ABSTRACT
There is an urgent need to find an environment friendly and sustainable technology for alternative energy due to rapid depletion of fossil fuel and industrialization. Microbial Fuel Cells (MFCs) have operational and functional advantages over the current technologies for energy generation from organic matter as it directly converts electricity from substrate at ambient temperature. However, MFCs are still unsuitable for high energy demands due to practical limitations. The overall performance of an MFC depends on microorganism, appropriate electrode materials, suitable MFC designs, and optimizing process parameters which would accelerate commercialization of this technology in near future. In this review, we put forth the recent developments on microorganism and electrode material that are critical for the generation of bioelectricity generation. This would give a comprehensive insight into the characteristics, options, modifications, and evaluations of these parameters and their effects on process development of MFCs.
KEYWORDS: electrode, electricity, microbial fuel cells (MFCs), microorganism, process development
Introduction
There is an urgent need to address the twin problems of the modern world that are energy insecurity and climate change caused by fossil fuel depletion and global warming respectively. The use of fossil fuels in recent years has hastened which led to a global energy crisis. Renewable energy is considered as a sustainable way to alleviate the current global warming crisis. However, efforts have been devoted to develop alternative mechanism for electricity generation. It is also desired that the electricity production mechanism should come from renewable resources without a net carbon dioxide emission.1,2 Microbial fuel cells (MFCs) offer new opportunities for the sustainable production of energy from waste water. MFCs generate sustainable power through utilization of different carbohydrates as well as on complex substrates present in wastewaters. Nevertheless, the commercialization of MFC is restricted due to low power generation along with its high cost. There are several challenges required to overcome in order to develop the performance and commercialization of MFCs.
Figure 1 illustrates a schematic diagram of a MFC for producing electricity.3 The MFC consists of an anode, a cathode, an electrolyte medium which are connected with two electrodes, PEM and microorganisms.4,5 Microorganism and electrode are the main components of an MFC that could significantly affect its cost and performance. However, there is inadequate information available about the energy metabolism pathway (shown in Fig. 2) and nature of the microorganism which is used in anode.6 Therefore, it is essential to identify the key steps that would optimize the process parameter which will enhance the energy production through microbial fuel cell. It has been found that microorganism can easily adopt the different metabolic pathways for efficient power generation which depends on the operational parameters of the MFC. Electrode design is the biggest challenge for cost effective scalable MFCs.7 Presently, researchers are focused on designing the electrode material and its configuration for development of MFCs. Carbon materials are mostly used as electrode because they are non-corrosive in nature having the general properties of an electrode. However Bio-electrodes are used as a conductor as well as a carrier for bacteria, and exhibits some special surface characteristics of electrode materials.8,9 The modification of electrode materials has proved to be an effective way to improve the performance of MFCs. This change in the physical and chemical properties of an electrode provides better microbial attachment and electron transfer. The efficiency of MFCS can be increased through improving the bacterial adhesion and also electron transfer along with modification of the electrode surface. Therefore, this review explores the recent progress in microorganism manipulation along with electrode design for MFC.
Figure 1.
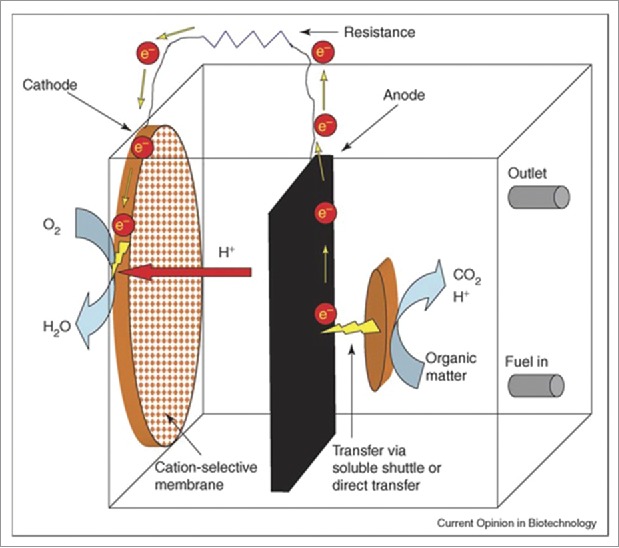
Generalized diagram of a microbial fuel cell.1
Figure 2.
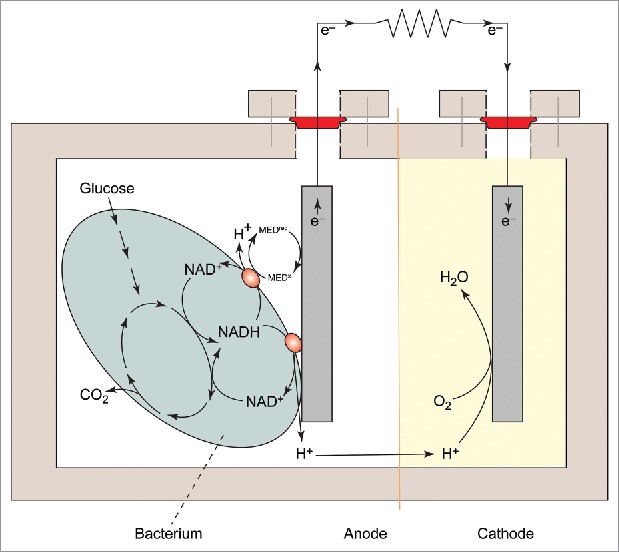
The working principle of energy metabolism pathway.6
Microorganism use in MFCs
Previously, microbial fuel cells were constructed with pure culture organisms that required the addition of a synthetic mediator which makes easier to facilitate electron transfer to the anode.10 Such types of microorganisms are possibly extraneous for electricity production from waste water effluent. However, additional synthetic mediators are not required. A number of microorganisms having electricity generating efficiency such as Geothrix species and Shewanella, can produce their own electron shuttles.11 It has been suggested that microorganism with own electron shuttle are advantageous as it can be positioned at a distance from the electrode and still can transfer electrons to the electrode surface.12 Geobacter species are advantageous due to presence of the ability to directly transfer electrons to electrodes, when competing for space on the anode of sediment microbial fuel cells. Similarly, Shewanella species are unable to generate electricity from waste organic matter. Since, the substrates they utilize are unlikely to be central extracellular intermediates in the anaerobic degradation of organic matter,11 therefore, Shewanella species can only incompletely oxidize lactate to acetate on electrodes, leading to inefficient electricity production.13,14
In past few years, one of the most exciting discovery in microbial fuel cell research was to design a microbial fuel cell which can produce electricity from the organic matter in aquatic sediments.15,16 This type of systems is now known as Benthic Unattended Generators or BUGs (http://www.nrl.navy.mil/code6900/bug/). This type of device (BUGs) is being designed to supply the power in remote locations. One of the remote location would be in the deepest of the ocean where it would be very expensive and technically not feasible to exchange traditional batteries.15,17 Thus Devices having similar design may be developed into efficient power electronic devices in distant terrestrial positions and could eventually be modified to harvest electricity from other sources such as compost piles, septic tanks and waste lagoons.
Lovley (2006) emphasized the recent development of a microbial fuel cell that can harvest electricity from the organic matter stored in marine sediments through the production of useful amounts of electricity in remote environments in feasible way. From his observation, it was found that self-sustaining microbial fuel cells can effectively convert a diverse range of waste organic matter or renewable biomass to electricity. However significant progress had recently been made to increase the power output to convert organic wastes to electricity. However, the substantial optimization is required for large scale electricity generation.1
In MFCs, the microorganisms which have been used include pure bacterial strains, e.g. Shewanella,18,19 and Geobacter13,20 species or, mixed culture, natural waste water source, brewery waste water, sediments of marine and lake6,21 However very few researches have been done so far on pre-genomic and genomic techniques such as16S rRNA based phylogeny analysis which could be used for efficient power generation. Furthermore, study of metagenomics along with phylogenetic analysis has provided important information in the structure and genetic potential of electrode-colonizing microbial communities. Post-genomic techniques such as meta-transcriptomics offer functional characterizations of electrode biofilm communities by quantifying gene expression levels. In addition, isotope-assisted phylogenetic analysis can provide further breakthrough about taxonomic information that may be helpful to understand the microbial metabolisms. An integrated knowledge of phylogenetic, electrochemical, metagenomic, and post-metagenomic techniques provides newer opportunities to understand the extracellular electron transport mechanisms which can eventually optimize the process parameters for power generation in microbial fuel cell.22
Identification of microorganisms
Utilization of biotechnological tools to understand the microbial processes normally starts with the discovery, investigation and understanding of naturally occurring microbial reactions. Molecular biology tools can help us, not only to gain better understanding of protein reactions; they also enable us to influence reaction properties. For efficient practical applications, modern biological techniques allow us to tailor these microbial reactions to optimize the desired functionality.23
The ability to produce high current densities by Geobacter sulfurreducens in microbial fuel cell has been made very popular in research community.24 The availability of complete genome sequence of Geobacter sulfurreducens,25 genetic system26,27 and the electron transfer ability to electrode has been proven that Geobacter sulfurreducens is a potential candidate for electricity generation in MFCs.24,28-30 The major pathway of electron transfer to a MFC anode is the direct extracellular electron transfer through cell membrane bound redox-proteins. Therefore, Shewanella oneidensis and Geobacter sulfurreducens have been extensively studied for extracellular respiration with an anode. These bacterial strains have ability to respire with solid extracellular electron acceptors, such as mineral oxides of Fe, Mn, and U, and are considered as model group of dissimilatory metal-reducing bacteria (DMRB).31,32 The electron transport chain of these organisms has a chain of redox-proteins (mostly cytochrome c-type) to conduct electrons across the cell envelope. However, the true pathway of the electron transport and the importance of specific proteins are still under investigation. Molecular biology techniques helped to clarify the essential electron transfer reaction steps and showed that engineered microorganisms can utilize complex fuels though generation of electricity.
Hererologous gene expression
The genomic study reveals that total of 42 and 111 membrane-associated c-type cytochromes are associated with S. oneidensis and G. sulfurreducens respectively.25,33 Involvement of high number of proteins in anaerobic respiration processes make these organisms very complicated for functional studies. Furthermore, the deletion mutations cannot result inoverall loss of function in the mutant since other cytochromes takeover and allow the reaction to happen. It is obvious that reported results from iron or manganese oxide reduction experiments are not necessarily similar for electron transfer to anodes. Bretschger et al. (2007) showed that knockout mutations of outer membrane reductases OmcA/MtrC in S. Oneidensis, knockouts of periplasmic proteins MtrA and MtrB only and reduced all three types of electron acceptors.34 Obviously, some of proteins play significant role in broad range of enzymatic pathways. However, they are very specific for certain reactions. knockout mechanisms also demonstrate that c-type cytochromes, are crucial for Fe(III) reduction or electron transfer to anodes. It is also important to express other cytochromes which are not directly involved in the respiration reaction. Kim et al. (2006) demonstrated that, G. Sulfur reducens c-type cytochromes, OmcG and OmcH are hypothesized to post-translation ally genetically modified microorganisms for BES 105 affect OmcB. Furthermore, knocking out OmcG and OmcH will reduce reduction rates indirectly. Therefore, OmcB is considered as essential for Fe(III) reduction in G. Sulfur reducens.35
Recently, Kimand Lovley (2008) demonstrated the possibility of similar function of expression control of OmcB, and their indirect involvement in the reduction reaction. He has also described for MacA, protein which is considered as a key-player in the electron transfer chain of G. Sulfur reducens.36 Kim (2008) demonstrated that gene for Omc F is a mono heme outer membrane c-type cytochrome in Geobacter sulphurreducens. Deletion of this gene can substantially decrease the current production. Previously it was also reported that inhibition of Fe(III) reduction in the OmcF-deficient mutant could be attributed to poor transcription of the gene for OmcB, an outer membrane c-type cytochrome which is required for Fe(III) reduction. However, a mutant in which omcB were deleted produced electricity as well as wild type. He also suggests that the requirement for OmcF is not required for optimal current production since OmcF is directly involved in extracellular electron transfer. However, OmcF is required for the appropriate transcription of other genes either directly or indirectly involved in electricity production.37
Recently Zheng et al. (2015) has developed a new approach to improve the membrane permeability of the bacteria by increasing the biosurfactant production from through genetic modification. It is also true that the efficiency of membrane permeability can be improved with biosurfactant which ultimately increases the transport across the membrane. Furthermore, researchers overexpressed the rhlAgene which is responsible for rhamnolipids(a biosurfactant) production. The bio surfactant directly influenced the overproduction of rhamnolipids from the electric bacteria such as Pseudomonas aeruginosa. The electron transport across the membrane was largely enhanced as the membrane permeability increased. The power output of the MFC catalyzed by this genetically engineered bacterium was enhanced up to about 2.5 times compared with the MFC with the parent strain.38,39
It was also reported that a number of nonspecific proteins are also involved in proper post translational folding and membrane localization of the terminal reductases. The transportation of respiratory proteins in the inner membrane of the bacteria is the first step. Second step involves the maturation of proteins with the help of cytochrome c maturation complexes which helps in proper attachment of heme molecules with the proteins within the periplasmic space.40 Lastly, they are transported over the outer membrane which might be facilitated by the Type II secretion system.41 In general, involvement of large number of c-type cytochromes and specific mechanism of transportation of proteins, electron transfer reactions to extracellular electron acceptors in S. oneidensis and G. Sulfur reducensare very complex that ultimately makes functional studies of individual enzymes more difficult. With the help of genetic engineering approach, E. coli is perfectly suitable for this task, because the wild-type strain has no intrinsic ability to reduce external electron acceptors and does not posse outer membrane c-type cytochromes.42 However, with the implementation of recent bio-molecular tools, it is easier for manipulating and screening E. coli cell lines. Those genes which are potentially responsible for enzyme function can be transferred into E. coli and studied for their expression, folding, localization, and in-vitro functionality. Stepwise addition of most promising proteins into the heterologous host, we may be able to build the minimum chain of proteins that will permit E. coli to respire with extra cellular terminal electron acceptors. With the help of minimal required protein chain is established in one E. coli cell line and the protein expression can be tuned and optimized to maximize the current generation.
Directed evolution
Directed evolution involves subjecting a given protein to a specific selection pressure (hence, directing the evolution of a desired trait). Random mutagenesis of a given gene using error prone PCR or mutagenesis of specific residues using primer oligonucleotides degenerates sequences through two methods. The above sequences are used to create a library of variants of the desired protein. With the help of biophysical, biochemical, or even electrochemical probes, the desired phenotype is screened for, usually via screening tools. Selection of desired mutants is based on their properties. In most cases the process is repeated for second and third rounds of selection. This process continues until sequencing of the DNA, analyzing the mutations, and studying the biochemical performance of a newly in vitro evolved enzyme. One of the common examples of directed evolution is evolution of glucose oxidase (GOx) (E. C. 1.1.3.4) enzyme from Aspergillus niger.
Gold/silver coating
It was already reported that the use of metallic nano-particles enhanced that electron turnover rate enhancement up to 8 folds as nano-particles act as nano-electrical connectors between active site of enzyme and an electrode.43 The enzyme used was FAD-reconstituted GOx, in which the FAD was modified with a gold nano-particle and then attached to an electrode. Its orientation relative to the electrode helped to achieve the best possible electron transfer. Different types of strategies have been adopted to maximize the efficiency of redox enzyme. It includes the encapsulation in conducting polymers44 through combining the nano-tubes with redox enzymes,45,46 or properly align of proteins with electrodes can be predicted through site directed mutagenesis.47 Several attempts have been made in the microbial world to coat bacteria with metal nano-particles (Fig. 3). One example is the biosynthesis of gold nano-particles (GNPs) (Fig. 4) assisted by E. coli where the application of bacterial/gold nano-particles hybrids was demonstrated in the direct electrochemistry of hemoglobin.48 A variety of bacteria have been used to induce architecture nanostructures (Fig. 3) made of metal nano-particles.49,50
Figure 3.
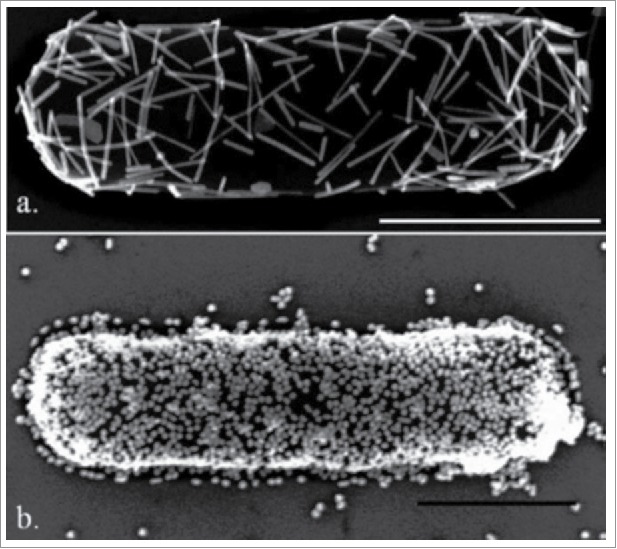
Gold nano-particles deposition on bacteria. (A) Percolating monolayer of nanorods (25 nm in diameter and 400 nm long) (B) Gold nano-spheres (45 nm diameter) on bacteria49
Figure 4.
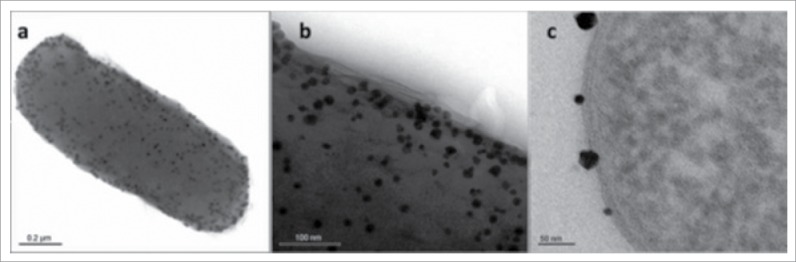
TEM images of gold-coated bacterium. (A) Isolated bacteria after 5 d of gold biosynthesis in water. (B) Closer view of bacteria. (C) Cross section of gold coated bacteria.22
Genetic analysis
A microarray analysis of Geobacter-based MFCs revealed significant up regulation of the genes involved in the production of the electrically conductive pili, known as microbial nanowires, and several outer-membrane c-type cytochromes. Genetic studies demonstrated that nanowires and the outer-membrane cytochrome, omcZ, were absolutely required for high-density power production. G. Sulphurreducens strains are adapted for faster extracellular electron transfer and also for transfer the electrons at significantly lower potentials than wild-type cells. Genome sequence of the adopted strains provides insight mechanisms behind self-optimization for power production. Furthermore, genome based in silico modeling can also provide important information toward optimization of power generation of microbial fuel cell rather than an empirical manner.51 A large team effort was dedicated to pinpoint the genes involved in electron transfer mechanisms. A very extensive study of forty six mutants of Shewanella oneidensis (S. oneidensis) MR-1 was done and tested for current production and metal oxide reduction.34 Surprisingly, out of 36 deletion mutants only 5 cytochrome deletion mutants have a limited ability to produce a current relative to the wild type. However, several cytochrome deletion mutants showed atleast 20% higher current values than the WT strain. They also conclude that different patterns of metal oxide reduction and current production indicate a highly complex picture of electron flow via MR-1 cytochromes.
Bacterial Pili
Bacteria having metal reducing ability can transfer electricity through conductive pili or “nanowires”.52 Recent studies have shown electrically conductive nanowires are not restricted to the metal reducing bacteria such as Shewanella and Geobacter. However, they are produced by oxygenic photosynthetic cyanobacteria and thermopile fermentative bacteria. It was also concluded that electricity generation in such bacteria are not only through the conductive nanowires, rather through some other cell organelles also.22 Fimbriae is an adhesive bacterial organelles which enable bacteria to target and to colonize specific host tissues. Usually, there are about 500 per cell and are as long as 1mm with diameters of about 7 nm. A large, highly diverse group of fimbriae of gram negative origin are already known. They have ability to bind heavy metals and heavy metal oxides.53 Binding of gold/silver nanoparticle to such peptides may lead to the development of real conducting nano wires, associated with gram negative bacteria.
Recent engineering approach
De Novo design
De Novo design mainly concerned with the process of introducing new elements into bacterial cells. In protein engineering de novo design relates to the assembly of peptides into predicted protein structures.54 Short gene fragments were inserted into the genes of the E. coli outer membrane proteins LamB, OmpA, Lpp- OmpA, PhoE which direct protein incorporation into the outer membrane of the cell. In the recent years, number of surface display systems have been developed, among those Lpp-ompA is the most popular system developed by G. Georgiou et al.55 As surface display systems evolved and became more robust, the number and variety of their uses have grown tremendously to include such exciting applications as proteins displays for bio sensing.56,57 It is believedthat glucose oxidase (GOx) can be displayed on the surface of bakers yeast (Saccharomyces cerevisiae).58 Primary metabolite is used directly by the displayed enzyme and the corresponding electron is delivered directly or indirectly by the enzyme to the electrode. In fact, this is a combined enzyme biofuel cell/microbial fuel cell system, in which the bacteria serves both as a micro factory for enzyme production and also as a stabilizing element.
Yeast surface display (YSD) of proteins
Yeast surface display is a powerful tool for displaying and engineering the affinity, specificity, and stability of antibodies, as well as other proteins. YSD tool is advantageous over other display technologies.59 Yeast cells can be sorted by employing Fluorescence Activated Cell Sorting (FACS) which creates quantitative discrimination between mutants. YSD allows eukaryotic expression of the heterologous target protein. however few application of YSD such as affinity maturation, protein engineering for improved production and stability, cell-based selections, epitope mapping, cDNA library screening, cell adhesion molecule engineering, and selections against non-biological targets. However, the use of modified yeast as anode compartment in microbial fuel cell has done recently. In addition, the application of engineered yeast has great advantages over unmodified yeast. It was also found that increased electromotive force (EMF) ca. 884 mV over unmodified yeast or purified GOx ca. 700 mV when the oxygen reducing enzyme laccase was present at the cathode compartment. One of the major advantages of GOx displaying yeast is the regeneration ability of the fuel cell.60
Unnatural amino acids incorporation
This technique involves selective incorporation of unnatural amino acids into proteins in E. coli,61 yeast62 and mammalian cells63 using orthogonal tRNA-aminoacyl synthetase pairs. These orthogonal pairs do not cross-react with endogenous components of the translational machinery of the host cell. However, the amber nonsense codon when used in E. Coli and yeast or TGA, the opal codon, when used in mammalian cells cannot recognize the desired unnatural amino acid and incorporate it into proteins in response to TAG. Recently, 3-amino tyrosine was incorporated into the 2 subunit of E. Coli ribonucleotide reductase, and it was successfully demonstrated that this 3-aminotyrosine served as a radical trap instead of one of the tyrosine radicals 730 or 731 separately. Therefore, it proves the putative electron transfer pathway in this important enzyme.64
Electrode materials
Carbonaceous materials are used widely because of their good biocompatibility, good chemical stability, high conductivity, and relatively low cost for MFC anodes. In the laboratory, graphite plates or sheets, carbon paper, and carbon cloth are the used for plain electrodes. In comparison with carbon sheets, carbon cloth is preferred as it is more flexible and much more porous which allows huge surface area for bacterial growth.However, it is excessively expensive to use for MFCs (ca. $1000/m2). A cheap carbon mesh material ($10−40/m2) was examined as a substantially cheap alternative to carbon paper and carbon cloth. Some hardly used fibrous materials, such as activated carbon cloth, graphite foil, and carbon fiber veil are also reported and comparatively evaluated for sulfide electrochemical oxidation in the anode of MFCs. Graphite or carbon felt is another fiber fabric that is much thicker and might loose its texture. But the space for bacterial growth is more than carbon cloth and graphite sheets, but the growth of bacteria is more likely to be controlled by the mass transfer of substrate and products on its inner surface. Similarly RVC can be used as packing material to fill the anode chamber. It is true that the more porous materials naturally produce more power per geometric surface area related to their smooth counterparts.
Similar to the biological filter, the anode chamber of the MFC can be completed with granular, irregularly shaped packing, small cubes of graphite or carbon felt that can also be used as a packing material of an MFC bed. In order to make the complete bed conductive, the granules must be tightly packed next to each other. The graphite brush anode is an ideal electrode that attains high porosities, high surface area and efficient current collection. Metal materials are much more conductive than carbon materials but it ruled out because of the non-corrosive condition for anode materials. So far, only stainless steel and titanium are used for anode material. Through studies it is found that the amount of electrical energy produced can increase 1,000 fold by using Mn4+-graphite electrode, made by mixing manganese sulfate with fine graphite powder. The anode made by mixing a sulfide-oxidizing Sb (V) complex and graphite paste which is 1.9 times higher than that of graphite paste anode where Sb (V) complex functioned as a mediator in anode chamber. Thus it can efficiently mediate electron transfer from bacteria to anode.65
Case studies for performance improvement of MFC
Effect of biofilm
Biofilm is a complex aggregated mass of microbial communities formed by self-immobilized attached growth on a solid substrate by the excretion of adhesive and protective matrix. It is also called extracellular polymeric substances (EPS). Biofilm plays a major role in the electrochemical process of bio-corrosion involving anodic reaction (ionization/oxidation) of the surface metal. In biofilms cell-to-cell contact is possible if high cell density can be created which helps to stimulate the electron transfer mechanism. Therefore the anode can play the role of the solid electron acceptor along with transformation of genetic information. Through research and studies, it is found that effectiveness of anodic biofilm formation enhanced the extracellular electron transfer in absence of mediators. Plain graphite plate (5 cm × 5 cm; 10 mm thick; soaked in de- ionized water for 24 h; pH 7) is used as anode for the development of biofilm.
Mohan and his coworkers(2008) fabricated three single chamber mediators less MFC of plain graphite electrodes with Nafion membrane and air cathode is fabricated in laboratory using ‘perplex’ material with proper leak proof sealing to maintain anaerobic microenvironment in the anode compartment. Anodes with variable biofilm coverage i.e. partially developed biofilm [Anode surface coverage (ASC) < 44%; 90 days] and fully developed biofilm [ASC < 96%; 180 days] placed separately in three MFCs and under the same operating conditions the performance was evaluated. The experiment was performed in presence of designed synthetic wastewater (DSW) and chemical wastewater (CW) as substrates and anaerobic mixed consortia was used as biocatalyst. Prior to feeding, wastewater pH was adjusted to pH 6. Current (I) was recorded by using digital multi-meter by connecting 100 Ω as external resistance in the open circuit and was monitored by connecting external resistances (100–30000 Ω) using a variable resistance box for polarization. Self-immobilized biofilm formed on the anode surface of the fuel cell influence both bioelectricity production and substrate degradation efficiency (Fig. 5).
Figure 5.
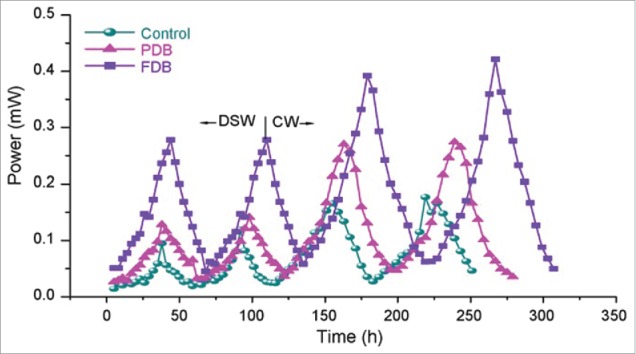
Power generation during the operation of MFC with the function of time (control, biofilm surface coverage, 0%; PDF, partially developed biofilm covered over ∼44% of anode surface; FDB, fully developed biofilm covered over ∼96% of anode surface; CW, chemical wastewater; DSW, design synthetic wastewater; operating of MFC, pH 6 at 28°C) [r2, 0.9918].66
It is evident from the Figure 5 that power generation potential depends on the extent of biofilm growth/coverage on the anode surface of the fuel cell. The performance of the fuel cell also depends on the nature of wastewater which was used as substrate in MFC. It has been found that significantly high power outputs were noticed for CW over DSWas substrate. The open circuit voltage (OCV) and current were found to be of 0.258V and 0.47mA, respectively at 100 Ωof external resistance in presence of DSW as feed (1.67 kg COD/m3-day). However, higher OCV of 0.274V and current of 0.52mA at 100 Ω were recorded in presence of CW as feed (1.81 kg COD/m3-day). The extended periods of time was 59 h and 71 h for DSW; CW respectively. The partially immobilized biofilm (PDB) showed comparatively higher performance with respect to power output over the control-MFC operation. The highest performance with respect to power output was noticed for fully immobilized biofilm (FDB) over the corresponding PDB and control operations. A maximum recorded OCV and current were 0.293V and 0.95mA at 100 Ω was recorded in presence of DSW as feed. However, relatively higher power outputs were observed with CW over DSW as feed. It is interesting to note that a marked drop in potential difference was observed after reaching maximum voltage which may be due to the metabolic hibernation.66
Effect of pretreatment and surface modification
Usually carbon cloth or carbon paper is expensive for MFCs so alternative inexpensive carbon mesh material was examined which is less expensive alternative to these materials for the anode material in an MFC. Pretreatment of the carbon mesh is needed to ensure suitable MFC performance. In first step, heating of the carbon mesh is needed in a muffle furnace (450°C for 30 min) which result in a maximum power density of 922 mW/m2 (46 W/m3) with this heat-treated anode, which produce 3% more power than that of mesh anode cleaned with acetone (893 mW/m2; 45 W/m3). This power density obtain with heating is just only 7% less than the carbon cloth treated at high temperature with ammonia gas process (988 mW/m2;49 W/m3). When the carbon mesh was treated with the ammonia gas process, power increased to1015mW/m2 (51 W/m3).Therefore, cleaned or heated surfaces process showed the decrease of atomic O/C ratio, which indicated removal of contaminants that obstructed with charge transfer. Along with this the ammonia gas treatment also increased the atomic N/C ratio which was due to nitrogen related functional groups that facilitated electron transfer. These results show that the anode in an MFC can be obtain from heat-treated carbon mesh materials and thus the costing is also reduced.
Treated carbon mesh anodes has improved electrochemical activities by K4Fe(CN)6 oxidation, and produced power densities which is less expensive than carbon cloth. The different anode treatment was observed which also decreased in the O/C ratio for heating, cleaning and ammonia gas treatments. It is also important that change in the electro chemically active surface area increased to 20 cm2 with cleaning. For further heat treatments or ammonia treated the active area is increased further to 54–58 cm2, with no difference in power density that could be credited to differences in effective area. The low cost and good performance of the carbon mesh is also encouraging as it allows close placement of the anodes with the cathodes. Closely placed carbon cloth electrode spacing, coupled with the use of a cloth separator, gives very high volumetric power densities. While the carbon mesh could substitute the carbon cloth as the anode, but it might not be possible to use it as a cathode.67
A phosphate buffer can increase the conductivity of the solution, and thus the ammonia gas treatment of a carbon cloth anode substantially increased the surface charge of the electrode, and thus the performance of MFC is improved. The carbon cloth cathode (0.5 mg/cm2) contained a 2Pt catalyst and four diffusion layers were used. Both electrodes had a projected surface area of 7 cm2. A thermo- gravimetric analyzer (TGA) was used to treat ammonia gas on carbon cloth. Single chamber air-cathode MFCs were constructed with the electrode spacing of 2 cm. The MFCs were inoculated with domestic wastewater and a phosphate buffer nutrient solution. The feed solution was replaced when the voltage dropped below 20 mV. Cell voltage across an external resistor was recorded using a multi meter with a data acquisition system. It has been found that maximum power production was reached after 150 h of operation in presence of untreated carbon cloth anode. However, comparatively less time (60 h) is required in presence of ammonia- treated carbon cloth anode (shown in Fig. 6). Using an ammonia treated anode, the maximum power density and volumetric power density were recorded as1970 mWm−2 and 115 w/m3 respectively. The columbic efficiency ranged from 30 to 60% (ammonia treated) depending on the current density with values 20% higher than those obtained from untreated anode and phosphate buffer. Along with that it also increased the surface charge from 0.38 to 3.99 meq m−2 at pH 7. This cause due to the presence of nitrogen-containing surface functional groups on the carbon cloth surface during the ammonium treatment.68
Figure 6.
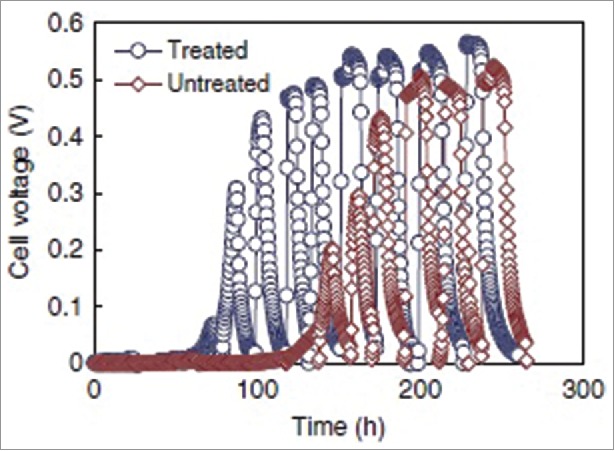
Enrichment with a mixed solution of phosphate buffered nutrient medium (50 mM) containing 1 g/L sodium acetate with domestic wastewater (50/50 v/v) for MFCs with different anodes. Each spike in power generation was followed by re-fueling of the reactor with new substrate, resulting in the next cycle of power generation.68
The ammonia treatment will be costlier if it is applied for large scale. There are three different treatment methods generally employed for enhanced power generation. They are carbon fiber brushes acid soaking (CF – A), heating (CF-H) and combination of both (CF-AH). A single chamber MFC was constructed where cathode (CF-C) used was carbon cloth (30% wet proof) containing 0.35mg.m−2 Pt catalyst and 20% domestic waste water was inoculated with MFC. Using Data acquisition system and online date monitoring the external resistance voltage was collected and recorded with a30min interval and polarization curve were measured. The surface of carbon fiber was analyzed by X-ray photoelectron spectra.
The lag time found for the MFC with acid-treated anodes (CF-A) and the heat-treated anodes (CF-H) was 140 and 240 h respectively. However, 200 h of lag phase is required for both the MFC control (CF-C) and the acid- and heat-treated MFCs (CF-AH) (Fig. 7). It is also revealed that pure acetone (CF – A) acid treatment, heat treatment (CF-H) and combined treatment (CF-AH)increased the output by 8%,25% and 34% respectively in comparison with control. However, highest columbic efficiency of 19.5% was obtained by using (CF-AH) treatment.Based on BET adsorption isotherms, before treatment (control) the measured surface area of the fibers on the anode was 7.11 m2g−1. Heat treatment increased the actual surface area by 6.94 times to 49.3 m2 g−1 compared with untreated fibers. Acid treatment alone increased the surface area by only 32.5%, while the area of the acid- and heat-treated anode had a surface area of 43.9 m2g−1. The increase of surface area during the heat treatment process was due to generation of cracks (Fig. 8). Treatment of carbon fiber materials by a simple acid and heat treatment process increased power production by 34% from 1020 to 1370 mW m−2. In addition, the average CE values increased from 14.6% to 19.5% due to increase in the current densities that resulted from reduced ohmic resistances. Heat treatment alone, however, increased power by 15% compared with the carbon mesh control. Considering costs and complexity of treatment, high-temperature ammonia gas treatment methods are absolutely not warranted for future practical applications.69
Figure 7.
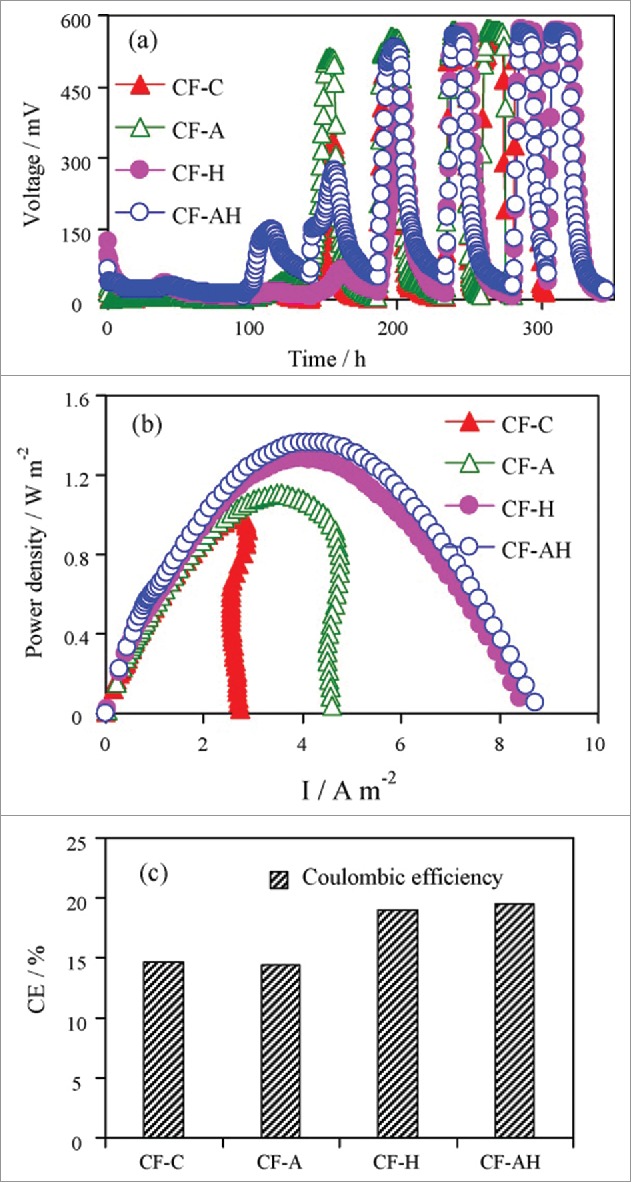
Performance of MFCs with anodes acid (CF-A), heat (CF-H), and acid- andheat-treated (CF-AH) anodes, compared with the control (CF-C): (A) voltage production, (B) power density, and (C) Coulombic efficiencies.69
Figure 8.
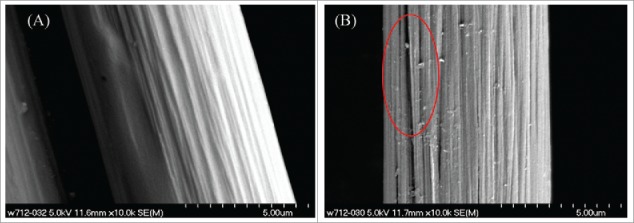
Electron microscope photograph of carbon fiber before (A) and after heat treatment (B).69
To scale up the MFC high power density is important factors which can be obtained by high surface areas and a porous structure which is examined through research. Brush anodes were made of carbon fibers cut to a set length and wound using an industrial brush. The brushes are twisted core consisting of two titanium wires with an estimated a surface area of 0.22 m2 or 18200 m2/m3, its volume for the small brush (95%porosity), and 1.06 m2 or 7170 m2/m3 brush volume for the larger brush (98% porosity) brush anodes were treated using ammonia gas and in some tests plain. Today carbon paper anodes were used for comparisons to brush anodes. The cathodes were made by applying platinum (0.5 mg/cm2 Pt) and four diffusion layers on a 30 wt % wet-proofed carbon cloth and in some experiments cathodes contained 40% cobalt tetra methyl phenyl porphyrin (CoTMPP, 1.2 mg/cm2) as the catalyst instead of Pt.
To improve power generation in microbial fuel cell (MFC) the surface modifications of anode materials are one of the most important factors. As the material used for anode is usually a limiting factor in power production in an MFC. Ideal anodic materials should be biocompatible, conductivity and chemically stable. Due to its conductivity and chemically stability, carbon-based anode materials are being used in a large scale including graphite, activated carbon, carbon paper, activated carbon fiber, carbon cloth, carbon nanotube, carbon brush, and carbon mesh. Many challenges have been made in change their surface that resulted in more efficient anode materials for power generation. In this study here to increase power density of membrane nitric acid soaking and EDA treatment were used to modify ACF (Activated carbon fiber felt) in a free single-chamber air-cathode microbial fuel cells. Cathode was made of carbon paper with Pt catalyst (0.5 mg/cm2) on the water-facing side and a poly-tetrafluoro ethylene (PTFE) diffusion layer on the air-facing side.
To examine a pond sediment was collected from a pond near University Mega Center campus of South China University of Technology and inoculated in the anode chamber which was a membrane free single-chamber air-cathode MFCs. When the voltage decreased to less than 0.05 V, the anode chamber solution was replaced. The surface morphologies of the ACF-A, ACF-N, and ACF-AT was examined by a scanning electron microscope electrodes before and after incubation. The XPS measurements were performed on an Axis Ultra DLD spectrometer and electrode potentials and Cell voltage was recorded using a multimeter with a data acquisition system.
The starter time for obtaining 0.42V was 216h for the MFC-N with the EDA-treated anode and 240h for the MFC-A with acid-treated anode. The control MFC required 440 h to achieve maximum repeatable voltages.The time to achieve maximum repeatable voltages of the MFC-A and MFC-N was shortened by 45% and 51% and maximum power densities of both was larger than that of the control MFC (1304 mW/m2). The acid treatment produced the power density of 2066 mW/m2, which was 58% larger than the control and EDA treatment increased power density by 25% reaching 1641 mW/m2. Thus it can be concluded that the membrane free single-chamber air-cathode microbial fuel cell can improve the maximum power densities by 25% and 58%, and thus shortened the start-up time by 51% and 45%, respectively using surface modified and unmodified anodes.70
Various electrodes
Logan et al. (2007) reported the two types of single-chambered MFCs to examine power production using brush electrodes cube-shaped MFCs (C-MFCs) and bottle-type MFCs containing a single side port (B-MFC). The voltage (V) across an external resistor (1000 Ω except as noted) in the MFC circuit was monitored at 30 min intervals using a multimeter and Current (I), power (P) and columbic efficiency was calculated. The maximum power produced by C-MFCs with brush anodes was 2400 mW/m2 at a current density of 0.82 mA/cm2. However, B-MFCs produced up to 1430 mW/m2 (2.3 W/m3). It has been reported that the power obtained from C-MFCs shows higher power density to the projected surface area of an electrode (cathode). However, 1970 mW/m2 was obtained using a smaller cube-type reactor (14 mL volume) which had a smaller electrode spacing (2 cm) than that used here (4 cm). From the above observation it can be concluded that brush anodes that have high surface areas and a porous structure which can produce high power densities.71
A unique nano-structured polyaniline (PANI)/meso porous TiO2 composite was synthesized and explored as an anode in E. coli microbial fuel cells(MFCs). Polyaniline (PANI) is a popular conducting polymer due to its simple synthesis process, good electrical conductivity, and environmental stability. It was also reported that a favorable nanostructure of a carbon nanotube/PANI composite anode improves the MFC performance, especially the power density. The nature of the catalytic mechanism of a MFC anode involves not only a bio-but also an electro catalytic process. An optimal nanostructure with high specific surface area favorable for both catalytic processes could play a critical role in improving the MFC power density. A new mesoporous TiO2 electrode material with uniform nano-pore distribution and high specific surface area was synthesized. In this work, we use this material to fabricate a unique nano-structured PANI/TiO2 composite for the MFC anode. The optimal composite is employed in an Escherichia coli MFC.72
The packing density of anodes in microbial fuel cells (MFCs) is an important parameter for maximum power output. In this regard, four different graphite fiber brush anode was studied in terms of carbon fiber length (brush diameter), the wire current collector gage and the number of brushes connected in parallel. The exo-electrogenic biofilm large anode to cathode surface area ratio is important for maximizing the power production. However the main goal was to minimize the anode sizes so that material costs are reduced. A better accepting of brush configuration (numbers and diameters) and number of current collectors will drive better design of larger reactors which allow more optimal use of materials. In addition, it also reduces the cost of the reactor.
Logan (2010) constructed a single-chamber, air-cathode MFCs where anode was treated at 450°C for 30 min before placing horizontally in the cylindrical chamber.73 The electrode spacing was set at 5 mm, measured from the front end of the brush anode to the inner face of the cathode. And four different reactor configurations; namely (C1-thick, C1, C3, and C6) were used for comparison of overall performance and power densities with different anode conditions. The C1 consisting of a single anode brush 25 mm in diameter made using two thick titanium wires (length = 60 mm, diameter = 1.5 mm, 15 gage, #2 grade; carbon fiber). The other three anodes all used a thinner titanium wire with a diameter of 0.8 mm (20 gage). Multiple anode configurations were also tested using three brush anodes, each with diameter of 12 mm (C3,), or six brushes each with diameter of 8 mm (C6,). The C3 and C6 anodes were connected externally by a single copper wire. Air-cathodes (projected surface area of 7 cm2, cathode-specific surface area of 25 m2/m3) which was wet-proofed (30%) carbon cloth (Type B-1B, E-TEK). The above cathode was coated with carbon black and platinum (0.5 mg Pt/cm2) using a nafion binder which was used on the electrolyte-facing side.
A carbon black base layer and four PTFE diffusion layers were placed on the air-facing side with an electrolyte of phosphate buffer nutrient solution. Polarization and power density curves was obtained by changing the external resistance (from 10 to 1000 Ω) and allowing three fed-batch cycles at each resistance of the biofilm to ensure reproducible power output.74 Two types of experiments were conducted following the initial polarization tests mentioned as brush anode disconnection, and brush anode removal. Current (I, mA) was calculated according to Ohm's Law and Chemical oxygen demand (COD, mg/L) was measured using standard methods. COD removals over an individual cycle were over 90% for all configurations and resistances. When brushes were disconnected, the decrease in power seemed to have occurred with the limited capacity of fewer anodes to sustain the maximum current which was compared with all brushes which was connected and COD removal, averaged 94 ± 2% for all setups. COD removal is typically >90% for MFCs with brushes anodes while using acetate. Multiple carbon brush anodes produced (1250 mW/m2) almost similar power densities to those obtained with single-brush systems (1150 ± 40 mW/m2), in spite of large differences in total brush anode surface areas. The use of more wires (current collectors) with the larger number of anodes thus decreases in power production. The thinner Ti enhance the MFC performance and thus saving material costs of Ti by more than 70% (in weight).Overall, anode coverage of the cathode was the most critical problem with respect to power production as removing anodes in the multiple anode systems which resulted in decreasing power.75
Conductive polymers are recently used for performance improvement of MFC. It contains a conjugated system of double bonds. Poly (3,4ethylenedioxythiophene) (PEDOT) is the most common conducting polymers as it is most conductive and stable polymer. Bayatsarmadi and his colleagues (2016) constructed a electrode where graphite papers (Fuel Cell Earth) were coated with PEDOT using Vacuum Vapor Phase Polymerization (VPP). The above coating process was performed at 45 mbar for 1 h. However, the blue deposited film of PEDOT was visible to the naked eyes. The morphological analysis of electrode was performed by scanning electron microscopy (Fig. 9). In the Fig. 9, it was revealed that a continuous thin layer of PEDOT over the porous graphite substrate was clearly visible. It was investigated that higher voltage, maximum power density and stability were found by using PEDOT coated on graphite electrodes.76
Figure 9.
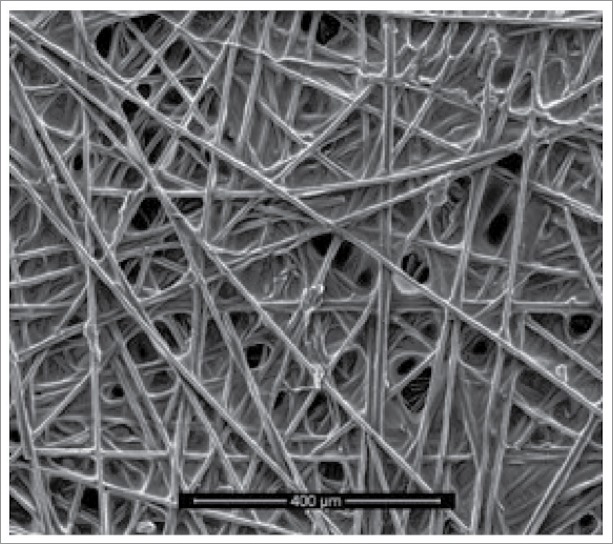
SEM image of a graphite paper coated with PEDOT using vacuum vapor phase polymerization.76
Thesuparungsikul et al. (2012) developed carbon nanotubes (CNT) based electrodes to improve MFC performance in terms of electricity generation. In this experiment, multi-walled carbon nanotubes (MWCNTs) with carboxyl groups were used to fabricate electrodes for single-chamber air-cathode MFCs. The morphology, electrical conductivity and specific surface area were used as key parameters for evaluation of quality of electrodes. The above parameters were measured by field emission scanning electron microscope, four-probe method and Brunauer-Emmerr-Teller method, respectively. The performance of MFCs equipped with MWCNT-based electrodes were identified as ideal candidates for replacing traditional carbon material used for MFC electrodes.77 Kong et al. (2016) developed a cost-effective and efficient electrochemical catalyst for the fuel cells electrode. A novel electrode La0.9Ca0.1Fe0.9Nb0.1O3-∂ (LCFNb) having good potentiality was synthesized for the symmetrical solid oxide fuel cells (SSOFC). It is observed that the Sc0.2Zr0.8O2-∂(SSZ) electrolyte supported symmetrical cells with impregnated LCFNb and LCFNb/SDC (Ce0.8Sm0.2O2-∂) electrodes showed relatively high power outputs with maximum power densities (MPDs). The maximum power output and MPDs were found to be 392 and 528.6 mW cm−2 at 850°C in dry H2, respectively which indicates the excellent electro-catalytic activity of LCFNb toward both hydrogen oxidation and oxygen reduction. It is interesting to note that the MPDs of LCFNb/SDC composite electrodes in presence of CO and syngas (CO: H2 ¼ 1:1) were almost identical in presence of H2. It is thus implied that LCFNb material showed similar catalytic activities to carbon monoxide compared with hydrogen.78
Conclusion
The electricity generation through MFCs is a promising technology which has received much attention globally among the researchers. However, many technological improvements are required to achieve stable power output. The microorganism and electrode are the main factors which are mainly affect the cost and performance of MFC. Proper optimization of the above factors can increase the application of MFC for commercialization. Therefore, intensive research on optimized microorganism and noble electrode are required to reduce the complexity of rate-limiting steps which are responsible for enhanced higher current output. Most of the literatures are recently focused to improve the performance of MFCs mainly on the reactor configurations and optimization of operational conditions. However limited research is concentrated on optimized microorganism and electrode of MFC. It can be concluded that commercial application of MFC can be increased through optimization of microorganism and invention of novel electrode which will provide a promising option for cost effective bioelectricity generation through MFCs.
Compliance with ethical standards
This article does not contain any studies with human participants or animals performed by any of the authors.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
Authors acknowledge to Dr. Gaurav Gupta, University of Manitoba, Canada, for English editing of this manuscript.
Funding
This material is based upon work supported by the National Institute of Technology, Agartala, India. The authors would like to acknowledge the National Institute of Technology, Agartala, Ministry of Human Resource and Development, Government of India for Fellowship.
References
- [1].Lovley DR. Microbial fuel cells: novel microbial physiologies and engineering approaches. Curr Opin Biotechnol 2006; 17:327-32; PMID:16679010; http://dx.doi.org/ 10.1016/j.copbio.2006.04.006 [DOI] [PubMed] [Google Scholar]
- [2].Davis F, Higson SP. Biofuel cells—recent advances and applications. Biosens Bioelectron 2007; 22:1224-35; PMID:16781864; http://dx.doi.org/ 10.1016/j.bios.2006.04.029 [DOI] [PubMed] [Google Scholar]
- [3].Wilkinson S. “Gastrobots”—benefits and challenges of microbial fuel cells in foodpowered robot applications. Autonomous Robots 2000; 9:99-111; http://dx.doi.org/ 10.1023/A:1008984516499 [DOI] [Google Scholar]
- [4].Cheng S, Liu H, Logan BE. Power densities using different cathode catalysts (Pt and CoTMPP) and polymer binders (Nafion and PTFE) in single chamber microbial fuel cells. Environ Sci Technol 2006; 40:364-9; PMID:16433373; http://dx.doi.org/ 10.1021/es0512071 [DOI] [PubMed] [Google Scholar]
- [5].Mathuriya AS, Yakhmi J. Microbial fuel cells–Applications for generation of electrical power and beyond. Crit Rev Microbiol 2014; 42:1-17; http://dx.doi.org/ 10.3109/1040841X.2014.905513 [DOI] [PubMed] [Google Scholar]
- [6].Rabaey K, Verstraete W. Microbial fuel cells: novel biotechnology for energy generation. Trends Biotechnol 2005; 23:291-8; PMID:15922081; http://dx.doi.org/ 10.1016/j.tibtech.2005.04.008 [DOI] [PubMed] [Google Scholar]
- [7].Rabaey K. Bioelectrochemical systems: from extracellular electron transfer to biotechnological application. Water Intelligence Online; IWA publishing, 2009. [Google Scholar]
- [8].Deng Q, Li X, Zuo J, Ling A, Logan BE. Power generation using an activated carbon fiber felt cathode in an upflow microbial fuel cell. J Power Sources 2010; 195:1130-5; http://dx.doi.org/ 10.1016/j.jpowsour.2009.08.092 [DOI] [Google Scholar]
- [9].Zhang F, Cheng S, Pant D, Van Bogaert G, Logan BE. Power generation using an activated carbon and metal mesh cathode in a microbial fuel cell. Electrochem Commun 2009; 11:2177-9; http://dx.doi.org/ 10.1016/j.elecom.2009.09.024 [DOI] [Google Scholar]
- [10].Gasteiger HA, Gu W, Makharia R, Mathias M, Sompalli B. Beginning-of-life MEA performance—efficiency loss contributions. Handbook of Fuel Cells; Wiley Online Library, 2003. [Google Scholar]
- [11].Lovley DR, Holmes DE, Nevin KP. Dissimilatory fe (iii) and mn (iv) reduction. Adv Microb Physiol 2004; 49:219-86; PMID:15518832; http://dx.doi.org/ 10.1016/S0065-2911(04)49005-5 [DOI] [PubMed] [Google Scholar]
- [12].Strycharz SM, Woodard TL, Johnson JP, Nevin KP, Sanford RA, Löffler FE, Lovley DR. Graphite electrode as a sole electron donor for reductive dechlorination of tetrachlorethene by Geobacter lovleyi. Appl Environ Microbiol 2008; 74:5943-7; PMID:18658278; http://dx.doi.org/ 10.1128/AEM.00961-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bond DR, Lovley DR. Electricity production by Geobacter sulfurreducens attached to electrodes. Appl Environ Microbiol 2003; 69:1548-55; PMID:12620842; http://dx.doi.org/ 10.1128/AEM.69.3.1548-1555.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Park D, Zeikus J. Impact of electrode composition on electricity generation in a single-compartment fuel cell using Shewanella putrefaciens. Appl Microbiol Biotechnol 2002; 59:58-61; PMID:12073132; http://dx.doi.org/ 10.1007/s00253-002-1089-2 [DOI] [PubMed] [Google Scholar]
- [15].Tender LM, Reimers CE, Stecher HA, Holmes DE, Bond DR, Lowy DA, Pilobello K, Fertig SJ, Lovley DR. Harnessing microbially generated power on the seafloor. Nat Biotechnol 2002; 20:821-5; PMID:12091916; http://dx.doi.org/ 10.1038/nbt716 [DOI] [PubMed] [Google Scholar]
- [16].Reimers CE, Tender LM, Fertig S, Wang W. Harvesting energy from the marine sediment-water interface. Environ Sci Technol 2001; 35:192-5; PMID:11352010; http://dx.doi.org/ 10.1021/es001223s [DOI] [PubMed] [Google Scholar]
- [17].Donovan C, Dewan A, Heo D, Beyenal H. Batteryless, wireless sensor powered by a sediment microbial fuel cell. Environ Sci Technol 2008; 42:8591-6; PMID:19068853; http://dx.doi.org/ 10.1021/es801763g [DOI] [PubMed] [Google Scholar]
- [18].Gorby YA, Yanina S, McLean JS, Rosso KM, Moyles D, Dohnalkova A, Beveridge TJ, Chang IS, Kim BH, Kim KS, et al.. Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proc Natl Acad Sci 2006; 103:11358-63; http://dx.doi.org/ 10.1073/pnas.0604517103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wang H, Wang G, Ling Y, Qian F, Song Y, Lu X, Chen S, Tong Y, Li Y. High power density microbial fuel cell with flexible 3D graphene–nickel foam as anode. Nanoscale 2013; 5:10283-90; PMID:24057049; http://dx.doi.org/ 10.1039/c3nr03487a [DOI] [PubMed] [Google Scholar]
- [20].Nevin KP, Richter H, Covalla S, Johnson J, Woodard T, Orloff A, Jia H, Zhang M, Lovley DR. Power output and columbic efficiencies from biofilms of Geobacter sulfurreducens comparable to mixed community microbial fuel cells. Environ Microbiol 2008; 10:2505-14; PMID:18564184; http://dx.doi.org/ 10.1111/j.1462-2920.2008.01675.x [DOI] [PubMed] [Google Scholar]
- [21].Feng Y, Wang X, Logan BE, Lee H. Brewery wastewater treatment using air-cathode microbial fuel cells. Appl Microbiol Biotechnol 2008; 78:873-80; PMID:18246346; http://dx.doi.org/ 10.1007/s00253-008-1360-2 [DOI] [PubMed] [Google Scholar]
- [22].Alfonta L. Genetically engineered microbial fuel cells. Electroanalysis 2010; 22:822-31; http://dx.doi.org/ 10.1002/elan.200980001 [DOI] [Google Scholar]
- [23].Uday USP, Bandyopadhyay TK, Bhunia B. Bioremediation and detoxification technology for treatment of Dye(s) from textile effluent. In Kumbasar EPA, Körlü AE, eds. Textile Wastewater Treatment; Croatia: Intechopen, 2016:75-92. [Google Scholar]
- [24].Mehta T, Childers SE, Glaven R, Lovley DR, Mester T. A putative multicopper protein secreted by an atypical type II secretion system involved in the reduction of insoluble electron acceptors in Geobacter sulfurreducens. Microbiology 2006; 152:2257-64; PMID:16849792; http://dx.doi.org/ 10.1099/mic.0.28864-0 [DOI] [PubMed] [Google Scholar]
- [25].Methe B, Nelson KE, Eisen J, Paulsen I, Nelson W, Heidelberg J, Wu D, Wu M, Ward N, Beanan MJ, et al.. Genome of Geobacter sulfurreducens: metal reduction in subsurface environments. Science 2003; 302:1967-9; PMID:14671304; http://dx.doi.org/ 10.1126/science.1088727 [DOI] [PubMed] [Google Scholar]
- [26].Lloyd JR, Leang C, Myerson ALH, Coppi MV, Cuifo S, Methe B, Sandler SJ, Lovley DR. Biochemical and genetic characterization of PpcA, a periplasmic c-type cytochrome in Geobacter sulfurreducens. Biochem J 2003; 369:153-61; PMID:12356333; http://dx.doi.org/ 10.1042/bj20020597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kim B-C, Leang C, Ding Y-HR, Glaven RH, Coppi MV, Lovley DR. OmcF, a putative c-type monoheme outer membrane cytochrome required for the expression of other outer membrane cytochromes in Geobacter sulfurreducens. J Bacteriol 2005; 187:4505-13; PMID:15968061; http://dx.doi.org/ 10.1128/JB.187.13.4505-4513.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Reguera G, McCarthy KD, Mehta T, Nicoll JS, Tuominen MT, Lovley DR. Extracellular electron transfer via microbial nanowires. Nature 2005; 435:1098-101; PMID:15973408; http://dx.doi.org/ 10.1038/nature03661 [DOI] [PubMed] [Google Scholar]
- [29].Mehta T, Coppi MV, Childers SE, Lovley DR. Outer membrane c-type cytochromes required for Fe (III) and Mn (IV) oxide reduction in Geobacter sulfurreducens. Appl Environ Microbiol 2005; 71:8634-41; PMID:16332857; http://dx.doi.org/ 10.1128/AEM.71.12.8634-8641.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Afkar E, Reguera G, Schiffer M, Lovley DR. A novel Geobacteraceae-specific outer membrane protein J (OmpJ) is essential for electron transport to Fe (III) and Mn (IV) oxides in Geobacter sulfurreducens. BMC Microbiol 2005; 5:1; PMID:15649330; http://dx.doi.org/ 10.1186/1471-2180-5-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Heidelberg JF, Paulsen IT, Nelson KE, Gaidos EJ, Nelson WC, Read TD, Eisen JA, Seshadri R, Ward N, Methe B, et al.. Genome sequence of the dissimilatory metal ion–reducing bacterium Shewanella oneidensis. Nat Biotechnol 2002; 20:1118-23; PMID:12368813; http://dx.doi.org/ 10.1038/nbt749 [DOI] [PubMed] [Google Scholar]
- [32].Weber KA, Achenbach LA, Coates JD. Microorganisms pumping iron: anaerobic microbial iron oxidation and reduction. Nat Rev Microbiol 2006; 4:752-64; PMID:16980937; http://dx.doi.org/ 10.1038/nrmicro1490 [DOI] [PubMed] [Google Scholar]
- [33].Myers J, Myers C. Overlapping role of the outer membrane cytochromes of Shewanella oneidensis MR-1 in the reduction of manganese (IV) oxide. Lett Appl Microbiol 2003; 37:21-5; PMID:12803550; http://dx.doi.org/ 10.1046/j.1472-765X.2003.01338.x [DOI] [PubMed] [Google Scholar]
- [34].Bretschger O, Obraztsova A, Sturm CA, Chang IS, Gorby YA, Reed SB, Culley DE, Reardon CL, Barua S, Romine MF, et al.. Current production and metal oxide reduction by Shewanella oneidensis MR-1 wild type and mutants. Appl Environ Microbiol 2007; 73:7003-12; PMID:17644630; http://dx.doi.org/ 10.1128/AEM.01087-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kim B-C, Qian X, Leang C, Coppi MV, Lovley DR. Two putative c-type multiheme cytochromes required for the expression of OmcB, an outer membrane protein essential for optimal Fe (III) reduction in Geobacter sulfurreducens. J Bacteriol 2006; 188:3138-42; PMID:16585776; http://dx.doi.org/ 10.1128/JB.188.8.3138-3142.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kim B-C, Lovley DR. Investigation of direct vs. indirect involvement of the c-type cytochrome MacA in Fe (III) reduction by Geobacter sulfurreducens. FEMS Microbiol Lett 2008; 286:39-44; PMID:18616590; http://dx.doi.org/ 10.1111/j.1574-6968.2008.01252.x [DOI] [PubMed] [Google Scholar]
- [37].Kim B-C, Postier BL, DiDonato RJ, Chaudhuri SK, Nevin KP, Lovley DR. Insights into genes involved in electricity generation in Geobacter sulfurreducens via whole genome microarray analysis of the OmcF-deficient mutant. Bioelectrochemistry 2008; 73:70-5; PMID:18538641; http://dx.doi.org/ 10.1016/j.bioelechem.2008.04.023 [DOI] [PubMed] [Google Scholar]
- [38].Zheng T, Xu Y-S, Yong X-Y, Li B, Yin D, Cheng Q-W, Yuan HR, Yong YC. Endogenously enhanced biosurfactant production promotes electricity generation from microbial fuel cells. Bioresour Technol 2015; 197:416-21; PMID:26356112; http://dx.doi.org/ 10.1016/j.biortech.2015.08.136 [DOI] [PubMed] [Google Scholar]
- [39].Van Gennip M, Christensen LD, Alhede M, Phipps R, Jensen PO, Christophersen L, Pamp SJ, Moser C, Mikkelsen PJ, Koh AY, et al.. Inactivation of the rhlA gene in Pseudomonas aeruginosa prevents rhamnolipid production, disabling the protection against polymorphonuclear leukocytes. APMIS 2009; 117:537-46; PMID:19594494; http://dx.doi.org/ 10.1111/j.1600-0463.2009.02466.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Feissner RE, Richard-Fogal CL, Frawley ER, Loughman JA, Earley KW, Kranz RG. Recombinant cytochromes c biogenesis systems I and II and analysis of haem delivery pathways in Escherichia coli. Mol Microbiol 2006; 60:563-77; PMID:16629661; http://dx.doi.org/ 10.1111/j.1365-2958.2006.05132.x [DOI] [PubMed] [Google Scholar]
- [41].Shi L, Chen B, Wang Z, Elias DA, Mayer MU, Gorby YA, Ni S, Lower BH, Kennedy DW, Wunschel DS, et al.. Isolation of a high-affinity functional protein complex between OmcA and MtrC: two outer membrane decaheme c-type cytochromes of Shewanella oneidensis MR-1. J Bacteriol 2006; 188:4705-14; PMID:16788180; http://dx.doi.org/ 10.1128/JB.01966-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Shi L, Deng S, Marshall MJ, Wang Z, Kennedy DW, Dohnalkova AC, Mottaz HM, Hill EA, Gorby YA, Beliaev AS, et al.. Direct involvement of type II secretion system in extracellular translocation of Shewanella oneidensis outer membrane cytochromes MtrC and OmcA. J Bacteriol 2008; 190:5512-6; PMID:18502849; http://dx.doi.org/ 10.1128/JB.00514-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Xiao Y, Patolsky F, Katz E, Hainfeld JF, Willner I. “Plugging into enzymes”: Nanowiring of redox enzymes by a gold nanoparticle. Science 2003; 299:1877-81; PMID:12649477; http://dx.doi.org/ 10.1126/science.1080664 [DOI] [PubMed] [Google Scholar]
- [44].Gregg BA, Heller A. Redox polymer films containing enzymes. 2. Glucose oxidase containing enzyme electrodes. J Phys Chem 1991; 95:5976-80; http://dx.doi.org/ 10.1021/j100168a047 [DOI] [Google Scholar]
- [45].Lin Y, Lu F, Wang J. Disposable carbon nanotube modified screen-printed biosensor for amperometric detection of organophosphorus pesticides and nerve agents. Electroanalysis 2004; 16:145-9; http://dx.doi.org/ 10.1002/elan.200302933 [DOI] [Google Scholar]
- [46].Patolsky F, Weizmann Y, Willner I. Long-range electrical contacting of redox enzymes by SWCNT connectors. Angew Chem Int Ed 2004; 43:2113-7; PMID:15083459; http://dx.doi.org/ 10.1002/anie.200353275 [DOI] [PubMed] [Google Scholar]
- [47].Pardo-Yissar V, Katz E, Willner I, Kotlyar AB, Sanders C, Lill H. Biomaterial engineered electrodes for bioelectronics. Faraday Discuss 2000; 116:119-34; http://dx.doi.org/ 10.1039/b001508n [DOI] [PubMed] [Google Scholar]
- [48].Du L, Jiang H, Liu X, Wang E. Biosynthesis of gold nanoparticles assisted by Escherichia coli DH5α and its application on direct electrochemistry of hemoglobin. Electrochem Commun 2007; 9:1165-70; http://dx.doi.org/ 10.1016/j.elecom.2007.01.007 [DOI] [Google Scholar]
- [49].Berry V, Gole A, Kundu S, Murphy CJ, Saraf RF. Deposition of CTAB-terminated nanorods on bacteria to form highly conducting hybrid systems. J Am Chem Soc 2005; 127:17600-1; PMID:16351078; http://dx.doi.org/ 10.1021/ja056428l [DOI] [PubMed] [Google Scholar]
- [50].He Y, Yuan J, Su F, Xing X, Shi G. Bacillus subtilis assisted assembly of gold nanoparticles into long conductive nodous ribbons. J Phys Chem B 2006; 110:17813-8; PMID:16956267; http://dx.doi.org/ 10.1021/jp063729o [DOI] [PubMed] [Google Scholar]
- [51].Holmes DE, Chaudhuri SK, Nevin KP, Mehta T, Methé BA, Liu A, Ward JE, Woodard TL, Webster J, Lovley DR. Microarray and genetic analysis of electron transfer to electrodes in Geobacter sulfurreducens. Environ Microbiol 2006; 8:1805-15; PMID:16958761; http://dx.doi.org/ 10.1111/j.1462-2920.2006.01065.x [DOI] [PubMed] [Google Scholar]
- [52].Mavrodi DV, Bonsall RF, Delaney SM, Soule MJ, Phillips G, Thomashow LS. Functional analysis of genes for biosynthesis of pyocyanin and phenazine-1-carboxamide from Pseudomonas aeruginosa PAO1. J Bacteriol 2001; 183:6454-65; PMID:11591691; http://dx.doi.org/ 10.1128/JB.183.21.6454-6465.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Hedegaard L, Klemm P. Type 1 fimbriae of Escherichia coli as carriers of heterologous antigenic sequences. Gene 1989; 85:115-24; PMID:2576014; http://dx.doi.org/ 10.1016/0378-1119(89)90471-X [DOI] [PubMed] [Google Scholar]
- [54].Gibney BR, Dutton PL. De novo design and synthesis of heme proteins. Adv Inorg Chem 2000; 51:409-56; http://dx.doi.org/ 10.1016/S0898-8838(00)51008-3 [DOI] [Google Scholar]
- [55].Georgiou G, Stathopoulos C, Daugherty PS, Nayak AR, Iverson BL, Curtiss III R. Display of heterologous proteins on the surface of microorganisms: from the screening of combinatorial libraries to live recombinant vaccines. Nat Biotechnol 1997; 15:29-34; PMID:9035102; http://dx.doi.org/ 10.1038/nbt0197-29 [DOI] [PubMed] [Google Scholar]
- [56].Samuelson P, Wernérus H, Svedberg M, Ståhl S. Staphylococcal surface display of metal-binding polyhistidyl peptides. Appl Environ Microbiol 2000; 66:1243-8; PMID:10698802; http://dx.doi.org/ 10.1128/AEM.66.3.1243-1248.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Dhillon J, Drew P, Porter A. Bacterial surface display of an anti-pollutant antibody fragment. Lett Appl Microbiol 1999; 28:350-4; PMID:10347888; http://dx.doi.org/ 10.1046/j.1365-2672.1999.00552.x [DOI] [PubMed] [Google Scholar]
- [58].Fishilevich S, Amir L, Fridman Y, Aharoni A, Alfonta L. Surface display of redox enzymes in microbial fuel cells. J Am Chem Soc 2009; 131:12052-3; PMID:19663383; http://dx.doi.org/ 10.1021/ja9042017 [DOI] [PubMed] [Google Scholar]
- [59].Pepper LR, Cho YK, Boder ET, Shusta EV. A decade of yeast surface display technology: where are we now? Comb Chem High Throughput Screen 2008; 11:127; PMID:18336206; http://dx.doi.org/ 10.2174/138620708783744516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Chao G, Lau WL, Hackel BJ, Sazinsky SL, Lippow SM, Wittrup KD. Isolating and engineering human antibodies using yeast surface display. Nat Protoc 2006; 1:755-68; PMID:17406305; http://dx.doi.org/ 10.1038/nprot.2006.94 [DOI] [PubMed] [Google Scholar]
- [61].Wang L, Brock A, Herberich B, Schultz PG. Expanding the genetic code of Escherichia coli. Science 2001; 292:498-500; PMID:11313494; http://dx.doi.org/ 10.1126/science.1060077 [DOI] [PubMed] [Google Scholar]
- [62].Chin JW, Cropp TA, Anderson JC, Mukherji M, Zhang Z, Schultz PG. An expanded eukaryotic genetic code. Science 2003; 301:964-7; PMID:12920298; http://dx.doi.org/ 10.1126/science.1084772 [DOI] [PubMed] [Google Scholar]
- [63].Zhang Z, Alfonta L, Tian F, Bursulaya B, Uryu S, King DS, Schultz PG. Selective incorporation of 5-hydroxytryptophan into proteins in mammalian cells. Proc Natl Acad Sci U S A 2004; 101:8882-7; PMID:15187228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Seyedsayamdost MR, Xie J, Chan CT, Schultz PG, Stubbe J. Site-specific insertion of 3-aminotyrosine into subunit α2 of E. coli ribonucleotide reductase: direct evidence for involvement of Y730 and Y731 in radical propagation. J Am Chem Soc 2007; 129:15060-71; PMID:17990884; http://dx.doi.org/ 10.1021/ja076043y [DOI] [PubMed] [Google Scholar]
- [65].Wei J, Liang P, Huang X. Recent progress in electrodes for microbial fuel cells. Bioresour Technol 2011; 102:9335-44; PMID:21855328; http://dx.doi.org/ 10.1016/j.biortech.2011.07.019 [DOI] [PubMed] [Google Scholar]
- [66].Mohan SV, Raghavulu SV, Sarma P. Influence of anodic biofilm growth on bioelectricity production in single chambered mediatorless microbial fuel cell using mixed anaerobic consortia. Biosen Bioelectron 2008; 24:41-7; PMID:18440217; http://dx.doi.org/ 10.1016/j.bios.2008.03.010 [DOI] [PubMed] [Google Scholar]
- [67].Wang X, Cheng S, Feng Y, Merrill MD, Saito T, Logan BE. Use of carbon mesh anodes and the effect of different pretreatment methods on power production in microbial fuel cells. Environ Sci Technol 2009; 43:6870-4; PMID:19764262; http://dx.doi.org/ 10.1021/es900997w [DOI] [PubMed] [Google Scholar]
- [68].Cheng S, Logan BE. Ammonia treatment of carbon cloth anodes to enhance power generation of microbial fuel cells. Electrochem Commun 2007; 9:492-6; http://dx.doi.org/ 10.1016/j.elecom.2006.10.023 [DOI] [Google Scholar]
- [69].Feng Y, Yang Q, Wang X, Logan BE. Treatment of carbon fiber brush anodes for improving power generation in air–cathode microbial fuel cells. J Power Sources 2010; 195:1841-4; http://dx.doi.org/ 10.1016/j.jpowsour.2009.10.030 [DOI] [Google Scholar]
- [70].Zhu N, Chen X, Zhang T, Wu P, Li P, Wu J. Improved performance of membrane free single-chamber air-cathode microbial fuel cells with nitric acid and ethylenediamine surface modified activated carbon fiber felt anodes. Bioresour Technol 2011; 102:422-6; PMID:20594833; http://dx.doi.org/ 10.1016/j.biortech.2010.06.046 [DOI] [PubMed] [Google Scholar]
- [71].Logan B, Cheng S, Watson V, Estadt G. Graphite fiber brush anodes for increased power production in air-cathode microbial fuel cells. Environ Sci Technol 2007; 41:3341-6; PMID:17539547; http://dx.doi.org/ 10.1021/es062644y [DOI] [PubMed] [Google Scholar]
- [72].Qiao Y, Bao S-J, Li CM, Cui X-Q, Lu Z-S, Guo J. Nanostructured polyaniline/titanium dioxide composite anode for microbial fuel cells. Acs Nano 2007; 2:113-9; http://dx.doi.org/ 10.1021/nn700102s [DOI] [PubMed] [Google Scholar]
- [73].Logan BE. Scaling up microbial fuel cells and other bioelectrochemical systems. Appl Microbiol Biotechnol 2010; 85:1665-71; PMID:20013119; http://dx.doi.org/ 10.1007/s00253-009-2378-9 [DOI] [PubMed] [Google Scholar]
- [74].Nam J-Y, Kim H-W, Lim K-H, Shin H-S, Logan BE. Variation of power generation at different buffer types and conductivities in single chamber microbial fuel cells. Biosens Bioelectron 2010; 25:1155-9; PMID:19896357; http://dx.doi.org/ 10.1016/j.bios.2009.10.005 [DOI] [PubMed] [Google Scholar]
- [75].Lanas V, Logan BE. Evaluation of multi-brush anode systems in microbial fuel cells. Bioresour Technol 2013; 148:379-85; PMID:24063821; http://dx.doi.org/ 10.1016/j.biortech.2013.08.154 [DOI] [PubMed] [Google Scholar]
- [76].Bayatsarmadi B, Peters A, Talemi P. Catalytic polymeric electrodes for direct borohydride fuel cells. J Power Sources 2016; 322:26-30; http://dx.doi.org/ 10.1016/j.jpowsour.2016.04.137 [DOI] [Google Scholar]
- [77].Thepsuparungsikul N, Phonthamachai N, Ng HY. Multi-walled carbon nanotubes as electrode material for microbial fuel cells. Water Sci Technol 2012; 65:1208-14; PMID:22437017; http://dx.doi.org/ 10.2166/wst.2012.956 [DOI] [PubMed] [Google Scholar]
- [78].Kong X, Zhou X, Tian Y, Wu X, Zhang J, Zuo W. Niobium doped lanthanum calcium ferrite perovskite as a novel electrode material for symmetrical solid oxide fuel cells. J Power Sources 2016; 326:35-42; http://dx.doi.org/ 10.1016/j.jpowsour.2016.06.111 [DOI] [Google Scholar]


