Abstract
Fear of cancer recurrence (FCR) is one of the most prevalent unmet psychosocial needs. This study aimed to confirm the cultural equivalence, reliability, and validity of the Korean version of Fear of Cancer Recurrence Inventory (K-FCRI). We conducted a forward–backward translation of the English version FCRI to Korean version through meticulous process including transcultural equivalence test. The psychometric property of the K-FCRI was then validated in 444 survivors from cancers at various sites. The Korean translation was accepted well by participants. There was a good cultural equivalence between the Korean version and the English version of FCRI. Confirmatory factor analysis supported the original seven-factor structure with slightly insufficient level of goodness-of-fit indices (comparative fit index = 0.900, non-normed fit index = 0.893, root mean square error of approximation = 0.060). The K-FCRI had high internal consistency (α = 0.85 for total scale and α = 0.77–0.87 for subscales) and test-retest reliability (r = 0.90 for total scale and r = 0.54–0.84 for subscales). The K-FCRI had significant correlations with the Korean version of Fear of Progression Questionnaire, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Version 3.0, Hospital Anxiety and Depression Scale, and Fatigue Severity Score, supporting the good construct validity and psychometric properties of K-FCRI. The K-FCRI was confirmed as a valid and reliable psychometric test for measuring FCR of Korean survivors from cancers at various sites.
Keywords: Fear, Neoplasms, Recurrence, Survivors, Validation Studies
Graphical Abstract
INTRODUCTION
Fear of cancer recurrence (FCR) is defined as the fear that cancer might return or progress in the same region or in another part of the body (1,2). People who have been diagnosed with cancer commonly suffer from various degrees of FCR. A study has reported that over 30% of ovarian cancer patients have experienced worry about cancer recurrence at least once a week, even after surviving for more than 2 years after completing cancer treatment (3). About 56% of breast cancer survivors have experienced moderate to severe FCR in another study (4). FCR is one of the most prevalent unmet psychosocial needs. It might be associated with psychological distress, functioning impairments, and increased use of health care resources (5,6). Therefore, evaluation of FCR seems essential for the care of cancer survivors. However, relatively few measures are available based on empirically supported theory of FCR (7).
Fear of Cancer Recurrence Inventory (FCRI), one of the strongest psychometrical tools that measures FCR for heterogeneous cancer populations, has been developed based on cognitive-behavioral conceptualization of FCR (7,8). It contains 42 items evaluating seven FCR components (triggers, severity, psychological distress, functioning impairments, insight, reassurance, and coping strategies). The original French version of FCRI was developed by Simard and Savard (8) using 600 French Canadians who survived breast, colon, prostate, or lung cancer. It has excellent level of internal consistency, reliability, and constructed validity (8). After that, an English version of FCRI has been validated (2).
In Korea, the 5-year survival rate of cancer patients was increased from 53.8% during 2001–2005 to 69.4% during 2008–2013, resulting in about 1,370,049 cancer survivors in 2013 (9). Several studies have found that the prevalence of psychological problem is substantially high in Korean cancer survivors (10,11,12). Therefore, the need for an instrument that adopts multi-dimensional approach to assess FCR of Korean cancer survivors is increasing. To the best of our knowledge, psychometrical instrument that encompasses comprehensive aspects of FCR of Koreans is currently unavailable. Therefore, the objective of the present study was to determine the cross-cultural equivalence of the Korean version of FCRI (K-FCRI) and examine its reliability and validity of K-FCRI using psychometric properties.
MATERIALS AND METHODS
We conducted the translation and validation of the K-FCRI through the following steps: 1) performing forward–backward translation of English version FCRI and pilot test, 2) establishing cross-cultural equivalence using a bilingual (both English and Korea) sample, and 3) measuring the psychometric properties in a large sample of Korean cancer survivors.
Translation and pilot test
The FCRI is a multidimensional questionnaire composed of seven subscale components of FCR: potential stimuli activating FCR (triggers), presence and severity of intrusive thoughts associated with FCR (severity), emotional disturbance associated with FCR (psychological distress), impact of FCR on important areas of functioning (functional impairments), self-criticism toward FCR intensity (insight), reassurance seeking such as thorough self-examination or repeated medical consultations (reassurance), and other strategies to cope with FCR (coping strategies) (8). Each item is rated on a Likert scale ranging from zero (‘not at all’ or ‘never’) to four (‘a great deal’ or ‘all the time’). A subscale score can be calculated for each subscale component. The total score is then calculated based on the scores of each subscale. Considering that the question for item 13 (“I believe that I am cured and the cancer will not come back”) is addressed in opposite direction of other questions, the response scale to item 13 is reversely put in the calculation of total score. Higher summary score of FCRI indicates higher levels of FCR. In addition, the severity subscale of the FCRI (also referred to as FCRI-short form) has an empirically validated cutoff score (≥ 13 points) for screening clinically significant level of FCR (13). Cronbach's alpha value for each seven subscales has been reported as follows in a previous validation study (8): trigger, α = 0.90; severity, α = 0.89, psychological distress, α = 0.86; functioning impairments, α = 0.91; insight, α = 0.80; reassurance, α = 0.75; and coping strategies, α = 0.89.
Initial translation of the FCRI from English version to Korean was done by a panel composed of three medical experts (one psychologist and two family physicians) who can speak both English and Korean fluently. Another bilingual psychologist who was blinded to the English version of FCRI backward translated the K-FCRI into English. A certified simultaneous Korean-English interpreter assessed the backward-translated English version FCRI as having maintained the semantics and meanings of the English version of FCRI.
Using the first-translated K-FCRI, a pilot test was conducted in 13 participants, including three cancer patients (breast, stomach, and thyroid), two patients without cancer, six physicians, and two nurses. All participating physicians and nurses had experience in cancer patient management. For the pilot test, the K-FCRI was self-administered to the 13 participants. A face-to-face interview was then performed by a well-trained research assistant to ask them whether any items of questions were confusing or difficult to answer. The first-translated K-FCRI was then revised accordingly to develop the final Korean version by the two initial translators considering the feedback and proposed changes based on the pilot test. The final Korean version was re-pilot tested in another five cancer patients (breast, two stomach, thyroid, and colon). They confirmed that the instructions, questions, and response options of the final K-FCRI could be clearly understood.
Cross-cultural validity and reliability
Thirty-two bilinguals composed of six cancer patients, eight nurses, and 18 physicians assessed the cultural equivalence of the K-FCRI and the original English versions FCRI. The mean score of these bilingual evaluators in assessing the level of fluency in both Korean and English was 6.1 in a self-rated 10-point scale (0 point: not at all to 10 point: perfectly).
We evaluated the language, similarity, and interpretability between the English version FCRI and the K-FCRI using counterbalanced design (13,14), for which participants were randomly assigned to begin with either the Korean version or with the original English version. Comparability of language refers to the formal similarity of words, phrases, and sentences. Similarity of interpretability refers to the degree to which the two versions engender the same response even though the wording is not the same. Similarity was quantified by Likert scale ranging from one (extremely comparable/extremely similar) to seven (not at all comparable/not at all similar). Question items acquiring a mean score of > 3 in any category or between 2.5 and 3 in interpretability were considered problematic and reviewed for possible correction (15). In our study, further correction was unnecessary because the mean scores for the comparability of language and similarity of interpretability were 1.34 and 1.49, respectively. Therefore, cultural equivalence of the FCRI between the Korean version and the English version was confirmed. To estimate the test-retest reliability of K-FCRI, we repeatedly administered it to 62 participants (14.0%) on two occasions with mean interval of 206 days (range, 25–444 days).
Measurement of psychometric properties
A self-administered questionnaire consisting of the K-FCRI and other instruments selected for validation of psychometric property of FCRI was given to 444 study participants. A trained research assistant supplemented the incompletely answered questions through additional face-to-face interview if necessary.
We assessed the psychometric properties of the K-FCRI in long-term cancer survivors who had visited cancer survivorship clinic for routine surveillance or care for health problems from September 2014 to December 2015. Of 603 cancer survivors who were contacted, 156 (25.9%) refused to participate in our study. The most common reasons for refusal were “too busy to complete the questionnaire” or “feel uncomfortable to be involved in a research.” Demographic characteristics of study participants were compared to those of nonparticipants using t-test or χ2 test. Although non-participants had slightly different distribution in age and cancer sites (P < 0.010) compared to participants, there was no significant difference in the time lapse since cancer diagnosis, distribution of sex, or treatment modality (Supplementary Table 1). Among 447 cancer survivors who provided written informed consent form, we excluded those who had missing data for more than 50% of question items (n = 1), answering with ‘0’ to all questions (n = 2) including one item that was reverse scored (item 13). Finally, data from 444 cancer survivors were included in our final analysis.
We selected four Korean version of instruments which have been previously validated for measuring psychological distress in Korean patients with cancer or other chronic diseases. They are Fear of Progression Questionnaire (FoP-Q), European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30, version 3.0), Hospital Anxiety and Depression Scale (HADS), and Fatigue Severity Score (FSS).
The FoP-Q consists of 43 statements regarding various concerns related to disease progression and coping with these concerns categorized into five subscales: affective reactions (13 items), partnership/family (7 items), occupation (7 items), loss of autonomy (7 items), and coping (9 items) (14). A five-point Likert scale was used for checking responses from 1 (never) to 5 (very often). It provides two total scores: one for FoP and the other for coping (14). In a study for validating the Korean version of FoP-Q, the Cronbach's alpha coefficient has been reported to be 0.92 for FoP total scale, 0.90 for affective reactions, 0.73 for partnership/family, 0.87 for occupation, 0.84 for loss of autonomy, and 0.68 for coping (14).
The 30-item questionnaire, EORTC QLQ-C30 was developed to assess health related quality of life of cancer patients, incorporating five functional scales (physical, cognitive, social, emotional, and role), symptom scale, and global quality of life scale (16). The score of each scale ranges from zero to 100. Lower functional score (≤ 33) indicates worse global health status and worse functional status while higher symptom score (≥ 66) indicates worse symptomatic status. The Cronbach's alpha coefficients for the Korean version of EORTC QLQ-C30 have been found to be greater than 0.70 for most subscales in a previous validation study except for cognitive functioning (α = 0.60) (16).
HADS is a 14-item questionnaire widely used to assess both dimensional and categorical aspects of anxiety and depression in cancer patients (17). Total summary score of HADS ranges from 0 to 21, with higher score indicating greater level of anxiety or depression. In a previous validation study for the Korean version of HADS, the Cronbach's alpha coefficient has been found to be 0.78 for anxiety subscale and 0.85 for depression subscale (18).
FSS is a 10-item questionnaire developed to assess the effect of fatigue on daily activities with seven-point response scale from 1 (strongly disagree) to 7 (strongly agree) (19). Higher score denotes more severe fatigue. In a previous validation study for the Korean version of FSS, the Cronbach's alpha coefficient for total FSS is 0.935, ranging from 0.925 to 0.932 for subscales (20).
Other measurements in this study included demographic and socioeconomic characteristics (marital status, education level, employment status, income level, religious activity) of participants, which were obtained using a self-administered questionnaire. We reviewed medical records to obtain information about cancer such as the site and stage of cancer treatment modality, status of metastases at the time of primary cancer diagnosis, cancer recurrence, second primary cancer, and family history of cancer.
Statistical analysis
We evaluated the difference of total K-FCRI score between different cancer sites by the analysis of variance (ANOVA) with post-hoc comparison, after adjusting for years since diagnosis, sex, and cancer stage. The reliability of K-FCRI was assessed by internal consistency and test-retest reliability. Internal consistency between items pertained to a subscale was estimated based on the Cronbach's alpha coefficient. Test-retest reliability was assessed by estimating intra-class correlations between responses to repeatedly addressed K-FCRI questionnaire on two different occasions. Construct validity of K-FCRI was evaluated by estimating convergent validity, concurrent criterion validity, and divergent validity.
We conducted a series of confirmatory factor analyses (CFAs) on the K-FCRI to assess divergent validity by examining whether the original 7-factor solution found for the English version FCRI could be replicated in K-FCRI (8). The tested model was planned with three levels following the original structure model: each of the 42 items, primary factors (7 subscales), and one secondary factor (FCR total score). Goodness-of-fit indices were used to assess the fitness of this model using chi-square likelihood ratio statistic (χ2), comparative fit index (CFI), non-normed fit index (NNFI), and root-mean-square error of approximation (RMSEA). The cut-off criteria for assessing goodness-of-fit were ≥ 0.90 for CFI, ≥ 0.95 for NNFI, and ≤ 0.06 for RMSEA (21).
Convergence validity was assessed by estimating Pearson's correlation coefficients between each subscale, between total summary score of K-FCRI and the score of each subscale of K-FCRI, and between each item and total summary score after correcting overlap. Concurrent criterion validity was evaluated by estimating Pearson's correlation coefficients between total K-FCRI score and scores of the Korean version of other psychometric instruments selected for this study such as FoP-Q, EORTC QLQ-C30, HADS, and FSS.
CFA was performed using Mplus version 6.1 (Muthén and Muthén, Los Angeles, CA, USA). All other analyses were performed using PASW Statistics 23.0 software (SPSS Inc., Chicago, IL, USA). The correlation strength was categorized according to the original validation study: weak (< 0.4), moderate (0.4–0.69), and strong (≥ 0.7) (22).
Ethics statement
Institutional Review Board (IRB) of Samsung Medical Center approved this study (IRB file number: SMC 2013-07-133). Informed consent was obtained from each participant.
RESULTS
The characteristics and score of the K-FCRI of the study population are summarized in Table 1. The mean age and mean survival time after cancer diagnosis were 55.3 years and 6.0 years, respectively. Most participants were married. Approximately half of these participants had received college or higher level of education with employed status. Around three quarters of these participants were involved in religious activities. The mean of total summary score of K-FCRI was 59.4. Among subscales of K-FCRI, the score for coping strategy was the highest, followed by trigger, severity, reassurance, and distress. The score for insight was the lowest.
Table 1. Demographic and socioeconomic characteristics of study participants.
| Characteristics | Total (n = 444) | Men (n = 157) | Women (n = 287) |
|---|---|---|---|
| Age, yr | 55.3 (10.3) | 58.5 (9.5) | 53.5 (10.3) |
| Age at cancer diagnosis, yr | 49.4 (10.2) | 52.6 (9.7) | 47.7 (10.0) |
| Time since cancer diagnosis, yr | 6.0 (4.2) | 6.0 (3.6) | 6.0 (4.5) |
| Marital status, % | |||
| Married/with partner | 80.9 | 89.8 | 76.0 |
| Single | 15.7 | 7.0 | 20.9 |
| Unknown | 3.4 | 3.2 | 3.1 |
| Education level, % | |||
| ≤ High school | 45.1 | 42.0 | 46.9 |
| ≥ College degree | 47.5 | 50.4 | 45.8 |
| Unknown | 7.4 | 7.6 | 7.3 |
| Employment, % | |||
| Retired/unemployed | 51.8 | 27.4 | 65.2 |
| Employed | 47.5 | 71.3 | 34.5 |
| Unknown | 0.7 | 1.2 | 0.3 |
| Monthly household income (Korean won), % | |||
| < 1,000,000 | 5.2 | 3.2 | 6.3 |
| 1,000,000–1,990,000 | 12.9 | 13.4 | 12.6 |
| 2,000,000–3,990,000 | 24.6 | 24.2 | 24.8 |
| More than 4,000,000 | 40.2 | 45.2 | 37.4 |
| Unknown | 17.1 | 14.0 | 18.9 |
| Religion | |||
| Do not have | 26.2 | 33.1 | 22.4 |
| Have, but no religious activity | 24.6 | 24.8 | 24.5 |
| Irregular activity | 16.7 | 17.2 | 16.4 |
| Regular activity | 32.5 | 24.8 | 36.7 |
| FCRI (range of score) | |||
| Total summary score (0–168) | 59.4 (24.3) | 54.0 (24.2) | 62.5 (23.8) |
| Trigger (0–32) | 13.3 (7.1) | 12.3 (7.4) | 13.8 (6.8) |
| Severity (0–36) | 12.5 (6.4) | 11.7 (6.4) | 12.9 (6.4) |
| Psychological distress (0–16) | 4.1 (3.9) | 3.5 (3.6) | 4.4 (4.0) |
| Coping strategies (0–36) | 18.5 (7.7) | 16.2 (7.7) | 19.8 (7.4) |
| Functioning impairments (0–24) | 4.9 (5.8) | 4.4 (5.5) | 5.1 (6.0) |
| Insight (0–12) | 1.4 (2.0) | 1.5 (1.9) | 1.3 (2.1) |
| Reassurance (0–12) | 4.9 (3.3) | 4.4 (3.5) | 5.2 (3.2) |
Values are presented as mean (SD).
FCRI = Fear of Cancer Recurrence Inventory, SD = standard deviation.
Cancer related information of participants is shown in Table 2. The sites of primary cancer were very diverse. Stomach and breast cancer patients occupied more than 50% of participants. Earlier stages of cancer were more common. More than half of these participants had stage I cancer. Most (96.1%) of these participants had undergone surgery for cancer treatment. At the time of primary cancer diagnosis, 1.3% had metastatic lesion. Among these cancer participants, 1.6% experienced recurrence of cancer and 3.9% already received a second primary cancer diagnosis. Total K-FCRI score of breast cancer patients was significantly higher than that of stomach cancer and lung cancer patients.
Table 2. Cancer related information of study participants according to primary cancer site.
| Cancer parameters | Total (n = 444) | Stomach (n = 173) | Breast (n = 112) | Lung (n = 42) | Thyroid (n = 37) | Other (n = 80) |
|---|---|---|---|---|---|---|
| Stage of primary cancer, % | ||||||
| I | 52.5 | 53.3 | 43.6 | 62.5 | 61.3 | 52.3 |
| II | 24.9 | 33.3 | 32.7 | 12.5 | 9.7 | 15.9 |
| III & IV | 19.0 | 13.3 | 14.5 | 25.0 | 29.0 | 25.0 |
| Unknown | 3.6 | 0 | 9.1 | 0 | 0 | 6.8 |
| Cancer treatment received, % | ||||||
| Surgery | 96.1 | 93.8 | 100 | 95.1 | 97.1 | 88.0 |
| Chemotherapy | 41.4 | 37.0 | 70.0 | 35.0 | 0 | 30.1 |
| Radiotherapy | 37.4 | 24.1 | 76.4 | 17.5 | 3.0 | 35.7 |
| Hormone therapy | 1.6 | - | 59.6 | - | - | - |
| Metastasis at diagnosis | 1.3 | 0.6 | 0 | 2.6 | 3.4 | 2.2 |
| Recurrence | 1.6 | 1.3 | 3.0 | 2.8 | 0 | 0 |
| Development of second primary cancer* | 3.9 | 1.1 | 8.9 | 2.4 | 0 | 5.0 |
| Family history of cancer | 47.5 | 43.9 | 50.0 | 40.5 | 45.9 | 56.3 |
| Total FCRI score† | 59.2 (24.5) | 57.2 (24.1)‡ | 67.0 (24.3)‡ | 52.9 (22.5)‡ | 56.7 (28.0) | 57.0 (23.2) |
Values are presented as percentage or mean (SD).
FCRI = Fear of Cancer Recurrence Inventory, SD = standard deviation, ANOVA = analysis of variance, FCR = fear of cancer recurrence.
*Cancer sites of second primary cancer (number of cases) were thyroid (n = 10), breast (n = 3), stomach (n = 2), lung (n = 1), and ovary cancer (n = 1). †The difference among cancer site obtained by the ANOVA with post hoc comparison after adjusted for year since diagnosis, sex and canter stage. ‡P < 0.05, breast cancer patients had a higher level of FCR.
The findings from CFA conducted in a series of two models to determine factor structure of the K-FCRI and assess discriminant validity on construct level are shown in Table 3. In the initial model (model A), the goodness-of-fit indices did not fully meet the criteria for adequate model fit (χ2 = 2,710.283, df = 812, CFI = 0.853, NNFI = 0.844, RMSEA = 0.073, 90% confidence interval = 0.070–0.076). In the next model (model B), modification indices were applied to free the parameters in the error covariance matrix, similar to the method used by Lebel et al. (2). The revised model showed improvement over the initial model. The original seven-factor structure remained. In addition, the same nine covariances were found, although items 13 and 14 were replaced by items 15 and 16. With the new adjustment, all goodness-of-fit indices were improved: RMSEA was improved from 0.073 to 0.060, CFI was improved from 0.853 to 0.900, and NNFI was improved from 0.844 to 0.893.
Table 3. Summary of results from CFAs for K-FCRI.
| Models | Study | χ2 | df | CFI | NNFI | RMSEA (90% CI) |
|---|---|---|---|---|---|---|
| Model A | Simard and Savard (2009) | 2,710.28 | 812 | 0.853 | 0.844 | 0.073 (0.070–0.076) |
| Model B | Shin et al. (2017) | 2,093.86 | 803 | 0.900 | 0.893 | 0.060 (0.057–0.063) |
CFA = confirmatory factor analysis, K-FCRI = Korean version of Fear of Cancer Recurrence Inventory, CFI = comparative fit index, NNFI = non-normed fit index, RMSEA = root-mean-square error of approximation, CI = confidence interval.
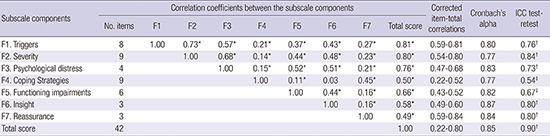
The reliability, convergence validity, and discrimination validity of K-FCRI are shown in Table 4. Corrected item-total correlations and Cronbach's alphas met the standards of convergence validity. The levels of corrected item-total correlations (r = 0.22 to 0.80) were neither less than 0.20 nor more than 0.80 (23). Cronbach's alpha values for total K-FCRI and subscales were 0.85 and 0.77–0.84, respectively, which were within acceptable to good levels. Test-retest reliability assessed by the intra-class correlation coefficient (ICC) between the responses to the repeatedly administered questionnaire was within reliable range: ICC for total K-FCRI was 0.90 (P < 0.001), ICC for subscales of FCRI ranged between 0.54 and 0.84 (P < 0.050). Four subscales (triggers, severity, psychological distress, and functioning impairments) had moderate to strong levels of correlations with total score, whereas coping strategies, insight, and reassurance subscales had low to moderate correlations with total summary score.
Table 4. Reliability and convergence validity of the K-FCRI.
| Subscale components | Correlation coefficients between the subscale components | Corrected | Cronbach's alpha | ICC test-retest | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. items | F1 | F2 | F3 | F4 | F5 | F6 | F7 | Total score | item-total correlations | |||
| F1. Triggers | 8 | 1.00 | 0.73* | 0.57* | 0.21* | 0.37* | 0.43* | 0.27* | 0.81* | 0.59-0.81 | 0.80 | 0.76† |
| F2. Severity | 9 | 1.00 | 0.68* | 0.14* | 0.44* | 0.48* | 0.23* | 0.80* | 0.54-0.80 | 0.77 | 0.84† | |
| F3. Psychological distress | 4 | 1.00 | 0.15* | 0.52* | 0.51* | 0.21* | 0.76* | 0.47-0.68 | 0.83 | 0.73† | ||
| F4. Coping Strategies | 9 | 1.00 | 0.11* | 0.03 | 0.45* | 0.50* | 0.22-0.52 | 0.77 | 0.54‡ | |||
| F5. Functioning impairments | 6 | 1.00 | 0.44* | 0.16* | 0.66* | 0.43-0.52 | 0.82 | 0.67‡ | ||||
| F6. Insight | 3 | 1.00 | 0.16* | 0.58* | 0.49-0.60 | 0.87 | 0.80† | |||||
| F7. Reassurance | 3 | 1.00 | 0.49* | 0.59-0.84 | 0.84 | 0.80† | ||||||
| Total score | 42 | 1.00 | 0.22-0.80 | 0.85 | 0.90† | |||||||
K-FCRI = Korean version of Fear of Cancer Recurrence Inventory, ICC = intra-class correlation coefficient.
*P < 0.00625 (Bonferroni correction applied); †P < 0.001; ‡P < 0.05.
The correlations between total summary score of K-FCRI and scores of FoP-Q, EORTC QLQ-C30, HADS-K, and FSS are shown in Table 5. Total score and subscale scores of FoP-Q showed significant positive correlations with the total score of K-FCRI. The correlation coefficient between K-FCRI with total score of FoP-Q was 0.73. There were significant inverse correlations between total summary score of K-FCRI and the scores of EORTC QLQ-C30 subscales. The highest correlation coefficient was found for emotional functioning scale. The inverse correlation between these tools indicated that cancer patients with high FCR might have poor quality of life because higher score of EORTC QLQ-C30 reflected better status. Total summary score of K-FCRI was positively correlated with anxiety category of HADS. However, there was no significant correlation between the total summary score of K-FCRI with the depression category of HADS. Total summary score of K-FCRI was positively correlated with total score of FSS. We presented K-FCRI in Supplementary Table 2.
Table 5. Correlation between the K-FCRI and other Korean version tools measuring psychological distress.
| Tools | Score range | Mean score (SD) | Correlation coefficient (r) with FCRI |
|---|---|---|---|
| FoP-Q | |||
| Affective reaction | 1–5 | 2.17 (0.80) | 0.65† |
| Partnership/family | 1–5 | 2.16 (0.74) | 0.56† |
| Occupation | 1–5 | 1.74 (0.83) | 0.47† |
| Loss of independence | 1–5 | 1.83 (0.62) | 0.57† |
| Total* | 1–20 | 7.73 (2.52) | 0.73† |
| Coping | 1–5 | 3.01 (0.82) | 0.30† |
| EORTC QLQ-C30 | |||
| Global health status | 0–100 | 66.60 (18.8) | −0.15† |
| Physical functioning | 0–100 | 78.90 (16.8) | −0.13† |
| Role functioning | 0–100 | 83.50 (22.4) | −0.21† |
| Emotional functioning | 0–100 | 76.90 (20.3) | −0.31† |
| Cognitive functioning | 0–100 | 74.60 (20.1) | −0.13† |
| Social functioning | 0–100 | 78.50 (24.6) | −0.21† |
| HADS | |||
| Anxiety | 0–21 | 5.40 (2.9) | 0.49† |
| Depression | 0–21 | 11.10 (3.3) | 0.02 |
| FSS, total | 1–70 | 2.99 (1.7) | 0.27† |
K-FCRI = Korean version of Fear of Cancer Recurrence Inventory, SD = standard deviation, FCRI = Fear of Cancer Recurrence Inventory, FoP-Q = fear of progression questionnaire, EORTC QLQ-C30 = European Organization for Research and Treatment of Cancer Quality of Life Questionnaire, HADS = Hospital Anxiety and Depression Scale, FSS = Fatigue Severity Score.
*Obtained by summation of mean values of four FoP-Q subscales except for coping scale. †P < 0.001.
DISCUSSION
In this comprehensive validation study, we demonstrated a cross-cultural equivalence and good psychometric properties of the K-FCRI, supporting that the K-FCRI is useful as a multi-dimensional instrument for assessing FCR of Korean cancer survivors. The distribution of mean score of K-FCRI in our study showed similar distributions for overall scales and subscales to those of the original French version (8) or the English version (2). The order of subscales by mean score in this study was also very similar to that of the original version of FCRI.
We obtained acceptable level of Cronbach's alpha (0.85) and corrected item-total correlation (0.22–0.80) for K-FCRI, although our estimates were slight lower than those obtained in the study for the original French-Canadian version (Cronbach's alpha: 0.95, item-total correlation: 0.26–0.82) (8). CFA showed that all goodness of fit indices of the K-FCRI met the required level of model fitness except for NNFI. The K-FCRI model fit was satisfactorily improved by fixing nine covariance parameters, although items 13 (“I believe that I am cured and that the cancer will not come back”) and 14 (“In your opinion, are you at risk of having a cancer recurrence?”) among the nine covariance parameters found in the study of Lebel et al. (2) were replaced by items 15 (“How often do you think about the possibility of cancer recurrence?”) and 16 (“How much time per day do you spend thinking about the possibility of cancer recurrence? “). These results confirmed the strong structure of FCRI even in a population with different language and culture. However, the slightly different finding regarding covariance parameters suggests that there might be redundancy among contents. Nevertheless, good construct validity of the K-FCRI assessed by convergent validity and concurrent criterion validity compensate the insufficient factorial analysis results, supporting that the K-FCRI is a usefulness tool to assess complex and multidimensional natures of FCR of Korean cancer survivors. Compared to the findings observed in the study with the original version FCRI in French-Canadian or English speaking population (0.26–0.82), each item of K-FCRI subscales showed similar levels of correlation (0.22–0.80) with total FCRI in Korean survivors (8). In general, each subscale of K-FCRI showed slightly weaker but similar correlation with total K-FCRI score than the correlations found in the original version of FCRI study.
However, the correlation between ‘coping strategies’ subscale and total FCRI was substantially different between the K-FCRI (r = 0.50) and the original French-Canadian version of FCRI (r = 0.74). In accordance with this, ‘coping measurement’ of FoP-Q had a weak correlation (r = 0.30) with the total score of FCRI in a Korean study (14). These findings indicate that coping strategies of Korean people might not have a close relation with fear of the disease. In addition, given the findings from a cross-cultural study showing that Korean cancer patients have worse health related quality of life with depressive coping than German or Japanese patients (24), it might be more difficult for Korean cancer patients to acquire adequate coping strategy for their FCR.
We evaluated the correlations of the K-FCRI with several psychometric measurement tools (EORTC QLQ-C30, HADS-anxiety, and HADS-depression) as done for the development of the original French-Canadian FCRI (8). We found that the K-FCRI had substantial correlations with those tools except for HADS-depression.
The correlation coefficients between the subscales of EORTC QLQ-C30 and K-FCRI (−0.21 to −0.13) were similar but slightly lower than those observed in the original FCRI study (−0.36 to −0.20). In both studies, global quality of life, role functioning, and social functioning subscales of EORTC QLQ-C30 had higher correlations with FCRI than physical functioning or cognitive functioning. The original French-Canadian FCRI had significant correlations with both HADS-anxiety and HADS-depression. It had moderate correlation with HADS-anxiety and low correlation with HADS-depression. In our study, the K-FCRI had no correlation with HADS-depression scale, while it had moderately high correlation with HADS-anxiety scale. This finding is similar to the findings of Shim et al. (14). These findings suggest that the K-FCRI might be more useful for assessing anxiety than for assessing depressiveness in Korean people. In addition, we evaluated the correlation of K-FCRI with the Korean version of FoP, a validated tool for measuring the fear of disease progression in Korean cancer patients. In the previous study for 112 Korean cancer patients, the FoP score of patients with recurrence of cancer was significantly different from that of patients without recurrence (14). Therefore, the moderate to strong correlation of the K-FCRI with FoP seems to support the usefulness of K-FCRI as a valid tool for measuring the fear of cancer progression.
The present study has some limitations. First, physicians and nurses were included as participants for cross-cultural validation because it was very hard to enroll bilingual (English–Korean) cancer patients. Thus, medical directives' experiences might have affected the translation. Second, FCR of people with advanced stage of cancer might not be adequately reflected to the translation process because a large portion of study participants has been diagnosed with relatively earlier stage of cancer. However, this issue does not seem to restrict the use of the K-FCRI given that the original French version of FCRI has been validated for use in cancer patients of a wide range of stages, including metastatic and recurrent cancers (8). Third, we could not establish the cut-off score for the K-FCRI to identify clinically significant FCR. Therefore, further clinical studies are needed.
In conclusion, this study confirmed that the K-FCRI could be used as a valid and reliable psychometric test to measure FCR of Korean cancers survivors with various cancer sites.
Footnotes
Funding: This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (2014R1A2A2A01002705).
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Conceptualization: Shin JY, Goo A, Ko H, Kim JH, Lim SU, Lee HK, Simard S, Song YM. Formal analysis: Shin JY, Goo A, Ko H, Kim JH, Lim SU, Lee HK, Simard S, Song YM. Investigation: Shin JY, Goo A, Ko H, Kim JH, Lim SU, Lee HK, Simard S, Song YM. Writing - original draft: Shin JY, Goo A, Ko H, Kim JH, Simard S, Song YM. Writing - review & editing: Shin JY, Goo A, Ko H, Kim JH, Lim SU, Lee HK, Simard S, Song YM.
Supplementary Materials
Distribution of participants and non-participants
K-FCRI
References
- 1.Vickberg SM. The Concerns About Recurrence Scale (CARS): a systematic measure of women's fears about the possibility of breast cancer recurrence. Ann Behav Med. 2003;25:16–24. doi: 10.1207/S15324796ABM2501_03. [DOI] [PubMed] [Google Scholar]
- 2.Lebel S, Simard S, Harris C, Feldstain A, Beattie S, McCallum M, Lefebvre M, Savard J, Devins GM. Empirical validation of the English version of the Fear of Cancer Recurrence Inventory. Qual Life Res. 2016;25:311–321. doi: 10.1007/s11136-015-1088-2. [DOI] [PubMed] [Google Scholar]
- 3.Stewart DE, Duff S, Wong F, Melancon C, Cheung AM. The views of ovarian cancer survivors on its cause, prevention, and recurrence. Medscape Womens Health. 2001;6:5. [PubMed] [Google Scholar]
- 4.van den Beuken-van Everdingen MH, Peters ML, de Rijke JM, Schouten HC, van Kleef M, Patijn J. Concerns of former breast cancer patients about disease recurrence: a validation and prevalence study. Psychooncology. 2008;17:1137–1145. doi: 10.1002/pon.1340. [DOI] [PubMed] [Google Scholar]
- 5.Crist JV, Grunfeld EA. Factors reported to influence fear of recurrence in cancer patients: a systematic review. Psychooncology. 2013;22:978–986. doi: 10.1002/pon.3114. [DOI] [PubMed] [Google Scholar]
- 6.Simard S, Thewes B, Humphris G, Dixon M, Hayden C, Mireskandari S, Ozakinci G. Fear of cancer recurrence in adult cancer survivors: a systematic review of quantitative studies. J Cancer Surviv. 2013;7:300–322. doi: 10.1007/s11764-013-0272-z. [DOI] [PubMed] [Google Scholar]
- 7.Thewes B, Butow P, Zachariae R, Christensen S, Simard S, Gotay C. Fear of cancer recurrence: a systematic literature review of self-report measures. Psychooncology. 2012;21:571–587. doi: 10.1002/pon.2070. [DOI] [PubMed] [Google Scholar]
- 8.Simard S, Savard J. Fear of Cancer Recurrence Inventory: development and initial validation of a multidimensional measure of fear of cancer recurrence. Support Care Cancer. 2009;17:241–251. doi: 10.1007/s00520-008-0444-y. [DOI] [PubMed] [Google Scholar]
- 9.Oh CM, Won YJ, Jung KW, Kong HJ, Cho H, Lee JK, Lee DH, Lee KH. Community of Population-Based Regional Cancer Registries. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2013. Cancer Res Treat. 2016;48:436–450. doi: 10.4143/crt.2016.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim SH, Son BH, Hwang SY, Han W, Yang JH, Lee S, Yun YH. Fatigue and depression in disease-free breast cancer survivors: prevalence, correlates, and association with quality of life. J Pain Symptom Manage. 2008;35:644–655. doi: 10.1016/j.jpainsymman.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Kim SH, Kang S, Kim YM, Kim BG, Seong SJ, Cha SD, Park CY, Yun YH. Prevalence and predictors of anxiety and depression among cervical cancer survivors in Korea. Int J Gynecol Cancer. 2010;20:1017–1024. doi: 10.1111/IGC.0b013e3181e4a704. [DOI] [PubMed] [Google Scholar]
- 12.Goo AJ, Song YM, Shin J, Ko H. Factors associated with depression assessed by the Patient Health Questionnaire-2 in long-term cancer survivors. Korean J Fam Med. 2016;37:228–234. doi: 10.4082/kjfm.2016.37.4.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simard S, Savard J. Screening and comorbidity of clinical levels of fear of cancer recurrence. J Cancer Surviv. 2015;9:481–491. doi: 10.1007/s11764-015-0424-4. [DOI] [PubMed] [Google Scholar]
- 14.Shim EJ, Shin YW, Oh DY, Hahm BJ. Increased fear of progression in cancer patients with recurrence. Gen Hosp Psychiatry. 2010;32:169–175. doi: 10.1016/j.genhosppsych.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 15.Sperber AD. Translation and validation of study instruments for cross-cultural research. Gastroenterology. 2004;126:S124–S128. doi: 10.1053/j.gastro.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 16.Yun YH, Park YS, Lee ES, Bang SM, Heo DS, Park SY, You CH, West K. Validation of the Korean version of the EORTC QLQ-C30. Qual Life Res. 2004;13:863–868. doi: 10.1023/B:QURE.0000021692.81214.70. [DOI] [PubMed] [Google Scholar]
- 17.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 18.Oh SM, Min KJ, Park DB. A study on the standardization of the Hospital Anxiety and Depression Scale for Koreans: a comparison of normal, depressed and anxious groups. J Korean Neuropsychiatr Assoc. 1999;38:289–296. [Google Scholar]
- 19.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 20.Chung KI, Song CH. Clinical usefulness of fatigue severity scale for patients with fatigue, and anxiety or depression. Korean J Psychosom Med. 2001;9:164–173. [Google Scholar]
- 21.Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Modeling. 1999;6:1–55. [Google Scholar]
- 22.Fleiss JL. Design and Analysis of Clinical Experiments. New York, NY: Wiley; 1999. [Google Scholar]
- 23.Tabachnick BG, Fidell LS. Using Multivariate Statistics. 4th ed. Boston, MA: Allyn and Bacon; 2001. [Google Scholar]
- 24.Shim EJ, Mehnert A, Koyama A, Cho SJ, Inui H, Paik NS, Koch U. Health-related quality of life in breast cancer: a cross-cultural survey of German, Japanese, and South Korean patients. Breast Cancer Res Treat. 2006;99:341–350. doi: 10.1007/s10549-006-9216-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Distribution of participants and non-participants
K-FCRI


