/NINDS: 1R01NS071049). Cases were compared across four cohorts of initial trauma center temperature (ITCT): <35.0°C [Very Low Temperature (VLT)]; 35.0–35.9°C [Low Temperature (LT)]; 36.0–37.9°C [Normal Temperature (NT)]; and ≥38.0°C [Elevated Temperature (ET)]. Multivariable analysis was performed adjusting for injury severity score, age, sex, race, ethnicity, blunt/penetrating trauma, and payment source. Adjusted odds ratios (aORs) with 95% confidence intervals (CI) for mortality were calculated. To evaluate non-mortality outcomes, deaths were excluded and the adjusted median increase in hospital length of stay (LOS), ICU LOS and total hospital charges were calculated for each ITCT group and compared to the NT group.
Results
22,925 cases were identified and cases with inter-facility transfer (7361, 32%), no EMS transport (1213, 5%), missing ITCT (2083, 9%), or missing demographic data (391, 2%) were excluded. Within this study cohort the aORs for death (compared to the NT group) were 2.41 (CI: 1.83–3.17) for VLT, 1.62 (CI: 1.37–1.93) for LT, and 1.86 (CI: 1.52–3.00) for ET. Similarly, trauma center (TC) LOS, ICU LOS, and total TC charges increased in all temperature groups when compared to NT.
Conclusion
In this large, statewide study of major TBI, both ETs and LTs immediately following prehospital transport were independently associated with higher mortality and with increased TC LOS, ICU LOS, and total TC charges. Further study is needed to identify the causes of abnormal body temperature during the prehospital interval and if in-field measures to prevent temperature variations might improve outcomes.
Keywords: Hyperthermia, Traumatic Brain Injury, Hypothermia, Mortality, Cost
Introduction
In 2010, Traumatic Brain Injury (TBI) led to over 1.7 million emergency department visits, 275,000 hospitalizations, and 50,000 deaths in the United States.1, 2 The lifetime cost of TBI sustained in the year 2000 alone was estimated to be over 60 billion US dollars3, 4 with more than 2% of the US population requiring long-term assistance as a result of TBI.5 Secondary brain injury is a major contributor to increased morbidity and mortality following TBI. Several factors have been identified as causing secondary brain injury during prehospital care including: hypotension, hypoxia, and hyperventilation.6–17 Through multiple pathophysiological mechanisms, both elevated body temperature and low body temperature could cause secondary brain injury with resulting increases in morbidity and mortality.18–23
Low body temperatures in the prehospital setting have long been known to be associated with poor outcomes in general trauma patients. In this population, multiple studies have reported that body temperature <35°C is associated with a marked increase in the adjusted odds of death when compared to patients with normal body temperature.20, 22, 24–26 However, the vast majority of available data on hypothermia in TBI has focused on therapeutic hypothermia as a modality to improve outcomes in the intensive care unit (ICU).27–29
Even less is known about the effect of elevated temperatures on TBI outcomes.18, 21, 23 Patients with severe TBI are known to frequently develop idiopathic elevated temperatures (“neurogenic fever”) during their hospital course. These elevated temperatures have been associated with poor outcomes and increased mortality.30–35 Although poorly understood, it is thought that temperature abnormalities in the ICU are a result of Central Nervous System (CNS) failure to regulate temperature following injury.36 However, these mechanisms that lead to fluctuations in body temperature during the hospital course may not be the primary cause of elevated temperatures identified on initial presentation to the ED.19 This is more likely due to environmental exposure that occurs from the time of injury until the patient arrives the hospital. However, very little is known about the incidence and outcomes of TBI patients who already have elevated body temperature by the end of their prehospital interval.
The purpose of the current study is to evaluate potential associations between body temperature immediately following prehospital transport and various outcomes in victims of major TBI.
Methods
Study Design
This study is a retrospective observational analysis of data contained in the Arizona State Trauma Registry (ASTR) and the Excellence in Prehospital Injury Care (EPIC) TBI database. The ASTR database contains information on all trauma patients cared for at level 1 trauma centers (TCs) in Arizona (total of 8 TCs) and was matched with prehospital data for participating EMS agencies transporting patients to one of the TCs. More than 90% of TBI patients in Arizona were cared for by agencies participating in the EPIC project. The details of the EPIC Study, a statewide, before/after, controlled evaluation of the impact of implementing the EMS TBI treatment guidelines (NIH/NINDS: 1R01NS071049; ClinicalTrials.gov: #NCT01339702), have been reported in detail elsewhere.37
Data Validity Efforts
The ASTR data validation tool, developed collaboratively by Arizona Department of Health Services (ADHS) staff and the trauma registry software vendor significantly increases the ASTR data quality. More than 800 data checks are performed per record for the full data set. Data checks include warning flags for blank fields, invalid entries, date and time errors, and other data logic errors. The Data and Quality Assurance (DQA) staff within ADHS run validation reports and the results are sent to the reporting hospitals so that the data can be updated, confirmed, and re-submitted to the ASTR with changes. The DQA section also performs statewide inter-rater reliability testing as a quality assurance tool to continuously improve on trauma data entry standardization and data reliability.
Study Population and Setting
Cases of moderate/severe (“major”) TBI in the State of Arizona, occurring between January 1, 2007 & December 31, 2012 were identified using the ASTR/EPIC database. In the EPIC Study, major TBI is defined as those patients with physical trauma who have trauma center diagnosis(es) consistent with TBI (either isolated or multisystem trauma that includes TBI) and meet at least one of the following definitions for moderate or severe TBI: a) Centers for Disease Control (CDC) Barell Matrix-Type 1, b) Head Region Severity Score (International Classification of Diseases-ICD-9) ≥3, and/or c) Abbreviated Injury Scale (AIS)-Head Region Severity Score ≥3.37 Cases were excluded if temperature on arrival to the TC was not recorded, temperature was recorded after a transfer from a non-TC to a TC, or if other important risk adjusters were missing. The included patients were cared for by more than 100 different EMS agencies. We are not aware of any attempt to specifically detect, prevent, or treat temperature abnormalities in the prehospital setting.
Human Subjects Review
The necessary regulatory approvals for EPIC have been obtained from the Arizona Department of Health Services (ADHS) and the State Attorney General. The University of Arizona Institutional Review Board and the ADHS Human Subjects Review Board have approved the project and publication of de-identified data.37
Statistical Analysis
All cases of major TBI in the EPIC/ASTR data set were evaluated. Those with an inter-facility transfer and those without a documented ITCT or missing important risk adjusters (e.g., race, ISS, payment source) were excluded. The unadjusted association between the continuous variable ITCT and mortality was first evaluated using a Lowess smoothing function, with the outcome transformed to logits (log odds), in order to assess whether body temperature was linearly related to the outcome in the logit, a key requirement for continuous variables in logistic regression. Fractional polynomial regression was used to find a transformation for ITCT as a continuous variable for logistic regression to satisfy the requirement of linearity in the logit. ITCT was also categorized using the following four commonly-used clinical cutoffs for abnormal body temperatures: very low temperatures (<35.0°C), low temperatures (35.0–35.9°C), normal temperatures (36.0–37.9°C), and elevated temperatures (≥38.0°C). Non-mortality outcomes were evaluated utilizing the sub-group of patients who survived. A severity-adjusted analysis (outlined below) was then used to compare mortality and non-morality outcomes between the four temperature-defined groups.
Measurements and Key Outcomes
The outcomes for this study were in-hospital mortality following the initial injury and other commonly reported non-mortality outcomes: TC length-of-stay, intensive care unit (ICU) length of stay, and total TC charges in United States Dollars ($).
Analysis
A multivariable risk adjustment analysis was performed comparing mortality between the very low temperature, low temperature and elevated temperature groups to that of the cases with normal temperature. The covariates for the severity adjusted analysis were chosen a priori, based on the known or suspected relationship (either directly or as a potential confounder) to the main outcome variable, mortality, and accounted for: injury severity scale (ISS), age, sex, race, ethnicity, trauma type (blunt vs. penetrating), and payment source (private, public, self, other) as describe elsewhere.37 The results of the logistic regression model are reported as adjusted odds ratios (aOR) with 95% Confidence Intervals (CI) for mortality among each group when compared to those in the NT group. Median regression was used to model the severity adjusted median difference in non-mortality outcomes between the very low temperature, low temperature, and elevated temperature groups to those cases with normal temperature after adjusting for ISS, age, sex, and trauma type. Statistical analyses were conducted using SAS v9.3 (SAS Institute, Inc., Cary, NC) and Stata v14 (StataCorp LP, College Station, TX).
Results
The EPIC TBI database contained 22,925 cases of major TBI, out of which 11,877 (51.8%) were included in the study. Of the 22,925 cases identified 2,083 (9.1%) were excluded due to missing ITCT data. An additional 7,361 (32.1%) cases were excluded because they were inter-facility transfers and 1213 (5.3%) due to transport by private vehicle. An additional 391 (1.7%) cases were excluded either due to missing information on race, ISS, or payment source leaving 11,877 cases included in this study. The demographic data for the study population stratified by ITCT group are shown in Table 1. Most cases (70.1%) were men and median age was 39 years. The majority (58.6%) had an ISS >15 and had a blunt mechanism of injury (95.6%). Patients excluded due to missing ITCT were more likely to be seriously injured (79.9% with an ISS > 15) and less likely to have blunt injury (85.6%).
Table 1.
Study Population Demographic Data
| Initial Trauma Center Temperature | |||||
|---|---|---|---|---|---|
| < 35°C | 35–35.9°C | 36–37.9°C | ≥ 38°C | Total TBI | |
| Total Patients | 473 (4.0%) | 2,256 (19.0%) | 8,971 (75.5%) | 177 (1.5%) | 11,877 |
| Male | 350 (73.9%) | 1,581 (70.0%) | 6,266 (69.8%) | 134 (75.7%) | 8,331 (70.1%) |
| Age in Years (Q1–Q3) | 36 (22–54) | 39 (22–58) | 39 (22–57) | 37 (20–55) | 39 (22–57) |
| Race | |||||
| Hispanic | 74 (15.6%) | 532 (23.5%) | 2,189 (24.4%) | 48 (27.1%) | 2,843 (23.9%) |
| White | 305 (64.4%) | 1419 (62.8%) | 5,555 (61.9%) | 101 (57.0%) | 7,380 (62.1%) |
| Other | 94 (19.8%) | 305 (13.5%) | 1,227 (13.6%) | 28 (15.8%) | 1,654 (13.9%) |
| Injury Severity Score (ISS) > 15 | 407 (86.0%) | 1,655 (73.3%) | 4,763 (53.0%) | 136 (76.8%) | 6,961 (58.6%) |
| Payer | |||||
| Public Insurance | 219 (46.3.%) | 1,095 (48.5%) | 4,092 (45.6%) | 79 (44.6%) | 5,485 (46.2%) |
| Private Insurance | 162 (34.2%) | 786 (34.8%) | 3,294 (36.7%) | 65 (36.7%) | 4,307 (%36.3) |
| Other Insurance | 92 (19.4%) | 375 (16.6%) | 1,585 (17.6%) | 33 (18.6%) | 2,085 (17.6%) |
| Blunt Trauma | 423 (89.4%) | 2,110 (93.5%) | 8,658 (96.5%) | 167 (94.3%) | 1,1358 (95.6%) |
| Mortality | 147 (31.0%) | 365 (16.1%) | 565 (6.2%) | 32 (18.0%) | 1,109 (9.3%) |
Figure 1 shows the plot of ITCT versus the unadjusted log odds (logit) of death using a Lowess smoothing function, which suggests a non-linear relationship between ITCT and the outcome in the logit scale. Fractional polynomial regression failed to find an adequate transformation of the continuous variable that was linearly associated with the log odds of death, a key requirement of logistic regression. Thus, ITCT categorized into 4 categories, based on commonly used clinical definitions of body temperature abnormalities, was used for all analyses.
Figure 1.
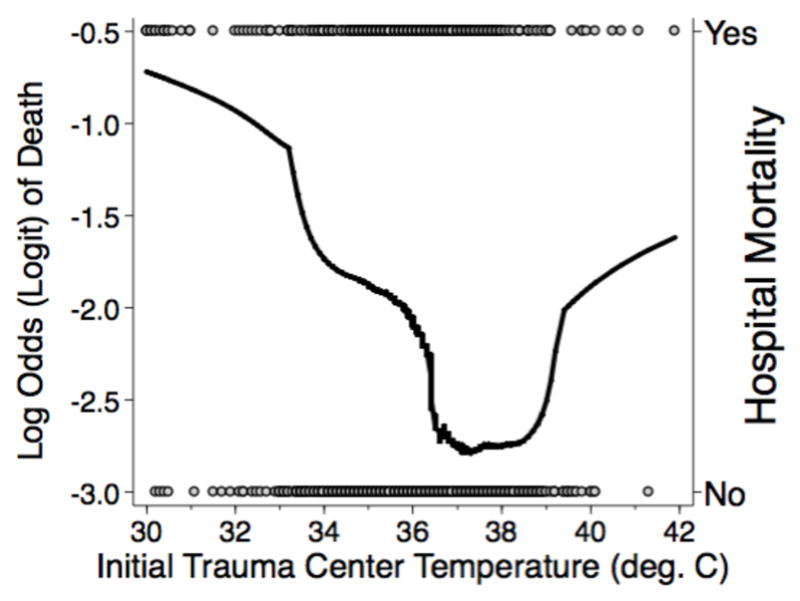
Lowess smoothing function for unadjusted mortality versus initial trauma center temperature
The normal temperature group accounted for 75.5% (n=8,971) of the total study population, while there were 2,256 (19.0%) in the low temperature group, 473 (4.0%) in very low temperature group and 177 (1.5%) in the elevated temperature group. Injury severity scores were higher in the elevated temperature, low temperature and very low temperature groups than in the normal temperature group. These differences were even more striking in patients with an ISS ≥ 25. The very low temperature group had more penetrating trauma (11.6%) cases compared to the other groups. The overall mortality in our study population was 9.3% (n=1,109). The crude mortality for each group is shown in Table 2. There was a significant increase in crude mortality across all temperature groups when compared to the normal temperature group (p <0.0001).
Table 2.
Crude and adjusted odds of mortality when temperature on arrival to a trauma center is above or below normal.
| Temperature | Mortality n / N (%) | Crude OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|---|
| >38°C | 32/177 (18.0%) | 3.28 (2.21–4.86) | 1.86 (1.15–3.00) |
| 36.0–37.9°C | 565/8971 (6.2%) | Referent | Referent |
| 35–35.9°C | 365/2256 (9.5%) | 2.87 (2.49–3.30) | 1.62 (1.37–1.93) |
| <35°C | 147/473 (31.0%) | 6.70 (5.42–8.29) | 2.41 (1.83–3.17) |
The crude and adjusted odds of mortality in each group is shown in Table 2. The adjusted odds of mortality differed significantly in the very low temperature (aOR 2.41, 95% CI 1.83–3.17), low temperature (aOR 1.62, 95% CI 1.37–1.93), and elevated temperature (aOR 1.86, 95% CI 1.15–3.00) group as compared to the normal temperature group.
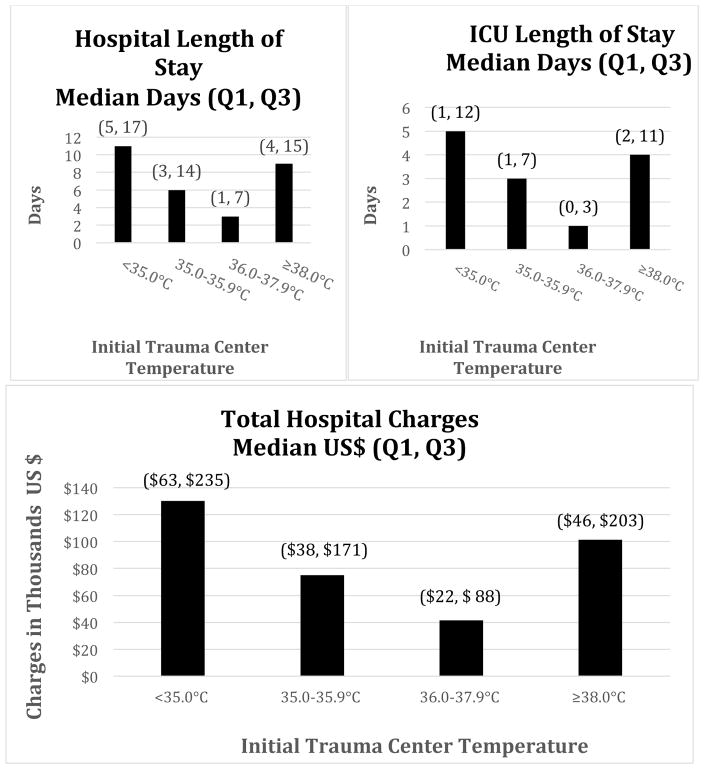
After excluding deaths, the association between ITCTs and crude non-mortality outcomes were calculated and are illustrated in Figure 2. This figure demonstrates the median hospital length of stay, ICU length of stay, and total hospital charges across all temperature groups. The median regression analysis provides the adjusted increases in median hospital length of stay, ICU length of stay, and TC total charges in all three groups compared with the normal temperature group (Table 3). All three groups had a significant increase in hospital length of stay and ICU length of stay compared to the normal temperature group (p values < 0.0001).
Figure 2.
Unadjusted non-mortality outcomes by initial trauma center temperature.
Values reported represent the raw or unadjusted median Hospital, ICU LOS or hospital charges with (Q1 = 25th Percentile and Q3 = 75th percentile).
Table 3.
Non-mortality Adjusted Median Regression Analysis.
| Temperature | Median Hospital LOS (95% CI) | Median ICU LOS (95% CI) | Median Hospital Charges (95% CI) |
|---|---|---|---|
| ≥ 38°C | 2.38 (1.94–2.81) | 1.29 (1.18–1.40) | $42,714 (36,853 – 48,574) |
| 36–37.9°C | Referent | Referent | Referent |
| 35–35.9°C | 1.07 (0.94–1.21) | 0.41 (0.38–0.45) | $11,599 (9,846 – 13,352) |
| < 35°C | 2.84 (2.52–3.13) | 1.29 (1.22–1.37) | $37,135 (33,214 –41,055) |
Values reported represent the adjusted increase in LOS or hospital charges with (95% Confidence Intervals) in each group when compared to the median value for the 36–37.9°C group.
Discussion
The negative impact of secondary insults on TBI outcome is well known. For example, hypoxia,9, 10, 12, 17, 38 hypotension,9, 12, 16, 17, 38 and hyperventilation (in intubated patients)14, 15, 38–40 are all associated with at least a doubling of mortality. While inhospital fever is strongly associated with the risk of death,31–33, 36 little is known about the impact of high temperatures occurring at the time of hospital arrival.
We found a significant association between abnormal initial trauma center temperature and poor outcomes in victims of major TBI. Since the temperatures were the initial ones obtained at the hospital, they likely reflect abnormalities that occurred during the prehospital interval. Although the association between hypothermia at the time of hospital arrival and increased mortality following TBI has been reported,20, 22, 24, 41, 42 we believe this is the first study to demonstrate this association across the entire range of presenting temperatures. Our findings show that increased body temperature occurring during the prehospital interval has an associated increased risk that is similar to the other the commonly-reported secondary insults (i.e., hypoxia, hypotension, and hyperventilation). In addition to the mortality findings, we identified a strong association between abnormal ITCTs and non-mortality outcomes with statistically significant increases in hospital length of stay, ICU length of stay and hospital charges in patients with either high or low temperatures. We have been unable to find any previous studies that reported an association between alterations in body temperature and healthcare resource utilization.
The causes of the abnormal temperatures observed in this study remain unclear. In ICU settings, thermo-dysregulation or infection are common causes of temperature abnormalities in TBI patients.31–33, 36 In this study interfacility transfers were excluded and the vast majority of cases in the EPIC population arrive at the hospital less than 30 minutes after the injury. Thus, given the brief amount of time that transpires between the injury event and arrival at the trauma center, in the prehospital setting, variations in body temperature are much more likely to be caused by exposure to environmental temperature extremes rather than underlying pathophysiological processes.
The attempt to show a linkage between environmental conditions and body temperature in trauma patients has led to mixed results. One TBI study that evaluated environmental temperatures and patient outcomes demonstrated no association between them.43 On the other hand, in both general trauma and TBI patients, some previous reports have demonstrated that the incidence of hypothermia is higher in the colder months of the year.20, 26 In addition, recent combat experience in Iraq and Afghanistan (predominantly warm climates) demonstrated that 7.4% of general trauma patients and as many as 47% of TBI patients had elevated temperatures on arrival at the forward aid stations.41, 44, 45
It is interesting that the prevalence of elevated temperatures in our study (177, 1.5%) was much lower than that of low temperatures (2256, 19.0%) or very low temperatures (473, 4.0%). Given the recent military literature described above and the relatively hot temperatures commonly encountered in Arizona (average summer high temperatures above 39°C), this finding was not anticipated. In part, this unexpected finding could be due to differences in injury location and prehospital care. For instance, civilian trauma patients may be more likely to be injured inside air-conditioned vehicles and transported in air-conditioned ambulances. These factors could mitigate an initial exposure to high environmental temperatures or increase the incidence of low temperatures.
Patients in the low and very low temperature groups had a significant increase in the adjusted odds of mortality when compared to those with a normal temperature. This is not a new finding in trauma patients. However, the incidence of hypothermia after sustaining a moderate or severe TBI was surprisingly high. In fact, 23% of patients had an initial temperature <36.0°C. Thus, since environmental exposure may be a key cause of temperature variations that occur during the initial care of trauma patients, it appears that hypothermia should be avoided if at all possible in the prehospital setting.
Similarly in patients with elevated temperatures, there was a clear increase in mortality and in poor non-mortality outcomes. This, in conjunction with multiple ICU studies where hyperthermia was associated with poor outcomes, makes a compelling argument that variations in body temperature in either direction from normal should be avoided in TBI.
While there may be some validity to the current recommendations aimed at treating low and high temperatures in trauma patients, the design of our study does not allow us to make conclusions about the potential effectiveness of such treatment. However, these findings do support future study of the effectiveness of such treatment. Our findings supply an important reminder that, even under the optimal conditions in a controlled ICU setting, inadvertent occurrence of hypothermia or hyperthermia is common and poses significant risks to TBI patients. While in-hospital treatment of hypothermia has been associated with improved outcomes following injury, this has not been demonstrated in patients with elevated temperatures.46 Therefore, any consideration of taking measures to prevent or treat body temperature abnormalities in the prehospital setting must carefully take into account the absence of demonstrated benefits and the potential risks. However, these finding do support future study of the effectiveness of such treatment and could help direct the future development of evidence-based guidelines for the field triage of patients with severe trauma.
Limitations
This study has several limitations. First, this is a retrospective, observational evaluation. Thus, it cannot be used to prove a causal effect of body temperature on outcome. Second, this study utilized CDC Barell Matrix among other criteria to identify patients with moderate or severe TBI. Use of diagnosis based inclusion criteria, may have introduced inclusion bias.37, 47 Additionally, by using this inclusion criteria, patients with other traumatic injuries were likely included in this study and the effect of temperature on TBI can not be isolated. Third, we do not know whether there were attempts to treat body temperature either in the prehospital or trauma center environments. Thus, we are not able to identify associations with treatment. Finally, temperatures were recorded at 8 different trauma centers across the state and we are not able to determine the method, accuracy, or exact time of the measurements. Because this study assumes that ITCT was measured with the initial set of vital signs at the trauma center and patients without an ITCT were excluded, it is possible that other patient care activities took precedence over the measurement of body temperature and measurement of ITCT was delayed. Given that patients without a measured ITCT (excluded cases) had a higher ISS and were more likely to have penetrating trauma, this seems likely and may have introduced selection bias.
Conclusion
In this statewide study of major TBI, both low and high initial trauma center body temperatures were associated with a significant increase in severity adjust mortality and poor non-mortality outcomes. Future work is needed to identify the cause of prehospital body temperature variation in patients with TBI and whether initiation of in-field measures to prevent temperature abnormalities is safe and effective.
Acknowledgments
Funding: Research reported in this publication was supported by the National Institute of Neurological Disorders And Stroke of the National Institutes of Health under Award Number R01NS071049. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21:375–8. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Faul M National Center for Injury Prevention and Control (U.S.) Traumatic brain injury in the United States emergency department visits, hospitalizations, and deaths, 2002–2006. 2010 [Google Scholar]
- 3.Finkelstein ECP, Miller TR. The incidence and economic burden of injuries in the United States. New York: Oxford University Press; 2006. [Google Scholar]
- 4.Corrigan JD, Selassie AW, Orman JA. The epidemiology of traumatic brain injury. J Head Trauma Rehabil. 2010;25:72–80. doi: 10.1097/HTR.0b013e3181ccc8b4. [DOI] [PubMed] [Google Scholar]
- 5.Thurman DJ, Alverson C, Dunn KA, Guerrero J, Sniezek JE. Traumatic brain injury in the United States: A public health perspective. J Head Trauma Rehabil. 1999;14:602–15. doi: 10.1097/00001199-199912000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Faul M, Wald MM, Rutland-Brown W, Sullivent EE, Sattin RW. Using a cost-benefit analysis to estimate outcomes of a clinical treatment guideline: testing the Brain Trauma Foundation guidelines for the treatment of severe traumatic brain injury. J Trauma. 2007;63:1271–8. doi: 10.1097/TA.0b013e3181493080. [DOI] [PubMed] [Google Scholar]
- 7.Robertson CS, Valadka AB, Hannay HJ, Contant CF, Gopinath SP, Cormio M, Uzura M, Grossman RG. Prevention of secondary ischemic insults after severe head injury. Crit Care Med. 1999;27:2086–95. doi: 10.1097/00003246-199910000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Davis DP, Idris AH, Sise MJ, Kennedy F, Eastman AB, Velky T, Vilke GM, Hoyt DB. Early ventilation and outcome in patients with moderate to severe traumatic brain injury. Crit Care Med. 2006;34:1202–8. doi: 10.1097/01.CCM.0000208359.74623.1C. [DOI] [PubMed] [Google Scholar]
- 9.Stocchetti N, Furlan A, Volta F. Hypoxemia and arterial hypotension at the accident scene in head injury. J Trauma. 1996;40:764–7. doi: 10.1097/00005373-199605000-00014. [DOI] [PubMed] [Google Scholar]
- 10.Chi JH, Knudson MM, Vassar MJ, McCarthy MC, Shapiro MB, Mallet S, Holcroft JJ, Moncrief H, Noble J, Wisner D, Kaups KL, Bennick LD, Manley GT. Prehospital hypoxia affects outcome in patients with traumatic brain injury: a prospective multicenter study. J Trauma. 2006;61:1134–41. doi: 10.1097/01.ta.0000196644.64653.d8. [DOI] [PubMed] [Google Scholar]
- 11.Badjatia N, Carney N, Crocco TJ, Fallat ME, Hennes HMA, Jagoda AS, Jernigan S, Letarte PB, Lerner EB, Moriarty TM, Pons PT, Sasser S, Scalea T, Schleien CL, Wright DW. Guidelines for Prehospital Management of Traumatic Brain Injury 2nd Edition. Prehospital Emergency Care. 2008;12:S1–S52. doi: 10.1080/10903120701732052. [DOI] [PubMed] [Google Scholar]
- 12.Chesnut RM, Marshall LF, Klauber MR, Blunt BA, Baldwin N, Eisenberg HM, Jane JA, Marmarou A, Foulkes MA. The role of secondary brain injury in determining outcome from severe head injury. J Trauma. 1993;34:216–22. doi: 10.1097/00005373-199302000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Davis DP, Dunford JV, Ochs M, Park K, Hoyt DB. The use of quantitative end-tidal capnometry to avoid inadvertent severe hyperventilation in patients with head injury after paramedic rapid sequence intubation. J Trauma. 2004;56:808–14. doi: 10.1097/01.ta.0000100217.05066.87. [DOI] [PubMed] [Google Scholar]
- 14.Davis DP, Dunford JV, Poste JC, Ochs M, Holbrook T, Fortlage D, Size MJ, Kennedy F, Hoyt DB. The impact of hypoxia and hyperventilation on outcome after paramedic rapid sequence intubation of severely head-injured patients. J Trauma. 2004;57:1–8. doi: 10.1097/01.ta.0000135503.71684.c8. discussion 8–10. [DOI] [PubMed] [Google Scholar]
- 15.Denninghoff KR, Griffin MJ, Bartolucci AA, Lobello SG, Fine PR. Emergent endotracheal intubation and mortality in traumatic brain injury. West J Emerg Med. 2008;9:184–9. [PMC free article] [PubMed] [Google Scholar]
- 16.Fearnside MR, Cook RJ, McDougall P, McNeil RJ. The Westmead Head Injury Project outcome in severe head injury. A comparative analysis of pre-hospital, clinical and CT variables. Br J Neurosurg. 1993;7:267–79. doi: 10.3109/02688699309023809. [DOI] [PubMed] [Google Scholar]
- 17.Spaite DW, Hu C, Bobrow BJ, Chikani V, Barnhart B, Gaither JB, Denninghoff KR, Adelson PD, Keim SM, Viscusi C, Mullins T, Sherrill D. The Effect of Combined Out-of-Hospital Hypotension and Hypoxia on Mortality in Major Traumatic Brain Injury. Ann Emerg Med. 2016;69:62–72. doi: 10.1016/j.annemergmed.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bouchama A, Knochel JP. Heat stroke. N Engl J Med. 2002;346:1978–88. doi: 10.1056/NEJMra011089. [DOI] [PubMed] [Google Scholar]
- 19.Gaither JB, Galson S, Curry M, Mhayamaguru M, Williams C, Keim SM, Bobrow BJ, Spaite DW. Environmental Hyperthermia in Prehospital Patients with Major Traumatic Brain Injury. The Journal of Emergency Medicine. 2015;49:375–381. doi: 10.1016/j.jemermed.2015.01.038. [DOI] [PubMed] [Google Scholar]
- 20.Ireland S, Endacott R, Cameron P, Fitzgerald M, Paul E. The incidence and significance of accidental hypothermia in major trauma--a prospective observational study. Resuscitation. 2011;82:300–6. doi: 10.1016/j.resuscitation.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 21.Jones TS, Liang AP, Kilbourne EM, Griffin MR, Patriarca PA, Wassilak SG, Mullan RJ, Herrick RF, Donnell HD, Choi K, Thacker SB. Morbidity and mortality associated with the July 1980 heat wave in St Louis and Kansas City, Mo. JAMA. 1982;247:3327–31. [PubMed] [Google Scholar]
- 22.Jurkovich GJ, Greiser WB, Luterman A, Curreri PW. Hypothermia in trauma victims: an ominous predictor of survival. J Trauma. 1987;27:1019–24. [PubMed] [Google Scholar]
- 23.Keim SM, Guisto JA, Sullivan JB. Environmental thermal stress. Ann Agric Environ Med. 2002;9:1–15. [PubMed] [Google Scholar]
- 24.Martin RS, Kilgo PD, Miller PR, Hoth JJ, Meredith JW, Chang MC. Injury-associated hypothermia: an analysis of the 2004 National Trauma Data Bank. Shock. 2005;24:114–8. doi: 10.1097/01.shk.0000169726.25189.b1. [DOI] [PubMed] [Google Scholar]
- 25.Strachan RD, Whittle IR, Miller JD. Hypothermia and severe head injury. Brain Injury. 1989;3:51–56. doi: 10.3109/02699058909008073. [DOI] [PubMed] [Google Scholar]
- 26.McHugh GS, Butcher I, Steyerberg EW, Lu J, Mushkudiani N, Marmarou A, Maas AI, Murray GD. Statistical approaches to the univariate prognostic analysis of the IMPACT database on traumatic brain injury. J Neurotrauma. 2007;24:251–8. doi: 10.1089/neu.2006.0026. [DOI] [PubMed] [Google Scholar]
- 27.Adelson PD, Wisniewski SR, Beca J, Brown SD, Bell M, Muizelaar JP, Okada P, Beers SR, Balasubramani GK, Hirtz D Consortium PTBI. Comparison of hypothermia and normothermia after severe traumatic brain injury in children (Cool Kids): a phase 3, randomised controlled trial. Lancet Neurol. 2013;12:546–53. doi: 10.1016/S1474-4422(13)70077-2. [DOI] [PubMed] [Google Scholar]
- 28.Saxena M, Andrews PJ, Cheng A, Deol K, Hammond N. Modest cooling therapies (35°C to 37.5°C) for traumatic brain injury. Cochrane Database Syst Rev. 2014;8:CD006811. doi: 10.1002/14651858.CD006811.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Childs C, Wieloch T, Lecky F, Machin G, Harris B, Stocchetti N. Report of a consensus meeting on human brain temperature after severe traumatic brain injury: its measurement and management during pyrexia. Front Neurol. 2010;1:146. doi: 10.3389/fneur.2010.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kilpatrick MM, Lowry DW, Firlik AD, Yonas H, Marion DW. Hyperthermia in the neurosurgical intensive care unit. Neurosurgery. 2000;47:850–5. doi: 10.1097/00006123-200010000-00011. discussion 855–6. [DOI] [PubMed] [Google Scholar]
- 31.Bao L, Chen D, Ding L, Ling W, Xu F. Fever burden is an independent predictor for prognosis of traumatic brain injury. PLoS One. 2014;9:e90956. doi: 10.1371/journal.pone.0090956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Natale JE, Joseph JG, Helfaer MA, Shaffner DH. Early hyperthermia after traumatic brain injury in children: risk factors, influence on length of stay, and effect on short-term neurologic status. Crit Care Med. 2000;28:2608–15. doi: 10.1097/00003246-200007000-00071. [DOI] [PubMed] [Google Scholar]
- 33.Li J, Jiang JY. Chinese Head Trauma Data Bank: effect of hyperthermia on the outcome of acute head trauma patients. J Neurotrauma. 2012;29:96–100. doi: 10.1089/neu.2011.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weisend MP, Feeney DM. The relationship between traumatic brain injury-induced changes in brain temperature and behavioral and anatomical outcome. J Neurosurg. 1994;80:120–32. doi: 10.3171/jns.1994.80.1.0120. [DOI] [PubMed] [Google Scholar]
- 35.Thompson HJ, Tkacs NC, Saatman KE, Raghupathi R, McIntosh TK. Hyperthermia following traumatic brain injury: a critical evaluation. Neurobiol Dis. 2003;12:163–73. doi: 10.1016/s0969-9961(02)00030-x. [DOI] [PubMed] [Google Scholar]
- 36.Thompson HJ, Pinto-Martin J, Bullock MR. Neurogenic fever after traumatic brain injury: an epidemiological study. J Neurol Neurosurg Psychiatry. 2003;74:614–9. doi: 10.1136/jnnp.74.5.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spaite DW, Bobrow BJ, Stolz U, Sherrill D, Chikani V, Barnhart B, Sotelo M, Gaither JB, Viscusi C, Adelson PD, Denninghoff KR. Evaluation of the impact of implementing the emergency medical services traumatic brain injury guidelines in Arizona: the Excellence in Prehospital Injury Care (EPIC) study methodology. Acad Emerg Med. 2014;21:818–30. doi: 10.1111/acem.12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Badjatia N, Carney N, Crocco TJ, Fallat ME, Hennes HM, Jagoda AS, Jernigan S, Letarte PB, Lerner EB, Moriarty TM, Pons PT, Sasser S, Scalea T, Schleien CL, Wright DW, Brain Trauma F Management BTFCfG. Guidelines for prehospital management of traumatic brain injury 2nd edition. Prehosp Emerg Care. 2008;12(Suppl 1):S1–52. doi: 10.1080/10903120701732052. [DOI] [PubMed] [Google Scholar]
- 39.Davis DP, Dunford JV, Ochs M, Park K, Hoyt DB. The use of quantitative end-tidal capnometry to avoid inadvertent severe hyperventilation in patients with head injury after paramedic rapid sequence intubation. Journal of Trauma-Injury Infection and Critical Care. 2004;56:808–814. doi: 10.1097/01.ta.0000100217.05066.87. [DOI] [PubMed] [Google Scholar]
- 40.Gaither JB, Spaite DW, Bobrow BJ, Denninghoff KR, Stolz U, Beskind DL, Meislin HW. Balancing the potential risks and benefits of out-of-hospital intubation in traumatic brain injury: the intubation/hyperventilation effect. Ann Emerg Med. 2012;60:732–6. doi: 10.1016/j.annemergmed.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 41.Wade CE, Salinas J, Eastridge BJ, McManus JG, Holcomb JB. Admission hypo- or hyperthermia and survival after trauma in civilian and military environments. Int J Emerg Med. 2011;4:35. doi: 10.1186/1865-1380-4-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shafi S, Elliott AC, Gentilello L. Is hypothermia simply a marker of shock and injury severity or an independent risk factor for mortality in trauma patients? Analysis of a large national trauma registry. J Trauma. 2005;59:1081–5. doi: 10.1097/01.ta.0000188647.03665.fd. [DOI] [PubMed] [Google Scholar]
- 43.Zheng D, Arima H, Heeley E, Karpin A, Heller G, Yang J, Chalmers J, Anderson CS. Ambient temperature and severity of intracerebral haemorrhage: the INTERACT1 study. Neuroepidemiology. 2014;42:169–73. doi: 10.1159/000358304. [DOI] [PubMed] [Google Scholar]
- 44.Dukes SF, Bridges E, Johantgen M. Occurrence of secondary insults of traumatic brain injury in patients transported by critical care air transport teams from Iraq/Afghanistan: 2003–2006. Mil Med. 2013;178:11–7. doi: 10.7205/milmed-d-12-00177. [DOI] [PubMed] [Google Scholar]
- 45.Hermstad E, Adams B. Traumatic brain injury complicated by environmental hyperthermia. J Emerg Trauma Shock. 2010;3:66–9. doi: 10.4103/0974-2700.58660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Axelrod YK, Diringer MN. Temperature management in acute neurologic disorders. Neurol Clin. 2008;26:585–603. xi. doi: 10.1016/j.ncl.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 47.Corrigan JD, Kreider S, Cuthbert J, Whyte J, Dams-O’Connor K, Faul M, Harrison-Felix C, Whiteneck G, Pretz CR. Components of traumatic brain injury severity indices. J Neurotrauma. 2014;31:1000–7. doi: 10.1089/neu.2013.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]